Volume 11, Issue 2 (March & April - Special Issue on COVID-19 2020)
BCN 2020, 11(2): 179-184 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ramezani M, Simani L, Karimialavijeh E, Rezaei O, Hajiesmaeili M, Pakdaman H. The Role of Anxiety and Cortisol in Outcomes of Patients With Covid-19. BCN 2020; 11 (2) :179-184
URL: http://bcn.iums.ac.ir/article-1-1771-en.html
URL: http://bcn.iums.ac.ir/article-1-1771-en.html
Mahtab Ramezani1 

 , Leila Simani *1
, Leila Simani *1 

 , Ehsan Karimialavijeh2
, Ehsan Karimialavijeh2 
 , Omidvar Rezaei1
, Omidvar Rezaei1 

 , Mohammadreza Hajiesmaeili3
, Mohammadreza Hajiesmaeili3 

 , Hossein Pakdaman4
, Hossein Pakdaman4 



 , Leila Simani *1
, Leila Simani *1 

 , Ehsan Karimialavijeh2
, Ehsan Karimialavijeh2 
 , Omidvar Rezaei1
, Omidvar Rezaei1 

 , Mohammadreza Hajiesmaeili3
, Mohammadreza Hajiesmaeili3 

 , Hossein Pakdaman4
, Hossein Pakdaman4 

1- Skull-Base Research Center, Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Department of Emergency Medicine, Sina Hospital, Tehran University of Medical Sciences, Tehran, Iran.
3- Anesthesiology Research Center, Loghman Hakim Medical Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4- Brain Mapping Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Department of Emergency Medicine, Sina Hospital, Tehran University of Medical Sciences, Tehran, Iran.
3- Anesthesiology Research Center, Loghman Hakim Medical Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4- Brain Mapping Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 590 kb]
| Abstract (HTML)
2.1. Statistical analysis
SSPS version 16.0 (SPSS, Inc., Chicago, IL, USA) was used for data analysis. A Chi-square test was applied for demographic variables, and the independent t-test was used to compare numeric data. To assess the differences between the surviving and dead patients in cortisol level and HADS score, we performed a Two-Way Analysis Of Variance (ANOVA) using the factors “outcome” and “comorbidity”. A correlation between HADS score and serum levels of cortisol was assessed by the Pearson correlation test and the results were presented as the mean±Standard Deviation (SD). A P-value < 0.05 was considered significant.
3. Results
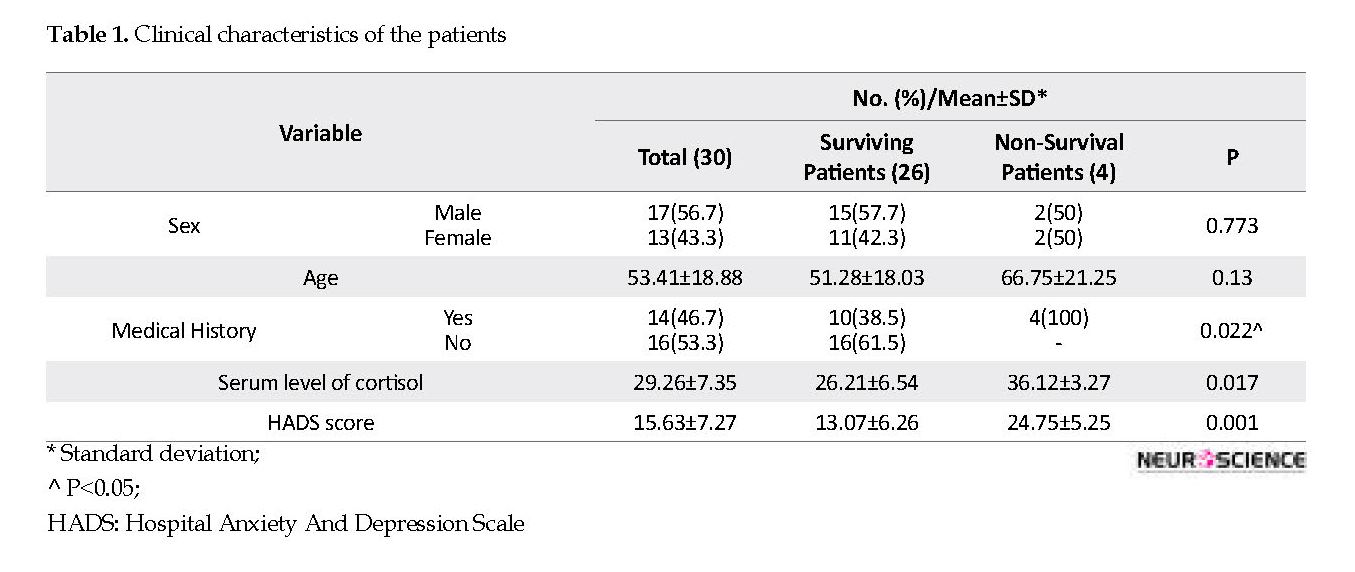
A total of 30 patients with COVID-19, 17 (56.7%) males and 13 (43.3%) females, were enrolled in this research. The mean age of the patients was 53.41±18.88 years. The serum level of cortisol and HADS score in COVID-19 patients are depicted in Table 1. Analysis of the HADS score and cortisol revealed a higher score of HADS in non-survival compared with surviving patients (p = 0.001). In addition, a higher serum level of cortisol was found in non-survival patients versus surviving patients (p=0.017).
Based on the two-way ANOVA test results, there was no statistically significant correlation between the outcome and comorbidity (p=0.278), and also no differences were found between the level of cortisol and HADS score (p=0.321). HADS score was found with a significant positive correlation with cortisol level (r; 0.842, P=0.004). There were no significant differences between sex and serum level of cortisol and HADS score (p=0.745 and p=0.912, respectively). Also, no significant correlation was observed between age and HADS score and cortisol level (p=0.684 and p=0.598, respectively).
4. Discussion
Our results revealed that although all participants had a mild to moderate presentation of COVID-19, patients with fatal outcome had higher serum cortisol levels and HADS score compared with the survived ones. This finding supports our primary hypothesis about the effect of stress and anxiety on the patient’s vulnerability to the infection.
The COVID-19 pandemic has increased concerns of widespread panic and anxiety (Haleem, Javaid, & Vaishya, 2020). Our lifestyle and habits are shifting dramatically, and COVID-19 pandemic has influenced all aspects of our lives (Kim & Su, 2020). Unlike other viruses, like influenza viruses and other infections, media has overemphasized the COVID-19 as a particular hazard that further has increased fear, tension, and anxiety (Kim & Su, 2020). The central nervous system, endocrine system, and immune system are closely linked (Seiler, Fagundes, & Christian, 2020). The inflammatory process may occur in response to psychological stress or other physical stressors by releasing neuropeptides or other inflammatory mediators (Black, 2002).
Central neuropeptides, particularly CRH induce a systemic response to stress by triggering neuroendocrinological pathways, such as HPA, sympathetic nervous system, and angiotensin, resulting in releasing stress hormones (i.e. corticosteroids, catecholamines, glucagons, growth hormones, and renin) (Reul, Labeur, Wiegers, & Linthorst, 1998). This process along with stress-induced cytokines causes Acute-Phase Response (APR) and the activation of acute-phase proteins, which are important inflammation mediators (Black, 2002). Norepinephrine in the central nervous system can also cause the APR by activating macrophages and releasing cytokines. The brain may activate the inflammatory process or inhibit it. The response to inflammation results in a subsequent psychological stress response. In addition, both stress and inflammation are mediated by the same neuropeptides (i.e. CRH). Cytokines evoked by either an inflammatory or stress response can cross similar somatosensory pathways to signal the brain (Black, 2002). Repeated episodes of acute or chronic psychogenic stress can cause chronic inflammatory changes in the brain and other organs.
Cohen et al. in their study noticed that psychological stress increased the risk of acute upper respiratory tract infections. They found that because of repeated stressful events, the failure of immune cells to control the inflammatory response increases the vulnerability to get common cold (Cohen et al., 1991). In another study, Cohen et al. demonstrated that prolonged stress alters the efficacy of cortisol to modulate immune responses due to reduced sensitivity of tissues to the cortisol (Cohen et al., 2012). A meta-analysis revealed that chronic stressful experiences could alter immune system responses, which might increase disease susceptibility. In this study, activation of the sympathetic nervous system via blockade of the beta-adrenergic endings, hormonal release following stressors, and behavioral factors were considered the possible mechanisms in immune dysregulation (Segerstrom & Miller, 2004).
A recent paper showed that infection with COVID-19 caused the release of pro-inflammatory cytokines, including interleukin (IL)-1b and IL-6 (Conti, Ronconi, Caraffa, Gallenga, Ross, Frydas, & Kritas, 2020). Furthermore, psychosocial stressors in response to this pandemic might increase psychiatric problems. More than 50 % of patients with severe acute respiratory syndrome and middle east respiratory syndrome suffered from psychological distress (Mak, Chu, Pan, Yiu, & Chan, 2009). In line with the mentioned studies, our findings revealed that sever COVID-19 infection outcomes are more prominent at a higher level of serum cortisol and HADS score. Although the psychological aspects of COVID-19 are poorly understood, infected patients may experience anxiety, depression, etc. Consequently, the emotional problems may decrease immune response and recovery. In general, all types of psychological supports should be systematically implemented to enhance psychoneuro immunity against COVID-19.
The first limitation of this study was the limited number of participants. Larger studies are needed to find the exact relationship between stress and severity of the COVID-19 infection. The second drawback of our study was no measuring serial cortisol levels during the course of the disease, and this may affect our results. Last but not least, we did not enroll sever COVID-19 patients because they were hemodynamically unstable and could not complete the HADS; however, these cases might provide more clues to illuminate the effect of stress on the disease severity.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences. The written informed consent was received from all participants.
Funding
This research received no specific grant from the public, commercial, or non-profit funding agencies.
Authors' contributions
Study concept and design: Leila Simani, and Mahtab Ramezani; Analysis and interpretation of data: Leila Simani, Mahtab Ramezani, and Ehsan Karimialavijeh; critical role in the acquisition of data and revised the manuscript: Omidvar Rezaei, Mohammadreza Hajiesmaeili, and Hossein Pakdaman. Writing-Review & Editing: Leila Simani, Mahtab Ramezani, and Ehsan Karimialavijeh
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the Clinical Research Development Unit of Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran forcooperation, and assistance throughout the study.
References
Asadi-Pooya, A. A., & Simani, L. (2020). Central nervous system manifestations of COVID-19: A systematic review. Journal of the Neurological Sciences, 116832. [DOI:10.1016/j.jns.2020.116832] [PMID]
Black, P. H. (2002). Stress and the inflammatory response: a review of neurogenic inflammation. Brain, Behavior, and Immunity, 16(6), 622-53. [DOI:10.1016/S0889-1591(02)00021-1]
Cohen, S., Janicki-Deverts, D., Doyle, W. J., Miller, G. E., Frank, E., Rabin, B. S., & Turner, R. B. (2012). Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proceedings of the National Academy of Sciences, 109(16), 5995-99. [DOI:10.1073/pnas.1118355109] [PMID] [PMCID]
Cohen, S., Tyrrell, D. A., & Smith, A. P. (1991). Psychological stress and susceptibility to the common cold. New England Journal Of Medicine, 325(9), 606-12. [DOI:10.1056/NEJM199108293250903] [PMID]
Conti, P., Ronconi, G., Caraffa, A., Gallenga, C., Ross, R., Frydas, I., & Kritas, S. (2020). Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. Journal of Biological Regulators and Homeostatic Agents, 34(2), 11-15.
Glaser, R., & Kiecolt-Glaser, J. K. (2005). Stress-induced immune dysfunction: implications for health. Nature Reviews Immunology, 5(3), 243-51. [DOI:10.1038/nri1571] [PMID]
Haleem, A., Javaid, M., & Vaishya, R. (2020). Effects of COVID 19 pandemic in daily life. Current Medicine Research and Practice. [Published online]. [DOI:10.1016/j.cmrp.2020.03.011] [PMID] [PMCID]
Kaviani, H., Seyfourian, H., Sharifi, V., & Ebrahimkhani, N. (2009). Reliability and validity of anxiety and depression hospital scales (HADS): Iranian patients with anxiety and depression disorders. Tehran University Medical Journal, 67(5), 379-85.
Kim, S. W., & Su, K.P. (2020). Using psychoneuroimmunity against COVID-19. Brain, Behavior, and Immunity. [Published online]. [DOI:10.1016/j.bbi.2020.03.025] [PMID] [PMCID]
Lyu, P., Liu, X., Zhang, R., Shi, L., & Gao, J. (2020). The performance of chest CT in evaluating the clinical severity of COVID-19 pneumonia: identifying critical cases based on CT characteristics. Investigative Radiology. [Published online] [DOI:10.1097/RLI.0000000000000689] [PMID] [PMCID]
Mak, I. W. C., Chu, C. M., Pan, P. C., Yiu, M. G. C., & Chan, V. L. (2009). Long-term psychiatric morbidities among SARS survivors. General Hospital Psychiatry, 31(4), 318-26. [DOI:10.1016/j.genhosppsych.2009.03.001] [PMID] [PMCID]
Reul, J. M., Labeur, M. S., Wiegers, G. J., & Linthorst, A. C. (1998). Altered Neuroimmunoendocrine Communication during a Condition of Chronically Increased Brain Corticotropin‐Releasing Hormone Drive a. Annals of the New York Academy of Sciences, 840(1), 444-55. [DOI:10.1111/j.1749-6632.1998.tb09583.x] [PMID]
Segerstrom, S. C., & Miller, G. E. (2004). Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychological bulletin, 130(4), 601. [DOI:10.1037/0033-2909.130.4.601] [PMID] [PMCID]
Seiler, A., Fagundes, C. P., & Christian, L. M. (2020). The impact of everyday stressors on the immune system and health stress challenges and immunity in space. Springer: Berlin, Heidelberg. pp. 71-92. [DOI:10.1007/978-3-030-16996-1_6]
Shi, Y., Wang, Y., Shao, C., Huang, J., Gan, J., & Huang, X, et al., (2020). COVID-19 infection: the perspectives on immune responses. Berlin, Germany: Nature Publishing Group. [DOI:10.1038/s41418-020-0530-3] [PMID] [PMCID]
Snaith, R. P. (2003). The hospital anxiety and depression scale. Health and Quality of Life Outcomes, 1(1), 29. [DOI:10.1186/1477-7525-1-29] [PMID] [PMCID]
Vanuytsel, T., van Wanrooy, S., Vanheel, H., Vanormelingen, C., Verschueren, S., & Houben, E., et al., (2014). Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut, 63(8), 1293-99. [DOI:10.1136/gutjnl-2013-305690] [PMID]
Xiang, Y. T., Yang, Y., Li, W., Zhang, L., Zhang, Q., Cheung, T., & Ng, C. H. (2020). Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. The Lancet Psychiatry, 7(3), 228-229. [DOI:10.1016/S2215-0366(20)30046-8]
Full-Text:
1. Introduction
The novel coronavirus has a wide range of disease severity among the patients. The Coronavirus Disease 2019 (COVID-19) causes viral damage to the cells and provokes a reactive storm of inflammation resulting in the multi-organ injury (Asadi-Pooya & Simani, 2020). Prior studies have demonstrated that psychological stress plays a vital role in the vulnerability, severity, and recurrence of acute respiratory infections (Cohen, Tyrrell, & Smith, 1991; Xiang et al., 2020). Stressful life events and perceived stress during the life lead to activation of the Hypothalamic-Pituitary-Adrenal (HPA) axis, as well as the Autonomic Nervous System (ANS) (Seiler, Fagundes, & Christian, 2020; Vanuytsel et al., 2014). As a result of HPA activation, Corticotropin-Releasing Hormone (CRH), cortisol, epinephrine, and norepinephrine levels increase in the serum (Glaser & Kiecolt-Glaser, 2005).
An increase in stress hormones exaggerates the inflammatory responses by suppressing the humoral and cellular immune system and altering the balance of pro-inflammatory cytokines (Cohen et al., 2012; Glaser & Kiecolt-Glaser, 2005). Recent studies have indicated changes in immune functions in patients with COVID-19 (Shi et al., 2020). We hypothesized that any lifetime stressful event in COVID-19 patients can be correlated with the severity of their illness. In this study, we evaluated the stress and anxiety during the lifetime of the COVID-19 patients and measured their serum cortisol levels to find any correlation between these factors and their outcomes.
2. Methods
This cross-sectional study was conducted on patients with a confirmed diagnosis of COVID-19 in a university-affiliated referral hospital in Tehran, Iran during March 2020. The inclusion criteria were the age of over 18 years, the onset of the illness within seven days of first symptoms, and mild to moderate COVID-19 based on the results of the Chest CT-Scan (Total CT Score < 10) (Lyu, Liu, Zhang, Shi, & Gao, 2020). The exclusion criteria were current use of corticosteroids, history of primary or secondary hypercortisolism or hypercortisolism, pregnancy, and no to consent to participate in the study. Informed consent was obtained from all participants and the complete medical history, clinical and biochemical evaluation, and cortisol measurement on the days of admission were recorded. We used the Persian version of the Hospital Anxiety and Depression Scale (HADS) to facilitate the early identification of both anxiety and depression simultaneously (Kaviani, Seyfourian, Sharifi, & Ebrahimkhani, 2009).
The HADS is a scale of 14 items, of which seven items are related to anxiety, and seven are related to depression. Each item on the questionnaire is scored from 0-3, which means that a person can score between 0 and 21 for either anxiety or depression. A score of 11 or higher demonstrates the probable presence (abnormal) of the mood disorder (Snaith, 2003).
The novel coronavirus has a wide range of disease severity among the patients. The Coronavirus Disease 2019 (COVID-19) causes viral damage to the cells and provokes a reactive storm of inflammation resulting in the multi-organ injury (Asadi-Pooya & Simani, 2020). Prior studies have demonstrated that psychological stress plays a vital role in the vulnerability, severity, and recurrence of acute respiratory infections (Cohen, Tyrrell, & Smith, 1991; Xiang et al., 2020). Stressful life events and perceived stress during the life lead to activation of the Hypothalamic-Pituitary-Adrenal (HPA) axis, as well as the Autonomic Nervous System (ANS) (Seiler, Fagundes, & Christian, 2020; Vanuytsel et al., 2014). As a result of HPA activation, Corticotropin-Releasing Hormone (CRH), cortisol, epinephrine, and norepinephrine levels increase in the serum (Glaser & Kiecolt-Glaser, 2005).
An increase in stress hormones exaggerates the inflammatory responses by suppressing the humoral and cellular immune system and altering the balance of pro-inflammatory cytokines (Cohen et al., 2012; Glaser & Kiecolt-Glaser, 2005). Recent studies have indicated changes in immune functions in patients with COVID-19 (Shi et al., 2020). We hypothesized that any lifetime stressful event in COVID-19 patients can be correlated with the severity of their illness. In this study, we evaluated the stress and anxiety during the lifetime of the COVID-19 patients and measured their serum cortisol levels to find any correlation between these factors and their outcomes.
2. Methods
This cross-sectional study was conducted on patients with a confirmed diagnosis of COVID-19 in a university-affiliated referral hospital in Tehran, Iran during March 2020. The inclusion criteria were the age of over 18 years, the onset of the illness within seven days of first symptoms, and mild to moderate COVID-19 based on the results of the Chest CT-Scan (Total CT Score < 10) (Lyu, Liu, Zhang, Shi, & Gao, 2020). The exclusion criteria were current use of corticosteroids, history of primary or secondary hypercortisolism or hypercortisolism, pregnancy, and no to consent to participate in the study. Informed consent was obtained from all participants and the complete medical history, clinical and biochemical evaluation, and cortisol measurement on the days of admission were recorded. We used the Persian version of the Hospital Anxiety and Depression Scale (HADS) to facilitate the early identification of both anxiety and depression simultaneously (Kaviani, Seyfourian, Sharifi, & Ebrahimkhani, 2009).
The HADS is a scale of 14 items, of which seven items are related to anxiety, and seven are related to depression. Each item on the questionnaire is scored from 0-3, which means that a person can score between 0 and 21 for either anxiety or depression. A score of 11 or higher demonstrates the probable presence (abnormal) of the mood disorder (Snaith, 2003).
2.1. Statistical analysis
SSPS version 16.0 (SPSS, Inc., Chicago, IL, USA) was used for data analysis. A Chi-square test was applied for demographic variables, and the independent t-test was used to compare numeric data. To assess the differences between the surviving and dead patients in cortisol level and HADS score, we performed a Two-Way Analysis Of Variance (ANOVA) using the factors “outcome” and “comorbidity”. A correlation between HADS score and serum levels of cortisol was assessed by the Pearson correlation test and the results were presented as the mean±Standard Deviation (SD). A P-value < 0.05 was considered significant.
3. Results
A total of 30 patients with COVID-19, 17 (56.7%) males and 13 (43.3%) females, were enrolled in this research. The mean age of the patients was 53.41±18.88 years. The serum level of cortisol and HADS score in COVID-19 patients are depicted in Table 1. Analysis of the HADS score and cortisol revealed a higher score of HADS in non-survival compared with surviving patients (p = 0.001). In addition, a higher serum level of cortisol was found in non-survival patients versus surviving patients (p=0.017).
Based on the two-way ANOVA test results, there was no statistically significant correlation between the outcome and comorbidity (p=0.278), and also no differences were found between the level of cortisol and HADS score (p=0.321). HADS score was found with a significant positive correlation with cortisol level (r; 0.842, P=0.004). There were no significant differences between sex and serum level of cortisol and HADS score (p=0.745 and p=0.912, respectively). Also, no significant correlation was observed between age and HADS score and cortisol level (p=0.684 and p=0.598, respectively).
4. Discussion
Our results revealed that although all participants had a mild to moderate presentation of COVID-19, patients with fatal outcome had higher serum cortisol levels and HADS score compared with the survived ones. This finding supports our primary hypothesis about the effect of stress and anxiety on the patient’s vulnerability to the infection.
The COVID-19 pandemic has increased concerns of widespread panic and anxiety (Haleem, Javaid, & Vaishya, 2020). Our lifestyle and habits are shifting dramatically, and COVID-19 pandemic has influenced all aspects of our lives (Kim & Su, 2020). Unlike other viruses, like influenza viruses and other infections, media has overemphasized the COVID-19 as a particular hazard that further has increased fear, tension, and anxiety (Kim & Su, 2020). The central nervous system, endocrine system, and immune system are closely linked (Seiler, Fagundes, & Christian, 2020). The inflammatory process may occur in response to psychological stress or other physical stressors by releasing neuropeptides or other inflammatory mediators (Black, 2002).
Central neuropeptides, particularly CRH induce a systemic response to stress by triggering neuroendocrinological pathways, such as HPA, sympathetic nervous system, and angiotensin, resulting in releasing stress hormones (i.e. corticosteroids, catecholamines, glucagons, growth hormones, and renin) (Reul, Labeur, Wiegers, & Linthorst, 1998). This process along with stress-induced cytokines causes Acute-Phase Response (APR) and the activation of acute-phase proteins, which are important inflammation mediators (Black, 2002). Norepinephrine in the central nervous system can also cause the APR by activating macrophages and releasing cytokines. The brain may activate the inflammatory process or inhibit it. The response to inflammation results in a subsequent psychological stress response. In addition, both stress and inflammation are mediated by the same neuropeptides (i.e. CRH). Cytokines evoked by either an inflammatory or stress response can cross similar somatosensory pathways to signal the brain (Black, 2002). Repeated episodes of acute or chronic psychogenic stress can cause chronic inflammatory changes in the brain and other organs.
Cohen et al. in their study noticed that psychological stress increased the risk of acute upper respiratory tract infections. They found that because of repeated stressful events, the failure of immune cells to control the inflammatory response increases the vulnerability to get common cold (Cohen et al., 1991). In another study, Cohen et al. demonstrated that prolonged stress alters the efficacy of cortisol to modulate immune responses due to reduced sensitivity of tissues to the cortisol (Cohen et al., 2012). A meta-analysis revealed that chronic stressful experiences could alter immune system responses, which might increase disease susceptibility. In this study, activation of the sympathetic nervous system via blockade of the beta-adrenergic endings, hormonal release following stressors, and behavioral factors were considered the possible mechanisms in immune dysregulation (Segerstrom & Miller, 2004).
A recent paper showed that infection with COVID-19 caused the release of pro-inflammatory cytokines, including interleukin (IL)-1b and IL-6 (Conti, Ronconi, Caraffa, Gallenga, Ross, Frydas, & Kritas, 2020). Furthermore, psychosocial stressors in response to this pandemic might increase psychiatric problems. More than 50 % of patients with severe acute respiratory syndrome and middle east respiratory syndrome suffered from psychological distress (Mak, Chu, Pan, Yiu, & Chan, 2009). In line with the mentioned studies, our findings revealed that sever COVID-19 infection outcomes are more prominent at a higher level of serum cortisol and HADS score. Although the psychological aspects of COVID-19 are poorly understood, infected patients may experience anxiety, depression, etc. Consequently, the emotional problems may decrease immune response and recovery. In general, all types of psychological supports should be systematically implemented to enhance psychoneuro immunity against COVID-19.
The first limitation of this study was the limited number of participants. Larger studies are needed to find the exact relationship between stress and severity of the COVID-19 infection. The second drawback of our study was no measuring serial cortisol levels during the course of the disease, and this may affect our results. Last but not least, we did not enroll sever COVID-19 patients because they were hemodynamically unstable and could not complete the HADS; however, these cases might provide more clues to illuminate the effect of stress on the disease severity.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences. The written informed consent was received from all participants.
Funding
This research received no specific grant from the public, commercial, or non-profit funding agencies.
Authors' contributions
Study concept and design: Leila Simani, and Mahtab Ramezani; Analysis and interpretation of data: Leila Simani, Mahtab Ramezani, and Ehsan Karimialavijeh; critical role in the acquisition of data and revised the manuscript: Omidvar Rezaei, Mohammadreza Hajiesmaeili, and Hossein Pakdaman. Writing-Review & Editing: Leila Simani, Mahtab Ramezani, and Ehsan Karimialavijeh
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the Clinical Research Development Unit of Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran forcooperation, and assistance throughout the study.
References
Asadi-Pooya, A. A., & Simani, L. (2020). Central nervous system manifestations of COVID-19: A systematic review. Journal of the Neurological Sciences, 116832. [DOI:10.1016/j.jns.2020.116832] [PMID]
Black, P. H. (2002). Stress and the inflammatory response: a review of neurogenic inflammation. Brain, Behavior, and Immunity, 16(6), 622-53. [DOI:10.1016/S0889-1591(02)00021-1]
Cohen, S., Janicki-Deverts, D., Doyle, W. J., Miller, G. E., Frank, E., Rabin, B. S., & Turner, R. B. (2012). Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proceedings of the National Academy of Sciences, 109(16), 5995-99. [DOI:10.1073/pnas.1118355109] [PMID] [PMCID]
Cohen, S., Tyrrell, D. A., & Smith, A. P. (1991). Psychological stress and susceptibility to the common cold. New England Journal Of Medicine, 325(9), 606-12. [DOI:10.1056/NEJM199108293250903] [PMID]
Conti, P., Ronconi, G., Caraffa, A., Gallenga, C., Ross, R., Frydas, I., & Kritas, S. (2020). Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. Journal of Biological Regulators and Homeostatic Agents, 34(2), 11-15.
Glaser, R., & Kiecolt-Glaser, J. K. (2005). Stress-induced immune dysfunction: implications for health. Nature Reviews Immunology, 5(3), 243-51. [DOI:10.1038/nri1571] [PMID]
Haleem, A., Javaid, M., & Vaishya, R. (2020). Effects of COVID 19 pandemic in daily life. Current Medicine Research and Practice. [Published online]. [DOI:10.1016/j.cmrp.2020.03.011] [PMID] [PMCID]
Kaviani, H., Seyfourian, H., Sharifi, V., & Ebrahimkhani, N. (2009). Reliability and validity of anxiety and depression hospital scales (HADS): Iranian patients with anxiety and depression disorders. Tehran University Medical Journal, 67(5), 379-85.
Kim, S. W., & Su, K.P. (2020). Using psychoneuroimmunity against COVID-19. Brain, Behavior, and Immunity. [Published online]. [DOI:10.1016/j.bbi.2020.03.025] [PMID] [PMCID]
Lyu, P., Liu, X., Zhang, R., Shi, L., & Gao, J. (2020). The performance of chest CT in evaluating the clinical severity of COVID-19 pneumonia: identifying critical cases based on CT characteristics. Investigative Radiology. [Published online] [DOI:10.1097/RLI.0000000000000689] [PMID] [PMCID]
Mak, I. W. C., Chu, C. M., Pan, P. C., Yiu, M. G. C., & Chan, V. L. (2009). Long-term psychiatric morbidities among SARS survivors. General Hospital Psychiatry, 31(4), 318-26. [DOI:10.1016/j.genhosppsych.2009.03.001] [PMID] [PMCID]
Reul, J. M., Labeur, M. S., Wiegers, G. J., & Linthorst, A. C. (1998). Altered Neuroimmunoendocrine Communication during a Condition of Chronically Increased Brain Corticotropin‐Releasing Hormone Drive a. Annals of the New York Academy of Sciences, 840(1), 444-55. [DOI:10.1111/j.1749-6632.1998.tb09583.x] [PMID]
Segerstrom, S. C., & Miller, G. E. (2004). Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychological bulletin, 130(4), 601. [DOI:10.1037/0033-2909.130.4.601] [PMID] [PMCID]
Seiler, A., Fagundes, C. P., & Christian, L. M. (2020). The impact of everyday stressors on the immune system and health stress challenges and immunity in space. Springer: Berlin, Heidelberg. pp. 71-92. [DOI:10.1007/978-3-030-16996-1_6]
Shi, Y., Wang, Y., Shao, C., Huang, J., Gan, J., & Huang, X, et al., (2020). COVID-19 infection: the perspectives on immune responses. Berlin, Germany: Nature Publishing Group. [DOI:10.1038/s41418-020-0530-3] [PMID] [PMCID]
Snaith, R. P. (2003). The hospital anxiety and depression scale. Health and Quality of Life Outcomes, 1(1), 29. [DOI:10.1186/1477-7525-1-29] [PMID] [PMCID]
Vanuytsel, T., van Wanrooy, S., Vanheel, H., Vanormelingen, C., Verschueren, S., & Houben, E., et al., (2014). Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut, 63(8), 1293-99. [DOI:10.1136/gutjnl-2013-305690] [PMID]
Xiang, Y. T., Yang, Y., Li, W., Zhang, L., Zhang, Q., Cheung, T., & Ng, C. H. (2020). Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. The Lancet Psychiatry, 7(3), 228-229. [DOI:10.1016/S2215-0366(20)30046-8]
Type of Study: Original |
Subject:
Clinical Neuroscience
Received: 2020/05/3 | Accepted: 2020/06/12 | Published: 2020/06/14
Received: 2020/05/3 | Accepted: 2020/06/12 | Published: 2020/06/14
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |