Volume 15, Issue 5 (September & October 2024)
BCN 2024, 15(5): 631-648 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rostami F, Esteki A, Sarabi-Jamab A. Interference Control Under the Stroop Task and Brain Oscillatory Activity among Internet Addicts Compared to Non-addicts. BCN 2024; 15 (5) :631-648
URL: http://bcn.iums.ac.ir/article-1-2596-en.html
URL: http://bcn.iums.ac.ir/article-1-2596-en.html
1- Department of Biomedical Engineering and Medical Physics, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Faculty of Governance, University of Tehran, Tehran, Iran.
2- Faculty of Governance, University of Tehran, Tehran, Iran.
Keywords: Stroop effect, Electroencephalographic (EEG) activity, Interference control, Internet addiction disorders (IADs), Time-frequency analysis
Full-Text [PDF 3751 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Nowadays, we are facing a new type of behavioral addiction named “internet addiction,” a significant issue of the information age. Since the development of cyberspace, the internet has been regarded as one of the most critical drivers of economic progress and social change (Barry et al., 2010; Omoyemiju & Popoola, 2021). However, with widespread access to the internet, as well as the occurrence of global challenges like COVID-19, people are heavily urged to use cyberspace, which in some cases turns into addictive behavior (King et al., 2011; Ozturk & Ayaz-Alkaya, 2021).
Previously, this newly defined addictive behavior was known as internet addiction disorder (IAD) or, in its specific form, internet gaming disorder (IGD) in the international classification of diseases (ICD-11) and the diagnostic and statistical manual for mental disorders (DSM-5).
IAD is a psychological and social disorder distinguished by the emergence of tolerance, symptoms of withdrawal, emotional disturbances, and the loss of social relationships (Gioia et al., 2021; West et al., 2005). Like other forms of behavioral addiction, e.g. gaming and social networks, as well as chemical addictions (e.g. alcohol and cocaine), IAD causes serious impairment in psychological and social functioning (Király et al., 2015; Park et al., 2020; Reed et al., 2019).
According to an increasing body of evidence, IAD may result in alternations in various cognitive functions, including perception (Pontes et al., 2015), attention (Gao et al., 2021; Heuer et al., 2021), memory (Zhou et al., 2016), and executive functions (Ioannidis et al., 2019; Zhou et al., 2016). Specifically, IADs are reported to lack the ability to perform inhibitory control, interference resolution, error processing, and decision-making (Park et al., 2020; Sun et al., 2009). Inhibitory control dysfunction has been suggested as an important feature of IAD (Antons & Matthias, 2020).
To cultivate inhibition control, people must learn to manage their focus, actions, thoughts, and feelings to overcome powerful internal or external impulses and choose the suitable or obligatory course of action. Comparatively, to the HC group, studies in the IAD group have generally reported reduced response-inhibition efficiency (Brand et al., 2016; Dong et al., 2011). Also, a few studies have shown a correlation between a decrease in inhibitory control and an increase in gaming time in cases of IGD (Kräplin et al., 2021). In accordance with the conflict monitoring theory, these impairments lead to a conflict between the stimulus presentation and the response display (Lustig et al., 2001).
The conflict monitoring method consists of a group of tasks that provide the possibility to investigate and monitor the interaction between incompatible responses (e.g. the simultaneous activation of incorrect and correct responses or the simultaneous activation of two rivalrous stimuli types) (Botvinick et al., 2004). The color-word Stroop task (Stroop, 1935) is considered one of the most widely known conflict monitoring tasks (Schmidt, 2019); participants are instructed to recognize the ink color of the stimulus based on whether the color word is congruent (e.g. yellow in yellow) or incongruent (e.g. yellow in green). Incongruent trials are generally associated with higher response times and error rates than congruent trials (MacLeod, 1991) due to Stroop’s interference effect, which arises from the different levels of conflict experienced in incongruent trials compared to congruent trials. Significant cognitive control is exhibited in response to highly conflictive stimuli, leading to decreased interference in subsequent high-conflict trials (Diamond, 2013).
The use of imaging methods such as functional magnetic resonance imaging (fMRI) and electroencephalography (EEG) showed that individuals with IAD have difficulties with their ability to control inhibitions and interference (Liu et al., 2016; Zhu et al., 2015). For example, Dong et al., (2010) observed that individuals with IAD exhibited disruptions in activity and connectivity within their prefrontal lobes, responsible for inhibitory control. Similar conclusions were drawn based on EEG studies (Ioannidis et al., 2019; Luijten et al., 2014). Compared to HCs, IAD individuals demonstrated altered amplitudes for the N2 and P3 components when detecting conflicts, suggesting that they are less capable of monitoring conflict and require greater attention when confronted with inhibition tasks (Dong et al., 2010). It has been found that individuals with IGD have a longer NoGo-N2 latency than their counterparts without IGD, possibly due to impaired response inhibition in individuals with IGD (Park et al., 2021). A study employing the Go/NoGo task to identify the source of activity found that individuals with IGD displayed reduced neural activity in the anterior cingulate cortex (ACC) and defective error processing, resulting in inadequate inhibition and decision-making abilities (Park et al., 2020).
There have been various studies conducted to examine the correlation between brain oscillations and addiction (Balconi & Finocchiaro, 2015; Balconi et al., 2015). According to previous studies, delta band responses are involved in signal detection and decision-making (Donoghue et al., 2022; Harmony, 2013), while theta functions are primarily concerned with inhibitory processes (Harper et al., 2014; Son et al., 2015). Earlier research has demonstrated that alterations in power in the theta frequency band are linked to cognitive conflict processes, with a marked rise in power observed in incongruent trials compared to congruent trials (Brittain et al., 2012; Tang et al., 2013). The results also show that theta power changes in relation to stimulus and response conflict in the Flanker task (Nigbur et al., 2012) (as well as a combination of a Stroop and Simon task) (Wang et al., 2014). Furthermore, enhanced theta effects are observed in frontal regions and were associated with the medial frontal cortex or ACC (Cavanagh et al., 2011; Hanslmayr et al., 2008; Nigbur et al., 2011). It has also been demonstrated that increased theta function may be associated with improved cognitive control under stimulus or response conflict conditions (Hanslmayr et al., 2008; Nigbur et al., 2011; van Steenbergen et al., 2012). Moreover, it has been found that for some specific types of substance abuse disorders (e.g. alcoholism), individuals showed significantly diminished delta and theta power during NoGo trials as compared with controls. The most significant decline was observed in the frontal region, indicating an incapacity to regulate inhibitory processes and accurately process information.
According to Balconi and Finocchiaro (2016), alterations in the frontal alpha band of IAD participants may demonstrate a deficit in inhibitory control, evidenced by inhibition and disengagement of task-relevant cortical regions. It was demonstrated by Wang and Griskova-Bulanova (2018) that Internet addiction test (IAT) scores were positively correlated with alpha power calculated from an eyes-closed EEG recording. Moreover, both higher frequency bands (beta and gamma) are related to response inhibition. In people with IAD, Choi et al. (2013) discovered that their absolute beta band power was decreased, which had previously been associated with activity-related impulsivity in attention-deficit/hyperactivity disorder patients (Snyder & Hall, 2006). According to Son et al. (2015), the IGD group has lower absolute beta power than the alcohol use disorder group and the HC group. Other studies have demonstrated that beta oscillations are important for interference control. The asymmetry of beta frequency band activity in the prefrontal hemisphere during rest was found to be correlated with participants’ capability to regulate interference in both the verbal and spatial versions of the Stroop task, as demonstrated by Ambrosini and Vallesi (2017).
Further, Wang et al. (2014) found that in a combined Stroop and Simon task, compared to the stimulus-stimulus conflict condition, the beta band power change was significantly smaller for the stimulus-response conflict condition. There has been a suggestion that the beta band suppresses proponent responses due to top-down inhibition (Liang et al., 2014). According to intracranial recordings, the medial prefrontal cortex displays a higher level of alpha and beta activity desynchronization when the individual is in an incongruent condition (Tafuro et al., 2019). There was also an increase in the absolute strength of the gamma band within IA. Moreover, it has been found that changes in the gamma band are associated with impulsivity (Barry et al., 2010).
Several objectives are pursued in an EEG study that assess interference and inhibitory control deficits in participants with IAD. This study examined whether interference control is related to event-related spectral perturbations (ERSPs) and whether these spectral dynamics are altered in participants with IAD. Thus, two samples of participants were selected from the IAD and HC groups, and time-frequency analyses were conducted subsequently. The event-related potential method can provide information about the time course of different frequency bands associated with specific cognitive function tasks (Engel & Fries, 2010). Using this analysis, the changes in power across all frequencies examined, as well as changes in power levels in response to task events, can be extracted (extracting the ERSP), thus demonstrating that neural oscillations have a direct connection to a wide range of cognitive processes (Herrmann et al., 2016). We expected a general reduction in neural resource activation in the IAD group compared to the HC group, considering the differences in ERSP variables of cognitive control in the IAD group. Additionally, we expect HC participants to demonstrate reduced left-hemisphere prefrontal asymmetry associated with interference control and Stroop effect.
2. Materials and Methods
Participant selection
The participants were recruited from universities in Tehran, Iran, through an online advertisement between October 2021 and April 2022. Their contact information was provided during the online survey completion using the IAT questionnaire. Upon receiving greater than 70 IAT scores (Young, 2009), they were invited to the laboratory for an IAD interview by a psychiatrist to exclude emotional disorders such as anxiety and depression. In the following step, the IAD cases were matched with the remaining non-IAD cases to create the final pool of 50%-50% of IAD cases and no-IAD (HC) cases. The participants all possessed normal or corrected-to-normal vision and were right-handed. No neurological disorders, including mental illness and brain injuries, were present in their history. Those participants with a history of substance use, as well as those with a previous hospitalization for psychiatric disorders or a history of therapy for central nervous system disorders, were excluded from the study. On the day of the experiment, participants were instructed not to consume caffeine, nicotine, or any drugs/substances. A final sample enrolled in this experiment included 42 individuals: 21 IAD participants (9 females) and 21 HC participants (10 females) with no significant difference in their mean age (IAD: 23.50±4.78 years vs HC: 24.60±4.21 years). Informed consent was obtained from all participants under the Declaration of Helsinki. One female participant from the HC group was excluded from the experiment due to technical problems with the EEG recording.
Demographic characteristics of participants
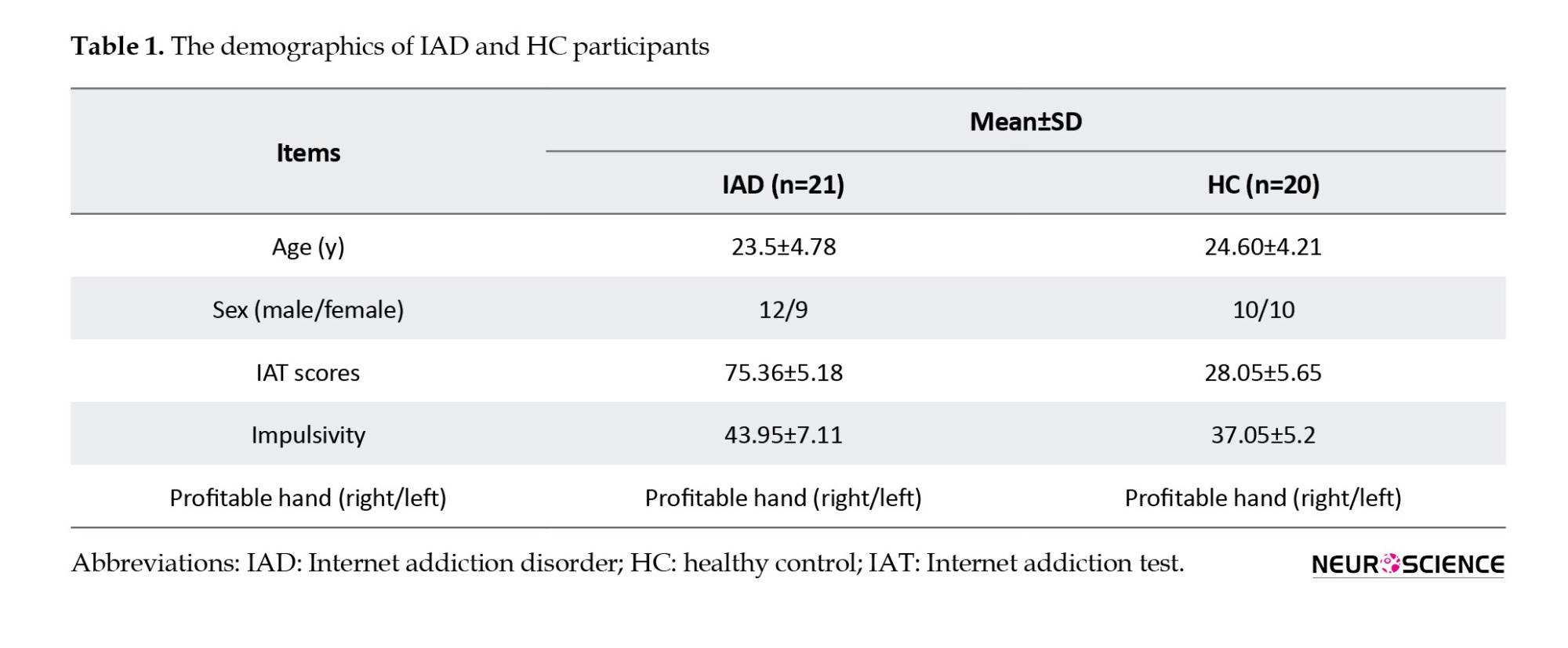
In addition to demographic data, e.g. age, gender, and education level, participants were asked to complete some questionnaires. The IAT questionnaire, designed by Kimberly Young (Alavi, 2010; Young, 2009), was used to assess the existence and level of internet addiction. The study included participants who spent at least 10 hours a day, 6 days a week, online and scored over 70 on the IAT. It was determined that HCs who used the internet less than 2 hours per day would be eligible for the study. Furthermore, HCs were evaluated with IAT criteria that were below 30. According to Table 1, specific information is provided for each sample. Anxiety scores were assessed by the Beck anxiety inventory (Beck et al., 1988; Kaviani & Mousavi, 2008), and only participants scoring less than 15 were chosen. Only participants with a depression score below 19 were included in the study, based on the Beck depression inventory (Beck et al., 1996; Ghassemzadeh et al., 2005). As part of the assessment of premeditation, sensation seeking, lack of perseverance, positive urgency, and negative urgency, the UPPS-P impulsivity score (Cyders et al., 2014; Jebraeili et al., 2019) was incorporated.
Experimental procedure
Stimuli and tasks
This study used the 4-choice Stroop task (288 trials with four equal runs) to investigate differences in interference control associated with internet addiction. In the Stroop task, participants are required to identify the color of the stimulus by pressing one of four keys on a computer keyboard (A=left middle finger, S=left index finger, G=right index finger, H=right middle finger). The response buttons on the keyboard are marked with color stickers (A=red, S=green, K=blue, L=yellow). Each trial displays one of the four colors (red, green, blue, and yellow). About 50% of color words are presented in congruent (same word color and meaning) trials, and 50% of color words are shown in incongruent (different word color and meaning) trials. Each run randomly displays 72 similar congruent and incongruent trials (Figure 1).
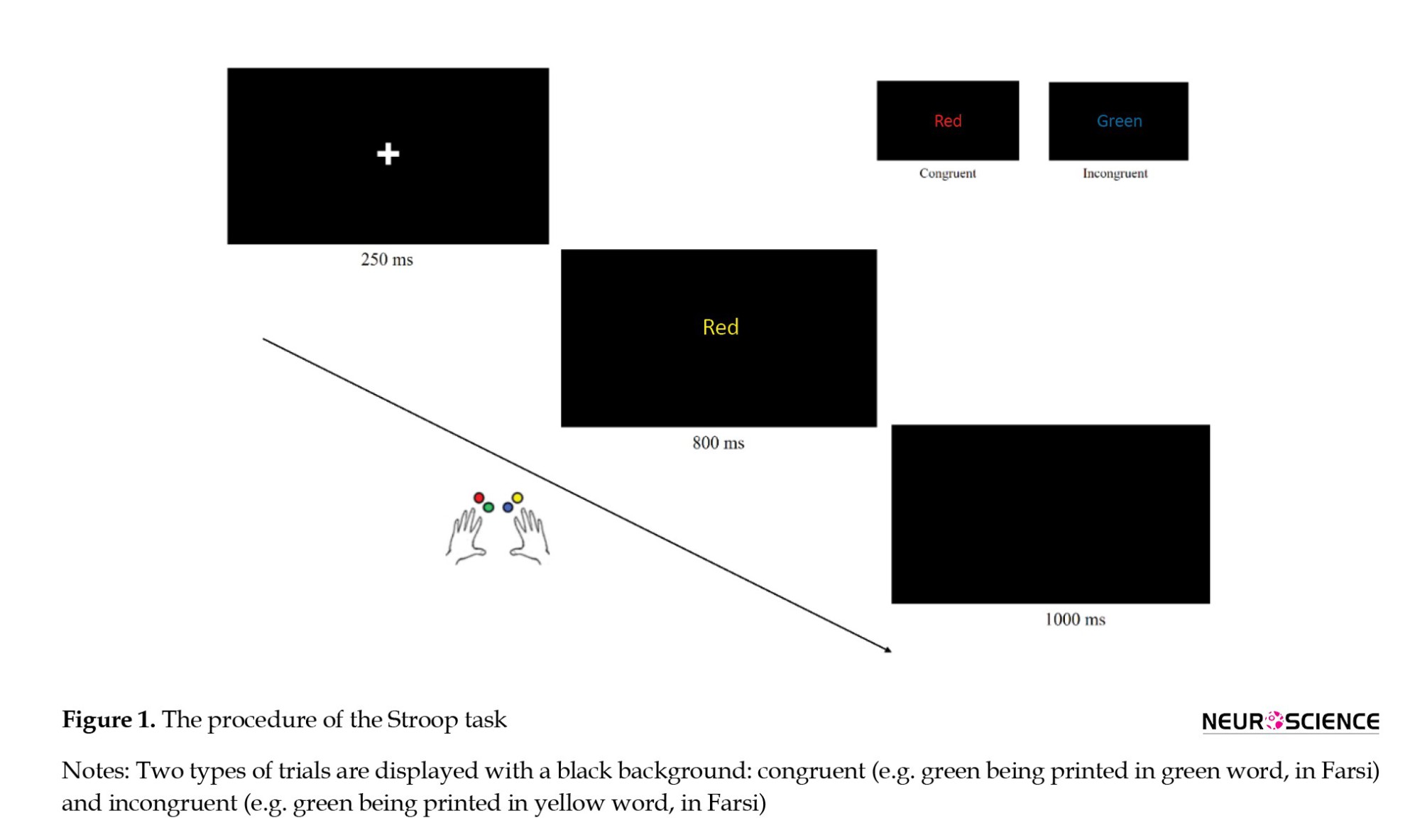
Before each block, participants were given instructions encompassing the number of runs and encouraging accurate and quick answers. A message on the screen urged the user to start running after pressing the space key. In the following step, a fixation cross was displayed on the monitor for 250 ms, a color word was displayed for 800 ms, and the response within 800 ms was recorded. Afterward, a black screen was shown for 1000 ms, and the subsequent trial began.
An electrically shielded room was used for this task, in which participants sat in chairs at a 60 cm distance from the viewing surface. A fixation cross was displayed on the screen at the center of the display before each stimulus. Before performing the main task, participants were trained on keyboard keys and the procedure. A training block comprises 16 trials. MATLAB’s psychophysics toolbox version 3 was used to perform the task.
Data acquisition and preprocessing
EEG was continuously recorded via a 64-channel g.tec g.HIamp (g.tec, Graz, Austria) with a 1200 Hz sampling rate and 10-10 international electrode placement system. The ground electrode was located on the forehead (Fpz), and the online reference electrode was located on the right mastoid. Impedance was maintained below 10 kΩ with all electrodes being passive.
Raw EEG data were imported to EEGLAB version 2021.0 (Delorme & Makeig, 2004) for standard preprocessing and analysis. An FIR Butterworth band pass filter was used to filter the EEG data between 0.5 and 40 Hz. After that, based on visual inspection, muscular and ocular artifacts trials were removed. Noisy channels were discarded subsequently. After that, independent component analysis (ICA) decomposition was done under the ICA calculation method by runica to remove non-brain sources from the EEG data (non-brain sources such as muscle contractions, eye blinks, gaze movements, and electromyography) (Jung et al., 2001; Makeig et al., 1997). Spherical interpolation methods replaced removed channels with weighted averages of adjacent channels. The clean EEG data were resampled to 256 Hz. Using this method, the results were extracted by epochs starting at 200 ms before stimulus onset and ending at 1500 ms after that. Epochs with amplitude exceeding ±100 μV were eliminated as an artifact from subsequent analyses.
ERSP analysis
A Morlet wavelet was employed to extract ERSP with linear separation between 2 and 40 Hz. Wavelet’s time window resolution is 500 ms, and the cycle number increases linearly from 2 to 20 cycles, corresponding to 2 and 40 Hz frequencies, respectively. The average power between -200 and 0 ms for each trial was used to perform baseline correction. This method enables us to capture all 128 channels and 107 time points from 100 ms up to 1500 ms throughout the channel-time-frequency domain. Through this method, edge artifacts caused by wavelet transforms are omitted, and stimuli-related activity is overlapped over trials. Approximately 15 ms of resolution are available, and a frequency range of 2 to 40 Hz is available.
In this study, we focused on power changes in the theta (4 to 7 Hz), alpha (8 to 12 Hz), beta (13 to 30 Hz), and gamma (31 to 40 Hz) frequency bands. A frequency band has been divided according to previous research (Hanslmayr et al., 2008; Nigbur et al., 2011). It was found that the beta frequency could be separated into two segments, with frequencies ranging from 13 to 19 Hz (beta1) and 20 to 30 Hz (beta2) (Engel & Fries, 2010). We averaged the time-frequency power estimates across trials for each participant, each condition, each block, each region, and within theta, alpha, beta1, beta2, and gamma frequency bands after obtaining wavelet representations of individual trials. The subject-averaged power changes were measured in the post-stimulus interval compared to the baseline (-200 to 0 ms). Compared with the event-related potential method, this approach provides information regarding the time course of various frequency bands’ involvement in specific cognitive tasks (Engel & Fries, 2010). By analyzing neural oscillations through this analysis, we can extract information regarding the power fluctuations at each frequency examined and their variations during time-based on the event at hand (extracting the ERSP), which allows us to identify cognitive processes associated with neural oscillations.
Statistical analysis
Behavioral data were analyzed using R (version 3.6.1, R Core Team, 2019) within the RStudio environment (version 1.2.5001). The Mean±SD for numerical variables were calculated when they were normally distributed, and the range was calculated for variables that were not normally distributed. A 2-sided significance threshold of less than 0.05 indicates statistical significance. A partial eta-squared (ηp2) was used to measure effect size, which represents the proportion of variance associated with an effect and the error variance associated with that effect. All post hoc analyses were corrected for type I errors by applying the Bonferroni correction.
The cluster-based random permutation test was also used to examine the differences in power between the two groups by comparing power amplitudes across trials within and between the two groups. (Maris & Oostenveld, 2007). The uncorrected P were determined by performing a straightforward permutation t test on each data point (for the wavelet data, electrode by frequency by time). Statistical power was increased by averaging the pre-selected frequencies (theta, alpha, beta 1, beta 2, and gamma) and time intervals (0 to 0.2 s) for each electrode in the wavelet analysis. This choice was made based on consistent patterns across all two groups and comparisons between groups. Then, neighboring data points that exceeded a predetermined significance level (5%) were grouped to form clusters. We added the t values within each cluster to compute cluster-level statistics, then constructed a null distribution assuming no differences between the groups. A histogram of the test statistics was constructed after 1000 permutations of the randomly assigned groups within participants. Based on the observed test statistic and the histogram, the proportion of random partitions resulting in a larger test statistic than the observed one was calculated. The Monte Carlo significance probability is defined as this proportion. In the case of significant differences between the two experimental conditions, the significance probability should be under the critical level (typically 0.05). It is also possible to calculate the significance probability for the second-most significant cluster-level statistic, the third-most significant statistic, etc. Using the Monte Carlo method is the only way to calculate the significance probability. This is because the Monte Carlo method can approximate (to any level of accuracy) the reference distribution for a permutation test. A significant cluster was determined by comparing the cluster-level test statistics to the null distribution, where clusters above or below the 2.5 percentile were considered significant. This method determined whether the ERSP Stroop effect for participants in HCs and IADs was significant by considering the congruency factor (incongruent versus congruent Stroop conditions). After comparing the ERSP Stroop effect between individuals with HC and those with IAD, we assessed whether internet addiction significantly modulated the impact. It was therefore examined whether there was an interaction between IAD and HC groups under congruent and incongruent conditions.
3. Results
Behavioral data
Demographic characteristics of the participants
Demographic results related to the IAT score and the scores derived from the questionnaires are presented in Table 1. Based on linear regression analysis, a significant positive linear relationship existed between the IAT and total impulsivity score (beta=1.6993, P<0.001).
Mean reaction times
In the mean reaction times (RTs) analysis, trials with incorrect responses and more or less than mean-RT±2SD were removed. It was found that the group had a significant effect on mean RT based on the two-way ANOVA (F(1, 140)=17.74, P=4.51e-05, ηp2=0.08843) due to considerably longer mean RTs in IADs than HCs (Figure 2). The main effect of congruity revealed that all participants experienced longer mean-RT in incongruent trials compared to congruent trials (F(1, 140)=46.76, P=2.29e-10, ηp2=0.22744).
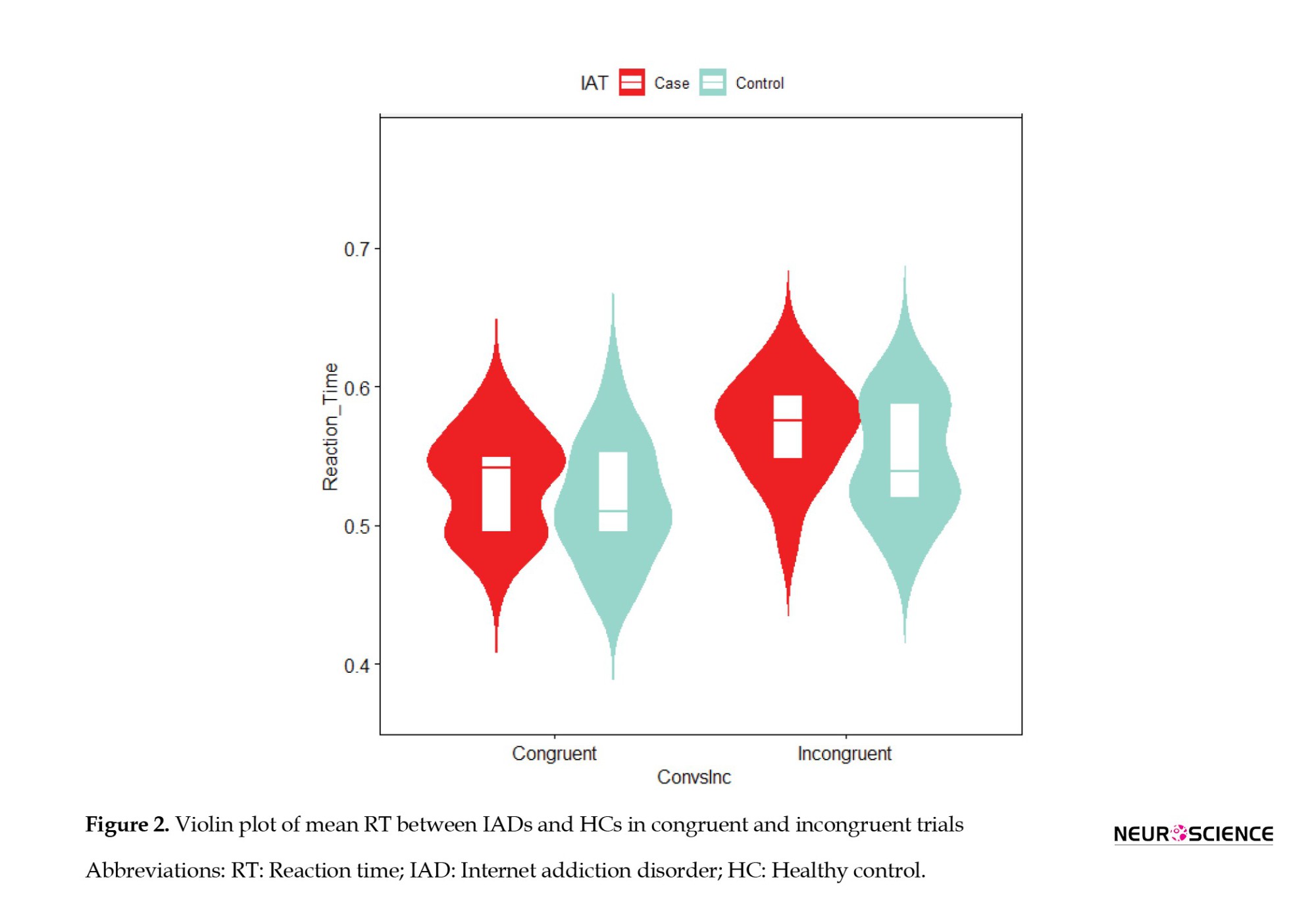
In the subgroup analysis, it was found that the group had a significant effect on congruent trials (F(1, 69)=5.524, P=0.0216, ηp2=0.07412), with IADs having the longest mean reaction times and HCs having the shortest. A significant difference was also observed between the incongruent trials (F(1, 69)=12.94, P=0.0006, ηp2=0.15744), as IADs had longer mean RTs than HCs. Congruent and incongruent trials were significantly different in IADs (F (1, 69)=31.491, P=3.87e-07, ηp2=0.31337) and in HCs (F(1, 69)=16.772, P=0.000113, ηp2=0.19554).
We also used the IES criterion (dividing the mean-RT by the accuracy [1–the proportion of errors]) to investigate the error rate between different conditions. The IES indicates a combination of speed and accuracy in response. Using a 2-way ANOVA, a significant group effect was found to be present (F(1, 140)=10.73, P=0.00133, ηp2=0.05875) due to the higher IES in IADs compared to HCs. The Wilcoxon rank-sum test showed a significant main effect of groups (Z=509, P=0.004852), with a moderate effect size (r=0.363) in incongruent trials, based on a greater error rate and mean reaction time of IADs than HCs in incongruent trials.
Scalp-based time-frequency analysis
Figure 3 shows the HC (Figure 3A) and IAD (Figure 3B) groups’ event-related spectral perturbation analyses. ERSP Stroop effects were found in the IAD group, specifically in the left hemisphere, but less specific to frequencies. As a result, a major cluster with significant ERSP Stroop effects was observed over most left frontal and left fronto-central channels within 200 and 800 ms. The same effect was seen in the frontal, fronto-central, and central channels within about 200 to 400 ms. An 800 to 1000 ms time window also showed this effect in beta1, beta2, and gamma frequency bands (Figure 3B). The left posterior channels also showed a second cluster of alpha, beta1, beta2, and gamma signals in time windows ranging between 200 and 400 ms and 600 and 1000 ms, respectively. IAD showed these effects due to a greater event-related power decrease for the incongruent condition than for the congruent condition.
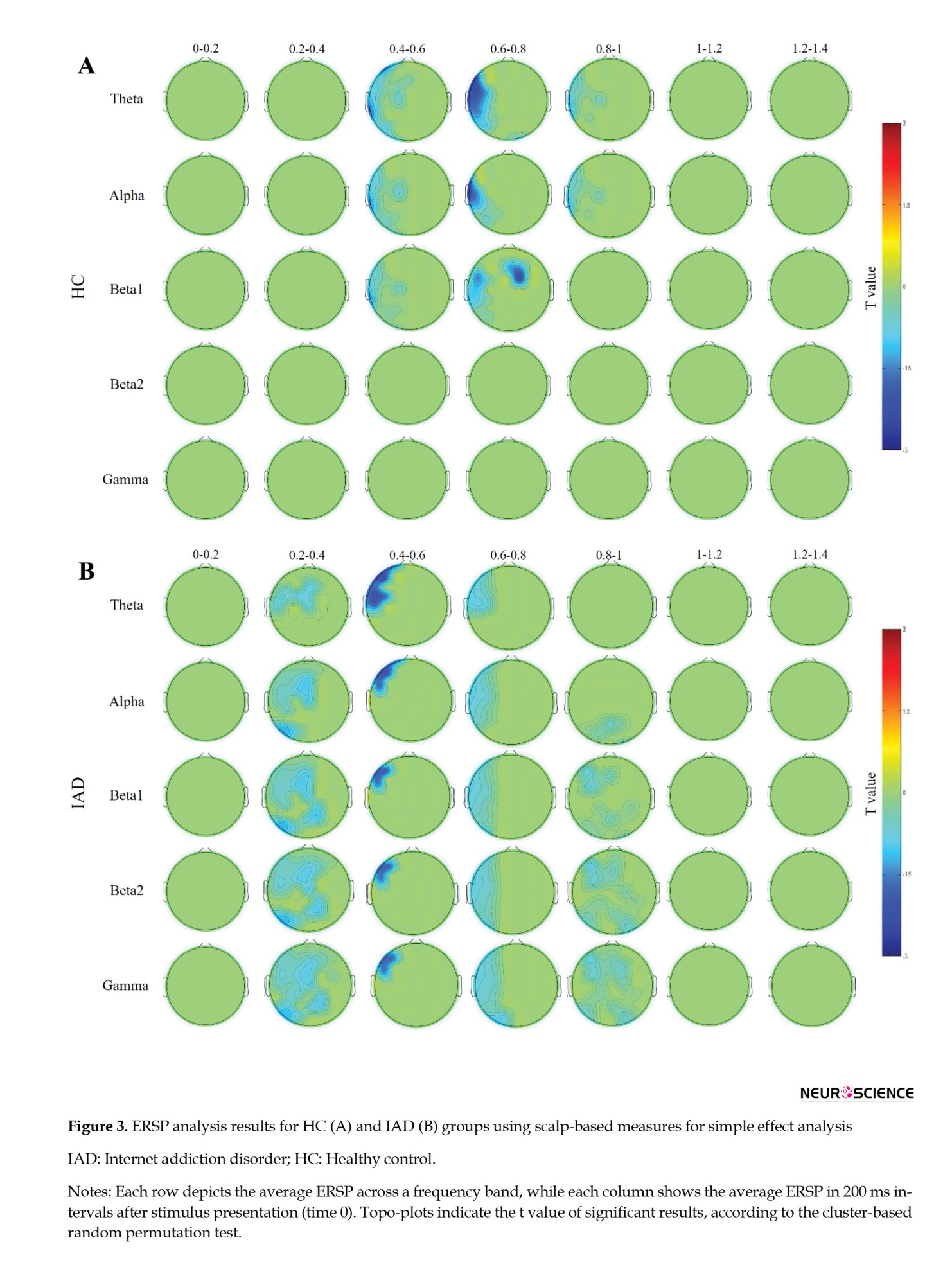
Based on the analysis of the HC group, several ERSP Stroop effects were identified, most of which affected the left hemisphere in the early time windows (Figure 3A). ERSP effects of Stroop were detected in the first prominent cluster involving theta, alpha, and beta1 frequencies over the frontal, fronto-central, and central channels with high left lateralization during a period ranging from 400 to 600 ms. As a first cluster, theta, alpha, and beta1 bands were also detected in the central, centroparietal, and parietal channels between 600 and 800 ms. Theta and alpha bands were observed over the fronto-central, central, and centro-parietal regions between 800 and 1000 ms as part of this cluster. A second cluster of beta1 bands occurred over the frontal and fronto-central channels during 600 to 800 ms.
A big cluster in the IAD group with ERSP Stroop effects at theta, alpha, beta, and gamma frequencies over electrodes spread across the left frontal region in a 200 to 1000 ms time window, including earlier samples. It was found that IAD participants experienced lower ERSP Stroop effects than participants in the HC group as a result of these effects. IAD participants showed a greater event-related power decline in the incongruent condition than HC participants since the weaker event-related power decreases were more visible on the left frontal electrodes under the incongruent condition. Additionally, an earlier time window ranged roughly from 200 to 400 ms and 600 to 1000 ms, showing a cluster of alpha, beta, and gamma frequencies over the left posterior channels (Figure 3B). In the congruent condition, the power increased more than in the incongruent condition in IAD compared to HC because there was a greater power increase for all frequency bands except theta.
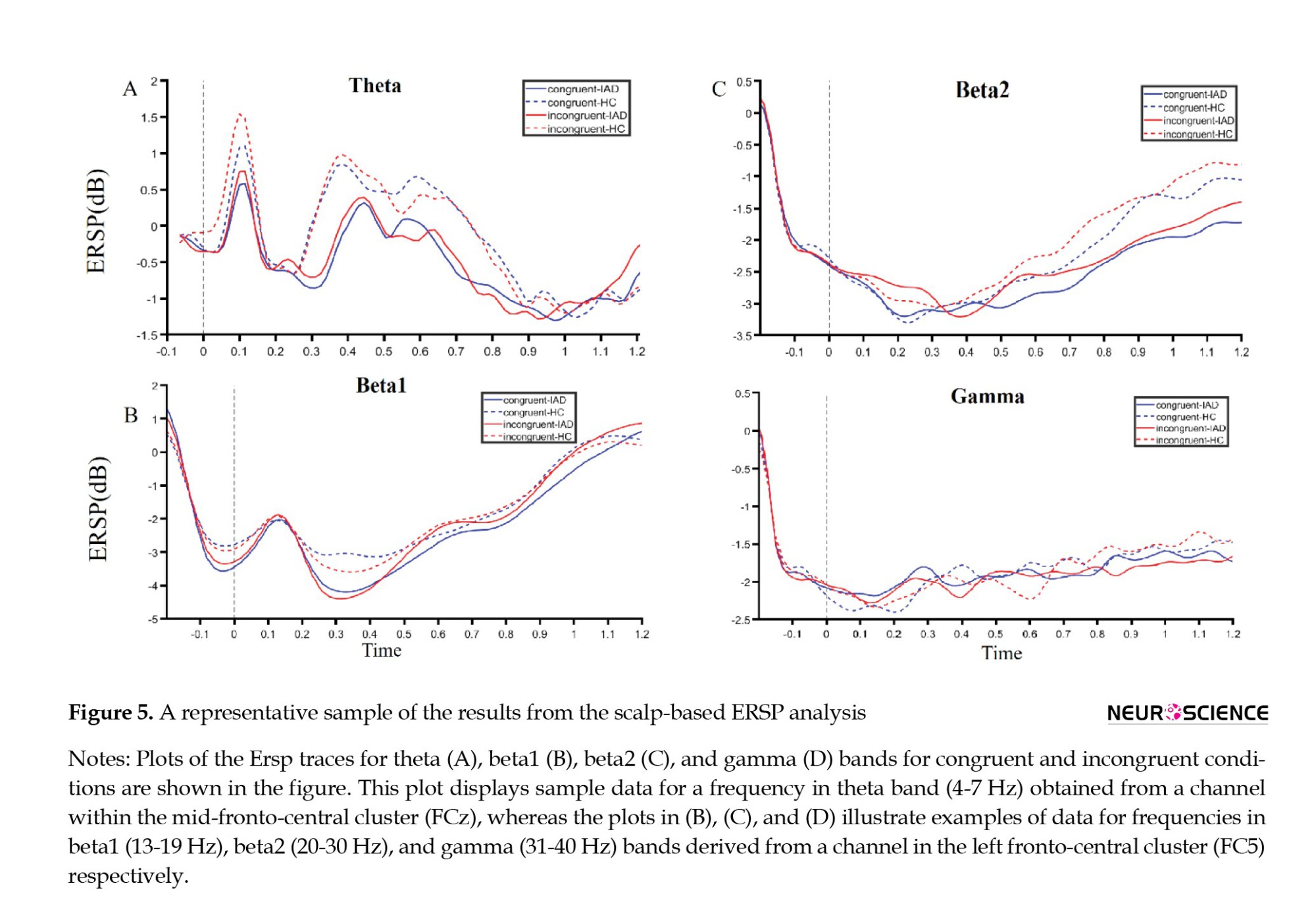
We also used scalp-based ERSP analysis to illustrate the interaction between the HC and IAD groups under congruent and incongruent conditions. The interaction analysis between HC and IAD groups in the congruent trials elicited several significant ERSP group effects, mostly involving theta band over frontal, fronto-central, centro-parietal, and parietal regions in relatively early time windows (Figures 4A and 5A). Over the entire left frontal hemisphere, a cluster showed significant ERSP group effects in theta from 400 to 850 ms. This cluster also included the ERSP group effects of the alpha band over left frontal and left fronto-central channels during temporal intervals of 400 to 800 ms. In a temporal window of 400 to 600 ms, we found a second cluster involving theta bands across the centro-parietal and parietal channels (Figures 4A and 5A).
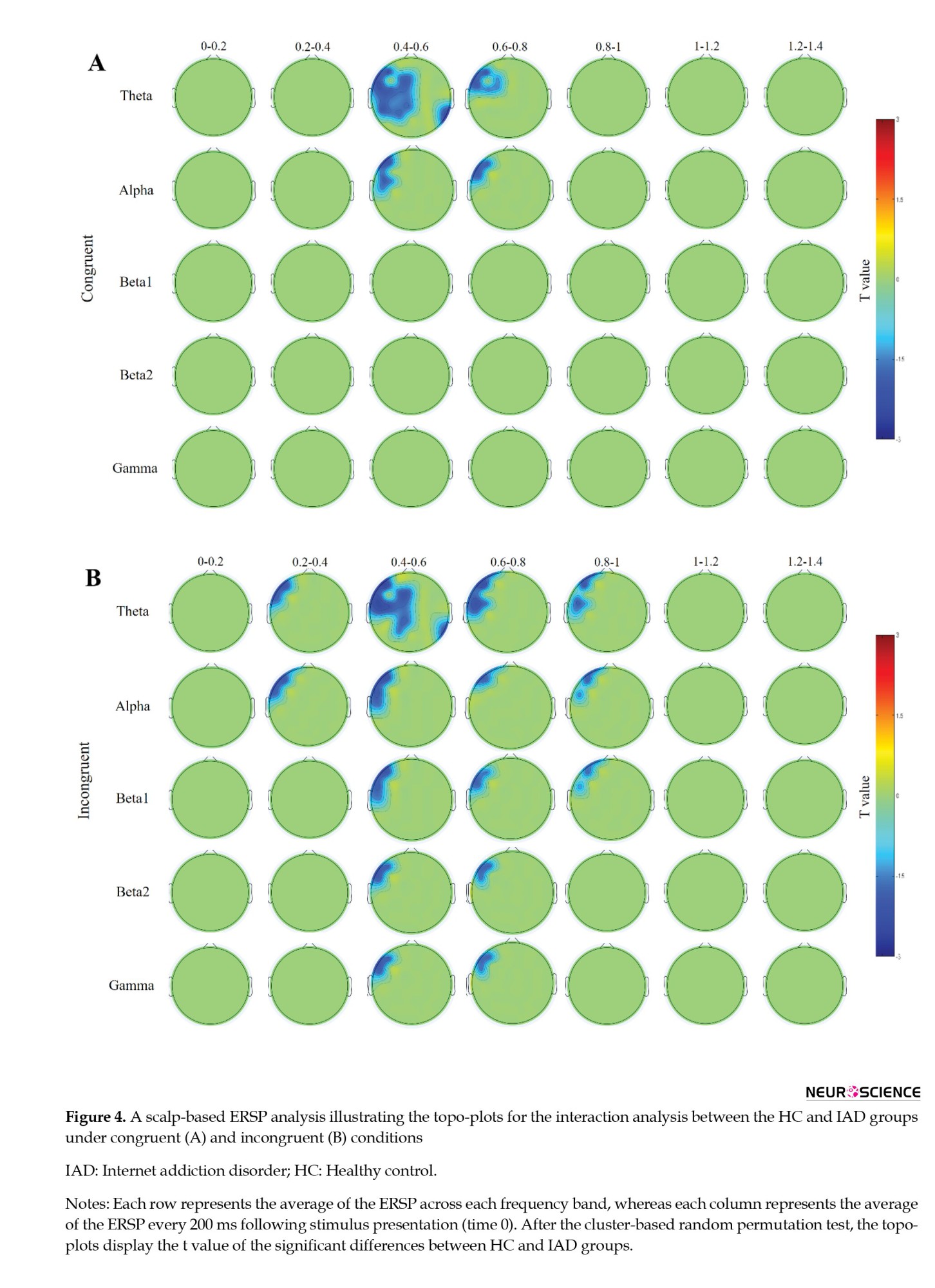
Interaction analysis between HC and IAD groups in the incongruent condition revealed a main significant ERSP group effect, mainly affecting theta and alpha bands over left frontal, frontocentral, and central channels, but a less clear interaction effect over time (Figures 4B and 5A). The first cluster of ERSP group effects was detected over the left frontal and fronto-central regions at a window between 200 and 1000 ms. Additionally, it was observed in the beta1 band during 400 and 1000 ms (Figures 4B and 5B), as well as beta2 and gamma frequencies in the period between 400 and 800 ms (Figures 4B and 5C, D). However, this effect was less time- and frequency-specific. Furthermore, we recognized a second cluster that involved a theta band over frontal, fronto-central, central, and centro-parietal regions with no lateralization in a temporal window roughly spanning 400 to 600 ms (Figure 4B). The IAD group experienced a greater event-related decline in power than the HC group, causing all these effects.
4. Discussion
In our study, interference control spectral correlates were compared between IAD and HC participants, and differences were found. For this purpose, EEG activity was recorded from IAD and HC groups during the color-word Stroop task, and the time-frequency dynamics were analyzed to estimate the underlying neural activity. Further, we were interested in examining whether the left portion of the PFC is involved in this cognitive process. Results indicated that participants with IAD displayed diminished conflict detection and response selection abilities against HCs, as measured by theta band indices. Furthermore, IAD participants displayed diminished conflict detection and resolution compared to HC participants, as revealed by damage interaction dynamics between beta2 and gamma bands in the fronto-centro-temporal regions.
Our behavioral findings showed that all participants had longer RTs and made more errors when their trials were congruent compared to incongruent, which is consistent with Stroop-related interference in response time and error rate. Individuals with IAD had a greater IES and reaction time than HCs; however, there was no difference in overall error rate between the two groups. It follows previous studies that reported significant differences in mean reaction times when using the computerized Stroop task (Dong et al., 2011). Following these results, studies (Ko et al., 2014; Dong et al., 2011) have noted impaired inhibitory control in individuals with IAD when performing the Go/NoGo and Stroop tasks. There is growing evidence suggesting that internet addiction may be caused by a deficit in inhibitory control (Kräplin et al., 2021; Wegmann et al., 2020). There has also been evidence of similar results regarding behavioral addiction as well as substance abuse in prior studies (Choi et al., 2014; Dong et al., 2010). We also found that participants who suffer from IAD demonstrate higher impulsivity and decreased inhibition, as evidenced by the elevated UPPS-P score and lower IES score. This finding is consistent with a previous study in which the development of IAD is positively correlated with impulsivity scores (Khanbabaei et al., 2022). Thus, IAD occurrence is associated with impulsive personality traits and impaired cognitive functioning.
Our electrophysiological results indicate significant ERSP Stroop effects at theta, alpha, beta, and gamma frequencies over the left frontal region in IADs. Most likely, IADs experienced a more significant event-related loss of power at left frontal electrodes when subjected to the incongruent condition than HCs and more frequencies were used over a greater number of channels at an earlier and more sustained period (Tang et al., 2013). According to these findings, there is a group-dependent modulation of ERSP Stroop effects. Compared to HCs, IADs showed spectral indicators of interference control earlier, wider, and more unspecific.
Compared with HC participants, the IAD group experienced decreased EEG power at frequencies ranging from alpha to gamma within a time window spanning 200 to 1000 ms. Recent research suggests that excessive internet use has the greatest impact on the gamma oscillation of the parietal, central region at 300 ms after stimuli at 40-50 Hz (Yu et al., 2009). These findings are consistent with our findings that high-band EEG power decreased earlier for IADs than HCs. Gamma synchrony is believed to be a neurophysiological process that regulates information coding and integration in the brain (Fries et al., 2007). Thus, excessive internet use impacts the processing and integration of information in the brain.
Stroop effects were observed as a result of ERSP. According to Figure 3, participants in HC primarily participated in slow-wave frequencies, with Stroop-dependent modulations occurring around the time of the response on the left hemisphere and eventually spreading over to the left frontal region when the window closes to the response. In the IAD group, individuals employed all frequency bands (from theta to gamma) primarily, and this activity was initiated shortly after stimulus presentation, engaging electrodes spread across the left scalp. Following this perspective, the IAD participants showed lower EEG power during the early time window close to stimulus presentation, reflecting impairments in executive function. In other words, they could not successfully inhibit their brain activation for stimulus conflict detection.
A general integrative role has been demonstrated for theta band activity in regulating brain activity when considered in light of spectral dynamics for cognitive tasks (Tafuro et al., 2019). Theta band oscillations are a common indicator of conflict resolution and interference control, demonstrating the requirement for cognitive control (Cohen, 2014). It has previously been reported that in incongruent conditions, theta band responses are enhanced in the frontal and fronto-central areas (Qi et al., 2022; Tang et al., 2013). The incongruent trials of the Stroop task were reported to display extended phase coupling between the ACC and the left prefrontal cortex (Hanslmayr et al., 2008). Theta amplitude during incongruent trials was proposed to be sensitive to conflict detection and response selection in a study conducted by Qi et al. (2022). In a study on the Stroop task, there was no increase in event-related theta magnitude when participants looked at stimuli with no response, suggesting a possible link between response inhibition and theta response (Tang et al., 2013). In light of these results, we anticipate finding evidence of theta band involvement in the early temporal window, which would be accompanied by decreased theta power in the left frontal and left frontocentral regions for the incongruent condition. Our study found that the IAD group recruited theta band at a lower level during an earlier time frame than the HC group in the left frontal and left fronto-central channels. In other words, theta power decreased significantly more for IADs than HCs under incongruent conditions in an earlier time window. Considering that event-related theta power plays an essential role in conflict detection and response selection, it is likely that conflict detection capability was diminished sooner in IADs following stimulus presentation than in HC groups.
Compared with HCs, IAD participants showed an overrepresentation of beta2 and gamma bands. As a result of a comprehensive ERSP analysis, it was found that IAD participants displayed a significant ERSP Stroop effect at beta2 and gamma frequencies when compared to HC participants. It was found that bilateral dorsal frontal and parietal cortex areas were involved during time windows between 200 and 400 ms and 800 and 1000 ms without evidence of hemispheric asymmetry during these behavior patterns. Our findings are consistent with other studies showing that patients with IGD do not show dynamic interactions between the fronto-centro-temporal regions at rest or when performing cognitive tasks (Park et al., 2021). In the case of IADs, high-frequency bands are likely involved in conflict detection and resolution during the early (200 to 400 ms) and late (800 to 1000 ms) periods. Consequently, IADs need additional resources to resolve conflict detection and resolution issues.
We concluded that interference control processes are modulated group-dependent based on the contrast analysis between the HC and IAD groups (Figure 4). IADs showed a stronger correlation in spectral behavior earlier and were more widely spread. Within 200 to 1000 ms time windows, several significant group-dependent modulations of ERSP across central and frontal regions existed. Our analysis of the ERSP on IAD and HC participants under congruent and incongruent conditions indicates that the theta band exhibits the greatest contrast between the two groups. Previous research has demonstrated that changes in theta band are associated with conflict processing. In congruent and incongruent conditions, theta band activity is suppressed earlier (200 ms after stimulus presentation) since IADs are less sensitive to conflict stimulus detection than HCs. It was also found that IADs experienced significant ERSP effects on beta2 and gamma frequencies compared to HCs in several electrodes situated throughout the left frontal and left fronto-central regions during a time window of mainly 600 to 800 ms under incongruent conditions. However, these ERSP effects were not observed when congruent conditions were present. The pattern of results indicates that incongruent conditions led to contrast changes in the high-frequency bands, which are associated with conflict detection and resolution. In other words, the ability of IADs to detect and resolve conflict is diminished in incongruent conditions compared to HC participants.
Although motor processes cannot be ruled out as a possible contributor to our findings regarding theta and beta1 bands, we cannot exclude the possibility that they might be more than simple correlates of motor outcomes. Our first finding was that power reduction was primarily located in the left hemisphere despite the task being bimanual. In addition, while we did not conduct response-locked ERSP analyses, we did not observe a correlation between the event-related decline in power at high frequencies and reaction times. It is important to note that the maximum decreases in theta and beta1 power occurred around the same time in both groups and conditions, even though IAD participants’ RTs were slower in both conditions, particularly in the incongruent condition. A third issue is that even if we consider that the differences between two groups in the Stroop effects are attributed to the reduction of RTs shown by IADs, this does not clarify the over-recruitment observed in both spatial and spectral regions due to group differences.
We believe this study is one of the first to use cluster-based random permutation tests to investigate oscillatory dynamics in conflict processing for IAD groups. As a result of our research, we found that in participants with IAD, neural oscillations differed primarily in relation to the impairment of theta-related cognitive processes, as well as beta2 and gamma-related cognitive processes. We could differentiate multiple processes with partially or completely overlapping frequency features or timescales using a wavelet transform. This decomposition results from a decomposition of the oscillatory activity across different frequency bands. These oscillatory responses reflect the interactions between neuronal networks operating on different timescales or frequencies during task performance. It should also be noted that this study has some limitations. The first limitation of the study was that we could not compare IADs with substance use disorders (SUD), as well as other behavioral addiction disorders, such as gambling disorders. Additionally, we used general stimuli during the Stroop task, which may be extended in future studies by using different tasks to identify various types of conflicts between stimulus and response. Moreover, EEG analysis at the sensor level is poor in spatial resolution and does not reveal the source of brain activity. Research into the localization of EEG sources and fMRI with high spatial resolution could effectively answer these questions in the future.
5. Conclusion
This study used Stroop tasks to examine the potential time-frequency dynamics of EEG activity and interference control and inhibitory control impairments in IAD. These findings suggest that neural oscillations differ primarily in relation to impairments in cognitive processes related to theta as well as beta2 and gamma. Based on our findings, participants with IAD displayed reduced conflict detection and response selection compared to HCs, as measured by theta band indices. In addition, they also displayed diminished conflict resolution, as demonstrated by altered interaction dynamics across beta2 and gamma bands. In consequence, this can facilitate our understanding of the neural mechanisms responsible for interference control and inhibitory control deficits in IAD, as well as suggest future interventions that might be effective. By understanding these mechanisms, it would be possible to develop neuromodulation therapies that would benefit the clinical patient and reduce cognitive deficits in people with IAD.
Ethical Considerations
Compliance with ethical guidelines
All research procedures involving human participants followed the ethical standards established by the Institutional Research Committee in accordance with the 1964 Helsinki Declaration. This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (Code: IR.SBMU.MSP.REC.1397.638). Each participant in the study provided informed written consent to participate.
Funding
The study was partially supported by a grant from Shahid Beheshti University of Medical Sciences (Grant No.: 16674). This article was extracted from the PhD dissertation of Farzad Rostami, approved by the Department of Biomedical Engineering and Medical Physics, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran. (Code: 15921).
Authors' contributions
Conceptualization, methodology and validation: All authors; Data acquisition, investigation, validation, visualization, and writing the original draft: Farzad Rostami; Software, data analysis, review, and editing: Atiye Sarabi-Jamab and Farzad Rostami; Project administration, and resources: Ali Esteki; Supervision: Atiye Sarabi-Jamab and Ali Esteki.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are very grateful for the collaboration of the National Brain Mapping Lab (NBML), Tehran, Iran, for providing data recording services.
References
Nowadays, we are facing a new type of behavioral addiction named “internet addiction,” a significant issue of the information age. Since the development of cyberspace, the internet has been regarded as one of the most critical drivers of economic progress and social change (Barry et al., 2010; Omoyemiju & Popoola, 2021). However, with widespread access to the internet, as well as the occurrence of global challenges like COVID-19, people are heavily urged to use cyberspace, which in some cases turns into addictive behavior (King et al., 2011; Ozturk & Ayaz-Alkaya, 2021).
Previously, this newly defined addictive behavior was known as internet addiction disorder (IAD) or, in its specific form, internet gaming disorder (IGD) in the international classification of diseases (ICD-11) and the diagnostic and statistical manual for mental disorders (DSM-5).
IAD is a psychological and social disorder distinguished by the emergence of tolerance, symptoms of withdrawal, emotional disturbances, and the loss of social relationships (Gioia et al., 2021; West et al., 2005). Like other forms of behavioral addiction, e.g. gaming and social networks, as well as chemical addictions (e.g. alcohol and cocaine), IAD causes serious impairment in psychological and social functioning (Király et al., 2015; Park et al., 2020; Reed et al., 2019).
According to an increasing body of evidence, IAD may result in alternations in various cognitive functions, including perception (Pontes et al., 2015), attention (Gao et al., 2021; Heuer et al., 2021), memory (Zhou et al., 2016), and executive functions (Ioannidis et al., 2019; Zhou et al., 2016). Specifically, IADs are reported to lack the ability to perform inhibitory control, interference resolution, error processing, and decision-making (Park et al., 2020; Sun et al., 2009). Inhibitory control dysfunction has been suggested as an important feature of IAD (Antons & Matthias, 2020).
To cultivate inhibition control, people must learn to manage their focus, actions, thoughts, and feelings to overcome powerful internal or external impulses and choose the suitable or obligatory course of action. Comparatively, to the HC group, studies in the IAD group have generally reported reduced response-inhibition efficiency (Brand et al., 2016; Dong et al., 2011). Also, a few studies have shown a correlation between a decrease in inhibitory control and an increase in gaming time in cases of IGD (Kräplin et al., 2021). In accordance with the conflict monitoring theory, these impairments lead to a conflict between the stimulus presentation and the response display (Lustig et al., 2001).
The conflict monitoring method consists of a group of tasks that provide the possibility to investigate and monitor the interaction between incompatible responses (e.g. the simultaneous activation of incorrect and correct responses or the simultaneous activation of two rivalrous stimuli types) (Botvinick et al., 2004). The color-word Stroop task (Stroop, 1935) is considered one of the most widely known conflict monitoring tasks (Schmidt, 2019); participants are instructed to recognize the ink color of the stimulus based on whether the color word is congruent (e.g. yellow in yellow) or incongruent (e.g. yellow in green). Incongruent trials are generally associated with higher response times and error rates than congruent trials (MacLeod, 1991) due to Stroop’s interference effect, which arises from the different levels of conflict experienced in incongruent trials compared to congruent trials. Significant cognitive control is exhibited in response to highly conflictive stimuli, leading to decreased interference in subsequent high-conflict trials (Diamond, 2013).
The use of imaging methods such as functional magnetic resonance imaging (fMRI) and electroencephalography (EEG) showed that individuals with IAD have difficulties with their ability to control inhibitions and interference (Liu et al., 2016; Zhu et al., 2015). For example, Dong et al., (2010) observed that individuals with IAD exhibited disruptions in activity and connectivity within their prefrontal lobes, responsible for inhibitory control. Similar conclusions were drawn based on EEG studies (Ioannidis et al., 2019; Luijten et al., 2014). Compared to HCs, IAD individuals demonstrated altered amplitudes for the N2 and P3 components when detecting conflicts, suggesting that they are less capable of monitoring conflict and require greater attention when confronted with inhibition tasks (Dong et al., 2010). It has been found that individuals with IGD have a longer NoGo-N2 latency than their counterparts without IGD, possibly due to impaired response inhibition in individuals with IGD (Park et al., 2021). A study employing the Go/NoGo task to identify the source of activity found that individuals with IGD displayed reduced neural activity in the anterior cingulate cortex (ACC) and defective error processing, resulting in inadequate inhibition and decision-making abilities (Park et al., 2020).
There have been various studies conducted to examine the correlation between brain oscillations and addiction (Balconi & Finocchiaro, 2015; Balconi et al., 2015). According to previous studies, delta band responses are involved in signal detection and decision-making (Donoghue et al., 2022; Harmony, 2013), while theta functions are primarily concerned with inhibitory processes (Harper et al., 2014; Son et al., 2015). Earlier research has demonstrated that alterations in power in the theta frequency band are linked to cognitive conflict processes, with a marked rise in power observed in incongruent trials compared to congruent trials (Brittain et al., 2012; Tang et al., 2013). The results also show that theta power changes in relation to stimulus and response conflict in the Flanker task (Nigbur et al., 2012) (as well as a combination of a Stroop and Simon task) (Wang et al., 2014). Furthermore, enhanced theta effects are observed in frontal regions and were associated with the medial frontal cortex or ACC (Cavanagh et al., 2011; Hanslmayr et al., 2008; Nigbur et al., 2011). It has also been demonstrated that increased theta function may be associated with improved cognitive control under stimulus or response conflict conditions (Hanslmayr et al., 2008; Nigbur et al., 2011; van Steenbergen et al., 2012). Moreover, it has been found that for some specific types of substance abuse disorders (e.g. alcoholism), individuals showed significantly diminished delta and theta power during NoGo trials as compared with controls. The most significant decline was observed in the frontal region, indicating an incapacity to regulate inhibitory processes and accurately process information.
According to Balconi and Finocchiaro (2016), alterations in the frontal alpha band of IAD participants may demonstrate a deficit in inhibitory control, evidenced by inhibition and disengagement of task-relevant cortical regions. It was demonstrated by Wang and Griskova-Bulanova (2018) that Internet addiction test (IAT) scores were positively correlated with alpha power calculated from an eyes-closed EEG recording. Moreover, both higher frequency bands (beta and gamma) are related to response inhibition. In people with IAD, Choi et al. (2013) discovered that their absolute beta band power was decreased, which had previously been associated with activity-related impulsivity in attention-deficit/hyperactivity disorder patients (Snyder & Hall, 2006). According to Son et al. (2015), the IGD group has lower absolute beta power than the alcohol use disorder group and the HC group. Other studies have demonstrated that beta oscillations are important for interference control. The asymmetry of beta frequency band activity in the prefrontal hemisphere during rest was found to be correlated with participants’ capability to regulate interference in both the verbal and spatial versions of the Stroop task, as demonstrated by Ambrosini and Vallesi (2017).
Further, Wang et al. (2014) found that in a combined Stroop and Simon task, compared to the stimulus-stimulus conflict condition, the beta band power change was significantly smaller for the stimulus-response conflict condition. There has been a suggestion that the beta band suppresses proponent responses due to top-down inhibition (Liang et al., 2014). According to intracranial recordings, the medial prefrontal cortex displays a higher level of alpha and beta activity desynchronization when the individual is in an incongruent condition (Tafuro et al., 2019). There was also an increase in the absolute strength of the gamma band within IA. Moreover, it has been found that changes in the gamma band are associated with impulsivity (Barry et al., 2010).
Several objectives are pursued in an EEG study that assess interference and inhibitory control deficits in participants with IAD. This study examined whether interference control is related to event-related spectral perturbations (ERSPs) and whether these spectral dynamics are altered in participants with IAD. Thus, two samples of participants were selected from the IAD and HC groups, and time-frequency analyses were conducted subsequently. The event-related potential method can provide information about the time course of different frequency bands associated with specific cognitive function tasks (Engel & Fries, 2010). Using this analysis, the changes in power across all frequencies examined, as well as changes in power levels in response to task events, can be extracted (extracting the ERSP), thus demonstrating that neural oscillations have a direct connection to a wide range of cognitive processes (Herrmann et al., 2016). We expected a general reduction in neural resource activation in the IAD group compared to the HC group, considering the differences in ERSP variables of cognitive control in the IAD group. Additionally, we expect HC participants to demonstrate reduced left-hemisphere prefrontal asymmetry associated with interference control and Stroop effect.
2. Materials and Methods
Participant selection
The participants were recruited from universities in Tehran, Iran, through an online advertisement between October 2021 and April 2022. Their contact information was provided during the online survey completion using the IAT questionnaire. Upon receiving greater than 70 IAT scores (Young, 2009), they were invited to the laboratory for an IAD interview by a psychiatrist to exclude emotional disorders such as anxiety and depression. In the following step, the IAD cases were matched with the remaining non-IAD cases to create the final pool of 50%-50% of IAD cases and no-IAD (HC) cases. The participants all possessed normal or corrected-to-normal vision and were right-handed. No neurological disorders, including mental illness and brain injuries, were present in their history. Those participants with a history of substance use, as well as those with a previous hospitalization for psychiatric disorders or a history of therapy for central nervous system disorders, were excluded from the study. On the day of the experiment, participants were instructed not to consume caffeine, nicotine, or any drugs/substances. A final sample enrolled in this experiment included 42 individuals: 21 IAD participants (9 females) and 21 HC participants (10 females) with no significant difference in their mean age (IAD: 23.50±4.78 years vs HC: 24.60±4.21 years). Informed consent was obtained from all participants under the Declaration of Helsinki. One female participant from the HC group was excluded from the experiment due to technical problems with the EEG recording.
Demographic characteristics of participants
In addition to demographic data, e.g. age, gender, and education level, participants were asked to complete some questionnaires. The IAT questionnaire, designed by Kimberly Young (Alavi, 2010; Young, 2009), was used to assess the existence and level of internet addiction. The study included participants who spent at least 10 hours a day, 6 days a week, online and scored over 70 on the IAT. It was determined that HCs who used the internet less than 2 hours per day would be eligible for the study. Furthermore, HCs were evaluated with IAT criteria that were below 30. According to Table 1, specific information is provided for each sample. Anxiety scores were assessed by the Beck anxiety inventory (Beck et al., 1988; Kaviani & Mousavi, 2008), and only participants scoring less than 15 were chosen. Only participants with a depression score below 19 were included in the study, based on the Beck depression inventory (Beck et al., 1996; Ghassemzadeh et al., 2005). As part of the assessment of premeditation, sensation seeking, lack of perseverance, positive urgency, and negative urgency, the UPPS-P impulsivity score (Cyders et al., 2014; Jebraeili et al., 2019) was incorporated.

Experimental procedure
Stimuli and tasks
This study used the 4-choice Stroop task (288 trials with four equal runs) to investigate differences in interference control associated with internet addiction. In the Stroop task, participants are required to identify the color of the stimulus by pressing one of four keys on a computer keyboard (A=left middle finger, S=left index finger, G=right index finger, H=right middle finger). The response buttons on the keyboard are marked with color stickers (A=red, S=green, K=blue, L=yellow). Each trial displays one of the four colors (red, green, blue, and yellow). About 50% of color words are presented in congruent (same word color and meaning) trials, and 50% of color words are shown in incongruent (different word color and meaning) trials. Each run randomly displays 72 similar congruent and incongruent trials (Figure 1).

Before each block, participants were given instructions encompassing the number of runs and encouraging accurate and quick answers. A message on the screen urged the user to start running after pressing the space key. In the following step, a fixation cross was displayed on the monitor for 250 ms, a color word was displayed for 800 ms, and the response within 800 ms was recorded. Afterward, a black screen was shown for 1000 ms, and the subsequent trial began.
An electrically shielded room was used for this task, in which participants sat in chairs at a 60 cm distance from the viewing surface. A fixation cross was displayed on the screen at the center of the display before each stimulus. Before performing the main task, participants were trained on keyboard keys and the procedure. A training block comprises 16 trials. MATLAB’s psychophysics toolbox version 3 was used to perform the task.
Data acquisition and preprocessing
EEG was continuously recorded via a 64-channel g.tec g.HIamp (g.tec, Graz, Austria) with a 1200 Hz sampling rate and 10-10 international electrode placement system. The ground electrode was located on the forehead (Fpz), and the online reference electrode was located on the right mastoid. Impedance was maintained below 10 kΩ with all electrodes being passive.
Raw EEG data were imported to EEGLAB version 2021.0 (Delorme & Makeig, 2004) for standard preprocessing and analysis. An FIR Butterworth band pass filter was used to filter the EEG data between 0.5 and 40 Hz. After that, based on visual inspection, muscular and ocular artifacts trials were removed. Noisy channels were discarded subsequently. After that, independent component analysis (ICA) decomposition was done under the ICA calculation method by runica to remove non-brain sources from the EEG data (non-brain sources such as muscle contractions, eye blinks, gaze movements, and electromyography) (Jung et al., 2001; Makeig et al., 1997). Spherical interpolation methods replaced removed channels with weighted averages of adjacent channels. The clean EEG data were resampled to 256 Hz. Using this method, the results were extracted by epochs starting at 200 ms before stimulus onset and ending at 1500 ms after that. Epochs with amplitude exceeding ±100 μV were eliminated as an artifact from subsequent analyses.
ERSP analysis
A Morlet wavelet was employed to extract ERSP with linear separation between 2 and 40 Hz. Wavelet’s time window resolution is 500 ms, and the cycle number increases linearly from 2 to 20 cycles, corresponding to 2 and 40 Hz frequencies, respectively. The average power between -200 and 0 ms for each trial was used to perform baseline correction. This method enables us to capture all 128 channels and 107 time points from 100 ms up to 1500 ms throughout the channel-time-frequency domain. Through this method, edge artifacts caused by wavelet transforms are omitted, and stimuli-related activity is overlapped over trials. Approximately 15 ms of resolution are available, and a frequency range of 2 to 40 Hz is available.
In this study, we focused on power changes in the theta (4 to 7 Hz), alpha (8 to 12 Hz), beta (13 to 30 Hz), and gamma (31 to 40 Hz) frequency bands. A frequency band has been divided according to previous research (Hanslmayr et al., 2008; Nigbur et al., 2011). It was found that the beta frequency could be separated into two segments, with frequencies ranging from 13 to 19 Hz (beta1) and 20 to 30 Hz (beta2) (Engel & Fries, 2010). We averaged the time-frequency power estimates across trials for each participant, each condition, each block, each region, and within theta, alpha, beta1, beta2, and gamma frequency bands after obtaining wavelet representations of individual trials. The subject-averaged power changes were measured in the post-stimulus interval compared to the baseline (-200 to 0 ms). Compared with the event-related potential method, this approach provides information regarding the time course of various frequency bands’ involvement in specific cognitive tasks (Engel & Fries, 2010). By analyzing neural oscillations through this analysis, we can extract information regarding the power fluctuations at each frequency examined and their variations during time-based on the event at hand (extracting the ERSP), which allows us to identify cognitive processes associated with neural oscillations.
Statistical analysis
Behavioral data were analyzed using R (version 3.6.1, R Core Team, 2019) within the RStudio environment (version 1.2.5001). The Mean±SD for numerical variables were calculated when they were normally distributed, and the range was calculated for variables that were not normally distributed. A 2-sided significance threshold of less than 0.05 indicates statistical significance. A partial eta-squared (ηp2) was used to measure effect size, which represents the proportion of variance associated with an effect and the error variance associated with that effect. All post hoc analyses were corrected for type I errors by applying the Bonferroni correction.
The cluster-based random permutation test was also used to examine the differences in power between the two groups by comparing power amplitudes across trials within and between the two groups. (Maris & Oostenveld, 2007). The uncorrected P were determined by performing a straightforward permutation t test on each data point (for the wavelet data, electrode by frequency by time). Statistical power was increased by averaging the pre-selected frequencies (theta, alpha, beta 1, beta 2, and gamma) and time intervals (0 to 0.2 s) for each electrode in the wavelet analysis. This choice was made based on consistent patterns across all two groups and comparisons between groups. Then, neighboring data points that exceeded a predetermined significance level (5%) were grouped to form clusters. We added the t values within each cluster to compute cluster-level statistics, then constructed a null distribution assuming no differences between the groups. A histogram of the test statistics was constructed after 1000 permutations of the randomly assigned groups within participants. Based on the observed test statistic and the histogram, the proportion of random partitions resulting in a larger test statistic than the observed one was calculated. The Monte Carlo significance probability is defined as this proportion. In the case of significant differences between the two experimental conditions, the significance probability should be under the critical level (typically 0.05). It is also possible to calculate the significance probability for the second-most significant cluster-level statistic, the third-most significant statistic, etc. Using the Monte Carlo method is the only way to calculate the significance probability. This is because the Monte Carlo method can approximate (to any level of accuracy) the reference distribution for a permutation test. A significant cluster was determined by comparing the cluster-level test statistics to the null distribution, where clusters above or below the 2.5 percentile were considered significant. This method determined whether the ERSP Stroop effect for participants in HCs and IADs was significant by considering the congruency factor (incongruent versus congruent Stroop conditions). After comparing the ERSP Stroop effect between individuals with HC and those with IAD, we assessed whether internet addiction significantly modulated the impact. It was therefore examined whether there was an interaction between IAD and HC groups under congruent and incongruent conditions.
3. Results
Behavioral data
Demographic characteristics of the participants
Demographic results related to the IAT score and the scores derived from the questionnaires are presented in Table 1. Based on linear regression analysis, a significant positive linear relationship existed between the IAT and total impulsivity score (beta=1.6993, P<0.001).
Mean reaction times
In the mean reaction times (RTs) analysis, trials with incorrect responses and more or less than mean-RT±2SD were removed. It was found that the group had a significant effect on mean RT based on the two-way ANOVA (F(1, 140)=17.74, P=4.51e-05, ηp2=0.08843) due to considerably longer mean RTs in IADs than HCs (Figure 2). The main effect of congruity revealed that all participants experienced longer mean-RT in incongruent trials compared to congruent trials (F(1, 140)=46.76, P=2.29e-10, ηp2=0.22744).

In the subgroup analysis, it was found that the group had a significant effect on congruent trials (F(1, 69)=5.524, P=0.0216, ηp2=0.07412), with IADs having the longest mean reaction times and HCs having the shortest. A significant difference was also observed between the incongruent trials (F(1, 69)=12.94, P=0.0006, ηp2=0.15744), as IADs had longer mean RTs than HCs. Congruent and incongruent trials were significantly different in IADs (F (1, 69)=31.491, P=3.87e-07, ηp2=0.31337) and in HCs (F(1, 69)=16.772, P=0.000113, ηp2=0.19554).
We also used the IES criterion (dividing the mean-RT by the accuracy [1–the proportion of errors]) to investigate the error rate between different conditions. The IES indicates a combination of speed and accuracy in response. Using a 2-way ANOVA, a significant group effect was found to be present (F(1, 140)=10.73, P=0.00133, ηp2=0.05875) due to the higher IES in IADs compared to HCs. The Wilcoxon rank-sum test showed a significant main effect of groups (Z=509, P=0.004852), with a moderate effect size (r=0.363) in incongruent trials, based on a greater error rate and mean reaction time of IADs than HCs in incongruent trials.
Scalp-based time-frequency analysis
Figure 3 shows the HC (Figure 3A) and IAD (Figure 3B) groups’ event-related spectral perturbation analyses. ERSP Stroop effects were found in the IAD group, specifically in the left hemisphere, but less specific to frequencies. As a result, a major cluster with significant ERSP Stroop effects was observed over most left frontal and left fronto-central channels within 200 and 800 ms. The same effect was seen in the frontal, fronto-central, and central channels within about 200 to 400 ms. An 800 to 1000 ms time window also showed this effect in beta1, beta2, and gamma frequency bands (Figure 3B). The left posterior channels also showed a second cluster of alpha, beta1, beta2, and gamma signals in time windows ranging between 200 and 400 ms and 600 and 1000 ms, respectively. IAD showed these effects due to a greater event-related power decrease for the incongruent condition than for the congruent condition.

Based on the analysis of the HC group, several ERSP Stroop effects were identified, most of which affected the left hemisphere in the early time windows (Figure 3A). ERSP effects of Stroop were detected in the first prominent cluster involving theta, alpha, and beta1 frequencies over the frontal, fronto-central, and central channels with high left lateralization during a period ranging from 400 to 600 ms. As a first cluster, theta, alpha, and beta1 bands were also detected in the central, centroparietal, and parietal channels between 600 and 800 ms. Theta and alpha bands were observed over the fronto-central, central, and centro-parietal regions between 800 and 1000 ms as part of this cluster. A second cluster of beta1 bands occurred over the frontal and fronto-central channels during 600 to 800 ms.
A big cluster in the IAD group with ERSP Stroop effects at theta, alpha, beta, and gamma frequencies over electrodes spread across the left frontal region in a 200 to 1000 ms time window, including earlier samples. It was found that IAD participants experienced lower ERSP Stroop effects than participants in the HC group as a result of these effects. IAD participants showed a greater event-related power decline in the incongruent condition than HC participants since the weaker event-related power decreases were more visible on the left frontal electrodes under the incongruent condition. Additionally, an earlier time window ranged roughly from 200 to 400 ms and 600 to 1000 ms, showing a cluster of alpha, beta, and gamma frequencies over the left posterior channels (Figure 3B). In the congruent condition, the power increased more than in the incongruent condition in IAD compared to HC because there was a greater power increase for all frequency bands except theta.
We also used scalp-based ERSP analysis to illustrate the interaction between the HC and IAD groups under congruent and incongruent conditions. The interaction analysis between HC and IAD groups in the congruent trials elicited several significant ERSP group effects, mostly involving theta band over frontal, fronto-central, centro-parietal, and parietal regions in relatively early time windows (Figures 4A and 5A). Over the entire left frontal hemisphere, a cluster showed significant ERSP group effects in theta from 400 to 850 ms. This cluster also included the ERSP group effects of the alpha band over left frontal and left fronto-central channels during temporal intervals of 400 to 800 ms. In a temporal window of 400 to 600 ms, we found a second cluster involving theta bands across the centro-parietal and parietal channels (Figures 4A and 5A).

Interaction analysis between HC and IAD groups in the incongruent condition revealed a main significant ERSP group effect, mainly affecting theta and alpha bands over left frontal, frontocentral, and central channels, but a less clear interaction effect over time (Figures 4B and 5A). The first cluster of ERSP group effects was detected over the left frontal and fronto-central regions at a window between 200 and 1000 ms. Additionally, it was observed in the beta1 band during 400 and 1000 ms (Figures 4B and 5B), as well as beta2 and gamma frequencies in the period between 400 and 800 ms (Figures 4B and 5C, D). However, this effect was less time- and frequency-specific. Furthermore, we recognized a second cluster that involved a theta band over frontal, fronto-central, central, and centro-parietal regions with no lateralization in a temporal window roughly spanning 400 to 600 ms (Figure 4B). The IAD group experienced a greater event-related decline in power than the HC group, causing all these effects.

4. Discussion
In our study, interference control spectral correlates were compared between IAD and HC participants, and differences were found. For this purpose, EEG activity was recorded from IAD and HC groups during the color-word Stroop task, and the time-frequency dynamics were analyzed to estimate the underlying neural activity. Further, we were interested in examining whether the left portion of the PFC is involved in this cognitive process. Results indicated that participants with IAD displayed diminished conflict detection and response selection abilities against HCs, as measured by theta band indices. Furthermore, IAD participants displayed diminished conflict detection and resolution compared to HC participants, as revealed by damage interaction dynamics between beta2 and gamma bands in the fronto-centro-temporal regions.
Our behavioral findings showed that all participants had longer RTs and made more errors when their trials were congruent compared to incongruent, which is consistent with Stroop-related interference in response time and error rate. Individuals with IAD had a greater IES and reaction time than HCs; however, there was no difference in overall error rate between the two groups. It follows previous studies that reported significant differences in mean reaction times when using the computerized Stroop task (Dong et al., 2011). Following these results, studies (Ko et al., 2014; Dong et al., 2011) have noted impaired inhibitory control in individuals with IAD when performing the Go/NoGo and Stroop tasks. There is growing evidence suggesting that internet addiction may be caused by a deficit in inhibitory control (Kräplin et al., 2021; Wegmann et al., 2020). There has also been evidence of similar results regarding behavioral addiction as well as substance abuse in prior studies (Choi et al., 2014; Dong et al., 2010). We also found that participants who suffer from IAD demonstrate higher impulsivity and decreased inhibition, as evidenced by the elevated UPPS-P score and lower IES score. This finding is consistent with a previous study in which the development of IAD is positively correlated with impulsivity scores (Khanbabaei et al., 2022). Thus, IAD occurrence is associated with impulsive personality traits and impaired cognitive functioning.
Our electrophysiological results indicate significant ERSP Stroop effects at theta, alpha, beta, and gamma frequencies over the left frontal region in IADs. Most likely, IADs experienced a more significant event-related loss of power at left frontal electrodes when subjected to the incongruent condition than HCs and more frequencies were used over a greater number of channels at an earlier and more sustained period (Tang et al., 2013). According to these findings, there is a group-dependent modulation of ERSP Stroop effects. Compared to HCs, IADs showed spectral indicators of interference control earlier, wider, and more unspecific.
Compared with HC participants, the IAD group experienced decreased EEG power at frequencies ranging from alpha to gamma within a time window spanning 200 to 1000 ms. Recent research suggests that excessive internet use has the greatest impact on the gamma oscillation of the parietal, central region at 300 ms after stimuli at 40-50 Hz (Yu et al., 2009). These findings are consistent with our findings that high-band EEG power decreased earlier for IADs than HCs. Gamma synchrony is believed to be a neurophysiological process that regulates information coding and integration in the brain (Fries et al., 2007). Thus, excessive internet use impacts the processing and integration of information in the brain.
Stroop effects were observed as a result of ERSP. According to Figure 3, participants in HC primarily participated in slow-wave frequencies, with Stroop-dependent modulations occurring around the time of the response on the left hemisphere and eventually spreading over to the left frontal region when the window closes to the response. In the IAD group, individuals employed all frequency bands (from theta to gamma) primarily, and this activity was initiated shortly after stimulus presentation, engaging electrodes spread across the left scalp. Following this perspective, the IAD participants showed lower EEG power during the early time window close to stimulus presentation, reflecting impairments in executive function. In other words, they could not successfully inhibit their brain activation for stimulus conflict detection.
A general integrative role has been demonstrated for theta band activity in regulating brain activity when considered in light of spectral dynamics for cognitive tasks (Tafuro et al., 2019). Theta band oscillations are a common indicator of conflict resolution and interference control, demonstrating the requirement for cognitive control (Cohen, 2014). It has previously been reported that in incongruent conditions, theta band responses are enhanced in the frontal and fronto-central areas (Qi et al., 2022; Tang et al., 2013). The incongruent trials of the Stroop task were reported to display extended phase coupling between the ACC and the left prefrontal cortex (Hanslmayr et al., 2008). Theta amplitude during incongruent trials was proposed to be sensitive to conflict detection and response selection in a study conducted by Qi et al. (2022). In a study on the Stroop task, there was no increase in event-related theta magnitude when participants looked at stimuli with no response, suggesting a possible link between response inhibition and theta response (Tang et al., 2013). In light of these results, we anticipate finding evidence of theta band involvement in the early temporal window, which would be accompanied by decreased theta power in the left frontal and left frontocentral regions for the incongruent condition. Our study found that the IAD group recruited theta band at a lower level during an earlier time frame than the HC group in the left frontal and left fronto-central channels. In other words, theta power decreased significantly more for IADs than HCs under incongruent conditions in an earlier time window. Considering that event-related theta power plays an essential role in conflict detection and response selection, it is likely that conflict detection capability was diminished sooner in IADs following stimulus presentation than in HC groups.
Compared with HCs, IAD participants showed an overrepresentation of beta2 and gamma bands. As a result of a comprehensive ERSP analysis, it was found that IAD participants displayed a significant ERSP Stroop effect at beta2 and gamma frequencies when compared to HC participants. It was found that bilateral dorsal frontal and parietal cortex areas were involved during time windows between 200 and 400 ms and 800 and 1000 ms without evidence of hemispheric asymmetry during these behavior patterns. Our findings are consistent with other studies showing that patients with IGD do not show dynamic interactions between the fronto-centro-temporal regions at rest or when performing cognitive tasks (Park et al., 2021). In the case of IADs, high-frequency bands are likely involved in conflict detection and resolution during the early (200 to 400 ms) and late (800 to 1000 ms) periods. Consequently, IADs need additional resources to resolve conflict detection and resolution issues.
We concluded that interference control processes are modulated group-dependent based on the contrast analysis between the HC and IAD groups (Figure 4). IADs showed a stronger correlation in spectral behavior earlier and were more widely spread. Within 200 to 1000 ms time windows, several significant group-dependent modulations of ERSP across central and frontal regions existed. Our analysis of the ERSP on IAD and HC participants under congruent and incongruent conditions indicates that the theta band exhibits the greatest contrast between the two groups. Previous research has demonstrated that changes in theta band are associated with conflict processing. In congruent and incongruent conditions, theta band activity is suppressed earlier (200 ms after stimulus presentation) since IADs are less sensitive to conflict stimulus detection than HCs. It was also found that IADs experienced significant ERSP effects on beta2 and gamma frequencies compared to HCs in several electrodes situated throughout the left frontal and left fronto-central regions during a time window of mainly 600 to 800 ms under incongruent conditions. However, these ERSP effects were not observed when congruent conditions were present. The pattern of results indicates that incongruent conditions led to contrast changes in the high-frequency bands, which are associated with conflict detection and resolution. In other words, the ability of IADs to detect and resolve conflict is diminished in incongruent conditions compared to HC participants.
Although motor processes cannot be ruled out as a possible contributor to our findings regarding theta and beta1 bands, we cannot exclude the possibility that they might be more than simple correlates of motor outcomes. Our first finding was that power reduction was primarily located in the left hemisphere despite the task being bimanual. In addition, while we did not conduct response-locked ERSP analyses, we did not observe a correlation between the event-related decline in power at high frequencies and reaction times. It is important to note that the maximum decreases in theta and beta1 power occurred around the same time in both groups and conditions, even though IAD participants’ RTs were slower in both conditions, particularly in the incongruent condition. A third issue is that even if we consider that the differences between two groups in the Stroop effects are attributed to the reduction of RTs shown by IADs, this does not clarify the over-recruitment observed in both spatial and spectral regions due to group differences.
We believe this study is one of the first to use cluster-based random permutation tests to investigate oscillatory dynamics in conflict processing for IAD groups. As a result of our research, we found that in participants with IAD, neural oscillations differed primarily in relation to the impairment of theta-related cognitive processes, as well as beta2 and gamma-related cognitive processes. We could differentiate multiple processes with partially or completely overlapping frequency features or timescales using a wavelet transform. This decomposition results from a decomposition of the oscillatory activity across different frequency bands. These oscillatory responses reflect the interactions between neuronal networks operating on different timescales or frequencies during task performance. It should also be noted that this study has some limitations. The first limitation of the study was that we could not compare IADs with substance use disorders (SUD), as well as other behavioral addiction disorders, such as gambling disorders. Additionally, we used general stimuli during the Stroop task, which may be extended in future studies by using different tasks to identify various types of conflicts between stimulus and response. Moreover, EEG analysis at the sensor level is poor in spatial resolution and does not reveal the source of brain activity. Research into the localization of EEG sources and fMRI with high spatial resolution could effectively answer these questions in the future.
5. Conclusion
This study used Stroop tasks to examine the potential time-frequency dynamics of EEG activity and interference control and inhibitory control impairments in IAD. These findings suggest that neural oscillations differ primarily in relation to impairments in cognitive processes related to theta as well as beta2 and gamma. Based on our findings, participants with IAD displayed reduced conflict detection and response selection compared to HCs, as measured by theta band indices. In addition, they also displayed diminished conflict resolution, as demonstrated by altered interaction dynamics across beta2 and gamma bands. In consequence, this can facilitate our understanding of the neural mechanisms responsible for interference control and inhibitory control deficits in IAD, as well as suggest future interventions that might be effective. By understanding these mechanisms, it would be possible to develop neuromodulation therapies that would benefit the clinical patient and reduce cognitive deficits in people with IAD.
Ethical Considerations
Compliance with ethical guidelines
All research procedures involving human participants followed the ethical standards established by the Institutional Research Committee in accordance with the 1964 Helsinki Declaration. This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (Code: IR.SBMU.MSP.REC.1397.638). Each participant in the study provided informed written consent to participate.
Funding
The study was partially supported by a grant from Shahid Beheshti University of Medical Sciences (Grant No.: 16674). This article was extracted from the PhD dissertation of Farzad Rostami, approved by the Department of Biomedical Engineering and Medical Physics, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran. (Code: 15921).
Authors' contributions
Conceptualization, methodology and validation: All authors; Data acquisition, investigation, validation, visualization, and writing the original draft: Farzad Rostami; Software, data analysis, review, and editing: Atiye Sarabi-Jamab and Farzad Rostami; Project administration, and resources: Ali Esteki; Supervision: Atiye Sarabi-Jamab and Ali Esteki.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are very grateful for the collaboration of the National Brain Mapping Lab (NBML), Tehran, Iran, for providing data recording services.
References
Alavi, S. (2010). [Psychometric properties of Young internet addiction test (Persian)]. International Journal of Behavioral Sciences, 4(3), 183-189. [Link]
Ambrosini, E., & Vallesi, A. (2017). Domain-general Stroop performance and hemispheric asymmetries: A resting-state EEG study. Journal of Cognitive Neuroscience, 29(5), 769-779. [DOI:10.1162/jocn_a_01076] [PMID]
Antons, S., & Matthias, B. (2020). Inhibitory control and problematic Internet-pornography use-The important balancing role of the insula. Journal of Behavioral Addictions, 9(1), 58-70. [DOI:10.1556/2006.2020.00010] [PMID] [PMCID]
Balconi, M., & Finocchiaro, R. (2015). Decisional impairments in cocaine addiction, reward bias, and cortical oscillation "unbalance". Neuropsychiatric Disease and Treatment, 11, 777–786.[DOI:10.2147/NDT.S79696] [PMID] [PMCID]
Balconi, M., & Finocchiaro, R. (2016). Deficit in rewarding mechanisms and prefrontal left/right cortical effect in vulnerability for internet addiction. Acta Neuropsychiatrica, 28(5), 272-285. [DOI:10.1017/neu.2016.9] [PMID]
Balconi, M., Finocchiaro, R., & Canavesio, Y. (2015). Left hemispheric imbalance and reward mechanisms affect gambling behavior: The contribution of the metacognition and cortical brain oscillations. Clinical EEG and Neuroscience, 46(3), 197-207. [DOI:10.1177/1550059413513261] [PMID]
Barry, R. J., Clarke, A. R., Hajos, M., McCarthy, R., Selikowitz, M., & Dupuy, F. E. (2010). Resting-state EEG gamma activity in children with attention-deficit/hyperactivity disorder. Clinical Neurophysiology, 121(11), 1871-1877. [DOI:10.1016/j.clinph.2010.04.022] [PMID]
Beck, A. T., Epstein, N., Brown, G., & Steer, R. A. (1988). An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology, 56(6), 893-897. [DOI:10.1037/0022-006X.56.6.893] [PMID]
Beck, A. T., Steer, R. A., & Brown, G. K. (1996). Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation.
Botvinick, M. M., Cohen, J. D., & Carter, C. S. (2004). Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences, 8(12), 539-546. [DOI:10.1016/j.tics.2004.10.003] [PMID]
Brand, M., Young, K. S., Laier, C., Wölfling, K., & Potenza, M. N. (2016). Integrating psychological and neurobiological considerations regarding the development and maintenance of specific Internet-use disorders: An Interaction of Person-Affect-Cognition-Execution (I-PACE) model. Neuroscience & Biobehavioral Reviews, 71, 252-266. [DOI:10.1016/j.neubiorev.2016.08.033] [PMID]
Brittain, J. S., Watkins, K. E., Joundi, R. A., Ray, N. J., Holland, P., & Green, A. L., et al. (2012). A role for the subthalamic nucleus in response inhibition during conflict. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 32(39), 13396–13401. [DOI:10.1523/JNEUROSCI.2259-12.2012] [PMID] [PMCID]
Cavanagh, J. F., Wiecki, T. V., Cohen, M. X., Figueroa, C. M., Samanta, J., & Sherman, S. J., et al. (2011). Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nature Neuroscience, 14(11), 1462-1467. [DOI:10.1038/nn.2925] [PMID] [PMCID]
Choi, J. S., Park, S. M., Lee, J., Hwang, J. Y., Jung, H. Y., & Choi, S. W., et al. (2013). Resting-state beta and gamma activity in Internet addiction. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 89(3), 328–333. [DOI:10.1016/j.ijpsycho.2013.06.007] [PMID]
Choi, J. S., Park, S. M., Roh, M. S., Lee, J. Y., Park, C. B., & Hwang, J. Y., et al. (2014). Dysfunctional inhibitory control and impulsivity in Internet addiction. Psychiatry Research, 215(2), 424-428. [DOI:10.1016/j.psychres.2013.12.001] [PMID]
Cohen, M. X. (2014). A neural microcircuit for cognitive conflict detection and signaling. Trends in Neurosciences, 37(9), 480-490. [DOI:10.1016/j.tins.2014.06.004] [PMID]
Cyders, M. A., Littlefield, A. K., Coffey, S., & Karyadi, K. A. (2014). Examination of a short English version of the UPPS-P Impulsive Behavior Scale. Addictive Behaviors, 39(9), 1372-1376. [DOI:10.1016/j.addbeh.2014.02.013] [PMID] [PMCID]
Delorme, A., & Makeig, S. (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9-21. [DOI:10.1016/j.jneumeth.2003.10.009] [PMID]
Diamond, A. (2013). Executive functions. Annual Review of Psychology, 64, 135–168. [DOI:10.1146/annurev-psych-113011-143750] [PMID] [PMCID]
Dong, G., Zhou, H., & Zhao, X. (2010). Impulse inhibition in people with Internet addiction disorder: Electrophysiological evidence from a Go/NoGo study. Neuroscience Letters, 485(2), 138–142. [DOI:10.1016/j.neulet.2010.09.002] [PMID]
Dong, G., Zhou, H., & Zhao, X. (2011). Male Internet addicts show impaired executive control ability: Evidence from a color-word Stroop task. Neuroscience Letters, 499(2), 114-118. [DOI:10.1016/j.neulet.2011.05.047] [PMID]
Donoghue, T., Schaworonkow, N., & Voytek, B. (2022). Methodological considerations for studying neural oscillations. The European Journal of Neuroscience, 55(11-12), 3502–3527.[DOI:10.1111/ejn.15361] [PMID] [PMCID]
Engel, A. K., & Fries, P. (2010). Beta-band oscillations-signalling the status quo? Current Opinion in Neurobiology, 20(2), 156-165. [DOI:10.1016/j.conb.2010.02.015] [PMID]
Fries, P., Nikolić, D., & Singer, W. (2007). The gamma cycle. Trends in Neurosciences, 30(7), 309-316. [DOI:10.1016/j.tins.2007.05.005] [PMID]
Gao, X., Zhang, M., Yang, Z., Wen, M., Huang, H., & Zheng, R., et al. (2021). Structural and functional brain abnormalities in internet gaming disorder and attention-deficit/hyperactivity disorder: A comparative meta-analysis. Frontiers in Psychiatry, 12, 679437. [DOI:10.3389/fpsyt.2021.679437] [PMID] [PMCID]
Ghassemzadeh, H., Mojtabai, R., Karamghadiri, N., & Ebrahimkhani, N. (2005). Psychometric properties of a Persian-language version of the Beck Depression Inventory-Second edition: BBDI-II-PERSIAN. Depression and Anxiety, 21(4), 185-192. [DOI:10.1002/da.20070] [PMID]
Gioia, F., Rega, V., & Boursier, V. (2021). Problematic internet use and emotional dysregulation among young people: A literature review. Clinical Neuropsychiatry, 18(1), 41–54. [PMID]
Hanslmayr, S., Pastötter, B., Bäuml, K. H., Gruber, S., Wimber, M., & Klimesch, W. (2008). The electrophysiological dynamics of interference during the Stroop task. Journal of Cognitive Neuroscience, 20(2), 215-225. [DOI:10.1162/jocn.2008.20020] [PMID]
Harmony, T. (2013). The functional significance of delta oscillations in cognitive processing. Frontiers in Integrative Neuroscience, 7, 83. [DOI:10.3389/fnint.2013.00083] [PMID] [PMCID]
Harper, J., Malone, S. M., & Bernat, E. M. (2014). Theta and delta band activity explain N2 and P3 ERP component activity in a go/no-go task. Clinical Neurophysiology, 125(1), 124-132. [DOI:10.1016/j.clinph.2013.06.025] [PMID] [PMCID]
Herrmann, C. S., Strüber, D., Helfrich, R. F., & Engel, A. K. (2016). EEG oscillations: From correlation to causality. International Journal of Psychophysiology, 103, 12-21. [DOI:10.1016/j.ijpsycho.2015.02.003] [PMID]
Heuer, A., Mennig, M., Schubö, A., & Barke, A. (2021). Impaired disengagement of attention from computer-related stimuli in Internet Gaming Disorder: Behavioral and electrophysiological evidence. Journal of Behavioral Addictions, 10(1), 77-87. [DOI:10.1556/2006.2020.00100] [PMID] [PMCID]
Ioannidis, K., Hook, R., Goudriaan, A. E., Vlies, S., Fineberg, N. A., & Grant, J. E., et al. (2019). Cognitive deficits in problematic internet use: Meta-analysis of 40 studies. The British Journal of Psychiatry, 215(5), 639-646. [DOI:10.1192/bjp.2019.3] [PMID] [PMCID]
Jebraeili, H., Moradi, A., & Habibi, M. (2019). Psychometric properties of Persian short version of the five factor impulsive behavior scale. Journal of Research and Health, 9(6), 516-524. [DOI:10.32598/jrh.9.6.516]
Jung, T. P., Makeig, S., McKeown, M. J., Bell, A. J., Lee, T. W., & Sejnowski, T. J. (2001). Imaging brain dynamics using independent component analysis. Proceedings of the IEEE. Institute of Electrical and Electronics Engineers, 89(7), 1107–1122. [DOI:10.1109/5.939827] [PMID] [PMCID]
Kaviani, H., & Mousavi, A. (2008). [Psychometric properties of the Persian version of Beck Anxiety Inventory (BAI) (Persian)]. Tehran University Medical Journal, 66(2), 136-140. [Link]
Khanbabaei, S., Abdollahi, M. H., & Shahgholian, M. (2022). The predictive role of working memory and impulsivity in internet addiction, an investigation about the mediating role of time perception. Personality and Individual Differences, 185, 111280. [DOI:10.1016/j.paid.2021.111280]
King, D. L., Delfabbro, P. H., Griffiths, M. D., & Gradisar, M. (2011). Assessing clinical trials of Internet addiction treatment: A systematic review and CONSORT evaluation. Clinical Psychology Review, 31(7), 1110-1116. [DOI:10.1016/j.cpr.2011.06.009] [PMID]
Király, O., Griffiths, M. D., & Demetrovics, Z. (2015). Internet gaming disorder and the DSM-5: Conceptualization, debates, and controversies. Current Addiction Reports, 2(3), 254-262. [DOI:10.1007/s40429-015-0066-7]
Ko, C. H., Hsieh, T. J., Chen, C. Y., Yen, C. F., Chen, C. S., & Yen, J. Y., et al. (2014). Altered brain activation during response inhibition and error processing in subjects with Internet gaming disorder: A functional magnetic imaging study. European Archives of Psychiatry and Clinical Neuroscience, 264(8), 661–672. [PMID]
Kräplin, A., Scherbaum, S., Kraft, E. M., Rehbein, F., Bühringer, G., & Goschke, T., et al. (2021). The role of inhibitory control and decision-making in the course of Internet gaming disorder. Journal of Behavioral Addictions, 9(4), 990-1001. [DOI:10.1556/2006.2020.00076] [PMID] [PMCID]
Liang, W. K., Lo, M. T., Yang, A. C., Peng, C. K., Cheng, S. K., & Tseng, P., et al. (2014). Revealing the brain's adaptability and the transcranial direct current stimulation facilitating effect in inhibitory control by multiscale entropy. NeuroImage, 90, 218-234. [DOI:10.1016/j.neuroimage.2013.12.048] [PMID]
Liu, J., Li, W., Zhou, S., Zhang, L., Wang, Z., & Zhang, Y., et al. (2016). Functional characteristics of the brain in college students with internet gaming disorder. Brain Imaging and Behavior, 10(1), 60-67. [DOI:10.1007/s11682-015-9364-x] [PMID]
Luijten, M., Machielsen, M. W., Veltman, D. J., Hester, R., de Haan, L., & Franken, I. H. (2014). Systematic review of ERP and fMRI studies investigating inhibitory control and error processing in people with substance dependence and behavioural addictions. Journal of Psychiatry and Neuroscience, 39(3), 149-169. [DOI:10.1503/jpn.130052] [PMID] [PMCID]
Lustig, C., Hasher, L., & Tonev, S. T. (2001). Inhibitory control over the present and the past. European Journal of Cognitive Psychology, 13(1-2), 107-122. [Link]
MacLeod, C. M. (1991). Half a century of research on the Stroop effect: An integrative review. Psychological Bulletin, 109(2), 163-203. [DOI:10.1037/0033-2909.109.2.163] [PMID]
Makeig, S., Jung, T. P., Bell, A. J., Ghahremani, D., & Sejnowski, T. J. (1997). Blind separation of auditory event-related brain responses into independent components. Proceedings of the National Academy of Sciences of the United States of America, 94(20), 10979–10984. [DOI:10.1073/pnas.94.20.10979] [PMID] [PMCID]
Maris, E., & Oostenveld, R. (2007). Nonparametric statistical testing of EEG-and MEG-data. Journal of Neuroscience Methods, 164(1), 177-190. [DOI:10.1016/j.jneumeth.2007.03.024] [PMID]
Nigbur, R., Cohen, M. X., Ridderinkhof, K. R., & Stürmer, B. (2012). Theta dynamics reveal domain-specific control over stimulus and response conflict. Journal of Cognitive Neuroscience, 24(5), 1264-1274. [DOI:10.1162/jocn_a_00128] [PMID]
Nigbur, R., Ivanova, G., & Stürmer, B. (2011). Theta power as a marker for cognitive interference. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 122(11), 2185–2194. [DOI:10.1016/j.clinph.2011.03.030] [PMID]
Omoyemiju, M. A., & Popoola, B. I. (2021). Prevalence of internet addiction among university students in Nigeria. British Journal of Guidance & Counselling, 49(1), 132-139. [DOI:10.1080/03069885.2020.1729339]
Ozturk, F. O., & Ayaz-Alkaya, S. (2021). Internet addiction and psychosocial problems among adolescents during the COVID-19 pandemic: A cross-sectional study. Archives of Psychiatric Nursing, 35(6), 595-601. [DOI:10.1016/j.apnu.2021.08.007] [PMID] [PMCID]
Park, M., Jung, M. H., Lee, J., Choi, A. R., Chung, S. J., & Kim, B., et al. (2020). Neurophysiological and cognitive correlates of error processing deficits in Internet gaming disorder. Cerebral Cortex, 30(9), 4914-4921. [DOI:10.1093/cercor/bhaa083] [PMID]
Park, M., Yoo, S. Y., Lee, J. Y., Koo, J. W., Kang, U. G., & Choi, J. S. (2021). Relationship between Resting-State Alpha coherence and cognitive control in individuals with internet gaming disorder: A multimodal approach based on resting-state electroencephalography and event-related potentials. Brain Sciences, 11(12), 1635. [DOI:10.3390/brainsci11121635] [PMID] [PMCID]
Pontes, H. M., Szabo, A., & Griffiths, M. D. (2015). The impact of Internet-based specific activities on the perceptions of Internet addiction, quality of life, and excessive usage: A cross-sectional study. Addictive Behaviors Reports, 1, 19-25. [DOI:10.1016/j.abrep.2015.03.002] [PMID] [PMCID]
Qi, Y., Liu, Y., Yan, Z., Hu, S., Zhang, X., & Zhao, J., et al. (2022). Slow-Wave EEG activity correlates with impaired inhibitory control in internet addiction disorder. International Journal of Environmental Research and Public Health, 19(5), 2686. [DOI:10.3390/ijerph19052686] [PMID] [PMCID]
Reed, G. M., First, M. B., Kogan, C. S., Hyman, S. E., Gureje, O., & Gaebel, W., et al. (2019). Innovations and changes in the ICD-11 classification of mental, behavioural and neurodevelopmental disorders. World Psychiatry, 18(1), 3-19. [DOI:10.1002/wps.20611] [PMID] [PMCID]
Schmidt, J. R. (2019). Evidence against conflict monitoring and adaptation: An updated review. Psychonomic Bulletin & Review, 26(3), 753-771. [DOI:10.3758/s13423-018-1520-z] [PMID]
Snyder, S. M., & Hall, J. R. (2006). A meta-analysis of quantitative EEG power associated with attention-deficit hyperactivity disorder. Journal of Clinical Neurophysiology, 23(5), 440–455. [DOI:10.1097/01.wnp.0000221363.12503.78] [PMID]
Son, K. L., Choi, J. S., Lee, J., Park, S. M., Lim, J. A., & Lee, J. Y., et al. (2015). Neurophysiological features of Internet gaming disorder and alcohol use disorder: A resting-state EEG study. Translational Psychiatry, 5(9), e628. [DOI:10.1038/tp.2015.124] [PMID] [PMCID]
Stroop, J. R. (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology, 18(6), 643–662.[DOI:10.1037/h0054651]
Sun, D. L., Chen, Z. J., Ma, N., Zhang, X. C., Fu, X. M., & Zhang, D. R. (2009). Decision-making and prepotent response inhibition functions in excessive internet users. CNS Spectrums, 14(2), 75–81. [DOI:10.1017/S1092852900000225] [PMID]
Tafuro, A., Ambrosini, E., Puccioni, O., & Vallesi, A. (2019). Brain oscillations in cognitive control: A cross-sectional study with a spatial stroop task. Neuropsychologia, 133, 107190. [DOI:10.1016/j.neuropsychologia.2019.107190] [PMID]
Tang, D., Hu, L., & Chen, A. (2013). The neural oscillations of conflict adaptation in the human frontal region. Biological Psychology, 93(3), 364-372. [DOI:10.1016/j.biopsycho.2013.03.004] [PMID]
van Steenbergen, H., Band, G. P., & Hommel, B. (2012). Reward valence modulates conflict-driven attentional adaptation: Electrophysiological evidence. Biological Psychology, 90(3), 234-241. [DOI:10.1016/j.biopsycho.2012.03.018] [PMID]
Wang, G. Y., & Griskova-Bulanova, I. (2018). Electrophysiological activity is associated with vulnerability of Internet addiction in non-clinical population. Addictive Behaviors, 84, 33-39. [DOI:10.1016/j.addbeh.2018.03.025] [PMID]
Wang, K., Li, Q., Zheng, Y., Wang, H., & Liu, X. (2014). Temporal and spectral profiles of stimulus-stimulus and stimulus-response conflict processing. NeuroImage, 89, 280-288. [DOI:10.1016/j.neuroimage.2013.11.045] [PMID]
Wegmann, E., Müller, S. M., Turel, O., & Brand, M. (2020). Interactions of impulsivity, general executive functions, and specific inhibitory control explain symptoms of social-networks-use disorder: An experimental study. Scientific Reports, 10(1), 3866. [DOI:10.1038/s41598-020-60819-4] [PMID] [PMCID]
West, R., Jakubek, K., Wymbs, N., Perry, M., & Moore, K. (2005). Neural correlates of conflict processing. Experimental Brain Research, 167(1), 38-48. [DOI:10.1007/s00221-005-2366-y] [PMID]
Young, K. S. (2009). Internet addiction: The emergence of a new clinical disorder. CyberPsychology & Behavior, 1(3). [DOI:10.1089/cpb.1998.1.237]
Yu, H., Zhao, X., Li, N., Wang, M., & Zhou, P. (2009). Effect of excessive Internet use on the time-frequency characteristic of EEG. Progress in Natural Science, 19(10), 1383-1387. [DOI:10.1016/j.pnsc.2008.11.015]
Type of Study: Original |
Subject:
Clinical Neuroscience
Received: 2022/11/9 | Accepted: 2022/12/10 | Published: 2024/09/1
Received: 2022/11/9 | Accepted: 2022/12/10 | Published: 2024/09/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








