Volume 14, Issue 5 (September & October 2023)
BCN 2023, 14(5): 549-564 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Keshtgar Z, Chalabianloo G, Esmaeili N. Probable Neuropsychological and Cognitive Complications Due to Cytokine Storm in Patients With COVID-19. BCN 2023; 14 (5) :549-564
URL: http://bcn.iums.ac.ir/article-1-2075-en.html
URL: http://bcn.iums.ac.ir/article-1-2075-en.html
1- Department of Neuroscience, School of Advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran.
2- Department of Neuroscience, School of Educational Sciences and Psychology, Azarbaijan Shahid Madani University, Tabriz, Iran.
3- Department of Hematology & Oncology, School of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran.
2- Department of Neuroscience, School of Educational Sciences and Psychology, Azarbaijan Shahid Madani University, Tabriz, Iran.
3- Department of Hematology & Oncology, School of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran.
Keywords: Neuropsychological complications, Cognitive impairments, Neuroinvasin, Routes of dissemination, Cytokine storm, Coronavirus, COVID-19
Full-Text [PDF 2487 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Coronaviruses are a large family of viruses responsible for diseases in mammals and birds (Liu et al., 2020). Like Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS), coronaviruses (CoVs) lead to severe respiratory diseases in humans. Some types of these viruses, including MERS-CoV, 229E, NL63, OC43, HKU1, and SARS-CoV, may have adverse neuropsychiatric impacts in humans (Myint, 1995; Matoba et al., 2015) (Table 1).
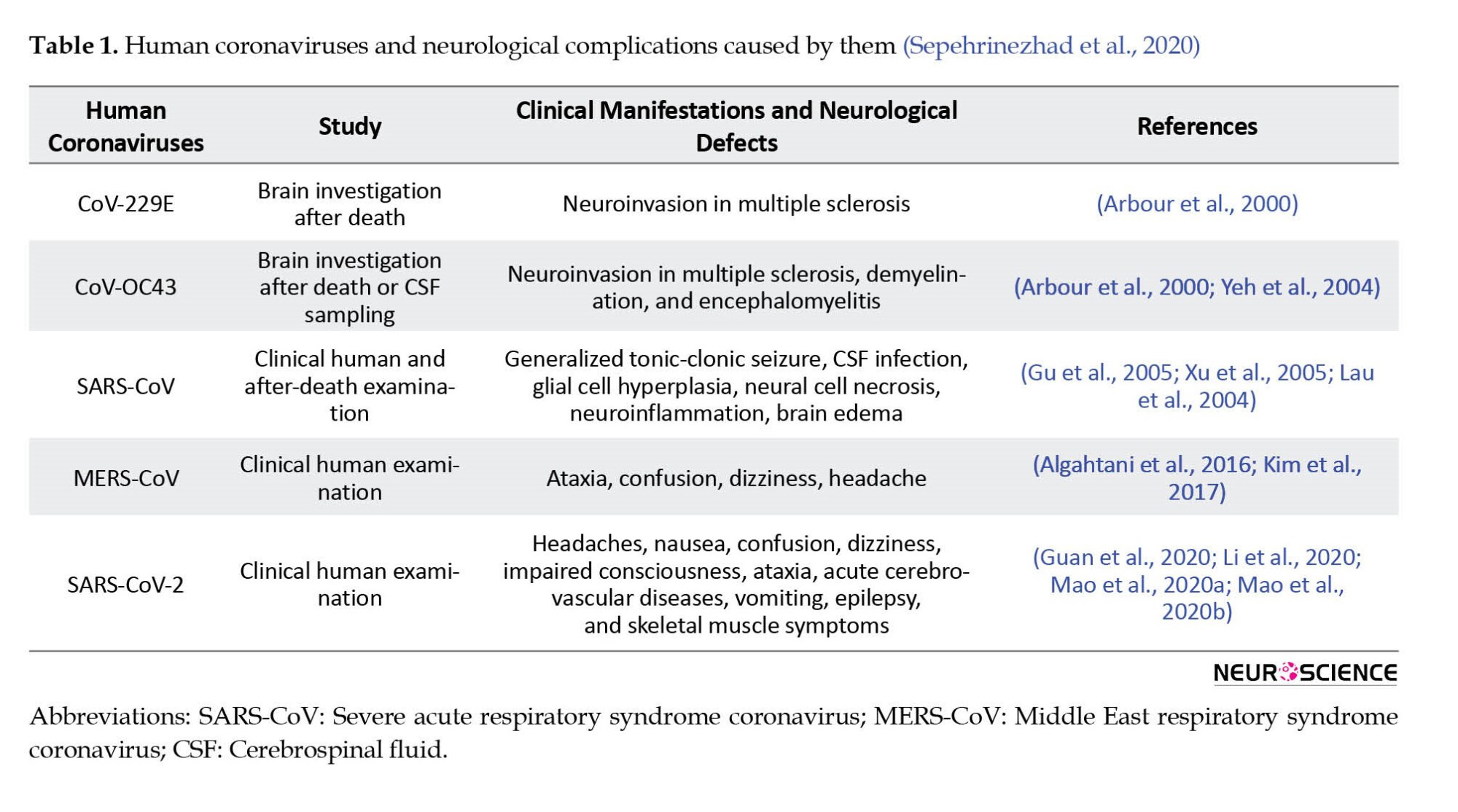
As a result of SARS infection, most SARS patients reported poor attention, memory impairments, insomnia, anxiety, and depression symptoms, indicating cognitive impairments after SARS infection (Sheng et al., 2005a). Despite physical recovery, these psychiatric symptoms may remain in patients with SARS (Tsang et al., 2004).
The first report of SARS-CoV-2 (also known as COVID-19) occurred in China in December 2019 (Lam et al., 2020). According to the World Health Organization (WHO), new coronavirus diseases are considered pandemics.
Like MERS and SARS, COVID-19 is characterized by shortness of breath, respiratory problems, cough, fatigue, sore throat, and fever. The patients also complain about headaches, nausea, dizziness, vertigo, and vomiting (Jiang et al., 2020). Evidence shows that MERS-CoV, SARS-CoV, and COVID-19 can cause acute respiratory distress syndrome (ARDS) and defile other members and cell types throughout the disease, such as the mucosa of the intestinal tract, renal tubular cells, lymphocytes, reticuloendothelial cells, and nerve cells (Kuiken et al., 2003; Leung et al., 2003; Peiris et al., 2003; Wu et al., 2020; Zhang et al., 2020).
Although coronaviruses mainly infect humans via the digestive and respiratory systems, SARS-CoV-2 infection can affect the nervous system indirectly, particularly through neurodegenerative diseases (Rhie et al., 2020). Researchers have recently found a strong correlation between gut microbiota, neuroinflammation, and neurological diseases. Lin et al. suggest that SARS-CoV-2 can affect gut mucosa cells, causing inflammation, and dysbiosis, and ultimately leading to neuroinflammation and neurodegeneration (Lin et al., 2018) as meningoencephalitis, various viral-associated necrotizing encephalitides, and secondary cytokine-induced acute necrotizing syndromes (Filatov et al., 2020; Poyiadji et al., 2020; Moriguchi et al., 2020). These reports highlight the effect of medical care during this pandemic. This makes COVID-19 another challenge for neurologists, clinical neuroscientists, and neuropsychologists (Zhao et al., 2020; Papa et al., 2020; Waldman et al., 2020; Khosravani et al., 2020).
Because the structure and pathogenesis of most coronaviruses (CoVs) are the same (St-Jean et al., 2004; Butler et al., 2006; Yuan et al., 2017) and it is unclear how COVID-19 works; therefore, it is essential to investigate the involvement of other organs (e.g. central nervous system [CNS]) and the relationship between psychological factors, inflammation, and cognitive functions, especially due to the new nature of the virus.
According to this research, cognitive deficits and inflammation are linked. Further exploration of the possible interrelationships among COVID-19 inflammation, cognitive function, and psychological factors is necessary to provide future research information. This issue is discussed in this study.
The articles used in this study were searched by keywords, such as cytokine storm and COVID-19, COVID-19 and executive dysfunction, cognitive disorder and COVID-19, CNS and COVID-19, coronavirus, and neuroinvasion. The keywords were searched in international databases, such as Science Direct, Scopus, PubMed, Embase, and Web of Science using the preferred reporting items for systematic reviews and meta-analysis (PRISMA) checklist. All observational studies published between December 2019 and April 2021 in peer-reviewed journals in any language, including cross-sectional, cohort, case-control, and case reports, were assessed in this study. The search result was 106 articles. Firstly, search engine results were evaluated for thematic relevance to select the documents. Then, after reviewing the titles, the abstract was evaluated in terms of relevance to the purpose. Finally, 73 articles were selected and thoroughly studied. Notes were taken of selected documents. The material collected about COVID-19, the stages of infection by this virus, its effect on the nervous system and neurological symptoms, the cytokine storm caused by this infection, and the possible cognitive consequences caused by this virus in patients were divided and summarized. Other articles were not checked due to their limited relevance to the topic under discussion.
Symptoms and consequences of CoV infection
The SARS-CoV-2 infection appears in three stages. At the early stages of the disease, the replication of the virus is high, which causes pyrexia, cough, and general discomfort for several days. Patients may develop high fever, hypoxemia, and respiratory signs that progress to bilateral pneumonia during the second stage. However, lab testing indicates that viral replication declines near the end of this stage (Peiris et al., 2003). About 20% of patients develop SARS at the last stage of the infection, which can be fatal (Nicholls et al., 2003; Van et al., 2014). The SARS may cause an inflammatory response in the host (known as a “cytokine storm”) that causes alveolar damage and severe hypoxemia, resulting in fatal secondary sepsis during the final stage.
Recently, studies conducted by Chen et al. have shown that SARS-CoV-2 infection leads to lymphocytopenia (low CD4+ and CD8+ T cell counts with average B cell counts), elevated cytokine levels of interleukin (IL)-6, IL-2R, IL-10, tumor necrosis factor-alpha (TNF-α), and CC chemokine ligand 2 (CCL2), as well as decreased interferon (IFN)- γ expression in CD4+ T cells. With mild symptoms of COVID-19, IL-6, IL-2R, IL-10, and TNF-α levels rise slightly or remain close to normal, but with severe symptoms, they rise (Figure 1). Scientists claim a connection between the cytokine storm and severe COVID-19, as observed in cases of sARS-CoV infection (Chen et al., 2019; Davidson et al., 2015).
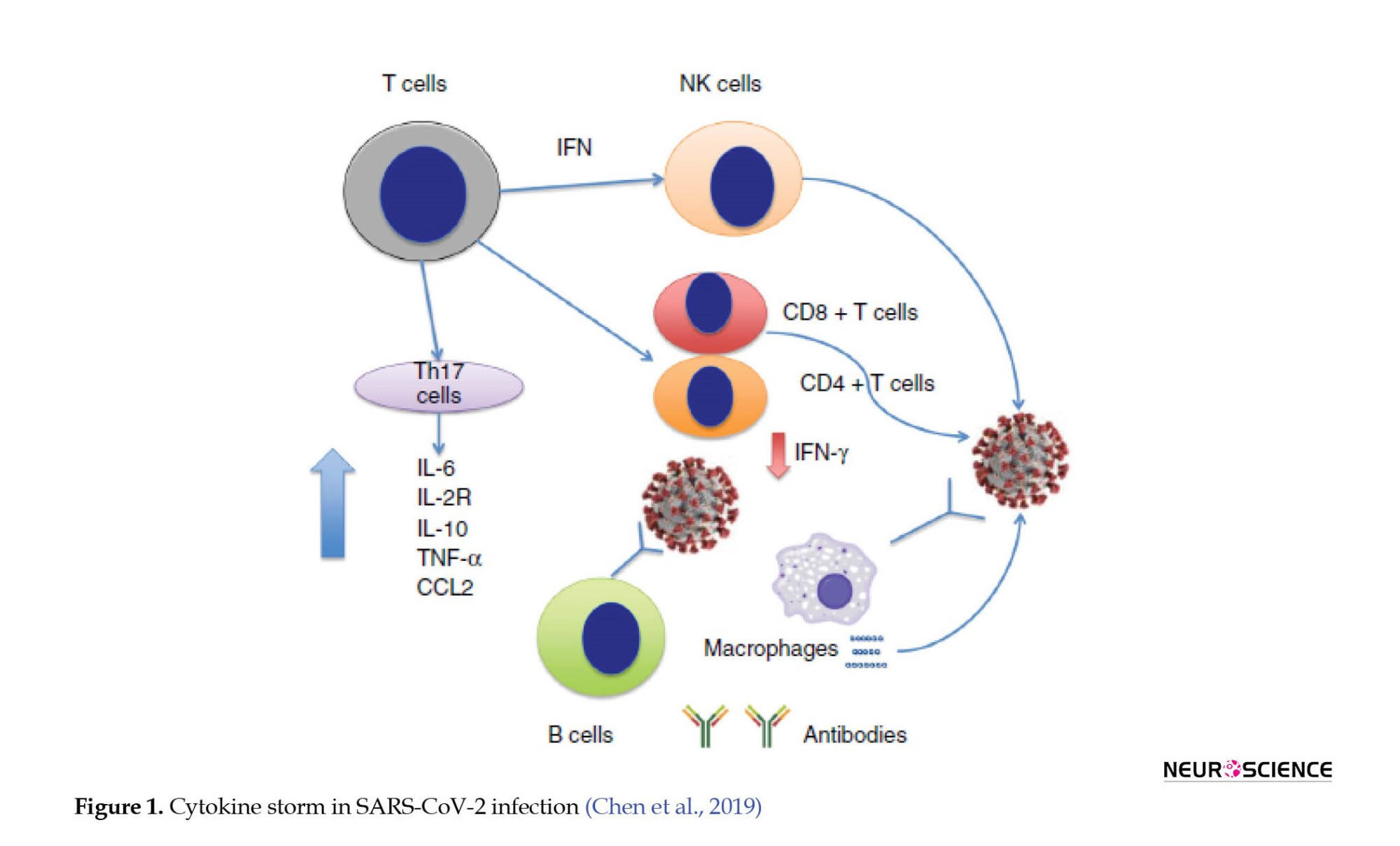
A persistent inflammatory, immunosuppressive, and catabolic syndrome (PICS) may result following infection, indicating a disturbance in the immune system. These conditions are probably caused by COVID-19 infection and the resulting cytokine storm, which is caused by the continuous release of endogenous alarmins or danger-associated molecular patterns from infected organs (Hawkins et al., 2018). Unfortunately, no definitive knowledge is available regarding chronic inflammation in survivors of COVID-19; however, this theory is credible given the correlation between severe infection and persistent inflammatory, immunosuppressive, and catabolic syndrome (PICS).
Brain invasion by severe acute respiratory SARS-CoV-2 and neurological symptoms: Possible mechanisms of transmission
Neural pathway
Despite the lack of evidence for SARS-CoV-2 invasion into the CNS, viral particles have been found in the cerebrospinal fluid (CSF) of a patient with meningoencephalitis (Wu et al., 2020) and in the frontal lobe of a patient with Parkinson’s disease with symptoms, such as fever and confusion at the time of admission (Paniz-Mondolfi et al., 2020). Animal studies have shown that two types of human coronaviruses (HCoV, HCoV-OC43) and SARS-CoV-1 enter the CNS through the olfactory nerve and the trigeminal nerve in the olfactory epithelium (Desforges et al., 2020). SARS-CoV-1 has been shown to infect the brainstem through the olfactory bulb and piriform cortex in transgenic mice expressing human angiotensin-converting enzyme 2 (ACE2) (Netland et al., 2008). Human coronavirus OC43 (HCoV-OC43) can also infect the olfactory bulb of mice and spread through nerve pathways to the brain and spinal cord (St-Jean et al., 2004; Desforges et al., 2020). A full brain infection of HCV-OC43 may occur within 7 days and nerve cells (neurons and glial cells) can be influenced by the virus (St-Jean et al., 2004). The flu A virus can also reach the respiratory centers in the brainstem via the vagus nerve (Pivot et al., 2001).
Pathway via the blood
The SARS-CoV-1, HCoV-OC43, and SARS-CoV-2 viruses can also infect the brain through the bloodstream (Desforges et al., 2020). After a viral infection of the respiratory tract, large amounts of cytokines and chemokines are released, causing the permeability of the blood-brain barrier (BBB) that can allow SARS-CoV-1 to spread (McCray et al., 2007). MERS-CoV can also infect the CNS using the leukocytes as Trojan horses because dipeptidyl-peptidase 4 (DPP4) is the MERS-CoV receptor expressed by activated leukocytes (Zhao et al., 2015). The hypothesis that MERS-CoV reaches the brain via the hematogenous pathway is confirmed by symptoms, such as seizure, encephalitis, and stroke, 2 to 3 weeks after acute respiratory distress syndrome (ARDS) (Kim et al., 2017). On the other hand, the hypothesis that infection spreads via the bloodstream is controversial during the first few days after infection. SARS-CoV-1 and MERS-CoV are not found in non-neurons in the brain, indicating a more direct route for these viruses to reach the brain (Li et al., 2020).
Target cells
Like the SARS-CoV-1 virus, the SARS-CoV-2 cellular receptor is the ACE2 (Wrapp et al., 2020; Hoffmann et al., 2020). This enzyme is highly expressed in various organs. The entry of the virus through the olfactory bulb causes inflammatory reactions and cytokine storms, eventually leading to anosmia and encephalitis. When the virus enters the eye, it causes conjunctivitis in the eyes, spreads through the tears, and is transmitted to various organs through the nasolacrimal system. Infection of the heart with this virus can lead to necrotic lipid formation and if ruptured, it can cause blood clots and myocardial infarction. The virus enters the kidney using the ACE2 receptors, causing deposition of the extracellular matrix, fibrosis, diuresis, and proliferation of kidney cells, resulting in acute kidney injury. (Harmer et al., 2002). Viral affects the liver by activating kupffer cells, which trigger an inflammatory reaction and activate hepatic stellate cells and hepatocytes, leading to pyroptosis and fibrosis. In the lungs, the virus causes damage to the walls of the alveolar cells and the formation of debris that causes thickening of the alveolar cell walls, lung damage, and shortness of breath that are common in COVID-19 (Figure 2) (Hamming et al., 2004).

Also, ACE2-mRNA and protein have been discovered in nuclei involved in the central regulation of cardiovascular function, such as the cardio‐respiratory neurons of the brainstem and the motor cortex, hypothalamus, and raphe (Harmer et al., 2002; Hamming et al., 2004; Xia & Lazartigues, 2008). It is yet to be determined what types of cells express ACE2 (Xia & Lazartigues, 2008), but animal studies show that this enzyme is mainly expressed by neurons; therefore, neurons may be directly infected with SARS-CoV-1 and SARS-CoV-2.
Cytokine storm and central nervous system pathophysiology
HCoV infection may affect the CNS in two ways, including viral replication in infected cells or neuro immunopathology. Activation of the immune system and macrophages by the SARS-CoV-2 causes the secretion of large amounts of cytokines and chemokines, which systemic inflammation caused by the cytokine storm leads to brain damage (Xu et al., 2005; Li et al., 2016b). In the same way, studies have shown increased interleukin (IL-) 6, 8, and 10 and of TNF-α in the blood of patients with SARS-CoV-2 (Figure 3) (Li et al., 2016b; Chen et al., 2019; Chen et al., 2020b). Also, inflammation in these patients, in addition to respiratory failure, causes hypoxic encephalopathy, which indicates the effect of SARS-CoV-2 on the CNS (Chen et al., 2019).
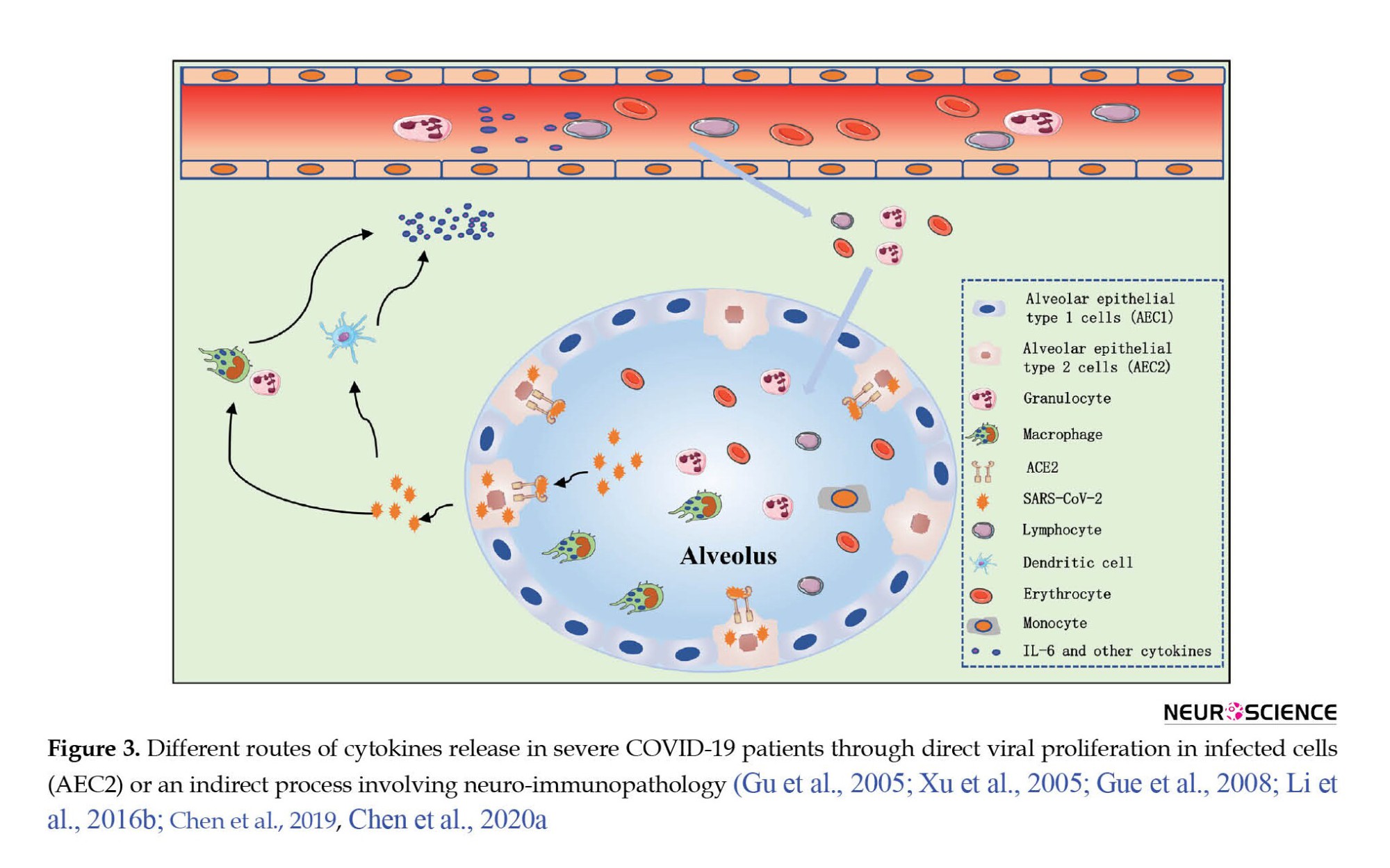
Examination of patients with severe COVID-19 infection compared to patients with moderate infection, and in patients who died than survivors shows that inflammation plays a crucial role in disease progression and death after SARS-CoV-2 infection (Chen et al., 2020a). Furthermore, peripheral lymphopenia was observed in most COVID-19 patients irrespective of their clinical severity (Chen et al., 2019).
Post-mortem evaluation of brain tissue of patients who died from SARS-CoV-1 infection indicates foci of necrotic cell death, edema, glial scar, and infiltrated immune cells (macrophages and T lymphocytes) (Figure 3) (Gu et al., 2005; Xu et al., 2005; Guo et al., 2008). Therefore, HCoV infection can cause inflammation of the brain, but underlying mechanisms need further explanations.
To further investigate the effect of SARS-CoV-1 infection on the brain, expressing the Human ACE was examined in transgenic mice, and the results indicated that the virus invades the brain (Doobay et al., 2007; McCray et al., 2007; Netland et al., 2008). Because SARS-CoV-1 viral particles are detected in olfactory bulbs (the most infected region) and deeper brain areas, including the piriform and infralimbic cortices, basal ganglia, dorsal raphe, thalamic nuclei, and brainstem (Netland et al., 2008). Contrary to human studies, brain inflammation was observed in these mice without alteration in astrocyte density and increased microglia cell density (Netland et al., 2008) and marked increases in IL-6, CCL2, IFN-γ and CCL12 levels following infection with SARS-CoV-1 (McCray et al., 2007). Therefore, these studies confirm the hypothesis of brain inflammation caused by HCoV infection.
Mice infected with John Howard Muller (JHM) strain of mouse hepatitis virus (MHV) through intranasal inoculation show rapid activation of microglia cells, phagocytosis of infected cells, and secretion of cytokines, including, IFN-α/β, CCL2, TNFα, and IL-6 (Wheeler et al., 2018). Thus, regardless of the virus strain, the brain’s immune response to the CoV infection is similar, indicating the critical role of microglia in the early stages of the immune response against the virus. Because the reduced microglia increase the proliferation and spread of the virus in the brain of infected mice (Lichanska et al., 1999; Wheeler et al., 2018). Microglia also activates T lymphocytes after infection (Wheeler et al., 2018). For example, by increasing the permeability of the blood-brain barrier (BBB) by metalloproteinases secreted by neutrophils and macrophages, CD4+ and CD8+ T lymphocytes can affect the CNS (Skinner et al., 2019). Subsequent studies of JHM of mouse hepatitis virus (MHV) inoculation also show both types of lymphocytes and increased cell infiltration in the brains of these mice (Phares et al., 2013). CD8+ T cells play a crucial role in controlling and clearing the virus, but T cells enhance the immune response by releasing IFN-γ and stimulating the expression of major histocompatibility complex (MHC) proteins in microglial cells (Wheeler et al., 2018). Such mechanisms are likely to be stimulated to increase viral clearance since it requires both major histocompatibility complex (MHC) 1 and 2 expression by immunocompetent cells (Skinner et al., 2019). After JHMV infection, an inflammatory reaction is initiated by two cytokines.
Evidence shows that C-X-C motif chemokine 10 (CXCL10) acts as a sentinel in the CNS because its release attracts T lymphocytes (Skinner et al., 2019), while IL-21 can improve both B and T lymphocyte responses (Phares et al., 2013). Additionally, CD4+ regulatory T cells (Treg) are activated when the inflammatory response reaches its peak and modulates the immune response (de Aquino et al., 2013). Therefore, Treg is essential to prevent damage caused by neuroinflammation and virus replication in CNS (de Aquino et al., 2013; Quarantelli, 2015). Therefore, evidence suggests that inhibiting inflammation of the brain can prevent or reduce CoV-induced neuropathologies.
After brain damage or infection, the first cells to be activated to maintain brain homeostasis and minimize tissue destruction are microglia (Hanisch & Kettenmann, 2007; Ransohoff & Perry, 2009), which produce and release several inflammatory chemomediators (pro-inflammatory cytokines and chemokines) (Ransohoff & Perry, 2009; Chatterjee et al., 2013), modulate inflammatory responses between the CNS and the peripheral immune system and as phagocytosis, they can clear damaged cells and cellular debris and present antigens to T lymphocytes (Wheeler et al., 2018). After brain homeostasis is reestablished, the activity of microglia decreases. Therefore, the consequences of overreacting microglia following SARS-CoV-2 infection can be particularly harmful since cytokine storm (a severely inflammatory immune response) is associated with COVID-19.
Cognitive functions after cytokine storm in COVID-19
The brain’s immune cells (microglia and cytokines) produce high levels of pro-inflammatory factors in various brain regions (Russo et al., 2010), which are harmful to brain cells. Acute or chronic inflammatory processes that induce the release of neurotoxic products such as reactive oxygen species (ROS) and certain damaging enzymes can damage brain tissue (Blasko et al., 2004; Raz & Rodrigue, 2006). Limbic and associated brain structures, such as hippocampi, prefrontal cortex, and basal ganglia (structures that play essential roles in cognitive processes, such as memory, attention, emotion, and perception) have more enzymes involved in an inflammatory response than primary motor or sensory cortices; therefore, these areas may be more affected by the destructive effects of inflammatory processes (Raz & Rodrigue, 2006; Wang et al., 2015).
Plenty of studies have shown that inflammation activation leads to cognitive deficits, suggesting a prominent role of IL-1β, IL-6, IL-18, and TNF-α (Duarte et al., 2017; Magalhaes et al., 2018; Shen et al., 2019; Chakrabarty et al., 2019). On the other hand, neurocognitive impairments are common in patients with viral infections because inflammation may also be present after clearing the virus (Peiris et al., 2003). For example, a follow-up interval ranging from 6 and 39 months after recovery from SARS and MERS showed impairment in memory, attention, concentration, or mental processing speed in more than 15% of patients (Sheng et al., 2005b; Lam et al., 2009; Bechter, 2013; Kepinska et al., 2020; Rogers et al., 2020).
Therefore, due to increased T helper (Th)-1 cytokines (IL-1β, IL-6, IL-8, IFN-γ, TNF-α, C-X-C motif chemokine 10 [CXCL10], and CCL2), and Th-2 cytokines (IL-4, IL-10, and IL-1 receptor antagonist) in the serum of COVID-19 patients (Channappanavar & Perlman, 2017; Chen et al., 2019, Chen et al., 2020a; Coperchini et al., 2020; Mazza et al., 2021), the possible relationship between inflammatory status and cognitive function in patients with COVID-19 should be investigated.
A functional neuroimaging study showed that increased IL-6 reduced the functional connectivity between the striatum and ventromedial prefrontal cortex (vmPFC), weakened prefrontal cortex control over the striatum, causing cognitive dysfunction, anhedonia, verbal memory deficit, and motor slowing (Lin et al., 2020; Felger et al., 2016). Studies in the elderly also indicate a positive relationship between plasma IL-6 level and cognitive deficits, such as prospective memory, working memory, executive functioning, processing speed, attention, orientation, immediate verbal recall, delayed recall or psychomotor speed, semantic fluency (Simpson et al., 2013; Heringa et al., 2014).
Increased TNF-α levels can cause neuronal damage directly via activation of apoptosis and increased glutamate-mediated excitotoxicity (Bortolato et al., 2015; Muneer, 2016; Chakrabarty et al., 2019), and inhibit hippocampal long-term potentiation. Also, elevated TNF-α receptor signaling may lead to brain atrophy, hippocampal neuronal lack, and increased risk of cognitive deficits, such as impaired verbal memory, learning, synaptic plasticity, and inhibitory control (Sudheimer et al., 2014; Bortolato et al., 2015; Chakrabarty et al., 2019).
Higher than normal levels of IL-8 cause impaired memory and motor function, as well as slower cognitive and perceptual speed (Baune et al., 2008; Alley et al., 2008).
Studies showed that IL-1β plays a key role in developing hippocampal-dependent learning and memory (Balschun et al., 2003; del Rey et al., 2013), but overexpression of IL-1β in the hippocampus can also negatively affect spatial memory (Moore et al., 2009; Spulber et al., 2009b).
Also, various studies showed increased pro-inflammatory (IL-1β, IL-6, TNF-α) and anti-inflammatory cytokines (IL-1ra, IL-10) in the CSF and plasma of patients with depression, schizophrenia, bipolar disorder, and Alzheimer (Levine et al., 1999; Dentino et al., 1999; Kim et al., 2007). In these patients, previous studies have found a correlation between peripheral IL-8, TNF-α, CCL2, CCL4, and brain thickness (Wan et al., 2017; Poletti et al., 2019), of IL-1β, IL-9, CCL5 with brain glutamate, N-acetyl aspartate, and myo-inositol levels (Poletti et al., 2020), and of IL-8, IL-10, TNF-α, IFN-γ with white matter (WM) microstructure, with levels of inflammatory cytokines being inversely related with measures of WM integrity (Benedetti et al., 2016). Also, a relationship is observed between this WM phenotype and cognitive impairments, such as working memory, verbal memory, inhibitory control, executive function, information processing speed, attention, and psychomotor coordination (Saczynski et al., 2010; Belarbi et al., 2012; Gabbita et al., 2012; Li et al., 2013; Sahin et al., 2015; Rosenblat et al., 2015; Poletti et al., 2015; Felger et al., 2016). Therefore, due to increased inflammatory cytokines in patients with COVID-19 and their effects on structural and functional brain connectivities, we can expect the mentioned cognitive impairments in these patients as well.
Few studies have examined cognitive function in these patients using cognitive tasks. For example, studies conducted by Almeria et al using test de Aprendizaje verbal Espa~na Complutense with three lists for the learning, interference, and recognition to assess verbal memory; visual reproduction of the Wechsler memory scale–IV), digits forward and backward, letter and numbers, trail making test A and B (TMT), symbol digit modalities test, Stroop, phonetic and semantic fluency and Boston naming test from the NEURONORMA project (NN) showed that COVID-19 patients acquired lower scores in memory domains, attention, semantic fluency in working memory, and mental flexibility, and phonetic fluency, and executive function subtests (Almeria et al., 2020).
Zhou et al. have conducted a study to evaluate COVID-19’s effects on cognitive functions and its association with inflammatory markers in recovered patients. They evaluated the cognitive functions of 29 subjects by the iPad-based online neuropsychological tests, including the trail-making test (TMT), sign coding test, continuous performance test (CPT), and digital span test (DST). In their study, only some parts of the continuous performance test (CPT) showed significant group differences, indicating cognitive deficits, especially sustained attention domain in patients recovered from COVID-19 (Zhou et al., 2020).
In a sample of 130 patients, Mazza et al. examined cognitive functions using the brief assessment of cognition in Schizophrenia (Mazza et al., 2021), a broad battery evaluating verbal memory, verbal fluency, working memory (digit sequencing), selective attention and processing speed (symbol coding), psychomotor coordination (token motor task), and executive functions (Tower of London). According to previous studies, regardless of illness severity, most COVID-19 survivors showed cognitive impairments after 3 months, 78% of the sample showed poor performances in all the investigated domains. Executive functions and psychomotor coordination were impaired in 50% and 57% of the sample; information processing, verbal fluency, and working memory were impaired in around 30% of the sample (Mazza et al., 2021). The cognitive deficits observed in these patients were affected by systemic inflammation, which confirms the relationship between inflammation and cognitive deficits.
Therefore, it can be concluded that COVID-19 causes systemic inflammation and cognitive deficits in patients.
As a result of neuroinflammation, neurotransmitter metabolism, neuroplasticity, and brain structure and function are altered, the microglia are activated, and the hypothalamic-pituitary-adrenal (HPA) axis is dysregulated, which is the main component of the endocrinal system of the stress response and may affect cognition, memory task, and behavior (Tay et al., 2018). Evidence shows that psychological stressors may increase the production of proinflammatory cytokines, such as IL-1, IL-6, TNF-a, and IFN-g in humans and experimental animals. These cytokines can stimulate the HPA axis and lead to cognitive impairments caused by stress (Ownby, 2010). Therefore, inflammation may act as a mediator of stress-induced cognitive dysfunction; therefore, with the increase of those factors, the probability of cognitive deficits due to stress increases.
Stress triggers an immune response via the HPA axis, the system involved in releasing the cortisol hormone. Numerous studies demonstrate that high cortisol levels lead to memory problems through effects on the hippocampal (Ownby, 2010), and stress-induced hippocampal damage has been demonstrated in animal experiments (Sapolsky, 1996).
On the other hand, a large number of recovered COVID-19 patients, healthy people, and medical staff usually suffer from stress for a short period but may experience prolonged psychophysical symptoms, such as depression, anxiety, and fear (Wang et al., 2020; Huang et al., 2020; Balachandar, 2020). Studies also show that in specific situations, the brain’s immune system may be chronically triggered. Especially, it is mentioned that chronic stress, trauma, neurodegenerative diseases, and aging cause changes in microglia’s nature and protective capabilities (known as “microglial priming”) (Norden et al., 2015; Fonken et al., 2018), which is associated with high release of pro-inflammatory cytokines, such as TNF-a and IL-6, chemokines and reactive oxygen species (ROS) (Ransohoff & Perry, 2009; Chatterjee et al., 2013) for a long time. Increased microglial activity can cause extended lesions and impaired brain function (Norden et al., 2015). Therefore, systemic inflammation caused by stress in COVID-19 patients may explain the pathogenesis of cognitive dysfunction, especially memory impairment in these patients.
2. Conclusion
Evidence demonstrates that the SARS-CoV-2 virus can infect the brain and lead to neurological complications in patients. In this state, the brain’s immune cells (microglia and cytokines) produce high levels of pro-inflammatory factors in various brain regions (Russo et al., 2010), which can damage brain cells.
Several studies have concluded that systemic inflammation accompanied by a significant release of cytokines (known as “cytokine storm”) causes brain damage due to SARS-CoV-1 (Xu et al., 2005; Li et al., 2016b). In such a way that recent studies show an increase in the level of proinflammatory cytokines, such as IL-1,6,8,10 and TNF-α factors in the brains of people with COVID-19 (Channappanavar & Perlman, 2017; Chen et al., 2019, Chen et al., 2020a). Studies show that higher than normal levels of IL-8 cause impaired memory and motor function, as well as slower cognitive and perceptual speed (Baune et al., 2008; Alley et al., 2008; Li et al., 2013; Mun et al., 2016).
Also, other results indicate a positive relationship between plasma IL-6 level and cognitive deficits, such as prospective memory, working memory, executive functioning, processing speed, attention, orientation, immediate verbal recall, delayed recall or psychomotor speed, semantic fluency (Li et al., 2013; Simpson et al., 2013; Heringa et al., 2014; Rosenblat et al., 2015; Mun et al., 2016).
Considering that limbic and associated brain structures, such as the amygdala, anterior cingulate cortex, hippocampi, prefrontal cortex, and basal ganglia (structures that play essential roles in cognitive processes like memory, attention, emotion, and perception) contain many enzymes and cytokines involved in an inflammatory response (Raz & Rodrigue, 2006; Sudheimer et al., 2014; Rosenblat et al., 2015; Wang et al., 2015; Bortolato et al., 2015; Elias et al., 2017; Acuff et al., 2018); therefore, according to the available evidence, there is a probability of occurrence of these cognitive defects in these patients, which needs further investigation.
On the other hand, various cognitive defects, such as attention, working memory, executive function, processing speed impairments, anhedonia, and motor slowing deficit disorder have been observed following increased levels of cytokines, such as TNF-α and IL-6 in major depressive disorder, bipolar disorder, and schizophrenia patients (Saczynski et al., 2010; Belarbi et al., 2012; Gabbita et al., 2012; Li et al., 2013; Sahin et al., 2015; Rosenblat et al., 2015; Felger et al., 2016; Kępińska et al., 2020). Therefore, these results can confirm previous results based on the occurrence of these cognitive impairments in these patients following cytokine storms.
According to the above evidence based on the occurrence of cytokine storms and their effects in areas involved in cognitive processes, such as limbic and associated brain structures, we can expect cognitive deficits in cured patients with COVID-19. Therefore, the possible association between inflammatory status and cognitive function in patients with COVID-19 should be investigated over a long period to provide the best therapeutic strategies for COVID-19 survivors (Benedetti et al., 2020b).
Therefore, it is suggested that electrophysiological tools, such as quantitative electroencephalography, event-related potential, neurofeedback, and electrical stimulation of the brain, including transcranial stimulation with direct electric current, transcranial random noise stimulation, and transcranial electrical stimulation with alternating current, be used in future to evaluate and intervene effectively to improve cognitive abilities, such as memory, learning, attention, processing speed in COVID-19 recoveries (Chabot et al., 2001; Monastra et al., 2002; Vourvopoulos & Badia. 2016; Moon et al., 2019; Harris et al., 2020).
Also, modulation of pro and anti-inflammatory cytokines may prevent cognitive impairment and improve brain function (Torres et al., 2010).
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Study design, Drafting the manuscript: Gholamreza Chalabianloo and Zahra Keshtgar; Review and editing: Gholamreza Chalabianloo and Niloofar Esmaeili; Final approval: All authors.
Conflict of interest
All authors declared no conflict of interest.
Reference
Acuff, H. E., Versace, A., Bertocci, M. A., Ladouceur, C. D., Hanford, L. C., & Manelis, A., et al. (2018). Association of neuroimaging measures of emotion processing and regulation neural circuitries with symptoms of bipolar disorder in offspring at risk for bipolar disorder. JAMA Psychiatry, 75(12), 1241-1251. [DOI:10.1001/jamapsychiatry.2018.2318] [PMID]
Algahtani, H., Subahi, A., & Shirah, B. (2016). Neurological complications of Middle East respiratory syndrome coronavirus: A report of two cases and review of the literature. Case Reports in Neurological Medicine, 2016, 3502683.[DOI:10.1155/2016/3502683] [PMID]
Alley, D. E., Crimmins, E. M., Karlamangla, A., Hu, P., & Seeman, T. E. (2008). Inflammation and rate of cognitive change in high-functioning older adults. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 63(1), 50-55. [DOI:10.1093/gerona/63.1.50] [PMID]
Almeria, M., Cejudo, J. C., Sotoca, J., Deus, J., & Krupinski, J. (2020). Cognitive profile following COVID-19 infection: Clinical predictors leading to neuropsychological impairment. Brain, Behavior, & Immunity-Health, 9, 100163. [DOI:10.1016/j.bbih.2020.100163] [PMID]
Arbour, N., Day, R., Newcombe, J., & Talbot, P. J. (2000). Neuroinvasion by human respiratory coronaviruses. Journal of Virology, 74(19), 8913-8921. [DOI:10.1128/JVI.74.19.8913-8921.2000] [PMID]
Balachandar, V., Mahalaxmi, I., Subramaniam, M., Kaavya, J., Senthil Kumar, N., & Laldinmawii, G., et al. (2020). Follow-up studies in COVID-19 recovered patients-is it mandatory?. The Science of the Total Environment, 729, 139021. [PMID]
Balschun, D., Randolf, A., Pitossi, F., Schneider, H., Del Rey, A., & Besedovsky, H. O. (2003). Hippocampal interleukin‐1β gene expression during long‐term potentiation decays with age. Annals of the New York Academy of Sciences, 992, 1–8. [DOI:10.1111/j.1749-6632.2003.tb03132.x] [PMID]
Baune, B. T., Ponath, G., Golledge, J., Varga, G., Arolt, V., & Rothermundt, M., et al. (2008). Association between IL-8 cytokine and cognitive performance in an elderly general population-the MEMO-Study. Neurobiology of Aging, 29(6), 937-944. [PMID]
Belarbi, K., Jopson, T., Tweedie, D., Arellano, C., Luo, W., & Greig, N. H., et al. (2012). TNF-α protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. Journal of Neuroinflammation, 9, 23. [DOI:10.1186/1742-2094-9-23] [PMID]
Bechter, K. (2013). Virus infection as a cause of inflammation in psychiatric disorders. Modern Trends in Pharmacopsychiatry, 28, 49–60. [PMID]
Benedetti, F., Mazza, M., Cavalli, G., Ciceri, F., Dagna, L., & Rovere-Querini, P. (2021). Can cytokine blocking prevent depression in covid-19 survivors?. Journal of Neuroimmune Pharmacology, 16(1), 1–3. [DOI:10.1007/s11481-020-09966-z] [PMID]
Benedetti, F., Poletti, S., Hoogenboezem, T. A., Mazza, E., Ambrée, O., & de Wit, H., et al. (2016). Inflammatory cytokines influence measures of white matter integrity in bipolar disorder. Journal of Affective Disorders, 202, 1-9. [DOI:10.1016/j.jad.2016.05.047]
Blasko, I., Beer, R., Bigl, M., Apelt, J., Franz, G., & Rudzki, D., et al. (2004). Experimental traumatic brain injury in rats stimulates the expression, production and activity of Alzheimer’s disease β-secretase (BACE-1). Journal of Neural Transmission, 111(4), 523–536. [PMID]
Bortolato, B., Carvalho, A. F., Soczynska, J. K., Perini, G. I., & McIntyre, R. S. (2015). The involvement of TNF-α in cognitive dysfunction associated with major depressive disorder: An opportunity for domain specific treatments. Current Neuropharmacology, 13(5), 558-576. [DOI:10.2174/1570159X13666150630171433] [PMID]
Butler, N., Pewe, L., Trandem, K., & Perlman, S. (2006). Murine encephalitis caused by HCoV-OC43, a human coronavirus with broad species specificity, is partly immune-mediated. Virology, 347(2), 410-421. [DOI:10.1016/j.virol.2005.11.044] [PMID]
Chabot, R. J., di Michele, F., Prichep, L., & John, E. R. (2001).The clinical role of computerized EEG in the evaluation and treatment of learning and attention disorders in children and adolescents. The Journal of Neuropsychiatry and Clinical Neurosciences, 13(2), 171-186. [DOI:10.1176/jnp.13.2.171] [PMID]
Chakrabarty, T., Torres, I. J., Bond, D. J., & Yatham, L. N. (2019).Inflammatory cytokines and cognitive functioning in early-stage bipolar I disorder. Journal of Affective Disorders, 245, 679-685. [DOI:10.1016/j.jad.2018.11.018] [PMID]
Channappanavar, R., & Perlman, S. (2017). Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Seminars in Immunopathology, 39(5), 529–539. [DOI:10.1007/s00281-017-0629-x] [PMID]
Chatterjee, D., Biswas, K., Nag, S., Ramachandra, S. G., & Das Sarma, J. (2013). Microglia play a major role in direct viral-induced demyelination. Clinical and Developmental Immunology, 2013, 510396. [DOI:10.1155/2013/510396] [PMID]
Chen, G., Wu, D., Guo, W., Cao, Y., Huang, D., & Wang, H., et al. (2020). Clinical and immunological features of severe and moderate coronavirus disease 2019. The Journal of Clinical Investigation, 130(5), 2620–2629. [PMID]
Chen, T., Wu, D., Chen, H., Yan, W., Yang, D., & Chen, G., et al. (2020). Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ (Clinical Research ed.), 368, m1091. [DOI:10.1136/bmj.m1091] [PMID]
Chen, N., Zhou, M., Dong, X., Qu, J., Gong, F., & Han, Y., et al. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet, 395(10223), 507-513. [DOI:10.1016/S0140-6736(20)30211-7] [PMID]
Coperchini, F., Chiovato, L., Croce, L., Magri, F., & Rotondi, M. (2020). The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine & Growth Factor Reviews, 53, 25-32. [DOI:10.1016/j.cytogfr.2020.05.003] [PMID]
Davidson, S., Maini, M. K., & Wack, A. (2015). Disease-promoting effects of type I interferons in viral, bacterial, and coinfections. Journal of Interferon & Cytokine Research, 35(4), 252-264. [PMID]
de Aquino, M. T., Puntambekar, S. S., Savarin, C., Bergmann, C. C., Phares, T. W., & Hinton, D. R., et al. (2013). Role of CD25+ CD4+ T cells in acute and persistent coronavirus infection of the central nervous system. Virology, 447(1-2), 112-120. [DOI:10.1016/j.virol.2013.08.030] [PMID]
del Rey, A., Balschun, D., Wetzel, W., Randolf, A., & Besedovsky, H. O. (2013). A cytokine network involving brain-borne IL-1β, IL-1ra, IL-18, IL-6, and TNFα operates during long-term potentiation and learning. Brain, Behavior, and Immunity, 33, 15-23. [DOI:10.1016/j.bbi.2013.05.011] [PMID]
Dentino, A. N., Pieper, C. F., Rao, M. K., Currie, M. S., Harris, T., & Blazer, D. G., et al. (1999). Association of interleukin‐6 and other biologic variables with depression in older people living in the community. Journal of the American Geriatrics Society, 47(1), 6-11. [DOI:10.1111/j.1532-5415.1999.tb01894.x] [PMID]
Desforges, M., Le Coupanec, A., Dubeau, P., Bourgouin, A., Lajoie, L., & Dubé, M., et al. (2019). Human coronaviruses and other respiratory viruses: Underestimated opportunistic pathogens of the central nervous system? Viruses, 12(1), 14. [DOI:10.3390/v12010014] [PMID]
Doobay, M. F., Talman, L. S., Obr, T. D., Tian, X., Davisson, R. L., & Lazartigues, E. (2007). Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 292(1), R373-R381. [PMID]
Duarte, P. O., Duarte, M. G. F., Pelichek, A., Pfrimer, K., Ferriolli, E., & Moriguti, J. C., et al. (2017). Cardiovascular risk factors and inflammatory activity among centenarians with and without dementia. Aging Clinical and Experimental Research, 29(3), 411-417. [DOI:10.1007/s40520-016-0603-9] [PMID]
Elias, L. R., Miskowiak, K. W., Vale, A. M., Köhler, C. A., Kjærstad, H. L., & Stubbs, B., et al. (2017). Cognitive impairment in euthymic pediatric bipolar disorder: A systematic review and meta-analysis. Journal of the American Academy of Child & Adolescent Psychiatry, 56(4), 286-296. [DOI:10.1016/j.jaac.2017.01.008] [PMID]
Felger, J. C., Li, Z., Haroon, E., Woolwine, B. J., Jung, M. Y., & Hu, X., et al. (2016). Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Molecular Psychiatry, 21(10), 1358-1365. [DOI:10.1038/mp.2015.168] [PMID]
Filatov, A., Sharma, P., Hindi, F., & Espinosa, P. S. (2020). Neurological complications of coronavirus disease (COVID-19): Encephalopathy. Cureus, 12(3). [DOI:10.7759/cureus.7352]
Fonken, L. K., Frank, M. G., Gaudet, A. D., & Maier, S. F. (2018). Stress and aging act through common mechanisms to elicit neuroinflammatory priming. Brain, Behavior, and Immunity, 73, 133-148. [DOI:10.1016/j.bbi.2018.07.012] [PMID]
Gabbita, S. P., Srivastava, M. K., Eslami, P., Johnson, M. F., Kobritz, N. K., & Tweedie, D., et al. (2012). Early intervention with a small molecule inhibitor for tumor nefosis factor-α prevents cognitive deficits in a triple transgenic mouse model of Alzheimer’s disease. Journal of Neuroinflammation, 9, 99. [DOI:10.1186/1742-2094-9-99] [PMID]
Gu, J., Gong, E., Zhang, B., Zheng, J., Gao, Z., & Zhong, Y., et al. (2005). Multiple organ infection and the pathogenesis of SARS. The Journal of experimental medicine, 202(3), 415–424.[DOI:10.1084/jem.20050828] [PMID]
Guan, W. J., Ni, Z. Y., Hu, Y., Liang, W. H., Ou, C. Q., & He, J. X., et al. (2020). Clinical characteristics of coronavirus disease 2019 in China. The New England Journal of Medicine, 382, 1708-1720. [Link]
Guo, Y., Korteweg, C., McNutt, M. A., & Gu, J. (2008). Pathogenetic mechanisms of severe acute respiratory syndrome. Virus Research, 133(1), 4-12. [DOI:10.1016/j.virusres.2007.01.022] [PMID]
Hamming, I., Timens, W., Bulthuis, M. L., Lely, A. T., Navis, G., & van Goor, H. (2004). Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology, 203(2), 631-637. [DOI:10.1002/path.1570] [PMID]
Hanisch, U. K., & Kettenmann, H. (2007). Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nature Neuroscience, 10(11), 1387-1394. [DOI:10.1038/nn1997] [PMID]
Harris, A. M., Jacoby, O., Remington, R. W., Becker, S. I., & Mattingley, J. B. (2020). Behavioral and electrophysiological evidence for a dissociation between working memory capacity and feature-based attention. Cortex; A Journal Devoted to the Study of the Nervous System and Behavior, 129, 158–174. [DOI:10.1016/j.cortex.2020.04.009] [PMID]
Harmer, D., Gilbert, M., Borman, R., & Clark, K. L. (2002). Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Letters, 532(1-2), 107-110. [DOI:10.1016/S0014-5793(02)03640-2] [PMID]
Hawkins, R. B., Raymond, S. L., Stortz, J. A., Horiguchi, H., Brakenridge, S. C., & Gardner, A., et al. (2018). Chronic critical illness and the persistent inflammation, immunosuppression, and catabolism syndrome. Frontiers in Immunology, 9, 1511.[DOI:10.3389/fimmu.2018.01511] [PMID]
Heringa, S. M., van den Berg, E., Reijmer, Y. D., Nijpels, G., Stehouwer, C. D., & Schalkwijk, C. G., et al. (2014). Markers of low-grade inflammation and endothelial dysfunction are related to reduced information processing speed and executive functioning in an older population-the Hoorn study. Psychoneuroendocrinology, 40, 108-118. [DOI:10.1016/j.psyneuen.2013.11.011] [PMID]
Hoffmann, M., Kleine-Weber, H., Schroeder, S., Krüger, N., Herrler, T., & Erichsen, S., et al. (2020). SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181(2), 271–280.e8. [DOI:10.1016/j.cell.2020.02.052] [PMID]
Huang, J. Z., Han, M. F., Luo, T. D., Ren, A. K., & Zhou, X. P. (2020). [Mental health survey of 230 medical staff in a tertiary infectious disease hospital for COVID-19 (Chinese)]. Chinese Journal of Industrial Hygiene and Occupational Diseases, 38(3), 192–195. [PMID]
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., & Hu, Y., et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England), 395(10223), 497–506. [DOI:10.1016/S0140-6736(20)30183-5] [PMID]
Jiang, F., Deng, L., Zhang, L., Cai, Y., Cheung, C. W., & Xia, Z. (2020). Review of the clinical characteristics of coronavirus disease 2019 (COVID-19). Journal of General Internal Medicine, 35(5), 1545–1549. [DOI:10.1007/s11606-020-05762-w] [PMID]
Kępińska, A. P., Iyegbe, C. O., Vernon, A. C., Yolken, R., Murray, R. M., & Pollak, T. A. (2020). Schizophrenia and influenza at the centenary of the 1918-1919 Spanish influenza pandemic: mechanisms of psychosis risk. Frontiers in psychiatry, 11, 72. [DOI:10.3389/fpsyt.2020.00072] [PMID]
Khosravani, H., Rajendram, P., Notario, L., Chapman, M. G., & Menon, B. K. (2020). Protected code stroke: Hyperacute stroke management during the coronavirus disease 2019 (COVID-19) pandemic. Stroke, 51(6), 1891-1895. [DOI:10.1161/STROKEAHA.120.029838] [PMID]
Kim, Y. K., Jung, H. G., Myint, A. M., Kim, H., & Park, S. H. (2007). Imbalance between pro-inflammatory and anti-inflammatory cytokines in bipolar disorder. Journal of Affective Disorders, 104(1-3), 91-95. [DOI:10.1016/j.jad.2007.02.018] [PMID]
Kim, J. E., Heo, J. H., Kim, H. O., Song, S. H., Park, S. S., & Park, T. H., et al. (2017). Neurological complications during treatment of middle east respiratory syndrome. Journal of Clinical Neurology, 13(3), 227-233. [DOI:10.3988/jcn.2017.13.3.227] [PMID]
Kinsinger, L. S., Anderson, C., Kim, J., Larson, M., Chan, S. H., & King, H. A., et al. (2017). Implementation of lung cancer screening in the Veterans Health Administration. JAMA Internal Medicine, 177(3), 399-406. [DOI:10.1001/jamainternmed.2016.9022] [PMID]
Kuiken, T., Fouchier, R. A., Schutten, M., Rimmelzwaan, G. F., van Amerongen, G., & van Riel, D., et al. (2003). Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet (London, England), 362(9380), 263–270. [DOI:10.1016/S0140-6736(03)13967-0] [PMID]
Lam, T. T., Jia, N., Zhang, Y. W., Shum, M. H., Jiang, J. F., & Zhu, H. C., et al. (2020). IIdentifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature, 583(7815), 282–285. [PMID]
Leung, W. K., To, K. F., Chan, P. K., Chan, H. L., Wu, A. K., & Lee, N., et al. (2003). Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology, 125(4), 1011-1017. [PMID]
Lam, M. H., Wing, Y. K., Yu, M. W., Leung, C. M., Ma, R. C., & Kong, A. P., et al. (2009). Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: Long-term follow-up. Archives of Internal Medicine, 169(22), 2142-2147. [DOI:10.1001/archinternmed.2009.384] [PMID]
Lau, K. K., Yu, W. C., Chu, C. M., Lau, S. T., Sheng, B., & Yuen, K. Y. (2004). Possible central nervous system infection by SARS coronavirus. Emerging Infectious Diseases, 10(2), 342–344.[DOI:10.3201/eid1002.030638] [PMID]
Levine, J., Barak, Y., Chengappa, K. N. R., Rapoport, A., Rebey, M., & Barak, V. (1999). Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology, 40(4), 171-176. [PMID]
Li, Y. C., Bai, W. Z., & Hashikawa, T. (2020). The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. Journal of Medical Virology, 92(6), 552-555. [DOI:10.1002/jmv.25728] [PMID]
Li, Y., Li, H., Fan, R., Wen, B., Zhang, J., & Cao, X., et al. (2016). Coronavirus infections in the central nervous system and respiratory tract show distinct features in hospitalized children. Intervirology, 59(3), 163-169. [DOI:10.1159/000453066] [PMID]
Li, B. H., Zhang, L. L., Yin, Y. W., Pi, Y., Guo, L., & Yang, Q. W., et al. (2013). Association between interleukin-1α C (− 889) T polymorphism and Alzheimer’s disease: A meta-analysis including 12,817 subjects. Journal of Neural Transmission, 120(3), 497-506. [DOI:10.1007/s00702-012-0867-y] [PMID]
Lichanska, A. M., Browne, C. M., Henkel, G. W., Murphy, K. M., Ostrowski, M. C., & McKercher, S. R., et al.(1999). Differentiation of the mononuclear phagocyte system during mouse embryogenesis: The role of transcription factor PU. 1. Blood, 94(1), 127–138. [DOI:10.1182/blood.V94.1.127.413k07_127_138] [PMID]
Lin, K., Shao, R., Wang, R., Lu, W., Zou, W., & Chen, K., et al. (2020). Inflammation, brain structure and cognition interrelations among individuals with differential risks for bipolar disorder. Brain, Behavior, and Immunity, 83, 192-199. [DOI:10.1016/j.bbi.2019.10.010] [PMID]
Lin, L., Zheng, L. J., & Zhang, L. J. (2018). Neuroinflammation, gut microbiome, and Alzheimer’s disease. Molecular Neurobiology, 55(11), 8243-8250. [DOI:10.1007/s12035-018-0983-2] [PMID]
Liu, Z., Xiao, X., Wei, X., Li, J., Yang, J., & Tan, H., et al. (2020). Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS‐CoV‐2. Journal of Medical Virology, 92(6), 595-601. [DOI:10.1002/jmv.25726] [PMID]
Magalhães, R. C., Pimenta, L. P., Barbosa, I. G., Moreira, J. M., de Barros, J. L. V. M., & Teixeira, A. L., et al. (2018). Inflammatory molecules and neurotrophic factors as biomarkers of neuropsychomotor development in preterm neonates: A Systematic Review. International Journal of Developmental Neuroscience, 65, 29-37. [DOI:10.1016/j.ijdevneu.2017.10.006] [PMID]
Mao, L., Jin, H., Wang, M., Hu, Y., Chen, S., & He, Q., et al. (2020). Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurology, 77(6), 683-690. [DOI:10.1001/jamaneurol.2020.1127] [PMID]
Mao, L., Wang, M., Chen, S., He, Q., Chang, J., & Hong, C., et al. (2020). Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: A retrospective case series study. MedRxiv. [DOI:10.1101/2020.02.22.20026500]
Matoba, Y., Abiko, C., Ikeda, T., Aoki, Y., Suzuki, Y., & Yahagi, K., et al. (2015). Detection of the human coronavirus 229E, HKU1, NL63, and OC43 between 2010 and 2013 in Yamagata, Japan. Japanese Journal of Infectious Diseases, 68(2), 138-141. [DOI:10.7883/yoken.JJID.2014.266] [PMID]
Mazza, M. G., Palladini, M., De Lorenzo, R., Magnaghi, C., Poletti, S., & Furlan, R., et al. (2021). Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain, Behavior, and Immunity, 94, 138–147. [DOI:10.1016/j.bbi.2021.02.021] [PMID]
McCray, P. B., Jr, Pewe, L., Wohlford-Lenane, C., Hickey, M., Manzel, L., & Shi, L., et al. (2007). Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. Journal of Virology, 81(2), 813-821. [DOI:10.1128/JVI.02012-06] [PMID]
Monastra, V. J., Monastra, D. M., & George, S. (2002). The effects of stimulant therapy, EEG biofeedback, and parenting style on the primary symptoms of attention-deficit/hyperactivity disorder. Applied Psychophysiology and Biofeedback, 27(4), 231-249. [DOI:10.1023/A:1021018700609] [PMID]
Moon, S. Y., Kim, M., Hwang, W. J., Lee, T. Y., & Kwon, J. S. (2019). A pilot study investigating the effect of transcranial direct current stimulation on the electrophysiological correlates of working memory in patients with schizophrenia. Psychiatry Research. Neuroimaging, 284, 9-12. [DOI:10.1016/j.pscychresns.2018.12.014] [PMID]
Moore, A. H., Wu, M., Shaftel, S. S., Graham, K. A., & O'Banion, M. K. (2009). Sustained expression of interleukin-1β in mouse hippocampus impairs spatial memory. Neuroscience, 164(4), 1484-1495. [DOI:10.1016/j.neuroscience.2009.08.073] [PMID]
Moriguchi, T., Harii, N., Goto, J., Harada, D., Sugawara, H., & Takamino, J., et al. (2020). A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. International Journal of Infectious Diseases, 94, 55–58. [DOI:10.1016/j.ijid.2020.03.062] [PMID]
Mun, M. J., Kim, J. H., Choi, J. Y., & Jang, W. C. (2016). Genetic polymorphisms of interleukin genes and the risk of Alzheimer’s disease: An update meta-analysis. Meta Gene, 8, 1-10. [DOI:10.1016/j.mgene.2016.01.001] [PMID]
Muneer, A. (2016). Bipolar disorder: Role of inflammation and the development of disease biomarkers. Psychiatry Investigation, 13(1), 18-33. [DOI:10.4306/pi.2016.13.1.18] [PMID]
Myint, S. H. (1995). Human coronavirus infections. In: S. G. Siddell (Ed.), The coronaviridae (pp. 389-401).Boston: Springer. [DOI:10.1007/978-1-4899-1531-3_18]
Netland, J., Meyerholz, D. K., Moore, S., Cassell, M., & Perlman, S. (2008). Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. Journal of Virology, 82(15), 7264-7275. [DOI:10.1128/JVI.00737-08] [PMID]
Nicholls, J., Dong, X. P., Jiang, G., & Peiris, M. (2003). SARS: Clinical virology and pathogenesis. Respirology, 8 Suppl(Suppl 1), S6–S8.[DOI:10.1046/j.1440-1843.2003.00517.x] [PMID]
Norden, D. M., Muccigrosso, M. M., & Godbout, J. P. (2015). Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology, 96(Pt A), 29–41. [DOI:10.1016/j.neuropharm.2014.10.028] [PMID]
Ownby, R. L. (2010). Neuroinflammation and cognitive aging. Current Psychiatry Reports, 12(1), 39–45. [PMID]
Paniz-Mondolfi, A., Bryce, C., Grimes, Z., Gordon, R. E., Reidy, J., & Lednicky, J., et al. (2020). Central nervous system involvement by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). Journal of Medical Virology, 92(7), 699-702. [DOI:10.1002/jmv.25915] [PMID]
Papa, S. M., Brundin, P., Fung, V. S. C., Kang, U. J., Burn, D. J., & Colosimo, C., et al. (2020). Impact of the COVID-19 pandemic on Parkinson’s disease and movement disorders. Movement Disorders Clinical Practice, 7(4), 357–360. [DOI:10.1002/mdc3.12953] [PMID]
Peiris, J. S., Chu, C. M., Cheng, V. C., Chan, K. S., Hung, I. F., & Poon, L. L., et al. (2003). Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: A prospective study. Lancet (London, England), 361(9371), 1767–1772. [DOI:10.1016/S0140-6736(03)13412-5] [PMID]
Peiris, J. S., Lai, S. T., Poon, L. L., Guan, Y., Yam, L. Y., & Lim, W., et al. (2003). Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet (London, England), 361(9366), 1319–1325. [DOI:10.1016/S0140-6736(03)13077-2] [PMID]
Phares, T. W., Stohlman, S. A., & Bergmann, C. C. (2013). Intrathecal humoral immunity to encephalitic RNA viruses. Viruses, 5(2), 732-752. [DOI:10.3390/v5020732] [PMID]
Pivot, X., Raymond, E., Laguerre, B., Degardin, M., Cals, L., & Armand, J. P., et al. (2001). Pemetrexed disodium in recurrent locally advanced or metastatic squamous cell carcinoma of the head and neck. British Journal of Cancer, 85(5), 649-655. [DOI:10.1054/bjoc.2001.2010] [PMID]
Poletti, S., Bollettini, I., Mazza, E., Locatelli, C., Radaelli, D., & Vai, B., et al. (2015). Cognitive performances associate with measures of white matter integrity in bipolar disorder. Journal of Affective Disorders, 174, 342-352. [DOI:10.1016/j.jad.2014.12.030] [PMID]
Poletti, S., Leone, G., Hoogenboezem, T. A., Ghiglino, D., Vai, B., & de Wit, H., et al. (2019). Markers of neuroinflammation influence measures of cortical thickness in bipolar depression. Psychiatry Research. Neuroimaging, 285, 64-66. [PMID]
Poletti, S., Mazza, M. G., Vai, B., Lorenzi, C., Colombo, C., & Benedetti, F. (2020). Proinflammatory cytokines predict brain metabolite concentrations in the anterior cingulate cortex of patients with bipolar disorder. Frontiers in Psychiatry, 11, 590095. [DOI:10.3389/fpsyt.2020.590095] [PMID]
Poyiadji, N., Shahin, G., Noujaim, D., Stone, M., Patel, S., & Griffith, B. (2020). COVID-19-associated acute hemorrhagic necrotizing encephalopathy: Imaging features. Radiology, 296(2), E119–E120. [PMID]
Raz, N., & Rodrigue, K. M. (2006). Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neuroscience & Biobehavioral Reviews, 30(6), 730-748. [DOI:10.1016/j.neubiorev.2006.07.001] [PMID]
Ransohoff, R. M., & Perry, V. H. (2009). Microglial physiology: Unique stimuli, specialized responses. Annual Review of Immunology, 27, 119-145. [PMID]
Rhie, S. J., Jung, E. Y., & Shim, I. (2020). The role of neuroinflammation on pathogenesis of affective disorders. Journal of Exercise Rehabilitation, 16(1), 2-9. [DOI:10.12965/jer.2040016.008] [PMID]
Rogers, J. P., Chesney, E., Oliver, D., Pollak, T. A., McGuire, P., & Fusar-Poli, P., et al. (2020). Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. The Lancet Psychiatry, 7(7), 611-627. [DOI:10.1016/S2215-0366(20)30203-0] [PMID]
Rosenblat, J. D., Brietzke, E., Mansur, R. B., Maruschak, N. A., Lee, Y., & McIntyre, R. S. (2015). Inflammation as a neurobiological substrate of cognitive impairment in bipolar disorder: evidence, pathophysiology and treatment implications. Journal of Affective Disorders, 188, 149-159. [DOI:10.1016/j.jad.2015.08.058] [PMID]
Russo, I., Barlati, S., & Bosetti, F. (2011). Effects of neuroinflammation on the regenerative capacity of brain stem cells. Journal of Neurochemistry, 116(6), 947-956. [DOI:10.1111/j.1471-4159.2010.07168.x] [PMID]
Saczynski, J. S., Beiser, A., Seshadri, S., Auerbach, S., Wolf, P. A., & Au, R. (2010). Depressive symptoms and risk of dementia: The framingham heart study. Neurology, 75(1), 35-41. [DOI:10.1212/WNL.0b013e3181e62138] [PMID]
Quarantelli, M. (2015). MRI/MRS in neuroinflammation: methodology and applications. Clinical and translational imaging, 3(6), 475-489. [DOI:10.1007/s40336-015-0142-y] [PMID]
Şahin, T. D., Karson, A., Balcı, F., Yazır, Y., Bayramgürler, D., & Utkan, T. (2015). TNF-alpha inhibition prevents cognitive decline and maintains hippocampal BDNF levels in the unpredictable chronic mild stress rat model of depression. Behavioural Brain Research, 292, 233-240. [DOI:10.1016/j.bbr.2015.05.062] [PMID]
Sapolsky, R. M. (1996). Why stress is bad for your brain. Science, 273(5276), 749-750. [DOI:10.1126/science.273.5276.749] [PMID]
Sepehrinezhad, A., Shahbazi, A., & Negah, S. S. (2020). COVID-19 virus may have neuroinvasive potential and cause neurological complications: A perspective review. Journal of Neurovirology, 26(3), 324–329. [DOI:10.1007/s13365-020-00851-2] [PMID]
Shen, X. N., Niu, L. D., Wang, Y. J., Cao, X. P., Liu, Q., & Tan, L., et al. (2019). Inflammatory markers in Alzheimer’s disease and mild cognitive impairment: a meta-analysis and systematic review of 170 studies. Journal of Neurology, Neurosurgery & Psychiatry, 90(5), 590-598. [DOI:10.1136/jnnp-2018-319148] [PMID]
Sheng, B., Cheng, S. K., Lau, K. K., Li, H. L., & Chan, E. L. (2005). The effects of disease severity, use of corticosteroids and social factors on neuropsychiatric complaints in severe acute respiratory syndrome (SARS) patients at acute and convalescent phases. European Psychiatry, 20(3), 236-242. [DOI:10.1016/j.eurpsy.2004.06.023] [PMID]
Sheng, W. H., Chiang, B. L., Chang, S. C., Ho, H. N., Wang, J. T., & Chen, Y. C., et al. (2005). Clinical manifestations and inflammatory cytokine responses in patients with severe acute respiratory syndrome. Journal of the Formosan Medical Association= Taiwan yi zhi, 104(10), 715-723.
Simpson, E. E., Hodkinson, C. F., Maylor, E. A., McCormack, J. M., Rae, G., & Strain, S., et al. (2013). Intracellular cytokine production and cognition in healthy older adults. Psychoneuroendocrinology, 38(10), 2196-2208. [DOI:10.1016/j.psyneuen.2013.04.007] [PMID]
Skinner, D., Marro, B. S., & Lane, T. E. (2019). Chemokine CXCL10 and coronavirus-induced neurologic disease. Viral Immunology, 32(1), 25-37. [DOI:10.1089/vim.2018.0073] [PMID]
Spulber, S., Mateos, L., Oprica, M., Cedazo-Minguez, A., Bartfai, T., & Winblad, B., et al. (2009). Impaired long term memory consolidation in transgenic mice overexpressing the human soluble form of IL-1ra in the brain. Journal of Neuroimmunology, 208(1-2), 46-53. [DOI:10.1016/j.jneuroim.2009.01.010] [PMID]
St-Jean, J. R., Jacomy, H., Desforges, M., Vabret, A., Freymuth, F., & Talbot, P. J. (2004). Human respiratory coronavirus OC43: Genetic stability and neuroinvasion. Journal of Virology, 78(16), 8824-8834. [DOI:10.1128/JVI.78.16.8824-8834.2004] [PMID]
Sudheimer, K. D., O'Hara, R., Spiegel, D., Powers, B., Kraemer, H. C., & Neri, E., et al. (2014). Cortisol, cytokines, and hippocampal volume interactions in the elderly. Frontiers in Aging Neuroscience, 6, 153. [DOI:10.3389/fnagi.2014.00153] [PMID]
Tay, T. L., Béchade, C., D’Andrea, I., St-Pierre, M. K., Henry, M. S., & Roumier, A., et al. (2018). Microglia gone rogue: impacts on psychiatric disorders across the lifespan. Frontiers in Molecular Neuroscience, 10, 421. [DOI:10.3389/fnmol.2017.00421] [PMID]
Torres, I. J., DeFreitas, V. G., DeFreitas, C. M., Kauer-Sant'Anna, M., Bond, D. J., & Honer, W. G., et al. (2010). Neurocognitive functioning in patients with bipolar I disorder recently recovered from a first manic episode. The Journal of clinical psychiatry, 71(9), 1234-1242. [DOI:10.4088/JCP.08m04997yel] [PMID]
Tsang, H. W., Scudds, R. J., & Chan, E. Y. (2004). Psychosocial impact of SARS. Emerging Infectious Diseases, 10(7), 1326–1327.[PMID]
van den Brand, J. M., Haagmans, B. L., van Riel, D., Osterhaus, A. D., & Kuiken, T. (2014). The pathology and pathogenesis of experimental severe acute respiratory syndrome and influenza in animal models. Journal of Comparative Pathology, 151(1), 83-112. [DOI:10.1016/j.jcpa.2014.01.004] [PMID]
Vourvopoulos, A., & Bermúdez I Badia, S. (2016). Motor priming in virtual reality can augment motor-imagery training efficacy in restorative brain-computer interaction: A within-subject analysis. Journal of Neuroengineering and Rehabilitation, 13(1), 69. [DOI:10.1186/s12984-016-0173-2] [PMID]
Waldman, G., Mayeux, R., Claassen, J., Agarwal, S., Willey, J., & Anderson, E., et al. (2020). Preparing a neurology department for SARS-CoV-2 (COVID-19): Early experiences at Columbia University Irving Medical Center and the New York Presbyterian Hospital in New York City. Neurology, 94(20), 886-891. [DOI:10.1212/WNL.0000000000009519] [PMID]
Wang, J., Gu, B. J., Masters, C. L., & Wang, Y. J. (2017). A systemic view of Alzheimer disease-insights from amyloid-β metabolism beyond the brain. Nature Reviews Neurology, 13(10), 612. [DOI:10.1038/nrneurol.2017.147] [PMID]
Wang, W. Y., Tan, M. S., Yu, J. T., & Tan, L. (2015). Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Annals of Translational Medicine, 3(10), 136. [PMID]
Wang, X. M., Walitt, B., Saligan, L., Tiwari, A. F., Cheung, C. W., & Zhang, Z. J. (2015). Chemobrain: A critical review and causal hypothesis of link between cytokines and epigenetic reprogramming associated with chemotherapy. Cytokine, 72(1), 86-96. [DOI:10.1016/j.cyto.2014.12.006] [PMID]
Wang, C., Pan, R., Wan, X., Tan, Y., Xu, L., & Ho, C. S., et al. (2020). Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. International Journal of Environmental Research and Public Health, 17(5), 1729. [DOI:10.3390/ijerph17051729] [PMID]
Werry, E. L., Bright, F. M., Piguet, O., Ittner, L. M., Halliday, G. M., & Hodges, J. R., et al. (2019). Recent developments in TSPO PET imaging as a biomarker of neuroinflammation in neurodegenerative disorders. International Journal of Molecular Sciences, 20(13), 3161. [DOI:10.3390/ijms20133166] [PMID]
Wheeler, D. L., Sariol, A., Meyerholz, D. K., & Perlman, S. (2018). Microglia are required for protection against lethal coronavirus encephalitis in mice. The Journal of Clinical Investigation, 128(3), 931-943. [DOI:10.1172/JCI97229] [PMID]
Wrapp, D., Wang, N., Corbett, K. S., Goldsmith, J. A., Hsieh, C. L., & Abiona, O., et al. (2020). Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science, 367(6483), 1260-1263. [DOI:10.1126/science.abb2507] [PMID]
Wu, Y., Xu, X., Yang, L., Liu, C., & Yang, C. (2020). Nervous system damage after COVID-19 infection: Presence or absence?. Brain, Behavior, and Immunity, 87, 55. [DOI:10.1016/j.bbi.2020.04.043] [PMID]
Xia, H., & Lazartigues, E. (2008). Angiotensin‐converting enzyme 2 in the brain: Properties and future directions. Journal of Neurochemistry, 107(6), 1482-1494. [DOI:10.1111/j.1471-4159.2008.05723.x] [PMID]
Xu, J., Zhong, S., Liu, J., Li, L., Li, Y., & Wu, X., et al. (2005). Detection of severe acute respiratory syndrome coronavirus in the brain: Potential role of the chemokine mig in pathogenesis. Clinical Infectious Diseases, 41(8), 1089-1096. [DOI:10.1086/444461] [PMID]
Yeh, E. A., Collins, A., Cohen, M. E., Duffner, P. K., & Faden, H. (2004). Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics, 113(1), e73-e76. [DOI:10.1542/peds.113.1.e73] [PMID]
Yuan, Y., Cao, D., Zhang, Y., Ma, J., Qi, J., & Wang, Q., et al.(2017). Cryo-EM structures of MERS-CoV and SARS-CoV spike glycoproteins reveal the dynamic receptor binding domains. Nature Communications, 8, 15092. [DOI:10.1038/ncomms15092] [PMID]
Zhang, C., Shi, L., & Wang, F. S. (2020). Liver injury in COVID-19: Management and challenges. The Lancet Gastroenterology & Hepatology, 5(5), 428-430. [DOI:10.1016/S2468-1253(20)30057-1] [PMID]
Zhao, G., Jiang, Y., Qiu, H., Gao, T., Zeng, Y., & Guo, Y., et al. (2015). Multi-organ damage in human dipeptidyl peptidase 4 transgenic mice infected with Middle East respiratory syndrome-coronavirus. PLoS One, 10(12), e0145561. [DOI:10.1371/journal.pone.0145561] [PMID]
Zhao, J., Rudd, A., & Liu, R. (2020). Challenges and potential solutions of stroke care during the coronavirus disease 2019 (COVID-19) outbreak. Stroke, 51(5), 1356–1357. [DOI:10.1161/STROKEAHA.120.029701] [PMID]
Zhou, H., Lu, S., Chen, J., Wei, N., Wang, D., & Lyu, H., et al. (2020). The landscape of cognitive function in recovered COVID-19 patients. Journal of Psychiatric Research, 129, 98-102. [DOI:10.1016/j.jpsychires.2020.06.022] [PMID]
Coronaviruses are a large family of viruses responsible for diseases in mammals and birds (Liu et al., 2020). Like Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS), coronaviruses (CoVs) lead to severe respiratory diseases in humans. Some types of these viruses, including MERS-CoV, 229E, NL63, OC43, HKU1, and SARS-CoV, may have adverse neuropsychiatric impacts in humans (Myint, 1995; Matoba et al., 2015) (Table 1).

As a result of SARS infection, most SARS patients reported poor attention, memory impairments, insomnia, anxiety, and depression symptoms, indicating cognitive impairments after SARS infection (Sheng et al., 2005a). Despite physical recovery, these psychiatric symptoms may remain in patients with SARS (Tsang et al., 2004).
The first report of SARS-CoV-2 (also known as COVID-19) occurred in China in December 2019 (Lam et al., 2020). According to the World Health Organization (WHO), new coronavirus diseases are considered pandemics.
Like MERS and SARS, COVID-19 is characterized by shortness of breath, respiratory problems, cough, fatigue, sore throat, and fever. The patients also complain about headaches, nausea, dizziness, vertigo, and vomiting (Jiang et al., 2020). Evidence shows that MERS-CoV, SARS-CoV, and COVID-19 can cause acute respiratory distress syndrome (ARDS) and defile other members and cell types throughout the disease, such as the mucosa of the intestinal tract, renal tubular cells, lymphocytes, reticuloendothelial cells, and nerve cells (Kuiken et al., 2003; Leung et al., 2003; Peiris et al., 2003; Wu et al., 2020; Zhang et al., 2020).
Although coronaviruses mainly infect humans via the digestive and respiratory systems, SARS-CoV-2 infection can affect the nervous system indirectly, particularly through neurodegenerative diseases (Rhie et al., 2020). Researchers have recently found a strong correlation between gut microbiota, neuroinflammation, and neurological diseases. Lin et al. suggest that SARS-CoV-2 can affect gut mucosa cells, causing inflammation, and dysbiosis, and ultimately leading to neuroinflammation and neurodegeneration (Lin et al., 2018) as meningoencephalitis, various viral-associated necrotizing encephalitides, and secondary cytokine-induced acute necrotizing syndromes (Filatov et al., 2020; Poyiadji et al., 2020; Moriguchi et al., 2020). These reports highlight the effect of medical care during this pandemic. This makes COVID-19 another challenge for neurologists, clinical neuroscientists, and neuropsychologists (Zhao et al., 2020; Papa et al., 2020; Waldman et al., 2020; Khosravani et al., 2020).
Because the structure and pathogenesis of most coronaviruses (CoVs) are the same (St-Jean et al., 2004; Butler et al., 2006; Yuan et al., 2017) and it is unclear how COVID-19 works; therefore, it is essential to investigate the involvement of other organs (e.g. central nervous system [CNS]) and the relationship between psychological factors, inflammation, and cognitive functions, especially due to the new nature of the virus.
According to this research, cognitive deficits and inflammation are linked. Further exploration of the possible interrelationships among COVID-19 inflammation, cognitive function, and psychological factors is necessary to provide future research information. This issue is discussed in this study.
The articles used in this study were searched by keywords, such as cytokine storm and COVID-19, COVID-19 and executive dysfunction, cognitive disorder and COVID-19, CNS and COVID-19, coronavirus, and neuroinvasion. The keywords were searched in international databases, such as Science Direct, Scopus, PubMed, Embase, and Web of Science using the preferred reporting items for systematic reviews and meta-analysis (PRISMA) checklist. All observational studies published between December 2019 and April 2021 in peer-reviewed journals in any language, including cross-sectional, cohort, case-control, and case reports, were assessed in this study. The search result was 106 articles. Firstly, search engine results were evaluated for thematic relevance to select the documents. Then, after reviewing the titles, the abstract was evaluated in terms of relevance to the purpose. Finally, 73 articles were selected and thoroughly studied. Notes were taken of selected documents. The material collected about COVID-19, the stages of infection by this virus, its effect on the nervous system and neurological symptoms, the cytokine storm caused by this infection, and the possible cognitive consequences caused by this virus in patients were divided and summarized. Other articles were not checked due to their limited relevance to the topic under discussion.
Symptoms and consequences of CoV infection
The SARS-CoV-2 infection appears in three stages. At the early stages of the disease, the replication of the virus is high, which causes pyrexia, cough, and general discomfort for several days. Patients may develop high fever, hypoxemia, and respiratory signs that progress to bilateral pneumonia during the second stage. However, lab testing indicates that viral replication declines near the end of this stage (Peiris et al., 2003). About 20% of patients develop SARS at the last stage of the infection, which can be fatal (Nicholls et al., 2003; Van et al., 2014). The SARS may cause an inflammatory response in the host (known as a “cytokine storm”) that causes alveolar damage and severe hypoxemia, resulting in fatal secondary sepsis during the final stage.
Recently, studies conducted by Chen et al. have shown that SARS-CoV-2 infection leads to lymphocytopenia (low CD4+ and CD8+ T cell counts with average B cell counts), elevated cytokine levels of interleukin (IL)-6, IL-2R, IL-10, tumor necrosis factor-alpha (TNF-α), and CC chemokine ligand 2 (CCL2), as well as decreased interferon (IFN)- γ expression in CD4+ T cells. With mild symptoms of COVID-19, IL-6, IL-2R, IL-10, and TNF-α levels rise slightly or remain close to normal, but with severe symptoms, they rise (Figure 1). Scientists claim a connection between the cytokine storm and severe COVID-19, as observed in cases of sARS-CoV infection (Chen et al., 2019; Davidson et al., 2015).

A persistent inflammatory, immunosuppressive, and catabolic syndrome (PICS) may result following infection, indicating a disturbance in the immune system. These conditions are probably caused by COVID-19 infection and the resulting cytokine storm, which is caused by the continuous release of endogenous alarmins or danger-associated molecular patterns from infected organs (Hawkins et al., 2018). Unfortunately, no definitive knowledge is available regarding chronic inflammation in survivors of COVID-19; however, this theory is credible given the correlation between severe infection and persistent inflammatory, immunosuppressive, and catabolic syndrome (PICS).
Brain invasion by severe acute respiratory SARS-CoV-2 and neurological symptoms: Possible mechanisms of transmission
Neural pathway
Despite the lack of evidence for SARS-CoV-2 invasion into the CNS, viral particles have been found in the cerebrospinal fluid (CSF) of a patient with meningoencephalitis (Wu et al., 2020) and in the frontal lobe of a patient with Parkinson’s disease with symptoms, such as fever and confusion at the time of admission (Paniz-Mondolfi et al., 2020). Animal studies have shown that two types of human coronaviruses (HCoV, HCoV-OC43) and SARS-CoV-1 enter the CNS through the olfactory nerve and the trigeminal nerve in the olfactory epithelium (Desforges et al., 2020). SARS-CoV-1 has been shown to infect the brainstem through the olfactory bulb and piriform cortex in transgenic mice expressing human angiotensin-converting enzyme 2 (ACE2) (Netland et al., 2008). Human coronavirus OC43 (HCoV-OC43) can also infect the olfactory bulb of mice and spread through nerve pathways to the brain and spinal cord (St-Jean et al., 2004; Desforges et al., 2020). A full brain infection of HCV-OC43 may occur within 7 days and nerve cells (neurons and glial cells) can be influenced by the virus (St-Jean et al., 2004). The flu A virus can also reach the respiratory centers in the brainstem via the vagus nerve (Pivot et al., 2001).
Pathway via the blood
The SARS-CoV-1, HCoV-OC43, and SARS-CoV-2 viruses can also infect the brain through the bloodstream (Desforges et al., 2020). After a viral infection of the respiratory tract, large amounts of cytokines and chemokines are released, causing the permeability of the blood-brain barrier (BBB) that can allow SARS-CoV-1 to spread (McCray et al., 2007). MERS-CoV can also infect the CNS using the leukocytes as Trojan horses because dipeptidyl-peptidase 4 (DPP4) is the MERS-CoV receptor expressed by activated leukocytes (Zhao et al., 2015). The hypothesis that MERS-CoV reaches the brain via the hematogenous pathway is confirmed by symptoms, such as seizure, encephalitis, and stroke, 2 to 3 weeks after acute respiratory distress syndrome (ARDS) (Kim et al., 2017). On the other hand, the hypothesis that infection spreads via the bloodstream is controversial during the first few days after infection. SARS-CoV-1 and MERS-CoV are not found in non-neurons in the brain, indicating a more direct route for these viruses to reach the brain (Li et al., 2020).
Target cells
Like the SARS-CoV-1 virus, the SARS-CoV-2 cellular receptor is the ACE2 (Wrapp et al., 2020; Hoffmann et al., 2020). This enzyme is highly expressed in various organs. The entry of the virus through the olfactory bulb causes inflammatory reactions and cytokine storms, eventually leading to anosmia and encephalitis. When the virus enters the eye, it causes conjunctivitis in the eyes, spreads through the tears, and is transmitted to various organs through the nasolacrimal system. Infection of the heart with this virus can lead to necrotic lipid formation and if ruptured, it can cause blood clots and myocardial infarction. The virus enters the kidney using the ACE2 receptors, causing deposition of the extracellular matrix, fibrosis, diuresis, and proliferation of kidney cells, resulting in acute kidney injury. (Harmer et al., 2002). Viral affects the liver by activating kupffer cells, which trigger an inflammatory reaction and activate hepatic stellate cells and hepatocytes, leading to pyroptosis and fibrosis. In the lungs, the virus causes damage to the walls of the alveolar cells and the formation of debris that causes thickening of the alveolar cell walls, lung damage, and shortness of breath that are common in COVID-19 (Figure 2) (Hamming et al., 2004).

Also, ACE2-mRNA and protein have been discovered in nuclei involved in the central regulation of cardiovascular function, such as the cardio‐respiratory neurons of the brainstem and the motor cortex, hypothalamus, and raphe (Harmer et al., 2002; Hamming et al., 2004; Xia & Lazartigues, 2008). It is yet to be determined what types of cells express ACE2 (Xia & Lazartigues, 2008), but animal studies show that this enzyme is mainly expressed by neurons; therefore, neurons may be directly infected with SARS-CoV-1 and SARS-CoV-2.
Cytokine storm and central nervous system pathophysiology
HCoV infection may affect the CNS in two ways, including viral replication in infected cells or neuro immunopathology. Activation of the immune system and macrophages by the SARS-CoV-2 causes the secretion of large amounts of cytokines and chemokines, which systemic inflammation caused by the cytokine storm leads to brain damage (Xu et al., 2005; Li et al., 2016b). In the same way, studies have shown increased interleukin (IL-) 6, 8, and 10 and of TNF-α in the blood of patients with SARS-CoV-2 (Figure 3) (Li et al., 2016b; Chen et al., 2019; Chen et al., 2020b). Also, inflammation in these patients, in addition to respiratory failure, causes hypoxic encephalopathy, which indicates the effect of SARS-CoV-2 on the CNS (Chen et al., 2019).

Examination of patients with severe COVID-19 infection compared to patients with moderate infection, and in patients who died than survivors shows that inflammation plays a crucial role in disease progression and death after SARS-CoV-2 infection (Chen et al., 2020a). Furthermore, peripheral lymphopenia was observed in most COVID-19 patients irrespective of their clinical severity (Chen et al., 2019).
Post-mortem evaluation of brain tissue of patients who died from SARS-CoV-1 infection indicates foci of necrotic cell death, edema, glial scar, and infiltrated immune cells (macrophages and T lymphocytes) (Figure 3) (Gu et al., 2005; Xu et al., 2005; Guo et al., 2008). Therefore, HCoV infection can cause inflammation of the brain, but underlying mechanisms need further explanations.
To further investigate the effect of SARS-CoV-1 infection on the brain, expressing the Human ACE was examined in transgenic mice, and the results indicated that the virus invades the brain (Doobay et al., 2007; McCray et al., 2007; Netland et al., 2008). Because SARS-CoV-1 viral particles are detected in olfactory bulbs (the most infected region) and deeper brain areas, including the piriform and infralimbic cortices, basal ganglia, dorsal raphe, thalamic nuclei, and brainstem (Netland et al., 2008). Contrary to human studies, brain inflammation was observed in these mice without alteration in astrocyte density and increased microglia cell density (Netland et al., 2008) and marked increases in IL-6, CCL2, IFN-γ and CCL12 levels following infection with SARS-CoV-1 (McCray et al., 2007). Therefore, these studies confirm the hypothesis of brain inflammation caused by HCoV infection.
Mice infected with John Howard Muller (JHM) strain of mouse hepatitis virus (MHV) through intranasal inoculation show rapid activation of microglia cells, phagocytosis of infected cells, and secretion of cytokines, including, IFN-α/β, CCL2, TNFα, and IL-6 (Wheeler et al., 2018). Thus, regardless of the virus strain, the brain’s immune response to the CoV infection is similar, indicating the critical role of microglia in the early stages of the immune response against the virus. Because the reduced microglia increase the proliferation and spread of the virus in the brain of infected mice (Lichanska et al., 1999; Wheeler et al., 2018). Microglia also activates T lymphocytes after infection (Wheeler et al., 2018). For example, by increasing the permeability of the blood-brain barrier (BBB) by metalloproteinases secreted by neutrophils and macrophages, CD4+ and CD8+ T lymphocytes can affect the CNS (Skinner et al., 2019). Subsequent studies of JHM of mouse hepatitis virus (MHV) inoculation also show both types of lymphocytes and increased cell infiltration in the brains of these mice (Phares et al., 2013). CD8+ T cells play a crucial role in controlling and clearing the virus, but T cells enhance the immune response by releasing IFN-γ and stimulating the expression of major histocompatibility complex (MHC) proteins in microglial cells (Wheeler et al., 2018). Such mechanisms are likely to be stimulated to increase viral clearance since it requires both major histocompatibility complex (MHC) 1 and 2 expression by immunocompetent cells (Skinner et al., 2019). After JHMV infection, an inflammatory reaction is initiated by two cytokines.
Evidence shows that C-X-C motif chemokine 10 (CXCL10) acts as a sentinel in the CNS because its release attracts T lymphocytes (Skinner et al., 2019), while IL-21 can improve both B and T lymphocyte responses (Phares et al., 2013). Additionally, CD4+ regulatory T cells (Treg) are activated when the inflammatory response reaches its peak and modulates the immune response (de Aquino et al., 2013). Therefore, Treg is essential to prevent damage caused by neuroinflammation and virus replication in CNS (de Aquino et al., 2013; Quarantelli, 2015). Therefore, evidence suggests that inhibiting inflammation of the brain can prevent or reduce CoV-induced neuropathologies.
After brain damage or infection, the first cells to be activated to maintain brain homeostasis and minimize tissue destruction are microglia (Hanisch & Kettenmann, 2007; Ransohoff & Perry, 2009), which produce and release several inflammatory chemomediators (pro-inflammatory cytokines and chemokines) (Ransohoff & Perry, 2009; Chatterjee et al., 2013), modulate inflammatory responses between the CNS and the peripheral immune system and as phagocytosis, they can clear damaged cells and cellular debris and present antigens to T lymphocytes (Wheeler et al., 2018). After brain homeostasis is reestablished, the activity of microglia decreases. Therefore, the consequences of overreacting microglia following SARS-CoV-2 infection can be particularly harmful since cytokine storm (a severely inflammatory immune response) is associated with COVID-19.
Cognitive functions after cytokine storm in COVID-19
The brain’s immune cells (microglia and cytokines) produce high levels of pro-inflammatory factors in various brain regions (Russo et al., 2010), which are harmful to brain cells. Acute or chronic inflammatory processes that induce the release of neurotoxic products such as reactive oxygen species (ROS) and certain damaging enzymes can damage brain tissue (Blasko et al., 2004; Raz & Rodrigue, 2006). Limbic and associated brain structures, such as hippocampi, prefrontal cortex, and basal ganglia (structures that play essential roles in cognitive processes, such as memory, attention, emotion, and perception) have more enzymes involved in an inflammatory response than primary motor or sensory cortices; therefore, these areas may be more affected by the destructive effects of inflammatory processes (Raz & Rodrigue, 2006; Wang et al., 2015).
Plenty of studies have shown that inflammation activation leads to cognitive deficits, suggesting a prominent role of IL-1β, IL-6, IL-18, and TNF-α (Duarte et al., 2017; Magalhaes et al., 2018; Shen et al., 2019; Chakrabarty et al., 2019). On the other hand, neurocognitive impairments are common in patients with viral infections because inflammation may also be present after clearing the virus (Peiris et al., 2003). For example, a follow-up interval ranging from 6 and 39 months after recovery from SARS and MERS showed impairment in memory, attention, concentration, or mental processing speed in more than 15% of patients (Sheng et al., 2005b; Lam et al., 2009; Bechter, 2013; Kepinska et al., 2020; Rogers et al., 2020).
Therefore, due to increased T helper (Th)-1 cytokines (IL-1β, IL-6, IL-8, IFN-γ, TNF-α, C-X-C motif chemokine 10 [CXCL10], and CCL2), and Th-2 cytokines (IL-4, IL-10, and IL-1 receptor antagonist) in the serum of COVID-19 patients (Channappanavar & Perlman, 2017; Chen et al., 2019, Chen et al., 2020a; Coperchini et al., 2020; Mazza et al., 2021), the possible relationship between inflammatory status and cognitive function in patients with COVID-19 should be investigated.
A functional neuroimaging study showed that increased IL-6 reduced the functional connectivity between the striatum and ventromedial prefrontal cortex (vmPFC), weakened prefrontal cortex control over the striatum, causing cognitive dysfunction, anhedonia, verbal memory deficit, and motor slowing (Lin et al., 2020; Felger et al., 2016). Studies in the elderly also indicate a positive relationship between plasma IL-6 level and cognitive deficits, such as prospective memory, working memory, executive functioning, processing speed, attention, orientation, immediate verbal recall, delayed recall or psychomotor speed, semantic fluency (Simpson et al., 2013; Heringa et al., 2014).
Increased TNF-α levels can cause neuronal damage directly via activation of apoptosis and increased glutamate-mediated excitotoxicity (Bortolato et al., 2015; Muneer, 2016; Chakrabarty et al., 2019), and inhibit hippocampal long-term potentiation. Also, elevated TNF-α receptor signaling may lead to brain atrophy, hippocampal neuronal lack, and increased risk of cognitive deficits, such as impaired verbal memory, learning, synaptic plasticity, and inhibitory control (Sudheimer et al., 2014; Bortolato et al., 2015; Chakrabarty et al., 2019).
Higher than normal levels of IL-8 cause impaired memory and motor function, as well as slower cognitive and perceptual speed (Baune et al., 2008; Alley et al., 2008).
Studies showed that IL-1β plays a key role in developing hippocampal-dependent learning and memory (Balschun et al., 2003; del Rey et al., 2013), but overexpression of IL-1β in the hippocampus can also negatively affect spatial memory (Moore et al., 2009; Spulber et al., 2009b).
Also, various studies showed increased pro-inflammatory (IL-1β, IL-6, TNF-α) and anti-inflammatory cytokines (IL-1ra, IL-10) in the CSF and plasma of patients with depression, schizophrenia, bipolar disorder, and Alzheimer (Levine et al., 1999; Dentino et al., 1999; Kim et al., 2007). In these patients, previous studies have found a correlation between peripheral IL-8, TNF-α, CCL2, CCL4, and brain thickness (Wan et al., 2017; Poletti et al., 2019), of IL-1β, IL-9, CCL5 with brain glutamate, N-acetyl aspartate, and myo-inositol levels (Poletti et al., 2020), and of IL-8, IL-10, TNF-α, IFN-γ with white matter (WM) microstructure, with levels of inflammatory cytokines being inversely related with measures of WM integrity (Benedetti et al., 2016). Also, a relationship is observed between this WM phenotype and cognitive impairments, such as working memory, verbal memory, inhibitory control, executive function, information processing speed, attention, and psychomotor coordination (Saczynski et al., 2010; Belarbi et al., 2012; Gabbita et al., 2012; Li et al., 2013; Sahin et al., 2015; Rosenblat et al., 2015; Poletti et al., 2015; Felger et al., 2016). Therefore, due to increased inflammatory cytokines in patients with COVID-19 and their effects on structural and functional brain connectivities, we can expect the mentioned cognitive impairments in these patients as well.
Few studies have examined cognitive function in these patients using cognitive tasks. For example, studies conducted by Almeria et al using test de Aprendizaje verbal Espa~na Complutense with three lists for the learning, interference, and recognition to assess verbal memory; visual reproduction of the Wechsler memory scale–IV), digits forward and backward, letter and numbers, trail making test A and B (TMT), symbol digit modalities test, Stroop, phonetic and semantic fluency and Boston naming test from the NEURONORMA project (NN) showed that COVID-19 patients acquired lower scores in memory domains, attention, semantic fluency in working memory, and mental flexibility, and phonetic fluency, and executive function subtests (Almeria et al., 2020).
Zhou et al. have conducted a study to evaluate COVID-19’s effects on cognitive functions and its association with inflammatory markers in recovered patients. They evaluated the cognitive functions of 29 subjects by the iPad-based online neuropsychological tests, including the trail-making test (TMT), sign coding test, continuous performance test (CPT), and digital span test (DST). In their study, only some parts of the continuous performance test (CPT) showed significant group differences, indicating cognitive deficits, especially sustained attention domain in patients recovered from COVID-19 (Zhou et al., 2020).
In a sample of 130 patients, Mazza et al. examined cognitive functions using the brief assessment of cognition in Schizophrenia (Mazza et al., 2021), a broad battery evaluating verbal memory, verbal fluency, working memory (digit sequencing), selective attention and processing speed (symbol coding), psychomotor coordination (token motor task), and executive functions (Tower of London). According to previous studies, regardless of illness severity, most COVID-19 survivors showed cognitive impairments after 3 months, 78% of the sample showed poor performances in all the investigated domains. Executive functions and psychomotor coordination were impaired in 50% and 57% of the sample; information processing, verbal fluency, and working memory were impaired in around 30% of the sample (Mazza et al., 2021). The cognitive deficits observed in these patients were affected by systemic inflammation, which confirms the relationship between inflammation and cognitive deficits.
Therefore, it can be concluded that COVID-19 causes systemic inflammation and cognitive deficits in patients.
As a result of neuroinflammation, neurotransmitter metabolism, neuroplasticity, and brain structure and function are altered, the microglia are activated, and the hypothalamic-pituitary-adrenal (HPA) axis is dysregulated, which is the main component of the endocrinal system of the stress response and may affect cognition, memory task, and behavior (Tay et al., 2018). Evidence shows that psychological stressors may increase the production of proinflammatory cytokines, such as IL-1, IL-6, TNF-a, and IFN-g in humans and experimental animals. These cytokines can stimulate the HPA axis and lead to cognitive impairments caused by stress (Ownby, 2010). Therefore, inflammation may act as a mediator of stress-induced cognitive dysfunction; therefore, with the increase of those factors, the probability of cognitive deficits due to stress increases.
Stress triggers an immune response via the HPA axis, the system involved in releasing the cortisol hormone. Numerous studies demonstrate that high cortisol levels lead to memory problems through effects on the hippocampal (Ownby, 2010), and stress-induced hippocampal damage has been demonstrated in animal experiments (Sapolsky, 1996).
On the other hand, a large number of recovered COVID-19 patients, healthy people, and medical staff usually suffer from stress for a short period but may experience prolonged psychophysical symptoms, such as depression, anxiety, and fear (Wang et al., 2020; Huang et al., 2020; Balachandar, 2020). Studies also show that in specific situations, the brain’s immune system may be chronically triggered. Especially, it is mentioned that chronic stress, trauma, neurodegenerative diseases, and aging cause changes in microglia’s nature and protective capabilities (known as “microglial priming”) (Norden et al., 2015; Fonken et al., 2018), which is associated with high release of pro-inflammatory cytokines, such as TNF-a and IL-6, chemokines and reactive oxygen species (ROS) (Ransohoff & Perry, 2009; Chatterjee et al., 2013) for a long time. Increased microglial activity can cause extended lesions and impaired brain function (Norden et al., 2015). Therefore, systemic inflammation caused by stress in COVID-19 patients may explain the pathogenesis of cognitive dysfunction, especially memory impairment in these patients.
2. Conclusion
Evidence demonstrates that the SARS-CoV-2 virus can infect the brain and lead to neurological complications in patients. In this state, the brain’s immune cells (microglia and cytokines) produce high levels of pro-inflammatory factors in various brain regions (Russo et al., 2010), which can damage brain cells.
Several studies have concluded that systemic inflammation accompanied by a significant release of cytokines (known as “cytokine storm”) causes brain damage due to SARS-CoV-1 (Xu et al., 2005; Li et al., 2016b). In such a way that recent studies show an increase in the level of proinflammatory cytokines, such as IL-1,6,8,10 and TNF-α factors in the brains of people with COVID-19 (Channappanavar & Perlman, 2017; Chen et al., 2019, Chen et al., 2020a). Studies show that higher than normal levels of IL-8 cause impaired memory and motor function, as well as slower cognitive and perceptual speed (Baune et al., 2008; Alley et al., 2008; Li et al., 2013; Mun et al., 2016).
Also, other results indicate a positive relationship between plasma IL-6 level and cognitive deficits, such as prospective memory, working memory, executive functioning, processing speed, attention, orientation, immediate verbal recall, delayed recall or psychomotor speed, semantic fluency (Li et al., 2013; Simpson et al., 2013; Heringa et al., 2014; Rosenblat et al., 2015; Mun et al., 2016).
Considering that limbic and associated brain structures, such as the amygdala, anterior cingulate cortex, hippocampi, prefrontal cortex, and basal ganglia (structures that play essential roles in cognitive processes like memory, attention, emotion, and perception) contain many enzymes and cytokines involved in an inflammatory response (Raz & Rodrigue, 2006; Sudheimer et al., 2014; Rosenblat et al., 2015; Wang et al., 2015; Bortolato et al., 2015; Elias et al., 2017; Acuff et al., 2018); therefore, according to the available evidence, there is a probability of occurrence of these cognitive defects in these patients, which needs further investigation.
On the other hand, various cognitive defects, such as attention, working memory, executive function, processing speed impairments, anhedonia, and motor slowing deficit disorder have been observed following increased levels of cytokines, such as TNF-α and IL-6 in major depressive disorder, bipolar disorder, and schizophrenia patients (Saczynski et al., 2010; Belarbi et al., 2012; Gabbita et al., 2012; Li et al., 2013; Sahin et al., 2015; Rosenblat et al., 2015; Felger et al., 2016; Kępińska et al., 2020). Therefore, these results can confirm previous results based on the occurrence of these cognitive impairments in these patients following cytokine storms.
According to the above evidence based on the occurrence of cytokine storms and their effects in areas involved in cognitive processes, such as limbic and associated brain structures, we can expect cognitive deficits in cured patients with COVID-19. Therefore, the possible association between inflammatory status and cognitive function in patients with COVID-19 should be investigated over a long period to provide the best therapeutic strategies for COVID-19 survivors (Benedetti et al., 2020b).
Therefore, it is suggested that electrophysiological tools, such as quantitative electroencephalography, event-related potential, neurofeedback, and electrical stimulation of the brain, including transcranial stimulation with direct electric current, transcranial random noise stimulation, and transcranial electrical stimulation with alternating current, be used in future to evaluate and intervene effectively to improve cognitive abilities, such as memory, learning, attention, processing speed in COVID-19 recoveries (Chabot et al., 2001; Monastra et al., 2002; Vourvopoulos & Badia. 2016; Moon et al., 2019; Harris et al., 2020).
Also, modulation of pro and anti-inflammatory cytokines may prevent cognitive impairment and improve brain function (Torres et al., 2010).
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Study design, Drafting the manuscript: Gholamreza Chalabianloo and Zahra Keshtgar; Review and editing: Gholamreza Chalabianloo and Niloofar Esmaeili; Final approval: All authors.
Conflict of interest
All authors declared no conflict of interest.
Reference
Acuff, H. E., Versace, A., Bertocci, M. A., Ladouceur, C. D., Hanford, L. C., & Manelis, A., et al. (2018). Association of neuroimaging measures of emotion processing and regulation neural circuitries with symptoms of bipolar disorder in offspring at risk for bipolar disorder. JAMA Psychiatry, 75(12), 1241-1251. [DOI:10.1001/jamapsychiatry.2018.2318] [PMID]
Algahtani, H., Subahi, A., & Shirah, B. (2016). Neurological complications of Middle East respiratory syndrome coronavirus: A report of two cases and review of the literature. Case Reports in Neurological Medicine, 2016, 3502683.[DOI:10.1155/2016/3502683] [PMID]
Alley, D. E., Crimmins, E. M., Karlamangla, A., Hu, P., & Seeman, T. E. (2008). Inflammation and rate of cognitive change in high-functioning older adults. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 63(1), 50-55. [DOI:10.1093/gerona/63.1.50] [PMID]
Almeria, M., Cejudo, J. C., Sotoca, J., Deus, J., & Krupinski, J. (2020). Cognitive profile following COVID-19 infection: Clinical predictors leading to neuropsychological impairment. Brain, Behavior, & Immunity-Health, 9, 100163. [DOI:10.1016/j.bbih.2020.100163] [PMID]
Arbour, N., Day, R., Newcombe, J., & Talbot, P. J. (2000). Neuroinvasion by human respiratory coronaviruses. Journal of Virology, 74(19), 8913-8921. [DOI:10.1128/JVI.74.19.8913-8921.2000] [PMID]
Balachandar, V., Mahalaxmi, I., Subramaniam, M., Kaavya, J., Senthil Kumar, N., & Laldinmawii, G., et al. (2020). Follow-up studies in COVID-19 recovered patients-is it mandatory?. The Science of the Total Environment, 729, 139021. [PMID]
Balschun, D., Randolf, A., Pitossi, F., Schneider, H., Del Rey, A., & Besedovsky, H. O. (2003). Hippocampal interleukin‐1β gene expression during long‐term potentiation decays with age. Annals of the New York Academy of Sciences, 992, 1–8. [DOI:10.1111/j.1749-6632.2003.tb03132.x] [PMID]
Baune, B. T., Ponath, G., Golledge, J., Varga, G., Arolt, V., & Rothermundt, M., et al. (2008). Association between IL-8 cytokine and cognitive performance in an elderly general population-the MEMO-Study. Neurobiology of Aging, 29(6), 937-944. [PMID]
Belarbi, K., Jopson, T., Tweedie, D., Arellano, C., Luo, W., & Greig, N. H., et al. (2012). TNF-α protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. Journal of Neuroinflammation, 9, 23. [DOI:10.1186/1742-2094-9-23] [PMID]
Bechter, K. (2013). Virus infection as a cause of inflammation in psychiatric disorders. Modern Trends in Pharmacopsychiatry, 28, 49–60. [PMID]
Benedetti, F., Mazza, M., Cavalli, G., Ciceri, F., Dagna, L., & Rovere-Querini, P. (2021). Can cytokine blocking prevent depression in covid-19 survivors?. Journal of Neuroimmune Pharmacology, 16(1), 1–3. [DOI:10.1007/s11481-020-09966-z] [PMID]
Benedetti, F., Poletti, S., Hoogenboezem, T. A., Mazza, E., Ambrée, O., & de Wit, H., et al. (2016). Inflammatory cytokines influence measures of white matter integrity in bipolar disorder. Journal of Affective Disorders, 202, 1-9. [DOI:10.1016/j.jad.2016.05.047]
Blasko, I., Beer, R., Bigl, M., Apelt, J., Franz, G., & Rudzki, D., et al. (2004). Experimental traumatic brain injury in rats stimulates the expression, production and activity of Alzheimer’s disease β-secretase (BACE-1). Journal of Neural Transmission, 111(4), 523–536. [PMID]
Bortolato, B., Carvalho, A. F., Soczynska, J. K., Perini, G. I., & McIntyre, R. S. (2015). The involvement of TNF-α in cognitive dysfunction associated with major depressive disorder: An opportunity for domain specific treatments. Current Neuropharmacology, 13(5), 558-576. [DOI:10.2174/1570159X13666150630171433] [PMID]
Butler, N., Pewe, L., Trandem, K., & Perlman, S. (2006). Murine encephalitis caused by HCoV-OC43, a human coronavirus with broad species specificity, is partly immune-mediated. Virology, 347(2), 410-421. [DOI:10.1016/j.virol.2005.11.044] [PMID]
Chabot, R. J., di Michele, F., Prichep, L., & John, E. R. (2001).The clinical role of computerized EEG in the evaluation and treatment of learning and attention disorders in children and adolescents. The Journal of Neuropsychiatry and Clinical Neurosciences, 13(2), 171-186. [DOI:10.1176/jnp.13.2.171] [PMID]
Chakrabarty, T., Torres, I. J., Bond, D. J., & Yatham, L. N. (2019).Inflammatory cytokines and cognitive functioning in early-stage bipolar I disorder. Journal of Affective Disorders, 245, 679-685. [DOI:10.1016/j.jad.2018.11.018] [PMID]
Channappanavar, R., & Perlman, S. (2017). Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Seminars in Immunopathology, 39(5), 529–539. [DOI:10.1007/s00281-017-0629-x] [PMID]
Chatterjee, D., Biswas, K., Nag, S., Ramachandra, S. G., & Das Sarma, J. (2013). Microglia play a major role in direct viral-induced demyelination. Clinical and Developmental Immunology, 2013, 510396. [DOI:10.1155/2013/510396] [PMID]
Chen, G., Wu, D., Guo, W., Cao, Y., Huang, D., & Wang, H., et al. (2020). Clinical and immunological features of severe and moderate coronavirus disease 2019. The Journal of Clinical Investigation, 130(5), 2620–2629. [PMID]
Chen, T., Wu, D., Chen, H., Yan, W., Yang, D., & Chen, G., et al. (2020). Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ (Clinical Research ed.), 368, m1091. [DOI:10.1136/bmj.m1091] [PMID]
Chen, N., Zhou, M., Dong, X., Qu, J., Gong, F., & Han, Y., et al. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet, 395(10223), 507-513. [DOI:10.1016/S0140-6736(20)30211-7] [PMID]
Coperchini, F., Chiovato, L., Croce, L., Magri, F., & Rotondi, M. (2020). The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine & Growth Factor Reviews, 53, 25-32. [DOI:10.1016/j.cytogfr.2020.05.003] [PMID]
Davidson, S., Maini, M. K., & Wack, A. (2015). Disease-promoting effects of type I interferons in viral, bacterial, and coinfections. Journal of Interferon & Cytokine Research, 35(4), 252-264. [PMID]
de Aquino, M. T., Puntambekar, S. S., Savarin, C., Bergmann, C. C., Phares, T. W., & Hinton, D. R., et al. (2013). Role of CD25+ CD4+ T cells in acute and persistent coronavirus infection of the central nervous system. Virology, 447(1-2), 112-120. [DOI:10.1016/j.virol.2013.08.030] [PMID]
del Rey, A., Balschun, D., Wetzel, W., Randolf, A., & Besedovsky, H. O. (2013). A cytokine network involving brain-borne IL-1β, IL-1ra, IL-18, IL-6, and TNFα operates during long-term potentiation and learning. Brain, Behavior, and Immunity, 33, 15-23. [DOI:10.1016/j.bbi.2013.05.011] [PMID]
Dentino, A. N., Pieper, C. F., Rao, M. K., Currie, M. S., Harris, T., & Blazer, D. G., et al. (1999). Association of interleukin‐6 and other biologic variables with depression in older people living in the community. Journal of the American Geriatrics Society, 47(1), 6-11. [DOI:10.1111/j.1532-5415.1999.tb01894.x] [PMID]
Desforges, M., Le Coupanec, A., Dubeau, P., Bourgouin, A., Lajoie, L., & Dubé, M., et al. (2019). Human coronaviruses and other respiratory viruses: Underestimated opportunistic pathogens of the central nervous system? Viruses, 12(1), 14. [DOI:10.3390/v12010014] [PMID]
Doobay, M. F., Talman, L. S., Obr, T. D., Tian, X., Davisson, R. L., & Lazartigues, E. (2007). Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 292(1), R373-R381. [PMID]
Duarte, P. O., Duarte, M. G. F., Pelichek, A., Pfrimer, K., Ferriolli, E., & Moriguti, J. C., et al. (2017). Cardiovascular risk factors and inflammatory activity among centenarians with and without dementia. Aging Clinical and Experimental Research, 29(3), 411-417. [DOI:10.1007/s40520-016-0603-9] [PMID]
Elias, L. R., Miskowiak, K. W., Vale, A. M., Köhler, C. A., Kjærstad, H. L., & Stubbs, B., et al. (2017). Cognitive impairment in euthymic pediatric bipolar disorder: A systematic review and meta-analysis. Journal of the American Academy of Child & Adolescent Psychiatry, 56(4), 286-296. [DOI:10.1016/j.jaac.2017.01.008] [PMID]
Felger, J. C., Li, Z., Haroon, E., Woolwine, B. J., Jung, M. Y., & Hu, X., et al. (2016). Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Molecular Psychiatry, 21(10), 1358-1365. [DOI:10.1038/mp.2015.168] [PMID]
Filatov, A., Sharma, P., Hindi, F., & Espinosa, P. S. (2020). Neurological complications of coronavirus disease (COVID-19): Encephalopathy. Cureus, 12(3). [DOI:10.7759/cureus.7352]
Fonken, L. K., Frank, M. G., Gaudet, A. D., & Maier, S. F. (2018). Stress and aging act through common mechanisms to elicit neuroinflammatory priming. Brain, Behavior, and Immunity, 73, 133-148. [DOI:10.1016/j.bbi.2018.07.012] [PMID]
Gabbita, S. P., Srivastava, M. K., Eslami, P., Johnson, M. F., Kobritz, N. K., & Tweedie, D., et al. (2012). Early intervention with a small molecule inhibitor for tumor nefosis factor-α prevents cognitive deficits in a triple transgenic mouse model of Alzheimer’s disease. Journal of Neuroinflammation, 9, 99. [DOI:10.1186/1742-2094-9-99] [PMID]
Gu, J., Gong, E., Zhang, B., Zheng, J., Gao, Z., & Zhong, Y., et al. (2005). Multiple organ infection and the pathogenesis of SARS. The Journal of experimental medicine, 202(3), 415–424.[DOI:10.1084/jem.20050828] [PMID]
Guan, W. J., Ni, Z. Y., Hu, Y., Liang, W. H., Ou, C. Q., & He, J. X., et al. (2020). Clinical characteristics of coronavirus disease 2019 in China. The New England Journal of Medicine, 382, 1708-1720. [Link]
Guo, Y., Korteweg, C., McNutt, M. A., & Gu, J. (2008). Pathogenetic mechanisms of severe acute respiratory syndrome. Virus Research, 133(1), 4-12. [DOI:10.1016/j.virusres.2007.01.022] [PMID]
Hamming, I., Timens, W., Bulthuis, M. L., Lely, A. T., Navis, G., & van Goor, H. (2004). Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology, 203(2), 631-637. [DOI:10.1002/path.1570] [PMID]
Hanisch, U. K., & Kettenmann, H. (2007). Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nature Neuroscience, 10(11), 1387-1394. [DOI:10.1038/nn1997] [PMID]
Harris, A. M., Jacoby, O., Remington, R. W., Becker, S. I., & Mattingley, J. B. (2020). Behavioral and electrophysiological evidence for a dissociation between working memory capacity and feature-based attention. Cortex; A Journal Devoted to the Study of the Nervous System and Behavior, 129, 158–174. [DOI:10.1016/j.cortex.2020.04.009] [PMID]
Harmer, D., Gilbert, M., Borman, R., & Clark, K. L. (2002). Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Letters, 532(1-2), 107-110. [DOI:10.1016/S0014-5793(02)03640-2] [PMID]
Hawkins, R. B., Raymond, S. L., Stortz, J. A., Horiguchi, H., Brakenridge, S. C., & Gardner, A., et al. (2018). Chronic critical illness and the persistent inflammation, immunosuppression, and catabolism syndrome. Frontiers in Immunology, 9, 1511.[DOI:10.3389/fimmu.2018.01511] [PMID]
Heringa, S. M., van den Berg, E., Reijmer, Y. D., Nijpels, G., Stehouwer, C. D., & Schalkwijk, C. G., et al. (2014). Markers of low-grade inflammation and endothelial dysfunction are related to reduced information processing speed and executive functioning in an older population-the Hoorn study. Psychoneuroendocrinology, 40, 108-118. [DOI:10.1016/j.psyneuen.2013.11.011] [PMID]
Hoffmann, M., Kleine-Weber, H., Schroeder, S., Krüger, N., Herrler, T., & Erichsen, S., et al. (2020). SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181(2), 271–280.e8. [DOI:10.1016/j.cell.2020.02.052] [PMID]
Huang, J. Z., Han, M. F., Luo, T. D., Ren, A. K., & Zhou, X. P. (2020). [Mental health survey of 230 medical staff in a tertiary infectious disease hospital for COVID-19 (Chinese)]. Chinese Journal of Industrial Hygiene and Occupational Diseases, 38(3), 192–195. [PMID]
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., & Hu, Y., et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England), 395(10223), 497–506. [DOI:10.1016/S0140-6736(20)30183-5] [PMID]
Jiang, F., Deng, L., Zhang, L., Cai, Y., Cheung, C. W., & Xia, Z. (2020). Review of the clinical characteristics of coronavirus disease 2019 (COVID-19). Journal of General Internal Medicine, 35(5), 1545–1549. [DOI:10.1007/s11606-020-05762-w] [PMID]
Kępińska, A. P., Iyegbe, C. O., Vernon, A. C., Yolken, R., Murray, R. M., & Pollak, T. A. (2020). Schizophrenia and influenza at the centenary of the 1918-1919 Spanish influenza pandemic: mechanisms of psychosis risk. Frontiers in psychiatry, 11, 72. [DOI:10.3389/fpsyt.2020.00072] [PMID]
Khosravani, H., Rajendram, P., Notario, L., Chapman, M. G., & Menon, B. K. (2020). Protected code stroke: Hyperacute stroke management during the coronavirus disease 2019 (COVID-19) pandemic. Stroke, 51(6), 1891-1895. [DOI:10.1161/STROKEAHA.120.029838] [PMID]
Kim, Y. K., Jung, H. G., Myint, A. M., Kim, H., & Park, S. H. (2007). Imbalance between pro-inflammatory and anti-inflammatory cytokines in bipolar disorder. Journal of Affective Disorders, 104(1-3), 91-95. [DOI:10.1016/j.jad.2007.02.018] [PMID]
Kim, J. E., Heo, J. H., Kim, H. O., Song, S. H., Park, S. S., & Park, T. H., et al. (2017). Neurological complications during treatment of middle east respiratory syndrome. Journal of Clinical Neurology, 13(3), 227-233. [DOI:10.3988/jcn.2017.13.3.227] [PMID]
Kinsinger, L. S., Anderson, C., Kim, J., Larson, M., Chan, S. H., & King, H. A., et al. (2017). Implementation of lung cancer screening in the Veterans Health Administration. JAMA Internal Medicine, 177(3), 399-406. [DOI:10.1001/jamainternmed.2016.9022] [PMID]
Kuiken, T., Fouchier, R. A., Schutten, M., Rimmelzwaan, G. F., van Amerongen, G., & van Riel, D., et al. (2003). Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet (London, England), 362(9380), 263–270. [DOI:10.1016/S0140-6736(03)13967-0] [PMID]
Lam, T. T., Jia, N., Zhang, Y. W., Shum, M. H., Jiang, J. F., & Zhu, H. C., et al. (2020). IIdentifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature, 583(7815), 282–285. [PMID]
Leung, W. K., To, K. F., Chan, P. K., Chan, H. L., Wu, A. K., & Lee, N., et al. (2003). Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology, 125(4), 1011-1017. [PMID]
Lam, M. H., Wing, Y. K., Yu, M. W., Leung, C. M., Ma, R. C., & Kong, A. P., et al. (2009). Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: Long-term follow-up. Archives of Internal Medicine, 169(22), 2142-2147. [DOI:10.1001/archinternmed.2009.384] [PMID]
Lau, K. K., Yu, W. C., Chu, C. M., Lau, S. T., Sheng, B., & Yuen, K. Y. (2004). Possible central nervous system infection by SARS coronavirus. Emerging Infectious Diseases, 10(2), 342–344.[DOI:10.3201/eid1002.030638] [PMID]
Levine, J., Barak, Y., Chengappa, K. N. R., Rapoport, A., Rebey, M., & Barak, V. (1999). Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology, 40(4), 171-176. [PMID]
Li, Y. C., Bai, W. Z., & Hashikawa, T. (2020). The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. Journal of Medical Virology, 92(6), 552-555. [DOI:10.1002/jmv.25728] [PMID]
Li, Y., Li, H., Fan, R., Wen, B., Zhang, J., & Cao, X., et al. (2016). Coronavirus infections in the central nervous system and respiratory tract show distinct features in hospitalized children. Intervirology, 59(3), 163-169. [DOI:10.1159/000453066] [PMID]
Li, B. H., Zhang, L. L., Yin, Y. W., Pi, Y., Guo, L., & Yang, Q. W., et al. (2013). Association between interleukin-1α C (− 889) T polymorphism and Alzheimer’s disease: A meta-analysis including 12,817 subjects. Journal of Neural Transmission, 120(3), 497-506. [DOI:10.1007/s00702-012-0867-y] [PMID]
Lichanska, A. M., Browne, C. M., Henkel, G. W., Murphy, K. M., Ostrowski, M. C., & McKercher, S. R., et al.(1999). Differentiation of the mononuclear phagocyte system during mouse embryogenesis: The role of transcription factor PU. 1. Blood, 94(1), 127–138. [DOI:10.1182/blood.V94.1.127.413k07_127_138] [PMID]
Lin, K., Shao, R., Wang, R., Lu, W., Zou, W., & Chen, K., et al. (2020). Inflammation, brain structure and cognition interrelations among individuals with differential risks for bipolar disorder. Brain, Behavior, and Immunity, 83, 192-199. [DOI:10.1016/j.bbi.2019.10.010] [PMID]
Lin, L., Zheng, L. J., & Zhang, L. J. (2018). Neuroinflammation, gut microbiome, and Alzheimer’s disease. Molecular Neurobiology, 55(11), 8243-8250. [DOI:10.1007/s12035-018-0983-2] [PMID]
Liu, Z., Xiao, X., Wei, X., Li, J., Yang, J., & Tan, H., et al. (2020). Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS‐CoV‐2. Journal of Medical Virology, 92(6), 595-601. [DOI:10.1002/jmv.25726] [PMID]
Magalhães, R. C., Pimenta, L. P., Barbosa, I. G., Moreira, J. M., de Barros, J. L. V. M., & Teixeira, A. L., et al. (2018). Inflammatory molecules and neurotrophic factors as biomarkers of neuropsychomotor development in preterm neonates: A Systematic Review. International Journal of Developmental Neuroscience, 65, 29-37. [DOI:10.1016/j.ijdevneu.2017.10.006] [PMID]
Mao, L., Jin, H., Wang, M., Hu, Y., Chen, S., & He, Q., et al. (2020). Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurology, 77(6), 683-690. [DOI:10.1001/jamaneurol.2020.1127] [PMID]
Mao, L., Wang, M., Chen, S., He, Q., Chang, J., & Hong, C., et al. (2020). Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: A retrospective case series study. MedRxiv. [DOI:10.1101/2020.02.22.20026500]
Matoba, Y., Abiko, C., Ikeda, T., Aoki, Y., Suzuki, Y., & Yahagi, K., et al. (2015). Detection of the human coronavirus 229E, HKU1, NL63, and OC43 between 2010 and 2013 in Yamagata, Japan. Japanese Journal of Infectious Diseases, 68(2), 138-141. [DOI:10.7883/yoken.JJID.2014.266] [PMID]
Mazza, M. G., Palladini, M., De Lorenzo, R., Magnaghi, C., Poletti, S., & Furlan, R., et al. (2021). Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain, Behavior, and Immunity, 94, 138–147. [DOI:10.1016/j.bbi.2021.02.021] [PMID]
McCray, P. B., Jr, Pewe, L., Wohlford-Lenane, C., Hickey, M., Manzel, L., & Shi, L., et al. (2007). Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. Journal of Virology, 81(2), 813-821. [DOI:10.1128/JVI.02012-06] [PMID]
Monastra, V. J., Monastra, D. M., & George, S. (2002). The effects of stimulant therapy, EEG biofeedback, and parenting style on the primary symptoms of attention-deficit/hyperactivity disorder. Applied Psychophysiology and Biofeedback, 27(4), 231-249. [DOI:10.1023/A:1021018700609] [PMID]
Moon, S. Y., Kim, M., Hwang, W. J., Lee, T. Y., & Kwon, J. S. (2019). A pilot study investigating the effect of transcranial direct current stimulation on the electrophysiological correlates of working memory in patients with schizophrenia. Psychiatry Research. Neuroimaging, 284, 9-12. [DOI:10.1016/j.pscychresns.2018.12.014] [PMID]
Moore, A. H., Wu, M., Shaftel, S. S., Graham, K. A., & O'Banion, M. K. (2009). Sustained expression of interleukin-1β in mouse hippocampus impairs spatial memory. Neuroscience, 164(4), 1484-1495. [DOI:10.1016/j.neuroscience.2009.08.073] [PMID]
Moriguchi, T., Harii, N., Goto, J., Harada, D., Sugawara, H., & Takamino, J., et al. (2020). A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. International Journal of Infectious Diseases, 94, 55–58. [DOI:10.1016/j.ijid.2020.03.062] [PMID]
Mun, M. J., Kim, J. H., Choi, J. Y., & Jang, W. C. (2016). Genetic polymorphisms of interleukin genes and the risk of Alzheimer’s disease: An update meta-analysis. Meta Gene, 8, 1-10. [DOI:10.1016/j.mgene.2016.01.001] [PMID]
Muneer, A. (2016). Bipolar disorder: Role of inflammation and the development of disease biomarkers. Psychiatry Investigation, 13(1), 18-33. [DOI:10.4306/pi.2016.13.1.18] [PMID]
Myint, S. H. (1995). Human coronavirus infections. In: S. G. Siddell (Ed.), The coronaviridae (pp. 389-401).Boston: Springer. [DOI:10.1007/978-1-4899-1531-3_18]
Netland, J., Meyerholz, D. K., Moore, S., Cassell, M., & Perlman, S. (2008). Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. Journal of Virology, 82(15), 7264-7275. [DOI:10.1128/JVI.00737-08] [PMID]
Nicholls, J., Dong, X. P., Jiang, G., & Peiris, M. (2003). SARS: Clinical virology and pathogenesis. Respirology, 8 Suppl(Suppl 1), S6–S8.[DOI:10.1046/j.1440-1843.2003.00517.x] [PMID]
Norden, D. M., Muccigrosso, M. M., & Godbout, J. P. (2015). Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology, 96(Pt A), 29–41. [DOI:10.1016/j.neuropharm.2014.10.028] [PMID]
Ownby, R. L. (2010). Neuroinflammation and cognitive aging. Current Psychiatry Reports, 12(1), 39–45. [PMID]
Paniz-Mondolfi, A., Bryce, C., Grimes, Z., Gordon, R. E., Reidy, J., & Lednicky, J., et al. (2020). Central nervous system involvement by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). Journal of Medical Virology, 92(7), 699-702. [DOI:10.1002/jmv.25915] [PMID]
Papa, S. M., Brundin, P., Fung, V. S. C., Kang, U. J., Burn, D. J., & Colosimo, C., et al. (2020). Impact of the COVID-19 pandemic on Parkinson’s disease and movement disorders. Movement Disorders Clinical Practice, 7(4), 357–360. [DOI:10.1002/mdc3.12953] [PMID]
Peiris, J. S., Chu, C. M., Cheng, V. C., Chan, K. S., Hung, I. F., & Poon, L. L., et al. (2003). Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: A prospective study. Lancet (London, England), 361(9371), 1767–1772. [DOI:10.1016/S0140-6736(03)13412-5] [PMID]
Peiris, J. S., Lai, S. T., Poon, L. L., Guan, Y., Yam, L. Y., & Lim, W., et al. (2003). Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet (London, England), 361(9366), 1319–1325. [DOI:10.1016/S0140-6736(03)13077-2] [PMID]
Phares, T. W., Stohlman, S. A., & Bergmann, C. C. (2013). Intrathecal humoral immunity to encephalitic RNA viruses. Viruses, 5(2), 732-752. [DOI:10.3390/v5020732] [PMID]
Pivot, X., Raymond, E., Laguerre, B., Degardin, M., Cals, L., & Armand, J. P., et al. (2001). Pemetrexed disodium in recurrent locally advanced or metastatic squamous cell carcinoma of the head and neck. British Journal of Cancer, 85(5), 649-655. [DOI:10.1054/bjoc.2001.2010] [PMID]
Poletti, S., Bollettini, I., Mazza, E., Locatelli, C., Radaelli, D., & Vai, B., et al. (2015). Cognitive performances associate with measures of white matter integrity in bipolar disorder. Journal of Affective Disorders, 174, 342-352. [DOI:10.1016/j.jad.2014.12.030] [PMID]
Poletti, S., Leone, G., Hoogenboezem, T. A., Ghiglino, D., Vai, B., & de Wit, H., et al. (2019). Markers of neuroinflammation influence measures of cortical thickness in bipolar depression. Psychiatry Research. Neuroimaging, 285, 64-66. [PMID]
Poletti, S., Mazza, M. G., Vai, B., Lorenzi, C., Colombo, C., & Benedetti, F. (2020). Proinflammatory cytokines predict brain metabolite concentrations in the anterior cingulate cortex of patients with bipolar disorder. Frontiers in Psychiatry, 11, 590095. [DOI:10.3389/fpsyt.2020.590095] [PMID]
Poyiadji, N., Shahin, G., Noujaim, D., Stone, M., Patel, S., & Griffith, B. (2020). COVID-19-associated acute hemorrhagic necrotizing encephalopathy: Imaging features. Radiology, 296(2), E119–E120. [PMID]
Raz, N., & Rodrigue, K. M. (2006). Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neuroscience & Biobehavioral Reviews, 30(6), 730-748. [DOI:10.1016/j.neubiorev.2006.07.001] [PMID]
Ransohoff, R. M., & Perry, V. H. (2009). Microglial physiology: Unique stimuli, specialized responses. Annual Review of Immunology, 27, 119-145. [PMID]
Rhie, S. J., Jung, E. Y., & Shim, I. (2020). The role of neuroinflammation on pathogenesis of affective disorders. Journal of Exercise Rehabilitation, 16(1), 2-9. [DOI:10.12965/jer.2040016.008] [PMID]
Rogers, J. P., Chesney, E., Oliver, D., Pollak, T. A., McGuire, P., & Fusar-Poli, P., et al. (2020). Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. The Lancet Psychiatry, 7(7), 611-627. [DOI:10.1016/S2215-0366(20)30203-0] [PMID]
Rosenblat, J. D., Brietzke, E., Mansur, R. B., Maruschak, N. A., Lee, Y., & McIntyre, R. S. (2015). Inflammation as a neurobiological substrate of cognitive impairment in bipolar disorder: evidence, pathophysiology and treatment implications. Journal of Affective Disorders, 188, 149-159. [DOI:10.1016/j.jad.2015.08.058] [PMID]
Russo, I., Barlati, S., & Bosetti, F. (2011). Effects of neuroinflammation on the regenerative capacity of brain stem cells. Journal of Neurochemistry, 116(6), 947-956. [DOI:10.1111/j.1471-4159.2010.07168.x] [PMID]
Saczynski, J. S., Beiser, A., Seshadri, S., Auerbach, S., Wolf, P. A., & Au, R. (2010). Depressive symptoms and risk of dementia: The framingham heart study. Neurology, 75(1), 35-41. [DOI:10.1212/WNL.0b013e3181e62138] [PMID]
Quarantelli, M. (2015). MRI/MRS in neuroinflammation: methodology and applications. Clinical and translational imaging, 3(6), 475-489. [DOI:10.1007/s40336-015-0142-y] [PMID]
Şahin, T. D., Karson, A., Balcı, F., Yazır, Y., Bayramgürler, D., & Utkan, T. (2015). TNF-alpha inhibition prevents cognitive decline and maintains hippocampal BDNF levels in the unpredictable chronic mild stress rat model of depression. Behavioural Brain Research, 292, 233-240. [DOI:10.1016/j.bbr.2015.05.062] [PMID]
Sapolsky, R. M. (1996). Why stress is bad for your brain. Science, 273(5276), 749-750. [DOI:10.1126/science.273.5276.749] [PMID]
Sepehrinezhad, A., Shahbazi, A., & Negah, S. S. (2020). COVID-19 virus may have neuroinvasive potential and cause neurological complications: A perspective review. Journal of Neurovirology, 26(3), 324–329. [DOI:10.1007/s13365-020-00851-2] [PMID]
Shen, X. N., Niu, L. D., Wang, Y. J., Cao, X. P., Liu, Q., & Tan, L., et al. (2019). Inflammatory markers in Alzheimer’s disease and mild cognitive impairment: a meta-analysis and systematic review of 170 studies. Journal of Neurology, Neurosurgery & Psychiatry, 90(5), 590-598. [DOI:10.1136/jnnp-2018-319148] [PMID]
Sheng, B., Cheng, S. K., Lau, K. K., Li, H. L., & Chan, E. L. (2005). The effects of disease severity, use of corticosteroids and social factors on neuropsychiatric complaints in severe acute respiratory syndrome (SARS) patients at acute and convalescent phases. European Psychiatry, 20(3), 236-242. [DOI:10.1016/j.eurpsy.2004.06.023] [PMID]
Sheng, W. H., Chiang, B. L., Chang, S. C., Ho, H. N., Wang, J. T., & Chen, Y. C., et al. (2005). Clinical manifestations and inflammatory cytokine responses in patients with severe acute respiratory syndrome. Journal of the Formosan Medical Association= Taiwan yi zhi, 104(10), 715-723.
Simpson, E. E., Hodkinson, C. F., Maylor, E. A., McCormack, J. M., Rae, G., & Strain, S., et al. (2013). Intracellular cytokine production and cognition in healthy older adults. Psychoneuroendocrinology, 38(10), 2196-2208. [DOI:10.1016/j.psyneuen.2013.04.007] [PMID]
Skinner, D., Marro, B. S., & Lane, T. E. (2019). Chemokine CXCL10 and coronavirus-induced neurologic disease. Viral Immunology, 32(1), 25-37. [DOI:10.1089/vim.2018.0073] [PMID]
Spulber, S., Mateos, L., Oprica, M., Cedazo-Minguez, A., Bartfai, T., & Winblad, B., et al. (2009). Impaired long term memory consolidation in transgenic mice overexpressing the human soluble form of IL-1ra in the brain. Journal of Neuroimmunology, 208(1-2), 46-53. [DOI:10.1016/j.jneuroim.2009.01.010] [PMID]
St-Jean, J. R., Jacomy, H., Desforges, M., Vabret, A., Freymuth, F., & Talbot, P. J. (2004). Human respiratory coronavirus OC43: Genetic stability and neuroinvasion. Journal of Virology, 78(16), 8824-8834. [DOI:10.1128/JVI.78.16.8824-8834.2004] [PMID]
Sudheimer, K. D., O'Hara, R., Spiegel, D., Powers, B., Kraemer, H. C., & Neri, E., et al. (2014). Cortisol, cytokines, and hippocampal volume interactions in the elderly. Frontiers in Aging Neuroscience, 6, 153. [DOI:10.3389/fnagi.2014.00153] [PMID]
Tay, T. L., Béchade, C., D’Andrea, I., St-Pierre, M. K., Henry, M. S., & Roumier, A., et al. (2018). Microglia gone rogue: impacts on psychiatric disorders across the lifespan. Frontiers in Molecular Neuroscience, 10, 421. [DOI:10.3389/fnmol.2017.00421] [PMID]
Torres, I. J., DeFreitas, V. G., DeFreitas, C. M., Kauer-Sant'Anna, M., Bond, D. J., & Honer, W. G., et al. (2010). Neurocognitive functioning in patients with bipolar I disorder recently recovered from a first manic episode. The Journal of clinical psychiatry, 71(9), 1234-1242. [DOI:10.4088/JCP.08m04997yel] [PMID]
Tsang, H. W., Scudds, R. J., & Chan, E. Y. (2004). Psychosocial impact of SARS. Emerging Infectious Diseases, 10(7), 1326–1327.[PMID]
van den Brand, J. M., Haagmans, B. L., van Riel, D., Osterhaus, A. D., & Kuiken, T. (2014). The pathology and pathogenesis of experimental severe acute respiratory syndrome and influenza in animal models. Journal of Comparative Pathology, 151(1), 83-112. [DOI:10.1016/j.jcpa.2014.01.004] [PMID]
Vourvopoulos, A., & Bermúdez I Badia, S. (2016). Motor priming in virtual reality can augment motor-imagery training efficacy in restorative brain-computer interaction: A within-subject analysis. Journal of Neuroengineering and Rehabilitation, 13(1), 69. [DOI:10.1186/s12984-016-0173-2] [PMID]
Waldman, G., Mayeux, R., Claassen, J., Agarwal, S., Willey, J., & Anderson, E., et al. (2020). Preparing a neurology department for SARS-CoV-2 (COVID-19): Early experiences at Columbia University Irving Medical Center and the New York Presbyterian Hospital in New York City. Neurology, 94(20), 886-891. [DOI:10.1212/WNL.0000000000009519] [PMID]
Wang, J., Gu, B. J., Masters, C. L., & Wang, Y. J. (2017). A systemic view of Alzheimer disease-insights from amyloid-β metabolism beyond the brain. Nature Reviews Neurology, 13(10), 612. [DOI:10.1038/nrneurol.2017.147] [PMID]
Wang, W. Y., Tan, M. S., Yu, J. T., & Tan, L. (2015). Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Annals of Translational Medicine, 3(10), 136. [PMID]
Wang, X. M., Walitt, B., Saligan, L., Tiwari, A. F., Cheung, C. W., & Zhang, Z. J. (2015). Chemobrain: A critical review and causal hypothesis of link between cytokines and epigenetic reprogramming associated with chemotherapy. Cytokine, 72(1), 86-96. [DOI:10.1016/j.cyto.2014.12.006] [PMID]
Wang, C., Pan, R., Wan, X., Tan, Y., Xu, L., & Ho, C. S., et al. (2020). Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. International Journal of Environmental Research and Public Health, 17(5), 1729. [DOI:10.3390/ijerph17051729] [PMID]
Werry, E. L., Bright, F. M., Piguet, O., Ittner, L. M., Halliday, G. M., & Hodges, J. R., et al. (2019). Recent developments in TSPO PET imaging as a biomarker of neuroinflammation in neurodegenerative disorders. International Journal of Molecular Sciences, 20(13), 3161. [DOI:10.3390/ijms20133166] [PMID]
Wheeler, D. L., Sariol, A., Meyerholz, D. K., & Perlman, S. (2018). Microglia are required for protection against lethal coronavirus encephalitis in mice. The Journal of Clinical Investigation, 128(3), 931-943. [DOI:10.1172/JCI97229] [PMID]
Wrapp, D., Wang, N., Corbett, K. S., Goldsmith, J. A., Hsieh, C. L., & Abiona, O., et al. (2020). Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science, 367(6483), 1260-1263. [DOI:10.1126/science.abb2507] [PMID]
Wu, Y., Xu, X., Yang, L., Liu, C., & Yang, C. (2020). Nervous system damage after COVID-19 infection: Presence or absence?. Brain, Behavior, and Immunity, 87, 55. [DOI:10.1016/j.bbi.2020.04.043] [PMID]
Xia, H., & Lazartigues, E. (2008). Angiotensin‐converting enzyme 2 in the brain: Properties and future directions. Journal of Neurochemistry, 107(6), 1482-1494. [DOI:10.1111/j.1471-4159.2008.05723.x] [PMID]
Xu, J., Zhong, S., Liu, J., Li, L., Li, Y., & Wu, X., et al. (2005). Detection of severe acute respiratory syndrome coronavirus in the brain: Potential role of the chemokine mig in pathogenesis. Clinical Infectious Diseases, 41(8), 1089-1096. [DOI:10.1086/444461] [PMID]
Yeh, E. A., Collins, A., Cohen, M. E., Duffner, P. K., & Faden, H. (2004). Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics, 113(1), e73-e76. [DOI:10.1542/peds.113.1.e73] [PMID]
Yuan, Y., Cao, D., Zhang, Y., Ma, J., Qi, J., & Wang, Q., et al.(2017). Cryo-EM structures of MERS-CoV and SARS-CoV spike glycoproteins reveal the dynamic receptor binding domains. Nature Communications, 8, 15092. [DOI:10.1038/ncomms15092] [PMID]
Zhang, C., Shi, L., & Wang, F. S. (2020). Liver injury in COVID-19: Management and challenges. The Lancet Gastroenterology & Hepatology, 5(5), 428-430. [DOI:10.1016/S2468-1253(20)30057-1] [PMID]
Zhao, G., Jiang, Y., Qiu, H., Gao, T., Zeng, Y., & Guo, Y., et al. (2015). Multi-organ damage in human dipeptidyl peptidase 4 transgenic mice infected with Middle East respiratory syndrome-coronavirus. PLoS One, 10(12), e0145561. [DOI:10.1371/journal.pone.0145561] [PMID]
Zhao, J., Rudd, A., & Liu, R. (2020). Challenges and potential solutions of stroke care during the coronavirus disease 2019 (COVID-19) outbreak. Stroke, 51(5), 1356–1357. [DOI:10.1161/STROKEAHA.120.029701] [PMID]
Zhou, H., Lu, S., Chen, J., Wei, N., Wang, D., & Lyu, H., et al. (2020). The landscape of cognitive function in recovered COVID-19 patients. Journal of Psychiatric Research, 129, 98-102. [DOI:10.1016/j.jpsychires.2020.06.022] [PMID]
Type of Study: Review |
Subject:
Cognitive Neuroscience
Received: 2021/01/23 | Accepted: 2023/06/28 | Published: 2023/09/1
Received: 2021/01/23 | Accepted: 2023/06/28 | Published: 2023/09/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








