Volume 15, Issue 2 (March & April 2024)
BCN 2024, 15(2): 261-272 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Jain A, Dhir N, Singh H, Sharma A R, Medhi B, Prakash A. Disrupting Maternal Behavior and Inducing Cannibalism Due to Valproic Acid: An Unexplored Insight. BCN 2024; 15 (2) :261-272
URL: http://bcn.iums.ac.ir/article-1-2503-en.html
URL: http://bcn.iums.ac.ir/article-1-2503-en.html
1- Experimental Pharmacology Laboratory, Neurobehavioral Research Laboratory, Department of Pharmacology, PGIMER, Chandigarh, India.
2- Department of Neurology, PGIMER, Chandigarh, India.
2- Department of Neurology, PGIMER, Chandigarh, India.
Full-Text [PDF 1739 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Various preclinical and clinical studies are required to understand the disease pathology of autism spectrum disorder (ASD), which leads to abnormal neuro-developmental manifestation in affected individuals, and discover new drugs to manage the symptoms (Al Dera & Pharmacotherapy, 2022; Gileadi et al., 2021; Jain et al., 2019; Rylaarsdam & Guemez-Gamboa, 2019; Wang & Doering, 2015; Won et al., 2013). The ASD model can be developed with different chemicals like propionic acid, 2,3,7,8-tetrachlorodibenzodioxin, chlorpyrifos, polyinosinic: Polycytidylic acid (poly I: C), and valproic acid (VPA) (Guo et al., 2018; Lan et al., 2017; MacFabe et al., 2007; Reisinger et al., 2015; Schneider & Przewłocki, 2005). Among these, VPA is predominantly used because it inhibits histone deacetylase, causing neural tube defects (Kawanai et al., 2016). VPA is an antiepileptic agent with varied adverse effects in the pregnant population (Alsdorf & Wyszynski, 2005). VPA causes behavioral abnormalities, delays neuronal development, and alters various gene expressions, leading to ASD (Kawanai et al., 2016). It becomes readily available in the in ine after VPA’s oral administration in pregnant women. It crosses the placental barrier from the apical (maternal interfacing) syncytiotrophoblast plasma membrane to the circulation of basal (fetal facing). As a result, VPA accumulates, and the concentration is three-fold in the embryonic circulation, resulting in teratogenic effects (Lloyd, 2013; Vajda, 2012). The teratogenic effects are cleft lip and palate, fetal valproate syndrome, genitourinary defects, autism, etc. (Alsdorf & Wyszynski, 2005). However, apart from its teratogenic effects, the effect of VPA on the mother has not been investigated yet (Lloyd, 2013).
VPA can disrupt maternal behavior during pregnancy or post-birth. VPA’s potential to cause autism in rats was first reported by Schneider et al. in 2005 (Schneider & Przewłocki, 2005). However, VPA’s mechanism for causing autism is unclear. Nevertheless, a study hypothesizes that VPA inhibits histone deacetylase post-administration in rodents (Kawanai et al., 2016). VPA causes teratogenic effects in rodents, such as teeth malformation, tail kink, delayed/abnormal growth, autism, etc. (Ruhela et al., 2017).
The current study was an observation during the development and validation of an experimental model of VPA in Wistar rats. Apart from teratogenicity, the behavioral alterations among the dams are still unexplored and under-reported in the VPA model. Cannibalism is a behavioral abnormality common among animals but more evident in laboratory rodents (Lane-Petter, 1968). Cannibalism is massacring and ingesting an organism’s specific part or whole (Fouilloux et al., 2019). Various reasons that could lead to cannibalistic activity among rodents are human handling, malnutrition, infant care, environmental conditions, noise decibels, home cage conditions, and so on (Lane-Petter, 1968).
Additionally, specific drugs administered during pregnancy can induce cannibalism post-birth (Schardein et al., 1978). Even though much research has focused on different types of autism models, it is necessary to report the VPA’s cannibalistic effects in rodent models. The effect of VPA in inducing maternal cannibalism has not yet been fully established. VPA can cause total loss of pregnancy/miscarriage in rodents. After all, the total number of pups cannibalized by dams is unreported. Hence, the observational study aims to estimate maternal cannibalistic behavior after VPA-induced ASD model development and validation in Wistar rats. Additionally, VPA’s potency to cause miscarriage (total/complete loss of pregnancy) in pregnant Wistar rats is also reported in this manuscript.
2. Materials and Methods
Animals and housing conditions
Male and non-pregnant female Wistar rats around 8-10 weeks of age, weighing 180 to 280 g, were used in this study. Animals (3-4 per cage) were randomly housed in individually ventilated propylene cages (30×18 cm) under a 12-hour light-dark cycle. The temperature and humidity were maintained at 21±2ºC and 55%±2%, respectively. The rats had access to food, water, and ad libitum throughout the experiment. The hygiene conditions of the animal vivarium were maintained according to the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines. The experiments were conducted in the experimental pharmacology laboratory, Neurobehavioral Research Laboratory, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India. All procedures were performed according to animal research: Reporting of in vivo experiments (ARRIVE) guidelines (Percie du Sert et al., 2020).
Acclimatization and mating
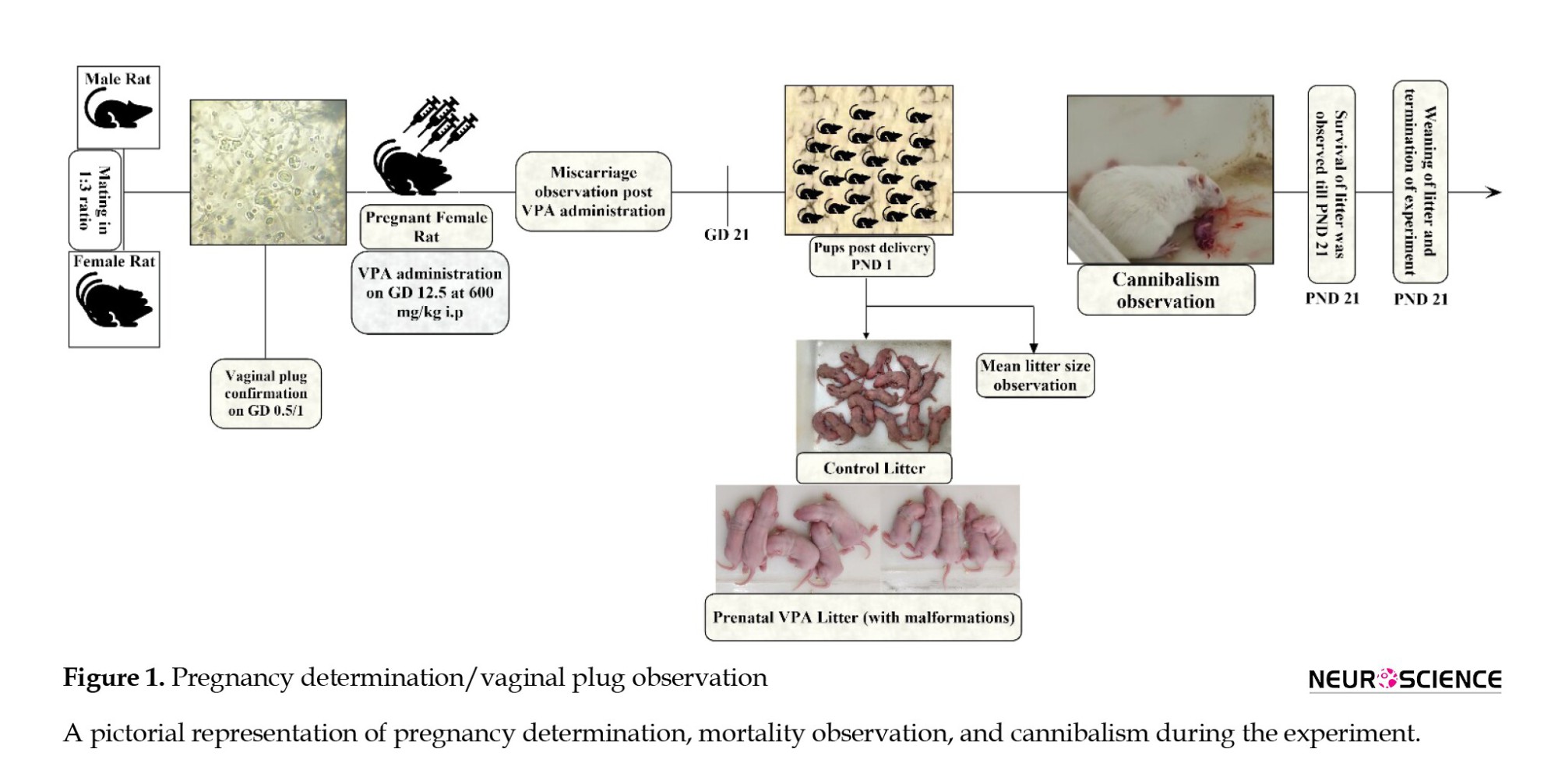
All animals were acclimatized for one week. All female rats (n=25) were observed for the estrus phase and allowed to mate in a 1:3 ratio (Figure 1).
Blinding and randomization
The female Wistar rats post-copulation were randomized into two groups (control & VPA). On gestation day (GD) 12.5, both groups received their respective injections (Figure 1). Blinded investigators performed the treatment, behavioral observation, and data analysis in all study procedures.
Pregnancy determination
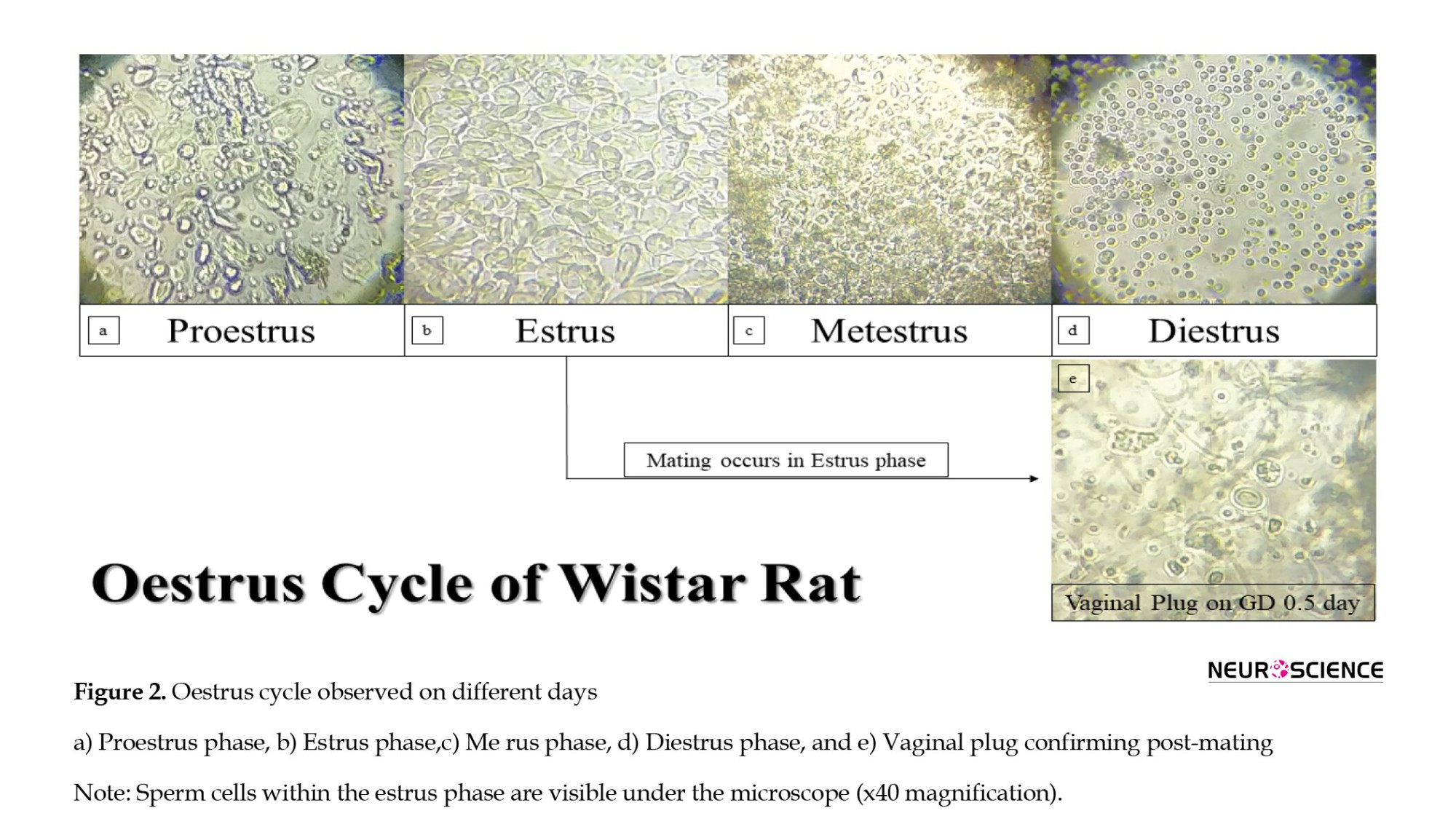
Initially, the female rat was handheld by an experimenter, and another individual using a 200-µL pipette drew inside-out in the female rat’s vagina about 60-100 µL of 0.9% saline. The vaginal fluid (vaginal smear) with 0.9% saline was placed on the glass slide and observed under the microscope at 40x magnification. If the smear depicted both nucleated epithelial and cornified cells (estrus phase) with spermatozoa (sperm cells), the pregnancy was confirmed as the gestational day (Elnahas et al., 2021) 0.5/1 day varied from animal to animal (Figures 1 and 2) (Marcondes et al., 2002).
VPA administration
Pregnant female Wistar rats (n=10) at GD 12.5 days received valproic acid sodium salt (CAS: 1069-66-5) at 600 mg/kg, intraperitoneally (IP) dissolved in 0.9% saline. The volume was prepared as 250 mg/mL. Similarly, control dams (n=10) received an equal volume of saline at GD 12.5 days (Ruhela et al., 2017). Administration and route of VPA (600 mg/kg, IP) was chosen as it has been shown to cause higher significance of ASD symptoms than other doses (300, 400, 500 mg/kg) (Chaliha et al., 2020). Five Wistar female rats were excluded from the cannibalism observation as they underwent miscarriage (complete loss of pregnancy) after VPA administration.
Weight observation
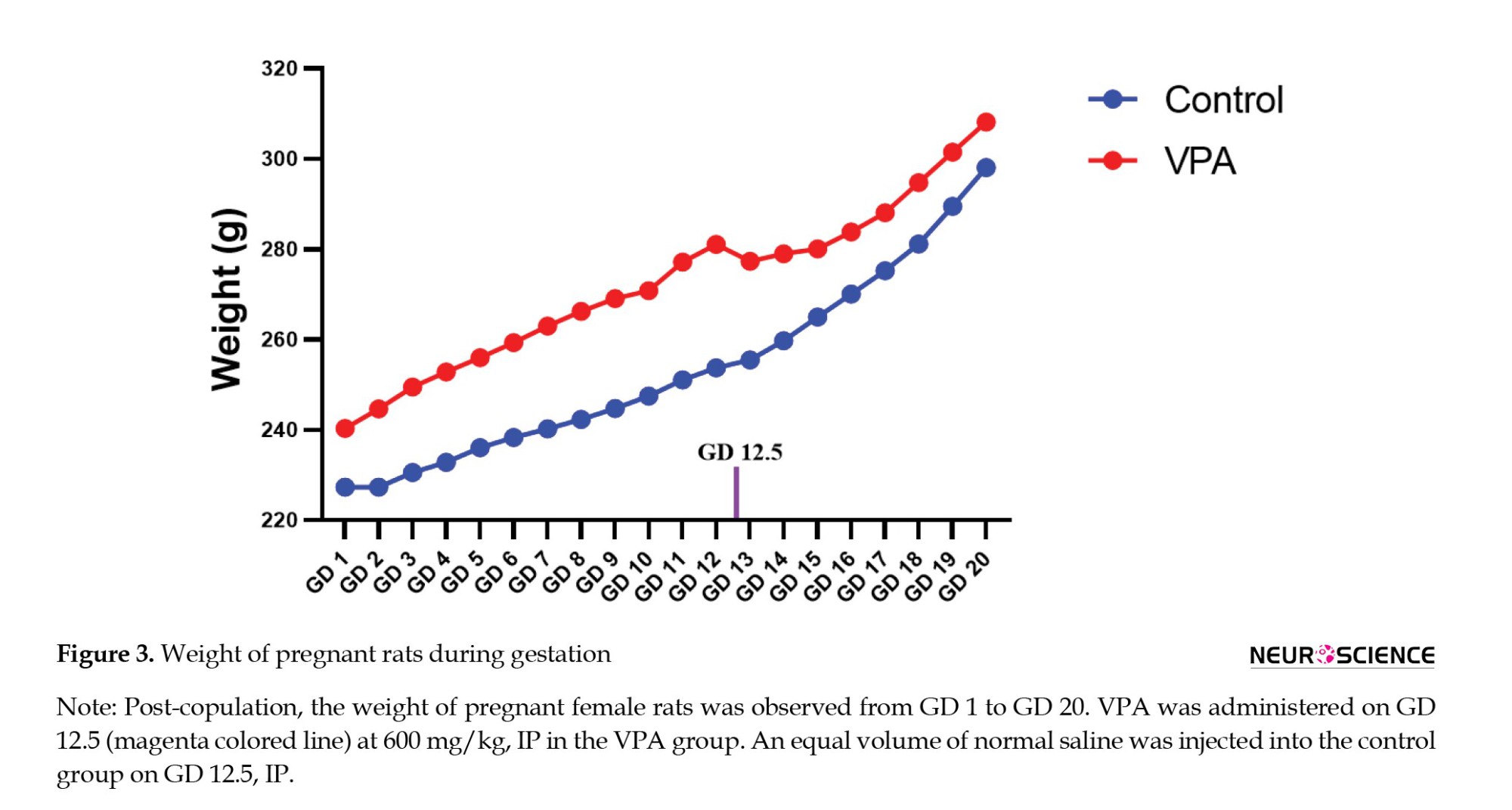
The weight of all pregnant female rats was observed from day 1 of copulation till day 20. Post-VPA administration rats with weight reduction were observed until GD day 20 for any delivery of offspring. If no pups were delivered, then they were excluded from the study.
Miscarriage observation
Miscarriage was considered when there was a complete loss of pregnancy. All females post-mating and post-VPA administration were examined for any miscarriage. Miscarriage was confirmed by substantial weight reduction and no pups delivery till GD 21-23. Furthermore, blind investigators held the animal post-VPA administration and palpated the rat’s abdomen to check for any pup’s sensation. The investigation was performed only in animals undergoing immediate weight loss post-VPA administration.
Total pups’ delivery observation
The number of pups delivered to each parental female rat was observed. All delivered pups were housed with their respective mothers, and the total count of male/female pups was also observed during the study.
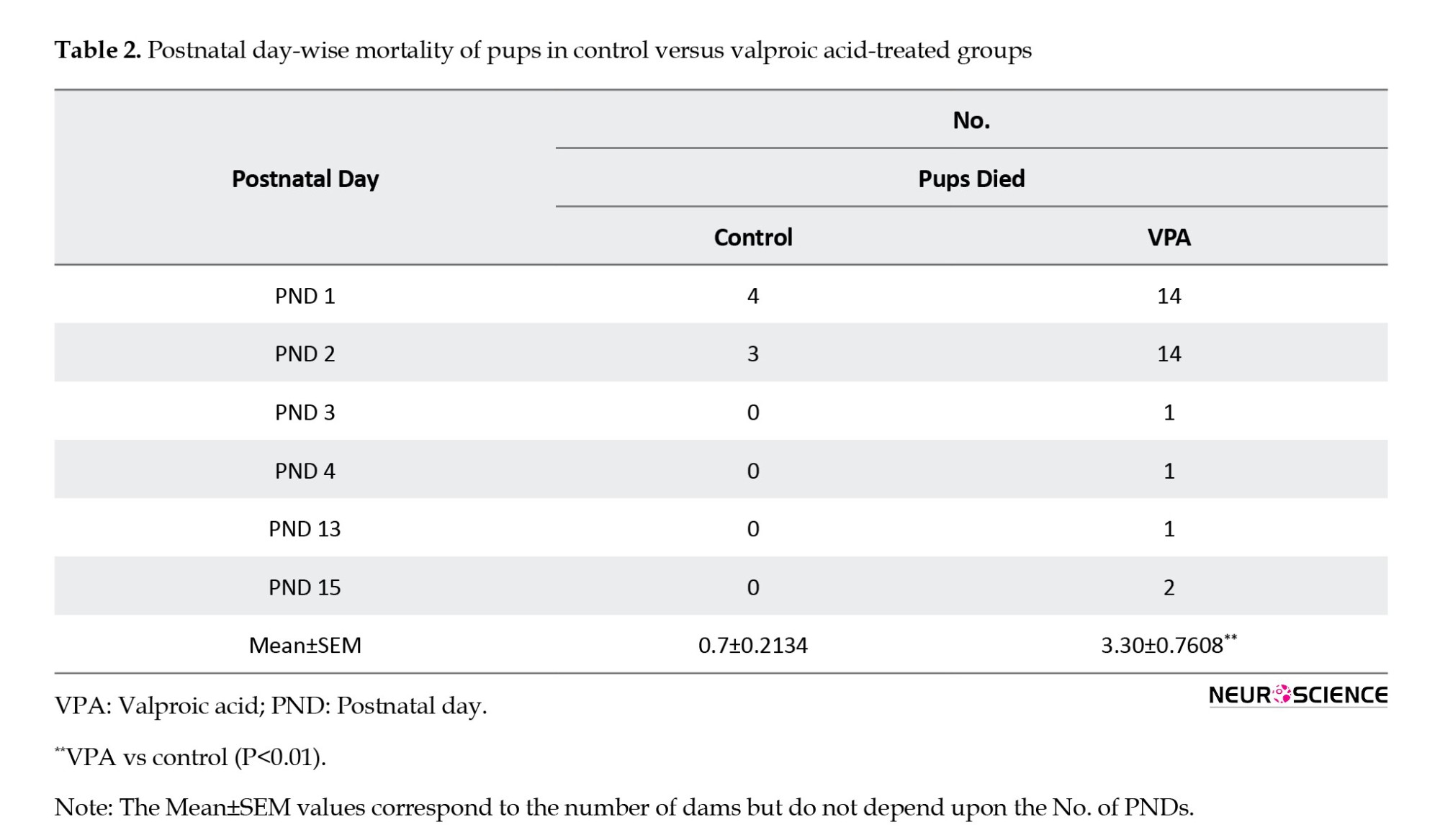
Mortality observation
All delivered pups were observed for mortality (death by cannibalism, death by birth, or death post-birth) until postnatal day (PND) 21.
Natural death
The death of all delivered pups, apart from maternal cannibalism, was considered a natural death (death on delivery/death post-delivery but not cannibalized).
Mortality percentage
The mortality (including cannibalism and natural death) percentage was calculated by Equation 1:
1. (Number of animals died/Total number of delivered pups)×100.
Cannibalism observation
Dams were observed for cannibalistic behavior until weaning (PND 21). During the late gestation period, we increased our visits (06:00 to 22.30 O’clock at every 15-minute interval) to the animal house (Central Unit for Animals in PGIMER) to observe the delivery, litter counts respective to each dam, male/female ratio, aggressive behavior (Davis, 1933), and maternal cannibalistic behavior. No video recordings were made during this study. We tried to minimize human handling during the delivery and post-delivery. Only one maternal cannibalization (VPA-treated) was recorded with a video camera, and the representative image is shown in this manuscript.
Cannibalism observation on malformed pups
Maternal cannibalism was observed depending on the pups’ malformation. Post-delivery malformation was immediately observed.
Timeline of the experiment
Figure 1 shows the experimental setup and timeline. Female rats were mated with male rats (1:3 ratio). After microscopic examination, the vaginal plug was confirmed as GD 0.5/1 day. On GD 12.5, VPA was administered to pregnant Wistar rats at 600 mg/kg IP, and control pregnant rats received an equal volume of saline. Until GD 21, miscarriage (complete pregnancy loss) was observed, and miscarriage females were excluded. The first day of the pups’ delivery was considered PND 1. A female rat cannibalizing its pup was video recorded with a smartphone. All study observations were carried out until weaning, and post-completion data analysis was performed.
Statistical analysis
GraphPad Prism software, version 9, was used for data analysis. Numerical data were expressed as the standard error of the mean (Mean±SEM/SEp). Student t or t with Welch’s correction was conducted for parametric observations to compare two groups/within-group means. The Mann-Whitney was conducted for non-parametric data. The two-sided Fisher exact, odds ratio, and relative risk were calculated for the pup’s death/cannibalization in both control and VPA groups.
Additionally, the Fisher exact was used for miscarriage analysis. The relative risk of 95% CI was calculated using the Koopman asymptomatic score, and the odds ratio of 95% CI was calculated using the Baptista-Pike method. Survival analysis was performed using the Mantel-Cox. The Kaplan-Meier curve depicted the results and calculated the day-wise mortality/cannibalization for both control and VPA groups. The standard P<0.05 was considered significant.
3. Results
Pregnancy determination/vaginal plug observation
All female rats were observed for the presence of sperm cells in the vagina. Figure 2 shows a) The proestrus phase, b) The estrus phase, c) The me rus phase, d) The diestrus phase, and e) The confirmation of sperm cells in the vaginal plug. Pregnancy can be confirmed only during the estrus phase (epithelial and cornified cells with spermatozoa – sperm cells), as shown in Figure 2e.
Weight observation
All females’ body weight post-copulation was monitored from GD 1 to GD 20 (Figure 3). No significant differences were observed between VPA and control groups.
Effect of VPA on miscarriage
We examined the effect of VPA on miscarriage from GD 1 to GD 21 by weight reduction. Prenatally injected VPA pregnant female (33.33%) rats were subjected to miscarriage. No miscarriage was observed in the control groups.
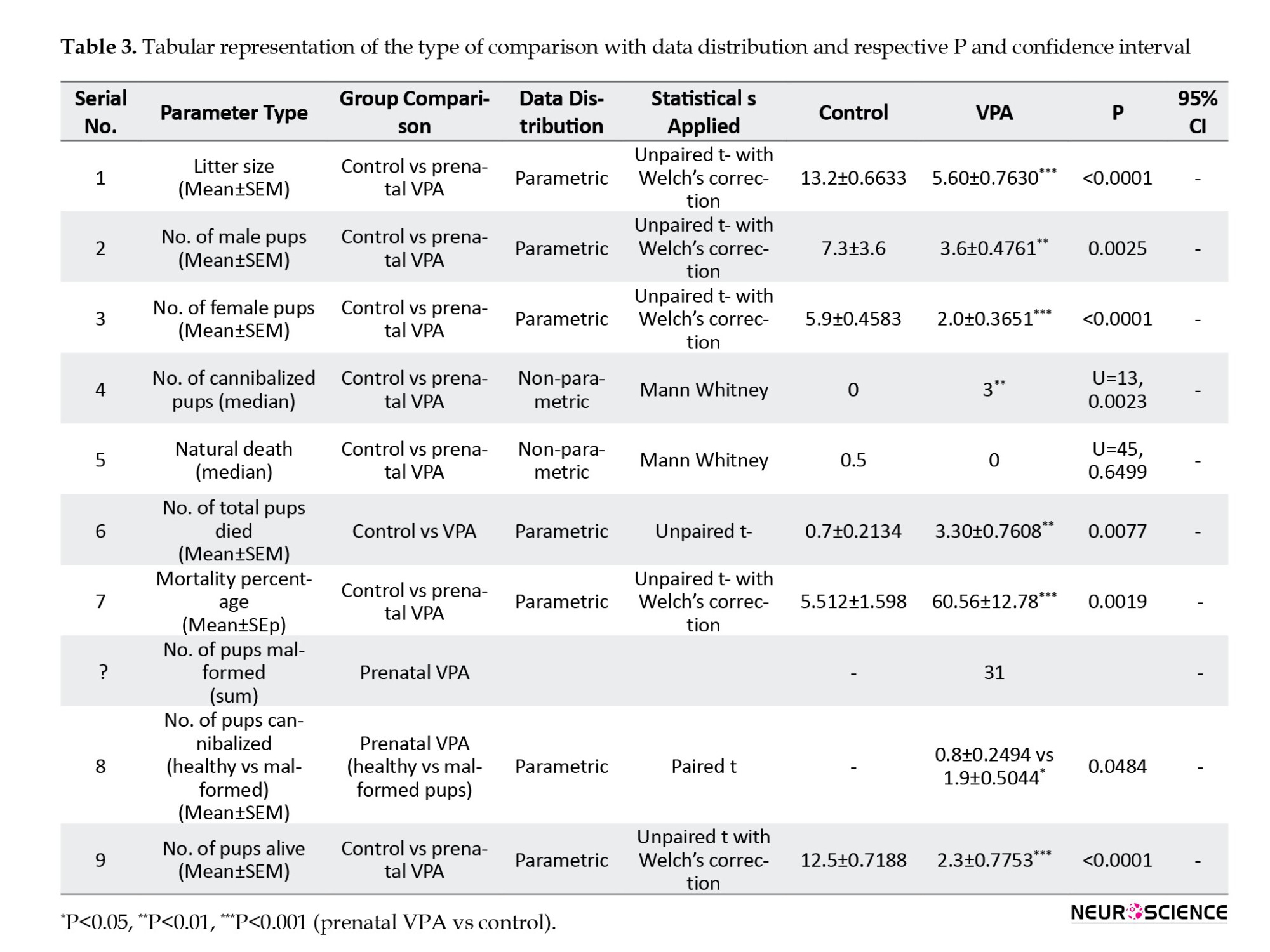
VPA-treated rats and reduced litter size
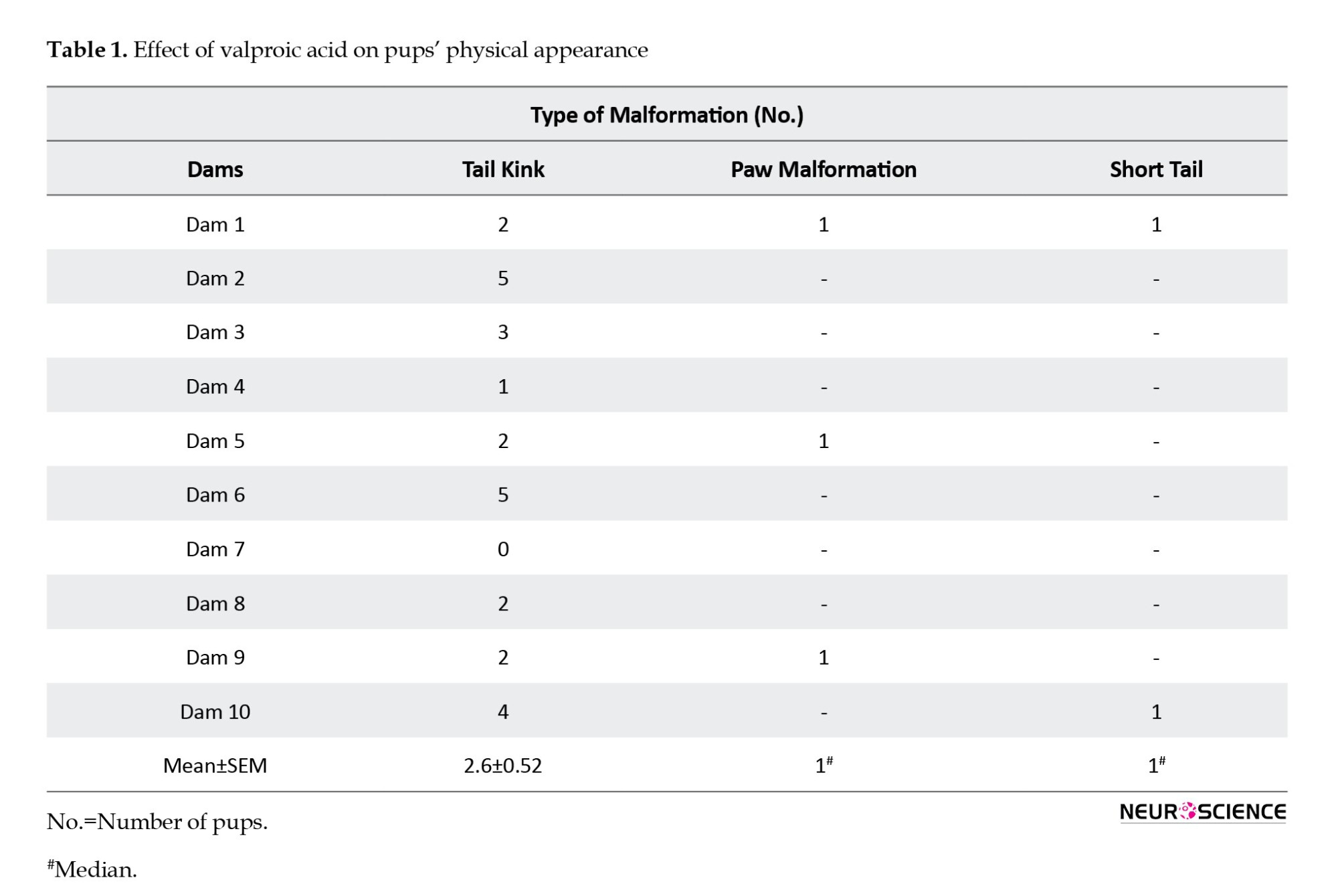
To observe the effect of VPA on litter size, the VPA-treated and control dams’ litter size was calculated. Table 1 indicates that VPA-treated female dams delivered reduced litter size (P<0.0001) compared to their female control counterparts, and the data were statistically significant. We also observed the birth rate of male and female pups in the control and prenatally VPA-treated pups. Table 1 indicates that VPA-treated dams delivered significantly reduced male (P=0.0025)/female pups (P<0.0001) compared to control dams.
Prenatal VPA-treated pups showing a substantial increase in mortality
We observed that the mortality rate significantly increased in the prenatally VPA-treated pups (P=0.0077) compared to control pups (Table 1). Moreover, no significant difference was observed in natural death among the prenatal VPA-treated and control pups. However, the mortality percentage increased significantly in the prenatally VPA-treated (P=0.0019) group compared to the control pups (Table 1).
VPA-treated maternal rats manifesting increased cannibalistic behavior
We examined the cannibalistic behavior under the influence of VPA postnatally. As illustrated in Figure 1 and Table 1, VPA-treated maternal rats exhibited significantly (P=0.0023) increased acts of cannibalism compared to the control females. Similarly, we examined the effect of maternal cannibalism in the VPA-treated group on healthy vs malformed pups. Table 1 indicates that malformed pups (P=0.0484) were subjected to significantly increased cannibalism by maternal females compared to healthy pups.
VPA depicting significant differences in alive pups
Prenatal VPA dams (P<0.0001) had significantly reduced lively pups (Table 1) compared to control.
VPA causing malformation in offspring
Prenatal exposure to VPA showed different malformations (tail kink, paw malformation, and short tail) post-birth (Table 1).
Survival analysis
Survival analysis showed that prenatal administration of VPA (P=0.0288) causes a decreased litter survival rate compared to control pups (Figure 4a, Tables 2 and 3).
The difference was found to be statistically significant. These results indicate that prenatal VPA decreases the survival rate of delivered pups either by cannibalism or by natural death.
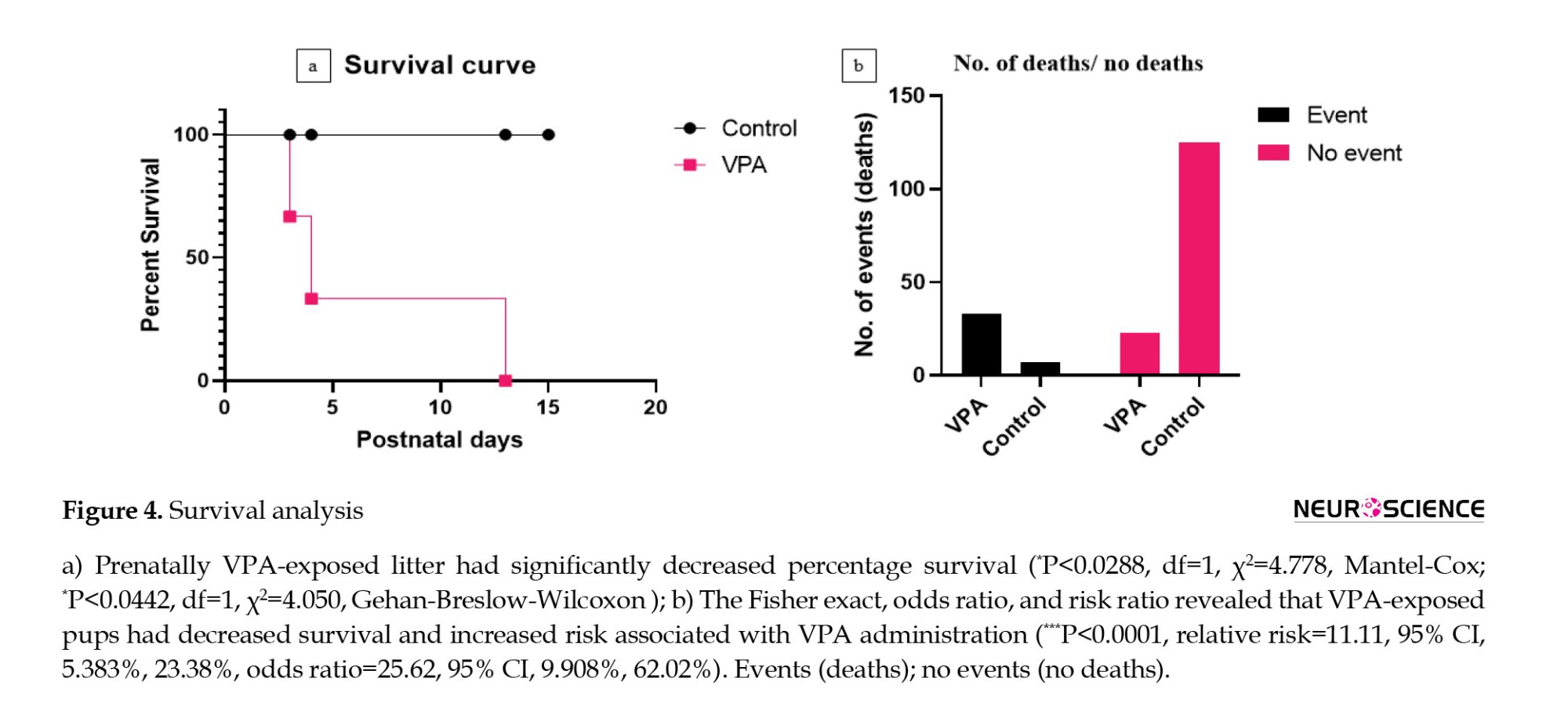
The risk associated with prenatal VPA administration
The outcome of events (death) was calculated. The Fisher exact revealed that the mortality associated with prenatal VPA pups was significant (P<0.0001) more than that of control pups (Figure 4b, Tables 2 and 3).
4. Discussion
The current study is the first to explore the effect of VPA on maternal cannibalism and investigate male/female and malformed litter cannibalism.
Different studies have reported rodent cannibalism, and some have reported it for possible reasons (Abel & Biology, 1979; Buntin et al., 1984). Additionally, Lane-Petter (1968) reported that cannibalism is more common in rats and mice. This same study stated that genetic factors might contribute to this cannibalistic behavior (Lane-Petter, 1968). Schardein et al., (1978) reported that maternal rats treated with a teratogen during gestation exhibited cannibalistic behavior. Moreover, these rats cannibalize malformed pups rather than normal pups (Schardein et al., 1978). Corresponding to this, Wyszynski et al. (2005) observed that human pregnant females exposed to valproate cause an increased risk of malformations in their offspring.
On the other hand, analogous to the previous study, Ruhela et al. (2017) observed anatomical malformations in prenatally VPA-administered Wistar rats. Compared to the above studies, our findings reported similar observations that female rats cannibalize malformed pups compared to healthy pups (Figure 1, Table 3). However, a study has reported that the chemical modification of VPA can prevent malformation in the murine model (Jazayeri et al., 2020). Okada et al. reported the teratogenic effects of VPA in clinical aspects and different preclinical models (Okada et al., 2004). Besides, no information was mentioned about cannibalism (Jazayeri et al., 2020).
Cannibalism can be caused due to the disruption in maternal behavior. Gaffori and Le Moal (1979) stated that thiamine induces disruption in normal maternal behavior and causes spontaneous abortion in pregnant female rats (Ba, 2013). Another study observed that ventral mesencephalic tegmentum lesions cause disruption in maternal behavior, which results in cannibalism. The study also observed that the mothers did not nurse their litter (Gaffori and Le Moal, 1979).
Additionally, Perez-Laso et al. (2008) investigated the effect of olfactory bulbectomy on maternal behavior disruption, which is relatable to previous findings (Schwartz et al., 1976). Apart from this, another study states that maternal behavior disruption can occur during gestation if female rats are exposed to extreme environmental stress conditions (Perez-Laso et al., 2008). Libbin and Person (1979) portrayed that cannibalism can be avoided by minimal human handling, limiting the change of beddings, and constantly acclimatizing the pregnant rats to human touch during the gestation period. Another study states that cannibalism could be prevented if maternal rats had not fasted and freely accessible food and water during gestation (Fox, 1975). However, our results were contradictory. Although we did not abstain female rats from food and water during gestation, it still resulted in cannibalism (Figure 1, Table 3). Serrano et al. (1991) explained the prevention of cannibalism by performing cesarean sectioning in pregnant rats on GD 21. Helander and Bergh (1980) investigated the prevention of cannibalism after neonatal surgery. They described that litter post-surgeries must be cleaned for blood spots or clots and sutured off open wounds, which prevented cannibalism successfully.
Mohan (1974) observed the effect of starvation on age-dependent cannibalism at Bangalore University. His findings revealed that at 3-3.9 months, female rats delivered a mean litter size (Mean±SD 11.3±1.40) (Mohan, 1974). However, our mean litter size (Mean±SEM 16.50±0.7782) was significantly higher as there was no starvation (Table 3). Nevertheless, we observed lower cannibalism in our control group, contrasting the above study. The probable reason for lower cannibalism is that no VPA was administered in the control rats. Mennella & Moltz (1988) have observed infanticide in rats. They stated that females synthesize low-volatility chemo-signal during pregnancy, protecting males from cannibalizing the delivered pups (Mennella & Moltz, 1988; Helander and Bergh, 1980). Another study noted that infanticide could be due to genetic, developmental, and hormonal imbalances (Svare et al., 1981). Discordant to the above study, we separated males and females post-copulation, so we did not observe any of these findings.
Porter (1968) reported a high chance of pre-weaning loss in laboratory animals. They observed the benefits of separating the mother and its litter or placing individual mothers in a separate cage, which resulted in preventing cannibalism. Supporting this study, Darlene et al. observed that the pre-weaning loss was around 33%, depending upon the intervention. However, they also observed that their mothers provided survival pups with good care and nursing (DeSantis & Schmaltz, 1984). Our findings were similar to those of the above study; the survived pups were provided adequate care and nursing regardless of malformation or healthy pups in control and prenatally VPA-treated groups (Table 2 – number of alive pups).
Komariah et al. observed the teratogenic effect of VPA (250 mg/kg per oral) (Abel & Biology) on GD 10, 13, and 16 in Sprague Dawley rats. They reported that administering VPA on GD 10 and 13 showed decreased mean litter size compared to GD 16. In addition, they also observed birth weight, body weight, and growth rate, and their data were statistically significant. Another study examined the effects of VPA at different doses at 0, 150, 200, 300, 400, and 600 mg/kg per oral on GD 7-18. This study concluded that 600 mg/kg was significantly toxic and resulted in pups’ mortality (Vorhees, 1987). Our study had different observations as the route of administration was IP on GD 12.5 at a 600 mg/kg dose. Our study reported reduced litter size and a higher incidence of mortality in the prenatally VPA-treated group (Table 3).
To the best of our knowledge, no study has dug deep into concepts of cannibalism associated with VPA exposure in preclinical aspects. The study is the first to report the potential of VPA to cause maternal behavior disruption, thereby causing aggressive behavior toward cage-mates and their litter. Finally, miscarriage and cannibalism are significant drawbacks in the preclinical model. We have reported the litter size, VPA effect on mortality, maternal cannibalistic behavior, average pups’ survival, the difference among maternal cannibalistic behavior on malformed vs healthy pups, amount of miscarriage, and survival analysis of pups. In addition, we reported the odds and risk ratios and percentage of deaths associated with VPA post-pregnancy.
Translational outcome
Valproic acid has been a significant concern for pregnant females resistant to other antiepileptic drugs. The current study outcome highlights that VPA induces maternal behavior disruption in rodents during gestation. Similarly, few case reports depict patients’ abnormal behavior/irritability/agitation due to valproic acid administration. Thus, VPA should be the least preferred drug or not be used in pregnant females suffering from seizures as it might be responsible for causing aggressive behavior in such cases. However, the current study is a pilot and needs to be explored in a large sample size.
5. Conclusion
To conclude, valproic acid at a dose of 600 mg/kg IP on GD 12.5 has a marked cannibalistic effect on pregnant rat dams’ post-parturition. In addition, VPA significantly reduced the litter size, increased cannibalism of malformed pups, decreased pups’ survival rate, and increased the chances of miscarriage upon administration. The mechanism behind cannibalism could be the disruption of maternal behavior. The cause might be histone deacetylase inhibition, resulting in downregulation of the NFκB signaling pathway. Besides, serotonin and gamma-aminobutyric acid have played a significant role in the aggressive behavior of the female rats. The rationale behind maternal behavior disruption and cannibalism is yet to be explored.
Moreover, the relationship between cannibalism and maternal behavior disruption is still an enigma. The current study raises some queries that could be explored shortly. Is the VPA preclinical model a gold standard model for ASD? Does it possess face validity, construct validity, and predictive validity? The VPA model is the most widely used model for ASD. Still, many insights have not been scrutinised as many observations have been unreported.
Study limitations
Some studies have reported the role of neurotransmitters (serotonin, dopamine, gamma-aminobutyric acid) in male/female behavior disruption or aggressive behavior associated with neurotransmitters. However, we are currently delineating the levels of different neurotransmitters in the maternal rat brain on molecular aspects. It may provide novel insights behind cannibalism.
Ethical Considerations
Compliance with ethical guidelines
All animals in this study received proper care in compliance with ARRIVE guidelines. The Institutional Ethics Committee (IAEC), PGIMER, Chandigarh, India, approved all experiments (IAEC NO.: 106/IAEC/727, dated: 4/02/2020). The Central Small Animal Research Facility, PGIMER, Chandigarh, provided the animals used in this study (approved by CPCSEA – Reg. No.: 47/GO/Re-SL/Bi-S/99/CPCSEA, Dated: 05/31/2016).
Funding
Indian Council of Medical Research (ICMR-SRF) provided a Senior Research Fellowship (SRF) (letter No: 45/45/2019-PHA/BMS, dated 07/23/2019.
Authors' contributions
Conceptualization: Ashish Jain and Ajay Prakash; Data extraction: Ashish Jain; Validation: Ashish Jain and Ajay Prakash; Analysis: Ashish Jain, Neha Dhir, Harvinder Singh, Bikash Medhi and Ajay Prakash; Writing the initial draft: Ashish Jain; Review, editing and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors acknowledge the Indian Council of Medical Research (ICMR) for providing manpower (Ashish Jain). The authors acknowledge the Postgraduate Institute of Medical Education and Research (PGIMER) for providing infrastructures and laboratory facilities within the institute.
References
Abel E. L. (1979). Effects of alcohol withdrawal and undernutrition of cannibalism of rat pups. Behavioral and Neural Biology, 25(3), 411–413. [DOI:10.1016/S0163-1047(79)90492-8] [PMID]
Al Dera H. (2022). Cellular and molecular mechanisms underlying autism spectrum disorders and associated comorbidities: A pathophysiological review. Biomedicine & Pharmacotherapy, 148, 112688. [DOI:10.1016/j.biopha.2022.112688] [PMID]
Alsdorf, R., & Wyszynski, D. F. (2005). Teratogenicity of sodium valproate. Expert Opinion on Drug Safety, 4(2), 345–353. [DOI:10.1517/14740338.4.2.345] [PMID]
Bâ A. (2013). Perinatal thiamine deficiency-induced spontaneous abortion and pup-killing responses in rat dams. Nutritional Neuroscience, 16(2), 69–77. [DOI:10.1179/1476830512Y.0000000032] [PMID]
Buntin, J. D., Jaffe, S., & Lisk, R. D. (1984). Changes in responsiveness to newborn pups in pregnant, nulliparous golden hamsters. Physiology & Behavior, 32(3), 437–439. [DOI:10.1016/0031-9384(84)90259-2] [PMID]
Chaliha, D., Albrecht, M., Vaccarezza, M., Takechi, R., Lam, V., & Al-Salami, H., et al. (2020). A systematic review of the valproic-acid-induced rodent model of autism. Developmental Neuroscience, 42(1), 12–48. [DOI:10.1159/000509109] [PMID]
Davis, F. C. (1933). The measurement of aggressive behavior in laboratory rats. The Pedagogical Seminary and Journal of Genetic Psychology, 43(1), 213-217. [DOI:10.1080/08856559.1933.10533127]
DeSantis, D. T., & Schmaltz, L. W. (1984). The mother-litter relationship in developmental rat studies: Cannibalism vs caring. Developmental Psychobiology, 17(3), 255–262. [DOI:10.1002/dev.420170306] [PMID]
Elnahas, E. M., Abuelezz, S. A., Mohamad, M. I., Nabil, M. M., Abdelraouf, S. M., & Bahaa, N., et al. (2021). Validation of prenatal versus postnatal valproic acid rat models of autism: A behavioral and neurobiological study. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 108, 110185. [DOI:10.1016/j.pnpbp.2020.110185] [PMID]
Fouilloux, C., Ringler, E., & Rojas, B. (2019). Cannibalism. Current Biology, 29(24), R1295-r1297. [DOI:10.1016/j.cub.2019.09.068] [PMID]
Fox, L. R. (1975). Cannibalism in natural populations. Annual Reviews, 6(1), 87-106. [DOI:10.1146/annurev.es.06.110175.000511]
Gaffori, O., & Le Moal, M. (1979). Disruption of maternal behavior and appearance of cannibalism after ventral mesencephalic tegmentum lesions. Physiology & Behavior, 23(2), 317–323. [DOI:10.1016/0031-9384(79)90373-1] [PMID]
Gileadi, T. E., Swamy, A. K., Hore, Z., Horswell, S., Ellegood, J., & Mohan, C., et al. (2021). Effects of low-dose gestational TCDD exposure on behavior and on hippocampal neuron morphology and gene expression in mice. Environmental Health Perspectives, 129(5), 57002. [DOI:10.1289/EHP7352] [PMID] [PMCID]
Guo, Z., Xie, H. Q., Zhang, P., Luo, Y., Xu, T., & Liu, Y., et al. (2018). Dioxins as potential risk factors for autism spectrum disorder. Environment International, 121(Pt 1), 906–915. [DOI:10.1016/j.envint.2018.10.028] [PMID]
Helander, H. F., & Bergh, A. (1980). How to avoid maternal cannibalism after neonatal surgery in rats. Experientia, 36(11), 1295–1296. [DOI:10.1007/BF01969597] [PMID]
Jain, R. A., Prakash, A., & Medhi, B. (2019). Newer potential pharmacological targets for autism spectrum disorder. Indian Journal of Pharmacology, 51(4), 284–286. [DOI:10.4103/ijp.IJP_518_19] [PMID] [PMCID]
Jazayeri, D., Braine, E., McDonald, S., Dworkin, S., Powell, K. L., & Griggs, K., et al. (2020). A rat model of valproate teratogenicity from chronic oral treatment during pregnancy. Epilepsia, 61(6), 1291–1300. [DOI:10.1111/epi.16536] [PMID]
Kawanai, T., Ago, Y., Watanabe, R., Inoue, A., Taruta, A., & Onaka, Y., et al. (2016). Prenatal exposure to histone deacetylase inhibitors affects gene expression of autism-related molecules and delays neuronal maturation. Neurochemical Research, 41(10), 2574–2584. [DOI:10.1007/s11064-016-1969-y] [PMID]
Komariah, K., Kiranadi, B., Winarto, A., Manalu, W., & Handharyani, E. Valproic acid administration in pregnant rats affects litter size, birth weight, and postnatal growth of the offspring. International Journal of Sciences, 33(3), 174-186. [Link]
Lan, A., Kalimian, M., Amram, B., & Kofman, O. (2017). Prenatal chlorpyrifos leads to autism-like deficits in C57Bl6/J mice. Environmental Health, 16(1), 43. [DOI:10.1186/s12940-017-0251-3] [PMID] [PMCID]
Lane-Petter, W. (1968). Parental injuries to offspring: Cannibalism in rats and mice. Journal of the Royal Society of Medicine, 61(12), 1295-1296. [DOI:10.1177/003591576806101228]
Libbin, R. M., & Person, P. (1979). Neonatal rat surgery: Avoiding maternal cannibalism. Science, 206(4414), 66. [DOI:10.1126/science.482926] [PMID]
Lloyd, K. A. (2013). A scientific review: Mechanisms of valproate-mediated teratogenesis. Bioscience Horizons, 6, hzt003. [DOI:10.1093/biohorizons/hzt003]
MacFabe, D. F., Cain, D. P., Rodriguez-Capote, K., Franklin, A. E., Hoffman, J. E., & Boon, F., et al. (2007). Neurobiological effects of intraventricular propionic acid in rats: Possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behavioural Brain Research, 176(1), 149–169. [DOI:10.1016/j.bbr.2006.07.025] [PMID]
Marcondes, F. K., Bianchi, F. J., & Tanno, A. P. (2002). Determination of the estrous cycle phases of rats: Some helpful considerations. Brazilian Journal of Biology, 62(4A), 609–614. [DOI:10.1590/s1519-69842002000400008] [PMID]
Mennella JA, Moltz H. (1988). Infanticide in rats: Male strategy and female counter-strategy. Physiology & Behavior. 42(1):19-28. [DOI: 10.1016/0031-9384(88)90254-5]
Mohan C. (1974). Age-dependent cannibalism in a colony of albino rats. Laboratory Animals, 8(1), 83–84. [DOI:10.1258/002367774780943869] [PMID]
Okada, A., Kurihara, H., Aoki, Y., Bialer, M., & Fujiwara, M. (2004). Amidic modification of valproic acid reduces skeletal teratogenicity in mice. Birth defects research. Part B, Developmental and Reproductive Toxicology, 71(1), 47–53. [DOI:10.1002/bdrb.10057] [PMID]
Percie du Sert, N., Hurst, V., Ahluwalia, A., Alam, S., Avey, M. T., & Baker, M., et al. (2020). The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Journal of Cerebral Blood Flow and Metabolism, 40(9), 1769–1777. [DOI:10.1177/0271678X20943823] [PMID] [PMCID]
Pérez-Laso, C., Segovia, S., Martín, J. L., Ortega, E., Gómez, F., & Del Cerro, M. C. (2008). Environmental prenatal stress alters sexual dimorphism of maternal behavior in rats. Behavioural Brain Research, 187(2), 284–288. [DOI:10.1016/j.bbr.2007.09.029] [PMID]
Porter, G. (1968). Parental injuries to offspring: Pre-weaning loss of laboratory animals. SAGE Journal, 61(12), 1255-1334. [DOI:10.1177/003591576806101227]
Reisinger, S., Khan, D., Kong, E., Berger, A., Pollak, A., & Pollak, D. D. (2015). The poly(I:C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacology & Therapeutics, 149, 213–226. [DOI:10.1016/j.pharmthera.2015.01.001] [PMID]
Ruhela, R. K., Sarma, P., Soni, S., Prakash, A., & Medhi, B. (2017). Congenital malformation and autism spectrum disorder: Insight from a rat model of autism spectrum disorder. Indian Journal of Pharmacology, 49(3), 243–249. [DOI:10.4103/ijp.IJP_183_17] [PMID] [PMCID]
Rylaarsdam, L., & Guemez-Gamboa, A. (2019). Genetic causes and modifiers of autism spectrum disorder. Frontiers in Cellular Neuroscience, 13, 385. [DOI:10.3389/fncel.2019.00385] [PMID] [PMCID]
Schardein, J. L., Petrere, J. A., Hentz, D. L., Camp, R. D., & Kurtz, S. M. (1978). Cannibalistic traits observed in rats treated with a teratogen. Laboratory Animals, 12(2), 81–83. [DOI:10.1258/002367778780953080] [PMID]
Schardein, J. L., Petrere, J. A., Hentz, D. L., Camp, R. D., & Kurtz, S. M. (1978). Cannibalistic traits observed in rats treated with a teratogen. Laboratory Animals, 12(2), 81–83. [DOI:10.1258/002367778780953080] [PMID]
Schneider, T., & Przewłocki, R. (2005). Behavioral alterations in rats prenatally exposed to valproic acid: Animal model of autism. Neuropsychopharmacology, 30(1), 80–89. [DOI:10.1038/sj.npp.1300518] [PMID]
Schwartz, E., & Rowe, F. A. (1976). Olfactory bulbectomy: Influences on maternal behavior in primiparous and multiparous rats. Physiology & Behavior, 17(6), 879–883. [DOI:10.1016/0031-9384(76)90002-0] [PMID]
Serrano, J., Esahli, H., Larsson, L., & Zetterström, R. (1991). Experimental intestinal obstruction in rats. Studies on structure and disaccharidase activities. European Journal of Pediatric Surger, 1(2), 92–96. [DOI:10.1055/s-2008-1042467] [PMID]
Svare, B., & Mann, M. (1981). Infanticide: Genetic, developmental and hormonal influences in mice. Physiology & Behavior, 27(5), 921–927. [DOI:10.1016/0031-9384(81)90062-7] [PMID]
Vajda F. (2012). Dose issues in antiepileptic therapy. Journal of Clinical Neuroscience, 19(11), 1475–1477. [DOI:10.1016/j.jocn.2012.05.003] [PMID]
Vorhees C. V. (1987). Teratogenicity and developmental toxicity of valproic acid in rats. Teratology, 35(2), 195–202. [DOI:10.1002/tera.1420350205] [PMID]
Wang, H., & Doering, L. C. (2015). Autism spectrum disorders: Emerging mechanisms and mechanism-based treatment. Frontiers in Cellular Neuroscience, 9, 183. [DOI:10.3389/fncel.2015.00183] [PMID] [PMCID]
Won, H., Mah, W., & Kim, E. (2013). Autism spectrum disorder causes, mechanisms, and treatments: Focus on neuronal synapses. Frontiers in Molecular Neuroscience, 6, 19. [DOI:10.3389/fnmol.2013.00019] [PMID] [PMCID]
Wyszynski, D. F., Nambisan, M., Surve, T., Alsdorf, R. M., Smith, C. R., & Holmes, L. B., et al. (2005). Increased rate of major malformations in offspring exposed to valproate during pregnancy. Neurology, 64(6), 961–965. [DOI:10.1212/01.WNL.0000154516.43630.C5] [PMID]
Various preclinical and clinical studies are required to understand the disease pathology of autism spectrum disorder (ASD), which leads to abnormal neuro-developmental manifestation in affected individuals, and discover new drugs to manage the symptoms (Al Dera & Pharmacotherapy, 2022; Gileadi et al., 2021; Jain et al., 2019; Rylaarsdam & Guemez-Gamboa, 2019; Wang & Doering, 2015; Won et al., 2013). The ASD model can be developed with different chemicals like propionic acid, 2,3,7,8-tetrachlorodibenzodioxin, chlorpyrifos, polyinosinic: Polycytidylic acid (poly I: C), and valproic acid (VPA) (Guo et al., 2018; Lan et al., 2017; MacFabe et al., 2007; Reisinger et al., 2015; Schneider & Przewłocki, 2005). Among these, VPA is predominantly used because it inhibits histone deacetylase, causing neural tube defects (Kawanai et al., 2016). VPA is an antiepileptic agent with varied adverse effects in the pregnant population (Alsdorf & Wyszynski, 2005). VPA causes behavioral abnormalities, delays neuronal development, and alters various gene expressions, leading to ASD (Kawanai et al., 2016). It becomes readily available in the in ine after VPA’s oral administration in pregnant women. It crosses the placental barrier from the apical (maternal interfacing) syncytiotrophoblast plasma membrane to the circulation of basal (fetal facing). As a result, VPA accumulates, and the concentration is three-fold in the embryonic circulation, resulting in teratogenic effects (Lloyd, 2013; Vajda, 2012). The teratogenic effects are cleft lip and palate, fetal valproate syndrome, genitourinary defects, autism, etc. (Alsdorf & Wyszynski, 2005). However, apart from its teratogenic effects, the effect of VPA on the mother has not been investigated yet (Lloyd, 2013).
VPA can disrupt maternal behavior during pregnancy or post-birth. VPA’s potential to cause autism in rats was first reported by Schneider et al. in 2005 (Schneider & Przewłocki, 2005). However, VPA’s mechanism for causing autism is unclear. Nevertheless, a study hypothesizes that VPA inhibits histone deacetylase post-administration in rodents (Kawanai et al., 2016). VPA causes teratogenic effects in rodents, such as teeth malformation, tail kink, delayed/abnormal growth, autism, etc. (Ruhela et al., 2017).
The current study was an observation during the development and validation of an experimental model of VPA in Wistar rats. Apart from teratogenicity, the behavioral alterations among the dams are still unexplored and under-reported in the VPA model. Cannibalism is a behavioral abnormality common among animals but more evident in laboratory rodents (Lane-Petter, 1968). Cannibalism is massacring and ingesting an organism’s specific part or whole (Fouilloux et al., 2019). Various reasons that could lead to cannibalistic activity among rodents are human handling, malnutrition, infant care, environmental conditions, noise decibels, home cage conditions, and so on (Lane-Petter, 1968).
Additionally, specific drugs administered during pregnancy can induce cannibalism post-birth (Schardein et al., 1978). Even though much research has focused on different types of autism models, it is necessary to report the VPA’s cannibalistic effects in rodent models. The effect of VPA in inducing maternal cannibalism has not yet been fully established. VPA can cause total loss of pregnancy/miscarriage in rodents. After all, the total number of pups cannibalized by dams is unreported. Hence, the observational study aims to estimate maternal cannibalistic behavior after VPA-induced ASD model development and validation in Wistar rats. Additionally, VPA’s potency to cause miscarriage (total/complete loss of pregnancy) in pregnant Wistar rats is also reported in this manuscript.
2. Materials and Methods
Animals and housing conditions
Male and non-pregnant female Wistar rats around 8-10 weeks of age, weighing 180 to 280 g, were used in this study. Animals (3-4 per cage) were randomly housed in individually ventilated propylene cages (30×18 cm) under a 12-hour light-dark cycle. The temperature and humidity were maintained at 21±2ºC and 55%±2%, respectively. The rats had access to food, water, and ad libitum throughout the experiment. The hygiene conditions of the animal vivarium were maintained according to the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines. The experiments were conducted in the experimental pharmacology laboratory, Neurobehavioral Research Laboratory, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India. All procedures were performed according to animal research: Reporting of in vivo experiments (ARRIVE) guidelines (Percie du Sert et al., 2020).
Acclimatization and mating
All animals were acclimatized for one week. All female rats (n=25) were observed for the estrus phase and allowed to mate in a 1:3 ratio (Figure 1).

Blinding and randomization
The female Wistar rats post-copulation were randomized into two groups (control & VPA). On gestation day (GD) 12.5, both groups received their respective injections (Figure 1). Blinded investigators performed the treatment, behavioral observation, and data analysis in all study procedures.
Pregnancy determination
Initially, the female rat was handheld by an experimenter, and another individual using a 200-µL pipette drew inside-out in the female rat’s vagina about 60-100 µL of 0.9% saline. The vaginal fluid (vaginal smear) with 0.9% saline was placed on the glass slide and observed under the microscope at 40x magnification. If the smear depicted both nucleated epithelial and cornified cells (estrus phase) with spermatozoa (sperm cells), the pregnancy was confirmed as the gestational day (Elnahas et al., 2021) 0.5/1 day varied from animal to animal (Figures 1 and 2) (Marcondes et al., 2002).

VPA administration
Pregnant female Wistar rats (n=10) at GD 12.5 days received valproic acid sodium salt (CAS: 1069-66-5) at 600 mg/kg, intraperitoneally (IP) dissolved in 0.9% saline. The volume was prepared as 250 mg/mL. Similarly, control dams (n=10) received an equal volume of saline at GD 12.5 days (Ruhela et al., 2017). Administration and route of VPA (600 mg/kg, IP) was chosen as it has been shown to cause higher significance of ASD symptoms than other doses (300, 400, 500 mg/kg) (Chaliha et al., 2020). Five Wistar female rats were excluded from the cannibalism observation as they underwent miscarriage (complete loss of pregnancy) after VPA administration.
Weight observation
The weight of all pregnant female rats was observed from day 1 of copulation till day 20. Post-VPA administration rats with weight reduction were observed until GD day 20 for any delivery of offspring. If no pups were delivered, then they were excluded from the study.
Miscarriage observation
Miscarriage was considered when there was a complete loss of pregnancy. All females post-mating and post-VPA administration were examined for any miscarriage. Miscarriage was confirmed by substantial weight reduction and no pups delivery till GD 21-23. Furthermore, blind investigators held the animal post-VPA administration and palpated the rat’s abdomen to check for any pup’s sensation. The investigation was performed only in animals undergoing immediate weight loss post-VPA administration.
Total pups’ delivery observation
The number of pups delivered to each parental female rat was observed. All delivered pups were housed with their respective mothers, and the total count of male/female pups was also observed during the study.
Mortality observation
All delivered pups were observed for mortality (death by cannibalism, death by birth, or death post-birth) until postnatal day (PND) 21.
Natural death
The death of all delivered pups, apart from maternal cannibalism, was considered a natural death (death on delivery/death post-delivery but not cannibalized).
Mortality percentage
The mortality (including cannibalism and natural death) percentage was calculated by Equation 1:
1. (Number of animals died/Total number of delivered pups)×100.
Cannibalism observation
Dams were observed for cannibalistic behavior until weaning (PND 21). During the late gestation period, we increased our visits (06:00 to 22.30 O’clock at every 15-minute interval) to the animal house (Central Unit for Animals in PGIMER) to observe the delivery, litter counts respective to each dam, male/female ratio, aggressive behavior (Davis, 1933), and maternal cannibalistic behavior. No video recordings were made during this study. We tried to minimize human handling during the delivery and post-delivery. Only one maternal cannibalization (VPA-treated) was recorded with a video camera, and the representative image is shown in this manuscript.
Cannibalism observation on malformed pups
Maternal cannibalism was observed depending on the pups’ malformation. Post-delivery malformation was immediately observed.
Timeline of the experiment
Figure 1 shows the experimental setup and timeline. Female rats were mated with male rats (1:3 ratio). After microscopic examination, the vaginal plug was confirmed as GD 0.5/1 day. On GD 12.5, VPA was administered to pregnant Wistar rats at 600 mg/kg IP, and control pregnant rats received an equal volume of saline. Until GD 21, miscarriage (complete pregnancy loss) was observed, and miscarriage females were excluded. The first day of the pups’ delivery was considered PND 1. A female rat cannibalizing its pup was video recorded with a smartphone. All study observations were carried out until weaning, and post-completion data analysis was performed.
Statistical analysis
GraphPad Prism software, version 9, was used for data analysis. Numerical data were expressed as the standard error of the mean (Mean±SEM/SEp). Student t or t with Welch’s correction was conducted for parametric observations to compare two groups/within-group means. The Mann-Whitney was conducted for non-parametric data. The two-sided Fisher exact, odds ratio, and relative risk were calculated for the pup’s death/cannibalization in both control and VPA groups.
Additionally, the Fisher exact was used for miscarriage analysis. The relative risk of 95% CI was calculated using the Koopman asymptomatic score, and the odds ratio of 95% CI was calculated using the Baptista-Pike method. Survival analysis was performed using the Mantel-Cox. The Kaplan-Meier curve depicted the results and calculated the day-wise mortality/cannibalization for both control and VPA groups. The standard P<0.05 was considered significant.
3. Results
Pregnancy determination/vaginal plug observation
All female rats were observed for the presence of sperm cells in the vagina. Figure 2 shows a) The proestrus phase, b) The estrus phase, c) The me rus phase, d) The diestrus phase, and e) The confirmation of sperm cells in the vaginal plug. Pregnancy can be confirmed only during the estrus phase (epithelial and cornified cells with spermatozoa – sperm cells), as shown in Figure 2e.
Weight observation
All females’ body weight post-copulation was monitored from GD 1 to GD 20 (Figure 3). No significant differences were observed between VPA and control groups.

Effect of VPA on miscarriage
We examined the effect of VPA on miscarriage from GD 1 to GD 21 by weight reduction. Prenatally injected VPA pregnant female (33.33%) rats were subjected to miscarriage. No miscarriage was observed in the control groups.
VPA-treated rats and reduced litter size
To observe the effect of VPA on litter size, the VPA-treated and control dams’ litter size was calculated. Table 1 indicates that VPA-treated female dams delivered reduced litter size (P<0.0001) compared to their female control counterparts, and the data were statistically significant. We also observed the birth rate of male and female pups in the control and prenatally VPA-treated pups. Table 1 indicates that VPA-treated dams delivered significantly reduced male (P=0.0025)/female pups (P<0.0001) compared to control dams.

Prenatal VPA-treated pups showing a substantial increase in mortality
We observed that the mortality rate significantly increased in the prenatally VPA-treated pups (P=0.0077) compared to control pups (Table 1). Moreover, no significant difference was observed in natural death among the prenatal VPA-treated and control pups. However, the mortality percentage increased significantly in the prenatally VPA-treated (P=0.0019) group compared to the control pups (Table 1).
VPA-treated maternal rats manifesting increased cannibalistic behavior
We examined the cannibalistic behavior under the influence of VPA postnatally. As illustrated in Figure 1 and Table 1, VPA-treated maternal rats exhibited significantly (P=0.0023) increased acts of cannibalism compared to the control females. Similarly, we examined the effect of maternal cannibalism in the VPA-treated group on healthy vs malformed pups. Table 1 indicates that malformed pups (P=0.0484) were subjected to significantly increased cannibalism by maternal females compared to healthy pups.
VPA depicting significant differences in alive pups
Prenatal VPA dams (P<0.0001) had significantly reduced lively pups (Table 1) compared to control.
VPA causing malformation in offspring
Prenatal exposure to VPA showed different malformations (tail kink, paw malformation, and short tail) post-birth (Table 1).
Survival analysis
Survival analysis showed that prenatal administration of VPA (P=0.0288) causes a decreased litter survival rate compared to control pups (Figure 4a, Tables 2 and 3).


The difference was found to be statistically significant. These results indicate that prenatal VPA decreases the survival rate of delivered pups either by cannibalism or by natural death.

The risk associated with prenatal VPA administration
The outcome of events (death) was calculated. The Fisher exact revealed that the mortality associated with prenatal VPA pups was significant (P<0.0001) more than that of control pups (Figure 4b, Tables 2 and 3).
4. Discussion
The current study is the first to explore the effect of VPA on maternal cannibalism and investigate male/female and malformed litter cannibalism.
Different studies have reported rodent cannibalism, and some have reported it for possible reasons (Abel & Biology, 1979; Buntin et al., 1984). Additionally, Lane-Petter (1968) reported that cannibalism is more common in rats and mice. This same study stated that genetic factors might contribute to this cannibalistic behavior (Lane-Petter, 1968). Schardein et al., (1978) reported that maternal rats treated with a teratogen during gestation exhibited cannibalistic behavior. Moreover, these rats cannibalize malformed pups rather than normal pups (Schardein et al., 1978). Corresponding to this, Wyszynski et al. (2005) observed that human pregnant females exposed to valproate cause an increased risk of malformations in their offspring.
On the other hand, analogous to the previous study, Ruhela et al. (2017) observed anatomical malformations in prenatally VPA-administered Wistar rats. Compared to the above studies, our findings reported similar observations that female rats cannibalize malformed pups compared to healthy pups (Figure 1, Table 3). However, a study has reported that the chemical modification of VPA can prevent malformation in the murine model (Jazayeri et al., 2020). Okada et al. reported the teratogenic effects of VPA in clinical aspects and different preclinical models (Okada et al., 2004). Besides, no information was mentioned about cannibalism (Jazayeri et al., 2020).
Cannibalism can be caused due to the disruption in maternal behavior. Gaffori and Le Moal (1979) stated that thiamine induces disruption in normal maternal behavior and causes spontaneous abortion in pregnant female rats (Ba, 2013). Another study observed that ventral mesencephalic tegmentum lesions cause disruption in maternal behavior, which results in cannibalism. The study also observed that the mothers did not nurse their litter (Gaffori and Le Moal, 1979).
Additionally, Perez-Laso et al. (2008) investigated the effect of olfactory bulbectomy on maternal behavior disruption, which is relatable to previous findings (Schwartz et al., 1976). Apart from this, another study states that maternal behavior disruption can occur during gestation if female rats are exposed to extreme environmental stress conditions (Perez-Laso et al., 2008). Libbin and Person (1979) portrayed that cannibalism can be avoided by minimal human handling, limiting the change of beddings, and constantly acclimatizing the pregnant rats to human touch during the gestation period. Another study states that cannibalism could be prevented if maternal rats had not fasted and freely accessible food and water during gestation (Fox, 1975). However, our results were contradictory. Although we did not abstain female rats from food and water during gestation, it still resulted in cannibalism (Figure 1, Table 3). Serrano et al. (1991) explained the prevention of cannibalism by performing cesarean sectioning in pregnant rats on GD 21. Helander and Bergh (1980) investigated the prevention of cannibalism after neonatal surgery. They described that litter post-surgeries must be cleaned for blood spots or clots and sutured off open wounds, which prevented cannibalism successfully.
Mohan (1974) observed the effect of starvation on age-dependent cannibalism at Bangalore University. His findings revealed that at 3-3.9 months, female rats delivered a mean litter size (Mean±SD 11.3±1.40) (Mohan, 1974). However, our mean litter size (Mean±SEM 16.50±0.7782) was significantly higher as there was no starvation (Table 3). Nevertheless, we observed lower cannibalism in our control group, contrasting the above study. The probable reason for lower cannibalism is that no VPA was administered in the control rats. Mennella & Moltz (1988) have observed infanticide in rats. They stated that females synthesize low-volatility chemo-signal during pregnancy, protecting males from cannibalizing the delivered pups (Mennella & Moltz, 1988; Helander and Bergh, 1980). Another study noted that infanticide could be due to genetic, developmental, and hormonal imbalances (Svare et al., 1981). Discordant to the above study, we separated males and females post-copulation, so we did not observe any of these findings.
Porter (1968) reported a high chance of pre-weaning loss in laboratory animals. They observed the benefits of separating the mother and its litter or placing individual mothers in a separate cage, which resulted in preventing cannibalism. Supporting this study, Darlene et al. observed that the pre-weaning loss was around 33%, depending upon the intervention. However, they also observed that their mothers provided survival pups with good care and nursing (DeSantis & Schmaltz, 1984). Our findings were similar to those of the above study; the survived pups were provided adequate care and nursing regardless of malformation or healthy pups in control and prenatally VPA-treated groups (Table 2 – number of alive pups).
Komariah et al. observed the teratogenic effect of VPA (250 mg/kg per oral) (Abel & Biology) on GD 10, 13, and 16 in Sprague Dawley rats. They reported that administering VPA on GD 10 and 13 showed decreased mean litter size compared to GD 16. In addition, they also observed birth weight, body weight, and growth rate, and their data were statistically significant. Another study examined the effects of VPA at different doses at 0, 150, 200, 300, 400, and 600 mg/kg per oral on GD 7-18. This study concluded that 600 mg/kg was significantly toxic and resulted in pups’ mortality (Vorhees, 1987). Our study had different observations as the route of administration was IP on GD 12.5 at a 600 mg/kg dose. Our study reported reduced litter size and a higher incidence of mortality in the prenatally VPA-treated group (Table 3).
To the best of our knowledge, no study has dug deep into concepts of cannibalism associated with VPA exposure in preclinical aspects. The study is the first to report the potential of VPA to cause maternal behavior disruption, thereby causing aggressive behavior toward cage-mates and their litter. Finally, miscarriage and cannibalism are significant drawbacks in the preclinical model. We have reported the litter size, VPA effect on mortality, maternal cannibalistic behavior, average pups’ survival, the difference among maternal cannibalistic behavior on malformed vs healthy pups, amount of miscarriage, and survival analysis of pups. In addition, we reported the odds and risk ratios and percentage of deaths associated with VPA post-pregnancy.
Translational outcome
Valproic acid has been a significant concern for pregnant females resistant to other antiepileptic drugs. The current study outcome highlights that VPA induces maternal behavior disruption in rodents during gestation. Similarly, few case reports depict patients’ abnormal behavior/irritability/agitation due to valproic acid administration. Thus, VPA should be the least preferred drug or not be used in pregnant females suffering from seizures as it might be responsible for causing aggressive behavior in such cases. However, the current study is a pilot and needs to be explored in a large sample size.
5. Conclusion
To conclude, valproic acid at a dose of 600 mg/kg IP on GD 12.5 has a marked cannibalistic effect on pregnant rat dams’ post-parturition. In addition, VPA significantly reduced the litter size, increased cannibalism of malformed pups, decreased pups’ survival rate, and increased the chances of miscarriage upon administration. The mechanism behind cannibalism could be the disruption of maternal behavior. The cause might be histone deacetylase inhibition, resulting in downregulation of the NFκB signaling pathway. Besides, serotonin and gamma-aminobutyric acid have played a significant role in the aggressive behavior of the female rats. The rationale behind maternal behavior disruption and cannibalism is yet to be explored.
Moreover, the relationship between cannibalism and maternal behavior disruption is still an enigma. The current study raises some queries that could be explored shortly. Is the VPA preclinical model a gold standard model for ASD? Does it possess face validity, construct validity, and predictive validity? The VPA model is the most widely used model for ASD. Still, many insights have not been scrutinised as many observations have been unreported.
Study limitations
Some studies have reported the role of neurotransmitters (serotonin, dopamine, gamma-aminobutyric acid) in male/female behavior disruption or aggressive behavior associated with neurotransmitters. However, we are currently delineating the levels of different neurotransmitters in the maternal rat brain on molecular aspects. It may provide novel insights behind cannibalism.
Ethical Considerations
Compliance with ethical guidelines
All animals in this study received proper care in compliance with ARRIVE guidelines. The Institutional Ethics Committee (IAEC), PGIMER, Chandigarh, India, approved all experiments (IAEC NO.: 106/IAEC/727, dated: 4/02/2020). The Central Small Animal Research Facility, PGIMER, Chandigarh, provided the animals used in this study (approved by CPCSEA – Reg. No.: 47/GO/Re-SL/Bi-S/99/CPCSEA, Dated: 05/31/2016).
Funding
Indian Council of Medical Research (ICMR-SRF) provided a Senior Research Fellowship (SRF) (letter No: 45/45/2019-PHA/BMS, dated 07/23/2019.
Authors' contributions
Conceptualization: Ashish Jain and Ajay Prakash; Data extraction: Ashish Jain; Validation: Ashish Jain and Ajay Prakash; Analysis: Ashish Jain, Neha Dhir, Harvinder Singh, Bikash Medhi and Ajay Prakash; Writing the initial draft: Ashish Jain; Review, editing and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors acknowledge the Indian Council of Medical Research (ICMR) for providing manpower (Ashish Jain). The authors acknowledge the Postgraduate Institute of Medical Education and Research (PGIMER) for providing infrastructures and laboratory facilities within the institute.
References
Abel E. L. (1979). Effects of alcohol withdrawal and undernutrition of cannibalism of rat pups. Behavioral and Neural Biology, 25(3), 411–413. [DOI:10.1016/S0163-1047(79)90492-8] [PMID]
Al Dera H. (2022). Cellular and molecular mechanisms underlying autism spectrum disorders and associated comorbidities: A pathophysiological review. Biomedicine & Pharmacotherapy, 148, 112688. [DOI:10.1016/j.biopha.2022.112688] [PMID]
Alsdorf, R., & Wyszynski, D. F. (2005). Teratogenicity of sodium valproate. Expert Opinion on Drug Safety, 4(2), 345–353. [DOI:10.1517/14740338.4.2.345] [PMID]
Bâ A. (2013). Perinatal thiamine deficiency-induced spontaneous abortion and pup-killing responses in rat dams. Nutritional Neuroscience, 16(2), 69–77. [DOI:10.1179/1476830512Y.0000000032] [PMID]
Buntin, J. D., Jaffe, S., & Lisk, R. D. (1984). Changes in responsiveness to newborn pups in pregnant, nulliparous golden hamsters. Physiology & Behavior, 32(3), 437–439. [DOI:10.1016/0031-9384(84)90259-2] [PMID]
Chaliha, D., Albrecht, M., Vaccarezza, M., Takechi, R., Lam, V., & Al-Salami, H., et al. (2020). A systematic review of the valproic-acid-induced rodent model of autism. Developmental Neuroscience, 42(1), 12–48. [DOI:10.1159/000509109] [PMID]
Davis, F. C. (1933). The measurement of aggressive behavior in laboratory rats. The Pedagogical Seminary and Journal of Genetic Psychology, 43(1), 213-217. [DOI:10.1080/08856559.1933.10533127]
DeSantis, D. T., & Schmaltz, L. W. (1984). The mother-litter relationship in developmental rat studies: Cannibalism vs caring. Developmental Psychobiology, 17(3), 255–262. [DOI:10.1002/dev.420170306] [PMID]
Elnahas, E. M., Abuelezz, S. A., Mohamad, M. I., Nabil, M. M., Abdelraouf, S. M., & Bahaa, N., et al. (2021). Validation of prenatal versus postnatal valproic acid rat models of autism: A behavioral and neurobiological study. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 108, 110185. [DOI:10.1016/j.pnpbp.2020.110185] [PMID]
Fouilloux, C., Ringler, E., & Rojas, B. (2019). Cannibalism. Current Biology, 29(24), R1295-r1297. [DOI:10.1016/j.cub.2019.09.068] [PMID]
Fox, L. R. (1975). Cannibalism in natural populations. Annual Reviews, 6(1), 87-106. [DOI:10.1146/annurev.es.06.110175.000511]
Gaffori, O., & Le Moal, M. (1979). Disruption of maternal behavior and appearance of cannibalism after ventral mesencephalic tegmentum lesions. Physiology & Behavior, 23(2), 317–323. [DOI:10.1016/0031-9384(79)90373-1] [PMID]
Gileadi, T. E., Swamy, A. K., Hore, Z., Horswell, S., Ellegood, J., & Mohan, C., et al. (2021). Effects of low-dose gestational TCDD exposure on behavior and on hippocampal neuron morphology and gene expression in mice. Environmental Health Perspectives, 129(5), 57002. [DOI:10.1289/EHP7352] [PMID] [PMCID]
Guo, Z., Xie, H. Q., Zhang, P., Luo, Y., Xu, T., & Liu, Y., et al. (2018). Dioxins as potential risk factors for autism spectrum disorder. Environment International, 121(Pt 1), 906–915. [DOI:10.1016/j.envint.2018.10.028] [PMID]
Helander, H. F., & Bergh, A. (1980). How to avoid maternal cannibalism after neonatal surgery in rats. Experientia, 36(11), 1295–1296. [DOI:10.1007/BF01969597] [PMID]
Jain, R. A., Prakash, A., & Medhi, B. (2019). Newer potential pharmacological targets for autism spectrum disorder. Indian Journal of Pharmacology, 51(4), 284–286. [DOI:10.4103/ijp.IJP_518_19] [PMID] [PMCID]
Jazayeri, D., Braine, E., McDonald, S., Dworkin, S., Powell, K. L., & Griggs, K., et al. (2020). A rat model of valproate teratogenicity from chronic oral treatment during pregnancy. Epilepsia, 61(6), 1291–1300. [DOI:10.1111/epi.16536] [PMID]
Kawanai, T., Ago, Y., Watanabe, R., Inoue, A., Taruta, A., & Onaka, Y., et al. (2016). Prenatal exposure to histone deacetylase inhibitors affects gene expression of autism-related molecules and delays neuronal maturation. Neurochemical Research, 41(10), 2574–2584. [DOI:10.1007/s11064-016-1969-y] [PMID]
Komariah, K., Kiranadi, B., Winarto, A., Manalu, W., & Handharyani, E. Valproic acid administration in pregnant rats affects litter size, birth weight, and postnatal growth of the offspring. International Journal of Sciences, 33(3), 174-186. [Link]
Lan, A., Kalimian, M., Amram, B., & Kofman, O. (2017). Prenatal chlorpyrifos leads to autism-like deficits in C57Bl6/J mice. Environmental Health, 16(1), 43. [DOI:10.1186/s12940-017-0251-3] [PMID] [PMCID]
Lane-Petter, W. (1968). Parental injuries to offspring: Cannibalism in rats and mice. Journal of the Royal Society of Medicine, 61(12), 1295-1296. [DOI:10.1177/003591576806101228]
Libbin, R. M., & Person, P. (1979). Neonatal rat surgery: Avoiding maternal cannibalism. Science, 206(4414), 66. [DOI:10.1126/science.482926] [PMID]
Lloyd, K. A. (2013). A scientific review: Mechanisms of valproate-mediated teratogenesis. Bioscience Horizons, 6, hzt003. [DOI:10.1093/biohorizons/hzt003]
MacFabe, D. F., Cain, D. P., Rodriguez-Capote, K., Franklin, A. E., Hoffman, J. E., & Boon, F., et al. (2007). Neurobiological effects of intraventricular propionic acid in rats: Possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behavioural Brain Research, 176(1), 149–169. [DOI:10.1016/j.bbr.2006.07.025] [PMID]
Marcondes, F. K., Bianchi, F. J., & Tanno, A. P. (2002). Determination of the estrous cycle phases of rats: Some helpful considerations. Brazilian Journal of Biology, 62(4A), 609–614. [DOI:10.1590/s1519-69842002000400008] [PMID]
Mennella JA, Moltz H. (1988). Infanticide in rats: Male strategy and female counter-strategy. Physiology & Behavior. 42(1):19-28. [DOI: 10.1016/0031-9384(88)90254-5]
Mohan C. (1974). Age-dependent cannibalism in a colony of albino rats. Laboratory Animals, 8(1), 83–84. [DOI:10.1258/002367774780943869] [PMID]
Okada, A., Kurihara, H., Aoki, Y., Bialer, M., & Fujiwara, M. (2004). Amidic modification of valproic acid reduces skeletal teratogenicity in mice. Birth defects research. Part B, Developmental and Reproductive Toxicology, 71(1), 47–53. [DOI:10.1002/bdrb.10057] [PMID]
Percie du Sert, N., Hurst, V., Ahluwalia, A., Alam, S., Avey, M. T., & Baker, M., et al. (2020). The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Journal of Cerebral Blood Flow and Metabolism, 40(9), 1769–1777. [DOI:10.1177/0271678X20943823] [PMID] [PMCID]
Pérez-Laso, C., Segovia, S., Martín, J. L., Ortega, E., Gómez, F., & Del Cerro, M. C. (2008). Environmental prenatal stress alters sexual dimorphism of maternal behavior in rats. Behavioural Brain Research, 187(2), 284–288. [DOI:10.1016/j.bbr.2007.09.029] [PMID]
Porter, G. (1968). Parental injuries to offspring: Pre-weaning loss of laboratory animals. SAGE Journal, 61(12), 1255-1334. [DOI:10.1177/003591576806101227]
Reisinger, S., Khan, D., Kong, E., Berger, A., Pollak, A., & Pollak, D. D. (2015). The poly(I:C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacology & Therapeutics, 149, 213–226. [DOI:10.1016/j.pharmthera.2015.01.001] [PMID]
Ruhela, R. K., Sarma, P., Soni, S., Prakash, A., & Medhi, B. (2017). Congenital malformation and autism spectrum disorder: Insight from a rat model of autism spectrum disorder. Indian Journal of Pharmacology, 49(3), 243–249. [DOI:10.4103/ijp.IJP_183_17] [PMID] [PMCID]
Rylaarsdam, L., & Guemez-Gamboa, A. (2019). Genetic causes and modifiers of autism spectrum disorder. Frontiers in Cellular Neuroscience, 13, 385. [DOI:10.3389/fncel.2019.00385] [PMID] [PMCID]
Schardein, J. L., Petrere, J. A., Hentz, D. L., Camp, R. D., & Kurtz, S. M. (1978). Cannibalistic traits observed in rats treated with a teratogen. Laboratory Animals, 12(2), 81–83. [DOI:10.1258/002367778780953080] [PMID]
Schardein, J. L., Petrere, J. A., Hentz, D. L., Camp, R. D., & Kurtz, S. M. (1978). Cannibalistic traits observed in rats treated with a teratogen. Laboratory Animals, 12(2), 81–83. [DOI:10.1258/002367778780953080] [PMID]
Schneider, T., & Przewłocki, R. (2005). Behavioral alterations in rats prenatally exposed to valproic acid: Animal model of autism. Neuropsychopharmacology, 30(1), 80–89. [DOI:10.1038/sj.npp.1300518] [PMID]
Schwartz, E., & Rowe, F. A. (1976). Olfactory bulbectomy: Influences on maternal behavior in primiparous and multiparous rats. Physiology & Behavior, 17(6), 879–883. [DOI:10.1016/0031-9384(76)90002-0] [PMID]
Serrano, J., Esahli, H., Larsson, L., & Zetterström, R. (1991). Experimental intestinal obstruction in rats. Studies on structure and disaccharidase activities. European Journal of Pediatric Surger, 1(2), 92–96. [DOI:10.1055/s-2008-1042467] [PMID]
Svare, B., & Mann, M. (1981). Infanticide: Genetic, developmental and hormonal influences in mice. Physiology & Behavior, 27(5), 921–927. [DOI:10.1016/0031-9384(81)90062-7] [PMID]
Vajda F. (2012). Dose issues in antiepileptic therapy. Journal of Clinical Neuroscience, 19(11), 1475–1477. [DOI:10.1016/j.jocn.2012.05.003] [PMID]
Vorhees C. V. (1987). Teratogenicity and developmental toxicity of valproic acid in rats. Teratology, 35(2), 195–202. [DOI:10.1002/tera.1420350205] [PMID]
Wang, H., & Doering, L. C. (2015). Autism spectrum disorders: Emerging mechanisms and mechanism-based treatment. Frontiers in Cellular Neuroscience, 9, 183. [DOI:10.3389/fncel.2015.00183] [PMID] [PMCID]
Won, H., Mah, W., & Kim, E. (2013). Autism spectrum disorder causes, mechanisms, and treatments: Focus on neuronal synapses. Frontiers in Molecular Neuroscience, 6, 19. [DOI:10.3389/fnmol.2013.00019] [PMID] [PMCID]
Wyszynski, D. F., Nambisan, M., Surve, T., Alsdorf, R. M., Smith, C. R., & Holmes, L. B., et al. (2005). Increased rate of major malformations in offspring exposed to valproate during pregnancy. Neurology, 64(6), 961–965. [DOI:10.1212/01.WNL.0000154516.43630.C5] [PMID]
Type of Study: Original |
Subject:
Behavioral Neuroscience
Received: 2022/06/30 | Accepted: 2022/08/9 | Published: 2024/03/1
Received: 2022/06/30 | Accepted: 2022/08/9 | Published: 2024/03/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








