Volume 15, Issue 3 (May & Jun 2024)
BCN 2024, 15(3): 317-332 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Soleymani M B, Sangchooli A, Ebrahimpoor M, Najafi M A, Vosoughi Vahdat B, Shahbabaie A, et al . Temporal Dynamics of Neural Response to Drug Cues in abstinent Methamphetamine Users. BCN 2024; 15 (3) :317-332
URL: http://bcn.iums.ac.ir/article-1-2035-en.html
URL: http://bcn.iums.ac.ir/article-1-2035-en.html
Mohamad Bagher Soleymani1 

 , Arshiya Sangchooli2
, Arshiya Sangchooli2 

 , Mitra Ebrahimpoor3
, Mitra Ebrahimpoor3 

 , Mohamad Amin Najafi1
, Mohamad Amin Najafi1 

 , Bijan Vosoughi Vahdat1
, Bijan Vosoughi Vahdat1 

 , Alireza Shahbabaie2
, Alireza Shahbabaie2 

 , Mohammad Ali Oghabian3
, Mohammad Ali Oghabian3 

 , Hamed Ekhtiari *2
, Hamed Ekhtiari *2 




 , Arshiya Sangchooli2
, Arshiya Sangchooli2 

 , Mitra Ebrahimpoor3
, Mitra Ebrahimpoor3 

 , Mohamad Amin Najafi1
, Mohamad Amin Najafi1 

 , Bijan Vosoughi Vahdat1
, Bijan Vosoughi Vahdat1 

 , Alireza Shahbabaie2
, Alireza Shahbabaie2 

 , Mohammad Ali Oghabian3
, Mohammad Ali Oghabian3 

 , Hamed Ekhtiari *2
, Hamed Ekhtiari *2 


1- Department of Medical Engineering, School of Electrical Engineering, Sharif University of Technology, Tehran, Iran.
2- Iranian National Center for Addiction Studies (INCAS), Tehran University of Medical Sciences, Tehran, Iran.
3- Neuroimaging and Analysis Group, Imam Hospital Complex, Tehran University of Medical Sciences, Tehran, Iran.
2- Iranian National Center for Addiction Studies (INCAS), Tehran University of Medical Sciences, Tehran, Iran.
3- Neuroimaging and Analysis Group, Imam Hospital Complex, Tehran University of Medical Sciences, Tehran, Iran.
Keywords: Cue reactivity, Addiction, Methamphetamine, Functional magnetic resonance imaging (fMRI), Craving
Full-Text [PDF 1743 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Addictive disorders are increasingly significant causes of mortality and morbidity worldwide (Merikangas & McClair, 2012; NIDA, 2015; Robbins & Clark, 2015; Whiteford et al., 2015). Recently, there have been attempts to better conceptualize these disorders neuro-cognitively (Volkow et al., 2011) and develop clinically useful biomarkers on this basis (Moeller & Paulus, 2018). Long recognized as a central process in addiction (Robinson & Berridge, 1993; Wise, 1988), craving appears as a key symptom of substance use disorders (SUDs) in the diagnostic and statistical manual of mental disorders, fifth edition (APA, 2013).
Substance cue presentation is the conventional method for controlled craving induction (Reynolds & Monti, 2013) and it has been widely incorporated into functional neuroimaging studies of craving (Garrison & Potenza, 2014; Ekhtiari et al., 2016). Functional magnetic resonance imaging (fMRI) literature on cue-reactivity and craving has matured sufficiently to allow for qualitative (Yalachkov et al., 2012) and quantitative (Chase et al., 2011; Kühn & Gallinat, 2011) reviews that analyze brain activation across cue-reactivity studies, even for specific SUDs (Engelmann et al., 2012; Schacht et al., 2013).
Multiple brain regions underlie cue-induced craving, including the anterior cingulate cortex (Kühn & Gallinat, 2011), ventral striatum and amygdala (Kühn & Gallinat, 2011; Chase et al., 2011), the orbitofrontal cortex (Chase et al., 2011), and other regions of the prefrontal cortex (Wilson et al., 2004), the insula (Noori et al., 2016), and the cerebellum (Moreno-Rius & Miquel, 2017). However, while these findings have raised hopes of clinical translation, no activation pattern has been consistent enough for clinical utility (Tiffany & Wray, 2012). Many potential causes of inconsistency in psychiatric neuroimaging have been outlined (Lui et al., 2016; Milham et al., 2017; Whelan & Garavan, 2014), and in the cue-reactivity literature, heterogeneity might be due to study design, drug use patterns, craving regulation (Jasinska et al., 2014), and urge intensity (Wilson & Sayette, 2015).
One problem is that event-related and conventional blocked design studies of cue reactivity usually consider the average overall neural reaction to drug cue exposures alternating with neutral cue presentation, assuming a stable response across blocks that becomes easier to detect by analyzing the entirety of the signal at once (Hartwell et al., 2011; Van Hedger et al., 2018; Limbrick-Oldfield et al., 2017; Schacht et al., 2011). This approach fails to account that cue-induced craving is comprised of interacting stages unfolding over seconds and minutes. These include exposure to drug cues, top-down or bottom-up attention, implicit and explicit salience processing, subjective craving and an appetitive/compulsive state, executive control mechanisms employed to regulate the craving state, and ultimately either abstinence or drug consumption. This process has been referred to as the cue-induced craving pipeline (Ekhtiari et al., 2016b). Also drug cue-reactivity likely causes fatigue and habituation during the task due to the affective/appetitive salience of drug cues. The habituation of brain activation to various emotionally salient cues has been reported previously in other contexts (Phan et al., 2003; Wright et al., 2001).
Thus, the inconsistent results of cue-reactivity studies might partly be due to this framework of task design and analysis. A recent fMRI cue-reactivity study in 65 individuals with methamphetamine use disorder (MUD) demonstrated that while many brain regions display relatively static activation, regions such as the ventromedial prefrontal cortex (vmPFC), amygdala, and ventral striatum show a dynamic and generally decreasing habituation response across time. These results were replicated in two separate samples as well (Ekhtiari et al., 2020). Another study with prolonged drug-cue exposure reported an initially increasing and later decreasing left amygdala activation, associated with changes in induced subjective craving. Furthermore, the dorsal anterior cingulate cortex showed increasing activity only as craving began to decrease, consistent with assumptions about its prominent role in the top-down inhibition of craving (Murphy et al., 2017). These preliminary findings suggest that considering the changes that occur during the neural cue-response across time can provide us with important information on the stages of cue-induced craving as they unfold, and help recognize and account for the effects of habituation and fatigue. We hope to show the importance of further investigations into the temporal character of cue-induced craving as it might help in the wider effort to develop a clearer picture of the temporal character of the brain craving response and its stages, and could ultimately improve our understanding of the neural underpinnings of cue-induced craving and developing valid fMRI biomarkers in addiction medicine.
Accordingly, we recruited abstinent individuals with MUD who underwent a drug cue-reactivity task. We used a conventional blocked design, but compared brain activations across temporally distinct intervals (the dynamic analysis) in addition to a conventional analysis of activation across all blocks (the static analysis). The goal was to examine the differences between dynamic and static analysis results and explore the temporal dynamics of brain activations (i.e. early responding or delayed responding) that are lost during static analysis.
2. Materials and Methods
Study participants
A total of 32 abstinent (mean abstinence duration=17.63±15.78 days), male methamphetamine smokers (mean age=30.47±5.46 years; age range=22–43 years) were recruited. The participants had no moderate to severe traumatic brain injury, past, or current major neurologic disorder, or history of any disorder from the diagnostic and statistical manual of mental disorders, fourth edition, text revision, axis i (APA, 2000), except for SUD.
All participants met the diagnostic and statistical manual of mental disorders, fourth edition, text revision criteria for methamphetamine dependence and were recruited from Omid-e-Javid, an abstinence-based residential center affiliated with Tehran Welfare Organization in Tehran City, Iran. The subjects were treated only by abstinence under observation, and no medications were used. All subjects reported methamphetamine use at least six days a week in the last month before entering the treatment program and were screened to ensure negative urine toxicology for any drug (except nicotine) for at least a week before study enrolment. All participants provided written informed consent before enrollment. An independent Ethics Committee at Tehran University of Medical Sciences reviewed and approved the study protocol and the consent form.
Stimuli and study procedure
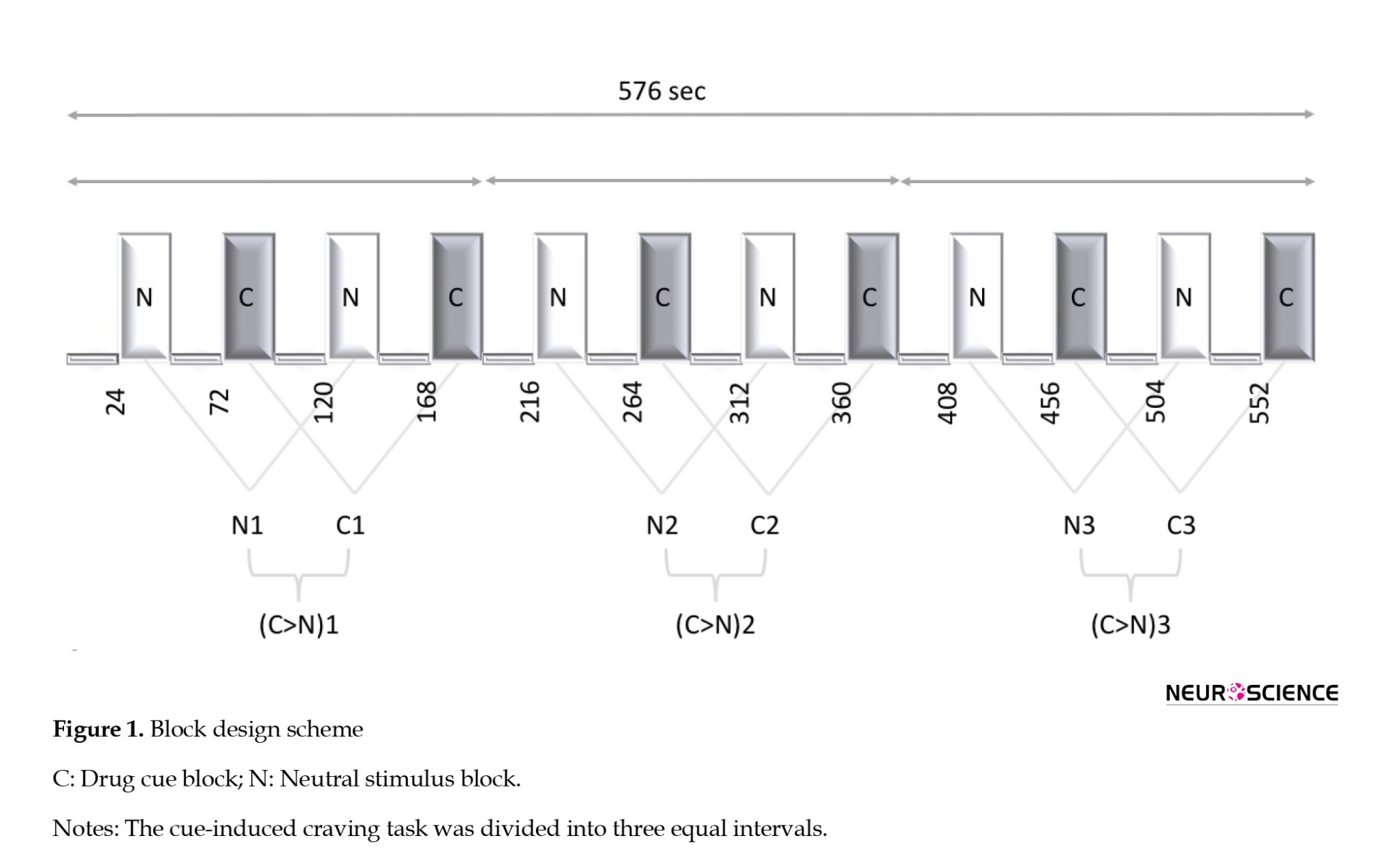
We utilized a cue-induced craving task based on a previous study (Ekhtiari et al., 2010). The task consisted of six meth-cue blocks and six neutral-cue blocks, each followed by a rest block. Blocks contained four visual stimuli, and each stimulus was presented for 6 s. The complete run (consisting of rest, neutral, rest, and drug cue blocks) was repeated six times (96 s); therefore, the cue-induced craving task took 576 s to complete. Overall, the participants viewed 24 meth-related images and 24 neutral images. The meth stimuli included pictures of meth, paraphernalia, and individuals smoking or preparing meth. The neutral stimuli included nature scenes selected from non-copyrighted images on the internet and were psycho-physically matched to drug-cue images (Ekhtiari et al., 2010).
Image acquisition procedure
Imaging was performed with a 3T magnetic resonance imaging (MRI) system (Siemens Tim Trio whole-body MRI system, Siemens Medical Solutions, Erlangen, Germany). An MRI scanner with an eight-channel head coil was used to acquire T1-weighted 3D anatomical images by a magnetization-prepared rapid gradient-echo sequence, with the following parameters: Repetition time (TR)=1800 ms, time to echo (TE)=3.4 ms, the field of view=256×256 mm, flip angle=7°, and 1 mm3 voxels parameters. Functional imaging using a standard T2* weighted echo-planar imaging sequence was performed with the following parameters: TR=3000 ms, TE=30 ms, matrix=64×64, flip angle=90°, field of view=192 mm, in-plane resolution of 3 mm2, and slice thickness 3 mm. A total of 196 continuous echo-planar imaging volumes were acquired in each session of the fMRI.
Data pre-processing procedure
Image preprocessing was conducted in FEAT (Woolrich et al., 2001), part of the functional magnetic resonance imaging of the Brain Software Library (Smith et al., 2004). The following preprocessing steps were applied for each subject: The first four volumes were discarded due to the T1 none-equilibrium effect, motion correction with MCFLIRT, B0 unwarping with field map images, brain extraction using BET, spatial smoothing with a Gaussian kernel of full-width half-maximum 6 mm and high-pass temporal filter with Gaussian-weighted least-squares straight-line fitting with σ=100 s. Subject-specific data were registered to the MNI152, 2 mm3 standard space template (Montreal Neurological Institute, Montreal, QC, Canada) and the fMRI data was transformed into standard space using the registration transformation matrices.
Statistical analysis
To address the study question, we divided the functional data (576 s) into three separate intervals, each comprised of two consecutive runs (Figure 1). A craving > neutral contrast was defined as the contrast of interest within each interval and parameter estimates for the contrasts were estimated with a general linear model using SPM12 (SPM, 2023).
The results were then entered into a second-level analysis based on a repeated measures analysis of variance (ANOVA) design. The main contrast was compared across intervals with an F test. Regions with a positive ANOVA test were termed dynamically active. To compare the three intervals in dynamically active regions, a series of post hoc t-tests were performed, using F test results as a binary mask to exclude dynamically inactive regions. The statistical maps from the group-level F test were considered thresholds based on a cluster significance threshold of P=0.05 after masking. A set of areas were considered a priori regions of interest based on the Harvard-Oxford cortical and subcortical structural atlases in FSL, including the left (l-) and right (r-) caudate, ventral striatum (vent-striatum), amygdala, posterior insula (post-insula), and anterior insula (ant-insula), middle frontal gyrus (MFG), superior frontal gyrus (SFG), inferior frontal gyrus (IFG), dorsal anterior cingulate (dorsal-ACC), rostral anterior cingulate (rostral-ACC), and ventromedial prefrontal cortex (vmPFC) (Figure 2).

Conventional (static) analysis, where complete fMRI time series were analyzed at once, was also performed so the results could be compared with those from the main analysis. Contrast images (craving>neutral) obtained from each subject entered a group analysis. Activated brain areas were determined using one-sample t-tests within each region of interest, and were termed statically active.
3. Results
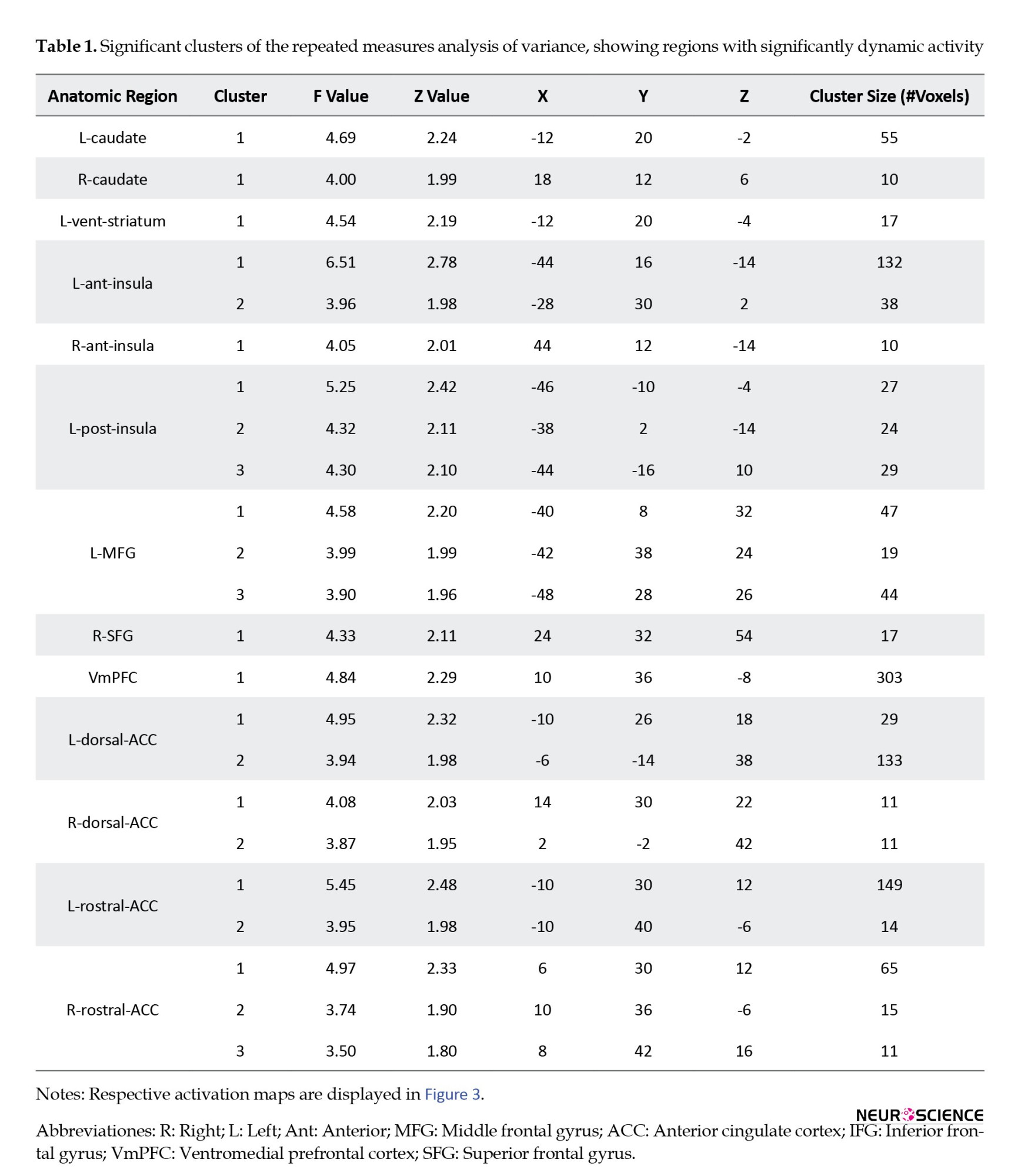
Regarding imaging analysis results, group-level F test results (Table 1 and Figure 3) showed dynamic activity in several brain regions: L-caudate, r-caudate, l-vent-striatum, l-ant-insula, r-ant-insula, l-post-insula, l-MFG, r-SFG, vmPFC, l-dorsal-ACC, r-dorsal-ACC, l-rostral-ACC, r-rostral-ACC. Figure 4 illustrates the changes in the activation pattern of intervals based on cluster mean values for each significant cluster.

The results of the three post hoc tests on dynamically active regions for pairwise comparisons of activity between intervals are shown in Table 2. All of these regions showed significantly higher activity in the first and second intervals than in the third interval. This suggests an initial activation and later deactivation of these regions during the task (Figure 4, Table 2).

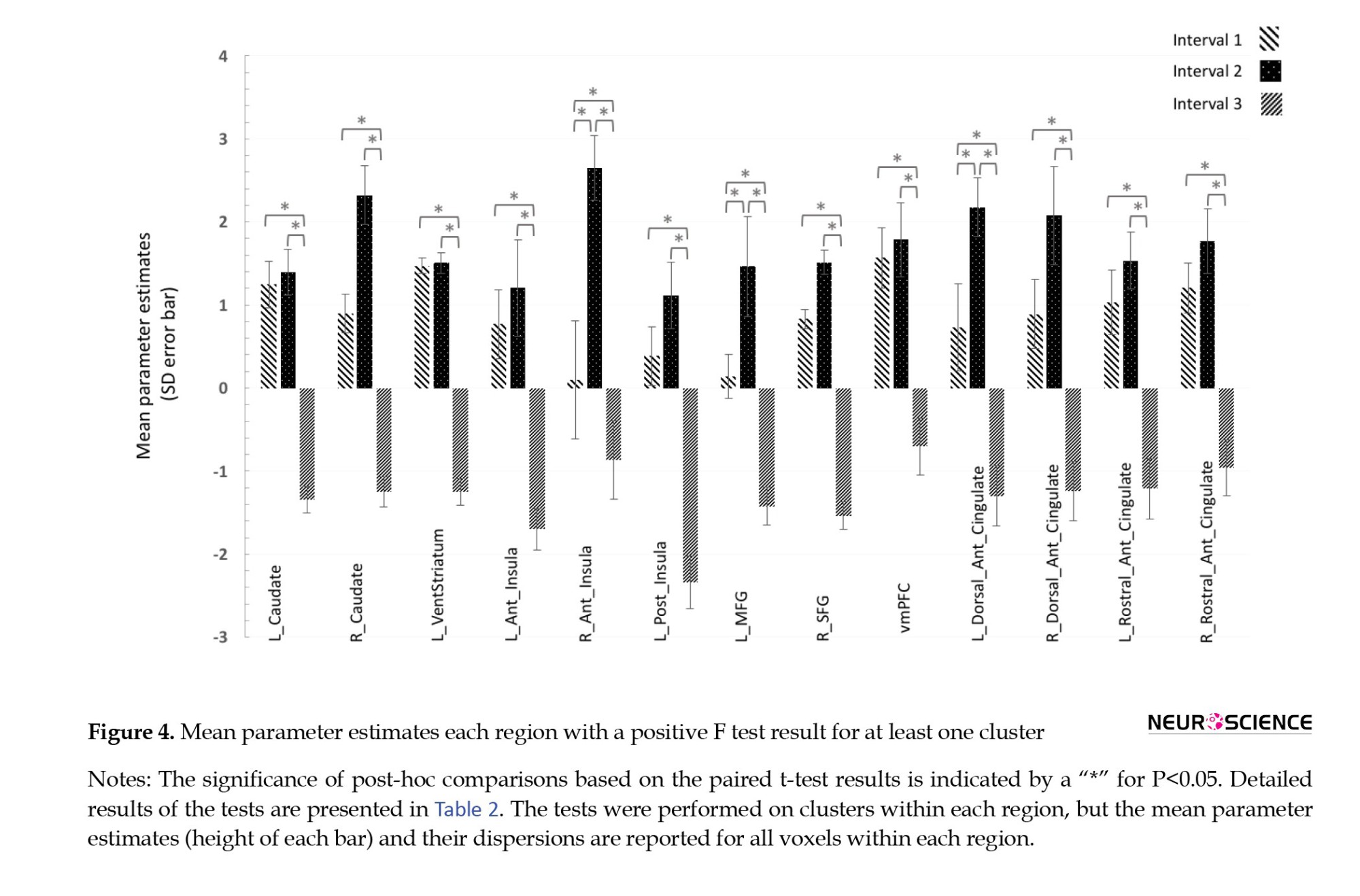
Concerning the comparison of the first two intervals, two groups of dynamically activated regions were separated. The r-ant-insula, l-MFG, and l-dorsal-ACC had significantly higher activations in the second compared to the first interval (Figure 5), while for other regions the first and second intervals had no significant difference. It was assumed that the former group responded to the presented cues with a relative delay, while the regions in the latter group had no further increase in activity moving from the first to the second interval; therefore, they were early responders. This second group included l-caudate, r-caudate, l-vent-striatum, l-ant-insula, l-post-insula, r-SFG, vmPFC, r-dorsal-ACC, l-rostral-ACC, and r-rostral-ACC.

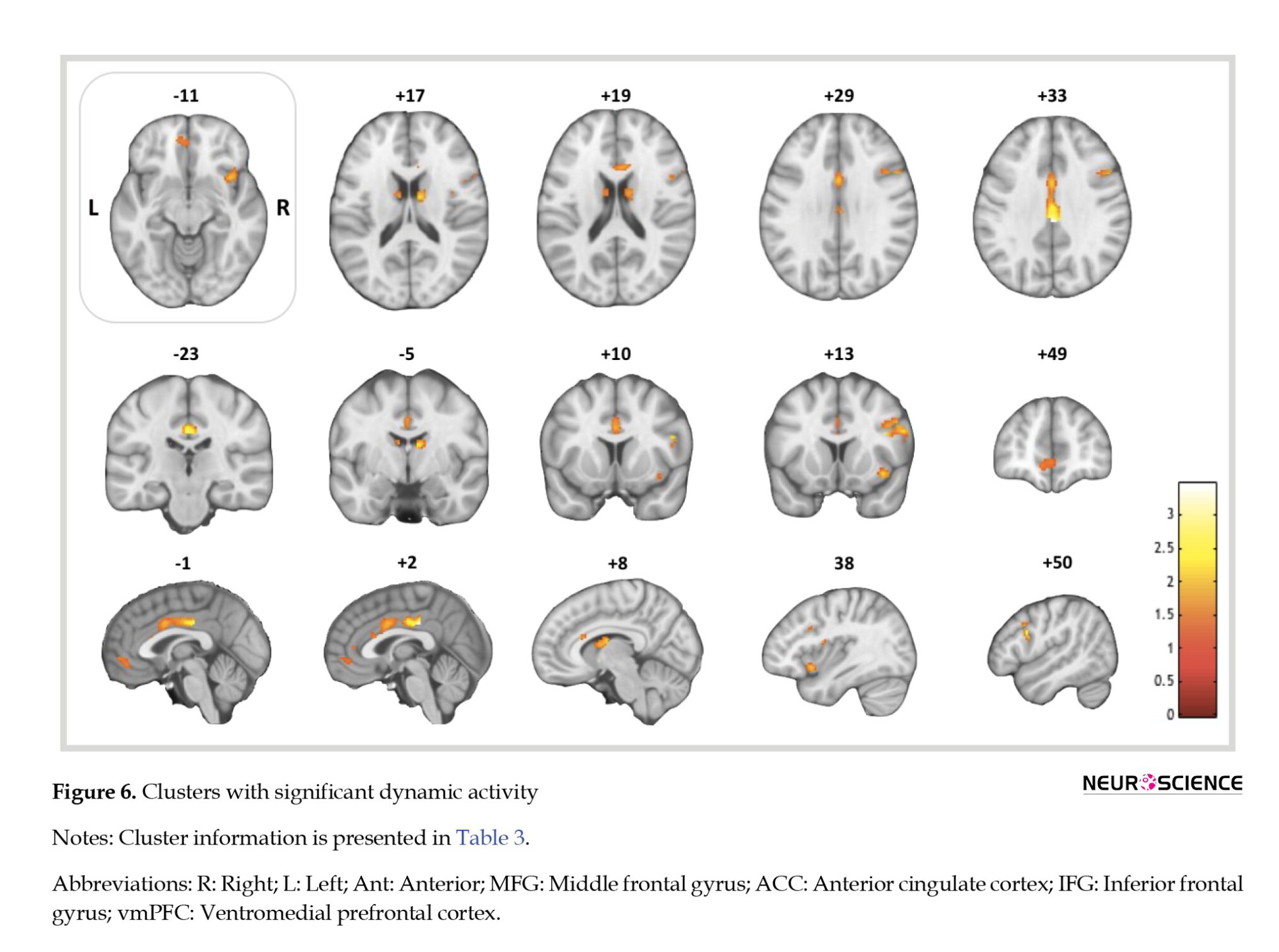
As for the conventional analysis, one sample t-test results showed several activated regions, including the l-caudate, r-caudate, r-ant-insula, r-MFG, r-IFG, vmPFC, l-dorsal-ACC, and r-dorsal-acc. Table 3 portrays the blood-oxygen-level-dependent (BOLD-response to craving > neutral contrast and Figure 6 presents the corresponding activation maps.

4. Discussion
This preliminary study explores a novel and simple method of analysis in a classic blocked design visual cue-reactivity task in methamphetamine users. We sought to assess whether a static analysis (by the conventional averaging of the signal for such blocked design studies) might miss meaningful regional activations and temporal patterns discovered by a simple dynamic analysis, using a comparison of signal across distinct intervals. While newer cue-reactivity paradigms, such as continuous cue exposure (long presentation blocks) may have better validity than classical blocked designs (Murphy et al., 2017). This study investigated whether dynamic changes in fMRI signal will occur with the brief pictorial cue presentations of a typical blocked design paradigm, as these comprise the majority of cue-reactivity fMRI studies. This is similar to a recent, more sophisticated fMRI study of drug cue reactivity in individuals with MUD (Ekhtiari et al., 2020).
Dynamic versus static analysis
Only 6 regions, namely l-caudate and r-caudate nuclei, l-dorsal-ACC, r-dorsal-ACC, vmPFC, and r-ant-insula showed static and dynamic activity. As most regions showed an increase in signal from the first to the second interval and a relative drop in the last interval, the discrepancy suggests that in static t-tests, BOLD signal changes may have canceled each other out in the regions with static, but not dynamic, activity. These seven regions include the l-vent-striatum, l-ant-insula and l-post-insula, l-MFG, r-SFG, l-rostral-ACC, and r-rostral-ACC. All these regions have been implicated in drug cue-reactivity research. The ventral striatum is activated with perceptions of appetitive value (Haber & Knutson, 2010), and recent meta-analyses have confirmed its activation during cue-induced craving (Chase et al., 2011; Kühn & Gallinat, 2011). Some other studies of methamphetamine cue-reactivity may have failed to detect ventral striatal activation because of their static analyses (Huang et al., 2018; Yin et al., 2012). The insula has a central role in interoception (Naqvi & Bechara, 2009) and salience processing (Liu et al., 2011). However, compared to the ventral striatum, the data regarding insular activation in cue-reactivity studies seems to have been less consistent. One meta-analysis of cue-reactivity studies failed to detect insular activation (Chase et al., 2011), and others noted activity only within the right insula (Kühn & Gallinat, 2011) or anterior insula (Tang et al., 2012). We also observed static activation within the right anterior Insula, and all other regions of the insular cortices had only dynamic activation. Those activations would have been missed without the dynamic analysis, which might have been the case in previous studies.
Prefrontal cortical regions have been widely implicated in drug cue reactivity (Wilson et al., 2004). The SFG might have a role in drug-related attentional processes (Hopfinger et al., 2000). Several meta-analyses (Chase et al., 2011; Noori et al., 2016; Schacht et al., 2011) have previously identified SFG activation in drug > control cue contrasts. The lack of dynamic analysis may have contributed to the failure to detect an SFG activation in one cue-reactivity study in MUD subjects (Yin et al., 2012). The MFG has more evidence supporting its role in cue-reactivity and overlaps with the dorsolateral prefrontal cortex (DLPFC) area. The DLPFC has been implicated in the inhibitory control of drug-related behavioral responses (Koob & Volkow, 2010). We expected to observe a dynamic MFG activation as subjects began to inhibit their craving later during the task, and activation was identified only in the dynamic analysis. While many meta-analyses have reported a DLPFC or MFG activation in cue-reactivity and craving (Chase et al., 2011; Kühn & Gallinat, 2011; Noori et al., 2016; Schacht et al., 2013; Tang et al., 2012), the three studies of individuals with MUD (Huang et al., 2018; Malcolm et al., 2016; Yin et al., 2012) failed to do so, potentially due to static analyses.
Meanwhile, the static analysis revealed expected activity in only two of the four ACC-related regions, the dorsal left and right ACCs. The ACC is involved in several central processes related to drug craving, including attentional bias (Luijten et al., 2011), goal setting and error processing (Goldstein et al., 2007), conflict monitoring (Lütcke & Frahm, 2008), self-referential processing (Moeller et al., 2014), emotion regulation (Goldstein et al., 2007), and salience (Seeley et al., 2007). Most meta-analyses (Engelmann et al., 2012; Kühn & Gallinat, 2011; Noori et al., 2016; Schacht et al., 2011; Tang et al., 2012) and all of the three methamphetamine cue-reactivity studies that were previously mentioned (Huang et al., 2018; Malcolm et al., 2016; Yin et al., 2012) reported ACC activation in cue-reactivity and craving reactions. As these studies and others have mostly not made a rostral/dorsal ACC division, it remains unclear why only the dorsal ACC had a dynamic activation. Particularly, the dynamic analysis seems to have captured a wider ACC activity than was seen with the static analysis.
Conversely, the r-IFG and r-MFG (unlike l-MFG) showed a significant overall activation but no dynamic activity over time according to ANOVA. These regions had a significant but sustained activation across the three intervals. In the case of r-MFG, static activity without dynamic activity was unexpected, as the MFG (and DLPFC) would hypothetically activate only in the final stages of the cue-induced craving process for craving inhibition and perhaps top-down attention. However, lateral asymmetry in MFG activation has been noted in several cue-reactivity studies before (Augustus Diggs et al., 2013; Nestor, et al., 2011; Sun et al., 2012) and a functional difference between the two MFGs is possible. The IFG is another region that we expected to activate dynamically, considering its role in response inhibition (Prisciandaro et al., 2014) and emotion regulation (Goldstein et al., 2007). The main reason for the unexpected lack of dynamic activity in the r-MFG and r-IFG was the flat and stable activation trends of these regions, perhaps because they are involved in providing an executive control tone, rather than acute inhibition. Even though the stable activation of these regions meant that their activation could be reliably found by a static analysis, a more powerful dynamic analysis of activation trends, with a longer task and higher temporal resolution, would probably have found these regional activations as well.
We demonstrate that discrepancies in regional activation patterns across studies of cue reactivity can also to be observed between our two analytic methods, and some unexpected results could be explained using a simple dynamic method. These suggest that, in addition to differences in study design, static analyses in original studies might have distorted regional activation patterns in each study differently and lead to discrepancies. This differential distortion is reasonable, considering the differences in temporal activation patterns that a dynamic model of craving would suggest is the case. Other causes further complicating the picture provided by a static analysis might be the differences in hemodynamic responses of various brain regions, and the length and number of blocks and cue presentations. This alteration of detected activations, as an artifact of static signal analysis, has been mostly overlooked as a potential cause of heterogeneity (Jasinska et al., 2014).
Temporal activation patterns
Another group of noteworthy observations are the patterns of signal change across the three intervals. These patterns were disregarded in the static analytical approach.
Most regions with dynamic activity showed no difference in activation between the first two intervals, suggesting a relatively sudden increase which declines by the third interval. The caudate nuclei are involved in habitual motor responses observed in SUDs (McClernon et al., 2009) and the relevant procedural memory (Volkow et al., 2006). The ventral striatum is involved in different aspects of reward-related processing (Haber & Knutson, 2010), like salience attribution (Koob & Volkow, 2016), motivation (David et al., 2007), and reward prediction (O’Doherty et al., 2004). The vmPFC is also involved in reward processing. It is activated by exposure to primary rewards (Haber & Knutson, 2010) and reward cues (Bray & O’Doherty, 2007; Gottfried et al., 2003). The SFG is involved in attentional processes (Hopfinger et al., 2000).
Three dynamically active regions, namely the l-MFG, r-ant-insula, and l-dorsal-ACC were observed to have a significantly greater BOLD signal contrast in the second interval compared to the first interval. This suggests a relatively delayed activation in the course of cue-response. The l-MFG’s late activation pattern is in line with its role in inhibitory control (Koob & Volkow, 2010) as abstinent patients inhibit their cue-induced craving response after it is initiated, and l-MFG activation has been more commonly reported in meta-analyses of cue-reactivity studies (Chase et al., 2011; Kühn & Gallinat, 2011; Schacht et al., 2013).
The insula and ACC had both regions with early activation and regions with late activation. This could be due to the complexities of the role these regions play in the cue-reactivity pipeline. In the insula’s case, all activated regions except for the r-ant-insula followed the same early activation pattern. The insula’s involvement in salience attribution (Ekhtiari et al., 2016b), interoception (Naqvi & Bechara, 2009), and subjective craving (Garavan, 2010) place it in the middle of the cue-induced craving pipeline. The two insulae might have somewhat differentiated functions, as there is some evidence for the lateral asymmetry of insulae’s role in addictive processes (Craig, 2010; Naqvi et al., 2007; Paulus et al., 2005). Regarding the ACC, every part, except for the l-dorsal-ACC, displayed an early activation. The ACC is involved in processes associated with both the earlier attention (Luijten et al., 2011), goal setting (Goldstein et al., 2007), salience (Seeley et al., 2007), and later (self-referential processing (Moeller et al., 2014) and emotion regulation (Goldstein et al., 2007) stages of the cue-reactivity process. Considering ACC’s many functions, it is more difficult to find specific temporal correspondences between ACC activation and any stage of the pipeline as we did for other regions, especially considering the methodological limitations of our exploratory study.
Overall, temporal activation patterns for most regions of interest fit expectations based on the cue-induced craving pipeline and previous research. While there were unexpected activation patterns, some of the irregularities could be explained by considering that the cue-induced craving pipeline is not completely linear. For example, the top-down attentional role of executive control regions, such as the prefrontal cortex and ACC might only become significant after the induction of craving and as a result of the patient’s attempt at suppressing the induced craving response.
Regions of interest with no activation
Finally, the l-IFG, r-vent-striatum, r-post-insula, l-SFG, and both amygdalae did not show activation in any of the analyses. Considering the observed activations in their opposite-hemisphere pair, the lack of activation in the first four regions could be due to hemispherically asymmetric activity; however, in the amygdala’s case, no one-sided static or dynamic activation was observed. This was significant as many clinical and preclinical studies confirm the amygdala’s roles in cue-induced craving, integration of cue-related information, and influencing relapse and drug-taking behavior (Buffalari & See, 2010; Li et al., 2008). Amygdalar activation is affected by pharmacological (Fox et al., 2012; Xu et al., 2014; Young et al., 2014) and psycho-social (McClernon et al., 2007; Wiers et al., 2015) interventions as well, and this modulation of amygdalar activity has been suggested to be crucial to treatment.
Amygdalar activation has been reported in several meta-analyses of reactivity to drug cues (Chase et al., 2011; Kühn & Gallinat, 2011; Noori et al., 2016). Some one-drug meta-analyses (Engelmann et al., 2012; Schacht et al., 2013) and methamphetamine cue-reactivity studies (Huang et al., 2018; Malcolm et al., 2016; Yin et al., 2012) have failed to detect amygdalar activation. We expected to do so with either of our two analytical approaches. In a study with sustained stimulus presentation and dynamic analysis, subjective craving across was correlated with left amygdala activation better than any other regional activation, and authors suggest that signal averaging might be one reason that many opioid cue-reactivity studies do not report amygdalar activation (Murphy et al., 2017). Future research might include various factors that affect amygdalar activation, and consider the cue-reactivities of various amygdalar sub-compartments. For instance, the basolateral amygdala is specifically involved in addictive processes (Wassum & Izquierdo, 2015).
5. Conclusion
Overall, the results suggest that a temporally dynamic analysis might reveal theoretically plausible activations that a static analysis would have failed to detect, possibly due to certain activations not surviving signal averaging across time. This demonstrates that static analysis might be deficient in answering simple questions about region activation. Also, several interesting spatial patterns of dynamic activity emerged that seem to have been mostly overlooked in the extant literature and provide new avenues for investigation, such as the laterality of dynamic and static activation and the different dynamic activations in the rostral and dorsal anterior cingulate cortices. Our analysis uncovered temporal patterns of activity across regions of interest that mostly conformed to our a priori predictions based on their roles in a dynamic model of cue-induced craving. These patterns cannot be uncovered by conventional analysis.
The results justify more extensive investigations based on a conception of cue-induced craving as a multi-staged and temporally dynamic process, potentially yielding replicable results and promoting a dynamic view of cue-induced craving, or better elucidating the effects of habituation in these studies. Hopefully, studies with more robust methodologies adopting sophisticated techniques used recently in other fields such as resting-state fMRI analysis will help investigate the under-studied temporality of craving and cue reactivity.
Study limitations and future studies
This exploratory study lacked a control group and only male patients with MUD were included. Studies with controls, other SUDs or behavioral addictions, and female participants are necessary to test the limits of our approach and its generalizability.
Further, attempts could be made to separate the hypothesized steps of the cue-induced craving pipeline and study activation patterns corresponding to each. Design features could be altered for increased ecological validity, and approaches, such as the continuous cue presentation utilized by Murphy et al., (2017) may better elicit the desired craving response.
We used only three intervals and our total task duration might not have been long enough to be divided. Future research could involve longer-duration tasks to capture more of the induced craving, more temporal intervals, and overlapping intervals to attain a finer view of signal change. Our approach is measuring fatigue since it is unclear whether a cohesive craving response is induced across the three entireties of the task. Blocked-design studies with sufficient power are required to disentangle the effects of fatigue and habituation in blocked-design tasks from the temporal stages of the craving response. Lastly, our participants were treatment-seeking abstinent individuals with MUD. These specifications limit the generalizability of our findings, as even treatment-seeking status has been shown to influence cue reactivity (Wilson et al., 2004).
Ethical Considerations
Compliance with ethical guidelines
This study was registered by Iranian Registry of Clinical Trials (IRCT) (Code: IRCT2012102011172N1).
Funding
This project was funded by a grant to Hamed Ekhtiariand Mohammad Ali Ohgabian from Tehran University of Medical Sciences (Grant No.: 91-02-98-17925).
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declare no potential conflicts of interests.
Acknowledgments
The authors thank all the patients who took part in this study.
References
Addictive disorders are increasingly significant causes of mortality and morbidity worldwide (Merikangas & McClair, 2012; NIDA, 2015; Robbins & Clark, 2015; Whiteford et al., 2015). Recently, there have been attempts to better conceptualize these disorders neuro-cognitively (Volkow et al., 2011) and develop clinically useful biomarkers on this basis (Moeller & Paulus, 2018). Long recognized as a central process in addiction (Robinson & Berridge, 1993; Wise, 1988), craving appears as a key symptom of substance use disorders (SUDs) in the diagnostic and statistical manual of mental disorders, fifth edition (APA, 2013).
Substance cue presentation is the conventional method for controlled craving induction (Reynolds & Monti, 2013) and it has been widely incorporated into functional neuroimaging studies of craving (Garrison & Potenza, 2014; Ekhtiari et al., 2016). Functional magnetic resonance imaging (fMRI) literature on cue-reactivity and craving has matured sufficiently to allow for qualitative (Yalachkov et al., 2012) and quantitative (Chase et al., 2011; Kühn & Gallinat, 2011) reviews that analyze brain activation across cue-reactivity studies, even for specific SUDs (Engelmann et al., 2012; Schacht et al., 2013).
Multiple brain regions underlie cue-induced craving, including the anterior cingulate cortex (Kühn & Gallinat, 2011), ventral striatum and amygdala (Kühn & Gallinat, 2011; Chase et al., 2011), the orbitofrontal cortex (Chase et al., 2011), and other regions of the prefrontal cortex (Wilson et al., 2004), the insula (Noori et al., 2016), and the cerebellum (Moreno-Rius & Miquel, 2017). However, while these findings have raised hopes of clinical translation, no activation pattern has been consistent enough for clinical utility (Tiffany & Wray, 2012). Many potential causes of inconsistency in psychiatric neuroimaging have been outlined (Lui et al., 2016; Milham et al., 2017; Whelan & Garavan, 2014), and in the cue-reactivity literature, heterogeneity might be due to study design, drug use patterns, craving regulation (Jasinska et al., 2014), and urge intensity (Wilson & Sayette, 2015).
One problem is that event-related and conventional blocked design studies of cue reactivity usually consider the average overall neural reaction to drug cue exposures alternating with neutral cue presentation, assuming a stable response across blocks that becomes easier to detect by analyzing the entirety of the signal at once (Hartwell et al., 2011; Van Hedger et al., 2018; Limbrick-Oldfield et al., 2017; Schacht et al., 2011). This approach fails to account that cue-induced craving is comprised of interacting stages unfolding over seconds and minutes. These include exposure to drug cues, top-down or bottom-up attention, implicit and explicit salience processing, subjective craving and an appetitive/compulsive state, executive control mechanisms employed to regulate the craving state, and ultimately either abstinence or drug consumption. This process has been referred to as the cue-induced craving pipeline (Ekhtiari et al., 2016b). Also drug cue-reactivity likely causes fatigue and habituation during the task due to the affective/appetitive salience of drug cues. The habituation of brain activation to various emotionally salient cues has been reported previously in other contexts (Phan et al., 2003; Wright et al., 2001).
Thus, the inconsistent results of cue-reactivity studies might partly be due to this framework of task design and analysis. A recent fMRI cue-reactivity study in 65 individuals with methamphetamine use disorder (MUD) demonstrated that while many brain regions display relatively static activation, regions such as the ventromedial prefrontal cortex (vmPFC), amygdala, and ventral striatum show a dynamic and generally decreasing habituation response across time. These results were replicated in two separate samples as well (Ekhtiari et al., 2020). Another study with prolonged drug-cue exposure reported an initially increasing and later decreasing left amygdala activation, associated with changes in induced subjective craving. Furthermore, the dorsal anterior cingulate cortex showed increasing activity only as craving began to decrease, consistent with assumptions about its prominent role in the top-down inhibition of craving (Murphy et al., 2017). These preliminary findings suggest that considering the changes that occur during the neural cue-response across time can provide us with important information on the stages of cue-induced craving as they unfold, and help recognize and account for the effects of habituation and fatigue. We hope to show the importance of further investigations into the temporal character of cue-induced craving as it might help in the wider effort to develop a clearer picture of the temporal character of the brain craving response and its stages, and could ultimately improve our understanding of the neural underpinnings of cue-induced craving and developing valid fMRI biomarkers in addiction medicine.
Accordingly, we recruited abstinent individuals with MUD who underwent a drug cue-reactivity task. We used a conventional blocked design, but compared brain activations across temporally distinct intervals (the dynamic analysis) in addition to a conventional analysis of activation across all blocks (the static analysis). The goal was to examine the differences between dynamic and static analysis results and explore the temporal dynamics of brain activations (i.e. early responding or delayed responding) that are lost during static analysis.
2. Materials and Methods
Study participants
A total of 32 abstinent (mean abstinence duration=17.63±15.78 days), male methamphetamine smokers (mean age=30.47±5.46 years; age range=22–43 years) were recruited. The participants had no moderate to severe traumatic brain injury, past, or current major neurologic disorder, or history of any disorder from the diagnostic and statistical manual of mental disorders, fourth edition, text revision, axis i (APA, 2000), except for SUD.
All participants met the diagnostic and statistical manual of mental disorders, fourth edition, text revision criteria for methamphetamine dependence and were recruited from Omid-e-Javid, an abstinence-based residential center affiliated with Tehran Welfare Organization in Tehran City, Iran. The subjects were treated only by abstinence under observation, and no medications were used. All subjects reported methamphetamine use at least six days a week in the last month before entering the treatment program and were screened to ensure negative urine toxicology for any drug (except nicotine) for at least a week before study enrolment. All participants provided written informed consent before enrollment. An independent Ethics Committee at Tehran University of Medical Sciences reviewed and approved the study protocol and the consent form.
Stimuli and study procedure
We utilized a cue-induced craving task based on a previous study (Ekhtiari et al., 2010). The task consisted of six meth-cue blocks and six neutral-cue blocks, each followed by a rest block. Blocks contained four visual stimuli, and each stimulus was presented for 6 s. The complete run (consisting of rest, neutral, rest, and drug cue blocks) was repeated six times (96 s); therefore, the cue-induced craving task took 576 s to complete. Overall, the participants viewed 24 meth-related images and 24 neutral images. The meth stimuli included pictures of meth, paraphernalia, and individuals smoking or preparing meth. The neutral stimuli included nature scenes selected from non-copyrighted images on the internet and were psycho-physically matched to drug-cue images (Ekhtiari et al., 2010).
Image acquisition procedure
Imaging was performed with a 3T magnetic resonance imaging (MRI) system (Siemens Tim Trio whole-body MRI system, Siemens Medical Solutions, Erlangen, Germany). An MRI scanner with an eight-channel head coil was used to acquire T1-weighted 3D anatomical images by a magnetization-prepared rapid gradient-echo sequence, with the following parameters: Repetition time (TR)=1800 ms, time to echo (TE)=3.4 ms, the field of view=256×256 mm, flip angle=7°, and 1 mm3 voxels parameters. Functional imaging using a standard T2* weighted echo-planar imaging sequence was performed with the following parameters: TR=3000 ms, TE=30 ms, matrix=64×64, flip angle=90°, field of view=192 mm, in-plane resolution of 3 mm2, and slice thickness 3 mm. A total of 196 continuous echo-planar imaging volumes were acquired in each session of the fMRI.
Data pre-processing procedure
Image preprocessing was conducted in FEAT (Woolrich et al., 2001), part of the functional magnetic resonance imaging of the Brain Software Library (Smith et al., 2004). The following preprocessing steps were applied for each subject: The first four volumes were discarded due to the T1 none-equilibrium effect, motion correction with MCFLIRT, B0 unwarping with field map images, brain extraction using BET, spatial smoothing with a Gaussian kernel of full-width half-maximum 6 mm and high-pass temporal filter with Gaussian-weighted least-squares straight-line fitting with σ=100 s. Subject-specific data were registered to the MNI152, 2 mm3 standard space template (Montreal Neurological Institute, Montreal, QC, Canada) and the fMRI data was transformed into standard space using the registration transformation matrices.
Statistical analysis
To address the study question, we divided the functional data (576 s) into three separate intervals, each comprised of two consecutive runs (Figure 1). A craving > neutral contrast was defined as the contrast of interest within each interval and parameter estimates for the contrasts were estimated with a general linear model using SPM12 (SPM, 2023).

The results were then entered into a second-level analysis based on a repeated measures analysis of variance (ANOVA) design. The main contrast was compared across intervals with an F test. Regions with a positive ANOVA test were termed dynamically active. To compare the three intervals in dynamically active regions, a series of post hoc t-tests were performed, using F test results as a binary mask to exclude dynamically inactive regions. The statistical maps from the group-level F test were considered thresholds based on a cluster significance threshold of P=0.05 after masking. A set of areas were considered a priori regions of interest based on the Harvard-Oxford cortical and subcortical structural atlases in FSL, including the left (l-) and right (r-) caudate, ventral striatum (vent-striatum), amygdala, posterior insula (post-insula), and anterior insula (ant-insula), middle frontal gyrus (MFG), superior frontal gyrus (SFG), inferior frontal gyrus (IFG), dorsal anterior cingulate (dorsal-ACC), rostral anterior cingulate (rostral-ACC), and ventromedial prefrontal cortex (vmPFC) (Figure 2).

Conventional (static) analysis, where complete fMRI time series were analyzed at once, was also performed so the results could be compared with those from the main analysis. Contrast images (craving>neutral) obtained from each subject entered a group analysis. Activated brain areas were determined using one-sample t-tests within each region of interest, and were termed statically active.
3. Results
Regarding imaging analysis results, group-level F test results (Table 1 and Figure 3) showed dynamic activity in several brain regions: L-caudate, r-caudate, l-vent-striatum, l-ant-insula, r-ant-insula, l-post-insula, l-MFG, r-SFG, vmPFC, l-dorsal-ACC, r-dorsal-ACC, l-rostral-ACC, r-rostral-ACC. Figure 4 illustrates the changes in the activation pattern of intervals based on cluster mean values for each significant cluster.


The results of the three post hoc tests on dynamically active regions for pairwise comparisons of activity between intervals are shown in Table 2. All of these regions showed significantly higher activity in the first and second intervals than in the third interval. This suggests an initial activation and later deactivation of these regions during the task (Figure 4, Table 2).


Concerning the comparison of the first two intervals, two groups of dynamically activated regions were separated. The r-ant-insula, l-MFG, and l-dorsal-ACC had significantly higher activations in the second compared to the first interval (Figure 5), while for other regions the first and second intervals had no significant difference. It was assumed that the former group responded to the presented cues with a relative delay, while the regions in the latter group had no further increase in activity moving from the first to the second interval; therefore, they were early responders. This second group included l-caudate, r-caudate, l-vent-striatum, l-ant-insula, l-post-insula, r-SFG, vmPFC, r-dorsal-ACC, l-rostral-ACC, and r-rostral-ACC.

As for the conventional analysis, one sample t-test results showed several activated regions, including the l-caudate, r-caudate, r-ant-insula, r-MFG, r-IFG, vmPFC, l-dorsal-ACC, and r-dorsal-acc. Table 3 portrays the blood-oxygen-level-dependent (BOLD-response to craving > neutral contrast and Figure 6 presents the corresponding activation maps.


4. Discussion
This preliminary study explores a novel and simple method of analysis in a classic blocked design visual cue-reactivity task in methamphetamine users. We sought to assess whether a static analysis (by the conventional averaging of the signal for such blocked design studies) might miss meaningful regional activations and temporal patterns discovered by a simple dynamic analysis, using a comparison of signal across distinct intervals. While newer cue-reactivity paradigms, such as continuous cue exposure (long presentation blocks) may have better validity than classical blocked designs (Murphy et al., 2017). This study investigated whether dynamic changes in fMRI signal will occur with the brief pictorial cue presentations of a typical blocked design paradigm, as these comprise the majority of cue-reactivity fMRI studies. This is similar to a recent, more sophisticated fMRI study of drug cue reactivity in individuals with MUD (Ekhtiari et al., 2020).
Dynamic versus static analysis
Only 6 regions, namely l-caudate and r-caudate nuclei, l-dorsal-ACC, r-dorsal-ACC, vmPFC, and r-ant-insula showed static and dynamic activity. As most regions showed an increase in signal from the first to the second interval and a relative drop in the last interval, the discrepancy suggests that in static t-tests, BOLD signal changes may have canceled each other out in the regions with static, but not dynamic, activity. These seven regions include the l-vent-striatum, l-ant-insula and l-post-insula, l-MFG, r-SFG, l-rostral-ACC, and r-rostral-ACC. All these regions have been implicated in drug cue-reactivity research. The ventral striatum is activated with perceptions of appetitive value (Haber & Knutson, 2010), and recent meta-analyses have confirmed its activation during cue-induced craving (Chase et al., 2011; Kühn & Gallinat, 2011). Some other studies of methamphetamine cue-reactivity may have failed to detect ventral striatal activation because of their static analyses (Huang et al., 2018; Yin et al., 2012). The insula has a central role in interoception (Naqvi & Bechara, 2009) and salience processing (Liu et al., 2011). However, compared to the ventral striatum, the data regarding insular activation in cue-reactivity studies seems to have been less consistent. One meta-analysis of cue-reactivity studies failed to detect insular activation (Chase et al., 2011), and others noted activity only within the right insula (Kühn & Gallinat, 2011) or anterior insula (Tang et al., 2012). We also observed static activation within the right anterior Insula, and all other regions of the insular cortices had only dynamic activation. Those activations would have been missed without the dynamic analysis, which might have been the case in previous studies.
Prefrontal cortical regions have been widely implicated in drug cue reactivity (Wilson et al., 2004). The SFG might have a role in drug-related attentional processes (Hopfinger et al., 2000). Several meta-analyses (Chase et al., 2011; Noori et al., 2016; Schacht et al., 2011) have previously identified SFG activation in drug > control cue contrasts. The lack of dynamic analysis may have contributed to the failure to detect an SFG activation in one cue-reactivity study in MUD subjects (Yin et al., 2012). The MFG has more evidence supporting its role in cue-reactivity and overlaps with the dorsolateral prefrontal cortex (DLPFC) area. The DLPFC has been implicated in the inhibitory control of drug-related behavioral responses (Koob & Volkow, 2010). We expected to observe a dynamic MFG activation as subjects began to inhibit their craving later during the task, and activation was identified only in the dynamic analysis. While many meta-analyses have reported a DLPFC or MFG activation in cue-reactivity and craving (Chase et al., 2011; Kühn & Gallinat, 2011; Noori et al., 2016; Schacht et al., 2013; Tang et al., 2012), the three studies of individuals with MUD (Huang et al., 2018; Malcolm et al., 2016; Yin et al., 2012) failed to do so, potentially due to static analyses.
Meanwhile, the static analysis revealed expected activity in only two of the four ACC-related regions, the dorsal left and right ACCs. The ACC is involved in several central processes related to drug craving, including attentional bias (Luijten et al., 2011), goal setting and error processing (Goldstein et al., 2007), conflict monitoring (Lütcke & Frahm, 2008), self-referential processing (Moeller et al., 2014), emotion regulation (Goldstein et al., 2007), and salience (Seeley et al., 2007). Most meta-analyses (Engelmann et al., 2012; Kühn & Gallinat, 2011; Noori et al., 2016; Schacht et al., 2011; Tang et al., 2012) and all of the three methamphetamine cue-reactivity studies that were previously mentioned (Huang et al., 2018; Malcolm et al., 2016; Yin et al., 2012) reported ACC activation in cue-reactivity and craving reactions. As these studies and others have mostly not made a rostral/dorsal ACC division, it remains unclear why only the dorsal ACC had a dynamic activation. Particularly, the dynamic analysis seems to have captured a wider ACC activity than was seen with the static analysis.
Conversely, the r-IFG and r-MFG (unlike l-MFG) showed a significant overall activation but no dynamic activity over time according to ANOVA. These regions had a significant but sustained activation across the three intervals. In the case of r-MFG, static activity without dynamic activity was unexpected, as the MFG (and DLPFC) would hypothetically activate only in the final stages of the cue-induced craving process for craving inhibition and perhaps top-down attention. However, lateral asymmetry in MFG activation has been noted in several cue-reactivity studies before (Augustus Diggs et al., 2013; Nestor, et al., 2011; Sun et al., 2012) and a functional difference between the two MFGs is possible. The IFG is another region that we expected to activate dynamically, considering its role in response inhibition (Prisciandaro et al., 2014) and emotion regulation (Goldstein et al., 2007). The main reason for the unexpected lack of dynamic activity in the r-MFG and r-IFG was the flat and stable activation trends of these regions, perhaps because they are involved in providing an executive control tone, rather than acute inhibition. Even though the stable activation of these regions meant that their activation could be reliably found by a static analysis, a more powerful dynamic analysis of activation trends, with a longer task and higher temporal resolution, would probably have found these regional activations as well.
We demonstrate that discrepancies in regional activation patterns across studies of cue reactivity can also to be observed between our two analytic methods, and some unexpected results could be explained using a simple dynamic method. These suggest that, in addition to differences in study design, static analyses in original studies might have distorted regional activation patterns in each study differently and lead to discrepancies. This differential distortion is reasonable, considering the differences in temporal activation patterns that a dynamic model of craving would suggest is the case. Other causes further complicating the picture provided by a static analysis might be the differences in hemodynamic responses of various brain regions, and the length and number of blocks and cue presentations. This alteration of detected activations, as an artifact of static signal analysis, has been mostly overlooked as a potential cause of heterogeneity (Jasinska et al., 2014).
Temporal activation patterns
Another group of noteworthy observations are the patterns of signal change across the three intervals. These patterns were disregarded in the static analytical approach.
Most regions with dynamic activity showed no difference in activation between the first two intervals, suggesting a relatively sudden increase which declines by the third interval. The caudate nuclei are involved in habitual motor responses observed in SUDs (McClernon et al., 2009) and the relevant procedural memory (Volkow et al., 2006). The ventral striatum is involved in different aspects of reward-related processing (Haber & Knutson, 2010), like salience attribution (Koob & Volkow, 2016), motivation (David et al., 2007), and reward prediction (O’Doherty et al., 2004). The vmPFC is also involved in reward processing. It is activated by exposure to primary rewards (Haber & Knutson, 2010) and reward cues (Bray & O’Doherty, 2007; Gottfried et al., 2003). The SFG is involved in attentional processes (Hopfinger et al., 2000).
Three dynamically active regions, namely the l-MFG, r-ant-insula, and l-dorsal-ACC were observed to have a significantly greater BOLD signal contrast in the second interval compared to the first interval. This suggests a relatively delayed activation in the course of cue-response. The l-MFG’s late activation pattern is in line with its role in inhibitory control (Koob & Volkow, 2010) as abstinent patients inhibit their cue-induced craving response after it is initiated, and l-MFG activation has been more commonly reported in meta-analyses of cue-reactivity studies (Chase et al., 2011; Kühn & Gallinat, 2011; Schacht et al., 2013).
The insula and ACC had both regions with early activation and regions with late activation. This could be due to the complexities of the role these regions play in the cue-reactivity pipeline. In the insula’s case, all activated regions except for the r-ant-insula followed the same early activation pattern. The insula’s involvement in salience attribution (Ekhtiari et al., 2016b), interoception (Naqvi & Bechara, 2009), and subjective craving (Garavan, 2010) place it in the middle of the cue-induced craving pipeline. The two insulae might have somewhat differentiated functions, as there is some evidence for the lateral asymmetry of insulae’s role in addictive processes (Craig, 2010; Naqvi et al., 2007; Paulus et al., 2005). Regarding the ACC, every part, except for the l-dorsal-ACC, displayed an early activation. The ACC is involved in processes associated with both the earlier attention (Luijten et al., 2011), goal setting (Goldstein et al., 2007), salience (Seeley et al., 2007), and later (self-referential processing (Moeller et al., 2014) and emotion regulation (Goldstein et al., 2007) stages of the cue-reactivity process. Considering ACC’s many functions, it is more difficult to find specific temporal correspondences between ACC activation and any stage of the pipeline as we did for other regions, especially considering the methodological limitations of our exploratory study.
Overall, temporal activation patterns for most regions of interest fit expectations based on the cue-induced craving pipeline and previous research. While there were unexpected activation patterns, some of the irregularities could be explained by considering that the cue-induced craving pipeline is not completely linear. For example, the top-down attentional role of executive control regions, such as the prefrontal cortex and ACC might only become significant after the induction of craving and as a result of the patient’s attempt at suppressing the induced craving response.
Regions of interest with no activation
Finally, the l-IFG, r-vent-striatum, r-post-insula, l-SFG, and both amygdalae did not show activation in any of the analyses. Considering the observed activations in their opposite-hemisphere pair, the lack of activation in the first four regions could be due to hemispherically asymmetric activity; however, in the amygdala’s case, no one-sided static or dynamic activation was observed. This was significant as many clinical and preclinical studies confirm the amygdala’s roles in cue-induced craving, integration of cue-related information, and influencing relapse and drug-taking behavior (Buffalari & See, 2010; Li et al., 2008). Amygdalar activation is affected by pharmacological (Fox et al., 2012; Xu et al., 2014; Young et al., 2014) and psycho-social (McClernon et al., 2007; Wiers et al., 2015) interventions as well, and this modulation of amygdalar activity has been suggested to be crucial to treatment.
Amygdalar activation has been reported in several meta-analyses of reactivity to drug cues (Chase et al., 2011; Kühn & Gallinat, 2011; Noori et al., 2016). Some one-drug meta-analyses (Engelmann et al., 2012; Schacht et al., 2013) and methamphetamine cue-reactivity studies (Huang et al., 2018; Malcolm et al., 2016; Yin et al., 2012) have failed to detect amygdalar activation. We expected to do so with either of our two analytical approaches. In a study with sustained stimulus presentation and dynamic analysis, subjective craving across was correlated with left amygdala activation better than any other regional activation, and authors suggest that signal averaging might be one reason that many opioid cue-reactivity studies do not report amygdalar activation (Murphy et al., 2017). Future research might include various factors that affect amygdalar activation, and consider the cue-reactivities of various amygdalar sub-compartments. For instance, the basolateral amygdala is specifically involved in addictive processes (Wassum & Izquierdo, 2015).
5. Conclusion
Overall, the results suggest that a temporally dynamic analysis might reveal theoretically plausible activations that a static analysis would have failed to detect, possibly due to certain activations not surviving signal averaging across time. This demonstrates that static analysis might be deficient in answering simple questions about region activation. Also, several interesting spatial patterns of dynamic activity emerged that seem to have been mostly overlooked in the extant literature and provide new avenues for investigation, such as the laterality of dynamic and static activation and the different dynamic activations in the rostral and dorsal anterior cingulate cortices. Our analysis uncovered temporal patterns of activity across regions of interest that mostly conformed to our a priori predictions based on their roles in a dynamic model of cue-induced craving. These patterns cannot be uncovered by conventional analysis.
The results justify more extensive investigations based on a conception of cue-induced craving as a multi-staged and temporally dynamic process, potentially yielding replicable results and promoting a dynamic view of cue-induced craving, or better elucidating the effects of habituation in these studies. Hopefully, studies with more robust methodologies adopting sophisticated techniques used recently in other fields such as resting-state fMRI analysis will help investigate the under-studied temporality of craving and cue reactivity.
Study limitations and future studies
This exploratory study lacked a control group and only male patients with MUD were included. Studies with controls, other SUDs or behavioral addictions, and female participants are necessary to test the limits of our approach and its generalizability.
Further, attempts could be made to separate the hypothesized steps of the cue-induced craving pipeline and study activation patterns corresponding to each. Design features could be altered for increased ecological validity, and approaches, such as the continuous cue presentation utilized by Murphy et al., (2017) may better elicit the desired craving response.
We used only three intervals and our total task duration might not have been long enough to be divided. Future research could involve longer-duration tasks to capture more of the induced craving, more temporal intervals, and overlapping intervals to attain a finer view of signal change. Our approach is measuring fatigue since it is unclear whether a cohesive craving response is induced across the three entireties of the task. Blocked-design studies with sufficient power are required to disentangle the effects of fatigue and habituation in blocked-design tasks from the temporal stages of the craving response. Lastly, our participants were treatment-seeking abstinent individuals with MUD. These specifications limit the generalizability of our findings, as even treatment-seeking status has been shown to influence cue reactivity (Wilson et al., 2004).
Ethical Considerations
Compliance with ethical guidelines
This study was registered by Iranian Registry of Clinical Trials (IRCT) (Code: IRCT2012102011172N1).
Funding
This project was funded by a grant to Hamed Ekhtiariand Mohammad Ali Ohgabian from Tehran University of Medical Sciences (Grant No.: 91-02-98-17925).
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declare no potential conflicts of interests.
Acknowledgments
The authors thank all the patients who took part in this study.
References
American Psychiatric Association, DSM-5 Task Force. (2013). Diagnostic and statistical manual of mental disorders: DSM-5™. Washington, D.C: American Psychiatric Publishing, Inc. [DOI:10.1176/appi.books.9780890425596]
American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association. [Link]
Bray, S., & O'Doherty, J. (2007). Neural coding of reward-prediction error signals during classical conditioning with attractive faces. Journal of Neurophysiology, 97(4), 3036-3045. [DOI:10.1152/jn.01211.2006] [PMID]
Buffalari, D. M., & See, R. E. (2010). Amygdala mechanisms of Pavlovian psychostimulant conditioning and relapse. Current Topics in Behavioral Neurosciences, 3, 73–99.[DOI:10.1007/7854_2009_18] [PMID]
Chase, H. W., Eickhoff, S. B., Laird, A. R., & Hogarth, L. (2011). The neural basis of drug stimulus processing and craving: An activation likelihood estimation meta-analysis. Biological Psychiatry, 70(8), 785-793. [DOI:10.1016/j.biopsych.2011.05.025] [PMID] [PMCID]
Craig A. D. (2010). The sentient self. Brain Structure & Function, 214(5-6), 563-577. [DOI:10.1007/s00429-010-0248-y] [PMID]
David, S. P., Munafò, M. R., Johansen-Berg, H., Mackillop, J., Sweet, L. H., & Cohen, R. A., et al. (2007). Effects of acute nicotine abstinence on cue-elicited ventral striatum/nucleus accumbens activation in female cigarette smokers: A functional magnetic resonance imaging study. Brain Imaging and Behavior, 1(3-4), 43-57. [DOI:10.1007/s11682-007-9004-1] [PMID] [PMCID]
Ekhtiari, H., Alam-Mehrjerdi, Z., Nouri, M., George, S., & Mokri, A. (2010). Designing and evaluation of reliability and validity of visual cue-induced craving assessment task for methamphetamine smokers. Basic and Clinical Neuroscience, 1(4), 34-37. [Link]
Ekhtiari, H., Faghiri, A., Oghabian, M. A., & Paulus, M. P. (2016). Functional neuroimaging for addiction medicine: From mechanisms to practical considerations. Progress in Brain Research, 224, 129–153. [DOI:10.1016/bs.pbr.2015.10.001] [PMID]
Ekhtiari, H., Nasseri, P., Yavari, F., Mokri, A., & Monterosso, J. (2016). Neuroscience of drug craving for addiction medicine: From circuits to therapies. Progress in Brain Research, 223, 115-141. [DOI:10.1016/bs.pbr.2015.10.002] [PMID]
Ekhtiari, H., Kuplicki, R., Aupperle, R. P., & Paulus, M. P. (2020). It is never as good the second time around: Brain areas involved in salience processing habituate during repeated drug cue exposure in methamphetamine and opioid users. BioRxiv, 1-20. [DOI:10.1101/2020.04.18.036368]
Engelmann, J. M., Versace, F., Robinson, J. D., Minnix, J. A., Lam, C. Y., & Cui, Y., et al. (2012). Neural substrates of smoking cue reactivity: A meta-analysis of fMRI studies. NeuroImage, 60(1), 252-262. [DOI:10.1016/j.neuroimage.2011.12.024] [PMID] [PMCID]
Fox, H. C., Seo, D., Tuit, K., Hansen, J., Kimmerling, A., & Morgan, P. T., et al. (2012). Guanfacine effects on stress, drug craving and prefrontal activation in cocaine-dependent individuals: Preliminary findings. Journal of Psychopharmacology (Oxford, England), 26(7), 958-972. [DOI:10.1177/0269881111430746] [PMID] [PMCID]
Garavan, H. (2010). Insula and drug cravings. Brain Structure & Function, 214(5-6), 593-601. [DOI:10.1007/s00429-010-0259-8] [PMID]
Garrison, K. A., & Potenza, M. N. (2014). Neuroimaging and Biomarkers in Addiction Treatment. Current Psychiatry Reports, 16(12), 513. [DOI:10.1007/s11920-014-0513-5] [PMID] [PMCID]
Goldstein, R. Z., Tomasi, D., Rajaram, S., Cottone, L. A., Zhang, L., & Maloney, T., et al. (2007). Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience, 144(4), 1153-1159. [DOI:10.1016/j.neuroscience.2006.11.024] [PMID] [PMCID]
Gottfried, J. A., O'Doherty, J., & Dolan, R. J. (2003). Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science (New York, N.Y.), 301(5636), 1104-1107. [DOI:10.1126/science.1087919] [PMID]
Haber, S. N., & Knutson, B. (2010). The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 35(1), 4-26. [DOI:10.1038/npp.2009.129] [PMID] [PMCID]
Hartwell, K. J., Johnson, K. A., Li, X., Myrick, H., LeMatty, T., & George, M. S., et al. (2011). Neural correlates of craving and resisting craving for tobacco in nicotine dependent smokers. Addiction Biology, 16(4), 654-666. [DOI:10.1111/j.1369-1600.2011.00340.x] [PMID] [PMCID]
Van Hedger, K., Keedy, S. K., Mayo, L. M., Heilig, M., & de Wit, H. (2018). Neural responses to cues paired with methamphetamine in healthy volunteers. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 43(8), 1732–1737.[DOI:10.1038/s41386-017-0005-5] [PMID] [PMCID]
Hopfinger, J. B., Buonocore, M. H., & Mangun, G. R. (2000). The neural mechanisms of top-down attentional control. Nature Neuroscience, 3(3), 284-291. [DOI:10.1038/72999] [PMID]
Huang, S., Zhang, Z., Dai, Y., Zhang, C., Yang, C., & Fan, L., et al. (2018). Craving responses to methamphetamine and sexual visual cues in individuals with methamphetamine use disorder after long-term drug rehabilitation. Frontiers in Psychiatry, 9, 145. [DOI:10.3389/fpsyt.2018.00145] [PMID] [PMCID]
Jasinska, A. J., Stein, E. A., Kaiser, J., Naumer, M. J., & Yalachkov, Y. (2014). Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neuroscience and Biobehavioral Reviews, 38, 1–16. [DOI:10.1016/j.neubiorev.2013.10.013] [PMID] [PMCID]
Koob, G. F., & Volkow, N. D. (2010). Neurocircuitry of addiction. Neuropsychopharmacology, 35(1), 217-238. [DOI:10.1038/npp.2009.110] [PMID] [PMCID]
Koob, G. F., & Volkow, N. D. (2016). Neurobiology of addiction: A neurocircuitry analysis. The Lancet. Psychiatry, 3(8), 760-773. [DOI:10.1016/S2215-0366(16)00104-8] [PMID]
Kühn, S., & Gallinat, J. (2011). Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. The European Journal of Neuroscience, 33(7), 1318-1326. [DOI:10.1111/j.1460-9568.2010.07590.x] [PMID]
Li, Y. Q., Li, F. Q., Wang, X. Y., Wu, P., Zhao, M., & Xu, C. M., et al. (2008). Central amygdala extracellular signal-regulated kinase signaling pathway is critical to incubation of opiate craving. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 28(49), 13248-13257. [DOI:10.1523/JNEUROSCI.3027-08.2008] [PMID] [PMCID]
Limbrick-Oldfield, E. H., Mick, I., Cocks, R. E., McGonigle, J., Sharman, S. P., & Goldstone, A. P., et al. (2017). Neural substrates of cue reactivity and craving in gambling disorder. Translational Psychiatry, 7(1), e992. [DOI:10.1038/tp.2016.256] [PMID] [PMCID]
Liu, X., Hairston, J., Schrier, M., & Fan, J. (2011). Common and distinct networks underlying reward valence and processing stages: A meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 35(5), 1219-1236. [DOI:10.1016/j.neubiorev.2010.12.012] [PMID] [PMCID]
Lui, S., Zhou, X. J., Sweeney, J. A., & Gong, Q. (2016). Psychoradiology: The frontier of neuroimaging in psychiatry. Radiology, 281(2), 357-372. [DOI:10.1148/radiol.2016152149] [PMID] [PMCID]
Luijten, M., Veltman, D. J., van den Brink, W., Hester, R., Field, M., & Smits, M., et al. (2011). Neurobiological substrate of smoking-related attentional bias. NeuroImage, 54(3), 2374-2381. [DOI:10.1016/j.neuroimage.2010.09.064] [PMID]
Lütcke, H., & Frahm, J. (2008). Lateralized anterior cingulate function during error processing and conflict monitoring as revealed by high-resolution fMRI. Cerebral Cortex (New York, N.Y.: 1991), 18(3), 508-515. [DOI:10.1093/cercor/bhm090] [PMID]
Malcolm, R., Myrick, H., Li, X., Henderson, S., Brady, K. T., & George, M. S., et al. (2016). Regional brain activity in abstinent methamphetamine dependent males following cue exposure. Journal of Drug Abuse, 2(1), 16. [DOI:10.21767/2471-853X.100016] [PMID] [PMCID]
McClernon, F. J., Hiott, F. B., Liu, J., Salley, A. N., Behm, F. M., & Rose, J. E. (2007). Selectively reduced responses to smoking cues in amygdala following extinction-based smoking cessation: results of a preliminary functional magnetic resonance imaging study. Addiction Biology, 12(3-4), 503-512. [DOI:10.1111/j.1369-1600.2007.00075.x] [PMID]
McClernon, F. J., Kozink, R. V., Lutz, A. M., & Rose, J. E. (2009). 24-hr smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology, 204(1), 25-35. [DOI:10.1007/s00213-008-1436-9] [PMID] [PMCID]
Merikangas, K. R., & McClair, V. L. (2012). Epidemiology of substance use disorders. Human Genetics, 131(6), 779-789. [DOI:10.1007/s00439-012-1168-0] [PMID] [PMCID]
Milham, M. P., Craddock, R. C., & Klein, A. (2017). Clinically useful brain imaging for neuropsychiatry: How can we get there? Depression and Anxiety, 34(7), 578-587. [DOI:10.1002/da.22627] [PMID]
Moeller, S. J., Konova, A. B., Parvaz, M. A., Tomasi, D., Lane, R. D., & Fort, C., et al. (2014). Functional, structural, and emotional correlates of impaired insight in cocaine addiction. JAMA Psychiatry, 71(1), 61-70. [DOI:10.1001/jamapsychiatry.2013.2833] [PMID] [PMCID]
Moeller, S. J., & Paulus, M. P. (2018). Toward biomarkers of the addicted human brain: Using neuroimaging to predict relapse and sustained abstinence in substance use disorder. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 80(Pt B), 143–154. [DOI:10.1016/j.pnpbp.2017.03.003] [PMID] [PMCID]
Moreno-Rius, J., & Miquel, M. (2017). The cerebellum in drug craving. Drug and Alcohol Dependence, 173, 151-158. [DOI:10.1016/j.drugalcdep.2016.12.028] [PMID]
Murphy, A., Lubman, D. I., McKie, S., Bijral, P. S., Peters, L. A., & Faiz, Q., et al. (2017). Time-dependent neuronal changes associated with craving in opioid dependence: An fMRI study. Addiction Biology, 23(5), 1168–1178. [DOI:10.1111/adb.12554] [PMID] [PMCID]
Naqvi, N. H., & Bechara, A. (2009). The hidden island of addiction: The insula. Trends in Neurosciences, 32(1), 56-67. [DOI:10.1016/j.tins.2008.09.009] [PMID] [PMCID]
Naqvi, N. H., Rudrauf, D., Damasio, H., & Bechara, A. (2007). Damage to the insula disrupts addiction to cigarette smoking. Science, 315(5811), 531-534. [DOI:10.1126/science.1135926] [PMID] [PMCID]
Nestor, L., McCabe, E., Jones, J., Clancy, L., & Garavan, H. (2011). Differences in “bottom-up” and “top-down” neural activity in current and former cigarette smokers: Evidence for neural substrates which may promote nicotine abstinence through increased cognitive control. NeuroImage, 56(4), 2258-2275. [DOI:10.1016/j.neuroimage.2011.03.054] [PMID]
National Institute on Drug Abuse. (2015). Nationwide trends. United States: National Institute on Drug Abuse. [Link]
Noori, H. R., Cosa Linan, A., & Spanagel, R. (2016). Largely overlapping neuronal substrates of reactivity to drug, gambling, food and sexual cues: A comprehensive meta-analysis. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology, 26(9), 1419-1430. [DOI:10.1016/j.euroneuro.2016.06.013] [PMID]
O'Doherty, J., Dayan, P., Schultz, J., Deichmann, R., Friston, K., & Dolan, R. J. (2004). Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science (New York, N.Y.), 304(5669), 452-454. [DOI:10.1126/science.1094285] [PMID]
Phan, K. L., Liberzon, I., Welsh, R. C., Britton, J. C., & Taylor, S. F. (2003). Habituation of rostral anterior cingulate cortex to repeated emotionally salient pictures. Neuropsychopharmacology, 28(7), 1344-1350. [DOI:10.1038/sj.npp.1300186] [PMID]
Paulus, M. P., Tapert, S. F., & Schuckit, M. A. (2005). Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Archives of General Psychiatry, 62(7), 761-768. [DOI:10.1001/archpsyc.62.7.761] [PMID]
Prisciandaro, J. J., Joseph, J. E., Myrick, H., McRae-Clark, A. L., Henderson, S., & Pfeifer, J., et al. (2014). The relationship between years of cocaine use and brain activation to cocaine and response inhibition cues. Addiction (Abingdon, England), 109(12), 2062-2070. [DOI:10.1111/add.12666] [PMID] [PMCID]
Reynolds, E. K., & Monti, P. M. (2013). The cue reactivity paradigm in addiction research. In: J. MacKillop, & H. de Wit (Eds.), The Wiley-Blackwell Handbook of addiction psychopharmacology (pp. 381-410). New Jersey: John Wiley & Sons, Ltd. [Link]
Robbins, T., & Clark, L. (2015). Behavioral addictions. Current Opinion in Neurobiology, 30, 66-72. [DOI:10.1016/j.conb.2014.09.005] [PMID]
Robinson, T. E., & Berridge, K. C. (1993). The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research. Brain Research Reviews, 18(3), 247-291. [DOI:10.1016/0165-0173(93)90013-P] [PMID]
Schacht, J. P., Anton, R. F., & Myrick, H. (2013). Functional neuroimaging studies of alcohol cue reactivity: A quantitative meta-analysis and systematic review. Addiction Biology, 18(1), 121-133. [DOI:10.1111/j.1369-1600.2012.00464.x] [PMID] [PMCID]
Schacht, J. P., Anton, R. F., Randall, P. K., Li, X., Henderson, S., & Myrick, H. (2011). Stability of fMRI striatal response to alcohol cues: A hierarchical linear modeling approach. NeuroImage, 56(1), 61-68. [DOI:10.1016/j.neuroimage.2011.02.004] [PMID] [PMCID]
Seeley, W. W., Menon, V., Schatzberg, A. F., Keller, J., Glover, G. H., & Kenna, H., et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27(9), 2349–2356. [DOI:10.1523/JNEUROSCI.5587-06.2007] [PMID] [PMCID]
Smith, S. M., Jenkinson, M., Woolrich, M. W., Beckmann, C. F., Behrens, T. E., & Johansen-Berg, H., et al. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23 (Suppl 1), S208–S219. [DOI:10.1016/j.neuroimage.2004.07.051] [PMID]
Sun, Y., Ying, H., Seetohul, R. M., Xuemei, W., Ya, Z., & Qian, L., et al. (2012). Brain fMRI study of crave induced by cue pictures in online game addicts (male adolescents). Behavioural Brain Research, 233(2), 563-576. [DOI:10.1016/j.bbr.2012.05.005] [PMID]
Tang, D. W., Fellows, L. K., Small, D. M., & Dagher, A. (2012). Food and drug cues activate similar brain regions: A meta-analysis of functional MRI studies. Physiology & Behavior, 106(3), 317-324. [DOI:10.1016/j.physbeh.2012.03.009] [PMID]
Tiffany, S. T., & Wray, J. M. (2012). The clinical significance of drug craving. Annals of the New York Academy of Sciences, 1248, 1-17. [DOI:10.1111/j.1749-6632.2011.06298.x] [PMID] [PMCID]
Volkow, N. D., Baler, R. D., & Goldstein, R. Z. (2011). Addiction: Pulling at the neural threads of social behaviors. Neuron, 69(4), 599-602. [DOI:10.1016/j.neuron.2011.01.027] [PMID] [PMCID]
Volkow, N. D., Wang, G. J., Telang, F., Fowler, J. S., Logan, J., & Childress, A. R., et al. (2006). Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 26(24), 6583–6588. [DOI:10.1523/JNEUROSCI.1544-06.2006] [PMID] [PMCID]
Wassum, K. M., & Izquierdo, A. (2015). The basolateral amygdala in reward learning and addiction. Neuroscience and Biobehavioral Reviews, 57, 271-283. [DOI:10.1016/j.neubiorev.2015.08.017] [PMID] [PMCID]
Whelan, R., & Garavan, H. (2014). When optimism hurts: Inflated predictions in psychiatric neuroimaging. Biological Psychiatry, 75(9), 746-748. [DOI:10.1016/j.biopsych.2013.05.014] [PMID]
Whiteford, H. A., Ferrari, A. J., Degenhardt, L., Feigin, V., & Vos, T. (2015). The global burden of mental, neurological and substance use disorders: An analysis from the global burden of disease study 2010. Plos One, 10(2), e0116820. [DOI:10.1371/journal.pone.0116820] [PMID] [PMCID]
Wiers, C. E., Stelzel, C., Gladwin, T. E., Park, S. Q., Pawelczack, S., & Gawron, C. K., et al. (2015). Effects of cognitive bias modification training on neural alcohol cue reactivity in alcohol dependence. The American Journal of Psychiatry, 172(4), 335-343. [DOI:10.1176/appi.ajp.2014.13111495] [PMID]
Wilson, S. J., & Sayette, M. A. (2015). Neuroimaging craving: Urge intensity matters. Addiction (Abingdon, England), 110(2), 195-203. [DOI:10.1111/add.12676] [PMID] [PMCID]
Wilson, S. J., Sayette, M. A., & Fiez, J. A. (2004). Prefrontal responses to drug cues: A neurocognitive analysis. Nature Neuroscience, 7(3), 211-214. [DOI:10.1038/nn1200] [PMID] [PMCID]
Wise, R. A. (1988). The neurobiology of craving: Implications for the understanding and treatment of addiction. Journal of Abnormal Psychology, 97(2), 118–132. [DOI:10.1037/0021-843X.97.2.118] [PMID] [PMCID]
Woolrich, M. W., Ripley, B. D., Brady, M., & Smith, S. M. (2001).Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage, 14(6), 1370-1386. [DOI:10.1006/nimg.2001.0931] [PMID]
Wright, C. I., Fischer, H., Whalen, P. J., McInerney, S. C., Shin, L. M., & Rauch, S. L. (2001). Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport, 12(2), 379-383. [DOI:10.1097/00001756-200102120-00039] [PMID]
Xu, X., Clark, U. S., David, S. P., Mulligan, R. C., Knopik, V. S., & McGeary, J., et al. (2014). Effects of nicotine deprivation and replacement on BOLD-fMRI response to smoking cues as a function of DRD4 VNTR genotype. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 16(7), 939-947. [DOI:10.1093/ntr/ntu010] [PMID] [PMCID]
Yalachkov, Y., Kaiser, J., & Naumer, M. J. (2012). Functional neuroimaging studies in addiction: Multisensory drug stimuli and neural cue reactivity. Neuroscience & Biobehavioral Reviews, 36(2), 825-835. [DOI:10.1016/j.neubiorev.2011.12.004] [PMID]
Yin, J. J., Ma, S. H., Xu, K., Wang, Z. X., Le, H. B., & Huang, J. Z., et al. (2012). Functional magnetic resonance imaging of methamphetamine craving. Clinical Imaging, 36(6), 695-701. [DOI:10.1016/j.clinimag.2012.02.006] [PMID]
Young, K. A., Franklin, T. R., Roberts, D. C., Jagannathan, K., Suh, J. J., & Wetherill, R. R., et al. (2014). Nipping cue reactivity in the bud: Baclofen prevents limbic activation elicited by subliminal drug cues. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 34(14), 5038-5043. [DOI:10.1523/JNEUROSCI.4977-13.2014] [PMID] [PMCID]
Type of Study: Original |
Subject:
Cognitive Neuroscience
Received: 2020/12/17 | Accepted: 2021/05/23 | Published: 2024/05/1
Received: 2020/12/17 | Accepted: 2021/05/23 | Published: 2024/05/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





