Volume 16 - Special Issue on Cognitive Sciences
BCN 2025, 16 - Special Issue on Cognitive Sciences: 251-264 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Aliyari H, Hosseinian M, Menhaj M B, Sahraei H, Shabani M, Kazemi M. Effect of High-voltage Electrical Field Exposure on Neurobiological Factors and Visual Working Memory of Macaques. BCN 2025; 16 (S1) :251-264
URL: http://bcn.iums.ac.ir/article-1-2675-en.html
URL: http://bcn.iums.ac.ir/article-1-2675-en.html
Hamed Aliyari *1 

 , Mohsen Hosseinian2
, Mohsen Hosseinian2 
 , Mohammad Bagher Menhaj2
, Mohammad Bagher Menhaj2 

 , Hedayat Sahraei3
, Hedayat Sahraei3 

 , Mohsen Shabani4
, Mohsen Shabani4 
 , Masoomeh Kazemi3
, Masoomeh Kazemi3 




 , Mohsen Hosseinian2
, Mohsen Hosseinian2 
 , Mohammad Bagher Menhaj2
, Mohammad Bagher Menhaj2 

 , Hedayat Sahraei3
, Hedayat Sahraei3 

 , Mohsen Shabani4
, Mohsen Shabani4 
 , Masoomeh Kazemi3
, Masoomeh Kazemi3 


1- Department of Psychiatry, University of Texas Southwestern Medical Center, Dallas, Texas.
2- Department of Electrical Engineering, School of Electrical, Computer & Biomedical Engineering, Amirkabir University of Technology, Tehran, Iran.
3- Neuroscience Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran.
4- Institute for Cognitive and Brain Sciences, Shahid Beheshti University, Tehran, Iran.
2- Department of Electrical Engineering, School of Electrical, Computer & Biomedical Engineering, Amirkabir University of Technology, Tehran, Iran.
3- Neuroscience Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran.
4- Institute for Cognitive and Brain Sciences, Shahid Beheshti University, Tehran, Iran.
Keywords: High-voltage electric fields, Visual working memory, Spiking neural network (SNN), Monkeys (Macaques)
Full-Text [PDF 1974 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
To prevent the potential effects of magnetic and electric fields generated by power transmission lines on human health, it is necessary to conduct calculations (Aliyari et al., 2019a; Aliyari et al., 2019a; Di et al., 2019; Pretorius, 2006) when installing these lines near cities and villages. In recent years, numerous studies have investigated the effects of low-frequency electromagnetic radiation on the nervous system and memory performance in humans and animals. The rhesus macaque, which shares 98% of its genes with humans, has been used as an important animal model in scientific studies (Kazemi et al., 2018; Squire, 1992; Stevens et al., 1993). Furthermore, cognitive-behavioral analyses have become a state-of-the-art research method using this animal model (Cook et al., 2002; Draper et al., 2005; Lowenthal et al., 2007).
It is necessary to calculate the fields in the surrounding areas to prevent potential health effects on humans from the electric and magnetic fields generated by power transmission lines near power generation centers, cities, and villages. In a recent study, neural network modeling was employed with biological, behavioral, and anatomical examinations to investigate the effects of a high-voltage (HV) electric field (400 kV transmission line) on two male rhesus macaques (Aliyari et al., 2019b; Aliyari et al., 2019b; Braicu et al., 2017; Ushie et al., 2017).
Visual memory (Brady et al., 2011) is an essential cognitive factor substantially fostered through learning. As a significant cognitive factor, the importance of memory has been valued by neurologists, artificial intelligence, and brain engineering specialists (Aliyari et al., 2018; Chudler & Bergsman, 2016). Experimental research has dramatically expanded our understanding of the brain, particularly with regard to the role of the hippocampus, amygdala, and prefrontal cortex in memory, decision-making, and other mental processes. However, it is essential to note that various factors limit this research. For example, it may be difficult to study certain human brain regions or functions due to ethical concerns or technical limitations. While helpful in exploring certain aspects of brain function, animal models may not always accurately represent human brain function.
Additionally, experimental designs may not always fully capture the complexity of real-world scenarios. Despite these limitations, experimental research remains critical for advancing our understanding of the brain and developing new treatments for neurological and psychiatric disorders. (Aliyari et al., 2018; Laakso et al., 1995; Soininen et al., 1994; Tekieh et al., 2017; Videbech & Ravnkilde, 2004). The precise examination of neuron behavior requires costly equipment and considerable effort, and the techniques used for this purpose are not always applicable. The first thing in studying memory formation in the brain is to determine the parts of the brain and neurons involved in memory development (Rose et al., 2009). After understanding the relevant neurons and areas involved in the formation of memory, the activity of each neuron and region must be measured/predicted, and interpreted. It is impossible to study the brain using the invasive methods that yield the most precise results, and the experimental approach is challenged radically. Therefore, these studies are inevitably conducted on mammals with the most genetic similarity to humans, but these studies are not flawless either (Engel et al., 2005).
Experimental research is a valuable tool for studying the brain, but it has limitations that necessitate the development of alternative methods. Computational and modeling approaches have emerged as important tools for understanding brain function, using computer simulations and mathematical models to study complex systems and processes in the brain. These approaches can provide insights that may not be easily observed through experimental approaches alone and can be used to test hypotheses and make predictions about brain function, potentially leading to new treatments for neurological and psychiatric disorders (Aliyari et al., 2018; Markram, 2012; Van der Velde, 2010). Computational models have become increasingly popular among researchers due to their ability to create precise models of complex systems, such as those found in the brain, which may not be easily studied through experimental methods. With these models, researchers can study element behavior and parameters that may not be able to be studied experimentally. Given these advantages, computational models have become an essential tool in studying complex systems and processes in the brain (Churchland & Sejnowski, 2016; Loh et al., 2007; Menon, 2011).
Also, the neural connection is one of the chief characteristics of the brains of the vertebrae. Through this connection, many neurons are linked to one another through axons and dendrites, which affect other neurons and are affected by them via the axons and dendrites. The junction between two neurons is called a synapse. There are typically synaptic connections between one neuron and several thousand other neurons, and that particular neuron receives synapses from those several thousand other neurons. Synapses are among the most crucial brain structures due to numerous reasons. Synapses are highly organized and stable structures from a biological perspective, and they play a pivotal role in information transmission and processing, enabling the brain to learn, memorize, and adapt. Synaptic disorders can lead to a range of brain and psychological disorders, given the critical role synapses play in neurotransmission. Clinically, synapses are the primary targets for medicinal treatments and healthcare interventions to address these disorders (Maass, 1997; Seung, 2003; Takac & Knott, 2015; Taylor, 2009; Tsodyks et al., 2006).
This study aims to investigate the impact of HV electric fields on the behavior of simulated neural models of the hippocampus, given its crucial role in learning and memory. To validate the results, the behavioral, blood, hormonal, gene, and cognitive observations from experimental examinations of two rhesus macaques exposed to similar electric fields will be compared. The research question holds significant importance as it can provide insights into the potential effects of HV electric fields on the brain and contribute to a better understanding of the associated risks with power transmission lines. It is important to note that the study adheres to all applicable international, national, and institutional guidelines for the care and use of animals.
2. Materials and Methods
Animal subjects
The study used two adult male rhesus macaques (Macaca mulatta) aged 4 to 5 years, with an average weight of 4 kg. The macaques were kept in a controlled environment for 12 months to adapt. The room where the animals were kept had appropriate lighting, temperature, and humidity and followed all ethical and international rules for the animals’ transportation, location, and maintenance (Aliyari et al., 2019a; Aliyari et al., 2019b; Aliyari et al., 2019a).
HV electric field exposure
In this experiment, one of the rhesus macaques was exposed to a simulated HV electric field of 3 kV/m for 4 hours a day for a month, while the other macaque was kept outside the field as a control sample. The HV electric field used in the experiment had a frequency of 50 Hz and was simulated in the laboratory using two metal sheets measuring 2×2 m. The sheets were placed by a crane at the bottom and on top of a polytetrafluoroethylene (PTFE) primate cage measuring 1×1×1 m, with a distance of 2 m between them. The sheets were exposed to a voltage of 6 kV to create a uniform 3kV/m field in the cage (Aliyari et al., 2019b).
Behavioral analysis
Behavioral analyses were conducted on both macaques before, during, and after the application of electric field simulations and were recorded on camera.
Blood analysis
In addition to behavioral analyses, 5 mL of blood was collected from each macaque and used to measure the concentrations of sodium and potassium blood electrolytes and the expression of the NMDA receptor gene. Blood samples were collected before the macaques were included in the research, after applying electric field simulations, and during the recovery phase. The expression of the NMDA receptor gene was determined using the RT-PCR method, and blood lymphocyte cells were obtained before and after applying electric field simulations and during the recovery phase. Additionally, the blood concentrations of sodium and potassium ions were measured during the same phases.
MRI analysis
An MRI-assisted analysis of the anatomy of the hippocampus and amygdala was conducted using DICOM LiteBox before and after the application of electric field simulations (Aliyari et al., 2018; Tekieh et al., 2017; Van Ooijen et al., 2005).
Neural modeling
The neural model was simulated using MATLAB software, version 2016b (Aliyari et al., 2023; Aliyari et al., 2018; Aliyari et al., 2015; Izhikevich, 2003).
Recovery phase
During the recovery phase, both macaques, one of which had been exposed to the HV electric field and the other kept in a non-exposed environment, were kept in the same previous place and situation without exposure to the electric field.
Cognitive tests have four phases, as represented below.
Phase one is the visual memory experiment. The visual memory recording device was placed in front of the primate, and a reward was randomly put in one of the dishes. After 30 s, the dish on the moving stand was provided to the animal. The macaque was allowed a single attempt to access the reward, and he would be deprived if he failed to open the right dish. Consequently, the animal had to focus and pay attention to the reward. This test was repeated thrice daily before the primate’s eyes (Constantinidis & Procyk, 2004; Kazemi et al., 2018).
In phase two, a visual memory experiment was conducted with the macaques. Peanuts were placed in one of two covered dishes in front of the animal, and the dish with the peanuts was presented to the animal after a 60-s delay. The process was explained to the animal before the experiment.
Phase three is the visual working memory experiment. Peanuts (the reward) were put in one of the covered dishes before the primate’s eyes, and a curtain was placed between the primate and the reward (dish) for 30 seconds. Following this period, the dish was presented to the animal after closing the curtain. The animal had only one chance to access the reward, so he had to pay attention, concentrate, and memorize (Kazemi et al., 2022; Kazemi et al., 2018).
Phase four also involves the visual working memory experiment. Peanuts (the reward) were put in one of the covered dishes before the animal’s eyes. A curtain was placed between the primate and the dish for 60 s, after which the dish was presented to the animal, and the procedure continued as described (Constantinidis & Procyk, 2004; Kazemi et al., 2022; Kazemi et al., 2018).
Spiking neural network (SNN) models of hippocampus
Neural network
Neural networks have various functions, including selecting input, adjusting gain, reducing turbulence, and selectively reinforcing activity. These functions form the basis of simple and complex models of primary visual cortex cells, such as short-term and hybrid memory models. The fire rate and spiking models of the neural network models have received significant attention from researchers. The fire rate model features neurons that produce fire rates instead of action potentials, making it a more cost-effective and simpler model to analyze mathematically. However, it has limitations, such as not analyzing fire duration and correlation in networks with high simultaneity. The spiking model can present more biological details due to its parameters and ability to analyze fire time and correlations, but it is more computationally expensive. Both models have strengths and weaknesses, and the choice depends on the research question being addressed (Aliyari et al., 2018; Demuth et al., 2014; Starzyk et al., 2012; Xu & Li, 2016; Zhang et al., 2018).
Spiking model
Modeling neuron populations often involves generating populations using basic computational neuron models and connecting them using synaptic models. Combining a wide range of neural and synaptic models makes it possible to describe the behavior of different brain regions with physiological details such as synaptic currents and their dynamics, neural receptors, and ion channels. This condition results in a computational model that provides insights into the complex processes and interactions within the brain (Izhikevich, 2003; Pillow et al., 2005).
Figure 1 illustrates the structure of a neural network consisting of three pyramidal neuron pools and one inhibitory neuron pool. All neurons in the network are interconnected, but the synaptic strength of the neurons within each neuron pool is higher than the connections between the pools.
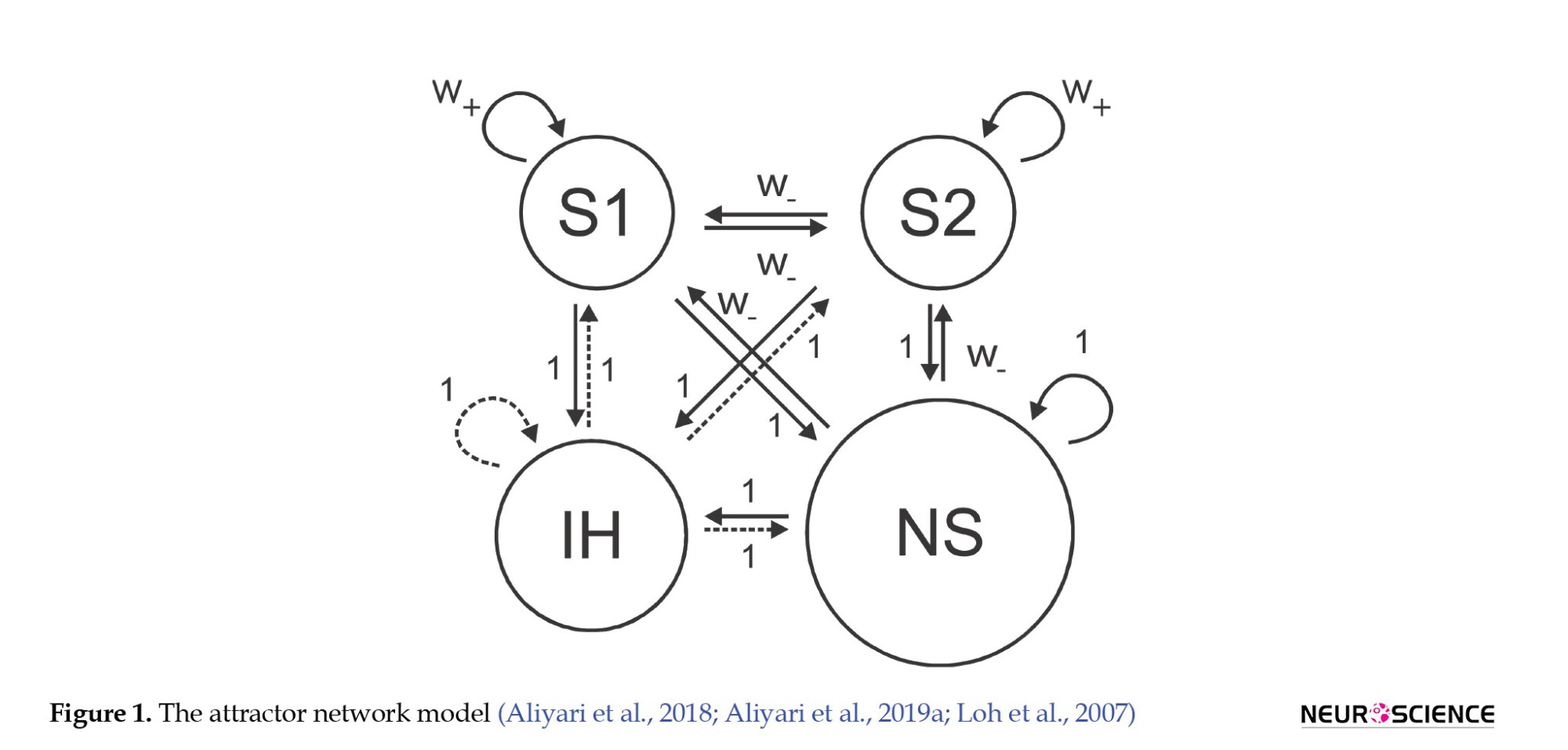
The Figure 2 displays the synaptic strength of the neurons and the connections between the neuron pools, with solid lines representing excitatory connections and dashed lines representing inhibitory connections (Brunel & Wang, 2001; Loh et al., 2007).
The S1 and S2 neuron pools are attractor neurons, meaning they can maintain a stable activity state without input. The NS neuron pool describes the activities of other pyramidal neurons in the brain’s cortex region. In contrast, the IH (hyperpolarization-activated current neurons) are inhibitory neurons that can modulate and control the other neurons’ activity (Brunel, 2000; Brunel & Wang, 2003; Compte et al., 2000).
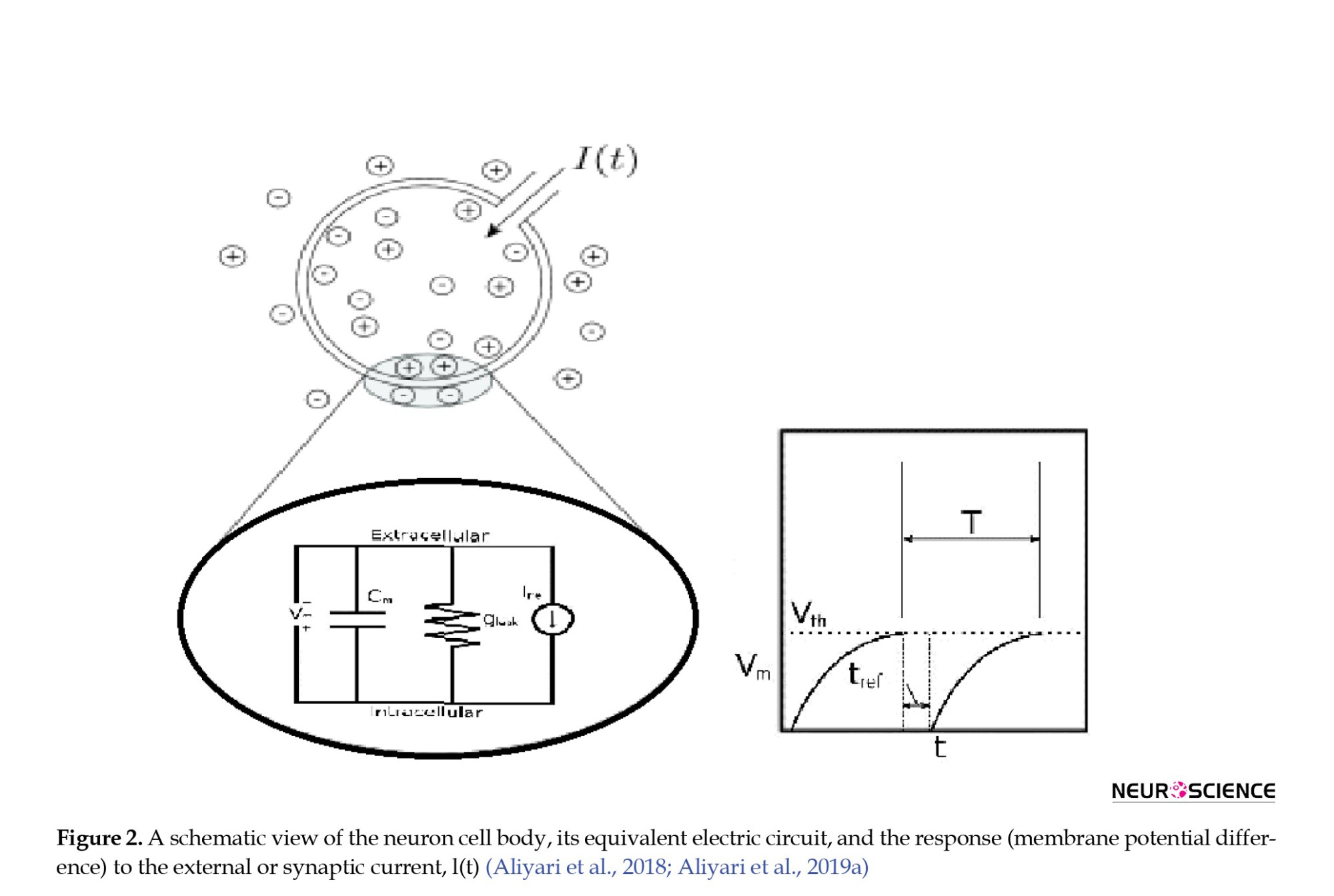
A simple yet efficient LIF (leaky integrate-and-fire) model was utilized to describe the voltage dynamics of neurons in the cortex, with AMPA, NMDA, and GABA receptor currents serving as the synaptic currents. Each neuron in the network receives four currents: off-network AMPA receptor currents, AMPA receptor currents from intra-network excitatory neurons, NMDA receptor currents from intra-network excitatory neurons, and GABA receptor currents from intra-network inhibitory neurons. The off-network AMPA receptor currents reflect the activity of neurons in other parts of the cortex that provide input to the network. In this model, each neuron receives 800 synapses with off-network AMPA receptors, contributing to the neurons’ background activity. Furthermore, each neuron receives 400 synapses from excitatory neurons with AMPA and NMDA receptors and 100 synapses from inhibitory neurons with GABA receptors. In the self-stimulatory state, the inhibitory current from the network’s inhibitory synapses overcomes the excitatory current from the excitatory synapses. However, in a stable state, this process is reversed. The LIF equation 1 is used to calculate the cortex voltage (Aliyari et al., 2018; Izhikevich, 2007).
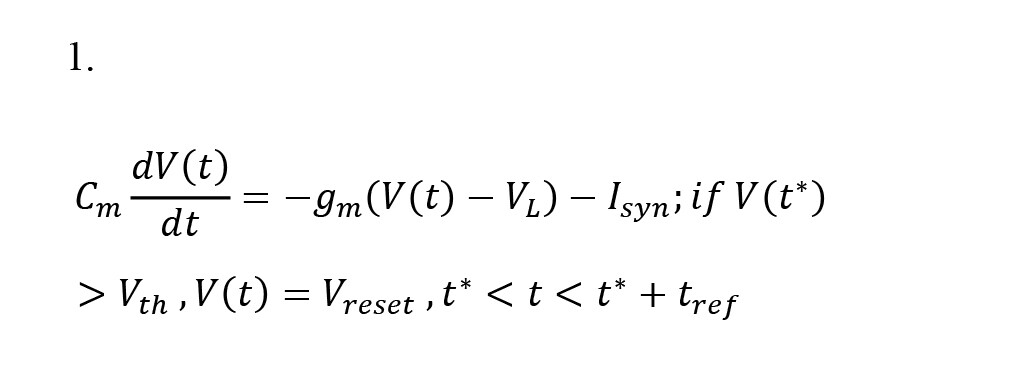
, where V, VL, Vreset, Vth, Cm, and gm denote cortex voltage, neuron resting voltage, neuron recovery potential, neuron fire threshold voltage, membrane capacitance, and membrane electrical conductivity, respectively.
The values of the parameters in the LIF model may differ for excitatory and inhibitory neurons. Moreover, Isyn represents the synaptic current received from the neuron, which can be one of four types, as mentioned earlier. The varying gate model was used to describe the different synaptic receptor currents, which are as follows:
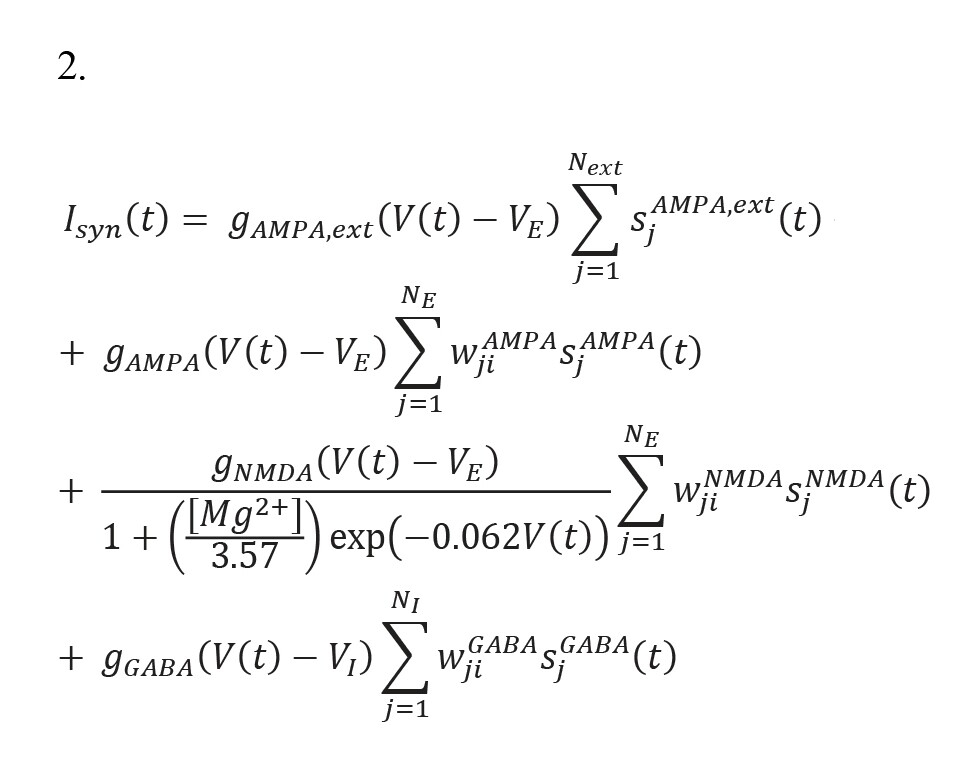
, where s(AMPA,ext), sAMPA, sNMDA, and sGABA, and the synaptic current gate variables correspond to the off-network AMPA receptors and intra-network (internal) AMPA, NMDA, and GABA receptors. In addition, g(AMPA,ext), gAMPA, gNMDA, and gGABA show the maximum electrical conductivity of the corresponding receptors. Moreover, VE and VI denote the resting voltages of the excitatory and inhibitory neurons, respectively. Mg++ refers to the concentration of intra-neuron magnesium in the NMDA channels, and wji shows the synaptic weights of the connections between the neurons and neuron pools. The following equations are used to describe the variables regulating the current flowing through the synapses.
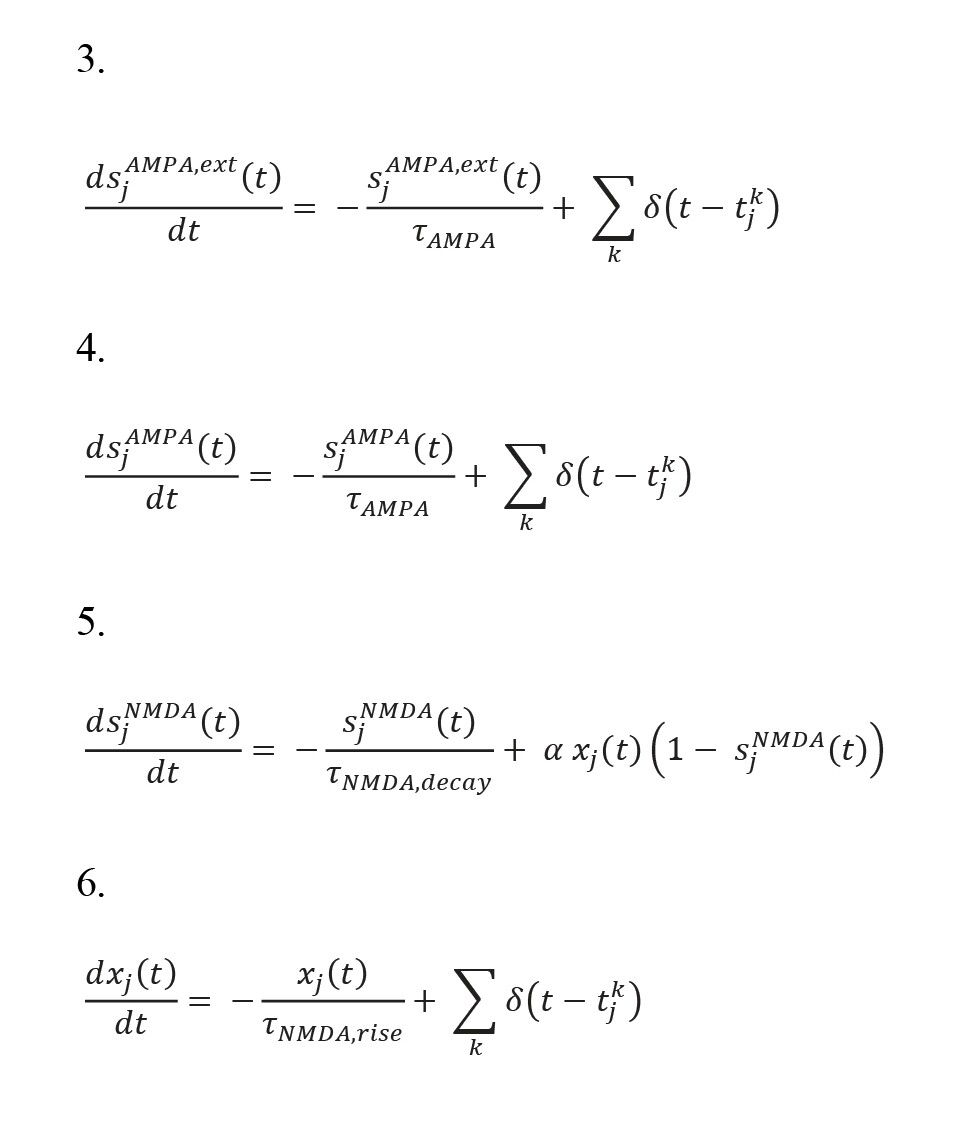
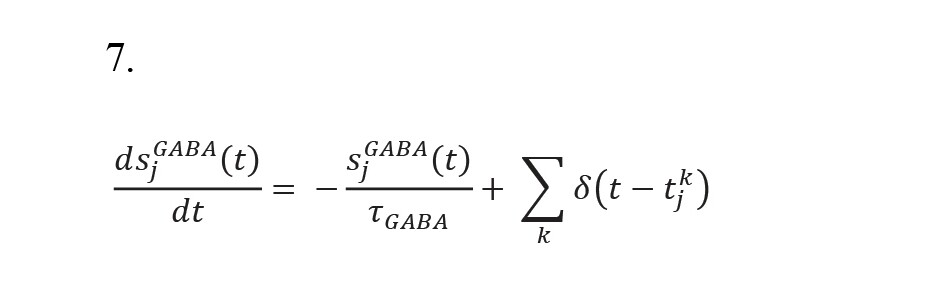
In addition, the time constants of the GABA, NMDA, and AMPA receptor gates are denoted by τGABA, τ(NMDA, decay), and τAMPA, respectively. τ(NMDA, decay) also affects the time constant of the rise of the NMDA receptor gate, and δ(t) is the Dirac function, which activates all fractions of the related receptors according to the potential. In this network, the S1 and S2 neuron pools represent two memories that switch from self-stimulatory mode to stable mode and vice versa with external excitation (Brunel, 2000; Brunel & Wang, 2001; Fourcaud & Brunel, 2002).
3. Results
Experimental phase results
Cognitive tests were conducted before and after applying electric field simulations to examine two important cognitive factors: visual memory and visual working memory performance. For these tests, a device was designed to record the visual memory behavior (visible) and visual working memory performance (behind a curtain). The device comprised two opaque dishes (each with a valve that opened in one direction). The reward (peanut) was put in a dish that was not visible to the primate and was on a moving stand (Kazemi et al., 2018). The experiment started after 17 hours of hunger.
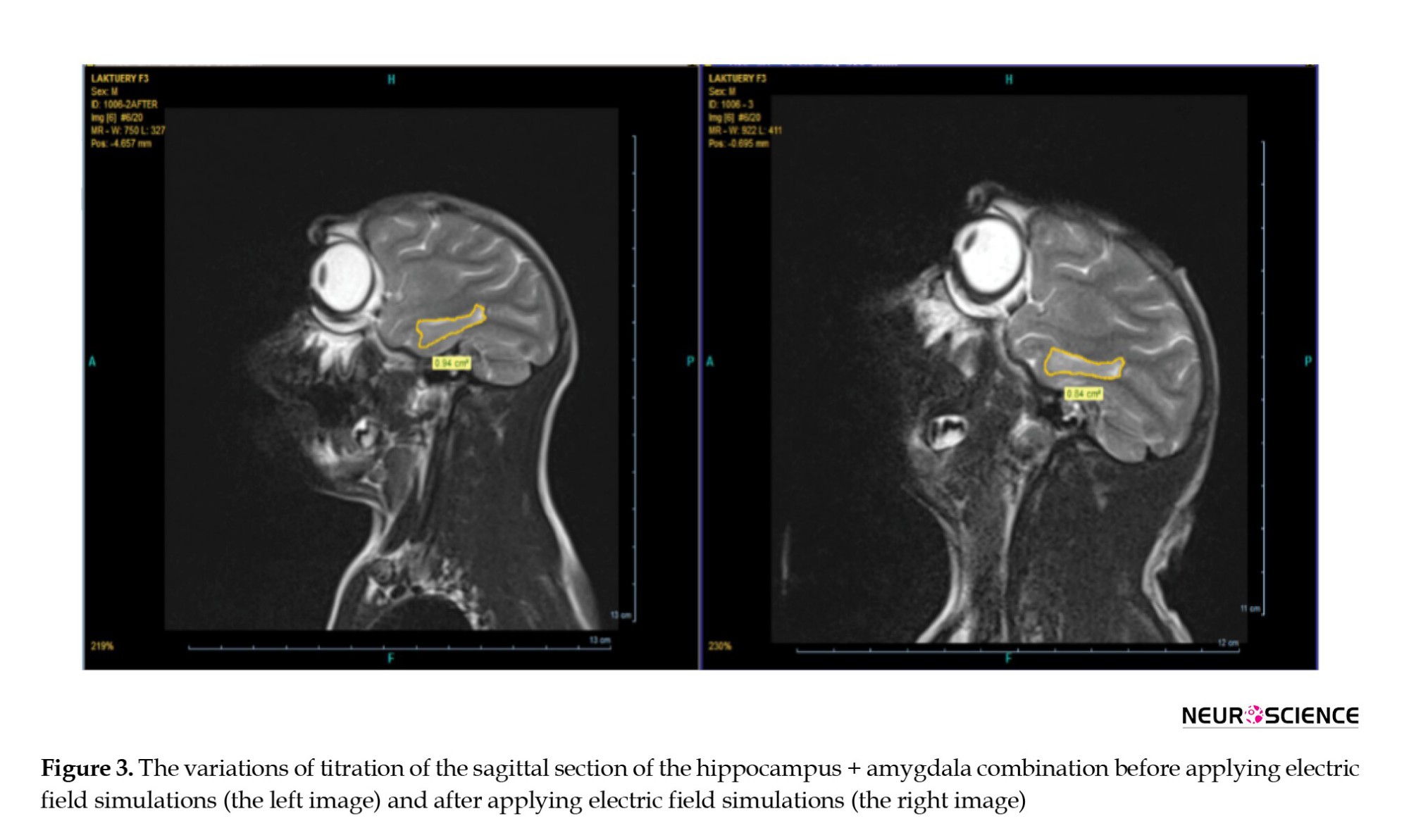
These variations are revealed by studying the hippocampus and amygdala MRI images in Figure 3 concerning the brain anatomy of the primate exposed to the HV field. This field reduced the size of the amygdala and hippocampus (memory and learning) of the primate by 10.5% following the treatment, but no considerable change was observed in the control sample. On the one hand, based on the MRI result, we perceived the primate’s memory decline. On the other hand, we observed the same results from cognitive tests. Therefore, we expect to observe the same results on the SNN model of the hippocampus (decreasing on the raster-grams diagram).
One important research goal is to understand the cognitive effects of HV fields on organisms, including humans. Memory and learning are crucial cognitive factors that these fields can influence. Therefore, researchers have studied the effects of electromagnetic fields on critical biological processes such as cell proliferation, ion exchanges (such as sodium and potassium ions), nerve repair, production of free radicals, and hormonal changes. The research results suggest that the expression of the glucocorticoid receptor gene plays a significant role in visual working memory. However, the effective doses of electromagnetic fields may vary depending on the organism. Additionally, variations in the levels of some membrane and intracellular proteins, such as the NMDA receptor gene, can significantly affect memory and learning (Beale et al., 1997; Crasson, 2003; Salunke et al., 2014).
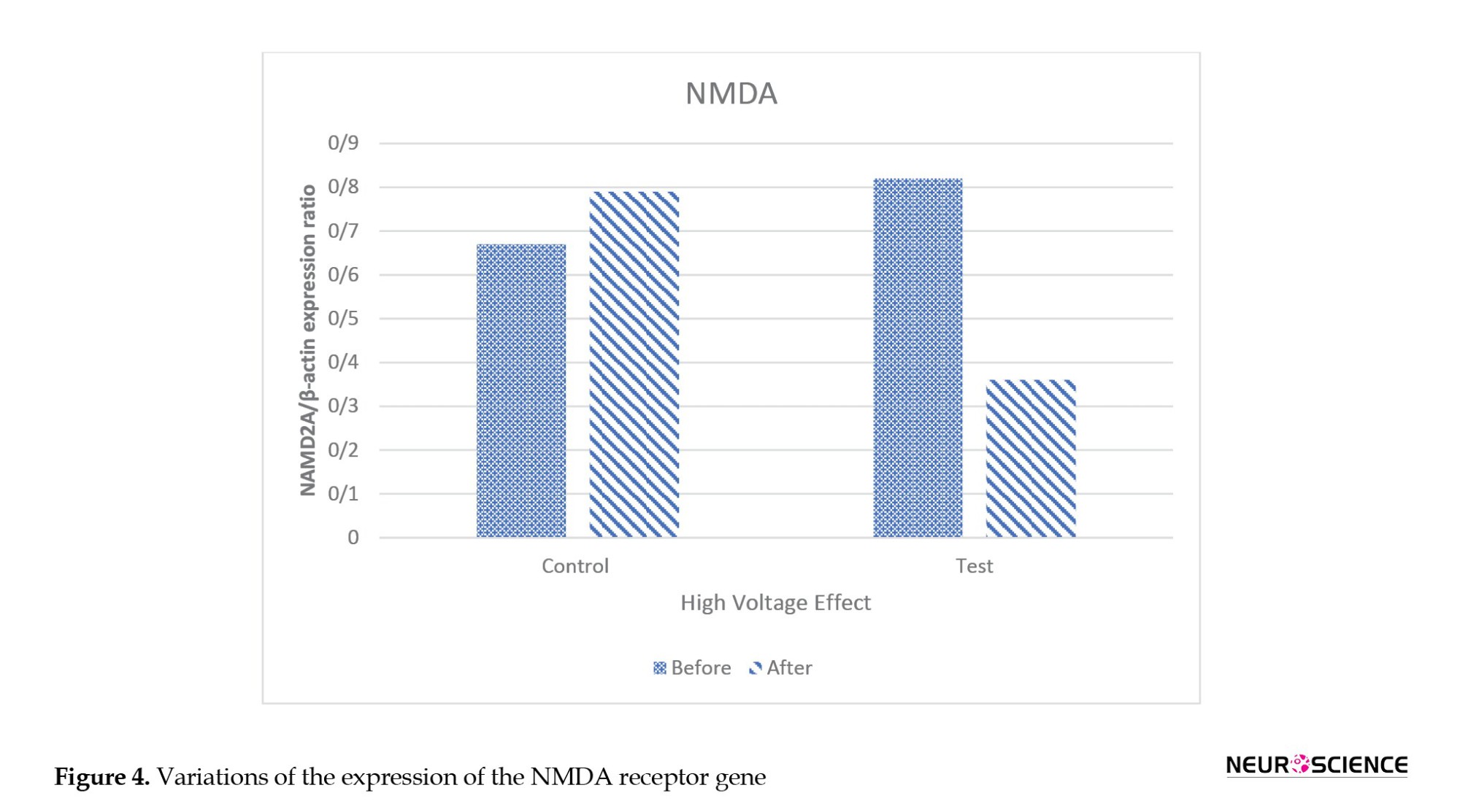
Figure 4 shows the variations in the expression of the NMDA receptor gene. As seen, the expression of this gene in the primates exposed to the HV field decreased by approximately 56%, but it increased by 18% in the control primates.
Sodium is the most important ion in the extracellular fluid and is valuable because it retains water. Different concentrations of this electrolyte have numerous functions in the body. The nerve and muscle impulses are transferred through the pumping of sodium when potassium is discharged from a cell. Sodium and potassium effectively and substantially influence the transmission of nerve impulses (Ehrenstein & Lecar, 1972).
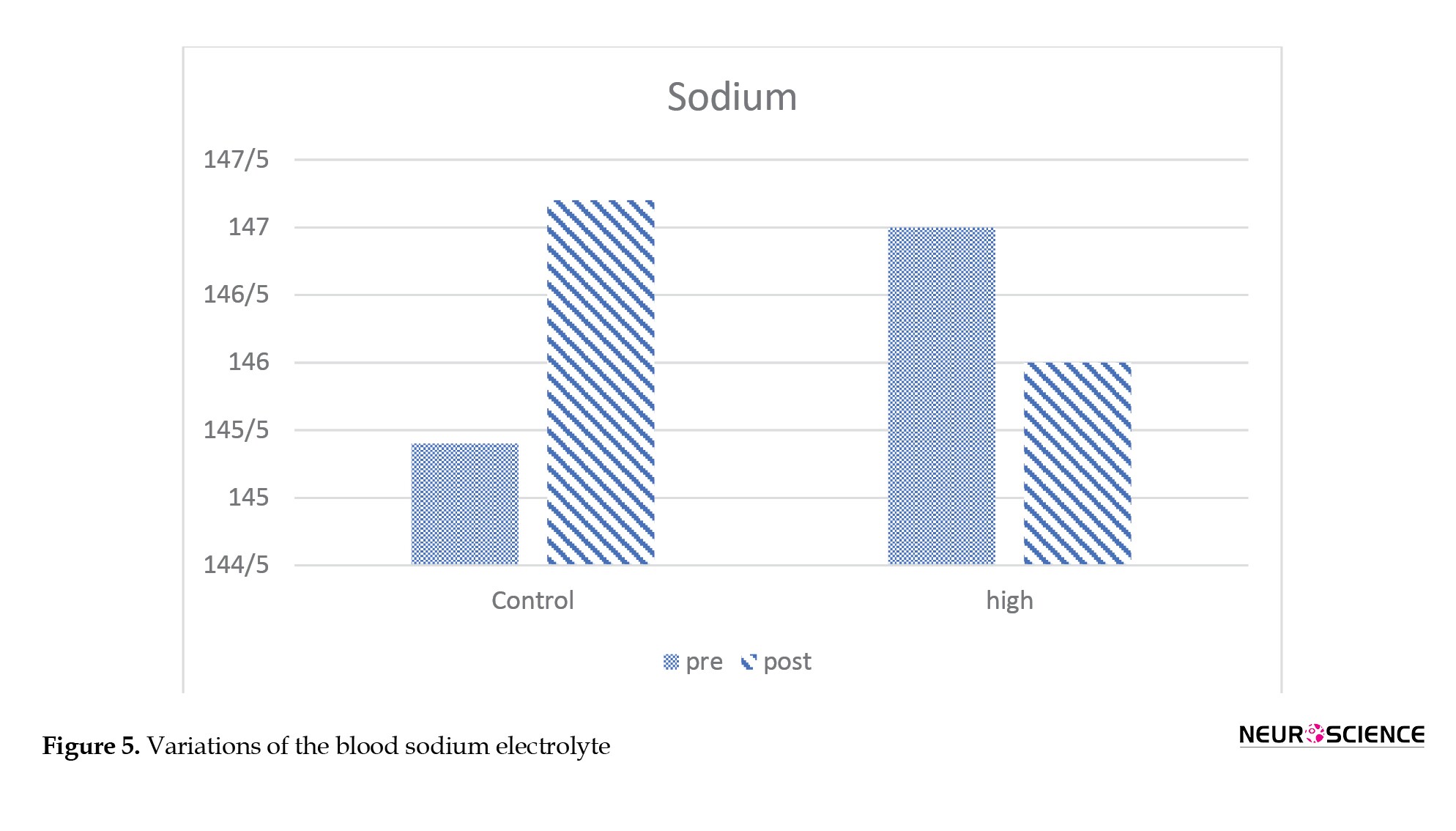
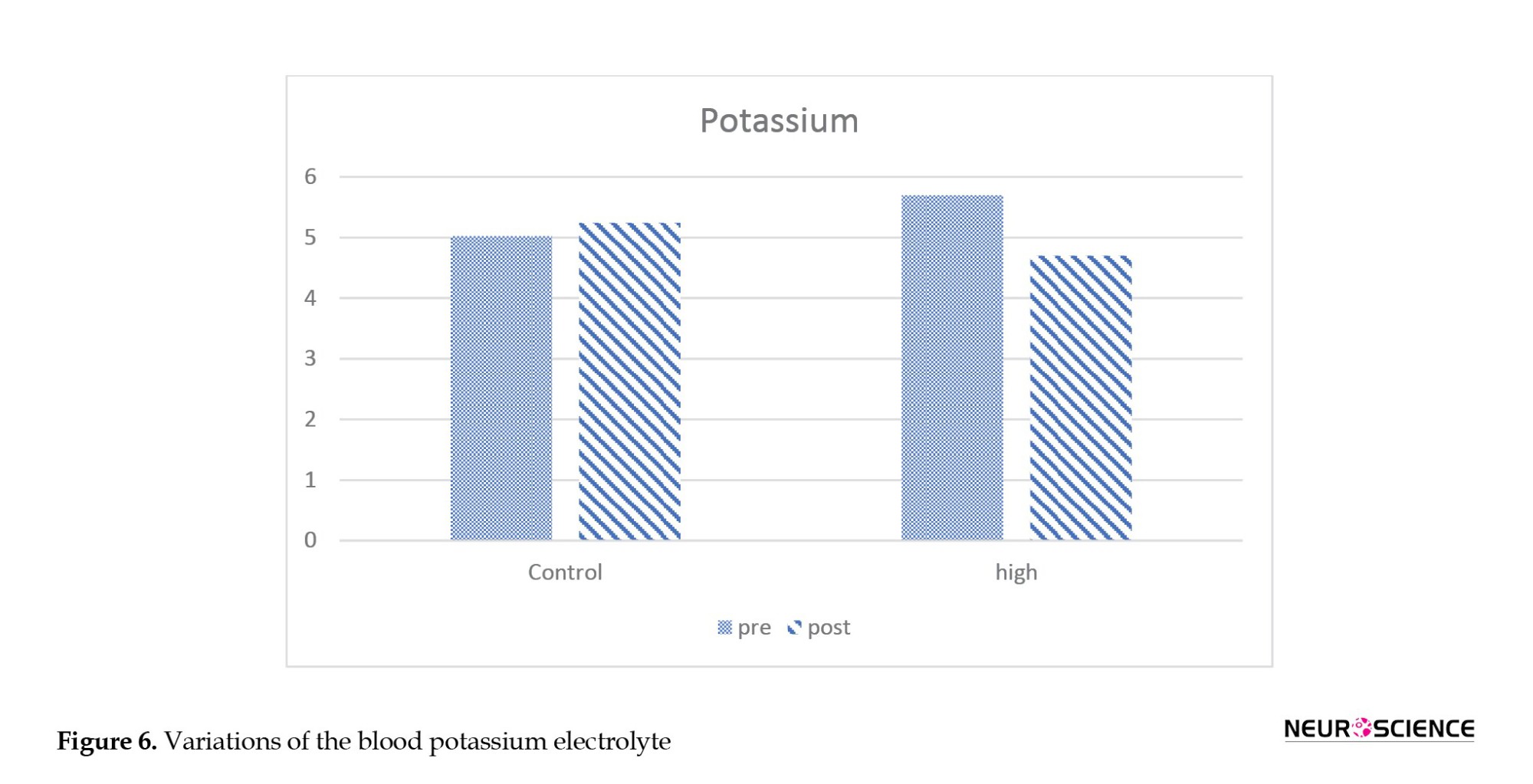
Figures 5 and 6 present the blood electrolyte and potassium and sodium ion levels. These parameters show a descending trend in the primate exposed to the HV field compared to the control sample.
SNN model phase result
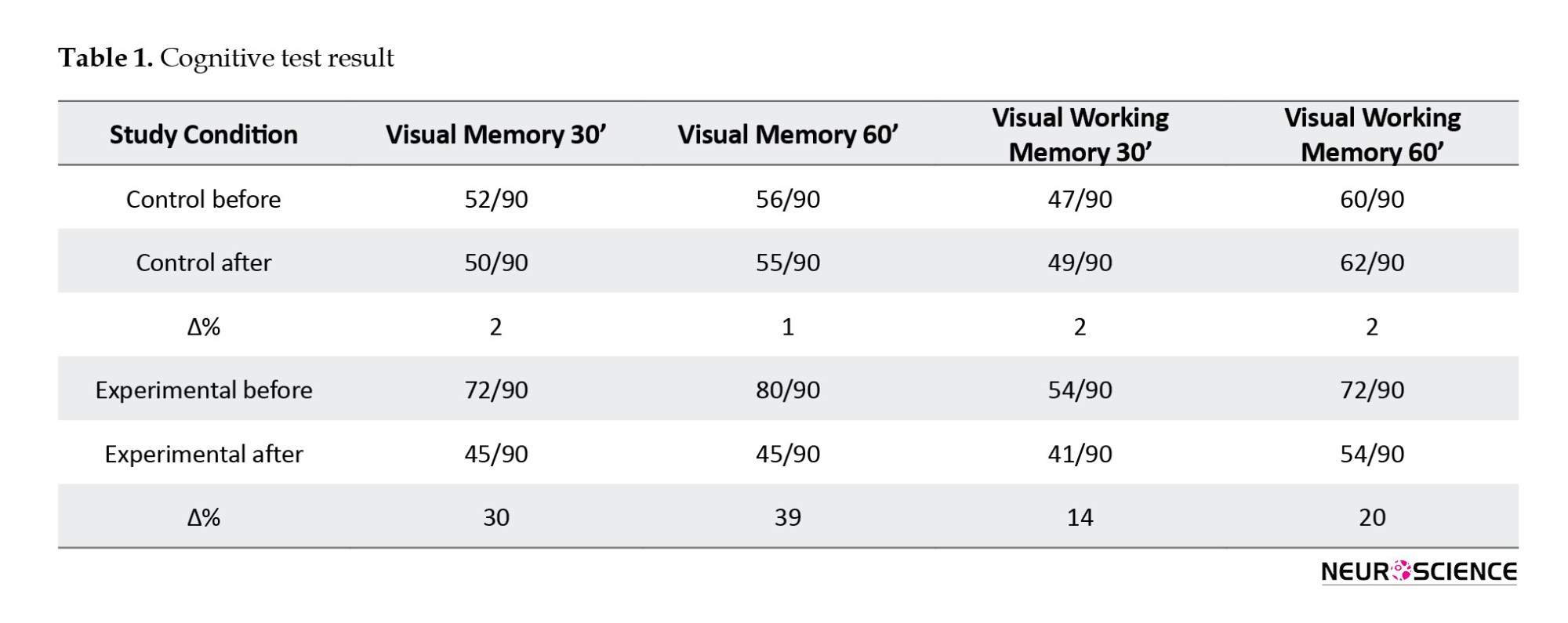
According to the research results, in the primate exposed to the HV field, the decrease in the hippocampus decreased the concentration of NMDA, sodium, and potassium (Figures 4, 5, and 6). In addition, a drastic reduction in performance was observed in the cognitive tests (Table 1) conducted in the first week after applying electric field simulations. Now, it should be determined whether the same result is obtained with the simulated model (the same result is expected from the SNN model, Which means experimental phase results are used to evaluate SNN model results).
The activity of NMDA receptors is an essential factor influencing memory performance in the hippocampus. Various studies have revealed that the performance of these receptors impairs visual working memory. According to the research data, the concentration of NMDA in the primate’s blood exposed to the HV field decreased. In the simulated model, the activity of the NMDA receptors declined. As a result, the conductivity of the NMDA receptors decreased, and the excitatory current (and its mean level) also reduced. Hence,


The result of this process is a decrease in the activity of the cortex (13), which is in line with the reduction in the activity of the memory of the primate. Figure 7 shows the raster gram and the mean fire rate of the corresponding neurons and pools (Guo et al., 2012; Kleine et al., 2003). In this simulation, the number of neurons in each pool was multiplied by 4 to observe the NMDA receptors’ effect. As seen in Figure 7, the activity of the neuron populations decreased with a decrease in the activity of the NMDA receptors. Hence, the memory performance is expected to decline in this state (As expected from the experimental phase).
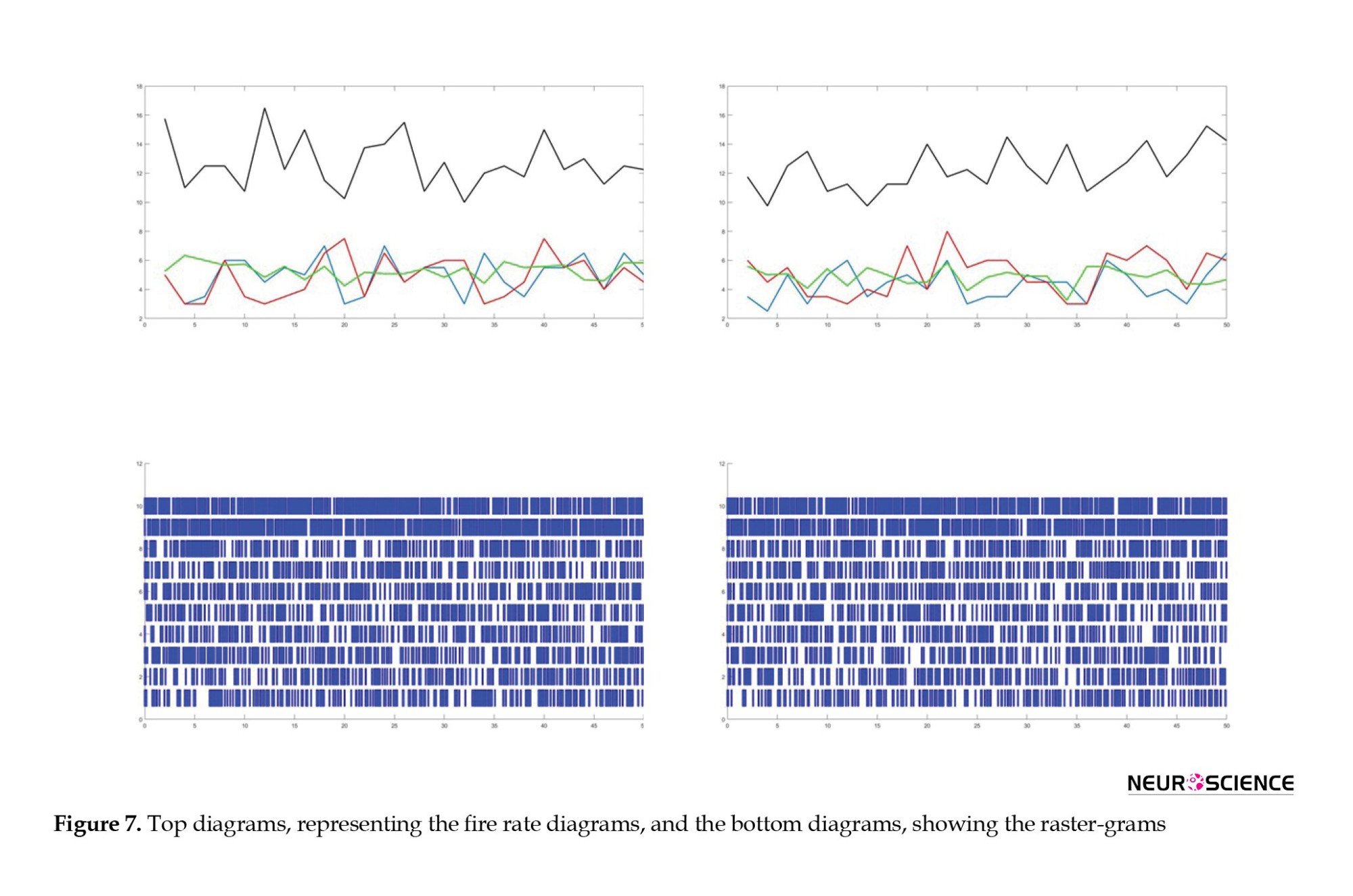
The images on the left depict the raster-gram and fire rate diagrams of all neurons in the primary state without any changes and with zero electric fields. The images on the right show the raster-gram and fire rate diagrams of all neurons exposed to a 3kV/m electric field, which decreased the NMDA conductivity.
Based on the experimental phase, exposure to HV fields led to a reduction in the hippocampus and amygdala volumes and a decrease in the concentration of NMDA. Furthermore, cognitive tests conducted on primates exposed to these fields revealed decreased visual working memory. Additionally, the concentrations of sodium and potassium also declined, which could make transmitting messages difficult and cause the performance of the simulated neural networks model to decrease. On the other hand, a decrease in cortex activity may indicate cognitive, emotional, or sensory disorders depending on the region. For example, studies using applied magnetic resonance imaging (MRI) techniques have shown that a decrease in the dorsolateral prefrontal cortex activity impairs visual memory. In contrast, a decrease in the activity of the dorsomedial prefrontal cortex and the amygdala is associated with depression and a lack of reaction to negative emotional stimuli. The simulated model could also explain the impairment of memory, the decrease in emotional responses, and the onset of depression (Figure 7).
It could be stated that the simulation results and the cognitive, hormonal, blood, and genetic test results follow the decrease in brain functionality, memory functions, and learning (In other words, the SNN model is evaluated by experiment results). Therefore, exposure to HV towers (due to living or working conditions) and electric fields may reduce brain performance, memory, and learning.
4. Discussion
The objective of the present research was to investigate the effect of HV towers on the brain. To achieve this objective, a rhesus macaque was exposed to a 3kV/m HV electric field for four hours a day for one month, and cognitive, blood, and anatomic variations (MRI) were recorded and analyzed. In this study, two male rhesus macaques were used. Additionally, a neural simulation model was created to investigate the performance of the hippocampus in the brain. Changes were made to the model depending on the conditions of certain elements in each state, and the results were obtained from the model. The SNN model results were evaluated based on the experimental results (Aliyari et al., 2019a; Carpenter & Sage, 2007; Chaddock et al., 2010; Kim et al., 2016).
The results obtained using the cognitive elements of the rhesus macaque revealed that the 3 kV/m HV electric field impaired the visual memory of the samples, and the duration of applying electric field simulations effectively contributed to this damage. This is because, in the recovery phase, the cognitive test results improve by distancing from using the electric field simulations phase. The exposure of the primate to 4 hours of treatment a day for a month caused this level of damage. Hence, if the hours of exposure to the HV field increase, the impacts will be more severe and difficult to reverse. Various studies have been carried out on this subject because different results are obtained depending on applying electric field simulations duration and the experiment’s frequency, severity, and time. Moreover, the hippocampus and amygdala play primary and secondary roles in memory, respectively (Aliyari et al., 2015; Aliyari et al., 2018; Aliyari et al.; Aliyaria et al., 2019; Malin & McGaugh, 2006). For example, when the hippocampus neurons are damaged, the patient develops Alzheimer disease (Carpenter, 2013; Vitvitsky et al., 2012).
In the titration examinations of the levels of the hippocampus and amygdala in the primate exposed to HV electric fields, a decreasing trend of variations was observed, which substantially contributes to memory impairment. Glutamate is an excitatory neurotransmitter and a vital source of other receptors. The effects of this neurotransmitter are exerted via membrane receptors known as the ionotropic and metatropic receptors. The NMDA receptors provide a slow synaptic response and contribute to the genesis of the brain, learning, and memory. In addition, the potassium, sodium, and calcium ions properly travel through these receptors. Examining the primates exposed to the HV field revealed a decrease in the expression of the NMDA receptor gene, which could be another cause of the reduction of visual memory and its performance. Furthermore, the decrease in the expression of the NMDA receptor gene reduces the flow of sodium and potassium ions. The sodium, potassium, and calcium ion elements substantially affect memory. Another result of this research was the decrease in sodium and potassium ion concentrations, which could be another cause of the decrease in visual memory and performance.
The NMDA neural receptors are among the important factors influencing the model’s performance in modeling cortex neural models. According to the research results, the variations of the NMDA element in the experimental group sample (i.e. the primate exposed to the HV field) reduced the concentration of this element (Di et al., 2019; Kazemi et al., 2018; Monyer et al., 1994; Salunke et al., 2014; Wang & Kriegstein, 2008). Similar results were obtained from the experimental model. In other words, a decrease was seen in the performance of the cortex neural network (impairment of visual memory). Hence, the results of the neural simulation and experimental models suggest that proximity to HV fields reduces memory performance. Thus, the performance of the SNN model is evaluated using experiment results.
The results of examining the primates can be generalized to humans. Moreover, it could be stated that by adopting some characteristics of the metrics of the resulting model, it is possible to obtain satisfactory results from examining the effects of HV fields on humans.
Finally, the experimental results agree with the results of the neural simulation modeling.
5. Conclusion
Our findings proved that prolonged exposure to HV electric fields can lead to significant neurobiological changes in macaques, including reduced NMDA receptor gene expression, altered blood sodium and potassium ion levels, and structural changes in the hippocampus and amygdala. It also can cause deficits in visual working memory. These results, supported by computational modeling, suggest that chronic exposure to HV fields may pose risks to brain function. Future studies should explore these effects in humans and investigate potential protective strategies.
Ethical Considerations
Compliance with ethical guidelines
This study was conducted in full compliance with international ethical standards for animal research. The study protocol was approved by the Institutional Review Board (IRB) of Baqiyatallah University of Medical Sciences, Tehran, Iran. All necessary measures were taken to ensure the well-being of the macaques, following institutional and international guidelines.
Funding
This research was financial supported by the Neuroscience Research Center at Baqiyatallah University of Medical Sciences, Tehran, Iran, and the Electrical Engineering Department at Amirkabir University of Technology, Tehran, Iran, and the Department of Electrical, Biomedical, and Mechatronics Engineering at QIAU (Cognitive Science Lab).
Authors' contributions
Conceptualization, study design, supervision, review and editing: Hamed Aliyari; Data collection, methodology, and statistical analysis: Mohsen Hosseinian; Neural modeling, computational analysis, and software implementation: Mohammad Bagher Menhaj: Biological assessments, experimental design, and ethical compliance oversight: Hedayat Sahraei; MRI analysis, neuroimaging interpretation, and result validation: Mohsen Shabani; Literature review, data interpretation, and manuscript drafting: Masoomeh Kazemi; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would also like to thank the staff who contributed to the study at each institution. Their assistance was invaluable in completing this research.
References
Aliyari, H., Golabi, S., Sahraei, H., Sahraei, M., Minaei-Bidgoli, B., & Daliri, M. R., et al. (2023). Perceived stress and cognition function quantification in a scary video game: An electroencephalogram features and biochemical measures. Basic and Clinical Neuroscience, 14(2), 297–309. [DOI:10.32598/bcn.2022.3811.1] [PMID]
Aliyari, H., Hosseinian, S., Sahraei, H., & Menhaj, M. (2019). Effect of proximity to high voltage fields: Results of the neural network model and experimental model with macaques. International Journal of Environmental Science and Technology, 16, 4315-4326. [Link]
Aliyari, H., Hosseinian, S., Sahraei, H., & Menhaj, M. (2019). Effect of proximity to high voltage fields: Results of the neural network model and experimental model with macaques. International Journal of Environmental Science and Technology, 16, 4315-4326. [DOI:10.1007/s13762-018-1830-8]
Aliyari, H., Hosseinian, S., Sahraei, H., & Menhaj, M. (2019). Effect of proximity to high voltage fields: Results of the neural network model and experimental model with macaques. International Journal of Environmental Science and Technology, 16(8), 4315-4326. [DOI:10.1007/s13762-018-1830-8]
Aliyari, H., Hosseinian, S. H., Menhaj, M. B., & Sahraei, H. (2019a). Analysis of the effects of high voltage transmission line on human stress and attention through electroencephalography (EEG). Iranian Journal of Science and Technology, Transactions of Electrical Engineering, 43, 211-218. [DOI:10.1007/s40998-018-0151-8]
Aliyari, H., Hosseinian, S. H., Menhaj, M. B., & Sahraei, H. (2019). Analysis of the effects of high voltage transmission line on human stress and attention through electroencephalography (EEG). Iranian Journal of Science and Technology, Transactions of Electrical Engineering, 43(1), 211-218. [DOI:10.1007/s40998-018-0151-8]
Aliyari, H., Kazemi, M., Tekieh, E., Salehi, M., Sahraei, H., & Daliri, M. R., et al. (2015). The effects of FIFA 2015 computer games on changes in cognitive, hormonal and brain waves functions of young men volunteers. Basic and Clinical Neuroscience, 6(3), 193-201. [PMID]
Aliyari, H., Sahraei, H., Daliri, M. R., Minaei Bidgoli, B., Kazemi, M., & Agaei, H., et al. (2018). The beneficial or harmful effects of computer game stress on cognitive functions of players. Basic and Clinical Neuroscience, 9(3), 177-186. [DOI:10.29252/nirp.bcn.9.3.177] [PMID]
Aliyari, H., Sahraei, H., Erfani, M., Mohammadi, M., Kazemi, M., & Daliri, M. R., et al. (2018). Alterations of cognitive functions following violent and football video games in young male volunteers: By studying brain waves. Basic and Clinical Neuroscience Journal, 11(3), 1-25. [Link]
Aliyaria, H., Sahraeib, H., Erfanid, M., Tekiehb, E., Salehib, M., & Kazemib, M., et al. (2019). The impacts of video games on cognitive function and cortisol levels in young female volunteers. Journal of Experimental and Clinical Neuroscience, 6(1), 1-5. [Link]
Beale, I., Pearce, N., Conroy, D., Henning, M., & Murrell, K. (1997). Psychological effects of chronic exposure to 50 Hz magnetic fields in humans living near extra high voltage transmission lines. Bioelectromagnetics, 18(8), 584-594. [DOI:10.1002/(SICI)1521-186X(1997)18:8<584::AID-BEM7>3.0.CO;2-Z]
Brady, T. F., Konkle, T., & Alvarez, G. A. (2011). A review of visual memory capacity: Beyond individual items and toward structured representations. Journal of Vision, 11(5), 4. [DOI:10.1167/11.5.4] [PMID]
Braicu, Ș., Czumbil, L., Șteț, D., & Micu, D. (2017). Evaluation of the electric and magnetic field near high voltage power lines. In: S. Vlad & N. Roman (Eds), International Conference on Advancements of Medicine and Health Care through Technology, Cluj-Napoca, Romania, 12th - 15th October 2016. [DOI:10.1007/978-3-319-52875-5_32]
Brunel, N. (2000). Dynamics of sparsely connected networks of excitatory and inhibitory spiking neurons. Journal of Computational Neuroscience, 8(3), 183-208. [DOI:10.1023/A:1008925309027] [PMID]
Brunel, N., & Wang, X. J. (2001). Effects of neuromodulation in a cortical network model of object working memory dominated by recurrent inhibition. Journal of Computational Neuroscience, 11(1), 63-85. [DOI:10.1023/A:1011204814320] [PMID]
Brunel, N., & Wang, X. J. (2003). What determines the frequency of fast network oscillations with irregular neural discharges? I. Synaptic dynamics and excitation inhibition balance. Journal of Neurophysiology, 90(1), 415-430. [DOI:10.1152/jn.01095.2002] [PMID]
Carpenter, D. & Sage, C. (2007) BioInitiative report: A rationale for a biologically-based public exposure standard for electromagnetic fields (ELF and RF). [Link]
Carpenter, D. O. (2013). Human disease resulting from exposure to electromagnetic fields. Reviews on Environmental Health, 28(4), 159-172. [DOI:10.1515/reveh-2013-0016] [PMID]
Chaddock, L., Erickson, K. I., Prakash, R. S., Kim, J. S., Voss, M. W., & VanPatter, M., et al. (2010). A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Research, 1358, 172-183. [DOI:10.1016/j.brainres.2010.08.049] [PMID]
Chudler, E. H., & Bergsman, K. C. (2016). Brains-computers-machines: Neural engineering in science classrooms. CBE Life Sciences Education, 15(1), fe1. [DOI:10.1187/cbe.15-11-0242] [PMID]
Churchland, P. S., & Sejnowski, T. J. (2016). The computational brain. Massachusetts: MIT Press. [DOI:10.7551/mitpress/11207.001.0001]
Compte, A., Brunel, N., Goldman Rakic, P. S., & Wang, X. J. (2000). Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cerebral Cortex, 10(9), 910-923. [DOI:10.1093/cercor/10.9.910] [PMID]
Constantinidis, C., & Procyk, E. (2004). The primate working memory networks. Cognitive, Affective, & Behavioral Neuroscience, 4(4), 444-465. [DOI:10.3758/CABN.4.4.444] [PMID]
Cook, C. M., Thomas, A. W., & Prato, F. S. (2002). Human electrophysiological and cognitive effects of exposure to ELF magnetic and ELF modulated RF and microwave fields: A review of recent studies. Bioelectromagnetics, 23(2), 144–157. [DOI:10.1002/bem.107] [PMID]
Crasson, M. (2003). 50 60 Hz electric and magnetic field effects on cognitive function in humans: A review. Radiation Protection Dosimetry, 106(4), 333–340. [DOI:10.1093/oxfordjournals.rpd.a006369] [PMID]
Demuth, H. B., Beale, M. H., De Jess, O., & Hagan, M. T. (2014). Neural network design. Cramlington: Martin Hagan. [Link]
Di, G., Kim, H., Xu, Y., Kim, J., & Gu, X. (2019). A comparative study on influences of static electric field and power frequency electric field on cognition in mice. Environmental Toxicology and Pharmacology, 66, 91-95. [DOI:10.1016/j.etap.2019.01.001] [PMID]
Draper, G., Vincent, T., Kroll, M. E., & Swanson, J. (2005). Childhood cancer in relation to distance from high voltage power lines in England and Wales: A case-control study. BMJ, 330(7503), 1290. [DOI:10.1136/bmj.330.7503.1290] [PMID]
Ehrenstein, G., & Lecar, H. (1972). The mechanism of signal transmission in nerve axons. Annual Review of Biophysics and Bioengineering, 1, 347-368. [DOI:10.1146/annurev.bb.01.060172.002023] [PMID]
Engel, A. K., Moll, C. K., Fried, I., & Ojemann, G. A. (2005). Invasive recordings from the human brain: Clinical insights and beyond. Nature Reviews Neuroscience, 6(1), 35-47. [DOI:10.1038/nrn1585] [PMID]
Fourcaud, N., & Brunel, N. (2002). Dynamics of the firing probability of noisy integrate and fire neurons. Neural Computation, 14(9), 2057-2110. [DOI:10.1162/089976602320264015] [PMID]
Guo, D., Wang, Q., & Perc, M. (2012). Complex synchronous behavior in interneuronal networks with delayed inhibitory and fast electrical synapses. Physical Review. E, Statistical, Nonlinear, and Soft Matter Physics, 85(6 Pt 1), 061905.[DOI:10.1103/PhysRevE.85.061905] [PMID]
Izhikevich, E. M. (2003). Simple model of spiking neurons. IEEE Transactions on Neural Networks, 14(6), 1569-1572. [DOI:10.1109/TNN.2003.820440] [PMID]
Izhikevich, E. M. (2007). Dynamical systems in neuroscience: The geometry of excitability and bursting. Massachusetts: MIT Press. [DOI:10.7551/mitpress/2526.001.0001]
Kazemi, M., Aliyari, H., Golabi, S., Tekieh, E., Tavakoli, H., & Saberi, M., et al. (2022). Improvement of cognitive indicators in male monkeys exposed to extremely low frequency electromagnetic fields. Archives of Razi Institute, 77(1), 503-511. [PMID]
Kazemi, M., Sahraei, H., Aliyari, H., Tekieh, E., Saberi, M., & Tavacoli, H., et al. (2018). Effects of the extremely low frequency electromagnetic fields on NMDA receptor gene expression and visual working memory in male rhesus macaques. Basic and Clinical Neuroscience, 9(3), 167-176. [DOI:10.29252/nirp.bcn.9.3.167] [PMID]
Kim, K. E., Park, S. K., Nam, S. Y., Han, T. J., & Cho, I. Y. (2016). Potential therapeutic mechanism of extremely low frequency high voltage electric fields in cells. Technology and Health Care, 24(3), 415-427. [DOI:10.3233/THC-151119] [PMID]
Kleine, J. F., Guan, Y., & Büttner, U. (2003). Saccade related neurons in the primate fastigial nucleus: What do they encode? Journal of Neurophysiology, 90(5), 3137-3154. [DOI:10.1152/jn.00021.2003] [PMID]
Laakso, M. P., Soininen, H., Partanen, K., Helkala, E. L., Hartikainen, P., & Vainio, P., et al. (1995). Volumes of hippocampus, amygdala and frontal lobes in the MRI based diagnosis of early Alzheimer’s disease: Correlation with memory functions. Journal of Neural Transmission: Parkinson’s Disease and Dementia Section, 9(1), 73-86. [DOI:10.1007/BF02252964] [PMID]
Loh, M., Rolls, E. T., & Deco, G. (2007). A dynamical systems hypothesis of schizophrenia. PLoS Computational Biology, 3(11), e228. [DOI:10.1371/journal.pcbi.0030228] [PMID]
Lowenthal, R. M., Tuck, D., & Bray, I. (2007). Residential exposure to electric power transmission lines and risk of lymphoproliferative and myeloproliferative disorders: A case-control study. Internal Medicine Journal, 37(9), 614–619.[DOI:10.1111/j.1445-5994.2007.01389.x] [PMID]
Maass, W. (1997). Networks of spiking neurons: The third generation of neural network models. Neural Networks, 10(9), 1659-1671. [DOI:10.1016/S0893-6080(97)00011-7]
Malin, E. L., & McGaugh, J. L. (2006). Differential involvement of the hippocampus, anterior cingulate cortex, and basolateral amygdala in memory for context and footshock. Proceedings of the National Academy of Sciences, 103(6), 1959-1963. [DOI:10.1073/pnas.0510890103] [PMID]
Markram, H. (2012). The human brain project. Scientific American, 306(6), 50-55. [DOI:10.1038/scientificamerican0612-50] [PMID]
Menon, V. (2011). Large scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15(10), 483-506. [DOI:10.1016/j.tics.2011.08.003] [PMID]
Monyer, H., Burnashev, N., Laurie, D. J., Sakmann, B., & Seeburg, P. H. (1994). Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron, 12(3), 529-540. [DOI:10.1016/0896-6273(94)90210-0] [PMID]
Pillow, J. W., Paninski, L., Uzzell, V. J., Simoncelli, E. P., & Chichilnisky, E. (2005). Prediction and decoding of retinal ganglion cell responses with a probabilistic spiking model. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 25(47), 11003–11013. [DOI:10.1523/JNEUROSCI.3305-05.2005] [PMID]
Pretorius, P. (2006). Electric and magnetic fields from overhead power lines: A summary of technical and biological aspects. Sandton: Eskom Holdings Ltd. [Link]
Rose, S. A., Feldman, J. F., & Jankowski, J. J. (2009). A cognitive approach to the development of early language. Child Development, 80(1), 134–150. [DOI:10.1111/j.1467-8624.2008.01250.x] [PMID]
Salunke, B. P., Umathe, S. N., & Chavan, J. G. (2014). Involvement of NMDA receptor in low frequency magnetic field induced anxiety in mice. Electromagnetic Biology and Medicine, 33(4), 312-326. [DOI:10.3109/15368378.2013.839453] [PMID]
Seung, H. S. (2003). Learning in spiking neural networks by reinforcement of stochastic synaptic transmission. Neuron, 40(6), 1063–1073. [DOI:10.1016/S0896-6273(03)00761-X] [PMID]
Soininen, H. S., Partanen, K., Pitkänen, A., Vainio, P., Hänninen, T., & Hallikainen, M., et al. (1994). Volumetric MRI analysis of the amygdala and the hippocampus in subjects with age associated memory impairment: Correlation to visual and verbal memory. Neurology, 44(9), 1660-1668. [DOI:10.1212/WNL.44.9.1660] [PMID]
Squire, L. R. (1992). Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychological Review, 99(2), 195-231. [DOI:10.1037/0033-295X.99.2.195] [PMID]
Starzyk, J. A., Graham, J. T., Raif, P., & Tan, A. H. (2012). Motivated learning for the development of autonomous systems. Cognitive Systems Research, 14(1), 10-25. [DOI:10.1016/j.cogsys.2010.12.009]
Stevens, J. C., Shipley, L. A., Cashman, J. R., Vandenbranden, M., & Wrighton, S. A. (1993). Comparison of human and rhesus monkey in vitro phase I and phase II hepatic drug metabolism activities. Drug Metabolism and Disposition, 21(5), 753-760. [DOI:10.1016/S0090-9556(25)08157-7] [PMID]
Takac, M., & Knott, A. (2015). A neural network model of episode representations in working memory. Cognitive Computation, 7(5), 509-525. [DOI:10.1007/s12559-015-9330-3]
Taylor, J. G. (2009). Cognitive computation. Cognitive Computation, 1(1), 4-16. [Link]
Tekieh, E., Riahi, E., Kazemi, M., Sahraei, H., Tavakoli, H., & Aliyary, H., et al. (2017). Role of basal stress hormones and amygdala dimensions in stress coping strategies of male rhesus monkeys in response to a hazard reward conflict. Iranian Journal of Basic Medical Sciences, 20(8), 951-957. [PMID]
Tsodyks, M., Pawelzik, K., & Markram, H. (1998). Neural networks with dynamic synapses. Neural computation, 10(4), 821–835. [DOI:10.1162/089976698300017502] [PMID]
Ushie, P., Pekene, D., Obi, E., & Ukhurebor, K. (2017). Investigation of field induced effect of high voltage transmission line in Calabar South, Nigeria. Physical Science International Journal, 15(1), 1-9. [DOI:10.9734/PSIJ/2017/32022]
Van der Velde, F. (2010). Where artificial intelligence and neuroscience meet: The search for grounded architectures of cognition. Advances in Artificial Intelligence, 2010, 5. [DOI:10.1155/2010/918062]
Van Ooijen, P., ten Bhömer, P., Blecourt, M., Roosjen, R., van Dam, R., & Oudkerk, M. (2005). DICOM storage into PACS of out hospital CD ROMs-a half year experience report. International Congress Series, 1281, 883-887. [DOI:10.1016/j.ics.2005.03.114]
Videbech, P., & Ravnkilde, B. (2004). Hippocampal volume and depression: A meta analysis of MRI studies. The American Journal of Psychiatry, 161(11), 1957–1966. [DOI:10.1176/appi.ajp.161.11.1957] [PMID]
Vitvitsky, V. M., Garg, S. K., Keep, R. F., Albin, R. L., & Banerjee, R. (2012). Na+ and K+ ion imbalances in Alzheimer’s disease. Biochimica et Biophysica acta, 1822(11), 1671–1681. [DOI:10.1016/j.bbadis.2012.07.004] [PMID]
Wang, M. D., & Kriegstein, A. R. (2008). GABA regulates excitatory synapse formation in the neocortex via NMDA receptor activation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 28(21), 5547–5558.[DOI:10.1523/JNEUROSCI.5599-07.2008] [PMID]
Xu, C., & Li, P. (2016). Dynamics in four neuron bidirectional associative memory networks with inertia and multiple delays. Cognitive Computation, 8(1), 78-104. [DOI:10.1007/s12559-015-9344-x]
Zhang, S., Huang, K., Zhang, R., & Hussain, A. (2018). Learning from few samples with memory network. Cognitive Computation, 10(1), 15-22. [DOI:10.1007/s12559-017-9507-z]
To prevent the potential effects of magnetic and electric fields generated by power transmission lines on human health, it is necessary to conduct calculations (Aliyari et al., 2019a; Aliyari et al., 2019a; Di et al., 2019; Pretorius, 2006) when installing these lines near cities and villages. In recent years, numerous studies have investigated the effects of low-frequency electromagnetic radiation on the nervous system and memory performance in humans and animals. The rhesus macaque, which shares 98% of its genes with humans, has been used as an important animal model in scientific studies (Kazemi et al., 2018; Squire, 1992; Stevens et al., 1993). Furthermore, cognitive-behavioral analyses have become a state-of-the-art research method using this animal model (Cook et al., 2002; Draper et al., 2005; Lowenthal et al., 2007).
It is necessary to calculate the fields in the surrounding areas to prevent potential health effects on humans from the electric and magnetic fields generated by power transmission lines near power generation centers, cities, and villages. In a recent study, neural network modeling was employed with biological, behavioral, and anatomical examinations to investigate the effects of a high-voltage (HV) electric field (400 kV transmission line) on two male rhesus macaques (Aliyari et al., 2019b; Aliyari et al., 2019b; Braicu et al., 2017; Ushie et al., 2017).
Visual memory (Brady et al., 2011) is an essential cognitive factor substantially fostered through learning. As a significant cognitive factor, the importance of memory has been valued by neurologists, artificial intelligence, and brain engineering specialists (Aliyari et al., 2018; Chudler & Bergsman, 2016). Experimental research has dramatically expanded our understanding of the brain, particularly with regard to the role of the hippocampus, amygdala, and prefrontal cortex in memory, decision-making, and other mental processes. However, it is essential to note that various factors limit this research. For example, it may be difficult to study certain human brain regions or functions due to ethical concerns or technical limitations. While helpful in exploring certain aspects of brain function, animal models may not always accurately represent human brain function.
Additionally, experimental designs may not always fully capture the complexity of real-world scenarios. Despite these limitations, experimental research remains critical for advancing our understanding of the brain and developing new treatments for neurological and psychiatric disorders. (Aliyari et al., 2018; Laakso et al., 1995; Soininen et al., 1994; Tekieh et al., 2017; Videbech & Ravnkilde, 2004). The precise examination of neuron behavior requires costly equipment and considerable effort, and the techniques used for this purpose are not always applicable. The first thing in studying memory formation in the brain is to determine the parts of the brain and neurons involved in memory development (Rose et al., 2009). After understanding the relevant neurons and areas involved in the formation of memory, the activity of each neuron and region must be measured/predicted, and interpreted. It is impossible to study the brain using the invasive methods that yield the most precise results, and the experimental approach is challenged radically. Therefore, these studies are inevitably conducted on mammals with the most genetic similarity to humans, but these studies are not flawless either (Engel et al., 2005).
Experimental research is a valuable tool for studying the brain, but it has limitations that necessitate the development of alternative methods. Computational and modeling approaches have emerged as important tools for understanding brain function, using computer simulations and mathematical models to study complex systems and processes in the brain. These approaches can provide insights that may not be easily observed through experimental approaches alone and can be used to test hypotheses and make predictions about brain function, potentially leading to new treatments for neurological and psychiatric disorders (Aliyari et al., 2018; Markram, 2012; Van der Velde, 2010). Computational models have become increasingly popular among researchers due to their ability to create precise models of complex systems, such as those found in the brain, which may not be easily studied through experimental methods. With these models, researchers can study element behavior and parameters that may not be able to be studied experimentally. Given these advantages, computational models have become an essential tool in studying complex systems and processes in the brain (Churchland & Sejnowski, 2016; Loh et al., 2007; Menon, 2011).
Also, the neural connection is one of the chief characteristics of the brains of the vertebrae. Through this connection, many neurons are linked to one another through axons and dendrites, which affect other neurons and are affected by them via the axons and dendrites. The junction between two neurons is called a synapse. There are typically synaptic connections between one neuron and several thousand other neurons, and that particular neuron receives synapses from those several thousand other neurons. Synapses are among the most crucial brain structures due to numerous reasons. Synapses are highly organized and stable structures from a biological perspective, and they play a pivotal role in information transmission and processing, enabling the brain to learn, memorize, and adapt. Synaptic disorders can lead to a range of brain and psychological disorders, given the critical role synapses play in neurotransmission. Clinically, synapses are the primary targets for medicinal treatments and healthcare interventions to address these disorders (Maass, 1997; Seung, 2003; Takac & Knott, 2015; Taylor, 2009; Tsodyks et al., 2006).
This study aims to investigate the impact of HV electric fields on the behavior of simulated neural models of the hippocampus, given its crucial role in learning and memory. To validate the results, the behavioral, blood, hormonal, gene, and cognitive observations from experimental examinations of two rhesus macaques exposed to similar electric fields will be compared. The research question holds significant importance as it can provide insights into the potential effects of HV electric fields on the brain and contribute to a better understanding of the associated risks with power transmission lines. It is important to note that the study adheres to all applicable international, national, and institutional guidelines for the care and use of animals.
2. Materials and Methods
Animal subjects
The study used two adult male rhesus macaques (Macaca mulatta) aged 4 to 5 years, with an average weight of 4 kg. The macaques were kept in a controlled environment for 12 months to adapt. The room where the animals were kept had appropriate lighting, temperature, and humidity and followed all ethical and international rules for the animals’ transportation, location, and maintenance (Aliyari et al., 2019a; Aliyari et al., 2019b; Aliyari et al., 2019a).
HV electric field exposure
In this experiment, one of the rhesus macaques was exposed to a simulated HV electric field of 3 kV/m for 4 hours a day for a month, while the other macaque was kept outside the field as a control sample. The HV electric field used in the experiment had a frequency of 50 Hz and was simulated in the laboratory using two metal sheets measuring 2×2 m. The sheets were placed by a crane at the bottom and on top of a polytetrafluoroethylene (PTFE) primate cage measuring 1×1×1 m, with a distance of 2 m between them. The sheets were exposed to a voltage of 6 kV to create a uniform 3kV/m field in the cage (Aliyari et al., 2019b).
Behavioral analysis
Behavioral analyses were conducted on both macaques before, during, and after the application of electric field simulations and were recorded on camera.
Blood analysis
In addition to behavioral analyses, 5 mL of blood was collected from each macaque and used to measure the concentrations of sodium and potassium blood electrolytes and the expression of the NMDA receptor gene. Blood samples were collected before the macaques were included in the research, after applying electric field simulations, and during the recovery phase. The expression of the NMDA receptor gene was determined using the RT-PCR method, and blood lymphocyte cells were obtained before and after applying electric field simulations and during the recovery phase. Additionally, the blood concentrations of sodium and potassium ions were measured during the same phases.
MRI analysis
An MRI-assisted analysis of the anatomy of the hippocampus and amygdala was conducted using DICOM LiteBox before and after the application of electric field simulations (Aliyari et al., 2018; Tekieh et al., 2017; Van Ooijen et al., 2005).
Neural modeling
The neural model was simulated using MATLAB software, version 2016b (Aliyari et al., 2023; Aliyari et al., 2018; Aliyari et al., 2015; Izhikevich, 2003).
Recovery phase
During the recovery phase, both macaques, one of which had been exposed to the HV electric field and the other kept in a non-exposed environment, were kept in the same previous place and situation without exposure to the electric field.
Cognitive tests have four phases, as represented below.
Phase one is the visual memory experiment. The visual memory recording device was placed in front of the primate, and a reward was randomly put in one of the dishes. After 30 s, the dish on the moving stand was provided to the animal. The macaque was allowed a single attempt to access the reward, and he would be deprived if he failed to open the right dish. Consequently, the animal had to focus and pay attention to the reward. This test was repeated thrice daily before the primate’s eyes (Constantinidis & Procyk, 2004; Kazemi et al., 2018).
In phase two, a visual memory experiment was conducted with the macaques. Peanuts were placed in one of two covered dishes in front of the animal, and the dish with the peanuts was presented to the animal after a 60-s delay. The process was explained to the animal before the experiment.
Phase three is the visual working memory experiment. Peanuts (the reward) were put in one of the covered dishes before the primate’s eyes, and a curtain was placed between the primate and the reward (dish) for 30 seconds. Following this period, the dish was presented to the animal after closing the curtain. The animal had only one chance to access the reward, so he had to pay attention, concentrate, and memorize (Kazemi et al., 2022; Kazemi et al., 2018).
Phase four also involves the visual working memory experiment. Peanuts (the reward) were put in one of the covered dishes before the animal’s eyes. A curtain was placed between the primate and the dish for 60 s, after which the dish was presented to the animal, and the procedure continued as described (Constantinidis & Procyk, 2004; Kazemi et al., 2022; Kazemi et al., 2018).
Spiking neural network (SNN) models of hippocampus
Neural network
Neural networks have various functions, including selecting input, adjusting gain, reducing turbulence, and selectively reinforcing activity. These functions form the basis of simple and complex models of primary visual cortex cells, such as short-term and hybrid memory models. The fire rate and spiking models of the neural network models have received significant attention from researchers. The fire rate model features neurons that produce fire rates instead of action potentials, making it a more cost-effective and simpler model to analyze mathematically. However, it has limitations, such as not analyzing fire duration and correlation in networks with high simultaneity. The spiking model can present more biological details due to its parameters and ability to analyze fire time and correlations, but it is more computationally expensive. Both models have strengths and weaknesses, and the choice depends on the research question being addressed (Aliyari et al., 2018; Demuth et al., 2014; Starzyk et al., 2012; Xu & Li, 2016; Zhang et al., 2018).
Spiking model
Modeling neuron populations often involves generating populations using basic computational neuron models and connecting them using synaptic models. Combining a wide range of neural and synaptic models makes it possible to describe the behavior of different brain regions with physiological details such as synaptic currents and their dynamics, neural receptors, and ion channels. This condition results in a computational model that provides insights into the complex processes and interactions within the brain (Izhikevich, 2003; Pillow et al., 2005).
Figure 1 illustrates the structure of a neural network consisting of three pyramidal neuron pools and one inhibitory neuron pool. All neurons in the network are interconnected, but the synaptic strength of the neurons within each neuron pool is higher than the connections between the pools.

The Figure 2 displays the synaptic strength of the neurons and the connections between the neuron pools, with solid lines representing excitatory connections and dashed lines representing inhibitory connections (Brunel & Wang, 2001; Loh et al., 2007).

The S1 and S2 neuron pools are attractor neurons, meaning they can maintain a stable activity state without input. The NS neuron pool describes the activities of other pyramidal neurons in the brain’s cortex region. In contrast, the IH (hyperpolarization-activated current neurons) are inhibitory neurons that can modulate and control the other neurons’ activity (Brunel, 2000; Brunel & Wang, 2003; Compte et al., 2000).
A simple yet efficient LIF (leaky integrate-and-fire) model was utilized to describe the voltage dynamics of neurons in the cortex, with AMPA, NMDA, and GABA receptor currents serving as the synaptic currents. Each neuron in the network receives four currents: off-network AMPA receptor currents, AMPA receptor currents from intra-network excitatory neurons, NMDA receptor currents from intra-network excitatory neurons, and GABA receptor currents from intra-network inhibitory neurons. The off-network AMPA receptor currents reflect the activity of neurons in other parts of the cortex that provide input to the network. In this model, each neuron receives 800 synapses with off-network AMPA receptors, contributing to the neurons’ background activity. Furthermore, each neuron receives 400 synapses from excitatory neurons with AMPA and NMDA receptors and 100 synapses from inhibitory neurons with GABA receptors. In the self-stimulatory state, the inhibitory current from the network’s inhibitory synapses overcomes the excitatory current from the excitatory synapses. However, in a stable state, this process is reversed. The LIF equation 1 is used to calculate the cortex voltage (Aliyari et al., 2018; Izhikevich, 2007).
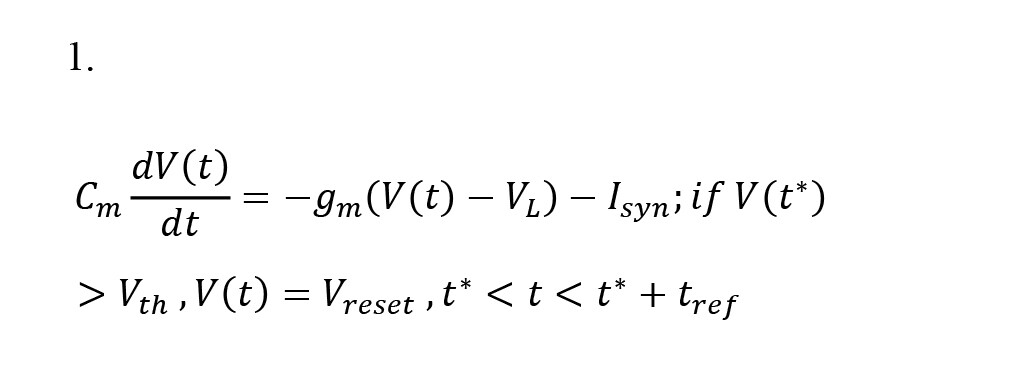
, where V, VL, Vreset, Vth, Cm, and gm denote cortex voltage, neuron resting voltage, neuron recovery potential, neuron fire threshold voltage, membrane capacitance, and membrane electrical conductivity, respectively.
The values of the parameters in the LIF model may differ for excitatory and inhibitory neurons. Moreover, Isyn represents the synaptic current received from the neuron, which can be one of four types, as mentioned earlier. The varying gate model was used to describe the different synaptic receptor currents, which are as follows:
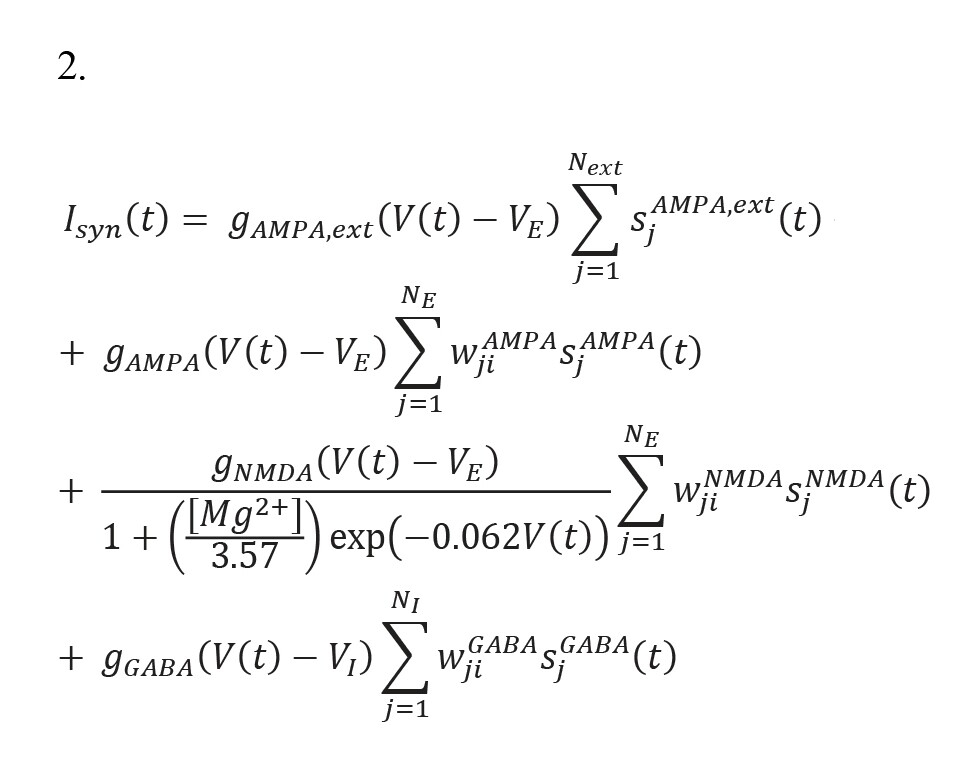
, where s(AMPA,ext), sAMPA, sNMDA, and sGABA, and the synaptic current gate variables correspond to the off-network AMPA receptors and intra-network (internal) AMPA, NMDA, and GABA receptors. In addition, g(AMPA,ext), gAMPA, gNMDA, and gGABA show the maximum electrical conductivity of the corresponding receptors. Moreover, VE and VI denote the resting voltages of the excitatory and inhibitory neurons, respectively. Mg++ refers to the concentration of intra-neuron magnesium in the NMDA channels, and wji shows the synaptic weights of the connections between the neurons and neuron pools. The following equations are used to describe the variables regulating the current flowing through the synapses.

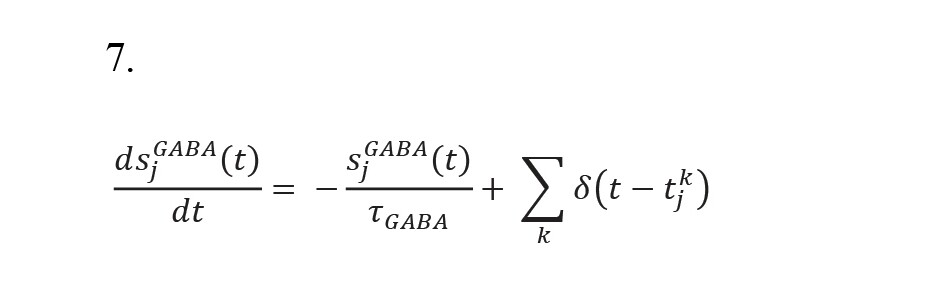
In addition, the time constants of the GABA, NMDA, and AMPA receptor gates are denoted by τGABA, τ(NMDA, decay), and τAMPA, respectively. τ(NMDA, decay) also affects the time constant of the rise of the NMDA receptor gate, and δ(t) is the Dirac function, which activates all fractions of the related receptors according to the potential. In this network, the S1 and S2 neuron pools represent two memories that switch from self-stimulatory mode to stable mode and vice versa with external excitation (Brunel, 2000; Brunel & Wang, 2001; Fourcaud & Brunel, 2002).
3. Results
Experimental phase results
Cognitive tests were conducted before and after applying electric field simulations to examine two important cognitive factors: visual memory and visual working memory performance. For these tests, a device was designed to record the visual memory behavior (visible) and visual working memory performance (behind a curtain). The device comprised two opaque dishes (each with a valve that opened in one direction). The reward (peanut) was put in a dish that was not visible to the primate and was on a moving stand (Kazemi et al., 2018). The experiment started after 17 hours of hunger.
These variations are revealed by studying the hippocampus and amygdala MRI images in Figure 3 concerning the brain anatomy of the primate exposed to the HV field. This field reduced the size of the amygdala and hippocampus (memory and learning) of the primate by 10.5% following the treatment, but no considerable change was observed in the control sample. On the one hand, based on the MRI result, we perceived the primate’s memory decline. On the other hand, we observed the same results from cognitive tests. Therefore, we expect to observe the same results on the SNN model of the hippocampus (decreasing on the raster-grams diagram).

One important research goal is to understand the cognitive effects of HV fields on organisms, including humans. Memory and learning are crucial cognitive factors that these fields can influence. Therefore, researchers have studied the effects of electromagnetic fields on critical biological processes such as cell proliferation, ion exchanges (such as sodium and potassium ions), nerve repair, production of free radicals, and hormonal changes. The research results suggest that the expression of the glucocorticoid receptor gene plays a significant role in visual working memory. However, the effective doses of electromagnetic fields may vary depending on the organism. Additionally, variations in the levels of some membrane and intracellular proteins, such as the NMDA receptor gene, can significantly affect memory and learning (Beale et al., 1997; Crasson, 2003; Salunke et al., 2014).
Figure 4 shows the variations in the expression of the NMDA receptor gene. As seen, the expression of this gene in the primates exposed to the HV field decreased by approximately 56%, but it increased by 18% in the control primates.

Sodium is the most important ion in the extracellular fluid and is valuable because it retains water. Different concentrations of this electrolyte have numerous functions in the body. The nerve and muscle impulses are transferred through the pumping of sodium when potassium is discharged from a cell. Sodium and potassium effectively and substantially influence the transmission of nerve impulses (Ehrenstein & Lecar, 1972).
Figures 5 and 6 present the blood electrolyte and potassium and sodium ion levels. These parameters show a descending trend in the primate exposed to the HV field compared to the control sample.

SNN model phase result
According to the research results, in the primate exposed to the HV field, the decrease in the hippocampus decreased the concentration of NMDA, sodium, and potassium (Figures 4, 5, and 6). In addition, a drastic reduction in performance was observed in the cognitive tests (Table 1) conducted in the first week after applying electric field simulations. Now, it should be determined whether the same result is obtained with the simulated model (the same result is expected from the SNN model, Which means experimental phase results are used to evaluate SNN model results).


The activity of NMDA receptors is an essential factor influencing memory performance in the hippocampus. Various studies have revealed that the performance of these receptors impairs visual working memory. According to the research data, the concentration of NMDA in the primate’s blood exposed to the HV field decreased. In the simulated model, the activity of the NMDA receptors declined. As a result, the conductivity of the NMDA receptors decreased, and the excitatory current (and its mean level) also reduced. Hence,


The result of this process is a decrease in the activity of the cortex (13), which is in line with the reduction in the activity of the memory of the primate. Figure 7 shows the raster gram and the mean fire rate of the corresponding neurons and pools (Guo et al., 2012; Kleine et al., 2003). In this simulation, the number of neurons in each pool was multiplied by 4 to observe the NMDA receptors’ effect. As seen in Figure 7, the activity of the neuron populations decreased with a decrease in the activity of the NMDA receptors. Hence, the memory performance is expected to decline in this state (As expected from the experimental phase).

The images on the left depict the raster-gram and fire rate diagrams of all neurons in the primary state without any changes and with zero electric fields. The images on the right show the raster-gram and fire rate diagrams of all neurons exposed to a 3kV/m electric field, which decreased the NMDA conductivity.
Based on the experimental phase, exposure to HV fields led to a reduction in the hippocampus and amygdala volumes and a decrease in the concentration of NMDA. Furthermore, cognitive tests conducted on primates exposed to these fields revealed decreased visual working memory. Additionally, the concentrations of sodium and potassium also declined, which could make transmitting messages difficult and cause the performance of the simulated neural networks model to decrease. On the other hand, a decrease in cortex activity may indicate cognitive, emotional, or sensory disorders depending on the region. For example, studies using applied magnetic resonance imaging (MRI) techniques have shown that a decrease in the dorsolateral prefrontal cortex activity impairs visual memory. In contrast, a decrease in the activity of the dorsomedial prefrontal cortex and the amygdala is associated with depression and a lack of reaction to negative emotional stimuli. The simulated model could also explain the impairment of memory, the decrease in emotional responses, and the onset of depression (Figure 7).
It could be stated that the simulation results and the cognitive, hormonal, blood, and genetic test results follow the decrease in brain functionality, memory functions, and learning (In other words, the SNN model is evaluated by experiment results). Therefore, exposure to HV towers (due to living or working conditions) and electric fields may reduce brain performance, memory, and learning.
4. Discussion
The objective of the present research was to investigate the effect of HV towers on the brain. To achieve this objective, a rhesus macaque was exposed to a 3kV/m HV electric field for four hours a day for one month, and cognitive, blood, and anatomic variations (MRI) were recorded and analyzed. In this study, two male rhesus macaques were used. Additionally, a neural simulation model was created to investigate the performance of the hippocampus in the brain. Changes were made to the model depending on the conditions of certain elements in each state, and the results were obtained from the model. The SNN model results were evaluated based on the experimental results (Aliyari et al., 2019a; Carpenter & Sage, 2007; Chaddock et al., 2010; Kim et al., 2016).
The results obtained using the cognitive elements of the rhesus macaque revealed that the 3 kV/m HV electric field impaired the visual memory of the samples, and the duration of applying electric field simulations effectively contributed to this damage. This is because, in the recovery phase, the cognitive test results improve by distancing from using the electric field simulations phase. The exposure of the primate to 4 hours of treatment a day for a month caused this level of damage. Hence, if the hours of exposure to the HV field increase, the impacts will be more severe and difficult to reverse. Various studies have been carried out on this subject because different results are obtained depending on applying electric field simulations duration and the experiment’s frequency, severity, and time. Moreover, the hippocampus and amygdala play primary and secondary roles in memory, respectively (Aliyari et al., 2015; Aliyari et al., 2018; Aliyari et al.; Aliyaria et al., 2019; Malin & McGaugh, 2006). For example, when the hippocampus neurons are damaged, the patient develops Alzheimer disease (Carpenter, 2013; Vitvitsky et al., 2012).
In the titration examinations of the levels of the hippocampus and amygdala in the primate exposed to HV electric fields, a decreasing trend of variations was observed, which substantially contributes to memory impairment. Glutamate is an excitatory neurotransmitter and a vital source of other receptors. The effects of this neurotransmitter are exerted via membrane receptors known as the ionotropic and metatropic receptors. The NMDA receptors provide a slow synaptic response and contribute to the genesis of the brain, learning, and memory. In addition, the potassium, sodium, and calcium ions properly travel through these receptors. Examining the primates exposed to the HV field revealed a decrease in the expression of the NMDA receptor gene, which could be another cause of the reduction of visual memory and its performance. Furthermore, the decrease in the expression of the NMDA receptor gene reduces the flow of sodium and potassium ions. The sodium, potassium, and calcium ion elements substantially affect memory. Another result of this research was the decrease in sodium and potassium ion concentrations, which could be another cause of the decrease in visual memory and performance.
The NMDA neural receptors are among the important factors influencing the model’s performance in modeling cortex neural models. According to the research results, the variations of the NMDA element in the experimental group sample (i.e. the primate exposed to the HV field) reduced the concentration of this element (Di et al., 2019; Kazemi et al., 2018; Monyer et al., 1994; Salunke et al., 2014; Wang & Kriegstein, 2008). Similar results were obtained from the experimental model. In other words, a decrease was seen in the performance of the cortex neural network (impairment of visual memory). Hence, the results of the neural simulation and experimental models suggest that proximity to HV fields reduces memory performance. Thus, the performance of the SNN model is evaluated using experiment results.
The results of examining the primates can be generalized to humans. Moreover, it could be stated that by adopting some characteristics of the metrics of the resulting model, it is possible to obtain satisfactory results from examining the effects of HV fields on humans.
Finally, the experimental results agree with the results of the neural simulation modeling.
5. Conclusion
Our findings proved that prolonged exposure to HV electric fields can lead to significant neurobiological changes in macaques, including reduced NMDA receptor gene expression, altered blood sodium and potassium ion levels, and structural changes in the hippocampus and amygdala. It also can cause deficits in visual working memory. These results, supported by computational modeling, suggest that chronic exposure to HV fields may pose risks to brain function. Future studies should explore these effects in humans and investigate potential protective strategies.
Ethical Considerations
Compliance with ethical guidelines
This study was conducted in full compliance with international ethical standards for animal research. The study protocol was approved by the Institutional Review Board (IRB) of Baqiyatallah University of Medical Sciences, Tehran, Iran. All necessary measures were taken to ensure the well-being of the macaques, following institutional and international guidelines.
Funding
This research was financial supported by the Neuroscience Research Center at Baqiyatallah University of Medical Sciences, Tehran, Iran, and the Electrical Engineering Department at Amirkabir University of Technology, Tehran, Iran, and the Department of Electrical, Biomedical, and Mechatronics Engineering at QIAU (Cognitive Science Lab).
Authors' contributions
Conceptualization, study design, supervision, review and editing: Hamed Aliyari; Data collection, methodology, and statistical analysis: Mohsen Hosseinian; Neural modeling, computational analysis, and software implementation: Mohammad Bagher Menhaj: Biological assessments, experimental design, and ethical compliance oversight: Hedayat Sahraei; MRI analysis, neuroimaging interpretation, and result validation: Mohsen Shabani; Literature review, data interpretation, and manuscript drafting: Masoomeh Kazemi; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would also like to thank the staff who contributed to the study at each institution. Their assistance was invaluable in completing this research.
References
Aliyari, H., Golabi, S., Sahraei, H., Sahraei, M., Minaei-Bidgoli, B., & Daliri, M. R., et al. (2023). Perceived stress and cognition function quantification in a scary video game: An electroencephalogram features and biochemical measures. Basic and Clinical Neuroscience, 14(2), 297–309. [DOI:10.32598/bcn.2022.3811.1] [PMID]
Aliyari, H., Hosseinian, S., Sahraei, H., & Menhaj, M. (2019). Effect of proximity to high voltage fields: Results of the neural network model and experimental model with macaques. International Journal of Environmental Science and Technology, 16, 4315-4326. [Link]
Aliyari, H., Hosseinian, S., Sahraei, H., & Menhaj, M. (2019). Effect of proximity to high voltage fields: Results of the neural network model and experimental model with macaques. International Journal of Environmental Science and Technology, 16, 4315-4326. [DOI:10.1007/s13762-018-1830-8]
Aliyari, H., Hosseinian, S., Sahraei, H., & Menhaj, M. (2019). Effect of proximity to high voltage fields: Results of the neural network model and experimental model with macaques. International Journal of Environmental Science and Technology, 16(8), 4315-4326. [DOI:10.1007/s13762-018-1830-8]
Aliyari, H., Hosseinian, S. H., Menhaj, M. B., & Sahraei, H. (2019a). Analysis of the effects of high voltage transmission line on human stress and attention through electroencephalography (EEG). Iranian Journal of Science and Technology, Transactions of Electrical Engineering, 43, 211-218. [DOI:10.1007/s40998-018-0151-8]
Aliyari, H., Hosseinian, S. H., Menhaj, M. B., & Sahraei, H. (2019). Analysis of the effects of high voltage transmission line on human stress and attention through electroencephalography (EEG). Iranian Journal of Science and Technology, Transactions of Electrical Engineering, 43(1), 211-218. [DOI:10.1007/s40998-018-0151-8]
Aliyari, H., Kazemi, M., Tekieh, E., Salehi, M., Sahraei, H., & Daliri, M. R., et al. (2015). The effects of FIFA 2015 computer games on changes in cognitive, hormonal and brain waves functions of young men volunteers. Basic and Clinical Neuroscience, 6(3), 193-201. [PMID]
Aliyari, H., Sahraei, H., Daliri, M. R., Minaei Bidgoli, B., Kazemi, M., & Agaei, H., et al. (2018). The beneficial or harmful effects of computer game stress on cognitive functions of players. Basic and Clinical Neuroscience, 9(3), 177-186. [DOI:10.29252/nirp.bcn.9.3.177] [PMID]
Aliyari, H., Sahraei, H., Erfani, M., Mohammadi, M., Kazemi, M., & Daliri, M. R., et al. (2018). Alterations of cognitive functions following violent and football video games in young male volunteers: By studying brain waves. Basic and Clinical Neuroscience Journal, 11(3), 1-25. [Link]
Aliyaria, H., Sahraeib, H., Erfanid, M., Tekiehb, E., Salehib, M., & Kazemib, M., et al. (2019). The impacts of video games on cognitive function and cortisol levels in young female volunteers. Journal of Experimental and Clinical Neuroscience, 6(1), 1-5. [Link]
Beale, I., Pearce, N., Conroy, D., Henning, M., & Murrell, K. (1997). Psychological effects of chronic exposure to 50 Hz magnetic fields in humans living near extra high voltage transmission lines. Bioelectromagnetics, 18(8), 584-594. [DOI:10.1002/(SICI)1521-186X(1997)18:8<584::AID-BEM7>3.0.CO;2-Z]
Brady, T. F., Konkle, T., & Alvarez, G. A. (2011). A review of visual memory capacity: Beyond individual items and toward structured representations. Journal of Vision, 11(5), 4. [DOI:10.1167/11.5.4] [PMID]
Braicu, Ș., Czumbil, L., Șteț, D., & Micu, D. (2017). Evaluation of the electric and magnetic field near high voltage power lines. In: S. Vlad & N. Roman (Eds), International Conference on Advancements of Medicine and Health Care through Technology, Cluj-Napoca, Romania, 12th - 15th October 2016. [DOI:10.1007/978-3-319-52875-5_32]
Brunel, N. (2000). Dynamics of sparsely connected networks of excitatory and inhibitory spiking neurons. Journal of Computational Neuroscience, 8(3), 183-208. [DOI:10.1023/A:1008925309027] [PMID]
Brunel, N., & Wang, X. J. (2001). Effects of neuromodulation in a cortical network model of object working memory dominated by recurrent inhibition. Journal of Computational Neuroscience, 11(1), 63-85. [DOI:10.1023/A:1011204814320] [PMID]
Brunel, N., & Wang, X. J. (2003). What determines the frequency of fast network oscillations with irregular neural discharges? I. Synaptic dynamics and excitation inhibition balance. Journal of Neurophysiology, 90(1), 415-430. [DOI:10.1152/jn.01095.2002] [PMID]
Carpenter, D. & Sage, C. (2007) BioInitiative report: A rationale for a biologically-based public exposure standard for electromagnetic fields (ELF and RF). [Link]
Carpenter, D. O. (2013). Human disease resulting from exposure to electromagnetic fields. Reviews on Environmental Health, 28(4), 159-172. [DOI:10.1515/reveh-2013-0016] [PMID]
Chaddock, L., Erickson, K. I., Prakash, R. S., Kim, J. S., Voss, M. W., & VanPatter, M., et al. (2010). A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Research, 1358, 172-183. [DOI:10.1016/j.brainres.2010.08.049] [PMID]
Chudler, E. H., & Bergsman, K. C. (2016). Brains-computers-machines: Neural engineering in science classrooms. CBE Life Sciences Education, 15(1), fe1. [DOI:10.1187/cbe.15-11-0242] [PMID]
Churchland, P. S., & Sejnowski, T. J. (2016). The computational brain. Massachusetts: MIT Press. [DOI:10.7551/mitpress/11207.001.0001]
Compte, A., Brunel, N., Goldman Rakic, P. S., & Wang, X. J. (2000). Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cerebral Cortex, 10(9), 910-923. [DOI:10.1093/cercor/10.9.910] [PMID]
Constantinidis, C., & Procyk, E. (2004). The primate working memory networks. Cognitive, Affective, & Behavioral Neuroscience, 4(4), 444-465. [DOI:10.3758/CABN.4.4.444] [PMID]
Cook, C. M., Thomas, A. W., & Prato, F. S. (2002). Human electrophysiological and cognitive effects of exposure to ELF magnetic and ELF modulated RF and microwave fields: A review of recent studies. Bioelectromagnetics, 23(2), 144–157. [DOI:10.1002/bem.107] [PMID]
Crasson, M. (2003). 50 60 Hz electric and magnetic field effects on cognitive function in humans: A review. Radiation Protection Dosimetry, 106(4), 333–340. [DOI:10.1093/oxfordjournals.rpd.a006369] [PMID]
Demuth, H. B., Beale, M. H., De Jess, O., & Hagan, M. T. (2014). Neural network design. Cramlington: Martin Hagan. [Link]
Di, G., Kim, H., Xu, Y., Kim, J., & Gu, X. (2019). A comparative study on influences of static electric field and power frequency electric field on cognition in mice. Environmental Toxicology and Pharmacology, 66, 91-95. [DOI:10.1016/j.etap.2019.01.001] [PMID]
Draper, G., Vincent, T., Kroll, M. E., & Swanson, J. (2005). Childhood cancer in relation to distance from high voltage power lines in England and Wales: A case-control study. BMJ, 330(7503), 1290. [DOI:10.1136/bmj.330.7503.1290] [PMID]
Ehrenstein, G., & Lecar, H. (1972). The mechanism of signal transmission in nerve axons. Annual Review of Biophysics and Bioengineering, 1, 347-368. [DOI:10.1146/annurev.bb.01.060172.002023] [PMID]
Engel, A. K., Moll, C. K., Fried, I., & Ojemann, G. A. (2005). Invasive recordings from the human brain: Clinical insights and beyond. Nature Reviews Neuroscience, 6(1), 35-47. [DOI:10.1038/nrn1585] [PMID]
Fourcaud, N., & Brunel, N. (2002). Dynamics of the firing probability of noisy integrate and fire neurons. Neural Computation, 14(9), 2057-2110. [DOI:10.1162/089976602320264015] [PMID]
Guo, D., Wang, Q., & Perc, M. (2012). Complex synchronous behavior in interneuronal networks with delayed inhibitory and fast electrical synapses. Physical Review. E, Statistical, Nonlinear, and Soft Matter Physics, 85(6 Pt 1), 061905.[DOI:10.1103/PhysRevE.85.061905] [PMID]
Izhikevich, E. M. (2003). Simple model of spiking neurons. IEEE Transactions on Neural Networks, 14(6), 1569-1572. [DOI:10.1109/TNN.2003.820440] [PMID]
Izhikevich, E. M. (2007). Dynamical systems in neuroscience: The geometry of excitability and bursting. Massachusetts: MIT Press. [DOI:10.7551/mitpress/2526.001.0001]
Kazemi, M., Aliyari, H., Golabi, S., Tekieh, E., Tavakoli, H., & Saberi, M., et al. (2022). Improvement of cognitive indicators in male monkeys exposed to extremely low frequency electromagnetic fields. Archives of Razi Institute, 77(1), 503-511. [PMID]
Kazemi, M., Sahraei, H., Aliyari, H., Tekieh, E., Saberi, M., & Tavacoli, H., et al. (2018). Effects of the extremely low frequency electromagnetic fields on NMDA receptor gene expression and visual working memory in male rhesus macaques. Basic and Clinical Neuroscience, 9(3), 167-176. [DOI:10.29252/nirp.bcn.9.3.167] [PMID]
Kim, K. E., Park, S. K., Nam, S. Y., Han, T. J., & Cho, I. Y. (2016). Potential therapeutic mechanism of extremely low frequency high voltage electric fields in cells. Technology and Health Care, 24(3), 415-427. [DOI:10.3233/THC-151119] [PMID]
Kleine, J. F., Guan, Y., & Büttner, U. (2003). Saccade related neurons in the primate fastigial nucleus: What do they encode? Journal of Neurophysiology, 90(5), 3137-3154. [DOI:10.1152/jn.00021.2003] [PMID]
Laakso, M. P., Soininen, H., Partanen, K., Helkala, E. L., Hartikainen, P., & Vainio, P., et al. (1995). Volumes of hippocampus, amygdala and frontal lobes in the MRI based diagnosis of early Alzheimer’s disease: Correlation with memory functions. Journal of Neural Transmission: Parkinson’s Disease and Dementia Section, 9(1), 73-86. [DOI:10.1007/BF02252964] [PMID]
Loh, M., Rolls, E. T., & Deco, G. (2007). A dynamical systems hypothesis of schizophrenia. PLoS Computational Biology, 3(11), e228. [DOI:10.1371/journal.pcbi.0030228] [PMID]
Lowenthal, R. M., Tuck, D., & Bray, I. (2007). Residential exposure to electric power transmission lines and risk of lymphoproliferative and myeloproliferative disorders: A case-control study. Internal Medicine Journal, 37(9), 614–619.[DOI:10.1111/j.1445-5994.2007.01389.x] [PMID]
Maass, W. (1997). Networks of spiking neurons: The third generation of neural network models. Neural Networks, 10(9), 1659-1671. [DOI:10.1016/S0893-6080(97)00011-7]
Malin, E. L., & McGaugh, J. L. (2006). Differential involvement of the hippocampus, anterior cingulate cortex, and basolateral amygdala in memory for context and footshock. Proceedings of the National Academy of Sciences, 103(6), 1959-1963. [DOI:10.1073/pnas.0510890103] [PMID]
Markram, H. (2012). The human brain project. Scientific American, 306(6), 50-55. [DOI:10.1038/scientificamerican0612-50] [PMID]
Menon, V. (2011). Large scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15(10), 483-506. [DOI:10.1016/j.tics.2011.08.003] [PMID]
Monyer, H., Burnashev, N., Laurie, D. J., Sakmann, B., & Seeburg, P. H. (1994). Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron, 12(3), 529-540. [DOI:10.1016/0896-6273(94)90210-0] [PMID]
Pillow, J. W., Paninski, L., Uzzell, V. J., Simoncelli, E. P., & Chichilnisky, E. (2005). Prediction and decoding of retinal ganglion cell responses with a probabilistic spiking model. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 25(47), 11003–11013. [DOI:10.1523/JNEUROSCI.3305-05.2005] [PMID]
Pretorius, P. (2006). Electric and magnetic fields from overhead power lines: A summary of technical and biological aspects. Sandton: Eskom Holdings Ltd. [Link]
Rose, S. A., Feldman, J. F., & Jankowski, J. J. (2009). A cognitive approach to the development of early language. Child Development, 80(1), 134–150. [DOI:10.1111/j.1467-8624.2008.01250.x] [PMID]
Salunke, B. P., Umathe, S. N., & Chavan, J. G. (2014). Involvement of NMDA receptor in low frequency magnetic field induced anxiety in mice. Electromagnetic Biology and Medicine, 33(4), 312-326. [DOI:10.3109/15368378.2013.839453] [PMID]
Seung, H. S. (2003). Learning in spiking neural networks by reinforcement of stochastic synaptic transmission. Neuron, 40(6), 1063–1073. [DOI:10.1016/S0896-6273(03)00761-X] [PMID]
Soininen, H. S., Partanen, K., Pitkänen, A., Vainio, P., Hänninen, T., & Hallikainen, M., et al. (1994). Volumetric MRI analysis of the amygdala and the hippocampus in subjects with age associated memory impairment: Correlation to visual and verbal memory. Neurology, 44(9), 1660-1668. [DOI:10.1212/WNL.44.9.1660] [PMID]
Squire, L. R. (1992). Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychological Review, 99(2), 195-231. [DOI:10.1037/0033-295X.99.2.195] [PMID]
Starzyk, J. A., Graham, J. T., Raif, P., & Tan, A. H. (2012). Motivated learning for the development of autonomous systems. Cognitive Systems Research, 14(1), 10-25. [DOI:10.1016/j.cogsys.2010.12.009]
Stevens, J. C., Shipley, L. A., Cashman, J. R., Vandenbranden, M., & Wrighton, S. A. (1993). Comparison of human and rhesus monkey in vitro phase I and phase II hepatic drug metabolism activities. Drug Metabolism and Disposition, 21(5), 753-760. [DOI:10.1016/S0090-9556(25)08157-7] [PMID]
Takac, M., & Knott, A. (2015). A neural network model of episode representations in working memory. Cognitive Computation, 7(5), 509-525. [DOI:10.1007/s12559-015-9330-3]
Taylor, J. G. (2009). Cognitive computation. Cognitive Computation, 1(1), 4-16. [Link]
Tekieh, E., Riahi, E., Kazemi, M., Sahraei, H., Tavakoli, H., & Aliyary, H., et al. (2017). Role of basal stress hormones and amygdala dimensions in stress coping strategies of male rhesus monkeys in response to a hazard reward conflict. Iranian Journal of Basic Medical Sciences, 20(8), 951-957. [PMID]
Tsodyks, M., Pawelzik, K., & Markram, H. (1998). Neural networks with dynamic synapses. Neural computation, 10(4), 821–835. [DOI:10.1162/089976698300017502] [PMID]
Ushie, P., Pekene, D., Obi, E., & Ukhurebor, K. (2017). Investigation of field induced effect of high voltage transmission line in Calabar South, Nigeria. Physical Science International Journal, 15(1), 1-9. [DOI:10.9734/PSIJ/2017/32022]
Van der Velde, F. (2010). Where artificial intelligence and neuroscience meet: The search for grounded architectures of cognition. Advances in Artificial Intelligence, 2010, 5. [DOI:10.1155/2010/918062]
Van Ooijen, P., ten Bhömer, P., Blecourt, M., Roosjen, R., van Dam, R., & Oudkerk, M. (2005). DICOM storage into PACS of out hospital CD ROMs-a half year experience report. International Congress Series, 1281, 883-887. [DOI:10.1016/j.ics.2005.03.114]
Videbech, P., & Ravnkilde, B. (2004). Hippocampal volume and depression: A meta analysis of MRI studies. The American Journal of Psychiatry, 161(11), 1957–1966. [DOI:10.1176/appi.ajp.161.11.1957] [PMID]
Vitvitsky, V. M., Garg, S. K., Keep, R. F., Albin, R. L., & Banerjee, R. (2012). Na+ and K+ ion imbalances in Alzheimer’s disease. Biochimica et Biophysica acta, 1822(11), 1671–1681. [DOI:10.1016/j.bbadis.2012.07.004] [PMID]
Wang, M. D., & Kriegstein, A. R. (2008). GABA regulates excitatory synapse formation in the neocortex via NMDA receptor activation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 28(21), 5547–5558.[DOI:10.1523/JNEUROSCI.5599-07.2008] [PMID]
Xu, C., & Li, P. (2016). Dynamics in four neuron bidirectional associative memory networks with inertia and multiple delays. Cognitive Computation, 8(1), 78-104. [DOI:10.1007/s12559-015-9344-x]
Zhang, S., Huang, K., Zhang, R., & Hussain, A. (2018). Learning from few samples with memory network. Cognitive Computation, 10(1), 15-22. [DOI:10.1007/s12559-017-9507-z]
Type of Study: Original |
Subject:
Cognitive Neuroscience
Received: 2023/03/8 | Accepted: 2023/07/25 | Published: 2025/03/18
Received: 2023/03/8 | Accepted: 2023/07/25 | Published: 2025/03/18
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





