Volume 15, Issue 6 (November & December 2024)
BCN 2024, 15(6): 833-842 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mehmandoust M, Raeesi S, Hashemi R, Bidkhori M, Namazi Shabestari A, Dashti F, et al . Synbiotic Supplementation and Its Effects on Cognition in Alzheimer’s Patients: A Double-blind Study. BCN 2024; 15 (6) :833-842
URL: http://bcn.iums.ac.ir/article-1-2541-en.html
URL: http://bcn.iums.ac.ir/article-1-2541-en.html
Synbiotic Supplementation and Its Effects on Cognition in Alzheimer’s Patients: A Double-blind Study
Mahdieh Mehmandoust1 

 , Shima Raeesi2
, Shima Raeesi2 

 , Rezvan Hashemi2
, Rezvan Hashemi2 

 , Mohammad Bidkhori3
, Mohammad Bidkhori3 

 , Alireza Namazi Shabestari1
, Alireza Namazi Shabestari1 

 , Fatemeh Dashti4
, Fatemeh Dashti4 

 , Farzaneh Asoudeh4
, Farzaneh Asoudeh4 

 , Zahra Vahabi *2
, Zahra Vahabi *2 




 , Shima Raeesi2
, Shima Raeesi2 

 , Rezvan Hashemi2
, Rezvan Hashemi2 

 , Mohammad Bidkhori3
, Mohammad Bidkhori3 

 , Alireza Namazi Shabestari1
, Alireza Namazi Shabestari1 

 , Fatemeh Dashti4
, Fatemeh Dashti4 

 , Farzaneh Asoudeh4
, Farzaneh Asoudeh4 

 , Zahra Vahabi *2
, Zahra Vahabi *2 


1- Department of Geriatric Medicine, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran.
2- Department of Geriatric Medicine, Ziaeian Hospital, Tehran University of Medical Sciences, Tehran, Iran.
3- Department of Epidemiology and Biostatistics, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran.
4- Department of Clinical Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran.
2- Department of Geriatric Medicine, Ziaeian Hospital, Tehran University of Medical Sciences, Tehran, Iran.
3- Department of Epidemiology and Biostatistics, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran.
4- Department of Clinical Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran.
Keywords: Synbiotics, Cognitive function, Activity of daily living, Elderly, Alzheimer’s disease (AD)
Full-Text [PDF 661 kb]
| Abstract (HTML)
Full-Text:
Introduction
Dementia is a chronic syndrome characterized by cognitive and behavioral symptoms, which may include short-term memory impairment and problems related to orientation, language, attention and perception (Ruiz-Gonzalez et al., 2021; Leszek et al., 2016). According to the world Alzheimer reports, more than 50 million individuals live with dementia throughout the world, and it is projected to affect almost 150 million people by 2050 (Patterson, 2018; Parra-Rodriguez & Welsh-Bohmer, 2020). Dementia could be associated with genetics, increasing age, low level of education, cardiovascular risk factors, and an unhealthy lifestyle (Patterson, 2018). Oxidative stress, chronic neuroinflammation, and gut microbiota alternations could also be related to the pathophysiology of dementia. Because dementia has a significant deleterious impact on the patients and their caregivers’ quality of life and exerts a substantial economic burden on the healthcare system, using practical preventive and therapeutic strategies and optimal care management is vital (D’Souza et al., 2010; van Middelaar et al., 2018; Moreira et al., 2020).
Recently published studies have demonstrated that the composition of gut microbiota and its bidirectional signaling pathways with the brain, called the gut-brain axis, might have a substantial role in the pathophysiology of neurocognitive disorders, including dementia (Aizawa et al., 2016; Dinan & Cryan, 2017; Vogt et al., 2017). Consequently, enhancing beneficial bacteria in the gut by probiotics administration as a microbiome-based therapy could be advantageous for those patients through different pathways, including augmented short-chain fatty acid production, improved immune system function, diminished inflammation, and the level of oxidative stress (Cheng et al., 2019; Hajifaraji et al., 2018; Maldonado Galdeano et al., 2019; Parmar, 2016; Zheng et al., 2019; Markowiak-Kopeć & Śliżewska, 2020). Also, some evidence has shown that probiotics could raise physical and cognitive function (Shimizu, 2018; Ton et al., 2020). Most studies are inconclusive, with inconsistent results (Buigues et al., 2016; Leblhuber et al., 2018), and more research is needed to narrow this gap. The current study aimed to evaluate the effect of synbiotics on the cognition and physical function of older adults with dementia.
2. Materials and Methods
Study population
The participants of this 12-week randomized, double-blind trial were community-dwelling adults over 60 recently diagnosed with mild to moderate dementia at the Memory Clinic of Ziaeian Hospital, Tehran City, Iran. This hospital is a medical and educational center affiliated with the Tehran University of Medical Sciences (TUMS). A neurologist and geriatrician have confirmed the diagnosis of dementia following the National Institute on Aging and Alzheimer Association (NIA-AA) criteria (McKhann et al., 2011). Regarding the wide range of symptoms in different types of dementia, only patients with Alzheimer’s disease (AD) were selected for the study. The exclusion criteria were infection at the time of assessment; severe co-morbidities; gastrointestinal diseases or surgery; metabolic, immunosuppressive, and psychiatric disorders; alcohol or drug abuse; and taking antibiotics, probiotic supplements, or other drugs consumption of probiotic products in the last three months. Patients with new conditions diminishing their cognition or physical function (e.g. delirium or stroke) over the study period or requiring antibiotics were also excluded. Accordingly, 60 patients were enrolled in the study between August 2019 and February 2021.
Study design and intervention
The standard formula for parallel clinical trials was used to calculate the study’s sample size. Based on previous studies (Kobayashi et al., 2019) and considering type I error (α) of 0.05 and type II error (β) of 0.2 (power=80%), 30 participants are needed in each group.
The random allocation method was accomplished through an online data center website (Sealed Envelope Ltd., 2019). Finally, 30 participants were assigned to the intervention and placebo groups by block randomization. All patients received the medications for AD and two capsules daily for 12 weeks.
Both synbiotics capsules (GeriLact®) and placebos were produced by Zist Takhmir Company, Tehran, Iran. GeriLact ® is a gluten-free synbiotic (probiotic and prebiotic) formulation that contains 109 CFU of 7 bacterial strains: Lactobacillus rhamnosus, Lactobacillus bulgaricus, Lactobacillus casei, Lactobacillus acidophilus, Bifidobacterium breve, Bifidobacterium longum, and Streptococcus thermophilus plus fructooligosaccharides as prebiotic. The placebo capsules contained 500 mg maltodextrin and were identical to the synbiotics capsules in taste and physical appearance. All participants were asked to continue their routine lifestyle and not add any new nutritional supplements throughout the study period. Patients’ adherence was monitored weekly during the study by weekly phone calls.
Data collection
Demographic data, including age, gender, education, occupation, dementia risk factors, co-morbidities, and medications, were recorded. A dietician educated caregivers to document dietary intakes by a 3-day food record at the study’s beginning and end. Dietary analysis was processed by nutritionist IV software (First Databank, San Bruno, CA). The cognition was evaluated at the baseline and end of the study using the Persian version of the mini-mental state examination (MMSE) (Seyedian et al., 2007), and functional status was measured using the Barthel index (Hormozi et al., 2019).
Fasting blood samples were collected to measure fasting blood sugar (FBS), triglycerides (TG), total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), serum albumin (Alb), and the serum level of 1, 25(OH) vitamin D. Height was measured in meters using a wall tape in a standing position without shoes. Participants’ weight (in kg) was assessed using a digital scale while minimally clothed at the study’s onset and end. Body mass index (BMI) was calculated as weight in kg divided by height in meters squared.
Statistical analysis
General characteristics of the study participants were reported using descriptive statistics (Mean±SD, and percentage). The Kolmogorov-Smirnov test was applied to ensure the normal distribution of variables. Log transformation was used in the case of variables with a non-normal distribution. Data on differences in macronutrient and micronutrient intakes between the two groups were also compared using independent samples student t test. Repeated measures analysis of variance (or its non-parametric equivalent) was applied to determine probiotic supplementations’ effect on metabolic profiles, cognitive status, and functional performance. All statistical analyses were done using the SPSS software, version 24 (SPSS Inc., Chicago, Illinois, USA), and P<0.05 were considered statistically significant.
3. Results
Of the 60 participants in the study, 8 were excluded due to death or the occurrence of new delirium and stroke during the study period. So, 52 participants (placebo group [n=27] and probiotic group [n=25]) finished the study.
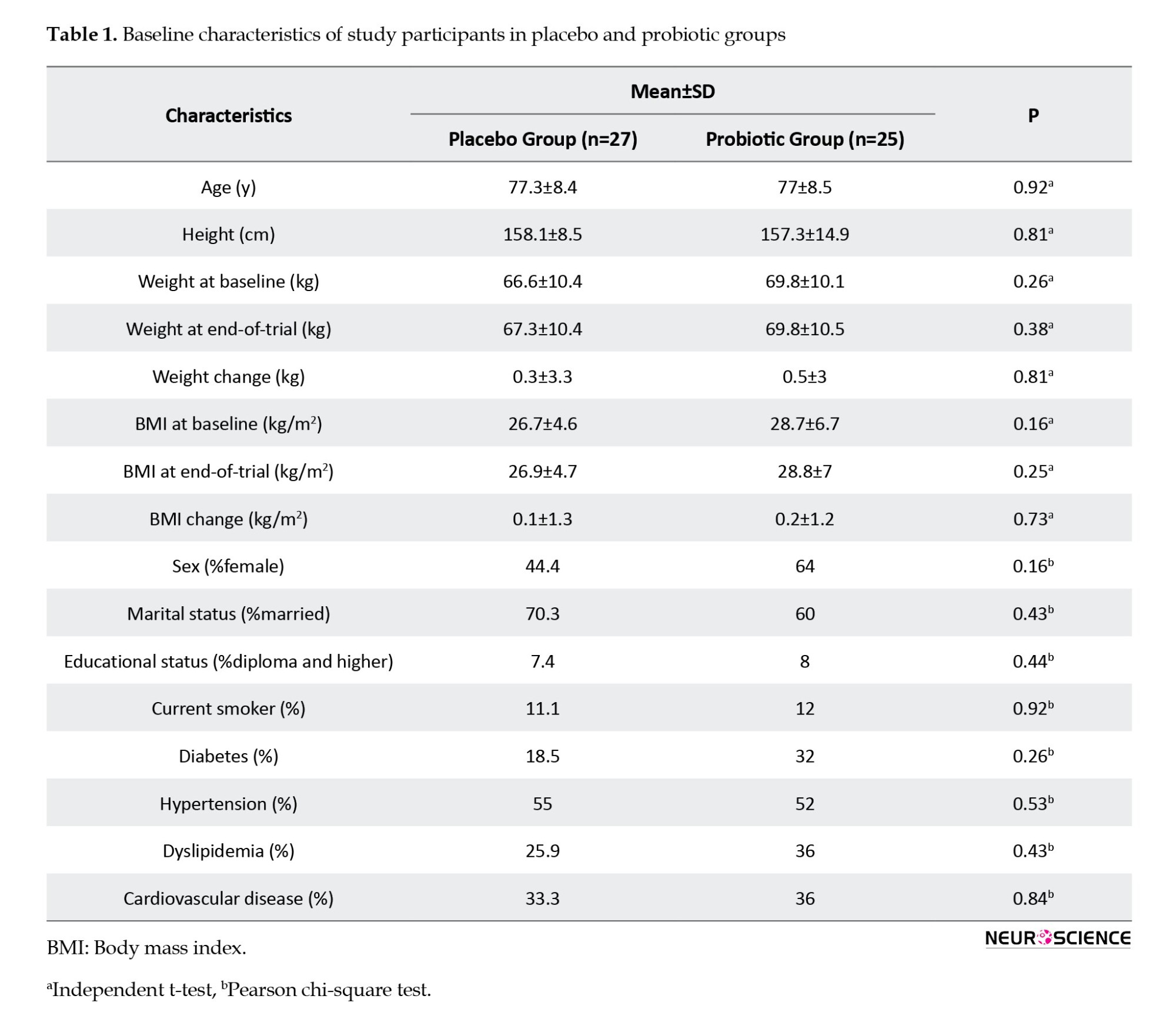
Participants’ characteristics are described in Table 1. The mean age in both groups was about 77 years. There is no significant difference in demographic features between the two groups, including age, sex, education, and marital status. The most common underlying diseases were hypertension, diabetes mellitus, dyslipidemia, and cardiovascular disease. Cardiometabolic factors and BMI were also not substantially different.
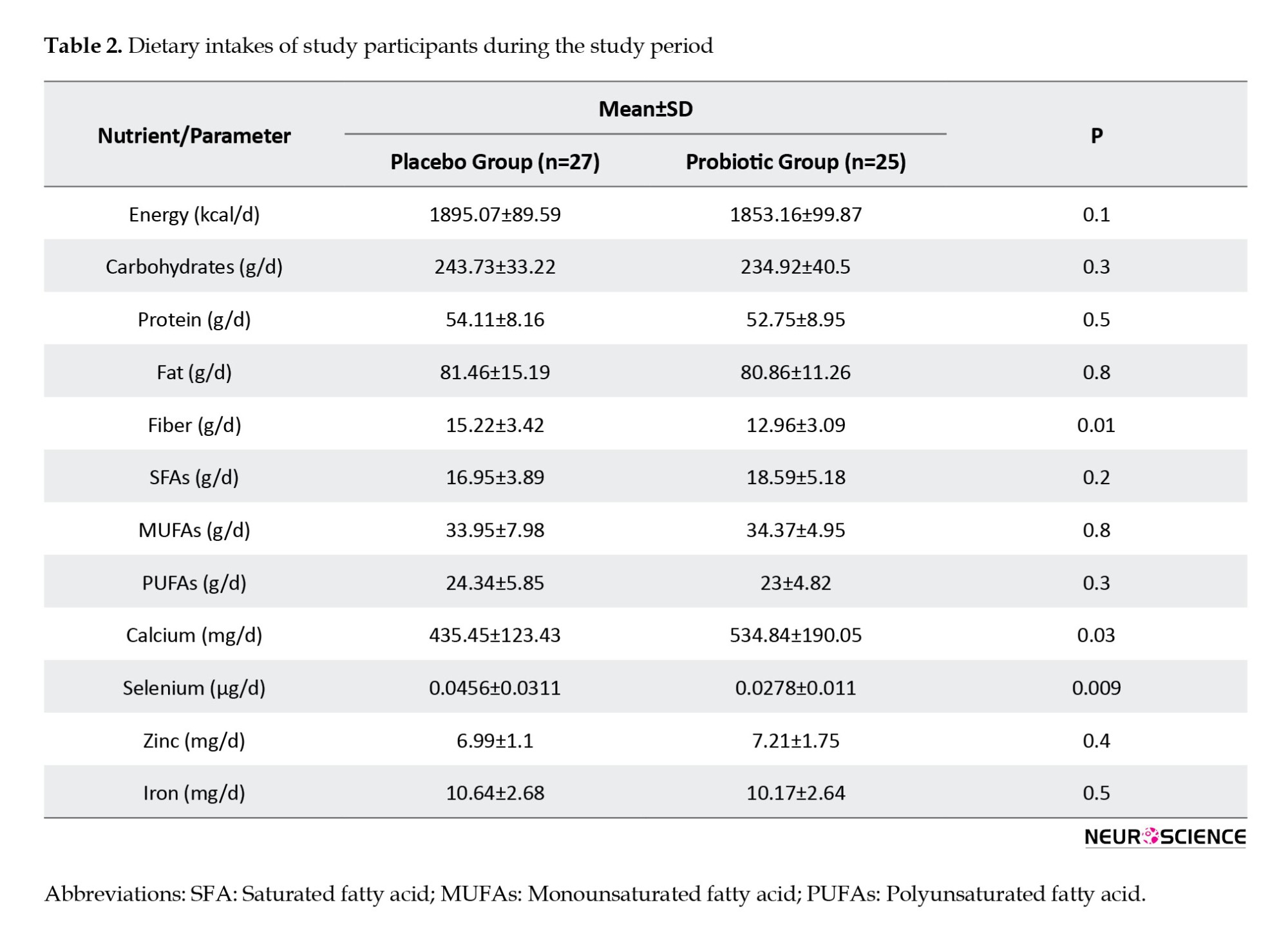
Comparing nutritional assessment between the two groups, we found that total dietary intakes of fiber (P=0.01), calcium (P=0.03), and selenium (P≤0.01) were significantly different between subjects in both groups (Table 2).
After 12 weeks, neither participant’s weight nor BMI changed. A similar trend was found in metabolic factors (including FBS, lipid profiles, and serum Alb level), as shown in Table 3. HDL-c serum concentrations dropped slightly more in the placebo group (-6.3±14.3 mg/dL) than in the probiotic group (-0.3±7.2 mg/dL). Serum levels of 25(OH) vitamin D increased in participants who consumed probiotic supplements (8.9±18.6 ng/dL), whereas it decreased in patients in the placebo group (-1.1±16.2 ng/dL).
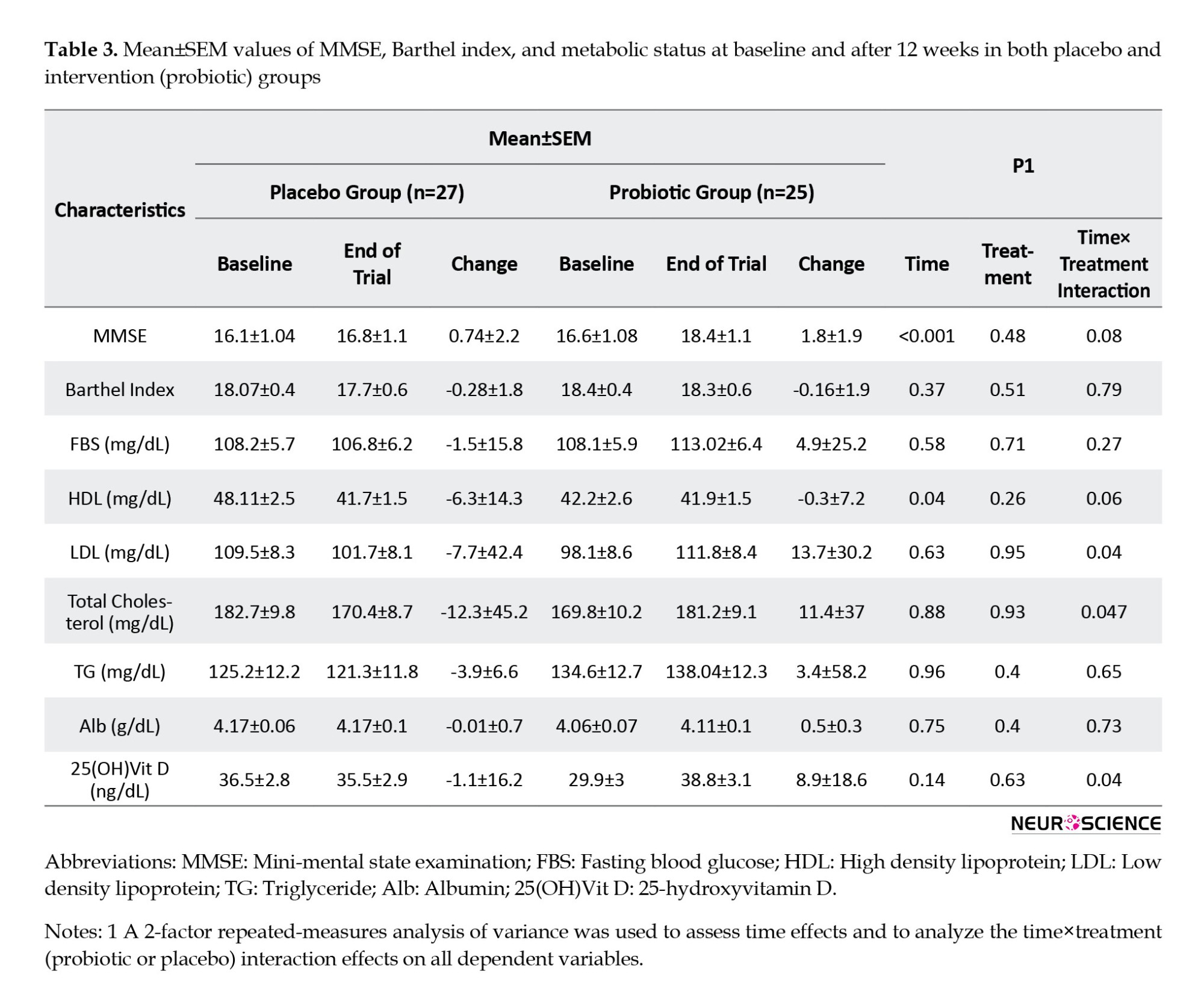
However, we observed significant time effects between baseline and week 12 for MMSE score and serum levels of HDL-c. It means that among participants in the probiotic group, MMSE scores increased more than those in the placebo group (placebo group: 0.74±2.2, probiotic group: 1.8±1.9; P≤0.001) and HDL-c serum concentrations (placebo group: -6.3±14.3 mg/dL, probiotic group: -0.3±7.2 mg/dL; P=0.04) decreased in the placebo group more than those in the probiotic group over the time. In addition, there were significant time pretreatment interaction effects for the serum concentrations of LDL-c, total cholesterol, and 25(OH)D. Such that LDL-c (placebo group: -7.7±42.4 mg/dL, probiotic group:13.7±30.2 mg/dL; P=0.04) and total cholesterol (placebo group: -12.3± 45.2 mg/dL, probiotic group: 11.4±37 mg/dL; P=0.04) concentrations over the intervention period decreased in placebo group while increased in the probiotic group. However, after a 12-week intervention, serum levels of 25(OH)D (probiotic group: 8.9±18.6 ng/dL placebo group: -1.1±16.2 ng/dL; P=0.04) increased in the participants who consumed probiotics supplements whereas decreased in the placebo group.
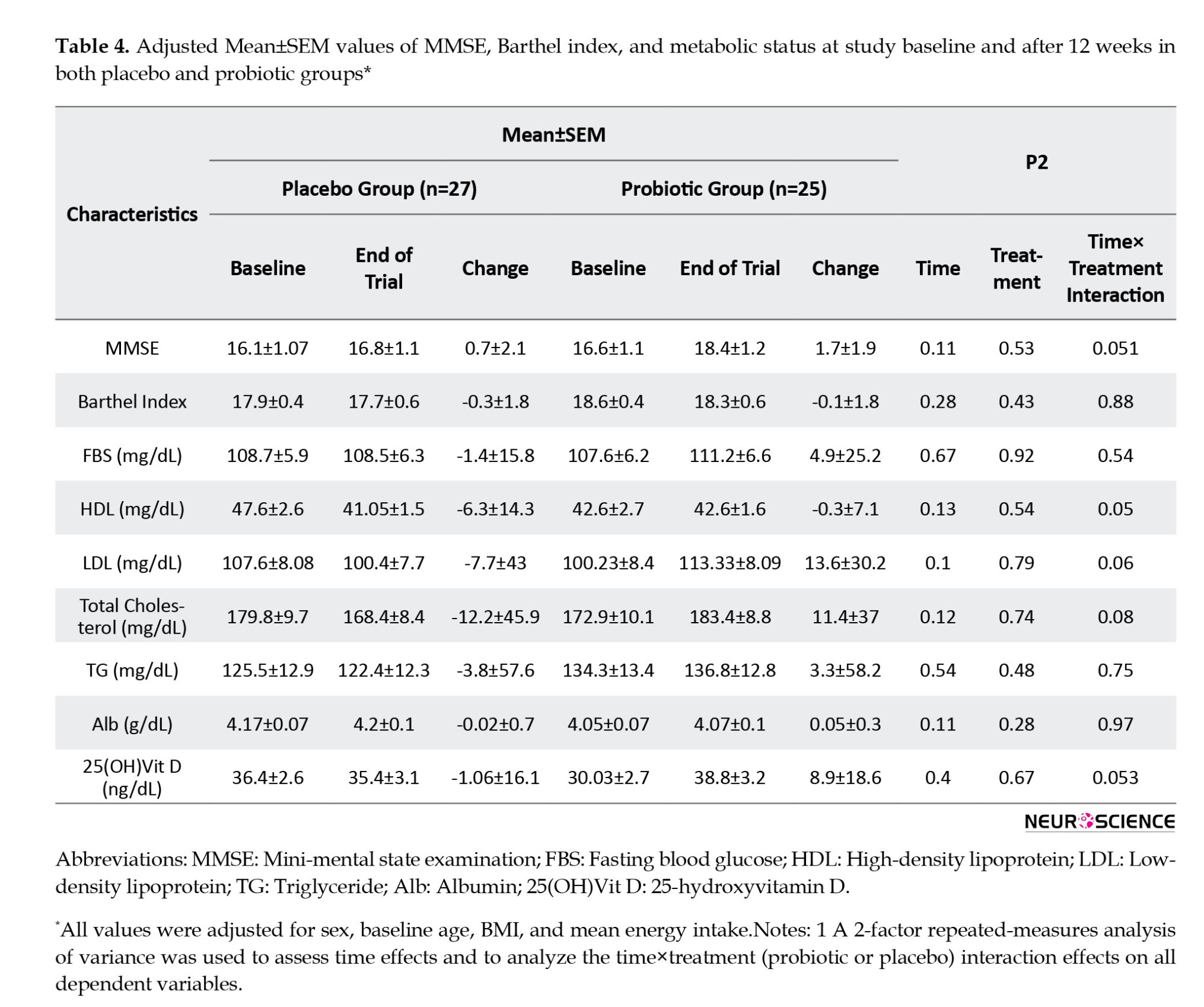
At baseline, the MMSE score in both groups was almost the same (16.6±5.4 in the probiotic group vs 16.1±5.4 in the placebo group). However, we observed significant time effects between baseline and week 12 for the MMSE score. It means that among participants in the probiotic group, MMSE scores increased more than those in the placebo group (placebo group: 0.74±2.2 vs probiotic group: 1.8±1.9), but the difference between the two groups was not statistically significant (P=0.53). At the end of the study, the cognitive function of both groups deteriorated, and only one person who received probiotics experienced cognitive improvement, which was not statistically significant. There were no significant differences in TG, FBS, and serum Alb between the two groups at baseline or the end of the study. After adjusting for covariates such as gender, age, and BMI at the beginning of the study, as well as the mean energy intake, we found no significant differences in our results (Table 4).
As dementia progresses, gradual loss of cognitive function negatively impacts a patient’s physical performance. At the beginning of the study, the mean Barthel index scores in the intervention and control groups were 18.5±1.9 and 18.1±2.6, respectively. After 12 weeks, functional loss was less significant in probiotic recipients (P=0.43) (Table 3).
4. Discussion
Dementia is the most prevalent and burdensome neurodegenerative disease, accounting for a remarkable morbidity and mortality rate in older adults (Hormozi et al., 2019; Barnes & Yaffe, 2011). As the world population is aging, finding an effective way to prevent and treat dementia is considered a global health challenge. Recently, probiotics as live microorganisms received significant attention due to their potential beneficial properties in cognitive abilities (Amirani et al., 2020; Kim et al., 2019). Probiotics might affect the central nervous system (CNS) through several direct and indirect pathways (Wang, 2018) and change CNS biochemistry by impacting brain-derived neurotrophic factors, serotonin, gamma-aminobutyric acid, and dopamine. Therefore, its properties could modify human behavior and cognition (Rahimlou et al., 2022; Ranuh et al., 2019; Park et al., 2014; Liu et al., 2020). Likewise, probiotics can inhibit the release of inflammatory cytokines and diminish oxidative stress by increasing antioxidants, including superoxide dismutase and glutathione peroxidase (D’Souza et al., 2010; Bezkorovainy, 2001; Desbonnet et al., 2008; Karimi & Pena, 2003). Another potential advantage of probiotics is altering gut microbiota composition and elevating the diversity of beneficial bacteria. Probiotics increased short-chain fatty acid and tryptophan production, indirectly affecting CNS function (Wang, 2018). The present study aimed to evaluate the effect of probiotic supplementation on cognitive function, physical performance, metabolic factors, vitamin D, and Alb levels in older patients with AD. Our findings demonstrated that supplementation with probiotics for 12-week had no considerable effect on these parameters before or after adjustment.
These findings confirm the results from prospective studies (Leblhuber et al., 2018; Krüger et al., 2021) that no improvement in the cognitive function of dementia patients was reported after taking probiotics. Our results for physical performance with probiotics aligned with two other prospective studies (Buigues et al., 2016; Mañé et al., 2011). In contrast to our results, in a study on 27 older adults, administered probiotic containing Bifidobacterium breve A1 for 24 weeks showed a considerable improvement in cognitive function and reduced the risk of dementia compared to the control group (Kobayashi et al., 2019). In the same way, another clinical trial revealed such a beneficial effect (Ton et al., 2020). The small sample size, lack of a placebo group, and difference in strains and doses of administrated probiotics might explain these discrepancies. More studies need to shed further light on understanding the effect of supplementation with probiotics on cognitive behavior.
Another outcome of this study is that taking supplemental probiotics does not affect serum levels of vitamin D, Alb, FBS, and lipid profile. This finding is similar to the studies that did not report a significant effect of probiotics on these parameters (Akkasheh et al., 2016; Romijn et al., 2017, Pu et al., 2017). However, it differed from a clinical trial that showed that supplementation with probiotics for 12 weeks had a beneficial effect on some of the metabolic parameters, including cholesterol and triglyceride levels among 79 patients with AD (Tamtaji et al., 2019). In addition, Kobayashi et al., (2019) reported a remarkable effect on triglyceride after 200 mL/d of probiotic milk consumption for 12 weeks (Kobayashi et al., 2019). An interventional study investigated a considerable rise in plasma Alb levels by taking for 12 weeks in patients with memory complaints (Kobayashi et al., 2019). Moreover, another trial evaluating the effects of probiotic administration on 127 Canadian otherwise healthy hypercholesterolemia adults demonstrated a significant increase in serum vitamin D concentrations after 9 weeks (Jones et al., 2013). The conflicting results of published clinical trials could be related to their methodology, including different intervention periods, analysis methods, and potential covariates.
Furthermore, using different doses and strains of probiotics may have contributed to the inconsistent findings. There is no common consent about the most effective dose and strain of probiotics (Guarner et al., 2012). This issue needs further exploration to be addressed.
Our study has several limitations. We could neither conduct stool analysis to evaluate the bacteria alteration in the gut nor assess the effect of probiotic administration on inflammatory cytokines due to resource limitations. Participation in this trial was declined due to the COVID-19 pandemic. Finally, probiotics supplements contain several strains of bacteria with different doses; thus, we could not find which strains had a favorable effect among dementia patients in the present clinical trial.
5. Conclusion
In conclusion, this clinical trial study revealed that supplementation with probiotics for 12 weeks had no favorable effect on cognitive function, activities of daily living, lipid profiles, vitamin D concentration, and serum Alb level in older patients with AD. Further studies with larger sample sizes and a more extended follow-up period are needed to clarify the impact of probiotic supplementation on older people.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Tehran University of Medical Sciences, Tehran, Iran (Code: IR.TUMS.VCR.REC.1398.132) and was registered by the Iranian Registry of Clinical Trials (IRCT) (Code: 20191008045024N1). Informed consent was obtained from all participants’ caregivers.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization: Mahdieh Mehmandoust and Alireza Namazi Shabestari; Methodology: Mahdieh Mehmandoust, Shima Raeesi and Mohammad Bidkhori; Software: Mohammad Bidkhori; Resources: Alireza Namazi Shabestari; Formal analysis: Fatemeh Dashti; Supervision: Zahra Vahabi; Writing the original draft: Mahdieh Mehmandoust, and Farzaneh Asoudeh; Review and editing: Shima Raeesi, Rezvan Hashemi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors want to thank all participants for their assistance in this project
References
Dementia is a chronic syndrome characterized by cognitive and behavioral symptoms, which may include short-term memory impairment and problems related to orientation, language, attention and perception (Ruiz-Gonzalez et al., 2021; Leszek et al., 2016). According to the world Alzheimer reports, more than 50 million individuals live with dementia throughout the world, and it is projected to affect almost 150 million people by 2050 (Patterson, 2018; Parra-Rodriguez & Welsh-Bohmer, 2020). Dementia could be associated with genetics, increasing age, low level of education, cardiovascular risk factors, and an unhealthy lifestyle (Patterson, 2018). Oxidative stress, chronic neuroinflammation, and gut microbiota alternations could also be related to the pathophysiology of dementia. Because dementia has a significant deleterious impact on the patients and their caregivers’ quality of life and exerts a substantial economic burden on the healthcare system, using practical preventive and therapeutic strategies and optimal care management is vital (D’Souza et al., 2010; van Middelaar et al., 2018; Moreira et al., 2020).
Recently published studies have demonstrated that the composition of gut microbiota and its bidirectional signaling pathways with the brain, called the gut-brain axis, might have a substantial role in the pathophysiology of neurocognitive disorders, including dementia (Aizawa et al., 2016; Dinan & Cryan, 2017; Vogt et al., 2017). Consequently, enhancing beneficial bacteria in the gut by probiotics administration as a microbiome-based therapy could be advantageous for those patients through different pathways, including augmented short-chain fatty acid production, improved immune system function, diminished inflammation, and the level of oxidative stress (Cheng et al., 2019; Hajifaraji et al., 2018; Maldonado Galdeano et al., 2019; Parmar, 2016; Zheng et al., 2019; Markowiak-Kopeć & Śliżewska, 2020). Also, some evidence has shown that probiotics could raise physical and cognitive function (Shimizu, 2018; Ton et al., 2020). Most studies are inconclusive, with inconsistent results (Buigues et al., 2016; Leblhuber et al., 2018), and more research is needed to narrow this gap. The current study aimed to evaluate the effect of synbiotics on the cognition and physical function of older adults with dementia.
2. Materials and Methods
Study population
The participants of this 12-week randomized, double-blind trial were community-dwelling adults over 60 recently diagnosed with mild to moderate dementia at the Memory Clinic of Ziaeian Hospital, Tehran City, Iran. This hospital is a medical and educational center affiliated with the Tehran University of Medical Sciences (TUMS). A neurologist and geriatrician have confirmed the diagnosis of dementia following the National Institute on Aging and Alzheimer Association (NIA-AA) criteria (McKhann et al., 2011). Regarding the wide range of symptoms in different types of dementia, only patients with Alzheimer’s disease (AD) were selected for the study. The exclusion criteria were infection at the time of assessment; severe co-morbidities; gastrointestinal diseases or surgery; metabolic, immunosuppressive, and psychiatric disorders; alcohol or drug abuse; and taking antibiotics, probiotic supplements, or other drugs consumption of probiotic products in the last three months. Patients with new conditions diminishing their cognition or physical function (e.g. delirium or stroke) over the study period or requiring antibiotics were also excluded. Accordingly, 60 patients were enrolled in the study between August 2019 and February 2021.
Study design and intervention
The standard formula for parallel clinical trials was used to calculate the study’s sample size. Based on previous studies (Kobayashi et al., 2019) and considering type I error (α) of 0.05 and type II error (β) of 0.2 (power=80%), 30 participants are needed in each group.
The random allocation method was accomplished through an online data center website (Sealed Envelope Ltd., 2019). Finally, 30 participants were assigned to the intervention and placebo groups by block randomization. All patients received the medications for AD and two capsules daily for 12 weeks.
Both synbiotics capsules (GeriLact®) and placebos were produced by Zist Takhmir Company, Tehran, Iran. GeriLact ® is a gluten-free synbiotic (probiotic and prebiotic) formulation that contains 109 CFU of 7 bacterial strains: Lactobacillus rhamnosus, Lactobacillus bulgaricus, Lactobacillus casei, Lactobacillus acidophilus, Bifidobacterium breve, Bifidobacterium longum, and Streptococcus thermophilus plus fructooligosaccharides as prebiotic. The placebo capsules contained 500 mg maltodextrin and were identical to the synbiotics capsules in taste and physical appearance. All participants were asked to continue their routine lifestyle and not add any new nutritional supplements throughout the study period. Patients’ adherence was monitored weekly during the study by weekly phone calls.
Data collection
Demographic data, including age, gender, education, occupation, dementia risk factors, co-morbidities, and medications, were recorded. A dietician educated caregivers to document dietary intakes by a 3-day food record at the study’s beginning and end. Dietary analysis was processed by nutritionist IV software (First Databank, San Bruno, CA). The cognition was evaluated at the baseline and end of the study using the Persian version of the mini-mental state examination (MMSE) (Seyedian et al., 2007), and functional status was measured using the Barthel index (Hormozi et al., 2019).
Fasting blood samples were collected to measure fasting blood sugar (FBS), triglycerides (TG), total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), serum albumin (Alb), and the serum level of 1, 25(OH) vitamin D. Height was measured in meters using a wall tape in a standing position without shoes. Participants’ weight (in kg) was assessed using a digital scale while minimally clothed at the study’s onset and end. Body mass index (BMI) was calculated as weight in kg divided by height in meters squared.
Statistical analysis
General characteristics of the study participants were reported using descriptive statistics (Mean±SD, and percentage). The Kolmogorov-Smirnov test was applied to ensure the normal distribution of variables. Log transformation was used in the case of variables with a non-normal distribution. Data on differences in macronutrient and micronutrient intakes between the two groups were also compared using independent samples student t test. Repeated measures analysis of variance (or its non-parametric equivalent) was applied to determine probiotic supplementations’ effect on metabolic profiles, cognitive status, and functional performance. All statistical analyses were done using the SPSS software, version 24 (SPSS Inc., Chicago, Illinois, USA), and P<0.05 were considered statistically significant.
3. Results
Of the 60 participants in the study, 8 were excluded due to death or the occurrence of new delirium and stroke during the study period. So, 52 participants (placebo group [n=27] and probiotic group [n=25]) finished the study.
Participants’ characteristics are described in Table 1. The mean age in both groups was about 77 years. There is no significant difference in demographic features between the two groups, including age, sex, education, and marital status. The most common underlying diseases were hypertension, diabetes mellitus, dyslipidemia, and cardiovascular disease. Cardiometabolic factors and BMI were also not substantially different.

Comparing nutritional assessment between the two groups, we found that total dietary intakes of fiber (P=0.01), calcium (P=0.03), and selenium (P≤0.01) were significantly different between subjects in both groups (Table 2).

After 12 weeks, neither participant’s weight nor BMI changed. A similar trend was found in metabolic factors (including FBS, lipid profiles, and serum Alb level), as shown in Table 3. HDL-c serum concentrations dropped slightly more in the placebo group (-6.3±14.3 mg/dL) than in the probiotic group (-0.3±7.2 mg/dL). Serum levels of 25(OH) vitamin D increased in participants who consumed probiotic supplements (8.9±18.6 ng/dL), whereas it decreased in patients in the placebo group (-1.1±16.2 ng/dL).

However, we observed significant time effects between baseline and week 12 for MMSE score and serum levels of HDL-c. It means that among participants in the probiotic group, MMSE scores increased more than those in the placebo group (placebo group: 0.74±2.2, probiotic group: 1.8±1.9; P≤0.001) and HDL-c serum concentrations (placebo group: -6.3±14.3 mg/dL, probiotic group: -0.3±7.2 mg/dL; P=0.04) decreased in the placebo group more than those in the probiotic group over the time. In addition, there were significant time pretreatment interaction effects for the serum concentrations of LDL-c, total cholesterol, and 25(OH)D. Such that LDL-c (placebo group: -7.7±42.4 mg/dL, probiotic group:13.7±30.2 mg/dL; P=0.04) and total cholesterol (placebo group: -12.3± 45.2 mg/dL, probiotic group: 11.4±37 mg/dL; P=0.04) concentrations over the intervention period decreased in placebo group while increased in the probiotic group. However, after a 12-week intervention, serum levels of 25(OH)D (probiotic group: 8.9±18.6 ng/dL placebo group: -1.1±16.2 ng/dL; P=0.04) increased in the participants who consumed probiotics supplements whereas decreased in the placebo group.
At baseline, the MMSE score in both groups was almost the same (16.6±5.4 in the probiotic group vs 16.1±5.4 in the placebo group). However, we observed significant time effects between baseline and week 12 for the MMSE score. It means that among participants in the probiotic group, MMSE scores increased more than those in the placebo group (placebo group: 0.74±2.2 vs probiotic group: 1.8±1.9), but the difference between the two groups was not statistically significant (P=0.53). At the end of the study, the cognitive function of both groups deteriorated, and only one person who received probiotics experienced cognitive improvement, which was not statistically significant. There were no significant differences in TG, FBS, and serum Alb between the two groups at baseline or the end of the study. After adjusting for covariates such as gender, age, and BMI at the beginning of the study, as well as the mean energy intake, we found no significant differences in our results (Table 4).

As dementia progresses, gradual loss of cognitive function negatively impacts a patient’s physical performance. At the beginning of the study, the mean Barthel index scores in the intervention and control groups were 18.5±1.9 and 18.1±2.6, respectively. After 12 weeks, functional loss was less significant in probiotic recipients (P=0.43) (Table 3).
4. Discussion
Dementia is the most prevalent and burdensome neurodegenerative disease, accounting for a remarkable morbidity and mortality rate in older adults (Hormozi et al., 2019; Barnes & Yaffe, 2011). As the world population is aging, finding an effective way to prevent and treat dementia is considered a global health challenge. Recently, probiotics as live microorganisms received significant attention due to their potential beneficial properties in cognitive abilities (Amirani et al., 2020; Kim et al., 2019). Probiotics might affect the central nervous system (CNS) through several direct and indirect pathways (Wang, 2018) and change CNS biochemistry by impacting brain-derived neurotrophic factors, serotonin, gamma-aminobutyric acid, and dopamine. Therefore, its properties could modify human behavior and cognition (Rahimlou et al., 2022; Ranuh et al., 2019; Park et al., 2014; Liu et al., 2020). Likewise, probiotics can inhibit the release of inflammatory cytokines and diminish oxidative stress by increasing antioxidants, including superoxide dismutase and glutathione peroxidase (D’Souza et al., 2010; Bezkorovainy, 2001; Desbonnet et al., 2008; Karimi & Pena, 2003). Another potential advantage of probiotics is altering gut microbiota composition and elevating the diversity of beneficial bacteria. Probiotics increased short-chain fatty acid and tryptophan production, indirectly affecting CNS function (Wang, 2018). The present study aimed to evaluate the effect of probiotic supplementation on cognitive function, physical performance, metabolic factors, vitamin D, and Alb levels in older patients with AD. Our findings demonstrated that supplementation with probiotics for 12-week had no considerable effect on these parameters before or after adjustment.
These findings confirm the results from prospective studies (Leblhuber et al., 2018; Krüger et al., 2021) that no improvement in the cognitive function of dementia patients was reported after taking probiotics. Our results for physical performance with probiotics aligned with two other prospective studies (Buigues et al., 2016; Mañé et al., 2011). In contrast to our results, in a study on 27 older adults, administered probiotic containing Bifidobacterium breve A1 for 24 weeks showed a considerable improvement in cognitive function and reduced the risk of dementia compared to the control group (Kobayashi et al., 2019). In the same way, another clinical trial revealed such a beneficial effect (Ton et al., 2020). The small sample size, lack of a placebo group, and difference in strains and doses of administrated probiotics might explain these discrepancies. More studies need to shed further light on understanding the effect of supplementation with probiotics on cognitive behavior.
Another outcome of this study is that taking supplemental probiotics does not affect serum levels of vitamin D, Alb, FBS, and lipid profile. This finding is similar to the studies that did not report a significant effect of probiotics on these parameters (Akkasheh et al., 2016; Romijn et al., 2017, Pu et al., 2017). However, it differed from a clinical trial that showed that supplementation with probiotics for 12 weeks had a beneficial effect on some of the metabolic parameters, including cholesterol and triglyceride levels among 79 patients with AD (Tamtaji et al., 2019). In addition, Kobayashi et al., (2019) reported a remarkable effect on triglyceride after 200 mL/d of probiotic milk consumption for 12 weeks (Kobayashi et al., 2019). An interventional study investigated a considerable rise in plasma Alb levels by taking for 12 weeks in patients with memory complaints (Kobayashi et al., 2019). Moreover, another trial evaluating the effects of probiotic administration on 127 Canadian otherwise healthy hypercholesterolemia adults demonstrated a significant increase in serum vitamin D concentrations after 9 weeks (Jones et al., 2013). The conflicting results of published clinical trials could be related to their methodology, including different intervention periods, analysis methods, and potential covariates.
Furthermore, using different doses and strains of probiotics may have contributed to the inconsistent findings. There is no common consent about the most effective dose and strain of probiotics (Guarner et al., 2012). This issue needs further exploration to be addressed.
Our study has several limitations. We could neither conduct stool analysis to evaluate the bacteria alteration in the gut nor assess the effect of probiotic administration on inflammatory cytokines due to resource limitations. Participation in this trial was declined due to the COVID-19 pandemic. Finally, probiotics supplements contain several strains of bacteria with different doses; thus, we could not find which strains had a favorable effect among dementia patients in the present clinical trial.
5. Conclusion
In conclusion, this clinical trial study revealed that supplementation with probiotics for 12 weeks had no favorable effect on cognitive function, activities of daily living, lipid profiles, vitamin D concentration, and serum Alb level in older patients with AD. Further studies with larger sample sizes and a more extended follow-up period are needed to clarify the impact of probiotic supplementation on older people.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Tehran University of Medical Sciences, Tehran, Iran (Code: IR.TUMS.VCR.REC.1398.132) and was registered by the Iranian Registry of Clinical Trials (IRCT) (Code: 20191008045024N1). Informed consent was obtained from all participants’ caregivers.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization: Mahdieh Mehmandoust and Alireza Namazi Shabestari; Methodology: Mahdieh Mehmandoust, Shima Raeesi and Mohammad Bidkhori; Software: Mohammad Bidkhori; Resources: Alireza Namazi Shabestari; Formal analysis: Fatemeh Dashti; Supervision: Zahra Vahabi; Writing the original draft: Mahdieh Mehmandoust, and Farzaneh Asoudeh; Review and editing: Shima Raeesi, Rezvan Hashemi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors want to thank all participants for their assistance in this project
References
Aizawa, E., Tsuji, H., Asahara, T., Takahashi, T., Teraishi, T., & Yoshida, S., et al. (2016). Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. Journal of Affective Disorders, 202, 254-257. [DOI:10.1016/j.jad.2016.05.038] [PMID]
Akasheh, G., Kashani-Poor, Z., Tajabadi-Ebrahimi, M., Jafari, P., Akbari, H., & Taghizadeh, M., et al. (2016). Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition, 32(3), 315-320. [DOI:10.1016/j.nut.2015.09.003] [PMID]
Alzheimer’s Disease International. (2018). World Alzheimer Report 2018. The State of the Art of Dementia Research: New Frontiers. London: Alzheimer’s Disease International. [Link]
Amirani, E., Milajerdi, A., Mirzaei, H., Jamilian, H., Mansournia, M. A., & Hallajzadeh, J., et al. (2020). The effects of probiotic supplementation on mental health, biomarkers of inflammation and oxidative stress in patients with psychiatric disorders: A systematic review and meta-analysis of randomized controlled trials. Complementary Therapies in Medicine, 49, 102361. [DOI:10.1016/j.ctim.2020.102361] [PMID]
Barnes, D. E., & Yaffe, K. (2011). The projected effect of risk factor reduction on Alzheimer’s disease prevalence. The Lancet Neurology, 10(9), 819-828. [DOI:10.1016/S1474-4422(11)70072-2] [PMID]
Bezkorovainy, A. (2001). Probiotics: Determinants of survival and growth in the gut. The American Journal of Clinical Nutrition, 73(2), 399s-405s. [DOI:10.1093/ajcn/73.2.399s] [PMID]
Buigues, C., Fernández-Garrido, J., Pruimboom, L., Hoogland, A. J., Navarro-Martínez, R., & Martínez-Martínez, M., et al. (2016). Effect of a prebiotic formulation on frailty syndrome: A randomized, double-blind clinical trial. International Journal of Molecular Science, 17(6), 932. [DOI:10.3390/ijms17060932] [PMID]
Cheng, L. H., Liu, Y. W., Wu, C. C., Wang, S., & Tsai, Y. C. (2019). Psychobiotics in mental health, neurodegenerative and neurodevelopmental disorders. Journal of Food and Drug Analysis, 27(3), 632-648. [DOI:10.1016/j.jfda.2019.01.002] [PMID]
Desbonnet, L., Garrett, L., Clarke, G., Bienenstock, J., & Dinan, T. G. (2008). The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. Journal of Psychiatric Research, 43(2), 164-174. [DOI:10.1016/j.jpsychires.2008.03.009] [PMID]
Dinan, T. G., & Cryan, J. F. (2017). Brain-gut-microbiota axis and mental health. Psychosomatic Medicine, 79(8), 920-926. [DOI:10.1097/PSY.0000000000000519] [PMID]
D’'Souza, A., Fordjour, L., Ahmad, A., Cai, C., Kumar, D., & Valencia, G., et al. (2010). Effects of probiotics, prebiotics, and synbiotics on messenger RNA expression of caveolin-1, NOS, and genes regulating oxidative stress in the terminal ileum of formula-fed neonatal rats. Pediatric Research, 67(5), 526-531. [DOI:10.1203/PDR.0b013e3181d4ff2b] [PMID]
Guarner, F., Khan, A. G., Garisch, J., Eliakim, R., Gangl, A., & Thomson, A., et al. (2012). World gastroenterology organisation global guidelines: probiotics and prebiotics october 2011. Journal of Clinical Gastroenterology, 46(6),468-481. [DOI:10.1097/MCG.0b013e3182549092] [PMID]
Hajifaraji, M., Jahanjou, F., Abbasalizadeh, F., Aghamohammadzadeh, N., Abbasi, M. M., & Dolatkhah, N. (2018). Effect of probiotic supplements in women with gestational diabetes mellitus on inflammation and oxidative stress biomarkers: A randomized clinical trial. Asia Pacific Journal of Clinical Nutrition, 27(3), 581-591. [PMID]
Hormozi, S., Alizadeh-Khoei, M., Sharifi, F., Taati, F., Aminalroaya, R., & Fadaee, S., et al. (2019). Iranian version of barthel index: validity and reliability in outpatients’ elderly. International Journal of Preventive Medicine, 10, 130. [DOI:10.4103/ijpvm.IJPVM_579_18] [PMID]
Jones, M. L., Martoni, C. J., & Prakash, S. (2013). Oral supplementation with probiotic L. reuteri NCIMB 30242 increases mean circulating 25-hydroxyvitamin D: A post hoc analysis of a randomized controlled trial. The Journal of Clinical Endocrinology & Metabolism, 98(7), 2944-2951. [DOI:10.1210/jc.2012-4262] [PMID]
Karimi, O., & Pena, A. S. (2003). Probiotics: Isolated bacteria strain or mixtures of different strains? Two different approaches in the use of probiotics as therapeutics. Drugs of Today (Barcelona, Spain: 1998), 39(8), 565-597. [DOI:10.1358/dot.2003.39.8.799406] [PMID
Kim, S. K., Guevarra, R. B., Kim, Y. T., Kwon, J., Kim, H., & Cho, J. H., et al. (2019). Role of probiotics in human gut microbiome-associated diseases. Journal of Microbiology and Biotechnology, 29(9), 1335–1340. [DOI:10.4014/jmb.1906.06064] [PMID]
Kobayashi, Y., Kuhara, T., Oki, M., & Xiao, J. Z. (2019). Effects of Bifidobacterium breve A1 on the cognitive function of older adults with memory complaints: a randomised, double-blind, placebo-controlled trial. Beneficial Microbes, 10(5), 511-520. [DOI:10.3920/BM2018.0170] [PMID]
Kobayashi, Y., Kinoshita, T., Matsumoto, A., Yoshino, K., Saito, I., & Xiao, J. Z. (2019). Bifidobacterium breve A1 supplementation improved cognitive decline in older adults with mild cognitive impairment: An open-label, single-arm study. The Journal of Prevention of Alzheimer’s Disease, 6(1), 70–75.[DOI:10.14283/jpad.2018.32] [PMID]
Krüger, J. F., Hillesheim, E., Pereira, A. C. S. N., Camargo, C. Q., & Rabito, E. I. (2021). Probiotics for dementia: A systematic review and meta-analysis of randomized controlled trials. Nutrition Reviews, 79(2), 160-170. [DOI:10.1093/nutrit/nuaa037] [PMID]
Leszek, J., Barreto, G. E., Gąsiorowski, K., Koutsouraki, E., Ávila-Rodrigues, M., & Aliev, G. (2016). Inflammatory mechanisms and oxidative stress as key factors responsible for progression of neurodegeneration: Role of brain innate immune system. CNS & Neurological Disorders Drug Targets, 15(3), 329–336. [DOI:10.2174/1871527315666160202125914] [PMID]
Leblhuber, F., Steiner, K., Schuetz, B., Fuchs, D., & Gostner, J. M. (2018). Probiotic supplementation in patients with Alzheimer’s dementia-an explorative intervention study. Current Alzheimer Research, 15(12), 1106-1113. [DOI:10.2174/1389200219666180813144834] [PMID]
Liu, G., Chong, H. X., Chung, F. Y., Li, Y., & Liong, M. T. (2020). Lactobacillus plantarum DR7 modulated bowel movement and gut microbiota associated with dopamine and serotonin pathways in stressed adults. International Journal of Molecular Sciences, 21(13), 4608. [DOI:10.3390/ijms21134608] [PMID]
Łuc, M., Misiak, B., Pawłowski, M., Stańczykiewicz, B., Zabłocka, A., & Szcześniak, D., et al. (2021). Gut microbiota in dementia. Critical review of novel findings and their potential application. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 104, 110039. [DOI:10.1016/j.pnpbp.2020.110039] [PMID]
Maldonado Galdeano, C., Cazorla, S. I., Lemme Dumit, J. M., Vélez, E., & Perdigón, G. (2019). Beneficial effects of probiotic consumption on the immune system. Annals of Nutrition and Metabolism, 74(2), 115-124. [DOI:10.1159/000496426] [PMID]
Mañé, J., Pedrosa, E., Lorén, V., Gassull, M. A., Espadaler, J., & Cuñé, J., et al. (2011). A mixture of Lactobacillus plantarum CECT 7315 and CECT 7316 enhances systemic immunity in elderly subjects. A dose-response, double-blind, placebo-controlled, randomized pilot trial. Nutricion Hospitalaria, 26(1), 228-235. [PMID]
Markowiak-Kopeć, P., & Śliżewska, K. (2020). The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients, 12(4), 1107. [DOI:10.3390/nu12041107] [PMID]
McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R., Jr, & Kawas, C. H., et al. (2011). The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7(3), 263-269. [DOI:10.1016/j.jalz.2011.03.005] [PMID]
Ministry of Health and Medical Education (MOHME). (2019). Iranian Registry of Clinical Trials. Tehran: Ministry of Health and Medical Education. [Link]
Moreira, S. C., Jansen, A. K., & Silva, F. M. (2020). Dietary interventions and cognition of Alzheimer’s disease patients: A systematic review of randomized controlled trial. Dementia & Neuropsychologia, 14(03), 258-282. [DOI:10.1590/1980-57642020dn14-030008] [PMID]
Parra-Rodriguez, M. A., & Welsh-Bohmer, K. A. (2020). Public Health: Engaging people in ADRD research. Paper presented at: Alzheimer's Association International Conference, Netherlands, Amsterdam, July 27-31; 2020. [Link]
Parmar, A. (2016). Gut-brain axis, psychobiotics, and mental health. Asian Journal of Psychiatry, 22, 84-85. [DOI:10.1016/j.ajp.2016.05.004] [PMID]
Park, S. Y., Lee, J. W., & Lim, S. D. (2014). The probiotic characteristics and GABA production of Lactobacillus plantarum K154 isolated from kimchi. Food Science and Biotechnology, 23, 1951-1957. [DOI:10.1007/s10068-014-0266-2]
Pu, F., Guo, Y., Li, M., Zhu, H., Wang, S., & Shen, X., et al. (2017). Yogurt supplemented with probiotics can protect the healthy elderly from respiratory infections: A randomized controlled open-label trial. Clinical Interventions in Aging, 12, 1223–1231.[DOI:10.2147/CIA.S141518] [PMID]
Rahimlou, M., Hosseini, S. A., Majdinasab, N., Haghighizadeh, M. H., & Husain, D. (2022). Effects of long-term administration of Multi-Strain Probiotic on circulating levels of BDNF, NGF, IL-6 and mental health in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled trial. Nutritional Neuroscience, 25(2), 411-422. [DOI:10.1080/1028415X.2020.1758887] [PMID]
Ranuh, R., Athiyyah, A. F., Darma, A., Risky, V. P., Riawan, W., & Surono, I. S., et al. (2019). Effect of the probiotic Lactobacillus plantarum IS-10506 on BDNF and 5HT stimulation: Role of intestinal microbiota on the gut-brain axis. Iranian Journal of Microbiology, 11(2), 145-150. [DOI:10.18502/ijm.v11i2.1077] [PMID]
Romijn, A. R., Rucklidge, J. J., Kuijer, R. G., & Frampton, C. (2017). A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. The Australian and New Zealand journal of psychiatry, 51(8), 810–821. [DOI:10.1177/0004867416686694] [PMID]
Ruiz-Gonzalez, C., Roman, P., Rueda-Ruzafa, L., Rodriguez-Arrastia, M., & Cardona, D. (2021). Effects of probiotics supplementation on dementia and cognitive impairment: a systematic review and meta-analysis of preclinical and clinical studies. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 108, 110189. [DOI:10.1016/j.pnpbp.2020.110189] [PMID]
Sealed Envelope Ltd. (2019). Randomisation and online databases for clinical trials. Retrieved from: [Link]
Shimizu, Y. (2018). Gut microbiota in common elderly diseases affecting activities of daily living. World Journal of Gastroenterology, 24(42), 4750-8. [DOI:10.3748/wjg.v24.i42.4750] [PMID]
Seyedian, M., Fallah, M., Norouzian, M., Nejat, S. A. H. A. R. N. A. Z., Delavar, A., & Ghasemzadeh, H. (2007). Validity of the farsi version of mini-mental state examination. Journal of Medical Council of Islamic Republic of Iran, 25(4), 408-414. [Link]
Tamtaji, O. R., Heidari-Soureshjani, R., Mirhosseini, N., Kouchaki, E., Bahmani, F., & Aghadavod, E., et al. (2019). Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: A randomized, double-blind, controlled trial. Clinical Nutrition, 38(6), 2569-2575. [DOI:10.1016/j.clnu.2018.11.034] [PMID]
Ton, A. M. M., Campagnaro, B. P., Alves, G. A., Aires, R., Côco, L. Z., & Arpini, C. M., et al. (2020). Oxidative stress and dementia in Alzheimer’s patients: Effects of synbiotic supplementation. Oxidative Medicine and Cellular Longevity, 2020, 2638703. [DOI:10.1155/2020/2638703] [PMID]
Van Middelaar, T., Hoevenaar-Blom, M. P., van Gool, W. A., Moll van Charante, E. P., van Dalen, J. W., & Deckers, K., et al. (2018). Modifiable dementia risk score to study heterogeneity in treatment effect of a dementia prevention trial: A post hoc analysis in the preDIVA trial using the LIBRA index. Alzheimer’s Research & Therapy, 10(1), 62. [DOI:10.1186/s13195-018-0389-4] [PMID]
Vogt, N. M., Kerby, R. L., Dill-McFarland, K. A., Harding, S. J., Merluzzi, A. P., & Johnson, S. C., et al. (2017). Gut microbiome alterations in Alzheimer’s disease. Scientific Reports, 7(1), 13537. [DOI:10.1038/s41598-017-13601-y] [PMID]
Wang, H. (2018). Effects of probiotics on central nervous system functions in humans [PhD dissertation]. Tübingen: Universität Tübingen. [Link]
Zheng, H. J., Guo, J., Jia, Q., Huang, Y. S., Huang, W. J., & Zhang, W., et al. (2019). The effect of probiotic and synbiotic supplementation on biomarkers of inflammation and oxidative stress in diabetic patients: A systematic review and meta-analysis of randomized controlled trials. Pharmacological Research, 142, 303-313. [DOI:10.1016/j.phrs.2019.02.016] [PMID]
Type of Study: Original |
Subject:
Clinical Neuroscience
Received: 2022/09/9 | Accepted: 2023/05/22 | Published: 2024/11/1
Received: 2022/09/9 | Accepted: 2023/05/22 | Published: 2024/11/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





