Volume 14, Issue 5 (September & October 2023)
BCN 2023, 14(5): 565-584 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Uzunoglu-Ozyurek E, Önal G, Dökmeci S. Investigating the Therapeutics Effects of Oral Cavity Derived Stem Cells on Neurodegenerative Diseases: A Systematic Review. BCN 2023; 14 (5) :565-584
URL: http://bcn.iums.ac.ir/article-1-1926-en.html
URL: http://bcn.iums.ac.ir/article-1-1926-en.html
1- Department of Endodontics, Dental Faculty, Hacettepe University, Ankara, Turkey.
2- Department of Medical Biology, Medical Faculty, Hacettepe University, Ankara, Turkey.
2- Department of Medical Biology, Medical Faculty, Hacettepe University, Ankara, Turkey.
Full-Text [PDF 961 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Stem cells can differentiate into multiple cell types and replicate. There are various types of stem cells, such as adipose-derived stem cells, bone-marrow-derived mesenchymal stem cells, embryonic stem cells, induced pluripotent stem cells, umbilical cord stem cells, and oral cavity-derived stem cells (Dulak et al., 2015). Oral cavity-derived stem cells (OCDSCs) are adult stem cells that can be isolated from the dental pulp of both permanent and primary teeth, and from the periodontal ligament, gingiva, maxillary sinus mucosa, and periapical lesions (Al-Habib & Huang, 2019; Marrelli et al., 2015). All dental, oral, and craniofacial structures are formed during development by neural crest-derived and or mesenchymal cells; therefore, stem cells derived from these structures have the potential to differentiate into neuronal cell lines (Heng et al., 2016; Raza et al., 2018). Dental pulp stem cells could differentiate into glial cells and neurons using both in vitro and in vivo models (Gronthos et al., 2002; Miura et al., 2003). They have strong repair capacity, high proliferation rate, low immunogenicity, greater neuronal differentiation capacity (Sakai et al., 2012), better plasticity, and more potential to treat neurological diseases (NDs) (de Almeida et al., 2011) compared with other adult stem cells (Gronthos et al., 2000). Thus, tooth-derived stem cells might play a role in treating NDs, such as Alzheimer disease (AD), amyotrophic lateral sclerosis (ALS), Huntington disease (HD), and Parkinson disease (PD) (Genç et al., 2017; Mita et al., 2015; Snyder et al., 2011; Zhang et al., 2018).
Persistent loss of structure and or function that causes the death of neurons are features of NDs. There are many in vivo and in vitro studies, showing that tooth-derived stem cells prevent and repair neuronal damage (Arthur et al., 2008; Ellis et al., 2014; Kiraly et al., 2011; Kiraly et al., 2009). There are systematic reviews evaluating the effect of mesenchymal (Riecke et al., 2015; Wang et al., 2015) or induced pluripotent stem cells (Zhang et al., 2018) transplantation to animal models of different NDs. Systematic reviews provide a clear and comprehensive overview of the available evidence on a particular topic. Furthermore, studies could be reviewed extensively to reveal research gaps in the current data. They may raise methodological concerns in research methods that can be used in the current field to improve future work (Eagly & Wood, 1994) and can be used to identify questions that existing evidence provides clear answers; therefore, they do not require further investigation (Chalmers & Glasziou, 2009). According to the literature, the effect of OCDSCs on NDs, modeled either in vitro with cell cultures or in vivo with animal models was not systematically reviewed. Therefore, this study reviews such studies to conclude the potential effects of OCDSCs on the recovery or therapy of NDs to compile the experimental methods and animal or cell culture models used in these studies, reveal the areas that need further research, and elaborate on the design of future studies.
2. Materials and Methods
Data sources and the literature search strategy
The preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (Moher et al., 2009) were followed up during this systematic review. An extensive electronic search was conducted in PubMed, Scopus, and Web of Science databases to identify articles published in all languages. The main question was whether OCDSCs repair or protect neurons in NDs. For the structured review question, the sample, phenomenon of interest, design, evaluation, and research type strategy were used as follows:
1. Sample: Animal models representing NDs or neuronal cell cultures are exposed to agents to simulate NDs.
2. Phenomenon of interest: Adapting OCDSCs to animal models with one of the abovementioned diseases or co-culturing OCDSCs with neuronal cells.
3. Design: Quantitative design with different biological and physical experiments was used and the results were compared with the control (animals or cell cultures) groups.
4. Evaluation: The effect of OCDSCs on repair or protection of neurons in NDs.
5. Research type: The research types were original quantitative research articles in English. Editorials, abstracts in proceedings, reviews, and expert opinions were excluded.
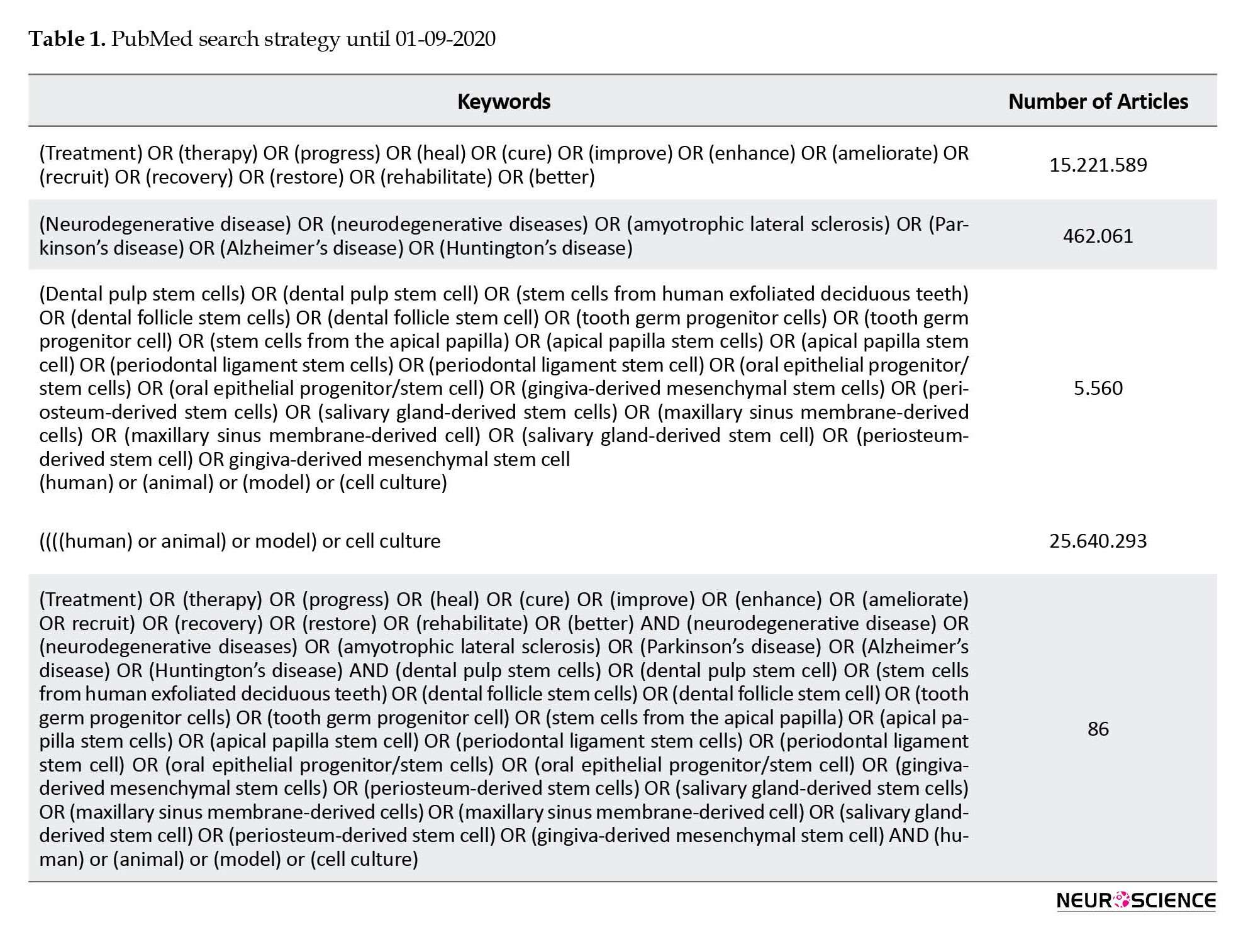
Accordingly, manuscripts published up to April 2021 were evaluated. Table 1 shows the search terms and their combinations. Keyword combinations were modified on every database scan. The references of included articles were also manually checked to find additional articles that were not revealed through electronic search.
Screening and selection of the studies
Initially, two authors independently reviewed the titles that emerged due to electronic searches, and it was decided which publications were relevant. Eligible studies were identified by careful examination of their abstracts. The entire article was evaluated if the information obtained from the title and abstract scanning did not provide sufficient data about the article’s status. The included studies fulfilled the following criteria:
In vitro studies evaluated oral-cavity-derived stem cells on the progress of simulated NDs;
In vivo studies transplanted oral-cavity-derived stem cells (or their secretome) to animal models with NDs.
Consensus of two referees was sought to include the articles. Studies using mesenchymal stem cells derived from oral cavity tissues, such as mature or immature teeth, oral mucosa, salivary glands, maxillary sinus mucosa, or buccal fat pad were included. Mesenchymal stem cells are identified with the following criteria:1) Plastic adherence of the isolated cells in culture; 2) Expression of cluster of differentiation (CD) markers, such as CD73, CD90, and CD105 in >95% of the culture with absent expression of markers, including CD11B or CD14, CD19 or CD79A, CD34, CD45, and human leukocyte antigen-DR in >95% of the culture; and 3) The capacity to differentiate into adipocytes chondrocytes and osteocytes (Dominici et al., 2006). Reviews and other studies (studies that used mesenchymal stem cells other than oral cavity-derived ones and only reported the neurogenic differentiation ability of OCDSCs) that did not meet such criteria were not included in the current study.
Data extraction
The full texts of all included studies were accessed, and two reviewers extracted the data simultaneously according to a standardized baseline. The parameters obtained from the publication were authors, publication year, journal name, type of OCDSCs, type of NDs, number of cells’ passages, type of neuronal cell culture, type of animal, type of transplantation method, performed experiments, and primary outcomes of each study. Characterization methods of stem cells with antigens specific to mesenchymal stem cells or lineage differentiation were also extracted.
Assessment of risk of bias and synthesis evidence
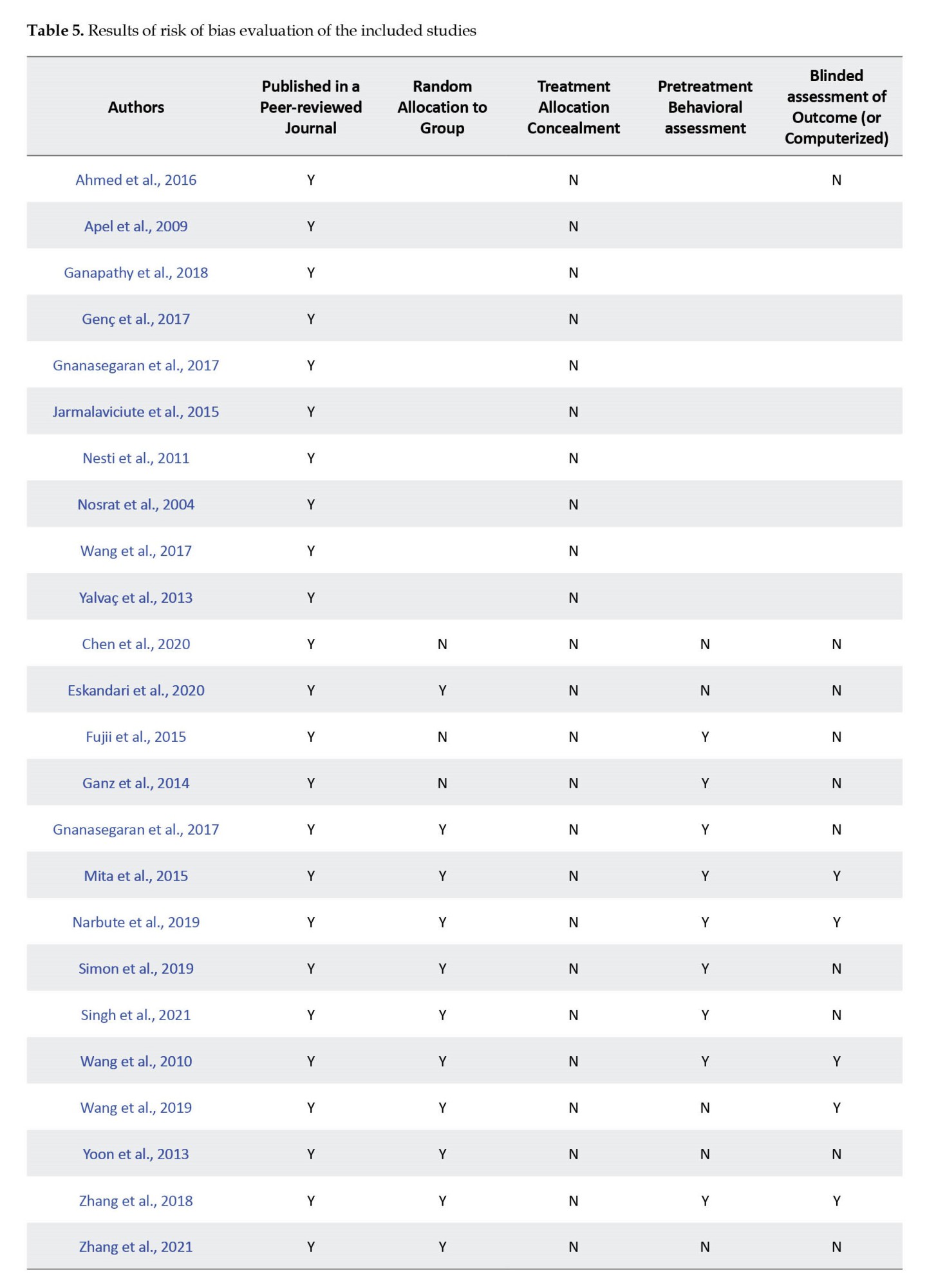
The risk of bias evaluation was made by considering previous studies and modifying related parameters from these studies (Fliefel et al., 2018; Zhang et al., 2018). The assessment was based on the description of the following parameters for the quality assessment of the study for in vivo studies as follows: 1) Published in a peer-reviewed journal; 2) Random allocation to group; 3) Treatment allocation concealment; 4) Pretreatment behavioral assessment; 5) Blinded assessment of outcome (or computerized); 6) Reporting of a sample size calculation; 7) Assessment of ≥2 outcomes; 8) Compliance with animal welfare regulations; 9) Ethics committee approval; and 10) Statement of a potential conflict of interest. If the parameter was included in the article, the article received “Y” denoting “yes” for that parameter; however, if the parameter was not included, the article received “N” indicating “no” for that parameter. Articles reporting 1-4, 5-7, and 8-10 parameters were classified as having high, medium, and low risk of bias, respectively.
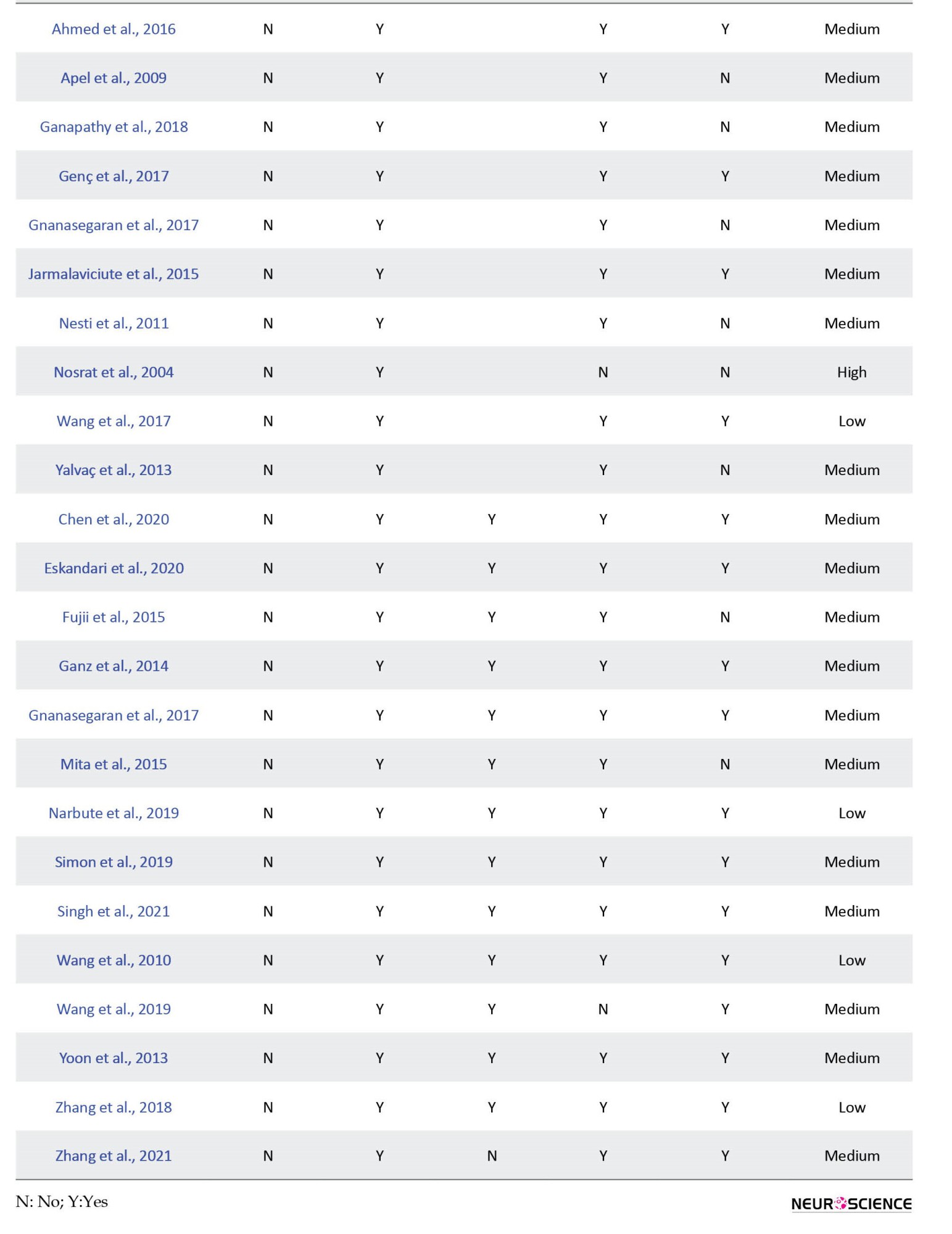
For in-vitro studies, the risk of bias was assessed via a modified 6-point-item checklist from previous studies (Fliefel et al., 2018; Malinowski et al., 2020), including the following items: 1) Published in a peer-reviewed journal; 2) Blinded assessment of outcome (detection bias); 3) Reporting sample size calculation; 4) Assessment of ≥2 outcomes (performance bias); 5) Ethics committee approval; and 6) Statement of potential conflict of interest (funding bias). The articles reporting 1-2, 3-4, and 5-6 parameters were classified as having high, medium, and low risk of bias, respectively. Two reviewers carried out the evaluations independently, and disagreements were resolved through consensus. Each included study (both in vivo and in vitro) was analyzed for similarities to perform a meta-analysis. If the data was heterogeneous, a synthesis of evidence was performed as in previous studies (de Vos et al., 2014; Swart et al., 2012; van Tulder et al., 2003):
1. Strong evidence: If there were 2 or more high-quality studies and or consistent findings across all studies (≥75% of studies reported consistent results).
2. Moderate evidence: If there were one high-quality study and 2 or more low-quality studies with consistent findings across all studies (≥75% of the studies reported consistent results).
3. Limited evidence: If there were one low-quality study.
4. Conflicting evidence: If there were inconsistent findings across multiple studies (<75% of studies reported consistent findings).
5. No evidence: If there was no study.
3. Results
Literature search
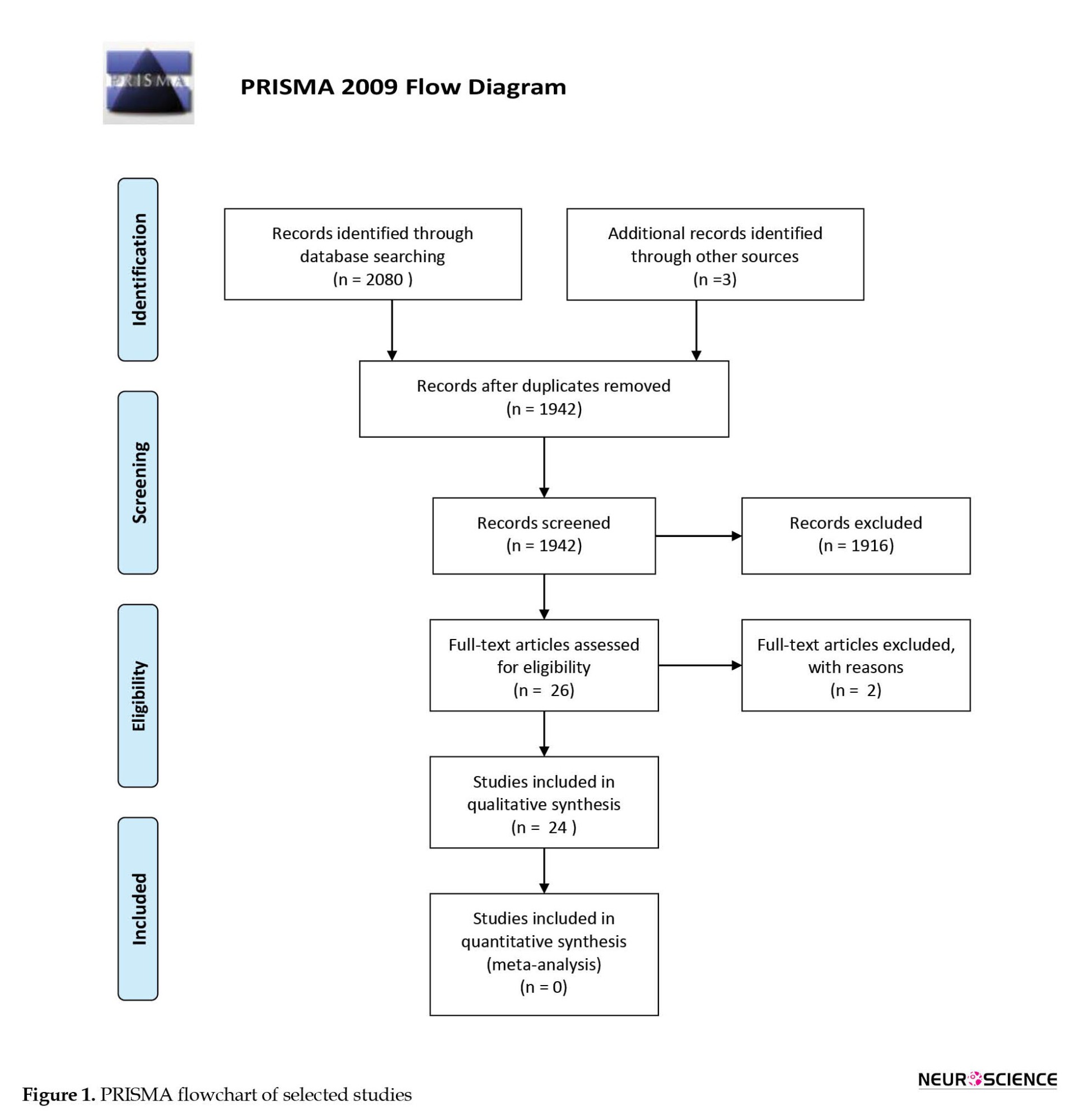
A comprehensive electronic search retrieved 2080 studies (PubMed=86, Scopus=1878, Web of Science=116), and the manual search retrieved three studies (Ganz et al., 2014; Nosrat et al., 2004; Yoon et al., 2013). Duplicates were removed and 1942 studies remained. The screening of the remaining titles and abstracts revealed 26 studies that met the inclusion criteria (Ahmed et al., 2016; Apel et al., 2009; Chen et al., 2020; Eskandari et al., 2020; Fujii et al., 2015; Ganapathy et al., 2018; Ganz et al., 2014; Genç et al., 2017; Gnanasegaran et al., 2017; Gnanasegaran et al., 2017; Jarmalaviciute et al., 2015; Mita et al., 2015; Narbute et al., 2019; Nesti et al., 2011; Nosrat et al., 2004; Simon et al., 2019; Singh et al., 2021; Testa et al., 2012; Venugopal et al., 2018; Wang et al., 2017; Wang et al., 2010; Wang et al., 2019; Yalvac et al., 2013; Yoon et al., 2013; Zhang et al., 2018; Zhang et al., 2021); however after complete text evaluation, 2 studies were discarded as one of these studies used the mixture of bone-marrow derived stem cells and dental pulp stem cells during experiments (Venugopal et al., 2018) and the other study applied amyloid-beta peptide to neuro-differentiated dental pulp stem cells instead of original neuronal cells (Testa et al., 2012). The PRISMA flow chart presents an overview of the study selection procedure (Figure 1).
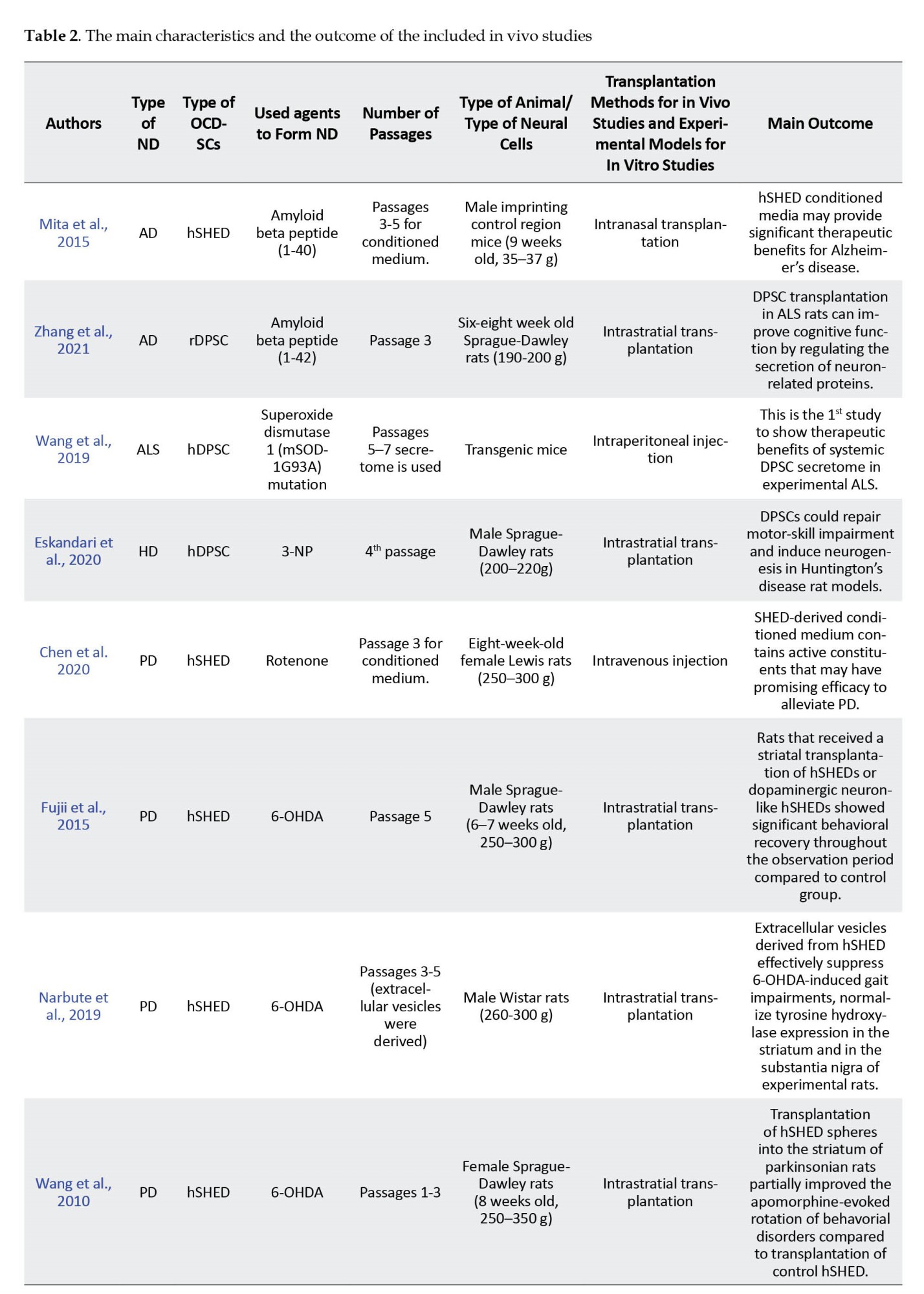
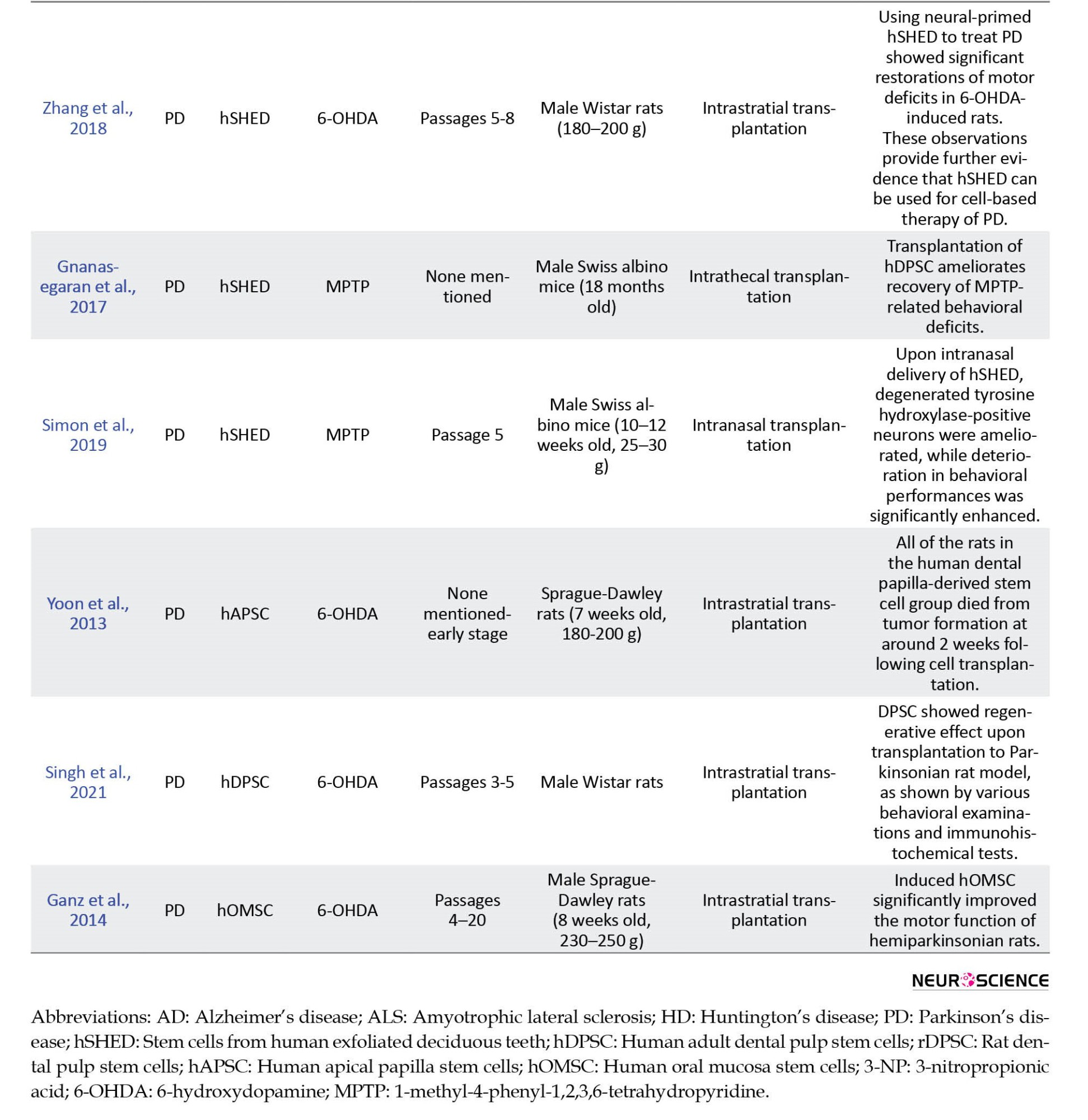
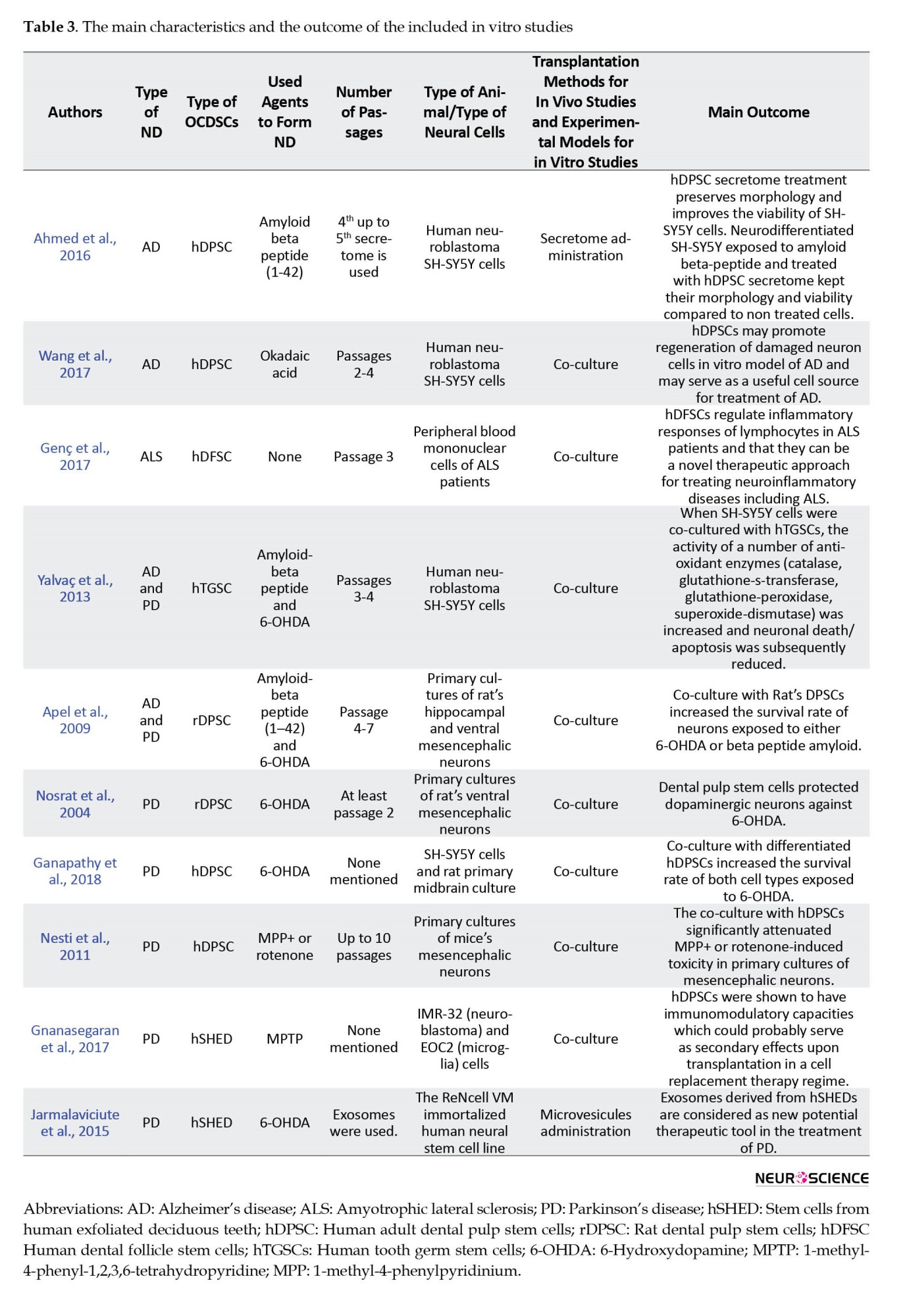
A total of 14 of the included studies were in vivo (Chen et al., 2020; Eskandari et al., 2020; Fujii et al., 2015; Ganz et al., 2014; Gnanasegaran et al., 2017; Mita et al., 2015; Narbute et al., 2019; Simon et al., 2019; Singh et al., 2021; Wang et al., 2010; Wang et al., 2019; Yoon et al., 2013; Zhang et al., 2018; Zhang et al., 2021), while the remaining 10 were in vitro (Ahmed et al., 2016; Apel et al., 2009; Ganapathy et al., 2018; Genç et al., 2017; Gnanasegaran et al., 2017; Jarmalaviciute et al., 2015; Nesti et al., 2011; Nosrat et al., 2004; Wang et al., 2017; Yalvac et al., 2013). The main characteristics and the outcome of the included studies are presented in Table 2 and 3 for in vivo and in vitro studies, respectively.
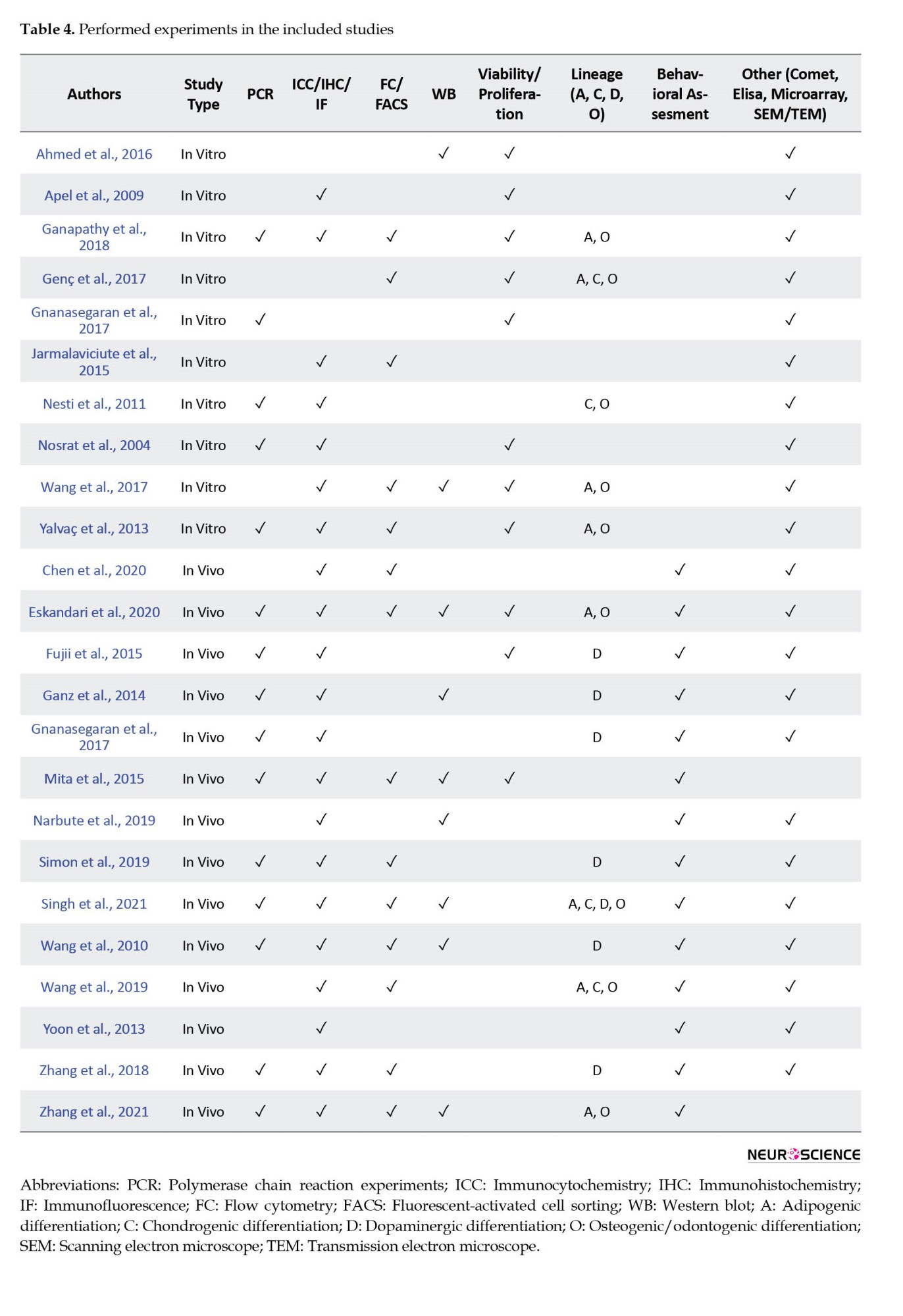
Experimental techniques used in the included studies took place in Table 4.
Neurodegenerative diseases: Risk of bias and synthesis of evidence
Various parameters, such as the number of used cells or amount of cell suspensions during experiments, the number of agents used to induce NDs, the type of transplantation, the type of animals, or neuronal cell cultures, showed diversity among studies. Because of these heterogeneous data, meta-analysis was not conducted in vivo and in vitro studies. Instead of meta-analysis, the risk of bias was analyzed, and the evidence synthesis was performed.
Parkinson disease (PD)
PD was modeled in 7 in vitro studies (Apel et al., 2009; Ganapathy et al., 2018; Gnanasegaran et al., 2017; Jarmalaviciute et al., 2015; Nesti et al., 2011; Nosrat et al., 2004; Yalvac et al., 2013). Of these studies, 1 article revealed high bias (Nosrat et al., 2004) while the remaining demonstrated medium bias (Apel et al., 2009; Ganapathy et al., 2018; Gnanasegaran et al., 2017; Jarmalaviciute et al., 2015; Nesti et al., 2011; Yalvac et al., 2013). All articles reported that OCDSCs (Apel et al., 2009; Ganapathy et al., 2018; Gnanasegaran et al., 2017; Nesti et al., 2011; Nosrat et al., 2004; Yalvac et al., 2013) or their vesicles (Jarmalaviciute et al., 2015) protected dopaminergic neurons, increased their survival rate which was previously exposed to chemical agents to simulate PD via decreasing their apoptosis [according to Jarmalaviciute et al., 2015 exosomes of stem cells from human exfoliated deciduous teeth (SHEDs) suppressed 6-OHDA-induced apoptosis approximately by 80% during the study], secreting anti-inflammatory cytokines and revealing antioxidant enzyme activity (Ganapathy et al., 2018; Gnanasegaran et al., 2017; Nesti et al., 2011; Yalvac et al., 2013). Moderate evidence was found (provided by six studies with low quality and consistent findings in all studies) because ≥75% of the studies reported consistently that the OCDSCs were efficient in vitro against PD-inducing chemicals.
PD was modeled in 10 in vivo studies (Chen et al., 2020; Fujii et al., 2015; Ganz et al., 2014; Gnanasegaran et al., 2017; Narbute et al., 2019; Simon et al., 2019; Singh et al., 2021; Wang et al., 2010; Yoon et al., 2013; Zhang et al., 2018). Three of the studies showed low bias (Narbute et al., 2019; Wang et al., 2010; Zhang et al., 2018) while the remaining seven revealed medium bias (Chen et al., 2020; Fujii et al., 2015; Ganz et al., 2014; Gnanasegaran et al., 2017; Simon et al., 2019; Singh et al., 2021; Yoon et al., 2013). Strong evidence was found regarding the recovery potential of OCDSCs for animal models with PD. Three studies provided it with high quality and ≥75% of the studies reported consistent findings. In 7 of the in vivo studies, OCDSCs were switched to dopaminergic neuronal differentiation (Fujii et al., 2015; Ganz et al., 2014; Gnanasegaran et al., 2017; Simon et al., 2019; Singh et al., 2021; Wang et al., 2010; Zhang et al., 2018). In these studies, tyrosine hydroxylase (TH; rate-limiting enzyme of catecholamine synthesis) positive cells were 40% to 83%. Apomorphine (Narbute et al., 2019; Singh et al., 2021; Wang et al., 2010; Yoon et al., 2013; Zhang et al., 2018), amphetamine (Ganz et al., 2014), or methamphetamine (Fujii et al., 2015) were used during behavioral assessment of parkinsonian animal models. Although different methods were used during behavioral assessment, Ganz et al., (2014) reported that animals treated with oral mucosa stem cells switched to dopaminergic differentiation fully recovered, reaching up to 99.4% of their performance before administering 6-OHDA. The following periods for behavioral assessment were between 2 to 8 weeks (Fujii et al., 2015; Ganz et al., 2014; Gnanasegaran et al., 2017; Narbute et al., 2019; Simon et al., 2019; Singh et al., 2021; Wang et al., 2010); however, Zhang et al. (2018) followed animals up to 16 weeks. Significant recovery was reported in all studies using behavioral assessment tests, except for the study of Yoon et al., (2013). They reported tumor formation after transplantation of dental papilla-derived stem cells. This could be because of the transplantation of early passages contrary to the remaining studies. Furthermore, the anti-inflammatory potential of transplanted OCDSCs was shown several times (Chen et al., 2020; Gnanasegaran et al., 2017; Zhang et al., 2018) with the expression of anti-inflammatory factors, such as IL2, IL4, IL6, and TNF-b.
Alzheimer disease (AD)
AD was induced in 4 in vitro studies (Ahmed et al., 2016; Apel et al., 2009; Wang et al., 2017; Yalvac et al., 2013). Three in vitro studies revealed medium bias (Ahmed et al., 2016; Apel et al., 2009; Yalvac et al., 2013), while the remaining article was low bias (Wang et al., 2017). All studies reported that OCDSCs (Apel et al., 2009; Wang et al., 2017; Yalvac et al., 2013) or their secretome (Ahmed et al., 2016) protected neurons and increased their survival rate which were previously exposed to chemical agent to simulate AD. As a result, moderate evidence was found (provided by 1 study with high quality and three with low quality and consistent findings in all studies).
Two in vivo studies (Mita et al., 2015; Zhang et al., 2021) with medium bias evaluated the effects of OCDSCs on AD and both studies reported that transplantation of OCDSCs resulted in substantially improved cognitive function. Moderate evidence was found regarding OCDSCs on the recovery of AD.
Amyotrophic lateral sclerosis (ALS)
One in vitro study with medium bias showed the effect of OCDSCs on peripheral blood mononuclear cells obtained from ALS patients (Genç et al., 2017) and 1 in vivo study also with medium bias modeled ALS via transgenic mice with superoxide dismutase 1 (mSOD1G93A) mutation (Wang et al., 2019). Both studies reported therapeutic effects for OCDSCs. Limited evidence was found for in vivo and in vitro studies regarding OCDSCs on the recovery of the ALS disease.
Huntington disease (HD)
HD was modeled in 1 in vivo study with 3-nitro propionic acid (Eskandari et al., 2020). The neurons increased, and inflammatory cytokine expression decreased following dental pulp stem cell transplantation (Eskandari et al., 2020). Limited evidence was found for in vivo studies. HD was not modeled in vitro, hence, no evidence was found for in vitro studies.
4. Discussion
NDs, such as AD, ALS, HD, and PD, are among the deteriorating disorders. Their progress worsens with time because the regeneration capacity of neurons and glial cells is restricted (Vishwakarma et al., 2014). A great deal of existing conventional medications have limited efficiency in the treatment of these diseases and provide only symptomatic relief. Therefore, there is a considerable effort to find alternative therapeutic approaches for NDs’ treatment, such as stem cell application. Mesenchymal stem cells (MSCs) that present in many tissues, such as bone marrow, skin, placenta, adipose tissues, umbilical cord, and oral-dental tissues could maintain their replicative capacity for prolonged periods in vitro compared to embryonic stem cells (Shamir et al., 2015) and could be used to treat NDs (Sherman et al., 2019; Song et al., 2018; Sugaya & Vaidya, 2018). The isolation of MSCs from autologous sources could avoid immune rejection and ethical concerns (Shamir et al., 2015). Bone marrow-derived MSCs (BM-MSCs) are accepted as the practical gold standard (Riecke et al., 2015), and several pre-clinical and clinical studies have revealed promising results during the treatment of NDs with these cells (Riecke et al., 2015; Sherman et al., 2019). Nonetheless, BM-MSCs isolation is an excruciating surgical procedure, and the differentiation capacity and proliferation rate of BM-MSCs correlates with the donor age (Huang, Gronthos & Shi, 2009; Stenderup et al., 2003; Wu et al., 2015). In this context, various OCDSCs, such as stem cells from the apical papilla, SHEDs, periodontal ligament stem cells, dental follicle MSCs (DF-MSCs), dental pulp MSCs (DP-MSCs), and MSCs from the gingiva have been identified (Al-Habib & Huang, 2019; Marrelli et al., 2015). A significant advantage of these OCDSCs is their easy isolation procedure by relatively non-invasive methods (Pisciotta et al., 2015). Their ex vivo expansion, self-renewal, and multilineage differentiation capacities, neurogenic potential, and potent anti-inflammatory and immunomodulatory properties are better compared to BM-MSCs (Govindasamy et al., 2010; Ibarretxe et al., 2012; Sakai et al., 2012; Tomar et al., 2010). They can differentiate into different cell types, such as adipocytes, chondrocytes, islet cells, neurons, odontoblasts, osteoblasts, and induced pluripotent stem cells (iPSCs) using classic reprogramming factors (Luo et al., 2018). DP-MSCs had promising potential as an iPSCs source and cell banking (Tamaoki et al., 2010). There is an increase in cryopreserved teeth in tooth banks for future regenerative medical therapies (Yen & Sharpe, 2008).
DP-MSCs express neuronal markers, product, and secrete neurotrophic growth factors, such as brain-derived neurotrophic factor, ciliary neurotrophic factor, fibroblast growth factor, glial-cell-derived growth factor, nerve growth factor, and differentiate into functionally active neurons dopaminergic-like cells, oligodendrocytes, and Schwann cells (Luo et al., 2018). The secretion of these factors is essential in boosting neuronal rescue and survival, neurite outgrowth, and guidance both in vitro and in vivo (Chun et al., 2016; Gnanasegaran et al., 2018; Gnanasegaran et al., 2017) and in stimulating neurogenesis after transplantation in the hippocampus (Ganz et al., 2014; Narbute et al., 2019; Simon et al., 2019). In this context, it is essential to note that BM-MSCs and DP-MSCs derive from the mesoderm and neural crest. DP-MSCs were the most preferred cells in included in vitro studies (Ahmed et al., 2016; Apel et al., 2009; Ganapathy et al., 2018; Nesti et al., 20111; Nosrat et al., 2004; Wang et al., 2017), while SHEDs were the most preferred cells in in-vivo studies (Chen et al., 2020; Fujii et al., 2015; Gnanasegaran et al., 2017; Mita et al., 2015; Narbute et al., 2019; Simon et al., 2019; Wang et al., 2010; Zhang et al., 2018). One explanation could be that the DP-MSCs lost their plasticity through passaging, while SHEDs retained this feature (Govindasamy et al., 2010; Nakamura et al., 2009). Furthermore, the proliferation rate of DP-MSCs and BM-MSCs was significantly lower than SHEDs (Nakamura et al., 2009).
Several reviews were published regarding the effect of OCDSCs on the treatment of different systemic diseases, such as diabetes mellitus, spinal cord injury, AD, PD, and cardiovascular diseases (Chalisserry et al., 2017; Luo et al., 2018; Mortada et al., 2018; Stanko et al., 2018; Wang et al., 2019; Yamada et al., 2019). According to the literature, the effects of BM-MSCs on NDs were systematically reviewed several times (Peng et al., 2015; Riecke et al., 2015; Wang et al., 2015). This systematic review was performed to compile the results of the in vitro and in vivo studies conducted in this field and to reveal the possible limitations. A total of 24 studies were included in this review, regarding AD, ALS, HD, and PD. Meanwhile, ALS is a progressive, incurable ND that targets motoneurons. Genç et al., (2017) reported that DF-MSCs caused an increase in the number of CD4+FoxP3+ regu
of lymphocytes. Also, DF-MSCs increased the apoptotic effect of lymphocytes in ALS patients while increasing cell survival in healthy individuals. Furthermore, Wang et al. (2019) reported that the administration of d DP-MSCs conditioned medium systemically from symptom onset until end-stage of ALS significantly increased the survival of transgenic mice. However, a limited number of studies regarding the effect of OCDSCs on the recovery of the ALS disease made it difficult to conclude ultimately. Further studies regarding the impact of OCDSCs on the ALS disease are needed. The same result is valid for HD. This autosomal dominant ND detects an unstable expansion of CAG repeats in the coding region of the Huntingtin gene IT15 (MacDonald et al., 1993). Modeling HD is complex (Carter & Chan, 2012), and there is only 1 in vivo study regarding the effect of OCDSCs on the progress of HD (Eskandari et al., 2020). The authors concluded that DP-MSCs could repair motor-skill impairment and induce neurogenesis in animal models. Furthermore, Snyder et al., (2011) reported that DP-MSCs from Huntington monkeys retain adult stem cell properties. DP-MSCs isolated from individuals with genetic disorders, such as HD could be considered a personal source of stem cells when considering therapeutic purposes. For personalized medicine, evaluating the stem cell properties of OCDSCs of patients with these NDs is essential. It has been reported that healthy donors- and relapsing-remitting multiple sclerosis patients-derived periodontal ligament stem cells showed similar expression of surface antigen markers, differentiation capacities, and cell proliferation rate (Diomede et al., 2017). OCDSCs could serve as a potential autologous stem cell niche during stem cell therapy of NDs.
Compared to ALS and HD, more studies searched the effect of OCDSCs on AD and PD. Accordingly, PD was induced 17 times (10 in vivo and 7 in vitro studies) according to the results of present review (Apel et al., 2009; Chen et al., 2020; Fujii et al., 2015; Ganapathy et al., 2018; Ganz et al., 2014; Gnanasegaran et al., 2017; Gnanasegaran et al., 2017; Jarmalaviciute et al., 2015; Narbute et al., 2019; Nesti et al., 2011; Nosrat et al., 2004; Simon et al., 2019; Singh et al., 2021; Wang et al., 2010; Yalvac et al., 2013; Yoon et al., 2013; Zhang et al., 2018). Although PD is the second most prevalent ND after AD (Riecke et al., 2015; Zhang et al., 2018), there was more information regarding the effect of OCDSCs on PD than AD. Symptoms of PD include bradykinesia, freezing, muscle rigidity, postural instability, resting tremor, and abnormalities in cognition, mood, and speech (Riecke et al., 2015). SHEDs were mainly used for neuroprotection in PD animal models (Arthur et al., 2008; Chen et al., 2020; Fujii et al., 2015; Gnanasegaran et al., 2017; Narbute et al., 2019; Simon et al., 2019; Wang et al., 2010; Zhang et al., 2018). Transplanted SHEDs restored dopaminergic neuron functions (Gnanasegaran et al., 2017) and promoted survival (Wang et al., 2010). Significant recovery was observed in behavioral deficits following transplantation of SHEDs in PD animal models (Chen et al., 2020; Fujii et al., 2015; Ganz et al., 2014; Gnanasegaran et al., 2017; Simon et al., 2019; Wang et al., 2010; Zhang et al., 2018). Furthermore, extracellular vesicles from SHEDs effectively suppress 6-OHDA-induced gait impairments and normalize tyrosine hydroxylase expression (Narbute et al., 2019). OCDSCs and their exosomes also showed neuro-immunomodulatory activity (Gnanasegaran et al., 2017; Jarmalaviciute et al., 2015; Yalvac et al., 2013) and increased the survival rate of neuronal cells in cellular PD models (Apel et al., 2009; Ganapathy et al., 2018; Yalvac et al., 2013). On the other hand, Yoon et al., 2013 reported the death of all rats because of tumors following transplantation of early-stage human dental papilla-derived stem cells. Switching cells to neurogenic differentiation or injecting their secretome might be advantageous compared to using cells when considering neurodegenerative improvement.
AD is a progressive ND caused by the deposition of insoluble β-amyloid peptides in the brain, the intracellular neurofibrillary tangles, and the loss of neurons (Wang et al., 2015). AD was induced in 6 studies (2 in vivo and 4 in vitro) according to the results of this systematic review (Ahmed et al., 2016; Apel et al., 2009; Mita et al., 2015; Wang et al., 2017; Yalvac et al., 2013; Zhang et al., 2021). OCDSCs reduced β-amyloid peptide-induced cytotoxicity and apoptosis in the AD cellular models (Apel et al., 2009; Yalvac et al., 2013). Furthermore, it has also been reported that β-amyloid peptide cytotoxicity was significantly reduced by increasing cell viability in cells treated with DP-MSC secretome compared to untreated cells (Ahmed et al., 2016). In addition, the endogenous survival factor Bcl-2 was stimulated by the DP-MSCs secretome, while the release of the apoptotic regulator Bax was decreased (Ahmed et al., 2016). SHEDs administered intranasally to the AD mouse model have been reported to cause significantly improved cognitive function by affecting factors involved in axonal elongation, suppression of inflammation, microglial regulation, neuroprotection, and neurotransmission (Mita et al., 2015). However, moderate evidence was found both for in vitro and in vivo studies.
Risk of bias evaluation revealed that 78.6% of in vivo and 80% of in vitro studies had a medium bias. Among 24 studies, there was only 1 in vitro study with high bias. Strong evidence was found only for in vivo studies that evaluated the OCDSCs’ effect on PD. Sample size calculation and treatment allocation concealment were missing parameters in all studies. Sample size calculation could be performed in future studies; blinded assessment is essential for preventing reporting bias. The other limitation is the period of studies. Behavioral assessment tests continued for an average of up to 4 weeks (Eskandari et al., 2020; Narbute et al., 2019; Simon et al., 2019; Singh et al., 2021), while the in vitro co-culture studies lasted as short as 24 h (Apel et al., 2009; Nesti et al., 2011; Yalvac et al., 2013). The long-term therapeutic effect of OCDSCs and their survival could also be studied in future studies. The quantity of transplanted cells to the animal models and the quantity of cells co-cultured with commercial neuronal cells showed diversity in the included studies. Small starting materials could affect the used quantity in these studies. However, this also limited comparisons and interpretations of the results. Chen et al. (2020) injected SHEDs-conditioned medium (SHEDs-CM) at 4 different concentrations and they concluded that 400 mg/mL of SHEDs-CM treatment did not reveal further improvement than the 100 mg/mL treatment group. Other studies could be designed to indicate the optimum cell quantity needed during these studies. It is also important to mention that toxins that were used for inducing ND models, such as 6-OHDA, MPTP, and β-amyloid peptide, did not completely represent the real pathological mechanisms that occur in patients (Mohamed et al., 2019). The efficiency of OCDSCs could be tested on patient-derived iPSCs or induced neuronal stem cells. Co-culture studies could be done with patient-derived induced neuronal stem cells instead of commercial neuronal cell lines. Furthermore, novel technologies such as genome-editing techniques, 3D organoids, 3D cell cultures, and organ-on-a-chip could be used for modeling these NDs (Mohamed et al., 2019) and more conclusive results regarding therapeutic effects of OCDSCs on NDs could be obtained.
5. Conclusion
Considering the limitations of the included studies, OCDSCs and their secretome yield potential for NDs based on their neural crest origin and neuronal characteristics in vitro and in vivo. Further in vitro and in vivo studies with low bias risk, reproducible animal models, and optimal chemical doses will be needed for all types of NDs before clinical implications.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and methodology: Emel Uzunoglu-Ozyurek; Investigation: Emel Uzunoglu-Ozyurek and Gizem Önal; Writing and original draft: Emel Uzunoglu-Ozyurek; Supervision: Serap Dökmec; Writing, review, editing and finding resources: All authors.
Conflict of interest
The authors declared no conflict of interest
References
Ahmed, N.el-M., Murakami, M., Hirose, Y., & Nakashima, M. (2016). Therapeutic potential of dental pulp stem cell secretome for alzheimer’s disease treatment: An in vitro study. Stem Cells International, 2016, 8102478. [DOI:10.1155/2016/8102478] [PMID]
Al-Habib, M., & Huang, G. T. (2019). Dental mesenchymal stem cells: Dental pulp stem cells, periodontal ligament stem cells, apical papilla stem cells, and primary teeth stem cells-isolation, characterization, and expansion for tissue engineering. Methods in Molecular Biology, 1922, 59-76. [DOI:10.1007/978-1-4939-9012-2_7] [PMID]
Apel, C., Forlenza, O. V., de Paula, V. J., Talib, L. L., Denecke, B., & Eduardo, C. P., et al. (2009). The neuroprotective effect of dental pulp cells in models of Alzheimer’s and Parkinson’s disease. Journal of Neural Transmission (Vienna, Austria: 1996), 116(1), 71–78. [DOI:10.1007/s00702-008-0135-3] [PMID]
Arthur, A., Rychkov, G., Shi, S., Koblar, S. A., & Gronthos, S. (2008). Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells, 26(7), 1787-1795. [DOI:10.1634/stemcells.2007-0979] [PMID]
Carter, R. L., & Chan, A. W. (2012). Pluripotent stem cells models for Huntington’s disease: Prospects and challenges. Journal of Genetics and Genomics, 39(6), 253-259. [DOI:10.1016/j.jgg.2012.04.006] [PMID]
Chalisserry, E. P., Nam, S. Y., Park, S. H., & Anil, S. (2017). Therapeutic potential of dental stem cells. Journal of Tissue Engineering, 8, 2041731417702531. [DOI:10.1177/2041731417702531] [PMID]
Chalmers, I., & Glasziou, P. (2009). Avoidable waste in the production and reporting of research evidence. Lancet (London, England), 374(9683), 86-89. [DOI:10.1016/S0140-6736(09)60329-9] [PMID]
Chen, Y. R., Lai, P. L., Chien, Y., Lee, P. H., Lai, Y. H., & Ma, H. I., et al. (2020). Improvement of impaired motor functions by human dental exfoliated deciduous teeth stem cell-derived factors in a rat model of parkinson’s disease. International Journal of Molecular Sciences, 21(11), 3807. [DOI:10.3390/ijms21113807] [PMID]
Chun, S. Y., Soker, S., Jang, Y. J., Kwon, T. G., & Yoo, E. S. (2016).Differentiation of human dental pulp stem cells into dopaminergic neuron-like cells in vitro. Journal of Korean Medical Science, 31(2), 171-177. [DOI:10.3346/jkms.2016.31.2.171] [PMID]
de Almeida, F. M., Marques, S. A., Ramalho Bdos, S., Rodrigues, R. F., Cadilhe, D. V., & Furtado, D., et al. (2011). Human dental pulp cells: A new source of cell therapy in a mouse model of compressive spinal cord injury. Journal of Neurotrauma, 28(9), 1939-1949. [DOI:10.1089/neu.2010.1317] [PMID]
de Vos, R. J., Windt, J., & Weir, A. (2014). Strong evidence against platelet-rich plasma injections for chronic lateral epicondylar tendinopathy: A systematic review. British Journal of Sports Medicine, 48(12), 952-956. [DOI:10.1136/bjsports-2013-093281] [PMID]
Diomede, F., Rajan, T. S., D'Aurora, M., Bramanti, P., Merciaro, I., & Marchisio, M., et al. (2017). Stemness characteristics of periodontal ligament stem cells from donors and multiple sclerosis patients: A comparative study. Stem Cells International, 2017, 1606125. [DOI:10.1155/2017/1606125] [PMID]
Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., & Krause, D., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8(4), 315-317. [DOI:10.1080/14653240600855905] [PMID]
Dulak, J., Szade, K., Szade, A., Nowak, W., & Józkowicz, A. (2015). Adult stem cells: Hopes and hypes of regenerative medicine. Acta Biochimica Polonica, 62(3), 329-337. [DOI:10.18388/abp.2015_1023] [PMID]
Eagly, A. H., & Wood, W. (1994). Using research syntheses to plan future research. In H. Cooper & L. V. Hedges (Eds.), The handbook of research synthesis (pp. 485-500). New York: Russell Sage Foundation. [Link]
Ellis, K. M., O'Carroll, D. C., Lewis, M. D., Rychkov, G. Y., & Koblar, S. A. (2014). Neurogenic potential of dental pulp stem cells isolated from murine incisors. Stem Cell Research & Therapy, 5(1), 30. [DOI:10.1186/scrt419] [PMID]
Eskandari, N., Boroujeni, M. E., Abdollahifar, M. A., Piryaei, A., Khodagholi, F., & Mirbehbahani, S. H., et al. (2021). Transplantation of human dental pulp stem cells compensates for striatal atrophy and modulates neuro-inflammation in 3-nitropropionic acid rat model of Huntington’s disease. Neuroscience Research, 170, 133–144. [DOI:10.1016/j.neures.2020.12.002] [PMID]
Fliefel, R., Ehrenfeld, M., & Otto, S. (2018). Induced pluripotent stem cells (iPSCs) as a new source of bone in reconstructive surgery: A systematic review and meta-analysis of preclinical studies. Journal of Tissue Engineering and Regenerative Medicine, 12(7), 1780-1797. [DOI:10.1002/term.2697] [PMID]
Fujii, H., Matsubara, K., Sakai, K., Ito, M., Ohno, K., & Ueda, M., et al. (2015). Dopaminergic differentiation of stem cells from human deciduous teeth and their therapeutic benefits for Parkinsonian rats. Brain Research, 1613, 59-72. [DOI:10.1016/j.brainres.2015.04.001] [PMID]
Ganapathy, K., Datta, I., & Bhonde, R. (2019). Astrocyte-like cells differentiated from dental pulp stem cells protect dopaminergic neurons against 6-hydroxydopamine toxicity. Molecular Neurobiology, 56(6), 4395–4413. [DOI:10.1007/s12035-018-1367-3] [PMID]
Ganz, J., Arie, I., Buch, S., Zur, T. B., Barhum, Y., & Pour, S., et al. (2014). Dopaminergic-like neurons derived from oral mucosa stem cells by developmental cues improve symptoms in the hemi-parkinsonian rat model. PLoS One, 9(6), e100445. [DOI:10.1371/journal.pone.0100445] [PMID]
Genç, D., Zibandeh, N., Uluç, K., Koytak, P. K., Gökalp, M., & Tanridag, T., et al. (2017). Dental follicle mesenchymal stem cells enhance CD4+ Foxp3+ regulatory T cells in the lymphocytes of amyotrophic lateral sclerosis patients. Clinical and Experimental Health Sciences, 7(3), 85-90. [Link]
Gnanasegaran, N., Govindasamy, V., Kathirvaloo, P., Musa, S., & Abu Kasim, N. H. (2018). Effects of cell cycle phases on the induction of dental pulp stem cells toward dopaminergic-like cells. Journal of Tissue Engineering and Regenerative Medicine, 12(2), e881-e893. [DOI:10.1002/term.2401] [PMID]
Gnanasegaran, N., Govindasamy, V., Mani, V., & Abu Kasim, N. H. (2017). Neuroimmunomodulatory properties of DPSCs in an in vitro model of Parkinson’s disease. IUBMB Life, 69(9), 689-699. [DOI:10.1002/iub.1655] [PMID]
Gnanasegaran, N., Govindasamy, V., Simon, C., Gan, Q. F., Vincent-Chong, V. K., & Mani, V., et al. (2017). Effect of dental pulp stem cells in MPTP-induced old-aged mice model. European Journal of Clinical Investigation, 47(6), 403-414. [DOI:10.1111/eci.12753] [PMID]
Govindasamy, V., Abdullah, A. N., Ronald, V. S., Musa, S., Ab Aziz, Z. A., & Zain, R. B., et al. (2010). Inherent differential propensity of dental pulp stem cells derived from human deciduous and permanent teeth. Journal of Endodontics, 36(9), 1504-1515. [DOI:10.1016/j.joen.2010.05.006] [PMID]
Gronthos, S., Brahim, J., Li, W., Fisher, L. W., Cherman, N., & Boyde, A., et al. (2002). Stem cell properties of human dental pulp stem cells. Journal of Dental Research, 81(8), 531-535. [DOI:10.1177/154405910208100806] [PMID]
Gronthos, S., Mankani, M., Brahim, J., Robey, P. G., & Shi, S. (2000). Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America, 97(25), 13625–13630. [DOI:10.1073/pnas.240309797] [PMID]
Heng, B. C., Lim, L. W., Wu, W., & Zhang, C. (2016). An overview of protocols for the neural induction of dental and oral stem cells in vitro. Tissue Engineering Part B: Reviews, 22(3), 220-250. [DOI:10.1089/ten.teb.2015.0488] [PMID]
Huang, G. T., Gronthos, S., & Shi, S. (2009). Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. Journal of Dental Research, 88(9), 792-806. [DOI:10.1177/0022034509340867] [PMID]
Ibarretxe, G., Crende, O., Aurrekoetxea, M., García-Murga, V., Etxaniz, J., & Unda, F. (2012). Neural crest stem cells from dental tissues: A new hope for dental and neural regeneration. Stem Cells International, 2012, 103503. [DOI:10.1155/2012/103503] [PMID]
Jarmalavičiūtė, A., Tunaitis, V., Pivoraitė, U., Venalis, A., & Pivoriūnas, A. (2015). Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine-induced apoptosis. Cytotherapy, 17(7), 932-939. [DOI:10.1016/j.jcyt.2014.07.013] [PMID]
Király, M., Kádár, K., Horváthy, D. B., Nardai, P., Rácz, G. Z., & Lacza, Z., et al. (2011). Integration of neuronally predifferentiated human dental pulp stem cells into rat brain in vivo. Neurochemistry International, 59(3), 371-381. [DOI:10.1016/j.neuint.2011.01.006] [PMID]
Király, M., Porcsalmy, B., Pataki, A., Kádár, K., Jelitai, M., & Molnár, B., et al. (2009). Simultaneous PKC and cAMP activation induces differentiation of human dental pulp stem cells into functionally active neurons. Neurochemistry International, 55(5), 323-332. [DOI:10.1016/j.neuint.2009.03.017] [PMID]
Luo, L., He, Y., Wang, X., Key, B., Lee, B. H., & Li, H., et al. (2018). Potential roles of dental pulp stem cells in neural regeneration and repair. Stem Cells International, 2018, 1731289. [DOI:10.1155/2018/1731289] [PMID]
MacDonald, M. E., Ambrose, C. M., Duyao, M. P., Myers, R. H., Lin, C., & Srinidhi, L., et al. (1993). A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s disease collaborative research group. Cell, 72(6), 971-983. [DOI:10.1016/0092-8674(93)90585-E] [PMID]
Malinowski, B., Musiała, N., & Wiciński, M. (2020). Metformin’s modulatory effects on miRNAs function in cancer stem cells-A systematic review. Cells, 9(6), 1401. [DOI:10.3390/cells9061401] [PMID]
Marrelli, M., Paduano, F., & Tatullo, M. (2015). Human periapical cyst-mesenchymal stem cells differentiate into neuronal cells. Journal of Dental Research, 94(6), 843-852. [DOI:10.1177/0022034515570316] [PMID]
Mita, T., Furukawa-Hibi, Y., Takeuchi, H., Hattori, H., Yamada, K., & Hibi, H., et al. (2015). Conditioned medium from the stem cells of human dental pulp improves cognitive function in a mouse model of Alzheimer’s disease. Behavioural Brain Research, 293, 189-197. [DOI:10.1016/j.bbr.2015.07.043] [PMID]
Miura, M., Gronthos, S., Zhao, M., Lu, B., Fisher, L. W., & Robey, P. G., et al. (2003). SHED: Stem cells from human exfoliated deciduous teeth. Proceedings of the National Academy of Sciences of the United States of America, 100(10), 5807-5812. [DOI:10.1073/pnas.0937635100] [PMID]
Mohamed, N. V., Larroquette, F., Beitel, L. K., Fon, E. A., & Durcan, T. M. (2019). One step into the future: New iPSC tools to advance research in parkinson’s disease and neurological disorders. Journal of Parkinson’s Disease, 9(2), 265-281. [DOI:10.3233/JPD-181515] [PMID]
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., & PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine, 151(4), 264–W64. [DOI:10.7326/0003-4819-151-4-200908180-00135] [PMID]
Mortada, I., Mortada, R., & Al Bazzal, M. (2018). Dental pulp stem cells and the management of neurological diseases: An update. Journal of Neuroscience Research, 96(2), 265-272. [DOI:10.1002/jnr.24122] [PMID]
Nakamura, S., Yamada, Y., Katagiri, W., Sugito, T., Ito, K., & Ueda, M. (2009). Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. Journal of Endodontics, 35(11), 1536-1542. [DOI:10.1016/j.joen.2009.07.024] [PMID]
Narbute, K., Piļipenko, V., Pupure, J., Dzirkale, Z., Jonavičė, U., & Tunaitis, V., et al. (2019). Intranasal administration of extracellular vesicles derived from human teeth stem cells improve motor symptoms and normalize tyrosine hydroxylase expression in the substantia nigra and striatum of the 6‐hydroxydopamine‐treated rats. Stem Cells Translational Medicine, 8(5), 490-499. [DOI:10.1002/sctm.18-0162] [PMID]
Nesti, C., Pardini, C., Barachini, S., D’Alessandro, D., Siciliano, G., & Murri, L., et al. (2011). Human dental pulp stem cells protect mouse dopaminergic neurons against MPP+ or rotenone. Brain Research, 1367, 94-102. [DOI:10.1016/j.brainres.2010.09.042] [PMID]
Nosrat, I. V., Smith, C. A., Mullally, P., Olson, L., & Nosrat, C. A. (2004). Dental pulp cells provide neurotrophic support for dopaminergic neurons and differentiate into neurons in vitro; implications for tissue engineering and repair in the nervous system. The European Journal of Neuroscience, 19(9), 2388-2398. [DOI:10.1111/j.0953-816X.2004.03314.x] [PMID]
Peng, W., Sun, J., Sheng, C., Wang, Z., Wang, Y., & Zhang, C., et al. (2015). Systematic review and meta-analysis of efficacy of mesenchymal stem cells on locomotor recovery in animal models of traumatic brain injury. Stem Cell Research & Therapy, 6(1), 47. [DOI:10.1186/s13287-015-0034-0] [PMID]
Pisciotta, A., Carnevale, G., Meloni, S., Riccio, M., De Biasi, S., & Gibellini, L., et al. (2015). Human dental pulp stem cells (hDPSCs): Isolation, enrichment and comparative differentiation of two sub-populations. BMC Developmental Biology, 15, 14. [DOI:10.1186/s12861-015-0065-x] [PMID]
Raza, S. S., Wagner, A. P., Hussain, Y. S., & Khan, M. A. (2018). Mechanisms underlying dental-derived stem cell-mediated neurorestoration in neurodegenerative disorders. Stem Cell Research & Therapy, 9(1), 245 [DOI:10.1186/s13287-018-1005-z] [PMID]
Riecke, J., Johns, K. M., Cai, C., Vahidy, F. S., Parsha, K., & Furr-Stimming, E., et al. (2015). A meta-analysis of mesenchymal stem cells in animal models of parkinson’s disease. Stem Cells and Development, 24(18), 2082-2090. [DOI:10.1089/scd.2015.0127] [PMID]
Sakai, K., Yamamoto, A., Matsubara, K., Nakamura, S., Naruse, M., & Yamagata, M., et al. (2012). Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. Journal of Clinical Investigation, 122(1), 80-90. [DOI:10.1172/JCI59251] [PMID]
Shamir, C., Venugopal, C., & Dhanushkodi, A. (2015). Dental pulp stem cells for treating neurodegenerative diseases. Neural Regeneration Research, 10(12), 1910-1911. [DOI:10.4103/1673-5374.169629] [PMID]
Sherman, L. S., Romagano, M. P., Williams, S. F., & Rameshwar, P. (2019). Mesenchymal stem cell therapies in brain disease. Seminars in Cell and Developmental Biology, 95, 111-119.[DOI:10.1016/j.semcdb.2019.03.003]
Simon, C., Gan, Q. F., Kathivaloo, P., Mohamad, N. A., Dhamodharan, J., & Krishnan, A., et al. (2019). Deciduous DPSCs ameliorate MPTP-mediated neurotoxicity, sensorimotor coordination and olfactory function in parkinsonian mice. International Journal of Molecular Sciences, 20(3), 568. [DOI:10.3390/ijms20030568] [PMID]
Singh, M., Jain, M., Bose, S., Halder, A., Nag, T. C., & Dinda, A. K., et al. (2021). 22(R)-hydroxycholesterol for dopaminergic neuronal specification of MSCs and amelioration of Parkinsonian symptoms in rats. Cell Death Discovery, 7(1), 13. [DOI:10.1038/s41420-020-00351-6] [PMID]
Snyder, B. R., Cheng, P. H., Yang, J., Yang, S. H., Huang, A. H., & Chan, A. W. (2011). Characterization of dental pulp stem/stromal cells of Huntington monkey tooth germs. BMC Cell Biology, 12, 39. [DOI:10.1186/1471-2121-12-39] [PMID]
Song, C. G., Zhang, Y. Z., Wu, H. N., Cao, X. L., Guo, C. J., & Li, Y. Q., et al. (2018). Stem cells: A promising candidate to treat neurological disorders. Neural Regeneration Research, 13(7), 1294-1304. [DOI:10.4103/1673-5374.235085] [PMID]
Stanko, P., Altanerova, U., Jakubechova, J., Repiska, V., & Altaner, C. (2018). Dental mesenchymal stem/stromal cells and their exosomes. Stem Cells International, 2018, 8973613. [DOI:10.1155/2018/8973613] [PMID]
Stenderup, K., Justesen, J., Clausen, C., & Kassem, M. (2003). Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone, 33(6), 919-926. [DOI:10.1016/j.bone.2003.07.005] [PMID]
Sugaya, K., & Vaidya, M. (2018). Stem cell therapies for neurodegenerative diseases. Advances in Experimental Medicine and Biology, 1056, 61-84. [DOI:10.1007/978-3-319-74470-4_5] [PMID]
Swart, N. M., van Linschoten, R., Bierma-Zeinstra, S. M., & van Middelkoop, M. (2012). The additional effect of orthotic devices on exercise therapy for patients with patellofemoral pain syndrome: A systematic review. British Journal of Sports Medicine, 46(8), 570-577. [DOI:10.1136/bjsm.2010.080218] [PMID]
Tamaoki, N., Takahashi, K., Tanaka, T., Ichisaka, T., Aoki, H., & Takeda-Kawaguchi, T., et al. (2010). Dental pulp cells for induced pluripotent stem cell banking. Journal of Dental Research, 89(8), 773-778. [DOI:10.1177/0022034510366846] [PMID]
Testa, G., Gamba, P., Di Scipio, F., Sprio, A. E., Salamone, P., & Gargiulo, S., et al. (2012). Potentiation of amyloid-beta peptide neurotoxicity in human dental-pulp neuron-like cells by the membrane lipid peroxidation product 4-hydroxynonenal. Free Radical Biology & Medicine, 53(9), 1708-1717. [DOI:10.1016/j.freeradbiomed.2012.08.581] [PMID]
Tomar, G. B., Srivastava, R. K., Gupta, N., Barhanpurkar, A. P., Pote, S. T., & Jhaveri, H. M., et al. (2010). Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochemical and Biophysical Research Communications, 393(3), 377-383. [DOI:10.1016/j.bbrc.2010.01.126] [PMID]
van Tulder, M., Furlan, A., Bombardier, C., Bouter, L., & Editorial Board of the Cochrane Collaboration Back Review Group (2003). Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine (Phila Pa 1976), 28(12), 1290-1299. [DOI:10.1097/01.BRS.0000065484.95996.AF] [PMID]
Venugopal, C., K, S., Rai, K. S., Pinnelli, V. B., Kutty, B. M., & Dhanushkodi, A. (2018). Neuroprotection by human dental pulp mesenchymal stem cells: from billions to nano. Current Gene Therapy, 18(5), 307-323. [DOI:10.2174/1566523218666180913152615] [PMID]
Vishwakarma, S. K., Bardia, A., Tiwari, S. K., Paspala, S. A., & Khan, A. A. (2014). Current concept in neural regeneration research: NSCs isolation, characterization and transplantation in various neurodegenerative diseases and stroke: A review. Journal of Advanced Research, 5(3), 277-294. [DOI:10.1016/j.jare.2013.04.005] [PMID]
Wang, D., Wang, Y., Tian, W., & Pan, J. (2019). Advances of tooth-derived stem cells in neural diseases treatments and nerve tissue regeneration. Cell Proliferation, 52(3), e12572. [DOI:10.1111/cpr.12572] [PMID]
Wang, F., Jia, Y., Liu, J., Zhai, J., Cao, N., & Yue, W., et al. (2017). Dental pulp stem cells promote regeneration of damaged neuron cells on the cellular model of Alzheimer’s disease. Cell Biology International, 41(6), 639-650. [DOI:10.1002/cbin.10767] [PMID]
Wang, J., Wang, X., Sun, Z., Wang, X., Yang, H., & Shi, S., et al. (2010). Stem cells from human-exfoliated deciduous teeth can differentiate into dopaminergic neuron-like cells. Stem Cells and Development, 19(9), 1375-1383. [DOI:10.1089/scd.2009.0258] [PMID]
Wang, J., Zuzzio, K., & Walker, C. L. (2019). Systemic dental pulp stem cell secretome therapy in a mouse model of amyotrophic lateral sclerosis. Brain sciences, 9(7), 165. [DOI:10.3390/brainsci9070165] [PMID]
Wang, Z., Peng, W., Zhang, C., Sheng, C., Huang, W., & Wang, Y., et al. (2015). Effects of stem cell transplantation on cognitive decline in animal models of Alzheimer’s disease: A systematic review and meta-analysis. Scientific Reports, 5, 12134. [DOI:10.1038/srep12134] [PMID]
Wu, W., Zhou, J., Xu, C. T., Zhang, J., Jin, Y. J., & Sun, G. L. (2015). Derivation and growth characteristics of dental pulp stem cells from patients of different ages. Molecular Medicine Reports, 12(4), 5127-5134. [DOI:10.3892/mmr.2015.4106] [PMID]
Yalvaç, M. E., Yarat, A., Mercan, D., Rizvanov, A. A., Palotás, A., & Şahin, F. (2013). Characterization of the secretome of human tooth germ stem cells (hTGSCs) reveals neuro-protection by fine-tuning micro-environment. Brain, Behavior, and Immunity, 32, 122-130. [DOI:10.1016/j.bbi.2013.03.007] [PMID]
Yamada, Y., Nakamura-Yamada, S., Kusano, K., & Baba, S. (2019). Clinical Potential and Current Progress of Dental Pulp Stem Cells for Various Systemic Diseases in Regenerative Medicine: A Concise Review. International Journal of Molecular Sciences, 20(5), 1132. [DOI:10.3390/ijms20051132] [PMID]
Yen, A. H., & Sharpe, P. T. (2008). Stem cells and tooth tissue engineering. Cell and Tissue Research, 331(1), 359-372. [DOI:10.1007/s00441-007-0467-6] [PMID]
Yoon, H. H., Min, J., Shin, N., Kim, Y. H., Kim, J. M., & Hwang, Y. S., et al. (2013). Are human dental papilla-derived stem cell and human brain-derived neural stem cell transplantations suitable for treatment of Parkinson’s disease?. Neural Regeneration Research, 8(13), 1190-1200. [PMID]
Zhang, N., Lu, X., Wu, S., Li, X., Duan, J., & Chen, C., et al. (2018). Intrastriatal transplantation of stem cells from human exfoliated deciduous teeth reduces motor defects in Parkinsonian rats. Cytotherapy, 20(5), 670-686. [DOI:10.1016/j.jcyt.2018.02.371] [PMID]
Zhang, Y., Ge, M., Hao, Q., & Dong, B. (2018). Induced pluripotent stem cells in rat models of Parkinson’s disease: A systematic review and meta-analysis. Biomedical Reports, 8(3), 289-296. [DOI:10.3892/br.2018.1049]
Zhang, X. M., Ouyang, Y. J., Yu, B. Q., Li, W., Yu, M. Y., & Li, J. Y., et al. (2021). Therapeutic potential of dental pulp stem cell transplantation in a rat model of Alzheimer’s disease. Neural Regeneration Research, 16(5), 893-898. [DOI:10.4103/1673-5374.297088] [PMID]
Stem cells can differentiate into multiple cell types and replicate. There are various types of stem cells, such as adipose-derived stem cells, bone-marrow-derived mesenchymal stem cells, embryonic stem cells, induced pluripotent stem cells, umbilical cord stem cells, and oral cavity-derived stem cells (Dulak et al., 2015). Oral cavity-derived stem cells (OCDSCs) are adult stem cells that can be isolated from the dental pulp of both permanent and primary teeth, and from the periodontal ligament, gingiva, maxillary sinus mucosa, and periapical lesions (Al-Habib & Huang, 2019; Marrelli et al., 2015). All dental, oral, and craniofacial structures are formed during development by neural crest-derived and or mesenchymal cells; therefore, stem cells derived from these structures have the potential to differentiate into neuronal cell lines (Heng et al., 2016; Raza et al., 2018). Dental pulp stem cells could differentiate into glial cells and neurons using both in vitro and in vivo models (Gronthos et al., 2002; Miura et al., 2003). They have strong repair capacity, high proliferation rate, low immunogenicity, greater neuronal differentiation capacity (Sakai et al., 2012), better plasticity, and more potential to treat neurological diseases (NDs) (de Almeida et al., 2011) compared with other adult stem cells (Gronthos et al., 2000). Thus, tooth-derived stem cells might play a role in treating NDs, such as Alzheimer disease (AD), amyotrophic lateral sclerosis (ALS), Huntington disease (HD), and Parkinson disease (PD) (Genç et al., 2017; Mita et al., 2015; Snyder et al., 2011; Zhang et al., 2018).
Persistent loss of structure and or function that causes the death of neurons are features of NDs. There are many in vivo and in vitro studies, showing that tooth-derived stem cells prevent and repair neuronal damage (Arthur et al., 2008; Ellis et al., 2014; Kiraly et al., 2011; Kiraly et al., 2009). There are systematic reviews evaluating the effect of mesenchymal (Riecke et al., 2015; Wang et al., 2015) or induced pluripotent stem cells (Zhang et al., 2018) transplantation to animal models of different NDs. Systematic reviews provide a clear and comprehensive overview of the available evidence on a particular topic. Furthermore, studies could be reviewed extensively to reveal research gaps in the current data. They may raise methodological concerns in research methods that can be used in the current field to improve future work (Eagly & Wood, 1994) and can be used to identify questions that existing evidence provides clear answers; therefore, they do not require further investigation (Chalmers & Glasziou, 2009). According to the literature, the effect of OCDSCs on NDs, modeled either in vitro with cell cultures or in vivo with animal models was not systematically reviewed. Therefore, this study reviews such studies to conclude the potential effects of OCDSCs on the recovery or therapy of NDs to compile the experimental methods and animal or cell culture models used in these studies, reveal the areas that need further research, and elaborate on the design of future studies.
2. Materials and Methods
Data sources and the literature search strategy
The preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (Moher et al., 2009) were followed up during this systematic review. An extensive electronic search was conducted in PubMed, Scopus, and Web of Science databases to identify articles published in all languages. The main question was whether OCDSCs repair or protect neurons in NDs. For the structured review question, the sample, phenomenon of interest, design, evaluation, and research type strategy were used as follows:
1. Sample: Animal models representing NDs or neuronal cell cultures are exposed to agents to simulate NDs.
2. Phenomenon of interest: Adapting OCDSCs to animal models with one of the abovementioned diseases or co-culturing OCDSCs with neuronal cells.
3. Design: Quantitative design with different biological and physical experiments was used and the results were compared with the control (animals or cell cultures) groups.
4. Evaluation: The effect of OCDSCs on repair or protection of neurons in NDs.
5. Research type: The research types were original quantitative research articles in English. Editorials, abstracts in proceedings, reviews, and expert opinions were excluded.
Accordingly, manuscripts published up to April 2021 were evaluated. Table 1 shows the search terms and their combinations. Keyword combinations were modified on every database scan. The references of included articles were also manually checked to find additional articles that were not revealed through electronic search.

Screening and selection of the studies
Initially, two authors independently reviewed the titles that emerged due to electronic searches, and it was decided which publications were relevant. Eligible studies were identified by careful examination of their abstracts. The entire article was evaluated if the information obtained from the title and abstract scanning did not provide sufficient data about the article’s status. The included studies fulfilled the following criteria:
In vitro studies evaluated oral-cavity-derived stem cells on the progress of simulated NDs;
In vivo studies transplanted oral-cavity-derived stem cells (or their secretome) to animal models with NDs.
Consensus of two referees was sought to include the articles. Studies using mesenchymal stem cells derived from oral cavity tissues, such as mature or immature teeth, oral mucosa, salivary glands, maxillary sinus mucosa, or buccal fat pad were included. Mesenchymal stem cells are identified with the following criteria:1) Plastic adherence of the isolated cells in culture; 2) Expression of cluster of differentiation (CD) markers, such as CD73, CD90, and CD105 in >95% of the culture with absent expression of markers, including CD11B or CD14, CD19 or CD79A, CD34, CD45, and human leukocyte antigen-DR in >95% of the culture; and 3) The capacity to differentiate into adipocytes chondrocytes and osteocytes (Dominici et al., 2006). Reviews and other studies (studies that used mesenchymal stem cells other than oral cavity-derived ones and only reported the neurogenic differentiation ability of OCDSCs) that did not meet such criteria were not included in the current study.
Data extraction
The full texts of all included studies were accessed, and two reviewers extracted the data simultaneously according to a standardized baseline. The parameters obtained from the publication were authors, publication year, journal name, type of OCDSCs, type of NDs, number of cells’ passages, type of neuronal cell culture, type of animal, type of transplantation method, performed experiments, and primary outcomes of each study. Characterization methods of stem cells with antigens specific to mesenchymal stem cells or lineage differentiation were also extracted.
Assessment of risk of bias and synthesis evidence
The risk of bias evaluation was made by considering previous studies and modifying related parameters from these studies (Fliefel et al., 2018; Zhang et al., 2018). The assessment was based on the description of the following parameters for the quality assessment of the study for in vivo studies as follows: 1) Published in a peer-reviewed journal; 2) Random allocation to group; 3) Treatment allocation concealment; 4) Pretreatment behavioral assessment; 5) Blinded assessment of outcome (or computerized); 6) Reporting of a sample size calculation; 7) Assessment of ≥2 outcomes; 8) Compliance with animal welfare regulations; 9) Ethics committee approval; and 10) Statement of a potential conflict of interest. If the parameter was included in the article, the article received “Y” denoting “yes” for that parameter; however, if the parameter was not included, the article received “N” indicating “no” for that parameter. Articles reporting 1-4, 5-7, and 8-10 parameters were classified as having high, medium, and low risk of bias, respectively.
For in-vitro studies, the risk of bias was assessed via a modified 6-point-item checklist from previous studies (Fliefel et al., 2018; Malinowski et al., 2020), including the following items: 1) Published in a peer-reviewed journal; 2) Blinded assessment of outcome (detection bias); 3) Reporting sample size calculation; 4) Assessment of ≥2 outcomes (performance bias); 5) Ethics committee approval; and 6) Statement of potential conflict of interest (funding bias). The articles reporting 1-2, 3-4, and 5-6 parameters were classified as having high, medium, and low risk of bias, respectively. Two reviewers carried out the evaluations independently, and disagreements were resolved through consensus. Each included study (both in vivo and in vitro) was analyzed for similarities to perform a meta-analysis. If the data was heterogeneous, a synthesis of evidence was performed as in previous studies (de Vos et al., 2014; Swart et al., 2012; van Tulder et al., 2003):
1. Strong evidence: If there were 2 or more high-quality studies and or consistent findings across all studies (≥75% of studies reported consistent results).
2. Moderate evidence: If there were one high-quality study and 2 or more low-quality studies with consistent findings across all studies (≥75% of the studies reported consistent results).
3. Limited evidence: If there were one low-quality study.
4. Conflicting evidence: If there were inconsistent findings across multiple studies (<75% of studies reported consistent findings).
5. No evidence: If there was no study.
3. Results
Literature search
A comprehensive electronic search retrieved 2080 studies (PubMed=86, Scopus=1878, Web of Science=116), and the manual search retrieved three studies (Ganz et al., 2014; Nosrat et al., 2004; Yoon et al., 2013). Duplicates were removed and 1942 studies remained. The screening of the remaining titles and abstracts revealed 26 studies that met the inclusion criteria (Ahmed et al., 2016; Apel et al., 2009; Chen et al., 2020; Eskandari et al., 2020; Fujii et al., 2015; Ganapathy et al., 2018; Ganz et al., 2014; Genç et al., 2017; Gnanasegaran et al., 2017; Gnanasegaran et al., 2017; Jarmalaviciute et al., 2015; Mita et al., 2015; Narbute et al., 2019; Nesti et al., 2011; Nosrat et al., 2004; Simon et al., 2019; Singh et al., 2021; Testa et al., 2012; Venugopal et al., 2018; Wang et al., 2017; Wang et al., 2010; Wang et al., 2019; Yalvac et al., 2013; Yoon et al., 2013; Zhang et al., 2018; Zhang et al., 2021); however after complete text evaluation, 2 studies were discarded as one of these studies used the mixture of bone-marrow derived stem cells and dental pulp stem cells during experiments (Venugopal et al., 2018) and the other study applied amyloid-beta peptide to neuro-differentiated dental pulp stem cells instead of original neuronal cells (Testa et al., 2012). The PRISMA flow chart presents an overview of the study selection procedure (Figure 1).

A total of 14 of the included studies were in vivo (Chen et al., 2020; Eskandari et al., 2020; Fujii et al., 2015; Ganz et al., 2014; Gnanasegaran et al., 2017; Mita et al., 2015; Narbute et al., 2019; Simon et al., 2019; Singh et al., 2021; Wang et al., 2010; Wang et al., 2019; Yoon et al., 2013; Zhang et al., 2018; Zhang et al., 2021), while the remaining 10 were in vitro (Ahmed et al., 2016; Apel et al., 2009; Ganapathy et al., 2018; Genç et al., 2017; Gnanasegaran et al., 2017; Jarmalaviciute et al., 2015; Nesti et al., 2011; Nosrat et al., 2004; Wang et al., 2017; Yalvac et al., 2013). The main characteristics and the outcome of the included studies are presented in Table 2 and 3 for in vivo and in vitro studies, respectively.



Experimental techniques used in the included studies took place in Table 4.

Neurodegenerative diseases: Risk of bias and synthesis of evidence
Various parameters, such as the number of used cells or amount of cell suspensions during experiments, the number of agents used to induce NDs, the type of transplantation, the type of animals, or neuronal cell cultures, showed diversity among studies. Because of these heterogeneous data, meta-analysis was not conducted in vivo and in vitro studies. Instead of meta-analysis, the risk of bias was analyzed, and the evidence synthesis was performed.


Parkinson disease (PD)
PD was modeled in 7 in vitro studies (Apel et al., 2009; Ganapathy et al., 2018; Gnanasegaran et al., 2017; Jarmalaviciute et al., 2015; Nesti et al., 2011; Nosrat et al., 2004; Yalvac et al., 2013). Of these studies, 1 article revealed high bias (Nosrat et al., 2004) while the remaining demonstrated medium bias (Apel et al., 2009; Ganapathy et al., 2018; Gnanasegaran et al., 2017; Jarmalaviciute et al., 2015; Nesti et al., 2011; Yalvac et al., 2013). All articles reported that OCDSCs (Apel et al., 2009; Ganapathy et al., 2018; Gnanasegaran et al., 2017; Nesti et al., 2011; Nosrat et al., 2004; Yalvac et al., 2013) or their vesicles (Jarmalaviciute et al., 2015) protected dopaminergic neurons, increased their survival rate which was previously exposed to chemical agents to simulate PD via decreasing their apoptosis [according to Jarmalaviciute et al., 2015 exosomes of stem cells from human exfoliated deciduous teeth (SHEDs) suppressed 6-OHDA-induced apoptosis approximately by 80% during the study], secreting anti-inflammatory cytokines and revealing antioxidant enzyme activity (Ganapathy et al., 2018; Gnanasegaran et al., 2017; Nesti et al., 2011; Yalvac et al., 2013). Moderate evidence was found (provided by six studies with low quality and consistent findings in all studies) because ≥75% of the studies reported consistently that the OCDSCs were efficient in vitro against PD-inducing chemicals.
PD was modeled in 10 in vivo studies (Chen et al., 2020; Fujii et al., 2015; Ganz et al., 2014; Gnanasegaran et al., 2017; Narbute et al., 2019; Simon et al., 2019; Singh et al., 2021; Wang et al., 2010; Yoon et al., 2013; Zhang et al., 2018). Three of the studies showed low bias (Narbute et al., 2019; Wang et al., 2010; Zhang et al., 2018) while the remaining seven revealed medium bias (Chen et al., 2020; Fujii et al., 2015; Ganz et al., 2014; Gnanasegaran et al., 2017; Simon et al., 2019; Singh et al., 2021; Yoon et al., 2013). Strong evidence was found regarding the recovery potential of OCDSCs for animal models with PD. Three studies provided it with high quality and ≥75% of the studies reported consistent findings. In 7 of the in vivo studies, OCDSCs were switched to dopaminergic neuronal differentiation (Fujii et al., 2015; Ganz et al., 2014; Gnanasegaran et al., 2017; Simon et al., 2019; Singh et al., 2021; Wang et al., 2010; Zhang et al., 2018). In these studies, tyrosine hydroxylase (TH; rate-limiting enzyme of catecholamine synthesis) positive cells were 40% to 83%. Apomorphine (Narbute et al., 2019; Singh et al., 2021; Wang et al., 2010; Yoon et al., 2013; Zhang et al., 2018), amphetamine (Ganz et al., 2014), or methamphetamine (Fujii et al., 2015) were used during behavioral assessment of parkinsonian animal models. Although different methods were used during behavioral assessment, Ganz et al., (2014) reported that animals treated with oral mucosa stem cells switched to dopaminergic differentiation fully recovered, reaching up to 99.4% of their performance before administering 6-OHDA. The following periods for behavioral assessment were between 2 to 8 weeks (Fujii et al., 2015; Ganz et al., 2014; Gnanasegaran et al., 2017; Narbute et al., 2019; Simon et al., 2019; Singh et al., 2021; Wang et al., 2010); however, Zhang et al. (2018) followed animals up to 16 weeks. Significant recovery was reported in all studies using behavioral assessment tests, except for the study of Yoon et al., (2013). They reported tumor formation after transplantation of dental papilla-derived stem cells. This could be because of the transplantation of early passages contrary to the remaining studies. Furthermore, the anti-inflammatory potential of transplanted OCDSCs was shown several times (Chen et al., 2020; Gnanasegaran et al., 2017; Zhang et al., 2018) with the expression of anti-inflammatory factors, such as IL2, IL4, IL6, and TNF-b.
Alzheimer disease (AD)
AD was induced in 4 in vitro studies (Ahmed et al., 2016; Apel et al., 2009; Wang et al., 2017; Yalvac et al., 2013). Three in vitro studies revealed medium bias (Ahmed et al., 2016; Apel et al., 2009; Yalvac et al., 2013), while the remaining article was low bias (Wang et al., 2017). All studies reported that OCDSCs (Apel et al., 2009; Wang et al., 2017; Yalvac et al., 2013) or their secretome (Ahmed et al., 2016) protected neurons and increased their survival rate which were previously exposed to chemical agent to simulate AD. As a result, moderate evidence was found (provided by 1 study with high quality and three with low quality and consistent findings in all studies).
Two in vivo studies (Mita et al., 2015; Zhang et al., 2021) with medium bias evaluated the effects of OCDSCs on AD and both studies reported that transplantation of OCDSCs resulted in substantially improved cognitive function. Moderate evidence was found regarding OCDSCs on the recovery of AD.
Amyotrophic lateral sclerosis (ALS)
One in vitro study with medium bias showed the effect of OCDSCs on peripheral blood mononuclear cells obtained from ALS patients (Genç et al., 2017) and 1 in vivo study also with medium bias modeled ALS via transgenic mice with superoxide dismutase 1 (mSOD1G93A) mutation (Wang et al., 2019). Both studies reported therapeutic effects for OCDSCs. Limited evidence was found for in vivo and in vitro studies regarding OCDSCs on the recovery of the ALS disease.
Huntington disease (HD)
HD was modeled in 1 in vivo study with 3-nitro propionic acid (Eskandari et al., 2020). The neurons increased, and inflammatory cytokine expression decreased following dental pulp stem cell transplantation (Eskandari et al., 2020). Limited evidence was found for in vivo studies. HD was not modeled in vitro, hence, no evidence was found for in vitro studies.
4. Discussion
NDs, such as AD, ALS, HD, and PD, are among the deteriorating disorders. Their progress worsens with time because the regeneration capacity of neurons and glial cells is restricted (Vishwakarma et al., 2014). A great deal of existing conventional medications have limited efficiency in the treatment of these diseases and provide only symptomatic relief. Therefore, there is a considerable effort to find alternative therapeutic approaches for NDs’ treatment, such as stem cell application. Mesenchymal stem cells (MSCs) that present in many tissues, such as bone marrow, skin, placenta, adipose tissues, umbilical cord, and oral-dental tissues could maintain their replicative capacity for prolonged periods in vitro compared to embryonic stem cells (Shamir et al., 2015) and could be used to treat NDs (Sherman et al., 2019; Song et al., 2018; Sugaya & Vaidya, 2018). The isolation of MSCs from autologous sources could avoid immune rejection and ethical concerns (Shamir et al., 2015). Bone marrow-derived MSCs (BM-MSCs) are accepted as the practical gold standard (Riecke et al., 2015), and several pre-clinical and clinical studies have revealed promising results during the treatment of NDs with these cells (Riecke et al., 2015; Sherman et al., 2019). Nonetheless, BM-MSCs isolation is an excruciating surgical procedure, and the differentiation capacity and proliferation rate of BM-MSCs correlates with the donor age (Huang, Gronthos & Shi, 2009; Stenderup et al., 2003; Wu et al., 2015). In this context, various OCDSCs, such as stem cells from the apical papilla, SHEDs, periodontal ligament stem cells, dental follicle MSCs (DF-MSCs), dental pulp MSCs (DP-MSCs), and MSCs from the gingiva have been identified (Al-Habib & Huang, 2019; Marrelli et al., 2015). A significant advantage of these OCDSCs is their easy isolation procedure by relatively non-invasive methods (Pisciotta et al., 2015). Their ex vivo expansion, self-renewal, and multilineage differentiation capacities, neurogenic potential, and potent anti-inflammatory and immunomodulatory properties are better compared to BM-MSCs (Govindasamy et al., 2010; Ibarretxe et al., 2012; Sakai et al., 2012; Tomar et al., 2010). They can differentiate into different cell types, such as adipocytes, chondrocytes, islet cells, neurons, odontoblasts, osteoblasts, and induced pluripotent stem cells (iPSCs) using classic reprogramming factors (Luo et al., 2018). DP-MSCs had promising potential as an iPSCs source and cell banking (Tamaoki et al., 2010). There is an increase in cryopreserved teeth in tooth banks for future regenerative medical therapies (Yen & Sharpe, 2008).
DP-MSCs express neuronal markers, product, and secrete neurotrophic growth factors, such as brain-derived neurotrophic factor, ciliary neurotrophic factor, fibroblast growth factor, glial-cell-derived growth factor, nerve growth factor, and differentiate into functionally active neurons dopaminergic-like cells, oligodendrocytes, and Schwann cells (Luo et al., 2018). The secretion of these factors is essential in boosting neuronal rescue and survival, neurite outgrowth, and guidance both in vitro and in vivo (Chun et al., 2016; Gnanasegaran et al., 2018; Gnanasegaran et al., 2017) and in stimulating neurogenesis after transplantation in the hippocampus (Ganz et al., 2014; Narbute et al., 2019; Simon et al., 2019). In this context, it is essential to note that BM-MSCs and DP-MSCs derive from the mesoderm and neural crest. DP-MSCs were the most preferred cells in included in vitro studies (Ahmed et al., 2016; Apel et al., 2009; Ganapathy et al., 2018; Nesti et al., 20111; Nosrat et al., 2004; Wang et al., 2017), while SHEDs were the most preferred cells in in-vivo studies (Chen et al., 2020; Fujii et al., 2015; Gnanasegaran et al., 2017; Mita et al., 2015; Narbute et al., 2019; Simon et al., 2019; Wang et al., 2010; Zhang et al., 2018). One explanation could be that the DP-MSCs lost their plasticity through passaging, while SHEDs retained this feature (Govindasamy et al., 2010; Nakamura et al., 2009). Furthermore, the proliferation rate of DP-MSCs and BM-MSCs was significantly lower than SHEDs (Nakamura et al., 2009).
Several reviews were published regarding the effect of OCDSCs on the treatment of different systemic diseases, such as diabetes mellitus, spinal cord injury, AD, PD, and cardiovascular diseases (Chalisserry et al., 2017; Luo et al., 2018; Mortada et al., 2018; Stanko et al., 2018; Wang et al., 2019; Yamada et al., 2019). According to the literature, the effects of BM-MSCs on NDs were systematically reviewed several times (Peng et al., 2015; Riecke et al., 2015; Wang et al., 2015). This systematic review was performed to compile the results of the in vitro and in vivo studies conducted in this field and to reveal the possible limitations. A total of 24 studies were included in this review, regarding AD, ALS, HD, and PD. Meanwhile, ALS is a progressive, incurable ND that targets motoneurons. Genç et al., (2017) reported that DF-MSCs caused an increase in the number of CD4+FoxP3+ regu
of lymphocytes. Also, DF-MSCs increased the apoptotic effect of lymphocytes in ALS patients while increasing cell survival in healthy individuals. Furthermore, Wang et al. (2019) reported that the administration of d DP-MSCs conditioned medium systemically from symptom onset until end-stage of ALS significantly increased the survival of transgenic mice. However, a limited number of studies regarding the effect of OCDSCs on the recovery of the ALS disease made it difficult to conclude ultimately. Further studies regarding the impact of OCDSCs on the ALS disease are needed. The same result is valid for HD. This autosomal dominant ND detects an unstable expansion of CAG repeats in the coding region of the Huntingtin gene IT15 (MacDonald et al., 1993). Modeling HD is complex (Carter & Chan, 2012), and there is only 1 in vivo study regarding the effect of OCDSCs on the progress of HD (Eskandari et al., 2020). The authors concluded that DP-MSCs could repair motor-skill impairment and induce neurogenesis in animal models. Furthermore, Snyder et al., (2011) reported that DP-MSCs from Huntington monkeys retain adult stem cell properties. DP-MSCs isolated from individuals with genetic disorders, such as HD could be considered a personal source of stem cells when considering therapeutic purposes. For personalized medicine, evaluating the stem cell properties of OCDSCs of patients with these NDs is essential. It has been reported that healthy donors- and relapsing-remitting multiple sclerosis patients-derived periodontal ligament stem cells showed similar expression of surface antigen markers, differentiation capacities, and cell proliferation rate (Diomede et al., 2017). OCDSCs could serve as a potential autologous stem cell niche during stem cell therapy of NDs.
Compared to ALS and HD, more studies searched the effect of OCDSCs on AD and PD. Accordingly, PD was induced 17 times (10 in vivo and 7 in vitro studies) according to the results of present review (Apel et al., 2009; Chen et al., 2020; Fujii et al., 2015; Ganapathy et al., 2018; Ganz et al., 2014; Gnanasegaran et al., 2017; Gnanasegaran et al., 2017; Jarmalaviciute et al., 2015; Narbute et al., 2019; Nesti et al., 2011; Nosrat et al., 2004; Simon et al., 2019; Singh et al., 2021; Wang et al., 2010; Yalvac et al., 2013; Yoon et al., 2013; Zhang et al., 2018). Although PD is the second most prevalent ND after AD (Riecke et al., 2015; Zhang et al., 2018), there was more information regarding the effect of OCDSCs on PD than AD. Symptoms of PD include bradykinesia, freezing, muscle rigidity, postural instability, resting tremor, and abnormalities in cognition, mood, and speech (Riecke et al., 2015). SHEDs were mainly used for neuroprotection in PD animal models (Arthur et al., 2008; Chen et al., 2020; Fujii et al., 2015; Gnanasegaran et al., 2017; Narbute et al., 2019; Simon et al., 2019; Wang et al., 2010; Zhang et al., 2018). Transplanted SHEDs restored dopaminergic neuron functions (Gnanasegaran et al., 2017) and promoted survival (Wang et al., 2010). Significant recovery was observed in behavioral deficits following transplantation of SHEDs in PD animal models (Chen et al., 2020; Fujii et al., 2015; Ganz et al., 2014; Gnanasegaran et al., 2017; Simon et al., 2019; Wang et al., 2010; Zhang et al., 2018). Furthermore, extracellular vesicles from SHEDs effectively suppress 6-OHDA-induced gait impairments and normalize tyrosine hydroxylase expression (Narbute et al., 2019). OCDSCs and their exosomes also showed neuro-immunomodulatory activity (Gnanasegaran et al., 2017; Jarmalaviciute et al., 2015; Yalvac et al., 2013) and increased the survival rate of neuronal cells in cellular PD models (Apel et al., 2009; Ganapathy et al., 2018; Yalvac et al., 2013). On the other hand, Yoon et al., 2013 reported the death of all rats because of tumors following transplantation of early-stage human dental papilla-derived stem cells. Switching cells to neurogenic differentiation or injecting their secretome might be advantageous compared to using cells when considering neurodegenerative improvement.
AD is a progressive ND caused by the deposition of insoluble β-amyloid peptides in the brain, the intracellular neurofibrillary tangles, and the loss of neurons (Wang et al., 2015). AD was induced in 6 studies (2 in vivo and 4 in vitro) according to the results of this systematic review (Ahmed et al., 2016; Apel et al., 2009; Mita et al., 2015; Wang et al., 2017; Yalvac et al., 2013; Zhang et al., 2021). OCDSCs reduced β-amyloid peptide-induced cytotoxicity and apoptosis in the AD cellular models (Apel et al., 2009; Yalvac et al., 2013). Furthermore, it has also been reported that β-amyloid peptide cytotoxicity was significantly reduced by increasing cell viability in cells treated with DP-MSC secretome compared to untreated cells (Ahmed et al., 2016). In addition, the endogenous survival factor Bcl-2 was stimulated by the DP-MSCs secretome, while the release of the apoptotic regulator Bax was decreased (Ahmed et al., 2016). SHEDs administered intranasally to the AD mouse model have been reported to cause significantly improved cognitive function by affecting factors involved in axonal elongation, suppression of inflammation, microglial regulation, neuroprotection, and neurotransmission (Mita et al., 2015). However, moderate evidence was found both for in vitro and in vivo studies.
Risk of bias evaluation revealed that 78.6% of in vivo and 80% of in vitro studies had a medium bias. Among 24 studies, there was only 1 in vitro study with high bias. Strong evidence was found only for in vivo studies that evaluated the OCDSCs’ effect on PD. Sample size calculation and treatment allocation concealment were missing parameters in all studies. Sample size calculation could be performed in future studies; blinded assessment is essential for preventing reporting bias. The other limitation is the period of studies. Behavioral assessment tests continued for an average of up to 4 weeks (Eskandari et al., 2020; Narbute et al., 2019; Simon et al., 2019; Singh et al., 2021), while the in vitro co-culture studies lasted as short as 24 h (Apel et al., 2009; Nesti et al., 2011; Yalvac et al., 2013). The long-term therapeutic effect of OCDSCs and their survival could also be studied in future studies. The quantity of transplanted cells to the animal models and the quantity of cells co-cultured with commercial neuronal cells showed diversity in the included studies. Small starting materials could affect the used quantity in these studies. However, this also limited comparisons and interpretations of the results. Chen et al. (2020) injected SHEDs-conditioned medium (SHEDs-CM) at 4 different concentrations and they concluded that 400 mg/mL of SHEDs-CM treatment did not reveal further improvement than the 100 mg/mL treatment group. Other studies could be designed to indicate the optimum cell quantity needed during these studies. It is also important to mention that toxins that were used for inducing ND models, such as 6-OHDA, MPTP, and β-amyloid peptide, did not completely represent the real pathological mechanisms that occur in patients (Mohamed et al., 2019). The efficiency of OCDSCs could be tested on patient-derived iPSCs or induced neuronal stem cells. Co-culture studies could be done with patient-derived induced neuronal stem cells instead of commercial neuronal cell lines. Furthermore, novel technologies such as genome-editing techniques, 3D organoids, 3D cell cultures, and organ-on-a-chip could be used for modeling these NDs (Mohamed et al., 2019) and more conclusive results regarding therapeutic effects of OCDSCs on NDs could be obtained.
5. Conclusion
Considering the limitations of the included studies, OCDSCs and their secretome yield potential for NDs based on their neural crest origin and neuronal characteristics in vitro and in vivo. Further in vitro and in vivo studies with low bias risk, reproducible animal models, and optimal chemical doses will be needed for all types of NDs before clinical implications.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and methodology: Emel Uzunoglu-Ozyurek; Investigation: Emel Uzunoglu-Ozyurek and Gizem Önal; Writing and original draft: Emel Uzunoglu-Ozyurek; Supervision: Serap Dökmec; Writing, review, editing and finding resources: All authors.
Conflict of interest
The authors declared no conflict of interest
References
Ahmed, N.el-M., Murakami, M., Hirose, Y., & Nakashima, M. (2016). Therapeutic potential of dental pulp stem cell secretome for alzheimer’s disease treatment: An in vitro study. Stem Cells International, 2016, 8102478. [DOI:10.1155/2016/8102478] [PMID]
Al-Habib, M., & Huang, G. T. (2019). Dental mesenchymal stem cells: Dental pulp stem cells, periodontal ligament stem cells, apical papilla stem cells, and primary teeth stem cells-isolation, characterization, and expansion for tissue engineering. Methods in Molecular Biology, 1922, 59-76. [DOI:10.1007/978-1-4939-9012-2_7] [PMID]
Apel, C., Forlenza, O. V., de Paula, V. J., Talib, L. L., Denecke, B., & Eduardo, C. P., et al. (2009). The neuroprotective effect of dental pulp cells in models of Alzheimer’s and Parkinson’s disease. Journal of Neural Transmission (Vienna, Austria: 1996), 116(1), 71–78. [DOI:10.1007/s00702-008-0135-3] [PMID]
Arthur, A., Rychkov, G., Shi, S., Koblar, S. A., & Gronthos, S. (2008). Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells, 26(7), 1787-1795. [DOI:10.1634/stemcells.2007-0979] [PMID]
Carter, R. L., & Chan, A. W. (2012). Pluripotent stem cells models for Huntington’s disease: Prospects and challenges. Journal of Genetics and Genomics, 39(6), 253-259. [DOI:10.1016/j.jgg.2012.04.006] [PMID]
Chalisserry, E. P., Nam, S. Y., Park, S. H., & Anil, S. (2017). Therapeutic potential of dental stem cells. Journal of Tissue Engineering, 8, 2041731417702531. [DOI:10.1177/2041731417702531] [PMID]
Chalmers, I., & Glasziou, P. (2009). Avoidable waste in the production and reporting of research evidence. Lancet (London, England), 374(9683), 86-89. [DOI:10.1016/S0140-6736(09)60329-9] [PMID]
Chen, Y. R., Lai, P. L., Chien, Y., Lee, P. H., Lai, Y. H., & Ma, H. I., et al. (2020). Improvement of impaired motor functions by human dental exfoliated deciduous teeth stem cell-derived factors in a rat model of parkinson’s disease. International Journal of Molecular Sciences, 21(11), 3807. [DOI:10.3390/ijms21113807] [PMID]
Chun, S. Y., Soker, S., Jang, Y. J., Kwon, T. G., & Yoo, E. S. (2016).Differentiation of human dental pulp stem cells into dopaminergic neuron-like cells in vitro. Journal of Korean Medical Science, 31(2), 171-177. [DOI:10.3346/jkms.2016.31.2.171] [PMID]
de Almeida, F. M., Marques, S. A., Ramalho Bdos, S., Rodrigues, R. F., Cadilhe, D. V., & Furtado, D., et al. (2011). Human dental pulp cells: A new source of cell therapy in a mouse model of compressive spinal cord injury. Journal of Neurotrauma, 28(9), 1939-1949. [DOI:10.1089/neu.2010.1317] [PMID]
de Vos, R. J., Windt, J., & Weir, A. (2014). Strong evidence against platelet-rich plasma injections for chronic lateral epicondylar tendinopathy: A systematic review. British Journal of Sports Medicine, 48(12), 952-956. [DOI:10.1136/bjsports-2013-093281] [PMID]
Diomede, F., Rajan, T. S., D'Aurora, M., Bramanti, P., Merciaro, I., & Marchisio, M., et al. (2017). Stemness characteristics of periodontal ligament stem cells from donors and multiple sclerosis patients: A comparative study. Stem Cells International, 2017, 1606125. [DOI:10.1155/2017/1606125] [PMID]
Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., & Krause, D., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8(4), 315-317. [DOI:10.1080/14653240600855905] [PMID]
Dulak, J., Szade, K., Szade, A., Nowak, W., & Józkowicz, A. (2015). Adult stem cells: Hopes and hypes of regenerative medicine. Acta Biochimica Polonica, 62(3), 329-337. [DOI:10.18388/abp.2015_1023] [PMID]
Eagly, A. H., & Wood, W. (1994). Using research syntheses to plan future research. In H. Cooper & L. V. Hedges (Eds.), The handbook of research synthesis (pp. 485-500). New York: Russell Sage Foundation. [Link]
Ellis, K. M., O'Carroll, D. C., Lewis, M. D., Rychkov, G. Y., & Koblar, S. A. (2014). Neurogenic potential of dental pulp stem cells isolated from murine incisors. Stem Cell Research & Therapy, 5(1), 30. [DOI:10.1186/scrt419] [PMID]
Eskandari, N., Boroujeni, M. E., Abdollahifar, M. A., Piryaei, A., Khodagholi, F., & Mirbehbahani, S. H., et al. (2021). Transplantation of human dental pulp stem cells compensates for striatal atrophy and modulates neuro-inflammation in 3-nitropropionic acid rat model of Huntington’s disease. Neuroscience Research, 170, 133–144. [DOI:10.1016/j.neures.2020.12.002] [PMID]
Fliefel, R., Ehrenfeld, M., & Otto, S. (2018). Induced pluripotent stem cells (iPSCs) as a new source of bone in reconstructive surgery: A systematic review and meta-analysis of preclinical studies. Journal of Tissue Engineering and Regenerative Medicine, 12(7), 1780-1797. [DOI:10.1002/term.2697] [PMID]
Fujii, H., Matsubara, K., Sakai, K., Ito, M., Ohno, K., & Ueda, M., et al. (2015). Dopaminergic differentiation of stem cells from human deciduous teeth and their therapeutic benefits for Parkinsonian rats. Brain Research, 1613, 59-72. [DOI:10.1016/j.brainres.2015.04.001] [PMID]
Ganapathy, K., Datta, I., & Bhonde, R. (2019). Astrocyte-like cells differentiated from dental pulp stem cells protect dopaminergic neurons against 6-hydroxydopamine toxicity. Molecular Neurobiology, 56(6), 4395–4413. [DOI:10.1007/s12035-018-1367-3] [PMID]
Ganz, J., Arie, I., Buch, S., Zur, T. B., Barhum, Y., & Pour, S., et al. (2014). Dopaminergic-like neurons derived from oral mucosa stem cells by developmental cues improve symptoms in the hemi-parkinsonian rat model. PLoS One, 9(6), e100445. [DOI:10.1371/journal.pone.0100445] [PMID]
Genç, D., Zibandeh, N., Uluç, K., Koytak, P. K., Gökalp, M., & Tanridag, T., et al. (2017). Dental follicle mesenchymal stem cells enhance CD4+ Foxp3+ regulatory T cells in the lymphocytes of amyotrophic lateral sclerosis patients. Clinical and Experimental Health Sciences, 7(3), 85-90. [Link]
Gnanasegaran, N., Govindasamy, V., Kathirvaloo, P., Musa, S., & Abu Kasim, N. H. (2018). Effects of cell cycle phases on the induction of dental pulp stem cells toward dopaminergic-like cells. Journal of Tissue Engineering and Regenerative Medicine, 12(2), e881-e893. [DOI:10.1002/term.2401] [PMID]
Gnanasegaran, N., Govindasamy, V., Mani, V., & Abu Kasim, N. H. (2017). Neuroimmunomodulatory properties of DPSCs in an in vitro model of Parkinson’s disease. IUBMB Life, 69(9), 689-699. [DOI:10.1002/iub.1655] [PMID]
Gnanasegaran, N., Govindasamy, V., Simon, C., Gan, Q. F., Vincent-Chong, V. K., & Mani, V., et al. (2017). Effect of dental pulp stem cells in MPTP-induced old-aged mice model. European Journal of Clinical Investigation, 47(6), 403-414. [DOI:10.1111/eci.12753] [PMID]
Govindasamy, V., Abdullah, A. N., Ronald, V. S., Musa, S., Ab Aziz, Z. A., & Zain, R. B., et al. (2010). Inherent differential propensity of dental pulp stem cells derived from human deciduous and permanent teeth. Journal of Endodontics, 36(9), 1504-1515. [DOI:10.1016/j.joen.2010.05.006] [PMID]
Gronthos, S., Brahim, J., Li, W., Fisher, L. W., Cherman, N., & Boyde, A., et al. (2002). Stem cell properties of human dental pulp stem cells. Journal of Dental Research, 81(8), 531-535. [DOI:10.1177/154405910208100806] [PMID]
Gronthos, S., Mankani, M., Brahim, J., Robey, P. G., & Shi, S. (2000). Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America, 97(25), 13625–13630. [DOI:10.1073/pnas.240309797] [PMID]
Heng, B. C., Lim, L. W., Wu, W., & Zhang, C. (2016). An overview of protocols for the neural induction of dental and oral stem cells in vitro. Tissue Engineering Part B: Reviews, 22(3), 220-250. [DOI:10.1089/ten.teb.2015.0488] [PMID]
Huang, G. T., Gronthos, S., & Shi, S. (2009). Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. Journal of Dental Research, 88(9), 792-806. [DOI:10.1177/0022034509340867] [PMID]
Ibarretxe, G., Crende, O., Aurrekoetxea, M., García-Murga, V., Etxaniz, J., & Unda, F. (2012). Neural crest stem cells from dental tissues: A new hope for dental and neural regeneration. Stem Cells International, 2012, 103503. [DOI:10.1155/2012/103503] [PMID]
Jarmalavičiūtė, A., Tunaitis, V., Pivoraitė, U., Venalis, A., & Pivoriūnas, A. (2015). Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine-induced apoptosis. Cytotherapy, 17(7), 932-939. [DOI:10.1016/j.jcyt.2014.07.013] [PMID]
Király, M., Kádár, K., Horváthy, D. B., Nardai, P., Rácz, G. Z., & Lacza, Z., et al. (2011). Integration of neuronally predifferentiated human dental pulp stem cells into rat brain in vivo. Neurochemistry International, 59(3), 371-381. [DOI:10.1016/j.neuint.2011.01.006] [PMID]
Király, M., Porcsalmy, B., Pataki, A., Kádár, K., Jelitai, M., & Molnár, B., et al. (2009). Simultaneous PKC and cAMP activation induces differentiation of human dental pulp stem cells into functionally active neurons. Neurochemistry International, 55(5), 323-332. [DOI:10.1016/j.neuint.2009.03.017] [PMID]
Luo, L., He, Y., Wang, X., Key, B., Lee, B. H., & Li, H., et al. (2018). Potential roles of dental pulp stem cells in neural regeneration and repair. Stem Cells International, 2018, 1731289. [DOI:10.1155/2018/1731289] [PMID]
MacDonald, M. E., Ambrose, C. M., Duyao, M. P., Myers, R. H., Lin, C., & Srinidhi, L., et al. (1993). A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s disease collaborative research group. Cell, 72(6), 971-983. [DOI:10.1016/0092-8674(93)90585-E] [PMID]
Malinowski, B., Musiała, N., & Wiciński, M. (2020). Metformin’s modulatory effects on miRNAs function in cancer stem cells-A systematic review. Cells, 9(6), 1401. [DOI:10.3390/cells9061401] [PMID]
Marrelli, M., Paduano, F., & Tatullo, M. (2015). Human periapical cyst-mesenchymal stem cells differentiate into neuronal cells. Journal of Dental Research, 94(6), 843-852. [DOI:10.1177/0022034515570316] [PMID]
Mita, T., Furukawa-Hibi, Y., Takeuchi, H., Hattori, H., Yamada, K., & Hibi, H., et al. (2015). Conditioned medium from the stem cells of human dental pulp improves cognitive function in a mouse model of Alzheimer’s disease. Behavioural Brain Research, 293, 189-197. [DOI:10.1016/j.bbr.2015.07.043] [PMID]
Miura, M., Gronthos, S., Zhao, M., Lu, B., Fisher, L. W., & Robey, P. G., et al. (2003). SHED: Stem cells from human exfoliated deciduous teeth. Proceedings of the National Academy of Sciences of the United States of America, 100(10), 5807-5812. [DOI:10.1073/pnas.0937635100] [PMID]
Mohamed, N. V., Larroquette, F., Beitel, L. K., Fon, E. A., & Durcan, T. M. (2019). One step into the future: New iPSC tools to advance research in parkinson’s disease and neurological disorders. Journal of Parkinson’s Disease, 9(2), 265-281. [DOI:10.3233/JPD-181515] [PMID]
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., & PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine, 151(4), 264–W64. [DOI:10.7326/0003-4819-151-4-200908180-00135] [PMID]
Mortada, I., Mortada, R., & Al Bazzal, M. (2018). Dental pulp stem cells and the management of neurological diseases: An update. Journal of Neuroscience Research, 96(2), 265-272. [DOI:10.1002/jnr.24122] [PMID]
Nakamura, S., Yamada, Y., Katagiri, W., Sugito, T., Ito, K., & Ueda, M. (2009). Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. Journal of Endodontics, 35(11), 1536-1542. [DOI:10.1016/j.joen.2009.07.024] [PMID]
Narbute, K., Piļipenko, V., Pupure, J., Dzirkale, Z., Jonavičė, U., & Tunaitis, V., et al. (2019). Intranasal administration of extracellular vesicles derived from human teeth stem cells improve motor symptoms and normalize tyrosine hydroxylase expression in the substantia nigra and striatum of the 6‐hydroxydopamine‐treated rats. Stem Cells Translational Medicine, 8(5), 490-499. [DOI:10.1002/sctm.18-0162] [PMID]
Nesti, C., Pardini, C., Barachini, S., D’Alessandro, D., Siciliano, G., & Murri, L., et al. (2011). Human dental pulp stem cells protect mouse dopaminergic neurons against MPP+ or rotenone. Brain Research, 1367, 94-102. [DOI:10.1016/j.brainres.2010.09.042] [PMID]
Nosrat, I. V., Smith, C. A., Mullally, P., Olson, L., & Nosrat, C. A. (2004). Dental pulp cells provide neurotrophic support for dopaminergic neurons and differentiate into neurons in vitro; implications for tissue engineering and repair in the nervous system. The European Journal of Neuroscience, 19(9), 2388-2398. [DOI:10.1111/j.0953-816X.2004.03314.x] [PMID]
Peng, W., Sun, J., Sheng, C., Wang, Z., Wang, Y., & Zhang, C., et al. (2015). Systematic review and meta-analysis of efficacy of mesenchymal stem cells on locomotor recovery in animal models of traumatic brain injury. Stem Cell Research & Therapy, 6(1), 47. [DOI:10.1186/s13287-015-0034-0] [PMID]
Pisciotta, A., Carnevale, G., Meloni, S., Riccio, M., De Biasi, S., & Gibellini, L., et al. (2015). Human dental pulp stem cells (hDPSCs): Isolation, enrichment and comparative differentiation of two sub-populations. BMC Developmental Biology, 15, 14. [DOI:10.1186/s12861-015-0065-x] [PMID]
Raza, S. S., Wagner, A. P., Hussain, Y. S., & Khan, M. A. (2018). Mechanisms underlying dental-derived stem cell-mediated neurorestoration in neurodegenerative disorders. Stem Cell Research & Therapy, 9(1), 245 [DOI:10.1186/s13287-018-1005-z] [PMID]
Riecke, J., Johns, K. M., Cai, C., Vahidy, F. S., Parsha, K., & Furr-Stimming, E., et al. (2015). A meta-analysis of mesenchymal stem cells in animal models of parkinson’s disease. Stem Cells and Development, 24(18), 2082-2090. [DOI:10.1089/scd.2015.0127] [PMID]
Sakai, K., Yamamoto, A., Matsubara, K., Nakamura, S., Naruse, M., & Yamagata, M., et al. (2012). Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. Journal of Clinical Investigation, 122(1), 80-90. [DOI:10.1172/JCI59251] [PMID]
Shamir, C., Venugopal, C., & Dhanushkodi, A. (2015). Dental pulp stem cells for treating neurodegenerative diseases. Neural Regeneration Research, 10(12), 1910-1911. [DOI:10.4103/1673-5374.169629] [PMID]
Sherman, L. S., Romagano, M. P., Williams, S. F., & Rameshwar, P. (2019). Mesenchymal stem cell therapies in brain disease. Seminars in Cell and Developmental Biology, 95, 111-119.[DOI:10.1016/j.semcdb.2019.03.003]
Simon, C., Gan, Q. F., Kathivaloo, P., Mohamad, N. A., Dhamodharan, J., & Krishnan, A., et al. (2019). Deciduous DPSCs ameliorate MPTP-mediated neurotoxicity, sensorimotor coordination and olfactory function in parkinsonian mice. International Journal of Molecular Sciences, 20(3), 568. [DOI:10.3390/ijms20030568] [PMID]
Singh, M., Jain, M., Bose, S., Halder, A., Nag, T. C., & Dinda, A. K., et al. (2021). 22(R)-hydroxycholesterol for dopaminergic neuronal specification of MSCs and amelioration of Parkinsonian symptoms in rats. Cell Death Discovery, 7(1), 13. [DOI:10.1038/s41420-020-00351-6] [PMID]
Snyder, B. R., Cheng, P. H., Yang, J., Yang, S. H., Huang, A. H., & Chan, A. W. (2011). Characterization of dental pulp stem/stromal cells of Huntington monkey tooth germs. BMC Cell Biology, 12, 39. [DOI:10.1186/1471-2121-12-39] [PMID]
Song, C. G., Zhang, Y. Z., Wu, H. N., Cao, X. L., Guo, C. J., & Li, Y. Q., et al. (2018). Stem cells: A promising candidate to treat neurological disorders. Neural Regeneration Research, 13(7), 1294-1304. [DOI:10.4103/1673-5374.235085] [PMID]
Stanko, P., Altanerova, U., Jakubechova, J., Repiska, V., & Altaner, C. (2018). Dental mesenchymal stem/stromal cells and their exosomes. Stem Cells International, 2018, 8973613. [DOI:10.1155/2018/8973613] [PMID]
Stenderup, K., Justesen, J., Clausen, C., & Kassem, M. (2003). Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone, 33(6), 919-926. [DOI:10.1016/j.bone.2003.07.005] [PMID]
Sugaya, K., & Vaidya, M. (2018). Stem cell therapies for neurodegenerative diseases. Advances in Experimental Medicine and Biology, 1056, 61-84. [DOI:10.1007/978-3-319-74470-4_5] [PMID]
Swart, N. M., van Linschoten, R., Bierma-Zeinstra, S. M., & van Middelkoop, M. (2012). The additional effect of orthotic devices on exercise therapy for patients with patellofemoral pain syndrome: A systematic review. British Journal of Sports Medicine, 46(8), 570-577. [DOI:10.1136/bjsm.2010.080218] [PMID]
Tamaoki, N., Takahashi, K., Tanaka, T., Ichisaka, T., Aoki, H., & Takeda-Kawaguchi, T., et al. (2010). Dental pulp cells for induced pluripotent stem cell banking. Journal of Dental Research, 89(8), 773-778. [DOI:10.1177/0022034510366846] [PMID]
Testa, G., Gamba, P., Di Scipio, F., Sprio, A. E., Salamone, P., & Gargiulo, S., et al. (2012). Potentiation of amyloid-beta peptide neurotoxicity in human dental-pulp neuron-like cells by the membrane lipid peroxidation product 4-hydroxynonenal. Free Radical Biology & Medicine, 53(9), 1708-1717. [DOI:10.1016/j.freeradbiomed.2012.08.581] [PMID]
Tomar, G. B., Srivastava, R. K., Gupta, N., Barhanpurkar, A. P., Pote, S. T., & Jhaveri, H. M., et al. (2010). Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochemical and Biophysical Research Communications, 393(3), 377-383. [DOI:10.1016/j.bbrc.2010.01.126] [PMID]
van Tulder, M., Furlan, A., Bombardier, C., Bouter, L., & Editorial Board of the Cochrane Collaboration Back Review Group (2003). Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine (Phila Pa 1976), 28(12), 1290-1299. [DOI:10.1097/01.BRS.0000065484.95996.AF] [PMID]
Venugopal, C., K, S., Rai, K. S., Pinnelli, V. B., Kutty, B. M., & Dhanushkodi, A. (2018). Neuroprotection by human dental pulp mesenchymal stem cells: from billions to nano. Current Gene Therapy, 18(5), 307-323. [DOI:10.2174/1566523218666180913152615] [PMID]
Vishwakarma, S. K., Bardia, A., Tiwari, S. K., Paspala, S. A., & Khan, A. A. (2014). Current concept in neural regeneration research: NSCs isolation, characterization and transplantation in various neurodegenerative diseases and stroke: A review. Journal of Advanced Research, 5(3), 277-294. [DOI:10.1016/j.jare.2013.04.005] [PMID]
Wang, D., Wang, Y., Tian, W., & Pan, J. (2019). Advances of tooth-derived stem cells in neural diseases treatments and nerve tissue regeneration. Cell Proliferation, 52(3), e12572. [DOI:10.1111/cpr.12572] [PMID]
Wang, F., Jia, Y., Liu, J., Zhai, J., Cao, N., & Yue, W., et al. (2017). Dental pulp stem cells promote regeneration of damaged neuron cells on the cellular model of Alzheimer’s disease. Cell Biology International, 41(6), 639-650. [DOI:10.1002/cbin.10767] [PMID]
Wang, J., Wang, X., Sun, Z., Wang, X., Yang, H., & Shi, S., et al. (2010). Stem cells from human-exfoliated deciduous teeth can differentiate into dopaminergic neuron-like cells. Stem Cells and Development, 19(9), 1375-1383. [DOI:10.1089/scd.2009.0258] [PMID]
Wang, J., Zuzzio, K., & Walker, C. L. (2019). Systemic dental pulp stem cell secretome therapy in a mouse model of amyotrophic lateral sclerosis. Brain sciences, 9(7), 165. [DOI:10.3390/brainsci9070165] [PMID]
Wang, Z., Peng, W., Zhang, C., Sheng, C., Huang, W., & Wang, Y., et al. (2015). Effects of stem cell transplantation on cognitive decline in animal models of Alzheimer’s disease: A systematic review and meta-analysis. Scientific Reports, 5, 12134. [DOI:10.1038/srep12134] [PMID]
Wu, W., Zhou, J., Xu, C. T., Zhang, J., Jin, Y. J., & Sun, G. L. (2015). Derivation and growth characteristics of dental pulp stem cells from patients of different ages. Molecular Medicine Reports, 12(4), 5127-5134. [DOI:10.3892/mmr.2015.4106] [PMID]
Yalvaç, M. E., Yarat, A., Mercan, D., Rizvanov, A. A., Palotás, A., & Şahin, F. (2013). Characterization of the secretome of human tooth germ stem cells (hTGSCs) reveals neuro-protection by fine-tuning micro-environment. Brain, Behavior, and Immunity, 32, 122-130. [DOI:10.1016/j.bbi.2013.03.007] [PMID]
Yamada, Y., Nakamura-Yamada, S., Kusano, K., & Baba, S. (2019). Clinical Potential and Current Progress of Dental Pulp Stem Cells for Various Systemic Diseases in Regenerative Medicine: A Concise Review. International Journal of Molecular Sciences, 20(5), 1132. [DOI:10.3390/ijms20051132] [PMID]
Yen, A. H., & Sharpe, P. T. (2008). Stem cells and tooth tissue engineering. Cell and Tissue Research, 331(1), 359-372. [DOI:10.1007/s00441-007-0467-6] [PMID]
Yoon, H. H., Min, J., Shin, N., Kim, Y. H., Kim, J. M., & Hwang, Y. S., et al. (2013). Are human dental papilla-derived stem cell and human brain-derived neural stem cell transplantations suitable for treatment of Parkinson’s disease?. Neural Regeneration Research, 8(13), 1190-1200. [PMID]
Zhang, N., Lu, X., Wu, S., Li, X., Duan, J., & Chen, C., et al. (2018). Intrastriatal transplantation of stem cells from human exfoliated deciduous teeth reduces motor defects in Parkinsonian rats. Cytotherapy, 20(5), 670-686. [DOI:10.1016/j.jcyt.2018.02.371] [PMID]
Zhang, Y., Ge, M., Hao, Q., & Dong, B. (2018). Induced pluripotent stem cells in rat models of Parkinson’s disease: A systematic review and meta-analysis. Biomedical Reports, 8(3), 289-296. [DOI:10.3892/br.2018.1049]
Zhang, X. M., Ouyang, Y. J., Yu, B. Q., Li, W., Yu, M. Y., & Li, J. Y., et al. (2021). Therapeutic potential of dental pulp stem cell transplantation in a rat model of Alzheimer’s disease. Neural Regeneration Research, 16(5), 893-898. [DOI:10.4103/1673-5374.297088] [PMID]
Type of Study: Review |
Subject:
Cellular and molecular Neuroscience
Received: 2020/09/9 | Accepted: 2023/05/20 | Published: 2023/09/1
Received: 2020/09/9 | Accepted: 2023/05/20 | Published: 2023/09/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








