Volume 13, Issue 1 (January & February 2022)
BCN 2022, 13(1): 15-24 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Borjkhani H, Borjkhani M, Sharif M A. Investigating the Cocaine-induced Reduction of Potassium Current on the Generation of Action Potentials Using a Computational Model. BCN 2022; 13 (1) :15-24
URL: http://bcn.iums.ac.ir/article-1-1541-en.html
URL: http://bcn.iums.ac.ir/article-1-1541-en.html
1- School of Engineering Sciences, University of Tehran, Tehran, Iran.
2- Department of Electrical Engineering, School of Industrial Technologies, Urmia University of Technology, Urmia, Iran.
2- Department of Electrical Engineering, School of Industrial Technologies, Urmia University of Technology, Urmia, Iran.
Full-Text [PDF 1505 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Addiction is a chronic disease that has various negative consequences. Cocaine consumption is one of the pervasive forms of addiction. The use of drugs, such as cocaine, can lead to maladaptive behavioral changes. Many researchers have shown that these changes are due to the intervention of drugs in the plasticity mechanism of the brain (Nestler, 2001; Kauer, & Malenka, 2007; Lüscher 2013; Kutlu, & Gould 2016; Borjkhani, Bahrami, & Janahmadi, 2018; Borjkhani, et al., 2018; Preston, Brown, & Wagner, 2019). Some researchers consider that drugs of abuse can form a pathological memory, called the memory of addiction (Boening 2001; Kelley 2004; Nestler, 2013).
Among the involvement of different brain regions in the addiction process, the hippocampus has an integral role in forming addiction memories and triggering relapse during withdrawal times (Caffino, et al., 2014,; Kutlu, & Gould, 2016; Preston, et al. 2019). It has been shown both empirically and computationally that one reason for the formation of addiction memory is the increased excitability of neurons (Cohen, Doze, & Madison, 1992; Kauer, & Malenka 2007; Borjkhani, Mahdavi, & Bahrami, 2014; van Huijstee & Mansvelder 2015; Shukla, et al. 2017; Borjkhani, et al. 2018; Borjkhani, et al. 2018a; Borjkhani, et al. 2018b).
Excitability of the neurons can be changed by drug intervention in N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors mediated currents that some researchers investigated (Trujillo 2002; Bao, Kang et al., 2007; Kauer, & Malenka 2007; Zweifel, Argilli, E., Bonci, & Palmiter, 2008; Zachariou, Alexander , Coombes, & Christodoulou2013; Borjkhani, et al. 2018; Borjkhani, et al. 2018). Meanwhile, the missing issue is the role of potassium currents in changing cell excitabilities. It has been observed that the consumption of cocaine increases the excitability of the hippocampal neurons by inhibiting the potassium current (Chen, Lin, Lin, & Tsai, 2006). Cocaine consumption provokes action potential bursts in neurons (Chen, et al., 2006). Moreover, it has been suggested that the emergence of the burst is associated with cocaine’s inhibitory effects on the delayed rectifying K current (Chen, et al., 2006). Therefore, one of the mechanisms by which cocaine changes neurons’ excitabilities and innervates in memory formation is the reduction of potassium current. So, based on these observations, we want to computationally investigate potassium current reduction affects the produced action potentials in the hippocampus. This research can reveal new dimensions of the formation of cellular addiction.
In the next section, the implemented computational model is introduced. Then the simulation results are presented, and, finally, potential applications of the proposed model are discussed.
2. Methods
To analyze the cocaine effects on action potential generation in hippocampal neurons, we used a modified version of the ‘Hodgkin–Huxley style’ formulation (Hodgkin & Huxley, 1952). The equation denoting voltage variation of the neuron with ionic current equations is as follows:
.jpg)
Here, INa is the transient sodium current, INap denotes the persistent sodium current, IKdr shows the delayed rectifier potassium current, IA demonstrates the A-type potassium current, IM exhibits the
muscarinic-sensitive potassium current, ICa illustrates the High-voltage calcium current, IC describes the
fast calcium-activated potassium current, IsAHP displays the slow calcium-activated potassium current,
IL indicates the leak current, IAMPA represents the AMPAR mediated current, and INMDA refers to the NMDAR mediated current.
Transient sodium current (INa) can be described using the following equations (Golomb, Yue, & Yaari., 2006):
.jpg)
Here, m and h are gating variables and 𝜏h is the time constant. Persistent sodium current can be described by the following equation (Golomb, et al., 2006):
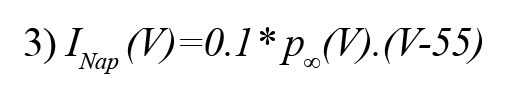
The delayed rectifier potassium current (IKdr ) can be represented by the following equations (Golomb, et al., 2006):
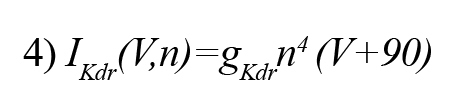
Here, n is the gating variable and gKdr = 6 mS/cm2 is the default conductance of the channel. The A-type potassium current (IA) can be demonstrated by the following equations (Golomb, et al., 2006):
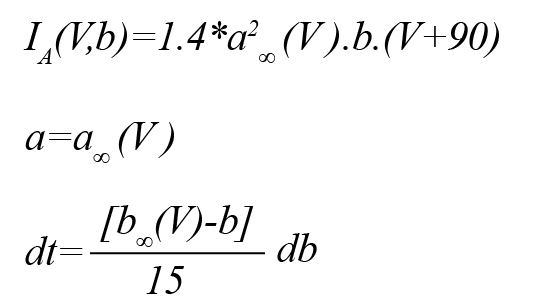
The muscarinic-sensitive potassium current (Im) is denoted by the below equation (Golomb, et al., 2006):
.jpg)
The High-voltage calcium current (ICa) can be shown by the following equation (Golomb, et al., 2006):

The fast calcium-activated potassium current (IC) is described by the following equation (Golomb, et al., 2006):

Here [Ca2+]j is the calcium concentration in the neuron; the slow calcium-activated potassium current (IsAHP) can be shown by this equation (Golomb, et al., 2006):

The leak current (IL) can be represented by this formula (Golomb, et al., 2006):
.jpg)
This equation can show the dynamics of calcium concentration inside the neuron:
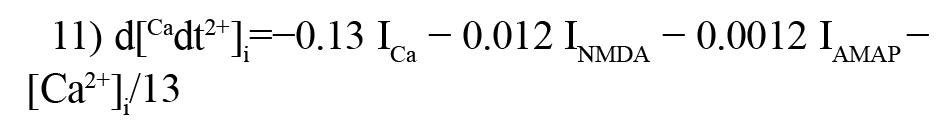
The AMPA current (IAMAP) is modeled by the following formula (Destexhe, Destexhe, Mainen, & Sejnowski 1998; Borjkhani, et al., 2018):
.jpg)
, where mAMPA is the gating variable of the receptor, aAMPA = 1.1 m𝑀/ms and 𝛽AMPA = 0.67 m𝑀/ ms are the opening and closing rate of the receptor, respectively, and Gst is the synaptic glutamate or the stimulatory signal which activates the receptor.
The NMDA current (INMDA) can be represented by the following formula (Destexhe, et al,, & Sejnowski 1998; Borjkhani, et al., 2018):
.jpg)
Here, mNMDA is the gating variable of the receptor, Mg, denotes the dynamics for Mg2+ blocking of the receptor, [Mg2+]0 = 2 mM is the concentration of Mg2+in the extracellular fluid, gVD is the receptor’s conductance dependent to the voltage, k = 0.0007 and V0 = −100 mv are constants, and 𝜏g= 0.05 ms is a time constant. αNMDA= 0.14 Mm/ms and 𝛽NMDA =0.03 mM/ms are the opening and closing rates of the receptor.
The following formula can describe the activation function of gating variables in the equations:
.jpg)
Here the units for the voltages are millivolts.
3. Results
Simulation results are presented in this section. All simulations have been performed in Matlab 2018a software. A stimulatory signal (assumed to be the glutamate) with an amplitude of 0.1 mM and a frequency of 10 Hz with a pulse duration of 10 ms affects the AMPARs and NMDARs (Gst in equations 12 and 13). The stimulatory signal is shown in Figure 1.
.jpg)
Since cocaine dose-dependently attenuates the potassium current in empirical observations (Chen, et al., 2006), in the simulations, the conductance of potassium current gradually increases from 0 to 10, and the bifurcation diagram is shown in Figure 2.
.jpg)
Here, by changing the conductance, the peaks of the action potentials are calculated. Between 2 to 8 values of the delayed rectifier potassium conductance (gKdr), the model has multi-stability, and the voltage has several peak values. These multi-peaks may represent a chaotic behavior. Further decrease of the conductance from 2 or increase of the conductance from 8 leads to semi-stable peak values for generated action potentials.
For further analysis, action potentials for different values of gkdr and their related phase spaces are calculated and represented through Figures 3, 4, 5, 6, 7. Phase spaces are calculated by plotting the Vn+1 against Vn.
Here Vn and Vn+1 are the values of the action potentials at the time of n and n+1, respectively. Figure 3 shows the produced action potentials and related phase space for gkdr = 0.01. It can be seen that regular spiking patterns exist, and reconstructed space denotes a limit cycle.
.jpg)
The left panel illustrates the produced action potentials, and the right panel the related phase space.
By increasing the conductance to 1 (Figure 4.), the firing frequency increases, and spiking patterns can be seen here. The produced action potentials follow the frequency of the stimulatory signal. Furthermore, the phase space demonstrates a normal limit cycle.
.jpg)
The left panel denotes the produced action potentials, and the right panel shows the related phase space.
By changing the conductance to the gKdr=4, burst-type action potentials emerge (Figure 5).
.jpg)
The reconstructed phase space of the signal shows that the chaotic regime appears. As seen, the generated potentials are different. Some of the potentials are single spikes, and some have burst. Also, the phase space shows that multiple limit cycles emerge that may show chaotic behaviors.
The left panel denotes the produced action potentials, and the right panel the related phase space.
By increasing the conductance (gkdr) to 6, more irregularities emerge in the generated action potentials (Figure 6).
.jpg)
These behaviors may represent chaotic behavior or may lead to chaos.
The left panel shows the produced action potentials, and the right panel the related phase space.
Figure 7 shows the action potentials and reconstructed phase space for gkdr = 10. It can be seen that chaotic oscillations diminish, and regular spiking patterns emerge again. In this case, we see the regular
The left panel denotes the produced action potentials, and the right panel the related phase space.
.jpg)
4. Discussion
Despite many studies in addiction, lots of questions have remained unanswered. Cocaine interacts with several transporters, receptors, and channels (Kobayashi, Nishizawa, Iwamura, & Ikeda, 2007). In this paper, the effect of reducing the conductivity of potassium channels (which can be due to the consumption of cocaine) in the generation of action potentials was investigated. For this purpose, a computational model was used that could generate the burst-type action potentials. The study of potassium current is critical because, in some experimental research, this current is affected by the consumption of cocaine. It has been shown that cocaine consumption blocks the calcium-dependent K+ channel in the hippocampus neurons. Besides, it has been suggested that cocaine is related to the blocking of Ca2+-dependent K+ channels that may broaden the action potential and cause repetitive firing of neurons (Premkumar, 2005). In addition to demonstrating this empirical finding using a computational model, we state that chaotic behaviors are also observed in the process of reducing potassium current. Besides that, decreasing the potassium currents in simulations elicits burst action potentials reported in empirical experiments (Chen, et al., 2006).
The simulation results show that by decreasing the potassium current for a fixed stimulatory signal, burst-type action potentials can be generated. With a further reduction of potassium conductance, produced action potentials exhibit non-linear and even chaotic behaviors. The appearance of chaotic patterns has been seen in many diseases and can contribute to forming abnormal and addictive memories, which requires further studies, both in computational and empirical research. The fact that cocaine inhibition of GIRK (the G protein-coupled inwardly-rectifying potassium) channels may involve in its toxic effects (Kobayashi, et al., 2007) can relate to emerging chaotic oscillations which were observed in the simulations. Since cocaine has diverse harmful effects such as seizures, cardiac arrhythmias, and sudden death (Lathers, Tyau, Spino, & Agarwal, 1988; O’leary and Hancox 2010), and the potassium channels play an essential role in creating these obstacles, the proposed model can help the researchers investigate these effects, too.
Ethical Considerations
Compliance with ethical guidelines
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflicts of interest.
Acknowledgments
???
Refrences
Bao, G., Kang, L., Li, H., Li, Y., Pu, L., Xia, P., et al. (2007). Morphine and heroin differentially modulate in vivo hippocampal LTP in opiate-dependent rat. Neuropsychopharmacology, 32(8), 1738-49. [DOI:10.1038/sj.npp.1301308] [PMID]
Boening, J. (2001). Neurobiology of an addiction memory. Journal of Neural Transmission, 108(6), 755-65. [DOI:10.1007/s007020170050] [PMID]
Borjkhani, M., Bahrami, F., & Janahmadi, M. (2018). Assessing the effects of opioids on pathological memory by a computational model. Basic and Clinical Neuroscience, 9(4), 275-88. [DOI:10.32598/bcn.9.4.275] [PMID] [PMCID]
Borjkhani, M., Bahrami, F., & Janahmadi, M. (2018a). Computational modeling of opioid-induced synaptic plasticity in hippocampus. PLoS One, 13(3), e0193410. [DOI:10.1371/journal.pone.0193410] [PMID] [PMCID]
Borjkhani, M., Bahrami, F., & Janahmadi, M. (2018b). Formation of opioid-induced memory and its prevention: A computational study. Frontiers in Computational Neuroscience, 12, 63. [DOI:10.3389/fncom.2018.00063] [PMID] [PMCID]
Borjkhani, M., Mahdavi, A., & Bahrami, F. (2014). A mathematical model for neuron astrocytes interactions in hippocampus during addiction. Paper presented at The 21th Iranian Conference on Biomedical Engineering (ICBME), Tehran, Iran, 26-28 November 2014. [DOI:10.1109/ICBME.2014.7043940]
Caffino, L., Frankowska, M., Giannotti, G., Miszkiel, J., Sadakierska-Chudy, A., Racagni, G., et al. (2014). “Cocaine-induced glutamate receptor trafficking is abrogated by extinction training in the rat hippocampus. Pharmacological Reports, 66(2), 198-204. [DOI:10.1016/j.pharep.2013.09.002] [PMID]
Chen, Y. H., Lin, C. H., Lin, P. L., & Tsai, M. C. (2006). Cocaine elicits action potential bursts in a central snail neuron: The role of delayed rectifying K+ current. Neuroscience, 138(1), 257-80. [DOI:10.1016/j.neuroscience.2005.11.006] [PMID]
Cohen, G. A., Doze, V. A., & Madison, D. V. (1992). Opioid inhibition of GABA release from presynaptic terminals of rat hippocampal interneurons. Neuron, 9(2), 325-35. [DOI:10.1016/0896-6273(92)90171-9]
Destexhe, A., Mainen, Z. F., & Sejnowski, T. J. (1998). Kinetic models of synaptic transmission. Methods in Neuronal Modeling, 2, 1-25. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.219.3728&rep=rep1&type=pdf
Golomb, D., Yue, C., & Yaari, Y. (2006). Contribution of persistent Na+ current and M-type K+ current to somatic bursting in CA1 pyramidal cells: Combined experimental and modeling study. Journal of Neurophysiology, 96(4), 1912-26. [DOI:10.1152/jn.00205.2006] [PMID]
Hodgkin, A. L., & Huxley, A. F. (1952). A quantitative description of membrane current and its application to conduction and excitation in nerve. The Journal of Physiology, 117(4), 500-44. [DOI:10.1113/jphysiol.1952.sp004764] [PMID] [PMCID]
Kauer, J. A., & Malenka, R. C. (2007). Synaptic plasticity and addiction. Nature Reviews Neuroscience, 8(11), 844-58. [DOI:10.1038/nrn2234] [PMID]
Kelley, A. E. (2004). Memory and addiction: Shared neural circuitry and molecular mechanisms. Neuron, 44(1), 161-79. [DOI:10.1016/j.neuron.2004.09.016] [PMID]
Kobayashi, T., Nishizawa, D., Iwamura, T., & Ikeda, K. (2007). Inhibition by cocaine of G protein-activated inwardly rectifying K+ channels expressed in Xenopus oocytes. Toxicology in Vitro, 21(4), 656-64. [DOI:10.1016/j.tiv.2007.01.009] [PMID]
Kutlu, M. G., & Gould, T. J. (2016). Effects of drugs of abuse on hippocampal plasticity and hippocampus-dependent learning and memory: Contributions to development and maintenance of addiction. Learning & Memory, 23(10), 515-33. [DOI:10.1101/lm.042192.116] [PMID] [PMCID]
Lathers, C. M., Tyau, L. S., Spino, M. M., & Agarwal, I. (1988). Cocaine-induced seizures, arrhythmias and sudden death. The Journal of Clinical Pharmacology, 28(7), 584-93. [DOI:10.1002/j.1552-4604.1988.tb03181.x] [PMID]
Lüscher, C. (2013). Drug-evoked synaptic plasticity causing addictive behavior. The Journal of Neuroscience, 33(45): 17641-6. [DOI:10.1523/JNEUROSCI.3406-13.2013] [PMID] [PMCID]
Nestler, E. J. (2001). Molecular basis of long-term plasticity underlying addiction. Nature Reviews Neuroscience, 2(2), 119-28. [DOI:10.1038/35053570] [PMID]
Nestler, E. J. (2013). Cellular basis of memory for addiction. Dialogues in Clinical Neuroscience, 15(4), 431-43. [DOI:10.31887/DCNS.2013.15.4/enestler] [PMID] [PMCID]
O’leary, M. E., & Hancox, J. C. (2010). Role of voltage-gated sodium, potassium and calcium channels in the development of cocaine-associated cardiac arrhythmias. British Journal of Clinical Pharmacology, 69(5), 427-42. [DOI:10.1111/j.1365-2125.2010.03629.x] [PMID] [PMCID]
Premkumar, L. (2005). Block of a Ca 2+-activated potassium channel by cocaine. The Journal of Membrane Biology, 204(3), 129-36. [DOI:10.1007/s00232-005-0755-6] [PMID]
Preston, C. J., Brown, K. A., & Wagner, J. J. (2019). Cocaine conditioning induces persisting changes in ventral hippocampus synaptic transmission, long-term potentiation, and radial arm maze performance in the mouse. Neuropharmacology, 150, 27-37. [DOI:10.1016/j.neuropharm.2019.02.033] [PMID]
Shukla, A., Beroun, A., Panopoulou, M., Neumann, P. A., Grant, S. G., Olive, M. F., et al. (2017). Calcium permeable AMPA receptors and silent synapses in cocaine conditioned place preference. The EMBO Journal, 36(4), 458-74. [DOI:10.15252/embj.201695465] [PMID] [PMCID]
Trujillo, K. A. (2002). The neurobiology of opiate tolerance, dependence and sensitization: Mechanisms of NMDA receptor-dependent synaptic plasticity. Neurotoxicity Research, 4(4), 373-91. [DOI:10.1080/10298420290023954] [PMID]
van Huijstee, A. N., & Mansvelder, H. D. (2015). Glutamatergic synaptic plasticity in the mesocorticolimbic system in addiction. Frontiers in Cellular Neuroscience, 8, 466. [DOI:10.3389/fncel.2014.00466] [PMID] [PMCID]
Zachariou, M., Alexander, S. P., Coombes, S., & Christodoulou, C. (2013). A biophysical model of endocannabinoid-mediated short term depression in hippocampal inhibition. PloS One, 8(3), e58926. [DOI:10.1371/journal.pone.0058926] [PMID] [PMCID]
Zweifel, L. S., Argilli, E., Bonci, A., & Palmiter, R. D. (2008). Role of NMDA receptors in dopamine neurons for plasticity and addictive behaviors. Neuron, 59(3), 486-96. [DOI:10.1016/j.neuron.2008.05.028] [PMID] [PMCID]
Addiction is a chronic disease that has various negative consequences. Cocaine consumption is one of the pervasive forms of addiction. The use of drugs, such as cocaine, can lead to maladaptive behavioral changes. Many researchers have shown that these changes are due to the intervention of drugs in the plasticity mechanism of the brain (Nestler, 2001; Kauer, & Malenka, 2007; Lüscher 2013; Kutlu, & Gould 2016; Borjkhani, Bahrami, & Janahmadi, 2018; Borjkhani, et al., 2018; Preston, Brown, & Wagner, 2019). Some researchers consider that drugs of abuse can form a pathological memory, called the memory of addiction (Boening 2001; Kelley 2004; Nestler, 2013).
Among the involvement of different brain regions in the addiction process, the hippocampus has an integral role in forming addiction memories and triggering relapse during withdrawal times (Caffino, et al., 2014,; Kutlu, & Gould, 2016; Preston, et al. 2019). It has been shown both empirically and computationally that one reason for the formation of addiction memory is the increased excitability of neurons (Cohen, Doze, & Madison, 1992; Kauer, & Malenka 2007; Borjkhani, Mahdavi, & Bahrami, 2014; van Huijstee & Mansvelder 2015; Shukla, et al. 2017; Borjkhani, et al. 2018; Borjkhani, et al. 2018a; Borjkhani, et al. 2018b).
Excitability of the neurons can be changed by drug intervention in N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors mediated currents that some researchers investigated (Trujillo 2002; Bao, Kang et al., 2007; Kauer, & Malenka 2007; Zweifel, Argilli, E., Bonci, & Palmiter, 2008; Zachariou, Alexander , Coombes, & Christodoulou2013; Borjkhani, et al. 2018; Borjkhani, et al. 2018). Meanwhile, the missing issue is the role of potassium currents in changing cell excitabilities. It has been observed that the consumption of cocaine increases the excitability of the hippocampal neurons by inhibiting the potassium current (Chen, Lin, Lin, & Tsai, 2006). Cocaine consumption provokes action potential bursts in neurons (Chen, et al., 2006). Moreover, it has been suggested that the emergence of the burst is associated with cocaine’s inhibitory effects on the delayed rectifying K current (Chen, et al., 2006). Therefore, one of the mechanisms by which cocaine changes neurons’ excitabilities and innervates in memory formation is the reduction of potassium current. So, based on these observations, we want to computationally investigate potassium current reduction affects the produced action potentials in the hippocampus. This research can reveal new dimensions of the formation of cellular addiction.
In the next section, the implemented computational model is introduced. Then the simulation results are presented, and, finally, potential applications of the proposed model are discussed.
2. Methods
To analyze the cocaine effects on action potential generation in hippocampal neurons, we used a modified version of the ‘Hodgkin–Huxley style’ formulation (Hodgkin & Huxley, 1952). The equation denoting voltage variation of the neuron with ionic current equations is as follows:
.jpg)
Here, INa is the transient sodium current, INap denotes the persistent sodium current, IKdr shows the delayed rectifier potassium current, IA demonstrates the A-type potassium current, IM exhibits the
muscarinic-sensitive potassium current, ICa illustrates the High-voltage calcium current, IC describes the
fast calcium-activated potassium current, IsAHP displays the slow calcium-activated potassium current,
IL indicates the leak current, IAMPA represents the AMPAR mediated current, and INMDA refers to the NMDAR mediated current.
Transient sodium current (INa) can be described using the following equations (Golomb, Yue, & Yaari., 2006):
.jpg)
Here, m and h are gating variables and 𝜏h is the time constant. Persistent sodium current can be described by the following equation (Golomb, et al., 2006):
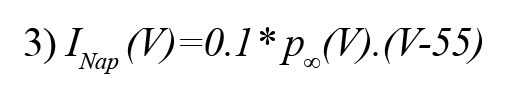
The delayed rectifier potassium current (IKdr ) can be represented by the following equations (Golomb, et al., 2006):
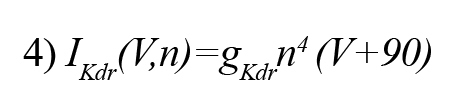
Here, n is the gating variable and gKdr = 6 mS/cm2 is the default conductance of the channel. The A-type potassium current (IA) can be demonstrated by the following equations (Golomb, et al., 2006):
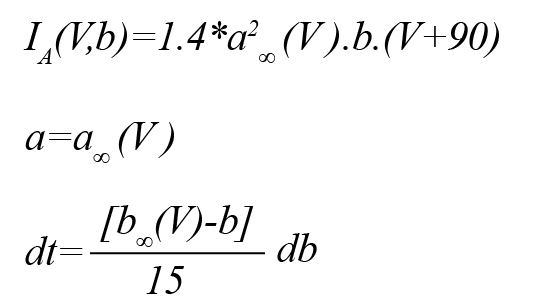
The muscarinic-sensitive potassium current (Im) is denoted by the below equation (Golomb, et al., 2006):
.jpg)
The High-voltage calcium current (ICa) can be shown by the following equation (Golomb, et al., 2006):

The fast calcium-activated potassium current (IC) is described by the following equation (Golomb, et al., 2006):

Here [Ca2+]j is the calcium concentration in the neuron; the slow calcium-activated potassium current (IsAHP) can be shown by this equation (Golomb, et al., 2006):

The leak current (IL) can be represented by this formula (Golomb, et al., 2006):
.jpg)
This equation can show the dynamics of calcium concentration inside the neuron:
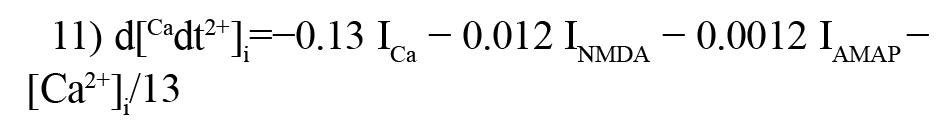
The AMPA current (IAMAP) is modeled by the following formula (Destexhe, Destexhe, Mainen, & Sejnowski 1998; Borjkhani, et al., 2018):
.jpg)
, where mAMPA is the gating variable of the receptor, aAMPA = 1.1 m𝑀/ms and 𝛽AMPA = 0.67 m𝑀/ ms are the opening and closing rate of the receptor, respectively, and Gst is the synaptic glutamate or the stimulatory signal which activates the receptor.
The NMDA current (INMDA) can be represented by the following formula (Destexhe, et al,, & Sejnowski 1998; Borjkhani, et al., 2018):
.jpg)
Here, mNMDA is the gating variable of the receptor, Mg, denotes the dynamics for Mg2+ blocking of the receptor, [Mg2+]0 = 2 mM is the concentration of Mg2+in the extracellular fluid, gVD is the receptor’s conductance dependent to the voltage, k = 0.0007 and V0 = −100 mv are constants, and 𝜏g= 0.05 ms is a time constant. αNMDA= 0.14 Mm/ms and 𝛽NMDA =0.03 mM/ms are the opening and closing rates of the receptor.
The following formula can describe the activation function of gating variables in the equations:
.jpg)
Here the units for the voltages are millivolts.
3. Results
Simulation results are presented in this section. All simulations have been performed in Matlab 2018a software. A stimulatory signal (assumed to be the glutamate) with an amplitude of 0.1 mM and a frequency of 10 Hz with a pulse duration of 10 ms affects the AMPARs and NMDARs (Gst in equations 12 and 13). The stimulatory signal is shown in Figure 1.
.jpg)
Since cocaine dose-dependently attenuates the potassium current in empirical observations (Chen, et al., 2006), in the simulations, the conductance of potassium current gradually increases from 0 to 10, and the bifurcation diagram is shown in Figure 2.
.jpg)
Here, by changing the conductance, the peaks of the action potentials are calculated. Between 2 to 8 values of the delayed rectifier potassium conductance (gKdr), the model has multi-stability, and the voltage has several peak values. These multi-peaks may represent a chaotic behavior. Further decrease of the conductance from 2 or increase of the conductance from 8 leads to semi-stable peak values for generated action potentials.
For further analysis, action potentials for different values of gkdr and their related phase spaces are calculated and represented through Figures 3, 4, 5, 6, 7. Phase spaces are calculated by plotting the Vn+1 against Vn.
Here Vn and Vn+1 are the values of the action potentials at the time of n and n+1, respectively. Figure 3 shows the produced action potentials and related phase space for gkdr = 0.01. It can be seen that regular spiking patterns exist, and reconstructed space denotes a limit cycle.
.jpg)
The left panel illustrates the produced action potentials, and the right panel the related phase space.
By increasing the conductance to 1 (Figure 4.), the firing frequency increases, and spiking patterns can be seen here. The produced action potentials follow the frequency of the stimulatory signal. Furthermore, the phase space demonstrates a normal limit cycle.
.jpg)
The left panel denotes the produced action potentials, and the right panel shows the related phase space.
By changing the conductance to the gKdr=4, burst-type action potentials emerge (Figure 5).
.jpg)
The reconstructed phase space of the signal shows that the chaotic regime appears. As seen, the generated potentials are different. Some of the potentials are single spikes, and some have burst. Also, the phase space shows that multiple limit cycles emerge that may show chaotic behaviors.
The left panel denotes the produced action potentials, and the right panel the related phase space.
By increasing the conductance (gkdr) to 6, more irregularities emerge in the generated action potentials (Figure 6).
.jpg)
These behaviors may represent chaotic behavior or may lead to chaos.
The left panel shows the produced action potentials, and the right panel the related phase space.
Figure 7 shows the action potentials and reconstructed phase space for gkdr = 10. It can be seen that chaotic oscillations diminish, and regular spiking patterns emerge again. In this case, we see the regular
The left panel denotes the produced action potentials, and the right panel the related phase space.
.jpg)
4. Discussion
Despite many studies in addiction, lots of questions have remained unanswered. Cocaine interacts with several transporters, receptors, and channels (Kobayashi, Nishizawa, Iwamura, & Ikeda, 2007). In this paper, the effect of reducing the conductivity of potassium channels (which can be due to the consumption of cocaine) in the generation of action potentials was investigated. For this purpose, a computational model was used that could generate the burst-type action potentials. The study of potassium current is critical because, in some experimental research, this current is affected by the consumption of cocaine. It has been shown that cocaine consumption blocks the calcium-dependent K+ channel in the hippocampus neurons. Besides, it has been suggested that cocaine is related to the blocking of Ca2+-dependent K+ channels that may broaden the action potential and cause repetitive firing of neurons (Premkumar, 2005). In addition to demonstrating this empirical finding using a computational model, we state that chaotic behaviors are also observed in the process of reducing potassium current. Besides that, decreasing the potassium currents in simulations elicits burst action potentials reported in empirical experiments (Chen, et al., 2006).
The simulation results show that by decreasing the potassium current for a fixed stimulatory signal, burst-type action potentials can be generated. With a further reduction of potassium conductance, produced action potentials exhibit non-linear and even chaotic behaviors. The appearance of chaotic patterns has been seen in many diseases and can contribute to forming abnormal and addictive memories, which requires further studies, both in computational and empirical research. The fact that cocaine inhibition of GIRK (the G protein-coupled inwardly-rectifying potassium) channels may involve in its toxic effects (Kobayashi, et al., 2007) can relate to emerging chaotic oscillations which were observed in the simulations. Since cocaine has diverse harmful effects such as seizures, cardiac arrhythmias, and sudden death (Lathers, Tyau, Spino, & Agarwal, 1988; O’leary and Hancox 2010), and the potassium channels play an essential role in creating these obstacles, the proposed model can help the researchers investigate these effects, too.
Ethical Considerations
Compliance with ethical guidelines
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflicts of interest.
Acknowledgments
???
Refrences
Bao, G., Kang, L., Li, H., Li, Y., Pu, L., Xia, P., et al. (2007). Morphine and heroin differentially modulate in vivo hippocampal LTP in opiate-dependent rat. Neuropsychopharmacology, 32(8), 1738-49. [DOI:10.1038/sj.npp.1301308] [PMID]
Boening, J. (2001). Neurobiology of an addiction memory. Journal of Neural Transmission, 108(6), 755-65. [DOI:10.1007/s007020170050] [PMID]
Borjkhani, M., Bahrami, F., & Janahmadi, M. (2018). Assessing the effects of opioids on pathological memory by a computational model. Basic and Clinical Neuroscience, 9(4), 275-88. [DOI:10.32598/bcn.9.4.275] [PMID] [PMCID]
Borjkhani, M., Bahrami, F., & Janahmadi, M. (2018a). Computational modeling of opioid-induced synaptic plasticity in hippocampus. PLoS One, 13(3), e0193410. [DOI:10.1371/journal.pone.0193410] [PMID] [PMCID]
Borjkhani, M., Bahrami, F., & Janahmadi, M. (2018b). Formation of opioid-induced memory and its prevention: A computational study. Frontiers in Computational Neuroscience, 12, 63. [DOI:10.3389/fncom.2018.00063] [PMID] [PMCID]
Borjkhani, M., Mahdavi, A., & Bahrami, F. (2014). A mathematical model for neuron astrocytes interactions in hippocampus during addiction. Paper presented at The 21th Iranian Conference on Biomedical Engineering (ICBME), Tehran, Iran, 26-28 November 2014. [DOI:10.1109/ICBME.2014.7043940]
Caffino, L., Frankowska, M., Giannotti, G., Miszkiel, J., Sadakierska-Chudy, A., Racagni, G., et al. (2014). “Cocaine-induced glutamate receptor trafficking is abrogated by extinction training in the rat hippocampus. Pharmacological Reports, 66(2), 198-204. [DOI:10.1016/j.pharep.2013.09.002] [PMID]
Chen, Y. H., Lin, C. H., Lin, P. L., & Tsai, M. C. (2006). Cocaine elicits action potential bursts in a central snail neuron: The role of delayed rectifying K+ current. Neuroscience, 138(1), 257-80. [DOI:10.1016/j.neuroscience.2005.11.006] [PMID]
Cohen, G. A., Doze, V. A., & Madison, D. V. (1992). Opioid inhibition of GABA release from presynaptic terminals of rat hippocampal interneurons. Neuron, 9(2), 325-35. [DOI:10.1016/0896-6273(92)90171-9]
Destexhe, A., Mainen, Z. F., & Sejnowski, T. J. (1998). Kinetic models of synaptic transmission. Methods in Neuronal Modeling, 2, 1-25. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.219.3728&rep=rep1&type=pdf
Golomb, D., Yue, C., & Yaari, Y. (2006). Contribution of persistent Na+ current and M-type K+ current to somatic bursting in CA1 pyramidal cells: Combined experimental and modeling study. Journal of Neurophysiology, 96(4), 1912-26. [DOI:10.1152/jn.00205.2006] [PMID]
Hodgkin, A. L., & Huxley, A. F. (1952). A quantitative description of membrane current and its application to conduction and excitation in nerve. The Journal of Physiology, 117(4), 500-44. [DOI:10.1113/jphysiol.1952.sp004764] [PMID] [PMCID]
Kauer, J. A., & Malenka, R. C. (2007). Synaptic plasticity and addiction. Nature Reviews Neuroscience, 8(11), 844-58. [DOI:10.1038/nrn2234] [PMID]
Kelley, A. E. (2004). Memory and addiction: Shared neural circuitry and molecular mechanisms. Neuron, 44(1), 161-79. [DOI:10.1016/j.neuron.2004.09.016] [PMID]
Kobayashi, T., Nishizawa, D., Iwamura, T., & Ikeda, K. (2007). Inhibition by cocaine of G protein-activated inwardly rectifying K+ channels expressed in Xenopus oocytes. Toxicology in Vitro, 21(4), 656-64. [DOI:10.1016/j.tiv.2007.01.009] [PMID]
Kutlu, M. G., & Gould, T. J. (2016). Effects of drugs of abuse on hippocampal plasticity and hippocampus-dependent learning and memory: Contributions to development and maintenance of addiction. Learning & Memory, 23(10), 515-33. [DOI:10.1101/lm.042192.116] [PMID] [PMCID]
Lathers, C. M., Tyau, L. S., Spino, M. M., & Agarwal, I. (1988). Cocaine-induced seizures, arrhythmias and sudden death. The Journal of Clinical Pharmacology, 28(7), 584-93. [DOI:10.1002/j.1552-4604.1988.tb03181.x] [PMID]
Lüscher, C. (2013). Drug-evoked synaptic plasticity causing addictive behavior. The Journal of Neuroscience, 33(45): 17641-6. [DOI:10.1523/JNEUROSCI.3406-13.2013] [PMID] [PMCID]
Nestler, E. J. (2001). Molecular basis of long-term plasticity underlying addiction. Nature Reviews Neuroscience, 2(2), 119-28. [DOI:10.1038/35053570] [PMID]
Nestler, E. J. (2013). Cellular basis of memory for addiction. Dialogues in Clinical Neuroscience, 15(4), 431-43. [DOI:10.31887/DCNS.2013.15.4/enestler] [PMID] [PMCID]
O’leary, M. E., & Hancox, J. C. (2010). Role of voltage-gated sodium, potassium and calcium channels in the development of cocaine-associated cardiac arrhythmias. British Journal of Clinical Pharmacology, 69(5), 427-42. [DOI:10.1111/j.1365-2125.2010.03629.x] [PMID] [PMCID]
Premkumar, L. (2005). Block of a Ca 2+-activated potassium channel by cocaine. The Journal of Membrane Biology, 204(3), 129-36. [DOI:10.1007/s00232-005-0755-6] [PMID]
Preston, C. J., Brown, K. A., & Wagner, J. J. (2019). Cocaine conditioning induces persisting changes in ventral hippocampus synaptic transmission, long-term potentiation, and radial arm maze performance in the mouse. Neuropharmacology, 150, 27-37. [DOI:10.1016/j.neuropharm.2019.02.033] [PMID]
Shukla, A., Beroun, A., Panopoulou, M., Neumann, P. A., Grant, S. G., Olive, M. F., et al. (2017). Calcium permeable AMPA receptors and silent synapses in cocaine conditioned place preference. The EMBO Journal, 36(4), 458-74. [DOI:10.15252/embj.201695465] [PMID] [PMCID]
Trujillo, K. A. (2002). The neurobiology of opiate tolerance, dependence and sensitization: Mechanisms of NMDA receptor-dependent synaptic plasticity. Neurotoxicity Research, 4(4), 373-91. [DOI:10.1080/10298420290023954] [PMID]
van Huijstee, A. N., & Mansvelder, H. D. (2015). Glutamatergic synaptic plasticity in the mesocorticolimbic system in addiction. Frontiers in Cellular Neuroscience, 8, 466. [DOI:10.3389/fncel.2014.00466] [PMID] [PMCID]
Zachariou, M., Alexander, S. P., Coombes, S., & Christodoulou, C. (2013). A biophysical model of endocannabinoid-mediated short term depression in hippocampal inhibition. PloS One, 8(3), e58926. [DOI:10.1371/journal.pone.0058926] [PMID] [PMCID]
Zweifel, L. S., Argilli, E., Bonci, A., & Palmiter, R. D. (2008). Role of NMDA receptors in dopamine neurons for plasticity and addictive behaviors. Neuron, 59(3), 486-96. [DOI:10.1016/j.neuron.2008.05.028] [PMID] [PMCID]
Type of Study: Original |
Subject:
Computational Neuroscience
Received: 2019/07/1 | Accepted: 2020/08/5 | Published: 2022/01/1
Received: 2019/07/1 | Accepted: 2020/08/5 | Published: 2022/01/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








