Volume 14, Issue 5 (September & October 2023)
BCN 2023, 14(5): 631-646 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ashraf R, Abdoli B, Khosrowabadi R, Farsi A, Pineda J A. The Effect of Modeling Methods on Mirror Neuron Activity and a Motor Skill Acquisition and Retention. BCN 2023; 14 (5) :631-646
URL: http://bcn.iums.ac.ir/article-1-2099-en.html
URL: http://bcn.iums.ac.ir/article-1-2099-en.html
The Effect of Modeling Methods on Mirror Neuron Activity and a Motor Skill Acquisition and Retention
1- Department of Behavioral and Cognitive Science in Sport, Faculty of Sport Science and Health, Shahid Beheshti University, Tehran, Iran.
2- Institute for Cognitive and Brain Sciences, Shahid Beheshti University, Tehran, Iran.
3- Department of Cognitive Science and Neuroscience, University of California, La Jolla, United States of America.
2- Institute for Cognitive and Brain Sciences, Shahid Beheshti University, Tehran, Iran.
3- Department of Cognitive Science and Neuroscience, University of California, La Jolla, United States of America.
Full-Text [PDF 667 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Observational learning or learning from a model is a particular type of perceptual learning and its usefulness has been shown in motor learning (Schmidt et al., 2011; Alhajri et al., 2018). Learning from a model is a crucial component of Bandura’s cognitive-social theory and refers to behavioral, cognitive, and emotional changes resulting from viewing models (Schunk, 2012). Observational learning is used as an effective way of learning simple and complex motor skills (Wulf et al., 2010). Studies show that observational training can play a critical and unique part in learning, especially when combined with physical training (Shebilskem et al. 1992; Shea et al. 2000). Also, neuroimaging studies show that a set of common neural structures are activated in both action execution and action observation (Gallese & Goldman, 1998; Jeannerod, 1994). A review of studies related to observational learning shows that many factors are involved in the effectiveness of observational interventions (Ste-Marie et al., 2012). Factors, such as observation and task characteristics are crucial to consider when observing interventions. The related literature suggests that researchers are interested in this crucial factor in observational interventions that “who” is the most useful model for observation (Ste-Marie et al., 2012). Common types of modeling used by researchers include observing others or observing themselves. In observing others, the skilled model, the novice model, and the learning model are examined. Another approach is to use self-modeling techniques (Ste-Marie et al., 2011). Self-modeling is a form of observational learning with the distinction that the observed and the observer are the same person, that is, they observe their executive behavior and then repeat the intended behavior (Dowrick, 2012a; Dowrick, 2012b).
Factors in self-modeling suggest that this model can be an optimal model for learning skills. This can be examined from several perspectives. From Holmes and Calmels’ neurological perspective (Holmes & Calmels, 2008), self-modeling can be more functional than observing other persons in terms of neural activation between action execution and observation. From a psychological point of view, self-modeling is also a desirable model. In this respect, psychological constructs, such as self-efficacy and other self-regulation processes are initiated by observing mastery experiences (Ste-Marie et al., 2011). Bandura’s cognitive-social theory also supports self-observation as a desirable model. According to this theory, observing the model does not ensure that acquisition will take place, or that the acquired behavior will occur later. In other words, many factors affect vicarious learning and acquired behaviors; one of which is the similarity of a model with an observer. Similarity is a crucial factor in assessing suitability and shaping beliefs. The more similar observers are to models, the more likely the observers will consider similar actions socially appropriate for themselves to perform. Besides, based on this theory, the model-observer similarity leads to the enhancement of the attention and retention processes of observation learning, thereby increasing the learning benefits through the observation process. According to this theory, the highest level of model-observer similarity happens while a person is in their model (Schunk, 2012). Based on Schmidt et al. (2011), Bandura’s theory, which was developed to explain the acquisition of social behaviors, seems not to be appropriate for understanding motor skills learning. However, recent advances provide new insights, specifically about observational learning (Schmidt et al., 2011). The discovery of a mirror neuron system in the brain has stimulated a great deal of research into the possibility that a particular neural mechanism is a basis for observational learning (Schmidt et al., 2011). These mirror neurons are a class of cortical neurons that discharge both when a person performs a certain motor action and when one sees the same motor action performed by others. These neurons were first discovered in the prefrontal cortex of monkeys by Italian scientists at the University of Parma (Di Pellegrino et al., 1992) in an accidental discovery. Following their discovery, some other researchers concluded that the human brain also has mirror neurons (Rosenbaum, 2009). The discovery of a mirror neuron system is a critical finding in observational learning. This system is thought to play a crucial function in understanding the actions of others and may be responsible for our ability to learn by observing and imitating the actions of others and can underpin observational learning mechanisms (Cattaneo & Rizzolatti, 2009; Van Gog et al., 2009). That is, these neurons are the basis for the perceptual-motor conversion mechanism and enable the visual information to be converted into motor commands. Thanks to such visual-motor conversions, humans can learn how to perform a particular action based on the information derived from the model. These conversions allow the observer to repeat the actions represented by the model (Lago Rodriguez et al., 2014).
In humans, the mirror neuron system operates differently based on the observed forms of motor behavior. Activity is greatly decreased when observing a movement that is biomechanically impossible to perform (Stevens et al., 2000) or when the observed movement is not part of the observer’s motor repertoires (Buccino et al., 2004). Likewise, the level of expertise also affects the involvement of this system (Calvo-Merino et al., 2004) which is a significant issue for the development of applications for observational use. In addition to finding cortical areas associated with the mirror neuron system, the level of activity of these neurons is also of interest to researchers. Studies (Buccino et al., 2004; Stevens et al., 2000) showed that the activity of the mirror neuron system can be due to the degree of adaptation between the observed actions and the observer’s motor abilities. Growing evidence shows that the activity of this system depends on observational motor experiences of a given action (Kim et al., 2011). Among the methods used to infer the activity of mirror neurons is Mu rhythm (also called sensory-motor rhythm). Mu rhythm is one of the dominant brain waves of about 8-13 Hz. These oscillations are limited to short periods of 0.5 to 2 s and can occur in the human sensory-motor cortex in the absence of movement. The results of the meta-analysis of 85 studies on 1 707 participants showed that Mu rhythm can be strongly inferred as a function of the human mirror system (Fox et al., 2015).
In a few studies, the activity of mirror neurons was investigated in the observation of motor and sports skills, and interesting results were obtained. For example, Calvo-Merino et al. found that when dancers see a similar style of dance, the activity of mirror neurons is greater than when they see a different style of dance. Kim et al. also examined the difference in mirror neuron activity during observation between the skilled archer group and the non-archer group. They concluded that the mirror neuron activity was more in the skilled archers’ group than in the novice group while watching the archery. Despite the importance of these neurons as the underlying mechanisms of observational learning, few studies have been conducted on the observational learning of motor and sports skills and have solely focused on observing others (Calvo-Merino et al., 2004; Kim et al., 2011). Although self-modeling is theoretically and psychologically better than other modeling methods mentioned (Bandura’s cognitive-social theory and Zimmerman’s self-regulation theory), the neural underpinning of this method has not been investigated. Also, the pattern of activity of these neurons along with learning sports skills is unclear which indicates a need for a laboratory study. That is, given the importance of the mirror neuron activity, this crucial factor during observing motor and sports skills has not been compared between self-modeling, skilled model, and learning model. The results of this study from the perspective of the mirror neuron system as a neural basis of observational learning can provide strong evidence for this crucial variable in motor learning, specifically in observational learning, i.e. the “who” is the most useful model for observation.
2. Materials and Methods
Participants
Forty-five healthy volunteer male students (aged 19.4±0.37 years) participated in the study. They were not aware of the specific purpose of the study. Informed consent was obtained from all the participants before starting the experiment. All participants had normal vision and right-sighted eyes and no familiarity with the research task. Participants were asked not to observe and practice outside the training protocol from the pre-test to the retention test. Therefore, in addition to the two cases mentioned, the individual’s request to quit the study was the exclusion criterion in this research. Participants were randomly assigned to one of the self-modeling, learning model, and skilled model groups.
Motor task
The task was to put the golf ball and guide the ball to the hole to gain a maximum score and the rating system was based on the accuracy of the putting. The target was a circle with a radius of five cm, located 4 m away from the participants. Fourteen concentric circles with radii 10,15, 20, 25, …, and 75 cm were traced around the target, and these circles were labeled with their points. These circles were used as a measure of the accuracy of the puts. If the ball was placed in area A (goal, which is the same hole in the golf matches), it would score 150 points, and by placing the ball in other areas, respectively B=140, C=130, D=120,… O=10 and outside of the area, they got 0 points (Badami et al., 2012). These concentric circles were used to measure the accuracy of the golf putting.
Data collection tools
A Sony DSCW830 20.1 MP digital camera with 20.1 Megapixel (effective) plus 8 x zoom and several pixels (Gross) which is approx. 20.4 megapixels and 720p MP4 HD movie mode was used to record golf putting with an angle of 45 degrees from the front view. The 15-inch Acer laptop (Aspire ES1-533-C7TG) was used to show the recorded videos to the participants.
Electroencephalography (EEG) recording
EEG was recorded using Psychlab EEG, a product of Contact Precision Instruments Company with a sampling rate of 256 Hz via Psychlab Data Acquisition software. To measure Mu rhythm suppression, the Mu rhythm at baseline, and observation conditions at C3, C4, and Cz area were calculated. Before calculating changes in mu power, in the pre-processing step, the EEG LAB toolbox version 14.1.1 was installed on the MATLAB software, version R2016a to eliminate the noise caused by blinking, eye movement, head movement, etc. Mu powers were calculated based on the Fourier transform technique (FFT). The suppression of mu rhythm was calculated as the ratio of mu power in the experimental condition (for example, observation) to mu power in the baseline condition which is well-known as the mirror neuron activity. This ratio is calculated to control for variability in absolute power as a result of individual differences such as skull thickness and electrode resistance. A log transformation was also used for the analysis because the data ratios are inherently abnormal as a result of the low range. A log ratio of value <0 indicates suppression of mu rhythm, a value=0 indicates no suppression and a value >0 indicates an increase.
Observational training
The experiment consisted of three periods, pre-test period, acquisition period, and retention period. After the initial training, the participants performed 10 trials (golf putting) as a pre-test and the results were recorded. The acquisition period consisted of six sessions and each session had six blocks of 10 trials. Participants at each session and before the start of each block saw a video related to their group and then performed physical training. The results of the final block of the sixth session were considered as the acquisition test score. Seven days (according to Schmidt et al. (2011), the retention interval is commonly considered 24 hours or more) after completing the acquisition test, the retention test was performed with 10 puts as well as the recording of electrical brain waves by observing 10 puts for a third time. The conditions of the golf putting test and recording of the brain electrical waves were similar to the previous two periods. In this way, the putting test was first performed and then the brain waves were recorded.
Procedure
Pre-training considerations, instructions, and initial training for the golf putting, including putting alignment, putting stance and ball position, keeping eyes over the ball, the putting grip, the putting stroke, etc. were presented to the participant. The participants’ performance of the self-modeling group was videotaped for the first acquisition session. Before the first acquisition physical training session, participants observed 10 times (in the early stages of learning, the emphasis is more on observation; for reviews, see Magill and Anderson (2010) their group films, then they continued training independently in their group. After filming 10 skilled golfer puts, the best performance was selected as a skilled model group movie to produce a skilled model movie. To prepare the movie for the learning model, four people were selected from the non-statistical population, then they practiced in six sessions similar to the research groups among which the person who acquired the natural acquisition pattern was chosen as a learning model. The movie of the self-modeling group was also a pre-test movie in the early acquisition. In each session, the acquisition was updated with filming, and in each session, the previous session films were played.
Statistical analyses
In the dependent variable of golf putting accuracy, the mean put accuracy was calculated based on the system traced around the hole; the closer the golf ball was to the hole, the higher the score obtained by 10 puts.
To investigate the effect of independent variables on golf put acquisition and retention, three (self-modeling, skilled model, and learning model) in three (pre-test, acquisition, and retention periods) mixed ANOVA were used and the last factor was a repeated measure. The same test was performed on the activity of the dependent variable mu rhythm. Bonferroni post hoc test was used and the significance level was 0.05. The data were analyzed with the SPSS software, version 25.
3. Results
Table 1 presents the Mean±SD of the accuracy of the participant’s golf putting in all three groups and all three experimental periods.
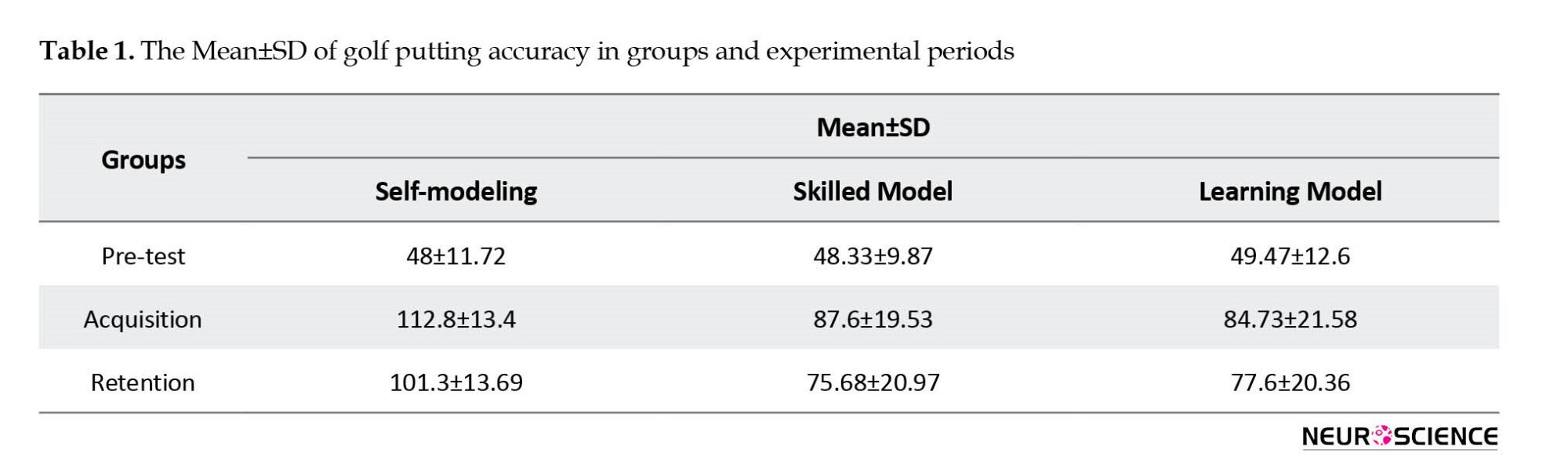
Table 2 presents the Mean±SD of the data related to the mu rhythm suppression in all three groups, and all three experimental periods.

Mu rhythm activity
The results of the mixed analysis of variance (ANOVA) indicated that the main effect of group (F(2, 42)=15.005; P=0.001; η2=0.601) and periods (F(2, 84)=13.95; P=0.001; η2=0.249) variables on mu rhythm suppression is significant. However, the main effect of the brain regions variable approaches significance (F(2, 84)=2.866; P=0.063; η2=0.064). Results of interaction between variables showed that interaction between groups and periods is significant (F(3.48, 42)=4.849; P=0.003; η2=0.188), and a marginally significant interaction is observed between group and brain regions (F(4, 42)=2.461; P=0.051; η2=0.105). However, the interaction between periods and brain regions (F (138.158, 3.289)=3.289; P=0.955; η2=0.003) is not the interaction between period variables, brain regions, and group (F(4, 42)=1.34; P=0.239; η2=0.06).
Due to the significant interaction between group variables and experimental periods, the main effect of period variables was neglected and a one-way ANOVA test was performed to investigate the effect of modeling type on mu rhythm suppression for each period.
As shown in Figure 1, the results of this test showed that in the pre-test period, a significant difference is observed in the mu rhythm suppression between the three groups of self-modeling, learning model and skilled model in all three brain regions C3 (F(2, 42)=8.077, P=0.001), C4 (F(2, 42)=6.967, P=0.002), and Cz (F(2, 42)=7.505, P=0.002).
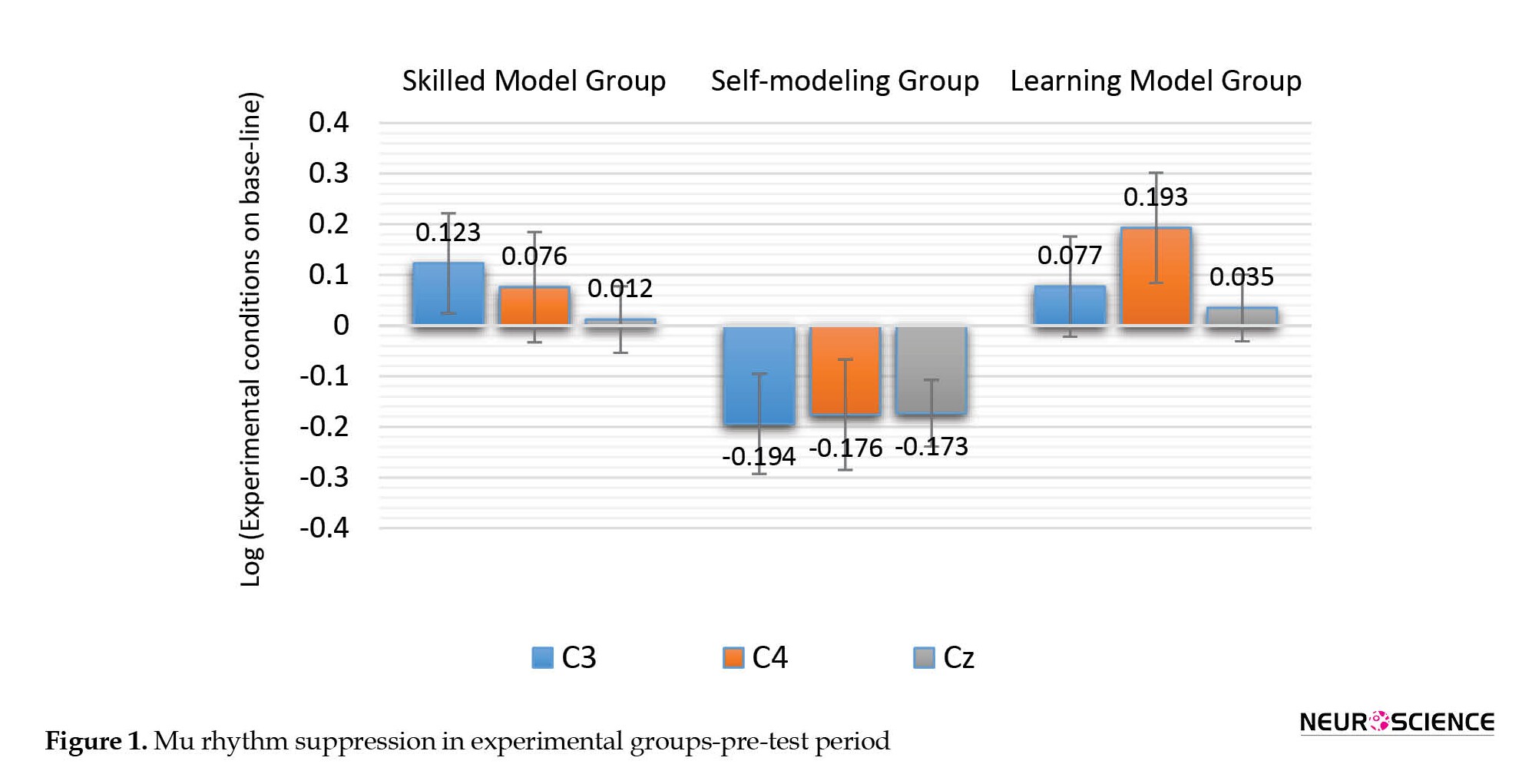
Bonferroni post hoc test was used to investigate the significant differences between the groups, which showed that the self-modeling group had higher mu rhythm suppression in the C3 -0.194±0.197, C4 -0.176±0.355, and Cz -0.173±0.183 regions than the mu rhythm suppression in skilled model groups in C3 0.123±0.191, C4 0.076±0.247 and Cz 0.194±0.197 and the in learning model in C3 0.077±0.279, C4 0.193±0.205 and Cz 0.035±0.15; however, the skilled model group and the learning model group had no significant difference in the mu rhythm suppression.
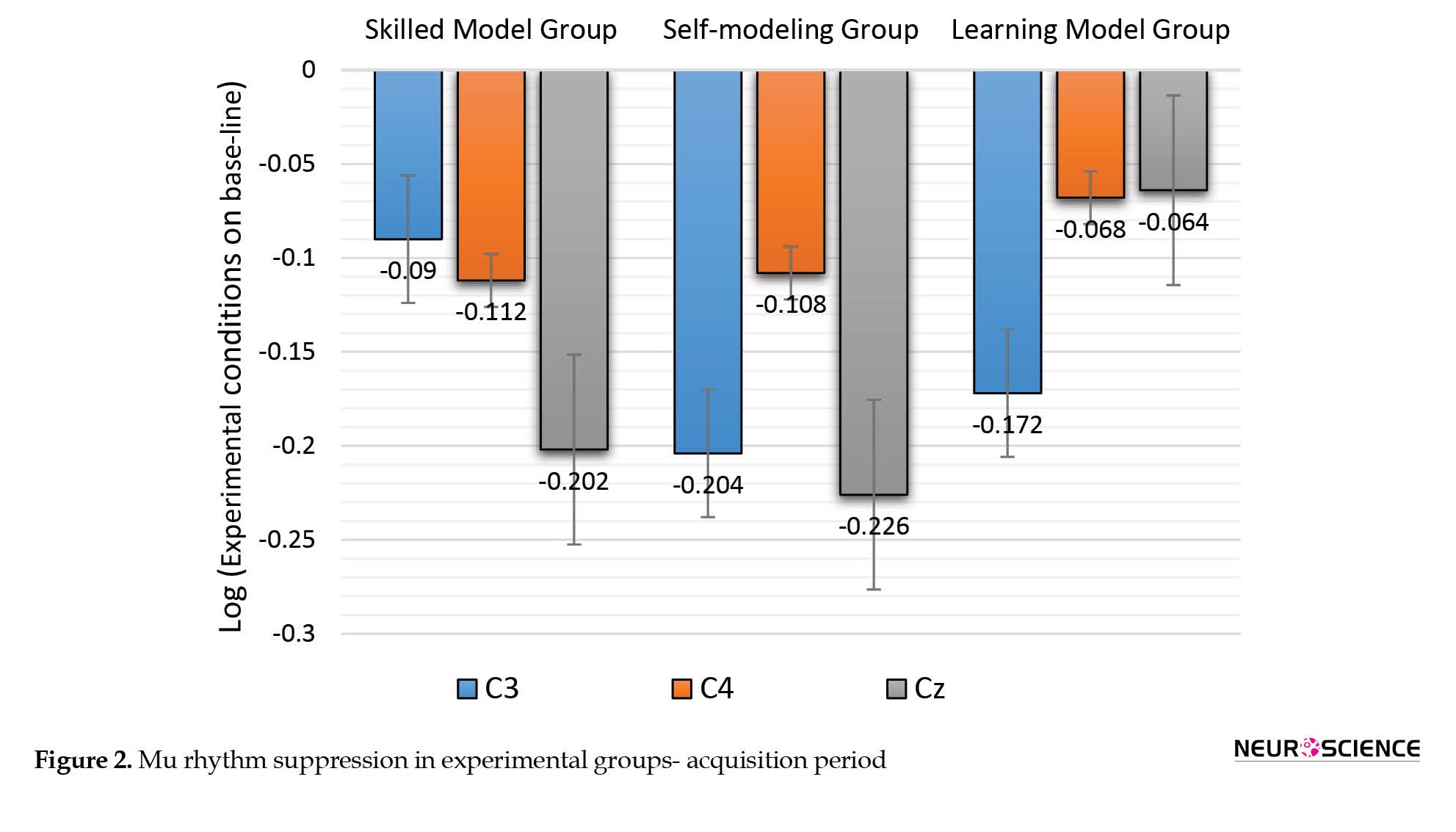
As shown in Figure 2, the results of this test showed that in the pre-test period, a significant difference is observed in the mu rhythm suppression between the three groups of self-modeling, learning model, and skilled model in all three brain regions, in the acquisition period, a one-way ANOVA test showed no significant difference between the experimental groups in C3 (F(2, 42)=1.976, P=0.151), C4 (F(2, 42)=0.211, P=0.811) and only approached significance at Cz (F(2, 42)=2.975, P=0.062) regions.
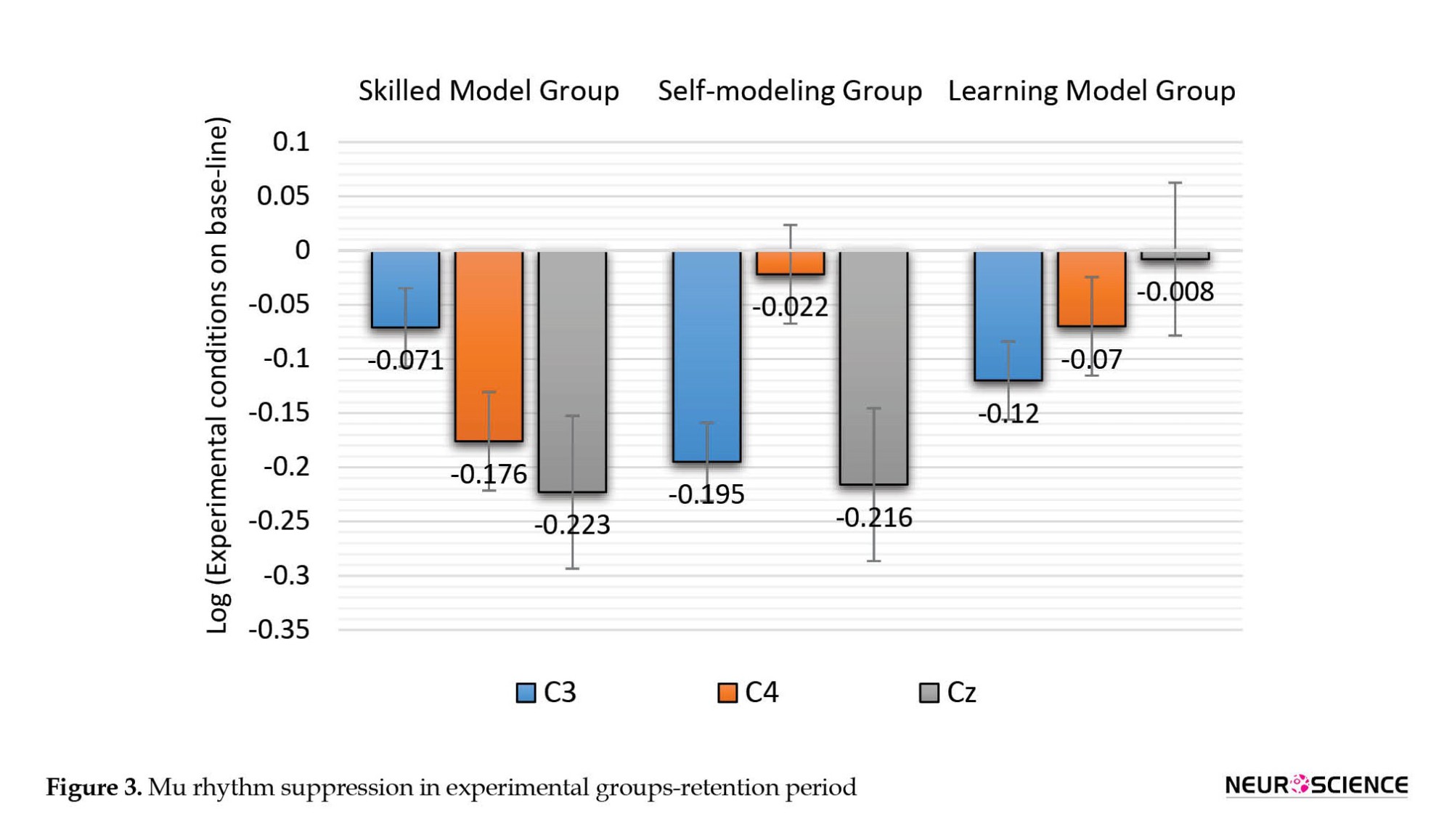
This test was also used in the retention period, which showed that the difference between the experimental groups in C3 (F(2, 42)=1.325, P=0.277), and C4 (F(2, 42)=2.411, P=0.102) regions was not significant but in the Cz (F(2, 42)=3.574, P=0.037) region, it was significant. Figure 3 shows these results. Bonferroni post hoc test with alpha adjustment based on the number of groups was used to investigate the significant differences between groups in the Cz region and showed no significant difference between groups.
Learning group
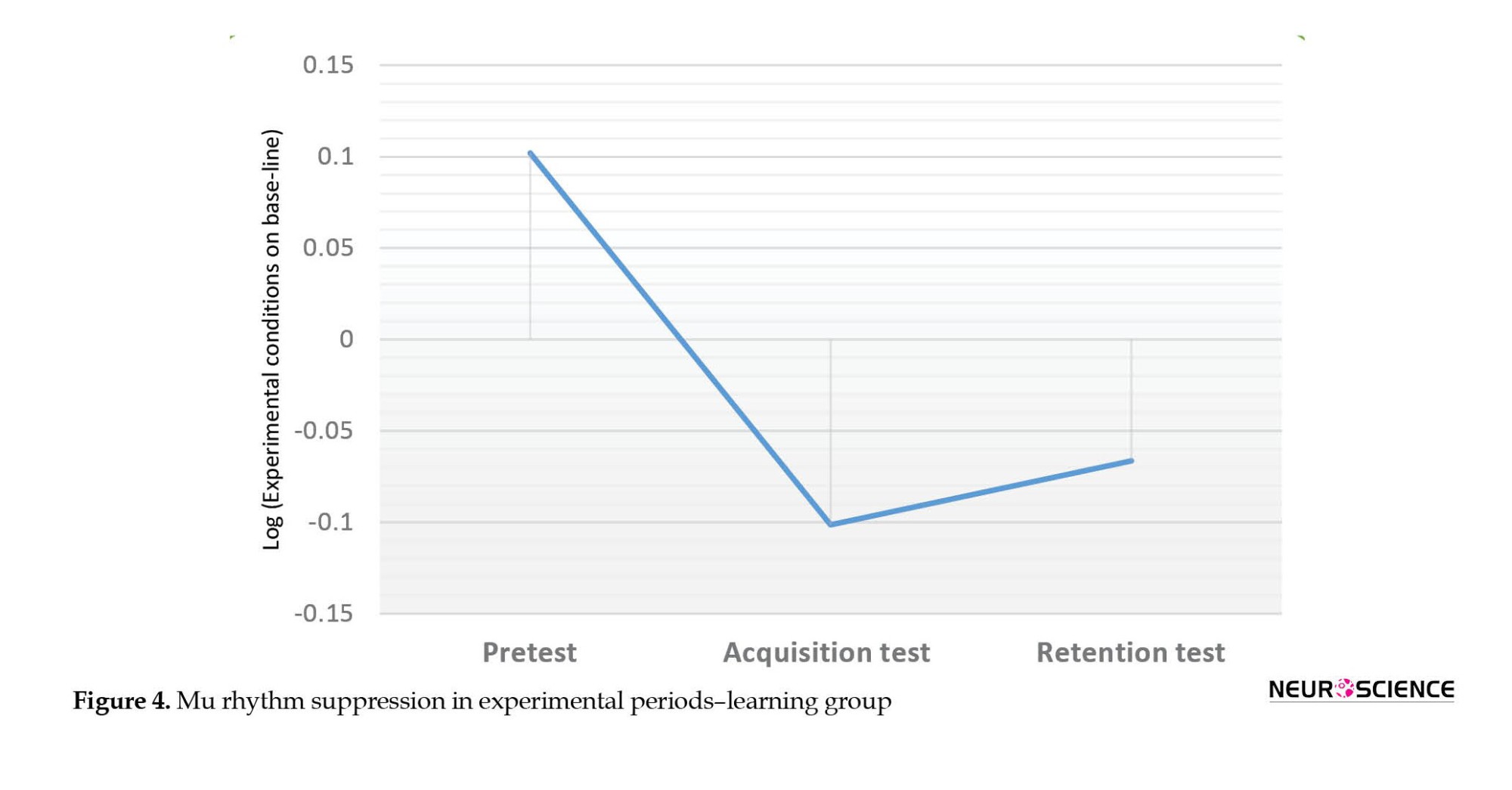
The results of two-factor ANOVA test showed that the main effect of periods on mu rhythm suppression was significant (F2, 28)=7.74; P=0.002; η2=0.356) and main effect of brain regions (F2, 28)=2.85; P=0.075; η2=0.169) and also interaction of periods with brain regions (F(2.71, 37.97)=1.522; P=0.208; η2=0.098) was not significant. Figure 4 shows these results.
The Bonferroni post hoc test showed that the mu rhythm suppression in the acquisition test (P=0.004) and the retention test (P=0.014) was significantly higher than the pre-test and no significant difference was observed between the acquisition and the retention test.
Self-modeling group
The results of two-factor ANOVA test indicated that the main effect of the periods on the mu rhythm suppression (F(1.44, 20.28)= 0.388; P=0.617; η2=0.027), main effect of brain regions (F2, 28)=1.015; P=0.147; η2=0.128), and also interaction of periods with brain regions (F(4, 56)=1.133; P=0.15; η2=0.075) was not significant. Figure 5 shows these results.

Skilled group
The results of two-factor ANOVA showed that the main effect of periods on mu rhythm suppression was significant (F(1.355, 18.98)=7.74; P=0.001; η2=0.544) and main effect of brain regions (F2, 28)=3.157; P=0.057; η2=0.184) and also interaction of periods with brain regions (F(4, 56)=0.185; P=0.945; η2=0.013) was not significant. Figure 6 shows these results. The Bonferroni post hoc test showed that the mu rhythm suppression in the acquisition test (P=0.001) and the retention test (P=0.002) was significantly higher than the pre-test and no significant difference was observed between the acquisition and the retention test.

Golf putting task
The mixed ANOVA test was performed to measure the effect of three types of intervention (self-modeling, skilled model, and learning model) on the obtained scores from the mean of the golf putting in three periods (pre-test, acquisition, and retention). Figure 7 shows these results. The main effect of the periods (F(1.35, 56.87)=189.51, P=0.001, η2=0.819), the main effect of the group (F(2, 42)=7.86; P=0.001; η2=0.27), and interaction between the modeling and the periods (F(2.7, 56.87)=189.515, P=0.001, η2=0.288) were significant.
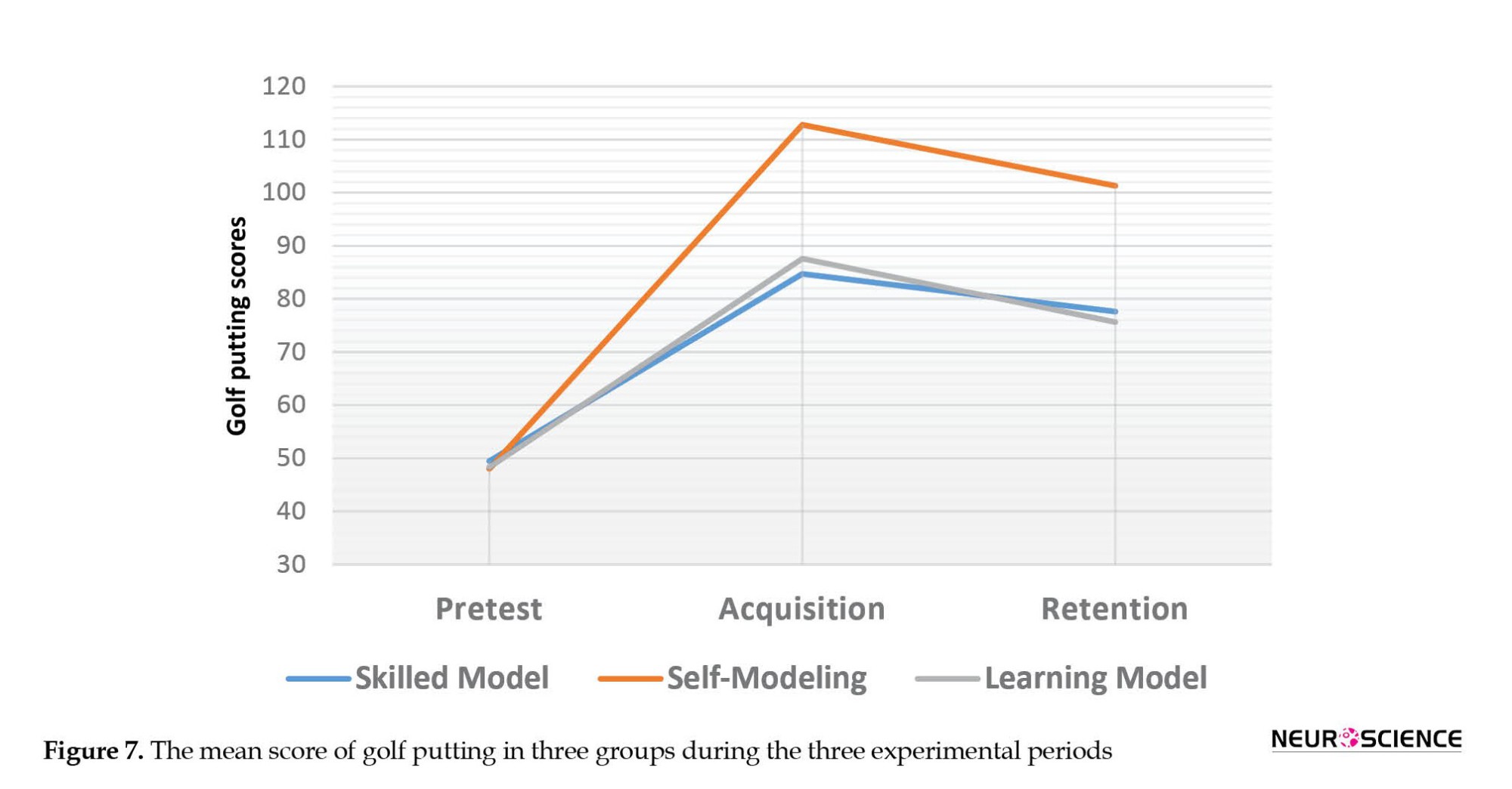
For this reason, a one-way ANOVA between groups was performed to examine the effect of modeling on the mean score of golf putting for each period. No significant difference was observed between the mean scores of the three groups in the pre-test (F(2, 42)=0.07; P=0.93). During the acquisition period, the difference between the groups was significant (F(2, 42)=10.45; P=0.001). Bonferroni post hoc test showed the self-modeling group with a higher score (Mean±SD 112.8±13.4) than the skilled model group (Mean±SD 87.6±19.53), and the learning model group (Mean±SD 84.73±21.58); however, no significant difference was observed between the skilled model group and the learning model group. During the retention period, although the mean score of the groups was slightly decreased, the results were similar to the results of the acquisition period and the mean scores of the self-modeling group (Mean±SD 101.28±13.6) had a significant difference with the skilled model group (Mean±SD 75.67±20.97), and the learning model group (Mean±SD 77.6±20.36).
Thus, to investigate the effect of periods, for each group, repeated measures ANOVA test was performed to compare the scores of the golf putting in the pre-test, acquisition, and retention periods. In the learning group, a significant effect was observed on the period (F(1.161, 16.261)=26.981; P=0.001; η2=0.658). Bonferroni post hoc test showed that the mean scores in all three periods were significantly different and significant progress was observed from the pre-test to the acquisition period (MD:35.262), as well as, a significant decrease was observed from acquisition to retention periods (MD:-7.33); however, despite this decrease, the difference between the pre-test and retention periods (mean differences (MD): 28.13) was significant.
The results of this test for the self-modeling group showed that the main effect of the period was significant (F2, 28)=159.235; P=0.001; η2=0.919). The Bonferroni post hoc test also showed that the mean scores in all three periods differed significantly, i.e. significant progress was made from the pre-test to acquisition periods (MD: 64.8) as well as a significant decrease was observed from acquisition to retention periods (MD: -11.353); however, despite this decrease, the difference between the pre-test and the retention periods (MD: 53.26) was significant.
The results of this test for the skilled model group were similar to those in the previous two groups. Thus, the main effect of the period variable was significant (F (1.201, 16.819)=51.268; P=0.001; η2=0.786). Bonferroni post hoc test also showed that the mean scores in all three periods were significantly different and in this group putting mean scores significantly increased from pre-test to acquisition periods (MD:39.26) as well as significantly decreased from acquisition to retention periods (MD:-11.933); however, despite this decrease, the difference between the pre-test and retention periods (MD:27.33) was significant.
4. Discussion
This study was conducted to investigate the effect of self-modeling, skilled model, and learning model on mu rhythm suppressions as an index of mirror neuron activity and learning of golf putting skill. In the pre-test period, mu rhythm suppression in the three brain regions studied was higher in the self-modeling group than in the skilled model and learning model groups. In the acquisition and retention periods, no significant difference was observed between the experimental groups in the mu rhythm suppression in the three brain regions studied. In the learning and skilled groups, the mu rhythm suppression increased significantly from the pre-test to the acquisition test period, and this increase was significant up to the retention period. In the self-modeling group, no significant difference was observed between the three experimental periods in the rate of mu rhythm suppression.
In the present study, one of the crucial variables of observational learning, namely the type of model was addressed. The effect of self-modeling, skilled model, and learning model on the acquisition and retention of golf putting skills was investigated. The results showed that all three groups had a significant improvement in this skill, although a significant decrease was observed in the retention test. Among the three groups, the self-modeling group had more progress than the other two groups, so that a significant difference was observed between them in both the acquisition test and the retention test. However, no significant difference was observed between the skilled model and the learning model groups at either stage. One of the results of this study was the significant effect of self-tuning on the acquisition and retention of golf putting skills. Because this modeling method was better than the other two methods, both in the acquisition test and in the retention test. This result is consistent with the results of other studies, such as Onate et al. (2005) ; Clark and Ste-marie (2007). These results are theoretically consistent with several perspectives. One of the critical theories about the optimal effect of self-modeling on learning is Bandura’s cognitive-social theory. From the point of view of this theory, a critical factor in vicarious learning is the similarity of model and observer; in this way, the more similar the observers are to the models, the more appropriate the actions of the models are for themselves. Also, according to this theory of model and observer similarity, the processes of attention and retention, which are part of the basic stages of observational learning, are strengthened, which is beneficial for learning through observation (Schunk, 2012). Therefore, it seems that in this study, self-modeling, which has the highest model-observer similarity and has led to better learning than the other two methods, has benefited from the similarity advantages mentioned in Bandura’s theory. According to Clark and Ste-Marie’s opinions, individuals by observing their performance engage in mental processes, such as performance assessment, which is useful for learning. Golf putting findings are consistent with Zimmerman’s theory of self-regulation, which states that psychological structures such as self-efficacy and other self-regulatory processes are triggered by the observation of mastery experiences. According to this theory, self-modeling is effective in self-regulation. Self-regulation also leads to improved self-satisfaction, which in turn has a positive effect on other self-regulatory processes, such as intrinsic motivation and self-efficacy, leading to better performance (Clark and Ste-marie, 2007). Therefore, it seems that observing the performance and progress in golf putting skill in the participants of the self-modeling group, has led to more belief in the ability to perform this skill, as well as increased task interest and motivation, and ultimately led to better learning.
Given that mu rhythm suppression in the C3, C4, and Cz regions indicates the activity of mirror neurons in the sensory-motor cortex, these results can be translated into observational learning language, as follows:
When a motor skill learner is a beginner, by:
1. Observing a beginner model, his/her mirror neurons become less active.
2. Observing a skilled model, his/her mirror neurons become less active.
3. Observing his/her performance, the activity of the mirror neurons becomes greater.
The critical result of this study was the different effects of three modeling methods (self, skilled, and learning) on the activity of mirror neurons in the pre-test period. As such, when participants were novice golf putting, only the mirror neurons of the self-modeling group were more active and no difference was observed between the skilled model and the learning model.
The section on the difference between the activity of mirror neurons in the skilled and learning models groups can be compared with some of the existing studies. This result is consistent with part of the results of the studies conducted by Calvo-Merino et al., (2004), Orgs et al. (2008), Kim et al., (2011) and Ashraf et al. (2019).
A part of Calvo-Merino’s study was related to the control group that observed both ballet and capoeira dance types. The results of this part showed no effect of the stimulus type (observing skilled ballet motion pictures and observing skilled capoeira motion pictures) on the mirror neuron activity of novice participants. This means that the participants had less mirror neuron activity when they saw the movements of the skilled people. In a similar study but with a different instrument, namely the recording of the brain electrical waves, Orgs et al also found that suppression of mu rhythm was less effective in non-skilled observing dance movements. Kim et al. also found that the novice archery participant’s mirror neuron activity was lower while observing skilled archers.
The reason for the lower activity of mirror neurons at this period can be attributed to the participant of motor repertoire by observing the skillful and learning model consistent with the mentioned studies. In this regard, evidence shows that the only actions that are involved in our motor repertoire affect the activity of the mirror neuron system (Stevens et al., 2000).
It has also been suggested that the activation of the mirror neuron system is related to the degree of adaptation between the observed actions and the observer’s motor capability (Kim et al., 2011). Observing a model affects the activity of the mirror neurons when the observer finds a similarity between the observed model’s execution and motor capability.
Therefore, it seems that participants in the skilled and learning model groups had less mirror neuron activity due to the lack of model-driven movement in their motor repertoire. In other words, because they were not proficient in the golf putting skill and had not yet learned the skill, the mirror neurons were also less active.
A very significant result from the pre-test period was more suppression of mu rhythm in the self-modeling group. Several studies can be more or less associated with this result. For example, Gastaut and Bert (1954) reported that when a person sees himself on the screen, the mu rhythm is blocked. Also, studies have shown that mirror neurons were affected by their observation even though they did not record electrical waves in the brain.
Coulson et al. (2006a) by self-modeling technique, Coulson et al. attempted to improve the smile deficits after nerve paralysis in two participants and achieved a significant improvement after two weeks. Seeing oneself smile on the videotape may guide mirror neurons to facilitate imitation. Coulson et al. (2006b) conducted another study with 10 participants with the same aim and method and achieved similar results, such as improved response speed, smile quality, and so on. In the mentioned study, participants’ smiles were automated as a result of the self-modeling intervention and it was stated that the possible explanation for the rapid acquisition of this skill is the activation of mirror neurons. In another study, Oberman et al. (2008) examined the modulation of mu suppression in autistic children and the control group in response to familiar and unfamiliar stimuli. The results of this study revealed that mu suppression is sensitive to the familiarity of the presented films. This means that the suppression of the mu rhythm was more pronounced during observing a familiar hand compared to an unfamiliar hand. Also, with his hand, the suppression of the mu rhythm increased. Thus, it seems that the mirror neuron system is not only the neural basis of observational learning but also the fundamental process of self-modeling. These results are consistent with the results of the present study that by self-observing in the pre-test period, the activity of the mirror neurons is increased.
These results are theoretically related to part of Bandura’s cognitive-social learning theory. According to this theory, the more alike observers are to the models, the more likely observers will consider similar actions appropriate for them to execute. Besides, model-observer similarity results in enhanced observation and retention processes of observational learning, which in turn increases the benefits of learning through the observation process. According to this theory, the highest model-observer similarity occurs when a person is a model himself (Schunk, 2012). Therefore, it seems that this is what happened in the present study in the pre-test period. In other words, the participants in the self-modeling group paid more attention to the model (themselves) than the other two groups by observing themselves during golf putting skills.
As mentioned, the activity of the mirror neurons in acquisition and retention periods was not significantly different between the three experimental groups. In other words, no difference was observed in the mirror neuron activity when the participants became somewhat proficient in golf putting and saw different models. This result is consistent with the literature on mirror neurons. Cattaneo & Rizzolatti, 2009 have stated that the activity of the mirror system is related to the observer’s motor experiences of a given action. In other words, when the observer has a motor experience from the observed motion, the mirror neurons are more active. Also, evidence shows that observing the motor actions present in the observer’s motor repertoire leads to a stronger activation of mirror neurons compared to observing new motor behaviors (Gazzaniga, 2009). Kim et al. also suggested that the activation of the mirror neuron system is related to the degree of adaptation between the observed actions and the observer’s motor capability (Kim et al., 2011). That is when observing a model affects the activity of the mirror neurons when the observer finds a similarity between the observed model’s execution and its capability. These principles are consistent with the results of the present study and are part of the results of the studies conducted by Calvo-Merino et al., (2004), Orgs et al. (2008), and Kim et al., (2011).
Therefore, based on the principles mentioned and as our results reveal, it can be concluded that the participants in acquisition and retention periods are somewhat proficient in golf putting skills, and therefore the activity of mirror neurons in the motor-sensory area is increased. In other words, after six sessions of training, the golf putting skill have been learned and become part of their motor repertoire and they have found a great similarity between the performance of the skilled model (skilled, self-skilled, novice model that turned to skilled) and their ability to perform the same movement. As a result of this similarity, the activity of mirror neurons has also increased. The other part of the acquisition and retention periods finding is related to a significant increase in mirror neuron activity from the pre-test to the acquisition and retention periods, although this increase occurred in the skilled and learning model groups. In other words, the pattern of mirror neurons’ activity has changed along with the learning of golf putting skills. Studies in this area have involved the learning process by measuring the activity of mirror neurons; therefore, it would be very useful to compare their results with the results of the present study. Cross et al. (2006) showed that mirror neuron activity increases with the learning of new dance sequences. Cross et al. (2008) also found that after the training period, participants’ brain activity in the mirror-related regions increased as they observed or saw sequences they performed. Nakano et al. (2013) concluded that in the five-trial practice of the ball-rotation task, suppression of mu rhythm in the last attempts increased and performance improved. These results are consistent with the results of our study. The results of these studies along with the results of studies on the theoretical foundations of mirror neurons are interpreted as the plasticity of the mirror system. This means that the mirror system is modified by motor experiences (Gazzaniga, 2009). Therefore, based on such backgrounds and theoretical foundations, we can also deduce from this study that six sessions of 60 observing and physical training trials of golf putting skill can result in a sufficient experience in the mentioned skill and this experience can result in plasticity and more activity of mirror neurons.
Looking at the results of the two behavioral (golf putting actions) and neuroscience (mu rhythm suppression) sections of golf putting skill learning with three different model types, a critical point emerges. This new result in the field of mirror neurons is accompanied by more activity of mirror neurons in the pre-test period in the self-modeling group with better learning in this group than the other two groups. As reported, the self-modeling group had learned better the golf putting skills and it was associated with more mirror neuron activity in the pre-test period. It is logical to claim that it occurred during the acquisition period or at least during the first few sessions of the acquisition period. This means that during the acquisition period and model observation between the blocks of physical training, mirror neurons were more active than the other two groups.
Theoretical backgrounds of the studies of mirror neurons suggest that when individuals are novices in the observed motor skills, mirror neurons are less responsive. In contrast, the level of mastery is highly influenced by the activity of these neurons, and with the increase of mastery, the activity of these neurons also increases. As a result of these comparisons, we concluded that this information is inconsistent with the crucial result of this study. On the other side, the results of modeling studies are mainly in favor of self-modeling. The results are also theoretically supported by several neurological (Holmes & Calmels, 2008) and psychological studies (Bandura’s cognitive-social learning theory and Zimmerman’s self-regulation theory). Most importantly, the results of the present study are consistent with these results and theoretical foundations, a crucial issue that has not been addressed from the perspective of mirror neurons.
5. Conclusion
These results are distinct from the studies that focus on already combined psychological (model-observer similarity in Bandura’s theory) and mirror neuron perspectives (the influence of expertise level on the activity of these neurons) and can be interpreted as an “overcoming model-observer similarity on level of expertise”. This means that due to more model-observer similarity, the participants in the self-modeling group have more mirror neuron activation despite lacking mastery in early periods. This may be due to more attention to this group or factors, such as triggering structures, such as self-efficacy and task interest (though none of these were examined).
Also, since the anatomical locations of the C3, C4, and Cz electrodes are related to the supplementary motor area and sensory-motor area and their activity is related to the mirror neurons, the supplementary area has a mediating role in the communication in the different parts of the mirror system. Based on the results of the present study, these areas were more involved in the self-modeling group in the early periods of golf skill training which ultimately benefited this group’s learning. The results of this study can have critical training considerations in motor learning, especially observational learning. These results can provide strong evidence for this crucial variable in motor learning and more specifically in observational learning that “who” is the most useful model for observation and can highlight the role of model-observer similarity in observational learning.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles are considered in this article. The participants were informed of the purpose of the research and its implementation stages. They were also assured about the confidentiality of their information and were free to leave the study whenever they wished, and if desired, the research results would be available to them. A written consent has been obtained from the participants. Principles of the Helsinki Convention were also observed. In addition, this article has received permission to carry out research activities from the ethics-research committee of Institute for Cognitive and Brain Sciences, Shahid Beheshti University (Code: IR.SBU.ICBS).
Funding
The paper was extracted from the PhD dissertation of Ramin Ashraf, Behrouz Abdoli, Reza Khosrowabadi, Alireza Farsi and Jaime A Pineda, approved by Department of Behavioral and Cognitive Science in Sport, Faculty of Sport Sciences and Health, Shahid Beheshti University.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are grateful to the Laboratory Center of Brain and Cognitive Sciences Institute of Shahid Beheshti University, the Laboratory of Cognitive and Behavioral Sciences in Sports, and all the students participating in the research.
References
Alhajri, N., Hodges, N. J., Zwicker, J. G., & Virji-Babul, N. (2018). Mu suppression is sensitive to observational practice but results in different patterns of activation in comparison with physical practice. Neural Plasticity, 2018, 8309483. [DOI:10.1155/2018/8309483] [PMID]
Ashraf, R., Abdoli, B., Khosrowabadi, R., & Farsi, A. (2019). [Effects of model type on mirror neurons activity during observation of a motor skill (Persian)]. Advances in Cognitive Science, 21(2), 132-140. [DOI:10.30699/icss.21.2.132]
Badami, R., VaezMousavi, M., Wulf, G., & Namazizadeh, M. (2012). Feedback about more accurate versus less accurate trials: Differential effects on self-confidence and activation. Research Quarterly for Exercise and Sport, 83(2), 196-203. [PMID]
Buccino, G., Lui, F., Canessa, N., Patteri, I., Lagravinese, G., & Benuzzi, F., et al. (2004). Neural circuits involved in the recognition of actions performed by nonconspecifics: An fMRI study. Journal of Cognitive Neuroscience, 16(1), 114-126. [DOI:10.1162/089892904322755601] [PMID]
Calvo-Merino, B., Glaser, D. E., Grèzes, J., Passingham, R. E., & Haggard, P. (2005). Action observation and acquired motor skills: An FMRI study with expert dancers. Cerebral Cortex, 15(8), 1243-1249. [DOI:10.1093/cercor/bhi007] [PMID]
Cattaneo, L., & Rizzolatti, G. (2009). The mirror neuron system. Archives of Neurology, 66(5), 557-560. [DOI:10.1001/archneurol.2009.41] [PMID]
Clark, S. E., & Ste-Marie, D. M. (2007). The impact of self-as-a-model interventions on children’s self-regulation of learning and swimming performance. Journal of Sports Sciences, 25, 577-586. [DOI:10.1080/02640410600947090] [PMID]
Clark, S. E., Ste-Marie, D. M., & Martini, R. (2006). The thought processes underlying self-as-a-model interventions: An exploratory study. Psychology of Sport and Exercise, 7(4), 381-386. [DOI:10.1016/j.psychsport.2005.09.001]
Coulson, S. E., Adams, R. D., O’Dwyer, N. J., & Croxson, G. R. (2006). Physiotherapy rehabilitation of the smile after long-term facial nerve palsy using video self-modeling and implementation intentions. Otolaryngology-Head and Neck Surgery, 134(1), 48-55. [DOI:10.1016/j.otohns.2005.09.010] [PMID]
Coulson, S. E., Adams, R. D., O’Dwyer, N. J., & Croxson, G. R. (2006). Use of video self-modelling and implementation intentions following facial nerve paralysis. International Journal of Therapy and Rehabilitation, 13(1), 30-35. [DOI:10.12968/ijtr.2006.13.1.21349]
Cross, E. S., Hamilton, A. F. D. C., & Grafton, S. T. (2006). Building a motor simulation de novo: Observation of dance by dancers. Neuroimage, 31(3), 1257-1267. [DOI:10.1016/j.neuroimage.2006.01.033] [PMID]
Cross, E. S., Kraemer, D. J., Hamilton, A. F. D. C., Kelley, W. M., & Grafton, S. T. (2008). Sensitivity of the action observation network to physical and observational learning. Cerebral Cortex, 19(2), 315-326. [DOI:10.1093/cercor/bhn083] [PMID]
di Pellegrino, G., Fadiga, L., Fogassi, L., Gallese, V., & Rizzolatti, G. (1992). Understanding motor events: A neurophysiological study. Experimental Brain Research, 91(1), 176-180. [DOI:10.1007/BF00230027] [PMID]
Dowrick, P. W. (2012). Self-model theory: Learning from the future. Wiley Interdisciplinary Reviews: Cognitive Science, 3(2), 215-230. [DOI:10.1002/wcs.1156] [PMID]
Dowrick, P. W. (2012). Self-modeling: Expanding the theories of learning. Psychology in the Schools, 49(1), 30-41. [DOI:10.1002/pits.20613]
Fox, N. A., Bakermans-Kranenburg, M. J., Yoo, K. H., Bowman, L. C., Cannon, E. N., & Vanderwert, R. E., et al. (2016). Assessing human mirror activity with EEG mu rhythm: A meta-analysis. Psychological Bulletin, 142(3), 291-313. [DOI:10.1037/bul0000031] [PMID]
Gallese, V., & Goldman, A. (1998). Mirror neurons and the simulation theory of mind-reading. Trends in Cognitive Sciences, 2(12), 493-501. [DOI:10.1016/S1364-6613(98)01262-5] [PMID]
Gastaut, H. J., & Bert, J. (1954). EEG changes during cinematographic presentation (Moving picture activation of the EEG). Electroencephalography and Clinical Neurophysiology, 6, 433-444. [DOI:10.1016/0013-4694(54)90058-9] [PMID]
Gazzaniga, M. S. (2009). The cognitive neurosciences. Massachusetts: MIT Press. [DOI:10.7551/mitpress/8029.001.0001]
Holmes, P., & Calmels, C. (2008). A neuroscientific review of imagery and observation use in sport. Journal of Motor Behavior, 40(5), 433-445. [DOI:10.3200/JMBR.40.5.433-445] [PMID]
Jeannerod, M. (1994). The representing brain: Neural correlates of motor intention and imagery. Behavioral and Brain Sciences, 17(2), 187–202. [Link]
Kim, Y. T., Seo, J. H., Song, H. J., Yoo, D. S., Lee, H. J.,& Lee, J., et al. (2011). Neural correlates related to action observation in expert archers. Behavioural Brain Research, 223(2), 342-347. [DOI:10.1016/j.bbr.2011.04.053] [PMID]
Lago-Rodríguez, A., Cheeran, B., Koch, G., Hortobagy, T., & Fernandez-del-Olmo, M. (2014). The role of mirror neurons in observational motor learning: An integrative review. European Journal of Human Movement, 32, 82-103. [Link]
Magill, R., & Anderson, D. (2010). Motor learning and control: oncepts and applications. New York: McGraw-Hill Publishing. [Link]
Nakano, H., Osumi, M., Ueta, K., Kodama, T., & Morioka, S. (2013). Changes in electroencephalographic activity during observation, preparation, and execution of a motor learning task. International Journal of Neuroscience, 123(12), 866-875. [DOI:10.3109/00207454.2013.813509] [PMID]
Oberman, L. M., Ramachandran, V. S., & Pineda, J. A. (2008). Modulation of mu suppression in children with autism spectrum disorders in response to familiar or unfamiliar stimuli: The mirror neuron hypothesis. Neuropsychologia, 46(5), 1558-1565. [DOI:10.1016/j.neuropsychologia.2008.01.010] [PMID]
Onate, J. A., Guskiewicz, K. M., Marshall, S. W., Giuliani, C., Yu, B., & Garrett, W. E. (2005). Instruction of jump-landing technique using videotape feedback: Altering lower extremity motion patterns. The American Journal of Sports Medicine, 33(6), 831-842. [DOI:10.1177/0363546504271499] [PMID]
Orgs, G., Dombrowski, J. H., Heil, M., & Jansen-Osmann, P. (2008). Expertise in dance modulates alpha/beta event‐related desynchronization during action observation. The European Journal of Neuroscience, 27(12), 3380–3384. [DOI:10.1111/j.1460-9568.2008.06271.x] [PMID]
Rosenbaum, D. A. (2009). Human motor control. Massachusetts: Academic press. [Link]
Schmidt, R. A., Lee, T. D., Winstein, C., Wulf, G., & Zelaznik, H. (2011). Motor control and learning: A Behavioral emphasis. Champaign: Human Kinetics. [Link]
Schunk, D. H. (2012). Learning theories an educational perspective. Boston, MA: Pearson Education Inc. [Link]
Shea, C. H., Wright, D. L., Wulf, G., & Whitacre, C. (2000). Physical and observational practice afford unique learning opportunities. Journal of Motor Behavior, 32(1), 27-36. [DOI:10.1080/00222890009601357] [PMID]
Shebilskem, W. L., Regian, J. W., Arthur, W., & Jordan, J. A. (1992). A dyadic protocol for training complex skills. Human Factors, 34(3), 369-374. [DOI:10.1177/001872089203400309]
Ste-Marie, D. M., Law, B., Rymal, A. M., Jenny, O., Hall, C., & McCullagh, P. (2012). Observation interventions for motor skill learning and performance: An applied model for the use of observation. International Review of Sport and Exercise Psychology, 5(2), 145-176. [DOI:10.1080/1750984X.2012.665076]
Ste-Marie, D. M., Vertes, K., Rymal, A. M., & Martini, R. (2011).Feedforward self-modeling enhances skill acquisition in children learning trampoline skills. Frontiers in Psychology, 2, 155. [DOI:10.3389/fpsyg.2011.00155] [PMID]
Stevens, J. A., Fonlupt, P., Shiffrar, M., & Decety, J. (2000). New aspects of motion perception: Selective neural encoding of apparent human movements. Neuroreport, 11(1), 109-115. [DOI:10.1097/00001756-200001170-00022] [PMID]
Van Gog, T., Paas, F., Marcus, N., Ayres, P., & Sweller, J. (2009). The mirror neuron system and observational learning: Implications for the effectiveness of dynamic visualizations. Educational Psychology Review, 21(1), 21-30. [DOI:10.1007/s10648-008-9094-3]
Wulf, G., Shea, C., & Lewthwaite, R. (2010). Motor skill learning and performance: A review of influential factors. Medical Education, 44(1), 75-84. [DOI:10.1111/j.1365-2923.2009.03421.x] [PMID]
Observational learning or learning from a model is a particular type of perceptual learning and its usefulness has been shown in motor learning (Schmidt et al., 2011; Alhajri et al., 2018). Learning from a model is a crucial component of Bandura’s cognitive-social theory and refers to behavioral, cognitive, and emotional changes resulting from viewing models (Schunk, 2012). Observational learning is used as an effective way of learning simple and complex motor skills (Wulf et al., 2010). Studies show that observational training can play a critical and unique part in learning, especially when combined with physical training (Shebilskem et al. 1992; Shea et al. 2000). Also, neuroimaging studies show that a set of common neural structures are activated in both action execution and action observation (Gallese & Goldman, 1998; Jeannerod, 1994). A review of studies related to observational learning shows that many factors are involved in the effectiveness of observational interventions (Ste-Marie et al., 2012). Factors, such as observation and task characteristics are crucial to consider when observing interventions. The related literature suggests that researchers are interested in this crucial factor in observational interventions that “who” is the most useful model for observation (Ste-Marie et al., 2012). Common types of modeling used by researchers include observing others or observing themselves. In observing others, the skilled model, the novice model, and the learning model are examined. Another approach is to use self-modeling techniques (Ste-Marie et al., 2011). Self-modeling is a form of observational learning with the distinction that the observed and the observer are the same person, that is, they observe their executive behavior and then repeat the intended behavior (Dowrick, 2012a; Dowrick, 2012b).
Factors in self-modeling suggest that this model can be an optimal model for learning skills. This can be examined from several perspectives. From Holmes and Calmels’ neurological perspective (Holmes & Calmels, 2008), self-modeling can be more functional than observing other persons in terms of neural activation between action execution and observation. From a psychological point of view, self-modeling is also a desirable model. In this respect, psychological constructs, such as self-efficacy and other self-regulation processes are initiated by observing mastery experiences (Ste-Marie et al., 2011). Bandura’s cognitive-social theory also supports self-observation as a desirable model. According to this theory, observing the model does not ensure that acquisition will take place, or that the acquired behavior will occur later. In other words, many factors affect vicarious learning and acquired behaviors; one of which is the similarity of a model with an observer. Similarity is a crucial factor in assessing suitability and shaping beliefs. The more similar observers are to models, the more likely the observers will consider similar actions socially appropriate for themselves to perform. Besides, based on this theory, the model-observer similarity leads to the enhancement of the attention and retention processes of observation learning, thereby increasing the learning benefits through the observation process. According to this theory, the highest level of model-observer similarity happens while a person is in their model (Schunk, 2012). Based on Schmidt et al. (2011), Bandura’s theory, which was developed to explain the acquisition of social behaviors, seems not to be appropriate for understanding motor skills learning. However, recent advances provide new insights, specifically about observational learning (Schmidt et al., 2011). The discovery of a mirror neuron system in the brain has stimulated a great deal of research into the possibility that a particular neural mechanism is a basis for observational learning (Schmidt et al., 2011). These mirror neurons are a class of cortical neurons that discharge both when a person performs a certain motor action and when one sees the same motor action performed by others. These neurons were first discovered in the prefrontal cortex of monkeys by Italian scientists at the University of Parma (Di Pellegrino et al., 1992) in an accidental discovery. Following their discovery, some other researchers concluded that the human brain also has mirror neurons (Rosenbaum, 2009). The discovery of a mirror neuron system is a critical finding in observational learning. This system is thought to play a crucial function in understanding the actions of others and may be responsible for our ability to learn by observing and imitating the actions of others and can underpin observational learning mechanisms (Cattaneo & Rizzolatti, 2009; Van Gog et al., 2009). That is, these neurons are the basis for the perceptual-motor conversion mechanism and enable the visual information to be converted into motor commands. Thanks to such visual-motor conversions, humans can learn how to perform a particular action based on the information derived from the model. These conversions allow the observer to repeat the actions represented by the model (Lago Rodriguez et al., 2014).
In humans, the mirror neuron system operates differently based on the observed forms of motor behavior. Activity is greatly decreased when observing a movement that is biomechanically impossible to perform (Stevens et al., 2000) or when the observed movement is not part of the observer’s motor repertoires (Buccino et al., 2004). Likewise, the level of expertise also affects the involvement of this system (Calvo-Merino et al., 2004) which is a significant issue for the development of applications for observational use. In addition to finding cortical areas associated with the mirror neuron system, the level of activity of these neurons is also of interest to researchers. Studies (Buccino et al., 2004; Stevens et al., 2000) showed that the activity of the mirror neuron system can be due to the degree of adaptation between the observed actions and the observer’s motor abilities. Growing evidence shows that the activity of this system depends on observational motor experiences of a given action (Kim et al., 2011). Among the methods used to infer the activity of mirror neurons is Mu rhythm (also called sensory-motor rhythm). Mu rhythm is one of the dominant brain waves of about 8-13 Hz. These oscillations are limited to short periods of 0.5 to 2 s and can occur in the human sensory-motor cortex in the absence of movement. The results of the meta-analysis of 85 studies on 1 707 participants showed that Mu rhythm can be strongly inferred as a function of the human mirror system (Fox et al., 2015).
In a few studies, the activity of mirror neurons was investigated in the observation of motor and sports skills, and interesting results were obtained. For example, Calvo-Merino et al. found that when dancers see a similar style of dance, the activity of mirror neurons is greater than when they see a different style of dance. Kim et al. also examined the difference in mirror neuron activity during observation between the skilled archer group and the non-archer group. They concluded that the mirror neuron activity was more in the skilled archers’ group than in the novice group while watching the archery. Despite the importance of these neurons as the underlying mechanisms of observational learning, few studies have been conducted on the observational learning of motor and sports skills and have solely focused on observing others (Calvo-Merino et al., 2004; Kim et al., 2011). Although self-modeling is theoretically and psychologically better than other modeling methods mentioned (Bandura’s cognitive-social theory and Zimmerman’s self-regulation theory), the neural underpinning of this method has not been investigated. Also, the pattern of activity of these neurons along with learning sports skills is unclear which indicates a need for a laboratory study. That is, given the importance of the mirror neuron activity, this crucial factor during observing motor and sports skills has not been compared between self-modeling, skilled model, and learning model. The results of this study from the perspective of the mirror neuron system as a neural basis of observational learning can provide strong evidence for this crucial variable in motor learning, specifically in observational learning, i.e. the “who” is the most useful model for observation.
2. Materials and Methods
Participants
Forty-five healthy volunteer male students (aged 19.4±0.37 years) participated in the study. They were not aware of the specific purpose of the study. Informed consent was obtained from all the participants before starting the experiment. All participants had normal vision and right-sighted eyes and no familiarity with the research task. Participants were asked not to observe and practice outside the training protocol from the pre-test to the retention test. Therefore, in addition to the two cases mentioned, the individual’s request to quit the study was the exclusion criterion in this research. Participants were randomly assigned to one of the self-modeling, learning model, and skilled model groups.
Motor task
The task was to put the golf ball and guide the ball to the hole to gain a maximum score and the rating system was based on the accuracy of the putting. The target was a circle with a radius of five cm, located 4 m away from the participants. Fourteen concentric circles with radii 10,15, 20, 25, …, and 75 cm were traced around the target, and these circles were labeled with their points. These circles were used as a measure of the accuracy of the puts. If the ball was placed in area A (goal, which is the same hole in the golf matches), it would score 150 points, and by placing the ball in other areas, respectively B=140, C=130, D=120,… O=10 and outside of the area, they got 0 points (Badami et al., 2012). These concentric circles were used to measure the accuracy of the golf putting.
Data collection tools
A Sony DSCW830 20.1 MP digital camera with 20.1 Megapixel (effective) plus 8 x zoom and several pixels (Gross) which is approx. 20.4 megapixels and 720p MP4 HD movie mode was used to record golf putting with an angle of 45 degrees from the front view. The 15-inch Acer laptop (Aspire ES1-533-C7TG) was used to show the recorded videos to the participants.
Electroencephalography (EEG) recording
EEG was recorded using Psychlab EEG, a product of Contact Precision Instruments Company with a sampling rate of 256 Hz via Psychlab Data Acquisition software. To measure Mu rhythm suppression, the Mu rhythm at baseline, and observation conditions at C3, C4, and Cz area were calculated. Before calculating changes in mu power, in the pre-processing step, the EEG LAB toolbox version 14.1.1 was installed on the MATLAB software, version R2016a to eliminate the noise caused by blinking, eye movement, head movement, etc. Mu powers were calculated based on the Fourier transform technique (FFT). The suppression of mu rhythm was calculated as the ratio of mu power in the experimental condition (for example, observation) to mu power in the baseline condition which is well-known as the mirror neuron activity. This ratio is calculated to control for variability in absolute power as a result of individual differences such as skull thickness and electrode resistance. A log transformation was also used for the analysis because the data ratios are inherently abnormal as a result of the low range. A log ratio of value <0 indicates suppression of mu rhythm, a value=0 indicates no suppression and a value >0 indicates an increase.
Observational training
The experiment consisted of three periods, pre-test period, acquisition period, and retention period. After the initial training, the participants performed 10 trials (golf putting) as a pre-test and the results were recorded. The acquisition period consisted of six sessions and each session had six blocks of 10 trials. Participants at each session and before the start of each block saw a video related to their group and then performed physical training. The results of the final block of the sixth session were considered as the acquisition test score. Seven days (according to Schmidt et al. (2011), the retention interval is commonly considered 24 hours or more) after completing the acquisition test, the retention test was performed with 10 puts as well as the recording of electrical brain waves by observing 10 puts for a third time. The conditions of the golf putting test and recording of the brain electrical waves were similar to the previous two periods. In this way, the putting test was first performed and then the brain waves were recorded.
Procedure
Pre-training considerations, instructions, and initial training for the golf putting, including putting alignment, putting stance and ball position, keeping eyes over the ball, the putting grip, the putting stroke, etc. were presented to the participant. The participants’ performance of the self-modeling group was videotaped for the first acquisition session. Before the first acquisition physical training session, participants observed 10 times (in the early stages of learning, the emphasis is more on observation; for reviews, see Magill and Anderson (2010) their group films, then they continued training independently in their group. After filming 10 skilled golfer puts, the best performance was selected as a skilled model group movie to produce a skilled model movie. To prepare the movie for the learning model, four people were selected from the non-statistical population, then they practiced in six sessions similar to the research groups among which the person who acquired the natural acquisition pattern was chosen as a learning model. The movie of the self-modeling group was also a pre-test movie in the early acquisition. In each session, the acquisition was updated with filming, and in each session, the previous session films were played.
Statistical analyses
In the dependent variable of golf putting accuracy, the mean put accuracy was calculated based on the system traced around the hole; the closer the golf ball was to the hole, the higher the score obtained by 10 puts.
To investigate the effect of independent variables on golf put acquisition and retention, three (self-modeling, skilled model, and learning model) in three (pre-test, acquisition, and retention periods) mixed ANOVA were used and the last factor was a repeated measure. The same test was performed on the activity of the dependent variable mu rhythm. Bonferroni post hoc test was used and the significance level was 0.05. The data were analyzed with the SPSS software, version 25.
3. Results
Table 1 presents the Mean±SD of the accuracy of the participant’s golf putting in all three groups and all three experimental periods.

Table 2 presents the Mean±SD of the data related to the mu rhythm suppression in all three groups, and all three experimental periods.

Mu rhythm activity
The results of the mixed analysis of variance (ANOVA) indicated that the main effect of group (F(2, 42)=15.005; P=0.001; η2=0.601) and periods (F(2, 84)=13.95; P=0.001; η2=0.249) variables on mu rhythm suppression is significant. However, the main effect of the brain regions variable approaches significance (F(2, 84)=2.866; P=0.063; η2=0.064). Results of interaction between variables showed that interaction between groups and periods is significant (F(3.48, 42)=4.849; P=0.003; η2=0.188), and a marginally significant interaction is observed between group and brain regions (F(4, 42)=2.461; P=0.051; η2=0.105). However, the interaction between periods and brain regions (F (138.158, 3.289)=3.289; P=0.955; η2=0.003) is not the interaction between period variables, brain regions, and group (F(4, 42)=1.34; P=0.239; η2=0.06).
Due to the significant interaction between group variables and experimental periods, the main effect of period variables was neglected and a one-way ANOVA test was performed to investigate the effect of modeling type on mu rhythm suppression for each period.
As shown in Figure 1, the results of this test showed that in the pre-test period, a significant difference is observed in the mu rhythm suppression between the three groups of self-modeling, learning model and skilled model in all three brain regions C3 (F(2, 42)=8.077, P=0.001), C4 (F(2, 42)=6.967, P=0.002), and Cz (F(2, 42)=7.505, P=0.002).

Bonferroni post hoc test was used to investigate the significant differences between the groups, which showed that the self-modeling group had higher mu rhythm suppression in the C3 -0.194±0.197, C4 -0.176±0.355, and Cz -0.173±0.183 regions than the mu rhythm suppression in skilled model groups in C3 0.123±0.191, C4 0.076±0.247 and Cz 0.194±0.197 and the in learning model in C3 0.077±0.279, C4 0.193±0.205 and Cz 0.035±0.15; however, the skilled model group and the learning model group had no significant difference in the mu rhythm suppression.
As shown in Figure 2, the results of this test showed that in the pre-test period, a significant difference is observed in the mu rhythm suppression between the three groups of self-modeling, learning model, and skilled model in all three brain regions, in the acquisition period, a one-way ANOVA test showed no significant difference between the experimental groups in C3 (F(2, 42)=1.976, P=0.151), C4 (F(2, 42)=0.211, P=0.811) and only approached significance at Cz (F(2, 42)=2.975, P=0.062) regions.

This test was also used in the retention period, which showed that the difference between the experimental groups in C3 (F(2, 42)=1.325, P=0.277), and C4 (F(2, 42)=2.411, P=0.102) regions was not significant but in the Cz (F(2, 42)=3.574, P=0.037) region, it was significant. Figure 3 shows these results. Bonferroni post hoc test with alpha adjustment based on the number of groups was used to investigate the significant differences between groups in the Cz region and showed no significant difference between groups.

Learning group
The results of two-factor ANOVA test showed that the main effect of periods on mu rhythm suppression was significant (F2, 28)=7.74; P=0.002; η2=0.356) and main effect of brain regions (F2, 28)=2.85; P=0.075; η2=0.169) and also interaction of periods with brain regions (F(2.71, 37.97)=1.522; P=0.208; η2=0.098) was not significant. Figure 4 shows these results.
The Bonferroni post hoc test showed that the mu rhythm suppression in the acquisition test (P=0.004) and the retention test (P=0.014) was significantly higher than the pre-test and no significant difference was observed between the acquisition and the retention test.

Self-modeling group
The results of two-factor ANOVA test indicated that the main effect of the periods on the mu rhythm suppression (F(1.44, 20.28)= 0.388; P=0.617; η2=0.027), main effect of brain regions (F2, 28)=1.015; P=0.147; η2=0.128), and also interaction of periods with brain regions (F(4, 56)=1.133; P=0.15; η2=0.075) was not significant. Figure 5 shows these results.

Skilled group
The results of two-factor ANOVA showed that the main effect of periods on mu rhythm suppression was significant (F(1.355, 18.98)=7.74; P=0.001; η2=0.544) and main effect of brain regions (F2, 28)=3.157; P=0.057; η2=0.184) and also interaction of periods with brain regions (F(4, 56)=0.185; P=0.945; η2=0.013) was not significant. Figure 6 shows these results. The Bonferroni post hoc test showed that the mu rhythm suppression in the acquisition test (P=0.001) and the retention test (P=0.002) was significantly higher than the pre-test and no significant difference was observed between the acquisition and the retention test.

Golf putting task
The mixed ANOVA test was performed to measure the effect of three types of intervention (self-modeling, skilled model, and learning model) on the obtained scores from the mean of the golf putting in three periods (pre-test, acquisition, and retention). Figure 7 shows these results. The main effect of the periods (F(1.35, 56.87)=189.51, P=0.001, η2=0.819), the main effect of the group (F(2, 42)=7.86; P=0.001; η2=0.27), and interaction between the modeling and the periods (F(2.7, 56.87)=189.515, P=0.001, η2=0.288) were significant.

For this reason, a one-way ANOVA between groups was performed to examine the effect of modeling on the mean score of golf putting for each period. No significant difference was observed between the mean scores of the three groups in the pre-test (F(2, 42)=0.07; P=0.93). During the acquisition period, the difference between the groups was significant (F(2, 42)=10.45; P=0.001). Bonferroni post hoc test showed the self-modeling group with a higher score (Mean±SD 112.8±13.4) than the skilled model group (Mean±SD 87.6±19.53), and the learning model group (Mean±SD 84.73±21.58); however, no significant difference was observed between the skilled model group and the learning model group. During the retention period, although the mean score of the groups was slightly decreased, the results were similar to the results of the acquisition period and the mean scores of the self-modeling group (Mean±SD 101.28±13.6) had a significant difference with the skilled model group (Mean±SD 75.67±20.97), and the learning model group (Mean±SD 77.6±20.36).
Thus, to investigate the effect of periods, for each group, repeated measures ANOVA test was performed to compare the scores of the golf putting in the pre-test, acquisition, and retention periods. In the learning group, a significant effect was observed on the period (F(1.161, 16.261)=26.981; P=0.001; η2=0.658). Bonferroni post hoc test showed that the mean scores in all three periods were significantly different and significant progress was observed from the pre-test to the acquisition period (MD:35.262), as well as, a significant decrease was observed from acquisition to retention periods (MD:-7.33); however, despite this decrease, the difference between the pre-test and retention periods (mean differences (MD): 28.13) was significant.
The results of this test for the self-modeling group showed that the main effect of the period was significant (F2, 28)=159.235; P=0.001; η2=0.919). The Bonferroni post hoc test also showed that the mean scores in all three periods differed significantly, i.e. significant progress was made from the pre-test to acquisition periods (MD: 64.8) as well as a significant decrease was observed from acquisition to retention periods (MD: -11.353); however, despite this decrease, the difference between the pre-test and the retention periods (MD: 53.26) was significant.
The results of this test for the skilled model group were similar to those in the previous two groups. Thus, the main effect of the period variable was significant (F (1.201, 16.819)=51.268; P=0.001; η2=0.786). Bonferroni post hoc test also showed that the mean scores in all three periods were significantly different and in this group putting mean scores significantly increased from pre-test to acquisition periods (MD:39.26) as well as significantly decreased from acquisition to retention periods (MD:-11.933); however, despite this decrease, the difference between the pre-test and retention periods (MD:27.33) was significant.
4. Discussion
This study was conducted to investigate the effect of self-modeling, skilled model, and learning model on mu rhythm suppressions as an index of mirror neuron activity and learning of golf putting skill. In the pre-test period, mu rhythm suppression in the three brain regions studied was higher in the self-modeling group than in the skilled model and learning model groups. In the acquisition and retention periods, no significant difference was observed between the experimental groups in the mu rhythm suppression in the three brain regions studied. In the learning and skilled groups, the mu rhythm suppression increased significantly from the pre-test to the acquisition test period, and this increase was significant up to the retention period. In the self-modeling group, no significant difference was observed between the three experimental periods in the rate of mu rhythm suppression.
In the present study, one of the crucial variables of observational learning, namely the type of model was addressed. The effect of self-modeling, skilled model, and learning model on the acquisition and retention of golf putting skills was investigated. The results showed that all three groups had a significant improvement in this skill, although a significant decrease was observed in the retention test. Among the three groups, the self-modeling group had more progress than the other two groups, so that a significant difference was observed between them in both the acquisition test and the retention test. However, no significant difference was observed between the skilled model and the learning model groups at either stage. One of the results of this study was the significant effect of self-tuning on the acquisition and retention of golf putting skills. Because this modeling method was better than the other two methods, both in the acquisition test and in the retention test. This result is consistent with the results of other studies, such as Onate et al. (2005) ; Clark and Ste-marie (2007). These results are theoretically consistent with several perspectives. One of the critical theories about the optimal effect of self-modeling on learning is Bandura’s cognitive-social theory. From the point of view of this theory, a critical factor in vicarious learning is the similarity of model and observer; in this way, the more similar the observers are to the models, the more appropriate the actions of the models are for themselves. Also, according to this theory of model and observer similarity, the processes of attention and retention, which are part of the basic stages of observational learning, are strengthened, which is beneficial for learning through observation (Schunk, 2012). Therefore, it seems that in this study, self-modeling, which has the highest model-observer similarity and has led to better learning than the other two methods, has benefited from the similarity advantages mentioned in Bandura’s theory. According to Clark and Ste-Marie’s opinions, individuals by observing their performance engage in mental processes, such as performance assessment, which is useful for learning. Golf putting findings are consistent with Zimmerman’s theory of self-regulation, which states that psychological structures such as self-efficacy and other self-regulatory processes are triggered by the observation of mastery experiences. According to this theory, self-modeling is effective in self-regulation. Self-regulation also leads to improved self-satisfaction, which in turn has a positive effect on other self-regulatory processes, such as intrinsic motivation and self-efficacy, leading to better performance (Clark and Ste-marie, 2007). Therefore, it seems that observing the performance and progress in golf putting skill in the participants of the self-modeling group, has led to more belief in the ability to perform this skill, as well as increased task interest and motivation, and ultimately led to better learning.
Given that mu rhythm suppression in the C3, C4, and Cz regions indicates the activity of mirror neurons in the sensory-motor cortex, these results can be translated into observational learning language, as follows:
When a motor skill learner is a beginner, by:
1. Observing a beginner model, his/her mirror neurons become less active.
2. Observing a skilled model, his/her mirror neurons become less active.
3. Observing his/her performance, the activity of the mirror neurons becomes greater.
The critical result of this study was the different effects of three modeling methods (self, skilled, and learning) on the activity of mirror neurons in the pre-test period. As such, when participants were novice golf putting, only the mirror neurons of the self-modeling group were more active and no difference was observed between the skilled model and the learning model.
The section on the difference between the activity of mirror neurons in the skilled and learning models groups can be compared with some of the existing studies. This result is consistent with part of the results of the studies conducted by Calvo-Merino et al., (2004), Orgs et al. (2008), Kim et al., (2011) and Ashraf et al. (2019).
A part of Calvo-Merino’s study was related to the control group that observed both ballet and capoeira dance types. The results of this part showed no effect of the stimulus type (observing skilled ballet motion pictures and observing skilled capoeira motion pictures) on the mirror neuron activity of novice participants. This means that the participants had less mirror neuron activity when they saw the movements of the skilled people. In a similar study but with a different instrument, namely the recording of the brain electrical waves, Orgs et al also found that suppression of mu rhythm was less effective in non-skilled observing dance movements. Kim et al. also found that the novice archery participant’s mirror neuron activity was lower while observing skilled archers.
The reason for the lower activity of mirror neurons at this period can be attributed to the participant of motor repertoire by observing the skillful and learning model consistent with the mentioned studies. In this regard, evidence shows that the only actions that are involved in our motor repertoire affect the activity of the mirror neuron system (Stevens et al., 2000).
It has also been suggested that the activation of the mirror neuron system is related to the degree of adaptation between the observed actions and the observer’s motor capability (Kim et al., 2011). Observing a model affects the activity of the mirror neurons when the observer finds a similarity between the observed model’s execution and motor capability.
Therefore, it seems that participants in the skilled and learning model groups had less mirror neuron activity due to the lack of model-driven movement in their motor repertoire. In other words, because they were not proficient in the golf putting skill and had not yet learned the skill, the mirror neurons were also less active.
A very significant result from the pre-test period was more suppression of mu rhythm in the self-modeling group. Several studies can be more or less associated with this result. For example, Gastaut and Bert (1954) reported that when a person sees himself on the screen, the mu rhythm is blocked. Also, studies have shown that mirror neurons were affected by their observation even though they did not record electrical waves in the brain.
Coulson et al. (2006a) by self-modeling technique, Coulson et al. attempted to improve the smile deficits after nerve paralysis in two participants and achieved a significant improvement after two weeks. Seeing oneself smile on the videotape may guide mirror neurons to facilitate imitation. Coulson et al. (2006b) conducted another study with 10 participants with the same aim and method and achieved similar results, such as improved response speed, smile quality, and so on. In the mentioned study, participants’ smiles were automated as a result of the self-modeling intervention and it was stated that the possible explanation for the rapid acquisition of this skill is the activation of mirror neurons. In another study, Oberman et al. (2008) examined the modulation of mu suppression in autistic children and the control group in response to familiar and unfamiliar stimuli. The results of this study revealed that mu suppression is sensitive to the familiarity of the presented films. This means that the suppression of the mu rhythm was more pronounced during observing a familiar hand compared to an unfamiliar hand. Also, with his hand, the suppression of the mu rhythm increased. Thus, it seems that the mirror neuron system is not only the neural basis of observational learning but also the fundamental process of self-modeling. These results are consistent with the results of the present study that by self-observing in the pre-test period, the activity of the mirror neurons is increased.
These results are theoretically related to part of Bandura’s cognitive-social learning theory. According to this theory, the more alike observers are to the models, the more likely observers will consider similar actions appropriate for them to execute. Besides, model-observer similarity results in enhanced observation and retention processes of observational learning, which in turn increases the benefits of learning through the observation process. According to this theory, the highest model-observer similarity occurs when a person is a model himself (Schunk, 2012). Therefore, it seems that this is what happened in the present study in the pre-test period. In other words, the participants in the self-modeling group paid more attention to the model (themselves) than the other two groups by observing themselves during golf putting skills.
As mentioned, the activity of the mirror neurons in acquisition and retention periods was not significantly different between the three experimental groups. In other words, no difference was observed in the mirror neuron activity when the participants became somewhat proficient in golf putting and saw different models. This result is consistent with the literature on mirror neurons. Cattaneo & Rizzolatti, 2009 have stated that the activity of the mirror system is related to the observer’s motor experiences of a given action. In other words, when the observer has a motor experience from the observed motion, the mirror neurons are more active. Also, evidence shows that observing the motor actions present in the observer’s motor repertoire leads to a stronger activation of mirror neurons compared to observing new motor behaviors (Gazzaniga, 2009). Kim et al. also suggested that the activation of the mirror neuron system is related to the degree of adaptation between the observed actions and the observer’s motor capability (Kim et al., 2011). That is when observing a model affects the activity of the mirror neurons when the observer finds a similarity between the observed model’s execution and its capability. These principles are consistent with the results of the present study and are part of the results of the studies conducted by Calvo-Merino et al., (2004), Orgs et al. (2008), and Kim et al., (2011).
Therefore, based on the principles mentioned and as our results reveal, it can be concluded that the participants in acquisition and retention periods are somewhat proficient in golf putting skills, and therefore the activity of mirror neurons in the motor-sensory area is increased. In other words, after six sessions of training, the golf putting skill have been learned and become part of their motor repertoire and they have found a great similarity between the performance of the skilled model (skilled, self-skilled, novice model that turned to skilled) and their ability to perform the same movement. As a result of this similarity, the activity of mirror neurons has also increased. The other part of the acquisition and retention periods finding is related to a significant increase in mirror neuron activity from the pre-test to the acquisition and retention periods, although this increase occurred in the skilled and learning model groups. In other words, the pattern of mirror neurons’ activity has changed along with the learning of golf putting skills. Studies in this area have involved the learning process by measuring the activity of mirror neurons; therefore, it would be very useful to compare their results with the results of the present study. Cross et al. (2006) showed that mirror neuron activity increases with the learning of new dance sequences. Cross et al. (2008) also found that after the training period, participants’ brain activity in the mirror-related regions increased as they observed or saw sequences they performed. Nakano et al. (2013) concluded that in the five-trial practice of the ball-rotation task, suppression of mu rhythm in the last attempts increased and performance improved. These results are consistent with the results of our study. The results of these studies along with the results of studies on the theoretical foundations of mirror neurons are interpreted as the plasticity of the mirror system. This means that the mirror system is modified by motor experiences (Gazzaniga, 2009). Therefore, based on such backgrounds and theoretical foundations, we can also deduce from this study that six sessions of 60 observing and physical training trials of golf putting skill can result in a sufficient experience in the mentioned skill and this experience can result in plasticity and more activity of mirror neurons.
Looking at the results of the two behavioral (golf putting actions) and neuroscience (mu rhythm suppression) sections of golf putting skill learning with three different model types, a critical point emerges. This new result in the field of mirror neurons is accompanied by more activity of mirror neurons in the pre-test period in the self-modeling group with better learning in this group than the other two groups. As reported, the self-modeling group had learned better the golf putting skills and it was associated with more mirror neuron activity in the pre-test period. It is logical to claim that it occurred during the acquisition period or at least during the first few sessions of the acquisition period. This means that during the acquisition period and model observation between the blocks of physical training, mirror neurons were more active than the other two groups.
Theoretical backgrounds of the studies of mirror neurons suggest that when individuals are novices in the observed motor skills, mirror neurons are less responsive. In contrast, the level of mastery is highly influenced by the activity of these neurons, and with the increase of mastery, the activity of these neurons also increases. As a result of these comparisons, we concluded that this information is inconsistent with the crucial result of this study. On the other side, the results of modeling studies are mainly in favor of self-modeling. The results are also theoretically supported by several neurological (Holmes & Calmels, 2008) and psychological studies (Bandura’s cognitive-social learning theory and Zimmerman’s self-regulation theory). Most importantly, the results of the present study are consistent with these results and theoretical foundations, a crucial issue that has not been addressed from the perspective of mirror neurons.
5. Conclusion
These results are distinct from the studies that focus on already combined psychological (model-observer similarity in Bandura’s theory) and mirror neuron perspectives (the influence of expertise level on the activity of these neurons) and can be interpreted as an “overcoming model-observer similarity on level of expertise”. This means that due to more model-observer similarity, the participants in the self-modeling group have more mirror neuron activation despite lacking mastery in early periods. This may be due to more attention to this group or factors, such as triggering structures, such as self-efficacy and task interest (though none of these were examined).
Also, since the anatomical locations of the C3, C4, and Cz electrodes are related to the supplementary motor area and sensory-motor area and their activity is related to the mirror neurons, the supplementary area has a mediating role in the communication in the different parts of the mirror system. Based on the results of the present study, these areas were more involved in the self-modeling group in the early periods of golf skill training which ultimately benefited this group’s learning. The results of this study can have critical training considerations in motor learning, especially observational learning. These results can provide strong evidence for this crucial variable in motor learning and more specifically in observational learning that “who” is the most useful model for observation and can highlight the role of model-observer similarity in observational learning.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles are considered in this article. The participants were informed of the purpose of the research and its implementation stages. They were also assured about the confidentiality of their information and were free to leave the study whenever they wished, and if desired, the research results would be available to them. A written consent has been obtained from the participants. Principles of the Helsinki Convention were also observed. In addition, this article has received permission to carry out research activities from the ethics-research committee of Institute for Cognitive and Brain Sciences, Shahid Beheshti University (Code: IR.SBU.ICBS).
Funding
The paper was extracted from the PhD dissertation of Ramin Ashraf, Behrouz Abdoli, Reza Khosrowabadi, Alireza Farsi and Jaime A Pineda, approved by Department of Behavioral and Cognitive Science in Sport, Faculty of Sport Sciences and Health, Shahid Beheshti University.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are grateful to the Laboratory Center of Brain and Cognitive Sciences Institute of Shahid Beheshti University, the Laboratory of Cognitive and Behavioral Sciences in Sports, and all the students participating in the research.
References
Alhajri, N., Hodges, N. J., Zwicker, J. G., & Virji-Babul, N. (2018). Mu suppression is sensitive to observational practice but results in different patterns of activation in comparison with physical practice. Neural Plasticity, 2018, 8309483. [DOI:10.1155/2018/8309483] [PMID]
Ashraf, R., Abdoli, B., Khosrowabadi, R., & Farsi, A. (2019). [Effects of model type on mirror neurons activity during observation of a motor skill (Persian)]. Advances in Cognitive Science, 21(2), 132-140. [DOI:10.30699/icss.21.2.132]
Badami, R., VaezMousavi, M., Wulf, G., & Namazizadeh, M. (2012). Feedback about more accurate versus less accurate trials: Differential effects on self-confidence and activation. Research Quarterly for Exercise and Sport, 83(2), 196-203. [PMID]
Buccino, G., Lui, F., Canessa, N., Patteri, I., Lagravinese, G., & Benuzzi, F., et al. (2004). Neural circuits involved in the recognition of actions performed by nonconspecifics: An fMRI study. Journal of Cognitive Neuroscience, 16(1), 114-126. [DOI:10.1162/089892904322755601] [PMID]
Calvo-Merino, B., Glaser, D. E., Grèzes, J., Passingham, R. E., & Haggard, P. (2005). Action observation and acquired motor skills: An FMRI study with expert dancers. Cerebral Cortex, 15(8), 1243-1249. [DOI:10.1093/cercor/bhi007] [PMID]
Cattaneo, L., & Rizzolatti, G. (2009). The mirror neuron system. Archives of Neurology, 66(5), 557-560. [DOI:10.1001/archneurol.2009.41] [PMID]
Clark, S. E., & Ste-Marie, D. M. (2007). The impact of self-as-a-model interventions on children’s self-regulation of learning and swimming performance. Journal of Sports Sciences, 25, 577-586. [DOI:10.1080/02640410600947090] [PMID]
Clark, S. E., Ste-Marie, D. M., & Martini, R. (2006). The thought processes underlying self-as-a-model interventions: An exploratory study. Psychology of Sport and Exercise, 7(4), 381-386. [DOI:10.1016/j.psychsport.2005.09.001]
Coulson, S. E., Adams, R. D., O’Dwyer, N. J., & Croxson, G. R. (2006). Physiotherapy rehabilitation of the smile after long-term facial nerve palsy using video self-modeling and implementation intentions. Otolaryngology-Head and Neck Surgery, 134(1), 48-55. [DOI:10.1016/j.otohns.2005.09.010] [PMID]
Coulson, S. E., Adams, R. D., O’Dwyer, N. J., & Croxson, G. R. (2006). Use of video self-modelling and implementation intentions following facial nerve paralysis. International Journal of Therapy and Rehabilitation, 13(1), 30-35. [DOI:10.12968/ijtr.2006.13.1.21349]
Cross, E. S., Hamilton, A. F. D. C., & Grafton, S. T. (2006). Building a motor simulation de novo: Observation of dance by dancers. Neuroimage, 31(3), 1257-1267. [DOI:10.1016/j.neuroimage.2006.01.033] [PMID]
Cross, E. S., Kraemer, D. J., Hamilton, A. F. D. C., Kelley, W. M., & Grafton, S. T. (2008). Sensitivity of the action observation network to physical and observational learning. Cerebral Cortex, 19(2), 315-326. [DOI:10.1093/cercor/bhn083] [PMID]
di Pellegrino, G., Fadiga, L., Fogassi, L., Gallese, V., & Rizzolatti, G. (1992). Understanding motor events: A neurophysiological study. Experimental Brain Research, 91(1), 176-180. [DOI:10.1007/BF00230027] [PMID]
Dowrick, P. W. (2012). Self-model theory: Learning from the future. Wiley Interdisciplinary Reviews: Cognitive Science, 3(2), 215-230. [DOI:10.1002/wcs.1156] [PMID]
Dowrick, P. W. (2012). Self-modeling: Expanding the theories of learning. Psychology in the Schools, 49(1), 30-41. [DOI:10.1002/pits.20613]
Fox, N. A., Bakermans-Kranenburg, M. J., Yoo, K. H., Bowman, L. C., Cannon, E. N., & Vanderwert, R. E., et al. (2016). Assessing human mirror activity with EEG mu rhythm: A meta-analysis. Psychological Bulletin, 142(3), 291-313. [DOI:10.1037/bul0000031] [PMID]
Gallese, V., & Goldman, A. (1998). Mirror neurons and the simulation theory of mind-reading. Trends in Cognitive Sciences, 2(12), 493-501. [DOI:10.1016/S1364-6613(98)01262-5] [PMID]
Gastaut, H. J., & Bert, J. (1954). EEG changes during cinematographic presentation (Moving picture activation of the EEG). Electroencephalography and Clinical Neurophysiology, 6, 433-444. [DOI:10.1016/0013-4694(54)90058-9] [PMID]
Gazzaniga, M. S. (2009). The cognitive neurosciences. Massachusetts: MIT Press. [DOI:10.7551/mitpress/8029.001.0001]
Holmes, P., & Calmels, C. (2008). A neuroscientific review of imagery and observation use in sport. Journal of Motor Behavior, 40(5), 433-445. [DOI:10.3200/JMBR.40.5.433-445] [PMID]
Jeannerod, M. (1994). The representing brain: Neural correlates of motor intention and imagery. Behavioral and Brain Sciences, 17(2), 187–202. [Link]
Kim, Y. T., Seo, J. H., Song, H. J., Yoo, D. S., Lee, H. J.,& Lee, J., et al. (2011). Neural correlates related to action observation in expert archers. Behavioural Brain Research, 223(2), 342-347. [DOI:10.1016/j.bbr.2011.04.053] [PMID]
Lago-Rodríguez, A., Cheeran, B., Koch, G., Hortobagy, T., & Fernandez-del-Olmo, M. (2014). The role of mirror neurons in observational motor learning: An integrative review. European Journal of Human Movement, 32, 82-103. [Link]
Magill, R., & Anderson, D. (2010). Motor learning and control: oncepts and applications. New York: McGraw-Hill Publishing. [Link]
Nakano, H., Osumi, M., Ueta, K., Kodama, T., & Morioka, S. (2013). Changes in electroencephalographic activity during observation, preparation, and execution of a motor learning task. International Journal of Neuroscience, 123(12), 866-875. [DOI:10.3109/00207454.2013.813509] [PMID]
Oberman, L. M., Ramachandran, V. S., & Pineda, J. A. (2008). Modulation of mu suppression in children with autism spectrum disorders in response to familiar or unfamiliar stimuli: The mirror neuron hypothesis. Neuropsychologia, 46(5), 1558-1565. [DOI:10.1016/j.neuropsychologia.2008.01.010] [PMID]
Onate, J. A., Guskiewicz, K. M., Marshall, S. W., Giuliani, C., Yu, B., & Garrett, W. E. (2005). Instruction of jump-landing technique using videotape feedback: Altering lower extremity motion patterns. The American Journal of Sports Medicine, 33(6), 831-842. [DOI:10.1177/0363546504271499] [PMID]
Orgs, G., Dombrowski, J. H., Heil, M., & Jansen-Osmann, P. (2008). Expertise in dance modulates alpha/beta event‐related desynchronization during action observation. The European Journal of Neuroscience, 27(12), 3380–3384. [DOI:10.1111/j.1460-9568.2008.06271.x] [PMID]
Rosenbaum, D. A. (2009). Human motor control. Massachusetts: Academic press. [Link]
Schmidt, R. A., Lee, T. D., Winstein, C., Wulf, G., & Zelaznik, H. (2011). Motor control and learning: A Behavioral emphasis. Champaign: Human Kinetics. [Link]
Schunk, D. H. (2012). Learning theories an educational perspective. Boston, MA: Pearson Education Inc. [Link]
Shea, C. H., Wright, D. L., Wulf, G., & Whitacre, C. (2000). Physical and observational practice afford unique learning opportunities. Journal of Motor Behavior, 32(1), 27-36. [DOI:10.1080/00222890009601357] [PMID]
Shebilskem, W. L., Regian, J. W., Arthur, W., & Jordan, J. A. (1992). A dyadic protocol for training complex skills. Human Factors, 34(3), 369-374. [DOI:10.1177/001872089203400309]
Ste-Marie, D. M., Law, B., Rymal, A. M., Jenny, O., Hall, C., & McCullagh, P. (2012). Observation interventions for motor skill learning and performance: An applied model for the use of observation. International Review of Sport and Exercise Psychology, 5(2), 145-176. [DOI:10.1080/1750984X.2012.665076]
Ste-Marie, D. M., Vertes, K., Rymal, A. M., & Martini, R. (2011).Feedforward self-modeling enhances skill acquisition in children learning trampoline skills. Frontiers in Psychology, 2, 155. [DOI:10.3389/fpsyg.2011.00155] [PMID]
Stevens, J. A., Fonlupt, P., Shiffrar, M., & Decety, J. (2000). New aspects of motion perception: Selective neural encoding of apparent human movements. Neuroreport, 11(1), 109-115. [DOI:10.1097/00001756-200001170-00022] [PMID]
Van Gog, T., Paas, F., Marcus, N., Ayres, P., & Sweller, J. (2009). The mirror neuron system and observational learning: Implications for the effectiveness of dynamic visualizations. Educational Psychology Review, 21(1), 21-30. [DOI:10.1007/s10648-008-9094-3]
Wulf, G., Shea, C., & Lewthwaite, R. (2010). Motor skill learning and performance: A review of influential factors. Medical Education, 44(1), 75-84. [DOI:10.1111/j.1365-2923.2009.03421.x] [PMID]
Type of Study: Original |
Subject:
Cognitive Neuroscience
Received: 2021/02/12 | Accepted: 2023/07/27 | Published: 2023/09/1
Received: 2021/02/12 | Accepted: 2023/07/27 | Published: 2023/09/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








