Volume 13, Issue 2 (March & April 2022)
BCN 2022, 13(2): 257-268 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Haghshenas Bilehsavar S, Batouli S A H, Soukhtanlou M, Alavi S, Oghabian M A. Different Olfactory Perception in Heroin Addicts Using Functional Magnetic Resonance Imaging. BCN 2022; 13 (2) :257-268
URL: http://bcn.iums.ac.ir/article-1-1736-en.html
URL: http://bcn.iums.ac.ir/article-1-1736-en.html
Shirin Haghshenas Bilehsavar1 
 , Seyed Amir Hossein Batouli1
, Seyed Amir Hossein Batouli1 

 , Mohammad Soukhtanlou2
, Mohammad Soukhtanlou2 
 , Sasan Alavi3
, Sasan Alavi3 
 , Mohammad Ali Oghabian *
, Mohammad Ali Oghabian * 
 4
4

 , Seyed Amir Hossein Batouli1
, Seyed Amir Hossein Batouli1 

 , Mohammad Soukhtanlou2
, Mohammad Soukhtanlou2 
 , Sasan Alavi3
, Sasan Alavi3 
 , Mohammad Ali Oghabian *
, Mohammad Ali Oghabian * 
 4
4
1- Department of Neuroimaging and Analysis, Tehran University of Medical Sciences, Tehran, Iran.
2- Department of Psychology and Education, Alborz Campus, University of Tehran, Tehran, Iran.
3- Department of Addiction, School of Behavioural Sciences and Mental Health (Institute of Tehran Psychiatry), Iran University of Medical Sciences, Tehran, Iran.
4- Department of Neuroscience and Addiction Studies, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran.
2- Department of Psychology and Education, Alborz Campus, University of Tehran, Tehran, Iran.
3- Department of Addiction, School of Behavioural Sciences and Mental Health (Institute of Tehran Psychiatry), Iran University of Medical Sciences, Tehran, Iran.
4- Department of Neuroscience and Addiction Studies, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran.
Keywords: Drug addiction, Olfactory perception, Functional Magnetic Resonance Imaging, Lingual gyrus, Cerebellum
Full-Text [PDF 1133 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Adverse effects of addiction on the brain
Addiction is a mental disorder with cognitive, clinical, and social adverse effects, and recently its rising prevalence has been observed in the communities. Drugs affect the functional brain networks, for example, by altering the level of neurotransmitters or by over-exciting the brain’s reward system (Goldestein & Volkow, 2002), resulting in a craving for drug abuse (Goldestein & Volkow, 2002). Drug addiction is an acute relapsing disorder with the following characteristics: compulsion for drug abuse, disability in limiting the amount of consumption, and failure to control negative emotions (Koob & Volkow, 2010). Addiction happens mostly in 3 stages: binge or intoxication, withdrawal or negative effect, and mental preoccupation or anticipation. In these stages, alterations are observed in the neurotransmitters and neuromodulators of the brain, such as in the mesocorticolimbic dopaminergic system, the corticotropin-releasing hormone (CRH) of the amygdala, and the level of glutamate in the corticostriatal pathway. All these facts show that addictive behavior is the result of changes in the molecular, synaptic, and network levels of the brain (Spiga, Mulas, Piras & Diana, 2014).
Addiction could be in the form of drug dependency or behaviors such as gambling or gaming. The range of addictive disorders is so vast that sometimes an impulse control disorder, such as pathologic gambling, could also be included (Goudriaan, De Ruiter, van den Brink, Oosterlaan, & Veltman, 2010); however, different addictive disorders have common neurobiological factors. Now, besides gambling, many kinds of behavioral addiction have presented, like gaming addiction, sexual addiction, shopping addiction, pornography addiction, internet addiction, compulsive hoarding, and food addiction. Although behavioral addictions have specific diagnostic factors, drug addiction and behavioral addiction have many similarities in neurochemical and brain activation patterns.
These findings suggest that human societies face more complex addiction problems that require a more accurate and complete understanding of this disease to find effective prevention and treatment methods. Furthermore, addiction is a complex disorder under the influence of an interplay between genetic and environmental factors (Sachdev et al., 2013), and many neurobiological alterations are still unidentified (Goldestein & Volkow, 2002). Research studies are still conducted to understand better the neurobiology of addiction, which leads to successful treatment or its prevention. Among the many methods, neuroimaging approaches are the strongest to reveal the mechanisms of addiction. In recent decades, studies have been conducted using fMRI to understand the brain network involved in addiction better (Goudriaan et al., 2010; Bragulat, Dzemidzic, M., Talavage, Davidson, Oconnor, & Kareken, 2008; London, Kohno, Morales & Ballard, 2015; Goldstein et al., 2007). Today, with new neuroimaging techniques and methods, we are almost convinced that heroin causes noticeable structural changes in the brain. By a diffusion tensor imaging (DTI) study, abnormal white matter microstructure is seen in heroin users according to the duration of heroin-dependent, which can be associated with impaired decision-making (Qiu et al. 2013). A DTI study in permanent heroin users reported disrupted white matter integrity in the frontal white matter (Liu et al. 2008). Another study by fMRI and voxel-based morphometry (VBM) verifies grey matter atrophy, particularly in the frontal and cingulate areas (Liu et al. 2009).
However, these studies are few, and we are not entirely sure how much of these structural changes have directly caused functional alteration in heroin users. Previous studies by fMRI (task-based and resting-state) and positron emission tomography (PET) suggest a wide range of functional alterations in heroin abusers. Functional connectivity in the ventral anterior cingulate cortex (VACC) in a task-based fMRI study shows a significant decrease in heroin users (Wang et al. 2010). Activation in the default mode network (DMN) by rest fMRI is considerably different in heroin addicts compared to the normal group (Hu et al. 2012). Other studies suggest the abnormal functional organization of the DMN that may cause impaired cognitive control in heroin addicts (Ma et al. 2011). In a PET study, activation in the lateral orbitofrontal cortex (LOFC) as an important area in human decision-making and behavior in heroin users is significantly different from healthy group and methadone users (Ersche et al. 2006). However, all functional alterations in the whole brain and its bases have not been fully identified in heroin abusers.
In our previous projects, we attempted to examine the effective connectivity network of treatments such as methadone with fMRI (Zare Sadeghi et al., 2017). After our recent study that attempted to understand olfaction by fMRI better, another study seemed necessary to understand addiction better by olfactory perception differences as a necessary but less well-known factor in neurodegenerative disorders (Vedaei, Oghabian, Firouznia & Harirchian, 2016).
Disorders of olfaction
Olfaction is one of the sensory systems. It has been the topic of research in recent years due to its associations with many disorders such as neurodegenerative diseases, mood disorders such as depression, brain tumors, traumas, schizophrenia, and anorexia nervosa (Lombion-Pouthier et al., 2006). There have been many reports on the alteration of the olfactory perception in disorders such as Alzheimer disease, Parkinson disease, Lewy body dementia, PARK8 [individuals with mutations in the LRRK2 gene], Huntington disease, motor neuron disease, and Friedreich ataxia (Huttenbrink, Hummel, Berg & Hahner, 2013). These associations are now even used as a predictor of the diseases such as Alzheimer and Parkinson disease, as there are reports that 90% of such patients do show early signs of olfaction disorders. Olfactory function in Parkinson patients could be diagnosed with TDI and some other tests (Casjens et al., 2013). These studies illustrate that approximately 75% of IPD (idiopathic Parkinson disease) patients regarding the aging face decreasing in olfaction in their TDI as hyposmia and anosmia.
Olfaction in addicts
The olfactory system is different from other senses considering the human’s emotions and mood. This status could be due to direct pathways from the olfactory system to the limbic system (Lombion-Pouthier et al., 2006). The olfactory disorder has been studied in many diseases with respect to behavior changes. In this regard, drug addicts are among the most important patients whose olfaction should be studied. In the study by Sandrine lombain (Lombion-Pouthier et al., 2006), a lower score for odor discrimination and identification was observed in drug and alcohol addicts compared to healthy individuals. Similar findings were observed in another study, which showed deteriorated olfactory perception in alcoholics regarding the discrimination, identification, and threshold (Shear et al., 1992).
Olfaction is associated with some cognitive abilities of the brain, such as memory and emotion processing (Cerf-Ducastel & Murphy, 2006), and therefore studying olfactory ability in addiction would add information on the mechanism of addiction. A few behavioral studies on the olfactory perception of addicts versus normal controls showed a significant difference in odor identification, as well as a lower detection threshold in addicts (Lombion-Pouthier et al., 2006). These findings indicate the association of addiction with olfactory disorders. In studies on addicts to cannabis or alcohol, it was shown that, similar to general cognitive abilities, the olfactory perception could also be deteriorated due to drug addiction. In particular, these studies reported that addiction is associated with a decline in limbic system activities that impairs perceiving pleasant odors and the resulting pleasure. Studies have reported that brain neurons could influence the cannabinoid production process, which has a significant role in the olfactory bulb as a retrograde messenger (Walter et al., 2017) and therefore affect the GABA(γ-aminobutyric acid)ergic system.
In one fMRI study on the olfactory perception of alcoholic addicts, compared to other types of odors, smelling alcohol was associated with increased activation in a few brain areas, including the nucleus accumbens (Bragulat et al., 2008).
Aims of the study
Although there are behavioral studies on the olfactory perception of addicts, we have not found any fMRI study which tests the neural mechanisms of odor perception in heroin addicts and its differences from normal controls. In particular, understanding the neural mechanisms of the brain of drug addicts in response to smelling non-craving odors would be an interesting idea to gain more information on the relevant brain mechanisms.
In this study, we recruited 20 heroin addicts and 20 normal as controls. We presented odor stimuli and assessed them with fMRI to find differences in their brain activities when perceiving different odors. The results of this study would help find a new missing component in the brain function of addicted patients to design more accurate brain circuitry for these patients to fill the treatment or prevention gap of addiction or even design new treatment methods.
2. Materials and Methods
Study participants
This study was performed by the Ethics Committee of Tehran University of Medical Sciences. All participants gave their consent during the initial interview after being informed about the main study objectives and signed their consent form on the test day. Forty right-handed male subjects were included in this study. Of these, 20 were normal healthy participants (control group; Mean±SD age: 27.6±4.5 years; range: 22-38 years), and 20 were heroin-dependent subjects (addict group; Mean±SD age: 30.9±6.0 years; range: 20-41 years old). The Mean±SD duration of drug abuse in the addict group was 5.53±4.06 years. The two groups did not differ in age (Pt test >0.05), and education (Pt test >0.05) and were selected from the same socioeconomic class.
All the abstinent heroin subjects were members of “Congress 60,” an organization that manages networks of centers that treat abuse of substances in Iran by abstinent-based methods. All participants had negative urine tests for opiate and stimulant drugs at least 90 days before the scanning session based on their treatment center report (according to the center’s policy, urine check was carried out twice a week) and were rechecked during the study at the time before image acquisition for each subject. All heroin-dependent subjects met the diagnostic and statistical manual of mental disorders (DSM) IV-TR (Ref.) criteria for heroin dependence (before participating in the treatment program).
The mental and physical health of the participants was tested by a physician at the imaging center using our questionnaire and based on the inclusion/exclusion criteria of the International Consortium for Brain Mapping (ICBM) (1). None of the heroin dependents and healthy controls reported a head trauma or had nasal or sinus complaints. The participants were excluded due to any current or past chronic or acute neurologic or internal disorders, medicine consumption, surgery, or trauma; being overweight (over 100 kg); having a serious family history of any disease; being claustrophobic, or having implants or any other metal objects in the body. Smoking was not an exclusion criterion.
Behavioural olfactory test
The standardized psychophysical olfactory test, the “sniffing stick” test, was performed on all participants before the fMRI session. This battery of tests consisted of odor threshold (T), discrimination (D), and identification (I) parts. The total score (TDI) was determined by the sum of the three parts scores (T+D+I) (12). For this behavioral assessment, the odorants were presented via a pen-like dispensing device. The examiner removes the pen’s cap and places it 2 cm away from both nostrils for approximately 3 s. The threshold test is conducted with phenyl ethyl alcohol or n-butanol in a triple-forced-choice paradigm. Three pens are presented to the participants in a randomized manner. Only one of these contains the odorant, while the two others contain only the diluents. Each participant is then asked to determine the pen that smells different from the others.
A particular odorant concentration will only be correctly identified if the pen containing the odorant is recognized twice in a row. Following this, the next higher dilution step is presented. If this is also correctly identified twice, the next higher dilution step is presented until the participant makes an incorrect decision, in which case, the next lowest dilution step is presented. If the participant cannot identify this level of concentration, the next lower dilution step is presented. This pattern is continued until the participant correctly identifies a dilution step. The test is completed when seven reversal points are passed, and the smell threshold (T) is defined as the mean of the last four staircase reversal points. For the odor discrimination test (D), 16 pen triplets are presented to each participant: two pens smell the same, and the remaining pens contained a different odorant.
The participants should determine which of the three pens smells differently. During the odor threshold and odor discrimination tests, the participants are blindfolded to prevent visual recognition of the pen’s odorants. The odor identification test is conducted via the use of 16 common odorants. Each participant receives a multiple-choice card and is asked to pick the term that describes the presented odorant. For all three parts of the test (threshold, discrimination, and identification), the participants’ scores range from 1 to 16. Therefore, a total score is determined by summing the results obtained (TDI score). Accordingly, olfactory function are classified in terms of anosmia (TDI<16), hyposmia (1630). The results of this test ensure that all participants have a normal olfactory system. A score of ≥30 is utilized to identify participants that are appropriate for the fMRI session.
In our study, the average scores of addicts for this test were as follows: the mean threshold, 8.17; the mean discrimination, 10.03; the mean identification, 12.15; and the mean total score, 30.3. For controls, the scores were as follows: the mean threshold, 9.55; the mean discrimination, 11.90; the mean identification, 13.9, and the total mean score, 35.36. The two-sample two-tailed t test showed the two groups were significantly different in discrimination (P<0.05), identification (P<0.001), and the total score (P<0.001), but not in the threshold (P>0.05).
Imaging
Magnetic Resonance Imaging (MRI) of the brain was carried out using a SIEMENS 3 Tesla MRI scanner (MAGNETOM Trio; Siemens Healthcare GmbH, Federal Republic of Germany) with a 32-channel head coil at the Medical Imaging Center, Imam Khomeini Hospital, Tehran, Iran. During the scanning procedure, foam cushions were used to minimize head movements within the coil. Functional T2*-weighted images were collected using blood oxygen level-dependent (BOLD) contrast (TR [repetition time]=3000 ms, TE [time to echo]=30 ms, flip angle=90 degrees, FOV [field of view]=192 mm2, matrix size=64×64, voxel size=3×3×3 mm, and slice gap=0 mm). Before the functional scan, a T1-weighted anatomical image was acquired, using a gradient echo pulse sequence (TR=1800 ms, TE=3.44 ms, flip angle=7 degrees, voxel size=1×1×1 mm, FOV=256 mm2, matrix size=256×256, and slice gap=0 mm).
fMRI paradigm
Olfactory stimulation was administered using a Magconcept olfactometer (USA, 2010) with a continuous airflow rate (2 L/min). The olfactometer consists of three main parts: a positive air pressure device that was used in the control room, a nasal mask, and a delivery system consisting of some capsules with the odorants. The parts of the olfactometer placed in the magnet room were made with diamagnetic materials to avoid any uniformity disturbances of the magnetic field and a decline in the signal-to-noise ratio. This system was controlled by a computer program to permit choosing of, and switching between, different types of stimulation. Odor and air delivery timing, stimulus frequency, and choice of a specific odorant for stimulus presentation within any task are controlled by the software.
The odorant presentation model we used was a block design consisting of two alternating patterns. A 15-s odor presentation (eucalyptus; Nature’s Alchemy Co. 100% pure natural essential oil), followed by a rest period of 45 s (odorless air), constituted these blocks. To assure that the resting period only contains pure air and not being polluted by any odorant molecules of the stimulus phase, the duration of this block was selected to be longer. This alternation of activation and resting was repeated for 10 rounds (a total of 600 s). The pure air and odorant conveyed to the participant’s nose had similar pressure and temperature.
Data analysis
Before processing, the analysis was performed using the fMRI Expert Analysis Tool (FEAT), part of FMRIB’s Software Library (FSL, http://www.fmrib.ox.ac.uk/fsl) version 5.0.9. Pre-processing steps included motion correction using FSL-MCFLIRT (Motion Correction from FMRIB’s Linear Image Registration Tool), skull-stripping for removal of non-brain tissue using FSL-BET (Brain Extraction Tool), slice-timing correction using Fourier-space time-series phase-shifting, normalization of the functional images to the standard Montreal Neurological Institute (MNI) brain atlas in two steps: 1) co-registration of each individual’s functional images to his high-resolution T1-weighted scan, using FLIRT (FMRIB’s Linear Image Registration) and 7 degrees of freedom (DoF), 2) Linear registration of the structural T1 images to the MNI space, with 12 DoF; using a Gaussian kernel of 6.0 mm FWHM for spatial smoothing, multiplicative mean intensity normalization of the volume at each time point, and high pass temporal filtering (Gaussian-weighted least-squares straight-line fitting, with sigma=60.0 s).
In the first-level analysis, the parametric statistical analysis was based on a general linear model (GLM) and performed using FSL-FEAT (version 6.0.0). The FILM (FMRIB Improved Linear Model) pre-whitening was used for statistical analysis of the fMRI time-series to make the statistical approaches valid and maximally efficient, which devoted a “z-score” to the corresponding BOLD signal. The individual GLM analyses were performed by creating a boxcar function of tasks (different conditions) against rest, being convolved with a canonical hemodynamic response function and its temporal derivatives. As explained above, registration of the estimated function map to the corresponding structural image and ultimately the MNI space was also carried out.
For higher-level analysis, the group-level analysis was performed using FLAME (FMRIB’s local analysis of mixed effects) to estimate within-group averages and between-group comparisons. Cluster thresholding was performed to reveal the significantly activated clusters. The criteria for identification of significantly active clusters was a voxel-level probability threshold of z>2.3; a false discovery rate (PFDR<0.05) was also used to correct for multiple comparisons. For a higher assurance of the findings, we selected a more stringent P<0.005, corresponding to an effect size of z>2.6.
3. Results
Within-group activations
Initially, the average brain activations of the two groups in response to the odor stimulation were estimated. Details of these activations, including size, coordinates, and the maximum z-value of the activation clusters, are presented in Table 1.
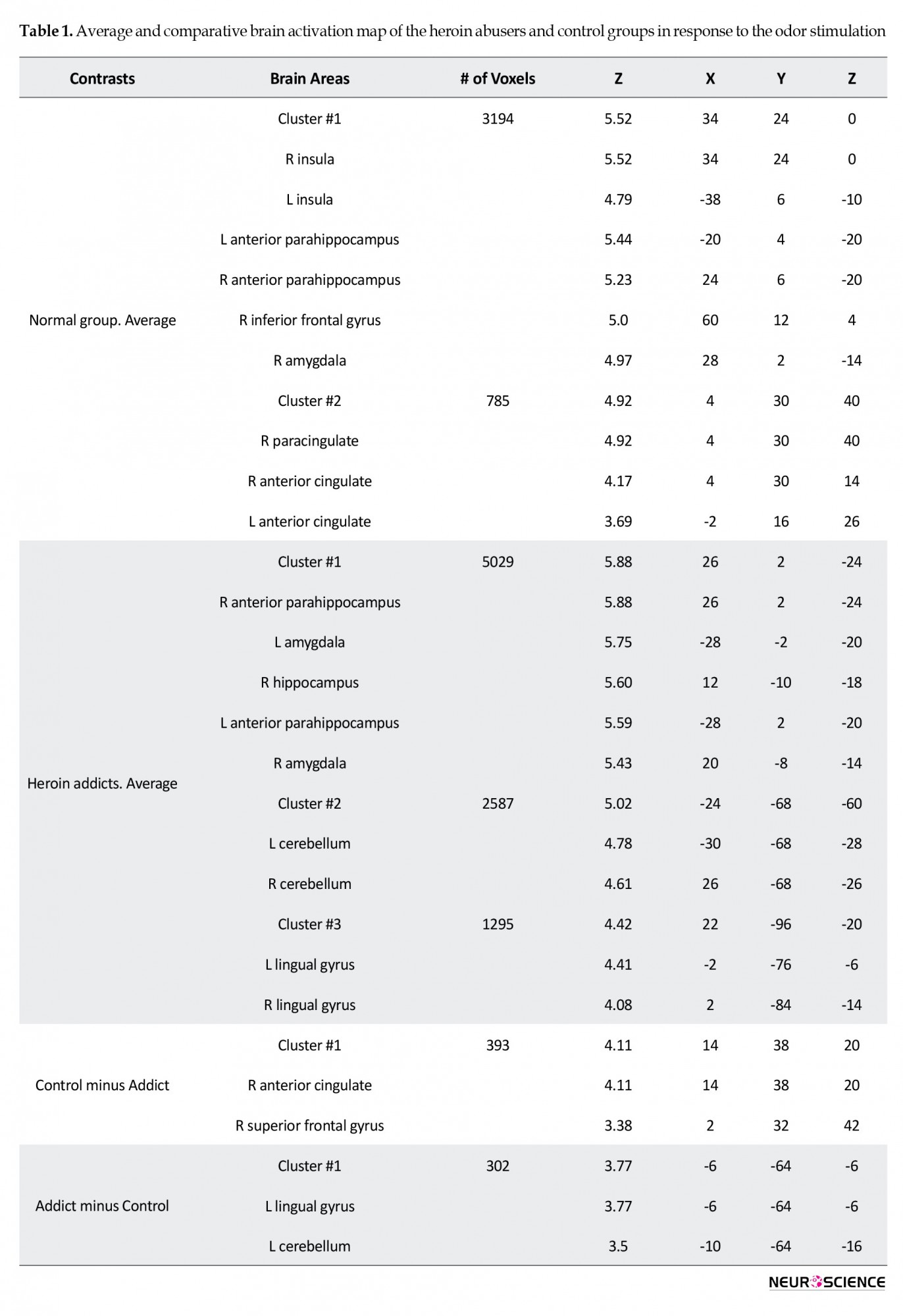
In the control group, two activation clusters were observed. Cluster 1 (number of voxels=3194, z=5.52) showed six active brain areas: the bilateral insula, bilateral anterior parahippocampus, right inferior frontal gyrus, and right amygdala. The second cluster (number of voxels=785, z=4.92) also showed activity of the right paracingulate and bilateral anterior cingulate.
The addicted group, on average, showed three activation clusters. The first cluster (number of voxels=5029, z=5.88) were bilateral anterior parahippocampus, bilateral amygdala, and right hippocampus; the second cluster (number of voxels=2587, z=5.02) included bilateral cerebellum, and the third cluster (number of voxels=1295, z=4.42) showed active bilateral lingual gyrus. The maps of these activations are provided in Figure 1.
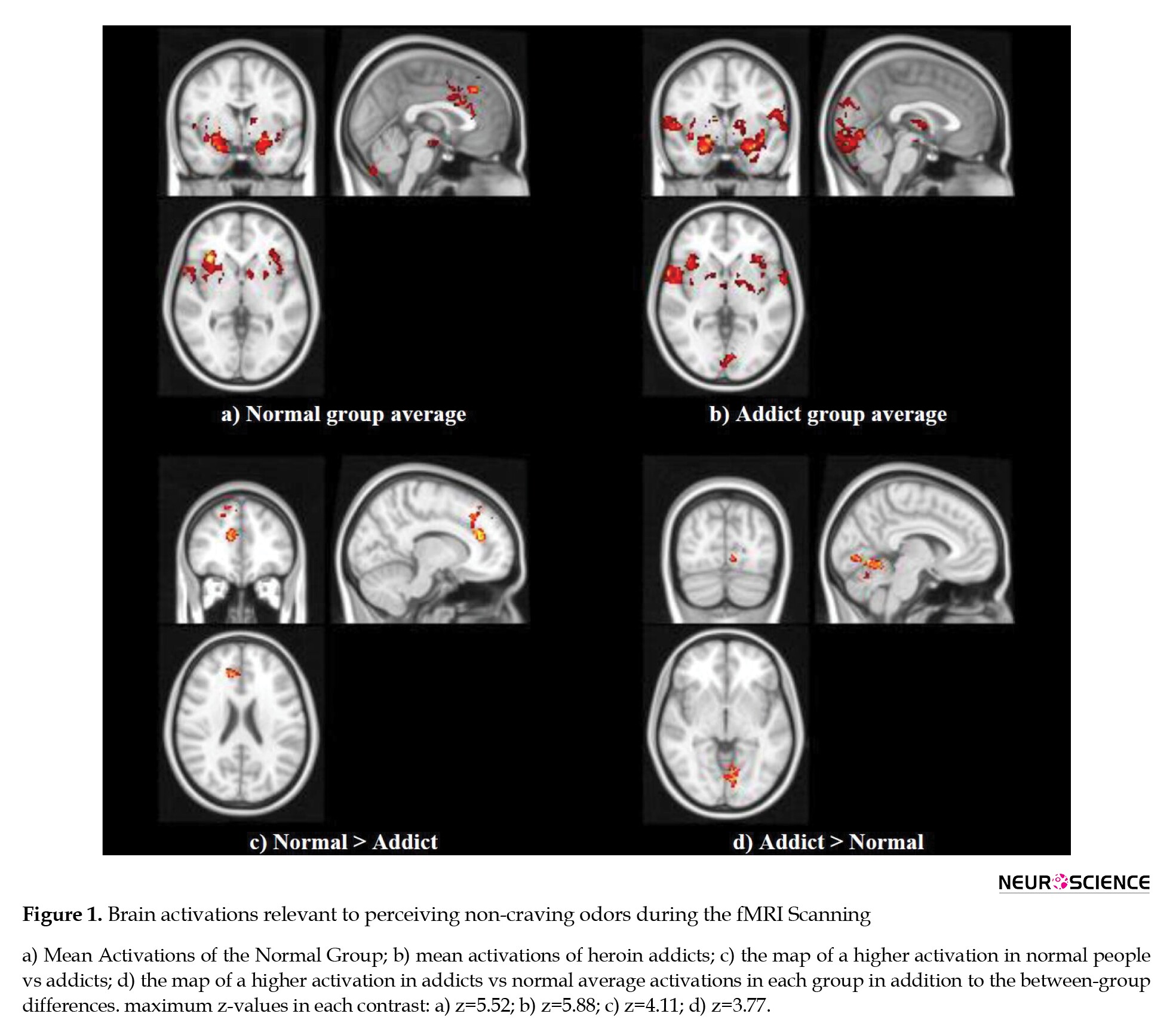
Between-group contrasts
The activation maps of the two groups were contrasted to find any statistically significant differences between them. Accordingly, two brain areas (number of voxels=393, z=4.11) showed higher activation in the control group: the right anterior cingulate and right superior frontal gyrus. On the other hand, two regions of the brain (number of voxels=302, z=3.77) were more active in addicts: the left lingual gyrus and left cerebellum. Details are provided in Table 1.
4. Discussions
Summary of the results
In this study, using a salient pleasant odor as the non-craving stimulant during the fMRI scanning, we investigated the brain areas involved in olfactory perception in the healthy normal and heroin addicts. The active brain areas in olfactory perception in the controls were similar to the previous findings of fMRI or PET/SPECT studies. However, there were differences between the pattern and intensity of the activations of the two groups, as will be discussed below.
Brain olfactory network
The active brain areas in the controls during olfactory perception included bilateral insula, bilateral anterior parahippocampus, bilateral anterior cingulate, right inferior frontal gyrus, right amygdala, and right paracingulate. A similar brain network in this function is also observed in previous studies (Herz, Eliassen, Beland & Souza, 2003). Details of these activations are discussed below.
Insula is connected to the primary olfactory cortex and the areas such as the piriform cortex and the amygdala, and it has shown activation in prolonged olfactory stimulation, as well as in the discrimination of odors (Vedaei, et al., 2016). There are previous reports on the role of the parahippocampus in identifying the known odors (Herz, etal., 2004). This area, along with the amygdala, is shown to have a significant activation in recalling odors (Herz, etal., 2004). Orbitofrontal cortex (OFC) is at the ventral surface of the frontal lobe, and the studies on the association of brain lesions and olfactory disorders have shown that the right OFC has a more dominant role in this function (Zatorre & Jones-Gotman,1991; Zald & Pardo, 2000); an exception is the unpleasant odors in which the left OFC shows a higher activation (Zatorre & Jones-Gotman,1991; Zald & Pardo, 2000). The posterior areas of the inferior frontal gyrus are also involved in olfaction (Zald & Pardo, 1999). Observing the activity of this area in this function is very troublesome and needs a high-resolution MRI due to its proximity to the ocular muscles (Zald & Pardo, 1999); albeit our results have shown that this area is active. The posterior and medial parts of the inferior frontal gyrus, such as the piriform cortex, show activation in response to the sniffing function (Sobel 1998).
Amygdala was also observed to be active in this study. This area is part of the primary olfactory cortex (Zald & Pardo, 1999; Gonzalez et al., 2006), which along with the piriform cortex, has some white matter connections to the olfactory areas. This area has a role in the recognition of odors as well as in emotional reactions to odors, and the level of amygdala activation is (Zald & Pardo, 1999) associated with the odor intensity (Vedaei, et al., 2016). In previous studies (Zatorre 1992, Zald & Pardo, 1999), it was reported that the left amygdala does not show significant activation when confronting unpleasant odors, and its activation is mostly associated with pleasant ones (Koob & Volkow 2010). This function could be one reason for observing unpleasant olfactory delusion in epileptic patients with lesions invading the amygdala, and these patients very rarely show a hallucination of pleasant odors (Zald & Pardo, 1999).
The cingulate cortex was also active in this study. The involvement of the anterior cingulate in this function is illustrated using PET scanning many years ago (Cerf Ducastel & Murphy, 2006; Savic, Gulyas, Larsson, & Roland 2000, dade 2002). In recent fMRI studies with pleasant odors, the activation of medial OFC and pregenual cingulate has been illustrated; the inferior frontal gyrus (IFG) was also active when the intensity of odors was elevated (Bragulat et al., 2008; Chiaravalloti et al., 2015). Studies have shown that involvement in emotional comparison or perception is one main reason for the activation of medial OFC and the pregenual cingulate (Chiaravalloti et al., 2015; Gonzalez et al., 2006). These regions, as the secondary olfactory areas, are therefore active when the sensory information is still under processing at the primary olfactory areas, also when the participant is processing the odor as being pleasant or unpleasant.
Olfactory perception in addiction
Our behavioral test showed a mean TDI score of above 30 for both groups, which shows normal odor perception. However, addicts showed significantly lower scores than the normal group. Observing behavioral differences between the two groups supports the differences in their fMRI activations.
Addiction, as a mental disorder, affects the learning and memory of humans. Studies have shown that odors, more than any other sensory stimulation, stimulate emotions and excitement (Chiaravalloti et al., 2015). One reason for this difference between the olfactory sense and other senses would be that it is only the olfactory system that has direct white matter connections to the amygdala, which is the area for emotional memory (Chiaravalloti et al., 2015). This unique characteristic that has been used as a tool for studying the addition in this study showed that addicts have a lower activation in the right anterior cingulate and right superior frontal gyrus. The ACC and OFC as part of the limbic system are expected to show a declined activation in response to neutral stimuli. That is why researchers investigate the associations between the ACC and medial orbitofrontal cortex (mOFC), albeit the studies have mostly separately studied these two areas in the rewarding process and the salience attribution, such as the studies by Garavanet al (2000) and Kaufman (2003). Nevertheless, the studies have recently investigated the OFC and ACC in the emotion and inhibitory control tasks, using the Stroop test in addicts.
These studies have shown a declined activation in the right ACC and mOFC in neutral stimulation in cocaine addicts. The correlations between these two areas correspond with their joint activation in such functions in drug addicts (Goldstein et al., 2007). In our study, the olfactory system has a close association with the emotional reactions, and the ACC has a role in the emotional system and regulating emotional responses, in particular in cooperation with the medial prefrontal cortex (MPFC) in the reward system (Goldstein et al., 2007), we have observed its low activation in neutral olfactory stimuli.
Also, addiction is associated with alterations in brain structure and function. The observed declined activation in the right ACC and right SFG in the addicts could be associated with the lower gray matter (GM) density of these brain regions. Previous studies on heroin addicts and comparing them to controls showed a declined GM volume in addicts in the right PFC and the left SMA and bilateral anterior cingulate. These areas, particularly the prefrontal and cingulate cortices, play a significant role in cognitive control (Huttenbrink, et al., 2013). In addition to heroin addicts, similar findings on the decline of the frontal lobe GM volume is observed in alcohol, cocaine, and methamphetamine addicts (Huttenbrink, et al., 2013). The volumetric and resting-state fMRI studies have also illustrated a lower GM density in the right DLPFC in heroin addicts, as well as a declined functional connectivity between the right DLPFC and the left inferior parietal lobe (Goldstein et al., 2007; Zald & Pardo, 2000). The volume of particular brain regions also showed a negative correlation with the time interval for heroin abuse (Goldstein et al., 2007; Zald & Pardo, 2000). As a result, the declined volume of the cingulate cortex and the right PFC in addicts could be a reason for the declined activation of the right cingulate and right SFG (as well as their functional connectivity) in addicts in this study.
Our results showed a different activation in the left lingual gyrus and the left cerebellum. The cerebellum has an established role in olfaction, and numerous reports support that (Vedaei, Oghabian, Firouznia & Harirchian, 2016). Because of the cerebellum role in attention, it was expected to see a lower activation in this area, similar to the report on testing olfactory perception in aging using fMRI (Ferdon & Murphy, 2003). Albeit, there are stronger reports on the cerebellum role in sniffing than passive smelling (Herz et al., 2004). In our results, the cerebellum showed considerable activity in addicts. One reason for this finding could be that addicts are probably more involved in sniffing and recognizing smells than controls Herz et al., 2004). The involvement of the cerebellum in olfaction has been shown in many previous reports (Cerf-Ducastel & Murphy, 2006; Savic, 2000; Ferdan & Murphy, 2003). These abilities of the cerebellum to be involved in motor functions or recognition memory have recently been highlighted (Cerf-Ducastel & Murphy, 2006; Andreasen et al., 1999).
The involvement of the lingual gyrus in olfaction has not been reported so much, but in a few fMRI studies, there are reports about it. In a study on young participants, brain regions involved in the recognition memory of odors were tested (Cerf-Ducastel & Murphy, 2006). For this test, olfactory stimulations were presented in 3 steps, and the results showed the involvement of the right hippocampus, parahippocampus, lingual and fusiform gyri, and medial frontal gyrus in the olfactory recognition memory. Besides, the right hemisphere was more involved in this function. The involvement of the lingual gyrus in olfactory perception has been shown in previous studies on healthy controls using the FDG-PET imaging (Chiaravalloti et al., 2015).
Although the previous studies on the olfactory system, such as the fMRI studies on alcohol addicts along with using alcohol as a strong stimulating odor, have shown the higher involvement of brain areas such as the nucleus accumbens and the OFC (as parts of the reward system) (Bragulat et al., 2008) compared to the controls, most of these studies which used odor stimulations only or in combination with other stimuli such as visual cues, aimed to create a craving. However, our results showed that in normal people, two brain structures of the limbic system (right ACC and right SFG), which are involved in emotion processing, showed an elevated activation compared to addicts. On the other hand, addicts showed higher activation in the brain regions involved in olfaction processing, and the lingual gyrus and cerebellum were two of the brain regions that showed a higher activation even in response to the non-craving odors.
The olfactory sensory in humans has shown associations with emotions and memory, the mechanism of which is not very clear; however, its association with neurodegenerative diseases is a fact. There are signs of olfactory disorder in patients with Parkinson disease years before the signs of movement disorder; such findings could also be helpful regarding the prevention of behavioral disorders, such as drug consumption. Observing differences in the patterns of brain activation when perceiving odors could be used as a biomarker for drug addiction, albeit this association could be either a cause or consequence of the addictive behavior, which needs further study. Also, such a pattern of brain activity could be used to follow a patient during the recovery process or to assess the chances of relapse in these patients.
Study strengths and limitations
Our study investigated a novel question on the mechanism of olfactory perception in heroin addicts. We selected a high Tesla MR machine, as well as established stimulation task and analysis methods to answer this question. However, the study suffered from many limitations. It would be advantageous to have a larger group of participants.
5. Conclusion
Our findings suggest that the olfactory perception is differently performed in the brain of normal group and heroin addicts, even in processing non-craving odors. Regarding the craving as a transient phase, it may be possible to use salient odors, such as eucalyptus, to pass this phase. In particular, the brain regions involved in higher-level cognitive functions are less active in addicts when presented with odors, whereas the cerebellum and lingual gyrus seem to be more involved in such processing in addicts. Therefore, according to the results of this study and previous studies, further structural and functional studies in areas such as the cerebellum and lingual gyrus as subtraction areas about addiction in healthy and addicted individuals are required. These areas may be used as target sites for screening high-risk individuals by non-craving odors in the future.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles are considered in this article. The participants were informed of the purpose of the research and its implementation stages. They were also assured about the confidentiality of their information and were free to leave the study whenever they wished, and if desired, the research results would be available to them.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
Andreasen, N. C., Oleary, D. S., Paradiso, S., Cizadlo, T., Arndt, S., Watkins, G. L., et al. (1999). The cerebellum plays a role in conscious episodic memory retrieval. Human Brain Mapping, 8(4), 226-234. [DOI: 10.1002/(SICI)1097-0193(1999)8] [PMID] [PMCID]
Bragulat, V., Dzemidzic, M., Talavage, T., Davidson, D., Oconnor, S. J., & Kareken, D. A. (2008). Alcohol sensitizes cerebral responses to the odors of alcoholic drinks: An fMRI study. Alcoholism: Clinical and Experimental Research, 32(7), 1124-1134. [DOI:10.1111/j.1530-0277.2008.00693.x] [PMID] [PMCID]
Casjens, S., Eckert, A., Woitalla, D., Ellrichmann, G., Turewicz, M., Stephan, C., et al. (2013). Diagnostic value of the impairment of olfaction in Parkinsons disease. PLoS One, 8(5), e64735. [DOI:10.1371/journal.pone.0064735] [PMID] [PMCID]
Cerf-Ducastel, B., & Murphy, C. (2006). Neural substrates of cross-modal olfactory recognition memory: An fMRI study. NeuroImage, 31(1), 386-396. [DOI:10.1016/j.neuroimage.2005.11.009] [PMID]
Chiaravalloti, A., Pagani, M., Micarelli, A., Di Pietro, B., Genovesi, G., & Alessandrini, M., et al. (2015). Cortical activity during olfactory stimulation in multiple chemical sensitivity: A 18F-FDG PET/CT study. European Journal of Nuclear Medicine and Molecular Imaging, 42(5), 733-740. [DOI:10.1007/s00259-014-2969-2] [PMID]
Dade, L. A., Zatorre, R. J., & Jones‐Gotman, M. (2002). Olfactory learning: Convergent findings from lesion and brain imaging studies in humans. Brain, 125(1), 86-101. [DOI:10.1093/brain/awf003] [PMID]
Ersche, K. D., Fletcher, P. C., Roiser, J. P., Fryer, T.D., London, M., & Robbins, T.W, et al. (2006). Differences in orbitofrontal activation during decision-making between methadone-maintained opiate users, heroin users and healthy volunteers. Psychopharmacology, 188(3):364-373. [DOI:10.1007/s00213-006-0515-z] [PMID] [PMCID]
Ferdon, S., & Murphy, C. (2003). The cerebellum and olfaction in the aging brain: A functional magnetic resonance imaging study. NeuroImage, 20(1), 12-21. [DOI:10.1016/S1053-8119(03)00276-3] [PMID]
Garavan, H., Pankiewicz, J., Bloom, A., Cho, J. K., Sperry, L., & Ross, T. J., et al. (2000). Cue-Induced cocaine craving: Neuroanatomical specificity for drug users and drug stimuli. American Journal of Psychiatry, 157(11), 1789-1798. [DOI:10.1176/appi.ajp.157.11.1789] [PMID]
Goldstein, R., Tomasi, D., Rajaram, S., Cottone, L. A., Zhang, L., & Maloney, T., et al. (2007). Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience, 144(4), 1153-1159. [DOI:10.1016/j.neuroscience.2006.11.024] [PMID] [PMCID]
Goldstein, R. Z., & Volkow, N. D. (2002). Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry, 159(10), 1642-1652. [DOI:10.1176/appi.ajp.159.10.1642] [PMID] [PMCID]
González, J., Barros-Loscertales, A., Pulvermüller, F., Meseguer, V., Sanjuán, A., & Belloch, V., et al. (2006). Reading cinnamon activates olfactory brain regions. NeuroImage, 32(2), 906-912. [DOI:10.1016/j.neuroimage.2006.03.037] [PMID]
Goudriaan, A. E., De Ruiter, M. B., van den Brink, W., Oosterlaan, J., & Veltman, D. J. (2010). Brain activation patterns associated with cue reactivity and craving in abstinent problem gamblers, heavy smokers and healthy controls: An fMRI study. Addiction Biology, 15(4), 491-503. [DOI:10.1111/j.1369-1600.2010.00242.x] [PMID] [PMCID]
Herz, R. S., Eliassen, J., Beland, S., & Souza, T. (2004). Neuroimaging evidence for the emotional potency of odor-evoked memory. Neuropsychologia, 42(3), 371-378. [DOI:10.1016/j.neuropsychologia.2003.08.009] [PMID]
Hu, W., Fu, X., Qian, R., Wei, X., Ji, X., & Niu, C. (2012). Changes in the default mode network in the prefrontal lobe, posterior cingulated cortex and hippocampus of heroin users. Neural Regeneration Reseasrch, 7(18), 1386-1391. [DOI:10.3969/j.issn.1673-5374.2012.18.004] [PMID] [PMCID]
Hüttenbrink, K., Hummel, T., Berg, D., Gasser, T., & Hähner, A. (2013). Olfactory dysfunction: Common in later life and early warning of neurodegenerative disease. Deutsches Ärzteblatt International, 110(1-2), 1-7. [DOI:10.3238/arztebl.2013.0001] [PMID] [PMCID]
Kaufman, J. N., Ross, T. J., Stein, E. A., & Garavan, H. (2003). Cingulate hypoactivity in cocaine users during a go-no-go task as revealed by event-related functional magnetic resonance imaging. Journal of Neuroscience, 23(21), 7839-7843. [DOI:10.1523/JNEUROSCI.23-21-07839.2003] [PMID] [PMCID]
Koob, G. F., & Volkow, N. D. (2010). Erratum: Neurocircuitry of Addiction. Neuropsychopharmacology, 35(4), 1051-1051. [DOI:10.1038/npp.2010.4] [PMCID]
Liu, H., Hao, Y., Kaneko, Y., Ouyang, X., Zhang, Y., & Xu, L., et al. (2009). Frontal and cingulate gray matter volume reduction in heroin dependence: Optimized voxel-based morphometry. Psychiatry Clinical Neurosciences, 63(4):563-568. [DOI:10.1111/j.1440-1819.2009.01989.x] [PMID]
Liu, H., Li, L., Hao, Y., Cao, D., Xu, L., & Rohrbaugh, R., et al. (2008). Disrupted white matter integrity in heroin dependence: A controlled study utilizing diffusion tensor imaging. American Journal of Drug and Alcohol Abuse, 34(5):562-575. [DOI:10.1080/00952990802295238] [PMID]
Lombion-Pouthier, S., Vandel, P., Nezelof, S., Haffen, E., & Millot, J. L. (2006). Odor perception in patients with mood disorders. Journal of Affective Disorders, 90(2-3), 187-191. [DOI:10.1016/j.jad.2005.11.012] [PMID]
London, E. D., Kohno, M., Morales, A. M., & Ballard, M. E. (2015). Chronic methamphetamine abuse and corticostriatal deficits revealed by neuroimaging. Brain Research, 1628(Pt 8), 174-185. [DOI:10.1016/j.brainres.2014.10.044] [PMID] [PMCID]
Ma, N., Liu, Y., Fu, X. M., Li, N., Wang, C. X., & Zhang, H., et al. (2011). Abnormal brain default-mode network functional connectivity in drug addicts. PLoS One, 6(1), e16560. [DOI:10.1371/journal.pone.0016560] [PMID] [PMCID]
Qiu, Y., Jiang, G., Su, H., Lv, X., Zhang, X., & Tian, J., et al. (2013). Progressive white matter microstructure damage in male chronic heroin dependent individuals: A DTI and TBSS study. PLoS One, 8(5), e63212. [DOI:10.1371/journal.pone.0063212] [PMID] [PMCID]
Sachdev, P. S., Lipnicki, D. M., Crawford, J., Reppermund, S., Kochan, N. A., & Trollor, J. N., et al. (2013). Factors predicting reversion from mild cognitive impairment to normal cognitive functioning: A population-based study. PLoS One, 8(3), e59649. [DOI:10.1371/journal.pone.0059649] [PMID] [PMCID]
Zare Sadeghi, A., Jafari, A. H., Oghabian, M. A., Salighehrad, H. R., Batouli, S. A. H., & Raminfard, S., et al. (2017). Changes in effective connectivity network patterns in drug abusers, treated with different methods. Basic and Clinical Neuroscience, 8(4), 285-298. [DOI:10.18869/nirp.bcn.8.4.285] [PMID] [PMCID]
Savic, I., Gulyas, B., Larsson, M., & Roland, P. (2000). Olfactory functions are mediated by parallel and hierarchical processing. Neuron, 26(3), 735-745. [DOI:10.1016/S0896-6273(00)81209-X]
Shear, P. K., Butters, N., Jernigan, T. L., Ditraglia, G. M., Irwin, M., & Schuckit, M. A., et al. (1992). Olfactory loss in alcoholics: Correlations with cortical and subcortical MRI indices. Alcohol, 9(3), 247-255. [DOI:10.1016/0741-8329(92)90061-E] [PMID]
Sobel, N., Prabhakaran, V., Hartley, C. A., Desmond, J. E., Zhao, Z., Glover, G. H., et al. (1998). Odorant-induced and sniff-induced activation in the cerebellum of the human. Journal of Neuroscience, 18(21), 8990-9001. [DOI:10.1523/JNEUROSCI.18-21-08990.1998] [PMID] [PMCID]
Spiga, S., Mulas, G., Piras, F., & Diana, M. (2014). The “addicted” spine. Frontiers in Neuroanatomy, 8, 110. [DOI:10.3389/fnana.2014.00110] [PMID] [PMCID]
Vedaei, F., Oghabian, M. A., Firouznia, K., Harirchian, M. H., Lotfi, Y., & Fakhri, M. (2016). The human olfactory system: Cortical brain mapping using fMRI. Iranian Journal of Radiology, 14(2), e16250. [DOI:10.5812/iranjradiol.16250]
Walter, C., Oertel, B. G., Felden, L., Nöth, U., Vermehren, J., & Deichmann, R., et al. (2017). Effects of oral Δ9-tetrahydrocannabinol on the cerebral processing of olfactory input in healthy non-addicted subjects. European Journal of Clinical Pharmacology, 73(12), 1579- 1587. [DOI:10.1007/s00228-017-2331-2] [PMID]
Wang, W., Wang, Y. R., Qin, W., Yuan, K., Tian, J., & Li, Q., et al. (2010). Changes in functional connectivity of ventral anterior cingulate cortex in heroin abusers. Chinese Medical Journal, 123(12), 1582-1588. [PMID]
Zald, D. H., & Pardo, J. V. (2000). Functional neuroimaging of the olfactory system in humans. International Journal of Psychophysiology, 36(2), 165-181. [DOI:10.1016/S0167-8760(99)00110-5] [PMID]
Zatorre, R. J., & Jones-Gotman, M. (1991). Human olfactory discrimination after unilateral frontal or temporal lobectomy. Brain, 114, 71-84. [DOI:10.1093/oxfordjournals.brain.a101868]
Adverse effects of addiction on the brain
Addiction is a mental disorder with cognitive, clinical, and social adverse effects, and recently its rising prevalence has been observed in the communities. Drugs affect the functional brain networks, for example, by altering the level of neurotransmitters or by over-exciting the brain’s reward system (Goldestein & Volkow, 2002), resulting in a craving for drug abuse (Goldestein & Volkow, 2002). Drug addiction is an acute relapsing disorder with the following characteristics: compulsion for drug abuse, disability in limiting the amount of consumption, and failure to control negative emotions (Koob & Volkow, 2010). Addiction happens mostly in 3 stages: binge or intoxication, withdrawal or negative effect, and mental preoccupation or anticipation. In these stages, alterations are observed in the neurotransmitters and neuromodulators of the brain, such as in the mesocorticolimbic dopaminergic system, the corticotropin-releasing hormone (CRH) of the amygdala, and the level of glutamate in the corticostriatal pathway. All these facts show that addictive behavior is the result of changes in the molecular, synaptic, and network levels of the brain (Spiga, Mulas, Piras & Diana, 2014).
Addiction could be in the form of drug dependency or behaviors such as gambling or gaming. The range of addictive disorders is so vast that sometimes an impulse control disorder, such as pathologic gambling, could also be included (Goudriaan, De Ruiter, van den Brink, Oosterlaan, & Veltman, 2010); however, different addictive disorders have common neurobiological factors. Now, besides gambling, many kinds of behavioral addiction have presented, like gaming addiction, sexual addiction, shopping addiction, pornography addiction, internet addiction, compulsive hoarding, and food addiction. Although behavioral addictions have specific diagnostic factors, drug addiction and behavioral addiction have many similarities in neurochemical and brain activation patterns.
These findings suggest that human societies face more complex addiction problems that require a more accurate and complete understanding of this disease to find effective prevention and treatment methods. Furthermore, addiction is a complex disorder under the influence of an interplay between genetic and environmental factors (Sachdev et al., 2013), and many neurobiological alterations are still unidentified (Goldestein & Volkow, 2002). Research studies are still conducted to understand better the neurobiology of addiction, which leads to successful treatment or its prevention. Among the many methods, neuroimaging approaches are the strongest to reveal the mechanisms of addiction. In recent decades, studies have been conducted using fMRI to understand the brain network involved in addiction better (Goudriaan et al., 2010; Bragulat, Dzemidzic, M., Talavage, Davidson, Oconnor, & Kareken, 2008; London, Kohno, Morales & Ballard, 2015; Goldstein et al., 2007). Today, with new neuroimaging techniques and methods, we are almost convinced that heroin causes noticeable structural changes in the brain. By a diffusion tensor imaging (DTI) study, abnormal white matter microstructure is seen in heroin users according to the duration of heroin-dependent, which can be associated with impaired decision-making (Qiu et al. 2013). A DTI study in permanent heroin users reported disrupted white matter integrity in the frontal white matter (Liu et al. 2008). Another study by fMRI and voxel-based morphometry (VBM) verifies grey matter atrophy, particularly in the frontal and cingulate areas (Liu et al. 2009).
However, these studies are few, and we are not entirely sure how much of these structural changes have directly caused functional alteration in heroin users. Previous studies by fMRI (task-based and resting-state) and positron emission tomography (PET) suggest a wide range of functional alterations in heroin abusers. Functional connectivity in the ventral anterior cingulate cortex (VACC) in a task-based fMRI study shows a significant decrease in heroin users (Wang et al. 2010). Activation in the default mode network (DMN) by rest fMRI is considerably different in heroin addicts compared to the normal group (Hu et al. 2012). Other studies suggest the abnormal functional organization of the DMN that may cause impaired cognitive control in heroin addicts (Ma et al. 2011). In a PET study, activation in the lateral orbitofrontal cortex (LOFC) as an important area in human decision-making and behavior in heroin users is significantly different from healthy group and methadone users (Ersche et al. 2006). However, all functional alterations in the whole brain and its bases have not been fully identified in heroin abusers.
In our previous projects, we attempted to examine the effective connectivity network of treatments such as methadone with fMRI (Zare Sadeghi et al., 2017). After our recent study that attempted to understand olfaction by fMRI better, another study seemed necessary to understand addiction better by olfactory perception differences as a necessary but less well-known factor in neurodegenerative disorders (Vedaei, Oghabian, Firouznia & Harirchian, 2016).
Disorders of olfaction
Olfaction is one of the sensory systems. It has been the topic of research in recent years due to its associations with many disorders such as neurodegenerative diseases, mood disorders such as depression, brain tumors, traumas, schizophrenia, and anorexia nervosa (Lombion-Pouthier et al., 2006). There have been many reports on the alteration of the olfactory perception in disorders such as Alzheimer disease, Parkinson disease, Lewy body dementia, PARK8 [individuals with mutations in the LRRK2 gene], Huntington disease, motor neuron disease, and Friedreich ataxia (Huttenbrink, Hummel, Berg & Hahner, 2013). These associations are now even used as a predictor of the diseases such as Alzheimer and Parkinson disease, as there are reports that 90% of such patients do show early signs of olfaction disorders. Olfactory function in Parkinson patients could be diagnosed with TDI and some other tests (Casjens et al., 2013). These studies illustrate that approximately 75% of IPD (idiopathic Parkinson disease) patients regarding the aging face decreasing in olfaction in their TDI as hyposmia and anosmia.
Olfaction in addicts
The olfactory system is different from other senses considering the human’s emotions and mood. This status could be due to direct pathways from the olfactory system to the limbic system (Lombion-Pouthier et al., 2006). The olfactory disorder has been studied in many diseases with respect to behavior changes. In this regard, drug addicts are among the most important patients whose olfaction should be studied. In the study by Sandrine lombain (Lombion-Pouthier et al., 2006), a lower score for odor discrimination and identification was observed in drug and alcohol addicts compared to healthy individuals. Similar findings were observed in another study, which showed deteriorated olfactory perception in alcoholics regarding the discrimination, identification, and threshold (Shear et al., 1992).
Olfaction is associated with some cognitive abilities of the brain, such as memory and emotion processing (Cerf-Ducastel & Murphy, 2006), and therefore studying olfactory ability in addiction would add information on the mechanism of addiction. A few behavioral studies on the olfactory perception of addicts versus normal controls showed a significant difference in odor identification, as well as a lower detection threshold in addicts (Lombion-Pouthier et al., 2006). These findings indicate the association of addiction with olfactory disorders. In studies on addicts to cannabis or alcohol, it was shown that, similar to general cognitive abilities, the olfactory perception could also be deteriorated due to drug addiction. In particular, these studies reported that addiction is associated with a decline in limbic system activities that impairs perceiving pleasant odors and the resulting pleasure. Studies have reported that brain neurons could influence the cannabinoid production process, which has a significant role in the olfactory bulb as a retrograde messenger (Walter et al., 2017) and therefore affect the GABA(γ-aminobutyric acid)ergic system.
In one fMRI study on the olfactory perception of alcoholic addicts, compared to other types of odors, smelling alcohol was associated with increased activation in a few brain areas, including the nucleus accumbens (Bragulat et al., 2008).
Aims of the study
Although there are behavioral studies on the olfactory perception of addicts, we have not found any fMRI study which tests the neural mechanisms of odor perception in heroin addicts and its differences from normal controls. In particular, understanding the neural mechanisms of the brain of drug addicts in response to smelling non-craving odors would be an interesting idea to gain more information on the relevant brain mechanisms.
In this study, we recruited 20 heroin addicts and 20 normal as controls. We presented odor stimuli and assessed them with fMRI to find differences in their brain activities when perceiving different odors. The results of this study would help find a new missing component in the brain function of addicted patients to design more accurate brain circuitry for these patients to fill the treatment or prevention gap of addiction or even design new treatment methods.
2. Materials and Methods
Study participants
This study was performed by the Ethics Committee of Tehran University of Medical Sciences. All participants gave their consent during the initial interview after being informed about the main study objectives and signed their consent form on the test day. Forty right-handed male subjects were included in this study. Of these, 20 were normal healthy participants (control group; Mean±SD age: 27.6±4.5 years; range: 22-38 years), and 20 were heroin-dependent subjects (addict group; Mean±SD age: 30.9±6.0 years; range: 20-41 years old). The Mean±SD duration of drug abuse in the addict group was 5.53±4.06 years. The two groups did not differ in age (Pt test >0.05), and education (Pt test >0.05) and were selected from the same socioeconomic class.
All the abstinent heroin subjects were members of “Congress 60,” an organization that manages networks of centers that treat abuse of substances in Iran by abstinent-based methods. All participants had negative urine tests for opiate and stimulant drugs at least 90 days before the scanning session based on their treatment center report (according to the center’s policy, urine check was carried out twice a week) and were rechecked during the study at the time before image acquisition for each subject. All heroin-dependent subjects met the diagnostic and statistical manual of mental disorders (DSM) IV-TR (Ref.) criteria for heroin dependence (before participating in the treatment program).
The mental and physical health of the participants was tested by a physician at the imaging center using our questionnaire and based on the inclusion/exclusion criteria of the International Consortium for Brain Mapping (ICBM) (1). None of the heroin dependents and healthy controls reported a head trauma or had nasal or sinus complaints. The participants were excluded due to any current or past chronic or acute neurologic or internal disorders, medicine consumption, surgery, or trauma; being overweight (over 100 kg); having a serious family history of any disease; being claustrophobic, or having implants or any other metal objects in the body. Smoking was not an exclusion criterion.
Behavioural olfactory test
The standardized psychophysical olfactory test, the “sniffing stick” test, was performed on all participants before the fMRI session. This battery of tests consisted of odor threshold (T), discrimination (D), and identification (I) parts. The total score (TDI) was determined by the sum of the three parts scores (T+D+I) (12). For this behavioral assessment, the odorants were presented via a pen-like dispensing device. The examiner removes the pen’s cap and places it 2 cm away from both nostrils for approximately 3 s. The threshold test is conducted with phenyl ethyl alcohol or n-butanol in a triple-forced-choice paradigm. Three pens are presented to the participants in a randomized manner. Only one of these contains the odorant, while the two others contain only the diluents. Each participant is then asked to determine the pen that smells different from the others.
A particular odorant concentration will only be correctly identified if the pen containing the odorant is recognized twice in a row. Following this, the next higher dilution step is presented. If this is also correctly identified twice, the next higher dilution step is presented until the participant makes an incorrect decision, in which case, the next lowest dilution step is presented. If the participant cannot identify this level of concentration, the next lower dilution step is presented. This pattern is continued until the participant correctly identifies a dilution step. The test is completed when seven reversal points are passed, and the smell threshold (T) is defined as the mean of the last four staircase reversal points. For the odor discrimination test (D), 16 pen triplets are presented to each participant: two pens smell the same, and the remaining pens contained a different odorant.
The participants should determine which of the three pens smells differently. During the odor threshold and odor discrimination tests, the participants are blindfolded to prevent visual recognition of the pen’s odorants. The odor identification test is conducted via the use of 16 common odorants. Each participant receives a multiple-choice card and is asked to pick the term that describes the presented odorant. For all three parts of the test (threshold, discrimination, and identification), the participants’ scores range from 1 to 16. Therefore, a total score is determined by summing the results obtained (TDI score). Accordingly, olfactory function are classified in terms of anosmia (TDI<16), hyposmia (16
In our study, the average scores of addicts for this test were as follows: the mean threshold, 8.17; the mean discrimination, 10.03; the mean identification, 12.15; and the mean total score, 30.3. For controls, the scores were as follows: the mean threshold, 9.55; the mean discrimination, 11.90; the mean identification, 13.9, and the total mean score, 35.36. The two-sample two-tailed t test showed the two groups were significantly different in discrimination (P<0.05), identification (P<0.001), and the total score (P<0.001), but not in the threshold (P>0.05).
Imaging
Magnetic Resonance Imaging (MRI) of the brain was carried out using a SIEMENS 3 Tesla MRI scanner (MAGNETOM Trio; Siemens Healthcare GmbH, Federal Republic of Germany) with a 32-channel head coil at the Medical Imaging Center, Imam Khomeini Hospital, Tehran, Iran. During the scanning procedure, foam cushions were used to minimize head movements within the coil. Functional T2*-weighted images were collected using blood oxygen level-dependent (BOLD) contrast (TR [repetition time]=3000 ms, TE [time to echo]=30 ms, flip angle=90 degrees, FOV [field of view]=192 mm2, matrix size=64×64, voxel size=3×3×3 mm, and slice gap=0 mm). Before the functional scan, a T1-weighted anatomical image was acquired, using a gradient echo pulse sequence (TR=1800 ms, TE=3.44 ms, flip angle=7 degrees, voxel size=1×1×1 mm, FOV=256 mm2, matrix size=256×256, and slice gap=0 mm).
fMRI paradigm
Olfactory stimulation was administered using a Magconcept olfactometer (USA, 2010) with a continuous airflow rate (2 L/min). The olfactometer consists of three main parts: a positive air pressure device that was used in the control room, a nasal mask, and a delivery system consisting of some capsules with the odorants. The parts of the olfactometer placed in the magnet room were made with diamagnetic materials to avoid any uniformity disturbances of the magnetic field and a decline in the signal-to-noise ratio. This system was controlled by a computer program to permit choosing of, and switching between, different types of stimulation. Odor and air delivery timing, stimulus frequency, and choice of a specific odorant for stimulus presentation within any task are controlled by the software.
The odorant presentation model we used was a block design consisting of two alternating patterns. A 15-s odor presentation (eucalyptus; Nature’s Alchemy Co. 100% pure natural essential oil), followed by a rest period of 45 s (odorless air), constituted these blocks. To assure that the resting period only contains pure air and not being polluted by any odorant molecules of the stimulus phase, the duration of this block was selected to be longer. This alternation of activation and resting was repeated for 10 rounds (a total of 600 s). The pure air and odorant conveyed to the participant’s nose had similar pressure and temperature.
Data analysis
Before processing, the analysis was performed using the fMRI Expert Analysis Tool (FEAT), part of FMRIB’s Software Library (FSL, http://www.fmrib.ox.ac.uk/fsl) version 5.0.9. Pre-processing steps included motion correction using FSL-MCFLIRT (Motion Correction from FMRIB’s Linear Image Registration Tool), skull-stripping for removal of non-brain tissue using FSL-BET (Brain Extraction Tool), slice-timing correction using Fourier-space time-series phase-shifting, normalization of the functional images to the standard Montreal Neurological Institute (MNI) brain atlas in two steps: 1) co-registration of each individual’s functional images to his high-resolution T1-weighted scan, using FLIRT (FMRIB’s Linear Image Registration) and 7 degrees of freedom (DoF), 2) Linear registration of the structural T1 images to the MNI space, with 12 DoF; using a Gaussian kernel of 6.0 mm FWHM for spatial smoothing, multiplicative mean intensity normalization of the volume at each time point, and high pass temporal filtering (Gaussian-weighted least-squares straight-line fitting, with sigma=60.0 s).
In the first-level analysis, the parametric statistical analysis was based on a general linear model (GLM) and performed using FSL-FEAT (version 6.0.0). The FILM (FMRIB Improved Linear Model) pre-whitening was used for statistical analysis of the fMRI time-series to make the statistical approaches valid and maximally efficient, which devoted a “z-score” to the corresponding BOLD signal. The individual GLM analyses were performed by creating a boxcar function of tasks (different conditions) against rest, being convolved with a canonical hemodynamic response function and its temporal derivatives. As explained above, registration of the estimated function map to the corresponding structural image and ultimately the MNI space was also carried out.
For higher-level analysis, the group-level analysis was performed using FLAME (FMRIB’s local analysis of mixed effects) to estimate within-group averages and between-group comparisons. Cluster thresholding was performed to reveal the significantly activated clusters. The criteria for identification of significantly active clusters was a voxel-level probability threshold of z>2.3; a false discovery rate (PFDR<0.05) was also used to correct for multiple comparisons. For a higher assurance of the findings, we selected a more stringent P<0.005, corresponding to an effect size of z>2.6.
3. Results
Within-group activations
Initially, the average brain activations of the two groups in response to the odor stimulation were estimated. Details of these activations, including size, coordinates, and the maximum z-value of the activation clusters, are presented in Table 1.

In the control group, two activation clusters were observed. Cluster 1 (number of voxels=3194, z=5.52) showed six active brain areas: the bilateral insula, bilateral anterior parahippocampus, right inferior frontal gyrus, and right amygdala. The second cluster (number of voxels=785, z=4.92) also showed activity of the right paracingulate and bilateral anterior cingulate.
The addicted group, on average, showed three activation clusters. The first cluster (number of voxels=5029, z=5.88) were bilateral anterior parahippocampus, bilateral amygdala, and right hippocampus; the second cluster (number of voxels=2587, z=5.02) included bilateral cerebellum, and the third cluster (number of voxels=1295, z=4.42) showed active bilateral lingual gyrus. The maps of these activations are provided in Figure 1.

Between-group contrasts
The activation maps of the two groups were contrasted to find any statistically significant differences between them. Accordingly, two brain areas (number of voxels=393, z=4.11) showed higher activation in the control group: the right anterior cingulate and right superior frontal gyrus. On the other hand, two regions of the brain (number of voxels=302, z=3.77) were more active in addicts: the left lingual gyrus and left cerebellum. Details are provided in Table 1.
4. Discussions
Summary of the results
In this study, using a salient pleasant odor as the non-craving stimulant during the fMRI scanning, we investigated the brain areas involved in olfactory perception in the healthy normal and heroin addicts. The active brain areas in olfactory perception in the controls were similar to the previous findings of fMRI or PET/SPECT studies. However, there were differences between the pattern and intensity of the activations of the two groups, as will be discussed below.
Brain olfactory network
The active brain areas in the controls during olfactory perception included bilateral insula, bilateral anterior parahippocampus, bilateral anterior cingulate, right inferior frontal gyrus, right amygdala, and right paracingulate. A similar brain network in this function is also observed in previous studies (Herz, Eliassen, Beland & Souza, 2003). Details of these activations are discussed below.
Insula is connected to the primary olfactory cortex and the areas such as the piriform cortex and the amygdala, and it has shown activation in prolonged olfactory stimulation, as well as in the discrimination of odors (Vedaei, et al., 2016). There are previous reports on the role of the parahippocampus in identifying the known odors (Herz, etal., 2004). This area, along with the amygdala, is shown to have a significant activation in recalling odors (Herz, etal., 2004). Orbitofrontal cortex (OFC) is at the ventral surface of the frontal lobe, and the studies on the association of brain lesions and olfactory disorders have shown that the right OFC has a more dominant role in this function (Zatorre & Jones-Gotman,1991; Zald & Pardo, 2000); an exception is the unpleasant odors in which the left OFC shows a higher activation (Zatorre & Jones-Gotman,1991; Zald & Pardo, 2000). The posterior areas of the inferior frontal gyrus are also involved in olfaction (Zald & Pardo, 1999). Observing the activity of this area in this function is very troublesome and needs a high-resolution MRI due to its proximity to the ocular muscles (Zald & Pardo, 1999); albeit our results have shown that this area is active. The posterior and medial parts of the inferior frontal gyrus, such as the piriform cortex, show activation in response to the sniffing function (Sobel 1998).
Amygdala was also observed to be active in this study. This area is part of the primary olfactory cortex (Zald & Pardo, 1999; Gonzalez et al., 2006), which along with the piriform cortex, has some white matter connections to the olfactory areas. This area has a role in the recognition of odors as well as in emotional reactions to odors, and the level of amygdala activation is (Zald & Pardo, 1999) associated with the odor intensity (Vedaei, et al., 2016). In previous studies (Zatorre 1992, Zald & Pardo, 1999), it was reported that the left amygdala does not show significant activation when confronting unpleasant odors, and its activation is mostly associated with pleasant ones (Koob & Volkow 2010). This function could be one reason for observing unpleasant olfactory delusion in epileptic patients with lesions invading the amygdala, and these patients very rarely show a hallucination of pleasant odors (Zald & Pardo, 1999).
The cingulate cortex was also active in this study. The involvement of the anterior cingulate in this function is illustrated using PET scanning many years ago (Cerf Ducastel & Murphy, 2006; Savic, Gulyas, Larsson, & Roland 2000, dade 2002). In recent fMRI studies with pleasant odors, the activation of medial OFC and pregenual cingulate has been illustrated; the inferior frontal gyrus (IFG) was also active when the intensity of odors was elevated (Bragulat et al., 2008; Chiaravalloti et al., 2015). Studies have shown that involvement in emotional comparison or perception is one main reason for the activation of medial OFC and the pregenual cingulate (Chiaravalloti et al., 2015; Gonzalez et al., 2006). These regions, as the secondary olfactory areas, are therefore active when the sensory information is still under processing at the primary olfactory areas, also when the participant is processing the odor as being pleasant or unpleasant.
Olfactory perception in addiction
Our behavioral test showed a mean TDI score of above 30 for both groups, which shows normal odor perception. However, addicts showed significantly lower scores than the normal group. Observing behavioral differences between the two groups supports the differences in their fMRI activations.
Addiction, as a mental disorder, affects the learning and memory of humans. Studies have shown that odors, more than any other sensory stimulation, stimulate emotions and excitement (Chiaravalloti et al., 2015). One reason for this difference between the olfactory sense and other senses would be that it is only the olfactory system that has direct white matter connections to the amygdala, which is the area for emotional memory (Chiaravalloti et al., 2015). This unique characteristic that has been used as a tool for studying the addition in this study showed that addicts have a lower activation in the right anterior cingulate and right superior frontal gyrus. The ACC and OFC as part of the limbic system are expected to show a declined activation in response to neutral stimuli. That is why researchers investigate the associations between the ACC and medial orbitofrontal cortex (mOFC), albeit the studies have mostly separately studied these two areas in the rewarding process and the salience attribution, such as the studies by Garavanet al (2000) and Kaufman (2003). Nevertheless, the studies have recently investigated the OFC and ACC in the emotion and inhibitory control tasks, using the Stroop test in addicts.
These studies have shown a declined activation in the right ACC and mOFC in neutral stimulation in cocaine addicts. The correlations between these two areas correspond with their joint activation in such functions in drug addicts (Goldstein et al., 2007). In our study, the olfactory system has a close association with the emotional reactions, and the ACC has a role in the emotional system and regulating emotional responses, in particular in cooperation with the medial prefrontal cortex (MPFC) in the reward system (Goldstein et al., 2007), we have observed its low activation in neutral olfactory stimuli.
Also, addiction is associated with alterations in brain structure and function. The observed declined activation in the right ACC and right SFG in the addicts could be associated with the lower gray matter (GM) density of these brain regions. Previous studies on heroin addicts and comparing them to controls showed a declined GM volume in addicts in the right PFC and the left SMA and bilateral anterior cingulate. These areas, particularly the prefrontal and cingulate cortices, play a significant role in cognitive control (Huttenbrink, et al., 2013). In addition to heroin addicts, similar findings on the decline of the frontal lobe GM volume is observed in alcohol, cocaine, and methamphetamine addicts (Huttenbrink, et al., 2013). The volumetric and resting-state fMRI studies have also illustrated a lower GM density in the right DLPFC in heroin addicts, as well as a declined functional connectivity between the right DLPFC and the left inferior parietal lobe (Goldstein et al., 2007; Zald & Pardo, 2000). The volume of particular brain regions also showed a negative correlation with the time interval for heroin abuse (Goldstein et al., 2007; Zald & Pardo, 2000). As a result, the declined volume of the cingulate cortex and the right PFC in addicts could be a reason for the declined activation of the right cingulate and right SFG (as well as their functional connectivity) in addicts in this study.
Our results showed a different activation in the left lingual gyrus and the left cerebellum. The cerebellum has an established role in olfaction, and numerous reports support that (Vedaei, Oghabian, Firouznia & Harirchian, 2016). Because of the cerebellum role in attention, it was expected to see a lower activation in this area, similar to the report on testing olfactory perception in aging using fMRI (Ferdon & Murphy, 2003). Albeit, there are stronger reports on the cerebellum role in sniffing than passive smelling (Herz et al., 2004). In our results, the cerebellum showed considerable activity in addicts. One reason for this finding could be that addicts are probably more involved in sniffing and recognizing smells than controls Herz et al., 2004). The involvement of the cerebellum in olfaction has been shown in many previous reports (Cerf-Ducastel & Murphy, 2006; Savic, 2000; Ferdan & Murphy, 2003). These abilities of the cerebellum to be involved in motor functions or recognition memory have recently been highlighted (Cerf-Ducastel & Murphy, 2006; Andreasen et al., 1999).
The involvement of the lingual gyrus in olfaction has not been reported so much, but in a few fMRI studies, there are reports about it. In a study on young participants, brain regions involved in the recognition memory of odors were tested (Cerf-Ducastel & Murphy, 2006). For this test, olfactory stimulations were presented in 3 steps, and the results showed the involvement of the right hippocampus, parahippocampus, lingual and fusiform gyri, and medial frontal gyrus in the olfactory recognition memory. Besides, the right hemisphere was more involved in this function. The involvement of the lingual gyrus in olfactory perception has been shown in previous studies on healthy controls using the FDG-PET imaging (Chiaravalloti et al., 2015).
Although the previous studies on the olfactory system, such as the fMRI studies on alcohol addicts along with using alcohol as a strong stimulating odor, have shown the higher involvement of brain areas such as the nucleus accumbens and the OFC (as parts of the reward system) (Bragulat et al., 2008) compared to the controls, most of these studies which used odor stimulations only or in combination with other stimuli such as visual cues, aimed to create a craving. However, our results showed that in normal people, two brain structures of the limbic system (right ACC and right SFG), which are involved in emotion processing, showed an elevated activation compared to addicts. On the other hand, addicts showed higher activation in the brain regions involved in olfaction processing, and the lingual gyrus and cerebellum were two of the brain regions that showed a higher activation even in response to the non-craving odors.
The olfactory sensory in humans has shown associations with emotions and memory, the mechanism of which is not very clear; however, its association with neurodegenerative diseases is a fact. There are signs of olfactory disorder in patients with Parkinson disease years before the signs of movement disorder; such findings could also be helpful regarding the prevention of behavioral disorders, such as drug consumption. Observing differences in the patterns of brain activation when perceiving odors could be used as a biomarker for drug addiction, albeit this association could be either a cause or consequence of the addictive behavior, which needs further study. Also, such a pattern of brain activity could be used to follow a patient during the recovery process or to assess the chances of relapse in these patients.
Study strengths and limitations
Our study investigated a novel question on the mechanism of olfactory perception in heroin addicts. We selected a high Tesla MR machine, as well as established stimulation task and analysis methods to answer this question. However, the study suffered from many limitations. It would be advantageous to have a larger group of participants.
5. Conclusion
Our findings suggest that the olfactory perception is differently performed in the brain of normal group and heroin addicts, even in processing non-craving odors. Regarding the craving as a transient phase, it may be possible to use salient odors, such as eucalyptus, to pass this phase. In particular, the brain regions involved in higher-level cognitive functions are less active in addicts when presented with odors, whereas the cerebellum and lingual gyrus seem to be more involved in such processing in addicts. Therefore, according to the results of this study and previous studies, further structural and functional studies in areas such as the cerebellum and lingual gyrus as subtraction areas about addiction in healthy and addicted individuals are required. These areas may be used as target sites for screening high-risk individuals by non-craving odors in the future.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles are considered in this article. The participants were informed of the purpose of the research and its implementation stages. They were also assured about the confidentiality of their information and were free to leave the study whenever they wished, and if desired, the research results would be available to them.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
Andreasen, N. C., Oleary, D. S., Paradiso, S., Cizadlo, T., Arndt, S., Watkins, G. L., et al. (1999). The cerebellum plays a role in conscious episodic memory retrieval. Human Brain Mapping, 8(4), 226-234. [DOI: 10.1002/(SICI)1097-0193(1999)8] [PMID] [PMCID]
Bragulat, V., Dzemidzic, M., Talavage, T., Davidson, D., Oconnor, S. J., & Kareken, D. A. (2008). Alcohol sensitizes cerebral responses to the odors of alcoholic drinks: An fMRI study. Alcoholism: Clinical and Experimental Research, 32(7), 1124-1134. [DOI:10.1111/j.1530-0277.2008.00693.x] [PMID] [PMCID]
Casjens, S., Eckert, A., Woitalla, D., Ellrichmann, G., Turewicz, M., Stephan, C., et al. (2013). Diagnostic value of the impairment of olfaction in Parkinsons disease. PLoS One, 8(5), e64735. [DOI:10.1371/journal.pone.0064735] [PMID] [PMCID]
Cerf-Ducastel, B., & Murphy, C. (2006). Neural substrates of cross-modal olfactory recognition memory: An fMRI study. NeuroImage, 31(1), 386-396. [DOI:10.1016/j.neuroimage.2005.11.009] [PMID]
Chiaravalloti, A., Pagani, M., Micarelli, A., Di Pietro, B., Genovesi, G., & Alessandrini, M., et al. (2015). Cortical activity during olfactory stimulation in multiple chemical sensitivity: A 18F-FDG PET/CT study. European Journal of Nuclear Medicine and Molecular Imaging, 42(5), 733-740. [DOI:10.1007/s00259-014-2969-2] [PMID]
Dade, L. A., Zatorre, R. J., & Jones‐Gotman, M. (2002). Olfactory learning: Convergent findings from lesion and brain imaging studies in humans. Brain, 125(1), 86-101. [DOI:10.1093/brain/awf003] [PMID]
Ersche, K. D., Fletcher, P. C., Roiser, J. P., Fryer, T.D., London, M., & Robbins, T.W, et al. (2006). Differences in orbitofrontal activation during decision-making between methadone-maintained opiate users, heroin users and healthy volunteers. Psychopharmacology, 188(3):364-373. [DOI:10.1007/s00213-006-0515-z] [PMID] [PMCID]
Ferdon, S., & Murphy, C. (2003). The cerebellum and olfaction in the aging brain: A functional magnetic resonance imaging study. NeuroImage, 20(1), 12-21. [DOI:10.1016/S1053-8119(03)00276-3] [PMID]
Garavan, H., Pankiewicz, J., Bloom, A., Cho, J. K., Sperry, L., & Ross, T. J., et al. (2000). Cue-Induced cocaine craving: Neuroanatomical specificity for drug users and drug stimuli. American Journal of Psychiatry, 157(11), 1789-1798. [DOI:10.1176/appi.ajp.157.11.1789] [PMID]
Goldstein, R., Tomasi, D., Rajaram, S., Cottone, L. A., Zhang, L., & Maloney, T., et al. (2007). Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience, 144(4), 1153-1159. [DOI:10.1016/j.neuroscience.2006.11.024] [PMID] [PMCID]
Goldstein, R. Z., & Volkow, N. D. (2002). Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry, 159(10), 1642-1652. [DOI:10.1176/appi.ajp.159.10.1642] [PMID] [PMCID]
González, J., Barros-Loscertales, A., Pulvermüller, F., Meseguer, V., Sanjuán, A., & Belloch, V., et al. (2006). Reading cinnamon activates olfactory brain regions. NeuroImage, 32(2), 906-912. [DOI:10.1016/j.neuroimage.2006.03.037] [PMID]
Goudriaan, A. E., De Ruiter, M. B., van den Brink, W., Oosterlaan, J., & Veltman, D. J. (2010). Brain activation patterns associated with cue reactivity and craving in abstinent problem gamblers, heavy smokers and healthy controls: An fMRI study. Addiction Biology, 15(4), 491-503. [DOI:10.1111/j.1369-1600.2010.00242.x] [PMID] [PMCID]
Herz, R. S., Eliassen, J., Beland, S., & Souza, T. (2004). Neuroimaging evidence for the emotional potency of odor-evoked memory. Neuropsychologia, 42(3), 371-378. [DOI:10.1016/j.neuropsychologia.2003.08.009] [PMID]
Hu, W., Fu, X., Qian, R., Wei, X., Ji, X., & Niu, C. (2012). Changes in the default mode network in the prefrontal lobe, posterior cingulated cortex and hippocampus of heroin users. Neural Regeneration Reseasrch, 7(18), 1386-1391. [DOI:10.3969/j.issn.1673-5374.2012.18.004] [PMID] [PMCID]
Hüttenbrink, K., Hummel, T., Berg, D., Gasser, T., & Hähner, A. (2013). Olfactory dysfunction: Common in later life and early warning of neurodegenerative disease. Deutsches Ärzteblatt International, 110(1-2), 1-7. [DOI:10.3238/arztebl.2013.0001] [PMID] [PMCID]
Kaufman, J. N., Ross, T. J., Stein, E. A., & Garavan, H. (2003). Cingulate hypoactivity in cocaine users during a go-no-go task as revealed by event-related functional magnetic resonance imaging. Journal of Neuroscience, 23(21), 7839-7843. [DOI:10.1523/JNEUROSCI.23-21-07839.2003] [PMID] [PMCID]
Koob, G. F., & Volkow, N. D. (2010). Erratum: Neurocircuitry of Addiction. Neuropsychopharmacology, 35(4), 1051-1051. [DOI:10.1038/npp.2010.4] [PMCID]
Liu, H., Hao, Y., Kaneko, Y., Ouyang, X., Zhang, Y., & Xu, L., et al. (2009). Frontal and cingulate gray matter volume reduction in heroin dependence: Optimized voxel-based morphometry. Psychiatry Clinical Neurosciences, 63(4):563-568. [DOI:10.1111/j.1440-1819.2009.01989.x] [PMID]
Liu, H., Li, L., Hao, Y., Cao, D., Xu, L., & Rohrbaugh, R., et al. (2008). Disrupted white matter integrity in heroin dependence: A controlled study utilizing diffusion tensor imaging. American Journal of Drug and Alcohol Abuse, 34(5):562-575. [DOI:10.1080/00952990802295238] [PMID]
Lombion-Pouthier, S., Vandel, P., Nezelof, S., Haffen, E., & Millot, J. L. (2006). Odor perception in patients with mood disorders. Journal of Affective Disorders, 90(2-3), 187-191. [DOI:10.1016/j.jad.2005.11.012] [PMID]
London, E. D., Kohno, M., Morales, A. M., & Ballard, M. E. (2015). Chronic methamphetamine abuse and corticostriatal deficits revealed by neuroimaging. Brain Research, 1628(Pt 8), 174-185. [DOI:10.1016/j.brainres.2014.10.044] [PMID] [PMCID]
Ma, N., Liu, Y., Fu, X. M., Li, N., Wang, C. X., & Zhang, H., et al. (2011). Abnormal brain default-mode network functional connectivity in drug addicts. PLoS One, 6(1), e16560. [DOI:10.1371/journal.pone.0016560] [PMID] [PMCID]
Qiu, Y., Jiang, G., Su, H., Lv, X., Zhang, X., & Tian, J., et al. (2013). Progressive white matter microstructure damage in male chronic heroin dependent individuals: A DTI and TBSS study. PLoS One, 8(5), e63212. [DOI:10.1371/journal.pone.0063212] [PMID] [PMCID]
Sachdev, P. S., Lipnicki, D. M., Crawford, J., Reppermund, S., Kochan, N. A., & Trollor, J. N., et al. (2013). Factors predicting reversion from mild cognitive impairment to normal cognitive functioning: A population-based study. PLoS One, 8(3), e59649. [DOI:10.1371/journal.pone.0059649] [PMID] [PMCID]
Zare Sadeghi, A., Jafari, A. H., Oghabian, M. A., Salighehrad, H. R., Batouli, S. A. H., & Raminfard, S., et al. (2017). Changes in effective connectivity network patterns in drug abusers, treated with different methods. Basic and Clinical Neuroscience, 8(4), 285-298. [DOI:10.18869/nirp.bcn.8.4.285] [PMID] [PMCID]
Savic, I., Gulyas, B., Larsson, M., & Roland, P. (2000). Olfactory functions are mediated by parallel and hierarchical processing. Neuron, 26(3), 735-745. [DOI:10.1016/S0896-6273(00)81209-X]
Shear, P. K., Butters, N., Jernigan, T. L., Ditraglia, G. M., Irwin, M., & Schuckit, M. A., et al. (1992). Olfactory loss in alcoholics: Correlations with cortical and subcortical MRI indices. Alcohol, 9(3), 247-255. [DOI:10.1016/0741-8329(92)90061-E] [PMID]
Sobel, N., Prabhakaran, V., Hartley, C. A., Desmond, J. E., Zhao, Z., Glover, G. H., et al. (1998). Odorant-induced and sniff-induced activation in the cerebellum of the human. Journal of Neuroscience, 18(21), 8990-9001. [DOI:10.1523/JNEUROSCI.18-21-08990.1998] [PMID] [PMCID]
Spiga, S., Mulas, G., Piras, F., & Diana, M. (2014). The “addicted” spine. Frontiers in Neuroanatomy, 8, 110. [DOI:10.3389/fnana.2014.00110] [PMID] [PMCID]
Vedaei, F., Oghabian, M. A., Firouznia, K., Harirchian, M. H., Lotfi, Y., & Fakhri, M. (2016). The human olfactory system: Cortical brain mapping using fMRI. Iranian Journal of Radiology, 14(2), e16250. [DOI:10.5812/iranjradiol.16250]
Walter, C., Oertel, B. G., Felden, L., Nöth, U., Vermehren, J., & Deichmann, R., et al. (2017). Effects of oral Δ9-tetrahydrocannabinol on the cerebral processing of olfactory input in healthy non-addicted subjects. European Journal of Clinical Pharmacology, 73(12), 1579- 1587. [DOI:10.1007/s00228-017-2331-2] [PMID]
Wang, W., Wang, Y. R., Qin, W., Yuan, K., Tian, J., & Li, Q., et al. (2010). Changes in functional connectivity of ventral anterior cingulate cortex in heroin abusers. Chinese Medical Journal, 123(12), 1582-1588. [PMID]
Zald, D. H., & Pardo, J. V. (2000). Functional neuroimaging of the olfactory system in humans. International Journal of Psychophysiology, 36(2), 165-181. [DOI:10.1016/S0167-8760(99)00110-5] [PMID]
Zatorre, R. J., & Jones-Gotman, M. (1991). Human olfactory discrimination after unilateral frontal or temporal lobectomy. Brain, 114, 71-84. [DOI:10.1093/oxfordjournals.brain.a101868]
Type of Study: Original |
Subject:
Computational Neuroscience
Received: 2020/03/22 | Accepted: 2020/05/9 | Published: 2022/03/1
Received: 2020/03/22 | Accepted: 2020/05/9 | Published: 2022/03/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





