Volume 11, Issue 4 (July & August - Special Issue on Memory, Reward & Stress 2020)
BCN 2020, 11(4): 413-422 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Nozari M, Nahavandi A, Zeinivand M, Eslami Gharaati M, Godarzi M, Ahmadi M et al . Ibuprofen Protection Against Restrained Chronic Stress-induced Depression in Male Rats. BCN 2020; 11 (4) :413-422
URL: http://bcn.iums.ac.ir/article-1-1472-en.html
URL: http://bcn.iums.ac.ir/article-1-1472-en.html
Masoumeh Nozari1 

 , Arezo Nahavandi2
, Arezo Nahavandi2 

 , Motahareh Zeinivand *3
, Motahareh Zeinivand *3 

 , Maryam Eslami Gharaati3
, Maryam Eslami Gharaati3 

 , Mina Godarzi3
, Mina Godarzi3 
 , Mohammad Ahmadi4
, Mohammad Ahmadi4 
 , Nida Jamali-Raeufy3
, Nida Jamali-Raeufy3 




 , Arezo Nahavandi2
, Arezo Nahavandi2 

 , Motahareh Zeinivand *3
, Motahareh Zeinivand *3 

 , Maryam Eslami Gharaati3
, Maryam Eslami Gharaati3 

 , Mina Godarzi3
, Mina Godarzi3 
 , Mohammad Ahmadi4
, Mohammad Ahmadi4 
 , Nida Jamali-Raeufy3
, Nida Jamali-Raeufy3 


1- Neuroscience Research Center, Institute of Neuropharmacology, Kerman University of Medical Sciences, Kerman, Iran.
2- Department of Neuroscience, Faculty of Advanced Technologies in Medicine, Iran University of Medical Sciences, Tehran, Iran.
3- Department of Physiology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
4- Neuroscience Research Center, Iran University of Medical Sciences, Tehran, Iran.
2- Department of Neuroscience, Faculty of Advanced Technologies in Medicine, Iran University of Medical Sciences, Tehran, Iran.
3- Department of Physiology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
4- Neuroscience Research Center, Iran University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 845 kb]
| Abstract (HTML)
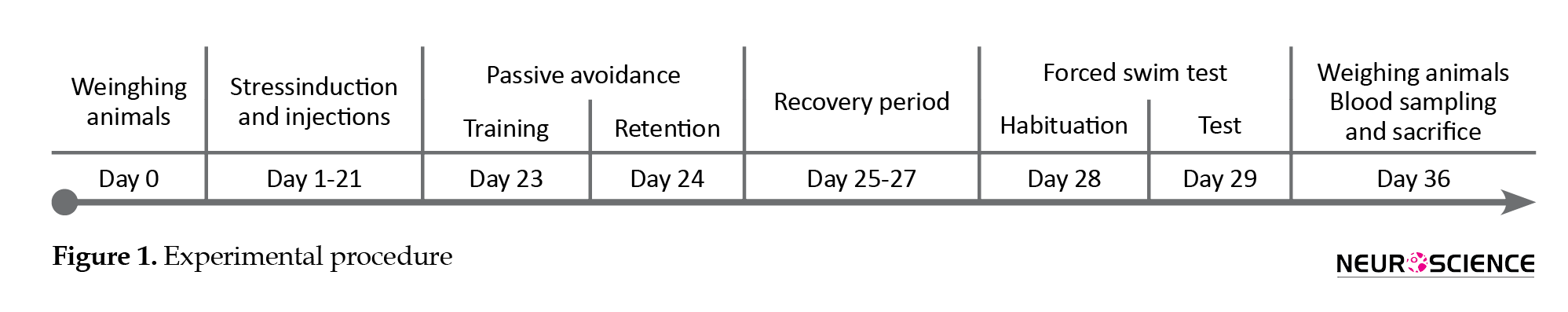
2.2. Experimental procedures
As illustrated in Figure 1, the animals were weighed before any handling (on day 0). The animals of the stress group were daily injected with ibuprofen or vehicle for 21 consecutive days (day 1-21). Also, 30 minutes after each injection, they were restrained for 6 hours (8 AM to 2 PM) with the standard rat Plexiglas restrainers (Campos, Fogaça, Aguiar, & Guimaraes, 2013). While the restrained rats were completely immobile with no access to food and water, the rats of the sham group were not subjected to stress and returned to their home cages after the vehicle injection. On day 23, the animals were trained for passive avoidance in the shuttle box and tested on day 24.
On day 28, the subjects were habituated for the FST that was performed on day 29. Ultimately, on day 36, the animals were weighed and deeply anesthetized with ketamine. The blood samples were taken for the assessments of IL-6 and corticosterone plasma levels with the Enzyme-Linked Immunosorbent Assay (ELISA) method. The animals were euthanized afterward. All the experiments were carried out by two observers who were blinded to the treatments.
2.3. Passive Avoidance Memory test
The custom-built shuttle box was made of two wooden compartments (each, 30×20×20 cm) separated by a guillotine door (20×15 cm). One of the boxes was equipped with a 20 W lamp, but the other one was dark and had a grid floor (3 mm diameter, 10 mm distance). For habituation, the animals were placed in the bright part and the guillotine door was opened after five seconds. Once the animal passed through the door, it was closed and the animal was returned to the home cage, after 20 seconds. Thirty minutes later, one trial training session was performed similar to the habituation procedure, but once the animal entered the dark compartment, it received a foot-shock (50 Hz, 1 mA, 3 s). Then, the animal was returned to the home cage. On the next day, the animal was placed in the bright compartment and the guillotine door was opened after 20 seconds. The latency time to enter the dark compartment was measured with a stopwatch; the cut off time was 300 seconds (Hosseinzadeh, Roshan, & Pourasghar, 2013; Barzegar et sl., 2015).
2.4. Forced Swimming test
Pretest: The animals were individually placed at a depth of 18 cm in a transparent cylinder (80 cm height, 30 cm diameter) filled with 24°C water, for 15 minutes. Then, they were dried with a towel and returned to their cages.
Swim test: At the same conditions as the pretest, the animals were placed in the cylinder, and the video was recorded. After five minutes, they were dried and returned to their cages. Cumulative immobility duration was measured by two observers who were blinded to the treatments (Cryan, Valentino, & Lucki, 2005; Slattery & Cryan, 2012).
2.5. IL-6 ELISA
The animals were recovered for a week to avoid transient increments in the IL-6 levels because of the behavioral experiments. Next, the rats were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg), then, the heart apex was exposed and blood samples (1 mL) were taken. The samples were centrifuged (10 min, 664×g), and the plasma was stored at -20°C. The plasma IL-6 levels were quantified using commercially available ELISA kit (eBioscience, Vienna, Austria), according to manufacturer’s instructions.
2.6. Corticosterone ELISA
The rat blood samples were centrifuged at 664×g for 10 minutes. The sera were immediately separated and stored at -80oC until the examination. The corticosterone level was measured using the ELISA kit (Zellbio GmbH, Germany), according to the manufacturer's protocol.
2.7. Transmission electron microscopy
Two rats of each group were anesthetized with the intraperitoneal injection of ketamine-xylazine combination (10:1) and perfused intracardially with 0.9% sodium chloride, 10X phosphate-buffered saline, and 4% paraformaldehyde. Then, they were decapitated and their brains removed. After tissue processing, 5-µm paraffin-embedded sections were provided. These structures become visualized in the electron microscope at the magnifications range of 50 to 1000000 times. The protocols given hereafter can be used for light microscopy and transmission electron microscopy.
2.8. Statistical analysis
Values were expressed as Mean±Standard deviation. The SigmaPlot-14 (Systat Software Inc.) was used to analyze the obtained data. To analyze variations among three or more groups, the one-way analysis of variance followed by the Tukey test for post hoc analysis was performed. The P-value of less than 0.05 (P<0.05) was considered statistically significant.
3. Results
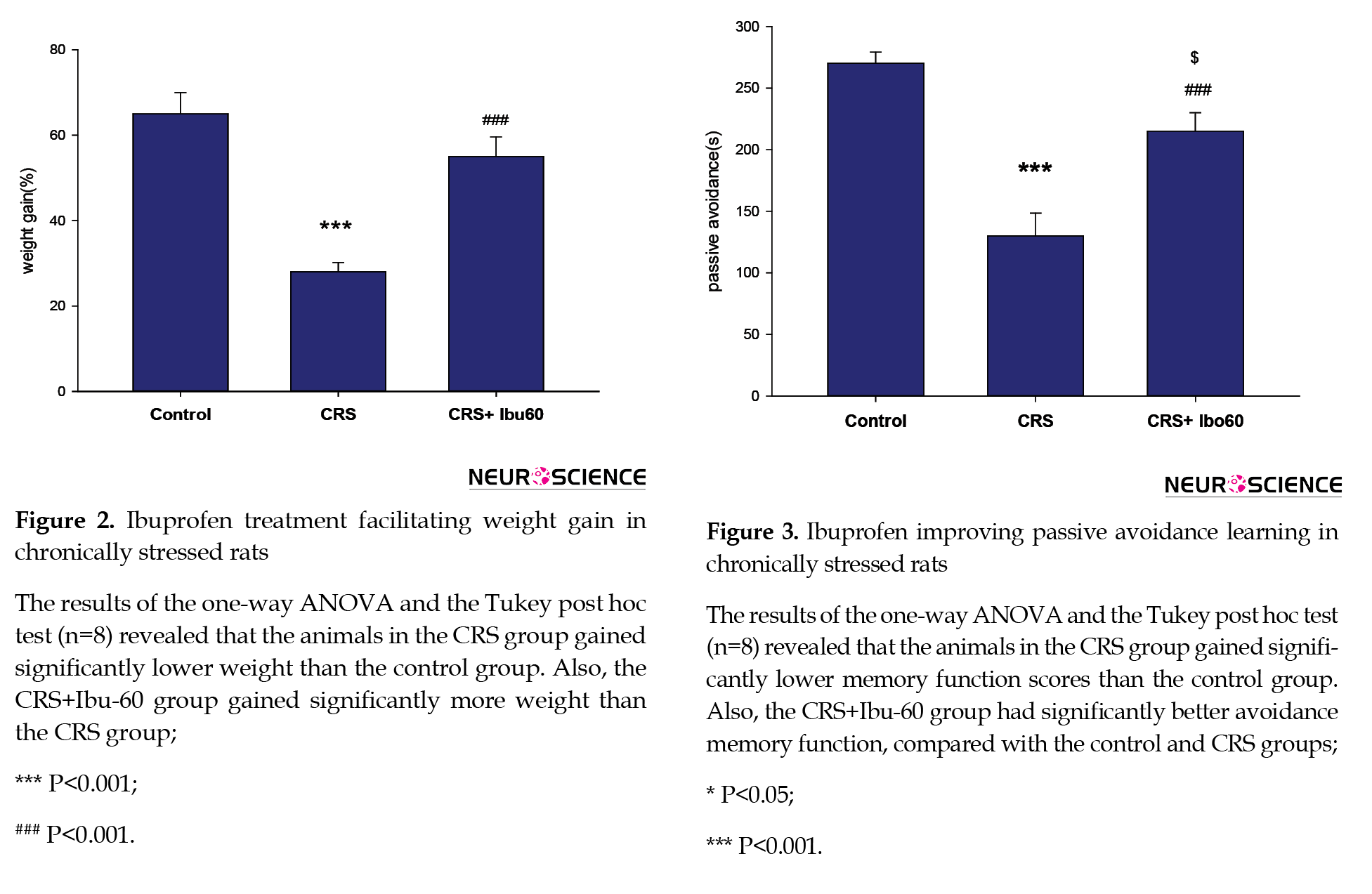
3.1. Ibuprofen protects against stress-induced weight loss
According to Figure 2, chronic stress disturbs normal weight gain, and ibuprofen is preventive against these changes. The stress group had the smallest weight gain, while the CRS+Ibu-60 group showed a high mean of weight gain. The CRS+Ibu-60 group had significantly higher weight gain, compared with the stress group (F6, 35= 6.46, P<0.001).
3.2. Ibuprofen improves stress-induced learning deficits
Chronic stress impairs learning processes and negatively impacts learning avoidance (Chida, Sudo, Mori, & Kubo, 2006; Radahmadi, Alaei, Sharifi, & Hosseini, 2013). Here, chronically stressed animals that were treated with a high dose of Ibuprofen (60 mg/kg) significantly improved avoidance performance. Compared with the control (P<0.05) and CRS (P<0.001) groups, the CRS+Ibu-60 group had significantly (F6, 35= 15.60, P<0.001) better performance in avoidance memory (Figure 3).
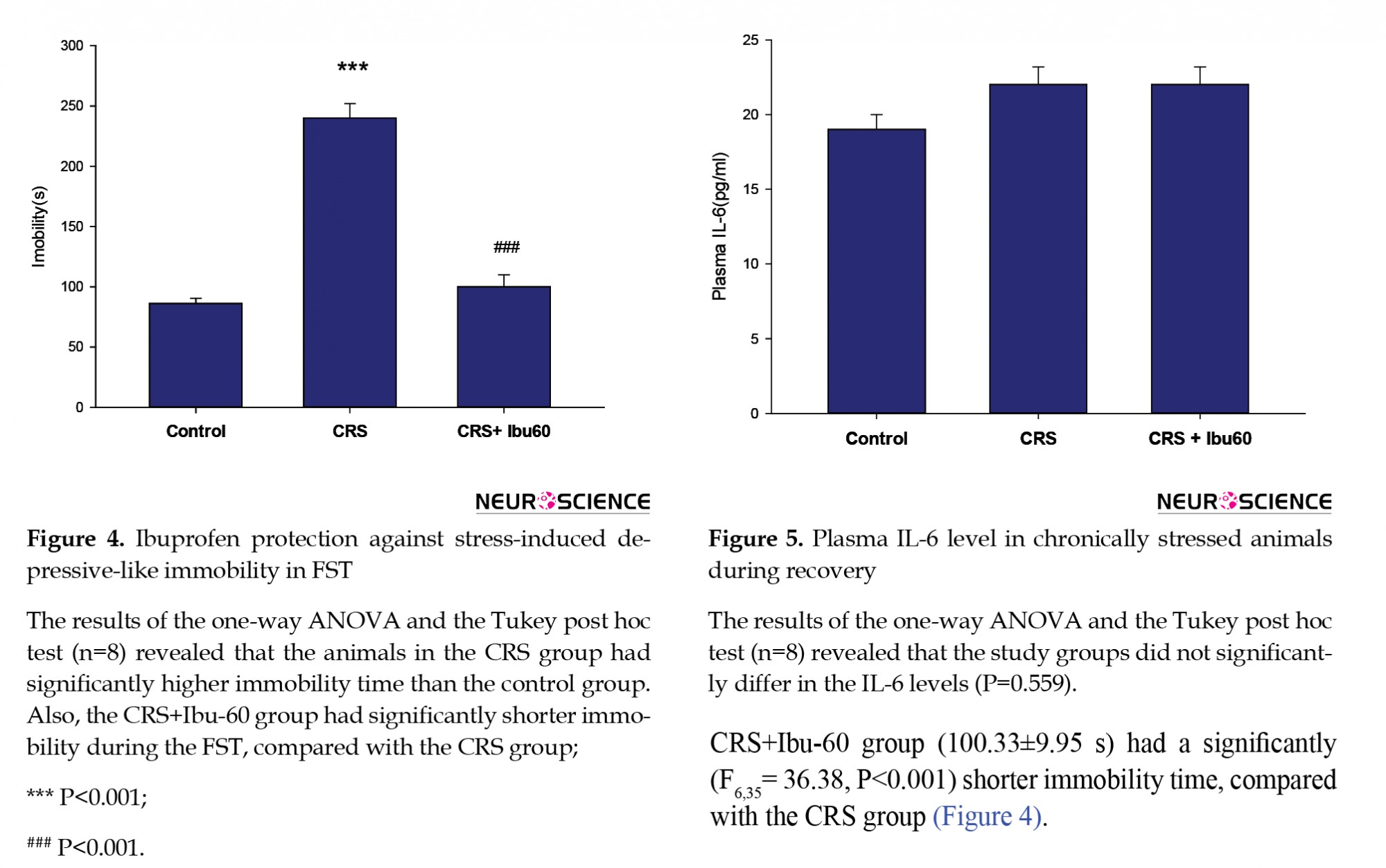
3.3. Ibuprofen prevents depressive-like immobility in the FST
The FST is one of the most frequently used tests for both the assessments of depressive-like behavior in rodents and the antidepressant-like properties of the pharmacological agents (Cryan, Valentino, & Lucki, 2005; Slattery & Cryan, 2012). Compared with the control group (217.83±25.40 s), the CRS group showed a higher immobility time (86.50±5.49 s, P<0.001). However, the CRS+Ibu-60 group (100.33±9.95 s) had a significantly (F6,35= 36.38, P<0.001) shorter immobility time, compared with the CRS group (Figure 4).
3.4. Plasma IL-6 level
The one-way ANOVA did not indicate any significant difference in the plasma levels of IL-6 between the study groups (F6,35=0.824, P=0.559; Figure 5).
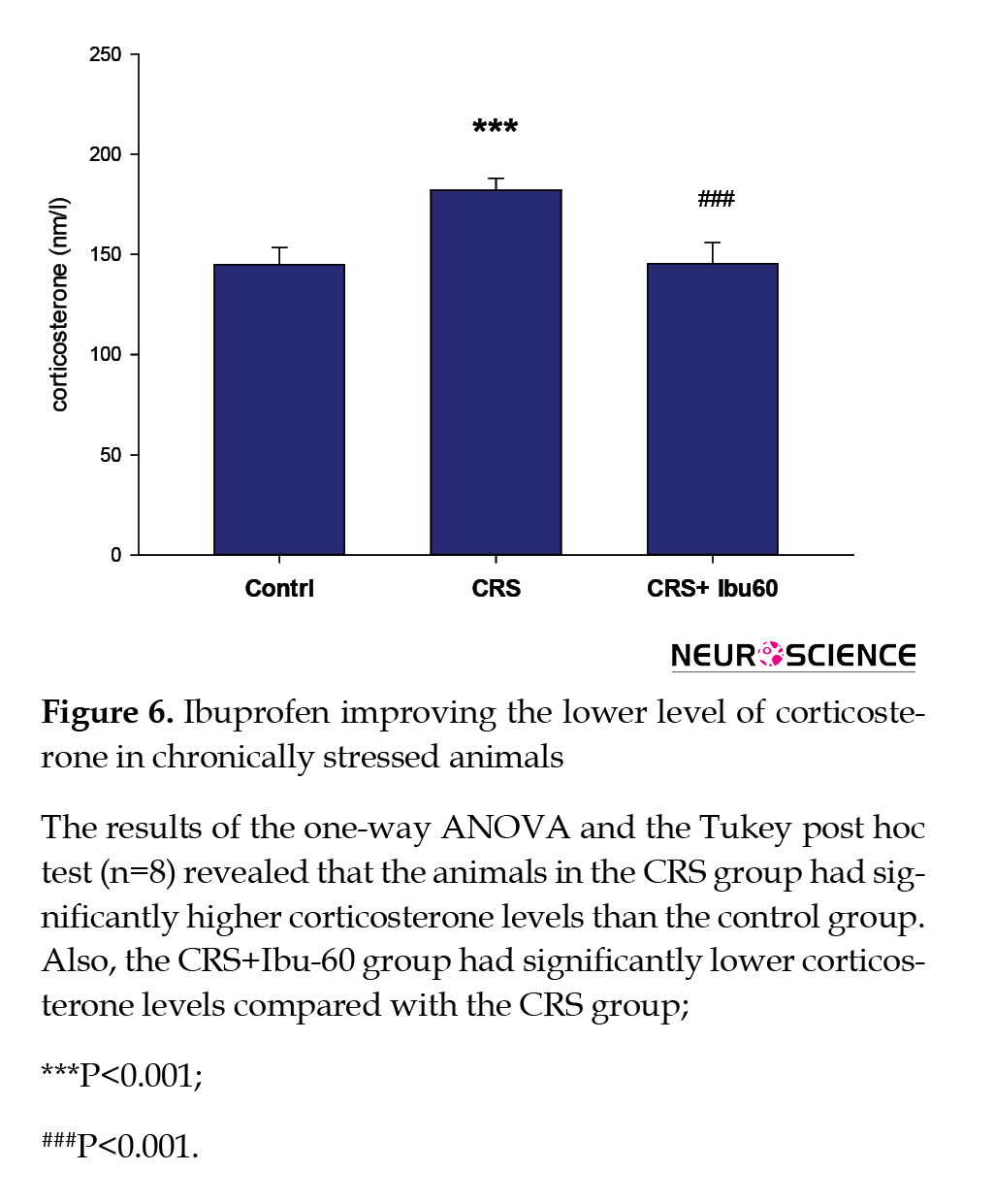
3.5. Plasma corticosterone level
Compared with the control group (217.83±25.40 s), the CRS group had a significantly higher plasma corticosterone level (86.50±5.49 s, P<0.001). However, the corticosterone plasma level of the CRS+Ibu-60 group (100.33±9.95 s ) was significantly (F6,35=33.38, P<0.001) lower than the CRS group (Figure 6).
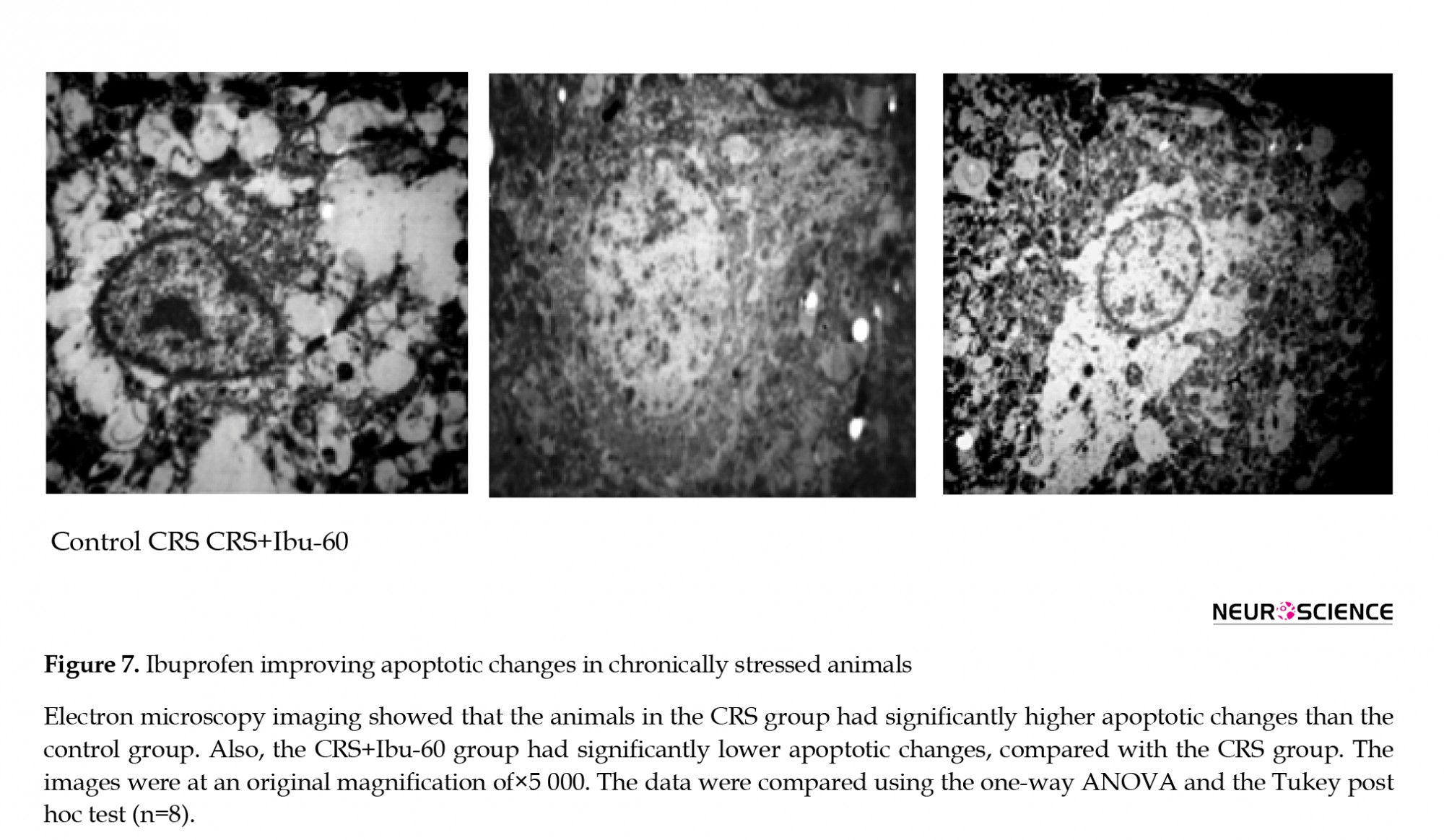
3.6. Ibuprofen protects against stress-induced apoptotic changes
The transmission electron microscopy results of the CA1 region showed apoptotic changes, including the chromatin margination and dissolution in the cell nucleus of stressed rats. However, we found the large and round nuclei and the intracellular organelles within the cytoplasm of both the control and CRS+Ibu-60 groups (F6, 35= 36.27, P<0.001; Figure 7).
4. Discussion
The present study is the first research investigating the preventive effect of ibuprofen on chronic stress-induced depressive-like behavior in rats. Our results provided a better insight into the stress-induced depressive-like behaviors and examined the potential of ibuprofen as a preventive medication during chronic stress. In the present study, a dose-dependent manner of ibuprofen administration showed that the highest dose of ibuprofen (60 mg) had the best efficiency, and prevented all the stress-induced symptoms. However, the moderate dose (30 mg) just improved the immobility behavior during the FST. Also, the lowest dose of ibuprofen (15 mg) had no detectable effect on any measurements. Therefore, we focused on the highest dose of ibuprofen (60 mg/kg) in the following. It should be emphasized that ibuprofen plays an anti-inflammatory role in higher doses, which is seen over the weeks following the administration.
Ibuprofen plays a role both as an anti-inflammatory and a neuroprotective agent in conditions, such as Parkinson and Alzheimer diseases (Wilkinson et al., 2012). Also, chronic ibuprofen administration can reverse depressive behavior in the Parkinson disease model of the rats (Zaminelli et al., 2014). Restraint-induced stress results in oxidative stress, inflammation, and depressive-like symptoms (Gárate et al., 2013). Ibuprofen as a non-specific COX inhibitor may protect against restraint-induced chronic stress via the blockage of this process (Mancuso et al., 2007). In response to stressors, there is a fundamental, dynamic, and reciprocal relationship between the corticosterone and the immune system (Bowers, Bilbo, Dhabhar, & Nelson, 2008). Following the stressors, pro-inflammatory cytokines, such as tumor necrosis factor-α increase, and result in the overactivity of the HPA axis. The increased corticosterone would suppress the high levels of inflammatory modulators (Leakey et al., 1994). Consequently, both the corticosterone and the inflammatory modulators rise (Besedovsky et al., 1991).
The normal body weight gain of the rats was reduced by the CRS paradigm; this result is in line with the previous reports about the impaired weight gain induced by depression following the CRS (Christiansen, Olesen, Wörtwein, & Woldbye, 2011). Chronic restraint stress increases the activity of the HPA axis and induces the signs and symptoms of depression, such as decreased appetite and reduced body weight gain (Kaestner et al., 2005).
In the present study, we found an increased immobility behavior in the CRS group, compared with the control and CRS+Ibu-60 groups. Immobility behavior could be translated into behavioral despair (Cryan & Mombereau, 2004). The restraint-induced chronic stress and depression result in the various modifications of the brain, including decreased dopamine level and reduced hippocampal volume and plasticity, which are almost the result of neuroinflammation (Lee et al., 2013).
Abnormal HPA axis activity is correlated with stress-related conditions, such as mental depression (Chang et al., 2009). In such conditions, the HPA activity is usually exaggerated (Chang et al., 2009). As mentioned earlier, the activity of the inflammatory modulators increased HPA activity. It seems that the high dose of ibuprofen has interrupted this loop and decreased behavioral despair. The high dose of ibuprofen could improve the passive avoidance task in the rat depression model. Hippocampus is the main organ involved in learning and memory and is susceptible to inflammation, apoptosis, and damage following the mental depression. The hippocampus function is best evaluated by the passive avoidance task (Ho, Sommers, & Lucki, 2013). Our findings indicated a normal passive avoidance task in the CRS+Ibu-60 group, compared with the control group. Observing a functional hippocampus in the rats of both intact group and CRS+Ibu-60 group indicates the preventive effect of Ibu 60 on hippocampal damage in stressful situations.
We found no significant increase in plasma IL-6 levels in our stress-induced model. Explaining this result, it is suggested that the time of sampling has been overlapped with the decrement of IL-6 by the first surge of corticosterone. The short course of stress induction (21 days) in our model may be another possible reason. It seems that the rats required more duration and the time of the CST to develop high levels of IL-6. Several studies have shown that chronic neuro-inflammatory and depressive-like behavior are essential for the elevation of plasma IL-6 (Voorhees et al., 2013). Another study confirmed the IL-6 increase following 28 days of restraint-induced stress in mice (Biesmans et al., 2015). It seems that 21 days of restraint stress is not an appropriate protocol for the IL-6 studies. Possibly, if we continued this phase, we could see the plasma IL-6 increment. The three-week protocol carried out in the present study explains why we could not find a significant increase in IL-6.
The electron microscope findings showed the signs of apoptotic changes in the CA1 area of the CRS group. Mental depression as a neuro-inflammatory process is associated with apoptosis, and the hippocampus is one of the most susceptible organs for such changes (Zunszain, Anacker, Cattaneo, Carvalho, & Pariante, 2011). Although there are reports about the apoptotic effect of ibuprofen in various conditions, a study revealed that all the NSAIDs, including ibuprofen, inhibit the nitric oxide-induced apoptosis in a non-cyclooxygenase-dependent manner in chondrocytes (Borutaite & Brown, 2005). The NSAIDs inhibit the nitric oxide-induced apoptosis and the differentiation of articular chondrocytes independent of cyclooxygenase activity (Borutaite & Brown, 2005).
Currently, there are controversial discussions regarding the efficacy of NSAIDs against depression. In the present study, we did not investigate the efficiency of ibuprofen as an antidepressant-like drug, but we rather tried to determine the protective value of chronic ibuprofen administration against depression during a chronic restrained stress period. Based on our results, the chronic and simultaneous administration of ibuprofen during stress dose-dependently prevents the metabolic, cognitive, and behavioral consequences of the chronic restraint stress in rats.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles were considered in this article.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed equally in preparing all parts of the research.
Conflict of interest
The authors declared no conflict of interest.
References
Barzegar, S., Komaki, A., Shahidi, S., Sarihi, A., Mirazi, N., & Salehi, I. (2015). Effects of cannabinoid and glutamate receptor antagonists and their interactions on learning and memory in male rats. Pharmacology Biochemistry and Behavior, 131, 87-90. [DOI:10.1016/j.pbb.2015.02.005] [PMID]
Besedovsky, H. O., Del Rey, A., Klusman, I., Furukawa, H., Arditi, G. M., & Kabiersch, A. (1991). Cytokines as modulators of the hypothalamus-pituitary-adrenal axis. The Journal of Steroid Biochemistry and Molecular Biology, 40(4-6), 613-8. [DOI:10.1016/0960-0760(91)90284-C]
Biesmans, S., Bouwknecht, J. A., Ver Donck, L., Langlois, X., Acton, P. D., & De Haes, P., et al. (2015). Peripheral administration of tumor necrosis factor-alpha induces neuroinflammation and sickness but not depressive-like behavior in mice. BioMed Research International, 2015. [DOI:10.1155/2015/716920] [PMID] [PMCID]
Borutaite, V. & Brown, G. (2005). What else has to happen for nitric oxide to induce cell death? Portland Press Limited, 716920 http://downloads.hindawi.com/journals/bmri/2015/716920.pdf
Bowers, S. L., Bilbo, S. D., Dhabhar, F. S., & Nelson, R. J. (2008). Stressor-specific alterations in corticosterone and immune responses in mice. Brain Behavior & Immunity, 22(1), 105-13. [DOI:10.1016/j.bbi.2007.07.012] [PMID] [PMCID]
Buchanan, K. L. (2000). Stress and the evolution of condition-dependent signals. Trends in Ecology & Evolution, 15(4), 156-60. [DOI:10.1016/S0169-5347(99)01812-1]
Campos, A. C., Fogaça, M. V., Aguiar, D. C., & Guimaraes, F. S. (2013). Animal models of anxiety disorders and stress. Revista Brasileira de Psiquiatria, 35(2), S101-11. [DOI:10.1590/1516-4446-2013-1139] [PMID]
Chang, L., Sundaresh, S., Elliott, J., Anton, P. A., Baldi, P., & Licudine, A. (2009). Dysregulation of the Hypothalamic‐Pituitary‐Adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterology & Motility 21(2), 149-59. [DOI:10.1111/j.1365-2982.2008.01171.x] [PMID] [PMCID]
Chida, Y., Sudo, N., Mori, J., & Kubo, C. (2006). Social isolation stress impairs passive avoidance learning in Senescence-Accelerated Mouse (SAM). Brain Research, 1067(1), 201-8. [DOI:10.1016/j.brainres.2005.10.042] [PMID]
Christiansen, S. H., Olesen, M. V., Wörtwein, G., & Woldbye, D. P. D. (2011). Fluoxetine reverts chronic restraint stress-induced depression-like behaviour and increases neuropeptide Y and galanin expression in mice. Behavioural Brain Research, 216(2), 585-91. [DOI:10.1016/j.bbr.2010.08.044] [PMID]
Cryan, J. F., & Mombereau, C. (2004). In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Molecular Psychiatry, 9(4), 326-7. [DOI:10.1038/sj.mp.4001457] [PMID]
Cryan, J. F., Valentino, R. J., & Lucki, I. (2005). Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neuroscience & Biobehavioral Reviews, 29(4-5), 547-69. [DOI:10.1016/j.neubiorev.2005.03.008] [PMID]
de la Puente, B., Romero-Alejo, E., Vela, J. M., Merlos, M., Zamanillo, D., & Portillo-Salido, E. (2015). Changes in saccharin preference behavior as a primary outcome to evaluate pain and analgesia in acetic acid-induced visceral pain in mice. Journal of Pain Research, 8, 663-73. [DOI:10.2147/JPR.S91230] [PMID] [PMCID]
Gárate, I., Garcia-Bueno, B., Madrigal, J. L. M., Caso, J. R., Alou, L., & Gomez-Lus, M. L. et al. (2013). Stress-induced neuroinflammation: role of the Toll-like receptor-4 pathway. Biological Psychiatry, 73(1), 32-43. [DOI:10.1016/j.biopsych.2012.07.005] [PMID]
Gárate, I., Garcia-Bueno, B., Madrigal, J. L. M., Caso, J. R., Alou, L., Gomez-Lus, M. L., et al. (2013). Stress-induced neuroinflammation: Role of the Toll-like receptor-4 pathway. Biological Psychiatry, 73(1), 32-43. [DOI:10.1016/j.biopsych.2012.07.005] [PMID]
García-Bueno, B., Caso, J. R., & Leza, J. C. (2008). Stress as a neuroinflammatory condition in brain: damaging and protective mechanisms. Neuroscience & Biobehavioral Reviews, 32(6), 1136-51. [DOI:10.1016/j.neubiorev.2008.04.001] [PMID]
Grippo, A. J., Scotti M. A. (2013). Stress and neuroinflammation. Modern Trends in Pharmacopsychiatry, 28, 20-32. [DOI:10.1159/000343965] [PMID]
Hammen, C. (2005). Stress and depression. Annual Review of Clinical Psychology, 1, 293-319. [DOI:10.1146/annurev.clinpsy.1.102803.143938] [PMID]
Ho, N., Sommers, M. S., & Lucki, I. (2013). Effects of diabetes on hippocampal neurogenesis: links to cognition and depression. Neuroscience & Biobehavioral Reviews, 37(8), 1346-62. [DOI:10.1016/j.neubiorev.2013.03.010] [PMID] [PMCID]
Hosseinzadeh, S., Roshan, V. D., & Pourasghar, M. (2013). Effects of intermittent aerobic training on passive avoidance test (shuttle box) and stress markers in the dorsal hippocampus of wistar rats exposed to administration of homocysteine. Iranian Journal of Psychiatry and Behavioral Sciences, 7(1), 37-44. [PMCID] [PMID]
Kaestner, F., Hettich, M., Peters, M., Sibrowski, W., Hetzel, G., & Ponath, G., et al. (2005). Different activation patterns of proinflammatory cytokines in melancholic and non-melancholic major depression are associated with HPA axis activity. Journal of Affective Disorders, 87(2-3), 305-11. [DOI:10.1016/j.jad.2005.03.012] [PMID]
Köhler, O., Petersen, L., Mors, O., & Gasse, C. (2015). Inflammation and depression: combined use of selective serotonin reuptake inhibitors and NSAIDs or paracetamol and psychiatric outcomes. Brain and Behavior, 5(8), e00338. [DOI:10.1002/brb3.338] [PMID] [PMCID]
Leakey, J. E., Chen, S. H. U., Manjgaladze, M., Turturro, A., Duffy, P. H., Pipkin, J. L., & Hart, R. W. (1994). Role of Glucocorticoids and “Caloric Stress” in Modulating the Effects of Caloric Restriction in Rodents a. Annals of the New York Academy of Sciences, 719(1), 171-94. [DOI:10.1111/j.1749-6632.1994.tb56828.x] [PMID]
Lee, B., Sur, B., Park, J., Kim, S. H., Kwon, S., & Yeom, M., et al. (2013). Chronic administration of baicalein decreases depression-like behavior induced by repeated restraint stress in rats. The Korean Journal Of Physiology & Pharmacology, 17(5), 393-403. [DOI:10.4196/kjpp.2013.17.5.393] [PMID] [PMCID]
Mancuso, C., Scapagini, G., Curro, D., Giuffrida Stella, A. M., De Marco, C., & Butterfield, D. A., et al. (2007). Mitochondrial dysfunction, free radical generation and cellular stress response in neurodegenerative disorders. Front Biosci, 12(1), 1107-23. [DOI:10.2741/2130] [PMID]
Mehrpouya, S., Nahavandi, A., Khojasteh, F., Soleimani, M., Ahmadi, M., & Barati, M. (2015). Iron administration prevents BDNF decrease and depressive-like behavior following chronic stress. Brain Research, 1596, 79-87. [DOI:10.1016/j.brainres.2014.10.057] [PMID]
Miller, A. H., & Raison, C. L. (2016). The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nature Reviews Immunology, 16(1), 22. [DOI:10.1038/nri.2015.5] [PMID] [PMCID]
Munhoz, C. D., Garcia-Bueno, B., Madrigal, J. L. M., Lepsch, L. B., Scavone, C., & Leza, J. C. (2008). Stress-induced neuroinflammation: mechanisms and new pharmacological targets. Brazilian Journal of Medical and Biological Research, 41(12), 1037-46. [DOI:10.1590/S0100-879X2008001200001] [PMID]
Radahmadi, M., Alaei, H., Sharifi, M. R., & Hosseini, N. (2013). The effect of synchronized running activity with chronic stress on passive avoidance learning and body weight in rats. International Journal of Preventive Medicine, 4(4), 430-7.[DOI:10.5812/asjsm.34532] [PMID] [PMCID]
Raison, C. L., & Miller, A. H. (2013). Role of inflammation in depression: implications for phenomenology, pathophysiology and treatment. In Inflammation in Psychiatry, 28, 33-48. [DOI:10.1159/000343966] [PMID]
Rao, P., & Knaus, E. E. (2008). Evolution of Nonsteroidal Anti-inflammatory Drugs (NSAIDs): Cyclooxygenase (COX) inhibition and beyond. Journal of Pharmacy & Pharmaceutical Sciences, 11(2), 81s-110s. [DOI:10.18433/J3T886] [PMID]
Rivat, C., Becker, C., Blugeot, A., Zeau, B., Mauborgne, A., Pohl, M., & Benoliel, J. J. (2010). Chronic stress induces transient spinal neuroinflammation, triggering sensory hypersensitivity and long-lasting anxiety-induced hyperalgesia. Pain, 150(2), 358-68. [DOI:10.1016/j.pain.2010.05.031] [PMID]
Slattery, D. A., & Cryan, J. F. (2012). Using the rat forced swim test to assess antidepressant-like activity in rodents. Nature Protocols, 7(6), 1009-014. [DOI:10.1038/nprot.2012.044] [PMID]
Voorhees, J. L., Tarr, A. J., Wohleb, E. S., Godbout, J. P., Mo, X., & Sheridan, J. F., et al. (2013). Prolonged restraint stress increases IL-6, reduces IL-10, and causes persistent depressive-like behavior that is reversed by recombinant IL-10. PloS One, 8(3), e58488. [DOI:10.1371/journal.pone.0058488] [PMID] [PMCID]
Wilkinson, B. L., Cramer, P. E., Varvel, N. H., Reed-Geaghan, E., Jiang, Q., & Szabo, A., et al. (2012). Ibuprofen attenuates oxidative damage through NOX2 inhibition in Alzheimer's disease. Neurobiology of Aging, 33(1), 197-e21. [DOI:10.1016/j.neurobiolaging.2010.06.014] [PMID] [PMCID]
Zaminelli, T., Gradowski, R. W., Bassani, T. B., Barbiero, J. K., Santiago, R. M., & Maria-Ferreira, D., et al. (2014). Antidepressant and antioxidative effect of ibuprofen in the rotenone model of Parkinson’s disease. Neurotoxicity Research, 26(4), 351-62. [DOI:10.1007/s12640-014-9467-y] [PMID]
Zunszain, P. A., Anacker, C., Cattaneo, A., Carvalho, L. A., & Pariante, C. M. (2011). Glucocorticoids, cytokines and brain abnormalities in depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35(3), 722-9. [DOI:10.1016/j.pnpbp.2010.04.011] [PMID] [PMCID]
Full-Text:
1. Introduction
Stress is defined as an imbalance in homeostasis. Every unknown new situation may activate the stress response that modulates the homeostasis. The stress response is the organism’s intrinsic reaction to physically or psychologically disturbing factors (Garcia-Bueno et al., 2008). Short-term stress reactions have evolved as adaptive strategies to foster survival (Buchanan, 2000; Miller & Raison, 2016). However, long-term ongoing stress may lead to mental and physical health problems (Munhoz et al., 2008). Therefore, neuropsychiatric conditions are expected to follow long-lasting chronic stress (Rivat et al., 2010). Repeated, unpredictable, and chronic stress is the critical trigger factor for depression (Hammen, 2005; Grippo & Scotti, 2013) and inflammatory response, within the brain and periphery (Garate et al., 2013; Grippo & Scotti, 2013; Raison & Miller, 2013). The reciprocal link between the immune and central nervous systems plays a key role in stress-induced neuroinflammation and depression (Grippo & Scotti, 2013). The upregulation of Cyclooxygenase 2 (COX-2) is proposed as one of the underlying mechanisms of stress-induced depression (Munhoz et al., 2008; Garate et al., 2013). Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) are agents that exert the anti-inflammatory and analgesic effects through the inhibition of the COX (Rao & Knaus, 2008). Although the efficacy of the NSAIDs as an anti-depressive and anti-inflammatory adjuvant (Köhler, Petersen, Mors, & Gasse, 2015) is well established, the protective potential of NSAIDs against chronic stress has partially become clear.
Administering the ibuprofen (as a prominent NSAID and nonselective cyclooxygenase inhibitor), we aimed to prevent the stress-induced behavioral, cognitive, and weight alterations following a chronic restraint stress model in rats. In the present study, the passive avoidance learning task and the shuttle box were used as an indicator of stress-induced cognitive impairments. Also, we used the Forced Swimming Test (FST) to measure depressive-like behaviors. Finally, the plasma level of interleukin 6 (IL-6) was measured, because the IL-6 is an indicator of inflammation in depressed patients; the plasma level of IL-6 increases after the chronic stress test in rodents (Mehrpouya et al., 2015).
2. Materials & Methods
2.1. Animals and treatments
A total number of 40 adult male Wistar rats with the weight range of 200 to 250 g, and the age range of 7 to 9 weeks (Pasteur Institute of Iran, Karaj, Iran) were kept under the standard light-dark cycle (12 h:12 h), temperature (21°C -25°C), and humidity (45-55%) with free access to food and water. All handling procedures complied with the international guidelines for the care and use of laboratory animals (EU Directive 2010/63/EU for animal experiments) and approved by the Ethics Committee of Iran University of Medical Sciences. Also, the experiments complied with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines for reporting experiments involving animals.
For behavioral experiments, all animals were transported to the test room, one hour before the test. Using a completely randomized design, the animals were categorized. They received sham (non-stress+vehicle), or stress and the intraperitoneal injection of ibuprofen (Ibuprofen; Damavand Darou, Tehran, Iran) in the volume of 15, 30, or 60 mg per each kilogram of body weight. Ibuprofen was dissolved in distilled water before the injection (de la Puente et al., 2015). The doses were based on previous studies (Zaminelli et al., 2014). Table 1 summarizes the treatments for each group of the study animals. (As the sign and symptoms were not responsive to 15 and 30 mg/kg of ibuprofen, we didn’t mention them later on.)
Stress is defined as an imbalance in homeostasis. Every unknown new situation may activate the stress response that modulates the homeostasis. The stress response is the organism’s intrinsic reaction to physically or psychologically disturbing factors (Garcia-Bueno et al., 2008). Short-term stress reactions have evolved as adaptive strategies to foster survival (Buchanan, 2000; Miller & Raison, 2016). However, long-term ongoing stress may lead to mental and physical health problems (Munhoz et al., 2008). Therefore, neuropsychiatric conditions are expected to follow long-lasting chronic stress (Rivat et al., 2010). Repeated, unpredictable, and chronic stress is the critical trigger factor for depression (Hammen, 2005; Grippo & Scotti, 2013) and inflammatory response, within the brain and periphery (Garate et al., 2013; Grippo & Scotti, 2013; Raison & Miller, 2013). The reciprocal link between the immune and central nervous systems plays a key role in stress-induced neuroinflammation and depression (Grippo & Scotti, 2013). The upregulation of Cyclooxygenase 2 (COX-2) is proposed as one of the underlying mechanisms of stress-induced depression (Munhoz et al., 2008; Garate et al., 2013). Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) are agents that exert the anti-inflammatory and analgesic effects through the inhibition of the COX (Rao & Knaus, 2008). Although the efficacy of the NSAIDs as an anti-depressive and anti-inflammatory adjuvant (Köhler, Petersen, Mors, & Gasse, 2015) is well established, the protective potential of NSAIDs against chronic stress has partially become clear.
Administering the ibuprofen (as a prominent NSAID and nonselective cyclooxygenase inhibitor), we aimed to prevent the stress-induced behavioral, cognitive, and weight alterations following a chronic restraint stress model in rats. In the present study, the passive avoidance learning task and the shuttle box were used as an indicator of stress-induced cognitive impairments. Also, we used the Forced Swimming Test (FST) to measure depressive-like behaviors. Finally, the plasma level of interleukin 6 (IL-6) was measured, because the IL-6 is an indicator of inflammation in depressed patients; the plasma level of IL-6 increases after the chronic stress test in rodents (Mehrpouya et al., 2015).
2. Materials & Methods
2.1. Animals and treatments
A total number of 40 adult male Wistar rats with the weight range of 200 to 250 g, and the age range of 7 to 9 weeks (Pasteur Institute of Iran, Karaj, Iran) were kept under the standard light-dark cycle (12 h:12 h), temperature (21°C -25°C), and humidity (45-55%) with free access to food and water. All handling procedures complied with the international guidelines for the care and use of laboratory animals (EU Directive 2010/63/EU for animal experiments) and approved by the Ethics Committee of Iran University of Medical Sciences. Also, the experiments complied with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines for reporting experiments involving animals.
For behavioral experiments, all animals were transported to the test room, one hour before the test. Using a completely randomized design, the animals were categorized. They received sham (non-stress+vehicle), or stress and the intraperitoneal injection of ibuprofen (Ibuprofen; Damavand Darou, Tehran, Iran) in the volume of 15, 30, or 60 mg per each kilogram of body weight. Ibuprofen was dissolved in distilled water before the injection (de la Puente et al., 2015). The doses were based on previous studies (Zaminelli et al., 2014). Table 1 summarizes the treatments for each group of the study animals. (As the sign and symptoms were not responsive to 15 and 30 mg/kg of ibuprofen, we didn’t mention them later on.)
2.2. Experimental procedures
As illustrated in Figure 1, the animals were weighed before any handling (on day 0). The animals of the stress group were daily injected with ibuprofen or vehicle for 21 consecutive days (day 1-21). Also, 30 minutes after each injection, they were restrained for 6 hours (8 AM to 2 PM) with the standard rat Plexiglas restrainers (Campos, Fogaça, Aguiar, & Guimaraes, 2013). While the restrained rats were completely immobile with no access to food and water, the rats of the sham group were not subjected to stress and returned to their home cages after the vehicle injection. On day 23, the animals were trained for passive avoidance in the shuttle box and tested on day 24.
On day 28, the subjects were habituated for the FST that was performed on day 29. Ultimately, on day 36, the animals were weighed and deeply anesthetized with ketamine. The blood samples were taken for the assessments of IL-6 and corticosterone plasma levels with the Enzyme-Linked Immunosorbent Assay (ELISA) method. The animals were euthanized afterward. All the experiments were carried out by two observers who were blinded to the treatments.
2.3. Passive Avoidance Memory test
The custom-built shuttle box was made of two wooden compartments (each, 30×20×20 cm) separated by a guillotine door (20×15 cm). One of the boxes was equipped with a 20 W lamp, but the other one was dark and had a grid floor (3 mm diameter, 10 mm distance). For habituation, the animals were placed in the bright part and the guillotine door was opened after five seconds. Once the animal passed through the door, it was closed and the animal was returned to the home cage, after 20 seconds. Thirty minutes later, one trial training session was performed similar to the habituation procedure, but once the animal entered the dark compartment, it received a foot-shock (50 Hz, 1 mA, 3 s). Then, the animal was returned to the home cage. On the next day, the animal was placed in the bright compartment and the guillotine door was opened after 20 seconds. The latency time to enter the dark compartment was measured with a stopwatch; the cut off time was 300 seconds (Hosseinzadeh, Roshan, & Pourasghar, 2013; Barzegar et sl., 2015).
2.4. Forced Swimming test
Pretest: The animals were individually placed at a depth of 18 cm in a transparent cylinder (80 cm height, 30 cm diameter) filled with 24°C water, for 15 minutes. Then, they were dried with a towel and returned to their cages.
Swim test: At the same conditions as the pretest, the animals were placed in the cylinder, and the video was recorded. After five minutes, they were dried and returned to their cages. Cumulative immobility duration was measured by two observers who were blinded to the treatments (Cryan, Valentino, & Lucki, 2005; Slattery & Cryan, 2012).
2.5. IL-6 ELISA
The animals were recovered for a week to avoid transient increments in the IL-6 levels because of the behavioral experiments. Next, the rats were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg), then, the heart apex was exposed and blood samples (1 mL) were taken. The samples were centrifuged (10 min, 664×g), and the plasma was stored at -20°C. The plasma IL-6 levels were quantified using commercially available ELISA kit (eBioscience, Vienna, Austria), according to manufacturer’s instructions.
2.6. Corticosterone ELISA
The rat blood samples were centrifuged at 664×g for 10 minutes. The sera were immediately separated and stored at -80oC until the examination. The corticosterone level was measured using the ELISA kit (Zellbio GmbH, Germany), according to the manufacturer's protocol.
2.7. Transmission electron microscopy
Two rats of each group were anesthetized with the intraperitoneal injection of ketamine-xylazine combination (10:1) and perfused intracardially with 0.9% sodium chloride, 10X phosphate-buffered saline, and 4% paraformaldehyde. Then, they were decapitated and their brains removed. After tissue processing, 5-µm paraffin-embedded sections were provided. These structures become visualized in the electron microscope at the magnifications range of 50 to 1000000 times. The protocols given hereafter can be used for light microscopy and transmission electron microscopy.
2.8. Statistical analysis
Values were expressed as Mean±Standard deviation. The SigmaPlot-14 (Systat Software Inc.) was used to analyze the obtained data. To analyze variations among three or more groups, the one-way analysis of variance followed by the Tukey test for post hoc analysis was performed. The P-value of less than 0.05 (P<0.05) was considered statistically significant.
3. Results
3.1. Ibuprofen protects against stress-induced weight loss
According to Figure 2, chronic stress disturbs normal weight gain, and ibuprofen is preventive against these changes. The stress group had the smallest weight gain, while the CRS+Ibu-60 group showed a high mean of weight gain. The CRS+Ibu-60 group had significantly higher weight gain, compared with the stress group (F6, 35= 6.46, P<0.001).
3.2. Ibuprofen improves stress-induced learning deficits
Chronic stress impairs learning processes and negatively impacts learning avoidance (Chida, Sudo, Mori, & Kubo, 2006; Radahmadi, Alaei, Sharifi, & Hosseini, 2013). Here, chronically stressed animals that were treated with a high dose of Ibuprofen (60 mg/kg) significantly improved avoidance performance. Compared with the control (P<0.05) and CRS (P<0.001) groups, the CRS+Ibu-60 group had significantly (F6, 35= 15.60, P<0.001) better performance in avoidance memory (Figure 3).
3.3. Ibuprofen prevents depressive-like immobility in the FST
The FST is one of the most frequently used tests for both the assessments of depressive-like behavior in rodents and the antidepressant-like properties of the pharmacological agents (Cryan, Valentino, & Lucki, 2005; Slattery & Cryan, 2012). Compared with the control group (217.83±25.40 s), the CRS group showed a higher immobility time (86.50±5.49 s, P<0.001). However, the CRS+Ibu-60 group (100.33±9.95 s) had a significantly (F6,35= 36.38, P<0.001) shorter immobility time, compared with the CRS group (Figure 4).
3.4. Plasma IL-6 level
The one-way ANOVA did not indicate any significant difference in the plasma levels of IL-6 between the study groups (F6,35=0.824, P=0.559; Figure 5).
3.5. Plasma corticosterone level
Compared with the control group (217.83±25.40 s), the CRS group had a significantly higher plasma corticosterone level (86.50±5.49 s, P<0.001). However, the corticosterone plasma level of the CRS+Ibu-60 group (100.33±9.95 s ) was significantly (F6,35=33.38, P<0.001) lower than the CRS group (Figure 6).
3.6. Ibuprofen protects against stress-induced apoptotic changes
The transmission electron microscopy results of the CA1 region showed apoptotic changes, including the chromatin margination and dissolution in the cell nucleus of stressed rats. However, we found the large and round nuclei and the intracellular organelles within the cytoplasm of both the control and CRS+Ibu-60 groups (F6, 35= 36.27, P<0.001; Figure 7).
4. Discussion
The present study is the first research investigating the preventive effect of ibuprofen on chronic stress-induced depressive-like behavior in rats. Our results provided a better insight into the stress-induced depressive-like behaviors and examined the potential of ibuprofen as a preventive medication during chronic stress. In the present study, a dose-dependent manner of ibuprofen administration showed that the highest dose of ibuprofen (60 mg) had the best efficiency, and prevented all the stress-induced symptoms. However, the moderate dose (30 mg) just improved the immobility behavior during the FST. Also, the lowest dose of ibuprofen (15 mg) had no detectable effect on any measurements. Therefore, we focused on the highest dose of ibuprofen (60 mg/kg) in the following. It should be emphasized that ibuprofen plays an anti-inflammatory role in higher doses, which is seen over the weeks following the administration.
Ibuprofen plays a role both as an anti-inflammatory and a neuroprotective agent in conditions, such as Parkinson and Alzheimer diseases (Wilkinson et al., 2012). Also, chronic ibuprofen administration can reverse depressive behavior in the Parkinson disease model of the rats (Zaminelli et al., 2014). Restraint-induced stress results in oxidative stress, inflammation, and depressive-like symptoms (Gárate et al., 2013). Ibuprofen as a non-specific COX inhibitor may protect against restraint-induced chronic stress via the blockage of this process (Mancuso et al., 2007). In response to stressors, there is a fundamental, dynamic, and reciprocal relationship between the corticosterone and the immune system (Bowers, Bilbo, Dhabhar, & Nelson, 2008). Following the stressors, pro-inflammatory cytokines, such as tumor necrosis factor-α increase, and result in the overactivity of the HPA axis. The increased corticosterone would suppress the high levels of inflammatory modulators (Leakey et al., 1994). Consequently, both the corticosterone and the inflammatory modulators rise (Besedovsky et al., 1991).
The normal body weight gain of the rats was reduced by the CRS paradigm; this result is in line with the previous reports about the impaired weight gain induced by depression following the CRS (Christiansen, Olesen, Wörtwein, & Woldbye, 2011). Chronic restraint stress increases the activity of the HPA axis and induces the signs and symptoms of depression, such as decreased appetite and reduced body weight gain (Kaestner et al., 2005).
In the present study, we found an increased immobility behavior in the CRS group, compared with the control and CRS+Ibu-60 groups. Immobility behavior could be translated into behavioral despair (Cryan & Mombereau, 2004). The restraint-induced chronic stress and depression result in the various modifications of the brain, including decreased dopamine level and reduced hippocampal volume and plasticity, which are almost the result of neuroinflammation (Lee et al., 2013).
Abnormal HPA axis activity is correlated with stress-related conditions, such as mental depression (Chang et al., 2009). In such conditions, the HPA activity is usually exaggerated (Chang et al., 2009). As mentioned earlier, the activity of the inflammatory modulators increased HPA activity. It seems that the high dose of ibuprofen has interrupted this loop and decreased behavioral despair. The high dose of ibuprofen could improve the passive avoidance task in the rat depression model. Hippocampus is the main organ involved in learning and memory and is susceptible to inflammation, apoptosis, and damage following the mental depression. The hippocampus function is best evaluated by the passive avoidance task (Ho, Sommers, & Lucki, 2013). Our findings indicated a normal passive avoidance task in the CRS+Ibu-60 group, compared with the control group. Observing a functional hippocampus in the rats of both intact group and CRS+Ibu-60 group indicates the preventive effect of Ibu 60 on hippocampal damage in stressful situations.
We found no significant increase in plasma IL-6 levels in our stress-induced model. Explaining this result, it is suggested that the time of sampling has been overlapped with the decrement of IL-6 by the first surge of corticosterone. The short course of stress induction (21 days) in our model may be another possible reason. It seems that the rats required more duration and the time of the CST to develop high levels of IL-6. Several studies have shown that chronic neuro-inflammatory and depressive-like behavior are essential for the elevation of plasma IL-6 (Voorhees et al., 2013). Another study confirmed the IL-6 increase following 28 days of restraint-induced stress in mice (Biesmans et al., 2015). It seems that 21 days of restraint stress is not an appropriate protocol for the IL-6 studies. Possibly, if we continued this phase, we could see the plasma IL-6 increment. The three-week protocol carried out in the present study explains why we could not find a significant increase in IL-6.
The electron microscope findings showed the signs of apoptotic changes in the CA1 area of the CRS group. Mental depression as a neuro-inflammatory process is associated with apoptosis, and the hippocampus is one of the most susceptible organs for such changes (Zunszain, Anacker, Cattaneo, Carvalho, & Pariante, 2011). Although there are reports about the apoptotic effect of ibuprofen in various conditions, a study revealed that all the NSAIDs, including ibuprofen, inhibit the nitric oxide-induced apoptosis in a non-cyclooxygenase-dependent manner in chondrocytes (Borutaite & Brown, 2005). The NSAIDs inhibit the nitric oxide-induced apoptosis and the differentiation of articular chondrocytes independent of cyclooxygenase activity (Borutaite & Brown, 2005).
Currently, there are controversial discussions regarding the efficacy of NSAIDs against depression. In the present study, we did not investigate the efficiency of ibuprofen as an antidepressant-like drug, but we rather tried to determine the protective value of chronic ibuprofen administration against depression during a chronic restrained stress period. Based on our results, the chronic and simultaneous administration of ibuprofen during stress dose-dependently prevents the metabolic, cognitive, and behavioral consequences of the chronic restraint stress in rats.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles were considered in this article.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed equally in preparing all parts of the research.
Conflict of interest
The authors declared no conflict of interest.
References
Barzegar, S., Komaki, A., Shahidi, S., Sarihi, A., Mirazi, N., & Salehi, I. (2015). Effects of cannabinoid and glutamate receptor antagonists and their interactions on learning and memory in male rats. Pharmacology Biochemistry and Behavior, 131, 87-90. [DOI:10.1016/j.pbb.2015.02.005] [PMID]
Besedovsky, H. O., Del Rey, A., Klusman, I., Furukawa, H., Arditi, G. M., & Kabiersch, A. (1991). Cytokines as modulators of the hypothalamus-pituitary-adrenal axis. The Journal of Steroid Biochemistry and Molecular Biology, 40(4-6), 613-8. [DOI:10.1016/0960-0760(91)90284-C]
Biesmans, S., Bouwknecht, J. A., Ver Donck, L., Langlois, X., Acton, P. D., & De Haes, P., et al. (2015). Peripheral administration of tumor necrosis factor-alpha induces neuroinflammation and sickness but not depressive-like behavior in mice. BioMed Research International, 2015. [DOI:10.1155/2015/716920] [PMID] [PMCID]
Borutaite, V. & Brown, G. (2005). What else has to happen for nitric oxide to induce cell death? Portland Press Limited, 716920 http://downloads.hindawi.com/journals/bmri/2015/716920.pdf
Bowers, S. L., Bilbo, S. D., Dhabhar, F. S., & Nelson, R. J. (2008). Stressor-specific alterations in corticosterone and immune responses in mice. Brain Behavior & Immunity, 22(1), 105-13. [DOI:10.1016/j.bbi.2007.07.012] [PMID] [PMCID]
Buchanan, K. L. (2000). Stress and the evolution of condition-dependent signals. Trends in Ecology & Evolution, 15(4), 156-60. [DOI:10.1016/S0169-5347(99)01812-1]
Campos, A. C., Fogaça, M. V., Aguiar, D. C., & Guimaraes, F. S. (2013). Animal models of anxiety disorders and stress. Revista Brasileira de Psiquiatria, 35(2), S101-11. [DOI:10.1590/1516-4446-2013-1139] [PMID]
Chang, L., Sundaresh, S., Elliott, J., Anton, P. A., Baldi, P., & Licudine, A. (2009). Dysregulation of the Hypothalamic‐Pituitary‐Adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterology & Motility 21(2), 149-59. [DOI:10.1111/j.1365-2982.2008.01171.x] [PMID] [PMCID]
Chida, Y., Sudo, N., Mori, J., & Kubo, C. (2006). Social isolation stress impairs passive avoidance learning in Senescence-Accelerated Mouse (SAM). Brain Research, 1067(1), 201-8. [DOI:10.1016/j.brainres.2005.10.042] [PMID]
Christiansen, S. H., Olesen, M. V., Wörtwein, G., & Woldbye, D. P. D. (2011). Fluoxetine reverts chronic restraint stress-induced depression-like behaviour and increases neuropeptide Y and galanin expression in mice. Behavioural Brain Research, 216(2), 585-91. [DOI:10.1016/j.bbr.2010.08.044] [PMID]
Cryan, J. F., & Mombereau, C. (2004). In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Molecular Psychiatry, 9(4), 326-7. [DOI:10.1038/sj.mp.4001457] [PMID]
Cryan, J. F., Valentino, R. J., & Lucki, I. (2005). Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neuroscience & Biobehavioral Reviews, 29(4-5), 547-69. [DOI:10.1016/j.neubiorev.2005.03.008] [PMID]
de la Puente, B., Romero-Alejo, E., Vela, J. M., Merlos, M., Zamanillo, D., & Portillo-Salido, E. (2015). Changes in saccharin preference behavior as a primary outcome to evaluate pain and analgesia in acetic acid-induced visceral pain in mice. Journal of Pain Research, 8, 663-73. [DOI:10.2147/JPR.S91230] [PMID] [PMCID]
Gárate, I., Garcia-Bueno, B., Madrigal, J. L. M., Caso, J. R., Alou, L., & Gomez-Lus, M. L. et al. (2013). Stress-induced neuroinflammation: role of the Toll-like receptor-4 pathway. Biological Psychiatry, 73(1), 32-43. [DOI:10.1016/j.biopsych.2012.07.005] [PMID]
Gárate, I., Garcia-Bueno, B., Madrigal, J. L. M., Caso, J. R., Alou, L., Gomez-Lus, M. L., et al. (2013). Stress-induced neuroinflammation: Role of the Toll-like receptor-4 pathway. Biological Psychiatry, 73(1), 32-43. [DOI:10.1016/j.biopsych.2012.07.005] [PMID]
García-Bueno, B., Caso, J. R., & Leza, J. C. (2008). Stress as a neuroinflammatory condition in brain: damaging and protective mechanisms. Neuroscience & Biobehavioral Reviews, 32(6), 1136-51. [DOI:10.1016/j.neubiorev.2008.04.001] [PMID]
Grippo, A. J., Scotti M. A. (2013). Stress and neuroinflammation. Modern Trends in Pharmacopsychiatry, 28, 20-32. [DOI:10.1159/000343965] [PMID]
Hammen, C. (2005). Stress and depression. Annual Review of Clinical Psychology, 1, 293-319. [DOI:10.1146/annurev.clinpsy.1.102803.143938] [PMID]
Ho, N., Sommers, M. S., & Lucki, I. (2013). Effects of diabetes on hippocampal neurogenesis: links to cognition and depression. Neuroscience & Biobehavioral Reviews, 37(8), 1346-62. [DOI:10.1016/j.neubiorev.2013.03.010] [PMID] [PMCID]
Hosseinzadeh, S., Roshan, V. D., & Pourasghar, M. (2013). Effects of intermittent aerobic training on passive avoidance test (shuttle box) and stress markers in the dorsal hippocampus of wistar rats exposed to administration of homocysteine. Iranian Journal of Psychiatry and Behavioral Sciences, 7(1), 37-44. [PMCID] [PMID]
Kaestner, F., Hettich, M., Peters, M., Sibrowski, W., Hetzel, G., & Ponath, G., et al. (2005). Different activation patterns of proinflammatory cytokines in melancholic and non-melancholic major depression are associated with HPA axis activity. Journal of Affective Disorders, 87(2-3), 305-11. [DOI:10.1016/j.jad.2005.03.012] [PMID]
Köhler, O., Petersen, L., Mors, O., & Gasse, C. (2015). Inflammation and depression: combined use of selective serotonin reuptake inhibitors and NSAIDs or paracetamol and psychiatric outcomes. Brain and Behavior, 5(8), e00338. [DOI:10.1002/brb3.338] [PMID] [PMCID]
Leakey, J. E., Chen, S. H. U., Manjgaladze, M., Turturro, A., Duffy, P. H., Pipkin, J. L., & Hart, R. W. (1994). Role of Glucocorticoids and “Caloric Stress” in Modulating the Effects of Caloric Restriction in Rodents a. Annals of the New York Academy of Sciences, 719(1), 171-94. [DOI:10.1111/j.1749-6632.1994.tb56828.x] [PMID]
Lee, B., Sur, B., Park, J., Kim, S. H., Kwon, S., & Yeom, M., et al. (2013). Chronic administration of baicalein decreases depression-like behavior induced by repeated restraint stress in rats. The Korean Journal Of Physiology & Pharmacology, 17(5), 393-403. [DOI:10.4196/kjpp.2013.17.5.393] [PMID] [PMCID]
Mancuso, C., Scapagini, G., Curro, D., Giuffrida Stella, A. M., De Marco, C., & Butterfield, D. A., et al. (2007). Mitochondrial dysfunction, free radical generation and cellular stress response in neurodegenerative disorders. Front Biosci, 12(1), 1107-23. [DOI:10.2741/2130] [PMID]
Mehrpouya, S., Nahavandi, A., Khojasteh, F., Soleimani, M., Ahmadi, M., & Barati, M. (2015). Iron administration prevents BDNF decrease and depressive-like behavior following chronic stress. Brain Research, 1596, 79-87. [DOI:10.1016/j.brainres.2014.10.057] [PMID]
Miller, A. H., & Raison, C. L. (2016). The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nature Reviews Immunology, 16(1), 22. [DOI:10.1038/nri.2015.5] [PMID] [PMCID]
Munhoz, C. D., Garcia-Bueno, B., Madrigal, J. L. M., Lepsch, L. B., Scavone, C., & Leza, J. C. (2008). Stress-induced neuroinflammation: mechanisms and new pharmacological targets. Brazilian Journal of Medical and Biological Research, 41(12), 1037-46. [DOI:10.1590/S0100-879X2008001200001] [PMID]
Radahmadi, M., Alaei, H., Sharifi, M. R., & Hosseini, N. (2013). The effect of synchronized running activity with chronic stress on passive avoidance learning and body weight in rats. International Journal of Preventive Medicine, 4(4), 430-7.[DOI:10.5812/asjsm.34532] [PMID] [PMCID]
Raison, C. L., & Miller, A. H. (2013). Role of inflammation in depression: implications for phenomenology, pathophysiology and treatment. In Inflammation in Psychiatry, 28, 33-48. [DOI:10.1159/000343966] [PMID]
Rao, P., & Knaus, E. E. (2008). Evolution of Nonsteroidal Anti-inflammatory Drugs (NSAIDs): Cyclooxygenase (COX) inhibition and beyond. Journal of Pharmacy & Pharmaceutical Sciences, 11(2), 81s-110s. [DOI:10.18433/J3T886] [PMID]
Rivat, C., Becker, C., Blugeot, A., Zeau, B., Mauborgne, A., Pohl, M., & Benoliel, J. J. (2010). Chronic stress induces transient spinal neuroinflammation, triggering sensory hypersensitivity and long-lasting anxiety-induced hyperalgesia. Pain, 150(2), 358-68. [DOI:10.1016/j.pain.2010.05.031] [PMID]
Slattery, D. A., & Cryan, J. F. (2012). Using the rat forced swim test to assess antidepressant-like activity in rodents. Nature Protocols, 7(6), 1009-014. [DOI:10.1038/nprot.2012.044] [PMID]
Voorhees, J. L., Tarr, A. J., Wohleb, E. S., Godbout, J. P., Mo, X., & Sheridan, J. F., et al. (2013). Prolonged restraint stress increases IL-6, reduces IL-10, and causes persistent depressive-like behavior that is reversed by recombinant IL-10. PloS One, 8(3), e58488. [DOI:10.1371/journal.pone.0058488] [PMID] [PMCID]
Wilkinson, B. L., Cramer, P. E., Varvel, N. H., Reed-Geaghan, E., Jiang, Q., & Szabo, A., et al. (2012). Ibuprofen attenuates oxidative damage through NOX2 inhibition in Alzheimer's disease. Neurobiology of Aging, 33(1), 197-e21. [DOI:10.1016/j.neurobiolaging.2010.06.014] [PMID] [PMCID]
Zaminelli, T., Gradowski, R. W., Bassani, T. B., Barbiero, J. K., Santiago, R. M., & Maria-Ferreira, D., et al. (2014). Antidepressant and antioxidative effect of ibuprofen in the rotenone model of Parkinson’s disease. Neurotoxicity Research, 26(4), 351-62. [DOI:10.1007/s12640-014-9467-y] [PMID]
Zunszain, P. A., Anacker, C., Cattaneo, A., Carvalho, L. A., & Pariante, C. M. (2011). Glucocorticoids, cytokines and brain abnormalities in depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35(3), 722-9. [DOI:10.1016/j.pnpbp.2010.04.011] [PMID] [PMCID]
Type of Study: Original |
Subject:
Behavioral Neuroscience
Received: 2019/04/8 | Accepted: 2019/10/15 | Published: 2020/07/1
Received: 2019/04/8 | Accepted: 2019/10/15 | Published: 2020/07/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |