Volume 11, Issue 6 (November & December 2020)
BCN 2020, 11(6): 795-804 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Zeinivand M, Nahavandi A, Baluchnejadmojarad T, Roghani M, Golab F. Dalteparin as a Novel Therapeutic Agent to Prevent Diabetic Encephalopathy by Targeting Oxidative Stress and Inflammation. BCN 2020; 11 (6) :795-804
URL: http://bcn.iums.ac.ir/article-1-1471-en.html
URL: http://bcn.iums.ac.ir/article-1-1471-en.html
Motahareh Zeinivand1 

 , Arezo Nahavandi *2
, Arezo Nahavandi *2 

 , Tourandokht Baluchnejadmojarad2
, Tourandokht Baluchnejadmojarad2 

 , Mehrdad Roghani3
, Mehrdad Roghani3 

 , Fereshteh Golab4
, Fereshteh Golab4 




 , Arezo Nahavandi *2
, Arezo Nahavandi *2 

 , Tourandokht Baluchnejadmojarad2
, Tourandokht Baluchnejadmojarad2 

 , Mehrdad Roghani3
, Mehrdad Roghani3 

 , Fereshteh Golab4
, Fereshteh Golab4 


1- Department of Physiology, School of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran.
2- Department of Physiology, School of Medicine, Iran University of Medical Sciences, Iran, Iran.
3- Department of Physiology, School of Medicine, Shahed University, Tehran, Iran.
4- Cellular and Molecular Research Center, Iran University of Medical Sciences, Tehran, Iran.
2- Department of Physiology, School of Medicine, Iran University of Medical Sciences, Iran, Iran.
3- Department of Physiology, School of Medicine, Shahed University, Tehran, Iran.
4- Cellular and Molecular Research Center, Iran University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 763 kb]
| Abstract (HTML)
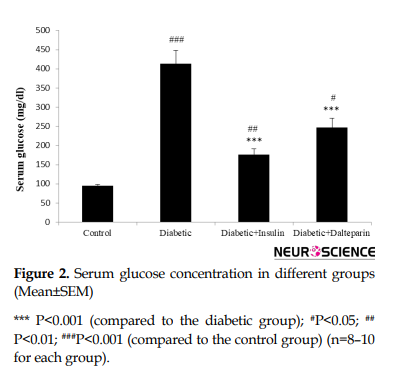
Moreover, induction of diabetes significantly increased serum glucose level compared to the control group (df: 3, F=33.05; P<0.001) (Figure 2).
These changes were diminished by dalteparin therapy (P<0.01) and insulin therapy (P<0.05). Additionally, no significant correlation was observed between the insulin- and dalteparin-treated groups. This indicates that dalteparin has anti-diabetic effects.
3.2. Diabetes induction affects iron and ferritin levels in the hippocampus
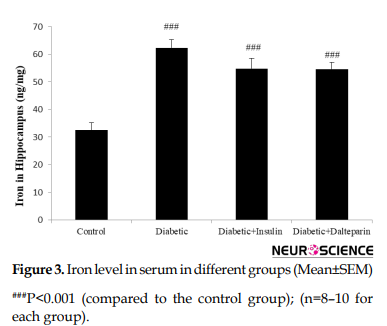
We assessed the effect of diabetes on hippocampal iron homeostasis. Induction of diabetes over 8 weeks caused iron accumulation in the hippocampus compared to the control group (df: 3, F=30.03; P<0.001) (Figure 3).
Treatment with dalteparin and insulin showed no difference in hippocampal iron levels compared to diabetic rats.
To confirm these alterations, the ferritin level was measured as the main parameter for intracellular iron level in the hippocampus. Induction of diabetes significantly increased ferritin level compared to the control group (df: 3, F=124.02; P<0.001) (Figure 4).
These changes decreased as a result of treatment with dalteparin (P<0.001) and insulin (P<0.01) compared to the diabetic group. Besides, the results are indicative of the better efficacy of dalteparin than insulin in reducing ferritin level and restoring it to control levels (P<0.01).
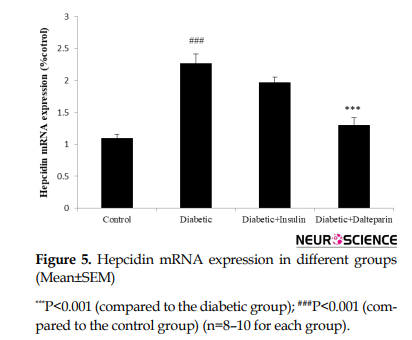
We measured the protein expression of key components in hippocampal iron homeostasis, hepcidin. Compared to the control, diabetes caused a significant increase in hepcidin in the hippocampus following the induction of diabetes over 8 weeks (df: 3, F=12.5; P<0.01) (Figure 5).
Treatment with dalteparin significantly boosted STL compared to the diabetic group (P<0.05) and insulin group (P<0.05), indicating that dalteparin reduced diabetic-induced memory impairment. No differences were found between groups in Initial Latency (IL) (data are not shown).
4. Discussion
The primary purpose of the current study was to find out whether dalteparin (an anti-hepcidin agent) might prevent the cognitive dysfunction caused by type 1 diabetes or not. The findings indicated a better control of diabetes by dalteparin than by insulin. Dalteparin has both antioxidant and anti-inflammatory effects on diabetic subjects. In T1DM, poor performance was reported for STZ-induced diabetic rats in passive avoidance functions (Nasri, Roghani, Baluchnejadmojarad, Balvardi, & Rabani, 2012). The partially positive impact of dalteparin on the improvement of memory could be related to its ability in improving chronic hyperglycemia, which is linked to most problems of diabetes, including cognitive dysfunctions (Kalalian-Moghaddam et al., 2013). Dalteparin can also inhibit hepatic gluconeogenesis (Vecchi et al., 2014). According to study results, the oral glucose tolerance test indicates an increase in serum hepcidin levels and a decrease in serum iron concentrations in healthy individuals (Chand, Singh, Pendharkar, & Petrov, 2018). Scientists have suggested that glucose probably plays a modulatory role in serum iron concentrations. On the other hand, the anti-hepcidin properties of dalteparin may exert anti-diabetic effects.
In agreement with other studies, the present study showed a significant elevation in the hippocampal MDA of diabetic rats. Sustained hyperglycemia is believed to be the main moderator for the elevated production of reactive oxygen species (Di Marco et al., 2015). The increased MDA concentration shows oxidative injury (Baluchnejadmojarad et al., 2017), which is involved in the development of a diabetic impaired brain (Baluchnejadmojarad et al., 2017). Iron is the main factor involved in the production of reactive oxygen species through the Fenton reaction, indicating that excessive iron is too toxic for neurons (Gong et al., 2016). Moreover, lipid and protein molecules in the cell membrane and intracellular structures may be impaired by the catalysis of hydroxyl radicals by iron and the generation of highly active free radicals (Hatunic et al., 2010). Cognitive impairment in diabetic individuals has been reported to be associated with iron overload in the brain and oxidative damage (Ward, Zucca et al. 2014; Garton et al., 2016). A study showed that iron played a pathophysiologic role in neurodegenerative conditions (Ward et al., 2014), AD (Smith, Harris, Sayre, & Perry, 1997), PD (Parkinson Disease) (Berg et al., 2001), and many other neurodegenerative dysfunctions (Dwyer et al., 2009). The present research indicated a reduction in MDA levels in the brain of dalteparin-treated rats compared with other diabetic groups, indicating the better antioxidant effect of dalteparin than insulin. Unlike dalteparin, insulin failed to control oxidative stress.
Hepcidin is the main moderator of systemic iron homeostasis (Vela 2018). Hepcidin hinders the expression of iron-uptake proteins in the brain (Du, Qian, Luo, Yung, & Ke, 2015). The uptake proteins interfere with “Fpn1-mediated” iron release in both astrocytes and neurons (Du et al., 2015). Down-regulation of Fpn1 by hepcidin elevates intracellular iron concentration and probably reduces the expression of uptake proteins. Any disturbance in the expression of iron transport protein causes an iron imbalance in the brain (Gong et al., 2016). In T1DM, up-regulation of hepcidin occurs due to iron overload following oxidative stress and inflammatory process (Ganz & Nemeth, 2012). In the pathogenesis of diabetes, iron plays a diabetic and a causal role in the failure of beta-cell, insulin resistance, and tissue dysfunction (Backe, Moen, Ellervik, Hansen, & Mandrup-Poulsen, 2016). In the current study, dalteparin seems to have ameliorated cognitive dysfunction by decreasing the expression of hepcidin as a result of reduced iron load.
Hepcidin gene expression terminates the overexpression of the hepcidin gene at low levels of iron (Poli et al., 2014). This study was the first to report the decreased expression of the hepcidin gene by dalteparin in T1DM. Unlike dalteparin, insulin could not reduce hepcidin expression in T1DM, which might explain why cognitive parameters were exacerbated in the insulin group. Dalteparin has been reported to have inhibitory effects on the expression of hepcidin during inflammatory conditions (Poli et al., 2014). In line with the findings of the present study, other studies have shown that insulin directly regulates hepcidin, and hepcidin is considerably involved in iron overload in STZ-induced diabetic rats (Wang, Li, Jiang, Shi, Shen, & Li 2014). Hyperglycemia may also play a role in the regulation of hepcidin (Aigner et al., 2013). Oral glucose tolerance test has recently been reported to increase serum hepcidin even in healthy individuals (Aigner et al., 2013). Another finding of the current study was the increased iron level in hippocampal tissue in T1DM. However, dalteparin could not modify this iron overload. Iron (extracellular iron) sedimentation of tissue in T1DM is mainly caused by impaired blood-brain barrier due to inflammation (Vela, 2018). On the other hand, iron sediment in the tissue must be cleaned by hepcidin, which is inhibited by dalteparin. Therefore, high levels of iron in hippocampal tissue cannot be regulated by dalteparin.
Furthermore, dalteparin reduced hippocampal ferritin levels, which is contrary to the above results. To explain this contradiction, it should be noted that ferritin is the major parameter of “intercellular iron level”. Hepcidin elevates intracellular iron and dalteparin hinders hepcidin, thereby preventing the increase of intracellular iron. Epidemiologic and clinical studies have shown that diabetic people are more vulnerable to AD (Crane et al., 2013). STZ-induced diabetes in rats causes inflammatory conditions and elevates pro-inflammatory and inflammatory cytokines. Neurovascular inflammation is mostly caused by tumor necrosis factor-alpha and IL-6, which contributes to synaptotoxicity and neurodegeneration (Matrone, Djelloul, Taglialatela, & Perrone, 2015). Also, strong clues indicate the modulatory activities of hepcidin following brain inflammatory conditions (Vela, 2018). IL-6 regulation hepcidin synthesis and dalteparin can suppress IL-6 levels in inflammatory conditions, which reduces the increase in hepcidin (Zhang et al., 2017). It is conspicuous that both local and or systemic hepcidin are elevated during brain inflammation, which consequently brings about iron overload (Zhang et al., 2017). Hepcidin functions as a double-edged sword that reduces intercellular iron overload through iron transfer to intracellular space to deal with inflammation (Zhao et al., 2018).
In conclusion, treatment with dalteparin prevents cognitive impairments in STZ-induced diabetic rats. Hence, it may be suggested as a therapeutic agent to control the adverse effects of T1DM.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles are considered in this article. The researchers observed the principles of the Helsinki Declaration and the principles of using laboratory animals as suggested in the National Institutes of Health Guide for Care and Use of Laboratory Animals over the course of the study.
Funding
The Iran University of Medical Sciences financially supported this research.
Authors' contributions
Methodology: Motahareh Zeinivand; Conceptualization and supervisor: Arezo Nahavandi; Investigation: Tourandokht Baluchnejad Mojarrad, Mehrdad Roghani, and Fereshteh Golab.
Conflict of interest
The authors declared no conflict of interest.
Full-Text:
1. Introduction
Researchers have recently proposed the term type 3 diabetes for Alzheimer Disease (AD) owing to the molecular and cellular features shared among type 1 diabetes, diabetes-related memory dysfunction, and cognitive impairment in the elderly. Chronic diabetes is associated with impaired learning, memory loss, attention dysfunction, and dementia (Kumar Datusalia & Sunder Sharma, 2016). Enhanced oxidative stress and inflammatory condition in the diabetic brain are also considered important pathogenic factors in the cognitive decline of diabetes (Ma et al., 2015). Recently, it has been shown that diabetes is an inflammatory condition associated with iron abnormalities and increased oxidative damage (Van Campenhout et al., 2006). Iron homeostasis is a main factor in sustaining the function of the Central Nervous System (CNS) and an essential element involved in the modulation of oxygen transport, neurotransmission, myelination, and neuronal metabolism (Murray & Lynch, 1998). However, iron imbalance and atypical build-up in the CNS are indicative of neuroinflammation and are involved in several neurodegenerative disorders (Ward, Zucca, Duyn, Crichton, & Zecca, 2014). A lot of evidence supports the idea that iron regularly builds up in the brain with age increase, oxidative stress, cell death, and neurotoxicity (Murray & Lynch 1998). Iron promotes the free radical formation and oxidative stress, which can trigger a cascade of harmful events that lead to neurodegeneration (Ke & Qian, 2007). Hepcidin is the primary regulator of iron homeostasis in the body and is mainly expressed in the liver (Nicolas et al., 2001). Many studies have also reported a large distribution of this peptide in the brain (Wang et al., 2008). Recent research has shown that iron dysmetabolism in the brain can be managed by the manipulation of hepcidin because the production of hepcidin in the brain has been reported to influence cellular iron transport (Xiong et al., 2016; Zhou et al., 2017). Yet, the presence or absence of inflammation appears to dictate the harmful vs. useful properties of hepcidin in the brain (Vela, 2018). In peripheral organs, the elevated level of hepcidin will lead to a lower iron level by controlling intestinal iron absorption, iron recycling in macrophages, iron mobilization from hepatic stores, and iron utilization in bone marrow cells (Park, Valore, Waring, & Ganz, 2001). A recent investigation showed that manipulation hepcidin has a role in reducing iron in the brain by down-regulating iron-transport proteins (Vela 2018). Thus, we hypothesized that an anti-hepcidin (dalteparin) agent might stop iron accumulation and decrease iron-induced oxidative stress. The current research tested this hypothesis and showed that treatment of diabetic rats with dalteparin decreased hepcidin expression and prevented the increased iron content and reactive oxygen species in the brain of diabetic rats.
2. Methods
2.1. Study design
This experimental research aimed to compare the effect of dalteparin (anti-hepcidin) and insulin treatment on cognitive dysfunction, learning, and memory impairment due to type 1 diabetes. The study protocol was approved by Iran University of Medical Sciences. The researchers followed the principles of the Helsinki Declaration and the principles of using laboratory animals as proposed in the National Institutes of Health Guide for Care and Use of Laboratory Animals during the study. Most of the materials were prepared from Sigma-Aldrich Company, Germany. The relevant company is given for each material.
2.2. Study animals
Male Wistar rats (n = 40) with a weight range of 200–250 g were obtained from the Laboratory Animal Breeding Center of Iran University of Medical Sciences and randomly classified into four groups (10 animals in each group) of control (healthy animals without treatment), diabetic group, diabetic + insulin, and diabetic + dalteparin groups. All animals were kept in special cages for a minimum of 2 weeks before the start of the experiment to adapt to the environment. They were provided with water and food ad libitum (temperature 21°C±1°C; 12-h light/dark cycle). All behavioral tests were conducted from 10:00 AM to 2:00 PM at room temperature. The study groups included control, diabetic, insulin-treated diabetic, and dalteparin-treated diabetic. Diabetes was induced by a single intraperitoneal administration of 60 mg/kg Streptozotocin (STZ) (Sigma Aldrich, USA) which was freshly dissolved in cold normal saline. One week following STZ administration, fasting blood samples were taken from the tail vein overnight and collected under light ether anesthesia. Serum glucose concentrations were measured by the glucose oxidase technique (Zistshimi, Tehran). Only rats with a fasting serum glucose level above 250 mg/dL were considered to be diabetic. Intraperitoneal administration of dalteparin (100 mg/kg/d) was initiated 1 week after STZ injection for 8 weeks. The dose of dalteparin was chosen based on previous research on the anti-inflammation and antioxidant effects of dalteparin (Farajdokht et al., 2015). Insulin (1.5 IU/ kg/d) (Caret, Bougnères, et al. 1996) was administered subcutaneously 1 week after STZ injection in the insulin group for 8 weeks.
2.3. Behavioral evaluations
Behavioral tests, including the Y-maze task and passive avoidance test, were performed 8 weeks after the induction of type 1 diabetes.
2.4.Y-maze test
Y-maze paradigm is a reliable and non-invasive behavioral test that is used to measure spatial working memory in rodents through evaluation of spontaneous alternation behavior (Kalalian-Moghaddam, Baluchnejadmojarad, Roghani, Goshadrou, & Ronaghi, 2013). The Y-maze comprises 3 arms and an equilateral triangular central area. All rats were tested once in a randomized order. They were placed at the end of 1 arm and permitted to make a move freely through the maze for 8 minutes. An arm entry was counted when the rat’s hind paws were in the arm completely. Alternation was defined as consecutive entries into the 3 arms on the overlapping triplet sets (i.e. A, B, C or B, C, A, etc.). The number of maximum spontaneous alternations was then determined as the total number of arm entries minus 2, and the percentage was computed as the ratio of actual/possible alternations (defined as the total number of arm entries minus 2). Besides, the total number of arm entries was utilized as an index for general locomotive activity. The maze was cleaned with 70% ethanol between sessions to eliminate the odor cues of compounds. The Y-maze task was performed on day 51 after STZ administration.
2.5. Passive Avoidance test
This test was performed by the shuttle box apparatus (BPT Co., Tehran) based on a former study with some changes applied (Roghani, Joghataie, Jalili, & Baluchnejadmojarad, 2006). The shuttle box consists of two compartments, one lighted chamber, and one dark chamber, with a grid floor connected by a guillotine door. Electric shock was applied by an isolated stimulator. On the first and second days, each rat was put into the apparatus and left there for 5 min to explore the chambers and adapt to the environment. During the acquisition test (the third day), the rats were put in the lighted chamber and after 5 min adaptation period, the guillotine door was opened and the latency to enter the dark chamber was recorded. Following the rat’s entry into the dark chamber, the door was closed and an electric foot shock (1 mA, 1 s) was applied to the grid floor. On the acquisition day, the Initial Latency (IL) of entry into the dark chamber was recorded, and rats with ILs of above 60 s were excluded from the study. Twenty-four hours later, a retention test was conducted and the interval between placement in the lighted chamber and entry into the dark chamber was calculated as Step-Through Latency (STL up to a maximum of 480 s as a cut-off point). Passive avoidance task was performed from day 52 to day 55 after STZ administration.
2.6. Determination of hippocampal oxidative stress
At the end of week 8 following STZ administration, hippocampal tissue was dissected out separately and 10% homogenate was prepared in ice-cold normal saline containing 0.1% Triton X100 and protease inhibitor cocktail. The obtained supernatant was aliquoted and kept at -70°C for the following experiments (Baluchnejadmojarad, Kiasalari, Afshin-Majd, Ghasemi, & Roghani, 2017). Malondialdehyde (MDA) level, the biological marker of lipid peroxidation due to oxidative stress, was determined in the supernatant as described previously (Kalalian-Moghaddam et al., 2013). To determine MDA concentration, trichloroacetic acid and TBARS (Thiobarbituric acid reactive substances) reagent were added to the supernatant and then mixed and incubated at boiling water for 90 min. The samples were centrifuged at 1000 g for 10 min after cooling on ice, and the absorbance rate of the supernatant was read at 532 nm. The findings were obtained on the standard curve of tetraethoxypropane.
2.7. Serum interleukin-6 measurements
Having conducted the last behavioral tests, the animals were deeply anesthetized and sacrificed from 10:00 to 12:00 AM. Then, their blood samples were collected, and serum Interleukin (IL)-6 concentration was determined by Enzyme-Linked Immunosorbent Assay (ELISA) kits (eBioscience) based on the manufacturer’s instructions.
2.8. Hippocampal iron and ferritin measurement
After performing the last behavioral tests, the animals were deeply anesthetized and sacrificed between 10:00 and 12:00 AM. The hippocampal tissue was then dissected out separately, and the acquired supernatant was aliquoted and kept at -70°C for the following experiments. Next, the concentration of iron and ferritin in the tissue sample was determined by Enzyme-Linked Immunosorbent Assay (ELISA) kits (eBioscience) based on the manufacturer’s guidelines.
2.9. Real-Time PCR
Hippocampal total RNA was isolated by Trizol Reagent (Roche) and alcohol-chloroform protocol according to the manufacturer’s instructions. Total RNA was reverse transcribed in a 25_l reaction using QuantiTec reverse transcription cDNA kits (Qiagen). Reactions were analyzed by QuantiFast SYBR Green PCR kit and a special primer pair for hepcidin cDNA and genomic DNA sequences of beta-actin. Primers were as follows: hepcidin forward 5/- TTGCGATACCAATGCAGAAGAG-3/and reverse 5/-AATTGTTACAGCATTTACAGCAGAAGA-3/, for the _-actin forward 5/- AGGCCCAGAGCAAGAGAGGTA-3/and reverse 5/-TCTCCATGTCGTCCCAGTTG-3/.
2.10. Statistical analysis
Data were analyzed by SPSS (version 21.0) and reported as the mean and standard error. One-way ANOVA and Bonferroni post hoc tests were used to compare the data. P<0.05 was considered significant for all analyses.
3. Results
3.1. Diabetic induced condition affecting body weight and serum glucose level
Induction of diabetes significantly decreased the body weight compared to the control group (df: 3, F=55.70; P<0.001). These alterations were reduced by dalteparin therapy (P<0.05) and insulin therapy (P<0.05). Further, no significant correlation was found between the insulin- and dalteparin-treated groups. This finding suggests that diabetes is strongly associated with lower body weight (Figure 1).
Researchers have recently proposed the term type 3 diabetes for Alzheimer Disease (AD) owing to the molecular and cellular features shared among type 1 diabetes, diabetes-related memory dysfunction, and cognitive impairment in the elderly. Chronic diabetes is associated with impaired learning, memory loss, attention dysfunction, and dementia (Kumar Datusalia & Sunder Sharma, 2016). Enhanced oxidative stress and inflammatory condition in the diabetic brain are also considered important pathogenic factors in the cognitive decline of diabetes (Ma et al., 2015). Recently, it has been shown that diabetes is an inflammatory condition associated with iron abnormalities and increased oxidative damage (Van Campenhout et al., 2006). Iron homeostasis is a main factor in sustaining the function of the Central Nervous System (CNS) and an essential element involved in the modulation of oxygen transport, neurotransmission, myelination, and neuronal metabolism (Murray & Lynch, 1998). However, iron imbalance and atypical build-up in the CNS are indicative of neuroinflammation and are involved in several neurodegenerative disorders (Ward, Zucca, Duyn, Crichton, & Zecca, 2014). A lot of evidence supports the idea that iron regularly builds up in the brain with age increase, oxidative stress, cell death, and neurotoxicity (Murray & Lynch 1998). Iron promotes the free radical formation and oxidative stress, which can trigger a cascade of harmful events that lead to neurodegeneration (Ke & Qian, 2007). Hepcidin is the primary regulator of iron homeostasis in the body and is mainly expressed in the liver (Nicolas et al., 2001). Many studies have also reported a large distribution of this peptide in the brain (Wang et al., 2008). Recent research has shown that iron dysmetabolism in the brain can be managed by the manipulation of hepcidin because the production of hepcidin in the brain has been reported to influence cellular iron transport (Xiong et al., 2016; Zhou et al., 2017). Yet, the presence or absence of inflammation appears to dictate the harmful vs. useful properties of hepcidin in the brain (Vela, 2018). In peripheral organs, the elevated level of hepcidin will lead to a lower iron level by controlling intestinal iron absorption, iron recycling in macrophages, iron mobilization from hepatic stores, and iron utilization in bone marrow cells (Park, Valore, Waring, & Ganz, 2001). A recent investigation showed that manipulation hepcidin has a role in reducing iron in the brain by down-regulating iron-transport proteins (Vela 2018). Thus, we hypothesized that an anti-hepcidin (dalteparin) agent might stop iron accumulation and decrease iron-induced oxidative stress. The current research tested this hypothesis and showed that treatment of diabetic rats with dalteparin decreased hepcidin expression and prevented the increased iron content and reactive oxygen species in the brain of diabetic rats.
2. Methods
2.1. Study design
This experimental research aimed to compare the effect of dalteparin (anti-hepcidin) and insulin treatment on cognitive dysfunction, learning, and memory impairment due to type 1 diabetes. The study protocol was approved by Iran University of Medical Sciences. The researchers followed the principles of the Helsinki Declaration and the principles of using laboratory animals as proposed in the National Institutes of Health Guide for Care and Use of Laboratory Animals during the study. Most of the materials were prepared from Sigma-Aldrich Company, Germany. The relevant company is given for each material.
2.2. Study animals
Male Wistar rats (n = 40) with a weight range of 200–250 g were obtained from the Laboratory Animal Breeding Center of Iran University of Medical Sciences and randomly classified into four groups (10 animals in each group) of control (healthy animals without treatment), diabetic group, diabetic + insulin, and diabetic + dalteparin groups. All animals were kept in special cages for a minimum of 2 weeks before the start of the experiment to adapt to the environment. They were provided with water and food ad libitum (temperature 21°C±1°C; 12-h light/dark cycle). All behavioral tests were conducted from 10:00 AM to 2:00 PM at room temperature. The study groups included control, diabetic, insulin-treated diabetic, and dalteparin-treated diabetic. Diabetes was induced by a single intraperitoneal administration of 60 mg/kg Streptozotocin (STZ) (Sigma Aldrich, USA) which was freshly dissolved in cold normal saline. One week following STZ administration, fasting blood samples were taken from the tail vein overnight and collected under light ether anesthesia. Serum glucose concentrations were measured by the glucose oxidase technique (Zistshimi, Tehran). Only rats with a fasting serum glucose level above 250 mg/dL were considered to be diabetic. Intraperitoneal administration of dalteparin (100 mg/kg/d) was initiated 1 week after STZ injection for 8 weeks. The dose of dalteparin was chosen based on previous research on the anti-inflammation and antioxidant effects of dalteparin (Farajdokht et al., 2015). Insulin (1.5 IU/ kg/d) (Caret, Bougnères, et al. 1996) was administered subcutaneously 1 week after STZ injection in the insulin group for 8 weeks.
2.3. Behavioral evaluations
Behavioral tests, including the Y-maze task and passive avoidance test, were performed 8 weeks after the induction of type 1 diabetes.
2.4.Y-maze test
Y-maze paradigm is a reliable and non-invasive behavioral test that is used to measure spatial working memory in rodents through evaluation of spontaneous alternation behavior (Kalalian-Moghaddam, Baluchnejadmojarad, Roghani, Goshadrou, & Ronaghi, 2013). The Y-maze comprises 3 arms and an equilateral triangular central area. All rats were tested once in a randomized order. They were placed at the end of 1 arm and permitted to make a move freely through the maze for 8 minutes. An arm entry was counted when the rat’s hind paws were in the arm completely. Alternation was defined as consecutive entries into the 3 arms on the overlapping triplet sets (i.e. A, B, C or B, C, A, etc.). The number of maximum spontaneous alternations was then determined as the total number of arm entries minus 2, and the percentage was computed as the ratio of actual/possible alternations (defined as the total number of arm entries minus 2). Besides, the total number of arm entries was utilized as an index for general locomotive activity. The maze was cleaned with 70% ethanol between sessions to eliminate the odor cues of compounds. The Y-maze task was performed on day 51 after STZ administration.
2.5. Passive Avoidance test
This test was performed by the shuttle box apparatus (BPT Co., Tehran) based on a former study with some changes applied (Roghani, Joghataie, Jalili, & Baluchnejadmojarad, 2006). The shuttle box consists of two compartments, one lighted chamber, and one dark chamber, with a grid floor connected by a guillotine door. Electric shock was applied by an isolated stimulator. On the first and second days, each rat was put into the apparatus and left there for 5 min to explore the chambers and adapt to the environment. During the acquisition test (the third day), the rats were put in the lighted chamber and after 5 min adaptation period, the guillotine door was opened and the latency to enter the dark chamber was recorded. Following the rat’s entry into the dark chamber, the door was closed and an electric foot shock (1 mA, 1 s) was applied to the grid floor. On the acquisition day, the Initial Latency (IL) of entry into the dark chamber was recorded, and rats with ILs of above 60 s were excluded from the study. Twenty-four hours later, a retention test was conducted and the interval between placement in the lighted chamber and entry into the dark chamber was calculated as Step-Through Latency (STL up to a maximum of 480 s as a cut-off point). Passive avoidance task was performed from day 52 to day 55 after STZ administration.
2.6. Determination of hippocampal oxidative stress
At the end of week 8 following STZ administration, hippocampal tissue was dissected out separately and 10% homogenate was prepared in ice-cold normal saline containing 0.1% Triton X100 and protease inhibitor cocktail. The obtained supernatant was aliquoted and kept at -70°C for the following experiments (Baluchnejadmojarad, Kiasalari, Afshin-Majd, Ghasemi, & Roghani, 2017). Malondialdehyde (MDA) level, the biological marker of lipid peroxidation due to oxidative stress, was determined in the supernatant as described previously (Kalalian-Moghaddam et al., 2013). To determine MDA concentration, trichloroacetic acid and TBARS (Thiobarbituric acid reactive substances) reagent were added to the supernatant and then mixed and incubated at boiling water for 90 min. The samples were centrifuged at 1000 g for 10 min after cooling on ice, and the absorbance rate of the supernatant was read at 532 nm. The findings were obtained on the standard curve of tetraethoxypropane.
2.7. Serum interleukin-6 measurements
Having conducted the last behavioral tests, the animals were deeply anesthetized and sacrificed from 10:00 to 12:00 AM. Then, their blood samples were collected, and serum Interleukin (IL)-6 concentration was determined by Enzyme-Linked Immunosorbent Assay (ELISA) kits (eBioscience) based on the manufacturer’s instructions.
2.8. Hippocampal iron and ferritin measurement
After performing the last behavioral tests, the animals were deeply anesthetized and sacrificed between 10:00 and 12:00 AM. The hippocampal tissue was then dissected out separately, and the acquired supernatant was aliquoted and kept at -70°C for the following experiments. Next, the concentration of iron and ferritin in the tissue sample was determined by Enzyme-Linked Immunosorbent Assay (ELISA) kits (eBioscience) based on the manufacturer’s guidelines.
2.9. Real-Time PCR
Hippocampal total RNA was isolated by Trizol Reagent (Roche) and alcohol-chloroform protocol according to the manufacturer’s instructions. Total RNA was reverse transcribed in a 25_l reaction using QuantiTec reverse transcription cDNA kits (Qiagen). Reactions were analyzed by QuantiFast SYBR Green PCR kit and a special primer pair for hepcidin cDNA and genomic DNA sequences of beta-actin. Primers were as follows: hepcidin forward 5/- TTGCGATACCAATGCAGAAGAG-3/and reverse 5/-AATTGTTACAGCATTTACAGCAGAAGA-3/, for the _-actin forward 5/- AGGCCCAGAGCAAGAGAGGTA-3/and reverse 5/-TCTCCATGTCGTCCCAGTTG-3/.
2.10. Statistical analysis
Data were analyzed by SPSS (version 21.0) and reported as the mean and standard error. One-way ANOVA and Bonferroni post hoc tests were used to compare the data. P<0.05 was considered significant for all analyses.
3. Results
3.1. Diabetic induced condition affecting body weight and serum glucose level
Induction of diabetes significantly decreased the body weight compared to the control group (df: 3, F=55.70; P<0.001). These alterations were reduced by dalteparin therapy (P<0.05) and insulin therapy (P<0.05). Further, no significant correlation was found between the insulin- and dalteparin-treated groups. This finding suggests that diabetes is strongly associated with lower body weight (Figure 1).
Moreover, induction of diabetes significantly increased serum glucose level compared to the control group (df: 3, F=33.05; P<0.001) (Figure 2).
These changes were diminished by dalteparin therapy (P<0.01) and insulin therapy (P<0.05). Additionally, no significant correlation was observed between the insulin- and dalteparin-treated groups. This indicates that dalteparin has anti-diabetic effects.
3.2. Diabetes induction affects iron and ferritin levels in the hippocampus
We assessed the effect of diabetes on hippocampal iron homeostasis. Induction of diabetes over 8 weeks caused iron accumulation in the hippocampus compared to the control group (df: 3, F=30.03; P<0.001) (Figure 3).
Treatment with dalteparin and insulin showed no difference in hippocampal iron levels compared to diabetic rats.
To confirm these alterations, the ferritin level was measured as the main parameter for intracellular iron level in the hippocampus. Induction of diabetes significantly increased ferritin level compared to the control group (df: 3, F=124.02; P<0.001) (Figure 4).
These changes decreased as a result of treatment with dalteparin (P<0.001) and insulin (P<0.01) compared to the diabetic group. Besides, the results are indicative of the better efficacy of dalteparin than insulin in reducing ferritin level and restoring it to control levels (P<0.01).
We measured the protein expression of key components in hippocampal iron homeostasis, hepcidin. Compared to the control, diabetes caused a significant increase in hepcidin in the hippocampus following the induction of diabetes over 8 weeks (df: 3, F=12.5; P<0.01) (Figure 5).
Furthermore, treatment with dalteparin significantly reduced diabetic-induced alterations in hepcidin level (P<0.01), suggesting that dalteparin is associated with decreased hepcidin content in the brain.
3.3. Dalteparin prevents inflammation and oxidative stress in the hippocampus
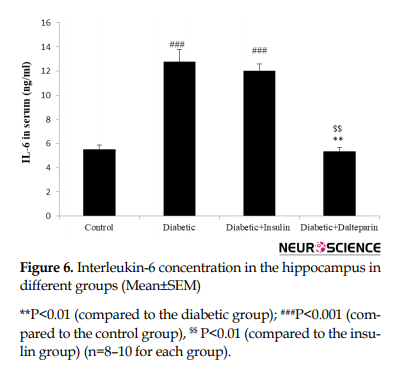
Induction of diabetes during 8 weeks significantly increased hippocampal IL-6 level (df: 3, F=35.36; P<0.001) (Figure 6).
3.3. Dalteparin prevents inflammation and oxidative stress in the hippocampus
Induction of diabetes during 8 weeks significantly increased hippocampal IL-6 level (df: 3, F=35.36; P<0.001) (Figure 6).
These changes were low as a result of treatment with dalteparin compared to the diabetic and insulin groups (P<0.01). There was also a significant correlation between hippocampal iron content and IL-6 level. Furthermore, the iron content in the brain highly affects neuroinflammation.
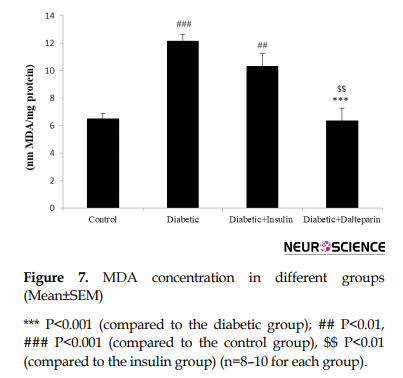
In addition to neuroinflammation and oxidative stress markers, hippocampal MDA levels significantly increased in the diabetic group compared to the control group (df: 3, F=16.84; P<0.001) (Figure 7), which was significantly diminished by the treatment with dalteparin (P<0.001) in comparison with the insulin therapy.
In addition to neuroinflammation and oxidative stress markers, hippocampal MDA levels significantly increased in the diabetic group compared to the control group (df: 3, F=16.84; P<0.001) (Figure 7), which was significantly diminished by the treatment with dalteparin (P<0.001) in comparison with the insulin therapy.
3.4. Dalteparin prevents memory impairments
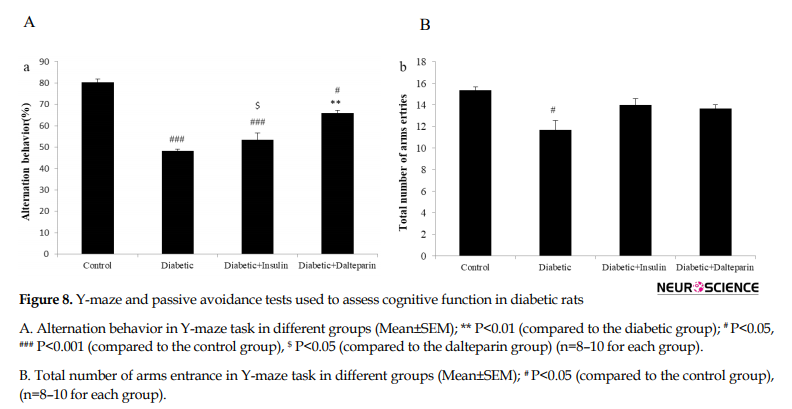
Y-maze and passive avoidance tests were used to assess cognitive function in diabetic rats. In the Y-maze task which indicated short-term spatial recognition memory, a significant reduction was found in alternation behavior (%) after induction of diabetes compared to the control group (df: 3, F=55.32; P<0.001) (Figure 8A).
Y-maze and passive avoidance tests were used to assess cognitive function in diabetic rats. In the Y-maze task which indicated short-term spatial recognition memory, a significant reduction was found in alternation behavior (%) after induction of diabetes compared to the control group (df: 3, F=55.32; P<0.001) (Figure 8A).
This effect was significantly attenuated by the treatment with dalteparin (P<0.001). These findings indicate the better impact of dalteparin compared to insulin in facilitating learning and restoring memory function. Moreover, the total number of arm entries in Y-maze showed that the diabetic group significantly spent less time in arm entries than the control group (df: 3, F=8.82; P<0.05) (Figure 8B), indicating that treatment with dalteparin had no significant impact on the locomotors activity.
In the passive avoidance task, a significant decrease was observed in Step-Through Latency (STL) after the induction of diabetes in comparison with the control group (df: 3, F=7.58; P<0.001) (Figure 9).
In the passive avoidance task, a significant decrease was observed in Step-Through Latency (STL) after the induction of diabetes in comparison with the control group (df: 3, F=7.58; P<0.001) (Figure 9).
Treatment with dalteparin significantly boosted STL compared to the diabetic group (P<0.05) and insulin group (P<0.05), indicating that dalteparin reduced diabetic-induced memory impairment. No differences were found between groups in Initial Latency (IL) (data are not shown).
4. Discussion
The primary purpose of the current study was to find out whether dalteparin (an anti-hepcidin agent) might prevent the cognitive dysfunction caused by type 1 diabetes or not. The findings indicated a better control of diabetes by dalteparin than by insulin. Dalteparin has both antioxidant and anti-inflammatory effects on diabetic subjects. In T1DM, poor performance was reported for STZ-induced diabetic rats in passive avoidance functions (Nasri, Roghani, Baluchnejadmojarad, Balvardi, & Rabani, 2012). The partially positive impact of dalteparin on the improvement of memory could be related to its ability in improving chronic hyperglycemia, which is linked to most problems of diabetes, including cognitive dysfunctions (Kalalian-Moghaddam et al., 2013). Dalteparin can also inhibit hepatic gluconeogenesis (Vecchi et al., 2014). According to study results, the oral glucose tolerance test indicates an increase in serum hepcidin levels and a decrease in serum iron concentrations in healthy individuals (Chand, Singh, Pendharkar, & Petrov, 2018). Scientists have suggested that glucose probably plays a modulatory role in serum iron concentrations. On the other hand, the anti-hepcidin properties of dalteparin may exert anti-diabetic effects.
In agreement with other studies, the present study showed a significant elevation in the hippocampal MDA of diabetic rats. Sustained hyperglycemia is believed to be the main moderator for the elevated production of reactive oxygen species (Di Marco et al., 2015). The increased MDA concentration shows oxidative injury (Baluchnejadmojarad et al., 2017), which is involved in the development of a diabetic impaired brain (Baluchnejadmojarad et al., 2017). Iron is the main factor involved in the production of reactive oxygen species through the Fenton reaction, indicating that excessive iron is too toxic for neurons (Gong et al., 2016). Moreover, lipid and protein molecules in the cell membrane and intracellular structures may be impaired by the catalysis of hydroxyl radicals by iron and the generation of highly active free radicals (Hatunic et al., 2010). Cognitive impairment in diabetic individuals has been reported to be associated with iron overload in the brain and oxidative damage (Ward, Zucca et al. 2014; Garton et al., 2016). A study showed that iron played a pathophysiologic role in neurodegenerative conditions (Ward et al., 2014), AD (Smith, Harris, Sayre, & Perry, 1997), PD (Parkinson Disease) (Berg et al., 2001), and many other neurodegenerative dysfunctions (Dwyer et al., 2009). The present research indicated a reduction in MDA levels in the brain of dalteparin-treated rats compared with other diabetic groups, indicating the better antioxidant effect of dalteparin than insulin. Unlike dalteparin, insulin failed to control oxidative stress.
Hepcidin is the main moderator of systemic iron homeostasis (Vela 2018). Hepcidin hinders the expression of iron-uptake proteins in the brain (Du, Qian, Luo, Yung, & Ke, 2015). The uptake proteins interfere with “Fpn1-mediated” iron release in both astrocytes and neurons (Du et al., 2015). Down-regulation of Fpn1 by hepcidin elevates intracellular iron concentration and probably reduces the expression of uptake proteins. Any disturbance in the expression of iron transport protein causes an iron imbalance in the brain (Gong et al., 2016). In T1DM, up-regulation of hepcidin occurs due to iron overload following oxidative stress and inflammatory process (Ganz & Nemeth, 2012). In the pathogenesis of diabetes, iron plays a diabetic and a causal role in the failure of beta-cell, insulin resistance, and tissue dysfunction (Backe, Moen, Ellervik, Hansen, & Mandrup-Poulsen, 2016). In the current study, dalteparin seems to have ameliorated cognitive dysfunction by decreasing the expression of hepcidin as a result of reduced iron load.
Hepcidin gene expression terminates the overexpression of the hepcidin gene at low levels of iron (Poli et al., 2014). This study was the first to report the decreased expression of the hepcidin gene by dalteparin in T1DM. Unlike dalteparin, insulin could not reduce hepcidin expression in T1DM, which might explain why cognitive parameters were exacerbated in the insulin group. Dalteparin has been reported to have inhibitory effects on the expression of hepcidin during inflammatory conditions (Poli et al., 2014). In line with the findings of the present study, other studies have shown that insulin directly regulates hepcidin, and hepcidin is considerably involved in iron overload in STZ-induced diabetic rats (Wang, Li, Jiang, Shi, Shen, & Li 2014). Hyperglycemia may also play a role in the regulation of hepcidin (Aigner et al., 2013). Oral glucose tolerance test has recently been reported to increase serum hepcidin even in healthy individuals (Aigner et al., 2013). Another finding of the current study was the increased iron level in hippocampal tissue in T1DM. However, dalteparin could not modify this iron overload. Iron (extracellular iron) sedimentation of tissue in T1DM is mainly caused by impaired blood-brain barrier due to inflammation (Vela, 2018). On the other hand, iron sediment in the tissue must be cleaned by hepcidin, which is inhibited by dalteparin. Therefore, high levels of iron in hippocampal tissue cannot be regulated by dalteparin.
Furthermore, dalteparin reduced hippocampal ferritin levels, which is contrary to the above results. To explain this contradiction, it should be noted that ferritin is the major parameter of “intercellular iron level”. Hepcidin elevates intracellular iron and dalteparin hinders hepcidin, thereby preventing the increase of intracellular iron. Epidemiologic and clinical studies have shown that diabetic people are more vulnerable to AD (Crane et al., 2013). STZ-induced diabetes in rats causes inflammatory conditions and elevates pro-inflammatory and inflammatory cytokines. Neurovascular inflammation is mostly caused by tumor necrosis factor-alpha and IL-6, which contributes to synaptotoxicity and neurodegeneration (Matrone, Djelloul, Taglialatela, & Perrone, 2015). Also, strong clues indicate the modulatory activities of hepcidin following brain inflammatory conditions (Vela, 2018). IL-6 regulation hepcidin synthesis and dalteparin can suppress IL-6 levels in inflammatory conditions, which reduces the increase in hepcidin (Zhang et al., 2017). It is conspicuous that both local and or systemic hepcidin are elevated during brain inflammation, which consequently brings about iron overload (Zhang et al., 2017). Hepcidin functions as a double-edged sword that reduces intercellular iron overload through iron transfer to intracellular space to deal with inflammation (Zhao et al., 2018).
In conclusion, treatment with dalteparin prevents cognitive impairments in STZ-induced diabetic rats. Hence, it may be suggested as a therapeutic agent to control the adverse effects of T1DM.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles are considered in this article. The researchers observed the principles of the Helsinki Declaration and the principles of using laboratory animals as suggested in the National Institutes of Health Guide for Care and Use of Laboratory Animals over the course of the study.
Funding
The Iran University of Medical Sciences financially supported this research.
Authors' contributions
Methodology: Motahareh Zeinivand; Conceptualization and supervisor: Arezo Nahavandi; Investigation: Tourandokht Baluchnejad Mojarrad, Mehrdad Roghani, and Fereshteh Golab.
Conflict of interest
The authors declared no conflict of interest.
Reference
Aigner, E., Felder, T. K., Oberkofler, H., Hahne, P., Auer, S., & Soyal, S., et al. (2013). Glucose acts as a regulator of serum iron by increasing serum hepcidin concentrations. The Journal of Nutritional Biochemistry, 24(1), 112-7. [DOI:10.1016/j.jnutbio.2012.02.017]
Backe, M. B., Moen, I. W., Ellervik, C., Hansen, J. B., & Mandrup-Poulsen, T. (2016). Iron regulation of pancreatic beta-cell functions and oxidative stress. Annual Review of Nutrition, 36, 241-73. [DOI:10.1146/annurev-nutr-071715-050939]
Baluchnejadmojarad, T., Kiasalari, Z., Afshin-Majd, S., Ghasemi, Z., & Roghani, M. (2017). S-allyl cysteine ameliorates cognitive deficits in streptozotocin-diabetic rats via suppression of oxidative stress, inflammation, and acetylcholinesterase. European Journal of Pharmacology, 794, 69-76. [DOI:10.1016/j.ejphar.2016.11.033]
Berg, D., Gerlach, M., Youdim, M. B. H., Double, K. L., Zecca L., & Riederer, P., et al. (2001). Brain iron pathways and their relevance to Parkinson’s disease. Journal of Neurochemistry, 79(2), 225-36. [DOI:10.1046/j.1471-4159.2001.00608.x]
Caret, J. C., Bougnères, P. F., & European Prediabetes Study Group. (1996). Treatment of prediabetic patients with insulin: Experience and future. Hormones, 45(Suppl 1), 44-7. [DOI:10.1159/000184829]
Chand, S. K., Singh, R. G., Pendharkar, S. A., & Petrov, M. S. (2018). Iron: A strong element in the pathogenesis of chronic hyperglycaemia after acute pancreatitis. Biological Trace Element Research, 183(1), 71-9. [DOI:10.1007/s12011-017-1131-y] [PMID]
Crane, P. K., Walker, R., Hubbard, R. A., Li, G., Nathan, D. M., & Zheng, H., et al. (2013). Glucose levels and risk of dementia. The New England Journal of Medicine, 369(6), 540-8. [DOI:10.1056/NEJMoa1215740] [PMID] [PMCID]
Di Marco, E., Jha, J. C., Sharma, A., Wilkinson-Berka, J. L., Jandeleit-Dahm, K. A., & de Haan, J. B. (2015). Are reactive oxygen species still the basis for diabetic complications? Clinical Science, 129(2), 199-216. [DOI:10.1042/CS20150093]
Du, F., Qian, Z. M., Luo, Q., Yung W. H., & Ke, Y. (2015). Hepcidin suppresses brain iron accumulation by downregulating iron transport proteins in iron-overloaded rats. Molecular Neurobiology, 52(1), 101-14. [DOI:10.1007/s12035-014-8847-x]
Dwyer, B. E., Zacharski, L. R., Balestra, D. J., Lerner, A. J., Perry, G., & Zhu, X., et al. (2009). Getting the iron out: Phlebotomy for Alzheimer’s disease? Medical Hypotheses, 72(5), 504-9. [DOI:10.1016/j.mehy.2008.12.029] [PMID] [PMCID]
Farajdokht, F., Soleimani, M., Mehrpouya, S., Barati, M., & Nahavandi, A. (2015). The role of hepcidin in chronic mild stress-induced depression. Neuroscience Letters, 588, 120-4. [DOI:10.1016/j.neulet.2015.01.008]
Ganz, T., & Nemeth, E. (2012). Hepcidin and iron homeostasis. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 1823(9), 1434-43. [DOI:10.1016/j.bbamcr.2012.01.014] [PMID] [PMCID]
Garton, T., Keep, R. F., Hua, Y., & Xi, G. (2016). Brain iron overload following intracranial haemorrhage. Stroke and Vascular Neurology, 1(4), 172-84. [DOI:10.1136/svn-2016-000042] [PMID] [PMCID]
Gong, J., Du, F., Qian, Z. M., Luo, Q. Q., Sheng, Y., & Yung, W. H., et al. (2016). Pre-treatment of rats with ad-hepcidin prevents iron-induced oxidative stress in the brain. Free Radical Biology and Medicine, 90, 126-32. [DOI:10.1016/j.freeradbiomed.2015.11.016]
Hatunic, M., Finucane, F. M., Brennan, A. M., Norris, S., Pacini, G., & Nolan, J. J. (2010). Effect of iron overload on glucose metabolism in patients with hereditary hemochromatosis. Metabolism, 59(3), 380-4. [DOI:10.1016/j.metabol.2009.08.006]
Kalalian-Moghaddam, H., Baluchnejadmojarad, T., Roghani, M., Goshadrou, F., & Ronaghi, A. (2013). Hippocampal synaptic plasticity restoration and anti-apoptotic effect underlie berberine improvement of learning and memory in streptozotocin-diabetic rats. European Journal of Pharmacology, 698(1-3), 259-66. [DOI:10.1016/j.ejphar.2012.10.020]
Ke, Y., & Qian, Z. M. (2007). Brain iron metabolism: Neurobiology and neurochemistry. Progress in Neurobiology, 83(3), 149-73. [DOI:10.1016/j.pneurobio.2007.07.009]
Kumar Datusalia, A., & Sunder Sharma, Sh. (2016). NF-κB inhibition resolves cognitive deficits in experimental type 2 diabetes mellitus through CREB and glutamate/GABA neurotransmitters pathway. Current Neurovascular Research, 13(1), 22-32. [DOI:10.2174/1567202612666151030104810]
Ma, P., Mao, X. Y., Li, X. L., Ma, Y., Qiao, Y. D., & Liu, Z. Q., et al. (2015). Baicalin alleviates diabetesassociated cognitive deficits via modulation of mitogen-activated protein kinase signaling, brainderived neurotrophic factor and apoptosis. Molecular Medicine Reports, 12(4), 6377-83. [DOI:10.3892/mmr.2015.4219]
Matrone, C., Djelloul, M., Taglialatela, G., & Perrone, L. (2015). Inflammatory risk factors and pathologies promoting Alzheimer’s disease progression: Is RAGE the key. Histology and Histopathology, 30(2), 125-39. [DOI:10.14670/HH-30.125] [PMID]
Murray, C. A., & Lynch, M. A. (1998). Evidence that increased hippocampal expression of the cytokine interleukin-1β is a common trigger for age-and stress-induced impairments in long-term potentiation. Journal of Neuroscience, 18(8), 2974-81. [DOI:10.1523/JNEUROSCI.18-08-02974.1998] [PMCID]
Nasri, S., Roghani, M., Baluchnejadmojarad, T., Balvardi, M., & Rabani, T. (2012). Chronic cyanidin‐3‐glucoside administration improves short‐term spatial recognition memory but not passive avoidance learning and memory in streptozotocin‐diabetic rats. Phytotherapy Research, 26(8), 1205-10. [DOI:10.1002/ptr.3702] [PMID]
Nicolas, G., Bennoun, M., Devaux, I., Beaumont, C., Grandchamp, B., & Kahn, A., et al. (2001). Lack of hepcidin gene expression and severe tissue iron overload in Upstream Stimulatory Factor 2 (USF2) knockout mice. Proceedings of the National Academy of Sciences of the United States of America, 98(15), 8780-5. [DOI:10.1073/pnas.151179498] [PMID] [PMCID]
Park, C. H., Valore, E. V., Waring, A. J., & Ganz, T. (2001). Hepcidin, a urinary antimicrobial peptide synthesized in the liver. Journal of Biological Chemistry, 276(11), 7806-10. [DOI:10.1074/jbc.M008922200] [PMID]
Poli, M., Poli, M., Asperti, M., Ruzzenenti, P., Regoni, M., & Arosio, P. (2014). Hepcidin antagonists for potential treatments of disorders with hepcidin excess. Frontiers in Pharmacology, 5, 86. [DOI:10.3389/fphar.2014.00086] [PMID] [PMCID]
Roghani, M., Joghataie, M. T., Jalili, M. R., & Baluchnejadmojarad, T. (2006). Time course of changes in passive avoidance and Y-maze performance in male diabetic rats. Iranian Biomedical Journal, 10(2), 99-104. http://ibj.pasteur.ac.ir/article-1-355-en.html
Smith, M. A., Harris, P. L., Sayre, L. M., & Perry, G. (1997). Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proceedings of the National Academy of Sciences of the United States of America, 94(18), 9866-8. [DOI:10.1073/pnas.94.18.9866] [PMID] [PMCID]
Van Campenhout, A., Van Campenhout, C., Lagrou, A. R., Abrams, P., Moorkens, G., & Van Gaal, L., et al. (2006). Impact of diabetes mellitus on the relationships between iron‐, inflammatory‐and oxidative stress status. Diabetes/Metabolism Research and Reviews, 22(6), 444-54. [DOI:10.1002/dmrr.635] [PMID]
Vecchi, C., Montosi, G., Garuti, C., Corradini, E., Sabelli, M., & Canali, S., et al. (2014). Gluconeogenic signals regulate iron homeostasis via hepcidin in mice. Gastroenterology, 146(4), 1060-9.E3. [DOI:10.1053/j.gastro.2013.12.016] [PMID] [PMCID]
Vela, D. (2018). The dual role of hepcidin in brain iron load and inflammation. Frontiers in Neuroscience, 12, 740. [DOI:10.3389/fnins.2018.00740] [PMID] [PMCID]
Wang, H., Li, H., Jiang, X., Shi, W., Shen, Z., & Li, M. (2014). Hepcidin is directly regulated by insulin and plays an important role in iron overload in streptozotocin-induced diabetic rats. Diabetes, 63(5), 1506-18. [DOI:10.2337/db13-1195] [PMID]
Wang, Q., Du, F., Qian, Z. M., Ge, X. H., Zhu, L., & Yung, W. H., et al. (2008). Lipopolysaccharide induces a significant increase in expression of iron regulatory hormone hepcidin in the cortex and substantia nigra in rat brain. Endocrinology, 149(8), 3920-5. [DOI:10.1210/en.2007-1626] [PMID] [PMCID]
Ward, R. J., Zucca, F. A., Duyn, J. H., Crichton, R. R., & Zecca, L. (2014). The role of iron in brain ageing and neurodegenerative disorders. The Lancet Neurology, 13(10), 1045-60. [DOI:10.1016/S1474-4422(14)70117-6]
Xiong, X. Y., Liu, L., Wang, F. X., Yang, Y. R., Hao, J. W., & Wang, P. F., et al. (2016). Toll-Like Receptor 4/MyD88–Mediated signaling of hepcidin expression causing brain iron accumulation, oxidative injury, and cognitive impairment after intracerebral hemorrhage. Circulation, 134(14), 1025-38. [DOI:10.1161/CIRCULATIONAHA.116.021881] [PMID]
Zhang, F. L., Hou, H. M., Yin, Z. N., Chang, L., Li, F. M., & Chen, Y. J., et al. (2017). Impairment of hepcidin upregulation by lipopolysaccharide in the interleukin-6 knockout mouse brain. Frontiers in Molecular Neuroscience, 10, 367. [DOI:10.3389/fnmol.2017.00367] [PMID] [PMCID]
Zhao, Y., Xin, Z., Li, N., Chang, Sh, Chen, Y., & Geng, L., et al. (2018). Nano-liposomes of lycopene reduces ischemic brain damage in rodents by regulating iron metabolism. Free Radical Biology and Medicine, 124, 1-11. [DOI:10.1016/j.freeradbiomed.2018.05.082]
Zhou, Y. F., Zhang, C., Yang, G., Qian, Z. M., Zhang, M. W., & Ma, J., et al. (2017). Hepcidin protects neuron from hemin-mediated injury by reducing iron. Frontiers in Physiology, 8, 332. [DOI:10.3389/fphys.2017.00332] [PMID]
Aigner, E., Felder, T. K., Oberkofler, H., Hahne, P., Auer, S., & Soyal, S., et al. (2013). Glucose acts as a regulator of serum iron by increasing serum hepcidin concentrations. The Journal of Nutritional Biochemistry, 24(1), 112-7. [DOI:10.1016/j.jnutbio.2012.02.017]
Backe, M. B., Moen, I. W., Ellervik, C., Hansen, J. B., & Mandrup-Poulsen, T. (2016). Iron regulation of pancreatic beta-cell functions and oxidative stress. Annual Review of Nutrition, 36, 241-73. [DOI:10.1146/annurev-nutr-071715-050939]
Baluchnejadmojarad, T., Kiasalari, Z., Afshin-Majd, S., Ghasemi, Z., & Roghani, M. (2017). S-allyl cysteine ameliorates cognitive deficits in streptozotocin-diabetic rats via suppression of oxidative stress, inflammation, and acetylcholinesterase. European Journal of Pharmacology, 794, 69-76. [DOI:10.1016/j.ejphar.2016.11.033]
Berg, D., Gerlach, M., Youdim, M. B. H., Double, K. L., Zecca L., & Riederer, P., et al. (2001). Brain iron pathways and their relevance to Parkinson’s disease. Journal of Neurochemistry, 79(2), 225-36. [DOI:10.1046/j.1471-4159.2001.00608.x]
Caret, J. C., Bougnères, P. F., & European Prediabetes Study Group. (1996). Treatment of prediabetic patients with insulin: Experience and future. Hormones, 45(Suppl 1), 44-7. [DOI:10.1159/000184829]
Chand, S. K., Singh, R. G., Pendharkar, S. A., & Petrov, M. S. (2018). Iron: A strong element in the pathogenesis of chronic hyperglycaemia after acute pancreatitis. Biological Trace Element Research, 183(1), 71-9. [DOI:10.1007/s12011-017-1131-y] [PMID]
Crane, P. K., Walker, R., Hubbard, R. A., Li, G., Nathan, D. M., & Zheng, H., et al. (2013). Glucose levels and risk of dementia. The New England Journal of Medicine, 369(6), 540-8. [DOI:10.1056/NEJMoa1215740] [PMID] [PMCID]
Di Marco, E., Jha, J. C., Sharma, A., Wilkinson-Berka, J. L., Jandeleit-Dahm, K. A., & de Haan, J. B. (2015). Are reactive oxygen species still the basis for diabetic complications? Clinical Science, 129(2), 199-216. [DOI:10.1042/CS20150093]
Du, F., Qian, Z. M., Luo, Q., Yung W. H., & Ke, Y. (2015). Hepcidin suppresses brain iron accumulation by downregulating iron transport proteins in iron-overloaded rats. Molecular Neurobiology, 52(1), 101-14. [DOI:10.1007/s12035-014-8847-x]
Dwyer, B. E., Zacharski, L. R., Balestra, D. J., Lerner, A. J., Perry, G., & Zhu, X., et al. (2009). Getting the iron out: Phlebotomy for Alzheimer’s disease? Medical Hypotheses, 72(5), 504-9. [DOI:10.1016/j.mehy.2008.12.029] [PMID] [PMCID]
Farajdokht, F., Soleimani, M., Mehrpouya, S., Barati, M., & Nahavandi, A. (2015). The role of hepcidin in chronic mild stress-induced depression. Neuroscience Letters, 588, 120-4. [DOI:10.1016/j.neulet.2015.01.008]
Ganz, T., & Nemeth, E. (2012). Hepcidin and iron homeostasis. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 1823(9), 1434-43. [DOI:10.1016/j.bbamcr.2012.01.014] [PMID] [PMCID]
Garton, T., Keep, R. F., Hua, Y., & Xi, G. (2016). Brain iron overload following intracranial haemorrhage. Stroke and Vascular Neurology, 1(4), 172-84. [DOI:10.1136/svn-2016-000042] [PMID] [PMCID]
Gong, J., Du, F., Qian, Z. M., Luo, Q. Q., Sheng, Y., & Yung, W. H., et al. (2016). Pre-treatment of rats with ad-hepcidin prevents iron-induced oxidative stress in the brain. Free Radical Biology and Medicine, 90, 126-32. [DOI:10.1016/j.freeradbiomed.2015.11.016]
Hatunic, M., Finucane, F. M., Brennan, A. M., Norris, S., Pacini, G., & Nolan, J. J. (2010). Effect of iron overload on glucose metabolism in patients with hereditary hemochromatosis. Metabolism, 59(3), 380-4. [DOI:10.1016/j.metabol.2009.08.006]
Kalalian-Moghaddam, H., Baluchnejadmojarad, T., Roghani, M., Goshadrou, F., & Ronaghi, A. (2013). Hippocampal synaptic plasticity restoration and anti-apoptotic effect underlie berberine improvement of learning and memory in streptozotocin-diabetic rats. European Journal of Pharmacology, 698(1-3), 259-66. [DOI:10.1016/j.ejphar.2012.10.020]
Ke, Y., & Qian, Z. M. (2007). Brain iron metabolism: Neurobiology and neurochemistry. Progress in Neurobiology, 83(3), 149-73. [DOI:10.1016/j.pneurobio.2007.07.009]
Kumar Datusalia, A., & Sunder Sharma, Sh. (2016). NF-κB inhibition resolves cognitive deficits in experimental type 2 diabetes mellitus through CREB and glutamate/GABA neurotransmitters pathway. Current Neurovascular Research, 13(1), 22-32. [DOI:10.2174/1567202612666151030104810]
Ma, P., Mao, X. Y., Li, X. L., Ma, Y., Qiao, Y. D., & Liu, Z. Q., et al. (2015). Baicalin alleviates diabetesassociated cognitive deficits via modulation of mitogen-activated protein kinase signaling, brainderived neurotrophic factor and apoptosis. Molecular Medicine Reports, 12(4), 6377-83. [DOI:10.3892/mmr.2015.4219]
Matrone, C., Djelloul, M., Taglialatela, G., & Perrone, L. (2015). Inflammatory risk factors and pathologies promoting Alzheimer’s disease progression: Is RAGE the key. Histology and Histopathology, 30(2), 125-39. [DOI:10.14670/HH-30.125] [PMID]
Murray, C. A., & Lynch, M. A. (1998). Evidence that increased hippocampal expression of the cytokine interleukin-1β is a common trigger for age-and stress-induced impairments in long-term potentiation. Journal of Neuroscience, 18(8), 2974-81. [DOI:10.1523/JNEUROSCI.18-08-02974.1998] [PMCID]
Nasri, S., Roghani, M., Baluchnejadmojarad, T., Balvardi, M., & Rabani, T. (2012). Chronic cyanidin‐3‐glucoside administration improves short‐term spatial recognition memory but not passive avoidance learning and memory in streptozotocin‐diabetic rats. Phytotherapy Research, 26(8), 1205-10. [DOI:10.1002/ptr.3702] [PMID]
Nicolas, G., Bennoun, M., Devaux, I., Beaumont, C., Grandchamp, B., & Kahn, A., et al. (2001). Lack of hepcidin gene expression and severe tissue iron overload in Upstream Stimulatory Factor 2 (USF2) knockout mice. Proceedings of the National Academy of Sciences of the United States of America, 98(15), 8780-5. [DOI:10.1073/pnas.151179498] [PMID] [PMCID]
Park, C. H., Valore, E. V., Waring, A. J., & Ganz, T. (2001). Hepcidin, a urinary antimicrobial peptide synthesized in the liver. Journal of Biological Chemistry, 276(11), 7806-10. [DOI:10.1074/jbc.M008922200] [PMID]
Poli, M., Poli, M., Asperti, M., Ruzzenenti, P., Regoni, M., & Arosio, P. (2014). Hepcidin antagonists for potential treatments of disorders with hepcidin excess. Frontiers in Pharmacology, 5, 86. [DOI:10.3389/fphar.2014.00086] [PMID] [PMCID]
Roghani, M., Joghataie, M. T., Jalili, M. R., & Baluchnejadmojarad, T. (2006). Time course of changes in passive avoidance and Y-maze performance in male diabetic rats. Iranian Biomedical Journal, 10(2), 99-104. http://ibj.pasteur.ac.ir/article-1-355-en.html
Smith, M. A., Harris, P. L., Sayre, L. M., & Perry, G. (1997). Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proceedings of the National Academy of Sciences of the United States of America, 94(18), 9866-8. [DOI:10.1073/pnas.94.18.9866] [PMID] [PMCID]
Van Campenhout, A., Van Campenhout, C., Lagrou, A. R., Abrams, P., Moorkens, G., & Van Gaal, L., et al. (2006). Impact of diabetes mellitus on the relationships between iron‐, inflammatory‐and oxidative stress status. Diabetes/Metabolism Research and Reviews, 22(6), 444-54. [DOI:10.1002/dmrr.635] [PMID]
Vecchi, C., Montosi, G., Garuti, C., Corradini, E., Sabelli, M., & Canali, S., et al. (2014). Gluconeogenic signals regulate iron homeostasis via hepcidin in mice. Gastroenterology, 146(4), 1060-9.E3. [DOI:10.1053/j.gastro.2013.12.016] [PMID] [PMCID]
Vela, D. (2018). The dual role of hepcidin in brain iron load and inflammation. Frontiers in Neuroscience, 12, 740. [DOI:10.3389/fnins.2018.00740] [PMID] [PMCID]
Wang, H., Li, H., Jiang, X., Shi, W., Shen, Z., & Li, M. (2014). Hepcidin is directly regulated by insulin and plays an important role in iron overload in streptozotocin-induced diabetic rats. Diabetes, 63(5), 1506-18. [DOI:10.2337/db13-1195] [PMID]
Wang, Q., Du, F., Qian, Z. M., Ge, X. H., Zhu, L., & Yung, W. H., et al. (2008). Lipopolysaccharide induces a significant increase in expression of iron regulatory hormone hepcidin in the cortex and substantia nigra in rat brain. Endocrinology, 149(8), 3920-5. [DOI:10.1210/en.2007-1626] [PMID] [PMCID]
Ward, R. J., Zucca, F. A., Duyn, J. H., Crichton, R. R., & Zecca, L. (2014). The role of iron in brain ageing and neurodegenerative disorders. The Lancet Neurology, 13(10), 1045-60. [DOI:10.1016/S1474-4422(14)70117-6]
Xiong, X. Y., Liu, L., Wang, F. X., Yang, Y. R., Hao, J. W., & Wang, P. F., et al. (2016). Toll-Like Receptor 4/MyD88–Mediated signaling of hepcidin expression causing brain iron accumulation, oxidative injury, and cognitive impairment after intracerebral hemorrhage. Circulation, 134(14), 1025-38. [DOI:10.1161/CIRCULATIONAHA.116.021881] [PMID]
Zhang, F. L., Hou, H. M., Yin, Z. N., Chang, L., Li, F. M., & Chen, Y. J., et al. (2017). Impairment of hepcidin upregulation by lipopolysaccharide in the interleukin-6 knockout mouse brain. Frontiers in Molecular Neuroscience, 10, 367. [DOI:10.3389/fnmol.2017.00367] [PMID] [PMCID]
Zhao, Y., Xin, Z., Li, N., Chang, Sh, Chen, Y., & Geng, L., et al. (2018). Nano-liposomes of lycopene reduces ischemic brain damage in rodents by regulating iron metabolism. Free Radical Biology and Medicine, 124, 1-11. [DOI:10.1016/j.freeradbiomed.2018.05.082]
Zhou, Y. F., Zhang, C., Yang, G., Qian, Z. M., Zhang, M. W., & Ma, J., et al. (2017). Hepcidin protects neuron from hemin-mediated injury by reducing iron. Frontiers in Physiology, 8, 332. [DOI:10.3389/fphys.2017.00332] [PMID]
Type of Study: Original |
Subject:
Cellular and molecular Neuroscience
Received: 2019/04/8 | Accepted: 2019/06/25 | Published: 2020/11/1
Received: 2019/04/8 | Accepted: 2019/06/25 | Published: 2020/11/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |