Volume 16, Issue 2 (March & April 2025)
BCN 2025, 16(2): 429-440 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ghadiry F, Amra B, Feizi A, Vafaei Shahi M. Evaluating the Validity of Sleep Disturbance Scale for Children in Attention-deficit/Hyperactivity Disorder. BCN 2025; 16 (2) :429-440
URL: http://bcn.iums.ac.ir/article-1-2698-en.html
URL: http://bcn.iums.ac.ir/article-1-2698-en.html
1- Sleep Ward, Department Internal Medicine, Isfahan University of Medical Sciences, Isfahan, Iran.
2- Pulmonary and Sleep Ward, Department of Internal Medicine, Isfahan University Of Medical Sciences, Isfahan, Iran.
3- Department of Epidemiology and Biostatistics, School of Health, Isfahan University of Medical Sciences, Isfahan, Iran. & Cardiac Rehabilitation Research Center, Cardiovascular Research Institute, Isfahan University of Medical Sciences, Isfahan, Iran.
4- Department of Psychiatry, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran.
2- Pulmonary and Sleep Ward, Department of Internal Medicine, Isfahan University Of Medical Sciences, Isfahan, Iran.
3- Department of Epidemiology and Biostatistics, School of Health, Isfahan University of Medical Sciences, Isfahan, Iran. & Cardiac Rehabilitation Research Center, Cardiovascular Research Institute, Isfahan University of Medical Sciences, Isfahan, Iran.
4- Department of Psychiatry, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran.
Keywords: Attention deficit disorder with hyperactivity (ADHD), Sleep-wake disorders, Surveys and questionnaires
Full-Text [PDF 774 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder characterized by inattention, hyperactivity, and impulsivity, which are pervasive, impairing, and otherwise age-inappropriate (Banaschewski et al., 2017). Difficulty regulating emotions or executive function are the main problems in patients with ADHD (Asherson et al., 2016).
ADHD is the most common behavioral disorder in childhood and adolescence, affecting about 3% to 5% of children before the age of seven. This complication is more common in children in elementary school and during puberty, and many patients get better with age (Thomas et al., 2015). It can be important to keep in mind that untreated hyperactivity is dangerous. Previous studies have shown that 25% to 40% of children with ADHD have not been treated (Singh et al., 2015).
The cause of most patients with ADHD is not yet clear, but it is thought to be a multifactorial disease with a genetic origin and related to the environment. The diagnosis of ADHD is a challenging issue due to assessments of normal levels of inattention, hyperactivity, and impulsivity, but this disease is mainly diagnosed by diagnostic and statistical manual of mental disorders-4th (DSM-IV) criteria (Dalsgaard et al., 2015; Hanć & Cortese, 2018).
Sleep disorders are common among patients with ADHD, which can be due to primary sleep disorders or side effects from stimulant medications (Vélez-Galarraga et al., 2016). Sleep disturbance could cause severe daily fatigue, mood problems, problems in attention and concentration, behavioral problems and disorders, as well as damage to physical health and reduced quality of life (QoL). The diagnosis of sleep disorder in patients with ADHD is a challenging issue (Cappe et al., 2017; Durmuş et al., 2017).
Polysomnography is recognized as the gold standard for objectively assessing sleep quality and difficulties (Ferreira et al., 2009), but its high cost and time-intensive nature limit its suitability for screening. In contrast, the sleep disturbance scale for children (SDSC), widely used for assessing sleep problems in children, offers a practical and cost-effective alternative. This informant-rated questionnaire, often completed by parents, evaluates specific sleep disorders and provides a comprehensive measure of overall sleep disturbance (Miano et al., 2016; Marriner et al., 2017; Van der Heijden et al., 2018). This study aimed to assess the validity of SDSC in children with ADHD.
2. Materials and Methods
This cross-sectional study was conducted between 2020 and 2021 at Khorshid Hospital, affiliated with Isfahan University of Medical Sciences, Isfahan City, Iran. The study involved 204 children diagnosed with ADHD and 202 healthy children as a control group. The study protocol was approved by the Research Committee and the Ethics Committee of Isfahan University of Medical Sciences.
Participants were recruited through a convenience sampling approach. The ADHD group consisted of children aged 6-15 years, newly diagnosed with ADHD by psychiatrists using the DSM-IV criteria. The exclusion criteria included psychiatric medication use within the past month, any sleep disorder, or previously diagnosed psychiatric disorders. Notably, the exclusion of participants with sleep disorders aimed to ensure that observed sleep disturbances were more likely associated with ADHD rather than co-occurring sleep disorders. All children in the case group underwent a psychiatric interview to exclude additional psychiatric disorders.
The control group, comprising 202 children without psychiatric disorders, was recruited from the same geographical area from schools, community centers, and pediatric clinics. The absence of psychiatric disorders in the control group was determined through a structured interview conducted by trained clinicians.
Participants’ demographic data, including gender, child educational grade, parents’ educational grade, and parents’ age, were collected at the study’s outset. Subsequently, parents completed the SDSC, a Likert-type rating scale designed by Bruni et al. (Bruni et al., 1996). It was translated into Persian in 2014 and had previously been validated (Saffari et al., 2014). The SDSC comprises 26 questions examining various sleep disorder domains, including disorders of initiating and maintaining sleep (DIMS), sleep disorder of breathing (SDB), the disorder of arousal (DA), sleep-wake transition disorders (SWTD), disorders of excessive somnolence (DOES), and sleep hyperhidrosis (SHY) (Romeo et al., 2013).
The results of the two groups were compared to determine the validity of the content of the shortened questionnaire by calculating the content validity ratio and content validity index, the construct validity of the shortened questionnaire by correlation methods and confirmatory factor analysis, the validity of the structure from convergence and differential validity, and the predictive validity of the SDSC.
The study involved participants completing the questionnaire at the onset and again two weeks later. These responses underwent a meticulous analysis through factor analysis to discern patterns and assess discriminative items related to ADHD. The questionnaire’s outcomes were then synthesized, allowing for a comprehensive comparison between the two groups. This comparative analysis aimed to determine the content validity of the questionnaire using the content validity ratio and content validity index. Additionally, the study assessed the construct validity through correlation methods and confirmatory factor analysis. We also examined the validity of the structure via convergence and differential validity and explored the predictive validity of the SDSC. Notably, the intraclass correlation coefficient (ICC) was calculated based on repeated measurements to assess the test re-test reliability of the questionnaire.
The obtained data were entered into SPSS software, version 24 (SPSS Inc., Chicago, IL). Quantitative data were reported as Mean±SD, and qualitative data as frequency distribution (percentage). The independent t-test and chi-square test were used to analyze the data. P<0.05 were considered significant.
3. Results
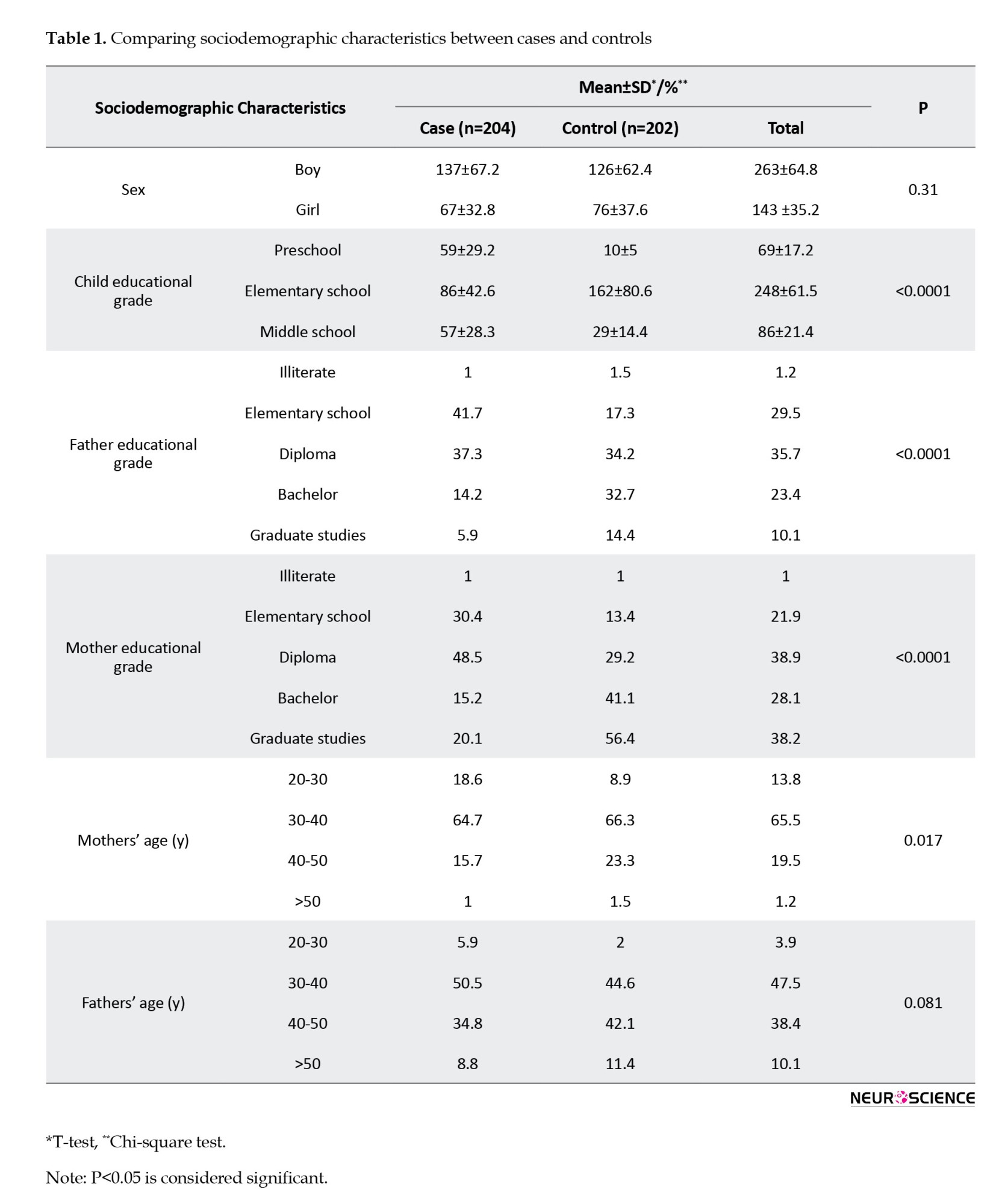
Table 1 details the sociodemographic characteristics of the study participants, consisting of 204 ADHD patients and 202 healthy controls. The male frequency was higher, with boys/girls ratios of 2.04/1 and 1.66/1 in the case and control groups, respectively. Notably, no significant differences were observed in age and sex between the two groups (P>0.05). However, there were significant distinctions in the educational levels of children and parents (P<0.05). Additionally, a significant difference was noted in mothers’ age but not fathers’ age.
The mean total score of the SDSC was significantly elevated in ADHD patients compared to controls (68.1±20.4 vs 57.3±18.2; P<0.05). Significant differences were also observed in specific subscales, including DIMS (21.6±7.5 versus 18.1±6.9; P<0.001), SDB (5.5±3.5 vs 4.9±2.5; P=0.006), DA (5.9±4.0 vs 4.4±2.7; P< 0.001), SWTD (17.8±6.6 vs 14.6±6.2; P<0.001) and SHY (4.5±3.5 vs 3.6±3.1; P=0.013), while no significant difference was found in the DOES (12.2±6.1 vs 11.4±5.9; P=0.208) subscale score (Table 2).

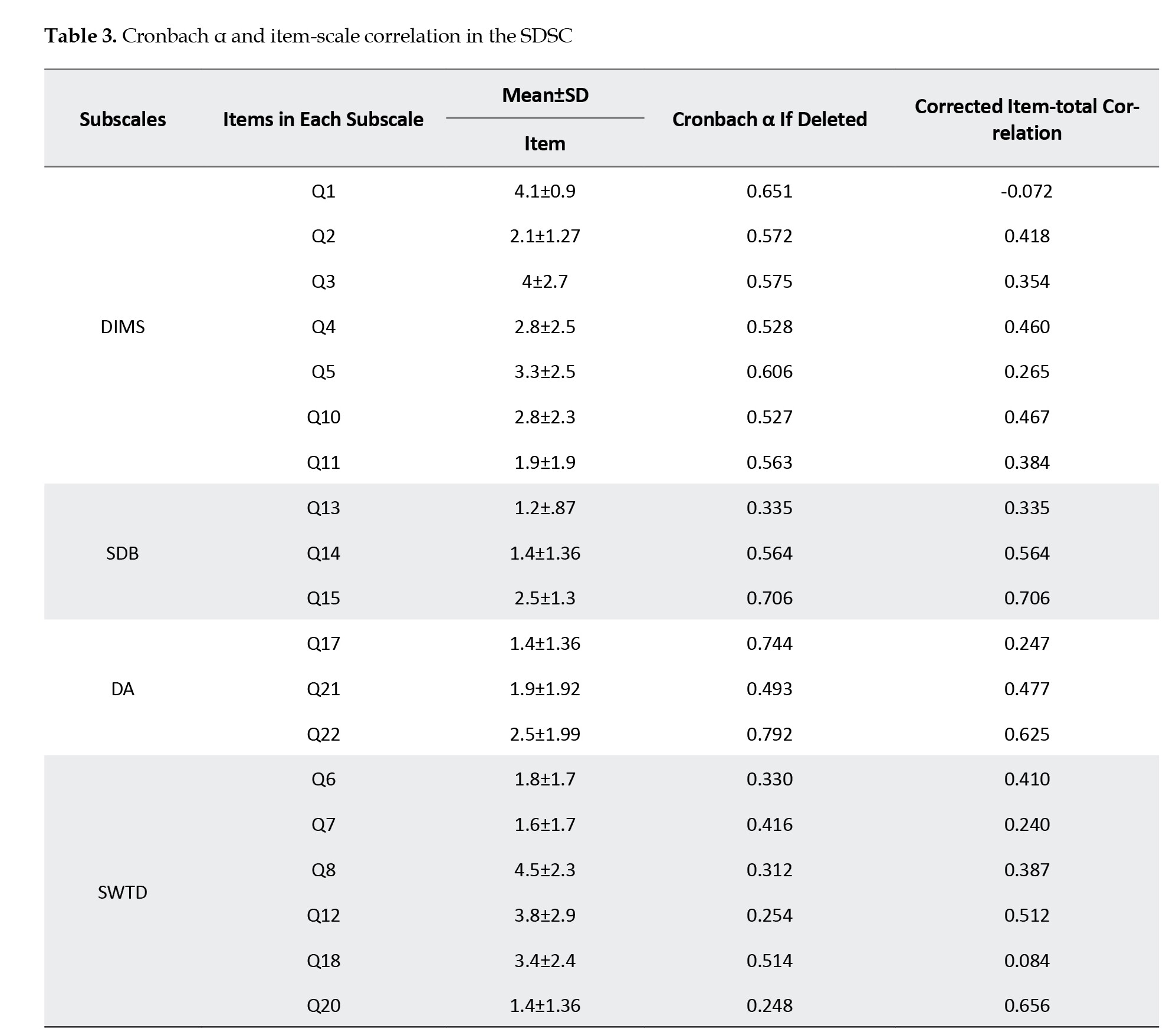
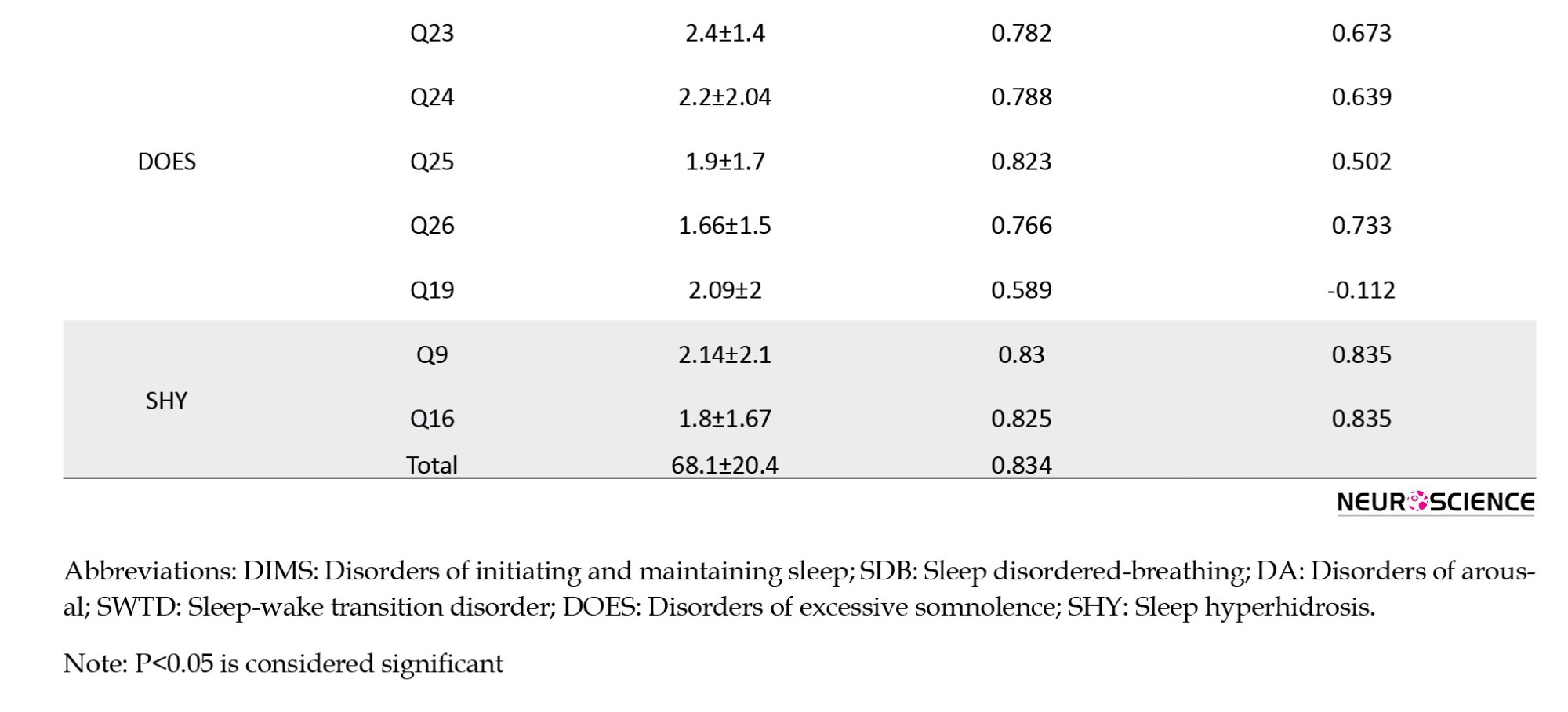
The SDSC demonstrated good internal consistency (the Cronbach α=0.83). Removal of all items, except for items 9 (Cronbach α=0.83), 16 (Cronbach α=0.83), and 25 (Cronbach α=0.82), resulted in comparable internal consistency. The corrected item-total correlation ranged from -0.112 to 0.89, as shown in Table 3. The highest mean corrected item-total correlation was observed for items 9 (falling sleep sweating) and 16 (night sweating) related to the SHY subscale (mean=0.835) (Table 3).
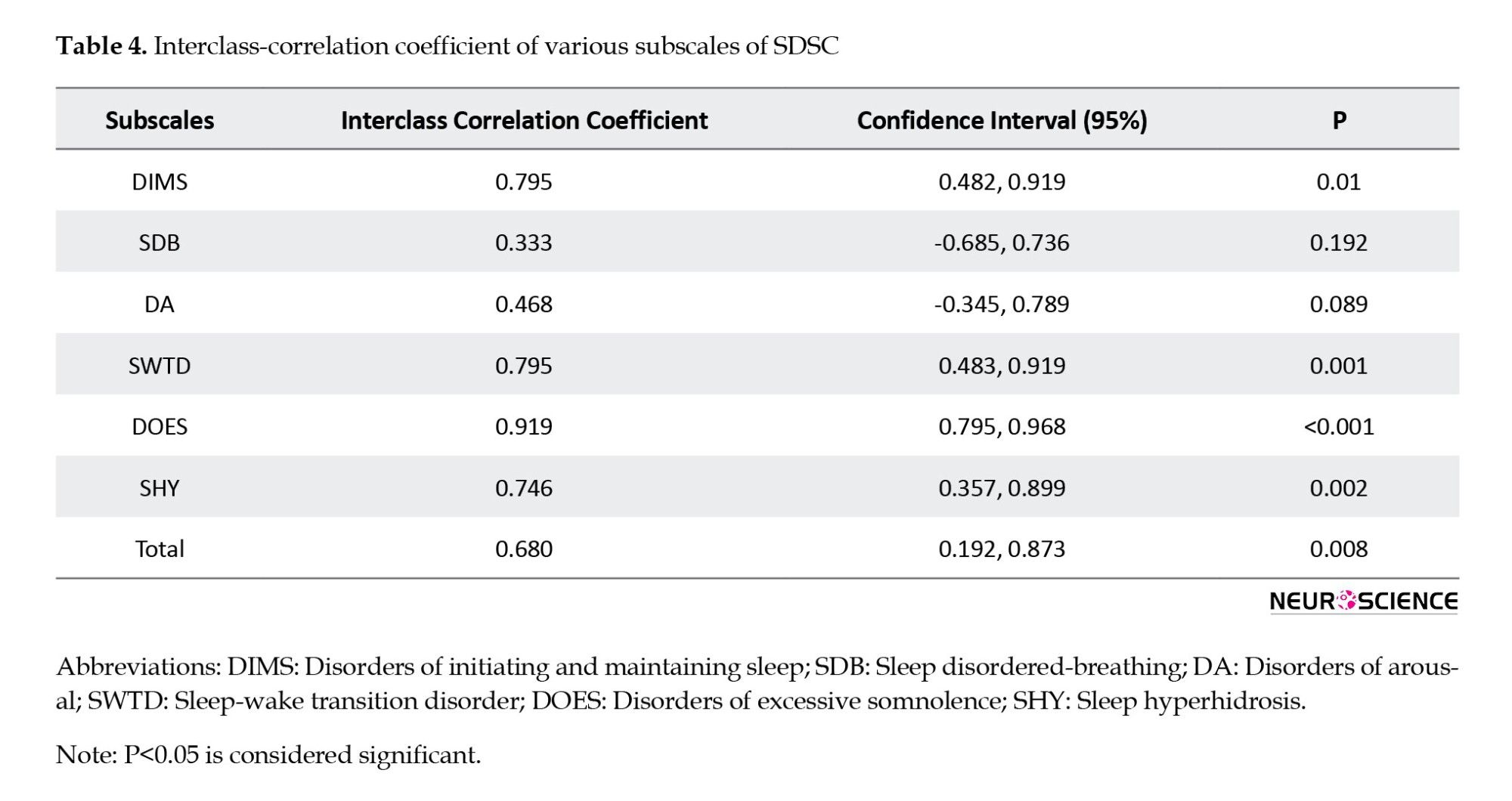
The test re-test ICC for SDSC subscales ranged from 0.33 to 0.919. Despite lower reliability in SDB (0.333) and DA (0.468) subscales, the overall SDSC exhibited good test re-test reliability with an ICC of 0.68 (P=0.008) (Table 4).
Convergent validity, assessed by the Pearson correlation coefficient (Table 5), revealed positive and statistically significant correlations (P<0.001) across all measures, indicating good convergent validity for the SDSC.

Table 6 presents the sensitivity, specificity, and area under the curve (AUC) for the SDSC total and its subscales.
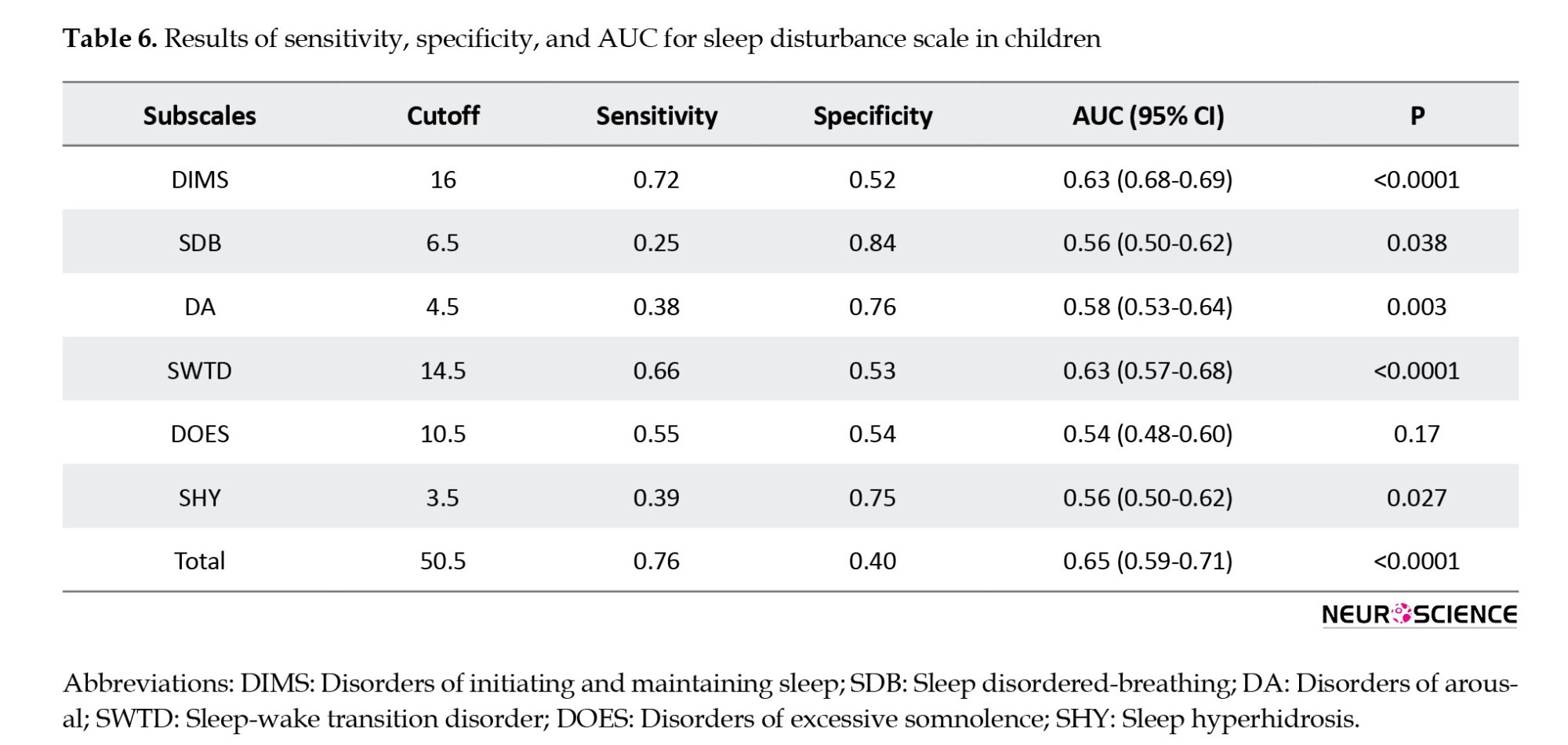
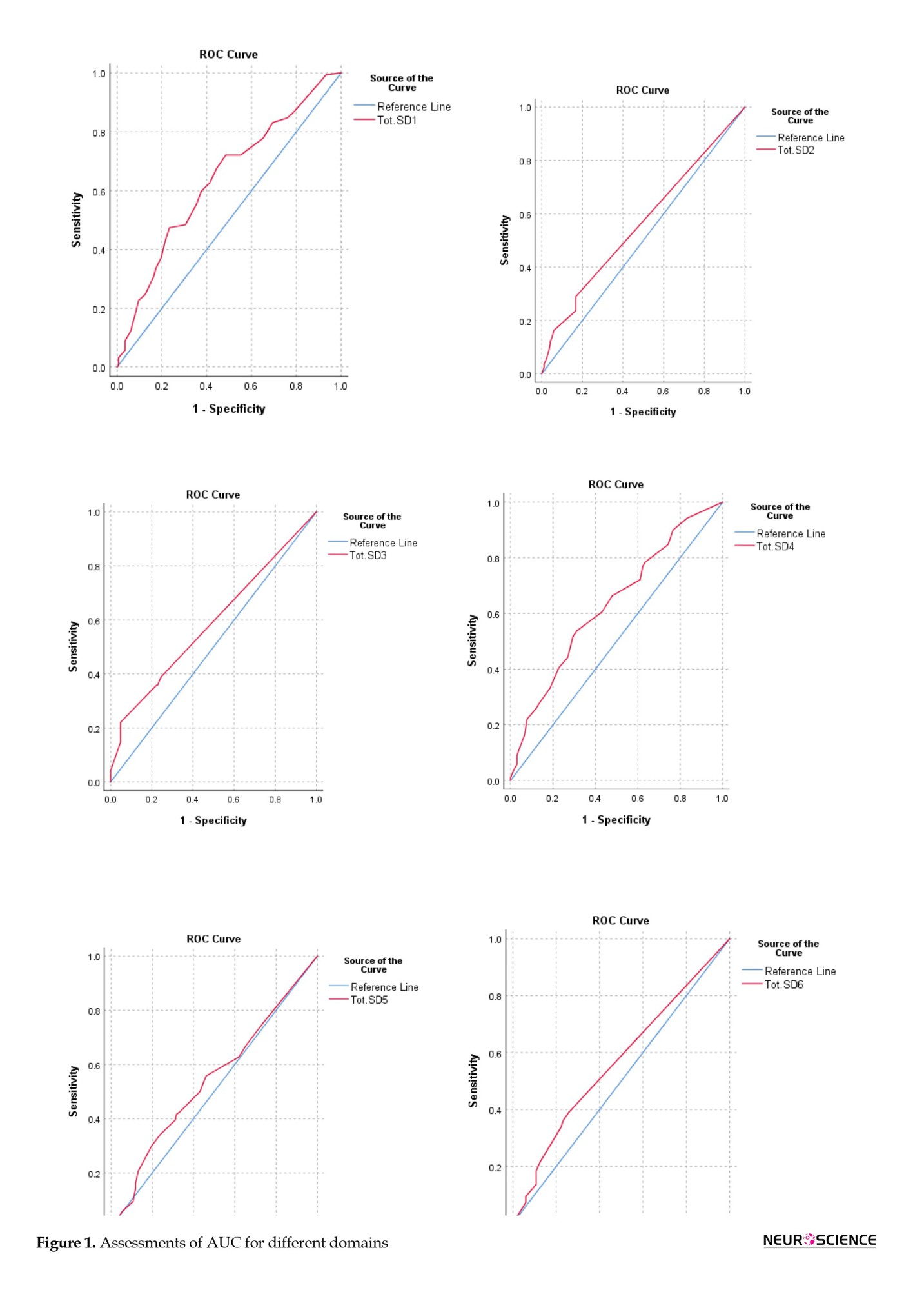
While AUCs for all subscales were below optimal levels (ranging from 0.54 to 0.63), the DMIS subscale showed the highest sensitivity at a cutoff score 16. The SDSC’s overall sensitivity and specificity at a cutoff score 50.5 were 76% and 40%, respectively, with an AUC of 0.65, indicating a 65% probability of accurately predicting sleep disturbance (Figure 1).
4. Discussion
This study underscores the critical role of addressing sleep disorders in individuals with ADHD, employing the SDSC for diagnosis. Patients with ADHD exhibited significantly higher SDSC scores, with the total score boasting a 76% sensitivity at a cutoff point of 50.5. The study delved into specific domains, revealing DIMS with the highest sensitivity and SDB with the highest specificity.
These findings highlight the effectiveness of SDSC in diagnosing sleep disorders within the ADHD population. Recent data has emphasized the significant role of sleep disorders in individuals diagnosed with ADHD (Silvestri et al., 2009; Vizzini et al., 2019; Bijlenga et al., 2019).Our study aligns with Wajszilber et al. (2018), stressing that sleep problems are reported in an estimated 25%–50% of individuals with ADHD, and diagnosing these issues could be crucial for providing proper treatments (Wajszilber et al., 2018). Furthermore, our research aligns with Craig et al. (2020), stressing the clinical importance of using appropriate questionnaires to diagnose sleep disorders in individuals with ADHD (Craig et al., 2020).
Sleep disturbances can influence cognitive functioning, connecting high-quality sleep with improved cognition in children and adolescents (de Bruin et al., 2015; Rey et al., 2020). On the other hand, disrupted sleep may result in cognitive deficits, affecting alertness, vigilance, attention tasks, and executive functions, especially in neurodevelopmental disorders such as ADHD (Killgore, 2010; Kirszenblat & van Swinderen, 2015).
Based on our study results, SDSC exhibits a 76% sensitivity in diagnosing sleep disorders. In Australia, Mancini and colleagues (2019) involved 307 children with ADHD who completed SDSC. They found that it was an effective diagnostic tool for sleep disorders in children with ADHD, with acceptable sensitivity. They reported sensitivities ranging from 46.2% to 72.8% in different domains of SDSC, and a total SDSC score cutoff of 48 showed 75% sensitivity (Mancini et al., 2019). Similarly, assessments by Ohi and colleagues in 2021 revealed positive correlations between Polygenic risk scores for ADHD and both initiating and DIMS disorders, as well as excessive somnolence. They also demonstrated that corrected total scores of SDSC could serve as a dependable factor in diagnosing sleep disorders with 70.6% sensitivity (Ohi et al., 2021). These findings align with our study, reinforcing the effectiveness of SDSC in diagnosing sleep disorders in ADHD patients.
We evaluated cutoff points for various SDSC domains, reporting their sensitivity and specificity. Our data revealed that DIMS had the highest sensitivity with a cutoff point of 16, and the SDB domain exhibited the highest specificity with a cutoff point 6.5. Thieux et al. evaluated SDSC and its various domains to diagnose sleep disorders in ADHD patients. Their findings concurred with ours, highlighting that DIMS, SDB, and DA domains demonstrate the highest sensitivity and specificity (Thieux et al., 2022).
The SDSC exhibits robust internal consistency. Our analysis of corrected item-total correlation identified specific items, particularly items 9 (falling asleep sweating) and 16 (night sweating), with the highest mean correlation, emphasizing the clinical importance of monitoring symptoms like night sweating when assessing sleep quality in children with ADHD (Kaplan et al., 1987).
Test re-test reliability is a critical aspect of assessing the stability and consistency of a measurement tool over time (Aldridge et al., 2017). our analysis revealed a relatively lower reliability in SDB and DA subscales. This finding suggests potential variability or sensitivity to external factors influencing these specific dimensions of sleep disturbances in children with ADHD. Further investigation is needed to understand these factors and enhance the measurement of these dimensions. Despite variations in specific subscales, the overall SDSC demonstrates favorable test re-test reliability, reinforcing its practicality for longitudinal assessments of sleep disturbances in children with ADHD.
Furthermore, the AUC, summarizing diagnostic accuracy (Eusebi, 2013), reflects the discrimination between individuals with and without the condition. In our study, an AUC of 0.65 suggests a moderate level of accuracy in predicting sleep disturbances using the SDSC. While not optimal, this accuracy level still provides valuable insights into the likelihood of sleep challenges in children with ADHD.
The clinical significance lies in the potential impact of diagnosing sleep disorders in ADHD patients using SDSC and implementing proper treatments, which could significantly improve the disease course. Further investigations are necessary to discern whether these disorders are linked to ADHD therapeutic strategies or independent conditions. Another advantage of SDSC is its simplicity, requiring minimal time, and parents can conveniently fill out the questionnaire in psychiatry offices’ waiting rooms. However, our study has limitations. The cross-sectional design limits our ability to establish causation between ADHD and sleep disturbances. Future research with longitudinal approaches could offer a more nuanced understanding of the dynamic relationship between these variables. Additionally, the reliance on subjective parental perceptions for assessing sleep disturbance severity introduces potential biases. Incorporating objective measurements like polysomnography could enhance the precision of our findings. It is important to note that while our study used the same terms for the 6 subscales of SDSC as in previous research, SDSC primarily provides trait/symptom dimensions rather than definitive sleep disorder diagnoses. This characteristic should be considered in the result interpretation. Furthermore, the study’s restricted population and the absence of comparisons with other questionnaires suggest the need for further research.
5. Conclusion
Patients with ADHD exhibited significantly higher SDSC scores compared to controls. The total SDSC score demonstrated a 76% sensitivity. Moreover, we noted significantly elevated scores in various domains of SDSC among ADHD patients. According to our findings, the DIMS domain had the highest sensitivity, and the SDB domain had the highest specificity. These results hold substantial clinical importance for the diagnosis and treatment of sleep disorders in individuals with ADHD.
Ethical Considerations
Compliance with ethical guidelines
All procedures performed in the study were in accordance with the ethical standards of the Isfahan University of Medical Science Research Committee, Isfahan, Iran, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all parents of participants included in the study.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Cnonceptualization, methodology, validation and writing: Faranak Ghadiry and Babak Amra; Data acquisition: Mohsen Vafaei Shahi; Software and data analysis: Avat Feizi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The author would like to extend thanks to all participants in this study.
References
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder characterized by inattention, hyperactivity, and impulsivity, which are pervasive, impairing, and otherwise age-inappropriate (Banaschewski et al., 2017). Difficulty regulating emotions or executive function are the main problems in patients with ADHD (Asherson et al., 2016).
ADHD is the most common behavioral disorder in childhood and adolescence, affecting about 3% to 5% of children before the age of seven. This complication is more common in children in elementary school and during puberty, and many patients get better with age (Thomas et al., 2015). It can be important to keep in mind that untreated hyperactivity is dangerous. Previous studies have shown that 25% to 40% of children with ADHD have not been treated (Singh et al., 2015).
The cause of most patients with ADHD is not yet clear, but it is thought to be a multifactorial disease with a genetic origin and related to the environment. The diagnosis of ADHD is a challenging issue due to assessments of normal levels of inattention, hyperactivity, and impulsivity, but this disease is mainly diagnosed by diagnostic and statistical manual of mental disorders-4th (DSM-IV) criteria (Dalsgaard et al., 2015; Hanć & Cortese, 2018).
Sleep disorders are common among patients with ADHD, which can be due to primary sleep disorders or side effects from stimulant medications (Vélez-Galarraga et al., 2016). Sleep disturbance could cause severe daily fatigue, mood problems, problems in attention and concentration, behavioral problems and disorders, as well as damage to physical health and reduced quality of life (QoL). The diagnosis of sleep disorder in patients with ADHD is a challenging issue (Cappe et al., 2017; Durmuş et al., 2017).
Polysomnography is recognized as the gold standard for objectively assessing sleep quality and difficulties (Ferreira et al., 2009), but its high cost and time-intensive nature limit its suitability for screening. In contrast, the sleep disturbance scale for children (SDSC), widely used for assessing sleep problems in children, offers a practical and cost-effective alternative. This informant-rated questionnaire, often completed by parents, evaluates specific sleep disorders and provides a comprehensive measure of overall sleep disturbance (Miano et al., 2016; Marriner et al., 2017; Van der Heijden et al., 2018). This study aimed to assess the validity of SDSC in children with ADHD.
2. Materials and Methods
This cross-sectional study was conducted between 2020 and 2021 at Khorshid Hospital, affiliated with Isfahan University of Medical Sciences, Isfahan City, Iran. The study involved 204 children diagnosed with ADHD and 202 healthy children as a control group. The study protocol was approved by the Research Committee and the Ethics Committee of Isfahan University of Medical Sciences.
Participants were recruited through a convenience sampling approach. The ADHD group consisted of children aged 6-15 years, newly diagnosed with ADHD by psychiatrists using the DSM-IV criteria. The exclusion criteria included psychiatric medication use within the past month, any sleep disorder, or previously diagnosed psychiatric disorders. Notably, the exclusion of participants with sleep disorders aimed to ensure that observed sleep disturbances were more likely associated with ADHD rather than co-occurring sleep disorders. All children in the case group underwent a psychiatric interview to exclude additional psychiatric disorders.
The control group, comprising 202 children without psychiatric disorders, was recruited from the same geographical area from schools, community centers, and pediatric clinics. The absence of psychiatric disorders in the control group was determined through a structured interview conducted by trained clinicians.
Participants’ demographic data, including gender, child educational grade, parents’ educational grade, and parents’ age, were collected at the study’s outset. Subsequently, parents completed the SDSC, a Likert-type rating scale designed by Bruni et al. (Bruni et al., 1996). It was translated into Persian in 2014 and had previously been validated (Saffari et al., 2014). The SDSC comprises 26 questions examining various sleep disorder domains, including disorders of initiating and maintaining sleep (DIMS), sleep disorder of breathing (SDB), the disorder of arousal (DA), sleep-wake transition disorders (SWTD), disorders of excessive somnolence (DOES), and sleep hyperhidrosis (SHY) (Romeo et al., 2013).
The results of the two groups were compared to determine the validity of the content of the shortened questionnaire by calculating the content validity ratio and content validity index, the construct validity of the shortened questionnaire by correlation methods and confirmatory factor analysis, the validity of the structure from convergence and differential validity, and the predictive validity of the SDSC.
The study involved participants completing the questionnaire at the onset and again two weeks later. These responses underwent a meticulous analysis through factor analysis to discern patterns and assess discriminative items related to ADHD. The questionnaire’s outcomes were then synthesized, allowing for a comprehensive comparison between the two groups. This comparative analysis aimed to determine the content validity of the questionnaire using the content validity ratio and content validity index. Additionally, the study assessed the construct validity through correlation methods and confirmatory factor analysis. We also examined the validity of the structure via convergence and differential validity and explored the predictive validity of the SDSC. Notably, the intraclass correlation coefficient (ICC) was calculated based on repeated measurements to assess the test re-test reliability of the questionnaire.
The obtained data were entered into SPSS software, version 24 (SPSS Inc., Chicago, IL). Quantitative data were reported as Mean±SD, and qualitative data as frequency distribution (percentage). The independent t-test and chi-square test were used to analyze the data. P<0.05 were considered significant.
3. Results
Table 1 details the sociodemographic characteristics of the study participants, consisting of 204 ADHD patients and 202 healthy controls. The male frequency was higher, with boys/girls ratios of 2.04/1 and 1.66/1 in the case and control groups, respectively. Notably, no significant differences were observed in age and sex between the two groups (P>0.05). However, there were significant distinctions in the educational levels of children and parents (P<0.05). Additionally, a significant difference was noted in mothers’ age but not fathers’ age.

The mean total score of the SDSC was significantly elevated in ADHD patients compared to controls (68.1±20.4 vs 57.3±18.2; P<0.05). Significant differences were also observed in specific subscales, including DIMS (21.6±7.5 versus 18.1±6.9; P<0.001), SDB (5.5±3.5 vs 4.9±2.5; P=0.006), DA (5.9±4.0 vs 4.4±2.7; P< 0.001), SWTD (17.8±6.6 vs 14.6±6.2; P<0.001) and SHY (4.5±3.5 vs 3.6±3.1; P=0.013), while no significant difference was found in the DOES (12.2±6.1 vs 11.4±5.9; P=0.208) subscale score (Table 2).

The SDSC demonstrated good internal consistency (the Cronbach α=0.83). Removal of all items, except for items 9 (Cronbach α=0.83), 16 (Cronbach α=0.83), and 25 (Cronbach α=0.82), resulted in comparable internal consistency. The corrected item-total correlation ranged from -0.112 to 0.89, as shown in Table 3. The highest mean corrected item-total correlation was observed for items 9 (falling sleep sweating) and 16 (night sweating) related to the SHY subscale (mean=0.835) (Table 3).


The test re-test ICC for SDSC subscales ranged from 0.33 to 0.919. Despite lower reliability in SDB (0.333) and DA (0.468) subscales, the overall SDSC exhibited good test re-test reliability with an ICC of 0.68 (P=0.008) (Table 4).

Convergent validity, assessed by the Pearson correlation coefficient (Table 5), revealed positive and statistically significant correlations (P<0.001) across all measures, indicating good convergent validity for the SDSC.

Table 6 presents the sensitivity, specificity, and area under the curve (AUC) for the SDSC total and its subscales.

While AUCs for all subscales were below optimal levels (ranging from 0.54 to 0.63), the DMIS subscale showed the highest sensitivity at a cutoff score 16. The SDSC’s overall sensitivity and specificity at a cutoff score 50.5 were 76% and 40%, respectively, with an AUC of 0.65, indicating a 65% probability of accurately predicting sleep disturbance (Figure 1).

4. Discussion
This study underscores the critical role of addressing sleep disorders in individuals with ADHD, employing the SDSC for diagnosis. Patients with ADHD exhibited significantly higher SDSC scores, with the total score boasting a 76% sensitivity at a cutoff point of 50.5. The study delved into specific domains, revealing DIMS with the highest sensitivity and SDB with the highest specificity.
These findings highlight the effectiveness of SDSC in diagnosing sleep disorders within the ADHD population. Recent data has emphasized the significant role of sleep disorders in individuals diagnosed with ADHD (Silvestri et al., 2009; Vizzini et al., 2019; Bijlenga et al., 2019).Our study aligns with Wajszilber et al. (2018), stressing that sleep problems are reported in an estimated 25%–50% of individuals with ADHD, and diagnosing these issues could be crucial for providing proper treatments (Wajszilber et al., 2018). Furthermore, our research aligns with Craig et al. (2020), stressing the clinical importance of using appropriate questionnaires to diagnose sleep disorders in individuals with ADHD (Craig et al., 2020).
Sleep disturbances can influence cognitive functioning, connecting high-quality sleep with improved cognition in children and adolescents (de Bruin et al., 2015; Rey et al., 2020). On the other hand, disrupted sleep may result in cognitive deficits, affecting alertness, vigilance, attention tasks, and executive functions, especially in neurodevelopmental disorders such as ADHD (Killgore, 2010; Kirszenblat & van Swinderen, 2015).
Based on our study results, SDSC exhibits a 76% sensitivity in diagnosing sleep disorders. In Australia, Mancini and colleagues (2019) involved 307 children with ADHD who completed SDSC. They found that it was an effective diagnostic tool for sleep disorders in children with ADHD, with acceptable sensitivity. They reported sensitivities ranging from 46.2% to 72.8% in different domains of SDSC, and a total SDSC score cutoff of 48 showed 75% sensitivity (Mancini et al., 2019). Similarly, assessments by Ohi and colleagues in 2021 revealed positive correlations between Polygenic risk scores for ADHD and both initiating and DIMS disorders, as well as excessive somnolence. They also demonstrated that corrected total scores of SDSC could serve as a dependable factor in diagnosing sleep disorders with 70.6% sensitivity (Ohi et al., 2021). These findings align with our study, reinforcing the effectiveness of SDSC in diagnosing sleep disorders in ADHD patients.
We evaluated cutoff points for various SDSC domains, reporting their sensitivity and specificity. Our data revealed that DIMS had the highest sensitivity with a cutoff point of 16, and the SDB domain exhibited the highest specificity with a cutoff point 6.5. Thieux et al. evaluated SDSC and its various domains to diagnose sleep disorders in ADHD patients. Their findings concurred with ours, highlighting that DIMS, SDB, and DA domains demonstrate the highest sensitivity and specificity (Thieux et al., 2022).
The SDSC exhibits robust internal consistency. Our analysis of corrected item-total correlation identified specific items, particularly items 9 (falling asleep sweating) and 16 (night sweating), with the highest mean correlation, emphasizing the clinical importance of monitoring symptoms like night sweating when assessing sleep quality in children with ADHD (Kaplan et al., 1987).
Test re-test reliability is a critical aspect of assessing the stability and consistency of a measurement tool over time (Aldridge et al., 2017). our analysis revealed a relatively lower reliability in SDB and DA subscales. This finding suggests potential variability or sensitivity to external factors influencing these specific dimensions of sleep disturbances in children with ADHD. Further investigation is needed to understand these factors and enhance the measurement of these dimensions. Despite variations in specific subscales, the overall SDSC demonstrates favorable test re-test reliability, reinforcing its practicality for longitudinal assessments of sleep disturbances in children with ADHD.
Furthermore, the AUC, summarizing diagnostic accuracy (Eusebi, 2013), reflects the discrimination between individuals with and without the condition. In our study, an AUC of 0.65 suggests a moderate level of accuracy in predicting sleep disturbances using the SDSC. While not optimal, this accuracy level still provides valuable insights into the likelihood of sleep challenges in children with ADHD.
The clinical significance lies in the potential impact of diagnosing sleep disorders in ADHD patients using SDSC and implementing proper treatments, which could significantly improve the disease course. Further investigations are necessary to discern whether these disorders are linked to ADHD therapeutic strategies or independent conditions. Another advantage of SDSC is its simplicity, requiring minimal time, and parents can conveniently fill out the questionnaire in psychiatry offices’ waiting rooms. However, our study has limitations. The cross-sectional design limits our ability to establish causation between ADHD and sleep disturbances. Future research with longitudinal approaches could offer a more nuanced understanding of the dynamic relationship between these variables. Additionally, the reliance on subjective parental perceptions for assessing sleep disturbance severity introduces potential biases. Incorporating objective measurements like polysomnography could enhance the precision of our findings. It is important to note that while our study used the same terms for the 6 subscales of SDSC as in previous research, SDSC primarily provides trait/symptom dimensions rather than definitive sleep disorder diagnoses. This characteristic should be considered in the result interpretation. Furthermore, the study’s restricted population and the absence of comparisons with other questionnaires suggest the need for further research.
5. Conclusion
Patients with ADHD exhibited significantly higher SDSC scores compared to controls. The total SDSC score demonstrated a 76% sensitivity. Moreover, we noted significantly elevated scores in various domains of SDSC among ADHD patients. According to our findings, the DIMS domain had the highest sensitivity, and the SDB domain had the highest specificity. These results hold substantial clinical importance for the diagnosis and treatment of sleep disorders in individuals with ADHD.
Ethical Considerations
Compliance with ethical guidelines
All procedures performed in the study were in accordance with the ethical standards of the Isfahan University of Medical Science Research Committee, Isfahan, Iran, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all parents of participants included in the study.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Cnonceptualization, methodology, validation and writing: Faranak Ghadiry and Babak Amra; Data acquisition: Mohsen Vafaei Shahi; Software and data analysis: Avat Feizi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The author would like to extend thanks to all participants in this study.
References
Aldridge, V. K., Dovey, T. M., & Wade, A. (2017). Assessing test-retest reliability of psychological measures. European Psychologist, 22(4), 207-218. [DOI:10.1027/1016-9040/a000298]
Asherson, P., Buitelaar, J., Faraone, S. V., & Rohde, L. A. (2016). Adult attention-deficit hyperactivity disorder: Key conceptual issues. The Lancet Psychiatry, 3(6), 568-578. [DOI:10.1016/S2215-0366(16)30032-3] [PMID]
Banaschewski, T., Becker, K., Döpfner, M., Holtmann, M., Rösler, M., & Romanos, M. (2017). Attention-deficit/hyperactivity disorder: a current overview. Deutsches Ärzteblatt International, 114(9), 149-159. [DOI:10.3238/arztebl.2017.0149] [PMID]
Bijlenga, D., Vollebregt, M. A., Kooij, J. J. S., & Arns, M. (2019). The role of the circadian system in the etiology and pathophysiology of ADHD: Time to redefine ADHD?. Attention Deficit and Hyperactivity Disorders, 11(1), 5–19. [PMID]
Bruni, O., Ottaviano, S., Guidetti, V., Romoli, M., Innocenzi, M., & Cortesi, F., et al. (1996). The Sleep Disturbance Scale for Children (SDSC). Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. Journal of Sleep Research, 5(4), 251–261. [DOI:10.1111/j.1365-2869.1996.00251.x] [PMID]
Cappe, E., Bolduc, M., Rougé, M. C., Saiag, M. C., & Delorme, R. (2017). Quality of life, psychological characteristics, and adjustment in parents of children with Attention-Deficit/Hyperactivity Disorder. Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 26(5), 1283–1294. [DOI:10.1007/s11136-016-1446-8] [PMID]
Craig, S. G., Weiss, M. D., Hudec, K. L., & Gibbins, C. (2020). The functional impact of sleep disorders in children with ADHD. Journal of Attention Disorders, 24(4), 499-508. [DOI:10.1177/1087054716685840] [PMID]
de Bruin, E. J., Dewald-Kaufmann, J. F., Oort, F. J., Bögels, S. M., & Meijer, A. M. (2015). Differential effects of online insomnia treatment on executive functions in adolescents. Sleep Medicine, 16(4), 510–520. [DOI:10.1016/j.sleep.2014.12.009] [PMID]
Dalsgaard, S., Leckman, J. F., Mortensen, P. B., Nielsen, H. S., & Simonsen, M. (2015). Effect of drugs on the risk of injuries in children with attention deficit hyperactivity disorder: A prospective cohort study. The Lancet Psychiatry, 2(8), 702-709. [DOI:10.1016/S2215-0366(15)00271-0] [PMID]
Durmuş, F. B., Arman, A. R., & Ayaz, A. B. (2017). Chronotype and its relationship with sleep disorders in children with attention deficit hyperactivity disorder. Chronobiology International, 34(7), 886-894. [DOI:10.1080/07420528.2017.1329207] [PMID]
Eusebi, P. (2013). Diagnostic accuracy measures. Cerebrovascular Diseases, 36(4), 267-272. [DOI:10.1159/000353863] [PMID]
Ferreira, V. R., Carvalho, L. B., Ruotolo, F., de Morais, J. F., Prado, L. B., & Prado, G. F. (2009). Sleep disturbance scale for children: translation, cultural adaptation, and validation. Sleep Medicine, 10(4), 457–463. [DOI:10.1016/j.sleep.2008.03.018] [PMID]
Hanć, T., & Cortese, S. (2018). Attention deficit/hyperactivity-disorder and obesity: A review and model of current hypotheses explaining their comorbidity. Neuroscience & Biobehavioral Reviews, 92, 16-28. [DOI:10.1016/j.neubiorev.2018.05.017] [PMID]
Kaplan, B. J., McNicol, J., Conte, R. A., & Moghadam, H. K. (1987). Sleep disturbance in preschool-aged hyperactive and nonhyperactive children. Pediatrics, 80(6), 839-844. [DOI:10.1542/peds.80.6.839] [PMID]
Killgore W. D. (2010). Effects of sleep deprivation on cognition. Progress in Brain Research, 185, 105–129. [PMID]
Kirszenblat, L., & van Swinderen, B. (2015). The Yin and Yang of Sleep and Attention. Trends in Neurosciences, 38(12), 776–786. [DOI:10.1016/j.tins.2015.10.001] [PMID]
Mancini, V. O., Rudaizky, D., Pearcy, B. T. D., Marriner, A., Pestell, C. F., & Gomez, R., et al. (2019). Factor structure of the sleep disturbance scale for children (SDSC) in those with attention deficit and hyperactivity disorder (ADHD). Sleep Medicine: X, 1, 100006. [DOI:10.1016/j.sleepx.2019.100006] [PMID]
Marriner, A. M., Pestell, C., Bayliss, D. M., McCann, M., & Bucks, R. S. (2017). Confirmatory factor analysis of the Sleep Disturbance Scale for Children (SDSC) in a clinical sample of children and adolescents. Journal of Sleep Research, 26(5), 587–594. [PMID]
Miano, S., Esposito, M., Foderaro, G., Ramelli, G. P., Pezzoli, V., & Manconi, M. (2016). Sleep-related disorders in children with attention-deficit hyperactivity disorder: Preliminary results of a full sleep assessment study. CNS Neuroscience & Therapeutics, 22(11), 906–914. [DOI:10.1111/cns.12573] [PMID]
Ohi, K., Ochi, R., Noda, Y., Wada, M., Sugiyama, S., & Nishi, A., et al. (2021). Polygenic risk scores for major psychiatric and neurodevelopmental disorders contribute to sleep disturbance in childhood: Adolescent Brain Cognitive Development (ABCD) Study. Translational Psychiatry, 11(1), 187. [DOI:10.1038/s41398-021-01308-8] [PMID]
Rey, A. E., Guignard-Perret, A., Imler-Weber, F., Garcia-Larrea, L., & Mazza, S. (2020). Improving sleep, cognitive functioning and academic performance with sleep education at school in children. Learning and Instruction, 65, 101270. [DOI:10.1016/j.learninstruc.2019.101270]
Romeo, D. M., Bruni, O., Brogna, C., Ferri, R., Galluccio, C., & De Clemente, V., et al. (2013). Application of the sleep disturbance scale for children (SDSC) in preschool age. European Journal of Paediatric Neurology: EJPN: Official Journal of the European Paediatric Neurology Society, 17(4), 374–382. [DOI:10.1016/j.ejpn.2012.12.009] [PMID]
Saffari, M., Gholamrezaei, A., Saneian, H., Attari, A., & Bruni, O. (2014). Linguistic validation of the Sleep Disturbance Scale for Children (SDSC) in Iranian children with Persian language. Sleep Medicine, 15(8), 998-1001. [DOI:10.1016/j.sleep.2014.03.021] [PMID]
Silvestri, R., Gagliano, A., Aricò, I., Calarese, T., Cedro, C., & Bruni, O., et al. (2009). Sleep disorders in children with Attention-Deficit/Hyperactivity Disorder (ADHD) recorded overnight by video-polysomnography. Sleep Medicine, 10(10), 1132–1138. [PMID]
Singh, A., Yeh, C. J., Verma, N., & Das, A. K. (2015). Overview of attention deficit hyperactivity disorder in young children. Health Psychology Research, 3(2), 2115. [DOI:10.4081/hpr.2015.2115] [PMID]
Thieux, M., Duca, M., Putois, B., Herbillon, V., Cottone, C., & Parmeggiani, A., et al. (2022). Sleep disorders and ADHD symptoms in children and adolescents with typical absence seizures: An observational study. Epilepsy & Behavior: E&B, 128, 108513. [DOI:10.1016/j.yebeh.2021.108513] [PMID]
Thomas, R., Sanders, S., Doust, J., Beller, E., & Glasziou, P. (2015). Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics, 135(4), e994–e1001. [DOI:10.1542/peds.2014-3482] [PMID]
van der Heijden, K. B., Stoffelsen, R. J., Popma, A., & Swaab, H. (2018). Sleep, chronotype, and sleep hygiene in children with attention-deficit/hyperactivity disorder, autism spectrum disorder, and controls. European Child & Adolescent Psychiatry, 27(1), 99–111. [PMID]
Vélez-Galarraga, R., Guillén-Grima, F., Crespo-Eguílaz, N., & Sánchez-Carpintero, R. (2016). Prevalence of sleep disorders and their relationship with core symptoms of inattention and hyperactivity in children with attention-deficit/hyperactivity disorder. European Journal of Paediatric Neurology: EJPN: Official Journal of the European Paediatric Neurology Society, 20(6), 925–937. [DOI:10.1016/j.ejpn.2016.07.004] [PMID]
Vizzini, L., Popovic, M., Zugna, D., Vitiello, B., Trevisan, M., & Pizzi, C., et al. (2019). Maternal anxiety, depression and sleep disorders before and during pregnancy, and preschool ADHD symptoms in the NINFEA birth cohort study. Epidemiology and Psychiatric Sciences, 28(5), 521–531. [DOI:10.1017/S2045796018000185] [PMID]
Wajszilber, D., Santiseban, J. A., & Gruber, R. (2018). Sleep disorders in patients with ADHD: Impact and management challenges. Nature and Science of Sleep, 10, 453-480. [DOI:10.2147/NSS.S163074] [PMID]
Type of Study: Original |
Subject:
Clinical Neuroscience
Received: 2023/04/29 | Accepted: 2024/03/2 | Published: 2025/03/1
Received: 2023/04/29 | Accepted: 2024/03/2 | Published: 2025/03/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








