Volume 15, Issue 3 (May & Jun 2024)
BCN 2024, 15(3): 301-316 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Adavi H, Kowsar R, Radahmadi M, Alaei H. Comparing Various Types of Chronic Psychological Stress on Cognitive Functions and Behaviors in Rats. BCN 2024; 15 (3) :301-316
URL: http://bcn.iums.ac.ir/article-1-2689-en.html
URL: http://bcn.iums.ac.ir/article-1-2689-en.html
Comparing Various Types of Chronic Psychological Stress on Cognitive Functions and Behaviors in Rats
1- Department of Animal Sciences, Faculty of Agriculture, Isfahan University of Technology, Isfahan, Iran.
2- Department of Physiology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran.
2- Department of Physiology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran.
Full-Text [PDF 1557 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Psychological stress is a common term to describe the processes that contribute to the onset and continuation of various mental and physical conditions. These stress types enable higher-order brain structures to provide additional interpretation of perceived danger (Fuchs & Flügge, 2003). Additionally, they need cortical processing and depend on former experiences or current activations. Moreover, the psychological information would be assembled within limbic circuits (hippocampus, amygdala, and prefrontal cortex) to induce neuroendocrine and behavioral responses (Fuchs & Flügge, 2003). Therefore, it is a common daily occurrence in today’s society that people experience various types of stress (Alkadhi, 2013). Stress activates the hypothalamic pituitary adrenal axis and the secretion of glucocorticoids or corticosteroids in rodents like corticosterone (CORT) (Radahmadi et al., 2015), which can affect cognitive functions, such as learning, memory, and mood states (Abou-Hany et al., 2018; Khani et al., 2018). Psychological stress could be categorized based on timing and type (Dastgerdi et al., 2020; Radahmadi et al., 2017). The effect of stress on physiological and psychological procedures is recognized by its stimulation aspects (Crestani, 2016). Some brain structures, including the limbic system and amygdala, are involved in causing various types of stress (Herman et al., 2005). Therefore, stress may result in a variety of behavioral issues (Hodgson et al., 2004; Watson et al., 2005). Nowadays, the most prevalent stress types in different societies are crowding, relocation, isolation, and restraint stress (emotional stress). Previous studies have reported that crowding, relocation, isolation, and restraint stress have destructive effects on physiological systems and behaviors (Chotiwat & Harris, 2006; Dastgerdi et al., 2017; Davenport et al., 2008; Eid et al., 2010; Hodgson et al., 2004; Watson et al., 2005). Social isolation stress has commonly coincided with anxiety-like behaviors, cognitive impairments, reduced social interactions, and weight loss (Qin et al., 2011). While stress has been indicated as beneficial or harmful to neural health, in some cases it has not affected neural health (Radahmadi et al., 2013). Therefore, stress may exhibit paradoxical effects on cognitive functions and behaviors (Schwabe & Wolf, 2013), depending on the secretion of stress hormones at different levels. Compared to physical stress, psychological stress affects the physiological system more adversely due to the interactions of the limbic system. However, the most prevalent psychological stress types in society that lead to such adverse effects are not indicated. Hence, understanding the effects of various types of prevalent social stress on cognitive performance and behavior is important. The current study investigates the effects of four major types of psychological stress (crowding, relocation, isolation, and restraint) on locomotor activity, learning, memory, anxiety-like behaviors, body weight differences (BWDs), serum CORT levels, and their correlations in rats.
2. Materials and Methods
Study animals
A total of 40 male Wistar rats, aged approximately 3 months (weight=250−300 g), were obtained from the Isfahan Royan Institute for the experiments. The rats were housed in similar cages with 42×27×15 cm3 dimensions (Tajhiz Gostar Omid Iranian Co., Tehran, Iran) under controlled conditions (light on from 07:00 to 19:00, 50%±5% humidity, and 23±2°C room temperature) and ere given ad libitum water. The study was approved by the Ethics Committee of Animal Use at the Isfahan University of Medical Sciences. After a 1-week adaptation period, the animals were randomly assigned to five equal groups (n=8) as follows: Control (Co), crowding stress (Cro-St), relocation stress (Rel-St), isolation stress (Iso-St), and restraint stress (Res-St). During the test period, the rats in the Co group were handled similarly to those in other groups. All behavioral tests were accomplished between 14:00 and 16:00 on day 21 (Figure 1).
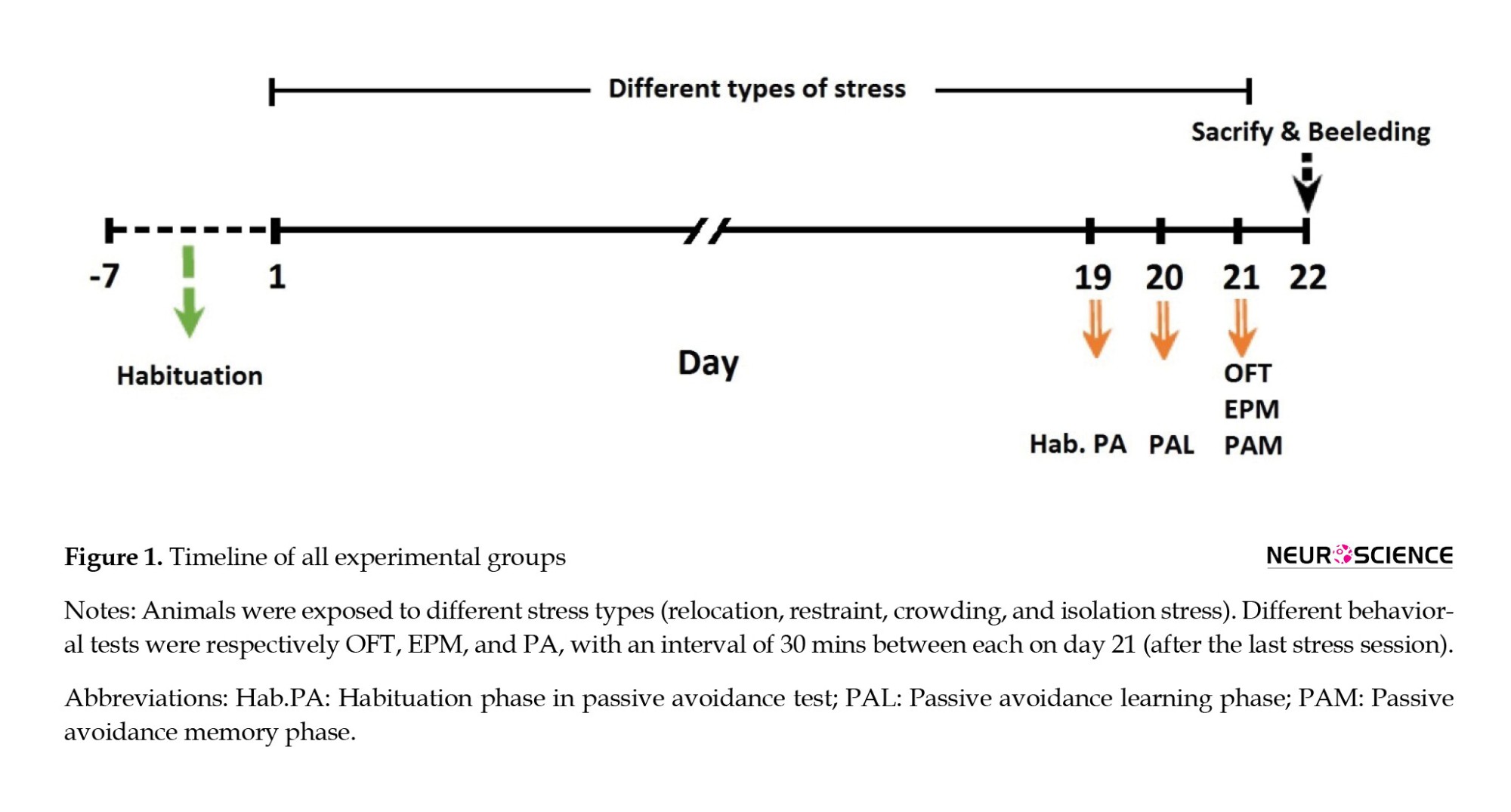
In this study, different behavioral tests, including the open field test (OFT), elevated plus maze test (EPM), and passive avoidance (PA) test were performed, respectively. To investigate the lasting effects of exposure to chronic stress on different behavioral variables, 30-min intervals were given between behavioral tests on day 21, after the last stress session. As shown in Figure 1, the passive avoidance test was performed as the last behavioral test in all experimental groups. Therefore, the received shock in the shuttle box did not affect the animals’ anxiety-like behaviors. All measured parameters in the OFT and EPM tests had changed due to the lasting effects of chronic stress.
Experimental procedures
Stress paradigm
Crowding stress was generated by increasing the population density in specified areas (Eid et al., 2010). In other words, inadequate space for the same number of subjects leads to crowding stress (Calhoun, 1973). Therefore, twice the original number of rats were placed in a normal cage (i.e. 8 rats instead of 4) to increase the population density and generate crowding stress (Eid et al., 2010). During the relocation stress, the animals experienced environmental changes and displacement disturbances as the new condition was felt as a threat to their lives (Watson et al., 2005). Accordingly, the second group was relocated to a new cage with an unfamiliar conspecific to generate relocation stress. Also, to induce isolation stress, the animals were placed in different cages separately (individual housing) before being placed in their home cage (group housing). During the stress period, each rat was placed in a cage in isolated conditions without other mates ( Khani et al., 2018; Song et al., 2021). Finally, in the last experimental stress group, the rats were placed in plexiglas cylindrical restrainers to generate restraint stress (Adachi et al., 2021), commonly characterized by either physical or physiological types of stressful stimuli (Dastgerdi et al., 2017; Sunanda et al., 2000). All of these stress types were induced for 21 consecutive days (6 h/day, from 08:00 to 14:00).
Behavioral paradigm
Passive avoidance test
PA was used to assess learning, memory, memory consolidation, and locomotor activity (Vohora et al., 2000). The PA apparatus (64×25×35 cm3) contained two identical rooms (light and dark, 32×25×35 cm3) with grid floors and a sliding door. A stimulator was used to administer electric shocks to the floor. The apparatus habituation impacts the performance of behavioral tasks negatively. The passive avoidance test includes three phases of habituation, learning, and memory. Based on the common protocols in several studies, on day 19, each rat was placed in the apparatus for 300 s (habituation) to diminish the novelty effects of the PA apparatus (Tatem et al., 2014). On day 20, the rats were placed in the lightroom individually (learning phase). The sliding door was raised after 10 s. As the rat fully entered the darkroom, this door was closed and a single electric shock (0.5 mA, 50 V, 3 s; once) was delivered to the animal’s foot (Huang et al., 2013). The initial latency (IL) to cross through the darkroom (the pre-shock latency) was recorded on day 20. On the next day (day 21), the memory phase was performed and the latency of entrance to the darkroom was measured up to a maximum delay of 300 s. The memory experiment was terminated if the rat did not enter the darkroom within 300 s. If the rat avoided the darkroom entry and stayed in the lightroom, a positive response was recorded (Dastgerdi et al., 2018). The total dark stay time was attributed to memory consolidation or storage of new information (Dastgerdi et al., 2018). The number of entries to the darkroom was interpreted as locomotor activity (Vohora et al., 2000). Also, the difference between the IL and latency after a day was considered the occurrence of learning (Dastgerdi et al., 2018). The animal’s ability to remember the foot shock was attributed to memory acquisition.
Elevated plus maze test
EPM test is commonly used to assess stress levels and anxiety-like behaviors (Walf & Frye, 2007). In this study, the EPM apparatus comprised a black opaque Plexiglas structure, elevated 70 cm above the ground. The apparatus consisted of two open arms (60×10×10 cm) and two closed arms (60×10×30 cm), extended from the central platform (10×10 cm). On day 21, each animal was separately placed in the center of the EPM apparatus, facing the open arms. According to the EPM criteria for anxious behaviors, an expert recorded the number of open arm entries (OAE) and the total time spent in the open arms (OAT) within 300 s (Foldi et al., 2019). The Equations 1 and 2 were used to calculate the percentage of OAE
1. (OAE%=[OAE/Total entries to the open and closed Arms]×100)
2. (OAT%=[OAT/300]×100) (Serafim et al., 2012).
Open field test
Another experiment to assess mobility and anxiety-like behaviors is OFT (Hines & Minton, 2012). The OFT equipment consists of a box-shaped platform (90×90×60 cm3), including painted grids that mark the floor with square crossings. In this experiment, the apparatus was placed in a silent room with no stressful stimulation. On day 21, the rats were placed separately at the center of the device before the test. Their activities within 300 s were recorded by a mounted video camera that had tracking software (Ranjbar et al., 2017). Each animal was only tested once in this apparatus. The number of passages through the center of the platform and the total distance traveled on this platform were recorded as indices for anxiety-like behaviors and locomotor activity (Ranjbar et al., 2017). After each experiment, the rat was removed from the apparatus. Then, the square was wiped with a cotton towel (soaked in 70% alcohol) to eliminate the odorant signals (Quillfeldt, 2016).
Determination of serum CORT levels
On day 22, the rats were anesthetized with an intraperitoneal injection of urethane (1.5 g/kg; Sigma-Aldrich Chemical Co., USA), and then sacrificed between 16:00 to 17:00. The blood samples were taken from the animal’s trunk. Subsequently, the serum was separated by centrifugation (6000 rpm, 20 min) to be stored at -80°C until the analyses. The serum CORT levels were measured using a commercial enzyme-linked immunosorbent assay CORT kit (Zellbio Co., Germany). The detection limit for the rat CORT was set to 0.1–20 ng/mL and sensitivity at 0.05 ng/mL (coefficient of variation percentage [C.V.%] for the intra- and inter-assay was less than 10% and 12%, respectively).
Body weight differences
The body weight was measured on day 1 and day 21. The difference between the final and initial weight for each animal was calculated by Equation 3:

Statistical analysis
All data were analyzed using the one-way analysis of variance, followed by the Tukey post hoc test for multiple groups. In addition, the paired sample t-test was used to compare the IL and the latency after a day (within groups). Using the Pearson correlation analysis (coefficient of determination [R2]), the correlation analyses of behavioral tests and BWDs with serum CORT levels were investigated. Furthermore, all data were estimated as Mean±SE of the mean. Meanwhile, a P<0.05 was considered statistically significant. The calculations were performed using the SPSS software, version 26 (IBM SPSS Inc., Chicago, USA).
3. Results
Effects of stress on the passive avoidance test
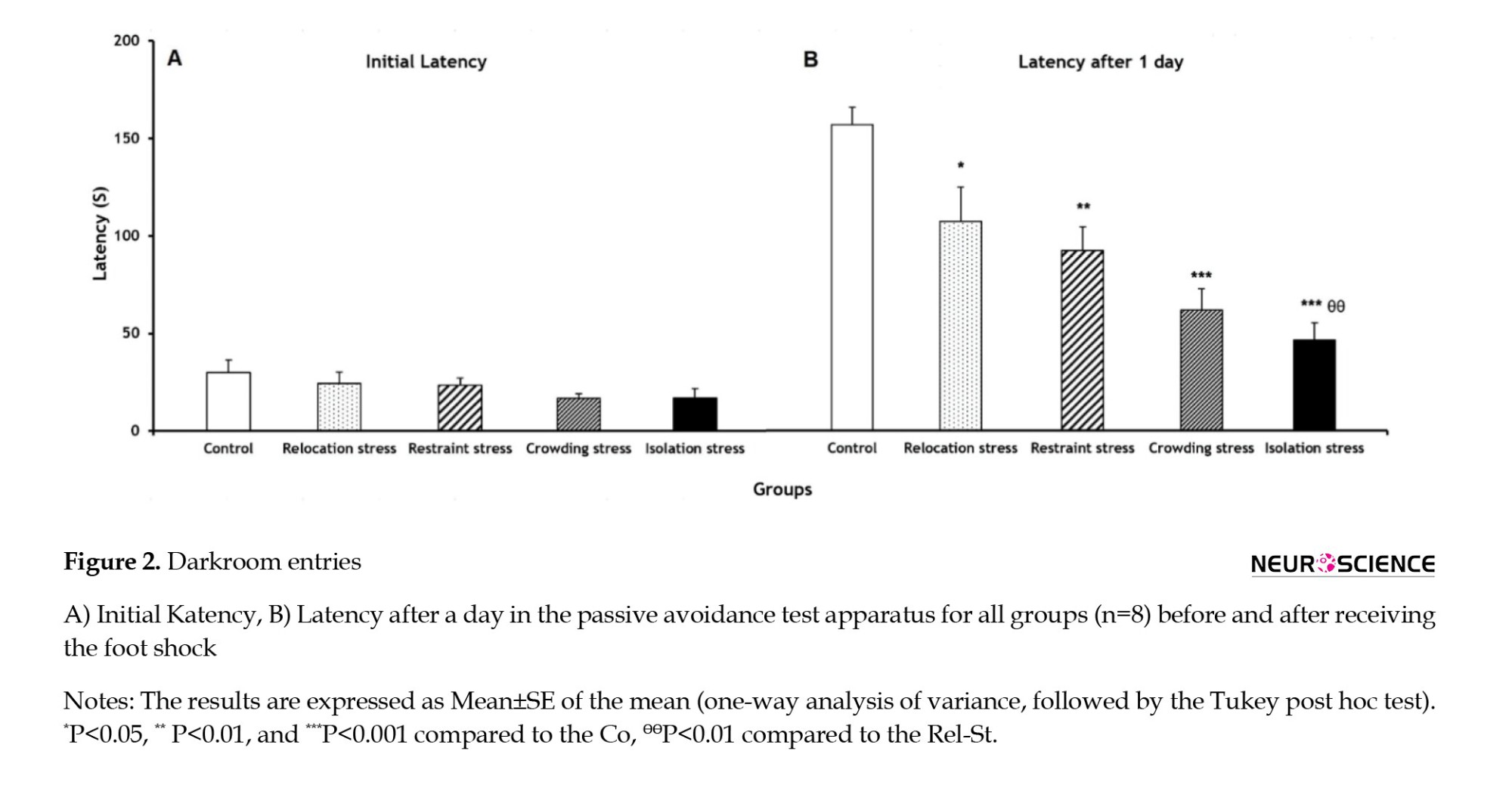
As shown in Figure 2A, IL had no significant difference in the experimental groups. The latency of entrance to the darkroom after a day was significantly lower in the Rel-St, Res-St, Res-St, Cro-St, and Iso-St groups (P<0.05, P<0.01, P<0.001, and P<0.001, respectively) compared to the Co group (Figure 2B); accordingly, memory impairment occurred as a result of different stress types, particularly in the Cro-St and Iso-St groups. Also, the latency after a day in the Iso-St group was significantly lower (P<0.01) compared to the Rel-St group (Figure 2B).
IL and latency after a day were analyzed using a paired-sample t test to evaluate the within-group latency changes. Significant differences were observed between IL and the latency after a day in all experimental groups (Co group: P<0.001; Rel-St, Res-St, and Cro-St groups: P<0.01; Iso-St group: P<0.05). These results indicated the occurrence of learning in these groups (Figure 3). However, the lowest and highest degrees of learning occurred in the Iso-St and Co groups, respectively.

The total dark stay time was significantly higher in the Res-St, Cro-St, and Iso-St groups (P<0.05, P<0.01, and P<0.001, respectively). However, compared to the and Co and Rel-St group, it was significantly higher only in the Iso-St group (P<0.05) (Figure 4A).
The number of entries to the darkroom had no significant differences in the Rel-St, Res-St, and Cro-St groups compared to the Co group. However, it was significantly lower in the Iso-St group (P<0.05) compared to the Co group. These results suggested locomotor activity reduction in the PA apparatus due to isolation stress (Figure 4B).

Effects of stress on the elevated plus maze test
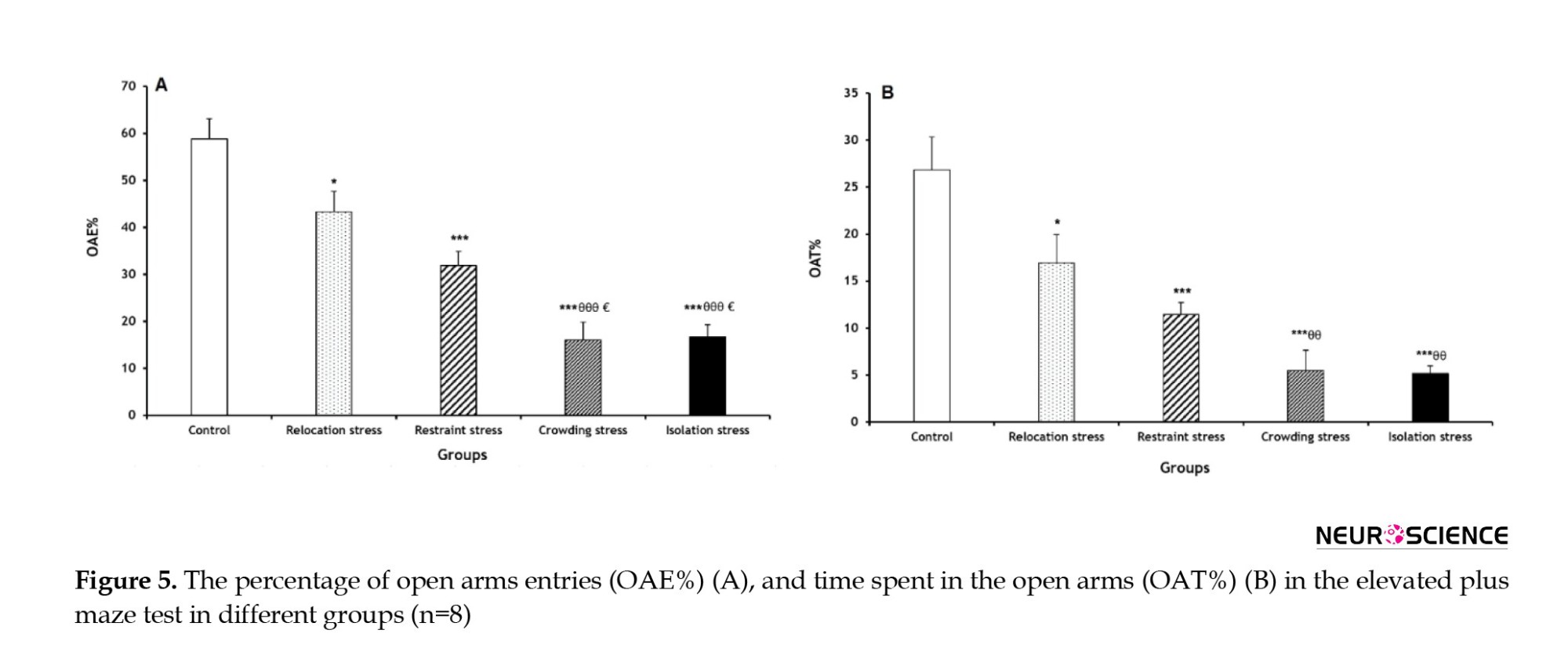
The OAE% decreased significantly in all stress groups (Rel-St group: P<0.05; other stress groups: P<0.001) compared to the Co group. Furthermore, OAE% had significant decreases in the Cro-St and Iso-St groups (both P<0.001) compared to the Rel-St group. Similarly, OAE% was significantly lower in the Cro-St and Iso-St groups (both P<0.05) compared to the Res-St group (Figure 5A).
A significant reduction of OAT% was observed in all stress groups (Rel-St group: P<0.05; other stress groups: P<0.001) compared to the Co group. Furthermore, OAE% in the Cro-St and Iso-St groups showed significant decreases (both P<0.01) compared to the Rel-St group (Figure 5B).
Effects of stress on the open field test
The number of entries to the platform’s center was significantly lower in the Cro-St and Iso-St groups (both P<0.01) compared to the Co group (Figure 6A). Also, this value had a significant decrease in the Cro-St and Iso-St groups (both P<0.01) in comparison with the Rel-St group (Figure 6A), although it was significantly lower in the Cro-St and Iso-St groups (both P<0.05) compared to the Res-St group.
The time spent in the central area of the OFT platform showed a significant decrease only in the Iso-St group (P<0.05) compared to the Co group (Figure 6B).

A significant decrease in the total distance traveled was observed in all stress groups (Res-St, Rel-St, and Cro-St groups: P<0.01; Iso-St group: P<0.001) compared to the Co group (Figure 6C). However, the highest decrease in exploration activities was observed in the Iso-St group in comparison with other stress groups.
Effects of stress on serum CORT levels
Serum CORT levels increased in all stress groups (Rel-St group: P<0.05; Res-St group: P<0.01; Cro-St and Iso-St groups: P<0.001) compared to the Co group. Furthermore, the serum CORT levels showed significant enhancement in the Iso-St group (P<0.05) compared to the Rel-St group (Figure 7).

Effects of stress on body weight differences
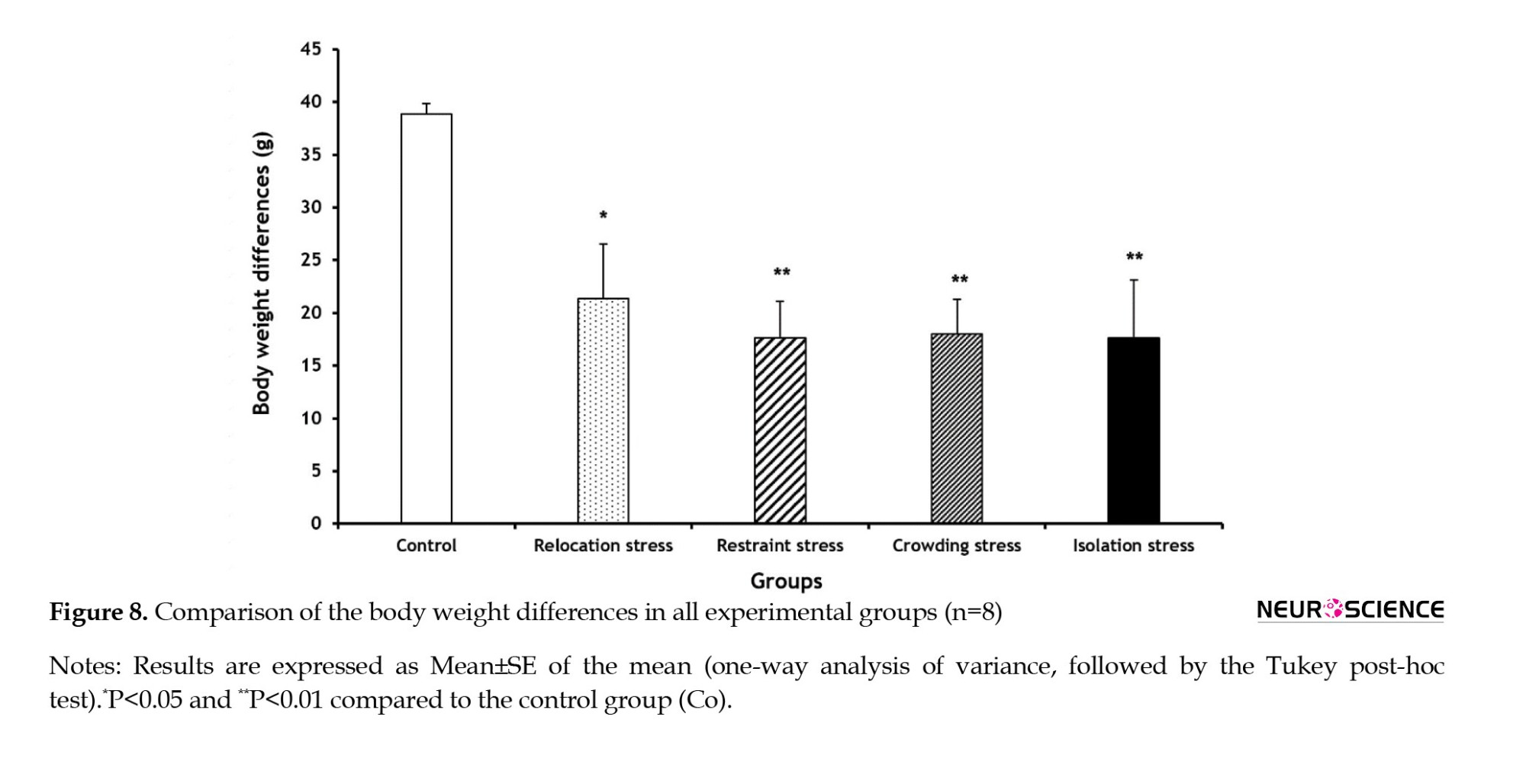
Compared to the Co group, the BWDs significantly declined in all stress groups (Rel-St group: P<0.05; other stress groups: P<0.01) compared to the Co group (Figure 8).
Correlation analyses of behavioral tests and body weight differences with serum CORT levels
In the correlation analysis of the PA test data, the latency after a day exhibited no significant correlation with serum CORT levels in the Co group (R2=0.2327). However, they had significant negative correlations in the Rel-St, Res-St, Cro-St, and Iso-St groups (R2=0.5934, 0.2619, 0.5945, and 0.6616, respectively; P<0.05) (Figure 9A). These findings supported the proposition that the serum CORT levels should be involved in the memory impairment in the Cro-St and Iso-St groups, as per the PA test.
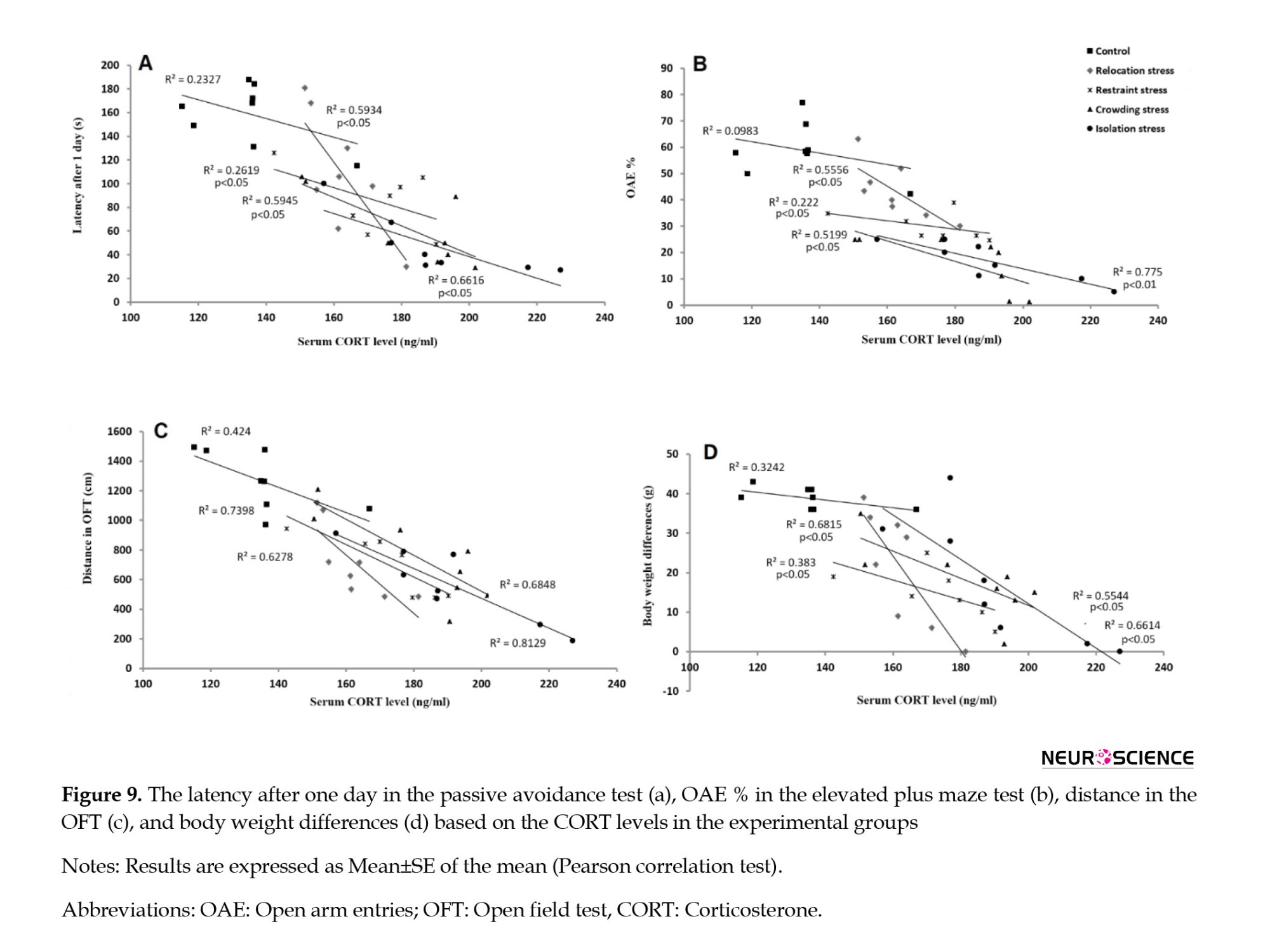
In the correlation analysis of the EPM test data, the OAE% presented no significant correlation with serum CORT levels in the Co group (R2=0.983). However, they had significant negative correlations in the Rel-St, Res-St, and Cro-St groups (R2=0.5556, 0.2220, 0.5945, and 0.5199, respectively; P<0.05) and Iso-St group (R2=0.775; P<0.01) (Figure 9B). These findings supported the proposition that serum CORT levels should be involved in anxiety-like behaviors in all stress groups, as per the EPM test.
In the correlation analysis of the OFT data, the total distance traveled revealed no significant correlation with serum CORT levels in the Co group (R2=0.424). According to Figure 9C, there were significant negative correlations in the Rel-St, Res-St, and Cro-St groups (R2=0.6278, 0.7398, R2=0.6848, respectively; P<0.05 in all groups) and Iso-St group (R2=0.8129; P<0.01). These findings supported the proposition that serum CORT levels should be involved in locomotor activity in all stress groups, as per the OFT.
Another correlation analysis between the BWD data and serum CORT levels exhibited no significant correlation in the Co group (R2=0.3242). However, significant negative correlations were observed in the Rel-St, Res-St, Cro-St, and Iso-St groups (R2=0.6815, 0.383, 0.5544, and 0.6614, respectively; P<0.05) (Figure 9D). These findings supported the proposition of serum CORT levels should be involved in body weight loss under stress.
4. Discussion
This study investigated the effects of four major types of psychological stress, namely crowding, relocation, isolation, and restraint (emotional stress), on learning, memory, memory consolidation, locomotor activity, anxiety-like behaviors, and BWDs in rats, as well as the correlations between their results.
According to the present PA data, learning occurred at different levels in all experimental groups. However, the lowest degree of learning occurred under the isolation stress. In line with current findings, learning was reported to have occurred under stress (Dastgerdi et al., 2018) because stress influences the onset and intensity of learning as a cognitive brain function (Rafah Sami, 2009). Although stress did not impede learning, it impaired brain performance byblocking the changes regarding habit memory formation (Schwabe et al., 2010). The impact of stress on learning has nevertheless been controversial. Based on different studies, stress either adversely or positively affected learning, or did not affect it at all depending on various high and low ranges of stress curve (distress and eustress, respectively) (Joëls et al., 2006; Rudland et al., 2020; Salehi et al., 2010). Moreover, stress altered the equilibrium between multiple underlying systems involved in learning and memory (Vogel & Schwabe, 2016).
According to other PA findings, memory was impaired in all chronic stress conditions, and memory consolidation was impaired by restraint, crowding, and especially isolation stress. Further findings related to the hormonal levels indicate that stress-driven memory deficits mainly occurred because of the changes in the CORT levels. Meanwhile, the correlations between our findings in the PA test with the serum CORT levels verify this proposition. Previous studies have stated chronic stress as an inevitable phenomenon that has impaired memory through the secretion of stress hormones (e.g. CORT) and other neurochemical factors (Jeong et al., 2006; Sandi & Pinelo-Nava, 2007; Sunanda et al., 2000). In addition, the comparison between previous studies demonstrated that isolation stress was more destructive to memory processing compared to restraint stress (Hosseini Dastgerdi et al., 2021; Khani et al., 2022). Other findings in this study suggested that cognitive performance strongly corresponds with social density (the average conspecific encounter rate in an animal population). A research study indicated that social density was influenced by the physical area and availability of resources in the habitat (Love & Zelikowsky, 2020). In humans and rodents, social stress could be triggered by interpersonal encounters, arguments, and fights (Love & Zelikowsky, 2020). In comparison to moderate levels of social density, extremely low or high levels of social density create a situation, in which the nervous system may not efficiently handle stress, especially chronic stress. With the increase in population, the behavior of individuals changes. Crowding increases the stress level as the competition for limited resources exacerbates and leads to increased aggression (Agrell et al., 1995).
According to the result of the PA test, locomotor activity was decreased in the subjects enduring isolation stress. However, the effect of stress on locomotor activity remains paradoxical as reduced (Sestakova et al., 2013) or increased locomotor activity (Weiss et al., 2000) is discussed in various studies. In these studies, besides behavioral assessment methods, stress duration and type have influenced the results concerning locomotor activity as well (Ranjbar et al., 2016). Along with altered secretion of hormones like glucocorticoids (Miranda & Oliveira, 2015), different mechanisms might be involved in stress-related memory impairment and behavioral changes. This includes the secretion of neurotransmitters (serotonin, dopamine, and norepinephrine) (Brenes et al., 2008; Dalesman & Lukowiak, 2011) and brain morphological changes (reduced expression of new neurons, synaptic proteins, dendritic density and length of neurons) (Bianchi et al., 2006).
The findings of the EPM test showed a significant reduction in the time spent in the EPM open arm and the number of entries to the open arm in all stress groups. The crowding and isolation stress increased anxiety-like behaviors more than other types of stress. Another study demonstrated that animals’ behaviors on the EPM platform were influenced by the stress type (Nazeri et al., 2017). In the present study, the increased serum CORT levels seemed to elevate anxiety-like behaviors. In line with other studies, the correlations between EPM findings and serum CORT levels confirmed that CORT has an influential role in causing anxiety-like behaviors. As such, longer and continuous periods of social isolation induce a cascade of negative behaviors in animal models, humans, and neural mechanisms, facilitating this shift (Love & Zelikowsky, 2020). The network organization of structural connectomes will begin to differ in stress conditions related to social isolation. For instance, some measures of the network structure, such as modularity (i.e. the strength of network division into modules) and small-worldness (the degree a network could be cluster-organized) decrease, indicating greater homogeneous connections (Liu et al., 2016). These changes depend on the outcome of the disrupted inter-hemispheric and inter-modular connections in the dorsolateral orbitofrontal cortex (Liu et al., 2016). Other studies have confirmed the association of social isolation with decreased myelination, altered dendritic development, decreased plasticity in the prefrontal cortex (Makinodan et al., 2012; Medendorp et al., 2018), and changes in the prefrontal cortex connectivity (Hermes et al., 2011), in particular concerning the prefrontal cortex-amygdala circuit (Castillo-Gómez et al., 2017). Also, the serotonergic fiber density in the inferior colliculus is a factor that is reduced by developmental isolation (Keesom et al., 2017). Moreover, chronic isolation stress induces a phenotype with similar aspects to anxiety, depression, and social withdrawal in adult rodents (Ieraci et al., 2016; Liu et al., 2012; Scaccianoce et al., 2006).
Based on the present OFT findings, the exploration activity was decreased in the subjects of the Iso-St and Cro-St groups that exhibited anxiety-like behaviors. Therefore, isolation and crowding stress types were more destructive than the other stress groups in this study. The locomotor activity decreased in all stress groups, especially in the Iso-St group. The changes in locomotor activity on the OFT platform were related to the serum CORT levels and were accordingly confirmed by the correlation analysis of the OFT findings with serum CORT. Another study reported that crowded housing (for mice) reduced exploration, locomotor activity, and anxiety-like behaviors in the OFT and EPM tests (Reiss et al., 2007). Furthermore, environmental factors seem to affect the expression of behavioral phenotypes. Therefore, social housing, as a stress factor, could affect psychological reactivity significantly. Also, there were locomotor activity differences in response to various stress types in the OFT and PA tests. Some behaviors, like locomotor activity, originate from certain brain areas; hence, their evaluation requires specific behavioral methods. In other words, specific behavioral tests should be considered for different behavioral assessments. Moreover, the open field is a more specific test for the evaluation of the passive avoidance test.
Based on current findings regarding hormonal changes, different stress types, particularly crowding and isolation stress, increased serum CORT levels more significantly than other stress types. According to previous studies, adrenal gland weight increased with the greater population density, indicating a probable increase in adrenal function (Love & Zelikowsky, 2020). In addition, isolation, restraint, relocation, and crowding stress increased CORT levels in rats (Djordjevic et al., 2003; Khani et al., 2018; Radahmadi et al., 2020). In another study, isolation stress strongly affected behavior but did not enhance plasma CORT levels, which were induced by other stressors (Scaccianoce et al., 2006). These differences might be related to the methodology, age, gender, physical area, as well as stress duration, and type (Radahmadi et al., 2017; Ranjbar et al., 2016). It is critical to determine the time when social isolation level and duration begin to have detrimental effects on the subject (Love & Zelikowsky, 2020). The role of other variables, such as the group size and housing duration, should be considered as well (Van Loo et al., 2001).
According to BWD findings, all types of chronic stress decreased body weight gain significantly. A relationship between weight changes and CORT levels should be well noted as the BWD findings and serum CORT correlation confirmed it. Meanwhile, some metabolic processes are mediated by glucocorticoids; thus, psychological stress could lead to body weight loss (Qin et al., 2011; van der Kooij et al., 2018). However, it was previously indicated that crowding increased adiposity without weight gain (Lin et al., 2015). Concerning stress-related body weight loss, a study has reported that epinephrine and norepinephrine stimulated hormone-sensitive lipase, whereas cortisol increased lipid cell sensitivity to epinephrine and norepinephrine (Lafontan & Langin, 2009). The secretion of corticotrophin-releasing hormones due to stress could decrease food ingestion and body weight (Heinrichs et al., 2001). The BWDs were related to stress exposure (Ranjbar et al., 2016). In another research study, various types of chronic stress, except chronic restraint stress, induced body weight loss because of the stress exposure (Marin et al., 2007). Finally, the potential effects of stress caused by social density could be highlighted as a neuroendocrine stress response, regulated or deregulated by the hypothalamic pituitary adrenal axis, as well as behavioral and neural alterations that are either primary or secondary responses to psychogenic stress (Love & Zelikowsky, 2020). However, understanding those brain mechanisms concerning chronic social stress that have such subserving adaptive functions should be of primary concern. This is because social stress is the major cause of stress stimuli in humans that lead to psychopathology. However, further cellular, biochemical, and structural research is needed to explain its underlying physiological mechanisms.
5. Conclusion
Overall, learning occurred at different levels in all experimental groups although the lowest level of learning occurred under isolation stress conditions. The crowding and isolation stress, as two models of social density stress, had further destructive effects on the impairment of cognitive functions in comparison with the relocation and emotional stress. As such, these stress models severely impair learning, memory, memory consolidation, locomotor activity, and body weight. The crowding and isolation stress increased anxiety-like behaviors and serum CORT levels more than other types of stress, (i.e. relocation and restraint stress). Thus, stress, which was caused by social density (housing density: Crowding and spatial isolation), led to the most negative effects on memory and mood, probably due to different CORT levels, as the main stress hormone. Finally, high or low populations of social density may create a condition, in which the nervous system could not efficiently handle stress, at chronic levels in particular.
Ethical Considerations
Compliance with ethical guidelines
All experiments were approved by the Research and Ethics Committee of Isfahan University of Medical Sciences (Code: IR.MUI.Research.REC.1399.677).
Funding
This work was partly supported by the Isfahan University of Technology.
Authors' contributions
Conceptualization, study design, methodology and writing: Maryam Radahmadi; Experiments: Hamed Adavi; Logistic support: Maryam Radahmadi and Rasul Kowsar; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The the authors thank the Isfahan University of Medical Sciences for the support.
References
Psychological stress is a common term to describe the processes that contribute to the onset and continuation of various mental and physical conditions. These stress types enable higher-order brain structures to provide additional interpretation of perceived danger (Fuchs & Flügge, 2003). Additionally, they need cortical processing and depend on former experiences or current activations. Moreover, the psychological information would be assembled within limbic circuits (hippocampus, amygdala, and prefrontal cortex) to induce neuroendocrine and behavioral responses (Fuchs & Flügge, 2003). Therefore, it is a common daily occurrence in today’s society that people experience various types of stress (Alkadhi, 2013). Stress activates the hypothalamic pituitary adrenal axis and the secretion of glucocorticoids or corticosteroids in rodents like corticosterone (CORT) (Radahmadi et al., 2015), which can affect cognitive functions, such as learning, memory, and mood states (Abou-Hany et al., 2018; Khani et al., 2018). Psychological stress could be categorized based on timing and type (Dastgerdi et al., 2020; Radahmadi et al., 2017). The effect of stress on physiological and psychological procedures is recognized by its stimulation aspects (Crestani, 2016). Some brain structures, including the limbic system and amygdala, are involved in causing various types of stress (Herman et al., 2005). Therefore, stress may result in a variety of behavioral issues (Hodgson et al., 2004; Watson et al., 2005). Nowadays, the most prevalent stress types in different societies are crowding, relocation, isolation, and restraint stress (emotional stress). Previous studies have reported that crowding, relocation, isolation, and restraint stress have destructive effects on physiological systems and behaviors (Chotiwat & Harris, 2006; Dastgerdi et al., 2017; Davenport et al., 2008; Eid et al., 2010; Hodgson et al., 2004; Watson et al., 2005). Social isolation stress has commonly coincided with anxiety-like behaviors, cognitive impairments, reduced social interactions, and weight loss (Qin et al., 2011). While stress has been indicated as beneficial or harmful to neural health, in some cases it has not affected neural health (Radahmadi et al., 2013). Therefore, stress may exhibit paradoxical effects on cognitive functions and behaviors (Schwabe & Wolf, 2013), depending on the secretion of stress hormones at different levels. Compared to physical stress, psychological stress affects the physiological system more adversely due to the interactions of the limbic system. However, the most prevalent psychological stress types in society that lead to such adverse effects are not indicated. Hence, understanding the effects of various types of prevalent social stress on cognitive performance and behavior is important. The current study investigates the effects of four major types of psychological stress (crowding, relocation, isolation, and restraint) on locomotor activity, learning, memory, anxiety-like behaviors, body weight differences (BWDs), serum CORT levels, and their correlations in rats.
2. Materials and Methods
Study animals
A total of 40 male Wistar rats, aged approximately 3 months (weight=250−300 g), were obtained from the Isfahan Royan Institute for the experiments. The rats were housed in similar cages with 42×27×15 cm3 dimensions (Tajhiz Gostar Omid Iranian Co., Tehran, Iran) under controlled conditions (light on from 07:00 to 19:00, 50%±5% humidity, and 23±2°C room temperature) and ere given ad libitum water. The study was approved by the Ethics Committee of Animal Use at the Isfahan University of Medical Sciences. After a 1-week adaptation period, the animals were randomly assigned to five equal groups (n=8) as follows: Control (Co), crowding stress (Cro-St), relocation stress (Rel-St), isolation stress (Iso-St), and restraint stress (Res-St). During the test period, the rats in the Co group were handled similarly to those in other groups. All behavioral tests were accomplished between 14:00 and 16:00 on day 21 (Figure 1).

In this study, different behavioral tests, including the open field test (OFT), elevated plus maze test (EPM), and passive avoidance (PA) test were performed, respectively. To investigate the lasting effects of exposure to chronic stress on different behavioral variables, 30-min intervals were given between behavioral tests on day 21, after the last stress session. As shown in Figure 1, the passive avoidance test was performed as the last behavioral test in all experimental groups. Therefore, the received shock in the shuttle box did not affect the animals’ anxiety-like behaviors. All measured parameters in the OFT and EPM tests had changed due to the lasting effects of chronic stress.
Experimental procedures
Stress paradigm
Crowding stress was generated by increasing the population density in specified areas (Eid et al., 2010). In other words, inadequate space for the same number of subjects leads to crowding stress (Calhoun, 1973). Therefore, twice the original number of rats were placed in a normal cage (i.e. 8 rats instead of 4) to increase the population density and generate crowding stress (Eid et al., 2010). During the relocation stress, the animals experienced environmental changes and displacement disturbances as the new condition was felt as a threat to their lives (Watson et al., 2005). Accordingly, the second group was relocated to a new cage with an unfamiliar conspecific to generate relocation stress. Also, to induce isolation stress, the animals were placed in different cages separately (individual housing) before being placed in their home cage (group housing). During the stress period, each rat was placed in a cage in isolated conditions without other mates ( Khani et al., 2018; Song et al., 2021). Finally, in the last experimental stress group, the rats were placed in plexiglas cylindrical restrainers to generate restraint stress (Adachi et al., 2021), commonly characterized by either physical or physiological types of stressful stimuli (Dastgerdi et al., 2017; Sunanda et al., 2000). All of these stress types were induced for 21 consecutive days (6 h/day, from 08:00 to 14:00).
Behavioral paradigm
Passive avoidance test
PA was used to assess learning, memory, memory consolidation, and locomotor activity (Vohora et al., 2000). The PA apparatus (64×25×35 cm3) contained two identical rooms (light and dark, 32×25×35 cm3) with grid floors and a sliding door. A stimulator was used to administer electric shocks to the floor. The apparatus habituation impacts the performance of behavioral tasks negatively. The passive avoidance test includes three phases of habituation, learning, and memory. Based on the common protocols in several studies, on day 19, each rat was placed in the apparatus for 300 s (habituation) to diminish the novelty effects of the PA apparatus (Tatem et al., 2014). On day 20, the rats were placed in the lightroom individually (learning phase). The sliding door was raised after 10 s. As the rat fully entered the darkroom, this door was closed and a single electric shock (0.5 mA, 50 V, 3 s; once) was delivered to the animal’s foot (Huang et al., 2013). The initial latency (IL) to cross through the darkroom (the pre-shock latency) was recorded on day 20. On the next day (day 21), the memory phase was performed and the latency of entrance to the darkroom was measured up to a maximum delay of 300 s. The memory experiment was terminated if the rat did not enter the darkroom within 300 s. If the rat avoided the darkroom entry and stayed in the lightroom, a positive response was recorded (Dastgerdi et al., 2018). The total dark stay time was attributed to memory consolidation or storage of new information (Dastgerdi et al., 2018). The number of entries to the darkroom was interpreted as locomotor activity (Vohora et al., 2000). Also, the difference between the IL and latency after a day was considered the occurrence of learning (Dastgerdi et al., 2018). The animal’s ability to remember the foot shock was attributed to memory acquisition.
Elevated plus maze test
EPM test is commonly used to assess stress levels and anxiety-like behaviors (Walf & Frye, 2007). In this study, the EPM apparatus comprised a black opaque Plexiglas structure, elevated 70 cm above the ground. The apparatus consisted of two open arms (60×10×10 cm) and two closed arms (60×10×30 cm), extended from the central platform (10×10 cm). On day 21, each animal was separately placed in the center of the EPM apparatus, facing the open arms. According to the EPM criteria for anxious behaviors, an expert recorded the number of open arm entries (OAE) and the total time spent in the open arms (OAT) within 300 s (Foldi et al., 2019). The Equations 1 and 2 were used to calculate the percentage of OAE
1. (OAE%=[OAE/Total entries to the open and closed Arms]×100)
2. (OAT%=[OAT/300]×100) (Serafim et al., 2012).
Open field test
Another experiment to assess mobility and anxiety-like behaviors is OFT (Hines & Minton, 2012). The OFT equipment consists of a box-shaped platform (90×90×60 cm3), including painted grids that mark the floor with square crossings. In this experiment, the apparatus was placed in a silent room with no stressful stimulation. On day 21, the rats were placed separately at the center of the device before the test. Their activities within 300 s were recorded by a mounted video camera that had tracking software (Ranjbar et al., 2017). Each animal was only tested once in this apparatus. The number of passages through the center of the platform and the total distance traveled on this platform were recorded as indices for anxiety-like behaviors and locomotor activity (Ranjbar et al., 2017). After each experiment, the rat was removed from the apparatus. Then, the square was wiped with a cotton towel (soaked in 70% alcohol) to eliminate the odorant signals (Quillfeldt, 2016).
Determination of serum CORT levels
On day 22, the rats were anesthetized with an intraperitoneal injection of urethane (1.5 g/kg; Sigma-Aldrich Chemical Co., USA), and then sacrificed between 16:00 to 17:00. The blood samples were taken from the animal’s trunk. Subsequently, the serum was separated by centrifugation (6000 rpm, 20 min) to be stored at -80°C until the analyses. The serum CORT levels were measured using a commercial enzyme-linked immunosorbent assay CORT kit (Zellbio Co., Germany). The detection limit for the rat CORT was set to 0.1–20 ng/mL and sensitivity at 0.05 ng/mL (coefficient of variation percentage [C.V.%] for the intra- and inter-assay was less than 10% and 12%, respectively).
Body weight differences
The body weight was measured on day 1 and day 21. The difference between the final and initial weight for each animal was calculated by Equation 3:

Statistical analysis
All data were analyzed using the one-way analysis of variance, followed by the Tukey post hoc test for multiple groups. In addition, the paired sample t-test was used to compare the IL and the latency after a day (within groups). Using the Pearson correlation analysis (coefficient of determination [R2]), the correlation analyses of behavioral tests and BWDs with serum CORT levels were investigated. Furthermore, all data were estimated as Mean±SE of the mean. Meanwhile, a P<0.05 was considered statistically significant. The calculations were performed using the SPSS software, version 26 (IBM SPSS Inc., Chicago, USA).
3. Results
Effects of stress on the passive avoidance test
As shown in Figure 2A, IL had no significant difference in the experimental groups. The latency of entrance to the darkroom after a day was significantly lower in the Rel-St, Res-St, Res-St, Cro-St, and Iso-St groups (P<0.05, P<0.01, P<0.001, and P<0.001, respectively) compared to the Co group (Figure 2B); accordingly, memory impairment occurred as a result of different stress types, particularly in the Cro-St and Iso-St groups. Also, the latency after a day in the Iso-St group was significantly lower (P<0.01) compared to the Rel-St group (Figure 2B).

IL and latency after a day were analyzed using a paired-sample t test to evaluate the within-group latency changes. Significant differences were observed between IL and the latency after a day in all experimental groups (Co group: P<0.001; Rel-St, Res-St, and Cro-St groups: P<0.01; Iso-St group: P<0.05). These results indicated the occurrence of learning in these groups (Figure 3). However, the lowest and highest degrees of learning occurred in the Iso-St and Co groups, respectively.

The total dark stay time was significantly higher in the Res-St, Cro-St, and Iso-St groups (P<0.05, P<0.01, and P<0.001, respectively). However, compared to the and Co and Rel-St group, it was significantly higher only in the Iso-St group (P<0.05) (Figure 4A).
The number of entries to the darkroom had no significant differences in the Rel-St, Res-St, and Cro-St groups compared to the Co group. However, it was significantly lower in the Iso-St group (P<0.05) compared to the Co group. These results suggested locomotor activity reduction in the PA apparatus due to isolation stress (Figure 4B).

Effects of stress on the elevated plus maze test
The OAE% decreased significantly in all stress groups (Rel-St group: P<0.05; other stress groups: P<0.001) compared to the Co group. Furthermore, OAE% had significant decreases in the Cro-St and Iso-St groups (both P<0.001) compared to the Rel-St group. Similarly, OAE% was significantly lower in the Cro-St and Iso-St groups (both P<0.05) compared to the Res-St group (Figure 5A).
A significant reduction of OAT% was observed in all stress groups (Rel-St group: P<0.05; other stress groups: P<0.001) compared to the Co group. Furthermore, OAE% in the Cro-St and Iso-St groups showed significant decreases (both P<0.01) compared to the Rel-St group (Figure 5B).

Effects of stress on the open field test
The number of entries to the platform’s center was significantly lower in the Cro-St and Iso-St groups (both P<0.01) compared to the Co group (Figure 6A). Also, this value had a significant decrease in the Cro-St and Iso-St groups (both P<0.01) in comparison with the Rel-St group (Figure 6A), although it was significantly lower in the Cro-St and Iso-St groups (both P<0.05) compared to the Res-St group.
The time spent in the central area of the OFT platform showed a significant decrease only in the Iso-St group (P<0.05) compared to the Co group (Figure 6B).

A significant decrease in the total distance traveled was observed in all stress groups (Res-St, Rel-St, and Cro-St groups: P<0.01; Iso-St group: P<0.001) compared to the Co group (Figure 6C). However, the highest decrease in exploration activities was observed in the Iso-St group in comparison with other stress groups.
Effects of stress on serum CORT levels
Serum CORT levels increased in all stress groups (Rel-St group: P<0.05; Res-St group: P<0.01; Cro-St and Iso-St groups: P<0.001) compared to the Co group. Furthermore, the serum CORT levels showed significant enhancement in the Iso-St group (P<0.05) compared to the Rel-St group (Figure 7).

Effects of stress on body weight differences
Compared to the Co group, the BWDs significantly declined in all stress groups (Rel-St group: P<0.05; other stress groups: P<0.01) compared to the Co group (Figure 8).

Correlation analyses of behavioral tests and body weight differences with serum CORT levels
In the correlation analysis of the PA test data, the latency after a day exhibited no significant correlation with serum CORT levels in the Co group (R2=0.2327). However, they had significant negative correlations in the Rel-St, Res-St, Cro-St, and Iso-St groups (R2=0.5934, 0.2619, 0.5945, and 0.6616, respectively; P<0.05) (Figure 9A). These findings supported the proposition that the serum CORT levels should be involved in the memory impairment in the Cro-St and Iso-St groups, as per the PA test.

In the correlation analysis of the EPM test data, the OAE% presented no significant correlation with serum CORT levels in the Co group (R2=0.983). However, they had significant negative correlations in the Rel-St, Res-St, and Cro-St groups (R2=0.5556, 0.2220, 0.5945, and 0.5199, respectively; P<0.05) and Iso-St group (R2=0.775; P<0.01) (Figure 9B). These findings supported the proposition that serum CORT levels should be involved in anxiety-like behaviors in all stress groups, as per the EPM test.
In the correlation analysis of the OFT data, the total distance traveled revealed no significant correlation with serum CORT levels in the Co group (R2=0.424). According to Figure 9C, there were significant negative correlations in the Rel-St, Res-St, and Cro-St groups (R2=0.6278, 0.7398, R2=0.6848, respectively; P<0.05 in all groups) and Iso-St group (R2=0.8129; P<0.01). These findings supported the proposition that serum CORT levels should be involved in locomotor activity in all stress groups, as per the OFT.
Another correlation analysis between the BWD data and serum CORT levels exhibited no significant correlation in the Co group (R2=0.3242). However, significant negative correlations were observed in the Rel-St, Res-St, Cro-St, and Iso-St groups (R2=0.6815, 0.383, 0.5544, and 0.6614, respectively; P<0.05) (Figure 9D). These findings supported the proposition of serum CORT levels should be involved in body weight loss under stress.
4. Discussion
This study investigated the effects of four major types of psychological stress, namely crowding, relocation, isolation, and restraint (emotional stress), on learning, memory, memory consolidation, locomotor activity, anxiety-like behaviors, and BWDs in rats, as well as the correlations between their results.
According to the present PA data, learning occurred at different levels in all experimental groups. However, the lowest degree of learning occurred under the isolation stress. In line with current findings, learning was reported to have occurred under stress (Dastgerdi et al., 2018) because stress influences the onset and intensity of learning as a cognitive brain function (Rafah Sami, 2009). Although stress did not impede learning, it impaired brain performance byblocking the changes regarding habit memory formation (Schwabe et al., 2010). The impact of stress on learning has nevertheless been controversial. Based on different studies, stress either adversely or positively affected learning, or did not affect it at all depending on various high and low ranges of stress curve (distress and eustress, respectively) (Joëls et al., 2006; Rudland et al., 2020; Salehi et al., 2010). Moreover, stress altered the equilibrium between multiple underlying systems involved in learning and memory (Vogel & Schwabe, 2016).
According to other PA findings, memory was impaired in all chronic stress conditions, and memory consolidation was impaired by restraint, crowding, and especially isolation stress. Further findings related to the hormonal levels indicate that stress-driven memory deficits mainly occurred because of the changes in the CORT levels. Meanwhile, the correlations between our findings in the PA test with the serum CORT levels verify this proposition. Previous studies have stated chronic stress as an inevitable phenomenon that has impaired memory through the secretion of stress hormones (e.g. CORT) and other neurochemical factors (Jeong et al., 2006; Sandi & Pinelo-Nava, 2007; Sunanda et al., 2000). In addition, the comparison between previous studies demonstrated that isolation stress was more destructive to memory processing compared to restraint stress (Hosseini Dastgerdi et al., 2021; Khani et al., 2022). Other findings in this study suggested that cognitive performance strongly corresponds with social density (the average conspecific encounter rate in an animal population). A research study indicated that social density was influenced by the physical area and availability of resources in the habitat (Love & Zelikowsky, 2020). In humans and rodents, social stress could be triggered by interpersonal encounters, arguments, and fights (Love & Zelikowsky, 2020). In comparison to moderate levels of social density, extremely low or high levels of social density create a situation, in which the nervous system may not efficiently handle stress, especially chronic stress. With the increase in population, the behavior of individuals changes. Crowding increases the stress level as the competition for limited resources exacerbates and leads to increased aggression (Agrell et al., 1995).
According to the result of the PA test, locomotor activity was decreased in the subjects enduring isolation stress. However, the effect of stress on locomotor activity remains paradoxical as reduced (Sestakova et al., 2013) or increased locomotor activity (Weiss et al., 2000) is discussed in various studies. In these studies, besides behavioral assessment methods, stress duration and type have influenced the results concerning locomotor activity as well (Ranjbar et al., 2016). Along with altered secretion of hormones like glucocorticoids (Miranda & Oliveira, 2015), different mechanisms might be involved in stress-related memory impairment and behavioral changes. This includes the secretion of neurotransmitters (serotonin, dopamine, and norepinephrine) (Brenes et al., 2008; Dalesman & Lukowiak, 2011) and brain morphological changes (reduced expression of new neurons, synaptic proteins, dendritic density and length of neurons) (Bianchi et al., 2006).
The findings of the EPM test showed a significant reduction in the time spent in the EPM open arm and the number of entries to the open arm in all stress groups. The crowding and isolation stress increased anxiety-like behaviors more than other types of stress. Another study demonstrated that animals’ behaviors on the EPM platform were influenced by the stress type (Nazeri et al., 2017). In the present study, the increased serum CORT levels seemed to elevate anxiety-like behaviors. In line with other studies, the correlations between EPM findings and serum CORT levels confirmed that CORT has an influential role in causing anxiety-like behaviors. As such, longer and continuous periods of social isolation induce a cascade of negative behaviors in animal models, humans, and neural mechanisms, facilitating this shift (Love & Zelikowsky, 2020). The network organization of structural connectomes will begin to differ in stress conditions related to social isolation. For instance, some measures of the network structure, such as modularity (i.e. the strength of network division into modules) and small-worldness (the degree a network could be cluster-organized) decrease, indicating greater homogeneous connections (Liu et al., 2016). These changes depend on the outcome of the disrupted inter-hemispheric and inter-modular connections in the dorsolateral orbitofrontal cortex (Liu et al., 2016). Other studies have confirmed the association of social isolation with decreased myelination, altered dendritic development, decreased plasticity in the prefrontal cortex (Makinodan et al., 2012; Medendorp et al., 2018), and changes in the prefrontal cortex connectivity (Hermes et al., 2011), in particular concerning the prefrontal cortex-amygdala circuit (Castillo-Gómez et al., 2017). Also, the serotonergic fiber density in the inferior colliculus is a factor that is reduced by developmental isolation (Keesom et al., 2017). Moreover, chronic isolation stress induces a phenotype with similar aspects to anxiety, depression, and social withdrawal in adult rodents (Ieraci et al., 2016; Liu et al., 2012; Scaccianoce et al., 2006).
Based on the present OFT findings, the exploration activity was decreased in the subjects of the Iso-St and Cro-St groups that exhibited anxiety-like behaviors. Therefore, isolation and crowding stress types were more destructive than the other stress groups in this study. The locomotor activity decreased in all stress groups, especially in the Iso-St group. The changes in locomotor activity on the OFT platform were related to the serum CORT levels and were accordingly confirmed by the correlation analysis of the OFT findings with serum CORT. Another study reported that crowded housing (for mice) reduced exploration, locomotor activity, and anxiety-like behaviors in the OFT and EPM tests (Reiss et al., 2007). Furthermore, environmental factors seem to affect the expression of behavioral phenotypes. Therefore, social housing, as a stress factor, could affect psychological reactivity significantly. Also, there were locomotor activity differences in response to various stress types in the OFT and PA tests. Some behaviors, like locomotor activity, originate from certain brain areas; hence, their evaluation requires specific behavioral methods. In other words, specific behavioral tests should be considered for different behavioral assessments. Moreover, the open field is a more specific test for the evaluation of the passive avoidance test.
Based on current findings regarding hormonal changes, different stress types, particularly crowding and isolation stress, increased serum CORT levels more significantly than other stress types. According to previous studies, adrenal gland weight increased with the greater population density, indicating a probable increase in adrenal function (Love & Zelikowsky, 2020). In addition, isolation, restraint, relocation, and crowding stress increased CORT levels in rats (Djordjevic et al., 2003; Khani et al., 2018; Radahmadi et al., 2020). In another study, isolation stress strongly affected behavior but did not enhance plasma CORT levels, which were induced by other stressors (Scaccianoce et al., 2006). These differences might be related to the methodology, age, gender, physical area, as well as stress duration, and type (Radahmadi et al., 2017; Ranjbar et al., 2016). It is critical to determine the time when social isolation level and duration begin to have detrimental effects on the subject (Love & Zelikowsky, 2020). The role of other variables, such as the group size and housing duration, should be considered as well (Van Loo et al., 2001).
According to BWD findings, all types of chronic stress decreased body weight gain significantly. A relationship between weight changes and CORT levels should be well noted as the BWD findings and serum CORT correlation confirmed it. Meanwhile, some metabolic processes are mediated by glucocorticoids; thus, psychological stress could lead to body weight loss (Qin et al., 2011; van der Kooij et al., 2018). However, it was previously indicated that crowding increased adiposity without weight gain (Lin et al., 2015). Concerning stress-related body weight loss, a study has reported that epinephrine and norepinephrine stimulated hormone-sensitive lipase, whereas cortisol increased lipid cell sensitivity to epinephrine and norepinephrine (Lafontan & Langin, 2009). The secretion of corticotrophin-releasing hormones due to stress could decrease food ingestion and body weight (Heinrichs et al., 2001). The BWDs were related to stress exposure (Ranjbar et al., 2016). In another research study, various types of chronic stress, except chronic restraint stress, induced body weight loss because of the stress exposure (Marin et al., 2007). Finally, the potential effects of stress caused by social density could be highlighted as a neuroendocrine stress response, regulated or deregulated by the hypothalamic pituitary adrenal axis, as well as behavioral and neural alterations that are either primary or secondary responses to psychogenic stress (Love & Zelikowsky, 2020). However, understanding those brain mechanisms concerning chronic social stress that have such subserving adaptive functions should be of primary concern. This is because social stress is the major cause of stress stimuli in humans that lead to psychopathology. However, further cellular, biochemical, and structural research is needed to explain its underlying physiological mechanisms.
5. Conclusion
Overall, learning occurred at different levels in all experimental groups although the lowest level of learning occurred under isolation stress conditions. The crowding and isolation stress, as two models of social density stress, had further destructive effects on the impairment of cognitive functions in comparison with the relocation and emotional stress. As such, these stress models severely impair learning, memory, memory consolidation, locomotor activity, and body weight. The crowding and isolation stress increased anxiety-like behaviors and serum CORT levels more than other types of stress, (i.e. relocation and restraint stress). Thus, stress, which was caused by social density (housing density: Crowding and spatial isolation), led to the most negative effects on memory and mood, probably due to different CORT levels, as the main stress hormone. Finally, high or low populations of social density may create a condition, in which the nervous system could not efficiently handle stress, at chronic levels in particular.
Ethical Considerations
Compliance with ethical guidelines
All experiments were approved by the Research and Ethics Committee of Isfahan University of Medical Sciences (Code: IR.MUI.Research.REC.1399.677).
Funding
This work was partly supported by the Isfahan University of Technology.
Authors' contributions
Conceptualization, study design, methodology and writing: Maryam Radahmadi; Experiments: Hamed Adavi; Logistic support: Maryam Radahmadi and Rasul Kowsar; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The the authors thank the Isfahan University of Medical Sciences for the support.
References
Abou-Hany, H. O., Atef, H., Said, E., Elkashef, H. A., & Salem, H. A. (2018). Crocin mediated amelioration of oxidative burden and inflammatory cascade suppresses diabetic nephropathy progression in diabetic rats. Chemico-Biological Interactions, 284, 90-100. [DOI:10.1016/j.cbi.2018.02.001] [PMID]
Adachi, N., Sakhri, F. Z., Ikemoto, H., Ohashi, Y., Kato, M., & Inoue, T., et al. (2021). Kamikihito rescued depressive-like behaviors and hippocampus neurogenesis in chronic restraint stress rats. Journal of Traditional and Complementary Medicine, 12(2), 172–179. [DOI:10.1016/j.jtcme.2021.08.001] [PMID] [PMCID]
Agrell, J., Erlinge, S., Nelson, J., Nilsson, C., & Persson, I. (1995).Delayed density-dependence in a small-rodent population. Proceedings. Biological Sciences, 262(1363), 65–70. [DOI:10.1098/rspb.1995.0177] [PMID]
Alkadhi, K. (2013). Brain physiology and pathophysiology in mental stress. International Scholarly Research Notices, 2013, 1-23. [Link]
Bianchi, M., Fone, K. F., Azmi, N., Heidbreder, C. A., Hagan, J. J., & Marsden, C. A. (2006). Isolation rearing induces recognition memory deficits accompanied by cytoskeletal alterations in rat hippocampus. The European Journal of Neuroscience, 24(10), 2894–2902. [DOI:10.1111/j.1460-9568.2006.05170.x] [PMID]
Brenes, J. C., Rodríguez, O., & Fornaguera, J. (2008). Differential effect of environment enrichment and social isolation on depressive-like behavior, spontaneous activity and serotonin and norepinephrine concentration in prefrontal cortex and ventral striatum. Pharmacology Biochemistry and Behavior, 89(1), 85-93. [DOI:10.1016/j.pbb.2007.11.004] [PMID]
Calhoun, J. B. (1973). Death squared: the explosive growth and demise of a mouse population. Proceedings of the Royal Society of Medicine, 66(1P2), 80-88. [DOI:10.1177/00359157730661P202]
Castillo-Gómez, E., Pérez-Rando, M., Bellés, M., Gilabert-Juan, J., Llorens, J. V., & Carceller, H., et al. (2017). Early social isolation stress and perinatal NMDA receptor antagonist treatment induce changes in the structure and neurochemistry of inhibitory neurons of the adult amygdala and prefrontal cortex. eNeuro, 4(2), ENEURO.0034-17.2017. [DOI:10.1523/ENEURO.0034-17.2017] [PMID] [PMCID]
Chotiwat, C., & Harris, R. B. (2006). Increased anxiety-like behavior during the post-stress period in mice exposed to repeated restraint stress. Hormones and Behavior, 50(3), 489-495. [DOI:10.1016/j.yhbeh.2006.06.007] [PMID]
Crestani, C. C. (2016). Emotional stress and cardiovascular complications in animal models: A review of the influence of stress type. Frontiers in Physiology, 7, 251. [DOI:10.3389/fphys.2016.00251] [PMID] [PMCID]
Dalesman, S., & Lukowiak, K. (2011). Social snails: The effect of social isolation on cognition is dependent on environmental context. The Journal of Experimental Biology, 214(Pt 24), 4179–4185.[DOI:10.1242/jeb.064857] [PMID]
Dastgerdi, A. H., Radahmadi, M., Pourshanazari, A. A., & Dastgerdi, H. H. (2017). Effects of crocin on learning and memory in rats under chronic restraint stress with special focus on the hippocampal and frontal cortex corticosterone levels. Advanced Biomedical Research, 6, 157. [DOI:10.4103/abr.abr_107_17] [PMID] [PMCID]
Dastgerdi, H. H., Radahmadi, M., & Reisi, P. (2020). Comparative study of the protective effects of crocin and exercise on long-term potentiation of CA1 in rats under chronic unpredictable stress. Life Sciences, 256, 118018. [DOI:10.1016/j.lfs.2020.118018] [PMID]
Dastgerdi, H. H., Radahmadi, M., Reisi, P., & Dastgerdi, A. H. (2018). Effect of crocin, exercise, and crocin-accompanied exercise on learning and memory in rats under chronic unpredictable stress. Advanced Biomedical Research, 7, 137. [DOI:10.4103/abr.abr_153_18] [PMID] [PMCID]
Davenport, M. D., Lutz, C. K., Tiefenbacher, S., Novak, M. A., & Meyer, J. S. (2008). A rhesus monkey model of self-injury: effects of relocation stress on behavior and neuroendocrine function. Biological Psychiatry, 63(10), 990-996. [DOI:10.1016/j.biopsych.2007.10.025] [PMID] [PMCID]
Djordjevic, J., Cvijic, G., & Davidovic, V. (2003). Different activation of ACTH and corticosterone release in response to various stressors in rats. Physiological Research, 52(1), 67-72. [DOI:10.33549/physiolres.930273] [PMID]
Eid, F., Helal, E. G., & Taha, N. M. (2010). Effect of crowding stress and/or sulpiride treatment on some physiological and histological parameters in female albino rats. The Egyptian Journal of Hospital Medicine, 41(1), 566-589. [Link]
Foldi, C. J., Eyles, D. W., McGrath, J. J., & Burne, T. H. J. (2019).Increasing paternal age alters anxiety-related behaviour in adult mice. Genes, Brain and Behavior, 18(2), e12522. [DOI:10.1111/gbb.12522] [PMID]
Fuchs, E., & Flügge, G. (2003). Chronic social stress: Effects on limbic brain structures. Physiology & Behavior, 79(3), 417-427. [DOI:10.1016/S0031-9384(03)00161-6] [PMID]
Heinrichs, S. C., Li, D. L., & Iyengar, S. (2001). Corticotropin-releasing factor (CRF) or CRF binding-protein ligand inhibitor administration suppresses food intake in mice and elevates body temperature in rats. Brain Research, 900(2), 177-185. [DOI:10.1016/S0006-8993(01)02286-7] [PMID]
Herman, J. P., Ostrander, M. M., Mueller, N. K., & Figueiredo, H. (2005). Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 29(8), 1201-1213. [DOI:10.1016/j.pnpbp.2005.08.006] [PMID]
Hermes, G., Li, N., Duman, C., & Duman, R. (2011). Post-weaning chronic social isolation produces profound behavioral dysregulation with decreases in prefrontal cortex synaptic-associated protein expression in female rats. Physiology & Behavior, 104(2), 354-359. [DOI:10.1016/j.physbeh.2010.12.019] [PMID] [PMCID]
Hines, T. J., Minton, B. R., & Zrull, M. (2012). Effects of environmental enrichment on rat behavior in the open field test. Paper presented at: Proceedings of the National Conference on Undergraduate Research (NCUR), Weber State University, Ogden, Utah, March 29 – 31, 2012. [Link]
Hodgson, N., Freedman, V. A., Granger, D. A., & Erno, A. (2004). Biobehavioral correlates of relocation in the frail elderly: Salivary cortisol, affect, and cognitive function. Journal of the American Geriatrics Society, 52(11), 1856-1862. [DOI:10.1111/j.1532-5415.2004.52505.x] [PMID]
Hosseini Dastgerdi, A., Radahmadi, M., & Pourshanazari, A. A. (2021). Comparing the effects of crocin at different doses on excitability and long-term potentiation in the CA1 area, as well as the electroencephalogram responses of rats under chronic stress. Metabolic Brain Disease, 36(7), 1879-1887. [DOI:10.1007/s11011-021-00747-y] [PMID]
Huang, A. C., Shyu, B. C., Hsiao, S., Chen, T. C., & He, A. B. (2013). Neural substrates of fear conditioning, extinction, and spontaneous recovery in passive avoidance learning: A c-fos study in rats. Behavioural Brain Research, 237, 23-31. [DOI:10.1016/j.bbr.2012.09.024] [PMID]
Ieraci, A., Mallei, A., & Popoli, M. (2016). Social isolation stress induces anxious-depressive-like behavior and alterations of neuroplasticity-related genes in adult male mice. Neural plasticity, 2016, 6212983. [DOI:10.1155/2016/6212983] [PMID] [PMCID]
Jeong, Y. H., Park, C. H., Yoo, J., Shin, K. Y., Ahn, S. M., & Kim, H. S., et al. (2006). Chronic stress accelerates learning and memory impairments and increases amyloid deposition in APPV717I-CT100 transgenic mice, an Alzheimer’s disease model. FASEB Journal, 20(6), 729-731. [DOI:10.1096/fj.05-4265fje] [PMID]
Joëls, M., Pu, Z., Wiegert, O., Oitzl, M. S., & Krugers, H. J. (2006).Learning under stress: How does it work? Trends in Cognitive Sciences, 10(4), 152-158. [DOI:10.1016/j.tics.2006.02.002] [PMID]
Keesom, S. M., Finton, C. J., Sell, G. L., & Hurley, L. M. (2017).Early-life social isolation influences mouse ultrasonic vocalizations during male-male social encounters. Plos One, 12(1), e0169705. [DOI:10.1371/journal.pone.0169705] [PMID] [PMCID]
Khani, F., Radahmadi, M., & Alaei, H. (2022). The protective effects of crocin on input-output functions and long-term potentiation of hippocampal CA1 area in rats exposed to chronic social isolated stress. Basic and Clinical Neuroscience, 13(2), 165-174. [DOI:10.32598/bcn.2022.2346.2] [PMID] [PMCID]
Khani, F., Radahmadi, M., Alaei, H., & Jafari, E. (2018). Effects of crocin on cognitive and spatial memories in rats under chronic isolation stress. Physiology and Pharmacology, 22(4), 254-268. [Link]
Lafontan, M., & Langin, D. (2009). Lipolysis and lipid mobilization in human adipose tissue. Progress in Lipid Research, 48(5), 275-297. [DOI:10.1016/j.plipres.2009.05.001] [PMID]
Lin, E. J., Sun, M., Choi, E. Y., Magee, D., Stets, C. W., & During, M. J. (2015). Social overcrowding as a chronic stress model that increases adiposity in mice. Psychoneuroendocrinology, 51, 318-330. [DOI:10.1016/j.psyneuen.2014.10.007] [PMID] [PMCID]
Liu, C., Li, Y., Edwards, T. J., Kurniawan, N. D., Richards, L. J., & Jiang, T. (2016). Altered structural connectome in adolescent socially isolated mice. Neuroimage, 139, 259-270. [DOI:10.1016/j.neuroimage.2016.06.037] [PMID]
Liu, J., Dietz, K., DeLoyht, J. M., Pedre, X., Kelkar, D., & Kaur, J., et al. (2012). Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nature neuroscience, 15(12), 1621-1623. [DOI:10.1038/nn.3263] [PMID] [PMCID]
Love, J., & Zelikowsky, M. (2020). Stress varies along the social density continuum. Frontiers in Systems Neuroscience, 14, 582985. [DOI:10.3389/fnsys.2020.582985] [PMID] [PMCID]
Makinodan, M., Rosen, K. M., Ito, S., & Corfas, G. (2012). A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science (New York, N.Y.), 337(6100), 1357–1360. [DOI:10.1126/science.1220845] [PMID] [PMCID]
Marin, M. T., Cruz, F. C., & Planeta, C. S. (2007). Chronic restraint or variable stresses differently affect the behavior, corticosterone secretion and body weight in rats. Physiology & Behavior, 90(1), 29-35. [DOI:10.1016/j.physbeh.2006.08.021] [PMID]
Medendorp, W. E., Petersen, E. D., Pal, A., Wagner, L. M., Myers, A. R., & Hochgeschwender, U., et al. (2018). Altered behavior in mice socially isolated during adolescence corresponds with immature dendritic spine morphology and impaired plasticity in the prefrontal cortex. Frontiers in Behavioral Neuroscience, 12, 87. [DOI:10.3389/fnbeh.2018.00087] [PMID] [PMCID]
Miranda, A. M., & Oliveira, T. G. (2015). Lipids under stress-a lipidomic approach for the study of mood disorders. Bioessays, 37(11), 1226-1235. [DOI:10.1002/bies.201500070] [PMID]
Nazeri, M., Ebrahimi, A., Aghaei, I., Ghotbi Ravandi, S., & Shabani, M. (2017). Psychological stress has a higher rate of developing addictive behaviors compared to physical stress in rat offspring. EXCLI Journal, 16, 903–913. [PMID]
Qin, M., Xia, Z., Huang, T., & Smith, C. B. (2011). Effects of chronic immobilization stress on anxiety-like behavior and basolateral amygdala morphology in Fmr1 knockout mice. Neuroscience, 194, 282-290. [DOI:10.1016/j.neuroscience.2011.06.047] [PMID] [PMCID]
Quillfeldt, J. A. (2016). Behavioral methods to study learning and memory in rats. In: M. Andersen, & S. Tufik (Eds.), Rodent Model as Tools in Ethical Biomedical Research (pp. 271-311). Cham: Springer. [Link]
Radahmadi, M., Alaei, H., Sharifi, M. R., & Hosseini, N. (2013). The effect of synchronized forced running with chronic stress on short, mid, and long-term memory in rats. Asian Journal of Sports Medicine, 4(1), 54-62. [DOI:10.5812/asjsm.34532] [PMID] [PMCID]
Radahmadi, M., Alaei, H., Sharifi, M. R., & Hosseini, N. (2015). Effects of different timing of stress on corticosterone, BDNF and memory in male rats. Physiology & Behavior, 139, 459-467. [DOI:10.1016/j.physbeh.2014.12.004] [PMID]
Radahmadi, M., Hosseini Dastgerdi, A., Fallah, N., & Alaei, H. (2017). The effects of acute, sub-chronic and chronic psychical stress on the brain electrical activity in male rats. Physiology and Pharmacology, 21(3), 185-192. [Link]
Radahmadi, M., Hosseini Dastgerdi, A., & Pourshanazari, A. A. (2020). Effects of crocin on locomotor activity as well as novel object recognition and object location memories in chronic restraint stressed rats. Physiology and Pharmacology, 24(2), 123-132. [DOI:10.32598/ppj.24.2.80]
Rafah Sami, A. (2009). Effect of exercise on spatial learning and memory in male diabetic rats. International Journal of Diabetes and Metabolism, 17(3), 93–98. [DOI:10.1159/000497679]
Ranjbar, H., Radahmadi, M., Alaei, H., Reisi, P., & Karimi, S. (2016). The effect of basolateral amygdala nucleus lesion on memory under acute, mid and chronic stress in male rats. Turkish Journal of Medical Sciences, 46(6), 1915-1925. [DOI:10.3906/sag-1507-7] [PMID]
Ranjbar, H., Radahmadi, M., Reisi, P., & Alaei, H. (2017). Effects of electrical lesion of basolateral amygdala nucleus on rat anxiety-like behaviour under acute, sub-chronic, and chronic stresses. Clinical and Experimental Pharmacology and Physiology, 44(4), 470-479. [DOI:10.1111/1440-1681.12727] [PMID]
Reiss, D., Wolter-Sutter, A., Krezel, W., & Ouagazzal, A. M. (2007). Effects of social crowding on emotionality and expression of hippocampal nociceptin/orphanin FQ system transcripts in mice. Behavioural Brain Research, 184(2), 167–173.[DOI:10.1016/j.bbr.2007.07.010] [PMID]
Rudland, J. R., Golding, C., & Wilkinson, T. J. (2020). The stress paradox: How stress can be good for learning. Medical Education, 54(1), 40-45. [DOI:10.1111/medu.13830] [PMID]
Salehi, B., Cordero, M. I., & Sandi, C. (2010). Learning under stress: The inverted-U-shape function revisited. Learning & Memory, 17(10), 522-530. [DOI:10.1101/lm.1914110] [PMID]
Sandi, C., & Pinelo-Nava, M. T. (2007). Stress and memory: Behavioral effects and neurobiological mechanisms. Neural Plasticity, 2007, 78970. [DOI:10.1155/2007/78970] [PMID] [PMCID]
Scaccianoce, S., Del Bianco, P., Paolone, G., Caprioli, D., Modafferi, A. M., & Nencini, P., et al. (2006). Social isolation selectively reduces hippocampal brain-derived neurotrophic factor without altering plasma corticosterone. Behavioural Brain Research, 168(2), 323-325. [DOI:10.1016/j.bbr.2005.04.024] [PMID]
Schwabe, L., Schächinger, H., de Kloet, E. R., & Oitzl, M. S. (2010). Corticosteroids operate as a switch between memory systems. Journal of Cognitive Neuroscience, 22(7), 1362-1372. [DOI:10.1162/jocn.2009.21278] [PMID]
Schwabe, L., & Wolf, O. T. (2013). Stress and multiple memory systems: From ‘thinking’to ‘doing’. Trends in Cognitive Sciences, 17(2), 60-68. [DOI:10.1016/j.tics.2012.12.001] [PMID]
Serafim, K. R., Gianlorenço, A. C., Daher, F. P., & Mattioli, R. (2012). H1-histamine receptors in the amygdala are involved in emotional memory but do not mediate anxiety-related behaviors in mice submitted to EPM testing. Brain Research Bulletin, 89(1-2), 1-7. [DOI:10.1016/j.brainresbull.2012.06.009] [PMID]
Sestakova, N., Puzserova, A., Kluknavsky, M., & Bernatova, I. (2013). Determination of motor activity and anxiety-related behaviour in rodents: Methodological aspects and role of nitric oxide. Interdisciplinary Toxicology, 6(3), 126-135. [DOI:10.2478/intox-2013-0020] [PMID] [PMCID]
Song, M. K., Lee, J. H., & Kim, Y. J. (2021). Effect of chronic handling and social isolation on emotion and cognition in adolescent rats. Physiology & Behavior, 237, 113440. [DOI:10.1016/j.physbeh.2021.113440] [PMID]
Sunanda, Rao, B. S., & Raju, T. R. (2000). Chronic restraint stress impairs acquisition and retention of spatial memory tasks in rats. Current Science, 79(11), 1581-1584. [Link]
Tatem, K. S., Quinn, J. L., Phadke, A., Yu, Q., Gordish-Dressman, H., & Nagaraju, K. (2014). Behavioral and locomotor measurements using an open field activity monitoring system for skeletal muscle diseases. Journal of Visualized Experiments: JoVE, (91), 51785. [DOI:10.3791/51785] [PMID] [PMCID]
van der Kooij, M. A., Jene, T., Treccani, G., Miederer, I., Hasch, A., & Voelxen, N., et al. (2018). Chronic social stress-induced hyperglycemia in mice couples individual stress susceptibility to impaired spatial memory. Proceedings of the National Academy of Sciences of the United States of America, 115(43), E10187–E10196. [DOI:10.1073/pnas.1804412115] [PMID] [PMCID]
Van Loo, P. L., Mol, J. A., Koolhaas, J. M., Van Zutphen, B. F., & Baumans, V. (2001). Modulation of aggression in male mice: Influence of group size and cage size. Physiology & Behavior, 72(5), 675-683. [DOI:10.1016/S0031-9384(01)00425-5] [PMID]
Vogel, S., & Schwabe, L. (2016). Learning and memory under stress: implications for the classroom. NPJ Science of Learning, 1, 16011. [DOI:10.1038/npjscilearn.2016.11] [PMID] [PMCID]
Vohora, D., Pal, S., & Pillai, K. (2000). Effect of locomotor activity on the passive avoidance test for the evaluation of cognitive function. Indian Journal of Pharmacology, 32(3), 242-245. [Link]
Walf, A. A., & Frye, C. A. (2007). The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nature Protocols, 2(2), 322–328. [DOI:10.1038/nprot.2007.44] [PMID] [PMCID]
Type of Study: Original |
Subject:
Behavioral Neuroscience
Received: 2023/04/9 | Accepted: 2023/05/8 | Published: 2024/05/1
Received: 2023/04/9 | Accepted: 2023/05/8 | Published: 2024/05/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








