Volume 15, Issue 1 (January & February 2024)
BCN 2024, 15(1): 89-100 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Babaee M, Atashgar K, Amini Harandi A, Yousefi A. Prediction of Stroke After the COVID-19 Infection. BCN 2024; 15 (1) :89-100
URL: http://bcn.iums.ac.ir/article-1-2247-en.html
URL: http://bcn.iums.ac.ir/article-1-2247-en.html
1- Faculty of Industrial Engineering, Malek Ashtar University of Technology, Tehran, Iran.
2- Brain Mapping Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Department of Neurology, Shohadaye Tajrish Hospital, Shahid Beheshti University of Medical Science, Tehran, Iran.
2- Brain Mapping Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Department of Neurology, Shohadaye Tajrish Hospital, Shahid Beheshti University of Medical Science, Tehran, Iran.
Full-Text [PDF 623 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Numerous studies have been published on the COVID-19 pandemic. There have been reports of stroke occurrences in patients with this virus, showing inconsistent results. The connection between coronaviruses as endemic diseases and various neurological disorders is being closely examined (Matías-Guiu et al., 2020). A historical analysis of different types of coronaviruses by Trejo-Gabriel-Galán (2020), indicates that the three known types of coronaviruses associated with acute respiratory symptoms and high mortality rates are linked to neurological complications. These include the SARS-CoV virus in 2003, the MERS-CoV virus in 2012, and COVID-19, which emerged in December 2019 in Wuhan, China. A review article documented 5 cases of stroke among 206 patients in Singapore and another 5 cases among 664 Taiwanese patients during the 2003 epidemic (Trejo-Gabriel-Galán, 2020). The literature indicates several neurological advents, including cerebrovascular accidents in patients with COVID-19. Due to the possibility of the virus entering into the nerve cells, by several mechanisms, such as interfering with coagulation or invasion of vessel walls, in addition to stroke, other neurological manifestations, such as dizziness, anosmia, impaired consciousness, seizures (Ling et al., 2020), Guillain–Barre syndrome (Hua et al., 2020), encephalitis (Pilotto et al., 2020), hyposmia (Lechien et al., 2020) and headache have also been observed in some patients with COVID-19. The virus can cause strokes by impairing coagulation when it enters the heart and brain. However, strokes are not a primary manifestation of COVID-19, indicating that patients with stroke may present with COVID-19 even in the absence of respiratory symptoms (Trejo-Gabriel-Galán, 2020).
Many deaths associated with COVID-19 occur in patients with cardiovascular and cerebrovascular conditions (Viguier et al., 2020). In some cases, the coronavirus invades the central nervous system instead of the respiratory system. A study of four patients in Sakarya, Turkey, who experienced strokes concurrent with a COVID-19 diagnosis, indicated that cerebrovascular diseases can occur simultaneously and independently of the COVID-19 process (TunÇ et al., 2020). According to another study in Wuhan, China (Jin et al., 2020), COVID-19 also may involve the nervous system, and the patients whose nervous system is involved may not be easily diagnosed in the early stages of the disease. This study led to the development of clinical guidelines aimed at helping neurologists diagnose the infection earlier and protect treatment staff.
Although neurological involvement is not common in COVID-19, symptoms such as encephalopathy, impaired consciousness, and skeletal muscle injury may indicate acute cerebrovascular disease (Ling et al., 2020). Evidence suggests that there is a 2.5-fold increase in the risk of developing severe COVID-19 in patients with a history of stroke (Lee et al., 2020), prompting clinicians to be vigilant for COVID-19 in cases of cerebrovascular accidents. Scientific reports emphasize the need for research to enhance our understanding of the neurological implications of COVID-19 (Avula et al., 2020). Nonetheless, numerous studies have confirmed that comorbidities such as cancer, diabetes, cardiovascular disorders, and hypertension increase the risk of developing more severe COVID-19 and mortality (Wu et al., 2020; Wang et al., 2020; Guan et al., 2020). Several studies have investigated the link between stroke and COVID-19: A retrospective study on 11 patients with coronavirus pneumonia, who were diagnosed with stroke based on neurological symptoms and confirmed by imaging, showed that the incidence of stroke in COVID-19 patients was significantly higher than the average level in the general population. The incidence of stroke increased compared to the period before the pandemic in China (Chen et al., 2020). A study was conducted in India on 3923 patients with COVID-19, of whom 62 cases were patients with stroke and the majority had carotid territory infarcts. The logistic regression model indicated that a low Glasgow coma score (GCS) and the need for respiratory support were predictors of in-hospital mortality (Sundar et al., 2021). A study in the United Kingdom examined the risk factors, assessed the incidence, described clinical radiological manifestations, and evaluated the outcome of strokes in COVID-19 patients. Data were collected from studies reporting >5 strokes in COVID-19 patients. The findings suggested that acute cerebrovascular disease is not rare in COVID-19 patients, especially those with a history of vascular risk factors, and cerebral thrombosis and/or thromboembolism could be potential causes of this condition (Nannoni et al., 2021).
Studies have shown that although stroke is not a common complication of COVID-19, its occurrence often results in significant complications and mortality. Stroke in patients with COVID-19 was related to older age, comorbidities, and disease severity (Siow et al., 2021).
The aim of the present study was to predict the incidence of stroke in patients with COVID-19 using logistic regression model analysis and identify the most important significant factors according to clinical features and effective blood markers.
2. Materials and Methods
Data collection
The data collection method for patients with confirmed COVID-19 in this study utilized a non-random, prospective registration approach. Confirmation of COVID-19 in these patients was achieved through polymerase chain reaction (PCR) testing and evidence of positive lung involvement. This study analyzed the demographic information, clinical characteristics, and laboratory parameters of 128 patients with COVID-19 who were referred to Shohada-e-Tajrish Hospital (Tehran, Iran) from March to September 2020. The data were obtained from the emergency department (ED), reports of the intensive care unit (ICU), and reports of the admission department of the hospital.
The classification analysis revealed the following results: 1) 28 of the patients (21.87%) exhibited stroke symptoms, 2) 52 patients (42%) were females with an average age of 57.109±15.97, ranging from 18 to 87 years, 3) The mean age of patients with ischemic stroke was 57.96±16.82 and 4) The mean age of patients without ischemic stroke was 56.87±15.8.
Patients were included in this study after laboratory confirmation of COVID-19. Clinical features were defined following consultations with neurologists who were part of the treatment team for patients with stroke and COVID-19. After diagnosis, the treatment was followed according to the severity of the disease and naturally was different for each patient, but in general, the following protocol was performed for all patients:
Vitamin D3 supplementation, famotidine 40 mL taken twice daily, systemic corticosteroids, naproxen, and respiratory treatment, if needed (patients with stroke needed oxygen therapy and ventilator more frequently than others).
Variables
Demographic variables, including age and sex, the onset date of COVID-19 symptoms, the date of a positive COVID-19 test, ventilator dependency, comorbidities/risk factors, and laboratory tests including white blood cell (WBC) count, neutrophil count, lymphocyte count (count×109/L, reference value (RV): 0.9–5.2), platelet count (count×109/L, RV: 150–400), C-reactive protein (mg/L, RV: 1–5), blood urea nitrogen, creatinine (U/L, RV: 20–170), alanine transaminase (ALT), aspartate transaminase (AST), and lactate dehydrogenase (LDH) were collected for analysis. We analyzed comorbidities, including hypertension (systemic blood pressure higher than 140/90 mm Hg), current smoking status, diabetes (fasting blood glucose >126 mg/dL on two separate tests, HbA1c >6,5%, blood glucose levels >200 mg after oral glucose overload or blood glucose levels >200 mg/dL with diabetes symptoms), chronic kidney disease, active neoplasm, and rheumatological disease. Given their significance in neurological disorders, cardiovascular or cerebrovascular diseases, such as ischemic heart disease, atrial fibrillation, carotid stenosis, cardiac ejection fraction <40%, and history of stroke/transient ischemic attack (TIA) were considered as comorbidities (Wang et al., 2020; Chen et al., 2020). Table 1 displays the demographic variables, clinical features, comorbidities, and laboratory test results. Table 1 shows demographic variables, clinical features, comorbidities, and laboratory test results.

Statistical analysis
In this research identification of important and influential variables on stroke incidence was analyzed using logistic regression analysis. This method is capable of leading physicians to predict the incidence of stroke in patients with COVID-19. Ordinal and qualitative variables are defined in this approach as numbered classes. In this analysis, single mean imputation was used to manage missing data (Papageorgiou et al., 2018). Hosmer and Lemeshow’s test was also used to investigate the model’s goodness of fit. Statistical analyses were conducted using SPSS software, version 26, with results reported in terms of OR, P<0.05, and 95% CI.
3. Results
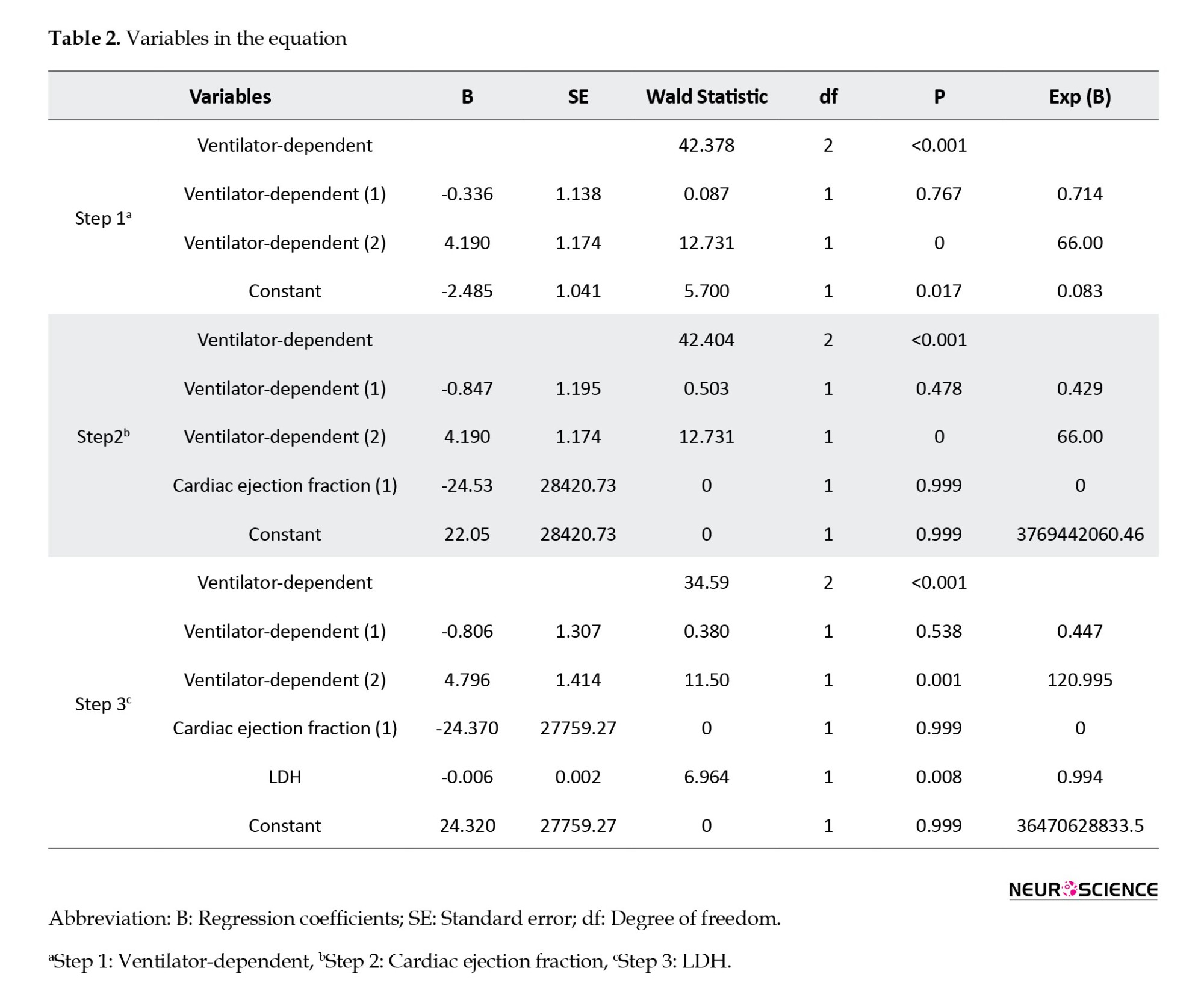
The analysis of stroke patients revealed that, on average, stroke occurred 5 days (ranging from 0 to 17 days) after the onset of COVID-19 symptoms. The Wald statistic examines the significance of the presence of each independent variable in the model, which we can find out through its significance level. The Wald statistic functions similarly to the t-statistic in linear regression. The interpretation is such that if P<0.05 for a variable, it suggests that the inclusion of that variable in the model is meaningful and its effect is statistically significant. Exp (B) known as the OR, reflects the ratio of the odds of an event occurring to the odds of it not occurring. An OR<1 suggests that as the values of the independent variable increase, the probability of the event occurring decreases (negative effect). Conversely, an OR>1 indicates that as the values of the independent variable increase, the probability of the event occurring also increases (positive effect).
Ventilator dependency, cardiac ejection fraction <40, and LDH were associated with the occurrence of stroke and could predict its occurrence. The regression model illustrating the relationship between strokes and significant variables is presented as Equation 1:
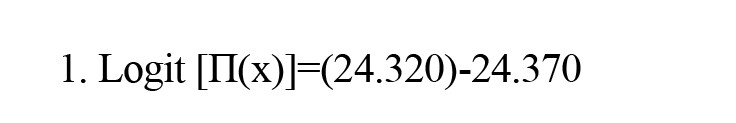
(Cardiac ejection fraction: NO)+4.796 (Ventilator dependent: YES)-0.806 (Ventilator dependent: NO)-0.006 (LDH)
In Equation 2, “cardiac ejection fraction: No” is denoted by x1, “ventilator dependent: Yes” is addressed by x2, “ventilator dependent: NO“ is indicated by x3, and LDH is presented by x4:

In Equation 3, Π(x) is the predicted value for the model output in the range of 0 to 1.

This model clearly identifies cardiac ejection fraction, ventilator dependency, and LDH as independent variables, with a constant coefficient (i.e. intercept) of the functional model being 24.320. According to Table 2, in the final analysis, these three variables remained significant in the model, with the P for ventilator dependency and LDH being <0.05, making them statistically significant, whereas the cardiac ejection fraction is considered clinically significant and important.
As detailed in Table 2, the Exp (B) value for ventilator use exceeds one (120.995), indicating that an increase in the probability of ventilator use is associated with a higher risk of stroke. This underscores a positive correlation between ventilator use and the incidence of stroke.
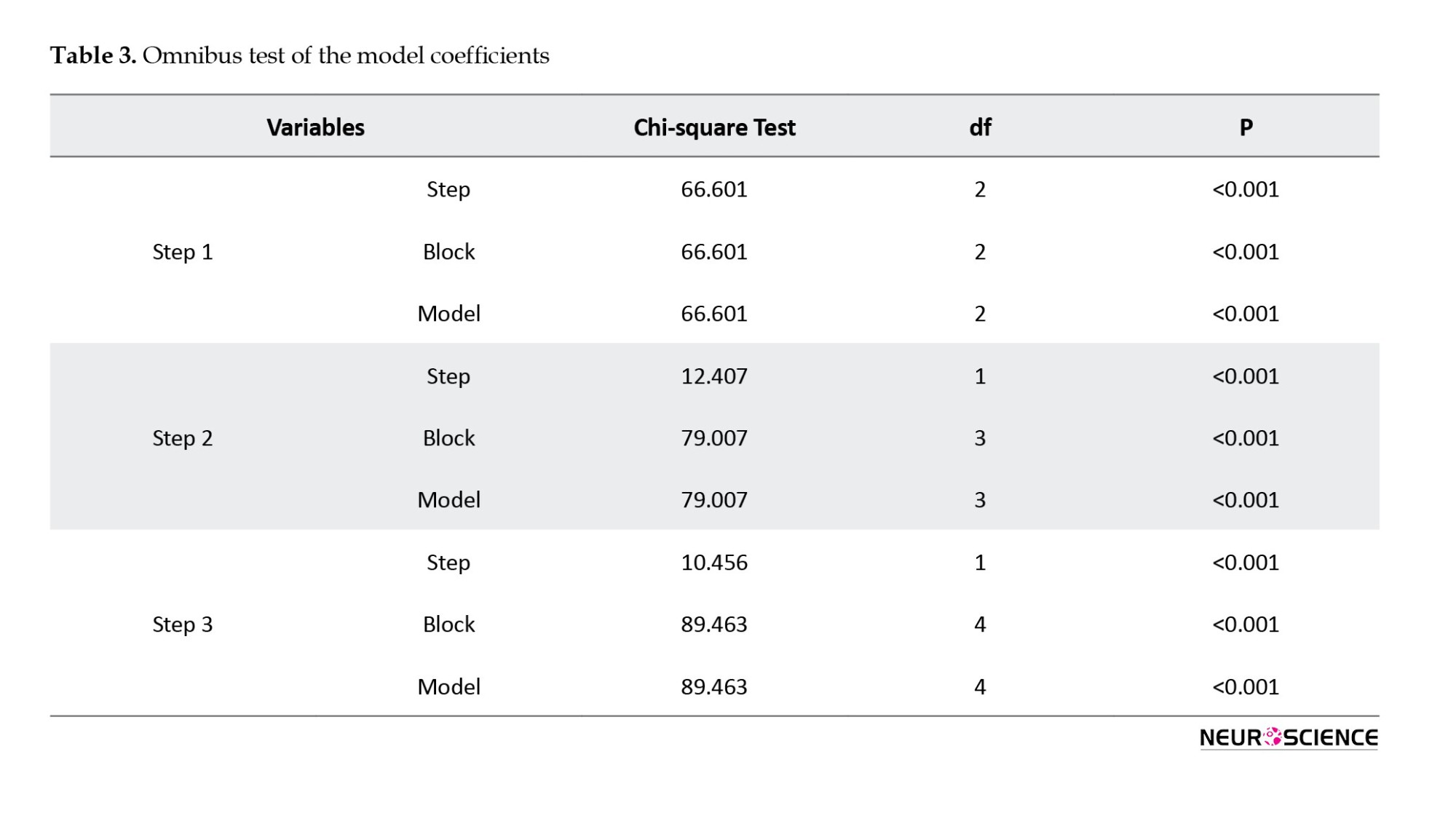
The results of the omnibus test showed the model’s acceptable predictive capability. Table 3 addresses the results of the omnibus test at the third stage. As shown in Table 3, the fitness of the proposed model is deemed acceptable showing significance at an error level of <0.05.
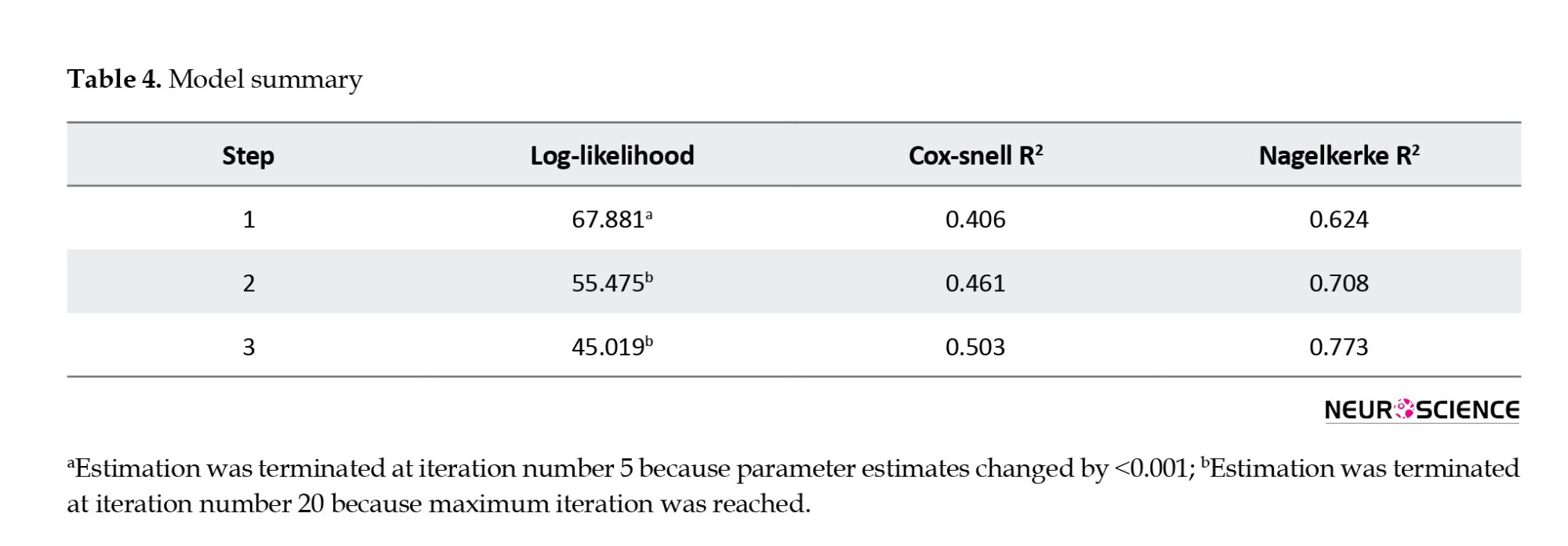
The Cox & Snell and Nagelkerke coefficients, as shown in Table 4, demonstrate that the independent variables in this study possess a relatively high capacity to explain the variance in the dependent variable (stroke). According to Table 4, the independent variables account for between 50.3% and 77.3% of the variability in stroke (the dependent variable).
The Hasmer-Lemeshow test was used to analyze the goodness of the proposed logistic regression model. According to Table 5, the significance level was >0.05, suggesting that the model fits well. This implies that the independent variables are effective in predicting variations in the dependent variable.
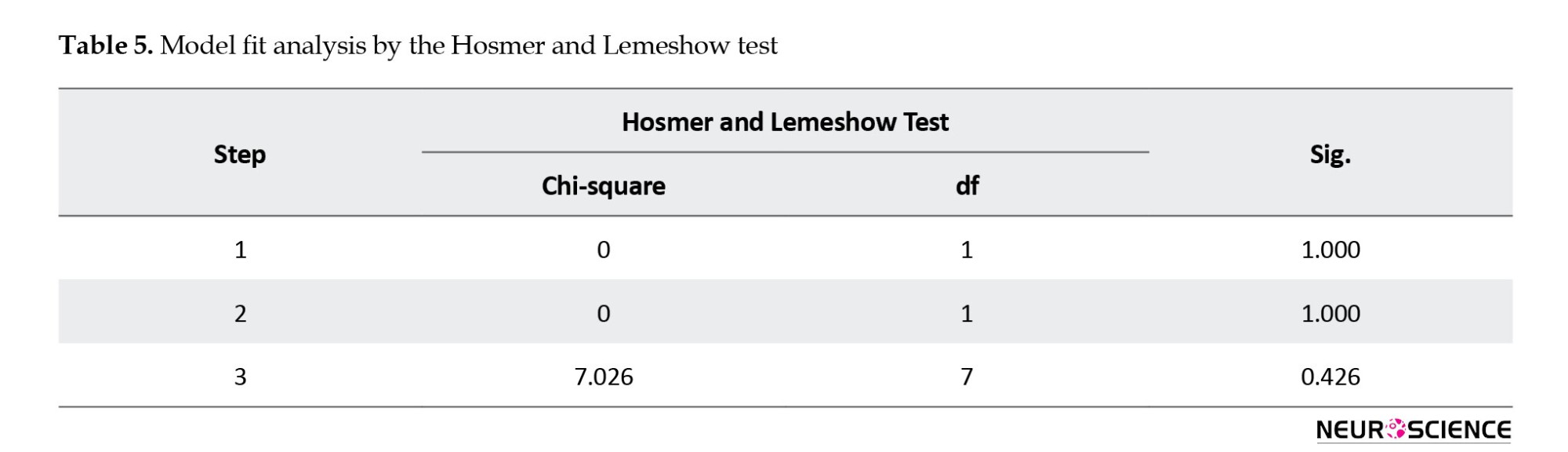
Table 6 reveals that the model’s classification accuracy stands at 93.8%. This means that the model can predict changes in the dependent variable (stroke incidence) with 93.8% confidence.
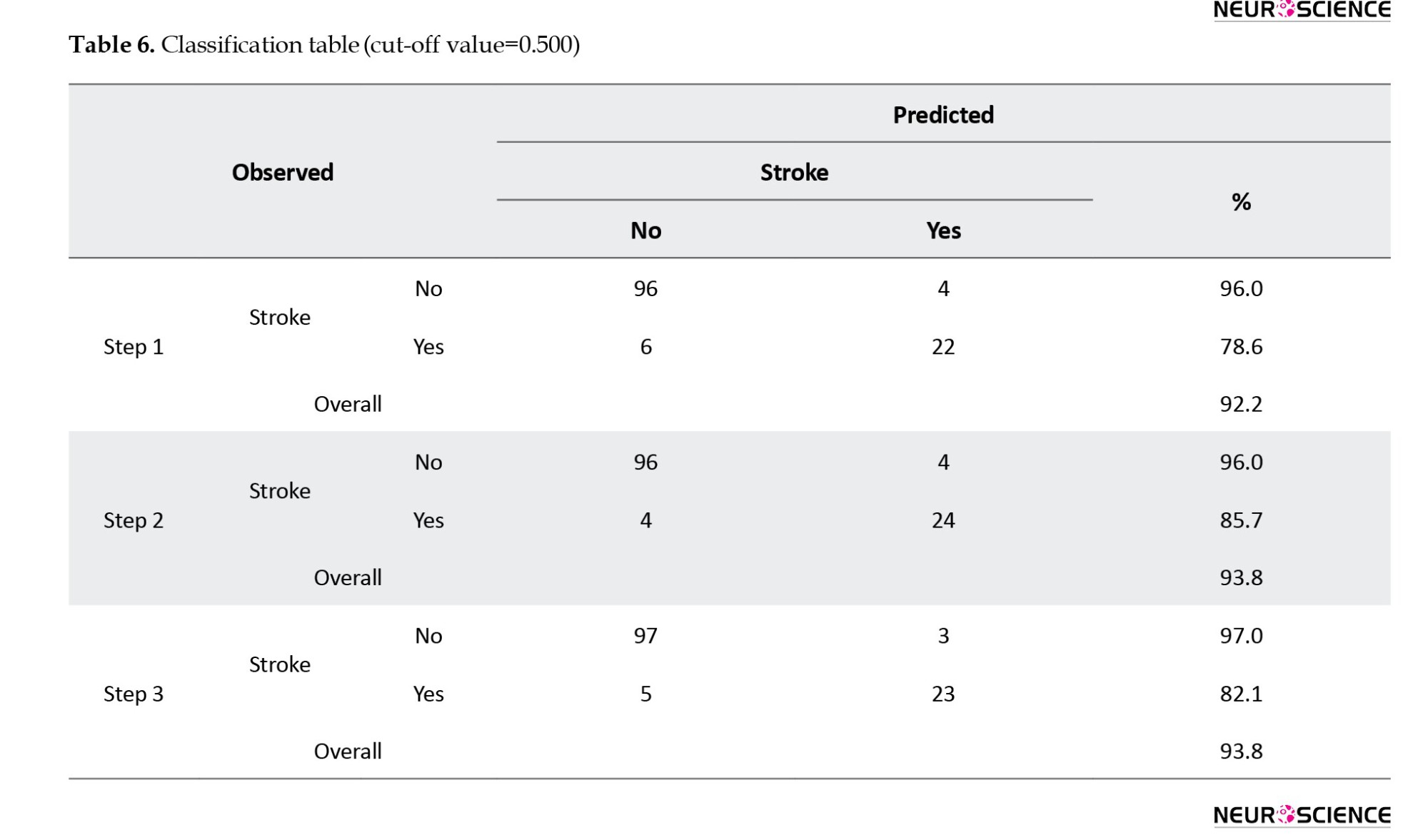
Due to the limitations of this study in collecting more data to investigate the generalizability of the model, 1000 samples were drawn from the original population of 128 subjects using replication and simple random sampling. This constitutes a bootstrap sample. The estimated coefficients were applied to this dataset. The ROC curve for the new data is illustrated in Figure 1, displaying an area under the curve (AUC) of 0.977 with approximately a 95% confidence interval. The AUC is significantly distinct from 0.5, indicating that the proposed logistic regression model significantly outperforms chance classification, as evidenced by a p-value of <0.001.
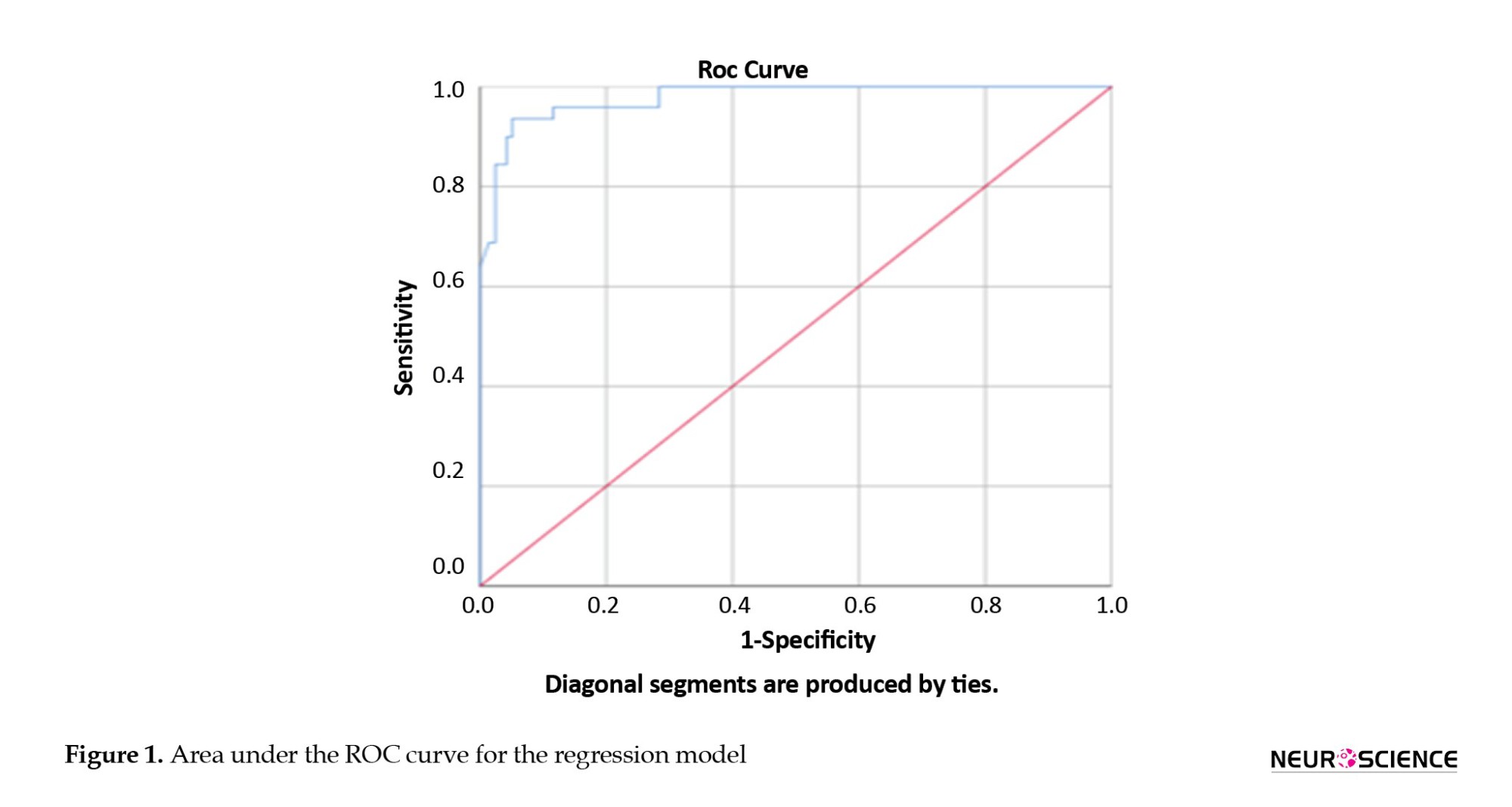
Table 7 presents the classification of bootstrap data in both classes.
Table 8 indicates that the area under the ROC curve AUC was 97%.
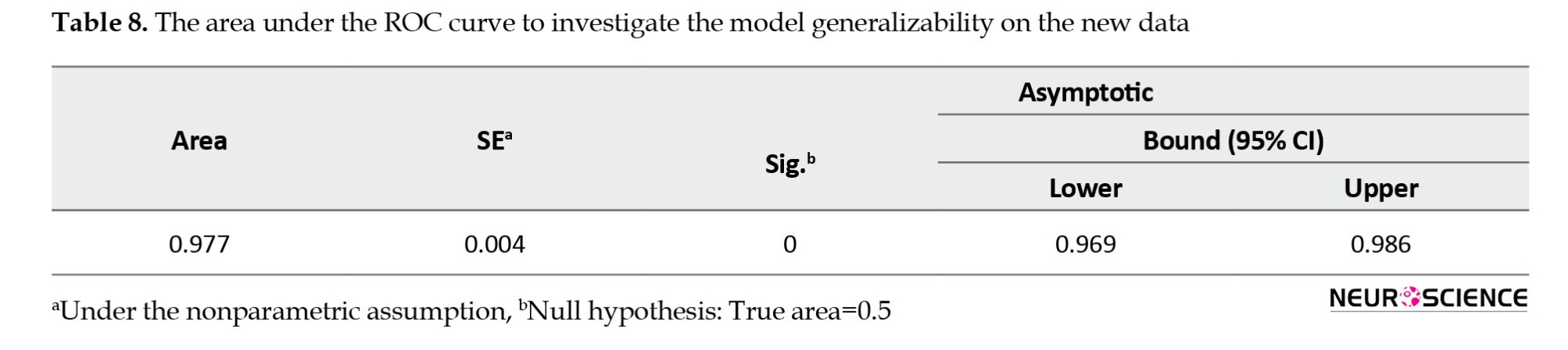
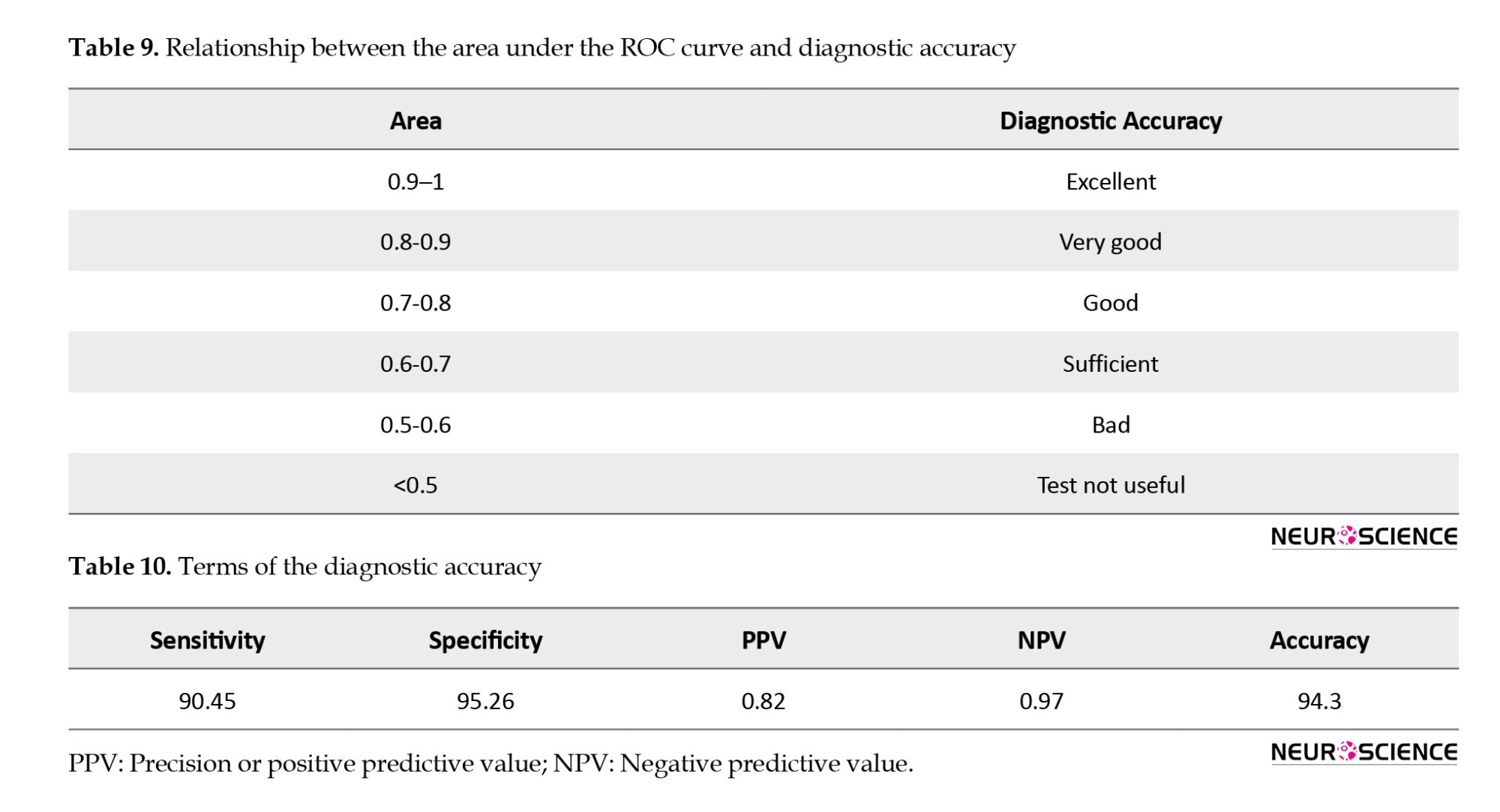
Table 9 also indicates the relationship between the AUC and diagnostic accuracy terms (Hosmer & Lemeshow, 2000). The proposed model showed an excellent capability of discrimination.
Table 10 presents the diagnostic accuracy terms, including sensitivity, specificity, and precision on the new data. The simultaneous attainment of optimal values for both sensitivity and specificity suggests that the model possesses satisfactory discriminative ability.
4. Discussion
While ischemic stroke is recognized as a complex complication of COVID-19, the specifics of this condition have not been fully elucidated. Severe COVID-19 can lead to the activation of endothelial and mononuclear cells, resulting in coagulation and thrombin production. A study from Johann-China (Beyrouti et al., 2020) observed neurological complications in 36% of 214 COVID-19 patients, noting ischemic strokes were more frequent in patients with severe COVID-19 compared to those with non-severe conditions (5.7% vs 0.8%) (Beyrouti et al., 2020). At the beginning of the COVID-19 pandemic, many articles have addressed the increased stroke risk among COVID-19 patients (Beyrouti et al., 2020; Wu et al., 2020; Steardo et al., 2020; Oxley et al., 2020; Zhang et al., 2020).
This research introduced a logistic functional model to predict the likelihood of stroke in COVID-19 patients. The study’s findings underscore the significance of ventilator use and the presence of a cardiac ejection fraction <40 and elevated LDH levels as independent predictors of stroke. The results are supported by a multicenter and multinational study involving 17,799 hospitalized SARS-CoV-2 patients, which investigated stroke risk and related factors in SARS-CoV-2. This extensive study, encompassing North and South America and various countries from Europe, Asia, and Oceania, found that mechanical ventilation and ischemic heart conditions are predictive of stroke (Shahjouei et al., 2020). To the best of the authors’ knowledge, this is the first study proposing a functional model to predict stroke incidence in COVID-19 patients, especially in Iran.
The sensitivity and specificity high values achieved by the proposed model demonstrate its proficiency in accurately diagnosing both stroke occurrences and non-stroke cases. Several studies have examined the relationship between stroke risk and respiratory infections (Arabi et al., 2015; Algahtani et al., 2016; Madjid et al., 2009). The likelihood of experiencing a stroke following an infection appears to be minimal, approximately 1% (Shahjouei et al., 2020), Also, according to the United States National Readmissions Database, based on data from 46,000 patients with influenza, the risk of stroke was 0.3% (Yandrapalli et al., 2018).
Patients with comorbidities, inflammatory markers, or those requiring vasopressors are more inclined to undergo mechanical ventilation (Goyal et al., 2020).
Identification of important characteristics in the occurrence of stroke can be beneficial for earlier diagnosis. The limited number of patients is one of the limitations of the present study. Further studies can lead researchers to develop the proposed model of this research. It is suggested that incorporating additional clinical factors could enhance the model’s development. We recommend that different techniques, such as genetic algorithms or machine learning can be used in this field of research.
5. Conclusion
This research introduced a logistic functional model to predict the incidence of stroke in COVID-19 patients. The literature indicates that stroke in COVID-19 patients often results in mortality. Numerical analysis of the results revealed that the proposed logistic regression model predicts stroke incidence with 93.75% accuracy and an AUC of 97%. The findings showed that ventilator dependence, a cardiac ejection fraction of <40, and elevated LDH are associated with the risk of stroke. The logistic model proposed in this study is effective in guiding physicians toward appropriate treatment strategies. Identifying significant clinical factors using the proposed model can aid in the early diagnosis of potential stroke cases.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This study was supported by the Malek Ashtar University of Technology and Shohada-e-Tajrish Hospital affiliated with the Shahid Beheshti University of Medical Sciences.
Authors' contributions
Conceptualization, investigation, software calculation and writing: Mahsa Babaee; Data collection: Ali Amini Harandi and Atefeh Yousefi; Data analysis: Mahsa Babaee and Ali Amini Harandi; Supervision: Karim Atashgar and Ali Amini Harandi.
Conflict of interest
The authors declared no conflicts of interests.
References
Algahtani, H., Subahi, A., & Shirah, B. (2016). Neurological complications of Middle East Respiratory Syndrome coronavirus: A report of two cases and review of the literature. Case Reports in Neurological Medicine, 2016, 3502683. [DOI:10.1155/2016/3502683] [PMID]
Avula, A., Nalleballeb, K., Narulaa, N., Sapozhnikov, S., Dandu, V., & Toom, S., et al. (2020). COVID-19 presenting as stroke. Brain, Behavior, and Immunity, 87, 115-119. [DOI:10.1016/j.bbi.2020.04.077] [PMID]
Arabi, Y. M., Harthi, A., Hussein, J., Bouchama, A., Johani, S., & Hajeer, A. H., et al. (2015). Severe neurologic syndrome associated with Middle East respiratory syndrome coronavirus (MERS - CoV). Infection, 43(4), 495-501. [DOI:10.1007/s15010-015-0720-y] [PMID]
Beyrouti, R., Adams, M. E., Benjamin, L., Cohen, H., Farmer, S. F., & Goh, Y. Y., et al. (2020). Characteristics of ischaemic stroke associated with COVID-19. Journal of Neurology, Neurosurgery, and Psychiatry, 91(8), 889–891. [DOI:10.1136/jnnp-2020-323586] [PMID]
Chen, J., Wu, Y., Chen, Z., Yi, B., Zhang, L., & Yin, C., et al. (2020). High incidence of stroke in COVID-19 patients. Aging, 12(22), 22390-22398. [DOI:10.18632/aging.104092]
Chen, N., Zhou, M., Dong, X., Qu, J., Gong, F., & Han, Y., et al. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet, 395(10223), 507-513. [DOI:10.1016/S0140-6736(20)30211-7] [PMID]
Goyal, P., Choi, J. J., Pinheiro, L. C., Schenck, E. J., Chen, R., & Jabri, A., et al. (2020). Clinical characteristics of covid-19 in New York City. The New England Journal of Medicine, 382(24), 2372–2374. [DOI:10.1056/NEJMc2010419] [PMID]
Guan, W. J., Ni, Z. Y., Hu, Y., Liang, W. H., Ou, C. Q., & He, J. X., et al. (2020). Clinical characteristics of coronavirus disease 2019 in China. The New England Journal of Medicine, 382(18), 1708–1720. [DOI:10.1056/NEJMoa2002032] [PMID]
Hosmer, D.W., & Lemeshow, S. (2000). Applied logistic regression. New Jersey: John Wiley & Sons,156-64. [Link]
Hua, Z., Shen, D., Zhou, H., Liu, J., & Chen, S. (2020). Guillain-Barré syndrome associated with SARS-CoV-2 infection: Causality or coincidence? The Lancet. Neurology, 19(5), 383–384.[DOI:10.1016/S1474-4422(20)30109-5] [PMID]
Jin, H., Hong, C., Chen, S., Zhou, Y., Wang, Y., & Mao, L., et al. (2020). Consensus for prevention and management of coronavirus disease 2019 (COVID-19) for neurologists. Stroke & Vascular Neurology, 5(2), 146–151. [DOI:10.1136/svn-2020-000382] [PMID]
Lechien, J. R., Chiesa-Estomba, C. M., De Siati, D. R., Horoi, M., Le Bon, S. D., & Rodriguez, A., et al. (2020). Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID- 19): A multicenter European study. European Archives of Oto-Rhino-Laryngology: Official Journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS): Affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery, 277(8), 2251–2261. [DOI:10.1007/s00405-020-05965-1] [PMID]
Lee, M., Chen, C. S., & Ovbiagele, B. (2020). Covert COVID-19 complications: Continuing the use of evidence-based drugs to minimize potentially lethal indirect effects of the pandemic in stroke patients. Journal of The Neurological Sciences, 414, 116883. [DOI:10.1016/j.jns.2020.116883] [PMID]
Ling, M., Jin, H., Wang, M., Hu, Y., Chen, S., & He, Q., et al. (2020). Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurology, 77(6), 683–690. [DOI:10.1001/jamaneurol.2020.1127] [PMID]
Madjid, M., Curkendall, S., & Blumentals, W. A. (2009). The influence of oseltamivir treatment on the risk of stroke after influenza infection. Cardiology, 113(2), 98-107. [DOI:10.1159/000172796] [PMID]
Matías-Guiu, J., Gomez-Pinedo, U., Montero-Escribano, P., Gomez-Iglesias, P., Porta-Etessam, J., & Matias-Guiu, J. A. (2020). Should we expect neurological symptoms in the SARS-CoV-2 epidemic? Neurologia, 35(3), 170–175. [DOI:10.1016/j.nrl.2020.03.001] [PMID]
Nannoni, S., de Groot, R., Bell, S., & Markus, H. S. (2021). Stroke in COVID-19: A systematic review and meta-analysis. International Journal of Stroke: Official Journal of The International Stroke Society, 16(2), 137–149. [DOI:10.1177/1747493020972922] [PMID]
Oxley, T. J., Mocco, J., Majidi, S., Kellner, C. P., Shoirah, H., & Singh, I. P., et al. (2020). Large-Vessel stroke as a presenting feature of covid-19 in the young. The New England Journal of Medicine, 382(20), e60. [DOI:10.1056/NEJMc2009787] [PMID]
Papageorgiou, G., Grant, S. W., Takkenberg, J. J. M., & Mokhles, M. M. (2018). Statistical primer: How to deal with missing data in scientific research? Interactive Cardiovascular and Thoracic Surgery, 27(2), 153-158. [DOI:10.1093/icvts/ivy102] [PMID]
Pilotto, A., Odolini, S., Masciocchi, S., Comelli, A., Volonghi, I., Gazzina, S., Nocivelli, S., Pezzini, A., Focà, E., Caruso, A., Leonardi, M., Pasolini, M., P., Gasparotti, R., Castelli, F., Ashton, N., J., Blennow, K., Zetterberg, H., Padovani, A. (2020). Steroid-responsive encephalitis in Covid-19 disease. Annals of Neurology, 88(2), 423–427. [DOI:10.1002/ana.25783] [PMID]
Shahjouei, S., Naderi, S., Li, J., Khan, A., Chaudhary, D., & Farahmand, G., et al. (2020). Risk of stroke in hospitalized SARS-CoV-2 infected patients: A multinational study. EBioMedicine, 59, 102939. [DOI:10.1016/j.ebiom.2020.102939] [PMID]
Siow, I., Lee, K. S., Zhang, J. J. Y., Saffari, S. E., Ng, A., & Young, B. (2021). Stroke as a Neurological Complication of COVID-19: A systematic review and meta-analysis of incidence, outcomes and predictors. Journal of Stroke and Cerebrovascular Diseases: The Official Journal of National Stroke Association, 30(3), 105549. [DOI:10.1016/j.jstrokecerebrovasdis.2020.105549] [PMID]
Steardo, L., Steardo, L., Jr, Zorec, R., & Verkhratsky, A. (2020). Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiologica (Oxford, England), 229(3), e13473. [DOI:10.1111/apha.13473] [PMID]
Sundar, U., Karnik, N. D., Mukhopadhyay, A., Darole, P., Kolte, S., & Bansal, A., et al. (2021). COVID-19 Associated Stroke-A single centre experience. The Journal of The Association of Physicians of India, 69(6), 11–12. [DOI:10.1101/2021.02.15.21249420]
TunÇ, A., ÜnlÜbaŞ, Y., Alemdar, M., & AkyÜz, E. (2020). Coexistence of COVID-19 and acute ischemic stroke report of four cases. Journal of Clinical Neuroscience: Official Journal of the Neurosurgical Society of Australasia, 77, 227–229. [DOI:10.1016/j.jocn.2020.05.018] [PMID]
Trejo-Gabriel-Galán J. M. (2020). Stroke as a complication and prognostic factor of COVID-19. Neurología, 35(5), 318-322. [DOI:10.1016/j.nrl.2020.04.015] [PMID]
Viguier, A., Delamarre, L., Duplantier, J., Olivot, J. M., & Bonneville, F. (2020). Acute ischemic stroke complicating common carotid artery thrombosis during a severe COVID-19 infection. Journal of Neuroradiology = Journal de neuroradiologie, 47(5), 393–394. [DOI:10.1016/j.neurad.2020.04.003] [PMID]
Wang, D., Hu, B., Hu, C., Zhu, F., Liu, X., Zhang, J., Wang, B., Xiang, H., Cheng, Z., Xiong, Y., Zhao, Y., Li, Y., Wang, X., Peng, Z. (2020). Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA, 323(11), 1061–1069. [DOI:10.1001/jama.2020.1585] [PMID]
Wu, C., Chen, X., Cai, Y., Xia, J., Zhou, X., & Xu, S., et al. (2020). Risk factors associated with acute respiratory distress síndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Internal Medicine, 180(7), 934–943. [DOI:10.1001/jamainternmed.2020.0994] [PMID]
Wu, Y., Xu, X., Chen, Z., Duan, J., Hashimoto, K., & Yang, L., et al. (2020). Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain, Behavior, and Immunity, 87, 18–22. [DOI:10.1016/j.bbi.2020.03.031] [PMID]
Yandrapalli, S., Aronow, W. S., & Frishman, W. H. (2018). Readmissions in adult patients following hospitalization for influenza: A nationwide cohort study. Annals of Translational medicine, 6(16), 318. [DOI:10.21037/atm.2018.07.18] [PMID]
Zhang, Y., Xiao, M., Zhang, S., Xia, P., Cao, W., & Jiang, W., et al. (2020). Coagulopathy and antiphospholipid antibodies in patients with Covid-19. The New England Journal of Medicine, 382(17), e38. [DOI:10.1056/NEJMc2007575] [PMID]
Numerous studies have been published on the COVID-19 pandemic. There have been reports of stroke occurrences in patients with this virus, showing inconsistent results. The connection between coronaviruses as endemic diseases and various neurological disorders is being closely examined (Matías-Guiu et al., 2020). A historical analysis of different types of coronaviruses by Trejo-Gabriel-Galán (2020), indicates that the three known types of coronaviruses associated with acute respiratory symptoms and high mortality rates are linked to neurological complications. These include the SARS-CoV virus in 2003, the MERS-CoV virus in 2012, and COVID-19, which emerged in December 2019 in Wuhan, China. A review article documented 5 cases of stroke among 206 patients in Singapore and another 5 cases among 664 Taiwanese patients during the 2003 epidemic (Trejo-Gabriel-Galán, 2020). The literature indicates several neurological advents, including cerebrovascular accidents in patients with COVID-19. Due to the possibility of the virus entering into the nerve cells, by several mechanisms, such as interfering with coagulation or invasion of vessel walls, in addition to stroke, other neurological manifestations, such as dizziness, anosmia, impaired consciousness, seizures (Ling et al., 2020), Guillain–Barre syndrome (Hua et al., 2020), encephalitis (Pilotto et al., 2020), hyposmia (Lechien et al., 2020) and headache have also been observed in some patients with COVID-19. The virus can cause strokes by impairing coagulation when it enters the heart and brain. However, strokes are not a primary manifestation of COVID-19, indicating that patients with stroke may present with COVID-19 even in the absence of respiratory symptoms (Trejo-Gabriel-Galán, 2020).
Many deaths associated with COVID-19 occur in patients with cardiovascular and cerebrovascular conditions (Viguier et al., 2020). In some cases, the coronavirus invades the central nervous system instead of the respiratory system. A study of four patients in Sakarya, Turkey, who experienced strokes concurrent with a COVID-19 diagnosis, indicated that cerebrovascular diseases can occur simultaneously and independently of the COVID-19 process (TunÇ et al., 2020). According to another study in Wuhan, China (Jin et al., 2020), COVID-19 also may involve the nervous system, and the patients whose nervous system is involved may not be easily diagnosed in the early stages of the disease. This study led to the development of clinical guidelines aimed at helping neurologists diagnose the infection earlier and protect treatment staff.
Although neurological involvement is not common in COVID-19, symptoms such as encephalopathy, impaired consciousness, and skeletal muscle injury may indicate acute cerebrovascular disease (Ling et al., 2020). Evidence suggests that there is a 2.5-fold increase in the risk of developing severe COVID-19 in patients with a history of stroke (Lee et al., 2020), prompting clinicians to be vigilant for COVID-19 in cases of cerebrovascular accidents. Scientific reports emphasize the need for research to enhance our understanding of the neurological implications of COVID-19 (Avula et al., 2020). Nonetheless, numerous studies have confirmed that comorbidities such as cancer, diabetes, cardiovascular disorders, and hypertension increase the risk of developing more severe COVID-19 and mortality (Wu et al., 2020; Wang et al., 2020; Guan et al., 2020). Several studies have investigated the link between stroke and COVID-19: A retrospective study on 11 patients with coronavirus pneumonia, who were diagnosed with stroke based on neurological symptoms and confirmed by imaging, showed that the incidence of stroke in COVID-19 patients was significantly higher than the average level in the general population. The incidence of stroke increased compared to the period before the pandemic in China (Chen et al., 2020). A study was conducted in India on 3923 patients with COVID-19, of whom 62 cases were patients with stroke and the majority had carotid territory infarcts. The logistic regression model indicated that a low Glasgow coma score (GCS) and the need for respiratory support were predictors of in-hospital mortality (Sundar et al., 2021). A study in the United Kingdom examined the risk factors, assessed the incidence, described clinical radiological manifestations, and evaluated the outcome of strokes in COVID-19 patients. Data were collected from studies reporting >5 strokes in COVID-19 patients. The findings suggested that acute cerebrovascular disease is not rare in COVID-19 patients, especially those with a history of vascular risk factors, and cerebral thrombosis and/or thromboembolism could be potential causes of this condition (Nannoni et al., 2021).
Studies have shown that although stroke is not a common complication of COVID-19, its occurrence often results in significant complications and mortality. Stroke in patients with COVID-19 was related to older age, comorbidities, and disease severity (Siow et al., 2021).
The aim of the present study was to predict the incidence of stroke in patients with COVID-19 using logistic regression model analysis and identify the most important significant factors according to clinical features and effective blood markers.
2. Materials and Methods
Data collection
The data collection method for patients with confirmed COVID-19 in this study utilized a non-random, prospective registration approach. Confirmation of COVID-19 in these patients was achieved through polymerase chain reaction (PCR) testing and evidence of positive lung involvement. This study analyzed the demographic information, clinical characteristics, and laboratory parameters of 128 patients with COVID-19 who were referred to Shohada-e-Tajrish Hospital (Tehran, Iran) from March to September 2020. The data were obtained from the emergency department (ED), reports of the intensive care unit (ICU), and reports of the admission department of the hospital.
The classification analysis revealed the following results: 1) 28 of the patients (21.87%) exhibited stroke symptoms, 2) 52 patients (42%) were females with an average age of 57.109±15.97, ranging from 18 to 87 years, 3) The mean age of patients with ischemic stroke was 57.96±16.82 and 4) The mean age of patients without ischemic stroke was 56.87±15.8.
Patients were included in this study after laboratory confirmation of COVID-19. Clinical features were defined following consultations with neurologists who were part of the treatment team for patients with stroke and COVID-19. After diagnosis, the treatment was followed according to the severity of the disease and naturally was different for each patient, but in general, the following protocol was performed for all patients:
Vitamin D3 supplementation, famotidine 40 mL taken twice daily, systemic corticosteroids, naproxen, and respiratory treatment, if needed (patients with stroke needed oxygen therapy and ventilator more frequently than others).
Variables
Demographic variables, including age and sex, the onset date of COVID-19 symptoms, the date of a positive COVID-19 test, ventilator dependency, comorbidities/risk factors, and laboratory tests including white blood cell (WBC) count, neutrophil count, lymphocyte count (count×109/L, reference value (RV): 0.9–5.2), platelet count (count×109/L, RV: 150–400), C-reactive protein (mg/L, RV: 1–5), blood urea nitrogen, creatinine (U/L, RV: 20–170), alanine transaminase (ALT), aspartate transaminase (AST), and lactate dehydrogenase (LDH) were collected for analysis. We analyzed comorbidities, including hypertension (systemic blood pressure higher than 140/90 mm Hg), current smoking status, diabetes (fasting blood glucose >126 mg/dL on two separate tests, HbA1c >6,5%, blood glucose levels >200 mg after oral glucose overload or blood glucose levels >200 mg/dL with diabetes symptoms), chronic kidney disease, active neoplasm, and rheumatological disease. Given their significance in neurological disorders, cardiovascular or cerebrovascular diseases, such as ischemic heart disease, atrial fibrillation, carotid stenosis, cardiac ejection fraction <40%, and history of stroke/transient ischemic attack (TIA) were considered as comorbidities (Wang et al., 2020; Chen et al., 2020). Table 1 displays the demographic variables, clinical features, comorbidities, and laboratory test results. Table 1 shows demographic variables, clinical features, comorbidities, and laboratory test results.

Statistical analysis
In this research identification of important and influential variables on stroke incidence was analyzed using logistic regression analysis. This method is capable of leading physicians to predict the incidence of stroke in patients with COVID-19. Ordinal and qualitative variables are defined in this approach as numbered classes. In this analysis, single mean imputation was used to manage missing data (Papageorgiou et al., 2018). Hosmer and Lemeshow’s test was also used to investigate the model’s goodness of fit. Statistical analyses were conducted using SPSS software, version 26, with results reported in terms of OR, P<0.05, and 95% CI.
3. Results
The analysis of stroke patients revealed that, on average, stroke occurred 5 days (ranging from 0 to 17 days) after the onset of COVID-19 symptoms. The Wald statistic examines the significance of the presence of each independent variable in the model, which we can find out through its significance level. The Wald statistic functions similarly to the t-statistic in linear regression. The interpretation is such that if P<0.05 for a variable, it suggests that the inclusion of that variable in the model is meaningful and its effect is statistically significant. Exp (B) known as the OR, reflects the ratio of the odds of an event occurring to the odds of it not occurring. An OR<1 suggests that as the values of the independent variable increase, the probability of the event occurring decreases (negative effect). Conversely, an OR>1 indicates that as the values of the independent variable increase, the probability of the event occurring also increases (positive effect).
Ventilator dependency, cardiac ejection fraction <40, and LDH were associated with the occurrence of stroke and could predict its occurrence. The regression model illustrating the relationship between strokes and significant variables is presented as Equation 1:
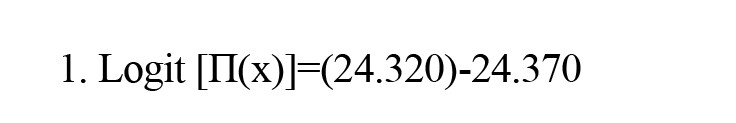
(Cardiac ejection fraction: NO)+4.796 (Ventilator dependent: YES)-0.806 (Ventilator dependent: NO)-0.006 (LDH)
In Equation 2, “cardiac ejection fraction: No” is denoted by x1, “ventilator dependent: Yes” is addressed by x2, “ventilator dependent: NO“ is indicated by x3, and LDH is presented by x4:

In Equation 3, Π(x) is the predicted value for the model output in the range of 0 to 1.

This model clearly identifies cardiac ejection fraction, ventilator dependency, and LDH as independent variables, with a constant coefficient (i.e. intercept) of the functional model being 24.320. According to Table 2, in the final analysis, these three variables remained significant in the model, with the P for ventilator dependency and LDH being <0.05, making them statistically significant, whereas the cardiac ejection fraction is considered clinically significant and important.
As detailed in Table 2, the Exp (B) value for ventilator use exceeds one (120.995), indicating that an increase in the probability of ventilator use is associated with a higher risk of stroke. This underscores a positive correlation between ventilator use and the incidence of stroke.

The results of the omnibus test showed the model’s acceptable predictive capability. Table 3 addresses the results of the omnibus test at the third stage. As shown in Table 3, the fitness of the proposed model is deemed acceptable showing significance at an error level of <0.05.

The Cox & Snell and Nagelkerke coefficients, as shown in Table 4, demonstrate that the independent variables in this study possess a relatively high capacity to explain the variance in the dependent variable (stroke). According to Table 4, the independent variables account for between 50.3% and 77.3% of the variability in stroke (the dependent variable).

The Hasmer-Lemeshow test was used to analyze the goodness of the proposed logistic regression model. According to Table 5, the significance level was >0.05, suggesting that the model fits well. This implies that the independent variables are effective in predicting variations in the dependent variable.

Table 6 reveals that the model’s classification accuracy stands at 93.8%. This means that the model can predict changes in the dependent variable (stroke incidence) with 93.8% confidence.

Due to the limitations of this study in collecting more data to investigate the generalizability of the model, 1000 samples were drawn from the original population of 128 subjects using replication and simple random sampling. This constitutes a bootstrap sample. The estimated coefficients were applied to this dataset. The ROC curve for the new data is illustrated in Figure 1, displaying an area under the curve (AUC) of 0.977 with approximately a 95% confidence interval. The AUC is significantly distinct from 0.5, indicating that the proposed logistic regression model significantly outperforms chance classification, as evidenced by a p-value of <0.001.

Table 7 presents the classification of bootstrap data in both classes.

Table 8 indicates that the area under the ROC curve AUC was 97%.

Table 9 also indicates the relationship between the AUC and diagnostic accuracy terms (Hosmer & Lemeshow, 2000). The proposed model showed an excellent capability of discrimination.
Table 10 presents the diagnostic accuracy terms, including sensitivity, specificity, and precision on the new data. The simultaneous attainment of optimal values for both sensitivity and specificity suggests that the model possesses satisfactory discriminative ability.

4. Discussion
While ischemic stroke is recognized as a complex complication of COVID-19, the specifics of this condition have not been fully elucidated. Severe COVID-19 can lead to the activation of endothelial and mononuclear cells, resulting in coagulation and thrombin production. A study from Johann-China (Beyrouti et al., 2020) observed neurological complications in 36% of 214 COVID-19 patients, noting ischemic strokes were more frequent in patients with severe COVID-19 compared to those with non-severe conditions (5.7% vs 0.8%) (Beyrouti et al., 2020). At the beginning of the COVID-19 pandemic, many articles have addressed the increased stroke risk among COVID-19 patients (Beyrouti et al., 2020; Wu et al., 2020; Steardo et al., 2020; Oxley et al., 2020; Zhang et al., 2020).
This research introduced a logistic functional model to predict the likelihood of stroke in COVID-19 patients. The study’s findings underscore the significance of ventilator use and the presence of a cardiac ejection fraction <40 and elevated LDH levels as independent predictors of stroke. The results are supported by a multicenter and multinational study involving 17,799 hospitalized SARS-CoV-2 patients, which investigated stroke risk and related factors in SARS-CoV-2. This extensive study, encompassing North and South America and various countries from Europe, Asia, and Oceania, found that mechanical ventilation and ischemic heart conditions are predictive of stroke (Shahjouei et al., 2020). To the best of the authors’ knowledge, this is the first study proposing a functional model to predict stroke incidence in COVID-19 patients, especially in Iran.
The sensitivity and specificity high values achieved by the proposed model demonstrate its proficiency in accurately diagnosing both stroke occurrences and non-stroke cases. Several studies have examined the relationship between stroke risk and respiratory infections (Arabi et al., 2015; Algahtani et al., 2016; Madjid et al., 2009). The likelihood of experiencing a stroke following an infection appears to be minimal, approximately 1% (Shahjouei et al., 2020), Also, according to the United States National Readmissions Database, based on data from 46,000 patients with influenza, the risk of stroke was 0.3% (Yandrapalli et al., 2018).
Patients with comorbidities, inflammatory markers, or those requiring vasopressors are more inclined to undergo mechanical ventilation (Goyal et al., 2020).
Identification of important characteristics in the occurrence of stroke can be beneficial for earlier diagnosis. The limited number of patients is one of the limitations of the present study. Further studies can lead researchers to develop the proposed model of this research. It is suggested that incorporating additional clinical factors could enhance the model’s development. We recommend that different techniques, such as genetic algorithms or machine learning can be used in this field of research.
5. Conclusion
This research introduced a logistic functional model to predict the incidence of stroke in COVID-19 patients. The literature indicates that stroke in COVID-19 patients often results in mortality. Numerical analysis of the results revealed that the proposed logistic regression model predicts stroke incidence with 93.75% accuracy and an AUC of 97%. The findings showed that ventilator dependence, a cardiac ejection fraction of <40, and elevated LDH are associated with the risk of stroke. The logistic model proposed in this study is effective in guiding physicians toward appropriate treatment strategies. Identifying significant clinical factors using the proposed model can aid in the early diagnosis of potential stroke cases.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This study was supported by the Malek Ashtar University of Technology and Shohada-e-Tajrish Hospital affiliated with the Shahid Beheshti University of Medical Sciences.
Authors' contributions
Conceptualization, investigation, software calculation and writing: Mahsa Babaee; Data collection: Ali Amini Harandi and Atefeh Yousefi; Data analysis: Mahsa Babaee and Ali Amini Harandi; Supervision: Karim Atashgar and Ali Amini Harandi.
Conflict of interest
The authors declared no conflicts of interests.
References
Algahtani, H., Subahi, A., & Shirah, B. (2016). Neurological complications of Middle East Respiratory Syndrome coronavirus: A report of two cases and review of the literature. Case Reports in Neurological Medicine, 2016, 3502683. [DOI:10.1155/2016/3502683] [PMID]
Avula, A., Nalleballeb, K., Narulaa, N., Sapozhnikov, S., Dandu, V., & Toom, S., et al. (2020). COVID-19 presenting as stroke. Brain, Behavior, and Immunity, 87, 115-119. [DOI:10.1016/j.bbi.2020.04.077] [PMID]
Arabi, Y. M., Harthi, A., Hussein, J., Bouchama, A., Johani, S., & Hajeer, A. H., et al. (2015). Severe neurologic syndrome associated with Middle East respiratory syndrome coronavirus (MERS - CoV). Infection, 43(4), 495-501. [DOI:10.1007/s15010-015-0720-y] [PMID]
Beyrouti, R., Adams, M. E., Benjamin, L., Cohen, H., Farmer, S. F., & Goh, Y. Y., et al. (2020). Characteristics of ischaemic stroke associated with COVID-19. Journal of Neurology, Neurosurgery, and Psychiatry, 91(8), 889–891. [DOI:10.1136/jnnp-2020-323586] [PMID]
Chen, J., Wu, Y., Chen, Z., Yi, B., Zhang, L., & Yin, C., et al. (2020). High incidence of stroke in COVID-19 patients. Aging, 12(22), 22390-22398. [DOI:10.18632/aging.104092]
Chen, N., Zhou, M., Dong, X., Qu, J., Gong, F., & Han, Y., et al. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet, 395(10223), 507-513. [DOI:10.1016/S0140-6736(20)30211-7] [PMID]
Goyal, P., Choi, J. J., Pinheiro, L. C., Schenck, E. J., Chen, R., & Jabri, A., et al. (2020). Clinical characteristics of covid-19 in New York City. The New England Journal of Medicine, 382(24), 2372–2374. [DOI:10.1056/NEJMc2010419] [PMID]
Guan, W. J., Ni, Z. Y., Hu, Y., Liang, W. H., Ou, C. Q., & He, J. X., et al. (2020). Clinical characteristics of coronavirus disease 2019 in China. The New England Journal of Medicine, 382(18), 1708–1720. [DOI:10.1056/NEJMoa2002032] [PMID]
Hosmer, D.W., & Lemeshow, S. (2000). Applied logistic regression. New Jersey: John Wiley & Sons,156-64. [Link]
Hua, Z., Shen, D., Zhou, H., Liu, J., & Chen, S. (2020). Guillain-Barré syndrome associated with SARS-CoV-2 infection: Causality or coincidence? The Lancet. Neurology, 19(5), 383–384.[DOI:10.1016/S1474-4422(20)30109-5] [PMID]
Jin, H., Hong, C., Chen, S., Zhou, Y., Wang, Y., & Mao, L., et al. (2020). Consensus for prevention and management of coronavirus disease 2019 (COVID-19) for neurologists. Stroke & Vascular Neurology, 5(2), 146–151. [DOI:10.1136/svn-2020-000382] [PMID]
Lechien, J. R., Chiesa-Estomba, C. M., De Siati, D. R., Horoi, M., Le Bon, S. D., & Rodriguez, A., et al. (2020). Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID- 19): A multicenter European study. European Archives of Oto-Rhino-Laryngology: Official Journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS): Affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery, 277(8), 2251–2261. [DOI:10.1007/s00405-020-05965-1] [PMID]
Lee, M., Chen, C. S., & Ovbiagele, B. (2020). Covert COVID-19 complications: Continuing the use of evidence-based drugs to minimize potentially lethal indirect effects of the pandemic in stroke patients. Journal of The Neurological Sciences, 414, 116883. [DOI:10.1016/j.jns.2020.116883] [PMID]
Ling, M., Jin, H., Wang, M., Hu, Y., Chen, S., & He, Q., et al. (2020). Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurology, 77(6), 683–690. [DOI:10.1001/jamaneurol.2020.1127] [PMID]
Madjid, M., Curkendall, S., & Blumentals, W. A. (2009). The influence of oseltamivir treatment on the risk of stroke after influenza infection. Cardiology, 113(2), 98-107. [DOI:10.1159/000172796] [PMID]
Matías-Guiu, J., Gomez-Pinedo, U., Montero-Escribano, P., Gomez-Iglesias, P., Porta-Etessam, J., & Matias-Guiu, J. A. (2020). Should we expect neurological symptoms in the SARS-CoV-2 epidemic? Neurologia, 35(3), 170–175. [DOI:10.1016/j.nrl.2020.03.001] [PMID]
Nannoni, S., de Groot, R., Bell, S., & Markus, H. S. (2021). Stroke in COVID-19: A systematic review and meta-analysis. International Journal of Stroke: Official Journal of The International Stroke Society, 16(2), 137–149. [DOI:10.1177/1747493020972922] [PMID]
Oxley, T. J., Mocco, J., Majidi, S., Kellner, C. P., Shoirah, H., & Singh, I. P., et al. (2020). Large-Vessel stroke as a presenting feature of covid-19 in the young. The New England Journal of Medicine, 382(20), e60. [DOI:10.1056/NEJMc2009787] [PMID]
Papageorgiou, G., Grant, S. W., Takkenberg, J. J. M., & Mokhles, M. M. (2018). Statistical primer: How to deal with missing data in scientific research? Interactive Cardiovascular and Thoracic Surgery, 27(2), 153-158. [DOI:10.1093/icvts/ivy102] [PMID]
Pilotto, A., Odolini, S., Masciocchi, S., Comelli, A., Volonghi, I., Gazzina, S., Nocivelli, S., Pezzini, A., Focà, E., Caruso, A., Leonardi, M., Pasolini, M., P., Gasparotti, R., Castelli, F., Ashton, N., J., Blennow, K., Zetterberg, H., Padovani, A. (2020). Steroid-responsive encephalitis in Covid-19 disease. Annals of Neurology, 88(2), 423–427. [DOI:10.1002/ana.25783] [PMID]
Shahjouei, S., Naderi, S., Li, J., Khan, A., Chaudhary, D., & Farahmand, G., et al. (2020). Risk of stroke in hospitalized SARS-CoV-2 infected patients: A multinational study. EBioMedicine, 59, 102939. [DOI:10.1016/j.ebiom.2020.102939] [PMID]
Siow, I., Lee, K. S., Zhang, J. J. Y., Saffari, S. E., Ng, A., & Young, B. (2021). Stroke as a Neurological Complication of COVID-19: A systematic review and meta-analysis of incidence, outcomes and predictors. Journal of Stroke and Cerebrovascular Diseases: The Official Journal of National Stroke Association, 30(3), 105549. [DOI:10.1016/j.jstrokecerebrovasdis.2020.105549] [PMID]
Steardo, L., Steardo, L., Jr, Zorec, R., & Verkhratsky, A. (2020). Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiologica (Oxford, England), 229(3), e13473. [DOI:10.1111/apha.13473] [PMID]
Sundar, U., Karnik, N. D., Mukhopadhyay, A., Darole, P., Kolte, S., & Bansal, A., et al. (2021). COVID-19 Associated Stroke-A single centre experience. The Journal of The Association of Physicians of India, 69(6), 11–12. [DOI:10.1101/2021.02.15.21249420]
TunÇ, A., ÜnlÜbaŞ, Y., Alemdar, M., & AkyÜz, E. (2020). Coexistence of COVID-19 and acute ischemic stroke report of four cases. Journal of Clinical Neuroscience: Official Journal of the Neurosurgical Society of Australasia, 77, 227–229. [DOI:10.1016/j.jocn.2020.05.018] [PMID]
Trejo-Gabriel-Galán J. M. (2020). Stroke as a complication and prognostic factor of COVID-19. Neurología, 35(5), 318-322. [DOI:10.1016/j.nrl.2020.04.015] [PMID]
Viguier, A., Delamarre, L., Duplantier, J., Olivot, J. M., & Bonneville, F. (2020). Acute ischemic stroke complicating common carotid artery thrombosis during a severe COVID-19 infection. Journal of Neuroradiology = Journal de neuroradiologie, 47(5), 393–394. [DOI:10.1016/j.neurad.2020.04.003] [PMID]
Wang, D., Hu, B., Hu, C., Zhu, F., Liu, X., Zhang, J., Wang, B., Xiang, H., Cheng, Z., Xiong, Y., Zhao, Y., Li, Y., Wang, X., Peng, Z. (2020). Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA, 323(11), 1061–1069. [DOI:10.1001/jama.2020.1585] [PMID]
Wu, C., Chen, X., Cai, Y., Xia, J., Zhou, X., & Xu, S., et al. (2020). Risk factors associated with acute respiratory distress síndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Internal Medicine, 180(7), 934–943. [DOI:10.1001/jamainternmed.2020.0994] [PMID]
Wu, Y., Xu, X., Chen, Z., Duan, J., Hashimoto, K., & Yang, L., et al. (2020). Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain, Behavior, and Immunity, 87, 18–22. [DOI:10.1016/j.bbi.2020.03.031] [PMID]
Yandrapalli, S., Aronow, W. S., & Frishman, W. H. (2018). Readmissions in adult patients following hospitalization for influenza: A nationwide cohort study. Annals of Translational medicine, 6(16), 318. [DOI:10.21037/atm.2018.07.18] [PMID]
Zhang, Y., Xiao, M., Zhang, S., Xia, P., Cao, W., & Jiang, W., et al. (2020). Coagulopathy and antiphospholipid antibodies in patients with Covid-19. The New England Journal of Medicine, 382(17), e38. [DOI:10.1056/NEJMc2007575] [PMID]
Type of Study: Original |
Subject:
Computational Neuroscience
Received: 2021/07/30 | Accepted: 2022/04/8 | Published: 2024/01/1
Received: 2021/07/30 | Accepted: 2022/04/8 | Published: 2024/01/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








