Volume 16 - Special Issue on Cognitive Sciences
BCN 2025, 16 - Special Issue on Cognitive Sciences: 353-366 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Kord M, Fath-Abadi J, Gharibzadeh S, Khosrowabadi R. Enhancing EEG Components in Adolescents With ADHD Using Transcranial Electrical Stimulation: A Randomized-active Controlled Study. BCN 2025; 16 (S1) :353-366
URL: http://bcn.iums.ac.ir/article-1-2620-en.html
URL: http://bcn.iums.ac.ir/article-1-2620-en.html
1- Institute for Cognitive and Brain Sciences, Shahid Beheshti University, Tehran, Iran.
2- Department of Psychology, School of Educational Sciences and Psychology, Shahid Beheshti University, Tehran, Iran.
3- Department of Educational Measurement and Evaluation, Faculty of Educational Sciences and Psychology, University of Tehran, Tehran, Iran.
2- Department of Psychology, School of Educational Sciences and Psychology, Shahid Beheshti University, Tehran, Iran.
3- Department of Educational Measurement and Evaluation, Faculty of Educational Sciences and Psychology, University of Tehran, Tehran, Iran.
Keywords: Electroencephalography (EEG), Transcranial electrical Stimulation, Attention-deficit/hyperactivity disorder (ADHD)
Full-Text [PDF 1417 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Attention deficit hyperactivity disorder (ADHD) is a childhood-onset psychiatric disorder characterized by disproportionate levels of developmental inattention, impulsivity, and hyperactivity (APA, 2013). The global prevalence rate of the disorder is 5.3% in children (Mannuzza et al., 2003) and 3.4% in adults (Fayyad et al., 2007).
Despite various research on this disorder, uncertainty persists regarding the causes of the disorder, as it is heterogeneous and changes drastically at an individual level. Sustained attention, inhibitory control, and executive functions are often affected. Specifically, Walcott et al. (2005) showed that response inhibition and working memory are impaired in most people with ADHD. Previous research has also shown that executive function defi-cits, particularly working memory, are highly correlated with academic dysfunction (Irwin et al., 2022). Although drug treatments have proved effective against the disorder’s main symptoms, they have a limited effect on cognitive deficits, especially executive dysfunction, calling for more research on treat-ments that target cognitive deficits (Irwin et al., 2022).
Working memory refers to the active, top-down process of manipulating information stored in short-term memory. It includes functions implicated in the temporal lobe prefrontal cortex that directs behavior by updating, processing, and manipulating the time/sequence of information in short-term memory (Lara & Wallis, 2015). Working memory acts as an interface between the environment and long-term memory. It is the basis of a set of learning skills, including note-taking, listening comprehension, and following instructions. Working memory also supports functions such as impulse control (Maraver et al., 2016), cooperation with others, dynamic decoding of social information (Phillips et al., 2007), and tolerance of delayed gratification (McInnes et al., 2003; Aliyari et al., 2018), all of which are impaired in ADHD.
In recent years, non-invasive brain stimulation has been introduced as a new treatment method for disorders with a neurocognitive basis. According to the specific evidence provided by functional magnetic resonance imaging studies, regions that are closer to the skull surface can be the target of non-invasive brain stimulation interventions. Although many studies show the effectiveness of transcranial direct current stimulation (tDCS) in improving working memory, this effect is still uncertain. These conflicting results seem to be due to differences in study design, stimulation protocol, and inter-individual differences (Jantz, et al., 2016).
Several studies have provided support for the effectiveness of electrical stimulation with random noise flow (tRNS) in boosting cognitive functions, including perceptual learning (Fertonani et al., 2011), number discrimination (Chick, 2014), and mathematics learning (Snowball et al., 2013). Brauer et al. (2018) did not report better performance on a go/no-go task after stimulating the right inferior frontal cortex region using tRNS. In another study, Bruit-Abi et al. (2018) showed that three sessions of tRNS over the left dorsolateral prefrontal cortex reduced participants’ reaction time on the go/no-go task but did not affect their accuracy.
So far, only one study has compared the efficacy of the two electrical stimulation techniques, i.e. tDCS and tRNS, in improving working memory in healthy individuals. Based on the results from this study, three sessions of 10-min tDCS over the left dorsolateral prefrontal cortex area led to improved performance on the 2-back test. However, treatment with tRNS did not yield the same performance outcome, seemingly due to the larger electrode size used in the tRNS treatment (Mulquiney et al., 2011).
In general, several studies have shown that defects in executive functions of inhibitory control and working memory adversely affect self-management behavior, causing behavioral symptoms in people with ADHD. Given the importance of executive dysfunction in ADHD, several studies have attempted to identify the neurological and biological correlates of inhibitory control and working memory deficits in people with ADHD (Zhao et al., 2010; Martel et al., 2011; Sonuga-Barke et al., 2010).
Considering the significant role of working memory in ADHD and the need to provide new, low-cost, and comprehensive treatments, we aimed to investigate the efficacy of tDCS and tRNS in ameliorating working memory and inhibitory control in individuals with ADHD.
2. Materials and Methods
Study participants
The statistical sample included 45 adolescents with ADHD, aged 13 to 17. They were randomly selected from 110 ADHD cases referred to Baharan Psychiatric Hospital of Zahedan University of Medical Sciences, Zahedan City, Iran, in the second half of 2018 and 2019. The inclusion criteria were as follows: Meeting the DSM-5 criteria for ADHD along with a diagnosis of ADHD by a psychologist and a psychiatrist, aged 13 to 17, willingness to provide informed consent (of both participants and their parents), right-handedness, being a male gender. The exclusion criteria were as follows: History of seizures and epilepsy, any blow to the head, history of psychiatric disorders, unwillingness to participate at any time during the experiment, and unbearable discomfort or difficulty when receiving transcranial electrical stimulation (tES).
Participants were randomly assigned to three groups, each including 15 members: The tDCS group, the tRNS group, and the control group. To ensure that participants in the control group experience the same effect as participants in the tDCS and tRNS groups, we applied sham transcranial stimulation to participants in the control group. Each person in the experimental group received 5 sessions of electrical stimulation with an interval of 24 hours between sessions. Electroencephalography (EEG) recording and cognitive assessment were done before the intervention, immediately after the intervention, and one week after the intervention.
Study measures
Measuring the side effects of transcranial stimulation with direct electric current
This questionnaire includes 7 items, each referring to a particular effect reported or possibly experienced by those receiving tDCS. They include headache, dizziness, heartburn, itchy head, feeling confused, drowsiness, and nausea. An item titled “other” was also added to ensure participants could still report how they felt even if none of the items on the questionnaire matched their experience with tDCS (Najati et al., 2020).
Cognitive rehabilitation task
In this study and in line with previous research (Westwood et al., 2022), the n-back cognitive rehabilitation task was used along with transcranial stimulation to increase working memory capacity. In this task, a sequence of stimuli is displayed on the screen one after another, and the participants are required to compare the current stimulus with the one that appeared in the n-trials back in the sequence and press the response key if they match.
Dual n-back is a task variation in which two types of stimuli (visual-spatial and auditory) are presented simultaneously (Heinzel et al., 2017). Research in neurology has shown that cognitive training using dual n-back often increases brain activity in the left and right prefrontal areas, especially the left posterior-lateral area, which is implicated in executive functions of working memory, including updating, shifting, and inhibition. This tool has been used in many studies, and its effectiveness has been shown (Haq Nazari et al., 2022).
Digit span test
In the direct digit span memory test, lists of 3 to 9 digits are orally presented, each at a time, and participants are asked to repeat the digits in the same order they hear them. In the reverse digit span memory test, however, lists of 2 to 8 digits are presented, and participants must repeat the digits on each list in reverse order. Aminzadeh and Hasanabadi (2012) have reported a reliability score of 0.8 and 0.68 for the direct and reverse versions of the task, respectively. Gathercole et al., 2004) reported a test re-test reliability score of 0.81 for the direct digit memory test, and Thompson and Gathercole (2006) reported a test re-test reliability score of 0.71 for the reverse digit memory test. Also, he calculated the reliability model of the memory of direct and reverse digits through retesting, respectively, 0.84 and 0.60. (Aliyari et al., 2019).
EEG data recording and analysis
EEG data were collected using a 21-channel contact instrument psych lab EEG amplifier (Medinateb, 2025). The electrode impedances were kept below 5 k Ω. The EEG signals were recorded with a sampling frequency of 500 Hz. The electrode placed at the right earlobe served as the reference. Moreover, the electrode on the left mastoid region was applied as the ground. Subsequently, using the EEGLAB toolbox, we performed standard preprocessing, including band-pass filtering (1-40 Hz), running ICA, and reducing sampling frequency to 256 Hz to remove noise and artifacts from EEG data.
Based on their absolute power, the preprocessed data were divided into 9 components: Delta (1-4 Hz), theta (4-8 Hz), alpha (8-13 Hz), alpha1(8-10 Hz), alpha2 (11-13 Hz), beta (13-21 Hz), beta1 (13-21 Hz), beta2 (19-30 Hz).), and gamma (30-40). The data were analyzed using a two-way ANOVA with frequency channel as within-group and stimulation type as between-group factors. The t-test was applied to explore statistically significant differences between group means (Schomer & da Silva, 2005; Shabani et al., 2024).
tES device (NeuroStim2)
This device was launched by the Research & Development team of Medina Teb company in 2015. This device has two completely separate channels and can apply various electrical stimulation patterns with the highest quality. NeuroStim2 has two channels that are electrically isolated from each other, and each channel can be set independently to apply separate stimulations.
Transcranial brain stimulation is provided in the form of two electrodes placed on the target areas on the surface of the head with a weak current of 1 to 2 mA. After about 5 minutes, this weak current passes through the surface of the skull. It affects the activity of nerve cells in the area where the electrodes are placed and the subcortical regions connected to them (Weber et al., 2014).
Study procedure
Participants were first homogenized based on results from the Connors questionnaire (parent and teacher forms), their performance on the working memory scale of the Wechsler test (digit span, number-letter sequence), and their age. They were then randomly assigned to one of the three groups: The tDCS, the tRNS, and the sham control group. Before the intervention, a resting state EEG recording was done, and the digit span test was administered. Participants received 5 sessions of electrical stimulation with an interval of 24 hours between sessions. To explore the transfer effect of the intervention, behavioral and psychological tests (digit span task) were administered one week after the end of the intervention.
3. Results
Behavioral data: Digit span test
Descriptive statistics of the digit span test scores are provided in Table 1.
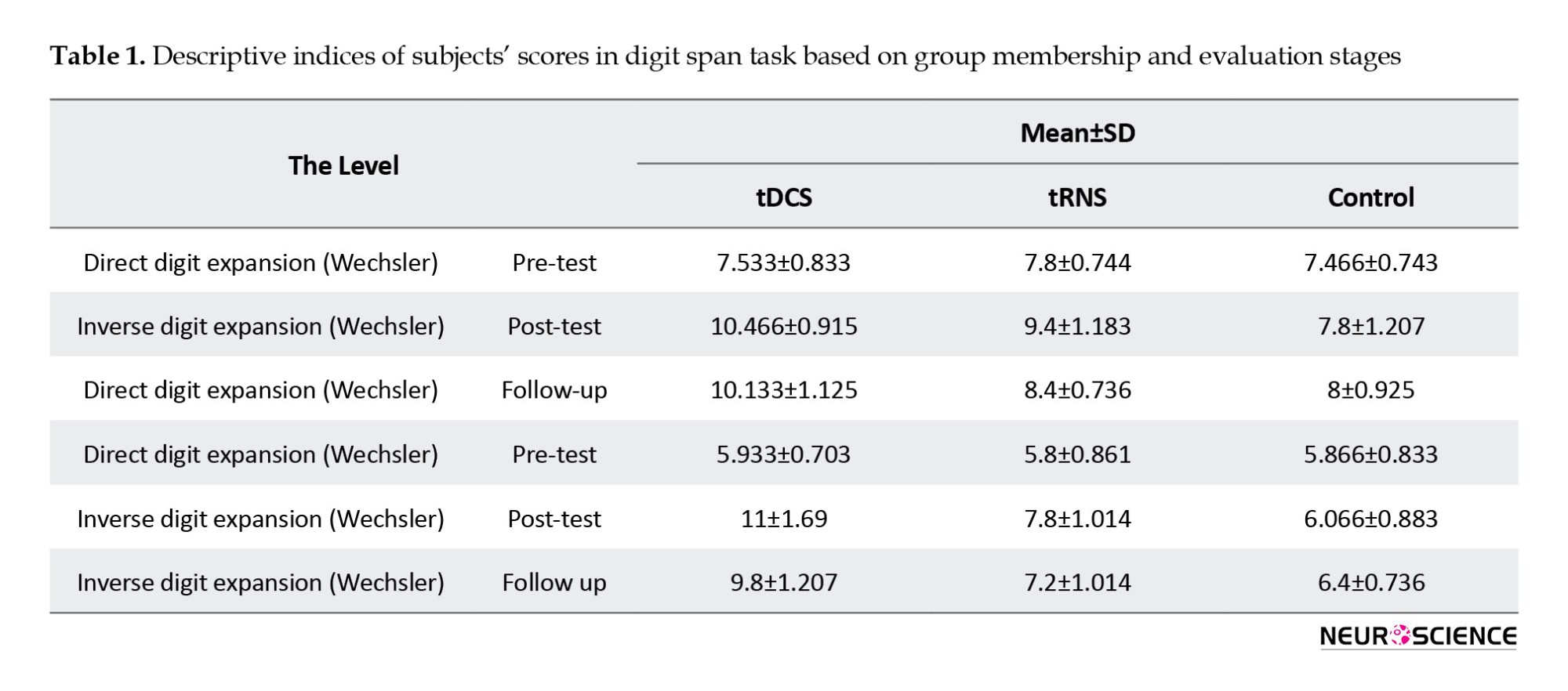
A mixed-design analysis of variance was applied to mean scores on the digit span test to investigate the effectiveness of tDCS and tRNS on working memory functioning in adolescents with ADHD. The results are shown in Table 2.
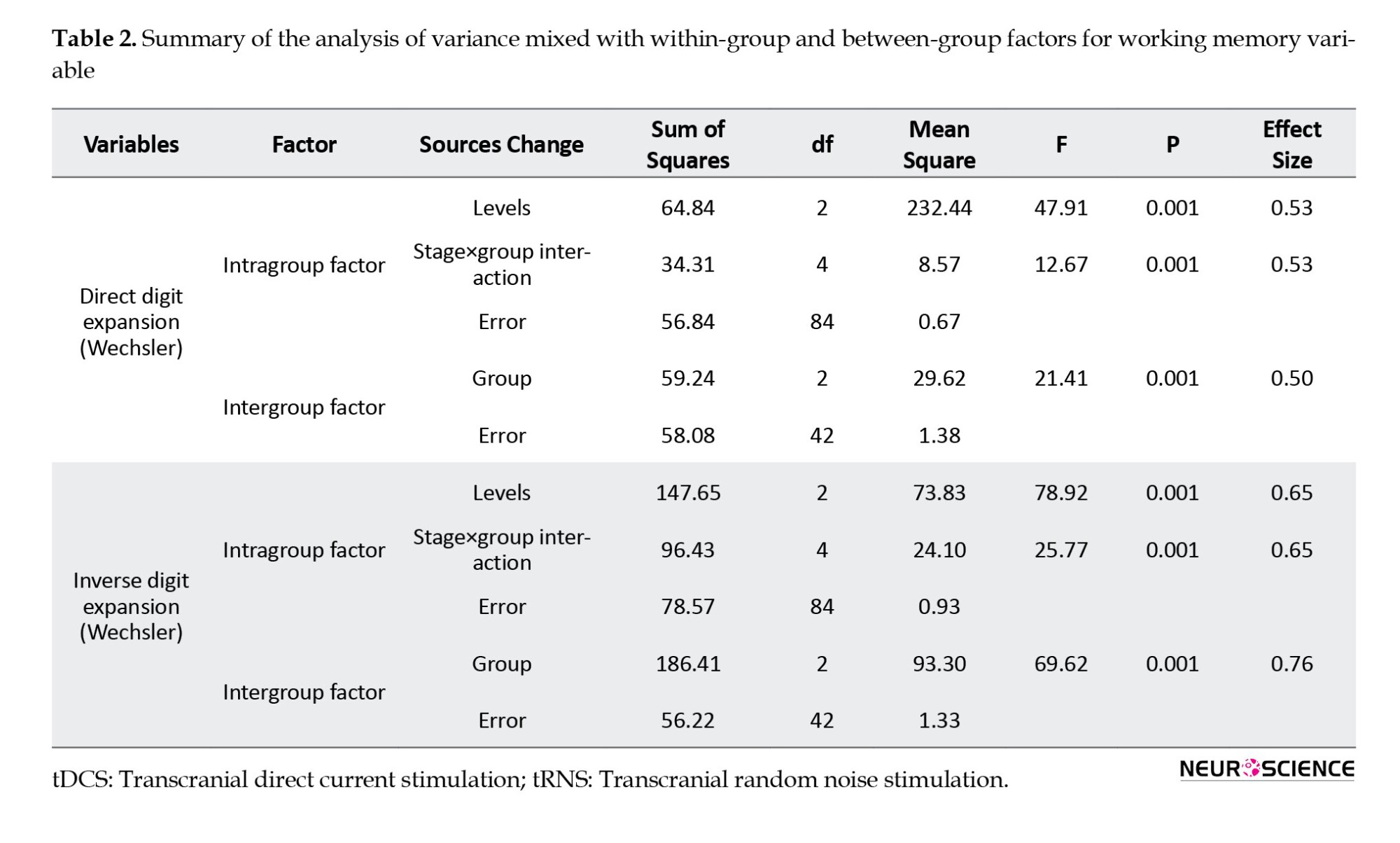
As shown in Table 2, the F value observed for the effect of the intervention stage (pre-test, post-test, and follow-up) was significant at the 0.01 level for all components of working memory (direct and reverse digit span). As a result, there is an essential difference between scores on all components of working memory at pre-test, post-test, and 1-week follow-up. Regarding the between-group factor of stimulation type (tDCS, tRNS, and sham stimulation), the analysis revealed statistical significance between mean scores on all components of working memory (P<0.01). The interaction effect between the intervention stage and stimulation type was also significant for all working memory components (P<0.001).
The Bonferroni post hoc test results to compare the pairwise differences between intervention stages and the Tukey post hoc test to compare the pairwise differences between stimulation types are presented in Tables 3 and 4.
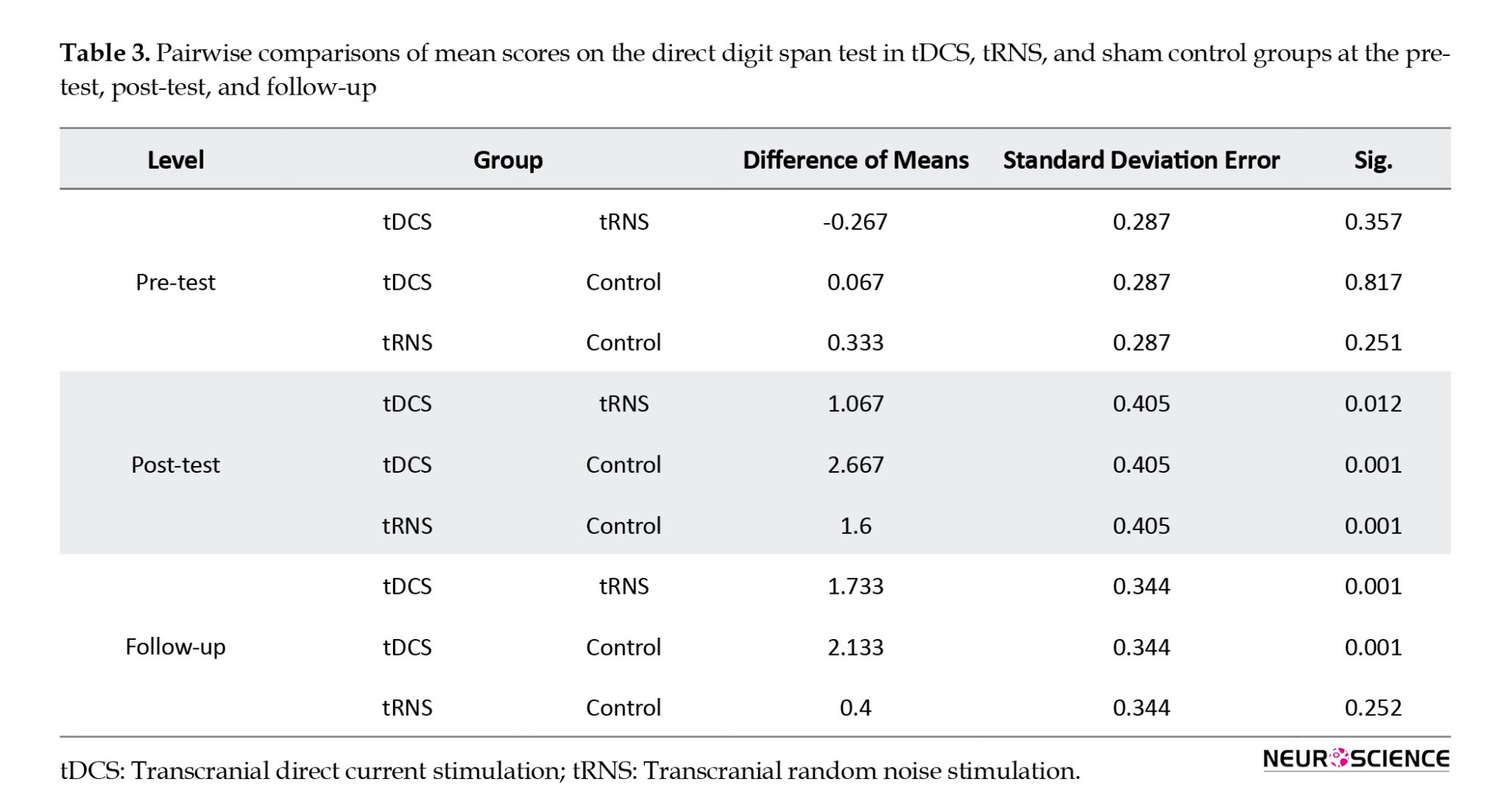
Table 3 presents no significant differences between tDCS, tRNS, and sham control at pre-test (P>0.05). In the post-test, however, participants in the tDCS and tRNS groups achieved significantly higher scores than those in the sham control group (P<0.001). The difference between scores in the tDCS and the tRNS groups was also statistically significant at post-test (P<0.001), with the tDCS group getting better scores. The tDCS group had significantly higher scores at follow-up than the tRNS and sham control groups. However, no significant difference was found between the tRNS and control groups (P>0.05).
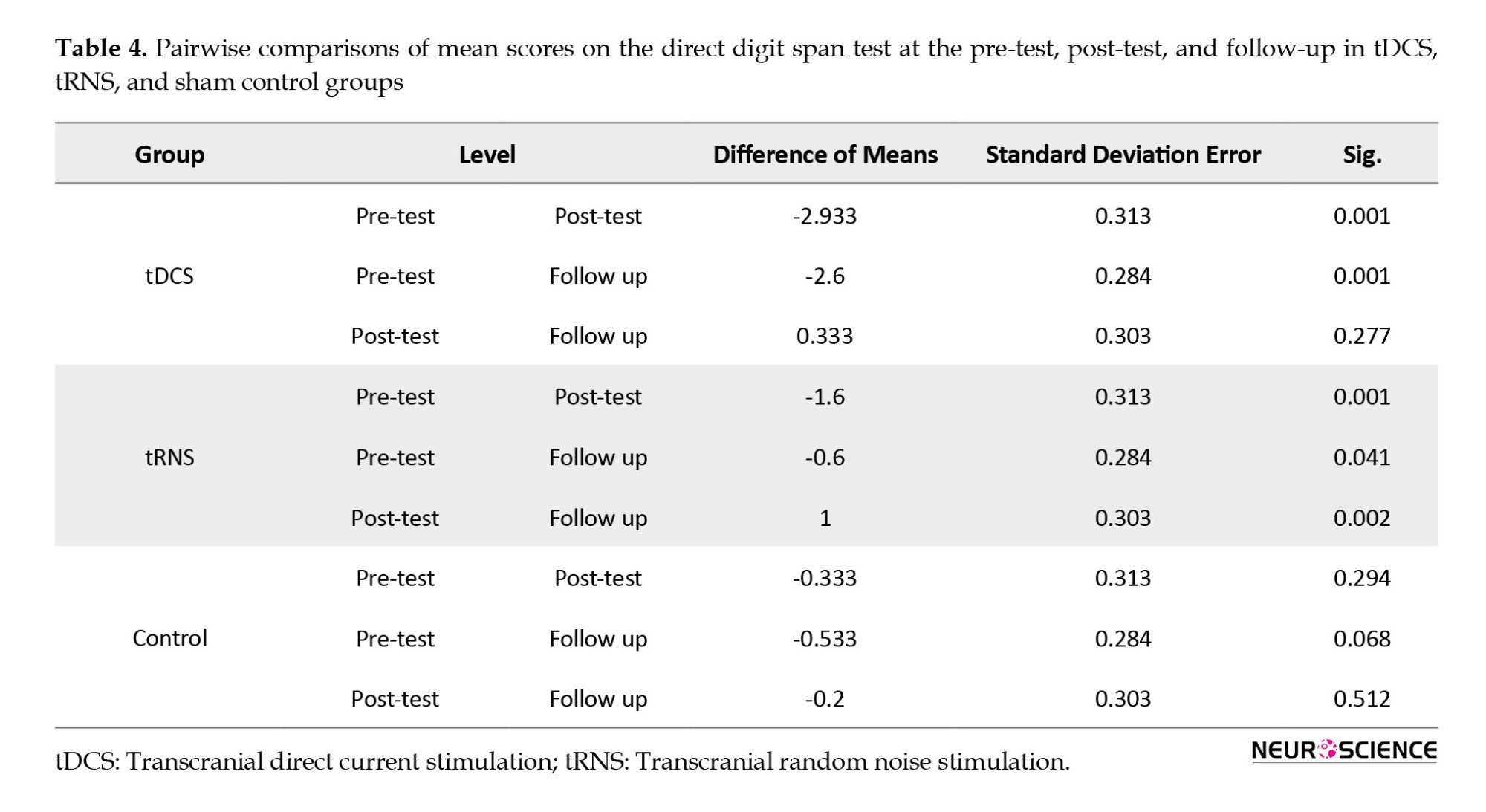
Table 5 indicates no significant differences between reverse digit span scores in tDCS, tRNS, and sham control groups at pre-test scores (P>0.05). However, a significant difference was observed between the groups during the post-test and follow-up. The tDCS and tRNS groups got significantly higher scores than those in the control group (P<0.001). In addition, the results showed that the tDCS group performed significantly better than the tRNS group on the reverse digit span test (P<0.001).
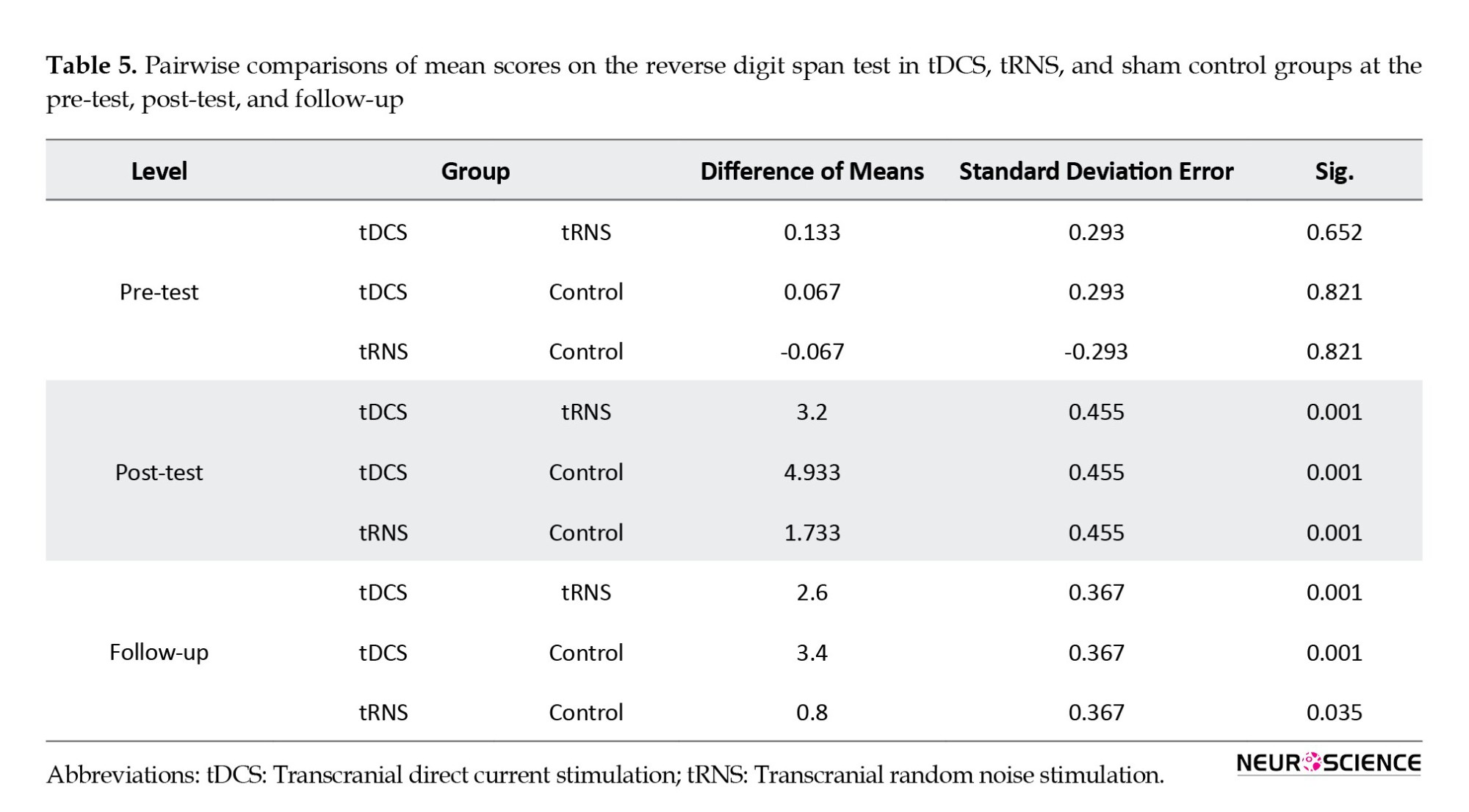
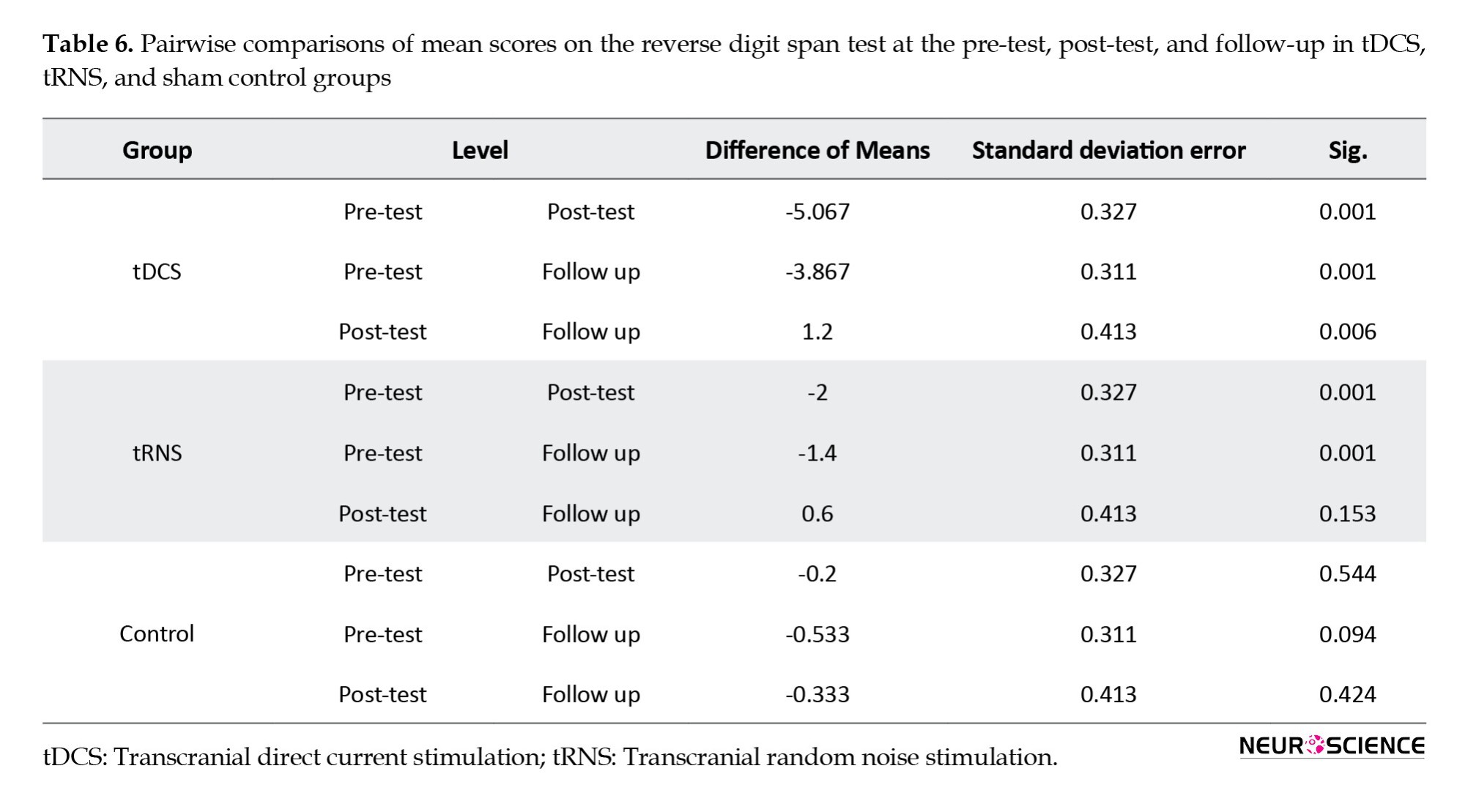
Results from pairwise analysis on mean scores on the reverse digit span test (Table 6) showed a significant difference between the pre-test and post-test (P<0.001) and pre-test and follow-up stages in the tDCS group (P<0.001). However, no significant difference between the post-test and follow-up (P>0.05) was found. In the tRNS group, the results showed significant differences at pre-test, post-test, and follow-up (P<0.05). No significant differences were found between intervention stages in the control group (P>0.05).
Data analysis of brain signals (EEG)
This study analyzed brain wave patterns over time. Different scores are presented at various time points per second for ten brain wave rhythms. These figures facilitate understanding the changes in the participants’ brain waves during treatment. The pattern of brain waves was examined in the tRNS, the tDCS, and the sham control group. The results are presented in Table 7.
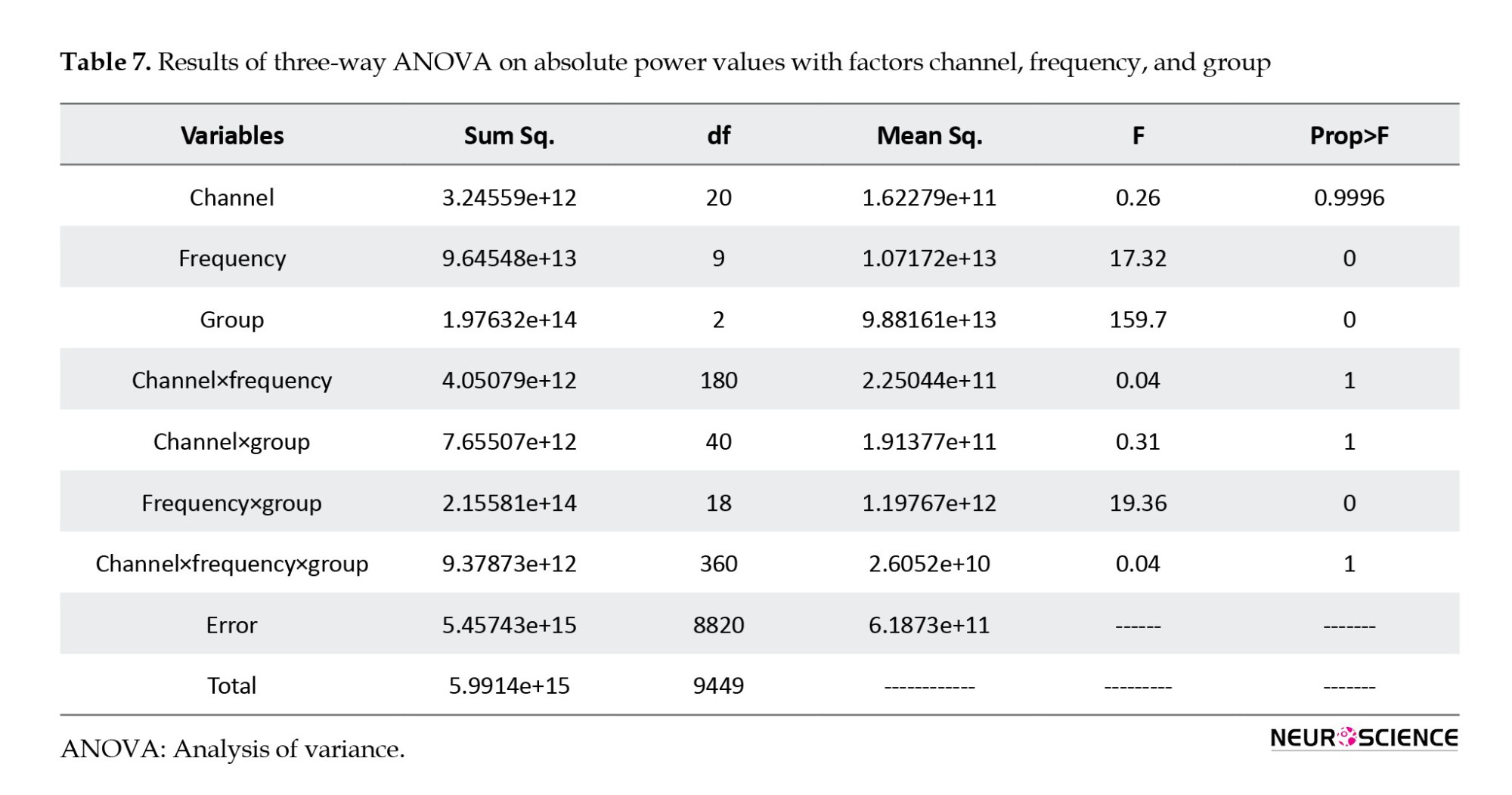
Table 7 outlines the results of a three-way analysis of variance (ANOVA) for absolute power values using the factors channel, frequency, and group. A significant difference was found in the group and frequency factors, and based on this, subsequent t tests were taken. The intragroup post hoc t-test for comparing the pre-test and post-test shows the absolute power in each group.
In the following, the within-group post hoc t-test was done to compare absolute power values at the pre-test and post-test in each group. Significant channels in each group are presented in Table 8.
.jpg)
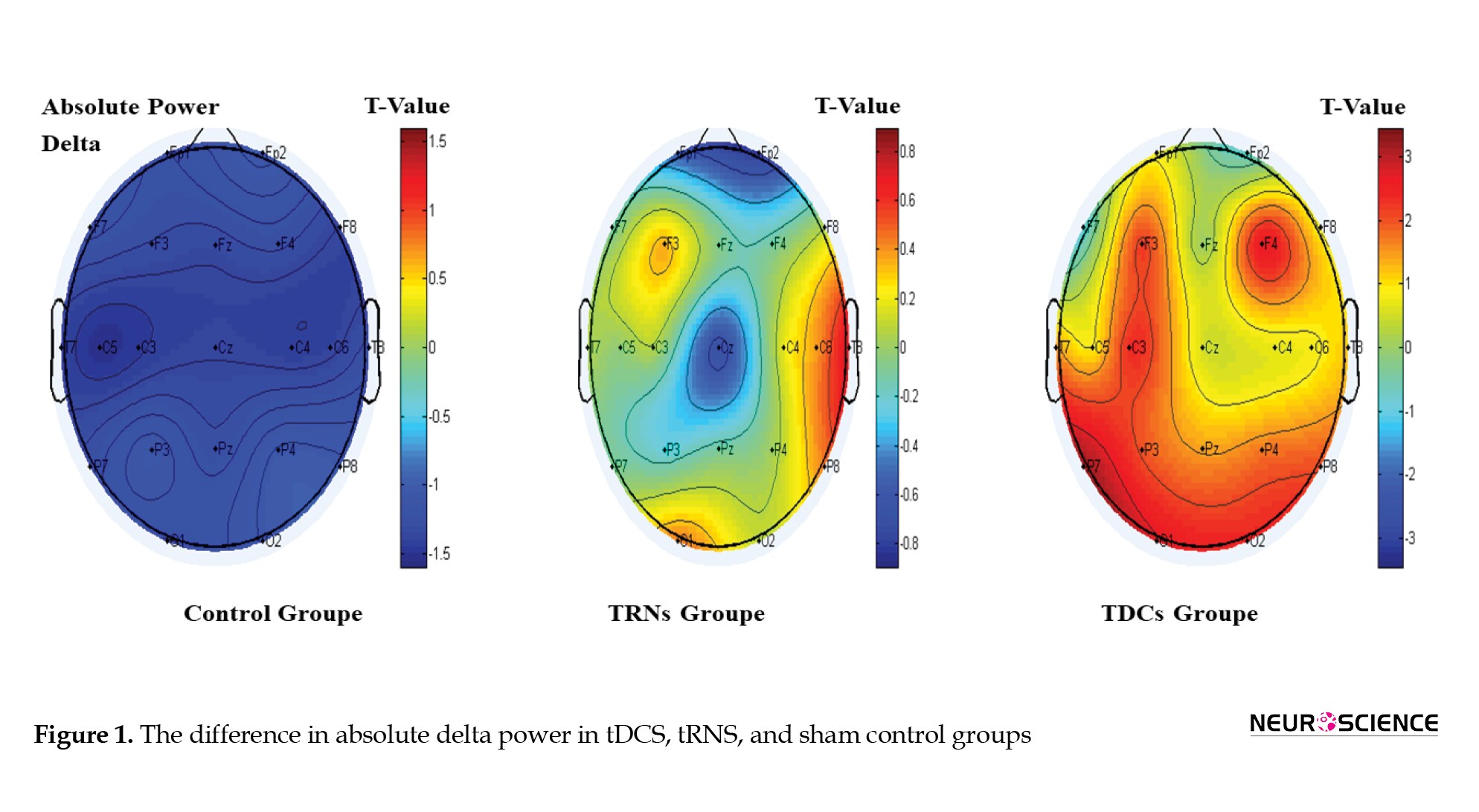
The brain map diagram of the difference in absolute delta power between the three groups is shown in Figure 1. In this diagram, the closer the color of the brain channels to blue, the higher the absolute power of the wave. As shown in the Figure, the temporal areas in the tRNS group and the occipital and frontal areas in the tDCS group have the most activity in absolute delta power.
The pattern of brain waves in the tRNS group differed from that in the tDCS and control groufips. A mixed design 2-way repeated measure analysis of variance (2-way ANOVA 2×3) was applied to channel×frequency and group.
As seen in Table 9, there is a significant difference between the group and the channel. To better understand the intergroup significance, a t-test was performed between groups in different frequencies and channels.
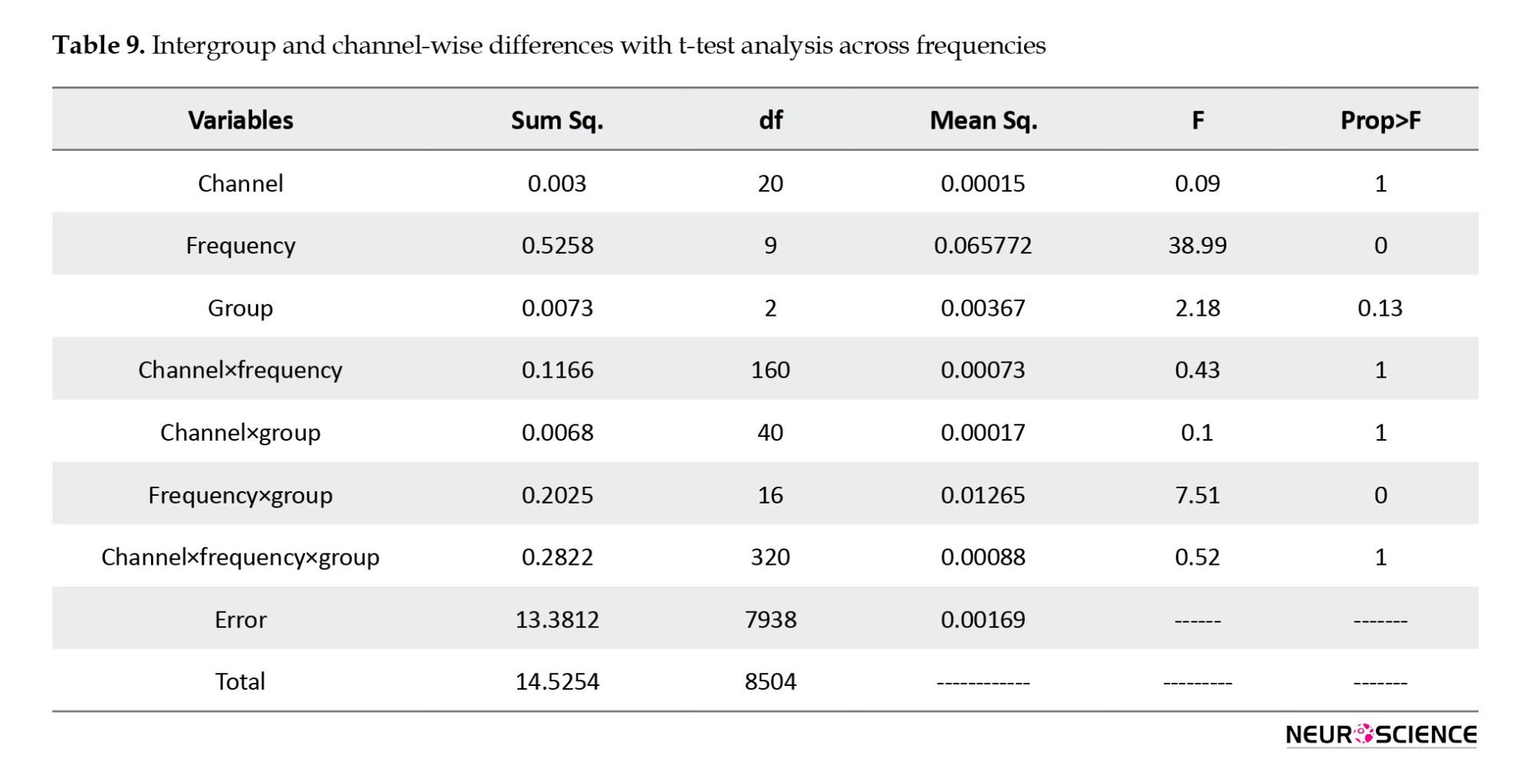
According to Table 10, the most significant effect has occurred in the absolute power and the difference in theta and delta frequencies.
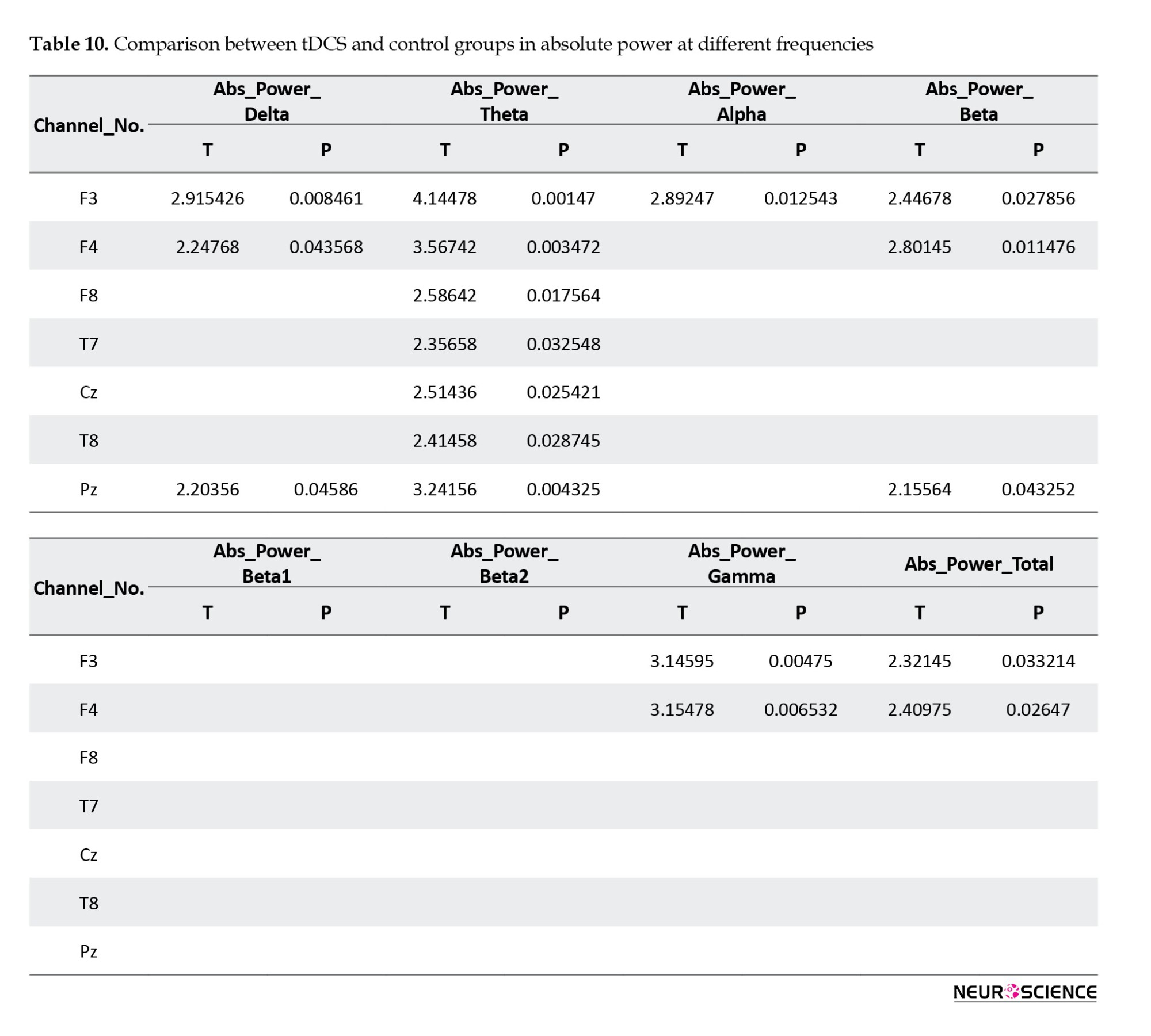
Figure 2 shows the theta frequency comparison in two groups and its difference. As can be seen, the absolute power in the central areas was significantly different in the two groups. There is a difference in the final comparison of the frontal, central, and occipital channels.
4. Discussion
In the current study, we aimed to see how an intervention program of brain stimulation and working memory training could help adolescents with ADHD enhance their working memory performance. Further, we sought to compare the efficacy of two different stimulation techniques, i.e. tDCS and tRNS, in boosting working memory. The results indicated the effectiveness of electrical stimulation (tDCS) on working memory. In the following, we will discuss these results in detail.
Promising findings are obtained from studies on the effectiveness of tDCS in the treatment of ADHD. To date, 8 studies have been conducted using tDCS in children and adolescents with ADHD: 5 randomized, double-blind, sham-controlled trials (Pern-Christensen et al., 2014; Munz et al., 2015; Nejati et al., 2020; Sotnikova et al., 2017; Moein et al., 2022), two randomized, single-blind, sham-controlled trials (Sultaninejad et al., 2019; Breitling et al., 2016) and one open-label randomized controlled trial with matching participants (Bandira et al., 2016). These studies have mainly focused on memory consolidation, working memory, and inhibitory control using different tDCS protocols over the posterior lateral prefrontal cortex.
In a pilot study, Bandira et al. (2016) investigated the effects of applying anode stimulation on the left posterolateral prefrontal cortex in 9 children and adolescents with ADHD. The anode electrode was placed on the F3 area, and the cathode electrode was placed on the upper area of the right eye. Stimulation was performed daily in five consecutive 30-min sessions. During each session, stimulation was performed at an intensity of 2 mA (except for the first and last minute of stimulation, when the current was reduced to 1 mA). Importantly, to activate the posterolateral prefrontal cortex, the participants were asked to participate in a card-matching game by matching pictures and making connections between them. The effects of anodal stimulation on several executive functions, including working memory and attention (assessed with the digit span subtest of the Wechsler III), inhibitory control (evaluated with the Nepsey II subtest), visual working memory and visual attention (assessed with the chair test), and visual attention (assessed with attention task) were explored.
These tests were performed before the first and after the last stimulation session. In addition, at the end of the last session, parents were asked to evaluate their children’s overall clinical improvement during the treatment process. Participants were asked about any side effects during or after treatment at the end of each stimulation session. Mild and moderate levels of headache, neck pain, itching, burning, and tingling sensation at the location of the anode, local redness, and drowsiness were often observed as side effects. In addition, a mild level of shock was also reported. Compared to the 1-mA current mainly used in other studies, the higher stimulation intensity was the cause of the discomfort. Overall, improvements were also observed in parents’ reports, except for worsening behavior in one child (the child also had oppositional defiant disorder). Notably, the absence of a sham control group does not allow for a thorough evaluation of the treatment’s efficacy. Furthermore, as both participants and parents were aware of the stimulation conditions, the occurrence of placebo effects cannot be excluded (Shabani et al., 2022).
Unlike the previous study used a double-blind, randomized crossover design with a sham control group to evaluate the effects of anode stimulation over the left posterolateral prefrontal cortex on working memory and the clinical course of ADHD. The logical reason for using anode stimulation in this brain region to improve working memory was to observe the decrease in the activity of this region in people with ADHD and the possibility of improving working memory performance in healthy participants by stimulating the anode of the left posterolateral prefrontal cortex. Fifteen teenagers with ADHD participated in the study. Each participant received either anode or sham stimulation for 5 days with a 2-week interval between the two treatment sessions. A 1-mA current was applied for either 20 minutes (anode stimulation) or 23 seconds (sham stimulation) using the anode electrode in the region (F3) and the cathode electrode on the top of the head (Cz). Electrical stimulation with direct current was applied while participants performed a computer task based on the n-back working memory paradigm. In the evaluation session, the assessment of participants’ task performance was combined with the amount of motor activity to evaluate the main symptoms, i.e. attention, hyperactivity, and impulsivity. In addition, working memory performance and parents’ reports of the severity of symptoms were also evaluated at the beginning of the stimulation, on the fifth day of stimulation, and one week after the end of stimulation. All participants completed the test, and the participants tolerated the protocol well. Tingling and slight itching under electrodes were the most common side effects. Only one participant developed a headache. Anodal stimulation improved symptoms of ADHD compared to sham stimulation. Compared to the baseline, a long-term reduction in inattention and hyperactivity was observed 7 days after the treatment ended, with no significant effect on impulsivity. Interestingly, in another study (Sotnikova et al., 2017), the authors reported the results of a functional magnetic resonance imaging study performed during the first session of anodic or sham stimulation while doing the n-back task. Compared to sham stimulation, anode stimulation stimulated more of the sub-electrode region, i.e. the left posterolateral prefrontal cortex, as well as the ipsilateral Barrington nucleus, sensorimotor area, and precuneus regions, suggesting that stimulation of the left posterolateral prefrontal cortex likely affects the entire network. Neurologically related to working memory function is effective. However, the limited sample size of these studies only confirmed that transcranial stimulation with direct current can be used to reduce the symptoms of people with ADHD, and more studies are still needed to verify the effectiveness of this method.
Non-pharmacological treatment options using non-invasive brain stimulation are helpful in this regard. One important reason for the use of non-invasive brain stimulation in the treatment of ADHD comes from studies showing that abnormal excitability of the cerebral cortex in ADHD is due to reduced motor inhibition (Buchman et al., 2003), as well as studies showing that two groups of ADHD drugs work by altering cortical excitability (Gilbert et al., 2006). Therefore, considering that non-invasive brain stimulation can affect the excitability of the cerebral cortex, it can be suggested as an effective alternative to drugs. Behavioral deficits in patients with ADHD can be attributed to defective inhibitory processes that lead to dysfunctional executive control, impulsive and hyperactive behavior (inhibition-based model), or deficits in motivation and reward processing (functional disorder model) (Sonoga-Barke, 2010).
It is suggested that for future research, other cognitive variables be investigated in different age groups, not only in ADHD but also in other (developmental) disorders. Moreover, using measurement tools such as fMRI in future research can help better discover the neural foundations of disorders.
One of the limitations of this research is the lack of access to female participants.
5. Conclusion
This study demonstrated the efficacy of electrical stimulation of prefrontal areas in improving working memory in adolescents with ADHD. The results showed that tDCS had a beneficial effect on working memory performance in the early stages. This effect was previously identified with theta/beta ratio and other predictors. Our study showed that tDCS and tRNS affect working memory differently, with tDCS stimulation being more effective. There is evidence that multi-session tDCS intervention improves memory performance and that these effects are maintained for weeks to months after stimulation. This finding suggests that repeated application of tDCS can enhance neural plasticity during stimulation.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles are considered in this article. The participants were informed of the purpose of the research and its implementation stages. They were also assured about the confidentiality of their information and were free to leave the study whenever they wished, and if desired, the research results would be available to them. A written consent has been obtained from the subjects. Principles of the Helsinki Convention were also observed. This study was approved by the Ethics Committee of Shahid Beheshti University, Tehran, Iran (Code: IR.SBU.REC.1399.068).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interception of the results and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors extend their sincere gratitude to Behzad Rigi Koteh, Assistant Professor of Clinical Psychology at Zahedan University of Medical Sciences, and Zahra Ghiyasi, Psychiatrist and Assistant Professor at Zahedan University of Medical Sciences, for their invaluable support and contribution to this research.
References
Attention deficit hyperactivity disorder (ADHD) is a childhood-onset psychiatric disorder characterized by disproportionate levels of developmental inattention, impulsivity, and hyperactivity (APA, 2013). The global prevalence rate of the disorder is 5.3% in children (Mannuzza et al., 2003) and 3.4% in adults (Fayyad et al., 2007).
Despite various research on this disorder, uncertainty persists regarding the causes of the disorder, as it is heterogeneous and changes drastically at an individual level. Sustained attention, inhibitory control, and executive functions are often affected. Specifically, Walcott et al. (2005) showed that response inhibition and working memory are impaired in most people with ADHD. Previous research has also shown that executive function defi-cits, particularly working memory, are highly correlated with academic dysfunction (Irwin et al., 2022). Although drug treatments have proved effective against the disorder’s main symptoms, they have a limited effect on cognitive deficits, especially executive dysfunction, calling for more research on treat-ments that target cognitive deficits (Irwin et al., 2022).
Working memory refers to the active, top-down process of manipulating information stored in short-term memory. It includes functions implicated in the temporal lobe prefrontal cortex that directs behavior by updating, processing, and manipulating the time/sequence of information in short-term memory (Lara & Wallis, 2015). Working memory acts as an interface between the environment and long-term memory. It is the basis of a set of learning skills, including note-taking, listening comprehension, and following instructions. Working memory also supports functions such as impulse control (Maraver et al., 2016), cooperation with others, dynamic decoding of social information (Phillips et al., 2007), and tolerance of delayed gratification (McInnes et al., 2003; Aliyari et al., 2018), all of which are impaired in ADHD.
In recent years, non-invasive brain stimulation has been introduced as a new treatment method for disorders with a neurocognitive basis. According to the specific evidence provided by functional magnetic resonance imaging studies, regions that are closer to the skull surface can be the target of non-invasive brain stimulation interventions. Although many studies show the effectiveness of transcranial direct current stimulation (tDCS) in improving working memory, this effect is still uncertain. These conflicting results seem to be due to differences in study design, stimulation protocol, and inter-individual differences (Jantz, et al., 2016).
Several studies have provided support for the effectiveness of electrical stimulation with random noise flow (tRNS) in boosting cognitive functions, including perceptual learning (Fertonani et al., 2011), number discrimination (Chick, 2014), and mathematics learning (Snowball et al., 2013). Brauer et al. (2018) did not report better performance on a go/no-go task after stimulating the right inferior frontal cortex region using tRNS. In another study, Bruit-Abi et al. (2018) showed that three sessions of tRNS over the left dorsolateral prefrontal cortex reduced participants’ reaction time on the go/no-go task but did not affect their accuracy.
So far, only one study has compared the efficacy of the two electrical stimulation techniques, i.e. tDCS and tRNS, in improving working memory in healthy individuals. Based on the results from this study, three sessions of 10-min tDCS over the left dorsolateral prefrontal cortex area led to improved performance on the 2-back test. However, treatment with tRNS did not yield the same performance outcome, seemingly due to the larger electrode size used in the tRNS treatment (Mulquiney et al., 2011).
In general, several studies have shown that defects in executive functions of inhibitory control and working memory adversely affect self-management behavior, causing behavioral symptoms in people with ADHD. Given the importance of executive dysfunction in ADHD, several studies have attempted to identify the neurological and biological correlates of inhibitory control and working memory deficits in people with ADHD (Zhao et al., 2010; Martel et al., 2011; Sonuga-Barke et al., 2010).
Considering the significant role of working memory in ADHD and the need to provide new, low-cost, and comprehensive treatments, we aimed to investigate the efficacy of tDCS and tRNS in ameliorating working memory and inhibitory control in individuals with ADHD.
2. Materials and Methods
Study participants
The statistical sample included 45 adolescents with ADHD, aged 13 to 17. They were randomly selected from 110 ADHD cases referred to Baharan Psychiatric Hospital of Zahedan University of Medical Sciences, Zahedan City, Iran, in the second half of 2018 and 2019. The inclusion criteria were as follows: Meeting the DSM-5 criteria for ADHD along with a diagnosis of ADHD by a psychologist and a psychiatrist, aged 13 to 17, willingness to provide informed consent (of both participants and their parents), right-handedness, being a male gender. The exclusion criteria were as follows: History of seizures and epilepsy, any blow to the head, history of psychiatric disorders, unwillingness to participate at any time during the experiment, and unbearable discomfort or difficulty when receiving transcranial electrical stimulation (tES).
Participants were randomly assigned to three groups, each including 15 members: The tDCS group, the tRNS group, and the control group. To ensure that participants in the control group experience the same effect as participants in the tDCS and tRNS groups, we applied sham transcranial stimulation to participants in the control group. Each person in the experimental group received 5 sessions of electrical stimulation with an interval of 24 hours between sessions. Electroencephalography (EEG) recording and cognitive assessment were done before the intervention, immediately after the intervention, and one week after the intervention.
Study measures
Measuring the side effects of transcranial stimulation with direct electric current
This questionnaire includes 7 items, each referring to a particular effect reported or possibly experienced by those receiving tDCS. They include headache, dizziness, heartburn, itchy head, feeling confused, drowsiness, and nausea. An item titled “other” was also added to ensure participants could still report how they felt even if none of the items on the questionnaire matched their experience with tDCS (Najati et al., 2020).
Cognitive rehabilitation task
In this study and in line with previous research (Westwood et al., 2022), the n-back cognitive rehabilitation task was used along with transcranial stimulation to increase working memory capacity. In this task, a sequence of stimuli is displayed on the screen one after another, and the participants are required to compare the current stimulus with the one that appeared in the n-trials back in the sequence and press the response key if they match.
Dual n-back is a task variation in which two types of stimuli (visual-spatial and auditory) are presented simultaneously (Heinzel et al., 2017). Research in neurology has shown that cognitive training using dual n-back often increases brain activity in the left and right prefrontal areas, especially the left posterior-lateral area, which is implicated in executive functions of working memory, including updating, shifting, and inhibition. This tool has been used in many studies, and its effectiveness has been shown (Haq Nazari et al., 2022).
Digit span test
In the direct digit span memory test, lists of 3 to 9 digits are orally presented, each at a time, and participants are asked to repeat the digits in the same order they hear them. In the reverse digit span memory test, however, lists of 2 to 8 digits are presented, and participants must repeat the digits on each list in reverse order. Aminzadeh and Hasanabadi (2012) have reported a reliability score of 0.8 and 0.68 for the direct and reverse versions of the task, respectively. Gathercole et al., 2004) reported a test re-test reliability score of 0.81 for the direct digit memory test, and Thompson and Gathercole (2006) reported a test re-test reliability score of 0.71 for the reverse digit memory test. Also, he calculated the reliability model of the memory of direct and reverse digits through retesting, respectively, 0.84 and 0.60. (Aliyari et al., 2019).
EEG data recording and analysis
EEG data were collected using a 21-channel contact instrument psych lab EEG amplifier (Medinateb, 2025). The electrode impedances were kept below 5 k Ω. The EEG signals were recorded with a sampling frequency of 500 Hz. The electrode placed at the right earlobe served as the reference. Moreover, the electrode on the left mastoid region was applied as the ground. Subsequently, using the EEGLAB toolbox, we performed standard preprocessing, including band-pass filtering (1-40 Hz), running ICA, and reducing sampling frequency to 256 Hz to remove noise and artifacts from EEG data.
Based on their absolute power, the preprocessed data were divided into 9 components: Delta (1-4 Hz), theta (4-8 Hz), alpha (8-13 Hz), alpha1(8-10 Hz), alpha2 (11-13 Hz), beta (13-21 Hz), beta1 (13-21 Hz), beta2 (19-30 Hz).), and gamma (30-40). The data were analyzed using a two-way ANOVA with frequency channel as within-group and stimulation type as between-group factors. The t-test was applied to explore statistically significant differences between group means (Schomer & da Silva, 2005; Shabani et al., 2024).
tES device (NeuroStim2)
This device was launched by the Research & Development team of Medina Teb company in 2015. This device has two completely separate channels and can apply various electrical stimulation patterns with the highest quality. NeuroStim2 has two channels that are electrically isolated from each other, and each channel can be set independently to apply separate stimulations.
Transcranial brain stimulation is provided in the form of two electrodes placed on the target areas on the surface of the head with a weak current of 1 to 2 mA. After about 5 minutes, this weak current passes through the surface of the skull. It affects the activity of nerve cells in the area where the electrodes are placed and the subcortical regions connected to them (Weber et al., 2014).
Study procedure
Participants were first homogenized based on results from the Connors questionnaire (parent and teacher forms), their performance on the working memory scale of the Wechsler test (digit span, number-letter sequence), and their age. They were then randomly assigned to one of the three groups: The tDCS, the tRNS, and the sham control group. Before the intervention, a resting state EEG recording was done, and the digit span test was administered. Participants received 5 sessions of electrical stimulation with an interval of 24 hours between sessions. To explore the transfer effect of the intervention, behavioral and psychological tests (digit span task) were administered one week after the end of the intervention.
3. Results
Behavioral data: Digit span test
Descriptive statistics of the digit span test scores are provided in Table 1.

A mixed-design analysis of variance was applied to mean scores on the digit span test to investigate the effectiveness of tDCS and tRNS on working memory functioning in adolescents with ADHD. The results are shown in Table 2.

As shown in Table 2, the F value observed for the effect of the intervention stage (pre-test, post-test, and follow-up) was significant at the 0.01 level for all components of working memory (direct and reverse digit span). As a result, there is an essential difference between scores on all components of working memory at pre-test, post-test, and 1-week follow-up. Regarding the between-group factor of stimulation type (tDCS, tRNS, and sham stimulation), the analysis revealed statistical significance between mean scores on all components of working memory (P<0.01). The interaction effect between the intervention stage and stimulation type was also significant for all working memory components (P<0.001).
The Bonferroni post hoc test results to compare the pairwise differences between intervention stages and the Tukey post hoc test to compare the pairwise differences between stimulation types are presented in Tables 3 and 4.

Table 3 presents no significant differences between tDCS, tRNS, and sham control at pre-test (P>0.05). In the post-test, however, participants in the tDCS and tRNS groups achieved significantly higher scores than those in the sham control group (P<0.001). The difference between scores in the tDCS and the tRNS groups was also statistically significant at post-test (P<0.001), with the tDCS group getting better scores. The tDCS group had significantly higher scores at follow-up than the tRNS and sham control groups. However, no significant difference was found between the tRNS and control groups (P>0.05).

Table 5 indicates no significant differences between reverse digit span scores in tDCS, tRNS, and sham control groups at pre-test scores (P>0.05). However, a significant difference was observed between the groups during the post-test and follow-up. The tDCS and tRNS groups got significantly higher scores than those in the control group (P<0.001). In addition, the results showed that the tDCS group performed significantly better than the tRNS group on the reverse digit span test (P<0.001).

Results from pairwise analysis on mean scores on the reverse digit span test (Table 6) showed a significant difference between the pre-test and post-test (P<0.001) and pre-test and follow-up stages in the tDCS group (P<0.001). However, no significant difference between the post-test and follow-up (P>0.05) was found. In the tRNS group, the results showed significant differences at pre-test, post-test, and follow-up (P<0.05). No significant differences were found between intervention stages in the control group (P>0.05).

Data analysis of brain signals (EEG)
This study analyzed brain wave patterns over time. Different scores are presented at various time points per second for ten brain wave rhythms. These figures facilitate understanding the changes in the participants’ brain waves during treatment. The pattern of brain waves was examined in the tRNS, the tDCS, and the sham control group. The results are presented in Table 7.

Table 7 outlines the results of a three-way analysis of variance (ANOVA) for absolute power values using the factors channel, frequency, and group. A significant difference was found in the group and frequency factors, and based on this, subsequent t tests were taken. The intragroup post hoc t-test for comparing the pre-test and post-test shows the absolute power in each group.
In the following, the within-group post hoc t-test was done to compare absolute power values at the pre-test and post-test in each group. Significant channels in each group are presented in Table 8.
.jpg)
The brain map diagram of the difference in absolute delta power between the three groups is shown in Figure 1. In this diagram, the closer the color of the brain channels to blue, the higher the absolute power of the wave. As shown in the Figure, the temporal areas in the tRNS group and the occipital and frontal areas in the tDCS group have the most activity in absolute delta power.

The pattern of brain waves in the tRNS group differed from that in the tDCS and control groufips. A mixed design 2-way repeated measure analysis of variance (2-way ANOVA 2×3) was applied to channel×frequency and group.
As seen in Table 9, there is a significant difference between the group and the channel. To better understand the intergroup significance, a t-test was performed between groups in different frequencies and channels.

According to Table 10, the most significant effect has occurred in the absolute power and the difference in theta and delta frequencies.

Figure 2 shows the theta frequency comparison in two groups and its difference. As can be seen, the absolute power in the central areas was significantly different in the two groups. There is a difference in the final comparison of the frontal, central, and occipital channels.

4. Discussion
In the current study, we aimed to see how an intervention program of brain stimulation and working memory training could help adolescents with ADHD enhance their working memory performance. Further, we sought to compare the efficacy of two different stimulation techniques, i.e. tDCS and tRNS, in boosting working memory. The results indicated the effectiveness of electrical stimulation (tDCS) on working memory. In the following, we will discuss these results in detail.
Promising findings are obtained from studies on the effectiveness of tDCS in the treatment of ADHD. To date, 8 studies have been conducted using tDCS in children and adolescents with ADHD: 5 randomized, double-blind, sham-controlled trials (Pern-Christensen et al., 2014; Munz et al., 2015; Nejati et al., 2020; Sotnikova et al., 2017; Moein et al., 2022), two randomized, single-blind, sham-controlled trials (Sultaninejad et al., 2019; Breitling et al., 2016) and one open-label randomized controlled trial with matching participants (Bandira et al., 2016). These studies have mainly focused on memory consolidation, working memory, and inhibitory control using different tDCS protocols over the posterior lateral prefrontal cortex.
In a pilot study, Bandira et al. (2016) investigated the effects of applying anode stimulation on the left posterolateral prefrontal cortex in 9 children and adolescents with ADHD. The anode electrode was placed on the F3 area, and the cathode electrode was placed on the upper area of the right eye. Stimulation was performed daily in five consecutive 30-min sessions. During each session, stimulation was performed at an intensity of 2 mA (except for the first and last minute of stimulation, when the current was reduced to 1 mA). Importantly, to activate the posterolateral prefrontal cortex, the participants were asked to participate in a card-matching game by matching pictures and making connections between them. The effects of anodal stimulation on several executive functions, including working memory and attention (assessed with the digit span subtest of the Wechsler III), inhibitory control (evaluated with the Nepsey II subtest), visual working memory and visual attention (assessed with the chair test), and visual attention (assessed with attention task) were explored.
These tests were performed before the first and after the last stimulation session. In addition, at the end of the last session, parents were asked to evaluate their children’s overall clinical improvement during the treatment process. Participants were asked about any side effects during or after treatment at the end of each stimulation session. Mild and moderate levels of headache, neck pain, itching, burning, and tingling sensation at the location of the anode, local redness, and drowsiness were often observed as side effects. In addition, a mild level of shock was also reported. Compared to the 1-mA current mainly used in other studies, the higher stimulation intensity was the cause of the discomfort. Overall, improvements were also observed in parents’ reports, except for worsening behavior in one child (the child also had oppositional defiant disorder). Notably, the absence of a sham control group does not allow for a thorough evaluation of the treatment’s efficacy. Furthermore, as both participants and parents were aware of the stimulation conditions, the occurrence of placebo effects cannot be excluded (Shabani et al., 2022).
Unlike the previous study used a double-blind, randomized crossover design with a sham control group to evaluate the effects of anode stimulation over the left posterolateral prefrontal cortex on working memory and the clinical course of ADHD. The logical reason for using anode stimulation in this brain region to improve working memory was to observe the decrease in the activity of this region in people with ADHD and the possibility of improving working memory performance in healthy participants by stimulating the anode of the left posterolateral prefrontal cortex. Fifteen teenagers with ADHD participated in the study. Each participant received either anode or sham stimulation for 5 days with a 2-week interval between the two treatment sessions. A 1-mA current was applied for either 20 minutes (anode stimulation) or 23 seconds (sham stimulation) using the anode electrode in the region (F3) and the cathode electrode on the top of the head (Cz). Electrical stimulation with direct current was applied while participants performed a computer task based on the n-back working memory paradigm. In the evaluation session, the assessment of participants’ task performance was combined with the amount of motor activity to evaluate the main symptoms, i.e. attention, hyperactivity, and impulsivity. In addition, working memory performance and parents’ reports of the severity of symptoms were also evaluated at the beginning of the stimulation, on the fifth day of stimulation, and one week after the end of stimulation. All participants completed the test, and the participants tolerated the protocol well. Tingling and slight itching under electrodes were the most common side effects. Only one participant developed a headache. Anodal stimulation improved symptoms of ADHD compared to sham stimulation. Compared to the baseline, a long-term reduction in inattention and hyperactivity was observed 7 days after the treatment ended, with no significant effect on impulsivity. Interestingly, in another study (Sotnikova et al., 2017), the authors reported the results of a functional magnetic resonance imaging study performed during the first session of anodic or sham stimulation while doing the n-back task. Compared to sham stimulation, anode stimulation stimulated more of the sub-electrode region, i.e. the left posterolateral prefrontal cortex, as well as the ipsilateral Barrington nucleus, sensorimotor area, and precuneus regions, suggesting that stimulation of the left posterolateral prefrontal cortex likely affects the entire network. Neurologically related to working memory function is effective. However, the limited sample size of these studies only confirmed that transcranial stimulation with direct current can be used to reduce the symptoms of people with ADHD, and more studies are still needed to verify the effectiveness of this method.
Non-pharmacological treatment options using non-invasive brain stimulation are helpful in this regard. One important reason for the use of non-invasive brain stimulation in the treatment of ADHD comes from studies showing that abnormal excitability of the cerebral cortex in ADHD is due to reduced motor inhibition (Buchman et al., 2003), as well as studies showing that two groups of ADHD drugs work by altering cortical excitability (Gilbert et al., 2006). Therefore, considering that non-invasive brain stimulation can affect the excitability of the cerebral cortex, it can be suggested as an effective alternative to drugs. Behavioral deficits in patients with ADHD can be attributed to defective inhibitory processes that lead to dysfunctional executive control, impulsive and hyperactive behavior (inhibition-based model), or deficits in motivation and reward processing (functional disorder model) (Sonoga-Barke, 2010).
It is suggested that for future research, other cognitive variables be investigated in different age groups, not only in ADHD but also in other (developmental) disorders. Moreover, using measurement tools such as fMRI in future research can help better discover the neural foundations of disorders.
One of the limitations of this research is the lack of access to female participants.
5. Conclusion
This study demonstrated the efficacy of electrical stimulation of prefrontal areas in improving working memory in adolescents with ADHD. The results showed that tDCS had a beneficial effect on working memory performance in the early stages. This effect was previously identified with theta/beta ratio and other predictors. Our study showed that tDCS and tRNS affect working memory differently, with tDCS stimulation being more effective. There is evidence that multi-session tDCS intervention improves memory performance and that these effects are maintained for weeks to months after stimulation. This finding suggests that repeated application of tDCS can enhance neural plasticity during stimulation.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles are considered in this article. The participants were informed of the purpose of the research and its implementation stages. They were also assured about the confidentiality of their information and were free to leave the study whenever they wished, and if desired, the research results would be available to them. A written consent has been obtained from the subjects. Principles of the Helsinki Convention were also observed. This study was approved by the Ethics Committee of Shahid Beheshti University, Tehran, Iran (Code: IR.SBU.REC.1399.068).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interception of the results and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors extend their sincere gratitude to Behzad Rigi Koteh, Assistant Professor of Clinical Psychology at Zahedan University of Medical Sciences, and Zahra Ghiyasi, Psychiatrist and Assistant Professor at Zahedan University of Medical Sciences, for their invaluable support and contribution to this research.
References
Aliyari, H., Hosseinian, S., Sahraei, H., & Menhaj, M. (2019). Effect of proximity to high voltage fields: Results of the neural network model and experimental model with macaques. International Journal of Environmental Science and Technology, 16, 4315-4326. [Link]
Aliyari, H., Hosseinian, S. H., Menhaj, M. B., & Sahraei, H. (2019). Analysis of the effects of high voltage transmission line on human stress and attention through electroencephalography (EEG). Iranian Journal of Science and Technology, Transactions of Electrical Engineering, 43, 211-218. [Link]
Aliyari, H., Sahraei, H., Daliri, M. R., Minaei Bidgoli, B., Kazemi, M., & Agaei, H., et al. (2018). The beneficial or harmful effects of computer game stress on cognitive functions of players. Basic and Clinical Neuroscience, 9(3), 177-186. [DOI:10.29252/nirp.bcn.9.3.177] [PMID]
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM 5®). Washington: American Psychiatric Publishing. [DOI:10.1176/appi.books.9780890425596]
Aminzadeh, A., & Hassanabadi, H. (2012). [Based skills in children with mathematics disability and co-morbid mathematics and reading disability (Persian)]. Journal of Developmental Psychology, 8(31), 235–245. [Link]
Bandeira, I. D., Guimarães, R. S., Jagersbacher, J. G., Barretto, T. L., de Jesus-Silva, J. R., Santos, S. N., Argollo, N., & Lucena, R. (2016). Transcranial Direct Current Stimulation in Children and Adolescents With Attention-Deficit/Hyperactivity Disorder (ADHD): A Pilot Study. Journal of Child Neurology, 31(7), 918–924. [PMID]
Blair, C., & Ursache, A. (2011). A bidirectional model of executive functions and self regulation. In R. Baumeister & K. Vohs (Eds.), Handbook of self regulation: Research, theory, and applications (pp. 300-320). New York: Guilford Publications. [Link]
Brauer, H., Kadish, N. E., Pedersen, A., Siniatchkin, M., & Moliadze, V. (2018). No modulatory effects when stimulating the right inferior frontal gyrus with continuous 6 Hz tACS and tRNS on Response Inhibition: A Behavioral Study. Neural Plasticity, 2018, 3156796. [PMID]
Breitling, C., Zaehle, T., Dannhauer, M., Bonath, B., Tegelbeckers, J., & Flechtner, H. H., et al. (2016). Improving Interference Control in ADHD Patients with Transcranial Direct Current Stimulation (tDCS). Frontiers in Cellular Neuroscience, 10, 72. [DOI:10.3389/fncel.2016.00072] [PMID]
Buchmann, J., Wolters, A., Haessler, F., Bohne, S., Nordbeck, R., & Kunesch, E. (2003). Disturbed transcallosally mediated motor inhibition in children with attention deficit hyperactivity disorder (ADHD). Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 114(11), 2036–2042. [DOI:10.1016/s1388-2457(03)00208-6] [PMID]
Chick, C. F. (2014). Basic mechanisms of numerical processing: Cross-modal number comparisons and symbolic versus nonsymbolic numerosity in the intraparietal sulcus. Journal of Neuroscience, 34(5), 1567-1569. [Link]
Fayyad, J., De Graaf, R., Kessler, R., Alonso, J., Angermeyer, M., & Demyttenaere, K., et al.(2007). Cross national prevalence and correlates of adult attention deficit/hyperactivity disorder. The British Journal of Psychiatry: The Journal of Mental Science, 190, 402–409. [DOI:10.1192/bjp.bp.106.034389] [PMID]
Fertonani, A., Pirulli, C., & Miniussi, C. (2011). Random noise stimulation improves neuroplasticity in perceptual learning. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31(43), 15416–15423. [PMID]
Gathercole, S. E., Pickering, S. J., Knight, C., & Stegmann, Z. (2004). Working memory skills and educational attainment: Evidence from national curriculum assessments at 7 and 14 years of age. Applied Cognitive Psychology: The Official Journal of the Society for Applied Research in Memory and Cognition, 18(1), 1-16. [DOI:10.1002/acp.934]
Gilbert, D. L., Ridel, K. R., Sallee, F. R., Zhang, J., Lipps, T. D., & Wassermann, E. M. (2006). Comparison of the inhibitory and excitatory effects of ADHD medications methylphenidate and atomoxetine on motor cortex. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 31(2), 442–449. [DOI:10.1038/sj.npp.1300806] [PMID]
Heinzel, S., Rimpel, J., Stelzel, C., & Rapp, M. A. (2017). Transfer Effects to a Multimodal Dual-Task after Working Memory Training and Associated Neural Correlates in Older Adults - A Pilot Study. Frontiers in Human Neuroscience, 11, 85. [PMID]
Haq Nazari, F., Nejati, V., & Pouratmad, H. R. (2022). The effectiveness of working memory enhancement through computer-based training on sustained attention in students. Scientific and Research Journal of Rehabilitation Medicine, 11(2), 2-13. [Link]
Irwin, L. N., Soto, E. F., Chan, E. S. M., Miller, C. E., Carrington-Forde, S., & Groves, N. B., et al. (2021). Activities of daily living and working memory in pediatric attention deficit/hyperactivity disorder (ADHD). Child Neuropsychology, 27(4), 468-490. [DOI:10.1080/09297049.2020.1866521] [PMID]
Jantz, T. K., Katz, B., & Reuter-Lorenz, P. A. (2016). Uncertainty and promise: The effects of transcranial direct current stimulation on working memory. Current Behavioral Neuroscience Reports, 3, 109-121. [Link]
Lara, A. H., & Wallis, J. D. (2015). The role of prefrontal cortex in working memory: A mini review. Frontiers in Systems Neuroscience, 9, 173. [DOI:10.3389/fnsys.2015.00173] [PMID]
Martel, M. M., Roberts, B., Gremillion, M., von Eye, A., & Nigg, J. T. (2011). External validation of bifactor model of ADHD: explaining heterogeneity in psychiatric comorbidity, cognitive control, and personality trait profiles within DSM-IV ADHD. Journal of Abnormal Child Psychology, 39(8), 1111–1123. [DOI:10.1007/s10802-011-9538-y] [PMID]
Maraver, M. J., Bajo, M. T., & Gomez-Ariza, C. J. (2016). Training on Working Memory and Inhibitory Control in Young Adults. Frontiers in Human Neuroscience, 10, 588. [PMID]
Moein, N., Rostami, R., Mohamadi, R., Zomorrodi, R., Nitsche, M., & Ostadi, A., et al. (2022). Electrophysiological correlates of stuttering severity: An ERP study. Journal of Clinical Neuroscience, 101, 80-88. [DOI:10.1016/j.jocn.2022.03.021] [PMID]
Munz, M. T., Prehn-Kristensen, A., Thielking, F., Mölle, M., Göder, R., & Baving, L. (2015). Slow oscillating transcranial direct current stimulation during non-rapid eye movement sleep improves behavioral inhibition in attention-deficit/hyperactivity disorder. Frontiers in Cellular Neuroscience, 9, 307. [PMID]
Mulquiney, P. G., Hoy, K. E., Daskalakis, Z. J., & Fitzgerald, P. B. (2011). Improving working memory: Exploring the effect of transcranial random noise stimulation and transcranial direct current stimulation on the dorsolateral prefrontal cortex. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 122(12), 2384–2389. [PMID]
Nejati, V., Salehinejad, M. A., Nitsche, M. A., Najian, A., & Javadi, A. H. (2020). Transcranial Direct Current Stimulation Improves Executive Dysfunctions in ADHD: Implications for Inhibitory Control, Interference Control, Working Memory, and Cognitive Flexibility. Journal of Attention Disorders, 24(13), 1928–1943. [DOI:10.1177/1087054717730611] [PMID]
Prehn-Kristensen, A., Munz, M., Göder, R., Wilhelm, I., Korr, K., Vahl, W., Wiesner, C. D., & Baving, L. (2014). Transcranial oscillatory direct current stimulation during sleep improves declarative memory consolidation in children with attention-deficit/hyperactivity disorder to a level comparable to healthy controls. Brain Stimulation, 7(6), 793–799. [DOI:10.1016/j.brs.2014.07.036] [PMID]
Phillips, S. M., Moore, D. R., & Tang, J. E. (2007). A critical examination of dietary protein requirements, benefits, and excesses in athletes. International Journal of Sport Nutrition and Exercise Metabolism, 17(Suppl. 1), S58-S76. [DOI:10.1123/ijsnem.17.s1.s58] [PMID]
Shabani, M., Salehi, J., & Khosrowabadi, R. (2024). Gender effect on neural correlates of autobiographical false memories for brand images. Basic and Clinical Neuroscience, 15(1), 117–130. [PMID]
Shabani, M., Salehi, J., & Khosrowabadi, R. (2022). Comparing autobiographical brand images and neutral images regarding false memory formation. Basic and Clinical Neuroscience, 13(4), 489–499. [DOI:10.32598/bcn.2021.2275.2] [PMID]
Schomer, D. L., & Lopes da Silva, F. H. (2005). *Niedermeyer's Electroencephalography. In E. Niedermeyer ·(Ed.), Basic Principles, Clinical Applications, and Related Fields. Pennsylvania: Lippincott Williams & Wilkins. [Link]
Snowball, A., Tachtsidis, I., Popescu, T., Thompson, J., Delazer, M., & Zamarian, L., et al. (2013). Long-term enhancement of brain function and cognition using cognitive training and brain stimulation. Current Biology: CB, 23(11), 987–992. [PMID]
Soltaninejad, Z., Nejati, V., & Ekhtiari, H. (2019). Effect of Anodal and Cathodal Transcranial Direct Current Stimulation on DLPFC on Modulation of Inhibitory Control in ADHD. Journal of Attention Disorders, 23(4), 325–332. [PMID]
Sonuga-Barke, E., Bitsakou, P., & Thompson, M. (2010). Beyond the dual pathway model: evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 49(4), 345–355. [DOI:10.1016/j.jaac.2009.12.018] [PMID]
Sotnikova, A., Soff, C., Tagliazucchi, E., Becker, K., & Siniatchkin, M. (2017). Transcranial Direct Current Stimulation Modulates Neuronal Networks in Attention Deficit Hyperactivity Disorder. Brain Topography, 30(5), 656–672. [PMID]
Thompson, H. L., & Gathercole, S. E. (2006). Executive functions and achievements in school: Shifting, updating, inhibition, and working memory. Quarterly Journal of Experimental Psychology, 59*(4), 745–756. [DOI: 10.1080/17470210500162854] [PMID]
Walcott, C. M., & Landau, S. (2004). The relation between disinhibition and emotion regulation in boys with attention deficit/hyperactivity disorder. Journal of Clinical Child and Adolescent Psychology, 33(4), 772-782. [DOI:10.1207/s15374424jccp3304_12] [PMID]
Weber, M. J., Messing, S. B., Rao, H., Detre, J. A., & Thompson Schill, S. L. (2014). Prefrontal transcranial direct current stimulation alters activation and connectivity in cortical and subcortical reward systems: A tDCS fMRI study. Human Brain Mapping, 35(8), 3673-3686. [DOI:10.1002/hbm.22429] [PMID]
Westwood, S. J., Bozhilova, N., Criaud, M., Lam, S. L., Lukito, S., & Wallace Hanlon, S., et al. (2021). The effect of transcranial direct current stimulation (tDCS) combined with cognitive training on EEG spectral power in adolescent boys with ADHD: A double blind, randomized, sham controlled trial. IBRO Neuroscience Reports, 12, 55-64. [DOI:10.1016/j.ibneur.2021.12.005] [PMID]
Type of Study: Original |
Subject:
Cognitive Neuroscience
Received: 2022/12/8 | Accepted: 2023/04/10 | Published: 2025/03/18
Received: 2022/12/8 | Accepted: 2023/04/10 | Published: 2025/03/18
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








