Volume 15, Issue 2 (March & April 2024)
BCN 2024, 15(2): 211-220 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Tirgar F, Azizi Z, Hadjighassem M. A Novel Approach for Mucosal and Bulbar Olfactory Ensheathing Cells Isolation Based on the Non-adherent Subculture Technique. BCN 2024; 15 (2) :211-220
URL: http://bcn.iums.ac.ir/article-1-2390-en.html
URL: http://bcn.iums.ac.ir/article-1-2390-en.html
1- Department of Neuroscience and Addiction Studies, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran.
2- Department of Molecular Medicine, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran.
2- Department of Molecular Medicine, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran.
Keywords: Olfactory ensheathing cells, Cell isolation, Cell transplantation, Olfactory bulb, Olfactory mucosa
Full-Text [PDF 1052 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Currently, there are an increasing number of studies that utilize isolated olfactory ensheathing cells (OECs) for transplantation in different pathologies, such as spinal cord injury (Feron et al., 2005; Mackay-Sim et al., 2008; Tabakow et al., 2013) and amyotrophic lateral sclerosis (Chen et al., 2012) with some promising clinical results. The high migration potency, neurotrophic factors secretion, inherent pathotropism toward inflammatory cues, and non-invasive accessible source of OECs, as glial cells, make them appropriate for cell therapies (Hashemi et al., 2016; Lankford, et al., 2008; Pellitteri et al., 2016; Su et al., 2013). According to previous studies, there are two primary sources for isolating OECs: Olfactory bulb (OB) in the brain and olfactory mucosa (OM) in the nasal cavity.
Isolation and purification of specific cell types from a heterogeneous population of different cells are essential for in vitro and in vivo studies and clinical cell-based therapies. According to the researcher’s demand, various techniques are applied for primary OEC isolation (Reshamwala et al., 2020). The high purity and viability of isolated cells are critical issues that should be considered. In addition to several other cell types that accompany OB and OM, cell contamination during the isolation procedure may cause tumor mass, indicating the importance of purification of OECs before transplantation to avoid unwanted side effects (Dlouhy et al., 2014; Woodworth et al., 2019). Based on adherence properties, different cells exert various attachment behaviors. For example, fibroblasts have a high affinity to attach to the surface, whereas this affinity is lower in OECs (Reshamwala et al., 2020). Cell adhesion molecules are essential in interacting with the cells and the culture surface (Abdal Dayem & Cho, 2018).
Accordingly, it will help achieve a novel, non-invasive OECs isolation strategy with high efficiency and less expensive than the previous studies. In this manner, we successfully introduced a new rapid and cost-effective approach for the isolation and purification of OECs from rat OM as efficiently as OB, along with the difference between the two sources.
2. Materials and Methods
Study animals
OECs were isolated from OM and OB of 12 neonatal Wistar rats. All efforts were made to reduce animal suffering and minimize the number of rats used for these tests. The rats were housed in cages in groups of four, and their access to food and water was controlled every day. The animal’s cages were kept at 23±1°C under a 12/12-h light-dark cycle, which began at 7:00 AM. The experimental procedures for the treatment of animals were conducted in accordance with the Ethics Committee of Tehran University of Medical Sciences.
Primary OECs isolation
OECs were isolated from OM and OB of rats utilizing the previous original protocol with modifications (Ramón-Cueto & Nieto-Sampedro, 1992). Briefly, animals were anesthetized using ketamine hydrochloride (100 mg/kg, Alfasan Co., Woerden, Netherlands) and xylazine (10 mg/kg, Alfasan Co., Woerden, Netherlands). To obtain OECs from OBs, we removed the skull bones from the top and harvested the OBs. Collected OBs were rinsed with sterile, cold phosphate-buffered saline (PBS) and then transferred to a dish containing 5% antibiotic/antimycotic (Gibco, Carlsbad, CA, USA). The whole isolation procedure should take less than 10 minutes. At first, the samples were thoroughly washed with PBS so that the external blood was gently washed from the sample, and with the help of a razor, the internal blood vessels were gently separated.
OECs in OB are located in the bulb’s two external layers, typically termed olfactory nerve glomerular layers (ONGLs). Employing a stereo microscope instrument, the ONGLs were separated by dissection forceps. Next, ONGL underwent mechanical and chemical homogenization. For this purpose, we used a scalpel blade for chopping and then 0.25% trypsin EDTA (Gibco, Carlsbad, CA, USA) for chemical dissociation. Trypsinization lasted about 15 minutes at 37°C. Subsequently, we added Dulbecco’s modified eagle medium/nutrient mixture F12 (DMEM/F12; Gibco, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA, USA) to end the trypsinization. The isolated heterogeneous samples were centrifuged for 10 minutes at 1200 rpm; the cell pellet was transferred to an uncoated petri dish and incubated with DMEM/F12 medium supplemented with 10% FBS and 1% antibiotic/antimycotic (Gibco, Carlsbad, CA, USA). Based on differential adhesion properties among astrocytes, fibroblasts, and OECs, the latter cells have a low affinity to attach to the uncoated surface. Therefore, OECs were easily separated from other cells by transferring the supernatant to another uncoated Petri dish at 36-h post isolation. We repeated this procedure for another 24 h to eliminate the cell contamination and achieve high purification. Finally, after 60 h, the supernatant containing isolated OECs was transferred to a coated flask and incubated at 37°C under 90% humidity containing 5% CO2, replacing the medium every two days. We performed the same protocol for isolating OECs from OM, except we isolated the cells from the posterior part of the nasal cavity near the septum, and the epithelium was dissociated from the lamina propria. All images from both preparations were taken by light inverted microscope (Labomed TCM 400, CA, USA) at different time points.
Immunocytofluorescent
After 21 days, the cells were characterized with immunofluorescent using a selective marker, nerve growth factor receptor p75 (1:200, mouse monoclonal anti-NGFR p75 primary antibody, Santa Cruz, Biotechnology Inc., USA). About 3×105 OECs were seeded in a 6-well plate to achieve this goal. On the next day, the cells were fixed by 4% paraformaldehyde, and then 10% bovine serum albumin (BSA, Sigma-Aldrich St. Louis, MO, USA) and 0.3% Triton X-100 (ATOCEL, Austria) were added for 45 minutes. Afterward, the cells were incubated with anti-NGFR p75 primary antibody overnight at 4°C. Incubation with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG secondary antibody (1:200 Biorbyt Ltd, Cambridge, UK) was performed for 1 h at room temperature. As a nuclear stainer, we used 4,6-diamidino-2-phenylindole (DAPI; 1.5 µg/mL, Santa Cruz, Biotechnology Inc., USA). Eventually, NGFR p75 expression was detected by an inverted fluorescence microscope (OPTIKA Srl, Ponteranica BG, Italy) and quantified by ImageJ software, version 1.52 (National Institute of Health, USA).
Western blotting
Western blot analysis was performed to detect S100β as a selective marker for OECs in three independent samples. Briefly, total protein extract was obtained, and protein concentration was measured using the well-established Bradford assay. Total protein (50 µg) was loaded on an SDS-PAGE and transferred onto a PVDF membrane (Amersham, GE Healthcare, Piscataway, USA). Blocking was carried out using 5% BSA for 60 min. Blots were incubated with S100β primary antibody (1:1000, rabbit monoclonal anti-S100β primary antibody (Abcam, Cambridge, MA, USA) overnight at 4°C. Next, blots were washed with Tris-buffered saline-Tween 20 (TBS-T) thrice and incubated with secondary antibody (1:5000, Goat polyclonal anti-rabbit IgG H&L horseradish peroxidase (HRP) secondary antibody (Abcam, Cambridge, MA, USA) for 90 min at room temperature. Finally, S100β detection was performed using a chemiluminescence detection kit (Pars Tous Biotechnology, Mashhad, Iran). Anti-β-actin primary antibody (1:2500, rabbit polyclonal anti-β-actin primary antibody, PADZA Co., Tehran, Iran) was used as a loading control. The S100β amounts were quantified to their corresponding β-actin levels using ImageJ software.
Cell viability
To assess whether the isolation protocol affects cell survival, OECs viability was evaluated by 3-(4,5-dimethylthiazol2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich, Sigma-Aldrich, Saint Louis, MO, USA) assays. A total number of 5×103 OECs were seeded in a 96-well plate at different time points (7, 14, and 28 days post-isolation). MTT (0.5 mg/mL) was added in the next day, and OECs were incubated for 4 h at 37°C. Following the removal of MTT, DMSO (100 μL) was added. Finally, absorbance was obtained at 570 nm utilizing an ELISA reader (Bio-Tek Instruments Inc., VT, USA).
Statistical analysis
GraphPad Prism software, version 6.01 (GraphPad Software, Inc., CA, USA) was used for statistical analysis. Data were analyzed using a t-test for comparing two groups and one-way ANOVA for comparing more than two groups, followed by the Tukey post hoc test. All data were presented as Mean±SD (n=3), and P<0.05 was considered significant.
3. Results
Morphology of isolated OB- and OM-derived OECs
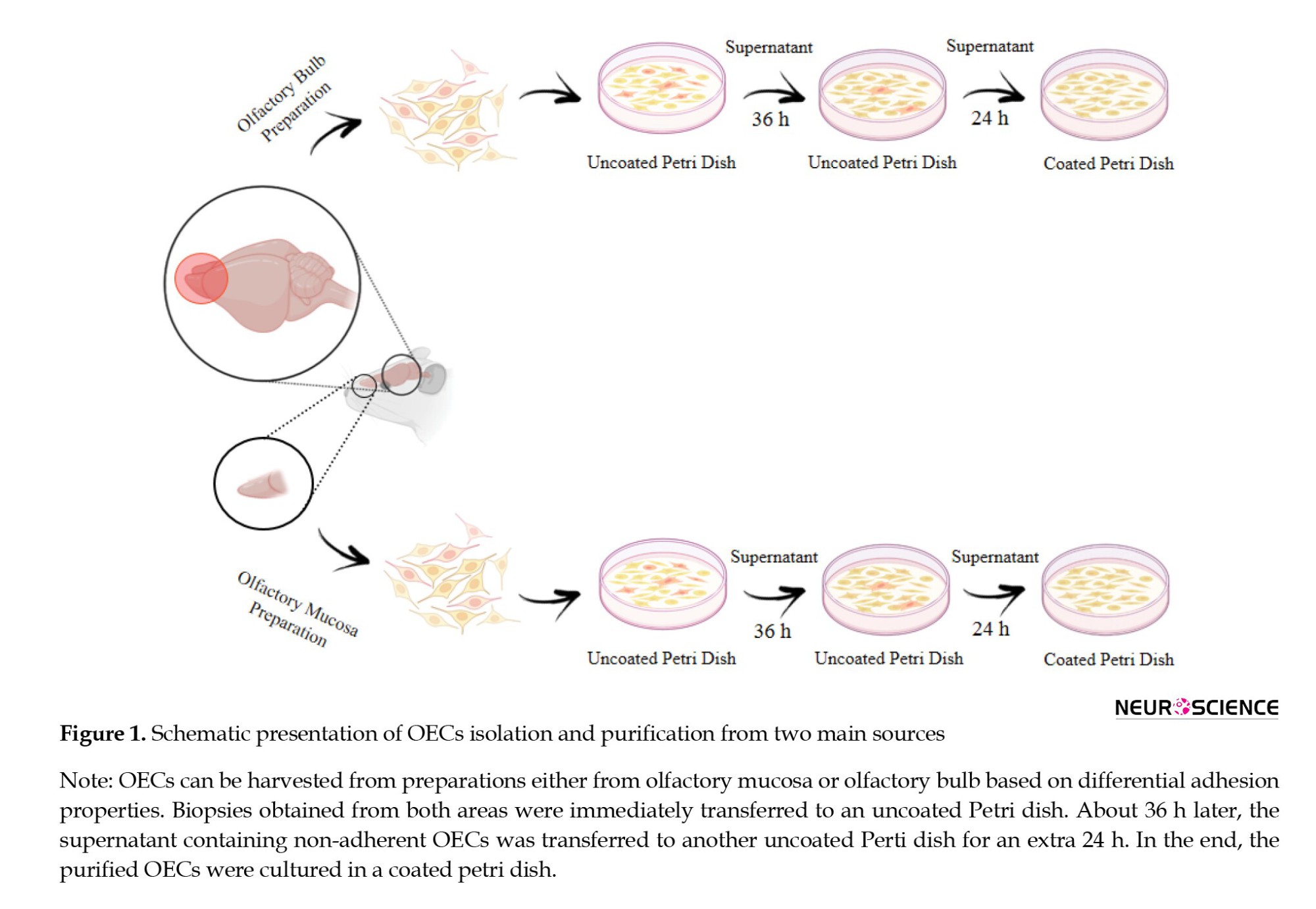
According to our suggested protocol, OECs were successfully isolated from rat OB and OM (Figure 1).
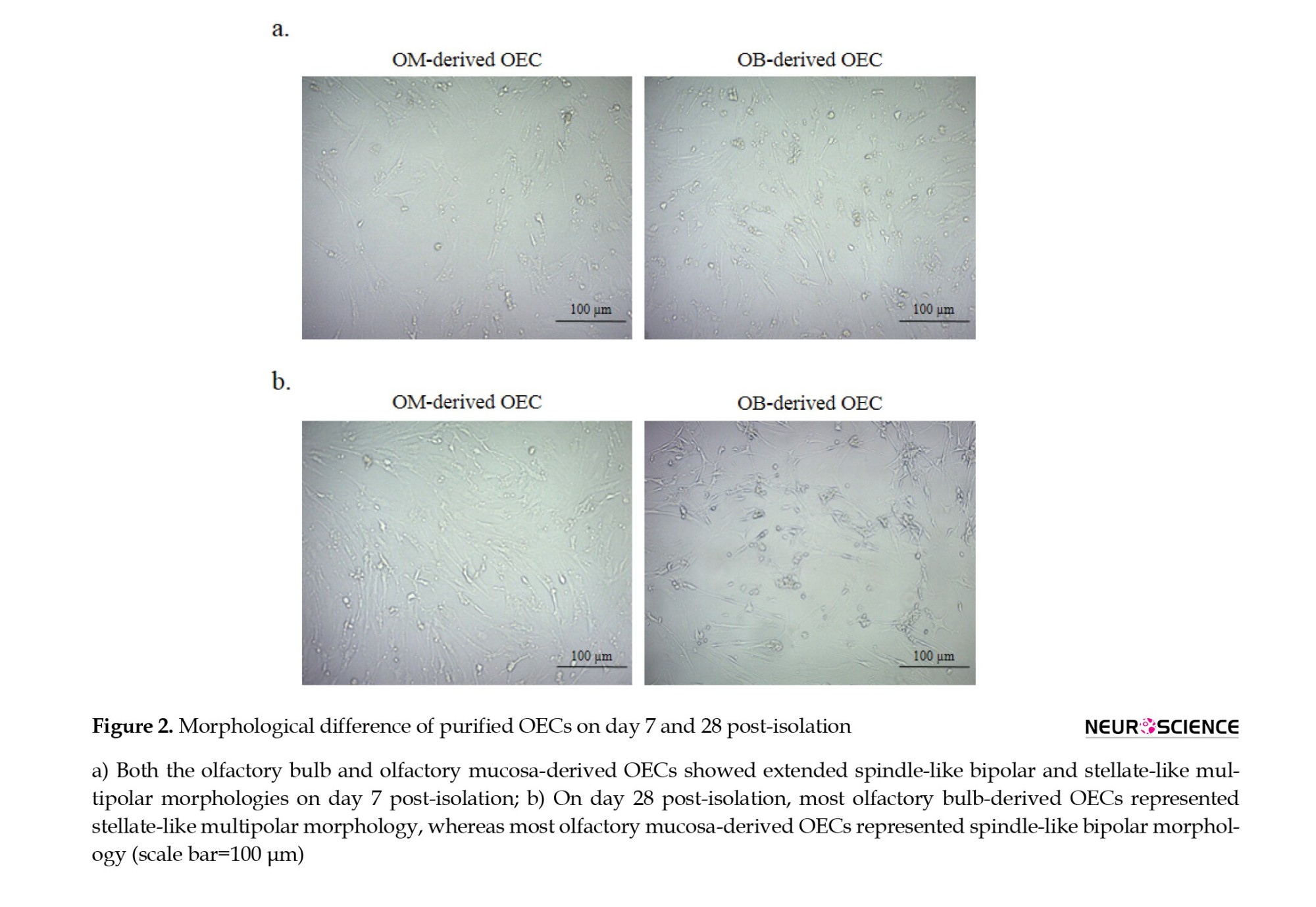
As illustrated in Figure 2, isolated OECs at different time points had normal growth and proliferation rates. The OECs on day 7 post-isolation procedure have spindle- to satellite-like shapes. At this time, both OB-derived and OM-derived OECs exhibited the same morphology (Figure 2a). However, the difference became apparent at day 28 post-isolation. At this time, most OB-derived OECs showed stellate-like multipolar morphology with short processes, unlike most OM-derived OECs, which exhibited spindle-like bipolar morphology (Figure 2b).
Characterization of isolated OB- and OM-derived OECs
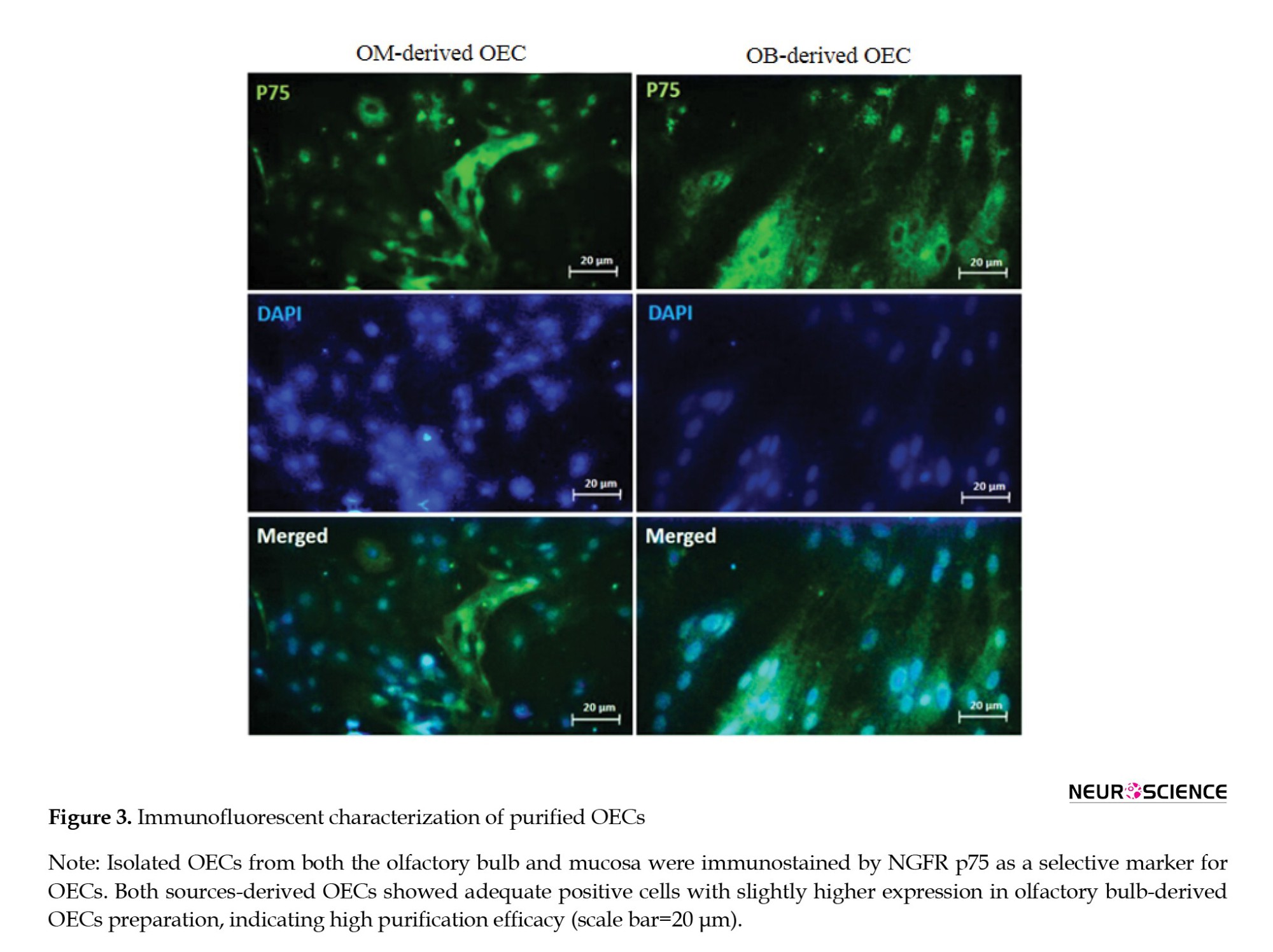
Immunofluorescence analysis visualized the expression of NGFR p75 as a selective marker for OECs. OM-derived OECs highly expressed NGFR P75 and OB-derived OECs (Figure 3). This protocol guaranteed the high purity of OECs-derived from both sources. It should be noted that the NGFR P75 expression was higher in OB-derived OECs.
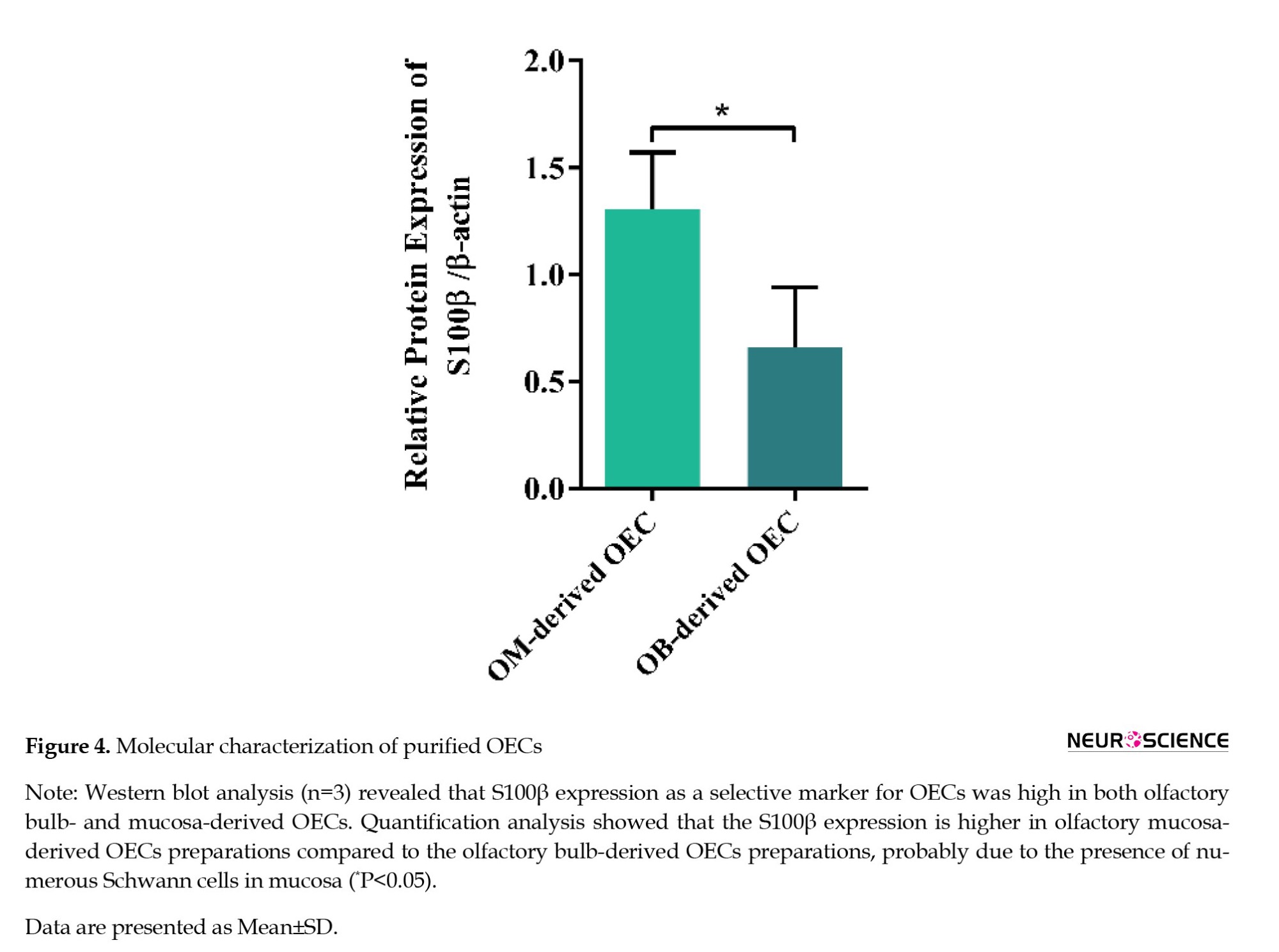
OECs characterization was further verified by western blot against S100β protein, another specific marker for OECs. S100β protein expression was high in both OB- and OM-derived OECs. However, this expression increased in OM-derived OECs (Figure 4a). Quantification of band densities highlighted the significant difference between the two OEC populations from different origins (P<0.05, t=2.9, df=4) (Figure 4b).
Taken together, based on the principle that the adhesion potential of OECs is lower than that of fibroblasts and astrocytes, OECs were successfully screened for adhesive fibroblasts and astrocytes from both OB and OM of rats.
Cell viability of isolated OB- and OM-derived OECs
The MTT assay was performed to evaluate the OECs viability at different time points. Our results demonstrated that cell viability was near 100% in both OB- and OM-derived OEC cultures on day 7 and day 14 post-isolation. On day 28, there was a decrease in cell viability in OB-derived OECs culture. However, the reduction was not significant, indicating that OB-derived primary OECs may be slightly more vulnerable to death compared to the OM-derived OECs in primary cultures (P>0.05) (Figure 5).
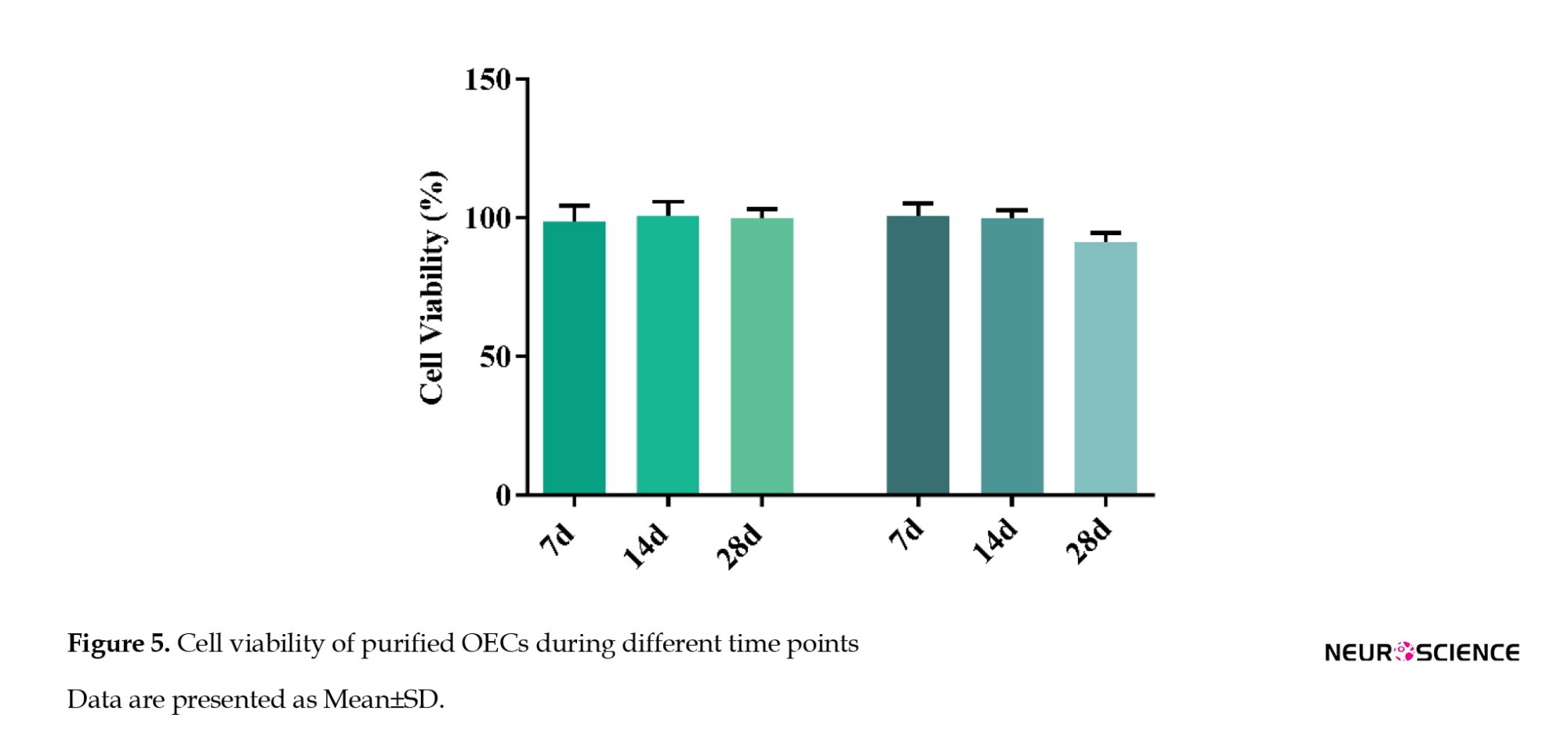
Our new approach demonstrated that isolating OECs from both sources may not significantly alter the cell until at least 28 days post-isolation.
4. Discussion
Recently, olfactory ensheathing cells have been considered alternative cell therapy in numerous diseases. The regeneration potency of OECs (Doucette, 1990), secretion of neurotrophic and guidance factors (Barnett & Riddell, 2004; Barton et al., 2017), phagocytosis ability (Su et al., 2013), migration capability (Hashemi et al., 2016), and restorative potential combined with an accessible non-invasive source of OECs make them suitable for transplantation studies (Carvalho et al., 2019). In this manner, autologous graft strategy may exert some promise due to overcoming ethical issues (Granger et al., 2012; Muñoz-Quiles et al., 2009; Tabakow et al., 2013).
OECs, as glial-like cells, support and convoy the olfactory axons extending from the nasal mucosa to the olfactory bulb in their natural location (Carvalho et al., 2019). Either OM or OB can be considered an OECs source. Hence, OECs can be isolated from both, although other cell populations exist in each preparation (Yao et al., 2018). One of the major challenges of these methods is purification. There are different techniques to screen cells, such as their size, but sometimes different cell types in a population have nearly the same size, so we need an alternative method to separate them. There is little evidence that addresses this point of view.
Previous studies reported different isolation methods that harvested the OECs from OB or OM. OECs purification from a heterogeneous cell population is the most considerable issue before OEC transplantation studies. However, the isolation methods and their outcome show some controversy and variability based on the origin of the OECs. Among different methods for OECs purification, techniques based on cell-specific markers and isolation due to the differential adhesion properties are more frequently used. Usually, the isolation of OECs from OB employs immunofluorescent-based combined with differential adherence-based purification, while the isolation of OECs from OM utilizes enrichment of the environment (Reshamwala et al., 2020). The immuno-purification method relies on the expression of NGFR p75 as the most common and well-characterized selective hallmark of OECs or other relative markers such as S100β (Bianco et al., 2004; Franceschini & Barnett, 1996; Gong et al., 1994; Novikova et al., 2012; Torres-Espín et al., 2013). It should be noted that there would be cell contamination in isolated OECs preparations, whether from the OB or OM source, due to trigeminal nerve Schwann cells. In our study, as shown in Figure 4, S100β expression was higher in OM-derived OECs, probably due to the presence of numerous Schwann cells in the mucosa (Ziege et al., 2013).
The latter one, differential adhesion properties, is based on different time points in which different cell types must be attached to the surface according to their properties. This method was first published by Nash et al. (2001) to purify OB-derived OECs, and later, several studies utilized it with or without modifications (Huang et al., 2008). Kueh et al. (2011) used another method in which she and her colleagues immediately seeded the primary cells into a poly-D-lysine (PDL) coated dish. Every 3 days, the medium was changed, and the supernatant was collected and replated for the first two mediums changing. On the other hand, in another study, Rubio et al. (2008) demonstrated that OECs from primate’s bulb could be screened without subculturing in an uncoated plate (Rubio et al., 2008). Among all studies applying OECs as cell-based therapy, OB-derived OECs were the most commonly used due to their higher purification efficacy compared to the purity of OM-OECs. However, it would be more valuable to use OM-derived OECs in clinics due to their non-invasive accessible source (Ekberg & St John, 2015; Franceschini & Barnett, 1996; Gorrie et al., 2010; Lima et al., 2006). Only one study compared OECs with two different sources and reported no significant difference in outcome among the two OECs populations (Mayeur et al., 2013). According to their result, focusing on isolated OECs from OM rather than OB would be helpful due to their non-invasive accessible source.
It should be noted that in all studies employing differential adhesion methods, OB-derived OECs were used (Reshamwala et al., 2020). No study mentioned the difference between the OM and OB-derived-OECs based on the cell differential adhesion method. Therefore, in this study, we reported for the first time that differential adhesion protocol with some specific modifications, including time points for subculturing and the speed of centrifugation, could be used for isolating OECs from both OB and OM with high efficiency. According to the principle that the adhesion potential of OEC is lower than that of fibroblasts and astrocytes, we plated the harvested cells into an uncoated plate first for 36 h and the next step for 24 additional hours. There have been many trials and errors to get the best outcome. Using our suggested method with the optimum time points, OECs were screened for adhesive fibroblasts and astrocytes, and the purification efficacy was high in both OB- and OM-derived samples. Interestingly, our follow-up analysis revealed that our isolation method did not affect the cell viability of primary OECs. It should be noted that OB-derived OECs were purer than OM-derived OECs, as NGFR p75 expression was higher in OB-derived preparations while S100β expression was higher in OM-derived preparations. In contrast, OM-derived OECs had a higher viability percentage over time, indicating they are less vulnerable to cell death in primary cell preparations. Considering all aspects, OM-derived OECs are preferred for transplantation studies from a clinical point of view according to their non-invasive isolation method.
5. Conclusion
In conclusion, our results from the current study suggest a novel procedure for isolating OECs from both natural sources, the olfactory bulb and the olfactory mucosa. The purification process for harvested OECs is favored over other protocols due to its simplicity, efficacy, cost, and time saving. Additionally, OM-derived OECs isolated based on differential adhesion strategy had high purification and viability, so they can be considered a non-invasive source for cell-based therapy in the future.
Ethical Considerations
Compliance with ethical guidelines
The experimental procedures for treating animals were conducted following the Ethics Committee of Tehran University of Medical Sciences (Code: IR.TUMS.VCR.REC.1396.3859).
Funding
This study was supported by grants from the National Institute for Medical Research Development (NIMAD) (Grant No.: 957121), and the Tehran University of Medical Sciences (Grant No.: 36385).
Authors' contributions
Conceptualization, study design, methodology and writing: Mahmoudreza Hadjighassem and Fatemeh Tirgar; Experiments: Fatemeh Tirgar; Data interpretation: Zahra Azizi, Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank National Institute for Medical Research Development (NIMAD) and the Tehran University of Medical Sciences (TUMS) for their financial support.
References
Abdal Dayem, A., Lee, S., Y Choi, H., & Cho, S. G. (2018). The impact of adhesion molecules on the in vitro culture and differentiation of stem cells. Biotechnology Journal, 13(2), 10.1002/biot.201700575. [DOI:10.1002/biot.201700575] [PMID]
Barnett, S. C., & Riddell, J. S. (2004). Olfactory ensheathing cells (OECs) and the treatment of CNS injury: Advantages and possible caveats. Journal of Anatomy, 204(1), 57–67. [DOI:10.1111/j.1469-7580.2004.00257.x] [PMID] [PMCID]
Barton, M. J., John, J. S., Clarke, M., Wright, A., & Ekberg, J. (2017). The glia response after peripheral nerve injury: A comparison between schwann cells and olfactory ensheathing cells and their uses for neural regenerative therapies. International Journal of Molecular Sciences, 18(2), 287. [DOI:10.3390/ijms18020287] [PMID] [PMCID]
Bianco, J. I., Perry, C., Harkin, D. G., Mackay-Sim, A., & Féron, F. (2004). Neurotrophin 3 promotes purification and proliferation of olfactory ensheathing cells from human nose. Glia, 45(2), 111–123. [DOI:10.1002/glia.10298] [PMID]
Carvalho, L. A., Teng, J., Fleming, R. L., Tabet, E. I., Zinter, M., & de Melo Reis, R. A., et al. (2019). Olfactory ensheathing cells: A trojan horse for glioma gene therapy. Journal of the National Cancer Institute, 111(3), 283–291 [DOI:10.1093/jnci/djy138] [PMID] [PMCID]
Chen, L., Chen, D., Xi, H., Wang, Q., Liu, Y., & Zhang, F., et al. (2012). Olfactory ensheathing cell neurorestorotherapy for amyotrophic lateral sclerosis patients: Benefits from multiple transplantations. Cell Transplantation, 21(Suppl 1), S65–S77. [DOI:10.3727/096368912X633789] [PMID]
Dlouhy, B. J., Awe, O., Rao, R. C., Kirby, P. A., & Hitchon, P. W. (2014). Autograft-derived spinal cord mass following olfactory mucosal cell transplantation in a spinal cord injury patient: Case report. Journal of Neurosurgery. Spine, 21(4), 618–622. [DOI:10.3171/2014.5.SPINE13992] [PMID]
Doucette R. (1990). Glial influences on axonal growth in the primary olfactory system. Glia, 3(6), 433–449. [DOI:10.1002/glia.440030602] [PMID]
Ekberg, J. A., & St John, J. A. (2015). Olfactory ensheathing cells for spinal cord repair: Crucial differences between subpopulations of the glia. Neural Regeneration Research, 10(9), 1395–1396. [DOI:10.4103/1673-5374.165504] [PMID] [PMCID]
Féron, F., Perry, C., Cochrane, J., Licina, P., Nowitzke, A., & Urquhart, S., et al. (2005). Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain, 128(Pt 12), 2951–2960. [DOI:10.1093/brain/awh657] [PMID]
Franceschini, I. A., & Barnett, S. C. (1996). Low-affinity NGF-receptor and E-N-CAM expression define two types of olfactory nerve ensheathing cells that share a common lineage. Developmental Biology, 173(1), 327–343. [DOI:10.1006/dbio.1996.0027] [PMID]
Gong, Q., Bailey, M. S., Pixley, S. K., Ennis, M., Liu, W., & Shipley, M. T. (1994). Localization and regulation of low affinity nerve growth factor receptor expression in the rat olfactory system during development and regeneration. The Journal of Comparative Neurology, 344(3), 336–348. [DOI:10.1002/cne.903440303] [PMID]
Gorrie, C. A., Hayward, I., Cameron, N., Kailainathan, G., Nandapalan, N., & Sutharsan, R., et al. (2010). Effects of human OEC-derived cell transplants in rodent spinal cord contusion injury. Brain Research, 1337, 8–20. [DOI:10.1016/j.brainres.2010.04.019] [PMID]
Granger, N., Blamires, H., Franklin, R. J., & Jeffery, N. D. (2012). Autologous olfactory mucosal cell transplants in clinical spinal cord injury: A randomized double-blinded trial in a canine translational model. Brain, 135(Pt 11), 3227–3237. [DOI:10.1093/brain/aws268] [PMID] [PMCID]
Hashemi, M., Fallah, A., Aghayan, H. R., Arjmand, B., Yazdani, N., & Verdi, J., et al. (2016). A new approach in gene therapy of glioblastoma multiforme: Human olfactory ensheathing cells as a novel carrier for suicide gene delivery. Molecular Neurobiology, 53(8), 5118–5128. [DOI:10.1007/s12035-015-9412-y] [PMID]
Huang, Z. H., Wang, Y., Cao, L., Su, Z. D., Zhu, Y. L., & Chen, Y. Z., et al. (2008). Migratory properties of cultured olfactory ensheathing cells by single-cell migration assay. Cell Research, 18(4), 479–490. [DOI:10.1038/cr.2008.38] [PMID]
Kueh, J. L., Raisman, G., Li, Y., Stevens, R., & Li, D. (2011). Comparison of bulbar and mucosal olfactory ensheathing cells using FACS and simultaneous antigenic bivariate cell cycle analysis. Glia, 59(11), 1658–1671. [DOI:10.1002/glia.21213] [PMID]
Lankford, K. L., Sasaki, M., Radtke, C., & Kocsis, J. D. (2008). Olfactory ensheathing cells exhibit unique migratory, phagocytic, and myelinating properties in the X-irradiated spinal cord not shared by Schwann cells. Glia, 56(15), 1664–1678.[DOI:10.1002/glia.20718] [PMID]
Lima, C., Pratas-Vital, J., Escada, P., Hasse-Ferreira, A., Capucho, C., & Peduzzi, J. D. (2006). Olfactory mucosa autografts in human spinal cord injury: A pilot clinical study. The Journal of Spinal Cord Medicine, 29(3), 191–206. [DOI:10.1080/10790268.2006.11753874] [PMID] [PMCID]
Mackay-Sim, A., Féron, F., Cochrane, J., Bassingthwaighte, L., Bayliss, C., & Davies, W., et al. (2008). Autologous olfactory ensheathing cell transplantation in human paraplegia: A 3-year clinical trial. Brain, 131(Pt 9), 2376–2386. [DOI:10.1093/brain/awn173] [PMID] [PMCID]
Mayeur, A., Duclos, C., Honoré, A., Gauberti, M., Drouot, L., & do Rego, J. C., et al. (2013). Potential of olfactory ensheathing cells from different sources for spinal cord repair. Plos One, 8(4), e62860. [DOI:10.1371/journal.pone.0062860] [PMID] [PMCID]
Muñoz-Quiles, C., Santos-Benito, F. F., Llamusí, M. B., & Ramón-Cueto, A. (2009). Chronic spinal injury repair by olfactory bulb ensheathing glia and feasibility for autologous therapy. Journal of Neuropathology and Experimental Neurology, 68(12), 1294–1308. [DOI:10.1097/NEN.0b013e3181c34bbe] [PMID] [PMCID]
Nash, H. H., Borke, R. C., & Anders, J. J. (2001). New method of purification for establishing primary cultures of ensheathing cells from the adult olfactory bulb. Glia, 34(2), 81–87.[DOI:10.1002/glia.1043] [PMID]
Novikova, L. N., Lobov, S., Wiberg, M., & Novikov, L. N. (2011). Efficacy of olfactory ensheathing cells to support regeneration after spinal cord injury is influenced by method of culture preparation. Experimental Neurology, 229(1), 132–142. [DOI:10.1016/j.expneurol.2010.09.021] [PMID]
Pellitteri, R., Cova, L., Zaccheo, D., Silani, V., & Bossolasco, P. (2016). Phenotypic modulation and neuroprotective effects of olfactory ensheathing cells: A promising tool for cell therapy. Stem Cell Reviews and Reports, 12(2), 224–234. [DOI:10.1007/s12015-015-9635-3] [PMID]
Ramón-Cueto, A., & Nieto-Sampedro, M. (1992). Glial cells from adult rat olfactory bulb: Immunocytochemical properties of pure cultures of ensheathing cells. Neuroscience, 47(1), 213–220. [DOI:10.1016/0306-4522(92)90134-N] [PMID]
Reshamwala, R., Shah, M., Belt, L., Ekberg, J. A. K., & St John, J. A. (2020). Reliable cell purification and determination of cell purity: Crucial aspects of olfactory ensheathing cell transplantation for spinal cord repair. Neural Regeneration Research, 15(11), 2016–2026. [DOI:10.4103/1673-5374.282218] [PMID] [PMCID]
Rubio, M. P., Muñoz-Quiles, C., & Ramón-Cueto, A. (2008). Adult olfactory bulbs from primates provide reliable ensheathing glia for cell therapy. Glia, 56(5), 539–551. [DOI:10.1002/glia.20635] [PMID]
Su, Z., Chen, J., Qiu, Y., Yuan, Y., Zhu, F., & Zhu, Y.,et al. (2013). Olfactory ensheathing cells: The primary innate immunocytes in the olfactory pathway to engulf apoptotic olfactory nerve debris. Glia, 61(4), 490–503. [DOI:10.1002/glia.22450] [PMID]
Tabakow, P., Jarmundowicz, W., Czapiga, B., Fortuna, W., Miedzybrodzki, R., & Czyz, M., et al. (2013). Transplantation of autologous olfactory ensheathing cells in complete human spinal cord injury. Cell Transplantation, 22(9), 1591–1612. [DOI:10.3727/096368912X663532] [PMID]
Toft, A., Tomé, M., Lindsay, S. L., Barnett, S. C., & Riddell, J. S. (2012). Transplant-mediated repair properties of rat olfactory mucosal OM-I and OM-II sphere-forming cells. Journal of Neuroscience Research, 90(3), 619–631. [DOI:10.1002/jnr.22789] [PMID]
Torres-Espín, A., Hernández, J., & Navarro, X. (2013). Gene expression changes in the injured spinal cord following transplantation of mesenchymal stem cells or olfactory ensheathing cells. Plos One, 8(10), e76141. [DOI:10.1371/journal.pone.0076141] [PMID] [PMCID]
Woodworth, C. F., Jenkins, G., Barron, J., & Hache, N. (2019). Intramedullary cervical spinal mass after stem cell transplantation using an olfactory mucosal cell autograft. CMAJ, 191(27), E761-E764. [DOI:10.1503/cmaj.181696] [PMID] [PMCID]
Yao, R., Murtaza, M., Velasquez, J. T., Todorovic, M., Rayfield, A., & Ekberg, J., et al. (2018). Olfactory ensheathing cells for spinal cord injury: Sniffing out the issues. Cell Transplantation, 27(6), 879-889. [DOI:10.1177/0963689718779353] [PMID] [PMCID]
Ziege, S., Baumgärtner, W., & Wewetzer, K. (2013). Toward defining the regenerative potential of olfactory mucosa: Establishment of Schwann cell-free adult canine olfactory ensheathing cell preparations suitable for transplantation. Cell Transplantation, 22(2), 355-367. [DOI:10.3727/096368912X656108] [PMID]
Currently, there are an increasing number of studies that utilize isolated olfactory ensheathing cells (OECs) for transplantation in different pathologies, such as spinal cord injury (Feron et al., 2005; Mackay-Sim et al., 2008; Tabakow et al., 2013) and amyotrophic lateral sclerosis (Chen et al., 2012) with some promising clinical results. The high migration potency, neurotrophic factors secretion, inherent pathotropism toward inflammatory cues, and non-invasive accessible source of OECs, as glial cells, make them appropriate for cell therapies (Hashemi et al., 2016; Lankford, et al., 2008; Pellitteri et al., 2016; Su et al., 2013). According to previous studies, there are two primary sources for isolating OECs: Olfactory bulb (OB) in the brain and olfactory mucosa (OM) in the nasal cavity.
Isolation and purification of specific cell types from a heterogeneous population of different cells are essential for in vitro and in vivo studies and clinical cell-based therapies. According to the researcher’s demand, various techniques are applied for primary OEC isolation (Reshamwala et al., 2020). The high purity and viability of isolated cells are critical issues that should be considered. In addition to several other cell types that accompany OB and OM, cell contamination during the isolation procedure may cause tumor mass, indicating the importance of purification of OECs before transplantation to avoid unwanted side effects (Dlouhy et al., 2014; Woodworth et al., 2019). Based on adherence properties, different cells exert various attachment behaviors. For example, fibroblasts have a high affinity to attach to the surface, whereas this affinity is lower in OECs (Reshamwala et al., 2020). Cell adhesion molecules are essential in interacting with the cells and the culture surface (Abdal Dayem & Cho, 2018).
Accordingly, it will help achieve a novel, non-invasive OECs isolation strategy with high efficiency and less expensive than the previous studies. In this manner, we successfully introduced a new rapid and cost-effective approach for the isolation and purification of OECs from rat OM as efficiently as OB, along with the difference between the two sources.
2. Materials and Methods
Study animals
OECs were isolated from OM and OB of 12 neonatal Wistar rats. All efforts were made to reduce animal suffering and minimize the number of rats used for these tests. The rats were housed in cages in groups of four, and their access to food and water was controlled every day. The animal’s cages were kept at 23±1°C under a 12/12-h light-dark cycle, which began at 7:00 AM. The experimental procedures for the treatment of animals were conducted in accordance with the Ethics Committee of Tehran University of Medical Sciences.
Primary OECs isolation
OECs were isolated from OM and OB of rats utilizing the previous original protocol with modifications (Ramón-Cueto & Nieto-Sampedro, 1992). Briefly, animals were anesthetized using ketamine hydrochloride (100 mg/kg, Alfasan Co., Woerden, Netherlands) and xylazine (10 mg/kg, Alfasan Co., Woerden, Netherlands). To obtain OECs from OBs, we removed the skull bones from the top and harvested the OBs. Collected OBs were rinsed with sterile, cold phosphate-buffered saline (PBS) and then transferred to a dish containing 5% antibiotic/antimycotic (Gibco, Carlsbad, CA, USA). The whole isolation procedure should take less than 10 minutes. At first, the samples were thoroughly washed with PBS so that the external blood was gently washed from the sample, and with the help of a razor, the internal blood vessels were gently separated.
OECs in OB are located in the bulb’s two external layers, typically termed olfactory nerve glomerular layers (ONGLs). Employing a stereo microscope instrument, the ONGLs were separated by dissection forceps. Next, ONGL underwent mechanical and chemical homogenization. For this purpose, we used a scalpel blade for chopping and then 0.25% trypsin EDTA (Gibco, Carlsbad, CA, USA) for chemical dissociation. Trypsinization lasted about 15 minutes at 37°C. Subsequently, we added Dulbecco’s modified eagle medium/nutrient mixture F12 (DMEM/F12; Gibco, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA, USA) to end the trypsinization. The isolated heterogeneous samples were centrifuged for 10 minutes at 1200 rpm; the cell pellet was transferred to an uncoated petri dish and incubated with DMEM/F12 medium supplemented with 10% FBS and 1% antibiotic/antimycotic (Gibco, Carlsbad, CA, USA). Based on differential adhesion properties among astrocytes, fibroblasts, and OECs, the latter cells have a low affinity to attach to the uncoated surface. Therefore, OECs were easily separated from other cells by transferring the supernatant to another uncoated Petri dish at 36-h post isolation. We repeated this procedure for another 24 h to eliminate the cell contamination and achieve high purification. Finally, after 60 h, the supernatant containing isolated OECs was transferred to a coated flask and incubated at 37°C under 90% humidity containing 5% CO2, replacing the medium every two days. We performed the same protocol for isolating OECs from OM, except we isolated the cells from the posterior part of the nasal cavity near the septum, and the epithelium was dissociated from the lamina propria. All images from both preparations were taken by light inverted microscope (Labomed TCM 400, CA, USA) at different time points.
Immunocytofluorescent
After 21 days, the cells were characterized with immunofluorescent using a selective marker, nerve growth factor receptor p75 (1:200, mouse monoclonal anti-NGFR p75 primary antibody, Santa Cruz, Biotechnology Inc., USA). About 3×105 OECs were seeded in a 6-well plate to achieve this goal. On the next day, the cells were fixed by 4% paraformaldehyde, and then 10% bovine serum albumin (BSA, Sigma-Aldrich St. Louis, MO, USA) and 0.3% Triton X-100 (ATOCEL, Austria) were added for 45 minutes. Afterward, the cells were incubated with anti-NGFR p75 primary antibody overnight at 4°C. Incubation with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG secondary antibody (1:200 Biorbyt Ltd, Cambridge, UK) was performed for 1 h at room temperature. As a nuclear stainer, we used 4,6-diamidino-2-phenylindole (DAPI; 1.5 µg/mL, Santa Cruz, Biotechnology Inc., USA). Eventually, NGFR p75 expression was detected by an inverted fluorescence microscope (OPTIKA Srl, Ponteranica BG, Italy) and quantified by ImageJ software, version 1.52 (National Institute of Health, USA).
Western blotting
Western blot analysis was performed to detect S100β as a selective marker for OECs in three independent samples. Briefly, total protein extract was obtained, and protein concentration was measured using the well-established Bradford assay. Total protein (50 µg) was loaded on an SDS-PAGE and transferred onto a PVDF membrane (Amersham, GE Healthcare, Piscataway, USA). Blocking was carried out using 5% BSA for 60 min. Blots were incubated with S100β primary antibody (1:1000, rabbit monoclonal anti-S100β primary antibody (Abcam, Cambridge, MA, USA) overnight at 4°C. Next, blots were washed with Tris-buffered saline-Tween 20 (TBS-T) thrice and incubated with secondary antibody (1:5000, Goat polyclonal anti-rabbit IgG H&L horseradish peroxidase (HRP) secondary antibody (Abcam, Cambridge, MA, USA) for 90 min at room temperature. Finally, S100β detection was performed using a chemiluminescence detection kit (Pars Tous Biotechnology, Mashhad, Iran). Anti-β-actin primary antibody (1:2500, rabbit polyclonal anti-β-actin primary antibody, PADZA Co., Tehran, Iran) was used as a loading control. The S100β amounts were quantified to their corresponding β-actin levels using ImageJ software.
Cell viability
To assess whether the isolation protocol affects cell survival, OECs viability was evaluated by 3-(4,5-dimethylthiazol2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich, Sigma-Aldrich, Saint Louis, MO, USA) assays. A total number of 5×103 OECs were seeded in a 96-well plate at different time points (7, 14, and 28 days post-isolation). MTT (0.5 mg/mL) was added in the next day, and OECs were incubated for 4 h at 37°C. Following the removal of MTT, DMSO (100 μL) was added. Finally, absorbance was obtained at 570 nm utilizing an ELISA reader (Bio-Tek Instruments Inc., VT, USA).
Statistical analysis
GraphPad Prism software, version 6.01 (GraphPad Software, Inc., CA, USA) was used for statistical analysis. Data were analyzed using a t-test for comparing two groups and one-way ANOVA for comparing more than two groups, followed by the Tukey post hoc test. All data were presented as Mean±SD (n=3), and P<0.05 was considered significant.
3. Results
Morphology of isolated OB- and OM-derived OECs
According to our suggested protocol, OECs were successfully isolated from rat OB and OM (Figure 1).

As illustrated in Figure 2, isolated OECs at different time points had normal growth and proliferation rates. The OECs on day 7 post-isolation procedure have spindle- to satellite-like shapes. At this time, both OB-derived and OM-derived OECs exhibited the same morphology (Figure 2a). However, the difference became apparent at day 28 post-isolation. At this time, most OB-derived OECs showed stellate-like multipolar morphology with short processes, unlike most OM-derived OECs, which exhibited spindle-like bipolar morphology (Figure 2b).

Characterization of isolated OB- and OM-derived OECs
Immunofluorescence analysis visualized the expression of NGFR p75 as a selective marker for OECs. OM-derived OECs highly expressed NGFR P75 and OB-derived OECs (Figure 3). This protocol guaranteed the high purity of OECs-derived from both sources. It should be noted that the NGFR P75 expression was higher in OB-derived OECs.

OECs characterization was further verified by western blot against S100β protein, another specific marker for OECs. S100β protein expression was high in both OB- and OM-derived OECs. However, this expression increased in OM-derived OECs (Figure 4a). Quantification of band densities highlighted the significant difference between the two OEC populations from different origins (P<0.05, t=2.9, df=4) (Figure 4b).
Taken together, based on the principle that the adhesion potential of OECs is lower than that of fibroblasts and astrocytes, OECs were successfully screened for adhesive fibroblasts and astrocytes from both OB and OM of rats.

Cell viability of isolated OB- and OM-derived OECs
The MTT assay was performed to evaluate the OECs viability at different time points. Our results demonstrated that cell viability was near 100% in both OB- and OM-derived OEC cultures on day 7 and day 14 post-isolation. On day 28, there was a decrease in cell viability in OB-derived OECs culture. However, the reduction was not significant, indicating that OB-derived primary OECs may be slightly more vulnerable to death compared to the OM-derived OECs in primary cultures (P>0.05) (Figure 5).

Our new approach demonstrated that isolating OECs from both sources may not significantly alter the cell until at least 28 days post-isolation.
4. Discussion
Recently, olfactory ensheathing cells have been considered alternative cell therapy in numerous diseases. The regeneration potency of OECs (Doucette, 1990), secretion of neurotrophic and guidance factors (Barnett & Riddell, 2004; Barton et al., 2017), phagocytosis ability (Su et al., 2013), migration capability (Hashemi et al., 2016), and restorative potential combined with an accessible non-invasive source of OECs make them suitable for transplantation studies (Carvalho et al., 2019). In this manner, autologous graft strategy may exert some promise due to overcoming ethical issues (Granger et al., 2012; Muñoz-Quiles et al., 2009; Tabakow et al., 2013).
OECs, as glial-like cells, support and convoy the olfactory axons extending from the nasal mucosa to the olfactory bulb in their natural location (Carvalho et al., 2019). Either OM or OB can be considered an OECs source. Hence, OECs can be isolated from both, although other cell populations exist in each preparation (Yao et al., 2018). One of the major challenges of these methods is purification. There are different techniques to screen cells, such as their size, but sometimes different cell types in a population have nearly the same size, so we need an alternative method to separate them. There is little evidence that addresses this point of view.
Previous studies reported different isolation methods that harvested the OECs from OB or OM. OECs purification from a heterogeneous cell population is the most considerable issue before OEC transplantation studies. However, the isolation methods and their outcome show some controversy and variability based on the origin of the OECs. Among different methods for OECs purification, techniques based on cell-specific markers and isolation due to the differential adhesion properties are more frequently used. Usually, the isolation of OECs from OB employs immunofluorescent-based combined with differential adherence-based purification, while the isolation of OECs from OM utilizes enrichment of the environment (Reshamwala et al., 2020). The immuno-purification method relies on the expression of NGFR p75 as the most common and well-characterized selective hallmark of OECs or other relative markers such as S100β (Bianco et al., 2004; Franceschini & Barnett, 1996; Gong et al., 1994; Novikova et al., 2012; Torres-Espín et al., 2013). It should be noted that there would be cell contamination in isolated OECs preparations, whether from the OB or OM source, due to trigeminal nerve Schwann cells. In our study, as shown in Figure 4, S100β expression was higher in OM-derived OECs, probably due to the presence of numerous Schwann cells in the mucosa (Ziege et al., 2013).
The latter one, differential adhesion properties, is based on different time points in which different cell types must be attached to the surface according to their properties. This method was first published by Nash et al. (2001) to purify OB-derived OECs, and later, several studies utilized it with or without modifications (Huang et al., 2008). Kueh et al. (2011) used another method in which she and her colleagues immediately seeded the primary cells into a poly-D-lysine (PDL) coated dish. Every 3 days, the medium was changed, and the supernatant was collected and replated for the first two mediums changing. On the other hand, in another study, Rubio et al. (2008) demonstrated that OECs from primate’s bulb could be screened without subculturing in an uncoated plate (Rubio et al., 2008). Among all studies applying OECs as cell-based therapy, OB-derived OECs were the most commonly used due to their higher purification efficacy compared to the purity of OM-OECs. However, it would be more valuable to use OM-derived OECs in clinics due to their non-invasive accessible source (Ekberg & St John, 2015; Franceschini & Barnett, 1996; Gorrie et al., 2010; Lima et al., 2006). Only one study compared OECs with two different sources and reported no significant difference in outcome among the two OECs populations (Mayeur et al., 2013). According to their result, focusing on isolated OECs from OM rather than OB would be helpful due to their non-invasive accessible source.
It should be noted that in all studies employing differential adhesion methods, OB-derived OECs were used (Reshamwala et al., 2020). No study mentioned the difference between the OM and OB-derived-OECs based on the cell differential adhesion method. Therefore, in this study, we reported for the first time that differential adhesion protocol with some specific modifications, including time points for subculturing and the speed of centrifugation, could be used for isolating OECs from both OB and OM with high efficiency. According to the principle that the adhesion potential of OEC is lower than that of fibroblasts and astrocytes, we plated the harvested cells into an uncoated plate first for 36 h and the next step for 24 additional hours. There have been many trials and errors to get the best outcome. Using our suggested method with the optimum time points, OECs were screened for adhesive fibroblasts and astrocytes, and the purification efficacy was high in both OB- and OM-derived samples. Interestingly, our follow-up analysis revealed that our isolation method did not affect the cell viability of primary OECs. It should be noted that OB-derived OECs were purer than OM-derived OECs, as NGFR p75 expression was higher in OB-derived preparations while S100β expression was higher in OM-derived preparations. In contrast, OM-derived OECs had a higher viability percentage over time, indicating they are less vulnerable to cell death in primary cell preparations. Considering all aspects, OM-derived OECs are preferred for transplantation studies from a clinical point of view according to their non-invasive isolation method.
5. Conclusion
In conclusion, our results from the current study suggest a novel procedure for isolating OECs from both natural sources, the olfactory bulb and the olfactory mucosa. The purification process for harvested OECs is favored over other protocols due to its simplicity, efficacy, cost, and time saving. Additionally, OM-derived OECs isolated based on differential adhesion strategy had high purification and viability, so they can be considered a non-invasive source for cell-based therapy in the future.
Ethical Considerations
Compliance with ethical guidelines
The experimental procedures for treating animals were conducted following the Ethics Committee of Tehran University of Medical Sciences (Code: IR.TUMS.VCR.REC.1396.3859).
Funding
This study was supported by grants from the National Institute for Medical Research Development (NIMAD) (Grant No.: 957121), and the Tehran University of Medical Sciences (Grant No.: 36385).
Authors' contributions
Conceptualization, study design, methodology and writing: Mahmoudreza Hadjighassem and Fatemeh Tirgar; Experiments: Fatemeh Tirgar; Data interpretation: Zahra Azizi, Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank National Institute for Medical Research Development (NIMAD) and the Tehran University of Medical Sciences (TUMS) for their financial support.
References
Abdal Dayem, A., Lee, S., Y Choi, H., & Cho, S. G. (2018). The impact of adhesion molecules on the in vitro culture and differentiation of stem cells. Biotechnology Journal, 13(2), 10.1002/biot.201700575. [DOI:10.1002/biot.201700575] [PMID]
Barnett, S. C., & Riddell, J. S. (2004). Olfactory ensheathing cells (OECs) and the treatment of CNS injury: Advantages and possible caveats. Journal of Anatomy, 204(1), 57–67. [DOI:10.1111/j.1469-7580.2004.00257.x] [PMID] [PMCID]
Barton, M. J., John, J. S., Clarke, M., Wright, A., & Ekberg, J. (2017). The glia response after peripheral nerve injury: A comparison between schwann cells and olfactory ensheathing cells and their uses for neural regenerative therapies. International Journal of Molecular Sciences, 18(2), 287. [DOI:10.3390/ijms18020287] [PMID] [PMCID]
Bianco, J. I., Perry, C., Harkin, D. G., Mackay-Sim, A., & Féron, F. (2004). Neurotrophin 3 promotes purification and proliferation of olfactory ensheathing cells from human nose. Glia, 45(2), 111–123. [DOI:10.1002/glia.10298] [PMID]
Carvalho, L. A., Teng, J., Fleming, R. L., Tabet, E. I., Zinter, M., & de Melo Reis, R. A., et al. (2019). Olfactory ensheathing cells: A trojan horse for glioma gene therapy. Journal of the National Cancer Institute, 111(3), 283–291 [DOI:10.1093/jnci/djy138] [PMID] [PMCID]
Chen, L., Chen, D., Xi, H., Wang, Q., Liu, Y., & Zhang, F., et al. (2012). Olfactory ensheathing cell neurorestorotherapy for amyotrophic lateral sclerosis patients: Benefits from multiple transplantations. Cell Transplantation, 21(Suppl 1), S65–S77. [DOI:10.3727/096368912X633789] [PMID]
Dlouhy, B. J., Awe, O., Rao, R. C., Kirby, P. A., & Hitchon, P. W. (2014). Autograft-derived spinal cord mass following olfactory mucosal cell transplantation in a spinal cord injury patient: Case report. Journal of Neurosurgery. Spine, 21(4), 618–622. [DOI:10.3171/2014.5.SPINE13992] [PMID]
Doucette R. (1990). Glial influences on axonal growth in the primary olfactory system. Glia, 3(6), 433–449. [DOI:10.1002/glia.440030602] [PMID]
Ekberg, J. A., & St John, J. A. (2015). Olfactory ensheathing cells for spinal cord repair: Crucial differences between subpopulations of the glia. Neural Regeneration Research, 10(9), 1395–1396. [DOI:10.4103/1673-5374.165504] [PMID] [PMCID]
Féron, F., Perry, C., Cochrane, J., Licina, P., Nowitzke, A., & Urquhart, S., et al. (2005). Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain, 128(Pt 12), 2951–2960. [DOI:10.1093/brain/awh657] [PMID]
Franceschini, I. A., & Barnett, S. C. (1996). Low-affinity NGF-receptor and E-N-CAM expression define two types of olfactory nerve ensheathing cells that share a common lineage. Developmental Biology, 173(1), 327–343. [DOI:10.1006/dbio.1996.0027] [PMID]
Gong, Q., Bailey, M. S., Pixley, S. K., Ennis, M., Liu, W., & Shipley, M. T. (1994). Localization and regulation of low affinity nerve growth factor receptor expression in the rat olfactory system during development and regeneration. The Journal of Comparative Neurology, 344(3), 336–348. [DOI:10.1002/cne.903440303] [PMID]
Gorrie, C. A., Hayward, I., Cameron, N., Kailainathan, G., Nandapalan, N., & Sutharsan, R., et al. (2010). Effects of human OEC-derived cell transplants in rodent spinal cord contusion injury. Brain Research, 1337, 8–20. [DOI:10.1016/j.brainres.2010.04.019] [PMID]
Granger, N., Blamires, H., Franklin, R. J., & Jeffery, N. D. (2012). Autologous olfactory mucosal cell transplants in clinical spinal cord injury: A randomized double-blinded trial in a canine translational model. Brain, 135(Pt 11), 3227–3237. [DOI:10.1093/brain/aws268] [PMID] [PMCID]
Hashemi, M., Fallah, A., Aghayan, H. R., Arjmand, B., Yazdani, N., & Verdi, J., et al. (2016). A new approach in gene therapy of glioblastoma multiforme: Human olfactory ensheathing cells as a novel carrier for suicide gene delivery. Molecular Neurobiology, 53(8), 5118–5128. [DOI:10.1007/s12035-015-9412-y] [PMID]
Huang, Z. H., Wang, Y., Cao, L., Su, Z. D., Zhu, Y. L., & Chen, Y. Z., et al. (2008). Migratory properties of cultured olfactory ensheathing cells by single-cell migration assay. Cell Research, 18(4), 479–490. [DOI:10.1038/cr.2008.38] [PMID]
Kueh, J. L., Raisman, G., Li, Y., Stevens, R., & Li, D. (2011). Comparison of bulbar and mucosal olfactory ensheathing cells using FACS and simultaneous antigenic bivariate cell cycle analysis. Glia, 59(11), 1658–1671. [DOI:10.1002/glia.21213] [PMID]
Lankford, K. L., Sasaki, M., Radtke, C., & Kocsis, J. D. (2008). Olfactory ensheathing cells exhibit unique migratory, phagocytic, and myelinating properties in the X-irradiated spinal cord not shared by Schwann cells. Glia, 56(15), 1664–1678.[DOI:10.1002/glia.20718] [PMID]
Lima, C., Pratas-Vital, J., Escada, P., Hasse-Ferreira, A., Capucho, C., & Peduzzi, J. D. (2006). Olfactory mucosa autografts in human spinal cord injury: A pilot clinical study. The Journal of Spinal Cord Medicine, 29(3), 191–206. [DOI:10.1080/10790268.2006.11753874] [PMID] [PMCID]
Mackay-Sim, A., Féron, F., Cochrane, J., Bassingthwaighte, L., Bayliss, C., & Davies, W., et al. (2008). Autologous olfactory ensheathing cell transplantation in human paraplegia: A 3-year clinical trial. Brain, 131(Pt 9), 2376–2386. [DOI:10.1093/brain/awn173] [PMID] [PMCID]
Mayeur, A., Duclos, C., Honoré, A., Gauberti, M., Drouot, L., & do Rego, J. C., et al. (2013). Potential of olfactory ensheathing cells from different sources for spinal cord repair. Plos One, 8(4), e62860. [DOI:10.1371/journal.pone.0062860] [PMID] [PMCID]
Muñoz-Quiles, C., Santos-Benito, F. F., Llamusí, M. B., & Ramón-Cueto, A. (2009). Chronic spinal injury repair by olfactory bulb ensheathing glia and feasibility for autologous therapy. Journal of Neuropathology and Experimental Neurology, 68(12), 1294–1308. [DOI:10.1097/NEN.0b013e3181c34bbe] [PMID] [PMCID]
Nash, H. H., Borke, R. C., & Anders, J. J. (2001). New method of purification for establishing primary cultures of ensheathing cells from the adult olfactory bulb. Glia, 34(2), 81–87.[DOI:10.1002/glia.1043] [PMID]
Novikova, L. N., Lobov, S., Wiberg, M., & Novikov, L. N. (2011). Efficacy of olfactory ensheathing cells to support regeneration after spinal cord injury is influenced by method of culture preparation. Experimental Neurology, 229(1), 132–142. [DOI:10.1016/j.expneurol.2010.09.021] [PMID]
Pellitteri, R., Cova, L., Zaccheo, D., Silani, V., & Bossolasco, P. (2016). Phenotypic modulation and neuroprotective effects of olfactory ensheathing cells: A promising tool for cell therapy. Stem Cell Reviews and Reports, 12(2), 224–234. [DOI:10.1007/s12015-015-9635-3] [PMID]
Ramón-Cueto, A., & Nieto-Sampedro, M. (1992). Glial cells from adult rat olfactory bulb: Immunocytochemical properties of pure cultures of ensheathing cells. Neuroscience, 47(1), 213–220. [DOI:10.1016/0306-4522(92)90134-N] [PMID]
Reshamwala, R., Shah, M., Belt, L., Ekberg, J. A. K., & St John, J. A. (2020). Reliable cell purification and determination of cell purity: Crucial aspects of olfactory ensheathing cell transplantation for spinal cord repair. Neural Regeneration Research, 15(11), 2016–2026. [DOI:10.4103/1673-5374.282218] [PMID] [PMCID]
Rubio, M. P., Muñoz-Quiles, C., & Ramón-Cueto, A. (2008). Adult olfactory bulbs from primates provide reliable ensheathing glia for cell therapy. Glia, 56(5), 539–551. [DOI:10.1002/glia.20635] [PMID]
Su, Z., Chen, J., Qiu, Y., Yuan, Y., Zhu, F., & Zhu, Y.,et al. (2013). Olfactory ensheathing cells: The primary innate immunocytes in the olfactory pathway to engulf apoptotic olfactory nerve debris. Glia, 61(4), 490–503. [DOI:10.1002/glia.22450] [PMID]
Tabakow, P., Jarmundowicz, W., Czapiga, B., Fortuna, W., Miedzybrodzki, R., & Czyz, M., et al. (2013). Transplantation of autologous olfactory ensheathing cells in complete human spinal cord injury. Cell Transplantation, 22(9), 1591–1612. [DOI:10.3727/096368912X663532] [PMID]
Toft, A., Tomé, M., Lindsay, S. L., Barnett, S. C., & Riddell, J. S. (2012). Transplant-mediated repair properties of rat olfactory mucosal OM-I and OM-II sphere-forming cells. Journal of Neuroscience Research, 90(3), 619–631. [DOI:10.1002/jnr.22789] [PMID]
Torres-Espín, A., Hernández, J., & Navarro, X. (2013). Gene expression changes in the injured spinal cord following transplantation of mesenchymal stem cells or olfactory ensheathing cells. Plos One, 8(10), e76141. [DOI:10.1371/journal.pone.0076141] [PMID] [PMCID]
Woodworth, C. F., Jenkins, G., Barron, J., & Hache, N. (2019). Intramedullary cervical spinal mass after stem cell transplantation using an olfactory mucosal cell autograft. CMAJ, 191(27), E761-E764. [DOI:10.1503/cmaj.181696] [PMID] [PMCID]
Yao, R., Murtaza, M., Velasquez, J. T., Todorovic, M., Rayfield, A., & Ekberg, J., et al. (2018). Olfactory ensheathing cells for spinal cord injury: Sniffing out the issues. Cell Transplantation, 27(6), 879-889. [DOI:10.1177/0963689718779353] [PMID] [PMCID]
Ziege, S., Baumgärtner, W., & Wewetzer, K. (2013). Toward defining the regenerative potential of olfactory mucosa: Establishment of Schwann cell-free adult canine olfactory ensheathing cell preparations suitable for transplantation. Cell Transplantation, 22(2), 355-367. [DOI:10.3727/096368912X656108] [PMID]
Type of Study: Original |
Subject:
Cellular and molecular Neuroscience
Received: 2021/01/16 | Accepted: 2022/04/12 | Published: 2024/03/1
Received: 2021/01/16 | Accepted: 2022/04/12 | Published: 2024/03/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








