Volume 15, Issue 6 (November & December 2024)
BCN 2024, 15(6): 735-744 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mosaed R, Akhavan Rezayat A, Rohani B, Ayati Afin A, Najmeddin F, Amini S, et al . Effects of Hypertonic Sodium Lactate on Intracranial Pressure in Patients With Traumatic Brain Injury: A Systematic Review and Meta-analysis on Clinical Trial Studies. BCN 2024; 15 (6) :735-744
URL: http://bcn.iums.ac.ir/article-1-2437-en.html
URL: http://bcn.iums.ac.ir/article-1-2437-en.html
Reza Mosaed1 

 , Arash Akhavan Rezayat2
, Arash Akhavan Rezayat2 

 , Behnaz Rohani3
, Behnaz Rohani3 
 , Aida Ayati Afin3
, Aida Ayati Afin3 

 , Farhad Najmeddin1
, Farhad Najmeddin1 

 , Shahideh Amini1
, Shahideh Amini1 
 , Maryam Taghizadeh-Ghehi4
, Maryam Taghizadeh-Ghehi4 

 , Mohamad Afshar Ardalan5
, Mohamad Afshar Ardalan5 
 , Atabak Najafi6
, Atabak Najafi6 
 , Mojtaba Mojtahedzadeh *1
, Mojtaba Mojtahedzadeh *1 




 , Arash Akhavan Rezayat2
, Arash Akhavan Rezayat2 

 , Behnaz Rohani3
, Behnaz Rohani3 
 , Aida Ayati Afin3
, Aida Ayati Afin3 

 , Farhad Najmeddin1
, Farhad Najmeddin1 

 , Shahideh Amini1
, Shahideh Amini1 
 , Maryam Taghizadeh-Ghehi4
, Maryam Taghizadeh-Ghehi4 

 , Mohamad Afshar Ardalan5
, Mohamad Afshar Ardalan5 
 , Atabak Najafi6
, Atabak Najafi6 
 , Mojtaba Mojtahedzadeh *1
, Mojtaba Mojtahedzadeh *1 


1- Department of Clinical Pharmacy, School of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran.
2- Health Policy Research Center, Institute of Health, Shiraz University of Medical Sciences, Shiraz, Iran.
3- Student Research Committee, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
4- Research Center for Rational Use of Drugs, Tehran University of Medical Sciences, Tehran, Iran.
5- Department of Internal Medicine, Faculty of Medicine, Aja University of Medical Sciences, Tehran, Iran.
6- Department of Anesthesiology and Critical Care Medicine, School of Medicine, Sina Hospital, Tehran University of Medical Sciences, Tehran, Iran.
2- Health Policy Research Center, Institute of Health, Shiraz University of Medical Sciences, Shiraz, Iran.
3- Student Research Committee, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
4- Research Center for Rational Use of Drugs, Tehran University of Medical Sciences, Tehran, Iran.
5- Department of Internal Medicine, Faculty of Medicine, Aja University of Medical Sciences, Tehran, Iran.
6- Department of Anesthesiology and Critical Care Medicine, School of Medicine, Sina Hospital, Tehran University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 784 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Traumatic brain injury (TBI) is one of the most important causes of cerebral dysfunction, and it is the reason for more than 30% of post-injury mortalities (Cassidy et al., 2004; Herbert et al., 2017). Increased intracranial pressure (ICP) following TBI is the main predisposing factor for death in the patients. Hyperosmolar therapy could ameliorate this condition (Fenn III & Sierra, 2019; Gerber et al., 2013). However, osmotherapy has been debated as the treatment of choice for these patients, considering the complex and nonselective nature of osmotic mediators’ effects (Bergersen, 2015). The mechanism attributed to hyperosmolar agents is that they increase the oncotic movement of water into the intravascular space and decrease the blood viscosity. As a result, hyperosmolar agents reduce the cerebral intracellular fluid and increase the cerebral blood flow (CBF) (Berthet et al., 2012).
Mannitol and hypertonic saline (HTS) solutions have been used as hyperosmolar therapy in patients with elevated ICP (Francony et al., 2008). Traditionally, mannitol has been recommended as the first choice of osmotherapy in TBI (Maas et al., 1997). However, hypotension, especially in the hypovolemic state, and rebound rise in ICP are the main drawbacks of mannitol (Anonymous, 2000; White et al., 2006). In refractory cases of increased ICP following mannitol administration, HTS increases brain oxygenation and reduces ICP (Ogden et al., 2005). Moreover, HTS has a more pronounced and longer-lasting effect than mannitol on elevated ICP and causes no rebound increase in ICP (Kerwin et al., 2009). Hence, some recent studies report HTS as the superior hyperosmolar agent (Mangat et al., 2020). No clear recommendation was made in a recently published meta-analysis regarding the choice of hypertonic saline or mannitol in treating patients with TBI-induced elevated ICP (Fatima et al., 2019). However, some concerns exist about nephrotoxic effects and metabolic acidosis following high chloride-containing solutions. Chloride as a nonorganic anion in sodium solution has no role in osmotic therapy, and previous studies have suggested replacing chloride with other organic anions.
Another hyperosmolar solution studied in patients with elevated ICP is hypertonic sodium lactate (HSL) (Ichai et al., 2013). Lactate, as an organic anion, is a suitable energy source for brain cells, and sodium lactate might work not only as a hypertonic agent but also as a metabolic fuel for the brain (Ichai et al., 2009; Leverve & Mustafa, 2002; Rice et al., 2002). HSL could decrease ICP through the osmotic effect of hypertonic sodium and the cerebral vasodilator effect of lactate (Gordon et al., 2008). Ichai et al. mentioned that HSL’s effect was even more potent than mannitol in reducing ICP in some trials (Ichai et al., 2009). Notably, in a recent study on patients with TBI-induced elevated ICP, Arifianto et al. (2018) introduced HSL as a choice for fluid therapy. Considering HSL’s beneficial roles in TBI, in this study, we systematically reviewed the articles investigating HSL’s therapeutic role in patients with TBI.
2. Materials and Methods
Data sources and search strategy
We performed our search in electronic databases, including PubMed, Embase, Scopus, and Web of Science, for studies investigating the effect of hyperosmolar agents on increased ICP in patients with TBI. Two independent investigators (Arash Akhavan Rezayat, and Behnaz Rohani) searched the relevant published articles from January 2000 to December 2020. We searched the literature applying a different combination of our keywords, including TBI, hypertonic agents, and their related terms. PubMed search strategy that consisted of various concepts of our keywords was as follows:
((“Brain injury”) OR (“brain injuries”) OR (“brain injuries, traumatic”) OR (“brain injuries, diffuse”) OR (“brain concussion”) OR (“head injury”) OR (“head injuries”) OR (“head injuries, penetrating”) OR (“cerebrovascular trauma”) OR (“craniocerebral trauma injuries”) OR (“cerebral hemorrhage, traumatic”) OR (“TBI”)) AND ((“hypertonic saline”) OR (“saline solution, hypertonic”) OR (“hypertonic sodium”) OR (“sodium chloride”) OR (“lactate”) OR (“sodium lactate”) OR (“hypertonic sodium lactate”) OR (“hyperosmolar sodium lactate”) OR (“mannitol”) OR (“osmotic diuretic”) OR (“osmotic diuretics”) OR (“diuretics, osmotic”)).
We also searched for grey literature using Open Grey, ProQuest Dissertations, and theses full texts.
Inclusion and exclusion criteria
All published randomized control trials in English literature involving our keywords were included for further evaluation. Our inclusion criteria were as follows: All patients were older than 16, suffered from TBI, and had ICP above 20 mm Hg. Animal studies, case reports, and studies on patients with liver and renal failure, cardiac dysfunction, or hypovolemic shock were excluded. We also excluded studies that did not directly compare the effects of hypertonic saline or mannitol on elevated ICP or did not provide sufficient primary data.
Study selection
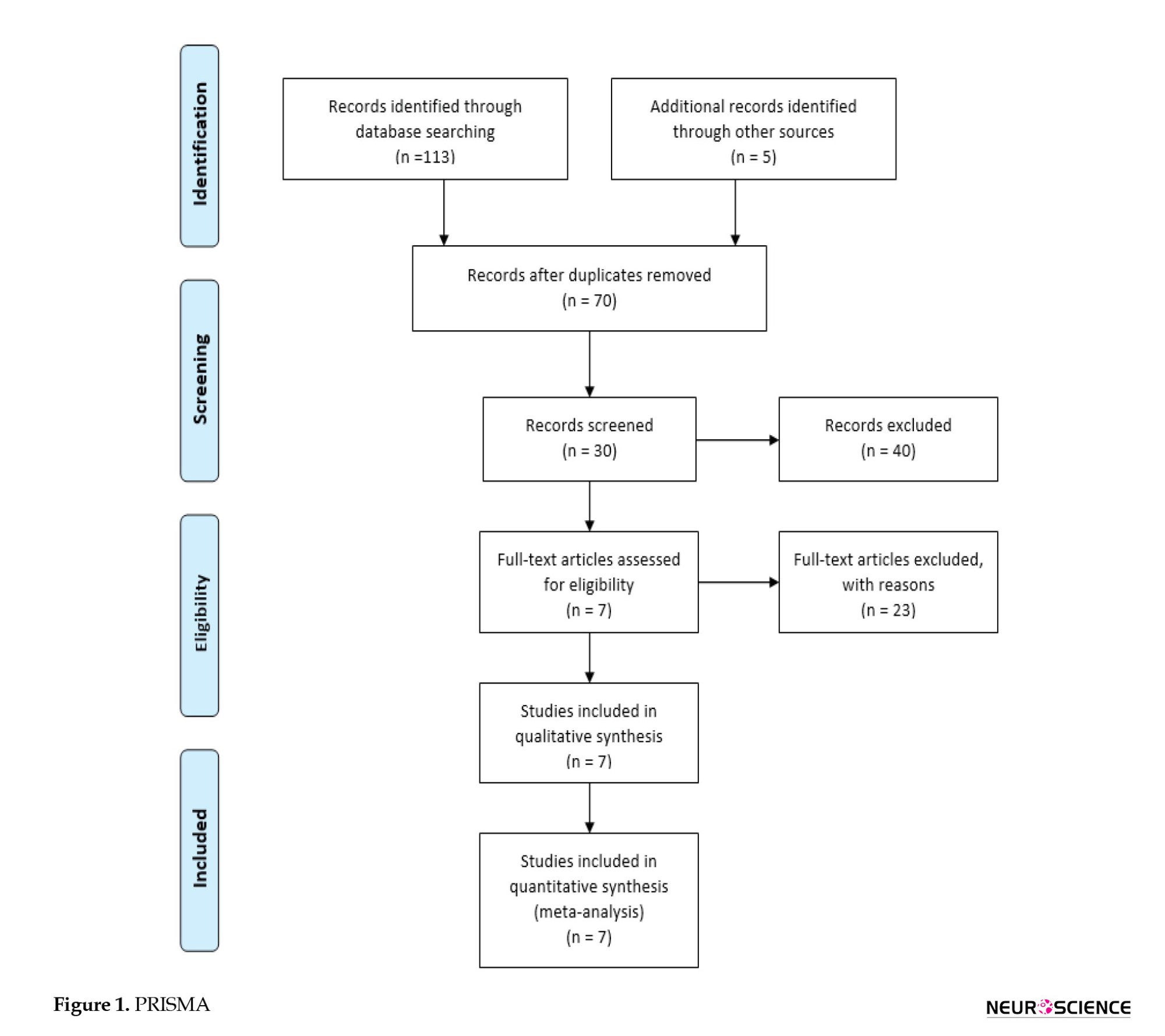
After checking for duplications, two investigators independently reviewed all studies’ titles and abstracts to meet our inclusion and exclusion criteria. Any disagreement was discussed with the third reviewer. The flow diagram of the selection process of the studies is presented in Figure 1.
Data extraction
We extracted the information on the first author, publication time, country, study design, patient number, mean age, gender ratio, and interventions. The other reviewer resolved any disagreements on the selected studies.
Demographic data, methods, interventions, and outcomes were extracted from the included manuscripts and documented.
Data analysis
Data from included studies were pooled using a random-effect model. All continuous data were summarized as the standard difference in Means±SE and analyzed using comprehensive meta-analysis. The inconsistency index (I2) was used to measure heterogeneity, with I2>50% indicating substantial heterogeneity. P<0.05 was considered statistically significant.
3. Results
Selection process results
Our initial search with the predefined search strategy proceeded in 34 studies from the Scopus database and 32 publications from the PubMed database. Furthermore, 32 additional publications were included by searching the Web of Science. Also, reviewing Embase added 15 articles to the studies above. Removing duplicates resulted in 70 studies. The title and abstract screening excluded 40 studies. Subsequently, 30 remaining full texts were assessed; 7 studies did not provide enough information, 10 articles were irrelevant, and 6 were animal studies. Finally, 7 studies (Fenn III & Sierra, 2019; Gerber et al., 2013; Halestrap & Price, 1999; Herbert et al., 2017; Holloway et al., 2007; Ichai et al., 2009; Ichai et al., 2013) were eligible for this systematic review, of which 3 studies (Gerber et al., 2013; Halestrap & Price, 1999; Ichai et al., 2009), comprising ICP between before and after injection of HSL, were eligible for a meta-analysis. The selection process is detailed in Figure 1 PRISMA (the preferred reporting items for systematic reviews and meta-analyses) flow chart.
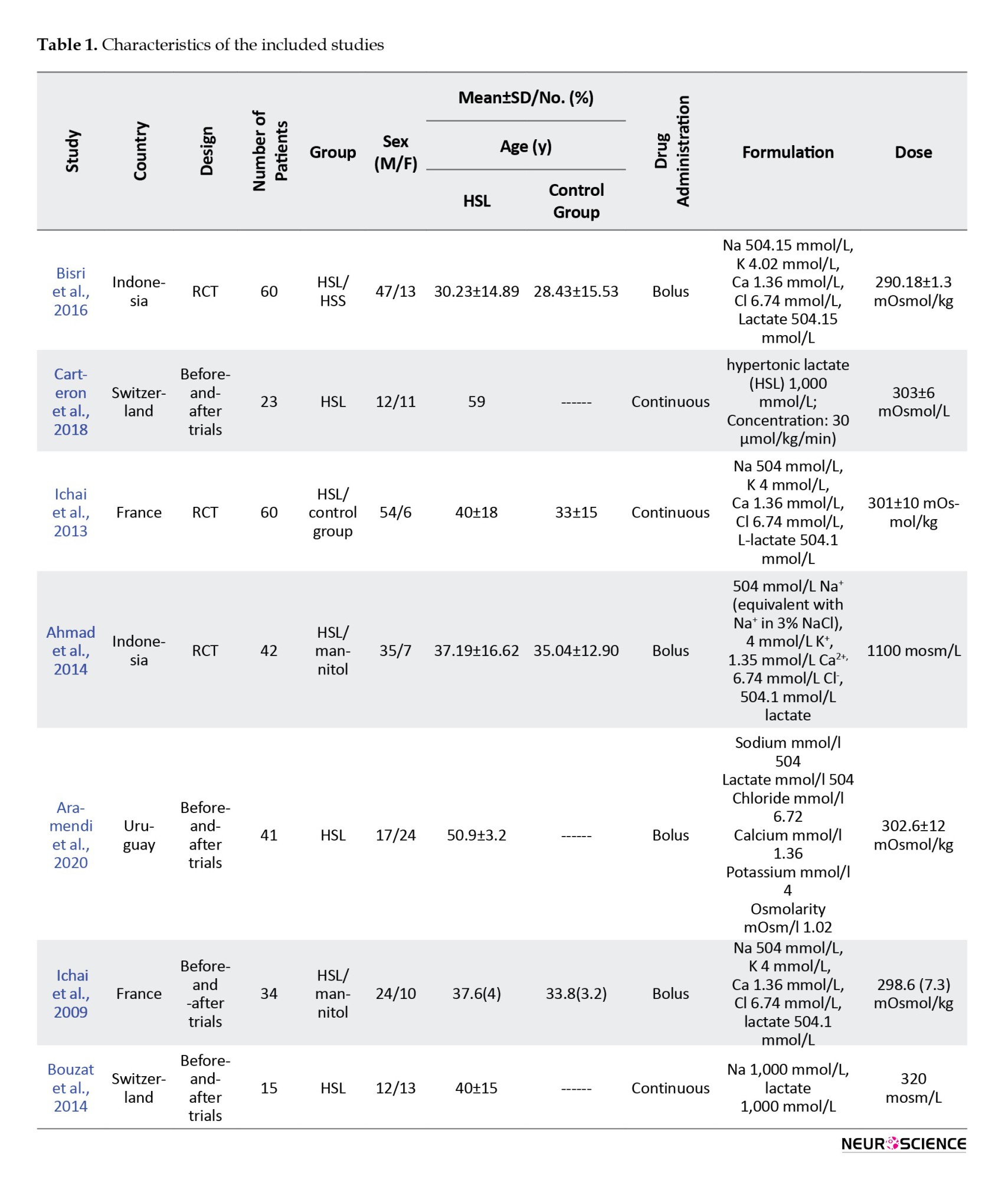
Characteristics of the included studies
Of 138 initially reviewed papers, 131 were removed, and 7 articles were assessed for eligibility using the Newcastle-OTTAWA scale. Studies from France, Switzerland, and Indonesia were evenly distributed, with two articles from each country. One study was conducted in Uruguay. Four studies (Fenn III & Sierra, 2019; Gerber et al., 2013; Herbert et al., 2017; Ichai et al., 2013) (were randomized trials, of which 3 (Aramendi et al., 2020; Holloway et al., 2007; Ichai et al., 2009) were before-and-after trials. The total number of patients from the initial 7 articles was 275, and 98 were included in the final 3 analyzed studies. Two included studies (Halestrap & Price, 1999; Ichai et al., 2009) (were prospective interventional, and 1 (Gerber et al., 2013) was a prospective open randomized study.
The meta-analysis was carried out for ICP reduction according to data reported in 3 articles. Drug administration characteristics in 4 publications (Aramendi et al., 2020; Bisri et al., 2016; Ichai et al., 2009; Muhammad & Hanna, 2014) were bolus, while the other 3 studies utilized the continuous form. Study characteristics are presented in Table 1.
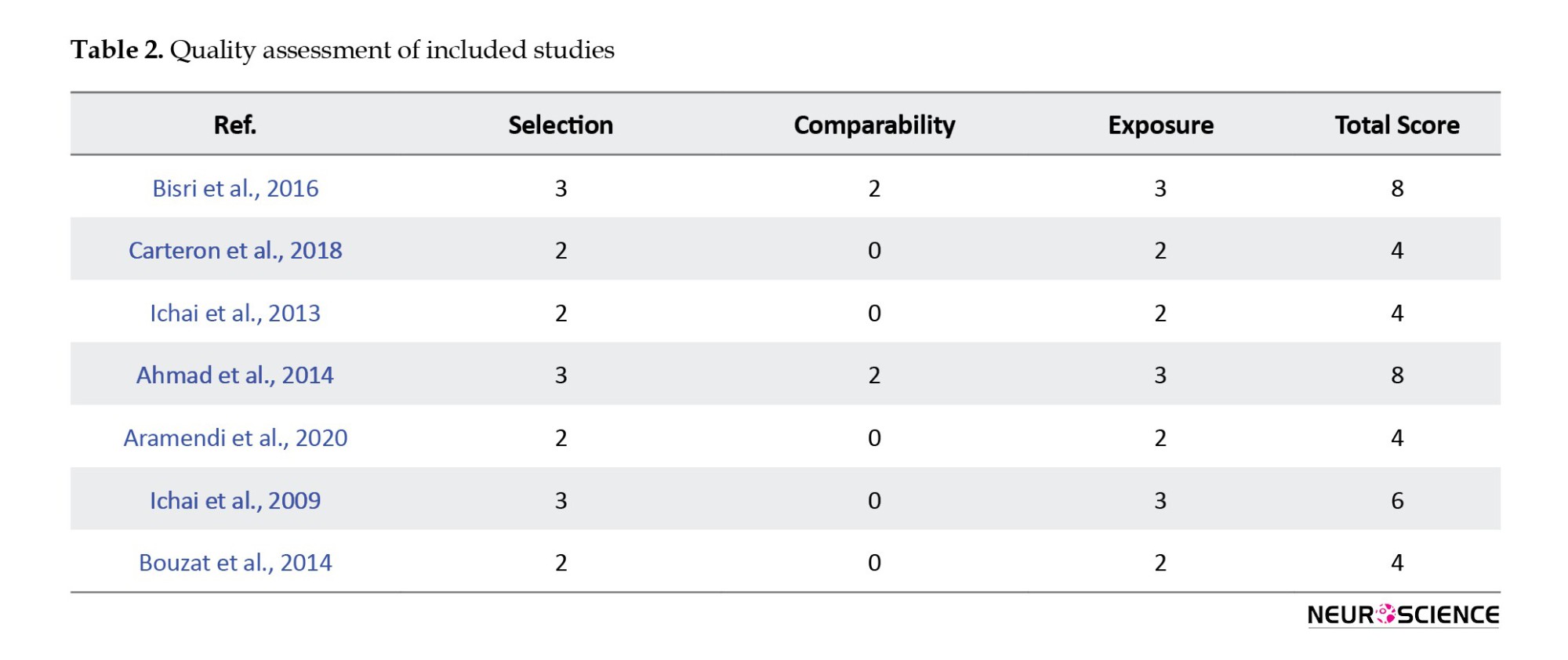
Critical appraisal
The risk of bias assessment of our final articles is demonstrated in Table 2. Of the 7 included studies, 5 (Aramendi et al., 2020; Bouzat et al., 2014; Carteron et al., 2018; Ichai et al., 2009; Ichai et al., 2013) had an overall moderate risk of bias, and 2 (Bisri et al., 2016; Muhammad & Hanna, 2014) had a high-quality risk of bias.
Study outcomes
The pooled meta-analysis revealed a significant effect on ICP reduction in the use of HSL. Details were reported separately, including ICP, sodium osmolality, and neurological outcomes.
ICP
Five trials (Aramendi et al., 2020; Bouzat et al., 2014; Carteron et al., 2018; Ichai et al., 2009; Ichai et al., 2013) reported information on ICP. A study by Ichai et al., (2009) that compared ICP reduction using mannitol and sodium lactate showed a significantly lower ICP with sodium lactate infusion (group effect P=0.0161). Other before-and-after trials also reported positive effects on ICP. Carteron et al., (2018) reported ICP reduction after three hours in comparison with baseline (8±6 vs 10±5 mm Hg), P<0.01). Aramendi et al., (2020) showed a significant decrease in ICP after 30 and 60 minutes compared to the preinfusion state (P=0.0001). Another study by Bouzat et al., (2014) assessed the cerebral metabolic effects of HSL. In this study, HL therapy positively affected both ICP reduction and brain glutamate during three hours of infusion. Another study by Ichai et al., (2013) showed a reduction in raised ICP episodes in comparison with the control group during the 48-h study: 23 vs 53 episodes (P<0.05).
Serum sodium and serum osmolality
In two studies (Carteron et al., 2018; Ichai et al., 2013), sodium osmolality was increased between the two groups. In one study by Ichai et al., (2013), there was a significantly higher cumulative sodium intake and output between two groups of case and control (0.9% saline solution) in 48 h (P<0.01). Also, Carteron et al., (2018) reported a significant increase in sodium and osmolarity in a 3-h HS infusion (146±3 vs 152±3 mmol/L; P<0.001 and 303±6 vs 314±7 mOsmol/L; P<0.001). In another study by Ichai et al., (2009), there was no significant difference between mannitol and lactate therapy. In another trial by Bisri et al., (2016), serum sodium and osmolality levels were increased within 15 minutes in the HSS group compared to the HSL group, which remained unchanged (P<0.001).
Neurological outcomes
In 4 studies, the Glasgow outcome scale (GOS) was used for neurologic function assessment (Bouzat et al., 2014; Carteron et al., 2018; Ichai et al., 2009; Ichai et al., 2013). Ichai et al., (2013) assessed GOS for HSL and the control group after 6 months. Although survivors had poorer neurological outcomes among the control group (9 versus 4), there was no significant difference between the two groups (P=0.14). Carteron et al., (2018), with the use of a 3-h HSL solution, reported ten patients with good neurological recovery (GOS 4 or 5) at 6 months (out of 20 survivors and 3 deaths). Another study by Ichai et al., (2009) reported better outcomes using HSL compared with mannitol. Bouzat et al., (2014) reported 15 patients receiving a 3-h of HSL infusion, of which 9 had good recovery of neurologic function (GOS 4 or 5), 6 patients with GOS 3 did not have a good outcome (including 2 with severe disability), and 1 patient with GOS 2 and 3 with GOS 1 had died.
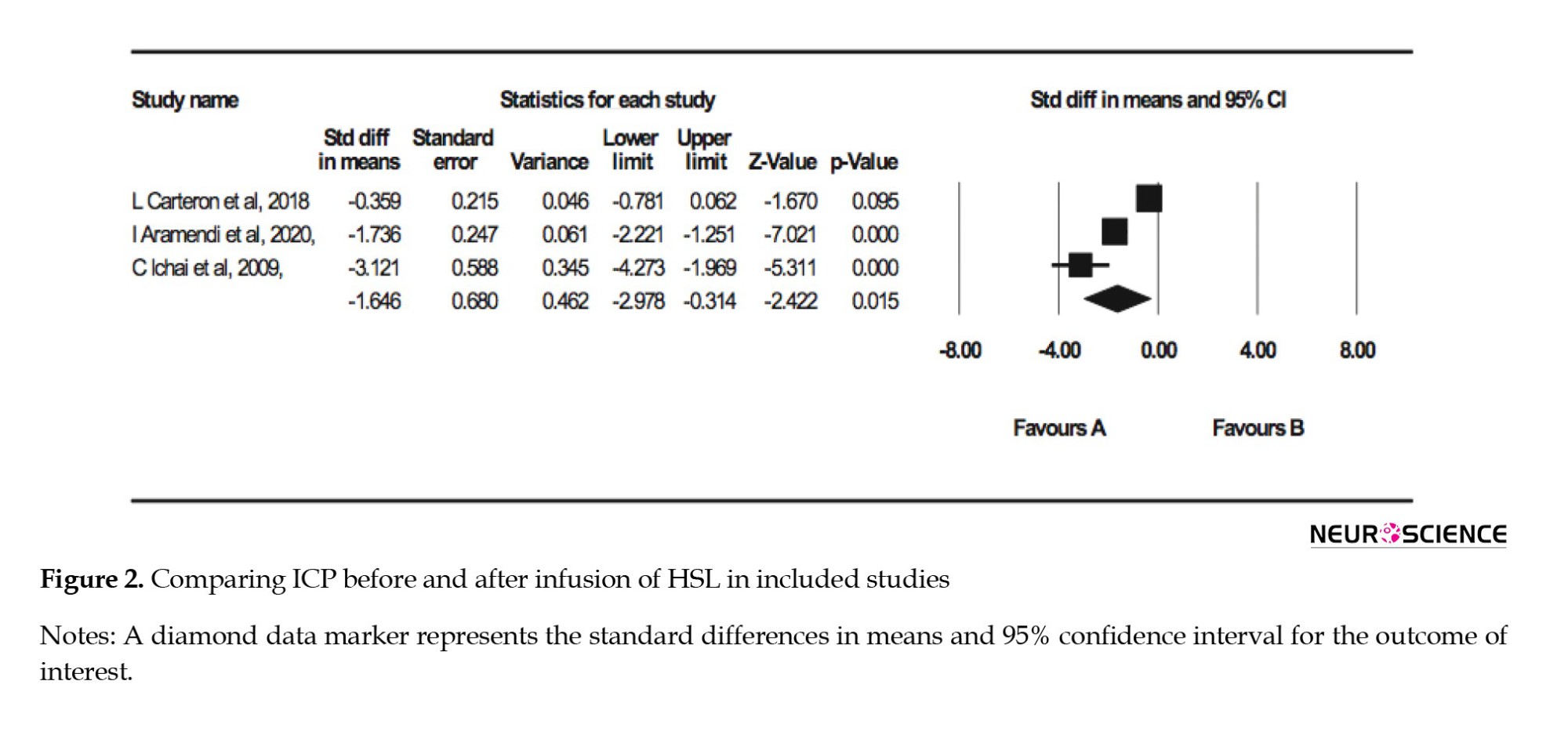
Meta-analysis
The forest plot of the random meta-analysis of three articles approved data on reduced ICP in using HSL in traumatic brain injuries (P=0.015). Details are presented in Figure 2.
4. Discussion
According to the controversies on the effect of hyperosmolar agents, we reviewed the articles investigating the role of HSL in managing TBI.
The backbones of the therapeutic goal during TBI are maintaining sufficient CBF and energy supply for the injured brain (Anonymous, 2000; Aramendi et al., 2020). The brain’s glucose use may be impaired after acute injury, leading to a poor outcome (Jalloh et al., 2013; Ichai et al., 2009). One of the suitable alternative substrates investigated in vitro and in vivo for over twenty years is lactate (Jourdain et al., 2016). To adjust to the shortage of glucose, the primary energy source for the brain, neurons start to increase the lactate’s uptake and usage (Gallagher et al., 2009; Glenn et al., 2015; Jalloh et al., 2013). Then, lactate can replace the missing glucose in different pathways, including protein synthesis and redox protection (Barros, 2013). Recent animal studies (Berthet et al., 2012; Jourdain et al., 2016; Roumes et al., 2020) have mentioned the potential role of lactate therapy in brain injury based on its capacity to act as an energy substrate. Astrocytes and oligodendrocytes start to increase lactate production, which could eventually be exported to the neurons when neurons are under stress and energy shortage. Accordingly, lactate’s presence seems necessary for the neurons’ survival after brain injuries (Mächler et al., 2016; Magistretti & Allaman, 2018). It has also been reported that the systemic administration of lactate in animal models could enhance cerebral angiogenesis. This relatively long-term effect of the lactate is through the hydroxycarboxylic acid receptor 1 (HCAR1) (Morland et al., 2017). Roumes et al., (2020) discussed the lactate’s ability to reduce brain lesions after hypoxia-ischemia in neonatal rats. He further exhibited that the co-injection of lactate and a lactate dehydrogenase inhibitor completely inhibits the neuroprotective effects.
The energy substrate role of lactate also does such prevention. Lactate can also modulate CBF through different mechanisms connected with CBF and brain energy turnover. Its action regulates the Nicotinamide-adenine dinucleotide (NADH/NAD+) redox ratio in the glycolysis cycle and through HCAR1 and cyclic AMP formation (cAMP) (Bergersen, 2015). Astrocytes and lactate can act as regulators for vessel diameter. An increase in the extracellular level of lactate causes the accumulation of prostaglandin E2, eventually leading to vasodilation (Gordon et al., 2008). Our meta-analysis found that ICP decreases significantly before and after administrating the HSL among patients with TBI. It is well known that elevated ICP and cerebral hypoperfusion after the TBI for even 5 minutes are associated with poorer neurological prognosis (Brain, 2007; Stein et al., 2011). As mentioned, lactate is responsible for vasodilation and could decrease cerebral vascular resistance. This reduction accordingly might increase CBF, which is related to a better outcome after TBI (Stein et al., 2011). Despite being less hypertonic than mannitol because of its carriers’ nature, HSL could alter the ICP more powerfully than mannitol (Halestrap & Price, 1999; Ichai et al., 2009). Although lactate gets metabolized quickly, the inorganic ions in the lactate solution are non-metabolizable. Intracellular chloride efflux will compensate for the excessive sodium ions that remain in extracellular space after administrating HSL to sustain the charges’ neutrality on both sides of the plasma membrane. Consequently, water will follow the chloride ions to the extracellular space, regulating the cellular volume and preventing cellular swelling (Ichai et al., 2009). Notably, a clinical trial evaluating the effects of hypertonic lactate on patients with brain injury recorded an increase in systemic sodium levels and osmolarity, all remaining within the normal range (Ichai et al., 2009).
Lactate could also prevent cellular swelling because of its capacity to act as an energy substrate for the brain and its monocarboxylate carriers in the blood-brain barrier (Holloway et al., 2007; Maran et al., 1994; Pellerin et al., 2005; Rice et al., 2002). During TBI, an increase in lactate levels could appear in two situations, including normal and low oxygen pressure. Such an increase in normal pressure oxygen conditions implicates aerobic glycolysis and lactate oxidation. However, this increase in oxygen-deficient settings suggests anaerobic glycolysis with a poorer prognosis (Carpenter et al., 2015; Sala et al., 2013). In a recent study, all patients with acute brain injury (ABI) and normal ICP and cerebral perfusion pressure (CPP) showed improved brain perfusion after hypertonic lactate administration (Bouzat et al., 2014). In another recent animal study, administrating HSL three hours after diffuse TBI seemed beneficial and improved brain metabolism, oxygenation, and perfusion. The group treated with HSL showed fewer mitochondrial changes and reduced lactate levels within their brain cells than the control group (Millet et al., 2018). Lactate could also have a beneficial role in a variety of non-traumatic lesions like hypoxia-ischemia (HI) and thrombotic events. In a recent study by Tassinari et al. (2020) on neonatal rats with HI, intraperitoneal administration of high doses of lactate showed positive effects on different functional aspects of the brain. He showed that administering high lactate doses lacks serious complications and could ameliorate neurological reflexes and decrease brain lesions’ size. They also reported sensory-motor neuron recovery in the subjected rats. During ischemia lactate levels in the brain are relatively high, and it is to no purpose to add more lactate via systemic administration.
Nonetheless, an animal study by Berthet et al. reported lactate levels within normal ranges of 15 to 60 minutes after transient middle cerebral artery occlusion (Berthet et al., 2012). He stated that both adenosine triphosphate (ATP) shortage and increased energy demand happen during reperfusion after ischemia, emphasizing the pivotal role of the lactate produced during the anaerobic cycle. Such growth in energy demand could justify the depletion of extracellular lactate and the advantages of administrating it (Berthet et al., 2012). Consequently, systemic lactate administration seems to be a favorable option for the TBI management course, whether with or without ischemia. Interestingly, a recent clinical trial assessing HSL’s effects on cognitive functions argues that HSL could boost neurologic recovery in patients with mild brain injury (Maas et al., 1997).
Altogether, HSL seems to be a reasonable substitute for fluid resuscitation after brain injury. However, Dienel et al. (2016) have challenged the effectiveness of lactate and addressed their concerns about its administration complications. They argued that extracellular lactate could decrease the pH of the neurons and astrocytes, consequently increasing NADH production. A rise in NADH levels could interfere with the activity of pH-sensitive enzymes such as phosphofructokinase. Low pH levels inhibit phosphofructokinase activity, which has an important regulatory role during glycolysis and could impede the cycle. Increased levels of NADH could also reduce the accessibility of NAD+ during glycolysis. He also argued that this lactate amount could also tamper with the pentose phosphate pathway and oxidative stress management in neurons.
As we previously mentioned, despite the probable side effects of lactate, we cannot disregard its inevitable role in elevated ICP management. Even so, lactate administration should be done with caution and properly evaluated. Considering the lack of sufficient clinical trials, controversial evidence, and the potential capability of lactate therapy in managing patients with TBI, we strongly encourage fellow researchers to further assess other aspects of lactate therapy in TBI.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Study design: Reza Mosaed, Mojtaba Mojtahedzadeh, Arash Akhavan Rezayat, and Farhad Najmeddin; Methodology: Behnaz Rohani and Aida Ayati Afin; Data collection: Arash Akhavan Rezayat, Behnaz Rohani, and Aida Ayati Afin; Statistical analysis: Arash Akhavan Rezayat; Writing the initial draft: Maryam Taghizadeh-Ghehi, Shahideh Amini, and Mohamad Afshar Ardalan; Final approval; All authors.
Conflict of interest
The authors declared no conflict of interest.
References
Traumatic brain injury (TBI) is one of the most important causes of cerebral dysfunction, and it is the reason for more than 30% of post-injury mortalities (Cassidy et al., 2004; Herbert et al., 2017). Increased intracranial pressure (ICP) following TBI is the main predisposing factor for death in the patients. Hyperosmolar therapy could ameliorate this condition (Fenn III & Sierra, 2019; Gerber et al., 2013). However, osmotherapy has been debated as the treatment of choice for these patients, considering the complex and nonselective nature of osmotic mediators’ effects (Bergersen, 2015). The mechanism attributed to hyperosmolar agents is that they increase the oncotic movement of water into the intravascular space and decrease the blood viscosity. As a result, hyperosmolar agents reduce the cerebral intracellular fluid and increase the cerebral blood flow (CBF) (Berthet et al., 2012).
Mannitol and hypertonic saline (HTS) solutions have been used as hyperosmolar therapy in patients with elevated ICP (Francony et al., 2008). Traditionally, mannitol has been recommended as the first choice of osmotherapy in TBI (Maas et al., 1997). However, hypotension, especially in the hypovolemic state, and rebound rise in ICP are the main drawbacks of mannitol (Anonymous, 2000; White et al., 2006). In refractory cases of increased ICP following mannitol administration, HTS increases brain oxygenation and reduces ICP (Ogden et al., 2005). Moreover, HTS has a more pronounced and longer-lasting effect than mannitol on elevated ICP and causes no rebound increase in ICP (Kerwin et al., 2009). Hence, some recent studies report HTS as the superior hyperosmolar agent (Mangat et al., 2020). No clear recommendation was made in a recently published meta-analysis regarding the choice of hypertonic saline or mannitol in treating patients with TBI-induced elevated ICP (Fatima et al., 2019). However, some concerns exist about nephrotoxic effects and metabolic acidosis following high chloride-containing solutions. Chloride as a nonorganic anion in sodium solution has no role in osmotic therapy, and previous studies have suggested replacing chloride with other organic anions.
Another hyperosmolar solution studied in patients with elevated ICP is hypertonic sodium lactate (HSL) (Ichai et al., 2013). Lactate, as an organic anion, is a suitable energy source for brain cells, and sodium lactate might work not only as a hypertonic agent but also as a metabolic fuel for the brain (Ichai et al., 2009; Leverve & Mustafa, 2002; Rice et al., 2002). HSL could decrease ICP through the osmotic effect of hypertonic sodium and the cerebral vasodilator effect of lactate (Gordon et al., 2008). Ichai et al. mentioned that HSL’s effect was even more potent than mannitol in reducing ICP in some trials (Ichai et al., 2009). Notably, in a recent study on patients with TBI-induced elevated ICP, Arifianto et al. (2018) introduced HSL as a choice for fluid therapy. Considering HSL’s beneficial roles in TBI, in this study, we systematically reviewed the articles investigating HSL’s therapeutic role in patients with TBI.
2. Materials and Methods
Data sources and search strategy
We performed our search in electronic databases, including PubMed, Embase, Scopus, and Web of Science, for studies investigating the effect of hyperosmolar agents on increased ICP in patients with TBI. Two independent investigators (Arash Akhavan Rezayat, and Behnaz Rohani) searched the relevant published articles from January 2000 to December 2020. We searched the literature applying a different combination of our keywords, including TBI, hypertonic agents, and their related terms. PubMed search strategy that consisted of various concepts of our keywords was as follows:
((“Brain injury”) OR (“brain injuries”) OR (“brain injuries, traumatic”) OR (“brain injuries, diffuse”) OR (“brain concussion”) OR (“head injury”) OR (“head injuries”) OR (“head injuries, penetrating”) OR (“cerebrovascular trauma”) OR (“craniocerebral trauma injuries”) OR (“cerebral hemorrhage, traumatic”) OR (“TBI”)) AND ((“hypertonic saline”) OR (“saline solution, hypertonic”) OR (“hypertonic sodium”) OR (“sodium chloride”) OR (“lactate”) OR (“sodium lactate”) OR (“hypertonic sodium lactate”) OR (“hyperosmolar sodium lactate”) OR (“mannitol”) OR (“osmotic diuretic”) OR (“osmotic diuretics”) OR (“diuretics, osmotic”)).
We also searched for grey literature using Open Grey, ProQuest Dissertations, and theses full texts.
Inclusion and exclusion criteria
All published randomized control trials in English literature involving our keywords were included for further evaluation. Our inclusion criteria were as follows: All patients were older than 16, suffered from TBI, and had ICP above 20 mm Hg. Animal studies, case reports, and studies on patients with liver and renal failure, cardiac dysfunction, or hypovolemic shock were excluded. We also excluded studies that did not directly compare the effects of hypertonic saline or mannitol on elevated ICP or did not provide sufficient primary data.
Study selection
After checking for duplications, two investigators independently reviewed all studies’ titles and abstracts to meet our inclusion and exclusion criteria. Any disagreement was discussed with the third reviewer. The flow diagram of the selection process of the studies is presented in Figure 1.

Data extraction
We extracted the information on the first author, publication time, country, study design, patient number, mean age, gender ratio, and interventions. The other reviewer resolved any disagreements on the selected studies.
Demographic data, methods, interventions, and outcomes were extracted from the included manuscripts and documented.
Data analysis
Data from included studies were pooled using a random-effect model. All continuous data were summarized as the standard difference in Means±SE and analyzed using comprehensive meta-analysis. The inconsistency index (I2) was used to measure heterogeneity, with I2>50% indicating substantial heterogeneity. P<0.05 was considered statistically significant.
3. Results
Selection process results
Our initial search with the predefined search strategy proceeded in 34 studies from the Scopus database and 32 publications from the PubMed database. Furthermore, 32 additional publications were included by searching the Web of Science. Also, reviewing Embase added 15 articles to the studies above. Removing duplicates resulted in 70 studies. The title and abstract screening excluded 40 studies. Subsequently, 30 remaining full texts were assessed; 7 studies did not provide enough information, 10 articles were irrelevant, and 6 were animal studies. Finally, 7 studies (Fenn III & Sierra, 2019; Gerber et al., 2013; Halestrap & Price, 1999; Herbert et al., 2017; Holloway et al., 2007; Ichai et al., 2009; Ichai et al., 2013) were eligible for this systematic review, of which 3 studies (Gerber et al., 2013; Halestrap & Price, 1999; Ichai et al., 2009), comprising ICP between before and after injection of HSL, were eligible for a meta-analysis. The selection process is detailed in Figure 1 PRISMA (the preferred reporting items for systematic reviews and meta-analyses) flow chart.
Characteristics of the included studies
Of 138 initially reviewed papers, 131 were removed, and 7 articles were assessed for eligibility using the Newcastle-OTTAWA scale. Studies from France, Switzerland, and Indonesia were evenly distributed, with two articles from each country. One study was conducted in Uruguay. Four studies (Fenn III & Sierra, 2019; Gerber et al., 2013; Herbert et al., 2017; Ichai et al., 2013) (were randomized trials, of which 3 (Aramendi et al., 2020; Holloway et al., 2007; Ichai et al., 2009) were before-and-after trials. The total number of patients from the initial 7 articles was 275, and 98 were included in the final 3 analyzed studies. Two included studies (Halestrap & Price, 1999; Ichai et al., 2009) (were prospective interventional, and 1 (Gerber et al., 2013) was a prospective open randomized study.
The meta-analysis was carried out for ICP reduction according to data reported in 3 articles. Drug administration characteristics in 4 publications (Aramendi et al., 2020; Bisri et al., 2016; Ichai et al., 2009; Muhammad & Hanna, 2014) were bolus, while the other 3 studies utilized the continuous form. Study characteristics are presented in Table 1.

Critical appraisal
The risk of bias assessment of our final articles is demonstrated in Table 2. Of the 7 included studies, 5 (Aramendi et al., 2020; Bouzat et al., 2014; Carteron et al., 2018; Ichai et al., 2009; Ichai et al., 2013) had an overall moderate risk of bias, and 2 (Bisri et al., 2016; Muhammad & Hanna, 2014) had a high-quality risk of bias.

Study outcomes
The pooled meta-analysis revealed a significant effect on ICP reduction in the use of HSL. Details were reported separately, including ICP, sodium osmolality, and neurological outcomes.
ICP
Five trials (Aramendi et al., 2020; Bouzat et al., 2014; Carteron et al., 2018; Ichai et al., 2009; Ichai et al., 2013) reported information on ICP. A study by Ichai et al., (2009) that compared ICP reduction using mannitol and sodium lactate showed a significantly lower ICP with sodium lactate infusion (group effect P=0.0161). Other before-and-after trials also reported positive effects on ICP. Carteron et al., (2018) reported ICP reduction after three hours in comparison with baseline (8±6 vs 10±5 mm Hg), P<0.01). Aramendi et al., (2020) showed a significant decrease in ICP after 30 and 60 minutes compared to the preinfusion state (P=0.0001). Another study by Bouzat et al., (2014) assessed the cerebral metabolic effects of HSL. In this study, HL therapy positively affected both ICP reduction and brain glutamate during three hours of infusion. Another study by Ichai et al., (2013) showed a reduction in raised ICP episodes in comparison with the control group during the 48-h study: 23 vs 53 episodes (P<0.05).
Serum sodium and serum osmolality
In two studies (Carteron et al., 2018; Ichai et al., 2013), sodium osmolality was increased between the two groups. In one study by Ichai et al., (2013), there was a significantly higher cumulative sodium intake and output between two groups of case and control (0.9% saline solution) in 48 h (P<0.01). Also, Carteron et al., (2018) reported a significant increase in sodium and osmolarity in a 3-h HS infusion (146±3 vs 152±3 mmol/L; P<0.001 and 303±6 vs 314±7 mOsmol/L; P<0.001). In another study by Ichai et al., (2009), there was no significant difference between mannitol and lactate therapy. In another trial by Bisri et al., (2016), serum sodium and osmolality levels were increased within 15 minutes in the HSS group compared to the HSL group, which remained unchanged (P<0.001).
Neurological outcomes
In 4 studies, the Glasgow outcome scale (GOS) was used for neurologic function assessment (Bouzat et al., 2014; Carteron et al., 2018; Ichai et al., 2009; Ichai et al., 2013). Ichai et al., (2013) assessed GOS for HSL and the control group after 6 months. Although survivors had poorer neurological outcomes among the control group (9 versus 4), there was no significant difference between the two groups (P=0.14). Carteron et al., (2018), with the use of a 3-h HSL solution, reported ten patients with good neurological recovery (GOS 4 or 5) at 6 months (out of 20 survivors and 3 deaths). Another study by Ichai et al., (2009) reported better outcomes using HSL compared with mannitol. Bouzat et al., (2014) reported 15 patients receiving a 3-h of HSL infusion, of which 9 had good recovery of neurologic function (GOS 4 or 5), 6 patients with GOS 3 did not have a good outcome (including 2 with severe disability), and 1 patient with GOS 2 and 3 with GOS 1 had died.
Meta-analysis
The forest plot of the random meta-analysis of three articles approved data on reduced ICP in using HSL in traumatic brain injuries (P=0.015). Details are presented in Figure 2.

4. Discussion
According to the controversies on the effect of hyperosmolar agents, we reviewed the articles investigating the role of HSL in managing TBI.
The backbones of the therapeutic goal during TBI are maintaining sufficient CBF and energy supply for the injured brain (Anonymous, 2000; Aramendi et al., 2020). The brain’s glucose use may be impaired after acute injury, leading to a poor outcome (Jalloh et al., 2013; Ichai et al., 2009). One of the suitable alternative substrates investigated in vitro and in vivo for over twenty years is lactate (Jourdain et al., 2016). To adjust to the shortage of glucose, the primary energy source for the brain, neurons start to increase the lactate’s uptake and usage (Gallagher et al., 2009; Glenn et al., 2015; Jalloh et al., 2013). Then, lactate can replace the missing glucose in different pathways, including protein synthesis and redox protection (Barros, 2013). Recent animal studies (Berthet et al., 2012; Jourdain et al., 2016; Roumes et al., 2020) have mentioned the potential role of lactate therapy in brain injury based on its capacity to act as an energy substrate. Astrocytes and oligodendrocytes start to increase lactate production, which could eventually be exported to the neurons when neurons are under stress and energy shortage. Accordingly, lactate’s presence seems necessary for the neurons’ survival after brain injuries (Mächler et al., 2016; Magistretti & Allaman, 2018). It has also been reported that the systemic administration of lactate in animal models could enhance cerebral angiogenesis. This relatively long-term effect of the lactate is through the hydroxycarboxylic acid receptor 1 (HCAR1) (Morland et al., 2017). Roumes et al., (2020) discussed the lactate’s ability to reduce brain lesions after hypoxia-ischemia in neonatal rats. He further exhibited that the co-injection of lactate and a lactate dehydrogenase inhibitor completely inhibits the neuroprotective effects.
The energy substrate role of lactate also does such prevention. Lactate can also modulate CBF through different mechanisms connected with CBF and brain energy turnover. Its action regulates the Nicotinamide-adenine dinucleotide (NADH/NAD+) redox ratio in the glycolysis cycle and through HCAR1 and cyclic AMP formation (cAMP) (Bergersen, 2015). Astrocytes and lactate can act as regulators for vessel diameter. An increase in the extracellular level of lactate causes the accumulation of prostaglandin E2, eventually leading to vasodilation (Gordon et al., 2008). Our meta-analysis found that ICP decreases significantly before and after administrating the HSL among patients with TBI. It is well known that elevated ICP and cerebral hypoperfusion after the TBI for even 5 minutes are associated with poorer neurological prognosis (Brain, 2007; Stein et al., 2011). As mentioned, lactate is responsible for vasodilation and could decrease cerebral vascular resistance. This reduction accordingly might increase CBF, which is related to a better outcome after TBI (Stein et al., 2011). Despite being less hypertonic than mannitol because of its carriers’ nature, HSL could alter the ICP more powerfully than mannitol (Halestrap & Price, 1999; Ichai et al., 2009). Although lactate gets metabolized quickly, the inorganic ions in the lactate solution are non-metabolizable. Intracellular chloride efflux will compensate for the excessive sodium ions that remain in extracellular space after administrating HSL to sustain the charges’ neutrality on both sides of the plasma membrane. Consequently, water will follow the chloride ions to the extracellular space, regulating the cellular volume and preventing cellular swelling (Ichai et al., 2009). Notably, a clinical trial evaluating the effects of hypertonic lactate on patients with brain injury recorded an increase in systemic sodium levels and osmolarity, all remaining within the normal range (Ichai et al., 2009).
Lactate could also prevent cellular swelling because of its capacity to act as an energy substrate for the brain and its monocarboxylate carriers in the blood-brain barrier (Holloway et al., 2007; Maran et al., 1994; Pellerin et al., 2005; Rice et al., 2002). During TBI, an increase in lactate levels could appear in two situations, including normal and low oxygen pressure. Such an increase in normal pressure oxygen conditions implicates aerobic glycolysis and lactate oxidation. However, this increase in oxygen-deficient settings suggests anaerobic glycolysis with a poorer prognosis (Carpenter et al., 2015; Sala et al., 2013). In a recent study, all patients with acute brain injury (ABI) and normal ICP and cerebral perfusion pressure (CPP) showed improved brain perfusion after hypertonic lactate administration (Bouzat et al., 2014). In another recent animal study, administrating HSL three hours after diffuse TBI seemed beneficial and improved brain metabolism, oxygenation, and perfusion. The group treated with HSL showed fewer mitochondrial changes and reduced lactate levels within their brain cells than the control group (Millet et al., 2018). Lactate could also have a beneficial role in a variety of non-traumatic lesions like hypoxia-ischemia (HI) and thrombotic events. In a recent study by Tassinari et al. (2020) on neonatal rats with HI, intraperitoneal administration of high doses of lactate showed positive effects on different functional aspects of the brain. He showed that administering high lactate doses lacks serious complications and could ameliorate neurological reflexes and decrease brain lesions’ size. They also reported sensory-motor neuron recovery in the subjected rats. During ischemia lactate levels in the brain are relatively high, and it is to no purpose to add more lactate via systemic administration.
Nonetheless, an animal study by Berthet et al. reported lactate levels within normal ranges of 15 to 60 minutes after transient middle cerebral artery occlusion (Berthet et al., 2012). He stated that both adenosine triphosphate (ATP) shortage and increased energy demand happen during reperfusion after ischemia, emphasizing the pivotal role of the lactate produced during the anaerobic cycle. Such growth in energy demand could justify the depletion of extracellular lactate and the advantages of administrating it (Berthet et al., 2012). Consequently, systemic lactate administration seems to be a favorable option for the TBI management course, whether with or without ischemia. Interestingly, a recent clinical trial assessing HSL’s effects on cognitive functions argues that HSL could boost neurologic recovery in patients with mild brain injury (Maas et al., 1997).
Altogether, HSL seems to be a reasonable substitute for fluid resuscitation after brain injury. However, Dienel et al. (2016) have challenged the effectiveness of lactate and addressed their concerns about its administration complications. They argued that extracellular lactate could decrease the pH of the neurons and astrocytes, consequently increasing NADH production. A rise in NADH levels could interfere with the activity of pH-sensitive enzymes such as phosphofructokinase. Low pH levels inhibit phosphofructokinase activity, which has an important regulatory role during glycolysis and could impede the cycle. Increased levels of NADH could also reduce the accessibility of NAD+ during glycolysis. He also argued that this lactate amount could also tamper with the pentose phosphate pathway and oxidative stress management in neurons.
As we previously mentioned, despite the probable side effects of lactate, we cannot disregard its inevitable role in elevated ICP management. Even so, lactate administration should be done with caution and properly evaluated. Considering the lack of sufficient clinical trials, controversial evidence, and the potential capability of lactate therapy in managing patients with TBI, we strongly encourage fellow researchers to further assess other aspects of lactate therapy in TBI.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Study design: Reza Mosaed, Mojtaba Mojtahedzadeh, Arash Akhavan Rezayat, and Farhad Najmeddin; Methodology: Behnaz Rohani and Aida Ayati Afin; Data collection: Arash Akhavan Rezayat, Behnaz Rohani, and Aida Ayati Afin; Statistical analysis: Arash Akhavan Rezayat; Writing the initial draft: Maryam Taghizadeh-Ghehi, Shahideh Amini, and Mohamad Afshar Ardalan; Final approval; All authors.
Conflict of interest
The authors declared no conflict of interest.
References
Ahmad, M. R., & Hanna, H. (2014). Effect of equiosmolar solutions of hypertonic sodium lactate versus mannitol in craniectomy patients with moderate traumatic brain injury. Medical Journal of Indonesia, 23(1), 30-5. [Link]
Aramendi, I., Stolovas, A., Mendaña, S., Barindelli, A., Manzanares, W., & Biestro, A. (2021). Effect of half-molar sodium lactate infusion on biochemical parameters in critically ill patients. Medicina Intensiva, 45(7), 421–430. [PMID]
Arifianto, M. R., Ma’ruf, A. Z., Ibrahim, A., & Bajamal, A. H. (2018). Role of hypertonic sodium lactate in traumatic brain injury management. Asian Journal of Neurosurgery, 13(4), 971-975. [DOI:10.4103/ajns.AJNS_10_17] [PMID]
Barros, L. F. (2013). Metabolic signaling by lactate in the brain. Trends in Neurosciences, 36(7), 396-404. [DOI:10.1016/j.tins.2013.04.002] [PMID]
Bergersen, L. H. (2015). Lactate transport and signaling in the brain: Potential therapeutic targets and roles in body-Brain interaction. Journal of Cerebral Blood Flow & Metabolism, 35(2), 176-185. [DOI:10.1038/jcbfm.2014.206] [PMID]
Berthet, C., Castillo, X., Magistretti, P. J., & Hirt, L. (2012). New evidence of neuroprotection by lactate after transient focal cerebral ischaemia: extended benefit after intracerebroventricular injection and efficacy of intravenous administration. Cerebrovascular Diseases, 34(5-6), 329-335. [DOI:10.1159/000343657] [PMID]
Bisri, T., Utomo, B. A., & Fuadi, I. (2016). Exogenous lactate infusion improved neurocognitive function of patients with mild traumatic brain injury. Asian Journal of Neurosurgery, 11(2), 151–159. [DOI:10.4103/1793-5482.145375] [PMID]
Bouzat, P., Sala, N., Suys, T., Zerlauth, J. B., Marques-Vidal, P., & Feihl, F., et al. (2014). Cerebral metabolic effects of exogenous lactate supplementation on the injured human brain. Intensive Care Medicine, 40(3), 412-421. [DOI:10.1007/s00134-013-3203-6] [PMID]
Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care, AANS/CNS, & Bratton, S. L., et al. (2007). Guidelines for the management of severe traumatic brain injury. IX. Cerebral perfusion thresholds. Journal of Neurotrauma, 24 (Suppl 1), S59–S64. [PMID]
Carpenter, K. L., Jalloh, I., & Hutchinson, P. J. (2015). Glycolysis and the significance of lactate in traumatic brain injury. Frontiers in Neuroscience, 9, 112. [DOI:10.3389/fnins.2015.00112] [PMID]
Carteron, L., Solari, D., Patet, C., Quintard, H., Miroz, J. P., & Bloch, J., et al. (2018). Hypertonic lactate to improve cerebral perfusion and glucose availability after acute brain injury. Critical Care Medicine, 46(10), 1649-1655. [DOI:10.1097/CCM.0000000000003274] [PMID]
Cassidy, J. D., Carroll, L. J., Peloso, P. M., Borg, J., von Holst, H., & Holm, L., et al. (2004). Incidence, risk factors and prevention of mild traumatic brain injury: Results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Journal of Rehabilitation Medicine, (43 Suppl), 28–60.[DOI:10.1080/16501960410023732] [PMID]
Fatima, N., Ayyad, A., Shuaib, A., & Saqqur, M. (2019). Hypertonic solutions in traumatic brain injury: A systematic review and meta-analysis. Asian Journal of Neurosurgery, 14(2), 382-391. [DOI:10.4103/ajns.AJNS_8_19] [PMID]
Fenn, N. E., 3rd, & Sierra, C. M. (2019). Hyperosmolar therapy for severe traumatic brain injury in pediatrics: A review of the literature. The Journal of Pediatric Pharmacology and Therapeutics, 24(6), 465-472. [DOI:10.5863/1551-6776-24.6.465] [PMID]
Francony, G., Fauvage, B., Falcon, D., Canet, C., Dilou, H., & Lavagne, P., et al. (2008). Equimolar doses of mannitol and hypertonic saline in the treatment of increased intracranial pressure. Critical Care Medicine, 36(3), 795-800. [DOI:10.1097/CCM.0B013E3181643B41] [PMID]
Gallagher, C. N., Carpenter, K. L., Grice, P., Howe, D. J., Mason, A., & Timofeev, I., et al. (2009). The human brain utilizes lactate via the tricarboxylic acid cycle: A 13C-labelled microdialysis and high-resolution nuclear magnetic resonance study. Brain, 132(10), 2839-2849. [DOI:10.1093/brain/awp202] [PMID]
Gerber, L. M., Chiu, Y. L., Carney, N., Härtl, R., & Ghajar, J. (2013). Marked reduction in mortality in patients with severe traumatic brain injury. Journal of Neurosurgery, 119(6), 1583-1590. [DOI:10.3171/2013.8.JNS13276] [PMID]
Glenn, T. C., Martin, N. A., Horning, M. A., McArthur, D. L., Hovda, D. A., & Vespa, P., et al. (2015). Lactate: Brain fuel in human traumatic brain injury: A comparison with normal healthy control subjects. Journal of Neurotrauma, 32(11), 820-832. [DOI:10.1089/neu.2014.3483] [PMID]
Gordon, G. R., Choi, H. B., Rungta, R. L., Ellis-Davies, G. C., & MacVicar, B. A. (2008). Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature, 456(7223), 745-749. [DOI:10.1038/nature07525] [PMID]
Halestrap, A. P., & PRICE, N. T. (1999). The proton-linked monocarboxylate transporter (MCT) family: Structure, function and regulation. The Biochemical Journal, 343(Pt 2), 281–299. [PMID]
Herbert, J. P., Guillotte, A. R., Hammer, R. D., & Litofsky, N. S. (2017). Coagulopathy in the setting of mild traumatic brain injury: Truths and consequences. Brain Sciences, 7(7), 92. [DOI:10.3390/brainsci7070092] [PMID]
Holloway, R., Zhou, Z., Harvey, H. B., Levasseur, J. E., Rice, A. C., & Sun, D., et al. (2007). Effect of lactate therapy upon cognitive deficits after traumatic brain injury in the rat. Acta Neurochirurgica, 149(9), 919-927. [DOI:10.1007/s00701-007-1241-y] [PMID]
Ichai, C., Armando, G., Orban, J. C., Berthier, F., Rami, L., & Samat-Long, C., et al. (2009). Sodium lactate versus mannitol in the treatment of intracranial hypertensive episodes in severe traumatic brain-injured patients. Intensive Care Medicine, 35(3), 471-479. [DOI:10.1007/s00134-008-1283-5] [PMID]
Ichai, C., Payen, J. F., Orban, J. C., Quintard, H., Roth, H., & Legrand, R., et al. (2013). Half-molar sodium lactate infusion to prevent intracranial hypertensive episodes in severe traumatic brain injured patients: A randomized controlled trial. Intensive Care Medicine, 39(8), 1413-1422. [DOI:10.1007/s00134-013-2978-9] [PMID]
Jalloh, I., Helmy, A., Shannon, R. J., Gallagher, C. N., Menon, D. K., & Carpenter, K. L., et al. (2013). Lactate uptake by the injured human brain: Evidence from an arteriovenous gradient and cerebral microdialysis study. Journal of Neurotrauma, 30(24), 2031-2037. [DOI:10.1089/neu.2013.2947] [PMID]
Jourdain, P., Allaman, I., Rothenfusser, K., Fiumelli, H., Marquet, P., & Magistretti, P. J. (2016). L-Lactate protects neurons against excitotoxicity: Implication of an ATP-mediated signaling cascade. Scientific Reports, 6, 21250. [DOI:10.1038/srep21250] [PMID]
Kerwin, A. J., Schinco, M. A., Tepas, J. J., 3rd, Renfro, W. H., Vitarbo, E. A., & Muehlberger, M. (2009). The use of 23.4% hypertonic saline for the management of elevated intracranial pressure in patients with severe traumatic brain injury: A pilot study. The Journal of Trauma, 67(2), 277–282. [DOI:10.1097/TA.0b013e3181acc726] [PMID]
Leverve, X. M., & Mustafa, I. (2002). Lactate: A key metabolite in the intercellular metabolic interplay. Critical Care, 6(4), 284-285. [DOI:10.1186/cc1509] [PMID]
Maas, A. I., Dearden, M., Teasdale, G. M., Braakman, R., Cohadon, F., & Iannotti, F., et al. (1997). EBIC-guidelines for management of severe head injury in adults. Acta Neurochirurgica, 139(4), 286-294. [DOI:10.1007/BF01808823] [PMID]
Mächler, P., Wyss, M. T., Elsayed, M., Stobart, J., Gutierrez, R., & von Faber-Castell, A., et al. (2016). In vivo evidence for a lactate gradient from astrocytes to neurons. Cell Metabolism, 23(1), 94-102. [DOI:10.1016/j.cmet.2015.10.010] [PMID]
Magistretti, P. J., & Allaman, I. (2018). Lactate in the brain: From metabolic end-product to signalling molecule. Nature Reviews Neuroscience, 19(4), 235-249. [DOI:10.1038/nrn.2018.19] [PMID]
Mangat, H. S., Wu, X., Gerber, L. M., Schwarz, J. T., Fakhar, M., & Murthy, S. B., et al. (2020). Hypertonic saline is superior to mannitol for the combined effect on intracranial pressure and cerebral perfusion pressure burdens in patients with severe traumatic brain injury. Neurosurgery, 86(2), 221-230. [DOI:10.1093/neuros/nyz046] [PMID]
Maran, A., Cranston, I., Lomas, J., Macdonald, I., & Amiel, S. A. (1994). Protection by lactate of cerebral function during hypoglycaemia. Lancet, 343(8888), 16-20. [DOI:10.1016/S0140-6736(94)90876-1] [PMID]
Millet, A., Cuisinier, A., Bouzat, P., Batandier, C., Lemasson, B., & Stupar, V., et al. (2018). Hypertonic sodium lactate reverses brain oxygenation and metabolism dysfunction after traumatic brain injury. British Journal of Anaesthesia, 120(6), 1295–1303. [DOI:10.1016/j.bja.2018.01.025] [PMID]
Morland, C., Andersson, K. A., Haugen, Ø. P., Hadzic, A., Kleppa, L., & Gille, A., et al. (2017). Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nature Communications, 8, 15557. [DOI:10.1038/ncomms15557] [PMID]
Muhammad, R. A., & Hanna, H. (2014). Effect of equiosmolar solutions of hypertonic sodium lactate versus mannitol in craniectomy patients with moderate traumatic brain injury. Medical Journal of Indonesia, 23(1). [DOI:10.13181/mji.v23i1.686]
Ogden, A. T., Mayer, S. A., & Connolly, E. S., Jr (2005). Hyperosmolar agents in neurosurgical practice: The evolving role of hypertonic saline. Neurosurgery, 57(2), 207-215. [DOI:10.1227/01.NEU.0000166533.79031.D8] [PMID]
Pellerin, L., Bergersen, L. H., Halestrap, A. P., & Pierre, K. (2005). Cellular and subcellular distribution of monocarboxylate transporters in cultured brain cells and in the adult brain. Journal of Neuroscience Research, 79(1‐2), 55-64. [DOI:10.1002/jnr.20307] [PMID]
Rice, A. C., Zsoldos, R., Chen, T., Wilson, M. S., Alessandri, B., & Hamm, R. J., et al. (2002). Lactate administration attenuates cognitive deficits following traumatic brain injury. Brain Research, 928(1-2), 156-159. [DOI:10.1016/S0006-8993(01)03299-1]
Roumes, H., Dumont, U., Sanchez, S., Mazuel, L., Blanc, J., & Raffard, G., et al. (2021). Neuroprotective role of lactate in rat neonatal hypoxia-ischemia. Journal of cerebral Blood Flow and Metabolism, 41(2), 342–358. [DOI:10.1177/0271678X20908355] [PMID]
Sala, N., Suys, T., Zerlauth, J. B., Bouzat, P., Messerer, M., & Bloch, J., et al. (2013). Cerebral extracellular lactate increase is predominantly nonischemic in patients with severe traumatic brain injury. Journal of Cerebral Blood Flow & Metabolism, 33(11), 1815-1822. [DOI:10.1038/jcbfm.2013.142] [PMID]
Stein, D. M., Hu, P. F., Brenner, M., Sheth, K. N., Liu, K. H., & Xiong, W., et al. (2011). Brief episodes of intracranial hypertension and cerebral hypoperfusion are associated with poor functional outcome after severe traumatic brain injury. The Journal of Trauma, 71(2), 364–374. [DOI:10.1097/TA.0b013e31822820da] [PMID]
Tassinari, I. D., Andrade, M. K. G., da Rosa, L. A., Hoff, M. L. M., Nunes, R. R., & Vogt, E. L., et al. (2020). Lactate Administration reduces brain injury and ameliorates behavioral outcomes following neonatal hypoxia-ischemia. Neuroscience, 448, 191-205. [DOI:10.1016/j.neuroscience.2020.09.006] [PMID]
Type of Study: Review |
Subject:
Clinical Neuroscience
Received: 2022/03/11 | Accepted: 2022/06/25 | Published: 2024/11/1
Received: 2022/03/11 | Accepted: 2022/06/25 | Published: 2024/11/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





