Volume 16, Issue 5 (September & October 2025)
BCN 2025, 16(5): 965-974 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Manorenj S, Jawalkar S. Clinical Efficacy and Safety of Dual Versus Single Antiplatelet Therapy in Lacunar Stroke. BCN 2025; 16 (5) :965-974
URL: http://bcn.iums.ac.ir/article-1-1972-en.html
URL: http://bcn.iums.ac.ir/article-1-1972-en.html
1- Department of Neurology, Deccan College of Medical Sciences, Princess Esra Hospital, Hyderabad, India.
Full-Text [PDF 739 kb]
| Abstract (HTML)
Full-Text:
Introduction
Ischemic stroke accounts for the major cause of disability and death among adults. Statistics have shown that every 20 seconds, one Indian suffers a brain stroke, and every year it debilitates around 1.54 million Indians. (Sentinel, 2019). Small vessel disease or lacunar stroke is the second common subtype of stroke in India, comprising 18% (Banerjee & Das, 2016) of ischemic stroke and 20%-30% of stroke worldwide (Moran et al., 2012). Antiplatelet therapy has been proven to play a crucial role in the secondary prevention of stroke. Notably, low-dose aspirin is effective in minimizing ischemic events in patients above a certain risk threshold (Antithrombotic Trialists' Collaboration, 2002). A study on clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE) has indicated the efficacy of clopidogrel over aspirin in ischemic stroke patients (CAPRIE Steering Committee, 1996).
Clopidogrel in high-risk patients with acute nondisabling cerebrovascular events (CHANCE) trial, which administered dual antiplatelet therapy (DAPT) versus 75 mg aspirin monotherapy within 24 hours of presentation in Chinese patients with minor stroke (National Institute of Health Stroke scale [NIHSS] <4) or transient ischemic attack (TIA) for 3 weeks after the index event, has proved a reduction in stroke recurrence at 90 days (8.2% versus 11.7%, P<0.001) without an associated increase in haemorrhagic stroke (Wang et al., 2013). However, the adequacy and risks of DAPT have not yet been evaluated specifically for lacunar stroke, though 56% cases in the CHANCE trial had lacunar stroke. The platelet-oriented inhibition in new TIA and minor ischemic stroke (POINT) trial evaluated a similar regimen of DAPT compared to aspirin monotherapy (50-325 mg). Still, the treatment lasted for an extended period of 3 months rather than 3 weeks (Johnston et al., 2013), and the POINT study inferred that those who received a combination of clopidogrel and aspirin had a lower risk of major ischemic events but a higher risk of major hemorrhage at 90 days than those who received aspirin alone.
In the MATCH trial (management of atherothrombosis with clopidogrel in high-risk patients), the combination of clopidogrel and aspirin was compared with clopidogrel alone in patients with recent ischemic stroke or TIA with at least one additional vascular risk factor (Diener et al., 2004). In their study, more than half of the patients (53%) had been diagnosed with small vessel disease, which was the cause of their qualifying event. The MATCH study concluded that DAPT in high-risk patients could lead to a non-significant decline in major vascular events coupled with an increased risk of major bleeding. The SPS3 trial (secondary prevention of small subcortical strokes) found no benefit in adding clopidogrel to aspirin, especially when compared with placebo, in patients with a recent small vessel lacunar stroke who were already taking aspirin at the time of their index event (Benavente et al., 2011).
The recently published guidelines endorse the use of single antiplatelet therapy without a fixed dose regimen for acute ischemic stroke. In contrast, DAPT for 3 weeks is recommended in patients with minor stroke or TIA. The available data on the effect of dual antiplatelet treatment with aspirin plus clopidogrel vs single antiplatelet therapy with aspirin alone and their doses in lacunar stroke among the Indian population is rather inadequate. Accordingly, our study aimed to determine the effectiveness, safety, and tolerability of dual versus single antiplatelet therapy in the treatment of patients with recent lacunar stroke, among the local population.
Materials and Methods
Study design
This research was a prospective, single-center study conducted at the Department of Neurology at our institute over 12 months, from December 2018 to December 2019. Patients with clinically evident acute cerebral infarction (CI) or TIA who had a lacunar stroke were enrolled in the study. Lacunar infarctions are defined as small subcortical infarcts with a size less than 15 mm in diameter seen in the deep cerebral white matter, basal ganglia, or pons, presumed to result from the occlusion of a single small perforating artery supplying the subcortical areas of the brain, caused by occlusion of a penetrating artery from a large cerebral artery, most commonly from the circle of Willis (Feekes et al., 2005).
Study participants
During the study period, patients with possible clinical ischemic cerebrovascular events during their period of hospitalization were subjected to computed tomography (CT) or magnetic resonance imaging (MRI) of the head and neck. The diagnosis was made using the trial of ORG 10172 in acute stroke treatment [TOAST] criteria for lacunar stroke. TOAST criteria (1993) consisted of 1) a traditional lacunar syndrome without cortical signs, 2) supporting features such as hypertension and diabetes mellitus, 3) the lack of an infarct explaining deficits on computed tomography CT/MRI examination or a subcortical lesion less than 15 mm in diameter, and 4) the absence of features that suggest a high likelihood of cardioembolism or embolism from upstream arterial stenosis being greater than 50% (Adams et al., 1993).
The eligibility of a patient was determined by the following inclusive criteria: Patients aged 18-80 years, a diagnosis of an acute CI or TIA, and a confirmed lacunar infarction as evidenced by CT/MRI of the brain. The exclusion criteria included large-area cerebral infarction or haemorrhagic infarct, cardiogenic brain embolism, cortical infarction, current peptic ulceration or history of systemic bleeding, systemic diseases like terminal malignancy or serious renal or liver disease, known contraindication to clopidogrel or aspirin, major surgery or trauma in the past 3 months or planning operation in the near future, gastrointestinal disorders or inability to obtain oral drugs and discontinuation of the study drug before testing. Baseline characteristics like age, sex, smoking history, alcohol use, and vascular risk factors, including diabetes mellitus, hyperlipidaemia, hypertension, TIA, and coronary heart disease, were assessed at baseline.
Study procedure
The patients were randomly categorized into 4 groups. Group 1 received aspirin 150 mg (A150), group 2 aspirin 150 mg plus clopidogrel 75 mg (A150+C75), group 3 aspirin 75 mg plus clopidogrel 75 mg (A75+C75), and group 4 aspirin 75 mg (A75) once daily. They were diligently followed up on for 90 days. A loading dose of antiplatelet agents was not administered to patients in the study groups. Neurological deficit was measured using the NIHSS stroke scale, and functional status was assessed using the modified Rankin scale (mRS) at baseline and subsequent follow-up. All four groups received atorvastatin 80 mg, along with their respective antihypertensive and diabetic medications. All patients were required to return at 90 days for a consultation with the neurophysician. If not feasible, follow-up evaluations were conducted by telephonic contact with the patient/her family doctor, or occasionally a caregiver. Patients were instructed to report immediately if they detected an external bleeding tendency, a worsening headache, or a new deficit. Tolerability was decided based on minor adverse reactions. Data were collected using a proforma that consisted of a demographic profile, risk factors, details about drugs, clinical profile, and radiological profile, as well as their respective groups, endpoints, and follow-ups (2 weeks, 1 month, 2 months, and 3 months). During contacts with the patients, details regarding the occurrence of possible outcome events, changes in trial medication, and adverse events were earnestly recorded.
Endpoints
Our focus was largely on the main endpoints. Endpoints were the recurrence of stroke, cardiovascular death, and bleeding events. The efficacy of the drug was determined by clinical improvement or any recurrence at follow-up. Intracranial and extracranial bleeding tendencies, and the tolerability of the drug, determined the safety of the drug.
Statistical analysis
Data obtained in the study were subjected to statistical analysis using SPSS software, version 18.0 (IBM) and were tabulated in a Microsoft Excel spreadsheet. A two-tailed probability value <0.05 was considered significant. All confidence intervals (CI) were set at 95%. All data were shown as the Mean±SD. Differences in baseline factors (sex, age, clinical characteristics, and risk factors) among the groups were compared using the 2x2 Fisher exact test for significance. Multivariate analysis included adjustments for age and sex. Analysis of efficacy and safety data was calculated using a 95% CI of the difference in proportions.
Results
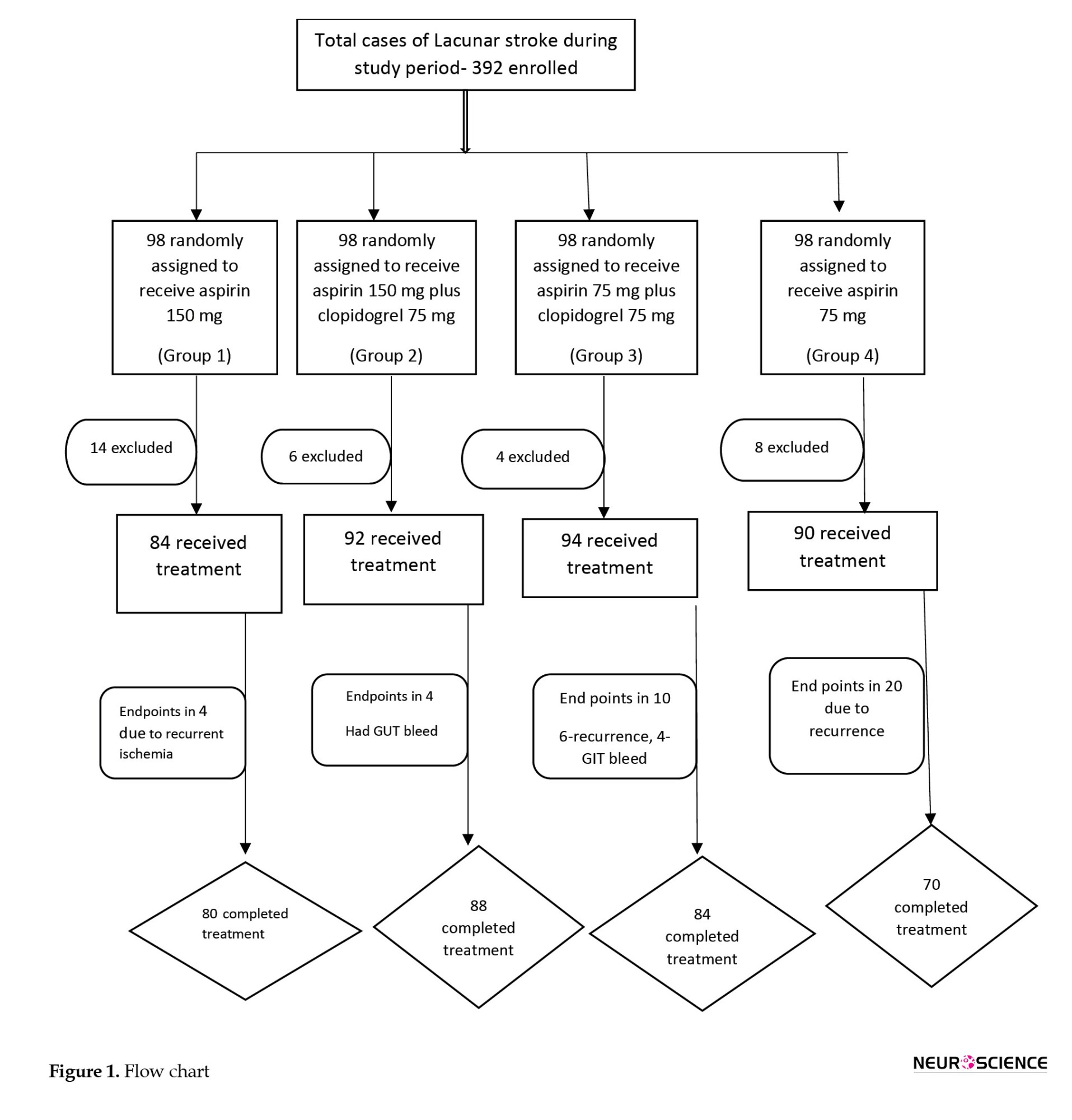
Of the 392 patients diagnosed with lacunar stroke, 360 were included in the study (Figure 1) and were followed for 90 days. Participants were randomly assigned to 4 groups. A total of 84 patients received aspirin 150 mg (group 1: A150) alone, 92 patients received aspirin 150 mg plus clopidogrel 75 mg (group 2: A150+C75), 94 patients received aspirin 75 mg plus clopidogrel 75 mg (group 3: A75+C75), and the remaining 90 patients were treated with aspirin 75 mg alone (group 4: A75).
Baseline characteristics
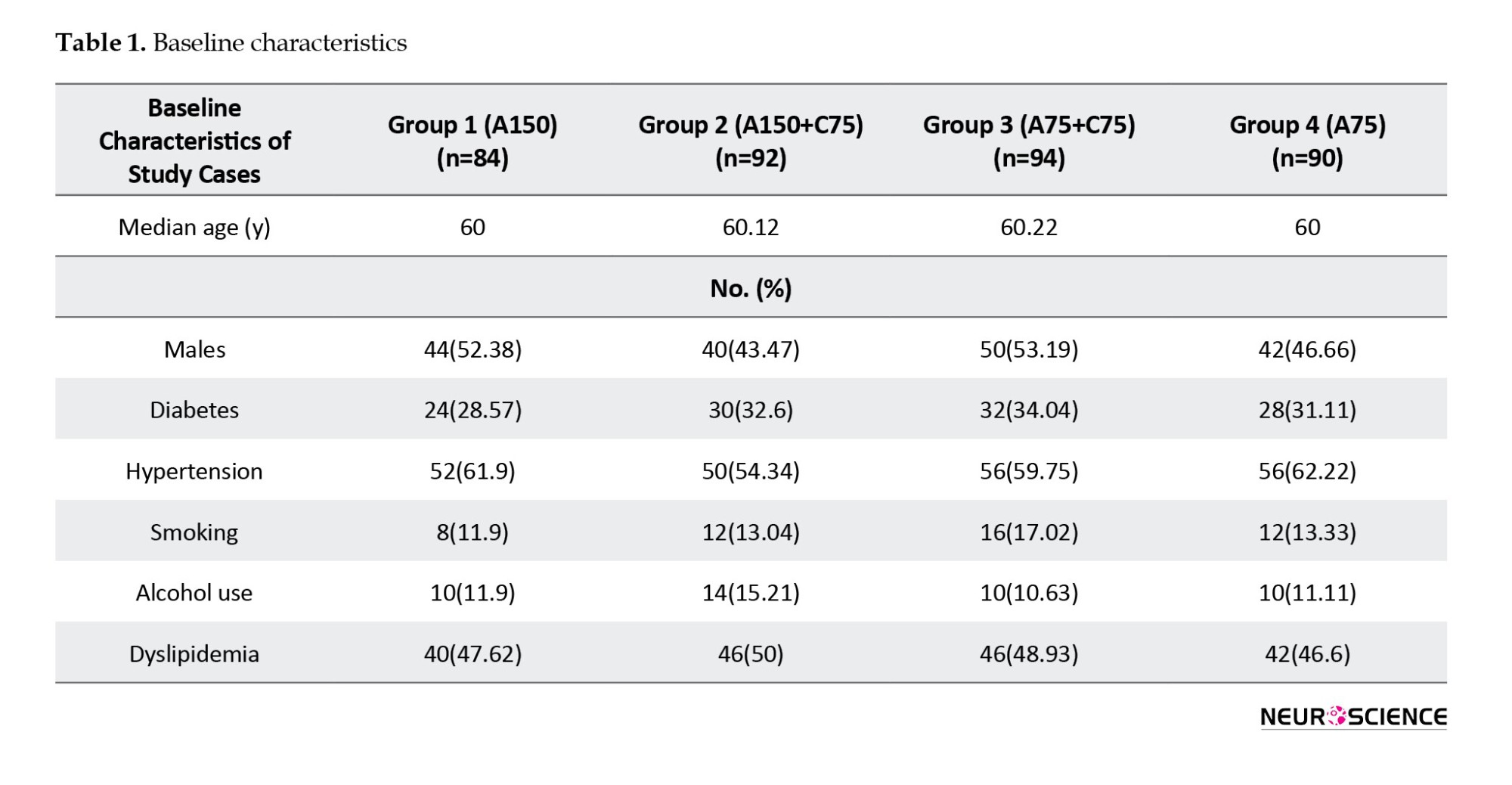
The patients’ ages ranged between 24 and 80 years, with a mean age of 57.8±14.1 years. A total of 188 patients (52.2%) were males. The most prevalent risk factors among these 4 groups were hypertension (62.2%), diabetes mellitus (33.3%), smoking (4.4%), alcohol consumption (11.1%), and tobacco use (7.7%). There were no significant differences among these risk factors, nor among individual groups (Table 1).
Clinical characteristics
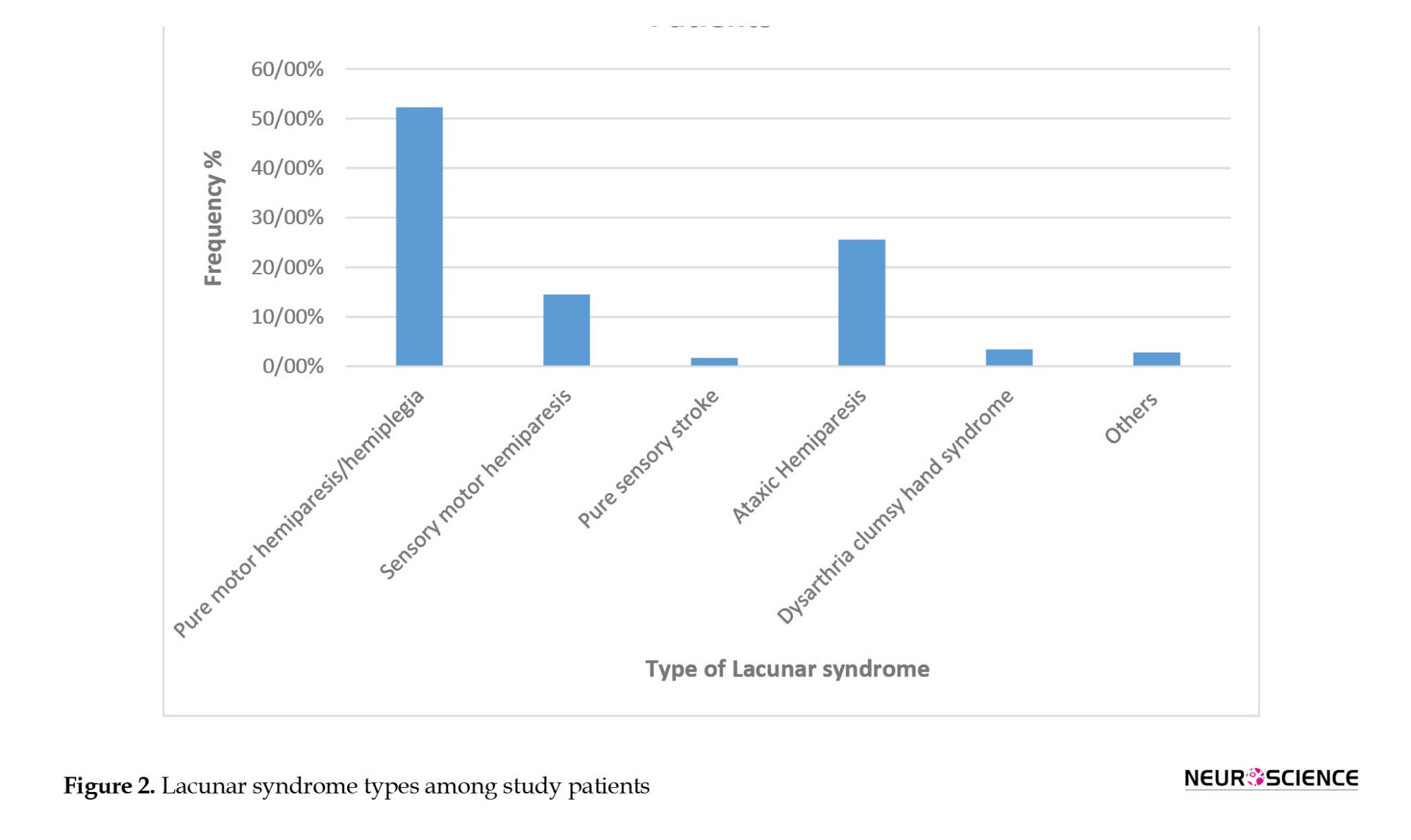
A majority of patients belonged to the mild (NIHSS ≤8) to moderate (NIHSS, 9-15) stroke scale range. There were no significant differences among individual groups. Baseline NIHSS scores ranged from 2 to 17, with mRS scores of 1-4 in both groups. At 90 days, mRS scores were 0-1 in all groups. The Mean±SD baseline NIHSS was 7.92±4.05, and the mean mRS was 3.18±0.77 among the groups. A majority of the patients had a baseline NIHSS score of 8 and an mRS score of 3. Pure motor hemiparesis was the most common type of lacunar syndrome observed, comprising 52.22% of patients, followed by ataxic hemiparesis in 25.55% of patients (Figure 2).
Efficacy and safety endpoints
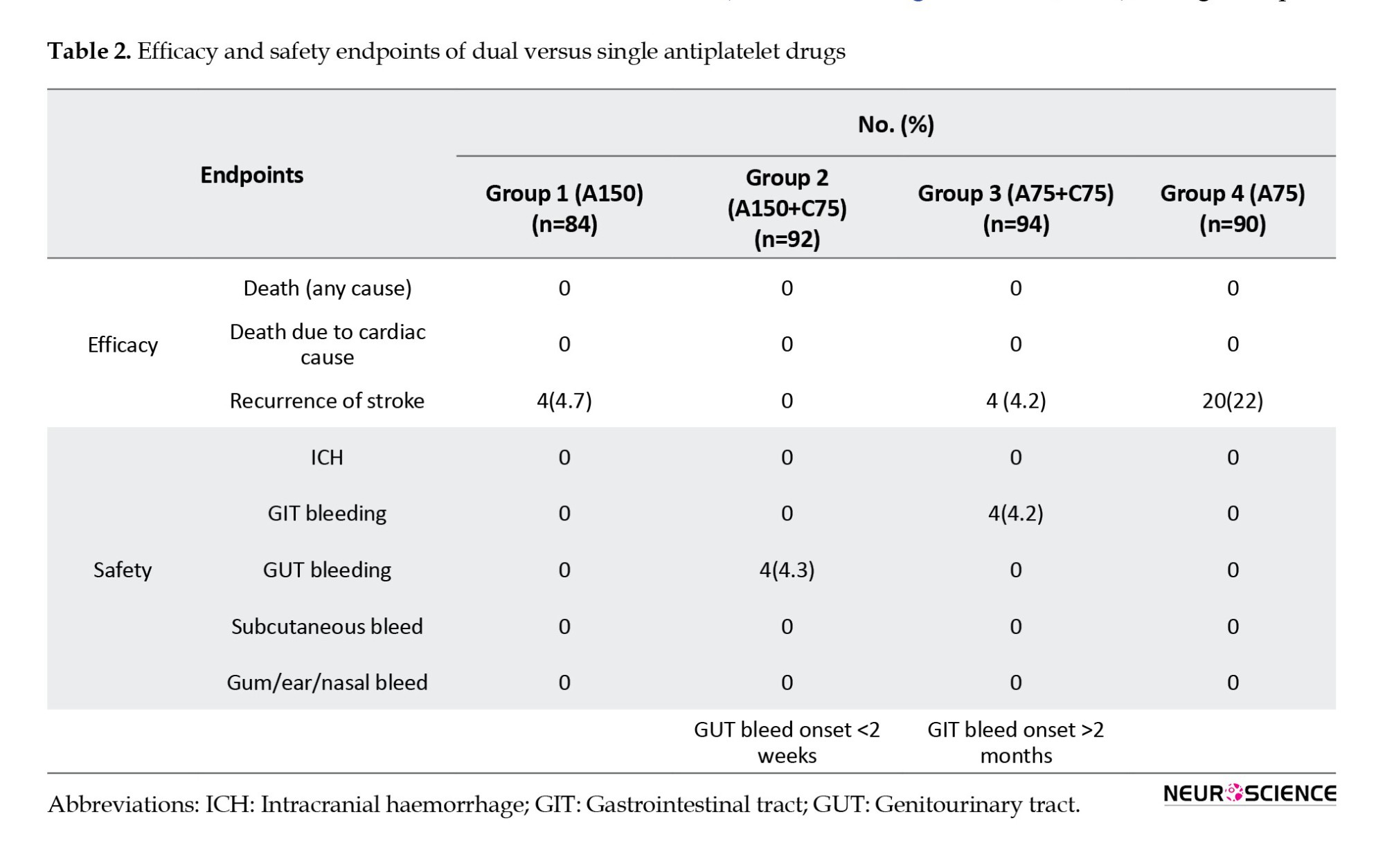
Among the 360 participants, recurrence of ischemic stroke occurred in 4 patients (4.7%) who were treated with 150 mg aspirin alone, 4(4.2%) receiving 75 mg aspirin and 75 mg clopidogrel, and a much higher number of 20(22%) in the group administered 75 mg aspirin alone. The 95% confidence interval (CI) was 2.6829% to 31.73551% with a P of 0.01 when patients in group 1 (A150) were compared with those in group 4 (A75). Similarly, the P was statistically significant when group 3 (A75 mg + C75) patients were compared with patients in group 4 (A75); the 95% confidence interval was 3.9439% to 32.1542%, and the P was 0.0114 (statistically significant). None of the patients on aspirin 150 mg plus clopidogrel 75 mg (group 2) developed recurrence of ischemic stroke.
There were haemorrhagic events among 4 recipients on DAPT (4.3%) in the aspirin 150 mg plus clopidogrel 75 mg group and 4 patients (4.2%) in the aspirin 75 mg plus clopidogrel 75 mg group compared to the aspirin-alone group (aspirin 150 mg [0%], aspirin 75 mg [0%]). The major haemorrhages observed were genitourinary tract bleeding among group 2 (aspirin 150 mg plus clopidogrel 75 mg) and gastrointestinal bleeding among group 3 (aspirin 75 mg plus clopidogrel 7 mg) (Table 2). No intracranial haemorrhage occurred in any of the 4 groups.
When comparing patients on DAPT (groups 2 [A150+C75] and 3 [A75+C75]) with those on single antiplatelet therapy (SAPT), groups 1 (A150) and 4 (A75), significant differences were noted in terms of the efficacy and safety of the planned antiplatelet regimen. DAPT was superior to SAPT in preventing the recurrence of ischemic stroke (P<0.0001). However, bleeding trends were noted in DAPT when compared to SAPT, and these differences were significant (P=0.005) (Table 3). The average time to recurrence of stroke was shorter in patients who were on aspirin 75 mg (8 days), while those on aspirin 150 mg developed recurrence of stroke in 52 days. Patients in group 3 (A 75+C75) who were on dual antiplatelets developed recurrence of stroke in an average of 59 days. Thus, patients on SAPT developed recurrence earlier than those on dual antiplatelets. We could not establish any relationship between recurrences of stroke and the progression time of lacunar stroke.

Discussion
It is noteworthy that in Japan, a country in Asia, a lower maintenance dose of clopidogrel (50 mg) is widely used for the prevention of vascular events (Asano et al., 2008). In contrast, in the United States and Europe (CAPRIE Steering Committee, 1996), 75 mg of clopidogrel is utilized. A Chinese study (Wang et al., 2013; Zuo et al., 2017) has demonstrated that DAPT is safer in preventing ischemic vascular events. Unfortunately, studies that compare the efficacy and safety of a combination of aspirin and clopidogrel over aspirin alone, in patients with lacunar stroke, continue to be meager. Most studies conducted in the past were focused on patients with all the subtypes of ischemic stroke, rather than a single subtype. To the best of our knowledge, there has been no study from India to ascertain the efficacy and optimal dosage of dual versus single antiplatelet therapy among Indians. Our data proved beyond doubt that a dual therapy with aspirin and clopidogrel over aspirin alone is more effective in preventing recurrent vascular events among Indian patients with lacunar stroke. Among the dual antiplatelet therapies, our study showed that aspirin 150 mg plus clopidogrel 75 mg was more efficacious than aspirin 75 mg plus clopidogrel 75 mg in preventing recurrent ischemic stroke. However, the dual therapy led to increased bleeding events when compared with the aspirin alone treatment, and the difference was statistically significant. Patients who received aspirin 75 mg plus clopidogrel 75 mg had fewer bleeding events than those in the aspirin 150 mg plus clopidogrel 75 mg group. Still, the disparity was found to be not statistically significant.
It was also seen that a higher dose of aspirin (150 mg) combined with clopidogrel 75 mg had resulted in major genitourinary tract bleeding in many cases, while aspirin 75 mg plus clopidogrel 75 mg led to gastrointestinal tract bleeding events in some. Genitourinary tract bleeding occurred by 2 weeks after initiation of aspirin 150 mg plus clopidogrel 75 mg regimen, while gastrointestinal bleeding occurred after 2 months of initiation of aspirin 75 mg plus clopidogrel 75 mg regimen. These results indicate that aspirin 75 mg plus clopidogrel 75 mg is safer and can be administered for longer durations compared to aspirin 150 mg plus clopidogrel 75 mg, particularly among the subset of the Indian population. The aggregate of our data also shows that the dual therapy, especially aspirin 75 mg plus clopidogrel 75 mg, is more effective and safer in the early treatment of patients with lacunar stroke.
The CHANCE trial (Wang et al., 2013) conducted among Chinese patients, they combined clopidogrel (initial dose 300 mg, followed by 75 mg/d for 90 days) and low-dose aspirin (75 mg/d for the first 3 weeks) was compared with placebo plus aspirin (75 mg/d for 90 days) in 5170 patients within 24 hours after the onset of minor ischemic stroke or high-risk TIA. The CHANCE trial demonstrated a significant reduction in stroke recurrence at 90 days without a corresponding increase in haemorrhagic stroke (Wang et al., 2013). However, the efficacy and risks of DAPT were not evaluated specifically for lacunar stroke. Similar findings were noted in our study, which showed that aspirin 75 mg plus clopidogrel 75 mg was superior to and more effective than single antiplatelet therapy (aspirin 150 mg or aspirin 75 mg) in terms of efficacy. In the present study, patients were not given the loading dose of clopidogrel or aspirin, and haemorrhagic complications developed at 2 months after initiation of DAPT (aspirin 75 mg plus clopidogrel 75 mg), indicating better tolerability and safety of the drug compared to Chinese patients.
SPS3 randomized controlled trials (RCT) (Benavente et al., 2011) found no benefit from adding clopidogrel to aspirin compared with placebo in patients (3020 patients) with a recent small vessel lacunar stroke taking aspirin at the time of their index event. In SPS3, the aspirin dose was 325 mg, and the clopidogrel dose was 75 mg. However, the median time from the qualifying event to enrolment in the SPS3 trial was more than 40 days. Hence, the results might have led to an underestimation of the therapy’s benefit, especially in the early post-stroke period (Benavente et al., 2011). In the present study, we used aspirin 150 mg plus clopidogrel 75 mg, as well as aspirin 75 mg plus clopidogrel 75 mg. We found that DAPT was potentially more beneficial compared to the SPS3 trial. This result may be due to the change in aspirin dosage in SPS3, where 325 mg of aspirin was sufficient to prevent recurrence in lacunar stroke, and the addition of clopidogrel did not provide any benefit. Juxtaposing SPS3 with the present study, it can be inferred that the addition of clopidogrel was beneficial in patients with an aspirin dose of 150 mg or 75 mg in preventing the recurrence of Lacunar stroke. We also found that aspirin alone in either 150 mg or 75 mg was not superior to DAPT in preventing recurrence of Ischemic events in patients with Lacunar stroke.
Although ours is the first prospective study with its primary focus on dual versus single antiplatelet therapy in lacunar stroke from India and second in the world (the SPS3 trial solely focused on lacunar stroke), it is not without its own limitations. Firstly, it was a single-center hospital-based study design with a small number of patients in each group. In contrast, large-scale sample studies are needed for further confirmation of our findings. Secondly, the findings regarding the efficacy and safety of aspirin plus clopidogrel were obtained within a short follow-up period of 90 days, when the patients were still under follow-up. Additionally, our study did not employ the front-loading method to measure the effect of clopidogrel and aspirin.
Nevertheless, our meticulous study yielded certain valuable, evidence-based findings. Our results have shown that the combination regimen of clopidogrel plus aspirin is more efficacious than aspirin alone for secondary stroke prevention in patients with lacunar stroke. The use of 150 mg aspirin plus 75 mg clopidogrel was found to be superior to aspirin alone and the aspirin 75 mg plus clopidogrel 75 mg regimen in reducing the incidence of ischemic stroke for patients with lacunar stroke, while there was an earlier and a higher rate of occurrence of haemorrhage in the aspirin 150 mg plus clopidogrel 75 mg group. And the study could decisively conclude that the 75 mg plus clopidogrel 75 mg regimen was the most promising approach in preventing the recurrence of ischemic stroke among patients with lacunar stroke in the subset of the South Indian population. Our study would certainly provide the much-needed direction for future studies that may contribute further to an area that urgently requires evidence-based research data. Although large-scale, randomized, multicenter, controlled trials are needed to confirm our findings among Indians, our study would certainly provide the necessary direction.
Conclusion
According to the study results, it can be concluded unequivocally that 150 mg aspirin plus 75 mg clopidogrel is superior to 75 mg aspirin plus 75 mg clopidogrel and aspirin alone (either 150 or 75 mg) in preventing stroke in patients with cerebral infarction or TIA due to lacunar stroke. The dual therapy of 150 mg aspirin plus 75 mg clopidogrel and 75 mg aspirin plus 75 mg clopidogrel resulted in a rather perturbing sequence of bleeding events. Accordingly, balancing between benefits and risks, it was inferred that aspirin 75 mg plus clopidogrel 75 mg is far superior and safer in preventing recurrent ischemic stroke among Indian patients with Lacunar stroke.
Ethical Considerations
Compliance with ethical guidelines
All the necessary information for conducting the study was collected prospectively, with the approval of the Ethics Committee of Deccan College of Medical Sciences, Hyderabad, India. Informed consent was secured from either the patients or their relatives before their inclusion in the study.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, and writing the original draft: Sandhya Manorenj; Visualization, and supervision: Srikanth Jawalkar; Review and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank the neurology staffs and, emergency team for helping in the study.
References
Ischemic stroke accounts for the major cause of disability and death among adults. Statistics have shown that every 20 seconds, one Indian suffers a brain stroke, and every year it debilitates around 1.54 million Indians. (Sentinel, 2019). Small vessel disease or lacunar stroke is the second common subtype of stroke in India, comprising 18% (Banerjee & Das, 2016) of ischemic stroke and 20%-30% of stroke worldwide (Moran et al., 2012). Antiplatelet therapy has been proven to play a crucial role in the secondary prevention of stroke. Notably, low-dose aspirin is effective in minimizing ischemic events in patients above a certain risk threshold (Antithrombotic Trialists' Collaboration, 2002). A study on clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE) has indicated the efficacy of clopidogrel over aspirin in ischemic stroke patients (CAPRIE Steering Committee, 1996).
Clopidogrel in high-risk patients with acute nondisabling cerebrovascular events (CHANCE) trial, which administered dual antiplatelet therapy (DAPT) versus 75 mg aspirin monotherapy within 24 hours of presentation in Chinese patients with minor stroke (National Institute of Health Stroke scale [NIHSS] <4) or transient ischemic attack (TIA) for 3 weeks after the index event, has proved a reduction in stroke recurrence at 90 days (8.2% versus 11.7%, P<0.001) without an associated increase in haemorrhagic stroke (Wang et al., 2013). However, the adequacy and risks of DAPT have not yet been evaluated specifically for lacunar stroke, though 56% cases in the CHANCE trial had lacunar stroke. The platelet-oriented inhibition in new TIA and minor ischemic stroke (POINT) trial evaluated a similar regimen of DAPT compared to aspirin monotherapy (50-325 mg). Still, the treatment lasted for an extended period of 3 months rather than 3 weeks (Johnston et al., 2013), and the POINT study inferred that those who received a combination of clopidogrel and aspirin had a lower risk of major ischemic events but a higher risk of major hemorrhage at 90 days than those who received aspirin alone.
In the MATCH trial (management of atherothrombosis with clopidogrel in high-risk patients), the combination of clopidogrel and aspirin was compared with clopidogrel alone in patients with recent ischemic stroke or TIA with at least one additional vascular risk factor (Diener et al., 2004). In their study, more than half of the patients (53%) had been diagnosed with small vessel disease, which was the cause of their qualifying event. The MATCH study concluded that DAPT in high-risk patients could lead to a non-significant decline in major vascular events coupled with an increased risk of major bleeding. The SPS3 trial (secondary prevention of small subcortical strokes) found no benefit in adding clopidogrel to aspirin, especially when compared with placebo, in patients with a recent small vessel lacunar stroke who were already taking aspirin at the time of their index event (Benavente et al., 2011).
The recently published guidelines endorse the use of single antiplatelet therapy without a fixed dose regimen for acute ischemic stroke. In contrast, DAPT for 3 weeks is recommended in patients with minor stroke or TIA. The available data on the effect of dual antiplatelet treatment with aspirin plus clopidogrel vs single antiplatelet therapy with aspirin alone and their doses in lacunar stroke among the Indian population is rather inadequate. Accordingly, our study aimed to determine the effectiveness, safety, and tolerability of dual versus single antiplatelet therapy in the treatment of patients with recent lacunar stroke, among the local population.
Materials and Methods
Study design
This research was a prospective, single-center study conducted at the Department of Neurology at our institute over 12 months, from December 2018 to December 2019. Patients with clinically evident acute cerebral infarction (CI) or TIA who had a lacunar stroke were enrolled in the study. Lacunar infarctions are defined as small subcortical infarcts with a size less than 15 mm in diameter seen in the deep cerebral white matter, basal ganglia, or pons, presumed to result from the occlusion of a single small perforating artery supplying the subcortical areas of the brain, caused by occlusion of a penetrating artery from a large cerebral artery, most commonly from the circle of Willis (Feekes et al., 2005).
Study participants
During the study period, patients with possible clinical ischemic cerebrovascular events during their period of hospitalization were subjected to computed tomography (CT) or magnetic resonance imaging (MRI) of the head and neck. The diagnosis was made using the trial of ORG 10172 in acute stroke treatment [TOAST] criteria for lacunar stroke. TOAST criteria (1993) consisted of 1) a traditional lacunar syndrome without cortical signs, 2) supporting features such as hypertension and diabetes mellitus, 3) the lack of an infarct explaining deficits on computed tomography CT/MRI examination or a subcortical lesion less than 15 mm in diameter, and 4) the absence of features that suggest a high likelihood of cardioembolism or embolism from upstream arterial stenosis being greater than 50% (Adams et al., 1993).
The eligibility of a patient was determined by the following inclusive criteria: Patients aged 18-80 years, a diagnosis of an acute CI or TIA, and a confirmed lacunar infarction as evidenced by CT/MRI of the brain. The exclusion criteria included large-area cerebral infarction or haemorrhagic infarct, cardiogenic brain embolism, cortical infarction, current peptic ulceration or history of systemic bleeding, systemic diseases like terminal malignancy or serious renal or liver disease, known contraindication to clopidogrel or aspirin, major surgery or trauma in the past 3 months or planning operation in the near future, gastrointestinal disorders or inability to obtain oral drugs and discontinuation of the study drug before testing. Baseline characteristics like age, sex, smoking history, alcohol use, and vascular risk factors, including diabetes mellitus, hyperlipidaemia, hypertension, TIA, and coronary heart disease, were assessed at baseline.
Study procedure
The patients were randomly categorized into 4 groups. Group 1 received aspirin 150 mg (A150), group 2 aspirin 150 mg plus clopidogrel 75 mg (A150+C75), group 3 aspirin 75 mg plus clopidogrel 75 mg (A75+C75), and group 4 aspirin 75 mg (A75) once daily. They were diligently followed up on for 90 days. A loading dose of antiplatelet agents was not administered to patients in the study groups. Neurological deficit was measured using the NIHSS stroke scale, and functional status was assessed using the modified Rankin scale (mRS) at baseline and subsequent follow-up. All four groups received atorvastatin 80 mg, along with their respective antihypertensive and diabetic medications. All patients were required to return at 90 days for a consultation with the neurophysician. If not feasible, follow-up evaluations were conducted by telephonic contact with the patient/her family doctor, or occasionally a caregiver. Patients were instructed to report immediately if they detected an external bleeding tendency, a worsening headache, or a new deficit. Tolerability was decided based on minor adverse reactions. Data were collected using a proforma that consisted of a demographic profile, risk factors, details about drugs, clinical profile, and radiological profile, as well as their respective groups, endpoints, and follow-ups (2 weeks, 1 month, 2 months, and 3 months). During contacts with the patients, details regarding the occurrence of possible outcome events, changes in trial medication, and adverse events were earnestly recorded.
Endpoints
Our focus was largely on the main endpoints. Endpoints were the recurrence of stroke, cardiovascular death, and bleeding events. The efficacy of the drug was determined by clinical improvement or any recurrence at follow-up. Intracranial and extracranial bleeding tendencies, and the tolerability of the drug, determined the safety of the drug.
Statistical analysis
Data obtained in the study were subjected to statistical analysis using SPSS software, version 18.0 (IBM) and were tabulated in a Microsoft Excel spreadsheet. A two-tailed probability value <0.05 was considered significant. All confidence intervals (CI) were set at 95%. All data were shown as the Mean±SD. Differences in baseline factors (sex, age, clinical characteristics, and risk factors) among the groups were compared using the 2x2 Fisher exact test for significance. Multivariate analysis included adjustments for age and sex. Analysis of efficacy and safety data was calculated using a 95% CI of the difference in proportions.
Results
Of the 392 patients diagnosed with lacunar stroke, 360 were included in the study (Figure 1) and were followed for 90 days. Participants were randomly assigned to 4 groups. A total of 84 patients received aspirin 150 mg (group 1: A150) alone, 92 patients received aspirin 150 mg plus clopidogrel 75 mg (group 2: A150+C75), 94 patients received aspirin 75 mg plus clopidogrel 75 mg (group 3: A75+C75), and the remaining 90 patients were treated with aspirin 75 mg alone (group 4: A75).

Baseline characteristics
The patients’ ages ranged between 24 and 80 years, with a mean age of 57.8±14.1 years. A total of 188 patients (52.2%) were males. The most prevalent risk factors among these 4 groups were hypertension (62.2%), diabetes mellitus (33.3%), smoking (4.4%), alcohol consumption (11.1%), and tobacco use (7.7%). There were no significant differences among these risk factors, nor among individual groups (Table 1).

Clinical characteristics
A majority of patients belonged to the mild (NIHSS ≤8) to moderate (NIHSS, 9-15) stroke scale range. There were no significant differences among individual groups. Baseline NIHSS scores ranged from 2 to 17, with mRS scores of 1-4 in both groups. At 90 days, mRS scores were 0-1 in all groups. The Mean±SD baseline NIHSS was 7.92±4.05, and the mean mRS was 3.18±0.77 among the groups. A majority of the patients had a baseline NIHSS score of 8 and an mRS score of 3. Pure motor hemiparesis was the most common type of lacunar syndrome observed, comprising 52.22% of patients, followed by ataxic hemiparesis in 25.55% of patients (Figure 2).

Efficacy and safety endpoints
Among the 360 participants, recurrence of ischemic stroke occurred in 4 patients (4.7%) who were treated with 150 mg aspirin alone, 4(4.2%) receiving 75 mg aspirin and 75 mg clopidogrel, and a much higher number of 20(22%) in the group administered 75 mg aspirin alone. The 95% confidence interval (CI) was 2.6829% to 31.73551% with a P of 0.01 when patients in group 1 (A150) were compared with those in group 4 (A75). Similarly, the P was statistically significant when group 3 (A75 mg + C75) patients were compared with patients in group 4 (A75); the 95% confidence interval was 3.9439% to 32.1542%, and the P was 0.0114 (statistically significant). None of the patients on aspirin 150 mg plus clopidogrel 75 mg (group 2) developed recurrence of ischemic stroke.
There were haemorrhagic events among 4 recipients on DAPT (4.3%) in the aspirin 150 mg plus clopidogrel 75 mg group and 4 patients (4.2%) in the aspirin 75 mg plus clopidogrel 75 mg group compared to the aspirin-alone group (aspirin 150 mg [0%], aspirin 75 mg [0%]). The major haemorrhages observed were genitourinary tract bleeding among group 2 (aspirin 150 mg plus clopidogrel 75 mg) and gastrointestinal bleeding among group 3 (aspirin 75 mg plus clopidogrel 7 mg) (Table 2). No intracranial haemorrhage occurred in any of the 4 groups.

When comparing patients on DAPT (groups 2 [A150+C75] and 3 [A75+C75]) with those on single antiplatelet therapy (SAPT), groups 1 (A150) and 4 (A75), significant differences were noted in terms of the efficacy and safety of the planned antiplatelet regimen. DAPT was superior to SAPT in preventing the recurrence of ischemic stroke (P<0.0001). However, bleeding trends were noted in DAPT when compared to SAPT, and these differences were significant (P=0.005) (Table 3). The average time to recurrence of stroke was shorter in patients who were on aspirin 75 mg (8 days), while those on aspirin 150 mg developed recurrence of stroke in 52 days. Patients in group 3 (A 75+C75) who were on dual antiplatelets developed recurrence of stroke in an average of 59 days. Thus, patients on SAPT developed recurrence earlier than those on dual antiplatelets. We could not establish any relationship between recurrences of stroke and the progression time of lacunar stroke.

Discussion
It is noteworthy that in Japan, a country in Asia, a lower maintenance dose of clopidogrel (50 mg) is widely used for the prevention of vascular events (Asano et al., 2008). In contrast, in the United States and Europe (CAPRIE Steering Committee, 1996), 75 mg of clopidogrel is utilized. A Chinese study (Wang et al., 2013; Zuo et al., 2017) has demonstrated that DAPT is safer in preventing ischemic vascular events. Unfortunately, studies that compare the efficacy and safety of a combination of aspirin and clopidogrel over aspirin alone, in patients with lacunar stroke, continue to be meager. Most studies conducted in the past were focused on patients with all the subtypes of ischemic stroke, rather than a single subtype. To the best of our knowledge, there has been no study from India to ascertain the efficacy and optimal dosage of dual versus single antiplatelet therapy among Indians. Our data proved beyond doubt that a dual therapy with aspirin and clopidogrel over aspirin alone is more effective in preventing recurrent vascular events among Indian patients with lacunar stroke. Among the dual antiplatelet therapies, our study showed that aspirin 150 mg plus clopidogrel 75 mg was more efficacious than aspirin 75 mg plus clopidogrel 75 mg in preventing recurrent ischemic stroke. However, the dual therapy led to increased bleeding events when compared with the aspirin alone treatment, and the difference was statistically significant. Patients who received aspirin 75 mg plus clopidogrel 75 mg had fewer bleeding events than those in the aspirin 150 mg plus clopidogrel 75 mg group. Still, the disparity was found to be not statistically significant.
It was also seen that a higher dose of aspirin (150 mg) combined with clopidogrel 75 mg had resulted in major genitourinary tract bleeding in many cases, while aspirin 75 mg plus clopidogrel 75 mg led to gastrointestinal tract bleeding events in some. Genitourinary tract bleeding occurred by 2 weeks after initiation of aspirin 150 mg plus clopidogrel 75 mg regimen, while gastrointestinal bleeding occurred after 2 months of initiation of aspirin 75 mg plus clopidogrel 75 mg regimen. These results indicate that aspirin 75 mg plus clopidogrel 75 mg is safer and can be administered for longer durations compared to aspirin 150 mg plus clopidogrel 75 mg, particularly among the subset of the Indian population. The aggregate of our data also shows that the dual therapy, especially aspirin 75 mg plus clopidogrel 75 mg, is more effective and safer in the early treatment of patients with lacunar stroke.
The CHANCE trial (Wang et al., 2013) conducted among Chinese patients, they combined clopidogrel (initial dose 300 mg, followed by 75 mg/d for 90 days) and low-dose aspirin (75 mg/d for the first 3 weeks) was compared with placebo plus aspirin (75 mg/d for 90 days) in 5170 patients within 24 hours after the onset of minor ischemic stroke or high-risk TIA. The CHANCE trial demonstrated a significant reduction in stroke recurrence at 90 days without a corresponding increase in haemorrhagic stroke (Wang et al., 2013). However, the efficacy and risks of DAPT were not evaluated specifically for lacunar stroke. Similar findings were noted in our study, which showed that aspirin 75 mg plus clopidogrel 75 mg was superior to and more effective than single antiplatelet therapy (aspirin 150 mg or aspirin 75 mg) in terms of efficacy. In the present study, patients were not given the loading dose of clopidogrel or aspirin, and haemorrhagic complications developed at 2 months after initiation of DAPT (aspirin 75 mg plus clopidogrel 75 mg), indicating better tolerability and safety of the drug compared to Chinese patients.
SPS3 randomized controlled trials (RCT) (Benavente et al., 2011) found no benefit from adding clopidogrel to aspirin compared with placebo in patients (3020 patients) with a recent small vessel lacunar stroke taking aspirin at the time of their index event. In SPS3, the aspirin dose was 325 mg, and the clopidogrel dose was 75 mg. However, the median time from the qualifying event to enrolment in the SPS3 trial was more than 40 days. Hence, the results might have led to an underestimation of the therapy’s benefit, especially in the early post-stroke period (Benavente et al., 2011). In the present study, we used aspirin 150 mg plus clopidogrel 75 mg, as well as aspirin 75 mg plus clopidogrel 75 mg. We found that DAPT was potentially more beneficial compared to the SPS3 trial. This result may be due to the change in aspirin dosage in SPS3, where 325 mg of aspirin was sufficient to prevent recurrence in lacunar stroke, and the addition of clopidogrel did not provide any benefit. Juxtaposing SPS3 with the present study, it can be inferred that the addition of clopidogrel was beneficial in patients with an aspirin dose of 150 mg or 75 mg in preventing the recurrence of Lacunar stroke. We also found that aspirin alone in either 150 mg or 75 mg was not superior to DAPT in preventing recurrence of Ischemic events in patients with Lacunar stroke.
Although ours is the first prospective study with its primary focus on dual versus single antiplatelet therapy in lacunar stroke from India and second in the world (the SPS3 trial solely focused on lacunar stroke), it is not without its own limitations. Firstly, it was a single-center hospital-based study design with a small number of patients in each group. In contrast, large-scale sample studies are needed for further confirmation of our findings. Secondly, the findings regarding the efficacy and safety of aspirin plus clopidogrel were obtained within a short follow-up period of 90 days, when the patients were still under follow-up. Additionally, our study did not employ the front-loading method to measure the effect of clopidogrel and aspirin.
Nevertheless, our meticulous study yielded certain valuable, evidence-based findings. Our results have shown that the combination regimen of clopidogrel plus aspirin is more efficacious than aspirin alone for secondary stroke prevention in patients with lacunar stroke. The use of 150 mg aspirin plus 75 mg clopidogrel was found to be superior to aspirin alone and the aspirin 75 mg plus clopidogrel 75 mg regimen in reducing the incidence of ischemic stroke for patients with lacunar stroke, while there was an earlier and a higher rate of occurrence of haemorrhage in the aspirin 150 mg plus clopidogrel 75 mg group. And the study could decisively conclude that the 75 mg plus clopidogrel 75 mg regimen was the most promising approach in preventing the recurrence of ischemic stroke among patients with lacunar stroke in the subset of the South Indian population. Our study would certainly provide the much-needed direction for future studies that may contribute further to an area that urgently requires evidence-based research data. Although large-scale, randomized, multicenter, controlled trials are needed to confirm our findings among Indians, our study would certainly provide the necessary direction.
Conclusion
According to the study results, it can be concluded unequivocally that 150 mg aspirin plus 75 mg clopidogrel is superior to 75 mg aspirin plus 75 mg clopidogrel and aspirin alone (either 150 or 75 mg) in preventing stroke in patients with cerebral infarction or TIA due to lacunar stroke. The dual therapy of 150 mg aspirin plus 75 mg clopidogrel and 75 mg aspirin plus 75 mg clopidogrel resulted in a rather perturbing sequence of bleeding events. Accordingly, balancing between benefits and risks, it was inferred that aspirin 75 mg plus clopidogrel 75 mg is far superior and safer in preventing recurrent ischemic stroke among Indian patients with Lacunar stroke.
Ethical Considerations
Compliance with ethical guidelines
All the necessary information for conducting the study was collected prospectively, with the approval of the Ethics Committee of Deccan College of Medical Sciences, Hyderabad, India. Informed consent was secured from either the patients or their relatives before their inclusion in the study.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, and writing the original draft: Sandhya Manorenj; Visualization, and supervision: Srikanth Jawalkar; Review and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank the neurology staffs and, emergency team for helping in the study.
References
Adams, H. P., Jr, Bendixen, B. H., Kappelle, L. J., Biller, J., Love, B. B., & Gordon, D. L., et al. (1993). Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke, 24(1), 35–41. [DOI:10.1161/01.STR.24.1.35] [PMID]
Antithrombotic Trialists' Collaboration. (2002). Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ (Clinical research ed.), 324(7329), 71–86. [DOI:10.1136/bmj.324.7329.71] [PMID]
Asano, T., Kobayashi, Y., Fukushima, K., Iwata, Y., Kitahara, H., & Ishio, N., et al. (2008). Safety and efficacy of low-dose clopidogrel in Japanese patients undergoing coronary stenting: preliminary 30-day clinical outcome. Circulation Journal: Official Journal of the Japanese Circulation Society, 72(10), 1707–1708. [DOI:10.1253/circj.CJ-08-0401] [PMID]
Banerjee, T. K., & Das, S. K. (2016). Fifty years of stroke researches in India. Annals of Indian Academy of Neurology, 19(1), 1–8. [DOI:10.4103/0972-2327.168631] [PMID]
Benavente, O. R., White, C. L., Pearce, L., Pergola, P., Roldan, A., & Benavente, M. F., et al. (2011). The secondary prevention of small subcortical strokes (SPS3) study. International Journal of Stroke: Official Journal of the International Stroke Society, 6(2), 164–175. [DOI:10.1111/j.1747-4949.2010.00573.x] [PMID]
CAPRIE Steering Committee. (1996). A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet (London, England), 348(9038), 1329–1339. [DOI:10.1016/S0140-6736(96)09457-3] [PMID]
Diener, H. C., Bogousslavsky, J., Brass, L. M., Cimminiello, C., Csiba, L., & Kaste, M., et al. (2004). Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): Randomised, double-blind, placebo-controlled trial. Lancet (London, England), 364(9431), 331–337. [DOI:10.1016/S0140-6736(04)16721-4] [PMID]
Feekes, J. A., Hsu, S. W., Chaloupka, J. C., & Cassell, M. D. (2005). Tertiary microvascular territories define lacunar infarcts in the basal ganglia. Annals of Neurology, 58(1), 18–30. [DOI:10.1002/ana.20505] [PMID]
Johnston, S. C., Easton, J. D., Farrant, M., Barsan, W., Battenhouse, H., & Conwit, R., et al. (2013). Platelet-oriented inhibition in new TIA and minor ischemic stroke (POINT) trial: Rationale and design. International Journal of Stroke: Official Journal of the International Stroke Society, 8(6), 479–483. [DOI:10.1111/ijs.12129] [PMID]
Moran, C., Phan, T. G., & Srikanth, V. K. (2012). Cerebral small vessel disease: A review of clinical, radiological, and histopathological phenotypes. International Journal of Stroke: Official Journal of the International Stroke Society, 7(1), 36–46. [DOI:10.1111/j.1747-4949.2011.00725.x] [PMID]
Sentinel. (2019). Every 20 Seconds, 1 Indian suffers a brain-stroke. Retrieved from: [Link]
Wang, Y., Wang, Y., Zhao, X., Liu, L., Wang, D., & Wang, C., et al. (2013). Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. The New England Journal of Medicine, 369(1), 11–19. [DOI:10.1056/NEJMoa1215340] [PMID]
Zuo, F. T., Liu, H., Wu, H. J., Su, N., Liu, J. Q., & Dong, A. Q. (2017). The effectiveness and safety of dual antiplatelet therapy in ischemic cerebrovascular disease with intracranial and extracranial arteriostenosis in Chinese patients: A randomized and controlled trail. Medicine, 96(1), e5497. [DOI:10.1097/MD.0000000000005497] [PMID]
Type of Study: Original |
Subject:
Clinical Neuroscience
Received: 2019/10/19 | Accepted: 2024/09/27 | Published: 2025/09/1
Received: 2019/10/19 | Accepted: 2024/09/27 | Published: 2025/09/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








