Volume 14, Issue 5 (September & October 2023)
BCN 2023, 14(5): 585-604 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Batouli S A H, Razavi F, Sisakhti M, Oghabian Z, Ahmadzade H, Tehrani Doost M. Examining the Dominant Presence of Brain Grey Matter in Autism During Functional Magnetic Resonance Imaging. BCN 2023; 14 (5) :585-604
URL: http://bcn.iums.ac.ir/article-1-2067-en.html
URL: http://bcn.iums.ac.ir/article-1-2067-en.html
Seyed Amir Hossein Batouli1 

 , Foroogh Razavi2
, Foroogh Razavi2 

 , Minoo Sisakhti2
, Minoo Sisakhti2 

 , Zeinab Oghabian2
, Zeinab Oghabian2 

 , Haady Ahmadzade1
, Haady Ahmadzade1 

 , Mehdi Tehrani Doost *1
, Mehdi Tehrani Doost *1 




 , Foroogh Razavi2
, Foroogh Razavi2 

 , Minoo Sisakhti2
, Minoo Sisakhti2 

 , Zeinab Oghabian2
, Zeinab Oghabian2 

 , Haady Ahmadzade1
, Haady Ahmadzade1 

 , Mehdi Tehrani Doost *1
, Mehdi Tehrani Doost *1 


1- Department of Neuroscience and Addiction Studies, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran.
2- Neuroimaging and Analysis Group, Research Center for Molecular and Cellular Imaging, Tehran University of Medical Sciences, Tehran, Iran.
2- Neuroimaging and Analysis Group, Research Center for Molecular and Cellular Imaging, Tehran University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 2409 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Autism is a cognitive impairment involved in a spectrum of heterogeneous neurodevelopmental disabilities. It is characterized by possible difficulties, including impaired social interactions, significantly isolated living conditions, language difficulties, degraded face recognition, abject empathy, and constrained and repetitive behaviors, thoughts, and actions (Spencer et al., 2011). There are usual therapeutic methods used to treat ASD, such as multiple pharmacological treatments and target maladaptive co-occurring conditions, behavioral interventions, and advanced adaptive skills (Zwaigenbaum et al., 2015); however, treatment is more successful if it begins in the earlier stages of the disease. Early diagnosis means an early intervention and treatment, showing the impacts earlier. The affected children benefit from this early treatment before they reach their maximum brain plasticity and neural development (Calderoni et al., 2016; Dawson et al., 2010) controlled trial to evaluate the efficacy of the early start Denver model (ESDM).
There is no generally accepted diagnostic biomarker for autism (Howsmon et al., 2017). A biomarker is a characteristic objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention. The new biomarkers are expected to optimize or improve the current behavioral ASD diagnosis and early recognition of the pathological conditions; however, none of the ASD biomarkers has yet represented enough accuracy and specificity to be construed as clinical utility (Walsh et al., 2011). Accordingly, researchers have tried neuroimaging biomarkers to achieve an earlier diagnosis and enable earlier intervention (Zheng et al., 2017).
Functional magnetic resonance imaging (fMRI) is a non-invasive method capable of identifying the pattern and extent of activity of brain areas during a cognitive function (Batouli & Sisakhti, 2020). The popularity of fMRI is primarily due to its widespread availability, non-invasiveness, low cost, and appropriate spatial resolution (Glover, 2011). It has shown many research applications, namely in studies relevant to imaging genetics (Sachdev et al., 2013), addiction (Zare Sadeghi et al., 2017), language (Alemi et al., 2018), memory (Batouli & Sisakhti, 2019), emotion regulation (Batouli & Saba, 2020), motor function (Batouli et al., 2016), sensory function (Parker et al., 2018), and vision (Schindler & Bartels, 2018). In addition, numerous reports are available on the clinical applications of fMRI. Examples include depression (Neufeld et al., 2018), bipolar disorder (Li et al., 2018), Alzheimer (Oghabian & Batouli, 2010), aging (Batouli et al., 2009), autism (He et al., 2018), epilepsy (Klugah-Brown et al., 2018), and coma (Tomaiuolo et al., 2016). It is also used as a biomarker for various diseases (Batouli et al., 2021; Batouli et al., 2020; Greicius et al., 2004), to monitor a therapy (Richards & Berninger, 2008) or to study the pharmacological efficacy (Wise & Preston, 2010).
In a literature review, we identified 18 studies that had reviewed the MRI studies on ASD. The studies were primarily focused on examining the findings on autism, aiming to improve the diagnosis accuracy. Among these studies, three articles were focused merely on the results of structural MRI in autism (Baribeau & Anagnostou, 2013; Pagnozzi et al., 2018; Palmen & van Engeland, 2004), two articles reviewed resting state fMRI works (Jack, 2018; Li et al., 2017), and among the 12 studies which examined the task-based fMRI studies on autism, 11 studies were old (more than six years ago) (Anagnostou & Taylor, 2011; Brambilla et al., 2004; Cody et al., 2002; Dichter, 2012; Mueller et al., 2012; Philip et al., 2012; Pina-Camacho et al., 2012; Stigler et al., 2011; Verhoeven et al., 2010; Voineagu & Yoo, 2013; Williams & Minshew, 2007), and only five articles provided biomarkers for autism diagnosis. Except for one study (Philip et al., 2012), none provided a map in which the fMRI findings on ASD were summarized. The study by Philip et al. (Philip et al., 2012) is an excellent attempt to provide neuroimaging biomarkers for ASD. In this meta-analysis of 95 fMRI papers on ASD, six categories of cognitive functions were of interest: motor, visual, executive, auditory, social, and complex cognition functions. Using the activation likelihood estimation algorithm, they provided maps in which the brain areas with higher and lower activation probabilities in the ASD group were compared to those of typically developing individuals. The maps and the primary brain areas of difference were provided for all the six groups of cognitive functions.
In using any diagnostic method for ASD, having access to the most reliable and frequent biomarkers is necessary. Accordingly, this study reviews the available task-based fMRI studies on ASD with the following objectives: identify the cognitive functions that are mainly studied in ASD patients; evaluate the potentials of fMRI for ASD diagnosis; and identify the differences of fMRI maps between ASD and normal individuals as diagnostic biomarkers. A biomarker in this study is defined as a brain area that shows a different activation pattern in fMRI in the ASD group compared to expected.
2. Materials and Methods
Data sources
The PubMed and Science Direct databases were searched to identify relevant studies with no date limitation. Different combinations (AND/OR) of the following keywords were used for the search: “Functional MRI”, “brain functionality”, “task-based fMRI”, “brain networks”, autism spectrum”, “ASD”, “PDD”, “pervasive developmental disorder”, “autistic”, and “asperger”. This search resulted in 651 papers. Due to the large number of studies in our initial search, a manual search of the references of the selected studies was not performed for missing documents. Our detailed search strategy is provided while registering for the study.
Study selection
The title, abstract, and full text of the identified manuscripts (if needed) were studied to select the appropriate reports. The studies with the following conditions were initially excluded: non-human studies; studies on diseases other than ASD or only on patients without average population; any imaging modality other than fMRI; and resting-state fMRI or functional/effective connectivity analyses. The inclusion criteria were as follows: 1) published in a peer-reviewed journal; 2) written in the English language; 3) a comparison between autistic and typically developed individuals using task-based fMRI; 3) having a fully and clearly explained methodology, and 4) reporting the brain areas with a different activation between the normal and ASD individuals in the considered fMRI stimulus.
These criteria resulted in 292 papers. The papers were categorized in terms of their evaluated cognitive function, and the number of documents in each category was as follows: language=26; facial expression=21; attention=17; reward=17; theory of mind (TOM)=16; social cognition=15; face processing=13; eye gaze=13; emotion=13; social interaction=11; working memory=11; decision making=9; sensory processing=9; executive function=8; inhibition=8; visuospatial processing=8; face detection=7; location detection=7; response monitoring=7; body motion=7; imitation=6; social perception=5; pain=5; mirror neurons=4; learning=4; voice processing=4; temporal discounting=2; self-face processing=2; counting=1; restricted interests=1; object recognition=1; judgment=1; mentalizing=1; physical condition=1; cognitive flexibility=1; geometric reasoning=1; analogical reasoning=1; self-evaluative processing=1; change detection=1; deviance detection=1; novelty detection=1; empathy processing=1; spatial working memory=1; gesture expression=1; and mental rotation=1.
Given the high number of papers, we selected four categories of the stimulus types with the highest number of studies. As a result, facial expression, face processing, and face detection papers were regarded as category one (n=41), social cognition, social interaction, social perception, and theory of mind studies were considered as category two (n=47), language was category three (n=26), and attention was selected as category four (n=17).
Quality check
The quality of the included studies was checked. In this process, each study was scored from 0 to 12, with a higher score showing a better quality. This assessment included questions on the following items: a) case definition being adequate (score: 0, 1, 2); b) how much the patient group represented the community of ASD (score: 0, 1); c) how the control group participants were selected (score: 0, 1, 2); d) adequate definition of the control group (score: 0, 1); e) how comparable were the patient and control groups regarding the statistical analysis (score: 0, 1, 2); f) how reliable were the imaging and neurocognitive instruments and task (score: 0, 1, 2); g) the patients and controls being assessed in a similar methodology (score: 0, 1); and h) how the non-participation rate of the study was (score: 0, 1). Based on this assessment and the scoring instructions, all the included papers received a score above 9.
Data extraction
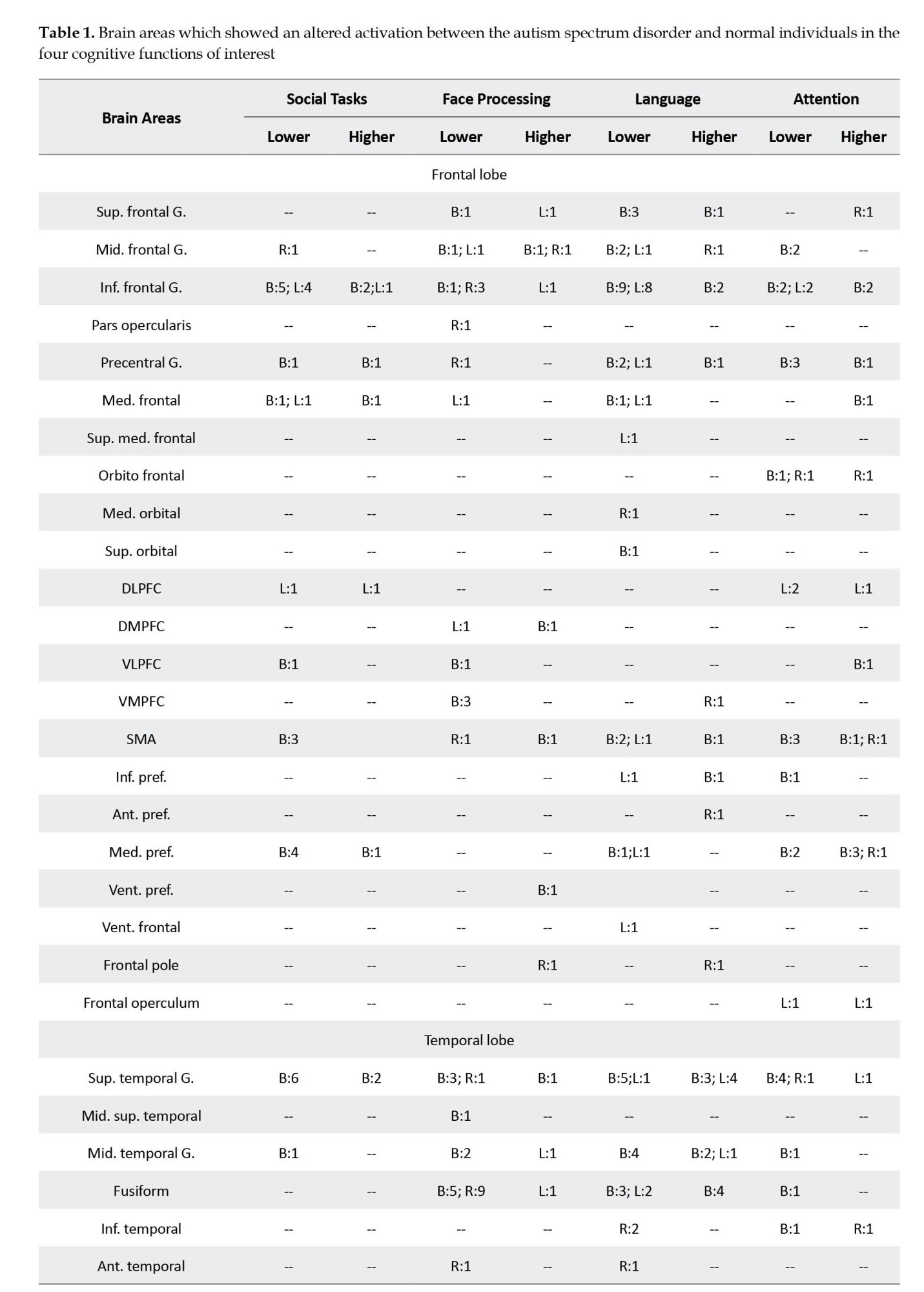
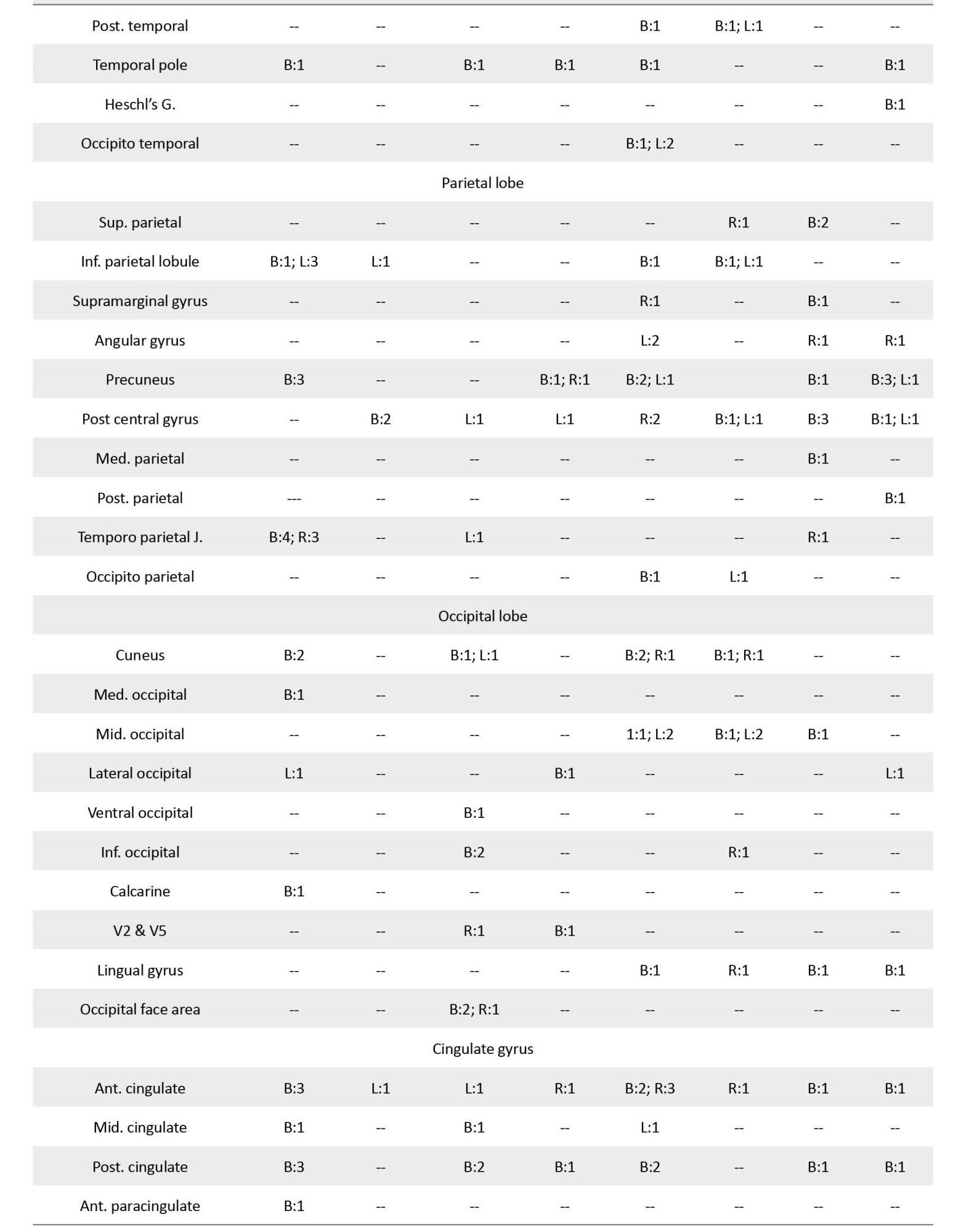
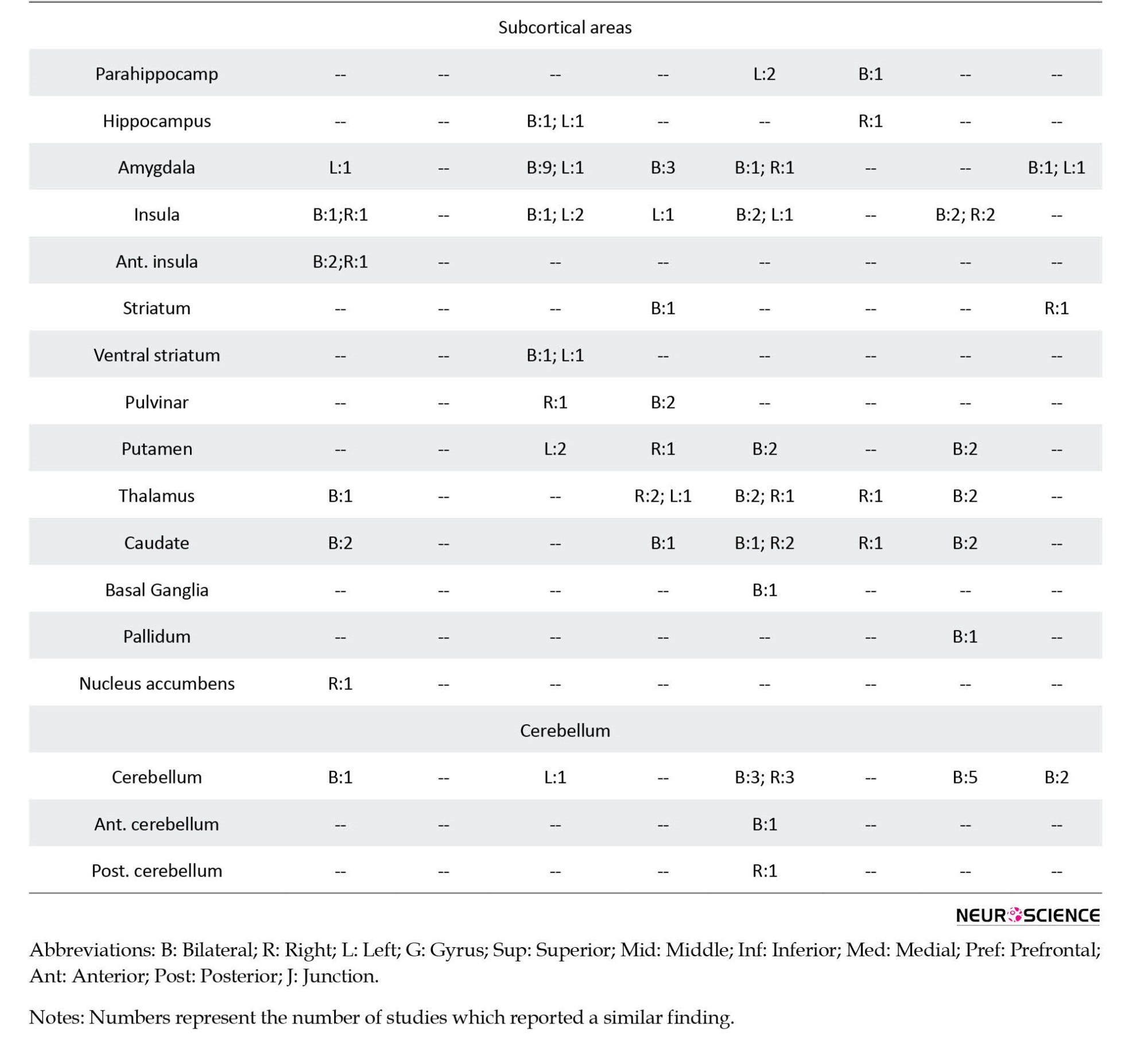
The following information was extracted from each article: I) study: authors, journal, originality, study design, number of sessions, and any intervention; II) participants: inclusion and exclusion criteria, number of cases and controls, age, gender, and handedness; III) data acquisition: type of MRI machine and head coil, details of the imaging protocol, and the fMRI task; IV) outcome measures: number and name of the outcome measures, the analysis software, other tests; and V) findings: results, conclusion, and the suggested biomarkers. As provided in Table 1, the primary information extracted from each study was the name of the brain areas that showed an altered (higher or lower) activation between the ASD and control groups during the cognitive tasks.
Data mapping
To visualize the brain structures that showed an altered activation between the normal and ASD groups in the four cognitive functions of interest, we prepared two maps for each of the cognitive functions. The resulting eight maps are illustrated in Figure 1. The maps in warm colors show the brain areas with higher activation in the ASD group. In comparison, the maps with cool colors demonstrate the brain structures with lower activation in ASD individuals compared to normal subjects. The intensity of colors in each map is also proportional to the number of reports for each structure. In other words, the maps provided in Figure 1 first illustrate the brain regions that showed a different activation between the ASD and normal individuals, and second, the brightness of the colors in the maps are in proportion to the number of reports on each region; a brighter yellow or a brighter blue shows that higher number of studies had reported changes in the activation of that particular brain area.
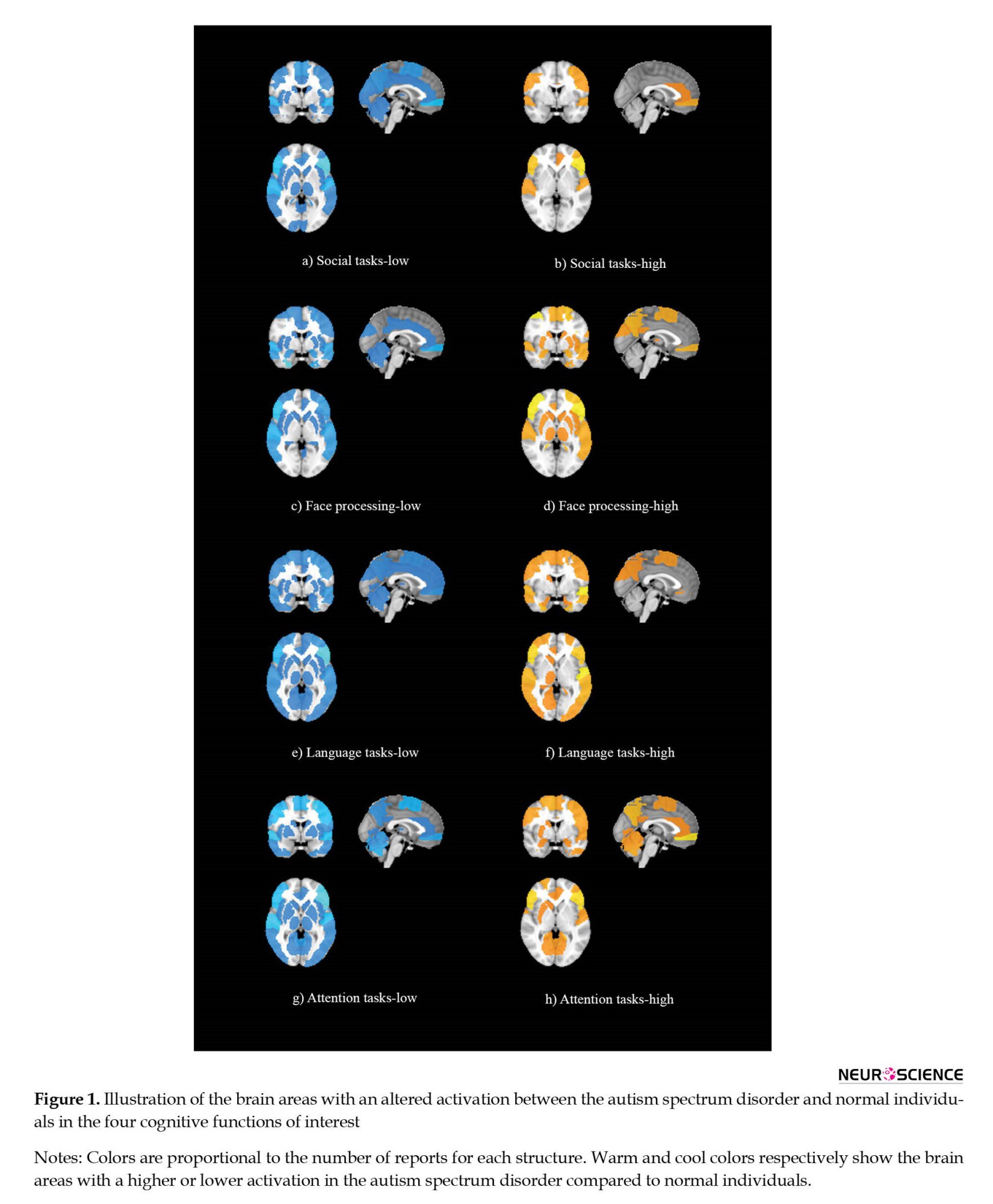
Preparing the maps was based on the methods of our previous study (Batouli & Saba, 2017; Razavi et al., 2021). Accordingly, a region of interest (ROI) was first created for each brain structure using the automated anatomical labeling atlas in the Montreal Neurologic Institute (MNI) space. It was performed using the “wfu pickatlas” toolbox in the MATLAB software (Maldjian et al., 2003). For structures whose ROI was unavailable in the automated anatomical labeling atlas, the creation and extraction of the ROIs were performed using the “Freeview” toolbox of the “Freesurfer” package software, version 5.3a). The global brain measures and the total lobar volumes from these maps were excluded better to understand the single brain areas with an altered activation. Next, the ROIs were added together to make a map, using codes written in the “SPM12” toolbox in the MATLAB software, version R2016a.v9.0) which showed the brain areas with lower or higher activation in the ASD group in any of those cognitive functions. As stated above, the intensity of colors here was proportional to the number of reports for each brain structure. The resulting maps are illustrated in Figure 1.
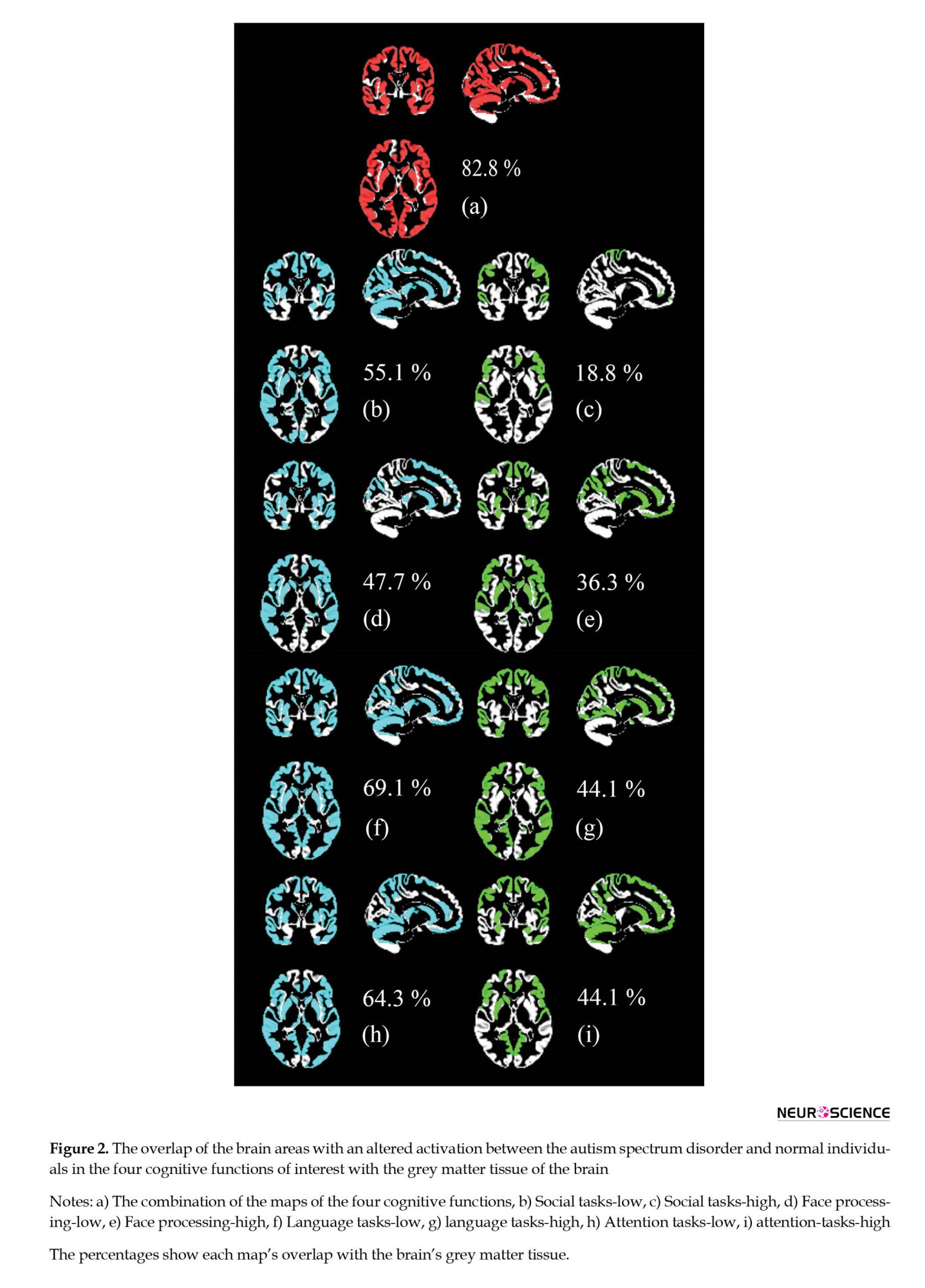
In addition to the maps which showed the areas of the brain with an altered activation, we identified the ratio of the grey matter of the brain that is showing a functional change in the ASD group. In other words, the aim was to estimate and illustrate the amount of overlap between the grey matter tissue of the brain and the brain areas with an altered activation in fMRI. First, the normalization of the ROI maps of Figure 1 to the MNI space was performed. Next, the MNI atlas (Montreal Neurologic Institute; MNI152 template) was segmented into its different tissue types, including grey matter (GM), white matter (WM), cerebrospinal fluid (CSF), and three other tissue types, using the “Segment” toolbox in “SPM12”. Finally, using a code in MATLAB, the number of voxels in the brain function maps which had an overlap with the grey matter tissue of the brain was counted and reported in proportion to the total number of voxels in the GM map. Hence, a ratio was estimated that illustrated the portion of the GM tissue of the brain which showed an altered activation in the cognitive functions of interest. These maps are illustrated in Figure 2.
3. Results
Summary of the included studies
There were 131 case-control studies in the four selected cognitive functions. A total of 2953 patients and 2799 typically developing individuals were evaluated. Of this population, around 86% were male and 35% of the studies included only male participants. Regarding the MRI machine, 73% of the studies used a 3 Tesla MRI scanner, and the rest used 1.5T, along with using 8, 12, or 32-channel head coils.
Around 79% of the studies were performed in one session. Other studies used 2, 3, 4, or even 5 sessions. The sessions involved preparation, MRI scanning, neurocognitive assessments, behavioral testing, and treatments and interventions. Only 3% of the studies had an intervention, including intranasal administration of oxytocin, pivotal response treatment, taking medications, and visualizing and verbalizing for language comprehension and thinking.
There were different criteria in the studies for the inclusion of patients, such as receiving a diagnosis of autism or Asperger syndrome from an independent clinician based on standard criteria of the Diagnostic and Statistical Manual of Mental Disorders, fourth edition; the autism diagnostic observational schedule-generic; autism diagnostic interview-revised; the social responsiveness scale; childhood autism rating scale; clinical impressions and experienced clinical judgment; and a series of clinical assessments that included a detailed developmental history, clinical interview and observation, medical workup, and cognitive testing. An IQ of 80 or above, measured by the Wechsler abbreviated intelligence scale, was necessary in some studies. The control groups were mainly composed of typically developing children or adolescents with no history of psychiatric or neurological disorders and they were matched with the patients in IQ, age, gender, race, and socioeconomic status of the family.
A summary of the findings of the studies is provided in Table 1. This table illustrates the brain areas with reports on a different activation between cases and controls in the four functions and the number of reports for each area. The areas with more than two reports on their different activation between the two groups are elaborated below in the order of their frequency of being reported. References of the selected 131 studies are provided as supplementary data.
Findings of the social tasks
A total of 30 brain areas showed a different activation between cases and controls during the processing of social tasks; 21 only showed lower activation in patients, 1 had a higher activation, and 8 areas had lower and higher reports. The brain areas with lower activation in patients were temporoparietal junction (n=13), inferior frontal gyrus (IFG; n=12), superior temporal gyrus (STG; n=12), medial prefrontal (n=8), supplementary motor area (SMA; n=6), precuneus (n=6), anterior cingulate cortex (ACC; n=6), posterior cingulate cortex (PCC; n=6), inferior parietal lobule (n=5), anterior insula (n=5), caudate (n=4), cuneus (n=4), medial frontal (n=3), and insula (n=3). Post central gyrus (n=4) was the only brain structure with a higher activation in patients.
Findings of the face processing tasks
A total of 40 brain regions showed a different activation between cases and controls when processing faces. Sixteen areas only showed a lower activation, 7 areas demonstrated only a higher activation, and 17 areas showed lower and higher activation. The areas with a lower activation included fusiform (n=19), amygdala (n=19), STG (n=7), ventromedial prefrontal cortex (VMPFC; n=6), occipital face area (n=5), IFG (n=5), inferior occipital (n=4), middle temporal gyrus (MTG; n=4), PCC (n=4), insula (n=4), middle frontal gyrus (MFG; n=3), cuneus (n=3), hippocampus (n=3), and ventral striatum (n=3). Higher activations in patients were observed in amygdala (n=6), pulvinar (n=4), thalamus (n=3), MFG (n=3), and precuneus (n=3).
Findings of the language tasks
A total of 48 brain regions showed a different activation level in the language tasks; meanwhile, 22 only had lower activation, 6 had higher, and 20 had higher and lower reports. The areas which showed a lower activation in patients in the language tasks were IFG (n=26), STG (n=11), cerebellum (n=9), MTG (n=8), fusiform (n=8), ACC (n=7), superior frontal gyrus (SFG; n=6), MFG (n=5), precentral gyrus (n=5), SMA (n=5), precuneus (n=5), cuneus (n=5), thalamus (n=5), occipito temporal gyrus (n=4), middle occipital gyrus (MOG; n=4), PCC (n=4), putamen (n=4), caudate (n=4), medial frontal gyrus (n=3), medial prefrontal gyrus (n=3), amygdala (n=3), and insula (n=3). The brain areas with a report on their higher activations in patients were STG (n=10), fusiform (n=8), MTG (n=5), IFG (n=4), MOG (n=4), posterior temporal gyrus (PTG; n=3), inferior parietal lobule (n=3), postcentral gyrus (n=3), and cuneus (n=3).
Findings of the attention tasks
A total of 39 regions showed differences in brain activations between the cases and controls; meanwhile, 14 areas were only lower in patients, 9 regions were only higher, and 16 areas had lower and higher reports. The areas with lower activation in patients were cerebellum (n=10), STG (n=9), IFG (n=6), precentral gyrus (n=6), SMA (n=6), postcentral gyrus (n=6), insula (n=6), MFG (n=4), medial prefrontal (n=4), superior parietal gyrus (SPG; n=4), putamen (n=4), thalamus (n=4), caudate (n=4), and orbitofrontal gyrus (n=3). The brain regions with higher activation in patients were medial prefrontal (n=7), precuneus (n=7), IFG (n=4), cerebellum (n=4), SMA (n=3), postcentral gyrus (n=3), and amygdala (n=3).
Overlap with the grey matter
As mentioned above, this study aimed to determine whether fMRI can reveal considerable data on ASD. A positive answer to this question would introduce fMRI as a potential diagnostic tool for ASD in future works. Accordingly, we assessed the overlap of the brain maps obtained for the four cognitive functions (illustrated in Figure 1) with the grey matter tissue of the brain, as explained in Materials and Methods. Figure 2 shows these overlaps as well as the percentage of the overlap.
The highest coverage of the GM was observed in the language tasks-low condition (69%); other estimates were attention tasks-low (64%), social tasks-low (55%), and face processing tasks-low (48%). These findings showed that a larger area of the brain showed a lower activation in the ASD individuals versus normal participants, compared to a higher activation. On the other hand, attention tasks-high and language tasks-high maps showed 44% coverage of the GM, 36% in the face processing-high, and 19% in social tasks-high maps.
When all eight brain maps in the four cognitive tasks were combined, 83% of the brain GM was covered, which suggests that fMRI is a strong tool in revealing signs of ASD.
4. Discussion
Summary of the results
This study reviewed the task-based fMRI studies on ASD aiming to assess the potential of fMRI in predicting or diagnosing ASD in the future. The severity of the difference between the fMRI measures of the normal and ASD groups could suggest the fMRI to be useful. By initially recruiting 292 studies and reviewing the results of 131 fMRI works in the four cognitive abilities of interest, 73 brain structures showed an altered brain function between the two groups. In addition, a combination of the results of the four cognitive functions showed that about 83% of brain GM shows an altered activation in ASD compared to normal individuals. Future research to diagnose ASD using automatic algorithms, such as machine learning, would benefit from the findings of this study.
Autism spectrum disorder diagnosis
The prevalence of ASD is 1 in 59 subjects in the US (Baio et al., 2018), and 1 in 132 individuals globally (Baxter et al., 2015) the burden of ASDs has been estimated for the global burden of disease study 2010 (GBD 2010. They are much higher than previously reported. This data reflects the growing knowledge about ASD and innovations in the diagnostic approaches which enhanced evaluation of its clinical symptoms (Sandin et al., 2014). The main characteristic of autism’s clinical manifestations is its significant heterogeneity; no two autism patients are alike, which means that each autistic individual shows unique phenotypic heterogeneity in the combination of symptom severity and comorbid conditions (anxiety, depression, social communication disorder, attention deficit hyperactivity disorder, epileptic disorders) (Jones & Lord, 2013). Early diagnosis would facilitate decisions for the selection of the best therapeutic method, improve the quality of life of children with ASD and their families (Pagnozzi et al., 2018), and lead to preventing notable economic and emotional costs to autistic people and their families. Despite all these, early diagnosis is challenging (Fein et al., 2013; Zwaigenbaum et al., 2015).
There are numerous undetermined questions about the causes and the pathophysiology of ASD (Li et al., 2017). To date, diagnostic evaluation of ASD is based on clinical observation and interviews with caregivers using some standardized instruments, including an autism diagnostic observational schedule (Steiner et al., 2012). The lack of knowledge on the exact neural basis of autism spectrum disorder and the absence of validated diagnosis measures throughout development is challenging. To date, diagnostic evaluation for autism requires a team of professionals from different disciplines, including a physician, speech therapist, cognitive therapist, and occupational therapist.
Since the first description of the disease in the early 1940s, researchers have been intensively trying to identify biological markers for ASD (Szpir, 2006). As mentioned before, the crucial challenges are the underlying biological heterogeneity of ASD. Nowadays, we can only reliably diagnose ASD based on the current standardized behavioral observations and psychometric tools (Steiner et al., 2012). Precise diagnostic assessments are required, as finding specific biomarkers for autism may reduce the variability of ASD diagnosis. Such methods are also helpful in evaluating treatment effects on the neurodevelopmental disorders.
Neuroimaging has already played a pivotal role in ASD characterization by the in vivo monitoring of the brain structure and function during the disorder (Retico et al., 2014). MRI is a safe, non-invasive, and powerful diagnostic tool for observing alterations in the brain’s structure and function. Moreover, fMRI techniques are essential in forming these diagnostic assessments (Li et al., 2017). fMRI is considered to provide the required biomarkers for exploring and diagnosing the severity of neurodevelopmental disorders, such as autism (Castellanos et al., 2013). Current research on structural and functional brain MRI indicates that identifying general biological alterations in most ASD patients requires large cohort sizes, and they often lead to numerous overlapping markers with those of other conditions and the public population, rather than a single marker (Voineagu & Yoo, 2013). As a result, more reliable algorithms are needed to use neuroimaging for purposes such as ASD diagnosis. Neuroimaging could be combined with machine learning methods for disease diagnosis or prediction (Andrews et al., 2018). These methods are already used for the ASD diagnosis. As an example, classification approaches using a support vector machine were used to characterize the structural changes of the brain in adults with ASD to discriminate them from the average population (Ecker et al., 2010). These methods have recently been reviewed (Andrews et al., 2018). In addition to separating patients and controls, these classifiers apply on a single-subject basis to aid diagnosis. This is done by assigning an abnormality score to each subject, quantifying the degree of pathology (Ingalhalikar et al., 2011). The current study aimed to initially show the potential of fMRI for ASD diagnosis along with machine learning algorithms and provide the biomarkers for fMRI in such works.
Face processing stimuli
The fusiform gyrus, as well as the amygdala, STG, VMPFC, occipital face area, IFG, IOG, MTG, PCC, insula, MFG, cuneus, hippocampus, and striatum showed lower activation in the ASD group when processing faces. Striatum is involved in processing rewards, and for individuals with high autistic traits, facial mimicry is associated with lower reward-related neural response (Hsu et al., 2018). Amygdala showed a lower activation during the attentional orienting triggered by eye gaze (Klapwijk et al., 2016), suggesting that impairments associated with gaze-triggered attentional orienting could be modified by treatments directed at amygdala activity (Sato et al, 2017). Decreased activation in the hippocampus also suggests problems integrating emotional information with declarative memory (Klapwijk et al., 2016).
Fusiform is vital in face processing (Grill-Spector et al., 2004) and its activation is associated with one’s face discrimination performance (Jiang et al., 2013). It is suggested that the phenotypic heterogeneity in face processing in ASD is mediated by neuronal selectivity to faces in this area (Jiang et al., 2013). Moreover, it is proposed that training on tasks that recruit face representation in this area could remediate face-processing differences (Jiang et al., 2013). Another study hypothesized that the abnormal activity of this area in autism could be due to inappropriate information acquisition during eye scanning (Pelphrey et al., 2007). Besides, ASD children differently recognize faces, as they focus more on feature-based than configured analyses, and the fusiform gyrus is associated with configural processing (Pelphrey et al., 2007). Also, fusiform face area activation depends on orientation toward the eyes during stimulus presentation (Zürcher et al., 2013); therefore, this reduced activation may be caused by atypical eye-gaze patterns towards faces.
MTG is involved in the processing of facial features and expressions (Critchley et al., 2000); meanwhile, it is also strongly modulated by top-down attentional mechanisms, hence, different attentional mechanisms concerning faces (Critchley et al., 2000) in ASD could be a reason for its declined activation. STG is active in tasks involving attributing intentions to moving geometric figures, and in social dysfunction in autism (Pelphrey et al., 2007). Other brain areas with a declined activation in ASD are also involved in face processing, such as the insula, a driving node in the salience network (Sridharan et al., 2008), PCC in acquiring facial familiarity (Kosaka et al., 2003), and IFG consistently being active during imitation, action observation, and intention understanding (Iacoboni, 2005).
Higher activations in individuals with ASD while processing faces were observed in the amygdala, pulvinar, thalamus, MFG, and precuneus. The pulvinar nucleus and amygdala have roles in rapid face processing (Hadjikhani et al., 2017). Higher activation in the subcortical areas, such as the amygdala, in children with ASD when looking at the eyes, is possibly related to their eye avoidance in daily life (Hadjikhani et al., 2017). The subcortical system in ASD overreacts to stimuli that should be considered positively engaging and socially rewarding (Hadjikhani et al., 2017), and for example, the amygdala hyper-responsiveness to direct gaze shows a neural indicator for heightened emotional arousal triggered by eye contact (Dalton et al., 2005). The thalamic hyperactivation is also hypothesized as one of the substrates of social cognition deficits in ASD, through its deregulatory impact on the dorsolateral prefrontal cortex (Hadjikhani et al., 2017). The activation of precuneus is modulated depending on attentional demands; therefore, its increased activation in ASD could be a compensatory mechanism (Wang et al., 2004). The amygdala reported higher, lower, and no difference in activation between the ASD and healthy individuals in face processing. The inconsistent findings could be due to the differences in attention to the faces (Klapwijk et al., 2016) or the type of tasks and stimuli (Pinal et al., 2014).
Language stimuli
Language function is impaired in ASD children. They have difficulty generating words relevant to a context or topic (Kenworthy et al., 2013) and processing mental states and words related to emotions (Moseley et al., 2015). This impairment is also observed in patients with a lesion in the motor system (Moseley et al., 2013). There is altered recruitment of reading-related neural resources in ASD children, weaknesses in the top-down modulation of semantic processing (Karten & Hirsch, 2015), and difficulties in using context to predict the final word of sentences (Catarino et al., 2011). Executive functions are critical for selecting, generating, and organizing words into sentences and sustaining meaningful conversations, and there are reports on impaired executive control of language in ASD (Kenworthy et al., 2013).
A total of 22 brain structures showed a declined activation in language processing in the ASD children, including IFG, STG, cerebellum, MTG, fusiform, ACC, SFG, MFG, precentral gyrus, SMA, precuneus, cuneus, thalamus, MOG, PCC, putamen, caudate, medial frontal gyrus, amygdala, and insula. These structures are involved in different aspects of the language processing. The thalamus's putamen and ventral lateral nucleus are involved in fluency-related activity (Kenworthy et al., 2013). In contrast, temporal regions are essential in auditory processing (Belin et al., 2000) particularly emotional prosody (Wildgruber et al., 2005). The ACC is activated in explicitly evaluating emotions (Bach et al., 2008). The thalamus has a role in language and verbal memory (Hebb & Ojemann, 2013), in addition to visual attention (Fan et al., 2005), suggestive of serial visual scanning during the initial steps of reading (Koyama et al., 2011). The left putamen has also been implicated in reading and language comprehension, especially in sub-lexical and lexical processing (Oberhuber et al., 2013).
The middle and superior frontal gyri are involved in executive functions (Moreno-López et al., 2012), attention, and working memory (Boisgueheneuc et al., 2006); therefore, they are related to the cognitive evaluation of the emotional content (Gebauer et al., 2014). LMFG is also reported to be a region involved in word retrieval during language production (Bednarz et al., 2017), and there are numerous reports on the activation of the LMFG during single-word reading, sentence reading, and lexical retrieval (Bednarz et al., 2017). This region is underactive in children with reading difficulty (Barquero et al., 2014). The lack of activation of the LMOG is previously shown in reading dysfunction, due to its role in reading intervention (Barquero et al., 2014). Frishkoff et al. (Frishkoff et al., 2004) also suggested that the anterior cingulate cortex discriminates congruous and incongruous words and its activity is sustained over time while processing semantic incongruities. In addition, the cerebellum has direct connections to Broca’s area, which enables it to facilitate verbal abilities (Harris et al., 2006).
Meanwhile, 9 brain structures showed an elevated activation in ASD individuals, including STG, fusiform, MTG, IFG, MOG, PTG, inferior parietal lobule, postcentral gyrus, and cuneus. The increased activation of brain areas in ASD is suggested to be a compensatory mechanism to aid in language comprehension (Murdaugh et al., 2016). There is evidence of recruiting more cortical resources during word generation in males and females with ASD (Beacher et al., 2012). Elevated fusiform activation has been shown in individuals with ASD to interpret the meaning of emotional words (Han et al., 2014). Greater activation of the cuneus is in line with the evidence of atypical reliance on the visual cortex in individuals with ASD (Chen et al., 2016), as the cuneus activation is related to visual perception and retrieving stored mental imagery of word stimuli (Kim et al., 2013). Individuals with ASD tend to recruit more visual cortex than IFG for word processing (Samson et al., 2012).
Another finding is using right hemisphere language-analogous regions in ASD to assist reading. This finding supports the idea that autism is related to early left-hemisphere dysfunction (Takeuchi et al., 2004). Studies suggest that ASD children are more likely to show right-hemisphere lateralization for language (Harris et al., 2006). For example, the right IFG has been more strongly implicated in emotional prosody processing than its contralateral homologs (Schirmer & Kotz, 2006). However, there are still disputes on whether the reversal of Broca’s asymmetry in the language is a predisposing factor toward language impairment or a compensatory neurodevelopmental response to language dysfunction in the left hemisphere (Harris et al., 2006), although the increased right hemisphere activity for the ASD group is mostly interpreted as reflecting more effortful processing (Tesink et al., 2009).
Commonly referred to as Broca’s area, the LIFG is involved in affective aspects of language processing, semantics, and visual memory (Beacher et al., 2012), in word processing and controlled retrieval and selection of semantic knowledge (Weikum et al., 2007), in unification operations required for binding single word information into larger structures (Fitzpatrick & Indefrey, 2009), in representing social knowledge and abstract social concepts (Zahn et al., 2007), and in attributing personality traits (Heberlein & Saxe, 2005). It also subserves verbal working memory by maintaining semantic representations (Bednarz et al., 2017). This area had 26 reports of decreased and 4 reports of higher activation in ASD. The activation of this area is dependent on the stimuli, for example, elevated activation of letter fluency vs category (Kenworthy et al., 2013), and attenuation of responsiveness to manipulations of semantic congruity (Beacher et al., 2012). In addition, the different activation of this region in ASD could be linked to other perceptual and expressive deficits in affective communication (Beacher et al., 2012).
Attention tasks
The neural mechanism of attention is different in ASD. The failure to orient attention toward salient stimuli is a fundamental problem in ASD that impairs social skills and learning (Klin et al., 2003). It could be due to the abnormalities in their functional brain maturation (Murphy et al., 2014), which is associated with clinical symptoms of ASD and inattention (Murphy et al., 2014). Neural circuitry of attention is driven by hyper-responsivity to salience (Murphy et al., 2017), and the observed differences may reflect compensatory mechanisms enabling normal behavioral performance (Rahko et al., 2015). In addition, over-focused attention in ASD results from hyperarousal, seen as disinhibiting the competing sensory information that leads to attentional shifts (Liss et al., 2006). Evidence shows that arousal regulation in ASD results from early deficits in disengaging attention (Rahko et al., 2015). ASD children are under-reactive to behaviorally-relevant stimuli. There are also findings of atypical functions of both top-down and bottom-up attention networks in these individuals, and they cannot filter irrelevant information (Keehn et al., 2016).
A total of 14 brain structures have been shown to have lower activation in patients with ASD, including cerebellum, STG, IFG, precentral gyrus, SMA, post-central gyrus, insula, MFG, medial prefrontal, SPG, putamen, thalamus, caudate, and orbitofrontal gyrus. The cerebellum plays an essential role in attentional processes (Keehn et al., 2016), and is implicated in the neuropathology of ASD (Fatemi et al., 2012). Caudate, STG, and SPG are involved in joint attention, facilitating attentional regulatory processes (Williams et al., 2005), which show deficits in ASD. STG also plays a role in utilizing social cues to orient attention. However, in ASD, this area may not be sensitive to the social meaning conveyed by eye gaze (Pelphrey et al., 2005). Finally, putamen responds when a stimulus is important, but not when the behavioral significance is removed (Greene et al., 2011).
On the contrary, 7 brain areas showed an elevated activation in ASD, including the medial prefrontal, precuneus, IFG, cerebellum, SMA, post-central gyrus, and amygdala. The activation of the visual cortex, particularly the precuneus, was significantly correlated with the severity of autistic symptoms (Ohta et al., 2012). Precuneus is implicated in spatial attention and anticipation of motor responses (Cavanna & Trimble, 2006), and the deficits in this area were positively correlated with the severity of social reciprocity and communication problems (Christakou et al., 2013). The increased response in children with ASD may reflect hyper-responsivity of bottom-up processing of salient visual stimuli (Murphy et al., 2017). This increase may be attributable to differences in the visual receptive fields in ASD. There is evidence of increased perifoveal population receptive fields in extrastriate cortex, associated with hyper-excitability of the visual cortex or poor peripheral spatial attention in individuals with ASD (Schwarzkopf et al., 2014).
Studies have indicated that the medial rostral PFC plays a role in attentional selection between stimulus-oriented and stimulus-independent thought (Gilbert et al., 2007). This region also involves multitasking and prospective memory (Simons et al., 2006). One suggestion based on these findings is that healthy individuals could modulate activity in their visual cortex according to the attentional demands of the task to a higher degree than the ASD group (Gilbert et al., 2008). This finding also suggests functional underconnectivity in ASD (Just et al., 2012), decreasing top-down modulation of sensory areas according to attentional demands (Gilbert et al., 2008). Moreover, the medial rostral PFC is involved in mentalizing (Gilbert et al., 2007), and its higher activation in ASD raises the possibility that these individuals recruit brain regions typically associated with mentalizing to perform other tasks, such as face perception (Pierce et al., 2001). As an example, the inability to suppress task-related activation of the medial prefrontal cortex is linked to distractibility in attention deficit hyperactivity disorder (Fassbender et al., 2009), and because of the high comorbidity of attention deficit hyperactivity disorder and autism (Simonoff et al., 2008), this may indicate a particular difficulty with distraction during the global condition (Gadgil et al., 2013).
5. Conclusion
This study, initially including 292 papers and studying 131 in detail, first showed which cognitive domains are currently mainly studied in ASD individuals. The most checked domains are the best candidates for diagnosis/prediction purposes. Also, the less studied cognitive functions could be suggestions for future works. Second, the study showed that a large part of the brain shows abnormalities in fMRI in ASD individuals compared to normal, suggesting fMRI's application in ASD diagnosis. For this aim, the study provided a comprehensive list of biomarkers for using task-based fMRI in the diagnosis/prediction of ASD, which included the brain structures that showed an altered activation in ASD and the frequency of reports for each structure. All the 73 brain areas illustrated in Figure 2 are ideal candidates for this purpose, with the brighter areas being stronger neuro-markers. FMRI imaging of an individual, and evaluating these biomarkers in methods such as machine-learning, could help assess an individual's risk of being in the ASD spectrum.
Study limitations
There are a few limitations to our work. Several studies may be missing during our database searches. Our inferences were based on 131 studies from 292, including many works that could result in more comprehensive deductions. Also, the methodology and participants of the selected studies were not identical, the factors that could have associations with the findings of a study. More detailed discussions could be provided on our findings, and finally, practically using the reported biomarkers for ASD diagnosis would be helpful as a validation algorithm.
Ethical Considerations
Compliance with ethical guidelines
This study was part of a systematic review that was registered at the International Prospective Register of Systematic Reviews (PROSPERO) (Code: PROSPERO_2017_CRD42017070975).
Funding
This study was financially supported by the Iranian Council for Cognitive Sciences and Technologies.
Authors' contributions
Conceptualization: Mehdi Tehrani Doost and Seyed Amir Hossein Batouli; Methodology: Foroogh Razavi, Zeinab Oghabian and Minoo Sisakhti; The original draft preparation: Minoo Sisakhti, Foroogh Razavi, Zeinab Oghabian and Haady Ahmadzade; Visualization, supervision and writing–review & editing: Mehdi Tehrani Doost and Seyed Amir Hossein Batouli; Project Administration and funding acquisition: Mehdi Tehrani Doost.
Conflict of interest
The authors declared no conflict of interest..
Acknowledgments
The authors appreciate the support from the Iranian Council for Cognitive Sciences and Technologies.
References
Alemi, R., Batouli, S. A. H., Behzad, E., Ebrahimpoor, M., & Oghabian, M. A. (2018). Not single brain areas but a network is involved in language: Applications in presurgical planning. Clinical Neurology and Neurosurgery, 165, 116-128. [DOI:10.1016/j.clineuro.2018.01.009] [PMID]
Anagnostou, E., & Taylor, M. J. (2011). Review of neuroimaging in autism spectrum disorders: What have we learned and where we go from here. Molecular Autism, 2(1), 4. [PMID]
Andrews, D. S., Marquand, A., Ecker, C., & McAlonan, G. (2018). Using pattern classification to identify brain imaging markers in autism spectrum disorder. Current Topics in Behavioral Neurosciences, 40, 413–436. [PMID]
Bach, D. R., Grandjean, D., Sander, D., Herdener, M., Strik, W. K., & Seifritz, E. (2008). The effect of appraisal level on processing of emotional prosody in meaningless speech. NeuroImage, 42(2), 919–927. [DOI:10.1016/j.neuroimage.2008.05.034] [PMID]
Baio, J., Wiggins, L., Christensen, D. L., Maenner, M. J., Daniels, J., & Warren, Z., et al. (2018). Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2014. Morbidity and Mortality Weekly Report. Surveillance Summaries (Washington, D.C. : 2002), 67(6), 1–23. [DOI:10.15585/mmwr.ss6706a1] [PMID]
Baribeau, D. A., & Anagnostou, E. (2013). A comparison of neuroimaging findings in childhood onset schizophrenia and autism spectrum disorder: A review of the literature. Frontiers in Psychiatry, 4, 175. [DOI:10.3389/fpsyt.2013.00175] [PMID]
Barquero, L. A., Davis, N., & Cutting, L. E. (2014). Neuroimaging of reading intervention: A systematic review and activation likelihood estimate meta-analysis. PloS One, 9(1), e83668-e83668. [DOI:10.1371/journal.pone.0083668] [PMID]
Batouli, S. A. H., Alemi, R., Khoshkhouy Delshad, H., & Oghabian, M. A. (2020). The influence of mental fatigue on the face and word encoding activations. Clinical Neurology and Neurosurgery, 189, 105626. [DOI:10.1016/j.clineuro.2019.105626] [PMID]
Batouli, S. A. H., Boroomand, A., Fakhri, M., Sikaroodi, H., Oghabian, M. A., & Firouznia, K. (2009). The effect of aging on resting state brain function: An fMRI study. Iranian Journal of Radiology, 6(3), 153-158. [Link]
Batouli, S. A., Hasani, N., Gheisari, S., Behzad, E., & Oghabian, M. A. (2016). Evaluation of the factors influencing brain language laterality in presurgical planning. Physica Medica, 32(10), 1201-1209. [DOI:10.1016/j.ejmp.2016.06.008] [PMID]
Batouli, S. A. H., & Saba, V. (2017). At least eighty percent of brain grey matter is modifiable by physical activity: A review study. Behavioural Brain Research, 332, 204–217. [DOI:10.1016/j.bbr.2017.06.002] [PMID]
Batouli, S. A. H., & Saba, V. (2020). Larger volume and a different activation of the brain in response to threat in military officers. Basic and Clinical Neuroscience, 11(5), 669-686. [DOI:10.32598/bcn.9.10.160] [PMID]
Batouli, S. A. H., & Sisakhti, M. (2019). Investigating a hypothesis on the mechanism of long-term memory storage. NeuroQuantology, 17(3), 60-79. [DOI:10.14704/nq.2019.17.3.1813]
Batouli, S. A. H., & Sisakhti, M. (2020). Some points to consider in a task-based fMRI study: A guideline for beginners. Frontiers in Biomedical Technologies, 7(1), 52-73. [Link]
Batouli, S. A. H., Sisakhti, M., Haghshenas, S., Dehghani, H., Sachdev, P., & Ekhtiari, H., et al. (2021). Iranian Brain Imaging Database: A neuropsychiatric database of healthy brain. Basic and Clinical Neuroscience, 12(1), 115–132. [DOI:10.32598/bcn.12.1.1774.2] [PMID]
Baxter, A. J., Brugha, T. S., Erskine, H. E., Scheurer, R. W., Vos, T., & Scott, J. G. (2015). The epidemiology and global burden of autism spectrum disorders. Psychological Medicine, 45(3), 601-613. [DOI:DOI: 10.1017/S003329171400172X] [PMID]
Beacher, F. D., Radulescu, E., Minati, L., Baron-Cohen, S., Lombardo, M. V., & Lai, M. C., et al. (2012). Sex differences and autism: brain function during verbal fluency and mental rotation. PloS One, 7(6), e38355. [DOI:10.1371/journal.pone.0038355] [PMID]
Bednarz, H. M., Maximo, J. O., Murdaugh, D. L., O'Kelley, S., & Kana, R. K. (2017). Decoding versus comprehension: Brain responses underlying reading comprehension in children with autism. Brain and Language, 169, 39-47. [PMID]
Belin, P., Zatorre, R. J., Lafaille, P., Ahad, P., & Pike, B. (2000). Voice-selective areas in human auditory cortex. Nature, 403(6767), 309-312. [DOI:10.1038/35002078] [PMID]
du Boisgueheneuc, F., Levy, R., Volle, E., Seassau, M., Duffau, H., & Kinkingnehun, S., et al. (2006). Functions of the left superior frontal gyrus in humans: A lesion study. Brain, 129(12), 3315-3328. [DOI:10.1093/brain/awl244] [PMID]
Brambilla, P., Hardan, A. Y., di Nemi, S. U., Caverzasi, E., Soares, J. C., & Perez, J., et al. (2004). The functional neuroanatomy of autism. Functional Neurology, 19(1), 9–17. [PMID]
Calderoni, S., Billeci, L., Narzisi, A., Brambilla, P., Retico, A., & Muratori, F. (2016). Rehabilitative interventions and brain plasticity in autism spectrum disorders: Focus on MRI-based studies. Frontiers in Neuroscience, 10, 139. [DOI:10.3389/fnins.2016.00139] [PMID]
Castellanos, F. X., Di Martino, A., Craddock, R. C., Mehta, A. D., & Milham, M. P. (2013). Clinical applications of the functional connectome. NeuroImage, 80, 527-540. [DOI:10.1016/j.neuroimage.2013.04.083] [PMID]
Catarino, A., Luke, L., Waldman, S., Andrade, A., Fletcher, P. C., & Ring, H. (2011). An fMRI investigation of detection of semantic incongruities in autistic spectrum conditions. The European Journal of Neuroscience, 33(3), 558–567. [DOI:10.1111/j.1460-9568.2010.07503.x] [PMID]
Cavanna, A. E., & Trimble, M. R. (2006). The precuneus: A review of its functional anatomy and behavioural correlates. Brain, 129(3), 564-583. [DOI:10.1093/brain/awl004] [PMID]
Chen, P. J., Gau, S. S., Lee, S. H., & Chou, T. L. (2016). Differences in age-dependent neural correlates of semantic processing between youths with autism spectrum disorder and typically developing youths. Autism Research, 9(12), 1263-1273. [DOI:10.1002/aur.1616] [PMID]
Christakou, A., Murphy, C. M., Chantiluke, K., Cubillo, A. I., Smith, A. B., & Giampietro, V., et al. (2013). Disorder-specific functional abnormalities during sustained attention in youth with Attention Deficit Hyperactivity Disorder (ADHD) and with Autism. Molecular Psychiatry, 18(2), 236-244. [DOI:10.1038/mp.2011.185] [PMID]
Cody, H., Pelphrey, K., & Piven, J. (2002). Structural and functional magnetic resonance imaging of autism. International Journal of Developmental Neuroscience, 20(3-5), 421–438. [PMID]
Critchley, H. D., Daly, E. M., Bullmore, E. T., Williams, S. C., Van Amelsvoort, T., & Robertson, D. M., et al. (2000). The functional neuroanatomy of social behaviour: Changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain, 123(11), 2203-2212. [DOI:10.1093/brain/123.11.2203] [PMID]
Critchley, H., Daly, E., Phillips, M., Brammer, M., Bullmore, E., & Williams, S., et al. (2000). Explicit and implicit neural mechanisms for processing of social information from facial expressions: A functional magnetic resonance imaging study. Human Brain Mapping, 9(2), 93-105. [PMID]
Dalton, K. M., Nacewicz, B. M., Johnstone, T., Schaefer, H. S., Gernsbacher, M. A., & Goldsmith, H. H., et al. (2005). Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience, 8(4), 519-526. [DOI:10.1038/nn1421] [PMID]
Dawson, G., Rogers, S., Munson, J., Smith, M., Winter, J., & Greenson, J., et al. (2010). Randomized, controlled trial of an intervention for toddlers with autism: The Early Start Denver Model. Pediatrics, 125(1), e17-e23. [DOI:10.1542/peds.2009-0958] [PMID]
Dichter, G. S. (2012). Functional magnetic resonance imaging of autism spectrum disorders. Dialogues in Clinical Neuroscience, 14(3), 319-351. [DOI:10.31887/DCNS.2012.14.3/gdichter] [PMID]
Ecker, C., Marquand, A., Mourão-Miranda, J., Johnston, P., Daly, E. M., & Brammer, M. J., et al. (2010). Describing the brain in autism in five dimensions-magnetic resonance imaging-assisted diagnosis of autism spectrum disorder using a multiparameter classification approach. The Journal of Neuroscience, 30(32), 10612 - 10623. [DOI:10.1523/JNEUROSCI.5413-09.2010] [PMID]
Fan, J., McCandliss, B. D., Fossella, J., Flombaum, J. I., & Posner, M. I. (2005). The activation of attentional networks. NeuroImage, 26(2), 471-479. [DOI:10.1016/j.neuroimage.2005.02.004] [PMID]
Fassbender, C., Zhang, H., Buzy, W. M., Cortes, C. R., Mizuiri, D., & Beckett, L., et al. (2009). A lack of default network suppression is linked to increased distractibility in ADHD. Brain Research, 1273, 114-128. [DOI:10.1016/j.brainres.2009.02.070] [PMID]
Fatemi, S. H., Aldinger, K. A., Ashwood, P., Bauman, M. L., Blaha, C. D., & Blatt, G. J., et al. (2012). Consensus paper: Pathological role of the cerebellum in autism. Cerebellum (London, England), 11(3), 777-807. [DOI:10.1007/s12311-012-0355-9] [PMID]
Fein, D., Barton, M., Eigsti, I. M., Kelley, E., Naigles, L., & Schultz, R. T., et al. (2013). Optimal outcome in individuals with a history of autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 54(2), 195-205. [DOI:10.1111/jcpp.12037] [PMID]
FitzPatrick, I., & Indefrey, P. (2010). Lexical competition in nonnative speech comprehension. Journal of Cognitive Neuroscience, 22(6), 1165–1178. [DOI:10.1162/jocn.2009.21301] [PMID]
Frishkoff, G. A., Tucker, D. M., Davey, C., & Scherg, M. (2004). Frontal and posterior sources of event-related potentials in semantic comprehension. Brain research. Cognitive Brain Research, 20(3), 329–354. [DOI:10.1016/j.cogbrainres.2004.02.009] [PMID]
Gadgil, M., Peterson, E., Tregellas, J., Hepburn, S., & Rojas, D. C. (2013). Differences in global and local level information processing in autism: An fMRI investigation. Psychiatry Research, 213(2), 115–121. [DOI:10.1016/j.pscychresns.2013.02.005] [PMID]
Gebauer, L., Skewes, J., Hørlyck, L., & Vuust, P. (2014). Atypical perception of affective prosody in Autism Spectrum Disorder. NeuroImage. Clinical, 6, 370–378. [DOI:10.1016/j.nicl.2014.08.025] [PMID]
Gilbert, S. J., Bird, G., Brindley, R., Frith, C. D., & Burgess, P. W. (2008). Atypical recruitment of medial prefrontal cortex in autism spectrum disorders: An fMRI study of two executive function tasks. Neuropsychologia, 46(9), 2281-2291. [DOI:10.1016/j.neuropsychologia.2008.03.025] [PMID]
Gilbert, S. J., Williamson, I. D., Dumontheil, I., Simons, J. S., Frith, C. D., & Burgess, P. W. (2007). Distinct regions of medial rostral prefrontal cortex supporting social and nonsocial functions. Social Cognitive and Affective Neuroscience, 2(3), 217-226. [DOI:10.1093/scan/nsm014] [PMID]
Glover G. H. (2011). Overview of functional magnetic resonance imaging. Neurosurgery Clinics of North America, 22(2), 133–vii. [DOI:10.1016/j.nec.2010.11.001] [PMID]
Greene, D. J., Colich, N., Iacoboni, M., Zaidel, E., Bookheimer, S. Y., & Dapretto, M. (2011). Atypical neural networks for social orienting in autism spectrum disorders. NeuroImage, 56(1), 354-362. [DOI:10.1016/j.neuroimage.2011.02.031] [PMID]
Greicius, M. D., Srivastava, G., Reiss, A. L., & Menon, V. (2004).Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America, 101(13), 4637-4642. [DOI:10.1073/pnas.0308627101] [PMID]
Grill-Spector, K., Knouf, N., & Kanwisher, N. (2004). The fusiform face area subserves face perception, not generic within-category identification. Nature Neuroscience, 7(5), 555-562. [DOI:10.1038/nn1224] [PMID]
Hadjikhani, N., Åsberg Johnels, J., Zürcher, N. R., Lassalle, A., Guillon, Q., & Hippolyte, L., et al. (2017). Look me in the eyes: constraining gaze in the eye-region provokes abnormally high subcortical activation in autism. Scientific Reports, 7(1), 3163. [DOI:10.1038/s41598-017-03378-5] [PMID]
Han, D. H., Yoo, H. J., Kim, B. N., McMahon, W., & Renshaw, P. F. (2014). Brain activity of adolescents with high functioning autism in response to emotional words and facial emoticons. Plos One, 9(3), e91214. [DOI:10.1371/journal.pone.0091214] [PMID]
Harris, G. J., Chabris, C. F., Clark, J., Urban, T., Aharon, I., & Steele, S., et al. (2006). Brain activation during semantic processing in autism spectrum disorders via functional magnetic resonance imaging. Brain and Cognition, 61(1), 54-68. [DOI:10.1016/j.bandc.2005.12.015] [PMID]
He, C., Chen, Y., Jian, T., Chen, H., Guo, X., & Wang, J., et al. (2018). Dynamic functional connectivity analysis reveals decreased variability of the default-mode network in developing autistic brain. Autism Research, 11(11), 1479–1493. [DOI:10.1002/aur.2020] [PMID]
Hebb, A. O., & Ojemann, G. A. (2013). The thalamus and language revisited. Brain and Language, 126(1), 99-108. [DOI:10.1016/j.bandl.2012.06.010] [PMID]
Heberlein, A. S., & Saxe, R. R. (2005). Dissociation between emotion and personality judgments: Convergent evidence from functional neuroimaging. NeuroImage, 28(4), 770-777. [PMID]
Howsmon, D. P., Kruger, U., Melnyk, S., James, S. J., & Hahn, J. (2017). Classification and adaptive behavior prediction of children with autism spectrum disorder based upon multivariate data analysis of markers of oxidative stress and DNA methylation. PLoS Computational Biology, 13(3), e1005385. [DOI:10.1371/journal.pcbi.1005385] [PMID]
Hsu, C. T., Neufeld, J., & Chakrabarti, B. (2018). Reduced reward-related neural response to mimicry in individuals with autism. The European Journal of Neuroscience, 47(6), 610-618. [DOI:10.1111/ejn.13620] [PMID]
Iacoboni, M. (2005). Neural mechanisms of imitation. Current Opinion in Neurobiology, 15(6), 632-637. [DOI:10.1016/j.conb.2005.10.010] [PMID]
Ingalhalikar, M., Parker, D., Bloy, L., Roberts, T. P., & Verma, R. (2011). Diffusion based abnormality markers of pathology: Toward learned diagnostic prediction of ASD. NeuroImage, 57(3), 918-927. [DOI:10.1016/j.neuroimage.2011.05.023] [PMID]
Jack, A. (2018). Neuroimaging in neurodevelopmental disorders: Focus on resting-state fMRI analysis of intrinsic functional brain connectivity. Current Opinion in Neurology, 31(2), 140–148. [DOI:10.1097/WCO.0000000000000536] [PMID]
Jiang, X., Bollich, A., Cox, P., Hyder, E., James, J., & Gowani, S. A., et al. (2013). A quantitative link between face discrimination deficits and neuronal selectivity for faces in autism. NeuroImage. Clinical, 2, 320–331. [DOI:10.1016/j.nicl.2013.02.002] [PMID]
Jones, R. M., & Lord, C. (2013). Diagnosing autism in neurobiological research studies. Behavioural Brain Research, 251, 113-124. [DOI:10.1016/j.bbr.2012.10.037] [PMID]
Just, M. A., Keller, T. A., Malave, V. L., Kana, R. K., & Varma, S. (2012). Autism as a neural systems disorder: A theory of frontal-posterior underconnectivity. Neuroscience and Biobehavioral Reviews, 36(4), 1292-1313. [DOI:10.1016/j.neubiorev.2012.02.007] [PMID]
Karten, A., & Hirsch, J. (2015). Brief report: Anomalous neural deactivations and functional connectivity during receptive language in autism spectrum disorder: A functional MRI study. Journal of Autism and Developmental Disorders, 45(6), 1905-1914. [DOI:10.1007/s10803-014-2344-y] [PMID]
Keehn, B., Nair, A., Lincoln, A. J., Townsend, J., & Müller, R. A. (2016). Under-reactive but easily distracted: An fMRI investigation of attentional capture in autism spectrum disorder. Developmental Cognitive Neuroscience, 17, 46-56. [DOI:10.1016/j.dcn.2015.12.002] [PMID]
Kenworthy, L., Wallace, G. L., Birn, R., Milleville, S. C., Case, L. K., & Bandettini, P. A., et al. (2013). Aberrant neural mediation of verbal fluency in autism spectrum disorders. Brain and Cognition, 83(2), 218-226. [DOI:10.1016/j.bandc.2013.08.003] [PMID]
Kim, S., Borst, G., Thompson, W. L., Hopkins, R. O., Kosslyn, S. M., & Squire, L. R. (2013). Sparing of spatial mental imagery in patients with hippocampal lesions. Learning & memory (Cold Spring Harbor, N.Y.), 20(11), 657–663. [DOI:10.1101/lm.031633.113] [PMID]
Klapwijk, E. T., Aghajani, M., Colins, O. F., Marijnissen, G. M., Popma, A., & van Lang, N. D., et al. (2016). Different brain responses during empathy in autism spectrum disorders versus conduct disorder and callous-unemotional traits. Journal of Child Psychology and Psychiatry, 57(6), 737-747. [DOI:10.1111/jcpp.12498] [PMID]
Klin, A., Jones, W., Schultz, R., & Volkmar, F. (2003). The enactive mind, or from actions to cognition: Lessons from autism. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 358(1430), 345–360. [DOI:10.1098/rstb.2002.1202] [PMID]
Klugah-Brown, B., Luo, C., He, H., Jiang, S., Armah, G. K., & Wu, Y., et al. (2019). Altered dynamic functional network connectivity in frontal lobe epilepsy. Brain Topography, 32(3), 394–404. [DOI:10.1007/s10548-018-0678-z] [PMID]
Kosaka, H., Omori, M., Iidaka, T., Murata, T., Shimoyama, T., & Okada, T., et al. (2003). Neural substrates participating in acquisition of facial familiarity: An fMRI study. NeuroImage, 20(3), 1734-1742. [PMID]
Koyama, M. S., Di Martino, A., Zuo, X. N., Kelly, C., Mennes, M., & Jutagir, D. R., et al. (2011). Resting-state functional connectivity indexes reading competence in children and adults. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 31(23), 8617–8624. [DOI:10.1523/JNEUROSCI.4865-10.2011] [PMID]
Li, D., Karnath, H. O., & Xu, X. (2017). Candidate biomarkers in children with autism spectrum disorder: A review of MRI studies. Neuroscience Bulletin, 33(2), 219–237. [DOI:10.1007/s12264-017-0118-1] [PMID]
Li, G., Liu, P., Andari, E., Zhang, A., & Zhang, K. (2018). The role of amygdala in patients with euthymic bipolar disorder during resting state. Frontiers in Psychiatry, 9, 445. [DOI:10.3389/fpsyt.2018.00445] [PMID]
Liss, M., Saulnier, C., Fein, D., & Kinsbourne, M. (2006). Sensory and attention abnormalities in autistic spectrum disorders. Autism, 10(2), 155-172. [DOI:10.1177/1362361306062021] [PMID]
Maldjian, J. A., Laurienti, P. J., Kraft, R. A., & Burdette, J. H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19(3), 1233–1239. [DOI:10.1016/S1053-8119(03)00169-1] [PMID]
Moreno-López, L., Catena, A., Fernández-Serrano, M. J., Delgado-Rico, E., Stamatakis, E. A., & Pérez-García, M., et al. (2012). Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug and Alcohol Dependence, 125(3), 208–214. [DOI:10.1016/j.drugalcdep.2012.02.012] [PMID]
Moseley, R. L., Mohr, B., Lombardo, M. V., Baron-Cohen, S., & Hauk, O., et al. (2013). Brain and behavioral correlates of action semantic deficits in autism. Frontiers in Human Neuroscience, 7, 725. [PMID]
Moseley, R. L., Shtyrov, Y., Mohr, B., Lombardo, M. V., Baron-Cohen, S., & Pulvermüller, F. (2015). Lost for emotion words: What motor and limbic brain activity reveals about autism and semantic theory. NeuroImage, 104, 413-422. [DOI:10.1016/j.neuroimage.2014.09.046] [PMID]
Mueller, S., Keeser, D., Reiser, M. F., Teipel, S., & Meindl, T. (2012). Functional and Structural MR imaging in neuropsychiatric disorders, part 2: Application in schizophrenia and autism. American Journal of Neuroradiology, 33(11), 2033 - 2037. [DOI:10.3174/ajnr.A2800] [PMID]
Murdaugh, D. L., Deshpande, H. D., & Kana, R. K. (2016). The impact of reading intervention on brain responses underlying language in children with autism. Autism Research, 9(1), 141-154. [DOI:10.1002/aur.1503] [PMID]
Murphy, C. M., Christakou, A., Daly, E. M., Ecker, C., Giampietro, V., & Brammer, M., et al. (2014). Abnormal functional activation and maturation of fronto-striato-temporal and cerebellar regions during sustained attention in autism spectrum disorder. The American Journal of Psychiatry, 171(10), 1107–1116. [DOI:10.1176/appi.ajp.2014.12030352] [PMID]
Murphy, E. R., Norr, M., Strang, J. F., Kenworthy, L., Gaillard, W. D., & Vaidya, C. J. (2017). Neural basis of visual attentional orienting in childhood autism spectrum disorders. Journal of Autism and Developmental Disorders, 47(1), 58-67. [DOI:10.1007/s10803-016-2928-9] [PMID]
Neufeld, N. H., Mulsant, B. H., Dickie, E. W., Meyers, B. S., Alexopoulos, G. S., & Rothschild, A. J., et al. (2018). Resting state functional connectivity in patients with remitted psychotic depression: A multi-centre STOP-PD study. EBioMedicine, 36, 446–453. [DOI:10.1016/j.ebiom.2018.09.025] [PMID]
Oberhuber, M., Parker Jones, , Hope, T. M., Prejawa, S., Seghier, M. L., & Green, D. W., et al. (2013). Functionally distinct contributions of the anterior and posterior putamen during sublexical and lexical reading. Frontiers in Human Neuroscience, 7, 787. [PMID]
Oghabian, M. A., Batouli, S. A., Norouzian, M., Ziaei, M., & Sikaroodi, H. (2010). Using functional Magnetic Resonance Imaging to differentiate between healthy aging subjects, Mild Cognitive Impairment, and Alzheimer's patients. Journal of Research in Medical Sciences : The Official Journal of Isfahan University of Medical Sciences, 15(2), 84–93. [PMID]
Ohta, H., Yamada, T., Watanabe, H., Kanai, C., Tanaka, E., & Ohno, T., et al. (2012). An fMRI study of reduced perceptual load-dependent modulation of task-irrelevant activity in adults with autism spectrum conditions. NeuroImage, 61(4), 1176-1187. [DOI:10.1016/j.neuroimage.2012.03.042] [PMID]
Pagnozzi, A. M., Conti, E., Calderoni, S., Fripp, J., & Rose, S. E. (2018). A systematic review of structural MRI biomarkers in autism spectrum disorder: A machine learning perspective. International Journal of Developmental Neuroscience, 71, 68-82. [PMID]
Palmen, S. J., & van Engeland, H. (2004). Review on structural neuroimaging findings in autism. Journal of Neural Transmission, 111(7), 903-929. [DOI:10.1007/s00702-003-0068-9] [PMID]
Parker, H., Hoad, C. L., Tucker, E., Costigan, C., Marciani, L., & Gowland, P., et al. (2018). Gastric motor and sensory function in health assessed by magnetic resonance imaging: Establishment of reference intervals for the Nottingham test meal in healthy subjects. Neurogastroenterology and Motility, 30(12), e13463. [DOI:10.1111/nmo.13463] [PMID]
Pelphrey, K. A., Morris, J. P., & McCarthy, G. (2005). Neural basis of eye gaze processing deficits in autism. Brain, 128(5), 1038-1048. [DOI:10.1093/brain/awh404] [PMID]
Pelphrey, K. A., Morris, J. P., McCarthy, G., & Labar, K. S. (2007). Perception of dynamic changes in facial affect and identity in autism. Social Cognitive and Affective Neuroscience, 2(2), 140-149. [DOI:10.1093/scan/nsm010] [PMID]
Philip, R. C., Dauvermann, M. R., Whalley, H. C., Baynham, K., Lawrie, S. M., & Stanfield, A. C. (2012). A systematic review and meta-analysis of the fMRI investigation of autism spectrum disorders. Neuroscience & Biobehavioral Reviews, 36(2), 901-942. [PMID]
Pierce, K., Müller, R. A., Ambrose, J., Allen, G., & Courchesne, E. (2001). Face processing occurs outside the fusiform ‘face area’ in autism: Evidence from functional MRI. Brain, 124(10), 2059-2073. [DOI:10.1093/brain/124.10.2059] [PMID]
Pina-Camacho, L., Villero, S., Fraguas, D., Boada, L., Janssen, J., & Navas-Sánchez, F. J., et al. (2012). Autism spectrum disorder: Does neuroimaging support the DSM-5 proposal for a symptom dyad? A systematic review of functional magnetic resonance imaging and diffusion tensor imaging studies. Journal of Autism and Developmental Disorders, 42(7), 1326-1341. [DOI:10.1007/s10803-011-1360-4] [PMID]
Pinal, D., Zurrón, M., & Díaz, F. (2014). Effects of load and maintenance duration on the time course of information encoding and retrieval in working memory: From perceptual analysis to post-categorization processes . Frontiers in Human Neuroscience, 8, 165. [PMID]
Rahko, J. S., Vuontela, V. A., Carlson, S., Nikkinen, J., Hurtig, T. M., & Kuusikko-Gauffin, S., et al. (2016). Attention and working memory in adolescents with autism spectrum disorder: A functional MRI study. Child Psychiatry and Human Development, 47(3), 503–517. [DOI:10.1007/s10578-015-0583-6] [PMID]
Razavi, F., Raminfard, S., Kalantar Hormozi, H., Sisakhti, M., & Batouli, S. A. H. (2021). A probabilistic atlas of the pineal gland in the standard space. Frontiers in Neuroinformatics, 15, 554229.[DOI:10.3389/fninf.2021.554229] [PMID]
Retico, A., Tosetti, M., Muratori, F., & Calderoni, S. (2014). Neuroimaging-based methods for autism identification: A possible translational application? Functional Neurology, 29(4), 231-239. [PMID]
Richards, T. L., & Berninger, V. W. (2008). Abnormal fMRI connectivity in children with dyslexia during a phoneme task: Before but not after treatment . Journal of Neurolinguistics, 21(4), 294-304. [DOI:10.1016/j.jneuroling.2007.07.002] [PMID]
Sachdev, P. S., Lee, T., Wen, W., Ames, D., Batouli, A. H., & Bowden, J., et al. (2013). The contribution of twins to the study of cognitive ageing and dementia: The older Australian Twins study. International Review of Psychiatry, 25(6), 738-747. [DOI:10.3109/09540261.2013.870137] [PMID]
Samson, F., Mottron, L., Soulières, I., & Zeffiro, T. A. (2012).Enhanced visual functioning in autism: An ALE meta-analysis. Human Brain Mapping, 33(7), 1553-1581. [PMID]
Sandin, S., Lichtenstein, P., Kuja-Halkola, R., Larsson, H., Hultman, C. M., & Reichenberg, A. (2014). The familial risk of autism. JAMA, 311(17), 1770-1777. [DOI:10.1001/jama.2014.4144] [PMID]
Sato, W., Kochiyama, T., Uono, S., Yoshimura, S., & Toichi, M. (2017). Neural mechanisms underlying conscious and unconscious gaze-triggered attentional orienting in autism spectrum disorder. Frontiers in Human Neuroscience, 11, 339. [DOI:10.3389/fnhum.2017.00339] [PMID]
Schindler, A., & Bartels, A. (2018). Human V6 integrates visual and extra-retinal cues during head-induced gaze shifts. IScience, 7, 191-197. [PMID]
Schirmer, A., & Kotz, S. (2006). Beyond the right hemisphere: Brain mechanisms mediating vocal emotional processing. Trends in Cognitive Sciences, 10(1), 24–30. [DOI:10.1016/j.tics.2005.11.009] [PMID]
Schwarzkopf, D. S., Anderson, E. J., de Haas, B., White, S. J., & Rees, G. (2014). Larger extrastriate population receptive fields in autism spectrum disorders. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 34(7), 2713-2724. [DOI:10.1523/JNEUROSCI.4416-13.2014] [PMID]
Simonoff, E., Pickles, A., Charman, T., Chandler, S., Loucas, T., & Baird, G. (2008). Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child & Adolescent Psychiatry, 47(8), 921-929. [PMID]
Simons, J. S., Schölvinck, M. L., Gilbert, S. J., Frith, C. D., & Burgess, P. W. (2006). Differential components of prospective memory?: Evidence from fMRI. Neuropsychologia, 44(8), 1388-1397. [PMID]
Spencer, M. D., Holt, R. J., Chura, L. R., Suckling, J., Calder, A. J., & Bullmore, E. T., et al. (2011). A novel functional brain imaging endophenotype of autism: The neural response to facial expression of emotion. Translational Psychiatry, 1(7), e19.[DOI:10.1038/tp.2011.18] [PMID]
Sridharan, D., Levitin, D. J., & Menon, V. (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America, 105(34), 12569-12574. [DOI:10.1073/pnas.0800005105] [PMID]
Steiner, A. M., Koegel, L. K., Koegel, R. L., & Ence, W. A. (2012). Issues and theoretical constructs regarding parent education for autism spectrum disorders. Journal of Autism and Developmental Disorders, 42(6), 1218-1227. [DOI:10.1007/s10803-011-1194-0] [PMID]
Stigler, K. A., McDonald, B. C., Anand, A., Saykin, A. J., & McDougle, C. J. (2011). Structural and functional magnetic resonance imaging of autism spectrum disorders. Brain Research, 1380, 146-161. [DOI:10.1016/j.brainres.2010.11.076] [PMID]
Szpir, M. (2006). Tracing the origins of autism: A spectrum of new studies. Environmental Health Perspectives, 114(7), A412-A418. [PMID]
Takeuchi, M., Harada, M., Matsuzaki, K., Nishitani, H., & Mori, K. (2004). Difference of signal change by a language task on autistic patients using functional MRI. The Journal of Medical Investigation : JMI, 51(1-2), 59-62. [DOI:10.2152/jmi.51.59] [PMID]
Tesink, C. M., Buitelaar, J. K., Petersson, K. M., van der Gaag, R. J., Kan, C. C., & Tendolkar, I., et al. (2009). Neural correlates of pragmatic language comprehension in autism spectrum disorders. Brain, 132(7), 1941-1952. [DOI:10.1093/brain/awp103] [PMID]
Tomaiuolo, F., Cecchetti, L., Gibson, R. M., Logi, F., Owen, A. M., & Malasoma, F., et al. (2016). Progression from vegetative to minimally conscious state is associated with changes in brain neural response to passive tasks: A longitudinal single-case functional MRI study. Journal of the International Neuropsychological Society, 22(6), 620-630. [DOI:DOI: 10.1017/S1355617716000485] [PMID]
Verhoeven, J. S., De Cock, P., Lagae, L., & Sunaert, S. (2010).Neuroimaging of autism. Neuroradiology, 52(1), 3-14. [DOI:10.1007/s00234-009-0583-y] [PMID]
Voineagu, I., & Yoo, H. (2013). Current progress and challenges in the search for autism biomarkers. Disease Markers, 35(1), 55–65. [DOI:10.1155/2013/476276] [PMID]
Walsh, P., Elsabbagh, M., Bolton, P., & Singh, I. (2011). In search of biomarkers for autism: scientific, social and ethical challenges. Nature Reviews. Neuroscience, 12(10), 603–612. [DOI:10.1038/nrn3113] [PMID]
Wang, A. T., Dapretto, M., Hariri, A. R., Sigman, M., & Bookheimer, S. Y. (2004). Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 43(4), 481-490. [DOI:10.1097/00004583-200404000-00015] [PMID]
Weikum, W. M., Vouloumanos, A., Navarra, J., Soto-Faraco, S., Sebastián-Gallés, N., & Werker, J. F. (2007). Visual language discrimination in infancy. Science, 316(5828), 1159. [DOI:10.1126/science.1137686] [PMID]
Wildgruber, D., Riecker, A., Hertrich, I., Erb, M., Grodd, W., & Ethofer, T., et al. (2005). Identification of emotional intonation evaluated by fMRI. NeuroImage, 24(4), 1233-1241. [PMID]
Williams, D. L., & Minshew, N. J. (2007). Understanding autism and related disorders: What has imaging taught us? Neuroimaging Clinics of North America, 17(4), 495-ix. [DOI:10.1016/j.nic.2007.07.007] [PMID]
Williams, J. H., Waiter, G. D., Perra, O., Perrett, D. I., & Whiten, A. (2005). An fMRI study of joint attention experience. NeuroImage, 25(1), 133-140. [DOI:10.1016/j.neuroimage.2004.10.047] [PMID]
Wise, R. G., & Preston, C. (2010). What is the value of human FMRI in CNS drug development? Drug Discovery Today, 15(21), 973-980. [DOI:10.1016/j.drudis.2010.08.016] [PMID]
Zahn, R., Moll, J., Krueger, F., Huey, E. D., Garrido, G., & Grafman, J. (2007). Social concepts are represented in the superior anterior temporal cortex. Proceedings of the National Academy of Sciences of the United States of America, 104(15), 6430–6435. [DOI:10.1073/pnas.0607061104] [PMID]
Zare Sadeghi, A., Jafari, A. H., Oghabian, M. A., Salighehrad, H. R., Batouli, S. A. H., & Raminfard, S., et al.(2017). Changes in effective connectivity network patterns in drug abusers, treated with different methods. Basic and Clinical Neuroscience, 8(4), 285-298. [DOI:10.18869/nirp.bcn.8.4.285] [PMID]
Zheng, Z., Zhang, L., Li, S., Zhao, F., Wang, Y., & Huang, L., et al. (2017). Association among obesity, overweight and autism spectrum disorder: A systematic review and meta-analysis. Scientific Reports, 7(1), 11697. [DOI:10.1038/s41598-017-12003-4] [PMID]
Zürcher, N. R., Donnelly, N., Rogier, O., Russo, B., Hippolyte, L., & Hadwin, J., et al. (2013). It’s all in the eyes: Subcortical and cortical activation during grotesqueness perception in autism. PloS One, 8(1), e54313. [DOI:10.1371/journal.pone.0054313] [PMID]
Zwaigenbaum, L., Bauman, M. L., Stone, W. L., Yirmiya, N., Estes, A., & Hansen, R. L., et al. (2015). Early identification of autism spectrum disorder: Recommendations for practice and research. Pediatrics, 136(Supplement 1), S10-40. [DOI:10.1542/peds.2014-3667C] [PMID]
Autism is a cognitive impairment involved in a spectrum of heterogeneous neurodevelopmental disabilities. It is characterized by possible difficulties, including impaired social interactions, significantly isolated living conditions, language difficulties, degraded face recognition, abject empathy, and constrained and repetitive behaviors, thoughts, and actions (Spencer et al., 2011). There are usual therapeutic methods used to treat ASD, such as multiple pharmacological treatments and target maladaptive co-occurring conditions, behavioral interventions, and advanced adaptive skills (Zwaigenbaum et al., 2015); however, treatment is more successful if it begins in the earlier stages of the disease. Early diagnosis means an early intervention and treatment, showing the impacts earlier. The affected children benefit from this early treatment before they reach their maximum brain plasticity and neural development (Calderoni et al., 2016; Dawson et al., 2010) controlled trial to evaluate the efficacy of the early start Denver model (ESDM).
There is no generally accepted diagnostic biomarker for autism (Howsmon et al., 2017). A biomarker is a characteristic objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention. The new biomarkers are expected to optimize or improve the current behavioral ASD diagnosis and early recognition of the pathological conditions; however, none of the ASD biomarkers has yet represented enough accuracy and specificity to be construed as clinical utility (Walsh et al., 2011). Accordingly, researchers have tried neuroimaging biomarkers to achieve an earlier diagnosis and enable earlier intervention (Zheng et al., 2017).
Functional magnetic resonance imaging (fMRI) is a non-invasive method capable of identifying the pattern and extent of activity of brain areas during a cognitive function (Batouli & Sisakhti, 2020). The popularity of fMRI is primarily due to its widespread availability, non-invasiveness, low cost, and appropriate spatial resolution (Glover, 2011). It has shown many research applications, namely in studies relevant to imaging genetics (Sachdev et al., 2013), addiction (Zare Sadeghi et al., 2017), language (Alemi et al., 2018), memory (Batouli & Sisakhti, 2019), emotion regulation (Batouli & Saba, 2020), motor function (Batouli et al., 2016), sensory function (Parker et al., 2018), and vision (Schindler & Bartels, 2018). In addition, numerous reports are available on the clinical applications of fMRI. Examples include depression (Neufeld et al., 2018), bipolar disorder (Li et al., 2018), Alzheimer (Oghabian & Batouli, 2010), aging (Batouli et al., 2009), autism (He et al., 2018), epilepsy (Klugah-Brown et al., 2018), and coma (Tomaiuolo et al., 2016). It is also used as a biomarker for various diseases (Batouli et al., 2021; Batouli et al., 2020; Greicius et al., 2004), to monitor a therapy (Richards & Berninger, 2008) or to study the pharmacological efficacy (Wise & Preston, 2010).
In a literature review, we identified 18 studies that had reviewed the MRI studies on ASD. The studies were primarily focused on examining the findings on autism, aiming to improve the diagnosis accuracy. Among these studies, three articles were focused merely on the results of structural MRI in autism (Baribeau & Anagnostou, 2013; Pagnozzi et al., 2018; Palmen & van Engeland, 2004), two articles reviewed resting state fMRI works (Jack, 2018; Li et al., 2017), and among the 12 studies which examined the task-based fMRI studies on autism, 11 studies were old (more than six years ago) (Anagnostou & Taylor, 2011; Brambilla et al., 2004; Cody et al., 2002; Dichter, 2012; Mueller et al., 2012; Philip et al., 2012; Pina-Camacho et al., 2012; Stigler et al., 2011; Verhoeven et al., 2010; Voineagu & Yoo, 2013; Williams & Minshew, 2007), and only five articles provided biomarkers for autism diagnosis. Except for one study (Philip et al., 2012), none provided a map in which the fMRI findings on ASD were summarized. The study by Philip et al. (Philip et al., 2012) is an excellent attempt to provide neuroimaging biomarkers for ASD. In this meta-analysis of 95 fMRI papers on ASD, six categories of cognitive functions were of interest: motor, visual, executive, auditory, social, and complex cognition functions. Using the activation likelihood estimation algorithm, they provided maps in which the brain areas with higher and lower activation probabilities in the ASD group were compared to those of typically developing individuals. The maps and the primary brain areas of difference were provided for all the six groups of cognitive functions.
In using any diagnostic method for ASD, having access to the most reliable and frequent biomarkers is necessary. Accordingly, this study reviews the available task-based fMRI studies on ASD with the following objectives: identify the cognitive functions that are mainly studied in ASD patients; evaluate the potentials of fMRI for ASD diagnosis; and identify the differences of fMRI maps between ASD and normal individuals as diagnostic biomarkers. A biomarker in this study is defined as a brain area that shows a different activation pattern in fMRI in the ASD group compared to expected.
2. Materials and Methods
Data sources
The PubMed and Science Direct databases were searched to identify relevant studies with no date limitation. Different combinations (AND/OR) of the following keywords were used for the search: “Functional MRI”, “brain functionality”, “task-based fMRI”, “brain networks”, autism spectrum”, “ASD”, “PDD”, “pervasive developmental disorder”, “autistic”, and “asperger”. This search resulted in 651 papers. Due to the large number of studies in our initial search, a manual search of the references of the selected studies was not performed for missing documents. Our detailed search strategy is provided while registering for the study.
Study selection
The title, abstract, and full text of the identified manuscripts (if needed) were studied to select the appropriate reports. The studies with the following conditions were initially excluded: non-human studies; studies on diseases other than ASD or only on patients without average population; any imaging modality other than fMRI; and resting-state fMRI or functional/effective connectivity analyses. The inclusion criteria were as follows: 1) published in a peer-reviewed journal; 2) written in the English language; 3) a comparison between autistic and typically developed individuals using task-based fMRI; 3) having a fully and clearly explained methodology, and 4) reporting the brain areas with a different activation between the normal and ASD individuals in the considered fMRI stimulus.
These criteria resulted in 292 papers. The papers were categorized in terms of their evaluated cognitive function, and the number of documents in each category was as follows: language=26; facial expression=21; attention=17; reward=17; theory of mind (TOM)=16; social cognition=15; face processing=13; eye gaze=13; emotion=13; social interaction=11; working memory=11; decision making=9; sensory processing=9; executive function=8; inhibition=8; visuospatial processing=8; face detection=7; location detection=7; response monitoring=7; body motion=7; imitation=6; social perception=5; pain=5; mirror neurons=4; learning=4; voice processing=4; temporal discounting=2; self-face processing=2; counting=1; restricted interests=1; object recognition=1; judgment=1; mentalizing=1; physical condition=1; cognitive flexibility=1; geometric reasoning=1; analogical reasoning=1; self-evaluative processing=1; change detection=1; deviance detection=1; novelty detection=1; empathy processing=1; spatial working memory=1; gesture expression=1; and mental rotation=1.
Given the high number of papers, we selected four categories of the stimulus types with the highest number of studies. As a result, facial expression, face processing, and face detection papers were regarded as category one (n=41), social cognition, social interaction, social perception, and theory of mind studies were considered as category two (n=47), language was category three (n=26), and attention was selected as category four (n=17).
Quality check
The quality of the included studies was checked. In this process, each study was scored from 0 to 12, with a higher score showing a better quality. This assessment included questions on the following items: a) case definition being adequate (score: 0, 1, 2); b) how much the patient group represented the community of ASD (score: 0, 1); c) how the control group participants were selected (score: 0, 1, 2); d) adequate definition of the control group (score: 0, 1); e) how comparable were the patient and control groups regarding the statistical analysis (score: 0, 1, 2); f) how reliable were the imaging and neurocognitive instruments and task (score: 0, 1, 2); g) the patients and controls being assessed in a similar methodology (score: 0, 1); and h) how the non-participation rate of the study was (score: 0, 1). Based on this assessment and the scoring instructions, all the included papers received a score above 9.
Data extraction
The following information was extracted from each article: I) study: authors, journal, originality, study design, number of sessions, and any intervention; II) participants: inclusion and exclusion criteria, number of cases and controls, age, gender, and handedness; III) data acquisition: type of MRI machine and head coil, details of the imaging protocol, and the fMRI task; IV) outcome measures: number and name of the outcome measures, the analysis software, other tests; and V) findings: results, conclusion, and the suggested biomarkers. As provided in Table 1, the primary information extracted from each study was the name of the brain areas that showed an altered (higher or lower) activation between the ASD and control groups during the cognitive tasks.



Data mapping
To visualize the brain structures that showed an altered activation between the normal and ASD groups in the four cognitive functions of interest, we prepared two maps for each of the cognitive functions. The resulting eight maps are illustrated in Figure 1. The maps in warm colors show the brain areas with higher activation in the ASD group. In comparison, the maps with cool colors demonstrate the brain structures with lower activation in ASD individuals compared to normal subjects. The intensity of colors in each map is also proportional to the number of reports for each structure. In other words, the maps provided in Figure 1 first illustrate the brain regions that showed a different activation between the ASD and normal individuals, and second, the brightness of the colors in the maps are in proportion to the number of reports on each region; a brighter yellow or a brighter blue shows that higher number of studies had reported changes in the activation of that particular brain area.

Preparing the maps was based on the methods of our previous study (Batouli & Saba, 2017; Razavi et al., 2021). Accordingly, a region of interest (ROI) was first created for each brain structure using the automated anatomical labeling atlas in the Montreal Neurologic Institute (MNI) space. It was performed using the “wfu pickatlas” toolbox in the MATLAB software (Maldjian et al., 2003). For structures whose ROI was unavailable in the automated anatomical labeling atlas, the creation and extraction of the ROIs were performed using the “Freeview” toolbox of the “Freesurfer” package software, version 5.3a). The global brain measures and the total lobar volumes from these maps were excluded better to understand the single brain areas with an altered activation. Next, the ROIs were added together to make a map, using codes written in the “SPM12” toolbox in the MATLAB software, version R2016a.v9.0) which showed the brain areas with lower or higher activation in the ASD group in any of those cognitive functions. As stated above, the intensity of colors here was proportional to the number of reports for each brain structure. The resulting maps are illustrated in Figure 1.
In addition to the maps which showed the areas of the brain with an altered activation, we identified the ratio of the grey matter of the brain that is showing a functional change in the ASD group. In other words, the aim was to estimate and illustrate the amount of overlap between the grey matter tissue of the brain and the brain areas with an altered activation in fMRI. First, the normalization of the ROI maps of Figure 1 to the MNI space was performed. Next, the MNI atlas (Montreal Neurologic Institute; MNI152 template) was segmented into its different tissue types, including grey matter (GM), white matter (WM), cerebrospinal fluid (CSF), and three other tissue types, using the “Segment” toolbox in “SPM12”. Finally, using a code in MATLAB, the number of voxels in the brain function maps which had an overlap with the grey matter tissue of the brain was counted and reported in proportion to the total number of voxels in the GM map. Hence, a ratio was estimated that illustrated the portion of the GM tissue of the brain which showed an altered activation in the cognitive functions of interest. These maps are illustrated in Figure 2.

3. Results
Summary of the included studies
There were 131 case-control studies in the four selected cognitive functions. A total of 2953 patients and 2799 typically developing individuals were evaluated. Of this population, around 86% were male and 35% of the studies included only male participants. Regarding the MRI machine, 73% of the studies used a 3 Tesla MRI scanner, and the rest used 1.5T, along with using 8, 12, or 32-channel head coils.
Around 79% of the studies were performed in one session. Other studies used 2, 3, 4, or even 5 sessions. The sessions involved preparation, MRI scanning, neurocognitive assessments, behavioral testing, and treatments and interventions. Only 3% of the studies had an intervention, including intranasal administration of oxytocin, pivotal response treatment, taking medications, and visualizing and verbalizing for language comprehension and thinking.
There were different criteria in the studies for the inclusion of patients, such as receiving a diagnosis of autism or Asperger syndrome from an independent clinician based on standard criteria of the Diagnostic and Statistical Manual of Mental Disorders, fourth edition; the autism diagnostic observational schedule-generic; autism diagnostic interview-revised; the social responsiveness scale; childhood autism rating scale; clinical impressions and experienced clinical judgment; and a series of clinical assessments that included a detailed developmental history, clinical interview and observation, medical workup, and cognitive testing. An IQ of 80 or above, measured by the Wechsler abbreviated intelligence scale, was necessary in some studies. The control groups were mainly composed of typically developing children or adolescents with no history of psychiatric or neurological disorders and they were matched with the patients in IQ, age, gender, race, and socioeconomic status of the family.
A summary of the findings of the studies is provided in Table 1. This table illustrates the brain areas with reports on a different activation between cases and controls in the four functions and the number of reports for each area. The areas with more than two reports on their different activation between the two groups are elaborated below in the order of their frequency of being reported. References of the selected 131 studies are provided as supplementary data.
Findings of the social tasks
A total of 30 brain areas showed a different activation between cases and controls during the processing of social tasks; 21 only showed lower activation in patients, 1 had a higher activation, and 8 areas had lower and higher reports. The brain areas with lower activation in patients were temporoparietal junction (n=13), inferior frontal gyrus (IFG; n=12), superior temporal gyrus (STG; n=12), medial prefrontal (n=8), supplementary motor area (SMA; n=6), precuneus (n=6), anterior cingulate cortex (ACC; n=6), posterior cingulate cortex (PCC; n=6), inferior parietal lobule (n=5), anterior insula (n=5), caudate (n=4), cuneus (n=4), medial frontal (n=3), and insula (n=3). Post central gyrus (n=4) was the only brain structure with a higher activation in patients.
Findings of the face processing tasks
A total of 40 brain regions showed a different activation between cases and controls when processing faces. Sixteen areas only showed a lower activation, 7 areas demonstrated only a higher activation, and 17 areas showed lower and higher activation. The areas with a lower activation included fusiform (n=19), amygdala (n=19), STG (n=7), ventromedial prefrontal cortex (VMPFC; n=6), occipital face area (n=5), IFG (n=5), inferior occipital (n=4), middle temporal gyrus (MTG; n=4), PCC (n=4), insula (n=4), middle frontal gyrus (MFG; n=3), cuneus (n=3), hippocampus (n=3), and ventral striatum (n=3). Higher activations in patients were observed in amygdala (n=6), pulvinar (n=4), thalamus (n=3), MFG (n=3), and precuneus (n=3).
Findings of the language tasks
A total of 48 brain regions showed a different activation level in the language tasks; meanwhile, 22 only had lower activation, 6 had higher, and 20 had higher and lower reports. The areas which showed a lower activation in patients in the language tasks were IFG (n=26), STG (n=11), cerebellum (n=9), MTG (n=8), fusiform (n=8), ACC (n=7), superior frontal gyrus (SFG; n=6), MFG (n=5), precentral gyrus (n=5), SMA (n=5), precuneus (n=5), cuneus (n=5), thalamus (n=5), occipito temporal gyrus (n=4), middle occipital gyrus (MOG; n=4), PCC (n=4), putamen (n=4), caudate (n=4), medial frontal gyrus (n=3), medial prefrontal gyrus (n=3), amygdala (n=3), and insula (n=3). The brain areas with a report on their higher activations in patients were STG (n=10), fusiform (n=8), MTG (n=5), IFG (n=4), MOG (n=4), posterior temporal gyrus (PTG; n=3), inferior parietal lobule (n=3), postcentral gyrus (n=3), and cuneus (n=3).
Findings of the attention tasks
A total of 39 regions showed differences in brain activations between the cases and controls; meanwhile, 14 areas were only lower in patients, 9 regions were only higher, and 16 areas had lower and higher reports. The areas with lower activation in patients were cerebellum (n=10), STG (n=9), IFG (n=6), precentral gyrus (n=6), SMA (n=6), postcentral gyrus (n=6), insula (n=6), MFG (n=4), medial prefrontal (n=4), superior parietal gyrus (SPG; n=4), putamen (n=4), thalamus (n=4), caudate (n=4), and orbitofrontal gyrus (n=3). The brain regions with higher activation in patients were medial prefrontal (n=7), precuneus (n=7), IFG (n=4), cerebellum (n=4), SMA (n=3), postcentral gyrus (n=3), and amygdala (n=3).
Overlap with the grey matter
As mentioned above, this study aimed to determine whether fMRI can reveal considerable data on ASD. A positive answer to this question would introduce fMRI as a potential diagnostic tool for ASD in future works. Accordingly, we assessed the overlap of the brain maps obtained for the four cognitive functions (illustrated in Figure 1) with the grey matter tissue of the brain, as explained in Materials and Methods. Figure 2 shows these overlaps as well as the percentage of the overlap.
The highest coverage of the GM was observed in the language tasks-low condition (69%); other estimates were attention tasks-low (64%), social tasks-low (55%), and face processing tasks-low (48%). These findings showed that a larger area of the brain showed a lower activation in the ASD individuals versus normal participants, compared to a higher activation. On the other hand, attention tasks-high and language tasks-high maps showed 44% coverage of the GM, 36% in the face processing-high, and 19% in social tasks-high maps.
When all eight brain maps in the four cognitive tasks were combined, 83% of the brain GM was covered, which suggests that fMRI is a strong tool in revealing signs of ASD.
4. Discussion
Summary of the results
This study reviewed the task-based fMRI studies on ASD aiming to assess the potential of fMRI in predicting or diagnosing ASD in the future. The severity of the difference between the fMRI measures of the normal and ASD groups could suggest the fMRI to be useful. By initially recruiting 292 studies and reviewing the results of 131 fMRI works in the four cognitive abilities of interest, 73 brain structures showed an altered brain function between the two groups. In addition, a combination of the results of the four cognitive functions showed that about 83% of brain GM shows an altered activation in ASD compared to normal individuals. Future research to diagnose ASD using automatic algorithms, such as machine learning, would benefit from the findings of this study.
Autism spectrum disorder diagnosis
The prevalence of ASD is 1 in 59 subjects in the US (Baio et al., 2018), and 1 in 132 individuals globally (Baxter et al., 2015) the burden of ASDs has been estimated for the global burden of disease study 2010 (GBD 2010. They are much higher than previously reported. This data reflects the growing knowledge about ASD and innovations in the diagnostic approaches which enhanced evaluation of its clinical symptoms (Sandin et al., 2014). The main characteristic of autism’s clinical manifestations is its significant heterogeneity; no two autism patients are alike, which means that each autistic individual shows unique phenotypic heterogeneity in the combination of symptom severity and comorbid conditions (anxiety, depression, social communication disorder, attention deficit hyperactivity disorder, epileptic disorders) (Jones & Lord, 2013). Early diagnosis would facilitate decisions for the selection of the best therapeutic method, improve the quality of life of children with ASD and their families (Pagnozzi et al., 2018), and lead to preventing notable economic and emotional costs to autistic people and their families. Despite all these, early diagnosis is challenging (Fein et al., 2013; Zwaigenbaum et al., 2015).
There are numerous undetermined questions about the causes and the pathophysiology of ASD (Li et al., 2017). To date, diagnostic evaluation of ASD is based on clinical observation and interviews with caregivers using some standardized instruments, including an autism diagnostic observational schedule (Steiner et al., 2012). The lack of knowledge on the exact neural basis of autism spectrum disorder and the absence of validated diagnosis measures throughout development is challenging. To date, diagnostic evaluation for autism requires a team of professionals from different disciplines, including a physician, speech therapist, cognitive therapist, and occupational therapist.
Since the first description of the disease in the early 1940s, researchers have been intensively trying to identify biological markers for ASD (Szpir, 2006). As mentioned before, the crucial challenges are the underlying biological heterogeneity of ASD. Nowadays, we can only reliably diagnose ASD based on the current standardized behavioral observations and psychometric tools (Steiner et al., 2012). Precise diagnostic assessments are required, as finding specific biomarkers for autism may reduce the variability of ASD diagnosis. Such methods are also helpful in evaluating treatment effects on the neurodevelopmental disorders.
Neuroimaging has already played a pivotal role in ASD characterization by the in vivo monitoring of the brain structure and function during the disorder (Retico et al., 2014). MRI is a safe, non-invasive, and powerful diagnostic tool for observing alterations in the brain’s structure and function. Moreover, fMRI techniques are essential in forming these diagnostic assessments (Li et al., 2017). fMRI is considered to provide the required biomarkers for exploring and diagnosing the severity of neurodevelopmental disorders, such as autism (Castellanos et al., 2013). Current research on structural and functional brain MRI indicates that identifying general biological alterations in most ASD patients requires large cohort sizes, and they often lead to numerous overlapping markers with those of other conditions and the public population, rather than a single marker (Voineagu & Yoo, 2013). As a result, more reliable algorithms are needed to use neuroimaging for purposes such as ASD diagnosis. Neuroimaging could be combined with machine learning methods for disease diagnosis or prediction (Andrews et al., 2018). These methods are already used for the ASD diagnosis. As an example, classification approaches using a support vector machine were used to characterize the structural changes of the brain in adults with ASD to discriminate them from the average population (Ecker et al., 2010). These methods have recently been reviewed (Andrews et al., 2018). In addition to separating patients and controls, these classifiers apply on a single-subject basis to aid diagnosis. This is done by assigning an abnormality score to each subject, quantifying the degree of pathology (Ingalhalikar et al., 2011). The current study aimed to initially show the potential of fMRI for ASD diagnosis along with machine learning algorithms and provide the biomarkers for fMRI in such works.
Face processing stimuli
The fusiform gyrus, as well as the amygdala, STG, VMPFC, occipital face area, IFG, IOG, MTG, PCC, insula, MFG, cuneus, hippocampus, and striatum showed lower activation in the ASD group when processing faces. Striatum is involved in processing rewards, and for individuals with high autistic traits, facial mimicry is associated with lower reward-related neural response (Hsu et al., 2018). Amygdala showed a lower activation during the attentional orienting triggered by eye gaze (Klapwijk et al., 2016), suggesting that impairments associated with gaze-triggered attentional orienting could be modified by treatments directed at amygdala activity (Sato et al, 2017). Decreased activation in the hippocampus also suggests problems integrating emotional information with declarative memory (Klapwijk et al., 2016).
Fusiform is vital in face processing (Grill-Spector et al., 2004) and its activation is associated with one’s face discrimination performance (Jiang et al., 2013). It is suggested that the phenotypic heterogeneity in face processing in ASD is mediated by neuronal selectivity to faces in this area (Jiang et al., 2013). Moreover, it is proposed that training on tasks that recruit face representation in this area could remediate face-processing differences (Jiang et al., 2013). Another study hypothesized that the abnormal activity of this area in autism could be due to inappropriate information acquisition during eye scanning (Pelphrey et al., 2007). Besides, ASD children differently recognize faces, as they focus more on feature-based than configured analyses, and the fusiform gyrus is associated with configural processing (Pelphrey et al., 2007). Also, fusiform face area activation depends on orientation toward the eyes during stimulus presentation (Zürcher et al., 2013); therefore, this reduced activation may be caused by atypical eye-gaze patterns towards faces.
MTG is involved in the processing of facial features and expressions (Critchley et al., 2000); meanwhile, it is also strongly modulated by top-down attentional mechanisms, hence, different attentional mechanisms concerning faces (Critchley et al., 2000) in ASD could be a reason for its declined activation. STG is active in tasks involving attributing intentions to moving geometric figures, and in social dysfunction in autism (Pelphrey et al., 2007). Other brain areas with a declined activation in ASD are also involved in face processing, such as the insula, a driving node in the salience network (Sridharan et al., 2008), PCC in acquiring facial familiarity (Kosaka et al., 2003), and IFG consistently being active during imitation, action observation, and intention understanding (Iacoboni, 2005).
Higher activations in individuals with ASD while processing faces were observed in the amygdala, pulvinar, thalamus, MFG, and precuneus. The pulvinar nucleus and amygdala have roles in rapid face processing (Hadjikhani et al., 2017). Higher activation in the subcortical areas, such as the amygdala, in children with ASD when looking at the eyes, is possibly related to their eye avoidance in daily life (Hadjikhani et al., 2017). The subcortical system in ASD overreacts to stimuli that should be considered positively engaging and socially rewarding (Hadjikhani et al., 2017), and for example, the amygdala hyper-responsiveness to direct gaze shows a neural indicator for heightened emotional arousal triggered by eye contact (Dalton et al., 2005). The thalamic hyperactivation is also hypothesized as one of the substrates of social cognition deficits in ASD, through its deregulatory impact on the dorsolateral prefrontal cortex (Hadjikhani et al., 2017). The activation of precuneus is modulated depending on attentional demands; therefore, its increased activation in ASD could be a compensatory mechanism (Wang et al., 2004). The amygdala reported higher, lower, and no difference in activation between the ASD and healthy individuals in face processing. The inconsistent findings could be due to the differences in attention to the faces (Klapwijk et al., 2016) or the type of tasks and stimuli (Pinal et al., 2014).
Language stimuli
Language function is impaired in ASD children. They have difficulty generating words relevant to a context or topic (Kenworthy et al., 2013) and processing mental states and words related to emotions (Moseley et al., 2015). This impairment is also observed in patients with a lesion in the motor system (Moseley et al., 2013). There is altered recruitment of reading-related neural resources in ASD children, weaknesses in the top-down modulation of semantic processing (Karten & Hirsch, 2015), and difficulties in using context to predict the final word of sentences (Catarino et al., 2011). Executive functions are critical for selecting, generating, and organizing words into sentences and sustaining meaningful conversations, and there are reports on impaired executive control of language in ASD (Kenworthy et al., 2013).
A total of 22 brain structures showed a declined activation in language processing in the ASD children, including IFG, STG, cerebellum, MTG, fusiform, ACC, SFG, MFG, precentral gyrus, SMA, precuneus, cuneus, thalamus, MOG, PCC, putamen, caudate, medial frontal gyrus, amygdala, and insula. These structures are involved in different aspects of the language processing. The thalamus's putamen and ventral lateral nucleus are involved in fluency-related activity (Kenworthy et al., 2013). In contrast, temporal regions are essential in auditory processing (Belin et al., 2000) particularly emotional prosody (Wildgruber et al., 2005). The ACC is activated in explicitly evaluating emotions (Bach et al., 2008). The thalamus has a role in language and verbal memory (Hebb & Ojemann, 2013), in addition to visual attention (Fan et al., 2005), suggestive of serial visual scanning during the initial steps of reading (Koyama et al., 2011). The left putamen has also been implicated in reading and language comprehension, especially in sub-lexical and lexical processing (Oberhuber et al., 2013).
The middle and superior frontal gyri are involved in executive functions (Moreno-López et al., 2012), attention, and working memory (Boisgueheneuc et al., 2006); therefore, they are related to the cognitive evaluation of the emotional content (Gebauer et al., 2014). LMFG is also reported to be a region involved in word retrieval during language production (Bednarz et al., 2017), and there are numerous reports on the activation of the LMFG during single-word reading, sentence reading, and lexical retrieval (Bednarz et al., 2017). This region is underactive in children with reading difficulty (Barquero et al., 2014). The lack of activation of the LMOG is previously shown in reading dysfunction, due to its role in reading intervention (Barquero et al., 2014). Frishkoff et al. (Frishkoff et al., 2004) also suggested that the anterior cingulate cortex discriminates congruous and incongruous words and its activity is sustained over time while processing semantic incongruities. In addition, the cerebellum has direct connections to Broca’s area, which enables it to facilitate verbal abilities (Harris et al., 2006).
Meanwhile, 9 brain structures showed an elevated activation in ASD individuals, including STG, fusiform, MTG, IFG, MOG, PTG, inferior parietal lobule, postcentral gyrus, and cuneus. The increased activation of brain areas in ASD is suggested to be a compensatory mechanism to aid in language comprehension (Murdaugh et al., 2016). There is evidence of recruiting more cortical resources during word generation in males and females with ASD (Beacher et al., 2012). Elevated fusiform activation has been shown in individuals with ASD to interpret the meaning of emotional words (Han et al., 2014). Greater activation of the cuneus is in line with the evidence of atypical reliance on the visual cortex in individuals with ASD (Chen et al., 2016), as the cuneus activation is related to visual perception and retrieving stored mental imagery of word stimuli (Kim et al., 2013). Individuals with ASD tend to recruit more visual cortex than IFG for word processing (Samson et al., 2012).
Another finding is using right hemisphere language-analogous regions in ASD to assist reading. This finding supports the idea that autism is related to early left-hemisphere dysfunction (Takeuchi et al., 2004). Studies suggest that ASD children are more likely to show right-hemisphere lateralization for language (Harris et al., 2006). For example, the right IFG has been more strongly implicated in emotional prosody processing than its contralateral homologs (Schirmer & Kotz, 2006). However, there are still disputes on whether the reversal of Broca’s asymmetry in the language is a predisposing factor toward language impairment or a compensatory neurodevelopmental response to language dysfunction in the left hemisphere (Harris et al., 2006), although the increased right hemisphere activity for the ASD group is mostly interpreted as reflecting more effortful processing (Tesink et al., 2009).
Commonly referred to as Broca’s area, the LIFG is involved in affective aspects of language processing, semantics, and visual memory (Beacher et al., 2012), in word processing and controlled retrieval and selection of semantic knowledge (Weikum et al., 2007), in unification operations required for binding single word information into larger structures (Fitzpatrick & Indefrey, 2009), in representing social knowledge and abstract social concepts (Zahn et al., 2007), and in attributing personality traits (Heberlein & Saxe, 2005). It also subserves verbal working memory by maintaining semantic representations (Bednarz et al., 2017). This area had 26 reports of decreased and 4 reports of higher activation in ASD. The activation of this area is dependent on the stimuli, for example, elevated activation of letter fluency vs category (Kenworthy et al., 2013), and attenuation of responsiveness to manipulations of semantic congruity (Beacher et al., 2012). In addition, the different activation of this region in ASD could be linked to other perceptual and expressive deficits in affective communication (Beacher et al., 2012).
Attention tasks
The neural mechanism of attention is different in ASD. The failure to orient attention toward salient stimuli is a fundamental problem in ASD that impairs social skills and learning (Klin et al., 2003). It could be due to the abnormalities in their functional brain maturation (Murphy et al., 2014), which is associated with clinical symptoms of ASD and inattention (Murphy et al., 2014). Neural circuitry of attention is driven by hyper-responsivity to salience (Murphy et al., 2017), and the observed differences may reflect compensatory mechanisms enabling normal behavioral performance (Rahko et al., 2015). In addition, over-focused attention in ASD results from hyperarousal, seen as disinhibiting the competing sensory information that leads to attentional shifts (Liss et al., 2006). Evidence shows that arousal regulation in ASD results from early deficits in disengaging attention (Rahko et al., 2015). ASD children are under-reactive to behaviorally-relevant stimuli. There are also findings of atypical functions of both top-down and bottom-up attention networks in these individuals, and they cannot filter irrelevant information (Keehn et al., 2016).
A total of 14 brain structures have been shown to have lower activation in patients with ASD, including cerebellum, STG, IFG, precentral gyrus, SMA, post-central gyrus, insula, MFG, medial prefrontal, SPG, putamen, thalamus, caudate, and orbitofrontal gyrus. The cerebellum plays an essential role in attentional processes (Keehn et al., 2016), and is implicated in the neuropathology of ASD (Fatemi et al., 2012). Caudate, STG, and SPG are involved in joint attention, facilitating attentional regulatory processes (Williams et al., 2005), which show deficits in ASD. STG also plays a role in utilizing social cues to orient attention. However, in ASD, this area may not be sensitive to the social meaning conveyed by eye gaze (Pelphrey et al., 2005). Finally, putamen responds when a stimulus is important, but not when the behavioral significance is removed (Greene et al., 2011).
On the contrary, 7 brain areas showed an elevated activation in ASD, including the medial prefrontal, precuneus, IFG, cerebellum, SMA, post-central gyrus, and amygdala. The activation of the visual cortex, particularly the precuneus, was significantly correlated with the severity of autistic symptoms (Ohta et al., 2012). Precuneus is implicated in spatial attention and anticipation of motor responses (Cavanna & Trimble, 2006), and the deficits in this area were positively correlated with the severity of social reciprocity and communication problems (Christakou et al., 2013). The increased response in children with ASD may reflect hyper-responsivity of bottom-up processing of salient visual stimuli (Murphy et al., 2017). This increase may be attributable to differences in the visual receptive fields in ASD. There is evidence of increased perifoveal population receptive fields in extrastriate cortex, associated with hyper-excitability of the visual cortex or poor peripheral spatial attention in individuals with ASD (Schwarzkopf et al., 2014).
Studies have indicated that the medial rostral PFC plays a role in attentional selection between stimulus-oriented and stimulus-independent thought (Gilbert et al., 2007). This region also involves multitasking and prospective memory (Simons et al., 2006). One suggestion based on these findings is that healthy individuals could modulate activity in their visual cortex according to the attentional demands of the task to a higher degree than the ASD group (Gilbert et al., 2008). This finding also suggests functional underconnectivity in ASD (Just et al., 2012), decreasing top-down modulation of sensory areas according to attentional demands (Gilbert et al., 2008). Moreover, the medial rostral PFC is involved in mentalizing (Gilbert et al., 2007), and its higher activation in ASD raises the possibility that these individuals recruit brain regions typically associated with mentalizing to perform other tasks, such as face perception (Pierce et al., 2001). As an example, the inability to suppress task-related activation of the medial prefrontal cortex is linked to distractibility in attention deficit hyperactivity disorder (Fassbender et al., 2009), and because of the high comorbidity of attention deficit hyperactivity disorder and autism (Simonoff et al., 2008), this may indicate a particular difficulty with distraction during the global condition (Gadgil et al., 2013).
5. Conclusion
This study, initially including 292 papers and studying 131 in detail, first showed which cognitive domains are currently mainly studied in ASD individuals. The most checked domains are the best candidates for diagnosis/prediction purposes. Also, the less studied cognitive functions could be suggestions for future works. Second, the study showed that a large part of the brain shows abnormalities in fMRI in ASD individuals compared to normal, suggesting fMRI's application in ASD diagnosis. For this aim, the study provided a comprehensive list of biomarkers for using task-based fMRI in the diagnosis/prediction of ASD, which included the brain structures that showed an altered activation in ASD and the frequency of reports for each structure. All the 73 brain areas illustrated in Figure 2 are ideal candidates for this purpose, with the brighter areas being stronger neuro-markers. FMRI imaging of an individual, and evaluating these biomarkers in methods such as machine-learning, could help assess an individual's risk of being in the ASD spectrum.
Study limitations
There are a few limitations to our work. Several studies may be missing during our database searches. Our inferences were based on 131 studies from 292, including many works that could result in more comprehensive deductions. Also, the methodology and participants of the selected studies were not identical, the factors that could have associations with the findings of a study. More detailed discussions could be provided on our findings, and finally, practically using the reported biomarkers for ASD diagnosis would be helpful as a validation algorithm.
Ethical Considerations
Compliance with ethical guidelines
This study was part of a systematic review that was registered at the International Prospective Register of Systematic Reviews (PROSPERO) (Code: PROSPERO_2017_CRD42017070975).
Funding
This study was financially supported by the Iranian Council for Cognitive Sciences and Technologies.
Authors' contributions
Conceptualization: Mehdi Tehrani Doost and Seyed Amir Hossein Batouli; Methodology: Foroogh Razavi, Zeinab Oghabian and Minoo Sisakhti; The original draft preparation: Minoo Sisakhti, Foroogh Razavi, Zeinab Oghabian and Haady Ahmadzade; Visualization, supervision and writing–review & editing: Mehdi Tehrani Doost and Seyed Amir Hossein Batouli; Project Administration and funding acquisition: Mehdi Tehrani Doost.
Conflict of interest
The authors declared no conflict of interest..
Acknowledgments
The authors appreciate the support from the Iranian Council for Cognitive Sciences and Technologies.
References
Alemi, R., Batouli, S. A. H., Behzad, E., Ebrahimpoor, M., & Oghabian, M. A. (2018). Not single brain areas but a network is involved in language: Applications in presurgical planning. Clinical Neurology and Neurosurgery, 165, 116-128. [DOI:10.1016/j.clineuro.2018.01.009] [PMID]
Anagnostou, E., & Taylor, M. J. (2011). Review of neuroimaging in autism spectrum disorders: What have we learned and where we go from here. Molecular Autism, 2(1), 4. [PMID]
Andrews, D. S., Marquand, A., Ecker, C., & McAlonan, G. (2018). Using pattern classification to identify brain imaging markers in autism spectrum disorder. Current Topics in Behavioral Neurosciences, 40, 413–436. [PMID]
Bach, D. R., Grandjean, D., Sander, D., Herdener, M., Strik, W. K., & Seifritz, E. (2008). The effect of appraisal level on processing of emotional prosody in meaningless speech. NeuroImage, 42(2), 919–927. [DOI:10.1016/j.neuroimage.2008.05.034] [PMID]
Baio, J., Wiggins, L., Christensen, D. L., Maenner, M. J., Daniels, J., & Warren, Z., et al. (2018). Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2014. Morbidity and Mortality Weekly Report. Surveillance Summaries (Washington, D.C. : 2002), 67(6), 1–23. [DOI:10.15585/mmwr.ss6706a1] [PMID]
Baribeau, D. A., & Anagnostou, E. (2013). A comparison of neuroimaging findings in childhood onset schizophrenia and autism spectrum disorder: A review of the literature. Frontiers in Psychiatry, 4, 175. [DOI:10.3389/fpsyt.2013.00175] [PMID]
Barquero, L. A., Davis, N., & Cutting, L. E. (2014). Neuroimaging of reading intervention: A systematic review and activation likelihood estimate meta-analysis. PloS One, 9(1), e83668-e83668. [DOI:10.1371/journal.pone.0083668] [PMID]
Batouli, S. A. H., Alemi, R., Khoshkhouy Delshad, H., & Oghabian, M. A. (2020). The influence of mental fatigue on the face and word encoding activations. Clinical Neurology and Neurosurgery, 189, 105626. [DOI:10.1016/j.clineuro.2019.105626] [PMID]
Batouli, S. A. H., Boroomand, A., Fakhri, M., Sikaroodi, H., Oghabian, M. A., & Firouznia, K. (2009). The effect of aging on resting state brain function: An fMRI study. Iranian Journal of Radiology, 6(3), 153-158. [Link]
Batouli, S. A., Hasani, N., Gheisari, S., Behzad, E., & Oghabian, M. A. (2016). Evaluation of the factors influencing brain language laterality in presurgical planning. Physica Medica, 32(10), 1201-1209. [DOI:10.1016/j.ejmp.2016.06.008] [PMID]
Batouli, S. A. H., & Saba, V. (2017). At least eighty percent of brain grey matter is modifiable by physical activity: A review study. Behavioural Brain Research, 332, 204–217. [DOI:10.1016/j.bbr.2017.06.002] [PMID]
Batouli, S. A. H., & Saba, V. (2020). Larger volume and a different activation of the brain in response to threat in military officers. Basic and Clinical Neuroscience, 11(5), 669-686. [DOI:10.32598/bcn.9.10.160] [PMID]
Batouli, S. A. H., & Sisakhti, M. (2019). Investigating a hypothesis on the mechanism of long-term memory storage. NeuroQuantology, 17(3), 60-79. [DOI:10.14704/nq.2019.17.3.1813]
Batouli, S. A. H., & Sisakhti, M. (2020). Some points to consider in a task-based fMRI study: A guideline for beginners. Frontiers in Biomedical Technologies, 7(1), 52-73. [Link]
Batouli, S. A. H., Sisakhti, M., Haghshenas, S., Dehghani, H., Sachdev, P., & Ekhtiari, H., et al. (2021). Iranian Brain Imaging Database: A neuropsychiatric database of healthy brain. Basic and Clinical Neuroscience, 12(1), 115–132. [DOI:10.32598/bcn.12.1.1774.2] [PMID]
Baxter, A. J., Brugha, T. S., Erskine, H. E., Scheurer, R. W., Vos, T., & Scott, J. G. (2015). The epidemiology and global burden of autism spectrum disorders. Psychological Medicine, 45(3), 601-613. [DOI:DOI: 10.1017/S003329171400172X] [PMID]
Beacher, F. D., Radulescu, E., Minati, L., Baron-Cohen, S., Lombardo, M. V., & Lai, M. C., et al. (2012). Sex differences and autism: brain function during verbal fluency and mental rotation. PloS One, 7(6), e38355. [DOI:10.1371/journal.pone.0038355] [PMID]
Bednarz, H. M., Maximo, J. O., Murdaugh, D. L., O'Kelley, S., & Kana, R. K. (2017). Decoding versus comprehension: Brain responses underlying reading comprehension in children with autism. Brain and Language, 169, 39-47. [PMID]
Belin, P., Zatorre, R. J., Lafaille, P., Ahad, P., & Pike, B. (2000). Voice-selective areas in human auditory cortex. Nature, 403(6767), 309-312. [DOI:10.1038/35002078] [PMID]
du Boisgueheneuc, F., Levy, R., Volle, E., Seassau, M., Duffau, H., & Kinkingnehun, S., et al. (2006). Functions of the left superior frontal gyrus in humans: A lesion study. Brain, 129(12), 3315-3328. [DOI:10.1093/brain/awl244] [PMID]
Brambilla, P., Hardan, A. Y., di Nemi, S. U., Caverzasi, E., Soares, J. C., & Perez, J., et al. (2004). The functional neuroanatomy of autism. Functional Neurology, 19(1), 9–17. [PMID]
Calderoni, S., Billeci, L., Narzisi, A., Brambilla, P., Retico, A., & Muratori, F. (2016). Rehabilitative interventions and brain plasticity in autism spectrum disorders: Focus on MRI-based studies. Frontiers in Neuroscience, 10, 139. [DOI:10.3389/fnins.2016.00139] [PMID]
Castellanos, F. X., Di Martino, A., Craddock, R. C., Mehta, A. D., & Milham, M. P. (2013). Clinical applications of the functional connectome. NeuroImage, 80, 527-540. [DOI:10.1016/j.neuroimage.2013.04.083] [PMID]
Catarino, A., Luke, L., Waldman, S., Andrade, A., Fletcher, P. C., & Ring, H. (2011). An fMRI investigation of detection of semantic incongruities in autistic spectrum conditions. The European Journal of Neuroscience, 33(3), 558–567. [DOI:10.1111/j.1460-9568.2010.07503.x] [PMID]
Cavanna, A. E., & Trimble, M. R. (2006). The precuneus: A review of its functional anatomy and behavioural correlates. Brain, 129(3), 564-583. [DOI:10.1093/brain/awl004] [PMID]
Chen, P. J., Gau, S. S., Lee, S. H., & Chou, T. L. (2016). Differences in age-dependent neural correlates of semantic processing between youths with autism spectrum disorder and typically developing youths. Autism Research, 9(12), 1263-1273. [DOI:10.1002/aur.1616] [PMID]
Christakou, A., Murphy, C. M., Chantiluke, K., Cubillo, A. I., Smith, A. B., & Giampietro, V., et al. (2013). Disorder-specific functional abnormalities during sustained attention in youth with Attention Deficit Hyperactivity Disorder (ADHD) and with Autism. Molecular Psychiatry, 18(2), 236-244. [DOI:10.1038/mp.2011.185] [PMID]
Cody, H., Pelphrey, K., & Piven, J. (2002). Structural and functional magnetic resonance imaging of autism. International Journal of Developmental Neuroscience, 20(3-5), 421–438. [PMID]
Critchley, H. D., Daly, E. M., Bullmore, E. T., Williams, S. C., Van Amelsvoort, T., & Robertson, D. M., et al. (2000). The functional neuroanatomy of social behaviour: Changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain, 123(11), 2203-2212. [DOI:10.1093/brain/123.11.2203] [PMID]
Critchley, H., Daly, E., Phillips, M., Brammer, M., Bullmore, E., & Williams, S., et al. (2000). Explicit and implicit neural mechanisms for processing of social information from facial expressions: A functional magnetic resonance imaging study. Human Brain Mapping, 9(2), 93-105. [PMID]
Dalton, K. M., Nacewicz, B. M., Johnstone, T., Schaefer, H. S., Gernsbacher, M. A., & Goldsmith, H. H., et al. (2005). Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience, 8(4), 519-526. [DOI:10.1038/nn1421] [PMID]
Dawson, G., Rogers, S., Munson, J., Smith, M., Winter, J., & Greenson, J., et al. (2010). Randomized, controlled trial of an intervention for toddlers with autism: The Early Start Denver Model. Pediatrics, 125(1), e17-e23. [DOI:10.1542/peds.2009-0958] [PMID]
Dichter, G. S. (2012). Functional magnetic resonance imaging of autism spectrum disorders. Dialogues in Clinical Neuroscience, 14(3), 319-351. [DOI:10.31887/DCNS.2012.14.3/gdichter] [PMID]
Ecker, C., Marquand, A., Mourão-Miranda, J., Johnston, P., Daly, E. M., & Brammer, M. J., et al. (2010). Describing the brain in autism in five dimensions-magnetic resonance imaging-assisted diagnosis of autism spectrum disorder using a multiparameter classification approach. The Journal of Neuroscience, 30(32), 10612 - 10623. [DOI:10.1523/JNEUROSCI.5413-09.2010] [PMID]
Fan, J., McCandliss, B. D., Fossella, J., Flombaum, J. I., & Posner, M. I. (2005). The activation of attentional networks. NeuroImage, 26(2), 471-479. [DOI:10.1016/j.neuroimage.2005.02.004] [PMID]
Fassbender, C., Zhang, H., Buzy, W. M., Cortes, C. R., Mizuiri, D., & Beckett, L., et al. (2009). A lack of default network suppression is linked to increased distractibility in ADHD. Brain Research, 1273, 114-128. [DOI:10.1016/j.brainres.2009.02.070] [PMID]
Fatemi, S. H., Aldinger, K. A., Ashwood, P., Bauman, M. L., Blaha, C. D., & Blatt, G. J., et al. (2012). Consensus paper: Pathological role of the cerebellum in autism. Cerebellum (London, England), 11(3), 777-807. [DOI:10.1007/s12311-012-0355-9] [PMID]
Fein, D., Barton, M., Eigsti, I. M., Kelley, E., Naigles, L., & Schultz, R. T., et al. (2013). Optimal outcome in individuals with a history of autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 54(2), 195-205. [DOI:10.1111/jcpp.12037] [PMID]
FitzPatrick, I., & Indefrey, P. (2010). Lexical competition in nonnative speech comprehension. Journal of Cognitive Neuroscience, 22(6), 1165–1178. [DOI:10.1162/jocn.2009.21301] [PMID]
Frishkoff, G. A., Tucker, D. M., Davey, C., & Scherg, M. (2004). Frontal and posterior sources of event-related potentials in semantic comprehension. Brain research. Cognitive Brain Research, 20(3), 329–354. [DOI:10.1016/j.cogbrainres.2004.02.009] [PMID]
Gadgil, M., Peterson, E., Tregellas, J., Hepburn, S., & Rojas, D. C. (2013). Differences in global and local level information processing in autism: An fMRI investigation. Psychiatry Research, 213(2), 115–121. [DOI:10.1016/j.pscychresns.2013.02.005] [PMID]
Gebauer, L., Skewes, J., Hørlyck, L., & Vuust, P. (2014). Atypical perception of affective prosody in Autism Spectrum Disorder. NeuroImage. Clinical, 6, 370–378. [DOI:10.1016/j.nicl.2014.08.025] [PMID]
Gilbert, S. J., Bird, G., Brindley, R., Frith, C. D., & Burgess, P. W. (2008). Atypical recruitment of medial prefrontal cortex in autism spectrum disorders: An fMRI study of two executive function tasks. Neuropsychologia, 46(9), 2281-2291. [DOI:10.1016/j.neuropsychologia.2008.03.025] [PMID]
Gilbert, S. J., Williamson, I. D., Dumontheil, I., Simons, J. S., Frith, C. D., & Burgess, P. W. (2007). Distinct regions of medial rostral prefrontal cortex supporting social and nonsocial functions. Social Cognitive and Affective Neuroscience, 2(3), 217-226. [DOI:10.1093/scan/nsm014] [PMID]
Glover G. H. (2011). Overview of functional magnetic resonance imaging. Neurosurgery Clinics of North America, 22(2), 133–vii. [DOI:10.1016/j.nec.2010.11.001] [PMID]
Greene, D. J., Colich, N., Iacoboni, M., Zaidel, E., Bookheimer, S. Y., & Dapretto, M. (2011). Atypical neural networks for social orienting in autism spectrum disorders. NeuroImage, 56(1), 354-362. [DOI:10.1016/j.neuroimage.2011.02.031] [PMID]
Greicius, M. D., Srivastava, G., Reiss, A. L., & Menon, V. (2004).Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America, 101(13), 4637-4642. [DOI:10.1073/pnas.0308627101] [PMID]
Grill-Spector, K., Knouf, N., & Kanwisher, N. (2004). The fusiform face area subserves face perception, not generic within-category identification. Nature Neuroscience, 7(5), 555-562. [DOI:10.1038/nn1224] [PMID]
Hadjikhani, N., Åsberg Johnels, J., Zürcher, N. R., Lassalle, A., Guillon, Q., & Hippolyte, L., et al. (2017). Look me in the eyes: constraining gaze in the eye-region provokes abnormally high subcortical activation in autism. Scientific Reports, 7(1), 3163. [DOI:10.1038/s41598-017-03378-5] [PMID]
Han, D. H., Yoo, H. J., Kim, B. N., McMahon, W., & Renshaw, P. F. (2014). Brain activity of adolescents with high functioning autism in response to emotional words and facial emoticons. Plos One, 9(3), e91214. [DOI:10.1371/journal.pone.0091214] [PMID]
Harris, G. J., Chabris, C. F., Clark, J., Urban, T., Aharon, I., & Steele, S., et al. (2006). Brain activation during semantic processing in autism spectrum disorders via functional magnetic resonance imaging. Brain and Cognition, 61(1), 54-68. [DOI:10.1016/j.bandc.2005.12.015] [PMID]
He, C., Chen, Y., Jian, T., Chen, H., Guo, X., & Wang, J., et al. (2018). Dynamic functional connectivity analysis reveals decreased variability of the default-mode network in developing autistic brain. Autism Research, 11(11), 1479–1493. [DOI:10.1002/aur.2020] [PMID]
Hebb, A. O., & Ojemann, G. A. (2013). The thalamus and language revisited. Brain and Language, 126(1), 99-108. [DOI:10.1016/j.bandl.2012.06.010] [PMID]
Heberlein, A. S., & Saxe, R. R. (2005). Dissociation between emotion and personality judgments: Convergent evidence from functional neuroimaging. NeuroImage, 28(4), 770-777. [PMID]
Howsmon, D. P., Kruger, U., Melnyk, S., James, S. J., & Hahn, J. (2017). Classification and adaptive behavior prediction of children with autism spectrum disorder based upon multivariate data analysis of markers of oxidative stress and DNA methylation. PLoS Computational Biology, 13(3), e1005385. [DOI:10.1371/journal.pcbi.1005385] [PMID]
Hsu, C. T., Neufeld, J., & Chakrabarti, B. (2018). Reduced reward-related neural response to mimicry in individuals with autism. The European Journal of Neuroscience, 47(6), 610-618. [DOI:10.1111/ejn.13620] [PMID]
Iacoboni, M. (2005). Neural mechanisms of imitation. Current Opinion in Neurobiology, 15(6), 632-637. [DOI:10.1016/j.conb.2005.10.010] [PMID]
Ingalhalikar, M., Parker, D., Bloy, L., Roberts, T. P., & Verma, R. (2011). Diffusion based abnormality markers of pathology: Toward learned diagnostic prediction of ASD. NeuroImage, 57(3), 918-927. [DOI:10.1016/j.neuroimage.2011.05.023] [PMID]
Jack, A. (2018). Neuroimaging in neurodevelopmental disorders: Focus on resting-state fMRI analysis of intrinsic functional brain connectivity. Current Opinion in Neurology, 31(2), 140–148. [DOI:10.1097/WCO.0000000000000536] [PMID]
Jiang, X., Bollich, A., Cox, P., Hyder, E., James, J., & Gowani, S. A., et al. (2013). A quantitative link between face discrimination deficits and neuronal selectivity for faces in autism. NeuroImage. Clinical, 2, 320–331. [DOI:10.1016/j.nicl.2013.02.002] [PMID]
Jones, R. M., & Lord, C. (2013). Diagnosing autism in neurobiological research studies. Behavioural Brain Research, 251, 113-124. [DOI:10.1016/j.bbr.2012.10.037] [PMID]
Just, M. A., Keller, T. A., Malave, V. L., Kana, R. K., & Varma, S. (2012). Autism as a neural systems disorder: A theory of frontal-posterior underconnectivity. Neuroscience and Biobehavioral Reviews, 36(4), 1292-1313. [DOI:10.1016/j.neubiorev.2012.02.007] [PMID]
Karten, A., & Hirsch, J. (2015). Brief report: Anomalous neural deactivations and functional connectivity during receptive language in autism spectrum disorder: A functional MRI study. Journal of Autism and Developmental Disorders, 45(6), 1905-1914. [DOI:10.1007/s10803-014-2344-y] [PMID]
Keehn, B., Nair, A., Lincoln, A. J., Townsend, J., & Müller, R. A. (2016). Under-reactive but easily distracted: An fMRI investigation of attentional capture in autism spectrum disorder. Developmental Cognitive Neuroscience, 17, 46-56. [DOI:10.1016/j.dcn.2015.12.002] [PMID]
Kenworthy, L., Wallace, G. L., Birn, R., Milleville, S. C., Case, L. K., & Bandettini, P. A., et al. (2013). Aberrant neural mediation of verbal fluency in autism spectrum disorders. Brain and Cognition, 83(2), 218-226. [DOI:10.1016/j.bandc.2013.08.003] [PMID]
Kim, S., Borst, G., Thompson, W. L., Hopkins, R. O., Kosslyn, S. M., & Squire, L. R. (2013). Sparing of spatial mental imagery in patients with hippocampal lesions. Learning & memory (Cold Spring Harbor, N.Y.), 20(11), 657–663. [DOI:10.1101/lm.031633.113] [PMID]
Klapwijk, E. T., Aghajani, M., Colins, O. F., Marijnissen, G. M., Popma, A., & van Lang, N. D., et al. (2016). Different brain responses during empathy in autism spectrum disorders versus conduct disorder and callous-unemotional traits. Journal of Child Psychology and Psychiatry, 57(6), 737-747. [DOI:10.1111/jcpp.12498] [PMID]
Klin, A., Jones, W., Schultz, R., & Volkmar, F. (2003). The enactive mind, or from actions to cognition: Lessons from autism. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 358(1430), 345–360. [DOI:10.1098/rstb.2002.1202] [PMID]
Klugah-Brown, B., Luo, C., He, H., Jiang, S., Armah, G. K., & Wu, Y., et al. (2019). Altered dynamic functional network connectivity in frontal lobe epilepsy. Brain Topography, 32(3), 394–404. [DOI:10.1007/s10548-018-0678-z] [PMID]
Kosaka, H., Omori, M., Iidaka, T., Murata, T., Shimoyama, T., & Okada, T., et al. (2003). Neural substrates participating in acquisition of facial familiarity: An fMRI study. NeuroImage, 20(3), 1734-1742. [PMID]
Koyama, M. S., Di Martino, A., Zuo, X. N., Kelly, C., Mennes, M., & Jutagir, D. R., et al. (2011). Resting-state functional connectivity indexes reading competence in children and adults. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 31(23), 8617–8624. [DOI:10.1523/JNEUROSCI.4865-10.2011] [PMID]
Li, D., Karnath, H. O., & Xu, X. (2017). Candidate biomarkers in children with autism spectrum disorder: A review of MRI studies. Neuroscience Bulletin, 33(2), 219–237. [DOI:10.1007/s12264-017-0118-1] [PMID]
Li, G., Liu, P., Andari, E., Zhang, A., & Zhang, K. (2018). The role of amygdala in patients with euthymic bipolar disorder during resting state. Frontiers in Psychiatry, 9, 445. [DOI:10.3389/fpsyt.2018.00445] [PMID]
Liss, M., Saulnier, C., Fein, D., & Kinsbourne, M. (2006). Sensory and attention abnormalities in autistic spectrum disorders. Autism, 10(2), 155-172. [DOI:10.1177/1362361306062021] [PMID]
Maldjian, J. A., Laurienti, P. J., Kraft, R. A., & Burdette, J. H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19(3), 1233–1239. [DOI:10.1016/S1053-8119(03)00169-1] [PMID]
Moreno-López, L., Catena, A., Fernández-Serrano, M. J., Delgado-Rico, E., Stamatakis, E. A., & Pérez-García, M., et al. (2012). Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug and Alcohol Dependence, 125(3), 208–214. [DOI:10.1016/j.drugalcdep.2012.02.012] [PMID]
Moseley, R. L., Mohr, B., Lombardo, M. V., Baron-Cohen, S., & Hauk, O., et al. (2013). Brain and behavioral correlates of action semantic deficits in autism. Frontiers in Human Neuroscience, 7, 725. [PMID]
Moseley, R. L., Shtyrov, Y., Mohr, B., Lombardo, M. V., Baron-Cohen, S., & Pulvermüller, F. (2015). Lost for emotion words: What motor and limbic brain activity reveals about autism and semantic theory. NeuroImage, 104, 413-422. [DOI:10.1016/j.neuroimage.2014.09.046] [PMID]
Mueller, S., Keeser, D., Reiser, M. F., Teipel, S., & Meindl, T. (2012). Functional and Structural MR imaging in neuropsychiatric disorders, part 2: Application in schizophrenia and autism. American Journal of Neuroradiology, 33(11), 2033 - 2037. [DOI:10.3174/ajnr.A2800] [PMID]
Murdaugh, D. L., Deshpande, H. D., & Kana, R. K. (2016). The impact of reading intervention on brain responses underlying language in children with autism. Autism Research, 9(1), 141-154. [DOI:10.1002/aur.1503] [PMID]
Murphy, C. M., Christakou, A., Daly, E. M., Ecker, C., Giampietro, V., & Brammer, M., et al. (2014). Abnormal functional activation and maturation of fronto-striato-temporal and cerebellar regions during sustained attention in autism spectrum disorder. The American Journal of Psychiatry, 171(10), 1107–1116. [DOI:10.1176/appi.ajp.2014.12030352] [PMID]
Murphy, E. R., Norr, M., Strang, J. F., Kenworthy, L., Gaillard, W. D., & Vaidya, C. J. (2017). Neural basis of visual attentional orienting in childhood autism spectrum disorders. Journal of Autism and Developmental Disorders, 47(1), 58-67. [DOI:10.1007/s10803-016-2928-9] [PMID]
Neufeld, N. H., Mulsant, B. H., Dickie, E. W., Meyers, B. S., Alexopoulos, G. S., & Rothschild, A. J., et al. (2018). Resting state functional connectivity in patients with remitted psychotic depression: A multi-centre STOP-PD study. EBioMedicine, 36, 446–453. [DOI:10.1016/j.ebiom.2018.09.025] [PMID]
Oberhuber, M., Parker Jones, , Hope, T. M., Prejawa, S., Seghier, M. L., & Green, D. W., et al. (2013). Functionally distinct contributions of the anterior and posterior putamen during sublexical and lexical reading. Frontiers in Human Neuroscience, 7, 787. [PMID]
Oghabian, M. A., Batouli, S. A., Norouzian, M., Ziaei, M., & Sikaroodi, H. (2010). Using functional Magnetic Resonance Imaging to differentiate between healthy aging subjects, Mild Cognitive Impairment, and Alzheimer's patients. Journal of Research in Medical Sciences : The Official Journal of Isfahan University of Medical Sciences, 15(2), 84–93. [PMID]
Ohta, H., Yamada, T., Watanabe, H., Kanai, C., Tanaka, E., & Ohno, T., et al. (2012). An fMRI study of reduced perceptual load-dependent modulation of task-irrelevant activity in adults with autism spectrum conditions. NeuroImage, 61(4), 1176-1187. [DOI:10.1016/j.neuroimage.2012.03.042] [PMID]
Pagnozzi, A. M., Conti, E., Calderoni, S., Fripp, J., & Rose, S. E. (2018). A systematic review of structural MRI biomarkers in autism spectrum disorder: A machine learning perspective. International Journal of Developmental Neuroscience, 71, 68-82. [PMID]
Palmen, S. J., & van Engeland, H. (2004). Review on structural neuroimaging findings in autism. Journal of Neural Transmission, 111(7), 903-929. [DOI:10.1007/s00702-003-0068-9] [PMID]
Parker, H., Hoad, C. L., Tucker, E., Costigan, C., Marciani, L., & Gowland, P., et al. (2018). Gastric motor and sensory function in health assessed by magnetic resonance imaging: Establishment of reference intervals for the Nottingham test meal in healthy subjects. Neurogastroenterology and Motility, 30(12), e13463. [DOI:10.1111/nmo.13463] [PMID]
Pelphrey, K. A., Morris, J. P., & McCarthy, G. (2005). Neural basis of eye gaze processing deficits in autism. Brain, 128(5), 1038-1048. [DOI:10.1093/brain/awh404] [PMID]
Pelphrey, K. A., Morris, J. P., McCarthy, G., & Labar, K. S. (2007). Perception of dynamic changes in facial affect and identity in autism. Social Cognitive and Affective Neuroscience, 2(2), 140-149. [DOI:10.1093/scan/nsm010] [PMID]
Philip, R. C., Dauvermann, M. R., Whalley, H. C., Baynham, K., Lawrie, S. M., & Stanfield, A. C. (2012). A systematic review and meta-analysis of the fMRI investigation of autism spectrum disorders. Neuroscience & Biobehavioral Reviews, 36(2), 901-942. [PMID]
Pierce, K., Müller, R. A., Ambrose, J., Allen, G., & Courchesne, E. (2001). Face processing occurs outside the fusiform ‘face area’ in autism: Evidence from functional MRI. Brain, 124(10), 2059-2073. [DOI:10.1093/brain/124.10.2059] [PMID]
Pina-Camacho, L., Villero, S., Fraguas, D., Boada, L., Janssen, J., & Navas-Sánchez, F. J., et al. (2012). Autism spectrum disorder: Does neuroimaging support the DSM-5 proposal for a symptom dyad? A systematic review of functional magnetic resonance imaging and diffusion tensor imaging studies. Journal of Autism and Developmental Disorders, 42(7), 1326-1341. [DOI:10.1007/s10803-011-1360-4] [PMID]
Pinal, D., Zurrón, M., & Díaz, F. (2014). Effects of load and maintenance duration on the time course of information encoding and retrieval in working memory: From perceptual analysis to post-categorization processes . Frontiers in Human Neuroscience, 8, 165. [PMID]
Rahko, J. S., Vuontela, V. A., Carlson, S., Nikkinen, J., Hurtig, T. M., & Kuusikko-Gauffin, S., et al. (2016). Attention and working memory in adolescents with autism spectrum disorder: A functional MRI study. Child Psychiatry and Human Development, 47(3), 503–517. [DOI:10.1007/s10578-015-0583-6] [PMID]
Razavi, F., Raminfard, S., Kalantar Hormozi, H., Sisakhti, M., & Batouli, S. A. H. (2021). A probabilistic atlas of the pineal gland in the standard space. Frontiers in Neuroinformatics, 15, 554229.[DOI:10.3389/fninf.2021.554229] [PMID]
Retico, A., Tosetti, M., Muratori, F., & Calderoni, S. (2014). Neuroimaging-based methods for autism identification: A possible translational application? Functional Neurology, 29(4), 231-239. [PMID]
Richards, T. L., & Berninger, V. W. (2008). Abnormal fMRI connectivity in children with dyslexia during a phoneme task: Before but not after treatment . Journal of Neurolinguistics, 21(4), 294-304. [DOI:10.1016/j.jneuroling.2007.07.002] [PMID]
Sachdev, P. S., Lee, T., Wen, W., Ames, D., Batouli, A. H., & Bowden, J., et al. (2013). The contribution of twins to the study of cognitive ageing and dementia: The older Australian Twins study. International Review of Psychiatry, 25(6), 738-747. [DOI:10.3109/09540261.2013.870137] [PMID]
Samson, F., Mottron, L., Soulières, I., & Zeffiro, T. A. (2012).Enhanced visual functioning in autism: An ALE meta-analysis. Human Brain Mapping, 33(7), 1553-1581. [PMID]
Sandin, S., Lichtenstein, P., Kuja-Halkola, R., Larsson, H., Hultman, C. M., & Reichenberg, A. (2014). The familial risk of autism. JAMA, 311(17), 1770-1777. [DOI:10.1001/jama.2014.4144] [PMID]
Sato, W., Kochiyama, T., Uono, S., Yoshimura, S., & Toichi, M. (2017). Neural mechanisms underlying conscious and unconscious gaze-triggered attentional orienting in autism spectrum disorder. Frontiers in Human Neuroscience, 11, 339. [DOI:10.3389/fnhum.2017.00339] [PMID]
Schindler, A., & Bartels, A. (2018). Human V6 integrates visual and extra-retinal cues during head-induced gaze shifts. IScience, 7, 191-197. [PMID]
Schirmer, A., & Kotz, S. (2006). Beyond the right hemisphere: Brain mechanisms mediating vocal emotional processing. Trends in Cognitive Sciences, 10(1), 24–30. [DOI:10.1016/j.tics.2005.11.009] [PMID]
Schwarzkopf, D. S., Anderson, E. J., de Haas, B., White, S. J., & Rees, G. (2014). Larger extrastriate population receptive fields in autism spectrum disorders. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 34(7), 2713-2724. [DOI:10.1523/JNEUROSCI.4416-13.2014] [PMID]
Simonoff, E., Pickles, A., Charman, T., Chandler, S., Loucas, T., & Baird, G. (2008). Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child & Adolescent Psychiatry, 47(8), 921-929. [PMID]
Simons, J. S., Schölvinck, M. L., Gilbert, S. J., Frith, C. D., & Burgess, P. W. (2006). Differential components of prospective memory?: Evidence from fMRI. Neuropsychologia, 44(8), 1388-1397. [PMID]
Spencer, M. D., Holt, R. J., Chura, L. R., Suckling, J., Calder, A. J., & Bullmore, E. T., et al. (2011). A novel functional brain imaging endophenotype of autism: The neural response to facial expression of emotion. Translational Psychiatry, 1(7), e19.[DOI:10.1038/tp.2011.18] [PMID]
Sridharan, D., Levitin, D. J., & Menon, V. (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America, 105(34), 12569-12574. [DOI:10.1073/pnas.0800005105] [PMID]
Steiner, A. M., Koegel, L. K., Koegel, R. L., & Ence, W. A. (2012). Issues and theoretical constructs regarding parent education for autism spectrum disorders. Journal of Autism and Developmental Disorders, 42(6), 1218-1227. [DOI:10.1007/s10803-011-1194-0] [PMID]
Stigler, K. A., McDonald, B. C., Anand, A., Saykin, A. J., & McDougle, C. J. (2011). Structural and functional magnetic resonance imaging of autism spectrum disorders. Brain Research, 1380, 146-161. [DOI:10.1016/j.brainres.2010.11.076] [PMID]
Szpir, M. (2006). Tracing the origins of autism: A spectrum of new studies. Environmental Health Perspectives, 114(7), A412-A418. [PMID]
Takeuchi, M., Harada, M., Matsuzaki, K., Nishitani, H., & Mori, K. (2004). Difference of signal change by a language task on autistic patients using functional MRI. The Journal of Medical Investigation : JMI, 51(1-2), 59-62. [DOI:10.2152/jmi.51.59] [PMID]
Tesink, C. M., Buitelaar, J. K., Petersson, K. M., van der Gaag, R. J., Kan, C. C., & Tendolkar, I., et al. (2009). Neural correlates of pragmatic language comprehension in autism spectrum disorders. Brain, 132(7), 1941-1952. [DOI:10.1093/brain/awp103] [PMID]
Tomaiuolo, F., Cecchetti, L., Gibson, R. M., Logi, F., Owen, A. M., & Malasoma, F., et al. (2016). Progression from vegetative to minimally conscious state is associated with changes in brain neural response to passive tasks: A longitudinal single-case functional MRI study. Journal of the International Neuropsychological Society, 22(6), 620-630. [DOI:DOI: 10.1017/S1355617716000485] [PMID]
Verhoeven, J. S., De Cock, P., Lagae, L., & Sunaert, S. (2010).Neuroimaging of autism. Neuroradiology, 52(1), 3-14. [DOI:10.1007/s00234-009-0583-y] [PMID]
Voineagu, I., & Yoo, H. (2013). Current progress and challenges in the search for autism biomarkers. Disease Markers, 35(1), 55–65. [DOI:10.1155/2013/476276] [PMID]
Walsh, P., Elsabbagh, M., Bolton, P., & Singh, I. (2011). In search of biomarkers for autism: scientific, social and ethical challenges. Nature Reviews. Neuroscience, 12(10), 603–612. [DOI:10.1038/nrn3113] [PMID]
Wang, A. T., Dapretto, M., Hariri, A. R., Sigman, M., & Bookheimer, S. Y. (2004). Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 43(4), 481-490. [DOI:10.1097/00004583-200404000-00015] [PMID]
Weikum, W. M., Vouloumanos, A., Navarra, J., Soto-Faraco, S., Sebastián-Gallés, N., & Werker, J. F. (2007). Visual language discrimination in infancy. Science, 316(5828), 1159. [DOI:10.1126/science.1137686] [PMID]
Wildgruber, D., Riecker, A., Hertrich, I., Erb, M., Grodd, W., & Ethofer, T., et al. (2005). Identification of emotional intonation evaluated by fMRI. NeuroImage, 24(4), 1233-1241. [PMID]
Williams, D. L., & Minshew, N. J. (2007). Understanding autism and related disorders: What has imaging taught us? Neuroimaging Clinics of North America, 17(4), 495-ix. [DOI:10.1016/j.nic.2007.07.007] [PMID]
Williams, J. H., Waiter, G. D., Perra, O., Perrett, D. I., & Whiten, A. (2005). An fMRI study of joint attention experience. NeuroImage, 25(1), 133-140. [DOI:10.1016/j.neuroimage.2004.10.047] [PMID]
Wise, R. G., & Preston, C. (2010). What is the value of human FMRI in CNS drug development? Drug Discovery Today, 15(21), 973-980. [DOI:10.1016/j.drudis.2010.08.016] [PMID]
Zahn, R., Moll, J., Krueger, F., Huey, E. D., Garrido, G., & Grafman, J. (2007). Social concepts are represented in the superior anterior temporal cortex. Proceedings of the National Academy of Sciences of the United States of America, 104(15), 6430–6435. [DOI:10.1073/pnas.0607061104] [PMID]
Zare Sadeghi, A., Jafari, A. H., Oghabian, M. A., Salighehrad, H. R., Batouli, S. A. H., & Raminfard, S., et al.(2017). Changes in effective connectivity network patterns in drug abusers, treated with different methods. Basic and Clinical Neuroscience, 8(4), 285-298. [DOI:10.18869/nirp.bcn.8.4.285] [PMID]
Zheng, Z., Zhang, L., Li, S., Zhao, F., Wang, Y., & Huang, L., et al. (2017). Association among obesity, overweight and autism spectrum disorder: A systematic review and meta-analysis. Scientific Reports, 7(1), 11697. [DOI:10.1038/s41598-017-12003-4] [PMID]
Zürcher, N. R., Donnelly, N., Rogier, O., Russo, B., Hippolyte, L., & Hadwin, J., et al. (2013). It’s all in the eyes: Subcortical and cortical activation during grotesqueness perception in autism. PloS One, 8(1), e54313. [DOI:10.1371/journal.pone.0054313] [PMID]
Zwaigenbaum, L., Bauman, M. L., Stone, W. L., Yirmiya, N., Estes, A., & Hansen, R. L., et al. (2015). Early identification of autism spectrum disorder: Recommendations for practice and research. Pediatrics, 136(Supplement 1), S10-40. [DOI:10.1542/peds.2014-3667C] [PMID]
Type of Study: Review |
Subject:
Clinical Neuroscience
Received: 2021/01/17 | Accepted: 2023/06/2 | Published: 2023/09/1
Received: 2021/01/17 | Accepted: 2023/06/2 | Published: 2023/09/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





