Volume 15, Issue 2 (March & April 2024)
BCN 2024, 15(2): 165-174 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Atak E S, Yıldız D, Kocatürk R R, Temizyürek A, Özcan Ö Ö, Ergüzel T T, et al . Therapeutic Targets of Probiotics in Parkinson Disease: A Systematic Review of Randomized Controlled Trials. BCN 2024; 15 (2) :165-174
URL: http://bcn.iums.ac.ir/article-1-2262-en.html
URL: http://bcn.iums.ac.ir/article-1-2262-en.html
Elif Sina Atak1 

 , Dilara Yıldız1
, Dilara Yıldız1 

 , Rümeysa Rabia Kocatürk1
, Rümeysa Rabia Kocatürk1 

 , Arzu Temizyürek2
, Arzu Temizyürek2 

 , Öznur Özge Özcan3
, Öznur Özge Özcan3 

 , Türker Tekin Ergüzel4
, Türker Tekin Ergüzel4 

 , Mesut Karahan *1
, Mesut Karahan *1 

 , Nevzat Tarhan5
, Nevzat Tarhan5 




 , Dilara Yıldız1
, Dilara Yıldız1 

 , Rümeysa Rabia Kocatürk1
, Rümeysa Rabia Kocatürk1 

 , Arzu Temizyürek2
, Arzu Temizyürek2 

 , Öznur Özge Özcan3
, Öznur Özge Özcan3 

 , Türker Tekin Ergüzel4
, Türker Tekin Ergüzel4 

 , Mesut Karahan *1
, Mesut Karahan *1 

 , Nevzat Tarhan5
, Nevzat Tarhan5 


1- Department of Nutrition and Dietetics, Faculty of Health Sciences, Üsküdar University, Istanbul, Turkey.
2- Department of Physiology, School of Medicine, Koç University, Istanbul, Turkey.
3- Department of Molecular Neuroscience, Health Sciences Institute, Üsküdar University, Istanbul, Turkey.
4- Department of Software Engineering, Faculty of Engineering and Natural Sciences, Üsküdar University, Istanbul, Turkey.
5- Department of Psychiatry, School of Medicine, Üskudar University, Istanbul, Turkey.
2- Department of Physiology, School of Medicine, Koç University, Istanbul, Turkey.
3- Department of Molecular Neuroscience, Health Sciences Institute, Üsküdar University, Istanbul, Turkey.
4- Department of Software Engineering, Faculty of Engineering and Natural Sciences, Üsküdar University, Istanbul, Turkey.
5- Department of Psychiatry, School of Medicine, Üskudar University, Istanbul, Turkey.
Full-Text [PDF 696 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Parkinson disease (PD) is a neurological disease whose prevalence is rapidly rising. The disease incidence and prevalence were reported to be 11–19 and 108–257 per 100000 in Europe, respectively. The loss of dopaminergic neurons in the substantia nigra and pars compacta, as well as the accumulation of tender folded-synuclein termed Lewy bodies in the intracytoplasmic space, are the clinical features of PD. Mitochondrial dysfunction, oxidative stress, increased inflammatory cytokines and free radicals, and genetic and environmental variables are associated with this clinical condition (Balestrino & Schapira, 2020). These factors commonly cause motor symptoms, including bradykinesia, tremor, and postural disorder. However, the clinical side also includes non-motor symptoms (Ding et al., 2017). The most common non-motor symptoms of PD are gastrointestinal (GI) dysfunction, such as constipation, bloating, abdominal pain, stool consistency, and bowel movements (Schrag et al., 2000). Many factors can be associated with neurodegenerative disorders, including gut microbiota and functions related to the blood-brain barrier (Shoemark & Allen, 2015). Probiotic supplementation has been reported to help relieve clinical and metabolic outcomes in PD (Parashar & Udayabanu, 2017). As a result, modulation of the gut microbiome could be a therapeutic target for PD.
Supplementing with probiotics reduces motor severity ratings (UPDRS), serum biomarkers, and body mass index (BMI) in people with PD (Tamtaji et al., 2019). However, there is a scarcity of evidence on the impact of probiotic supplementation on serum biomarkers in PD patients. Furthermore, only a few clinical studies have mentioned the influence of probiotics on PD management. This study aimed to investigate the effects of probiotic supplementation on BMI indices, gastrointestinal symptoms, and glutathione levels in people with PD.
2. Materials and Methods
Literature search
Electronic databases, including Scopus, PubMed, The Cochrane Library, Web of Science, Ovid-LWW, and ScienceDirect, were searched until April 2021. The following keywords and medical subject header (MeSH) phrases were used in the search strategy: “Probiotics,” “fermented foods,” “Lactobacillus,” and “Bifidobacterium,” combined with “Parkinson’s disease.” They were used with the boolean operators (‘AND’ and ‘OR’).
Inclusion and exclusion criteria
The selection process was conducted independently by two researchers. The inclusion and exclusion criteria were as follows: 1) Randomized controlled trials (RTCs) with placebo groups included patients with Parkinson, supplemented live bacteria as probiotics; 2) Relevant data about the study’s features and major outcomes, such as BMI, UPDRS, serum glutathione and gastrointestinal problems (constipation, bloating, abdominal pain, stool consistency, and bowel movements); 3) PD patients.
Irrelevant titles and abstracts, reviews, editorials, conference book chapters, books, letters, case reports, retracted articles, and case reports were included, and non-English articles and in vitro and in vivo studies were excluded. The articles that did not meet the inclusion criteria were also excluded.
Study selection
All ‘titles’ and ‘abstracts’ were recovered from the Zotero library to perform the research processes, and duplicate results were removed with Zotero reference manager software (Zotero, 2020). Then, the remaining ‘Titles’ and ‘Abstracts’ of studies were scanned for eligibility. The next stage was to go over the whole text of the remaining studies. The screening procedure was carried out separately by three researchers. There were no disagreements between the three researchers. The following data were documented using standardized tables: 1) Basic data, including first author, year of publication, research design, country, age, gender, weight, and BMI; 2) Interventions, including participant count, probiotic kind, methods, and study duration; 3) Outcomes of interest, including change in interested parameters in each article; and 4) Quality of the article.

Quality appraisal of included studies
The quality appraisal of included studies was assessed using the Jadad checklist (maximum 5 points) (Jadad et al., 1996). The following criteria were considered: 1) Randomization (2 points), 1 point if randomization is mentioned, 1 additional point if the method of randomization is appropriate or -1 point if it is inappropriate; 2) Blinding (2 points), 1 point if blinding is mentioned, 1 additional point if the method blinding is appropriate or -1 point if it is inappropriate; and 3) Withdrawals and dropouts (1 point), 1 point if the fate of all patients in the trial is known. Obtaining 4 to 5 points indicates good quality of studies.
3. Results
A total of 27395 records were identified using a search strategy from eight databases (PubMed, n=63; The Cochrane Library, n=50; Scopus, n=346; Web of Science, n=112 [Korean Journal Database, Web of Science Core Collection, SciELO citation index, Russian science citation index]; ScienceDirect, n=2791; and Ovid-LWW, n=24033). Then, 16309 records were removed because they were duplicates. So, 11086 studies were available for screening. Of those, reviews (n=3402), book chapters and encyclopedia (n=637), non-research publications (letters, conference, index, editorial, and abstract) (n=3119), case reports (n=295), article retraction (n=81), irrelevant titles and abstracts (n=3418) were excluded based on screening the ‘titles’ and ‘abstracts.’ Finally, 134 articles were selected for full-text reading. Of those, 129 studies were excluded for the following reasons: Non-English (n=11), animals and in vivo and in vitro (n=79), no probiotic treatment provided (n=16), no placebo (n=1), no PD (n=8), and no available full text (n=14). The inclusion and exclusion process led to the inclusion of 5 randomized control trials (Figure 1).
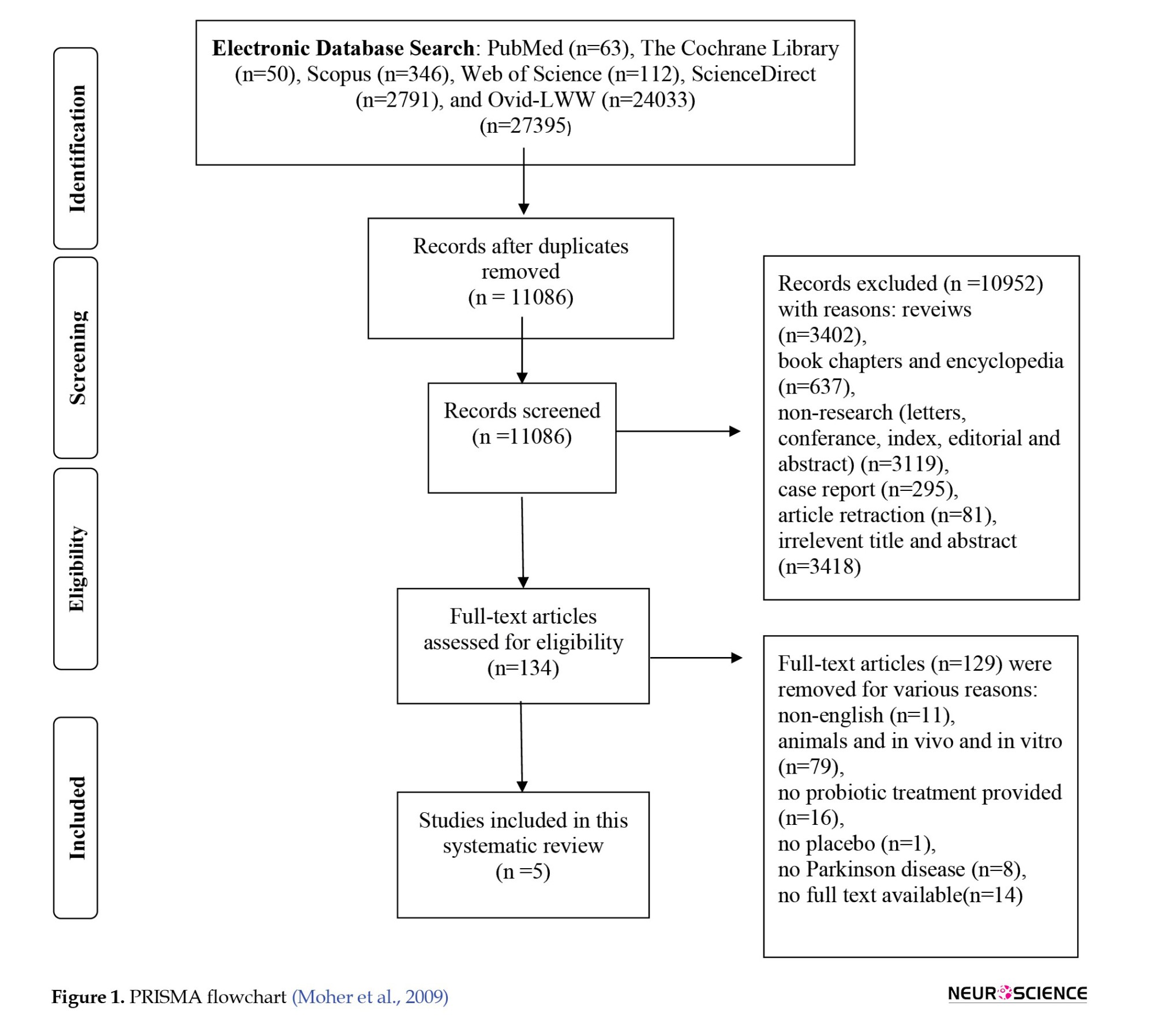
There was significant variation between research studies regarding the number of participants. The number of PD patients in the study ranged from 48 to 120, totaling 350. The supplement duration was in the range of 4-12 weeks (Table 1).
PD rating
Movement disorder society-sponsored revision of the unified Parkinson disease rating scale (MDS-UPDRS) was used to monitor the longitudinal course of PD, with a point range from 0 to 199 (Goetz et al., 2008). Higher scores show worsening outcomes of the disease. In two studies, UPDRS was used. In both studies, UPDRS scores were dropped after 8 weeks use of Lactobacillus Casei, Lactobacillus acidophilus, Bifidobacterium infantis, Lactobacillus lactis, Bifidobacterium longum supplement and 12 weeks use of L. acidophilus, Bifidobacterium bifidum, Lactobacillus reuteri, and Lactobacillus fermentum 2×109 CFU (colony-forming unit/g) (Tamtaji et al., 2019; Ibrahim et al., 2020). Also, the non-motor symptoms score (NMSS) is a scale that informs the severity of the PD patient’s non-motor symptoms. The score ranges from 0 to 360; higher scores define severe deterioration (Van Wamelen et al., 2021). Ibrahim et al. (2020) reported decreasing scores of NMSS after 8 weeks use of probiotics.
In other words, the probiotics used decreased these scales in PD, proving that probiotics may help by reducing the severity of the disease.
Oxidative stress
Glutathione (GSH) is a tripeptide amino acid and a powerful antioxidant in the cells and tissues of the body. It consists of the amino acids L-cysteine, L-glutamate, and glycine. Glutathione protects the organism by eliminating free radicals and reactive oxygen species (Smeyne & Smeyne, 2013). In two studies, GSH was measured. Tamtaji et al. (2019) reported that 12 weeks use of B. bifidum, L. acidophilus, L. fermentum, and L. reuteri (2×109 CFU/g) supplement increased GSH levels in plasma. Borzobadi et al. (2018) reported insignificant increases in GSH levels after 12 weeks use of B. bifidum, L. acidophilus, L. fermentum, and Lactobacillus reuter (8×109 CFU/d probiotic). Moreover, malondialdehyde (MDA) is a highly reactive molecule that is an oxidative stress biomarker. Tamtaji et al. (2019) reported decreases in MDA after 12 weeks of probiotic use, and inflammation biomarker C- reactive protein (CRP) remarkably dropped.
This finding supported the idea that probiotics can protect oxidative stress by increasing GSH levels and decreasing MDA and CRP levels.
Gastrointestinal system
Gastrointestinal problems are frequent in PD, and the whole GI tract is affected mainly by numerous complications (Fasano et al., 2015).
Ibrahim et al. (2020) reported increased bowel opening frequency (BOF) and gut transit time (GTT) decreased in PD patients after 8 weeks of usage of L. acidophilus, L. Casei, L. lactis, B. infantis, B. longum. In addition, following 4 weeks of using fermented milk containing several probiotic strains and prebiotic fiber, Barichella et al. (2016) reported an increase in complete bowel movement. After 4 weeks of using a probiotic pill containing 10 billion CFU of eight different commercially available bacterial strains (L. reuteri, L. acidophilus, Lactobacillus gasseri, B. bifidum, Lactobacillus rhamnosus, B. longum, Enterococcus faecium, Enterococcus faecalis), spontaneous bowel movements increased. Also, probiotic supplements increased stool consistency and decreased constipation. On the other hand, the quality of life-related to constipation decreased (Tan et al., 2020).
As a result, supplements containing probiotics helped to regulate GI problems regardless of the time of administration but did not affect the quality of life due to constipation. However, the data in existing studies are very limited.
Quality appraisal of included studies
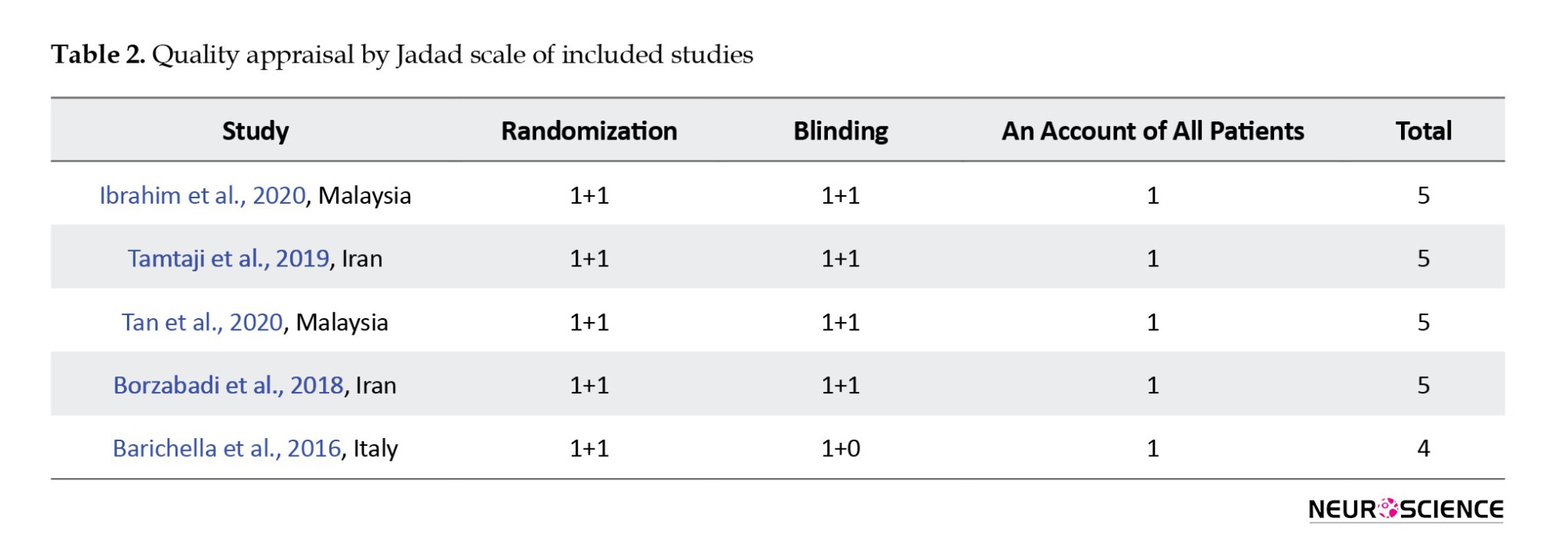
The average score obtained from the modified Jadad scale was calculated as 4.8 points (range 4-5 points). Five studies were classified as “good” methodological quality. Individual scores are shown in Table 2.
4. Discussion
This systematic review investigates the effects of probiotic/prebiotic consumption in PD patients, which is a priority study in this area. Despite little evidence, the included five trials in this systematic review of PD individuals recommended the beneficial effects of probiotics in the clinical setting. Also, GI functions are highly related to probiotics. However, the evidence obtained from the RTCs about PD is limited. Similar to our results, in a systematic review, certain probiotics are beneficial in treating GI tract problems. Specific probiotics have been demonstrated to alleviate irritable bowel syndrome and lower gastrointestinal symptoms, prevent diarrhea associated with antibiotics and H. pylori eradication therapy, and demonstrate appropriate safety (Hungin et al., 2018). Kesika et al. (2020) reported the effect of probiotics on Alzheimer disease and the gut-brain axis; it was found that probiotic consumption prevented inflammation, reduced oxidative stress, and improved memory in Alzheimer patients. Amirani et al. (2020) conducted a systematic study of the effects of probiotic supplementation on mental health, inflammatory biomarkers, and oxidative stress in psychiatric patients. Probiotic supplementation has a beneficial impact on the Hamilton depression rating scale, CRP, interleukin-10, and MDA levels but no effect on inflammation and oxidative stress indicators. Similarly, another study showed that GSH levels increased, and MDA and CRP levels dropped by using probiotics (Tamtaji et al., 2019).
Although with low evidence, data from three RCTs involving 161 individuals with Alzheimer disease who received Lactobacillus and Bifidobacterium strains from the diet showed no benefit of probiotic supplementation on cognitive functions (standardized mean difference, 0.56; 95% CI, -0.06%, 1.18%) (Krüger et al., 2021). The MDA-UPDRS and NMSS were improved after usage of probiotics (Ibrahim et al., 2020). In the chronic phase of PD, cognition and brain functions may be associated. On the other hand, probiotic supplementation improved plasma triglycerides, very low-density lipoprotein cholesterol, insulin resistance, and plasma MDA in another trial. There were no RCTs that looked at synbiotic supplementation or microbiota compositions. The evidence for probiotics and synbiotics in treating dementia and cognition is insufficient to justify their use in clinical practice (Krüger et al., 2021).
On the other hand, although probiotics have many benefits, they can also cause some complications. A systematic review assessed probiotic use problems in 60 case reports and 7 case series involving 93 individuals. Fungi were the most common infectious complication in 35(37.6%) cases, with Saccharomyces genus being the most common genus with 47(50.6%) cases, followed by Lactobacillus, Bifidobacterium, Bacillus, Pedioccocus, and Escherichia coli with 26(27.9%), 12(12.8%), 5(5.4%), 2(2.2%), and 1(1.1%) cases, respectively (Costa et al., 2018). Also, Rao et al. investigated brain fogginess (BF), gas, and bloating dependence with D-lactate in small intestinal bacterial overgrowth (SIBO). SIBO was more common in the BF group than in the non-BF group (68% vs 28%, P=0.05). Probiotics were used by everyone in the BF group. D-lactic acidosis was more common in the BF group than in the non-BF group (77% vs 25%, P=0.006). In 20/30 (66%) of the patients, BF was replicated. In 10/30 (33%) of patients with BF and 2/8 (25%) of those without, gastrointestinal transit was delayed. The results of the other metabolic tests were normal. However, BF was resolved, and gastrointestinal symptoms improved considerably (P=0.005) in 23/30 people after stopping probiotics and using antibiotics (77%). In a group without short bowel syndrome, BF, gas, and bloating may be linked to probiotic use, SIBO, and D-lactic acidosis (Rao et al., 2018). However, the probiotic does not include Neisseria streptococcus, Staphylococcus, or Hemophilus strains in the predominant organisms discovered by culture in duodenal aspirates. However, there is no data to confirm that the patients who applied are acidotic. It should be noted that many Lactobacilli and all Bifidobacteria exclusively create L-lactate and not D-lactate (Petrova et al., 2018; Quigley et al., 2018; Reid et al., 2019). Probiotics may trigger bacterial translocation and enterocyte damage in patients with acute pancreatitis, resulting in organ failure (Besselink et al., 2004; Besselink et al., 2009). Still, in a later study, it was found that probiotics did not cause any contraindications. Conversely, carbohydrates may worsen the situation with lactic acidosis caused by their fermentation (Bongaerts & Severijnen, 2016). Established tests and clinical guidelines in treating high-risk adults and children are essential, as probiotics can produce different effects in different health conditions (Sanders et al., 2016). More importantly, probiotic complications in PD are unknown, but the impact on neurological diseases may also be an indicator of PD.
Furthermore ,it is essential to emphasize that there are some limitations to consider when designing future interventions (1). Although this study was conducted through a wide literature search (27395 records), the studies that could be included in probiotic use in PD patients were low and insufficient. Therefore, the systematically compiled parameters are limited and unsuitable for comparison (2). The probiotic and the active bacteria in its content are stated in our study. Still, it is impossible to identify which bacteria can be better and provide more benefits to PD patients due to the lack of existing studies (3). Bacterial colony numbers vary from study to study, and since there is no standard yet, no interpretation of the amount of CFU could be made (4). Positive results in the researched area have been revealed so that this area can be pursued, and more profound research is needed (5). A comprehensive systematic review forms the basis of new studies on the amount of probiotic usage, duration, and variety of clinically supportive nutritional support for PD patients (6).
5. Conclusion
A growing body of data suggests that the gut-brain bidirectional connection and dysbiosis have a role in PD. A healthy gut microbiome may lower the chance of acquiring a variety of human diseases, including PD. α-synuclein build-up in PD has been shown to begin in the enteral nervous system and move to the central nervous system by trans-synaptic cell-to-cell transmission. As previously noted, creating a proinflammatory environment in dysbiosis can signal the brain via systemic routes and may cause a defective blood-brain barrier. Finally, it was suggested that an immune system imbalance in the host might be partially responsible for PD’s motor and non-motor symptoms. The hunt for early biomarkers and innovative treatment methods has accelerated due to an improved understanding of PD pathophysiology.
In this systematic review, probiotics used in PD are reviewed. As a result, various probiotic supplements were used for 4-12 weeks in PD rating, oxidative stress, and GI tract symptoms such as spontaneous bowel movement, GTT, BOF, abdominal pain, bloating, constipation, and improvements in each parameter were observed. Although the studies included in this study are of high methodological quality, the number of studies is insufficient. Therefore, some issues need to be included in the literature, from different probiotic strains, parameters, and duration of use to clinical studies of probiotics in PD.”
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.”
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to express their gratitude to the Üsküdar University and NP Brain Hospital for their support”
References
Amirani, E., Milajerdi, A., Mirzaei, H., Jamilian, H., Mansournia, M. A., & Hallajzadeh, J., et al. (2020). The effects of probiotic supplementation on mental health, biomarkers of inflammation and oxidative stress in patients with psychiatric disorders: A systematic review and meta-analysis of randomized controlled trials. Complementary Therapies in Medicine, 49, 102361. [DOI:10.1016/j.ctim.2020.102361] [PMID]
Balestrino, R., & Schapira, A. H. V. (2020). Parkinson disease. European Journal of Neurology, 27(1), 27-42. [DOI:10.1111/ene.14108] [PMID]
Barichella, M., Pacchetti, C., Bolliri, C., Cassani, E., Iorio, L., & Pusani, C., et al. (2016). Probiotics and prebiotic fiber for constipation associated with Parkinson disease: An RCT. Neurology, 87(12), 1274-1280. [DOI:10.1212/WNL.0000000000003127] [PMID]
Besselink, M. G., Timmerman, H. M., Buskens, E., Nieuwenhuijs, V. B., Akkermans, L. M., & Gooszen, H. G., et al. (2004). Probiotic prophylaxis in patients with predicted severe acute pancreatitis (PROPATRIA): Design and rationale of a double-blind, placebo-controlled randomised multicenter trial [ISRCTN38327949]. BMC Surgery, 4, 12. [DOI:10.1186/1471-2482-4-12] [PMID]
Besselink, M. G., van Santvoort, H. C., Renooij, W., de Smet, M. B., Boermeester, M. A., & Fischer, K., et al. (2009). Intestinal barrier dysfunction in a randomized trial of a specific probiotic composition in acute pancreatitis. Annals of Surgery, 250(5), 712-719. [DOI:10.1097/SLA.0b013e3181bce5bd] [PMID]
Bongaerts, G. P., & Severijnen, R. S. (2016). A reassessment of the PROPATRIA study and its implications for probiotic therapy. Nature Biotechnology, 34(1), 55-63. [DOI:10.1038/nbt.3436] [PMID]
Borzabadi, S., Oryan, S., Eidi, A., Aghadavod, E., Daneshvar Kakhaki, R., & Tamtaji, O. R., et al. (2018). The effects of probiotic supplementation on gene expression related to inflammation, insulin and lipid in patients with Parkinsonâ s disease: A randomized, double-blind, placebo-controlled trial. Archives of Iranian Medicine, 21(7), 289-295. [PMID]
Costa, R. L., Moreira, J., Lorenzo, A., & Lamas, C. C. (2018). Infectious complications following probiotic ingestion: a potentially underestimated problem? A systematic review of reports and case series. BMC Complementary and Alternative Medicine, 18(1), 329. [DOI:10.1186/s12906-018-2394-3] [PMID]
Ding, X., Gao, J., Xie, C., Xiong, B., Wu, S., & Cen, Z., et al. (2018). Prevalence and clinical correlation of dysphagia in Parkinson disease: A study on Chinese patients. European Journal of Clinical Nutrition, 72(1), 82-86. [DOI:10.1038/ejcn.2017.100] [PMID]
Fasano, A., Visanji, N. P., Liu, L. W., Lang, A. E., & Pfeiffer, R. F. (2015). Gastrointestinal dysfunction in Parkinson’s disease. The Lancet Neurology, 14(6), 625-639. [DOI:10.1016/S1474-4422(15)00007-1] [PMID]
Goetz, C. G., Tilley, B. C., Shaftman, S. R., Stebbins, G. T., Fahn, S., & Martinez-Martin, P., et al. (2008). Movement disorder society‐sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS‐UPDRS): Scale presentation and clinimetric testing results. Movement Disorders: Official Journal of the Movement Disorder Society, 23(15), 2129-2170. [PMID]
Hungin, A. P., Mulligan, C., Pot, B., Whorwell, P., Agréus, L., & Fracasso, P., et al. (2013). Systematic review: Probiotics in the management of lower gastrointestinal symptoms in clinical practice -- an evidence-based international guide. Alimentary Pharmacology & Therapeutics, 38(8), 864–886. [DOI:10.1111/apt.12460] [PMID]
Ibrahim, A., Ali, R. A. R., Manaf, M. R. A., Ahmad, N., Tajurruddin, F. W., & Qin, W. Z., et al. (2020). Multi-strain probiotics (Hexbio) containing MCP BCMC strains improved constipation and gut motility in Parkinson’s disease: A randomised controlled trial. Plos One, 15(12), e0244680. [DOI:10.1371/journal.pone.0244680] [PMID]
Jadad, A. R., Moore, R. A., Carroll, D., Jenkinson, C., Reynolds, D. J., & Gavaghan, D. J., et al. (1996). Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Controlled Clinical Trials, 17(1), 1-12. [DOI:10.1016/0197-2456(95)00134-4] [PMID]
Kesika, P., Suganthy, N., Sivamaruthi, B. S., & Chaiyasut, C. (2021). Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sciences, 264, 118627. [DOI:10.1016/j.lfs.2020.118627] [PMID]
Krüger, J. F., Hillesheim, E., Pereira, A. C. S. N., Camargo, C. Q., & Rabito, E. I. (2021). Probiotics for dementia: A systematic review and meta-analysis of randomized controlled trials. Nutrition Reviews, 79(2), 160-170. [DOI:10.1093/nutrit/nuaa037] [PMID]
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., & Prisma Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS medicine, 6(7), e1000097. [DOI: 10.1371/journal.pmed.1000097] [PMID]
Parashar, A., & Udayabanu, M. (2017). Gut microbiota: Implications in Parkinson’s disease. Parkinsonism & Related Disorders, 38, 1-7. [DOI:10.1016/j.parkreldis.2017.02.002] [PMID]
Petrova, M. I., Macklaim, J. M., Wuyts, S., Verhoeven, T., Vanderleyden, J., & Gloor, G. B., et al. (2018). Comparative genomic and phenotypic analysis of the vaginal probiotic lactobacillus rhamnosus GR-1. Frontiers in Microbiology, 9, 1278. [DOI:10.3389/fmicb.2018.01278] [PMID]
Quigley, E. M. M., Pot, B., & Sanders, M. E. (2018). ‘Brain Fogginess’ and D-Lactic Acidosis: Probiotics are not the cause. Clinical and Translational Gastroenterology, 9(9), 187. [DOI:10.1038/s41424-018-0057-9] [PMID]
Rao, S., S. S. C., Rehman, A., Yu, S., & Andino, N. M. (2018). Brain fogginess, gas and bloating: A link between SIBO, probiotics and metabolic acidosis. Clinical and Translational Gastroenterology, 9(6), 162. [DOI:10.1038/s41424-018-0030-7] [PMID]
Reid, G., Gadir, A. A., & Dhir, R. (2019). Probiotics: Reiterating what they are and what they are not. Frontiers in Microbiology, 10, 424. [DOI:10.3389/fmicb.2019.00424] [PMID]
Sanders, M. E., Merenstein, D. J., Ouwehand, A. C., Reid, G., Salminen, S., & Cabana, M. D., et al. (2016). Probiotic use in at-risk populations. Journal of the American Pharmacists Association: JAPhA, 56(6), 680-686. [DOI:10.1016/j.japh.2016.07.001] [PMID]
Schrag, A., Jahanshahi, M., & Quinn, N. (2000). What contributes to quality of life in patients with Parkinson’s disease?. Journal of Neurology, Neurosurgery & Psychiatry, 69(3), 308-312. [DOI:10.1136/jnnp.69.3.308] [PMID]
Shoemark, D. K., & Allen, S. J. (2015). The microbiome and disease: Reviewing the links between the oral microbiome, aging, and Alzheimer’s disease. Journal of Alzheimer’s Disease, 43(3), 725-738. [DOI:10.3233/JAD-141170] [PMID]
Smeyne, M., & Smeyne, R. J. (2013). Glutathione metabolism and Parkinson’s disease. Free Radical Biology and Medicine, 62, 13-25. [DOI:10.1016/j.freeradbiomed.2013.05.001] [PMID]
Tamtaji, O. R., Taghizadeh, M., Kakhaki, R. D., Kouchaki, E., Bahmani, F., Borzabadi, S.,... & Asemi, Z. (2019). Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Clinical Nutrition, 38(3), 1031-1035. [DOI:10.1016/j.clnu.2018.05.018] [PMID]
Tan, A. H., Lim, S. Y., Chong, K. K., Manap, M. A. A. A., Hor, J. W., & Lim, J. L., et al. (2021). Probiotics for constipation in Parkinson Disease: A randomized placebo-controlled study. Neurology, 96(5), e772-e782. [DOI:10.1212/WNL.0000000000010998]
Van Wamelen, D. J., Martinez-Martin, P., Weintraub, D., Schrag, A., Antonini, A., & Falup-Pecurariu, C., et al. (2021). The Non‐Motor Symptoms Scale in Parkinson’s disease: Validation and use. Acta Neurologica Scandinavica, 143(1), 3-12. [DOI:10.1111/ane.13336] [PMID]
Zotero. (2020). Zotero|your personal research assistant [internet]. Retrived from: [Link]
Parkinson disease (PD) is a neurological disease whose prevalence is rapidly rising. The disease incidence and prevalence were reported to be 11–19 and 108–257 per 100000 in Europe, respectively. The loss of dopaminergic neurons in the substantia nigra and pars compacta, as well as the accumulation of tender folded-synuclein termed Lewy bodies in the intracytoplasmic space, are the clinical features of PD. Mitochondrial dysfunction, oxidative stress, increased inflammatory cytokines and free radicals, and genetic and environmental variables are associated with this clinical condition (Balestrino & Schapira, 2020). These factors commonly cause motor symptoms, including bradykinesia, tremor, and postural disorder. However, the clinical side also includes non-motor symptoms (Ding et al., 2017). The most common non-motor symptoms of PD are gastrointestinal (GI) dysfunction, such as constipation, bloating, abdominal pain, stool consistency, and bowel movements (Schrag et al., 2000). Many factors can be associated with neurodegenerative disorders, including gut microbiota and functions related to the blood-brain barrier (Shoemark & Allen, 2015). Probiotic supplementation has been reported to help relieve clinical and metabolic outcomes in PD (Parashar & Udayabanu, 2017). As a result, modulation of the gut microbiome could be a therapeutic target for PD.
Supplementing with probiotics reduces motor severity ratings (UPDRS), serum biomarkers, and body mass index (BMI) in people with PD (Tamtaji et al., 2019). However, there is a scarcity of evidence on the impact of probiotic supplementation on serum biomarkers in PD patients. Furthermore, only a few clinical studies have mentioned the influence of probiotics on PD management. This study aimed to investigate the effects of probiotic supplementation on BMI indices, gastrointestinal symptoms, and glutathione levels in people with PD.
2. Materials and Methods
Literature search
Electronic databases, including Scopus, PubMed, The Cochrane Library, Web of Science, Ovid-LWW, and ScienceDirect, were searched until April 2021. The following keywords and medical subject header (MeSH) phrases were used in the search strategy: “Probiotics,” “fermented foods,” “Lactobacillus,” and “Bifidobacterium,” combined with “Parkinson’s disease.” They were used with the boolean operators (‘AND’ and ‘OR’).
Inclusion and exclusion criteria
The selection process was conducted independently by two researchers. The inclusion and exclusion criteria were as follows: 1) Randomized controlled trials (RTCs) with placebo groups included patients with Parkinson, supplemented live bacteria as probiotics; 2) Relevant data about the study’s features and major outcomes, such as BMI, UPDRS, serum glutathione and gastrointestinal problems (constipation, bloating, abdominal pain, stool consistency, and bowel movements); 3) PD patients.
Irrelevant titles and abstracts, reviews, editorials, conference book chapters, books, letters, case reports, retracted articles, and case reports were included, and non-English articles and in vitro and in vivo studies were excluded. The articles that did not meet the inclusion criteria were also excluded.
Study selection
All ‘titles’ and ‘abstracts’ were recovered from the Zotero library to perform the research processes, and duplicate results were removed with Zotero reference manager software (Zotero, 2020). Then, the remaining ‘Titles’ and ‘Abstracts’ of studies were scanned for eligibility. The next stage was to go over the whole text of the remaining studies. The screening procedure was carried out separately by three researchers. There were no disagreements between the three researchers. The following data were documented using standardized tables: 1) Basic data, including first author, year of publication, research design, country, age, gender, weight, and BMI; 2) Interventions, including participant count, probiotic kind, methods, and study duration; 3) Outcomes of interest, including change in interested parameters in each article; and 4) Quality of the article.

Quality appraisal of included studies
The quality appraisal of included studies was assessed using the Jadad checklist (maximum 5 points) (Jadad et al., 1996). The following criteria were considered: 1) Randomization (2 points), 1 point if randomization is mentioned, 1 additional point if the method of randomization is appropriate or -1 point if it is inappropriate; 2) Blinding (2 points), 1 point if blinding is mentioned, 1 additional point if the method blinding is appropriate or -1 point if it is inappropriate; and 3) Withdrawals and dropouts (1 point), 1 point if the fate of all patients in the trial is known. Obtaining 4 to 5 points indicates good quality of studies.
3. Results
A total of 27395 records were identified using a search strategy from eight databases (PubMed, n=63; The Cochrane Library, n=50; Scopus, n=346; Web of Science, n=112 [Korean Journal Database, Web of Science Core Collection, SciELO citation index, Russian science citation index]; ScienceDirect, n=2791; and Ovid-LWW, n=24033). Then, 16309 records were removed because they were duplicates. So, 11086 studies were available for screening. Of those, reviews (n=3402), book chapters and encyclopedia (n=637), non-research publications (letters, conference, index, editorial, and abstract) (n=3119), case reports (n=295), article retraction (n=81), irrelevant titles and abstracts (n=3418) were excluded based on screening the ‘titles’ and ‘abstracts.’ Finally, 134 articles were selected for full-text reading. Of those, 129 studies were excluded for the following reasons: Non-English (n=11), animals and in vivo and in vitro (n=79), no probiotic treatment provided (n=16), no placebo (n=1), no PD (n=8), and no available full text (n=14). The inclusion and exclusion process led to the inclusion of 5 randomized control trials (Figure 1).

There was significant variation between research studies regarding the number of participants. The number of PD patients in the study ranged from 48 to 120, totaling 350. The supplement duration was in the range of 4-12 weeks (Table 1).
PD rating
Movement disorder society-sponsored revision of the unified Parkinson disease rating scale (MDS-UPDRS) was used to monitor the longitudinal course of PD, with a point range from 0 to 199 (Goetz et al., 2008). Higher scores show worsening outcomes of the disease. In two studies, UPDRS was used. In both studies, UPDRS scores were dropped after 8 weeks use of Lactobacillus Casei, Lactobacillus acidophilus, Bifidobacterium infantis, Lactobacillus lactis, Bifidobacterium longum supplement and 12 weeks use of L. acidophilus, Bifidobacterium bifidum, Lactobacillus reuteri, and Lactobacillus fermentum 2×109 CFU (colony-forming unit/g) (Tamtaji et al., 2019; Ibrahim et al., 2020). Also, the non-motor symptoms score (NMSS) is a scale that informs the severity of the PD patient’s non-motor symptoms. The score ranges from 0 to 360; higher scores define severe deterioration (Van Wamelen et al., 2021). Ibrahim et al. (2020) reported decreasing scores of NMSS after 8 weeks use of probiotics.
In other words, the probiotics used decreased these scales in PD, proving that probiotics may help by reducing the severity of the disease.
Oxidative stress
Glutathione (GSH) is a tripeptide amino acid and a powerful antioxidant in the cells and tissues of the body. It consists of the amino acids L-cysteine, L-glutamate, and glycine. Glutathione protects the organism by eliminating free radicals and reactive oxygen species (Smeyne & Smeyne, 2013). In two studies, GSH was measured. Tamtaji et al. (2019) reported that 12 weeks use of B. bifidum, L. acidophilus, L. fermentum, and L. reuteri (2×109 CFU/g) supplement increased GSH levels in plasma. Borzobadi et al. (2018) reported insignificant increases in GSH levels after 12 weeks use of B. bifidum, L. acidophilus, L. fermentum, and Lactobacillus reuter (8×109 CFU/d probiotic). Moreover, malondialdehyde (MDA) is a highly reactive molecule that is an oxidative stress biomarker. Tamtaji et al. (2019) reported decreases in MDA after 12 weeks of probiotic use, and inflammation biomarker C- reactive protein (CRP) remarkably dropped.
This finding supported the idea that probiotics can protect oxidative stress by increasing GSH levels and decreasing MDA and CRP levels.
Gastrointestinal system
Gastrointestinal problems are frequent in PD, and the whole GI tract is affected mainly by numerous complications (Fasano et al., 2015).
Ibrahim et al. (2020) reported increased bowel opening frequency (BOF) and gut transit time (GTT) decreased in PD patients after 8 weeks of usage of L. acidophilus, L. Casei, L. lactis, B. infantis, B. longum. In addition, following 4 weeks of using fermented milk containing several probiotic strains and prebiotic fiber, Barichella et al. (2016) reported an increase in complete bowel movement. After 4 weeks of using a probiotic pill containing 10 billion CFU of eight different commercially available bacterial strains (L. reuteri, L. acidophilus, Lactobacillus gasseri, B. bifidum, Lactobacillus rhamnosus, B. longum, Enterococcus faecium, Enterococcus faecalis), spontaneous bowel movements increased. Also, probiotic supplements increased stool consistency and decreased constipation. On the other hand, the quality of life-related to constipation decreased (Tan et al., 2020).
As a result, supplements containing probiotics helped to regulate GI problems regardless of the time of administration but did not affect the quality of life due to constipation. However, the data in existing studies are very limited.
Quality appraisal of included studies
The average score obtained from the modified Jadad scale was calculated as 4.8 points (range 4-5 points). Five studies were classified as “good” methodological quality. Individual scores are shown in Table 2.

4. Discussion
This systematic review investigates the effects of probiotic/prebiotic consumption in PD patients, which is a priority study in this area. Despite little evidence, the included five trials in this systematic review of PD individuals recommended the beneficial effects of probiotics in the clinical setting. Also, GI functions are highly related to probiotics. However, the evidence obtained from the RTCs about PD is limited. Similar to our results, in a systematic review, certain probiotics are beneficial in treating GI tract problems. Specific probiotics have been demonstrated to alleviate irritable bowel syndrome and lower gastrointestinal symptoms, prevent diarrhea associated with antibiotics and H. pylori eradication therapy, and demonstrate appropriate safety (Hungin et al., 2018). Kesika et al. (2020) reported the effect of probiotics on Alzheimer disease and the gut-brain axis; it was found that probiotic consumption prevented inflammation, reduced oxidative stress, and improved memory in Alzheimer patients. Amirani et al. (2020) conducted a systematic study of the effects of probiotic supplementation on mental health, inflammatory biomarkers, and oxidative stress in psychiatric patients. Probiotic supplementation has a beneficial impact on the Hamilton depression rating scale, CRP, interleukin-10, and MDA levels but no effect on inflammation and oxidative stress indicators. Similarly, another study showed that GSH levels increased, and MDA and CRP levels dropped by using probiotics (Tamtaji et al., 2019).
Although with low evidence, data from three RCTs involving 161 individuals with Alzheimer disease who received Lactobacillus and Bifidobacterium strains from the diet showed no benefit of probiotic supplementation on cognitive functions (standardized mean difference, 0.56; 95% CI, -0.06%, 1.18%) (Krüger et al., 2021). The MDA-UPDRS and NMSS were improved after usage of probiotics (Ibrahim et al., 2020). In the chronic phase of PD, cognition and brain functions may be associated. On the other hand, probiotic supplementation improved plasma triglycerides, very low-density lipoprotein cholesterol, insulin resistance, and plasma MDA in another trial. There were no RCTs that looked at synbiotic supplementation or microbiota compositions. The evidence for probiotics and synbiotics in treating dementia and cognition is insufficient to justify their use in clinical practice (Krüger et al., 2021).
On the other hand, although probiotics have many benefits, they can also cause some complications. A systematic review assessed probiotic use problems in 60 case reports and 7 case series involving 93 individuals. Fungi were the most common infectious complication in 35(37.6%) cases, with Saccharomyces genus being the most common genus with 47(50.6%) cases, followed by Lactobacillus, Bifidobacterium, Bacillus, Pedioccocus, and Escherichia coli with 26(27.9%), 12(12.8%), 5(5.4%), 2(2.2%), and 1(1.1%) cases, respectively (Costa et al., 2018). Also, Rao et al. investigated brain fogginess (BF), gas, and bloating dependence with D-lactate in small intestinal bacterial overgrowth (SIBO). SIBO was more common in the BF group than in the non-BF group (68% vs 28%, P=0.05). Probiotics were used by everyone in the BF group. D-lactic acidosis was more common in the BF group than in the non-BF group (77% vs 25%, P=0.006). In 20/30 (66%) of the patients, BF was replicated. In 10/30 (33%) of patients with BF and 2/8 (25%) of those without, gastrointestinal transit was delayed. The results of the other metabolic tests were normal. However, BF was resolved, and gastrointestinal symptoms improved considerably (P=0.005) in 23/30 people after stopping probiotics and using antibiotics (77%). In a group without short bowel syndrome, BF, gas, and bloating may be linked to probiotic use, SIBO, and D-lactic acidosis (Rao et al., 2018). However, the probiotic does not include Neisseria streptococcus, Staphylococcus, or Hemophilus strains in the predominant organisms discovered by culture in duodenal aspirates. However, there is no data to confirm that the patients who applied are acidotic. It should be noted that many Lactobacilli and all Bifidobacteria exclusively create L-lactate and not D-lactate (Petrova et al., 2018; Quigley et al., 2018; Reid et al., 2019). Probiotics may trigger bacterial translocation and enterocyte damage in patients with acute pancreatitis, resulting in organ failure (Besselink et al., 2004; Besselink et al., 2009). Still, in a later study, it was found that probiotics did not cause any contraindications. Conversely, carbohydrates may worsen the situation with lactic acidosis caused by their fermentation (Bongaerts & Severijnen, 2016). Established tests and clinical guidelines in treating high-risk adults and children are essential, as probiotics can produce different effects in different health conditions (Sanders et al., 2016). More importantly, probiotic complications in PD are unknown, but the impact on neurological diseases may also be an indicator of PD.
Furthermore ,it is essential to emphasize that there are some limitations to consider when designing future interventions (1). Although this study was conducted through a wide literature search (27395 records), the studies that could be included in probiotic use in PD patients were low and insufficient. Therefore, the systematically compiled parameters are limited and unsuitable for comparison (2). The probiotic and the active bacteria in its content are stated in our study. Still, it is impossible to identify which bacteria can be better and provide more benefits to PD patients due to the lack of existing studies (3). Bacterial colony numbers vary from study to study, and since there is no standard yet, no interpretation of the amount of CFU could be made (4). Positive results in the researched area have been revealed so that this area can be pursued, and more profound research is needed (5). A comprehensive systematic review forms the basis of new studies on the amount of probiotic usage, duration, and variety of clinically supportive nutritional support for PD patients (6).
5. Conclusion
A growing body of data suggests that the gut-brain bidirectional connection and dysbiosis have a role in PD. A healthy gut microbiome may lower the chance of acquiring a variety of human diseases, including PD. α-synuclein build-up in PD has been shown to begin in the enteral nervous system and move to the central nervous system by trans-synaptic cell-to-cell transmission. As previously noted, creating a proinflammatory environment in dysbiosis can signal the brain via systemic routes and may cause a defective blood-brain barrier. Finally, it was suggested that an immune system imbalance in the host might be partially responsible for PD’s motor and non-motor symptoms. The hunt for early biomarkers and innovative treatment methods has accelerated due to an improved understanding of PD pathophysiology.
In this systematic review, probiotics used in PD are reviewed. As a result, various probiotic supplements were used for 4-12 weeks in PD rating, oxidative stress, and GI tract symptoms such as spontaneous bowel movement, GTT, BOF, abdominal pain, bloating, constipation, and improvements in each parameter were observed. Although the studies included in this study are of high methodological quality, the number of studies is insufficient. Therefore, some issues need to be included in the literature, from different probiotic strains, parameters, and duration of use to clinical studies of probiotics in PD.”
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.”
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to express their gratitude to the Üsküdar University and NP Brain Hospital for their support”
References
Amirani, E., Milajerdi, A., Mirzaei, H., Jamilian, H., Mansournia, M. A., & Hallajzadeh, J., et al. (2020). The effects of probiotic supplementation on mental health, biomarkers of inflammation and oxidative stress in patients with psychiatric disorders: A systematic review and meta-analysis of randomized controlled trials. Complementary Therapies in Medicine, 49, 102361. [DOI:10.1016/j.ctim.2020.102361] [PMID]
Balestrino, R., & Schapira, A. H. V. (2020). Parkinson disease. European Journal of Neurology, 27(1), 27-42. [DOI:10.1111/ene.14108] [PMID]
Barichella, M., Pacchetti, C., Bolliri, C., Cassani, E., Iorio, L., & Pusani, C., et al. (2016). Probiotics and prebiotic fiber for constipation associated with Parkinson disease: An RCT. Neurology, 87(12), 1274-1280. [DOI:10.1212/WNL.0000000000003127] [PMID]
Besselink, M. G., Timmerman, H. M., Buskens, E., Nieuwenhuijs, V. B., Akkermans, L. M., & Gooszen, H. G., et al. (2004). Probiotic prophylaxis in patients with predicted severe acute pancreatitis (PROPATRIA): Design and rationale of a double-blind, placebo-controlled randomised multicenter trial [ISRCTN38327949]. BMC Surgery, 4, 12. [DOI:10.1186/1471-2482-4-12] [PMID]
Besselink, M. G., van Santvoort, H. C., Renooij, W., de Smet, M. B., Boermeester, M. A., & Fischer, K., et al. (2009). Intestinal barrier dysfunction in a randomized trial of a specific probiotic composition in acute pancreatitis. Annals of Surgery, 250(5), 712-719. [DOI:10.1097/SLA.0b013e3181bce5bd] [PMID]
Bongaerts, G. P., & Severijnen, R. S. (2016). A reassessment of the PROPATRIA study and its implications for probiotic therapy. Nature Biotechnology, 34(1), 55-63. [DOI:10.1038/nbt.3436] [PMID]
Borzabadi, S., Oryan, S., Eidi, A., Aghadavod, E., Daneshvar Kakhaki, R., & Tamtaji, O. R., et al. (2018). The effects of probiotic supplementation on gene expression related to inflammation, insulin and lipid in patients with Parkinsonâ s disease: A randomized, double-blind, placebo-controlled trial. Archives of Iranian Medicine, 21(7), 289-295. [PMID]
Costa, R. L., Moreira, J., Lorenzo, A., & Lamas, C. C. (2018). Infectious complications following probiotic ingestion: a potentially underestimated problem? A systematic review of reports and case series. BMC Complementary and Alternative Medicine, 18(1), 329. [DOI:10.1186/s12906-018-2394-3] [PMID]
Ding, X., Gao, J., Xie, C., Xiong, B., Wu, S., & Cen, Z., et al. (2018). Prevalence and clinical correlation of dysphagia in Parkinson disease: A study on Chinese patients. European Journal of Clinical Nutrition, 72(1), 82-86. [DOI:10.1038/ejcn.2017.100] [PMID]
Fasano, A., Visanji, N. P., Liu, L. W., Lang, A. E., & Pfeiffer, R. F. (2015). Gastrointestinal dysfunction in Parkinson’s disease. The Lancet Neurology, 14(6), 625-639. [DOI:10.1016/S1474-4422(15)00007-1] [PMID]
Goetz, C. G., Tilley, B. C., Shaftman, S. R., Stebbins, G. T., Fahn, S., & Martinez-Martin, P., et al. (2008). Movement disorder society‐sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS‐UPDRS): Scale presentation and clinimetric testing results. Movement Disorders: Official Journal of the Movement Disorder Society, 23(15), 2129-2170. [PMID]
Hungin, A. P., Mulligan, C., Pot, B., Whorwell, P., Agréus, L., & Fracasso, P., et al. (2013). Systematic review: Probiotics in the management of lower gastrointestinal symptoms in clinical practice -- an evidence-based international guide. Alimentary Pharmacology & Therapeutics, 38(8), 864–886. [DOI:10.1111/apt.12460] [PMID]
Ibrahim, A., Ali, R. A. R., Manaf, M. R. A., Ahmad, N., Tajurruddin, F. W., & Qin, W. Z., et al. (2020). Multi-strain probiotics (Hexbio) containing MCP BCMC strains improved constipation and gut motility in Parkinson’s disease: A randomised controlled trial. Plos One, 15(12), e0244680. [DOI:10.1371/journal.pone.0244680] [PMID]
Jadad, A. R., Moore, R. A., Carroll, D., Jenkinson, C., Reynolds, D. J., & Gavaghan, D. J., et al. (1996). Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Controlled Clinical Trials, 17(1), 1-12. [DOI:10.1016/0197-2456(95)00134-4] [PMID]
Kesika, P., Suganthy, N., Sivamaruthi, B. S., & Chaiyasut, C. (2021). Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sciences, 264, 118627. [DOI:10.1016/j.lfs.2020.118627] [PMID]
Krüger, J. F., Hillesheim, E., Pereira, A. C. S. N., Camargo, C. Q., & Rabito, E. I. (2021). Probiotics for dementia: A systematic review and meta-analysis of randomized controlled trials. Nutrition Reviews, 79(2), 160-170. [DOI:10.1093/nutrit/nuaa037] [PMID]
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., & Prisma Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS medicine, 6(7), e1000097. [DOI: 10.1371/journal.pmed.1000097] [PMID]
Parashar, A., & Udayabanu, M. (2017). Gut microbiota: Implications in Parkinson’s disease. Parkinsonism & Related Disorders, 38, 1-7. [DOI:10.1016/j.parkreldis.2017.02.002] [PMID]
Petrova, M. I., Macklaim, J. M., Wuyts, S., Verhoeven, T., Vanderleyden, J., & Gloor, G. B., et al. (2018). Comparative genomic and phenotypic analysis of the vaginal probiotic lactobacillus rhamnosus GR-1. Frontiers in Microbiology, 9, 1278. [DOI:10.3389/fmicb.2018.01278] [PMID]
Quigley, E. M. M., Pot, B., & Sanders, M. E. (2018). ‘Brain Fogginess’ and D-Lactic Acidosis: Probiotics are not the cause. Clinical and Translational Gastroenterology, 9(9), 187. [DOI:10.1038/s41424-018-0057-9] [PMID]
Rao, S., S. S. C., Rehman, A., Yu, S., & Andino, N. M. (2018). Brain fogginess, gas and bloating: A link between SIBO, probiotics and metabolic acidosis. Clinical and Translational Gastroenterology, 9(6), 162. [DOI:10.1038/s41424-018-0030-7] [PMID]
Reid, G., Gadir, A. A., & Dhir, R. (2019). Probiotics: Reiterating what they are and what they are not. Frontiers in Microbiology, 10, 424. [DOI:10.3389/fmicb.2019.00424] [PMID]
Sanders, M. E., Merenstein, D. J., Ouwehand, A. C., Reid, G., Salminen, S., & Cabana, M. D., et al. (2016). Probiotic use in at-risk populations. Journal of the American Pharmacists Association: JAPhA, 56(6), 680-686. [DOI:10.1016/j.japh.2016.07.001] [PMID]
Schrag, A., Jahanshahi, M., & Quinn, N. (2000). What contributes to quality of life in patients with Parkinson’s disease?. Journal of Neurology, Neurosurgery & Psychiatry, 69(3), 308-312. [DOI:10.1136/jnnp.69.3.308] [PMID]
Shoemark, D. K., & Allen, S. J. (2015). The microbiome and disease: Reviewing the links between the oral microbiome, aging, and Alzheimer’s disease. Journal of Alzheimer’s Disease, 43(3), 725-738. [DOI:10.3233/JAD-141170] [PMID]
Smeyne, M., & Smeyne, R. J. (2013). Glutathione metabolism and Parkinson’s disease. Free Radical Biology and Medicine, 62, 13-25. [DOI:10.1016/j.freeradbiomed.2013.05.001] [PMID]
Tamtaji, O. R., Taghizadeh, M., Kakhaki, R. D., Kouchaki, E., Bahmani, F., Borzabadi, S.,... & Asemi, Z. (2019). Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Clinical Nutrition, 38(3), 1031-1035. [DOI:10.1016/j.clnu.2018.05.018] [PMID]
Tan, A. H., Lim, S. Y., Chong, K. K., Manap, M. A. A. A., Hor, J. W., & Lim, J. L., et al. (2021). Probiotics for constipation in Parkinson Disease: A randomized placebo-controlled study. Neurology, 96(5), e772-e782. [DOI:10.1212/WNL.0000000000010998]
Van Wamelen, D. J., Martinez-Martin, P., Weintraub, D., Schrag, A., Antonini, A., & Falup-Pecurariu, C., et al. (2021). The Non‐Motor Symptoms Scale in Parkinson’s disease: Validation and use. Acta Neurologica Scandinavica, 143(1), 3-12. [DOI:10.1111/ane.13336] [PMID]
Zotero. (2020). Zotero|your personal research assistant [internet]. Retrived from: [Link]
Type of Study: Review |
Subject:
Clinical Neuroscience
Received: 2021/03/7 | Accepted: 2021/10/26 | Published: 2024/03/1
Received: 2021/03/7 | Accepted: 2021/10/26 | Published: 2024/03/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





