Volume 15, Issue 5 (September & October 2024)
BCN 2024, 15(5): 659-670 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Naderi Tehrani M, Hamidi G A, Heydari A, Nasrollahi S, Aghighi F, Salami M. Effect of Acute Administration of Caffeine on Neuropathic Pain and the Role of Nitric Oxide Pathway in an Animal Model of Chronic Constriction Injury. BCN 2024; 15 (5) :659-670
URL: http://bcn.iums.ac.ir/article-1-2701-en.html
URL: http://bcn.iums.ac.ir/article-1-2701-en.html
Monireh Naderi Tehrani1 

 , Gholam Ali Hamidi1
, Gholam Ali Hamidi1 

 , Azhdar Heydari1
, Azhdar Heydari1 

 , Saeedeh Nasrollahi1
, Saeedeh Nasrollahi1 

 , Fatemeh Aghighi1
, Fatemeh Aghighi1 

 , Mahmoud Salami *1
, Mahmoud Salami *1 




 , Gholam Ali Hamidi1
, Gholam Ali Hamidi1 

 , Azhdar Heydari1
, Azhdar Heydari1 

 , Saeedeh Nasrollahi1
, Saeedeh Nasrollahi1 

 , Fatemeh Aghighi1
, Fatemeh Aghighi1 

 , Mahmoud Salami *1
, Mahmoud Salami *1 


1- Physiology Research Center, Institute for Basic Sciences, Kashan University of Medical Sciences, Kashan, Iran.
Keywords: Neuropathic pain, Caffeine, L-NAME, L-Arginine, Nitric oxide (NO), Adenosine receptors (ARs)
Full-Text [PDF 997 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Neuropathic pain (NP) is a chronic disease that results from damage to the peripheral and central nervous system (Jensen et al., 2011). It can cause complex changes in the cognitive and emotional functions (Jensen et al., 2011). NP is characterized by allodynia, pain caused by an innocuous stimulus, and hyperalgesia, an exaggerated response to a painful stimulus (Treede et al., 2008). Tumors, metabolic disorders, viral infections, and injury to the peripheral or central nervous system are considered causes of NP (Cervero et al., 2003). The main drug treatments for NP include opioids, anticonvulsants, antidepressants, and topical agents (Attal, 2012). However, treatments have adverse side effects, including tolerance and physical dependence without complete pain relief (Grzanna et al., 2005). Therefore, further pharmacological interventions are necessary to relieve these side effects.
Caffeine is related to the purine alkaloid family and is the main active constituent in tea, coffee, and energy drinks (Nieber, 2017). Due to its mild stimulant effect, caffeine is the most widely consumed psychoactive substance in Western countries (Fredholm et al., 1999). Evidence suggests that some doses of caffeine have antinociceptive effects (Person et al., 1985; Wu, et al., 2006). Also, one of the non-selective antagonists of adenosine receptors (ARs) is caffeine (Tchekalarova et al., 2010). Adenosine has four G protein-coupled receptors: A2B, A1, A2A, and A3 (McGaraughty et al., 2005). The antinociceptive effect of caffeine is probably due to antagonism of the A2A receptors (Wu, et al., 2006; Zhang, 2001). Nitric oxide (NO) is synthesized from L-arginine by different isoforms of NO synthase (NOS) (Akula et al., 2008). NOS displays three isoforms: endothelial, neuronal, and inducible isoforms (Garthwaite, 2008). The finding suggests that NO plays a complex role in pain modulation (Basbaum et al., 2009; Schaible & Richter, 2004). On the other hand, it is known that caffeine can modulate NO production (Kayir & Uzbay, 2004; López-Muñoz et al., 1996). Whereas activation of the A2A receptors stimulates NO production, activation of the A1 receptor decreases NO production. It is suggested that the effects of caffeine on NO synthesis may be due to antagonism of the ARs (Bruce et al., 2002). However, it seems that the action mechanism of caffeine is not only through antagonism with ARs, but some other mechanisms like the NO-cGMP pathway can be involved too (Kayir & Uzbay, 2004; Orrú et al., 2013). Therefore, our study investigated the effects of acute caffeine administration on the NP threshold. The rule of the NO pathway was also considered.
2. Materials and Methods
Animals
A total of 88 male Wistar rats weighing 220–250 g were used in this study. Three or four rats were placed in every cage and kept at a 12 h light/dark cycle at a temperature of 22±2 °C and humidity-controlled of 55±5%. Water and food were available ad libitum. All experiments were carried out in accordance with Directive 2010/63/EU on the protection of animals used for scientific purposes and approved by the Ethics Committee of Kashan University of Medical Sciences.
Drugs
Caffeine, L-NAME, and L-arginine were purchased from Sigma-Aldrich Company. The drugs were diluted with normal saline and administered intraperitoneally.
Neuropathic pain model
We used the animal model of chronic constriction injury (CCI) described previously (Bennett & Xie, 1988). The subjects were anesthetized with a mixture of xylazine (10 mg/kg) and ketamine (50 mg/kg) (Verdi et al., 2013). The common portion of the left sciatic nerve was exposed and separated from the adjacent connective tissue. Four ligatures (4-0 intestinal chrome) were loosely tied around the nerve so as not to interrupt blood circulation through the superficial epineural vessels. After surgery, every rat was separately placed in a cage (Verdi et al., 2013).
Animal groups
The study protocol included 11 groups of rats comprising 8 animals in each group. Three control groups have entered the study. One group served as age-matched non-ligation control (CON). Two control groups of rats were subjected to the sciatic nerve ligation with (CCI+saline) or without (CCI) saline administration. The sham group (sham) experienced similar surgery, except that the left sciatic nerve was exposed with no ligation. Three groups of CCI animals received 10 (Caf.10), 50 (Caf.50), and 100 (Caf.100) mg/kg caffeine; two groups of rats were administered 100 mg/kg L-arginine (L-ARG) and 30 mg/kg L-NAME (L-NAME).
Also, two groups of animals were pre-treated with 30 mg/kg L-NAME (L-NAME+Caf) and 100 mg/kg L-arginine (L-ARG+Caf) 30 minutes before administration of the effective dose of caffeine (50 mg/kg) (Pottabathini et al., 2015). The same groups of animals were introduced to all tests.
Allodynia and hyperalgesia
The mechanical and cold stimulations were applied with von Frey filaments and acetone, respectively. Radiant heat was used as thermal stimulation to induce a hyperalgesia test. The medial plantar surface of the left hind paw was used to apply the stimulations.
Experimental design
The animals were introduced to the behavioral tests 4, 7, 14, 21, and 28 days after surgery. The subjects got used to the test environment at least 15 minutes before beginning the test (Hamidi et al., 2006).
Assessment of heat hyperalgesia
A plantar test device was used to evaluate paw withdrawal delay in response to radiant heat (Ugo Basile, Varese, Italy). As a heat source, an infrared light was transmitted from under the mid-plantar surface of the left hind paw. The delay (seconds) between the onset of the thermal stimulus and paw withdrawal was considered thermal withdrawal latency (Banafshe et al., 2012; Bennett & Xie, 1988). A 22-second cut-off time was used to avoid any damage to the tissue. Each trial was performed alternately three times with an interval of 5-10 minutes to prevent sensitization for the injured and non-injured paw in the control group (Banafshe et al., 2012).
Assessment of mechanical allodynia
Mechanical allodynia was evaluated as described previously (Chen et al., 2018; Mohammadifar et al., 2021). The effects of weak stimulation of von Frey fibers (force ranging from less than 2 to 60 g) were studied to assess the involvement of low-threshold fibers in nociceptive behavior before and after CCI. These stimulations are considered harmless because they usually provoke activity in low-threshold mechanoreceptors. The rats were placed on a mesh floor (0.8×0.8 cm cell), shielded by a transparent plastic box (18×18×25 cm), and allowed to explore for 15 minutes or when exploratory behavior finished. Sequences of von Frey fiber stimulation were applied in a rising order of forces to the hind paw plantar surface. The von Frey filament stimulation was applied in three consecutive trials, pressing down on the hind paw until the animal withdrew its paw or the filament bent. Paw lifting was ignored due to natural locomotor behavior. The withdrawal threshold was calculated for the smallest fiber size that induced at least two withdrawal responses through three consecutive trials using the same filament. Each stimulation was applied for approximately 1 s with an interstimulus interval of 5 s.
Assessment of cold allodynia (acetone test)
The modified acetone spray test was used for cold allodynia assessment (Yoon et al., 1994). While the rats stood on the perforated floor, 250 μL of acetone was sprayed onto the plantar skin using a smooth needle attached to a syringe. The trial was repeated 5 times (with an interval of 3 minutes) to the left paw. Withdrawal frequency is determined as a percentage (number of withdrawals/number of trials ×100) (Banafshe et al., 2014; Seltzer et al., 1990).
Statistical analysis
The obtained data were analyzed by a 2-way analysis of variance (ANOVA). The Tukey test was applied as post hoc. Between-group differences were considered significant with a P<0.05. The data are presented as Mean±SEM.
3. Results
Heat hyperalgesia in the CCI group
With the application of the radiant heat on the hind paw, the animals lifted their feet and showed aversive behaviors such as shaking, trapping, or licking the affected paw. Figure 1A reports the heat hyperalgesia after CCI injury in the control groups. Statistical analysis indicated a significant difference between the testing groups (F(3, 28)=99, P=0.0001). The sham group displayed an analogous withdrawal latency with the CON animals. Also, no significant difference was evident between the behavior of the CCI and CCI+saline groups. The CCI group significantly decreased the withdrawal latency in response to heat stimulation on days 4, 7, 21, 28 (P<0.001), and 14 (P<0.01), compared to the sham group.
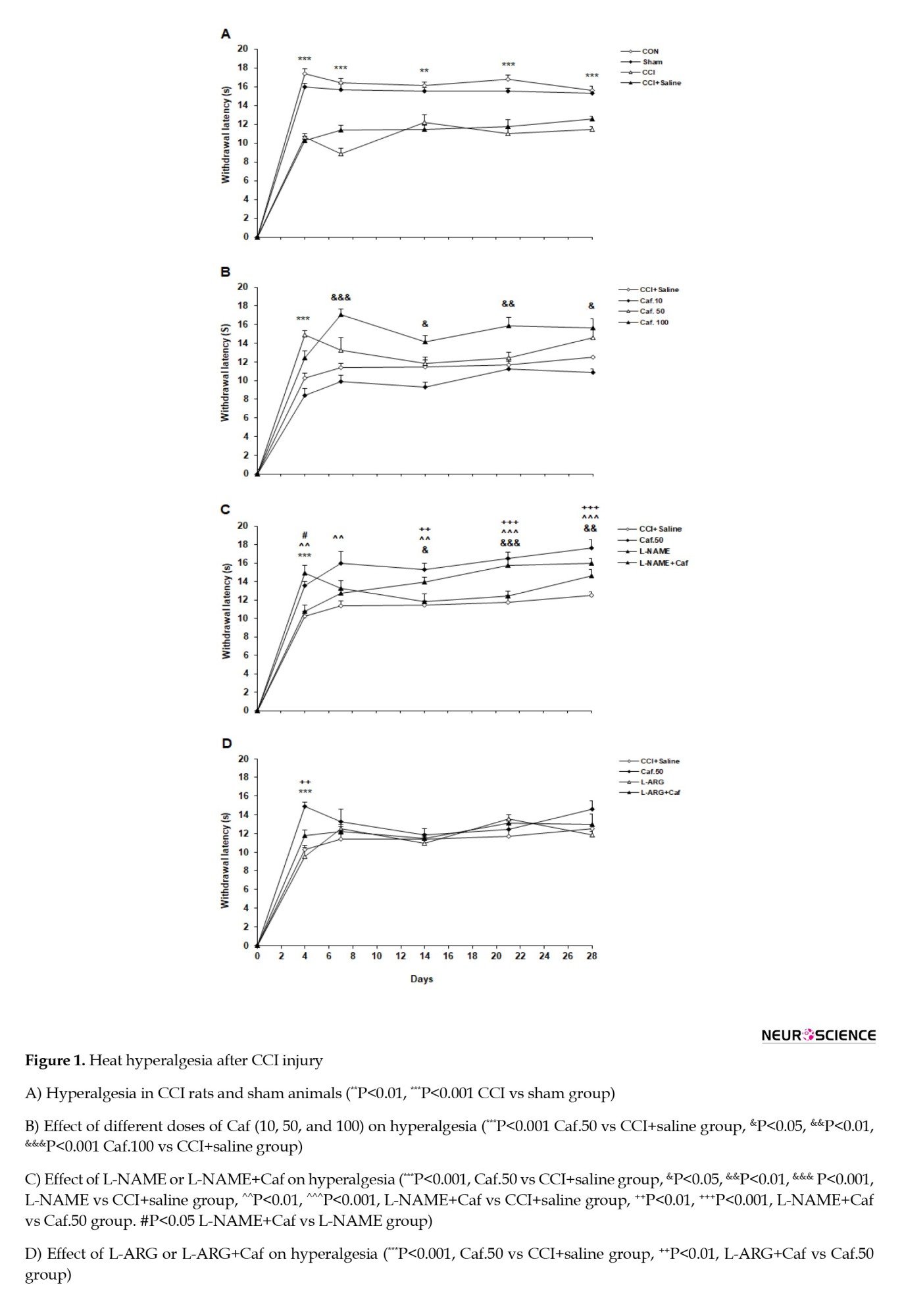
The effect of caffeine on the heat hyperalgesia
A considerable difference was found between the caffeine and vehicle-treated groups (F(3, 28)=52.818, P=0.0001). The Caf.10 group resembled the CCI+saline group in the heat hyperalgesia test. The withdrawal latency increased at day 4 in the Caf.50 rats (P<0.001). The maximum effect of caffeine was observed in the dose of 100 mg/kg, where the Caf.100 group showed a considerably increased withdrawal latency on days 7 (P<0.001), 14, 28 (P<0.05), and 21 (P<0.01) compared to the CCI+saline group. We selected the dose of 50 mg/kg as the minimum effective concentration of caffeine to investigate the possible role of the NO-cGMP signaling pathway. Figure 1B illustrates the acute administration of caffeine on the pain threshold in heat hyperalgesia.
The effect of NO-cGMP signaling pathway on heat hyperalgesia
General statistics indicated a possible involvement of the NO-cGMP signaling pathway in the heat hyperalgesia test (F(3, 28)=35.78, P=0.0001). Treatment with L-NAME significantly attenuated hyperalgesia on days 14 (P<0.05), 21 (P<0.001), and 28 (P<0.01) in the L-NAME group compared to the CCI+saline group. Furthermore, L-NAME+Caf significantly reduced hyperalgesia on days 4 (P<0.01), 7 (P<0.01), 14 (P<0.01), 21, and 28 (P<0.001). Moreover, compared with the Caf.50 group, the L-NAME+Caf group reduced the heat hyperalgesia on days 14 (P<0.01), 21, and 28 (P<0.001). A significant variation was also found between the L-NAME+Caf and the L-NAME groups on day 4 (P<0.05). Figure 1C shows the effect of L-NAME or L-NAME+Caf on heat hyperalgesia. The data analysis showed that L-arginine or L-arginine+caffeine significantly influenced the heat hyperalgesia threshold (F(3, 28)=26.312, P<0.001, Figure 1D). The animals treated with L-ARG or L-ARG+Caf showed no significant difference in paw withdrawal latency compared to the CCI+saline group. The L-ARG+Caf group showed significantly reduced withdrawal latency on day 4 (P<0.01) compared to the Caf.50 group. L-ARG+Caf revealed no significant difference with the L-ARG group.
Mechanical allodynia
Before surgery, the subjects rarely responded, even to the intense von Frey filament. Still, after the CCI procedure, the ipsilateral hind paw was sensitive to mechanical stimuli even with weak stimulations.
The statistical analysis showed a substantial variation between the testing groups (F(3, 28)=11.96, P=0.0001). The CON and sham groups showed an analogous pattern of behavior in the mechanical allodynia test. The response to the mechanical stimulus was markedly increased in the CCI compared to sham rats on days 21 (P<0.01) and 28 (P<0.01). The CCI and CCI+saline groups also showed a similar response to this test. Figure 2A compares the results of the mechanical allodynia test in different groups.
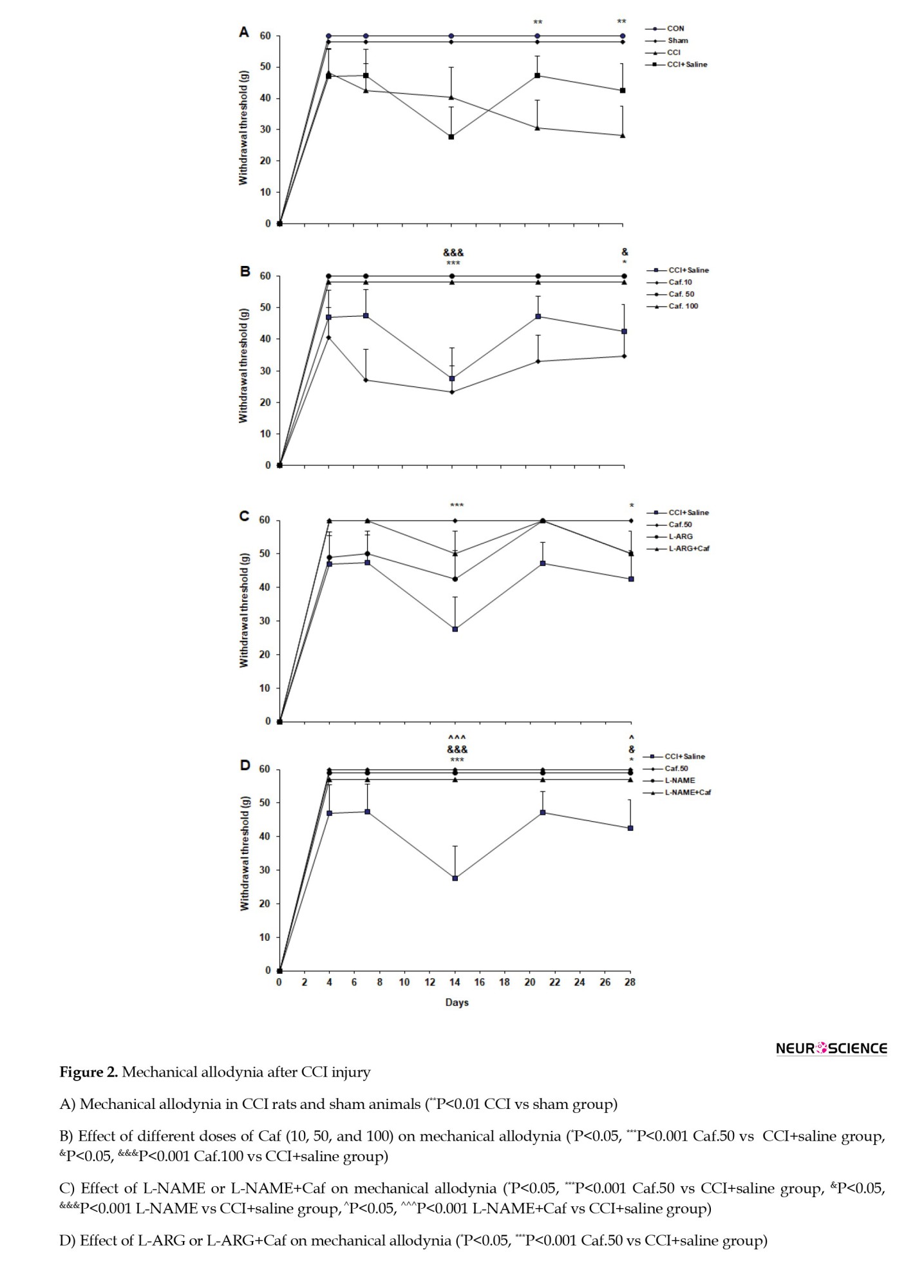
The effect of caffeine on the mechanical allodynia
A generally significant difference was evident between the caffeine-administered groups’ responses to the mechanical allodynia test (F(3, 28)=11, P=0.0001). The Caf.10 group showed a negligible difference from the CCI+saline group in the withdrawal threshold. However, the withdrawal threshold was increased in the Caf.50 and Caf.100 groups on days 14 (P<0.001) and 28 (P<0.05), compared with the CCI+saline group (Figure 2B).
The effect of NO-cGMP signaling pathway on the mechanical allodynia
Analysis of variance indicated a significant behavioral variation when applying L-NAME (F(3, 28)=10.09, P=0.0001). The L-NAME and L-NAME+Caf groups significantly attenuated the response to the allodynia test on days 14 (P<0.001) and 28 (P<0.05) in comparison to the CCI+saline group. However, the L-NAME+Caf group showed no difference in the paw withdrawal threshold with both L-NAME and Caf.50 rats (Figure 2C).
Statistics showed that L-arginine does not considerably influence the mechanical allodynia (F(3, 28)=4.08, P=0.03).
The animals treated with L-ARG or L-ARG+Caf did not show a significant difference in paw withdrawal threshold compared to the CCI+saline group. Moreover, there was no significant difference between the L-ARG+Caf and L-ARG and Caf.50 groups (Figure 2D).
Cold allodynia
While the animals were not responsive to the acetone application before surgery, the ipsilateral hind paw showed a high sensitivity to the acetone test after the CCI procedure. The acetone application caused rats to rapidly withdraw the affected foot (with a delay of about 0.2–0.3 s) and then shake, trap, or lick it.
Statistical analysis showed a significant difference between the groups testing for the cold allodynia (F(3, 28)=151.6, P=0.0001). The results indicate that the difference in allodynia between the CON and sham groups is not significant. However, an increased withdrawal frequency was observed in the CCI compared to sham groups on testing days 4, 7, 14, and 21 (P<0.001). The CCI and CCI+saline groups similarly behaved in the cold allodynia test. Figure 3A illustrates the cold allodynia in the control groups.
The effect of caffeine on the cold allodynia
The data analysis showed a substantial variation between different groups under caffeine treatment (F(3, 28)=35.78, P=0.0001). The Caf.10 rats increased the withdrawal frequency on days 14 (P<0.05), 21 (P<0.001), and 28 (P<0.01), compared with the CCI+saline group. However, the other caffeine-treated Caf.50 and Caf.100 groups responded similarly to the cold allodynia, as did the CCI+saline group (Figure 3B).
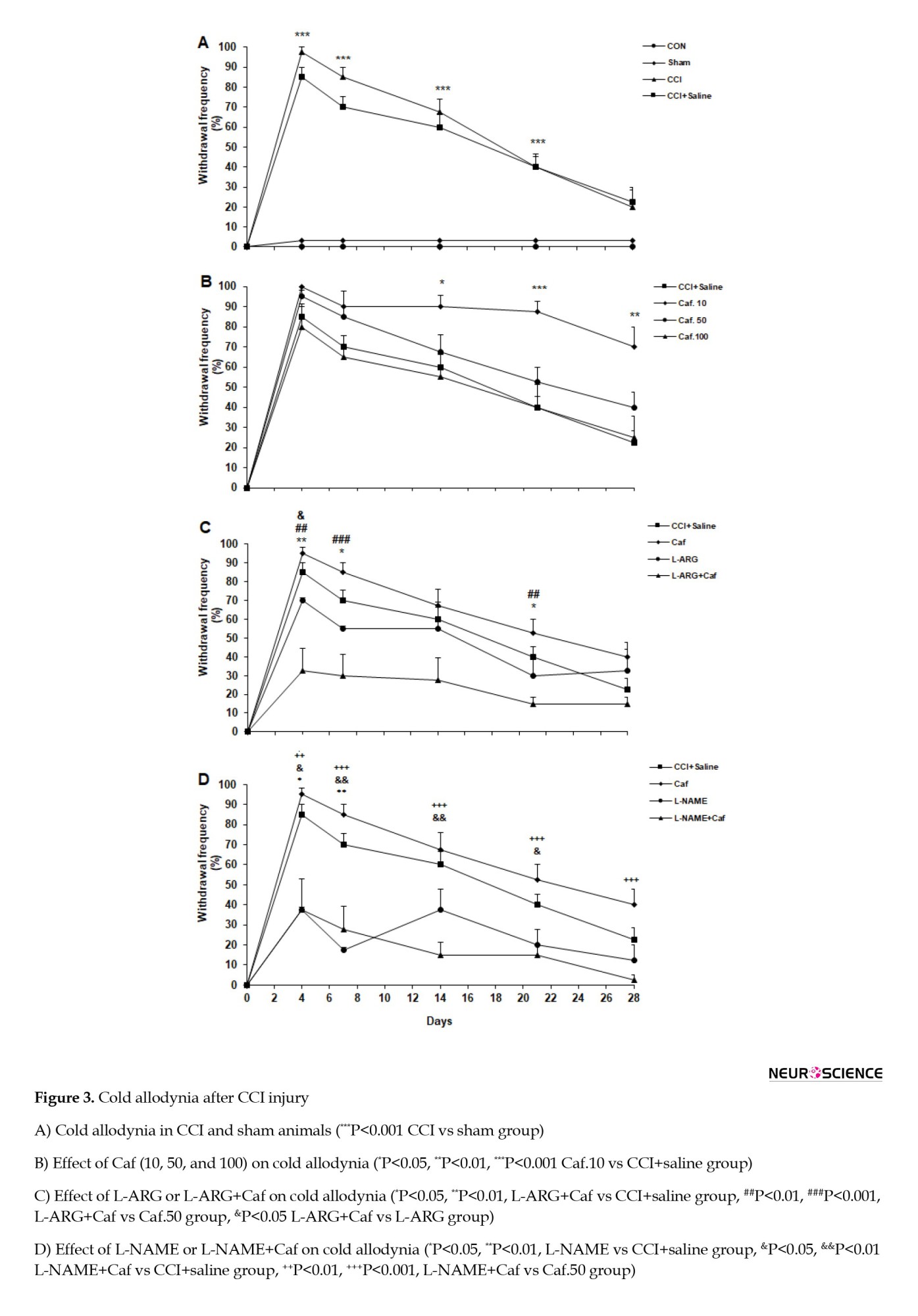
The effect of NO-cGMP signaling pathway on the cold allodynia
The statistical evaluation showed the effect of L-ARG or L-ARG+Caf on cold allodynia. Analysis of variance showed that L-arginine administration significantly underlies the withdrawal frequency in the cold allodynia testing (F(3, 28)=10.08, P=0.0001). The L-ARG group showed no significant difference in withdrawal frequency compared to the CCI+saline group. The L-ARG+Caf animals noticeably reduced the withdrawal frequency on days 4 (P<0.01), 7 (P<0.05), and 21 (P<0.05) compared to the CCI+saline group. The L-ARG+Caf rats appeared to have a significant reduction in the withdrawal frequency on days 4 (P<0.01), 7 (P<0.001), and 21 (P<0.01) compared to the Caf.50 group. Also, the L-ARG+Caf animals displayed a marked reduced withdrawal frequency on day 4 (P<0.05) compared to the L-ARG group (Figure 3C).
We found that the L-NAME application effectively affected the mechanical allodynia (F(3, 28)=20.02, P=0.0001). Treatment with L-NAME significantly attenuated the withdrawal frequency on days 4 (P<0.05) and 7 (P<0.01) in the L-NAME compared to the CCI+saline group. Also, the L-NAME+Caf group significantly reduced the withdrawal frequency on days 4 (P<0.05), 7 (P<0.01), 14 (P<0.01), and 21 (P<0.05) compared to the CCI+saline group. Moreover, L-NAME+Caf significantly reduced allodynia on days 4 and 21 (P<0.01), 7, 14, and 28 (P<0.001) compared with the Caf.50 group (Figure 3D).
4. Discussion
The finding of this study indicated that caffeine at a dose of 10 displays a pronociceptive effect. Conversely, the doses of 50 and 100 of caffeine show antinociceptive effects. Whereas L-NAME alone or pre-treated with caffeine has an antinociceptive impact, L-ARG pre-treatment with caffeine increased heat hyperalgesia and decreased cold allodynia.
Caffeine is an antagonist of ARs, and its main targets are A1 and A2A subtype receptors (Carrillo & Benitez, 2000). Adenosine displays its analgesic role by activating A1 receptors in the nervous system. On the other hand, the A2A receptor shows both pronociceptive and antinociceptive effects (Sawynok, 2016; Vincenzi et al., 2020; Yamamoto et al., 2003; Zahn et al., 2007). A1 receptors are found on peripheral sensory nerve endings in the spinal cord’s dorsal horn and supraspinal structures involved in pain processing (Vincenzi et al., 2020). A2 receptors are located on inflammatory and immune cells and are considered targets for inflammatory and immune conditions (Sawynok, 2016). In the CNS, they are expressed in pre- and post-synaptic neurons in the brain (Antonioli et al., 2014; Popoli & Pepponi, 2012). The results of this study show that the lowest dose of caffeine had a pronociceptive effect and increased the cold allodynia. Consistent with our findings, it is shown that 1 to 10 mg/kg of caffeine increases hyperalgesia and allodynia (Esser & Sawynok, 2000; Wu, et al., 2006). Moreover, heat hyperalgesia was exacerbated in the A1 receptors knockout mice (Wu et al., 2005). The hyperalgesic effects of the minimum dose of caffeine may be due to the antagonizing effect on A1 receptors (Sawynok et al., 2008; Sawynok et al., 2010). We showed that acute caffeine administration at 50 and 100 mg/kg doses reduces heat hyperalgesia and mechanical allodynia. Numerous studies confirm our results in that some doses of caffeine show antinociceptive effects (López et al., 2006; Shapiro, 2008; Wu, et al., 2006). Blockade of A2A receptors is one of the proposed mechanisms of analgesic effects of caffeine. Consistently, hypoalgesia was reported in mice lacking the A2A receptors (Ledent et al., 1997). Both central and peripheral A2A receptors are involved in pain facilitation and play a crucial pronociceptive role (Sawynok, 2016). It has been proposed that caffeine at a 100 mg/kg dose has a more inhibitory effect on A2A receptors. In this context, caffeine revealed a dose-dependent anti-hyperalgesia, and the maximal effect was achieved at a dose of 100 mg/kg. Overall, our findings suggest that the effect of caffeine on hyperalgesia is partly due to its dose-dependent effect on A1 receptors or A2A receptors (Sawynok et al., 2010; Wu, et al., 2006). Caffeine can modulate pain mainly through antagonism of ARs (Davis & Green, 2009; Massey et al., 1994; Ribeiro & Sebastiao, 2010). Activation of A2A receptors leads to increased NO production; therefore, high-dose caffeine’s antagonizing effect (50 and 100 mg/kg) may lead to decreased NO production (Esmaili & Heydari, 2019). Some evidence indicates that NO is involved in the development of N (Chauhan et al., 2018; Miller et al., 2014; Zhao et al., 2018). To further investigate the role of the NO-cGMP pathway, we applied L-arginine as NO releasers and L-NAME (non-selective NOS inhibitor). The administration of L-NAME before caffeine decreased susceptibilities to heat hyperalgesia and cold and mechanical allodynia. Also, L-arginine administration before caffeine increased heat hyperalgesia. These findings suggest that the pain attenuation effects of L-NAME might be mediated through an inhibitory effect on NOS isoforms and, subsequently, NO production. Another proposed mechanism to explain the antihyperalgesia effect of caffeine is the reduction of cyclooxygenase (COX-2) and prostaglandin E (PGE2). In this context, caffeine inhibits the synthesis of PGE2 and COX-2 in rat microglial cells (Fiebich et al., 2000). In addition, caffeine potentiates the antihyperalgesic effects of nonsteroidal anti-inflammatory drugs (NSAIDs) (Abou-Atme etal., 2019). Hyperalgesia is mainly associated with increased expression of COX-2 and local production of PGE2 (Li et al., 2018). The inhibitory effect of caffeine on COX-2 and reduced synthesis of PGE2 may contribute to the antihyperalgesic effect of caffeine (Fiebich et al., 2000). Interestingly, there is an interaction between NO and PGE2, so NO increases the production of PGE2 (Ilari et al., 2020).
5. Conclusion
In conclusion, our findings suggest that decreased NO production in the presence of caffeine may contribute to reducing PGE2 and subsequent antihyperalgesia. The NO-cGMP pathway’s involvement in modifying pain threshold might be a possible mechanism for caffeine. Elucidating the cause of the opposite effects of different doses of caffeine on pain requires further research.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Kashan University of Medical Sciences (KAUMS), Kahsan, Iran (Code: I.R.KAUMS.MEDNT.REC.1397.040).
Funding
The Deputy of Research of Kashan University of Medical Sciences (KAUMS) supported this study by a grant to Gholam Ali Hamidi (Grant No.: 97065).
Authors' contributions
Conceptualization and methodology: Azhdar Heydari; Supervision and funding administration: Gholam Ali Hamidi; Investigation: Monireh Naderi Tehrani; Data collection: Monireh Naderi Tehrani and Saeedeh Nasrollahi; Data analysis: Fatemeh Aghighi and Mahmoud Salami; Writing the original draft: Monireh Naderi Tehrani; Review and editing: Mahmoud Salami and Fatemeh Aghighi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors highly appreciate the Deputy of Research of Kashan University of Medical Sciences (KAUMS), Kahsan, Iran, for financial support.
References
Neuropathic pain (NP) is a chronic disease that results from damage to the peripheral and central nervous system (Jensen et al., 2011). It can cause complex changes in the cognitive and emotional functions (Jensen et al., 2011). NP is characterized by allodynia, pain caused by an innocuous stimulus, and hyperalgesia, an exaggerated response to a painful stimulus (Treede et al., 2008). Tumors, metabolic disorders, viral infections, and injury to the peripheral or central nervous system are considered causes of NP (Cervero et al., 2003). The main drug treatments for NP include opioids, anticonvulsants, antidepressants, and topical agents (Attal, 2012). However, treatments have adverse side effects, including tolerance and physical dependence without complete pain relief (Grzanna et al., 2005). Therefore, further pharmacological interventions are necessary to relieve these side effects.
Caffeine is related to the purine alkaloid family and is the main active constituent in tea, coffee, and energy drinks (Nieber, 2017). Due to its mild stimulant effect, caffeine is the most widely consumed psychoactive substance in Western countries (Fredholm et al., 1999). Evidence suggests that some doses of caffeine have antinociceptive effects (Person et al., 1985; Wu, et al., 2006). Also, one of the non-selective antagonists of adenosine receptors (ARs) is caffeine (Tchekalarova et al., 2010). Adenosine has four G protein-coupled receptors: A2B, A1, A2A, and A3 (McGaraughty et al., 2005). The antinociceptive effect of caffeine is probably due to antagonism of the A2A receptors (Wu, et al., 2006; Zhang, 2001). Nitric oxide (NO) is synthesized from L-arginine by different isoforms of NO synthase (NOS) (Akula et al., 2008). NOS displays three isoforms: endothelial, neuronal, and inducible isoforms (Garthwaite, 2008). The finding suggests that NO plays a complex role in pain modulation (Basbaum et al., 2009; Schaible & Richter, 2004). On the other hand, it is known that caffeine can modulate NO production (Kayir & Uzbay, 2004; López-Muñoz et al., 1996). Whereas activation of the A2A receptors stimulates NO production, activation of the A1 receptor decreases NO production. It is suggested that the effects of caffeine on NO synthesis may be due to antagonism of the ARs (Bruce et al., 2002). However, it seems that the action mechanism of caffeine is not only through antagonism with ARs, but some other mechanisms like the NO-cGMP pathway can be involved too (Kayir & Uzbay, 2004; Orrú et al., 2013). Therefore, our study investigated the effects of acute caffeine administration on the NP threshold. The rule of the NO pathway was also considered.
2. Materials and Methods
Animals
A total of 88 male Wistar rats weighing 220–250 g were used in this study. Three or four rats were placed in every cage and kept at a 12 h light/dark cycle at a temperature of 22±2 °C and humidity-controlled of 55±5%. Water and food were available ad libitum. All experiments were carried out in accordance with Directive 2010/63/EU on the protection of animals used for scientific purposes and approved by the Ethics Committee of Kashan University of Medical Sciences.
Drugs
Caffeine, L-NAME, and L-arginine were purchased from Sigma-Aldrich Company. The drugs were diluted with normal saline and administered intraperitoneally.
Neuropathic pain model
We used the animal model of chronic constriction injury (CCI) described previously (Bennett & Xie, 1988). The subjects were anesthetized with a mixture of xylazine (10 mg/kg) and ketamine (50 mg/kg) (Verdi et al., 2013). The common portion of the left sciatic nerve was exposed and separated from the adjacent connective tissue. Four ligatures (4-0 intestinal chrome) were loosely tied around the nerve so as not to interrupt blood circulation through the superficial epineural vessels. After surgery, every rat was separately placed in a cage (Verdi et al., 2013).
Animal groups
The study protocol included 11 groups of rats comprising 8 animals in each group. Three control groups have entered the study. One group served as age-matched non-ligation control (CON). Two control groups of rats were subjected to the sciatic nerve ligation with (CCI+saline) or without (CCI) saline administration. The sham group (sham) experienced similar surgery, except that the left sciatic nerve was exposed with no ligation. Three groups of CCI animals received 10 (Caf.10), 50 (Caf.50), and 100 (Caf.100) mg/kg caffeine; two groups of rats were administered 100 mg/kg L-arginine (L-ARG) and 30 mg/kg L-NAME (L-NAME).
Also, two groups of animals were pre-treated with 30 mg/kg L-NAME (L-NAME+Caf) and 100 mg/kg L-arginine (L-ARG+Caf) 30 minutes before administration of the effective dose of caffeine (50 mg/kg) (Pottabathini et al., 2015). The same groups of animals were introduced to all tests.
Allodynia and hyperalgesia
The mechanical and cold stimulations were applied with von Frey filaments and acetone, respectively. Radiant heat was used as thermal stimulation to induce a hyperalgesia test. The medial plantar surface of the left hind paw was used to apply the stimulations.
Experimental design
The animals were introduced to the behavioral tests 4, 7, 14, 21, and 28 days after surgery. The subjects got used to the test environment at least 15 minutes before beginning the test (Hamidi et al., 2006).
Assessment of heat hyperalgesia
A plantar test device was used to evaluate paw withdrawal delay in response to radiant heat (Ugo Basile, Varese, Italy). As a heat source, an infrared light was transmitted from under the mid-plantar surface of the left hind paw. The delay (seconds) between the onset of the thermal stimulus and paw withdrawal was considered thermal withdrawal latency (Banafshe et al., 2012; Bennett & Xie, 1988). A 22-second cut-off time was used to avoid any damage to the tissue. Each trial was performed alternately three times with an interval of 5-10 minutes to prevent sensitization for the injured and non-injured paw in the control group (Banafshe et al., 2012).
Assessment of mechanical allodynia
Mechanical allodynia was evaluated as described previously (Chen et al., 2018; Mohammadifar et al., 2021). The effects of weak stimulation of von Frey fibers (force ranging from less than 2 to 60 g) were studied to assess the involvement of low-threshold fibers in nociceptive behavior before and after CCI. These stimulations are considered harmless because they usually provoke activity in low-threshold mechanoreceptors. The rats were placed on a mesh floor (0.8×0.8 cm cell), shielded by a transparent plastic box (18×18×25 cm), and allowed to explore for 15 minutes or when exploratory behavior finished. Sequences of von Frey fiber stimulation were applied in a rising order of forces to the hind paw plantar surface. The von Frey filament stimulation was applied in three consecutive trials, pressing down on the hind paw until the animal withdrew its paw or the filament bent. Paw lifting was ignored due to natural locomotor behavior. The withdrawal threshold was calculated for the smallest fiber size that induced at least two withdrawal responses through three consecutive trials using the same filament. Each stimulation was applied for approximately 1 s with an interstimulus interval of 5 s.
Assessment of cold allodynia (acetone test)
The modified acetone spray test was used for cold allodynia assessment (Yoon et al., 1994). While the rats stood on the perforated floor, 250 μL of acetone was sprayed onto the plantar skin using a smooth needle attached to a syringe. The trial was repeated 5 times (with an interval of 3 minutes) to the left paw. Withdrawal frequency is determined as a percentage (number of withdrawals/number of trials ×100) (Banafshe et al., 2014; Seltzer et al., 1990).
Statistical analysis
The obtained data were analyzed by a 2-way analysis of variance (ANOVA). The Tukey test was applied as post hoc. Between-group differences were considered significant with a P<0.05. The data are presented as Mean±SEM.
3. Results
Heat hyperalgesia in the CCI group
With the application of the radiant heat on the hind paw, the animals lifted their feet and showed aversive behaviors such as shaking, trapping, or licking the affected paw. Figure 1A reports the heat hyperalgesia after CCI injury in the control groups. Statistical analysis indicated a significant difference between the testing groups (F(3, 28)=99, P=0.0001). The sham group displayed an analogous withdrawal latency with the CON animals. Also, no significant difference was evident between the behavior of the CCI and CCI+saline groups. The CCI group significantly decreased the withdrawal latency in response to heat stimulation on days 4, 7, 21, 28 (P<0.001), and 14 (P<0.01), compared to the sham group.

The effect of caffeine on the heat hyperalgesia
A considerable difference was found between the caffeine and vehicle-treated groups (F(3, 28)=52.818, P=0.0001). The Caf.10 group resembled the CCI+saline group in the heat hyperalgesia test. The withdrawal latency increased at day 4 in the Caf.50 rats (P<0.001). The maximum effect of caffeine was observed in the dose of 100 mg/kg, where the Caf.100 group showed a considerably increased withdrawal latency on days 7 (P<0.001), 14, 28 (P<0.05), and 21 (P<0.01) compared to the CCI+saline group. We selected the dose of 50 mg/kg as the minimum effective concentration of caffeine to investigate the possible role of the NO-cGMP signaling pathway. Figure 1B illustrates the acute administration of caffeine on the pain threshold in heat hyperalgesia.
The effect of NO-cGMP signaling pathway on heat hyperalgesia
General statistics indicated a possible involvement of the NO-cGMP signaling pathway in the heat hyperalgesia test (F(3, 28)=35.78, P=0.0001). Treatment with L-NAME significantly attenuated hyperalgesia on days 14 (P<0.05), 21 (P<0.001), and 28 (P<0.01) in the L-NAME group compared to the CCI+saline group. Furthermore, L-NAME+Caf significantly reduced hyperalgesia on days 4 (P<0.01), 7 (P<0.01), 14 (P<0.01), 21, and 28 (P<0.001). Moreover, compared with the Caf.50 group, the L-NAME+Caf group reduced the heat hyperalgesia on days 14 (P<0.01), 21, and 28 (P<0.001). A significant variation was also found between the L-NAME+Caf and the L-NAME groups on day 4 (P<0.05). Figure 1C shows the effect of L-NAME or L-NAME+Caf on heat hyperalgesia. The data analysis showed that L-arginine or L-arginine+caffeine significantly influenced the heat hyperalgesia threshold (F(3, 28)=26.312, P<0.001, Figure 1D). The animals treated with L-ARG or L-ARG+Caf showed no significant difference in paw withdrawal latency compared to the CCI+saline group. The L-ARG+Caf group showed significantly reduced withdrawal latency on day 4 (P<0.01) compared to the Caf.50 group. L-ARG+Caf revealed no significant difference with the L-ARG group.
Mechanical allodynia
Before surgery, the subjects rarely responded, even to the intense von Frey filament. Still, after the CCI procedure, the ipsilateral hind paw was sensitive to mechanical stimuli even with weak stimulations.
The statistical analysis showed a substantial variation between the testing groups (F(3, 28)=11.96, P=0.0001). The CON and sham groups showed an analogous pattern of behavior in the mechanical allodynia test. The response to the mechanical stimulus was markedly increased in the CCI compared to sham rats on days 21 (P<0.01) and 28 (P<0.01). The CCI and CCI+saline groups also showed a similar response to this test. Figure 2A compares the results of the mechanical allodynia test in different groups.

The effect of caffeine on the mechanical allodynia
A generally significant difference was evident between the caffeine-administered groups’ responses to the mechanical allodynia test (F(3, 28)=11, P=0.0001). The Caf.10 group showed a negligible difference from the CCI+saline group in the withdrawal threshold. However, the withdrawal threshold was increased in the Caf.50 and Caf.100 groups on days 14 (P<0.001) and 28 (P<0.05), compared with the CCI+saline group (Figure 2B).
The effect of NO-cGMP signaling pathway on the mechanical allodynia
Analysis of variance indicated a significant behavioral variation when applying L-NAME (F(3, 28)=10.09, P=0.0001). The L-NAME and L-NAME+Caf groups significantly attenuated the response to the allodynia test on days 14 (P<0.001) and 28 (P<0.05) in comparison to the CCI+saline group. However, the L-NAME+Caf group showed no difference in the paw withdrawal threshold with both L-NAME and Caf.50 rats (Figure 2C).
Statistics showed that L-arginine does not considerably influence the mechanical allodynia (F(3, 28)=4.08, P=0.03).
The animals treated with L-ARG or L-ARG+Caf did not show a significant difference in paw withdrawal threshold compared to the CCI+saline group. Moreover, there was no significant difference between the L-ARG+Caf and L-ARG and Caf.50 groups (Figure 2D).
Cold allodynia
While the animals were not responsive to the acetone application before surgery, the ipsilateral hind paw showed a high sensitivity to the acetone test after the CCI procedure. The acetone application caused rats to rapidly withdraw the affected foot (with a delay of about 0.2–0.3 s) and then shake, trap, or lick it.
Statistical analysis showed a significant difference between the groups testing for the cold allodynia (F(3, 28)=151.6, P=0.0001). The results indicate that the difference in allodynia between the CON and sham groups is not significant. However, an increased withdrawal frequency was observed in the CCI compared to sham groups on testing days 4, 7, 14, and 21 (P<0.001). The CCI and CCI+saline groups similarly behaved in the cold allodynia test. Figure 3A illustrates the cold allodynia in the control groups.
The effect of caffeine on the cold allodynia
The data analysis showed a substantial variation between different groups under caffeine treatment (F(3, 28)=35.78, P=0.0001). The Caf.10 rats increased the withdrawal frequency on days 14 (P<0.05), 21 (P<0.001), and 28 (P<0.01), compared with the CCI+saline group. However, the other caffeine-treated Caf.50 and Caf.100 groups responded similarly to the cold allodynia, as did the CCI+saline group (Figure 3B).

The effect of NO-cGMP signaling pathway on the cold allodynia
The statistical evaluation showed the effect of L-ARG or L-ARG+Caf on cold allodynia. Analysis of variance showed that L-arginine administration significantly underlies the withdrawal frequency in the cold allodynia testing (F(3, 28)=10.08, P=0.0001). The L-ARG group showed no significant difference in withdrawal frequency compared to the CCI+saline group. The L-ARG+Caf animals noticeably reduced the withdrawal frequency on days 4 (P<0.01), 7 (P<0.05), and 21 (P<0.05) compared to the CCI+saline group. The L-ARG+Caf rats appeared to have a significant reduction in the withdrawal frequency on days 4 (P<0.01), 7 (P<0.001), and 21 (P<0.01) compared to the Caf.50 group. Also, the L-ARG+Caf animals displayed a marked reduced withdrawal frequency on day 4 (P<0.05) compared to the L-ARG group (Figure 3C).
We found that the L-NAME application effectively affected the mechanical allodynia (F(3, 28)=20.02, P=0.0001). Treatment with L-NAME significantly attenuated the withdrawal frequency on days 4 (P<0.05) and 7 (P<0.01) in the L-NAME compared to the CCI+saline group. Also, the L-NAME+Caf group significantly reduced the withdrawal frequency on days 4 (P<0.05), 7 (P<0.01), 14 (P<0.01), and 21 (P<0.05) compared to the CCI+saline group. Moreover, L-NAME+Caf significantly reduced allodynia on days 4 and 21 (P<0.01), 7, 14, and 28 (P<0.001) compared with the Caf.50 group (Figure 3D).
4. Discussion
The finding of this study indicated that caffeine at a dose of 10 displays a pronociceptive effect. Conversely, the doses of 50 and 100 of caffeine show antinociceptive effects. Whereas L-NAME alone or pre-treated with caffeine has an antinociceptive impact, L-ARG pre-treatment with caffeine increased heat hyperalgesia and decreased cold allodynia.
Caffeine is an antagonist of ARs, and its main targets are A1 and A2A subtype receptors (Carrillo & Benitez, 2000). Adenosine displays its analgesic role by activating A1 receptors in the nervous system. On the other hand, the A2A receptor shows both pronociceptive and antinociceptive effects (Sawynok, 2016; Vincenzi et al., 2020; Yamamoto et al., 2003; Zahn et al., 2007). A1 receptors are found on peripheral sensory nerve endings in the spinal cord’s dorsal horn and supraspinal structures involved in pain processing (Vincenzi et al., 2020). A2 receptors are located on inflammatory and immune cells and are considered targets for inflammatory and immune conditions (Sawynok, 2016). In the CNS, they are expressed in pre- and post-synaptic neurons in the brain (Antonioli et al., 2014; Popoli & Pepponi, 2012). The results of this study show that the lowest dose of caffeine had a pronociceptive effect and increased the cold allodynia. Consistent with our findings, it is shown that 1 to 10 mg/kg of caffeine increases hyperalgesia and allodynia (Esser & Sawynok, 2000; Wu, et al., 2006). Moreover, heat hyperalgesia was exacerbated in the A1 receptors knockout mice (Wu et al., 2005). The hyperalgesic effects of the minimum dose of caffeine may be due to the antagonizing effect on A1 receptors (Sawynok et al., 2008; Sawynok et al., 2010). We showed that acute caffeine administration at 50 and 100 mg/kg doses reduces heat hyperalgesia and mechanical allodynia. Numerous studies confirm our results in that some doses of caffeine show antinociceptive effects (López et al., 2006; Shapiro, 2008; Wu, et al., 2006). Blockade of A2A receptors is one of the proposed mechanisms of analgesic effects of caffeine. Consistently, hypoalgesia was reported in mice lacking the A2A receptors (Ledent et al., 1997). Both central and peripheral A2A receptors are involved in pain facilitation and play a crucial pronociceptive role (Sawynok, 2016). It has been proposed that caffeine at a 100 mg/kg dose has a more inhibitory effect on A2A receptors. In this context, caffeine revealed a dose-dependent anti-hyperalgesia, and the maximal effect was achieved at a dose of 100 mg/kg. Overall, our findings suggest that the effect of caffeine on hyperalgesia is partly due to its dose-dependent effect on A1 receptors or A2A receptors (Sawynok et al., 2010; Wu, et al., 2006). Caffeine can modulate pain mainly through antagonism of ARs (Davis & Green, 2009; Massey et al., 1994; Ribeiro & Sebastiao, 2010). Activation of A2A receptors leads to increased NO production; therefore, high-dose caffeine’s antagonizing effect (50 and 100 mg/kg) may lead to decreased NO production (Esmaili & Heydari, 2019). Some evidence indicates that NO is involved in the development of N (Chauhan et al., 2018; Miller et al., 2014; Zhao et al., 2018). To further investigate the role of the NO-cGMP pathway, we applied L-arginine as NO releasers and L-NAME (non-selective NOS inhibitor). The administration of L-NAME before caffeine decreased susceptibilities to heat hyperalgesia and cold and mechanical allodynia. Also, L-arginine administration before caffeine increased heat hyperalgesia. These findings suggest that the pain attenuation effects of L-NAME might be mediated through an inhibitory effect on NOS isoforms and, subsequently, NO production. Another proposed mechanism to explain the antihyperalgesia effect of caffeine is the reduction of cyclooxygenase (COX-2) and prostaglandin E (PGE2). In this context, caffeine inhibits the synthesis of PGE2 and COX-2 in rat microglial cells (Fiebich et al., 2000). In addition, caffeine potentiates the antihyperalgesic effects of nonsteroidal anti-inflammatory drugs (NSAIDs) (Abou-Atme etal., 2019). Hyperalgesia is mainly associated with increased expression of COX-2 and local production of PGE2 (Li et al., 2018). The inhibitory effect of caffeine on COX-2 and reduced synthesis of PGE2 may contribute to the antihyperalgesic effect of caffeine (Fiebich et al., 2000). Interestingly, there is an interaction between NO and PGE2, so NO increases the production of PGE2 (Ilari et al., 2020).
5. Conclusion
In conclusion, our findings suggest that decreased NO production in the presence of caffeine may contribute to reducing PGE2 and subsequent antihyperalgesia. The NO-cGMP pathway’s involvement in modifying pain threshold might be a possible mechanism for caffeine. Elucidating the cause of the opposite effects of different doses of caffeine on pain requires further research.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Kashan University of Medical Sciences (KAUMS), Kahsan, Iran (Code: I.R.KAUMS.MEDNT.REC.1397.040).
Funding
The Deputy of Research of Kashan University of Medical Sciences (KAUMS) supported this study by a grant to Gholam Ali Hamidi (Grant No.: 97065).
Authors' contributions
Conceptualization and methodology: Azhdar Heydari; Supervision and funding administration: Gholam Ali Hamidi; Investigation: Monireh Naderi Tehrani; Data collection: Monireh Naderi Tehrani and Saeedeh Nasrollahi; Data analysis: Fatemeh Aghighi and Mahmoud Salami; Writing the original draft: Monireh Naderi Tehrani; Review and editing: Mahmoud Salami and Fatemeh Aghighi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors highly appreciate the Deputy of Research of Kashan University of Medical Sciences (KAUMS), Kahsan, Iran, for financial support.
References
Abou-Atme, Y. S., Melis, M., & Zawawi, K. H. (2019). Efficacy and safety of acetaminophen and caffeine for the management of acute dental pain: A systematic review. The Saudi Dental Journal, 31(4), 417-423. [DOI:10.1016/j.sdentj.2019.04.008] [PMID] [PMCID]
Akula, K. K., Dhir, A., & Kulkarni, S. K. (2008). Nitric oxide signaling pathway in the anticonvulsant effect of adenosine against pentylenetetrazol-induced seizure threshold in mice. European Journal of Pharmacology, 587(1-3), 129-134. [DOI:10.1016/j.ejphar.2008.03.038] [PMID]
Antonioli, L., Csóka, B., Fornai, M., Colucci, R., Kókai, E., & Blandizzi, C., et al. (2014). Adenosine and inflammation: What’s new on the horizon? Drug Discovery Today, 19(8), 1051-1068. [DOI:10.1016/j.drudis.2014.02.010] [PMID]
Attal, N. (2012). Neuropathic pain: Mechanisms, therapeutic approach, and interpretation of clinical trials. Continuum (Minneapolis, Minn.), 18(1), 161–175. [DOI:10.1212/01.CON.0000411564.41709.2d] [PMID]
Banafshe, H. R., Hamidi, G. A., Noureddini, M., Mirhashemi, S. M., Mokhtari, R., & Shoferpour, M. (2014). Effect of curcumin on diabetic peripheral neuropathic pain: Possible involvement of opioid system. European Journal of Pharmacology, 723, 202-206. [DOI:10.1016/j.ejphar.2013.11.033] [PMID]
Banafshe, H. R., Mesdaghinia, A., Arani, M. N., Ramezani, M. H., Heydari, A., & Hamidi, G. A. (2012). Lithium attenuates pain-related behavior in a rat model of neuropathic pain: possible involvement of opioid system. Pharmacology Biochemistry and Behavior, 100(3), 425-430. [DOI:10.1016/j.pbb.2011.10.004] [PMID]
Basbaum, A. I., Bautista, D. M., Scherrer, G., & Julius, D. (2009). Cellular and molecular mechanisms of pain. Cell, 139(2), 267-284. [DOI:10.1016/j.cell.2009.09.028] [PMID] [PMCID]
Bennett, G. J., & Xie, Y. K. (1988). A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain, 33(1), 87-107. [DOI:10.1016/0304-3959(88)90209-6] [PMID]
Bruce, C., Yates, D. H., & Thomas, P. S. (2002). Caffeine decreases exhaled nitric oxide. Thorax, 57(4), 361-363. [DOI:10.1136/thorax.57.4.361] [PMID] [PMCID]
Carrillo, J. A., & Benitez, J. (2000). Clinically significant pharmacokinetic interactions between dietary caffeine and medications. Clinical Pharmacokinetics, 39(2), 127-153. [DOI:10.2165/00003088-200039020-00004] [PMID]
Cervero, F., Laird, J. M., & García-Nicas, E. (2003). Secondary hyperalgesia and presynaptic inhibition: An update. European Journal of Pain (London, England), 7(4), 345–351. [DOI:10.1016/S1090-3801(03)00047-8] [PMID]
Chauhan, P., Sheng, W. S., Hu, S., Prasad, S., & Lokensgard, J. R. (2018). Nitrosative damage during retrovirus infection-induced neuropathic pain. Journal of Neuroinflammation, 15(1), 66. [DOI:10.1186/s12974-018-1107-7] [PMID] [PMCID]
Chen, H., Hu, Y., Xie, K., Chen, Y., Wang, H., & Bian, Y., et al. (2018). Effect of autophagy on allodynia, hyperalgesia and astrocyte activation in a rat model of neuropathic pain. International Journal of Molecular Medicine, 42(4), 2009-2019. [DOI:10.3892/ijmm.2018.3763] [PMID] [PMCID]
Davis, J., & Green, J. M. (2009). Caffeine and anaerobic performance: Ergogenic value and mechanisms of action. Sports Medicine (Auckland, N.Z.), 39(10), 813–832. [DOI:10.2165/11317770-000000000-00000] [PMID]
Esmaili, Z., & Heydari, A. (2019). Effect of acute caffeine administration on PTZ-induced seizure threshold in mice: Involvement of adenosine receptors and NO-cGMP signaling pathway. Epilepsy Research, 149, 1–8. [DOI:10.1016/j.eplepsyres.2018.10.013] [PMID]
Esser, M. J., & Sawynok, J. (2000). Caffeine blockade of the thermal antihyperalgesic effect of acute amitriptyline in a rat model of neuropathic pain. European Journal of Pharmacology, 399(2-3), 131-139. [DOI:10.1016/S0014-2999(00)00336-8] [PMID]
Fiebich, B. L., Lieb, K., Hüll, M., Aicher, B., van Ryn, J., & Pairet, M., et al. (2000). Effects of caffeine and paracetamol alone or in combination with acetylsalicylic acid on prostaglandin E2 synthesis in rat microglial cells. Neuropharmacology, 39(11), 2205-2213. [DOI:10.1016/S0028-3908(00)00045-9] [PMID]
Fredholm, B. B., Bättig, K., Holmén, J., Nehlig, A., & Zvartau, E. E. (1999). Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacological Reviews, 51(1), 83-133. [PMID]
Garthwaite, J. (2008). Concepts of neural nitric oxide-mediated transmission. European Journal of Neuroscience, 27(11), 2783-2802. [DOI:10.1111/j.1460-9568.2008.06285.x] [PMID] [PMCID]
Grzanna, R., Lindmark, L., & Frondoza, C. G. (2005). Ginger-an herbal medicinal product with broad anti-inflammatory actions. Journal of Medicinal Food, 8(2), 125-132. [DOI:10.1089/jmf.2005.8.125] [PMID]
Hamidi, G. A., Manaheji, H., Janahmadi, M., Noorbakhsh, S. M., & Salami, M. (2006). Co-administration of MK-801 and morphine attenuates neuropathic pain in rat. Physiology & Behavior, 88(4-5), 628-635. [DOI:10.1016/j.physbeh.2006.05.017] [PMID]
Ilari, S., Dagostino, C., Malafoglia, V., Lauro, F., Giancotti, L. A., & Spila, A., et al. (2020). Protective effect of antioxidants in nitric oxide/cox-2 interaction during inflammatory pain: The role of nitration. Antioxidants, 9(12), 1284. [DOI:10.3390/antiox9121284] [PMID] [PMCID]
Jensen, T. S., Baron, R., Haanpää, M., Kalso, E., Loeser, J. D., & Rice, A. S. C., et al. (2011). A new definition of neuropathic pain. Pain, 152(10), 2204–2205. [DOI:10.1016/j.pain.2011.06.017] [PMID]
Kayir, H., & Uzbay, I. T. (2004). Evidence for the role of nitric oxide in caffeine-induced locomotor activity in mice. Psychopharmacology, 172(1), 11-15. [DOI:10.1007/s00213-003-1625-5] [PMID]
Ledent, C., Vaugeois, J. M., Schiffmann, S. N., Pedrazzini, T., El Yacoubi, M., & Vanderhaeghen, J. J., et al. (1997). Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A 2a receptor. Nature, 388(6643), 674-678. [DOI:10.1038/41771] [PMID]
Li, Q. B., Chang, L., Ye, F., Luo, Q. H., Tao, Y. X., & Shu, H. H. (2018). Role of spinal cyclooxygenase-2 and prostaglandin E2 in fentanyl-induced hyperalgesia in rats. British Journal of Anaesthesia, 120(4), 827-835. [DOI:10.1016/j.bja.2017.11.103] [PMID] [PMCID]
López-Muñoz, F. J., Castañeda-Hernández, G., Flores-Murrieta, F. J., & Granados-Soto, V. (1996). Effect of caffeine coadministration and of nitric oxide synthesis inhibition on the antinociceptive action of ketorolac. European Journal of Pharmacology, 308(3), 275-277. [DOI:10.1016/0014-2999(96)00320-2] [PMID]
López, J. R., Domínguez-Ramírez, A. M., Cook, H. J., Bravo, G., Díaz-Reval, M. I., & Déciga-Campos, M., et al. (2006). Enhancement of antinociception by co-administration of ibuprofen and caffeine in arthritic rats. European Journal of Pharmacology, 544(1-3), 31-38. [DOI:10.1016/j.ejphar.2006.06.041] [PMID]
Massey, L. K., Bergman, E. A., Wise, K., & Sherrard, D. J. (1994). Interactions between dietary caffeine and calcium on calcium and bone metabolism in older women. Journal of the American College of Nutrition, 13(6), 592-596. [DOI:10.1080/07315724.1994.10718453] [PMID]
McGaraughty, S., Cowart, M., Jarvis, M. F., & Berman, R. F. (2005). Anticonvulsant and antinociceptive actions of novel adenosine kinase inhibitors. Current Topics in Medicinal Chemistry, 5(1), 43-58. [DOI:10.2174/1568026053386845] [PMID]
Miller, R. E., Miller, R. J., & Malfait, A. M. (2014). Osteoarthritis joint pain: The cytokine connection. Cytokine, 70(2), 185-193. [DOI:10.1016/j.cyto.2014.06.019] [PMID] [PMCID]
Mohammadifar, M., Aarabi, M. H., Aghighi, F., Kazemi, M., Vakili, Z., & Memarzadeh, M. R., et al. (2021). Anti-osteoarthritis potential of peppermint and rosemary essential oils in a nanoemulsion form: Behavioral, biochemical, and histopathological evidence. BMC Complementary Medicine and Therapies, 21(1), 57. [DOI:10.1186/s12906-021-03236-y] [PMID] [PMCID]
Nieber, K. (2017). The impact of coffee on health. Planta Medica, 83(16), 1256-1263. [DOI:10.1055/s-0043-115007] [PMID]
Orrú, M., Guitart, X., Karcz-Kubicha, M., Solinas, M., Justinova, Z., & Barodia, S. K., et al. (2013). Psychostimulant pharmacological profile of paraxanthine, the main metabolite of caffeine in humans. Neuropharmacology, 67, 476-484. [DOI:10.1016/j.neuropharm.2012.11.029] [PMID] [PMCID]
Person, D. L., Kissin, I., Brown, P. T., Xavier, A. V., Vinik, H. R., & Bradley, E. L. (1985). Morphine--caffeine analgesic interaction in rats. Anesthesia and Analgesia, 64(9), 851-856. [DOI:10.1213/00000539-198509000-00002] [PMID]
Popoli, P., & Pepponi, R. (2012). Potential therapeutic relevance of adenosine A2B and A2A receptors in the central nervous system. CNS & Neurological Disorders-Drug Targets, 11(6), 664-674. [DOI:10.2174/187152712803581100] [PMID]
Pottabathini, R., Kumar, A., Bhatnagar, A., & Garg, S. (2015). Possible involvement of nitric oxide modulatory mechanism in the protective effect of retigabine against spinal nerve ligation-induced neuropathic pain. Cellular and Molecular Neurobiology, 35(1), 137-146. [DOI:10.1007/s10571-014-0105-2] [PMID]
Ribeiro, J. A., & Sebastiao, A. M. (2010). Caffeine and adenosine. Journal of Alzheimer’s Disease, 20(Suppl 1), S3–S15. [DOI:10.3233/JAD-2010-1379] [PMID]
Sawynok, J. (2016). Adenosine receptor targets for pain. Neuroscience, 338, 1-18. [DOI:10.1016/j.neuroscience.2015.10.031] [PMID]
Sawynok, J., Reid, A. R., & Fredholm, B. B. (2008). Caffeine reverses antinociception by amitriptyline in wild type mice but not in those lacking adenosine A1 receptors. Neuroscience Letters, 440(2), 181-184. [DOI:10.1016/j.neulet.2008.05.074] [PMID]
Sawynok, J., Reid, A. R., & Fredholm, B. B. (2010). Caffeine reverses antinociception by oxcarbazepine by inhibition of adenosine A1 receptors: Insights using knockout mice. Neuroscience Letters, 473(3), 178-181. [DOI:10.1016/j.neulet.2010.02.028] [PMID]
Schaible, H. G., & Richter, F. (2004). Pathophysiology of pain. Langenbeck’s Archives of Surgery, 389(4), 237-243. [DOI:10.1007/s00423-004-0468-9] [PMID]
Seltzer, Z., Dubner, R., & Shir, Y. (1990). A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain, 43(2), 205-218. [DOI:10.1016/0304-3959(90)91074-S] [PMID]
Shapiro, R. E. (2008). Caffeine and headaches. Current Pain and Headache Reports, 12(4), 311-315. [DOI:10.1007/s11916-008-0052-z] [PMID]
Tchekalarova, J., Kubová, H., & Mareš, P. (2010). Postnatal period of caffeine treatment and time of testing modulate the effect of acute caffeine on cortical epileptic afterdischarges in rats. Brain Research, 1356, 121-129. [DOI:10.1016/j.brainres.2010.07.107] [PMID]
Treede, R. D., Jensen, T. S., Campbell, J. N., Cruccu, G., Dostrovsky, J. O., & Griffin, J. W., et al. (2008). Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology, 70(18), 1630–1635. [DOI:10.1212/01.wnl.0000282763.29778.59] [PMID]
Verdi, J., Jafari-Sabet, M., Mokhtari, R., Mesdaghinia, A., & Banafshe, H. R. (2013). The effect of progesterone on expression and development of neuropathic pain in a rat model of peripheral neuropathy. European Journal of Pharmacology, 699(1-3), 207-212. [DOI:10.1016/j.ejphar.2012.11.052] [PMID]
Vincenzi, F., Pasquini, S., Borea, P. A., & Varani, K. (2020). Targeting adenosine receptors: A potential pharmacological avenue for acute and chronic pain. International Journal of Molecular Sciences, 21(22), 8710. [DOI:10.3390/ijms21228710] [PMID] [PMCID]
Wu, W. P., Hao, J. X., Fredholm, B. B., Wiesenfeld-Hallin, Z., & Xu, X. J. (2006). Effect of acute and chronic administration of caffeine on pain-like behaviors in rats with partial sciatic nerve injury. Neuroscience Letters, 402(1-2), 164-166. [DOI:10.1016/j.neulet.2006.03.065] [PMID]
Wu, W. P., Hao, J. X., Halldner, L., Lövdahl, C., DeLander, G. E., & Wiesenfeld-Hallin, Z., et al. (2005). Increased nociceptive response in mice lacking the adenosine A1 receptor. Pain, 113(3), 395-404. [DOI:10.1016/j.pain.2004.11.020] [PMID]
Yamamoto, S., Nakanishi, O., Matsui, T., Shinohara, N., Kinoshita, H., & Lambert, C., et al. (2003). Intrathecal adenosine A 1 receptor agonist attenuates hyperalgesia without inhibiting spinal glutamate release in the rat. Cellular and Molecular Neurobiology, 23(2), 175-185. [DOI:10.1023/A:1022997805525] [PMID]
Yoon, C., Wook, Y. Y., Sik, N. H., Ho, K. S., & Mo, C. J. (1994). Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain, 59(3), 369-376. [DOI:10.1016/0304-3959(94)90023-X] [PMID]
Zahn, P. K., Straub, H., Wenk, M., & Pogatzki-Zahn, E. M. (2007). Adenosine A1but Not A2aReceptor agonist reduces hyperalgesia caused by a surgical incision in rats: A pertussis toxin-sensitive G protein-dependent process. Anesthesiology, 107(5), 797–806. [DOI:10.1097/01.anes.0000286982.36342.3f] [PMID]
Type of Study: Original |
Subject:
Behavioral Neuroscience
Received: 2023/05/2 | Accepted: 2023/05/30 | Published: 2024/09/1
Received: 2023/05/2 | Accepted: 2023/05/30 | Published: 2024/09/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





