Volume 13, Issue 2 (March & April 2022)
BCN 2022, 13(2): 237-246 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Yaghoubi E, Shariat S V, Rashedi V, Ghanbari Jolfaei A. Repetitive Transcranial Magnetic Stimulation in Delirium: A Double-blind, Randomized, Sham-controlled, Pilot Study. BCN 2022; 13 (2) :237-246
URL: http://bcn.iums.ac.ir/article-1-1503-en.html
URL: http://bcn.iums.ac.ir/article-1-1503-en.html
1- Department of Psychiatry, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
2- Mental Health Research Center, Iran University of Medical Sciences, Tehran, Iran.
3- School of Behavioral Sciences and Mental Health (Tehran Institute of Psychiatry), Iran University of Medical Sciences, Tehran, Iran.
2- Mental Health Research Center, Iran University of Medical Sciences, Tehran, Iran.
3- School of Behavioral Sciences and Mental Health (Tehran Institute of Psychiatry), Iran University of Medical Sciences, Tehran, Iran.
Keywords: Delirium, Repetitive transcranial magnetic stimulation (rTMS), Randomized Controlled Trial
Full-Text [PDF 774 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Delirium is a fatal but potentially reversible disorder of the central nervous system. It is characterized by changes in cognition, behavior, perception, and emotion (Setters & Solberg, 2017; Zoremba & Coburn, 2019). This disorder has three types: hyperactive, hypoactive, and mixed (Morandi et al., 2017).
The prevalence of delirium varies in different countries and among patients in various wards. According to Ryan et al. study in south Ireland, the prevalence of delirium varies between 7% and 53% (Ryan et al., 2013). Delirium affects 22% of the elderly hospitalized patients in Iran ( Foroughan, Delbari, Said, AkbariKamrani, Rashedi, & Zandi, 2016). Sometimes the delirium’s side effects remain in patients over 65 years for months. In the USA, the imposing cost on hospitals for each delirious patient is estimated at around $2500, accounting for $6,9 billion annually. Moreover, by considering the incomplete recovery of some patients after hospital discharge, needing home care services, and nurseries costs, the above-remarked expenses are doubled (Devlin et al., 2008; Wei, Fearing, Sternberg, & Inouye, 2008).
The delirium’s pathophysiology is not entirely known. However, the neuroanatomical data from neuro-imaging and reports suggest that specific brain regions such as the prefrontal cortex, fusiform cortex, and basal ganglia may have a critical role in causing delirium symptoms. According to many studies, these parts have a significant role in the final common pathway (Maldonado, 2008; Veiga Fernandez & Cruz Jentoft, 2008) that may be in charge of the main symptoms of delirium (Trzepacz, 1999).
Decreasing cholinergic and increasing dopaminergic activity also have an important role in the etiology of delirium. The cholinergic system is essential in the cognition processes (Trzepacz, 2000). Also, it has been remarked that delirium is associated with abnormal activity and mostly hyperactivity of the brain’s dopaminergic system; an imbalance between the dopaminergic and cholinergic systems may play an important role in delirium etiology (Maldonado, 2008; Trzepacz, 2000; Veiga Fernandez, et al., 2008). In addition, in recent studies, it has been found that throughout the delirium phase, the activity of the dorsolateral prefrontal cortex (DLPFC) is decreased, and the consecutive disconnection of the DLPFC with the posterior cingulate cortex may cause delirium (Choi et al., 2012; Mannarelli et al., 2015).
Currently, delirium treatment is based on pharmacological and non-pharmacological conservative treatments. The pharmacological treatments include typical and atypical antipsychotics and benzodiazepines. However, these treatments usually affect the behavioral disturbances of the patients and are not efficient for the cognition and orientation issues. Moreover, it is less recommended to treat hypoactive delirium, which does not have agitation and behavioral disturbances (Michaud, Bullard, Harris, & Thomas, 2015).
Repetitive transcranial magnetic stimulation (rTMS) is among the modern treatments that earned FDA (USA Food and Drug Administration) approval for the treatment of some disorders such as major depressive disorder (Boggio et al., 2008). Several researchers have reported that the high-frequency rTMS causes increasing excitability in the left DLPFC (Miniussi & Ruzzoli, 2013; Sato et al., 2013). Moreover, we know that rTMS has a positive impact on optimizing the dopaminergic system, extending the cholinergic system function, and improving the relevant indexes in attention deficit hyperactivity disorder (ADHD) patients (Cho & Strafella, 2009; Kim et al., 2014; Nardone, Tezzon, Holler, Golaszewski, Trinka, & Brigo, 2014).
According to what was said, the limitations of conventional treatments, considering the cognition disturbances as the main symptom of delirious patients, and the positive effect of rTMS on cognitive functions, we hypothesized that rTMS could be effective in the treatment of delirium and thus designed this study. If the study results are positive, the treatment costs decrease because of shorter hospitalization stay, fewer side effects, and optimizing patient care services.
2. Materials and Methods
Type of study and participants
This research was a randomized, double-blind controlled clinical trial. The participants were enrolled in the study from different departments of Rasoul-e-Akram Hospital in Tehran City, Iran. They received a delirium diagnosis based upon both diagnostic criteria of The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) and the confusion assessment method (CAM) by an Associate Professor of Psychiatry. If the patients lacked decision-making capacity, their legal guardian would be asked to sign an informed consent letter after explaining the study. Finally, 30 patients older than 18 years who provided an informed consent form and had no contraindication for rTMS were admitted to the study. The exclusion criteria were being intubated, having a history of dementia, having symptoms of alcohol or substance withdrawal, suffering from abnormal neurological symptoms, a recent head trauma, or other neurosurgical conditions and cerebrovascular accidents.
Study interventions
The participants were randomly allocated into two groups of active intermittent theta-burst stimulation (iTBS) and a sham group. Each group had 15 patients (Figure 1).
.jpg)
We used a Magventure MagPro X100 (Adventure, Denmark), connected to a figure-of-eight formed double 70-mm coil. Individual motor threshold (MT) for right abductor pollicis brevis muscle was determined using single-pulse TMS (transcranial magnetic stimulation) with motor evoked potentials (MEP). Left DLPFC (F3) was mapped using a 10-20 EEG system. The intervention group received one session of 600 pulses at an intensity of 80% active motor threshold on the left DLPFC area with three pulses of 50 Hz bursts delivered at 5 Hz in 30 sets of 10 bursts in 8 seconds off. The severity of delirium was assessed 15 minutes before the intervention and 15 minutes after it with the Neelon and Champagne (NEECHAM) confusion scale. Three days later, the patients were assessed for a diagnosis of delirium according to the DSM-5 criteria and CAM. Both groups received their sessions at the exact timetable from 9:00 to 13:00. Disposable hats were provided for them, and after placement of the probe, the device was adjusted according to the safety guidelines. In the control group, the coil was held at a 3 cm distance from the scalp. To avoid any possible problems in transferring process of some patients into the experiment room, the rTMS device was moved into all patients’ rooms.
It has to be cited that the patients were not banned from the standard treatment for delirium. All patients received equal and routine treatment, including a 0.5 mg haloperidol tablet at night and a 5.0 mg haloperidol intramuscular injection in case of severe agitation.
Data gathering method
Basic information registry questionnaire
The questionnaire includes name, medical diagnosis report, type of delirium, days of hospitalization, age, sex, and the file number of the patient.
NEECHAM confusion scale and confusion assessment method
The NEECHAM confusion scale as a delirium screening tool is applied to evaluate delirium severity. This questionnaire contains three subscales of information processing (attention and alertness, verbal and motor response, and memory and orientation with 0 to 14 points), behavior (general appearance and posture, sensory-motor performance, and verbal responses with 0 to 10 points), and performance (vital signs, oxygen saturation level and urinary incontinence with 0 to 6 points). The patients are divided into 4 groups based on the total acquired scores. The scores may range from 0 (minimal function) to 30 (normal function). The total needed time for scoring is 5 to 10 minutes (Grover & Kate, 2012).
Van Rompaey studied 172 patients to compare two questionnaires of NEECHAM and confusion assessment method (CAM)-ICU. He stated that the NEECHAM could diagnose delirium better than CAM-ICU and its advantage is the capacity to evaluate the delirium severity. The sensitivity and specificity of NEECHAM were estimated to be 78% and 95%, respectively (Van Rompaey,Schuurmans,Shortridge-Baggett,Truijen,Elseviers,& Bossaert, 2008). Also, Jannati reported the validity and reliability of the NEECHAM Persian version (Sohrabi, Jannati, Bagheri Nesami, Yazdani Charaty, & Mazdarani, 2013).
CAM is a standardized tool that enables clinicians and researchers to diagnose delirium in clinical and research settings. The questionnaire consists of 4 main items: 1) acute change in mental status, 2) lack of attention, 3) thought disorder, and 4) changes in awareness and consciousness. CAM has a sensitivity of 94%-100%, specificity of 90%-95%, and high inter-rater reliability (Oh, Fong, Hshieh, & Inouye, 2017).
Statistical Analysis
All collected data were analyzed using SPSS 20. Based on the Shapiro-Wilk test, the study data were not normally distributed. So, the Wilcoxon signed-rank test was used to compare the two groups.
3. Results
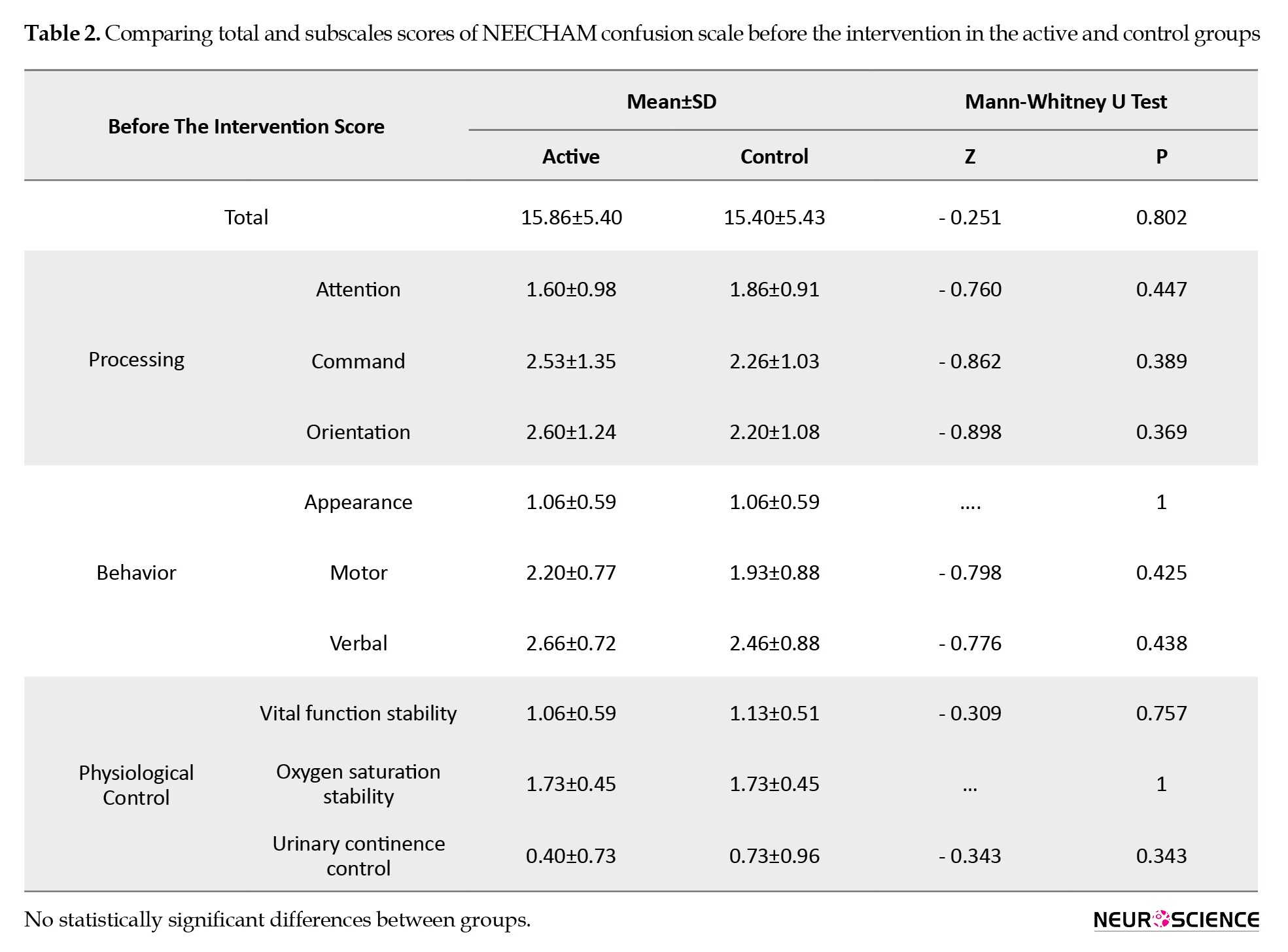
Thirty patients (18 males) were enrolled in the study. The Mean±SD age of the subjects was 65.7±7.66 years. There were no significant differences between groups in age and gender. Table 1 presents demographic data. Regarding the pretest scores of NEECHAM, there was no difference between the two groups (Table 2).
.jpg)
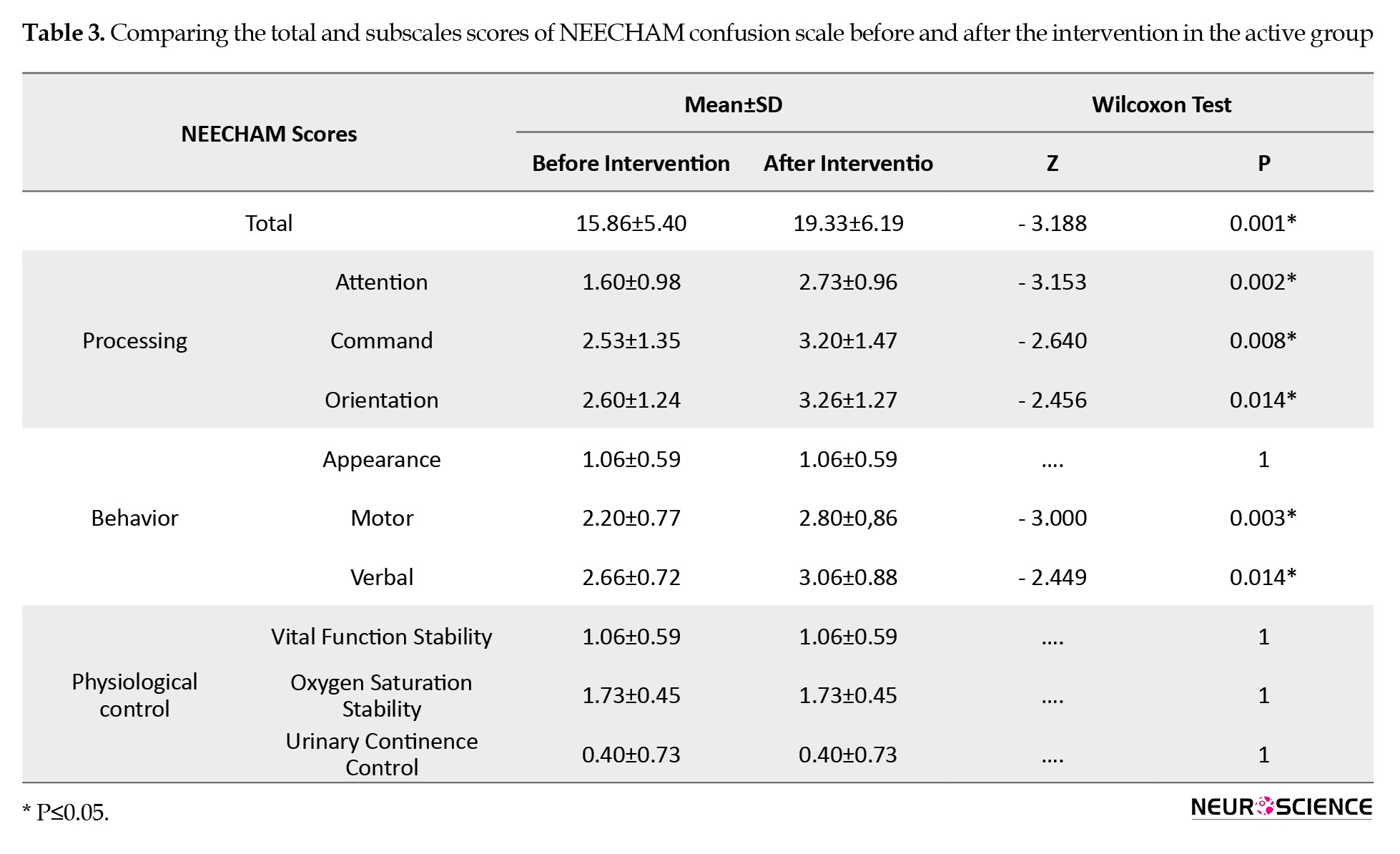
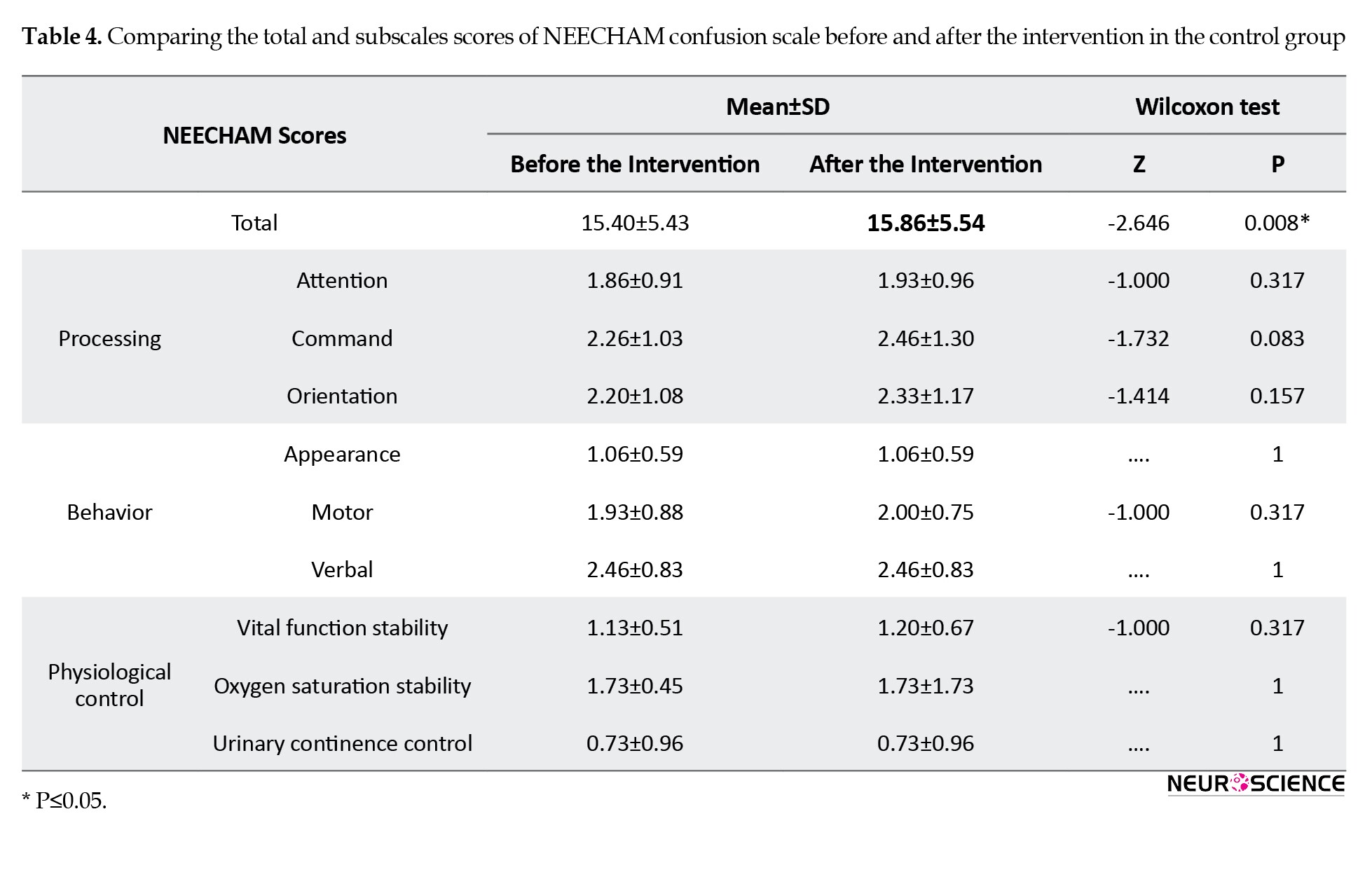
The subscale scores of NEECHAM before and after the intervention are shown in Tables 3 and 4.
In the active group, the total and subscale scores of NEECHAM significantly decreased after the intervention (P<0.05). Although no statistical difference was found in the control group in subscale scores of NEECHAM, the difference in the total scores before and after the sham intervention was statistically significant. The number of delirious patients was not different between the two groups three days after the intervention.
4. Discussion
According to this research, one session of rTMS on the left DLPFC resulted in a significant reduction of the total scores of NEECHAM and its subscale scores of attention, command, orientation, motor, and verbal responses. However, appearance, vital function stability, oxygen saturation, and urinary continence control were not significantly changed. Moreover, this intervention could not significantly reduce the delirium duration and frequency in the active group after 3 days compared to the controls.
The observed improvement in cognitive functions after one session of rTMS supported our hypothesis. This improvement can be feasible via the following phenomenon. Although the research on the effect of rTMS on delirium has not been carried out, some studies have shown the effect of rTMS on cognitive functions and some of the subscales of the NEECHAM, such as attention.
The imaging studies have elaborated on the involvement and role of the left DLPFC in executive functioning and, more specifically, in selective attention. The neurons of the DLPFC play a critical role in working memory and attention. The stimulation of this part via rTMS enhanced these fields (Mottaghy, Gangitano, Sparing, Krause, & Pascual-Leone, 2002). Furthermore, Vanderhasselt et al. reported the influence of high-frequency rTMS on the left DLPFC on improving the Stroop test scores among the volunteer healthy women (Vanderhasselt, De Raedt, Baeken, Leyman, & D’Haenen, 2006). Similarly, attention and motor function enhancement was seen in the control group in our study.
In addition, the research on the adult ADHD patients by the Bloch et al. showed that one session of high-frequency rTMS on the right DLPFC resulted in a significant advance in attention scores 10 minutes after the session. They reported a dopaminergic decrease in the prefrontal area in ADHD patients, and the rTMS causes dopamine release through prefrontal neurons (Bloch et al., 2010). This finding is consistent with our results, and the improvement of attention scores in the delirious patients 15 minutes after rTMS may be because of the increase in dopaminergic and cholinergic system activity.
In the current study, the improvement of memory scores in NEECHAM was also seen. The function of the prefrontal cortex in the long-term, short-term, and episodic memories was revealed in previous studies, particularly in encoding and retrieval tasks. Consistent with our findings, the rTMS’s significant influences on episodic memory have been debated by Sandrini et al. (Sandrini, Cappa, Rossi, Rossini, & Miniussi, 2003). In addition, the rTMS operation on DLPFC also leads to neurogenesis and the serotonin increase in the hippocampus, consequently improving emotional and cognition functionality (Juckel, Mendlin, & Jacobs, 1999).
On the other hand, we know that electroconvulsive therapy (ECT) can be an effective treatment for delirium, and through studying the ECT mechanism in treating delirium, we may be able to postulate a hypothesis about the mechanism of rTMS, too. Nielsen et al. put forward some hypotheses around the mechanism of ECT on delirium. One of them is the neuroendocrine-dysfunctional theory. According to the mentioned theory, the ECT improves the malfunction of the hypothalamus-pituitary-adrenal (HPA) axis, which is determined by the regulation of the cortisol level with its potential psychiatric features. Moreover, the ECT increases prolactin, adrenocorticotropin, and neuropeptide Y secretion (Nielsen, Olsen, Lauritsen, & Boesen, 2014). One session of high-frequency rTMS significantly influences the HPA axis like ECT, which may express the anti-delirium effects (Baeken et al., 2009). In addition, the extension of the hippocampus was observed one week after ECT via MRI, and the increase in hippocampus neurogenesis was also significant (Nielsen et al., 2014). Concordant with ECT effects, Juckel et al. showed that indirect action of TMS on the frontal cortex might cause long-term potentiation of neurons in the hippocampus (Juckel et al., 1999).
Movement was one of the other items of the NEECHAM questionnaire that significantly improved in our study. The rTMS effect on the improvement of the movement in disorders such as depression and especially Parkinson has been reported in previous studies. For example, applying the rTMS to the primary motor cortex and the DLPFC has improved movements and gait among patients with Parkinson (Helmich, Siebner, Bakker, Munchau, & Bloem, 2006). The stimulation of DLPFC may enhance motor conditions via the hyper direct pathway that connects the different regions of the brain, including the supplementary motor area, DLPFC, inferior frontal gyrus, and the sub-thalamic nucleus (Nambu, Tokuno, Inase, & Takada, 1997).
Most participants in our study were old people. While the dedicated studies into this age group are few, it has been shown that stimulation with high-frequency rTMS on the left or right DLPFC resulted in enhancement of cognitive function among the Alzheimer patients, and also some positive influences of rTMS were reported on the treatment of the depression of the old ages, the post-stroke depression, and the depression in patients with Parkinson (Cotelli et al., 2006; Epstein, Lah, Meador, Weissman, Gaitan, & Dihenia, 1996).
Although no statistical differences were found in the control group concerning the scores of NEECHAM’s subscales, the notable point of this study was the significant difference in the questionnaire’s total scores before and after the sham intervention in the control group that maybe is related to the research limitations. For example, this finding can be related to the fluctuating course of delirium symptoms that affects the results. One of the reasons for the NEECHAM score enhancement in the second evaluation is simply the time between the evaluation and talking to the patient. During this time, the patient becomes more conscious and gets better scores on the NEECHAM scale.
The other unexpected finding of this study was the unchanged number of the patients who remained delirious in both case and control groups after 3 days of interventions. This result can be due to various possible reasons. One can be the short-term effect of rTMS, and maybe for a general permanent improvement in the disorder, more rTMS sessions are needed. It should be cited that in previous studies that led to the enhancement of cognition profile among patients with depression, Alzheimer disease, and ADHD in the long-term, the number of rTMS sessions was more than one (Cao et al., 2018; Nadeau et al., 2014; Rabey, Dobronevsky, Aichenbaum, Gonen, Marton, & Khaigrekht, 2013). The other possibility is that the dual-mode variable (having or not having delirium) has insufficient sensitivity to address the cognitive modifications via one rTMS session. In other words, the severity of delirium level may decrease in the active group, but this reduction was not enough to exclude the symptoms completely. The other point that should be taken into consideration is that although having dementia was one of the excluding criteria, regarding the average age of participants (65.7 years old), there is the possibility of MCI (mild cognitive impairment) and its effects on cognition scores.
The small sample size was a limitation of this study. In addition, in the applied questionnaire of this study, there were physiologically related items such as vital signs, oxygen saturation level, and urinary incontinence that would not be expected to show significant modifications 15 minutes after the intervention. So, for the subsequent studies, it is recommended to consider the following issues: enlarging the sample size, using more rTMS sessions, and using the other questionnaires such as DOS, MSAS, and NEECHAM questionnaires. Thus, one session of rTMS on the left DLPFC can reduce the delirium severity in a short period, although it will not decrease the number of delirium cases three days after the intervention. It is necessary to carry out more studies to assess the rTMS influence on delirium severity more precisely, particularly by extending the treatment sessions and sample size.
Ethical Considerations
Compliance with ethical guidelines
The study was registered on the website of the Iranian Registry of Clinical Trials (IRCT.ir) (Code: IRCT20171223038019N1) and approved by the Ethics Committee of Iran University of Medical Sciences (Code: IR.IUMS.FMD.REC 1396.9411286017).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank all participants and their families, and hospital staff who contributed to the study.
References
Baeken, C., De Raedt, R., Leyman, L., Schiettecatte, J., Kaufman, L., Poppe, K., et al. (2009). The impact of one HF-rTMS session on mood and salivary cortisol in treatment resistant unipolar melancholic depressed patients. Journal of Affective Disorders, 113(1-2), 100-108. [DOI:10.1016/j.jad.2008.05.008] [PMID]
Bloch, Y., Harel, E. V., Aviram, S., Govezensky, J., Ratzoni, G., & Levkovitz, Y. (2010). Positive effects of repetitive transcranial magnetic stimulation on attention in ADHD Subjects: A randomized controlled pilot study. The World Journal of Biological Psychiatry, 11(5), 755-758. [DOI:10.3109/15622975.2010.484466] [PMID]
Boggio, P. S., Rigonatti, S. P., Ribeiro, R. B., Myczkowski, M. L., Nitsche, M. A., & Pascual-Leone, A., et al. (2008). A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. The International Journal of Neuropsychopharmacology, 11(2), 249-254. [DOI:10.1017/S1461145707007833] [PMID] [PMCID]
Cao, P., Xing, J., Cao, Y., Cheng, Q., Sun, X., & Kang, Q., et al. (2018). Clinical effects of repetitive transcranial magnetic stimulation combined with atomoxetine in the treatment of attention-deficit hyperactivity disorder. Neuropsychiatric Disease and Treatment, 14, 3231-3240. [DOI:10.2147/NDT.S182527] [PMID] [PMCID]
Cho, S. S., & Strafella, A. P. (2009). rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One, 4(8), e6725. [DOI:10.1371/journal.pone.0006725] [PMID] [PMCID]
Choi, S. H., Lee, H., Chung, T. S., Park, K. M., Jung, Y. C., & Kim, S. I., et al. (2012). Neural network functional connectivity during and after an episode of delirium. The American Journal of Psychiatry, 169(5), 498-507. [DOI:10.1176/appi.ajp.2012.11060976] [PMID]
Cotelli, M., Manenti, R., Cappa, S. F., Geroldi, C., Zanetti, O., & Rossini, P. M., et al. (2006). Effect of transcranial magnetic stimulation on action naming in patients with Alzheimer disease. Archives of Neurology, 63(11), 1602-1604. [DOI:10.1001/archneur.63.11.1602] [PMID]
Devlin, J. W., Fong, J. J., Howard, E. P., Skrobik, Y., McCoy, N., & Yasuda, C., et al. (2008). Assessment of delirium in the intensive care unit: Nursing practices and perceptions. American Journal of Critical Care, 17(6), 555-565. [DOI:10.4037/ajcc2008.17.6.555]
Epstein, C. M., Lah, J. J., Meador, K., Weissman, J. D., Gaitan, L. E., & Dihenia, B. (1996). Optimum stimulus parameters for lateralized suppression of speech with magnetic brain stimulation. Neurology, 47(6), 1590-1593. [DOI:10.1212/WNL.47.6.1590] [PMID]
Foroughan, M., Delbari, A., Said, S. E., AkbariKamrani, A. A., Rashedi, V., & Zandi, T. (2016). Risk factors and clinical aspects of delirium in elderly hospitalized patients in Iran. Aging Clinical and Experimental Research, 28(2), 313-319. [DOI:10.1007/s40520-015-0400-x] [PMID]
Grover, S., & Kate, N. (2012). Assessment scales for delirium: A review. World Journal of Psychiatry, 2(4), 58-70. [DOI:10.5498/wjp.v2.i4.58] [PMID] [PMCID]
Helmich, R. C., Siebner, H. R., Bakker, M., Munchau, A., & Bloem, B. R. (2006). Repetitive transcranial magnetic stimulation to improve mood and motor function in Parkinson’s disease. Journal of the Neurological Sciences, 248(1-2), 84-96. [DOI:10.1016/j.jns.2006.05.009] [PMID]
Sohrabi, M., Jannati, Y., Bagheri Nesami, M., Yazdani Charaty, J., & Mazdarani, S. (2013). [Incidence of delirium and associated factors before open heart surgery (Persian)] . Journal of Research Development in Nursing & Midwifery, 10(1), 33-42. https://nmj.goums.ac.ir/article-1-318-en.html
Juckel, G., Mendlin, A., & Jacobs, B. L. (1999). Electrical stimulation of rat medial prefrontal cortex enhances forebrain serotonin output: implications for electroconvulsive therapy and transcranial magnetic stimulation in depression. Neuropsychopharmacology, 21(3), 391-398. [DOI:10.1016/S0893-133X(98)00097-9] [PMID]
Kim, S. Y., Lee, D. W., Kim, H., Bang, E., Chae, J. H., & Choe, B. Y. (2014). Chronic repetitive transcranial magnetic stimulation enhances GABAergic and cholinergic metabolism in chronic unpredictable mild stress rat model: (1)H-NMR spectroscopy study at 11.7T. Neuroscience Letters, 572, 32-37. [DOI:10.1016/j.neulet.2014.04.033] [PMID]
Maldonado, J. R. (2008). Pathoetiological model of delirium: A comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Critical Care Clinics, 24(4), 789-856, ix. [DOI:10.1016/j.ccc.2008.06.004] [PMID]
Mannarelli, D., Pauletti, C., Grippo, A., Amantini, A., Augugliaro, V., Curra, A., et al. (2015). The role of the right dorsolateral prefrontal cortex in phasic alertness: Evidence from a contingent negative variation and repetitive transcranial magnetic stimulation study. Neural Plasticity, 2015, 410785. [DOI:10.1155/2015/410785] [PMID] [PMCID]
Michaud, C. J., Bullard, H. M., Harris, S. A., & Thomas, W. L. (2015). Impact of quetiapine treatment on duration of hypoactive delirium in critically Ill adults: A retrospective analysis. Pharmacotherapy, 35(8), 731-739. [DOI:10.1002/phar.1619] [PMID]
Miniussi, C., & Ruzzoli, M. (2013). [Transcranial stimulation and cognition (Persian)] . In: C. Miniussi, & M. Ruzzoli(Eds.), Handbook of clinical neurology (pp. 739-750). Amsterdam: Elsevier. [DOI:10.1016/B978-0-444-53497-2.00056-5] [PMID]
Morandi, A., Di Santo, S. G., Cherubini, A., Mossello, E., Meagher, D., & Mazzone, A., et al. (2017). Clinical features associated with delirium motor subtypes in older inpatients: Results of a multicenter study. The American Journal of Geriatric Psychiatry, 25(10), 1064-1071. [DOI:10.1016/j.jagp.2017.05.003] [PMID]
Mottaghy, F. M., Gangitano, M., Sparing, R., Krause, B. J., & Pascual-Leone, A. (2002). Segregation of areas related to visual working memory in the prefrontal cortex revealed by rTMS. Cerebral Cortex, 12(4), 369-375. [DOI:10.1093/cercor/12.4.369] [PMID]
Nadeau, S. E., Bowers, D., Jones, T. L., Wu, S. S., Triggs, W. J., & Heilman, K. M. (2014). Cognitive effects of treatment of depression with repetitive transcranial magnetic stimulation. Cognitive and Behavioral Neurology, 27(2), 77-87. [DOI:10.1097/WNN.0000000000000031] [PMID]
Nambu, A., Tokuno, H., Inase, M., & Takada, M. (1997). Corticosubthalamic input zones from forelimb representations of the dorsal and ventral divisions of the premotor cortex in the macaque monkey: Comparison with the input zones from the primary motor cortex and the supplementary motor area. Neuroscience Letter, 239(1), 13-16. [DOI:10.1016/S0304-3940(97)00877-X] [PMID]
Nardone, R., Tezzon, F., Holler, Y., Golaszewski, S., Trinka, E., & Brigo, F. (2014). Transcranial Magnetic Stimulation (TMS)/repetitive TMS in mild cognitive impairment and Alzheimer’s disease. Acta Neurologica Scandmavica, 129(6), 351-366. [DOI:10.1111/ane.12223] [PMID]
Nielsen, R. M., Olsen, K. S., Lauritsen, A. O., & Boesen, H. C. (2014). Electroconvulsive therapy as a treatment for protracted refractory delirium in the intensive care unit--five cases and a review. Journal of Critical Care, 29(5), 881.e1-6. [DOI:10.1016/j.jcrc.2014.05.012] [PMID]
Oh, E. S., Fong, T. G., Hshieh, T. T., & Inouye, S. K. (2017). Delirium in older persons: Advances in diagnosis and treatment. JAMA, 318(12), 1161-1174. [DOI:10.1001/jama.2017.12067] [PMID] [PMCID]
Rabey, J. M., Dobronevsky, E., Aichenbaum, S., Gonen, O., Marton, R. G., & Khaigrekht, M. (2013). Repetitive transcranial magnetic stimulation combined with cognitive training is a safe and effective modality for the treatment of Alzheimer’s disease: A randomized, double-blind study. Journal of Neural Transmission, 120(5), 813-819. [DOI:10.1007/s00702-012-0902-z] [PMID]
Ryan, D. J., O’Regan, N. A., Caoimh, R. O., Clare, J., O’Connor, M., & Leonard, M., et al. (2013). Delirium in an adult acute hospital population: Predictors, prevalence and detection. BMJ Open, 3(1), e001772. [DOI:10.1136/bmjopen-2012-001772] [PMID]
Sandrini, M., Cappa, S. F., Rossi, S., Rossini, P. M., & Miniussi, C. (2003). The role of prefrontal cortex in verbal episodic memory: rTMS evidence. Journal of Cognitive Neuroscience, 15(6), 855-861. [DOI:10.1162/089892903322370771] [PMID]
Sato, A., Torii, T., Nakahara, Y., Iwahashi, M., Itoh, Y., & Iramina, K. (2013). The impact of rTMS over the dorsolateral prefrontal cortex on cognitive processing. Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2013, 1988-1991. [DOI:10.1109/EMBC.2013.6609919] [PMID]
Setters, B., & Solberg, L. M. (2017). Delirium. Primary Care: Clinics in Office Practice, 44(3), 541-559. [DOI:10.1016/j.pop.2017.04.010] [PMID]
Trzepacz, P. T. (1999). Update on the neuropathogenesis of delirium. Dementia and Geriatric Cognitive Disorders, 10(5), 330-334. [DOI:10.1159/000017164] [PMID]
Trzepacz, P. T. (2000). Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Seminars in Clinical Neuropsychiatry, 5(2), 132-148. [DOI:10.153/SCNP00500132]
Van Rompaey, B., Schuurmans, M. J., Shortridge-Baggett, L. M., Truijen, S., Elseviers, M., & Bossaert, L. (2008). A comparison of the CAM-ICU and the NEECHAM Confusion Scale in intensive care delirium assessment: An observational study in non-intubated patients. Critical Care, 12(1), R16. [DOI:10.1186/cc6790] [PMID] [PMCID]
Vanderhasselt, M. A., De Raedt, R., Baeken, C., Leyman, L., & D’Haenen, H. (2006). The influence of rTMS over the left dorsolateral prefrontal cortex on Stroop task performance. Experimental Brain Research, 169(2), 279-282. [DOI:10.1007/s00221-005-0344-z] [PMID]
Veiga Fernandez, F., & Cruz Jentoft, A. J. (2008). [Delirium: Etiology and pathophysiology (Spanish)] . Revista Española de Geriatría y Gerontología, 43 Suppl 3, 4-12. [PMID]
Wei, L. A., Fearing, M. A., Sternberg, E. J., & Inouye, S. K. (2008). The confusion assessment method: A systematic review of current usage. Journal of the American Geriatrics Society, 56(5), 823-830. [DOI:10.1111/j.1532-5415.2008.01674.x] [PMID] [PMCID]
Zoremba, N., & Coburn, M. (2019). Acute Confusional States in Hospital. Deutsches Ärzteblatt International, 116(7), 101-106. [DOI:10.3238/arztebl.2019.0101] [PMID] [PMCID]
Delirium is a fatal but potentially reversible disorder of the central nervous system. It is characterized by changes in cognition, behavior, perception, and emotion (Setters & Solberg, 2017; Zoremba & Coburn, 2019). This disorder has three types: hyperactive, hypoactive, and mixed (Morandi et al., 2017).
The prevalence of delirium varies in different countries and among patients in various wards. According to Ryan et al. study in south Ireland, the prevalence of delirium varies between 7% and 53% (Ryan et al., 2013). Delirium affects 22% of the elderly hospitalized patients in Iran ( Foroughan, Delbari, Said, AkbariKamrani, Rashedi, & Zandi, 2016). Sometimes the delirium’s side effects remain in patients over 65 years for months. In the USA, the imposing cost on hospitals for each delirious patient is estimated at around $2500, accounting for $6,9 billion annually. Moreover, by considering the incomplete recovery of some patients after hospital discharge, needing home care services, and nurseries costs, the above-remarked expenses are doubled (Devlin et al., 2008; Wei, Fearing, Sternberg, & Inouye, 2008).
The delirium’s pathophysiology is not entirely known. However, the neuroanatomical data from neuro-imaging and reports suggest that specific brain regions such as the prefrontal cortex, fusiform cortex, and basal ganglia may have a critical role in causing delirium symptoms. According to many studies, these parts have a significant role in the final common pathway (Maldonado, 2008; Veiga Fernandez & Cruz Jentoft, 2008) that may be in charge of the main symptoms of delirium (Trzepacz, 1999).
Decreasing cholinergic and increasing dopaminergic activity also have an important role in the etiology of delirium. The cholinergic system is essential in the cognition processes (Trzepacz, 2000). Also, it has been remarked that delirium is associated with abnormal activity and mostly hyperactivity of the brain’s dopaminergic system; an imbalance between the dopaminergic and cholinergic systems may play an important role in delirium etiology (Maldonado, 2008; Trzepacz, 2000; Veiga Fernandez, et al., 2008). In addition, in recent studies, it has been found that throughout the delirium phase, the activity of the dorsolateral prefrontal cortex (DLPFC) is decreased, and the consecutive disconnection of the DLPFC with the posterior cingulate cortex may cause delirium (Choi et al., 2012; Mannarelli et al., 2015).
Currently, delirium treatment is based on pharmacological and non-pharmacological conservative treatments. The pharmacological treatments include typical and atypical antipsychotics and benzodiazepines. However, these treatments usually affect the behavioral disturbances of the patients and are not efficient for the cognition and orientation issues. Moreover, it is less recommended to treat hypoactive delirium, which does not have agitation and behavioral disturbances (Michaud, Bullard, Harris, & Thomas, 2015).
Repetitive transcranial magnetic stimulation (rTMS) is among the modern treatments that earned FDA (USA Food and Drug Administration) approval for the treatment of some disorders such as major depressive disorder (Boggio et al., 2008). Several researchers have reported that the high-frequency rTMS causes increasing excitability in the left DLPFC (Miniussi & Ruzzoli, 2013; Sato et al., 2013). Moreover, we know that rTMS has a positive impact on optimizing the dopaminergic system, extending the cholinergic system function, and improving the relevant indexes in attention deficit hyperactivity disorder (ADHD) patients (Cho & Strafella, 2009; Kim et al., 2014; Nardone, Tezzon, Holler, Golaszewski, Trinka, & Brigo, 2014).
According to what was said, the limitations of conventional treatments, considering the cognition disturbances as the main symptom of delirious patients, and the positive effect of rTMS on cognitive functions, we hypothesized that rTMS could be effective in the treatment of delirium and thus designed this study. If the study results are positive, the treatment costs decrease because of shorter hospitalization stay, fewer side effects, and optimizing patient care services.
2. Materials and Methods
Type of study and participants
This research was a randomized, double-blind controlled clinical trial. The participants were enrolled in the study from different departments of Rasoul-e-Akram Hospital in Tehran City, Iran. They received a delirium diagnosis based upon both diagnostic criteria of The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) and the confusion assessment method (CAM) by an Associate Professor of Psychiatry. If the patients lacked decision-making capacity, their legal guardian would be asked to sign an informed consent letter after explaining the study. Finally, 30 patients older than 18 years who provided an informed consent form and had no contraindication for rTMS were admitted to the study. The exclusion criteria were being intubated, having a history of dementia, having symptoms of alcohol or substance withdrawal, suffering from abnormal neurological symptoms, a recent head trauma, or other neurosurgical conditions and cerebrovascular accidents.
Study interventions
The participants were randomly allocated into two groups of active intermittent theta-burst stimulation (iTBS) and a sham group. Each group had 15 patients (Figure 1).
.jpg)
We used a Magventure MagPro X100 (Adventure, Denmark), connected to a figure-of-eight formed double 70-mm coil. Individual motor threshold (MT) for right abductor pollicis brevis muscle was determined using single-pulse TMS (transcranial magnetic stimulation) with motor evoked potentials (MEP). Left DLPFC (F3) was mapped using a 10-20 EEG system. The intervention group received one session of 600 pulses at an intensity of 80% active motor threshold on the left DLPFC area with three pulses of 50 Hz bursts delivered at 5 Hz in 30 sets of 10 bursts in 8 seconds off. The severity of delirium was assessed 15 minutes before the intervention and 15 minutes after it with the Neelon and Champagne (NEECHAM) confusion scale. Three days later, the patients were assessed for a diagnosis of delirium according to the DSM-5 criteria and CAM. Both groups received their sessions at the exact timetable from 9:00 to 13:00. Disposable hats were provided for them, and after placement of the probe, the device was adjusted according to the safety guidelines. In the control group, the coil was held at a 3 cm distance from the scalp. To avoid any possible problems in transferring process of some patients into the experiment room, the rTMS device was moved into all patients’ rooms.
It has to be cited that the patients were not banned from the standard treatment for delirium. All patients received equal and routine treatment, including a 0.5 mg haloperidol tablet at night and a 5.0 mg haloperidol intramuscular injection in case of severe agitation.
Data gathering method
Basic information registry questionnaire
The questionnaire includes name, medical diagnosis report, type of delirium, days of hospitalization, age, sex, and the file number of the patient.
NEECHAM confusion scale and confusion assessment method
The NEECHAM confusion scale as a delirium screening tool is applied to evaluate delirium severity. This questionnaire contains three subscales of information processing (attention and alertness, verbal and motor response, and memory and orientation with 0 to 14 points), behavior (general appearance and posture, sensory-motor performance, and verbal responses with 0 to 10 points), and performance (vital signs, oxygen saturation level and urinary incontinence with 0 to 6 points). The patients are divided into 4 groups based on the total acquired scores. The scores may range from 0 (minimal function) to 30 (normal function). The total needed time for scoring is 5 to 10 minutes (Grover & Kate, 2012).
Van Rompaey studied 172 patients to compare two questionnaires of NEECHAM and confusion assessment method (CAM)-ICU. He stated that the NEECHAM could diagnose delirium better than CAM-ICU and its advantage is the capacity to evaluate the delirium severity. The sensitivity and specificity of NEECHAM were estimated to be 78% and 95%, respectively (Van Rompaey,Schuurmans,Shortridge-Baggett,Truijen,Elseviers,& Bossaert, 2008). Also, Jannati reported the validity and reliability of the NEECHAM Persian version (Sohrabi, Jannati, Bagheri Nesami, Yazdani Charaty, & Mazdarani, 2013).
CAM is a standardized tool that enables clinicians and researchers to diagnose delirium in clinical and research settings. The questionnaire consists of 4 main items: 1) acute change in mental status, 2) lack of attention, 3) thought disorder, and 4) changes in awareness and consciousness. CAM has a sensitivity of 94%-100%, specificity of 90%-95%, and high inter-rater reliability (Oh, Fong, Hshieh, & Inouye, 2017).
Statistical Analysis
All collected data were analyzed using SPSS 20. Based on the Shapiro-Wilk test, the study data were not normally distributed. So, the Wilcoxon signed-rank test was used to compare the two groups.
3. Results
Thirty patients (18 males) were enrolled in the study. The Mean±SD age of the subjects was 65.7±7.66 years. There were no significant differences between groups in age and gender. Table 1 presents demographic data. Regarding the pretest scores of NEECHAM, there was no difference between the two groups (Table 2).
.jpg)

The subscale scores of NEECHAM before and after the intervention are shown in Tables 3 and 4.
In the active group, the total and subscale scores of NEECHAM significantly decreased after the intervention (P<0.05). Although no statistical difference was found in the control group in subscale scores of NEECHAM, the difference in the total scores before and after the sham intervention was statistically significant. The number of delirious patients was not different between the two groups three days after the intervention.


4. Discussion
According to this research, one session of rTMS on the left DLPFC resulted in a significant reduction of the total scores of NEECHAM and its subscale scores of attention, command, orientation, motor, and verbal responses. However, appearance, vital function stability, oxygen saturation, and urinary continence control were not significantly changed. Moreover, this intervention could not significantly reduce the delirium duration and frequency in the active group after 3 days compared to the controls.
The observed improvement in cognitive functions after one session of rTMS supported our hypothesis. This improvement can be feasible via the following phenomenon. Although the research on the effect of rTMS on delirium has not been carried out, some studies have shown the effect of rTMS on cognitive functions and some of the subscales of the NEECHAM, such as attention.
The imaging studies have elaborated on the involvement and role of the left DLPFC in executive functioning and, more specifically, in selective attention. The neurons of the DLPFC play a critical role in working memory and attention. The stimulation of this part via rTMS enhanced these fields (Mottaghy, Gangitano, Sparing, Krause, & Pascual-Leone, 2002). Furthermore, Vanderhasselt et al. reported the influence of high-frequency rTMS on the left DLPFC on improving the Stroop test scores among the volunteer healthy women (Vanderhasselt, De Raedt, Baeken, Leyman, & D’Haenen, 2006). Similarly, attention and motor function enhancement was seen in the control group in our study.
In addition, the research on the adult ADHD patients by the Bloch et al. showed that one session of high-frequency rTMS on the right DLPFC resulted in a significant advance in attention scores 10 minutes after the session. They reported a dopaminergic decrease in the prefrontal area in ADHD patients, and the rTMS causes dopamine release through prefrontal neurons (Bloch et al., 2010). This finding is consistent with our results, and the improvement of attention scores in the delirious patients 15 minutes after rTMS may be because of the increase in dopaminergic and cholinergic system activity.
In the current study, the improvement of memory scores in NEECHAM was also seen. The function of the prefrontal cortex in the long-term, short-term, and episodic memories was revealed in previous studies, particularly in encoding and retrieval tasks. Consistent with our findings, the rTMS’s significant influences on episodic memory have been debated by Sandrini et al. (Sandrini, Cappa, Rossi, Rossini, & Miniussi, 2003). In addition, the rTMS operation on DLPFC also leads to neurogenesis and the serotonin increase in the hippocampus, consequently improving emotional and cognition functionality (Juckel, Mendlin, & Jacobs, 1999).
On the other hand, we know that electroconvulsive therapy (ECT) can be an effective treatment for delirium, and through studying the ECT mechanism in treating delirium, we may be able to postulate a hypothesis about the mechanism of rTMS, too. Nielsen et al. put forward some hypotheses around the mechanism of ECT on delirium. One of them is the neuroendocrine-dysfunctional theory. According to the mentioned theory, the ECT improves the malfunction of the hypothalamus-pituitary-adrenal (HPA) axis, which is determined by the regulation of the cortisol level with its potential psychiatric features. Moreover, the ECT increases prolactin, adrenocorticotropin, and neuropeptide Y secretion (Nielsen, Olsen, Lauritsen, & Boesen, 2014). One session of high-frequency rTMS significantly influences the HPA axis like ECT, which may express the anti-delirium effects (Baeken et al., 2009). In addition, the extension of the hippocampus was observed one week after ECT via MRI, and the increase in hippocampus neurogenesis was also significant (Nielsen et al., 2014). Concordant with ECT effects, Juckel et al. showed that indirect action of TMS on the frontal cortex might cause long-term potentiation of neurons in the hippocampus (Juckel et al., 1999).
Movement was one of the other items of the NEECHAM questionnaire that significantly improved in our study. The rTMS effect on the improvement of the movement in disorders such as depression and especially Parkinson has been reported in previous studies. For example, applying the rTMS to the primary motor cortex and the DLPFC has improved movements and gait among patients with Parkinson (Helmich, Siebner, Bakker, Munchau, & Bloem, 2006). The stimulation of DLPFC may enhance motor conditions via the hyper direct pathway that connects the different regions of the brain, including the supplementary motor area, DLPFC, inferior frontal gyrus, and the sub-thalamic nucleus (Nambu, Tokuno, Inase, & Takada, 1997).
Most participants in our study were old people. While the dedicated studies into this age group are few, it has been shown that stimulation with high-frequency rTMS on the left or right DLPFC resulted in enhancement of cognitive function among the Alzheimer patients, and also some positive influences of rTMS were reported on the treatment of the depression of the old ages, the post-stroke depression, and the depression in patients with Parkinson (Cotelli et al., 2006; Epstein, Lah, Meador, Weissman, Gaitan, & Dihenia, 1996).
Although no statistical differences were found in the control group concerning the scores of NEECHAM’s subscales, the notable point of this study was the significant difference in the questionnaire’s total scores before and after the sham intervention in the control group that maybe is related to the research limitations. For example, this finding can be related to the fluctuating course of delirium symptoms that affects the results. One of the reasons for the NEECHAM score enhancement in the second evaluation is simply the time between the evaluation and talking to the patient. During this time, the patient becomes more conscious and gets better scores on the NEECHAM scale.
The other unexpected finding of this study was the unchanged number of the patients who remained delirious in both case and control groups after 3 days of interventions. This result can be due to various possible reasons. One can be the short-term effect of rTMS, and maybe for a general permanent improvement in the disorder, more rTMS sessions are needed. It should be cited that in previous studies that led to the enhancement of cognition profile among patients with depression, Alzheimer disease, and ADHD in the long-term, the number of rTMS sessions was more than one (Cao et al., 2018; Nadeau et al., 2014; Rabey, Dobronevsky, Aichenbaum, Gonen, Marton, & Khaigrekht, 2013). The other possibility is that the dual-mode variable (having or not having delirium) has insufficient sensitivity to address the cognitive modifications via one rTMS session. In other words, the severity of delirium level may decrease in the active group, but this reduction was not enough to exclude the symptoms completely. The other point that should be taken into consideration is that although having dementia was one of the excluding criteria, regarding the average age of participants (65.7 years old), there is the possibility of MCI (mild cognitive impairment) and its effects on cognition scores.
The small sample size was a limitation of this study. In addition, in the applied questionnaire of this study, there were physiologically related items such as vital signs, oxygen saturation level, and urinary incontinence that would not be expected to show significant modifications 15 minutes after the intervention. So, for the subsequent studies, it is recommended to consider the following issues: enlarging the sample size, using more rTMS sessions, and using the other questionnaires such as DOS, MSAS, and NEECHAM questionnaires. Thus, one session of rTMS on the left DLPFC can reduce the delirium severity in a short period, although it will not decrease the number of delirium cases three days after the intervention. It is necessary to carry out more studies to assess the rTMS influence on delirium severity more precisely, particularly by extending the treatment sessions and sample size.
Ethical Considerations
Compliance with ethical guidelines
The study was registered on the website of the Iranian Registry of Clinical Trials (IRCT.ir) (Code: IRCT20171223038019N1) and approved by the Ethics Committee of Iran University of Medical Sciences (Code: IR.IUMS.FMD.REC 1396.9411286017).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank all participants and their families, and hospital staff who contributed to the study.
References
Baeken, C., De Raedt, R., Leyman, L., Schiettecatte, J., Kaufman, L., Poppe, K., et al. (2009). The impact of one HF-rTMS session on mood and salivary cortisol in treatment resistant unipolar melancholic depressed patients. Journal of Affective Disorders, 113(1-2), 100-108. [DOI:10.1016/j.jad.2008.05.008] [PMID]
Bloch, Y., Harel, E. V., Aviram, S., Govezensky, J., Ratzoni, G., & Levkovitz, Y. (2010). Positive effects of repetitive transcranial magnetic stimulation on attention in ADHD Subjects: A randomized controlled pilot study. The World Journal of Biological Psychiatry, 11(5), 755-758. [DOI:10.3109/15622975.2010.484466] [PMID]
Boggio, P. S., Rigonatti, S. P., Ribeiro, R. B., Myczkowski, M. L., Nitsche, M. A., & Pascual-Leone, A., et al. (2008). A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. The International Journal of Neuropsychopharmacology, 11(2), 249-254. [DOI:10.1017/S1461145707007833] [PMID] [PMCID]
Cao, P., Xing, J., Cao, Y., Cheng, Q., Sun, X., & Kang, Q., et al. (2018). Clinical effects of repetitive transcranial magnetic stimulation combined with atomoxetine in the treatment of attention-deficit hyperactivity disorder. Neuropsychiatric Disease and Treatment, 14, 3231-3240. [DOI:10.2147/NDT.S182527] [PMID] [PMCID]
Cho, S. S., & Strafella, A. P. (2009). rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One, 4(8), e6725. [DOI:10.1371/journal.pone.0006725] [PMID] [PMCID]
Choi, S. H., Lee, H., Chung, T. S., Park, K. M., Jung, Y. C., & Kim, S. I., et al. (2012). Neural network functional connectivity during and after an episode of delirium. The American Journal of Psychiatry, 169(5), 498-507. [DOI:10.1176/appi.ajp.2012.11060976] [PMID]
Cotelli, M., Manenti, R., Cappa, S. F., Geroldi, C., Zanetti, O., & Rossini, P. M., et al. (2006). Effect of transcranial magnetic stimulation on action naming in patients with Alzheimer disease. Archives of Neurology, 63(11), 1602-1604. [DOI:10.1001/archneur.63.11.1602] [PMID]
Devlin, J. W., Fong, J. J., Howard, E. P., Skrobik, Y., McCoy, N., & Yasuda, C., et al. (2008). Assessment of delirium in the intensive care unit: Nursing practices and perceptions. American Journal of Critical Care, 17(6), 555-565. [DOI:10.4037/ajcc2008.17.6.555]
Epstein, C. M., Lah, J. J., Meador, K., Weissman, J. D., Gaitan, L. E., & Dihenia, B. (1996). Optimum stimulus parameters for lateralized suppression of speech with magnetic brain stimulation. Neurology, 47(6), 1590-1593. [DOI:10.1212/WNL.47.6.1590] [PMID]
Foroughan, M., Delbari, A., Said, S. E., AkbariKamrani, A. A., Rashedi, V., & Zandi, T. (2016). Risk factors and clinical aspects of delirium in elderly hospitalized patients in Iran. Aging Clinical and Experimental Research, 28(2), 313-319. [DOI:10.1007/s40520-015-0400-x] [PMID]
Grover, S., & Kate, N. (2012). Assessment scales for delirium: A review. World Journal of Psychiatry, 2(4), 58-70. [DOI:10.5498/wjp.v2.i4.58] [PMID] [PMCID]
Helmich, R. C., Siebner, H. R., Bakker, M., Munchau, A., & Bloem, B. R. (2006). Repetitive transcranial magnetic stimulation to improve mood and motor function in Parkinson’s disease. Journal of the Neurological Sciences, 248(1-2), 84-96. [DOI:10.1016/j.jns.2006.05.009] [PMID]
Sohrabi, M., Jannati, Y., Bagheri Nesami, M., Yazdani Charaty, J., & Mazdarani, S. (2013). [Incidence of delirium and associated factors before open heart surgery (Persian)] . Journal of Research Development in Nursing & Midwifery, 10(1), 33-42. https://nmj.goums.ac.ir/article-1-318-en.html
Juckel, G., Mendlin, A., & Jacobs, B. L. (1999). Electrical stimulation of rat medial prefrontal cortex enhances forebrain serotonin output: implications for electroconvulsive therapy and transcranial magnetic stimulation in depression. Neuropsychopharmacology, 21(3), 391-398. [DOI:10.1016/S0893-133X(98)00097-9] [PMID]
Kim, S. Y., Lee, D. W., Kim, H., Bang, E., Chae, J. H., & Choe, B. Y. (2014). Chronic repetitive transcranial magnetic stimulation enhances GABAergic and cholinergic metabolism in chronic unpredictable mild stress rat model: (1)H-NMR spectroscopy study at 11.7T. Neuroscience Letters, 572, 32-37. [DOI:10.1016/j.neulet.2014.04.033] [PMID]
Maldonado, J. R. (2008). Pathoetiological model of delirium: A comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Critical Care Clinics, 24(4), 789-856, ix. [DOI:10.1016/j.ccc.2008.06.004] [PMID]
Mannarelli, D., Pauletti, C., Grippo, A., Amantini, A., Augugliaro, V., Curra, A., et al. (2015). The role of the right dorsolateral prefrontal cortex in phasic alertness: Evidence from a contingent negative variation and repetitive transcranial magnetic stimulation study. Neural Plasticity, 2015, 410785. [DOI:10.1155/2015/410785] [PMID] [PMCID]
Michaud, C. J., Bullard, H. M., Harris, S. A., & Thomas, W. L. (2015). Impact of quetiapine treatment on duration of hypoactive delirium in critically Ill adults: A retrospective analysis. Pharmacotherapy, 35(8), 731-739. [DOI:10.1002/phar.1619] [PMID]
Miniussi, C., & Ruzzoli, M. (2013). [Transcranial stimulation and cognition (Persian)] . In: C. Miniussi, & M. Ruzzoli(Eds.), Handbook of clinical neurology (pp. 739-750). Amsterdam: Elsevier. [DOI:10.1016/B978-0-444-53497-2.00056-5] [PMID]
Morandi, A., Di Santo, S. G., Cherubini, A., Mossello, E., Meagher, D., & Mazzone, A., et al. (2017). Clinical features associated with delirium motor subtypes in older inpatients: Results of a multicenter study. The American Journal of Geriatric Psychiatry, 25(10), 1064-1071. [DOI:10.1016/j.jagp.2017.05.003] [PMID]
Mottaghy, F. M., Gangitano, M., Sparing, R., Krause, B. J., & Pascual-Leone, A. (2002). Segregation of areas related to visual working memory in the prefrontal cortex revealed by rTMS. Cerebral Cortex, 12(4), 369-375. [DOI:10.1093/cercor/12.4.369] [PMID]
Nadeau, S. E., Bowers, D., Jones, T. L., Wu, S. S., Triggs, W. J., & Heilman, K. M. (2014). Cognitive effects of treatment of depression with repetitive transcranial magnetic stimulation. Cognitive and Behavioral Neurology, 27(2), 77-87. [DOI:10.1097/WNN.0000000000000031] [PMID]
Nambu, A., Tokuno, H., Inase, M., & Takada, M. (1997). Corticosubthalamic input zones from forelimb representations of the dorsal and ventral divisions of the premotor cortex in the macaque monkey: Comparison with the input zones from the primary motor cortex and the supplementary motor area. Neuroscience Letter, 239(1), 13-16. [DOI:10.1016/S0304-3940(97)00877-X] [PMID]
Nardone, R., Tezzon, F., Holler, Y., Golaszewski, S., Trinka, E., & Brigo, F. (2014). Transcranial Magnetic Stimulation (TMS)/repetitive TMS in mild cognitive impairment and Alzheimer’s disease. Acta Neurologica Scandmavica, 129(6), 351-366. [DOI:10.1111/ane.12223] [PMID]
Nielsen, R. M., Olsen, K. S., Lauritsen, A. O., & Boesen, H. C. (2014). Electroconvulsive therapy as a treatment for protracted refractory delirium in the intensive care unit--five cases and a review. Journal of Critical Care, 29(5), 881.e1-6. [DOI:10.1016/j.jcrc.2014.05.012] [PMID]
Oh, E. S., Fong, T. G., Hshieh, T. T., & Inouye, S. K. (2017). Delirium in older persons: Advances in diagnosis and treatment. JAMA, 318(12), 1161-1174. [DOI:10.1001/jama.2017.12067] [PMID] [PMCID]
Rabey, J. M., Dobronevsky, E., Aichenbaum, S., Gonen, O., Marton, R. G., & Khaigrekht, M. (2013). Repetitive transcranial magnetic stimulation combined with cognitive training is a safe and effective modality for the treatment of Alzheimer’s disease: A randomized, double-blind study. Journal of Neural Transmission, 120(5), 813-819. [DOI:10.1007/s00702-012-0902-z] [PMID]
Ryan, D. J., O’Regan, N. A., Caoimh, R. O., Clare, J., O’Connor, M., & Leonard, M., et al. (2013). Delirium in an adult acute hospital population: Predictors, prevalence and detection. BMJ Open, 3(1), e001772. [DOI:10.1136/bmjopen-2012-001772] [PMID]
Sandrini, M., Cappa, S. F., Rossi, S., Rossini, P. M., & Miniussi, C. (2003). The role of prefrontal cortex in verbal episodic memory: rTMS evidence. Journal of Cognitive Neuroscience, 15(6), 855-861. [DOI:10.1162/089892903322370771] [PMID]
Sato, A., Torii, T., Nakahara, Y., Iwahashi, M., Itoh, Y., & Iramina, K. (2013). The impact of rTMS over the dorsolateral prefrontal cortex on cognitive processing. Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2013, 1988-1991. [DOI:10.1109/EMBC.2013.6609919] [PMID]
Setters, B., & Solberg, L. M. (2017). Delirium. Primary Care: Clinics in Office Practice, 44(3), 541-559. [DOI:10.1016/j.pop.2017.04.010] [PMID]
Trzepacz, P. T. (1999). Update on the neuropathogenesis of delirium. Dementia and Geriatric Cognitive Disorders, 10(5), 330-334. [DOI:10.1159/000017164] [PMID]
Trzepacz, P. T. (2000). Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Seminars in Clinical Neuropsychiatry, 5(2), 132-148. [DOI:10.153/SCNP00500132]
Van Rompaey, B., Schuurmans, M. J., Shortridge-Baggett, L. M., Truijen, S., Elseviers, M., & Bossaert, L. (2008). A comparison of the CAM-ICU and the NEECHAM Confusion Scale in intensive care delirium assessment: An observational study in non-intubated patients. Critical Care, 12(1), R16. [DOI:10.1186/cc6790] [PMID] [PMCID]
Vanderhasselt, M. A., De Raedt, R., Baeken, C., Leyman, L., & D’Haenen, H. (2006). The influence of rTMS over the left dorsolateral prefrontal cortex on Stroop task performance. Experimental Brain Research, 169(2), 279-282. [DOI:10.1007/s00221-005-0344-z] [PMID]
Veiga Fernandez, F., & Cruz Jentoft, A. J. (2008). [Delirium: Etiology and pathophysiology (Spanish)] . Revista Española de Geriatría y Gerontología, 43 Suppl 3, 4-12. [PMID]
Wei, L. A., Fearing, M. A., Sternberg, E. J., & Inouye, S. K. (2008). The confusion assessment method: A systematic review of current usage. Journal of the American Geriatrics Society, 56(5), 823-830. [DOI:10.1111/j.1532-5415.2008.01674.x] [PMID] [PMCID]
Zoremba, N., & Coburn, M. (2019). Acute Confusional States in Hospital. Deutsches Ärzteblatt International, 116(7), 101-106. [DOI:10.3238/arztebl.2019.0101] [PMID] [PMCID]
Type of Study: Original |
Subject:
Clinical Neuroscience
Received: 2019/05/14 | Accepted: 2020/11/14 | Published: 2022/03/1
Received: 2019/05/14 | Accepted: 2020/11/14 | Published: 2022/03/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








