Volume 14, Issue 6 (November & December 2023)
BCN 2023, 14(6): 813-826 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Abouee-Mehrizi A, Motalebi Kashani M, Rasoulzadeh Y, Mehdipour A, Nasirzadeh N, Shatouei-Gharenjeh O et al . Co-exposure to Toluene and Noise Made Synergistic and Antagonistic Effects on Some Neurotoxic Parameters in New Zealand White Rabbits. BCN 2023; 14 (6) :813-826
URL: http://bcn.iums.ac.ir/article-1-2130-en.html
URL: http://bcn.iums.ac.ir/article-1-2130-en.html
Amirreza Abouee-Mehrizi1 

 , Masoud Motalebi Kashani2
, Masoud Motalebi Kashani2 

 , Yahya Rasoulzadeh *3
, Yahya Rasoulzadeh *3 

 , Ahmad Mehdipour4
, Ahmad Mehdipour4 

 , Nafiseh Nasirzadeh5
, Nafiseh Nasirzadeh5 

 , Omid Shatouei-Gharenjeh1
, Omid Shatouei-Gharenjeh1 

 , Abbas Ebrahimi-Kalan6
, Abbas Ebrahimi-Kalan6 




 , Masoud Motalebi Kashani2
, Masoud Motalebi Kashani2 

 , Yahya Rasoulzadeh *3
, Yahya Rasoulzadeh *3 

 , Ahmad Mehdipour4
, Ahmad Mehdipour4 

 , Nafiseh Nasirzadeh5
, Nafiseh Nasirzadeh5 

 , Omid Shatouei-Gharenjeh1
, Omid Shatouei-Gharenjeh1 

 , Abbas Ebrahimi-Kalan6
, Abbas Ebrahimi-Kalan6 


1- Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran.
2- Department of Occupational Health & Safety, School of Health, Kashan University of Medical Sciences, Kashan, Iran.
3- Department of Occupational Health Engineering, Faculty of Health, Tabriz University of Medical Sciences, Tabriz, Iran.
4- Department of Tissue Engineering, Faculty of Advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran.
5- Department of Occupational Health Engineering, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran.
6- Department of Neurosciences and Cognitive, Neurosciences Research Center, Faculty of Advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran.
2- Department of Occupational Health & Safety, School of Health, Kashan University of Medical Sciences, Kashan, Iran.
3- Department of Occupational Health Engineering, Faculty of Health, Tabriz University of Medical Sciences, Tabriz, Iran.
4- Department of Tissue Engineering, Faculty of Advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran.
5- Department of Occupational Health Engineering, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran.
6- Department of Neurosciences and Cognitive, Neurosciences Research Center, Faculty of Advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran.
Full-Text [PDF 3688 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Noise is a fundamental property of different activities produced by changes in air pressure (Snyder, 2000). Excessive noise hurts various systems in the body, including the cardiovascular system, auditory system, psychological system, and neural system (Babisch, 2003; Brunoni et al., 2008). It has been proven that exposure to noise causes some disorders in the brain tissue, such as congestion, edema, blood vessel expansion, neuroglial hyperplasia, and mild atypia (Xue et al., 2014). Regrettably, the main cause or intensifying factors of many disasters and accidents, which could be related to the neurological and psychological disorders provoked by exposure to noise, such as enhancing the occurrence of violence and increasing workplace accidents (Toppila et al., 2009) may not be well detected or resolved.
Toluene is one of the most suitable solvents with various applications in the plastic, rubber, leather, adhesives, and pharmaceutical industries (Acton, 2013). Previous studies have demonstrated that exposure to toluene induces different diseases in various organs of the body, including the liver and kidneys (Schardein & Macina, 2006). The psychological and neurotoxic effects of toluene are complicated (Lowinson et al., 2005) and include the loss of balance, headache, weakness, fatigue, and joy feelings. In addition, toluene has a devastating effect on the nervous tissue of the brain (Dobbs, 2009).
There are many different physical and chemical stressors in the industry. Noise and toluene are some of the prevalent physical and chemical stressors in different workplaces. Previous studies have indicated that different stressors could deteriorate some brain disorders (Demır et al., 2017; Xiu et al., 2009).
The debate about the health effects of cocoa exposure to chemical and physical pollutants has been highlighted by many discussions about the interaction between different chemical and physical harmful agents (Sexton & Linder, 2011; Streffer et al., 2013). As stated by numerous reports and studies, many people in various occupations are not only exposed to noise (such as constructions, factories, nightclubs, and airports) (Tripathy, 2008) or only toluene (such as petrochemical, plastics factories, adhesive factories, and pharmaceutical factories) (Acton, 2013; Miller, 2002) but also simultaneously exposed to noise and toluene (Dobie, 2015; Rea & Patel, 2014). This indicates a need to understand various perceptions of neurotoxicity induced by simultaneous exposure to noise and toluene. Therefore, this experimental study assessed how co-exposure to toluene and noise changes biochemical factors and brain tissue in rabbits.
2. Materials and Methods
Animals and groups
Twenty-four healthy adult male white New Zealand rabbits (Pasteur Institute, Iran) weighing 2.83±0.41 kg were used in this experiment. Animals were kept on a 12:12 hour light/dark circulation period at controlled ambient temperature (21±2°C) and with a humidity of 50 to 70% while having access to food (standard rabbit pellet, Behparvar Feed Manufacturing Company, Tehran) and treated water. Animals were housed in the laboratory for 14 days before the experiments to adapt to the environmental conditions of the laboratory. They were randomly segregated into four groups (n=6 per group) including, noise exposure group (group 1), toluene exposure group (group 2), co-exposure to toluene and noise group (group 3), and control group (group 4). Exposure duration was 14 consecutive days (8 h/day from 9:00 AM to 5:00 PM) for all groups. The control group was placed in the exposure chamber with clean air flowing (toluene=0 ppm) and <50 dBA noise (background noise) with an exposure duration of 14 consecutive days. Then, group 1 was placed in the exposure chamber to receive 100±5 decibel (dBA) noise. After that, group 2 was placed in the same exposure to receive toluene (1000±50 ppm) for the exposure duration. Then, group 3 was placed in the exposure chamber and exposed to noise and toluene with the same specifications as groups 1 and 2 for the exposure duration. The temperature was 23±3°C and the relative humidity was 65%-80% inside the exposure chamber for all the experimental groups.
Exposure chamber
Animals in all groups (n=6) were exposed in the same exposure chamber with the dimensions of 50×60×90 cm3 made of clear and transparent polycarbonate sheets, which were coated with transparent polyethylene terephthalate (PET) adhesive tape. Specifications of the exposure chamber were chosen according to the animals’ welfare requirements (Gad, 2006) and based on the reverberant chamber features (Cobo et al., 2009; Moreno et al., 2000) to generate the same noise specifications inside the exposure chamber and to make noise intensity independent from distance in the chamber.
Noise exposure set-up
White noise at 100±5 dBA (70-20000 Hz) was produced by the White Noise Generator Software, version 1.3.12 and monitored by Cool Edit Pro Software, version 2.1 and Syntrillium Software version 2.0. The selected noise was amplified by a power amplifier (3030 W, multi tone) and transported to a loudspeaker. The loudspeaker was placed directly above the chamber on the chamber roof. The noise level was continuously measured using a sound level analyzer (TES 1358 sound analyzer real-time 1/1 & 1/3 octave band analyzer) in a 1/3 octave band during the exposure (groups 1 and 3). The sound level meter microphone was placed at the same level as the ears of the animals by checking the designed outlets (made of rubber tape and coated with PET adhesive tape) every 30 min in order to monitor noise specifications.
Toluene exposure set-up
Exposure to 1000±50 ppm toluene happened through its surface evaporation. In summary, constant generation of toluene vapors was done using an impinger (capacity of 250 mL) with the content of 100 mL toluene liquid (Merck KGaA, extra pure, Darmstadt, Germany) and via injecting 20 mL toluene into the impinger every 90 min on the periodical basis. The toluene concentration was analyzed during pilot studies using a calibrated humidity-resistant real-time instrument (Phocheck, Ion Science Ltd, Cambs UK 07-01782) and also charcoal absorbents with gas chromatography (model agilent 7890A GC system) established on the 1501 NIOSH method. Accordingly, changing the toluene concentration was negligible (5%) during the exposure time in the pilot and real experiments. According to the pilot experiments, the pure air flow rate was 30 L/min and the toluene vapor-contained air flow rate was 3 L/min. Moreover, before starting real exposures, the purity of the toluene vapors was confirmed by air sampling from the exposure chamber (active charcoal samples) and using a gas chromatography-mass spectrometry instrument (Agilent 6890/5973 GCMS). To obtain the target concentration of toluene constantly and continuously, a mixer chamber with the same materials and specifications as the exposure chamber with the dimensions of 50×50×20 cm3 was used before entering the air into the exposure chamber. To monitor and check toluene concentration, the same real-time instrument (Phocheck), which had been already calibrated and utilized in pilot experiments was used. Air samples were prepared from inside the exposure chamber through checking outlets designed on four sides in the surrounding walls of the exposure chamber (checking outlets).
Blood sampling
Blood samples were collected from the marginal vein of the ears of each animal in five stages, as mentioned in Figure 1.

Tissue sampling and processing
Rabbits were sacrificed after anesthesia with 35 mg/kg ketamine and 5 mg/kg xylazine (IM injection). The animals were sacrificed 14 days after the end of the exposure in each group. The whole brain consisting of the cerebral cortex, hippocampus, and cerebellum was dissected immediately after the animals were sacrificed and fixed in 10% formalin solution (formaldehyde solution, Merck KGaA, Darmstadt, Germany) at pH 7.2. After the fixation, paraffin blocks were prepared. Moreover, for biochemical analyses, the cerebral cortex of the brain tissues was immediately collected and kept at -80°C until the time of biochemical experiments. Dehydration and paraffin impregnation phases of the tissue samples were made by an autotechnicon device. Then, paraffin molding was carried out and the slides were prepared using a microtome with a typical diameter of 5 μ. Eventually, the slides were stained with hematoxylin and eosin (H&E) and histopathologic changes were assessed by light microscopy (Nikon Eclipse E100, Japan).
Homogeneity of brain tissue
First, 100 g of the cerebral cortex of the brain samples was weighed out of all the groups to measure the oxidant/antioxidant parameters of the brain tissue. Afterward, they were dissolved in 1 cc phosphate-buffered saline and homogenized by a homogenizer device (ultrasonic homogenizer Hielscher UIP-1500HD). Then, the samples were centrifuged by a centrifuge device at 1000 g at 4°C for 20 min. Required samples were taken from the clear supernatant liquid of the centrifuged samples using a sampler and placed in microtubes. The supernatant protein liquid of the cerebral cortex was measured using a commercial kit (Pars Azmoon Company, Karaj, Iran). Finally, the parameters in the supernatant liquid were normalized by measuring the amount of supernatant protein liquid of the brain tissue and valued per mg of tissue protein.
Determining oxidant and anti-oxidant factors in the cerebral cortex using the spectrophotometric method
Total antioxidant capacity (TAC) was measured by a Naxifer™ diagnosis kit (TAC assay kit, Navand Lab Kit, Urmia, Iran). The levels of glutathione peroxidase (GPx) were measured by a Biorexfars diagnosis kit (production code: BXC0551, Shiraz, Iran). Catalase (CAT) levels were measured by a Cayman chemical company kit (CAT assay kit, Item No. 707002), and superoxide dismutase (SOD) levels were measured by a non-enzymatic method Nasdox™ diagnosis kit (Navand Lab Kit, Urmia, Iran). All the measurements were done according to the instructions provided by the kit manufacturers; in addition, malondialdehyde (MDA) levels were measured using the thiobarbituric acid (TBA) method (Janero, 1990; Stewart & Bewley, 1980).
Determining the serum levels of brain-derived neurotrophic factor-α (BDNF-α) using the ELISA method
BDNF-α levels were measured by Rabbit ELISA kits (Hangzhou Eastbiopharm CO., LTD, Hangzhou, China) and based on the manufacturer’s instructions (Cat. No: CK-E91855). In this experiment, the State Fax 2100 ELISA plate reader (Awareness Technology, Inc., USA) and the State Fax 2600 ELISA plate washer (Awareness Technology, Inc., USA) were employed.
Statistical analysis
The generalized estimating equation (GEE ) statistical method was used to assess BDNF-α levels so that differences between different repeats in different groups were compared by SPSS software, version 25. Moreover, analysis of variance (ANOVA ) followed by Tukey’s post hoc test was used to compare the oxidant and anti-oxidant parameters and the mean brain tissue weight/body weight in different groups in Minitab software, Version 18. The statistical significance level was considered at 0.05. The logarithm of abnormal parameters was used for GEE statistical analysis.
3. Results
Biochemical changes
According to Figure 2E, TAC values in group 1 were significantly higher than those in groups 2-4. Figure 2 A, B, C & D shows CAT, GPx, SOD, and MDA levels in group 1.

Exposure to noise significantly decreased BDNF-α levels compared to the control and toluene exposure groups on the 14th day after exposure. Moreover, co-exposure to noise and toluene significantly increased BDNF-α levels compared to the noise and toluene exposure groups on the 3rd day after exposure. Furthermore, co-exposure to noise and toluene significantly decreased BDNF-α levels compared to the toluene exposure group on the 7th and 14th days after exposure (Figure 3).

Brain tissue weight changes
According to Figure 4, the average brain weight/body weight in the noise exposure group was significantly lower than that in the toluene exposure group.

Histopathological changes
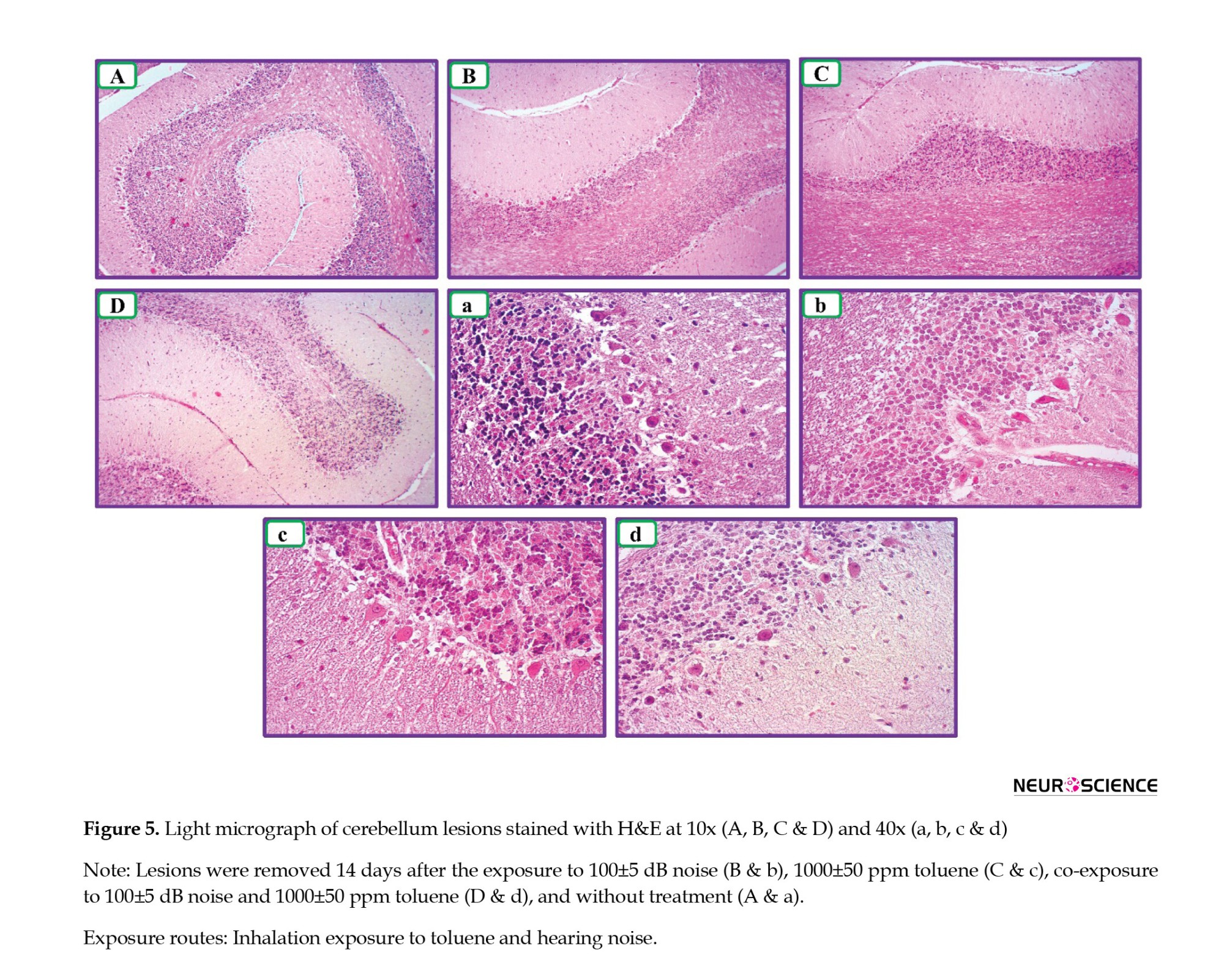
The cerebellum was in normal conditions in the control group. However, the population of Purkinje cells decreased in the cerebellum in group 1; in addition, pyknosis appeared in Purkinje cells in this group. The relative reduction in the number of cells was visible in the granular layer in groups 1 and 2. Granular cells not only reduced relatively but also appeared massively in group 2. Pathological changes were more specific in the cerebellum area in group 3. A decrease in the population of Purkinje cells, a decrease in the thickness of the granular layer, and a decrease in granule cells were some of the significant changes observed in the cerebellum in group 3 (Figure 5).
The cortex area in the frontal part of the brain was normal in the control group. Nevertheless, gliosis was detected in the prefrontal cortex area in groups 1-3 compared to the control group. The number of pyknotic cells in groups 1 and 3 appeared higher than group 2 in the prefrontal cortex area. Minor lymphocyte infiltration, which was possibly induced by the retrograde effect of toluene and transmitted to the frontal part of the brain cortex through olfactory nerves, was visible in the prefrontal cortex area in group 2. Some pathological changes were noticed in the prefrontal cortex area in group 3, such as disorganization in the cortex layer, relative reduction of pyramidal cells, vacuolization, and chromatolysis (Figure 6).

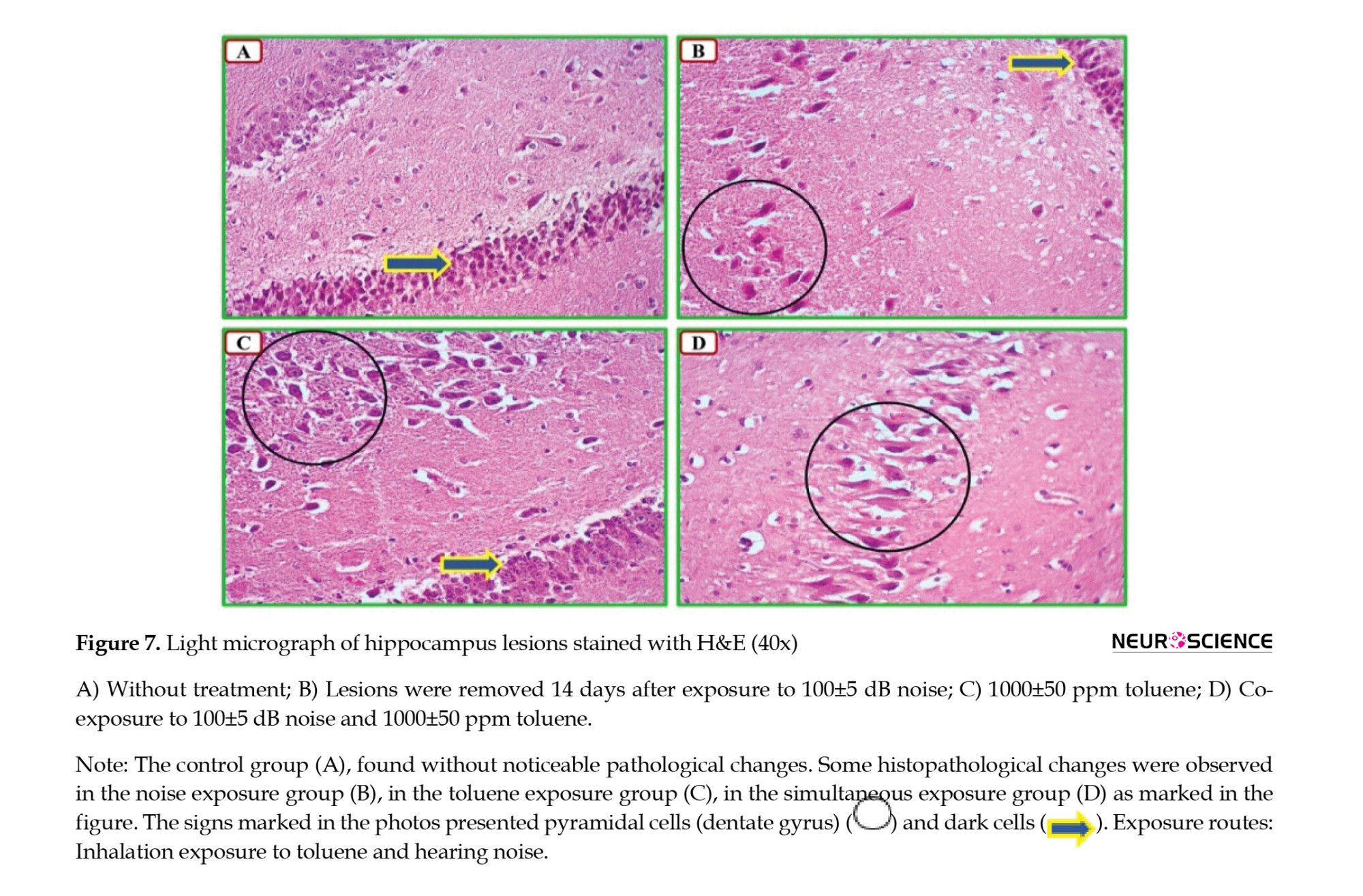
The light micrographs of the hippocampus area were normal in the control group. Dark cells were reduced in population and also were pyknotic in the hippocampus area in group 1, even more severely than in group 2. However, it was not remarkable compared to group 3. Moreover, the pyramidal cells in group 1 noticeably decreased in population compared to group 2 in the hippocampus area. But it was not remarkable compared to group 3. All the changes reported for group 1 also appeared in group 2 in the hippocampus area but were milder than in group 3 so the pathological changes appearing in group 3 were more severe than those in groups 1 and 2 (Figure 7).
4. Discussion
The results of this study showed that the noise caused an increase in TAC in the cerebral cortex. In a study on the effects of industrial noise stress on workers, the serum levels of CAT and TAC were significantly higher in workers exposed to noise stress (Bagheri Hosseinabadi et al., 2019). Increased SOD levels were reported by exposure to noise at 100 dB in the cerebral cortex of the rats’ brain (Samson et al., 2007); however, increased anti-oxidant factors after exposure to high levels of noise may be due to induced oxidative stress during noise exposure. Moreover, activating protective mechanisms in the body, such as producing TAC, GPx, SOD, and CAT as anti-oxidant factors and decreasing MDA levels as an oxidative factor are predictable during oxidative stress to prevent the excessive generation of free radicals (Rosenbaum et al., 1994). Furthermore, some of the previous studies have shown that toluene induces oxidative stress in several brain regions by producing reactive oxygen species (ROS) (Kodavanti et al., 2011). As a result, ROS can induce apoptosis in the brain (Redza-Dutordoir & Averill-Bates, 2016). Therefore, co-exposure to toluene and noise changed oxidant and anti-oxidant parameters in the brain tissue.
BDNF is a protein encoded by a gene called BDNF. This protein promotes the growth and development of the central and peripheral nervous system. It also triggers nerve synapses and establishes neural connections. It is most active in the hippocampus and the upper part of the brain. The highest production of BDNF is in the brain, the amount of which is different in brain damage, especially with environmental stress.
The current study revealed that co-exposure to toluene and noise increased the serum levels of BDNF; however, exposure to noise decreased BDNF serum levels compared to the control group. In the study on the effects of toluene inhalation exposure (5 ppm) for five days, a decrease in the relative mRNA expression of BDNF, and an increase in the mRNA expression levels of NF-κB were reported in the hippocampus of the infant mice during brain development (Win‐Shwe et al., 2012). In a study on the effects of chronic stress on BDNF levels, inducing 80 dB noise stress for five weeks made a significant reduction in hippocampal BDNF levels in rats (Taliaz et al., 2011). Moreover, another study demonstrated that acute stress caused oxidative stress and consequently, produced an increase in BDNF levels (Hacioglu et al., 2016). This means that there is a direct relationship between BDNF levels and oxidative stress indicators (Hacioglu et al., 2016). On the other hand, previous studies have shown that some stressors, such as noise initiate an increase in corticosterone levels (Laugero & Moberg, 2000; Lehmann et al., 2002; Şahin & Gümüşlü, 2007). Consequently, corticosterone can induce a decrease in BDNF levels (Jacobsen & Mork, 2006; Schaaf et al., 2000).
This study provides considerable insight into the combined effects of noise and toluene on neurological disorders. Based on the results of the present study related to oxidative stress induced after co-exposure to toluene and noise and according to the multiple combined stressors (Piggott et al., 2015), different synergistic and antagonistic effects induced by exposure to noise and toluene are provided in Appendices 1 and 2.
This study demonstrated that co-exposure to noise and toluene can induce different histopathological changes in the brain tissue, such as lymphocyte infiltration, a relative reduction in pyramidal cells, chromatolysis, and vacuolization. There were remarkable signs of inflammation in the co-exposure group, even more severe than the noise and toluene exposure groups. Based on the results obtained, it seems that even though there were no significant changes induced by noise exposure, there were some changes in the brain tissue, which if persisted, can be an important alarm. Induced mild atypia, edema, hyperemia, expansion of blood vessels, and glial cell hyperplasia were reported in several areas in the brain tissue in female rats by exposure to noise at 95 dB (4000 Hz) for two weeks (Xue et al., 2014). Makhlouf et al., (2014) reported a decrease in the thickness of the pyramidal cell layer of CA1 (cornu ammonis area 1), CA3 (cornu ammonis area 3), and also the granular cells, which exhibited degeneration and loss of many cells after exposure to 100 dB noise during four weeks (4 h/day) in male albino rats (Makhlouf et al., 2014). Abousetta et al. (2014) indicated that exposure to 100 dB white noise for 6 h/day during four consecutive weeks induced a decrease in the thickness of dentate gyrus (DG) granular cells and pyramidal cells with the loss and degeneration of numerous cells in male albino rats (Abousetta et al. 2014).
Enhanced glial fibrillary acidic protein (GFAP)-immunoreactive cytoplasm was demonstrated in the white matter of the cerebellum and in the dentate gyrus of the hippocampus by inhaling 1500 ppm toluene for four and seven days in the brain tissue of the rats (Gotohda et al., 2000). In addition, Gotohda et al., (2000) showed that chronic exposure to 3000 ppm toluene for 12 weeks caused severe degenerative changes, dark pyknotic nuclei, and small cytoplasm in neurons of different hippocampal regions in rats (Gotohda et al., 2000). Kanter (2008) demonstrated that exposure to 3000 ppm toluene for 12 weeks (8 h/day) induced dilated cisternae of endoplasmic reticulum, small cytoplasm, evidently inflamed mitochondria with deteriorated cristae in the hippocampus in male Wistar albino rats (Kanter, 2008). Tas et al., (2012) reported that exposure to a high dose of toluene (5200 mg/kg/gavage) for two weeks caused a significant increase in the immune reactivity of Bax in the cerebellum and the brain cortex (Tas et al., 2012). Demır et al., (2017) demonstrated that acute exposure of New Zealand rabbits to toluene by a single dose (876 mg/kg) intraperitoneal (IP) injection into the brain caused severe dilation of blood vessels, diffuse cell borders, vacuolar degeneration, severe degeneration of the compensation, perivascular demyelination, gliosis, and several necrosis and pyknotic cells (Demır et al., 2017). Moreover, a noticeable enhancement in the count of apoptotic cells in the cerebral granule layer was indicated by inhaling 1200-1800 ppm toluene for 6 h per day in rats (Dalgaard et al., 2001). Nevertheless, Seo et al. (2010) indicated that exposure to toluene at the dose of 500 mg/kg did not cause apoptosis in the hippocampus in mice (Seo et al., 2010). Considering the findings proven by previous studies and the results obtained from this study, noise, and toluene are some of the noticeable neurotoxic pollutants.
5. Conclusion
This study indicated that co-exposure to toluene and noise might induce neurotoxicity in white New Zealand rabbits. Histopathological experiments indicated that co-exposure to toluene and noise could aggravate neurotoxic effects. Co-exposure to noise and toluene induced some pathological changes in the cerebellum, hippocampus, and frontal section. Nonetheless, further studies are needed to determine the definite neurotoxic effects induced by co-exposure to toluene and noise.
Limitations
Although this study showed non-auditory changes induced by exposure to toluene and noise, neurotoxic effects induced by chronic exposure to noise and toluene were not identified. Furthermore, this study did not evaluate other biological and pathological factors during or after exposure. Therefore, further studies are necessary in order to enhance the results obtained from this work.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the National Ethics Committee of Tabriz University of Medical Sciences, for biomedical research in Iran (Code: IR.TBZMED.REC.1396.953).
Funding
This study was accomplished within the framework of the master’s thesis of Amirreza Abouee Mehrizi, approved by Department of Occupational Health Engineering, School of Health, Tabriz University of Medical Sciences (Code: 378).
Authors' contributions
Conceptualization, supervision, investigation, data analysis and writing: Amirreza Abouee-Mehrizi and Yahya Rasoulzadeh; Methodology: Amirreza Abouee-Mehrizi, Yahya Rasoulzadehand, and Masoud Motallebi-Kashani; Data collection: Amirreza Abouee-Mehrizi, Yahya Rasoulzadeh, and Ahmad Mehdipour; Nafiseh Nasirzadeh, Omid Shatouei-Gharenjeh and Abbas Ebrahimi-Kalan.
Conflict of interest
The authors declared no conflict of interest.
References
Abousetta, A., Makhlouf, N. A., & El-Beshbishy, R. A. (2014). The effects of concomitant Ginkgo intake on noise induced hippocampus injury. Possible auditory clinical correlate. Egyptian Journal of Ear, Nose, Throat and Allied Sciences 15(3), 231-239. [DOI:10.1016/j.ejenta.2014.05.003]
Acton, Q. A. (2013). Advances in toluene research and application: 2013 edition: Scholarlybrief. Atlanta: ScholarlyEditions. [Link]
Babisch, W. (2003). Stress hormones in the research on cardiovascular effects of noise. Noise & Health, 5(18), 1–11. [PMID]
Bagheri Hosseinabadi, M., Khanjani, N., Ebrahimi, M. H., Mirbadie, S. R., & Biganeh, J. (2019). The effects of industrial noise exposure on lipid peroxidation and antioxidant enzymes among workers. International Archives of Occupational and Environmental Health, 92(7), 1041–1046. [DOI:10.1007/s00420-019-01444-1] [PMID]
Brunoni, A. R., Lopes, M., & Fregni, F. (2008). A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: Implications for the role of neuroplasticity in depression. The International Journal of Neuropsychopharmacology, 11(8), 1169–1180. [DOI:10.1017/S1461145708009309] [PMID]
Cobo, P., Murillo-Cuesta, S., Cediel, R., Moreno, A., Lorenzo-García, P., & Varela-Nieto, I. (2009). Design of a reverberant chamber for noise exposure experiments with small animals. Applied Acoustics 70(8), 1034-1040. [DOI:10.1016/j.apacoust.2009.03.005]
Dalgaard, M., Hossaini, A., Hougaard, K. S., Hass, U., & Ladefoged, O. (2001). Developmental toxicity of toluene in male rats: Effects on semen quality, testis morphology, and apoptotic neurodegeneration. Archives of Toxicology, 75(2), 103–109. [DOI:10.1007/s002040000209] [PMID]
Demır, M., Cicek, M., Eser, N., Yoldaş, A., & Sısman, T. (2017). Effects of acute toluene toxicity on different regions of rabbit brain. Analytical Cellular Pathology, 2017, 2805370. [DOI:10.1155/2017/2805370] [PMID] [PMCID]
Dobbs, M. R. (2009). Clinical neurotoxicology E-book: Syndromes, substances, environments. Edinburgh: Elsevier Health Sciences. [Link]
Dobie, R. A. (2015). Medical-legal evaluation of hearing loss, third edition. San Diego: Plural Publishing, Incorporated. [Link]
Gad, S. C. (2006). Animal models in toxicology. Oxfordshire: Taylor & Francis. [DOI:10.1201/9781420014204]
Gotohda, T., Tokunaga, I., Kubo, S., Morita, K., Kitamura, O., & Eguchi, A. (2000). Effect of toluene inhalation on astrocytes and neurotrophic factor in rat brain. Forensic Science International, 113(1-3), 233–238. [DOI:10.1016/S0379-0738(00)00215-2] [PMID]
Hacioglu, G., Senturk, A., Ince, I., & Alver, A. (2016). Assessment of oxidative stress parameters of brain-derived neurotrophic factor heterozygous mice in acute stress model. Iranian Journal of Basic Medical Sciences, 19(4), 388–393. [PMID] [PMCID]
Jacobsen, J. P., & Mørk, A. (2006). Chronic corticosterone decreases brain-derived neurotrophic factor (BDNF) mRNA and protein in the hippocampus, but not in the frontal cortex, of the rat. Brain Research, 1110(1), 221–225. [DOI:10.1016/j.brainres.2006.06.077] [PMID]
Janero, D. R. (1990). Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radical Biology & Medicine, 9(6), 515–540. [DOI:10.1016/0891-5849(90)90131-2] [PMID]
Kanter, M. (2008). Nigella sativa and derived thymoquinone prevents hippocampal neurodegeneration after chronic toluene exposure in rats. Neurochemical Research, 33(3), 579–588. [DOI:10.1007/s11064-007-9481-z] [PMID]
Kodavanti, P. R., Royland, J. E., Richards, J. E., Besas, J., & Macphail, R. C. (2011). Toluene effects on oxidative stress in brain regions of young-adult, middle-age, and senescent Brown Norway rats. Toxicology and Applied Pharmacology, 256(3), 386–398. [DOI:10.1016/j.taap.2011.04.012] [PMID]
Laugero, K. D., & Moberg, G. P. (2000). Energetic response to repeated restraint stress in rapidly growing mice. American Journal of Physiology. Endocrinology and Metabolism, 279(1), E33–E43. [DOI:10.1152/ajpendo.2000.279.1.E33] [PMID]
Lehmann, J., Russig, H., Feldon, J., & Pryce, C. R. (2002). Effect of a single maternal separation at different pup ages on the corticosterone stress response in adult and aged rats. Pharmacology, Biochemistry, and Behavior, 73(1), 141–145. [DOI:10.1016/S0091-3057(02)00788-8] [PMID]
Lowinson, J. H. (Ed.). (2005). Substance abuse: A comprehensive textbook. Lippincott Williams & Wilkins. `
Makhlouf, N. A., El-Beshbishy, R. A., & Abousetta, A. (2014). Ginkgo modulates noise-induced hippocampal damage in male albino rats: A light and electron microscopic study. Egyptian Journal of Histology, 37(1), 159-174. [DOI:10.1097/01.EHX.0000444078.17248.ab]
Miller, R. L. (2002). The encyclopedia of addictive drugs. Bloomsbury: Bloomsbury Academic. [Link]
Moreno, A., Ruiz, J., & de la Colina, C. (2000). Re-visiting bolt’s criterion for homogeneous distribution of normal frequencies in rectangular enclosures. Madrid: Sociedad Española de Acústica. [Link]
Piggott, J. J., Townsend, C. R., & Matthaei, C. D. (2015). Reconceptualizing synergism and antagonism among multiple stressors. Ecology and Evolution, 5(7), 1538–1547. [DOI:10.1002/ece3.1465] [PMID] [PMCID]
Rea, W. J., & Patel, K. D. (2014). Reversibility of chronic disease and hypersensitivity,volume 2: The effects of environmental pollutants on the organ system. New York: CRC Press. [Link]
Redza-Dutordoir, M., & Averill-Bates, D. A. (2016). Activation of apoptosis signalling pathways by reactive oxygen species. Biochimica et Biophysica Acta, 1863(12), 2977–2992. [DOI:10.1016/j.bbamcr.2016.09.012] [PMID]
Rosenbaum, D. M., Kalberg, J., & Kessler, J. A. (1994). Superoxide dismutase ameliorates neuronal death from hypoxia in culture. Stroke, 25(4), 857–863. [DOI:10.1161/01.STR.25.4.857] [PMID]
Sahin, E., & Gümüşlü, S. (2007). Immobilization stress in rat tissues: Alterations in protein oxidation, lipid peroxidation and antioxidant defense system. Comparative biochemistry and physiology. Toxicology & Pharmacology, 144(4), 342–347. [DOI:10.1016/j.cbpc.2006.10.009] [PMID]
Samson, J., Sheeladevi, R., & Ravindran, R. (2007). Oxidative stress in brain and antioxidant activity of Ocimum sanctum in noise exposure. Neurotoxicology, 28(3), 679–685. [DOI:10.1016/j.neuro.2007.02.011] [PMID]
Schaaf, M. J., De Kloet, E. R., & Vreugdenhil, E. (2000). Corticosterone effects on BDNF expression in the hippocampus. Implications for memory formation. Stress, 3(3), 201–208. [DOI:10.3109/10253890009001124] [PMID]
Schardein, J. L., Macina, O. T. (2006). Human developmental toxicants: Aspects of toxicology and chemistry. Boca Raton: CRC Press. [DOI:10.1201/9781420006759]
Seo, H. S., Yang, M., Song, M. S., Kim, J. S., Kim, S. H., & Kim, J. C., et al. (2010). Toluene inhibits hippocampal neurogenesis in adult mice. Pharmacology, Biochemistry, and Behavior, 94(4), 588–594. [DOI:10.1016/j.pbb.2009.11.015] [PMID]
Sexton, K., & Linder, S. H. (2011). Cumulative risk assessment for combined health effects from chemical and nonchemical stressors. American Journal of Public Health, 101(Suppl 1), S81–S88. [DOI:10.2105/AJPH.2011.300118] [PMID] [PMCID]
Snyder, S. D. (2012). Active noise control primer. New York: Springer. [Link]
Stewart, R. R., & Bewley, J. D. (1980). Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiology, 65(2), 245–248. [DOI:10.1104/pp.65.2.245] [PMID] [PMCID]
Streffer, C., Bücker, J., Cansier, A., Cansier, D., Gethmann, C. F., & Guderian, R., et al. (2013). Environmental standards: Combined exposures and their effects on human beings and their environment. Berlin: Springer Berlin Heidelberg. [Link]
Taliaz, D., Loya, A., Gersner, R., Haramati, S., Chen, A., & Zangen, A. (2011). Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. The Journal of Neuroscience, 31(12), 4475–4483. [DOI:10.1523/JNEUROSCI.5725-10.2011] [PMID] [PMCID]
Tas, U., Ayan, M., Kuloglu, T., Suren, M., Cakıl, D., & Ozyurt, B., et al. (2012). Examination of apoptotic effects of high-dose toluene on the brain cortex and cerebellum tissue during the acute phase: An experimental study. European Journal of General Medicine 9(4), 235-240. [DOI:10.29333/ejgm/82437]
Toppila, E., Pyykkö, I., & Pääkkönen, R. (2009). Evaluation of the increased accident risk from workplace noise. International Journal of Occupational Safety and Ergonomics, 15(2), 155–162. [DOI:10.1080/10803548.2009.11076796] [PMID]
Tripathy, D. P. (2008). Noise pollution. New Delhi: APH Publishing. [Link]
WHO (2020). IARC monographs on the identification of carcinogenic hazards to humans. World Health Organisation. [Link]
Win-Shwe, T. T., Kunugita, N., Yoshida, Y., Nakajima, D., Tsukahara, S., & Fujimaki, H. (2012). Differential mRNA expression of neuroimmune markers in the hippocampus of infant mice following toluene exposure during brain developmental period. Journal of Applied Toxicology, 32(2), 126–134. [DOI:10.1002/jat.1643] [PMID]
Xiu, M. H., Hui, L., Dang, Y. F., Hou, T. D., Zhang, C. X., & Zheng, Y. L., et al. (2009). Decreased serum BDNF levels in chronic institutionalized schizophrenia on long-term treatment with typical and atypical antipsychotics. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 33(8), 1508–1512. [DOI:10.1016/j.pnpbp.2009.08.011] [PMID]
Xue, L., Zhang, D., Xiaokaiti·Yibulayin, Wang, T., & Shou, X. (2014). Effects of high frequency noise on female rat's multi-organ histology. Noise & Health, 16(71), 213–217. [DOI:10.4103/1463-1741.137048] [PMID]


Noise is a fundamental property of different activities produced by changes in air pressure (Snyder, 2000). Excessive noise hurts various systems in the body, including the cardiovascular system, auditory system, psychological system, and neural system (Babisch, 2003; Brunoni et al., 2008). It has been proven that exposure to noise causes some disorders in the brain tissue, such as congestion, edema, blood vessel expansion, neuroglial hyperplasia, and mild atypia (Xue et al., 2014). Regrettably, the main cause or intensifying factors of many disasters and accidents, which could be related to the neurological and psychological disorders provoked by exposure to noise, such as enhancing the occurrence of violence and increasing workplace accidents (Toppila et al., 2009) may not be well detected or resolved.
Toluene is one of the most suitable solvents with various applications in the plastic, rubber, leather, adhesives, and pharmaceutical industries (Acton, 2013). Previous studies have demonstrated that exposure to toluene induces different diseases in various organs of the body, including the liver and kidneys (Schardein & Macina, 2006). The psychological and neurotoxic effects of toluene are complicated (Lowinson et al., 2005) and include the loss of balance, headache, weakness, fatigue, and joy feelings. In addition, toluene has a devastating effect on the nervous tissue of the brain (Dobbs, 2009).
There are many different physical and chemical stressors in the industry. Noise and toluene are some of the prevalent physical and chemical stressors in different workplaces. Previous studies have indicated that different stressors could deteriorate some brain disorders (Demır et al., 2017; Xiu et al., 2009).
The debate about the health effects of cocoa exposure to chemical and physical pollutants has been highlighted by many discussions about the interaction between different chemical and physical harmful agents (Sexton & Linder, 2011; Streffer et al., 2013). As stated by numerous reports and studies, many people in various occupations are not only exposed to noise (such as constructions, factories, nightclubs, and airports) (Tripathy, 2008) or only toluene (such as petrochemical, plastics factories, adhesive factories, and pharmaceutical factories) (Acton, 2013; Miller, 2002) but also simultaneously exposed to noise and toluene (Dobie, 2015; Rea & Patel, 2014). This indicates a need to understand various perceptions of neurotoxicity induced by simultaneous exposure to noise and toluene. Therefore, this experimental study assessed how co-exposure to toluene and noise changes biochemical factors and brain tissue in rabbits.
2. Materials and Methods
Animals and groups
Twenty-four healthy adult male white New Zealand rabbits (Pasteur Institute, Iran) weighing 2.83±0.41 kg were used in this experiment. Animals were kept on a 12:12 hour light/dark circulation period at controlled ambient temperature (21±2°C) and with a humidity of 50 to 70% while having access to food (standard rabbit pellet, Behparvar Feed Manufacturing Company, Tehran) and treated water. Animals were housed in the laboratory for 14 days before the experiments to adapt to the environmental conditions of the laboratory. They were randomly segregated into four groups (n=6 per group) including, noise exposure group (group 1), toluene exposure group (group 2), co-exposure to toluene and noise group (group 3), and control group (group 4). Exposure duration was 14 consecutive days (8 h/day from 9:00 AM to 5:00 PM) for all groups. The control group was placed in the exposure chamber with clean air flowing (toluene=0 ppm) and <50 dBA noise (background noise) with an exposure duration of 14 consecutive days. Then, group 1 was placed in the exposure chamber to receive 100±5 decibel (dBA) noise. After that, group 2 was placed in the same exposure to receive toluene (1000±50 ppm) for the exposure duration. Then, group 3 was placed in the exposure chamber and exposed to noise and toluene with the same specifications as groups 1 and 2 for the exposure duration. The temperature was 23±3°C and the relative humidity was 65%-80% inside the exposure chamber for all the experimental groups.
Exposure chamber
Animals in all groups (n=6) were exposed in the same exposure chamber with the dimensions of 50×60×90 cm3 made of clear and transparent polycarbonate sheets, which were coated with transparent polyethylene terephthalate (PET) adhesive tape. Specifications of the exposure chamber were chosen according to the animals’ welfare requirements (Gad, 2006) and based on the reverberant chamber features (Cobo et al., 2009; Moreno et al., 2000) to generate the same noise specifications inside the exposure chamber and to make noise intensity independent from distance in the chamber.
Noise exposure set-up
White noise at 100±5 dBA (70-20000 Hz) was produced by the White Noise Generator Software, version 1.3.12 and monitored by Cool Edit Pro Software, version 2.1 and Syntrillium Software version 2.0. The selected noise was amplified by a power amplifier (3030 W, multi tone) and transported to a loudspeaker. The loudspeaker was placed directly above the chamber on the chamber roof. The noise level was continuously measured using a sound level analyzer (TES 1358 sound analyzer real-time 1/1 & 1/3 octave band analyzer) in a 1/3 octave band during the exposure (groups 1 and 3). The sound level meter microphone was placed at the same level as the ears of the animals by checking the designed outlets (made of rubber tape and coated with PET adhesive tape) every 30 min in order to monitor noise specifications.
Toluene exposure set-up
Exposure to 1000±50 ppm toluene happened through its surface evaporation. In summary, constant generation of toluene vapors was done using an impinger (capacity of 250 mL) with the content of 100 mL toluene liquid (Merck KGaA, extra pure, Darmstadt, Germany) and via injecting 20 mL toluene into the impinger every 90 min on the periodical basis. The toluene concentration was analyzed during pilot studies using a calibrated humidity-resistant real-time instrument (Phocheck, Ion Science Ltd, Cambs UK 07-01782) and also charcoal absorbents with gas chromatography (model agilent 7890A GC system) established on the 1501 NIOSH method. Accordingly, changing the toluene concentration was negligible (5%) during the exposure time in the pilot and real experiments. According to the pilot experiments, the pure air flow rate was 30 L/min and the toluene vapor-contained air flow rate was 3 L/min. Moreover, before starting real exposures, the purity of the toluene vapors was confirmed by air sampling from the exposure chamber (active charcoal samples) and using a gas chromatography-mass spectrometry instrument (Agilent 6890/5973 GCMS). To obtain the target concentration of toluene constantly and continuously, a mixer chamber with the same materials and specifications as the exposure chamber with the dimensions of 50×50×20 cm3 was used before entering the air into the exposure chamber. To monitor and check toluene concentration, the same real-time instrument (Phocheck), which had been already calibrated and utilized in pilot experiments was used. Air samples were prepared from inside the exposure chamber through checking outlets designed on four sides in the surrounding walls of the exposure chamber (checking outlets).
Blood sampling
Blood samples were collected from the marginal vein of the ears of each animal in five stages, as mentioned in Figure 1.

Tissue sampling and processing
Rabbits were sacrificed after anesthesia with 35 mg/kg ketamine and 5 mg/kg xylazine (IM injection). The animals were sacrificed 14 days after the end of the exposure in each group. The whole brain consisting of the cerebral cortex, hippocampus, and cerebellum was dissected immediately after the animals were sacrificed and fixed in 10% formalin solution (formaldehyde solution, Merck KGaA, Darmstadt, Germany) at pH 7.2. After the fixation, paraffin blocks were prepared. Moreover, for biochemical analyses, the cerebral cortex of the brain tissues was immediately collected and kept at -80°C until the time of biochemical experiments. Dehydration and paraffin impregnation phases of the tissue samples were made by an autotechnicon device. Then, paraffin molding was carried out and the slides were prepared using a microtome with a typical diameter of 5 μ. Eventually, the slides were stained with hematoxylin and eosin (H&E) and histopathologic changes were assessed by light microscopy (Nikon Eclipse E100, Japan).
Homogeneity of brain tissue
First, 100 g of the cerebral cortex of the brain samples was weighed out of all the groups to measure the oxidant/antioxidant parameters of the brain tissue. Afterward, they were dissolved in 1 cc phosphate-buffered saline and homogenized by a homogenizer device (ultrasonic homogenizer Hielscher UIP-1500HD). Then, the samples were centrifuged by a centrifuge device at 1000 g at 4°C for 20 min. Required samples were taken from the clear supernatant liquid of the centrifuged samples using a sampler and placed in microtubes. The supernatant protein liquid of the cerebral cortex was measured using a commercial kit (Pars Azmoon Company, Karaj, Iran). Finally, the parameters in the supernatant liquid were normalized by measuring the amount of supernatant protein liquid of the brain tissue and valued per mg of tissue protein.
Determining oxidant and anti-oxidant factors in the cerebral cortex using the spectrophotometric method
Total antioxidant capacity (TAC) was measured by a Naxifer™ diagnosis kit (TAC assay kit, Navand Lab Kit, Urmia, Iran). The levels of glutathione peroxidase (GPx) were measured by a Biorexfars diagnosis kit (production code: BXC0551, Shiraz, Iran). Catalase (CAT) levels were measured by a Cayman chemical company kit (CAT assay kit, Item No. 707002), and superoxide dismutase (SOD) levels were measured by a non-enzymatic method Nasdox™ diagnosis kit (Navand Lab Kit, Urmia, Iran). All the measurements were done according to the instructions provided by the kit manufacturers; in addition, malondialdehyde (MDA) levels were measured using the thiobarbituric acid (TBA) method (Janero, 1990; Stewart & Bewley, 1980).
Determining the serum levels of brain-derived neurotrophic factor-α (BDNF-α) using the ELISA method
BDNF-α levels were measured by Rabbit ELISA kits (Hangzhou Eastbiopharm CO., LTD, Hangzhou, China) and based on the manufacturer’s instructions (Cat. No: CK-E91855). In this experiment, the State Fax 2100 ELISA plate reader (Awareness Technology, Inc., USA) and the State Fax 2600 ELISA plate washer (Awareness Technology, Inc., USA) were employed.
Statistical analysis
The generalized estimating equation (GEE ) statistical method was used to assess BDNF-α levels so that differences between different repeats in different groups were compared by SPSS software, version 25. Moreover, analysis of variance (ANOVA ) followed by Tukey’s post hoc test was used to compare the oxidant and anti-oxidant parameters and the mean brain tissue weight/body weight in different groups in Minitab software, Version 18. The statistical significance level was considered at 0.05. The logarithm of abnormal parameters was used for GEE statistical analysis.
3. Results
Biochemical changes
According to Figure 2E, TAC values in group 1 were significantly higher than those in groups 2-4. Figure 2 A, B, C & D shows CAT, GPx, SOD, and MDA levels in group 1.

Exposure to noise significantly decreased BDNF-α levels compared to the control and toluene exposure groups on the 14th day after exposure. Moreover, co-exposure to noise and toluene significantly increased BDNF-α levels compared to the noise and toluene exposure groups on the 3rd day after exposure. Furthermore, co-exposure to noise and toluene significantly decreased BDNF-α levels compared to the toluene exposure group on the 7th and 14th days after exposure (Figure 3).

Brain tissue weight changes
According to Figure 4, the average brain weight/body weight in the noise exposure group was significantly lower than that in the toluene exposure group.

Histopathological changes
The cerebellum was in normal conditions in the control group. However, the population of Purkinje cells decreased in the cerebellum in group 1; in addition, pyknosis appeared in Purkinje cells in this group. The relative reduction in the number of cells was visible in the granular layer in groups 1 and 2. Granular cells not only reduced relatively but also appeared massively in group 2. Pathological changes were more specific in the cerebellum area in group 3. A decrease in the population of Purkinje cells, a decrease in the thickness of the granular layer, and a decrease in granule cells were some of the significant changes observed in the cerebellum in group 3 (Figure 5).

The cortex area in the frontal part of the brain was normal in the control group. Nevertheless, gliosis was detected in the prefrontal cortex area in groups 1-3 compared to the control group. The number of pyknotic cells in groups 1 and 3 appeared higher than group 2 in the prefrontal cortex area. Minor lymphocyte infiltration, which was possibly induced by the retrograde effect of toluene and transmitted to the frontal part of the brain cortex through olfactory nerves, was visible in the prefrontal cortex area in group 2. Some pathological changes were noticed in the prefrontal cortex area in group 3, such as disorganization in the cortex layer, relative reduction of pyramidal cells, vacuolization, and chromatolysis (Figure 6).

The light micrographs of the hippocampus area were normal in the control group. Dark cells were reduced in population and also were pyknotic in the hippocampus area in group 1, even more severely than in group 2. However, it was not remarkable compared to group 3. Moreover, the pyramidal cells in group 1 noticeably decreased in population compared to group 2 in the hippocampus area. But it was not remarkable compared to group 3. All the changes reported for group 1 also appeared in group 2 in the hippocampus area but were milder than in group 3 so the pathological changes appearing in group 3 were more severe than those in groups 1 and 2 (Figure 7).

4. Discussion
The results of this study showed that the noise caused an increase in TAC in the cerebral cortex. In a study on the effects of industrial noise stress on workers, the serum levels of CAT and TAC were significantly higher in workers exposed to noise stress (Bagheri Hosseinabadi et al., 2019). Increased SOD levels were reported by exposure to noise at 100 dB in the cerebral cortex of the rats’ brain (Samson et al., 2007); however, increased anti-oxidant factors after exposure to high levels of noise may be due to induced oxidative stress during noise exposure. Moreover, activating protective mechanisms in the body, such as producing TAC, GPx, SOD, and CAT as anti-oxidant factors and decreasing MDA levels as an oxidative factor are predictable during oxidative stress to prevent the excessive generation of free radicals (Rosenbaum et al., 1994). Furthermore, some of the previous studies have shown that toluene induces oxidative stress in several brain regions by producing reactive oxygen species (ROS) (Kodavanti et al., 2011). As a result, ROS can induce apoptosis in the brain (Redza-Dutordoir & Averill-Bates, 2016). Therefore, co-exposure to toluene and noise changed oxidant and anti-oxidant parameters in the brain tissue.
BDNF is a protein encoded by a gene called BDNF. This protein promotes the growth and development of the central and peripheral nervous system. It also triggers nerve synapses and establishes neural connections. It is most active in the hippocampus and the upper part of the brain. The highest production of BDNF is in the brain, the amount of which is different in brain damage, especially with environmental stress.
The current study revealed that co-exposure to toluene and noise increased the serum levels of BDNF; however, exposure to noise decreased BDNF serum levels compared to the control group. In the study on the effects of toluene inhalation exposure (5 ppm) for five days, a decrease in the relative mRNA expression of BDNF, and an increase in the mRNA expression levels of NF-κB were reported in the hippocampus of the infant mice during brain development (Win‐Shwe et al., 2012). In a study on the effects of chronic stress on BDNF levels, inducing 80 dB noise stress for five weeks made a significant reduction in hippocampal BDNF levels in rats (Taliaz et al., 2011). Moreover, another study demonstrated that acute stress caused oxidative stress and consequently, produced an increase in BDNF levels (Hacioglu et al., 2016). This means that there is a direct relationship between BDNF levels and oxidative stress indicators (Hacioglu et al., 2016). On the other hand, previous studies have shown that some stressors, such as noise initiate an increase in corticosterone levels (Laugero & Moberg, 2000; Lehmann et al., 2002; Şahin & Gümüşlü, 2007). Consequently, corticosterone can induce a decrease in BDNF levels (Jacobsen & Mork, 2006; Schaaf et al., 2000).
This study provides considerable insight into the combined effects of noise and toluene on neurological disorders. Based on the results of the present study related to oxidative stress induced after co-exposure to toluene and noise and according to the multiple combined stressors (Piggott et al., 2015), different synergistic and antagonistic effects induced by exposure to noise and toluene are provided in Appendices 1 and 2.
This study demonstrated that co-exposure to noise and toluene can induce different histopathological changes in the brain tissue, such as lymphocyte infiltration, a relative reduction in pyramidal cells, chromatolysis, and vacuolization. There were remarkable signs of inflammation in the co-exposure group, even more severe than the noise and toluene exposure groups. Based on the results obtained, it seems that even though there were no significant changes induced by noise exposure, there were some changes in the brain tissue, which if persisted, can be an important alarm. Induced mild atypia, edema, hyperemia, expansion of blood vessels, and glial cell hyperplasia were reported in several areas in the brain tissue in female rats by exposure to noise at 95 dB (4000 Hz) for two weeks (Xue et al., 2014). Makhlouf et al., (2014) reported a decrease in the thickness of the pyramidal cell layer of CA1 (cornu ammonis area 1), CA3 (cornu ammonis area 3), and also the granular cells, which exhibited degeneration and loss of many cells after exposure to 100 dB noise during four weeks (4 h/day) in male albino rats (Makhlouf et al., 2014). Abousetta et al. (2014) indicated that exposure to 100 dB white noise for 6 h/day during four consecutive weeks induced a decrease in the thickness of dentate gyrus (DG) granular cells and pyramidal cells with the loss and degeneration of numerous cells in male albino rats (Abousetta et al. 2014).
Enhanced glial fibrillary acidic protein (GFAP)-immunoreactive cytoplasm was demonstrated in the white matter of the cerebellum and in the dentate gyrus of the hippocampus by inhaling 1500 ppm toluene for four and seven days in the brain tissue of the rats (Gotohda et al., 2000). In addition, Gotohda et al., (2000) showed that chronic exposure to 3000 ppm toluene for 12 weeks caused severe degenerative changes, dark pyknotic nuclei, and small cytoplasm in neurons of different hippocampal regions in rats (Gotohda et al., 2000). Kanter (2008) demonstrated that exposure to 3000 ppm toluene for 12 weeks (8 h/day) induced dilated cisternae of endoplasmic reticulum, small cytoplasm, evidently inflamed mitochondria with deteriorated cristae in the hippocampus in male Wistar albino rats (Kanter, 2008). Tas et al., (2012) reported that exposure to a high dose of toluene (5200 mg/kg/gavage) for two weeks caused a significant increase in the immune reactivity of Bax in the cerebellum and the brain cortex (Tas et al., 2012). Demır et al., (2017) demonstrated that acute exposure of New Zealand rabbits to toluene by a single dose (876 mg/kg) intraperitoneal (IP) injection into the brain caused severe dilation of blood vessels, diffuse cell borders, vacuolar degeneration, severe degeneration of the compensation, perivascular demyelination, gliosis, and several necrosis and pyknotic cells (Demır et al., 2017). Moreover, a noticeable enhancement in the count of apoptotic cells in the cerebral granule layer was indicated by inhaling 1200-1800 ppm toluene for 6 h per day in rats (Dalgaard et al., 2001). Nevertheless, Seo et al. (2010) indicated that exposure to toluene at the dose of 500 mg/kg did not cause apoptosis in the hippocampus in mice (Seo et al., 2010). Considering the findings proven by previous studies and the results obtained from this study, noise, and toluene are some of the noticeable neurotoxic pollutants.
5. Conclusion
This study indicated that co-exposure to toluene and noise might induce neurotoxicity in white New Zealand rabbits. Histopathological experiments indicated that co-exposure to toluene and noise could aggravate neurotoxic effects. Co-exposure to noise and toluene induced some pathological changes in the cerebellum, hippocampus, and frontal section. Nonetheless, further studies are needed to determine the definite neurotoxic effects induced by co-exposure to toluene and noise.
Limitations
Although this study showed non-auditory changes induced by exposure to toluene and noise, neurotoxic effects induced by chronic exposure to noise and toluene were not identified. Furthermore, this study did not evaluate other biological and pathological factors during or after exposure. Therefore, further studies are necessary in order to enhance the results obtained from this work.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the National Ethics Committee of Tabriz University of Medical Sciences, for biomedical research in Iran (Code: IR.TBZMED.REC.1396.953).
Funding
This study was accomplished within the framework of the master’s thesis of Amirreza Abouee Mehrizi, approved by Department of Occupational Health Engineering, School of Health, Tabriz University of Medical Sciences (Code: 378).
Authors' contributions
Conceptualization, supervision, investigation, data analysis and writing: Amirreza Abouee-Mehrizi and Yahya Rasoulzadeh; Methodology: Amirreza Abouee-Mehrizi, Yahya Rasoulzadehand, and Masoud Motallebi-Kashani; Data collection: Amirreza Abouee-Mehrizi, Yahya Rasoulzadeh, and Ahmad Mehdipour; Nafiseh Nasirzadeh, Omid Shatouei-Gharenjeh and Abbas Ebrahimi-Kalan.
Conflict of interest
The authors declared no conflict of interest.
References
Abousetta, A., Makhlouf, N. A., & El-Beshbishy, R. A. (2014). The effects of concomitant Ginkgo intake on noise induced hippocampus injury. Possible auditory clinical correlate. Egyptian Journal of Ear, Nose, Throat and Allied Sciences 15(3), 231-239. [DOI:10.1016/j.ejenta.2014.05.003]
Acton, Q. A. (2013). Advances in toluene research and application: 2013 edition: Scholarlybrief. Atlanta: ScholarlyEditions. [Link]
Babisch, W. (2003). Stress hormones in the research on cardiovascular effects of noise. Noise & Health, 5(18), 1–11. [PMID]
Bagheri Hosseinabadi, M., Khanjani, N., Ebrahimi, M. H., Mirbadie, S. R., & Biganeh, J. (2019). The effects of industrial noise exposure on lipid peroxidation and antioxidant enzymes among workers. International Archives of Occupational and Environmental Health, 92(7), 1041–1046. [DOI:10.1007/s00420-019-01444-1] [PMID]
Brunoni, A. R., Lopes, M., & Fregni, F. (2008). A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: Implications for the role of neuroplasticity in depression. The International Journal of Neuropsychopharmacology, 11(8), 1169–1180. [DOI:10.1017/S1461145708009309] [PMID]
Cobo, P., Murillo-Cuesta, S., Cediel, R., Moreno, A., Lorenzo-García, P., & Varela-Nieto, I. (2009). Design of a reverberant chamber for noise exposure experiments with small animals. Applied Acoustics 70(8), 1034-1040. [DOI:10.1016/j.apacoust.2009.03.005]
Dalgaard, M., Hossaini, A., Hougaard, K. S., Hass, U., & Ladefoged, O. (2001). Developmental toxicity of toluene in male rats: Effects on semen quality, testis morphology, and apoptotic neurodegeneration. Archives of Toxicology, 75(2), 103–109. [DOI:10.1007/s002040000209] [PMID]
Demır, M., Cicek, M., Eser, N., Yoldaş, A., & Sısman, T. (2017). Effects of acute toluene toxicity on different regions of rabbit brain. Analytical Cellular Pathology, 2017, 2805370. [DOI:10.1155/2017/2805370] [PMID] [PMCID]
Dobbs, M. R. (2009). Clinical neurotoxicology E-book: Syndromes, substances, environments. Edinburgh: Elsevier Health Sciences. [Link]
Dobie, R. A. (2015). Medical-legal evaluation of hearing loss, third edition. San Diego: Plural Publishing, Incorporated. [Link]
Gad, S. C. (2006). Animal models in toxicology. Oxfordshire: Taylor & Francis. [DOI:10.1201/9781420014204]
Gotohda, T., Tokunaga, I., Kubo, S., Morita, K., Kitamura, O., & Eguchi, A. (2000). Effect of toluene inhalation on astrocytes and neurotrophic factor in rat brain. Forensic Science International, 113(1-3), 233–238. [DOI:10.1016/S0379-0738(00)00215-2] [PMID]
Hacioglu, G., Senturk, A., Ince, I., & Alver, A. (2016). Assessment of oxidative stress parameters of brain-derived neurotrophic factor heterozygous mice in acute stress model. Iranian Journal of Basic Medical Sciences, 19(4), 388–393. [PMID] [PMCID]
Jacobsen, J. P., & Mørk, A. (2006). Chronic corticosterone decreases brain-derived neurotrophic factor (BDNF) mRNA and protein in the hippocampus, but not in the frontal cortex, of the rat. Brain Research, 1110(1), 221–225. [DOI:10.1016/j.brainres.2006.06.077] [PMID]
Janero, D. R. (1990). Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radical Biology & Medicine, 9(6), 515–540. [DOI:10.1016/0891-5849(90)90131-2] [PMID]
Kanter, M. (2008). Nigella sativa and derived thymoquinone prevents hippocampal neurodegeneration after chronic toluene exposure in rats. Neurochemical Research, 33(3), 579–588. [DOI:10.1007/s11064-007-9481-z] [PMID]
Kodavanti, P. R., Royland, J. E., Richards, J. E., Besas, J., & Macphail, R. C. (2011). Toluene effects on oxidative stress in brain regions of young-adult, middle-age, and senescent Brown Norway rats. Toxicology and Applied Pharmacology, 256(3), 386–398. [DOI:10.1016/j.taap.2011.04.012] [PMID]
Laugero, K. D., & Moberg, G. P. (2000). Energetic response to repeated restraint stress in rapidly growing mice. American Journal of Physiology. Endocrinology and Metabolism, 279(1), E33–E43. [DOI:10.1152/ajpendo.2000.279.1.E33] [PMID]
Lehmann, J., Russig, H., Feldon, J., & Pryce, C. R. (2002). Effect of a single maternal separation at different pup ages on the corticosterone stress response in adult and aged rats. Pharmacology, Biochemistry, and Behavior, 73(1), 141–145. [DOI:10.1016/S0091-3057(02)00788-8] [PMID]
Lowinson, J. H. (Ed.). (2005). Substance abuse: A comprehensive textbook. Lippincott Williams & Wilkins. `
Makhlouf, N. A., El-Beshbishy, R. A., & Abousetta, A. (2014). Ginkgo modulates noise-induced hippocampal damage in male albino rats: A light and electron microscopic study. Egyptian Journal of Histology, 37(1), 159-174. [DOI:10.1097/01.EHX.0000444078.17248.ab]
Miller, R. L. (2002). The encyclopedia of addictive drugs. Bloomsbury: Bloomsbury Academic. [Link]
Moreno, A., Ruiz, J., & de la Colina, C. (2000). Re-visiting bolt’s criterion for homogeneous distribution of normal frequencies in rectangular enclosures. Madrid: Sociedad Española de Acústica. [Link]
Piggott, J. J., Townsend, C. R., & Matthaei, C. D. (2015). Reconceptualizing synergism and antagonism among multiple stressors. Ecology and Evolution, 5(7), 1538–1547. [DOI:10.1002/ece3.1465] [PMID] [PMCID]
Rea, W. J., & Patel, K. D. (2014). Reversibility of chronic disease and hypersensitivity,volume 2: The effects of environmental pollutants on the organ system. New York: CRC Press. [Link]
Redza-Dutordoir, M., & Averill-Bates, D. A. (2016). Activation of apoptosis signalling pathways by reactive oxygen species. Biochimica et Biophysica Acta, 1863(12), 2977–2992. [DOI:10.1016/j.bbamcr.2016.09.012] [PMID]
Rosenbaum, D. M., Kalberg, J., & Kessler, J. A. (1994). Superoxide dismutase ameliorates neuronal death from hypoxia in culture. Stroke, 25(4), 857–863. [DOI:10.1161/01.STR.25.4.857] [PMID]
Sahin, E., & Gümüşlü, S. (2007). Immobilization stress in rat tissues: Alterations in protein oxidation, lipid peroxidation and antioxidant defense system. Comparative biochemistry and physiology. Toxicology & Pharmacology, 144(4), 342–347. [DOI:10.1016/j.cbpc.2006.10.009] [PMID]
Samson, J., Sheeladevi, R., & Ravindran, R. (2007). Oxidative stress in brain and antioxidant activity of Ocimum sanctum in noise exposure. Neurotoxicology, 28(3), 679–685. [DOI:10.1016/j.neuro.2007.02.011] [PMID]
Schaaf, M. J., De Kloet, E. R., & Vreugdenhil, E. (2000). Corticosterone effects on BDNF expression in the hippocampus. Implications for memory formation. Stress, 3(3), 201–208. [DOI:10.3109/10253890009001124] [PMID]
Schardein, J. L., Macina, O. T. (2006). Human developmental toxicants: Aspects of toxicology and chemistry. Boca Raton: CRC Press. [DOI:10.1201/9781420006759]
Seo, H. S., Yang, M., Song, M. S., Kim, J. S., Kim, S. H., & Kim, J. C., et al. (2010). Toluene inhibits hippocampal neurogenesis in adult mice. Pharmacology, Biochemistry, and Behavior, 94(4), 588–594. [DOI:10.1016/j.pbb.2009.11.015] [PMID]
Sexton, K., & Linder, S. H. (2011). Cumulative risk assessment for combined health effects from chemical and nonchemical stressors. American Journal of Public Health, 101(Suppl 1), S81–S88. [DOI:10.2105/AJPH.2011.300118] [PMID] [PMCID]
Snyder, S. D. (2012). Active noise control primer. New York: Springer. [Link]
Stewart, R. R., & Bewley, J. D. (1980). Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiology, 65(2), 245–248. [DOI:10.1104/pp.65.2.245] [PMID] [PMCID]
Streffer, C., Bücker, J., Cansier, A., Cansier, D., Gethmann, C. F., & Guderian, R., et al. (2013). Environmental standards: Combined exposures and their effects on human beings and their environment. Berlin: Springer Berlin Heidelberg. [Link]
Taliaz, D., Loya, A., Gersner, R., Haramati, S., Chen, A., & Zangen, A. (2011). Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. The Journal of Neuroscience, 31(12), 4475–4483. [DOI:10.1523/JNEUROSCI.5725-10.2011] [PMID] [PMCID]
Tas, U., Ayan, M., Kuloglu, T., Suren, M., Cakıl, D., & Ozyurt, B., et al. (2012). Examination of apoptotic effects of high-dose toluene on the brain cortex and cerebellum tissue during the acute phase: An experimental study. European Journal of General Medicine 9(4), 235-240. [DOI:10.29333/ejgm/82437]
Toppila, E., Pyykkö, I., & Pääkkönen, R. (2009). Evaluation of the increased accident risk from workplace noise. International Journal of Occupational Safety and Ergonomics, 15(2), 155–162. [DOI:10.1080/10803548.2009.11076796] [PMID]
Tripathy, D. P. (2008). Noise pollution. New Delhi: APH Publishing. [Link]
WHO (2020). IARC monographs on the identification of carcinogenic hazards to humans. World Health Organisation. [Link]
Win-Shwe, T. T., Kunugita, N., Yoshida, Y., Nakajima, D., Tsukahara, S., & Fujimaki, H. (2012). Differential mRNA expression of neuroimmune markers in the hippocampus of infant mice following toluene exposure during brain developmental period. Journal of Applied Toxicology, 32(2), 126–134. [DOI:10.1002/jat.1643] [PMID]
Xiu, M. H., Hui, L., Dang, Y. F., Hou, T. D., Zhang, C. X., & Zheng, Y. L., et al. (2009). Decreased serum BDNF levels in chronic institutionalized schizophrenia on long-term treatment with typical and atypical antipsychotics. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 33(8), 1508–1512. [DOI:10.1016/j.pnpbp.2009.08.011] [PMID]
Xue, L., Zhang, D., Xiaokaiti·Yibulayin, Wang, T., & Shou, X. (2014). Effects of high frequency noise on female rat's multi-organ histology. Noise & Health, 16(71), 213–217. [DOI:10.4103/1463-1741.137048] [PMID]


Type of Study: Original |
Subject:
Cellular and molecular Neuroscience
Received: 2021/03/28 | Accepted: 2021/09/18 | Published: 2023/11/1
Received: 2021/03/28 | Accepted: 2021/09/18 | Published: 2023/11/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





