Volume 15, Issue 3 (May & Jun 2024)
BCN 2024, 15(3): 355-366 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Zeynali F, Raoufy M R, Gharakhanlou R. Investigating Olfactory Sensory Neurons Facilitation For Aerobic Exercise-induced Spatial Memory Improvement. BCN 2024; 15 (3) :355-366
URL: http://bcn.iums.ac.ir/article-1-2435-en.html
URL: http://bcn.iums.ac.ir/article-1-2435-en.html
1- Department of Exercise Physiology & Sport Sciences, Faculty of Humanities, Tarbiat Modares University, Tehran, Iran.
Full-Text [PDF 2112 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Exercise and physical activity are inescapable necessities for health (Bherer et al., 2013). Various studies have shown that physical activity plays an essential role in brain health (Bherer et al., 2013; Di Liegro et al., 2019). In the meantime, animal and human studies provide reasonable evidence that exercise improves cognitive performance and reduces the risk of dementia and cognitive impairment (Weuve et al., 2004). For example, our previous studies showed beneficial effects of exercise on Aβ-induced learning and memory impairment by up-regulation of the PGC-1α/FNDC5/BDNF pathway and increasing the Aβ clearance (Azimi et al., 2018; Khodadadi et al., 2018). While evidence supports the positive effects of exercise on cognitive performance, little is known regarding the mechanisms that may mediate this phenomenon.
Exercise increases the expression of neurotrophic factors in the brain and improves cognitive function by promoting neural plasticity, neurogenesis, and angiogenesis (Liu et al., 2019). Recently, it has been suggested that direct nerve stimulation to the brain during exercise may also be related to the beneficial effects of exercise on the brain (Liu et al., 2019). Exercise has been reported to promote rodents’ learning and memory performance and reverse memory and spatial learning impairment in old animals (Cefis et al., 2019). Therefore, several pathways are involved in these mechanisms, and exercise is a complex neurobiological modulator that involves interactions between the brain and the environment (Liu et al., 2019). However, there are still many barriers and challenges to modulating learning and memory in the exercise research trajectory.
The respiratory system plays a critical role in all types of exercise. Breathing through the nose and mouth during physical activity is controversial in exercise science, and extensive research has been done in this regard (Bennett et al., 2003). Breathing through the nose has many health benefits, particularly during exercise. These include heating, humidifying, and purifying/filtering the air in preparation for delivery to the lungs (Allen, 2017). Nose versus mouth breathing induces 50% more resistance to the air stream, causing 10% to 20% more oxygen uptake and improving the overall pulmonary volume (Allen, 2017). Also, previous research has shown changes in brain oscillatory activities and improved cognitive performance after yoga interventions associated with specific breathing exercises (breathing from the nose) (Desai et al., 2015). Therefore, breathing through the nose during exercise can affect brain function and behavior.
Breathing is one of the primary rhythms generated by the brain and is a critical signal in synchronizing brain activities (Bagur et al., 2021; Chi et al., 2016). Earlier investigations have demonstrated that the respiratory cycle has a critical role in information processing (Kepecs et al., 2006). Nasal airflow stimulates the olfactory sensory neurons (OSNs) linked to the olfactory bulb (OB) and other brain regions, like the entorhinal cortex, dentate gyrus, and CA1 in the hippocampus, prefrontal cortex, and amygdala (Grosmaitre et al., 2007). Local field potentials (LFPs) from the OB exhibit a prominent rhythm entrained by respiration which depends on nasal airflow, called respiration-entrained brain rhythm (Vanderwolf & Szechtman, 1987). Studies show that OB delivers the respiration-entrained brain rhythm to different brain regions (Bagur et al., 2021; Yanovsky et al., 2014). Also, different brain regions, especially the hippocampus, show reduced activity during intubation due to eliminating nasal airflow for a long time, and this might lead to cognitive disorders (Girard et al., 2010).
On the other hand, stimulation of the OSNs using air-puff leads to excitation of the OB, even when the animal cannot breathe intentionally through the nose, which improves working memory performance (Ghazvineh et al., 2021). The OSNs respond to the mechanical stimulation induced by airflow, which correlates with airflow intensity (Grosmaitre et al., 2007). Therefore, any changes in peripheral signals cause severe changes in the function of the nervous system, and nasal breathing can improve cognitive functions, specifically learning and memory, through entraining brain oscillations leading to synchronized network activity (Zelano et al., 2016).
Overall, exercise can non-invasively stimulate the OSNs by enhancing the nasal airflow intensity due to increased airflow and respiratory rate. Therefore, we hypothesized that aerobic exercise synchronizes oscillations in different brain regions by stimulating OSNs, which might effectively improve spatial memory during exercise. Accordingly, we destroyed the nasal epithelium to investigate the modulatory effect of OSNs on aerobic exercise-induced spatial memory improvement.
2. Material and Methods
Study animals
A total of 32 (8 rats in each group) adult male Wistar rats (weight=250-300 g) were obtained from the Pasteur Institute (Tehran, Iran). The rats were housed in standard cages in a climate-controlled room (temperature=23±2˚C with 50% to 55% humidity) with a 12 h light/dark cycle. Food and water were available sufficiently.
Surgery and LFPs recording
We used intraperitoneal injections of ketamine (100 mg/kg) and xylazine (10 mg/kg) for anesthetization in rats and confirmed anesthesia induction by the absence of the hind limb withdrawal reflex. After confirming the anesthesia, the animal’s head was in a flat skull position fixed inside the stereotaxic device (Narishige, Japan), and the scalp was cut along the midline of the skull surface. Then, stainless-steel recording electrodes (127 μm in diameter, A.M. System Inc., USA) were implanted unilaterally into the right hemisphere of the OB (anteroposterior=8.5 mm, mediolateral=-1 mm, dorsoventral=-1.5 mm) (Paxinos & Watson, 2006). Also, we used a stainless-steel screw as a reference and placed it at the right parietal bone. The scalp skin was treated with antibiotics (tetracycline). Small pins were connected to the electrodes and then inserted into a small socket. The whole structure was fixated on the skull with dental acrylic cement (Figure 1) (Dehdar et al., 2019).
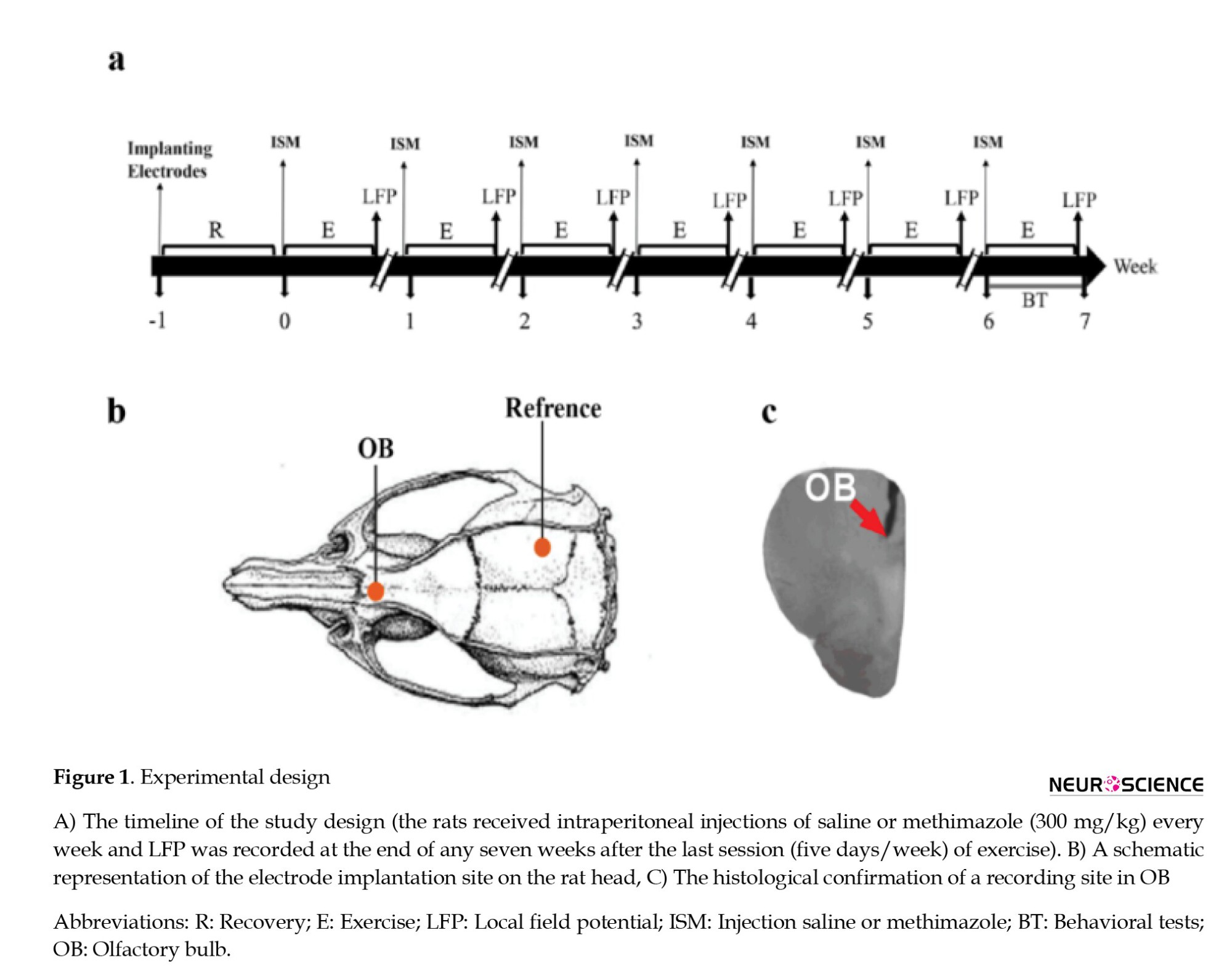
One week after surgery, the animals were divided into two exercise (exercise saline [ES] and exercise methimazole [EM]) and two control groups (control saline [CS] and control methimazole [CM]). At the beginning of each week, one exercise and one control group to destroy the OSNs received 300 mg/kg methimazole (Genter et al., 1995), and the other two groups received saline. Also, 30 min before methimazole injection, the animals were injected with thyroxine (10 µg) to prevent the effects of methimazole on metabolism (Bergman & Brittebo, 1999). At the end of each week, LFPs were recorded for 15 min. For this purpose, the socket, fixed to the animal’s head, was connected to a miniature buffer head-stage with high-input impedance (BIODAC-A, TRITA Health Tec., Tehran, Iran), via cables to a main AC coupled amplifier (1000 amplification) and the recording system (BIODAC-ESR18622, Health Tec., Tehran, Iran). Spontaneous LFPs of OB were recorded, low-pass filtered<500 Hz, digitized at 1 kHz, and stored for offline analyses with custom-written MATLAB routines (The Mathworks, Inc.). A video tracker was used to record the movement of rats to detect awake immobility periods (Dehdar et al., 2019). Also, after seven weeks of aerobic exercise to measure spatial memory, the Barnes behavioral test was performed (Figure 1).
Treadmill exercise
The rats were placed on the rodent treadmill (Tajhiz Gostar Omid Iranian, Tehran, Iran) for moderate regular exercise. Endurance training instructions included seven weeks of progressive running on a treadmill at a speed of 15-23 m/min for 15-50 min per day and five days per week. The training period was divided into three stages as follows: Familiarization, overload, and maintenance. In the familiarization phase, the rats were forced to run at the speed of 10-15 m/min for 15 min for five days. The animals that could not run were left out of the study. In the overload phase, the time increased to 30 min in the first week and reached 18 m/min in the second week. In the third and fourth weeks, the time reached 50 min, and the speed reached 20 m/min. In the fifth and sixth weeks, it maintained with increasing speed to 23 m/min. This protocol was a moderated exercise, and it is expressed that oxygen consumption in this situation is about 55%- 65% for the rats (Chae et al., 2011). The angle of inclination was 0%. The rats in the control groups received no exercise.
Behavioral tests
The Barnes maze was used to measure spatial memory in mice and rats. This maze is made of a circular plate made of black plexiglas, which is 92 cm in diameter. Around this maze, at a distance of 2 cm from the edge of the maze, 18 holes with a diameter of 8 cm have been installed. A black plexiglas escape box was located under one of the holes (target hole). On the familiarity day, the rats were allowed for 90 s to explore the field freely. After going into the target hole, the rats stayed in the escape box for 30 s. If they did not find the target hole, the experimenter would guide them to the target hole. The training phase included 4 trials per day for four consecutive days. One day after the last training session memory test was taken, and the time spent around the target hole was measured. After each trial to remove odor effects, the maze was carefully cleaned with a 70% alcohol solution. The Auto Vision software, version 2015 was used to analyze the Barnes behavioral test.
Cresyl violet histochemistry
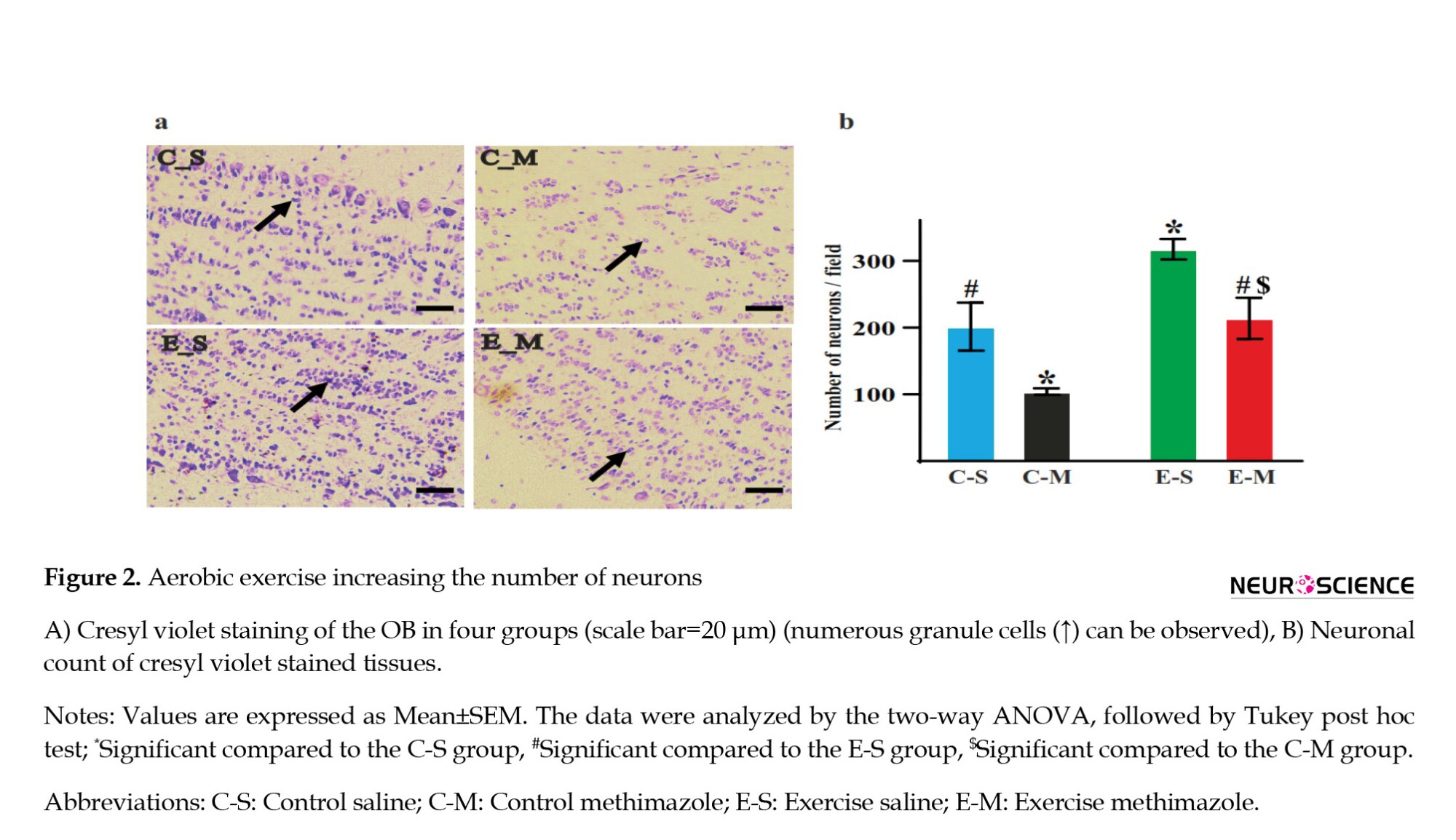
To elucidate the effects of methimazole on OSN destruction, the OB sections of the rats were stained with cresyl violet. In summary, the sections were mounted on gelatin-coated microscopy slides and were counterstained with 0.1% cresyl violet (Merck, Germany), and then dehydrated in increasing alcohol concentrations. The tissues were cleared in xylene, mounted, and then cover-slipped. All the samples were captured at 40× magnification, and the neuronal count was measured using the ImageJ software version 1.53e (number of neurons/filed), and statistical comparisons were made among the groups.
LFPs and statistical analysis
In this study, the power spectral density of OB signals was computed using the “pwelch” function in MATLAB sofware, version R2020a. We computed cross-correlation coefficient values for the filtered signals by MATLAB “xcorr” function, with the “coeff” option to normalize values between 1 and -1. For this purpose, respiration and OB signals were bandpass filtered in the respiratory frequencies range. Also, the statistical analysis was performed by GraphPad Prism software, version 8.4.3. (GraphPad Software, USA). The achieved results were compared by t-test, two-way, and three-way analysis of variance. Meanwhile, we used the Bonferroni test for the analysis of variance post hoc testing. We used the Cohen d test to measure effect size. The data are shown as Mean±SEM Also, P<0.05 were considered statistically significant.
3. Results
Destruction of the OSNs by methimazole injection
An apparent increase in the number of cells was evident. Histological images showed an increased number of neurons in the ES group compared to the CS group (Figure 2a). Also, statistically, the granule cell number increased in the ES group compared to other groups, indicating that exercise can increase the number of neurons (P=0.01, d=2.09). The granule cell population significantly decreased in the CM group, showing that methimazole could decrease the number of neurons (P=0.03, d=1.88) (Figure 2b). When we eliminated the OSNs, exercise could compensate for this decrease in the EM group (P=0.01, d=2.47) (Figure 2). The results show that exercise increases the number of neurons, and methimazole leads to the destruction of OSNs.
Increase in power spectral density with increased non-invasive stimulation of the OSNs by aerobic exercise
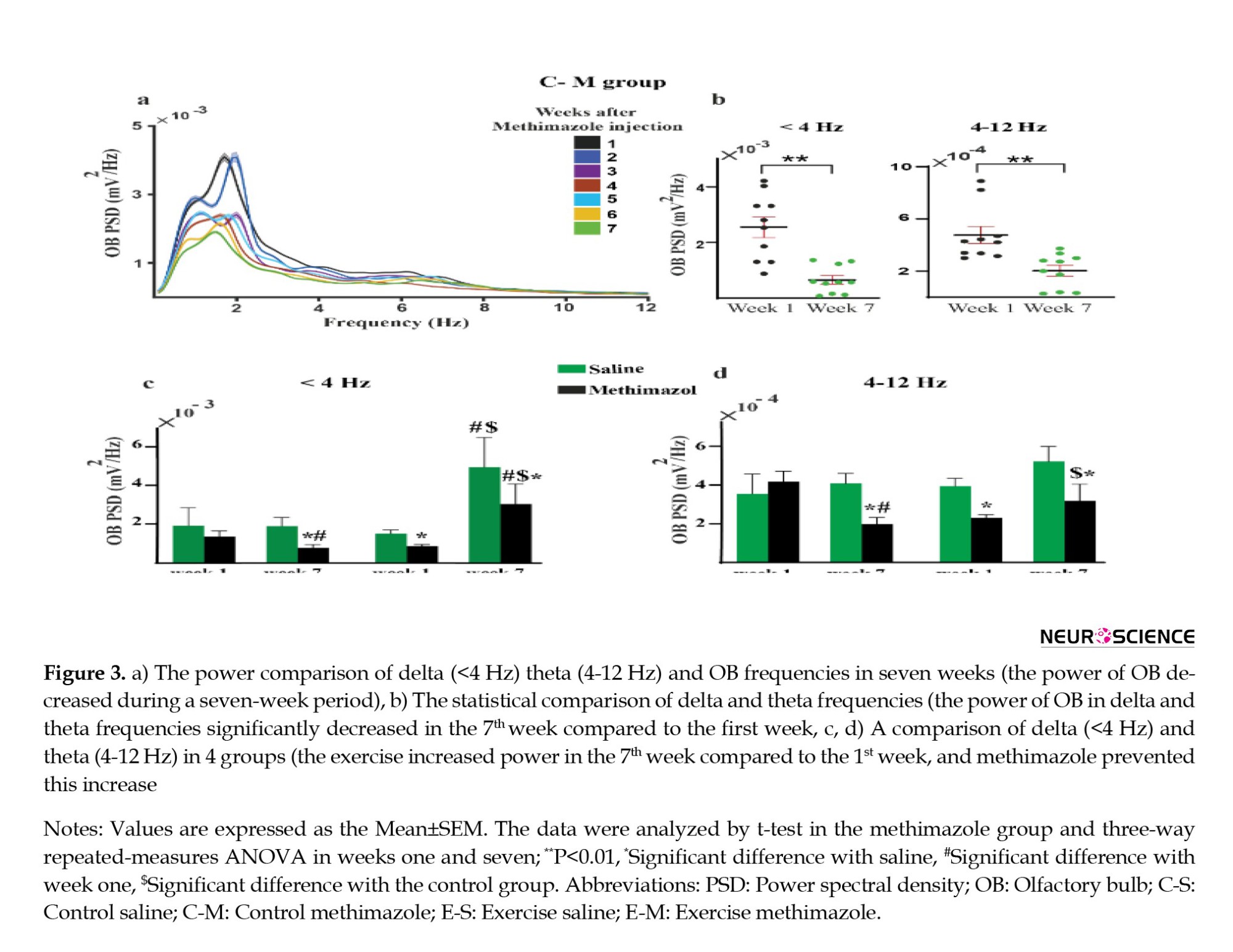
Power spectral density (PSD) analysis of LFPs showed a significant decrease in the mean delta (<4 Hz) and theta (4–12 Hz) power of OB in the CM group during seven weeks, and this decrease was significant between weeks one and seven (P delta=0.0025 and P theta=0.24) (Figures 3a and 3b). Comparison between weeks one and seven show that exercise increased the PSD in the ES and EM groups, while there was no significant difference in PSD between the first and seventh weeks in the CS group. Additionally, in week seven, a significant difference in PSD was observed between the ES and EM groups (Figures 3c and 3d). Thus, exercise improved the power of delta and theta frequencies. In comparison, methimazole injection prevented this increase.
Increase in respiration-OB synchronization by exercise
For the cross-correlation analysis to further explore the impact of exercise on the relationship between respiration and OB activity, we calculated cross-correlation by computing the mean correlation of respiration and OB LFPs.
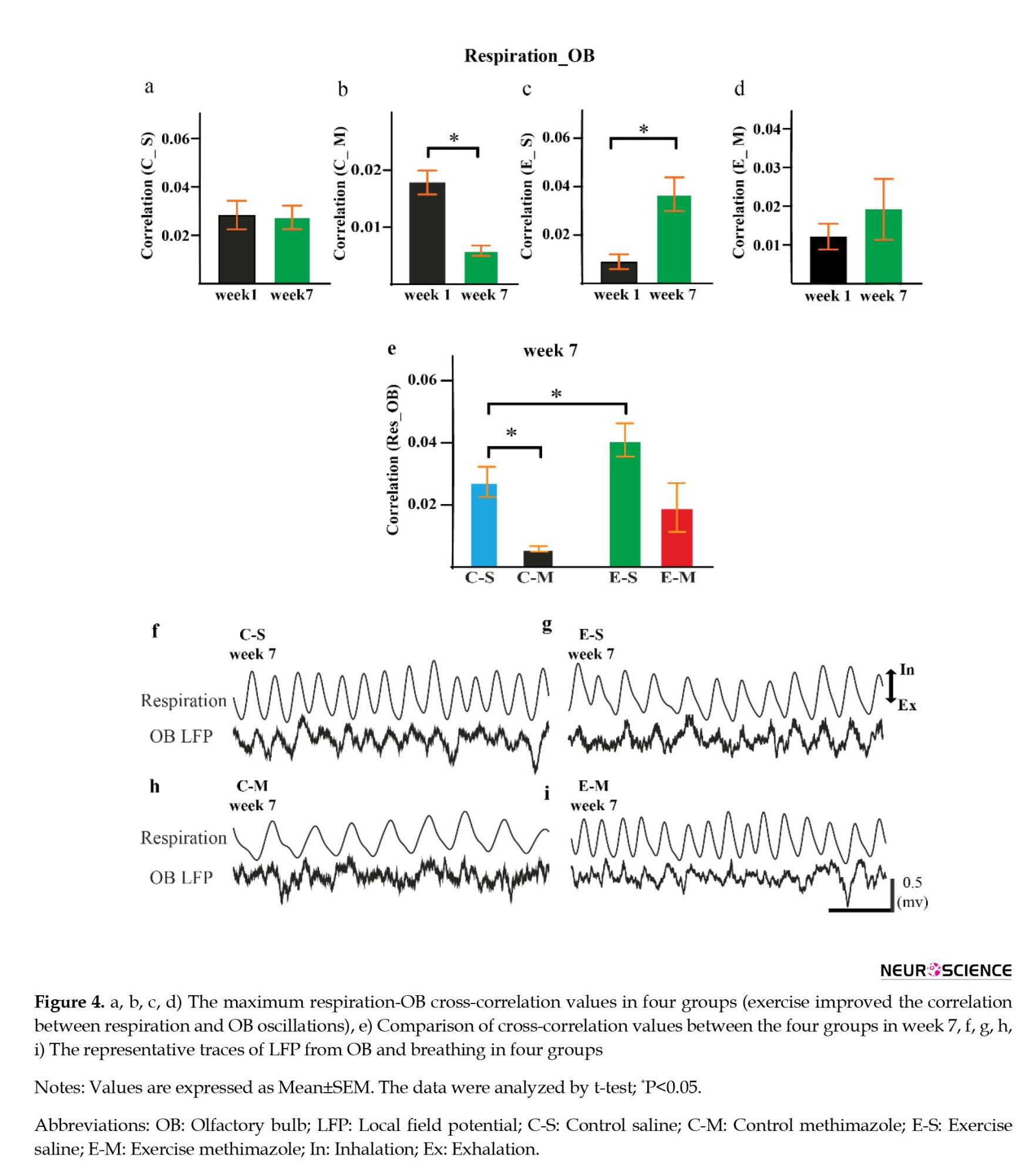
The mean correlation for the respiratory-OB filtered signals was significantly increased in the ES group (P=0.003, d=2.29) (Figure 4c). Methimazole decreased the cross-correlation in week seven compared to week one (P=0.0002, d=2.49) (Figure 4b). Meanwhile, there was a significant difference between the saline and methimazole control groups (Figure 4e). No significant cross-correlation difference was found between weeks one and seven in the CS group (P=0.9) (Figure 4a).
Also, representative traces of LFP from OB and breathing were shown in four groups. In the CS group, OB oscillation was coupled with animal respiration, while this coupling was not seen in the CM group (Figures 4f and 4g)
Destroyed OSNs impair learning and spatial memory
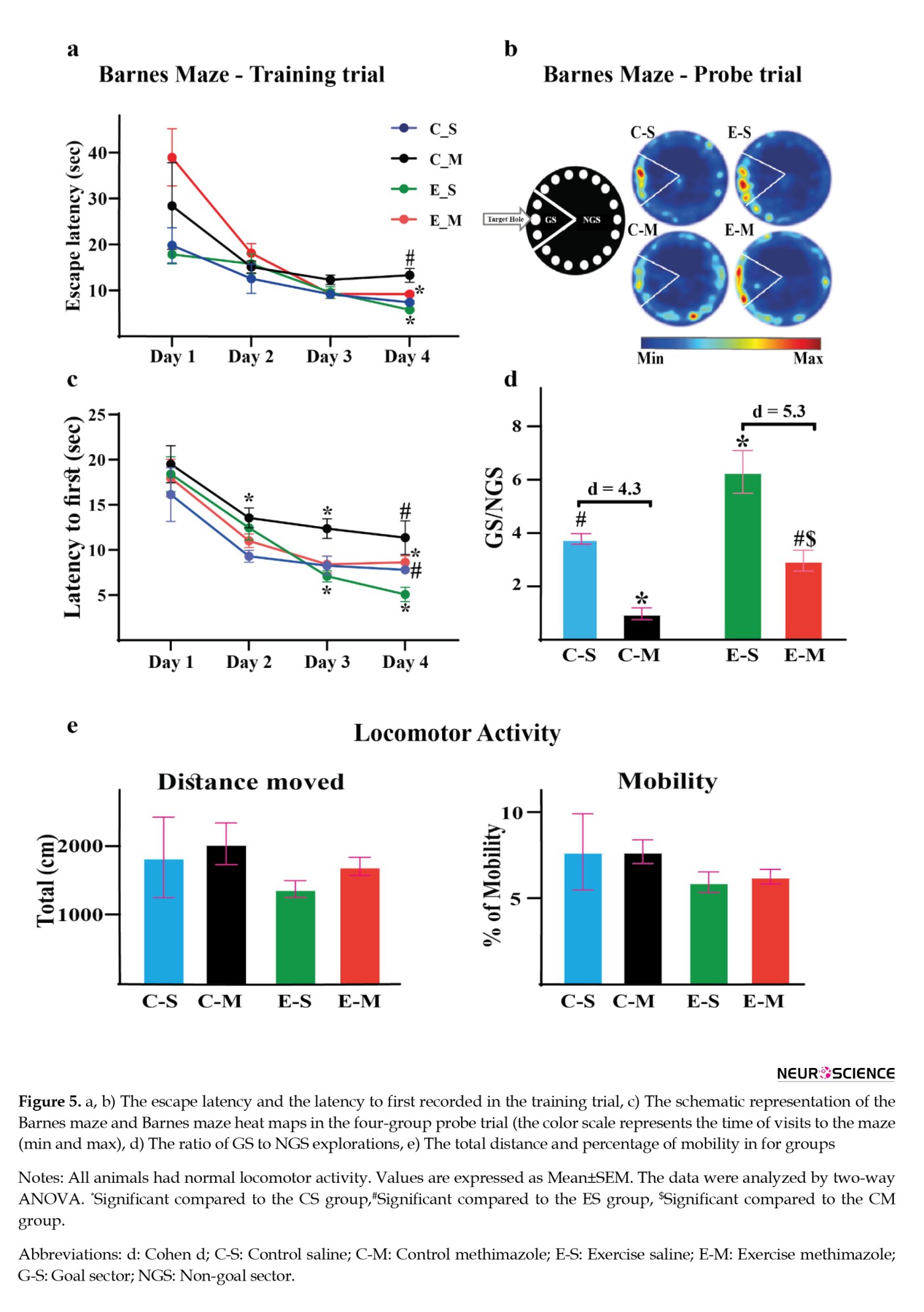
The Barnes behavioral test results in the training trial showed a significant difference in escape latency and latency to first between groups (a, b). Also, a significant difference in escape latency was observed between days one and seven in the exercise groups (Figures 5a and 5 b).
Statistical comparison of the Barnes behavioral test showed that the ratio of hole exploration frequency in the goal sector (GS) to hole exploration frequency in the non-goal sector (NGS) decreased in the CM group (P=0.002, d=4.3). In the ES group compared to the CS group, there was a significant increase in the goal sector preference (GS/NGS) (P=0.005, d=2.14) (Figure 5d). Also, In the ES group compared to the EM group, there was a significant increase in the GS/NGS (P=0.0002, d=5.3).
4. Discussion
Our results indicated that aerobic exercise improves spatial memory, consistent with previous reports (Inoue et al., 2015; Uysal et al., 2015; Wang & Wang, 2016). Stimulation of the OSNs during nasal respiration generated brain oscillations. OSNs are shown to respond to the chemical stimulus (odor) and are activated by nasal airflow (Lockmann et al., 2016). We presume that OSN stimulation enhances during exercise and increases connectivity in the OB and other brain regions improving spatial memory, and can be introduced as a probable mechanism. Therefore, we have used the methimazole injury model to investigate this mechanism. Methimazole is an olfactotoxic drug and disrupts the OSNs all over the epithelium by activating the apoptotic cascade (Sakamoto et al., 2007) without disturbing breathing rates during normal behaviors (Moberly et al., 2018). Our results indicated that the administration of methimazole reduces the number of neurons in the olfactory epithelium and the OB. Also, impairment of the OSNs is associated with decreased activity of OB at delta and theta frequency bands, as well as the coupling and synchronization between respiration and OB oscillations. Therefore, these results show that methimazole could destroy OSNs and reduce respiration-entrained brain oscillations. On the other hand, exercise increased the number of OB neurons and the synchrony between respiration and OB oscillations. To the best of our knowledge, this is the first report of improved spatial memory with aerobic exercise by modulating OB through stimulating OSNs.
During nasal breathing, OSNs fire rhythmically with respiration, and this activity is transported to OB cells (Heck et al., 2019). These OB oscillations generated during nasal respiration synchronize neural activities of the brain networks (Singer, 1993). Respiration-entrained brain oscillations emerge due to the phase-locking between olfactory neural oscillations and respiration cycles (Gretenkord et al., 2019). OSNs are sensitive to odors and mechanical stimulation from nasal airflow, and the entrainment of OB neuronal activity with respiration is independent of odor perception (Grosmaitre et al., 2007; Heck et al., 2019). Our result indicated that by destroying the OSNs (like breathing through mouth), the coupling between respiration and OB oscillations, and therefore, respiration-entrained brain oscillations were reduced, which was associated with a reduction in memory improvement induced by exercise. Nasal respiration modulates cognitive function while memory encoding and retrieval remain unchanged during mouth breathing (Zelano et al., 2016). Also, our previous study showed that stimulation of the OSNs using air-puff leads to excitation of the OB in conditions where the animal cannot breathe intentionally through the nose, and it can improve working memory performance (Ghazvineh et al., 2021). In addition, rhythmic nasal air puffing promotes brain activity and improves network connectivity in comatose patients (Salimi et al., 2022).
Besides, nasal breathing can modulate brain oscillations in nonolfactory areas, and respiration-entrained oscillations aid the integration of information and improve cognitive abilities (Zelano et al., 2016). Preceding works revealed that changes in breathing frequency could influence nonolfactory information processing (Tort et al., 2018; Tort et al., 2021). Also, differences in nasal airflow rate can affect olfactory processing (Esclassan et al., 2012; Hammer et al., 2021). Given that nasal airflow increases with respiratory intensity and rate and that aerobic exercise increases these parameters, we suggest that nasal airflow might have a role in memory improvements following aerobic exercise. The destruction of OSNs reverses this effect.
Previous evidence demonstrated that OB activity entrains regional oscillations in widespread brain areas, showing higher synchrony during cognitive performance (Tort et al., 2018). For instance, it has been reported that OB and hippocampus are synchronized in theta oscillation during reversal learning (Macrides et al., 1982). Therefore, OSNs may modulate OB oscillations through nasal respiration which in turn drives the changes throughout other brain regions. Also, the coupling between OB oscillations and other brain areas is correlated with cognitive functions (Salimi et al., 2019), and this coupling is crucial for information processing during the expression of behaviors (Fell & Axmacher, 2011). Eliminating the OB leads to cognitive impairments in attention, reference memory, delayed matching, reversal memory, and working memory (van Rijzingen et al., 1995). Therefore, respiration is a crucial neurobiological modulator in organizing cortical activity during memory processes in brain regions like the hippocampus and medial prefrontal cortex (Heck et al., 2019).
Aerobic exercise improved spatial memory; however, destroying OSNs reduced this effect which we believe might be due to the presence of respiration-entrained brain oscillation. This partial compensation in memory impairment is due to several parallel biological pathways recruited by exercise in affecting brain function. The present study results showed that nasal breathing improves spatial memory both at rest and aerobic exercise, and it was more significant following aerobic exercise. Additionally, spatial memory decreases when we eliminate the OSNs and respiration-entrained brain oscillation, like oral breathing. Therefore, our results suggest that nasal breathing is essential for cognitive function in rest and exercise. Aerobic exercise can be defined as a non-invasive mechanical stimulation method for the brain to restore cognitive deficits such as memory impairment.
5. Conclusion
The OB pathway is a probable mechanism for mediating the effects of exercise on spatial memory. These results provide new insight regarding the effect of OB activity on remote brain areas associated with cognition and behavior following exercise. However, the relative contribution of exercise to this mechanism is currently unclear, and further studies are needed.
Study limitations
This study faced some limitations in suggesting the entire mechanism. First, in this study, exercise had a compensatory effect when we destroyed the OSNs. It might be due to the role of other pathways in improving memory, such as increased neurogenesis and blood flow distribution in the olfactory epithelium, which we did not investigate in this study and should be evaluated in subsequent studies. Second, our study was limited to evaluating the long-term effects of exercise; however, it would be helpful for future studies that evaluate this pathway in short-term exercise. Third, since our recordings were not taken during exercise, we could not evaluate respiration intensity in this study, which needs further experiments and might provide interesting results.
Ethical Considerations
Compliance with ethical guidelines
The Ethics Committee of the Faculty of Medical Sciences, Tarbiat Modares University approved all animal protocols (Code: IR.MODARES.REC.1397.160).
Funding
The paper was extracted from the PhD dissertation of Farzaneh Zeynali, approved by the Department of Exercise Physiology & Sport Sciences, Faculty of Humanities, Tarbiat Modares University, Tehran, Iran.
Authors' contributions
All authors contributed equally in preparing all parts of the research.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank Morteza Mooziri for his invaluable assistance in editing this manuscript. Also, the authors would like to thank Fatemeh Hosseinpour, Jalaledin Noroozi, and Mohammad Valadi Athar for their technical assistance.
References
Exercise and physical activity are inescapable necessities for health (Bherer et al., 2013). Various studies have shown that physical activity plays an essential role in brain health (Bherer et al., 2013; Di Liegro et al., 2019). In the meantime, animal and human studies provide reasonable evidence that exercise improves cognitive performance and reduces the risk of dementia and cognitive impairment (Weuve et al., 2004). For example, our previous studies showed beneficial effects of exercise on Aβ-induced learning and memory impairment by up-regulation of the PGC-1α/FNDC5/BDNF pathway and increasing the Aβ clearance (Azimi et al., 2018; Khodadadi et al., 2018). While evidence supports the positive effects of exercise on cognitive performance, little is known regarding the mechanisms that may mediate this phenomenon.
Exercise increases the expression of neurotrophic factors in the brain and improves cognitive function by promoting neural plasticity, neurogenesis, and angiogenesis (Liu et al., 2019). Recently, it has been suggested that direct nerve stimulation to the brain during exercise may also be related to the beneficial effects of exercise on the brain (Liu et al., 2019). Exercise has been reported to promote rodents’ learning and memory performance and reverse memory and spatial learning impairment in old animals (Cefis et al., 2019). Therefore, several pathways are involved in these mechanisms, and exercise is a complex neurobiological modulator that involves interactions between the brain and the environment (Liu et al., 2019). However, there are still many barriers and challenges to modulating learning and memory in the exercise research trajectory.
The respiratory system plays a critical role in all types of exercise. Breathing through the nose and mouth during physical activity is controversial in exercise science, and extensive research has been done in this regard (Bennett et al., 2003). Breathing through the nose has many health benefits, particularly during exercise. These include heating, humidifying, and purifying/filtering the air in preparation for delivery to the lungs (Allen, 2017). Nose versus mouth breathing induces 50% more resistance to the air stream, causing 10% to 20% more oxygen uptake and improving the overall pulmonary volume (Allen, 2017). Also, previous research has shown changes in brain oscillatory activities and improved cognitive performance after yoga interventions associated with specific breathing exercises (breathing from the nose) (Desai et al., 2015). Therefore, breathing through the nose during exercise can affect brain function and behavior.
Breathing is one of the primary rhythms generated by the brain and is a critical signal in synchronizing brain activities (Bagur et al., 2021; Chi et al., 2016). Earlier investigations have demonstrated that the respiratory cycle has a critical role in information processing (Kepecs et al., 2006). Nasal airflow stimulates the olfactory sensory neurons (OSNs) linked to the olfactory bulb (OB) and other brain regions, like the entorhinal cortex, dentate gyrus, and CA1 in the hippocampus, prefrontal cortex, and amygdala (Grosmaitre et al., 2007). Local field potentials (LFPs) from the OB exhibit a prominent rhythm entrained by respiration which depends on nasal airflow, called respiration-entrained brain rhythm (Vanderwolf & Szechtman, 1987). Studies show that OB delivers the respiration-entrained brain rhythm to different brain regions (Bagur et al., 2021; Yanovsky et al., 2014). Also, different brain regions, especially the hippocampus, show reduced activity during intubation due to eliminating nasal airflow for a long time, and this might lead to cognitive disorders (Girard et al., 2010).
On the other hand, stimulation of the OSNs using air-puff leads to excitation of the OB, even when the animal cannot breathe intentionally through the nose, which improves working memory performance (Ghazvineh et al., 2021). The OSNs respond to the mechanical stimulation induced by airflow, which correlates with airflow intensity (Grosmaitre et al., 2007). Therefore, any changes in peripheral signals cause severe changes in the function of the nervous system, and nasal breathing can improve cognitive functions, specifically learning and memory, through entraining brain oscillations leading to synchronized network activity (Zelano et al., 2016).
Overall, exercise can non-invasively stimulate the OSNs by enhancing the nasal airflow intensity due to increased airflow and respiratory rate. Therefore, we hypothesized that aerobic exercise synchronizes oscillations in different brain regions by stimulating OSNs, which might effectively improve spatial memory during exercise. Accordingly, we destroyed the nasal epithelium to investigate the modulatory effect of OSNs on aerobic exercise-induced spatial memory improvement.
2. Material and Methods
Study animals
A total of 32 (8 rats in each group) adult male Wistar rats (weight=250-300 g) were obtained from the Pasteur Institute (Tehran, Iran). The rats were housed in standard cages in a climate-controlled room (temperature=23±2˚C with 50% to 55% humidity) with a 12 h light/dark cycle. Food and water were available sufficiently.
Surgery and LFPs recording
We used intraperitoneal injections of ketamine (100 mg/kg) and xylazine (10 mg/kg) for anesthetization in rats and confirmed anesthesia induction by the absence of the hind limb withdrawal reflex. After confirming the anesthesia, the animal’s head was in a flat skull position fixed inside the stereotaxic device (Narishige, Japan), and the scalp was cut along the midline of the skull surface. Then, stainless-steel recording electrodes (127 μm in diameter, A.M. System Inc., USA) were implanted unilaterally into the right hemisphere of the OB (anteroposterior=8.5 mm, mediolateral=-1 mm, dorsoventral=-1.5 mm) (Paxinos & Watson, 2006). Also, we used a stainless-steel screw as a reference and placed it at the right parietal bone. The scalp skin was treated with antibiotics (tetracycline). Small pins were connected to the electrodes and then inserted into a small socket. The whole structure was fixated on the skull with dental acrylic cement (Figure 1) (Dehdar et al., 2019).

One week after surgery, the animals were divided into two exercise (exercise saline [ES] and exercise methimazole [EM]) and two control groups (control saline [CS] and control methimazole [CM]). At the beginning of each week, one exercise and one control group to destroy the OSNs received 300 mg/kg methimazole (Genter et al., 1995), and the other two groups received saline. Also, 30 min before methimazole injection, the animals were injected with thyroxine (10 µg) to prevent the effects of methimazole on metabolism (Bergman & Brittebo, 1999). At the end of each week, LFPs were recorded for 15 min. For this purpose, the socket, fixed to the animal’s head, was connected to a miniature buffer head-stage with high-input impedance (BIODAC-A, TRITA Health Tec., Tehran, Iran), via cables to a main AC coupled amplifier (1000 amplification) and the recording system (BIODAC-ESR18622, Health Tec., Tehran, Iran). Spontaneous LFPs of OB were recorded, low-pass filtered<500 Hz, digitized at 1 kHz, and stored for offline analyses with custom-written MATLAB routines (The Mathworks, Inc.). A video tracker was used to record the movement of rats to detect awake immobility periods (Dehdar et al., 2019). Also, after seven weeks of aerobic exercise to measure spatial memory, the Barnes behavioral test was performed (Figure 1).
Treadmill exercise
The rats were placed on the rodent treadmill (Tajhiz Gostar Omid Iranian, Tehran, Iran) for moderate regular exercise. Endurance training instructions included seven weeks of progressive running on a treadmill at a speed of 15-23 m/min for 15-50 min per day and five days per week. The training period was divided into three stages as follows: Familiarization, overload, and maintenance. In the familiarization phase, the rats were forced to run at the speed of 10-15 m/min for 15 min for five days. The animals that could not run were left out of the study. In the overload phase, the time increased to 30 min in the first week and reached 18 m/min in the second week. In the third and fourth weeks, the time reached 50 min, and the speed reached 20 m/min. In the fifth and sixth weeks, it maintained with increasing speed to 23 m/min. This protocol was a moderated exercise, and it is expressed that oxygen consumption in this situation is about 55%- 65% for the rats (Chae et al., 2011). The angle of inclination was 0%. The rats in the control groups received no exercise.
Behavioral tests
The Barnes maze was used to measure spatial memory in mice and rats. This maze is made of a circular plate made of black plexiglas, which is 92 cm in diameter. Around this maze, at a distance of 2 cm from the edge of the maze, 18 holes with a diameter of 8 cm have been installed. A black plexiglas escape box was located under one of the holes (target hole). On the familiarity day, the rats were allowed for 90 s to explore the field freely. After going into the target hole, the rats stayed in the escape box for 30 s. If they did not find the target hole, the experimenter would guide them to the target hole. The training phase included 4 trials per day for four consecutive days. One day after the last training session memory test was taken, and the time spent around the target hole was measured. After each trial to remove odor effects, the maze was carefully cleaned with a 70% alcohol solution. The Auto Vision software, version 2015 was used to analyze the Barnes behavioral test.
Cresyl violet histochemistry
To elucidate the effects of methimazole on OSN destruction, the OB sections of the rats were stained with cresyl violet. In summary, the sections were mounted on gelatin-coated microscopy slides and were counterstained with 0.1% cresyl violet (Merck, Germany), and then dehydrated in increasing alcohol concentrations. The tissues were cleared in xylene, mounted, and then cover-slipped. All the samples were captured at 40× magnification, and the neuronal count was measured using the ImageJ software version 1.53e (number of neurons/filed), and statistical comparisons were made among the groups.
LFPs and statistical analysis
In this study, the power spectral density of OB signals was computed using the “pwelch” function in MATLAB sofware, version R2020a. We computed cross-correlation coefficient values for the filtered signals by MATLAB “xcorr” function, with the “coeff” option to normalize values between 1 and -1. For this purpose, respiration and OB signals were bandpass filtered in the respiratory frequencies range. Also, the statistical analysis was performed by GraphPad Prism software, version 8.4.3. (GraphPad Software, USA). The achieved results were compared by t-test, two-way, and three-way analysis of variance. Meanwhile, we used the Bonferroni test for the analysis of variance post hoc testing. We used the Cohen d test to measure effect size. The data are shown as Mean±SEM Also, P<0.05 were considered statistically significant.
3. Results
Destruction of the OSNs by methimazole injection
An apparent increase in the number of cells was evident. Histological images showed an increased number of neurons in the ES group compared to the CS group (Figure 2a). Also, statistically, the granule cell number increased in the ES group compared to other groups, indicating that exercise can increase the number of neurons (P=0.01, d=2.09). The granule cell population significantly decreased in the CM group, showing that methimazole could decrease the number of neurons (P=0.03, d=1.88) (Figure 2b). When we eliminated the OSNs, exercise could compensate for this decrease in the EM group (P=0.01, d=2.47) (Figure 2). The results show that exercise increases the number of neurons, and methimazole leads to the destruction of OSNs.

Increase in power spectral density with increased non-invasive stimulation of the OSNs by aerobic exercise
Power spectral density (PSD) analysis of LFPs showed a significant decrease in the mean delta (<4 Hz) and theta (4–12 Hz) power of OB in the CM group during seven weeks, and this decrease was significant between weeks one and seven (P delta=0.0025 and P theta=0.24) (Figures 3a and 3b). Comparison between weeks one and seven show that exercise increased the PSD in the ES and EM groups, while there was no significant difference in PSD between the first and seventh weeks in the CS group. Additionally, in week seven, a significant difference in PSD was observed between the ES and EM groups (Figures 3c and 3d). Thus, exercise improved the power of delta and theta frequencies. In comparison, methimazole injection prevented this increase.

Increase in respiration-OB synchronization by exercise
For the cross-correlation analysis to further explore the impact of exercise on the relationship between respiration and OB activity, we calculated cross-correlation by computing the mean correlation of respiration and OB LFPs.
The mean correlation for the respiratory-OB filtered signals was significantly increased in the ES group (P=0.003, d=2.29) (Figure 4c). Methimazole decreased the cross-correlation in week seven compared to week one (P=0.0002, d=2.49) (Figure 4b). Meanwhile, there was a significant difference between the saline and methimazole control groups (Figure 4e). No significant cross-correlation difference was found between weeks one and seven in the CS group (P=0.9) (Figure 4a).
Also, representative traces of LFP from OB and breathing were shown in four groups. In the CS group, OB oscillation was coupled with animal respiration, while this coupling was not seen in the CM group (Figures 4f and 4g)

Destroyed OSNs impair learning and spatial memory
The Barnes behavioral test results in the training trial showed a significant difference in escape latency and latency to first between groups (a, b). Also, a significant difference in escape latency was observed between days one and seven in the exercise groups (Figures 5a and 5 b).
Statistical comparison of the Barnes behavioral test showed that the ratio of hole exploration frequency in the goal sector (GS) to hole exploration frequency in the non-goal sector (NGS) decreased in the CM group (P=0.002, d=4.3). In the ES group compared to the CS group, there was a significant increase in the goal sector preference (GS/NGS) (P=0.005, d=2.14) (Figure 5d). Also, In the ES group compared to the EM group, there was a significant increase in the GS/NGS (P=0.0002, d=5.3).

4. Discussion
Our results indicated that aerobic exercise improves spatial memory, consistent with previous reports (Inoue et al., 2015; Uysal et al., 2015; Wang & Wang, 2016). Stimulation of the OSNs during nasal respiration generated brain oscillations. OSNs are shown to respond to the chemical stimulus (odor) and are activated by nasal airflow (Lockmann et al., 2016). We presume that OSN stimulation enhances during exercise and increases connectivity in the OB and other brain regions improving spatial memory, and can be introduced as a probable mechanism. Therefore, we have used the methimazole injury model to investigate this mechanism. Methimazole is an olfactotoxic drug and disrupts the OSNs all over the epithelium by activating the apoptotic cascade (Sakamoto et al., 2007) without disturbing breathing rates during normal behaviors (Moberly et al., 2018). Our results indicated that the administration of methimazole reduces the number of neurons in the olfactory epithelium and the OB. Also, impairment of the OSNs is associated with decreased activity of OB at delta and theta frequency bands, as well as the coupling and synchronization between respiration and OB oscillations. Therefore, these results show that methimazole could destroy OSNs and reduce respiration-entrained brain oscillations. On the other hand, exercise increased the number of OB neurons and the synchrony between respiration and OB oscillations. To the best of our knowledge, this is the first report of improved spatial memory with aerobic exercise by modulating OB through stimulating OSNs.
During nasal breathing, OSNs fire rhythmically with respiration, and this activity is transported to OB cells (Heck et al., 2019). These OB oscillations generated during nasal respiration synchronize neural activities of the brain networks (Singer, 1993). Respiration-entrained brain oscillations emerge due to the phase-locking between olfactory neural oscillations and respiration cycles (Gretenkord et al., 2019). OSNs are sensitive to odors and mechanical stimulation from nasal airflow, and the entrainment of OB neuronal activity with respiration is independent of odor perception (Grosmaitre et al., 2007; Heck et al., 2019). Our result indicated that by destroying the OSNs (like breathing through mouth), the coupling between respiration and OB oscillations, and therefore, respiration-entrained brain oscillations were reduced, which was associated with a reduction in memory improvement induced by exercise. Nasal respiration modulates cognitive function while memory encoding and retrieval remain unchanged during mouth breathing (Zelano et al., 2016). Also, our previous study showed that stimulation of the OSNs using air-puff leads to excitation of the OB in conditions where the animal cannot breathe intentionally through the nose, and it can improve working memory performance (Ghazvineh et al., 2021). In addition, rhythmic nasal air puffing promotes brain activity and improves network connectivity in comatose patients (Salimi et al., 2022).
Besides, nasal breathing can modulate brain oscillations in nonolfactory areas, and respiration-entrained oscillations aid the integration of information and improve cognitive abilities (Zelano et al., 2016). Preceding works revealed that changes in breathing frequency could influence nonolfactory information processing (Tort et al., 2018; Tort et al., 2021). Also, differences in nasal airflow rate can affect olfactory processing (Esclassan et al., 2012; Hammer et al., 2021). Given that nasal airflow increases with respiratory intensity and rate and that aerobic exercise increases these parameters, we suggest that nasal airflow might have a role in memory improvements following aerobic exercise. The destruction of OSNs reverses this effect.
Previous evidence demonstrated that OB activity entrains regional oscillations in widespread brain areas, showing higher synchrony during cognitive performance (Tort et al., 2018). For instance, it has been reported that OB and hippocampus are synchronized in theta oscillation during reversal learning (Macrides et al., 1982). Therefore, OSNs may modulate OB oscillations through nasal respiration which in turn drives the changes throughout other brain regions. Also, the coupling between OB oscillations and other brain areas is correlated with cognitive functions (Salimi et al., 2019), and this coupling is crucial for information processing during the expression of behaviors (Fell & Axmacher, 2011). Eliminating the OB leads to cognitive impairments in attention, reference memory, delayed matching, reversal memory, and working memory (van Rijzingen et al., 1995). Therefore, respiration is a crucial neurobiological modulator in organizing cortical activity during memory processes in brain regions like the hippocampus and medial prefrontal cortex (Heck et al., 2019).
Aerobic exercise improved spatial memory; however, destroying OSNs reduced this effect which we believe might be due to the presence of respiration-entrained brain oscillation. This partial compensation in memory impairment is due to several parallel biological pathways recruited by exercise in affecting brain function. The present study results showed that nasal breathing improves spatial memory both at rest and aerobic exercise, and it was more significant following aerobic exercise. Additionally, spatial memory decreases when we eliminate the OSNs and respiration-entrained brain oscillation, like oral breathing. Therefore, our results suggest that nasal breathing is essential for cognitive function in rest and exercise. Aerobic exercise can be defined as a non-invasive mechanical stimulation method for the brain to restore cognitive deficits such as memory impairment.
5. Conclusion
The OB pathway is a probable mechanism for mediating the effects of exercise on spatial memory. These results provide new insight regarding the effect of OB activity on remote brain areas associated with cognition and behavior following exercise. However, the relative contribution of exercise to this mechanism is currently unclear, and further studies are needed.
Study limitations
This study faced some limitations in suggesting the entire mechanism. First, in this study, exercise had a compensatory effect when we destroyed the OSNs. It might be due to the role of other pathways in improving memory, such as increased neurogenesis and blood flow distribution in the olfactory epithelium, which we did not investigate in this study and should be evaluated in subsequent studies. Second, our study was limited to evaluating the long-term effects of exercise; however, it would be helpful for future studies that evaluate this pathway in short-term exercise. Third, since our recordings were not taken during exercise, we could not evaluate respiration intensity in this study, which needs further experiments and might provide interesting results.
Ethical Considerations
Compliance with ethical guidelines
The Ethics Committee of the Faculty of Medical Sciences, Tarbiat Modares University approved all animal protocols (Code: IR.MODARES.REC.1397.160).
Funding
The paper was extracted from the PhD dissertation of Farzaneh Zeynali, approved by the Department of Exercise Physiology & Sport Sciences, Faculty of Humanities, Tarbiat Modares University, Tehran, Iran.
Authors' contributions
All authors contributed equally in preparing all parts of the research.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank Morteza Mooziri for his invaluable assistance in editing this manuscript. Also, the authors would like to thank Fatemeh Hosseinpour, Jalaledin Noroozi, and Mohammad Valadi Athar for their technical assistance.
References
Azimi, M., Gharakhanlou, R., Naghdi, N., Khodadadi, D., & Heysieattalab, S. (2018). Moderate treadmill exercise ameliorates amyloid-β-induced learning and memory impairment, possibly via increasing AMPK activity and up-regulation of the PGC-1α/FNDC5/BDNF pathway. Peptides, 102, 78-88. [DOI:10.1016/j.peptides.2017.12.027] [PMID]
Bagur, S., Lefort, J. M., Lacroix, M. M., de Lavilléon, G., Herry, C., & Chouvaeff, M., et al. (2021). Breathing-driven prefrontal oscillations regulate maintenance of conditioned-fear evoked freezing independently of initiation. Nature Communications, 12(1), 2605. [DOI:10.1038/s41467-021-22798-6] [PMID] [PMCID]
Bennett, W. D., Zeman, K. L., & Jarabek, A. M. (2003). Nasal contribution to breathing with exercise: Effect of race and gender. Journal of Applied Physiology, 95(2), 497-503. [DOI:10.1152/japplphysiol.00718.2002] [PMID]
Bergman, U., & Brittebo, E. B. (1999). Methimazole toxicity in rodents: covalent binding in the olfactory mucosa and detection of glial fibrillary acidic protein in the olfactory bulb. Toxicology and Applied Pharmacology, 155(2), 190–200. [DOI:10.1006/taap.1998.8590] [PMID]
Bherer, L., Erickson, K. I., & Liu-Ambrose, T. (2013). A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. Journal of Aging Research, 2013, 657508. [DOI:10.1155/2013/657508] [PMID] [PMCID]
Cefis, M., Prigent-Tessier, A., Quirié, A., Pernet, N., Marie, C., & Garnier, P. (2019). The effect of exercise on memory and BDNF signaling is dependent on intensity. Brain Structure and Function, 224(6), 1975-1985. [DOI:10.1007/s00429-019-01889-7] [PMID]
Chae, C. H., Jung, S. L., An, S. H., Jung, C. K., Nam, S. N., & Kim, H. T. (2011). Treadmill exercise suppresses muscle cell apoptosis by increasing nerve growth factor levels and stimulating p-phosphatidylinositol 3-kinase activation in the soleus of diabetic rats. Journal of Physiology and Biochemistry, 67(2), 235-241. [DOI:10.1007/s13105-010-0068-9] [PMID]
Dehdar, K., Mahdidoust, S., Salimi, M., Gholami-Mahtaj, L., Nazari, M., & Mohammadi, S., et al. (2019). Allergen-induced anxiety-like behavior is associated with disruption of medial prefrontal cortex-amygdala circuit. Scientific Reports, 9(1), 19586. [DOI:10.1038/s41598-019-55539-3] [PMID] [PMCID]
Desai, R., Tailor, A., & Bhatt, T. (2015). Effects of yoga on brain waves and structural activation: A review. Complementary Therapies in Clinical Practice, 21(2), 112-118. [DOI:10.1016/j.ctcp.2015.02.002] [PMID]
Di Liegro, C. M., Schiera, G., Proia, P., & Di Liegro, I. (2019). Physical activity and brain health. Genes, 10(9), 720. [DOI:10.3390/genes10090720] [PMID] [PMCID]
Esclassan, F., Courtiol, E., Thévenet, M., Garcia, S., Buonviso, N., & Litaudon, P. (2012). Faster, deeper, better: The impact of sniffing modulation on bulbar olfactory processing. Plos One, 7(7), e40927. [DOI:10.1371/journal.pone.0040927] [PMID] [PMCID]
Fell, J., & Axmacher, N. (2011). The role of phase synchronization in memory processes. Nature Reviews Neuroscience, 12(2), 105-118. [DOI:10.1038/nrn2979] [PMID]
Genter, M. B., Deamer, N. J., Blake, B. L., Wesley, D. S., & Levi, P. E. (1995). Olfactory toxicity of methimazole: Dose-response and structure-activity studies and characterization of flavin-containing monooxygenase activity in the Long-Evans rat olfactory mucosa. Toxicologic Pathology, 23(4), 477-486. [DOI:10.1177/019262339502300404] [PMID]
Ghazvineh, S., Salimi, M., Nazari, M., Garousi, M., Tabasi, F., & Dehdar, K., et al. (2021). Rhythmic air-puff into nasal cavity modulates activity across multiple brain areas: A non-invasive brain stimulation method to reduce ventilator-induced memory impairment. Respiratory Physiology & Neurobiology, 287, 103627. [DOI:10.1016/j.resp.2021.103627] [PMID]
Girard, T. D., Jackson, J. C., Pandharipande, P. P., Pun, B. T., Thompson, J. L., & Shintani, A. K., et al. (2010). Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Critical Care Medicine, 38(7), 1513-1520. [DOI:10.1097/CCM.0b013e3181e47be1] [PMID] [PMCID]
Gretenkord, S., Kostka, J. K., Hartung, H., Watznauer, K., Fleck, D., & Minier-Toribio, A., et al. (2019). Coordinated electrical activity in the olfactory bulb gates the oscillatory entrainment of entorhinal networks in neonatal mice. Plos Biology, 17(1), e2006994. [DOI:10.1371/journal.pbio.2006994] [PMID] [PMCID]
Grosmaitre, X., Santarelli, L. C., Tan, J., Luo, M., & Ma, M. (2007). Dual functions of mammalian olfactory sensory neurons as odor detectors and mechanical sensors. Nature Neuroscience, 10(3), 348-354. [DOI:10.1038/nn1856] [PMID] [PMCID]
Hammer, M., Schwale, C., Brankačk, J., Draguhn, A., & Tort, A. B. L. (2021). Theta-gamma coupling during REM sleep depends on breathing rate. Sleep, 44(12), zsab189. [DOI:10.1093/sleep/zsab189] [PMID]
Heck, D. H., Kozma, R., & Kay, L. M. (2019). The rhythm of memory: How breathing shapes memory function. Journal of Neurophysiology, 122(2), 563-571. [DOI:10.1152/jn.00200.2019] [PMID] [PMCID]
Inoue, K., Hanaoka, Y., Nishijima, T., Okamoto, M., Chang, H., & Saito, T., et al. (2015). Long-term mild exercise training enhances hippocampus-dependent memory in rats. International Journal of Sports Medicine, 36(4), 280-285. [DOI:10.1055/s-0034-1390465] [PMID]
Kepecs, A., Uchida, N., & Mainen, Z. F. (2006). The sniff as a unit of olfactory processing. Chemical Senses, 31(2), 167-179. [DOI:10.1093/chemse/bjj016] [PMID]
Khodadadi, D., Gharakhanlou, R., Naghdi, N., Salimi, M., Azimi, M., & Shahed, A., et al. (2018). Treadmill exercise ameliorates spatial learning and memory deficits through improving the clearance of peripheral and central amyloid-beta levels. Neurochemical Research, 43(8), 1561-1574. [DOI:10.1007/s11064-018-2571-2] [PMID]
Liu, Y., Yan, T., Chu, J. M., Chen, Y., Dunnett, S., & Ho, Y. S., et al. (2019). The beneficial effects of physical exercise in the brain and related pathophysiological mechanisms in neurodegenerative diseases. Laboratory Investigation, 99(7), 943-957. [DOI:10.1038/s41374-019-0232-y] [PMID]
Lockmann, A. L., Laplagne, D. A., Leão, R. N., & Tort, A. B. (2016). A respiration-coupled rhythm in the rat hippocampus independent of theta and slow oscillations. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 36(19), 5338–5352. [DOI:10.1523/JNEUROSCI.3452-15.2016] [PMID] [PMCID]
Macrides, F., Eichenbaum, H. B., & Forbes, W. B. (1982). Temporal relationship between sniffing and the limbic theta rhythm during odor discrimination reversal learning. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 2(12), 1705–1717. [DOI:10.1523/JNEUROSCI.02-12-01705.1982] [PMID] [PMCID]
Moberly, A. H., Schreck, M., Bhattarai, J. P., Zweifel, L. S., Luo, W., & Ma, M. (2018). Olfactory inputs modulate respiration-related rhythmic activity in the prefrontal cortex and freezing behavior. Nature Communications, 9(1), 1528. [DOI:10.1038/s41467-018-03988-1] [PMID] [PMCID]
Paxinos, G., & Watson, C. (2006). The rat brain in stereotaxic coordinates. Amsterdam: Elsevier. [Link]
Sakamoto, T., Kondo, K., Kashio, A., Suzukawa, K., & Yamasoba, T. (2007). Methimazole-induced cell death in rat olfactory receptor neurons occurs via apoptosis triggered through mitochondrial cytochrome c-mediated caspase-3 activation pathway. Journal of Neuroscience Research, 85(3), 548-557. [DOI:10.1002/jnr.21155] [PMID]
Salimi, M., Ghazvineh, S., Zare, M., Parsazadegan, T., Dehdar, K., & Nazari, M., et al. (2019). Distraction of olfactory bulb-medial prefrontal cortex circuit may induce anxiety-like behavior in allergic rhinitis. PloS One, 14(9), e0221978. [DOI:10.1371/journal.pone.0221978] [PMID] [PMCID]
Salimi, M., Javadi, A. H., Nazari, M., Bamdad, S., Tabasi, F., & Parsazadegan, T., et al. (2022). Nasal Air Puff Promotes Default Mode Network Activity in Mechanically Ventilated Comatose Patients: A Noninvasive Brain Stimulation Approach. Neuromodulation: Journal of the International Neuromodulation Society, 25(8), 1351–1363.[DOI:10.1016/j.neurom.2021.11.003] [PMID]
Singer, W. (1993). Synchronization of cortical activity and its putative role in information processing and learning. Annual Review of Physiology, 55(1), 349-374. [DOI:10.1146/annurev.ph.55.030193.002025] [PMID]
Tort, A. B. L., Brankačk, J., & Draguhn, A. (2018). Respiration-entrained brain rhythms are global but often overlooked. Trends in Neurosciences, 41(4), 186-197. [DOI:10.1016/j.tins.2018.01.007] [PMID]
Tort, A. B. L., Hammer, M., Zhang, J., Brankačk, J., & Draguhn, A. (2021). Temporal relations between cortical network oscillations and breathing frequency during REM sleep. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 41(24), 5229–5242. [DOI:10.1523/JNEUROSCI.3067-20.2021] [PMID] [PMCID]
Uysal, N., Kiray, M., Sisman, A. R., Camsari, U. M., Gencoglu, C., & Baykara, B., et al. (2015). Effects of voluntary and involuntary exercise on cognitive functions, and VEGF and BDNF levels in adolescent rats. Biotechnic & Histochemistry, 90(1), 55-68. [DOI:10.3109/10520295.2014.946968] [PMID]
van Rijzingen, I. M., Gispen, W. H., & Spruijt, B. M. (1995). Olfactory bulbectomy temporarily impairs Morris maze performance: An ACTH (4-9) analog accellerates return of function. Physiology & Behavior, 58(1), 147-152. [DOI:10.1016/0031-9384(95)00032-E] [PMID]
Vanderwolf, C., & Szechtman, H. (1987). Electrophysiological correlates of stereotyped sniffing in rats injected with apomorphine. Pharmacology Biochemistry and Behavior, 26(2), 299-304. [DOI:10.1016/0091-3057(87)90122-5] [PMID]
Wang, X. Q., & Wang, G. W. (2016). Effects of treadmill exercise intensity on spatial working memory and long-term memory in rats. Life Sciences, 149, 96-103. [DOI:10.1016/j.lfs.2016.02.070] [PMID]
Weuve, J., Kang, J. H., Manson, J. E., Breteler, M. M., Ware, J. H., & Grodstein, F. (2004). Physical activity, including walking, and cognitive function in older women. JAMA, 292(12), 1454–1461. [DOI:10.1001/jama.292.12.1454] [PMID]
Type of Study: Original |
Subject:
Cognitive Neuroscience
Received: 2022/03/7 | Accepted: 2022/05/1 | Published: 2024/05/1
Received: 2022/03/7 | Accepted: 2022/05/1 | Published: 2024/05/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








