Volume 15, Issue 1 (January & February 2024)
BCN 2024, 15(1): 109-116 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Gholipour D, Shahraki M, Saravani M, Payandeh A, Eslahi H. The Effect of Omega-3 Supplementation on Serum Levels of Antioxidant Status in Patients With Bipolar Disease: A Randomized Double-blind Controlled Clinical Trial. BCN 2024; 15 (1) :109-116
URL: http://bcn.iums.ac.ir/article-1-2490-en.html
URL: http://bcn.iums.ac.ir/article-1-2490-en.html
1- Student Research Committee, Zahedan University of Medical Sciences, Zahedan, Iran.
2- Department of Nutrition, School of Medicine, Zahedan University of Medical Sciences, Zahedan, Iran.
3- Cellular and Molecular Research Center, Resistant Tuberculosis Institute, Zahedan University of Medical Sciences, Zahedan, Iran.
4- Community Nursing Research Center, Zahedan University of Medical Sciences, Zahedan, Iran.
2- Department of Nutrition, School of Medicine, Zahedan University of Medical Sciences, Zahedan, Iran.
3- Cellular and Molecular Research Center, Resistant Tuberculosis Institute, Zahedan University of Medical Sciences, Zahedan, Iran.
4- Community Nursing Research Center, Zahedan University of Medical Sciences, Zahedan, Iran.
Full-Text [PDF 581 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Bipolar disorder (BD) is a chronic disorder affecting 1% of people worldwide and causes disability in young people (Vieta et al., 2018). This disorder is associated with comorbid systemic illnesses, morbidity, faulted function, and a high risk of suicide and mortality (Patel et al., 2018). The disease is characterized by mood fluctuations, such as mania, hypomania, mixed episodes, and depression (Lokeshu et al., 2020). Several factors are involved in the etiology of the disorders. Oxidative stress is an effective factor in the progression of BD (Anderson & Maes, 2015). Mood stabilizers have antioxidant properties protecting brain cells from dysfunction and apoptosis (Jiménez‐Fernández et al., 2021). Oxidative stress is significantly higher in patients with BD compared to the general population (Anderson & Maes, 2015). Oxidative stress diminishes neuroplasticity and neurogenesis while increasing apoptosis and neurodegeneration in patients with BD and depression (Fornito et al., 2007; Maes et al., 2009). Several studies have reported changes in antioxidant enzymes among patients with BD (Cudney et al., 2014; de Sousa et al., 2014; Mansur et al., 2016). Studies have reported that antioxidant supplementation reduces symptom burden in patients with BD (Berk et al., 2013; Sommer et al., 2014). Several enzymes and factors are involved in the prevention of progressions of the disorder, such as superoxide dismutase (SOD), catalase (CAT), and total antioxidant capacity (TAC) (Jahangard et al., 2019; Rahsepar & Mohammadpour, 2021; Siwek et al., 2013). Hence, supplementation of antioxidant structures helps to improve antioxidant balance and may be beneficial in patients with BD.
Omega-3 fatty acids play a vital role in the body and participate in regulating the active transport of amino acids by the cell membrane, the function of sodium channels as well as starting the response of rhodopsin to visual stimuli (Djuricic & Calder, 2021). Omega-3 fatty acids change the balance of reductive and oxidative species and have roles in glucose and lipid metabolism (Amos et al., 2019; Sarris et al., 2011). Lagarde and Calzada reported the antioxidant properties of polyunsaturated fatty acids (Guillot et al., 2009). Omega-3 fatty acids have been suggested as antioxidants in the brain (Yavin et al., 2002). A review article concluded that omega-3 fatty acids have antioxidant properties (Giordano & Visioli, 2014). Parellada et al. did not report significant effects of omega-3 on antioxidant status (Parellada et al., 2017). Avramovic and others reported positive effects of omega-3 fatty acids in increasing SOD and decreasing lipid peroxidation (Avramovic et al., 2012). Barbora Katrenčíková et al investigated markers of oxidative stress and antioxidant enzymes in people with depressive disorder and reported that omega-3 may diminish oxidative stress (Katrenčíková et al., 2021).
In sum, BD imbalances oxidant-antioxidant balance, and omega-3 supplementation may improve the balance in patients with BD. In addition, examining the intake and metabolism of omega-3 supplementation has provided clues that may be crucial for treating BD (Saunders et al., 2016). Thus, omega-3 supplementation is beneficial in patients with BD compared to the population. This study was conducted to evaluate the effects of omega-3 supplementation on serum levels of antioxidant status in patients with BD by examining the serum concentrations of CAT, SOD and TAC.
2. Materials and Methods
Participants
This randomized clinical trial study was conducted on 56 men and women with BD referred to Baharan Hospital (Zahedan City-Iran) in 2021. The inclusion criteria included male and female patients aged 18-65 years without a change in the medication schedule, without a history of taking omega-3 supplementation and having literacy and consent to participate in the study. The exclusion criteria included patients with schizophrenia, cardiac disorders, and mental retardation, and programming for surgery and patients who changed drug dosages disagreed to participate in the study and were allergic to drugs. Informed consent was received at the beginning of the study.
Randomization and allocation
A total of 56 patients were randomly divided into two homogenous groups. The patients were allocated into groups using stratified permuted block randomization. The patients were classified based on age and gender. Quadruple blocks (both groups and two replications for each group) were randomly selected from permutes and assigned to groups with the help of R software, version 4.0.2. Both groups were matched in terms of sex and age based on frequency matching and with the help of stratified permuted block randomization. Double blinding was conducted and people distributing supplements and intervening and technicians registering outcomes had no information for the study. Groups included omega-3 (14 men and 14 women) and placebo (14 men and 14 women). The patients in the omega-3 and the placebo groups daily received 2 g of omega-3 in gel form and 2 g of paraffin, respectively. Omega-3 and paraffin were prepared by Barij-essence Company (Kashan City-Iran). This study lasted for two months. The patients and lab staff responsible for lab analysis had no information about supplements and interventions. We also collected the data for demographic characteristics. We also collected the data for demographic characteristics. The sample size was calculated based on previous studies (Safari, 2010) and based on the Equation 1.
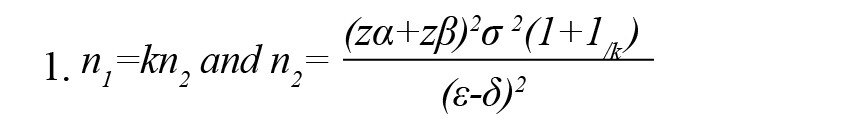
Where, n1 and n2 show the number of samples in omega-3 and placebo groups, respectively. Also, κ, α, 1-β, σ2, є, and δ show the number of samples in omega-3 to placebo, probability of type 1 error, statistical power, the mixed variance of groups, real difference, and clinical effective limit, respectively.
Assessment of antioxidant status
At the beginning and end of the study, blood samples (5 mL) were collected to investigate the serum concentrations of SOD, CAT, and TAC at 8 AM, and transferred into microtubes. The blood samples were centrifuged at 3500 rpm and serum was stored at -70ºC. SOD and CAT activities and TAC were measured by commercial kits (Kiazist, Iran) as suggested by the manufacturer. To investigate SOD activity, tetrazolium salt was used to detect superoxide radicals generated by xanthine oxidase and hypoxanthine. To investigate CAT, the enzymatic conversion of methanol to formaldehyde was used in the presence of an optimal concentration of hydrogen peroxide (H2O2).
Data analysis
Data related to demographic information were reported as frequency. The data for the antioxidant stratus were investigated for normality. Since the data were normal, we analyzed them by the parametric procedure of t-test. The data are reported as Mean±SD.
3. Results
Demographic data
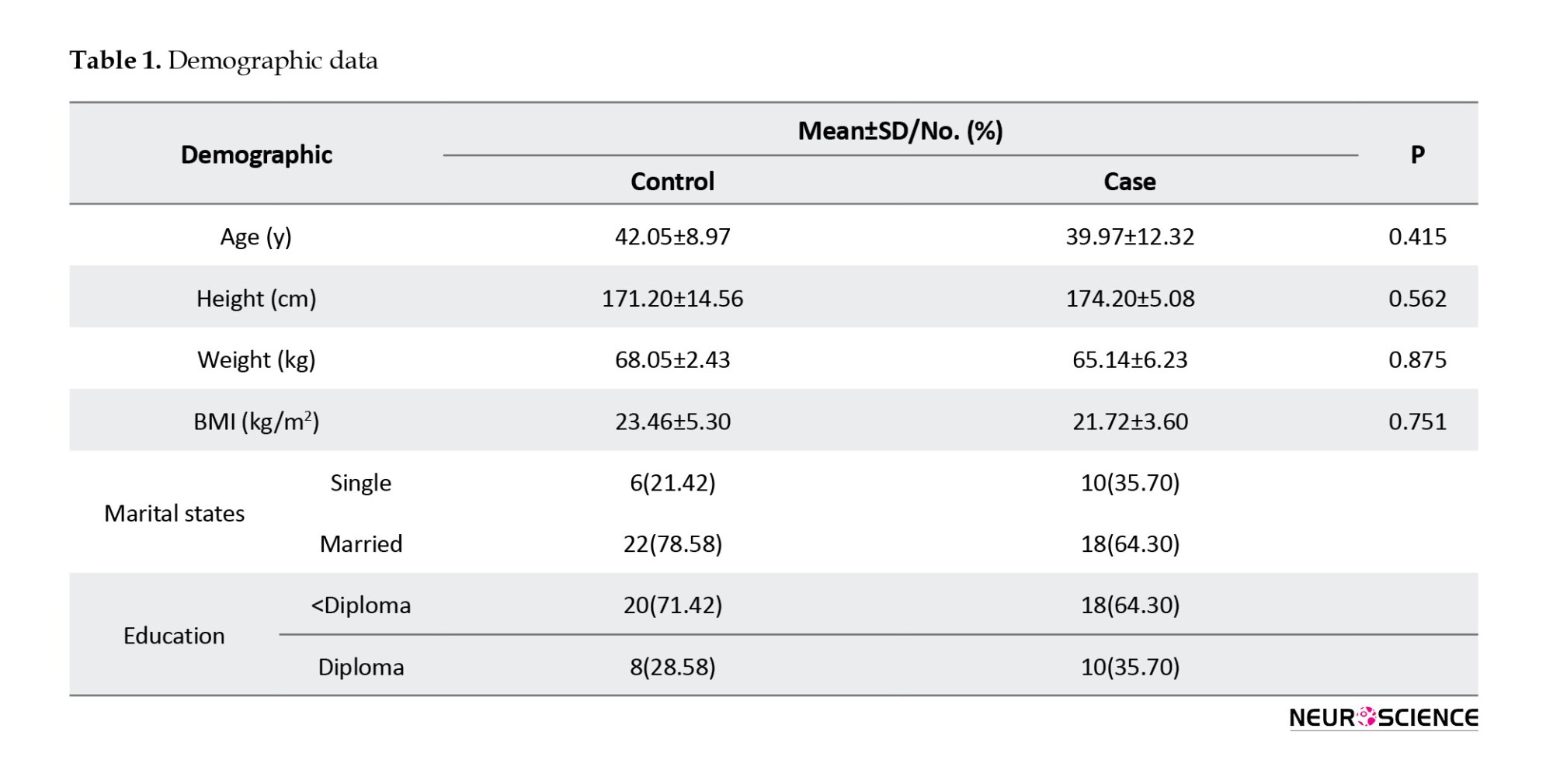
Table 1 presents the results of the demographic characteristics. The results showed no significant difference between groups in terms of age (P=0.415), height (P=0.562), weight (P=0.875), and body mass index (BMI) (P=0.751). The groups were similar in gender. The mean age was 42.05±8.97 years in the placebo group and 39.97±12.32 years in the case group. The mean of height was 174.20±14.56 cm and 179.20±5.08 cm in the placebo and omega-3 groups, respectively. The mean of weight was 68.05±2.43 kg and 65.14±6.23 kg in the case and control groups, respectively. In both groups, most patients were married and had educational levels lower than a diploma. No significant differences were observed between groups (P>0.05) affecting antioxidant results.
Antioxidant status
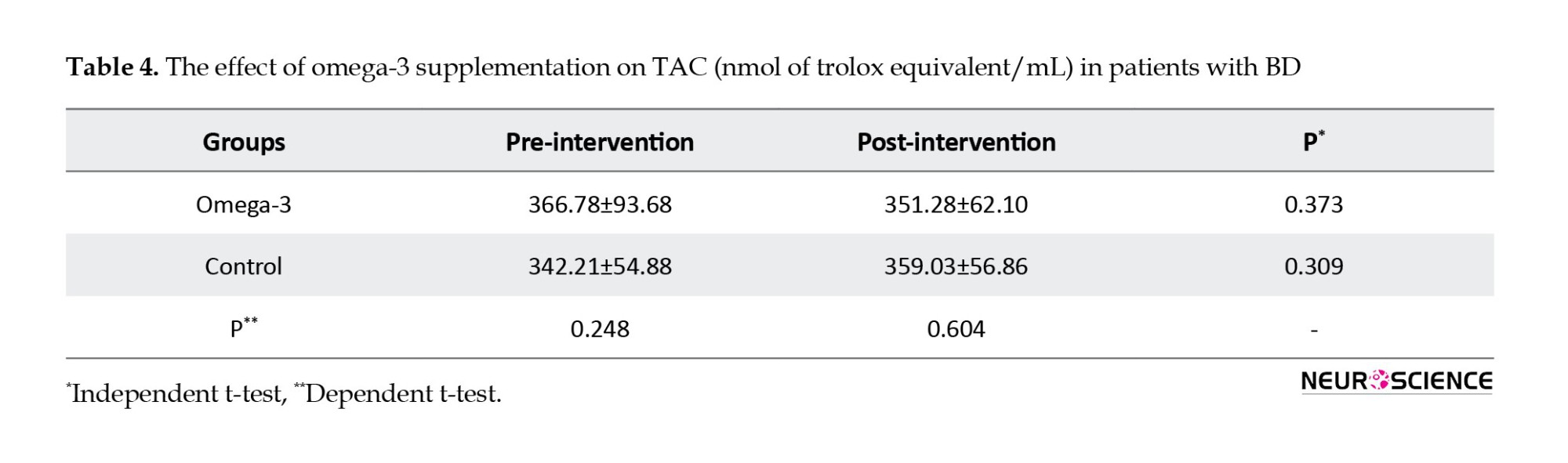
Tables 2, 3 and 4 present the results of the effects of omega-3 supplementation on activities of SOD and CAT and TAC.
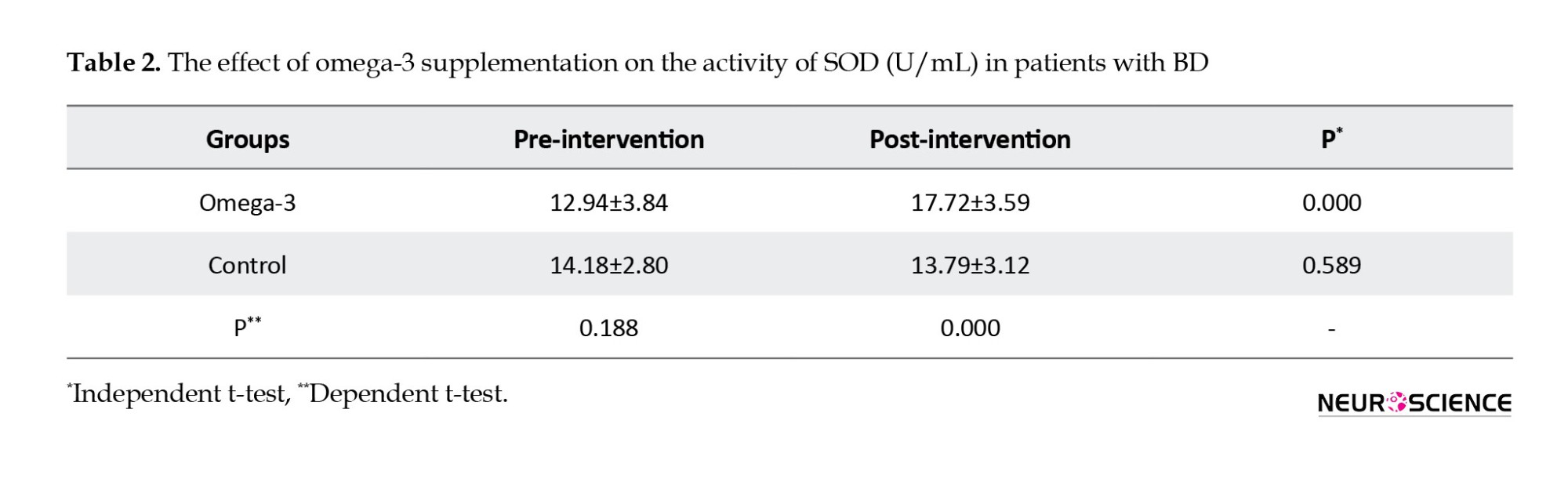
The results showed that omega-3 supplementation significantly increased activities of SOD (P=0.000) and CAT (P=0.000) in post-intervention compared to pre-intervention.
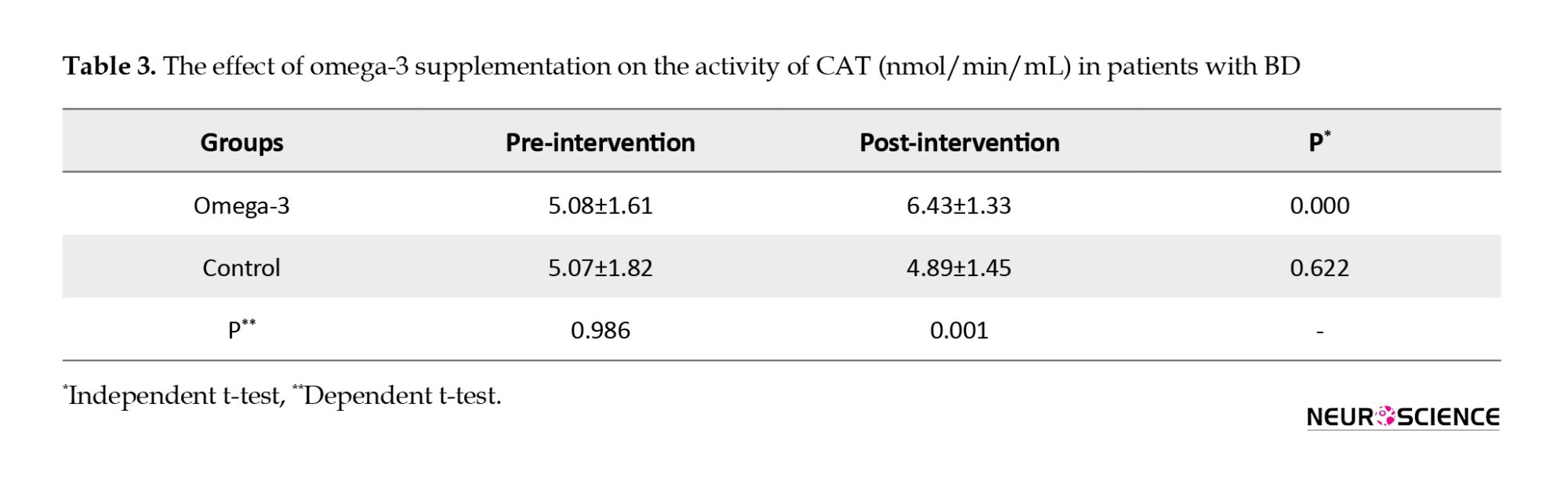
It also increased activities of SOD (P=0.000) and CAT (P=0.000) compared to the control group in post-intervention. Omega-3 supplementation significantly increased activities of SOD (21%) and CAT (27%) in post-intervention compared to pre-intervention. Omega-3 supplementation did not have significant effects on TAC in post-intervention compared to pre-intervention (P=0.373) and compared to the control group (P=0.604).
4. Discussion
This study was conducted to investigate the effects of omega-3 supplementation on the activities of SOD and CAT and also TAC. Serval studies have reported the positive effects of omega-3 on bipolar depressive symptoms (Sarris et al., 2011), the severity of brain dysfunction (Patrick & Ames, 2015) as well as monotherapy and adjunct therapy in patients with BD (Rutkofsky et al., 2017).
The results showed that omega-3 supplementation increased the activities of SOD and CAT in patients with BD. The results are consistent with the results reported by previous studies on the positive effects of omega-3 supplementation in improving SOD activity (Garrel et al., 2012; Zararsiz et al., 2011). The results are also consistent with animal studies that showed omega-3 supplementation significantly increased SOD activity in the brains of old rats (Avramovic et al., 2012). Barbora Katrenčíková et al investigated oxidative stress markers and antioxidant enzymes in people with depressive disorder and reported that omega-3 may diminish oxidative stress (Katrenčíková et al., 2021) that their results are partly consistent with our results. They did not report the positive effects of omega-3 on SOD activity in healthy control. This means that omega-3 may have beneficial effects on patients with psychological disorders. The results are also in contrast with the results reported by Parellada et al. who showed that omega-3 supplementation did not have significant effects on antioxidant status in patients with autism spectrum disorders (Parellada et al., 2017). The difference between our results and others may be attributed to the studied patients. SOD converts superoxide radicals to hydrogen peroxide (H2O2) and mitochondrial SOD comprises 60% of total antioxidants. However, CAT is an iron-containing enzyme in peroxisomes, lysosomes, and mitochondria (Mahadik et al., 2006). A possible mechanism of omega-3 is via increasing the anti-oxidative intracellular defense system (Sivrioglu et al., 2007). Omega-3 is known to have antioxidant properties via suppressing lipid peroxidation (da Silva et al., 2016). The mechanism of action of omega-3 supplements in increasing activities of SOD and CAT is not clear. However, mechanistic studies have shown that omega-3 supplementation improves antioxidant activity by affecting syntaxin-3, a molecule effector in cell membrane expansion (Darios & Davletov, 2006). It also improves antioxidant status by decreasing the production of reactive oxygen species (Iraz et al., 2005). In sum, omega-3 influences CAT and SOD via mentioned possible mechanisms. Compared to the placebo group, omega-3 supplementation increased the activities of SOD and CAT. The disorder decreases the activities of SOD and CAT and omega-3 supplement compensates antioxidant faults and improves antioxidant status in terms of activities of SOD and CAT. However, the results showed that omega-3 supplementation had no significant effect on TAC. The results regarding the effects of the omega-3 supplement on TAC contradict those reported by others (Heshmati et al., 2019; Sepidarkish et al., 2020; Taghizadeh et al., 2017). It was expected that omega-3 supplementation can increase TAC but no such result was observed. Although omega-3 supplementation improved the activities of SOD and TAC, it did not show significant effects on TAC, which can be attributed to different efficiency and mechanisms of omega-3 on various antioxidant parameters.
5. Conclusion
In sum, omega-3 supplementation increased the activities of CAT and SOD but had no significant effect on TAC. Improving antioxidant enzyme activities in patients with BD can be beneficial and can alleviate the severity of the disease. We suggest omega-3 supplementation in patients with BD alongside common drugs after clinical future studies.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethical Committee of Zahedan University of Medical Sciences (Code: IR.ZAMUS.REC.1400,381). The study was registered by the Iranian Registry of Clinical Trials (IRCT) (Code: IRCT20211220053469N2).
Funding
This research was supported by the research project (No.: 10504), funded by the Student Research Committee of Zahedan University of Medical Sciences.
Authors' contributions
Conceptualization and supervision: Hadi Eslahi and Dina Gholipour; Methodology: Mansour Shahraki and Mohsen Saravani; Data collection: Hadi Eslahi; Investigation: Hadi Eslahi and Dina Gholipour; Data analysis: Abolfazl Payandeh; The original draft preparation: Hadi Eslahi and Dina Gholipour; Review & editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors appreciate Zahedan University of Medical Sciences for approving the study and also all people who participated in this study.
References
Amos, D., Cook, C., & Santanam, N. (2019). Omega 3 rich diet modulates energy metabolism via GPR120-Nrf2 crosstalk in a novel antioxidant mouse model. Biochimica et biophysica acta. Molecular and Cell Biology of Lipids, 1864(4), 466–488. [DOI:10.1016/j.bbalip.2019.01.002] [PMID] [PMCID]
Anderson, G., & Maes, M. (2015). Bipolar disorder: Role of immune-inflammatory cytokines, oxidative and nitrosative stress and tryptophan catabolites. Current Psychiatry Reports, 17(2), 8. [DOI:10.1007/s11920-014-0541-1] [PMID]
Avramovic, N., Dragutinovic, V., Krstic, D., Colovic, M., Trbovic, A., & de Luka, S., et al. (2012). The effects of omega 3 fatty acid supplementation on brain tissue oxidative status in aged wistar rats. Hippokratia, 16(3), 241–245. [PMID] [PMCID]
Berk, M., Malhi, G. S., Gray, L. J., & Dean, O. M. (2013). The promise of N-acetylcysteine in neuropsychiatry. Trends in Pharmacological Sciences, 34(3), 167–177. [DOI:10.1016/j.tips.2013.01.001] [PMID]
Cudney, L. E., Sassi, R. B., Behr, G. A., Streiner, D. L., Minuzzi, L., & Moreira, J. C., et al. (2014). Alterations in circadian rhythms are associated with increased lipid peroxidation in females with bipolar disorder. The International Journal of Neuropsychopharmacology, 17(5), 715–722. [DOI:10.1017/S1461145713001740] [PMID]
da Silva, E. P., Jr, Nachbar, R. T., Levada-Pires, A. C., Hirabara, S. M., & Lambertucci, R. H. (2016). Omega-3 fatty acids differentially modulate enzymatic anti-oxidant systems in skeletal muscle cells. Cell Stress & Chaperones, 21(1), 87–95. [DOI:10.1007/s12192-015-0642-8] [PMID] [PMCID]
Darios, F., & Davletov, B. (2006). Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3. Nature, 440(7085), 813–817. [DOI:10.1038/nature04598] [PMID]
de Sousa, R. T., Zarate, C. A., Jr, Zanetti, M. V., Costa, A. C., Talib, L. L., & Gattaz, W. F., et al. (2014). Oxidative stress in early stage bipolar disorder and the association with response to lithium. Journal of Psychiatric Research, 50, 36–41.[DOI:10.1016/j.jpsychires.2013.11.011] [PMID] [PMCID]
Djuricic, I., & Calder, P. C. (2021). Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids on human health: An update for 2021. Nutrients, 13(7), 2421 [DOI:10.3390/nu13072421] [PMID] [PMCID]
Fornito, A., Malhi, G. S., Lagopoulos, J., Ivanovski, B., Wood, S. J., & Velakoulis, D., et al. (2007). In vivo evidence for early neurodevelopmental anomaly of the anterior cingulate cortex in bipolar disorder. Acta Psychiatrica Scandinavica, 116(6), 467–472. [DOI:10.1111/j.1600-0447.2007.01069.x] [PMID]
Garrel, C., Alessandri, J. M., Guesnet, P., & Al-Gubory, K. H. (2012). Omega-3 fatty acids enhance mitochondrial superoxide dismutase activity in rat organs during post-natal development. The International Journal of Biochemistry & Cell Biology, 44(1), 123–131. [DOI:10.1016/j.biocel.2011.10.007] [PMID]
Giordano, E., & Visioli, F. (2014). Long-chain omega 3 fatty acids: Molecular bases of potential antioxidant actions. Prostaglandins, Leukotrienes, and Essential Fatty Acids, 90(1), 1–4.[DOI:10.1016/j.plefa.2013.11.002] [PMID]
Guillot, N., Caillet, E., Laville, M., Calzada, C., Lagarde, M., & Véricel, E. (2009). Increasing intakes of the long-chain omega-3 docosahexaenoic acid: Effects on platelet functions and redox status in healthy men. FASEB Journal, 23(9), 2909–2916. [DOI:10.1096/fj.09-133421] [PMID]
Heshmati, J., Morvaridzadeh, M., Maroufizadeh, S., Akbari, A., Yavari, M., & Amirinejad, A., et al. (2019). Omega-3 fatty acids supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacological Research, 149, 104462. [DOI:10.1016/j.phrs.2019.104462] [PMID]
Iraz, M., Erdogan, H., Ozyurt, B., Ozugurlu, F., Ozgocmen, S., & Fadillioglu, E. (2005). Brief communication: Omega-3 essential fatty acid supplementation and erythrocyte oxidant/antioxidant status in rats. Annals of Clinical and Laboratory Science, 35(2), 169–173. [PMID]
Jahangard, L., Yasrebifar, F., Haghighi, M., Ranjbar, A., & Mehrpooya, M. (2019). Influence of adjuvant Coenzyme Q10 on inflammatory and oxidative stress biomarkers in patients with bipolar disorders during the depressive episode. Molecular Biology Reports, 46(5), 5333–5343. [DOI:10.1007/s11033-019-04989-z] [PMID]
Jiménez-Fernández, S., Gurpegui, M., Garrote-Rojas, D., Gutiérrez-Rojas, L., Carretero, M. D., & Correll, C. U. (2021). Oxidative stress parameters and antioxidants in patients with bipolar disorder: Results from a meta-analysis comparing patients, including stratification by polarity and euthymic status, with healthy controls. Bipolar Disorders, 23(2), 117–129.[DOI:10.1111/bdi.12980] [PMID]
Katrenčíková, B., Vaváková, M., Paduchová, Z., Nagyová, Z., Garaiova, I., & Muchová, J., et al. (2021). Oxidative stress markers and antioxidant enzymes in children and adolescents with depressive disorder and impact of omega-3 fatty acids in randomised clinical trial. Antioxidants, 10(8), 1256. [DOI:10.3390/antiox10081256] [PMID] [PMCID]
Lokeshu, T., Lakshmi, V., Kumari, V. J., Bhargavi, C., & Sindhu, P. M. (2020). Plasma antioxidant levels in bipolar disorder. Haya, 5(10), 208-214 [DOI:10.36348/sjls.2020.v05i10.003]
Maes, M., Yirmyia, R., Noraberg, J., Brene, S., Hibbeln, J., & Perini, G., et al. (2009). The inflammatory & neurodegenerative (I&ND) hypothesis of depression: Leads for future research and new drug developments in depression. Metabolic Brain Disease, 24(1), 27–53. [DOI:10.1007/s11011-008-9118-1] [PMID]
Mahadik, S. P., Pillai, A., Joshi, S., & Foster, A. (2006). Prevention of oxidative stress-mediated neuropathology and improved clinical outcome by adjunctive use of a combination of antioxidants and omega-3 fatty acids in schizophrenia. International Review of Psychiatry, 18(2), 119–131. [DOI:10.1080/09540260600581993] [PMID]
Mansur, R. B., Rizzo, L. B., Santos, C. M., Asevedo, E., Cunha, G. R., & Noto, M. N., et al. (2016). Bipolar disorder course, impaired glucose metabolism and antioxidant enzymes activities: A preliminary report. Journal of Psychiatric Research, 80, 38–44. [DOI:10.1016/j.jpsychires.2016.05.014] [PMID]
Parellada, M., Llorente, C., Calvo, R., Gutierrez, S., Lázaro, L., & Graell, M., et al. (2017). Randomized trial of omega-3 for autism spectrum disorders: Effect on cell membrane composition and behavior. European Neuropsychopharmacology, 27(12), 1319–1330. [DOI:10.1016/j.euroneuro.2017.08.426] [PMID]
Patel, R. S., Virani, S., Saeed, H., Nimmagadda, S., Talukdar, J., & Youssef, N. A. (2018). Gender differences and comorbidities in US adults with bipolar disorder. Brain Sciences, 8(9), 168. [DOI:10.3390/brainsci8090168] [PMID] [PMCID]
Patrick, R. P., & Ames, B. N. (2015). Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: Relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB Journal, 29(6), 2207–2222. [DOI:10.1096/fj.14-268342] [PMID]
Rahsepar, S., & Mohammadpour, A. (2021). Oxidative stress and bipolar mood disorder: An important yet ambiguous relationship. Journal of Pharmaceutical Care, 9(4), 195-208. [DOI:10.18502/jpc.v9i4.8225]
Rutkofsky, I. H., Khan, A. S., Sahito, S., & Kumar, V. (2017). The psychoneuroimmunological role of omega-3 polyunsaturated fatty acids in major depressive disorder and bipolar disorder. Advances in Mind-Body Medicine, 31(3), 8-16. [Link]
Safari, M., Sadr, S., Mirabzadeh, K., & Saki, M. (2010). [The effect of omega3 and fluvoxamine on the depressive phase in bipolar disorder type I (Persian)]. Iranian Journal of Psychiatry and Clinical Psychology, 16(3), 316-317. [Link]
Sarris, J., Mischoulon, D., & Schweitzer, I. (2012). Omega-3 for bipolar disorder: Meta-analyses of use in mania and bipolar depression. The Journal of Clinical Psychiatry, 73(1), 81–86.[DOI:10.4088/JCP.10r06710] [PMID]
Saunders, E. F., Ramsden, C. E., Sherazy, M. S., Gelenberg, A. J., Davis, J. M., & Rapoport, S. I. (2016). Omega-3 and omega-6 polyunsaturated fatty acids in bipolar disorder: A review of biomarker and treatment studies. The Journal of Clinical Psychiatry, 77(10), e1301–e1308. [DOI:10.4088/JCP.15r09925] [PMID] [PMCID]
Sepidarkish, M., Akbari-Fakhrabadi, M., Daneshzad, E., Yavari, M., Rezaeinejad, M., & Morvaridzadeh, M., et al. (2020). Effect of omega-3 fatty acid plus vitamin E Co-Supplementation on oxidative stress parameters: A systematic review and meta-analysis. Clinical Nutrition, 39(4), 1019–1025. [DOI:10.1016/j.clnu.2019.05.004] [PMID]
Sivrioglu, E. Y., Kirli, S., Sipahioglu, D., Gursoy, B., & Sarandöl, E. (2007). The impact of omega-3 fatty acids, vitamins E and C supplementation on treatment outcome and side effects in schizophrenia patients treated with haloperidol: An open-label pilot study. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 31(7), 1493–1499. [DOI:10.1016/j.pnpbp.2007.07.004] [PMID]
Siwek, M., Sowa-Kućma, M., Dudek, D., Styczeń, K., Szewczyk, B., & Kotarska, K., et al. (2013). Oxidative stress markers in affective disorders. Pharmacological Reports, 65(6), 1558-1571. [DOI:10.1016/S1734-1140(13)71517-2]
Sommer, I. E., van Westrhenen, R., Begemann, M. J., de Witte, L. D., Leucht, S., & Kahn, R. S. (2014). Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: An update. Schizophrenia Bulletin, 40(1), 181–191.[DOI:10.1093/schbul/sbt139] [PMID] [PMCID]
Taghizadeh, M., Tamtaji, O. R., Dadgostar, E., Daneshvar Kakhaki, R., Bahmani, F., & Abolhassani, J., et al. (2017). The effects of omega-3 fatty acids and vitamin E co-supplementation on clinical and metabolic status in patients with Parkinson's disease: A randomized, double-blind, placebo-controlled trial. Neurochemistry International, 108, 183–189. [DOI:10.1016/j.neuint.2017.03.014] [PMID]
Vieta, E., Berk, M., Schulze, T. G., Carvalho, A. F., Suppes, T., & Calabrese, J. R., et al. (2018). Bipolar disorders. Nature Reviews. Disease Primers, 4, 18008. [DOI:10.1038/nrdp.2018.8] [PMID]
Yavin, E., Brand, A., & Green, P. (2002). Docosahexaenoic acid abundance in the brain: A biodevice to combat oxidative stress. Nutritional Neuroscience, 5(3), 149–157.[DOI:10.1080/10284150290003159] [PMID]
Zararsiz, I., Meydan, S., Sarsilmaz, M., Songur, A., Ozen, O. A., & Sogut, S. (2011). Protective effects of omega-3 essential fatty acids against formaldehyde-induced cerebellar damage in rats. Toxicology and Industrial Health, 27(6), 489–495.[DOI:10.1177/0748233710389852] [PMID]
Bipolar disorder (BD) is a chronic disorder affecting 1% of people worldwide and causes disability in young people (Vieta et al., 2018). This disorder is associated with comorbid systemic illnesses, morbidity, faulted function, and a high risk of suicide and mortality (Patel et al., 2018). The disease is characterized by mood fluctuations, such as mania, hypomania, mixed episodes, and depression (Lokeshu et al., 2020). Several factors are involved in the etiology of the disorders. Oxidative stress is an effective factor in the progression of BD (Anderson & Maes, 2015). Mood stabilizers have antioxidant properties protecting brain cells from dysfunction and apoptosis (Jiménez‐Fernández et al., 2021). Oxidative stress is significantly higher in patients with BD compared to the general population (Anderson & Maes, 2015). Oxidative stress diminishes neuroplasticity and neurogenesis while increasing apoptosis and neurodegeneration in patients with BD and depression (Fornito et al., 2007; Maes et al., 2009). Several studies have reported changes in antioxidant enzymes among patients with BD (Cudney et al., 2014; de Sousa et al., 2014; Mansur et al., 2016). Studies have reported that antioxidant supplementation reduces symptom burden in patients with BD (Berk et al., 2013; Sommer et al., 2014). Several enzymes and factors are involved in the prevention of progressions of the disorder, such as superoxide dismutase (SOD), catalase (CAT), and total antioxidant capacity (TAC) (Jahangard et al., 2019; Rahsepar & Mohammadpour, 2021; Siwek et al., 2013). Hence, supplementation of antioxidant structures helps to improve antioxidant balance and may be beneficial in patients with BD.
Omega-3 fatty acids play a vital role in the body and participate in regulating the active transport of amino acids by the cell membrane, the function of sodium channels as well as starting the response of rhodopsin to visual stimuli (Djuricic & Calder, 2021). Omega-3 fatty acids change the balance of reductive and oxidative species and have roles in glucose and lipid metabolism (Amos et al., 2019; Sarris et al., 2011). Lagarde and Calzada reported the antioxidant properties of polyunsaturated fatty acids (Guillot et al., 2009). Omega-3 fatty acids have been suggested as antioxidants in the brain (Yavin et al., 2002). A review article concluded that omega-3 fatty acids have antioxidant properties (Giordano & Visioli, 2014). Parellada et al. did not report significant effects of omega-3 on antioxidant status (Parellada et al., 2017). Avramovic and others reported positive effects of omega-3 fatty acids in increasing SOD and decreasing lipid peroxidation (Avramovic et al., 2012). Barbora Katrenčíková et al investigated markers of oxidative stress and antioxidant enzymes in people with depressive disorder and reported that omega-3 may diminish oxidative stress (Katrenčíková et al., 2021).
In sum, BD imbalances oxidant-antioxidant balance, and omega-3 supplementation may improve the balance in patients with BD. In addition, examining the intake and metabolism of omega-3 supplementation has provided clues that may be crucial for treating BD (Saunders et al., 2016). Thus, omega-3 supplementation is beneficial in patients with BD compared to the population. This study was conducted to evaluate the effects of omega-3 supplementation on serum levels of antioxidant status in patients with BD by examining the serum concentrations of CAT, SOD and TAC.
2. Materials and Methods
Participants
This randomized clinical trial study was conducted on 56 men and women with BD referred to Baharan Hospital (Zahedan City-Iran) in 2021. The inclusion criteria included male and female patients aged 18-65 years without a change in the medication schedule, without a history of taking omega-3 supplementation and having literacy and consent to participate in the study. The exclusion criteria included patients with schizophrenia, cardiac disorders, and mental retardation, and programming for surgery and patients who changed drug dosages disagreed to participate in the study and were allergic to drugs. Informed consent was received at the beginning of the study.
Randomization and allocation
A total of 56 patients were randomly divided into two homogenous groups. The patients were allocated into groups using stratified permuted block randomization. The patients were classified based on age and gender. Quadruple blocks (both groups and two replications for each group) were randomly selected from permutes and assigned to groups with the help of R software, version 4.0.2. Both groups were matched in terms of sex and age based on frequency matching and with the help of stratified permuted block randomization. Double blinding was conducted and people distributing supplements and intervening and technicians registering outcomes had no information for the study. Groups included omega-3 (14 men and 14 women) and placebo (14 men and 14 women). The patients in the omega-3 and the placebo groups daily received 2 g of omega-3 in gel form and 2 g of paraffin, respectively. Omega-3 and paraffin were prepared by Barij-essence Company (Kashan City-Iran). This study lasted for two months. The patients and lab staff responsible for lab analysis had no information about supplements and interventions. We also collected the data for demographic characteristics. We also collected the data for demographic characteristics. The sample size was calculated based on previous studies (Safari, 2010) and based on the Equation 1.
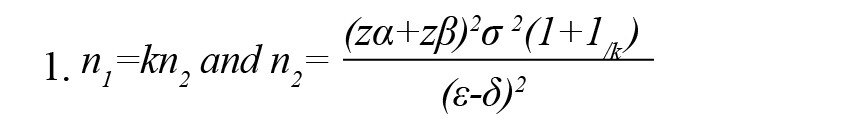
Where, n1 and n2 show the number of samples in omega-3 and placebo groups, respectively. Also, κ, α, 1-β, σ2, є, and δ show the number of samples in omega-3 to placebo, probability of type 1 error, statistical power, the mixed variance of groups, real difference, and clinical effective limit, respectively.
Assessment of antioxidant status
At the beginning and end of the study, blood samples (5 mL) were collected to investigate the serum concentrations of SOD, CAT, and TAC at 8 AM, and transferred into microtubes. The blood samples were centrifuged at 3500 rpm and serum was stored at -70ºC. SOD and CAT activities and TAC were measured by commercial kits (Kiazist, Iran) as suggested by the manufacturer. To investigate SOD activity, tetrazolium salt was used to detect superoxide radicals generated by xanthine oxidase and hypoxanthine. To investigate CAT, the enzymatic conversion of methanol to formaldehyde was used in the presence of an optimal concentration of hydrogen peroxide (H2O2).
Data analysis
Data related to demographic information were reported as frequency. The data for the antioxidant stratus were investigated for normality. Since the data were normal, we analyzed them by the parametric procedure of t-test. The data are reported as Mean±SD.
3. Results
Demographic data
Table 1 presents the results of the demographic characteristics. The results showed no significant difference between groups in terms of age (P=0.415), height (P=0.562), weight (P=0.875), and body mass index (BMI) (P=0.751). The groups were similar in gender. The mean age was 42.05±8.97 years in the placebo group and 39.97±12.32 years in the case group. The mean of height was 174.20±14.56 cm and 179.20±5.08 cm in the placebo and omega-3 groups, respectively. The mean of weight was 68.05±2.43 kg and 65.14±6.23 kg in the case and control groups, respectively. In both groups, most patients were married and had educational levels lower than a diploma. No significant differences were observed between groups (P>0.05) affecting antioxidant results.

Antioxidant status
Tables 2, 3 and 4 present the results of the effects of omega-3 supplementation on activities of SOD and CAT and TAC.

The results showed that omega-3 supplementation significantly increased activities of SOD (P=0.000) and CAT (P=0.000) in post-intervention compared to pre-intervention.

It also increased activities of SOD (P=0.000) and CAT (P=0.000) compared to the control group in post-intervention. Omega-3 supplementation significantly increased activities of SOD (21%) and CAT (27%) in post-intervention compared to pre-intervention. Omega-3 supplementation did not have significant effects on TAC in post-intervention compared to pre-intervention (P=0.373) and compared to the control group (P=0.604).

4. Discussion
This study was conducted to investigate the effects of omega-3 supplementation on the activities of SOD and CAT and also TAC. Serval studies have reported the positive effects of omega-3 on bipolar depressive symptoms (Sarris et al., 2011), the severity of brain dysfunction (Patrick & Ames, 2015) as well as monotherapy and adjunct therapy in patients with BD (Rutkofsky et al., 2017).
The results showed that omega-3 supplementation increased the activities of SOD and CAT in patients with BD. The results are consistent with the results reported by previous studies on the positive effects of omega-3 supplementation in improving SOD activity (Garrel et al., 2012; Zararsiz et al., 2011). The results are also consistent with animal studies that showed omega-3 supplementation significantly increased SOD activity in the brains of old rats (Avramovic et al., 2012). Barbora Katrenčíková et al investigated oxidative stress markers and antioxidant enzymes in people with depressive disorder and reported that omega-3 may diminish oxidative stress (Katrenčíková et al., 2021) that their results are partly consistent with our results. They did not report the positive effects of omega-3 on SOD activity in healthy control. This means that omega-3 may have beneficial effects on patients with psychological disorders. The results are also in contrast with the results reported by Parellada et al. who showed that omega-3 supplementation did not have significant effects on antioxidant status in patients with autism spectrum disorders (Parellada et al., 2017). The difference between our results and others may be attributed to the studied patients. SOD converts superoxide radicals to hydrogen peroxide (H2O2) and mitochondrial SOD comprises 60% of total antioxidants. However, CAT is an iron-containing enzyme in peroxisomes, lysosomes, and mitochondria (Mahadik et al., 2006). A possible mechanism of omega-3 is via increasing the anti-oxidative intracellular defense system (Sivrioglu et al., 2007). Omega-3 is known to have antioxidant properties via suppressing lipid peroxidation (da Silva et al., 2016). The mechanism of action of omega-3 supplements in increasing activities of SOD and CAT is not clear. However, mechanistic studies have shown that omega-3 supplementation improves antioxidant activity by affecting syntaxin-3, a molecule effector in cell membrane expansion (Darios & Davletov, 2006). It also improves antioxidant status by decreasing the production of reactive oxygen species (Iraz et al., 2005). In sum, omega-3 influences CAT and SOD via mentioned possible mechanisms. Compared to the placebo group, omega-3 supplementation increased the activities of SOD and CAT. The disorder decreases the activities of SOD and CAT and omega-3 supplement compensates antioxidant faults and improves antioxidant status in terms of activities of SOD and CAT. However, the results showed that omega-3 supplementation had no significant effect on TAC. The results regarding the effects of the omega-3 supplement on TAC contradict those reported by others (Heshmati et al., 2019; Sepidarkish et al., 2020; Taghizadeh et al., 2017). It was expected that omega-3 supplementation can increase TAC but no such result was observed. Although omega-3 supplementation improved the activities of SOD and TAC, it did not show significant effects on TAC, which can be attributed to different efficiency and mechanisms of omega-3 on various antioxidant parameters.
5. Conclusion
In sum, omega-3 supplementation increased the activities of CAT and SOD but had no significant effect on TAC. Improving antioxidant enzyme activities in patients with BD can be beneficial and can alleviate the severity of the disease. We suggest omega-3 supplementation in patients with BD alongside common drugs after clinical future studies.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethical Committee of Zahedan University of Medical Sciences (Code: IR.ZAMUS.REC.1400,381). The study was registered by the Iranian Registry of Clinical Trials (IRCT) (Code: IRCT20211220053469N2).
Funding
This research was supported by the research project (No.: 10504), funded by the Student Research Committee of Zahedan University of Medical Sciences.
Authors' contributions
Conceptualization and supervision: Hadi Eslahi and Dina Gholipour; Methodology: Mansour Shahraki and Mohsen Saravani; Data collection: Hadi Eslahi; Investigation: Hadi Eslahi and Dina Gholipour; Data analysis: Abolfazl Payandeh; The original draft preparation: Hadi Eslahi and Dina Gholipour; Review & editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors appreciate Zahedan University of Medical Sciences for approving the study and also all people who participated in this study.
References
Amos, D., Cook, C., & Santanam, N. (2019). Omega 3 rich diet modulates energy metabolism via GPR120-Nrf2 crosstalk in a novel antioxidant mouse model. Biochimica et biophysica acta. Molecular and Cell Biology of Lipids, 1864(4), 466–488. [DOI:10.1016/j.bbalip.2019.01.002] [PMID] [PMCID]
Anderson, G., & Maes, M. (2015). Bipolar disorder: Role of immune-inflammatory cytokines, oxidative and nitrosative stress and tryptophan catabolites. Current Psychiatry Reports, 17(2), 8. [DOI:10.1007/s11920-014-0541-1] [PMID]
Avramovic, N., Dragutinovic, V., Krstic, D., Colovic, M., Trbovic, A., & de Luka, S., et al. (2012). The effects of omega 3 fatty acid supplementation on brain tissue oxidative status in aged wistar rats. Hippokratia, 16(3), 241–245. [PMID] [PMCID]
Berk, M., Malhi, G. S., Gray, L. J., & Dean, O. M. (2013). The promise of N-acetylcysteine in neuropsychiatry. Trends in Pharmacological Sciences, 34(3), 167–177. [DOI:10.1016/j.tips.2013.01.001] [PMID]
Cudney, L. E., Sassi, R. B., Behr, G. A., Streiner, D. L., Minuzzi, L., & Moreira, J. C., et al. (2014). Alterations in circadian rhythms are associated with increased lipid peroxidation in females with bipolar disorder. The International Journal of Neuropsychopharmacology, 17(5), 715–722. [DOI:10.1017/S1461145713001740] [PMID]
da Silva, E. P., Jr, Nachbar, R. T., Levada-Pires, A. C., Hirabara, S. M., & Lambertucci, R. H. (2016). Omega-3 fatty acids differentially modulate enzymatic anti-oxidant systems in skeletal muscle cells. Cell Stress & Chaperones, 21(1), 87–95. [DOI:10.1007/s12192-015-0642-8] [PMID] [PMCID]
Darios, F., & Davletov, B. (2006). Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3. Nature, 440(7085), 813–817. [DOI:10.1038/nature04598] [PMID]
de Sousa, R. T., Zarate, C. A., Jr, Zanetti, M. V., Costa, A. C., Talib, L. L., & Gattaz, W. F., et al. (2014). Oxidative stress in early stage bipolar disorder and the association with response to lithium. Journal of Psychiatric Research, 50, 36–41.[DOI:10.1016/j.jpsychires.2013.11.011] [PMID] [PMCID]
Djuricic, I., & Calder, P. C. (2021). Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids on human health: An update for 2021. Nutrients, 13(7), 2421 [DOI:10.3390/nu13072421] [PMID] [PMCID]
Fornito, A., Malhi, G. S., Lagopoulos, J., Ivanovski, B., Wood, S. J., & Velakoulis, D., et al. (2007). In vivo evidence for early neurodevelopmental anomaly of the anterior cingulate cortex in bipolar disorder. Acta Psychiatrica Scandinavica, 116(6), 467–472. [DOI:10.1111/j.1600-0447.2007.01069.x] [PMID]
Garrel, C., Alessandri, J. M., Guesnet, P., & Al-Gubory, K. H. (2012). Omega-3 fatty acids enhance mitochondrial superoxide dismutase activity in rat organs during post-natal development. The International Journal of Biochemistry & Cell Biology, 44(1), 123–131. [DOI:10.1016/j.biocel.2011.10.007] [PMID]
Giordano, E., & Visioli, F. (2014). Long-chain omega 3 fatty acids: Molecular bases of potential antioxidant actions. Prostaglandins, Leukotrienes, and Essential Fatty Acids, 90(1), 1–4.[DOI:10.1016/j.plefa.2013.11.002] [PMID]
Guillot, N., Caillet, E., Laville, M., Calzada, C., Lagarde, M., & Véricel, E. (2009). Increasing intakes of the long-chain omega-3 docosahexaenoic acid: Effects on platelet functions and redox status in healthy men. FASEB Journal, 23(9), 2909–2916. [DOI:10.1096/fj.09-133421] [PMID]
Heshmati, J., Morvaridzadeh, M., Maroufizadeh, S., Akbari, A., Yavari, M., & Amirinejad, A., et al. (2019). Omega-3 fatty acids supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacological Research, 149, 104462. [DOI:10.1016/j.phrs.2019.104462] [PMID]
Iraz, M., Erdogan, H., Ozyurt, B., Ozugurlu, F., Ozgocmen, S., & Fadillioglu, E. (2005). Brief communication: Omega-3 essential fatty acid supplementation and erythrocyte oxidant/antioxidant status in rats. Annals of Clinical and Laboratory Science, 35(2), 169–173. [PMID]
Jahangard, L., Yasrebifar, F., Haghighi, M., Ranjbar, A., & Mehrpooya, M. (2019). Influence of adjuvant Coenzyme Q10 on inflammatory and oxidative stress biomarkers in patients with bipolar disorders during the depressive episode. Molecular Biology Reports, 46(5), 5333–5343. [DOI:10.1007/s11033-019-04989-z] [PMID]
Jiménez-Fernández, S., Gurpegui, M., Garrote-Rojas, D., Gutiérrez-Rojas, L., Carretero, M. D., & Correll, C. U. (2021). Oxidative stress parameters and antioxidants in patients with bipolar disorder: Results from a meta-analysis comparing patients, including stratification by polarity and euthymic status, with healthy controls. Bipolar Disorders, 23(2), 117–129.[DOI:10.1111/bdi.12980] [PMID]
Katrenčíková, B., Vaváková, M., Paduchová, Z., Nagyová, Z., Garaiova, I., & Muchová, J., et al. (2021). Oxidative stress markers and antioxidant enzymes in children and adolescents with depressive disorder and impact of omega-3 fatty acids in randomised clinical trial. Antioxidants, 10(8), 1256. [DOI:10.3390/antiox10081256] [PMID] [PMCID]
Lokeshu, T., Lakshmi, V., Kumari, V. J., Bhargavi, C., & Sindhu, P. M. (2020). Plasma antioxidant levels in bipolar disorder. Haya, 5(10), 208-214 [DOI:10.36348/sjls.2020.v05i10.003]
Maes, M., Yirmyia, R., Noraberg, J., Brene, S., Hibbeln, J., & Perini, G., et al. (2009). The inflammatory & neurodegenerative (I&ND) hypothesis of depression: Leads for future research and new drug developments in depression. Metabolic Brain Disease, 24(1), 27–53. [DOI:10.1007/s11011-008-9118-1] [PMID]
Mahadik, S. P., Pillai, A., Joshi, S., & Foster, A. (2006). Prevention of oxidative stress-mediated neuropathology and improved clinical outcome by adjunctive use of a combination of antioxidants and omega-3 fatty acids in schizophrenia. International Review of Psychiatry, 18(2), 119–131. [DOI:10.1080/09540260600581993] [PMID]
Mansur, R. B., Rizzo, L. B., Santos, C. M., Asevedo, E., Cunha, G. R., & Noto, M. N., et al. (2016). Bipolar disorder course, impaired glucose metabolism and antioxidant enzymes activities: A preliminary report. Journal of Psychiatric Research, 80, 38–44. [DOI:10.1016/j.jpsychires.2016.05.014] [PMID]
Parellada, M., Llorente, C., Calvo, R., Gutierrez, S., Lázaro, L., & Graell, M., et al. (2017). Randomized trial of omega-3 for autism spectrum disorders: Effect on cell membrane composition and behavior. European Neuropsychopharmacology, 27(12), 1319–1330. [DOI:10.1016/j.euroneuro.2017.08.426] [PMID]
Patel, R. S., Virani, S., Saeed, H., Nimmagadda, S., Talukdar, J., & Youssef, N. A. (2018). Gender differences and comorbidities in US adults with bipolar disorder. Brain Sciences, 8(9), 168. [DOI:10.3390/brainsci8090168] [PMID] [PMCID]
Patrick, R. P., & Ames, B. N. (2015). Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: Relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB Journal, 29(6), 2207–2222. [DOI:10.1096/fj.14-268342] [PMID]
Rahsepar, S., & Mohammadpour, A. (2021). Oxidative stress and bipolar mood disorder: An important yet ambiguous relationship. Journal of Pharmaceutical Care, 9(4), 195-208. [DOI:10.18502/jpc.v9i4.8225]
Rutkofsky, I. H., Khan, A. S., Sahito, S., & Kumar, V. (2017). The psychoneuroimmunological role of omega-3 polyunsaturated fatty acids in major depressive disorder and bipolar disorder. Advances in Mind-Body Medicine, 31(3), 8-16. [Link]
Safari, M., Sadr, S., Mirabzadeh, K., & Saki, M. (2010). [The effect of omega3 and fluvoxamine on the depressive phase in bipolar disorder type I (Persian)]. Iranian Journal of Psychiatry and Clinical Psychology, 16(3), 316-317. [Link]
Sarris, J., Mischoulon, D., & Schweitzer, I. (2012). Omega-3 for bipolar disorder: Meta-analyses of use in mania and bipolar depression. The Journal of Clinical Psychiatry, 73(1), 81–86.[DOI:10.4088/JCP.10r06710] [PMID]
Saunders, E. F., Ramsden, C. E., Sherazy, M. S., Gelenberg, A. J., Davis, J. M., & Rapoport, S. I. (2016). Omega-3 and omega-6 polyunsaturated fatty acids in bipolar disorder: A review of biomarker and treatment studies. The Journal of Clinical Psychiatry, 77(10), e1301–e1308. [DOI:10.4088/JCP.15r09925] [PMID] [PMCID]
Sepidarkish, M., Akbari-Fakhrabadi, M., Daneshzad, E., Yavari, M., Rezaeinejad, M., & Morvaridzadeh, M., et al. (2020). Effect of omega-3 fatty acid plus vitamin E Co-Supplementation on oxidative stress parameters: A systematic review and meta-analysis. Clinical Nutrition, 39(4), 1019–1025. [DOI:10.1016/j.clnu.2019.05.004] [PMID]
Sivrioglu, E. Y., Kirli, S., Sipahioglu, D., Gursoy, B., & Sarandöl, E. (2007). The impact of omega-3 fatty acids, vitamins E and C supplementation on treatment outcome and side effects in schizophrenia patients treated with haloperidol: An open-label pilot study. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 31(7), 1493–1499. [DOI:10.1016/j.pnpbp.2007.07.004] [PMID]
Siwek, M., Sowa-Kućma, M., Dudek, D., Styczeń, K., Szewczyk, B., & Kotarska, K., et al. (2013). Oxidative stress markers in affective disorders. Pharmacological Reports, 65(6), 1558-1571. [DOI:10.1016/S1734-1140(13)71517-2]
Sommer, I. E., van Westrhenen, R., Begemann, M. J., de Witte, L. D., Leucht, S., & Kahn, R. S. (2014). Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: An update. Schizophrenia Bulletin, 40(1), 181–191.[DOI:10.1093/schbul/sbt139] [PMID] [PMCID]
Taghizadeh, M., Tamtaji, O. R., Dadgostar, E., Daneshvar Kakhaki, R., Bahmani, F., & Abolhassani, J., et al. (2017). The effects of omega-3 fatty acids and vitamin E co-supplementation on clinical and metabolic status in patients with Parkinson's disease: A randomized, double-blind, placebo-controlled trial. Neurochemistry International, 108, 183–189. [DOI:10.1016/j.neuint.2017.03.014] [PMID]
Vieta, E., Berk, M., Schulze, T. G., Carvalho, A. F., Suppes, T., & Calabrese, J. R., et al. (2018). Bipolar disorders. Nature Reviews. Disease Primers, 4, 18008. [DOI:10.1038/nrdp.2018.8] [PMID]
Yavin, E., Brand, A., & Green, P. (2002). Docosahexaenoic acid abundance in the brain: A biodevice to combat oxidative stress. Nutritional Neuroscience, 5(3), 149–157.[DOI:10.1080/10284150290003159] [PMID]
Zararsiz, I., Meydan, S., Sarsilmaz, M., Songur, A., Ozen, O. A., & Sogut, S. (2011). Protective effects of omega-3 essential fatty acids against formaldehyde-induced cerebellar damage in rats. Toxicology and Industrial Health, 27(6), 489–495.[DOI:10.1177/0748233710389852] [PMID]
Type of Study: Original |
Subject:
Clinical Neuroscience
Received: 2022/06/13 | Accepted: 2022/10/31 | Published: 2024/01/1
Received: 2022/06/13 | Accepted: 2022/10/31 | Published: 2024/01/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








