Volume 15, Issue 3 (May & Jun 2024)
BCN 2024, 15(3): 343-354 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Aydıntuğ-Gürbüz T, Toprak F, Toprak S, Sözer S. The Effects of IGF1 and MGF on Neural Stem Cells in Hypoxic Conditions. BCN 2024; 15 (3) :343-354
URL: http://bcn.iums.ac.ir/article-1-2407-en.html
URL: http://bcn.iums.ac.ir/article-1-2407-en.html
1- Department of Genetics, Aziz Sancar Research Institute of Experimental Medicine, Iıstanbul University, Çapa Campus, Iıstanbul, Turkey.
2- Department of Neurosurgery, Haydarpaşa Numune Training and Research Hospital, Istanbul, Turkey.
2- Department of Neurosurgery, Haydarpaşa Numune Training and Research Hospital, Istanbul, Turkey.
Keywords: Neural stem cell (NSC), Hypoxia, Growth factors, Mechano growth factor (MGF), Insulin-like growth factor 1 (IGF-I)
Full-Text [PDF 2248 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
An ischemic stroke results from insufficient blood flow and causes cells in the tissue to be exposed to low oxygen (O2) levels (hypoxia). The effect of such changes and impairment of the normal cellular function of the tissue inevitably progresses to severe diseases, including stroke and death in humans (Kalogeris et al., 2012; Østergaard et al., 2016). Ischemic tissue damage has a complex pathophysiology that can affect many different cells and tissues. Such neurons require a constant supply of O2 to maintain ionic gradients across their membrane for impulse conduction and are vulnerable to hypoxic changes (Chamorro et al., 2016). The brain consumes 25% of the O2 requirement of the whole body, revealing O2 as one of the most critical factors for the brain (Steiner, 2019). The deficit in the vascular flow causes a decrease in the intracellular adenosine triphosphate levels that initiates apoptosis within 2-3 min after energy deprivation (Lee et al., 2000). The death of oligodendrocytes, astrocytes, endothelial cells, and various neuron types leads to the loss of regional tissue in the brain (Xu et al., 2020). Therefore, neuronal damage triggered by cerebral ischemia/reperfusion injury is the primary cause of the neurological disorder. In such cases, supporting neurogenesis is an essential factor for the success of the recovery of cerebral function. Neural stem cells (NSC) could regenerate the central nervous system with the ability to differentiate into astrocytes, neurons, and oligodendrocytes (Kriegstein & Alvarez-Buylla, 2009). Neurogenesis occurs in the specialized microenvironment of NSCs. Thus, the NSC niche plays an essential role in nerve regeneration and repair of damaged brain tissue (Decimo et al., 2012). Stem cells migrate to damaged areas (Boltze et al., 2020). Recent studies revealed that both endogenous and transplanted NSC could be activated by cerebral ischemia and have a role in neural regeneration (Zhang et al., 2019). Therefore, understanding the cellular biology of NSCs in the hypoxic environment in an in vitro ischemia model may provide new opportunities for controlling the expansion and proliferation of NSCs, which could lead to improving new therapies after ischemic stroke.
The O2 concentration of the niche is estimated to be 3% (Sharee Ghourichaee & Leach, 2016), whereas, in alveolar lung cells, it is 20% (Yee et al., 2016), and in arterial blood flow the rate is 10.5% to 13% (Williams, 1998). In some studies, low oxygen levels (~1% O2) have led to cellular damage and death of the quiescent NSC as detected in brain ischemia (Zhang et al., 2011). However, some findings suggest that the reduction of O2 levels in the microenvironment, for instance, 3% to 5%, regulates the biological properties of NSCs in vitro and initiates their proliferation and differentiation ability. The stroke studies performed in rodents and primates have increased neurogenesis in their brain’s sub-ventricular and sub-granular regions (Ceanga et al., 2021; Paredes et al., 2016).
Insulin-like growth factor-1 (IGF-I) is a neurotrophic factor for the repair and development of neurons, and numerous in vivo and in vitro studies revealed its effect on NSC development (Nieto-Estevez et al., 2016). IGF-I promotes cell proliferation, differentiation, and survival (Tunç et al., 2021). IGF-I creates different messenger ribonucleic acid variants due to alternative splicing in many tissues, especially in skeletal and cardiac muscle, liver, and brain (Oberbauer, 2013). At least three alternative splice variants of IGF-1 have been demonstrated. IGF-1 Ea, which acts systemically, IGF-1 Eb, and IGF-1 Ec, activated with mechanical damage and expressed in a mechano-sensitive manner, was named the mechano growth factor (MGF) (Bailes & Soloviev, 2021; Goldspink, 2005). MGF stimulates muscle stem cells to re-enter the cell cycle, initiate proliferation, and further support new muscle cells (Ates et al., 2007; Matheny et al., 2010). MGF expression has been detected in damaged tissue, including the brain and heart, after various stress conditions, such as ischemia (Bailes & Soloviev, 2021; Dluzniewska et al., 2005), particularly in the damage-resistant region. The endogenous MGF expression was also detected in proliferating cells in neurogenic niches and also hypoxic neuroblastoma cells in an in vitro ischemic stroke model (Canazza et al., 2014; Dluzniewska et al., 2005; Tang et al., 2017), which all had a role in neuroprotection (Aperghis et al., 2004; Dluzniewska et al., 2005). Furthermore, studies have shown that exogenous administration of MGF and IGF-1 reduces ischemic brain damage (Dluzniewska et al., 2005).
Transcription factor hypoxia-inducible factor-1 (HIF-1) is essential in regulating many hypoxia-activated genes across many different types of cells (Wiesener et al., 2003). HIF-1 binds to hypoxia-sensitive elements located in the promoter region of target genes and is thus a pleiotropic transcription factor that controls their transcription (Baddela et al., 2020). HIF-1 consists of HIF-1α and HIF-1β subunits. The mRNAs of HIF-1α and HIF-1β are constitutively expressed in cells (Wang & Semenza, 1995). However, HIF-1α protein expression is tightly regulated by changes in cellular oxygen and growth factors, including IGF-I, IGF-II, and angiotensin II (Masoud & Li, 2015). It is primarily regulated at the level of protein stability (Huang et al., 1996). In normal O2 levels (normoxia), HIF-1α is rapidly degraded via targeted ubiquitination, followed by its subsequent degradation by the proteasome (Salceda & Caro, 1997). In response to hypoxia, HIF-1α becomes rapidly stabilized, localized to the nucleus, and forms the HIF-1 complex with HIF-1β (Masoud & Li, 2015; Ziello et al., 2007). Recent studies have shown that, especially in tumors, the hypoxic cells exhibit resistance to apoptosis and these tumor cells were more aggressive, probably due to the overexpression of HIF-1α (Jing et al., 2019). Therefore, there has been an increase in the strategies to develop modalities that target HIF-1 activity and might become an alternative treatment strategy in cancerogenesis within recent years (Chau et al., 2005; Ma et al., 2020).
Although these studies showed a neuroprotective role for IGF-I and MGF, the function of IGF-I and MGF under varying oxygen levels is still not well understood. In this study, growth factors, IGF-I and MGF, are investigated on NSC under varying oxygen levels. Accordingly, an in vitro ischemia model was created by exposing adult hippocampal rat NSC to varying levels of O2, representing anoxia, hypoxia, and normoxia conditions. The effects of varying O2 levels on NSC gene expressions, specifically IGF-I, MGF, and HIF-1α, were investigated. The external administration of growth factors, IGF-I and MGF, to NSC exposed to such conditions are performed, and their effects on gene expressions and NSC proliferation are examined. This study is the first to reveal the effect of MGF and IGF-I in a hypoxic environment and further inquiries about their possible therapeutic application in such conditions.
2. Materials and Methods
NSC culture
Adult rat hippocampal NSCs line (Sigma-Aldrich, Merck, Germany) were cultured at a density of 6×105 cell/mL and 300 μL/well culture media in Poly-L-Ornithine (Sigma-Aldrich) (10 mg/mL) and Laminin (Sigma-Aldrich) (6 µg/mL) coated cell culture plate. NSC culture media contained B-27 supplement (Invitrogen), DMEM/F12 (Biochrom), 100 μg/mL streptomycin (1%), l-glutamine (200 mM), and FGF-2 (20 ng/mL) (PeproTech) as detailed explained elsewhere (Aydıntug-Gurbuz et al., 2023). FGF-2 was added fresh to the medium every time. NSCs were incubated at 37°C in 5% CO2. When NSCs reached 70%-80% confluence in the flask, the cells were transferred to the plates for the following experiments.
Oxygen exposure of NSCs
The Cell ASIC ONIX Microfluidic System (Merck) was applied to investigate the different O2 levels, including 0%, 3%, and 20%, and growth factors, including IGF-I and MGF on NSC in vitro, as summarized in Figure 1. M04S-03 microfluidic plates for cell ASIC ONIX (Merck) were coated with poly-l-ornithine (Sigma) and laminin (Sigma) as described previously (Croushore & Sweedler, 2013). This system provides the ability to control the environment of individual plates and provides simultaneous analysis of the living cells in vitro. Since it is designed to provide a dynamic cellular microenvironment, namely niche control, it is suitable for studies investigating extracellular factors’ effects on cells. NSCs were seeded at 1×104 cells per well, and the manufacturer applied a gravity-driven perfusion culture protocol. After 24 h of incubation, the stabilized cells were treated with growth factors following the experiment design. DMEM/F12 solutions were referred to as untreated. In external administration of growth factors, IGF-I 0.2 µg/mL or MGF 0.2 µg/mL were prepared and added to the relevant wells. The O2 levels were adjusted according to the experiment design as follows: Anoxia with 0% O2, hypoxia with 3% O2, and normoxia with 20% O2. Culture plates were connected to the Cell ASIC device with the manifold’s help, following the manufacturer’s instructions (Croushore & Sweedler, 2013). The experiments were set to 24 h in the software available on the computer. Readouts were analyzed with the program integrated into the software.
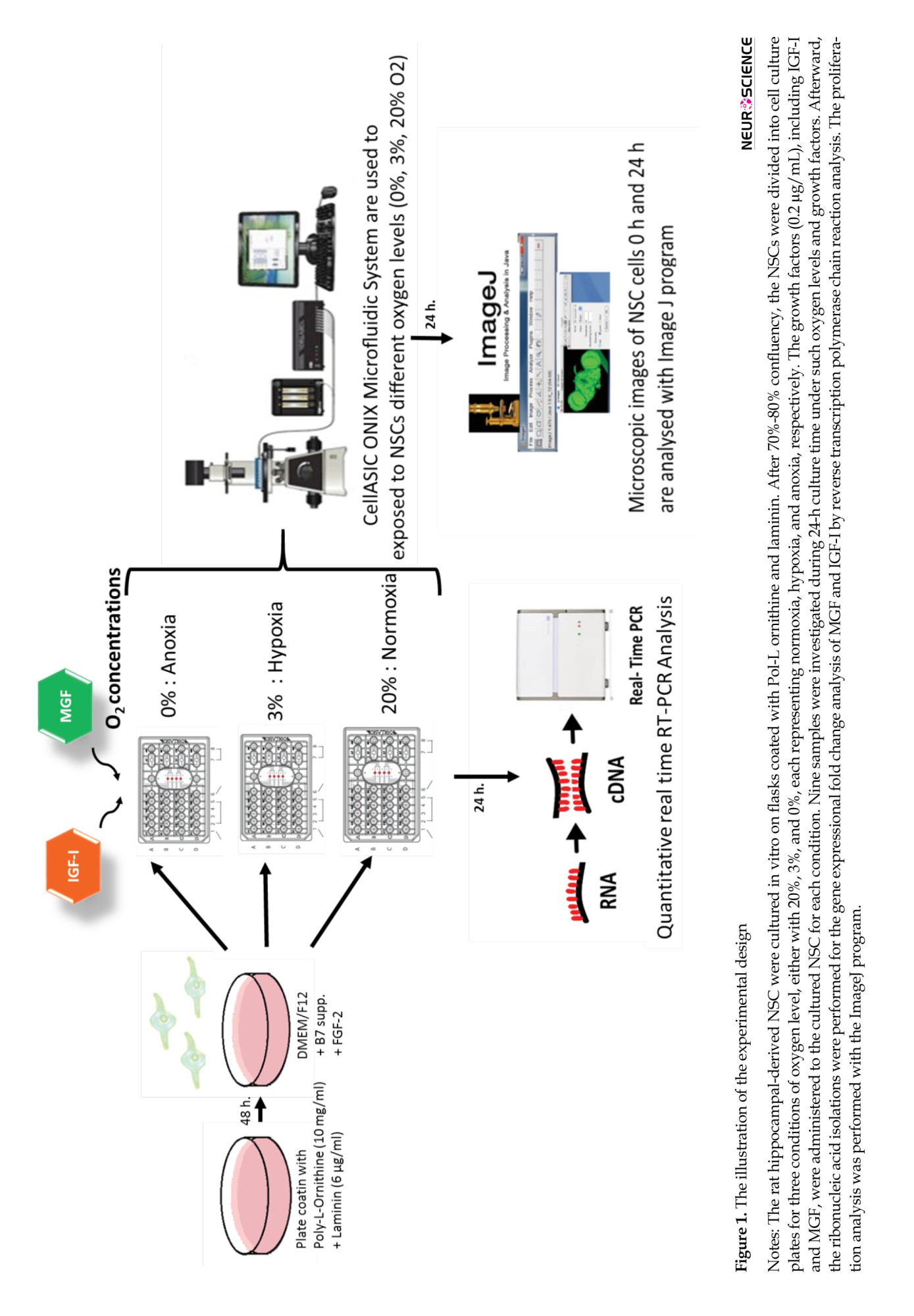
Cell proliferation assay
NSC-exposed growth factors, IGF-I and MGF, were analyzed after 24 h. The image program was used to measure cell proliferation. Phase-contrast images of NSCs were taken from the culture plate under an inverted microscope (Olympus CKX41) equipped with a digital camera with a 10x objective before starting the experiment (0 h) and after the experiment was concluded (24 h). The images were analyzed with the ImageJ software, version 1.8.0 (developed by W.S. Rasband, NIH). Since the culture time for NSC in defined conditions was estimated to be 24 h in this study, instead of counts of neurospheres, the effect of O2 levels and growth factors on NSC in the migration-proliferation without disturbing such O2 levels in culture conditions, the total area of the seeded cells was measured and compared. The analysis was performed on the images that could provide information for the functions of both migration and proliferation of NSC. By doing so, the area of the cells was measured with ImageJ. The calculations were performed using the Equation 1:
1.
Area of the cultured cells after 24 h/Area of the cultured cells in 0 h in defined conditions
Ribonucleic acid isolation, cDNA synthesis, and real-time polymerase chain reaction
Total RNA isolation was performed using a total RNA purification kit (Jena Bioscience) in NSC. SCRIPT cDNA Synthesis Kit (Jena Bioscience) was used for cDNA synthesis from 50 ng/µL RNA. IGF-1Ea’s forward primer was 5’-GCT TGC TCA CCT TTA CCA GC-3,’ and the reverse primer was 5’-AAG TGT ACT TCC TTC TGA GTC T-3’ with 130 base pairs (bp) in length. MGF’s forward primer was 5’-GCT TGC TCA CCT TTA CCA GC-3,’ and the reverse primer was 5’-AAG TGT ACT TCC TTT CCT TCT C-3’ (130 bp). HIF1-a forward primer was 5’ TGC TTG GTG CTG ATT TGT GA 3’and the reverse primer was 5’-GGT CAG ATG ATC AGA GTC CA-3’ (131 bp). GAPDH was applied as a housekeeping control. GAPDH’s forward primer was 5’GGT GTG AAC GGA TTT GGC CGT AT-3’and reverse primer 5’CTC AGC ACC AGC GTC ACC CCA TT3’ (129 bp).
Changes in each gene expression were detected with Sensi FAST SYBR No-ROX Kit (Bioline, UK) by the real-time quantitative RT-PCR (Light cycler 480, Roche Diagnostics). These specific primers were applied for amplification. The fluorescence emitted by the dye above the baseline signal was detected using the software in real time, recorded, and represented as the cycle threshold (CT). The arithmetic mean values of CTs, which were performed twice, were calculated for the statistical analysis. All samples were studied in duplicate. The samples were not treated with the growth factor, but the media were evaluated as the control sample. RNA samples directly isolated from a rat hippocampal region were also applied as positive controls in RT-PCR analysis.
Statistical analysis
The ΔΔCT method was used to determine the gene expression fold change with the results obtained from RT-PCR. CT values of target mRNAs were normalized according to the housekeeping GAPDH gene (Equation 2):
2. ΔCT=CTTarget–CTGAPDH
Some analysis for the target gene expression changes was performed by the Equation 3:
3. 2-ΔCT×100
For the fold change of target gene expressions analysis, the values were normalized to the control (Equation 4):
4. ΔΔCT=ΔCT–CTControl
and the Equation 5 was applied:
5. 2-ΔΔCT
The variables were used as Mean±SD and percentage and frequency values. Meanwhile, P<0.05 and P<0.01 levels were considered statistically significant.
3. Result
The initiation of neurogenesis after ischemia is the essential recovery opportunity for treatment success, and NSC is a crucial element in such post-ischemic repair. In this study, the external administration of the IGF-I and MGF was performed on NSC, exposed to various oxygen concentrations, including normoxia, hypoxia, and anoxia, with 20%, 3%, and 0%, respectively, as summarized in Table 1. During 24 h, their effects on the molecular function of NSC employing IGF-I and MGF and HIF-1α gene expression changes and NSC proliferation-migration rates were investigated (Figure 1).
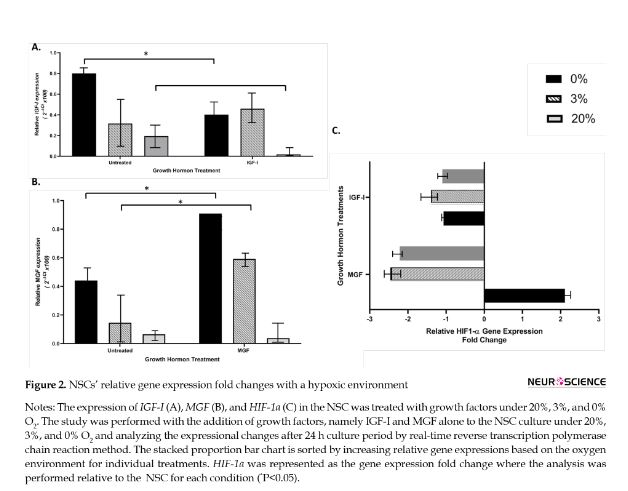
Low levels of oxygen induce IGF-I and MGF gene expressions
The quantitative analysis of real-time RT-PCR was performed to determine the changes in IGF-I and MGF expressions during 24 h of NSC culture exposed to different O2 concentrations with growth factors.
There was a significant change in IGF-I expression relative to oxygen levels. The highest rate that induced IGF-I expression was determined with the 0% O2 following hypoxic and normoxia concentrations, 0.8, 0.3, and 0.1, respectively. The external administration of IGF-I reduced the IGF-I expression to 0% and 20% O2 levels (P<0.05). The IGF-I application at the hypoxic level did not reveal any significant change in IGF-I expression (Figure 2A).
The MGF expression was determined to be affected by O2. The highest rate of MGF expression was determined with the 0% O2 following 3% and 20% O2 levels, 0.4, 0.1, and 0.01, respectively. There was a statistically significant difference in MGF expression between 0% and 20% O2 levels (P<0.05). The induction of MGF expression with lower O2 levels was even more significant after administration of MGF in NSC culture in such conditions (Figure 2B).
The analysis of HIF1-α expression in such conditions revealed that the application of IGF-I lowered its relative fold change expression in all O2 levels. The application of MGF achieved this downregulation with 20% and 3% O2 levels. In contrast, MGF application induces upregulation in the HIF1-α relative fold change expression at the anoxic level (Figure 2C).
IGF-I and MGF promotes NSCs proliferation-migration in varying levels of oxygen
In physiological O2 concentrations (3%-5%) of the brain, the cultured NSC increases the proliferation of and modulates to differentiate into neurons. Regarding the effect of oxygen levels on NSC proliferation-migration, we have applied a culture method by measuring the area of the cultured NSC from images at 0 and 24 h (Figure 3A-B). Comparing the area between time intervals provided evidence for the NSC’s proliferation–migration under such conditions. Although it was not statistically significant, there was a slight increase in proliferation-migration of NSC in untreated NSC within 24 h of culture at a 3% O2 level. IGF-I is known to support neuronal survival during development as well as neuronal damage. Meanwhile, IGF-I-treated NSC in anoxia and normoxia conditions revealed a statistically significant increase in proliferation-migration. However, hypoxia reduced this rate (Figure 3C). According to the literature, MGF is a mitogen for neural cells and has neuroprotective effects in the ischemic brain model. Although it is not statistically significant, we have found that MGF induces NSC proliferation-migration in all conditions within 24 h compared to untreated NSC (Figure 3C).

4. Discussion
In mammalian CNS, oxygen homeostasis plays an essential role in the proliferation and differentiation of NSCs. Even small changes in the O2 level might affect dynamic balance and disturb cellular physiology and survival by affecting signal transduction pathways that control cell proliferation, fate and survival, and tissue and organ morphogenesis and regeneration (Fathollahipour et al., 2018; Zhou et al., 2014). Although low O2 levels are associated with pathological conditions in various tissues, in the hippocampus, where NSCs are commonly located, the estimated O2 level is around 3%-4% from early embryogenesis. NSC maintains its stability in a hypoxic environment and this physiological hypoxia promotes the growth, survival, and maintenance of the multipotent properties of NSC (Harvey et al., 2004; Morrison et al., 2000; Studer et al., 2000). Although the consequence of the ischemia is detrimental to homeostasis, the studies on the role of NSC in ischemic conditions are still insufficient in the literature.
Furthermore, the role of growth factors in a hypoxic environment on NSC remains elusive. Intrathecal administration of IGF-I had a protective effect on neurons in rats after hypoxic ischemia (O’Kusky & Ye, 2012). MGF blocks the apoptosis of damaged myocytes and stem cells and can protect myocytes and neurons from hypoxia (Chiong et al., 2011; Yoshida & Delafontaine, 2020). Meanwhile, MGF is markedly more effective than IGF-I (Aperghis et al., 2004). Under these consequences, it might be relevant to consider NSC conditioned with growth factors that have stimulant effects as an effective modality in stroke treatment. Therefore, it is essential to clarify the role of varying O2 environments on NSC proliferation and the effects of growth factors. Based on these discoveries, IGF-I and MGF gene expressions in NSCs exposed to varying O2 levels could provide essential evidence regarding IGF-I and MGF growth factor roles in NSC and whether external application of IGF-I and MGF might provide any effect, including NSC proliferation that would benefit the treatment modalities of ischemic stroke.
There was a significant upregulation in IGF-I expression relative to oxygen levels. The 0% O2 levels induce the IGF-I expression at the highest compared to 3% O2 levels and 20% O2. This result is in line with the literature, where the effect of IGF-I has been induced in damaged brain regions after cerebral ischemia (O’Kusky & Ye, 2012). The external application of IGF-I has lowered its expression in 0% and 20% O2 conditions but saved the 3% O2. The proliferation-migration data supported this result by revealing the highest proliferation-migration rate within IGF-I application in 0% and 20% O2 conditions. Mild hypoxia enhances the proliferation of human NSCs (Santilli et al., 2010). These findings suggest that the 3% O2, the average oxygen level in the central nervous system, does not induce NSC proliferation, retain the cells in a state of quiescence, and IGF-I is ineffective in such an environment. In contrast, reduced oxygen levels such as 0% O2 occur in neuronal disorders like cerebral ischemia, transiently leading to NSC proliferation with induction of IGF-I expressions. The MGF expression relative to oxygen levels was similar to IGF-I, where the highest MGF expression was detected in the 0% O2, compared to 3% O2 and 20% O2 levels.
On the other hand, the MGF administration dramatically increased the MGF expression in 0% and 3% O2. The proliferation-migration rate with MGF external application revealed not significant, but the upregulated rate of NSC. These results were in line with the literature where MGF overexpression increases the number of neural progenitor cells and promotes neurogenesis in transgenic mice that constitutively overexpresses MGF from birth (Tang et al., 2017). It has also been previously found that exogenous MGF and IGF-I increase NSC proliferation during damage. Although MGF is known for its regenerative capability in skeletal muscle, mononucleated progenitors, and its neuroprotective effect in vivo and in vitro (Dluzniewska et al., 2005; Ates et al., 2007; Quesada et al., 2009). This study is the first finding revealing the MGF and IGF-I effect on varying O2 levels. At the same time, when MGF and IGF-I were administered externally to NSC exposed to anoxia and hypoxia, it was determined that these two growth factors positively affect the proliferation of NSC. The data might all provide a significant advantage and a valuable tool for growth hormones, IGF-I, and MGF-mediated NSC therapy in ischemic stroke as well as for neurodegenerative diseases like Parkinson’s disease, multiple sclerosis, and Alzheimer.
Furthermore, HIF-1-α induction correlated with changes in cellular oxygen and growth factors, including IGF-I, was also confirmed in this study, where relative HIF-1-α gene expression fold change was suppressed with IGF-I administration in varying O2 levels. Surprisingly, the MGF gene expression was increased by 0% O2. This study is the first evidence indicating the role of MGF in an anoxic environment. It might suggest that targeting HIF-1 as a novel small molecule inhibitors might be an attractive strategy for therapeutic development. This study was designed to search for the environmental effects within 24 hours. Prolonged and or intermittent exposure to hypoxia might reveal diverse findings since more complex pathways will be expected to enroll in such chronic condition (Khuu et al., 2019).
5. Conclusion
Overall, this study shed light on using exogenous MGF and IGF-I administration for their neuroprotective and proliferative effects on NSC for the harmful effects of ischemic stroke. More in vitro and in vivo intensive studies are essential to understand the role of growth factors for such treatment modalities in ischemia.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This work was supported by the Scientific Research Projects Coordination Unit of Istanbul University (Project No.: 58165).
Authors' contributions
Conceptualization: Tuğba Aydıntuğ-Gürbüz and Selçuk Sözer; Supervision: Selçuk Sözer; Materials, data collection and processing: Tuğba Aydıntuğ-Gürbüz, Selin Toprak and Selçuk Sözer; Data analysis and interpretation: Fatih Toprak, Selin Toprak and Selçuk Sözer; Investigation: Tuğba Aydıntuğ-Gürbüz, Fatih Toprak and Selin Toprak; Writing the original draft: Fatih Toprak, Selin Toprak and Selçuk Sözer; Review and editing: Selçuk Sözer and Fatih Toprak.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
In honor of Kenan Ates, rest in peace.
References
An ischemic stroke results from insufficient blood flow and causes cells in the tissue to be exposed to low oxygen (O2) levels (hypoxia). The effect of such changes and impairment of the normal cellular function of the tissue inevitably progresses to severe diseases, including stroke and death in humans (Kalogeris et al., 2012; Østergaard et al., 2016). Ischemic tissue damage has a complex pathophysiology that can affect many different cells and tissues. Such neurons require a constant supply of O2 to maintain ionic gradients across their membrane for impulse conduction and are vulnerable to hypoxic changes (Chamorro et al., 2016). The brain consumes 25% of the O2 requirement of the whole body, revealing O2 as one of the most critical factors for the brain (Steiner, 2019). The deficit in the vascular flow causes a decrease in the intracellular adenosine triphosphate levels that initiates apoptosis within 2-3 min after energy deprivation (Lee et al., 2000). The death of oligodendrocytes, astrocytes, endothelial cells, and various neuron types leads to the loss of regional tissue in the brain (Xu et al., 2020). Therefore, neuronal damage triggered by cerebral ischemia/reperfusion injury is the primary cause of the neurological disorder. In such cases, supporting neurogenesis is an essential factor for the success of the recovery of cerebral function. Neural stem cells (NSC) could regenerate the central nervous system with the ability to differentiate into astrocytes, neurons, and oligodendrocytes (Kriegstein & Alvarez-Buylla, 2009). Neurogenesis occurs in the specialized microenvironment of NSCs. Thus, the NSC niche plays an essential role in nerve regeneration and repair of damaged brain tissue (Decimo et al., 2012). Stem cells migrate to damaged areas (Boltze et al., 2020). Recent studies revealed that both endogenous and transplanted NSC could be activated by cerebral ischemia and have a role in neural regeneration (Zhang et al., 2019). Therefore, understanding the cellular biology of NSCs in the hypoxic environment in an in vitro ischemia model may provide new opportunities for controlling the expansion and proliferation of NSCs, which could lead to improving new therapies after ischemic stroke.
The O2 concentration of the niche is estimated to be 3% (Sharee Ghourichaee & Leach, 2016), whereas, in alveolar lung cells, it is 20% (Yee et al., 2016), and in arterial blood flow the rate is 10.5% to 13% (Williams, 1998). In some studies, low oxygen levels (~1% O2) have led to cellular damage and death of the quiescent NSC as detected in brain ischemia (Zhang et al., 2011). However, some findings suggest that the reduction of O2 levels in the microenvironment, for instance, 3% to 5%, regulates the biological properties of NSCs in vitro and initiates their proliferation and differentiation ability. The stroke studies performed in rodents and primates have increased neurogenesis in their brain’s sub-ventricular and sub-granular regions (Ceanga et al., 2021; Paredes et al., 2016).
Insulin-like growth factor-1 (IGF-I) is a neurotrophic factor for the repair and development of neurons, and numerous in vivo and in vitro studies revealed its effect on NSC development (Nieto-Estevez et al., 2016). IGF-I promotes cell proliferation, differentiation, and survival (Tunç et al., 2021). IGF-I creates different messenger ribonucleic acid variants due to alternative splicing in many tissues, especially in skeletal and cardiac muscle, liver, and brain (Oberbauer, 2013). At least three alternative splice variants of IGF-1 have been demonstrated. IGF-1 Ea, which acts systemically, IGF-1 Eb, and IGF-1 Ec, activated with mechanical damage and expressed in a mechano-sensitive manner, was named the mechano growth factor (MGF) (Bailes & Soloviev, 2021; Goldspink, 2005). MGF stimulates muscle stem cells to re-enter the cell cycle, initiate proliferation, and further support new muscle cells (Ates et al., 2007; Matheny et al., 2010). MGF expression has been detected in damaged tissue, including the brain and heart, after various stress conditions, such as ischemia (Bailes & Soloviev, 2021; Dluzniewska et al., 2005), particularly in the damage-resistant region. The endogenous MGF expression was also detected in proliferating cells in neurogenic niches and also hypoxic neuroblastoma cells in an in vitro ischemic stroke model (Canazza et al., 2014; Dluzniewska et al., 2005; Tang et al., 2017), which all had a role in neuroprotection (Aperghis et al., 2004; Dluzniewska et al., 2005). Furthermore, studies have shown that exogenous administration of MGF and IGF-1 reduces ischemic brain damage (Dluzniewska et al., 2005).
Transcription factor hypoxia-inducible factor-1 (HIF-1) is essential in regulating many hypoxia-activated genes across many different types of cells (Wiesener et al., 2003). HIF-1 binds to hypoxia-sensitive elements located in the promoter region of target genes and is thus a pleiotropic transcription factor that controls their transcription (Baddela et al., 2020). HIF-1 consists of HIF-1α and HIF-1β subunits. The mRNAs of HIF-1α and HIF-1β are constitutively expressed in cells (Wang & Semenza, 1995). However, HIF-1α protein expression is tightly regulated by changes in cellular oxygen and growth factors, including IGF-I, IGF-II, and angiotensin II (Masoud & Li, 2015). It is primarily regulated at the level of protein stability (Huang et al., 1996). In normal O2 levels (normoxia), HIF-1α is rapidly degraded via targeted ubiquitination, followed by its subsequent degradation by the proteasome (Salceda & Caro, 1997). In response to hypoxia, HIF-1α becomes rapidly stabilized, localized to the nucleus, and forms the HIF-1 complex with HIF-1β (Masoud & Li, 2015; Ziello et al., 2007). Recent studies have shown that, especially in tumors, the hypoxic cells exhibit resistance to apoptosis and these tumor cells were more aggressive, probably due to the overexpression of HIF-1α (Jing et al., 2019). Therefore, there has been an increase in the strategies to develop modalities that target HIF-1 activity and might become an alternative treatment strategy in cancerogenesis within recent years (Chau et al., 2005; Ma et al., 2020).
Although these studies showed a neuroprotective role for IGF-I and MGF, the function of IGF-I and MGF under varying oxygen levels is still not well understood. In this study, growth factors, IGF-I and MGF, are investigated on NSC under varying oxygen levels. Accordingly, an in vitro ischemia model was created by exposing adult hippocampal rat NSC to varying levels of O2, representing anoxia, hypoxia, and normoxia conditions. The effects of varying O2 levels on NSC gene expressions, specifically IGF-I, MGF, and HIF-1α, were investigated. The external administration of growth factors, IGF-I and MGF, to NSC exposed to such conditions are performed, and their effects on gene expressions and NSC proliferation are examined. This study is the first to reveal the effect of MGF and IGF-I in a hypoxic environment and further inquiries about their possible therapeutic application in such conditions.
2. Materials and Methods
NSC culture
Adult rat hippocampal NSCs line (Sigma-Aldrich, Merck, Germany) were cultured at a density of 6×105 cell/mL and 300 μL/well culture media in Poly-L-Ornithine (Sigma-Aldrich) (10 mg/mL) and Laminin (Sigma-Aldrich) (6 µg/mL) coated cell culture plate. NSC culture media contained B-27 supplement (Invitrogen), DMEM/F12 (Biochrom), 100 μg/mL streptomycin (1%), l-glutamine (200 mM), and FGF-2 (20 ng/mL) (PeproTech) as detailed explained elsewhere (Aydıntug-Gurbuz et al., 2023). FGF-2 was added fresh to the medium every time. NSCs were incubated at 37°C in 5% CO2. When NSCs reached 70%-80% confluence in the flask, the cells were transferred to the plates for the following experiments.
Oxygen exposure of NSCs
The Cell ASIC ONIX Microfluidic System (Merck) was applied to investigate the different O2 levels, including 0%, 3%, and 20%, and growth factors, including IGF-I and MGF on NSC in vitro, as summarized in Figure 1. M04S-03 microfluidic plates for cell ASIC ONIX (Merck) were coated with poly-l-ornithine (Sigma) and laminin (Sigma) as described previously (Croushore & Sweedler, 2013). This system provides the ability to control the environment of individual plates and provides simultaneous analysis of the living cells in vitro. Since it is designed to provide a dynamic cellular microenvironment, namely niche control, it is suitable for studies investigating extracellular factors’ effects on cells. NSCs were seeded at 1×104 cells per well, and the manufacturer applied a gravity-driven perfusion culture protocol. After 24 h of incubation, the stabilized cells were treated with growth factors following the experiment design. DMEM/F12 solutions were referred to as untreated. In external administration of growth factors, IGF-I 0.2 µg/mL or MGF 0.2 µg/mL were prepared and added to the relevant wells. The O2 levels were adjusted according to the experiment design as follows: Anoxia with 0% O2, hypoxia with 3% O2, and normoxia with 20% O2. Culture plates were connected to the Cell ASIC device with the manifold’s help, following the manufacturer’s instructions (Croushore & Sweedler, 2013). The experiments were set to 24 h in the software available on the computer. Readouts were analyzed with the program integrated into the software.

Cell proliferation assay
NSC-exposed growth factors, IGF-I and MGF, were analyzed after 24 h. The image program was used to measure cell proliferation. Phase-contrast images of NSCs were taken from the culture plate under an inverted microscope (Olympus CKX41) equipped with a digital camera with a 10x objective before starting the experiment (0 h) and after the experiment was concluded (24 h). The images were analyzed with the ImageJ software, version 1.8.0 (developed by W.S. Rasband, NIH). Since the culture time for NSC in defined conditions was estimated to be 24 h in this study, instead of counts of neurospheres, the effect of O2 levels and growth factors on NSC in the migration-proliferation without disturbing such O2 levels in culture conditions, the total area of the seeded cells was measured and compared. The analysis was performed on the images that could provide information for the functions of both migration and proliferation of NSC. By doing so, the area of the cells was measured with ImageJ. The calculations were performed using the Equation 1:
1.
Area of the cultured cells after 24 h/Area of the cultured cells in 0 h in defined conditions
Ribonucleic acid isolation, cDNA synthesis, and real-time polymerase chain reaction
Total RNA isolation was performed using a total RNA purification kit (Jena Bioscience) in NSC. SCRIPT cDNA Synthesis Kit (Jena Bioscience) was used for cDNA synthesis from 50 ng/µL RNA. IGF-1Ea’s forward primer was 5’-GCT TGC TCA CCT TTA CCA GC-3,’ and the reverse primer was 5’-AAG TGT ACT TCC TTC TGA GTC T-3’ with 130 base pairs (bp) in length. MGF’s forward primer was 5’-GCT TGC TCA CCT TTA CCA GC-3,’ and the reverse primer was 5’-AAG TGT ACT TCC TTT CCT TCT C-3’ (130 bp). HIF1-a forward primer was 5’ TGC TTG GTG CTG ATT TGT GA 3’and the reverse primer was 5’-GGT CAG ATG ATC AGA GTC CA-3’ (131 bp). GAPDH was applied as a housekeeping control. GAPDH’s forward primer was 5’GGT GTG AAC GGA TTT GGC CGT AT-3’and reverse primer 5’CTC AGC ACC AGC GTC ACC CCA TT3’ (129 bp).
Changes in each gene expression were detected with Sensi FAST SYBR No-ROX Kit (Bioline, UK) by the real-time quantitative RT-PCR (Light cycler 480, Roche Diagnostics). These specific primers were applied for amplification. The fluorescence emitted by the dye above the baseline signal was detected using the software in real time, recorded, and represented as the cycle threshold (CT). The arithmetic mean values of CTs, which were performed twice, were calculated for the statistical analysis. All samples were studied in duplicate. The samples were not treated with the growth factor, but the media were evaluated as the control sample. RNA samples directly isolated from a rat hippocampal region were also applied as positive controls in RT-PCR analysis.
Statistical analysis
The ΔΔCT method was used to determine the gene expression fold change with the results obtained from RT-PCR. CT values of target mRNAs were normalized according to the housekeeping GAPDH gene (Equation 2):
2. ΔCT=CTTarget–CTGAPDH
Some analysis for the target gene expression changes was performed by the Equation 3:
3. 2-ΔCT×100
For the fold change of target gene expressions analysis, the values were normalized to the control (Equation 4):
4. ΔΔCT=ΔCT–CTControl
and the Equation 5 was applied:
5. 2-ΔΔCT
The variables were used as Mean±SD and percentage and frequency values. Meanwhile, P<0.05 and P<0.01 levels were considered statistically significant.
3. Result
The initiation of neurogenesis after ischemia is the essential recovery opportunity for treatment success, and NSC is a crucial element in such post-ischemic repair. In this study, the external administration of the IGF-I and MGF was performed on NSC, exposed to various oxygen concentrations, including normoxia, hypoxia, and anoxia, with 20%, 3%, and 0%, respectively, as summarized in Table 1. During 24 h, their effects on the molecular function of NSC employing IGF-I and MGF and HIF-1α gene expression changes and NSC proliferation-migration rates were investigated (Figure 1).

Low levels of oxygen induce IGF-I and MGF gene expressions
The quantitative analysis of real-time RT-PCR was performed to determine the changes in IGF-I and MGF expressions during 24 h of NSC culture exposed to different O2 concentrations with growth factors.
There was a significant change in IGF-I expression relative to oxygen levels. The highest rate that induced IGF-I expression was determined with the 0% O2 following hypoxic and normoxia concentrations, 0.8, 0.3, and 0.1, respectively. The external administration of IGF-I reduced the IGF-I expression to 0% and 20% O2 levels (P<0.05). The IGF-I application at the hypoxic level did not reveal any significant change in IGF-I expression (Figure 2A).
The MGF expression was determined to be affected by O2. The highest rate of MGF expression was determined with the 0% O2 following 3% and 20% O2 levels, 0.4, 0.1, and 0.01, respectively. There was a statistically significant difference in MGF expression between 0% and 20% O2 levels (P<0.05). The induction of MGF expression with lower O2 levels was even more significant after administration of MGF in NSC culture in such conditions (Figure 2B).
The analysis of HIF1-α expression in such conditions revealed that the application of IGF-I lowered its relative fold change expression in all O2 levels. The application of MGF achieved this downregulation with 20% and 3% O2 levels. In contrast, MGF application induces upregulation in the HIF1-α relative fold change expression at the anoxic level (Figure 2C).

IGF-I and MGF promotes NSCs proliferation-migration in varying levels of oxygen
In physiological O2 concentrations (3%-5%) of the brain, the cultured NSC increases the proliferation of and modulates to differentiate into neurons. Regarding the effect of oxygen levels on NSC proliferation-migration, we have applied a culture method by measuring the area of the cultured NSC from images at 0 and 24 h (Figure 3A-B). Comparing the area between time intervals provided evidence for the NSC’s proliferation–migration under such conditions. Although it was not statistically significant, there was a slight increase in proliferation-migration of NSC in untreated NSC within 24 h of culture at a 3% O2 level. IGF-I is known to support neuronal survival during development as well as neuronal damage. Meanwhile, IGF-I-treated NSC in anoxia and normoxia conditions revealed a statistically significant increase in proliferation-migration. However, hypoxia reduced this rate (Figure 3C). According to the literature, MGF is a mitogen for neural cells and has neuroprotective effects in the ischemic brain model. Although it is not statistically significant, we have found that MGF induces NSC proliferation-migration in all conditions within 24 h compared to untreated NSC (Figure 3C).

4. Discussion
In mammalian CNS, oxygen homeostasis plays an essential role in the proliferation and differentiation of NSCs. Even small changes in the O2 level might affect dynamic balance and disturb cellular physiology and survival by affecting signal transduction pathways that control cell proliferation, fate and survival, and tissue and organ morphogenesis and regeneration (Fathollahipour et al., 2018; Zhou et al., 2014). Although low O2 levels are associated with pathological conditions in various tissues, in the hippocampus, where NSCs are commonly located, the estimated O2 level is around 3%-4% from early embryogenesis. NSC maintains its stability in a hypoxic environment and this physiological hypoxia promotes the growth, survival, and maintenance of the multipotent properties of NSC (Harvey et al., 2004; Morrison et al., 2000; Studer et al., 2000). Although the consequence of the ischemia is detrimental to homeostasis, the studies on the role of NSC in ischemic conditions are still insufficient in the literature.
Furthermore, the role of growth factors in a hypoxic environment on NSC remains elusive. Intrathecal administration of IGF-I had a protective effect on neurons in rats after hypoxic ischemia (O’Kusky & Ye, 2012). MGF blocks the apoptosis of damaged myocytes and stem cells and can protect myocytes and neurons from hypoxia (Chiong et al., 2011; Yoshida & Delafontaine, 2020). Meanwhile, MGF is markedly more effective than IGF-I (Aperghis et al., 2004). Under these consequences, it might be relevant to consider NSC conditioned with growth factors that have stimulant effects as an effective modality in stroke treatment. Therefore, it is essential to clarify the role of varying O2 environments on NSC proliferation and the effects of growth factors. Based on these discoveries, IGF-I and MGF gene expressions in NSCs exposed to varying O2 levels could provide essential evidence regarding IGF-I and MGF growth factor roles in NSC and whether external application of IGF-I and MGF might provide any effect, including NSC proliferation that would benefit the treatment modalities of ischemic stroke.
There was a significant upregulation in IGF-I expression relative to oxygen levels. The 0% O2 levels induce the IGF-I expression at the highest compared to 3% O2 levels and 20% O2. This result is in line with the literature, where the effect of IGF-I has been induced in damaged brain regions after cerebral ischemia (O’Kusky & Ye, 2012). The external application of IGF-I has lowered its expression in 0% and 20% O2 conditions but saved the 3% O2. The proliferation-migration data supported this result by revealing the highest proliferation-migration rate within IGF-I application in 0% and 20% O2 conditions. Mild hypoxia enhances the proliferation of human NSCs (Santilli et al., 2010). These findings suggest that the 3% O2, the average oxygen level in the central nervous system, does not induce NSC proliferation, retain the cells in a state of quiescence, and IGF-I is ineffective in such an environment. In contrast, reduced oxygen levels such as 0% O2 occur in neuronal disorders like cerebral ischemia, transiently leading to NSC proliferation with induction of IGF-I expressions. The MGF expression relative to oxygen levels was similar to IGF-I, where the highest MGF expression was detected in the 0% O2, compared to 3% O2 and 20% O2 levels.
On the other hand, the MGF administration dramatically increased the MGF expression in 0% and 3% O2. The proliferation-migration rate with MGF external application revealed not significant, but the upregulated rate of NSC. These results were in line with the literature where MGF overexpression increases the number of neural progenitor cells and promotes neurogenesis in transgenic mice that constitutively overexpresses MGF from birth (Tang et al., 2017). It has also been previously found that exogenous MGF and IGF-I increase NSC proliferation during damage. Although MGF is known for its regenerative capability in skeletal muscle, mononucleated progenitors, and its neuroprotective effect in vivo and in vitro (Dluzniewska et al., 2005; Ates et al., 2007; Quesada et al., 2009). This study is the first finding revealing the MGF and IGF-I effect on varying O2 levels. At the same time, when MGF and IGF-I were administered externally to NSC exposed to anoxia and hypoxia, it was determined that these two growth factors positively affect the proliferation of NSC. The data might all provide a significant advantage and a valuable tool for growth hormones, IGF-I, and MGF-mediated NSC therapy in ischemic stroke as well as for neurodegenerative diseases like Parkinson’s disease, multiple sclerosis, and Alzheimer.
Furthermore, HIF-1-α induction correlated with changes in cellular oxygen and growth factors, including IGF-I, was also confirmed in this study, where relative HIF-1-α gene expression fold change was suppressed with IGF-I administration in varying O2 levels. Surprisingly, the MGF gene expression was increased by 0% O2. This study is the first evidence indicating the role of MGF in an anoxic environment. It might suggest that targeting HIF-1 as a novel small molecule inhibitors might be an attractive strategy for therapeutic development. This study was designed to search for the environmental effects within 24 hours. Prolonged and or intermittent exposure to hypoxia might reveal diverse findings since more complex pathways will be expected to enroll in such chronic condition (Khuu et al., 2019).
5. Conclusion
Overall, this study shed light on using exogenous MGF and IGF-I administration for their neuroprotective and proliferative effects on NSC for the harmful effects of ischemic stroke. More in vitro and in vivo intensive studies are essential to understand the role of growth factors for such treatment modalities in ischemia.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This work was supported by the Scientific Research Projects Coordination Unit of Istanbul University (Project No.: 58165).
Authors' contributions
Conceptualization: Tuğba Aydıntuğ-Gürbüz and Selçuk Sözer; Supervision: Selçuk Sözer; Materials, data collection and processing: Tuğba Aydıntuğ-Gürbüz, Selin Toprak and Selçuk Sözer; Data analysis and interpretation: Fatih Toprak, Selin Toprak and Selçuk Sözer; Investigation: Tuğba Aydıntuğ-Gürbüz, Fatih Toprak and Selin Toprak; Writing the original draft: Fatih Toprak, Selin Toprak and Selçuk Sözer; Review and editing: Selçuk Sözer and Fatih Toprak.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
In honor of Kenan Ates, rest in peace.
References
Aperghis, M., Johnson, I. P., Cannon, J., Yang, S. Y., & Goldspink, G. (2004). Different levels of neuroprotection by two insulin-like growth factor-I splice variants. Brain Research, 1009(1-2), 213–218. [PMID]
Ates, K., Yang, S. Y., Orrell, R. W., Sinanan, A. C., Simons, P., & Solomon, A., et al. (2007). The IGF-I splice variant MGF increases progenitor cells in ALS, dystrophic, and normal muscle. FEBS Letters, 581(14), 2727–2732. [DOI:10.1016/j.febslet.2007.05.030] [PMID]
Gürbüz, T. A., Güleç, Ç., Toprak, F., Toprak, S. F., & Sozer, S. (2023). In Vitro investigation of insulin-like growth factor and mechano-growth factor on proliferation of neural stem cells in high glucose environment. Neurological Sciences and Neurophysiology, 40(1), 27-36. [Link]
Baddela, V. S., Sharma, A., Michaelis, M., & Vanselow, J. (2020).HIF1 driven transcriptional activity regulates steroidogenesis and proliferation of bovine granulosa cells. Scientific Reports, 10(1), 3906. [DOI:10.1038/s41598-020-60935-1] [PMID] [PMCID]
Bailes, J., & Soloviev, M. (2021). Insulin-Like Growth Factor-1 (IGF-1) and its monitoring in medical diagnostic and in sports. Biomolecules, 11(2), 217. [DOI:10.3390/biom11020217] [PMID] [PMCID]
Boltze, J., Didwischus, N., Li, S., Jolkkonen, J., & Taguchi, A. (2020). Increased migratory and homing abilities of neural and mesenchymal stem cell populations by transient cell modifications: Preclinical progress and clinical relevance. EBioMedicine, 60, 103022. [DOI:10.1016/j.ebiom.2020.103022] [PMID] [PMCID]
Canazza, A., Minati, L., Boffano, C., Parati, E., & Binks, S. (2014).Experimental models of brain ischemia: A review of techniques, magnetic resonance imaging, and investigational cell-based therapies. Frontiers in Neurology, 5, 19. [DOI:10.3389/fneur.2014.00019] [PMID] [PMCID]
Ceanga, M., Dahab, M., Witte, O. W., & Keiner, S. (2021). Adult Neurogenesis and Stroke: A tale of two neurogenic niches. Frontiers in Neuroscience, 15, 700297. [DOI:10.3389/fnins.2021.700297] [PMID] [PMCID]
Chamorro, Á., Dirnagl, U., Urra, X., & Planas, A. M. (2016). Neuroprotection in acute stroke: Targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. The Lancet. Neurology, 15(8), 869–881. [DOI:10.1016/S1474-4422(16)00114-9] [PMID]
Chau, N. M., Rogers, P., Aherne, W., Carroll, V., Collins, I., & McDonald, E., et al. (2005). Identification of novel small molecule inhibitors of hypoxia-inducible factor-1 that differentially block hypoxia-inducible factor-1 activity and hypoxia-inducible factor-1α induction in response to hypoxic stress and growth factors. Cancer Research, 65(11), 4918-4928. [DOI:10.1158/0008-5472.Can-04-4453] [PMID]
Chiong, M., Wang, Z. V., Pedrozo, Z., Cao, D. J., Troncoso, R., & Ibacache, M., et al. (2011). Cardiomyocyte death: Mechanisms and translational implications. Cell Death & Disease, 2(12), e244. [DOI:10.1038/cddis.2011.130] [PMID] [PMCID]
Croushore, C. A., & Sweedler, J. V. (2013). Microfluidic systems for studying neurotransmitters and neurotransmission. Lab on a Chip, 13(9), 1666–1676. [DOI:10.1039/c3lc41334a] [PMID] [PMCID]
Decimo, I., Bifari, F., Krampera, M., & Fumagalli, G. (2012).Neural stem cell niches in health and diseases. Current Pharmaceutical Design, 18(13), 1755–1783. [DOI:10.2174/138161212799859611] [PMID] [PMCID]
Dluzniewska, J., Sarnowska, A., Beresewicz, M., Johnson, I., Srai, S. K., & Ramesh, B., et al. (2005). A strong neuroprotective effect of the autonomous C-terminal peptide of IGF-1 Ec (MGF) in brain ischemia. FASEB Journal: Official Publication of The Federation of American Societies for Experimental Biology, 19(13), 1896–1898. [DOI:10.1096/fj.05-3786fje] [PMID]
Fathollahipour, S., Patil, P. S., & Leipzig, N. D. (2018). Oxygen regulation in development: Lessons from embryogenesis towards tissue engineering. Cells Tissues Organs, 205(5-6), 350-371. [DOI:10.1159/000493162] [PMID] [PMCID]
Goldspink, G. (2005). Research on mechano growth factor: its potential for optimizing physical training as well as misuse in doping. British Journal of Sports Medicine, 39(11), 787-788. [DOI:10.1136/bjsm.2004.015826] [PMID] [PMCID]
Harvey, A. J., Kind, K. L., Pantaleon, M., Armstrong, D. T., & Thompson, J. G. (2004). Oxygen-regulated gene expression in bovine blastocysts. Biology of Reproduction, 71(4), 1108–1119.[DOI:10.1095/biolreprod.104.028639] [PMID]
Huang, L. E., Arany, Z., Livingston, D. M., & Bunn, H. F. (1996).Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. The Journal of Biological Chemistry, 271(50), 32253–32259. [DOI:10.1074/jbc.271.50.32253] [PMID]
Jing, X., Yang, F., Shao, C., Wei, K., Xie, M., & Shen, H., et al. (2019). Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Molecular Cancer, 18(1), 157. [DOI:10.1186/s12943-019-1089-9] [PMID] [PMCID]
Kalogeris, T., Baines, C. P., Krenz, M., & Korthuis, R. J. (2012). Cell biology of ischemia/reperfusion injury. International Review of Cell and Molecular Biology, 298, 229–317. [DOI:10.1016/B978-0-12-394309-5.00006-7] [PMID] [PMCID]
Khuu, M. A., Pagan, C. M., Nallamothu, T., Hevner, R. F., Hodge, R. D., & Ramirez, J. M., et al. (2019). Intermittent hypoxia disrupts adult neurogenesis and synaptic plasticity in the dentate gyrus. The Journal of Neuroscience, 39(7), 1320-1331. [DOI:10.1523/jneurosci.1359-18.2018] [PMID] [PMCID]
Kriegstein, A., & Alvarez-Buylla, A. (2009). The glial nature of embryonic and adult neural stem cells. Annual Review of Neuroscience, 32, 149–184. [DOI:10.1146/annurev.neuro.051508.135600] [PMID] [PMCID]
Lee, J. M., Grabb, M. C., Zipfel, G. J., & Choi, D. W. (2000). Brain tissue responses to ischemia. The Journal of Clinical Investigation, 106(6), 723–731.[DOI:10.1172/JCI11003] [PMID] [PMCID]
Ma, Z., Xiang, X., Li, S., Xie, P., Gong, Q., & Goh, B. C., et al. (2020). Targeting hypoxia-inducible factor-1, for cancer treatment: Recent advances in developing small-molecule inhibitors from natural compounds. Seminars in Cancer Biology, 80, 379–390.[DOI:10.1016/j.semcancer.2020.09.011] [PMID]
Masoud, G. N., & Li, W. (2015). HIF-1alpha pathway: Role, regulation, and intervention for cancer therapy. Acta Pharmaceutica Sinica. B, 5(5), 378–389. [DOI:10.1016/j.apsb.2015.05.007] [PMID] [PMCID]
Matheny, R. W., Jr, Nindl, B. C., & Adamo, M. L. (2010). Minireview: Mechano-growth factor: A putative product of IGF-I gene expression involved in tissue repair and regeneration. Endocrinology, 151(3), 865-875. [DOI:10.1210/en.2009-1217] [PMID] [PMCID]
Morrison, S. J., Csete, M., Groves, A. K., Melega, W., Wold, B., & Anderson, D. J. (2000). Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 20(19), 7370–7376. [DOI:10.1523/JNEUROSCI.20-19-07370.2000] [PMID] [PMCID]
Nieto-Estévez, V., Defterali, Ç., & Vicario-Abejón, C. (2016). IGF-I: A key growth factor that regulates neurogenesis and synaptogenesis from embryonic to adult stages of the brain. Frontiers in Neuroscience, 10, 52. [DOI:10.3389/fnins.2016.00052] [PMID] [PMCID]
O’Kusky, J., & Ye, P. (2012). Neurodevelopmental effects of insulin-like growth factor signaling. Frontiers in Neuroendocrinology, 33(3), 230–251. [DOI:10.1016/j.yfrne.2012.06.002] [PMID] [PMCID]
Oberbauer, A. M. (2013). The regulation of IGF-1 gene transcription and splicing during development and aging. Frontiers in Endocrinology, 4, 39. [DOI:10.3389/fendo.2013.00039] [PMID] [PMCID]
Østergaard, L., Engedal, T. S., Moreton, F., Hansen, M. B., Wardlaw, J. M., & Dalkara, T., et al. (2016). Cerebral small vessel disease: Capillary pathways to stroke and cognitive decline. Journal of Cerebral Blood Flow and Metabolism, 36(2), 302-325. [DOI:10.1177/0271678X15606723] [PMID] [PMCID]
Paredes, M. F., Sorrells, S. F., Garcia-Verdugo, J. M., & Alvarez-Buylla, A. (2016). Brain size and limits to adult neurogenesis. The Journal of Comparative Neurology, 524(3), 646–664. [DOI:10.1002/cne.23896] [PMID] [PMCID]
Quesada, A., Micevych, P., & Handforth, A. (2009). C-terminal mechano growth factor protects dopamine neurons: A novel peptide that induces heme oxygenase-1. Experimental Neurology, 220(2), 255–266. [DOI:10.1016/j.expneurol.2009.08.029] [PMID]
Salceda, S., & Caro, J. (1997). Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes.The Journal of Biological Chemistry, 272(36), 22642–22647 [DOI:10.1074/jbc.272.36.22642] [PMID]
Santilli, G., Lamorte, G., Carlessi, L., Ferrari, D., Rota Nodari, L., & Binda, E., et al. (2010). Mild hypoxia enhances the proliferation and multipotency of human neural stem cells. PloS One, 5(1), e8575. [DOI:10.1371/journal.pone.0008575] [PMID] [PMCID]
Sharee Ghourichaee, S., & Leach, J. B., (2016). The effect of hypoxia and laminin-rich substrates on the proliferative behavior of human neural stem cells. Journal of Materials Chemistry. B, 4(20), 3509–3514. [DOI:10.1039/c5tb02701b] [PMID]
Steiner, P. (2019). Brain Fuel utilization in the developing brain. Annals of Nutrition & Metabolism, 75 (Suppl 1), 8–18. [DOI:10.1159/000508054] [PMID]
Studer, L., Csete, M., Lee, S. H., Kabbani, N., Walikonis, J., & Wold, B., et al. (2000). Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 20(19), 7377–7383. [DOI:10.1523/JNEUROSCI.20-19-07377.2000] [PMID] [PMCID]
Tang, J. J., Podratz, J. L., Lange, M., Scrable, H. J., Jang, M. H., & Windebank, A. J. (2017). Mechano growth factor, a splice variant of IGF-1, promotes neurogenesis in the aging mouse brain. Molecular Brain, 10(1), 23. [DOI:10.1186/s13041-017-0304-0] [PMID] [PMCID]
Tunç, B. S., Toprak, F., Toprak, S. F., & Sozer, S. (2021). In vitro investigation of growth factors including MGF and IGF-1 in neural stem cell activation, proliferation, and migration. Brain Research, 1759, 147366. [DOI:10.1016/j.brainres.2021.147366] [PMID]
Wang, G. L., & Semenza, G. L. (1995). Purification and characterization of hypoxia-inducible factor 1. The Journal of Biological Chemistry, 270(3), 1230–1237. [DOI:10.1074/jbc.270.3.1230] [PMID]
Wiesener, M. S., Jürgensen, J. S., Rosenberger, C., Scholze, C. K., Hörstrup, J. H., & Warnecke, C., et al. (2003). Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB Journal, 17(2), 271-273. [DOI:10.1096/fj.02-0445fje] [PMID]
Williams, A. J. (1998). ABC of oxygen: Assessing and interpreting arterial blood gases and acid-base balance. BMJ, 317(7167), 1213-1216. [DOI:10.1136/bmj.317.7167.1213] [PMID] [PMCID]
Xu, S., Lu, J., Shao, A., Zhang, J. H., & Zhang, J. (2020). Glial Cells: Role of the immune response in ischemic stroke. Frontiers in Immunology, 11, 294. [DOI:10.3389/fimmu.2020.00294] [PMID] [PMCID]
Yee, M., Gelein, R., Mariani, T. J., Lawrence, B. P., & O'Reilly, M. A. (2016). The oxygen environment at birth specifies the population of alveolar epithelial stem cells in the adult lung. Stem Cells, 34(5), 1396-1406. [DOI:10.1002/stem.2330] [PMID] [PMCID]
Yoshida, T., & Delafontaine, P. (2020). Mechanisms of IGF-1-Mediated regulation of skeletal muscle hypertrophy and atrophy. Cells, 9(9), 1970. [DOI:10.3390/cells9091970] [PMID] [PMCID]
Zhang, G. L., Zhu, Z. H., & Wang, Y. Z. (2019). Neural stem cell transplantation therapy for brain ischemic stroke: Review and perspectives. World Journal of Stem Cells, 11(10), 817–830.[DOI:10.4252/wjsc.v11.i10.817] [PMID] [PMCID]
Zhang, K., Zhu, L., & Fan, M. (2011). Oxygen, a Key Factor Regulating Cell Behavior during Neurogenesis and Cerebral Diseases. Frontiers in Molecular Neuroscience, 4, 5. [PMID]
Type of Study: Original |
Subject:
Cellular and molecular Neuroscience
Received: 2022/02/8 | Accepted: 2022/09/21 | Published: 2024/05/1
Received: 2022/02/8 | Accepted: 2022/09/21 | Published: 2024/05/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








