Volume 15, Issue 5 (September & October 2024)
BCN 2024, 15(5): 595-606 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Roohi N, Ahmadi M, Fathollahi Y, Shojaei A, Mirnajafi-Zadeh J. Comparing the Seizure-induced Impairment of Short-term Plasticity in Dorsal and Ventral Hippocampus in Kindled Mice. BCN 2024; 15 (5) :595-606
URL: http://bcn.iums.ac.ir/article-1-1796-en.html
URL: http://bcn.iums.ac.ir/article-1-1796-en.html
1- Department of Physiology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran.
Full-Text [PDF 1039 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Hippocampus is one of the most critical brain structures in learning and memory (Morris, 2007). Hippocampal-dependent synaptic plasticity is involved in some physiological and pathological functions that can be affected/impaired by conditions such as epilepsy (Krug et al., 1997). Epilepsy is one of the most prevalent neurological diseases that influence the patients’ individual and social quality of life and their cognitive functions (Dodrill, 1986; Kwan & Brodie, 2001). The effect of epileptic seizures on short-term and long-term synaptic plasticity can also be observed in chemical kindling (Palizvan et al., 2005), which develops by serial injections of a subconvulsive dose of pentylenetetrazol (PTZ), a GABAA receptor antagonist.
Short- and long-term synaptic plasticity, as the basic mechanisms involved in learning and memory (Martin & Morris, 2002; Morris, 2007), have been studied in the hippocampus. Paired-pulse facilitation or depression is a relatively simple form of short-term synaptic plasticity (Kamiya & Zucker, 1994). They can be observed when two stimuli are delivered to presynaptic afferent fibers at different intervals and the obtained response to the second pulse is higher or lower than the response to the first pulse, respectively. Evaluating the changes in short-term plasticity gives us insights about the activity of neural circuits following seizures and helps in understanding the pathophysiology of epileptic brain.
It is important to note that the characteristics and magnitude of synaptic plasticity in the dorsal and ventral hippocampus (VH) are different (Dougherty et al., 2012; Dubovyk & Manahan‐Vaughan, 2018). Recent experimental data highlight the idea of functional separation along the longitudinal axis of the hippocampus (dorsoventral or septotemporal), with diverse functions such as cognition and emotionality in the dorsal hippocampus (DH) and VH, respectively (Poppenk et al., 2013; Bannerman et al., 2014). This diversification along the longitudinal axis may be due to different extrinsic connectivity of dorsal and ventral hippocampal segments with other brain structures or different local neural circuits in the dorsal and ventral segments of the hippocampus. Based on early and recent electrophysiological and neurochemical studies, the dorsal and VH have different local network organization including principal cell properties, synaptic transmission, neurotransmitter receptors, and synaptic plasticity (for review please see (Papatheodoropoulos, 2018).
Accordingly, it is necessary to determine the seizure-induced changes in short-term synaptic plasticity in the dorsal and ventral parts of the hippocampus better to understand the effect of seizure on cognitive functions. Therefore, in the present study, we examined the changes in paired-pulse facilitation of field excitatory postsynaptic potentials (fEPSPs) recorded from the CA1 region in DH and VH following PTZ kindling in mice. We were curious to find out whether PTZ kindling has different effects on the two poles of the hippocampus.
2. Materials and Methods
Study animals
All experiments and manipulations conformed to the guidelines set by the animal care commission of the Faculty of Medical Sciences, Tarbiat Modares University, Iran. B6/J male mice (10-12 weeks old) from Pasteur Institute (Iran) were used in the experiments. Animals consisted of 9 kindled and 10 control mice. Before engaging in any experimental paradigms, the mice were given one week to acclimate to the facility, and then PTZ or saline injections were done every other day. Mice were maintained on a normal light cycle with access to Purina mouse chow and sterile water ad libitum throughout the study.
PTZ kindling
For kindling, a sub-threshold dose of 35 mg/kg PTZ was injected intraperitoneally (10 µL/g, IP) every 48 h. Following injection, the convulsive behavior of each subject was observed for 20 min. PTZ was freshly prepared in a sterile isotonic saline before injections. Control mice were injected with the identical saline solution without PTZ. The resultant seizures were classified as follows: stage 0, no response; stage 1, ear and facial twitching; stage 2, convulsive waves axially through the body; stage 3, myoclonic body jerk; stage 4, turn over into side position, tonic-clonic seizures; and stage 5, turn over into back position, generalized tonic-clonic seizures (Dhir, 2012). All animals behaviorally showed three consecutive stages, 4 or 5, to be considered fully kindled.
Field potential recording in the hippocampal slices
Twenty-four hours after the last PTZ or saline injection, the mice were anesthetized with carbon dioxide (CO2) and decapitated. The brain was carefully transferred into a chamber containing chilled artificial cerebrospinal fluid (aCSF) bubbled by carbogen (95% O2 and 5% CO2). The aCSF contained (in mM) NaCl 124, NaHCO3 26, KH2PO4 1.25, KCl 5, CaCl2 2, MgCl2 2.06, and D-glucose 10 and its pH was 7.3-7.4. Then, the right hemisphere of the brain was dissected. Transverse hippocampal slices were sectioned (400 μm thick) using a vibratome (VT 1200, Leica, Germany). The slices were placed in a recovery chamber filled with aCSF for at least 60 min at room temperature. Then, they moved to an interface-type recording chamber and maintained at 32 oC, at the interface between aCSF and warm, humidified oxygen gas. Slices were perfused at 1 mL/min with aCSF, which was aerated continuously with carbogen. The slices were allowed to incubate in this medium for 30 min before recording.
Field potentials were recorded from the CA1 stratum radiatum in the dorsal and VH. Stimulating electrodes (stainless steel, Teflon coated, A-M Systems, US) and recording electrodes (borosilicate glass, O.D.: 1.5 mm, I.D.: 0.86 mm, Sutter instrument, USA) were placed on the same layer. A reference electrode was also placed in the recording chamber. After electrode implantation, square stimulating pulses (200 μs, 0.1 Hz) were applied through a bipolar stimulating electrode by an isolator and constant current unit apparatus (S403J, Nihon-Kohden, Japan). Since the stimulation strength required to evoke fEPSP of a given magnitude can vary from experiment to experiment, we made a normalized scale for stimulation intensity. The full scale of stimulation intensities was divided into 10 steps according to the stimulation intensities required to elicit minimum and maximum fEPSP-slope. Extracellular potentials were recorded using a glass recording electrode (2-5 MΩ) filled with aCSF.
Signals were transferred to an amplifier (ME208300, Nihon-Kohden, Japan) through the recording electrode and visualized by custom-made software (Potentalize; ScienceBeam Co., Iran). The input/output curve was plotted to determine the test pulse intensity. To record paired-pulse responses, test pulse intensity was used throughout the experiments. Paired-pulse stimulations were made at the inter-pulse intervals of 20, 80, and 160 ms.
Statistical analysis
fEPSP parameters were analyzed using repeated measures 2-way ANOVA followed by Sidak multiple comparison test, paired-pulse data were analyzed unpaired t student test. The values are expressed as Mean±SEM of the mean. The number of slices (n) used in the control group was n=8 in dorsal hippocampal slices and n=7 in ventral hippocampal slices. In kindled group, n=5 in dorsal hippocampal slices and n=6 in ventral hippocampal slices. In each experimental group, all slices were taken from 3 mice.
Paired-pulse responses were quantified as the ratio of the second pulse evoked fEPSP to the first. All statistical analyses were conducted using GraphPad Prism software, version 7.03. To be considered significant, P<0.05 were used.
3. Results
PTZ kindling procedure
Subjects were handled and acclimatized for at least one week, and intraperitoneal PTZ injections were done every other day (saline was injected instead of PTZ in the control group). Approximately 80% of animals fulfilled the kindling criteria after 7-10 injections. The mean number of injections was 6.3±2.
Effect of kindling on single-pulse evoked fEPSPs
fEPSPs evoked at test pulse stimulation intensity were recorded from the CA1 area of dorsal and ventral hippocampal slices (Figure 1A). In the first step, we compared the minimum and maximum stimulus intensities needed for evoking fEPSP. Obtained data from whole slices (dorsal and VH) showed that the control group’s minimum and maximum stimulation intensities were 20.6±1.1 and 124.6±4.2 µA. Similar data were achieved in the kindled group so that the minimum intensity was 21.11±3.3 µA and the maximum intensity was 118.35±23.4 µA (n=18). More specifically, the minimum and maximum stimulation values for dorsal hippocampal slices in the control group were 20.7±0.7 and 127.1±3.8 µA (n=22), respectively, and in kindled group were 21.1±1.1 and 121.1±9.2 µA (n=9). The corresponding values for the ventral control slices were 20.6±0.4 and 122.1±5 µA (n=22), and for the ventral kindled slices were 21.1±1.1 and 115.6±6 µA (n=9). These data showed no significant difference in minimum and maximum stimulus intensities between control and kindled slices in the dorsal and VH.
Then, input/output curves were plotted using arbitrary units. From input/output curves, we found that in relatively high intensities (numbered 8-10), the fEPSP-slope in ventral hippocampal slices was greater than the fEPSP-slope in dorsal hippocampal slices in the control group (F(9, 351)=9.713, P<0.0001). However, there was no significant difference in fEPSP-area between dorsal and ventral hippocampal slices at different stimulation intensities in the control group (F(9, 522)=0.3849, P=0.9424) (Figure 1B). In the kindled group, the differences in input/output curves between dorsal and ventral hippocampal slices were opposite to what observed in control slices, i.e. in PTZ kindled animals, there was no significant difference in fEPSP-slope between dorsal and ventral parts of the hippocampal slices (F(9, 280)=0.07886, P=0.9999). Still, a significant difference was observed in the fEPSP-area between the two regions. There was a greater fEPSP-area in the ventral than dorsal hippocampal slices in the kindled group (F(9, 135)=2.008, P=0.0429) (Figure 1C).
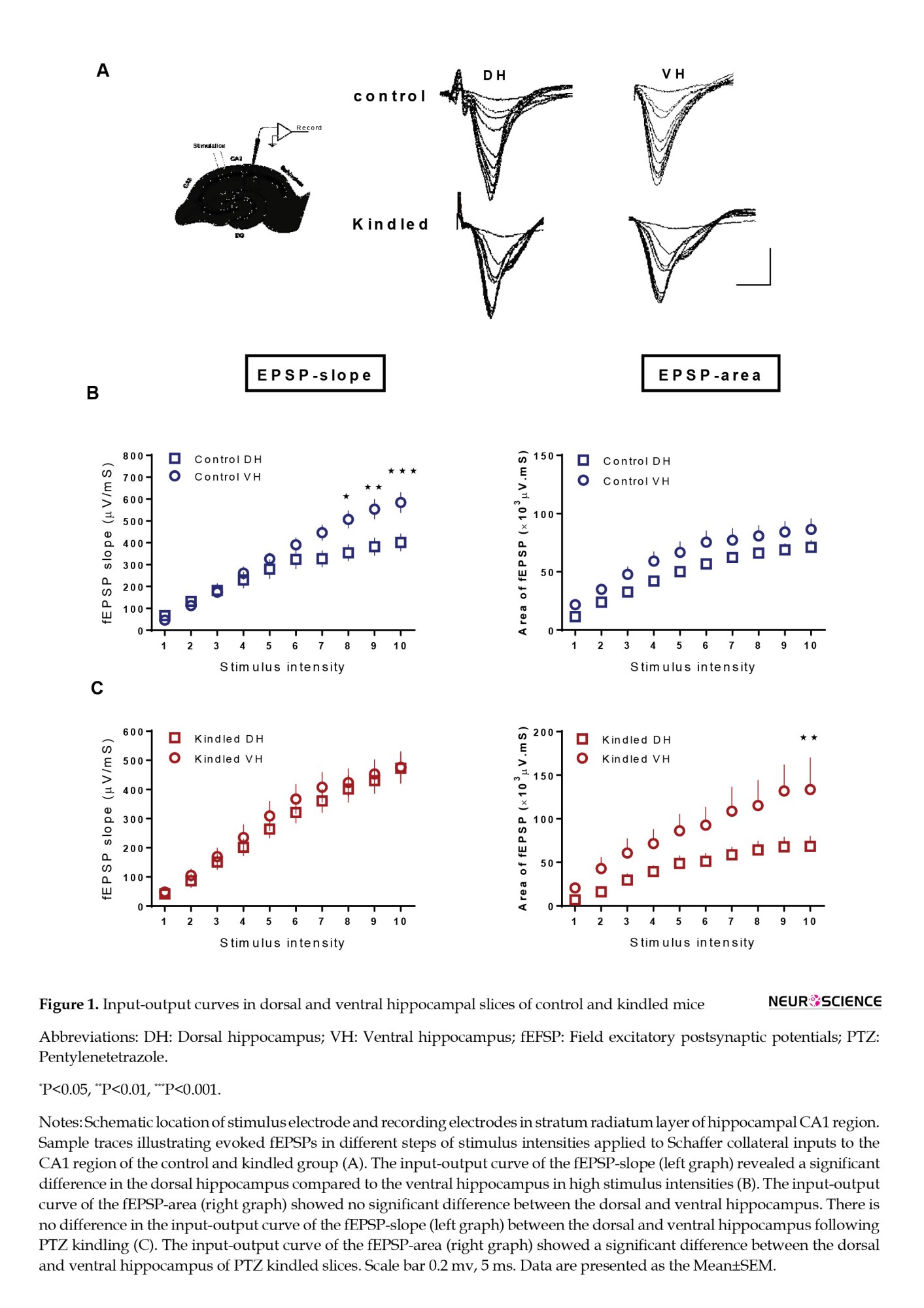
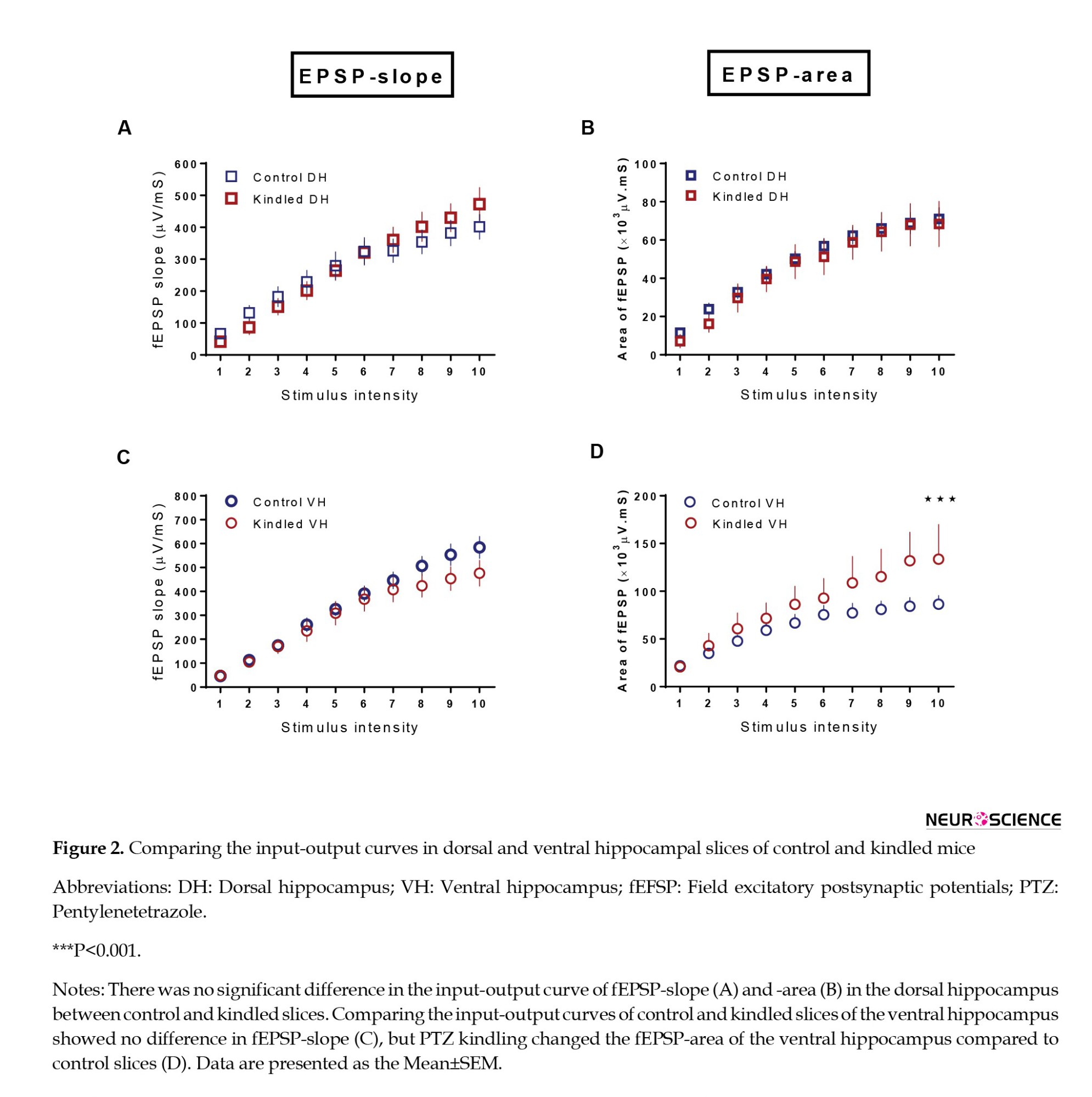
In the next step, we compared the input/output curves of fEPSP-slope and fEPSP-area in the dorsal and ventral hippocampal slices between control and kindled groups. PTZ kindling had no significant effect on the fEPSP-slope in dorsal (F(9, 324)=2.415, P=0.0115) and VH (F(9, 279)=2.080, P=0.0314) compared to the control group (Figures 2A, and C). Meanwhile, the fEPSP area significantly increased in the VH of the kindled group compared to the control group (F(1, 370)=13.49, P=0.0003) (Figure 2D). Kindling did not significantly affect the fEPSP-area in dorsal hippocampal slices (Figure 2B).
Effect of kindling on short-term synaptic plasticity
We examined the short-term plasticity of fEPSPs by calculating the paired-pulse ratio for fEPSP-slope or fEPSP-area in dorsal and ventral hippocampal slices of control and kindled groups. fEPSPs were evoked by stimulating the slices at test pulse intensity at inter-pulse intervals of 20, 80, and 160 ms. Test pulse intensities used to evoke fEPSPs in dorsal hippocampal slices in control and kindled groups were 61.0±2.7 and 58.8±5.2 µA, respectively. In ventral hippocampal slices, test pulse intensity was 59.02±2.6 µA in the control group and 56.5±3.9 µA in the kindled group. There was no significant difference in test pulse intensity between the control and kindled groups.
In the control group, there was a significant difference in paired-pulse facilitation of fEPSP-slope between dorsal and ventral hippocampal slices only at an inter-pulse interval of 20 ms (P=0.0194) (Figure 3B). When paired-pulse ratio was calculated for fEPSP-area, significant difference was observed in paired-pulse ratio (here as paired-pulse depression) between dorsal and VH of control slices at inter pulse-interval of 20 ms (P=0.0086) (Figure 3C). However, at longer inter pulse-intervals (80 and 160 ms), paired-pulse facilitation was observed in both dorsal and VH of control slices. There was a significant difference at inter-pulse intervals of 80 ms (P=0.0199) and 160 ms (P=0.0020) between dorsal and ventral hippocampal slices in the control group (Figure 3C).
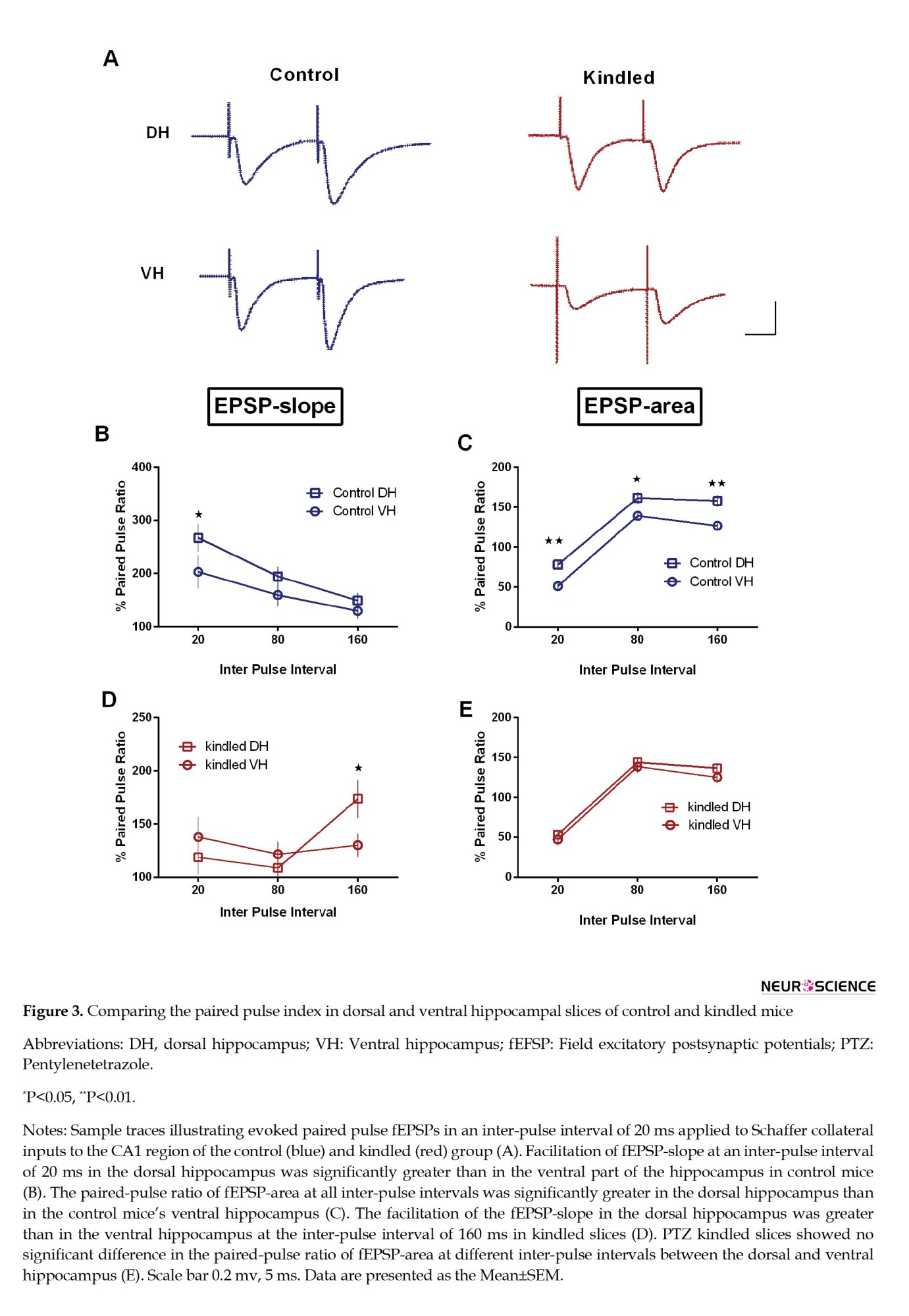
To find out the effect of PTZ kindling on short-term plasticity, we calculated the paired-pulse ratio for fEPSP-slope and fEPSP-area in dorsal and ventral hippocampal slices of PTZ kindled animals and compared them with the control group. Obtained results showed higher facilitation at an inter-pulse interval of 160 ms in the dorsal compared to the VH following PTZ kindling (P=0.05). At inter-pulse intervals of 20 and 80 ms, no significant difference was seen between the dorsal and VH in the fEPSP-slope (Figure 3D). Comparing the paired-pulse ratio for fEPSP-area at all inter-pulse intervals showed no difference between dorsal and VH in kindled slices (Figure 3E).
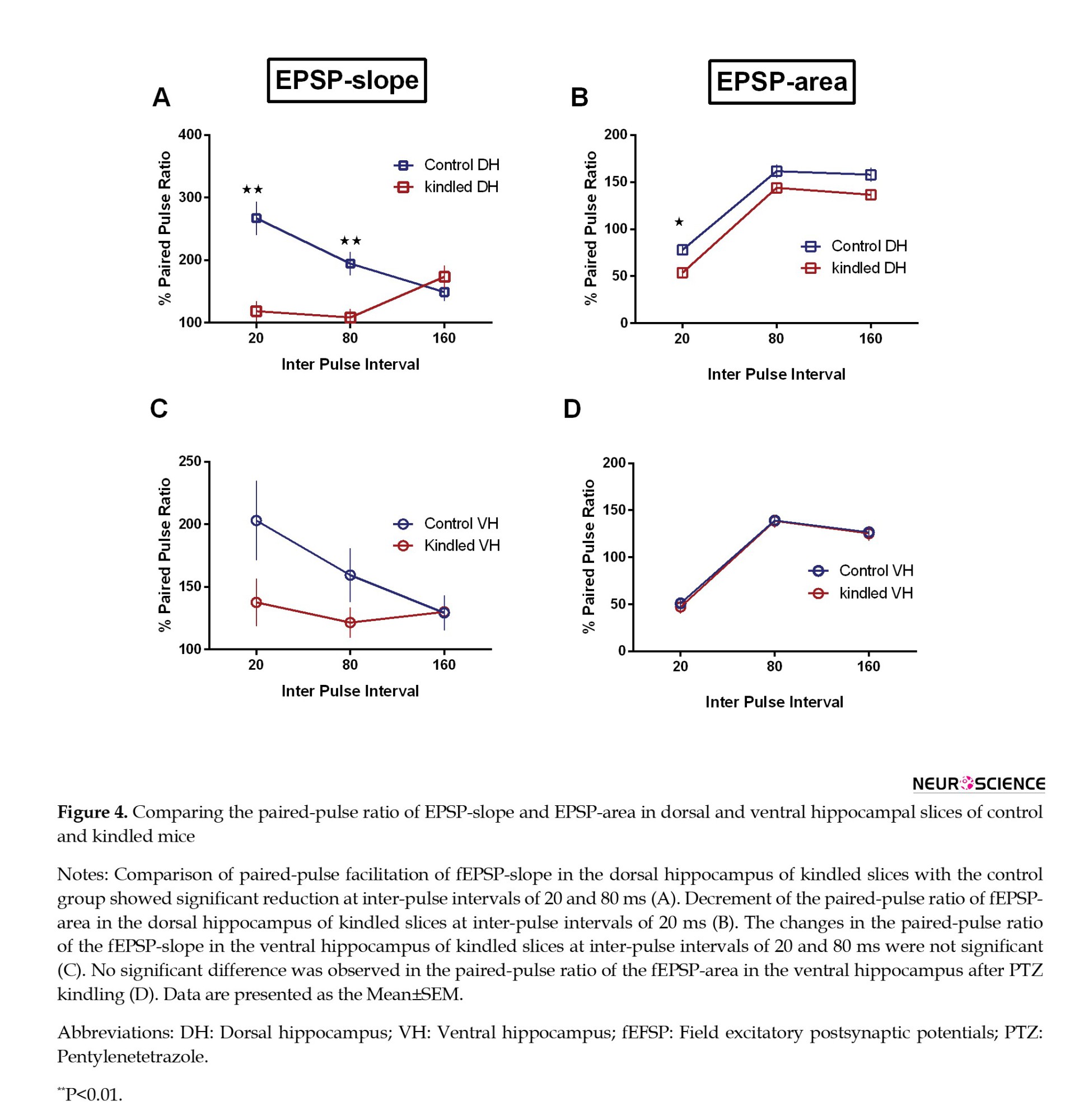
In comparing the slices of the control and kindled groups, a decrease in paired-pulse facilitation of fEPSP-slope was seen at inter-pulse intervals of 20 ms (P=0.002) and 80 ms (P=0.0062) in dorsal hippocampal slices of the kindled group. In addition, the paired-pulse ratio for the fEPSP-area was significantly decreased in dorsal hippocampal slices of kindled group only at an inter-pulse interval of 20 ms (P=0.0195) (Figures 4A and B). Kindling had no significant effect on the paired-pulse ratio in ventral hippocampal slices (Figures 4C and D). Like the control group, paired-pulse facilitation of fEPSP-area was observed at inter-pulse intervals of 80 and 160 ms in both dorsal and ventral slices of kindled mice.
4. Discussion
Obtained data showed that similar to previous reports (Fanselow & Dong, 2010; Dubovyk & Manahan‐Vaughan, 2018; Papatheodoropoulos, 2018), the neural excitability was higher in ventral compared to the DH in both control and kindled animals. Kindling-induced seizure significantly increased the excitability in the ventral but not in the dorsal hippocampal CA1 area. However, the significant effect of kindling on short-term plasticity was observed only in the DH.
Comparing fEPSP-slope and fEPSP-area between dorsal and ventral hippocampal slices following PTZ kindling
Our results indicated that changes in the slope and the area of fEPSPs are different in dorsal and ventral hippocampal slices of control and kindled groups. Paired-pulse facilitation or depression is usually assessed by measuring the slope of the fEPSP. It is generally assumed that the facilitation or depression of the fast, early phase of fEPSP slope results from presynaptic mechanisms (Zucker & Regehr, 2002). Nevertheless, the facilitation or depression of the slow late component of fEPSP (decaying phase of the fEPSP) assessed by measuring the area of the recorded fEPSP appears to require a more complicated interaction between both presynaptic and postsynaptic mechanisms (Davies et al., 1990; Nathan & Lambert, 1991; Davies & Collingridge, 1996). The slope and the area are distinct features of the fEPSP that reflect partially different cellular events. The fEPSP slope mainly depends on the number of activated synapses. In contrast, the fEPSP area depends not only on the activated synapses but also on the slow component of the postsynaptic potential produced by the significant contribution of NMDA receptors (Andersen et al., 1980; Forsythe & Westbrook, 1988; Andreasen et al., 1989; Zucker & Regehr, 2002; Pandis et al., 2006). In the present study, the fEPSP-slope was higher in the ventral than the DH in the control group. This condition may be due to the lower probability of neurotransmitter release in the DH (Papatheodoropoulos, 2018). It should be noted that the control animals showed a dorsoventral gradient of the GABAergic interneuron density along the hippocampus’s longitudinal axis. Therefore, a high density of GABAergic interneurons in the DH may be involved in the observed differences in evoked responses between the dorsal and VH (Czéh et al., 2013).
Interestingly, in the slices of kindled animals, the fEPSP-area was higher in the ventral than the DH. It may be explained according to the effect of seizure on postsynaptic components, mainly glutamate receptors. PTZ facilitates the activity of N-methyl-D-aspartate (NMDA) receptors by blocking the GABAA receptors (Thomsen & Dalby, 1998; Ekonomou & Angelatou, 1999; Zhu et al., 2015). Therefore, the higher effectiveness of PTZ on the fEPSP-area in the VH might be accounted for by the greater contribution of NMDA receptors in the postsynaptic response in the ventral compared to the DH (Pandis et al., 2006). NMDA receptors are more expressed in the VH (Dubovyk & Manahan‐Vaughan, 2018). In addition, NMDA receptors of the dorsal and VH differ in their subunit composition. Higher amounts of the NR2B subunit are expressed in the ventral pole of the hippocampus. These subunits are essential in PTZ kindling and result in prolonged decay time in NMDA receptor-mediated currents (Nabekura et al., 2002; Pandis et al., 2006; Zhu et al., 2015).
Facilitation of EPSP-slope and EPSP-area in DH and VH
We found that PTZ kindled slices displayed a significantly lower magnitude of paired-pulse facilitation of fEPSP-slope compared to the control slices at short inter-pulse intervals (20 ms). The reduction in paired-pulse facilitation was only significant in the dorsal part of the hippocampus. Taking into account that the magnitude of the facilitation is inversely related to the initial probability of release (Katz & Miledi, 1968; Zucker, 1989; Dobrunz & Stevens, 1997), it could be argued that the decrease in paired-pulse facilitation of fEPSP-slope in the kindled group may result from higher release probability following PTZ kindling. The same reduction pattern in paired-pulse facilitation of the fEPSP-slope was observed in the VH; however, it was not statistically significant. This non-significant difference in paired-pulse facilitation of the fEPSP-slope may be because of the masking effect of higher neural excitability of the VH in both the control and kindled group.
It is generally assumed that presynaptic mechanisms that involve a transient increase in the probability of transmitter release underlie the phenomenon of facilitation (Zucker & Regehr, 2002). A complex interaction between several biological and biophysical factors that influence the release of neurotransmitters and the postsynaptic responses may affect the facilitation of EPSP (Dutta Roy et al., 2014). The most generally accepted hypothesis to explain paired-pulse facilitation has been the “residual Ca2+ hypothesis” (Zucker, 1989; Kamiya & Zucker, 1994; Zucker & Regehr, 2002). According to this hypothesis, a portion of Ca2+ that enters during the first stimulus remains in the presynaptic terminal and adds to the calcium influx that occurs in response to the second stimulus, resulting in greater release of transmitter and greater postsynaptic response to the second stimulus. The effect of this transient increase in the intracellular concentration of calcium lasts a few hundred milliseconds. Predictably, the facilitation based on this presynaptic mechanism is maximal at short inter-pulse intervals and decays as the time interval increases between two stimuli.
When we used fEPSP-area in control animals to calculate paired-pulse indices, the changes in these indices differed from what was measured for fEPSP-slope. The paired-pulse index for the fEPSP-area showed facilitation optimally at an inter-pulse interval of 160 ms. In contrast, very low facilitation was observed in the paired-pulse index for the fEPSP-slope at this inter-pulse interval. Because paired-pulse index at an inter-pulse interval of 160 ms can be affected by activation of GABAB receptors, and at inter-pulse interval of 20 ms is modified by GABAA receptors activation (Papatheodoropoulos & Kostopoulos, 2000; Cutsuridis et al., 2010), the different patterns of paired-pulse indices at low (20 ms) and high (160 ms) inter-pulse intervals may be related to the action of these two receptors. FEPSP-slope is a fast phenomenon, while fEPSP-area is a function of both fast and slow components of synaptic responses. Therefore, paired-pulse facilitation measured based on fEPSP-slope or fEPSP-area may be differently affected by GABA receptors at low and high inter-pulse intervals (Papatheodoropoulos, 2018).
We should consider that GABAB receptors (pre- and post-synaptically) may be activated at IPI 160 ms, and their activation results in depression of the postsynaptic inhibition leading to facilitation of the postsynaptic excitatory potential n (Nathan & Lambert, 1991; Davies & Collingridge, 1996; Chalifoux & Carter, 2011). On the other hand, the inter-pulse interval of 160 ms is similar to the frequency of ≈6-7 Hz, i.e. the theta oscillation in the hippocampus (Buzsáki, 2002). These oscillations are associated with learning and memory as well as increased synaptic plasticity (Colgin, 2013), dominate during exploratory movement (Bland, 1986), and stimulation protocols based on the theta frequency are especially effective in inducing LTP in the hippocampal synapses (Larson & Munkácsy, 2015). More studies are needed to characterize the specific mechanisms of action of PTZ and its effects on short-term plasticity of CA1 region in kindled animals.
5. Conclusion
The available evidence indicates dorsoventral/long axis intrinsic diversification/segregation in excitability and neural network in the two poles of the hippocampus and the different sensibility to PTZ kindling, especially in short-term synaptic plasticity. Kindling-induced changes in short-term synaptic plasticity were significant only in dorsal hippocampal slices compared to the control group. The difference in the dorsal and VH responses may be related to the difference in the activity of their GABAergic neurons and, therefore, the changes in their ability to undergo synaptic plasticity. It is highlighted that enhancing our knowledge about the neuronal circuit and microcircuitry and the differences in control and disease conditions like epilepsy will help determine the mechanisms involved in memory and learning impairments of epileptic patients.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This study was supported by a grant from Iranian Cognitive Sciences & Technologies Council (Grant No.: #1848) and a grant from Tarbiat Modares University, Tehran, Iran (Grant No.: #IG-39709).
Authors' contributions
Conceptualization: Yaghoub Fathollahi and Javad Mirnajafi-Zadeh; Methodology: Yaghoub Fathollahi, Amir Shojaei, and Javad Mirnajafi-Zadeh; Investigation: Nahid Roohi and Mahboubeh Ahmadi; Writing the original draft: Nahid Roohi; Review, and editing: Javad Mirnajafi-Zadeh and Amir Shojaei; Supervision and funding acquisition: Javad Mirnajafi-Zadeh.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the Iranian Cognitive Sciences & Technologies Council for the support.
References
Hippocampus is one of the most critical brain structures in learning and memory (Morris, 2007). Hippocampal-dependent synaptic plasticity is involved in some physiological and pathological functions that can be affected/impaired by conditions such as epilepsy (Krug et al., 1997). Epilepsy is one of the most prevalent neurological diseases that influence the patients’ individual and social quality of life and their cognitive functions (Dodrill, 1986; Kwan & Brodie, 2001). The effect of epileptic seizures on short-term and long-term synaptic plasticity can also be observed in chemical kindling (Palizvan et al., 2005), which develops by serial injections of a subconvulsive dose of pentylenetetrazol (PTZ), a GABAA receptor antagonist.
Short- and long-term synaptic plasticity, as the basic mechanisms involved in learning and memory (Martin & Morris, 2002; Morris, 2007), have been studied in the hippocampus. Paired-pulse facilitation or depression is a relatively simple form of short-term synaptic plasticity (Kamiya & Zucker, 1994). They can be observed when two stimuli are delivered to presynaptic afferent fibers at different intervals and the obtained response to the second pulse is higher or lower than the response to the first pulse, respectively. Evaluating the changes in short-term plasticity gives us insights about the activity of neural circuits following seizures and helps in understanding the pathophysiology of epileptic brain.
It is important to note that the characteristics and magnitude of synaptic plasticity in the dorsal and ventral hippocampus (VH) are different (Dougherty et al., 2012; Dubovyk & Manahan‐Vaughan, 2018). Recent experimental data highlight the idea of functional separation along the longitudinal axis of the hippocampus (dorsoventral or septotemporal), with diverse functions such as cognition and emotionality in the dorsal hippocampus (DH) and VH, respectively (Poppenk et al., 2013; Bannerman et al., 2014). This diversification along the longitudinal axis may be due to different extrinsic connectivity of dorsal and ventral hippocampal segments with other brain structures or different local neural circuits in the dorsal and ventral segments of the hippocampus. Based on early and recent electrophysiological and neurochemical studies, the dorsal and VH have different local network organization including principal cell properties, synaptic transmission, neurotransmitter receptors, and synaptic plasticity (for review please see (Papatheodoropoulos, 2018).
Accordingly, it is necessary to determine the seizure-induced changes in short-term synaptic plasticity in the dorsal and ventral parts of the hippocampus better to understand the effect of seizure on cognitive functions. Therefore, in the present study, we examined the changes in paired-pulse facilitation of field excitatory postsynaptic potentials (fEPSPs) recorded from the CA1 region in DH and VH following PTZ kindling in mice. We were curious to find out whether PTZ kindling has different effects on the two poles of the hippocampus.
2. Materials and Methods
Study animals
All experiments and manipulations conformed to the guidelines set by the animal care commission of the Faculty of Medical Sciences, Tarbiat Modares University, Iran. B6/J male mice (10-12 weeks old) from Pasteur Institute (Iran) were used in the experiments. Animals consisted of 9 kindled and 10 control mice. Before engaging in any experimental paradigms, the mice were given one week to acclimate to the facility, and then PTZ or saline injections were done every other day. Mice were maintained on a normal light cycle with access to Purina mouse chow and sterile water ad libitum throughout the study.
PTZ kindling
For kindling, a sub-threshold dose of 35 mg/kg PTZ was injected intraperitoneally (10 µL/g, IP) every 48 h. Following injection, the convulsive behavior of each subject was observed for 20 min. PTZ was freshly prepared in a sterile isotonic saline before injections. Control mice were injected with the identical saline solution without PTZ. The resultant seizures were classified as follows: stage 0, no response; stage 1, ear and facial twitching; stage 2, convulsive waves axially through the body; stage 3, myoclonic body jerk; stage 4, turn over into side position, tonic-clonic seizures; and stage 5, turn over into back position, generalized tonic-clonic seizures (Dhir, 2012). All animals behaviorally showed three consecutive stages, 4 or 5, to be considered fully kindled.
Field potential recording in the hippocampal slices
Twenty-four hours after the last PTZ or saline injection, the mice were anesthetized with carbon dioxide (CO2) and decapitated. The brain was carefully transferred into a chamber containing chilled artificial cerebrospinal fluid (aCSF) bubbled by carbogen (95% O2 and 5% CO2). The aCSF contained (in mM) NaCl 124, NaHCO3 26, KH2PO4 1.25, KCl 5, CaCl2 2, MgCl2 2.06, and D-glucose 10 and its pH was 7.3-7.4. Then, the right hemisphere of the brain was dissected. Transverse hippocampal slices were sectioned (400 μm thick) using a vibratome (VT 1200, Leica, Germany). The slices were placed in a recovery chamber filled with aCSF for at least 60 min at room temperature. Then, they moved to an interface-type recording chamber and maintained at 32 oC, at the interface between aCSF and warm, humidified oxygen gas. Slices were perfused at 1 mL/min with aCSF, which was aerated continuously with carbogen. The slices were allowed to incubate in this medium for 30 min before recording.
Field potentials were recorded from the CA1 stratum radiatum in the dorsal and VH. Stimulating electrodes (stainless steel, Teflon coated, A-M Systems, US) and recording electrodes (borosilicate glass, O.D.: 1.5 mm, I.D.: 0.86 mm, Sutter instrument, USA) were placed on the same layer. A reference electrode was also placed in the recording chamber. After electrode implantation, square stimulating pulses (200 μs, 0.1 Hz) were applied through a bipolar stimulating electrode by an isolator and constant current unit apparatus (S403J, Nihon-Kohden, Japan). Since the stimulation strength required to evoke fEPSP of a given magnitude can vary from experiment to experiment, we made a normalized scale for stimulation intensity. The full scale of stimulation intensities was divided into 10 steps according to the stimulation intensities required to elicit minimum and maximum fEPSP-slope. Extracellular potentials were recorded using a glass recording electrode (2-5 MΩ) filled with aCSF.
Signals were transferred to an amplifier (ME208300, Nihon-Kohden, Japan) through the recording electrode and visualized by custom-made software (Potentalize; ScienceBeam Co., Iran). The input/output curve was plotted to determine the test pulse intensity. To record paired-pulse responses, test pulse intensity was used throughout the experiments. Paired-pulse stimulations were made at the inter-pulse intervals of 20, 80, and 160 ms.
Statistical analysis
fEPSP parameters were analyzed using repeated measures 2-way ANOVA followed by Sidak multiple comparison test, paired-pulse data were analyzed unpaired t student test. The values are expressed as Mean±SEM of the mean. The number of slices (n) used in the control group was n=8 in dorsal hippocampal slices and n=7 in ventral hippocampal slices. In kindled group, n=5 in dorsal hippocampal slices and n=6 in ventral hippocampal slices. In each experimental group, all slices were taken from 3 mice.
Paired-pulse responses were quantified as the ratio of the second pulse evoked fEPSP to the first. All statistical analyses were conducted using GraphPad Prism software, version 7.03. To be considered significant, P<0.05 were used.
3. Results
PTZ kindling procedure
Subjects were handled and acclimatized for at least one week, and intraperitoneal PTZ injections were done every other day (saline was injected instead of PTZ in the control group). Approximately 80% of animals fulfilled the kindling criteria after 7-10 injections. The mean number of injections was 6.3±2.
Effect of kindling on single-pulse evoked fEPSPs
fEPSPs evoked at test pulse stimulation intensity were recorded from the CA1 area of dorsal and ventral hippocampal slices (Figure 1A). In the first step, we compared the minimum and maximum stimulus intensities needed for evoking fEPSP. Obtained data from whole slices (dorsal and VH) showed that the control group’s minimum and maximum stimulation intensities were 20.6±1.1 and 124.6±4.2 µA. Similar data were achieved in the kindled group so that the minimum intensity was 21.11±3.3 µA and the maximum intensity was 118.35±23.4 µA (n=18). More specifically, the minimum and maximum stimulation values for dorsal hippocampal slices in the control group were 20.7±0.7 and 127.1±3.8 µA (n=22), respectively, and in kindled group were 21.1±1.1 and 121.1±9.2 µA (n=9). The corresponding values for the ventral control slices were 20.6±0.4 and 122.1±5 µA (n=22), and for the ventral kindled slices were 21.1±1.1 and 115.6±6 µA (n=9). These data showed no significant difference in minimum and maximum stimulus intensities between control and kindled slices in the dorsal and VH.
Then, input/output curves were plotted using arbitrary units. From input/output curves, we found that in relatively high intensities (numbered 8-10), the fEPSP-slope in ventral hippocampal slices was greater than the fEPSP-slope in dorsal hippocampal slices in the control group (F(9, 351)=9.713, P<0.0001). However, there was no significant difference in fEPSP-area between dorsal and ventral hippocampal slices at different stimulation intensities in the control group (F(9, 522)=0.3849, P=0.9424) (Figure 1B). In the kindled group, the differences in input/output curves between dorsal and ventral hippocampal slices were opposite to what observed in control slices, i.e. in PTZ kindled animals, there was no significant difference in fEPSP-slope between dorsal and ventral parts of the hippocampal slices (F(9, 280)=0.07886, P=0.9999). Still, a significant difference was observed in the fEPSP-area between the two regions. There was a greater fEPSP-area in the ventral than dorsal hippocampal slices in the kindled group (F(9, 135)=2.008, P=0.0429) (Figure 1C).

In the next step, we compared the input/output curves of fEPSP-slope and fEPSP-area in the dorsal and ventral hippocampal slices between control and kindled groups. PTZ kindling had no significant effect on the fEPSP-slope in dorsal (F(9, 324)=2.415, P=0.0115) and VH (F(9, 279)=2.080, P=0.0314) compared to the control group (Figures 2A, and C). Meanwhile, the fEPSP area significantly increased in the VH of the kindled group compared to the control group (F(1, 370)=13.49, P=0.0003) (Figure 2D). Kindling did not significantly affect the fEPSP-area in dorsal hippocampal slices (Figure 2B).

Effect of kindling on short-term synaptic plasticity
We examined the short-term plasticity of fEPSPs by calculating the paired-pulse ratio for fEPSP-slope or fEPSP-area in dorsal and ventral hippocampal slices of control and kindled groups. fEPSPs were evoked by stimulating the slices at test pulse intensity at inter-pulse intervals of 20, 80, and 160 ms. Test pulse intensities used to evoke fEPSPs in dorsal hippocampal slices in control and kindled groups were 61.0±2.7 and 58.8±5.2 µA, respectively. In ventral hippocampal slices, test pulse intensity was 59.02±2.6 µA in the control group and 56.5±3.9 µA in the kindled group. There was no significant difference in test pulse intensity between the control and kindled groups.
In the control group, there was a significant difference in paired-pulse facilitation of fEPSP-slope between dorsal and ventral hippocampal slices only at an inter-pulse interval of 20 ms (P=0.0194) (Figure 3B). When paired-pulse ratio was calculated for fEPSP-area, significant difference was observed in paired-pulse ratio (here as paired-pulse depression) between dorsal and VH of control slices at inter pulse-interval of 20 ms (P=0.0086) (Figure 3C). However, at longer inter pulse-intervals (80 and 160 ms), paired-pulse facilitation was observed in both dorsal and VH of control slices. There was a significant difference at inter-pulse intervals of 80 ms (P=0.0199) and 160 ms (P=0.0020) between dorsal and ventral hippocampal slices in the control group (Figure 3C).

To find out the effect of PTZ kindling on short-term plasticity, we calculated the paired-pulse ratio for fEPSP-slope and fEPSP-area in dorsal and ventral hippocampal slices of PTZ kindled animals and compared them with the control group. Obtained results showed higher facilitation at an inter-pulse interval of 160 ms in the dorsal compared to the VH following PTZ kindling (P=0.05). At inter-pulse intervals of 20 and 80 ms, no significant difference was seen between the dorsal and VH in the fEPSP-slope (Figure 3D). Comparing the paired-pulse ratio for fEPSP-area at all inter-pulse intervals showed no difference between dorsal and VH in kindled slices (Figure 3E).
In comparing the slices of the control and kindled groups, a decrease in paired-pulse facilitation of fEPSP-slope was seen at inter-pulse intervals of 20 ms (P=0.002) and 80 ms (P=0.0062) in dorsal hippocampal slices of the kindled group. In addition, the paired-pulse ratio for the fEPSP-area was significantly decreased in dorsal hippocampal slices of kindled group only at an inter-pulse interval of 20 ms (P=0.0195) (Figures 4A and B). Kindling had no significant effect on the paired-pulse ratio in ventral hippocampal slices (Figures 4C and D). Like the control group, paired-pulse facilitation of fEPSP-area was observed at inter-pulse intervals of 80 and 160 ms in both dorsal and ventral slices of kindled mice.

4. Discussion
Obtained data showed that similar to previous reports (Fanselow & Dong, 2010; Dubovyk & Manahan‐Vaughan, 2018; Papatheodoropoulos, 2018), the neural excitability was higher in ventral compared to the DH in both control and kindled animals. Kindling-induced seizure significantly increased the excitability in the ventral but not in the dorsal hippocampal CA1 area. However, the significant effect of kindling on short-term plasticity was observed only in the DH.
Comparing fEPSP-slope and fEPSP-area between dorsal and ventral hippocampal slices following PTZ kindling
Our results indicated that changes in the slope and the area of fEPSPs are different in dorsal and ventral hippocampal slices of control and kindled groups. Paired-pulse facilitation or depression is usually assessed by measuring the slope of the fEPSP. It is generally assumed that the facilitation or depression of the fast, early phase of fEPSP slope results from presynaptic mechanisms (Zucker & Regehr, 2002). Nevertheless, the facilitation or depression of the slow late component of fEPSP (decaying phase of the fEPSP) assessed by measuring the area of the recorded fEPSP appears to require a more complicated interaction between both presynaptic and postsynaptic mechanisms (Davies et al., 1990; Nathan & Lambert, 1991; Davies & Collingridge, 1996). The slope and the area are distinct features of the fEPSP that reflect partially different cellular events. The fEPSP slope mainly depends on the number of activated synapses. In contrast, the fEPSP area depends not only on the activated synapses but also on the slow component of the postsynaptic potential produced by the significant contribution of NMDA receptors (Andersen et al., 1980; Forsythe & Westbrook, 1988; Andreasen et al., 1989; Zucker & Regehr, 2002; Pandis et al., 2006). In the present study, the fEPSP-slope was higher in the ventral than the DH in the control group. This condition may be due to the lower probability of neurotransmitter release in the DH (Papatheodoropoulos, 2018). It should be noted that the control animals showed a dorsoventral gradient of the GABAergic interneuron density along the hippocampus’s longitudinal axis. Therefore, a high density of GABAergic interneurons in the DH may be involved in the observed differences in evoked responses between the dorsal and VH (Czéh et al., 2013).
Interestingly, in the slices of kindled animals, the fEPSP-area was higher in the ventral than the DH. It may be explained according to the effect of seizure on postsynaptic components, mainly glutamate receptors. PTZ facilitates the activity of N-methyl-D-aspartate (NMDA) receptors by blocking the GABAA receptors (Thomsen & Dalby, 1998; Ekonomou & Angelatou, 1999; Zhu et al., 2015). Therefore, the higher effectiveness of PTZ on the fEPSP-area in the VH might be accounted for by the greater contribution of NMDA receptors in the postsynaptic response in the ventral compared to the DH (Pandis et al., 2006). NMDA receptors are more expressed in the VH (Dubovyk & Manahan‐Vaughan, 2018). In addition, NMDA receptors of the dorsal and VH differ in their subunit composition. Higher amounts of the NR2B subunit are expressed in the ventral pole of the hippocampus. These subunits are essential in PTZ kindling and result in prolonged decay time in NMDA receptor-mediated currents (Nabekura et al., 2002; Pandis et al., 2006; Zhu et al., 2015).
Facilitation of EPSP-slope and EPSP-area in DH and VH
We found that PTZ kindled slices displayed a significantly lower magnitude of paired-pulse facilitation of fEPSP-slope compared to the control slices at short inter-pulse intervals (20 ms). The reduction in paired-pulse facilitation was only significant in the dorsal part of the hippocampus. Taking into account that the magnitude of the facilitation is inversely related to the initial probability of release (Katz & Miledi, 1968; Zucker, 1989; Dobrunz & Stevens, 1997), it could be argued that the decrease in paired-pulse facilitation of fEPSP-slope in the kindled group may result from higher release probability following PTZ kindling. The same reduction pattern in paired-pulse facilitation of the fEPSP-slope was observed in the VH; however, it was not statistically significant. This non-significant difference in paired-pulse facilitation of the fEPSP-slope may be because of the masking effect of higher neural excitability of the VH in both the control and kindled group.
It is generally assumed that presynaptic mechanisms that involve a transient increase in the probability of transmitter release underlie the phenomenon of facilitation (Zucker & Regehr, 2002). A complex interaction between several biological and biophysical factors that influence the release of neurotransmitters and the postsynaptic responses may affect the facilitation of EPSP (Dutta Roy et al., 2014). The most generally accepted hypothesis to explain paired-pulse facilitation has been the “residual Ca2+ hypothesis” (Zucker, 1989; Kamiya & Zucker, 1994; Zucker & Regehr, 2002). According to this hypothesis, a portion of Ca2+ that enters during the first stimulus remains in the presynaptic terminal and adds to the calcium influx that occurs in response to the second stimulus, resulting in greater release of transmitter and greater postsynaptic response to the second stimulus. The effect of this transient increase in the intracellular concentration of calcium lasts a few hundred milliseconds. Predictably, the facilitation based on this presynaptic mechanism is maximal at short inter-pulse intervals and decays as the time interval increases between two stimuli.
When we used fEPSP-area in control animals to calculate paired-pulse indices, the changes in these indices differed from what was measured for fEPSP-slope. The paired-pulse index for the fEPSP-area showed facilitation optimally at an inter-pulse interval of 160 ms. In contrast, very low facilitation was observed in the paired-pulse index for the fEPSP-slope at this inter-pulse interval. Because paired-pulse index at an inter-pulse interval of 160 ms can be affected by activation of GABAB receptors, and at inter-pulse interval of 20 ms is modified by GABAA receptors activation (Papatheodoropoulos & Kostopoulos, 2000; Cutsuridis et al., 2010), the different patterns of paired-pulse indices at low (20 ms) and high (160 ms) inter-pulse intervals may be related to the action of these two receptors. FEPSP-slope is a fast phenomenon, while fEPSP-area is a function of both fast and slow components of synaptic responses. Therefore, paired-pulse facilitation measured based on fEPSP-slope or fEPSP-area may be differently affected by GABA receptors at low and high inter-pulse intervals (Papatheodoropoulos, 2018).
We should consider that GABAB receptors (pre- and post-synaptically) may be activated at IPI 160 ms, and their activation results in depression of the postsynaptic inhibition leading to facilitation of the postsynaptic excitatory potential n (Nathan & Lambert, 1991; Davies & Collingridge, 1996; Chalifoux & Carter, 2011). On the other hand, the inter-pulse interval of 160 ms is similar to the frequency of ≈6-7 Hz, i.e. the theta oscillation in the hippocampus (Buzsáki, 2002). These oscillations are associated with learning and memory as well as increased synaptic plasticity (Colgin, 2013), dominate during exploratory movement (Bland, 1986), and stimulation protocols based on the theta frequency are especially effective in inducing LTP in the hippocampal synapses (Larson & Munkácsy, 2015). More studies are needed to characterize the specific mechanisms of action of PTZ and its effects on short-term plasticity of CA1 region in kindled animals.
5. Conclusion
The available evidence indicates dorsoventral/long axis intrinsic diversification/segregation in excitability and neural network in the two poles of the hippocampus and the different sensibility to PTZ kindling, especially in short-term synaptic plasticity. Kindling-induced changes in short-term synaptic plasticity were significant only in dorsal hippocampal slices compared to the control group. The difference in the dorsal and VH responses may be related to the difference in the activity of their GABAergic neurons and, therefore, the changes in their ability to undergo synaptic plasticity. It is highlighted that enhancing our knowledge about the neuronal circuit and microcircuitry and the differences in control and disease conditions like epilepsy will help determine the mechanisms involved in memory and learning impairments of epileptic patients.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This study was supported by a grant from Iranian Cognitive Sciences & Technologies Council (Grant No.: #1848) and a grant from Tarbiat Modares University, Tehran, Iran (Grant No.: #IG-39709).
Authors' contributions
Conceptualization: Yaghoub Fathollahi and Javad Mirnajafi-Zadeh; Methodology: Yaghoub Fathollahi, Amir Shojaei, and Javad Mirnajafi-Zadeh; Investigation: Nahid Roohi and Mahboubeh Ahmadi; Writing the original draft: Nahid Roohi; Review, and editing: Javad Mirnajafi-Zadeh and Amir Shojaei; Supervision and funding acquisition: Javad Mirnajafi-Zadeh.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the Iranian Cognitive Sciences & Technologies Council for the support.
References
Andersen, P., Silfvenius, H., Sundberg, S. H., & Sveen, O. (1980). A comparison of distal and proximal dendritic synapses on CA1 pyramids in guinea-pig hippocampal slices in vitro. The Journal of Physiology, 307, 273–299. [DOI:10.1113/jphysiol.1980.sp013435] [PMID] [PMCID]
Andreasen, M., Lambert, J. D., & Jensen, M. S. (1989). Effects of new non-N-methyl-D-aspartate antagonists on synaptic transmission in the in vitro rat hippocampus. The Journal of Physiology, 414, 317-336. [DOI:10.1113/jphysiol.1989.sp017690] [PMID] [PMCID]
Bannerman, D. M., Sprengel, R., Sanderson, D. J., McHugh, S. B., Rawlins, J. N., & Monyer, H., et al. (2014) Hippocampal synaptic plasticity, spatial memory and anxiety. Nature Reviews. Neuroscience, 15(3), 181–192. [DOI:10.1038/nrn3677] [PMID]
Bland, B. H. (1986). The physiology and pharmacology of hippocampal formation theta rhythms. Progress in Neurobiology, 26(1), 1–54. [DOI:10.1016/0301-0082(86)90019-5] [PMID]
Buzsáki, G. (2002). Theta oscillations in the hippocampus. Neuron, 33(3), 325–340. [DOI:10.1016/S0896-6273(02)00586-X] [PMID]
Chalifoux, J. R., & Carter, A. G. (2011). GABAB receptor modulation of synaptic function. Current Opinion in Neurobiology, 21(2), 339–344. [DOI:10.1016/j.conb.2011.02.004] [PMID] [PMCID]
Colgin, L. L. (2013). Mechanisms and functions of theta rhythms. Annual Review of Neuroscience, 36, 295–312. [DOI:10.1146/annurev-neuro-062012-170330] [PMID]
Cutsuridis, V., Graham, B., Cobb, S., & Vida I. (2010). Hippocampal microcircuits: A Computational Modeler's Resource Book. Berlin: Springer. [DOI:10.1007/978-1-4419-0996-1]
Czéh, B., Abrahám, H., Tahtakran, S., Houser, C. R., & Seress, L. (2013). Number and regional distribution of GAD65 mRNA-expressing interneurons in the rat hippocampal formation. Acta Biologica Hungarica, 64(4), 395–413. [DOI:10.1556/ABiol.64.2013.4.1] [PMID]
Davies, C. H., & Collingridge, G. L. (1996). Regulation of EPSPs by the synaptic activation of GABAB autoreceptors in rat hippocampus. The Journal of Physiology, 496(Pt 2)(Pt 2), 451–470.[DOI:10.1113/jphysiol.1996.sp021698] [PMID] [PMCID]
Davies, C. H., Davies, S. N., & Collingridge, G. L. (1990). Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. The Journal of Physiology, 424, 513-531. [DOI:10.1113/jphysiol.1990.sp018080] [PMID] [PMCID]
Dhir, A. (2012). Pentylenetetrazol (PTZ) kindling model of epilepsy. Current Protocols in Neuroscience, Chapter 9, Unit9.37.[DOI:10.1002/0471142301.ns0937s58] [PMID]
Dobrunz, L. E., & Stevens, C. F. (1997). Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron, 18(6), 995–1008. [DOI:10.1016/S0896-6273(00)80338-4] [PMID]
Dodrill, C. B. (1986). Correlates of generalized tonicclonic seizures with intellectual, neuropsychological, emotional, and social function in patients with epilepsy. Epilepsia, 27(4), 399–411. [DOI:10.1111/j.1528-1157.1986.tb03559.x] [PMID]
Dougherty, K. A., Islam, T., & Johnston, D. (2012). Intrinsic excitability of CA1 pyramidal neurones from the rat dorsal and ventral hippocampus. The Journal of Physiology, 590(22), 5707–5722. [DOI:10.1113/jphysiol.2012.242693] [PMID] [PMCID]
Dubovyk, V., & Manahan-Vaughan, D. (2018). Less means more: The magnitude of synaptic plasticity along the hippocampal dorso-ventral axis is inversely related to the expression levels of plasticity-related neurotransmitter receptors. Hippocampus, 28(2), 136–150. [DOI:10.1002/hipo.22816] [PMID] [PMCID]
Dutta Roy, R., Stefan, M. I., & Rosenmund, C. (2014). Biophysical properties of presynaptic short-term plasticity in hippocampal neurons: Insights from electrophysiology, imaging and mechanistic models. Frontiers in Cellular Neuroscience, 8, 141. [DOI:10.3389/fncel.2014.00141] [PMID] [PMCID]
Ekonomou, A., & Angelatou, F. (1999). Upregulation of NMDA receptors in hippocampus and cortex in the pentylenetetrazol-induced “kindling” model of epilepsy. Neurochemical Research, 24(12), 1515–1522. [DOI:10.1023/A:1021143813935] [PMID]
Fanselow, M. S., & Dong, H. W. (2010). Are the dorsal and ventral hippocampus functionally distinct structures? Neuron, 65(1), 7–19. [DOI:10.1016/j.neuron.2009.11.031] [PMID] [PMCID]
Forsythe, I. D., & Westbrook, G. L. (1988). Slow excitatory postsynaptic currents mediated by N-methyl-D-aspartate receptors on cultured mouse central neurones. The Journal of Physiology, 396, 515-533. [DOI:10.1113/jphysiol.1988.sp016975] [PMID] [PMCID]
Kamiya, H., & Zucker, R. S. (1994). Residual Ca 2+ and short-term synaptic plasticity. Nature, 371(6498), 603–606.[DOI:10.1038/371603a0] [PMID]
Katz, B., & Miledi, R. (1968).The role of calcium in neuromuscular facilitation. The Journal of Physiology, 195(2), 481–492.[DOI:10.1113/jphysiol.1968.sp008469] [PMID] [PMCID]
Krug, M., Koch, M., Grecksch, G., & Schulzeck, K. (1997). Pentylenetetrazol kindling changes the ability to induce potentiation phenomena in the hippocampal CA1 region. Physiology & Behavior, 62(4), 721–727. [DOI:10.1016/S0031-9384(97)00167-4] [PMID]
Kwan, P., & Brodie, M. J. (2001). Neuropsychological effects of epilepsy and antiepileptic drugs. Lancet, 357(9251), 216–222.[DOI:10.1016/S0140-6736(00)03600-X] [PMID]
Larson, J., & Munkácsy, E. (2015). Theta-burst LTP. Brain Research, 1621, 38-50. [DOI:10.1016/j.brainres.2014.10.034] [PMID] [PMCID]
Martin, S. J., & Morris, R. G. (2002). New life in an old idea: The synaptic plasticity and memory hypothesis revisited. Hippocampus, 12(5), 609–636. [DOI:10.1002/hipo.10107] [PMID]
Morris, R. (2007). Theories of hippocampal function. In P. Andersen, R. Morris, D. Amaral, T. Bliss & J. O'Keefe (Eds.), The hippocampus book (pp. 581–714). Oxford: Oxford University Press. [Link]
Nabekura, J., Ueno, T., Katsurabayashi, S., Furuta, A., Akaike, N., & Okada, M. (2002). Reduced NR2A expression and prolonged decay of NMDA receptor-mediated synaptic current in rat vagal motoneurons following axotomy. The Journal of Physiology, 539(Pt 3), 735–741. [DOI:10.1113/jphysiol.2001.013379] [PMID] [PMCID]
Nathan, T., & Lambert, J. D. (1991). Depression of the fast IPSP underlies paired-pulse facilitation in area CA1 of the rat hippocampus. Journal of Neurophysiology, 66(5), 1704–1715.[DOI:10.1152/jn.1991.66.5.1704] [PMID]
Palizvan, M. R., Fathollahi, Y., & Semnanian, S. (2005). Epileptogenic insult causes a shift in the form of long-term potentiation expression. Neuroscience, 134(2), 415–423. [DOI:10.1016/j.neuroscience.2005.04.016] [PMID]
Pandis, C., Sotiriou, E., Kouvaras, E., Asprodini, E., Papatheodoropoulos, C., & Angelatou, F. (2006). Differential expression of NMDA and AMPA receptor subunits in rat dorsal and ventral hippocampus. Neuroscience, 140(1), 163–175. [DOI:10.1016/j.neuroscience.2006.02.003] [PMID]
Papatheodoropoulos, C., & Kostopoulos, G. (2000). Dorsal-ventral differentiation of short-term synaptic plasticity in rat CA1 hippocampal region. Neuroscience Letters, 286(1), 57–60. [DOI:10.1016/S0304-3940(00)01084-3] [PMID]
Papatheodoropoulos, C. (2018). Electrophysiological evidence for long-axis intrinsic diversification of the hippocampus. Frontiers in Bioscience (Landmark edition), 23(1), 109–145. [DOI:10.2741/4584] [PMID]
Poppenk, J., Evensmoen, H. R., Moscovitch, M., & Nadel, L. (2013). Long-axis specialization of the human hippocampus. Trends in Cognitive Sciences, 17(5), 230–240. [DOI:10.1016/j.tics.2013.03.005] [PMID]
Thomsen, C., & Dalby, N. O. (1998). Roles of metabotropic glutamate receptor subtypes in modulation of pentylenetetrazole-induced seizure activity in mice. Neuropharmacology, 37(12), 1465–1473. [DOI:10.1016/S0028-3908(98)00138-5] [PMID]
Zhu, X., Dong, J., Shen, K., Bai, Y., Zhang, Y., & Lv, X., et al. (2015). NMDA receptor NR2B subunits contribute to PTZ-kindling-induced hippocampal astrocytosis and oxidative stress. Brain Research Bulletin, 114, 70–78. [DOI:10.1016/j.brainresbull.2015.04.002] [PMID]
Type of Study: Original |
Subject:
Cognitive Neuroscience
Received: 2020/05/21 | Accepted: 2020/08/9 | Published: 2024/09/1
Received: 2020/05/21 | Accepted: 2020/08/9 | Published: 2024/09/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








