Volume 16, Issue 4 (July & August 2025)
BCN 2025, 16(4): 737-750 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ashtiyani M, Moradi Birgani P, Soleimani M, Jameie B, Shahrokhi A, Deevband M R et al . Short-term Therapeutic Effects of Antigravity Treadmill Training on Brain Activities and Walking Capacity in Children With Cerebral Palsy. BCN 2025; 16 (4) :737-750
URL: http://bcn.iums.ac.ir/article-1-2314-en.html
URL: http://bcn.iums.ac.ir/article-1-2314-en.html
Meghdad Ashtiyani1 

 , Parmida Moradi Birgani2
, Parmida Moradi Birgani2 

 , Maryam Soleimani3
, Maryam Soleimani3 

 , Behnam Jameie4
, Behnam Jameie4 

 , Amin Shahrokhi3
, Amin Shahrokhi3 
 , Mohammad Reze Deevband5
, Mohammad Reze Deevband5 
 , Mohammad Mehdi Mirbagheri *6
, Mohammad Mehdi Mirbagheri *6 




 , Parmida Moradi Birgani2
, Parmida Moradi Birgani2 

 , Maryam Soleimani3
, Maryam Soleimani3 

 , Behnam Jameie4
, Behnam Jameie4 

 , Amin Shahrokhi3
, Amin Shahrokhi3 
 , Mohammad Reze Deevband5
, Mohammad Reze Deevband5 
 , Mohammad Mehdi Mirbagheri *6
, Mohammad Mehdi Mirbagheri *6 


1- Department of Biomedical Engineering and Medical Physics, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2- Department of Medical Physics and Biomedical Engineering, Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran.
3- Department of Basic Science, School of Rehabilitation Sciences, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
4- Neuroscience Research Centre (NRC), Iran University of Medical Sciences, Tehran, Iran.
5- Department of Biomedical Engineering and Medical Physics, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
6- Department of Medical Physics and Biomedical Engineering, Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran. & Department of Physical Medicine and Rehabilitation, Northwestern University Evanston, United States.
2- Department of Medical Physics and Biomedical Engineering, Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran.
3- Department of Basic Science, School of Rehabilitation Sciences, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
4- Neuroscience Research Centre (NRC), Iran University of Medical Sciences, Tehran, Iran.
5- Department of Biomedical Engineering and Medical Physics, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
6- Department of Medical Physics and Biomedical Engineering, Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran. & Department of Physical Medicine and Rehabilitation, Northwestern University Evanston, United States.
Keywords: Cerebral palsy (CP), Functional magnetic resonance imaging (fMRI), Anti-gravity treadmill, Gait
Full-Text [PDF 1224 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Cerebral palsy (CP), one of the most common causes of motor disability in children, is attributed to non-progressive disturbances occurring in the developing fetal or infant brain. Overall, the CP rate is between 2 and 3 per 1000 live births (Birgani et al., 2016) which increases to 40–100 in premature births or infants with very low birth weights (Weierink et al. 2013). CP belongs to the group of movement disorders that limit functional activities and reduce the quality of life, and also imposes an economic burden on societies. Hence, an effective intervention is needed to permanently improve their movement and balance, and as a result, to decrease their dependency.
Physical and occupational therapy (OT) usually starts in the first few years of life or soon after diagnosis. Despite their popularity, their outcomes are typically limited and short-lasting, indicating inadequate production of neuroplasticity (Palisano et al., 2012; Wiart et al., 2010). Therapeutic approaches, such as muscle injections, drugs, and surgical procedures, can reduce spasticity, but mostly have short-term effects and considerable side effects, and risk factors (Verrotti et al., 2006; Milla & Jackson, 1977; Koman et al., 1993; Albright, 1996).
An adequate, long-term, intensive, and systematic training is required for efficient neuroplasticity induction (Dietz, 2009), resulting in persistent improvement of balance and movement. In this regard, the robotic-assisted locomotor training (LOKOMAT) was developed and used to provide an intensive and systematic training. However, LOKOMAT training was limited and had short-term therapeutic effects since it mostly provided passive training (Draganski et al., 2004). Alternatively, active body weight-supported treadmill training (BWSTT) has been recently introduced to improve gait in individuals with CP. Some studies have shown that BWSTT was effective in enhancing gait speed (Mutlu et al., 2009; Cherng et al., 2007; Hesse et al., 1999), whereas others have reported no superiority of BWST versus conventional gait training (da Cunha et al., 2002; Franceschini et al., 2009). Although improvements were widely reported in gait endurance and speed (Booth et al., 2018), groin discomfort and skin irritation could be the restriction factors of BWSTT.
A recently developed anti-gravity treadmill (AlterG) (AlterG, 2025) can address the issues associated with BWSTT and facilitate treadmill walking because of its capacity to substantially reduce lower extremity weight bearing (Birgani et al., 2016; Rasooli et al., 2017). Using precise unweighting technology, the anti-gravity treadmill can help individuals walk actively on the treadmill with adjustable speed (Mutlu et al., 2009). This can trigger neural messaging in motor and sensory pathways, which may result in brain reorganization. If brain neuroplasticity is induced and correlated with clinical improvements, long-lasting effects would be expected.
The characterization of therapy-induced neuroplasticity, in addition to functional improvement, is of invaluable importance. This contributes to understanding the efficacy of the therapy and the underlying mechanisms of the disease, and in addition, ensures that clinically significant enhancement of functional outcomes is persistent. Accordingly, neuroimaging modalities, including structural and diffusion magnetic resonance imaging (MRI) and functional MRI (fMRI), are vital for identifying neuroplasticity (Birgani et al., 2016; Donabedian, 1966; Parvin et al., 2018; Krishnan et al., 2016; Reid et al., 2016). fMRI detects changes in blood flow (hemodynamic response) associated with neural activation of the brain (Heeger & Ress, 2002; Chen & Pike, 2009). fMRI uses blood-oxygen-level-dependent (BOLD) contrast and can measure BOLD responses of task-induced activities. One very popular modality used to measure neuroplasticity is task-based fMRI (t-fMRI), which is widely adopted for the identification of brain regions that are functionally involved in performing a specific task during data acquisition (Reid et al., 2016; Barch et al., 2013; Kornelsen & Stroman, 2004; Weiskopf et al., 2004).
Few studies to date have used task-based fMRI to investigate therapy-driven neuroplasticity in gait rehabilitation in children with spastic hemiplegic CP due to technical challenges (MacIntosh et al., 2004; Phillips et al., 2007). However, several small-scale studies have reported therapy-induced fMRI-detected changes in children with unilateral CP (UCP) (Phillips et al., 2007; Reid et al., 2015). Since fMRI acquisition requires subjects to remain fixed in a restricted space for a long period, motion artifacts in fMRI scans limit the feasibility of standard analysis in these studies, and even in studies on healthy pediatrics (Phillips et al., 2007; Reid et al., 2015; Bleyenheuft et al., 2015).
To overcome concerns regarding motion artifacts, subjects generally need to be sedated with an ideal sedative agent, which minimally hampers the neurophysiological effect of the administered sensory and motor stimulation (Wilke et al., 2003; Bernal et al., 2012). There are successful reports on the fMRI of sedated patients performed with passive tasks, including motion of the extremities (Souweidane et al., 1999; Rosazza et al., 2014; Ogg et al., 2009; Weiller et al., 1996). Passive movements could activate most of the cortical areas traditionally described in motor systems (Li et al., 2013; Azizi et al., 2018), such as the contralateral sensorimotor cortex, particularly the premotor cortex and parietal cortex (Rasooli et al., 2017). Accordingly, this study aimed to 1) Detect the signatures of ankle and knee passive movement tasks in the fMRI of children with CP, 2) Characterize these signatures, and 3) investigate the effects of AlterG training on these signatures, as well as on walking capacity in children with CP. We hypothesized that intensive and systematic AlterG training, which induces active walking, may result in enhancement of brain functional activities and long-term improvement of gait and balance impairments.
2. Materials and Methods
Study participants
Fourteen subjects with spastic hemiplegic CP (6–11 years old) were included in this study. They were randomly divided into 2 groups. The training group (5 females and 2 males; Mean±SD age 8.5±1.11 years, range 6 to 11 years) underwent AlterG training and the control group (5 females and 2 males; Mean±SD age 8.2±1.4 years, range 7 to 10 years) received OT. There was no significant difference in sex and age between the study and control groups. The inclusion criteria were hemiplegic, having spasticity in the lower limb (modified Ashworth >1), ability to stand independently for at least 30 s, and ambulatory. Meanwhile, the exclusion criteria were severe cognitive deficits, having received botulinum toxin injections within the past 2 months, and a history of surgery 6 months before training. All parents/guardians provided a written informed consent, and the study had ethical approval from the Tehran University of Medical Sciences (TUMS) Ethics Committee.
Training protocol
AlterG anti-gravity treadmill protocol
The AlterG anti-gravity treadmill (F320, AlterG® California USA) uses an inflatable tent surrounding the lower extremities to exert an upward force on the lower body and thereby reduce ground reaction forces in the lower limbs (Figure 1).
Cerebral palsy (CP), one of the most common causes of motor disability in children, is attributed to non-progressive disturbances occurring in the developing fetal or infant brain. Overall, the CP rate is between 2 and 3 per 1000 live births (Birgani et al., 2016) which increases to 40–100 in premature births or infants with very low birth weights (Weierink et al. 2013). CP belongs to the group of movement disorders that limit functional activities and reduce the quality of life, and also imposes an economic burden on societies. Hence, an effective intervention is needed to permanently improve their movement and balance, and as a result, to decrease their dependency.
Physical and occupational therapy (OT) usually starts in the first few years of life or soon after diagnosis. Despite their popularity, their outcomes are typically limited and short-lasting, indicating inadequate production of neuroplasticity (Palisano et al., 2012; Wiart et al., 2010). Therapeutic approaches, such as muscle injections, drugs, and surgical procedures, can reduce spasticity, but mostly have short-term effects and considerable side effects, and risk factors (Verrotti et al., 2006; Milla & Jackson, 1977; Koman et al., 1993; Albright, 1996).
An adequate, long-term, intensive, and systematic training is required for efficient neuroplasticity induction (Dietz, 2009), resulting in persistent improvement of balance and movement. In this regard, the robotic-assisted locomotor training (LOKOMAT) was developed and used to provide an intensive and systematic training. However, LOKOMAT training was limited and had short-term therapeutic effects since it mostly provided passive training (Draganski et al., 2004). Alternatively, active body weight-supported treadmill training (BWSTT) has been recently introduced to improve gait in individuals with CP. Some studies have shown that BWSTT was effective in enhancing gait speed (Mutlu et al., 2009; Cherng et al., 2007; Hesse et al., 1999), whereas others have reported no superiority of BWST versus conventional gait training (da Cunha et al., 2002; Franceschini et al., 2009). Although improvements were widely reported in gait endurance and speed (Booth et al., 2018), groin discomfort and skin irritation could be the restriction factors of BWSTT.
A recently developed anti-gravity treadmill (AlterG) (AlterG, 2025) can address the issues associated with BWSTT and facilitate treadmill walking because of its capacity to substantially reduce lower extremity weight bearing (Birgani et al., 2016; Rasooli et al., 2017). Using precise unweighting technology, the anti-gravity treadmill can help individuals walk actively on the treadmill with adjustable speed (Mutlu et al., 2009). This can trigger neural messaging in motor and sensory pathways, which may result in brain reorganization. If brain neuroplasticity is induced and correlated with clinical improvements, long-lasting effects would be expected.
The characterization of therapy-induced neuroplasticity, in addition to functional improvement, is of invaluable importance. This contributes to understanding the efficacy of the therapy and the underlying mechanisms of the disease, and in addition, ensures that clinically significant enhancement of functional outcomes is persistent. Accordingly, neuroimaging modalities, including structural and diffusion magnetic resonance imaging (MRI) and functional MRI (fMRI), are vital for identifying neuroplasticity (Birgani et al., 2016; Donabedian, 1966; Parvin et al., 2018; Krishnan et al., 2016; Reid et al., 2016). fMRI detects changes in blood flow (hemodynamic response) associated with neural activation of the brain (Heeger & Ress, 2002; Chen & Pike, 2009). fMRI uses blood-oxygen-level-dependent (BOLD) contrast and can measure BOLD responses of task-induced activities. One very popular modality used to measure neuroplasticity is task-based fMRI (t-fMRI), which is widely adopted for the identification of brain regions that are functionally involved in performing a specific task during data acquisition (Reid et al., 2016; Barch et al., 2013; Kornelsen & Stroman, 2004; Weiskopf et al., 2004).
Few studies to date have used task-based fMRI to investigate therapy-driven neuroplasticity in gait rehabilitation in children with spastic hemiplegic CP due to technical challenges (MacIntosh et al., 2004; Phillips et al., 2007). However, several small-scale studies have reported therapy-induced fMRI-detected changes in children with unilateral CP (UCP) (Phillips et al., 2007; Reid et al., 2015). Since fMRI acquisition requires subjects to remain fixed in a restricted space for a long period, motion artifacts in fMRI scans limit the feasibility of standard analysis in these studies, and even in studies on healthy pediatrics (Phillips et al., 2007; Reid et al., 2015; Bleyenheuft et al., 2015).
To overcome concerns regarding motion artifacts, subjects generally need to be sedated with an ideal sedative agent, which minimally hampers the neurophysiological effect of the administered sensory and motor stimulation (Wilke et al., 2003; Bernal et al., 2012). There are successful reports on the fMRI of sedated patients performed with passive tasks, including motion of the extremities (Souweidane et al., 1999; Rosazza et al., 2014; Ogg et al., 2009; Weiller et al., 1996). Passive movements could activate most of the cortical areas traditionally described in motor systems (Li et al., 2013; Azizi et al., 2018), such as the contralateral sensorimotor cortex, particularly the premotor cortex and parietal cortex (Rasooli et al., 2017). Accordingly, this study aimed to 1) Detect the signatures of ankle and knee passive movement tasks in the fMRI of children with CP, 2) Characterize these signatures, and 3) investigate the effects of AlterG training on these signatures, as well as on walking capacity in children with CP. We hypothesized that intensive and systematic AlterG training, which induces active walking, may result in enhancement of brain functional activities and long-term improvement of gait and balance impairments.
2. Materials and Methods
Study participants
Fourteen subjects with spastic hemiplegic CP (6–11 years old) were included in this study. They were randomly divided into 2 groups. The training group (5 females and 2 males; Mean±SD age 8.5±1.11 years, range 6 to 11 years) underwent AlterG training and the control group (5 females and 2 males; Mean±SD age 8.2±1.4 years, range 7 to 10 years) received OT. There was no significant difference in sex and age between the study and control groups. The inclusion criteria were hemiplegic, having spasticity in the lower limb (modified Ashworth >1), ability to stand independently for at least 30 s, and ambulatory. Meanwhile, the exclusion criteria were severe cognitive deficits, having received botulinum toxin injections within the past 2 months, and a history of surgery 6 months before training. All parents/guardians provided a written informed consent, and the study had ethical approval from the Tehran University of Medical Sciences (TUMS) Ethics Committee.
Training protocol
AlterG anti-gravity treadmill protocol
The AlterG anti-gravity treadmill (F320, AlterG® California USA) uses an inflatable tent surrounding the lower extremities to exert an upward force on the lower body and thereby reduce ground reaction forces in the lower limbs (Figure 1).
This allowed the body weight to be reduced up to 80% by 1% increments.
The AlterG training was performed for 45 min per session, 3 days/week for 8 weeks. In each session, the training started with a speed of 1 km/h and a body weight support of 50%. Then, the body weight support gradually decreased, and the speed increased as needed based on the subject’s ability and the discretion of the physical therapist who was responsible for the training. The subject was provided with necessary feedback by the trainer (Birgani et al., 2016; Rasooli et al., 2017; Azizi et al., 2018) with up to 45 minutes of training per session. The subject was evaluated before and after the 8-week training. The effects of training on the balance and postural stability was evaluated based on the Romberg test that was performed by using a posturography device. The parameters quantifying Center-of-Pressure (CoP).
OT training
A pediatric occupational therapist provided the control group with OT, with a focus on balance and gait training, at the rehabilitation center. OT training mostly concentrated on locomotion and was performed in 45 min sessions, 3 days per week for 8 weeks, similar to the training program of the training group.
Image acquisition
The participants underwent MRI scans before and after the 8-week training programs. All children were sedated under the supervision of pediatric anesthesiologists before undergoing MRI. The default sedation protocol involved general anesthesia with intravenous propofol administered at the lowest dose to keep the patient asleep after induction. For all subjects, oxygen saturation and heart rate were monitored by pulse-oximetry throughout the examination. Information regarding anesthesia, including the medications used for induction and maintenance, was recorded in the medical record.
Task description
t-fMRI is a non-invasive technique that allows for the identification of brain regions with altered activity due to the performance of given tasks. t-fMRI experiments obtained from high-resolution scans provide hundreds of thousands of longitudinal signals for each individual, corresponding to measurements of brain activity for each voxel of the brain throughout the experiment.
In this study, fMRI acquisition included two passive motor tasks for each leg: 1) Ankle plantarflexion to dorsiflexion movements over the range of motion (ROM) with 1 Hz frequency (Figure 2a), and 2) Knee flexion to extension movements over the ROM with 0.5 Hz frequency (Figure 2b).
The AlterG training was performed for 45 min per session, 3 days/week for 8 weeks. In each session, the training started with a speed of 1 km/h and a body weight support of 50%. Then, the body weight support gradually decreased, and the speed increased as needed based on the subject’s ability and the discretion of the physical therapist who was responsible for the training. The subject was provided with necessary feedback by the trainer (Birgani et al., 2016; Rasooli et al., 2017; Azizi et al., 2018) with up to 45 minutes of training per session. The subject was evaluated before and after the 8-week training. The effects of training on the balance and postural stability was evaluated based on the Romberg test that was performed by using a posturography device. The parameters quantifying Center-of-Pressure (CoP).
OT training
A pediatric occupational therapist provided the control group with OT, with a focus on balance and gait training, at the rehabilitation center. OT training mostly concentrated on locomotion and was performed in 45 min sessions, 3 days per week for 8 weeks, similar to the training program of the training group.
Image acquisition
The participants underwent MRI scans before and after the 8-week training programs. All children were sedated under the supervision of pediatric anesthesiologists before undergoing MRI. The default sedation protocol involved general anesthesia with intravenous propofol administered at the lowest dose to keep the patient asleep after induction. For all subjects, oxygen saturation and heart rate were monitored by pulse-oximetry throughout the examination. Information regarding anesthesia, including the medications used for induction and maintenance, was recorded in the medical record.
Task description
t-fMRI is a non-invasive technique that allows for the identification of brain regions with altered activity due to the performance of given tasks. t-fMRI experiments obtained from high-resolution scans provide hundreds of thousands of longitudinal signals for each individual, corresponding to measurements of brain activity for each voxel of the brain throughout the experiment.
In this study, fMRI acquisition included two passive motor tasks for each leg: 1) Ankle plantarflexion to dorsiflexion movements over the range of motion (ROM) with 1 Hz frequency (Figure 2a), and 2) Knee flexion to extension movements over the ROM with 0.5 Hz frequency (Figure 2b).
All passive tasks were performed by a trained biomedical engineer. A block design was used for fMRI studies with 24-s periods of rest alternating with 24-s periods of motor task for a total of 5 cycles.
Scanning parameters
fMRI data were acquired using a 3-Tesla GE MRI scanner with a standard head coil and single-shot gradient echo-planar imaging (matrix=64×64, echo time [TE]=30 ms, repetition time [TR]=3000 ms) to obtain 80 images with a 3 mm slice thickness. High-resolution anatomic T1-weighted images were obtained (matrix=192×192, TE=3.44 ms, TR=1800 ms) with a slice thickness of 1 mm. Structural and functional images were obtained in an axial direction parallel to the anterior/posterior commissure line.
fMRI processing
The preprocessing and statistical analysis of the fMRI data were calculated with fMRI of the brain (fMRIB) software library (FSL v6.02). The fMRI data were preprocessed using the standard steps, including realignment, brain extraction, motion correction, spatial smoothing, filtering, and denoising. Since the transformation of the functional data into standard space can affect functional analysis outcomes and group difference determinations, the standard space (MNI152 atlas) is registered to functional data (Hutchison et al., 2014). The region of interest (ROI) analysis used in this study included the primary motor cortex (M1), premotor cortex (PMC), supplementary motor area (SMA), precentral gyrus (PG) and corpus callosum (CC), which was selected from the Harvard-Oxford probabilistic atlas, and subsequently transformed to the individual’s native space.
The images were realigned and coregistered to the mean functional image from the first session. We followed the FMRIB Software Library (FSL) procedure to produce a non-brain mask for brain extraction. The movement parameters of the subjects were included in the individual analysis as covariates of no interest to reduce motion artifacts. The fMRI images were then smoothed with a Gaussian kernel by a 5×5×5 mm full width half maximum and a high-pass filter of 72 s. The primary goal of spatial smoothing is to enhance the signal-to-noise ratio and suppress spatial noise. Denoising was performed using multivariate exploratory linear optimized decomposition into independent components. Noisy components with voxels outside the brain were considered artifacts and removed from the data. Independent component analysis (ICA) was rerun for each subject’s temporally concatenated data across all sessions.
First-level individual statistical analyses were performed to calculate the significant brain areas, using the general linear model. Second-level random effects models were used to estimate brain activation for the separate contrasts (passive movements versus rest) and each group. Then, the test is used to detect significant differences in the training group, compared to the control group, for passive movements > rest, contrast. All contrasts were reported for clusters comprising at least 10 voxels and false discovery rate (FDR), P<0.05.
Clinical evaluation of gait and balance
The common clinical measures used to evaluate walking capacity included: 1) Ten-meter walk test (10 MWT) to assess walking speed (Ditunno et al., 2000) by measuring the duration of a 10-m walk; 2) 6-min walk test (6 MWT) to evaluate walking endurance (van Hedel et al., 2005), by measuring the distance walked in 6 min; 3) Timed-up-and-go (TUG) to assess balance and mobility (Bohannon, 2006), by measuring the duration of the required task, including standing, walking, and sitting.
3. Results
Therapeutic effects on functional brain activity
Individual results
The present results demonstrated that both ankle and knee passive motor tasks can activate the motor cortex in both the study and control groups. We could successfully detect the signatures of these tasks in the fMRI of CP children. We characterized these signatures in terms of activated voxels. Table 1 summarizes the pre- and post-results of the motor cortex activation analysis in terms of the number of active voxels for all tasks in both the study and control groups.

The therapeutic effects of the AlterG training on the activated voxels present several major points in the training group: 1) The passive tasks of the ankle resulted in greater motor cortex activation compared to those of the knee; 2) The pre-post activation changes in the motor cortex were observed for the passive ankle tasks of both sides in all but participant 4, in which the activation was elicited by the affected ankle after treatment; 3) In participants 1 and 2, the activation simultaneously increased in both the left and right hemispheres, in response to the passive movement of each ankle; 4) In participants 3 and 5, the activation increased in the less affected side but decreased in the more affected side; 5) In participant 6, passive movement of the more affected ankle resulted in an increase in the activation of the contralateral hemisphere and a decrease in the ipsilateral hemisphere. Whereas, the passive movement of the less affected ankle led to reduced activation of both hemispheres. Participant 7 was excluded due to the patient’s refusal to attend the second fMRI session.
In the control group, the pre-post activation improvement from OT training was seen mostly in participant 11. In contrast, the pre-post activation substantially reduced following the training. Participant 14 was excluded because of excessive head movement during fMRI acquisition.
To further analyze the pre-post activation changes in the motor cortex, we divided the motor cortex into distinct ROIs. Table 2 describes the distinct ROIs with significant activation alterations in response to therapy, along with the local maxima in Montreal Neurological Institute coordinates, as well as the most activated clusters for each participant.


The ROI-based analysis revealed that overall, PMC, PG, and CC showed significant enhancement in terms of activated voxels in most participants, while significant improvement in SMA and M1 was only observed in half of the subjects.
The details of the ROI analysis revealed several major points in the training group: 1) A significant increase in the activation of PMC and CC regardless of the affected sides; 2) In participants with increased activation in the PMC of one side, the more affected hemisphere showed more improvement; 3) In most participants with increased activation in the PMC of both sides, the enhancement was greater in the more affected side as compared to the less affected side; 4) In all participants but case 1, a significant increase was seen in the activation of M1, and in participants with increased activation of M1 in both hemispheres, the more affected side showed greater improvement; 5) All participants except case 5 revealed a significant increase in the activation of PG; 6) In participants 2, 3, and 5, a significant activation of SMA was observed; also, there was concurrent improvement in the PMC of both hemispheres as well.
In the control group, participants 8, 9, and 13 (half of the control group) showed little improvement, only in a limited number of regions, for which the training group demonstrated considerable improvement. Furthermore, in contrast to the training group, the activation enhancement was detected in the PMC of both sides of the remaining half, accompanied by similar changes in PG and SMA.
Group results
Figure 3 shows the average group results of significant activation changes following the completion of the 8-week training course for the ROI regions of both the study and control groups, as well as the group differences.
Scanning parameters
fMRI data were acquired using a 3-Tesla GE MRI scanner with a standard head coil and single-shot gradient echo-planar imaging (matrix=64×64, echo time [TE]=30 ms, repetition time [TR]=3000 ms) to obtain 80 images with a 3 mm slice thickness. High-resolution anatomic T1-weighted images were obtained (matrix=192×192, TE=3.44 ms, TR=1800 ms) with a slice thickness of 1 mm. Structural and functional images were obtained in an axial direction parallel to the anterior/posterior commissure line.
fMRI processing
The preprocessing and statistical analysis of the fMRI data were calculated with fMRI of the brain (fMRIB) software library (FSL v6.02). The fMRI data were preprocessed using the standard steps, including realignment, brain extraction, motion correction, spatial smoothing, filtering, and denoising. Since the transformation of the functional data into standard space can affect functional analysis outcomes and group difference determinations, the standard space (MNI152 atlas) is registered to functional data (Hutchison et al., 2014). The region of interest (ROI) analysis used in this study included the primary motor cortex (M1), premotor cortex (PMC), supplementary motor area (SMA), precentral gyrus (PG) and corpus callosum (CC), which was selected from the Harvard-Oxford probabilistic atlas, and subsequently transformed to the individual’s native space.
The images were realigned and coregistered to the mean functional image from the first session. We followed the FMRIB Software Library (FSL) procedure to produce a non-brain mask for brain extraction. The movement parameters of the subjects were included in the individual analysis as covariates of no interest to reduce motion artifacts. The fMRI images were then smoothed with a Gaussian kernel by a 5×5×5 mm full width half maximum and a high-pass filter of 72 s. The primary goal of spatial smoothing is to enhance the signal-to-noise ratio and suppress spatial noise. Denoising was performed using multivariate exploratory linear optimized decomposition into independent components. Noisy components with voxels outside the brain were considered artifacts and removed from the data. Independent component analysis (ICA) was rerun for each subject’s temporally concatenated data across all sessions.
First-level individual statistical analyses were performed to calculate the significant brain areas, using the general linear model. Second-level random effects models were used to estimate brain activation for the separate contrasts (passive movements versus rest) and each group. Then, the test is used to detect significant differences in the training group, compared to the control group, for passive movements > rest, contrast. All contrasts were reported for clusters comprising at least 10 voxels and false discovery rate (FDR), P<0.05.
Clinical evaluation of gait and balance
The common clinical measures used to evaluate walking capacity included: 1) Ten-meter walk test (10 MWT) to assess walking speed (Ditunno et al., 2000) by measuring the duration of a 10-m walk; 2) 6-min walk test (6 MWT) to evaluate walking endurance (van Hedel et al., 2005), by measuring the distance walked in 6 min; 3) Timed-up-and-go (TUG) to assess balance and mobility (Bohannon, 2006), by measuring the duration of the required task, including standing, walking, and sitting.
3. Results
Therapeutic effects on functional brain activity
Individual results
The present results demonstrated that both ankle and knee passive motor tasks can activate the motor cortex in both the study and control groups. We could successfully detect the signatures of these tasks in the fMRI of CP children. We characterized these signatures in terms of activated voxels. Table 1 summarizes the pre- and post-results of the motor cortex activation analysis in terms of the number of active voxels for all tasks in both the study and control groups.

The therapeutic effects of the AlterG training on the activated voxels present several major points in the training group: 1) The passive tasks of the ankle resulted in greater motor cortex activation compared to those of the knee; 2) The pre-post activation changes in the motor cortex were observed for the passive ankle tasks of both sides in all but participant 4, in which the activation was elicited by the affected ankle after treatment; 3) In participants 1 and 2, the activation simultaneously increased in both the left and right hemispheres, in response to the passive movement of each ankle; 4) In participants 3 and 5, the activation increased in the less affected side but decreased in the more affected side; 5) In participant 6, passive movement of the more affected ankle resulted in an increase in the activation of the contralateral hemisphere and a decrease in the ipsilateral hemisphere. Whereas, the passive movement of the less affected ankle led to reduced activation of both hemispheres. Participant 7 was excluded due to the patient’s refusal to attend the second fMRI session.
In the control group, the pre-post activation improvement from OT training was seen mostly in participant 11. In contrast, the pre-post activation substantially reduced following the training. Participant 14 was excluded because of excessive head movement during fMRI acquisition.
To further analyze the pre-post activation changes in the motor cortex, we divided the motor cortex into distinct ROIs. Table 2 describes the distinct ROIs with significant activation alterations in response to therapy, along with the local maxima in Montreal Neurological Institute coordinates, as well as the most activated clusters for each participant.


The ROI-based analysis revealed that overall, PMC, PG, and CC showed significant enhancement in terms of activated voxels in most participants, while significant improvement in SMA and M1 was only observed in half of the subjects.
The details of the ROI analysis revealed several major points in the training group: 1) A significant increase in the activation of PMC and CC regardless of the affected sides; 2) In participants with increased activation in the PMC of one side, the more affected hemisphere showed more improvement; 3) In most participants with increased activation in the PMC of both sides, the enhancement was greater in the more affected side as compared to the less affected side; 4) In all participants but case 1, a significant increase was seen in the activation of M1, and in participants with increased activation of M1 in both hemispheres, the more affected side showed greater improvement; 5) All participants except case 5 revealed a significant increase in the activation of PG; 6) In participants 2, 3, and 5, a significant activation of SMA was observed; also, there was concurrent improvement in the PMC of both hemispheres as well.
In the control group, participants 8, 9, and 13 (half of the control group) showed little improvement, only in a limited number of regions, for which the training group demonstrated considerable improvement. Furthermore, in contrast to the training group, the activation enhancement was detected in the PMC of both sides of the remaining half, accompanied by similar changes in PG and SMA.
Group results
Figure 3 shows the average group results of significant activation changes following the completion of the 8-week training course for the ROI regions of both the study and control groups, as well as the group differences.
The results revealed significant differences in brain activation for both groups (Figures 3a and 3b). However, the changes were observed in both hemispheres in the training group, whereas it was observed mostly in one of the hemispheres of the control group. Furthermore, the areas with significant changes were substantially larger in the training group than in the control group.
For a more precise description of these findings, the differences in the significant activation changes between the study and control groups were obtained. The results showed 16.1% more active voxels in the training group compared to the control group (Figure 3c).
Therapeutic effects on balance and gait impairment
Table 3 summarizes the average group results of the clinical measures of walking capacity and the percentage of changes after the administration of the 8-week training course for both the study and control groups.
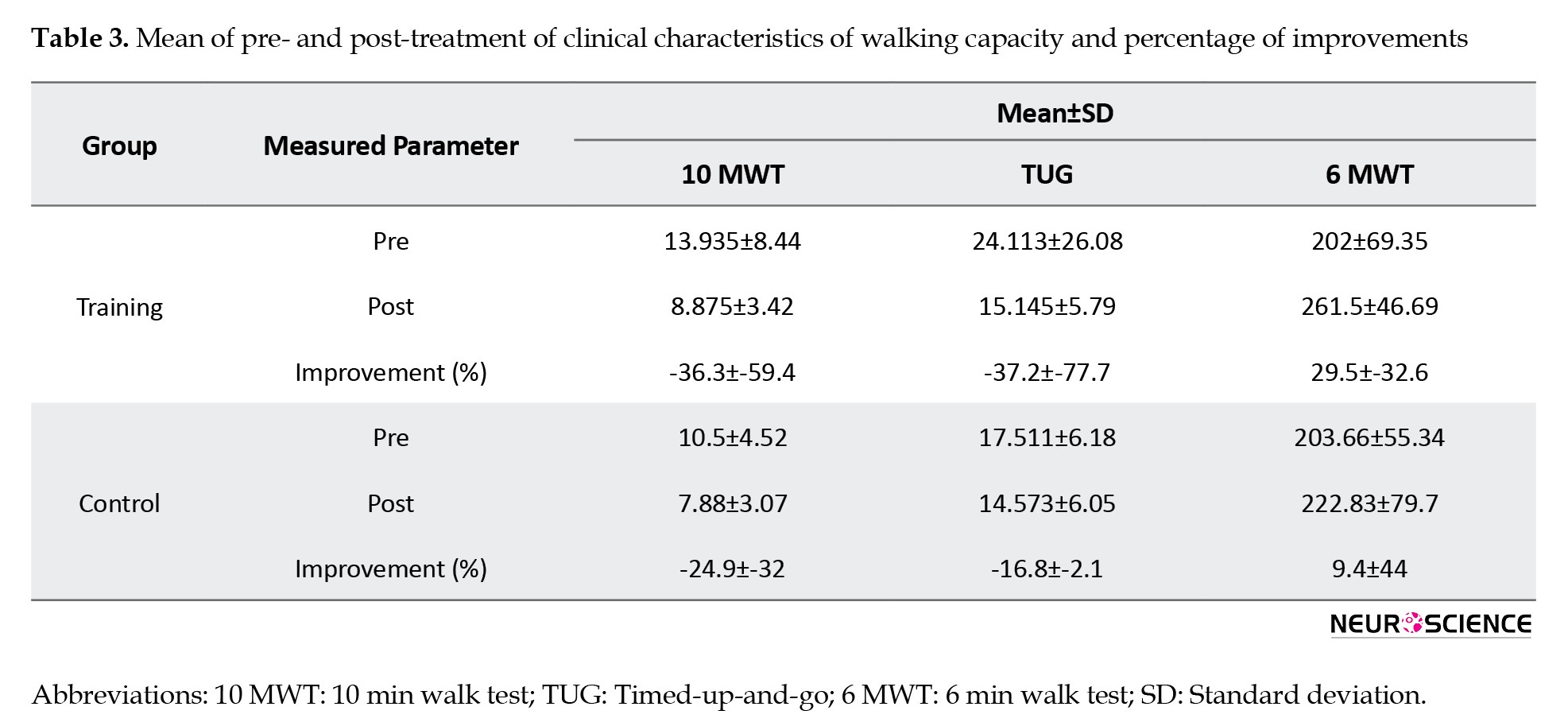
Walking speed increased by 36.3% and 24.9% for the study and control groups, respectively. Importantly, balance and mobility showed a much larger improvement in the training group compared to the control group (37.2% vs 16.8%). More importantly, walking endurance was enhanced three times more in the training group than in the control group.
4. Discussion
To the best of the authors’ knowledge, for the first time, this article characterizes the therapeutic effects of intensive AlterG training on brain cortical reorganization and walking capacity in children with CP using passive task-based fMRI under sedation. The objective was to study the responsiveness of motor cortical reorganization of children with hemiplegic CP after anti-gravity treadmill training versus over-ground walking OT. Passive movements included dorsiflexion and plantar flexion of both ankles and flexion-extension of both knees, which were carried out on all subjects before and after the 8-week training. This enabled us to investigate the intra-subject brain activation alterations induced by therapy, as well as the group comparison. The findings indicated a higher enhancement in both brain activity and walking capacity following the completion of AlterG in comparison to OT training, suggesting that AlterG training may be used as an effective therapeutic intervention for long-lasting improvement of gait and balance impairments in children with CP.
High sensitivity of task-based fMRI to motion artifacts could worsen in children with CP who neither cooperate to lie down immobile nor perform tasks correctly during fMRI acquisition. A similar study highlighted the difficulties of fMRI scanning in children with CP (Guzzetta et al., 2007). To address this issue, in this study, fMRI was acquired under sedation during which the passive tasks were applied. To the best of the authors’ knowledge, this was done for the first time; we found no evidence of sedative passive task-based fMRI in children with CP, although some studies investigated passive task-based fMRI in this patient population (Dinomais et al., 2013).
To consider the heterogeneity of the size and location of the brain lesions in CP participants included in this study, we used the ROI analyses to measure the therapy-induced fMRI-detected changes of brain activation between the motor cortices before and after training in each subject. The percentage of changes in the outcome measures was calculated for each subject in each study and control group. Then, the group average results were used to determine the impact of each intervention and compare the effectiveness of the interventions.
The activation patterns for different tasks were compared using the same data acquisition parameters, and analysis methods for both the intra- and inter-subject and group analyses on the FDR (P<0.05) corrected level were employed. Subsequent data analysis and comparisons are the first to suggest that motor cortical activation increases after 8 weeks of anti-gravity treadmill training and OT training. Moreover, the changes in therapy-driven motor cortical activation were more widely distributed with higher intensity in the training group compared to the control group. Passive movement tasks used in this study produced consistent activation in the motor system of both legs and demonstrated robust activation in M1, PMC, SMA, and PG of both hemispheres, as well as the CC, which connects the left and right cerebral hemispheres. This distribution of brain activity following sedative passive task-based fMRI is consistent with previous studies on sedative-free passive task-based fMRI movements in healthy adults (Ogg et al., 2009) the authors examined whether passive range of motion (ROM, adult stroke patients (Cho et al., 2016), healthy children (Drużbicki et al., 2013) and children with CP (Dinomais et al., 2013). This might imply that the supraspinal sensorimotor network for the neural control of walking can be assessed indirectly by these tasks.
According to Table 1, in 3 subjects of the training group (i.e. subjects 3, 5, and 6), the activation of motor areas induced by training increased in one hemisphere but decreased in the other. This was observed in only one task in one control patient (subject 9, left knee task). In subject 3, brain activation due to 3 tasks increased in the left hemisphere and CC, but decreased in the right hemisphere. In subject 5, for 3 tasks, brain activation increased in the contralateral side and decreased in the ipsilateral side and the CC. In subject 6, in the left knee task, brain activation increased in the contralateral side and decreased in the ipsilateral side and the CC. These contradictory activation changes in the hemispheres suggest that the investigated motor areas in one hemisphere may adaptively compensate for the other.
Studies that utilized anti-gravity treadmill training, BWST, and LOKOMAT for gait improvement mostly reported functional improvement rather than characterization of therapy-induced brain reorganization in children with CP (Birgani et al., 2016; Hesse et al., 1999; Drużbicki et al., 2013; Willoughby et al. 2009) with up to 45 minutes of training per session. The subject was evaluated before and after the 8-week training. The effects of training on the balance and postural stability was evaluated based on the Romberg test that was performed by using a posturography device. The parameters quantifying Center-of-Pressure (CoP. However, few studies have investigated the therapy-driven neuroplasticity in gait rehabilitation using BWST in adults with stroke and children with CP (Phillips et al., 2007; Dobkin et al., 2004; Yang et al., 2010). The hemodynamic response of the sensorimotor cortices following therapy has been reported to increase in some of these fMRI studies, while others showed that brain cortical activation decreased (Dinomais et al., 2013; Drużbicki et al., 2013). Furthermore, according to a limited number of small-scale fMRI investigations in children with UCP, increased contralateral activity may accompany functional gains. For instance, cluster-based S1–M1 voxel counts were increased after virtual reality therapy in three adults with UCP (Cho et al., 2016). However, our intra-subject analysis demonstrates both an increase and a decrease in motor cortical activation after therapy. This might be due to the initial severity of the sensorimotor impairments evident in participants.
Although both groups demonstrated an improvement in walking speed, TUG, and walking endurance, participants in the training group had much greater enhancement. This is concurrent with higher brain motor cortical reorganization induced in the training group. This implies that AlterG training may have the potential to promote effective neuroplasticity that can improve walking ability in children with CP.
5. Conclusion
The findings of this study demonstrate brain activation enhancement following the administration of the 8-week AlterG training in children with CP. This implies that AlterG training can be considered an effective physical intervention to improve walking capacity in children with CP. Our results also indicate that fMRI, performed with passive tasks, is an effective tool for detecting alterations in brain activity induced by physical activities in children with CP.
Study limitation
In this study, the therapeutic effects of AlterG training on brain functional activity and walking capacity were successfully characterized. While our results were promising with respect to the investigation of therapy-driven improvement of functional brain activities, our study had a few limitations. Firstly, a few patients could not complete the required training sessions due to the intensive treatment schedule.
Secondly, our results showed no significant correlation between these measurements (secondary aim), probably due to the limited sample size, which can mostly influence this aim, but not the primary ones. Our major objectives were firstly to examine the possibility of detecting the signatures of ankle and knee passive movement tasks in the fMRI of CP children, and if so, secondly to characterize these signatures, and finally to determine the potential therapeutic effects of the antigravity treadmill training on these signatures, and on balance and gait impairments. We could detect these signatures (objective 1), particularly for the ankle task, characterize them in terms of activated voxels (objective 2), and determine the therapeutic effects of training on the activated voxels (objective 3). Additionally, the findings revealed different therapeutic responses following the completion of training, consistent with the literature reporting a high inter-subject variability in brain structural and functional neuroplasticity due to several factors.
Finally, characterization of the intervention effects may not solely be achieved by the pre-post analyses and calculation of the average group results. Alternatively, since any intervention can have different effects on patients, the recovery patterns need to be identified to fully characterize the therapeutic effects of interventions and individualize treatment. This required a larger sample size and further data acquisition time points, considered in our ongoing studies
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Ethics Committee of Tehran University of Medical Sciences (TUMS), Tehran, Iran. This study was registered by the Iranian Registry of Clinical Trials (IRCT), Tehran, Iran (Code: IRCT2015121625568N1). All participants gave their written informed consent to participate in the study.
Funding
This study was extracted from the PhD dissertation of Meghdad Ashtiyani, approved by the Department of Biomedical Engineering and Medical Physics, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran (Registration No.: 365m)
Authors' contributions
Study design and investigation: Parmida Moradi Birgani and Mohammad Reze Deevband; Methodology: Meghdad Ashtiyani, Parmida Moradi Birgani, Amin Shahrokhi, and Mohammad Reze Deevband; Data acquisition, analysis and writing: Meghdad Ashtiyani and Parmida Moradi Birgani; Data interpretation: Meghdad Ashtiyani, Mohammad Mehdi Mirbagheri, Behnam Jameie and Maryam Soleimani; Review, editing, and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the participants for their corporations.
References
Albright A. L. (1996). Baclofen in the treatment of cerebral palsy. Journal of Child Neurology, 11(2), 77–83. [DOI:10.1177/088307389601100202] [PMID]
Azizi, S., Rasooli, A. H., Soleimani, M., Irani, A., Shahrokhi, A., & Mirbagheri, M. M. (2018). The impact of AlterG training on balance and structure of vestibulospinal tract in cerebral palsy children. Paper presented at: 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18-21 July 2018. [DOI:10.1109/EMBC.2018.8512772] [PMID]
Barch, D. M., Burgess, G. C., Harms, M. P., Petersen, S. E., Schlaggar, B. L., & Corbetta, M., et al. (2013). Function in the human connectome: Task-fMRI and individual differences in behavior. NeuroImage, 80, 169–189. [DOI:10.1016/j.neuroimage.2013.05.033] [PMID]
Bernal, B., Grossman, S., Gonzalez, R., & Altman, N. (2012). FMRI under sedation: What is the best choice in children?. Journal of Clinical Medicine Research, 4(6), 363-370. [PMID]
Birgani, P. M., Ashtiyani, M., Rasooli, A., Shahrokhnia, M., Shahrokhi, A., & Mirbagheri, M. M. (2016). Can an anti-gravity treadmill improve stability of children with cerebral palsy?. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual International Conference, 2016, 5465–5468. [DOI:10.1109/EMBC.2016.7591963] [PMID]
Bleyenheuft, Y., Dricot, L., Gilis, N., Kuo, H. C., Grandin, C., & Bleyenheuft, C., et al. (2015). Capturing neuroplastic changes after bimanual intensive rehabilitation in children with unilateral spastic cerebral palsy: A combined DTI, TMS and fMRI pilot study. Research in Developmental Disabilities, 43-44, 136–149. [DOI:10.1016/j.ridd.2015.06.014] [PMID]
Bohannon, R. W. (2006). Reference values for the timed up and go test: A descriptive meta-analysis. Journal of Geriatric Physical Therapy (2001), 29(2), 64–68. [DOI:10.1519/00139143-200608000-00004] [PMID]
Booth, A. T. C., Buizer, A. I., Meyns, P., Oude Lansink, I. L. B., Steenbrink, F., & van der Krogt, M. M. (2018). The efficacy of functional gait training in children and young adults with cerebral palsy: A systematic review and meta-analysis. Developmental Medicine and Child Neurology, 60(9), 866–883. [DOI:10.1111/dmcn.13708] [PMID]
Chen, J. J., & Pike, G. B. (2009). BOLD-specific cerebral blood volume and blood flow changes during neuronal activation in humans. NMR in Biomedicine, 22(10), 1054–1062. [DOI:10.1002/nbm.1411] [PMID]
Cherng, R. J., Liu, C. F., Lau, T. W., & Hong, R. B. (2007). Effect of treadmill training with body weight support on gait and gross motor function in children with spastic cerebral palsy. American Journal of Physical Medicine & Rehabilitation, 86(7), 548–555. [DOI:10.1097/PHM.0b013e31806dc302] [PMID]
Cho, C., Hwang, W., Hwang, S., & Chung, Y. (2016). Treadmill Training with Virtual Reality Improves Gait, Balance, and Muscle Strength in Children with Cerebral Palsy. The Tohoku Journal of Experimental Medicine, 238(3), 213–218. [DOI:10.1620/tjem.238.213] [PMID]
da Cunha, I. T., Jr, Lim, P. A., Qureshy, H., Henson, H., Monga, T., & Protas, E. J. (2002). Gait outcomes after acute stroke rehabilitation with supported treadmill ambulation training: A randomized controlled pilot study. Archives of physical Medicine and Rehabilitation, 83(9), 1258–1265. [DOI:10.1053/apmr.2002.34267] [PMID]
Dietz, V. (2009). Body weight supported gait training: From laboratory to clinical setting. Brain Research Bulletin, 78(1), I–VI. [DOI:10.1016/S0361-9230(08)00410-3] [PMID]
Dinomais, M., Chinier, E., Lignon, G., Richard, I., Ter Minassian, A., & Tich, S. N. (2013). The effect of video-guidance on passive movement in patients with cerebral palsy: fMRI study. Research in Developmental Disabilities, 34(10), 3487–3496. [DOI:10.1016/j.ridd.2013.07.008] [PMID]
Ditunno, J. F., Jr, Ditunno, P. L., Graziani, V., Scivoletto, G., Bernardi, M., & Castellano, V., et al. (2000). Walking index for spinal cord injury (WISCI): An international multicenter validity and reliability study. Spinal Cord, 38(4), 234–243. [DOI:10.1038/sj.sc.3100993] [PMID]
Draganski, B., Gaser, C., Busch, V., Schuierer, G., Bogdahn, U., & May, A. (2004). Neuroplasticity: Changes in grey matter induced by training. Nature, 427(6972), 311–312. [DOI:10.1038/427311a] [PMID]
Drużbicki, M., Rusek, W., Snela, S., Dudek, J., Szczepanik, M., & Zak, E., et al. (2013). Functional effects of robotic-assisted locomotor treadmill thearapy in children with cerebral palsy. Journal of Rehabilitation Medicine, 45(4), 358–363. [DOI:10.2340/16501977-1114] [PMID]
Dobkin, B. H., Firestine, A., West, M., Saremi, K., & Woods, R. (2004). Ankle dorsiflexion as an fMRI paradigm to assay motor control for walking during rehabilitation. NeuroImage, 23(1), 370–381. [DOI:10.1016/j.neuroimage.2004.06.008] [PMID]
Donabedian, A. (2005). Evaluating the quality of medical care. 1966. The Milbank Quarterly, 83(4), 691–729. [DOI:10.1111/j.1468-0009.2005.00397.x] [PMID]
Enzinger, C., Dawes, H., Johansen-Berg, H., Wade, D., Bogdanovic, M., & Collett, J., et al. (2009). Brain activity changes associated with treadmill training after stroke. Stroke, 40(7), 2460–2467. [DOI:10.1161/STROKEAHA.109.550053] [PMID]
Franceschini, M., Carda, S., Agosti, M., Antenucci, R., Malgrati, D., & Cisari, C., et al. (2009). Walking after stroke: What does treadmill training with body weight support add to overground gait training in patients early after stroke?: A single-blind, randomized, controlled trial. Stroke, 40(9), 3079–3085.[DOI:10.1161/STROKEAHA.109.555540] [PMID]
Guzzetta, A., Staudt, M., Petacchi, E., Ehlers, J., Erb, M., & Wilke, M., et al. (2007). Brain representation of active and passive hand movements in children. Pediatric Research, 61(4), 485–490. [DOI:10.1203/pdr.0b013e3180332c2e] [PMID]
Hesse, S., Konrad, M., & Uhlenbrock, D. (1999). Treadmill walking with partial body weight support versus floor walking in hemiparetic subjects. Archives of Physical Medicine and Rehabilitation, 80(4), 421–427. [DOI:10.1016/S0003-9993(99)90279-4] [PMID]
Heeger, D. J., & Ress, D. (2002). What does fMRI tell us about neuronal activity?. Nature Reviews. Neuroscience, 3(2), 142–151. [DOI:10.1038/nrn730] [PMID]
Hutchison, J. L., Hubbard, N. A., Brigante, R. M., Turner, M., Sandoval, T. I., & Hillis, G. A. J., et al. (2014). The efficiency of fMRI region of interest analysis methods for detecting group differences. Journal of Neuroscience Methods, 226, 57–65. [DOI:10.1016/j.jneumeth.2014.01.012] [PMID]
Kornelsen, J., & Stroman, P. W. (2004). fMRI of the lumbar spinal cord during a lower limb motor task. Magnetic Resonance in Medicine, 52(2), 411–414. [DOI:10.1002/mrm.20157] [PMID]
Krishnan, V., Kindig, M., & Mirbagheri, M. (2016). Robotic-assisted locomotor training enhances ankle performance in adults with incomplete spinal cord injury. Journal of Rehabilitation Medicine, 48(9), 781–786. [DOI:10.2340/16501977-2133] [PMID]
Koman, L. A., Mooney, J. F., 3rd, Smith, B., Goodman, A., & Mulvaney, T. (1993). Management of cerebral palsy with botulinum-A toxin: Preliminary investigation. Journal of Pediatric Orthopedics, 13(4), 489–495. [DOI:10.1097/01241398-199307000-00013] [PMID]
Li, W., Wait, S. D., Ogg, R. J., Scoggins, M. A., Zou, P., & Wheless, J., et al. (2013). Functional magnetic resonance imaging of the visual cortex performed in children under sedation to assist in presurgical planning. Journal of Neurosurgery. Pediatrics, 11(5), 543–546. [DOI:10.3171/2013.1.PEDS12401] [PMID]
Luft, A. R., Macko, R. F., Forrester, L. W., Villagra, F., Ivey, F., & Sorkin, J. D., et al. (2008). Treadmill exercise activates subcortical neural networks and improves walking after stroke: A randomized controlled trial. Stroke, 39(12), 3341–3350. [DOI:10.1161/STROKEAHA.108.527531] [PMID]
MacIntosh, B. J., Mraz, R., Baker, N., Tam, F., Staines, W. R., & Graham, S. J. (2004). Optimizing the experimental design for ankle dorsiflexion fMRI. NeuroImage, 22(4), 1619–1627.[DOI:10.1016/j.neuroimage.2004.03.035] [PMID]
Milla, P. J., & Jackson, A. D. (1977). A controlled trial of baclofen in children with cerebral palsy. The Journal of International Medical Research, 5(6), 398–404. [DOI:10.1177/030006057300100203] [PMID]
Mutlu, A., Krosschell, K., & Spira, D. G. (2009). Treadmill training with partial body-weight support in children with cerebral palsy: A systematic review. Developmental Medicine and Child Neurology, 51(4), 268–275. [DOI:10.1111/j.1469-8749.2008.03221.x] [PMID]
Ogg, R. J., Laningham, F. H., Clarke, D., Einhaus, S., Zou, P., & Tobias, M. E., et al. (2009). Passive range of motion functional magnetic resonance imaging localizing sensorimotor cortex in sedated children. Journal of Neurosurgery. Pediatrics, 4(4), 317–322. [PMID]
Palisano, R. J., Begnoche, D. M., Chiarello, L. A., Bartlett, D. J., McCoy, S. W., & Chang, H. J. (2012). Amount and focus of physical therapy and occupational therapy for young children with cerebral palsy. Physical & Occupational Therapy in Pediatrics, 32(4), 368–382. [DOI:10.3109/01942638.2012.715620] [PMID]
Parvin, S., Mehdinezhad, M., Taghiloo, A., Nourian, R., & Mirbagheri, M. M. (2018). The impact of repetitive transcranial magnetic stimulation on affected and unaffected sides of a child with hemiplegic cerebral palsy. Paper presented at: 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18-21 July 2018. [DOI:10.1109/EMBC.2018.8512877] [PMID]
Phillips, J. P., Sullivan, K. J., Burtner, P. A., Caprihan, A., Provost, B., & Bernitsky-Beddingfield, A. (2007). Ankle dorsiflexion fMRI in children with cerebral palsy undergoing intensive body-weight-supported treadmill training: A pilot study. Developmental Medicine and Child Neurology, 49(1), 39–44. [DOI:10.1017/S0012162207000102.x] [PMID]
Rasooli, A. H., Birgani, P. M., Azizi, S., Shahrokhi, A., & Mirbagheri, M. M. (2017). Therapeutic effects of an anti-gravity locomotor training (AlterG) on postural balance and cerebellum structure in children with Cerebral Palsy. Paper presented at: 2017 International Conference on Rehabilitation Robotics (ICORR), London, UK, 17-20 July 2017. [DOI:10.1109/ICORR.2017.8009229] [PMID]
Reid, L. B., Boyd, R. N., Cunnington, R., & Rose, S. E. (2016). Interpreting intervention induced neuroplasticity with fMRI: The case for multimodal imaging strategies. Neural Plasticity, 2016, 2643491. [DOI:10.1155/2016/2643491] [PMID]
Reid, L. B., Rose, S. E., & Boyd, R. N. (2015). Rehabilitation and neuroplasticity in children with unilateral cerebral palsy. Nature Reviews. Neurology, 11(7), 390–400. [DOI:10.1038/nrneurol.2015.97] [PMID]
Rosazza, C., Aquino, D., D'Incerti, L., Cordella, R., Andronache, A., & Zacà, D., et al. (2014). Preoperative mapping of the sensorimotor cortex: Comparative assessment of task-based and resting-state FMRI. Plos One, 9(6), e98860. [DOI:10.1371/journal.pone.0098860] [PMID]
Souweidane, M. M., Kim, K. H., McDowall, R., Ruge, M. I., Lis, E., & Krol, G., et al. (1999). Brain mapping in sedated infants and young children with passive-functional magnetic resonance imaging. Pediatric Neurosurgery, 30(2), 86–92. [DOI:10.1159/000028768] [PMID]
van Hedel, H. J., Wirz, M., & Dietz, V. (2005). Assessing walking ability in subjects with spinal cord injury: Validity and reliability of 3 walking tests. Archives of Physical Medicine and Rehabilitation, 86(2), 190–196. [DOI:10.1016/j.apmr.2004.02.010] [PMID]
Verrotti, A., Greco, R., Spalice, A., Chiarelli, F., & Iannetti, P. (2006). Pharmacotherapy of spasticity in children with cerebral palsy. Pediatric Neurology, 34(1), 1–6. [DOI:10.1016/j.pediatrneurol.2005.05.001] [PMID]
Weierink, L., Vermeulen, R. J., & Boyd, R. N. (2013). Brain structure and executive functions in children with cerebral palsy: A systematic review. Research in Developmental Disabilities, 34(5), 1678–1688. [DOI:10.1016/j.ridd.2013.01.035] [PMID]
Weiskopf, N., Scharnowski, F., Veit, R., Goebel, R., Birbaumer, N., & Mathiak, K. (2004). Self-regulation of local brain activity using real-time functional magnetic resonance imaging (fMRI). Journal of Physiology, Paris, 98(4-6), 357–373. [DOI:10.1016/j.jphysparis.2005.09.019] [PMID]
Wiart, L., Ray, L., Darrah, J., & Magill-Evans, J. (2010). Parents' perspectives on occupational therapy and physical therapy goals for children with cerebral palsy. Disability and Rehabilitation, 32(3), 248–258.[DOI:10.3109/09638280903095890] [PMID]
Wilke, M., Holland, S. K., Myseros, J. S., Schmithorst, V. J., & Ball, W. S., Jr (2003). Functional magnetic resonance imaging in pediatrics. Neuropediatrics, 34(5), 225–233.[DOI:10.1055/s-2003-43260] [PMID]
Willoughby, K. L., Dodd, K. J., & Shields, N. (2009). A systematic review of the effectiveness of treadmill training for children with cerebral palsy. Disability and Rehabilitation, 31(24), 1971–1979. [DOI:10.3109/09638280902874204] [PMID]
Weiller, C., Jüptner, M., Fellows, S., Rijntjes, M., Leonhardt, G., & Kiebel, S., et al. (1996). Brain representation of active and passive movements. NeuroImage, 4(2), 105–110. [DOI:10.1006/nimg.1996.0034] [PMID]
Yang, Y. R., Chen, I. H., Liao, K. K., Huang, C. C., & Wang, R. Y. (2010). Cortical reorganization induced by body weight-supported treadmill training in patients with hemiparesis of different stroke durations. Archives of Physical Medicine and Rehabilitation, 91(4), 513–518. [DOI:10.1016/j.apmr.2009.11.021] [PMID]
For a more precise description of these findings, the differences in the significant activation changes between the study and control groups were obtained. The results showed 16.1% more active voxels in the training group compared to the control group (Figure 3c).
Therapeutic effects on balance and gait impairment
Table 3 summarizes the average group results of the clinical measures of walking capacity and the percentage of changes after the administration of the 8-week training course for both the study and control groups.

Walking speed increased by 36.3% and 24.9% for the study and control groups, respectively. Importantly, balance and mobility showed a much larger improvement in the training group compared to the control group (37.2% vs 16.8%). More importantly, walking endurance was enhanced three times more in the training group than in the control group.
4. Discussion
To the best of the authors’ knowledge, for the first time, this article characterizes the therapeutic effects of intensive AlterG training on brain cortical reorganization and walking capacity in children with CP using passive task-based fMRI under sedation. The objective was to study the responsiveness of motor cortical reorganization of children with hemiplegic CP after anti-gravity treadmill training versus over-ground walking OT. Passive movements included dorsiflexion and plantar flexion of both ankles and flexion-extension of both knees, which were carried out on all subjects before and after the 8-week training. This enabled us to investigate the intra-subject brain activation alterations induced by therapy, as well as the group comparison. The findings indicated a higher enhancement in both brain activity and walking capacity following the completion of AlterG in comparison to OT training, suggesting that AlterG training may be used as an effective therapeutic intervention for long-lasting improvement of gait and balance impairments in children with CP.
High sensitivity of task-based fMRI to motion artifacts could worsen in children with CP who neither cooperate to lie down immobile nor perform tasks correctly during fMRI acquisition. A similar study highlighted the difficulties of fMRI scanning in children with CP (Guzzetta et al., 2007). To address this issue, in this study, fMRI was acquired under sedation during which the passive tasks were applied. To the best of the authors’ knowledge, this was done for the first time; we found no evidence of sedative passive task-based fMRI in children with CP, although some studies investigated passive task-based fMRI in this patient population (Dinomais et al., 2013).
To consider the heterogeneity of the size and location of the brain lesions in CP participants included in this study, we used the ROI analyses to measure the therapy-induced fMRI-detected changes of brain activation between the motor cortices before and after training in each subject. The percentage of changes in the outcome measures was calculated for each subject in each study and control group. Then, the group average results were used to determine the impact of each intervention and compare the effectiveness of the interventions.
The activation patterns for different tasks were compared using the same data acquisition parameters, and analysis methods for both the intra- and inter-subject and group analyses on the FDR (P<0.05) corrected level were employed. Subsequent data analysis and comparisons are the first to suggest that motor cortical activation increases after 8 weeks of anti-gravity treadmill training and OT training. Moreover, the changes in therapy-driven motor cortical activation were more widely distributed with higher intensity in the training group compared to the control group. Passive movement tasks used in this study produced consistent activation in the motor system of both legs and demonstrated robust activation in M1, PMC, SMA, and PG of both hemispheres, as well as the CC, which connects the left and right cerebral hemispheres. This distribution of brain activity following sedative passive task-based fMRI is consistent with previous studies on sedative-free passive task-based fMRI movements in healthy adults (Ogg et al., 2009) the authors examined whether passive range of motion (ROM, adult stroke patients (Cho et al., 2016), healthy children (Drużbicki et al., 2013) and children with CP (Dinomais et al., 2013). This might imply that the supraspinal sensorimotor network for the neural control of walking can be assessed indirectly by these tasks.
According to Table 1, in 3 subjects of the training group (i.e. subjects 3, 5, and 6), the activation of motor areas induced by training increased in one hemisphere but decreased in the other. This was observed in only one task in one control patient (subject 9, left knee task). In subject 3, brain activation due to 3 tasks increased in the left hemisphere and CC, but decreased in the right hemisphere. In subject 5, for 3 tasks, brain activation increased in the contralateral side and decreased in the ipsilateral side and the CC. In subject 6, in the left knee task, brain activation increased in the contralateral side and decreased in the ipsilateral side and the CC. These contradictory activation changes in the hemispheres suggest that the investigated motor areas in one hemisphere may adaptively compensate for the other.
Studies that utilized anti-gravity treadmill training, BWST, and LOKOMAT for gait improvement mostly reported functional improvement rather than characterization of therapy-induced brain reorganization in children with CP (Birgani et al., 2016; Hesse et al., 1999; Drużbicki et al., 2013; Willoughby et al. 2009) with up to 45 minutes of training per session. The subject was evaluated before and after the 8-week training. The effects of training on the balance and postural stability was evaluated based on the Romberg test that was performed by using a posturography device. The parameters quantifying Center-of-Pressure (CoP. However, few studies have investigated the therapy-driven neuroplasticity in gait rehabilitation using BWST in adults with stroke and children with CP (Phillips et al., 2007; Dobkin et al., 2004; Yang et al., 2010). The hemodynamic response of the sensorimotor cortices following therapy has been reported to increase in some of these fMRI studies, while others showed that brain cortical activation decreased (Dinomais et al., 2013; Drużbicki et al., 2013). Furthermore, according to a limited number of small-scale fMRI investigations in children with UCP, increased contralateral activity may accompany functional gains. For instance, cluster-based S1–M1 voxel counts were increased after virtual reality therapy in three adults with UCP (Cho et al., 2016). However, our intra-subject analysis demonstrates both an increase and a decrease in motor cortical activation after therapy. This might be due to the initial severity of the sensorimotor impairments evident in participants.
Although both groups demonstrated an improvement in walking speed, TUG, and walking endurance, participants in the training group had much greater enhancement. This is concurrent with higher brain motor cortical reorganization induced in the training group. This implies that AlterG training may have the potential to promote effective neuroplasticity that can improve walking ability in children with CP.
5. Conclusion
The findings of this study demonstrate brain activation enhancement following the administration of the 8-week AlterG training in children with CP. This implies that AlterG training can be considered an effective physical intervention to improve walking capacity in children with CP. Our results also indicate that fMRI, performed with passive tasks, is an effective tool for detecting alterations in brain activity induced by physical activities in children with CP.
Study limitation
In this study, the therapeutic effects of AlterG training on brain functional activity and walking capacity were successfully characterized. While our results were promising with respect to the investigation of therapy-driven improvement of functional brain activities, our study had a few limitations. Firstly, a few patients could not complete the required training sessions due to the intensive treatment schedule.
Secondly, our results showed no significant correlation between these measurements (secondary aim), probably due to the limited sample size, which can mostly influence this aim, but not the primary ones. Our major objectives were firstly to examine the possibility of detecting the signatures of ankle and knee passive movement tasks in the fMRI of CP children, and if so, secondly to characterize these signatures, and finally to determine the potential therapeutic effects of the antigravity treadmill training on these signatures, and on balance and gait impairments. We could detect these signatures (objective 1), particularly for the ankle task, characterize them in terms of activated voxels (objective 2), and determine the therapeutic effects of training on the activated voxels (objective 3). Additionally, the findings revealed different therapeutic responses following the completion of training, consistent with the literature reporting a high inter-subject variability in brain structural and functional neuroplasticity due to several factors.
Finally, characterization of the intervention effects may not solely be achieved by the pre-post analyses and calculation of the average group results. Alternatively, since any intervention can have different effects on patients, the recovery patterns need to be identified to fully characterize the therapeutic effects of interventions and individualize treatment. This required a larger sample size and further data acquisition time points, considered in our ongoing studies
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Ethics Committee of Tehran University of Medical Sciences (TUMS), Tehran, Iran. This study was registered by the Iranian Registry of Clinical Trials (IRCT), Tehran, Iran (Code: IRCT2015121625568N1). All participants gave their written informed consent to participate in the study.
Funding
This study was extracted from the PhD dissertation of Meghdad Ashtiyani, approved by the Department of Biomedical Engineering and Medical Physics, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran (Registration No.: 365m)
Authors' contributions
Study design and investigation: Parmida Moradi Birgani and Mohammad Reze Deevband; Methodology: Meghdad Ashtiyani, Parmida Moradi Birgani, Amin Shahrokhi, and Mohammad Reze Deevband; Data acquisition, analysis and writing: Meghdad Ashtiyani and Parmida Moradi Birgani; Data interpretation: Meghdad Ashtiyani, Mohammad Mehdi Mirbagheri, Behnam Jameie and Maryam Soleimani; Review, editing, and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the participants for their corporations.
References
Albright A. L. (1996). Baclofen in the treatment of cerebral palsy. Journal of Child Neurology, 11(2), 77–83. [DOI:10.1177/088307389601100202] [PMID]
Azizi, S., Rasooli, A. H., Soleimani, M., Irani, A., Shahrokhi, A., & Mirbagheri, M. M. (2018). The impact of AlterG training on balance and structure of vestibulospinal tract in cerebral palsy children. Paper presented at: 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18-21 July 2018. [DOI:10.1109/EMBC.2018.8512772] [PMID]
Barch, D. M., Burgess, G. C., Harms, M. P., Petersen, S. E., Schlaggar, B. L., & Corbetta, M., et al. (2013). Function in the human connectome: Task-fMRI and individual differences in behavior. NeuroImage, 80, 169–189. [DOI:10.1016/j.neuroimage.2013.05.033] [PMID]
Bernal, B., Grossman, S., Gonzalez, R., & Altman, N. (2012). FMRI under sedation: What is the best choice in children?. Journal of Clinical Medicine Research, 4(6), 363-370. [PMID]
Birgani, P. M., Ashtiyani, M., Rasooli, A., Shahrokhnia, M., Shahrokhi, A., & Mirbagheri, M. M. (2016). Can an anti-gravity treadmill improve stability of children with cerebral palsy?. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual International Conference, 2016, 5465–5468. [DOI:10.1109/EMBC.2016.7591963] [PMID]
Bleyenheuft, Y., Dricot, L., Gilis, N., Kuo, H. C., Grandin, C., & Bleyenheuft, C., et al. (2015). Capturing neuroplastic changes after bimanual intensive rehabilitation in children with unilateral spastic cerebral palsy: A combined DTI, TMS and fMRI pilot study. Research in Developmental Disabilities, 43-44, 136–149. [DOI:10.1016/j.ridd.2015.06.014] [PMID]
Bohannon, R. W. (2006). Reference values for the timed up and go test: A descriptive meta-analysis. Journal of Geriatric Physical Therapy (2001), 29(2), 64–68. [DOI:10.1519/00139143-200608000-00004] [PMID]
Booth, A. T. C., Buizer, A. I., Meyns, P., Oude Lansink, I. L. B., Steenbrink, F., & van der Krogt, M. M. (2018). The efficacy of functional gait training in children and young adults with cerebral palsy: A systematic review and meta-analysis. Developmental Medicine and Child Neurology, 60(9), 866–883. [DOI:10.1111/dmcn.13708] [PMID]
Chen, J. J., & Pike, G. B. (2009). BOLD-specific cerebral blood volume and blood flow changes during neuronal activation in humans. NMR in Biomedicine, 22(10), 1054–1062. [DOI:10.1002/nbm.1411] [PMID]
Cherng, R. J., Liu, C. F., Lau, T. W., & Hong, R. B. (2007). Effect of treadmill training with body weight support on gait and gross motor function in children with spastic cerebral palsy. American Journal of Physical Medicine & Rehabilitation, 86(7), 548–555. [DOI:10.1097/PHM.0b013e31806dc302] [PMID]
Cho, C., Hwang, W., Hwang, S., & Chung, Y. (2016). Treadmill Training with Virtual Reality Improves Gait, Balance, and Muscle Strength in Children with Cerebral Palsy. The Tohoku Journal of Experimental Medicine, 238(3), 213–218. [DOI:10.1620/tjem.238.213] [PMID]
da Cunha, I. T., Jr, Lim, P. A., Qureshy, H., Henson, H., Monga, T., & Protas, E. J. (2002). Gait outcomes after acute stroke rehabilitation with supported treadmill ambulation training: A randomized controlled pilot study. Archives of physical Medicine and Rehabilitation, 83(9), 1258–1265. [DOI:10.1053/apmr.2002.34267] [PMID]
Dietz, V. (2009). Body weight supported gait training: From laboratory to clinical setting. Brain Research Bulletin, 78(1), I–VI. [DOI:10.1016/S0361-9230(08)00410-3] [PMID]
Dinomais, M., Chinier, E., Lignon, G., Richard, I., Ter Minassian, A., & Tich, S. N. (2013). The effect of video-guidance on passive movement in patients with cerebral palsy: fMRI study. Research in Developmental Disabilities, 34(10), 3487–3496. [DOI:10.1016/j.ridd.2013.07.008] [PMID]
Ditunno, J. F., Jr, Ditunno, P. L., Graziani, V., Scivoletto, G., Bernardi, M., & Castellano, V., et al. (2000). Walking index for spinal cord injury (WISCI): An international multicenter validity and reliability study. Spinal Cord, 38(4), 234–243. [DOI:10.1038/sj.sc.3100993] [PMID]
Draganski, B., Gaser, C., Busch, V., Schuierer, G., Bogdahn, U., & May, A. (2004). Neuroplasticity: Changes in grey matter induced by training. Nature, 427(6972), 311–312. [DOI:10.1038/427311a] [PMID]
Drużbicki, M., Rusek, W., Snela, S., Dudek, J., Szczepanik, M., & Zak, E., et al. (2013). Functional effects of robotic-assisted locomotor treadmill thearapy in children with cerebral palsy. Journal of Rehabilitation Medicine, 45(4), 358–363. [DOI:10.2340/16501977-1114] [PMID]
Dobkin, B. H., Firestine, A., West, M., Saremi, K., & Woods, R. (2004). Ankle dorsiflexion as an fMRI paradigm to assay motor control for walking during rehabilitation. NeuroImage, 23(1), 370–381. [DOI:10.1016/j.neuroimage.2004.06.008] [PMID]
Donabedian, A. (2005). Evaluating the quality of medical care. 1966. The Milbank Quarterly, 83(4), 691–729. [DOI:10.1111/j.1468-0009.2005.00397.x] [PMID]
Enzinger, C., Dawes, H., Johansen-Berg, H., Wade, D., Bogdanovic, M., & Collett, J., et al. (2009). Brain activity changes associated with treadmill training after stroke. Stroke, 40(7), 2460–2467. [DOI:10.1161/STROKEAHA.109.550053] [PMID]
Franceschini, M., Carda, S., Agosti, M., Antenucci, R., Malgrati, D., & Cisari, C., et al. (2009). Walking after stroke: What does treadmill training with body weight support add to overground gait training in patients early after stroke?: A single-blind, randomized, controlled trial. Stroke, 40(9), 3079–3085.[DOI:10.1161/STROKEAHA.109.555540] [PMID]
Guzzetta, A., Staudt, M., Petacchi, E., Ehlers, J., Erb, M., & Wilke, M., et al. (2007). Brain representation of active and passive hand movements in children. Pediatric Research, 61(4), 485–490. [DOI:10.1203/pdr.0b013e3180332c2e] [PMID]
Hesse, S., Konrad, M., & Uhlenbrock, D. (1999). Treadmill walking with partial body weight support versus floor walking in hemiparetic subjects. Archives of Physical Medicine and Rehabilitation, 80(4), 421–427. [DOI:10.1016/S0003-9993(99)90279-4] [PMID]
Heeger, D. J., & Ress, D. (2002). What does fMRI tell us about neuronal activity?. Nature Reviews. Neuroscience, 3(2), 142–151. [DOI:10.1038/nrn730] [PMID]
Hutchison, J. L., Hubbard, N. A., Brigante, R. M., Turner, M., Sandoval, T. I., & Hillis, G. A. J., et al. (2014). The efficiency of fMRI region of interest analysis methods for detecting group differences. Journal of Neuroscience Methods, 226, 57–65. [DOI:10.1016/j.jneumeth.2014.01.012] [PMID]
Kornelsen, J., & Stroman, P. W. (2004). fMRI of the lumbar spinal cord during a lower limb motor task. Magnetic Resonance in Medicine, 52(2), 411–414. [DOI:10.1002/mrm.20157] [PMID]
Krishnan, V., Kindig, M., & Mirbagheri, M. (2016). Robotic-assisted locomotor training enhances ankle performance in adults with incomplete spinal cord injury. Journal of Rehabilitation Medicine, 48(9), 781–786. [DOI:10.2340/16501977-2133] [PMID]
Koman, L. A., Mooney, J. F., 3rd, Smith, B., Goodman, A., & Mulvaney, T. (1993). Management of cerebral palsy with botulinum-A toxin: Preliminary investigation. Journal of Pediatric Orthopedics, 13(4), 489–495. [DOI:10.1097/01241398-199307000-00013] [PMID]
Li, W., Wait, S. D., Ogg, R. J., Scoggins, M. A., Zou, P., & Wheless, J., et al. (2013). Functional magnetic resonance imaging of the visual cortex performed in children under sedation to assist in presurgical planning. Journal of Neurosurgery. Pediatrics, 11(5), 543–546. [DOI:10.3171/2013.1.PEDS12401] [PMID]
Luft, A. R., Macko, R. F., Forrester, L. W., Villagra, F., Ivey, F., & Sorkin, J. D., et al. (2008). Treadmill exercise activates subcortical neural networks and improves walking after stroke: A randomized controlled trial. Stroke, 39(12), 3341–3350. [DOI:10.1161/STROKEAHA.108.527531] [PMID]
MacIntosh, B. J., Mraz, R., Baker, N., Tam, F., Staines, W. R., & Graham, S. J. (2004). Optimizing the experimental design for ankle dorsiflexion fMRI. NeuroImage, 22(4), 1619–1627.[DOI:10.1016/j.neuroimage.2004.03.035] [PMID]
Milla, P. J., & Jackson, A. D. (1977). A controlled trial of baclofen in children with cerebral palsy. The Journal of International Medical Research, 5(6), 398–404. [DOI:10.1177/030006057300100203] [PMID]
Mutlu, A., Krosschell, K., & Spira, D. G. (2009). Treadmill training with partial body-weight support in children with cerebral palsy: A systematic review. Developmental Medicine and Child Neurology, 51(4), 268–275. [DOI:10.1111/j.1469-8749.2008.03221.x] [PMID]
Ogg, R. J., Laningham, F. H., Clarke, D., Einhaus, S., Zou, P., & Tobias, M. E., et al. (2009). Passive range of motion functional magnetic resonance imaging localizing sensorimotor cortex in sedated children. Journal of Neurosurgery. Pediatrics, 4(4), 317–322. [PMID]
Palisano, R. J., Begnoche, D. M., Chiarello, L. A., Bartlett, D. J., McCoy, S. W., & Chang, H. J. (2012). Amount and focus of physical therapy and occupational therapy for young children with cerebral palsy. Physical & Occupational Therapy in Pediatrics, 32(4), 368–382. [DOI:10.3109/01942638.2012.715620] [PMID]
Parvin, S., Mehdinezhad, M., Taghiloo, A., Nourian, R., & Mirbagheri, M. M. (2018). The impact of repetitive transcranial magnetic stimulation on affected and unaffected sides of a child with hemiplegic cerebral palsy. Paper presented at: 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18-21 July 2018. [DOI:10.1109/EMBC.2018.8512877] [PMID]
Phillips, J. P., Sullivan, K. J., Burtner, P. A., Caprihan, A., Provost, B., & Bernitsky-Beddingfield, A. (2007). Ankle dorsiflexion fMRI in children with cerebral palsy undergoing intensive body-weight-supported treadmill training: A pilot study. Developmental Medicine and Child Neurology, 49(1), 39–44. [DOI:10.1017/S0012162207000102.x] [PMID]
Rasooli, A. H., Birgani, P. M., Azizi, S., Shahrokhi, A., & Mirbagheri, M. M. (2017). Therapeutic effects of an anti-gravity locomotor training (AlterG) on postural balance and cerebellum structure in children with Cerebral Palsy. Paper presented at: 2017 International Conference on Rehabilitation Robotics (ICORR), London, UK, 17-20 July 2017. [DOI:10.1109/ICORR.2017.8009229] [PMID]
Reid, L. B., Boyd, R. N., Cunnington, R., & Rose, S. E. (2016). Interpreting intervention induced neuroplasticity with fMRI: The case for multimodal imaging strategies. Neural Plasticity, 2016, 2643491. [DOI:10.1155/2016/2643491] [PMID]
Reid, L. B., Rose, S. E., & Boyd, R. N. (2015). Rehabilitation and neuroplasticity in children with unilateral cerebral palsy. Nature Reviews. Neurology, 11(7), 390–400. [DOI:10.1038/nrneurol.2015.97] [PMID]
Rosazza, C., Aquino, D., D'Incerti, L., Cordella, R., Andronache, A., & Zacà, D., et al. (2014). Preoperative mapping of the sensorimotor cortex: Comparative assessment of task-based and resting-state FMRI. Plos One, 9(6), e98860. [DOI:10.1371/journal.pone.0098860] [PMID]
Souweidane, M. M., Kim, K. H., McDowall, R., Ruge, M. I., Lis, E., & Krol, G., et al. (1999). Brain mapping in sedated infants and young children with passive-functional magnetic resonance imaging. Pediatric Neurosurgery, 30(2), 86–92. [DOI:10.1159/000028768] [PMID]
van Hedel, H. J., Wirz, M., & Dietz, V. (2005). Assessing walking ability in subjects with spinal cord injury: Validity and reliability of 3 walking tests. Archives of Physical Medicine and Rehabilitation, 86(2), 190–196. [DOI:10.1016/j.apmr.2004.02.010] [PMID]
Verrotti, A., Greco, R., Spalice, A., Chiarelli, F., & Iannetti, P. (2006). Pharmacotherapy of spasticity in children with cerebral palsy. Pediatric Neurology, 34(1), 1–6. [DOI:10.1016/j.pediatrneurol.2005.05.001] [PMID]
Weierink, L., Vermeulen, R. J., & Boyd, R. N. (2013). Brain structure and executive functions in children with cerebral palsy: A systematic review. Research in Developmental Disabilities, 34(5), 1678–1688. [DOI:10.1016/j.ridd.2013.01.035] [PMID]
Weiskopf, N., Scharnowski, F., Veit, R., Goebel, R., Birbaumer, N., & Mathiak, K. (2004). Self-regulation of local brain activity using real-time functional magnetic resonance imaging (fMRI). Journal of Physiology, Paris, 98(4-6), 357–373. [DOI:10.1016/j.jphysparis.2005.09.019] [PMID]
Wiart, L., Ray, L., Darrah, J., & Magill-Evans, J. (2010). Parents' perspectives on occupational therapy and physical therapy goals for children with cerebral palsy. Disability and Rehabilitation, 32(3), 248–258.[DOI:10.3109/09638280903095890] [PMID]
Wilke, M., Holland, S. K., Myseros, J. S., Schmithorst, V. J., & Ball, W. S., Jr (2003). Functional magnetic resonance imaging in pediatrics. Neuropediatrics, 34(5), 225–233.[DOI:10.1055/s-2003-43260] [PMID]
Willoughby, K. L., Dodd, K. J., & Shields, N. (2009). A systematic review of the effectiveness of treadmill training for children with cerebral palsy. Disability and Rehabilitation, 31(24), 1971–1979. [DOI:10.3109/09638280902874204] [PMID]
Weiller, C., Jüptner, M., Fellows, S., Rijntjes, M., Leonhardt, G., & Kiebel, S., et al. (1996). Brain representation of active and passive movements. NeuroImage, 4(2), 105–110. [DOI:10.1006/nimg.1996.0034] [PMID]
Yang, Y. R., Chen, I. H., Liao, K. K., Huang, C. C., & Wang, R. Y. (2010). Cortical reorganization induced by body weight-supported treadmill training in patients with hemiparesis of different stroke durations. Archives of Physical Medicine and Rehabilitation, 91(4), 513–518. [DOI:10.1016/j.apmr.2009.11.021] [PMID]
Type of Study: Original |
Subject:
Clinical Neuroscience
Received: 2021/10/9 | Accepted: 2022/01/3 | Published: 2025/07/1
Received: 2021/10/9 | Accepted: 2022/01/3 | Published: 2025/07/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








