Volume 15, Issue 2 (March & April 2024)
BCN 2024, 15(2): 175-184 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Nafari A, Shojaei S, Jalili Khoshnood R, Ghajarzadeh M, Tafreshinejad A, Safari S et al . Myasthenia Gravis and COVID-19: A Systematic Review and Meta-analysis. BCN 2024; 15 (2) :175-184
URL: http://bcn.iums.ac.ir/article-1-2526-en.html
URL: http://bcn.iums.ac.ir/article-1-2526-en.html
Amirhossein Nafari1 

 , Seyedpouzhia Shojaei2
, Seyedpouzhia Shojaei2 

 , Reza Jalili Khoshnood3
, Reza Jalili Khoshnood3 

 , Mahsa Ghajarzadeh4
, Mahsa Ghajarzadeh4 

 , Arash Tafreshinejad3
, Arash Tafreshinejad3 

 , Saeid Safari *3
, Saeid Safari *3 

 , Omid Mirmosayyeb5
, Omid Mirmosayyeb5 




 , Seyedpouzhia Shojaei2
, Seyedpouzhia Shojaei2 

 , Reza Jalili Khoshnood3
, Reza Jalili Khoshnood3 

 , Mahsa Ghajarzadeh4
, Mahsa Ghajarzadeh4 

 , Arash Tafreshinejad3
, Arash Tafreshinejad3 

 , Saeid Safari *3
, Saeid Safari *3 

 , Omid Mirmosayyeb5
, Omid Mirmosayyeb5 


1- Department of Clinical Biochemistry, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran.
2- Critical Care Quality Improvement Research Center, Imam Hossein Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Functional Neurosurgery Research Center, Shohada Tajrish Neurosurgical Comprehensive Center of Excellence, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4- Multiple Sclerosis Research Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, Iran.
5- Isfahan Neurosciences Research Center, Isfahan University of Medical Sciences, Isfahan, Iran.
2- Critical Care Quality Improvement Research Center, Imam Hossein Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Functional Neurosurgery Research Center, Shohada Tajrish Neurosurgical Comprehensive Center of Excellence, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4- Multiple Sclerosis Research Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, Iran.
5- Isfahan Neurosciences Research Center, Isfahan University of Medical Sciences, Isfahan, Iran.
Full-Text [PDF 630 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
In December 2019, a new coronavirus emerged in China and rapidly spread worldwide, leading to a pandemic (Moghadasi et al., 2021). Fever, cough, and malaise are the most frequent clinical symptoms, while different factors such as the presence of underlying diseases, advanced age, and used medications play crucial roles in the prognosis of the COVID-19 infection (Li et al., 2021). Myasthenia gravis (MG) is an autoimmune disorder that affects the neuromuscular junction, and patients should use immune suppressors as the treatment (Hübers et al., 2020). Administration of immune suppressors predisposes these cases to severe form of the disease, and anti-virus treatments such as hydroxyl-chloroquine exacerbate MG (Anand et al., 2020; Gilhus et al., 2018).
Various studies have reported different rates of COVID-19 infection in patients with MG. Consequently, this systematic review and meta-analysis were designed to estimate the pooled prevalence of COVID-19 infection in patients with MG.
2. Materials and Methods
We systematically searched PubMed, Scopus, EMBASE, Web of Science, Google Scholar, and gray literature, including references to the included studies published before October 2021.
The search strategy was as follows:
((“Myasthenia gravis” AND “ocular”) OR “ocular myasthenia gravis” OR (“myasthenia gravis” AND “generalized”) OR (generalized myasthenia gravis) OR (“muscle-specific receptor tyrosine kinase myasthenia gravis”) OR (“muscle specific receptor tyrosine kinase myasthenia gravis”) OR (“muscle-specific tyrosine kinase antibody positive myasthenia gravis”) OR (“muscle specific tyrosine kinase antibody positive myasthenia gravis”) OR (“MuSK MG”) OR (“MuSK myasthenia gravis”) OR (“myasthenia gravis” AND “MuSK”) OR (“anti-MuSK myasthenia gravis”) OR (“anti MuSK myasthenia gravis”) OR (“myasthenia gravis” AND “anti-MuSK”)) AND (“COVID 19” OR “COVID-19 virus disease” OR “COVID 19 virus disease*” OR “COVID-19 virus disease*” OR (disease AND “COVID-19 Virus”) OR (“virus disease” AND COVID-19) OR “COVID-19 virus infection*” OR “COVID 19 virus infection” OR (infection AND “COVID-19 virus”) OR (“virus infection” AND COVID-19) OR “2019-nCoV infection” OR “2019 nCoV infection*” OR (infection AND 2019-nCoV) OR “coronavirus disease-19” OR “coronavirus disease 19” OR “2019 novel coronavirus disease” OR “2019 novel coronavirus infection” OR “2019-nCoV disease” OR “2019 nCoV disease” OR “2019-nCoV diseases” OR (disease AND 2019-nCoV) OR “COVID19” OR “coronavirus disease 2019” OR (“disease 2019” AND coronavirus) OR “SARS coronavirus 2 infection” OR “SARS-CoV-2 infection” OR (infection AND SARS-CoV-2) OR “SARS CoV 2 infection*” OR “COVID-19 pandemic*” OR “COVID 19 pandemic” OR (pandemic AND COVID-19))
Inclusion criteria
We included cross-sectional studies or case series reporting the incidence of COVID-19 infection, hospitalization, or mortality in individuals with MG.
Exclusion criteria
We excluded letters to the editor, case-control studies, and case reports. Data were extracted regarding the total number of participants, first author, publication year, country of origin, individuals with myasthenia gravis, symptoms, hospitalization, and death.
Risk of bias assessment
We assessed the risk of bias using the Newcastle-Ottawa scale (NOS) for cross-sectional studies (Modesti et al., 2016).
Statistical analysis
All statistical analyses were performed using STATA software, Version 14.0 (Stata Corp LP, College Station, TX, USA), employing random-effects models. We calculated inconsistency (I2) to determine heterogeneity.
3. Results
We found 253 articles utilizing a literature search; after excluding duplicates, 75 remained. Finally, 18 articles were selected for meta-analysis (Figure 1).

A total of 18 articles were included in the analysis, and their basic characteristics are presented in Table 1.


Figure 2 displays the pooled prevalence of COVID-19 infection in MG cases, which was 2% (95% CI, 1%-3%; I2=85%; P<0.001).

Figure 3 provides information on the pooled prevalence of hospitalization among individuals with COVID-19 infection, which was calculated to be 43% (95% CI, 26%, 60%; I2=97.6%; P<0.001).

Figure 4 shows the pooled prevalence of MG exacerbation among those with COVID-19 infection, which was 33% (95% CI, 20%, 46%; I2=92.6%; P<0.001).

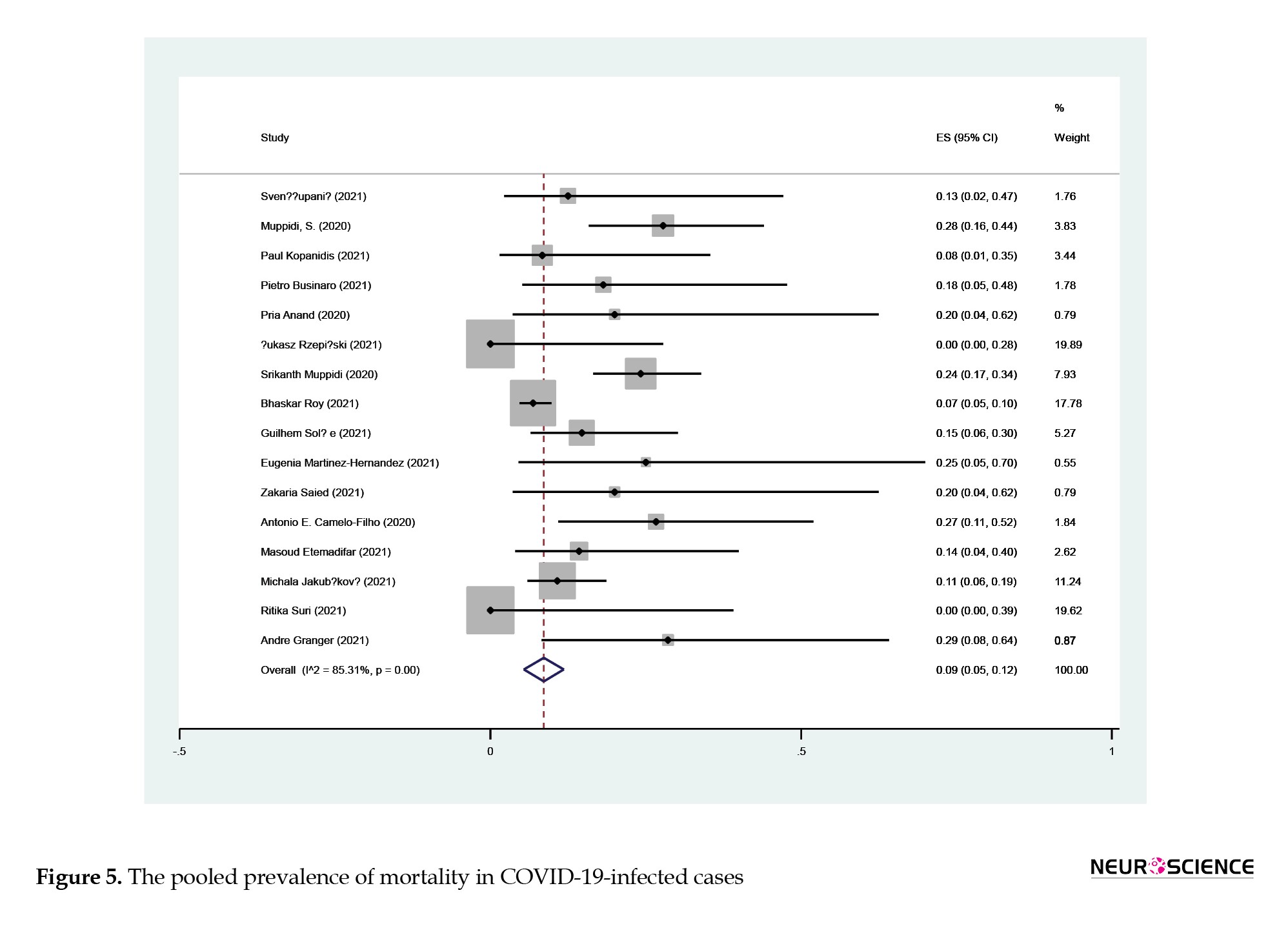
According to Figure 5, the pooled prevalence of mortality in infected cases was 9% (95% CI, 5%, 12%; I2:85.3%; P<0.001).
4. Discussion
To our understanding, this systematic review and meta-analysis is the first to evaluate the prevalence of COVID-19 infection in MG cases. The findings indicate that the pooled prevalence of COVID-19 infection in MG cases is 2%, the pooled hospitalization rate is 43%, disease exacerbation is 33%, and the pooled mortality rate is 9%.
Previous studies evaluating patients who received immunosuppressive agents demonstrated that using medications does not predispose patients to higher COVID-19 infection risk. A 2021 systematic review and meta-analysis reported that the pooled prevalence of COVID-19 in MS cases was 4%, and the pooled hospitalization rate was 10% (Moghadasi et al., 2021). Businaro et al. evaluated 162 MG patients and reported COVID-19 infection in 11. They found that the severity of MG was not related to the seriousness of COVID-19 infection (Businaro et al., 2021). Rein et al. reported three cases of COVID-19 infection and MG and reported favorable outcomes, and only one experience exacerbation of the disease (Rein et al., 2020).
Our results show that the pooled prevalence of disease exacerbation was 33%, which indicates that COVID-19 infection interferes with MG’s nature.
It is suggested that early administration of intravenous immunoglobulins or steroids could prevent complications in MG cases (International MG/COVID-19 Working Group et al., 2020).
Rzepiński et al. evaluated 30 MG cases who had no vaccination against COVID-19 and found that exacerbation of MG was presented in 11, which needed hospitalization (Rzepiński & Zawadka-Kunikowska, 2021). Muppidi et al. evaluated 91 MG patients who had COVID-19 infection and reported hospitalization, disease exacerbation, and mortality in 69%, 40%, and 22%, respectively (Muppidi et al., 2020). By including 3558 MG cases, Sole et al. reported 34 cases of COVID-19 infection, of whom 5 died due to illness. They found that disease severity was not associated with infection severity (Solé et al., 2021). Anand et al. described COVID-19 infection in 5 MG cases who were hospitalized and were immunosuppressed. Four had favorable outcomes, and mycophenolate mofetil was held in two cases (Anand et al., 2020).
It should be considered that patients with COVID-19 infection experience a wide range of neurological complications. Farsalinos et al. suggested that SARS-CoV-2 may interact with the nicotinic AChR, potentially leading to dysregulation of the cholinergic anti-inflammatory pathway (Farsalinos et al., 2020).
The International MG/COVID-19 Working Group suggested continuing medications in MG cases and medication changes or stops after consultation with the health care provider (International MG/COVID-19 Working Group et al., 2020).
This study holds several strengths. Firstly, it represents the pioneering systematic review and meta-analysis in this context. Secondly, we included all relevant research manuscripts in our analysis.
5. Conclusion
The findings derived from this systematic review and meta-analysis indicate that the pooled prevalence of COVID-19 infection in MG cases is 2%.
Ethical Considerations
Compliance with ethical guidelines
This article is a meta-analysis with no human or animal sample.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
Anand, P., Slama, M. C. C., Kaku, M., Ong, C., Cervantes-Arslanian, A. M., & Zhou, L., et al. (2020). COVID-19 in patients with myasthenia gravis. Muscle & Nerve, 62(2), 254–258. [DOI:10.1002/mus.26918] [PMID] [PMCID]
Businaro, P., Vaghi, G., Marchioni, E., Diamanti, L., Arceri, S., & Bini, P., et al. (2021). COVID-19 in patients with myasthenia gravis: Epidemiology and disease course. Muscle & Nerve, 64(2), 206–211. [DOI:10.1002/mus.27324] [PMID] [PMCID]
Camelo-Filho, A. E., Silva, A. M. S., Estephan, E. P., Zambon, A. A., Mendonça, R. H., & Souza, P. V. S., et al. (2020). Myasthenia gravis and COVID-19: Clinical characteristics and outcomes. Frontiers in Neurology, 11, 1053. [DOI:10.3389/fneur.2020.01053] [PMID] [PMCID]
Etemadifar, M., Akafzadeh-Savari, M., Salari, M., Akhavan Sigari, A., Ebrahimi-Pelarti, S., & Sedaghat, N., et al. (2021). Myasthenia gravis and coronavirus disease 2019: A report from Iran. Current Journal of Neurology, 20(3), 162–165. [DOI:10.18502/cjn.v20i3.7692] [PMID] [PMCID]
Farsalinos, K., Niaura, R., Le Houezec, J., Barbouni, A., Tsatsakis, A., & Kouretas, D., et al. (2020). Editorial: Nicotine and SARS-CoV-2: COVID-19 may be a disease of the nicotinic cholinergic system. Toxicology Reports, 7, 658–663. [DOI:10.1016/j.toxrep.2020.04.012] [PMID] [PMCID]
Gilhus, N. E., Romi, F., Hong, Y., & Skeie, G. O. (2018). Myasthenia gravis and infectious disease. Journal of Neurology, 265(6), 1251–1258. [DOI:10.1007/s00415-018-8751-9] [PMID]
Granger, A., Kwon, P., & Zakin, E. (2021). Characteristics and outcomes of myasthenia gravis patients with COVID-19-A case series (4807). Neurology, 96(15_supplement), 4807.[DOI:10.1212/WNL.96.15_supplement.4807]
Hübers, A., Lascano, A. M., & Lalive, P. H. (2020). Management of patients with generalised myasthenia gravis and COVID-19: Four case reports. Journal of Neurology, Neurosurgery, and Psychiatry, 91(10), 1124–1125. [DOI:10.1136/jnnp-2020-323565] [PMID]
International MG/COVID-19 Working Group, Jacob, S., Muppidi, S., Guidon, A., Guptill, J., & Hehir, M., et al. (2020). Guidance for the management of myasthenia gravis (MG) and Lambert-Eaton myasthenic syndrome (LEMS) during the COVID-19 pandemic. Journal of the Neurological Sciences, 412, 116803.[DOI:10.1016/j.jns.2020.116803] [PMID] [PMCID]
Kopanidis, P., Quirke, M., Buckley, C., & Leite, I. (2021). 031 COVID-19 disease outcomes in a UK myasthenia centre during the first year of the pandemic. BMJ Journals, 3(1), A1–A45. [DOI:10.1136/bmjno-2021-ANZAN.31]
Li, J., Huang, D. Q., Zou, B., Yang, H., Hui, W. Z., & Rui, F., et al. (2021). Epidemiology of COVID-19: A systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. Journal of Medical Virology, 93(3), 1449–1458. [DOI:10.1002/jmv.26424] [PMID] [PMCID]
Martinez-Hernandez, E., Esteller, D., Sepulveda, M., Llufriu, S., Guasp, M., & Cabrera, J. M., et al. (2021). Incidence and impact of COVID-19 in a cohort of patients with myasthenia gravis from Barcelona.(4170). Neurology, 96(15_supplement), 4807. [DOI:10.1212/WNL.96.15_supplement.4170]
Jakubikova, M., Tyblova, M., Tesar, A., Magda, H., Daniela, V., & Irena, R., et al. (2022). Predictive factors for a severe course of COVID-19 infection in myasthenia gravis patients with an overall impact on myasthenic outcome status and survival. European Journal of Neurology, 29(1), e7–e8. [PMID] [PMCID]
Modesti, P. A., Reboldi, G., Cappuccio, F. P., Agyemang, C., Remuzzi, G., & Rapi, S., et al. (2016). Panethnic differences in blood pressure in Europe: A systematic review and meta-analysis. Plos One, 11(1), e0147601. [DOI:10.1371/journal.pone.0147601] [PMID] [PMCID]
Moghadasi, A. N., Mirmosayyeb, O., Barzegar, M., Sahraian, M. A., & Ghajarzadeh, M. (2021). The prevalence of COVID-19 infection in patients with multiple sclerosis (MS): A systematic review and meta-analysis. Neurological Sciences, 42(8), 3093–3099. [DOI:10.1007/s10072-021-05373-1] [PMID] [PMCID]
Muppidi, S., Guptill, J. T., Jacob, S., Li, Y., Farrugia, M. E., & Guidon, A. C., et al. (2020). COVID-19-associated risks and effects in myasthenia gravis (CARE-MG). The Lancet Neurology, 19(12), 970-971. [DOI:10.1016/S1474-4422(20)30413-0]
Neykova, K. K., Milanova, M., & Ignatov, P. N. (2022). Myasthenia gravis and covid-19 in pregnancy: A review of the literature and case series report. The Journal of Maternal-Fetal & Neonatal Medicine, 35(25), 8308–8316. [DOI:10.1080/14767058.2021.1973418] [PMID]
Rein, N., Haham, N., Orenbuch-Harroch, E., Romain, M., Argov, Z., Vaknin-Dembinsky, A., & Gotkine, M. (2020). Description of 3 patients with myasthenia gravis and COVID-19. Journal of the Neurological Sciences, 417, 117053. [DOI:10.1016/j.jns.2020.117053] [PMID] [PMCID]
Roy, B., Kovvuru, S., Nalleballe, K., Onteddu, S. R., & Nowak, R. J. (2021). Electronic health record derived-impact of COVID-19 on myasthenia gravis. Journal of the Neurological Sciences, 423, 117362. [DOI:10.1016/j.jns.2021.117362]
Rzepiński, Ł., & Zawadka-Kunikowska, M. (2022). COVID-19 pandemic year in a sample of Polish myasthenia gravis patients: An observational study. Neurologia I neurochirurgia Polska, 56(1), 61–67. [DOI:10.5603/PJNNS.a2021.0054] [PMID]
Saied, Z., Rachdi, A., Thamlaoui, S., Nabli, F., Jeridi, C., & Baffoun, N., et al. (2021). Myasthenia gravis and COVID-19: A case series and comparison with literature. Acta Neurologica Scandinavica, 144(3), 334–340. [DOI:10.1111/ane.13440] [PMID] [PMCID]
Sarmiento-Monroy, J. C., Espinosa, G., Londoño, M. C., Meira, F., Caballol, B., & Llufriu, S., et al. (2021). A multidisciplinary registry of patients with autoimmune and immune-mediated diseases with symptomatic COVID-19 from a single center. Journal of Autoimmunity, 117, 102580. [DOI:10.1016/j.jaut.2020.102580] [PMID] [PMCID]
Solé, G., Mathis, S., Friedman, D., Salort-Campana, E., Tard, C., & Bouhour, F., et al. (2021). Impact of coronavirus disease 2019 in a french cohort of myasthenia gravis. Neurology, 96(16), e2109–e2120. [DOI:10.1212/WNL.0000000000011669] [PMID]
Suri, R., Chandok, A., Sripathi, N., & Grover, K. (2021). Case series of myasthenia gravis patients with COVID-19 infection (4523). Neurology, 96(15_supplement), 4807. [DOI:10.1212/WNL.96.15_supplement.4523]
Županić, S., Perić Šitum, M., Majdak, M., Karakaš, M., Bašić, S., & Sporiš, D. (2021). Case series of COVID-19 in patients with myasthenia gravis: A single institution experience. Acta Neurologica Belgica, 121(4), 1039–1044. [DOI:10.1007/s13760-021-01662-w] [PMID] [PMCID]
In December 2019, a new coronavirus emerged in China and rapidly spread worldwide, leading to a pandemic (Moghadasi et al., 2021). Fever, cough, and malaise are the most frequent clinical symptoms, while different factors such as the presence of underlying diseases, advanced age, and used medications play crucial roles in the prognosis of the COVID-19 infection (Li et al., 2021). Myasthenia gravis (MG) is an autoimmune disorder that affects the neuromuscular junction, and patients should use immune suppressors as the treatment (Hübers et al., 2020). Administration of immune suppressors predisposes these cases to severe form of the disease, and anti-virus treatments such as hydroxyl-chloroquine exacerbate MG (Anand et al., 2020; Gilhus et al., 2018).
Various studies have reported different rates of COVID-19 infection in patients with MG. Consequently, this systematic review and meta-analysis were designed to estimate the pooled prevalence of COVID-19 infection in patients with MG.
2. Materials and Methods
We systematically searched PubMed, Scopus, EMBASE, Web of Science, Google Scholar, and gray literature, including references to the included studies published before October 2021.
The search strategy was as follows:
((“Myasthenia gravis” AND “ocular”) OR “ocular myasthenia gravis” OR (“myasthenia gravis” AND “generalized”) OR (generalized myasthenia gravis) OR (“muscle-specific receptor tyrosine kinase myasthenia gravis”) OR (“muscle specific receptor tyrosine kinase myasthenia gravis”) OR (“muscle-specific tyrosine kinase antibody positive myasthenia gravis”) OR (“muscle specific tyrosine kinase antibody positive myasthenia gravis”) OR (“MuSK MG”) OR (“MuSK myasthenia gravis”) OR (“myasthenia gravis” AND “MuSK”) OR (“anti-MuSK myasthenia gravis”) OR (“anti MuSK myasthenia gravis”) OR (“myasthenia gravis” AND “anti-MuSK”)) AND (“COVID 19” OR “COVID-19 virus disease” OR “COVID 19 virus disease*” OR “COVID-19 virus disease*” OR (disease AND “COVID-19 Virus”) OR (“virus disease” AND COVID-19) OR “COVID-19 virus infection*” OR “COVID 19 virus infection” OR (infection AND “COVID-19 virus”) OR (“virus infection” AND COVID-19) OR “2019-nCoV infection” OR “2019 nCoV infection*” OR (infection AND 2019-nCoV) OR “coronavirus disease-19” OR “coronavirus disease 19” OR “2019 novel coronavirus disease” OR “2019 novel coronavirus infection” OR “2019-nCoV disease” OR “2019 nCoV disease” OR “2019-nCoV diseases” OR (disease AND 2019-nCoV) OR “COVID19” OR “coronavirus disease 2019” OR (“disease 2019” AND coronavirus) OR “SARS coronavirus 2 infection” OR “SARS-CoV-2 infection” OR (infection AND SARS-CoV-2) OR “SARS CoV 2 infection*” OR “COVID-19 pandemic*” OR “COVID 19 pandemic” OR (pandemic AND COVID-19))
Inclusion criteria
We included cross-sectional studies or case series reporting the incidence of COVID-19 infection, hospitalization, or mortality in individuals with MG.
Exclusion criteria
We excluded letters to the editor, case-control studies, and case reports. Data were extracted regarding the total number of participants, first author, publication year, country of origin, individuals with myasthenia gravis, symptoms, hospitalization, and death.
Risk of bias assessment
We assessed the risk of bias using the Newcastle-Ottawa scale (NOS) for cross-sectional studies (Modesti et al., 2016).
Statistical analysis
All statistical analyses were performed using STATA software, Version 14.0 (Stata Corp LP, College Station, TX, USA), employing random-effects models. We calculated inconsistency (I2) to determine heterogeneity.
3. Results
We found 253 articles utilizing a literature search; after excluding duplicates, 75 remained. Finally, 18 articles were selected for meta-analysis (Figure 1).

A total of 18 articles were included in the analysis, and their basic characteristics are presented in Table 1.


Figure 2 displays the pooled prevalence of COVID-19 infection in MG cases, which was 2% (95% CI, 1%-3%; I2=85%; P<0.001).

Figure 3 provides information on the pooled prevalence of hospitalization among individuals with COVID-19 infection, which was calculated to be 43% (95% CI, 26%, 60%; I2=97.6%; P<0.001).

Figure 4 shows the pooled prevalence of MG exacerbation among those with COVID-19 infection, which was 33% (95% CI, 20%, 46%; I2=92.6%; P<0.001).

According to Figure 5, the pooled prevalence of mortality in infected cases was 9% (95% CI, 5%, 12%; I2:85.3%; P<0.001).

4. Discussion
To our understanding, this systematic review and meta-analysis is the first to evaluate the prevalence of COVID-19 infection in MG cases. The findings indicate that the pooled prevalence of COVID-19 infection in MG cases is 2%, the pooled hospitalization rate is 43%, disease exacerbation is 33%, and the pooled mortality rate is 9%.
Previous studies evaluating patients who received immunosuppressive agents demonstrated that using medications does not predispose patients to higher COVID-19 infection risk. A 2021 systematic review and meta-analysis reported that the pooled prevalence of COVID-19 in MS cases was 4%, and the pooled hospitalization rate was 10% (Moghadasi et al., 2021). Businaro et al. evaluated 162 MG patients and reported COVID-19 infection in 11. They found that the severity of MG was not related to the seriousness of COVID-19 infection (Businaro et al., 2021). Rein et al. reported three cases of COVID-19 infection and MG and reported favorable outcomes, and only one experience exacerbation of the disease (Rein et al., 2020).
Our results show that the pooled prevalence of disease exacerbation was 33%, which indicates that COVID-19 infection interferes with MG’s nature.
It is suggested that early administration of intravenous immunoglobulins or steroids could prevent complications in MG cases (International MG/COVID-19 Working Group et al., 2020).
Rzepiński et al. evaluated 30 MG cases who had no vaccination against COVID-19 and found that exacerbation of MG was presented in 11, which needed hospitalization (Rzepiński & Zawadka-Kunikowska, 2021). Muppidi et al. evaluated 91 MG patients who had COVID-19 infection and reported hospitalization, disease exacerbation, and mortality in 69%, 40%, and 22%, respectively (Muppidi et al., 2020). By including 3558 MG cases, Sole et al. reported 34 cases of COVID-19 infection, of whom 5 died due to illness. They found that disease severity was not associated with infection severity (Solé et al., 2021). Anand et al. described COVID-19 infection in 5 MG cases who were hospitalized and were immunosuppressed. Four had favorable outcomes, and mycophenolate mofetil was held in two cases (Anand et al., 2020).
It should be considered that patients with COVID-19 infection experience a wide range of neurological complications. Farsalinos et al. suggested that SARS-CoV-2 may interact with the nicotinic AChR, potentially leading to dysregulation of the cholinergic anti-inflammatory pathway (Farsalinos et al., 2020).
The International MG/COVID-19 Working Group suggested continuing medications in MG cases and medication changes or stops after consultation with the health care provider (International MG/COVID-19 Working Group et al., 2020).
This study holds several strengths. Firstly, it represents the pioneering systematic review and meta-analysis in this context. Secondly, we included all relevant research manuscripts in our analysis.
5. Conclusion
The findings derived from this systematic review and meta-analysis indicate that the pooled prevalence of COVID-19 infection in MG cases is 2%.
Ethical Considerations
Compliance with ethical guidelines
This article is a meta-analysis with no human or animal sample.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
Anand, P., Slama, M. C. C., Kaku, M., Ong, C., Cervantes-Arslanian, A. M., & Zhou, L., et al. (2020). COVID-19 in patients with myasthenia gravis. Muscle & Nerve, 62(2), 254–258. [DOI:10.1002/mus.26918] [PMID] [PMCID]
Businaro, P., Vaghi, G., Marchioni, E., Diamanti, L., Arceri, S., & Bini, P., et al. (2021). COVID-19 in patients with myasthenia gravis: Epidemiology and disease course. Muscle & Nerve, 64(2), 206–211. [DOI:10.1002/mus.27324] [PMID] [PMCID]
Camelo-Filho, A. E., Silva, A. M. S., Estephan, E. P., Zambon, A. A., Mendonça, R. H., & Souza, P. V. S., et al. (2020). Myasthenia gravis and COVID-19: Clinical characteristics and outcomes. Frontiers in Neurology, 11, 1053. [DOI:10.3389/fneur.2020.01053] [PMID] [PMCID]
Etemadifar, M., Akafzadeh-Savari, M., Salari, M., Akhavan Sigari, A., Ebrahimi-Pelarti, S., & Sedaghat, N., et al. (2021). Myasthenia gravis and coronavirus disease 2019: A report from Iran. Current Journal of Neurology, 20(3), 162–165. [DOI:10.18502/cjn.v20i3.7692] [PMID] [PMCID]
Farsalinos, K., Niaura, R., Le Houezec, J., Barbouni, A., Tsatsakis, A., & Kouretas, D., et al. (2020). Editorial: Nicotine and SARS-CoV-2: COVID-19 may be a disease of the nicotinic cholinergic system. Toxicology Reports, 7, 658–663. [DOI:10.1016/j.toxrep.2020.04.012] [PMID] [PMCID]
Gilhus, N. E., Romi, F., Hong, Y., & Skeie, G. O. (2018). Myasthenia gravis and infectious disease. Journal of Neurology, 265(6), 1251–1258. [DOI:10.1007/s00415-018-8751-9] [PMID]
Granger, A., Kwon, P., & Zakin, E. (2021). Characteristics and outcomes of myasthenia gravis patients with COVID-19-A case series (4807). Neurology, 96(15_supplement), 4807.[DOI:10.1212/WNL.96.15_supplement.4807]
Hübers, A., Lascano, A. M., & Lalive, P. H. (2020). Management of patients with generalised myasthenia gravis and COVID-19: Four case reports. Journal of Neurology, Neurosurgery, and Psychiatry, 91(10), 1124–1125. [DOI:10.1136/jnnp-2020-323565] [PMID]
International MG/COVID-19 Working Group, Jacob, S., Muppidi, S., Guidon, A., Guptill, J., & Hehir, M., et al. (2020). Guidance for the management of myasthenia gravis (MG) and Lambert-Eaton myasthenic syndrome (LEMS) during the COVID-19 pandemic. Journal of the Neurological Sciences, 412, 116803.[DOI:10.1016/j.jns.2020.116803] [PMID] [PMCID]
Kopanidis, P., Quirke, M., Buckley, C., & Leite, I. (2021). 031 COVID-19 disease outcomes in a UK myasthenia centre during the first year of the pandemic. BMJ Journals, 3(1), A1–A45. [DOI:10.1136/bmjno-2021-ANZAN.31]
Li, J., Huang, D. Q., Zou, B., Yang, H., Hui, W. Z., & Rui, F., et al. (2021). Epidemiology of COVID-19: A systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. Journal of Medical Virology, 93(3), 1449–1458. [DOI:10.1002/jmv.26424] [PMID] [PMCID]
Martinez-Hernandez, E., Esteller, D., Sepulveda, M., Llufriu, S., Guasp, M., & Cabrera, J. M., et al. (2021). Incidence and impact of COVID-19 in a cohort of patients with myasthenia gravis from Barcelona.(4170). Neurology, 96(15_supplement), 4807. [DOI:10.1212/WNL.96.15_supplement.4170]
Jakubikova, M., Tyblova, M., Tesar, A., Magda, H., Daniela, V., & Irena, R., et al. (2022). Predictive factors for a severe course of COVID-19 infection in myasthenia gravis patients with an overall impact on myasthenic outcome status and survival. European Journal of Neurology, 29(1), e7–e8. [PMID] [PMCID]
Modesti, P. A., Reboldi, G., Cappuccio, F. P., Agyemang, C., Remuzzi, G., & Rapi, S., et al. (2016). Panethnic differences in blood pressure in Europe: A systematic review and meta-analysis. Plos One, 11(1), e0147601. [DOI:10.1371/journal.pone.0147601] [PMID] [PMCID]
Moghadasi, A. N., Mirmosayyeb, O., Barzegar, M., Sahraian, M. A., & Ghajarzadeh, M. (2021). The prevalence of COVID-19 infection in patients with multiple sclerosis (MS): A systematic review and meta-analysis. Neurological Sciences, 42(8), 3093–3099. [DOI:10.1007/s10072-021-05373-1] [PMID] [PMCID]
Muppidi, S., Guptill, J. T., Jacob, S., Li, Y., Farrugia, M. E., & Guidon, A. C., et al. (2020). COVID-19-associated risks and effects in myasthenia gravis (CARE-MG). The Lancet Neurology, 19(12), 970-971. [DOI:10.1016/S1474-4422(20)30413-0]
Neykova, K. K., Milanova, M., & Ignatov, P. N. (2022). Myasthenia gravis and covid-19 in pregnancy: A review of the literature and case series report. The Journal of Maternal-Fetal & Neonatal Medicine, 35(25), 8308–8316. [DOI:10.1080/14767058.2021.1973418] [PMID]
Rein, N., Haham, N., Orenbuch-Harroch, E., Romain, M., Argov, Z., Vaknin-Dembinsky, A., & Gotkine, M. (2020). Description of 3 patients with myasthenia gravis and COVID-19. Journal of the Neurological Sciences, 417, 117053. [DOI:10.1016/j.jns.2020.117053] [PMID] [PMCID]
Roy, B., Kovvuru, S., Nalleballe, K., Onteddu, S. R., & Nowak, R. J. (2021). Electronic health record derived-impact of COVID-19 on myasthenia gravis. Journal of the Neurological Sciences, 423, 117362. [DOI:10.1016/j.jns.2021.117362]
Rzepiński, Ł., & Zawadka-Kunikowska, M. (2022). COVID-19 pandemic year in a sample of Polish myasthenia gravis patients: An observational study. Neurologia I neurochirurgia Polska, 56(1), 61–67. [DOI:10.5603/PJNNS.a2021.0054] [PMID]
Saied, Z., Rachdi, A., Thamlaoui, S., Nabli, F., Jeridi, C., & Baffoun, N., et al. (2021). Myasthenia gravis and COVID-19: A case series and comparison with literature. Acta Neurologica Scandinavica, 144(3), 334–340. [DOI:10.1111/ane.13440] [PMID] [PMCID]
Sarmiento-Monroy, J. C., Espinosa, G., Londoño, M. C., Meira, F., Caballol, B., & Llufriu, S., et al. (2021). A multidisciplinary registry of patients with autoimmune and immune-mediated diseases with symptomatic COVID-19 from a single center. Journal of Autoimmunity, 117, 102580. [DOI:10.1016/j.jaut.2020.102580] [PMID] [PMCID]
Solé, G., Mathis, S., Friedman, D., Salort-Campana, E., Tard, C., & Bouhour, F., et al. (2021). Impact of coronavirus disease 2019 in a french cohort of myasthenia gravis. Neurology, 96(16), e2109–e2120. [DOI:10.1212/WNL.0000000000011669] [PMID]
Suri, R., Chandok, A., Sripathi, N., & Grover, K. (2021). Case series of myasthenia gravis patients with COVID-19 infection (4523). Neurology, 96(15_supplement), 4807. [DOI:10.1212/WNL.96.15_supplement.4523]
Županić, S., Perić Šitum, M., Majdak, M., Karakaš, M., Bašić, S., & Sporiš, D. (2021). Case series of COVID-19 in patients with myasthenia gravis: A single institution experience. Acta Neurologica Belgica, 121(4), 1039–1044. [DOI:10.1007/s13760-021-01662-w] [PMID] [PMCID]
Type of Study: Review |
Subject:
Clinical Neuroscience
Received: 2022/08/10 | Accepted: 2023/10/21 | Published: 2024/03/1
Received: 2022/08/10 | Accepted: 2023/10/21 | Published: 2024/03/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





