Volume 16 - Special Issue on Cognitive Sciences
BCN 2025, 16 - Special Issue on Cognitive Sciences: 309-322 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Moradi Birgani P, Ashtiyani M, Jameie S B, Shahrokhi A, Rahimian E, Deevband M R et al . Brain Functional Activity and Walking Capacity Enhancement in Children With Cerebral Palsy: A Pilot fMRI Study. BCN 2025; 16 (S1) :309-322
URL: http://bcn.iums.ac.ir/article-1-2548-en.html
URL: http://bcn.iums.ac.ir/article-1-2548-en.html
Parmida Moradi Birgani1 

 , Meghdad Ashtiyani2
, Meghdad Ashtiyani2 

 , Seyed Behnamedin Jameie3
, Seyed Behnamedin Jameie3 

 , Amin Shahrokhi4
, Amin Shahrokhi4 
 , Elham Rahimian5
, Elham Rahimian5 

 , Mohammad Reza Deevband2
, Mohammad Reza Deevband2 

 , M. Mehdi Mirbagheri *1
, M. Mehdi Mirbagheri *1 




 , Meghdad Ashtiyani2
, Meghdad Ashtiyani2 

 , Seyed Behnamedin Jameie3
, Seyed Behnamedin Jameie3 

 , Amin Shahrokhi4
, Amin Shahrokhi4 
 , Elham Rahimian5
, Elham Rahimian5 

 , Mohammad Reza Deevband2
, Mohammad Reza Deevband2 

 , M. Mehdi Mirbagheri *1
, M. Mehdi Mirbagheri *1 


1- Department of Medical Physics and Biomedical Engineering, Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran.
2- Department of Biomedical Engineering and Medical Physics, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Neuroscience Research Centre (NRC), Iran University of Medical Sciences, Tehran, Iran.
4- Department of Basic Science, School of Rehabilitation Sciences, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
5- Shefa Neuroscience Research Centre, Khatam Alanbia Hospital, Tehran, Iran.
2- Department of Biomedical Engineering and Medical Physics, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Neuroscience Research Centre (NRC), Iran University of Medical Sciences, Tehran, Iran.
4- Department of Basic Science, School of Rehabilitation Sciences, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
5- Shefa Neuroscience Research Centre, Khatam Alanbia Hospital, Tehran, Iran.
Keywords: Functional magnetic resonance imaging (fMRI), Cerebral palsy (CP), Correlation, Corpus callosum (CC), Gait
Full-Text [PDF 1894 kb]
| Abstract (HTML)
Azizi, S., Rasooli, A. H., Soleimani, M., Irani, A., Shahrokhi, A., & Mirbagheri, M. M. (2018). The impact of AlterG training on balance and structure of vestibulospinal tract in cerebral palsy children. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual International Conference, 2018, 2499–2502. [DOI:10.1109/EMBC.2018.8512772] [PMID]
Barch, D. M., Burgess, G. C., Harms, M. P., Petersen, S. E., Schlaggar, B. L., & Corbetta, M., et al. (2013). Function in the human connectome: Task-fMRI and individual differences in behavior. NeuroImage, 80, 169–189. [DOI:10.1016/j.neuroimage.2013.05.033] [PMID]
Bernal, B., Grossman, S., Gonzalez, R., & Altman, N. (2012). FMRI under sedation: What is the best choice in children?. Journal of Clinical Medicine Research, 4(6), 363–370. [DOI:10.4021/jocmr1047w] [PMID]
Bleyenheuft, Y., Dricot, L., Gilis, N., Kuo, H. C., Grandin, C., & Bleyenheuft, C., et al. 2015). Capturing neuroplastic changes after bimanual intensive rehabilitation in children with unilateral spastic cerebral palsy: A combined DTI, TMS and fMRI pilot study. Research in Developmental Disabilities, 43-44, 136–149. [DOI:10.1016/j.ridd.2015.06.014] [PMID]
Birgani, P. M., Ashtiyani, M., Rasooli, A., Shahrokhnia, M., Shahrokhi, A., & Mirbagheri, M. M. (2016). Can an anti-gravity treadmill improve stability of children with cerebral palsy?, Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual International Conference, 2016, 5465–5468.[DOI:10.1109/EMBC.2016.7591963] [PMID]
Bloom, J. S., & Hynd, G. W. (2005). The role of the corpus callosum in interhemispheric transfer of information: Excitation or inhibition?. Neuropsychology Review, 15(2), 59–71. [DOI:10.1007/s11065-005-6252-y] [PMID]
Bohannon R. W. (2006). Reference values for the timed up and go test: a descriptive meta-analysis. Journal of Geriatric Physical Therapy (2001), 29(2), 64–68. [DOI:10.1519/00139143-200608000-00004] [PMID]
Dan B. (2016). Beyond localizing neurology and psychology. Developmental Medicine and child Neurology, 58(1), 4. [DOI10.1111/dmcn.12985] [PMID]
Calmels C. (2020). Neural correlates of motor expertise: Extensive motor training and cortical changes. Brain Research, 1739, 146323. [DOI:10.1016/j.brainres.2019.146323] [PMID]
Carr L. J. (1996). Development and reorganization of descending motor pathways in children with hemiplegic cerebral palsy. Acta Paediatrica (Oslo, Norway: 1992). Supplement, 416, 53–57. [DOI:10.1111/j.1651-2227.1996.tb14278.x] [PMID]
Dan B. (2020). Cerebral palsy is a sensorimotor disorder. Developmental Medicine and Child Neurology, 62(7), 768. [DOI: 10.1111/dmcn.14542] [PMID]
Dan B. (2021). Intensive repetitive motor training: how does it work in children with cerebral palsy? Developmental Medicine and Child Neurology, 63(9), 1008. [DOI:10.1111/dmcn.14970] [PMID]
Dan, X., Hu, Y., Sun, J., Gao, L., Zhou, Y., & Ma, J., et al. (2021). Altered cerebellar resting-state functional connectivity in early-stage parkinson's disease patients with cognitive impairment. Frontiers in Neurology, 12, 678013. [DOI:10.3389/fneur.2021.678013] [PMID]
Davatzikos, C., Barzi, A., Lawrie, T., Hoon, A. H., Jr, & Melhem, E. R. (2003). Correlation of corpus callosal morphometry with cognitive and motor function in periventricular leukomalacia. Neuropediatrics, 34(5), 247–252. [DOI:10.1055/s-2003-43259] [PMID]
Dietz, V. (2009). Body weight supported gait training: from laboratory to clinical setting. Brain Research Bulletin, 78(1), I–VI. [DOI:10.1016/S0361-9230(08)00410-3] [PMID]
Dinomais, M., Chinier, E., Lignon, G., Richard, I., Ter Minassian, A., & Tich, S. N. (2013). The effect of video-guidance on passive movement in patients with cerebral palsy: fMRI study. Research in Developmental Disabilities, 34(10), 3487–3496. [DOI:10.1016/j.ridd.2013.07.008] [PMID]
Ditunno, J. F., Jr, Ditunno, P. L., Graziani, V., Scivoletto, G., Bernardi, M., & Castellano, V., et al. (2000). Walking index for spinal cord injury (WISCI): An international multicenter validity and reliability study. Spinal Cord, 38(4), 234–243.[DOI:10.1038/sj.sc.3100993] [PMID]
Dobkin, B. H., Firestine, A., West, M., Saremi, K., & Woods, R. (2004). Ankle dorsiflexion as an fMRI paradigm to assay motor control for walking during rehabilitation. NeuroImage, 23(1), 370–381. [DOI:10.1016/j.neuroimage.2004.06.008] [PMID]
Donabedian, A. (2005). Evaluating the quality of medical care. 1966. The Milbank Quarterly, 83(4), 691–729. [DOI:10.1111/j.1468-0009.2005.00397.x] [PMID]
Drużbicki, M., Rusek, W., Snela, S., Dudek, J., Szczepanik, M., & Zak, E., et al. (2013). Functional effects of robotic-assisted locomotor treadmill thearapy in children with cerebral palsy. Journal of Rehabilitation Medicine, 45(4), 358–363. [DOI:10.2340/16501977-1114] [PMID]
Esteban, O., Ciric, R., Finc, K., Blair, R. W., Markiewicz, C. J., & Moodie, C. A., t al. (2020). Analysis of task-based functional MRI data preprocessed with fMRIPrep. Nature Protocols, 15(7), 2186–2202 [DOI:10.1038/s41596-020-0327-3] [PMID]
Fabri, M., Pierpaoli, C., Barbaresi, P., & Polonara, G. (2014). Functional topography of the corpus callosum investigated by DTI and fMRI. World Journal of Radiology, 6(12), 895–906. [DOI:10.4329/wjr.v6.i12.895] [PMID]
Garvey, M. A., & Mall, V. (2008). Transcranial magnetic stimulation in children. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 119(5), 973–984. [DOI:10.1016/j.clinph.2007.11.048] [PMID]
Gawryluk, J. R., Mazerolle, E. L., & D'Arcy, R. C. (2014). Does functional MRI detect activation in white matter? A review of emerging evidence, issues, and future directions. Frontiers in Neuroscience, 8, 239. [DOI:10.3389/fnins.2014.00239] [PMID]
Hallett, M. (2007). Transcranial magnetic stimulation: A primer. Neuron, 55(2), 187–199. [DOI:10.1016/j.neuron.2007.06.026] [PMID]
Hesse, S., Konrad, M., & Uhlenbrock, D. (1999). Treadmill walking with partial body weight support versus floor walking in hemiparetic subjects. Archives of Physical Medicine and Rehabilitation, 80(4), 421–427. [DOI: 10.1016/s0003-9993(99)90279-4] [PMID]
Hosp, J. A., & Luft, A. R. (2011). Cortical plasticity during motor learning and recovery after ischemic stroke. Neural Plasticity, 2011, 871296.[DOI:10.1155/2011/871296] [PMID]
Hutchison, J. L., Hubbard, N. A., Brigante, R. M., Turner, M., Sandoval, T. I., & Hillis, G. A. J., et al. (2014). The efficiency of fMRI region of interest analysis methods for detecting group differences. Journal of Neuroscience Methods, 226, 57–65. [DOI:10.1016/j.jneumeth.2014.01.012] [PMID]
Krishnan, V., Kindig, M., & Mirbagheri, M. (2016). Robotic-assisted locomotor training enhances ankle performance in adults with incomplete spinal cord injury. Journal of Rehabilitation Medicine, 48(9), 781–786. [DOI:10.2340/16501977-2133] [PMID]
Kornelsen, J., & Stroman, P. W. (2004). fMRI of the lumbar spinal cord during a lower limb motor task. Magnetic Resonance in Medicine, 52(2), 411–414. [DOI:10.1002/mrm.20157] [PMID]
Lefaucheur, J. P., André-Obadia, N., Antal, A., Ayache, S. S., Baeken, C., Benninger, D. H., et al. (2014). Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 125(11), 2150–2206. [DOI:10.1016/j.clinph.2014.05.021] [PMID]
MacIntosh, B. J., Mraz, R., Baker, N., Tam, F., Staines, W. R., & Graham, S. J. (2004). Optimizing the experimental design for ankle dorsiflexion fMRI. NeuroImage, 22(4), 1619–1627. [DOI:10.1016/j.neuroimage.2004.03.035] [PMID]
Marzbani, H., Shahrokhi, A., Irani, A., Mehdinezhad, M., Kohanpour, M., & Mirbagheri, M. M. (2018). The Effects of Low Frequency Repetitive Transcranial Magnetic Stimulation on White Matter Structural Connectivity in Children with Cerebral Palsy. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual International Conference, 2018, 2491–2494. [DOI:10.1109/EMBC.2018.8512866] [PMID]
Navaei Lavasani, S., Mostaar, A., & Ashtiyani, M. (2018). Automatic prostate cancer segmentation using kinetic analysis in dynamic contrast-enhanced MRI. Journal Of Biomedical Physics & Engineering, 8(1), 107–116. [PMID]
Ogg, R. J., Laningham, F. H., Clarke, D., Einhaus, S., Zou, P., & Tobias, M. E., et al. (2009). Passive range of motion functional magnetic resonance imaging localizing sensorimotor cortex in sedated children. Journal of neurosurgery. Pediatrics, 4(4), 317–322. [DOI:10.3171/2009.4.PEDS08402] [PMID]
Palisano, R. J., Begnoche, D. M., Chiarello, L. A., Bartlett, D. J., McCoy, S. W., & Chang, H. J. (2012). Amount and focus of physical therapy and occupational therapy for young children with cerebral palsy. Physical & Occupational Therapy in Pediatrics, 32(4), 368–382. [DOI:10.3109/01942638.2012.715620] [PMID]
Parvin, S., Shahrokhi, A., Tafakhori, A., Irani, A., Rasteh, M., & Mirbagheri, M. M. (2018). Therapeutic effects of repetitive transcranial magnetic stimulation on corticospinal tract activities and neuromuscular properties in children with cerebral palsy. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual International Conference, 2018, 2218–2221. [DOI:10.1109/EMBC.2018.8512805] [PMID]
Parvin, S., Mehdinezhad, M., Taghiloo, A., Nourian, R., & Mirbagheri, M. M. (2018). The Impact of repetitive transcranial magnetic stimulation on affected and unaffected sides of a child with hemiplegic cerebral palsy. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual International Conference, 2018, 2523–2526. [DOI:10.1109/EMBC.2018.8512877] [PMID]
Pannek, K., Boyd, R. N., Fiori, S., Guzzetta, A., & Rose, S. E. (2014). Assessment of the structural brain network reveals altered connectivity in children with unilateral cerebral palsy due to periventricular white matter lesions. NeuroImage. Clinical, 5, 84–92. [DOI:10.1016/j.nicl.2014.05.018] [PMID]
Phillips, J. P., Sullivan, K. J., Burtner, P. A., Caprihan, A., Provost, B., & Bernitsky-Beddingfield, A. (2007). Ankle dorsiflexion fMRI in children with cerebral palsy undergoing intensive body-weight-supported treadmill training: A pilot study. Developmental Medicine and Child Neurology, 49(1), 39–44. [DOI:10.1017/s0012162207000102.x] [PMID]
Peterson, D. S., Gera, G., Horak, F. B., & Fling, B. W. (2017). Corpus callosum structural integrity is associated with postural control improvement in persons with multiple sclerosis who have minimal disability. Neurorehabilitation and Neural Repair, 31(4), 343-353. [DOI:10.1177/1545968316680]
Rademaker, K. J., Lam, J. N., Van Haastert, I. C., Uiterwaal, C. S., Lieftink, A. F., & Groenendaal, F., et al. (2004). Larger corpus callosum size with better motor performance in prematurely born children. Seminars in Perinatology, 28(4), 279–287. [DOI:10.1053/j.semperi.2004.08.005] [PMID]
Rasooli, A. H., Birgani, P. M., Azizi, S., Shahrokhi, A., & Mirbagheri, M. M. (2017). Therapeutic effects of an anti-gravity locomotor training (AlterG) on postural balance and cerebellum structure in children with Cerebral Palsy. IEEE. International Conference on Rehabilitation Robotics: [proceedings], 2017, 101–105. [DOI:10.1109/ICORR.2017.8009229] [PMID]
Reid, S. M., Ditchfield, M. R., Bracken, J., & Reddihough, D. S. (2015). Relationship between characteristics on magnetic resonance imaging and motor outcomes in children with cerebral palsy and white matter injury. Research in Developmental Disabilities, 45-46, 178–187. [DOI:10.1016/j.ridd.2015.07.030] [PMID]
Reid, L. B., Boyd, R. N., Cunnington, R., & Rose, S. E. (2016). Interpreting Intervention Induced Neuroplasticity with fMRI: The Case for Multimodal Imaging Strategies. Neural Plasticity, 2016, 2643491. [DOI:10.1155/2016/2643491] [PMID]
Reid, L. B., Rose, S. E., & Boyd, R. N. (2015). Rehabilitation and neuroplasticity in children with unilateral cerebral palsy. Nature Reviews. Neurology, 11(7), 390–400. [DOI:10.1038/nrneurol.2015.97] [PMID]
Rasooli, A. H., Ashtiyani, M., Birgani, P. M., Amiri, S., Mirmohammadi, P. & Deevband, M. R. (2018). MRI segmentation using Fuzzy C-means and radial basis function neural networks. Current Science, 115(2018), 1091-197. [DOI:10.18520/cs/v115/i6/1091-1097]
Rosazza, C., Aquino, D., D'Incerti, L., Cordella, R., Andronache, A., & Zacà, D., et al. (2014). Preoperative mapping of the sensorimotor cortex: comparative assessment of task-based and resting-state FMRI. Plos One, 9(6), e98860. [DOI:10.1371/journal.pone.0098860] [PMID]
Souweidane, M. M., Kim, K. H., McDowall, R., Ruge, M. I., Lis, E., & Krol, G., et al. (1999). Brain mapping in sedated infants and young children with passive-functional magnetic resonance imaging. Pediatric Neurosurgery, 30(2), 86–92.[DOI:10.1159/000028768] [PMID]
Takeuchi, N., Oouchida, Y., & Izumi, S. (2012). Motor control and neural plasticity through interhemispheric interactions. Neural Plasticity, 2012, 823285. [DOI:10.1155/2012/823285] [PMID]
van Hedel, H. J., Wirz, M., & Dietz, V. (2005). Assessing walking ability in subjects with spinal cord injury: validity and reliability of 3 walking tests. Archives of Physical Medicine and Rehabilitation, 86(2), 190–196. [DOI:10.1016/j.apmr.2004.02.010] [PMID]
Weierink, L., Vermeulen, R. J., & Boyd, R. N. (2013). Brain structure and executive functions in children with cerebral palsy: A systematic review. Research in Developmental Disabilities, 34(5), 1678–1688. [DOI:10.1016/j.ridd.2013.01.035] [PMID]
Weiskopf, N., Scharnowski, F., Veit, R., Goebel, R., Birbaumer, N., & Mathiak, K. (2004). Self-regulation of local brain activity using real-time functional magnetic resonance imaging (fMRI). Journal of Physiology, Paris, 98(4-6), 357–373. [DOI:10.1016/j.jphysparis.2005.09.019] [PMID]
Wilke, M., Holland, S. K., Myseros, J. S., Schmithorst, V. J., & Ball, W. S., Jr (2003). Functional magnetic resonance imaging in pediatrics. Neuropediatrics, 34(5), 225–233.[DOI:10.1055/s-2003-43260] [PMID]
Weiller, C., Jüptner, M., Fellows, S., Rijntjes, M., Leonhardt, G., & Kiebel, S., et al. (1996). Brain representation of active and passive movements. NeuroImage, 4(2), 105–110. [DOI:10.1006/nimg.1996.0034] [PMID]
Willoughby, K. L., Dodd, K. J., & Shields, N. (2009). A systematic review of the effectiveness of treadmill training for children with cerebral palsy. Disability and Rehabilitation, 31(24), 1971–1979. [DOI:10.3109/09638280902874204] [PMID]
Yang, Y. R., Chen, I. H., Liao, K. K., Huang, C. C., & Wang, R. Y. (2010). Cortical reorganization induced by body weight-supported treadmill training in patients with hemiparesis of different stroke durations. Archives of Physical Medicine and rehabilitation, 91(4), 513–518. [DOI:10.1016/j.apmr.2009.11.021] [PMID]
Full-Text:
1. Introduction
Functional deficits, including gait impairment in childhood, are mostly one of the consequences of cerebral palsy (CP), a permanent neurological disorder with a prevalence of 2-3 per 1000 live births, which results from a lesion or insult to the developing brain in early life (Dan, 2020; Weierink et al., 2013; Palisano et al., 2012). CP as a non-progressive brain disorder results in impaired gait and posture which in turn affect the developing musculoskeletal system. Spastic hemiplegic CP (HCP), as a common subtype of CP, is identified by unilateral involvement in the extremities (Carr, 1996; Dan, 2021).
Although medications, including Botulinum toxin and orthopedic surgery, have been used for children with CP, they have their pros and cons, including limited therapeutic efficiency and side effects, to name but a few. Rehabilitation, including occupational therapy (OT) and physical therapy, are the most prevalent treatment strategies for pediatrics with CP, which focus on strengthening muscles and joints mainly in a passive manner (Carr, 1996; Dietz, 1996). However, efficient treatment should be accompanied by adequate voluntary long-term intensive systematic training. The anti-gravity treadmill (AlterG system) allows the subject to perform voluntary gait and postural training. The AlterG can help individuals walk independently and actively using precise unweighting technology with adjustable speed. Successful treatment by triggering neural messaging in motor and sensory pathways is expected to cause brain neuroplasticity, and forming or activating specific neural pathways results in gait and postural improvement (Birgani et al., 2016; Azizi et al., 2018; Rasooli et al., 2017; Ashtiyani et al., 2020).
Specific neural pathway formation may also be expected from repetitive transcranial magnetic stimulation (rTMS), which directly increases the activity and strengths of neural tracts. However, deploying rTMS in line with OT may improve gait and function efficiency (Hallett, 2007; Garvey Mall, 2008; Bleyenheuft et al., 2015; Parvin et al., 2018).
Since CP is a consequence of brain injury, improvement in brain structure and function in line with consistent clinical enhancement may result in long-lasting treatment. Considering this, other than clinical assessment following treatment, investigation of therapy-induced neuroplasticity is of great value.
In this regard, advanced neuroimaging modalities, such as structural magnetic resonance imaging (MRI), functional MRI (fMRI), and task-based fMRI (t-fMRI), are used for neuroplasticity assessment (Birgani et al., 2016; Donabedian, 1966; Parvin et al., 2018; Krishnan & Kindig, 2016; Reid et al., 2016; Lavasani et al., 2018; Rasooli et al., 2018). The latter characterizes functionally involved brain regions while performing a specific task (Reid et al., 2016; Barch et al., 2013; Kornelsen & Stroman, 2004; Weiskopf et al., 2004). T-fMRI provides hundreds of thousands of signals, reflecting functional brain activity, resulting from each voxel of the brain (Bleyenheuft et al., 2015; Esteban et al., 2029; Phillips et al., 2007; Reid et al., 2015). This information helps us understand how the human brain is functionally organized into distinct subdivisions. Due to technical challenges in acquiring t-fMRI in children with spastic HCP, a limited number of studies have used it to explore gait rehabilitation-induced neuroplasticity in these children (Phillips et al., 2007; MacIntosh et al., 2004). Also, some small-scale studies have detected fMRI alternations following therapy in children with unilateral CP (Phillips et al., 2007; Reid et al., 2015).
The feasibility of standard processing steps of fMRI is mainly restricted by motion artifacts in children with CP, and studies on healthy children show that fMRI acquisition necessitates subjects remaining fixed for a long time (Bleyenheuft et al., 2015; Phillips et al., 2007; Reid et al., 2015). Sedation with the least impact on administrated motor and sensory stimulation is used to reduce head motions while imaging (Wilke et al., 2003; Bernal et al., 2012). Previous studies also yielded reasonable results in performing passive motor tasks during sedative fMRI (Souweidane et al., 1999; Rosazza et al., 2014; Ogg et al., 2009; Weiller et al., 1996).
Investigating the motor cortex as the source of most descending motor pathways and the corpus callosum (CC) as the largest interhemispheric commissure is of great interest in literature. In the case of prematurely born children or pediatrics with periventricular leukomalacia, motor performance is positively correlated with CC size (Pannek et al., 2014; Rademaker et al., 2004; Davatzikos et al., 2003). As an extensive inter-hemispheric communication line, CC plays a crucial role in motor control (Bloom et al., 2005). Reid et al., (2015) showed that poor gross motor function classification system (GMFCS) is related to severe axonal loss of CC measured by MRI. Also, Peterson et al. showed that postural control enhancement is associated with the structural integrity of CC in patients with multiple sclerosis (Peterson et al., 2017). Although, to date, fMRI studies have mainly investigated gray matter, recent studies also explored the functional organization of CC using t-fMRI (Gawryluk et al., 2014; Fabri et al., 2014). However, the effect of treatment on CC and motor cortex functional activities remains elusive.
Therefore, we aimed to explore the effects of OT, rTMS, and AlterG training on walking capacity and brain functional activities of the motor cortex and CC in children with CP and to determine the possible associations between these measurements in this pilot study.
2. Materials and Methods
Study participants
Twenty-one hemiplegic CP children (7-12 years old) with level II and level III GMFCS participated in this study. They were cooperative and capable of following instructions; the participants had no severe cognitive impairment, no botulinum toxin injections within 3 months of the study, no previous constraint therapy within 9 months, and no history of orthopedic surgery 6 months before training. Two subjects did not participate in the fMRI portion of the study, and the fMRI data of one subject was excluded due to excessive head motion.
Treatment protocols
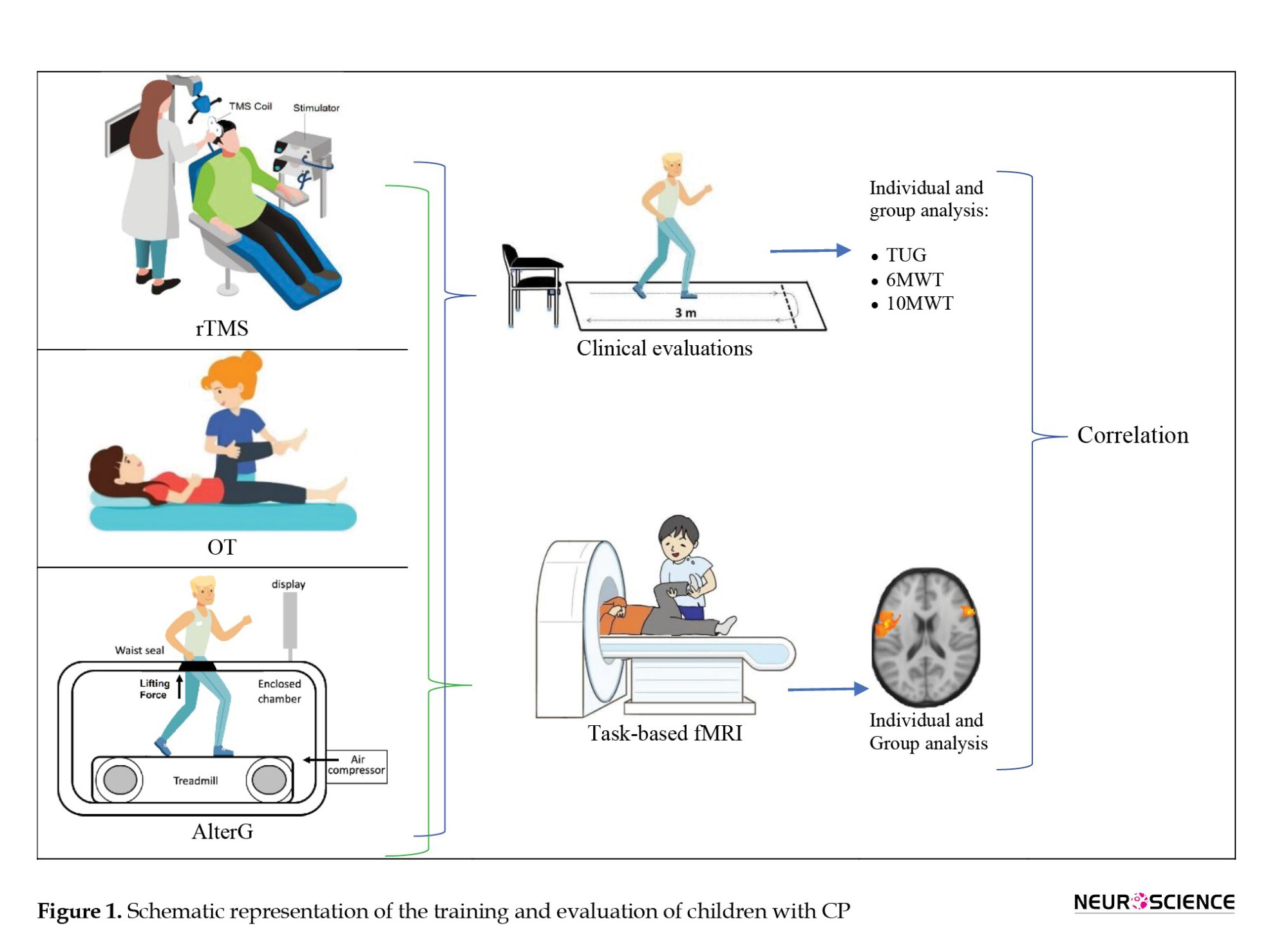
The participants were randomly divided into 3 groups: OT, rTMS, and AlterG. All participants were provided with 24 sessions of treatment. A schematic diagram of this study is shown in Figure 1.
OT
A pediatric occupational therapist provided OT training focused on reducing spasticity, gait training, coordination of agonist and antagonist, protective balance exercise, active-passive range of motion (ROM), and foot-eye coordination. Each participant received 24 sessions of 45 minutes of OT 3 days/week.
rTMS
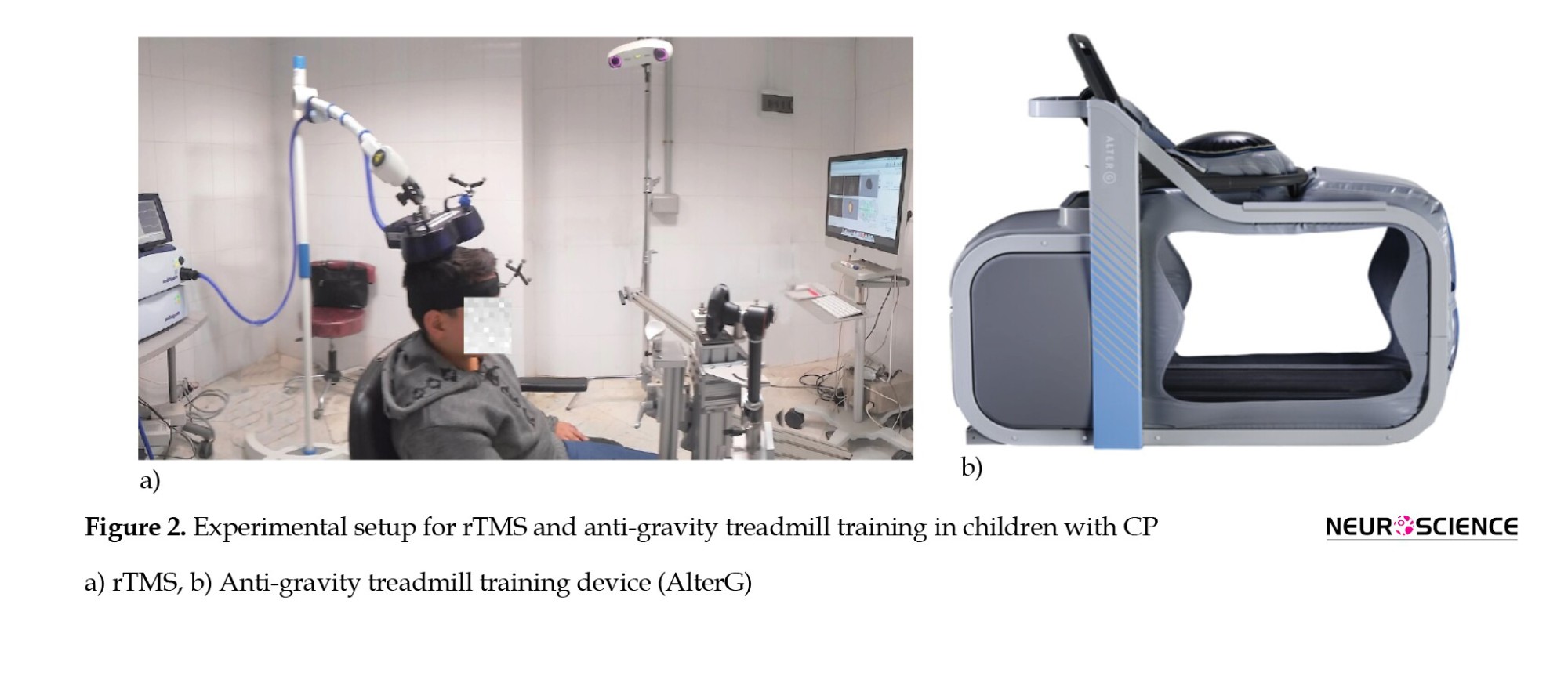
rTMS, as a non-invasive multisession treatment technique, deploys magnetic fields to stimulate nerve cells of the cerebral cortex by altering cortical plasticity (Lefaucheur et al., 2014). rTMS refers to the application of recurring TMS pulses to a specific brain region, which was performed by “Magstim Rapid2” (Magstim, UK). and its D70 air film coil. Depending on the rTMS frequency, it could increase or decrease cortical excitability by applying high-frequency or low-frequency, respectively. The former may have an excitatory effect on brain function and increased motor function, while the latter could suppress brain function. Due to the risk of seizure initiation in high-frequency rTMS, we applied low-frequency rTMS on the contralesional primary motor cortex (M1) to inhibit the unaffected motor cortex excitability, which may indirectly force the affected hemisphere to enhance its function. A Brainsight system compatible with Magstim is used to position the rTMS coil over the stimulation-targeted site. To prevent head movement, the head position was fixed using the device’s equipment (Figure 2a). The resting motor threshold (rMT) has been defined as the minimum intensity stimulus necessary to drive motor-evoked potentials (MEP) in the resting target muscle during single-pulse TMS (50 µV) of peak-to-peak MEP amplitude occurs in 50% of 10 stimulations. Electromyography active electrodes were placed on the unaffected leg to determine the MEP of the tibialis anterior (TA) relaxed muscle during single pulse TMS with the same treatment coil to acquire rMT in each participant. These resting motor thresholds were used to adjust the intensity of rTMS for each participant. One thousand two hundred rTMS pulses in the form of 120 trains with an inter-train interval of 1 second were applied to each subject for 24 sessions, 4 days/week (Marzbani et al., 2018). From the 13th session, the rTMS was followed by 45 minutes of OT.
Anti-gravity treadmill training
The anti-gravity treadmill (AlterG F320, CA USA) applies positive air pressure into an inflatable chamber that surrounds the lower extremities to uniformly reduce gravitational load and unweighed the participant to 20% of his body weight gradually in 1% increments (Figure 2b). The patients received 45-minute AlterG training 3 days/week for 8 weeks. In each session, the participants started walking at a speed of 1 km/h while unweighted to 50% of their body weight to warm up. As the speed increased, the body weight support gradually decreased based on the patients’ walking capacity on the treadmill and the trained physical therapist’s discretion to provide the participants with necessary feedback (Birgani et al., 2016; Azizi et al., 2018; Rasooli et al., 2017).
fMRI acquisition
Scanning was performed with a single-shot gradient echo-planar imaging pulse sequence and standard head coil using a GE 3-Tesla scanner. Eighty axial slices parallel to the posterior/anterior commissure line with a slice thickness of 3 mm were acquired using a repetition time (TR) of 3 s, echo time (TE) of 30 ms, a flip angle of 90°, and 64×64 matrix size. Following completion of the fMRI protocol, a T1-weighted image was acquired parallel to the posterior/anterior commissure line (resolution=1×1×1 mm, TR=1800 ms, TE=3.4 ms, matrix size=256×256).
Each participant underwent MRI scans pre- and post-treatments. The pediatric anesthesiologist sedated the participants before MRI data acquisition. The default protocol for sedation involved intravenous propofol administered at the lowest dose to keep the patient asleep after induction. Throughout the examination, heart rate and oxygen saturation were monitored for all subjects by pulse-oximetry. Information about the sedation was documented in the medical record.
Knee flexion/extension and ankle plantarflexion/dorsiflexion movements over the range of motion (ROM) with 0.5 Hz and 1 Hz frequencies were defined as two passive motor tasks for acquiring t-fMRI. A trained biomedical engineer performed all passive tasks. A block design of 24 s of rest alternating 24 s of motor task repeated for 5 cycles was used for fMRI acquisition
FMRI data processing
Statistical analysis and preprocessing of the functional data were performed with the fMRI of the brain (FMRIB) software library (FSL v6.02). Brain extraction, realignment, spatial smoothing, motion correction, denoising, and filtering as the standard preprocessing steps were applied to fMRI raw data. The brain images were coregistered and realigned to the mean functional image from the first session. The realigned images are skull-stripped with a brain extraction tool in FSL. To reduce motion artifacts, the subjects’ motion parameters were extracted and included as nuisance covariates in individual analysis. Then, the functional images were smoothed with a 5×5×5 mm3 full width at the half maximum Gaussian kernel and a high pass filter of 72 s. To enhance the signal-to-noise ratio, spatial smoothing was deployed. Voxels residing on the exterior of the brain were removed. Multivariate exploratory linear optimized decomposition into independent components (MELODIC) was performed for denoising. MELODIC uses independent component analysis to decompose 4D data sets into different temporal and spatial components.
fMRI data transformation into standard space may affect group differences and brain functional analysis outcomes. Hence, the reverse process of registering Montreal Neurological Institute (MNI152 atlas) standard space to brain functional data is performed (Hutchison et al., 2014). This study uses region of interest (ROI) analysis to cover the motor cortex and CC. The motor cortex includes brain regions related to planning (premotor cortex [PMC], execution in supplementary motor area [SMA], and precentral gyrus [PG]) and control of movements (primary motor cortex [M1]). These regions were selected from the Harvard-Oxford subcortical and cortical atlases and transformed into the individual’s native space.
First-level individual statistical analyses were performed using the general linear model (GLM) to calculate the significant brain regions. To estimate brain activation for the separate contrasts (rest Clinical evaluations
In this study, 3 common clinical characteristics were used to evaluate balance and movement, including a 6-minute walk test (6MWT), timed-up-and-go (TUG), and 10-m walk test (10MWT). Also, 6MWT evaluates a person’s walking endurance by measuring the distance walked in 6 minutes (van Hedel et al., 2005). In addition, TUG assesses both static and dynamic balance by measuring the time required for subsequently performing the following tasks: rising from a chair, walking 3 m, making a U-turn, walking back 3 m to the starting point, and sitting down on the chair while turning 180° (Bohannon, 2006). Also, 10MWT assesses functional mobility and gait by measuring the walking speed of a 10-m walk at the self-selected velocity (SSV) and fast velocity (FV) (Ditunno et al., 2000).
3. Results
Therapeutic effects on gait and balance impairment
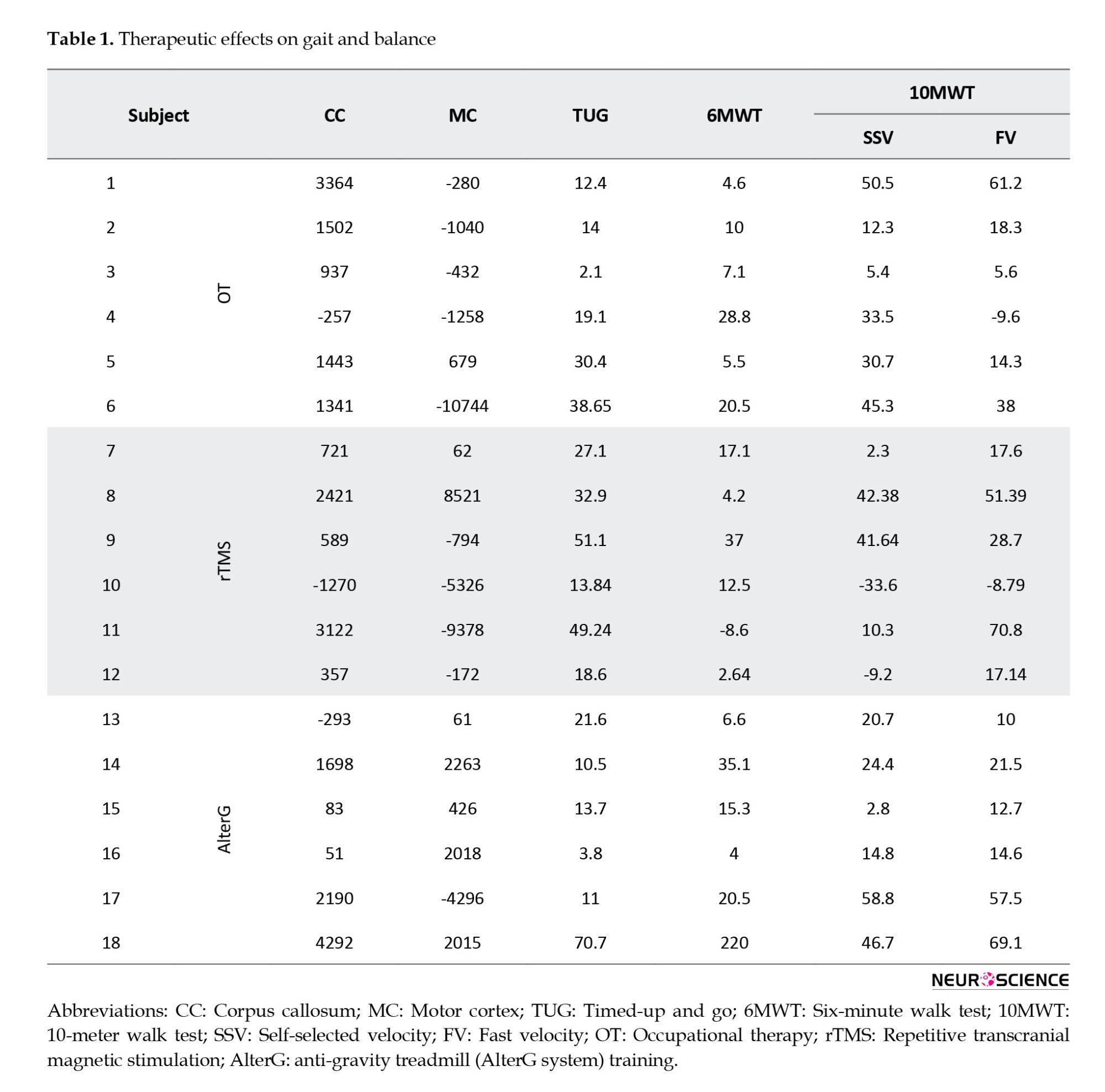
Table 1 summarizes the percentage of clinical characteristics improvements following 24 sessions of training courses for each subject. Clinical characteristics include TUG, 6MWT and 10MWT. In the OT group, TUG, 6MWT, and SSV improved in all subjects, and FV improved in all subjects except one. In the rTMS group, TUG improved in all subjects, while 6MWT and 10MWT improved in all subjects but one and two cases, respectively. In the AlterG group, all clinical parameters improved in all subjects.
Therapeutic effects on functional brain activity
This study investigated the functional activation in 2 brain areas: Motor cortex and CC, which connect two hemispheres. The motor cortex includes brain regions associated with planning (PMC), execution (SMA and PG), and control of movements (M1). Table 1 summarizes the significant difference between pre- and post-treatment in the motor cortex and CC regarding the number of activated voxels for all tasks and each subject. According to Table 1, in each group, the number of activated voxels in CC decreased in one subject and increased in others due to treatments. In the motor cortex, the number of activated voxels is a combination of increase and decrease following treatments.
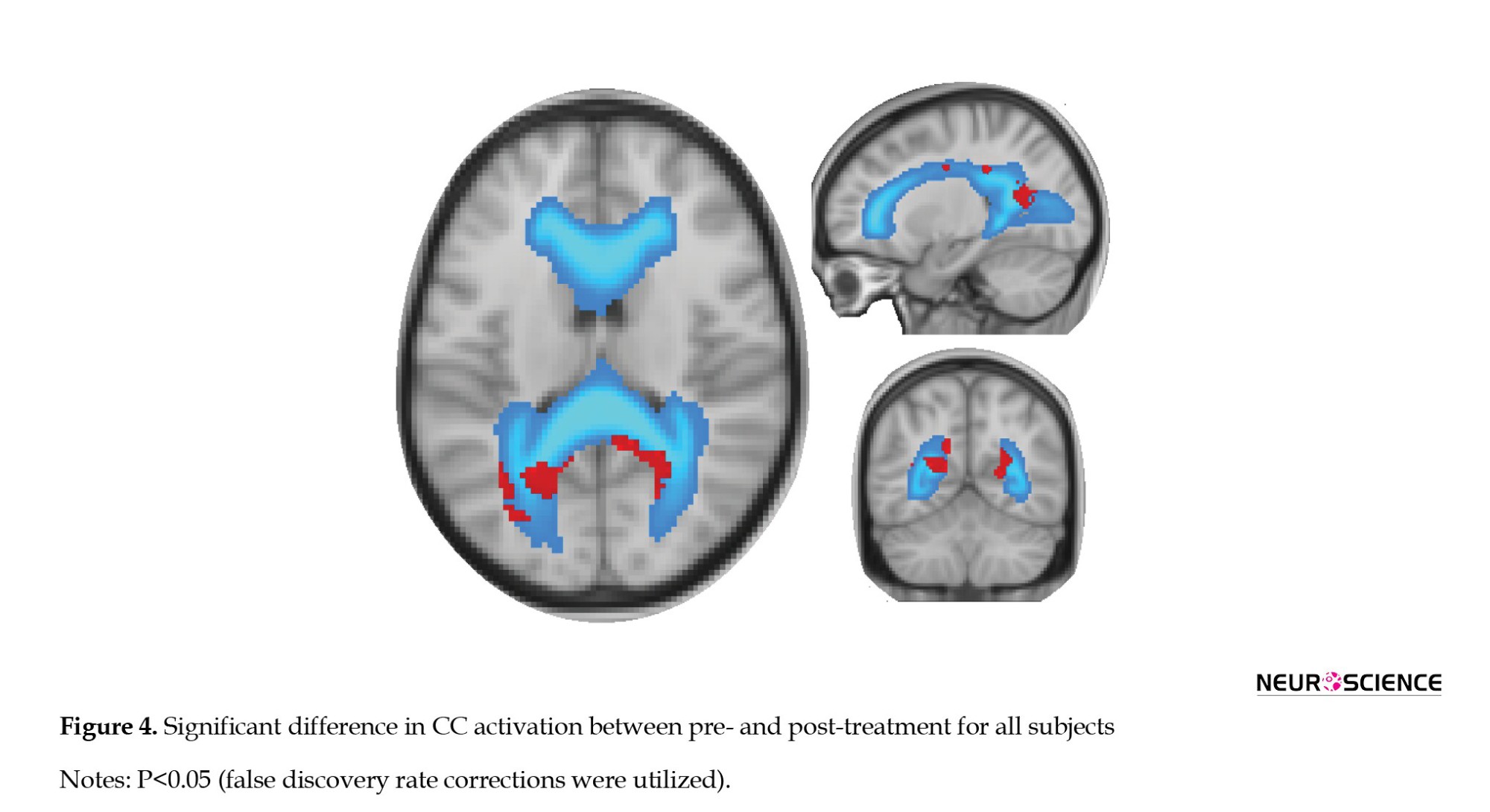
Figure 4 depicts a significant difference in CC activation between pre-treatment and post-treatment for all subjects and a P<0.05 (FDR corrections were applied).
Correlation results
According to Table 1, the difference in brain functional activity between pre- and post-treatment in the motor cortex is negative in 10 participants and positive in the others. Although in 6 subjects, the clinical characteristics improved following treatment, the number of activated voxels decreased in the motor cortex. Since the decreased cortical activation may not represent the deterioration in functional activity (Dan, 2021), the absolute value of motor cortex functional activity changes is used to investigate the possible correlation with clinical parameter improvements in Figure 3. In rTMS and OT groups, motor cortex functional activity does not correlate with clinical parameters. In the AlterG group, motor area functional activity significantly correlates only with FV (P<0.05). For all subjects, regardless of the type of treatment, there is no significant correlation between motor cortex activity and clinical parameters (Figure 3).
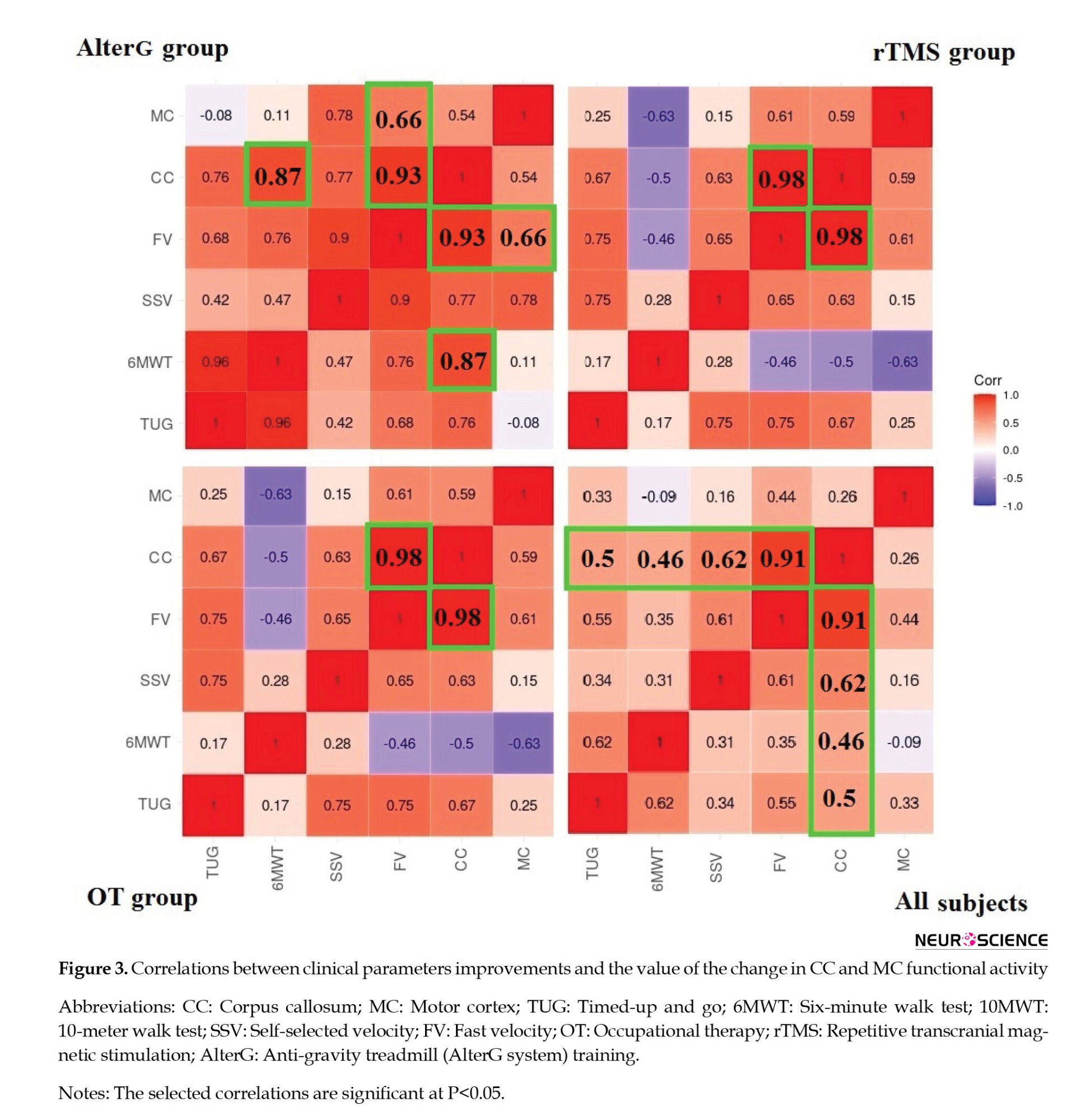
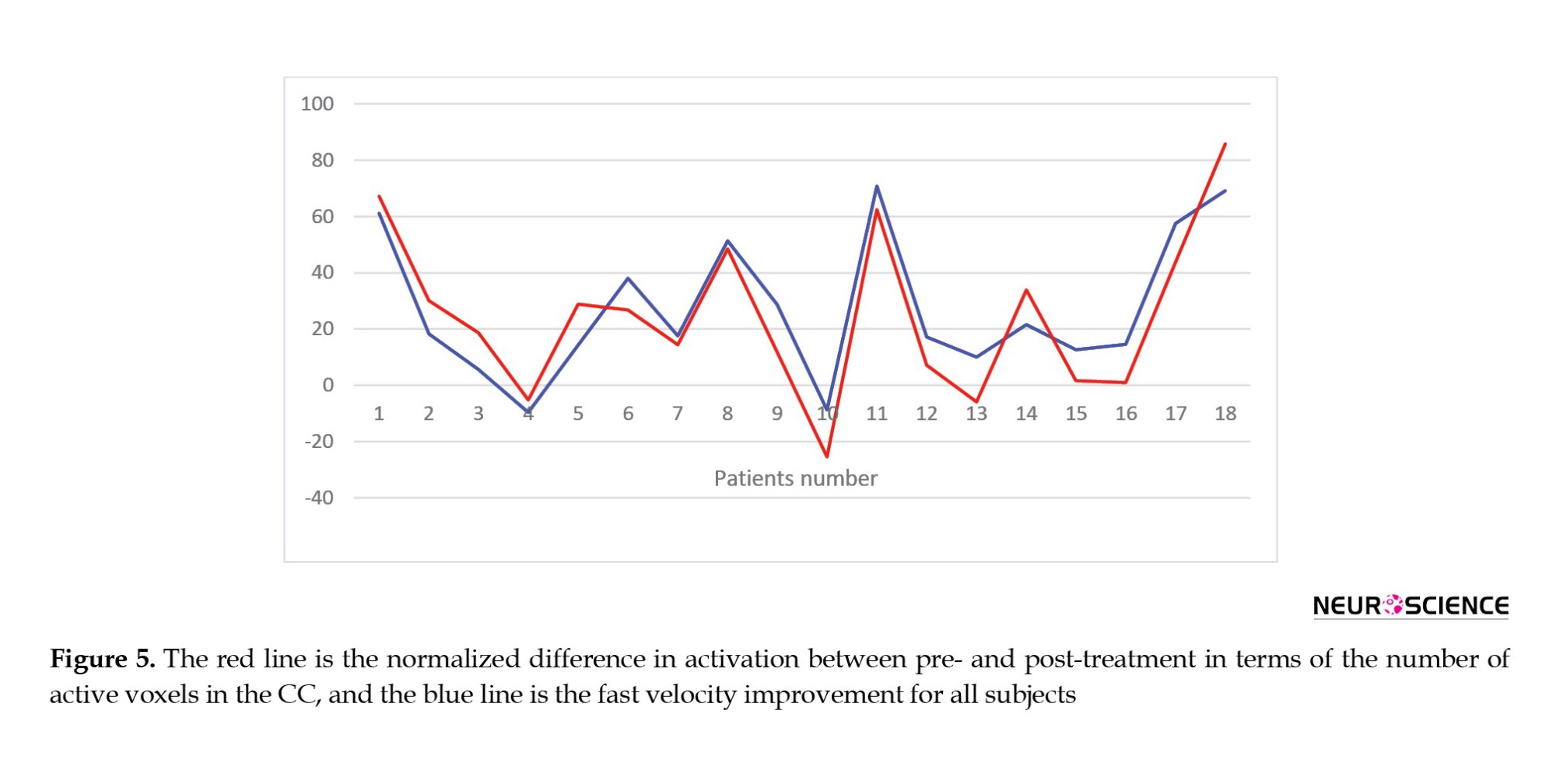
Figure 3 also shows the correlations between clinical improvements and the value of the functional activity changes in the CC. FV changes following treatment significantly correlate with CC functional activity alternations in all groups. However, SSV and 6MWT changes only correlate substantially with brain activity changes in the CC region following rTMS and AlterG training, respectively. Figure 5 shows all subjects’ CC functional activity alternations and FV changes.
4. Discussion
This pilot study explores the therapeutic effects of intensive OT, rTMS, and AlterG training on walking capacity and induced neuroplasticity indexed by brain activation alternations using passive task-based fMRI. Passive tasks include plantar flexion and dorsiflexion over the ROM of both ankles and flexion to extension over both knees, which were carried out pre- and post-treatments under sedation. To our knowledge, a limited number of studies investigated brain functional reorganization following gait rehabilitation in pediatrics with CP and adults with stroke (Phillips et al., 2007; Dobkin et al., 2004; Yang et al., 2010). The studies utilizing OT and Lokomat for enhancing walking capacity mainly focused on clinical improvement rather than brain reorganization assessment (Hesse et al., 1999; Drużbicki et al., 2013; Willoughby et al., 2009). Furthermore, the effect of treatment on interhemispheric relations and brain functional activities remains elusive. Therefore, we aimed to characterize the brain functional activity alternations following these treatments in children with spastic HCP in addition to assessment of walking capacity improvement. Also, we aimed to investigate the neural correlation of clinical improvement in terms of brain functional activity changes in specific brain regions in children with CP induced by the treatments, regardless of the type of treatment. Our findings showed improved walking capacity and brain functional activity following OT, rTMS, and AlterG training. Also, our results showed that the improvement in clinical measurements significantly correlates with changes in brain functional activity in selected regions.
The motor cortex is responsible for the planning, execution, and control of movements, and CC is considered the largest WM tract connecting the two hemispheres, which is critical for performing tasks that require interhemispheric interaction. Moreover, the homotopic connectivity between the motor cortices is believed to emerge through CC. The interhemispheric exchange role of CC could be inhibitory, excitatory, or a combination of both, which may alter through neurological diseases such as stroke and CP (Takeuchi et al., 2012). Additionally, previous studies have reported a positive correlation between CC in children with CP and motor performance (Pannek et al., 2014; Rademaker et al., 2004; Davatzikos et al., 2003). In this regard, assessments of interhemispheric relations through CC may unravel the neurophysiological underpinnings that modulate motor control and may result in introducing interventional therapies to improve motor function in children with CP. Although many studies investigate the activation of CC following different visual, motor, and tactile tasks using fMRI, none of them explore its therapy-driven alternations. Addressing this issue and considering the lesion heterogeneity in terms of location and size in our CP cohort, this pilot study, for the first time, investigates the therapy-induced brain functional activity alternation in the motor cortex and CC regions.
Our analysis showed both a decrease and an increase in brain activation after therapy. According to Table 1, in 6 subjects, the clinical characteristics improved following treatments while the number of activated voxels decreased in the motor cortex. This decrease in cortical activation may be evidence of less neural energy consumption following intensive training and is thought to be a gain in neural efficiency (Dan, 2021). Furthermore, learning specific tasks following intensive repetitive training may allow the tasks to be performed through memory-based processing by reorganizing the cortical representations of sensorimotor features, which has been suggested for children with CP (Dan, 2016). In line with our results, intensive motor training in a related study resulted in decreased and increased cortical hemodynamic response during task execution (Calmels, 2020). Also, the cortical activation of the sensorimotor area following treatment has reduced in some studies, while others showed increased cortical activation (Drużbicki et al., 2013; Dinomais et al., 2013). However, interpreting such alternations in cortical activity of the brain regions of interest is challenging.
As illustrated in Figure 3, alternations in the hemodynamic response of the motor cortex only correlate with FV in the AlterG group. However, the absence of these correlations is not equivalent to a lack of neural associations. The reasons for this condition stem primarily from the complex and non-linear association between the underlying mechanisms of these measures, which will be investigated in future studies.
On the other hand, according to Figure 3, alternations in CC functional activity in OT and rTMS groups significantly correlate with functional mobility and gait improvement indexed by FV. In the AlterG group, changes in CC functional activity significantly correlate with enhancements in functional mobility, gait, and walking endurance indexed by FV and 6MWT, respectively. Regardless of the type of treatment, CC functional activity changes significantly correlate with alternations of TUG and 6MWT.
Also, our analysis reveals a robust and significant correlation between alternations in CC functional activity and walking capacity improvement in terms of fast velocity, regardless of the treatment group. Figure 5 illustrates the point. This strong correlation aligns with studies suggesting that the modulation of interhemispheric communication supports the brain's ability to form new neuronal connections, compensate for impairments, and acquire new functions—key aspects of neural plasticity. (Takeuchi et al., 2012; Hosp, 2011). In children with CP, the less affected side of the brain tries to compensate for the weakness of the more affected side, and this amount of interaction can be mainly related to the relationship between the two hemispheres.
5. Conclusion
Our results indicate that intensive OT, rTMS, and AlterG training enhances brain activity and walking capacity in children with CP. OT, rTMS, and AlterG improve functional mobility and gait, while AlterG improves walking endurance. Regardless of the type of treatment, robust correlation between walking capacity improvement (FV) and CC activity changes were successfully characterized in children with hemiplegic CP (HCP). These results show that the improvement in gait function in children with CP may reflect an increase in brain functional activity in callosal neurons, revealing the rise in interhemispheric coupling.
Study limitations
Our study has some limitations. Firstly, some subjects could not complete the required training sessions due to the intensive training schedule. Secondly, some fMRI data could not be used due to excessive motion. Thirdly, despite the damping effect of sedation on brain activity, we successfully detected the brain activity and its changes following treatments. However, brain activity changes following treatment must be greater in practice than reported in this study. Finally, the characterization of the intervention effects may not be achieved solely by calculating the average group results and pre-post analyses. This condition required further time points and a larger sample size, which we have taken into consideration in our ongoing studies.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Ethics Committee of Tehran University of Medical Sciences (TUMS), Tehran, Iran. This study was registered in the Iranian Registry of Clinical Trials (IRCT) with the number IRCT2015121625568N1. All participants gave their written informed consent to participate in the study.
Funding
This research was supported by grants from the Ministry of Health and Medical Education and Tehran University of Medical Sciences (TUMS), Tehran, Iran.
Authors' contributions
Study design and investigation: Parmida Moradi Birgani, Meghdad Ashtiyani and M. Mehdi Mirbagheri; Methodology: Parmida Moradi Birgani, Meghdad Ashtiyani, M. Mehdi Mirbagheri, seyed. Behnamedin Jamei, and Mohammad Reza Deevband; Data analysis: Parmida Moradi Birgani, Meghdad Ashtiyani and M. Mehdi Mirbagheri; Data acquisition: Parmida Moradi Birgani, Meghdad Ashtiyani, M. Mehdi Mirbagheri, Elham Rahimian and Amin Shahrokhi; Writing the original draft: Parmida Moradi birgani, and Meghdad Ashtiyani; Data interpretation and final approval: All authors.
Acknowledgments
The authors would like to thank Ministry of Health and Medical Education and Tehran University of Medical Sciences for their support in conducting this study. The authors wish to thank Shahrokhnia, and Kohanpour, for their cooperation. The authors are also grateful to the children and families who participated in the research.
Conflict of interest
The authors expressed no conflict of interest.
References
Functional deficits, including gait impairment in childhood, are mostly one of the consequences of cerebral palsy (CP), a permanent neurological disorder with a prevalence of 2-3 per 1000 live births, which results from a lesion or insult to the developing brain in early life (Dan, 2020; Weierink et al., 2013; Palisano et al., 2012). CP as a non-progressive brain disorder results in impaired gait and posture which in turn affect the developing musculoskeletal system. Spastic hemiplegic CP (HCP), as a common subtype of CP, is identified by unilateral involvement in the extremities (Carr, 1996; Dan, 2021).
Although medications, including Botulinum toxin and orthopedic surgery, have been used for children with CP, they have their pros and cons, including limited therapeutic efficiency and side effects, to name but a few. Rehabilitation, including occupational therapy (OT) and physical therapy, are the most prevalent treatment strategies for pediatrics with CP, which focus on strengthening muscles and joints mainly in a passive manner (Carr, 1996; Dietz, 1996). However, efficient treatment should be accompanied by adequate voluntary long-term intensive systematic training. The anti-gravity treadmill (AlterG system) allows the subject to perform voluntary gait and postural training. The AlterG can help individuals walk independently and actively using precise unweighting technology with adjustable speed. Successful treatment by triggering neural messaging in motor and sensory pathways is expected to cause brain neuroplasticity, and forming or activating specific neural pathways results in gait and postural improvement (Birgani et al., 2016; Azizi et al., 2018; Rasooli et al., 2017; Ashtiyani et al., 2020).
Specific neural pathway formation may also be expected from repetitive transcranial magnetic stimulation (rTMS), which directly increases the activity and strengths of neural tracts. However, deploying rTMS in line with OT may improve gait and function efficiency (Hallett, 2007; Garvey Mall, 2008; Bleyenheuft et al., 2015; Parvin et al., 2018).
Since CP is a consequence of brain injury, improvement in brain structure and function in line with consistent clinical enhancement may result in long-lasting treatment. Considering this, other than clinical assessment following treatment, investigation of therapy-induced neuroplasticity is of great value.
In this regard, advanced neuroimaging modalities, such as structural magnetic resonance imaging (MRI), functional MRI (fMRI), and task-based fMRI (t-fMRI), are used for neuroplasticity assessment (Birgani et al., 2016; Donabedian, 1966; Parvin et al., 2018; Krishnan & Kindig, 2016; Reid et al., 2016; Lavasani et al., 2018; Rasooli et al., 2018). The latter characterizes functionally involved brain regions while performing a specific task (Reid et al., 2016; Barch et al., 2013; Kornelsen & Stroman, 2004; Weiskopf et al., 2004). T-fMRI provides hundreds of thousands of signals, reflecting functional brain activity, resulting from each voxel of the brain (Bleyenheuft et al., 2015; Esteban et al., 2029; Phillips et al., 2007; Reid et al., 2015). This information helps us understand how the human brain is functionally organized into distinct subdivisions. Due to technical challenges in acquiring t-fMRI in children with spastic HCP, a limited number of studies have used it to explore gait rehabilitation-induced neuroplasticity in these children (Phillips et al., 2007; MacIntosh et al., 2004). Also, some small-scale studies have detected fMRI alternations following therapy in children with unilateral CP (Phillips et al., 2007; Reid et al., 2015).
The feasibility of standard processing steps of fMRI is mainly restricted by motion artifacts in children with CP, and studies on healthy children show that fMRI acquisition necessitates subjects remaining fixed for a long time (Bleyenheuft et al., 2015; Phillips et al., 2007; Reid et al., 2015). Sedation with the least impact on administrated motor and sensory stimulation is used to reduce head motions while imaging (Wilke et al., 2003; Bernal et al., 2012). Previous studies also yielded reasonable results in performing passive motor tasks during sedative fMRI (Souweidane et al., 1999; Rosazza et al., 2014; Ogg et al., 2009; Weiller et al., 1996).
Investigating the motor cortex as the source of most descending motor pathways and the corpus callosum (CC) as the largest interhemispheric commissure is of great interest in literature. In the case of prematurely born children or pediatrics with periventricular leukomalacia, motor performance is positively correlated with CC size (Pannek et al., 2014; Rademaker et al., 2004; Davatzikos et al., 2003). As an extensive inter-hemispheric communication line, CC plays a crucial role in motor control (Bloom et al., 2005). Reid et al., (2015) showed that poor gross motor function classification system (GMFCS) is related to severe axonal loss of CC measured by MRI. Also, Peterson et al. showed that postural control enhancement is associated with the structural integrity of CC in patients with multiple sclerosis (Peterson et al., 2017). Although, to date, fMRI studies have mainly investigated gray matter, recent studies also explored the functional organization of CC using t-fMRI (Gawryluk et al., 2014; Fabri et al., 2014). However, the effect of treatment on CC and motor cortex functional activities remains elusive.
Therefore, we aimed to explore the effects of OT, rTMS, and AlterG training on walking capacity and brain functional activities of the motor cortex and CC in children with CP and to determine the possible associations between these measurements in this pilot study.
2. Materials and Methods
Study participants
Twenty-one hemiplegic CP children (7-12 years old) with level II and level III GMFCS participated in this study. They were cooperative and capable of following instructions; the participants had no severe cognitive impairment, no botulinum toxin injections within 3 months of the study, no previous constraint therapy within 9 months, and no history of orthopedic surgery 6 months before training. Two subjects did not participate in the fMRI portion of the study, and the fMRI data of one subject was excluded due to excessive head motion.
Treatment protocols
The participants were randomly divided into 3 groups: OT, rTMS, and AlterG. All participants were provided with 24 sessions of treatment. A schematic diagram of this study is shown in Figure 1.

OT
A pediatric occupational therapist provided OT training focused on reducing spasticity, gait training, coordination of agonist and antagonist, protective balance exercise, active-passive range of motion (ROM), and foot-eye coordination. Each participant received 24 sessions of 45 minutes of OT 3 days/week.
rTMS
rTMS, as a non-invasive multisession treatment technique, deploys magnetic fields to stimulate nerve cells of the cerebral cortex by altering cortical plasticity (Lefaucheur et al., 2014). rTMS refers to the application of recurring TMS pulses to a specific brain region, which was performed by “Magstim Rapid2” (Magstim, UK). and its D70 air film coil. Depending on the rTMS frequency, it could increase or decrease cortical excitability by applying high-frequency or low-frequency, respectively. The former may have an excitatory effect on brain function and increased motor function, while the latter could suppress brain function. Due to the risk of seizure initiation in high-frequency rTMS, we applied low-frequency rTMS on the contralesional primary motor cortex (M1) to inhibit the unaffected motor cortex excitability, which may indirectly force the affected hemisphere to enhance its function. A Brainsight system compatible with Magstim is used to position the rTMS coil over the stimulation-targeted site. To prevent head movement, the head position was fixed using the device’s equipment (Figure 2a). The resting motor threshold (rMT) has been defined as the minimum intensity stimulus necessary to drive motor-evoked potentials (MEP) in the resting target muscle during single-pulse TMS (50 µV) of peak-to-peak MEP amplitude occurs in 50% of 10 stimulations. Electromyography active electrodes were placed on the unaffected leg to determine the MEP of the tibialis anterior (TA) relaxed muscle during single pulse TMS with the same treatment coil to acquire rMT in each participant. These resting motor thresholds were used to adjust the intensity of rTMS for each participant. One thousand two hundred rTMS pulses in the form of 120 trains with an inter-train interval of 1 second were applied to each subject for 24 sessions, 4 days/week (Marzbani et al., 2018). From the 13th session, the rTMS was followed by 45 minutes of OT.
Anti-gravity treadmill training
The anti-gravity treadmill (AlterG F320, CA USA) applies positive air pressure into an inflatable chamber that surrounds the lower extremities to uniformly reduce gravitational load and unweighed the participant to 20% of his body weight gradually in 1% increments (Figure 2b). The patients received 45-minute AlterG training 3 days/week for 8 weeks. In each session, the participants started walking at a speed of 1 km/h while unweighted to 50% of their body weight to warm up. As the speed increased, the body weight support gradually decreased based on the patients’ walking capacity on the treadmill and the trained physical therapist’s discretion to provide the participants with necessary feedback (Birgani et al., 2016; Azizi et al., 2018; Rasooli et al., 2017).

fMRI acquisition
Scanning was performed with a single-shot gradient echo-planar imaging pulse sequence and standard head coil using a GE 3-Tesla scanner. Eighty axial slices parallel to the posterior/anterior commissure line with a slice thickness of 3 mm were acquired using a repetition time (TR) of 3 s, echo time (TE) of 30 ms, a flip angle of 90°, and 64×64 matrix size. Following completion of the fMRI protocol, a T1-weighted image was acquired parallel to the posterior/anterior commissure line (resolution=1×1×1 mm, TR=1800 ms, TE=3.4 ms, matrix size=256×256).
Each participant underwent MRI scans pre- and post-treatments. The pediatric anesthesiologist sedated the participants before MRI data acquisition. The default protocol for sedation involved intravenous propofol administered at the lowest dose to keep the patient asleep after induction. Throughout the examination, heart rate and oxygen saturation were monitored for all subjects by pulse-oximetry. Information about the sedation was documented in the medical record.
Knee flexion/extension and ankle plantarflexion/dorsiflexion movements over the range of motion (ROM) with 0.5 Hz and 1 Hz frequencies were defined as two passive motor tasks for acquiring t-fMRI. A trained biomedical engineer performed all passive tasks. A block design of 24 s of rest alternating 24 s of motor task repeated for 5 cycles was used for fMRI acquisition
FMRI data processing
Statistical analysis and preprocessing of the functional data were performed with the fMRI of the brain (FMRIB) software library (FSL v6.02). Brain extraction, realignment, spatial smoothing, motion correction, denoising, and filtering as the standard preprocessing steps were applied to fMRI raw data. The brain images were coregistered and realigned to the mean functional image from the first session. The realigned images are skull-stripped with a brain extraction tool in FSL. To reduce motion artifacts, the subjects’ motion parameters were extracted and included as nuisance covariates in individual analysis. Then, the functional images were smoothed with a 5×5×5 mm3 full width at the half maximum Gaussian kernel and a high pass filter of 72 s. To enhance the signal-to-noise ratio, spatial smoothing was deployed. Voxels residing on the exterior of the brain were removed. Multivariate exploratory linear optimized decomposition into independent components (MELODIC) was performed for denoising. MELODIC uses independent component analysis to decompose 4D data sets into different temporal and spatial components.
fMRI data transformation into standard space may affect group differences and brain functional analysis outcomes. Hence, the reverse process of registering Montreal Neurological Institute (MNI152 atlas) standard space to brain functional data is performed (Hutchison et al., 2014). This study uses region of interest (ROI) analysis to cover the motor cortex and CC. The motor cortex includes brain regions related to planning (premotor cortex [PMC], execution in supplementary motor area [SMA], and precentral gyrus [PG]) and control of movements (primary motor cortex [M1]). These regions were selected from the Harvard-Oxford subcortical and cortical atlases and transformed into the individual’s native space.
First-level individual statistical analyses were performed using the general linear model (GLM) to calculate the significant brain regions. To estimate brain activation for the separate contrasts (rest
In this study, 3 common clinical characteristics were used to evaluate balance and movement, including a 6-minute walk test (6MWT), timed-up-and-go (TUG), and 10-m walk test (10MWT). Also, 6MWT evaluates a person’s walking endurance by measuring the distance walked in 6 minutes (van Hedel et al., 2005). In addition, TUG assesses both static and dynamic balance by measuring the time required for subsequently performing the following tasks: rising from a chair, walking 3 m, making a U-turn, walking back 3 m to the starting point, and sitting down on the chair while turning 180° (Bohannon, 2006). Also, 10MWT assesses functional mobility and gait by measuring the walking speed of a 10-m walk at the self-selected velocity (SSV) and fast velocity (FV) (Ditunno et al., 2000).
3. Results
Therapeutic effects on gait and balance impairment
Table 1 summarizes the percentage of clinical characteristics improvements following 24 sessions of training courses for each subject. Clinical characteristics include TUG, 6MWT and 10MWT. In the OT group, TUG, 6MWT, and SSV improved in all subjects, and FV improved in all subjects except one. In the rTMS group, TUG improved in all subjects, while 6MWT and 10MWT improved in all subjects but one and two cases, respectively. In the AlterG group, all clinical parameters improved in all subjects.

Therapeutic effects on functional brain activity
This study investigated the functional activation in 2 brain areas: Motor cortex and CC, which connect two hemispheres. The motor cortex includes brain regions associated with planning (PMC), execution (SMA and PG), and control of movements (M1). Table 1 summarizes the significant difference between pre- and post-treatment in the motor cortex and CC regarding the number of activated voxels for all tasks and each subject. According to Table 1, in each group, the number of activated voxels in CC decreased in one subject and increased in others due to treatments. In the motor cortex, the number of activated voxels is a combination of increase and decrease following treatments.
Figure 4 depicts a significant difference in CC activation between pre-treatment and post-treatment for all subjects and a P<0.05 (FDR corrections were applied).
Correlation results
According to Table 1, the difference in brain functional activity between pre- and post-treatment in the motor cortex is negative in 10 participants and positive in the others. Although in 6 subjects, the clinical characteristics improved following treatment, the number of activated voxels decreased in the motor cortex. Since the decreased cortical activation may not represent the deterioration in functional activity (Dan, 2021), the absolute value of motor cortex functional activity changes is used to investigate the possible correlation with clinical parameter improvements in Figure 3. In rTMS and OT groups, motor cortex functional activity does not correlate with clinical parameters. In the AlterG group, motor area functional activity significantly correlates only with FV (P<0.05). For all subjects, regardless of the type of treatment, there is no significant correlation between motor cortex activity and clinical parameters (Figure 3).

Figure 3 also shows the correlations between clinical improvements and the value of the functional activity changes in the CC. FV changes following treatment significantly correlate with CC functional activity alternations in all groups. However, SSV and 6MWT changes only correlate substantially with brain activity changes in the CC region following rTMS and AlterG training, respectively. Figure 5 shows all subjects’ CC functional activity alternations and FV changes.

4. Discussion
This pilot study explores the therapeutic effects of intensive OT, rTMS, and AlterG training on walking capacity and induced neuroplasticity indexed by brain activation alternations using passive task-based fMRI. Passive tasks include plantar flexion and dorsiflexion over the ROM of both ankles and flexion to extension over both knees, which were carried out pre- and post-treatments under sedation. To our knowledge, a limited number of studies investigated brain functional reorganization following gait rehabilitation in pediatrics with CP and adults with stroke (Phillips et al., 2007; Dobkin et al., 2004; Yang et al., 2010). The studies utilizing OT and Lokomat for enhancing walking capacity mainly focused on clinical improvement rather than brain reorganization assessment (Hesse et al., 1999; Drużbicki et al., 2013; Willoughby et al., 2009). Furthermore, the effect of treatment on interhemispheric relations and brain functional activities remains elusive. Therefore, we aimed to characterize the brain functional activity alternations following these treatments in children with spastic HCP in addition to assessment of walking capacity improvement. Also, we aimed to investigate the neural correlation of clinical improvement in terms of brain functional activity changes in specific brain regions in children with CP induced by the treatments, regardless of the type of treatment. Our findings showed improved walking capacity and brain functional activity following OT, rTMS, and AlterG training. Also, our results showed that the improvement in clinical measurements significantly correlates with changes in brain functional activity in selected regions.
The motor cortex is responsible for the planning, execution, and control of movements, and CC is considered the largest WM tract connecting the two hemispheres, which is critical for performing tasks that require interhemispheric interaction. Moreover, the homotopic connectivity between the motor cortices is believed to emerge through CC. The interhemispheric exchange role of CC could be inhibitory, excitatory, or a combination of both, which may alter through neurological diseases such as stroke and CP (Takeuchi et al., 2012). Additionally, previous studies have reported a positive correlation between CC in children with CP and motor performance (Pannek et al., 2014; Rademaker et al., 2004; Davatzikos et al., 2003). In this regard, assessments of interhemispheric relations through CC may unravel the neurophysiological underpinnings that modulate motor control and may result in introducing interventional therapies to improve motor function in children with CP. Although many studies investigate the activation of CC following different visual, motor, and tactile tasks using fMRI, none of them explore its therapy-driven alternations. Addressing this issue and considering the lesion heterogeneity in terms of location and size in our CP cohort, this pilot study, for the first time, investigates the therapy-induced brain functional activity alternation in the motor cortex and CC regions.
Our analysis showed both a decrease and an increase in brain activation after therapy. According to Table 1, in 6 subjects, the clinical characteristics improved following treatments while the number of activated voxels decreased in the motor cortex. This decrease in cortical activation may be evidence of less neural energy consumption following intensive training and is thought to be a gain in neural efficiency (Dan, 2021). Furthermore, learning specific tasks following intensive repetitive training may allow the tasks to be performed through memory-based processing by reorganizing the cortical representations of sensorimotor features, which has been suggested for children with CP (Dan, 2016). In line with our results, intensive motor training in a related study resulted in decreased and increased cortical hemodynamic response during task execution (Calmels, 2020). Also, the cortical activation of the sensorimotor area following treatment has reduced in some studies, while others showed increased cortical activation (Drużbicki et al., 2013; Dinomais et al., 2013). However, interpreting such alternations in cortical activity of the brain regions of interest is challenging.
As illustrated in Figure 3, alternations in the hemodynamic response of the motor cortex only correlate with FV in the AlterG group. However, the absence of these correlations is not equivalent to a lack of neural associations. The reasons for this condition stem primarily from the complex and non-linear association between the underlying mechanisms of these measures, which will be investigated in future studies.
On the other hand, according to Figure 3, alternations in CC functional activity in OT and rTMS groups significantly correlate with functional mobility and gait improvement indexed by FV. In the AlterG group, changes in CC functional activity significantly correlate with enhancements in functional mobility, gait, and walking endurance indexed by FV and 6MWT, respectively. Regardless of the type of treatment, CC functional activity changes significantly correlate with alternations of TUG and 6MWT.
Also, our analysis reveals a robust and significant correlation between alternations in CC functional activity and walking capacity improvement in terms of fast velocity, regardless of the treatment group. Figure 5 illustrates the point. This strong correlation aligns with studies suggesting that the modulation of interhemispheric communication supports the brain's ability to form new neuronal connections, compensate for impairments, and acquire new functions—key aspects of neural plasticity. (Takeuchi et al., 2012; Hosp, 2011). In children with CP, the less affected side of the brain tries to compensate for the weakness of the more affected side, and this amount of interaction can be mainly related to the relationship between the two hemispheres.

5. Conclusion
Our results indicate that intensive OT, rTMS, and AlterG training enhances brain activity and walking capacity in children with CP. OT, rTMS, and AlterG improve functional mobility and gait, while AlterG improves walking endurance. Regardless of the type of treatment, robust correlation between walking capacity improvement (FV) and CC activity changes were successfully characterized in children with hemiplegic CP (HCP). These results show that the improvement in gait function in children with CP may reflect an increase in brain functional activity in callosal neurons, revealing the rise in interhemispheric coupling.
Study limitations
Our study has some limitations. Firstly, some subjects could not complete the required training sessions due to the intensive training schedule. Secondly, some fMRI data could not be used due to excessive motion. Thirdly, despite the damping effect of sedation on brain activity, we successfully detected the brain activity and its changes following treatments. However, brain activity changes following treatment must be greater in practice than reported in this study. Finally, the characterization of the intervention effects may not be achieved solely by calculating the average group results and pre-post analyses. This condition required further time points and a larger sample size, which we have taken into consideration in our ongoing studies.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Ethics Committee of Tehran University of Medical Sciences (TUMS), Tehran, Iran. This study was registered in the Iranian Registry of Clinical Trials (IRCT) with the number IRCT2015121625568N1. All participants gave their written informed consent to participate in the study.
Funding
This research was supported by grants from the Ministry of Health and Medical Education and Tehran University of Medical Sciences (TUMS), Tehran, Iran.
Authors' contributions
Study design and investigation: Parmida Moradi Birgani, Meghdad Ashtiyani and M. Mehdi Mirbagheri; Methodology: Parmida Moradi Birgani, Meghdad Ashtiyani, M. Mehdi Mirbagheri, seyed. Behnamedin Jamei, and Mohammad Reza Deevband; Data analysis: Parmida Moradi Birgani, Meghdad Ashtiyani and M. Mehdi Mirbagheri; Data acquisition: Parmida Moradi Birgani, Meghdad Ashtiyani, M. Mehdi Mirbagheri, Elham Rahimian and Amin Shahrokhi; Writing the original draft: Parmida Moradi birgani, and Meghdad Ashtiyani; Data interpretation and final approval: All authors.
Acknowledgments
The authors would like to thank Ministry of Health and Medical Education and Tehran University of Medical Sciences for their support in conducting this study. The authors wish to thank Shahrokhnia, and Kohanpour, for their cooperation. The authors are also grateful to the children and families who participated in the research.
Conflict of interest
The authors expressed no conflict of interest.
References
Ashtiyani, M., Moradi Birgani, P., Soleimani, M., Jameie, S. B., Shahrokhi, A., & Deevband, M. R., et al. (2020). Short-Term Therapeutic Effects of anti-gravity treadmill training on brain functional activities and walking capacity in children with cerebral palsy. Basic and Clinical Neuroscience. [Unpublished]. [Link]
Type of Study: Original |
Subject:
Cognitive Neuroscience
Received: 2022/09/8 | Accepted: 2022/12/28 | Published: 2025/03/18
Received: 2022/09/8 | Accepted: 2022/12/28 | Published: 2025/03/18
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





