Volume 15, Issue 1 (January & February 2024)
BCN 2024, 15(1): 61-72 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Pourmotahari F, Tabatabaei S M, Borumandnia N, Khadembashi N, Olazadeh K, Alavimajd H. A Study Over Brain Connectivity Network of Parkinson's Patients, Using Nonparametric Bayesian Model. BCN 2024; 15 (1) :61-72
URL: http://bcn.iums.ac.ir/article-1-2082-en.html
URL: http://bcn.iums.ac.ir/article-1-2082-en.html
Fatemeh Pourmotahari1 

 , Seyyed Mohammad Tabatabaei2
, Seyyed Mohammad Tabatabaei2 

 , Nasrin Borumandnia3
, Nasrin Borumandnia3 

 , Naghmeh Khadembashi4
, Naghmeh Khadembashi4 

 , Keyvan Olazadeh4
, Keyvan Olazadeh4 

 , Hamid Alavimajd *5
, Hamid Alavimajd *5 




 , Seyyed Mohammad Tabatabaei2
, Seyyed Mohammad Tabatabaei2 

 , Nasrin Borumandnia3
, Nasrin Borumandnia3 

 , Naghmeh Khadembashi4
, Naghmeh Khadembashi4 

 , Keyvan Olazadeh4
, Keyvan Olazadeh4 

 , Hamid Alavimajd *5
, Hamid Alavimajd *5 


1- Clinical Research and Development Center, Shahid Modarres Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Department of Medical Informatics, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
3- Urology and Nephrology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4- Department of English Language, School of Allied Medical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
5- Department of Biostatistics, School of Paramedical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Department of Medical Informatics, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
3- Urology and Nephrology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4- Department of English Language, School of Allied Medical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
5- Department of Biostatistics, School of Paramedical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 1556 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Parkinson is a chronic neurological system disorder that affects the dopaminergic, noradrenergic, cholinergic, and serotoninergic systems. It is the most common age-related neurological disease next to Alzheimer, with a prevalence of about 0.5% to 1% in the age range of 69-65 and 1% to 3% in people over 80 (Ahmadou et al., 2019; Nussbaum & Ellis, 2003). The risk factors for Parkinson disease (PD) are generally unknown, and old age, environmental factors, and genetic factors increase the risk of developing the disease (Kouli et al., 2018; Reeve et al., 2014). The clinical signs of Parkinson are characterized by motor and non-motor symptoms. Motor symptoms include rigidity, bradykinesia, akinesia, abnormal posture, and resting tremors. Non-motor symptoms such as autonomic, sleep, olfactory, psychiatric (depression, psychosis, hallucination, anxiety, and impulse control), and cognitive disorders are essential factors in patients’ disabilities that are referred to as the mechanisms of the initial stages of Parkinson diagnosis. Since cognitive and psychiatric disorders can reduce the daily function and quality of life of patients with PD, non-motor symptoms are of high clinical importance (Błaszczyk, 2016; Martinez‐Martin et al., 2011; Pellicano et al., 2007; Han et al., 2018; Painous & Marti, 2020). Among the psychiatric disorders, depression and anxiety have been particularly determined as risk factors for PD. The literature has also displayed that the underlying effects of depression and anxiety can appear many years before the incidence of motor symptoms (Behari et al., 2001; Bower et al., 2010; Lin et al., 2014; Shiba et al., 2000).
Resting-state functional connectivity (rsFC) studies are used to examine the pathophysiology of neurodegenerative disorders, including Parkinson’s. These studies use non-invasive functional magnetic resonance imaging (fMRI) to distinguish distinct patterns of brain connectivity between healthy and diseased individuals. Since many neurological disorders are associated with altered topological patterns of brain connectivity, rsFC studies can detect connections between different brain areas by recognizing this topological structure. Topology is defined as the study of features that describe how brain areas are arranged based on their interconnections. The use of these studies in Parkinson patients is important as it provides helpful information about functional and morphological changes, including motor and non-motor functions (Chen et al., 2020; Markošová et al., 2008; Stoessl, 2009; Tuovinen et al., 2018). In this regard, several studies have indicated alterations in brain connectivity in PD patients with cognitive disorders. For example, Gorges et al. assessed the brain connectivity networks using seed-based analyses. Compared with the control subjects, PD patients decreased functional communication within some regions of the default mode network (DMN). Baggio et al. reported decreased FC in PD patients between the dorsal attention network and right fronto insular areas using independent component analysis (Amboni et al., 2015; Baggio et al., 2015; Chen et al., 2017; Gorges et al., 2015).
Functional connectivity (FC) is determined based on the correlation patterns, using statistical methods such as Pearson correlation coefficient, mutual information, and partial correlation coefficient (Kim & Pan, 2015; Smith et al., 2011; Xiong et al., 1999). Moreover, there are other statistical methods for inferring functional relationships, including clustering models, multivariate models, graphical lasso models, and Bayesian models (Baumgartner et al., 2000; Cribben et al., 2012; Hyvärinen & Oja, 2000; Patel et al., 2006a; Patel et al., 2006b; Varoquaux et al., 2010).
Functional communication data at rest faces significant challenges: 1) The existence of correlations between the connectivity edges that are related to the features of the topological network and 2) The high number of parameters in the covariance matrix, specifically if the number of regions of interest (ROIs) is high. Although many functional studies have been performed on PD data, the analysis of functional correlation data without considering these characteristics does not seem appropriate. Accordingly, in this study, considering the characteristics of functional relationship data, the advanced nonparametric Bayesian model introduced by Chen et al. was used to evaluate the topological network structure in Parkinson patients (Chen et al., 2018).
2. Materials and Methods
Data acquisition
The resting-state fMRI data were obtained from the OpenfMRI dataset with the document ID ds000245. The scans acquisition protocol was obtained as follows: Repetition time (TR)=2500 ms, echo time (TE)=30 ms, 39 transverse slices with inter-slice interval=0.5 mm and thickness=3 mm, FOV=192 mm, matrix size=64×64, flip angle=80°. Resting-state fMRI scans were obtained for 8 minutes with eyes closed. T1-weighted images had a total time of 349 seconds.
Data processing
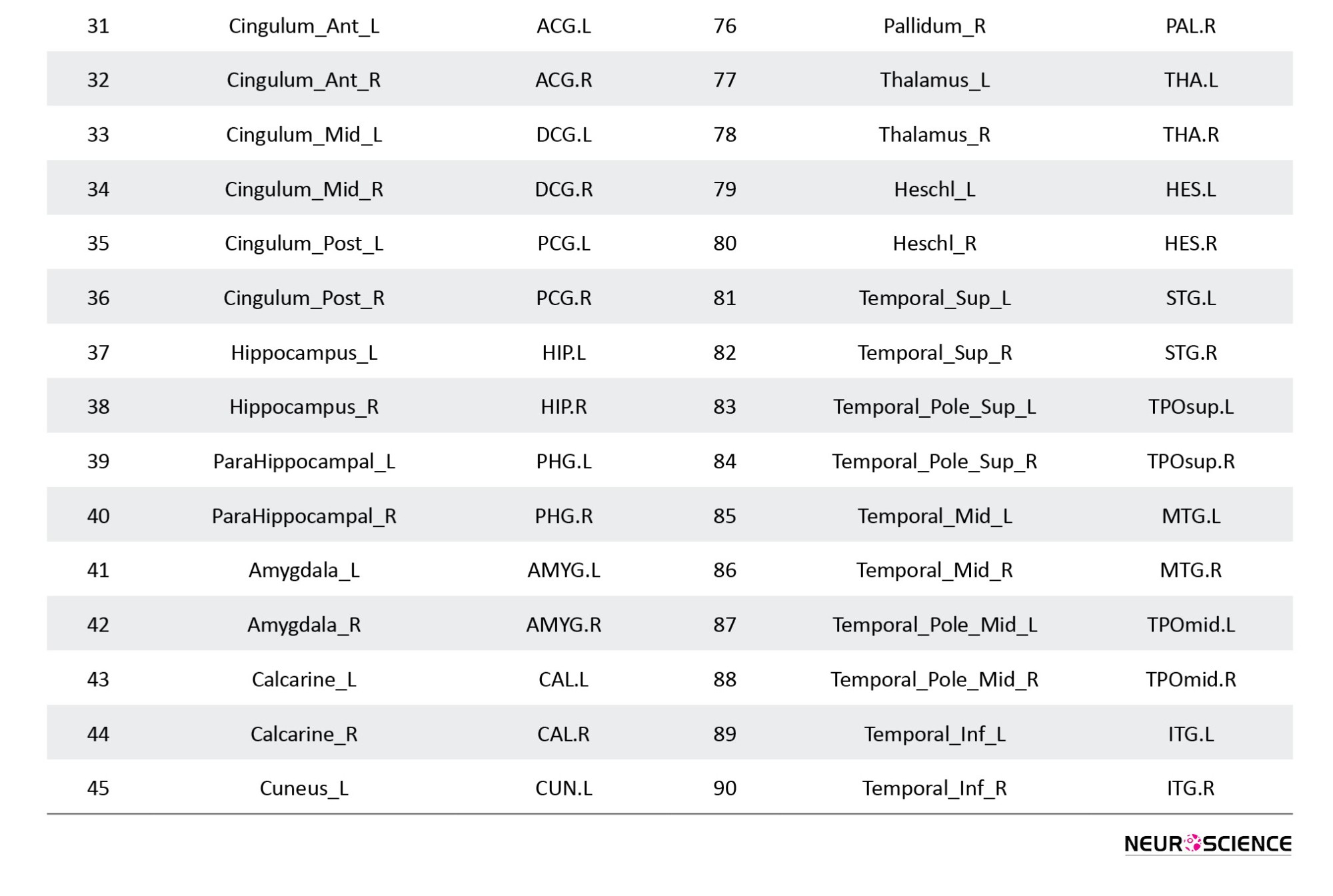
Pre-processing of resting-state fMRI scans was performed using FSL software, version 6.0.1. The first five volumes of each time course were removed due to the correction of the initial image heterogeneity and the adaptation of individuals to the surrounding conditions; hence, a total of 193 volumes per person was considered. Images were normalized with a voxel resolution of 2×2×2 mm3, and for smoothing, a gaussian filter with 6 mm FWHM was used. Then, the pre-processed images were divided into 90 desired areas, according to atlas AAl, using the WFU pickatlas toolbox in MATLAB R2019b software (Tzourio-Mazoyer et al., 2002). Fisher Z-transformed correlations were considered as the measurement index of the edges.
Statistical analysis
Statistical inference of brain FC was performed in two stages:
Step 1: A nonparametric Bayesian model was used to evaluate the topological structure of the brain network. To assess the network properties, including determining the number of clusters of brain regions and the correlation coefficient of clusters, first, the residual matrix RoN×E was calculated as Equation 1:

YN×E is the E-th sample Fisher’s Z transformed correlation for the N-th of the subject (1, ..., N). Each subject has V=90 areas and edges. is the design matrix of p-covariates and βP×E is the parameters estimation linking the covariates to the response.
Suppose represent the correlation matrix between regions of the brain. This matrix is a function of network structure and correlation parameters ρ=(ρ0,ρ1,…,ρk) .
The network topological structure-based correlation matrix is defined as Equation 2:

which is based on the correlation between the edges ei',j' (correlation between regions i and j, i≠j ) and ei',j'. ωi=Ck is considered as an indicator variable, to determine whether region i belongs to the cluster or not. If a pair of edges are in a cluster, then it can be assumed that ei,jei',j') (Equation 3):
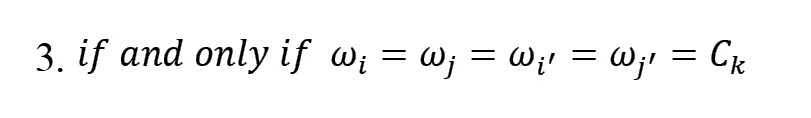
Finally, the posterior distributions ρ and ω are obtained using the markov monte carlo chain (MCMC) with 5000 iterations.
Step 2: To compare the pairwise association of 90 regions, the number of univariate tests is as high as =4005. Considering multiple comparisons, the network-based statistics (NBS) and false discovery rate (FDR) were performed to assess any significant pairwise connections between the patient and healthy groups. The NBS method uses a permutation test to examine the cluster difference of edges with a predefined threshold across the two groups. The FDR method examines the significant individual level of each edge in the two groups. P<0.05 was considered as a significant level. The analysis was performed through the NBS Connectome package in MATLAB software, version R2021b.
3. Results
Resting-state fMRI data included 11 Parkinson patients (six male) and 11 healthy individuals (six male), matched on sex ratio. The mean age was 64.36 years for the PD group and 63.73 years for the healthy group, in which, in terms of age distribution (P=0.83), there was no significant difference between the two groups.
Estimation of the number of clusters and their correlation coefficient was performed using a nonparametric Bayesian model to consider the specific characteristics of functional relationship data. According to what was previously explained in the theory of this model: 1) The regions within each cluster have a considerable functional relationship with each other and 2) The correlation coefficient of each cluster expresses the degree of pairwise correlation of regions within the cluster. Figure 1 shows the mean Fisher Z-transformed correlations of brain regions in both diseased and healthy groups.
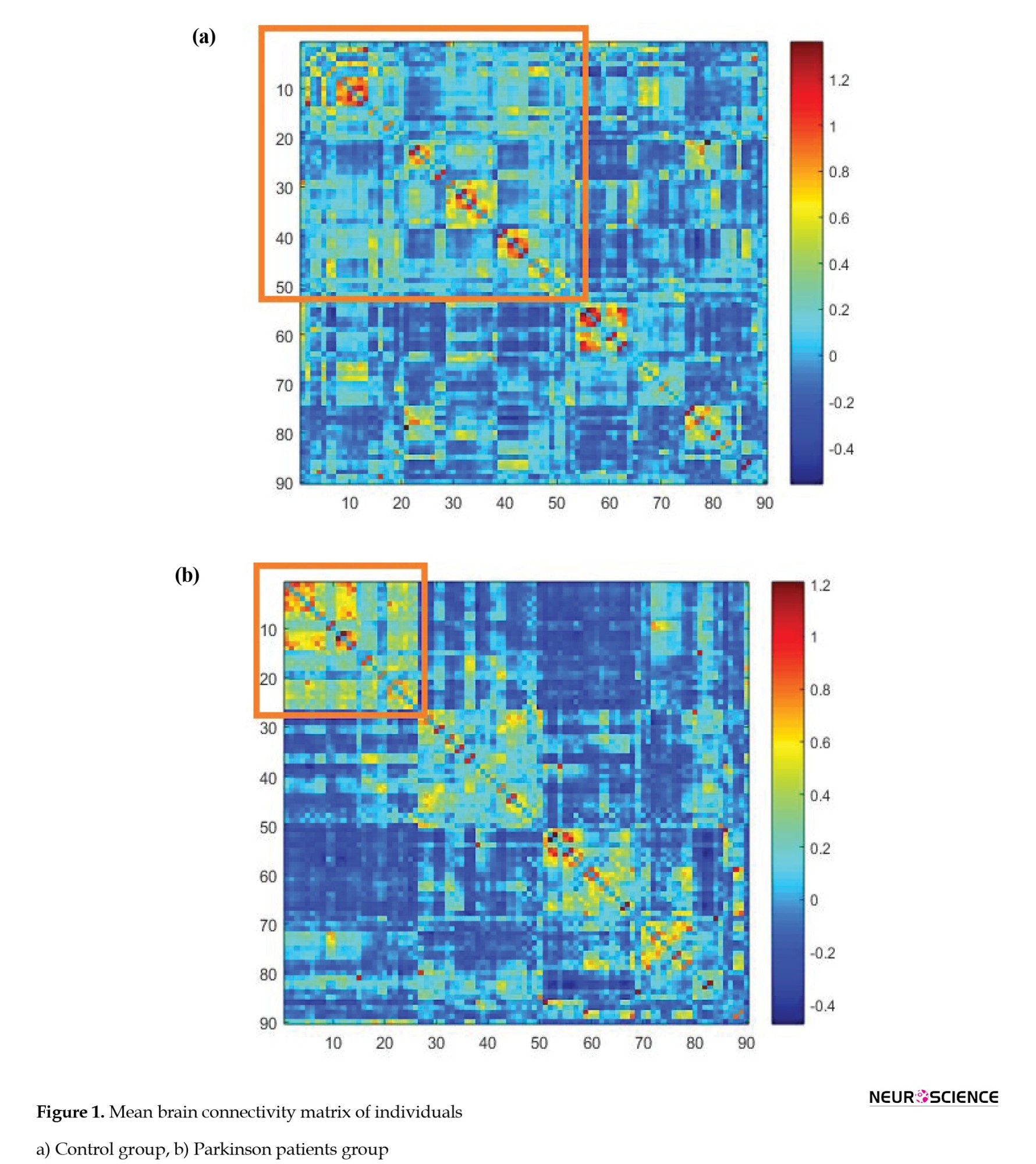
The areas in the diagram are arranged according to their placement in the clusters. The more correlated edges had a higher mean value in each cluster, indicating the correct detection of clustering by the nonparametric Bayesian model. Figure 2 shows a clustering of areas of the brain. The brain network regions of the two groups were divided into six clusters and identified by the same color.
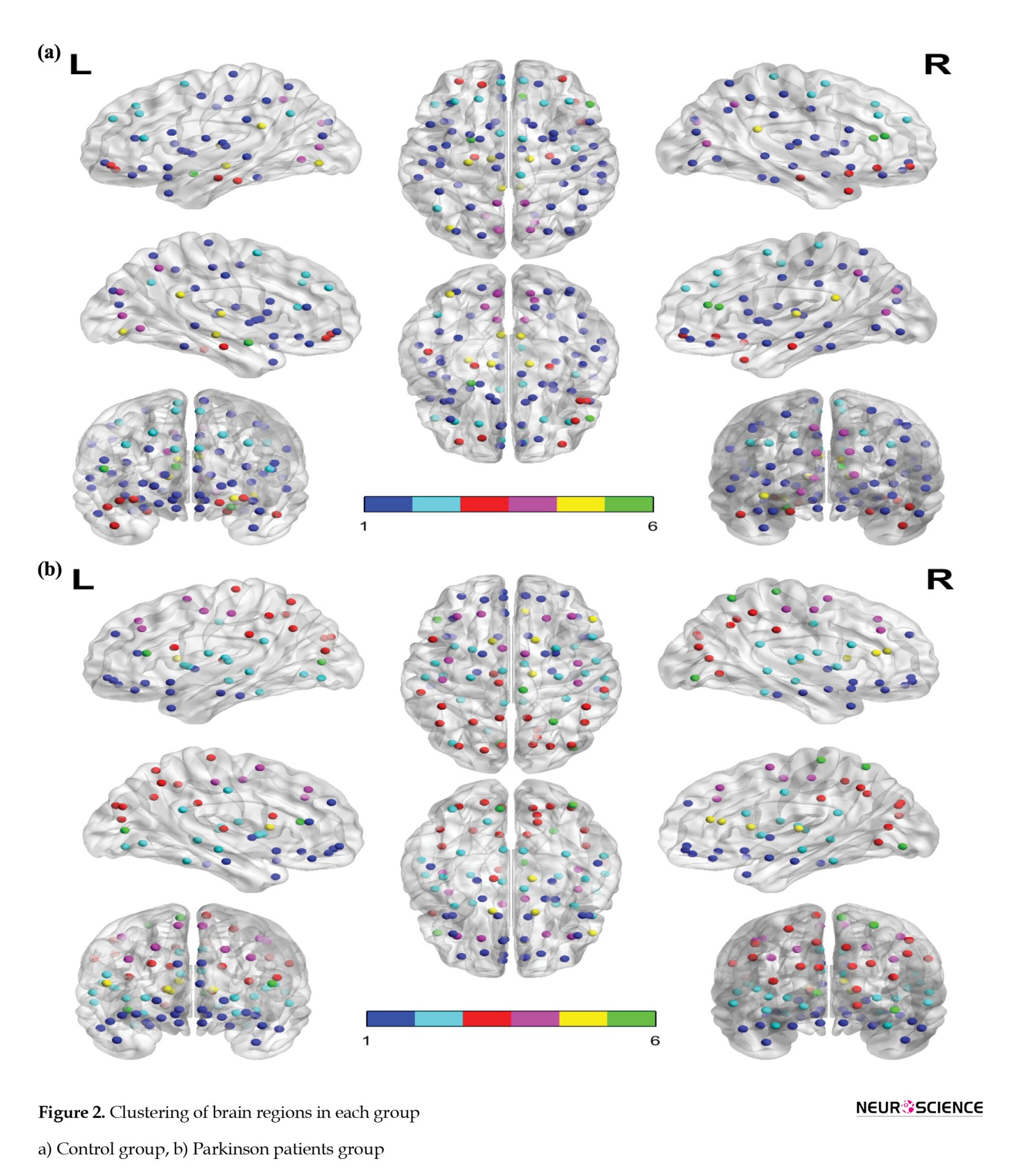
Table 1 shows the names of the areas in each cluster and the estimation of the correlation between the clusters by groups (further details on the full names of the regions are available in the Appendix). The largest cluster in the patient and the healthy group has 26 and 53 areas, respectively. Common areas of these clusters include right superior frontal gyrus (orbital part), left inferior frontal gyrus (orbital part), left olfactory cortex, right olfactory cortex, left middle frontal gyrus (orbital part), right middle frontal gyrus (orbital part), left gyrus rectus, right gyrus rectus, left anterior cingulate and paracingulate gyri, right amygdala, left pallidum, right pallidum, left temporal pole (superior), left temporal pole (middle), and right inferior temporal gyrus.
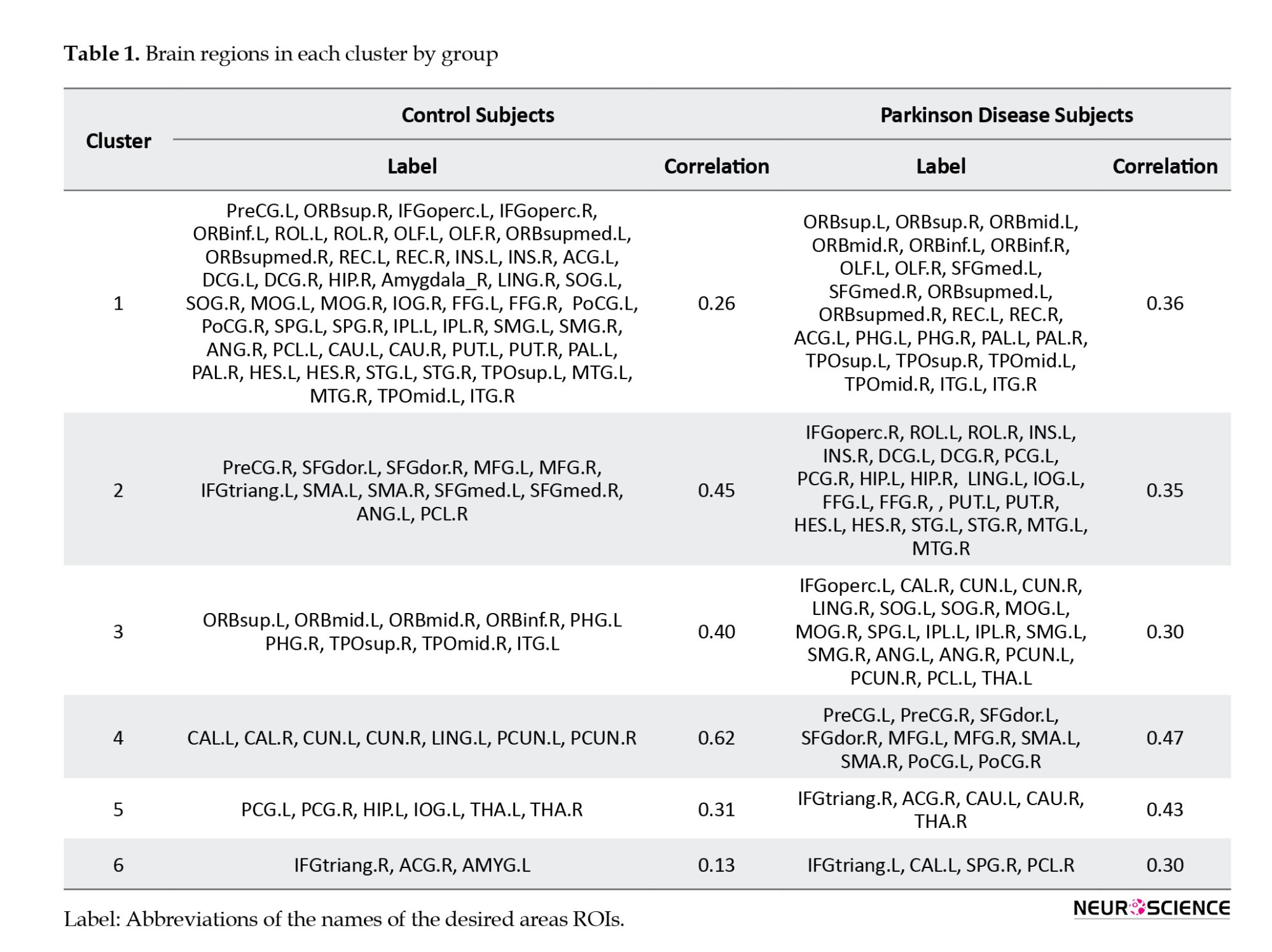
Although the clusters have common areas, the correlation between these areas is different in the two groups of sick and healthy. The correlation of this cluster was 0.36 in the patient group and 0.26 in the control group, which indicates that the relationship between the regions of this cluster in the patient group is stronger than that of the control group. The correlation coefficient results in clusters five and six also show stronger regional connectivity in the patient group. The correlation coefficients of the second, third, and fourth clusters in the patient group are 0.35, 0.30, and 0.47, respectively, which shows weaker regional connectivity of these clusters than their respective clusters in the control group.
To evaluate the cluster performance of the edges, the NBS method was used with the following settings: t=3.1, permutations=5000, and component size=extent. The results of this method did not show a statistically significant difference between the edges in the two groups of patients and healthy, which was similar to the results of the FDR method, with the P>0.05.
4. Discussion
This study used a nonparametric Bayesian method to evaluate brain connectivity between Parkinson patients and healthy groups. Different areas were assigned to clusters based on the similarity between these areas and variation from conventional clustering methods. Previous models often consider the structure of the dependence between the edges based on the spatial closeness, which depends on the characteristics of the topological network. Still, biologically, the correct structures of the topological network are not limited to being spatially adjacent, so it does not seem appropriate to use them. The nonparametric Bayesian model does the clustering of the brain regions based on the correlation between edges based on topological features.
According to the nonparametric Bayesian model, brain regions in both groups were divided into six clusters. Although there were common regions between the two groups of patients and healthy in each cluster, the intensity of the functional relationship between these regions differed in the two groups. In addition, the connectivity of some clusters in the control group was higher, while several clusters showed stronger FC in the patient group. In this regard, Chen et al. used a new statistical method to study the network topology of brain connectivity in Parkinson patients. In this study, the control group in the occipital and inferior temporal lobes had more substantial connections with the superior temporal lobes and insular than the patient group. However, the control group showed a weaker functional relationship than the patient group in several areas, including the insular right or superior frontal gyrus orbital (Chen et al., 2020).
Another study on brain connectivity in people with PD showed a decrease in functional communication between the amygdala and the inferior parietal lobule, lingual gyrus, and fusiform gyrus associated with the severity of hyposmia and cognitive performance. In this study, Parkinson patients in canonical networks such as high visual, primarily visual, executive control, visuospatial, salience, and DMN had a more functional relationship with areas outside these canonical networks than the control group (Yoneyama et al., 2018). Also, a study on whole-brain analysis of PDs with visual hallucinations showed that the disease-related effects influence the resting-state FC of posterior and paracentral brain regions (Hepp et al., 2017).
In the present study, decreased FC was identified in the medial superior frontal gyrus and the precuneus gyrus (both as critical parts of DMN) in some brain regions in PD patients. These alterations can affect cognitive processes such as visuospatial attention and episodic memory retrieval. The findings seem to be consistent with other research, which found different aspects of reduction connectivity in the DMN across PD patients (Shin et al., 2016).
In general, the results of the present study show fundamental differences between the two groups of patients and healthy in terms of areas in each cluster and their correlation coefficients. These results could provide a better understanding of the topological mechanism of PD. The findings of this study are in line with the results of several studies that show changes in the topological characteristics of Parkinson patients (Engels et al., 2018; Huang et al., 2019; Prajapati & Emerson, 2020; Shine et al., 2019). Sang et al. examined the brain topology network of Parkinson patients receiving anti-Parkinson therapy. This study reported changes in the topological organization of these patients and showed that anti-Parkinson therapy could affect the effectiveness of the brain network, ineffectively relieving Parkinson clinical symptoms (Sang et al., 2015).
A total of 4005 univariate tests are needed to compare the pairwise connectivity of 90 brain regions. In evaluating the significance of the connections between these areas, multiple comparison methods of FDR and NBS were used, but no significant relationship was found between the two groups. However, as previously reported, there were different topological features in the two groups. In this regard, Heidari et al. also examined the functional communication characteristics of Parkinson patients using variance components linear modeling. In this study, a decrease in the functional association of 10 pairs of ROIs was observed in Parkinson patients. However, considering the multiple comparison tests, the functional relationship of each couple of regions was not significantly different between the two groups (Heidari et al., 2019).
5. Conclusion
Given that rsFC studies identify communication patterns associated with phenotypes of neurological diseases, appropriate statistical tests to estimate the correlation patterns of FC data are of utmost importance. This study investigated the brain connectivity of Parkinson patients using an advanced nonparametric Bayesian model. The results of this model indicate that the characteristics of the brain functional network topology in Parkinson patients are different from the control group.
Limitations
A limitation of this study is the relatively small number of subjects in the patient and control groups. Although the Bayesian nonparametric model addressed shortcomings in FC data, the power of analysis could be improved in a larger sample size. Therefore, considering future studies with an increased sample size will help better understand the underlying brain connectivity network in PD. The other factor is hardware limitations which required high computational time to analyze multi-subject fMRI data.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (Code: IR.SBMU.RETECH.REC.1399.820).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and supervision: Fatemeh Pourmotahari and Hamid Alavimajd; Methodology: Fatemeh Pourmotahari, Nasrin Borumandnia and Seyyed Mohammad Tabatabaei; Data collection: Fatemeh Pourmotahari and Keyvan Olazadeh; Data analysis: Fatemeh Pourmotahari and Seyyed Mohammad Tabatabaei; Writing the original draft: Fatemeh Pourmotahari and Naghmeh Khadembashi; Review & editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would thank the users of OpenNeuro platform for sharing the fMRI data.
References
Ahmadou, T. M., Daouda, M. T., Aboulem, G., Mariam, J., Faouzi, B. M., & Touhami, A. A. O. (2019). Neurocognitive profile study of Parkinsonian patients by automatic analysis of Rey’s Complex Figure-A. Activitas Nervosa Superior Rediviva, 61(2), 75-80. [Link]
Amboni, M., Tessitore, A., Esposito, F., Santangelo, G., Picillo, M., & Vitale, C., et al. (2015). Resting-state functional connectivity associated with mild cognitive impairment in Parkinson’s disease. Journal of Neurology, 262(2), 425-434. [DOI:10.1007/s00415-014-7591-5] [PMID]
Baggio, H. C., Segura, B., Sala-Llonch, R., Marti, M. J., Valldeoriola, F., & Compta, Y., et al. (2015). Cognitive impairment and resting-state network connectivity in Parkinson’s disease. Human Brain Mapping, 36(1), 199-212. [DOI:10.1002/hbm.22622] [PMID]
Baumgartner, R., Ryner, L., Richter, W., Summers, R., Jarmasz, M., & Somorjai, R. (2000). Comparison of two exploratory data analysis methods for fMRI: Fuzzy clustering vs. principal component analysis. Magnetic Resonance Imaging, 18(1), 89–94. [DOI:10.1016/S0730-725X(99)00102-2] [PMID]
Behari, M., Srivastava, A. K., Das, R. R., & Pandey, R. M. (2001). Risk factors of Parkinson’s disease in Indian patients. Journal of the Neurological Sciences, 190(1-2), 49-55. [DOI:10.1016/S0022-510X(01)00578-0]
Błaszczyk, J. W. (2016). Parkinson’s disease and neurodegeneration: GABA-collapse hypothesis. Frontiers in Neuroscience, 10, 269. [DOI:10.3389/fnins.2016.00269] [PMID]
Bower, J. H., Grossardt, B. R., Maraganore, D. M., Ahlskog, J. E., Colligan, R. C., & Geda, Y. E., et al. (2010). Anxious personality predicts an increased risk of Parkinson’s disease. Movement Disorders, 25(13), 2105-2113. [DOI:10.1002/mds.23230] [PMID]
Chen, B., Wang, S., Sun, W., Shang, X., Liu, H., & Liu, G., et al. (2017). Functional and structural changes in gray matter of Parkinson’s disease patients with mild cognitive impairment. European Journal of Radiology, 93, 16-23. [DOI:10.1016/j.ejrad.2017.05.018] [PMID]
Chen, S., Bowman, F. D., & Xing, Y. (2020). Detecting and testing altered brain connectivity networks with k-partite network topology. Computational Statistics & Data Analysis, 141, 109-122. [DOI:10.1016/j.csda.2019.06.007] [PMID]
Chen, S., Xing, Y., Kang, J., Kochunov, P., & Hong, L. E. (2020). Bayesian modeling of dependence in brain connectivity data. Biostatistics (Oxford, England), 21(2), 269–286. [DOI:10.1093/biostatistics/kxy046] [PMID]
Cribben, I., Haraldsdottir, R., Atlas, L. Y., Wager, T. D., & Lindquist, M. A. (2012). Dynamic connectivity regression: Determining state-related changes in brain connectivity. Neuroimage, 61(4), 907-920. [DOI:10.1016/j.neuroimage.2012.03.070] [PMID]
Engels, G., McCoy, B., Vlaar, A., Theeuwes, J., Weinstein, H., & Scherder, E., et al. (2018). Clinical pain and functional network topology in Parkinson’s disease: A resting-state fMRI study. Journal of Neural Transmission (Vienna, Austria : 1996), 125(10), 1449-1459. [DOI:10.1007/s00702-018-1916-y] [PMID]
Gorges, M., Müller, H. P., Lulé, D., LANDSCAPE Consortium, Pinkhardt, E. H., Ludolph, A. C., & Kassubek, J. (2015). To rise and to fall: Functional connectivity in cognitively normal and cognitively impaired patients with Parkinson’s disease. Neurobiology of Aging, 36(4), 1727-1735. [DOI:10.1016/j.neurobiolaging.2014.12.026] [PMID]
Han, J. W., Ahn, Y. D., Kim, W. S., Shin, C. M., Jeong, S. J., & Song, Y. S., et al. (2018). Psychiatric manifestation in patients with Parkinson’s disease. Journal of Korean Medical Science, 33(47), e300. [DOI:10.3346/jkms.2018.33.e300] [PMID]
Heidari, S., Borumandnia, N., Khadembashi, N., & Alavimajd, H. (2019). Functional connectivity network analysis in brain regions using resting State-fMRI data with Parkinson’s disease. Activitas Nervosa Superior Rediviva, 61(3-4), 155-160. [Link]
Hepp, D. H., Foncke, E. M. J., Olde Dubbelink, K. T. E., van de Berg, W. D. J., Berendse, H. W., & Schoonheim, M. M. (2017).Loss of functional connectivity in patients with Parkinson disease and visual hallucinations. Radiology, 285(3), 896-903. [DOI:10.1148/radiol.2017170438] [PMID]
Huang, L. C., Wu, P. A., Lin, S. Z., Pang, C. Y., & Chen, S. Y. (2019). Graph theory and network topological metrics may be the potential biomarker in Parkinson’s disease. Journal of Clinical Neuroscience, 68, 235-242. [DOI:https://doi.org/10.1016/j.jocn.2019.07.082] [PMID]
Hyvärinen, A., & Oja, E. (2000). Independent component analysis: Algorithms and applications. Neural Networks, 13(4-5), 411-430. [DOI:10.1016/S0893-6080(00)00026-5]
Kim, J., Pan, W., & Alzheimer's Disease Neuroimaging Initiative (2015). Highly adaptive tests for group differences in brain functional connectivity. NeuroImage. Clinical, 9, 625–639.[PMID]
Kouli, A., Torsney, K. M., & Kuan, W. L. (2018). Parkinson’s disease: Etiology, neuropathology, and pathogenesis. In T. B. Stoker (Eds.) et. al., Parkinson’s Disease: Pathogenesis and clinical aspects. Codon Publications. [PMID]
Lin, H. L., Lin, H. C., & Chen, Y. H. (2014). Psychiatric diseases predated the occurrence of Parkinson disease: A retrospective cohort study. Annals of Epidemiology, 24(3), 206-213. [DOI:10.1016/j.annepidem.2013.12.010] [PMID]
Markošová, M., Franz, L., & Beňušková, Ľ. (2009). Topology of brain functional networks: Towards the role of genes. In: M. Köppen, N. Kasabov, & G. Coghill (Eds), Advances in Neuro-Information Processing. ICONIP 2008. Lecture Notes in Computer Science, vol 5506. Berlin: Springer. [Link]
Martinez-Martin, P., Rodriguez-Blazquez, C., Kurtis, M. M., Chaudhuri, K. R., & NMSS Validation Group (2011). The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Movement Disorders, 26(3), 399-406. [DOI:10.1002/mds.23462] [PMID]
Nussbaum, R. L., & Ellis, C. E. (2003). Alzheimer’s disease and Parkinson’s disease. The New England Journal of Medicine, 348(14), 1356–1364. [DOI:10.1056/NEJM2003ra020003] [PMID]
Painous, C., & Marti, M. J. (2020). Cognitive impairment in Parkinson’s disease: What we know so far. Research and Reviews in Parkinsonism, 7-17. [DOI:10.2147/JPRLS.S263041]
Patel, R. S., Bowman, F. D., & Rilling, J. K. (2006). A Bayesian approach to determining connectivity of the human brain. Human Brain Mapping, 27(3), 267-276. [DOI:10.1002/hbm.20182] [PMID]
Patel, R. S., Bowman, F. D., & Rilling, J. K. (2006). Determining hierarchical functional networks from auditory stimuli fMRI. Human Brain Mapping, 27(5), 462–470. [DOI:10.1002/hbm.20245] [PMID]
Pellicano, C., Benincasa, D., Pisani, V., Buttarelli, F. R., Giovannelli, M., & Pontieri, F. E. (2007). Prodromal non-motor symptoms of Parkinson’s disease. Neuropsychiatric Disease and Treatment, 3(1), 145–152. [DOI:10.2147/nedt.2007.3.1.145] [PMID]
Prajapati, R., & Emerson, I. A. (2020). Global and regional connectivity analysis of resting-state function MRI brain images using graph theory in Parkinson’s disease. The International Journal of Neuroscience, 131(2), 105–115. [DOI:10.1080/00207454.2020.1733559] [PMID]
Reeve, A., Simcox, E., & Turnbull, D. (2014). Ageing and Parkinson’s disease: Why is advancing age the biggest risk factor? Ageing Research Reviews, 14(100), 19–30. [DOI:10.1016/j.arr.2014.01.004] [PMID]
Sang, L., Zhang, J., Wang, L., Zhang, J., Zhang, Y., & Li, P., et al. (2015). Alteration of brain functional networks in early-stage Parkinson’s disease: A resting-state fMRI study. PloS One, 10(10), e0141815. [DOI:10.1371/journal.pone.0141815] [PMID]
Shiba, M., Bower, J. H., Maraganore, D. M., McDonnell, S. K., Peterson, B. J., & Ahlskog, J. E., et al. (2000). Anxiety disorders and depressive disorders preceding Parkinson’s disease: A case-control study. Movement Disorders: Official Journal of the Movement Disorder Society, 15(4), 669-677. [DOI:10.1002/1531-8257(200007)15:4<669::aid-mds1011>3.0.co;2-5] [PMID]
Shin, N. Y., Shin, Y. S., Lee, P. H., Yoon, U., Han, S., & Kim, D. J., et al. (2016). Different functional and microstructural changes depending on duration of mild cognitive impairment in Parkinson disease. American Journal of Neuroradiology, 37(5), 897-903. [DOI:10.3174/ajnr.A4626] [PMID]
Shine, J. M., Bell, P. T., Matar, E., Poldrack, R. A., Lewis, S. J. G., & Halliday, G. M., et al. (2019). Dopamine depletion alters macroscopic network dynamics in Parkinson’s disease. Brain : A Journal of Neurology, 142(4), 1024-1034. [DOI:10.1093/brain/awz034] [PMID]
Smith, S. M., Miller, K. L., Salimi-Khorshidi, G., Webster, M., Beckmann, C. F., & Nichols, T. E., et al. (2011). Network modelling methods for FMRI. Neuroimage, 54(2), 875-891. [DOI:10.1016/j.neuroimage.2010.08.063] [PMID]
Stoessl, A. J. (2009). Functional imaging studies of non-motoric manifestations of Parkinson’s disease. Parkinsonism & Related Disorders, 15(Suppl 3), S13–S16. [DOI:10.1016/S1353-8020(09)70771-0] [PMID]
Tuovinen, N., Seppi, K., de Pasquale, F., Müller, C., Nocker, M., & Schocke, M., et al. (2018). The reorganization of functional architecture in the early-stages of Parkinson’s disease. Parkinsonism & Related Disorders, 50, 61-68. [DOI:10.1016/j.parkreldis.2018.02.013] [PMID]
Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., & Delcroix, N., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage, 15(1), 273-289. [DOI:10.1006/nimg.2001.0978] [PMID]
Varoquaux, G., Gramfort, A., Poline, J. B., & Thirion, B. (2010). Brain covariance selection: Better individual functional connectivity models using population prior. Advances in Neural Information Processing Systems, 23. [Limk]
Xiong, J., Parsons, L. M., Gao, J. H., & Fox, P. T. (1999). Interregional connectivity to primary motor cortex revealed using MRI resting state images. Human Brain Mapping, 8(2-3), 151-156. [PMID]
Yoneyama, N., Watanabe, H., Kawabata, K., Bagarinao, E., Hara, K., & Tsuboi, T., et al. (2018). Severe hyposmia and aberrant functional connectivity in cognitively normal Parkinson’s disease. Plos One, 13(1), e0190072. [DOI:10.1371/journal.pone.0190072] [PMID]

Parkinson is a chronic neurological system disorder that affects the dopaminergic, noradrenergic, cholinergic, and serotoninergic systems. It is the most common age-related neurological disease next to Alzheimer, with a prevalence of about 0.5% to 1% in the age range of 69-65 and 1% to 3% in people over 80 (Ahmadou et al., 2019; Nussbaum & Ellis, 2003). The risk factors for Parkinson disease (PD) are generally unknown, and old age, environmental factors, and genetic factors increase the risk of developing the disease (Kouli et al., 2018; Reeve et al., 2014). The clinical signs of Parkinson are characterized by motor and non-motor symptoms. Motor symptoms include rigidity, bradykinesia, akinesia, abnormal posture, and resting tremors. Non-motor symptoms such as autonomic, sleep, olfactory, psychiatric (depression, psychosis, hallucination, anxiety, and impulse control), and cognitive disorders are essential factors in patients’ disabilities that are referred to as the mechanisms of the initial stages of Parkinson diagnosis. Since cognitive and psychiatric disorders can reduce the daily function and quality of life of patients with PD, non-motor symptoms are of high clinical importance (Błaszczyk, 2016; Martinez‐Martin et al., 2011; Pellicano et al., 2007; Han et al., 2018; Painous & Marti, 2020). Among the psychiatric disorders, depression and anxiety have been particularly determined as risk factors for PD. The literature has also displayed that the underlying effects of depression and anxiety can appear many years before the incidence of motor symptoms (Behari et al., 2001; Bower et al., 2010; Lin et al., 2014; Shiba et al., 2000).
Resting-state functional connectivity (rsFC) studies are used to examine the pathophysiology of neurodegenerative disorders, including Parkinson’s. These studies use non-invasive functional magnetic resonance imaging (fMRI) to distinguish distinct patterns of brain connectivity between healthy and diseased individuals. Since many neurological disorders are associated with altered topological patterns of brain connectivity, rsFC studies can detect connections between different brain areas by recognizing this topological structure. Topology is defined as the study of features that describe how brain areas are arranged based on their interconnections. The use of these studies in Parkinson patients is important as it provides helpful information about functional and morphological changes, including motor and non-motor functions (Chen et al., 2020; Markošová et al., 2008; Stoessl, 2009; Tuovinen et al., 2018). In this regard, several studies have indicated alterations in brain connectivity in PD patients with cognitive disorders. For example, Gorges et al. assessed the brain connectivity networks using seed-based analyses. Compared with the control subjects, PD patients decreased functional communication within some regions of the default mode network (DMN). Baggio et al. reported decreased FC in PD patients between the dorsal attention network and right fronto insular areas using independent component analysis (Amboni et al., 2015; Baggio et al., 2015; Chen et al., 2017; Gorges et al., 2015).
Functional connectivity (FC) is determined based on the correlation patterns, using statistical methods such as Pearson correlation coefficient, mutual information, and partial correlation coefficient (Kim & Pan, 2015; Smith et al., 2011; Xiong et al., 1999). Moreover, there are other statistical methods for inferring functional relationships, including clustering models, multivariate models, graphical lasso models, and Bayesian models (Baumgartner et al., 2000; Cribben et al., 2012; Hyvärinen & Oja, 2000; Patel et al., 2006a; Patel et al., 2006b; Varoquaux et al., 2010).
Functional communication data at rest faces significant challenges: 1) The existence of correlations between the connectivity edges that are related to the features of the topological network and 2) The high number of parameters in the covariance matrix, specifically if the number of regions of interest (ROIs) is high. Although many functional studies have been performed on PD data, the analysis of functional correlation data without considering these characteristics does not seem appropriate. Accordingly, in this study, considering the characteristics of functional relationship data, the advanced nonparametric Bayesian model introduced by Chen et al. was used to evaluate the topological network structure in Parkinson patients (Chen et al., 2018).
2. Materials and Methods
Data acquisition
The resting-state fMRI data were obtained from the OpenfMRI dataset with the document ID ds000245. The scans acquisition protocol was obtained as follows: Repetition time (TR)=2500 ms, echo time (TE)=30 ms, 39 transverse slices with inter-slice interval=0.5 mm and thickness=3 mm, FOV=192 mm, matrix size=64×64, flip angle=80°. Resting-state fMRI scans were obtained for 8 minutes with eyes closed. T1-weighted images had a total time of 349 seconds.
Data processing
Pre-processing of resting-state fMRI scans was performed using FSL software, version 6.0.1. The first five volumes of each time course were removed due to the correction of the initial image heterogeneity and the adaptation of individuals to the surrounding conditions; hence, a total of 193 volumes per person was considered. Images were normalized with a voxel resolution of 2×2×2 mm3, and for smoothing, a gaussian filter with 6 mm FWHM was used. Then, the pre-processed images were divided into 90 desired areas, according to atlas AAl, using the WFU pickatlas toolbox in MATLAB R2019b software (Tzourio-Mazoyer et al., 2002). Fisher Z-transformed correlations were considered as the measurement index of the edges.
Statistical analysis
Statistical inference of brain FC was performed in two stages:
Step 1: A nonparametric Bayesian model was used to evaluate the topological structure of the brain network. To assess the network properties, including determining the number of clusters of brain regions and the correlation coefficient of clusters, first, the residual matrix RoN×E was calculated as Equation 1:

YN×E is the E-th sample Fisher’s Z transformed correlation for the N-th of the subject (1, ..., N). Each subject has V=90 areas and edges. is the design matrix of p-covariates and βP×E is the parameters estimation linking the covariates to the response.
Suppose represent the correlation matrix between regions of the brain. This matrix is a function of network structure and correlation parameters ρ=(ρ0,ρ1,…,ρk) .
The network topological structure-based correlation matrix is defined as Equation 2:

which is based on the correlation between the edges ei',j' (correlation between regions i and j, i≠j ) and ei',j'. ωi=Ck is considered as an indicator variable, to determine whether region i belongs to the cluster or not. If a pair of edges are in a cluster, then it can be assumed that ei,jei',j') (Equation 3):
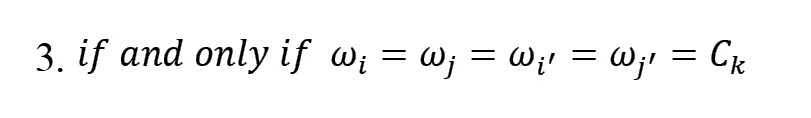
Finally, the posterior distributions ρ and ω are obtained using the markov monte carlo chain (MCMC) with 5000 iterations.
Step 2: To compare the pairwise association of 90 regions, the number of univariate tests is as high as =4005. Considering multiple comparisons, the network-based statistics (NBS) and false discovery rate (FDR) were performed to assess any significant pairwise connections between the patient and healthy groups. The NBS method uses a permutation test to examine the cluster difference of edges with a predefined threshold across the two groups. The FDR method examines the significant individual level of each edge in the two groups. P<0.05 was considered as a significant level. The analysis was performed through the NBS Connectome package in MATLAB software, version R2021b.
3. Results
Resting-state fMRI data included 11 Parkinson patients (six male) and 11 healthy individuals (six male), matched on sex ratio. The mean age was 64.36 years for the PD group and 63.73 years for the healthy group, in which, in terms of age distribution (P=0.83), there was no significant difference between the two groups.
Estimation of the number of clusters and their correlation coefficient was performed using a nonparametric Bayesian model to consider the specific characteristics of functional relationship data. According to what was previously explained in the theory of this model: 1) The regions within each cluster have a considerable functional relationship with each other and 2) The correlation coefficient of each cluster expresses the degree of pairwise correlation of regions within the cluster. Figure 1 shows the mean Fisher Z-transformed correlations of brain regions in both diseased and healthy groups.

The areas in the diagram are arranged according to their placement in the clusters. The more correlated edges had a higher mean value in each cluster, indicating the correct detection of clustering by the nonparametric Bayesian model. Figure 2 shows a clustering of areas of the brain. The brain network regions of the two groups were divided into six clusters and identified by the same color.

Table 1 shows the names of the areas in each cluster and the estimation of the correlation between the clusters by groups (further details on the full names of the regions are available in the Appendix). The largest cluster in the patient and the healthy group has 26 and 53 areas, respectively. Common areas of these clusters include right superior frontal gyrus (orbital part), left inferior frontal gyrus (orbital part), left olfactory cortex, right olfactory cortex, left middle frontal gyrus (orbital part), right middle frontal gyrus (orbital part), left gyrus rectus, right gyrus rectus, left anterior cingulate and paracingulate gyri, right amygdala, left pallidum, right pallidum, left temporal pole (superior), left temporal pole (middle), and right inferior temporal gyrus.

Although the clusters have common areas, the correlation between these areas is different in the two groups of sick and healthy. The correlation of this cluster was 0.36 in the patient group and 0.26 in the control group, which indicates that the relationship between the regions of this cluster in the patient group is stronger than that of the control group. The correlation coefficient results in clusters five and six also show stronger regional connectivity in the patient group. The correlation coefficients of the second, third, and fourth clusters in the patient group are 0.35, 0.30, and 0.47, respectively, which shows weaker regional connectivity of these clusters than their respective clusters in the control group.
To evaluate the cluster performance of the edges, the NBS method was used with the following settings: t=3.1, permutations=5000, and component size=extent. The results of this method did not show a statistically significant difference between the edges in the two groups of patients and healthy, which was similar to the results of the FDR method, with the P>0.05.
4. Discussion
This study used a nonparametric Bayesian method to evaluate brain connectivity between Parkinson patients and healthy groups. Different areas were assigned to clusters based on the similarity between these areas and variation from conventional clustering methods. Previous models often consider the structure of the dependence between the edges based on the spatial closeness, which depends on the characteristics of the topological network. Still, biologically, the correct structures of the topological network are not limited to being spatially adjacent, so it does not seem appropriate to use them. The nonparametric Bayesian model does the clustering of the brain regions based on the correlation between edges based on topological features.
According to the nonparametric Bayesian model, brain regions in both groups were divided into six clusters. Although there were common regions between the two groups of patients and healthy in each cluster, the intensity of the functional relationship between these regions differed in the two groups. In addition, the connectivity of some clusters in the control group was higher, while several clusters showed stronger FC in the patient group. In this regard, Chen et al. used a new statistical method to study the network topology of brain connectivity in Parkinson patients. In this study, the control group in the occipital and inferior temporal lobes had more substantial connections with the superior temporal lobes and insular than the patient group. However, the control group showed a weaker functional relationship than the patient group in several areas, including the insular right or superior frontal gyrus orbital (Chen et al., 2020).
Another study on brain connectivity in people with PD showed a decrease in functional communication between the amygdala and the inferior parietal lobule, lingual gyrus, and fusiform gyrus associated with the severity of hyposmia and cognitive performance. In this study, Parkinson patients in canonical networks such as high visual, primarily visual, executive control, visuospatial, salience, and DMN had a more functional relationship with areas outside these canonical networks than the control group (Yoneyama et al., 2018). Also, a study on whole-brain analysis of PDs with visual hallucinations showed that the disease-related effects influence the resting-state FC of posterior and paracentral brain regions (Hepp et al., 2017).
In the present study, decreased FC was identified in the medial superior frontal gyrus and the precuneus gyrus (both as critical parts of DMN) in some brain regions in PD patients. These alterations can affect cognitive processes such as visuospatial attention and episodic memory retrieval. The findings seem to be consistent with other research, which found different aspects of reduction connectivity in the DMN across PD patients (Shin et al., 2016).
In general, the results of the present study show fundamental differences between the two groups of patients and healthy in terms of areas in each cluster and their correlation coefficients. These results could provide a better understanding of the topological mechanism of PD. The findings of this study are in line with the results of several studies that show changes in the topological characteristics of Parkinson patients (Engels et al., 2018; Huang et al., 2019; Prajapati & Emerson, 2020; Shine et al., 2019). Sang et al. examined the brain topology network of Parkinson patients receiving anti-Parkinson therapy. This study reported changes in the topological organization of these patients and showed that anti-Parkinson therapy could affect the effectiveness of the brain network, ineffectively relieving Parkinson clinical symptoms (Sang et al., 2015).
A total of 4005 univariate tests are needed to compare the pairwise connectivity of 90 brain regions. In evaluating the significance of the connections between these areas, multiple comparison methods of FDR and NBS were used, but no significant relationship was found between the two groups. However, as previously reported, there were different topological features in the two groups. In this regard, Heidari et al. also examined the functional communication characteristics of Parkinson patients using variance components linear modeling. In this study, a decrease in the functional association of 10 pairs of ROIs was observed in Parkinson patients. However, considering the multiple comparison tests, the functional relationship of each couple of regions was not significantly different between the two groups (Heidari et al., 2019).
5. Conclusion
Given that rsFC studies identify communication patterns associated with phenotypes of neurological diseases, appropriate statistical tests to estimate the correlation patterns of FC data are of utmost importance. This study investigated the brain connectivity of Parkinson patients using an advanced nonparametric Bayesian model. The results of this model indicate that the characteristics of the brain functional network topology in Parkinson patients are different from the control group.
Limitations
A limitation of this study is the relatively small number of subjects in the patient and control groups. Although the Bayesian nonparametric model addressed shortcomings in FC data, the power of analysis could be improved in a larger sample size. Therefore, considering future studies with an increased sample size will help better understand the underlying brain connectivity network in PD. The other factor is hardware limitations which required high computational time to analyze multi-subject fMRI data.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (Code: IR.SBMU.RETECH.REC.1399.820).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and supervision: Fatemeh Pourmotahari and Hamid Alavimajd; Methodology: Fatemeh Pourmotahari, Nasrin Borumandnia and Seyyed Mohammad Tabatabaei; Data collection: Fatemeh Pourmotahari and Keyvan Olazadeh; Data analysis: Fatemeh Pourmotahari and Seyyed Mohammad Tabatabaei; Writing the original draft: Fatemeh Pourmotahari and Naghmeh Khadembashi; Review & editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would thank the users of OpenNeuro platform for sharing the fMRI data.
References
Ahmadou, T. M., Daouda, M. T., Aboulem, G., Mariam, J., Faouzi, B. M., & Touhami, A. A. O. (2019). Neurocognitive profile study of Parkinsonian patients by automatic analysis of Rey’s Complex Figure-A. Activitas Nervosa Superior Rediviva, 61(2), 75-80. [Link]
Amboni, M., Tessitore, A., Esposito, F., Santangelo, G., Picillo, M., & Vitale, C., et al. (2015). Resting-state functional connectivity associated with mild cognitive impairment in Parkinson’s disease. Journal of Neurology, 262(2), 425-434. [DOI:10.1007/s00415-014-7591-5] [PMID]
Baggio, H. C., Segura, B., Sala-Llonch, R., Marti, M. J., Valldeoriola, F., & Compta, Y., et al. (2015). Cognitive impairment and resting-state network connectivity in Parkinson’s disease. Human Brain Mapping, 36(1), 199-212. [DOI:10.1002/hbm.22622] [PMID]
Baumgartner, R., Ryner, L., Richter, W., Summers, R., Jarmasz, M., & Somorjai, R. (2000). Comparison of two exploratory data analysis methods for fMRI: Fuzzy clustering vs. principal component analysis. Magnetic Resonance Imaging, 18(1), 89–94. [DOI:10.1016/S0730-725X(99)00102-2] [PMID]
Behari, M., Srivastava, A. K., Das, R. R., & Pandey, R. M. (2001). Risk factors of Parkinson’s disease in Indian patients. Journal of the Neurological Sciences, 190(1-2), 49-55. [DOI:10.1016/S0022-510X(01)00578-0]
Błaszczyk, J. W. (2016). Parkinson’s disease and neurodegeneration: GABA-collapse hypothesis. Frontiers in Neuroscience, 10, 269. [DOI:10.3389/fnins.2016.00269] [PMID]
Bower, J. H., Grossardt, B. R., Maraganore, D. M., Ahlskog, J. E., Colligan, R. C., & Geda, Y. E., et al. (2010). Anxious personality predicts an increased risk of Parkinson’s disease. Movement Disorders, 25(13), 2105-2113. [DOI:10.1002/mds.23230] [PMID]
Chen, B., Wang, S., Sun, W., Shang, X., Liu, H., & Liu, G., et al. (2017). Functional and structural changes in gray matter of Parkinson’s disease patients with mild cognitive impairment. European Journal of Radiology, 93, 16-23. [DOI:10.1016/j.ejrad.2017.05.018] [PMID]
Chen, S., Bowman, F. D., & Xing, Y. (2020). Detecting and testing altered brain connectivity networks with k-partite network topology. Computational Statistics & Data Analysis, 141, 109-122. [DOI:10.1016/j.csda.2019.06.007] [PMID]
Chen, S., Xing, Y., Kang, J., Kochunov, P., & Hong, L. E. (2020). Bayesian modeling of dependence in brain connectivity data. Biostatistics (Oxford, England), 21(2), 269–286. [DOI:10.1093/biostatistics/kxy046] [PMID]
Cribben, I., Haraldsdottir, R., Atlas, L. Y., Wager, T. D., & Lindquist, M. A. (2012). Dynamic connectivity regression: Determining state-related changes in brain connectivity. Neuroimage, 61(4), 907-920. [DOI:10.1016/j.neuroimage.2012.03.070] [PMID]
Engels, G., McCoy, B., Vlaar, A., Theeuwes, J., Weinstein, H., & Scherder, E., et al. (2018). Clinical pain and functional network topology in Parkinson’s disease: A resting-state fMRI study. Journal of Neural Transmission (Vienna, Austria : 1996), 125(10), 1449-1459. [DOI:10.1007/s00702-018-1916-y] [PMID]
Gorges, M., Müller, H. P., Lulé, D., LANDSCAPE Consortium, Pinkhardt, E. H., Ludolph, A. C., & Kassubek, J. (2015). To rise and to fall: Functional connectivity in cognitively normal and cognitively impaired patients with Parkinson’s disease. Neurobiology of Aging, 36(4), 1727-1735. [DOI:10.1016/j.neurobiolaging.2014.12.026] [PMID]
Han, J. W., Ahn, Y. D., Kim, W. S., Shin, C. M., Jeong, S. J., & Song, Y. S., et al. (2018). Psychiatric manifestation in patients with Parkinson’s disease. Journal of Korean Medical Science, 33(47), e300. [DOI:10.3346/jkms.2018.33.e300] [PMID]
Heidari, S., Borumandnia, N., Khadembashi, N., & Alavimajd, H. (2019). Functional connectivity network analysis in brain regions using resting State-fMRI data with Parkinson’s disease. Activitas Nervosa Superior Rediviva, 61(3-4), 155-160. [Link]
Hepp, D. H., Foncke, E. M. J., Olde Dubbelink, K. T. E., van de Berg, W. D. J., Berendse, H. W., & Schoonheim, M. M. (2017).Loss of functional connectivity in patients with Parkinson disease and visual hallucinations. Radiology, 285(3), 896-903. [DOI:10.1148/radiol.2017170438] [PMID]
Huang, L. C., Wu, P. A., Lin, S. Z., Pang, C. Y., & Chen, S. Y. (2019). Graph theory and network topological metrics may be the potential biomarker in Parkinson’s disease. Journal of Clinical Neuroscience, 68, 235-242. [DOI:https://doi.org/10.1016/j.jocn.2019.07.082] [PMID]
Hyvärinen, A., & Oja, E. (2000). Independent component analysis: Algorithms and applications. Neural Networks, 13(4-5), 411-430. [DOI:10.1016/S0893-6080(00)00026-5]
Kim, J., Pan, W., & Alzheimer's Disease Neuroimaging Initiative (2015). Highly adaptive tests for group differences in brain functional connectivity. NeuroImage. Clinical, 9, 625–639.[PMID]
Kouli, A., Torsney, K. M., & Kuan, W. L. (2018). Parkinson’s disease: Etiology, neuropathology, and pathogenesis. In T. B. Stoker (Eds.) et. al., Parkinson’s Disease: Pathogenesis and clinical aspects. Codon Publications. [PMID]
Lin, H. L., Lin, H. C., & Chen, Y. H. (2014). Psychiatric diseases predated the occurrence of Parkinson disease: A retrospective cohort study. Annals of Epidemiology, 24(3), 206-213. [DOI:10.1016/j.annepidem.2013.12.010] [PMID]
Markošová, M., Franz, L., & Beňušková, Ľ. (2009). Topology of brain functional networks: Towards the role of genes. In: M. Köppen, N. Kasabov, & G. Coghill (Eds), Advances in Neuro-Information Processing. ICONIP 2008. Lecture Notes in Computer Science, vol 5506. Berlin: Springer. [Link]
Martinez-Martin, P., Rodriguez-Blazquez, C., Kurtis, M. M., Chaudhuri, K. R., & NMSS Validation Group (2011). The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Movement Disorders, 26(3), 399-406. [DOI:10.1002/mds.23462] [PMID]
Nussbaum, R. L., & Ellis, C. E. (2003). Alzheimer’s disease and Parkinson’s disease. The New England Journal of Medicine, 348(14), 1356–1364. [DOI:10.1056/NEJM2003ra020003] [PMID]
Painous, C., & Marti, M. J. (2020). Cognitive impairment in Parkinson’s disease: What we know so far. Research and Reviews in Parkinsonism, 7-17. [DOI:10.2147/JPRLS.S263041]
Patel, R. S., Bowman, F. D., & Rilling, J. K. (2006). A Bayesian approach to determining connectivity of the human brain. Human Brain Mapping, 27(3), 267-276. [DOI:10.1002/hbm.20182] [PMID]
Patel, R. S., Bowman, F. D., & Rilling, J. K. (2006). Determining hierarchical functional networks from auditory stimuli fMRI. Human Brain Mapping, 27(5), 462–470. [DOI:10.1002/hbm.20245] [PMID]
Pellicano, C., Benincasa, D., Pisani, V., Buttarelli, F. R., Giovannelli, M., & Pontieri, F. E. (2007). Prodromal non-motor symptoms of Parkinson’s disease. Neuropsychiatric Disease and Treatment, 3(1), 145–152. [DOI:10.2147/nedt.2007.3.1.145] [PMID]
Prajapati, R., & Emerson, I. A. (2020). Global and regional connectivity analysis of resting-state function MRI brain images using graph theory in Parkinson’s disease. The International Journal of Neuroscience, 131(2), 105–115. [DOI:10.1080/00207454.2020.1733559] [PMID]
Reeve, A., Simcox, E., & Turnbull, D. (2014). Ageing and Parkinson’s disease: Why is advancing age the biggest risk factor? Ageing Research Reviews, 14(100), 19–30. [DOI:10.1016/j.arr.2014.01.004] [PMID]
Sang, L., Zhang, J., Wang, L., Zhang, J., Zhang, Y., & Li, P., et al. (2015). Alteration of brain functional networks in early-stage Parkinson’s disease: A resting-state fMRI study. PloS One, 10(10), e0141815. [DOI:10.1371/journal.pone.0141815] [PMID]
Shiba, M., Bower, J. H., Maraganore, D. M., McDonnell, S. K., Peterson, B. J., & Ahlskog, J. E., et al. (2000). Anxiety disorders and depressive disorders preceding Parkinson’s disease: A case-control study. Movement Disorders: Official Journal of the Movement Disorder Society, 15(4), 669-677. [DOI:10.1002/1531-8257(200007)15:4<669::aid-mds1011>3.0.co;2-5] [PMID]
Shin, N. Y., Shin, Y. S., Lee, P. H., Yoon, U., Han, S., & Kim, D. J., et al. (2016). Different functional and microstructural changes depending on duration of mild cognitive impairment in Parkinson disease. American Journal of Neuroradiology, 37(5), 897-903. [DOI:10.3174/ajnr.A4626] [PMID]
Shine, J. M., Bell, P. T., Matar, E., Poldrack, R. A., Lewis, S. J. G., & Halliday, G. M., et al. (2019). Dopamine depletion alters macroscopic network dynamics in Parkinson’s disease. Brain : A Journal of Neurology, 142(4), 1024-1034. [DOI:10.1093/brain/awz034] [PMID]
Smith, S. M., Miller, K. L., Salimi-Khorshidi, G., Webster, M., Beckmann, C. F., & Nichols, T. E., et al. (2011). Network modelling methods for FMRI. Neuroimage, 54(2), 875-891. [DOI:10.1016/j.neuroimage.2010.08.063] [PMID]
Stoessl, A. J. (2009). Functional imaging studies of non-motoric manifestations of Parkinson’s disease. Parkinsonism & Related Disorders, 15(Suppl 3), S13–S16. [DOI:10.1016/S1353-8020(09)70771-0] [PMID]
Tuovinen, N., Seppi, K., de Pasquale, F., Müller, C., Nocker, M., & Schocke, M., et al. (2018). The reorganization of functional architecture in the early-stages of Parkinson’s disease. Parkinsonism & Related Disorders, 50, 61-68. [DOI:10.1016/j.parkreldis.2018.02.013] [PMID]
Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., & Delcroix, N., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage, 15(1), 273-289. [DOI:10.1006/nimg.2001.0978] [PMID]
Varoquaux, G., Gramfort, A., Poline, J. B., & Thirion, B. (2010). Brain covariance selection: Better individual functional connectivity models using population prior. Advances in Neural Information Processing Systems, 23. [Limk]
Xiong, J., Parsons, L. M., Gao, J. H., & Fox, P. T. (1999). Interregional connectivity to primary motor cortex revealed using MRI resting state images. Human Brain Mapping, 8(2-3), 151-156. [PMID]
Yoneyama, N., Watanabe, H., Kawabata, K., Bagarinao, E., Hara, K., & Tsuboi, T., et al. (2018). Severe hyposmia and aberrant functional connectivity in cognitively normal Parkinson’s disease. Plos One, 13(1), e0190072. [DOI:10.1371/journal.pone.0190072] [PMID]


Type of Study: Original |
Subject:
Clinical Neuroscience
Received: 2021/01/30 | Accepted: 2021/09/4 | Published: 2024/01/1
Received: 2021/01/30 | Accepted: 2021/09/4 | Published: 2024/01/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





