Volume 10, Issue 5 (September & October 2019)
BCN 2019, 10(5): 443-450 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Saberi Moghadam S, Samsami Khodadad F, Khazaeinezhad V. An Algorithmic Model of Decision Making in the Human Brain. BCN 2019; 10 (5) :443-450
URL: http://bcn.iums.ac.ir/article-1-1502-en.html
URL: http://bcn.iums.ac.ir/article-1-1502-en.html
1- Faculty of Engineering Modern Technologies, Amol University of Special Modern Technologies, Amol, Iran.
2- Department of Computer Engineering, Faculty of Engineering, Ayatollah Amoli Branch, Islamic Azad University, Amol, Iran.
2- Department of Computer Engineering, Faculty of Engineering, Ayatollah Amoli Branch, Islamic Azad University, Amol, Iran.
Keywords: Decision-making process, Model predictive control, Memory structure, Prefrontal cortex, Hippocampus
Full-Text [PDF 1993 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Decision-making is a recurrent, ubiquitous cognitive process and a consequential part of human behavior. A decision is made out of a set of items based on specific criteria. It is widely accepted in cognitive science that damage to the frontal lobe in the brain impairs one’s ability to make a decision (Ratcliff & Rouder, 1998). The researchers are still studying the relationship between thinking and automated human activities (Barraclough, Conroy, & Lee, 2004; Hasegawa, 2000; Wang, 2008), including the control of the decision-making process. By putting the prediction horizon and control horizon of a given control system together, we can take a step forward in increasing the trust and precision of decisions (Ramírez & Camacho, 2006). In our study, the intelligent control of decision-making process based on prior experience will improve brain functionality under a new conceptual control role. It results in the promotion of a learning model based on prior knowledge.
In recent years, many efforts have been made to design various decision-making models using different kinds of neural networks such as neural networks in distributed decision making using the Kalman filter series (Gers & Schmidhuber, 2000) and fuzzy models, models which are based on primary knowledge and experience. While the field of decision-making research continues to be vibrant, several researchers, including Dawes (e.g. Hastie and Dawes, 2001), Loewenstein (e.g. Camerer et al., 2005), and Mellers (2000) have proposed that the next phase of research in this area is likely to emerge from building on recent advances in the field of neuroscience. Since 1990, neuroscience methods and decision-making were combined to investigate the nature of decision-making, brain structures, and neurological mechanisms (Niwa & Ditterich, 2008; Romo, Hernández, Zainos, Lemus, & Brody, 2002; Wong & Wang, 2006). The primary objective of this article is to predict the correct decision making path and control of these complex mechanisms, which results from human cognitive planning.
The prefrontal cortex (PFC) and hippocampus are the most critical parts of the human brain for decision making. The decision-making process contains four steps. In the first step, some initial stimuli produced by sensory inputs, excite a set of hippocampal neurons as part of the neural system. In the second step, a set of secondary stimuli arrives in the hippocampus, and the stimulusdriven neural response is produced as initial information for two entry stimulus sets in the hippocampus. In the third step, the initial information is sent to PFC. The PFC determines the additional required information and retrieves complementary information from the hippocampus (Wang, 2008). In the last step, the PFC decides the proposed controlling process in this study. However, there is a mutual communication between PFC and hippocampus with neural connectivity. This neural wiring makes closed-loop neural circuits to generate a preferred decision.
2. Model Predictive Control
This paper is based on the concept of a Model Predictive Controller (MPC) presented by Enkavi et al. (2017). MPC is used for path planning (trajectory) of autonomous actions and formation control. MPC is a proper technique for optimizing the efficiency of control systems, The MPC is a proper technique for optimizing the trajectory control problem. The MPC Controller tries to minimize the cost in each sample time by taking the initial and current states, and the optimization process calculated from the current state in the next loop.
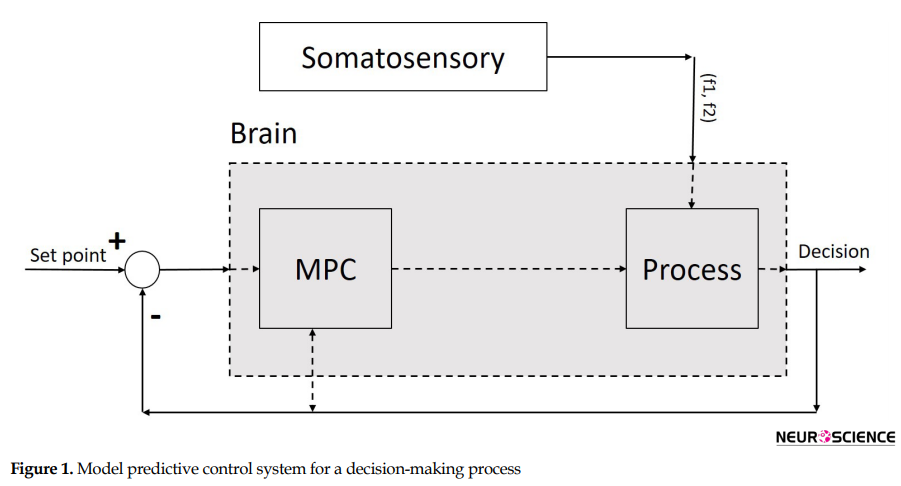
As seen in Figure 1, the MPC (controller) is used to control the process (part of the brain) to make an optimized decision on a sequence of decision items. We proposed the MPC for a class of nonlinear discrete-time systems using the constraint positively invariant sets (Figure 1). We construct the algorithm by two control modes: the state feedback mode for keeping the state (decision items) in a set (decision plan) and the MPC mode for steering the state to the set. A typical cost function in MPC in our nonlinear discrete-time control systems is as follows Formol 1, 2, 3, 4 & 5:
, where u (k), y (k),) and (k) denote the controller output, the process output, and the predicted process output, respectively at time instant k, r (k) is the desired output or the set points (desired decision items), and H (P) is the predicted horizon step. At time k, MPC solves an optimal control problem over a finite future horizon of Hp step and receives a new controller output sequence u(k+1) and repeats the optimization at time k+1 by minimization of J concerning u (k) and soon.
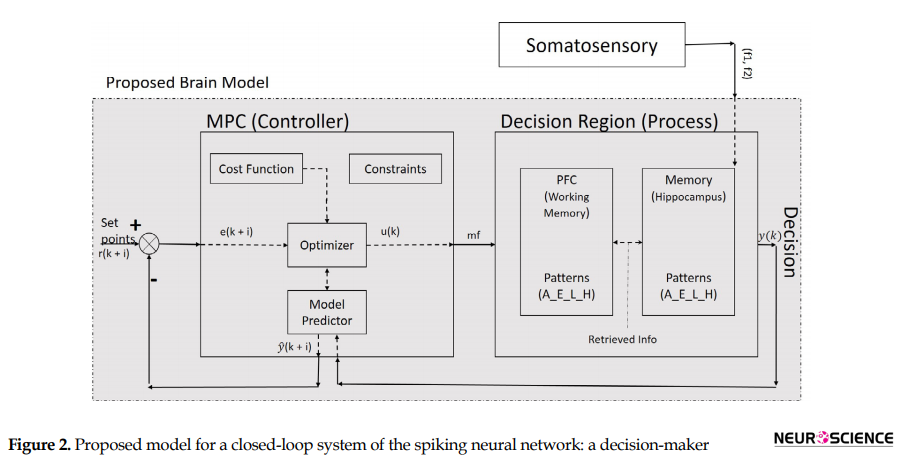
The main problem to be solved here is to find a model, which could choose proper decision items in the decision plan and steer it in a state space from its current location (status) to its goal. Status is the understanding of a goal in control iterations (Figure 2). The state space is all possible states in the problem environment.
3. The proposed model
Since the decision region (Figure 2) is related to the hippocampus and PFC (Weilbächer & Gluth, 2016), our process contains a set of neurons of both PFC and hippocampus. Likewise, the offered model contains a controller and set of the neural network as a process, which is interrelated and has nonlinear and dynamic parts. The model has a long-term memory (hippocampus), which maintains the classified sensory information (Enkavi et al., 2017). It can retrieve the classified information in PFC based on the properties of neurons. The input of the proposed process is sensory stimulation, and its output is a discrete signal, which contains decision item types. Therefore, the offered model with a dynamic property has a structure based on saved knowledge and experience which results in the next decision. In other words, the output of the MPC controller, as a controlling signal input of our process block (Figure 1) and changes in our neural network weights is based on experience analogous to human memory.
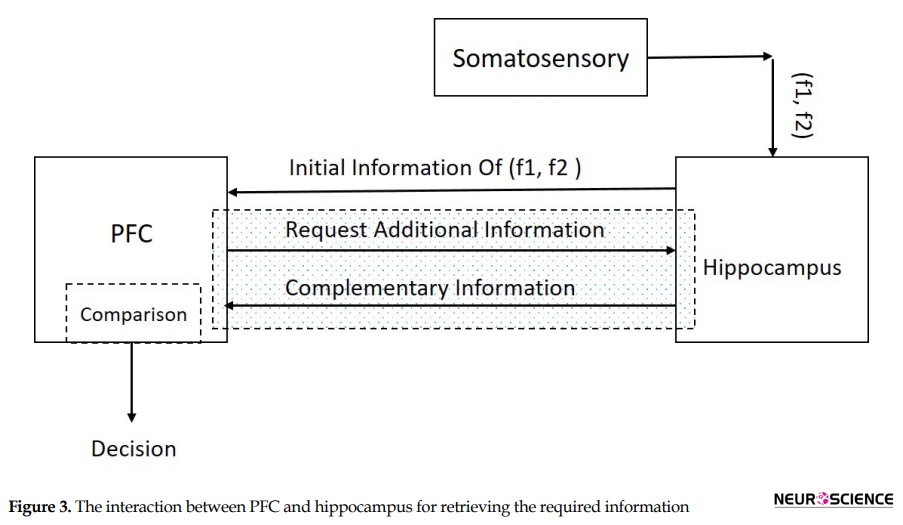
Suppose a sequence of decision items (desired set points) like (A-E-L-H) are created and classified based on forming cognitive planning. Figure 2 illustrates that decision-making could result from a comparison between the sensory stimuli of two frequencies (f1 and f2) in the specific area of the brain in the decision region (Haegens, Nácher, Luna, Romo, & Jensen, 2011). In this paper, the decision region is composed of the hippocampus and PFC and neural connectivity’s between them (Figure 3).
While some interactive signals interchange through the main parts of our model, the decision is made. The first part of our model, which takes the initial signal from the somatosensory system is hippocampus. Hippocampus is in pre-exited mode and waits for stimulation from somatosensory area. When f1 reaches the permanent memory in the hippocampus, the neural network transients to the exciting or loading mode (Wang, 2008). After a few seconds, the second sensory stimulus from somatosensory area (f2) reaches to the hippocampus. The first and secondary stimulus (f1 & f2) create a neural response in the hippocampus (Figure 3). The PFC would be aware of the situation by taking this neural response as initial information.
The PFC goes to comparison mode by receiving the initial information. The PFC based on this received information sends a request to hippocampus for additional information. Hippocampus responses with appropriate saved knowledge as complementary information to the working memory in the PFC. This mutual communication (request and response) is called a retrieval mechanism (the hachure region in Figure 3). The retrieved knowledge contains decision-items such (A-E-L-H). These patterns maintained in working memory as a set point. For example, item A is our first target in a decision plan. The decision plan is controlled by the output of the MPC system, as a controlling signal, which is called the modified frequency (MF).
The MPC controller works under constraints, and cost function needs to find the best-predicted items closest to the desired set points (decision plan [A-E-L-H]). Therefore, it generates multiple future scenarios in a systematic way where an optimizer comes into the MPC controller block. A copy of the decision-items (output of process) goes to the MPC and is compared to the desired set points. By solving an optimization problem, the MPC controller tries to minimize the difference between the desired set points and predicted items of decision items (A-E-L-H).
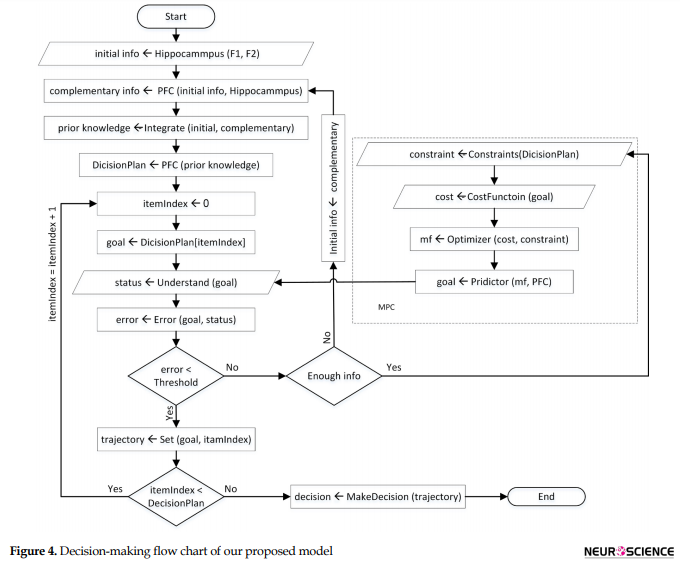
The output of the MPC block produces the future controlling signal over the prediction horizon and predict the MF, which drives brain response to make a correct decision. The cost function of this optimization problem is represented as a weighted squared sum of the predicted errors (Eq. 1). Moreover, MPC ensures that the MF and correct decision making stays within pre-determined limits (Eq. 5). These are referred to as constraints such as the range of amplitude and frequency of brain responses during the decision process. Generally, the MPC controller is solving the optimization problem over the prediction horizon while satisfying the constraints. The predicted item with the smallest cost function gives the optimal solution and therefore determines the optimal MF (outputs of MPC) that will get the predicted item as close as possible to the desired set points. Figure 4 shows the algorithmic modeling for the process of decision making. This flow chart represents our proposed model systematically.
In other words, a combination of model responses (saved knowledge), past controlling signal (MF), desired set points (decision plan), and process output create the future output process, and finally, the future decision is made. Therefore, in the control process, we can obtain a new desired set points signal in each feedback loop. It means that if we want to go from item (A) to (E), we choose (E) as the desired set points, and we try to reach decision item (E) based on prediction horizon and MPC control horizon. Then in the next closed loop, we choose a new-desired set point (L), and the purpose is to reach a new decision point.
4. Discussion
It is believed that damage to the brain frontal lobe may impair one’s ability to think and make decisions. The precise process of the prefrontal cortex is still unknown. In this paper, by offering a universal algorithmic model, we replaced the non-specific area of the brain with an MPC controller to mimic the frontal lobe function. To make a decision, we have assumed four main activities that may be part of the control design, including internal and external properties of neuronal subpopulations. MPC is planning to develop the model with more stability and robustness against disturbances and noise.
The proposed model has the predictive and control horizons to optimize future decision plan with higher accuracy compared with the other methods. Since the human brain has a set of neurons with corresponding complexity, it seems that the decision-making and cognitive process of the brain can solve this complexity in an advanced control block. Therefore, to reach this horizon, we tried to combine both hippocampus and prefrontal cortex parts (decision area) and consider this combination as a model process and take advantage of MPC for this model. In other words, model process is defined with the neural wiring between cortical and subcortical networks in a closed-loop control system. It is worth mentioning that the change of electrical properties of neural responses (frequency and amplitude) create some specific information in decision area. The change of these properties determines a decision signal in a comparison mode.
Ethical Considerations
Compliance with ethical guidelines
There is no ethical principle to be considered doing in this research.
Funding
This work was financially supported by the Amol University of Special Modern Technology, Amol, Iran (Grant No. 9820).
Authors' contributions
All authors contributed in preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
- Barraclough, D. J., Conroy, M. L., & Lee, D. (2004). Prefrontal cortex and decision making in a mixed-strategy game. Nature Neuroscience, 7(4), 404-10. [DOI:10.1038/nn1209] [PMID]
- Camerer, C., Loewenstein, G., & Prelec, D. Neuroeconomics: How neuroscience can inform economics. Journal of economic Literature. 2005; 43(1):9-64.
- Enkavi, A. Z., Weber, B., Zweyer, I., Wagner, J., Elger, C. E., & Weber, E. U., et al. (2017). Evidence for hippocampal dependence of value-based decisions. Scientific Reports, 7(1), 17738. [DOI:10.1038/s41598-017-18015-4] [PMID] [PMCID]
- Gers, F. A., & Schmidhuber, J. (2000). Recurrent nets that time and count. In Proceedings of the IEEE-INNS-ENNS International Joint Conference on Neural Networks. IJCNN 2000. Neural Computing: New Challenges and Perspectives for the New Millennium. Institute of Electrical and Electronics Engineers, 3(4), 189-94. [DOI:10.1109/IJCNN.2000.861302]
- Haegens, S., Nácher, V., Luna, R., Romo, R., & Jensen, O. (2011). α-Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. proceedings of the National Academy of Sciences, 108(48), 19377-82. [DOI:10.1073/pnas.1117190108] [PMID] [PMCID]
- Hasegawa, I. (2000). Neural mechanisms of memory retrieval: role of the prefrontal cortex. Reviews in the Neurosciences, 11(2-3), 113-26. [DOI:10.1515/REVNEURO.2000.11.2-3.113] [PMID]
- Hastie, R., & Dawes, R. (2001). The psychology of judgment and decision making. Thousand Oaks, CA: Sage.
- Hsu, M., Bhatt, M., Adolphs, R., Tranel, D., & Camerer, C. F. (2005). Neural systems responding to degrees of uncertainty in human decision-making. Science, 310(5754), 16801683.
- Niwa, M., & Ditterich, J. (2008). Perceptual decisions between multiple directions of visual motion. Journal of Neuroscience, 28(17), 4435-45. [DOI:10.1523/JNEUROSCI.5564-07.2008] [PMID] [PMCID]
- Mellers, B. A. (2000). Choice and the relative pleasure of consequences. Psychological Bulletin, 126(6), 910.
- Ramírez, D. R., & Camacho, E. F. (2006). Piecewise affinity of min-max MPC with bounded additive uncertainties and a quadratic criterion. Automatica, 42(2), 295-302. [DOI:10.1016/j.automatica.2005.09.009]
- Ratcliff, R., & Rouder, J. N. (1998). Modeling response times for two-choice decisions. Psychological Science, 9(5), 347-56. [DOI:10.1111/1467-9280.00067]
- Romo, R., Hernández, A., Zainos, A., Lemus, L., & Brody, C. D. (2002). Neuronal correlates of decision-making in secondary somatosensory cortex. Nature Neuroscience, 5(11), 1217. [DOI:10.1038/nn950] [PMID]
- Wang, X. J. (2008). Decision making in recurrent neuronal circuits. Neuron, 60(2), 215-34. [DOI:10.1016/j.neuron.2008.09.034] [PMID] [PMCID]
- Weilbächer, R. A., & Gluth, S. (2016). The interplay of hippocampus and ventromedial prefrontal cortex in memory-based decision making. Brain Sciences, 7(1), 4. [DOI:10.3390/brainsci7010004] [PMID] [PMCID]
- Wong, K. F., & Wang, X. J. (2006). A recurrent network mechanism of time integration in perceptual decisions. Journal of Neuroscience, 26(4), 1314-28. [DOI:10.1523/JNEUROSCI.3733-05.2006] [PMID] [PMCID]
Type of Study: Original |
Subject:
Computational Neuroscience
Received: 2019/05/13 | Accepted: 2019/08/17 | Published: 2019/09/1
Received: 2019/05/13 | Accepted: 2019/08/17 | Published: 2019/09/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |