Volume 15, Issue 4 (July & August 2024)
BCN 2024, 15(4): 499-508 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ahadi T, Noori I, Khalifeh Soltani S, Ghaboosi P, Raissi G R. Efficacy of Percutaneous vs Transcutaneous Posterior Tibial Nerve Stimulation in Overactive Bladder Syndrome: A Randomized Clinical Trial. BCN 2024; 15 (4) :499-508
URL: http://bcn.iums.ac.ir/article-1-2579-en.html
URL: http://bcn.iums.ac.ir/article-1-2579-en.html
Tannaz Ahadi *1 

 , Ismaeel Noori2
, Ismaeel Noori2 
 , Shayesteh Khalifeh Soltani2
, Shayesteh Khalifeh Soltani2 
 , Pouya Ghaboosi2
, Pouya Ghaboosi2 
 , Gholam Reza Raissi1
, Gholam Reza Raissi1 




 , Ismaeel Noori2
, Ismaeel Noori2 
 , Shayesteh Khalifeh Soltani2
, Shayesteh Khalifeh Soltani2 
 , Pouya Ghaboosi2
, Pouya Ghaboosi2 
 , Gholam Reza Raissi1
, Gholam Reza Raissi1 


1- Neuromusculoskeletal Research Centre, Firoozgar Hospital, Iran University of Medical Sciences, Tehran, Iran.
2- Neuromusculoskeletal Research Center, Iran University of Medical Sciences, Tehran, Iran.
2- Neuromusculoskeletal Research Center, Iran University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 1781 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Based on the International Continence Society (ICS), overactive bladder (OAB) is urinary urgency, with or without urge incontinence, usually with urinary frequency and nocturia, in the absence of urinary tract infection (UTI) or other pathologies that would explain these symptoms (Wein & Rovner, 2002). The overall prevalence rates of OAB in the United States are reported to be 16.0% and 16.9% for men and women, respectively. However, in another EPIC study in 5 countries (Canada, Germany, Sweden, Italy, and the UK), a similar prevalence of 11.8% was reported for men and women (Irwin et al., 2006; Stewart et al., 2003).
OAB is associated with significantly lower quality-of-life (QoL) scores, higher depression scores, poorer sleep quality, sexual dissatisfaction, and work productivity (Coyne et al., 2008; Stewart et al., 2003). ICS recommends that initial management should include lifestyle modification, bladder training, pelvic floor muscle training, antimuscarinics, anti-diuretic or α-blockers medications, and intermittent catheterization when the post-void residual volume (PVR) >30% of bladder capacity, which may differ based on symptoms severity and sex. Invasive therapies such as neuromodulation and botulinum toxin injections in case of initial management failure and markedly disrupted QoL are being considered (Lightner et al., 2019).
Neuromodulation can be accomplished by invasive implantable sacral nerve root stimulation systems with relatively high complication rates or less invasive perineal, perianal, or tibial nerve stimulation (Cooperberg & Stoller, 2005). Stoller described the percutaneous tibial nerve stimulation (PTNS) technique in the late 1990s as a treatment for OAB syndrome (Stoller, 1999). Posterior tibial nerve (PTN) is a mixed sensorimotor nerve originating from L4 to S3 lumbosacral nerve roots, while sacral S3-S4 spinal segments contribute to pelvic floor, bladder, and urethra autonomic and somatic innervations. Theoretically, PTN stimulation at the medial malleolus directly stimulates the upper sacral segments’ afferent fibers (S1-S2). Although the exact mechanism of the urinary inhibitory response generated by PTN stimulation is unclear, it may exert an inhibitory effect on spinothalamic tract neurons (Chung et al., 1984). More recently, specific spinal receptors and the micturition pathway’s central neuroplasticity are considered to be involved (van der Pal et al., 2006). Several reports show its effectiveness in treating OAB (Burton et al., 2012; Finazzi-Agrò et al., 2010; Gaziev et al., 2013; MacDiarmid et al., 2010; MacDiarmid & Staskin, 2009; Peters et al., 2009; Staskin et al., 2012; van Balken et al., 2001).
A 34-gauge needle electrode is inserted 4–5 cm cephalad to the medial malleolus, a neural access point for regulating bladder and pelvic floor function, for 20 Hz, 200 µs duration electrical stimulation. In an even less invasive manner, transcutaneous tibial nerve stimulation (TTNS) with two surface electrodes for electrical stimulation is suggested to be effective in OAB treatment (Ammi et al., 2014; Manríquez et al., 2016). This study evaluates the therapeutic effect of TTNS and compares it with the PTNS technique for OAB management.
2. Materials and Methods
We enrolled 44 patients of OAB who were diagnosed clinically based on the 2019 American Urological Association (AUA) guideline (Lightner et al., 2019). The inclusion criteria comprised patients older than 18 who had not used anticholinergic medication for one week before and during the study. All participants have received the initial treatment (lifestyle modification, pelvic floor muscle training with or without pharmacologic therapies). Participants were omitted if they were inflicted with these conditions: Diabetes mellitus, pregnancy or attempting to get pregnant, implanted pacemaker, active or recurrent (>4 times per year) UTI, and neurologic disease.
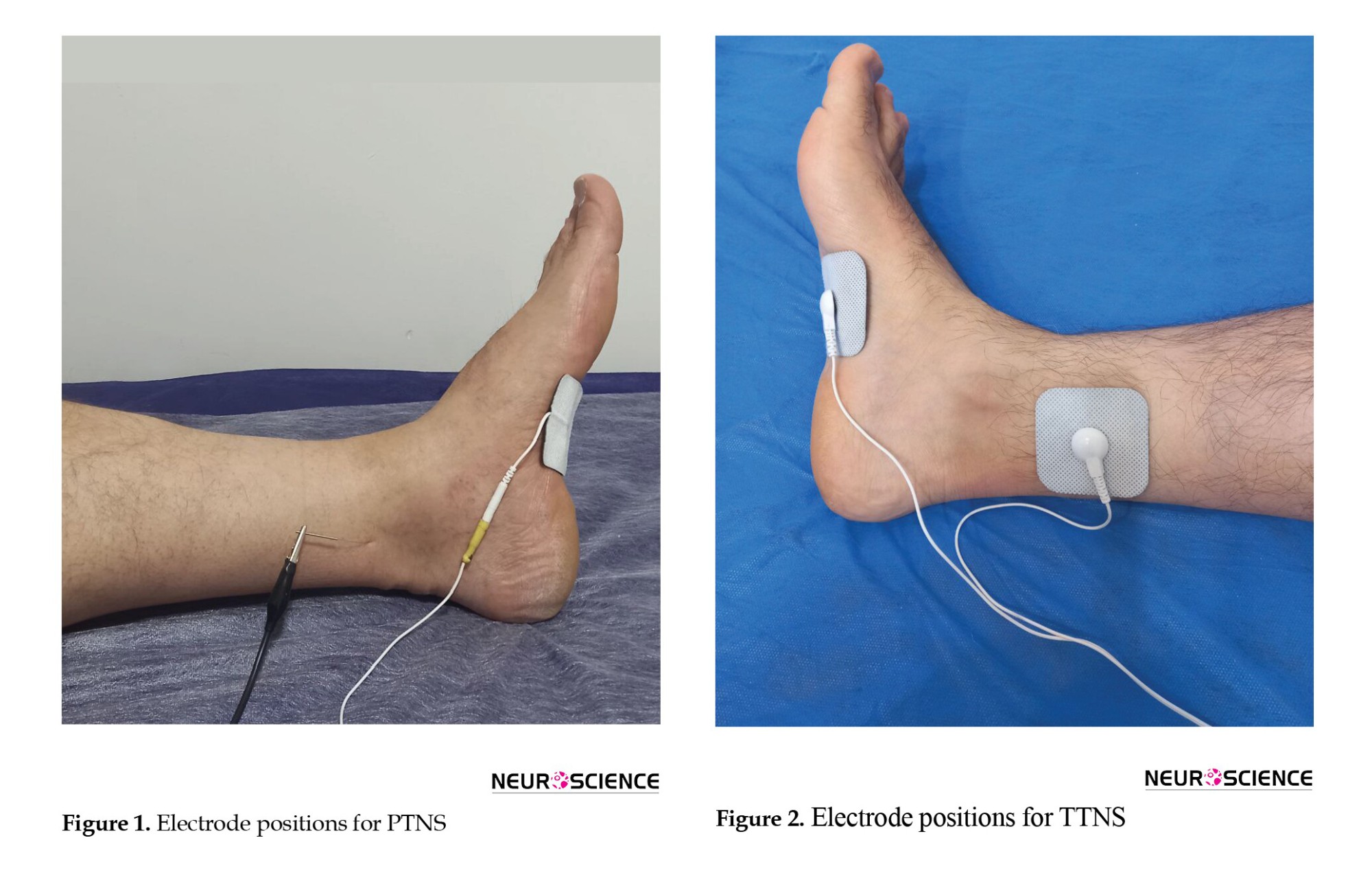
All participants were informed and provided with the consent form. They were randomly assigned to one of the two groups using a randomization table. In the PTNS group, a 34-gauge needle electrode was inserted 5 cm cephalad to the medial malleolus and posterior to the tibial bone (Figure 1). The needle electrode was connected to the active pole, and a surface reference electrode was placed on the medial malleolus. Bi-phasic constant current with 200 µs pulse width, 0.5-9 mA, and 20 Hz was applied to induce big toe plantar flexion for 30 minutes each session, thrice weekly for four weeks.
In the TTNS group, instead of needle insertion, an active surface electrode was used with the same stimulation parameters (Figure 2). The participants filled out the self-reported incontinence quality of life (I-QOL) questionnaire and OAB symptom score (OABSS) form before, 1 week, and 4 weeks after the termination of intervention sessions.
The I-QOL contains 22 items with a 5-point response scale, yielding a total and three subscale scores for avoidance and limiting behavior, psychosocial impacts, and social embarrassment (Patrick et al., 1999). The OABSS quantifies four symptoms of OAB in the past week, which include daytime frequency, night-time frequency, urgency, and urinary incontinence (Homma et al., 2006).
Like the data analyst, the data reviewer was blinded to the participant’s groups despite the physician involved in the therapy sessions.
The mixed analysis of variance (ANOVA) test was used for statistical analysis to compare intervention efficacy between groups. P<0.05 were considered statistically significant.
3. Results
We treated 44 patients, 41 females and 3 males, with no significant difference in mean age, sex, body mass index (BMI), and patients’ symptom duration between the two groups (Table 1).

OABSS questionnaire
In the within-group analysis, we observed a significant difference in this scaled score in all three intervals (before intervention vs week 1, before intervention vs week 4, and week 1 vs week 4) in both groups (P<0.001). Therefore, TTNS and PTNS effectively improved patients’ symptoms (Table 2).
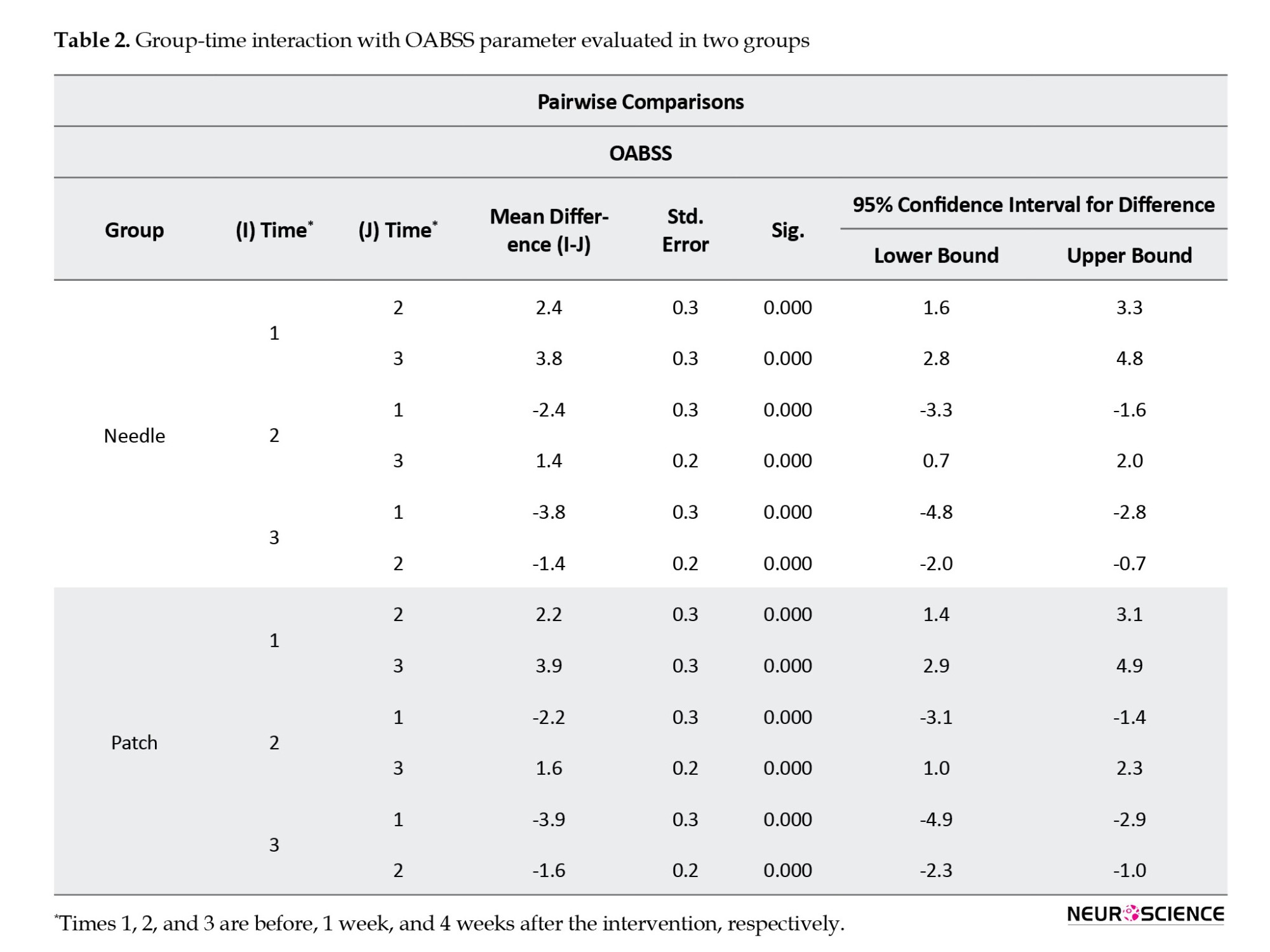
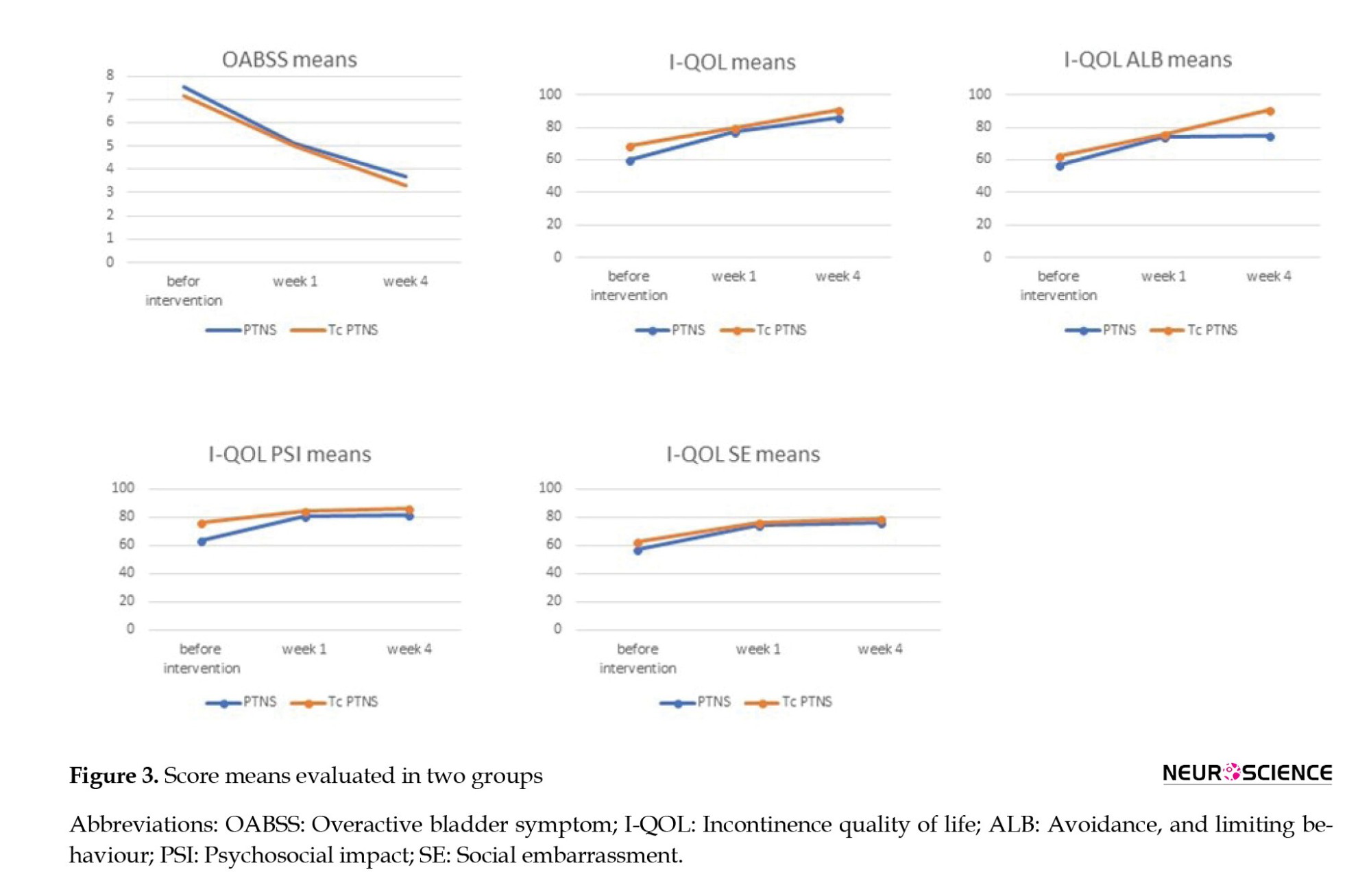
Between-group analysis showed no statistically significant difference between the changes in two groups at three intervals (P=0.79), which means both interventions have the same therapeutic effect (Figure 3).
I-QOL questionnaire
The total and three subscale scores for avoidance and limiting behavior, psychosocial impacts, and social embarrassment were analyzed separately. Within-group analysis of total scores showed that both interventions showed a statistically significant difference in three intervals of follow-up (P<0.001), which indicates both interventions were effective in QOL improvement (Table 3).
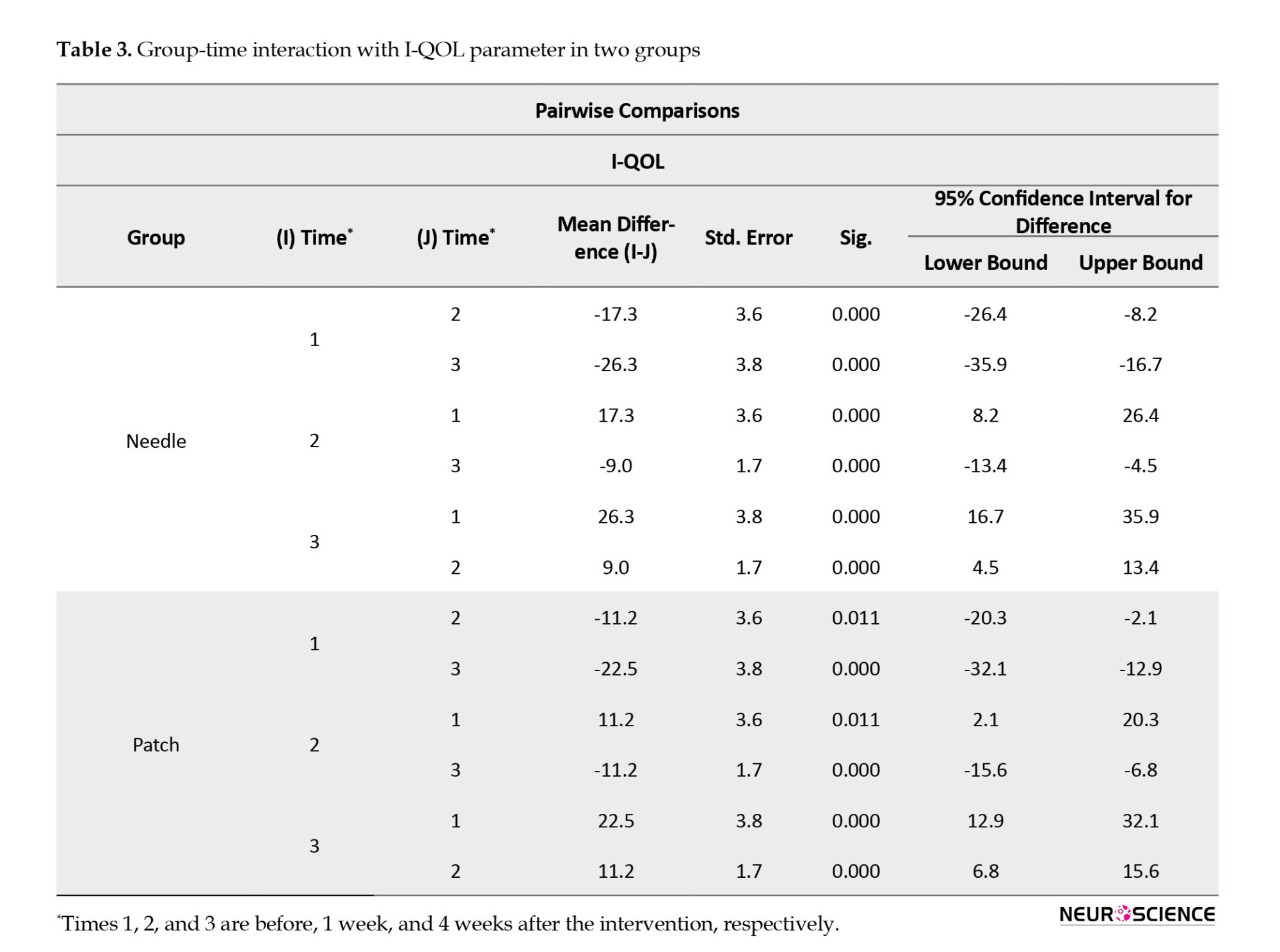
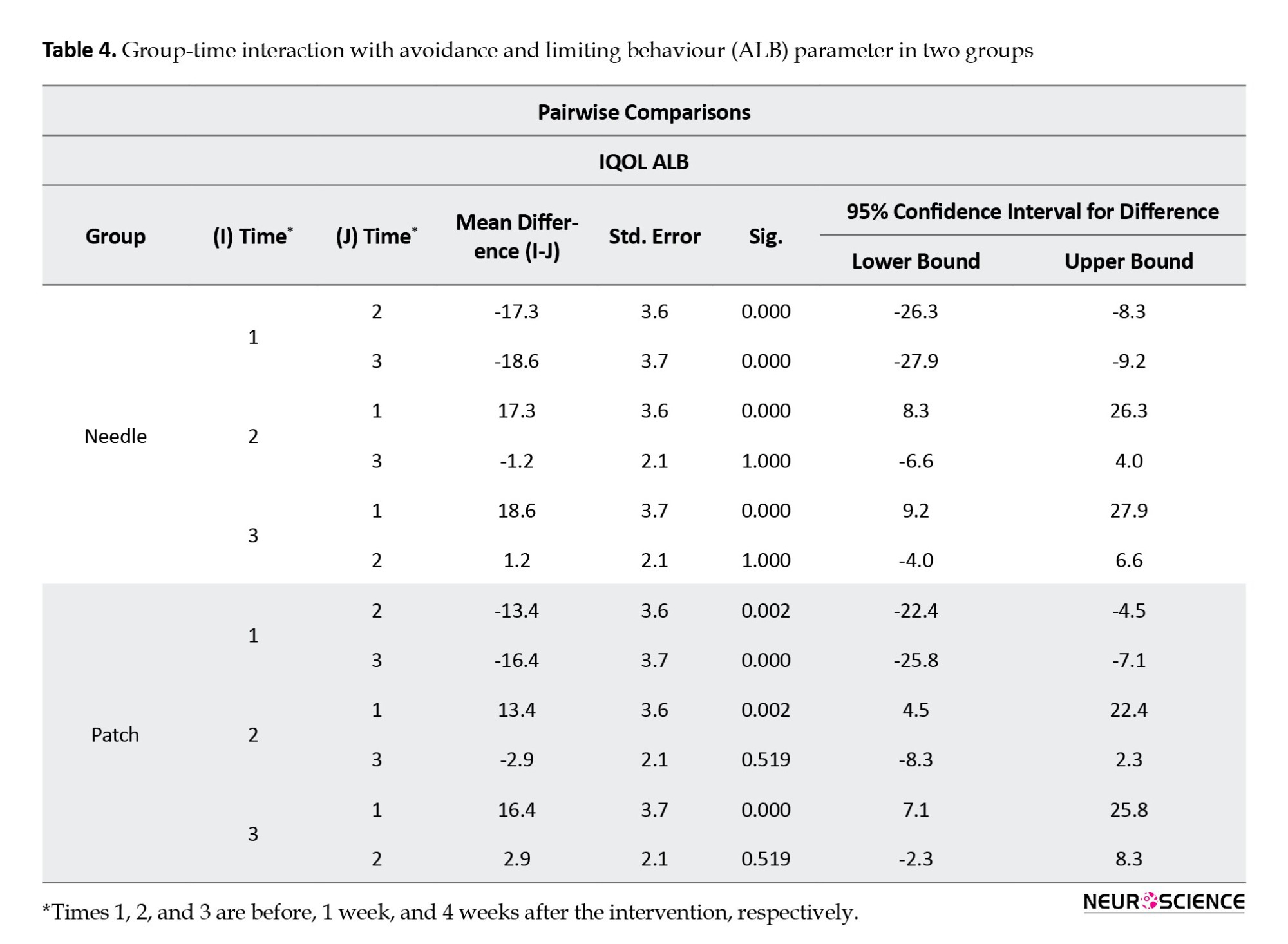
The result of the between-group analysis of the I-QOL questionnaire total scores also showed no statistically significant difference between the changes of the two groups at all time intervals (P=0.37), which means both interventions have acted similarly in refining patients’ QOL (Figure 3). We observed the same results as total scores in all three I-QOL questionnaire subscales (Tables 4, 5, and 6; Figure 3).


4. Discussion
OAB affects a significant proportion of the population with a weighty public health burden. Aside from psychological impacts and physical activity limitations, which affect the individual and the economic system for routine treatment and care, occupational productivity is also affected (Reynolds et al., 2016; Tang et al., 2014). Subjects with OAB had shorter times to disability than those without OAB (Wu et al., 2005).
Conventional first-line treatment includes behavioral and pharmacologic therapy (anticholinergic and ß3 agonists), which can induce better efficacy, lesser drug dosage, and side effects than pharmacologic treatment alone. PTNS, as a less invasive peripheral neuromodulator, is considered for the third-line treatment based on the AUA/SUFU guideline (Lightner et al., 2019).
In 1999, Yamanishi et al. used vaginal/perianal electrical stimulation to treat refractory OAB-induced incontinence and reported increased bladder capacity measured urodynamically (Yamanishi et al., 2000). Published reviews in 2005 and 2009 on PTNS clinical trials found it encouraging, less invasive, economical, and negligible risks in managing a wide range of pelvic floor dysfunction symptoms (Cooperberg & Stoller, 2005; MacDiarmid & Staskin, 2009). In 2012, Staskin et al. evaluated the aspects of PTNS, including effectiveness, adverse effects, and cost-effectiveness with other treatment options. They suggested that it should be considered early in the care algorithm of OAB patients (Staskin et al., 2012). Eventually, the evidence reported OAB PTNS therapy as level 1 in 2013 (Gaziev et al., 2013).
TTNS, an even less invasive intervention than PTNS, is also being investigated and reported effective in recent trials as a neuromodulation method for OAB treatment. Two studies reported that this method could provide greater benefits than behavioral therapy (Booth et al., 2018). Two 30-minute sessions of TTNS showed a similar outcome as 10 mg of extended-release oxybutynin after 12 weeks of treatment (Manríquez et al., 2016). In the study of Ammi et al., home-based daily TTNS for one month was successful in 53% of patients with previous failed anticholinergic therapy (Ammi et al., 2014), and interestingly, it could also be effective by being used once a week for 3 months (Moratalla Charcos et al., 2018). The number of therapy sessions per week and different stimulation characteristics that may alter the therapeutic result must be enlightened by further trials.
In line with our study, other researchers have reported encouraging results from comparing two methods of TNS for OAB treatment. In a review of 4 trials (142 patients) comparing PTNS and TTNS by Yang et al., (2021). TTNS showed the same effectiveness as PTNS for treating OAB patients (Yang et al., 2021). Similarly, a recent clinical trial compared PTNS and TTNS for OAB treatment in a 12-week therapy course and concluded the same effectiveness of these two methods (Ramírez‐García et al., 2020).
In this study, we found that TTNS and PTNS are both equally effective in OAB. We evaluated the effectiveness of 30 minutes of tibial nerve stimulation for 12 sessions, 3 times a week (4 weeks in total). All three symptoms of OAB (frequency, urgency, and incontinency) were improved one week after the treatment and kept the progressive trend to the fourth week after the intervention in both groups, together with the QOL improvements related to OAB-induced avoidance and limiting behavior, psychosocial impacts, and social embarrassment. Comparing the study of Ramírez‐García et al., (2020) with ours, 12 sessions of P/TTNS with similar stimulation parameters, but at different intervals (1 and 3 times a week), were effective in controlling OAB symptoms.
Considering the anticholinergic side effects of medications used in non-responders to behavioral therapy alone and the reported similar effects of PTNS and medication (Manríquez et al., 2016), it may be rational to investigate TTNS therapy as a second-line treatment after behavioral therapy.
Regarding the safety and feasibility of TTNS, it can also be suggested as a home-based modality for the initial treatment course or long-term maintenance therapy (Seth et al., 2018; Van Der Pal & Van Balken, 2006; Yoong et al., 2013). This method may extend its therapeutic effects while lessening the time-consuming process of inpatient PTNS and its burden on the patient and healthcare system. In the study of Martin-Garcia and Crampton, (2019) home-based TTNS is reported as effective as maintenance PTNS treatment for previous PTNS responders. Almost 50% of patients who completed inpatient sessions are said to discontinue long-term PTNS therapy (Gordon et al., 2020; Jung et al., 2020). Thus, it would be hypothesized that home-based TTNS may improve patient adherence to the treatment, which needs further investigation.
5. Conclusion
TTNS could effectively manage OAB symptoms in the same way as PTNS. The relatively equal effectiveness of both methods and the less invasive, more feasible process of TTNS make it an excellent option to be considered early in the management of OAB patients.
Study strengths and limitations
This RCT has a triple-blind design with properly matched controls. However, the long-term effectiveness of P/TTNS in OAB syndrome was not evaluated.
Ethical Considerations
Compliance with ethical guidelines
This clinical trial was approved by the Ethics Committee of Iran University of Medical Sciences, Tehran, Iran (Code: IR.IUMS.REC1395.94115240080). Also, the study was registered by Iranian Registry of Clinical Trials (IRCT) (Code: IRCT2016101730339N1).
Funding
This study was funded by Neuromuskuloskeletal Research Center, Iran University of Medical Sciences, Tehran, Iran.
Authors' contributions
Study design: Gholamreza Raissi and Tannaz Ahadi; Data interpretation: Ismaeel Noori and Shayesteh Khalifeh Soltani; Data analysis: Shayesteh Khalifeh Soltani: Data acquisition: Ismaeel Noori and Pouya Ghaboosi; Writing: Gholamreza Raissi, Shayesteh Khalifeh Soltani and Tannaz Ahadi; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to express their gratitude to all those who contributed to the success of this clinical trial. The patients and their families, all the colleagues and the PM&R Department staff.
References
Based on the International Continence Society (ICS), overactive bladder (OAB) is urinary urgency, with or without urge incontinence, usually with urinary frequency and nocturia, in the absence of urinary tract infection (UTI) or other pathologies that would explain these symptoms (Wein & Rovner, 2002). The overall prevalence rates of OAB in the United States are reported to be 16.0% and 16.9% for men and women, respectively. However, in another EPIC study in 5 countries (Canada, Germany, Sweden, Italy, and the UK), a similar prevalence of 11.8% was reported for men and women (Irwin et al., 2006; Stewart et al., 2003).
OAB is associated with significantly lower quality-of-life (QoL) scores, higher depression scores, poorer sleep quality, sexual dissatisfaction, and work productivity (Coyne et al., 2008; Stewart et al., 2003). ICS recommends that initial management should include lifestyle modification, bladder training, pelvic floor muscle training, antimuscarinics, anti-diuretic or α-blockers medications, and intermittent catheterization when the post-void residual volume (PVR) >30% of bladder capacity, which may differ based on symptoms severity and sex. Invasive therapies such as neuromodulation and botulinum toxin injections in case of initial management failure and markedly disrupted QoL are being considered (Lightner et al., 2019).
Neuromodulation can be accomplished by invasive implantable sacral nerve root stimulation systems with relatively high complication rates or less invasive perineal, perianal, or tibial nerve stimulation (Cooperberg & Stoller, 2005). Stoller described the percutaneous tibial nerve stimulation (PTNS) technique in the late 1990s as a treatment for OAB syndrome (Stoller, 1999). Posterior tibial nerve (PTN) is a mixed sensorimotor nerve originating from L4 to S3 lumbosacral nerve roots, while sacral S3-S4 spinal segments contribute to pelvic floor, bladder, and urethra autonomic and somatic innervations. Theoretically, PTN stimulation at the medial malleolus directly stimulates the upper sacral segments’ afferent fibers (S1-S2). Although the exact mechanism of the urinary inhibitory response generated by PTN stimulation is unclear, it may exert an inhibitory effect on spinothalamic tract neurons (Chung et al., 1984). More recently, specific spinal receptors and the micturition pathway’s central neuroplasticity are considered to be involved (van der Pal et al., 2006). Several reports show its effectiveness in treating OAB (Burton et al., 2012; Finazzi-Agrò et al., 2010; Gaziev et al., 2013; MacDiarmid et al., 2010; MacDiarmid & Staskin, 2009; Peters et al., 2009; Staskin et al., 2012; van Balken et al., 2001).
A 34-gauge needle electrode is inserted 4–5 cm cephalad to the medial malleolus, a neural access point for regulating bladder and pelvic floor function, for 20 Hz, 200 µs duration electrical stimulation. In an even less invasive manner, transcutaneous tibial nerve stimulation (TTNS) with two surface electrodes for electrical stimulation is suggested to be effective in OAB treatment (Ammi et al., 2014; Manríquez et al., 2016). This study evaluates the therapeutic effect of TTNS and compares it with the PTNS technique for OAB management.
2. Materials and Methods
We enrolled 44 patients of OAB who were diagnosed clinically based on the 2019 American Urological Association (AUA) guideline (Lightner et al., 2019). The inclusion criteria comprised patients older than 18 who had not used anticholinergic medication for one week before and during the study. All participants have received the initial treatment (lifestyle modification, pelvic floor muscle training with or without pharmacologic therapies). Participants were omitted if they were inflicted with these conditions: Diabetes mellitus, pregnancy or attempting to get pregnant, implanted pacemaker, active or recurrent (>4 times per year) UTI, and neurologic disease.
All participants were informed and provided with the consent form. They were randomly assigned to one of the two groups using a randomization table. In the PTNS group, a 34-gauge needle electrode was inserted 5 cm cephalad to the medial malleolus and posterior to the tibial bone (Figure 1). The needle electrode was connected to the active pole, and a surface reference electrode was placed on the medial malleolus. Bi-phasic constant current with 200 µs pulse width, 0.5-9 mA, and 20 Hz was applied to induce big toe plantar flexion for 30 minutes each session, thrice weekly for four weeks.
In the TTNS group, instead of needle insertion, an active surface electrode was used with the same stimulation parameters (Figure 2). The participants filled out the self-reported incontinence quality of life (I-QOL) questionnaire and OAB symptom score (OABSS) form before, 1 week, and 4 weeks after the termination of intervention sessions.

The I-QOL contains 22 items with a 5-point response scale, yielding a total and three subscale scores for avoidance and limiting behavior, psychosocial impacts, and social embarrassment (Patrick et al., 1999). The OABSS quantifies four symptoms of OAB in the past week, which include daytime frequency, night-time frequency, urgency, and urinary incontinence (Homma et al., 2006).
Like the data analyst, the data reviewer was blinded to the participant’s groups despite the physician involved in the therapy sessions.
The mixed analysis of variance (ANOVA) test was used for statistical analysis to compare intervention efficacy between groups. P<0.05 were considered statistically significant.
3. Results
We treated 44 patients, 41 females and 3 males, with no significant difference in mean age, sex, body mass index (BMI), and patients’ symptom duration between the two groups (Table 1).

OABSS questionnaire
In the within-group analysis, we observed a significant difference in this scaled score in all three intervals (before intervention vs week 1, before intervention vs week 4, and week 1 vs week 4) in both groups (P<0.001). Therefore, TTNS and PTNS effectively improved patients’ symptoms (Table 2).

Between-group analysis showed no statistically significant difference between the changes in two groups at three intervals (P=0.79), which means both interventions have the same therapeutic effect (Figure 3).
I-QOL questionnaire
The total and three subscale scores for avoidance and limiting behavior, psychosocial impacts, and social embarrassment were analyzed separately. Within-group analysis of total scores showed that both interventions showed a statistically significant difference in three intervals of follow-up (P<0.001), which indicates both interventions were effective in QOL improvement (Table 3).

The result of the between-group analysis of the I-QOL questionnaire total scores also showed no statistically significant difference between the changes of the two groups at all time intervals (P=0.37), which means both interventions have acted similarly in refining patients’ QOL (Figure 3). We observed the same results as total scores in all three I-QOL questionnaire subscales (Tables 4, 5, and 6; Figure 3).




4. Discussion
OAB affects a significant proportion of the population with a weighty public health burden. Aside from psychological impacts and physical activity limitations, which affect the individual and the economic system for routine treatment and care, occupational productivity is also affected (Reynolds et al., 2016; Tang et al., 2014). Subjects with OAB had shorter times to disability than those without OAB (Wu et al., 2005).
Conventional first-line treatment includes behavioral and pharmacologic therapy (anticholinergic and ß3 agonists), which can induce better efficacy, lesser drug dosage, and side effects than pharmacologic treatment alone. PTNS, as a less invasive peripheral neuromodulator, is considered for the third-line treatment based on the AUA/SUFU guideline (Lightner et al., 2019).
In 1999, Yamanishi et al. used vaginal/perianal electrical stimulation to treat refractory OAB-induced incontinence and reported increased bladder capacity measured urodynamically (Yamanishi et al., 2000). Published reviews in 2005 and 2009 on PTNS clinical trials found it encouraging, less invasive, economical, and negligible risks in managing a wide range of pelvic floor dysfunction symptoms (Cooperberg & Stoller, 2005; MacDiarmid & Staskin, 2009). In 2012, Staskin et al. evaluated the aspects of PTNS, including effectiveness, adverse effects, and cost-effectiveness with other treatment options. They suggested that it should be considered early in the care algorithm of OAB patients (Staskin et al., 2012). Eventually, the evidence reported OAB PTNS therapy as level 1 in 2013 (Gaziev et al., 2013).
TTNS, an even less invasive intervention than PTNS, is also being investigated and reported effective in recent trials as a neuromodulation method for OAB treatment. Two studies reported that this method could provide greater benefits than behavioral therapy (Booth et al., 2018). Two 30-minute sessions of TTNS showed a similar outcome as 10 mg of extended-release oxybutynin after 12 weeks of treatment (Manríquez et al., 2016). In the study of Ammi et al., home-based daily TTNS for one month was successful in 53% of patients with previous failed anticholinergic therapy (Ammi et al., 2014), and interestingly, it could also be effective by being used once a week for 3 months (Moratalla Charcos et al., 2018). The number of therapy sessions per week and different stimulation characteristics that may alter the therapeutic result must be enlightened by further trials.
In line with our study, other researchers have reported encouraging results from comparing two methods of TNS for OAB treatment. In a review of 4 trials (142 patients) comparing PTNS and TTNS by Yang et al., (2021). TTNS showed the same effectiveness as PTNS for treating OAB patients (Yang et al., 2021). Similarly, a recent clinical trial compared PTNS and TTNS for OAB treatment in a 12-week therapy course and concluded the same effectiveness of these two methods (Ramírez‐García et al., 2020).
In this study, we found that TTNS and PTNS are both equally effective in OAB. We evaluated the effectiveness of 30 minutes of tibial nerve stimulation for 12 sessions, 3 times a week (4 weeks in total). All three symptoms of OAB (frequency, urgency, and incontinency) were improved one week after the treatment and kept the progressive trend to the fourth week after the intervention in both groups, together with the QOL improvements related to OAB-induced avoidance and limiting behavior, psychosocial impacts, and social embarrassment. Comparing the study of Ramírez‐García et al., (2020) with ours, 12 sessions of P/TTNS with similar stimulation parameters, but at different intervals (1 and 3 times a week), were effective in controlling OAB symptoms.
Considering the anticholinergic side effects of medications used in non-responders to behavioral therapy alone and the reported similar effects of PTNS and medication (Manríquez et al., 2016), it may be rational to investigate TTNS therapy as a second-line treatment after behavioral therapy.
Regarding the safety and feasibility of TTNS, it can also be suggested as a home-based modality for the initial treatment course or long-term maintenance therapy (Seth et al., 2018; Van Der Pal & Van Balken, 2006; Yoong et al., 2013). This method may extend its therapeutic effects while lessening the time-consuming process of inpatient PTNS and its burden on the patient and healthcare system. In the study of Martin-Garcia and Crampton, (2019) home-based TTNS is reported as effective as maintenance PTNS treatment for previous PTNS responders. Almost 50% of patients who completed inpatient sessions are said to discontinue long-term PTNS therapy (Gordon et al., 2020; Jung et al., 2020). Thus, it would be hypothesized that home-based TTNS may improve patient adherence to the treatment, which needs further investigation.
5. Conclusion
TTNS could effectively manage OAB symptoms in the same way as PTNS. The relatively equal effectiveness of both methods and the less invasive, more feasible process of TTNS make it an excellent option to be considered early in the management of OAB patients.
Study strengths and limitations
This RCT has a triple-blind design with properly matched controls. However, the long-term effectiveness of P/TTNS in OAB syndrome was not evaluated.
Ethical Considerations
Compliance with ethical guidelines
This clinical trial was approved by the Ethics Committee of Iran University of Medical Sciences, Tehran, Iran (Code: IR.IUMS.REC1395.94115240080). Also, the study was registered by Iranian Registry of Clinical Trials (IRCT) (Code: IRCT2016101730339N1).
Funding
This study was funded by Neuromuskuloskeletal Research Center, Iran University of Medical Sciences, Tehran, Iran.
Authors' contributions
Study design: Gholamreza Raissi and Tannaz Ahadi; Data interpretation: Ismaeel Noori and Shayesteh Khalifeh Soltani; Data analysis: Shayesteh Khalifeh Soltani: Data acquisition: Ismaeel Noori and Pouya Ghaboosi; Writing: Gholamreza Raissi, Shayesteh Khalifeh Soltani and Tannaz Ahadi; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to express their gratitude to all those who contributed to the success of this clinical trial. The patients and their families, all the colleagues and the PM&R Department staff.
References
Ammi, M., Chautard, D., Brassart, E., Culty, T., Azzouzi, A. R., & Bigot, P. (2014). Transcutaneous posterior tibial nerve stimulation: evaluation of a therapeutic option in the management of anticholinergic refractory overactive bladder. International Urogynecology Journal, 25(8), 1065-1069. [DOI:10.1007/s00192-014-2359-0] [PMID]
Booth, J., Connelly, L., Dickson, S., Duncan, F., & Lawrence, M. (2018). The effectiveness of transcutaneous tibial nerve stimulation (TTNS) for adults with overactive bladder syndrome: A systematic review. Neurourology and Urodynamics, 37(2), 528-541. [DOI:10.1002/nau.23351] [PMID]
Burton, C., Sajja, A., & Latthe, P. M. (2012). Effectiveness of percutaneous posterior tibial nerve stimulation for overactive bladder: A systematic review and meta‐analysis. Neurourology and Urodynamics, 31(8), 1206-1216. [DOI:10.1002/nau.22251] [PMID]
Chung, J. M., Lee, K. H., Hori, Y., Endo, K., & Willis, W. D. (1984). Factors influencing peripheral nerve stimulation produced inhibition of primate spinothalamic tract cells. Pain, 19(3), 277-293. [PMID]
Cooperberg, M. R., & Stoller, M. L. (2005). Percutaneous neuromodulation. The Urologic Clinics of North America, 32(1), 71–vii. [DOI:10.1016/j.ucl.2004.09.007] [PMID]
Coyne, K. S., Sexton, C. C., Irwin, D. E., Kopp, Z. S., Kelleher, C. J., & Milsom, I. (2008). The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well‐being in men and women: Results from the EPIC study. BJU International, 101(11), 1388–1395. [DOI:10.1111/j.1464-410X.2008.07601.x] [PMID]
Finazzi-Agrò, E., Petta, F., Sciobica, F., Pasqualetti, P., Musco, S., & Bove, P. (2010). Percutaneous tibial nerve stimulation effects on detrusor overactivity incontinence are not due to a placebo effect: A randomized, double-blind, placebo controlled trial. The Journal of Urology, 184(5), 2001-2006. [DOI:10.1016/j.juro.2010.06.113] [PMID]
Gaziev, G., Topazio, L., Iacovelli, V., Asimakopoulos, A., Di Santo, A., & De Nunzio, C., et al. (2013). Percutaneous tibial nerve stimulation (PTNS) efficacy in the treatment of lower urinary tract dysfunctions: A systematic review. BMC Urology, 13, 61. [DOI:10.1186/1471-2490-13-61] [PMID]
Gordon, T., Merchant, M., Ramm, O., & Patel, M. (2021). Factors associated with long-term use of percutaneous tibial nerve stimulation for management of overactive bladder syndrome. Female Pelvic Medicine & Reconstructive Surgery, 27(7), 444–449.[DOI:10.1097/SPV.0000000000000911] [PMID]
Homma, Y., Yoshida, M., Seki, N., Yokoyama, O., Kakizaki, H., & Gotoh, M., et al. (2006). Symptom assessment tool for overactive bladder syndrome-overactive bladder symptom score. Urology, 68(2), 318-323. [DOI:10.1016/j.urology.2006.02.042] [PMID]
Irwin, D. E., Milsom, I., Hunskaar, S., Reilly, K., Kopp, Z., & Herschorn, S., et al. (2006). Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: Results of the EPIC study. European Urology, 50(6), 1306-1315. [DOI:10.1016/j.eururo.2006.09.019] [PMID]
Jung, C. E., Menefee, S. A., & Diwadkar, G. B. (2021). Percutaneous tibial nerve stimulation maintenance therapy for overactive bladder in women: Long-Term success rates and adherence. International Urogynecology Journal, 32(3), 617–625.[DOI:10.1007/s00192-020-04325-1] [PMID]
Lightner, D. J., Gomelsky, A., Souter, L., & Vasavada, S. P. (2019). Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline Amendment 2019. The Journal of Urology, 202(3), 558-563. [DOI:10.1097/ju.0000000000000309] [PMID]
MacDiarmid, S. A., Peters, K. M., Shobeiri, S. A., Wooldridge, L. S., Rovner, E. S., & Leong, F. C., et al. (2010). Long-term durability of percutaneous tibial nerve stimulation for the treatment of overactive bladder. The Journal of Urology, 183(1), 234-240. [DOI:10.1016/j.juro.2009.08.160] [PMID]
MacDiarmid, S. A., & Staskin, D. R. (2009). Percutaneous tibial nerve stimulation (PTNS): A literature-based assessment. Current Bladder Dysfunction Reports, 4(1), 29-33. [DOI:10.1007/s11884-009-0005-3]
Manríquez, V., Guzmán, R., Naser, M., Aguilera, A., Narvaez, S., & Castro, A., et al. (2016). Transcutaneous posterior tibial nerve stimulation versus extended release oxybutynin in overactive bladder patients. A prospective randomized trial. European Journal of Obstetrics & Gynecology and Reproductive Biology, 196, 6-10. [DOI:10.1016/j.ejogrb.2015.09.020] [PMID]
Martin-Garcia, M., & Crampton, J. (2019). A single-blind, randomized controlled trial to evaluate the effectiveness of transcutaneous tibial nerve stimulation (TTNS) in Overactive Bladder symptoms in women responders to percutaneous tibial nerve stimulation (PTNS). Physiotherapy, 105(4), 469-475. [DOI:10.1016/j.physio.2018.12.002] [PMID]
Moratalla Charcos, L. M., Planelles Gomez, J., García Mora, B., Santamaría Navarro, C., & Vidal Moreno, J. F. (2018). Efficacy and satisfaction with transcutaneous electrostimulation of the posterior tibial nerve in overactive bladder syndrome. Journal of Clinical Urology, 11(5), 331-338. [DOI:10.1177/2051415818776186]
Patrick, D. L., Martin, M. L., Bushnell, D. M., Yalcin, I., Wagner, T. H., & Buesching, D. P. (1999). Quality of life of women with urinary incontinence: Further development of the incontinence quality of life instrument (I-QOL) 1. Urology, 53(1), 71-76. [DOI:10.1016/S0090-4295(98)00454-3] [PMID]
Peters, K. M., Macdiarmid, S. A., Wooldridge, L. S., Leong, F. C., Shobeiri, S. A., & Rovner, E. S., et al. (2009). Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: Results from the overactive bladder innovative therapy trial. The Journal of Urology, 182(3), 1055-1061. [DOI:10.1016/j.juro.2009.05.045] [PMID]
Ramírez-García, I., Kauffmann, S., Blanco-Ratto, L., Carralero-Martínez, A., & Sánchez, E. (2021). Patient‐reported outcomes in the setting of a randomized control trial on the efficacy of transcutaneous stimulation of the posterior tibial nerve compared to percutaneous stimulation in idiopathic overactive bladder syndrome. Neurourology and Urodynamics, 40(1), 295–302. [DOI:10.1002/nau.24554] [PMID]
Reynolds, W. S., Fowke, J., & Dmochowski, R. (2016). The burden of overactive bladder on US public health. Current Bladder Dysfunction Reports, 11(1), 8-13. [DOI:10.1007/s11884-016-0344-9] [PMID]
Seth, J. H., Gonzales, G., Haslam, C., Pakzad, M., Vashisht, A., & Sahai, A., et al. (2018). Feasibility of using a novel non-invasive ambulatory tibial nerve stimulation device for the home-based treatment of overactive bladder symptoms. Translational Andrology and Urology, 7(6), 912-919. [DOI:10.21037/tau.2018.09.12] [PMID]
Staskin, D. R., Peters, K. M., MacDiarmid, S., Shore, N., & de Groat, W. C. (2012). Percutaneous tibial nerve stimulation: A clinically and cost effective addition to the overactive bladder algorithm of care. Current Urology Reports, 13(5), 327-334. [DOI:10.1007/s11934-012-0274-9] [PMID]
Stewart, W. F., Van Rooyen, J. B., Cundiff, G. W., Abrams, P., Herzog, A. R., & Corey, R., et al. (2003). Prevalence and burden of overactive bladder in the United States. World Journal of Urology, 20(6), 327-336. [DOI:10.1007/s00345-002-0301-4] [PMID]
Stoller, M. (1999). Afferent nerve stimulation for pelvic floor dysfunction. International Urogynecology Journal, 10(1), P99-P99. [Link]
Tang, D. H., Colayco, D. C., Khalaf, K. M., Piercy, J., Patel, V., & Globe, D., et al. (2014). Impact of urinary incontinence on healthcare resource utilization, health‐related quality of life and productivity in patients with overactive bladder. BJU International, 113(3), 484–491. [DOI:10.1111/bju.12505] [PMID]
van Balken, M. R., Vandoninck, V., Gisolf, K. W., Vergunst, H., Kiemeney, L. A., & Debruyne, F. M., et al. (2001). Posterior tibial nerve stimulation as neuromodulative treatment of lower urinary tract dysfunction. The Journal of Urology, 166(3), 914-918. [PMID]
van der Pal, F., Heesakkers, J. P., & Bemelmans, B. L. (2006). Current opinion on the working mechanisms of neuromodulation in the treatment of lower urinary tract dysfunction. Current Opinion in Urology, 16(4), 261-267. [DOI:10.1097/01.mou.0000232047.87803.1e] [PMID]
Van der Pal, F., van Balken, M. R., Heesakkers, J. P., Debruyne, F. M., & Bemelmans, B. L. (2006). Percutaneous tibial nerve stimulation in the treatment of refractory overactive bladder syndrome: Is maintenance treatment necessary? BJU International, 97(3), 547-550. [DOI:10.1111/j.1464-410X.2006.06055.x] [PMID]
Wein, A. J., & Rovner, E. S. (2002). Definition and epidemiology of overactive bladder. Urology, 60(5), 7-12. [DOI:10.1016/S0090-4295(02)01784-3] [PMID]
Wu, E. Q., Birnbaum, H., Marynchenko, M., Mareva, M., Williamson, T., & Mallett, D. (2005). Employees with overactive bladder: Work loss burden. Journal of Occupational and Environmental Medicine, 47(5), 439-446. [DOI:10.1097/01.jom.0000161744.21780.c1] [PMID]
Yamanishi, T., Yasuda, K., Sakakibara, R., Hattori, T., & Suda, S. (2000). Randomized, double-blind study of electrical stimulation for urinary incontinence due to detrusor overactivity. Urology, 55(3), 353-357. [DOI:10.1016/S0090-4295(99)00476-8] [PMID]
Type of Study: Original |
Subject:
Clinical Neuroscience
Received: 2022/10/31 | Accepted: 2023/03/15 | Published: 2024/07/20
Received: 2022/10/31 | Accepted: 2023/03/15 | Published: 2024/07/20
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





