Volume 14, Issue 6 (November & December 2023)
BCN 2023, 14(6): 857-866 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Sadeghinasab S, Moghadam A R E, Saki N, Bayat A, Saki G. Alterations in Diffusion Tensor Imaging-derived Indices of Auditory Pathway-related Fiber Tracts in Children With Sensorineural Hearing Loss. BCN 2023; 14 (6) :857-866
URL: http://bcn.iums.ac.ir/article-1-2410-en.html
URL: http://bcn.iums.ac.ir/article-1-2410-en.html
1- Department of Anatomical Sciences, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
2- Department of Anatomical Sciences, Faculty of Medicine, Baqiyatallah University of Medical Sciences, Tehran, Iran.
3- Hearing Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
2- Department of Anatomical Sciences, Faculty of Medicine, Baqiyatallah University of Medical Sciences, Tehran, Iran.
3- Hearing Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
Full-Text [PDF 1365 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Hearing loss is one of the most common diseases globally. There are two types of hearing loss: Conductive and sensorineural hearing loss (Zahnert, 2011). Sensorineural hearing loss (SNHL) is the most frequent congenital sensory deficit in children, affecting six in 1000 by age 18 (Huang et al., 2012). It mostly occurs as a result of changes in neural pathways and inner ear dysfunction caused by prenatal and postnatal infection (Chen & Oghalai, 2016). The prevalence of SNHL is about seven per 100,000 for younger children (Wellman et al., 2003), 75% of whom are younger than two years, 15% are 2–5 years, and 10% are older than five years (Koomen et al., 2003). However, SNHL is mainly associated with developmental delay and its underlying pathophysiological mechanisms are not yet well understood. Because sensory input plays a crucial role in human brain functionality, the early hearing deficit can affect the auditory cortex and its white matter fiber tracts, leading to cognitive decline in many domains, including language and learning (Oberg & Lukomski, 2011). Therefore, in order to prevent potential cognitive declines, and better treatment and assessment of cochlear implant candidacy, early detection of the brain microstructural changes is crucial (Seydell-Greenwald et al., 2014). With the continuous development of magnetic resonance technology, magnetic resonance imaging (MRI) has become a prominent modality to evaluate white matter lesions in the central nervous system. Diffusion tensor imaging (DTI) is an emerging MRI-based modality that can reveal microstructural changes in white matter pathways using specific parameters, such as mean diffusivity (MD) and axial diffusivity (AxD) which are assumed to reflect the breakdown of microstructural barriers and axonal damage (Qian et al., 2018). Radial diffusivity (RD) is specifically associated with myelin degeneration, and fractional anisotropy (FA) measures white matter integrity. DTI plays a key role in detecting early white matter changes associated with hearing loss (Tarabichi et al., 2018). The aim of the present study was to investigate the structural connectivity alterations in the brain white matter tracts of the auditory network in infants with bilateral profound SNHL who are under four years of age.
2. Materials and Methods
Subjects
A total of 22 subjects, including 11 children aged 1-4 years with SNHL leading to bilateral hearing loss and 11 age-matched controls were examined in this study. The patients were consecutively recruited. The healthy controls were recruited through local advertisements. Inclusion criteria were no structural abnormalities of the auditory system detected in conventional MRI images before DTI and no previous history of otological surgery. All subjects underwent audiometry, MRI, and DTI scanning (Table 1).

MRI protocol
Participants underwent a 3D T1-weighted BRAVO protocol, using a 1.5 T Optima MR 450w imager (GE, Milwaukee, WI, USA) with a 12-channel head coil. Whole-brain 3D T1-weighted images were obtained with the following imaging parameters: TR=1002 ms, TE=4.3 ms, voxel size=1×1×1 mm3, the field of view (FOV)=220 mm, flip angle=12°, and slice thickness=1 mm with no gaps. DTI data were obtained in the axial plane using a single-shot diffusion-weighted imaging sequence with 64 directions and the following parameters: b-value of 1000 s/mm2, TR=9275 ms, TE=79.3 ms, voxel size=1.09×1.09×2.4, 75 slices, slice thickness=2.4 mm, FOV=245 mm, and matrix size=256×256.
Image processing and tractography
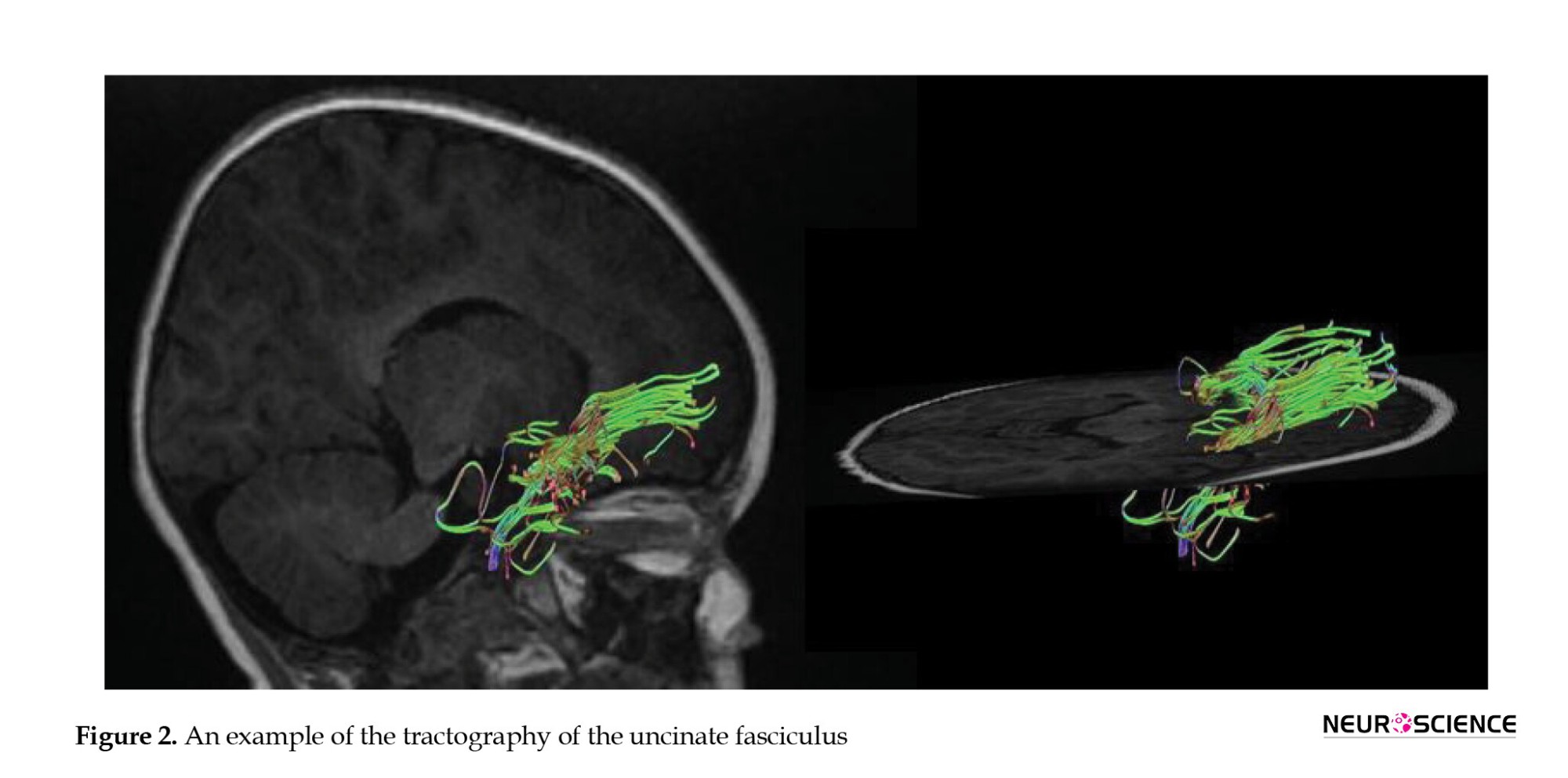
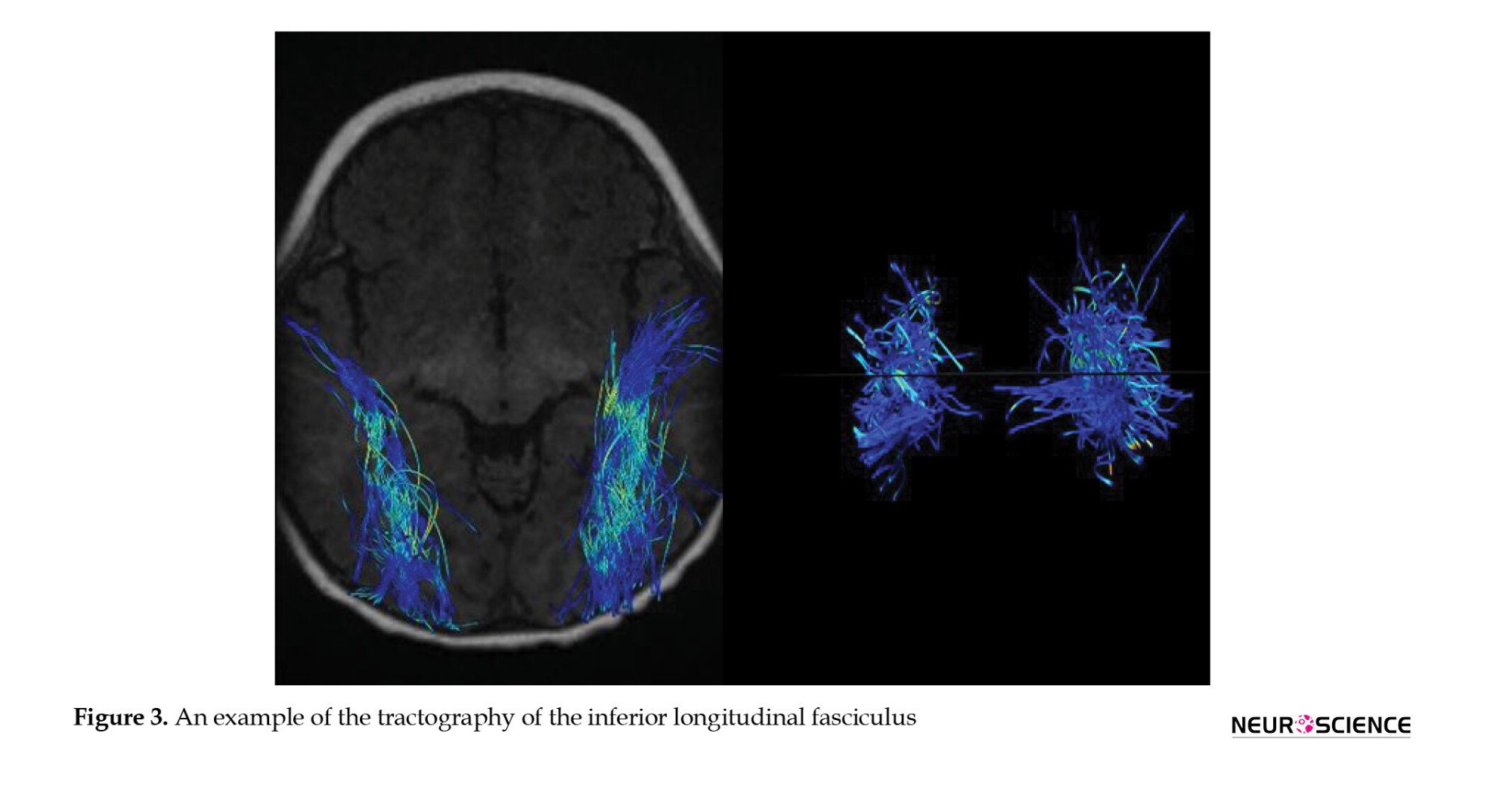
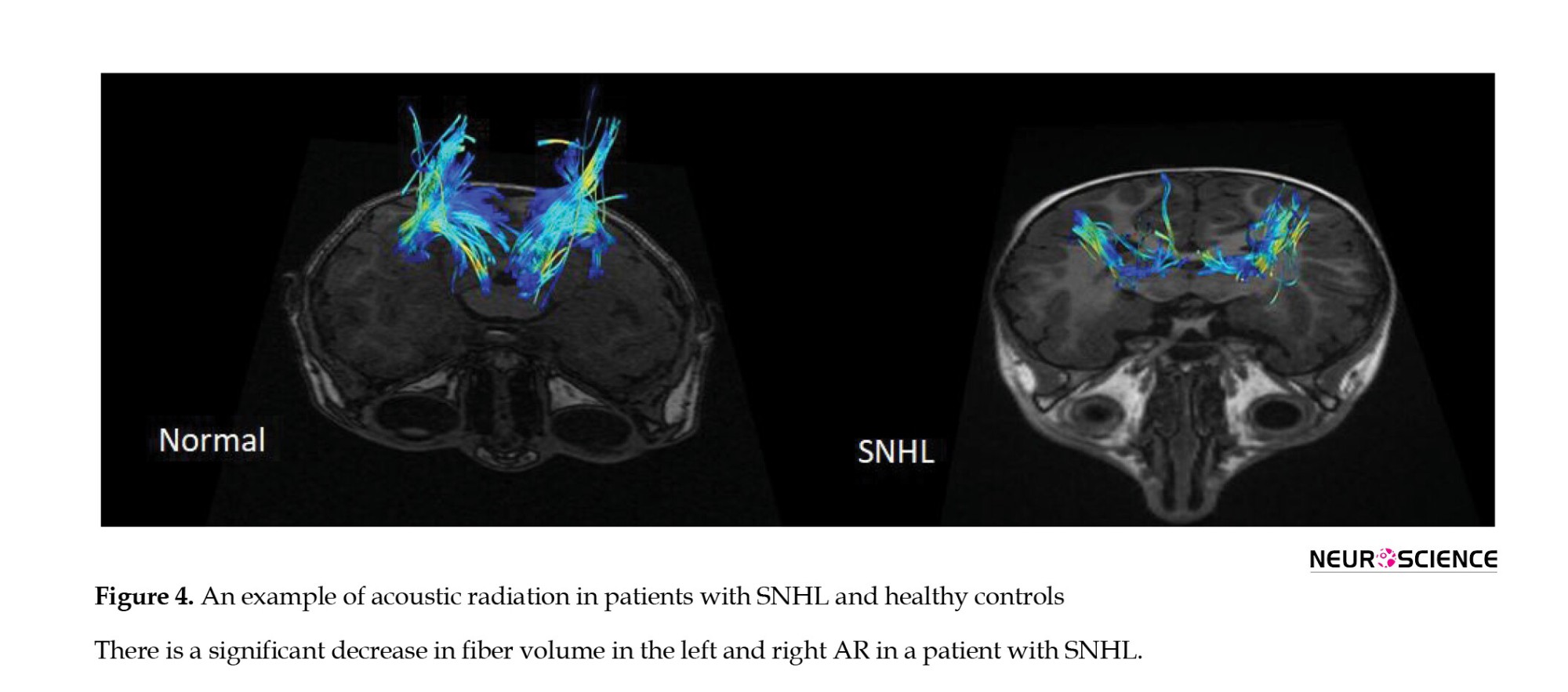
DTI studio (Latest×64 bit version of DTI Studio, Johns Hopkins University) was used for preprocessing and deterministic fiber tracking. The atlas-based tractography was performed. The three main auditory pathway-related fibers, including acoustic radiation (AR), uncinate fasciculus (UF), and inferior longitudinal fasciculus (ILF) were selected. The tractography was done with a step size of 0 mm, minimum fiber length of 30 mm, and turning angle threshold of 60 (Figures 1, 2, 3 and 4). The DTI-derived parameters, FA, MD, axial diffusivity (AxD), and radial diffusivity (RD) were extracted from each tract. Also, the mean length and diameter of all fiber tracts were calculated automatically using morphological assessment of Tensor.

Statistical analysis
Data were analyzed by SPSS software, version 20. The comparisons of demographic and clinical data between two the groups in terms of imaging parameters and proof of hypotheses were performed using the paired t-test. The threshold for establishing significance was set at P<0.05.
3. Results
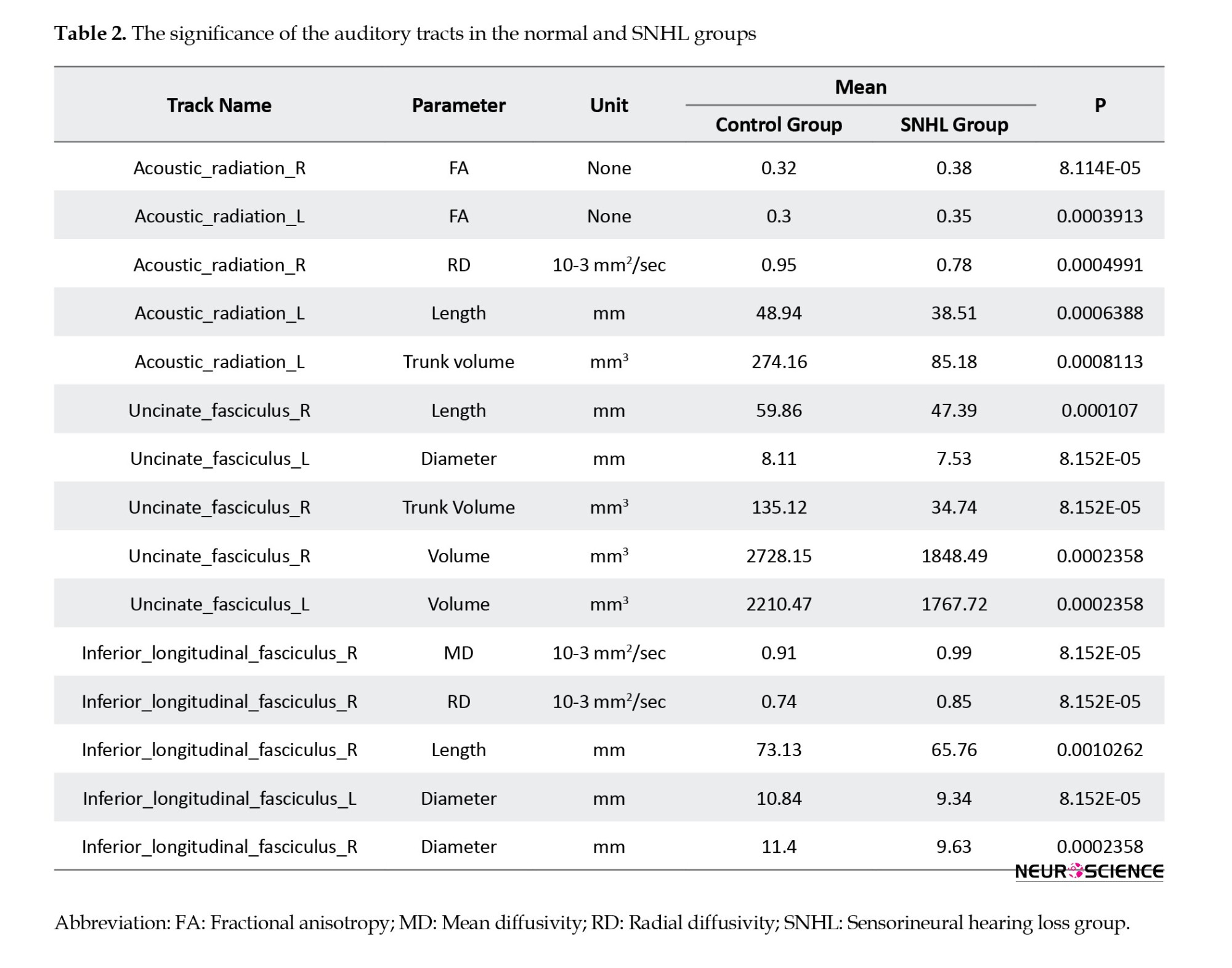
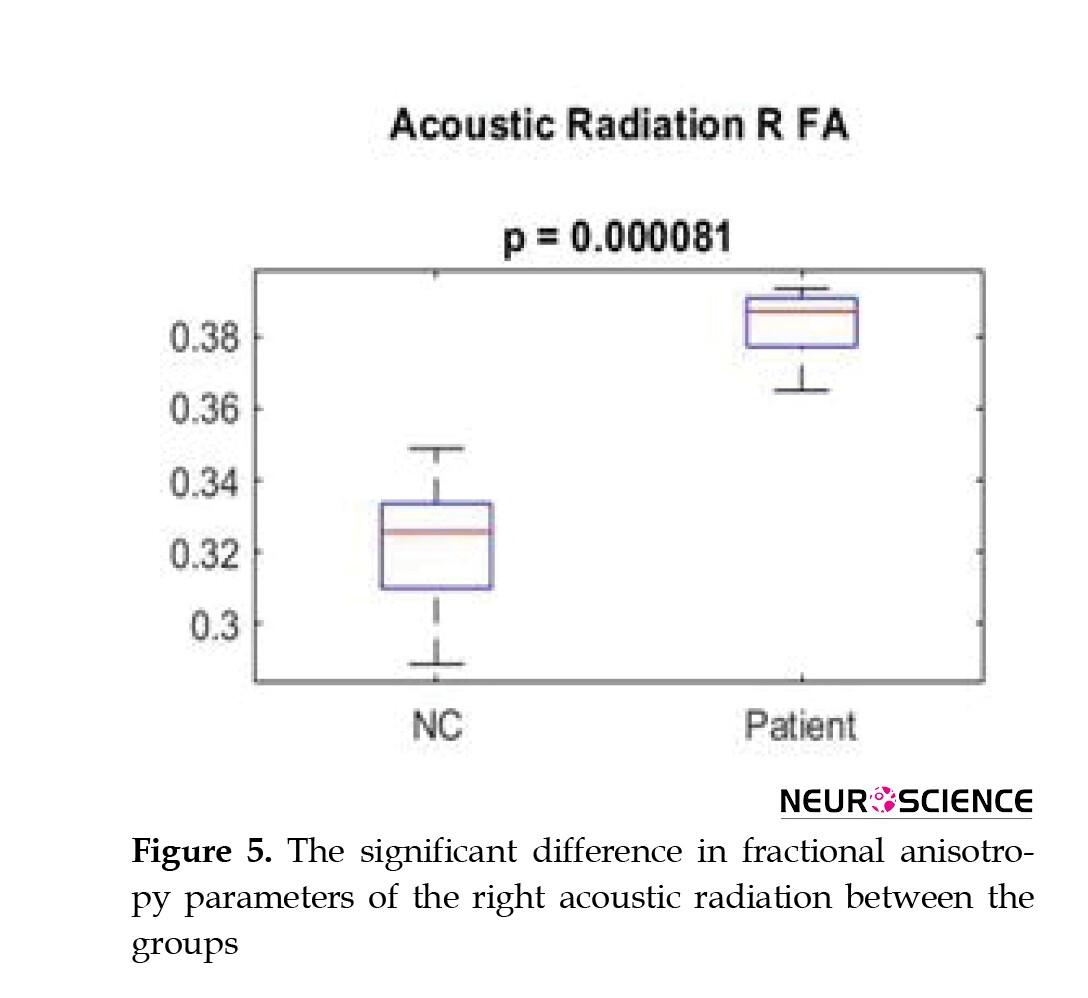
There was an increase in FA (P<0.0001) a decrease in RD (P<0.05) and a decrease in mean length and volume in the right and left AR tracts (P<0.0009). The decreased volume of right and left UF tracts (P<0.00025), including reduced trunk volume (P<0.0001) and reduced mean length (P<0.0001) were evident. A decrease in trunk volume and a decrease in the length (P<0.0011) and diameter of the right and left ILF (P<0.00024) as well as a decrease in FA and an increase in MD and RD parameters were seen in these tracts (P<0.0001) (Table 2, Figure 5).
4. Discussion
Radiological evaluation of children and adults with SNHL is usually performed by computed tomography (CT) or MRI to examine the temporal and retro-cochlear bone (Sterling et al., 2018). Although conventional and structured MRI is highly sensitive for the diagnosis of congenital anomalies, tumors of the internal auditory canal, and cochlear nerve anatomy, it does not provide information about the central auditory pathway (Glastonbury et al., 2002). DTI imaging is an MRI-based technique that can provide microstructural information about white matter connections in the central nervous system. The key to the usefulness of this imaging modality is the ability to detect changes and properties of white matter and brain tissue at the microscopic level. Disruption of biological barriers, such as myelin and cell membranes, which normally restrict the motion of water molecules, leads to measurable changes in the diffusion of water molecules (Uluǧ et al., 1999). Over the past five years, studies on the application of DTI in the evaluation of auditory white matter pathways have been increasing rapidly. The present study was done to evaluate the volume and characteristics of white matter in the auditory pathway-related fiber tracts in children with SNHL.
UF
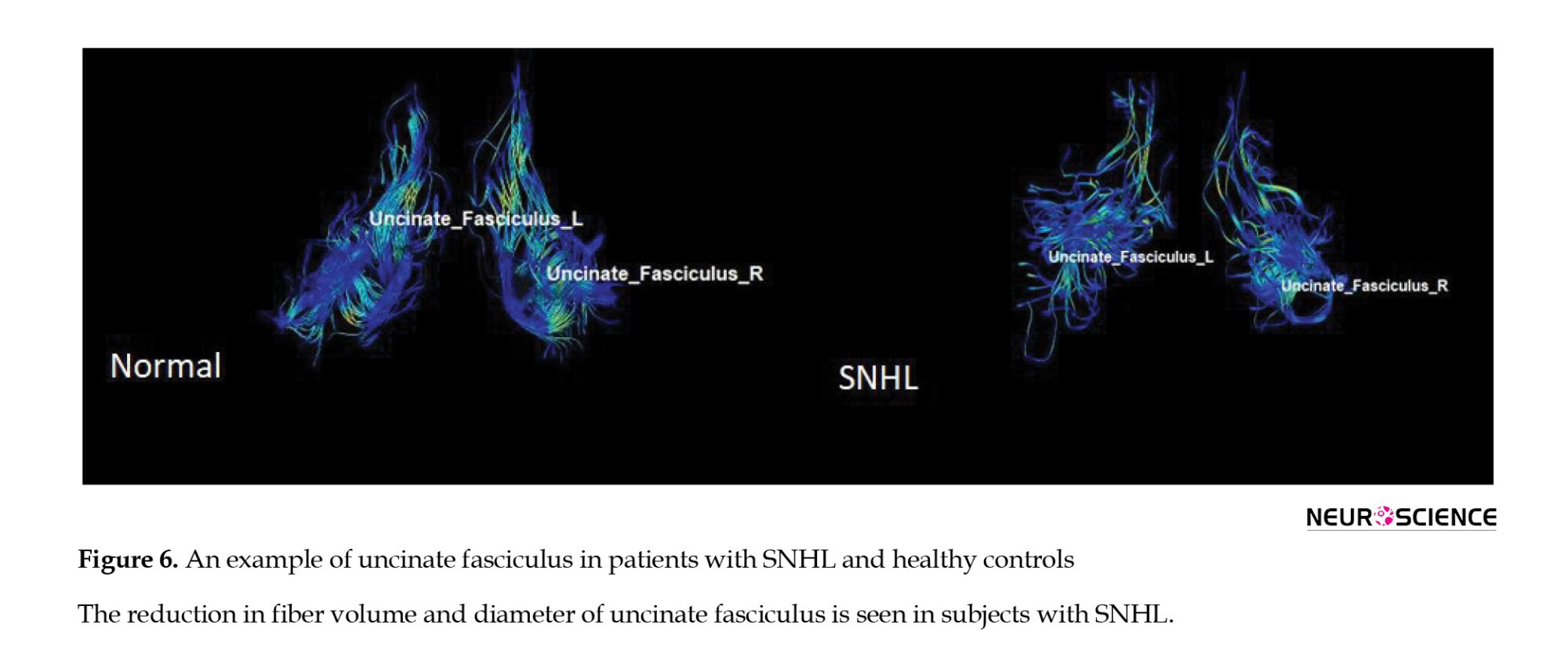
This area is the largest pathway of the association fiber tract, which connects the frontal lobe to the temporal lobe. This area is said to be part of the limbic system. It plays a major role in language production (Von Der Heide et al., 2013). However, various studies have suggested the role of this tract in the processing of auditory information (Chern et al., 2020). Many studies have shown a decrease in white matter integrity in UF in patients with SNHL (Croll et al., 2020). In a study on the combined DTI and FMRI, a decrease in FA in UF was found in patients with SNHL aged 1-4 years compared to the normal group (Wang & Fan, 2019; Wang et al., 2020). It should be noted that there are many studies, in which the FA parameter was not significant in differentiating the two groups (Huang et al., 2015). In the present study, which examined the volume and characteristics of UF, a decrease in total volume, trunk volume, and diameter, and also a decrease in length was seen in this tract, which indicates the loss of some nerve fibers due to atrophy in this area. It has been shown that this damage leads to a decrease in the proper functioning of the auditory sense, resulting in hearing impairment. AxD value increased in this area, indicating a decrease in myelin sheath of white matter, atrophy, and integrity in this area (Figure 6).
AR
AR contains a group of highly myelinated axons and is one of the main sensory pathways in the brain that transmit auditory information from the thalamus to the auditory cortex. More precisely, the central auditory pathway begins in the cochlear branch of the eighth nerve of the brain and sends auditory information through the internal capsule to the temporal cortex (Hernández-Zamora & Poblano, 2014). AR has been very difficult to study due to its complex anatomy. With the advent of DTI, the study of this neural pathway in three dimensions has been facilitated, but still, the tractography of this area is challenging (Langguth et al., 2010). In younger patients with congenital deafness, many studies have been performed on volumetric measurement of auditory-related areas (Shibata et al., 2007). However, there are few studies on microstructural alterations in AR in hearing-related diseases, especially SNHL. In addition, AR tractography may be potentially helpful in assessing the integrity of the auditory pathway before and after cochlear implantation. Previous studies have reported a decrease in FA of the AR in patients with SNHL (Huang et al., 2015). In the present study, which examined the volume and diffusion characteristics of AR, the FA parameter showed the highest level of significance in differentiating the two groups from each other. It should be noted that the total volume, trunk volume, and length of this tract decreased, which indicates the existence of a specific pathology in the field of hearing. A very interesting finding in this study was the increase in FA. It indicates an increase in the integrity of this tract, as well as a decrease in MD, AxD, and RD, suggesting an increase in myelin and increased integrity in this area. The cause of this phenomenon can be a compensatory effect against the atrophy of the auditory pathways. To compensate for the effect of the damage, this tract started to remyelinate to be the most efficient with the least volume. There are few studies in this field regarding the increase in FA in AR (Lin et al., 2008).
ILF
This fiber tract connects the occipital lobe to the anterior part of the temporal lobe. This fiber overlaps with the UF in the anterior part and participates in the transmission of auditory information to the orbitofrontal part of the brain. This fiber is one of the pathways related to visual and linguistic information processing. DTI in combination with magnetoencephalography studies has shown its role in auditory processes and voice-language learning tests (Shin et al., 2019). In other words, ILF integrates information from the auditory and speech areas in the cortex (Mcmaster et al., 2011). Some studies on SNHL have shown a reduction in FA of ILF (Huang et al., 2015; Wang & Fan 2019). In the present study, a decrease in overall volume, trunk volume, diameter, and length was observed in ILF, indicating a decrease in nerve fibers due to atrophy, leading to hearing disorders. MD, RD, and AxD values increased, indicating a reduction in myelin sheath, atrophy, and integrity in this area (Figure 7).

5. Conclusion
In conclusion, the present study showed that microstructural changes in the fiber tracts associated with the auditory pathway can distinguish SNHL from healthy subjects. This study provides DTI-based biomarkers for brain changes and allows us to better understand the pathophysiological changes of related diseases. DTI is an emerging tool for evaluating the affected white matter and compensatory action related to the auditory system in patients with SNHL.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Science (Code: IR.AJUMS.MEDICINE.REC.1399.016) and written informed consent was obtained from the parents of all participants before the study.
Funding
This paper was extracted from master's thesis of Samira Sadeghinasab, approved by Department of Anatomical Science, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, and was financially supported by the Research Deputy of Ahvaz Jundishapur University of Medical Sciences (Grant No.: HRC‐9911).
Authors' contributions
Conceptualization and supervision: Ghasem Saki and Ali Reza Eftekhari Moghadam; Methodology: Samira Sadeghi; Data collection: Samira Sadeghi, Nader Saki, and Arash Bayat; Data analysis: Ali Reza Eftekhari Moghadam and Samira Sadeghi; Funding acquisition and resources: Ghasem Saki, Nader Saki; Investigation and writing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the head of Medical Imaging Center of Golestan Hospital for its cooperation, and the patients, without whom, this research could not be conducted.
References
Chen, M. M., & Oghalai, J. S. (2016). Diagnosis and management of congenital sensorineural hearing loss. Current Treatment Options in Pediatrics, 2(3), 256–265. [DOI:10.1007/s40746-016-0056-6] [PMID] [PMCID]
Chern, A., Irace, A. L., & Golub, J. S. (2020). Mapping the brain effects of hearing loss: The matter of white matter. JAMA Otolaryngology-- Head & Neck Surgery, 146(11), 1043–1044. [DOI:10.1001/jamaoto.2020.2528] [PMID]
Croll, P. H., Vernooij, M. W., Reid, R. I., Goedegebure, A., Power, M. C., & Rigters, S. C., et al. (2020). Hearing loss and microstructural integrity of the brain in a dementia-free older population. Alzheimer’s and Dementia, 16(11): 1515-23. [DOI:10.1002/alz.12151] [PMID]
Glastonbury, C. M., Davidson, H. C., Harnsberger, H. R., Butler, J., Kertesz, T. R., & Shelton, C. (2002). Imaging findings of cochlear nerve deficiency. American Journal of Neuroradiology, 23(4), 635–643. [PMID] [PMCID]
Hernández-Zamora, E., & Poblano, A. (2014). [The auditory pathway: Levels of integration of information and principal neurotransmitters (Spanish)]. Gaceta Medica de Mexico, 150(5), 450–460. [PMID]
Huang, B. Y., Zdanski, C., & Castillo, M. (2012). Pediatric sensorineural hearing loss, part 2: Syndromic and acquired causes. American Journal of Neuroradiology, 33(3), 399–406. [DOI:10.3174/ajnr.A2499] [PMID] [PMCID]
Huang, L., Zheng, W., Wu, C., Wei, X., Wu, X., & Wang, Y., et al. (2015). Diffusion tensor imaging of the auditory neural pathway for clinical outcome of cochlear implantation in pediatric congenital sensorineural hearing loss patients. Plos One, 10(10), e0140643. [DOI:10.1371/journal.pone.0140643] [PMID] [PMCID]
Koomen, I., Grobbee, D. E., Roord, J. J., Donders, R., Jennekens-Schinkel, A., & van Furth, A. M. (2003). Hearing loss at school age in survivors of bacterial meningitis: Assessment, incidence, and prediction. Pediatrics, 112(5), 1049–1053. [DOI:10.1542/peds.112.5.1049] [PMID]
Langguth, B., Kleinjung, T., Landgrebe, M., de Ridder, D., & Hajak, G. (2010). rTMS for the treatment of tinnitus: The role of neuronavigation for coil positioning. Neurophysiologie Clinique, 40(1), 45–58. [DOI:10.1016/j.neucli.2009.03.001] [PMID]
Lin, Y., Wang, J., Wu, C., Wai, Y., Yu, J., & Ng, S. (2008). Diffusion tensor imaging of the auditory pathway in sensorineural hearing loss: Changes in radial diffusivity and diffusion anisotropy. Journal of Magnetic Resonance Imaging, 28(3), 598–603. [DOI:10.1002/jmri.21464] [PMID]
Oberg, E., & Lukomski, J. (2011). Executive functioning and the impact of a hearing loss: Performance-based measures and the Behavior Rating Inventory of Executive Function (BRIEF). Child Neuropsychology, 17(6), 521–545. [DOI:10.1080/09297049.2011.555760] [PMID]
Qian, Y., Zhong, S., Hu, G., Kang, H., Wang, L., & Lei, Y. (2018). Sudden Sensorineural Hearing Loss in Children: A Report of 75 Cases. Otology & neurotology: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology, 39(8), 1018–1024. [DOI:10.1097/MAO.0000000000001891] [PMID]
Seydell-Greenwald, Anna et al. 2014. “Diffusion Imaging of Auditory and Auditory-Limbic Connectivity in Tinnitus: Preliminary Evidence and Methodological Challenges.” Neural Plasticity 2014. [DOI:10.1155/2014/145943] [PMID]
Shibata, D. K. (2007). Differences in brain structure in deaf persons on MR imaging studied with voxel-based morphometry. American Journal of Neuroradiology, 28(2), 243-249. [PMID]
Shin, J., Rowley, J., Chowdhury, R., Jolicoeur, P., Klein, D., & Grova, C., et al. (2019). Inferior longitudinal fasciculus' role in visual processing and language comprehension: A combined MEG-DTI study. Frontiers in Neuroscience, 13, 875. [DOI:10.3389/fnins.2019.00875] [PMID] [PMCID]
Sterling, M. R., Lin, F. R., Jannat-Khah, D. P., Goman, A. M., Echeverria, S. E., & Safford, M. M. (2018). Hearing Loss Among Older Adults With Heart Failure in the United States: Data From the National Health and Nutrition Examination Survey. JAMA Otolaryngology-- Head & Neck Surgery, 144(3), 273–275. [DOI:10.1001/jamaoto.2017.2979] [PMID]
Tarabichi, O., Kozin, E. D., Kanumuri, V. V., Barber, S., Ghosh, S., & Sitek, K. R., et al. (2018). Diffusion tensor imaging of central auditory pathways in patients with sensorineural hearing loss: A systematic review. Otolaryngology--Head and Neck Surgery, 158(3), 432–442. [DOI:10.1177/0194599817739838] [PMID] [PMCID]
Uluğ, A. M., Moore, D. F., Bojko, A. S., & Zimmerman, R. D. (1999). Clinical use of diffusion-tensor imaging for diseases causing neuronal and axonal damage. American Journal of Neuroradiology, 20(6), 1044–1048. [PMID] [PMCID]
Von Der Heide, R. J., Skipper, L. M., Klobusicky, E., & Olson, I. R. (2013). Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain, 136(Pt 6), 1692–1707. [DOI:10.1093/brain/awt094] [PMID] [PMCID]
Wang, S., Chen, B., Yu, Y., Yang, H., Cui, W., & Li, J., et al. (2019). Alterations of structural and functional connectivity in profound sensorineural hearing loss infants within an early sensitive period: A combined DTI and fMRI study. Developmental Cognitive Neuroscience, 38, 100654. [DOI:10.1016/j.dcn.2019.100654] [PMID] [PMCID]
Wang, S., Chen, B., Yu, Y., Yang, H., Cui, W., & Li, J., et al. (2020). Corrigendum to "alterations of structural and functional connectivity in profound sensorineural hearing loss infants within an early sensitive period: A combined DTI and fMRI study" [Dev. Cogn. Neurosci. 38 (2019) 100654]. Developmental cognitive neuroscience, 41, 100689. [DOI::10.1016/j.dcn.2019.100689] [PMID]
Wellman, M. B., Sommer, D. D., & McKenna, J. (2003). Sensorineural hearing loss in postmeningitic children. Otology & Neurotology, 24(6), 907–912. [DOI:10.1097/00129492-200311000-00015] [PMID]
Zahnert T. (2011). The differential diagnosis of hearing loss. Deutsches Arzteblatt International, 108(25), 433–444. [DOI:10.3238/arztebl.2011.0433] [PMID] [PMCID]
Hearing loss is one of the most common diseases globally. There are two types of hearing loss: Conductive and sensorineural hearing loss (Zahnert, 2011). Sensorineural hearing loss (SNHL) is the most frequent congenital sensory deficit in children, affecting six in 1000 by age 18 (Huang et al., 2012). It mostly occurs as a result of changes in neural pathways and inner ear dysfunction caused by prenatal and postnatal infection (Chen & Oghalai, 2016). The prevalence of SNHL is about seven per 100,000 for younger children (Wellman et al., 2003), 75% of whom are younger than two years, 15% are 2–5 years, and 10% are older than five years (Koomen et al., 2003). However, SNHL is mainly associated with developmental delay and its underlying pathophysiological mechanisms are not yet well understood. Because sensory input plays a crucial role in human brain functionality, the early hearing deficit can affect the auditory cortex and its white matter fiber tracts, leading to cognitive decline in many domains, including language and learning (Oberg & Lukomski, 2011). Therefore, in order to prevent potential cognitive declines, and better treatment and assessment of cochlear implant candidacy, early detection of the brain microstructural changes is crucial (Seydell-Greenwald et al., 2014). With the continuous development of magnetic resonance technology, magnetic resonance imaging (MRI) has become a prominent modality to evaluate white matter lesions in the central nervous system. Diffusion tensor imaging (DTI) is an emerging MRI-based modality that can reveal microstructural changes in white matter pathways using specific parameters, such as mean diffusivity (MD) and axial diffusivity (AxD) which are assumed to reflect the breakdown of microstructural barriers and axonal damage (Qian et al., 2018). Radial diffusivity (RD) is specifically associated with myelin degeneration, and fractional anisotropy (FA) measures white matter integrity. DTI plays a key role in detecting early white matter changes associated with hearing loss (Tarabichi et al., 2018). The aim of the present study was to investigate the structural connectivity alterations in the brain white matter tracts of the auditory network in infants with bilateral profound SNHL who are under four years of age.
2. Materials and Methods
Subjects
A total of 22 subjects, including 11 children aged 1-4 years with SNHL leading to bilateral hearing loss and 11 age-matched controls were examined in this study. The patients were consecutively recruited. The healthy controls were recruited through local advertisements. Inclusion criteria were no structural abnormalities of the auditory system detected in conventional MRI images before DTI and no previous history of otological surgery. All subjects underwent audiometry, MRI, and DTI scanning (Table 1).

MRI protocol
Participants underwent a 3D T1-weighted BRAVO protocol, using a 1.5 T Optima MR 450w imager (GE, Milwaukee, WI, USA) with a 12-channel head coil. Whole-brain 3D T1-weighted images were obtained with the following imaging parameters: TR=1002 ms, TE=4.3 ms, voxel size=1×1×1 mm3, the field of view (FOV)=220 mm, flip angle=12°, and slice thickness=1 mm with no gaps. DTI data were obtained in the axial plane using a single-shot diffusion-weighted imaging sequence with 64 directions and the following parameters: b-value of 1000 s/mm2, TR=9275 ms, TE=79.3 ms, voxel size=1.09×1.09×2.4, 75 slices, slice thickness=2.4 mm, FOV=245 mm, and matrix size=256×256.
Image processing and tractography
DTI studio (Latest×64 bit version of DTI Studio, Johns Hopkins University) was used for preprocessing and deterministic fiber tracking. The atlas-based tractography was performed. The three main auditory pathway-related fibers, including acoustic radiation (AR), uncinate fasciculus (UF), and inferior longitudinal fasciculus (ILF) were selected. The tractography was done with a step size of 0 mm, minimum fiber length of 30 mm, and turning angle threshold of 60 (Figures 1, 2, 3 and 4). The DTI-derived parameters, FA, MD, axial diffusivity (AxD), and radial diffusivity (RD) were extracted from each tract. Also, the mean length and diameter of all fiber tracts were calculated automatically using morphological assessment of Tensor.




Statistical analysis
Data were analyzed by SPSS software, version 20. The comparisons of demographic and clinical data between two the groups in terms of imaging parameters and proof of hypotheses were performed using the paired t-test. The threshold for establishing significance was set at P<0.05.
3. Results
There was an increase in FA (P<0.0001) a decrease in RD (P<0.05) and a decrease in mean length and volume in the right and left AR tracts (P<0.0009). The decreased volume of right and left UF tracts (P<0.00025), including reduced trunk volume (P<0.0001) and reduced mean length (P<0.0001) were evident. A decrease in trunk volume and a decrease in the length (P<0.0011) and diameter of the right and left ILF (P<0.00024) as well as a decrease in FA and an increase in MD and RD parameters were seen in these tracts (P<0.0001) (Table 2, Figure 5).


4. Discussion
Radiological evaluation of children and adults with SNHL is usually performed by computed tomography (CT) or MRI to examine the temporal and retro-cochlear bone (Sterling et al., 2018). Although conventional and structured MRI is highly sensitive for the diagnosis of congenital anomalies, tumors of the internal auditory canal, and cochlear nerve anatomy, it does not provide information about the central auditory pathway (Glastonbury et al., 2002). DTI imaging is an MRI-based technique that can provide microstructural information about white matter connections in the central nervous system. The key to the usefulness of this imaging modality is the ability to detect changes and properties of white matter and brain tissue at the microscopic level. Disruption of biological barriers, such as myelin and cell membranes, which normally restrict the motion of water molecules, leads to measurable changes in the diffusion of water molecules (Uluǧ et al., 1999). Over the past five years, studies on the application of DTI in the evaluation of auditory white matter pathways have been increasing rapidly. The present study was done to evaluate the volume and characteristics of white matter in the auditory pathway-related fiber tracts in children with SNHL.
UF
This area is the largest pathway of the association fiber tract, which connects the frontal lobe to the temporal lobe. This area is said to be part of the limbic system. It plays a major role in language production (Von Der Heide et al., 2013). However, various studies have suggested the role of this tract in the processing of auditory information (Chern et al., 2020). Many studies have shown a decrease in white matter integrity in UF in patients with SNHL (Croll et al., 2020). In a study on the combined DTI and FMRI, a decrease in FA in UF was found in patients with SNHL aged 1-4 years compared to the normal group (Wang & Fan, 2019; Wang et al., 2020). It should be noted that there are many studies, in which the FA parameter was not significant in differentiating the two groups (Huang et al., 2015). In the present study, which examined the volume and characteristics of UF, a decrease in total volume, trunk volume, and diameter, and also a decrease in length was seen in this tract, which indicates the loss of some nerve fibers due to atrophy in this area. It has been shown that this damage leads to a decrease in the proper functioning of the auditory sense, resulting in hearing impairment. AxD value increased in this area, indicating a decrease in myelin sheath of white matter, atrophy, and integrity in this area (Figure 6).

AR
AR contains a group of highly myelinated axons and is one of the main sensory pathways in the brain that transmit auditory information from the thalamus to the auditory cortex. More precisely, the central auditory pathway begins in the cochlear branch of the eighth nerve of the brain and sends auditory information through the internal capsule to the temporal cortex (Hernández-Zamora & Poblano, 2014). AR has been very difficult to study due to its complex anatomy. With the advent of DTI, the study of this neural pathway in three dimensions has been facilitated, but still, the tractography of this area is challenging (Langguth et al., 2010). In younger patients with congenital deafness, many studies have been performed on volumetric measurement of auditory-related areas (Shibata et al., 2007). However, there are few studies on microstructural alterations in AR in hearing-related diseases, especially SNHL. In addition, AR tractography may be potentially helpful in assessing the integrity of the auditory pathway before and after cochlear implantation. Previous studies have reported a decrease in FA of the AR in patients with SNHL (Huang et al., 2015). In the present study, which examined the volume and diffusion characteristics of AR, the FA parameter showed the highest level of significance in differentiating the two groups from each other. It should be noted that the total volume, trunk volume, and length of this tract decreased, which indicates the existence of a specific pathology in the field of hearing. A very interesting finding in this study was the increase in FA. It indicates an increase in the integrity of this tract, as well as a decrease in MD, AxD, and RD, suggesting an increase in myelin and increased integrity in this area. The cause of this phenomenon can be a compensatory effect against the atrophy of the auditory pathways. To compensate for the effect of the damage, this tract started to remyelinate to be the most efficient with the least volume. There are few studies in this field regarding the increase in FA in AR (Lin et al., 2008).
ILF
This fiber tract connects the occipital lobe to the anterior part of the temporal lobe. This fiber overlaps with the UF in the anterior part and participates in the transmission of auditory information to the orbitofrontal part of the brain. This fiber is one of the pathways related to visual and linguistic information processing. DTI in combination with magnetoencephalography studies has shown its role in auditory processes and voice-language learning tests (Shin et al., 2019). In other words, ILF integrates information from the auditory and speech areas in the cortex (Mcmaster et al., 2011). Some studies on SNHL have shown a reduction in FA of ILF (Huang et al., 2015; Wang & Fan 2019). In the present study, a decrease in overall volume, trunk volume, diameter, and length was observed in ILF, indicating a decrease in nerve fibers due to atrophy, leading to hearing disorders. MD, RD, and AxD values increased, indicating a reduction in myelin sheath, atrophy, and integrity in this area (Figure 7).

5. Conclusion
In conclusion, the present study showed that microstructural changes in the fiber tracts associated with the auditory pathway can distinguish SNHL from healthy subjects. This study provides DTI-based biomarkers for brain changes and allows us to better understand the pathophysiological changes of related diseases. DTI is an emerging tool for evaluating the affected white matter and compensatory action related to the auditory system in patients with SNHL.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Science (Code: IR.AJUMS.MEDICINE.REC.1399.016) and written informed consent was obtained from the parents of all participants before the study.
Funding
This paper was extracted from master's thesis of Samira Sadeghinasab, approved by Department of Anatomical Science, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, and was financially supported by the Research Deputy of Ahvaz Jundishapur University of Medical Sciences (Grant No.: HRC‐9911).
Authors' contributions
Conceptualization and supervision: Ghasem Saki and Ali Reza Eftekhari Moghadam; Methodology: Samira Sadeghi; Data collection: Samira Sadeghi, Nader Saki, and Arash Bayat; Data analysis: Ali Reza Eftekhari Moghadam and Samira Sadeghi; Funding acquisition and resources: Ghasem Saki, Nader Saki; Investigation and writing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the head of Medical Imaging Center of Golestan Hospital for its cooperation, and the patients, without whom, this research could not be conducted.
References
Chen, M. M., & Oghalai, J. S. (2016). Diagnosis and management of congenital sensorineural hearing loss. Current Treatment Options in Pediatrics, 2(3), 256–265. [DOI:10.1007/s40746-016-0056-6] [PMID] [PMCID]
Chern, A., Irace, A. L., & Golub, J. S. (2020). Mapping the brain effects of hearing loss: The matter of white matter. JAMA Otolaryngology-- Head & Neck Surgery, 146(11), 1043–1044. [DOI:10.1001/jamaoto.2020.2528] [PMID]
Croll, P. H., Vernooij, M. W., Reid, R. I., Goedegebure, A., Power, M. C., & Rigters, S. C., et al. (2020). Hearing loss and microstructural integrity of the brain in a dementia-free older population. Alzheimer’s and Dementia, 16(11): 1515-23. [DOI:10.1002/alz.12151] [PMID]
Glastonbury, C. M., Davidson, H. C., Harnsberger, H. R., Butler, J., Kertesz, T. R., & Shelton, C. (2002). Imaging findings of cochlear nerve deficiency. American Journal of Neuroradiology, 23(4), 635–643. [PMID] [PMCID]
Hernández-Zamora, E., & Poblano, A. (2014). [The auditory pathway: Levels of integration of information and principal neurotransmitters (Spanish)]. Gaceta Medica de Mexico, 150(5), 450–460. [PMID]
Huang, B. Y., Zdanski, C., & Castillo, M. (2012). Pediatric sensorineural hearing loss, part 2: Syndromic and acquired causes. American Journal of Neuroradiology, 33(3), 399–406. [DOI:10.3174/ajnr.A2499] [PMID] [PMCID]
Huang, L., Zheng, W., Wu, C., Wei, X., Wu, X., & Wang, Y., et al. (2015). Diffusion tensor imaging of the auditory neural pathway for clinical outcome of cochlear implantation in pediatric congenital sensorineural hearing loss patients. Plos One, 10(10), e0140643. [DOI:10.1371/journal.pone.0140643] [PMID] [PMCID]
Koomen, I., Grobbee, D. E., Roord, J. J., Donders, R., Jennekens-Schinkel, A., & van Furth, A. M. (2003). Hearing loss at school age in survivors of bacterial meningitis: Assessment, incidence, and prediction. Pediatrics, 112(5), 1049–1053. [DOI:10.1542/peds.112.5.1049] [PMID]
Langguth, B., Kleinjung, T., Landgrebe, M., de Ridder, D., & Hajak, G. (2010). rTMS for the treatment of tinnitus: The role of neuronavigation for coil positioning. Neurophysiologie Clinique, 40(1), 45–58. [DOI:10.1016/j.neucli.2009.03.001] [PMID]
Lin, Y., Wang, J., Wu, C., Wai, Y., Yu, J., & Ng, S. (2008). Diffusion tensor imaging of the auditory pathway in sensorineural hearing loss: Changes in radial diffusivity and diffusion anisotropy. Journal of Magnetic Resonance Imaging, 28(3), 598–603. [DOI:10.1002/jmri.21464] [PMID]
Oberg, E., & Lukomski, J. (2011). Executive functioning and the impact of a hearing loss: Performance-based measures and the Behavior Rating Inventory of Executive Function (BRIEF). Child Neuropsychology, 17(6), 521–545. [DOI:10.1080/09297049.2011.555760] [PMID]
Qian, Y., Zhong, S., Hu, G., Kang, H., Wang, L., & Lei, Y. (2018). Sudden Sensorineural Hearing Loss in Children: A Report of 75 Cases. Otology & neurotology: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology, 39(8), 1018–1024. [DOI:10.1097/MAO.0000000000001891] [PMID]
Seydell-Greenwald, Anna et al. 2014. “Diffusion Imaging of Auditory and Auditory-Limbic Connectivity in Tinnitus: Preliminary Evidence and Methodological Challenges.” Neural Plasticity 2014. [DOI:10.1155/2014/145943] [PMID]
Shibata, D. K. (2007). Differences in brain structure in deaf persons on MR imaging studied with voxel-based morphometry. American Journal of Neuroradiology, 28(2), 243-249. [PMID]
Shin, J., Rowley, J., Chowdhury, R., Jolicoeur, P., Klein, D., & Grova, C., et al. (2019). Inferior longitudinal fasciculus' role in visual processing and language comprehension: A combined MEG-DTI study. Frontiers in Neuroscience, 13, 875. [DOI:10.3389/fnins.2019.00875] [PMID] [PMCID]
Sterling, M. R., Lin, F. R., Jannat-Khah, D. P., Goman, A. M., Echeverria, S. E., & Safford, M. M. (2018). Hearing Loss Among Older Adults With Heart Failure in the United States: Data From the National Health and Nutrition Examination Survey. JAMA Otolaryngology-- Head & Neck Surgery, 144(3), 273–275. [DOI:10.1001/jamaoto.2017.2979] [PMID]
Tarabichi, O., Kozin, E. D., Kanumuri, V. V., Barber, S., Ghosh, S., & Sitek, K. R., et al. (2018). Diffusion tensor imaging of central auditory pathways in patients with sensorineural hearing loss: A systematic review. Otolaryngology--Head and Neck Surgery, 158(3), 432–442. [DOI:10.1177/0194599817739838] [PMID] [PMCID]
Uluğ, A. M., Moore, D. F., Bojko, A. S., & Zimmerman, R. D. (1999). Clinical use of diffusion-tensor imaging for diseases causing neuronal and axonal damage. American Journal of Neuroradiology, 20(6), 1044–1048. [PMID] [PMCID]
Von Der Heide, R. J., Skipper, L. M., Klobusicky, E., & Olson, I. R. (2013). Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain, 136(Pt 6), 1692–1707. [DOI:10.1093/brain/awt094] [PMID] [PMCID]
Wang, S., Chen, B., Yu, Y., Yang, H., Cui, W., & Li, J., et al. (2019). Alterations of structural and functional connectivity in profound sensorineural hearing loss infants within an early sensitive period: A combined DTI and fMRI study. Developmental Cognitive Neuroscience, 38, 100654. [DOI:10.1016/j.dcn.2019.100654] [PMID] [PMCID]
Wang, S., Chen, B., Yu, Y., Yang, H., Cui, W., & Li, J., et al. (2020). Corrigendum to "alterations of structural and functional connectivity in profound sensorineural hearing loss infants within an early sensitive period: A combined DTI and fMRI study" [Dev. Cogn. Neurosci. 38 (2019) 100654]. Developmental cognitive neuroscience, 41, 100689. [DOI::10.1016/j.dcn.2019.100689] [PMID]
Wellman, M. B., Sommer, D. D., & McKenna, J. (2003). Sensorineural hearing loss in postmeningitic children. Otology & Neurotology, 24(6), 907–912. [DOI:10.1097/00129492-200311000-00015] [PMID]
Zahnert T. (2011). The differential diagnosis of hearing loss. Deutsches Arzteblatt International, 108(25), 433–444. [DOI:10.3238/arztebl.2011.0433] [PMID] [PMCID]
Type of Study: Original |
Subject:
Clinical Neuroscience
Received: 2022/02/10 | Accepted: 2023/04/3 | Published: 2023/11/1
Received: 2022/02/10 | Accepted: 2023/04/3 | Published: 2023/11/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








