Volume 15, Issue 5 (September & October 2024)
BCN 2024, 15(5): 569-582 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Motavalli A, Mahmoudi J, Majdi A, Sadigh-Eteghad S. Ask, and You Shall Receive: A Closer Look on Unsolved Consciousness Issue. BCN 2024; 15 (5) :569-582
URL: http://bcn.iums.ac.ir/article-1-1681-en.html
URL: http://bcn.iums.ac.ir/article-1-1681-en.html
1- Neurosciences Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Full-Text [PDF 1051 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Consciousness is one of the most intriguing topics in scientific and philosophical arguments. Living beings, especially human beings, experience phenomena and objects. Therefore, experiencing is a crucial part of this concept that paves the way to understanding consciousness better. Conscious experience is a first-person experience of the nature to which a third person does not have access. These features render consciousness one of the biggest unanswered questions in science (Jerath et al., 2015; Koch, 2018). Over the last few decades, research on consciousness has continued in philosophical, physical, and neuroscientific realms, leading to the emersion of diverse meanings related to consciousness (Jonkisz, 2012). Although there are numerous theoretical and empirical views about the concept of consciousness, there is no consensus regarding its meaning, leaving the term imprecise (Jonkisz, 2015).
The philosophical definition of consciousness varies from that of Descartes, “everything that is within us in such a way that we are immediately aware [conscii] of it…” (Jorgensen, 2010) to Sartre’s, as an existentialist, who defines it as “a being such that in its being, its being is in question insofar as this being implies a being other than itself” (Sartre, 2001). Quantum physics approaches the dilemma from another point of view and suggests that “the process of human c cannot be simulated classically.” Hameroff and Penrose. (2014) have offered “an orchestrated objective reduction (Orch OR) model” in which quantum computation occurs in the microtubule protein assemblies in brain neurons. In this model, every tubulin dimer represents a qubit, showing interaction with other qubits coulombically at a limited temperature, enabling it to possess quantum information processing (Behrman et al., 2006).
Neuroscientists deal with the issue from a different perspective. Consciousness is thought to illustrate the association between cortical structures, such as specific parts of the grey matter within neural operations in the brain, and subcortical regions in the upper brain stem, thalamus, hypothalamus, and basal forebrain (Blumenfeld, 2016; Laureys et al., 2004). Neurobiological theories such as the global neuronal workspace, recurrent processing theory, and information integration theory try to shed light on the unresolved issue of consciousness from its perspective (Lamme & Roelfsema, 2000). Still, no consensus exists about the exact meaning and features (Zeman, 2001). This review scrutinizes consciousness from cultural, historical, philosophical, and neuroscientific points of view.
Consciousness in the old and middle ages
Consciousness has long been part of human history, especially in its reflective form. Archeologists assume that the history of consciousness dates back as far as the Neolithic period, in which burial practices represent spiritual views about humans and life and offer signs for minimally reflective consciousness (Pearson, 1999). Others would believe that consciousness is a rather new concept in human history, dating back to the Homeric era, and those who lived earlier did not show any signs of reflective thought. Some scholars would claim that even Greek civilization had no words corresponding to “consciousness” (Wilkes, 1984). This view has been opposed by the fact that Plato famously believed that the existence of our minds precedes that of our body and outdates the body’s death. Accordingly, Aristotle would define “mind” as part of the body that recognizes and understands (Gennaro, 2016). Evidence also suggests that consciousness has been part of the culture of many civilizations. The Maya civilization was among the first to introduce the idea of consciousness to their general faith around 2000 BCE. Because consciousness contains internal and environmental triggers, the Mayans believed that consciousness should have existed from the beginning of human existence, having a major impact on their individual and social life (Calleman, 2004).
Primitive Indians attempted to describe human consciousness from a broader perspective (Kabat-Zinn, 2013). Indian philosopher Sri Aurobindo demonstrated that consciousness is not only about individual awareness but also comprises a distinctive state of energy (Wautischer, 2008). However, there are no specific clinical inquiries among Indian thoughts except the concept of Ayurveda (Feuerstein, 2001). Most Indian philosophers have consensus over the notion of samsara, meaning that living beings are reborn again in an infinite cycle, the final aim of which is to be released from the round sequence (Gupta, 2003; Matilal, 1986).
Human consciousness in Chinese thought consists of three divisions. The first section explains the cosmological aspect of consciousness, determining the objective world of being for the human. In the second part, the emphasis is on human distinction according to the reflection of the mind and heart in individual and social life. The third layer is about the correlation between politics and consciousness, where the human constructs an ideal political conviction relating to the concrete world (Ch’eng & Bunnin, 2002; Cua, 2003; Feng, 1983).
Self-awareness in Islamic philosophy ranges from Avicenna’s “flying man” as the soul having self-awareness (which is equal to the Cartesian cogito) to self-awareness without the substance of Abu’l-Barakāt al-Baghdādī and Yahya ibn Habash Suhrawardi (Kaukua, 2015). Avicenna considers two distinguished aspects of self-awareness: First, primitive self-awareness, which is equal to the concept of “flying man,” and second, reflexive self-awareness, meaning our awareness of cognizing objects other than ourselves (Black, 2008). Mullā Sadrā accepts the concept of the “flying man” and thus the notion of self-awareness it denotes. However, in his legacy, this concept has two important characteristics. Sadrā believes that self-awareness is a broader concept than the “flying man” and should incorporate both the intellectual and the sub-intellectual forms of mental existence. Besides, he believed that self-awareness is not distinguishable from other constituents of human experience (Kaukua, 2015).
Religion plays a pivotal role in some civilizations and has an important impact on believers’ way of thinking about life. Approximately every religion has some correlated beliefs about the concept of consciousness and reveals its strong regulations through the aid of consciousness (Khalid Ali & Sulam, 2018; Razak, 2012). However, its views on the self vary widely.
Consciousness in the modern era
The 17th century was probably the finest period for the evolution of the concept of consciousness relating to science and scientific views worldwide. Rene Descartes defined the meaning of thought (pensée) as “all that of which we are conscious as operating in us.” He explained two distinctive aspects related to consciousness (Churchland, 1996). By initiating a systematic method, he made a clear difference between consciousness’s physical and mental characteristics, described as Cartesian dualism. He opposed the existence of unconscious mental states. John Locke elaborated on the Cartesian view of consciousness. He claimed that human sensibility is necessary for his thoughts, saying that “he cannot think at any time, waking or sleeping, without being sensible of it.” His definition of a person is the same as consciousness. According to Locke, “[a person] is a thinking intelligent being, that has reason and reflection, and can consider itself as itself, the same thinking thing in different times and places; which it does only by that consciousness, which is inseparable from thinking, and as it seems to be essential to it: It being impossible for anyone to perceive, without perceiving, that he does perceive.” (Gennaro, 2016). Gottfried Wilhelm Leibniz was among the first to distinguish awareness (outer-directed consciousness) from self-awareness (self-consciousness), believing that consciousness could not arise from mere matter. Intriguingly, Leibniz would accept the notion of minimally conscious or unconscious mental states, calling them “petit perceptions.” Immanuel Kant perused the concept of consciousness from a philosophical view, distinguishing the philosophical side from the study of nature and scientific matter. He proposed two components of consciousness: apperception as an ability to be conscious of one’s spontaneous actions (understanding), and inner sense as the consciousness of everything in mind as opposed to apperception (sensibility) (Gennaro, 2016; Heinämaa & Reuter, 2009).
Friedrich Hegel strived to reunite consciousness with the world in a spiritual meaning structure in which the world is a spirit, and human consciousness is a subset of the world that can attain self-consciousness (Hegel, 1998). In the 19th century, Gustav Fechner opposed Descartes’ theory that mind and body were two prospects of a solitary entity; thus, mental procedures could be evaluated (Fechner, 1860; Herbart, 1824). One of the most convincing contemporary theories was explicated by William James, asserting that consciousness is like a flowing stream despite constant shifts and changes and maintains conceptual stability despite rapid alterations (James & CnpeReading, 2018). Sigmund Freud made an association between consciousness and human thoughts and actions. He conceived that the conscious mind depends on a further state of mind (subconscious) and concluded that there is a reciprocal correlation amid discrete states of mind (Mollon, 2014). The notion of the unconscious mind from Freud’s philosophical viewpoint is essential to ease the understanding of consciousness’s ambiguities. Modern theories generally pursue Freud’s psychologic perspectives, and with the aid of neuroscience, they introduce more convincing paths through specific neuroanatomical findings (Laureys et al., 2015). Edmund Husserl states that consciousness invariably includes a self-appearance (Für-sich-selbst-erscheinens). For Husserl, the pure I– the I of transcendental apperception –is not a ‘dead pole of identity’; it is an active living self continuously ‘appearing for itself’ (Moran, 2005). For Martin Heidegger, the doctrine of consciousness is different. Indeed, Heidegger does not possess such a doctrine, and he does not even use the term ‘consciousness’ in his masterpiece, being and time. Martin Heidegger’s concept of Dasein (human existence) encompasses subjectivity and self-consciousness, which interprets itself in the world. In his words, the self-[consciousness] is “given with our consciousness of objects” (Grove, 2004). In his being and nothingness, Sartre describes consciousness as a consciousness of objects and thus explains it in association with something else. In the same place, he stated, “consciousness is a being such that in its being, its being is in question insofar as this being implies a being other than itself.” For Sartre, there are two main types of consciousness, the pre-reflective and the reflective consciousness. There is no place for an ‘ I ‘ at the pre-reflective level, which is a non-observational self-acquaintance. However, at the reflective level, an awareness carries within itself the capacity to contemplate ourselves (Sartre, 2001).
Genetics and improvement in the diagnosis of congenital disorders reduced the complexities regarding clarifying consciousness states. David Chalmers, along with Frank Jackson, disputed that empirical and scientific descriptions are unable to explicate the meaning of consciousness; hence, without the existence of consciousness, the world could be similar to a hunting ground for wild zombies which act just in reaction to external triggers without having any conscious state of mind (Chalmers, 1996; Jackson, 1982). Thomas Nagel suspected whether the physical explanation of brain states could ever elucidate the secrets of the subjective experience of consciousness. He deduced that there should always be an interval between consciousness and scientific exposition of the brain (Nagel, 1997). John Searle distinguished between consciousness and other biological incidents and asserted that consciousness has a subjective nature, unlike other biological phenomena. However, the subjective part of consciousness does not deny the existence of an objective aspect (Searle, 2000). Dennett argued that qualia (instances of subjective, conscious experience) cannot exist as they are too incoherent and incompatible to be tangible. The model of parallelism in the brain is also a particular component of his consciousness view. He also claimed that consciousness is rooted in cultural structures derived from ancient Greek beliefs (Dennett, 2017).
Stuart Hameroff and Roger Penrose believed that the nature of consciousness and its position in the universe is ultimately unknown. Also, they asserted that consciousness relies on biologically lucid quantum operations in association with brain neurons and that these quantum actions modulate neuronal synaptic activities (Hameroff and Penrose, 2014). They proposed three versions of defining consciousness. The most crucial section explains consciousness as the consequence of distinct physical proceedings known as proto-conscious events. Proto-conscious events have been assimilated within living cellular frameworks, leading to brief states of mind called consciousness (Barlow, 2015).
Several contemporary neuroscientists assume a meaningful relationship between the utilization of energy in the brain and neural function and the processing of the data in the brain (Magistretti & Allaman, 2013). Consequently, the supplement of energy is an inseparable backbone of brain computational structure (Sterling & Laughlin, 2015). Robert Shulman and his coworkers have asserted an exact association between consciousness and energy through the brain (Shulman, 2013; Shulman et al., 2009). They revealed that by decreasing the response to an outer stimulus, for instance, in anesthesia, the metabolism of glucose in the brain also reduces. Therefore, energy consumption is vital for the brain’s consciousness.
Increasing attention has been paid to the concept of the brain process (Clarke & Sokoloff, 1994). There has been evidence that in a resting awake state of the brain and nonexistence of outer stimulation factors, the consumption of energy in particular parts of the brain called ‘dark energy’ is extremely low; however, the exact function of dark energy remains unclear (Morcom & Fletcher, 2007; Raichle, 2010).
Two major accepted theories are currently trying to explain consciousness: The global workspace theory (GW) and the integrated information (II) theory. According to the former, communicating but autonomous hubs throughout the brain are responsible for conscious experience. These torrents of unconscious parallel processing have restricted interaction with each other and strive for dissemination via a process called “winner-take-all,” during which several torrents exploit a single torrent for dissemination throughout the brain (Baars et al., 2013). According to the latter, which was proposed by Tononi in 2008, consciousness is the integration of information. In his model, sensory inputs from various sensory organs are integrated with cognitive mechanisms to bring about conscious experience. Further, Tononi introduced a concept known as “qualia space.” The concept assumes that several informational associations between different axes within qualia lead to a given conscious experience (Tononi, 2008). These two models primarily rely on the cortico-thalamic feedback loops. However, they neglect the vital role of the thalamus in this regard and overemphasize the role of the cortex (Jerath et al., 2015).
In what follows, we dig further into the meaning of consciousness in neuroscience.
Neuroanatomy and Neurophysiology of Consciousness
Basic neuroanatomy of consciousness
In medical terms, consciousness has two constituents: Awareness and arousal (wakefulness). Arousal, also defined as wakefulness, alludes to the level of consciousness or the ability to experience awareness. In contrast, awareness is defined as the content of consciousness or, as Nagel puts it, “the subjective character of experience” (Laureys, 2005). Arousal is related to the structures in the brain like hypothalamus and brainstem ascending reticular activating systems (ARAS), whereas awareness is associated with cortico-thalamic network connectivity and frontoparietal cortex (Di Perri et al., 2014; Laureys et al., 2000). Generally, a linear concordance exists between wakefulness and awareness, and along with an increase in one, the other also increases. However, in some conditions, the two aspects are dissociated. The minimally conscious and vegetative states (or unresponsive wakefulness syndrome-UWS) are the conditions during which wakefulness is preserved. Still, awareness is impaired to a different extent (Di Perri et al., 2014). Despite accumulating evidence, it is hard to point to structures that are “minimally sufficient and jointly necessary” for consciousness.
Limited subcortical regions are necessary for maintaining wakefulness, whereas cortical projection structures appear to provide perceptual contents of consciousness or awareness (Baars, 1995). In 1949, the first findings related to cerebral activities were recorded, and the role of ARAS, which is situated in the central thalamus and upper brain, and its relationship with brain activity were described (Moruzzi & Magoun, 1949; Neylan, 1995). The ARAS, a crucial component of consciousness, consists of numerous neural circuits originating from the brainstem’s reticular formation, projecting to the intralaminar nucleus of the thalamus and ending the cerebral cortex (Yeo et al., 2013). Other members of this system include the pedunculopontine nucleus, parabrachial nucleus, locus coeruleus, dorsal raphe, median raphe, hypothalamus, basal forebrain, and non-specific thalamic nuclei which take part in consciousness (Fuller et al., 2011). These structures and pathways stimulate awareness-related centers in the cerebral cortex through synapses in the basal forebrain and thalamus (Edlow et al., 2012). Despite having many neurons, subcortical areas of the brain make a different contribution to the concept of consciousness. The cerebellum has four times more neurons than the cortex (Herculano-Houzel, 2012). However, lesions of this region have little effect on the contents of consciousness (Lemon & Edgley, 2010).
On the other hand, evidence suggests that parts of the corticothalamic system are necessary for maintaining consciousness. Several undisputed examples of UWS demonstrate that massive cortical grey or white matter lesions commonly accompany the loss of consciousness. This frequently and significantly involves the thalamus (Posner et al., 2007). The evidence, when accompanied by the fact that patients with lesions of brain structures outside the corticothalamic system (e.g. spinal cord and cerebellum) remain conscious, becomes more compelling (Tononi et al., 2016). Here, another unresolved issue arises, positing the thalamus against other parts of the cortex. It has been proposed that the thalamus, particularly the intralaminar nuclei, might form a “centrencephalic system” responsible for a conscious state (Bogen, 1997). In this regard, patients with brain damage involving bilateral paramedian thalamic nuclei are often in an unresponsive state or minimally conscious state (Posner et al., 2007). However, evidence from other studies contradicts these findings and shows that the thalamus may not be necessary for consciousness. Thus, lesions involving the paramedian thalamic region and causing an unconscious state might involve projection from glutamatergic neurons in the parabrachial-precoeruleus complex of the brainstem. The latter is responsible for the stimulation of the cortex via a basal forebrain relay (Fuller et al., 2011). Other discussions regarding the role of primary areas vs higher level areas, ventral vs dorsal visual stream, posterior vs anterior (prefrontal) cortex, lateral fronto-parietal network vs (medial) default system, left vs right hemisphere, reentrant vs feed-forward connections, and superficial vs deep layers of cortex in a conscious experience exist. However, none of these are conclusive.
Cell types involved in consciousness
Neurosciences have always been preoccupied with whether specific cell types are directly responsible for conscious experience. Research has shown that specific cell types, such as the spindle neurons (also recognized as von Economo neurons) detected in layer 5 of the frontal lobe in species with bulky, complex brains, may be responsible, at least partially, for consciousness (Butti et al., 2013). On the other hand, others have indicated the important role of thin-tufted pyramidal cells in consciousness. These cells create corticocortical connections in layer 5A, and their interconnections are denser than those of the neurons in layer 6. To complicate the presented picture with more evidence, scientists have revealed that supra-granular pyramidal cells have denser interconnections with other neurons in the superficial cortical layers, possess more precise topography than those found in the infra-glandular layers, and are fire more specifically in response to stimuli than the latter layer (Harris & Shepherd, 2015; Markov et al., 2014; Zhang et al., 2014). What could be interpreted from the previous discussion is that neurons in the supra-granular layer are associated with a conscious experience (He & Raichle, 2009).
Electrophysiological correlates of consciousness
Alterations in neural activity do not inevitably associate with changes in conscious experience. From the electrophysiologic view, consciousness implies low frequency and desynchronized brain activity that ranges between 20 and 70 Hz. The parts of the brain involved in consciousness, like the nuclei of the thalamus, are connected with the cortex through the thalamocortical system. The oscillating loop is adjusted around 40 Hz during the information-flowing process. The neurons of the thalamic nuclei have been found to switch on particular cortical pyramidal cells and quiet other brain parts by activating gamma-aminobutyric acid (GABA) inhibitory interneurons. Consequently, consciousness is presumed to be feasible only when the 40 Hz electrical stream is established along with the brain circuits (Negrao & Viljoen, 2009)
Apart from the mentioned waves, which may play a role in consciousness, several super- or infra-slow waves (0.001-0.1 Hz) and slow waves (0.1-1 Hz) may also take part in the integration of faster frequencies and have a crucial role in maintaining a conscious state, integrating inputs from different sources and thus unifying consciousness. The slow and infra-slow waves are generated in the upper layers of higher-order brain regions such as the associative cortex, and their existence is thought to be a mandatory, non-sufficient prerequisite for consciousness. These waves are also called neural predisposition of the level/state of consciousness (NPC) as opposed to faster waves, which are neural correlates of consciousness (NCC), meaning that in normal conditions, slow/infra-slow waves provide “temporal basement” that works as “default-mode” for the coupling to faster waves (or cross-frequency coupling). Infra-slow and slow waves travel through the brain’s cortical layers and change direction when the animal is anesthetized. Also, when a given animal is unconscious, the association between these slow and fast waves breaks apart, causing “temporospatial fragmentation and isolation” (He & Raichle, 2009; Northoff, 2013; Northoff, 2017). What happens during unresponsive wakefulness syndrome (UWRS) is indeed an increase in the slow-wave power and a decrease in the faster-wave power or replacement of “temporospatial integration and nestedness” by “temporospatial fragmentation and isolation” (Northoff, 2017).
The NCC
The NCC is described as minimal neuronal operations mandatory for any specific conscious matter (Francis Crick & Koch, 1990; Koch, 2004). According to Chalmers, NCC “is a minimal neural system N such that there is a mapping from states of N to states of consciousness, where a given state of N is sufficient under conditions C, for the corresponding state of consciousness” (Chalmers, 1995).
There are typically two models of NCC. The content-specific NCC is defined as the minimal neural mechanisms and activities that define a certain phenomenal difference in conscious experience, such as places, colors, or thoughts. This could be artificially stimulated via specific techniques such as transcranial magnetic stimulation (TMS) when there are no real external stimuli or blocked via the exact mechanisms when there are real external phenomena (Koch et al., 2016). The full NCC can be explained as neural prerequisites associated with conscious experiences regardless of their certain contents. This, in general, has been described as the sum of the content-specific NCCs (Crick & Koch, 1990) (Figure 1A).
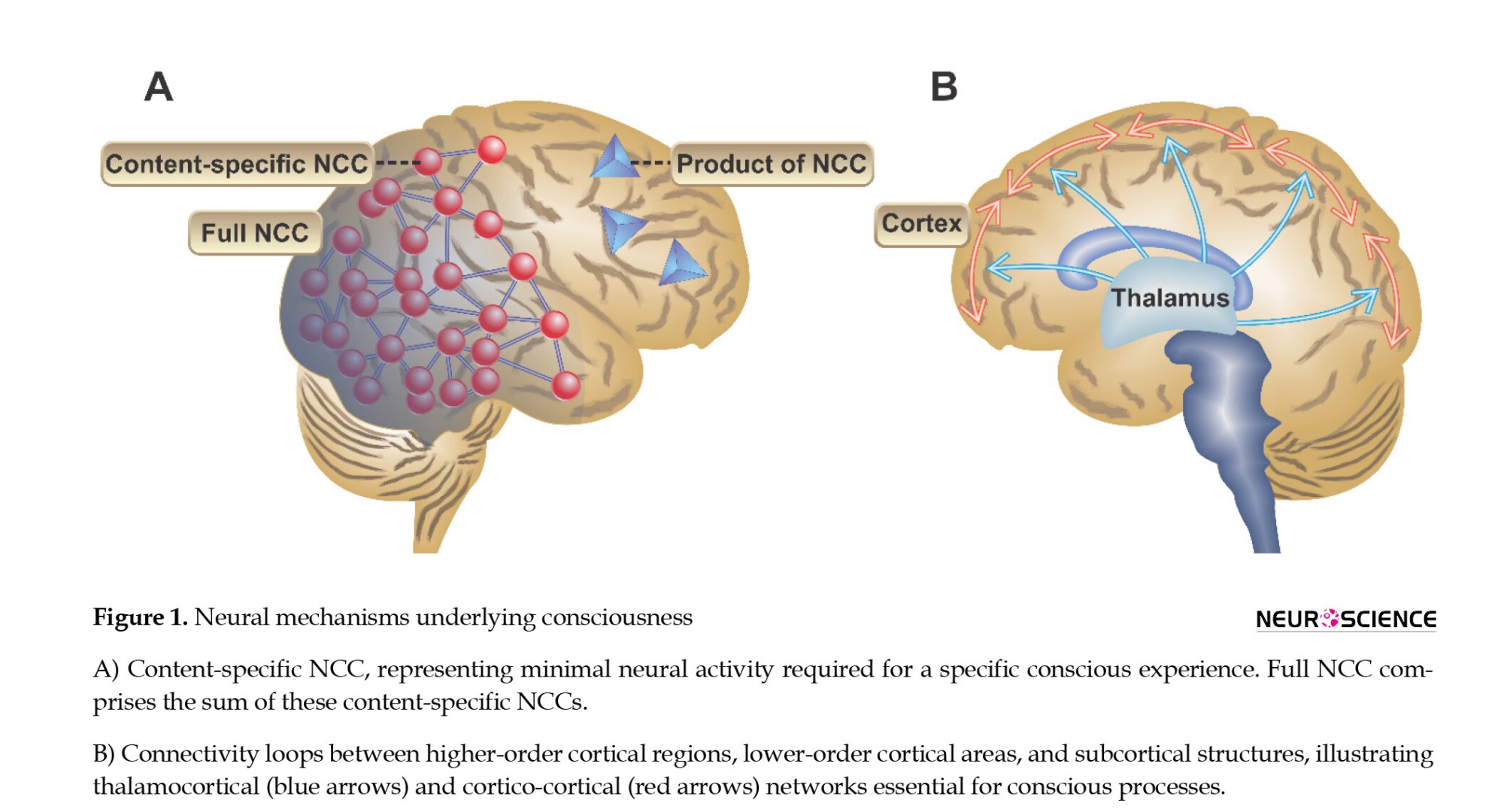
However, utmost efforts should be made to distinguish NCC from the necessary background factors that provide conditions to be conscious. Such factors include the activity of heterogeneous neuronal populations within the brainstem, hypothalamus, basal forebrain, blood-brain oxygen, glucose levels, and neurotransmitters facilitating a conscious experience (Koch et al., 2016).
When it aims to assess content-specific NCC, a given stimulus is presented to the brain, and its activity is compared using specific imaging modalities when this particular stimulus does not exist. For instance, it has been found that the introduction of visual stimuli triggers widespread brain activity in the brain’s frontoparietal and extra-striate occipital networks. These results have been substantiated by findings from a positron emission tomography showing that absolute cerebral blood flow decreases in both parietal and frontal cortices during sleep. Such a finding would confirm the crucial role of these regions in consciousness. On the other hand, assessment of the full NCC is achieved via the comparison between the brain activity in an awake healthy subject with the same person in a condition in which his or her consciousness is severely impaired, e.g. in dreamless sleep (Bai et al., 2010; Koch et al., 2016; Massimini et al., 2005). Such contrasting studies in healthy subjects indicate that full NCC is restricted to a temporal-parietal-occipital area in perceptual experiences and a frontal region in thought-like experiences. This compelling evidence suggests that the posterior cortical region can be assumed to be a “hot zone” for the NCC (Siclari et al., 2014).
What could be derived from both the NCC theories is that no specific part of the brain is directly responsible for conscious experience. On the contrary, it is assumed that a limited number of regions, particularly in the posterior cortical hot zone, are responsible for both full and content-specific NCC (Koch et al., 2016).
Loop connectivity between higher- and lower-order cortical regions and subcortical areas
Higher-order brain regions, particularly the prefrontal cortex, are involved in a range of higher cognitive operations known as decision-making functions. There is considerable verification that neural activity from the frontal cortex to sensory areas is more prognostic of conscious awareness (Crick & Koch, 2003). However, the latest research illuminates that posterior regions contribute more to the localization of consciousness than the frontal regions of the cortex. For instance, various patients have been reported with an average state of consciousness after severe frontal damage (Amberson, 1954).
The association of higher-order cortical regions with lower-order cortical areas and also with subcortical regions, i.e. thalamocortical and cortico-cortical networks, has been the basis for several models to explain consciousness (Figure 1B). This loop connectivity, which is also termed as reafferent, reverberant, recurrent, re-entrant, and feedback connectivity, explains that some higher-order loci in the cortex, i.e. association areas receive input from lower-order regions of the cortex and also subcortical regions. Accordingly, the feedback resulting from higher-order areas produces reverberating signals. These signals can provide the basis for consciousness in several ways. Such circulating signals prevent neural signals from rapid decay. Conversely, these circuits also amplify neural signals. Maintenance and amplification of neural signals in a large-scale network allow multiple cortical regions to access these representations and use these feedbacks as predictive signals to compare them with real-time feed-forward sensory data (Imas et al., 2005; Mashour, 2019).
However, not all things are apparent in this regard. For example, the role of some subcortical regions in consciousness in the aforementioned circuits is not thoroughly elucidated. Despite the association between cortex, basal ganglia, and thalamus, and their involvement in cognitive and motor operations (Alexander et al., 1986; McHaffie, et al., 2005), it remains debatable whether the basal ganglia contribute to consciousness directly, or they are connected using claustrum which is a crucial section in information gathering and its transformation into a conscious state (Crick & Koch, 2005). A recent study states that basal ganglia, cerebellum, and prefrontal cortex do not directly contribute to conscious experience (Koch et al., 2016).
Lateralization of consciousness
After several years of study, Gazzaniga and his colleagues found a significant association between the left hemisphere and consciousness. They suggested the idea of an “interpreter” (Volz & Gazzaniga, 2017), the main objective of which is consistent interpretations of the world and self. Ramachandran (1995) further suggested that the right hemisphere’s role is to recognize the inappropriate and incoherent information related to the left hemisphere. Like a detector, the right hemisphere obliges the left hemisphere to update and modify its beliefs (Gazzaniga, 1992). In healthy participants, data are transferred from the right hemisphere to the left, interpreted, and labeled. Findings showed that the “Interpreter” is situated in the left hemisphere, and it is reliant on both language and inferential thinking (Volz & Gazzaniga, 2017).
On the other hand, when split-brain patients are asked to point out the related cases, they point correctly with their left hand, but verbally, they cannot devise a suitable relationship and do not see any stimulus. The “interpreter” shows a vital prospect of consciousness and its localization, including the left anterior and mid-insula and dorsal caudate (Denny et al., 2012). The findings on split-brain patients assume that the corpus callosum, like the left hemisphere, has an essential influence on conscious experience. Corpus callosum involves both consciousness processing systems: synchronization (Engel & Singer, 2001) and integration (Tononi, 2004).
The global neuronal workspace
Bernard Baars first proposed this theory to provide a cognitive/computational model for consciousness (Baars, 1993). Accordingly, Dehaene et al. redefined the theory from the neural perspective as follows; “a state is conscious when and only when it (or its content) is present in the global neuronal workspace, making the state (content) globally accessible to multiple systems including long-term memory, motor, evaluation, attention, and perception systems.” Here, several terms and conditions should be clarified. First, there is the accessibility of system X to the information in system Y when X utilizes that information in its calculations/processing (Dehaene & Changeux, 2011; Dehaene et al., 1998; Dehaene & Naccache, 2001). In this regard, only states whose content is retrieved by the workspace (neurons with distant connections linking various systems, only if they demonstrate specific neural properties) are globally reachable to other systems and are considered conscious. Second, the workspace is not a solid neural structure but a swiftly evolving neural network. Third, the mere fact of accessibility is not enough to constitute the global workspace. Thus, workspace neurons should be in a sustained active condition, generating a recurrent activity between workspace systems (Dehaene & Naccache, 2001). However, the global neuronal workspace theory cannot sufficiently account for phenomenal consciousness as imaging results that disclose widespread activation during access consciousness are also the basis of phenomenal consciousness (Block, 2007).
Recurrent processing theory
This theory puts forward the effort to define perceptual consciousness based on a process independent of the workspace but dependent on recurrent activity in sensory areas. This recurrent activity is considered both necessary and sufficient for conscious experience and results from feedforward and feedback connections between highly interconnected sensory systems. In a four-stage state of normal visual processing (stage 1: Superficial feedforward processing, stage 2: Deep feedforward processing, stage 3: Superficial recurrent processing, and stage 4: Widespread recurrent processing), Lamme argued that recurrent processing in stage 3 is essential and enough for a conscious experience (Lamme, 2006; Lamme, 2010).
Higher-order theory (HOT)
This theory states that “one is in a conscious state if and only if one relevantly represents oneself as being in such a state,” meaning that one should be able to represent that state. This issue contrasts with first-order theories, which maintain that a mental state is considered conscious only by the neural or representational character of the perceptual condition (Rosenthal, 2002). Experiments performed on the prefrontal cortex provide a basis to test HOT (Dehaene & Changeux, 2011; Lau & Rosenthal, 2011). However, there are several criticisms against this theory, such as 1) prefrontal lesions do not distort awareness, and 2) prefrontal activity echoes attention but not awareness (Kouider et al., 2007; Tse et al., 2005).
Conclusion
The compelling concept of consciousness is one of the challenging discussions in human life from ancient human developments until modern neuroscientific progression. Based on cultural, religious, and social beliefs, there is usually a unique viewpoint about the origins of consciousness in different civilizations and philosophical schools. No consensus on the definite meaning of consciousness exists, though. Nevertheless, the latest neuroscientific developments have removed the misleading obstacles related to consciousness. The study of brain function and its functional and anatomical connections can uncover the location of consciousness in the brain, unraveling most of the unsolved issues related to states of consciousness. Neuroscientific efforts in determining the function of the brain and merging these findings with philosophical theories will bring about a more comprehensive perception of the notion of consciousness.
Ethical Considerations
Compliance with ethical guidelines
This article is a review study with no human or animal sample.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are grateful to the staff and director of the Neurosciences Research Center (NSRC), Tabriz University of Medical Sciences, Tabriz, Iran.
References
Consciousness is one of the most intriguing topics in scientific and philosophical arguments. Living beings, especially human beings, experience phenomena and objects. Therefore, experiencing is a crucial part of this concept that paves the way to understanding consciousness better. Conscious experience is a first-person experience of the nature to which a third person does not have access. These features render consciousness one of the biggest unanswered questions in science (Jerath et al., 2015; Koch, 2018). Over the last few decades, research on consciousness has continued in philosophical, physical, and neuroscientific realms, leading to the emersion of diverse meanings related to consciousness (Jonkisz, 2012). Although there are numerous theoretical and empirical views about the concept of consciousness, there is no consensus regarding its meaning, leaving the term imprecise (Jonkisz, 2015).
The philosophical definition of consciousness varies from that of Descartes, “everything that is within us in such a way that we are immediately aware [conscii] of it…” (Jorgensen, 2010) to Sartre’s, as an existentialist, who defines it as “a being such that in its being, its being is in question insofar as this being implies a being other than itself” (Sartre, 2001). Quantum physics approaches the dilemma from another point of view and suggests that “the process of human c cannot be simulated classically.” Hameroff and Penrose. (2014) have offered “an orchestrated objective reduction (Orch OR) model” in which quantum computation occurs in the microtubule protein assemblies in brain neurons. In this model, every tubulin dimer represents a qubit, showing interaction with other qubits coulombically at a limited temperature, enabling it to possess quantum information processing (Behrman et al., 2006).
Neuroscientists deal with the issue from a different perspective. Consciousness is thought to illustrate the association between cortical structures, such as specific parts of the grey matter within neural operations in the brain, and subcortical regions in the upper brain stem, thalamus, hypothalamus, and basal forebrain (Blumenfeld, 2016; Laureys et al., 2004). Neurobiological theories such as the global neuronal workspace, recurrent processing theory, and information integration theory try to shed light on the unresolved issue of consciousness from its perspective (Lamme & Roelfsema, 2000). Still, no consensus exists about the exact meaning and features (Zeman, 2001). This review scrutinizes consciousness from cultural, historical, philosophical, and neuroscientific points of view.
Consciousness in the old and middle ages
Consciousness has long been part of human history, especially in its reflective form. Archeologists assume that the history of consciousness dates back as far as the Neolithic period, in which burial practices represent spiritual views about humans and life and offer signs for minimally reflective consciousness (Pearson, 1999). Others would believe that consciousness is a rather new concept in human history, dating back to the Homeric era, and those who lived earlier did not show any signs of reflective thought. Some scholars would claim that even Greek civilization had no words corresponding to “consciousness” (Wilkes, 1984). This view has been opposed by the fact that Plato famously believed that the existence of our minds precedes that of our body and outdates the body’s death. Accordingly, Aristotle would define “mind” as part of the body that recognizes and understands (Gennaro, 2016). Evidence also suggests that consciousness has been part of the culture of many civilizations. The Maya civilization was among the first to introduce the idea of consciousness to their general faith around 2000 BCE. Because consciousness contains internal and environmental triggers, the Mayans believed that consciousness should have existed from the beginning of human existence, having a major impact on their individual and social life (Calleman, 2004).
Primitive Indians attempted to describe human consciousness from a broader perspective (Kabat-Zinn, 2013). Indian philosopher Sri Aurobindo demonstrated that consciousness is not only about individual awareness but also comprises a distinctive state of energy (Wautischer, 2008). However, there are no specific clinical inquiries among Indian thoughts except the concept of Ayurveda (Feuerstein, 2001). Most Indian philosophers have consensus over the notion of samsara, meaning that living beings are reborn again in an infinite cycle, the final aim of which is to be released from the round sequence (Gupta, 2003; Matilal, 1986).
Human consciousness in Chinese thought consists of three divisions. The first section explains the cosmological aspect of consciousness, determining the objective world of being for the human. In the second part, the emphasis is on human distinction according to the reflection of the mind and heart in individual and social life. The third layer is about the correlation between politics and consciousness, where the human constructs an ideal political conviction relating to the concrete world (Ch’eng & Bunnin, 2002; Cua, 2003; Feng, 1983).
Self-awareness in Islamic philosophy ranges from Avicenna’s “flying man” as the soul having self-awareness (which is equal to the Cartesian cogito) to self-awareness without the substance of Abu’l-Barakāt al-Baghdādī and Yahya ibn Habash Suhrawardi (Kaukua, 2015). Avicenna considers two distinguished aspects of self-awareness: First, primitive self-awareness, which is equal to the concept of “flying man,” and second, reflexive self-awareness, meaning our awareness of cognizing objects other than ourselves (Black, 2008). Mullā Sadrā accepts the concept of the “flying man” and thus the notion of self-awareness it denotes. However, in his legacy, this concept has two important characteristics. Sadrā believes that self-awareness is a broader concept than the “flying man” and should incorporate both the intellectual and the sub-intellectual forms of mental existence. Besides, he believed that self-awareness is not distinguishable from other constituents of human experience (Kaukua, 2015).
Religion plays a pivotal role in some civilizations and has an important impact on believers’ way of thinking about life. Approximately every religion has some correlated beliefs about the concept of consciousness and reveals its strong regulations through the aid of consciousness (Khalid Ali & Sulam, 2018; Razak, 2012). However, its views on the self vary widely.
Consciousness in the modern era
The 17th century was probably the finest period for the evolution of the concept of consciousness relating to science and scientific views worldwide. Rene Descartes defined the meaning of thought (pensée) as “all that of which we are conscious as operating in us.” He explained two distinctive aspects related to consciousness (Churchland, 1996). By initiating a systematic method, he made a clear difference between consciousness’s physical and mental characteristics, described as Cartesian dualism. He opposed the existence of unconscious mental states. John Locke elaborated on the Cartesian view of consciousness. He claimed that human sensibility is necessary for his thoughts, saying that “he cannot think at any time, waking or sleeping, without being sensible of it.” His definition of a person is the same as consciousness. According to Locke, “[a person] is a thinking intelligent being, that has reason and reflection, and can consider itself as itself, the same thinking thing in different times and places; which it does only by that consciousness, which is inseparable from thinking, and as it seems to be essential to it: It being impossible for anyone to perceive, without perceiving, that he does perceive.” (Gennaro, 2016). Gottfried Wilhelm Leibniz was among the first to distinguish awareness (outer-directed consciousness) from self-awareness (self-consciousness), believing that consciousness could not arise from mere matter. Intriguingly, Leibniz would accept the notion of minimally conscious or unconscious mental states, calling them “petit perceptions.” Immanuel Kant perused the concept of consciousness from a philosophical view, distinguishing the philosophical side from the study of nature and scientific matter. He proposed two components of consciousness: apperception as an ability to be conscious of one’s spontaneous actions (understanding), and inner sense as the consciousness of everything in mind as opposed to apperception (sensibility) (Gennaro, 2016; Heinämaa & Reuter, 2009).
Friedrich Hegel strived to reunite consciousness with the world in a spiritual meaning structure in which the world is a spirit, and human consciousness is a subset of the world that can attain self-consciousness (Hegel, 1998). In the 19th century, Gustav Fechner opposed Descartes’ theory that mind and body were two prospects of a solitary entity; thus, mental procedures could be evaluated (Fechner, 1860; Herbart, 1824). One of the most convincing contemporary theories was explicated by William James, asserting that consciousness is like a flowing stream despite constant shifts and changes and maintains conceptual stability despite rapid alterations (James & CnpeReading, 2018). Sigmund Freud made an association between consciousness and human thoughts and actions. He conceived that the conscious mind depends on a further state of mind (subconscious) and concluded that there is a reciprocal correlation amid discrete states of mind (Mollon, 2014). The notion of the unconscious mind from Freud’s philosophical viewpoint is essential to ease the understanding of consciousness’s ambiguities. Modern theories generally pursue Freud’s psychologic perspectives, and with the aid of neuroscience, they introduce more convincing paths through specific neuroanatomical findings (Laureys et al., 2015). Edmund Husserl states that consciousness invariably includes a self-appearance (Für-sich-selbst-erscheinens). For Husserl, the pure I– the I of transcendental apperception –is not a ‘dead pole of identity’; it is an active living self continuously ‘appearing for itself’ (Moran, 2005). For Martin Heidegger, the doctrine of consciousness is different. Indeed, Heidegger does not possess such a doctrine, and he does not even use the term ‘consciousness’ in his masterpiece, being and time. Martin Heidegger’s concept of Dasein (human existence) encompasses subjectivity and self-consciousness, which interprets itself in the world. In his words, the self-[consciousness] is “given with our consciousness of objects” (Grove, 2004). In his being and nothingness, Sartre describes consciousness as a consciousness of objects and thus explains it in association with something else. In the same place, he stated, “consciousness is a being such that in its being, its being is in question insofar as this being implies a being other than itself.” For Sartre, there are two main types of consciousness, the pre-reflective and the reflective consciousness. There is no place for an ‘ I ‘ at the pre-reflective level, which is a non-observational self-acquaintance. However, at the reflective level, an awareness carries within itself the capacity to contemplate ourselves (Sartre, 2001).
Genetics and improvement in the diagnosis of congenital disorders reduced the complexities regarding clarifying consciousness states. David Chalmers, along with Frank Jackson, disputed that empirical and scientific descriptions are unable to explicate the meaning of consciousness; hence, without the existence of consciousness, the world could be similar to a hunting ground for wild zombies which act just in reaction to external triggers without having any conscious state of mind (Chalmers, 1996; Jackson, 1982). Thomas Nagel suspected whether the physical explanation of brain states could ever elucidate the secrets of the subjective experience of consciousness. He deduced that there should always be an interval between consciousness and scientific exposition of the brain (Nagel, 1997). John Searle distinguished between consciousness and other biological incidents and asserted that consciousness has a subjective nature, unlike other biological phenomena. However, the subjective part of consciousness does not deny the existence of an objective aspect (Searle, 2000). Dennett argued that qualia (instances of subjective, conscious experience) cannot exist as they are too incoherent and incompatible to be tangible. The model of parallelism in the brain is also a particular component of his consciousness view. He also claimed that consciousness is rooted in cultural structures derived from ancient Greek beliefs (Dennett, 2017).
Stuart Hameroff and Roger Penrose believed that the nature of consciousness and its position in the universe is ultimately unknown. Also, they asserted that consciousness relies on biologically lucid quantum operations in association with brain neurons and that these quantum actions modulate neuronal synaptic activities (Hameroff and Penrose, 2014). They proposed three versions of defining consciousness. The most crucial section explains consciousness as the consequence of distinct physical proceedings known as proto-conscious events. Proto-conscious events have been assimilated within living cellular frameworks, leading to brief states of mind called consciousness (Barlow, 2015).
Several contemporary neuroscientists assume a meaningful relationship between the utilization of energy in the brain and neural function and the processing of the data in the brain (Magistretti & Allaman, 2013). Consequently, the supplement of energy is an inseparable backbone of brain computational structure (Sterling & Laughlin, 2015). Robert Shulman and his coworkers have asserted an exact association between consciousness and energy through the brain (Shulman, 2013; Shulman et al., 2009). They revealed that by decreasing the response to an outer stimulus, for instance, in anesthesia, the metabolism of glucose in the brain also reduces. Therefore, energy consumption is vital for the brain’s consciousness.
Increasing attention has been paid to the concept of the brain process (Clarke & Sokoloff, 1994). There has been evidence that in a resting awake state of the brain and nonexistence of outer stimulation factors, the consumption of energy in particular parts of the brain called ‘dark energy’ is extremely low; however, the exact function of dark energy remains unclear (Morcom & Fletcher, 2007; Raichle, 2010).
Two major accepted theories are currently trying to explain consciousness: The global workspace theory (GW) and the integrated information (II) theory. According to the former, communicating but autonomous hubs throughout the brain are responsible for conscious experience. These torrents of unconscious parallel processing have restricted interaction with each other and strive for dissemination via a process called “winner-take-all,” during which several torrents exploit a single torrent for dissemination throughout the brain (Baars et al., 2013). According to the latter, which was proposed by Tononi in 2008, consciousness is the integration of information. In his model, sensory inputs from various sensory organs are integrated with cognitive mechanisms to bring about conscious experience. Further, Tononi introduced a concept known as “qualia space.” The concept assumes that several informational associations between different axes within qualia lead to a given conscious experience (Tononi, 2008). These two models primarily rely on the cortico-thalamic feedback loops. However, they neglect the vital role of the thalamus in this regard and overemphasize the role of the cortex (Jerath et al., 2015).
In what follows, we dig further into the meaning of consciousness in neuroscience.
Neuroanatomy and Neurophysiology of Consciousness
Basic neuroanatomy of consciousness
In medical terms, consciousness has two constituents: Awareness and arousal (wakefulness). Arousal, also defined as wakefulness, alludes to the level of consciousness or the ability to experience awareness. In contrast, awareness is defined as the content of consciousness or, as Nagel puts it, “the subjective character of experience” (Laureys, 2005). Arousal is related to the structures in the brain like hypothalamus and brainstem ascending reticular activating systems (ARAS), whereas awareness is associated with cortico-thalamic network connectivity and frontoparietal cortex (Di Perri et al., 2014; Laureys et al., 2000). Generally, a linear concordance exists between wakefulness and awareness, and along with an increase in one, the other also increases. However, in some conditions, the two aspects are dissociated. The minimally conscious and vegetative states (or unresponsive wakefulness syndrome-UWS) are the conditions during which wakefulness is preserved. Still, awareness is impaired to a different extent (Di Perri et al., 2014). Despite accumulating evidence, it is hard to point to structures that are “minimally sufficient and jointly necessary” for consciousness.
Limited subcortical regions are necessary for maintaining wakefulness, whereas cortical projection structures appear to provide perceptual contents of consciousness or awareness (Baars, 1995). In 1949, the first findings related to cerebral activities were recorded, and the role of ARAS, which is situated in the central thalamus and upper brain, and its relationship with brain activity were described (Moruzzi & Magoun, 1949; Neylan, 1995). The ARAS, a crucial component of consciousness, consists of numerous neural circuits originating from the brainstem’s reticular formation, projecting to the intralaminar nucleus of the thalamus and ending the cerebral cortex (Yeo et al., 2013). Other members of this system include the pedunculopontine nucleus, parabrachial nucleus, locus coeruleus, dorsal raphe, median raphe, hypothalamus, basal forebrain, and non-specific thalamic nuclei which take part in consciousness (Fuller et al., 2011). These structures and pathways stimulate awareness-related centers in the cerebral cortex through synapses in the basal forebrain and thalamus (Edlow et al., 2012). Despite having many neurons, subcortical areas of the brain make a different contribution to the concept of consciousness. The cerebellum has four times more neurons than the cortex (Herculano-Houzel, 2012). However, lesions of this region have little effect on the contents of consciousness (Lemon & Edgley, 2010).
On the other hand, evidence suggests that parts of the corticothalamic system are necessary for maintaining consciousness. Several undisputed examples of UWS demonstrate that massive cortical grey or white matter lesions commonly accompany the loss of consciousness. This frequently and significantly involves the thalamus (Posner et al., 2007). The evidence, when accompanied by the fact that patients with lesions of brain structures outside the corticothalamic system (e.g. spinal cord and cerebellum) remain conscious, becomes more compelling (Tononi et al., 2016). Here, another unresolved issue arises, positing the thalamus against other parts of the cortex. It has been proposed that the thalamus, particularly the intralaminar nuclei, might form a “centrencephalic system” responsible for a conscious state (Bogen, 1997). In this regard, patients with brain damage involving bilateral paramedian thalamic nuclei are often in an unresponsive state or minimally conscious state (Posner et al., 2007). However, evidence from other studies contradicts these findings and shows that the thalamus may not be necessary for consciousness. Thus, lesions involving the paramedian thalamic region and causing an unconscious state might involve projection from glutamatergic neurons in the parabrachial-precoeruleus complex of the brainstem. The latter is responsible for the stimulation of the cortex via a basal forebrain relay (Fuller et al., 2011). Other discussions regarding the role of primary areas vs higher level areas, ventral vs dorsal visual stream, posterior vs anterior (prefrontal) cortex, lateral fronto-parietal network vs (medial) default system, left vs right hemisphere, reentrant vs feed-forward connections, and superficial vs deep layers of cortex in a conscious experience exist. However, none of these are conclusive.
Cell types involved in consciousness
Neurosciences have always been preoccupied with whether specific cell types are directly responsible for conscious experience. Research has shown that specific cell types, such as the spindle neurons (also recognized as von Economo neurons) detected in layer 5 of the frontal lobe in species with bulky, complex brains, may be responsible, at least partially, for consciousness (Butti et al., 2013). On the other hand, others have indicated the important role of thin-tufted pyramidal cells in consciousness. These cells create corticocortical connections in layer 5A, and their interconnections are denser than those of the neurons in layer 6. To complicate the presented picture with more evidence, scientists have revealed that supra-granular pyramidal cells have denser interconnections with other neurons in the superficial cortical layers, possess more precise topography than those found in the infra-glandular layers, and are fire more specifically in response to stimuli than the latter layer (Harris & Shepherd, 2015; Markov et al., 2014; Zhang et al., 2014). What could be interpreted from the previous discussion is that neurons in the supra-granular layer are associated with a conscious experience (He & Raichle, 2009).
Electrophysiological correlates of consciousness
Alterations in neural activity do not inevitably associate with changes in conscious experience. From the electrophysiologic view, consciousness implies low frequency and desynchronized brain activity that ranges between 20 and 70 Hz. The parts of the brain involved in consciousness, like the nuclei of the thalamus, are connected with the cortex through the thalamocortical system. The oscillating loop is adjusted around 40 Hz during the information-flowing process. The neurons of the thalamic nuclei have been found to switch on particular cortical pyramidal cells and quiet other brain parts by activating gamma-aminobutyric acid (GABA) inhibitory interneurons. Consequently, consciousness is presumed to be feasible only when the 40 Hz electrical stream is established along with the brain circuits (Negrao & Viljoen, 2009)
Apart from the mentioned waves, which may play a role in consciousness, several super- or infra-slow waves (0.001-0.1 Hz) and slow waves (0.1-1 Hz) may also take part in the integration of faster frequencies and have a crucial role in maintaining a conscious state, integrating inputs from different sources and thus unifying consciousness. The slow and infra-slow waves are generated in the upper layers of higher-order brain regions such as the associative cortex, and their existence is thought to be a mandatory, non-sufficient prerequisite for consciousness. These waves are also called neural predisposition of the level/state of consciousness (NPC) as opposed to faster waves, which are neural correlates of consciousness (NCC), meaning that in normal conditions, slow/infra-slow waves provide “temporal basement” that works as “default-mode” for the coupling to faster waves (or cross-frequency coupling). Infra-slow and slow waves travel through the brain’s cortical layers and change direction when the animal is anesthetized. Also, when a given animal is unconscious, the association between these slow and fast waves breaks apart, causing “temporospatial fragmentation and isolation” (He & Raichle, 2009; Northoff, 2013; Northoff, 2017). What happens during unresponsive wakefulness syndrome (UWRS) is indeed an increase in the slow-wave power and a decrease in the faster-wave power or replacement of “temporospatial integration and nestedness” by “temporospatial fragmentation and isolation” (Northoff, 2017).
The NCC
The NCC is described as minimal neuronal operations mandatory for any specific conscious matter (Francis Crick & Koch, 1990; Koch, 2004). According to Chalmers, NCC “is a minimal neural system N such that there is a mapping from states of N to states of consciousness, where a given state of N is sufficient under conditions C, for the corresponding state of consciousness” (Chalmers, 1995).
There are typically two models of NCC. The content-specific NCC is defined as the minimal neural mechanisms and activities that define a certain phenomenal difference in conscious experience, such as places, colors, or thoughts. This could be artificially stimulated via specific techniques such as transcranial magnetic stimulation (TMS) when there are no real external stimuli or blocked via the exact mechanisms when there are real external phenomena (Koch et al., 2016). The full NCC can be explained as neural prerequisites associated with conscious experiences regardless of their certain contents. This, in general, has been described as the sum of the content-specific NCCs (Crick & Koch, 1990) (Figure 1A).

However, utmost efforts should be made to distinguish NCC from the necessary background factors that provide conditions to be conscious. Such factors include the activity of heterogeneous neuronal populations within the brainstem, hypothalamus, basal forebrain, blood-brain oxygen, glucose levels, and neurotransmitters facilitating a conscious experience (Koch et al., 2016).
When it aims to assess content-specific NCC, a given stimulus is presented to the brain, and its activity is compared using specific imaging modalities when this particular stimulus does not exist. For instance, it has been found that the introduction of visual stimuli triggers widespread brain activity in the brain’s frontoparietal and extra-striate occipital networks. These results have been substantiated by findings from a positron emission tomography showing that absolute cerebral blood flow decreases in both parietal and frontal cortices during sleep. Such a finding would confirm the crucial role of these regions in consciousness. On the other hand, assessment of the full NCC is achieved via the comparison between the brain activity in an awake healthy subject with the same person in a condition in which his or her consciousness is severely impaired, e.g. in dreamless sleep (Bai et al., 2010; Koch et al., 2016; Massimini et al., 2005). Such contrasting studies in healthy subjects indicate that full NCC is restricted to a temporal-parietal-occipital area in perceptual experiences and a frontal region in thought-like experiences. This compelling evidence suggests that the posterior cortical region can be assumed to be a “hot zone” for the NCC (Siclari et al., 2014).
What could be derived from both the NCC theories is that no specific part of the brain is directly responsible for conscious experience. On the contrary, it is assumed that a limited number of regions, particularly in the posterior cortical hot zone, are responsible for both full and content-specific NCC (Koch et al., 2016).
Loop connectivity between higher- and lower-order cortical regions and subcortical areas
Higher-order brain regions, particularly the prefrontal cortex, are involved in a range of higher cognitive operations known as decision-making functions. There is considerable verification that neural activity from the frontal cortex to sensory areas is more prognostic of conscious awareness (Crick & Koch, 2003). However, the latest research illuminates that posterior regions contribute more to the localization of consciousness than the frontal regions of the cortex. For instance, various patients have been reported with an average state of consciousness after severe frontal damage (Amberson, 1954).
The association of higher-order cortical regions with lower-order cortical areas and also with subcortical regions, i.e. thalamocortical and cortico-cortical networks, has been the basis for several models to explain consciousness (Figure 1B). This loop connectivity, which is also termed as reafferent, reverberant, recurrent, re-entrant, and feedback connectivity, explains that some higher-order loci in the cortex, i.e. association areas receive input from lower-order regions of the cortex and also subcortical regions. Accordingly, the feedback resulting from higher-order areas produces reverberating signals. These signals can provide the basis for consciousness in several ways. Such circulating signals prevent neural signals from rapid decay. Conversely, these circuits also amplify neural signals. Maintenance and amplification of neural signals in a large-scale network allow multiple cortical regions to access these representations and use these feedbacks as predictive signals to compare them with real-time feed-forward sensory data (Imas et al., 2005; Mashour, 2019).
However, not all things are apparent in this regard. For example, the role of some subcortical regions in consciousness in the aforementioned circuits is not thoroughly elucidated. Despite the association between cortex, basal ganglia, and thalamus, and their involvement in cognitive and motor operations (Alexander et al., 1986; McHaffie, et al., 2005), it remains debatable whether the basal ganglia contribute to consciousness directly, or they are connected using claustrum which is a crucial section in information gathering and its transformation into a conscious state (Crick & Koch, 2005). A recent study states that basal ganglia, cerebellum, and prefrontal cortex do not directly contribute to conscious experience (Koch et al., 2016).
Lateralization of consciousness
After several years of study, Gazzaniga and his colleagues found a significant association between the left hemisphere and consciousness. They suggested the idea of an “interpreter” (Volz & Gazzaniga, 2017), the main objective of which is consistent interpretations of the world and self. Ramachandran (1995) further suggested that the right hemisphere’s role is to recognize the inappropriate and incoherent information related to the left hemisphere. Like a detector, the right hemisphere obliges the left hemisphere to update and modify its beliefs (Gazzaniga, 1992). In healthy participants, data are transferred from the right hemisphere to the left, interpreted, and labeled. Findings showed that the “Interpreter” is situated in the left hemisphere, and it is reliant on both language and inferential thinking (Volz & Gazzaniga, 2017).
On the other hand, when split-brain patients are asked to point out the related cases, they point correctly with their left hand, but verbally, they cannot devise a suitable relationship and do not see any stimulus. The “interpreter” shows a vital prospect of consciousness and its localization, including the left anterior and mid-insula and dorsal caudate (Denny et al., 2012). The findings on split-brain patients assume that the corpus callosum, like the left hemisphere, has an essential influence on conscious experience. Corpus callosum involves both consciousness processing systems: synchronization (Engel & Singer, 2001) and integration (Tononi, 2004).
The global neuronal workspace
Bernard Baars first proposed this theory to provide a cognitive/computational model for consciousness (Baars, 1993). Accordingly, Dehaene et al. redefined the theory from the neural perspective as follows; “a state is conscious when and only when it (or its content) is present in the global neuronal workspace, making the state (content) globally accessible to multiple systems including long-term memory, motor, evaluation, attention, and perception systems.” Here, several terms and conditions should be clarified. First, there is the accessibility of system X to the information in system Y when X utilizes that information in its calculations/processing (Dehaene & Changeux, 2011; Dehaene et al., 1998; Dehaene & Naccache, 2001). In this regard, only states whose content is retrieved by the workspace (neurons with distant connections linking various systems, only if they demonstrate specific neural properties) are globally reachable to other systems and are considered conscious. Second, the workspace is not a solid neural structure but a swiftly evolving neural network. Third, the mere fact of accessibility is not enough to constitute the global workspace. Thus, workspace neurons should be in a sustained active condition, generating a recurrent activity between workspace systems (Dehaene & Naccache, 2001). However, the global neuronal workspace theory cannot sufficiently account for phenomenal consciousness as imaging results that disclose widespread activation during access consciousness are also the basis of phenomenal consciousness (Block, 2007).
Recurrent processing theory
This theory puts forward the effort to define perceptual consciousness based on a process independent of the workspace but dependent on recurrent activity in sensory areas. This recurrent activity is considered both necessary and sufficient for conscious experience and results from feedforward and feedback connections between highly interconnected sensory systems. In a four-stage state of normal visual processing (stage 1: Superficial feedforward processing, stage 2: Deep feedforward processing, stage 3: Superficial recurrent processing, and stage 4: Widespread recurrent processing), Lamme argued that recurrent processing in stage 3 is essential and enough for a conscious experience (Lamme, 2006; Lamme, 2010).
Higher-order theory (HOT)
This theory states that “one is in a conscious state if and only if one relevantly represents oneself as being in such a state,” meaning that one should be able to represent that state. This issue contrasts with first-order theories, which maintain that a mental state is considered conscious only by the neural or representational character of the perceptual condition (Rosenthal, 2002). Experiments performed on the prefrontal cortex provide a basis to test HOT (Dehaene & Changeux, 2011; Lau & Rosenthal, 2011). However, there are several criticisms against this theory, such as 1) prefrontal lesions do not distort awareness, and 2) prefrontal activity echoes attention but not awareness (Kouider et al., 2007; Tse et al., 2005).
Conclusion
The compelling concept of consciousness is one of the challenging discussions in human life from ancient human developments until modern neuroscientific progression. Based on cultural, religious, and social beliefs, there is usually a unique viewpoint about the origins of consciousness in different civilizations and philosophical schools. No consensus on the definite meaning of consciousness exists, though. Nevertheless, the latest neuroscientific developments have removed the misleading obstacles related to consciousness. The study of brain function and its functional and anatomical connections can uncover the location of consciousness in the brain, unraveling most of the unsolved issues related to states of consciousness. Neuroscientific efforts in determining the function of the brain and merging these findings with philosophical theories will bring about a more comprehensive perception of the notion of consciousness.
Ethical Considerations
Compliance with ethical guidelines
This article is a review study with no human or animal sample.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are grateful to the staff and director of the Neurosciences Research Center (NSRC), Tabriz University of Medical Sciences, Tabriz, Iran.
References
Alexander, G. E., DeLong, M. R., & Strick, P. L. (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience, 9, 357–381. [DOI:10.1146/annurev.ne.09.030186.002041] [PMID]
Amberson, W. R. (1954). Epilepsy and the functional anatomy of the human brain. Wilder Penfield and Herbert Jasper. Little, Brown, Boston, 1954. 896 pp. Illus. + plates. $16.00. Science, 119(3097), 645. [Link]
Baars, B. J. (1993). A cognitive theory of consciousness. Cambridge: Cambridge University Press. [Link]
Baars, B. J. (1995). Tutorial commentary: Surprisingly small subcortical structures are needed for the state of waking consciousness, while cortical projection areas seem to provide perceptual contents of consciousness. Consciousness and Cognition, 4(2), 159–162. [DOI:10.1006/ccog.1995.1021] [PMID]
Baars, B. J., Franklin, S., & Ramsoy, T. Z. (2013). Global workspace dynamics: Cortical "binding and propagation" enables conscious contents. Frontiers in Psychology, 4, 200. [DOI:10.3389/fpsyg.2013.00200] [PMID]
Bai, X., Vestal, M., Berman, R., Negishi, M., Spann, M., & Vega, C., et al. (2010). Dynamic time course of typical childhood absence seizures: EEG, behavior, and functional magnetic resonance imaging. The Journal of Neuroscience: The Official Journal of The Society for Neuroscience, 30(17), 5884–5893. [DOI:10.1523/JNEUROSCI.5101-09.2010] [PMID] [PMCID]
Barlow, P. W. (2015). The natural history of consciousness, and the question of whether plants are conscious, in relation to the Hameroff-Penrose quantum-physical ‘Orch OR’ theory of universal consciousness. Communicative & Integrative Biology, 8(4), e1041696. [DOI:10.1080/19420889.2015.1041696] [PMID] [PMCID]
Behrman, E. C., Gaddam, K., Steck, J., & Skinner, S. (2006). Microtubules as a quantum Hopfield network. In J. A. Tuszynski (Ed.), The emerging physics of consciousness (pp. 351-370), Berlin: Springer. [DOI:10.1007/3-540-36723-3_10]
Black, D. L. (2008). Avicenna on self-awareness and knowing that one knows. In S. Rahman, T. Street, & H. Tahiri (Eds.), The Unity of Science in the Arabic Tradition. Logic, Epistemology, and The Unity of Science, vol 11. (pp. 63-87). Dordrecht: Springer. [DOI:10.1007/978-1-4020-8405-8_3]
Block, N. (2007). Consciousness, accessibility, and the mesh between psychology and neuroscience.The Behavioral and Brain Sciences, 30(5-6), 481–548. [DOI:10.1017/S0140525X07002786] [PMID]
Blumenfeld, H. (2016). Chapter 1 - Neuroanatomical basis of consciousness. In S. Laureys, O. Gosseries, & G. Tononi (Eds.), The Neurology of Conciousness (Second Edition) (pp. 3-29). San Diego: Academic Press. [DOI:10.1016/B978-0-12-800948-2.00001-7]
Bogen, J. E. (1997). Some neurophysiologic aspects of consciousness. Seminars in Neurology, 17(2), 95–103.[DOI:10.1055/s-2008-1040918] [PMID]
Butti, C., Santos, M., Uppal, N., & Hof, P. R. (2013). Von Economo neurons: Clinical and evolutionary perspectives. Cortex, 49(1), 312-326. [DOI:10.1016/j.cortex.2011.10.004] [PMID]
Calleman, C. J. (2004).The Mayan calendar and the transformation of consciousness.Vermont: Inner Traditions/Bear. [Link]
Ch’eng, C. Y., & Bunnin, N. (2002). Contemporary Chinese philosophy. Oxford: Blackwell Publishers Ltd. [DOI:10.1002/9780470753491]
Chalmers, D. J. (1995). Facing up to the problem of consciousness. Journal of Consciousness Studies, 2(3), 200-219. [Link]
Chalmers, D. J. (1997). The conscious mind: In search of a fundamental theory. Oxford: Oxford University Press. [Link]
Churchland, P. M. (1996). The engine of reason the seat of the soul: A philosophical journey into the brain. Cambridge (Mass.): MIT Press. [DOI:10.7551/mitpress/2758.001.0001]
Clarke, D., & Sokoloff, L. (1994). Circulation and energy metabolism of the brain. In G. J. Siegel, B. W. Agranoff, & R. W. Albers (Eds.), Basic neurochemistry: Molecular, cellular and medical aspects. Philadelphia: Lippincott-Raven. [Link]
Crick, F., & Koch, C. (1990). Toward a neurobiological theory of consciousness. Seminars in the Neurosciences, 2, 263-275. [Link]
Crick, F., & Koch, C. (2003). A framework for consciousness. Nature Neuroscience, 6(2), 119–126. [DOI:10.1038/nn0203-119] [PMID]
Crick, F. C., & Koch, C. (2005). What is the function of the claustrum? Philosophical transactions of the Royal Society of London. Series B, Biological Sciences, 360(1458), 1271-1279. [DOI:10.1098/rstb.2005.1661] [PMID] [PMCID]
Dehaene, S., & Changeux, J. P. (2011). Experimental and theoretical approaches to conscious processing. Neuron, 70(2), 200-227. [DOI:10.1016/j.neuron.2011.03.018] [PMID]
Dehaene, S., Kerszberg, M., & Changeux, J. P. (1998). A neuronal model of a global workspace in effortful cognitive tasks. Proceedings of the National Academy of Sciences of the United States of America, 95(24), 14529–14534. [DOI:10.1073/pnas.95.24.14529] [PMID] [PMCID]
Dehaene, S., & Naccache, L. (2001). Towards a cognitive neuroscience of consciousness: Basic evidence and a workspace framework. Cognition, 79(1-2), 1-37. [DOI:10.1016/S0010-0277(00)00123-2] [PMID]
Denny, B. T., Kober, H., Wager, T. D., & Ochsner, K. N. (2012). A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive Neuroscience, 24(8), 1742–1752. [DOI:10.1162/jocn_a_00233] [PMID] [PMCID]
Di Perri, C., Stender, J., Laureys, S., & Gosseries, O. (2014). Functional neuroanatomy of disorders of consciousness. Epilepsy & Behavior, 30, 28-32. [DOI:10.1016/j.yebeh.2013.09.014] [PMID]
Edlow, B. L., Takahashi, E., Wu, O., Benner, T., Dai, G., & Bu, L., et al. (2012). Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. Journal of Neuropathology & Experimental Neurology, 71(6), 531-546. [DOI:10.1097/NEN.0b013e3182588293] [PMID] [PMCID]
Engel, A. K., & Singer, W. (2001). Temporal binding and the neural correlates of sensory awareness. Trends in Cognitive Sciences, 5(1), 16–25. [DOI:10.1016/S1364-6613(00)01568-0] [PMID]
Feuerstein, G. (2001).The Yoga tradition: Its history, literature, philosophy, and practice. Arizona: Hohm Press. [Link]
Fuller, P. M., Sherman, D., Pedersen, N. P., Saper, C. B., & Lu, J. (2011). Reassessment of the structural basis of the ascending arousal system. The Journal of Comparative Neurology, 519(5), 933-956. [DOI:10.1002/cne.22559] [PMID] [PMCID]
Gazzaniga, M. S. (1992). Nature's mind: Biological roots of thinking, emotions, sexuality, language, and intelligence. New York: Basic Books. [Link]
Grove, P. (2004). Heidegger on subjectivity and self-consciousness. SATS, 5(1), 154-166. [DOI:10.1515/SATS.2004.154]
Hameroff, S., & Penrose, R. (2014). Consciousness in the universe: a review of the ‘Orch OR’ theory. Physics of Life Reviews, 11(1), 39–78. [DOI:10.1016/j.plrev.2013.08.002] [PMID]
Harris, K. D., & Shepherd, G. M. (2015). The neocortical circuit: Themes and variations. Nature Neuroscience, 18(2), 170–181.[DOI:10.1038/nn.3917] [PMID] [PMCID]
He, B. J., & Raichle, M. E. (2009). The fMRI signal, slow cortical potential and consciousness. Trends in Cognitive Sciences, 13(7), 302-309. [DOI:10.1016/j.tics.2009.04.004] [PMID] [PMCID]
Heinämaa, S., & Reuter, M. (2009). Psychology and philosophy: Inquiries into the soul from late scholasticism to contemporary thought. Dordrecht: Springer. [DOI:10.1007/978-1-4020-8582-6]
Herbart, J. F. (1824). [Psychologie als Wissenschaft: Neu gegründet auf Erfahrung, Metaphysik und Mathematik / 2 Analytischer Theil (German)]. Königsberg: A.W. Unzer. [Link]
Herculano-Houzel, S. (2012). The remarkable, yet not extraordinary, human brain as a scaled-up primate brain and its associated cost. Proceedings of the National Academy of Sciences of the United States of America, 109 Suppl 1(Suppl 1), 10661–10668.[DOI:10.1073/pnas.1201895109] [PMID]
Imas, O. A., Ropella, K. M., Ward, B. D., Wood, J. D., & Hudetz, A. G. (2005). Volatile anesthetics disrupt frontal-posterior recurrent information transfer at gamma frequencies in rat. Neuroscience Letters, 387(3), 145-150. [DOI:10.1016/j.neulet.2005.06.018] [PMID]
Jackson, F. (1982). Epiphenomenal qualia. Philosophical Quarterly, 32(127), 127-136. [DOI:10.2307/2960077]
Jerath, R., Crawford, M., & Barnes, V. (2015). A unified 3D default space consciousness model combining neurological and physiological processes that underlie conscious experience. Frontiers in Psychology, 6, 1204. [DOI:10.3389/fpsyg.2015.01204] [PMID]
Jonkisz, J. (2012). Consciousness: A four-fold taxonomy. Journal of Consciousness Studies, 19(11-12), 55-82. [Link]
Jonkisz, J. (2015). Consciousness: Individuated information in action. Frontiers in Psychology, 6, 1035. [DOI:10.3389/fpsyg.2015.01035] [PMID] [PMCID]
Jorgensen, L. M. (2010). Seventeenth-century theories of consciousness. Stanford: Stanford Encyclopedia of Philosophy. [Link]
Kabat-Zinn, J. (2013). Full catastrophe living: How to cope with stress, pain and illness using mindfulness meditation. London: Piatkus. [Link]
Kaukua, J. (2015). Self-Awareness in Islamic Philosophy. Cambridge University Press. [DOI:10.1017/CBO9781316105238]
Khalid Ali, K., & Sulam, M. (2018). The Paradigms of consciousness: A discourse. SHS Web of Conferences, 53, 1-10. [DOI:10.1051/shsconf/20185304003]
Koch, C. (2004). The quest for consciousness: A neurobiological approach. Englewood, CO: Roberts and Company Publishers. [Link]
Koch, C. (2018). What is consciousness? Nature, 557(7704), S8-s12. [DOI:10.1038/d41586-018-05097-x] [PMID]
Koch, C., Massimini, M., Boly, M., & Tononi, G. (2016). Neural correlates of consciousness: Progress and problems. Nature Reviews. Neuroscience, 17(5), 307–321. [DOI:10.1038/nrn.2016.22] [PMID]
Kouider, S., Dehaene, S., Jobert, A., & Le Bihan, D. (2007). Cerebral bases of subliminal and supraliminal priming during reading. Cerebral Cortex (New York, N.Y.: 1991), 17(9), 2019–2029. [DOI:10.1093/cercor/bhl110] [PMID]
Lamme, V. A. (2006). Towards a true neural stance on consciousness. Trends in Cognitive Sciences, 10(11), 494–501. [DOI:10.1016/j.tics.2006.09.001] [PMID]
Lamme, V. A. (2010). How neuroscience will change our view on consciousness. Cognitive Neuroscience, 1(3), 204–220.[DOI:10.1080/17588921003731586] [PMID]
Lamme, V. A., & Roelfsema, P. R. (2000). The distinct modes of vision offered by feedforward and recurrent processing. Trends in Neurosciences, 23(11), 571–579. [DOI:10.1016/S0166-2236(00)01657-X] [PMID]
Lau, H., & Rosenthal, D. (2011). Empirical support for higher-order theories of conscious awareness. Trends in Cognitive Sciences, 15(8), 365–373.[DOI:10.1016/j.tics.2011.05.009] [PMID]
Laureys, S. (2005). The neural correlate of (un)awareness: lessons from the vegetative state. Trends in Cognitive Sciences, 9(12), 556–559. [DOI:10.1016/j.tics.2005.10.010] [PMID]
Laureys, S., Faymonville, M. E., Luxen, A., Lamy, M., Franck, G., & Maquet, P. (2000). Restoration of thalamocortical connectivity after recovery from persistent vegetative state. Lancet, 355(9217), 1790-1791. [DOI:10.1016/S0140-6736(00)02271-6] [PMID]
Laureys, S., & Tononi, G. (2015). The neurology of consciousness: Cognitive neuroscience and neuropathology. Amsterdam: Elsevier Science. [Link]
Laureys, S., Owen, A. M., & Schiff, N. D. (2004). Brain function in coma, vegetative state, and related disorders. The Lancet. Neurology, 3(9), 537–546. [DOI:10.1016/S1474-4422(04)00852-X] [PMID]
Lemon, R. N., & Edgley, S. A. (2010). Life without a cerebellum. Brain, 133(Pt 3), 652-654. [DOI:10.1093/brain/awq030] [PMID]
Magistretti, P. J., & Allaman, I. (2013). Brain energy metabolism. In D. W. Pfaff (Ed.), Neuroscience in the 21st century. New York: Springer. [DOI:10.1007/978-1-4614-1997-6_56]
Markov, N. T., Vezoli, J., Chameau, P., Falchier, A., Quilodran, R., & Huissoud, C., et al. (2014). Anatomy of hierarchy: Feedforward and feedback pathways in macaque visual cortex. The Journal of Comparative Neurology, 522(1), 225–259. [DOI:10.1002/cne.23458] [PMID]
Mashour, G. A. (2019). Role of cortical feedback signalling in consciousness and anaesthetic-induced unconsciousness. British Journal of Anaesthesia, 123(4), 404-405. [DOI:10.1016/j.bja.2019.07.001]
Massimini, M., Ferrarelli, F., Huber, R., Esser, S. K., Singh, H., & Tononi, G. (2005). Breakdown of cortical effective connectivity during sleep. Science, 309(5744), 2228-2232. [DOI:10.1126/science.1117256] [PMID]
Matilal, B. K. (1991). Perception: An essay on classical Indian theories of knowledge. Oxford: Oxford University Press. [Link]
McHaffie, J. G., Stanford, T. R., Stein, B. E., Coizet, V., & Redgrave, P. (2005). Subcortical loops through the basal ganglia. Trends in Neurosciences, 28(8), 401–407. [DOI:10.1016/j.tins.2005.06.006] [PMID]
Mollon, P. (2014). Revisiting “analysis terminable and interminable” expressions of death instinct by patients and analyst.Psychoanalytic Inquiry, 34(1), 28-38. [DOI:10.1080/07351690.2014.859891]
Morcom, A. M., & Fletcher, P. C. (2007). Does the brain have a baseline? Why we should be resisting a rest. Neuroimage, 37(4), 1073-1082. [DOI:10.1016/j.neuroimage.2006.09.013]
Moruzzi, G., & Magoun, H. W. (1949). Brain stem reticular formation and activation of the EEG. Electroencephalography and Clinical Neurophysiology, 1(4), 455–473. [DOI:10.1016/0013-4694(49)90219-9] [PMID]
Nagel, T. (1974). What is it like to be a bat? The Philosophical Review, 83(4), 435-450. [DOI:10.2307/2183914]
Negrao, B. L., & Viljoen, M. (2009). Neural correlates of consciousness. African Journal of Psychiatry, 12(4), 265-269. [Link]
Neylan, T. C. (1995). Physiology of arousal: Moruzzi and Magoun’s ascending reticular activating system. The Journal of Neuropsychiatry and Clinical Neurosciences, 7(2), 250. [DOI:10.1176/jnp.7.2.250] [PMID]
Northoff, G. (2013). What the brain’s intrinsic activity can tell us about consciousness? A tri-dimensional view. Neuroscience & Biobehavioral Reviews, 37(4), 726-738. [DOI:10.1016/j.neubiorev.2012.12.004] [PMID]
Northoff, G. (2017). “Paradox of slow frequencies” - Are slow frequencies in upper cortical layers a neural predisposition of the level/state of consciousness (NPC)? Consciousness and Cognition, 54, 20-35. [DOI:10.1016/j.concog.2017.03.006] [PMID]
Posner, J. B., Saper, C. B., Schiff, N., & Plum, F. (2007). Plum and Posner’s diagnosis of stupor and coma. Oxford: Oxford University Press. [DOI:10.1093/med/9780195321319.001.0001]
Raichle, M. E. (2010). The brain’s dark energy. Scientific American, 302(3), 44-49. [DOI:10.1038/scientificamerican0310-44] [PMID]
Ramachandran, V. S. (1995). Anosognosia in parietal lobe syndrome. Consciousness and Cognition, 4(1), 22–51. [DOI:10.1006/ccog.1995.1002] [PMID]
Razak, M. A. A. (2011). Human nature: An islamic perspective. Journal of Islam in Asia (E-ISSN 2289-8077), 8, 251-274. [Link]
Rosenthal, D. M. (2002). Explaining consciousness. In D. J. Chalmers (Ed.), Philosophy of mind: Classical and contemporary readings(pp.109-131). New York: Oxford University Press. [Link]
Sartre, J. (2001). Being and nothingness: An essay in phenomenological ontology. New York: Citadel Press. [Link]
Searle, J. R. (2000). Consciousness. Annual Review of Neuroscience, 23, 557–578. [DOI:10.1146/annurev.neuro.23.1.557] [PMID]
Shulman, R. G. (2013). Brain imaging: What it can (and cannot) tell us about consciousness. Oxford: Oxford University Press. [DOI:10.1093/acprof:oso/9780199838721.001.0001]
Shulman, R. G., Hyder, F., & Rothman, D. L. (2009). Baseline brain energy supports the state of consciousness. Proceedings of the National Academy of Sciences of the United States of America, 106(27), 11096–11101. [DOI:10.1073/pnas.0903941106] [PMID]
Siclari, F., LaRocque, J. J., Bernardi, G., Postle, B. R., & Tononi, G. (2014). The neural correlates of consciousness in sleep: A no-task, within-state paradigm. BioRxiv, 012443. [Link]
Sterling, P., & Laughlin, S. (2015). Principles of neural design. Cambridge: MIT Press. [DOI:10.7551/mitpress/9780262028707.001.0001]
Tononi, G. (2004). An information integration theory of consciousness. BMC Neuroscience, 5, 42. [DOI:10.1186/1471-2202-5-42] [PMID]
Tononi, G. (2008). Consciousness as integrated information: A provisional manifesto. The Biological Bulletin, 215(3), 216–242.[DOI:10.2307/25470707] [PMID]
Tononi, G., Boly, M., Gosseries, O., & Laureys, S. (2016). The neurology of consciousness: An overview. In S. Laureys, O. Gosseries, & G. Tononi (Eds.), The neurology of conciousness (pp. 407-461). Cambridge: Academic Press. [DOI:10.1016/B978-0-12-800948-2.00025-X]
Tse, P. U., Martinez-Conde, S., Schlegel, A. A., & Macknik, S. L. (2005). Visibility, visual awareness, and visual masking of simple unattended targets are confined to areas in the occipital cortex beyond human V1/V2. Proceedings of the National Academy of Sciences of the United States of America, 102(47), 17178–17183. [DOI:10.1073/pnas.0508010102] [PMID]
Volz, L. J., & Gazzaniga, M. S. (2017). Interaction in isolation: 50 years of insights from split-brain research. Brain, 140(7), 2051-2060. [DOI:10.1093/brain/awx139] [PMID]
Wautischer, H. (2008). Ontology of consciousness: Percipient action. Cambridge, Mass.: MIT Press. [DOI:10.7551/mitpress/7415.001.0001]
Wilkes, K. V. (1984). Is consciousness important? British Journal for the Philosophy of Science, 35(3), 223-243. [DOI:10.1093/bjps/35.3.223]
Type of Study: Review |
Subject:
Cognitive Neuroscience
Received: 2019/12/28 | Accepted: 2020/07/6 | Published: 2024/09/1
Received: 2019/12/28 | Accepted: 2020/07/6 | Published: 2024/09/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








