Volume 15, Issue 4 (July & August 2024)
BCN 2024, 15(4): 553-560 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Zeinali M, Almasi Dooghaee M, Ziaee M, Haghi Ashtiani B. Evaluation of Relationship Between Laboratory, Electrodiagnostic, and Functional Parameters in Patients With Amyotrophic Lateral Sclerosis; A Cross Sectional Study. BCN 2024; 15 (4) :553-560
URL: http://bcn.iums.ac.ir/article-1-2178-en.html
URL: http://bcn.iums.ac.ir/article-1-2178-en.html
1- Department of Neurology, School of Medicine, Firoozgar Hospital, Iran University of Medical Sciences, Tehran, Iran.
Keywords: Amyotrophic lateral sclerosis (ALS), Motor unit, Function, Biomarkers, Muscle action potentials
Full-Text [PDF 517 kb]
| Abstract (HTML)
Full-Text:
Introduction
Amyotrophic lateral sclerosis (ALS) is the most common adult-onset motor neuron disease, with an incidence of 2-3 cases per 100000 per year (Kiernan et al., 2011). The average survival rate since the onset of symptoms is about 3-5 years (Armon, 1994). The prognosis is poor for most patients with ALS, particularly when the diagnosis is delayed (Miller & Appel, 2017).
Several markers have been identified for the functional assessment of ALS patients, which include age (Preux et al.,1996; Testa et al., 2004; Chio et al., 2002), symptoms of onset (Tysnes et al., 1991), body mass index (BMI), nutritional status (Stambler et al., 1998), as well as several biological and laboratory markers such as creatine kinase (CK) serum (Amrit & Anderson, 1974), serum creatinine (Cr) (Brooks et al., 2014), and serum albumin (Chio et al., 2014). A better understanding of these factors helps physicians and patients make more informed decisions based on their shared treatment goals (Albert et al., 1999). Also, clinical trials for ALS will be more applicable and easier to conduct as more disease progression markers are validated as outcome measures. Electrodiagnostic measures are another important tool for assessing motor neuron diseases, including ALS (Amin Lari et al., 2019). Measuring the rate of motor units’ loss is a good way to monitor disease progression (Grimaldi et al., 2017). Electrodiagnostic measures such as the motor unit number index (MUNIX) can help determine the number of lost motor units (Jacobsen et al., 2017; Nandedkar et al., 2010; Furtula et al., 2013).
Few studies have examined the relationship between the number of motor units and serologic biomarkers (e.g. CK, albumin, and Cr). We aimed to study the relationship between the laboratory findings, the number of motor units (based on electrodiagnostic studies), and the patient’s functional scores.
2. Materials and Methods
We conducted a cross-sectional study of patients with ALS diagnosed within two years before the study onset. The diagnosis was confirmed by the Awaji criteria, which are reliable criteria based on patient history, clinical examination, and electrodiagnostic testing. Patients with impaired renal function and high serum Cr, those functionally impaired due to medical conditions, and patients who refused to participate in the study were excluded.
Patients’ demographic features, time from symptom onset to diagnosis, and primary presenting symptoms were recorded based on history taking and clinical interviews. Laboratory investigations, including serum CK, Cr, blood urea nitrogen (BUN), and albumin, were undertaken at the central laboratory of Firoozgar Hospital upon enrollment. The MUNIX was calculated based on electromyographic (EMG) assessment of four muscles: Anterior tibialis, abductor digiti minimi, deltoid, and abductor pollicis brevis. This method uses compound motor action potential (CMAP) amplitude and surface electromyographic interference patterns (SIP) to calculate MUNIX, which measures the size and number of motor units. An experienced neurologist in neuromuscular disorders conducted the electromyography. All assessments were done by the same neurologist using the same EMG machine.
The main outcome of this study was the ALS functional rating scale (ALS-FRS), which measures self-sufficiency and disease progression in ALS patients. It is a 10-item inventory covering functional domains, including feeding, grooming, ambulation, and communication; the Persian version has been validated for use in Persian-speaking ALS patients (Afrakhteh et al., 2021). Each item is rated 0 to 4 based on the patient’s level of function. The scores were calculated, and relevant clinical assessments were performed by a neurologist.
Statistical analysis
The Pearson correlation coefficient was used to assess the association between demographic characteristics, clinical features, ALS-FRS, laboratory findings, and EMG findings. We used regression statistics to evaluate the strength of significant associations further. All statistical analyses were performed using SPSS software, version 16. A P<0.05 was considered significant.
Ethical considerations
This research was approved by the Ethics Committee at Iran University of Medical Sciences. We adhered to the principles for medical research on human subjects in accordance with the Helsinki Declaration. Informed consent was obtained from all included participants, having clarified that all the elements of this research, including clinical, electrodiagnostic, and laboratory assessments, were part of the routine care of their illness without any additional costs for the patient.
3. Results
Patients’ characteristics
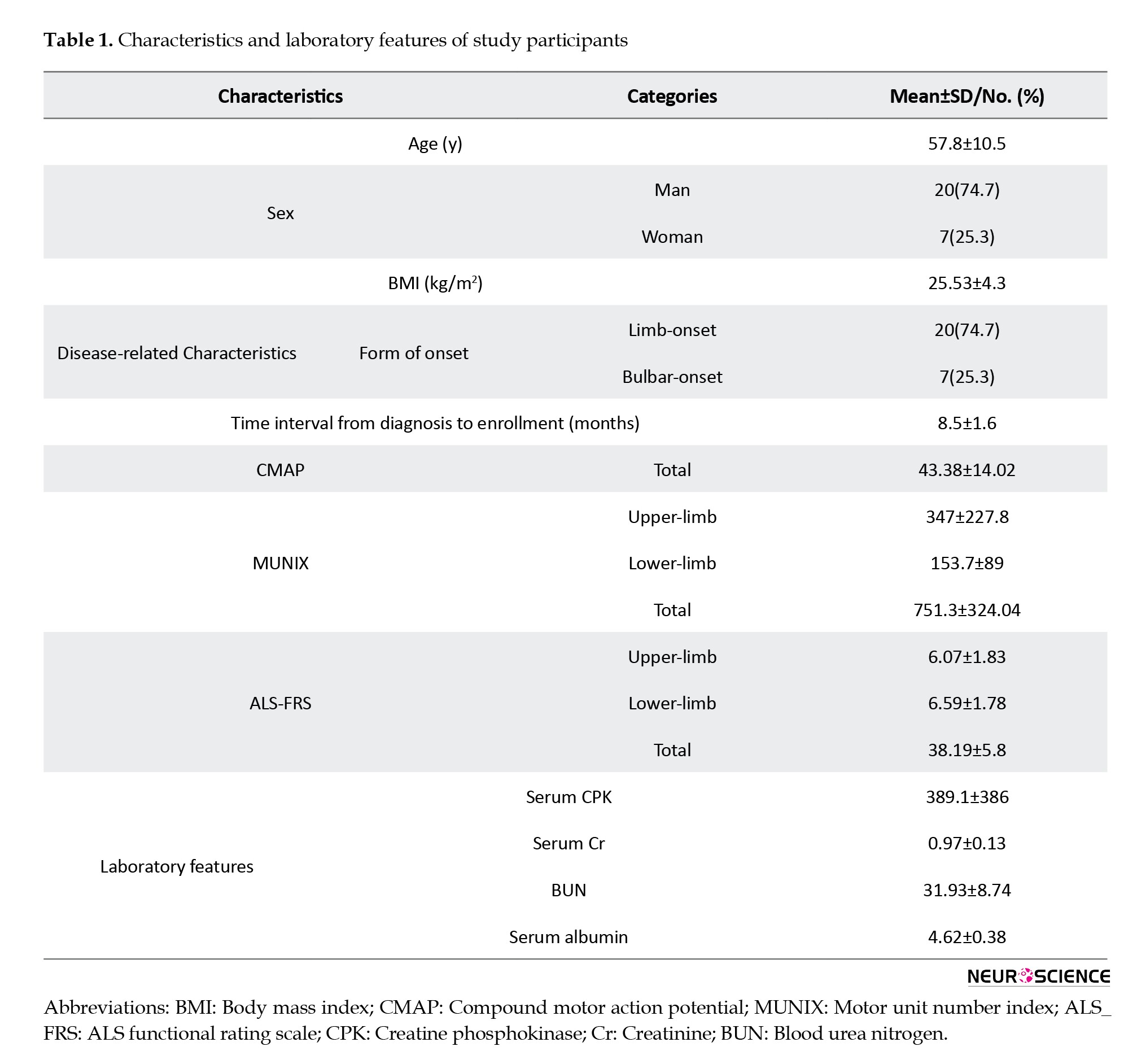
Twenty-seven patients were enrolled in this study. The mean age of patients was 57.8±10.5, ranging from 36 to 77 years. The majority of patients (20/27) were men. Most patients presented primarily with limb symptoms (20/27[74.7%]), whereas 7 had a bulbar-onset form. The mean interval between ALS diagnosis and enrollment in the study was 8.5±1.6 months. Clinical and laboratory features of the patients are presented in Table 1.
Relationships between study variables
There was no relationship between demographic factors and the primary study outcome (ALS-FRS). Serum albumin, Cr, and CK did not correlate with total ALS-FRS scores (Table 2). Higher serum Cr was associated with higher- and lower-limb MUNIX (P=0.04, the Pearson correlation: 0.39).

Higher BMI was significantly associated with lower total and limb-specific MUNIX scores (P=0.01, Pearson correlation coefficient=0.48). Furthermore, total CMAP was lower among patients with a higher BMI (P=0.02, the Pearson correlation coefficient=0.42). Patients with a limb-onset form of ALS had higher total and upper-limb MUNIX scores than those with a bulbar-onset disease (P=0.02, the Pearson correlation=0.44).
Total MUNIX scores, upper-limb MUNIX, and lower-limb MUNIX scores did not significantly correlate with total ALS-FR scores. However, higher- and lower-limb MUNIX was associated with a better lower limb level of functioning based on ALS-FRS (P=0.02, Pearson correlation: 0.44). The strength assessment of this association using regression analysis indicated that for every 1-point increase in MUNIX, ALS-FRS increased by 0.44 units.
4. Discussion
Our findings can be discussed in the context of three different dimensions: the relationship between laboratory markers and patient level of functioning, laboratory markers in relation to electrodiagnostic markers of disease activity, and the relationship between clinical and demographic characteristics with laboratory and electrodiagnostic markers of disease activity.
Cr is a marker of normal muscle cell metabolism, and a decrease in its serum level indicates a loss of muscle units (Brooks et al., 2014; van Eijk et al., 2018). We found no relationship between serum Cr and patient functioning based on ALS-FRS. A 2019 meta-analysis showed that higher serum Cr at baseline was associated with higher functional scores and that a sharper decline in serum Cr correlated with a more prominent decline in level of functioning (Lanznaster et al., 2019). Another study assessing the relationship between plasma biomarkers and function in 355 well-phenotype ALS patients reported a consistent relationship between serum Cr at all time points during follow-up (Mitsumoto et al., 2020). These findings are inconsistent with ours in that our study shows no relationship between ALS-FRS scores and serum Cr. The reason may be that we missed the association between Cr decline and level of functioning due to lack of longitudinal follow-up or the smaller number of participants in our study.
However, higher serum Cr levels were correlated with higher lower limb MUNIX scores, reflecting the number of active motor units in the lower limb. Moreover, we found that decreased lower-limb MUNIX correlated with lower-limb ALS-FRS scores. These findings are consistent with the notion that Cr is related to active muscle mass (Furtula et al., 2013; Freigang et al., 2021; Neuwirth et al., 2017). However, these findings were exclusively significant for the lower limb, and total MUNIX was not related to serum Cr. This issue may have been due to different clinical features of our patients or the small sample size.
Some studies have reported serum albumin decline as a prognostic factor and a predictor of the rate of disease progression (Chio et al., 2014; Verber et al., 2019; Ong et al., 2017). Interestingly, in some studies, only the decline of albumin and not one-time serum albumin levels were associated with disease progression (Xu et al., 2018). That is why we may not find any association between serum albumin and functional scores in this cross-sectional study.
CK has been a reliable and independent prognostic biomarker due to its association with ALS-frs scores in assessing treatment outcomes in ALS patients (Amrit & Anderson, 1974; Rafiq et al., 2016; Ilzecka & Stelmasiak, 2003). This enzyme is produced in greater quantities to provide energy for the metabolic stress imposed by ALS (Rafiq et al., 2016). In our study, the levels of this marker did not correlate with electrodiagnostic and functional indices.
In this study, patients with limb-onset ALS had a higher- and upper-limb MUNIX and ALS-FRS functional score compared to patients with a bulbar onset; however, this was unrelated to serum Cr, BUN, CPK, and level of functioning.
We also found that patients with higher BMI had lower MUNIX and CMAP scores; however, these factors were unrelated to ALS-FRS score and patient function. In contrast to our findings, most studies reported high BMI as a positive prognostic factor (Chio et al., 2014; Verber et al., 2019). According to these studies, BMI and MUNIX scores were not related, but BMI was linked to survival, functional score, and overall prognosis. Since BMI does not exclusively represent muscle mass, fat-free mass might be a more valid indicator of muscle bulk (Chio et al., 2014). A 2012 study states that low BMI and malnutrition have a neurotoxic effect, highlighting the role of BMI in the prognosis of ALS patients (Spencer & Palmer, 2012). Another study found that in people with a BMI below 30, a higher initial BMI predicts a slower rate of function decline. Still, the relationship is reversed in patients with BMIs over 30 (Reich-Slotky et al., 2013). Although the patients in our study had a mean BMI of 25, a higher BMI was associated with lower MUNIX and CMAP and did not correlate with ALS-FRS.
Age, gender, and disease duration had no significant relationships with laboratory, electrodiagnostic, and function findings.
Electrodiagnostic measures are common and reliable indicators of disease progression in ALS. The MUNIX index represents the number of active muscle units. In our study, cases with higher- and lower-limb MUNIX scores also had higher lower-limb ALS-FRS function scores. We did not find a significant relationship between other electrodiagnostic measures and functional scores. According to our results, the MUNIX score in the lower limb seems to be a more reliable measure for monitoring muscle function in these patients. A 2016 study concluded that the total MUNIX score significantly correlated with the ALS-FRS function score (Jacobsen et al., 2019). The same findings were repeated in another study (Gawel & Kuzma-Kozakiewicz, 2016). Also, a decrease in MUNIX has been associated with a significant reduction in the ALS-FRS score (Grimaldi et al., 2017; Jacobsen et al., 2019). Another study (Wirth et al., 2018) found that the MUNIX score significantly differed between lower and upper limb onset patients. Also, MUNIX decreased more rapidly in patients whose lower limbs were involved at disease onset.
We investigated the relationship between electrodiagnostic measures and laboratory indices to find a predicting measure and estimate the number of active motor units and their loss rate. As mentioned earlier, higher serum Cr levels, limb onset, lower BMI, and higher Cr-to-BMI ratio were associated with higher MUNIX scores, representing more active motor units.
Our results and a literature review suggest that biomarkers like Cr, MUNIX score, and BMI can be exploited to estimate the severity of the disease and assess and monitor the function level in ALS patients. Also, our study showed that serum Cr level and lower limb MUNIX score are significantly related, suggesting the benefit of Cr as a minimally invasive means to study the number of active motor units.
One of the strengths of our study was the simultaneous and multifaceted study of three aspects of disease activity together. However, the findings of this study are limited by the small sample size due to the low prevalence of ALS and pandemic conditions, which could affect the results and lead to an underestimation of significant correlations between the parameters. The lack of prospective follow-up due to the challenges during the COVID-19 pandemic is the other limitation of the study. In addition, some of the novel CSF and serum biomarkers that have been popularized within past years for evaluation in patients with ALS, including high-titer GM1 ganglioside antibody, endocrine markers like thyroid hormones, infection markers, and serum protein electrophoresis, are not included in this study.
5. Conclusion
Higher serum Cr was associated with higher- and lower-limb MUNIX in this study. Also, lower-limb MUNIX correlated with lower-limb ALS-FRS. Although there was no direct relationship between serum Cr and functional scores, the two were indirectly related. Our findings suggest that serum Cr may be a reliable and versatile biomarker for disease progression in ALS. Our results also show that lower-limb MUNIX may be an appropriate measure for the level of functioning in the lower limb. Further research with a multidimensional prospective design is needed to clarify the complex relationship between clinical and electrodiagnostic parameters and outcomes in ALS patients.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Iran University of Medical Sciences (Code: IUMS.FMD.REC.1399.767).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
Amyotrophic lateral sclerosis (ALS) is the most common adult-onset motor neuron disease, with an incidence of 2-3 cases per 100000 per year (Kiernan et al., 2011). The average survival rate since the onset of symptoms is about 3-5 years (Armon, 1994). The prognosis is poor for most patients with ALS, particularly when the diagnosis is delayed (Miller & Appel, 2017).
Several markers have been identified for the functional assessment of ALS patients, which include age (Preux et al.,1996; Testa et al., 2004; Chio et al., 2002), symptoms of onset (Tysnes et al., 1991), body mass index (BMI), nutritional status (Stambler et al., 1998), as well as several biological and laboratory markers such as creatine kinase (CK) serum (Amrit & Anderson, 1974), serum creatinine (Cr) (Brooks et al., 2014), and serum albumin (Chio et al., 2014). A better understanding of these factors helps physicians and patients make more informed decisions based on their shared treatment goals (Albert et al., 1999). Also, clinical trials for ALS will be more applicable and easier to conduct as more disease progression markers are validated as outcome measures. Electrodiagnostic measures are another important tool for assessing motor neuron diseases, including ALS (Amin Lari et al., 2019). Measuring the rate of motor units’ loss is a good way to monitor disease progression (Grimaldi et al., 2017). Electrodiagnostic measures such as the motor unit number index (MUNIX) can help determine the number of lost motor units (Jacobsen et al., 2017; Nandedkar et al., 2010; Furtula et al., 2013).
Few studies have examined the relationship between the number of motor units and serologic biomarkers (e.g. CK, albumin, and Cr). We aimed to study the relationship between the laboratory findings, the number of motor units (based on electrodiagnostic studies), and the patient’s functional scores.
2. Materials and Methods
We conducted a cross-sectional study of patients with ALS diagnosed within two years before the study onset. The diagnosis was confirmed by the Awaji criteria, which are reliable criteria based on patient history, clinical examination, and electrodiagnostic testing. Patients with impaired renal function and high serum Cr, those functionally impaired due to medical conditions, and patients who refused to participate in the study were excluded.
Patients’ demographic features, time from symptom onset to diagnosis, and primary presenting symptoms were recorded based on history taking and clinical interviews. Laboratory investigations, including serum CK, Cr, blood urea nitrogen (BUN), and albumin, were undertaken at the central laboratory of Firoozgar Hospital upon enrollment. The MUNIX was calculated based on electromyographic (EMG) assessment of four muscles: Anterior tibialis, abductor digiti minimi, deltoid, and abductor pollicis brevis. This method uses compound motor action potential (CMAP) amplitude and surface electromyographic interference patterns (SIP) to calculate MUNIX, which measures the size and number of motor units. An experienced neurologist in neuromuscular disorders conducted the electromyography. All assessments were done by the same neurologist using the same EMG machine.
The main outcome of this study was the ALS functional rating scale (ALS-FRS), which measures self-sufficiency and disease progression in ALS patients. It is a 10-item inventory covering functional domains, including feeding, grooming, ambulation, and communication; the Persian version has been validated for use in Persian-speaking ALS patients (Afrakhteh et al., 2021). Each item is rated 0 to 4 based on the patient’s level of function. The scores were calculated, and relevant clinical assessments were performed by a neurologist.
Statistical analysis
The Pearson correlation coefficient was used to assess the association between demographic characteristics, clinical features, ALS-FRS, laboratory findings, and EMG findings. We used regression statistics to evaluate the strength of significant associations further. All statistical analyses were performed using SPSS software, version 16. A P<0.05 was considered significant.
Ethical considerations
This research was approved by the Ethics Committee at Iran University of Medical Sciences. We adhered to the principles for medical research on human subjects in accordance with the Helsinki Declaration. Informed consent was obtained from all included participants, having clarified that all the elements of this research, including clinical, electrodiagnostic, and laboratory assessments, were part of the routine care of their illness without any additional costs for the patient.
3. Results
Patients’ characteristics
Twenty-seven patients were enrolled in this study. The mean age of patients was 57.8±10.5, ranging from 36 to 77 years. The majority of patients (20/27) were men. Most patients presented primarily with limb symptoms (20/27[74.7%]), whereas 7 had a bulbar-onset form. The mean interval between ALS diagnosis and enrollment in the study was 8.5±1.6 months. Clinical and laboratory features of the patients are presented in Table 1.

Relationships between study variables
There was no relationship between demographic factors and the primary study outcome (ALS-FRS). Serum albumin, Cr, and CK did not correlate with total ALS-FRS scores (Table 2). Higher serum Cr was associated with higher- and lower-limb MUNIX (P=0.04, the Pearson correlation: 0.39).

Higher BMI was significantly associated with lower total and limb-specific MUNIX scores (P=0.01, Pearson correlation coefficient=0.48). Furthermore, total CMAP was lower among patients with a higher BMI (P=0.02, the Pearson correlation coefficient=0.42). Patients with a limb-onset form of ALS had higher total and upper-limb MUNIX scores than those with a bulbar-onset disease (P=0.02, the Pearson correlation=0.44).
Total MUNIX scores, upper-limb MUNIX, and lower-limb MUNIX scores did not significantly correlate with total ALS-FR scores. However, higher- and lower-limb MUNIX was associated with a better lower limb level of functioning based on ALS-FRS (P=0.02, Pearson correlation: 0.44). The strength assessment of this association using regression analysis indicated that for every 1-point increase in MUNIX, ALS-FRS increased by 0.44 units.
4. Discussion
Our findings can be discussed in the context of three different dimensions: the relationship between laboratory markers and patient level of functioning, laboratory markers in relation to electrodiagnostic markers of disease activity, and the relationship between clinical and demographic characteristics with laboratory and electrodiagnostic markers of disease activity.
Cr is a marker of normal muscle cell metabolism, and a decrease in its serum level indicates a loss of muscle units (Brooks et al., 2014; van Eijk et al., 2018). We found no relationship between serum Cr and patient functioning based on ALS-FRS. A 2019 meta-analysis showed that higher serum Cr at baseline was associated with higher functional scores and that a sharper decline in serum Cr correlated with a more prominent decline in level of functioning (Lanznaster et al., 2019). Another study assessing the relationship between plasma biomarkers and function in 355 well-phenotype ALS patients reported a consistent relationship between serum Cr at all time points during follow-up (Mitsumoto et al., 2020). These findings are inconsistent with ours in that our study shows no relationship between ALS-FRS scores and serum Cr. The reason may be that we missed the association between Cr decline and level of functioning due to lack of longitudinal follow-up or the smaller number of participants in our study.
However, higher serum Cr levels were correlated with higher lower limb MUNIX scores, reflecting the number of active motor units in the lower limb. Moreover, we found that decreased lower-limb MUNIX correlated with lower-limb ALS-FRS scores. These findings are consistent with the notion that Cr is related to active muscle mass (Furtula et al., 2013; Freigang et al., 2021; Neuwirth et al., 2017). However, these findings were exclusively significant for the lower limb, and total MUNIX was not related to serum Cr. This issue may have been due to different clinical features of our patients or the small sample size.
Some studies have reported serum albumin decline as a prognostic factor and a predictor of the rate of disease progression (Chio et al., 2014; Verber et al., 2019; Ong et al., 2017). Interestingly, in some studies, only the decline of albumin and not one-time serum albumin levels were associated with disease progression (Xu et al., 2018). That is why we may not find any association between serum albumin and functional scores in this cross-sectional study.
CK has been a reliable and independent prognostic biomarker due to its association with ALS-frs scores in assessing treatment outcomes in ALS patients (Amrit & Anderson, 1974; Rafiq et al., 2016; Ilzecka & Stelmasiak, 2003). This enzyme is produced in greater quantities to provide energy for the metabolic stress imposed by ALS (Rafiq et al., 2016). In our study, the levels of this marker did not correlate with electrodiagnostic and functional indices.
In this study, patients with limb-onset ALS had a higher- and upper-limb MUNIX and ALS-FRS functional score compared to patients with a bulbar onset; however, this was unrelated to serum Cr, BUN, CPK, and level of functioning.
We also found that patients with higher BMI had lower MUNIX and CMAP scores; however, these factors were unrelated to ALS-FRS score and patient function. In contrast to our findings, most studies reported high BMI as a positive prognostic factor (Chio et al., 2014; Verber et al., 2019). According to these studies, BMI and MUNIX scores were not related, but BMI was linked to survival, functional score, and overall prognosis. Since BMI does not exclusively represent muscle mass, fat-free mass might be a more valid indicator of muscle bulk (Chio et al., 2014). A 2012 study states that low BMI and malnutrition have a neurotoxic effect, highlighting the role of BMI in the prognosis of ALS patients (Spencer & Palmer, 2012). Another study found that in people with a BMI below 30, a higher initial BMI predicts a slower rate of function decline. Still, the relationship is reversed in patients with BMIs over 30 (Reich-Slotky et al., 2013). Although the patients in our study had a mean BMI of 25, a higher BMI was associated with lower MUNIX and CMAP and did not correlate with ALS-FRS.
Age, gender, and disease duration had no significant relationships with laboratory, electrodiagnostic, and function findings.
Electrodiagnostic measures are common and reliable indicators of disease progression in ALS. The MUNIX index represents the number of active muscle units. In our study, cases with higher- and lower-limb MUNIX scores also had higher lower-limb ALS-FRS function scores. We did not find a significant relationship between other electrodiagnostic measures and functional scores. According to our results, the MUNIX score in the lower limb seems to be a more reliable measure for monitoring muscle function in these patients. A 2016 study concluded that the total MUNIX score significantly correlated with the ALS-FRS function score (Jacobsen et al., 2019). The same findings were repeated in another study (Gawel & Kuzma-Kozakiewicz, 2016). Also, a decrease in MUNIX has been associated with a significant reduction in the ALS-FRS score (Grimaldi et al., 2017; Jacobsen et al., 2019). Another study (Wirth et al., 2018) found that the MUNIX score significantly differed between lower and upper limb onset patients. Also, MUNIX decreased more rapidly in patients whose lower limbs were involved at disease onset.
We investigated the relationship between electrodiagnostic measures and laboratory indices to find a predicting measure and estimate the number of active motor units and their loss rate. As mentioned earlier, higher serum Cr levels, limb onset, lower BMI, and higher Cr-to-BMI ratio were associated with higher MUNIX scores, representing more active motor units.
Our results and a literature review suggest that biomarkers like Cr, MUNIX score, and BMI can be exploited to estimate the severity of the disease and assess and monitor the function level in ALS patients. Also, our study showed that serum Cr level and lower limb MUNIX score are significantly related, suggesting the benefit of Cr as a minimally invasive means to study the number of active motor units.
One of the strengths of our study was the simultaneous and multifaceted study of three aspects of disease activity together. However, the findings of this study are limited by the small sample size due to the low prevalence of ALS and pandemic conditions, which could affect the results and lead to an underestimation of significant correlations between the parameters. The lack of prospective follow-up due to the challenges during the COVID-19 pandemic is the other limitation of the study. In addition, some of the novel CSF and serum biomarkers that have been popularized within past years for evaluation in patients with ALS, including high-titer GM1 ganglioside antibody, endocrine markers like thyroid hormones, infection markers, and serum protein electrophoresis, are not included in this study.
5. Conclusion
Higher serum Cr was associated with higher- and lower-limb MUNIX in this study. Also, lower-limb MUNIX correlated with lower-limb ALS-FRS. Although there was no direct relationship between serum Cr and functional scores, the two were indirectly related. Our findings suggest that serum Cr may be a reliable and versatile biomarker for disease progression in ALS. Our results also show that lower-limb MUNIX may be an appropriate measure for the level of functioning in the lower limb. Further research with a multidimensional prospective design is needed to clarify the complex relationship between clinical and electrodiagnostic parameters and outcomes in ALS patients.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Iran University of Medical Sciences (Code: IUMS.FMD.REC.1399.767).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
Afrakhteh, M., Esmaeili, S., Shati, M., Shojaei, S. F., Bahadori, M., & Zamani, B., et al. (2021). Validating the Persian version of the amyotrophic lateral sclerosis-specific quality of life-revised instrument. Current Journal of Neurology, 20(1), 37–42. [DOI:10.18502/cjn.v20i1.6378]
Albert, S. M., Murphy, P. L., Del Bene, M. L., & Rowland, L. P. (1999). A prospective study of preferences and actual treatment choices in ALS. Neurology, 53(2), 278-283. [DOI:10.1212/WNL.53.2.278] [PMID]
Amin Lari, A., Ghavanini, A. A., & Bokaee, H. R. (2019). A review of electrophysiological studies of lower motor neuron involvement in amyotrophic lateral sclerosis. Neurological Sciences, 40(6), 1125-1136. [DOI:10.1007/s10072-019-03832-4]
Amrit, A. N., & Anderson, M. S. (1974). Serum creatine phosphokinase in amyotrophic lateral sclerosis: Correlation with sex, duration, and skeletal muscle biopsy. Neurology, 24(9), 834. [DOI:10.1212/WNL.24.9.834]
Brooks, B., Fischer, M., Sanjak, M., Holsten, S., Kandinov, B., & Bockenek, W., et al. (2014). Serum creatinine, a biomarker for muscle mass in Amyotrophic Lateral Sclerosis (ALS), predicts loss of ambulation measured by ALS Functional Rating Scale-Revised Walking Item Score (ALSFRS-Rw) (P4.085). Neurology, 82(10 Supplement), P4.085. [DOI:10.1212/WNL.82.10_supplement.P4.085]
Chio, A., Calvo, A., Bovio, G., Canosa,A., Bertuzzo, D., & Galmozzi, F, et al. (2014). Amyotrophic lateral sclerosis outcome measures and the role of albumin and creatinine: A population-based study. JAMA Neurology, 71(9), 1134–1142. [DOI:10.1001/jamaneurol.2014.1129] [PMID]
Chiò, A., Mora, G., Leone, M., Mazzini, L., Cocito, D., & Giordana, M. T., et al. (2002). Early symptom progression rate is related to ALS outcome: A prospective population-based study. Neurology, 59(1), 99-103. [DOI:10.1212/WNL.59.1.99] [PMID]
Freigang, M., Wurster, C. D., Hagenacker, T., Stolte, B., Weiler, M., & Kamm, C., et al. (2021). Serum creatine kinase and creatinine in adult spinal muscular atrophy under nusinersen treatment. Annals of Clinical and Translational Neurology, 8(5), 1049–1063. [DOI:10.1002/acn3.51340] [PMID]
Furtula, J., Johnsen, B., Christensen, P. B., Pugdahl, K., Bisgaard, C., & Christensen, M. K, et al. (2013). MUNIX and incremental stimulation MUNE in ALS patients and control subjects. Clinical Neurophysiology, 124(3), 610-618. [DOI:10.1016/j.clinph.2012.08.023]
Gawel, M., & Kuzma-Kozakiewicz, M. (2016). Does the MUNIX method reflect clinical dysfunction in amyotrophic lateral sclerosis: A practical experience. Medicine (Baltimore), 95(19), e3647. [DOI:10.1097/MD.0000000000003647]
Grimaldi, S., Duprat, L., Grapperon, A. M., Verschueren, A., Delmont, E., & Attarian, S. (2017). Global motor unit number index sum score for assessing the loss of lower motor neurons in amyotrophic lateral sclerosis. Muscle & Nerve, 56(2), 202-206. [DOI:10.1002/mus.25595]
Iłzecka, J., & Stelmasiak, Z. (2003). Creatine kinase activity in amyotrophic lateral sclerosis patients. Neurological Sciences: Official Journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology, 24(4), 286-287. [PMID]
Jacobsen, A. B., Bostock, H., Fuglsang-Frederiksen, A., Duez, L., Beniczky, S., & Moller, A. T., et al. (2017). Reproducibility, and sensitivity to motor unit loss in amyotrophic lateral sclerosis, of a novel MUNE method: MScanFit MUNE. Clinical Neurophysiology, 128(7), 1380-1388. [DOI:10.1016/j.clinph.2017.03.045]
Jacobsen, A. B., Bostock, H., & Tankisi, H. (2019). Following disease progression in motor neuron disorders with 3 motor unit number estimation methods. Muscle & Nerve, 59(1), 82-87. [DOI:10.1002/mus.26304]
Kiernan, M. C., Vucic, S., Cheah, B. C., Turner, M. R., Eisen, A., & Hardiman, O., et al. (2011). Amyotrophic lateral sclerosis. Lancet (London, England), 377(9769), 942–955. [DOI:10.1016/S0140-6736(10)61156-7] [PMID]
Lanznaster, D., Bejan-Angoulvant, T., Patin, F., Andres, C. R., Vourc’h, P., & Corcia P, et al. (2019). Plasma creatinine and amyotrophic lateral sclerosis prognosis: A systematic review and meta-analysis. Amyotrophic Lateral Sclerosis & Frontotemporal Degeneration, 20(3-4), 199-206. [DOI:10.1080/21678421.2019.1572192]
Miller, R. G., & Appel, S. H. (2017). Introduction to supplement: The current status of treatment for ALS. Amyotrophic Lateral Sclerosis & Frontotemporal Degeneration, 18(sup1), 1–4. [DOI:10.1080/21678421.2017.1361447] [PMID]
Mitsumoto, H., Garofalo, D. C, Santella, R. M., Sorenson, E. J., Oskarsson, B., Fernandes, J. A. M., et al. (2020). Plasma creatinine and oxidative stress biomarkers in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 21(3-4), 263-272. [DOI:10.1080/21678421.2020.1746810]
Nandedkar, S. D., Barkhaus, P. E., & Stalberg, E. V. (2010). Motor unit number index (MUNIX): Principle, method, and findings in healthy subjects and in patients with motor neuron disease. Muscle & Nerve, 42(5), 798-807. [DOI:10.1002/mus.21824]
Neuwirth, C., Barkhaus, P. E., Burkhardt, C., Castro, J., Czell, D., & de Carvalho, M., et al. (2017). Motor Unit Number Index (MUNIX) detects motor neuron loss in pre-symptomatic muscles in Amyotrophic Lateral Sclerosis. Clinical Neurophysiology, 128(3), 495-500. [DOI:10.1016/j.clinph.2016.11.026]
Ong, M. L., Tan, P. F., & Holbrook, J. D. (2017). Predicting functional decline and survival in amyotrophic lateral sclerosis. PloS One, 12(4), e0174925. [DOI:10.1371/journal.pone.0174925] [PMID]
Preux, P. M., Couratier, P., Boutros-Toni, F., Salle, J. Y., Tabaraud, F., & Bernet-Bernady, P., et al. (1996). Survival prediction in sporadic amyotrophic lateral sclerosis. Age and clinical form at onset are independent risk factors. Neuroepidemiology, 15(3), 153-160. [DOI:10.1159/000109902] [PMID]
Rafiq, M. K., Lee, E., Bradburn, M., McDermott, C. J., & Shaw, P. J. (2016). Creatine kinase enzyme level correlates positively with serum creatinine and lean body mass, and is a prognostic factor for survival in amyotrophic lateral sclerosis. European Journal of Neurology, 23(6), 1071-1078. [DOI:10.1111/ene.12995]
Reich-Slotky, R., Andrews, J., Cheng, B., Buchsbaum, R., Levy, D., & Kaufmann, P., et al. (2013). Body mass index (BMI) as predictor of ALSFRS-R score decline in ALS patients. Amyotrophic lateral Sclerosis & Frontotemporal Degeneration, 14(3), 212–216. [DOI:10.3109/21678421.2013.770028] [PMID]
Spencer, P. S., & Palmer, V. S. (2012). Interrelationships of undernutrition and neurotoxicity: Food for thought and research attention. Neurotoxicology, 33(3), 605-616. [DOI:10.1016/j.neuro.2012.02.015]
Stambler, N., Charatan, M., & Cedarbaum, J. M. (1998). Prognostic indicators of survival in ALS. ALS CNTF Treatment Study Group. Neurology, 50(1), 66–72. [DOI:10.1212/WNL.50.1.66] [PMID]
Testa, D., Lovati, R., Ferrarini, M., Salmoiraghi, F., & Filippini, G. (2004). Survival of 793 patients with amyotrophic lateral sclerosis diagnosed over a 28-year period. Amyotrophic Lateral Sclerosis and other Motor Neuron Disorders: Official Publication of the World Federation of Neurology, Research Group on Motor Neuron Diseases, 5(4), 208–212. [DOI:10.1080/14660820410021311] [PMID]
Tysnes, O. B., Vollset, S. E., & Aarli, J. A. (1991). Epidemiology of amyotrophic lateral sclerosis in Hordaland county, western Norway. Acta Neurologica Scandinavica, 83(5), 280–285.[DOI:10.1111/j.1600-0404.1991.tb04701.x] [PMID]
van Eijk R. P. A., Eijkemans, M. J. C., Ferguson, T. A., Nikolakopoulos, S., Veldink, J. H., & van den Berg, L. H. (2018). Monitoring disease progression with plasma creatinine in amyotrophic lateral sclerosis clinical trials. Journal of Neurology, Neurosurgery, and Psychiatry, 89(2), 156-161. [DOI:10.1136/jnnp-2017-317077]
Verber, N. S., Shepheard, S. R., Sassani, M., McDonough, H. E., Moore, S. A., & Alix, J. J. P., et al. (2019). Biomarkers in motor neuron disease: A state of the art review. Frontiers in Neurology, 10, 291. [DOI:10.3389/fneur.2019.00291] [PMID]
Wirth, A. M., Khomenko, A., Baldaranov, D., Kobor, I., Hsam, O., & Grimm, T, et al. (2018). Combinatory Biomarker Use of Cortical Thickness, MUNIX, and ALSFRS-R at Baseline and in Longitudinal Courses of Individual Patients With Amyotrophic Lateral Sclerosis. Frontiers in Neurology, 9, 614. [DOI:10.3389/fneur.2018.00614]
Type of Study: Original |
Subject:
Clinical Neuroscience
Received: 2021/05/28 | Accepted: 2021/07/10 | Published: 2024/07/20
Received: 2021/05/28 | Accepted: 2021/07/10 | Published: 2024/07/20
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








