Volume 14, Issue 2 (March & April 2023)
BCN 2023, 14(2): 297-310 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Aliyari H, Golabi S, Sahraei H, Sahraei M, Minaei-Bidgoli B, Daliri M R, et al . Perceived Stress and Cfognition Function Quantification in a Scary Video Game: An Electroencephalogram Features and Biochemical Measures. BCN 2023; 14 (2) :297-310
URL: http://bcn.iums.ac.ir/article-1-2326-en.html
URL: http://bcn.iums.ac.ir/article-1-2326-en.html
Hamed Aliyari1 

 , Sahar Golabi2
, Sahar Golabi2 

 , Hedayat Sahraei3
, Hedayat Sahraei3 

 , Mohammad Sahraei4
, Mohammad Sahraei4 

 , Behrouz Minaei-Bidgoli5
, Behrouz Minaei-Bidgoli5 

 , Mohammad Reza Daliri6
, Mohammad Reza Daliri6 

 , Reza Hazrati7
, Reza Hazrati7 

 , Hamed Tadayyoni8
, Hamed Tadayyoni8 

 , Masoomeh Kazemi *3
, Masoomeh Kazemi *3 




 , Sahar Golabi2
, Sahar Golabi2 

 , Hedayat Sahraei3
, Hedayat Sahraei3 

 , Mohammad Sahraei4
, Mohammad Sahraei4 

 , Behrouz Minaei-Bidgoli5
, Behrouz Minaei-Bidgoli5 

 , Mohammad Reza Daliri6
, Mohammad Reza Daliri6 

 , Reza Hazrati7
, Reza Hazrati7 

 , Hamed Tadayyoni8
, Hamed Tadayyoni8 

 , Masoomeh Kazemi *3
, Masoomeh Kazemi *3 


1- Department of Psychiatry, University of Texas Southwestern Medical Centre at Dallas, Dallas, United States.
2- Department of Medical Physiology, School of Medicine, Abadan University of Medical Sciences, Abadan, Iran.
3- Neuroscience Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran.
4- Department of Dentistry, School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran Iran.
5- Department of Computer Engineering, School of Computer Engineering, University of Science and Technology, Tehran, Iran.
6- Department of Electrical Engineering, School of Electrical Engineering, University of Science and Technology, Tehran, Iran.
7- CERVO Brain Research Center, Laval University, Quebec, Canada.
8- Faculty of Health Sciences, Ontario Tech University, Oshawa, Ontario, Canada.
2- Department of Medical Physiology, School of Medicine, Abadan University of Medical Sciences, Abadan, Iran.
3- Neuroscience Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran.
4- Department of Dentistry, School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran Iran.
5- Department of Computer Engineering, School of Computer Engineering, University of Science and Technology, Tehran, Iran.
6- Department of Electrical Engineering, School of Electrical Engineering, University of Science and Technology, Tehran, Iran.
7- CERVO Brain Research Center, Laval University, Quebec, Canada.
8- Faculty of Health Sciences, Ontario Tech University, Oshawa, Ontario, Canada.
Keywords: Brain-derived neurotrophic factor, Cognition, Cortisol, Salivary alpha-amylase, Oxytocin, Scary video game, Stress
Full-Text [PDF 780 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
It is indicated that negative environmental experiences can affect organisms’ homeostasis. This situation is called stress by some researchers (Dhama et al., 2019). Recently, research on the effects of video games on the stress system and cognitive potencies has attracted the researchers’ attention (Anderson et al., 2010; Porter & Goolkasian, 2019; Bavelier et al., 2011). Video games can affect the stress system (Téllez et al., 2023). It also was shown that different types of video games (gaming style) have different influences on the central nervous system (CNS) function (Trepte & Reinecke, 2010). Since games are used frequently by gamers, it is not surprising that the influence of game playing on the CNS function may be significant (Chonmaitree et al., 2008; Trepte & Reinecke, 2010). In this regard, playing video games is shown to have positive or negative effects on cognitive abilities (Aliyari et al., 2015; Aliyari et al., 2018a; Best, 2010; Boyle et al., 2011; Kaplan & Berman, 2010; Mastorakos & Ilias, 2003; Rabipour & Raz, 2012; Schultheiss & Stanton, 2009). Chronic stress can alter several body systems and promotes multiple health problems including neurological and neuropsychiatric disorders (Aliyari et al., 2018a; McKlveen et al., 2016). Stress is shown to activate the hypothalamus-pituitary-adrenal (HPA) axis and sympathetic nervous system. The activity of these systems results in glucocorticoid hormones and norepinephrine secretion. Experiments indicated that glucocorticoids can readily cross the barriers to be presented in the saliva. However, since norepinephrine cannot be detected in the saliva, it is postulated that salivary alpha-amylase concentration which is sensitive to the norepinephrine could be used as an indicator for the detection of the changes in norepinephrine concentration variations (Dhama et al., 2019). Activity in the brain stress systems leads to a change in the level of salivary cortisol and alpha-amylase (Diorio et al., 1993; Gatti & De Palo, 2011; Kreutz et al., 2004). As mentioned above, stress responses can produce diverse effects on the brain. So, cortisol and norepinephrine as stress biomarkers also affect cognitive abilities (Geoffroy et al., 2012; Lee et al., 2007; Maldonado et al., 2019). On the other hand, the brain stress system and oxytocin hormone (OT) are shown to have interaction in several ways. OT is implicated in the modulation of the HPA axis activity at several levels. Also, it was shown that OTergic neurons in the hypothalamus are activated during stressful experiences (Kotwica et al., 2004; McQuaid et al., 2016; Onaka, 2019). Moreover, OT has been implicated in a variety of cognitive processes and psychological stresses also may trigger the release of OT (Bendix et al., 2015; Wagner & Echterhoff, 2018). Studies also revealed that video games affect the endocrine system. Video games can change cortisol, norepinephrine, and OT secretion which in fact may lead to behavioral and cognitive impairments (Aliyari et al., 2015; Schultheiss & Stanton, 2009). Moreover, brain-derived neurotrophic factor (BDNF), has also a crucial role in neuronal and plastic development (Johnston, 2009). BDNF is also affected by stress. It has been shown that the BDNF expression in the brain is regulated by stress, and a role has been suggested for BDNF in recurrent depression, aging, and posttraumatic stress disorder (Licinio & Wong, 2002). It was revealed that in chronic stress situations, during which the levels of pro-inflammatory cytokines and glucocorticoids are consistently high, the BDNF gene is suppressed, resulting in brain atrophy and contributing to the development of mental disorders in predisposed subjects (Buselli et al., 2019). Based on these facts, about the relationship between stress and cognitive brain function, it is hypothesized that if the stress system is affected by a particular game, the person is unable to properly and timely make a decision (Aliyari et al., 2015).
One of the most important markers of brain activity is the electroencephalogram. (Bullmore & Sporns, 2009; Garrett, 2014). It is shown that electroencephalography (EEG) can be used for the evaluation of stress states and cognitive functions. In this regard, it is clear that the frontal lobe EEG is the best signal for the evaluation of the stress state (Al-Shargie, 2016). Studies showed that among the different waves of the EEG, alpha waves may better indicator of cognitive function (Bashashati et al., 2007). Any environmental factor that weakens the brain’s alpha band is considered a destructive stimulus of cognitive function (Destexhe et al., 2001; Fröhlich & McCormick, 2010). Accordingly, analysis of recorded EEG signals during a specific operation (e.g. playing a video game) could be considered the main indicator for the evaluation of brain (cognitive) function. Arousal, as a cognitive function, is a physiological and psychological condition of being awake. The arousal dimension of human emotions can be assessed from EEG signals using the alpha and beta signal strength (Guzel Aydin et al., 2016; Aliyari et al., 2020). Valence (positive, negative, or neutral) is an emotional state. It is one of the most important scientific concepts of the emotional experience. Valence refers to the forces that attract individuals to desirable objects and repel them from undesirable ones (Shuman et al., 2013). EEG is used for valence assessment (Wu, 2017). On the other hand, emotions (like arousal, and valence) should be regarded as an integral part of cognitive functions (Megalakaki et al., 2019).
Today, computer games play an important role in the quality of life (QOL) of children and adolescents. In addition, the time of playing computer games is increasing among players. The role of these games in the field of treatment, prevention of neurological disorders, and strengthening cognitive abilities has been specified. Considering the above sentences, the present study was designed to answer these questions: After playing a scary game, does the scary game cause changes in the activity of the stress system? Is this stress useful or harmful? Do the BDNF and OT levels change after scary games? Are these changes related to cognitive function weakness? Do arousal and valence change after playing the scary game?
2. Materials and Methods
Participants and study procedure
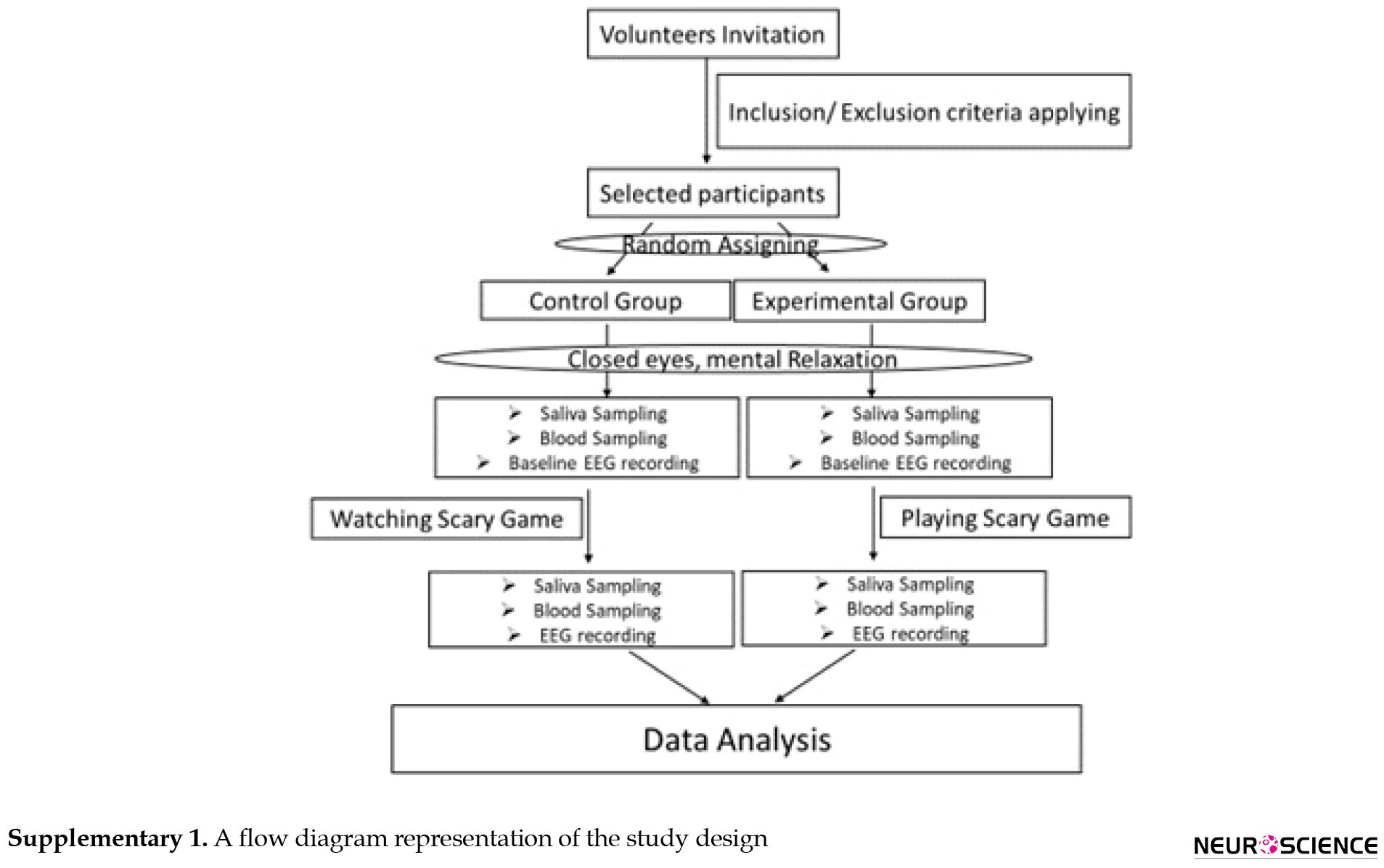
A total of thirty male students with an average age of twenty-six (age range from 21-31) were included in this analytical cross-sectional study. All participants were matched for study variables. The status of the participants was evaluated and confirmed in terms of contradicting the criteria for participation in the study including not having a history of any psychiatric or neurological disease, psychotropic medication, and addictive drug use. All eligible consciously agreed to participate in our study. We randomly grouped them into two control and experimental. They were not aware of which group they belonged to. The experimental group played a scary game in one round. The control participants were seated in front of the same computer in the same chair and hooked to accurately measuring equipment as the experimental group. They just looked at the scary game. A flow diagram of the study design is shown in Supplementary
Cortisol and alpha-amylase assay
The saliva samples were collected from all the participants before and just after the interventions (Kirschbaum & Hellhammer, 1989). Obtained samples (5 mL/person) were transferred to Falcon tubes and were maintained at -20°C. On the measurement day, samples first were melted at room temperature and after centrifuging at 3000 g for 5 minutes, 20 microliters of each sample were separated for testing using a cortisol ELISA kit (Cortisol ELISA KIT, Diagnostics Biochem Canada Inc, dbc), and sAA ELISA kit (Alpha-Amylase ELISA KIT, Diagnostics Biochem Canada Inc, dbc).
Oxytocin and brain-derived neurotrophic factor measurement
A total of 10 mL of blood was taken before and just after the intervention from the femoral artery of each subject and kept in heparin tubes of 10 mL volume and after centrifuging at 3000 g for 5 minutes, 20 microliters of each sample was separated to evaluate plasma levels of OT and BDNF using ELISA kits (MyBioSource; Catalog# MBS160452 and MyBioSource; Catalog# MBS824772 respectively) with specific protocols.
EEG recording: Stress, arousal, and valence assessment
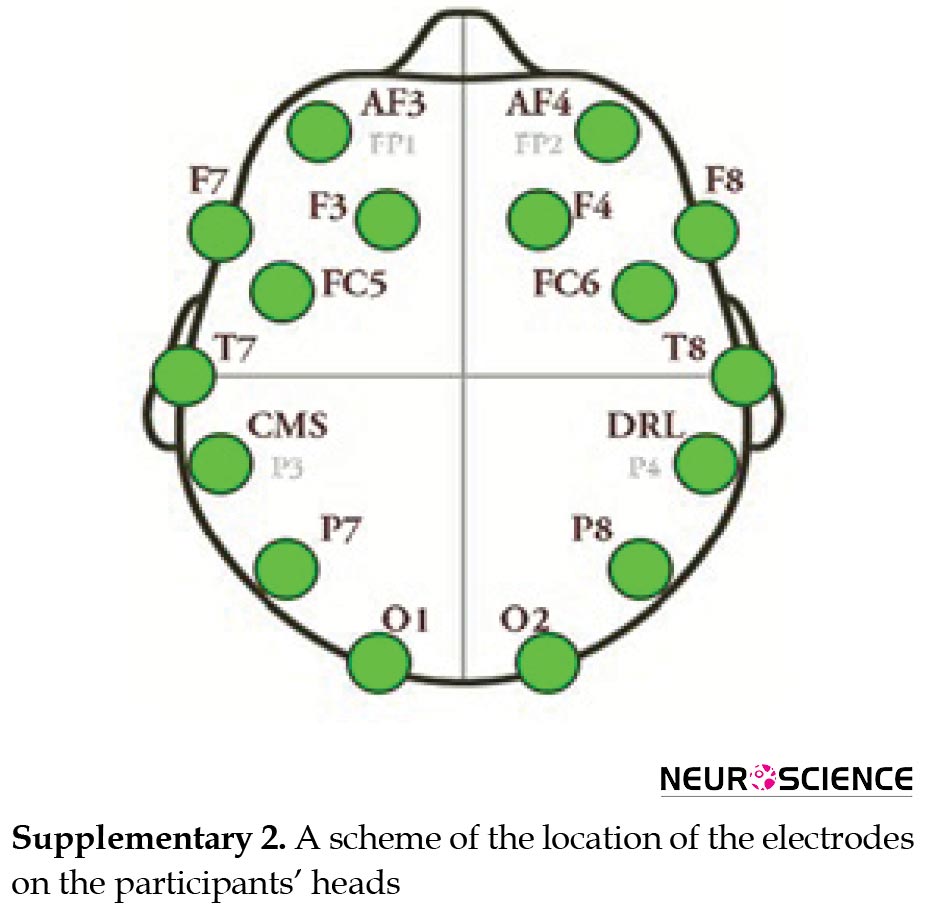
The brain waveforms were acquired by online EEG before (baseline, eyes closed) and during the interventions. For this purpose, we used the Emotive EPOC headset, a recently developed wireless EEG acquisition device. A total of 14 electrodes were placed on different locations of participants’ heads during the entire time of the intervention to record the changes in brain waves (Aliyari et al., 2018b). The subjects were asked to remain calm throughout the experiment and to keep their heads as stable as possible. Obtained data were filtered through artifact and noise removal. Required data were extracted and classified using machine learning algorithms. Eventually, the data were analyzed and the final algorithms were tested. A scheme of the location of the electrodes on the participants’ heads is shown in Supplementary 2.
It is indicated that negative environmental experiences can affect organisms’ homeostasis. This situation is called stress by some researchers (Dhama et al., 2019). Recently, research on the effects of video games on the stress system and cognitive potencies has attracted the researchers’ attention (Anderson et al., 2010; Porter & Goolkasian, 2019; Bavelier et al., 2011). Video games can affect the stress system (Téllez et al., 2023). It also was shown that different types of video games (gaming style) have different influences on the central nervous system (CNS) function (Trepte & Reinecke, 2010). Since games are used frequently by gamers, it is not surprising that the influence of game playing on the CNS function may be significant (Chonmaitree et al., 2008; Trepte & Reinecke, 2010). In this regard, playing video games is shown to have positive or negative effects on cognitive abilities (Aliyari et al., 2015; Aliyari et al., 2018a; Best, 2010; Boyle et al., 2011; Kaplan & Berman, 2010; Mastorakos & Ilias, 2003; Rabipour & Raz, 2012; Schultheiss & Stanton, 2009). Chronic stress can alter several body systems and promotes multiple health problems including neurological and neuropsychiatric disorders (Aliyari et al., 2018a; McKlveen et al., 2016). Stress is shown to activate the hypothalamus-pituitary-adrenal (HPA) axis and sympathetic nervous system. The activity of these systems results in glucocorticoid hormones and norepinephrine secretion. Experiments indicated that glucocorticoids can readily cross the barriers to be presented in the saliva. However, since norepinephrine cannot be detected in the saliva, it is postulated that salivary alpha-amylase concentration which is sensitive to the norepinephrine could be used as an indicator for the detection of the changes in norepinephrine concentration variations (Dhama et al., 2019). Activity in the brain stress systems leads to a change in the level of salivary cortisol and alpha-amylase (Diorio et al., 1993; Gatti & De Palo, 2011; Kreutz et al., 2004). As mentioned above, stress responses can produce diverse effects on the brain. So, cortisol and norepinephrine as stress biomarkers also affect cognitive abilities (Geoffroy et al., 2012; Lee et al., 2007; Maldonado et al., 2019). On the other hand, the brain stress system and oxytocin hormone (OT) are shown to have interaction in several ways. OT is implicated in the modulation of the HPA axis activity at several levels. Also, it was shown that OTergic neurons in the hypothalamus are activated during stressful experiences (Kotwica et al., 2004; McQuaid et al., 2016; Onaka, 2019). Moreover, OT has been implicated in a variety of cognitive processes and psychological stresses also may trigger the release of OT (Bendix et al., 2015; Wagner & Echterhoff, 2018). Studies also revealed that video games affect the endocrine system. Video games can change cortisol, norepinephrine, and OT secretion which in fact may lead to behavioral and cognitive impairments (Aliyari et al., 2015; Schultheiss & Stanton, 2009). Moreover, brain-derived neurotrophic factor (BDNF), has also a crucial role in neuronal and plastic development (Johnston, 2009). BDNF is also affected by stress. It has been shown that the BDNF expression in the brain is regulated by stress, and a role has been suggested for BDNF in recurrent depression, aging, and posttraumatic stress disorder (Licinio & Wong, 2002). It was revealed that in chronic stress situations, during which the levels of pro-inflammatory cytokines and glucocorticoids are consistently high, the BDNF gene is suppressed, resulting in brain atrophy and contributing to the development of mental disorders in predisposed subjects (Buselli et al., 2019). Based on these facts, about the relationship between stress and cognitive brain function, it is hypothesized that if the stress system is affected by a particular game, the person is unable to properly and timely make a decision (Aliyari et al., 2015).
One of the most important markers of brain activity is the electroencephalogram. (Bullmore & Sporns, 2009; Garrett, 2014). It is shown that electroencephalography (EEG) can be used for the evaluation of stress states and cognitive functions. In this regard, it is clear that the frontal lobe EEG is the best signal for the evaluation of the stress state (Al-Shargie, 2016). Studies showed that among the different waves of the EEG, alpha waves may better indicator of cognitive function (Bashashati et al., 2007). Any environmental factor that weakens the brain’s alpha band is considered a destructive stimulus of cognitive function (Destexhe et al., 2001; Fröhlich & McCormick, 2010). Accordingly, analysis of recorded EEG signals during a specific operation (e.g. playing a video game) could be considered the main indicator for the evaluation of brain (cognitive) function. Arousal, as a cognitive function, is a physiological and psychological condition of being awake. The arousal dimension of human emotions can be assessed from EEG signals using the alpha and beta signal strength (Guzel Aydin et al., 2016; Aliyari et al., 2020). Valence (positive, negative, or neutral) is an emotional state. It is one of the most important scientific concepts of the emotional experience. Valence refers to the forces that attract individuals to desirable objects and repel them from undesirable ones (Shuman et al., 2013). EEG is used for valence assessment (Wu, 2017). On the other hand, emotions (like arousal, and valence) should be regarded as an integral part of cognitive functions (Megalakaki et al., 2019).
Today, computer games play an important role in the quality of life (QOL) of children and adolescents. In addition, the time of playing computer games is increasing among players. The role of these games in the field of treatment, prevention of neurological disorders, and strengthening cognitive abilities has been specified. Considering the above sentences, the present study was designed to answer these questions: After playing a scary game, does the scary game cause changes in the activity of the stress system? Is this stress useful or harmful? Do the BDNF and OT levels change after scary games? Are these changes related to cognitive function weakness? Do arousal and valence change after playing the scary game?
2. Materials and Methods
Participants and study procedure
A total of thirty male students with an average age of twenty-six (age range from 21-31) were included in this analytical cross-sectional study. All participants were matched for study variables. The status of the participants was evaluated and confirmed in terms of contradicting the criteria for participation in the study including not having a history of any psychiatric or neurological disease, psychotropic medication, and addictive drug use. All eligible consciously agreed to participate in our study. We randomly grouped them into two control and experimental. They were not aware of which group they belonged to. The experimental group played a scary game in one round. The control participants were seated in front of the same computer in the same chair and hooked to accurately measuring equipment as the experimental group. They just looked at the scary game. A flow diagram of the study design is shown in Supplementary

Cortisol and alpha-amylase assay
The saliva samples were collected from all the participants before and just after the interventions (Kirschbaum & Hellhammer, 1989). Obtained samples (5 mL/person) were transferred to Falcon tubes and were maintained at -20°C. On the measurement day, samples first were melted at room temperature and after centrifuging at 3000 g for 5 minutes, 20 microliters of each sample were separated for testing using a cortisol ELISA kit (Cortisol ELISA KIT, Diagnostics Biochem Canada Inc, dbc), and sAA ELISA kit (Alpha-Amylase ELISA KIT, Diagnostics Biochem Canada Inc, dbc).
Oxytocin and brain-derived neurotrophic factor measurement
A total of 10 mL of blood was taken before and just after the intervention from the femoral artery of each subject and kept in heparin tubes of 10 mL volume and after centrifuging at 3000 g for 5 minutes, 20 microliters of each sample was separated to evaluate plasma levels of OT and BDNF using ELISA kits (MyBioSource; Catalog# MBS160452 and MyBioSource; Catalog# MBS824772 respectively) with specific protocols.
EEG recording: Stress, arousal, and valence assessment
The brain waveforms were acquired by online EEG before (baseline, eyes closed) and during the interventions. For this purpose, we used the Emotive EPOC headset, a recently developed wireless EEG acquisition device. A total of 14 electrodes were placed on different locations of participants’ heads during the entire time of the intervention to record the changes in brain waves (Aliyari et al., 2018b). The subjects were asked to remain calm throughout the experiment and to keep their heads as stable as possible. Obtained data were filtered through artifact and noise removal. Required data were extracted and classified using machine learning algorithms. Eventually, the data were analyzed and the final algorithms were tested. A scheme of the location of the electrodes on the participants’ heads is shown in Supplementary 2.
Feature extraction
Feature extraction can provide an efficient analysis. We use feature extraction for achieving a generalizable pattern for a large part of society. Feature extraction from EEG recording was done for mental stress, arousal, and valence indices.
Stress quantification
Stress quantification is very important for the design and analysis of video games, clinical interventions, and disease prevention (Al-Shargie et al., 2016). It was shown that the right hemispheres are more active in those who are socially anxious and stressed or at risk of social threats. We applied a non-parametric method to produce mental stress index using an EGG. According to previous studies and the source-based neurofeedback method, a correct procedure to determine the level of stress using EEG should explain by the ratio of brain activity between the two hemispheres in the forehead area (Aliyari et al., 2018a) (Equation 1):
.jpg)
Where F represents the frontal, 3/ 4 represents the location of the electrode, and α is the alpha band. According to the formulation for the stress index, the brain signals of participants were acquired and analyzed (Aliyari et al., 2018b).
Arousal quantification
We also measured the arousal level of subjects. One of the most important indicators of arousal cognitive ability is the beta wave-to-alpha wave ratio, which is shown in Equation 2:
.jpg)
Where (i) represents a subset of electrode counts. Channel selection is simply performed by using forehead and post-canal channels. These formations were selected because, at conscious and exciting status, the beta energy in the forehead elevates while the energy of the alpha band declines (Aliyari et al., 2020).
Valence quantification
Another indicator of emotion recognition is valence. This index explains the valence of a feeling based on whether it is positive or negative. So, if the index is a positive valence, the individual feelings are positive, and if the index is negative, the individual feelings will be negative. Some studies have shown that asymmetry in the alpha wave’s activity of the brain between the two hemispheres can be considered a good indicator to describe valence. However, these studies argued that the differences in the alpha activity are more derived from motivation (engaging with social activity following a social stimulus or keeping away from it) rather than the emotional valence of the person. Because the valence of feelings is directly related to the direction of motivation, we can use the following relation to calculate the valence (Aliyari et al., 2020) (Equation 3).
.jpg)
In which α and β indicate the power of the signal in the alpha (8-12 Hz) and beta (12-30 Hz) band, associated with the electrodes denoted by subscripts.
To get the power of signals in the specific bands of interest, initially, we pre-processed the EEG signals. The primary source of artifacts was basically due to the eye blinks/movements, heartbeats, muscle activities, and power line noise. By referencing the EEG signals and drawing out a band-pass filter, the power of the signals in alpha and beta bands were extracted and the artifacts components were removed. The level of arousal and valence in participants were defined using the power of EEG signals for specified electrodes in the alpha and beta bands (Aliyari et al., 2020).
Statistical analysis
First, all obtained data underwent tests of normality (Shapiro-Wilk). After determining the normal distribution of the data, repeated measures were applied to determine if there is any significant difference before and after (intragroup) the intervention in both control and experimental groups using SPSS software, version 21. P≤0.05 was defined as statistically significant. EEG data processing was performed using MATLAB software, version 7.0.1 Statistical Toolbox.
3. Results
Our results showed that the salivary cortisol (P=0.000214) and salivary alpha-amylase (P=0.00009596) levels significantly increased by playing the scary video game (Figure 1 and Figure 2 respectively).
.jpg)
.jpg)
The mean of oxytocin plasma levels showed an increasing trend in the scary video gameplay (P=0.01474) (Figure 3).
.jpg)
The mean of BDNF plasma levels had a decreasing trend by playing the scary video game (P=0.007813) (Figure 4).
.jpg)
The results of the EEG study on the experimental group suggested that the mean of the mental stress index was increased by the scary video gameplay (P=0.00003979) (Figure 5).
.jpg)
The mean arousal index in the experimental group showed an increasing trend in the scary video gameplay (P=0.00009989) (Figure 6).
.jpg)
Our EEG results from the experimental group also indicated that the mean of the valence index of players was reduced by the scary video game (***P<0.001) (Figure 7).
.jpg)
In the control group, all the mentioned items were compared before and after the intervention (watching the scary video game). Data analysis showed that there was no difference in any of the items before and after the intervention (The results are not shown).
4. Discussion
The present study revealed that scary game-playing increases perceived stress based on electroencephalography and biochemical evaluations. This type of game harms players’ cognitive functions. There are three types of stress including mental, emotional, and physical. Bio-signals had been extensively used by researchers in detecting human stress. EEG is also popular for detecting human brain signals and stress evaluation (Sulaiman et al., 2012). Our study showed that the perceived stress increases after the scary gameplay. We selected the prefrontal area in EEG analysis because the brain signals in this area are related to human emotion where stress is belonging to negative valence and high arousal (Sulaiman et al., 2012). Biochemical indicators of stress can also be affected by video games. We’ve shown that salivary biomarkers of stress increase under the influence of scary video games. Video games may act to affect cortisol secretion depending on the gaming style and age rating. Furthermore, the effect of video games on stress and fear systems are linked with cortisol secretion. So salivary cortisol can be a reliable estimate of stress-induced HPA activity (Aliyari et al., 2018b; Anderson et al., 2010; Dhama et al., 2019). The sympathetic-adrenal stress axis, which acts as the second axis responsive to stress, stimulates the sympathetic system, resulting in increased secretion of sAA as another stress biomarker (Aliyari et al., 2018a; Kirschbaum & Hellhammer, 1989). The findings of the present study revealed that the cortisol and sAA levels significantly increased, after playing scary video games with an age rating over eighteen. Results of the previous studies also showed that the level of stress is associated with the type of game (Aliyari et al., 2018b; Megalakaki et al., 2019; Wu, 2017). According to results obtained in the current study, the oxytocin plasma levels were lower after playing the scary game. Oxytocin is synthesized in the hypothalamus and secreted from the neurohypophysis (Lucas-Thompson & Holman, 2013; Ludwig & Leng, 2006). This neurohormone has a crucial role in social communication, happiness, and satisfaction, and can have therapeutic potential for neurological and psychiatric disorders. It was revealed that endogenous oxytocin levels are associated with maternal sensitivity, trust behaviors, and unanimity towards strangers. Furthermore, the administration of oxytocin by nasal spray promotes enhanced generosity, trust, empathy, positive communication, and helping behavior (McQuaid et al., 2016). Accordingly, oxytocin level increases to manage stress and anxiety against calamity (De Dreu, 2012; Lucas-Thompson & Holman, 2013). Oxytocin can compel anti-stress-like effects including reduction of blood pressure and cortisol levels (Uvnas-Moberg & Petersson, 2005). Previous studies demonstrated that oxytocin can modulate stress responses. Among them are studies that have shown stress responses in humans can be attenuated by exogenous oxytocin administration. For example, it was shown that intranasal administration of oxytocin reduced cortisol elevations elicited by a physical/ social stressor. Social interactions and social support are likely important components of the stress-attenuating effects of oxytocin (McQuaid et al., 2016). Also, it was suggested that OT reduces behavioral and neuroendocrine stress responses and dampens sympathetic nervous system activity (Gamer & Buchel, 2012). So, in line with previous findings, decreased oxytocin plasma levels in players of the scary game can be attributed to the increased salivary cortisol and alpha-amylase levels. Decreased plasma levels of oxytocin, alongside elevated levels of salivary cortisol and alpha-amylase, represent increased stress following the scary game. Many studies found that abnormal stress impairs cognitive capabilities such as attention, concentration, decision-making, and response speed (Megalakaki et al., 2019; Wu, 2017).Stress is known as an important regulator of cognition. Glucocorticoids affect cognitive functions through neural plasticity (Dayi et al., 2015). Cortisol, as an important glucocorticoid, was shown to have a significant effect on cognitive functioning (Russoniello et al., 2009). So, it can be concluded that scary video games as a strong stimulant, by affecting the secretion of oxytocin, cortisol, and alpha-amylase saliva, significantly affect the human nervous system. Changes in the secretion of cortisol, oxytocin, and alpha-amylase lead to dysfunction of the fear and stress systems. Environmental factors have many diverse effects on the human CNS. Various types of video games with specific playing styles, as important environmental factors, have different cognitive effects on audiences. Many studies found that abnormal stress (such as those that can happen with some video games) caused impaired cognitive capabilities such as attention, concentration, decision-making, and response speed. Playing scary and overwhelming games for a long time can cause irrecoverable negative consequences because the player is constantly overwhelmed by stress and fear. As a result, the HPA axis is always active. Thus, cortisol and alpha-amylase are secreted continually and abnormally. This type of created stress is called fear stress (Gordis et al., 2006; Tanaka et al., 2012). Also, BDNF serum levels were reduced after playing the scary game in our study. BDNF can play an important role in the pathophysiology of stress-related disorders, and its increased peripheral levels can be effective in protecting neurons exposed to stress. It was demonstrated that during chronic stress, the BDNF gene is down-regulated under the prolonged action of pro-inflammatory cytokines and glucocorticoids. This results in brain atrophy and contributes to the development of mental disorders in predisposed subjects. Several recent studies have shown that the peripheral (serum or plasma) levels of BDNF are lower in patients with mood disorders during manic/mixed and depressive episodes compared to healthy controls and that effective treatments can normalize them (Buselli et al., 2019). BDNF is involved in the reconstruction of brain neurons including neurons in the prefrontal cortex and hippocampus. Also, increased BDNF is related to the enhancement of synaptic connections and reinforcement of dendritic connections. BDNF has a substantial role in the learning, memory, and development of synaptic plasticity (Lu et al., 2013; Sweatt, 2001). It also contributes to the growth of new synapses and dendritic connections in the CNS (Lu et al., 2013). The observed BDNF reduction in our study could be due to increased levels of cortisol and alpha-amylases. It can be concluded that stress and fear in this study, disrupt cognitive functions by reducing the plasma level of BDNF, and BDNF reduction along with OT reduction weakens cognitive functions and positive behaviors among players (Jindal et al., 2010; Sweatt, 2001). On the other hand, the unusual elevation in cortisol secretion disrupts the glutamate system and damages the neural synapses leading to negative neurologic consequences such as loss of memory and learning, the presence of violent and anxious behaviors, and eventually depression. The increased extracellular glutamate concentration and excessive activity of ionotropic receptors lead to toxicity inside the neurons and damaged neurons and synapses in the hippocampus (Anderson et al., 2010; Liao et al., 2012; Williams, 2001). In another part of the present study, analysis of EEG revealed that the average perceive stress was significantly affected by the scary game, as mentioned above. Besides, the comparison of arousal and valence charts in the participants showed higher arousal and negative valence after the gameplay. It is interesting to note that arousal and valence were about two times higher after the game. However, stress was seven times higher after the game. Cortisol levels obtained from saliva samples have been accepted also as a reliable physical measure of emotions. In line with our results, it was shown previously that there is temporal connectivity between cortisol release and central alertness, as reflected in the waking EEGβ activity. Findings suggest the existence of connections between the mechanisms involved in the control of HPA axis activity and the activation processes of the brain, which undergo varying degrees of alertness (Chapotot, 1998). In line with our finding, given the considerable effects of cortisol on the neural circuitry underlying effectual decision-making, it was shown that higher stress reactivity might bias individuals to appraise ambiguous stimuli more negatively and further diminish regulatory capacity that may otherwise attenuate this negative bias during effective decision-making. This explanation is constant with our knowledge about the brain regions underlying negative valence bias and acute stress responses, namely the amygdala, which plays a central role in both processes. The high density of glucocorticoid receptors in the amygdala makes it especially sensitive to cortisol release after facing stress, thus this region is ideally positioned to bias appraisals of ambiguous stimuli toward a negative or threatening valence. We think that this increased amygdala activity paired with a more global shift in neural processing toward threat detection (Brown et al., 2017). Likewise, salivary alpha-amylase has associations with both arousal and valence. In an experienced fear study, salivary alpha-amylase showed a highly specific increase only for those participants who endorsed both emotional arousal and negative valence (Buchanan et al., 2010). In line with that, our study also showed that an elevation in salivary alpha-amylase was associated with an increase in arousal and a decrease in valence. It was revealed that OT had also differential effects on the activity of specific amygdala sub-regions. On the one hand, it attenuates activation in the lateral and dorsal parts of the anterior amygdala for afraid faces but raised activity for happy expressions, thus indicating a shift of the processing focus toward positive social stimuli. OT facilitates the social approach by reducing anxiety and neuroendocrine stress responses and has shown significant effects on anatomically distinct amygdala regions associated with valence processing (Gamer et al., 2010) which is in line with our results in the current study. Here we found that decreased OT levels were associated with increased arousal and decreased valence. BDNF is an important regulator of neural survival, development, function, and plasticity. BDNF is highly expressed in limbic structures and the cerebral cortex and has a pivotal role in learning, memory, anxiety-like behavior, and reward-related processes. It is associated with better cognitive potency and has positive effects on health and brain function. It is also associated with many mental disorders, like depression, anxiety, etc. (Jeon & Ha, 2017; Kim et al., 2018). According to BDNF reduction after playing the scary game in our study, and based on previous studies which revealed that BDNF synthesis occurs in regions that participate in emotional and cognitive functions (Phillips, 2017), reduction of the performance of individuals towards the fourth quarter of the valence excitatory axis is justifiable, and thus it can be said that the scary game kind of stress caused relatively high levels of arousal and negative valence because of fear. So, the results of our study, in line with previous studies confirm the importance of BDNF in the emotional and cognitive processes of the brain and show its importance in the relationship between stresses caused by scary video games and impaired emotional and cognitive functions. On the other hand, because of the level of change in the stress index and impairment of emotional and cognitive functions, the fear created by the scary game can be considered a destructive fear (Aliyari et al., 2018b; Toohey & Taylor, 2008).
We found elevated levels of salivary cortisol and alpha-amylase after the scary gameplay which is an indicator of stress and fear elevation among players. Moreover, decreased BDNF is associated with impaired emotional and cognitive functions among players. To control the abnormal activity of the stress-fear path in the nervous system, the secretion of oxytocin is decreased. Also, EEG analysis showed increased stress and arousal, and negative valence after playing the scary video game which confirms the destructive effect of the scary game on the emotional and cognitive processes of the nervous system. Our results along with the results obtained from similar studies provide a way for understanding the mental/physical effects of video games and can thus help with game modification and development. Depending on the goal of the game, a programmer could increase or decrease the amount of a certain variable (i.e. music, visuals, etc.) to increase or decrease the intended effect. So, designing a game that is physiologically tailored to the specific individual needs of humans, seems to be acceptable shortly. To strengthen positive cognitive and emotional indicators and weaken negative cognitive and emotional indicators of video games, video game pathophysiology, especially scary games, is essential for researchers.
Ethical Considerations
Compliance with ethical guidelines
All ethical standards were under the Declaration of Helsinki. Ethical approval to conduct the study was obtained from the Ethical Committee of Baqiyatallah University of Medical Sciences (Code: IR. BMSU.REC.12345).
Funding
This study was financially supported by the Neuroscience Research Center of Baqiyatallah University of Medical Sciences.
Authors' contributions
Conceptualization: Hamed Aliyari, Hedayat Sahraei and Masoomeh Kazemi; Methodology and investigation: Hamed Aliyari, Masoomeh Kazemi and Sahar Golabi; Data curation: Hamed Aliyari, Masoomeh Kazemi, Mohammad Sahraei; Formal analysis: Hamed Aliyari, Masoumeh Kazemi, Sahar Golabi, Hedayat Sahraei, Behrouz Minaei-Bidgoli and Mohammad Reza Daliri; Writing original draft: Hamed Aliyari and Sahar Golabi; Review & editing: Sahar Golabi and Reza Hazrati; Project administration and funding acquisition: Masoomeh Kazemi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
We express our gratitude to the Neuroscience Research Center of Baqiyatallah University of Medical Sciences, the Soft Technology Development Council, and the Neurogame Research Group.
References
Aliyari, H., Hosseinian, S. H., Menhaj, M. B., & Sahraei, H. (2018).Analysis of the effects of high-voltage transmission line on human stress and attention through electroencephalography (EEG). Iranian Journal of Science and Technology, Transactions of Electrical Engineering. 43, 211-218. [DOI:10.1007/s40998-018-0151-8]
Aliyari, H., Hosseinian, S. H., Sahraei, H., & Menhaj, M. B. (2019). Effect of proximity to high-voltage fields: Results of the neural network model and experimental model with macaques. International Journal of Environmental Science and Technology, 16, 4315-4326. [DOI: 10.1007/s40998-018-0151-8]
Aliyari, H., Kazemi, M., Tekieh, E., Salehi, M., Sahraei, H., & Daliri, M. R., et al. (2015). The effects of fifa 2015 computer games on changes in cognitive, hormonal and brain waves functions of young men volunteers. Basic and Clinical Neuroscience, 6(3), 193–201. [Link]
Aliyari, H., Sahraei, H., Daliri, M. R., Minaei-Bidgoli, B., Kazemi, M., & Agaei, H., et al. (2018). The beneficial or harmful effects of computer game stress on cognitive functions of players. Basic and Clinical Neuroscience, 9(3), 177–186. [DOI:10.29252/nirp.bcn.9.3.177] [PMID] [PMCID]
Aliyari, H., Sahraei, H., Erfani, M., Mohammadi, M., Kazemi, M., & Daliri, M. R., et al. (2020). Changes in cognitive functions following violent and football video games in young male volunteers by studying brain waves. Basic and Clinical Neuroscience, 11(3), 279–288. [DOI:10.32598/bcn.9.10.335] [PMID] [PMCID]
Anderson, C. A., Shibuya, A., Ihori, N., Swing, E. L., Bushman, B. J., & Sakamoto, A., et al. (2010). Violent video game effects on aggression, empathy, and prosocial behavior in eastern and western countries: A meta-analytic review. Psychological Bulletin, 136(2), 151–173. [DOI:10.1037/a0018251] [PMID]
Al-Shargie, F., Kiguchi, M., Badruddin, N., Dass, S. C., Hani, A. F. M., & Tang, T. B. (2016). Mental stress assessment using simultaneous measurement of EEG and fNIRS. Biomedical Optics Express, 7(10), 3882-3898. [DOI:10.1364/BOE.7.003882] [PMID]
Bashashati, A., Fatourechi, M., Ward, R. K., & Birch, G. E. (2007). A survey of signal processing algorithms in brain-computer interfaces based on electrical brain signals. Journal of Neural Engineering, 4(2), R32–R57. [DOI:10.1088/1741-2560/4/2/R03] [PMID]
Bavelier, D., Green, C. S., Han, D. H., Renshaw, P. F., Merzenich, M. M., & Gentile, D. A. (2011). Brains on video games. Nature Reviews. Neuroscience, 12(12), 763–768. [DOI:10.1038/nrn3135] [PMID] [PMCID]
Bendix, M., Uvnäs-Moberg, K., Petersson, M., Gustavsson, P., Svanborg, P., & Åsberg, M., et al. (2015). Plasma oxytocin and personality traits in psychiatric outpatients. Psychoneuroendocrinology, 57, 102–110. [DOI:10.1016/j.psyneuen.2015.04.003] [PMID]
Best J. R. (2010). Effects of physical activity on children's executive function: Contributions of experimental research on aerobic exercise. Developmental Review, 30(4), 331–551.[DOI:10.1016/j.dr.2010.08.001] [PMID] [PMCID]
Boyle, E., Connolly, T. M., & Hainey, T. (2011). The role of psychology in understanding the impact of computer games. Entertainment Computing, 2(2), 69-74. [DOI:10.1016/j.entcom.2010.12.002]
Brown, C. C., Raio, C. M., & Neta, M. (2017). Cortisol responses enhance negative valence perception for ambiguous facial expressions. Scientific Reports, 7(1), 15107. [DOI:10.1038/s41598-017-14846-3] [PMID] [PMCID]
Buchanan, T. W., Bibas, D., & Adolphs, R. (2010). Salivary α-amylase levels as a biomarker of experienced fear. Communicative & Integrative Biology, 3(6), 525–527. [DOI:10.4161/cib.3.6.12606] [PMID] [PMCID]
Bullmore, E., & Sporns, O. (2009). Complex brain networks: Graph theoretical analysis of structural and functional systems. Nature Reviews. Neuroscience, 10(3), 186–198. [DOI:10.1038/nrn2575] [PMID]
Buselli, R., Veltri, A., Baldanzi, S., Marino, R., Bonotti, A., & Chiumiento, M., et al. (2019). Plasma brain-derived neurotrophic factor (BDNF) and serum cortisol levels in a sample of workers exposed to occupational stress and suffering from adjustment disorders. Brain and Behavior, 9(7), e01298. [DOI:10.1002/brb3.1298] [PMID] [PMCID]
Chonmaitree, T., Revai, K., Grady, J. J., Clos, A., Patel, J. A., & Nair, S., et al. (2008). Viral upper respiratory tract infection and otitis media complication in young children. Clinical Infectious Diseases, 46(6), 815–823. [DOI:10.1086/528685] [PMID] [PMCID]
Dayi, A., Cetin, F., Sisman, A. R., Aksu, I., Tas, A., & Gönenc, S., et al. (2015). The effects of oxytocin on cognitive defect caused by chronic restraint stress applied to adolescent rats and on hippocampal VEGF and BDNF levels. Medical Science Monitor, 21, 69–75. [DOI:10.12659/MSM.893159] [PMID] [PMCID]
De Dreu, C. K. W. (2012). Oxytocin modulates the link between adult attachment and cooperation through reduced betrayal aversion. Psychoneuroendocrinology, 37(7), 871–880.[DOI:10.1016/j.psyneuen.2011.10.003] [PMID]
Destexhe, A., Rudolph, M., Fellous, J. M., & Sejnowski, T. J. (2001). Fluctuating synaptic conductances recreate in vivo-like activity in neocortical neurons. Neuroscience, 107(1), 13–24. [DOI:10.1016/S0306-4522(01)00344-X] [PMID]
Dhama, K., Latheef, S. K., Dadar, M., Samad, H. A., Munjal, A., & Khandia, R., et al. (2019). Biomarkers in stress related diseases/disorders: Diagnostic, prognostic, and therapeutic values. Frontiers in Molecular Biosciences, 6, 91. [DOI:10.3389/fmolb.2019.00091] [PMID] [PMCID]
Diorio, D., Viau, V., & Meaney, M. J. (1993). The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. The Journal of Neuroscience, 13(9), 3839–3847. [DOI:10.1523/JNEUROSCI.13-09-03839.1993] [PMID] [PMCID]
Al-Shargie, F., Kiguchi, M., Badruddin, N., Dass, S. C., Hani, A. F., & Tang, T. B. (2016). Mental stress assessment using simultaneous measurement of EEG and fNIRS. Biomedical Optics Express, 7(10), 3882–3898. [DOI:10.1364/BOE.7.003882] [PMID] [PMCID]
Chapotot, F., Gronfier, C., Jouny, C., Muzet, A., & Brandenberger, G. (1998). Cortisol secretion is related to electroencephalographic alertness in human subjects during daytime wakefulness. The Journal of Clinical Endocrinology and Metabolism, 83(12), 4263–4268. [DOI:10.1210/jc.83.12.4263] [PMID]
Fröhlich, F., & Mc Cormick, D. A. (2010). Endogenous electric fields may guide neocortical network activity. Neuron, 67(1), 129–143. [DOI:10.1016/j.neuron.2010.06.005] [PMID] [PMCID]
Gamer, M., & Büchel, C. (2012). Oxytocin specifically enhances valence-dependent parasympathetic responses. Psychoneuroendocrinology, 37(1), 87–93. [DOI:10.1016/j.psyneuen.2011.05.007] [PMID]
Gamer, M., Zurowski, B., & Büchel, C. (2010). Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proceedings of the National Academy of Sciences of the United States of America, 107(20), 9400–9405. [DOI:10.1073/pnas.1000985107] [PMID] [PMCID]
Garrett, B. (2014). Study guide to accompany bob garrett’s brain & behavior: An introduction to biological psychology. Newbury Park: Sage Publications. [Link]
Gatti, R., & De Palo, E. F. (2011). An update: Salivary hormones and physical exercise. Scandinavian Journal of Medicine & Science in Sports, 21(2), 157–169. [DOI:10.1111/j.1600-0838.2010.01252.x] [PMID]
Geoffroy, M. C., Hertzman, C., Li, L., & Power, C. (2012). Morning salivary cortisol and cognitive function in mid-life: Evidence from a population-based birth cohort. Psychological Medicine, 42(8), 1763–1773. [DOI:10.1017/S0033291711002704] [PMID]
Gordis, E. B., Granger, D. A., Susman, E. J., & Trickett, P. K. (2006). Asymmetry between salivary cortisol and alpha-amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology, 31(8), 976–987. [DOI:10.1016/j.psyneuen.2006.05.010] [PMID]
Guzel Aydin, S., Kaya, T., & Guler, H. (2016). Wavelet-based study of valence-arousal model of emotions on EEG signals with LabVIEW. Brain Informatics, 3(2), 109–117. [DOI:10.1007/s40708-016-0031-9] [PMID] [PMCID]
Jeon, Y. K., & Ha, C. H. (2017). The effect of exercise intensity on brain derived neurotrophic factor and memory in adolescents. Environmental Health and Preventive Medicine, 22(1), 27. [DOI:10.1186/s12199-017-0643-6] [PMID] [PMCID]
Jindal, R. D., Pillai, A. K., Mahadik, S. P., Eklund, K., Montrose, D. M., & Keshavan, M. S. (2010). Decreased BDNF in patients with antipsychotic naïve first episode schizophrenia. Schizophrenia Research, 119(1-3), 47–51. [DOI:10.1016/j.schres.2009.12.035] [PMID] [PMCID]
Johnston, M. V. (2009). Plasticity in the developing brain: implications for rehabilitation. Developmental Disabilities Research Reviews, 15(2), 94–101 [DOI:10.1002/ddrr.64] [PMID]
Kaplan, S., & Berman, M. G. (2010). Directed attention as a common resource for executive functioning and self-regulation. Perspectives on Psychological Science, 5(1), 43–57.[DOI:10.1177/1745691609356784] [PMID]
Kim, K. M., Choi, S. W., Lee, J., & Kim, J. W. (2018). EEG correlates associated with the severity of gambling disorder and serum BDNF levels in patients with gambling disorder. Journal of Behavioral Addictions, 7(2), 331–338. [DOI:10.1556/2006.7.2018.43] [PMID] [PMCID]
Kirschbaum, C., & Hellhammer, D. H. (1989). Salivary cortisol in psychobiological research: An overview. Neuropsychobiology, 22(3), 150-169. [DOI:10.1159/000118611] [PMID]
Kotwica, G., Kaminska, B., Franczak, A., Kurowicka, B., Staszkiewicz, J., & Skowronski, M. T., et al. (2004). The effect of oxytocin on cortisol and corticosterone secretion in cyclic gilts-in vivo and in vitro studies. Reprodution Biology, 4(1), 35-50. [Link]
Kreutz, G., Bongard, S., Rohrmann, S., Hodapp, V., & Grebe, D. (2004). Effects of choir singing or listening on secretory immunoglobulin A, cortisol, and emotional state. Journal of Behavioral Medicine, 27(6), 623–635. [DOI:10.1007/s10865-004-0006-9] [PMID]
Lee, B. K., Glass, T. A., Mc Atee, M. J., Wand, G. S., Bandeen-Roche, K., & Bolla, K. I., et al. (2007). Associations of salivary cortisol with cognitive function in the Baltimore memory study. Archives of General Psychiatry, 64(7), 810–818. [DOI:10.1001/archpsyc.64.7.810] [PMID]
Liao, L. D., Chen, C. Y., Wang, I. J., Chen, S. F., Li, S. Y., & Chen, B. W., et al. (2012). Gaming control using a wearable and wireless EEG-based brain-computer interface device with novel dry foam-based sensors. Journal of Neuroengineering and Rehabilitation, 9(1), 5. [DOI:10.1186/1743-0003-9-5] [PMID] [PMCID]
Licinio, J., & Wong, M. (2002). Brain-derived neurotrophic factor (BDNF) in stress and affective disorders. Molecular Psychiatry, 7(6), 519. [DOI:10.1038/sj.mp.4001211] [PMID]
Lu, B., Nagappan, G., Guan, X., Nathan, P. J., & Wren, P. (2013). BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nature Reviews. Neuroscience, 14(6), 401–416. [DOI:10.1038/nrn3505] [PMID]
Lucas-Thompson, R. G., & Holman, E. A. (2013). Environmental stress, oxytocin receptor gene (OXTR) polymorphism, and mental health following collective stress. Hormones and Behavior, 63(4), 615–624. [DOI:10.1016/j.yhbeh.2013.02.015] [PMID]
Ludwig, M., & Leng, G. (2006). Dendritic peptide release and peptide-dependent behaviours. Nature Reviews. Neuroscience, 7(2), 126–136. [DOI:10.1038/nrn1845] [PMID]
Maldonado, E. F., Nislin, M., Martínez-Escribano, A., Marín, L., Enguix, A., & Alamo, A., López, et al. (2019). Association of salivary alpha-amylase and salivary flow rate with working memory functioning in healthy children. Stress, 22(6), 670–678. [DOI:10.1080/10253890.2019.1611777] [PMID]
Mastorakos, G., & Ilias, I. (2003). Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Annals of the New York Academy of Sciences, 997, 136–149. [DOI:10.1196/annals.1290.016] [PMID]
McKlveen, J. M., Morano, R. L., Fitzgerald, M., Zoubovsky, S., Cassella, S. N., & Scheimann, J. R., et al. (2016). Chronic stress increases prefrontal inhibition: A mechanism for stress-induced prefrontal dysfunction. Biological Psychiatry, 80(10), 754–764. [DOI:10.1016/j.biopsych.2016.03.2101] [PMID] [PMCID]
McQuaid, R. J., McInnis, O. A., Paric, A., Al-Yawer, F., Matheson, K., & Anisman, H. (2016). Relations between plasma oxytocin and cortisol: The stress buffering role of social support. Neurobiology of Stress, 3, 52–60. [DOI:10.1016/j.ynstr.2016.01.001] [PMID] [PMCID]
Megalakaki, O., Ballenghein, U., & Baccino, T. (2019). Effects of valence and emotional intensity on the comprehension and memorization of texts. Frontiers in Psychology, 10, 179. [DOI:10.3389/fpsyg.2019.00179] [PMID] [PMCID]
Phillips C. (2017). Brain-Derived neurotrophic factor, depression, and physical activity: Making the neuroplastic connection. Neural Plasticity, 2017, 7260130. [DOI:10.1155/2017/7260130] [PMID] [PMCID]
Rabipour, S., & Raz, A. (2012). Training the brain: Fact and fad in cognitive and behavioral remediation. Brain and Cognition, 79(2), 159–179. [DOI:10.1016/j.bandc.2012.02.006] [PMID]
Russoniello, C. V., O’Brien, K., & Parks, J. M. (2009). The effectiveness of casual video games in improving mood and decreasing stress. Journal of CyberTherapy & Rehabilitation, 2(1), 53-66. [Link]
Schultheiss, O. C., & Stanton, S. J. (2009). Assessment of salivary hormones. In E. Harmon-Jones & J. S. Beer (Eds.), Methods in social neuroscience (pp. 17–44). New York: Guilford Press. [Link]
Sulaiman, N., Taib, M. N., Lias, S., Murat, Z. H., Aris, S. A. M., & Mustafa, M., et al. (2012). Development of EEG-based stress index. Paper presented at: 2012 International Conference on Biomedical Engineering. Penang, Malaysia, 28 February 2012. [DOI:10.1109/ICoBE.2012.6179059]
Téllez, A. M., Castro, L. A., & Tentori, M. (2023). Developing and Evaluating a virtual reality videogame using biofeedback for stress management in sports. Interacting with Computers, iwad025. [DOI:10.1093/iwc/iwad025]
Wu, S., Xu, X., Shu, L., & Hu, B. (2017). Estimation of valence of emotion using two frontal EEG channels. Paper presented at: 2017 IEEE International Conference on Bioinformatics and Biomedicine, Kansas City, USA, 16 November 2017. [DOI:10.1109/BIBM.2017.8217815]
Shuman, V., Sander, D., & Scherer, K. R. (2013). Levels of valence. Frontiers in Psychology, 4, 261. [DOI:10.3389/fpsyg.2013.00261]
Sweatt, J. D. (2001). The neuronal MAP kinase cascade: A biochemical signal integration system subserving synaptic plasticity and memory. Journal of Neurochemistry, 76(1), 1–10.[DOI:10.1046/j.1471-4159.2001.00054.x] [PMID]
Tanaka, Y., Ishitobi, Y., Maruyama, Y., Kawano, A., Ando, T., & Imanaga, J., et al. (2012). Salivary α-amylase and cortisol responsiveness following electrical stimulation stress in panic disorder patients. Neuroscience Research, 73(1), 80–84. [DOI:10.1016/j.neures.2012.01.006] [PMID]
Onaka, T., & Takayanagi, Y. (2019). Role of oxytocin in the control of stress and food intake. Journal of Neuroendocrinology, 31(3), e12700. [DOI:10.1111/jne.12700] [PMID] [PMCID]
Toohey, K., & Taylor, T. (2008). Mega events, fear, and risk: Terrorism at the olympic games. Journal of Sport Management, 22(4), 451-469. [DOI:10.1123/jsm.22.4.451]
Trepte, S., & Reinecke, L. (2010). Avatar creation and video game enjoyment: Effects of life-satisfaction, game competitiveness, and identification with the avatar. Journal of Media Psychology, 22(4), 171. [DOI:10.1027/1864-1105/a000022]
Uvnas-Moberg, K., & Petersson, M. (2005). [Oxytocin, a mediator of anti-stress, well-being, social interaction, growth and healing (German)]. Zeitschrift fur Psychosomatische Medizin und Psychotherapie, 51(1), 57–80. [DOI:10.13109/zptm.2005.51.1.57] [PMID]
Wagner, U., & Echterhoff, G. (2018). When does oxytocin affect human memory encoding? The role of social context and individual attachment style. Frontiers in Human Neuroscience, 12, 349. [DOI:10.3389/fnhum.2018.00349] [PMID] [PMCID]
Williams, J. A. (2001). Intracellular signaling mechanisms activated by cholecystokinin-regulating synthesis and secretion of digestive enzymes in pancreatic acinar cells. Annual Review of Physiology, 63, 77–97. [DOI:10.1146/annurev.physiol.63.1.77] [PMID]
Feature extraction can provide an efficient analysis. We use feature extraction for achieving a generalizable pattern for a large part of society. Feature extraction from EEG recording was done for mental stress, arousal, and valence indices.
Stress quantification
Stress quantification is very important for the design and analysis of video games, clinical interventions, and disease prevention (Al-Shargie et al., 2016). It was shown that the right hemispheres are more active in those who are socially anxious and stressed or at risk of social threats. We applied a non-parametric method to produce mental stress index using an EGG. According to previous studies and the source-based neurofeedback method, a correct procedure to determine the level of stress using EEG should explain by the ratio of brain activity between the two hemispheres in the forehead area (Aliyari et al., 2018a) (Equation 1):
.jpg)
Where F represents the frontal, 3/ 4 represents the location of the electrode, and α is the alpha band. According to the formulation for the stress index, the brain signals of participants were acquired and analyzed (Aliyari et al., 2018b).
Arousal quantification
We also measured the arousal level of subjects. One of the most important indicators of arousal cognitive ability is the beta wave-to-alpha wave ratio, which is shown in Equation 2:
.jpg)
Where (i) represents a subset of electrode counts. Channel selection is simply performed by using forehead and post-canal channels. These formations were selected because, at conscious and exciting status, the beta energy in the forehead elevates while the energy of the alpha band declines (Aliyari et al., 2020).
Valence quantification
Another indicator of emotion recognition is valence. This index explains the valence of a feeling based on whether it is positive or negative. So, if the index is a positive valence, the individual feelings are positive, and if the index is negative, the individual feelings will be negative. Some studies have shown that asymmetry in the alpha wave’s activity of the brain between the two hemispheres can be considered a good indicator to describe valence. However, these studies argued that the differences in the alpha activity are more derived from motivation (engaging with social activity following a social stimulus or keeping away from it) rather than the emotional valence of the person. Because the valence of feelings is directly related to the direction of motivation, we can use the following relation to calculate the valence (Aliyari et al., 2020) (Equation 3).
.jpg)
In which α and β indicate the power of the signal in the alpha (8-12 Hz) and beta (12-30 Hz) band, associated with the electrodes denoted by subscripts.
To get the power of signals in the specific bands of interest, initially, we pre-processed the EEG signals. The primary source of artifacts was basically due to the eye blinks/movements, heartbeats, muscle activities, and power line noise. By referencing the EEG signals and drawing out a band-pass filter, the power of the signals in alpha and beta bands were extracted and the artifacts components were removed. The level of arousal and valence in participants were defined using the power of EEG signals for specified electrodes in the alpha and beta bands (Aliyari et al., 2020).
Statistical analysis
First, all obtained data underwent tests of normality (Shapiro-Wilk). After determining the normal distribution of the data, repeated measures were applied to determine if there is any significant difference before and after (intragroup) the intervention in both control and experimental groups using SPSS software, version 21. P≤0.05 was defined as statistically significant. EEG data processing was performed using MATLAB software, version 7.0.1 Statistical Toolbox.
3. Results
Our results showed that the salivary cortisol (P=0.000214) and salivary alpha-amylase (P=0.00009596) levels significantly increased by playing the scary video game (Figure 1 and Figure 2 respectively).
.jpg)
.jpg)
The mean of oxytocin plasma levels showed an increasing trend in the scary video gameplay (P=0.01474) (Figure 3).
.jpg)
The mean of BDNF plasma levels had a decreasing trend by playing the scary video game (P=0.007813) (Figure 4).
.jpg)
The results of the EEG study on the experimental group suggested that the mean of the mental stress index was increased by the scary video gameplay (P=0.00003979) (Figure 5).
.jpg)
The mean arousal index in the experimental group showed an increasing trend in the scary video gameplay (P=0.00009989) (Figure 6).
.jpg)
Our EEG results from the experimental group also indicated that the mean of the valence index of players was reduced by the scary video game (***P<0.001) (Figure 7).
.jpg)
In the control group, all the mentioned items were compared before and after the intervention (watching the scary video game). Data analysis showed that there was no difference in any of the items before and after the intervention (The results are not shown).
4. Discussion
The present study revealed that scary game-playing increases perceived stress based on electroencephalography and biochemical evaluations. This type of game harms players’ cognitive functions. There are three types of stress including mental, emotional, and physical. Bio-signals had been extensively used by researchers in detecting human stress. EEG is also popular for detecting human brain signals and stress evaluation (Sulaiman et al., 2012). Our study showed that the perceived stress increases after the scary gameplay. We selected the prefrontal area in EEG analysis because the brain signals in this area are related to human emotion where stress is belonging to negative valence and high arousal (Sulaiman et al., 2012). Biochemical indicators of stress can also be affected by video games. We’ve shown that salivary biomarkers of stress increase under the influence of scary video games. Video games may act to affect cortisol secretion depending on the gaming style and age rating. Furthermore, the effect of video games on stress and fear systems are linked with cortisol secretion. So salivary cortisol can be a reliable estimate of stress-induced HPA activity (Aliyari et al., 2018b; Anderson et al., 2010; Dhama et al., 2019). The sympathetic-adrenal stress axis, which acts as the second axis responsive to stress, stimulates the sympathetic system, resulting in increased secretion of sAA as another stress biomarker (Aliyari et al., 2018a; Kirschbaum & Hellhammer, 1989). The findings of the present study revealed that the cortisol and sAA levels significantly increased, after playing scary video games with an age rating over eighteen. Results of the previous studies also showed that the level of stress is associated with the type of game (Aliyari et al., 2018b; Megalakaki et al., 2019; Wu, 2017). According to results obtained in the current study, the oxytocin plasma levels were lower after playing the scary game. Oxytocin is synthesized in the hypothalamus and secreted from the neurohypophysis (Lucas-Thompson & Holman, 2013; Ludwig & Leng, 2006). This neurohormone has a crucial role in social communication, happiness, and satisfaction, and can have therapeutic potential for neurological and psychiatric disorders. It was revealed that endogenous oxytocin levels are associated with maternal sensitivity, trust behaviors, and unanimity towards strangers. Furthermore, the administration of oxytocin by nasal spray promotes enhanced generosity, trust, empathy, positive communication, and helping behavior (McQuaid et al., 2016). Accordingly, oxytocin level increases to manage stress and anxiety against calamity (De Dreu, 2012; Lucas-Thompson & Holman, 2013). Oxytocin can compel anti-stress-like effects including reduction of blood pressure and cortisol levels (Uvnas-Moberg & Petersson, 2005). Previous studies demonstrated that oxytocin can modulate stress responses. Among them are studies that have shown stress responses in humans can be attenuated by exogenous oxytocin administration. For example, it was shown that intranasal administration of oxytocin reduced cortisol elevations elicited by a physical/ social stressor. Social interactions and social support are likely important components of the stress-attenuating effects of oxytocin (McQuaid et al., 2016). Also, it was suggested that OT reduces behavioral and neuroendocrine stress responses and dampens sympathetic nervous system activity (Gamer & Buchel, 2012). So, in line with previous findings, decreased oxytocin plasma levels in players of the scary game can be attributed to the increased salivary cortisol and alpha-amylase levels. Decreased plasma levels of oxytocin, alongside elevated levels of salivary cortisol and alpha-amylase, represent increased stress following the scary game. Many studies found that abnormal stress impairs cognitive capabilities such as attention, concentration, decision-making, and response speed (Megalakaki et al., 2019; Wu, 2017).Stress is known as an important regulator of cognition. Glucocorticoids affect cognitive functions through neural plasticity (Dayi et al., 2015). Cortisol, as an important glucocorticoid, was shown to have a significant effect on cognitive functioning (Russoniello et al., 2009). So, it can be concluded that scary video games as a strong stimulant, by affecting the secretion of oxytocin, cortisol, and alpha-amylase saliva, significantly affect the human nervous system. Changes in the secretion of cortisol, oxytocin, and alpha-amylase lead to dysfunction of the fear and stress systems. Environmental factors have many diverse effects on the human CNS. Various types of video games with specific playing styles, as important environmental factors, have different cognitive effects on audiences. Many studies found that abnormal stress (such as those that can happen with some video games) caused impaired cognitive capabilities such as attention, concentration, decision-making, and response speed. Playing scary and overwhelming games for a long time can cause irrecoverable negative consequences because the player is constantly overwhelmed by stress and fear. As a result, the HPA axis is always active. Thus, cortisol and alpha-amylase are secreted continually and abnormally. This type of created stress is called fear stress (Gordis et al., 2006; Tanaka et al., 2012). Also, BDNF serum levels were reduced after playing the scary game in our study. BDNF can play an important role in the pathophysiology of stress-related disorders, and its increased peripheral levels can be effective in protecting neurons exposed to stress. It was demonstrated that during chronic stress, the BDNF gene is down-regulated under the prolonged action of pro-inflammatory cytokines and glucocorticoids. This results in brain atrophy and contributes to the development of mental disorders in predisposed subjects. Several recent studies have shown that the peripheral (serum or plasma) levels of BDNF are lower in patients with mood disorders during manic/mixed and depressive episodes compared to healthy controls and that effective treatments can normalize them (Buselli et al., 2019). BDNF is involved in the reconstruction of brain neurons including neurons in the prefrontal cortex and hippocampus. Also, increased BDNF is related to the enhancement of synaptic connections and reinforcement of dendritic connections. BDNF has a substantial role in the learning, memory, and development of synaptic plasticity (Lu et al., 2013; Sweatt, 2001). It also contributes to the growth of new synapses and dendritic connections in the CNS (Lu et al., 2013). The observed BDNF reduction in our study could be due to increased levels of cortisol and alpha-amylases. It can be concluded that stress and fear in this study, disrupt cognitive functions by reducing the plasma level of BDNF, and BDNF reduction along with OT reduction weakens cognitive functions and positive behaviors among players (Jindal et al., 2010; Sweatt, 2001). On the other hand, the unusual elevation in cortisol secretion disrupts the glutamate system and damages the neural synapses leading to negative neurologic consequences such as loss of memory and learning, the presence of violent and anxious behaviors, and eventually depression. The increased extracellular glutamate concentration and excessive activity of ionotropic receptors lead to toxicity inside the neurons and damaged neurons and synapses in the hippocampus (Anderson et al., 2010; Liao et al., 2012; Williams, 2001). In another part of the present study, analysis of EEG revealed that the average perceive stress was significantly affected by the scary game, as mentioned above. Besides, the comparison of arousal and valence charts in the participants showed higher arousal and negative valence after the gameplay. It is interesting to note that arousal and valence were about two times higher after the game. However, stress was seven times higher after the game. Cortisol levels obtained from saliva samples have been accepted also as a reliable physical measure of emotions. In line with our results, it was shown previously that there is temporal connectivity between cortisol release and central alertness, as reflected in the waking EEGβ activity. Findings suggest the existence of connections between the mechanisms involved in the control of HPA axis activity and the activation processes of the brain, which undergo varying degrees of alertness (Chapotot, 1998). In line with our finding, given the considerable effects of cortisol on the neural circuitry underlying effectual decision-making, it was shown that higher stress reactivity might bias individuals to appraise ambiguous stimuli more negatively and further diminish regulatory capacity that may otherwise attenuate this negative bias during effective decision-making. This explanation is constant with our knowledge about the brain regions underlying negative valence bias and acute stress responses, namely the amygdala, which plays a central role in both processes. The high density of glucocorticoid receptors in the amygdala makes it especially sensitive to cortisol release after facing stress, thus this region is ideally positioned to bias appraisals of ambiguous stimuli toward a negative or threatening valence. We think that this increased amygdala activity paired with a more global shift in neural processing toward threat detection (Brown et al., 2017). Likewise, salivary alpha-amylase has associations with both arousal and valence. In an experienced fear study, salivary alpha-amylase showed a highly specific increase only for those participants who endorsed both emotional arousal and negative valence (Buchanan et al., 2010). In line with that, our study also showed that an elevation in salivary alpha-amylase was associated with an increase in arousal and a decrease in valence. It was revealed that OT had also differential effects on the activity of specific amygdala sub-regions. On the one hand, it attenuates activation in the lateral and dorsal parts of the anterior amygdala for afraid faces but raised activity for happy expressions, thus indicating a shift of the processing focus toward positive social stimuli. OT facilitates the social approach by reducing anxiety and neuroendocrine stress responses and has shown significant effects on anatomically distinct amygdala regions associated with valence processing (Gamer et al., 2010) which is in line with our results in the current study. Here we found that decreased OT levels were associated with increased arousal and decreased valence. BDNF is an important regulator of neural survival, development, function, and plasticity. BDNF is highly expressed in limbic structures and the cerebral cortex and has a pivotal role in learning, memory, anxiety-like behavior, and reward-related processes. It is associated with better cognitive potency and has positive effects on health and brain function. It is also associated with many mental disorders, like depression, anxiety, etc. (Jeon & Ha, 2017; Kim et al., 2018). According to BDNF reduction after playing the scary game in our study, and based on previous studies which revealed that BDNF synthesis occurs in regions that participate in emotional and cognitive functions (Phillips, 2017), reduction of the performance of individuals towards the fourth quarter of the valence excitatory axis is justifiable, and thus it can be said that the scary game kind of stress caused relatively high levels of arousal and negative valence because of fear. So, the results of our study, in line with previous studies confirm the importance of BDNF in the emotional and cognitive processes of the brain and show its importance in the relationship between stresses caused by scary video games and impaired emotional and cognitive functions. On the other hand, because of the level of change in the stress index and impairment of emotional and cognitive functions, the fear created by the scary game can be considered a destructive fear (Aliyari et al., 2018b; Toohey & Taylor, 2008).
We found elevated levels of salivary cortisol and alpha-amylase after the scary gameplay which is an indicator of stress and fear elevation among players. Moreover, decreased BDNF is associated with impaired emotional and cognitive functions among players. To control the abnormal activity of the stress-fear path in the nervous system, the secretion of oxytocin is decreased. Also, EEG analysis showed increased stress and arousal, and negative valence after playing the scary video game which confirms the destructive effect of the scary game on the emotional and cognitive processes of the nervous system. Our results along with the results obtained from similar studies provide a way for understanding the mental/physical effects of video games and can thus help with game modification and development. Depending on the goal of the game, a programmer could increase or decrease the amount of a certain variable (i.e. music, visuals, etc.) to increase or decrease the intended effect. So, designing a game that is physiologically tailored to the specific individual needs of humans, seems to be acceptable shortly. To strengthen positive cognitive and emotional indicators and weaken negative cognitive and emotional indicators of video games, video game pathophysiology, especially scary games, is essential for researchers.
Ethical Considerations
Compliance with ethical guidelines
All ethical standards were under the Declaration of Helsinki. Ethical approval to conduct the study was obtained from the Ethical Committee of Baqiyatallah University of Medical Sciences (Code: IR. BMSU.REC.12345).
Funding
This study was financially supported by the Neuroscience Research Center of Baqiyatallah University of Medical Sciences.
Authors' contributions
Conceptualization: Hamed Aliyari, Hedayat Sahraei and Masoomeh Kazemi; Methodology and investigation: Hamed Aliyari, Masoomeh Kazemi and Sahar Golabi; Data curation: Hamed Aliyari, Masoomeh Kazemi, Mohammad Sahraei; Formal analysis: Hamed Aliyari, Masoumeh Kazemi, Sahar Golabi, Hedayat Sahraei, Behrouz Minaei-Bidgoli and Mohammad Reza Daliri; Writing original draft: Hamed Aliyari and Sahar Golabi; Review & editing: Sahar Golabi and Reza Hazrati; Project administration and funding acquisition: Masoomeh Kazemi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
We express our gratitude to the Neuroscience Research Center of Baqiyatallah University of Medical Sciences, the Soft Technology Development Council, and the Neurogame Research Group.
References
Aliyari, H., Hosseinian, S. H., Menhaj, M. B., & Sahraei, H. (2018).Analysis of the effects of high-voltage transmission line on human stress and attention through electroencephalography (EEG). Iranian Journal of Science and Technology, Transactions of Electrical Engineering. 43, 211-218. [DOI:10.1007/s40998-018-0151-8]
Aliyari, H., Hosseinian, S. H., Sahraei, H., & Menhaj, M. B. (2019). Effect of proximity to high-voltage fields: Results of the neural network model and experimental model with macaques. International Journal of Environmental Science and Technology, 16, 4315-4326. [DOI: 10.1007/s40998-018-0151-8]
Aliyari, H., Kazemi, M., Tekieh, E., Salehi, M., Sahraei, H., & Daliri, M. R., et al. (2015). The effects of fifa 2015 computer games on changes in cognitive, hormonal and brain waves functions of young men volunteers. Basic and Clinical Neuroscience, 6(3), 193–201. [Link]
Aliyari, H., Sahraei, H., Daliri, M. R., Minaei-Bidgoli, B., Kazemi, M., & Agaei, H., et al. (2018). The beneficial or harmful effects of computer game stress on cognitive functions of players. Basic and Clinical Neuroscience, 9(3), 177–186. [DOI:10.29252/nirp.bcn.9.3.177] [PMID] [PMCID]
Aliyari, H., Sahraei, H., Erfani, M., Mohammadi, M., Kazemi, M., & Daliri, M. R., et al. (2020). Changes in cognitive functions following violent and football video games in young male volunteers by studying brain waves. Basic and Clinical Neuroscience, 11(3), 279–288. [DOI:10.32598/bcn.9.10.335] [PMID] [PMCID]
Anderson, C. A., Shibuya, A., Ihori, N., Swing, E. L., Bushman, B. J., & Sakamoto, A., et al. (2010). Violent video game effects on aggression, empathy, and prosocial behavior in eastern and western countries: A meta-analytic review. Psychological Bulletin, 136(2), 151–173. [DOI:10.1037/a0018251] [PMID]
Al-Shargie, F., Kiguchi, M., Badruddin, N., Dass, S. C., Hani, A. F. M., & Tang, T. B. (2016). Mental stress assessment using simultaneous measurement of EEG and fNIRS. Biomedical Optics Express, 7(10), 3882-3898. [DOI:10.1364/BOE.7.003882] [PMID]
Bashashati, A., Fatourechi, M., Ward, R. K., & Birch, G. E. (2007). A survey of signal processing algorithms in brain-computer interfaces based on electrical brain signals. Journal of Neural Engineering, 4(2), R32–R57. [DOI:10.1088/1741-2560/4/2/R03] [PMID]
Bavelier, D., Green, C. S., Han, D. H., Renshaw, P. F., Merzenich, M. M., & Gentile, D. A. (2011). Brains on video games. Nature Reviews. Neuroscience, 12(12), 763–768. [DOI:10.1038/nrn3135] [PMID] [PMCID]
Bendix, M., Uvnäs-Moberg, K., Petersson, M., Gustavsson, P., Svanborg, P., & Åsberg, M., et al. (2015). Plasma oxytocin and personality traits in psychiatric outpatients. Psychoneuroendocrinology, 57, 102–110. [DOI:10.1016/j.psyneuen.2015.04.003] [PMID]
Best J. R. (2010). Effects of physical activity on children's executive function: Contributions of experimental research on aerobic exercise. Developmental Review, 30(4), 331–551.[DOI:10.1016/j.dr.2010.08.001] [PMID] [PMCID]
Boyle, E., Connolly, T. M., & Hainey, T. (2011). The role of psychology in understanding the impact of computer games. Entertainment Computing, 2(2), 69-74. [DOI:10.1016/j.entcom.2010.12.002]
Brown, C. C., Raio, C. M., & Neta, M. (2017). Cortisol responses enhance negative valence perception for ambiguous facial expressions. Scientific Reports, 7(1), 15107. [DOI:10.1038/s41598-017-14846-3] [PMID] [PMCID]
Buchanan, T. W., Bibas, D., & Adolphs, R. (2010). Salivary α-amylase levels as a biomarker of experienced fear. Communicative & Integrative Biology, 3(6), 525–527. [DOI:10.4161/cib.3.6.12606] [PMID] [PMCID]
Bullmore, E., & Sporns, O. (2009). Complex brain networks: Graph theoretical analysis of structural and functional systems. Nature Reviews. Neuroscience, 10(3), 186–198. [DOI:10.1038/nrn2575] [PMID]
Buselli, R., Veltri, A., Baldanzi, S., Marino, R., Bonotti, A., & Chiumiento, M., et al. (2019). Plasma brain-derived neurotrophic factor (BDNF) and serum cortisol levels in a sample of workers exposed to occupational stress and suffering from adjustment disorders. Brain and Behavior, 9(7), e01298. [DOI:10.1002/brb3.1298] [PMID] [PMCID]
Chonmaitree, T., Revai, K., Grady, J. J., Clos, A., Patel, J. A., & Nair, S., et al. (2008). Viral upper respiratory tract infection and otitis media complication in young children. Clinical Infectious Diseases, 46(6), 815–823. [DOI:10.1086/528685] [PMID] [PMCID]
Dayi, A., Cetin, F., Sisman, A. R., Aksu, I., Tas, A., & Gönenc, S., et al. (2015). The effects of oxytocin on cognitive defect caused by chronic restraint stress applied to adolescent rats and on hippocampal VEGF and BDNF levels. Medical Science Monitor, 21, 69–75. [DOI:10.12659/MSM.893159] [PMID] [PMCID]
De Dreu, C. K. W. (2012). Oxytocin modulates the link between adult attachment and cooperation through reduced betrayal aversion. Psychoneuroendocrinology, 37(7), 871–880.[DOI:10.1016/j.psyneuen.2011.10.003] [PMID]
Destexhe, A., Rudolph, M., Fellous, J. M., & Sejnowski, T. J. (2001). Fluctuating synaptic conductances recreate in vivo-like activity in neocortical neurons. Neuroscience, 107(1), 13–24. [DOI:10.1016/S0306-4522(01)00344-X] [PMID]
Dhama, K., Latheef, S. K., Dadar, M., Samad, H. A., Munjal, A., & Khandia, R., et al. (2019). Biomarkers in stress related diseases/disorders: Diagnostic, prognostic, and therapeutic values. Frontiers in Molecular Biosciences, 6, 91. [DOI:10.3389/fmolb.2019.00091] [PMID] [PMCID]
Diorio, D., Viau, V., & Meaney, M. J. (1993). The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. The Journal of Neuroscience, 13(9), 3839–3847. [DOI:10.1523/JNEUROSCI.13-09-03839.1993] [PMID] [PMCID]
Al-Shargie, F., Kiguchi, M., Badruddin, N., Dass, S. C., Hani, A. F., & Tang, T. B. (2016). Mental stress assessment using simultaneous measurement of EEG and fNIRS. Biomedical Optics Express, 7(10), 3882–3898. [DOI:10.1364/BOE.7.003882] [PMID] [PMCID]
Chapotot, F., Gronfier, C., Jouny, C., Muzet, A., & Brandenberger, G. (1998). Cortisol secretion is related to electroencephalographic alertness in human subjects during daytime wakefulness. The Journal of Clinical Endocrinology and Metabolism, 83(12), 4263–4268. [DOI:10.1210/jc.83.12.4263] [PMID]
Fröhlich, F., & Mc Cormick, D. A. (2010). Endogenous electric fields may guide neocortical network activity. Neuron, 67(1), 129–143. [DOI:10.1016/j.neuron.2010.06.005] [PMID] [PMCID]
Gamer, M., & Büchel, C. (2012). Oxytocin specifically enhances valence-dependent parasympathetic responses. Psychoneuroendocrinology, 37(1), 87–93. [DOI:10.1016/j.psyneuen.2011.05.007] [PMID]
Gamer, M., Zurowski, B., & Büchel, C. (2010). Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proceedings of the National Academy of Sciences of the United States of America, 107(20), 9400–9405. [DOI:10.1073/pnas.1000985107] [PMID] [PMCID]
Garrett, B. (2014). Study guide to accompany bob garrett’s brain & behavior: An introduction to biological psychology. Newbury Park: Sage Publications. [Link]
Gatti, R., & De Palo, E. F. (2011). An update: Salivary hormones and physical exercise. Scandinavian Journal of Medicine & Science in Sports, 21(2), 157–169. [DOI:10.1111/j.1600-0838.2010.01252.x] [PMID]
Geoffroy, M. C., Hertzman, C., Li, L., & Power, C. (2012). Morning salivary cortisol and cognitive function in mid-life: Evidence from a population-based birth cohort. Psychological Medicine, 42(8), 1763–1773. [DOI:10.1017/S0033291711002704] [PMID]
Gordis, E. B., Granger, D. A., Susman, E. J., & Trickett, P. K. (2006). Asymmetry between salivary cortisol and alpha-amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology, 31(8), 976–987. [DOI:10.1016/j.psyneuen.2006.05.010] [PMID]
Guzel Aydin, S., Kaya, T., & Guler, H. (2016). Wavelet-based study of valence-arousal model of emotions on EEG signals with LabVIEW. Brain Informatics, 3(2), 109–117. [DOI:10.1007/s40708-016-0031-9] [PMID] [PMCID]
Jeon, Y. K., & Ha, C. H. (2017). The effect of exercise intensity on brain derived neurotrophic factor and memory in adolescents. Environmental Health and Preventive Medicine, 22(1), 27. [DOI:10.1186/s12199-017-0643-6] [PMID] [PMCID]
Jindal, R. D., Pillai, A. K., Mahadik, S. P., Eklund, K., Montrose, D. M., & Keshavan, M. S. (2010). Decreased BDNF in patients with antipsychotic naïve first episode schizophrenia. Schizophrenia Research, 119(1-3), 47–51. [DOI:10.1016/j.schres.2009.12.035] [PMID] [PMCID]
Johnston, M. V. (2009). Plasticity in the developing brain: implications for rehabilitation. Developmental Disabilities Research Reviews, 15(2), 94–101 [DOI:10.1002/ddrr.64] [PMID]
Kaplan, S., & Berman, M. G. (2010). Directed attention as a common resource for executive functioning and self-regulation. Perspectives on Psychological Science, 5(1), 43–57.[DOI:10.1177/1745691609356784] [PMID]
Kim, K. M., Choi, S. W., Lee, J., & Kim, J. W. (2018). EEG correlates associated with the severity of gambling disorder and serum BDNF levels in patients with gambling disorder. Journal of Behavioral Addictions, 7(2), 331–338. [DOI:10.1556/2006.7.2018.43] [PMID] [PMCID]
Kirschbaum, C., & Hellhammer, D. H. (1989). Salivary cortisol in psychobiological research: An overview. Neuropsychobiology, 22(3), 150-169. [DOI:10.1159/000118611] [PMID]
Kotwica, G., Kaminska, B., Franczak, A., Kurowicka, B., Staszkiewicz, J., & Skowronski, M. T., et al. (2004). The effect of oxytocin on cortisol and corticosterone secretion in cyclic gilts-in vivo and in vitro studies. Reprodution Biology, 4(1), 35-50. [Link]
Kreutz, G., Bongard, S., Rohrmann, S., Hodapp, V., & Grebe, D. (2004). Effects of choir singing or listening on secretory immunoglobulin A, cortisol, and emotional state. Journal of Behavioral Medicine, 27(6), 623–635. [DOI:10.1007/s10865-004-0006-9] [PMID]
Lee, B. K., Glass, T. A., Mc Atee, M. J., Wand, G. S., Bandeen-Roche, K., & Bolla, K. I., et al. (2007). Associations of salivary cortisol with cognitive function in the Baltimore memory study. Archives of General Psychiatry, 64(7), 810–818. [DOI:10.1001/archpsyc.64.7.810] [PMID]
Liao, L. D., Chen, C. Y., Wang, I. J., Chen, S. F., Li, S. Y., & Chen, B. W., et al. (2012). Gaming control using a wearable and wireless EEG-based brain-computer interface device with novel dry foam-based sensors. Journal of Neuroengineering and Rehabilitation, 9(1), 5. [DOI:10.1186/1743-0003-9-5] [PMID] [PMCID]
Licinio, J., & Wong, M. (2002). Brain-derived neurotrophic factor (BDNF) in stress and affective disorders. Molecular Psychiatry, 7(6), 519. [DOI:10.1038/sj.mp.4001211] [PMID]
Lu, B., Nagappan, G., Guan, X., Nathan, P. J., & Wren, P. (2013). BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nature Reviews. Neuroscience, 14(6), 401–416. [DOI:10.1038/nrn3505] [PMID]
Lucas-Thompson, R. G., & Holman, E. A. (2013). Environmental stress, oxytocin receptor gene (OXTR) polymorphism, and mental health following collective stress. Hormones and Behavior, 63(4), 615–624. [DOI:10.1016/j.yhbeh.2013.02.015] [PMID]
Ludwig, M., & Leng, G. (2006). Dendritic peptide release and peptide-dependent behaviours. Nature Reviews. Neuroscience, 7(2), 126–136. [DOI:10.1038/nrn1845] [PMID]
Maldonado, E. F., Nislin, M., Martínez-Escribano, A., Marín, L., Enguix, A., & Alamo, A., López, et al. (2019). Association of salivary alpha-amylase and salivary flow rate with working memory functioning in healthy children. Stress, 22(6), 670–678. [DOI:10.1080/10253890.2019.1611777] [PMID]
Mastorakos, G., & Ilias, I. (2003). Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Annals of the New York Academy of Sciences, 997, 136–149. [DOI:10.1196/annals.1290.016] [PMID]
McKlveen, J. M., Morano, R. L., Fitzgerald, M., Zoubovsky, S., Cassella, S. N., & Scheimann, J. R., et al. (2016). Chronic stress increases prefrontal inhibition: A mechanism for stress-induced prefrontal dysfunction. Biological Psychiatry, 80(10), 754–764. [DOI:10.1016/j.biopsych.2016.03.2101] [PMID] [PMCID]
McQuaid, R. J., McInnis, O. A., Paric, A., Al-Yawer, F., Matheson, K., & Anisman, H. (2016). Relations between plasma oxytocin and cortisol: The stress buffering role of social support. Neurobiology of Stress, 3, 52–60. [DOI:10.1016/j.ynstr.2016.01.001] [PMID] [PMCID]
Megalakaki, O., Ballenghein, U., & Baccino, T. (2019). Effects of valence and emotional intensity on the comprehension and memorization of texts. Frontiers in Psychology, 10, 179. [DOI:10.3389/fpsyg.2019.00179] [PMID] [PMCID]
Phillips C. (2017). Brain-Derived neurotrophic factor, depression, and physical activity: Making the neuroplastic connection. Neural Plasticity, 2017, 7260130. [DOI:10.1155/2017/7260130] [PMID] [PMCID]
Rabipour, S., & Raz, A. (2012). Training the brain: Fact and fad in cognitive and behavioral remediation. Brain and Cognition, 79(2), 159–179. [DOI:10.1016/j.bandc.2012.02.006] [PMID]
Russoniello, C. V., O’Brien, K., & Parks, J. M. (2009). The effectiveness of casual video games in improving mood and decreasing stress. Journal of CyberTherapy & Rehabilitation, 2(1), 53-66. [Link]
Schultheiss, O. C., & Stanton, S. J. (2009). Assessment of salivary hormones. In E. Harmon-Jones & J. S. Beer (Eds.), Methods in social neuroscience (pp. 17–44). New York: Guilford Press. [Link]
Sulaiman, N., Taib, M. N., Lias, S., Murat, Z. H., Aris, S. A. M., & Mustafa, M., et al. (2012). Development of EEG-based stress index. Paper presented at: 2012 International Conference on Biomedical Engineering. Penang, Malaysia, 28 February 2012. [DOI:10.1109/ICoBE.2012.6179059]
Téllez, A. M., Castro, L. A., & Tentori, M. (2023). Developing and Evaluating a virtual reality videogame using biofeedback for stress management in sports. Interacting with Computers, iwad025. [DOI:10.1093/iwc/iwad025]
Wu, S., Xu, X., Shu, L., & Hu, B. (2017). Estimation of valence of emotion using two frontal EEG channels. Paper presented at: 2017 IEEE International Conference on Bioinformatics and Biomedicine, Kansas City, USA, 16 November 2017. [DOI:10.1109/BIBM.2017.8217815]
Shuman, V., Sander, D., & Scherer, K. R. (2013). Levels of valence. Frontiers in Psychology, 4, 261. [DOI:10.3389/fpsyg.2013.00261]
Sweatt, J. D. (2001). The neuronal MAP kinase cascade: A biochemical signal integration system subserving synaptic plasticity and memory. Journal of Neurochemistry, 76(1), 1–10.[DOI:10.1046/j.1471-4159.2001.00054.x] [PMID]
Tanaka, Y., Ishitobi, Y., Maruyama, Y., Kawano, A., Ando, T., & Imanaga, J., et al. (2012). Salivary α-amylase and cortisol responsiveness following electrical stimulation stress in panic disorder patients. Neuroscience Research, 73(1), 80–84. [DOI:10.1016/j.neures.2012.01.006] [PMID]
Onaka, T., & Takayanagi, Y. (2019). Role of oxytocin in the control of stress and food intake. Journal of Neuroendocrinology, 31(3), e12700. [DOI:10.1111/jne.12700] [PMID] [PMCID]
Toohey, K., & Taylor, T. (2008). Mega events, fear, and risk: Terrorism at the olympic games. Journal of Sport Management, 22(4), 451-469. [DOI:10.1123/jsm.22.4.451]
Trepte, S., & Reinecke, L. (2010). Avatar creation and video game enjoyment: Effects of life-satisfaction, game competitiveness, and identification with the avatar. Journal of Media Psychology, 22(4), 171. [DOI:10.1027/1864-1105/a000022]
Uvnas-Moberg, K., & Petersson, M. (2005). [Oxytocin, a mediator of anti-stress, well-being, social interaction, growth and healing (German)]. Zeitschrift fur Psychosomatische Medizin und Psychotherapie, 51(1), 57–80. [DOI:10.13109/zptm.2005.51.1.57] [PMID]
Wagner, U., & Echterhoff, G. (2018). When does oxytocin affect human memory encoding? The role of social context and individual attachment style. Frontiers in Human Neuroscience, 12, 349. [DOI:10.3389/fnhum.2018.00349] [PMID] [PMCID]
Williams, J. A. (2001). Intracellular signaling mechanisms activated by cholecystokinin-regulating synthesis and secretion of digestive enzymes in pancreatic acinar cells. Annual Review of Physiology, 63, 77–97. [DOI:10.1146/annurev.physiol.63.1.77] [PMID]
Type of Study: Original |
Subject:
Cognitive Neuroscience
Received: 2021/10/24 | Accepted: 2022/01/12 | Published: 2023/03/1
Received: 2021/10/24 | Accepted: 2022/01/12 | Published: 2023/03/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |