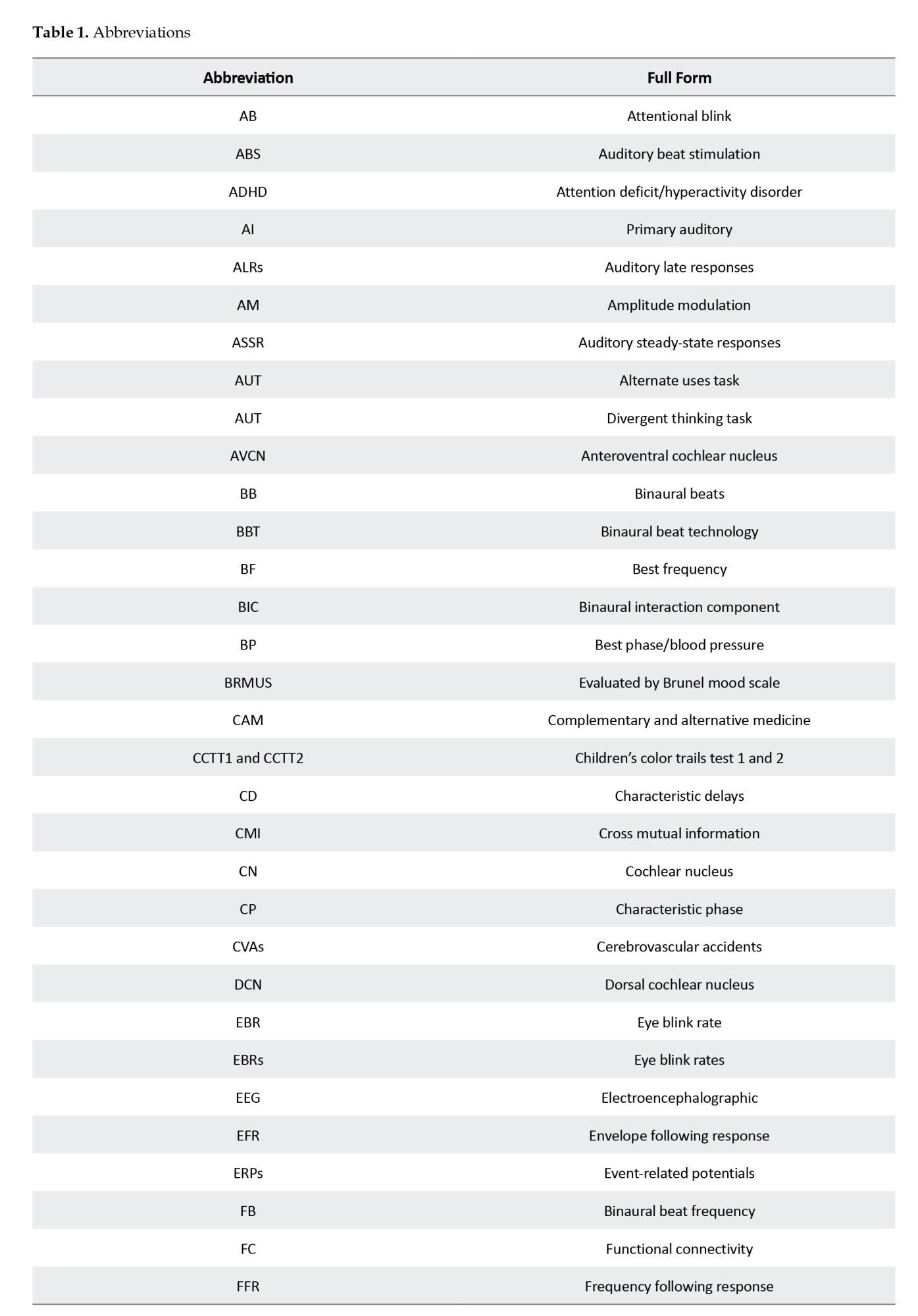
Volume 15, Issue 2 (March & April 2024)
BCN 2024, 15(2): 133-146 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mirmohamadi S, Norozpour Y, Zarrabian S. A Review of Binaural Beats and the Brain. BCN 2024; 15 (2) :133-146
URL: http://bcn.iums.ac.ir/article-1-2444-en.html
URL: http://bcn.iums.ac.ir/article-1-2444-en.html
1- Faculty of Medicine, Tehran Medical Sciences Branch, Islamic Azad University, Tehran, Iran.
2- Institute for Cognitive Science Studies (ICSS), Tehran, Iran.
3- Department of Anatomical Sciences & Cognitive Neuroscience, Faculty of Medicine, Tehran Medical Sciences Branch, Islamic Azad University, Tehran, Iran.
2- Institute for Cognitive Science Studies (ICSS), Tehran, Iran.
3- Department of Anatomical Sciences & Cognitive Neuroscience, Faculty of Medicine, Tehran Medical Sciences Branch, Islamic Azad University, Tehran, Iran.
Keywords: Auditory beat stimulation, Binaural beat, Monaural beat, Cognitive functions, Psychological effects
Full-Text [PDF 735 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Along with the advances in our knowledge about brain function, new methods are developed to improve cognitive functions and psychological states. Auditory beat stimulation (ABS) is one of these techniques that is claimed to induce brainwave entrainment, a frequency-following response of brainwaves. ABS has been considered for its potential applications in understanding and assessing auditory system responses, audiometric parameters, and mechanisms of sound localization (Kuwada et al., 1979). ABS can be applied as monaural beats (MBs) and binaural beats (BBs). To present MBs to a subject, two sinusoidal waves with close frequencies are instantaneously presented to both ears. When these waves are given to each ear separately, they are called BBs (Wernick & Starr 1968; Oster, 1973). MBs are also considered peripheral or objective beats, first distinguished by the cochlear, then forwarded to the brain stem, and afterward to the auditory cortex (AC). On the other hand, BBs are considered central or subjective beats, as the BB perception is formed from the distinctness in the phase of the waves that reach the right and left ears (Kuwada et al., 1979; Draganova et al., 2008). The superior olivary nucleus in the brainstem produces BB auditory responses when the ears are provided with two low-frequency tones (less than 1000 Hz) with steady intensities but different frequencies. Neurons of the inferior colliculus (IC) then recognize the variance in the interaural phase as a beat (Licklider et al., 1950; Perrott & Musicant, 1977; Schwarz & Taylor, 2005; Ross & Lopez, 2020). The beat frequency is halfway between the two carrier tones (Oster 1973; Pantev et al., 1996; Wahbeh et al., 2007; Pratt et al., 2009; Grose & Mamo 2012).
Experiments in animal models (Wernick & Starr 1968; Kuwada et al., 1979; Reale & Brugge, 1990; McAlpine et al., 1996; McAlpine et al., 1998; Spitzer & Semple, 1998) and humans (Barr et al., 1977; Starr et al., 1991; Lane et al., 1998) confirmed the involvement of structures in the auditory brainstem and cortex in ABS and BBs.
BB technology has been claimed to have benefits such as reduction in stress and anxiety, improvement in cognitive functions, including memory and attention, and improvements in other psychological states such as motivation and confidence (Wahbeh et al., 2007; Chaieb et al., 2015; Garcia-Argibay et al., 2019b).
Early reports show that the activity of the neurons in the auditory pathways of the brainstem follows the phase pattern of an incoming sound (Hink et al., 1980). Later research shows that BBs cause brainwave entrainment, which is a frequency-dependent EEG response to an external rhythmic stimulus (Karino et al., 2006; Huang & Charyton, 2008; Ozdamar et al., 2011; Seifi Ala et al., 2018). That is to say, similar EEG patterns during a cognitive function can be induced using synchronized pulsing stimuli (Tang et al., 2015; Lee et al., 2019).
For example, gamma frequencies (40 Hz) are reported to entrain brain oscillations; we already know that gamma oscillations play a key role in attention, feature binding, learning, and memory. In addition, BB stimulation has been reported to be beneficial for memory (Tallon-Baudry & Bertrand, 1999; Jensen et al., 2007; Tort et al., 2009; Kraus & Porubanova, 2015; Beauchene et al., 2017; Jirakittayakorn & Wongsawat, 2017a; Garcia-Argibay et al., 2019a), attention (Jensen et al., 2007; Lakatos et al., 2008; Kennel et al., 2010), vigilance (Chaieb et al., 2015), creativity (Reedijk et al., 2013), anxiety control (Le Scouarnec et al., 2001; Garcia-Argibay et al., 2019b), modulation of mood states (Chaieb et al., 2015; Jirakittayakorn & Wongsawat, 2017a), pain perception (Garcia-Argibay et al., 2019b), induction of meditation (Lavallee et al., 2011), and improvement of sleep quality (Chan et al., 2010; Bellesi et al., 2014; Besedovsky et al., 2017). Recent evidence also suggests that BB provokes cross-frequency connectivity patterns in the brain (Orozco Perez et al., 2020).
Based on the mentioned findings, it has been proposed that ABS, especially BBs, can be considered a digital drug and potential therapeutic method either alone or in combination with other ingestible drugs (Barratt et al., 2022). In this review study, we searched the PubMed and Google Scholar databases using the keywords “auditory beat stimulation,” “binaural beats,” “monaural beats,” and “brain” to look further into the mechanism of action and preclinical and clinical reports of BBs application and shed lights on BBs effects on the nervous system.
The concept of binaural beats and evoked potentials
BB concept was described by H. W. Dove in 1839. Later, it was claimed that rhythmic sensory stimulation, as an alternative to electrical stimulation, can entrain neural oscillations beyond the related brain areas (Walter & Walter, 1949). One year later, Licklider et al., (1950) assessed the frequency limits and outlined the BBs theory. They explained how synchronal activity in both auditory nerves resulted in the BB effect. Oster (1973) showed that BBs were detectable for carrier frequencies below 1000 Hz, and further research revealed that the brain cortex only encodes low carrier frequencies (Schwarz & Taylor, 2005).
Steady-state auditory responses (ASSRs) are evoked by acoustic beats. ASSRs originate at the brainstem, and the responses to stimulus rates (1-200 Hz) are recordable at the cortical level of the scalp (Dolphin, 1997; Picton et al., 2003). Prominent responses are observed when stimulus rates are near 40 Hz (Galambos et al., 1981; Herdman et al., 2002). Auditory thresholds (Picton et al., 2005) and the depth of anesthesia (Plourde, 2006) can also be measured using ASSRs.
The carrier frequency is also important in the outcome of BB stimulation. For example, a 40-Hz BB potential was observed following a 400-Hz carrier frequency but not when the carrier frequency was above 3 kHz (Schwarz & Taylor, 2005). Spectral analyses of the magnetic fields shows that BBs (4.00 or 6.66 Hz; carrier frequency 240 or 480 Hz) evoke small amplitude magnetic fields (Karino et al., 2006). BBs and acoustic beats have similar cortical processing as BBs (3 and 6 Hz), and amplitude-modulated acoustic beats (3 & 6 Hz; carrier frequency 250 &1000 Hz; 2000 ms duration; 1-s intervals) yield comparable cortical activity and perceptions (Pratt et al., 2010). The temporary auditory responses are separable from BB auditory illusion using binaural interaction component analysis (Ozdamar et al., 2011).
Binaural beat mechanism of action
Action potentials are fired when sound energy passes through the ears and cochlea and reaches the inner hair cells. Auditory nerve fibers carry the auditory information from the cochlea and join the vestibulocochlear nerve. The information enters the cochlear nucleus and bifurcates. The branches of the nerve fibers form synapses with stellate-, globular-, and spherical-bushy cells, which have specific temporal and spectral response properties (Wu & Oertel, 1984). The sound information then travels to either the superior olivary complex (projections of the bushy cells of the anteroventral cochlear nucleus) or to the IC (outputs of the stellate and dorsal cochlear nucleus cells) (Goldberg & Brownell, 1973). The superior olivary complex processes the data related to sound originating from both ears (Moore, 2012). The left and right IC make binaural interactions through commissural connections and the ascending pathway subnuclei. The information is then relayed to the medial geniculate nucleus and the AC. The processing of wave specifications depends on the integration time between the IC and the AC (Fitzpatrick et al., 2009; Bloom, 2013; Croom, 2014).
Auditory neurons discharge differently at various frequencies. For instance, synchronous discharges happen in response to low frequencies but not in response to shape synaptic summation. When the frequencies reach higher, neuronal discharges happen in turns. At middle frequencies, neurons respond in many volleys, and those involved in each volley fire synchronal, resulting in the appearance of beats (Licklider et al., 1950).
Kuwada et al., (1979) studied the interaural phase sensitivity of neurons using BBs and showed that many neurons phase-locked to the beat frequency. Other studies show that in addition to the primary AC, ASSRs responses to BBs can be recorded from the superior temporal, posterior parietal, and frontal cortices, which primarily originate in the AC, especially in response to gamma-frequency stimulation (Pastor et al., 2002). Recording of small amplitude magnetic fields following the application of low-frequency BBs (4.00-6.66 Hz) shows that BBs can synchronize the cortex activity (Karino et al., 2006; Pratt et al., 2009), and reports indicate that the interaural time difference that happens within early rising amplitude (20–25 ms) plays a key role in the prediction of perceived BB lateralization (Haywood & McAlpine, 2020). A comparison of the effects of BBs across four levels of subcortical, cortical, and scalp-level functional connectivity shows that BBs weakly entrain the cortex and generate cross-frequency connectivity patterns (Orozco Perez et al., 2020). Some other studies have recorded ERP N100 to BBs and amplitude modulation stimuli and reported separate processing sites for structure-based spatial processing and envelope-based level processing. The recorded N1 component also shows an age-related decline in magnitude (Ungan et al., 2019).
BBs effects have also been studied on the synchronization of the brain hemispheres. It has been reported that BBs frequencies (10 Hz, alpha; 4Hz, theta) increase alpha frequencies interhemispheric coherence and are interpreted as binaural integration rather than entrainment (Solcà et al., 2016).
Nevertheless, the responses to BBs are not always the same, and the brain areas respond differently to various beat frequencies. For example, the study by Karino et al. (2006) indicates that the application of four different BBs at 4.00- and 6.66-Hz (240-480 Hz; 10 min) induces ASSRs in the frontal region and also in the temporal and parietal areas but symmetry does not always occur. A magnetoencephalography (MEG) study shows right temporal responses to a 40-Hz BB after 1 s of BB application (Draganova et al., 2008). Another study reports that the presentation of 7- and 15-Hz BBs (15 min) increases left temporal delta power for the 7-Hz BB and gamma power for the 15-Hz BB (Lavallee et al., 2011). In a study on MEG, Chakalov et al. (2014) used 26-Hz BBs (250 Hz carrier tone; 500 ms) and reported a 26-Hz ASSR at the right parietal and left middle frontal regions. Also, left hemisphere dominance was observed in 3 Hz BB after 15 minutes and 15 Hz BB after 5 minutes. The right hemisphere dominance occurs in 10 Hz BB after 25 min. All brain areas are enhanced after a 6-Hz beat within 10 minutes. Differences are also observed in the frontal lobe, and responses are enhanced with 40 Hz beats, but 8 Hz and 25 Hz beats do not create any clear responses (Jirakittayakorn & Wongsawat, 2015). In a second study, Jirakittayakorn and Wongsawat (2017b) investigated theta activity responses (6-Hz BBs, 250 Hz carrier tone, 30 minutes) and reported that theta waves were observable in all cortex regions after 10 min of BB application. They also reported a meditation effect, measured by the Brunel mood scale.
Distribute processing across hemispheres has also been reported. For instance, BBs amplify the coherence of left and right auditory regions in contrast to MBs and resting state; it is inferred that the increased coherence selectively concerns the alpha band independent of BB frequency. These changes do not happen along with changes in amplitude (Solcà et al., 2016).
The effect of carrier tones has also been investigated in EEG studies. At the frontocentral region, BB stimulation (40 Hz) induces higher responses on a lower (400 Hz) carrier tone than a higher (3200 Hz) one (Schwarz & Taylor, 2005). Another study reports that the application of BBs (3 & 6 Hz; 250 & 1000 Hz; 2 s) generates left temporal ERPs that are more prominent for 250 Hz than 1000 Hz and similarly higher for 3 Hz than 6 Hz (Pratt et al., 2009).
A study on the differences in the perception of BBs, measured for 4, 8, 16, and 32 Hz (500-Hz carrier tone), reports that variability in perceiving BBs is due to the measurement plan (Grose et al., 2012).
EEG activity following BB stimulation
When BB is presented to a subject, two tones with close frequencies are presented to the left and right ears, and the brain perceives a third sound, the BB, that forms from integrating the presented signals. EEG alterations have been reported differently in the studies. To test if BB affects functional brain connectivity, Mujib et al. (2021) measured relative power, phase-locking value, and cross-mutual information in EEG recordings during delta (1 Hz), theta (5 Hz), alpha (10 Hz), and beta (20 Hz) band BB stimulations. The results show that the application of delta and alpha BB increased and decreased relative power in theta and beta bands, respectively. Theta BB stimulation also diminishes beta band relative power. No entrainment was reported, but the connectivity pattern showed variations. In another study, the frequency of the following responses was reported in delta, theta, and gamma bands but not in alpha and beta bands following exposure to BBs for ten 1-minute epochs (Vernon et al., 2014).
On the other hand, López et al. (2017) investigated brainwave entrainment. They reported no significant EEG spectral power changes for epochs of 3 min in theta (4.53 Hz), alpha (8.97 Hz), beta (17.93 Hz), gamma (34.49 Hz), or upper gamma (57.3 Hz) bands. Goodin et al. (2012) also studied brainwave entrainment following BB stimulation at beta and theta frequencies and reported that short presentation of BBs was insufficient to generate entrainment or alter vigilance.
Vernon et al. (2014) performed a limited recording of EEG (at T3 and T4) during the application of alpha (10 Hz) and beta (20 Hz) BBs. They reported greater beta activity in the left temporal region but no alteration in alpha activity. ACs show greater alpha-band synchrony after the application of alpha (10 Hz) and theta (4 Hz) BBs, which is a reflection of binaural integration (Solcà et al., 2016). Kasprzak et al. (2011) tested the brain’s ability to change its main activity frequency according to a dominant applied stimulus. They observed a significant decline in alpha rhythm (8–12 Hz) and simultaneous increment of narrow band share (9.9–10.1 Hz). They proposed that the blockade of alpha rhythm was due to the response of CNS to the acoustic stimulus and tuning to enhance the receipt of environmental information.
Studies have also investigated the effects of MBs and BBs on EEG power, phase patterns, and phase synchronization (Schwarz & Taylor, 2005; Becher et al., 2015; Derner et al., 2018). Prominent alterations have been observed at theta (5 Hz) range in the temporal regions, rhinal cortex, and hippocampus. The mediotemporal structure is important in memory function (Eichenbaum 2000). Fell et al. (2011) demonstrated that phase-related mechanisms, including phase synchronization, play a key role in long-term memory processing. In addition, a study in presurgical epilepsy patients shows that MB and BB stimulations alter brain activity power and synchronous phase (Becher et al., 2015). A recent study investigated the differential effects of MBs and BBs on phase synchronization and long-term memory performance. It has been reported that theta (5 Hz) range BB increased temporolateral phase synchronization while MB stimulation at the same frequency decreased mediotemporal phase synchronization. In addition, 5 Hz BB increases, and 5 Hz MBs diminishes both words and association memory. The results indicate that intracranial EEG phases change the threshold of neurons and neural activity, which may result in memory-related activity alterations within the required time window (Derner et al., 2021).
Animal models and our understanding of BBs
The experimental data from animal models help us better understand the basis of the BB mechanism of action. Early evidence came from the work by Kuwada et al. showing a phase-locked response in most cats’ IC cells to the BB frequency (Kuwada et al., 1979). The study by McAlpine et al. (1996) shows that in guinea pigs, single neurons in the IC process BBs at low frequencies, similar to other species. They also proposed that interaural-delay sensitivity differs for various frequencies and changes within each frequency band. Two years later, studies report that a system different from spatial processing of position is responsible for interaural phase responses, and convergence was reported from simple brainstem coincidence detectors (McAlpine et al., 1998; Spitzer & Semple, 1998). Reale et al. (1990) studied neurons in the primary AC in cats to evaluate the interaural-phase-difference sensitivity of the neurons using different stimulus frequencies (120 to 2500 Hz). Data showed a direct association between the interaural phase and beat frequency. Many of the studied cells responded equally to BBs, and an increase followed a rise in BB frequency in the action potentials of the neurons. However, after a certain frequency (35 Hz), the AC neurons could not follow the rate.
The cognitive effects of binaural beats
Table 1 summarizes the studies regarding auditory beat stimulation, indicating a rising trend for BBs application since 1947. With the rapid growth in cognitive sciences, BB stimulation has also become alluring to many researchers as a non-invasive method that may improve cognitive functions.


Application of 40-Hz gamma BB and MB for the assessment of attention (Flanker task) and working memory (Klingberg task) in high and low emotional participants shows that listening to BB at beta (15 Hz) range during the N-back task alters network connections and improves the accuracy of performance (Beauchene et al., 2017). Another study found that both BB and MB similarly enhance the speed of performance in the attention task, and their effects are also similar in high- and low-emotional participants (Engelbregt et al., 2019). A study on the attentional blink (AB) using MEG recordings shows that gamma (40-Hz) BB stimulation during training enhances the attentional blink task outcomes. However, the improvement is evident only after consolidation during sleep (Ross & Lopez, 2020). Using EEG recordings and a 5-minute presentation of 40 Hz BB, another study indicates that BB stimulation improves attention without neural entrainment (Engelbregt et al., 2021). The application of gamma-frequency (40 Hz) during a global-local task was reported to reduce the spotlight of attention (Colzato et al., 2017). A meta-analysis suggests that BBs positively affect attention (average effect size 0.58) (Garcia-Argibay et al., 2019a). On the other hand, beta-frequency (16 Hz) BB does not enhance sustained attention when measured by pupillary measures (Robison et al., 2021). The application of BB (20 min; 3 times a week; 3 weeks) does not reduce inattention in children and adolescents with attention-deficit/hyperactivity disorder (ADHD) (Kennel et al., 2010). However, parents of ADHD children reported improvement in homework problems during the 3 weeks of the study (Wiwatwongwana et al., 2016).
Application of BBs (beta, 16 and 24 Hz or theta/delta, 1.5 and 4 Hz; for 30 min) in visual vigilance task shows that beta-frequency BBs enhance psychomotor performance and increase the number of correct detections in comparison to theta or delta frequencies. Furthermore, the beta frequency is associated with less negative mood in the subjects under the study (Lane et al., 1998). Little et al. (1992) used the five-factor model to access participants’ personality traits and recorded EEG. They did not find any significant effects of theta (7 Hz) or beta (16 Hz) BBs applied during a psychomotor vigilance task, which needs the ability to remain focused and alert to stimuli over prolonged periods. Also, no correlations were observed between the stimulations and personality. The brief duration of exposure to BB stimulation might have had a key role in the observed results. However, Lane et al., (1998) applied BBs at beta (16 and 24 Hz) throughout a psychomotor vigilance task and reported improved performance in a vigilance task.
In a test of verbal memory, Wahbeh et al. (2007) reported that the application of BBs (7 Hz; 30 min) declined verbal memory recall. However, applying 5 Hz BBs (15 min; twice per day; 15 days) increased the number of words recalled (Ortiz et al., 2008). Beta-frequency (20 Hz) BBs in long-term memory tests produce better performance for recalled words and a higher sensitivity index in recognition tasks. However, theta-frequency (5 Hz) BBs diminish the performance of remembered words and the sensitivity index. Hence, depending on the frequency used, BBs positively or negatively affect long-term memory (Garcia-Argibay et al., 2019b). Chaieb et al. (2015) also mentioned that longer application of BBs affects verbal memory recall. BB stimulation at theta frequency (5 Hz) over a long time generated a coupling of brain activity and improved the capacity of immediate verbal memory (Ortiz et al., 2008). BBs have also been reported to have positive applications for neurological disorders and for older people to improve memory. For example, a study shows a significant increase in alpha frequency along with a significant decrease in reaction time in alpha (10 Hz; 15 min) and gamma (30 Hz; 15 min) frequencies (Mujib et al., 2021). In the N-back test, listening to beta-frequency (15 Hz) BB stimulation enhances accuracy and alters cortical network connection strengths (Beauchene et al., 2017). BB at beta (15 Hz) band also improves working memory by inducing beta activity in the brain (Beauchene et al., 2017).
To assess the effects of BBs on creativity, Reedijk et al. (2013) applied BBs at the alpha (10 Hz) and gamma (40 Hz) bands for 3 minutes before the divergent and convergent thinking tasks. Measurement of dopamine levels in the striatum using spontaneous eye blink rates (EBRs) showed that both applied BBs affected divergent but not convergent thinking. Alpha BBs enhance divergent thinking, mostly in subjects with low EBRs.
The psychological and physiological effects of binaural beats
BB stimulations have also been reported to have positive outcomes in pre-operative state-based anxiety (Padmanabhan et al., 2005; Weiland et al., 2011; Ungan et al., 2019; Ölçücü et al., 2021), anxiety in psychiatric outpatients (Yusim & Grigaitis, 2020), patients suffering from traumatic brain injury (Klepp & Summer, 2006), and dental anxiety (Menziletoglu et al., 2021). It has also been reported that BBs reduce pain scores associated with unit status reporting procedures (Ölçücü et al., 2021) and pain perception (Garcia-Argibay et al., 2019b). In a study on chronic pain patients, Gkolias et al.(2020) used theta (5 Hz) band BB to investigate if the resulting brain entrainment would decline pain perception and analgesic medication use and reported the effectiveness of BBs in the reduction of pain intensity, analgesic use, and stress. BB therapy has also been suggested as a therapeutic method that holds promise for the management of chronic pain in conditions like cancer (Zampi, 2016), for reducing morphine consumption in patients who underwent knee replacement (Tani et al., 2021), and for relieving pain in cases undergoing colonoscopy without sedation (Tani et al., 2022).
In patients with cataracts, BB stimulation reduces state-trait anxiety, heart rate, and systolic blood pressure during surgery, and it is suggested that BB-embedded musical intervention is beneficial over musical intervention alone (Wiwatwongwana et al., 2016). Theta BB also diminishes subsequent stress responses to an acute psychological stressor (Kelton et al., 2021). Listening to BB influences the power of both low-frequency, reflecting sympathetic and parasympathetic activity, and high-frequency, reflecting parasympathetic activity, components of heart rate variability, and LF/HF normalized powers, which was accompanied by a subjective report of relaxation (McConnell et al., 2014). Using the mood states questionnaire (McNair & Heuchert 2011), Wahbeh et al. (2007) reported an anxiety reduction. A study reports that theta-frequency (6 Hz; 250 Hz) BBs enhance the meditative state after 10 min of exposure, which can reduce stress (Jirakittayakorn and Wongsawat, 2017b). In dentistry, alpha-frequency (9.3 Hz; 200 Hz) BBs are also found helpful in reducing preoperative anxiety (Isik et al. 2017). Music with or without BB decreases anxiety and reduces systolic blood pressure. However, those who received BBs showed an additional decrease in heart rate and operative anxiety (Wiwatwongwana et al., 2016).
Lane et al., (1998) reported a decline in depression following the application of BB in the beta range (16 and 24 Hz), which indicates the association of beta BB with less negative mood (Chaieb et al., 2015).
Applying BBs in Parkinson disease (PD) normalizes EEG power and brain functional connectivity and improves working memory with no significant changes in the gait performance or anxiety level (Gálvez et al., 2018). A systematic scoping review also reports that non-invasive brain stimulation methods, such as BBs, change quantitative EEG in PD patients and recommends further research to confirm EEG as a biomarker (Costa et al., 2022).
BBs of theta and delta range during naps increase sleep stability, which can be considered a non-pharmacologic way of sleep treatment (Shumov et al., 2020). Subsequent reports also show that BBs enhance the activity of the parasympathetic part of the autonomic nervous system during naps (Bakaeva et al., 2021). Changing BBs in frequency from 8 to 1 Hz alleviates sleep initiation and maintenance difficulties in patients with chronic insomnia (Tang et al., 2015). Theta-range BBs also increase daytime alertness in subclinical insomnia (Bang et al., 2019). To discover new nonpharmacological methods for treating sleep disorders, Munoz et al. (2020) used a combination of real-time automatic sleep stage classification and a BB generator and showed that BBs improved sleep quality. BBs at the delta (3 Hz) band induce delta activity and increase the duration of non-rapid eye movement (NREM) in the third stage of sleep (Jirakittayakorn & Wongsawat, 2018). The BBs at theta (6 Hz) band have meditative effects and induce theta activity in the frontal and parietal-central regions (Jirakittayakorn and Wongsawat, 2017b). Subjects who used 6 Hz BB for 30 minutes before bedtime for 2 to 14 days showed a reduced hyperarousal state, which could contribute to sleep induction (Lee et al., 2022).
BB stimulation is used in the treatment of stroke, brain injury, tinnitus, dementia, and other cognitive deficits, and studies on the outcomes have provided evidence of the advantage of rehabilitation with music over one without music (David et al., 2010; Galińska, 2015). A summary of the application of BB stimulation has shown that listening to BBs for a suggested period can have the following effects on different behaviors:
1) BBs in the delta (0.5–4 Hz) pattern during sleep causes deeper stages of sleep (Jirakittayakorn & Wongsawat, 2018) and reduce mild anxiety (Le Scouarnec et al., 2001) and pre-operative state-based anxiety (Padmanabhan et al., 2005); 2) BBs in the theta pattern (4–7 Hz) improves meditation, creativity, REM sleep (Jirakittayakorn & Wongsawat, 2018), and relieves mild anxiety (Le Scouarnec et al., 2001; Wahbeh et al., 2007); 3) BBs in the alpha pattern (7–13 Hz) encourages relaxation (Jirakittayakorn & Wongsawat, 2018) and reduces anxiety (Weiland et al., 2011); 4) BBs in the beta pattern (13–30 Hz) promotes concentration and alertness but also increases anxiety, especially at the higher end of the range (Jirakittayakorn & Wongsawat, 2018); 5) BBs in the gamma pattern (30–50 Hz) enhances the maintenance of arousal (Jirakittayakorn & Wongsawat, 2018).
Possible complications of binaural beats
Some reports indicate that BBs’ repetitive nature could make people uncomfortable (Lee et al., 2019). In previous sections, we provided evidence that BBs have beneficial cognitive and non-cognitive effects. However, what goes undetected are the side effects. Until now, no study has reported any side effects during or after listening to BBs. However, keeping the sound level below the maximum safety level of 85 dB seems sensible, as they can cause hearing loss over time (Keith et al., 2008; Tuomi & Jellimann, 2009). Also, it is highly recommended that those with epilepsy consult with their doctors before trying BBs (Vernon et al., 2014).
Based on the alterations in behaviors mentioned in this review by some studies, it is warranted that more research be performed into the behavior alterations observed following intended changes (e.g. improvement of sleep behavior may also cause depression).
Binaural beats and future research directions
In addition to the duration of the stimulation (Chaieb et al., 2015), the choice of carrier tones for the stimulation should be considered, as reports indicate that lower carrier tones may result in more robust effects (Pratt et al., 2010). Some reports also suggest that adding background noise (pink or white) affects the processing of the BBs perception (Reedijk et al., 2013).
It must be considered that cognitive entrainment methods, including BBs, are not universal approaches, and we should consider the individual state.
The meta-analysis results also show that BBs positively affect cognition more than reducing anxiety and pain levels (Garcia-Argibay et al., 2019b). Hence, in designing a study frequency band, time under exposure and intervention time must be chosen to maximize the possible magnitude and direction of responses.
To sum up, the evidence discussed in this paper shows that auditory beats stimulation may be a useful tool for rehabilitation training to enhance both learning and training. However, we must solve the jigsaw puzzle by involving factors and the best configurations for each application. Hence, further research is warranted into the role of personality traits and factors such as duration, frequency, and carrier tones of the stimulation.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
Bakaeva, Z. V., Shumov, D. E., Yakunina, E. B., Starshinov, Y. P., Sveshnikov, D. S., & Torshin, V. I., et al. (2021). [The effect of music embedded with binaural beats on heart rate parameters during nap (Russian)]. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova, 121(4. Vyp. 2), 31–35. [DOI:10.17116/jnevro202112104231] [PMID]
Bang, Y. R., Choi, H. Y., & Yoon, I. Y. (2019). Minimal effects of binaural auditory beats for subclinical insomnia: A randomized double-blind controlled study. Journal of Clinical Psychopharmacology, 39(5), 499–503. [DOI:10.1097/JCP.0000000000001097] [PMID]
Barr, D. F., Mullin, T. A., & Herbert, P. S. (1977). Application of binaural beat phenomenon with aphasic patients. Archives of Otolaryngology, 103(4), 192–194. [DOI:10.1001/archotol.1977.00780210048003] [PMID]
Barratt, M. J., Maddox, A., Smith, N., Davis, J. L., Goold, L., & Winstock, A. R., et al. (2022). Who uses digital drugs? An international survey of 'binaural beat' consumers. Drug and Alcohol Review, 41(5), 1126–1130. [DOI:10.1111/dar.13464] [PMID]
Beauchene, C., Abaid, N., Moran, R., Diana, R. A., & Leonessa, A. (2017). The effect of binaural beats on verbal working memory and cortical connectivity. Journal of Neural Engineering, 14(2), 026014. [DOI:10.1088/1741-2552/aa5d67] [PMID]
Becher, A. K., Höhne, M., Axmacher, N., Chaieb, L., Elger, C. E., & Fell, J. (2015). Intracranial electroencephalography power and phase synchronization changes during monaural and binaural beat stimulation. The European Journal of Neuroscience, 41(2), 254–263. [DOI:10.1111/ejn.12760] [PMID]
Bellesi, M., Riedner, B. A., Garcia-Molina, G. N., Cirelli, C., & Tononi, G. (2014). Enhancement of sleep slow waves: Underlying mechanisms and practical consequences. Frontiers in Systems Neuroscience, 8, 208. [DOI:10.3389/fnsys.2014.00208] [PMID] [PMCID]
Besedovsky, L., Ngo, H. V., Dimitrov, S., Gassenmaier, C., Lehmann, R., & Born, J. (2017). Auditory closed-loop stimulation of EEG slow oscillations strengthens sleep and signs of its immune-supportive function. Nature Communications, 8(1), 1984. [DOI:10.1038/s41467-017-02170-3] [PMID] [PMCID]
Bloom, FE (2013).Fundamentals of Neuroscience. In LR. Squire, D. Berg, FE. Bloom, S. du Lac, A. Ghosh, & NC. Spitzer (Eds.), Fundamental neuroscience (fourth edition) [pp. 3-13]. San Diego: Academic Press. [DOI:10.1016/B978-0-12-385870-2.00001-9]
Chaieb, L., Wilpert, E. C., Reber, T. P., & Fell, J. (2015). Auditory beat stimulation and its effects on cognition and mood States. Frontiers in Psychiatry, 6, 70. [DOI:10.3389/fpsyt.2015.00070] [PMID] [PMCID]
Chakalov, I., Paraskevopoulos, E., Wollbrink, A., & Pantev, C. (2014). Mismatch negativity to acoustical illusion of beat: How and where the change detection takes place?. NeuroImage, 100, 337–346. [DOI:10.1016/j.neuroimage.2014.06.026] [PMID]
Chan, M. F., Chan, E. A., & Mok, E. (2010). Effects of music on depression and sleep quality in elderly people: A randomised controlled trial. Complementary Therapies in Medicine, 18(3-4), 150–159. [DOI:10.1016/j.ctim.2010.02.004] [PMID]
Colzato, L. S., Barone, H., Sellaro, R., & Hommel, B. (2017). More attentional focusing through binaural beats: Evidence from the global-local task. Psychological Research, 81(1), 271–277. [DOI:10.1007/s00426-015-0727-0] [PMID] [PMCID]
Costa, T. D. C., Godeiro Júnior, C., Silva, R. A. E., Dos Santos, S. F., Machado, D. G. D. S., & Andrade, S. M. (2022). The effects of non-invasive brain stimulation on quantitative EEG in patients with parkinson's disease: A systematic scoping review. Frontiers in Neurology, 13, 758452. [DOI:10.3389/fneur.2022.758452] [PMID] [PMCID]
Croom, A. M. (2014). Book review: Auditory neuroscience: Making sense of sound. Musicae Scientiae, 18(2):249-251 [DOI:10.1177/1029864914523548]
David, J. B., Naftali, A., & Katz, A. (2010). Tinntrain: A multifactorial treatment for tinnitus using binaural beats. The Hearing Journal, 63(11), 25-26, 28. [DOI:10.1097/01.HJ.0000390818.17619.65]
Derner, M., Chaieb, L., Dehnen, G., Reber, T. P., Borger, V., & Surges, R., et al. (2021). Auditory beat stimulation modulates memory-related single-neuron activity in the human medial temporal lobe. Brain Sciences, 11(3), 364. [DOI:10.3390/brainsci11030364] [PMID] [PMCID]
Derner, M., Chaieb, L., Surges, R., Staresina, B. P., & Fell, J. (2018). Modulation of item and source memory by auditory beat stimulation: A pilot study with intracranial EEG. Frontiers in Human Neuroscience, 12, 500. [DOI:10.3389/fnhum.2018.00500] [PMID] [PMCID]
Dolphin W. F. (1997). The envelope following response to multiple tone pair stimuli. Hearing Research, 110(1-2), 1–14. [DOI:10.1016/S0378-5955(97)00056-7] [PMID]
Draganova, R., Ross, B., Wollbrink, A., & Pantev, C. (2008). Cortical steady-state responses to central and peripheral auditory beats. Cerebral Cortex, 18(5), 1193–1200. [DOI:10.1093/cercor/bhm153] [PMID]
Eichenbaum H. (2000). A cortical-hippocampal system for declarative memory. Nature reviews. Neuroscience, 1(1), 41–50. [DOI:10.1038/35036213] [PMID]
Engelbregt, H., Barmentlo, M., Keeser, D., Pogarell, O., & Deijen, J. B. (2021). Effects of binaural and monaural beat stimulation on attention and EEG. Experimental Brain Research, 239(9), 2781–2791. [DOI:10.1007/s00221-021-06155-z] [PMID] [PMCID]
Engelbregt, H., Meijburg, N., Schulten, M., Pogarell, O., & Deijen, J. B. (2019). The effects of binaural and monoaural beat stimulation on cognitive functioning in subjects with different levels of emotionality. Advances in Cognitive Psychology, 15(3), 199–207. [DOI:10.5709/acp-0268-8] [PMID] [PMCID]
Fell, J., & Axmacher, N. (2011). The role of phase synchronization in memory processes. Nature Reviews. Neuroscience, 12(2), 105–118. [DOI:10.1038/nrn2979] [PMID]
Fitzpatrick, D. C., Roberts, J. M., Kuwada, S., Kim, D. O., & Filipovic, B. (2009). Processing temporal modulations in binaural and monaural auditory stimuli by neurons in the inferior colliculus and auditory cortex. Journal of the Association for Research in Otolaryngolog, 10(4), 579–593.[DOI:10.1007/s10162-009-0177-8] [PMID] [PMCID]
Galambos, R., Makeig, S., & Talmachoff, P. J. (1981). A 40-Hz auditory potential recorded from the human scalp. Proceedings of the National Academy of Sciences of the United States of America, 78(4), 2643–2647. [DOI:10.1073/pnas.78.4.2643] [PMID] [PMCID]
Galińska E. (2015). Music therapy in neurological rehabilitation settings. Psychiatria Polska, 49(4), 835–846. [DOI:10.12740/PP/25557] [PMID]
Gálvez, G., Recuero, M., Canuet, L., & Del-Pozo, F. (2018). Short-term effects of binaural beats on EEG power, functional connectivity, cognition, gait and anxiety in parkinson's disease. International Journal of Neural Systems, 28(5), 1750055. [DOI:10.1142/S0129065717500551] [PMID]
Garcia-Argibay, M., Santed, M. A., & Reales, J. M. (2019). Binaural auditory beats affect long-term memory. Psychological Research, 83(6), 1124–1136. [DOI:10.1007/s00426-017-0959-2] [PMID]
Garcia-Argibay, M., Santed, M. A., & Reales, J. M. (2019). Efficacy of binaural auditory beats in cognition, anxiety, and pain perception: A meta-analysis. Psychological Research, 83(2), 357–372. [DOI:10.1007/s00426-018-1066-8] [PMID]
Gkolias, V., Amaniti, A., Triantafyllou, A., Papakonstantinou, P., Kartsidis, P., & Paraskevopoulos, E., et al. (2020). Reduced pain and analgesic use after acoustic binaural beats therapy in chronic pain-A double-blind randomized control cross-over trial. European Journal of Pain, 24(9), 1716–1729. [DOI:10.1002/ejp.1615] [PMID]
Goldberg, J. M., & Brownell, W. E. (1973). Discharge characteristics of neurons in anteroventral and dorsal cochlear nuclei of cat. Brain Research, 64, 35–54. [DOI:10.1016/0006-8993(73)90169-8] [PMID]
Goodin, P., Ciorciari, J., Baker, K., Carey, A. M., Harper, M., & Kaufman, J. (2012). A high-density EEG investigation into steady state binaural beat stimulation. Plos One, 7(4), e34789. [DOI:10.1371/journal.pone.0034789] [PMID] [PMCID]
Grose, J. H., Buss, E., & Hall, J. W., 3rd (2012). Binaural beat salience. Hearing Research, 285(1-2), 40–45. [DOI:10.1016/j.heares.2012.01.012] [PMID] [PMCID]
Grose, J. H., & Mamo, S. K. (2012). Electrophysiological measurement of binaural beats: Effects of primary tone frequency and observer age. Ear and Hearing, 33(2), 187–194. [DOI:10.1097/AUD.0b013e318230bbbd] [PMID] [PMCID]
Haywood, N. R., & McAlpine, D. (2020). Estimating the perceptual weighting of interaural time difference cues in amplitude modulated binaural beats. The Journal of the Acoustical Society of America, 148(2), EL185. [DOI:10.1121/10.0001747] [PMID]
Herdman, A. T., Lins, O., Van Roon, P., Stapells, D. R., Scherg, M., & Picton, T. W. (2002). Intracerebral sources of human auditory steady-state responses. Brain Topography, 15(2), 69–86. [DOI:10.1023/A:1021470822922] [PMID]
Hink, R. F., Kodera, K., Yamada, O., Kaga, K., & Suzuki, J. (1980). Binaural interaction of a beating frequency-following response. Audiology, 19(1), 36–43. [DOI:10.3109/00206098009072647] [PMID]
Huang, T. L., & Charyton, C. (2008). A comprehensive review of the psychological effects of brainwave entrainment. Alternative Therapies in Health and Medicine, 14(5), 38–50. [PMID]
Isik, B. K., Esen, A., Büyükerkmen, B., Kilinç, A., & Menziletoglu, D. (2017). Effectiveness of binaural beats in reducing preoperative dental anxiety. The British Journal of Oral & Maxillofacial Surgery, 55(6), 571–574. [DOI:10.1016/j.bjoms.2017.02.014] [PMID]
Jensen, O., Kaiser, J., & Lachaux, J. P. (2007). Human gamma-frequency oscillations associated with attention and memory. Trends in Neurosciences, 30(7), 317–324. [DOI:10.1016/j.tins.2007.05.001] [PMID]
Jirakittayakorn, N., & Wongsawat, Y. (2015). The brain responses to different frequencies of binaural beat sounds on QEEG at cortical level. Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2015, 4687–4691. [DOI:10.1109/EMBC.2015.7319440] [PMID]
Jirakittayakorn, N., & Wongsawat, Y. (2017). Brain responses to 40-Hz binaural beat and effects on emotion and memory. International Journal of Psychophysiology, 120, 96–107. [DOI:10.1016/j.ijpsycho.2017.07.010] [PMID]
Jirakittayakorn, N., & Wongsawat, Y. (2017). Brain responses to a 6-Hz binaural beat: Effects on general theta rhythm and frontal midline theta activity. Frontiers in Neuroscience, 11, 365. [DOI:10.3389/fnins.2017.00365] [PMID] [PMCID]
Jirakittayakorn, N., & Wongsawat, Y. (2018). A novel insight of effects of a 3-Hz binaural beat on sleep stages during sleep. Frontiers in Human Neuroscience, 12, 387. [DOI:10.3389/fnhum.2018.00387] [PMID] [PMCID]
Karino, S., Yumoto, M., Itoh, K., Uno, A., Yamakawa, K., & Sekimoto, S., et al. (2006). Neuromagnetic responses to binaural beat in human cerebral cortex. Journal of Neurophysiology, 96(4), 1927–1938. [DOI:10.1152/jn.00859.2005] [PMID]
Kasprzak, C. (2011) Influence of binaural beats on EEG signal. Acta Physica Polonica A, 119, 986-990 [DOI:10.12693/APhysPolA.119.986]
Keith, S. E., Michaud, D. S., & Chiu, V. (2008). Evaluating the maximum playback sound levels from portable digital audio players. The Journal of the Acoustical Society of America, 123(6), 4227–4237. [DOI:10.1121/1.2904465] [PMID]
Kelton, K., Weaver, T. L., Willoughby, L., Kaufman, D., & Santowski, A. (2021). The Efficacy of Binaural Beats as a Stress-buffering Technique. Alternative Therapies in Health and Medicine, 27(4), 28–33. [PMID]
Kennel, S., Taylor, A. G., Lyon, D., & Bourguignon, C. (2010). Pilot feasibility study of binaural auditory beats for reducing symptoms of inattention in children and adolescents with attention-deficit/hyperactivity disorder. Journal of Pediatric Nursing, 25(1), 3–11. [DOI:10.1016/j.pedn.2008.06.010] [PMID]
Klepp, S. (2006) Effects of binaural-beat stimulation on recovery following traumatic brain injury: A pilot study. Subtle Energies and Energy Medicine, 17(2), 181. [Link]
Kraus, J., & Porubanova, M. (2015). The effect of binaural beats on working memory capacity. Studia Psychologica 57, 135-145. [DOI:10.21909/sp.2015.02.689]
Kuwada, S., Yin, T. C., & Wickesberg, R. E. (1979). Response of cat inferior colliculus neurons to binaural beat stimuli: Possible mechanisms for sound localization. Science, 206(4418), 586–588. [DOI:10.1126/science.493964] [PMID]
Lakatos, P., Karmos, G., Mehta, A. D., Ulbert, I., & Schroeder, C. E. (2008). Entrainment of neuronal oscillations as a mechanism of attentional selection. Science, 320(5872), 110–113. [DOI:10.1126/science.1154735] [PMID]
Lane, J. D., Kasian, S. J., Owens, J. E., & Marsh, G. R. (1998). Binaural auditory beats affect vigilance performance and mood. Physiology & Behavior, 63(2), 249–252. [DOI:10.1016/S0031-9384(97)00436-8] [PMID]
Lane, J. D., Kasian, S. J., Owens, J. E., & Marsh, G. R. (1998). Binaural auditory beats affect vigilance performance and mood. Physiology & Behavior, 63(2), 249–252. [DOI:10.1016/S0031-9384(97)00436-8] [PMID]
Lavallee, C. F., Koren, S. A., & Persinger, M. A. (2011). A quantitative electroencephalographic study of meditation and binaural beat entrainment. Journal of Alternative and Complementary Medicine, 17(4), 351–355. [DOI:10.1089/acm.2009.0691] [PMID]
Le Scouarnec, R. P., Poirier, R. M., Owens, J. E., Gauthier, J., Taylor, A. G., & Foresman, P. A. (2001). Use of binaural beat tapes for treatment of anxiety: A pilot study of tape preference and outcomes. Alternative Therapies in Health and Medicine, 7(1), 58–63. [PMID]
Lee, E., Bang, Y., Yoon, I. Y., & Choi, H. Y. (2022). Entrapment of binaural auditory beats in subjects with symptoms of insomnia. Brain Sciences, 12(3), 339. [DOI:10.3390/brainsci12030339] [PMID] [PMCID]
Lee, M., Song, C. B., Shin, G. H., & Lee, S. W. (2019). Possible effect of binaural beat combined with autonomous sensory meridian response for inducing sleep. Frontiers in Human Neuroscience, 13, 425. [DOI:10.3389/fnhum.2019.00425] [PMID] [PMCID]
Licklider, J. C. R., Webster, J. C., Hedlun, J. M. (1950) On the frequency limits of binaural beats. The Journal of the Acoustical Society of America, 22, 468-473. [DOI:10.1121/1.1906629]
Little, B. R., Lecci, L., & Watkinson, B. (1992). Personality and personal projects: Linking big five and PAC units of analysis. Journal of Personality, 60(2), 501–525. [DOI:10.1111/j.1467-6494.1992.tb00982.x] [PMID]
López-Caballero, F., & Escera, C. (2017). Binaural Beat: A Failure to Enhance EEG Power and Emotional Arousal. Frontiers in Human Neuroscience, 11, 557. [DOI:10.3389/fnhum.2017.00557] [PMID] [PMCID]
McAlpine, D., Jiang, D., & Palmer, A. R. (1996). Interaural delay sensitivity and the classification of low best-frequency binaural responses in the inferior colliculus of the guinea pig. Hearing Research, 97(1-2), 136–152. [DOI:10.1016/S0378-5955(96)80015-3] [PMID]
McAlpine, D., Jiang, D., Shackleton, T. M., & Palmer, A. R. (1998). Convergent input from brainstem coincidence detectors onto delay-sensitive neurons in the inferior colliculus. The Journal of Neuroscience, 18(15), 6026–6039. [DOI:10.1523/JNEUROSCI.18-15-06026.1998] [PMID] [PMCID]
McConnell, P. A., Froeliger, B., Garland, E. L., Ives, J. C., & Sforzo, G. A. (2014). Auditory driving of the autonomic nervous system: Listening to theta-frequency binaural beats post-exercise increases parasympathetic activation and sympathetic withdrawal. Frontiers in Psychology, 5, 1248. [DOI:10.3389/fpsyg.2014.01248] [PMID] [PMCID]
Heuchert, J. P., & McNair, D. M. (2011). Profile of mood states, 2nd edn. TM (POMS 2TM). San Diego: JvR Psychometrics Assessment Catalogue. [DOI:10.1037/t05057-000]
Menziletoglu, D., Guler, A. Y., Cayır, T., & Isik, B. K. (2021). Binaural beats or 432 Hz music? Which method is more effective for reducing preoperative dental anxiety? Medicina Oral, Patologia Oral y Cirugia Bucal, 26(1), e97–e101. [DOI:10.4317/medoral.24051] [PMID] [PMCID]
Moore, B. C. J. (2012). An introduction to the psychology of hearing. Leeds: Emerald. [Link]
Mujib, M. D., Hasan, M. A., Qazi, S. A., & Vuckovic, A. (2021). Understanding the neurological mechanism involved in enhanced memory recall task following binaural beat: A pilot study. Experimental Brain Research, 239(9), 2741–2754. [DOI:10.1007/s00221-021-06132-6] [PMID] [PMCID]
Munoz, J. P., & Rivera, L. A. (2020). Towards improving sleep quality using automatic sleep stage classification and binaural beats. Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2020, 4982–4985. [DOI:10.1109/EMBC44109.2020.9176385] [PMID]
Ölçücü, M. T., Yılmaz, K., Karamık, K., Okuducu, Y., Özsoy, Ç., & Aktaş, Y., et al. (2021). Effects of listening to binaural beats on anxiety levels and pain scores in male patients undergoing cystoscopy and ureteral stent removal: A randomized placebo-controlled trial. Journal of Endourology, 35(1), 54–61. [DOI:10.1089/end.2020.0353] [PMID]
Orozco Perez, H. D., Dumas, G., & Lehmann, A. (2020). Binaural beats through the auditory pathway: From brainstem to connectivity patterns. eNeuro, 7(2), ENEURO.0232-19.2020. [DOI:10.1523/ENEURO.0232-19.2020] [PMID] [PMCID]
Ortiz, T., Martínez, A. M., Fernández, A., Maestu, F., Campo, P., & Hornero, R., et al. (2008). Impact of auditory stimulation at a frequency of 5 Hz in verbal memory. Actas Espanolas de Psiquiatria, 36(6), 307–313. [PMID]
Oster, G. (1973). Auditory beats in the brain. Scientific American, 229(4), 94–102. [DOI:10.1038/scientificamerican1073-94] [PMID]
Ozdamar, O., Bohorquez, J., Mihajloski, T., Yavuz, E., & Lachowska, M. (2011). Auditory evoked responses to binaural beat illusion: Stimulus generation and the derivation of the binaural interaction component (BIC). Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2011, 830–833. [DOI:10.1109/IEMBS.2011.6090190] [PMID]
Padmanabhan, R., Hildreth, A. J., & Laws, D. (2005). A prospective, randomised, controlled study examining binaural beat audio and pre-operative anxiety in patients undergoing general anaesthesia for day case surgery. Anaesthesia, 60(9), 874–877. [DOI:10.1111/j.1365-2044.2005.04287.x] [PMID]
Pantev, C., Roberts, L. E., Elbert, T., Ross, B., & Wienbruch, C. (1996). Tonotopic organization of the sources of human auditory steady-state responses. Hearing Research, 101(1-2), 62–74. [DOI:10.1016/S0378-5955(96)00133-5] [PMID]
Pastor, M. A., Artieda, J., Arbizu, J., Marti-Climent, J. M., Peñuelas, I., & Masdeu, J. C. (2002). Activation of human cerebral and cerebellar cortex by auditory stimulation at 40 Hz. The Journal of Neuroscience, 22(23), 10501–10506. [DOI:10.1523/JNEUROSCI.22-23-10501.2002] [PMID] [PMCID]
Perrott, D. R., & Musicant, A. D. (1977). Rotating tones and binaural beats. The Journal of the Acoustical Society of America, 61(5), 1288–1292. [DOI:10.1121/1.381430] [PMID]
Picton, T. W., Dimitrijevic, A., Perez-Abalo, M. C., & Van Roon, P. (2005). Estimating audiometric thresholds using auditory steady-state responses. Journal of the American Academy of Audiology, 16(3), 140–156. [DOI:10.3766/jaaa.16.3.3] [PMID]
Picton, T. W., John, M. S., Dimitrijevic, A., & Purcell, D. (2003). Human auditory steady-state responses. International Journal of Audiology, 42(4), 177–219. [DOI:10.3109/14992020309101316] [PMID]
Plourde G. (2006). Auditory evoked potentials. Best practice & research. Clinical Anaesthesiology, 20(1), 129–139. [DOI:10.1016/j.bpa.2005.07.012] [PMID]
Pratt, H., Starr, A., Michalewski, H. J., Dimitrijevic, A., Bleich, N., & Mittelman, N. (2009). Cortical evoked potentials to an auditory illusion: Binaural beats. Clinical Neurophysiology, 120(8), 1514–1524. [DOI:10.1016/j.clinph.2009.06.014] [PMID] [PMCID]
Pratt, H., Starr, A., Michalewski, H. J., Dimitrijevic, A., Bleich, N., & Mittelman, N. (2010). A comparison of auditory evoked potentials to acoustic beats and to binaural beats. Hearing Research, 262(1-2), 34–44. [DOI:10.1016/j.heares.2010.01.013] [PMID]
Reale, R. A., & Brugge, J. F. (1990). Auditory cortical neurons are sensitive to static and continuously changing interaural phase cues. Journal of Neurophysiology, 64(4), 1247–1260. [DOI:10.1152/jn.1990.64.4.1247] [PMID]
Reedijk, S. A., Bolders, A., & Hommel, B. (2013). The impact of binaural beats on creativity. Frontiers in Human Neuroscience, 7, 786. [DOI:10.3389/fnhum.2013.00786] [PMID] [PMCID]
Robison, M. K., Obulasetty, M., Blais, C., Wingert, K. M., & Brewer, G. A. (2022). The effect of binaural beat stimulation on sustained attention. Psychological Research, 86(3), 808–822. [DOI:10.1007/s00426-021-01524-3] [PMID]
Ross, B., & Lopez, M. D. (2020). 40-Hz Binaural beats enhance training to mitigate the attentional blink. Scientific Reports, 10(1), 7002. [DOI:10.1038/s41598-020-63980-y] [PMID] [PMCID]
Schwarz, D. W., & Taylor, P. (2005). Human auditory steady state responses to binaural and monaural beats. Clinical Neurophysiology, 116(3), 658–668. [DOI:10.1016/j.clinph.2004.09.014] [PMID]
Seifi Ala, T., Ahmadi-Pajouh, M. A., & Nasrabadi. A. M. (2018) Cumulative effects of theta binaural beats on brain power and functional connectivity. Biomedical Signal Processing and Control 42, 242-252 [DOI:10.1016/j.bspc.2018.01.022]
Shumov, D. E., Yakovenko, I. A., Alipov, N. N., Bakaeva, Z. V., Yakunina, E. B., & Minyuk, A. N.,et al. (2020). [The effect of music containing binaural beats on daytime fall-asleep dynamics (Russian)]. Zhurnal Nevrologii i Psikhiatrii Imeni S.S. Korsakova, 120(2), 39–44. [DOI:10.17116/jnevro202012002139] [PMID]
Solcà, M., Mottaz, A., & Guggisberg, A. G. (2016). Binaural beats increase interhemispheric alpha-band coherence between auditory cortices. Hearing Research, 332, 233–237. [DOI:10.1016/j.heares.2015.09.011] [PMID]
Spitzer, M. W., & Semple, M. N. (1998). Transformation of binaural response properties in the ascending auditory pathway: Influence of time-varying interaural phase disparity. Journal of Neurophysiology, 80(6), 3062–3076. [DOI:10.1152/jn.1998.80.6.3062] [PMID]
Starr, A., McPherson, D., Patterson, J., Don, M., Luxford, W., & Shannon, R., et al. (1991). Absence of both auditory evoked potentials and auditory percepts dependent on timing cues. Brain, 114(Pt 3), 1157–1180. [DOI:10.1093/brain/114.3.1157] [PMID]
Tallon-Baudry, C., & Bertrand, O. (1999). Oscillatory gamma activity in humans and its role in object representation. Trends in Cognitive Sciences, 3(4), 151–162. [DOI:10.1016/S1364-6613(99)01299-1] [PMID]
Tang, H. Y., Vitiello, M. V., Perlis, M., & Riegel, B. (2015). Open-loop neurofeedback audiovisual stimulation: A pilot study of its potential for sleep induction in older adults. Applied Psychophysiology and Biofeedback, 40(3), 183–188. [DOI:10.1007/s10484-015-9285-x] [PMID] [PMCID]
Tani, A., Tartarisco, G., Vagheggini, G., Vaccaro, C., Campana, S., & Tomaiuolo, F. (2022). Binaural beats reduce feeling of pain and discomfort during colonoscopy procedure in not-sedated patients: A randomized control trial. Complementary Therapies in Clinical Practice, 48, 101605. [DOI:10.1016/j.ctcp.2022.101605] [PMID]
Tani, A., Vagheggini, G., Moretti, F., Del Colombo, V., Lehle, J., Campana, S., Labate, A., & Tomaiuolo, F. (2021). Binaural beats reduce postoperative morphine consumption in older adults after total knee replacement surgery. Alternative Therapies in Health and Medicine, 27(2), 27–30. [PMID]
Tort, A. B., Komorowski, R. W., Manns, J. R., Kopell, N. J., & Eichenbaum, H. (2009). Theta-gamma coupling increases during the learning of item-context associations. Proceedings of the National Academy of Sciences of the United States of America, 106(49), 20942–20947. [DOI:10.1073/pnas.0911331106] [PMID] [PMCID]
Tuomi, S., & Jellimann, M. (2009) Hear today-hearing loss tomorrow: A preliminary survey of the personal audio player user habits and knowledge of South African first-year university students. South African Family Practice, 51, 166-167 [DOI:10.1080/20786204.2009.10873835]
Ungan, P., Yagcioglu, S., & Ayik, E. (2019). Event-related potentials to single-cycle binaural beats and diotic amplitude modulation of a tone. Experimental Brain Research, 237(8), 1931–1945. [DOI:10.1007/s00221-019-05562-7] [PMID]
Vernon, D., Peryer, G., Louch, J., & Shaw, M. (2014). Tracking EEG changes in response to alpha and beta binaural beats. International Journal of Psychophysiology, 93(1), 134–139. [DOI:10.1016/j.ijpsycho.2012.10.008] [PMID]
Wahbeh, H., Calabrese, C., & Zwickey, H. (2007). Binaural beat technology in humans: A pilot study to assess psychologic and physiologic effects. Journal of Alternative and Complementary Medicine, 13(1), 25–32. [DOI:10.1089/acm.2006.6196] [PMID]
Walter, V. J., & Walter, W. G. (1949). The central effects of rhythmic sensory stimulation. Electroencephalography and Clinical Neurophysiology, 1(1), 57–86. [DOI:10.1016/0013-4694(49)90164-9] [PMID]
Weiland, T. J., Jelinek, G. A., Macarow, K. E., Samartzis, P., Brown, D. M., & Grierson, E. M., et al. (2011). Original sound compositions reduce anxiety in emergency department patients: a randomised controlled trial. The Medical Journal of Australia, 195(11-12), 694–698. [DOI:10.5694/mja10.10662] [PMID]
Wernick, J. S., & Starr, A. (1968). Binaural interaction in the superior olivary complex of the cat: an analysis of field potentials evoked by binaural-beat stimuli. Journal of Neurophysiology, 31(3), 428–441. [DOI:10.1152/jn.1968.31.3.428] [PMID]
Wiwatwongwana, D., Vichitvejpaisal, P., Thaikruea, L., Klaphajone, J., Tantong, A., & Wiwatwongwana, A., et al. (2016). The effect of music with and without binaural beat audio on operative anxiety in patients undergoing cataract surgery: A randomized controlled trial. Eye, 30(11), 1407–1414. [DOI:10.1038/eye.2016.160] [PMID] [PMCID]
Wu, S. H., & Oertel, D. (1984). Intracellular injection with horseradish peroxidase of physiologically characterized stellate and bushy cells in slices of mouse anteroventral cochlear nucleus. The Journal of Neuroscience, 4(6), 1577–1588. [DOI:10.1523/JNEUROSCI.04-06-01577.1984] [PMID] [PMCID]
Yusim, A., & Grigaitis, J. (2020). Efficacy of binaural beat meditation technology for treating anxiety symptoms: A pilot study. The Journal of Nervous and Mental Disease, 208(2), 155–160. [DOI:10.1097/NMD.0000000000001070] [PMID]
Zampi, D. D. (2016). Efficacy of theta binaural beats for the treatment of chronic pain [doctoral dissertation]. Prescott Valley: Northcentral University. [Link]
Along with the advances in our knowledge about brain function, new methods are developed to improve cognitive functions and psychological states. Auditory beat stimulation (ABS) is one of these techniques that is claimed to induce brainwave entrainment, a frequency-following response of brainwaves. ABS has been considered for its potential applications in understanding and assessing auditory system responses, audiometric parameters, and mechanisms of sound localization (Kuwada et al., 1979). ABS can be applied as monaural beats (MBs) and binaural beats (BBs). To present MBs to a subject, two sinusoidal waves with close frequencies are instantaneously presented to both ears. When these waves are given to each ear separately, they are called BBs (Wernick & Starr 1968; Oster, 1973). MBs are also considered peripheral or objective beats, first distinguished by the cochlear, then forwarded to the brain stem, and afterward to the auditory cortex (AC). On the other hand, BBs are considered central or subjective beats, as the BB perception is formed from the distinctness in the phase of the waves that reach the right and left ears (Kuwada et al., 1979; Draganova et al., 2008). The superior olivary nucleus in the brainstem produces BB auditory responses when the ears are provided with two low-frequency tones (less than 1000 Hz) with steady intensities but different frequencies. Neurons of the inferior colliculus (IC) then recognize the variance in the interaural phase as a beat (Licklider et al., 1950; Perrott & Musicant, 1977; Schwarz & Taylor, 2005; Ross & Lopez, 2020). The beat frequency is halfway between the two carrier tones (Oster 1973; Pantev et al., 1996; Wahbeh et al., 2007; Pratt et al., 2009; Grose & Mamo 2012).
Experiments in animal models (Wernick & Starr 1968; Kuwada et al., 1979; Reale & Brugge, 1990; McAlpine et al., 1996; McAlpine et al., 1998; Spitzer & Semple, 1998) and humans (Barr et al., 1977; Starr et al., 1991; Lane et al., 1998) confirmed the involvement of structures in the auditory brainstem and cortex in ABS and BBs.
BB technology has been claimed to have benefits such as reduction in stress and anxiety, improvement in cognitive functions, including memory and attention, and improvements in other psychological states such as motivation and confidence (Wahbeh et al., 2007; Chaieb et al., 2015; Garcia-Argibay et al., 2019b).
Early reports show that the activity of the neurons in the auditory pathways of the brainstem follows the phase pattern of an incoming sound (Hink et al., 1980). Later research shows that BBs cause brainwave entrainment, which is a frequency-dependent EEG response to an external rhythmic stimulus (Karino et al., 2006; Huang & Charyton, 2008; Ozdamar et al., 2011; Seifi Ala et al., 2018). That is to say, similar EEG patterns during a cognitive function can be induced using synchronized pulsing stimuli (Tang et al., 2015; Lee et al., 2019).
For example, gamma frequencies (40 Hz) are reported to entrain brain oscillations; we already know that gamma oscillations play a key role in attention, feature binding, learning, and memory. In addition, BB stimulation has been reported to be beneficial for memory (Tallon-Baudry & Bertrand, 1999; Jensen et al., 2007; Tort et al., 2009; Kraus & Porubanova, 2015; Beauchene et al., 2017; Jirakittayakorn & Wongsawat, 2017a; Garcia-Argibay et al., 2019a), attention (Jensen et al., 2007; Lakatos et al., 2008; Kennel et al., 2010), vigilance (Chaieb et al., 2015), creativity (Reedijk et al., 2013), anxiety control (Le Scouarnec et al., 2001; Garcia-Argibay et al., 2019b), modulation of mood states (Chaieb et al., 2015; Jirakittayakorn & Wongsawat, 2017a), pain perception (Garcia-Argibay et al., 2019b), induction of meditation (Lavallee et al., 2011), and improvement of sleep quality (Chan et al., 2010; Bellesi et al., 2014; Besedovsky et al., 2017). Recent evidence also suggests that BB provokes cross-frequency connectivity patterns in the brain (Orozco Perez et al., 2020).
Based on the mentioned findings, it has been proposed that ABS, especially BBs, can be considered a digital drug and potential therapeutic method either alone or in combination with other ingestible drugs (Barratt et al., 2022). In this review study, we searched the PubMed and Google Scholar databases using the keywords “auditory beat stimulation,” “binaural beats,” “monaural beats,” and “brain” to look further into the mechanism of action and preclinical and clinical reports of BBs application and shed lights on BBs effects on the nervous system.
The concept of binaural beats and evoked potentials
BB concept was described by H. W. Dove in 1839. Later, it was claimed that rhythmic sensory stimulation, as an alternative to electrical stimulation, can entrain neural oscillations beyond the related brain areas (Walter & Walter, 1949). One year later, Licklider et al., (1950) assessed the frequency limits and outlined the BBs theory. They explained how synchronal activity in both auditory nerves resulted in the BB effect. Oster (1973) showed that BBs were detectable for carrier frequencies below 1000 Hz, and further research revealed that the brain cortex only encodes low carrier frequencies (Schwarz & Taylor, 2005).
Steady-state auditory responses (ASSRs) are evoked by acoustic beats. ASSRs originate at the brainstem, and the responses to stimulus rates (1-200 Hz) are recordable at the cortical level of the scalp (Dolphin, 1997; Picton et al., 2003). Prominent responses are observed when stimulus rates are near 40 Hz (Galambos et al., 1981; Herdman et al., 2002). Auditory thresholds (Picton et al., 2005) and the depth of anesthesia (Plourde, 2006) can also be measured using ASSRs.
The carrier frequency is also important in the outcome of BB stimulation. For example, a 40-Hz BB potential was observed following a 400-Hz carrier frequency but not when the carrier frequency was above 3 kHz (Schwarz & Taylor, 2005). Spectral analyses of the magnetic fields shows that BBs (4.00 or 6.66 Hz; carrier frequency 240 or 480 Hz) evoke small amplitude magnetic fields (Karino et al., 2006). BBs and acoustic beats have similar cortical processing as BBs (3 and 6 Hz), and amplitude-modulated acoustic beats (3 & 6 Hz; carrier frequency 250 &1000 Hz; 2000 ms duration; 1-s intervals) yield comparable cortical activity and perceptions (Pratt et al., 2010). The temporary auditory responses are separable from BB auditory illusion using binaural interaction component analysis (Ozdamar et al., 2011).
Binaural beat mechanism of action
Action potentials are fired when sound energy passes through the ears and cochlea and reaches the inner hair cells. Auditory nerve fibers carry the auditory information from the cochlea and join the vestibulocochlear nerve. The information enters the cochlear nucleus and bifurcates. The branches of the nerve fibers form synapses with stellate-, globular-, and spherical-bushy cells, which have specific temporal and spectral response properties (Wu & Oertel, 1984). The sound information then travels to either the superior olivary complex (projections of the bushy cells of the anteroventral cochlear nucleus) or to the IC (outputs of the stellate and dorsal cochlear nucleus cells) (Goldberg & Brownell, 1973). The superior olivary complex processes the data related to sound originating from both ears (Moore, 2012). The left and right IC make binaural interactions through commissural connections and the ascending pathway subnuclei. The information is then relayed to the medial geniculate nucleus and the AC. The processing of wave specifications depends on the integration time between the IC and the AC (Fitzpatrick et al., 2009; Bloom, 2013; Croom, 2014).
Auditory neurons discharge differently at various frequencies. For instance, synchronous discharges happen in response to low frequencies but not in response to shape synaptic summation. When the frequencies reach higher, neuronal discharges happen in turns. At middle frequencies, neurons respond in many volleys, and those involved in each volley fire synchronal, resulting in the appearance of beats (Licklider et al., 1950).
Kuwada et al., (1979) studied the interaural phase sensitivity of neurons using BBs and showed that many neurons phase-locked to the beat frequency. Other studies show that in addition to the primary AC, ASSRs responses to BBs can be recorded from the superior temporal, posterior parietal, and frontal cortices, which primarily originate in the AC, especially in response to gamma-frequency stimulation (Pastor et al., 2002). Recording of small amplitude magnetic fields following the application of low-frequency BBs (4.00-6.66 Hz) shows that BBs can synchronize the cortex activity (Karino et al., 2006; Pratt et al., 2009), and reports indicate that the interaural time difference that happens within early rising amplitude (20–25 ms) plays a key role in the prediction of perceived BB lateralization (Haywood & McAlpine, 2020). A comparison of the effects of BBs across four levels of subcortical, cortical, and scalp-level functional connectivity shows that BBs weakly entrain the cortex and generate cross-frequency connectivity patterns (Orozco Perez et al., 2020). Some other studies have recorded ERP N100 to BBs and amplitude modulation stimuli and reported separate processing sites for structure-based spatial processing and envelope-based level processing. The recorded N1 component also shows an age-related decline in magnitude (Ungan et al., 2019).
BBs effects have also been studied on the synchronization of the brain hemispheres. It has been reported that BBs frequencies (10 Hz, alpha; 4Hz, theta) increase alpha frequencies interhemispheric coherence and are interpreted as binaural integration rather than entrainment (Solcà et al., 2016).
Nevertheless, the responses to BBs are not always the same, and the brain areas respond differently to various beat frequencies. For example, the study by Karino et al. (2006) indicates that the application of four different BBs at 4.00- and 6.66-Hz (240-480 Hz; 10 min) induces ASSRs in the frontal region and also in the temporal and parietal areas but symmetry does not always occur. A magnetoencephalography (MEG) study shows right temporal responses to a 40-Hz BB after 1 s of BB application (Draganova et al., 2008). Another study reports that the presentation of 7- and 15-Hz BBs (15 min) increases left temporal delta power for the 7-Hz BB and gamma power for the 15-Hz BB (Lavallee et al., 2011). In a study on MEG, Chakalov et al. (2014) used 26-Hz BBs (250 Hz carrier tone; 500 ms) and reported a 26-Hz ASSR at the right parietal and left middle frontal regions. Also, left hemisphere dominance was observed in 3 Hz BB after 15 minutes and 15 Hz BB after 5 minutes. The right hemisphere dominance occurs in 10 Hz BB after 25 min. All brain areas are enhanced after a 6-Hz beat within 10 minutes. Differences are also observed in the frontal lobe, and responses are enhanced with 40 Hz beats, but 8 Hz and 25 Hz beats do not create any clear responses (Jirakittayakorn & Wongsawat, 2015). In a second study, Jirakittayakorn and Wongsawat (2017b) investigated theta activity responses (6-Hz BBs, 250 Hz carrier tone, 30 minutes) and reported that theta waves were observable in all cortex regions after 10 min of BB application. They also reported a meditation effect, measured by the Brunel mood scale.
Distribute processing across hemispheres has also been reported. For instance, BBs amplify the coherence of left and right auditory regions in contrast to MBs and resting state; it is inferred that the increased coherence selectively concerns the alpha band independent of BB frequency. These changes do not happen along with changes in amplitude (Solcà et al., 2016).
The effect of carrier tones has also been investigated in EEG studies. At the frontocentral region, BB stimulation (40 Hz) induces higher responses on a lower (400 Hz) carrier tone than a higher (3200 Hz) one (Schwarz & Taylor, 2005). Another study reports that the application of BBs (3 & 6 Hz; 250 & 1000 Hz; 2 s) generates left temporal ERPs that are more prominent for 250 Hz than 1000 Hz and similarly higher for 3 Hz than 6 Hz (Pratt et al., 2009).
A study on the differences in the perception of BBs, measured for 4, 8, 16, and 32 Hz (500-Hz carrier tone), reports that variability in perceiving BBs is due to the measurement plan (Grose et al., 2012).
EEG activity following BB stimulation
When BB is presented to a subject, two tones with close frequencies are presented to the left and right ears, and the brain perceives a third sound, the BB, that forms from integrating the presented signals. EEG alterations have been reported differently in the studies. To test if BB affects functional brain connectivity, Mujib et al. (2021) measured relative power, phase-locking value, and cross-mutual information in EEG recordings during delta (1 Hz), theta (5 Hz), alpha (10 Hz), and beta (20 Hz) band BB stimulations. The results show that the application of delta and alpha BB increased and decreased relative power in theta and beta bands, respectively. Theta BB stimulation also diminishes beta band relative power. No entrainment was reported, but the connectivity pattern showed variations. In another study, the frequency of the following responses was reported in delta, theta, and gamma bands but not in alpha and beta bands following exposure to BBs for ten 1-minute epochs (Vernon et al., 2014).
On the other hand, López et al. (2017) investigated brainwave entrainment. They reported no significant EEG spectral power changes for epochs of 3 min in theta (4.53 Hz), alpha (8.97 Hz), beta (17.93 Hz), gamma (34.49 Hz), or upper gamma (57.3 Hz) bands. Goodin et al. (2012) also studied brainwave entrainment following BB stimulation at beta and theta frequencies and reported that short presentation of BBs was insufficient to generate entrainment or alter vigilance.
Vernon et al. (2014) performed a limited recording of EEG (at T3 and T4) during the application of alpha (10 Hz) and beta (20 Hz) BBs. They reported greater beta activity in the left temporal region but no alteration in alpha activity. ACs show greater alpha-band synchrony after the application of alpha (10 Hz) and theta (4 Hz) BBs, which is a reflection of binaural integration (Solcà et al., 2016). Kasprzak et al. (2011) tested the brain’s ability to change its main activity frequency according to a dominant applied stimulus. They observed a significant decline in alpha rhythm (8–12 Hz) and simultaneous increment of narrow band share (9.9–10.1 Hz). They proposed that the blockade of alpha rhythm was due to the response of CNS to the acoustic stimulus and tuning to enhance the receipt of environmental information.
Studies have also investigated the effects of MBs and BBs on EEG power, phase patterns, and phase synchronization (Schwarz & Taylor, 2005; Becher et al., 2015; Derner et al., 2018). Prominent alterations have been observed at theta (5 Hz) range in the temporal regions, rhinal cortex, and hippocampus. The mediotemporal structure is important in memory function (Eichenbaum 2000). Fell et al. (2011) demonstrated that phase-related mechanisms, including phase synchronization, play a key role in long-term memory processing. In addition, a study in presurgical epilepsy patients shows that MB and BB stimulations alter brain activity power and synchronous phase (Becher et al., 2015). A recent study investigated the differential effects of MBs and BBs on phase synchronization and long-term memory performance. It has been reported that theta (5 Hz) range BB increased temporolateral phase synchronization while MB stimulation at the same frequency decreased mediotemporal phase synchronization. In addition, 5 Hz BB increases, and 5 Hz MBs diminishes both words and association memory. The results indicate that intracranial EEG phases change the threshold of neurons and neural activity, which may result in memory-related activity alterations within the required time window (Derner et al., 2021).
Animal models and our understanding of BBs
The experimental data from animal models help us better understand the basis of the BB mechanism of action. Early evidence came from the work by Kuwada et al. showing a phase-locked response in most cats’ IC cells to the BB frequency (Kuwada et al., 1979). The study by McAlpine et al. (1996) shows that in guinea pigs, single neurons in the IC process BBs at low frequencies, similar to other species. They also proposed that interaural-delay sensitivity differs for various frequencies and changes within each frequency band. Two years later, studies report that a system different from spatial processing of position is responsible for interaural phase responses, and convergence was reported from simple brainstem coincidence detectors (McAlpine et al., 1998; Spitzer & Semple, 1998). Reale et al. (1990) studied neurons in the primary AC in cats to evaluate the interaural-phase-difference sensitivity of the neurons using different stimulus frequencies (120 to 2500 Hz). Data showed a direct association between the interaural phase and beat frequency. Many of the studied cells responded equally to BBs, and an increase followed a rise in BB frequency in the action potentials of the neurons. However, after a certain frequency (35 Hz), the AC neurons could not follow the rate.
The cognitive effects of binaural beats
Table 1 summarizes the studies regarding auditory beat stimulation, indicating a rising trend for BBs application since 1947. With the rapid growth in cognitive sciences, BB stimulation has also become alluring to many researchers as a non-invasive method that may improve cognitive functions.



Application of 40-Hz gamma BB and MB for the assessment of attention (Flanker task) and working memory (Klingberg task) in high and low emotional participants shows that listening to BB at beta (15 Hz) range during the N-back task alters network connections and improves the accuracy of performance (Beauchene et al., 2017). Another study found that both BB and MB similarly enhance the speed of performance in the attention task, and their effects are also similar in high- and low-emotional participants (Engelbregt et al., 2019). A study on the attentional blink (AB) using MEG recordings shows that gamma (40-Hz) BB stimulation during training enhances the attentional blink task outcomes. However, the improvement is evident only after consolidation during sleep (Ross & Lopez, 2020). Using EEG recordings and a 5-minute presentation of 40 Hz BB, another study indicates that BB stimulation improves attention without neural entrainment (Engelbregt et al., 2021). The application of gamma-frequency (40 Hz) during a global-local task was reported to reduce the spotlight of attention (Colzato et al., 2017). A meta-analysis suggests that BBs positively affect attention (average effect size 0.58) (Garcia-Argibay et al., 2019a). On the other hand, beta-frequency (16 Hz) BB does not enhance sustained attention when measured by pupillary measures (Robison et al., 2021). The application of BB (20 min; 3 times a week; 3 weeks) does not reduce inattention in children and adolescents with attention-deficit/hyperactivity disorder (ADHD) (Kennel et al., 2010). However, parents of ADHD children reported improvement in homework problems during the 3 weeks of the study (Wiwatwongwana et al., 2016).
Application of BBs (beta, 16 and 24 Hz or theta/delta, 1.5 and 4 Hz; for 30 min) in visual vigilance task shows that beta-frequency BBs enhance psychomotor performance and increase the number of correct detections in comparison to theta or delta frequencies. Furthermore, the beta frequency is associated with less negative mood in the subjects under the study (Lane et al., 1998). Little et al. (1992) used the five-factor model to access participants’ personality traits and recorded EEG. They did not find any significant effects of theta (7 Hz) or beta (16 Hz) BBs applied during a psychomotor vigilance task, which needs the ability to remain focused and alert to stimuli over prolonged periods. Also, no correlations were observed between the stimulations and personality. The brief duration of exposure to BB stimulation might have had a key role in the observed results. However, Lane et al., (1998) applied BBs at beta (16 and 24 Hz) throughout a psychomotor vigilance task and reported improved performance in a vigilance task.
In a test of verbal memory, Wahbeh et al. (2007) reported that the application of BBs (7 Hz; 30 min) declined verbal memory recall. However, applying 5 Hz BBs (15 min; twice per day; 15 days) increased the number of words recalled (Ortiz et al., 2008). Beta-frequency (20 Hz) BBs in long-term memory tests produce better performance for recalled words and a higher sensitivity index in recognition tasks. However, theta-frequency (5 Hz) BBs diminish the performance of remembered words and the sensitivity index. Hence, depending on the frequency used, BBs positively or negatively affect long-term memory (Garcia-Argibay et al., 2019b). Chaieb et al. (2015) also mentioned that longer application of BBs affects verbal memory recall. BB stimulation at theta frequency (5 Hz) over a long time generated a coupling of brain activity and improved the capacity of immediate verbal memory (Ortiz et al., 2008). BBs have also been reported to have positive applications for neurological disorders and for older people to improve memory. For example, a study shows a significant increase in alpha frequency along with a significant decrease in reaction time in alpha (10 Hz; 15 min) and gamma (30 Hz; 15 min) frequencies (Mujib et al., 2021). In the N-back test, listening to beta-frequency (15 Hz) BB stimulation enhances accuracy and alters cortical network connection strengths (Beauchene et al., 2017). BB at beta (15 Hz) band also improves working memory by inducing beta activity in the brain (Beauchene et al., 2017).
To assess the effects of BBs on creativity, Reedijk et al. (2013) applied BBs at the alpha (10 Hz) and gamma (40 Hz) bands for 3 minutes before the divergent and convergent thinking tasks. Measurement of dopamine levels in the striatum using spontaneous eye blink rates (EBRs) showed that both applied BBs affected divergent but not convergent thinking. Alpha BBs enhance divergent thinking, mostly in subjects with low EBRs.
The psychological and physiological effects of binaural beats
BB stimulations have also been reported to have positive outcomes in pre-operative state-based anxiety (Padmanabhan et al., 2005; Weiland et al., 2011; Ungan et al., 2019; Ölçücü et al., 2021), anxiety in psychiatric outpatients (Yusim & Grigaitis, 2020), patients suffering from traumatic brain injury (Klepp & Summer, 2006), and dental anxiety (Menziletoglu et al., 2021). It has also been reported that BBs reduce pain scores associated with unit status reporting procedures (Ölçücü et al., 2021) and pain perception (Garcia-Argibay et al., 2019b). In a study on chronic pain patients, Gkolias et al.(2020) used theta (5 Hz) band BB to investigate if the resulting brain entrainment would decline pain perception and analgesic medication use and reported the effectiveness of BBs in the reduction of pain intensity, analgesic use, and stress. BB therapy has also been suggested as a therapeutic method that holds promise for the management of chronic pain in conditions like cancer (Zampi, 2016), for reducing morphine consumption in patients who underwent knee replacement (Tani et al., 2021), and for relieving pain in cases undergoing colonoscopy without sedation (Tani et al., 2022).
In patients with cataracts, BB stimulation reduces state-trait anxiety, heart rate, and systolic blood pressure during surgery, and it is suggested that BB-embedded musical intervention is beneficial over musical intervention alone (Wiwatwongwana et al., 2016). Theta BB also diminishes subsequent stress responses to an acute psychological stressor (Kelton et al., 2021). Listening to BB influences the power of both low-frequency, reflecting sympathetic and parasympathetic activity, and high-frequency, reflecting parasympathetic activity, components of heart rate variability, and LF/HF normalized powers, which was accompanied by a subjective report of relaxation (McConnell et al., 2014). Using the mood states questionnaire (McNair & Heuchert 2011), Wahbeh et al. (2007) reported an anxiety reduction. A study reports that theta-frequency (6 Hz; 250 Hz) BBs enhance the meditative state after 10 min of exposure, which can reduce stress (Jirakittayakorn and Wongsawat, 2017b). In dentistry, alpha-frequency (9.3 Hz; 200 Hz) BBs are also found helpful in reducing preoperative anxiety (Isik et al. 2017). Music with or without BB decreases anxiety and reduces systolic blood pressure. However, those who received BBs showed an additional decrease in heart rate and operative anxiety (Wiwatwongwana et al., 2016).
Lane et al., (1998) reported a decline in depression following the application of BB in the beta range (16 and 24 Hz), which indicates the association of beta BB with less negative mood (Chaieb et al., 2015).
Applying BBs in Parkinson disease (PD) normalizes EEG power and brain functional connectivity and improves working memory with no significant changes in the gait performance or anxiety level (Gálvez et al., 2018). A systematic scoping review also reports that non-invasive brain stimulation methods, such as BBs, change quantitative EEG in PD patients and recommends further research to confirm EEG as a biomarker (Costa et al., 2022).
BBs of theta and delta range during naps increase sleep stability, which can be considered a non-pharmacologic way of sleep treatment (Shumov et al., 2020). Subsequent reports also show that BBs enhance the activity of the parasympathetic part of the autonomic nervous system during naps (Bakaeva et al., 2021). Changing BBs in frequency from 8 to 1 Hz alleviates sleep initiation and maintenance difficulties in patients with chronic insomnia (Tang et al., 2015). Theta-range BBs also increase daytime alertness in subclinical insomnia (Bang et al., 2019). To discover new nonpharmacological methods for treating sleep disorders, Munoz et al. (2020) used a combination of real-time automatic sleep stage classification and a BB generator and showed that BBs improved sleep quality. BBs at the delta (3 Hz) band induce delta activity and increase the duration of non-rapid eye movement (NREM) in the third stage of sleep (Jirakittayakorn & Wongsawat, 2018). The BBs at theta (6 Hz) band have meditative effects and induce theta activity in the frontal and parietal-central regions (Jirakittayakorn and Wongsawat, 2017b). Subjects who used 6 Hz BB for 30 minutes before bedtime for 2 to 14 days showed a reduced hyperarousal state, which could contribute to sleep induction (Lee et al., 2022).
BB stimulation is used in the treatment of stroke, brain injury, tinnitus, dementia, and other cognitive deficits, and studies on the outcomes have provided evidence of the advantage of rehabilitation with music over one without music (David et al., 2010; Galińska, 2015). A summary of the application of BB stimulation has shown that listening to BBs for a suggested period can have the following effects on different behaviors:
1) BBs in the delta (0.5–4 Hz) pattern during sleep causes deeper stages of sleep (Jirakittayakorn & Wongsawat, 2018) and reduce mild anxiety (Le Scouarnec et al., 2001) and pre-operative state-based anxiety (Padmanabhan et al., 2005); 2) BBs in the theta pattern (4–7 Hz) improves meditation, creativity, REM sleep (Jirakittayakorn & Wongsawat, 2018), and relieves mild anxiety (Le Scouarnec et al., 2001; Wahbeh et al., 2007); 3) BBs in the alpha pattern (7–13 Hz) encourages relaxation (Jirakittayakorn & Wongsawat, 2018) and reduces anxiety (Weiland et al., 2011); 4) BBs in the beta pattern (13–30 Hz) promotes concentration and alertness but also increases anxiety, especially at the higher end of the range (Jirakittayakorn & Wongsawat, 2018); 5) BBs in the gamma pattern (30–50 Hz) enhances the maintenance of arousal (Jirakittayakorn & Wongsawat, 2018).
Possible complications of binaural beats
Some reports indicate that BBs’ repetitive nature could make people uncomfortable (Lee et al., 2019). In previous sections, we provided evidence that BBs have beneficial cognitive and non-cognitive effects. However, what goes undetected are the side effects. Until now, no study has reported any side effects during or after listening to BBs. However, keeping the sound level below the maximum safety level of 85 dB seems sensible, as they can cause hearing loss over time (Keith et al., 2008; Tuomi & Jellimann, 2009). Also, it is highly recommended that those with epilepsy consult with their doctors before trying BBs (Vernon et al., 2014).
Based on the alterations in behaviors mentioned in this review by some studies, it is warranted that more research be performed into the behavior alterations observed following intended changes (e.g. improvement of sleep behavior may also cause depression).
Binaural beats and future research directions
In addition to the duration of the stimulation (Chaieb et al., 2015), the choice of carrier tones for the stimulation should be considered, as reports indicate that lower carrier tones may result in more robust effects (Pratt et al., 2010). Some reports also suggest that adding background noise (pink or white) affects the processing of the BBs perception (Reedijk et al., 2013).
It must be considered that cognitive entrainment methods, including BBs, are not universal approaches, and we should consider the individual state.
The meta-analysis results also show that BBs positively affect cognition more than reducing anxiety and pain levels (Garcia-Argibay et al., 2019b). Hence, in designing a study frequency band, time under exposure and intervention time must be chosen to maximize the possible magnitude and direction of responses.
To sum up, the evidence discussed in this paper shows that auditory beats stimulation may be a useful tool for rehabilitation training to enhance both learning and training. However, we must solve the jigsaw puzzle by involving factors and the best configurations for each application. Hence, further research is warranted into the role of personality traits and factors such as duration, frequency, and carrier tones of the stimulation.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
Bakaeva, Z. V., Shumov, D. E., Yakunina, E. B., Starshinov, Y. P., Sveshnikov, D. S., & Torshin, V. I., et al. (2021). [The effect of music embedded with binaural beats on heart rate parameters during nap (Russian)]. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova, 121(4. Vyp. 2), 31–35. [DOI:10.17116/jnevro202112104231] [PMID]
Bang, Y. R., Choi, H. Y., & Yoon, I. Y. (2019). Minimal effects of binaural auditory beats for subclinical insomnia: A randomized double-blind controlled study. Journal of Clinical Psychopharmacology, 39(5), 499–503. [DOI:10.1097/JCP.0000000000001097] [PMID]
Barr, D. F., Mullin, T. A., & Herbert, P. S. (1977). Application of binaural beat phenomenon with aphasic patients. Archives of Otolaryngology, 103(4), 192–194. [DOI:10.1001/archotol.1977.00780210048003] [PMID]
Barratt, M. J., Maddox, A., Smith, N., Davis, J. L., Goold, L., & Winstock, A. R., et al. (2022). Who uses digital drugs? An international survey of 'binaural beat' consumers. Drug and Alcohol Review, 41(5), 1126–1130. [DOI:10.1111/dar.13464] [PMID]
Beauchene, C., Abaid, N., Moran, R., Diana, R. A., & Leonessa, A. (2017). The effect of binaural beats on verbal working memory and cortical connectivity. Journal of Neural Engineering, 14(2), 026014. [DOI:10.1088/1741-2552/aa5d67] [PMID]
Becher, A. K., Höhne, M., Axmacher, N., Chaieb, L., Elger, C. E., & Fell, J. (2015). Intracranial electroencephalography power and phase synchronization changes during monaural and binaural beat stimulation. The European Journal of Neuroscience, 41(2), 254–263. [DOI:10.1111/ejn.12760] [PMID]
Bellesi, M., Riedner, B. A., Garcia-Molina, G. N., Cirelli, C., & Tononi, G. (2014). Enhancement of sleep slow waves: Underlying mechanisms and practical consequences. Frontiers in Systems Neuroscience, 8, 208. [DOI:10.3389/fnsys.2014.00208] [PMID] [PMCID]
Besedovsky, L., Ngo, H. V., Dimitrov, S., Gassenmaier, C., Lehmann, R., & Born, J. (2017). Auditory closed-loop stimulation of EEG slow oscillations strengthens sleep and signs of its immune-supportive function. Nature Communications, 8(1), 1984. [DOI:10.1038/s41467-017-02170-3] [PMID] [PMCID]
Bloom, FE (2013).Fundamentals of Neuroscience. In LR. Squire, D. Berg, FE. Bloom, S. du Lac, A. Ghosh, & NC. Spitzer (Eds.), Fundamental neuroscience (fourth edition) [pp. 3-13]. San Diego: Academic Press. [DOI:10.1016/B978-0-12-385870-2.00001-9]
Chaieb, L., Wilpert, E. C., Reber, T. P., & Fell, J. (2015). Auditory beat stimulation and its effects on cognition and mood States. Frontiers in Psychiatry, 6, 70. [DOI:10.3389/fpsyt.2015.00070] [PMID] [PMCID]
Chakalov, I., Paraskevopoulos, E., Wollbrink, A., & Pantev, C. (2014). Mismatch negativity to acoustical illusion of beat: How and where the change detection takes place?. NeuroImage, 100, 337–346. [DOI:10.1016/j.neuroimage.2014.06.026] [PMID]
Chan, M. F., Chan, E. A., & Mok, E. (2010). Effects of music on depression and sleep quality in elderly people: A randomised controlled trial. Complementary Therapies in Medicine, 18(3-4), 150–159. [DOI:10.1016/j.ctim.2010.02.004] [PMID]
Colzato, L. S., Barone, H., Sellaro, R., & Hommel, B. (2017). More attentional focusing through binaural beats: Evidence from the global-local task. Psychological Research, 81(1), 271–277. [DOI:10.1007/s00426-015-0727-0] [PMID] [PMCID]
Costa, T. D. C., Godeiro Júnior, C., Silva, R. A. E., Dos Santos, S. F., Machado, D. G. D. S., & Andrade, S. M. (2022). The effects of non-invasive brain stimulation on quantitative EEG in patients with parkinson's disease: A systematic scoping review. Frontiers in Neurology, 13, 758452. [DOI:10.3389/fneur.2022.758452] [PMID] [PMCID]
Croom, A. M. (2014). Book review: Auditory neuroscience: Making sense of sound. Musicae Scientiae, 18(2):249-251 [DOI:10.1177/1029864914523548]
David, J. B., Naftali, A., & Katz, A. (2010). Tinntrain: A multifactorial treatment for tinnitus using binaural beats. The Hearing Journal, 63(11), 25-26, 28. [DOI:10.1097/01.HJ.0000390818.17619.65]
Derner, M., Chaieb, L., Dehnen, G., Reber, T. P., Borger, V., & Surges, R., et al. (2021). Auditory beat stimulation modulates memory-related single-neuron activity in the human medial temporal lobe. Brain Sciences, 11(3), 364. [DOI:10.3390/brainsci11030364] [PMID] [PMCID]
Derner, M., Chaieb, L., Surges, R., Staresina, B. P., & Fell, J. (2018). Modulation of item and source memory by auditory beat stimulation: A pilot study with intracranial EEG. Frontiers in Human Neuroscience, 12, 500. [DOI:10.3389/fnhum.2018.00500] [PMID] [PMCID]
Dolphin W. F. (1997). The envelope following response to multiple tone pair stimuli. Hearing Research, 110(1-2), 1–14. [DOI:10.1016/S0378-5955(97)00056-7] [PMID]
Draganova, R., Ross, B., Wollbrink, A., & Pantev, C. (2008). Cortical steady-state responses to central and peripheral auditory beats. Cerebral Cortex, 18(5), 1193–1200. [DOI:10.1093/cercor/bhm153] [PMID]
Eichenbaum H. (2000). A cortical-hippocampal system for declarative memory. Nature reviews. Neuroscience, 1(1), 41–50. [DOI:10.1038/35036213] [PMID]
Engelbregt, H., Barmentlo, M., Keeser, D., Pogarell, O., & Deijen, J. B. (2021). Effects of binaural and monaural beat stimulation on attention and EEG. Experimental Brain Research, 239(9), 2781–2791. [DOI:10.1007/s00221-021-06155-z] [PMID] [PMCID]
Engelbregt, H., Meijburg, N., Schulten, M., Pogarell, O., & Deijen, J. B. (2019). The effects of binaural and monoaural beat stimulation on cognitive functioning in subjects with different levels of emotionality. Advances in Cognitive Psychology, 15(3), 199–207. [DOI:10.5709/acp-0268-8] [PMID] [PMCID]
Fell, J., & Axmacher, N. (2011). The role of phase synchronization in memory processes. Nature Reviews. Neuroscience, 12(2), 105–118. [DOI:10.1038/nrn2979] [PMID]
Fitzpatrick, D. C., Roberts, J. M., Kuwada, S., Kim, D. O., & Filipovic, B. (2009). Processing temporal modulations in binaural and monaural auditory stimuli by neurons in the inferior colliculus and auditory cortex. Journal of the Association for Research in Otolaryngolog, 10(4), 579–593.[DOI:10.1007/s10162-009-0177-8] [PMID] [PMCID]
Galambos, R., Makeig, S., & Talmachoff, P. J. (1981). A 40-Hz auditory potential recorded from the human scalp. Proceedings of the National Academy of Sciences of the United States of America, 78(4), 2643–2647. [DOI:10.1073/pnas.78.4.2643] [PMID] [PMCID]
Galińska E. (2015). Music therapy in neurological rehabilitation settings. Psychiatria Polska, 49(4), 835–846. [DOI:10.12740/PP/25557] [PMID]
Gálvez, G., Recuero, M., Canuet, L., & Del-Pozo, F. (2018). Short-term effects of binaural beats on EEG power, functional connectivity, cognition, gait and anxiety in parkinson's disease. International Journal of Neural Systems, 28(5), 1750055. [DOI:10.1142/S0129065717500551] [PMID]
Garcia-Argibay, M., Santed, M. A., & Reales, J. M. (2019). Binaural auditory beats affect long-term memory. Psychological Research, 83(6), 1124–1136. [DOI:10.1007/s00426-017-0959-2] [PMID]
Garcia-Argibay, M., Santed, M. A., & Reales, J. M. (2019). Efficacy of binaural auditory beats in cognition, anxiety, and pain perception: A meta-analysis. Psychological Research, 83(2), 357–372. [DOI:10.1007/s00426-018-1066-8] [PMID]
Gkolias, V., Amaniti, A., Triantafyllou, A., Papakonstantinou, P., Kartsidis, P., & Paraskevopoulos, E., et al. (2020). Reduced pain and analgesic use after acoustic binaural beats therapy in chronic pain-A double-blind randomized control cross-over trial. European Journal of Pain, 24(9), 1716–1729. [DOI:10.1002/ejp.1615] [PMID]
Goldberg, J. M., & Brownell, W. E. (1973). Discharge characteristics of neurons in anteroventral and dorsal cochlear nuclei of cat. Brain Research, 64, 35–54. [DOI:10.1016/0006-8993(73)90169-8] [PMID]
Goodin, P., Ciorciari, J., Baker, K., Carey, A. M., Harper, M., & Kaufman, J. (2012). A high-density EEG investigation into steady state binaural beat stimulation. Plos One, 7(4), e34789. [DOI:10.1371/journal.pone.0034789] [PMID] [PMCID]
Grose, J. H., Buss, E., & Hall, J. W., 3rd (2012). Binaural beat salience. Hearing Research, 285(1-2), 40–45. [DOI:10.1016/j.heares.2012.01.012] [PMID] [PMCID]
Grose, J. H., & Mamo, S. K. (2012). Electrophysiological measurement of binaural beats: Effects of primary tone frequency and observer age. Ear and Hearing, 33(2), 187–194. [DOI:10.1097/AUD.0b013e318230bbbd] [PMID] [PMCID]
Haywood, N. R., & McAlpine, D. (2020). Estimating the perceptual weighting of interaural time difference cues in amplitude modulated binaural beats. The Journal of the Acoustical Society of America, 148(2), EL185. [DOI:10.1121/10.0001747] [PMID]
Herdman, A. T., Lins, O., Van Roon, P., Stapells, D. R., Scherg, M., & Picton, T. W. (2002). Intracerebral sources of human auditory steady-state responses. Brain Topography, 15(2), 69–86. [DOI:10.1023/A:1021470822922] [PMID]
Hink, R. F., Kodera, K., Yamada, O., Kaga, K., & Suzuki, J. (1980). Binaural interaction of a beating frequency-following response. Audiology, 19(1), 36–43. [DOI:10.3109/00206098009072647] [PMID]
Huang, T. L., & Charyton, C. (2008). A comprehensive review of the psychological effects of brainwave entrainment. Alternative Therapies in Health and Medicine, 14(5), 38–50. [PMID]
Isik, B. K., Esen, A., Büyükerkmen, B., Kilinç, A., & Menziletoglu, D. (2017). Effectiveness of binaural beats in reducing preoperative dental anxiety. The British Journal of Oral & Maxillofacial Surgery, 55(6), 571–574. [DOI:10.1016/j.bjoms.2017.02.014] [PMID]
Jensen, O., Kaiser, J., & Lachaux, J. P. (2007). Human gamma-frequency oscillations associated with attention and memory. Trends in Neurosciences, 30(7), 317–324. [DOI:10.1016/j.tins.2007.05.001] [PMID]
Jirakittayakorn, N., & Wongsawat, Y. (2015). The brain responses to different frequencies of binaural beat sounds on QEEG at cortical level. Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2015, 4687–4691. [DOI:10.1109/EMBC.2015.7319440] [PMID]
Jirakittayakorn, N., & Wongsawat, Y. (2017). Brain responses to 40-Hz binaural beat and effects on emotion and memory. International Journal of Psychophysiology, 120, 96–107. [DOI:10.1016/j.ijpsycho.2017.07.010] [PMID]
Jirakittayakorn, N., & Wongsawat, Y. (2017). Brain responses to a 6-Hz binaural beat: Effects on general theta rhythm and frontal midline theta activity. Frontiers in Neuroscience, 11, 365. [DOI:10.3389/fnins.2017.00365] [PMID] [PMCID]
Jirakittayakorn, N., & Wongsawat, Y. (2018). A novel insight of effects of a 3-Hz binaural beat on sleep stages during sleep. Frontiers in Human Neuroscience, 12, 387. [DOI:10.3389/fnhum.2018.00387] [PMID] [PMCID]
Karino, S., Yumoto, M., Itoh, K., Uno, A., Yamakawa, K., & Sekimoto, S., et al. (2006). Neuromagnetic responses to binaural beat in human cerebral cortex. Journal of Neurophysiology, 96(4), 1927–1938. [DOI:10.1152/jn.00859.2005] [PMID]
Kasprzak, C. (2011) Influence of binaural beats on EEG signal. Acta Physica Polonica A, 119, 986-990 [DOI:10.12693/APhysPolA.119.986]
Keith, S. E., Michaud, D. S., & Chiu, V. (2008). Evaluating the maximum playback sound levels from portable digital audio players. The Journal of the Acoustical Society of America, 123(6), 4227–4237. [DOI:10.1121/1.2904465] [PMID]
Kelton, K., Weaver, T. L., Willoughby, L., Kaufman, D., & Santowski, A. (2021). The Efficacy of Binaural Beats as a Stress-buffering Technique. Alternative Therapies in Health and Medicine, 27(4), 28–33. [PMID]
Kennel, S., Taylor, A. G., Lyon, D., & Bourguignon, C. (2010). Pilot feasibility study of binaural auditory beats for reducing symptoms of inattention in children and adolescents with attention-deficit/hyperactivity disorder. Journal of Pediatric Nursing, 25(1), 3–11. [DOI:10.1016/j.pedn.2008.06.010] [PMID]
Klepp, S. (2006) Effects of binaural-beat stimulation on recovery following traumatic brain injury: A pilot study. Subtle Energies and Energy Medicine, 17(2), 181. [Link]
Kraus, J., & Porubanova, M. (2015). The effect of binaural beats on working memory capacity. Studia Psychologica 57, 135-145. [DOI:10.21909/sp.2015.02.689]
Kuwada, S., Yin, T. C., & Wickesberg, R. E. (1979). Response of cat inferior colliculus neurons to binaural beat stimuli: Possible mechanisms for sound localization. Science, 206(4418), 586–588. [DOI:10.1126/science.493964] [PMID]
Lakatos, P., Karmos, G., Mehta, A. D., Ulbert, I., & Schroeder, C. E. (2008). Entrainment of neuronal oscillations as a mechanism of attentional selection. Science, 320(5872), 110–113. [DOI:10.1126/science.1154735] [PMID]
Lane, J. D., Kasian, S. J., Owens, J. E., & Marsh, G. R. (1998). Binaural auditory beats affect vigilance performance and mood. Physiology & Behavior, 63(2), 249–252. [DOI:10.1016/S0031-9384(97)00436-8] [PMID]
Lane, J. D., Kasian, S. J., Owens, J. E., & Marsh, G. R. (1998). Binaural auditory beats affect vigilance performance and mood. Physiology & Behavior, 63(2), 249–252. [DOI:10.1016/S0031-9384(97)00436-8] [PMID]
Lavallee, C. F., Koren, S. A., & Persinger, M. A. (2011). A quantitative electroencephalographic study of meditation and binaural beat entrainment. Journal of Alternative and Complementary Medicine, 17(4), 351–355. [DOI:10.1089/acm.2009.0691] [PMID]
Le Scouarnec, R. P., Poirier, R. M., Owens, J. E., Gauthier, J., Taylor, A. G., & Foresman, P. A. (2001). Use of binaural beat tapes for treatment of anxiety: A pilot study of tape preference and outcomes. Alternative Therapies in Health and Medicine, 7(1), 58–63. [PMID]
Lee, E., Bang, Y., Yoon, I. Y., & Choi, H. Y. (2022). Entrapment of binaural auditory beats in subjects with symptoms of insomnia. Brain Sciences, 12(3), 339. [DOI:10.3390/brainsci12030339] [PMID] [PMCID]
Lee, M., Song, C. B., Shin, G. H., & Lee, S. W. (2019). Possible effect of binaural beat combined with autonomous sensory meridian response for inducing sleep. Frontiers in Human Neuroscience, 13, 425. [DOI:10.3389/fnhum.2019.00425] [PMID] [PMCID]
Licklider, J. C. R., Webster, J. C., Hedlun, J. M. (1950) On the frequency limits of binaural beats. The Journal of the Acoustical Society of America, 22, 468-473. [DOI:10.1121/1.1906629]
Little, B. R., Lecci, L., & Watkinson, B. (1992). Personality and personal projects: Linking big five and PAC units of analysis. Journal of Personality, 60(2), 501–525. [DOI:10.1111/j.1467-6494.1992.tb00982.x] [PMID]
López-Caballero, F., & Escera, C. (2017). Binaural Beat: A Failure to Enhance EEG Power and Emotional Arousal. Frontiers in Human Neuroscience, 11, 557. [DOI:10.3389/fnhum.2017.00557] [PMID] [PMCID]
McAlpine, D., Jiang, D., & Palmer, A. R. (1996). Interaural delay sensitivity and the classification of low best-frequency binaural responses in the inferior colliculus of the guinea pig. Hearing Research, 97(1-2), 136–152. [DOI:10.1016/S0378-5955(96)80015-3] [PMID]
McAlpine, D., Jiang, D., Shackleton, T. M., & Palmer, A. R. (1998). Convergent input from brainstem coincidence detectors onto delay-sensitive neurons in the inferior colliculus. The Journal of Neuroscience, 18(15), 6026–6039. [DOI:10.1523/JNEUROSCI.18-15-06026.1998] [PMID] [PMCID]
McConnell, P. A., Froeliger, B., Garland, E. L., Ives, J. C., & Sforzo, G. A. (2014). Auditory driving of the autonomic nervous system: Listening to theta-frequency binaural beats post-exercise increases parasympathetic activation and sympathetic withdrawal. Frontiers in Psychology, 5, 1248. [DOI:10.3389/fpsyg.2014.01248] [PMID] [PMCID]
Heuchert, J. P., & McNair, D. M. (2011). Profile of mood states, 2nd edn. TM (POMS 2TM). San Diego: JvR Psychometrics Assessment Catalogue. [DOI:10.1037/t05057-000]
Menziletoglu, D., Guler, A. Y., Cayır, T., & Isik, B. K. (2021). Binaural beats or 432 Hz music? Which method is more effective for reducing preoperative dental anxiety? Medicina Oral, Patologia Oral y Cirugia Bucal, 26(1), e97–e101. [DOI:10.4317/medoral.24051] [PMID] [PMCID]
Moore, B. C. J. (2012). An introduction to the psychology of hearing. Leeds: Emerald. [Link]
Mujib, M. D., Hasan, M. A., Qazi, S. A., & Vuckovic, A. (2021). Understanding the neurological mechanism involved in enhanced memory recall task following binaural beat: A pilot study. Experimental Brain Research, 239(9), 2741–2754. [DOI:10.1007/s00221-021-06132-6] [PMID] [PMCID]
Munoz, J. P., & Rivera, L. A. (2020). Towards improving sleep quality using automatic sleep stage classification and binaural beats. Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2020, 4982–4985. [DOI:10.1109/EMBC44109.2020.9176385] [PMID]
Ölçücü, M. T., Yılmaz, K., Karamık, K., Okuducu, Y., Özsoy, Ç., & Aktaş, Y., et al. (2021). Effects of listening to binaural beats on anxiety levels and pain scores in male patients undergoing cystoscopy and ureteral stent removal: A randomized placebo-controlled trial. Journal of Endourology, 35(1), 54–61. [DOI:10.1089/end.2020.0353] [PMID]
Orozco Perez, H. D., Dumas, G., & Lehmann, A. (2020). Binaural beats through the auditory pathway: From brainstem to connectivity patterns. eNeuro, 7(2), ENEURO.0232-19.2020. [DOI:10.1523/ENEURO.0232-19.2020] [PMID] [PMCID]
Ortiz, T., Martínez, A. M., Fernández, A., Maestu, F., Campo, P., & Hornero, R., et al. (2008). Impact of auditory stimulation at a frequency of 5 Hz in verbal memory. Actas Espanolas de Psiquiatria, 36(6), 307–313. [PMID]
Oster, G. (1973). Auditory beats in the brain. Scientific American, 229(4), 94–102. [DOI:10.1038/scientificamerican1073-94] [PMID]
Ozdamar, O., Bohorquez, J., Mihajloski, T., Yavuz, E., & Lachowska, M. (2011). Auditory evoked responses to binaural beat illusion: Stimulus generation and the derivation of the binaural interaction component (BIC). Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2011, 830–833. [DOI:10.1109/IEMBS.2011.6090190] [PMID]
Padmanabhan, R., Hildreth, A. J., & Laws, D. (2005). A prospective, randomised, controlled study examining binaural beat audio and pre-operative anxiety in patients undergoing general anaesthesia for day case surgery. Anaesthesia, 60(9), 874–877. [DOI:10.1111/j.1365-2044.2005.04287.x] [PMID]
Pantev, C., Roberts, L. E., Elbert, T., Ross, B., & Wienbruch, C. (1996). Tonotopic organization of the sources of human auditory steady-state responses. Hearing Research, 101(1-2), 62–74. [DOI:10.1016/S0378-5955(96)00133-5] [PMID]
Pastor, M. A., Artieda, J., Arbizu, J., Marti-Climent, J. M., Peñuelas, I., & Masdeu, J. C. (2002). Activation of human cerebral and cerebellar cortex by auditory stimulation at 40 Hz. The Journal of Neuroscience, 22(23), 10501–10506. [DOI:10.1523/JNEUROSCI.22-23-10501.2002] [PMID] [PMCID]
Perrott, D. R., & Musicant, A. D. (1977). Rotating tones and binaural beats. The Journal of the Acoustical Society of America, 61(5), 1288–1292. [DOI:10.1121/1.381430] [PMID]
Picton, T. W., Dimitrijevic, A., Perez-Abalo, M. C., & Van Roon, P. (2005). Estimating audiometric thresholds using auditory steady-state responses. Journal of the American Academy of Audiology, 16(3), 140–156. [DOI:10.3766/jaaa.16.3.3] [PMID]
Picton, T. W., John, M. S., Dimitrijevic, A., & Purcell, D. (2003). Human auditory steady-state responses. International Journal of Audiology, 42(4), 177–219. [DOI:10.3109/14992020309101316] [PMID]
Plourde G. (2006). Auditory evoked potentials. Best practice & research. Clinical Anaesthesiology, 20(1), 129–139. [DOI:10.1016/j.bpa.2005.07.012] [PMID]
Pratt, H., Starr, A., Michalewski, H. J., Dimitrijevic, A., Bleich, N., & Mittelman, N. (2009). Cortical evoked potentials to an auditory illusion: Binaural beats. Clinical Neurophysiology, 120(8), 1514–1524. [DOI:10.1016/j.clinph.2009.06.014] [PMID] [PMCID]
Pratt, H., Starr, A., Michalewski, H. J., Dimitrijevic, A., Bleich, N., & Mittelman, N. (2010). A comparison of auditory evoked potentials to acoustic beats and to binaural beats. Hearing Research, 262(1-2), 34–44. [DOI:10.1016/j.heares.2010.01.013] [PMID]
Reale, R. A., & Brugge, J. F. (1990). Auditory cortical neurons are sensitive to static and continuously changing interaural phase cues. Journal of Neurophysiology, 64(4), 1247–1260. [DOI:10.1152/jn.1990.64.4.1247] [PMID]
Reedijk, S. A., Bolders, A., & Hommel, B. (2013). The impact of binaural beats on creativity. Frontiers in Human Neuroscience, 7, 786. [DOI:10.3389/fnhum.2013.00786] [PMID] [PMCID]
Robison, M. K., Obulasetty, M., Blais, C., Wingert, K. M., & Brewer, G. A. (2022). The effect of binaural beat stimulation on sustained attention. Psychological Research, 86(3), 808–822. [DOI:10.1007/s00426-021-01524-3] [PMID]
Ross, B., & Lopez, M. D. (2020). 40-Hz Binaural beats enhance training to mitigate the attentional blink. Scientific Reports, 10(1), 7002. [DOI:10.1038/s41598-020-63980-y] [PMID] [PMCID]
Schwarz, D. W., & Taylor, P. (2005). Human auditory steady state responses to binaural and monaural beats. Clinical Neurophysiology, 116(3), 658–668. [DOI:10.1016/j.clinph.2004.09.014] [PMID]
Seifi Ala, T., Ahmadi-Pajouh, M. A., & Nasrabadi. A. M. (2018) Cumulative effects of theta binaural beats on brain power and functional connectivity. Biomedical Signal Processing and Control 42, 242-252 [DOI:10.1016/j.bspc.2018.01.022]
Shumov, D. E., Yakovenko, I. A., Alipov, N. N., Bakaeva, Z. V., Yakunina, E. B., & Minyuk, A. N.,et al. (2020). [The effect of music containing binaural beats on daytime fall-asleep dynamics (Russian)]. Zhurnal Nevrologii i Psikhiatrii Imeni S.S. Korsakova, 120(2), 39–44. [DOI:10.17116/jnevro202012002139] [PMID]
Solcà, M., Mottaz, A., & Guggisberg, A. G. (2016). Binaural beats increase interhemispheric alpha-band coherence between auditory cortices. Hearing Research, 332, 233–237. [DOI:10.1016/j.heares.2015.09.011] [PMID]
Spitzer, M. W., & Semple, M. N. (1998). Transformation of binaural response properties in the ascending auditory pathway: Influence of time-varying interaural phase disparity. Journal of Neurophysiology, 80(6), 3062–3076. [DOI:10.1152/jn.1998.80.6.3062] [PMID]
Starr, A., McPherson, D., Patterson, J., Don, M., Luxford, W., & Shannon, R., et al. (1991). Absence of both auditory evoked potentials and auditory percepts dependent on timing cues. Brain, 114(Pt 3), 1157–1180. [DOI:10.1093/brain/114.3.1157] [PMID]
Tallon-Baudry, C., & Bertrand, O. (1999). Oscillatory gamma activity in humans and its role in object representation. Trends in Cognitive Sciences, 3(4), 151–162. [DOI:10.1016/S1364-6613(99)01299-1] [PMID]
Tang, H. Y., Vitiello, M. V., Perlis, M., & Riegel, B. (2015). Open-loop neurofeedback audiovisual stimulation: A pilot study of its potential for sleep induction in older adults. Applied Psychophysiology and Biofeedback, 40(3), 183–188. [DOI:10.1007/s10484-015-9285-x] [PMID] [PMCID]
Tani, A., Tartarisco, G., Vagheggini, G., Vaccaro, C., Campana, S., & Tomaiuolo, F. (2022). Binaural beats reduce feeling of pain and discomfort during colonoscopy procedure in not-sedated patients: A randomized control trial. Complementary Therapies in Clinical Practice, 48, 101605. [DOI:10.1016/j.ctcp.2022.101605] [PMID]
Tani, A., Vagheggini, G., Moretti, F., Del Colombo, V., Lehle, J., Campana, S., Labate, A., & Tomaiuolo, F. (2021). Binaural beats reduce postoperative morphine consumption in older adults after total knee replacement surgery. Alternative Therapies in Health and Medicine, 27(2), 27–30. [PMID]
Tort, A. B., Komorowski, R. W., Manns, J. R., Kopell, N. J., & Eichenbaum, H. (2009). Theta-gamma coupling increases during the learning of item-context associations. Proceedings of the National Academy of Sciences of the United States of America, 106(49), 20942–20947. [DOI:10.1073/pnas.0911331106] [PMID] [PMCID]
Tuomi, S., & Jellimann, M. (2009) Hear today-hearing loss tomorrow: A preliminary survey of the personal audio player user habits and knowledge of South African first-year university students. South African Family Practice, 51, 166-167 [DOI:10.1080/20786204.2009.10873835]
Ungan, P., Yagcioglu, S., & Ayik, E. (2019). Event-related potentials to single-cycle binaural beats and diotic amplitude modulation of a tone. Experimental Brain Research, 237(8), 1931–1945. [DOI:10.1007/s00221-019-05562-7] [PMID]
Vernon, D., Peryer, G., Louch, J., & Shaw, M. (2014). Tracking EEG changes in response to alpha and beta binaural beats. International Journal of Psychophysiology, 93(1), 134–139. [DOI:10.1016/j.ijpsycho.2012.10.008] [PMID]
Wahbeh, H., Calabrese, C., & Zwickey, H. (2007). Binaural beat technology in humans: A pilot study to assess psychologic and physiologic effects. Journal of Alternative and Complementary Medicine, 13(1), 25–32. [DOI:10.1089/acm.2006.6196] [PMID]
Walter, V. J., & Walter, W. G. (1949). The central effects of rhythmic sensory stimulation. Electroencephalography and Clinical Neurophysiology, 1(1), 57–86. [DOI:10.1016/0013-4694(49)90164-9] [PMID]
Weiland, T. J., Jelinek, G. A., Macarow, K. E., Samartzis, P., Brown, D. M., & Grierson, E. M., et al. (2011). Original sound compositions reduce anxiety in emergency department patients: a randomised controlled trial. The Medical Journal of Australia, 195(11-12), 694–698. [DOI:10.5694/mja10.10662] [PMID]
Wernick, J. S., & Starr, A. (1968). Binaural interaction in the superior olivary complex of the cat: an analysis of field potentials evoked by binaural-beat stimuli. Journal of Neurophysiology, 31(3), 428–441. [DOI:10.1152/jn.1968.31.3.428] [PMID]
Wiwatwongwana, D., Vichitvejpaisal, P., Thaikruea, L., Klaphajone, J., Tantong, A., & Wiwatwongwana, A., et al. (2016). The effect of music with and without binaural beat audio on operative anxiety in patients undergoing cataract surgery: A randomized controlled trial. Eye, 30(11), 1407–1414. [DOI:10.1038/eye.2016.160] [PMID] [PMCID]
Wu, S. H., & Oertel, D. (1984). Intracellular injection with horseradish peroxidase of physiologically characterized stellate and bushy cells in slices of mouse anteroventral cochlear nucleus. The Journal of Neuroscience, 4(6), 1577–1588. [DOI:10.1523/JNEUROSCI.04-06-01577.1984] [PMID] [PMCID]
Yusim, A., & Grigaitis, J. (2020). Efficacy of binaural beat meditation technology for treating anxiety symptoms: A pilot study. The Journal of Nervous and Mental Disease, 208(2), 155–160. [DOI:10.1097/NMD.0000000000001070] [PMID]
Zampi, D. D. (2016). Efficacy of theta binaural beats for the treatment of chronic pain [doctoral dissertation]. Prescott Valley: Northcentral University. [Link]
Type of Study: Review |
Subject:
Behavioral Neuroscience
Received: 2022/03/18 | Accepted: 2022/06/29 | Published: 2024/03/1
Received: 2022/03/18 | Accepted: 2022/06/29 | Published: 2024/03/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








