Volume 15, Issue 4 (July & August 2024)
BCN 2024, 15(4): 509-518 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Olazadeh K, Borumandnia N, Habibi M, Alavi Majd H. Using Longitudinal Variance Components Models to Assess Hyper-connectivity in Severe Traumatic Brain Injury Patients. BCN 2024; 15 (4) :509-518
URL: http://bcn.iums.ac.ir/article-1-2319-en.html
URL: http://bcn.iums.ac.ir/article-1-2319-en.html
1- Department of Biostatistics, School of Allied Medical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Urology and Nephrology Research Centre, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Department of Biostatistics, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran.
2- Urology and Nephrology Research Centre, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Department of Biostatistics, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran.
Keywords: Traumatic brain injury (TBI), Hyper-connectivity, Functional magnetic resonance imaging (fMRI) neuroimaging, Longitudinal model of variance components, Cognitive function
Full-Text [PDF 721 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Functional magnetic resonance imaging (fMRI) is a non-invasive technique that has become a standard criterion for imaging human brain function in healthy and diseased populations (Eklund et al., 2017). One type of fMRI is resting imaging. This type of imaging was first used by Biswal et al. and is now a popular method in neuroscience (Biswal et al., 1995). One way to discover and study the brain mechanisms that underlie behavioral changes in individuals and patients is to look at brain networks (Bittencourt-Villalpando et al., 2021). Brain networks affect various functions, such as vision, cognition, or movement control (McTeague et al., 2016). One of these brain networks is functional connectivity (FC), which can lead to discovering patterns and connections between brain areas. Increasing or decreasing FC in the brain can be a way to early diagnosis or treatment of neurological diseases (Hart et al., 2018). fMRI allows indirect measurements of neural activity; therefore, this method of imaging can detect changes in functional brain communication associated with neuropathology, including brain damage (Anderson et al., 1995; Crosson et al., 1993). Traumatic brain injury (TBI) is one of the most important diseases indicated by the World Health Organization (WHO). It is one of the leading causes of death and disability worldwide and affects adults and children at all economic levels and in society (Levin et al., 1981). A 2010 study found that approximately 57 million people worldwide are hospitalized with brain injury problems each year. However, the exact relationship between life and disability caused by TBI is unclear (WHO, 2010). Recently, the use of resting fMRI data in patients with TBI has been expanding. The magnetic resonance imaging (MRI) data of people with TBI show abnormalities in the frontal lobes, temporal lobe, and laterals and enlarging the brain's ventricles (Crosson et al., 1993). Studies have shown that ventricular enlargement is one of the best indicators of the severity of brain damage (Henry-Feugeas et al., 2000). Cognitive control disorders have also been observed in fMRI data associated with moderate to severe TBI (Scheibel, 2017).
According to studies, FC has increased abnormally and excessively in people with moderate to severe TBI, and the consequences of this increased FC are still being investigated by neuroscientists (Roy et al., 2017). Also, fMRI data, which compare two groups of healthy people with a specific neurological disease collected longitudinally, have become popular nowadays (Esposito et al., 2013). Accordingly, this study uses the longitudinal model of Hart et al. on fMRI data of individuals with TBI, collected longitudinally. Then, it investigates the excessive increase in functional communication of people with TBI. Due to the different error structures, the longitudinal model used in this study can be more reliable than other similar longitudinal models (Hart et al., 2018).
2. Materials and Methods
The data used in this study were downloaded from the accessible Open fMRI site. This data has the access number “ds000220” on the site. The subjects who underwent brain imaging included 14 people with severe TBI. They were in the age range of 18 to 36 years. Severe brain injury was measured based on the Glasgow coma scale. Those with spinal cord injuries or other neurological diseases were excluded from the study. A control group was also selected, including 12 healthy individuals similar in age and literacy to the TBI group. The subjects who were finally chosen as the TBI group were followed up three months, six months, and twelve months after the brain injury, and all three times, brain imaging was performed. Healthy individuals also underwent brain imaging twice at three-month intervals. Brain imaging was performed using the Philips Achieva 3T or Siemens Magnetom Trio 3T devices. These scanners were used for neuroimaging subjects in the control and the TBI group. The subjects were asked to have the least amount of movement in scanners. Written consent is obtained from all individuals, and all data collection steps are approved by the Pennsylvania State University Research Support Office. Also, all methods and models used in this study were approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences.
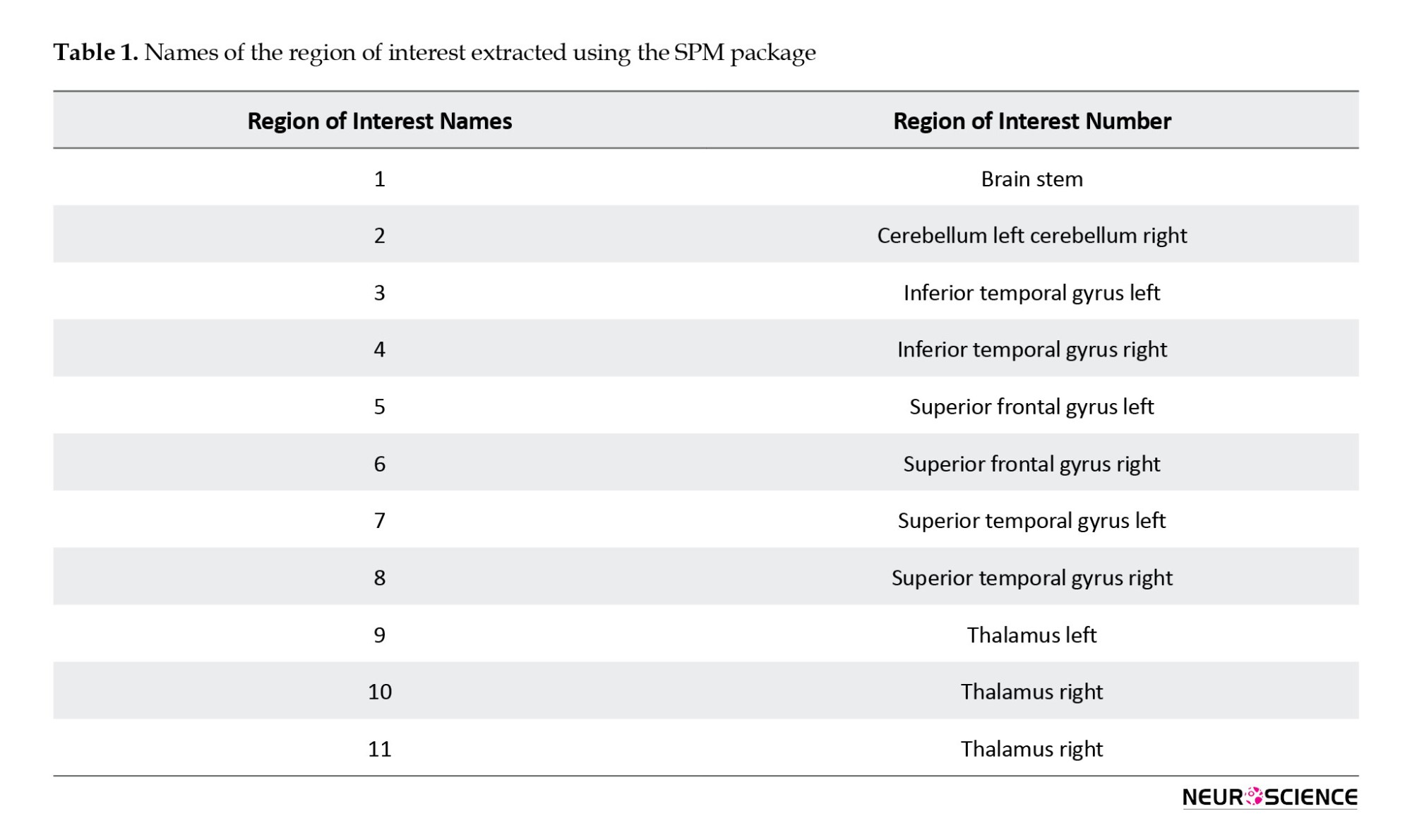
The image resolution of T1 was obtained with cerebral segregation weight with an isotropic spatial resolution of 1.0 mm. Echo planar imaging evaluated blood oxygen level-dependent responses for performance imaging. Imaging parameters for the echo planar imaging were 2000 ms/30 ms/90˚ (repeat time/echo time/flip angle), 240×240 mm square field of view, and 80, 80, 80 acquisition matrix with 4 mm thick axial cuts parallel to the second line It was media (Roy et al., 2017). All data preprocessing steps, including temporal correction, motion correction, matching, normalization, and spatial smoothing, were performed by the FSL software version 6.0.1. After using the FSL software, the output was entered into the MATLAB software, version 2019 software, and the SPM package version 12. The WFU-pickatlas module was used to exit the region of interest (ROI). A total of 11 ROI of each person's brain was extracted using the SPM package, in which brain damage had the most significant impact. The names of 11 ROIs were removed, and the corresponding number of each ROI is shown in Table 1.
Statistical inference
A longitudinal variance components model was used to compare FC in control and TBI groups (Hart et al., 2018). The longitudinal model used in this study has two basic properties. Using the longitudinal model of variance components, the automatic correlation of the data obtained from the fMRI time series, the conflict of covariance due to heterogeneity of individuals, and the clash of covariance due to the difference of each individual over time can be measured. By measuring the mentioned parameters, the results obtained from fitting the longitudinal model of the variance components will have minor errors. If we show the number of areas with P, we also offer the number of pairs ROI that are compared in two groups with Q, considering that in this study, 11 ROI have been selected, the total number of comparisons will be calculated in two groups of control and TBI using the relation (11)2. Therefore, 55 comparisons between pairs' ROI are 2 calculated in each group. Assuming β is a vector of length 2Q, Q will denote the first element by β0 which determines the group difference due to the FC baseline effect; accordingly, the difference in FC between the control group and the TBI group at the base time. We also show the second element, Q, with β1 which calculates the group difference in the longitudinal trend of FC, i.e. the difference in FC between the control and TBI groups over time using the longitudinal model of the variance components. The meaning of FC group difference in the baseline is to evaluate the difference in FC between the two TBI and control groups at baseline. This assessment is a difference in the degree of correlation between the ROI in the two groups of TBI and control at the base time, that is, the first scan taken from individuals. Also, the difference between the FC Longitudinal trend means that the difference in FC over time between the TBI and control groups is evaluated. This assessment is also a difference in the degree of correlation between ROI in the two groups of TBI and control over time. More information on the details of the longitudinal model of the variance components can be found in Hart et al. (Hart et al., 2018).
3. Results
The longitudinal model of the variance components tests the group difference based on the FC base time and the group difference in the FC longitudinal trend. The estimation of the main effects and longitudinal trend in the control group was determined with βHC. Assessment of the main effects and longitudinal trend in the intervention group was shown with βTBI. After fitting the longitudinal model of the variance components to the two groups, it was observed that no statistically significant difference between the control group and the TBI group due to the base time of FC and between pairs ROI. By fitting the longitudinal model of the variance components to the control and TBI groups, a statistically significant difference was observed between the control group and the TBI group in 3 pairs of ROIs in the longitudinal trend of FC. The pairs of regions, cerebellum left and cerebellum right, superior temporal gyrus left and superior temporal gyrus right, thalamus left and thalamus right had a statistically significant difference in FC over time between the control and TBI groups. Therefore, out of 55 ROI, in three pairs ROI, there was a statistically significant difference between the control group and the TBI group in the longitudinal trend of FC. Table 2 shows the complete information about the pair of substantial ROIs.
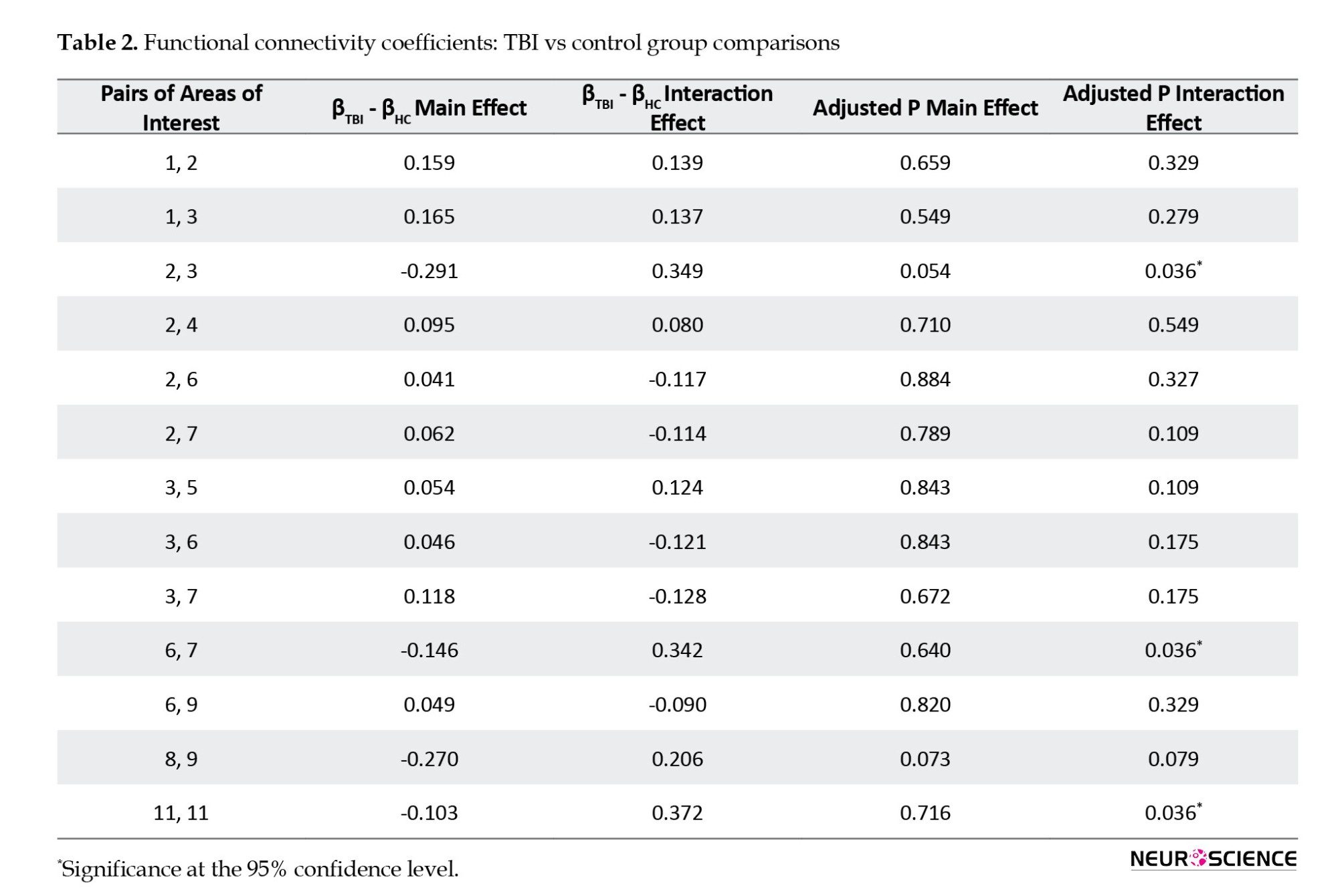
Figure 1 at the bottom left shows the difference between the estimates of the main effects and the longitudinal trend of FC in the control and TBI groups. Accordingly, in most pairs of ROIs, the difference between the estimation of the main effects and the longitudinal trend of FC was more significant in the TBI group than in the control group. The red circle points indicate a positive difference, and the blue circle points indicate a negative difference between the two groups. With these interpretations, in estimating the main effects of FC, the TBI group in the two pairs of regions 2 and 3 and 8 and 9 showed a decrease in FC compared to the control group. However, this difference was not statistically significant. The estimation of the effects of the longitudinal trend of FC also shows that in comparison with most pairs of ROIs, FC between the TBI group has increased compared to the control group. In Figure 1, at the bottom left, the top triangular diagram shows that the pairs of regions “2 and 3,” “6 and 7,” and “10 and 11” have bolder points than the other pairs of pants. This indicates an increase in FC in the TBI group compared to the control group over one year.
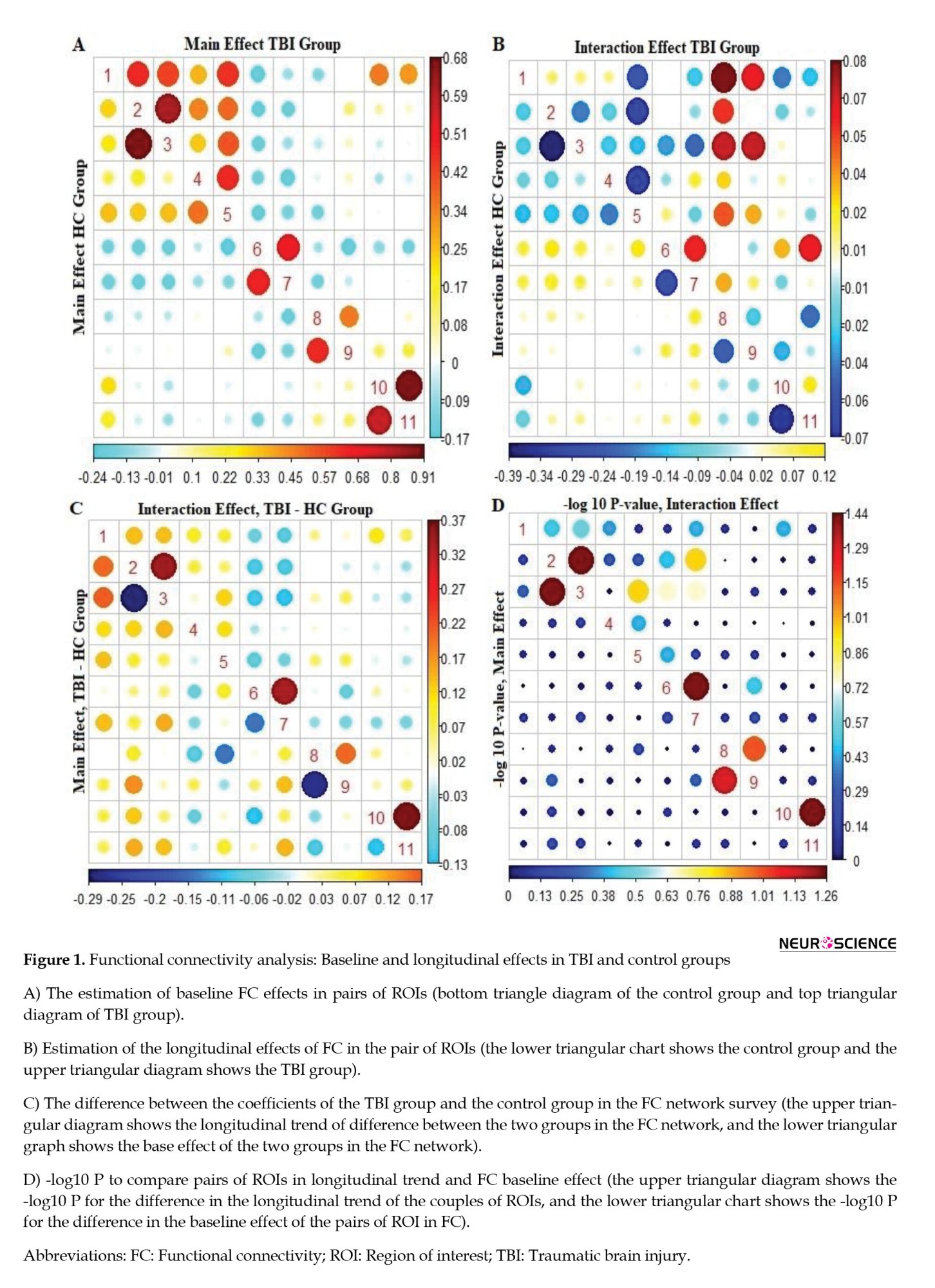
Figure 1, at the bottom right, shows the -log 10 P to compare the estimation of the main effects and the longitudinal trend of FC in the pair's ROI between the TBI and the control groups. Accordingly, in estimating the longitudinal trend of FC between the control group and TBI, pairs of regions “2 and 3,” “6 and 7,” and “10 and 11” have more prominent points than other points.
4. Discussion
This study applied the longitudinal model of variance components to fMRI neuroimaging data of subjects with severe TBI disease that were collected longitudinally. The data used in this study are unique in fMRI imaging studies due to the severity of brain damage and follow-up time. Also, due to the different error structures, the longitudinal model used in this study has better reliability. By fitting the longitudinal model of variance components, no statistically significant difference was observed in the baseline effect of FC between the TBI group and the control group. However, in the longitudinal course of FC between the three pairs of regions, hyper-connectivity was observed in the TBI group compared to the control group. The cerebellum left and cerebellum right regions, superior frontal gyrus left and superior frontal gyrus right, and the thalamus left and thalamus right regions were areas where hyper-connectivity was observed in the TBI group compared to the control group. The cerebellum left and cerebellum right regions are responsible for balancing and coordinating the body's muscles to perform a specific function in the brain. Eye movements and motor learning also function in the cerebellum left and cerebellum right areas (Ferrari et al., 2019). The superior frontal gyrus left and superior frontal gyrus right regions play a crucial role in human self-awareness and working memory (Boisgueheneuc et al., 2006). The thalamus left and thalamus right regions are also responsible for amplifying sensory signals in the brain. Other functions of the thalamus left and thalamus right include learning, episodic memory, sleep regulation, and wakefulness (Torrico & Munakomi, 2023).
This study only identified brain areas in people with severe TBI who have experienced an acute increase in FC in their areas of the brain. Numerous studies have addressed the issue of sharp FC increase in patients with moderate to severe TBI. In 2015, Sours et al. evaluated 77 individuals with mild TBI and 35 healthy individuals for the severity of FC under the thalamus cerebral cortex using the standard t test and the analysis of variance test. The results of their study showed that people with mild TBI experienced an excessive increase in FC beneath the various layers of the thalamus, the subcortices associated with sensory processing, and the default mode network. According to Sours et al., people with mild TBI showed that they experienced cognitive, neurological, behavioral, sensory, and physical disorders. Their results suggest that this hyper-connectivity may affect brain areas related to sensory, motor, and even auditory functions. The brain is trying to heal after an injury, and time must be given for a full recovery (Sours et al., 2015).
The present study results are also consistent with the study of Sours et al. In the present study, an acute increase in FC in the thalamus was observed as well. In the study of Sours et al., common statistical methods were used to examine group differences. In contrast, our study used a new longitudinal statistical model to examine group differences in baseline effect and FC longitudinal trend. In 2016, Iraji et al. followed up on 16 patients with mild TBI for 4 to 6 weeks after injury and were given fMRI at rest. A total of 24 people were also selected as the control group. In this study, it was concluded that mild TBI patients have higher FC than the control group. Iraji et al. concluded that concussion can alter FC and that the brains of people with mild TBI tend to be hyperactive to compensate for the pathophysiological abnormalities caused by the injury. This brain activity occurs in response to the problem created after a brain injury, and the brain increases these functional connections to compensate for the damage. This increase is too much for the affected areas responsible for executive functions and working memory (Iraji et al., 2016).
The results of our study are mainly similar to Iraji et al. because the areas in the present study that experienced an excessive increase in FC affect organizational performance and working memory. Meanwhile, the data used in our study are similar to the study of Iraji et al., but our data's follow-up time is one year and is more valid in terms of time. The model used in our study may provide stronger results due to the different error structure than the common methods used in the study by Iraji et al. In 2017, Bernier et al. examined FC at rest using fMRI images. They analyzed 14 people with moderate to severe TBI and 19 healthy people. They used a 264-area atlas to identify areas of the brain. Finally, they concluded that the FC in the group with TBI was much higher than the FC in the control group. Bernier et al. hypothesized that this hyper-connectivity after brain injury might have been due to dedifferentiation. Dedifferentiation means the loss of specialization and function in a specific network of the brain that occurs most often in old age (Bernier et al., 2017). The areas used in the study by Bernier et al. were different from our study. Also, the data used in their study were cross-sectional, while the data used in the present study were collected longitudinally. A new longitudinal model was used in our study, while in Bernier et al., common statistical methods such as t test and analysis of variance were used. However, the results of both studies showed a hyper-connectivity in the TBI group. In 2019, Konstantinou et al. examined the correlation between brain regions in two groups of people with moderate to severe TBI. There were 11 people in both groups. They found that those with moderate to severe TBI had impaired executive function, verbal memory, and visual memory, which were associated with differences in FC in the cerebral cortex (Konstantinou et al., 2019).
Our study also showed that hyper-connectivity occurs in areas of the brain that are responsible for executive functions. In a 2020 study by Lu et al., 27 people with moderate TBI and 43 healthy people with FC in several brain areas were compared using fMRI imaging. This study was cross-sectional and showed a decrease in FC in the superior frontal gyrus. The results of Lu et al.'s study showed that decreased FC in the thalamus and superior frontal gyrus caused headaches in people with mild TBI (Lu et al., 2020). The results of our study are different from those of Lu et al., as they observed a decrease in FC in the TBI group, while we observed hyper-connectivity in the TBI group. Their study is cross-sectional, while our study is longitudinal. In 2020, Lu et al. conducted another study on 53 patients with mild TBI and 37 healthy individuals. Resting fMRI was used to compare FC in the two groups. The results showed that hyper-connectivity in the right posterior insula and inferior frontal gyrus was observed in the mild TBI group compared to the control group (Lu et al., 2020). Their study was cross-sectional and common statistical methods, such as the Pearson correlation coefficient test have been used. However, longitudinal data have been used in the present study, and a newer statistical model has been fitted to the data. The results of both studies show an excessive increase in FC in many areas of the brain. In 2021, Sheth et al. analyzed 49 veterans with mild TBI and 25 veterans without cerebral palsy for FC using fMRI neuroimaging. They found that FC was significantly increased in veterans with mTBI compared with veterans without cerebral palsy. Sheth et al. concluded that this sharp increase in FC in veterans with mild TBI could be a mechanism for maintaining overall brain network function (Sheth et al., 2021). Also, in 2021, Amir et al. examined the FC network of resting fMRI neuroimaging among 27 patients with mild TBI and 26 in the control group. They observed hyper-connectivity in patients with mild TBI compared to the control group. Amir et al. argued that this hyper-connectivity could be due to post-injury protective mechanisms, which are nerve compensation in the brain system. There are also signs that this acute increase in FC may be causing psychological distress or headaches. The nature of this hyper-connectivity can depend on some factors, including age, pre-injury cognitive function, or nervous system (Amir et al., 2021). The results of Amir et al.'s study are in line with the present study. However, the differences between our study and Amir et al.'s study are in the length of the data and different statistical methods to examine the difference between FC in the two groups. There is still no convincing evidence for the claims of hyper-connectivity in patients with moderate to severe TBI. Researchers and neuroscientists need to investigate the nature and cause of this acute increase (Amir et al., 2021).
5. Conclusion
Using neuroimaging data from people with severe TBI, collected longitudinally and applying the longitudinal model of variance components for these data, due to the different nature of the error structure compared to similar models, can make the results of the error structure of this study highly cited. By fitting the longitudinal model of variance components, it was concluded that in the longitudinal trend of FC in the TBI group, hyper-connectivity compared to the control group was observed in the pairs of cerebellum left and cerebellum right, superior frontal gyrus left and superior frontal gyrus right, as well as thalamus left and thalamus right. This hyper-connectivity can affect people's cognitive functions, but the extent and manner of this effect have not been determined to date and require more detailed studies.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran. (Code: IR.SBMU.RETECH.REC.1397.606).
Funding
The paper was extracted from the master's thesis of Keyvan Olazadeh, approved by the Department of Biostatistics, School of Allied Medical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Authors' contributions
Study design and data extraction: Keyvan Olazadeh; Data analysis, data interpretation: Hamid Alavi Majd and Nasrin Borumndnia; Literature research: Mahin Habibi; Writing and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors sincerely thank the Open fMRI for providing the data.
References
Functional magnetic resonance imaging (fMRI) is a non-invasive technique that has become a standard criterion for imaging human brain function in healthy and diseased populations (Eklund et al., 2017). One type of fMRI is resting imaging. This type of imaging was first used by Biswal et al. and is now a popular method in neuroscience (Biswal et al., 1995). One way to discover and study the brain mechanisms that underlie behavioral changes in individuals and patients is to look at brain networks (Bittencourt-Villalpando et al., 2021). Brain networks affect various functions, such as vision, cognition, or movement control (McTeague et al., 2016). One of these brain networks is functional connectivity (FC), which can lead to discovering patterns and connections between brain areas. Increasing or decreasing FC in the brain can be a way to early diagnosis or treatment of neurological diseases (Hart et al., 2018). fMRI allows indirect measurements of neural activity; therefore, this method of imaging can detect changes in functional brain communication associated with neuropathology, including brain damage (Anderson et al., 1995; Crosson et al., 1993). Traumatic brain injury (TBI) is one of the most important diseases indicated by the World Health Organization (WHO). It is one of the leading causes of death and disability worldwide and affects adults and children at all economic levels and in society (Levin et al., 1981). A 2010 study found that approximately 57 million people worldwide are hospitalized with brain injury problems each year. However, the exact relationship between life and disability caused by TBI is unclear (WHO, 2010). Recently, the use of resting fMRI data in patients with TBI has been expanding. The magnetic resonance imaging (MRI) data of people with TBI show abnormalities in the frontal lobes, temporal lobe, and laterals and enlarging the brain's ventricles (Crosson et al., 1993). Studies have shown that ventricular enlargement is one of the best indicators of the severity of brain damage (Henry-Feugeas et al., 2000). Cognitive control disorders have also been observed in fMRI data associated with moderate to severe TBI (Scheibel, 2017).
According to studies, FC has increased abnormally and excessively in people with moderate to severe TBI, and the consequences of this increased FC are still being investigated by neuroscientists (Roy et al., 2017). Also, fMRI data, which compare two groups of healthy people with a specific neurological disease collected longitudinally, have become popular nowadays (Esposito et al., 2013). Accordingly, this study uses the longitudinal model of Hart et al. on fMRI data of individuals with TBI, collected longitudinally. Then, it investigates the excessive increase in functional communication of people with TBI. Due to the different error structures, the longitudinal model used in this study can be more reliable than other similar longitudinal models (Hart et al., 2018).
2. Materials and Methods
The data used in this study were downloaded from the accessible Open fMRI site. This data has the access number “ds000220” on the site. The subjects who underwent brain imaging included 14 people with severe TBI. They were in the age range of 18 to 36 years. Severe brain injury was measured based on the Glasgow coma scale. Those with spinal cord injuries or other neurological diseases were excluded from the study. A control group was also selected, including 12 healthy individuals similar in age and literacy to the TBI group. The subjects who were finally chosen as the TBI group were followed up three months, six months, and twelve months after the brain injury, and all three times, brain imaging was performed. Healthy individuals also underwent brain imaging twice at three-month intervals. Brain imaging was performed using the Philips Achieva 3T or Siemens Magnetom Trio 3T devices. These scanners were used for neuroimaging subjects in the control and the TBI group. The subjects were asked to have the least amount of movement in scanners. Written consent is obtained from all individuals, and all data collection steps are approved by the Pennsylvania State University Research Support Office. Also, all methods and models used in this study were approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences.
The image resolution of T1 was obtained with cerebral segregation weight with an isotropic spatial resolution of 1.0 mm. Echo planar imaging evaluated blood oxygen level-dependent responses for performance imaging. Imaging parameters for the echo planar imaging were 2000 ms/30 ms/90˚ (repeat time/echo time/flip angle), 240×240 mm square field of view, and 80, 80, 80 acquisition matrix with 4 mm thick axial cuts parallel to the second line It was media (Roy et al., 2017). All data preprocessing steps, including temporal correction, motion correction, matching, normalization, and spatial smoothing, were performed by the FSL software version 6.0.1. After using the FSL software, the output was entered into the MATLAB software, version 2019 software, and the SPM package version 12. The WFU-pickatlas module was used to exit the region of interest (ROI). A total of 11 ROI of each person's brain was extracted using the SPM package, in which brain damage had the most significant impact. The names of 11 ROIs were removed, and the corresponding number of each ROI is shown in Table 1.

Statistical inference
A longitudinal variance components model was used to compare FC in control and TBI groups (Hart et al., 2018). The longitudinal model used in this study has two basic properties. Using the longitudinal model of variance components, the automatic correlation of the data obtained from the fMRI time series, the conflict of covariance due to heterogeneity of individuals, and the clash of covariance due to the difference of each individual over time can be measured. By measuring the mentioned parameters, the results obtained from fitting the longitudinal model of the variance components will have minor errors. If we show the number of areas with P, we also offer the number of pairs ROI that are compared in two groups with Q, considering that in this study, 11 ROI have been selected, the total number of comparisons will be calculated in two groups of control and TBI using the relation (11)2. Therefore, 55 comparisons between pairs' ROI are 2 calculated in each group. Assuming β is a vector of length 2Q, Q will denote the first element by β0 which determines the group difference due to the FC baseline effect; accordingly, the difference in FC between the control group and the TBI group at the base time. We also show the second element, Q, with β1 which calculates the group difference in the longitudinal trend of FC, i.e. the difference in FC between the control and TBI groups over time using the longitudinal model of the variance components. The meaning of FC group difference in the baseline is to evaluate the difference in FC between the two TBI and control groups at baseline. This assessment is a difference in the degree of correlation between the ROI in the two groups of TBI and control at the base time, that is, the first scan taken from individuals. Also, the difference between the FC Longitudinal trend means that the difference in FC over time between the TBI and control groups is evaluated. This assessment is also a difference in the degree of correlation between ROI in the two groups of TBI and control over time. More information on the details of the longitudinal model of the variance components can be found in Hart et al. (Hart et al., 2018).
3. Results
The longitudinal model of the variance components tests the group difference based on the FC base time and the group difference in the FC longitudinal trend. The estimation of the main effects and longitudinal trend in the control group was determined with βHC. Assessment of the main effects and longitudinal trend in the intervention group was shown with βTBI. After fitting the longitudinal model of the variance components to the two groups, it was observed that no statistically significant difference between the control group and the TBI group due to the base time of FC and between pairs ROI. By fitting the longitudinal model of the variance components to the control and TBI groups, a statistically significant difference was observed between the control group and the TBI group in 3 pairs of ROIs in the longitudinal trend of FC. The pairs of regions, cerebellum left and cerebellum right, superior temporal gyrus left and superior temporal gyrus right, thalamus left and thalamus right had a statistically significant difference in FC over time between the control and TBI groups. Therefore, out of 55 ROI, in three pairs ROI, there was a statistically significant difference between the control group and the TBI group in the longitudinal trend of FC. Table 2 shows the complete information about the pair of substantial ROIs.

Figure 1 at the bottom left shows the difference between the estimates of the main effects and the longitudinal trend of FC in the control and TBI groups. Accordingly, in most pairs of ROIs, the difference between the estimation of the main effects and the longitudinal trend of FC was more significant in the TBI group than in the control group. The red circle points indicate a positive difference, and the blue circle points indicate a negative difference between the two groups. With these interpretations, in estimating the main effects of FC, the TBI group in the two pairs of regions 2 and 3 and 8 and 9 showed a decrease in FC compared to the control group. However, this difference was not statistically significant. The estimation of the effects of the longitudinal trend of FC also shows that in comparison with most pairs of ROIs, FC between the TBI group has increased compared to the control group. In Figure 1, at the bottom left, the top triangular diagram shows that the pairs of regions “2 and 3,” “6 and 7,” and “10 and 11” have bolder points than the other pairs of pants. This indicates an increase in FC in the TBI group compared to the control group over one year.

Figure 1, at the bottom right, shows the -log 10 P to compare the estimation of the main effects and the longitudinal trend of FC in the pair's ROI between the TBI and the control groups. Accordingly, in estimating the longitudinal trend of FC between the control group and TBI, pairs of regions “2 and 3,” “6 and 7,” and “10 and 11” have more prominent points than other points.
4. Discussion
This study applied the longitudinal model of variance components to fMRI neuroimaging data of subjects with severe TBI disease that were collected longitudinally. The data used in this study are unique in fMRI imaging studies due to the severity of brain damage and follow-up time. Also, due to the different error structures, the longitudinal model used in this study has better reliability. By fitting the longitudinal model of variance components, no statistically significant difference was observed in the baseline effect of FC between the TBI group and the control group. However, in the longitudinal course of FC between the three pairs of regions, hyper-connectivity was observed in the TBI group compared to the control group. The cerebellum left and cerebellum right regions, superior frontal gyrus left and superior frontal gyrus right, and the thalamus left and thalamus right regions were areas where hyper-connectivity was observed in the TBI group compared to the control group. The cerebellum left and cerebellum right regions are responsible for balancing and coordinating the body's muscles to perform a specific function in the brain. Eye movements and motor learning also function in the cerebellum left and cerebellum right areas (Ferrari et al., 2019). The superior frontal gyrus left and superior frontal gyrus right regions play a crucial role in human self-awareness and working memory (Boisgueheneuc et al., 2006). The thalamus left and thalamus right regions are also responsible for amplifying sensory signals in the brain. Other functions of the thalamus left and thalamus right include learning, episodic memory, sleep regulation, and wakefulness (Torrico & Munakomi, 2023).
This study only identified brain areas in people with severe TBI who have experienced an acute increase in FC in their areas of the brain. Numerous studies have addressed the issue of sharp FC increase in patients with moderate to severe TBI. In 2015, Sours et al. evaluated 77 individuals with mild TBI and 35 healthy individuals for the severity of FC under the thalamus cerebral cortex using the standard t test and the analysis of variance test. The results of their study showed that people with mild TBI experienced an excessive increase in FC beneath the various layers of the thalamus, the subcortices associated with sensory processing, and the default mode network. According to Sours et al., people with mild TBI showed that they experienced cognitive, neurological, behavioral, sensory, and physical disorders. Their results suggest that this hyper-connectivity may affect brain areas related to sensory, motor, and even auditory functions. The brain is trying to heal after an injury, and time must be given for a full recovery (Sours et al., 2015).
The present study results are also consistent with the study of Sours et al. In the present study, an acute increase in FC in the thalamus was observed as well. In the study of Sours et al., common statistical methods were used to examine group differences. In contrast, our study used a new longitudinal statistical model to examine group differences in baseline effect and FC longitudinal trend. In 2016, Iraji et al. followed up on 16 patients with mild TBI for 4 to 6 weeks after injury and were given fMRI at rest. A total of 24 people were also selected as the control group. In this study, it was concluded that mild TBI patients have higher FC than the control group. Iraji et al. concluded that concussion can alter FC and that the brains of people with mild TBI tend to be hyperactive to compensate for the pathophysiological abnormalities caused by the injury. This brain activity occurs in response to the problem created after a brain injury, and the brain increases these functional connections to compensate for the damage. This increase is too much for the affected areas responsible for executive functions and working memory (Iraji et al., 2016).
The results of our study are mainly similar to Iraji et al. because the areas in the present study that experienced an excessive increase in FC affect organizational performance and working memory. Meanwhile, the data used in our study are similar to the study of Iraji et al., but our data's follow-up time is one year and is more valid in terms of time. The model used in our study may provide stronger results due to the different error structure than the common methods used in the study by Iraji et al. In 2017, Bernier et al. examined FC at rest using fMRI images. They analyzed 14 people with moderate to severe TBI and 19 healthy people. They used a 264-area atlas to identify areas of the brain. Finally, they concluded that the FC in the group with TBI was much higher than the FC in the control group. Bernier et al. hypothesized that this hyper-connectivity after brain injury might have been due to dedifferentiation. Dedifferentiation means the loss of specialization and function in a specific network of the brain that occurs most often in old age (Bernier et al., 2017). The areas used in the study by Bernier et al. were different from our study. Also, the data used in their study were cross-sectional, while the data used in the present study were collected longitudinally. A new longitudinal model was used in our study, while in Bernier et al., common statistical methods such as t test and analysis of variance were used. However, the results of both studies showed a hyper-connectivity in the TBI group. In 2019, Konstantinou et al. examined the correlation between brain regions in two groups of people with moderate to severe TBI. There were 11 people in both groups. They found that those with moderate to severe TBI had impaired executive function, verbal memory, and visual memory, which were associated with differences in FC in the cerebral cortex (Konstantinou et al., 2019).
Our study also showed that hyper-connectivity occurs in areas of the brain that are responsible for executive functions. In a 2020 study by Lu et al., 27 people with moderate TBI and 43 healthy people with FC in several brain areas were compared using fMRI imaging. This study was cross-sectional and showed a decrease in FC in the superior frontal gyrus. The results of Lu et al.'s study showed that decreased FC in the thalamus and superior frontal gyrus caused headaches in people with mild TBI (Lu et al., 2020). The results of our study are different from those of Lu et al., as they observed a decrease in FC in the TBI group, while we observed hyper-connectivity in the TBI group. Their study is cross-sectional, while our study is longitudinal. In 2020, Lu et al. conducted another study on 53 patients with mild TBI and 37 healthy individuals. Resting fMRI was used to compare FC in the two groups. The results showed that hyper-connectivity in the right posterior insula and inferior frontal gyrus was observed in the mild TBI group compared to the control group (Lu et al., 2020). Their study was cross-sectional and common statistical methods, such as the Pearson correlation coefficient test have been used. However, longitudinal data have been used in the present study, and a newer statistical model has been fitted to the data. The results of both studies show an excessive increase in FC in many areas of the brain. In 2021, Sheth et al. analyzed 49 veterans with mild TBI and 25 veterans without cerebral palsy for FC using fMRI neuroimaging. They found that FC was significantly increased in veterans with mTBI compared with veterans without cerebral palsy. Sheth et al. concluded that this sharp increase in FC in veterans with mild TBI could be a mechanism for maintaining overall brain network function (Sheth et al., 2021). Also, in 2021, Amir et al. examined the FC network of resting fMRI neuroimaging among 27 patients with mild TBI and 26 in the control group. They observed hyper-connectivity in patients with mild TBI compared to the control group. Amir et al. argued that this hyper-connectivity could be due to post-injury protective mechanisms, which are nerve compensation in the brain system. There are also signs that this acute increase in FC may be causing psychological distress or headaches. The nature of this hyper-connectivity can depend on some factors, including age, pre-injury cognitive function, or nervous system (Amir et al., 2021). The results of Amir et al.'s study are in line with the present study. However, the differences between our study and Amir et al.'s study are in the length of the data and different statistical methods to examine the difference between FC in the two groups. There is still no convincing evidence for the claims of hyper-connectivity in patients with moderate to severe TBI. Researchers and neuroscientists need to investigate the nature and cause of this acute increase (Amir et al., 2021).
5. Conclusion
Using neuroimaging data from people with severe TBI, collected longitudinally and applying the longitudinal model of variance components for these data, due to the different nature of the error structure compared to similar models, can make the results of the error structure of this study highly cited. By fitting the longitudinal model of variance components, it was concluded that in the longitudinal trend of FC in the TBI group, hyper-connectivity compared to the control group was observed in the pairs of cerebellum left and cerebellum right, superior frontal gyrus left and superior frontal gyrus right, as well as thalamus left and thalamus right. This hyper-connectivity can affect people's cognitive functions, but the extent and manner of this effect have not been determined to date and require more detailed studies.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran. (Code: IR.SBMU.RETECH.REC.1397.606).
Funding
The paper was extracted from the master's thesis of Keyvan Olazadeh, approved by the Department of Biostatistics, School of Allied Medical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Authors' contributions
Study design and data extraction: Keyvan Olazadeh; Data analysis, data interpretation: Hamid Alavi Majd and Nasrin Borumndnia; Literature research: Mahin Habibi; Writing and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors sincerely thank the Open fMRI for providing the data.
References
Amir, J., Nair, J. K. R., Del Carpio-O'Donovan, R., Ptito, A., Chen, J. K., & Chankowsky, J., et al. (2021). Atypical resting state functional connectivity in mild traumatic brain injury. Brain and Behavior, 11(8), e2261. [PMID]
Anderson, C. V., Bigler, E. D., & Blatter, D. D. (1995). Frontal lobe lesions, diffuse damage, and neuropsychological functioning in traumatic brain-injured patients. Journal of Clinical and Experimental Neuropsychology, 17(6), 900-908. [DOI:10.1080/01688639508402438] [PMID]
Bernier, R. A., Roy, A., Venkatesan, U. M., Grossner, E. C., Brenner, E. K., & Hillary, F. G. (2017). Dedifferentiation does not account for hyperconnectivity after traumatic brain injury. Frontiers in Neurology, 8, 674. [PMID]
Biswal, B., Yetkin, F. Z., Haughton, V. M., & Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magnetic Resonance in Medicine, 34(4), 537- 541. [DOI:10.1002/mrm.1910340409] [PMID]
Bittencourt-Villalpando, M., van der Horn, H. J., Maurits, N. M., & van der Naalt, J. (2021). Disentangling the effects of age and mild traumatic brain injury on brain network connectivity: A resting state fMRI study. NeuroImage. Clinical, 29, 102534.[DOI:10.1016/j.nicl.2020.102534] [PMID]
Crosson, B., Sartor, K. J., Jenny, A. B., Nabors, N. A., & Moberg, P. J. (1993). Increased intrusions during verbal recall in traumatic and nontraumatic lesions of the temporal lobe. Neuropsychology, 7(2),193-208. [DOI:10.1037/0894-4105.7.2.193]
du Boisgueheneuc, F., Levy, R., Volle, E., Seassau, M., Duffau, H., & Kinkingnehun, S., et al. (2006). Functions of the left superior frontal gyrus in humans: A lesion study. Brain, 129(12), 3315-3328. [PMID]
Eklund, A., Lindquist, M. A., & Villani, M. (2017). A Bayesian heteroscedastic GLM with application to fMRI data with motion spikes. NeuroImage, 155, 354-369. [DOI:10.1016/j.neuroimage.2017.04.069] [PMID]
Esposito, R., Cilli, F., Pieramico, V., Ferretti, A., Macchia, A., & Tommasi, M., et al. (2013). Acute effects of modafinil on brain resting state networks in young healthy subjects. Plos One, 8(7), e69224. [DOI:10.1371/journal.pone.0069224] [PMID]
Ferrari, C., Ciricugno, A., Battelli, L., Grossman, E. D., & Cattaneo, Z. (2022). Distinct cerebellar regions for body motion discrimination. Social Cognitive and Affective Neuroscience, 17(1), 72–80. [DOI:10.1093/scan/nsz088] [PMID]
Hart, B., Cribben, I., Fiecas, M., & Alzheimer's Disease Neuroimaging Initiative (2018). A longitudinal model for functional connectivity networks using resting-state fMRI. NeuroImage, 178, 687-701. [DOI:10.1016/j.neuroimage.2018.05.071] [PMID]
Henry-Feugeas, M. C., Azouvi, P., Fontaine, A., Denys, P., Bussel, B., & Maaz, F., et al. (2000). MRI analysis of brain atrophy after severe closed-head injury: Relation to clinical status. Brain Injury, 14(7), 597-604. [DOI:10.1080/02699050050043962] [PMID]
Iraji, A., Chen, H., Wiseman, N., Welch, R. D., O'Neil, B. J., & Haacke, E. M., et al. (2016). Compensation through functional hyperconnectivity: A longitudinal connectome assessment of mild traumatic brain injury. Neural Plasticity, 2016, 4072402.[DOI:10.1155/2016/4072402] [PMID]
Konstantinou, N., Pettemeridou, E., Stamatakis, E. A., Seimenis, I., & Constantinidou, F. (2019). Altered resting functional connectivity is related to cognitive outcomes in males with moderate-severe traumatic brain injury. Frontiers in Neurology, 9, 1163. [DOI:10.3389/fneur.2018.01163] [PMID]
Levin, H. S., Meyers, C. A., Grossman, R. G., & Sarwar, M. (1981). Ventricular enlargement after closed head injury. Archives of Neurology, 38(10), 623-629. [DOI:10.1001/archneur.1981.00510100051007] [PMID]
Lu, L., Li, F., Chen, H., Wang, P., Zhang, H., & Chen, Y. C., et al. (2020). Functional connectivity dysfunction of insular subdivisions in cognitive impairment after acute mild traumatic brain injury. Brain Imaging and Behavior, 14(3), 941-948. [DOI:10.1007/s11682-020-00288-5] [PMID]
Lu, L., Li, F., Wang, P., Chen, H., Chen, Y. C., & Yin, X. (2020). Altered hypothalamic functional connectivity in post-traumatic headache after mild traumatic brain injury. The Journal of Headache and Pain, 21(1), 93. [DOI:10.1186/s10194-020-01164-9] [PMID]
McTeague, L. M., Goodkind, M. S., & Etkin, A. (2016). Transdiagnostic impairment of cognitive control in mental illness. Journal of Psychiatric Research, 83, 37-46. [DOI:10.1016/j.jpsychires.2016.08.001] [PMID]
Roy, A., Bernier, R. A., Wang, J., Benson, M., French, J. J., Jr, & Good, D. C., et al. (2017). The evolution of cost-efficiency in neural networks during recovery from traumatic brain injury. Plos One, 12(4), e0170541. [DOI:10.1371/journal.pone.0170541] [PMID]
Scheibel, R. S. (2017). Functional magnetic resonance imaging of cognitive control following traumatic brain injury. Frontiers in Neurology, 8, 352. [DOI:10.3389/fneur.2017.00352] [PMID]
Sheth, C., Rogowska, J., Legarreta, M., McGlade, E., & Yurgelun-Todd, D. (2021). Functional connectivity of the anterior cingulate cortex in Veterans with mild traumatic brain injury. Behavioural Brain Research, 396, 112882. [DOI:10.1016/j.bbr.2020.112882] [PMID]
Type of Study: Original |
Subject:
Clinical Neuroscience
Received: 2021/10/15 | Accepted: 2022/04/16 | Published: 2024/07/20
Received: 2021/10/15 | Accepted: 2022/04/16 | Published: 2024/07/20
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








