Volume 15, Issue 5 (September & October 2024)
BCN 2024, 15(5): 607-616 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rezvani Z, Khosrowabadi R, Seyedebrahimi A, Meftahi G, Hatef B. Stress Induction and Release: Electroencephalography Study on Brain Networks and Cortisol. BCN 2024; 15 (5) :607-616
URL: http://bcn.iums.ac.ir/article-1-1764-en.html
URL: http://bcn.iums.ac.ir/article-1-1764-en.html
Zahra Rezvani1 

 , Reza Khosrowabadi2
, Reza Khosrowabadi2 

 , Afrooz Seyedebrahimi2
, Afrooz Seyedebrahimi2 

 , Golam-Hossein Meftahi3
, Golam-Hossein Meftahi3 

 , Boshra Hatef *3
, Boshra Hatef *3 




 , Reza Khosrowabadi2
, Reza Khosrowabadi2 

 , Afrooz Seyedebrahimi2
, Afrooz Seyedebrahimi2 

 , Golam-Hossein Meftahi3
, Golam-Hossein Meftahi3 

 , Boshra Hatef *3
, Boshra Hatef *3 


1- Department of Computer Science, Faculty of Mathematical Sciences, Alzahra University, Tehran, Iran.
2- Institute for Cognitive and Brain Sciences, Shahid Beheshti University, Tehran, Iran.
3- Neuroscience Research Center, Baqiyatallah University of Medical Science, Tehran, Iran.
2- Institute for Cognitive and Brain Sciences, Shahid Beheshti University, Tehran, Iran.
3- Neuroscience Research Center, Baqiyatallah University of Medical Science, Tehran, Iran.
Keywords: Stress, Visual analog scale (VAS), Cortisol, Electroencephalography (EEG), Functional connectivity (FC)
Full-Text [PDF 1838 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Stressful events could crucially influence behavioral and cognitive performances. Stress is defined as a behavioral response to uncertain conditions in which the proper body responses appear in the form of chemical adjustment and physiological changes (Peters et al., 2017). These biological modifiers should have enough time to restore the normal state of homeostasis; otherwise, chronic stress would be anticipated (McEwen, 2007), an aggregator for many disorders (Yaribeygi et al., 2017).
Previous studies have reported two neuroendocrine systems for responding to stressful events: Activation of the hypothalamus-pituitary-adrenal axis that regulates the release of the glucocorticoid (GC) hormones (mainly cortisol in humans) and sympathetic-adrenal-medullary system that increases the sympathetic tone (Yaribeygi et al., 2017). The GC has many receptors in the brain that, following a stressful condition, could change brain activities (McEwen et al., 2015). The changes in brain activities could occur in the form of changes in neural oscillations (Putman et al., 2014; Hamid et al., 2010; Lewis et al., 2007). In comparison, the pattern of neural oscillations could be traced by measuring electroencephalography (EEG) signals in a millisecond range. Based on the EEG signal, interactions between pairs of electrodes could also be calculated (Schoffelen & Gross 2009; Khosrowabadi, 2018; Achard et al., 2006).
The interactions between various brain regions are crucial for normal brain functioning, which can be measured as synchrony between them. These interactions are called functional connectivity (FC) (Achard et al., 2006; Garmezy & Rutter, 1983). The FC approach has been used to study stress (Khosrowabadi et al., 2011; Khosrowabadi, 2018; Alonso et al., 2015). These studies have reported an inverted-u-shaped relationship between GC levels and cognitive performance in stressful conditions (Lupien et al., 2009). The GC receptors are activated in acute stress conditions, destabilizing the established synaptic connection (Hüther, 1998). Subsequently, the neural pathway between cortical and limbic systems is facilitated to trigger large-scale brain networks (Hermans et al., 2014). For instance, the neural system handled by the salience processing network is triggered and facilitates the relocation of the executive-control network (Hermans et al., 2014). This effect follows a bihemispheric autonomic model (BHAM) (Tegeler et al., 2015). The BHAM model relates the right hemisphere to the sympathetic response ‘fight or flight,’ and the left hemisphere responsible for the parasympathetic response ‘rest, digest, or freeze.’
Nevertheless, the mechanism of changes in the FC network of the brain during induction and release of stress still needs to be well understood. Therefore, this study used a whole-brain approach using EEG data to investigate changes in the FC network while exposed to acute stressors and after recovery. We aimed to test the BHAM model on these data. The hypothesis states that stress changes the FC of brain activity based on EEG. However, after 20 minutes of recovery, the changes return to pre-stress. In this study, 20 male subjects were recruited, and their psychological stress scores, salivary cortisol level, and EEG data were recorded before and after stress induction, as well as 20 minutes after recovery. Then, behavioral, physiological, and neurophysiological markers were statistically compared at the conditions mentioned.
2. Materials and Methods
Study participants
Twenty healthy male subjects with a Mean±SD age of 23.37±2.7 years were recruited from students of Baqiyatallah University of Medical Sciences for the study. All participants are right-handed. The inclusion criteria consisted of general physical and mental health, no smoking habit, no spine and cervicocephalic surgery, no neuropsychological medication usage, no regular exercise (at least three times a week), and no abnormal sleep pattern. Before the experiment, all participants signed a consent form approved by the Baqiyatallah University of Medical Sciences Ethical Board.
Experimental design
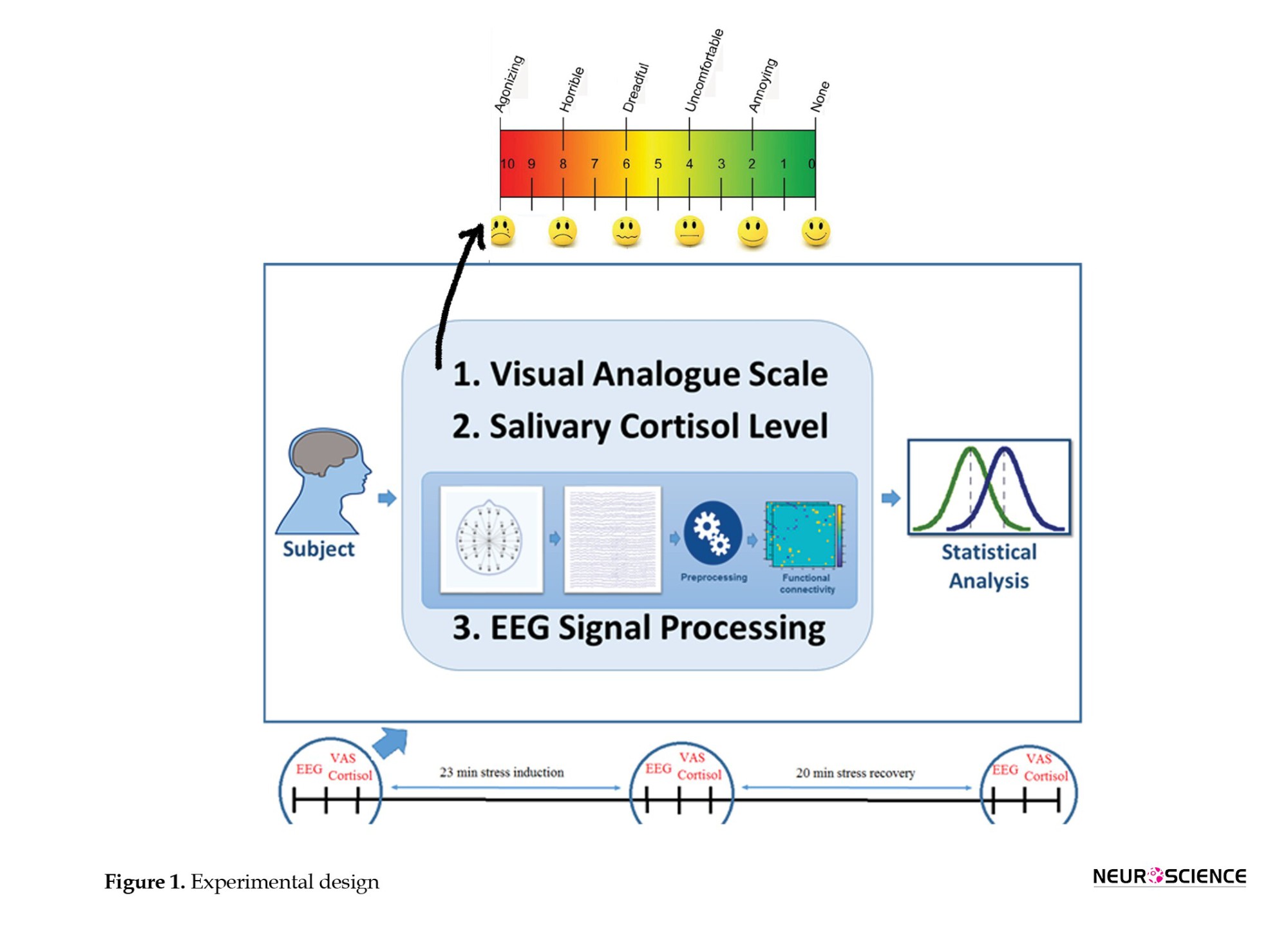
The experiment consisted of two phases, including 23 minutes of stress induction, 20 minutes of recovery, and three measurements (pre-stress, post-stress, and post-recovery). The subjects’ psychological stress scores were measured by the visual analog scale (VAS) (Hellhammer & Schubert, 2012). Their salivary cortisol level and EEG data were also recorded. The stress was induced by the Trier social stress test (TSST) (Kirschbaum et al., 1993), and recordings were performed before and after stress induction and after the recovery phase. The experimental design is illustrated in Figure 1.
TSST
TSST is a standardized protocol for generating moderate psychosocial stress in laboratory settings (Kirschbaum et al., 1993). The TSST consists of 3 minutes brief preparation period followed by 10 minutes test period in which the subject has to deliver a free speech for 2 minutes and perform a mental arithmetic task for 8 minutes in a standing position in front of two referees behind the desk. During his speech, referees with neutral faces only listened to him and warned him to continue when he stopped talking. After the first 2 minutes, the participant was asked to count down from 1022 to 13, and at each wrong subtraction, he was warned to start counting down from the beginning. The VAS questionnaire measured the perceived stress and anxiety scores, assessing the stress (self-reporting) before and after the test and 20 minutes after recovery.
Salivary cortisol level
Following the activation of the hypothalamic-pituitary-adrenal axis and sympathomedullary pathway (Baum & Contrada, 2010), cortisol is released from the adrenal gland into the bloodstream and spreads throughout the body. Changes in cortisol levels have been introduced as a standard stress index that could be measured using the blood or salivary test (Zigmond & Bloom, 1999; Dickerson & Kemeny, 2004). In this study, a salivary cortisol test was performed to confirm the results of TSST. The subjects were asked to eat nothing one hour before the test and wash their mouth right before it. An ELISA kit (IBL Company, made in Germany) was used in the following procedure. First, 0.5 mL of salivary sample was collected before and after stress induction as well as 20 minutes after recovery; then, the samples were frozen at -80 °C. The salivary cortisol levels were then measured from the frozen samples. A statistical analysis was then performed to compare data of the 3 conditions.
EEG data acquisition
EEG data were recorded using a 32-channel amplifier (Mitsar Co Ltd, EEG 202) positioned according to the standard international 10-20 system (one minute with closed eyes and one minute with open eyes). An EEG cap with 32 reusable sintered Ag/AgCl electrodes was applied, and the scalp skin beneath each electrode was kept clean with slight abrasion and cleaning with alcohol. An impedance check was performed, and resistances below 10 kΩ accepted. A bandpass of 0.1-70 Hz was considered during the recording, and the EEG data were recorded using a 12-bit digitizer with a sampling frequency of 256 Hz.
EEG data processing and analysis
A standard preprocessing was performed on the EEG data using the Matlab EEGlab toolbox. The preprocessing consisted of the following sessions: Conversion of the EEG data format readable in Matlab software, version 2017b, filtering unwanted noises using a bandpass FIR filter from 1 to 40 Hz, epoching the data to segments of 1 second, employing ADJUST plugin to remove artifacts based on ICA, and interpolation of bad channels detected by kurtosis of EEG data. Reference of all the electrodes of the average of all channels. After preprocessing, FC is considered as the temporal dependency between neuronal activations of pairs of electrodes (Lang et al., 2012). The FC network was estimated by taking the partial correlation between pairs of electrodes (Friston, 1994, Lang et al., 2012). In the context of brain networks, partial correlation is the correlation between the time series of two nodes after adjusting for the time series from all other network nodes as covariate factors (Wang et al., 2016). FC analysis was performed on the conventional frequency bands, including delta [1-4 Hz], theta [4-8 Hz], alpha [8-13 Hz], beta [13-30 Hz], and lower-gamma [30-40 Hz].
Statistical analysis
Statistical analysis was performed on the VAS, salivary cortisol, and FC maps measured before stress induction, after 23 minutes of inducing mental stress, and 20 minutes after the recovery. After checking the data normality with the Kolmogorov-Smirnov test, the statistical analysis was performed using two distinct paired t-tests for stress induction and recovery phases. Firstly, a paired t-test was applied to the data gathered before and after stress induction to identify how stress induction changes the VAS, salivary cortisol level, and pattern of brain connectivity. Subsequently, the correlation between relative changes in FC [for instance (FCafter stress-FCbefore)/FCbefore] and relative changes in cortisol [for example (Cortisolafter stress-Cortisolbefore)/Cortisolbefore] and VAS [for instance (VASafter stress-VASbefore)/VASbefore] was also computed on the data gathered after inducing stress and after recovery. Relative changes are calculated based on the difference between two conditions divided by the value of the initial condition. Finally, the statistical results were corrected for multiple comparisons effect using the false discovery rate (FDR) (Benjamini & Hochberg, 1995; Shaffer, 1995).
3. Results
Behavioral and physiological changes
The average changes in the VAS in three measurements are presented in Figure 2. The results showed that the VAS scores significantly increased by stress induction. As expected, the VAS scores also significantly decreased after the release of stressful condition (pre-stress: 1.1±1, post-stress: 3.2±2, post-recovery: 0.6±0.7) (F=22.91, P<0.0000 for significant different between post-stress and pre-stress and post-recovery).
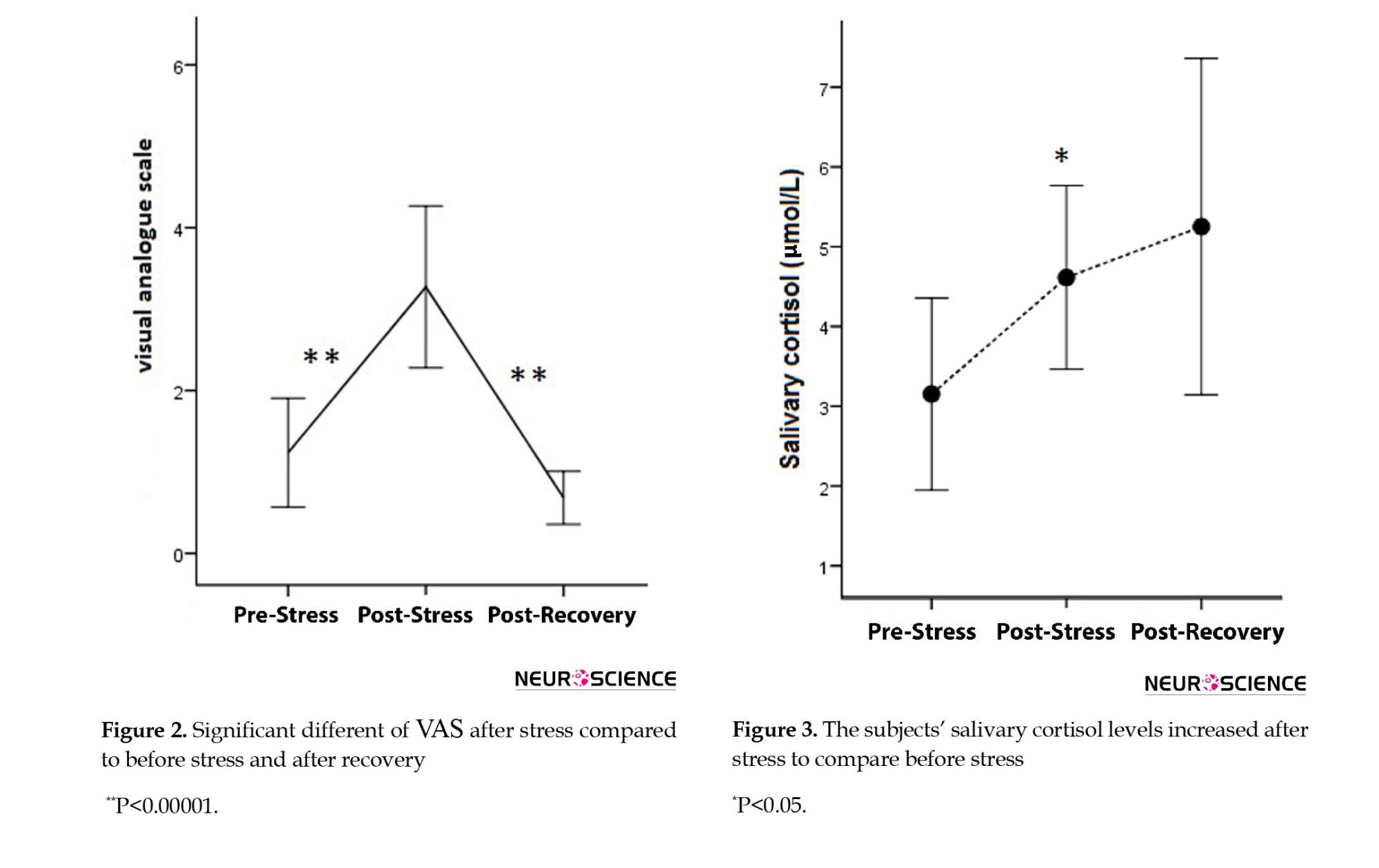
The salivary cortisol levels at three stages of the experiment (pre-stress: 2.5±1, post-stress: 4.4±2, post-recovery: 5.1±4 μm/dL) are also presented in Figure 3. The results showed a significant cortisol increase after mental stress induction (P<0.017). Although the cortisol level increased in the recovery phase, the alteration was not significant after the recovery.
Alteration of brain FC
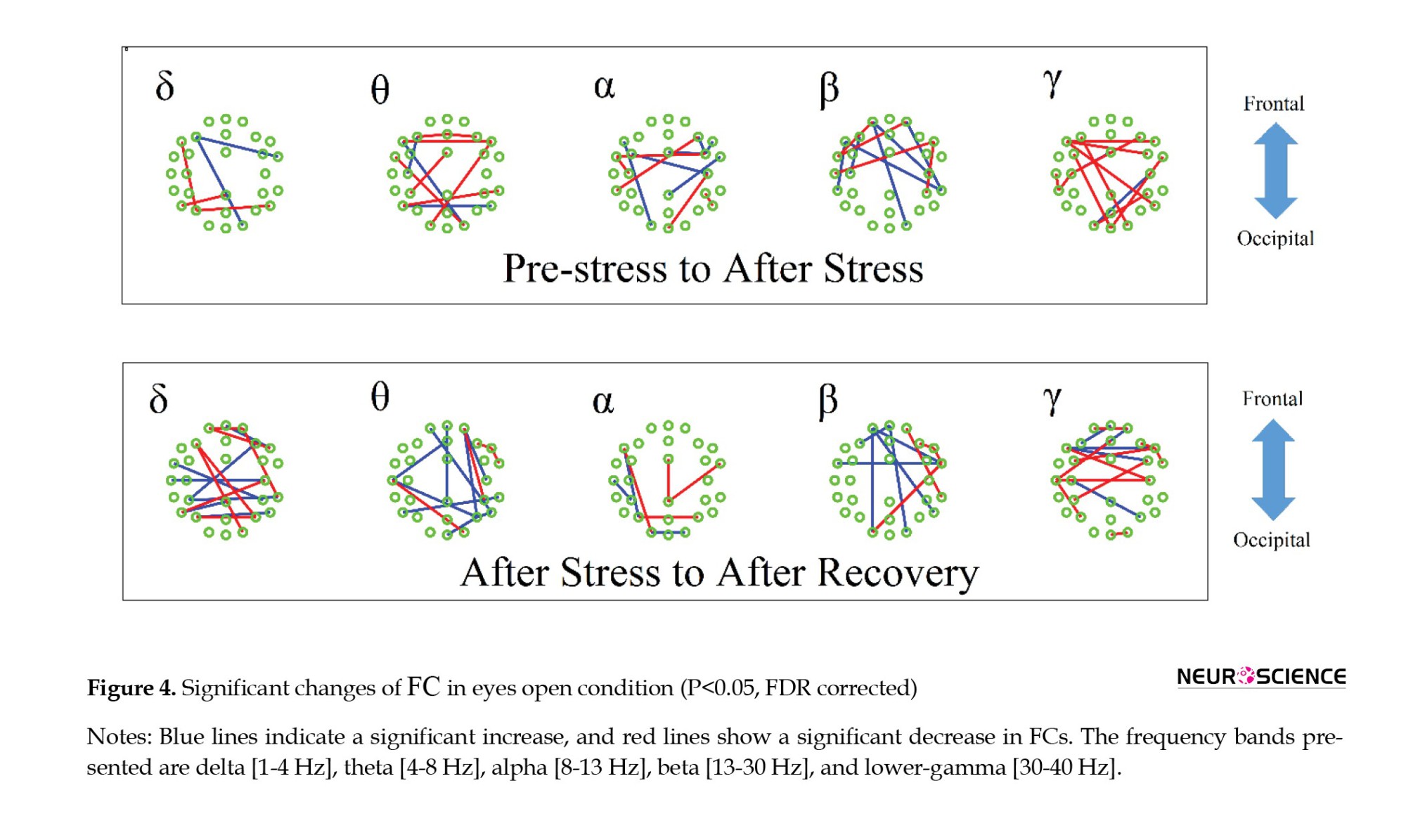
As described in the previous section, the subject-wise FC pattern was calculated based on synchrony between EEG signals of a pair of electrodes using a partial correlation approach (Baba et al., 2004). Subsequently, a statistical comparison was performed using the paired t-test. A correction for multiple comparisons was then performed, presenting the significant changes in Figure 4.

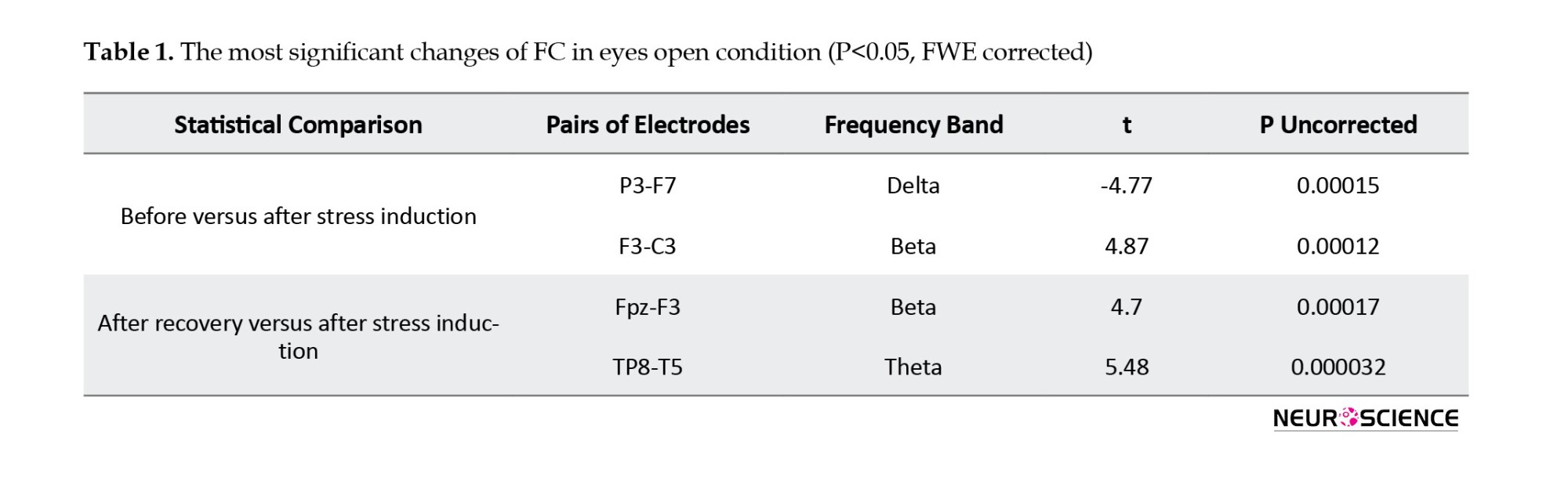
The results showed that stress induction mainly changes the frontotemporal functional connections, especially in delta, alpha, beta, and gamma bands (P<0.05, FDR corrected), observed primarily on the right hemisphere (Figure 4). In addition, 20 minutes of recovery causes a significant change in the temporal-parietal functional connections, especially in theta and beta bands (P<0.05, FDR corrected). In the recovery phase, FCs of the right frontal region increased while FCs in the right parietal region decreased in theta band. FCs of the left parietal region also increase in the beta band. Also, the most significant results (P<0.05, family-wise error (FWE) corrected using the Bonferroni method) are presented in Table 1.
Association between alteration of brain FCs, cortisol level, and VAS
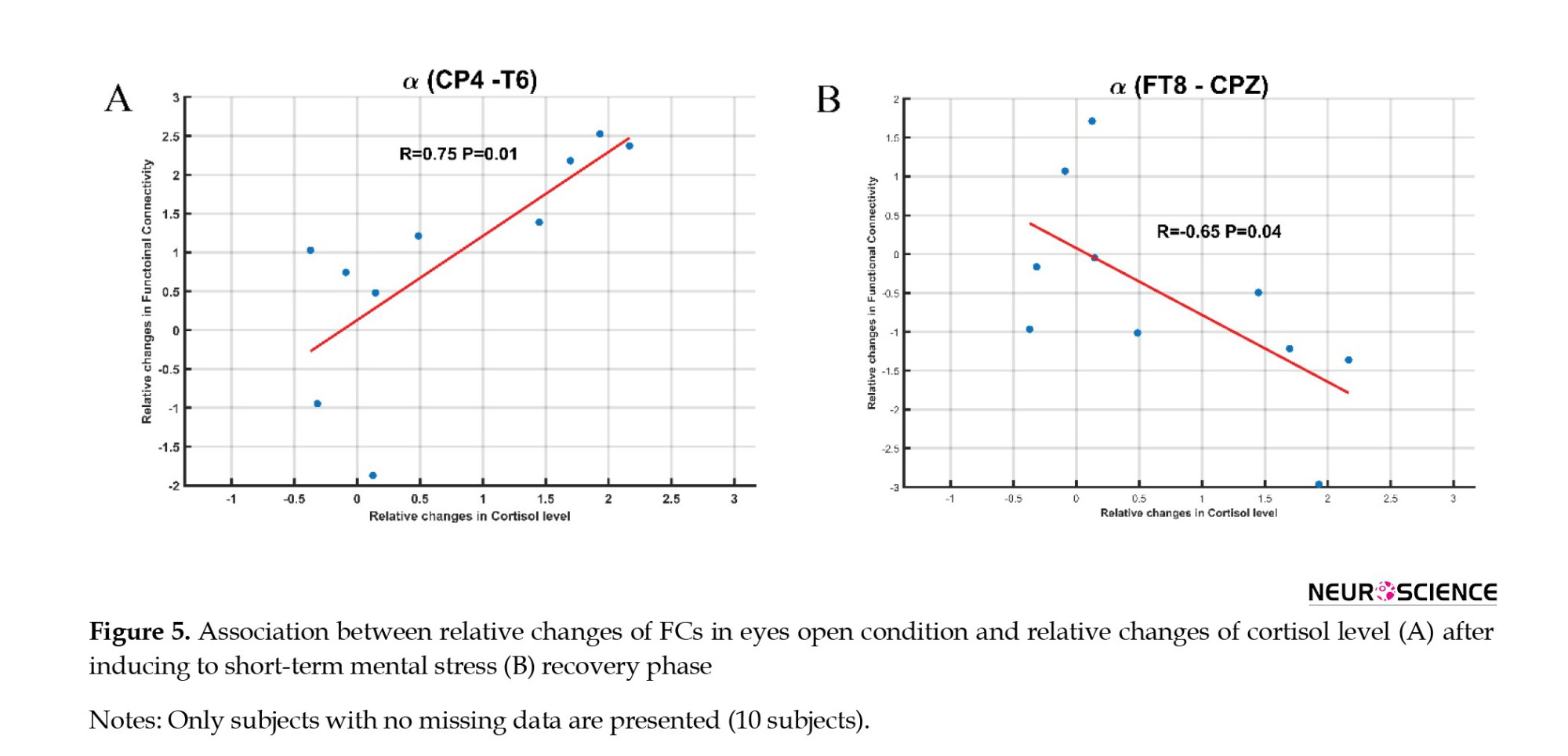
Relative changes in brain FC after stress induction and 20 minutes after the stress release showed a positive correlation with the related cortisol changes after stress induction. Changes in FC between CP4 and T6 in the alpha band (r=0.75, P=0.01) showed a positive correlation with changes in cortisol levels (Figure 5A). In addition, changes in FC between FT8 and CPZ in the alpha band (r=-0.65, P=0.04) also showed a negative correlation with changes in cortisol levels (Figure 5B).
4. Discussion
Our study aimed to evaluate the effect of short-term psychological stress and its release on the brain’s FC. Our findings demonstrate that stressful events change the frontotemporal connections, especially in the delta, alpha, beta, and gamma bands. Moreover, inter-hemispheric FCs of the right frontal regions are mainly decreased by inducing mental stress. In contrast, the interhemispheric FCs of the left frontal regions increase at the delta, theta, alpha, and beta bands and significantly decrease at the lower gamma band. Interestingly, even after 20 minutes of recovery, some reactivity occurred, and the temporal-parietal connections, especially at the theta and beta bands, significantly changed after the release of stressors. The release of stressors presents a recovery pattern of inter-hemispheric FCs mainly observed at the homologous inter-hemispheric FCs in frontocentral regions at the lower gamma band and temporal-parietal regions at the delta band. In addition, the significant changes in the FCs correlate with changes in cortisol and VAS.
The steroid hormone cortisol almost has receptors in every cell in the body that enable it to activate them depending on the cell type, including those in the brain, to regulate metabolism and restore homeostasis (McEwen, 2017). Cortisol could pass the blood-brain barrier and reach its related receptors in the cortex, limbic system, hippocampus, thalamus, and hypothalamus (Dallman, 2005). Hyperpolarization of the membrane has been suggested to be the primary mechanism for the fast effect of cortisol (15 to 20 min) (Makara & Haller 2001). Hyperpolarization is associated with neuronal silence, which could influence the connectivity of a neuronal network (Wrosch et al., 2017). In stressful conditions, this effect has been observed in several brain regions, including the hippocampus, amygdala, and the prefrontal cortex (PFC), and their interactions are influenced (Yaribeygi et al., 2017).
Consequently, cognitive functioning, emotional regulation, and self-regulatory behaviors will be affected (Ursin & Eriksen 2004; McEwen, 2007; Erfani et al., 2016; Yaribeygi et al., 2017). Moreover, it is also important to return to an initial state after removing the stressor to prevent overload stress, which may advance to chronic stress (McEwen, 2017). Since the autonomic nervous system is managed by hemispheric lateralization (Lee et al., 2014), the bihemispheric association must be critical in the recovery phase, which will be discussed in the following section.
Previous studies have shown that the salivary cortisol, VAS, heart rate variation features, the linear and non-linear features, and network features of EEG changed significantly following TSST (Ghahvehchi-Hosseini et al., 2018, Mohammadi Alireza et al., 2018, Lotfan et al., 2019). The relative alpha band power (8-10 Hz) increased after stress in the eye-closed mode in all channels in the same pattern of cortisol change (Ghahvehchi-Hosseini et al., 2018). Besides, the theta/beta ratio decreases (Putman et al., 2013), and asymmetry of functionality in the PFC rises towards the right hemisphere (Seo & Lee, 2010). Moreover, a decrease in alpha band power and an increase in beta band power and their associations with changes in heart rate variability and cortisol level have been reported (Hamid et al., 2010; Seo & Lee, 2010). The imaging study shows that the dorsolateral PFC activity decreases under short-term mental stress (Hermans et al., 2014). These findings demonstrate the complexity of the underlying mechanism that could influence widely distributed FCs in the brain network.
In the same line with the previous studies, we also observed that an active induction of mental stress could significantly decrease the right frontal inter-hemispheric FCs and increase the left frontal inter-hemispheric FCs at the delta, theta, alpha, and beta bands while reducing them at the lower gamma band. For survival, automatic responses must be suppressed after mentally stressful situations. Considering the role of the right side of the brain as the main manager of the sympathetic response (Lee et al., 2014), interactions of the right hemisphere with other parts of the brain must be decreased. One possible interpretation of this mechanism is that cortisol facilitates the hyperpolarization of the neural cell membranes, which is associated with neuronal silence and exerts a top-down inhibition on subcortical regions (Wager et al., 2008) such as the amygdala. Subsequently, the network of hypothalamus-pituitary and adrenal glands are activated, managed by the left hemisphere, to dampen the stress response. Therefore, the left frontal interactions should also increase in this study paradigm. Furthermore, significant correlations between changes in FCs and VAS and cortisol values suggest that the interhemispheric connectivity changes may be associated with the induced stress level.
On the other hand, the release of stressors also presents a recovery pattern of inter-hemispheric FCs. The inter-hemispheric FCs of the right frontal region increase at the delta, theta, and gamma bands, while FCs of the left frontal regions decrease at the delta and alpha bands. Moreover, the inter-hemispheric FCs of the right parietal regions also increase at the theta band, and the inter-hemispheric FCs of the left parietal region decrease at the beta band, which could be related to the reformation of the attention network during the stress release.
5. Conclusion
Our findings reveal that exposure to short-term psychological stress changes the cortisol level and brain FC pattern, which is a clear sign of the behavior measured by the VAS in this study. Based on the results, the bihemispheric association plays a vital role in adapting and coping with stressful conditions.
Study limitations
We cannot thoroughly exclude the variability of the FC measures based on the task-free EEG data in various stages of the experiment. However, paired comparisons in a controlled environment and implying a restricted threshold (FWE corrected P) could reduce the risk of over-interpretation. In addition, only male subjects were recruited in this study, making it inconceivable to apply the results to female subjects. Gender differences in response to stress are considered a major issue. Furthermore, this study might be performed with a larger sample size to improve the power of calculations. Also, investigating task-based EEG while subjects are exposed to stress stimuli could provide more information on the dynamic of FC changes.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Baqiyatallah University of Medical Science (Code: IR.BMSU.REC.1389.141).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and supervision: Reza Khosrowabadi and Boshra Hatef; Methodology: Zahra Rezvani; Data collection: Boshra Hatef and Golam-Hossein Meftahi; Data analysis: Zahra Rezvani and Afrooz Seyedebrahimi; Investigation, and writing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to extend their heartfelt thanks to all participants in the experiments for their invaluable contribution to this study.
References
Stressful events could crucially influence behavioral and cognitive performances. Stress is defined as a behavioral response to uncertain conditions in which the proper body responses appear in the form of chemical adjustment and physiological changes (Peters et al., 2017). These biological modifiers should have enough time to restore the normal state of homeostasis; otherwise, chronic stress would be anticipated (McEwen, 2007), an aggregator for many disorders (Yaribeygi et al., 2017).
Previous studies have reported two neuroendocrine systems for responding to stressful events: Activation of the hypothalamus-pituitary-adrenal axis that regulates the release of the glucocorticoid (GC) hormones (mainly cortisol in humans) and sympathetic-adrenal-medullary system that increases the sympathetic tone (Yaribeygi et al., 2017). The GC has many receptors in the brain that, following a stressful condition, could change brain activities (McEwen et al., 2015). The changes in brain activities could occur in the form of changes in neural oscillations (Putman et al., 2014; Hamid et al., 2010; Lewis et al., 2007). In comparison, the pattern of neural oscillations could be traced by measuring electroencephalography (EEG) signals in a millisecond range. Based on the EEG signal, interactions between pairs of electrodes could also be calculated (Schoffelen & Gross 2009; Khosrowabadi, 2018; Achard et al., 2006).
The interactions between various brain regions are crucial for normal brain functioning, which can be measured as synchrony between them. These interactions are called functional connectivity (FC) (Achard et al., 2006; Garmezy & Rutter, 1983). The FC approach has been used to study stress (Khosrowabadi et al., 2011; Khosrowabadi, 2018; Alonso et al., 2015). These studies have reported an inverted-u-shaped relationship between GC levels and cognitive performance in stressful conditions (Lupien et al., 2009). The GC receptors are activated in acute stress conditions, destabilizing the established synaptic connection (Hüther, 1998). Subsequently, the neural pathway between cortical and limbic systems is facilitated to trigger large-scale brain networks (Hermans et al., 2014). For instance, the neural system handled by the salience processing network is triggered and facilitates the relocation of the executive-control network (Hermans et al., 2014). This effect follows a bihemispheric autonomic model (BHAM) (Tegeler et al., 2015). The BHAM model relates the right hemisphere to the sympathetic response ‘fight or flight,’ and the left hemisphere responsible for the parasympathetic response ‘rest, digest, or freeze.’
Nevertheless, the mechanism of changes in the FC network of the brain during induction and release of stress still needs to be well understood. Therefore, this study used a whole-brain approach using EEG data to investigate changes in the FC network while exposed to acute stressors and after recovery. We aimed to test the BHAM model on these data. The hypothesis states that stress changes the FC of brain activity based on EEG. However, after 20 minutes of recovery, the changes return to pre-stress. In this study, 20 male subjects were recruited, and their psychological stress scores, salivary cortisol level, and EEG data were recorded before and after stress induction, as well as 20 minutes after recovery. Then, behavioral, physiological, and neurophysiological markers were statistically compared at the conditions mentioned.
2. Materials and Methods
Study participants
Twenty healthy male subjects with a Mean±SD age of 23.37±2.7 years were recruited from students of Baqiyatallah University of Medical Sciences for the study. All participants are right-handed. The inclusion criteria consisted of general physical and mental health, no smoking habit, no spine and cervicocephalic surgery, no neuropsychological medication usage, no regular exercise (at least three times a week), and no abnormal sleep pattern. Before the experiment, all participants signed a consent form approved by the Baqiyatallah University of Medical Sciences Ethical Board.
Experimental design
The experiment consisted of two phases, including 23 minutes of stress induction, 20 minutes of recovery, and three measurements (pre-stress, post-stress, and post-recovery). The subjects’ psychological stress scores were measured by the visual analog scale (VAS) (Hellhammer & Schubert, 2012). Their salivary cortisol level and EEG data were also recorded. The stress was induced by the Trier social stress test (TSST) (Kirschbaum et al., 1993), and recordings were performed before and after stress induction and after the recovery phase. The experimental design is illustrated in Figure 1.

TSST
TSST is a standardized protocol for generating moderate psychosocial stress in laboratory settings (Kirschbaum et al., 1993). The TSST consists of 3 minutes brief preparation period followed by 10 minutes test period in which the subject has to deliver a free speech for 2 minutes and perform a mental arithmetic task for 8 minutes in a standing position in front of two referees behind the desk. During his speech, referees with neutral faces only listened to him and warned him to continue when he stopped talking. After the first 2 minutes, the participant was asked to count down from 1022 to 13, and at each wrong subtraction, he was warned to start counting down from the beginning. The VAS questionnaire measured the perceived stress and anxiety scores, assessing the stress (self-reporting) before and after the test and 20 minutes after recovery.
Salivary cortisol level
Following the activation of the hypothalamic-pituitary-adrenal axis and sympathomedullary pathway (Baum & Contrada, 2010), cortisol is released from the adrenal gland into the bloodstream and spreads throughout the body. Changes in cortisol levels have been introduced as a standard stress index that could be measured using the blood or salivary test (Zigmond & Bloom, 1999; Dickerson & Kemeny, 2004). In this study, a salivary cortisol test was performed to confirm the results of TSST. The subjects were asked to eat nothing one hour before the test and wash their mouth right before it. An ELISA kit (IBL Company, made in Germany) was used in the following procedure. First, 0.5 mL of salivary sample was collected before and after stress induction as well as 20 minutes after recovery; then, the samples were frozen at -80 °C. The salivary cortisol levels were then measured from the frozen samples. A statistical analysis was then performed to compare data of the 3 conditions.
EEG data acquisition
EEG data were recorded using a 32-channel amplifier (Mitsar Co Ltd, EEG 202) positioned according to the standard international 10-20 system (one minute with closed eyes and one minute with open eyes). An EEG cap with 32 reusable sintered Ag/AgCl electrodes was applied, and the scalp skin beneath each electrode was kept clean with slight abrasion and cleaning with alcohol. An impedance check was performed, and resistances below 10 kΩ accepted. A bandpass of 0.1-70 Hz was considered during the recording, and the EEG data were recorded using a 12-bit digitizer with a sampling frequency of 256 Hz.
EEG data processing and analysis
A standard preprocessing was performed on the EEG data using the Matlab EEGlab toolbox. The preprocessing consisted of the following sessions: Conversion of the EEG data format readable in Matlab software, version 2017b, filtering unwanted noises using a bandpass FIR filter from 1 to 40 Hz, epoching the data to segments of 1 second, employing ADJUST plugin to remove artifacts based on ICA, and interpolation of bad channels detected by kurtosis of EEG data. Reference of all the electrodes of the average of all channels. After preprocessing, FC is considered as the temporal dependency between neuronal activations of pairs of electrodes (Lang et al., 2012). The FC network was estimated by taking the partial correlation between pairs of electrodes (Friston, 1994, Lang et al., 2012). In the context of brain networks, partial correlation is the correlation between the time series of two nodes after adjusting for the time series from all other network nodes as covariate factors (Wang et al., 2016). FC analysis was performed on the conventional frequency bands, including delta [1-4 Hz], theta [4-8 Hz], alpha [8-13 Hz], beta [13-30 Hz], and lower-gamma [30-40 Hz].
Statistical analysis
Statistical analysis was performed on the VAS, salivary cortisol, and FC maps measured before stress induction, after 23 minutes of inducing mental stress, and 20 minutes after the recovery. After checking the data normality with the Kolmogorov-Smirnov test, the statistical analysis was performed using two distinct paired t-tests for stress induction and recovery phases. Firstly, a paired t-test was applied to the data gathered before and after stress induction to identify how stress induction changes the VAS, salivary cortisol level, and pattern of brain connectivity. Subsequently, the correlation between relative changes in FC [for instance (FCafter stress-FCbefore)/FCbefore] and relative changes in cortisol [for example (Cortisolafter stress-Cortisolbefore)/Cortisolbefore] and VAS [for instance (VASafter stress-VASbefore)/VASbefore] was also computed on the data gathered after inducing stress and after recovery. Relative changes are calculated based on the difference between two conditions divided by the value of the initial condition. Finally, the statistical results were corrected for multiple comparisons effect using the false discovery rate (FDR) (Benjamini & Hochberg, 1995; Shaffer, 1995).
3. Results
Behavioral and physiological changes
The average changes in the VAS in three measurements are presented in Figure 2. The results showed that the VAS scores significantly increased by stress induction. As expected, the VAS scores also significantly decreased after the release of stressful condition (pre-stress: 1.1±1, post-stress: 3.2±2, post-recovery: 0.6±0.7) (F=22.91, P<0.0000 for significant different between post-stress and pre-stress and post-recovery).

The salivary cortisol levels at three stages of the experiment (pre-stress: 2.5±1, post-stress: 4.4±2, post-recovery: 5.1±4 μm/dL) are also presented in Figure 3. The results showed a significant cortisol increase after mental stress induction (P<0.017). Although the cortisol level increased in the recovery phase, the alteration was not significant after the recovery.

Alteration of brain FC
As described in the previous section, the subject-wise FC pattern was calculated based on synchrony between EEG signals of a pair of electrodes using a partial correlation approach (Baba et al., 2004). Subsequently, a statistical comparison was performed using the paired t-test. A correction for multiple comparisons was then performed, presenting the significant changes in Figure 4.

The results showed that stress induction mainly changes the frontotemporal functional connections, especially in delta, alpha, beta, and gamma bands (P<0.05, FDR corrected), observed primarily on the right hemisphere (Figure 4). In addition, 20 minutes of recovery causes a significant change in the temporal-parietal functional connections, especially in theta and beta bands (P<0.05, FDR corrected). In the recovery phase, FCs of the right frontal region increased while FCs in the right parietal region decreased in theta band. FCs of the left parietal region also increase in the beta band. Also, the most significant results (P<0.05, family-wise error (FWE) corrected using the Bonferroni method) are presented in Table 1.

Association between alteration of brain FCs, cortisol level, and VAS
Relative changes in brain FC after stress induction and 20 minutes after the stress release showed a positive correlation with the related cortisol changes after stress induction. Changes in FC between CP4 and T6 in the alpha band (r=0.75, P=0.01) showed a positive correlation with changes in cortisol levels (Figure 5A). In addition, changes in FC between FT8 and CPZ in the alpha band (r=-0.65, P=0.04) also showed a negative correlation with changes in cortisol levels (Figure 5B).

4. Discussion
Our study aimed to evaluate the effect of short-term psychological stress and its release on the brain’s FC. Our findings demonstrate that stressful events change the frontotemporal connections, especially in the delta, alpha, beta, and gamma bands. Moreover, inter-hemispheric FCs of the right frontal regions are mainly decreased by inducing mental stress. In contrast, the interhemispheric FCs of the left frontal regions increase at the delta, theta, alpha, and beta bands and significantly decrease at the lower gamma band. Interestingly, even after 20 minutes of recovery, some reactivity occurred, and the temporal-parietal connections, especially at the theta and beta bands, significantly changed after the release of stressors. The release of stressors presents a recovery pattern of inter-hemispheric FCs mainly observed at the homologous inter-hemispheric FCs in frontocentral regions at the lower gamma band and temporal-parietal regions at the delta band. In addition, the significant changes in the FCs correlate with changes in cortisol and VAS.
The steroid hormone cortisol almost has receptors in every cell in the body that enable it to activate them depending on the cell type, including those in the brain, to regulate metabolism and restore homeostasis (McEwen, 2017). Cortisol could pass the blood-brain barrier and reach its related receptors in the cortex, limbic system, hippocampus, thalamus, and hypothalamus (Dallman, 2005). Hyperpolarization of the membrane has been suggested to be the primary mechanism for the fast effect of cortisol (15 to 20 min) (Makara & Haller 2001). Hyperpolarization is associated with neuronal silence, which could influence the connectivity of a neuronal network (Wrosch et al., 2017). In stressful conditions, this effect has been observed in several brain regions, including the hippocampus, amygdala, and the prefrontal cortex (PFC), and their interactions are influenced (Yaribeygi et al., 2017).
Consequently, cognitive functioning, emotional regulation, and self-regulatory behaviors will be affected (Ursin & Eriksen 2004; McEwen, 2007; Erfani et al., 2016; Yaribeygi et al., 2017). Moreover, it is also important to return to an initial state after removing the stressor to prevent overload stress, which may advance to chronic stress (McEwen, 2017). Since the autonomic nervous system is managed by hemispheric lateralization (Lee et al., 2014), the bihemispheric association must be critical in the recovery phase, which will be discussed in the following section.
Previous studies have shown that the salivary cortisol, VAS, heart rate variation features, the linear and non-linear features, and network features of EEG changed significantly following TSST (Ghahvehchi-Hosseini et al., 2018, Mohammadi Alireza et al., 2018, Lotfan et al., 2019). The relative alpha band power (8-10 Hz) increased after stress in the eye-closed mode in all channels in the same pattern of cortisol change (Ghahvehchi-Hosseini et al., 2018). Besides, the theta/beta ratio decreases (Putman et al., 2013), and asymmetry of functionality in the PFC rises towards the right hemisphere (Seo & Lee, 2010). Moreover, a decrease in alpha band power and an increase in beta band power and their associations with changes in heart rate variability and cortisol level have been reported (Hamid et al., 2010; Seo & Lee, 2010). The imaging study shows that the dorsolateral PFC activity decreases under short-term mental stress (Hermans et al., 2014). These findings demonstrate the complexity of the underlying mechanism that could influence widely distributed FCs in the brain network.
In the same line with the previous studies, we also observed that an active induction of mental stress could significantly decrease the right frontal inter-hemispheric FCs and increase the left frontal inter-hemispheric FCs at the delta, theta, alpha, and beta bands while reducing them at the lower gamma band. For survival, automatic responses must be suppressed after mentally stressful situations. Considering the role of the right side of the brain as the main manager of the sympathetic response (Lee et al., 2014), interactions of the right hemisphere with other parts of the brain must be decreased. One possible interpretation of this mechanism is that cortisol facilitates the hyperpolarization of the neural cell membranes, which is associated with neuronal silence and exerts a top-down inhibition on subcortical regions (Wager et al., 2008) such as the amygdala. Subsequently, the network of hypothalamus-pituitary and adrenal glands are activated, managed by the left hemisphere, to dampen the stress response. Therefore, the left frontal interactions should also increase in this study paradigm. Furthermore, significant correlations between changes in FCs and VAS and cortisol values suggest that the interhemispheric connectivity changes may be associated with the induced stress level.
On the other hand, the release of stressors also presents a recovery pattern of inter-hemispheric FCs. The inter-hemispheric FCs of the right frontal region increase at the delta, theta, and gamma bands, while FCs of the left frontal regions decrease at the delta and alpha bands. Moreover, the inter-hemispheric FCs of the right parietal regions also increase at the theta band, and the inter-hemispheric FCs of the left parietal region decrease at the beta band, which could be related to the reformation of the attention network during the stress release.
5. Conclusion
Our findings reveal that exposure to short-term psychological stress changes the cortisol level and brain FC pattern, which is a clear sign of the behavior measured by the VAS in this study. Based on the results, the bihemispheric association plays a vital role in adapting and coping with stressful conditions.
Study limitations
We cannot thoroughly exclude the variability of the FC measures based on the task-free EEG data in various stages of the experiment. However, paired comparisons in a controlled environment and implying a restricted threshold (FWE corrected P) could reduce the risk of over-interpretation. In addition, only male subjects were recruited in this study, making it inconceivable to apply the results to female subjects. Gender differences in response to stress are considered a major issue. Furthermore, this study might be performed with a larger sample size to improve the power of calculations. Also, investigating task-based EEG while subjects are exposed to stress stimuli could provide more information on the dynamic of FC changes.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Baqiyatallah University of Medical Science (Code: IR.BMSU.REC.1389.141).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and supervision: Reza Khosrowabadi and Boshra Hatef; Methodology: Zahra Rezvani; Data collection: Boshra Hatef and Golam-Hossein Meftahi; Data analysis: Zahra Rezvani and Afrooz Seyedebrahimi; Investigation, and writing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to extend their heartfelt thanks to all participants in the experiments for their invaluable contribution to this study.
References
Achard, S., Salvador, R., Whitcher, B., Suckling, J., & Bullmore, E. (2006). A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 26(1), 63–72. [DOI:10.1523/JNEUROSCI.3874-05.2006] [PMID] [PMCID]
Alonso, J. F., Romero, S., Ballester, M. R., Antonijoan, R. M., & Mañanas, M. A. (2015). “Stress assessment based on EEG univariate features and functional connectivity measures.” Physiological Measurement, 36(7),1351-1365. [DOI:10.1088/0967-3334/36/7/1351] [PMID]
Baba, K., Shibata, R., & Sibuya, M. (2004). “Partial correlation and conditional correlation as measures of conditional independence.” Australian & New Zealand Journal of Statistics, 46(4), 657-664. [DOI:10.1111/j.1467-842X.2004.00360.x]
Contrada, R. J. (2010). The handbook of stress science: Biology, psychology, and health. New York: Springer Publishing Company.[Link]
Benjamini, Y., & Hochberg, Y. (1995). “Controlling the false discovery rate: A practical and powerful approach to multiple testing.” Journal of The Royal Statistical Society. Series B (Methodological), 57(1), 289-300. [DOI:10.1111/j.2517-6161.1995.tb02031.x]
Dallman, M. F. (2005). “Fast glucocorticoid actions on brain: Back to the future.” Frontiers in Neuroendocrinology, 26(3-4), 103-108. [DOI:10.1016/j.yfrne.2005.08.001] [PMID]
Dickerson, S. S., & Kemeny, M. E. (2004). “Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research.” Psychological Bulletin, 130(3), 355–391.[DOI:10.1037/0033-2909.130.3.355] [PMID]
Erfani, M., Sahraei, H., Bahari, Z., Meftahi, G. H., Hatef, B., & Mohammadi, A., et al. (2017). “Evaluation of the effect of time change in cognitive function in volunteers in Tehran.” Global Journal of Health Science, 9(2), 119. [DOI:10.5539/gjhs.v9n2p119]
Friston, K. J. (1994). “Functional and effective connectivity in neuroimaging: A synthesis.” Human Brain Mapping, 2(1-2), 56-78. [DOI:10.1002/hbm.460020107]
Garmezy, N., & Rutter, M. (1983). Stress, coping, and development in children. Maryland: Johns Hopkins University Press. [Link]
Ghahvehchi-Hosseini, F., Manshadi, E., Mohammadi, A., Jahromi, G., & Hatef, B. (2018). [Evaluation of the persistence effect acute social stress test on the alpha band power (Persian)]. Journal of Military Medicine, 20(5), 509-518. [Link]
Hamid, N. H., Sulaiman, N., Aris, S. A. M., Murat, Z. H., & Taib, M. N. (2010). Evaluation of human stress using EEG Power Spectrum. Paper presented at: 6th International Colloquium on Signal Processing & its Applications, Malacca, Malaysia, 21-23 May 2010. [DOI:10.1109/CSPA.2010.5545282]
Hellhammer, J., & Schubert, M. (2012). The physiological response to Trier Social Stress Test relates to subjective measures of stress during but not before or after the test. Psychoneuroendocrinology, 37(1), 119-124. [DOI:10.1016/j.psyneuen.2011.05.012] [PMID]
Hermans, E. J., Henckens, M. J., Joëls, M., & Fernández, G. (2014). Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends in Neurosciences, 37(6), 304-314. [DOI:10.1016/j.tins.2014.03.006] [PMID]
Hüther, G. (1998). “Stress and the adaptive self-organization of neuronal connectivity during early childhood.” International Journal of Developmental Neuroscience, 16(3-4), 297-306. [DOI:10.1016/S0736-5748(98)00023-9] [PMID]
Khosrowabadi, R. (2018). Stress and Perception of emotional stimuli: Long-term stress rewiring the brain. Basic and Clinical Neuroscience, 9(2), 107-120. [DOI:10.29252/nirp.bcn.9.2.107] [PMID] [PMCID]
Khosrowabadi, R., Quek, C., Ang, K. K., Tung, S. W., & Heijnen, M. (2011). A brain-computer interface for classifying EEG correlates of chronic mental stress. Paper presented at: The 2011 International Joint Conference on Neural Networks, San Jose, CA, USA, 31 July 2011 - 05 August 2011. [DOI:10.1109/IJCNN.2011.6033297]
Kirschbaum, C., Pirke, K. M., & Hellhammer, D. H. (1993). “The ‘Trier Social Stress Test’-a tool for investigating psychobiological stress responses in a laboratory setting.” Neuropsychobiology, 28(1-2), 76-81. [DOI:10.1159/000119004] [PMID]
Lang, E. W., Tomé, A. M., Keck, I. R., Górriz-Sáez, J. M., & Puntonet, C. G. (2012). Brain connectivity analysis: A short survey. Computational Intelligence and Neuroscience, 2012, 412512.[DOI:10.1155/2012/412512] [PMID] [PMCID]
Lee, S. W., Gerdes, L., Tegeler, C. L., Shaltout, H. A., & Tegeler, C. H. (2014). “A bihemispheric autonomic model for traumatic stress effects on health and behavior.” Frontiers in Psychology, 5, 843. [DOI:10.3389/fpsyg.2014.00843] [PMID]
Lewis, R. S., Weekes, N. Y., & Wang, T. H. (2007). The effect of a naturalistic stressor on frontal EEG asymmetry, stress, and health. Biological Psychology, 75(3), 239-247. [DOI:10.1016/j.biopsycho.2007.03.004] [PMID]
Lotfan, S., Shahyad, S., Khosrowabadi, R., Mohammadi, A., & Hatef, B. (2019). Support vector machine classification of brain states exposed to social stress test using EEG-based brain network measures. Biocybernetics and Biomedical Engineering, 39(1), 199-213. [DOI:10.1016/j.bbe.2018.10.008]
Lupien, S. J., McEwen, B. S., Gunnar, M. R., & Heim, C. (2009).“Effects of stress throughout the lifespan on the brain, behaviour and cognition.” Nature Reviews. Neuroscience, 10(6), 434–445. [DOI:10.1038/nrn2639] [PMID]
Makara, G. B., & Haller, J. (2001). Non-genomic effects of glucocorticoids in the neural system: Evidence, mechanisms and implications. Progress in Neurobiology, 65(4), 367-390. [DOI:10.1016/S0301-0082(01)00012-0] [PMID]
McEwen, B. S. (2007). Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Reviews, 87(3), 873-904. [DOI:10.1152/physrev.00041.2006] [PMID]
McEwen, B. S. (2017). Neurobiological and systemic effects of chronic stress. Chronic Stress (Thousand Oaks, Calif.), 1, 2470547017692328. [DOI:10.1177/2470547017692328] [PMID] [PMCID]
McEwen, B. S., Bowles, N. P., Gray, J. D., Hill, M. N., Hunter, R. G., & Karatsoreos, I. N., et al. (2015). Mechanisms of stress in the brain. Nature Neuroscience, 18(10), 1353–1363. [DOI:10.1038/nn.4086] [PMID] [PMCID]
Mohammadi, A., Emamgoli A., Shirinkalam, M., Meftahi, GH., Yagoobi, K., & Hatef, B. (2019). The persistent effect of acute psychosocial stress on heart rate variability. The Egyptian Heart Journal, 71(18). [Link]
Peters, A., McEwen, B. S., & Friston, K. (2017).“Uncertainty and stress: Why it causes diseases and how it is mastered by the brain.” Progress in Neurobiology, 156, 164–188. [DOI:10.1016/j.pneurobio.2017.05.004] [PMID]
Putman, P., Verkuil, B., Arias-Garcia, E., Pantazi, I., & van Schie, C. (2014).“EEG theta/beta ratio as a potential biomarker for attentional control and resilience against deleterious effects of stress on attention.” Cognitive, Affective & Behavioral Neuroscience, 14(2), 782–791. [PMID]
Putman, P., Verkuil, B., Arias-Garcia, E., Pantazi, I., & van Schie, C. (2014). EEG theta/beta ratio as a potential biomarker for attentional control and resilience against deleterious effects of stress on attention. Cognitive, Affective, & Behavioral Neuroscience, 14(2), 782-791. [DOI:10.3758/s13415-013-0238-7] [PMID]
Schoffelen, J. M., & Gross, J. (2009). Source connectivity analysis with MEG and EEG. Human Brain Mapping, 30(6), 1857-1865. [DOI:10.1002/hbm.20745] [PMID] [PMCID]
Seo, S. H., Lee, J. T., & Crisan, M. (2010). Stress and EEG. In M. Crisan (Ed.), Convergence and hybrid information technologies. London: IntechOpen. [Link]
Shaffer, J. P. (1995). Multiple hypothesis testing. Annual Review of Psychology, 46(1), 561-584. [DOI:10.1146/annurev.ps.46.020195.003021]
Tegeler, C. H., Shaltout, H. A., Tegeler, C. L., Gerdes, L., & Lee, S. W. (2015). Rightward dominance in temporal high-frequency electrical asymmetry corresponds to higher resting heart rate and lower baroreflex sensitivity in a heterogeneous population. Brain and Behavior, 5(6), e00343. [DOI:10.1002/brb3.343] [PMID] [PMCID]
Ursin, H., & Eriksen, H. R. (2004). The cognitive activation theory of stress. Psychoneuroendocrinology, 29(5), 567-592. [DOI:10.1016/S0306-4530(03)00091-X] [PMID]
Wager, T. D., Davidson, M. L., Hughes, B. L., Lindquist, M. A., & Ochsner, K. N. (2008). Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron, 59(6), 1037-1050. [DOI:10.1016/j.neuron.2008.09.006] [PMID] [PMCID]
Wang, Y., Kang, J., Kemmer, P. B., & Guo, Y. (2016). “An efficient and reliable statistical method for estimating functional connectivity in large scale brain networks using partial correlation. Frontiers in Neuroscience, 10, 123. [DOI:10.3389/fnins.2016.00123] [PMID]
Wrosch, J. K., Einem, V. V., Breininger, K., Dahlmanns, M., Maier, A., & Kornhuber, J., et al. (2017). Rewiring of neuronal networks during synaptic silencing. Scientific Reports, 7(1), 11724. [DOI:10.1038/s41598-017-11729-5] [PMID] [PMCID]
Type of Study: Original |
Subject:
Cognitive Neuroscience
Received: 2020/04/26 | Accepted: 2020/07/6 | Published: 2024/09/1
Received: 2020/04/26 | Accepted: 2020/07/6 | Published: 2024/09/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





