Volume 15, Issue 6 (November & December 2024)
BCN 2024, 15(6): 843-854 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mohammadi S, Ahmadlou S, Dargahi L, Zibaii M I, Ghasemi P, Asgari Taei A, et al . Pretreatment Effect of Photo-Biomodulation through Alterations of MicroRNAs (21, 124a) in a Rat Model of Ischemic Stroke. BCN 2024; 15 (6) :843-854
URL: http://bcn.iums.ac.ir/article-1-2574-en.html
URL: http://bcn.iums.ac.ir/article-1-2574-en.html
Sanaz Mohammadi1 

 , Salma Ahmadlou2
, Salma Ahmadlou2 

 , Leila Dargahi3
, Leila Dargahi3 

 , Mohammad Ismail Zibaii4
, Mohammad Ismail Zibaii4 

 , Pouria Ghasemi4
, Pouria Ghasemi4 

 , Afsaneh Asgari Taei5
, Afsaneh Asgari Taei5 

 , Andisheh Balouchi1
, Andisheh Balouchi1 
 , Mohammad Reza Bigdeli *6
, Mohammad Reza Bigdeli *6 




 , Salma Ahmadlou2
, Salma Ahmadlou2 

 , Leila Dargahi3
, Leila Dargahi3 

 , Mohammad Ismail Zibaii4
, Mohammad Ismail Zibaii4 

 , Pouria Ghasemi4
, Pouria Ghasemi4 

 , Afsaneh Asgari Taei5
, Afsaneh Asgari Taei5 

 , Andisheh Balouchi1
, Andisheh Balouchi1 
 , Mohammad Reza Bigdeli *6
, Mohammad Reza Bigdeli *6 


1- Department of Animal Sciences and Marine Biology, School of Life Sciences and Biotechnology, Shahid Beheshti University, Tehran, Iran.
2- Institute for Cognitive and Brain Sciences (ICBS), Shahid Beheshti University, Tehran, Iran.
3- Institute of Neuroscience and Cognition, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4- Laser and Plasma Research Institute, Shahid Beheshti University, Tehran, Iran.
5- Neurobiology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
6- Department of Animal Sciences and Marine Biology, Faculty of Life Sciences and Biotechnology, Shahid Beheshti University, Tehran, Iran.
2- Institute for Cognitive and Brain Sciences (ICBS), Shahid Beheshti University, Tehran, Iran.
3- Institute of Neuroscience and Cognition, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4- Laser and Plasma Research Institute, Shahid Beheshti University, Tehran, Iran.
5- Neurobiology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
6- Department of Animal Sciences and Marine Biology, Faculty of Life Sciences and Biotechnology, Shahid Beheshti University, Tehran, Iran.
Keywords: Ischemic stroke, Optogenetic stimulation, Ischemic tolerance, MicroRNA, Neuroprotection, Neurogenesis
Full-Text [PDF 1050 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Stroke is one of the leading causes of adult long-term disability; it has a significant negative economic and social impact on human society. Ischemic stroke is a non-communicable disease that is on the rise in both developed and developing nations. It happens when a blood vessel supplying the brain is blocked (Martinez & Peplow, 2017). By 2030, it is anticipated that many nations will join aging societies (1 in 5 people will be 65 or older), and regrettably, it is predicted that the number of people who suffer from stroke problems will rise every year (Sakai & Shichita, 2019). Most stroke survivors experience various neurological problems and disabilities that affect their long-term everyday activities. However, there is currently no specific treatment for ischemic stroke, necessitating a new viewpoint on the pathophysiological and molecular conditions (Panagal et al., 2019).
The primary neurogenic niches that contain neural progenitor cells (NPCs) and neuroblasts in the adult brain are the subventricular zone (SVZ) in the lateral ventricles and the subgranular zone (SGZ) in the hippocampus. According to Ohab and Carmichael (2008), the neurogenesis in the SVZ reacts to brain injuries, including stroke and traumatic brain injury. The striatum is anatomically close to the SVZ region and is known to send axons and dendrites there. Therefore, the neurogenesis process in the SVZ can be started by stimulating glutamatergic receptors in the striatum, resulting in the survival of neurons in the striatum. The striatum is, therefore, likely to be in the proper location next to the SVZ to influence cellular activity (Morimoto et al., 2011).
Today, the optogenetic technique, as a revolutionary method for stimulating specific brain areas, scrutinizing the role of specific neuron activity in brain function (Sayed Javad Javaheri et al., 2019). Some studies highlight that stimulating the direct flow of striatum cells in the chronic phase of cerebral ischemia leads to an amelioration pattern (Morimoto et al., 2011; Song et al., 2017). Besides, it has been reported that selective activation of glutamatergic neurons in the striatum area using an optogenetic technique causes cascading SVZ cellular responses by increasing regenerative activity and, eventually, triggers ischemic brain function (Song et al., 2017). Given the above findings, it can be assumed that the optical stimulating effect of striatum cells may operate by activating glutamatergic neurons in the striatum (Lu et al., 2019). Although glutamate is an excitatory neurotransmitter in the central nervous system, its over-accumulation in the extracellular matrix may show a cytotoxic effect and lead to the activation of cell death processes such as apoptosis. Apoptosis caused by focal ischemic stroke in a short period (30 to 60 minutes) is an outstanding feature of penumbra in ischemic stroke (Sun et al., 2017).
It has been revealed that microRNAs (miRs), as novel biomarkers, could be targeted in diagnosing and treating ischemic stroke. According to some studies, more than 20% of miRs dysregulate in ischemic stroke, and it can be presumed that they may act as mediators in the pathogenesis of stroke. These small non-coding RNAs are considered essential biological modulators that regulate the substantial signaling pathways in stroke pathology (Han et al., 2014; Liu et al., 2016). Among the reported miRs, miR-21 is an important one often elevated in various diseases and has been shown to play an essential role in cell proliferation and apoptosis (Sekar et al., 2016). It has been reported that the level of miR-21 in rodent models of ischemic stroke is relatively high, and it is a potent antiapoptotic in some biological systems (Liu et al., 2013). The expression of miR-21 protected neurons from cell death caused by hypoxia-activated microglia (Zhang et al., 2012). Under ischemic and reperfusion conditions, increased miR-21 expression via suppression of phosphatase and tensin homolog (PTEN) expression causes an upregulation of the Akt (protein kinase B signaling pathway) signaling, consequently inhibiting apoptosis by suppressing pro-apoptotic factors such as caspase-3 and increasing ratio of Bcl-2/Bax (Yang et al., 2014).
Another small non-coding RNA that dysregulates during stroke is miR-124a. Under physiological and pathophysiological conditions, the Notch signaling pathway regulates neurogenesis by controlling stem cell survival and determining cellular fate in the brain (Zhu et al., 2019). According to previous studies, during ischemic stroke, the Notch signaling pathway is activated in NPCs located in the SVZ region in the lateral ventricles, leading to the proliferation of NPCs (Kageyama et al., 2009; Zhu et al., 2019). It has been reported that miR-124a regulates neurogenesis in the SVZ region of the adult brain by suppressing SOX9 (Liu et al., 2011). The presence of miR-124a in NPCs significantly reduces JAG1 transcription and protein levels, leading to the inactivation of the Notch signaling pathway. It has been shown that in adult rats with stroke, reduced expression of miR-124a in NPCs of SVZ is inversely related to the activation of the Notch signaling pathway (Liu et al., 2011; Wang et al., 2019).
Thus, we propose that specific signatures of reducing infarct volume with optogenetic stimulation could be obtained from brain tissues and behavioral tests and used to identify biomarkers (miR-21, miR-124a) for diagnosis, prognosis, or even etiology of ischemic stroke. These findings show that increased expression of miR-21 protects against neuronal death in ischemia, and decreased expression of miR-124a leads to induction of neurogenesis in neurogenic niches. MiR-21 and miR-124a may be essential molecules for ischemic tolerance. Therefore, we aimed to answer whether pretreatment with optogenetic stimulation through modulating expression levels of miR-21 and miR-124a can restore neurological deficits and tissue injury in a rat model of transient middle cerebral artery occlusion (tMCAO).
2. Materials and Methods
Study animals and experimental design
The Institutional Animal Care and Use Committee of Shahid Beheshti University, Tehran, Iran, approved all animal procedures and ensured they adhered to NIH standards. Wistar adult male rats weighing 230–300 g were housed at 21±3 ºC on a 12-hour light/dark cycle with free access to enough food and drink. Figure 1 depicts the experimental layout for this study.

In this study, 24 rats were randomly divided into three groups: 1) Sham group (stereotaxic surgery with the injection of virus-carrying empty vector, optical cannula implantation, and MCAO surgery without suture thread insertion); 2) Stroke group (stereotaxic surgery with the injection of virus carrying empty vector, optical cannula implantation and tMCAO induction); 3) Pretreatment group (stereotaxic surgery with the injection of virus carrying opsin, optical cannula implantation, optogenetic stimulation for six days, and tMCAO induction).
Briefly, lentivirus suspension was injected into the striatum. On day 25 post-injection, optical fiber was implanted, and laser stimulation was carried out from day 26 to day 32 for 30 minutes daily. Finally, tMCAO surgery was performed on day 33. Evaluation of neurological functions and infarct volume were performed one day following the stroke.
Lentivirus injection
The lentiviral particles carrying pLenti-CaMKIIa-hChR2(H134R)-mCherry-WPRE, a gift from Karl Deisseroth (Zhang et al., 2007) were prepared according to our previous work Chavoshinezhad et al. (2021) Rats were anesthetized with intraperitoneal ketamine/xylazine (80/20 mg/kg body weight) injection. Then, 2.5 µL lentivirus suspension, at a titer of approximately 109 TU/ml, was injected into the right striatum (AP=0.36 mm, ML=3 mm, DV=-4.8 mm relative to the bregma) according to rat brain atlas of Paxinos and Watson (2007). We used a Hamilton syringe (0.05 μL/min) under stereotaxic surgery (Hamilton, Reno, Nevada). After the injection, the needle was maintained in the brain for an additional 10 min before it was withdrawn. Then, the animals were placed in a recovery chamber.
Optical fiber implantation and laser stimulation
On day 25, after the virus injection, animals underwent optical fiber cannula implantation. The ceramic LC fiber optic cannula was implanted into the right striatum; the exact coordination of virus injection was generated through a small burr hole with a drill. The implanted optical fiber was immobilized on the surface of the skull via dental cement. One day later, laser stimulation was performed once daily for 6 consecutive days, from 26 to 32 days after the virus injection. Each stimulation session was lasted for 30 minutes. Every session had 30 explosions, each containing 120 pulses. The parameters of lasers were controlled by LabVIEW software. Each 60-second stimulation cycle comprises 12 seconds stimulation phase and 48 seconds resting phase. In the stimulation phase, 473-nm solid-state laser pulses were administrated at 10 Hz. The laser powers were ~10-11 mW output at the tip of a 200 μm fiber. Laser waves are transmitted through optical fiber attached to a rotary joint patch cable (Thorlabs) so the rat can freely rotate in the chamber (Sayed Javad Javaheri et al., 2019).
tMCAO surgery
Twenty-four hours after the last stimulation, rats were anesthetized with an intraperitoneal injection of chloral hydrate (385 mg/kg, Merck, Germany). The protocol of MCAO surgery was described by Longa et al. (1989). The right middle cerebral artery was occluded by a 3–0 nylon suture. After 60 minutes, the suture was withdrawn to establish reperfusion of the ischemic areas.
Neurological and infarct volumes assessments
A neurological severity score (NSS) was conducted 24 hours after the reperfusion to evaluate neurological function. It was used for the estimation of injury caused by ischemic stroke and the assessment of recovery with laser stimulation. NSS was scaled from 0 to 18 (normal score 0; maximal deficit score 18). The neurological findings included raising the tail and evaluating motor function, sensory function, beam test, and reflex activity (Long et al., 2013).
For infarct volume assessment, triphenyl tetrazolium chloride (TTC) staining was carried out as a reliable histochemical indicator one day after MCAO. Animals were sacrificed, and brains were quickly removed and cut into coronal sections with 2 mm thickness. The sections were stained with 2% TTC solution. Then, they were incubated at 37 °C for 15 minutes. The stained brain sections were photographed and evaluated via ImageJ software, version1.50 and total, striatal, and hippocampal infarct volumes were calculated separately (Khaksar & Bigdeli, 2017). The volume was assessed using the Equation 1:
1. The Corrected Volume of Damaged Area=Left Hemisphere Volume - (Left Hemisphere Volume - Damaged Area Volume)
RNA extraction and quantitative polymerase chain reaction (qPCR)
One day after the stroke, 3 rats from the sham, stroke, and pretreatment groups were sacrificed. The right striatum and hippocampus tissues were immediately dissected on ice, snap-frozen in liquid nitrogen, and kept at −80 °C until qPCR analysis. Total RNA from the striatum and hippocampus were extracted via Trizol solution (Favorgen, Taiwan). NanoDrop 2000c UV-Vis spectrophotometer (Thermos Scientific, USA) was used to detect RNA quality. Reverse transcriptions of RNA to specific cDNA were performed via a cDNA synthesis kit (Smobio, Taiwan). The amplification was performed by a real-time PCR system (ABI, USA) using a SYBR Green master mix (Ampliqon, Denmark). The relative expression level of miR-21 and miR-124a was normalized to the U6 control, and fold change was calculated through the 2-ΔΔCt formula (Table 1).

Statistical analysis
All statistical analyses were performed with GraphPad Prism software, version 9.1 (GraphPad, Inc., La Jolla, CA). The volume of infarction was determined by the ImageJ software, version 1.50 and findings were analyzed by the t-test. The findings of NSS were analyzed using the nonparametric test (the Mann-Whitney test). One-way analysis of variance (ANOVA) followed by post hoc Tukey test was applied to compare molecular assessments between groups. All data are presented Mean±SEM, and statistically significant results were considered when P<0.05.
3. Results
Effect of optogenetic stimulation on neurological deficits
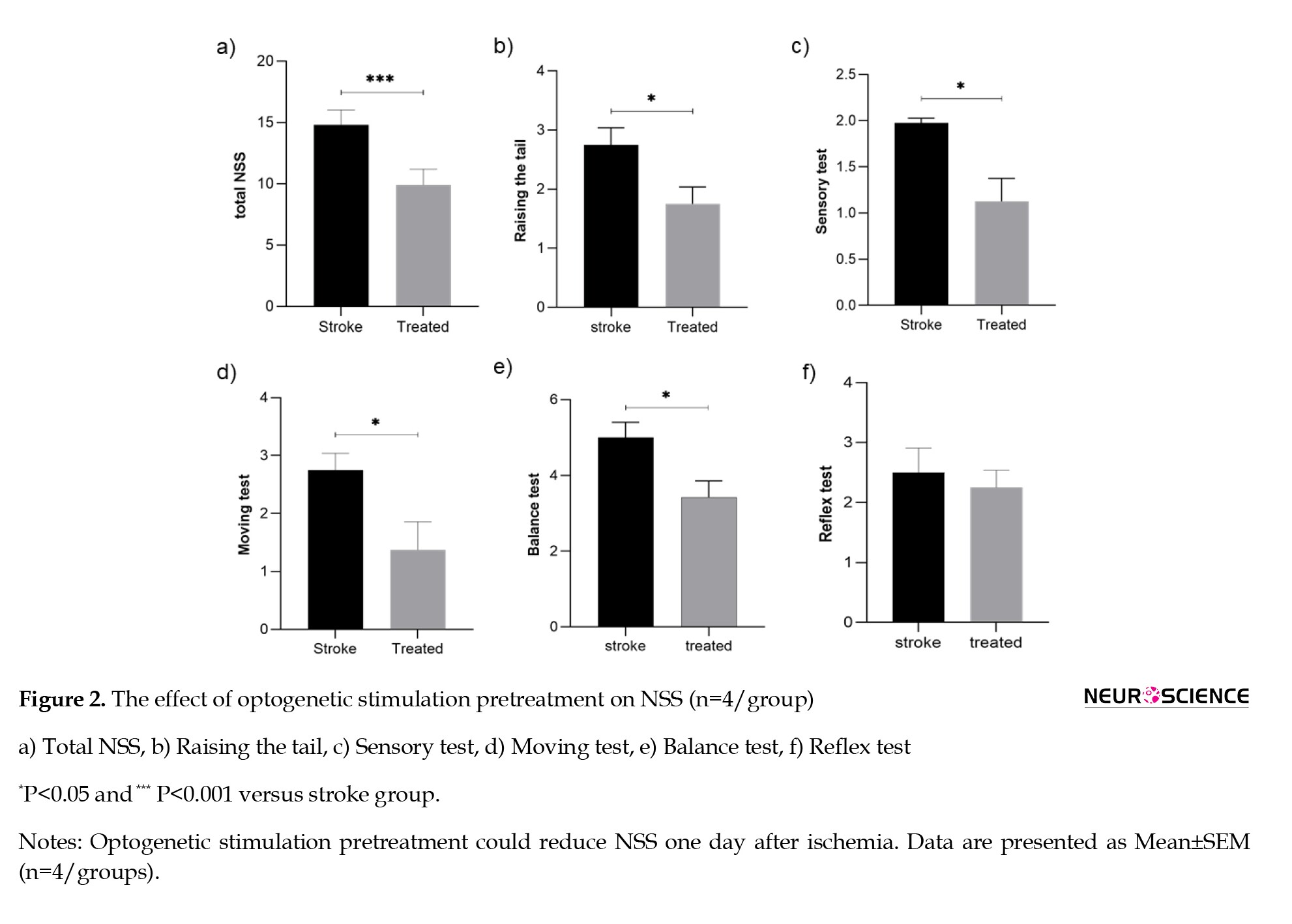
The statistical analysis revealed a significant difference in NSS scores between groups. As shown in Figure 2, optogenetic stimulation pretreatment could improve neurological deficits one day after ischemia compared to the stroke group (P<0.001, Figure 2a).
As seen in Figure 2, parts b–f, the Mann-Whitney analysis indicates that the raising of the tail and sensory, moving, and balance tests significantly reduced in the pretreatment of the optically stimulated group versus the stroke group (P<0.05).
Effect of optogenetic stimulation on infarct volumes
The results showed that in the group with light stimulation of glutamatergic neurons, total infarct volume decreased compared to the stroke group (P<0.01, Figure 3a). Furthermore, statistical analysis demonstrated that infarct volume significantly reduced in the ipsilateral hippocampus and striatum of the pretreated rats compared to stroke a group (Figures 3b and 3c, P <0.001 and P<0.01, respectively).

Effect of optogenetic stimulation on MiR-21 expression
One day after stroke induction, the levels of miR-21 in the striatum and hippocampus were evaluated by the qPCR analysis. One-way ANOVA followed by the Tukey test revealed that miR-21 transcripts in both regions of the MCAO group were not altered compared with the sham rats. Furthermore, the pretreatment with optogenetic stimulation increased the expression level of miR-21 in the striatum compared to the stroke (Figure 4a; P<0.001), while no significant change was found in the mRNA level of miR-21 in the hippocampus of the pretreated group compared to the stroke (Figure 4b; P>0.05).

Effect of optogenetic stimulation on MiR-124a expression changes
The real-time PCR technique used to evaluate miR-124a after optogenetic stimulation showed that the striatum expression level of miR-124a in the pretreatment group was decreased compared to the stroke group. Anyway, this difference between groups was not significant. There also was a significant increase (P<0.05) in the stroke group compared to the sham group. In the hippocampus, the expression level of miR-124a in the pretreatment group was significantly reduced (P<0.05) compared to the stroke group. Also, in the stroke group compared to the sham group, we see a significant increase (P<0.01) in the expression of this miRNA.
One-way ANOVA revealed that miR-124a levels in the striatum and hippocampus were reduced in optically stimulated rats compared with the corresponding stroke (Figures 5a and 5b). It was revealed that miR-124a transcripts in both regions of the MCAO group significantly increased compared with the sham rats (Figures 5a and 4b, P<0.05 and P<0.01, respectively). Moreover, the pretreatment with optogenetic stimulation decreased the expression level of miR-124a in the hippocampus compared to the stroke (Figure 5b; P<0.05), while the changes of miR-124a expression were not statistically significant in the striatum of the optically stimulated group compared to the stroke (Figure 5a).

4. Discussion
In the current study, we established that an animal model of ischemic stroke could develop ischemia tolerance when glutamatergic neurons in the striatum were optogenetically stimulated.
The evaluation of sensory-motor capabilities to assess the severity of the stroke or the stroke recovery process following therapy is one of the most scientific assessments of the stroke. According to the current study, glutamatergic neurons can be stimulated optogenetically to reduce neurological impairments. Based on earlier research, it was shown that optogenetic stimulation has a considerable impact on improving neurological impairments (Chavoshinezhad et al., 2021; Safial Hosseini et al., 2020; Shah et al., 2017). Only optogenetic stimulation is required to activate helpful pathways that aid recovery (Cheng et al., 2014). Optogenetic activation is an approach for maintaining protective neurons in the striatum and primary motor regions (Chen et al., 2017; Pendharkar et al., 2021).
Previous research has also demonstrated a correlation between the recovery of motor function and the migration of DCX+ neuroblasts from the SVZ to the peri-infarct region (Song et al., 2017). The forepaw sensorimotor ability and somatosensory cortical circuit function are both enhanced by optogenetic activation. These findings imply that an optogenetic strategy can rewire thalamocortical circuits and repair dysfunctional brain activity (Tennant et al., 2017). Thus, activating glutamatergic neurons and axons in the striatum may constitute the initial result of brain plasticity on stimulating striatal cells. It has been suggested that the striatum and the sensory-motor regions of the cortex and the hippocampus are anatomically connected indirectly, highlighting the importance of glutamatergic neurons in these interactions. Wang et al. (2016) showed that the striatum and the hippocampus were anatomically connected through the central nucleus of the amygdala. The cortico-striatal-thalamo-cortical (CSTC) network is a significant pathway connecting various brain regions (Rădulescu et al., 2017). Researchers looked at the glutamatergic circuits that mediate the indirect connection between the cortex and the striatum. They demonstrated that in these circuits, the prefrontal cortex activates the striatum by transmitting glutamatergic neurons, and the thalamus communicates with areas of the sensory-motor and prefrontal cortex (Buschman & Miller, 2014). These results may explain how activating glutamatergic neurons in the striatum may promote neurogenesis in the cortical or hippocampus’s neurogenic niche. The neurological function ratings in the pretreatment group likewise showed a significant decline. Recovery from neurological deficiencies is likely linked to lessening damage to nearby brain regions.
Interestingly, we found that light-induced striatum activation had significant results and an additive effect on the diminution of infarct volume. Compared to those not receiving light stimulation, we saw that the infarct volume in the striatum area was much smaller in the pretreatment group. According to Bo et al. (2018) and Lu et al. (2017), optogenetic activation of certain neurons during a stroke is neuroprotective and shrinks the infarct size.
Increasing the activity of the glutamate neurotransmitter and its various receptors through the optogenetic technique triggers neuroprotective molecules (Lerchundi et al., 2015), which are established to protect neurons in ischemia and hypoxia, eventually cell destruction in the striatum should be reduced (Cheng et al., 2014; Monteiro et al., 2021). These molecules protect neurons in ischemia and hypoxia. The rate of cell death in the hippocampus decreased due to the infarct volume. Because the striatum and the hippocampus are indirectly related, the glutamate accumulation in the hippocampus as a neurological niche effectively induces the effect of neuroprotection on neurogenesis as well. As a result, the infarct volume caused by optogenetic pretreatment of the striatum and hippocampus has been greatly reduced.
MicroRNAs are among the main epigenetic elements influencing many nervous system systems (Rink & Khanna, 2011). The potential of miRs as biomarkers for brain damage in ischemic stroke has been investigated (Eyileten et al., 2018; Mirzaei et al., 2018). According to a large body of research, miRs are thought to be important in many cellular alterations that occur after an ischemic stroke (Vilar-Bergua et al., 2016). MiR-21, often raised in many forms of stroke and engaged in antiapoptotic pathways, is regarded as a significant miR among ischemic stroke patients (Chen et al., 2008; Xu et al., 2014).
In particular, miR-21 has been demonstrated to be a strong antiapoptotic factor (Chan et al., 2005). Programmed cell death 4 (PDCD4) is an important functional target of the miR-21 in breast cancer cells (Papagiannakopoulos et al., 2008). The most important target genes for miR-21 are PDCD4, FASLG, and PTEN (MirTarBase database); studies have shown that all of them are highly effective in exacerbating apoptosis and inflammation (Buller et al., 2010; Gaudet et al., 2018; Young et al., 2014). Buller et al. showed that miR-21 would play a definitive role in the decrease of ischemic cell death by targeting FASLG 3′-UTR, which is a main cell death-inducing ligand of the Tumor necrosis factor-alpha (TNF-α) family (Buller et al., 2010). Tumor necrosis factor-alpha is a mediator of focal ischemic brain injury. It can act as a neuroprotective against stroke, and nerve and heart stem cells can be protected from stressful stimuli such as hypoxia and apoptosis (Chen et al., 2017; Shi et al., 2017).
In the current work, optogenetic stimulation of glutamatergic neurons in the striatum area elevated the expression of miR-21 in the pretreatment group compared to the stroke group. It is possible to conclude that up-regulation of miR-21 in the striatum by optogenetic stimulation increases neuroprotective processes and ultimately prevents cell death by ischemia based on the results of NSS and infarct volume in the striatum region. Even though miR-21 expression in the hippocampus region of the pretreatment group increased, the results did not indicate any relevance.
A bulk of studies show that miR-124 is the most extremely expressed miRNA in the central nervous system affiliated with the development of ischemic stroke (Wang et al., 2017). The plasma level of miR-124a can be used to diagnose ischemic brain damage (Laterza et al., 2009). After ischemia in rats, the plasma level of miR-124 was elevated, which offers its capacity as a biomarker for ischemic stroke (Weng et al., 2011). Some studies acknowledged that cell proliferation and promoting cell differentiation could be inhibited via miR-124 (Cai et al., 2012; Lang et al., 2012; Makeyev et al., 2007).
According to the findings of Liu et al., (2013), the brain level of inhibitory members of the apoptosis-stimulating proteins of the p53 family (iASPP) is a possible target of miR-124, reduced after ischemic stroke in vivo condition. Additionally, inhibition of miR-124 increased the level of iASPP and significantly decreased infarction in ischemia. Also, their study indicates that after ischemic stroke, neural cell death mediated by p53 can be nontranscriptionally regulated by suppressing the mechanism of endogenous cell death inhibitors by miR-124. Based on the study findings, we observed that the expression of miR-124a in NPCs in the hippocampus decreased significantly one day after ischemic stroke. In other words, because the hippocampal region is a neurogenic niche and NPCs differentiate in this region, pretreatment by optogenetic technique caused miR-124a to be down-regulated. The observed improvement in disease status was consistent with the results of stroke volume measurements. Understanding the interaction between miRNAs and the regulatory mechanisms in the adult brain after stroke could potentially provide new therapies to prevent neural cell death after ischemic stroke.
5. Conclusion
Optogenetics has become a potent method for controlling intracellular signaling cascades thanks to light-induced proteins. By assessing miR-21 and miR-124a expression levels and exciting glutamatergic neurons in an animal model of ischemic stroke, we looked into the potential pretreatment effect of the optogenetic technology. The present study’s findings suggest that up-regulation of miR-21, a neuroprotection marker, improves neurological abnormalities and decreases infarct volume in the striatum and hippocampus. Furthermore, it can be deduced from the downregulation of miR-124a that this gene contributes to neurogenesis, neuroprotection, and the differentiation of NPCs into neurons, all of which alleviate ischemia conditions. Because compulsive ischemia may raise the likelihood of blood clot formation or conditions needing surgery, such as carotid aneurysms, our finding shows that neuroprotection induced by optogenetics can be useful in producing ischemic tolerance. We, therefore, believed that using optogenetic techniques in the clinical setting could help reduce brain damage brought on by cerebral ischemia in situations of compulsive ischemia based on our findings and those of earlier investigations.
Ethical Considerations
Compliance with ethical guidelines
All the experimental protocols were approved by the Animal Research Ethics Committee at Shahid Beheshti University, Tehran, Iran. This project was conducted in accordance with NIH ethical guidelines.
Funding
This research was funded by the Cognitive Sciences and Technology Council (CSTC) of Tehran, Iran.
Authors' contributions
Conceptualization, supervision and funding acquisition: Mohammad Reza Bigdeli; Methodology: Salma Ahmadlou, Leila Dargahi and Mohammad Ismail Zibaii Investigation; Software, data Analysis, and writing the original draft: Sanaz Mohammadi, and Pouria Ghasemi; Review and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank Mir Hossein Nazari, Ahmad Ghorbani, and Elham Sadat Seyed Javad Javaheri for providing helpful assistance in optogenetics stimulation of animals, Sara Chavoshi Nezhad as experimental assistants in Neurobiology Research Center of Shahid Beheshti University of Medical Sciences, and all the experts who helped in this project.
References
Stroke is one of the leading causes of adult long-term disability; it has a significant negative economic and social impact on human society. Ischemic stroke is a non-communicable disease that is on the rise in both developed and developing nations. It happens when a blood vessel supplying the brain is blocked (Martinez & Peplow, 2017). By 2030, it is anticipated that many nations will join aging societies (1 in 5 people will be 65 or older), and regrettably, it is predicted that the number of people who suffer from stroke problems will rise every year (Sakai & Shichita, 2019). Most stroke survivors experience various neurological problems and disabilities that affect their long-term everyday activities. However, there is currently no specific treatment for ischemic stroke, necessitating a new viewpoint on the pathophysiological and molecular conditions (Panagal et al., 2019).
The primary neurogenic niches that contain neural progenitor cells (NPCs) and neuroblasts in the adult brain are the subventricular zone (SVZ) in the lateral ventricles and the subgranular zone (SGZ) in the hippocampus. According to Ohab and Carmichael (2008), the neurogenesis in the SVZ reacts to brain injuries, including stroke and traumatic brain injury. The striatum is anatomically close to the SVZ region and is known to send axons and dendrites there. Therefore, the neurogenesis process in the SVZ can be started by stimulating glutamatergic receptors in the striatum, resulting in the survival of neurons in the striatum. The striatum is, therefore, likely to be in the proper location next to the SVZ to influence cellular activity (Morimoto et al., 2011).
Today, the optogenetic technique, as a revolutionary method for stimulating specific brain areas, scrutinizing the role of specific neuron activity in brain function (Sayed Javad Javaheri et al., 2019). Some studies highlight that stimulating the direct flow of striatum cells in the chronic phase of cerebral ischemia leads to an amelioration pattern (Morimoto et al., 2011; Song et al., 2017). Besides, it has been reported that selective activation of glutamatergic neurons in the striatum area using an optogenetic technique causes cascading SVZ cellular responses by increasing regenerative activity and, eventually, triggers ischemic brain function (Song et al., 2017). Given the above findings, it can be assumed that the optical stimulating effect of striatum cells may operate by activating glutamatergic neurons in the striatum (Lu et al., 2019). Although glutamate is an excitatory neurotransmitter in the central nervous system, its over-accumulation in the extracellular matrix may show a cytotoxic effect and lead to the activation of cell death processes such as apoptosis. Apoptosis caused by focal ischemic stroke in a short period (30 to 60 minutes) is an outstanding feature of penumbra in ischemic stroke (Sun et al., 2017).
It has been revealed that microRNAs (miRs), as novel biomarkers, could be targeted in diagnosing and treating ischemic stroke. According to some studies, more than 20% of miRs dysregulate in ischemic stroke, and it can be presumed that they may act as mediators in the pathogenesis of stroke. These small non-coding RNAs are considered essential biological modulators that regulate the substantial signaling pathways in stroke pathology (Han et al., 2014; Liu et al., 2016). Among the reported miRs, miR-21 is an important one often elevated in various diseases and has been shown to play an essential role in cell proliferation and apoptosis (Sekar et al., 2016). It has been reported that the level of miR-21 in rodent models of ischemic stroke is relatively high, and it is a potent antiapoptotic in some biological systems (Liu et al., 2013). The expression of miR-21 protected neurons from cell death caused by hypoxia-activated microglia (Zhang et al., 2012). Under ischemic and reperfusion conditions, increased miR-21 expression via suppression of phosphatase and tensin homolog (PTEN) expression causes an upregulation of the Akt (protein kinase B signaling pathway) signaling, consequently inhibiting apoptosis by suppressing pro-apoptotic factors such as caspase-3 and increasing ratio of Bcl-2/Bax (Yang et al., 2014).
Another small non-coding RNA that dysregulates during stroke is miR-124a. Under physiological and pathophysiological conditions, the Notch signaling pathway regulates neurogenesis by controlling stem cell survival and determining cellular fate in the brain (Zhu et al., 2019). According to previous studies, during ischemic stroke, the Notch signaling pathway is activated in NPCs located in the SVZ region in the lateral ventricles, leading to the proliferation of NPCs (Kageyama et al., 2009; Zhu et al., 2019). It has been reported that miR-124a regulates neurogenesis in the SVZ region of the adult brain by suppressing SOX9 (Liu et al., 2011). The presence of miR-124a in NPCs significantly reduces JAG1 transcription and protein levels, leading to the inactivation of the Notch signaling pathway. It has been shown that in adult rats with stroke, reduced expression of miR-124a in NPCs of SVZ is inversely related to the activation of the Notch signaling pathway (Liu et al., 2011; Wang et al., 2019).
Thus, we propose that specific signatures of reducing infarct volume with optogenetic stimulation could be obtained from brain tissues and behavioral tests and used to identify biomarkers (miR-21, miR-124a) for diagnosis, prognosis, or even etiology of ischemic stroke. These findings show that increased expression of miR-21 protects against neuronal death in ischemia, and decreased expression of miR-124a leads to induction of neurogenesis in neurogenic niches. MiR-21 and miR-124a may be essential molecules for ischemic tolerance. Therefore, we aimed to answer whether pretreatment with optogenetic stimulation through modulating expression levels of miR-21 and miR-124a can restore neurological deficits and tissue injury in a rat model of transient middle cerebral artery occlusion (tMCAO).
2. Materials and Methods
Study animals and experimental design
The Institutional Animal Care and Use Committee of Shahid Beheshti University, Tehran, Iran, approved all animal procedures and ensured they adhered to NIH standards. Wistar adult male rats weighing 230–300 g were housed at 21±3 ºC on a 12-hour light/dark cycle with free access to enough food and drink. Figure 1 depicts the experimental layout for this study.

In this study, 24 rats were randomly divided into three groups: 1) Sham group (stereotaxic surgery with the injection of virus-carrying empty vector, optical cannula implantation, and MCAO surgery without suture thread insertion); 2) Stroke group (stereotaxic surgery with the injection of virus carrying empty vector, optical cannula implantation and tMCAO induction); 3) Pretreatment group (stereotaxic surgery with the injection of virus carrying opsin, optical cannula implantation, optogenetic stimulation for six days, and tMCAO induction).
Briefly, lentivirus suspension was injected into the striatum. On day 25 post-injection, optical fiber was implanted, and laser stimulation was carried out from day 26 to day 32 for 30 minutes daily. Finally, tMCAO surgery was performed on day 33. Evaluation of neurological functions and infarct volume were performed one day following the stroke.
Lentivirus injection
The lentiviral particles carrying pLenti-CaMKIIa-hChR2(H134R)-mCherry-WPRE, a gift from Karl Deisseroth (Zhang et al., 2007) were prepared according to our previous work Chavoshinezhad et al. (2021) Rats were anesthetized with intraperitoneal ketamine/xylazine (80/20 mg/kg body weight) injection. Then, 2.5 µL lentivirus suspension, at a titer of approximately 109 TU/ml, was injected into the right striatum (AP=0.36 mm, ML=3 mm, DV=-4.8 mm relative to the bregma) according to rat brain atlas of Paxinos and Watson (2007). We used a Hamilton syringe (0.05 μL/min) under stereotaxic surgery (Hamilton, Reno, Nevada). After the injection, the needle was maintained in the brain for an additional 10 min before it was withdrawn. Then, the animals were placed in a recovery chamber.
Optical fiber implantation and laser stimulation
On day 25, after the virus injection, animals underwent optical fiber cannula implantation. The ceramic LC fiber optic cannula was implanted into the right striatum; the exact coordination of virus injection was generated through a small burr hole with a drill. The implanted optical fiber was immobilized on the surface of the skull via dental cement. One day later, laser stimulation was performed once daily for 6 consecutive days, from 26 to 32 days after the virus injection. Each stimulation session was lasted for 30 minutes. Every session had 30 explosions, each containing 120 pulses. The parameters of lasers were controlled by LabVIEW software. Each 60-second stimulation cycle comprises 12 seconds stimulation phase and 48 seconds resting phase. In the stimulation phase, 473-nm solid-state laser pulses were administrated at 10 Hz. The laser powers were ~10-11 mW output at the tip of a 200 μm fiber. Laser waves are transmitted through optical fiber attached to a rotary joint patch cable (Thorlabs) so the rat can freely rotate in the chamber (Sayed Javad Javaheri et al., 2019).
tMCAO surgery
Twenty-four hours after the last stimulation, rats were anesthetized with an intraperitoneal injection of chloral hydrate (385 mg/kg, Merck, Germany). The protocol of MCAO surgery was described by Longa et al. (1989). The right middle cerebral artery was occluded by a 3–0 nylon suture. After 60 minutes, the suture was withdrawn to establish reperfusion of the ischemic areas.
Neurological and infarct volumes assessments
A neurological severity score (NSS) was conducted 24 hours after the reperfusion to evaluate neurological function. It was used for the estimation of injury caused by ischemic stroke and the assessment of recovery with laser stimulation. NSS was scaled from 0 to 18 (normal score 0; maximal deficit score 18). The neurological findings included raising the tail and evaluating motor function, sensory function, beam test, and reflex activity (Long et al., 2013).
For infarct volume assessment, triphenyl tetrazolium chloride (TTC) staining was carried out as a reliable histochemical indicator one day after MCAO. Animals were sacrificed, and brains were quickly removed and cut into coronal sections with 2 mm thickness. The sections were stained with 2% TTC solution. Then, they were incubated at 37 °C for 15 minutes. The stained brain sections were photographed and evaluated via ImageJ software, version1.50 and total, striatal, and hippocampal infarct volumes were calculated separately (Khaksar & Bigdeli, 2017). The volume was assessed using the Equation 1:
1. The Corrected Volume of Damaged Area=Left Hemisphere Volume - (Left Hemisphere Volume - Damaged Area Volume)
RNA extraction and quantitative polymerase chain reaction (qPCR)
One day after the stroke, 3 rats from the sham, stroke, and pretreatment groups were sacrificed. The right striatum and hippocampus tissues were immediately dissected on ice, snap-frozen in liquid nitrogen, and kept at −80 °C until qPCR analysis. Total RNA from the striatum and hippocampus were extracted via Trizol solution (Favorgen, Taiwan). NanoDrop 2000c UV-Vis spectrophotometer (Thermos Scientific, USA) was used to detect RNA quality. Reverse transcriptions of RNA to specific cDNA were performed via a cDNA synthesis kit (Smobio, Taiwan). The amplification was performed by a real-time PCR system (ABI, USA) using a SYBR Green master mix (Ampliqon, Denmark). The relative expression level of miR-21 and miR-124a was normalized to the U6 control, and fold change was calculated through the 2-ΔΔCt formula (Table 1).

Statistical analysis
All statistical analyses were performed with GraphPad Prism software, version 9.1 (GraphPad, Inc., La Jolla, CA). The volume of infarction was determined by the ImageJ software, version 1.50 and findings were analyzed by the t-test. The findings of NSS were analyzed using the nonparametric test (the Mann-Whitney test). One-way analysis of variance (ANOVA) followed by post hoc Tukey test was applied to compare molecular assessments between groups. All data are presented Mean±SEM, and statistically significant results were considered when P<0.05.
3. Results
Effect of optogenetic stimulation on neurological deficits
The statistical analysis revealed a significant difference in NSS scores between groups. As shown in Figure 2, optogenetic stimulation pretreatment could improve neurological deficits one day after ischemia compared to the stroke group (P<0.001, Figure 2a).
As seen in Figure 2, parts b–f, the Mann-Whitney analysis indicates that the raising of the tail and sensory, moving, and balance tests significantly reduced in the pretreatment of the optically stimulated group versus the stroke group (P<0.05).

Effect of optogenetic stimulation on infarct volumes
The results showed that in the group with light stimulation of glutamatergic neurons, total infarct volume decreased compared to the stroke group (P<0.01, Figure 3a). Furthermore, statistical analysis demonstrated that infarct volume significantly reduced in the ipsilateral hippocampus and striatum of the pretreated rats compared to stroke a group (Figures 3b and 3c, P <0.001 and P<0.01, respectively).

Effect of optogenetic stimulation on MiR-21 expression
One day after stroke induction, the levels of miR-21 in the striatum and hippocampus were evaluated by the qPCR analysis. One-way ANOVA followed by the Tukey test revealed that miR-21 transcripts in both regions of the MCAO group were not altered compared with the sham rats. Furthermore, the pretreatment with optogenetic stimulation increased the expression level of miR-21 in the striatum compared to the stroke (Figure 4a; P<0.001), while no significant change was found in the mRNA level of miR-21 in the hippocampus of the pretreated group compared to the stroke (Figure 4b; P>0.05).

Effect of optogenetic stimulation on MiR-124a expression changes
The real-time PCR technique used to evaluate miR-124a after optogenetic stimulation showed that the striatum expression level of miR-124a in the pretreatment group was decreased compared to the stroke group. Anyway, this difference between groups was not significant. There also was a significant increase (P<0.05) in the stroke group compared to the sham group. In the hippocampus, the expression level of miR-124a in the pretreatment group was significantly reduced (P<0.05) compared to the stroke group. Also, in the stroke group compared to the sham group, we see a significant increase (P<0.01) in the expression of this miRNA.
One-way ANOVA revealed that miR-124a levels in the striatum and hippocampus were reduced in optically stimulated rats compared with the corresponding stroke (Figures 5a and 5b). It was revealed that miR-124a transcripts in both regions of the MCAO group significantly increased compared with the sham rats (Figures 5a and 4b, P<0.05 and P<0.01, respectively). Moreover, the pretreatment with optogenetic stimulation decreased the expression level of miR-124a in the hippocampus compared to the stroke (Figure 5b; P<0.05), while the changes of miR-124a expression were not statistically significant in the striatum of the optically stimulated group compared to the stroke (Figure 5a).

4. Discussion
In the current study, we established that an animal model of ischemic stroke could develop ischemia tolerance when glutamatergic neurons in the striatum were optogenetically stimulated.
The evaluation of sensory-motor capabilities to assess the severity of the stroke or the stroke recovery process following therapy is one of the most scientific assessments of the stroke. According to the current study, glutamatergic neurons can be stimulated optogenetically to reduce neurological impairments. Based on earlier research, it was shown that optogenetic stimulation has a considerable impact on improving neurological impairments (Chavoshinezhad et al., 2021; Safial Hosseini et al., 2020; Shah et al., 2017). Only optogenetic stimulation is required to activate helpful pathways that aid recovery (Cheng et al., 2014). Optogenetic activation is an approach for maintaining protective neurons in the striatum and primary motor regions (Chen et al., 2017; Pendharkar et al., 2021).
Previous research has also demonstrated a correlation between the recovery of motor function and the migration of DCX+ neuroblasts from the SVZ to the peri-infarct region (Song et al., 2017). The forepaw sensorimotor ability and somatosensory cortical circuit function are both enhanced by optogenetic activation. These findings imply that an optogenetic strategy can rewire thalamocortical circuits and repair dysfunctional brain activity (Tennant et al., 2017). Thus, activating glutamatergic neurons and axons in the striatum may constitute the initial result of brain plasticity on stimulating striatal cells. It has been suggested that the striatum and the sensory-motor regions of the cortex and the hippocampus are anatomically connected indirectly, highlighting the importance of glutamatergic neurons in these interactions. Wang et al. (2016) showed that the striatum and the hippocampus were anatomically connected through the central nucleus of the amygdala. The cortico-striatal-thalamo-cortical (CSTC) network is a significant pathway connecting various brain regions (Rădulescu et al., 2017). Researchers looked at the glutamatergic circuits that mediate the indirect connection between the cortex and the striatum. They demonstrated that in these circuits, the prefrontal cortex activates the striatum by transmitting glutamatergic neurons, and the thalamus communicates with areas of the sensory-motor and prefrontal cortex (Buschman & Miller, 2014). These results may explain how activating glutamatergic neurons in the striatum may promote neurogenesis in the cortical or hippocampus’s neurogenic niche. The neurological function ratings in the pretreatment group likewise showed a significant decline. Recovery from neurological deficiencies is likely linked to lessening damage to nearby brain regions.
Interestingly, we found that light-induced striatum activation had significant results and an additive effect on the diminution of infarct volume. Compared to those not receiving light stimulation, we saw that the infarct volume in the striatum area was much smaller in the pretreatment group. According to Bo et al. (2018) and Lu et al. (2017), optogenetic activation of certain neurons during a stroke is neuroprotective and shrinks the infarct size.
Increasing the activity of the glutamate neurotransmitter and its various receptors through the optogenetic technique triggers neuroprotective molecules (Lerchundi et al., 2015), which are established to protect neurons in ischemia and hypoxia, eventually cell destruction in the striatum should be reduced (Cheng et al., 2014; Monteiro et al., 2021). These molecules protect neurons in ischemia and hypoxia. The rate of cell death in the hippocampus decreased due to the infarct volume. Because the striatum and the hippocampus are indirectly related, the glutamate accumulation in the hippocampus as a neurological niche effectively induces the effect of neuroprotection on neurogenesis as well. As a result, the infarct volume caused by optogenetic pretreatment of the striatum and hippocampus has been greatly reduced.
MicroRNAs are among the main epigenetic elements influencing many nervous system systems (Rink & Khanna, 2011). The potential of miRs as biomarkers for brain damage in ischemic stroke has been investigated (Eyileten et al., 2018; Mirzaei et al., 2018). According to a large body of research, miRs are thought to be important in many cellular alterations that occur after an ischemic stroke (Vilar-Bergua et al., 2016). MiR-21, often raised in many forms of stroke and engaged in antiapoptotic pathways, is regarded as a significant miR among ischemic stroke patients (Chen et al., 2008; Xu et al., 2014).
In particular, miR-21 has been demonstrated to be a strong antiapoptotic factor (Chan et al., 2005). Programmed cell death 4 (PDCD4) is an important functional target of the miR-21 in breast cancer cells (Papagiannakopoulos et al., 2008). The most important target genes for miR-21 are PDCD4, FASLG, and PTEN (MirTarBase database); studies have shown that all of them are highly effective in exacerbating apoptosis and inflammation (Buller et al., 2010; Gaudet et al., 2018; Young et al., 2014). Buller et al. showed that miR-21 would play a definitive role in the decrease of ischemic cell death by targeting FASLG 3′-UTR, which is a main cell death-inducing ligand of the Tumor necrosis factor-alpha (TNF-α) family (Buller et al., 2010). Tumor necrosis factor-alpha is a mediator of focal ischemic brain injury. It can act as a neuroprotective against stroke, and nerve and heart stem cells can be protected from stressful stimuli such as hypoxia and apoptosis (Chen et al., 2017; Shi et al., 2017).
In the current work, optogenetic stimulation of glutamatergic neurons in the striatum area elevated the expression of miR-21 in the pretreatment group compared to the stroke group. It is possible to conclude that up-regulation of miR-21 in the striatum by optogenetic stimulation increases neuroprotective processes and ultimately prevents cell death by ischemia based on the results of NSS and infarct volume in the striatum region. Even though miR-21 expression in the hippocampus region of the pretreatment group increased, the results did not indicate any relevance.
A bulk of studies show that miR-124 is the most extremely expressed miRNA in the central nervous system affiliated with the development of ischemic stroke (Wang et al., 2017). The plasma level of miR-124a can be used to diagnose ischemic brain damage (Laterza et al., 2009). After ischemia in rats, the plasma level of miR-124 was elevated, which offers its capacity as a biomarker for ischemic stroke (Weng et al., 2011). Some studies acknowledged that cell proliferation and promoting cell differentiation could be inhibited via miR-124 (Cai et al., 2012; Lang et al., 2012; Makeyev et al., 2007).
According to the findings of Liu et al., (2013), the brain level of inhibitory members of the apoptosis-stimulating proteins of the p53 family (iASPP) is a possible target of miR-124, reduced after ischemic stroke in vivo condition. Additionally, inhibition of miR-124 increased the level of iASPP and significantly decreased infarction in ischemia. Also, their study indicates that after ischemic stroke, neural cell death mediated by p53 can be nontranscriptionally regulated by suppressing the mechanism of endogenous cell death inhibitors by miR-124. Based on the study findings, we observed that the expression of miR-124a in NPCs in the hippocampus decreased significantly one day after ischemic stroke. In other words, because the hippocampal region is a neurogenic niche and NPCs differentiate in this region, pretreatment by optogenetic technique caused miR-124a to be down-regulated. The observed improvement in disease status was consistent with the results of stroke volume measurements. Understanding the interaction between miRNAs and the regulatory mechanisms in the adult brain after stroke could potentially provide new therapies to prevent neural cell death after ischemic stroke.
5. Conclusion
Optogenetics has become a potent method for controlling intracellular signaling cascades thanks to light-induced proteins. By assessing miR-21 and miR-124a expression levels and exciting glutamatergic neurons in an animal model of ischemic stroke, we looked into the potential pretreatment effect of the optogenetic technology. The present study’s findings suggest that up-regulation of miR-21, a neuroprotection marker, improves neurological abnormalities and decreases infarct volume in the striatum and hippocampus. Furthermore, it can be deduced from the downregulation of miR-124a that this gene contributes to neurogenesis, neuroprotection, and the differentiation of NPCs into neurons, all of which alleviate ischemia conditions. Because compulsive ischemia may raise the likelihood of blood clot formation or conditions needing surgery, such as carotid aneurysms, our finding shows that neuroprotection induced by optogenetics can be useful in producing ischemic tolerance. We, therefore, believed that using optogenetic techniques in the clinical setting could help reduce brain damage brought on by cerebral ischemia in situations of compulsive ischemia based on our findings and those of earlier investigations.
Ethical Considerations
Compliance with ethical guidelines
All the experimental protocols were approved by the Animal Research Ethics Committee at Shahid Beheshti University, Tehran, Iran. This project was conducted in accordance with NIH ethical guidelines.
Funding
This research was funded by the Cognitive Sciences and Technology Council (CSTC) of Tehran, Iran.
Authors' contributions
Conceptualization, supervision and funding acquisition: Mohammad Reza Bigdeli; Methodology: Salma Ahmadlou, Leila Dargahi and Mohammad Ismail Zibaii Investigation; Software, data Analysis, and writing the original draft: Sanaz Mohammadi, and Pouria Ghasemi; Review and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank Mir Hossein Nazari, Ahmad Ghorbani, and Elham Sadat Seyed Javad Javaheri for providing helpful assistance in optogenetics stimulation of animals, Sara Chavoshi Nezhad as experimental assistants in Neurobiology Research Center of Shahid Beheshti University of Medical Sciences, and all the experts who helped in this project.
References
Bo, B., Li, Y., Li, W., Wang, Y., & Tong, S. (2019). Optogenetic excitation of ipsilesional sensorimotor neurons is protective in acute ischemic stroke: A laser speckle imaging study. IEEE Transactions on Bio-Medical Engineering, 66(5), 1372–1379. [DOI:10.1109/TBME.2018.2872965] [PMID]
Buller, B., Liu, X., Wang, X., Zhang, R. L., Zhang, L., & Hozeska-Solgot, A., et al. (2010). MicroRNA‐21 protects neurons from ischemic death. The FEBS Journal, 277(20), 4299–4307. [DOI:10.1111/j.1742-4658.2010.07818.x] [PMID]
Buschman, T. J., & Miller, E. K. (2014). Goal-direction and top-down control. Philosophical Transactions of the Royal Society of London. Series B, Biological sciences, 369(1655), 20130471. [DOI:10.1098/rstb.2013.0471] [PMID]
Cai, B., Li, J., Wang, J., Luo, X., Ai, J., & Liu, Y., et al. (2012). microRNA‐124 regulates cardiomyocyte differentiation of bone marrow‐derived mesenchymal stem cells via targeting STAT3 signaling. Stem Cells (Dayton, Ohio), 30(8), 1746–1755. [DOI:10.1002/stem.1154] [PMID]
Chan, J. A., Krichevsky, A. M., & Kosik, K. S. (2005). MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Research, 65(14), 6029–6033. [DOI:10.1158/0008-5472.CAN-05-0137] [PMID]
Chavoshinezhad, S., Zibaii, M. I., Seyed Nazari, M. H., Ronaghi, A., Asgari Taei, A., & Ghorbani, A., et al. (2021). Optogenetic stimulation of entorhinal cortex reveals the implication of insulin signaling in adult rat’s hippocampal neurogenesis. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 111, 110344. [DOI:10.1016/j.pnpbp.2021.110344] [PMID]
Chen, R., Liu, Y., Su, Q., Yang, Y., Wang, L., & Ma, S., et al. (2017). Hypoxia stimulates proliferation of rat neural stem/progenitor cells by regulating mir-21: An in vitro study. Neuroscience Letters, 661, 71–76. [DOI:10.1016/j.neulet.2017.09.037] [PMID]
Chen, Y., Liu, W., Chao, T., Zhang, Y., Yan, X., & Gong, Y., et al. (2008). MicroRNA-21 down-regulates the expression of tumor suppressor PDCD4 in human glioblastoma cell T98G. Cancer Letters, 272(2), 197–205. [DOI:10.1016/j.canlet.2008.06.034] [PMID]
Cheng, M. Y., Wang, E. H., Woodson, W. J., Wang, S., Sun, G., & Lee, A. G., et al. (2014). Optogenetic neuronal stimulation promotes functional recovery after stroke. Proceedings of the National Academy of Sciences of the United States of America, 111(35), 12913–12918. [DOI:10.1073/pnas.1404109111] [PMID]
Eyileten, C., Wicik, Z., De Rosa, S., Mirowska-Guzel, D., Soplinska, A., & Indolfi, C., et al. (2018). MicroRNAs as diagnostic and prognostic biomarkers in ischemic stroke-a comprehensive review and bioinformatic analysis. Cells, 7(12), 249.[DOI:10.3390/cells7120249] [PMID]
Gaudet, A. D., Fonken, L. K., Watkins, L. R., Nelson, R. J., & Popovich, P. G. (2018). MicroRNAs: Roles in regulating neuroinflammation. The Neuroscientist : A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 24(3), 221–245. [DOI:10.1177/1073858417721150] [PMID]
Han, Z., Chen, F., Ge, X., Tan, J., Lei, P., & Zhang, J. (2014). miR-21 alleviated apoptosis of cortical neurons through promoting PTEN-Akt signaling pathway in vitro after experimental traumatic brain injury. Brain Research, 1582, 12–20. [DOI:10.1016/j.brainres.2014.07.045] [PMID]
Kageyama, R., Ohtsuka, T., Shimojo, H., & Imayoshi, I. (2009). Dynamic regulation of Notch signaling in neural progenitor cells. Current Opinion in Cell Biology, 21(6), 733–740. [DOI:10.1016/j.ceb.2009.08.009] [PMID]
Khaksar, S., & Bigdeli, M. R. (2017). Anti-excitotoxic effects of cannabidiol are partly mediated by enhancement of NCX2 and NCX3 expression in animal model of cerebral ischemia. European Journal of Pharmacology, 794, 270–279. [DOI:10.1016/j.ejphar.2016.11.011] [PMID]
Lang, Q., & Ling, C. (2012). MiR-124 suppresses cell proliferation in hepatocellular carcinoma by targeting PIK3CA. Biochemical and Biophysical Research Communications, 426(2), 247–252. [DOI:10.1016/j.bbrc.2012.08.075] [PMID]
Laterza, O. F., Lim, L., Garrett-Engele, P. W., Vlasakova, K., Muniappa, N., & Tanaka, W. K., et al. (2009). Plasma MicroRNAs as sensitive and specific biomarkers of tissue injury. Clinical Chemistry, 55(11), 1977–1983. [DOI:10.1373/clinchem.2009.131797] [PMID]
Lerchundi, R., Fernández-Moncada, I., Contreras-Baeza, Y., Sotelo-Hitschfeld, T., Mächler, P., & Wyss, M. T., et al. (2015). NH4+ triggers the release of astrocytic lactate via mitochondrial pyruvate shunting. Proceedings of the National Academy of Sciences of the United States of America, 112(35), 11090–11095. [DOI:10.1073/pnas.1508259112] [PMID]
Liu, F. J., Lim, K. Y., Kaur, P., Sepramaniam, S., Armugam, A., & Wong, P. T., et al. (2013). microRNAs involved in regulating spontaneous recovery in embolic stroke model. PloS One, 8(6), e66393. [DOI:10.1371/journal.pone.0066393] [PMID]
Liu, W., Chen, X., & Zhang, Y. (2016). Effects of microRNA-21 and microRNA-24 inhibitors on neuronal apoptosis in ischemic stroke. American Journal of Translational Research, 8(7), 3179–3187. [PMID]
Liu, X., Li, F., Zhao, S., Luo, Y., Kang, J., & Zhao, H., et al. (2013). MicroRNA-124-mediated regulation of inhibitory member of apoptosis-stimulating protein of p53 family in experimental stroke. Stroke, 44(7), 1973–1980. [DOI:10.1161/STROKEAHA.111.000613] [PMID]
Liu, X. S., Chopp, M., Zhang, R. L., Tao, T., Wang, X. L., & Kassis, H., et al. (2011). MicroRNA profiling in subventricular zone after stroke: MiR-124a regulates proliferation of neural progenitor cells through Notch signaling pathway. Plos One, 6(8), e23461.[DOI:10.1371/journal.pone.0023461] [PMID]
Long, J., Cai, L., Li, J., Zhang, L., Yang, H., & Wang, T. (2013). JNK3 involvement in nerve cell apoptosis and neurofunctional recovery after traumatic brain injury. Neural Regeneration Research, 8(16), 1491–1499. [PMID]
Longa, E. Z., Weinstein, P. R., Carlson, S., & Cummins, R. (1989). Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke, 20(1), 84–91. [DOI:10.1161/01.STR.20.1.84] [PMID]
Lu, C., Wu, X., Ma, H., Wang, Q., Wang, Y., & Luo, Y., et al. (2019). Optogenetic stimulation enhanced neuronal plasticities in motor recovery after ischemic stroke. Neural Plasticity, 2019, 5271573. [DOI:10.1155/2019/5271573] [PMID]
Lu, Y., Jiang, L., Li, W., Qu, M., Song, Y., & He, X., et al. (2017). Optogenetic inhibition of striatal neuronal activity improves the survival of transplanted neural stem cells and neurological outcomes after ischemic stroke in mice. Stem Cells International, 2017, 4364302. [DOI:10.1155/2017/4364302] [PMID]
Makeyev, E. V., Zhang, J., Carrasco, M. A., & Maniatis, T. (2007). The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Molecular Cell, 27(3), 435–448. [DOI:10.1016/j.molcel.2007.07.015] [PMID]
Martinez, B., & Peplow, P. V. (2017). Immunomodulators and microRNAs as neurorestorative therapy for ischemic stroke. Neural Regeneration Research, 12(6), 865–874. [DOI:10.4103/1673-5374.208540] [PMID]
Mirzaei, H., Momeni, F., Saadatpour, L., Sahebkar, A., Goodarzi, M., & Masoudifar, A., et al. (2018). MicroRNA: Relevance to stroke diagnosis, prognosis, and therapy. Journal of Cellular Physiology, 233(2), 856–865. [DOI:10.1002/jcp.25787] [PMID]
Pires Monteiro, S., Voogd, E., Muzzi, L., De Vecchis, G., Mossink, B., & Levers, M., et al. (2021). Neuroprotective effect of hypoxic preconditioning and neuronal activation in a in vitro human model of the ischemic penumbra. Journal of Neural Engineering, 18(3), 036016. [DOI:10.1088/1741-2552/abe68a] [PMID]
Morimoto, T., Yasuhara, T., Kameda, M., Baba, T., Kuramoto, S., & Kondo, A., et al. (2011). Striatal stimulation nurtures endogenous neurogenesis and angiogenesis in chronic-phase ischemic stroke rats. Cell Transplantation, 20(7), 1049–1064.[DOI:10.3727/096368910X544915] [PMID]
Ohab, J. J., & Carmichael, S. T. (2008). Poststroke neurogenesis: Emerging principles of migration and localization of immature neurons. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 14(4), 369–380. [DOI:10.1177/1073858407309545] [PMID]
Panagal, M., Biruntha, M., Vidhyavathi, R. M., Sivagurunathan, P., Senthilkumar, S. R., & Sekar, D. (2019). Dissecting the role of miR-21 in different types of stroke. Gene, 681, 69–72.[DOI:10.1016/j.gene.2018.09.048] [PMID]
Papagiannakopoulos, T., Shapiro, A., & Kosik, K. S. (2008). MicroRNA-21 targets a network of key tumor-suppressive pathways in glioblastoma cells. Cancer Research, 68(19), 8164–8172.[DOI:10.1158/0008-5472.CAN-08-1305] [PMID]
Paxinos, G., & Watson, C. (2007). The rat brain in stereotaxic coordinates. Massachusetts: Academic Press. [Link]
Pendharkar, A. V., Smerin, D., Gonzalez, L., Wang, E. H., Levy, S., & Wang, S., et al. (2021). Optogenetic stimulation reduces neuronal nitric oxide synthase expression after stroke. Translational Stroke Research, 12(2), 347–356. [DOI:10.1007/s12975-020-00831-y] [PMID]
Rădulescu, A., Herron, J., Kennedy, C., & Scimemi, A. (2017). Global and local excitation and inhibition shape the dynamics of the cortico-striatal-thalamo-cortical pathway. Scientific Reports, 7(1), 1-21. [DOI:10.1038/s41598-017-07527-8]
Rink, C., & Khanna, S. (2011). MicroRNA in ischemic stroke etiology and pathology. Physiological Genomics, 43(10), 521–528.[DOI:10.1152/physiolgenomics.00158.2010] [PMID]
SafialHosseini, Z., Bigdeli, M., Khaksar, S., & Aliaghaei, A. (2020). Allograft of sertoli cell transplantation in combination with memantine alleviates ischemia-induced tissue damages in an animal model of rat. Cell Journal, 22(3), 334–343. [PMID]
Sakai, S., & Shichita, T. (2019). Inflammation and neural repair after ischemic brain injury. Neurochemistry International, 130, 104316. [DOI:10.1016/j.neuint.2018.10.013] [PMID]
Sayed Javad Javaheri, E. S., Bigdeli, M. R., Zibaii, M. I., Dargahi, L., & Pouretemad, H. R. (2019). Optogenetic stimulation of the anterior cingulate cortex ameliorates autistic-like behaviors in rats induced by neonatal isolation, caudate putamen as a site for alteration. Neuromolecular Medicine, 21(2), 132–142. [DOI:10.1007/s12017-019-08526-w] [PMID]
Sekar, D., Venugopal, B., Sekar, P., & Ramalingam, K. (2016). Role of microRNA 21 in diabetes and associated/related diseases. Gene, 582(1), 14–18. [DOI:10.1016/j.gene.2016.01.039] [PMID]
Shah, A. M., Ishizaka, S., Cheng, M. Y., Wang, E. H., Bautista, A. R., & Levy, S., et al. (2017). Optogenetic neuronal stimulation of the lateral cerebellar nucleus promotes persistent functional recovery after stroke. Scientific Reports, 7(1), 1-11. [DOI:10.1038/srep46612]
Shi, B., Deng, W., Long, X., Zhao, R., Wang, Y., & Chen, W., et al. (2017). miR-21 increases c-kit+ cardiac stem cell proliferation in vitro through PTEN/PI3K/Akt signaling. PeerJ, 5, e2859.[DOI:10.7717/peerj.2859] [PMID]
Song, M., Yu, S. P., Mohamad, O., Cao, W., Wei, Z. Z., & Gu, X., et al. (2017). Optogenetic stimulation of glutamatergic neuronal activity in the striatum enhances neurogenesis in the subventricular zone of normal and stroke mice. Neurobiology of Disease, 98, 9–24.[DOI:10.1016/j.nbd.2016.11.005] [PMID]
Sun, F., Li, X., Duan, W. Q., Tian, W., Gao, M., & Yang, J., et al. (2017). Transforming growth factor‐β receptor III is a potential regulator of ischemia‐induced cardiomyocyte apoptosis. Journal of the American Heart Association, 6(6), e005357. [DOI:10.1161/JAHA.116.005357] [PMID]
Tennant, K. A., Taylor, S. L., White, E. R., & Brown, C. E. (2017). Optogenetic rewiring of thalamocortical circuits to restore function in the stroke injured brain. Nature Communications, 8, 15879. [DOI:10.1038/ncomms15879] [PMID]
Vilar-Bergua, A., Riba-Llena, I., Nafría, C., Bustamante, A., Llombart, V., & Delgado, P., et al. (2016). Blood and CSF biomarkers in brain subcortical ischemic vascular disease: Involved pathways and clinical applicability. Journal of Cerebral Blood Flow and Metabolism, 36(1), 55-71. [DOI:10.1038/jcbfm.2015.68] [PMID]
Wang, B., Chen, Y. C., Jiang, G., Ning, Q., Ma, L., & Chan, W. Y., et al. (2016). New learning and memory related pathways among the hippocampus, the amygdala and the ventromedial region of the striatum in rats. Journal of Chemical Neuroanatomy, 71, 13–19. [DOI:10.1016/j.jchemneu.2015.12.006] [PMID]
Wang, C., Wei, Z., Jiang, G., & Liu, H. (2017). Neuroprotective mechanisms of miR-124 activating PI3K/Akt signaling pathway in ischemic stroke. Experimental and Therapeutic Medicine, 13(6), 3315–3318. [DOI:10.3892/etm.2017.4424] [PMID]
Wang, J., Huang, Q., Ding, J., & Wang, X. (2019). Elevated serum levels of brain-derived neurotrophic factor and miR-124 in acute ischemic stroke patients and the molecular mechanism. 3 Biotech, 9(386), 1-6. [DOI:10.1007/s13205-019-1914-2]
Weng, H., Shen, C., Hirokawa, G., Ji, X., Takahashi, R., & Shimada, K., et al. (2011). Plasma miR-124 as a biomarker for cerebral infarction. Biomedical Research (Tokyo, Japan), 32(2), 135–141. [DOI:10.2220/biomedres.32.135] [PMID]
Xu, L. F., Wu, Z. P., Chen, Y., Zhu, Q. S., Hamidi, S., & Navab, R. (2014). MicroRNA-21 (miR-21) regulates cellular proliferation, invasion, migration, and apoptosis by targeting PTEN, RECK and Bcl-2 in lung squamous carcinoma, Gejiu City, China. PloS One, 9(8), e103698. [DOI:10.1371/journal.pone.0103698] [PMID]
Yang, Q., Yang, K., & Li, A. (2014). MicroRNA21 protects against ischemiareperfusion and hypoxiareperfusioninduced cardiocyte apoptosis via the phosphatase and tensin homolog/Aktdependent mechanism. Molecular Medicine Reports, 9(6), 2213–2220. [DOI:10.3892/mmr.2014.2068] [PMID]
Young, S. Z., Lafourcade, C. A., Platel, J. C., Lin, T. V., & Bordey, A. (2014). GABAergic striatal neurons project dendrites and axons into the postnatal subventricular zone leading to calcium activity. Frontiers in Cellular Neuroscience, 8, 10. [DOI:10.3389/fncel.2014.00010] [PMID]
Zhang, F., Wang, L. P., Brauner, M., Liewald, J. F., Kay, K., & Watzke, N., et al. (2007). Multimodal fast optical interrogation of neural circuitry. Nature, 446(7136), 633–639. [Link]
Type of Study: Original |
Subject:
Cellular and molecular Neuroscience
Received: 2022/10/25 | Accepted: 2024/02/10 | Published: 2024/11/1
Received: 2022/10/25 | Accepted: 2024/02/10 | Published: 2024/11/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





