Volume 15, Issue 4 (July & August 2024)
BCN 2024, 15(4): 489-498 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Jimenez J C, Ruiz Garcia R I, Cedillo-Ildefonso B, Hernandez D, Miranda F. Mecamylamine Reverses the Effects of Cytisine on the Oral Self-administration of Ethanol in Rats. BCN 2024; 15 (4) :489-498
URL: http://bcn.iums.ac.ir/article-1-2561-en.html
URL: http://bcn.iums.ac.ir/article-1-2561-en.html
Juan C Jimenez1 

 , Rosa I Ruiz Garcia1
, Rosa I Ruiz Garcia1 

 , Benita Cedillo-Ildefonso1
, Benita Cedillo-Ildefonso1 

 , David Hernandez1
, David Hernandez1 

 , Florencio Miranda *1
, Florencio Miranda *1 




 , Rosa I Ruiz Garcia1
, Rosa I Ruiz Garcia1 

 , Benita Cedillo-Ildefonso1
, Benita Cedillo-Ildefonso1 

 , David Hernandez1
, David Hernandez1 

 , Florencio Miranda *1
, Florencio Miranda *1 


1- Faculty of Higher Studies Iztacala, Universidad Nacional Autónoma de México, Mexico City, Mexico.
Keywords: Nicotinic acetylcholine receptors (nAchRs), Ethanol, Nucleus accumbens (nAcc), Ethanol self-administration
Full-Text [PDF 1550 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Ethyl alcohol or ethanol (EtOH) abuse is a major public health problem around the world (WHO, 2018), including in Mexico (ENCODAT, 2017). Therefore, studying the neurobiology of EtOH-related behaviors is of fundamental importance. The mesocorticolimbic dopamine (DA) system plays a crucial role in mediating the addictive effects of EtOH and other drugs of abuse. This system comprises Dopaminergic neurons in the ventral tegmental area (VTA) that project their axons to the nucleus accumbens (nAcc), prefrontal cortex, amygdala, and other limbic structures. EtOH interacts with several neurotransmission systems to produce its reinforcing effects, such as glutamate (Woodward, 2000), serotonin (Sari, 2013; Yan et al., 2005), norepinephrine (Weinshenker et al., 2000), glycine (Söderpalm et al., 2017), and gamma-aminobutyric acid (GABA) (Koob, 2004) systems. DA release into the nAcc by VTA presynaptic DA terminals is controlled by several other neurotransmitters, such as acetylcholine (Ach). Ach is released from cholinergic interneurons (iAch) within the nAcc and acts on nicotinic Ach receptors (nAchRs) and muscarinic Ach receptors. nAchRs are expressed on dopaminergic nerve endings. Optogenetic activation of iAch elicits DA release in the nAcc (Cachope et al., 2012) and could modulate the effects of drugs of abuse. For example, it has been reported that the nAchR antagonist mecamylamine reduces cocaine self-administration in rats (Hansen & Mark, 2007) and blocks the acquisition of reinforcement for remifentanil, a potent synthetic opioid (Crespo et al., 2006), nicotine-induced locomotor activity (Bevins & Besheer, 2001), and nicotine self-administration (Ding et al., 2021). In addition, it has also been reported that the nAchR partial agonist varenicline decreases methamphetamine intake in rats (Kangiser et al., 2018).
These data suggest that the activation or blockade of nAchRs in the nAcc could also modulate EtOH-related behaviors. Therefore, this study evaluated the effects of cytisine and mecamylamine, a nAchR agonist and antagonist, respectively, on operant oral EtOH self-administration in rats.
2. Materials and Methods
Subjects
Male Wistar rats weighing 250-300 g at the beginning of the experiment were used. The rats were individually housed in standard plastic rodent cages in a colony room maintained at 21±1 °C under a 12/12 h light/dark cycle (lights on at 6:00 AM). They had continuous access to water and food (Teklad LM485 Rat Diet from Harlan, Mexico City, Mexico). All experiments were conducted during the light phase (between 11:00 AM and 1:00 PM).
Apparatus
Training and testing were conducted in five modular operant test chambers (30.5 cm long by 24 cm wide by 25 cm high) housed inside sound-attenuating cabinets with a ventilation fan (MED-008-D1 model; Med Associates, St. Albans, VT). Each chamber was equipped with two 4.5-cm wide retractable levers elevated 6.5 cm above the floor of the chamber and a stimulus light located 6 cm above each lever. A force of 0.25 N was required to activate the microswitch, and responses were reinforced with access to water (0.01 mL) or EtOH (0.01 mL). The availability of liquids was signaled by a light on the wall in front of the drinking cup that would light up for the duration of liquid delivery. The chambers were connected to a PC equipped with software for programming sessions and data recording.
Drugs
Ethyl alcohol or ethanol (99.91%) was purchased from J. T. Baker (Mexico City, Mexico), and cytisine, (1R,5S)-1,2,3,4,5,6-hexahydro-1,5-methano-8H-pyrido[1,2-a][1,5]diazocin-8-one, and mecamylamine, (1R,2S,4S)-rel-N,2,3,3-tetramethylbicyclo [2.2.1] heptan-2-amine hydrochloride, were purchased from Tocris Bioscience (Ballwin, MO, USA). Ethanol was diluted in filtered tap water (12% v/v). Cytisine and mecamylamine were dissolved in saline. All drugs were prepared fresh daily.
Surgical procedure for intracerebral cannulation
Rats were anesthetized with a ketamine-xylazine mixture (22.5 mg/kg-112.5 mg/kg, IP) and placed in a stereotaxic frame (Stoelting, Wood Dale, Illinois, USA). A guide cannula (23 G, 22 mm long; BD Precision Glide, Becton Dickinson and Co., Mexico) was unilaterally implanted in the right hemisphere of the rat brain as unilateral implantation produces less damage to brain tissue than bilateral implantation. In addition, it has been reported that in some behavioral paradigms, no evidence is found for the superiority of one or other hemisphere (Schildein et al., 2002) 0.2 mm above the nAcc shell (AP +1.4 mm, ML 0.6 mm, DV 4.6 mm relative to bregma, 22° angle from vertical) according to the stereotaxic atlas of Paxinos and Watson (2007). After surgery, all animals were injected with an antibiotic (benzathine penicillin 300,000 Ul/kg IM) to prevent infection. The rats were allowed a 5-day postoperative recovery period.
Histology
After intra-nAcc administration of the cholinergic ligands, the rats were killed with a lethal dose of halothane (5%), and the brains were removed from the skull and preserved in 10% formaldehyde/saline solution for one week. The brains were frozen and cut into 300 μm serial coronal slices on a vibratome. The injection sites were verified under a light microscope.
Behavioral procedures
Access to one bottle of water or EtOH solution
Rats were trained for 7 days to drink water in 30 minutes. After this training, the rats were placed in the experimental cages, where they had access to one bottle of water or EtOH solution for 30 min.
Training and testing procedure for operant oral EtOH self-administration
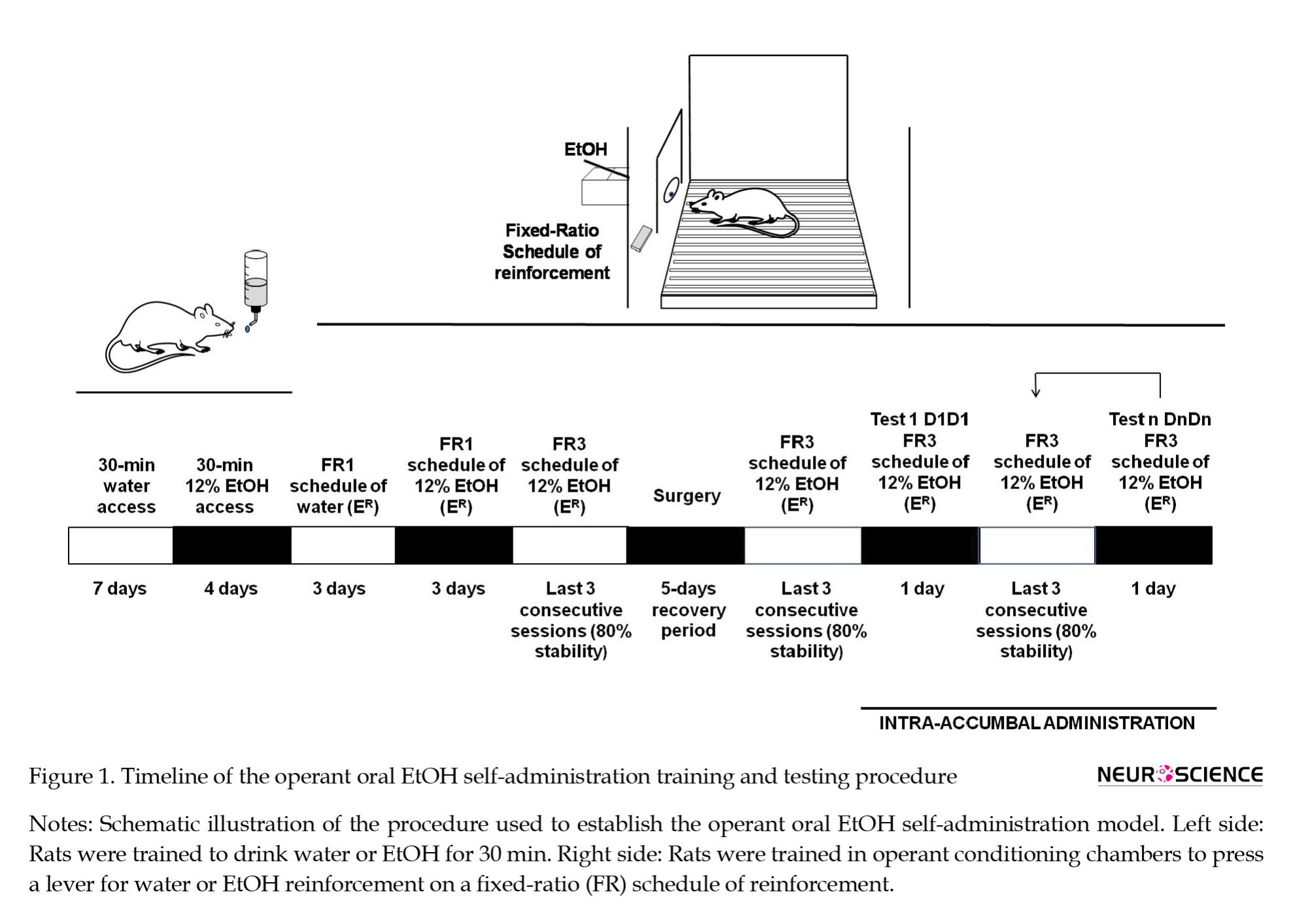
The timeline of the general procedure is shown in Figure 1. The training and testing procedures were similar to those previously described (Jimenez et al., 2022). This experimental procedure leads to a stable baseline of responses under the fixed ratio (FR) schedule of reinforcement of oral self-administration of EtOH. For the first 7 days, tap water availability was restricted to 30 min of access to one bottle of water in the home cages. For the next 4 days, the rats had 30 minutes of access to one bottle of 12% EtOH solution. After this training, the rats were water deprived for 24 h and then trained in operant conditioning chambers to press a lever for water reinforcement on a FR1 schedule in 30-min daily sessions for 3 consecutive days. After these training sessions, the rats were provided access to 12% EtOH solution on an FR1 reinforcement schedule for 3 straight days. After that, the number of responses in the FR schedule was increased to 3, i.e. each third response was reinforced with 12% EtOH (0.01 mL) until the response rate remained stable at 80% for 3 consecutive days. Once a stable response ratio was established, the effects of cholinergic receptor ligands were evaluated by injecting the rats with dose 1 of drug 1 and providing them access to 12% EtOH solution on an FR3 schedule of reinforcement for a 30-minute session. After this test, reinstatement sessions were performed under conditions identical to those the rats experienced before the test session. A cycle of 12% EtOH solution reinforcement on an FR3 schedule-test-12% EtOH solution reinforcement on an FR3 schedule was applied until all doses of the drugs were evaluated.
Acute effects of intra-nAcc administration of cholinergic receptor ligands on water intake
We conducted an initial experiment to investigate the acute effects of cytisine and mecamylamine on water intake. The effects of different doses of cytisine (0.0, 0.8, 1.6, and 3.2 µg) and mecamylamine (0.0, 1.25, 2.5, and 5.0 µg) were assessed in different groups of rats (n=6) according to the procedure described in the access to one bottle of water section. The doses of cytisine and mecamylamine were chosen according to previous behavioral studies in which these drugs had been injected either into the nAcc or the VTA (cytisine: Reavill & Stolerman, 1990; mecamylamine: Collins et al., 2016; Pratt & Kelley, 2004; Schildein et al., 2002).
Effects of intra-nAcc administration of a cholinergic receptor agonist and antagonist on operant oral EtOH self-administration
This experiment used a within-subjects design in which a group of rats (n=10) was trained according to the procedure described in the training and testing procedure for the operant oral EtOH self-administration section. After the response rate for 12% EtOH remained stable at 80% on an FR3 schedule for 3 consecutive days, the rats underwent implantation of a guide cannula according to the surgical procedure for intracerebral cannulation described above. After the surgery, the rats were allowed a 5-day recovery period. Following recovery, the rats were retrained to lever press for 12% EtOH solution reinforcement on an FR3 schedule until the response rate remained stable at 80% for 3 consecutive days. The effects of intra-nAcc administration of different doses of cytisine (0.0, 0.8, 1.6, and 3.2 µg), mecamylamine (0.0, 1.25, 2.5, and 5.0 µg), and coadministration of cytisine (3.2 µg) and mecamylamine (0.0, 1.25, 2.5, and 5.0 µg) were tested, with one dose being evaluated per test session. A pharmacological strategy to confirm if the cytisine is acting through nAchRs is to administrate an antagonist, such as mecamylamine, that binds to nAchRs. The drugs were administered by microinjection with calibrated polyethylene tubing connected to Hamilton syringes, and the animals were placed in the chambers for data recording 10 min later. The rate of infusion was 0.5 μL/min. After administering each tested dose, the rats were retrained to lever press for 12% EtOH solution reinforcement on an FR3 schedule until the response rate remained stable at 80% for 3 consecutive days. The dose to be tested was randomly chosen.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics 22 software. The data were expressed as the Mean±SE of water or EtOH intake (mL) or the Mean±SE number of lever presses. The data obtained during the evaluation of the acute effects of cytisine and mecamylamine on water intake were analyzed using a one-way analysis of variance (ANOVA). The mean number of levers pressed in the training and testing procedure for operant oral EtOH self-administration was analyzed using one-way ANOVA for repeated measures. When the ANOVA results were significant, the Bonferroni test (P<0.05) was used to perform post hoc comparisons.
3. Results
Acute effects of intra-accumbal administration of cholinergic receptor ligands on water intake
The results of this experiment revealed that neither cytisine (F(4, 29)=0.850, P=0.507) nor mecamylamine (F(4, 29)=0.486, P=0.746) altered water intake (data not shown).
Effects of intra-nAcc administration of cholinergic receptor agonist and antagonist on operant oral EtOH self-administration behavior
As shown in Table 1 parts A-C, training sessions on FR schedules of reinforcement produced stable responses; the response rate for water was stable on an FR1 schedule (F(2, 18)=2.180, P=0.142), the response rate for 12% EtOH was stable on an FR1 schedule (F(2, 18)=1.291, P=0.299), and the response rate for 12% ethanol was stable on an FR3 schedule (F(2, 18)=0.639, P=0.539). The response rate to 12% EtOH on an FR3 schedule after the 5-day postsurgical recovery period is shown in Table 1D. As noted, the FR3 schedule of reinforcement produced a stable response rate (F(2, 16)=0.129, P=0.880). Intra-nAcc administration of the nAchR antagonist, mecamylamine, decreased the operant oral self-administration of 12% EtOH (Figure 2A).
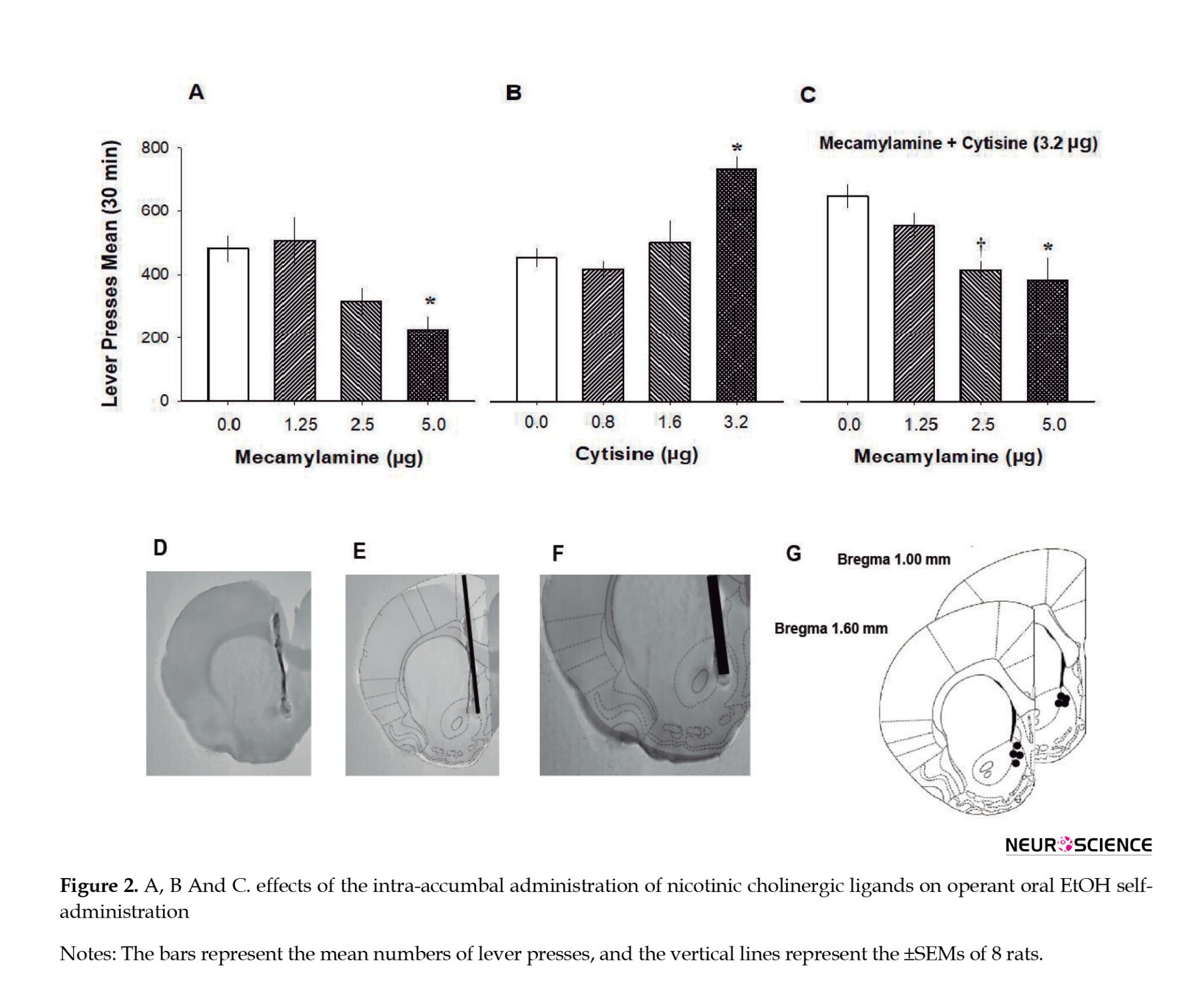
One-way ANOVA for repeated measures revealed significant differences (F(3, 21)=6.167, P=0.004). The Bonferroni test showed that the effect of 5.0 μg of mecamylamine was different from that of 0.0 and 1.25 μg of mecamylamine. Intra-accumbal administration of the nAchR agonist, cytisine, increased the operant oral self-administration of EtOH (Figure 2B). One-way ANOVA for repeated measures revealed significant differences (F(3, 21)=11.064, P=0.001). The Bonferroni test showed that the effect of 3.2 μg of cytisine was different from that of the other doses of cytisine. Administration of a fixed dose of nAchR agonist cytisine (3.2 µg) in combination with various doses of the nAchR antagonist mecamylamine produced a dose-dependent decrease in the operant oral self-administration of 12% EtOH (F(3, 21)=7.606, P=0.001). The Bonferroni test revealed that the effect of 5.0 µg of mecamylamine combined with cytisine (3.2 µg) was different from the effect of 0.0 and 1.25 µg of mecamylamine and that the effect of 2.5 µg of mecamylamine combined with 3.2 µg of cytisine was different from the effect of 0.0 of mecamylamine (Figure 2C). Figure 2D shows the histological localization of the injection sites in the shell of the nAcc. Rats in which the cannula was implanted outside the nAcc were excluded from the analyses (1/9).

4. Discussion
The present study aimed to examine the effects of intra-nAcc administration of a nAchR antagonist and agonist, i.e. mecamylamine and cytisine, respectively, on operant oral self-administration of EtOH in rats. We found that rats learned to lever-press for 12% EtOH reinforcement and showed a stable operant response rate before and after cannula implantation into the nAcc shell (Table 1) without initiating procedures for EtOH consumption, such as sucrose fading. In the present study, the operant oral EtOH self-administration was modeled as described in other studies (Blegen et al., 2018; Carnicella et al., 2011; Jimenez et al., 2022; Peana et al., 2014; Simms et al., 2010; Viudez-Martínez et al., 2018). This animal model of voluntary oral EtOH self-administration is a useful tool for studying the behavioral, neurochemical, and cellular mechanisms underlying the addictive properties of EtOH and other drugs of abuse (Blegen et al., 2018; de Siqueira Umpierrez et al., 2022; Fernandes et al., 2020; Haile et al., 2021). Before beginning the main experiments, the acute effects of intra-nAcc administration of mecamylamine and cytisine on water intake were studied in separate groups of rats in an initial experiment. We observed that neither mecamylamine nor cytisine altered drinking behavior at the doses used in this research.
In the current research, we also found that intra-nAcc administration of the nAchR antagonist mecamylamine reduced operant oral EtOH self-administration, while the nAchR agonist, cytisine, increased operant oral EtOH self-administration. The administration of mecamylamine reversed this effect. These observations suggest that nAchRs in the nAcc may be involved in modulating operant oral EtOH self-administration. Antagonists of specific receptor subtypes are often used to confirm the mechanism associated with agonist-induced changes in some behaviors. In the present study, the intra-nAcc administration of mecamylamine, a non-competitive nAchR antagonist, reduced the effects of cytisine on operant oral EtOH self-administration in rats. Although mecamylamine and cytisine are not selective for nAchRs, they are common pharmacological ligands employed in behavioral and neurobiological studies (Gotti & Clementi, 2021; Hendrickson et al., 2009).
The above behavioral results are consistent with previous reports demonstrating that nAchR ligands modulate some EtOH-induced behaviors. For instance, systemic administration of mecamylamine (0-8 mg/kg) reduces EtOH self-administration following a sucrose fading procedure in C57BL/6J mice (Ford et al., 2009). Similarly, systemic administration of mecamylamine (1.25, 2.5, and 5.0 mg/kg) reduces operant oral EtOH self-administration and blocks the deprivation-induced increase in alcohol consumption (Kuzmin et al., 2009). It has also been reported that intra-accumbal administration of mecamylamine reduces EtOH self-administration but not sucrose self-administration (Nadal et al., 1998). Systemic administration of mecamylamine (1.0 and 2.0 mg/kg) also significantly reduced EtOH consumption in a limited access procedure (Lê et al., 2000). Another study reported that mecamylamine reduced EtOH consumption and EtOH preference in a two-bottle choice test procedure after mecamylamine was administered intermittently and daily (Farook et al., 2009).
In the current research, intra-nAcc administration of the nAchR agonist cytisine, a partial agonist, at a dose of 3.2 µg increased operant oral EtOH self-administration. In contrast, it has been reported that intraperitoneal administration of cytisine at a dose of 3.0 mg/kg reduces EtOH consumption in a drinking-in-the-dark procedure in mice (Hendrickson et al., 2009). Other studies have also reported that cytisine decreases EtOH-related behaviors. For example, intraperitoneal cytisine administration (1.5 mg/kg) reduces EtOH in a preference test after 24 h of concurrent access to 15% and 30% EtOH (Bell et al., 2009). It has also been reported that intraperitoneal administration of cytisine (0.5 and 1.0 mg/kg) or varenicline (0.5 and 1.0 mg/kg) for three consecutive days reduces EtOH preference after continuous access to EtOH for four weeks in rats (Sotomayor-Zarate et al., 2013). Varenicline, a partial agonist of α4β2 nAchR, is a synthetic derivative of cytisine (Canu Boido & Sparatore, 1999) that has been shown to reduce EtOH intake. For example, acute subcutaneous administration of varenicline (1.0 or 2.0 mg/kg) dose-dependently attenuated oral EtOH self-administration and EtOH consumption in a two-bottle choice procedure in rats (Steensland et al., 2007). Additionally, it has been reported that intraperitoneal administration of varenicline (0.5, 1.0, and 2.0 mg/kg) dose-dependently reduces EtOH consumption in rats under a drinking-in-the-dark procedure. Still, it reduces saccharin intake (Kamens et al., 2018). In contrast with these studies, another study shows that systemic varenicline administration at lower doses (0.56 and 1.0 mg/kg) increases EtOH self-administration following a sucrose fading procedure in Lewis rats without affecting responses to food. However, higher doses of varenicline decrease the response to EtOH and food (Ginsburg & Lamb, 2013).
The reason for this discrepancy between our study of intra-nAcc administration of cytisine and some of the studies mentioned above is unclear. One possible hypothesis is differences in the route of drug administration. As noted above, systemic administration of nAchR agonists such as cytisine and varenicline reduces EtOH-related behaviors. In contrast, cytisine increases operant oral EtOH self-administration when directly administered into the nAcc in the present study. Systemic administration of the nAchR agonist cytisine can activate nAchRs expressed on different neurons in the brain reward system to reduce EtOH-related behaviors instead of increasing such behaviors. Also, α4β2 nAchRs (which are activated by Ach and nicotine and are involved in modulating the rewarding effects of EtOH) are expressed on dopaminergic neurons projecting to the nAcc and on local GABA interneurons in the VTA and modulate the firing patterns of DA neurons (Mameli-Engvall et al., 2006; Maurer & Schmidt, 2019). In addition, α4β2 nAchRs are also expressed on DA terminals in the nAcc (Feduccia et al., 2012; Grady et al., 2007). Therefore, it can be speculated that systemic administration of cytisine can activate α4β2 nAchRs expressed on all these neurons, including those expressed on the cell bodies of GABAergic interneurons in the VTA, to inhibit EtOH-related behaviors. In contrast, intra-nAcc administration of cytisine promotes EtOH-related behaviors, but this possibility remains to be evaluated. Another possible hypothesis accounting for the discrepancy between the abovementioned data and the present study’s results is cytisine’s pharmacological profile. Cytisine is an alkaloid with partial agonist activity at α4β2 nAchRs, and it has been shown to reduce rather than increase EtOH-related behaviors in several animal models (see above). A partial agonist does not produce the maximal response and can act as an antagonist in the presence of a full agonist, depending on the dose. It has been reported that cytisine (3.0 mg/kg) partially substitutes nicotine (59%) in rats trained to discriminate nicotine (0.6 mg/kg) from saline (Radchenko et al., 2015). In addition, it has also been reported that intraperitoneal administration of a high dose of cytisine (3.0 mg/kg) partially generalizes (23% of nicotine-appropriate lever presses) and antagonizes nicotine-induced discriminative stimulus effects in rats (LeSageet al., 2009). In general, the discrepancy between the effects of intra-nAcc administration of cytisine in our study and some of the data mentioned above may be related to differences in drug administration procedures, dosage regimen, or procedural variables.
Inhibition or activation of α4β2 nAchRs in the nAcc might be involved in modulating the operant oral self-administration of EtOH observed in the present study. This speculation is based on the following findings. First, the mesocorticolimbic DA system, particularly the projections from the VTA to the nAcc, is important for EtOH-related behaviors (Rodd et al., 2004; Weiss et al., 1993). Second, α4β2 nAchRs, which are expressed in DA terminals in the nAcc (Feduccia et al., 2012; Grady et al., 2007), play a modulatory role in DA release in the nAcc and, as a consequence, affect EtOH-related behaviors. DA release from VTA DA terminals in the nAcc is controlled by different receptors, such as D2-autoreceptors and α4β2 nAchRs. Some studies have shown that stimulation of those receptors modulates DA release in the nAcc. For instance, optogenetic stimulation of nAcc iAch produced the DA release in nAcc presynaptic DA terminals that express α4β2 nAchRs (Cachope et al., 2012). Furthermore, inhibition of iAch firing using an opioid agonist decreased the frequency of spontaneous DA transients in the nAcc (Yorgason et al., 2017). Consistent with this finding, behavioral studies involving manipulation of α4β2 nAchRs have provided additional support for the modulatory role of α4β2 nAchRs on EtOH-related behaviors. Nadal et al., (1998) reported that intra-accumbal administration of the α4β2 nAchR antagonist mecamylamine reduced EtOH self-administration but not sucrose self-administration in rats. In addition, α4β2 nAchRs are not only involved in EtOH-related behaviors but are also involved in drug abuse-related behaviors. For instance, it has been reported that pretreatment with mecamylamine (0.3, 1.0, and 3.0 mg/kg) reduces cocaine and nicotine self-administration in rats (Blokhina et al., 2005). Another study shows that mecamylamine reduces cocaine self-administration but not food self-administration (Levin et al., 2000). Although in these studies, the α4β2 nAchR antagonist mecamylamine was administered systematically, it could reach and block nAchRs expressed on different neurons in the brain reward system, including the nAcc, to reduce drug abuse-related behaviors. However, more research is needed before concluding the expression of α4β2 nAchRs in different regions of the brain reward system and their involvement in EtOH-related behaviors.
5. Conclusion
Our results show intra-accumbal administration of α4β2 nAchR ligands modulates operant oral EtOH self-administration. These data provide further evidence that α4β2 nAchRs may modulate drug abuse-induced behaviors, particularly the reinforcing effects of EtOH.
Ethical Considerations
Compliance with ethical guidelines
Animal care and handling procedures followed the Official Mexican Norm (NOM-062-ZOO-1999) entitled “technical specifications for the production, care, and use of laboratory animals.”
Funding
This study was supported by a grant from PAPIITUNAM, Universidad Nacional Autónoma de México, México City, México (Grant No.: IN305420).
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
Ethyl alcohol or ethanol (EtOH) abuse is a major public health problem around the world (WHO, 2018), including in Mexico (ENCODAT, 2017). Therefore, studying the neurobiology of EtOH-related behaviors is of fundamental importance. The mesocorticolimbic dopamine (DA) system plays a crucial role in mediating the addictive effects of EtOH and other drugs of abuse. This system comprises Dopaminergic neurons in the ventral tegmental area (VTA) that project their axons to the nucleus accumbens (nAcc), prefrontal cortex, amygdala, and other limbic structures. EtOH interacts with several neurotransmission systems to produce its reinforcing effects, such as glutamate (Woodward, 2000), serotonin (Sari, 2013; Yan et al., 2005), norepinephrine (Weinshenker et al., 2000), glycine (Söderpalm et al., 2017), and gamma-aminobutyric acid (GABA) (Koob, 2004) systems. DA release into the nAcc by VTA presynaptic DA terminals is controlled by several other neurotransmitters, such as acetylcholine (Ach). Ach is released from cholinergic interneurons (iAch) within the nAcc and acts on nicotinic Ach receptors (nAchRs) and muscarinic Ach receptors. nAchRs are expressed on dopaminergic nerve endings. Optogenetic activation of iAch elicits DA release in the nAcc (Cachope et al., 2012) and could modulate the effects of drugs of abuse. For example, it has been reported that the nAchR antagonist mecamylamine reduces cocaine self-administration in rats (Hansen & Mark, 2007) and blocks the acquisition of reinforcement for remifentanil, a potent synthetic opioid (Crespo et al., 2006), nicotine-induced locomotor activity (Bevins & Besheer, 2001), and nicotine self-administration (Ding et al., 2021). In addition, it has also been reported that the nAchR partial agonist varenicline decreases methamphetamine intake in rats (Kangiser et al., 2018).
These data suggest that the activation or blockade of nAchRs in the nAcc could also modulate EtOH-related behaviors. Therefore, this study evaluated the effects of cytisine and mecamylamine, a nAchR agonist and antagonist, respectively, on operant oral EtOH self-administration in rats.
2. Materials and Methods
Subjects
Male Wistar rats weighing 250-300 g at the beginning of the experiment were used. The rats were individually housed in standard plastic rodent cages in a colony room maintained at 21±1 °C under a 12/12 h light/dark cycle (lights on at 6:00 AM). They had continuous access to water and food (Teklad LM485 Rat Diet from Harlan, Mexico City, Mexico). All experiments were conducted during the light phase (between 11:00 AM and 1:00 PM).
Apparatus
Training and testing were conducted in five modular operant test chambers (30.5 cm long by 24 cm wide by 25 cm high) housed inside sound-attenuating cabinets with a ventilation fan (MED-008-D1 model; Med Associates, St. Albans, VT). Each chamber was equipped with two 4.5-cm wide retractable levers elevated 6.5 cm above the floor of the chamber and a stimulus light located 6 cm above each lever. A force of 0.25 N was required to activate the microswitch, and responses were reinforced with access to water (0.01 mL) or EtOH (0.01 mL). The availability of liquids was signaled by a light on the wall in front of the drinking cup that would light up for the duration of liquid delivery. The chambers were connected to a PC equipped with software for programming sessions and data recording.
Drugs
Ethyl alcohol or ethanol (99.91%) was purchased from J. T. Baker (Mexico City, Mexico), and cytisine, (1R,5S)-1,2,3,4,5,6-hexahydro-1,5-methano-8H-pyrido[1,2-a][1,5]diazocin-8-one, and mecamylamine, (1R,2S,4S)-rel-N,2,3,3-tetramethylbicyclo [2.2.1] heptan-2-amine hydrochloride, were purchased from Tocris Bioscience (Ballwin, MO, USA). Ethanol was diluted in filtered tap water (12% v/v). Cytisine and mecamylamine were dissolved in saline. All drugs were prepared fresh daily.
Surgical procedure for intracerebral cannulation
Rats were anesthetized with a ketamine-xylazine mixture (22.5 mg/kg-112.5 mg/kg, IP) and placed in a stereotaxic frame (Stoelting, Wood Dale, Illinois, USA). A guide cannula (23 G, 22 mm long; BD Precision Glide, Becton Dickinson and Co., Mexico) was unilaterally implanted in the right hemisphere of the rat brain as unilateral implantation produces less damage to brain tissue than bilateral implantation. In addition, it has been reported that in some behavioral paradigms, no evidence is found for the superiority of one or other hemisphere (Schildein et al., 2002) 0.2 mm above the nAcc shell (AP +1.4 mm, ML 0.6 mm, DV 4.6 mm relative to bregma, 22° angle from vertical) according to the stereotaxic atlas of Paxinos and Watson (2007). After surgery, all animals were injected with an antibiotic (benzathine penicillin 300,000 Ul/kg IM) to prevent infection. The rats were allowed a 5-day postoperative recovery period.
Histology
After intra-nAcc administration of the cholinergic ligands, the rats were killed with a lethal dose of halothane (5%), and the brains were removed from the skull and preserved in 10% formaldehyde/saline solution for one week. The brains were frozen and cut into 300 μm serial coronal slices on a vibratome. The injection sites were verified under a light microscope.
Behavioral procedures
Access to one bottle of water or EtOH solution
Rats were trained for 7 days to drink water in 30 minutes. After this training, the rats were placed in the experimental cages, where they had access to one bottle of water or EtOH solution for 30 min.
Training and testing procedure for operant oral EtOH self-administration
The timeline of the general procedure is shown in Figure 1. The training and testing procedures were similar to those previously described (Jimenez et al., 2022). This experimental procedure leads to a stable baseline of responses under the fixed ratio (FR) schedule of reinforcement of oral self-administration of EtOH. For the first 7 days, tap water availability was restricted to 30 min of access to one bottle of water in the home cages. For the next 4 days, the rats had 30 minutes of access to one bottle of 12% EtOH solution. After this training, the rats were water deprived for 24 h and then trained in operant conditioning chambers to press a lever for water reinforcement on a FR1 schedule in 30-min daily sessions for 3 consecutive days. After these training sessions, the rats were provided access to 12% EtOH solution on an FR1 reinforcement schedule for 3 straight days. After that, the number of responses in the FR schedule was increased to 3, i.e. each third response was reinforced with 12% EtOH (0.01 mL) until the response rate remained stable at 80% for 3 consecutive days. Once a stable response ratio was established, the effects of cholinergic receptor ligands were evaluated by injecting the rats with dose 1 of drug 1 and providing them access to 12% EtOH solution on an FR3 schedule of reinforcement for a 30-minute session. After this test, reinstatement sessions were performed under conditions identical to those the rats experienced before the test session. A cycle of 12% EtOH solution reinforcement on an FR3 schedule-test-12% EtOH solution reinforcement on an FR3 schedule was applied until all doses of the drugs were evaluated.

Acute effects of intra-nAcc administration of cholinergic receptor ligands on water intake
We conducted an initial experiment to investigate the acute effects of cytisine and mecamylamine on water intake. The effects of different doses of cytisine (0.0, 0.8, 1.6, and 3.2 µg) and mecamylamine (0.0, 1.25, 2.5, and 5.0 µg) were assessed in different groups of rats (n=6) according to the procedure described in the access to one bottle of water section. The doses of cytisine and mecamylamine were chosen according to previous behavioral studies in which these drugs had been injected either into the nAcc or the VTA (cytisine: Reavill & Stolerman, 1990; mecamylamine: Collins et al., 2016; Pratt & Kelley, 2004; Schildein et al., 2002).
Effects of intra-nAcc administration of a cholinergic receptor agonist and antagonist on operant oral EtOH self-administration
This experiment used a within-subjects design in which a group of rats (n=10) was trained according to the procedure described in the training and testing procedure for the operant oral EtOH self-administration section. After the response rate for 12% EtOH remained stable at 80% on an FR3 schedule for 3 consecutive days, the rats underwent implantation of a guide cannula according to the surgical procedure for intracerebral cannulation described above. After the surgery, the rats were allowed a 5-day recovery period. Following recovery, the rats were retrained to lever press for 12% EtOH solution reinforcement on an FR3 schedule until the response rate remained stable at 80% for 3 consecutive days. The effects of intra-nAcc administration of different doses of cytisine (0.0, 0.8, 1.6, and 3.2 µg), mecamylamine (0.0, 1.25, 2.5, and 5.0 µg), and coadministration of cytisine (3.2 µg) and mecamylamine (0.0, 1.25, 2.5, and 5.0 µg) were tested, with one dose being evaluated per test session. A pharmacological strategy to confirm if the cytisine is acting through nAchRs is to administrate an antagonist, such as mecamylamine, that binds to nAchRs. The drugs were administered by microinjection with calibrated polyethylene tubing connected to Hamilton syringes, and the animals were placed in the chambers for data recording 10 min later. The rate of infusion was 0.5 μL/min. After administering each tested dose, the rats were retrained to lever press for 12% EtOH solution reinforcement on an FR3 schedule until the response rate remained stable at 80% for 3 consecutive days. The dose to be tested was randomly chosen.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics 22 software. The data were expressed as the Mean±SE of water or EtOH intake (mL) or the Mean±SE number of lever presses. The data obtained during the evaluation of the acute effects of cytisine and mecamylamine on water intake were analyzed using a one-way analysis of variance (ANOVA). The mean number of levers pressed in the training and testing procedure for operant oral EtOH self-administration was analyzed using one-way ANOVA for repeated measures. When the ANOVA results were significant, the Bonferroni test (P<0.05) was used to perform post hoc comparisons.
3. Results
Acute effects of intra-accumbal administration of cholinergic receptor ligands on water intake
The results of this experiment revealed that neither cytisine (F(4, 29)=0.850, P=0.507) nor mecamylamine (F(4, 29)=0.486, P=0.746) altered water intake (data not shown).
Effects of intra-nAcc administration of cholinergic receptor agonist and antagonist on operant oral EtOH self-administration behavior
As shown in Table 1 parts A-C, training sessions on FR schedules of reinforcement produced stable responses; the response rate for water was stable on an FR1 schedule (F(2, 18)=2.180, P=0.142), the response rate for 12% EtOH was stable on an FR1 schedule (F(2, 18)=1.291, P=0.299), and the response rate for 12% ethanol was stable on an FR3 schedule (F(2, 18)=0.639, P=0.539). The response rate to 12% EtOH on an FR3 schedule after the 5-day postsurgical recovery period is shown in Table 1D. As noted, the FR3 schedule of reinforcement produced a stable response rate (F(2, 16)=0.129, P=0.880). Intra-nAcc administration of the nAchR antagonist, mecamylamine, decreased the operant oral self-administration of 12% EtOH (Figure 2A).

One-way ANOVA for repeated measures revealed significant differences (F(3, 21)=6.167, P=0.004). The Bonferroni test showed that the effect of 5.0 μg of mecamylamine was different from that of 0.0 and 1.25 μg of mecamylamine. Intra-accumbal administration of the nAchR agonist, cytisine, increased the operant oral self-administration of EtOH (Figure 2B). One-way ANOVA for repeated measures revealed significant differences (F(3, 21)=11.064, P=0.001). The Bonferroni test showed that the effect of 3.2 μg of cytisine was different from that of the other doses of cytisine. Administration of a fixed dose of nAchR agonist cytisine (3.2 µg) in combination with various doses of the nAchR antagonist mecamylamine produced a dose-dependent decrease in the operant oral self-administration of 12% EtOH (F(3, 21)=7.606, P=0.001). The Bonferroni test revealed that the effect of 5.0 µg of mecamylamine combined with cytisine (3.2 µg) was different from the effect of 0.0 and 1.25 µg of mecamylamine and that the effect of 2.5 µg of mecamylamine combined with 3.2 µg of cytisine was different from the effect of 0.0 of mecamylamine (Figure 2C). Figure 2D shows the histological localization of the injection sites in the shell of the nAcc. Rats in which the cannula was implanted outside the nAcc were excluded from the analyses (1/9).

4. Discussion
The present study aimed to examine the effects of intra-nAcc administration of a nAchR antagonist and agonist, i.e. mecamylamine and cytisine, respectively, on operant oral self-administration of EtOH in rats. We found that rats learned to lever-press for 12% EtOH reinforcement and showed a stable operant response rate before and after cannula implantation into the nAcc shell (Table 1) without initiating procedures for EtOH consumption, such as sucrose fading. In the present study, the operant oral EtOH self-administration was modeled as described in other studies (Blegen et al., 2018; Carnicella et al., 2011; Jimenez et al., 2022; Peana et al., 2014; Simms et al., 2010; Viudez-Martínez et al., 2018). This animal model of voluntary oral EtOH self-administration is a useful tool for studying the behavioral, neurochemical, and cellular mechanisms underlying the addictive properties of EtOH and other drugs of abuse (Blegen et al., 2018; de Siqueira Umpierrez et al., 2022; Fernandes et al., 2020; Haile et al., 2021). Before beginning the main experiments, the acute effects of intra-nAcc administration of mecamylamine and cytisine on water intake were studied in separate groups of rats in an initial experiment. We observed that neither mecamylamine nor cytisine altered drinking behavior at the doses used in this research.
In the current research, we also found that intra-nAcc administration of the nAchR antagonist mecamylamine reduced operant oral EtOH self-administration, while the nAchR agonist, cytisine, increased operant oral EtOH self-administration. The administration of mecamylamine reversed this effect. These observations suggest that nAchRs in the nAcc may be involved in modulating operant oral EtOH self-administration. Antagonists of specific receptor subtypes are often used to confirm the mechanism associated with agonist-induced changes in some behaviors. In the present study, the intra-nAcc administration of mecamylamine, a non-competitive nAchR antagonist, reduced the effects of cytisine on operant oral EtOH self-administration in rats. Although mecamylamine and cytisine are not selective for nAchRs, they are common pharmacological ligands employed in behavioral and neurobiological studies (Gotti & Clementi, 2021; Hendrickson et al., 2009).
The above behavioral results are consistent with previous reports demonstrating that nAchR ligands modulate some EtOH-induced behaviors. For instance, systemic administration of mecamylamine (0-8 mg/kg) reduces EtOH self-administration following a sucrose fading procedure in C57BL/6J mice (Ford et al., 2009). Similarly, systemic administration of mecamylamine (1.25, 2.5, and 5.0 mg/kg) reduces operant oral EtOH self-administration and blocks the deprivation-induced increase in alcohol consumption (Kuzmin et al., 2009). It has also been reported that intra-accumbal administration of mecamylamine reduces EtOH self-administration but not sucrose self-administration (Nadal et al., 1998). Systemic administration of mecamylamine (1.0 and 2.0 mg/kg) also significantly reduced EtOH consumption in a limited access procedure (Lê et al., 2000). Another study reported that mecamylamine reduced EtOH consumption and EtOH preference in a two-bottle choice test procedure after mecamylamine was administered intermittently and daily (Farook et al., 2009).
In the current research, intra-nAcc administration of the nAchR agonist cytisine, a partial agonist, at a dose of 3.2 µg increased operant oral EtOH self-administration. In contrast, it has been reported that intraperitoneal administration of cytisine at a dose of 3.0 mg/kg reduces EtOH consumption in a drinking-in-the-dark procedure in mice (Hendrickson et al., 2009). Other studies have also reported that cytisine decreases EtOH-related behaviors. For example, intraperitoneal cytisine administration (1.5 mg/kg) reduces EtOH in a preference test after 24 h of concurrent access to 15% and 30% EtOH (Bell et al., 2009). It has also been reported that intraperitoneal administration of cytisine (0.5 and 1.0 mg/kg) or varenicline (0.5 and 1.0 mg/kg) for three consecutive days reduces EtOH preference after continuous access to EtOH for four weeks in rats (Sotomayor-Zarate et al., 2013). Varenicline, a partial agonist of α4β2 nAchR, is a synthetic derivative of cytisine (Canu Boido & Sparatore, 1999) that has been shown to reduce EtOH intake. For example, acute subcutaneous administration of varenicline (1.0 or 2.0 mg/kg) dose-dependently attenuated oral EtOH self-administration and EtOH consumption in a two-bottle choice procedure in rats (Steensland et al., 2007). Additionally, it has been reported that intraperitoneal administration of varenicline (0.5, 1.0, and 2.0 mg/kg) dose-dependently reduces EtOH consumption in rats under a drinking-in-the-dark procedure. Still, it reduces saccharin intake (Kamens et al., 2018). In contrast with these studies, another study shows that systemic varenicline administration at lower doses (0.56 and 1.0 mg/kg) increases EtOH self-administration following a sucrose fading procedure in Lewis rats without affecting responses to food. However, higher doses of varenicline decrease the response to EtOH and food (Ginsburg & Lamb, 2013).
The reason for this discrepancy between our study of intra-nAcc administration of cytisine and some of the studies mentioned above is unclear. One possible hypothesis is differences in the route of drug administration. As noted above, systemic administration of nAchR agonists such as cytisine and varenicline reduces EtOH-related behaviors. In contrast, cytisine increases operant oral EtOH self-administration when directly administered into the nAcc in the present study. Systemic administration of the nAchR agonist cytisine can activate nAchRs expressed on different neurons in the brain reward system to reduce EtOH-related behaviors instead of increasing such behaviors. Also, α4β2 nAchRs (which are activated by Ach and nicotine and are involved in modulating the rewarding effects of EtOH) are expressed on dopaminergic neurons projecting to the nAcc and on local GABA interneurons in the VTA and modulate the firing patterns of DA neurons (Mameli-Engvall et al., 2006; Maurer & Schmidt, 2019). In addition, α4β2 nAchRs are also expressed on DA terminals in the nAcc (Feduccia et al., 2012; Grady et al., 2007). Therefore, it can be speculated that systemic administration of cytisine can activate α4β2 nAchRs expressed on all these neurons, including those expressed on the cell bodies of GABAergic interneurons in the VTA, to inhibit EtOH-related behaviors. In contrast, intra-nAcc administration of cytisine promotes EtOH-related behaviors, but this possibility remains to be evaluated. Another possible hypothesis accounting for the discrepancy between the abovementioned data and the present study’s results is cytisine’s pharmacological profile. Cytisine is an alkaloid with partial agonist activity at α4β2 nAchRs, and it has been shown to reduce rather than increase EtOH-related behaviors in several animal models (see above). A partial agonist does not produce the maximal response and can act as an antagonist in the presence of a full agonist, depending on the dose. It has been reported that cytisine (3.0 mg/kg) partially substitutes nicotine (59%) in rats trained to discriminate nicotine (0.6 mg/kg) from saline (Radchenko et al., 2015). In addition, it has also been reported that intraperitoneal administration of a high dose of cytisine (3.0 mg/kg) partially generalizes (23% of nicotine-appropriate lever presses) and antagonizes nicotine-induced discriminative stimulus effects in rats (LeSageet al., 2009). In general, the discrepancy between the effects of intra-nAcc administration of cytisine in our study and some of the data mentioned above may be related to differences in drug administration procedures, dosage regimen, or procedural variables.
Inhibition or activation of α4β2 nAchRs in the nAcc might be involved in modulating the operant oral self-administration of EtOH observed in the present study. This speculation is based on the following findings. First, the mesocorticolimbic DA system, particularly the projections from the VTA to the nAcc, is important for EtOH-related behaviors (Rodd et al., 2004; Weiss et al., 1993). Second, α4β2 nAchRs, which are expressed in DA terminals in the nAcc (Feduccia et al., 2012; Grady et al., 2007), play a modulatory role in DA release in the nAcc and, as a consequence, affect EtOH-related behaviors. DA release from VTA DA terminals in the nAcc is controlled by different receptors, such as D2-autoreceptors and α4β2 nAchRs. Some studies have shown that stimulation of those receptors modulates DA release in the nAcc. For instance, optogenetic stimulation of nAcc iAch produced the DA release in nAcc presynaptic DA terminals that express α4β2 nAchRs (Cachope et al., 2012). Furthermore, inhibition of iAch firing using an opioid agonist decreased the frequency of spontaneous DA transients in the nAcc (Yorgason et al., 2017). Consistent with this finding, behavioral studies involving manipulation of α4β2 nAchRs have provided additional support for the modulatory role of α4β2 nAchRs on EtOH-related behaviors. Nadal et al., (1998) reported that intra-accumbal administration of the α4β2 nAchR antagonist mecamylamine reduced EtOH self-administration but not sucrose self-administration in rats. In addition, α4β2 nAchRs are not only involved in EtOH-related behaviors but are also involved in drug abuse-related behaviors. For instance, it has been reported that pretreatment with mecamylamine (0.3, 1.0, and 3.0 mg/kg) reduces cocaine and nicotine self-administration in rats (Blokhina et al., 2005). Another study shows that mecamylamine reduces cocaine self-administration but not food self-administration (Levin et al., 2000). Although in these studies, the α4β2 nAchR antagonist mecamylamine was administered systematically, it could reach and block nAchRs expressed on different neurons in the brain reward system, including the nAcc, to reduce drug abuse-related behaviors. However, more research is needed before concluding the expression of α4β2 nAchRs in different regions of the brain reward system and their involvement in EtOH-related behaviors.
5. Conclusion
Our results show intra-accumbal administration of α4β2 nAchR ligands modulates operant oral EtOH self-administration. These data provide further evidence that α4β2 nAchRs may modulate drug abuse-induced behaviors, particularly the reinforcing effects of EtOH.
Ethical Considerations
Compliance with ethical guidelines
Animal care and handling procedures followed the Official Mexican Norm (NOM-062-ZOO-1999) entitled “technical specifications for the production, care, and use of laboratory animals.”
Funding
This study was supported by a grant from PAPIITUNAM, Universidad Nacional Autónoma de México, México City, México (Grant No.: IN305420).
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
Bell, R. L., Eiler, B. J., 2nd, Cook, J. B., & Rahman, S. (2009). Nicotinic receptor ligands reduce ethanol intake by high alcohol-drinking HAD-2 rats. Alcohol (Fayetteville, N.Y.), 43(8), 581-592. [DOI:10.1016/j.alcohol.2009.09.027] [PMID]
Bevins, R. A., & Besheer, J. (2001). Individual differences in rat locomotor activity are diminished by nicotine through stimulation of central nicotinic acetylcholine receptors. Physiology & Behavior, 72(1-2), 237-244. [DOI:10.1016/s0031-9384(00)00413-3] [PMID]
Blegen, M. B., da Silva E Silva, D., Bock, R., Morisot, N., Ron, D., & Alvarez, V. A. (2018). Alcohol operant self-administration: Investigating how alcohol-seeking behaviors predict drinking in mice using two operant approaches. Alcohol (Fayetteville, N.Y.), 67, 23-36. [DOI:10.1016/j.alcohol.2017.08.008] [PMID]
Blokhina, E. A., Kashkin, V. A., Zvartau, E. E., Danysz, W., & Bespalov, A. Y. (2005). Effects of nicotinic and NMDA receptor channel blockers on intravenous cocaine and nicotine self-administration in mice. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology, 15(2), 219-225. [DOI:10.1016/j.euroneuro.2004.07.005] [PMID]
Cachope, R., Mateo, Y., Mathur, B. N., Irving, J., Wang, H. L., & Morales, M., et al. (2012). Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: Setting the tone for reward processing. Cell Reports, 2(1), 33-41. [DOI:10.1016/j.celrep.2012.05.011] [PMID]
Canu Boido, C., & Sparatore, F. (1999). Synthesis and preliminary pharmacological evaluation of some cytisine derivatives. Farmaco (Societa chimica italiana: 1989), 54(7), 438-451. [DOI:10.1016/s0014-827x(99)00049-x] [PMID]
Carnicella, S., Yowell, Q. V., & Ron, D. (2011). Regulation of operant oral ethanol self-administration: A dose-response curve study in rats. Alcoholism, Clinical and Experimental Research, 35(1), 116-125. [DOI:10.1111/j.1530-0277.2010.01328.x] [PMID]
Collins, A. L., Aitken, T. J., Greenfield, V. Y., Ostlund, S. B., & Wassum, K. M. (2016). Nucleus accumbens acetylcholine receptors modulate dopamine and motivation. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 41(12), 2830-2838. [DOI:10.1038/npp.2016.81] [PMID]
Crespo, J. A., Sturm, K., Saria, A., & Zernig, G. (2006). Activation of muscarinic and nicotinic acetylcholine receptors in the nucleus accumbens core is necessary for the acquisition of drug reinforcement. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 26(22), 6004-6010. [DOI:10.1523/JNEUROSCI.4494-05.2006] [PMID]
de Siqueira Umpierrez, L., Freese, L., Almeida, F. B., Costa, P. A., Fernandes, P. R., & Nin, M. S., et al. (2022). Effects of neonatal dopaminergic lesion on oral cocaine self-administration in rats: Higher female vulnerability to cocaine consumption. Pharmacology, Biochemistry, and Behavior, 212, 173315. [DOI:10.1016/j.pbb.2021.173315] [PMID]
Ding, Z. M., Gao, Y., Sentir, A. M., & Tan, X. (2021). Self-Administration of cotinine in wistar rats: Comparisons to nicotine. The Journal of Pharmacology and Experimental Therapeutics, 376(3), 338-347. [DOI:10.1124/jpet.120.000367] [PMID]
ENCODAT (2017). [National survey on drug, alcohol and tobacco use 2016-2017 (Spanish)] Dragus report. Mexico City: Ministry of Health. [Link]
Farook, J. M., Lewis, B., Gaddis, J. G., Littleton, J. M., & Barron, S. (2009). Effects of mecamylamine on alcohol consumption and preference in male C57BL/6J mice. Pharmacology, 83(6), 379-384. [DOI:10.1159/000219488] [PMID]
Feduccia, A. A., Chatterjee, S., & Bartlett, S. E. (2012). Neuronal nicotinic acetylcholine receptors: neuroplastic changes underlying alcohol and nicotine addictions. Frontiers in Molecular Neuroscience, 5, 83. [DOI:10.3389/fnmol.2012.00083] [PMID]
Fernandes, P. R., Almeida, F. B., da Cunha, M. M. M. V., Feddern, C. F., Freese, L., & Barros, H. M. T. (2020). The effects of caffeine on alcohol oral self-administration behavior in rats. Physiology & Behavior, 223, 112966. [DOI:10.1016/j.physbeh.2020.112966] [PMID]
Ford, M. M., Fretwell, A. M., Nickel, J. D., Mark, G. P., Strong, M. N., & Yoneyama, N., et al.(2009). The influence of mecamylamine on ethanol and sucrose self-administration. Neuropharmacology, 57(3), 250-258. [DOI:10.1016/j.neuropharm.2009.05.012] [PMID]
Ginsburg, B. C., & Lamb, R. J. (2013). Effects of varenicline on ethanol- and food-maintained responding in a concurrent access procedure. Alcoholism, Clinical and Experimental Research, 37(7), 1228-1233. [DOI:10.1111/acer.12085] [PMID]
Gotti, C., & Clementi, F. (2021). Cytisine and cytisine derivatives. More than smoking cessation aids. Pharmacological Research, 170, 105700. [DOI:10.1016/j.phrs.2021.105700] [PMID]
Grady, S. R., Salminen, O., Laverty, D. C., Whiteaker, P., McIntosh, J. M., & Collins, A. C., et al. (2007). The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochemical Pharmacology, 74(8), 1235-1246. [DOI:10.1016/j.bcp.2007.07.032] [PMID]
Haile, C. N., Carper, B. A., Nolen, T. L., & Kosten, T. A. (2021).The GABAB receptor positive allosteric modulator ASP8062 reduces operant alcohol self-administration in male and female Sprague Dawley rats. Psychopharmacology, 238(9), 2587-2600. [DOI:10.1007/s00213-021-05881-0] [PMID]
Hansen, S. T., & Mark, G. P. (2007). The nicotinic acetylcholine receptor antagonist mecamylamine prevents escalation of cocaine self-administration in rats with extended daily access. Psychopharmacology, 194(1), 53-61. [DOI:10.1007/s00213-007-0822-z] [PMID]
Hendrickson, L. M., Zhao-Shea, R., & Tapper, A. R. (2009). Modulation of ethanol drinking-in-the-dark by mecamylamine and nicotinic acetylcholine receptor agonists in C57BL/6J mice. Psychopharmacology, 204(4), 563-572. [DOI:10.1007/s00213-009-1488-5] [PMID]
Jimenez, J. C., Cortés-Salazar, F., Cedillo-Ildefonso, B., & Miranda, F. (2022). [La administración sistémica e intra-accumbens del agonista 5-HT1B CP94253 modula la auto-administración oral de etanol en ratas (Spanish)]. Revista Argentina de Ciencias del Comportamiento, 14(1), 68-81. [Doi:10.32348/1852.4206.V14.N1.30138]
Kamens, H. M., Silva, C., Peck, C., & Miller, C. N. (2018). Varenicline modulates ethanol and saccharin consumption in adolescent male and female C57BL/6J mice. Brain Research Bulletin, 138, 20-25. [DOI:10.1016/j.brainresbull.2017.07.020] [PMID]
Kangiser, M. M., Dwoskin, L. P., Zheng, G., Crooks, P. A., & Stairs, D. J. (2018). Varenicline and GZ-793A differentially decrease methamphetamine self-administration under a multiple schedule of reinforcement in rats. Behavioural Pharmacology, 29(1), 87-97. [DOI:10.1097/FBP.0000000000000340] [PMID]
Koob G. F. (2004). A role for GABA mechanisms in the motivational effects of alcohol. Biochemical Pharmacology, 68(8), 1515-1525. [DOI:10.1016/j.bcp.2004.07.031] [PMID]
Kuzmin, A., Jerlhag, E., Liljequist, S., & Engel, J. (2009). Effects of subunit selective nACh receptors on operant ethanol self-administration and relapse-like ethanol-drinking behavior. Psychopharmacology, 203(1), 99-108. [DOI:10.1007/s00213-008-1375-5] [PMID]
Lê, A. D., Corrigall, W. A., Harding, J. W., Juzytsch, W., & Li, T. K. (2000). Involvement of nicotinic receptors in alcohol self-administration. Alcoholism, Clinical and Experimental Research, 24(2), 155-163. [DOI:10.1111/j.1530-0277.2000.tb04585.x] [PMID]
LeSage, M. G., Shelley, D., Ross, J. T., Carroll, F. I., & Corrigall, W. A. (2009). Effects of the nicotinic receptor partial agonists varenicline and cytisine on the discriminative stimulus effects of nicotine in rats. Pharmacology, Biochemistry, and Behavior, 91(3), 461-467. [DOI:10.1016/j.pbb.2008.08.024] [PMID]
Levin, E. D., Mead, T., Rezvani, A. H., Rose, J. E., Gallivan, C., & Gross, R. (2000). The nicotinic antagonist mecamylamine preferentially inhibits cocaine vs. food self-administration in rats. Physiology & Behavior, 71(5), 565-570. [DOI:10.1016/s0031-9384(00)00382-6] [PMID]
Mameli-Engvall, M., Evrard, A., Pons, S., Maskos, U., Svensson, T. H., & Changeux, J. P., et al. (2006). Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron, 50(6), 911–921. [DOI:10.1016/j.neuron.2006.05.007] [PMID]
Maurer, J. J., & Schmidt, H. D. (2019). Nicotine Addiction and Alpha4beta2* Nicotinic Acetylcholine Receptors. In: Preedy VR, editors. Neuroscience of nicotine: Mechanisms and treatment. Massachusetts: Academic Press. [DOI:10.1016/B978-0-12-813035-3.00031-9]
Nadal, R., Chappell, A. M., & Samson, H. H. (1998). Effects of nicotine and mecamylamine microinjections into the nucleus accumbens on ethanol and sucrose self-administration. Alcoholism, Clinical and Experimental Research, 22(6), 1190-1198. [DOI:10.1111/j.1530-0277.1998.tb03898.x] [PMID]
Paxinos, G., & Watson, C. (2007). The rat brain in stereotaxic co¬ordinates. Cambridge: AcademicPress. [Link]
Peana, A. T., Muggironi, G., Spina, L., Rosas, M., Kasture, S. B., Cotti, E., & Acquas, E. (2014). Effects of Withania somnifera on oral ethanol self-administration in rats. Behavioural Pharmacology, 25(7), 618-628. [DOI:10.1097/FBP.0000000000000078] [PMID]
Pratt, W. E., & Kelley, A. E. (2004). Nucleus accumbens acetylcholine regulates appetitive learning and motivation for food via activation of muscarinic receptors. Behavioral Neuroscience, 118(4), 730-739. [DOI:10.1037/0735-7044.118.4.730] [PMID]
Radchenko, E. V., Dravolina, O. A., & Bespalov, A. Y. (2015). Agonist and antagonist effects of cytisine in vivo. Neuropharmacology, 95, 206-214. [DOI:10.1016/j.neuropharm.2015.03.019] [PMID]
Reavill, C., & Stolerman, I. P. (1990). Locomotor activity in rats after administration of nicotinic agonists intracerebrally. British Journal of Pharmacology, 99(2), 273-278. [DOI:10.1111/j.1476-5381.1990.tb14693.x] [PMID]
Rodd, Z. A., Melendez, R. I., Bell, R. L., Kuc, K. A., Zhang, Y., & Murphy, J. M., et al. (2004). Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: evidence for involvement of dopamine neurons. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 24(5), 1050-1057. [DOI:10.1523/JNEUROSCI.1319-03.2004] [PMID]
Sari, Y. (2013). Role of 5-hydroxytryptamine 1B (5-HT1B) receptors in the regulation of ethanol intake in rodents. Journal of Psychopharmacology (Oxford, England), 27(1), 3-12. [DOI:10.1177/0269881112463126] [PMID]
Schildein, S., Huston, J. P., & Schwarting, R. K. (2002). Open field habituation learning is improved by nicotine and attenuated by mecamylamine administered posttrial into the nucleus accumbens. Neurobiology of Learning and Memory, 77(3), 277-290. [DOI:10.1006/nlme.2001.4017] [PMID]
Simms, J. A., Bito-Onon, J. J., Chatterjee, S., & Bartlett, S. E. (2010). Long-Evans rats acquire operant self-administration of 20% ethanol without sucrose fading. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 35(7), 1453-1463. [DOI:10.1038/npp.2010.15] [PMID]
Söderpalm, B., Lidö, H. H., & Ericson, M. (2017). The glycine receptor-A functionally important primary brain target of ethanol. Alcoholism, Clinical and Experimental Research, 41(11), 1816-1830. [DOI:10.1111/acer.13483] [PMID]
Sotomayor-Zárate, R., Gysling, K., Busto, U. E., Cassels, B. K., Tampier, L., & Quintanilla, M. E. (2013). Varenicline and cytisine: two nicotinic acetylcholine receptor ligands reduce ethanol intake in University of Chile bibulous rats. Psychopharmacology, 227(2), 287-298. [DOI:10.1007/s00213-013-2974-3] [PMID]
Steensland, P., Simms, J. A., Holgate, J., Richards, J. K., & Bartlett, S. E. (2007). Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proceedings of the National Academy of Sciences of the United States of America, 104(30), 12518-12523. [DOI:10.1073/pnas.0705368104] [PMID]
Viudez-Martínez, A., García-Gutiérrez, M. S., Navarrón, C. M., Morales-Calero, M. I., Navarrete, F., & Torres-Suárez, A. I., et al. (2018). Cannabidiol reduces ethanol consumption, motivation and relapse in mice. Addiction Biology, 23(1), 154-164. [DOI:10.1111/adb.12495] [PMID]
Weinshenker, D., Rust, N. C., Miller, N. S., & Palmiter, R. D. (2000). Ethanol-associated behaviors of mice lacking norepinephrine. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 20(9), 3157-3164. [DOI:10.1523/JNEUROSCI.20-09-03157.2000] [PMID]
Weiss, F., Lorang, M. T., Bloom, F. E., & Koob, G. F. (1993). Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: Genetic and motivational determinants. The Journal of Pharmacology and Experimental Therapeutics, 267(1), 250-258. [PMID]
Woodward J. J. (2000). Ethanol and NMDA receptor signaling. Critical Reviews in Neurobiology, 14(1), 69-89. [DOI:10.1080/08913810008443548] [PMID]
World Health Organization (2018). Global status report on alcohol 2018. Geneva: World Health Organization.
Type of Study: Original |
Subject:
Behavioral Neuroscience
Received: 2022/10/12 | Accepted: 2023/02/12 | Published: 2024/07/20
Received: 2022/10/12 | Accepted: 2023/02/12 | Published: 2024/07/20
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





