Volume 11, Issue 5 (September & October - Special Issue on Cognitive Neuroscience 2020)
BCN 2020, 11(5): 649-658 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mazhari S, Arjmand S, Eslami Shahrbabaki M, Karimi Ghoughari E. Comparing Copper Serum Level and Cognitive Functioning in Patients With Schizophrenia and Healthy Controls. BCN 2020; 11 (5) :649-658
URL: http://bcn.iums.ac.ir/article-1-1624-en.html
URL: http://bcn.iums.ac.ir/article-1-1624-en.html
1- Neuroscience Research Centre, Institute of Neuropharmacology, Kerman University of Medical Sciences, Kerman, Iran.
2- Department of Psychiatry, School of Medicine, Kerman University of Medical Sciences, Kerman, Iran.
2- Department of Psychiatry, School of Medicine, Kerman University of Medical Sciences, Kerman, Iran.
Keywords: Cognition, Copper, Magnesium, Schizophrenia, Trace elements, Brief Assessment of Cognition in Schizophrenia (BACS)
Full-Text [PDF 782 kb]
| Abstract (HTML)
In all cognitive domains of the BACS, both patient groups achieved significantly lower values than the healthy controls. Moreover, chronic patients obtained the minimum scores in all cognitive domains (Table 2).
To examine the effects of Cu and Mg serum levels on cognitive functions, the correlation coefficients were calculated between Cu and Mg levels and the BACS cognitive domains for the control and patient groups, separately. For Cu, the relevant results indicated that Cu levels had significant negative correlations with working memory (r=0.42, P=0.02), and executive function (r=0.40, P=0.03) in the control group. However, there were no significant correlations between Culevel and cognitive functioning in the patient groups. For Mg, the related data signified no significant correlations between Mg level and cognitive functions in any of the study groups (P>0.05 for all variables).
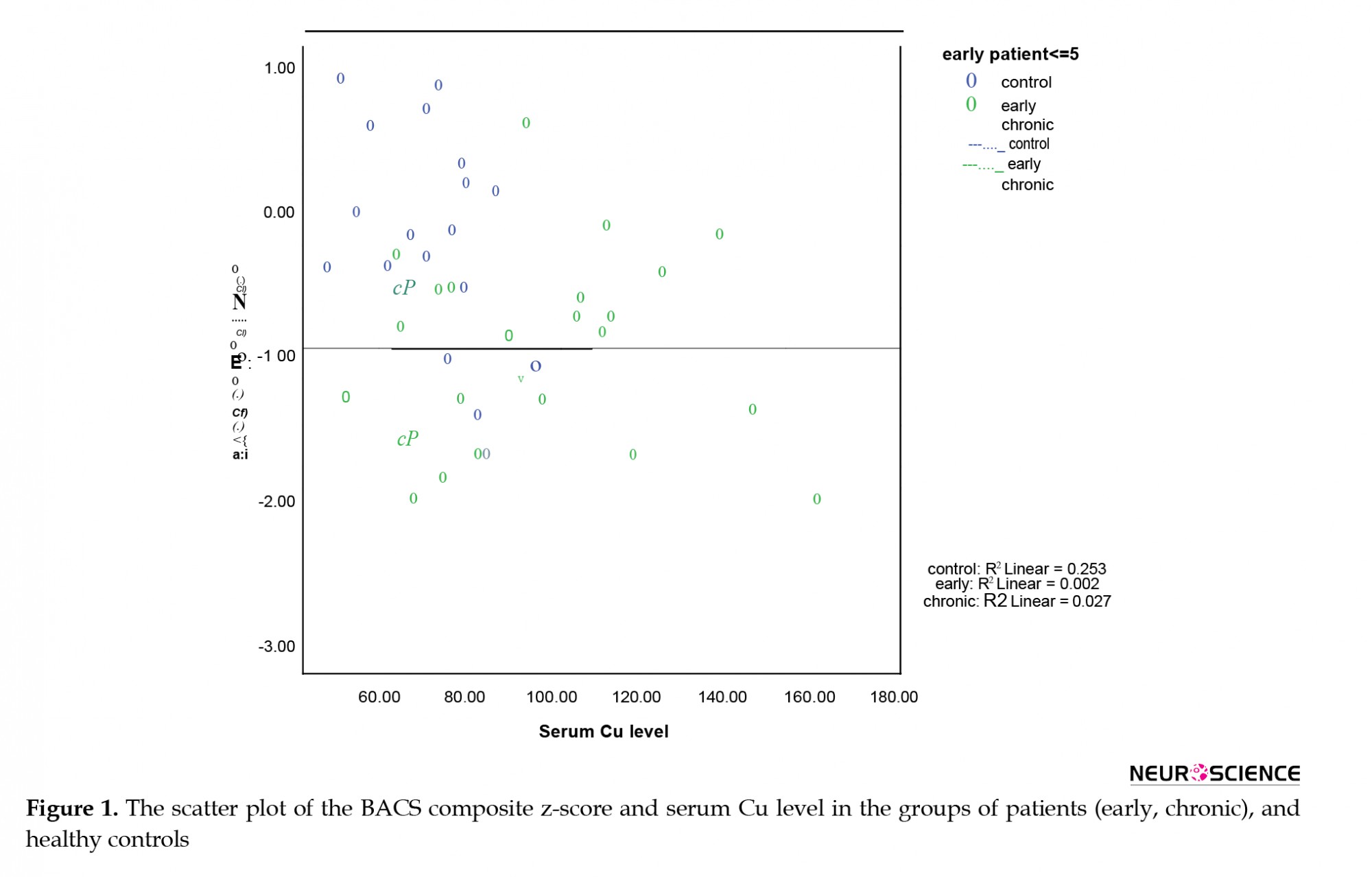
Next, we explored whether Cu serum levels could predict the cognitive performances of the study participants. The controls had significantly more years of education than the patients; thus, education was also considered as an independent variable. The collected results suggested an overall fit of the model (df=4, F=25.7, P<0.001). All variables were significant predictors of the BACS composite z-score (P<0.05). As per Table 3, increased educational level was associated with enhanced BACS composite z-score (i.e. the coefficient of the education represents the mean change of the BACS composite z-score). Additionally, the average difference in BACS between the control and patient groups was -1.23. Moreover, lower years of education, increased Cu serum level, and being diagnosed with SCZ were accompanied by reduced BACS composite z-scores. The interaction between the group type and Cu level was also significant (P=0.006); accordingly, the high Cu serum levels affected cognition in the control group and it is distinct from the patients (i.e. the coefficient of group*Cu means that the effect of Cu on BACS was different for the control and patient groups). This finding highlights that in the control group, increased Cu serum levels could predict faulty performances in the BACS cognitive domains, but not in patients with SCZ. Figure 1 provides a scatter plot of the correlation between Cu serum levels and the BACS composite z-scores. This plot demonstrates poor correlations between Cu and BACS composite z-score for both patient groups; however, it was nearly strong for the controls. Given the negative slope in Figure 1, the BACS composite z-score decreased, as Cu increased.
Finally, to examine the effect of clinical characteristics of patients on the Cu serum level in SCZ, the correlation coefficients (Spearman r) were evaluated. There was no significant correlation between serum Cu levels and PANSS positive, and negative symptoms (P>0.2). Furthermore, no correlation was observed between Cu serum levels and chlorpromazine equivalent dose (P=0.2).
4. Discussion
To the best of our knowledge, the present study was the first attempt to understand whether there is an association between the serum levels of two important trace elements whose functions seem to contribute to the pathophysiology of SCZ and their cognitive functioning.
We found significantly high levels of Cu, but not Mg, in the explored patients with SCZ, compared to the healthy control group. Numerous controversial reports are comparing the tissue and serum levels of several trace elements, including Cu and Mg in patients with SCZ and healthy individuals (Cai et al., 2015; Devanarayanan et al., 2016; Ordak et al., 2017).
The selected patients who met the present research inclusion criteria were all on antipsychotics. Our results were in-line with those of several studies on Cu serum levels in patients with SCZ (Devanarayanan et al., 2016; Ghanem et al., 2009; Rahman et al., 2009; Vidović et al., 2013; Yanik et al., 2004). Several lines of evidence have concluded that the elevation of plasma/serum Cu concentration may be due to treatment with various antipsychotics. A study has reported higher levels of Cu in patients receiving depot neuroleptics relative to those who were not on antipsychotics. However, a study reported increased Cu serum levels even in medication-free patients with SCZ (Devanarayanan et al., 2016). Contrarily, there is a report indicating lower levels of Cu in patients with SCZ (Liu et al., 2015); however, most findings have focused on the elevated levels of Cu. Further studies and meta-analyses are required to draw a more precise conclusion on the serum levels of Cu in patients with SCZ. Additionally, possible mechanisms by which antipsychotics might lead to excessive Cu serum levels should be further investigated.
Furthermore, no significant correlation was detected between the serum level of Cu and the cognitive functioning in patients with SCZ. In agreement with previous findings, Cu serum levels in healthy individuals were negatively correlated with their cognitive performance (composite z-score). The higher the Cu level, the worse cognitive performance in healthy participants. With a more meticulous look into each individual component of the BACS, Cu serum levels in the healthy control group exhibited a negative correlation; specifically, with the working memory and executive functioning.
It is suggested that an increased concentration of Cu could lead to cognitive decline in healthy individuals, owing to free Cu deregulation (Klevay, 2010). Additionally, Zhou et al. have reported decreased working memory and cognition in children, especially males; as the increased Cu serum level, exceeded the required amount for metabolic processes (Zhou et al., 2015). Another study has also stated that elevated Cu plasma level is linked to reduced cognitive functions in the elderly. Besides, they have discussed that the aggregation of amyloid beta-protein due to interactions with Cu as a reason for such findings (Gao et al., 2008).
In this regard, Salustri et al. have presented the interrelation between higher free Cu serum levels and reduced cognitive functions in healthy populations. They also argued that free Cu may act by disrupting the neurons of locus coeruleus (Salustri et al., 2010), the foremost site for the synthesis of the brain’s norepinephrine (Sara, 2009). On the other hand, studies exhibited the principal involvement of the locus coeruleus and noradrenergic system in cognitive processes and working memory (Berridge & Waterhouse, 2003; Borodovitsyna, Flamini, & Chandler, 2017; Sara, 2009).
Contrary to the healthy controls, no significant correlation was detected between the serum level of Cu and their cognitive functioning in patients with SCZ. Decreased DBH activity and noradrenergic transmission in patients with SCZ have been established a blunted catecholamine synthesis (Cubells & Zabetian, 2004; Rahman, Rahman, Rahman, & Kato, 2009). On the other hand, ionized Cu actively contributes as a ligand for the enzymatic activity of DBH. Moreover, an excessive amount of Cu may be engaged in the structure of DBH; accordingly, it boosts the conversion of dopamine into norepinephrine, with the final repercussion of decreased dopaminergic activity and increased synthesis of epinephrine from the noradrenergic neurons of the locus coeruleus. Therefore, we presumed that it might be an explanation to understand why excessive Cu is not attributed to declined cognitive functioning in patients with SCZ, unlike the healthy participants. It might be also induced by the pre-existing dysfunctional activity of locus coeruleus’ noradrenergic neurons in patients with SCZ despite the Cu-dependent disturbance of locus coeruleus neurons in healthy individuals. Another probable reason might be that in patients with SCZ, cognition is markedly impaired and Cu serum concentrations can hardly leave a noticeable impact on it; therefore, no significant association was observed in this regard.
Per previous findings (Devanarayanan et al., 2016; Ghanem et al., 2009), we found no significant correlation between higher Cu serum level and PANSS score. Thus, increased Cu concentration does not reflect the severity of SCZ.
Eventually, we observed no significant alternation in the Mg serum concentration; it may be as a result of receiving antipsychotics. Our data are in alignment with a study conducted by
Nechifor, Vaideanu, Palamaru, Borza, and Mindreci, (2004), i.e. indicative of lower erythrocyte; but not comparing Mg serum levels in healthy controls, which then got normalized after receiving treatment and during remission. A finding led to this deduction that increased intracellular Mg level is associated with enhanced GABAergic activity, and reduced glutamatergic and dopaminergic transmission (Ordak et al., 2017). In the face of these data, another contradictory finding has presented elevated platelet Mg concentration in suicidal patients with SCZ (Ruljancic, Mihanovic, Cepelak, & Bakliza, 2013). Ordak et al. (2017) highlighted that such inconsistencies may be due to extracellular measurements of Mg instead of intracellular and ionized ones. This is because only around 1% of the total Mg is distributed in the plasma.
5. Conclusion
Our study had several limitations. First, free (ionized) Cu can actively cross the blood-brain barrier and has a more crucial role in cognition than that of the bound one; thus, future studies are recommended measuring the ionized and non-ionized Cu levels. Moreover, we recommend performing a study on medicated and non- medicated patients to better recognize whether elevated Cu serum levels are related to antipsychotic therapy or not.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles are considered in this article. The participants were informed about the purpose of the research and its implementation stages; they were also assured about the confidentiality of their information; moreover, they were free to leave the study whenever they wished, and if desired, the research results would be available to them.
Funding
The current study was supported by the Neuroscience Research Center of Kerman University of Medical Sciences (Code: 95-38/KNRC).
Authors' contributions
Conceptualization, Investigation, Writing – review & editing: Shahrzad Mazhari, Elham Karimi Ghoughari, and Mahin Eslami Shahrbabaki; Data Collection: Shokouh Arjmand; All authors have contributed significantly, and all are in agreement with the content of the manuscript.
Conflict of interest
The authors declared no conflicts of interest.
References
Berridge, C. W., & Waterhouse, B. D. (2003). The locus coeruleus-noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Research Reviews, 42(1), 33-84. [DOI:10.1016/S0165-0173(03)00143-7]
Borodovitsyna, O., Flamini, M., & Chandler, D. (2017). Noradrenergic Modulation of Cognition in Health and Disease. Neural Plasticity, 2017, 1-14. [DOI:10.1155/2017/6031478] [PMID] [PMCID]
Cai, L., Chen, T., Yang, J., Zhou, K., Yan, X., & Chen, W., et al. (2015). Serum trace element differences between Schizophrenia patients and controls in the Han Chinese population. Scientific Reports, 5, 15013. [DOI:10.1038/srep15013] [PMID] [PMCID]
Cubells, J. F., & Zabetian, C. P. (2004). Human genetics of plasma dopamine β-hydroxylase activity: Applications to research in psychiatry and neurology. Psychopharmacology, 174(4), 463-76. [DOI:10.1007/s00213-004-1840-8] [PMID]
Cubells, Joseph F, Sun, X., Li, W., Bonsall, R. W., McGrath, J. A., & Avramopoulos, D., et al. (2011). Linkage analysis of plasma dopamine β-hydroxylase activity in families of patients with schizophrenia. Human Genetics, 130(5), 635-43. [DOI:10.1007/s00439-011-0989-6] [PMID] [PMCID]
de Jonge, J. C., Vinkers, C. H., Hulshoff Pol, H. E., & Marsman, A. (2017). GABAergic mechanisms in schizophrenia: linking postmortem and in vivo studies. Frontiers in Psychiatry, 8, 118. [DOI:10.3389/fpsyt.2017.00118] [PMID] [PMCID]
Devanarayanan, S., Nandeesha, H., Kattimani, S., Sarkar, S., & Jose, J. (2016). Elevated copper, hs C-reactive protein and dyslipidemia in drug free schizophrenia: Relation with psychopathology score. Asian Journal of Psychiatry, 24, 99-102. [DOI:10.1016/J.AJP.2016.08.025] [PMID]
Fraga, C. G. (2005). Relevance, essentiality and toxicity of trace elements in human health. Molecular Aspects of Medicine, 26(4-5), 235-44. [DOI:10.1016/j.mam.2005.07.013] [PMID]
Fryar-Williams, S., & Strobel, J. E. (2015). Biomarkers of a five-domain translational substrate for schizophrenia and schizoaffective psychosis. Biomarker Research, 3(1), 3. [DOI:10.1186/s40364-015-0028-1] [PMID] [PMCID]
Gao, S., Jin, Y., Unverzagt, F. W., Ma, F., Hall, K. S., & Murrell, J. R., et al. (2008). Trace element levels and cognitive function in rural elderly Chinese. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 63(6), 635-41. [DOI:10.1093/gerona/63.6.635] [PMID] [PMCID]
Ghanem, A. A., Ali, E. M., El-Bakary, A. A., El-Morsy, D., & Elkanishi, S. M., et al. (2009). Copper and Zinc levels in hair of both schizophrenic and depressed patients. Mansoura Journal of Forensic Medicine and Clinical Toxicology, 17(1), 89-102. [DOI:10.21608/mjfmct.2009.53299]
Green, M. F., & Harvey, P. D. (2014). Cognition in schizophrenia: Past, present, and future. Schizophrenia Research: Cognition, 1(1), e1-e9. [DOI:10.1016/j.scog.2014.02.001] [PMID] [PMCID]
Alvarez, C., & Amado, J. A. (2000). Higher levels of serum copper in schizophrenic patients treated with depot neuroleptics. Psychiatry Research, 94(1), 51-8. [DOI:10.1016/S0165-1781(00)00126-8]
Howes, O., McCutcheon, R., & Stone, J. (2015). Glutamate and dopamine in schizophrenia: An update for the 21st century. Journal of Psychopharmacology (Oxford, England), 29(2), 97- 115. [DOI:10.1177/0269881114563634] [PMID] [PMCID]
Javitt, D. C. (2010). Glutamatergic theories of schizophrenia. The Israel Journal of Psychiatry and Related Sciences, 47(1), 4-16. http://sites.oxy.edu/clint/physio/article/Glutamatergictheoriesofschizophrenia.pdf
Kay, S. R., Opler, L. A., & Lindenmayer, J. P. (1988). Reliability and validity of the positive and negative syndrome scale for schizophrenics. Psychiatry Research, 23(1), 99-110. [DOI:10.1016/0165-1781(88)90038-8]
Keefe, R., Goldberg, T. E., Harvey, P. D., Gold, J. M., Poe, M. P., & Coughenour, L. (2004). The Brief Assessment of Cognition in Schizophrenia: Reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophrenia Research, 68(2-3), 283- 97. [DOI:10.1016/j.schres.2003.09.011] [PMID]
Klevay, L. M. (2010). Copper and cognition. Clinical Neurophysiology, 121(12), 2177. [DOI:10.1016/j.clinph.2010.04.025] [PMID]
Lam, P. K., Kritz-Silverstein, D., Barrett Connor, E., Milne, D., Nielsen, F., & Gamst, A., et al. (2008). Plasma trace elements and cognitive function in older men and women: the Rancho Bernardo study. The Journal of Nutrition, Health & Aging, 12(1), 22-7. [DOI:10.1007/BF02982160] [PMID] [PMCID]
Lin, T., Liu, T., Lin, Y., Yan, L., Chen, Z., & Wang, J. (2017). Comparative study on serum levels of macro and trace elements in schizophrenia based on supervised learning methods. Journal of Trace Elements in Medicine and Biology, 43, 202-8. [DOI:10.1016/J.JTEMB.2017.03.010] [PMID]
Liu, T., Lu, Q. B., Yan, L., Guo, J., Feng, F., Qiu, J., & Wang, J. (2015). Comparative Study on Serum Levels of 10 Trace Elements in Schizophrenia. PloS One, 10(7), e0133622. [DOI:10.1371/journal.pone.0133622] [PMID] [PMCID]
Mazhari, S., Parvaresh, N., Eslami Shahrbabaki, M., Sadeghi, M. M., Nakhaee, N., & Keefe, R. S. E. (2014). Validation of the Persian version of the Brief Assessment of Cognition in Schizophrenia in patients with schizophrenia and healthy controls. Psychiatry and Clinical Neurosciences, 68(2), 160-6. [DOI:10.1111/pcn.12107] [PMID]
Nechifor, M., Vaideanu, C., Palamaru, I., Borza, C., & Mindreci, I. (2004). The influence of some antipsychotics on erythrocyte magnesium and plasma magnesium, calcium, copper and zinc in patients with paranoid Schizophrenia. Journal of the American College of Nutrition, 23(5), 549S-51S. [DOI:10.1080/07315724.2004.10719401] [PMID]
Ordak, M., Matras, J., Muszynska, E., Nasierowski, T., & Bujalska-Zadrozny, M. (2017). Magnesium in schizophrenia. Pharmacological Reports, 69(5), 929-934. [DOI:10.1016/j.pharep.2017.03.022] [PMID]
Osredkar, J., & Sustar, N. (2011). Copper and zinc, biological role and significance of copper/zinc imbalance. Journal of Clinical Toxicology, 3(2161), 0495. [DOI:10.4172/2161- 0495.S3-001]
Rahman, M. A., Azad, M. A. K., Hossain, M. I., Qusar, M. M. A. S., Bari, W., & Begum, F., et al. (2009). Zinc, manganese, calcium, copper, and cadmium level in scalp hair samples of schizophrenic patients. Biological Trace Element Research, 127(2), 102-8. [DOI:10.1007/s12011-008-8230-8] [PMID]
Rahman, M. K., Rahman, F., Rahman, T., & Kato, T. (2009). Dopamine-β-Hydroxylase (DBH), its cofactors and other biochemical parameters in the serum of neurological patients in Bangladesh. International Journal of Biomedical Science: IJBS, 5(4), 395-401. [DOI:10.1016/j.ijcard.2009.09.092] [PMID]
Ruljancic, N., Mihanovic, M., Cepelak, I., & Bakliza, A. (2013). Platelet and serum calcium and magnesium concentration in suicidal and non-suicidal schizophrenic patients. Psychiatry and Clinical Neurosciences, 67(3), 154-9. [DOI:10.1111/pcn.12038] [PMID]
Saha, S., Chant, D., Welham, J., & McGrath, J. (2005). A Systematic Review of the Prevalence of Schizophrenia. PLoS Medicine, 2(5), e141. [DOI:10.1371/journal.pmed.0020141] [PMID] [PMCID]
Salustri, C., Barbati, G., Ghidoni, R., Quintiliani, L., Ciappina, S., & Binetti, G., et al. (2010). Is cognitive function linked to serum free copper levels? A cohort study in a normal population. Clinical Neurophysiology, 121(4), 502-507. [DOI:10.1016/j.clinph.2009.11.090] [PMID]
Sara, S. J. (2009). The locus coeruleus and noradrenergic modulation of cognition. Nature Reviews Neuroscience, 10(3), 211-23. [DOI:10.1038/nrn2573] [PMID]
Smorgon, C., Mari, E., Atti, A. R., Dalla Nora, E., Zamboni, P. F., & Calzoni, F., et al. (2004). Trace elements and cognitive impairment: an elderly cohort study. Archives of Gerontology and Geriatrics, 38, 393-402.. [DOI:10.1016/j.archger.2004.04.050] [PMID]
Tang, S., Yao, B., Li, N., Lin, S., & Huang, Z. (2018). Association of dopamine beta- hydroxylase polymorphisms with Alzheimer’s disease, Parkinson’s disease and schizophrenia: Evidence based on currently available loci. Cellular Physiology and Biochemistry, 51(1), 411-28. [DOI:10.1159/000495238] [PMID]
Tripathi, A., Kar, S. K., & Shukla, R. (2018). Cognitive deficits in schizophrenia: understanding the biological correlates and remediation strategies. Clinical Psychopharmacology and Neuroscience, 16(1), 7-17. [DOI:10.9758/cpn.2018.16.1.7] [PMID] [PMCID]
van Os, J., & Kapur, S. (2009). Schizophrenia. The Lancet, 374(9690), 635-45. [DOI:10.1016/S0140-6736(09)60995-8]
Vendelboe, T. V., Harris, P., Zhao, Y., Walter, T. S., Harlos, K., & El Omari, K., et al. (2016). The crystal structure of human dopamine β-hydroxylase at 2.9 Å resolution. Science Advances, 2(4), e1500980. [DOI:10.1126/sciadv.1500980] [PMID] [PMCID]
Vidović, B., Đorđević, B., Milovanović, S., Škrivanj, S., Pavlović, Z., & Stefanović, A., et al. (2013). Selenium, zinc, and copper plasma levels in patients with schizophrenia: Relationship with metabolic risk factors. Biological Trace Element Research, 156(1-3), 22-8. [DOI:10.1007/s12011-013-9842-1] [PMID]
Woods, S. W. (2003). Woods, S. W. (2003). Chlorpromazine equivalent doses for the newer atypical antipsychotics. The Journal of Clinical Psychiatry, 64(6), 663-67. [DOI:10.4088/JCP.v64n0607] [PMID]
Yanik, M., Kocyigit, A., Tutkun, H., Vural, H., & Herken, H. (2004). Plasma manganese, selenium, zinc, copper, and iron concentrations in patients with schizophrenia. Biological Trace Element Research, 98(2), 109-18. [DOI:10.1385/BTER:98:2:109]
Zhou, G., Ji, X., Cui, N., Cao, S., Liu, C., & Liu, J. (2015). Association between serum copper status and working memory in schoolchildren. Nutrients, 7(9), 7185-96. [DOI:10.3390/nu7095331] [PMID] [PMCID]
Full-Text:
1. Introduction
chizophrenia (SCZ) is a severe debilitating neurodevelopmental disorder that affects emotion, thought, cognition, and behavior (Rahman et al., 2009). It affects roughly 1% of the population globally. Besides, it is recognized by heterogeneous symptoms, ranging from negative symptoms; psychotic symptoms; and disorganized behavior/speech, to the loss of goal-directed activities and impaired socio-educational functioning (Cai et al., 2015; Saha, Chant, Welham, & McGrath, 2005). Despite extensive research, the exact pathophysiology of SCZ remains unknown and not well-established; we only possess a sketchy picture displaying miscellaneous and disparate contributing factors, varying from genetic and metabolic to environmental ones (van Os & Kapur, 2009).
Within the last few years, cognitive deficits have been considered as a core feature of SCZ. Furthermore, numerous studies have focused on understanding the basis of cognitive decline to better manage the disorder and improve patients’ quality of life (Green & Harvey, 2014; Tripathi, Kar, & Shukla, 2018).
The altered amounts of trace elements have been linked to reduced cognitive functions in the elderly (Lam et al., 2008; Smorgon et al., 2004). Furthermore, previous studies reported that the serum or tissue levels of several trace elements are altered in patients with SCZ; they developed the trace element hypothesis of SCZ (Ghanem et al., 2009; Rahman et al., 2009; Yanik, Kocyigit, Tutkun, Vural, & Herken, 2004). Such findings were followed by a great body of interventions to search for possible biological markers representing the altered levels of trace elements in SCZ (Cai et al., 2015; Fryar-Williams & Strobel, 2015; Lin et al., 2017). However, the related data are controversial and there exist some ongoing debates about the contributing role of some trace elements in SCZ (Cai et al., 2015; Devanarayanan, Nandeesha, Kattimani, Sarkar, & Jose, 2016; Ordak, Matras, Muszynska, Nasierowski, & Bujalska-Zadrozny, 2017).
The main assumption in explaining the pathophysiology of SCZ is dysfunctional dopaminergic, glutamatergic, and GABAergic transduction (de Jonge, Vinkers, Hulshoff Pol, & Marsman, 2017; Howes, McCutcheon, & Stone, 2015; Javitt, 2010). Moreover, trace elements are responsible for numerous intricate biological processes and of great significance for the efficacious functioning of the cells (Fraga, 2005; Osredkar & Sustar, 2011). Copper (Cu) is a bivalent cation and an essential ligand for some metalloenzymes, including Dopamine β-Hydroxylase (DBH) (Vendelboe et al., 2016), i.e. a Cu-dependent oxygenase, a key component of catecholamine biosynthesis; and has been demonstrated to be impaired in SCZ (Cubells & Zabetian, 2004; Cubells et al., 2011; Osredkar & Sustar, 2011; Tang, Yao, Li, Lin, & Huang, 2018). Another bivalent cation, Magnesium (Mg), has been proven to impact GABAergic and glutamatergic transmission; both have been implicated dysfunctional in SCZ (Ordak et al., 2017).
Thus, this study aimed to investigate the possible correlation between cognitive functioning in patients with SCZ and their Cu and Mg serum levels. Previous study findings on the serum levels of Cu and Mg in patients with SCZ have been inconsistent and controversial (Cai et al., 2015; Devanarayanan et al., 2016; Ordak et al., 2017). Besides, to our knowledge, there are few, if any study to examine the association between the serum levels of these trace elements and cognitive functioning in SCZ.
Accordingly, the serum levels of Mg and Cu in patients with SCZ were measured. Then, a probable existing correlation between the serum profile of Cu and Mg and the cognitive functioning of patients with SCZ, as well as healthy participants were explored. For this purpose, a reliable and valid instrument, namely the Brief Assessment of Cognition in Schizophrenia (BACS), was used.
2. Materials and Methods
A group of 60 patients (48 males) was recruited through consecutive admissions to a psychiatric hospital in Kerman, Iran. The study patients were divided into two groups based on the chronicity of their illness: i) early patients (n=35, ≤5 years of illness initiation), and ii) chronic patients (n=25, >5 years of illness duration). Clinical symptoms were assessed by the Positive and Negative Syndrome Scale (PANSS) (Kay, Opler, & Lindenmayer, 1988). The study patients were all receiving antipsychotic medications and clinically stable at the time of the experiment. The Mean±SD chlorpromazine equivalent was 897.2 (498.8) mg (Woods, 2003).
The control group consisted of 30 healthy participants (24 males) without a personal or familial history of psychotic disorders. Head injury, neurological disorders, and substance abuse at the time of the experiment were the exclusion criteria for all study participants. The study was conducted under the principles of the Declaration of Helsinki for Biomedical Research. A written informed consent form was obtained from all research participants. The study was approved by the Ethics Committees of Kerman University of Medical Sciences (code: EC/95-38/KNRC).
Fasting blood samples (5mL) were collected from the antecubital vein of all study participants; we used a plastic syringe with a stainless-steel needle, between 7.00 AM to 9.00 AM. Each blood sample was poured into a metal-free plastic tube and clotted at room temperature for 30 minutes. Then, the serum was separated by centrifuging it at 3000 rpm for 15 minutes and preserved at -20°C until assayed. The concentrations of Cu and Mg were measured by atomic absorption spectrophotometer (PG instrument, AA500), and ion-selective electrode potentiometry (COBAS INTEGRA® 400 plus), respectively. Mg and Cu values were expressed in milligrams per deciliter (mg/dL) and micrograms per deciliter (ug/dL), respectively.
A Persian adaptation of the BACS was administered to assess cognitive functioning (Mazhari et al., 2014). The BACS, a performance-based cognitive assessment battery, is specifically designed to examine the cognitive function of SCZ, requiring 2.5 hours of testing (Keefe et al., 2004). The BACS is sensitive to cognitive impairment in SCZ, has high test-retest reliability, and is related to functional outcome (Keefe et al., 2004). The BACS assesses the 6 cognitive domains found to be consistently impaired, including verbal memory, verbal fluency, working memory, motor speed, attention, and executive function. The standardized z-scores from each test were summed; the composite score is the z-score of that sum. A lower score reflects greater cognitive impairment.
The obtained demographic data were compared between the groups using a combination of Chi-squared test and one-way Analysis of Variance (ANOVA). Spearman’s correlation coefficient was used to examine the correlation between Cu and Mg serum levels, and cognitive functioning. Backward multiple linear regression analysis was performed to explore the relationship between the BACS composite z-scores (dependent variable) and the independent variables, including the serum levels of Cu, the years of education, and the group. The variable of the group was coded as 0 (control group), 1 (early patients), and 2 (chronic patients). It was also offered as an ordinal variable to the multifactorial model.
3. Results
Table 1 lists the demographic and clinical characteristics of the study participants. The obtained results suggested differences in the mean age of the control and the patient groups; the mean age of the chronic patients was higher than that of the control and the early patient groups. Moreover, both patient groups had significantly fewer years of education than the controls. Both patient groups and the control group were well-matched for gender (P>0.05). The serum levels of Cu were significantly different between the three groups. Besides, both patient groups presented higher serum levels than that of the controls; the difference between the two patient groups was not significant. However, the serum levels of Mg were not significantly different between the control and both patients groups.
chizophrenia (SCZ) is a severe debilitating neurodevelopmental disorder that affects emotion, thought, cognition, and behavior (Rahman et al., 2009). It affects roughly 1% of the population globally. Besides, it is recognized by heterogeneous symptoms, ranging from negative symptoms; psychotic symptoms; and disorganized behavior/speech, to the loss of goal-directed activities and impaired socio-educational functioning (Cai et al., 2015; Saha, Chant, Welham, & McGrath, 2005). Despite extensive research, the exact pathophysiology of SCZ remains unknown and not well-established; we only possess a sketchy picture displaying miscellaneous and disparate contributing factors, varying from genetic and metabolic to environmental ones (van Os & Kapur, 2009).
Within the last few years, cognitive deficits have been considered as a core feature of SCZ. Furthermore, numerous studies have focused on understanding the basis of cognitive decline to better manage the disorder and improve patients’ quality of life (Green & Harvey, 2014; Tripathi, Kar, & Shukla, 2018).
The altered amounts of trace elements have been linked to reduced cognitive functions in the elderly (Lam et al., 2008; Smorgon et al., 2004). Furthermore, previous studies reported that the serum or tissue levels of several trace elements are altered in patients with SCZ; they developed the trace element hypothesis of SCZ (Ghanem et al., 2009; Rahman et al., 2009; Yanik, Kocyigit, Tutkun, Vural, & Herken, 2004). Such findings were followed by a great body of interventions to search for possible biological markers representing the altered levels of trace elements in SCZ (Cai et al., 2015; Fryar-Williams & Strobel, 2015; Lin et al., 2017). However, the related data are controversial and there exist some ongoing debates about the contributing role of some trace elements in SCZ (Cai et al., 2015; Devanarayanan, Nandeesha, Kattimani, Sarkar, & Jose, 2016; Ordak, Matras, Muszynska, Nasierowski, & Bujalska-Zadrozny, 2017).
The main assumption in explaining the pathophysiology of SCZ is dysfunctional dopaminergic, glutamatergic, and GABAergic transduction (de Jonge, Vinkers, Hulshoff Pol, & Marsman, 2017; Howes, McCutcheon, & Stone, 2015; Javitt, 2010). Moreover, trace elements are responsible for numerous intricate biological processes and of great significance for the efficacious functioning of the cells (Fraga, 2005; Osredkar & Sustar, 2011). Copper (Cu) is a bivalent cation and an essential ligand for some metalloenzymes, including Dopamine β-Hydroxylase (DBH) (Vendelboe et al., 2016), i.e. a Cu-dependent oxygenase, a key component of catecholamine biosynthesis; and has been demonstrated to be impaired in SCZ (Cubells & Zabetian, 2004; Cubells et al., 2011; Osredkar & Sustar, 2011; Tang, Yao, Li, Lin, & Huang, 2018). Another bivalent cation, Magnesium (Mg), has been proven to impact GABAergic and glutamatergic transmission; both have been implicated dysfunctional in SCZ (Ordak et al., 2017).
Thus, this study aimed to investigate the possible correlation between cognitive functioning in patients with SCZ and their Cu and Mg serum levels. Previous study findings on the serum levels of Cu and Mg in patients with SCZ have been inconsistent and controversial (Cai et al., 2015; Devanarayanan et al., 2016; Ordak et al., 2017). Besides, to our knowledge, there are few, if any study to examine the association between the serum levels of these trace elements and cognitive functioning in SCZ.
Accordingly, the serum levels of Mg and Cu in patients with SCZ were measured. Then, a probable existing correlation between the serum profile of Cu and Mg and the cognitive functioning of patients with SCZ, as well as healthy participants were explored. For this purpose, a reliable and valid instrument, namely the Brief Assessment of Cognition in Schizophrenia (BACS), was used.
2. Materials and Methods
A group of 60 patients (48 males) was recruited through consecutive admissions to a psychiatric hospital in Kerman, Iran. The study patients were divided into two groups based on the chronicity of their illness: i) early patients (n=35, ≤5 years of illness initiation), and ii) chronic patients (n=25, >5 years of illness duration). Clinical symptoms were assessed by the Positive and Negative Syndrome Scale (PANSS) (Kay, Opler, & Lindenmayer, 1988). The study patients were all receiving antipsychotic medications and clinically stable at the time of the experiment. The Mean±SD chlorpromazine equivalent was 897.2 (498.8) mg (Woods, 2003).
The control group consisted of 30 healthy participants (24 males) without a personal or familial history of psychotic disorders. Head injury, neurological disorders, and substance abuse at the time of the experiment were the exclusion criteria for all study participants. The study was conducted under the principles of the Declaration of Helsinki for Biomedical Research. A written informed consent form was obtained from all research participants. The study was approved by the Ethics Committees of Kerman University of Medical Sciences (code: EC/95-38/KNRC).
Fasting blood samples (5mL) were collected from the antecubital vein of all study participants; we used a plastic syringe with a stainless-steel needle, between 7.00 AM to 9.00 AM. Each blood sample was poured into a metal-free plastic tube and clotted at room temperature for 30 minutes. Then, the serum was separated by centrifuging it at 3000 rpm for 15 minutes and preserved at -20°C until assayed. The concentrations of Cu and Mg were measured by atomic absorption spectrophotometer (PG instrument, AA500), and ion-selective electrode potentiometry (COBAS INTEGRA® 400 plus), respectively. Mg and Cu values were expressed in milligrams per deciliter (mg/dL) and micrograms per deciliter (ug/dL), respectively.
A Persian adaptation of the BACS was administered to assess cognitive functioning (Mazhari et al., 2014). The BACS, a performance-based cognitive assessment battery, is specifically designed to examine the cognitive function of SCZ, requiring 2.5 hours of testing (Keefe et al., 2004). The BACS is sensitive to cognitive impairment in SCZ, has high test-retest reliability, and is related to functional outcome (Keefe et al., 2004). The BACS assesses the 6 cognitive domains found to be consistently impaired, including verbal memory, verbal fluency, working memory, motor speed, attention, and executive function. The standardized z-scores from each test were summed; the composite score is the z-score of that sum. A lower score reflects greater cognitive impairment.
The obtained demographic data were compared between the groups using a combination of Chi-squared test and one-way Analysis of Variance (ANOVA). Spearman’s correlation coefficient was used to examine the correlation between Cu and Mg serum levels, and cognitive functioning. Backward multiple linear regression analysis was performed to explore the relationship between the BACS composite z-scores (dependent variable) and the independent variables, including the serum levels of Cu, the years of education, and the group. The variable of the group was coded as 0 (control group), 1 (early patients), and 2 (chronic patients). It was also offered as an ordinal variable to the multifactorial model.
3. Results
Table 1 lists the demographic and clinical characteristics of the study participants. The obtained results suggested differences in the mean age of the control and the patient groups; the mean age of the chronic patients was higher than that of the control and the early patient groups. Moreover, both patient groups had significantly fewer years of education than the controls. Both patient groups and the control group were well-matched for gender (P>0.05). The serum levels of Cu were significantly different between the three groups. Besides, both patient groups presented higher serum levels than that of the controls; the difference between the two patient groups was not significant. However, the serum levels of Mg were not significantly different between the control and both patients groups.
In all cognitive domains of the BACS, both patient groups achieved significantly lower values than the healthy controls. Moreover, chronic patients obtained the minimum scores in all cognitive domains (Table 2).
To examine the effects of Cu and Mg serum levels on cognitive functions, the correlation coefficients were calculated between Cu and Mg levels and the BACS cognitive domains for the control and patient groups, separately. For Cu, the relevant results indicated that Cu levels had significant negative correlations with working memory (r=0.42, P=0.02), and executive function (r=0.40, P=0.03) in the control group. However, there were no significant correlations between Culevel and cognitive functioning in the patient groups. For Mg, the related data signified no significant correlations between Mg level and cognitive functions in any of the study groups (P>0.05 for all variables).
Next, we explored whether Cu serum levels could predict the cognitive performances of the study participants. The controls had significantly more years of education than the patients; thus, education was also considered as an independent variable. The collected results suggested an overall fit of the model (df=4, F=25.7, P<0.001). All variables were significant predictors of the BACS composite z-score (P<0.05). As per Table 3, increased educational level was associated with enhanced BACS composite z-score (i.e. the coefficient of the education represents the mean change of the BACS composite z-score). Additionally, the average difference in BACS between the control and patient groups was -1.23. Moreover, lower years of education, increased Cu serum level, and being diagnosed with SCZ were accompanied by reduced BACS composite z-scores. The interaction between the group type and Cu level was also significant (P=0.006); accordingly, the high Cu serum levels affected cognition in the control group and it is distinct from the patients (i.e. the coefficient of group*Cu means that the effect of Cu on BACS was different for the control and patient groups). This finding highlights that in the control group, increased Cu serum levels could predict faulty performances in the BACS cognitive domains, but not in patients with SCZ. Figure 1 provides a scatter plot of the correlation between Cu serum levels and the BACS composite z-scores. This plot demonstrates poor correlations between Cu and BACS composite z-score for both patient groups; however, it was nearly strong for the controls. Given the negative slope in Figure 1, the BACS composite z-score decreased, as Cu increased.
Finally, to examine the effect of clinical characteristics of patients on the Cu serum level in SCZ, the correlation coefficients (Spearman r) were evaluated. There was no significant correlation between serum Cu levels and PANSS positive, and negative symptoms (P>0.2). Furthermore, no correlation was observed between Cu serum levels and chlorpromazine equivalent dose (P=0.2).
4. Discussion
To the best of our knowledge, the present study was the first attempt to understand whether there is an association between the serum levels of two important trace elements whose functions seem to contribute to the pathophysiology of SCZ and their cognitive functioning.
We found significantly high levels of Cu, but not Mg, in the explored patients with SCZ, compared to the healthy control group. Numerous controversial reports are comparing the tissue and serum levels of several trace elements, including Cu and Mg in patients with SCZ and healthy individuals (Cai et al., 2015; Devanarayanan et al., 2016; Ordak et al., 2017).
The selected patients who met the present research inclusion criteria were all on antipsychotics. Our results were in-line with those of several studies on Cu serum levels in patients with SCZ (Devanarayanan et al., 2016; Ghanem et al., 2009; Rahman et al., 2009; Vidović et al., 2013; Yanik et al., 2004). Several lines of evidence have concluded that the elevation of plasma/serum Cu concentration may be due to treatment with various antipsychotics. A study has reported higher levels of Cu in patients receiving depot neuroleptics relative to those who were not on antipsychotics. However, a study reported increased Cu serum levels even in medication-free patients with SCZ (Devanarayanan et al., 2016). Contrarily, there is a report indicating lower levels of Cu in patients with SCZ (Liu et al., 2015); however, most findings have focused on the elevated levels of Cu. Further studies and meta-analyses are required to draw a more precise conclusion on the serum levels of Cu in patients with SCZ. Additionally, possible mechanisms by which antipsychotics might lead to excessive Cu serum levels should be further investigated.
Furthermore, no significant correlation was detected between the serum level of Cu and the cognitive functioning in patients with SCZ. In agreement with previous findings, Cu serum levels in healthy individuals were negatively correlated with their cognitive performance (composite z-score). The higher the Cu level, the worse cognitive performance in healthy participants. With a more meticulous look into each individual component of the BACS, Cu serum levels in the healthy control group exhibited a negative correlation; specifically, with the working memory and executive functioning.
It is suggested that an increased concentration of Cu could lead to cognitive decline in healthy individuals, owing to free Cu deregulation (Klevay, 2010). Additionally, Zhou et al. have reported decreased working memory and cognition in children, especially males; as the increased Cu serum level, exceeded the required amount for metabolic processes (Zhou et al., 2015). Another study has also stated that elevated Cu plasma level is linked to reduced cognitive functions in the elderly. Besides, they have discussed that the aggregation of amyloid beta-protein due to interactions with Cu as a reason for such findings (Gao et al., 2008).
In this regard, Salustri et al. have presented the interrelation between higher free Cu serum levels and reduced cognitive functions in healthy populations. They also argued that free Cu may act by disrupting the neurons of locus coeruleus (Salustri et al., 2010), the foremost site for the synthesis of the brain’s norepinephrine (Sara, 2009). On the other hand, studies exhibited the principal involvement of the locus coeruleus and noradrenergic system in cognitive processes and working memory (Berridge & Waterhouse, 2003; Borodovitsyna, Flamini, & Chandler, 2017; Sara, 2009).
Contrary to the healthy controls, no significant correlation was detected between the serum level of Cu and their cognitive functioning in patients with SCZ. Decreased DBH activity and noradrenergic transmission in patients with SCZ have been established a blunted catecholamine synthesis (Cubells & Zabetian, 2004; Rahman, Rahman, Rahman, & Kato, 2009). On the other hand, ionized Cu actively contributes as a ligand for the enzymatic activity of DBH. Moreover, an excessive amount of Cu may be engaged in the structure of DBH; accordingly, it boosts the conversion of dopamine into norepinephrine, with the final repercussion of decreased dopaminergic activity and increased synthesis of epinephrine from the noradrenergic neurons of the locus coeruleus. Therefore, we presumed that it might be an explanation to understand why excessive Cu is not attributed to declined cognitive functioning in patients with SCZ, unlike the healthy participants. It might be also induced by the pre-existing dysfunctional activity of locus coeruleus’ noradrenergic neurons in patients with SCZ despite the Cu-dependent disturbance of locus coeruleus neurons in healthy individuals. Another probable reason might be that in patients with SCZ, cognition is markedly impaired and Cu serum concentrations can hardly leave a noticeable impact on it; therefore, no significant association was observed in this regard.
Per previous findings (Devanarayanan et al., 2016; Ghanem et al., 2009), we found no significant correlation between higher Cu serum level and PANSS score. Thus, increased Cu concentration does not reflect the severity of SCZ.
Eventually, we observed no significant alternation in the Mg serum concentration; it may be as a result of receiving antipsychotics. Our data are in alignment with a study conducted by
Nechifor, Vaideanu, Palamaru, Borza, and Mindreci, (2004), i.e. indicative of lower erythrocyte; but not comparing Mg serum levels in healthy controls, which then got normalized after receiving treatment and during remission. A finding led to this deduction that increased intracellular Mg level is associated with enhanced GABAergic activity, and reduced glutamatergic and dopaminergic transmission (Ordak et al., 2017). In the face of these data, another contradictory finding has presented elevated platelet Mg concentration in suicidal patients with SCZ (Ruljancic, Mihanovic, Cepelak, & Bakliza, 2013). Ordak et al. (2017) highlighted that such inconsistencies may be due to extracellular measurements of Mg instead of intracellular and ionized ones. This is because only around 1% of the total Mg is distributed in the plasma.
5. Conclusion
Our study had several limitations. First, free (ionized) Cu can actively cross the blood-brain barrier and has a more crucial role in cognition than that of the bound one; thus, future studies are recommended measuring the ionized and non-ionized Cu levels. Moreover, we recommend performing a study on medicated and non- medicated patients to better recognize whether elevated Cu serum levels are related to antipsychotic therapy or not.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles are considered in this article. The participants were informed about the purpose of the research and its implementation stages; they were also assured about the confidentiality of their information; moreover, they were free to leave the study whenever they wished, and if desired, the research results would be available to them.
Funding
The current study was supported by the Neuroscience Research Center of Kerman University of Medical Sciences (Code: 95-38/KNRC).
Authors' contributions
Conceptualization, Investigation, Writing – review & editing: Shahrzad Mazhari, Elham Karimi Ghoughari, and Mahin Eslami Shahrbabaki; Data Collection: Shokouh Arjmand; All authors have contributed significantly, and all are in agreement with the content of the manuscript.
Conflict of interest
The authors declared no conflicts of interest.
References
Berridge, C. W., & Waterhouse, B. D. (2003). The locus coeruleus-noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Research Reviews, 42(1), 33-84. [DOI:10.1016/S0165-0173(03)00143-7]
Borodovitsyna, O., Flamini, M., & Chandler, D. (2017). Noradrenergic Modulation of Cognition in Health and Disease. Neural Plasticity, 2017, 1-14. [DOI:10.1155/2017/6031478] [PMID] [PMCID]
Cai, L., Chen, T., Yang, J., Zhou, K., Yan, X., & Chen, W., et al. (2015). Serum trace element differences between Schizophrenia patients and controls in the Han Chinese population. Scientific Reports, 5, 15013. [DOI:10.1038/srep15013] [PMID] [PMCID]
Cubells, J. F., & Zabetian, C. P. (2004). Human genetics of plasma dopamine β-hydroxylase activity: Applications to research in psychiatry and neurology. Psychopharmacology, 174(4), 463-76. [DOI:10.1007/s00213-004-1840-8] [PMID]
Cubells, Joseph F, Sun, X., Li, W., Bonsall, R. W., McGrath, J. A., & Avramopoulos, D., et al. (2011). Linkage analysis of plasma dopamine β-hydroxylase activity in families of patients with schizophrenia. Human Genetics, 130(5), 635-43. [DOI:10.1007/s00439-011-0989-6] [PMID] [PMCID]
de Jonge, J. C., Vinkers, C. H., Hulshoff Pol, H. E., & Marsman, A. (2017). GABAergic mechanisms in schizophrenia: linking postmortem and in vivo studies. Frontiers in Psychiatry, 8, 118. [DOI:10.3389/fpsyt.2017.00118] [PMID] [PMCID]
Devanarayanan, S., Nandeesha, H., Kattimani, S., Sarkar, S., & Jose, J. (2016). Elevated copper, hs C-reactive protein and dyslipidemia in drug free schizophrenia: Relation with psychopathology score. Asian Journal of Psychiatry, 24, 99-102. [DOI:10.1016/J.AJP.2016.08.025] [PMID]
Fraga, C. G. (2005). Relevance, essentiality and toxicity of trace elements in human health. Molecular Aspects of Medicine, 26(4-5), 235-44. [DOI:10.1016/j.mam.2005.07.013] [PMID]
Fryar-Williams, S., & Strobel, J. E. (2015). Biomarkers of a five-domain translational substrate for schizophrenia and schizoaffective psychosis. Biomarker Research, 3(1), 3. [DOI:10.1186/s40364-015-0028-1] [PMID] [PMCID]
Gao, S., Jin, Y., Unverzagt, F. W., Ma, F., Hall, K. S., & Murrell, J. R., et al. (2008). Trace element levels and cognitive function in rural elderly Chinese. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 63(6), 635-41. [DOI:10.1093/gerona/63.6.635] [PMID] [PMCID]
Ghanem, A. A., Ali, E. M., El-Bakary, A. A., El-Morsy, D., & Elkanishi, S. M., et al. (2009). Copper and Zinc levels in hair of both schizophrenic and depressed patients. Mansoura Journal of Forensic Medicine and Clinical Toxicology, 17(1), 89-102. [DOI:10.21608/mjfmct.2009.53299]
Green, M. F., & Harvey, P. D. (2014). Cognition in schizophrenia: Past, present, and future. Schizophrenia Research: Cognition, 1(1), e1-e9. [DOI:10.1016/j.scog.2014.02.001] [PMID] [PMCID]
Alvarez, C., & Amado, J. A. (2000). Higher levels of serum copper in schizophrenic patients treated with depot neuroleptics. Psychiatry Research, 94(1), 51-8. [DOI:10.1016/S0165-1781(00)00126-8]
Howes, O., McCutcheon, R., & Stone, J. (2015). Glutamate and dopamine in schizophrenia: An update for the 21st century. Journal of Psychopharmacology (Oxford, England), 29(2), 97- 115. [DOI:10.1177/0269881114563634] [PMID] [PMCID]
Javitt, D. C. (2010). Glutamatergic theories of schizophrenia. The Israel Journal of Psychiatry and Related Sciences, 47(1), 4-16. http://sites.oxy.edu/clint/physio/article/Glutamatergictheoriesofschizophrenia.pdf
Kay, S. R., Opler, L. A., & Lindenmayer, J. P. (1988). Reliability and validity of the positive and negative syndrome scale for schizophrenics. Psychiatry Research, 23(1), 99-110. [DOI:10.1016/0165-1781(88)90038-8]
Keefe, R., Goldberg, T. E., Harvey, P. D., Gold, J. M., Poe, M. P., & Coughenour, L. (2004). The Brief Assessment of Cognition in Schizophrenia: Reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophrenia Research, 68(2-3), 283- 97. [DOI:10.1016/j.schres.2003.09.011] [PMID]
Klevay, L. M. (2010). Copper and cognition. Clinical Neurophysiology, 121(12), 2177. [DOI:10.1016/j.clinph.2010.04.025] [PMID]
Lam, P. K., Kritz-Silverstein, D., Barrett Connor, E., Milne, D., Nielsen, F., & Gamst, A., et al. (2008). Plasma trace elements and cognitive function in older men and women: the Rancho Bernardo study. The Journal of Nutrition, Health & Aging, 12(1), 22-7. [DOI:10.1007/BF02982160] [PMID] [PMCID]
Lin, T., Liu, T., Lin, Y., Yan, L., Chen, Z., & Wang, J. (2017). Comparative study on serum levels of macro and trace elements in schizophrenia based on supervised learning methods. Journal of Trace Elements in Medicine and Biology, 43, 202-8. [DOI:10.1016/J.JTEMB.2017.03.010] [PMID]
Liu, T., Lu, Q. B., Yan, L., Guo, J., Feng, F., Qiu, J., & Wang, J. (2015). Comparative Study on Serum Levels of 10 Trace Elements in Schizophrenia. PloS One, 10(7), e0133622. [DOI:10.1371/journal.pone.0133622] [PMID] [PMCID]
Mazhari, S., Parvaresh, N., Eslami Shahrbabaki, M., Sadeghi, M. M., Nakhaee, N., & Keefe, R. S. E. (2014). Validation of the Persian version of the Brief Assessment of Cognition in Schizophrenia in patients with schizophrenia and healthy controls. Psychiatry and Clinical Neurosciences, 68(2), 160-6. [DOI:10.1111/pcn.12107] [PMID]
Nechifor, M., Vaideanu, C., Palamaru, I., Borza, C., & Mindreci, I. (2004). The influence of some antipsychotics on erythrocyte magnesium and plasma magnesium, calcium, copper and zinc in patients with paranoid Schizophrenia. Journal of the American College of Nutrition, 23(5), 549S-51S. [DOI:10.1080/07315724.2004.10719401] [PMID]
Ordak, M., Matras, J., Muszynska, E., Nasierowski, T., & Bujalska-Zadrozny, M. (2017). Magnesium in schizophrenia. Pharmacological Reports, 69(5), 929-934. [DOI:10.1016/j.pharep.2017.03.022] [PMID]
Osredkar, J., & Sustar, N. (2011). Copper and zinc, biological role and significance of copper/zinc imbalance. Journal of Clinical Toxicology, 3(2161), 0495. [DOI:10.4172/2161- 0495.S3-001]
Rahman, M. A., Azad, M. A. K., Hossain, M. I., Qusar, M. M. A. S., Bari, W., & Begum, F., et al. (2009). Zinc, manganese, calcium, copper, and cadmium level in scalp hair samples of schizophrenic patients. Biological Trace Element Research, 127(2), 102-8. [DOI:10.1007/s12011-008-8230-8] [PMID]
Rahman, M. K., Rahman, F., Rahman, T., & Kato, T. (2009). Dopamine-β-Hydroxylase (DBH), its cofactors and other biochemical parameters in the serum of neurological patients in Bangladesh. International Journal of Biomedical Science: IJBS, 5(4), 395-401. [DOI:10.1016/j.ijcard.2009.09.092] [PMID]
Ruljancic, N., Mihanovic, M., Cepelak, I., & Bakliza, A. (2013). Platelet and serum calcium and magnesium concentration in suicidal and non-suicidal schizophrenic patients. Psychiatry and Clinical Neurosciences, 67(3), 154-9. [DOI:10.1111/pcn.12038] [PMID]
Saha, S., Chant, D., Welham, J., & McGrath, J. (2005). A Systematic Review of the Prevalence of Schizophrenia. PLoS Medicine, 2(5), e141. [DOI:10.1371/journal.pmed.0020141] [PMID] [PMCID]
Salustri, C., Barbati, G., Ghidoni, R., Quintiliani, L., Ciappina, S., & Binetti, G., et al. (2010). Is cognitive function linked to serum free copper levels? A cohort study in a normal population. Clinical Neurophysiology, 121(4), 502-507. [DOI:10.1016/j.clinph.2009.11.090] [PMID]
Sara, S. J. (2009). The locus coeruleus and noradrenergic modulation of cognition. Nature Reviews Neuroscience, 10(3), 211-23. [DOI:10.1038/nrn2573] [PMID]
Smorgon, C., Mari, E., Atti, A. R., Dalla Nora, E., Zamboni, P. F., & Calzoni, F., et al. (2004). Trace elements and cognitive impairment: an elderly cohort study. Archives of Gerontology and Geriatrics, 38, 393-402.. [DOI:10.1016/j.archger.2004.04.050] [PMID]
Tang, S., Yao, B., Li, N., Lin, S., & Huang, Z. (2018). Association of dopamine beta- hydroxylase polymorphisms with Alzheimer’s disease, Parkinson’s disease and schizophrenia: Evidence based on currently available loci. Cellular Physiology and Biochemistry, 51(1), 411-28. [DOI:10.1159/000495238] [PMID]
Tripathi, A., Kar, S. K., & Shukla, R. (2018). Cognitive deficits in schizophrenia: understanding the biological correlates and remediation strategies. Clinical Psychopharmacology and Neuroscience, 16(1), 7-17. [DOI:10.9758/cpn.2018.16.1.7] [PMID] [PMCID]
van Os, J., & Kapur, S. (2009). Schizophrenia. The Lancet, 374(9690), 635-45. [DOI:10.1016/S0140-6736(09)60995-8]
Vendelboe, T. V., Harris, P., Zhao, Y., Walter, T. S., Harlos, K., & El Omari, K., et al. (2016). The crystal structure of human dopamine β-hydroxylase at 2.9 Å resolution. Science Advances, 2(4), e1500980. [DOI:10.1126/sciadv.1500980] [PMID] [PMCID]
Vidović, B., Đorđević, B., Milovanović, S., Škrivanj, S., Pavlović, Z., & Stefanović, A., et al. (2013). Selenium, zinc, and copper plasma levels in patients with schizophrenia: Relationship with metabolic risk factors. Biological Trace Element Research, 156(1-3), 22-8. [DOI:10.1007/s12011-013-9842-1] [PMID]
Woods, S. W. (2003). Woods, S. W. (2003). Chlorpromazine equivalent doses for the newer atypical antipsychotics. The Journal of Clinical Psychiatry, 64(6), 663-67. [DOI:10.4088/JCP.v64n0607] [PMID]
Yanik, M., Kocyigit, A., Tutkun, H., Vural, H., & Herken, H. (2004). Plasma manganese, selenium, zinc, copper, and iron concentrations in patients with schizophrenia. Biological Trace Element Research, 98(2), 109-18. [DOI:10.1385/BTER:98:2:109]
Zhou, G., Ji, X., Cui, N., Cao, S., Liu, C., & Liu, J. (2015). Association between serum copper status and working memory in schoolchildren. Nutrients, 7(9), 7185-96. [DOI:10.3390/nu7095331] [PMID] [PMCID]
Type of Study: Original |
Subject:
Cognitive Neuroscience
Received: 2019/10/7 | Accepted: 2020/04/13 | Published: 2020/09/1
Received: 2019/10/7 | Accepted: 2020/04/13 | Published: 2020/09/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








.png)
.png)
.png)