Volume 15, Issue 3 (May & Jun 2024)
BCN 2024, 15(3): 421-432 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Yadolahi F, Roostayi M M, Khalkhali Zavieh M, Rahimi A, Mehrpour M, Akbarzadeh Baghban A. Investigating Combined Balance Training and Transcranial Direct Current Stimulation for the Recovery of Postural Control Following Chronic Stroke: A Study Protocol. BCN 2024; 15 (3) :421-432
URL: http://bcn.iums.ac.ir/article-1-1442-en.html
URL: http://bcn.iums.ac.ir/article-1-1442-en.html
Fariba Yadolahi1 

 , Mohammad Mohsen Roostayi *2
, Mohammad Mohsen Roostayi *2 

 , Minoo Khalkhali Zavieh2
, Minoo Khalkhali Zavieh2 

 , Abas Rahimi1
, Abas Rahimi1 

 , Masoud Mehrpour3
, Masoud Mehrpour3 

 , Alireza Akbarzadeh Baghban4
, Alireza Akbarzadeh Baghban4 




 , Mohammad Mohsen Roostayi *2
, Mohammad Mohsen Roostayi *2 

 , Minoo Khalkhali Zavieh2
, Minoo Khalkhali Zavieh2 

 , Abas Rahimi1
, Abas Rahimi1 

 , Masoud Mehrpour3
, Masoud Mehrpour3 

 , Alireza Akbarzadeh Baghban4
, Alireza Akbarzadeh Baghban4 


1- Department of Physiotherapy, Physiotherapy Research Center, School of Rehabilitation, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Department of Physical Therapy, School of Rehabilitation Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Department of Neurology, Faculty of Medicine, Iran University of Medical Sciences, Tehran, Iran.
4- Department of Basic Sciences, Roteomics Research Center, School of Rehabilitation Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Department of Physical Therapy, School of Rehabilitation Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Department of Neurology, Faculty of Medicine, Iran University of Medical Sciences, Tehran, Iran.
4- Department of Basic Sciences, Roteomics Research Center, School of Rehabilitation Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Keywords: Transcranial direct current stimulation (tDCS), Chronic stroke, Motor cortex, Postural control, Complexity, Multiscale entropy
Full-Text [PDF 1554 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Stroke is the main cause of long-term disability in adults which affects the independence, social participation, and quality of life (QoL) of survivors. Stroke in low-to-middle-income countries is increasing and these countries are the most affected (Strong et al., 2007). Based on the results of a study in 2010, the annual incidence of first-ever stroke is 139 per 10 000 among the Iranian population which is significantly higher compared to most Western countries and also occurs at lower age ranges. The majority of individuals with stroke have postural deficits, moderate to severe walking disability, and reduced gait speeds (Azarpazhooh et al., 2010).
Balance impairment is considered a challenging issue for healthcare providers due to its high prevalence of falling, in addition to its physical and financial burden on society. An increased postural sway and asymmetrical weight distribution with the center of pressure (COP) shift toward the unaffected side, increased spontaneous postural sway, is characteristic of postural impairment in hemiplegic stroke patients (Roerdink et al., 2006; Geurts et al., 2005). Individuals with stroke also have sensory deficits, abnormal sensory reweighting, and muscle weakness. Rehabilitation methods which improve balance and balance-recovery reactions are crucial for reducing the cost of long-term care of stroke patients and to prevent such a load on the healthcare system (Harris et al., 2005). Balance training improves walking through effects on weight-bearing after stroke (de Haart et al., 2004; Yavuzer et al., 2006).
Previous studies have confirmed the limited effectiveness of sensory stimulation by transcutaneous electrical nerve stimulation, functional electrical stimulation, electromyography feedback, or body weight-supported treadmill training on balance and related activities of daily living in patients with stroke (Verheyden et al., 2013; Brewer et al., 2012). However, no particular physiotherapy approach was more successful than any other in the recovery of postural control and lower limb function (Geurts et al., 2005).
Evidence revealed that postural control is extremely influenced by the cerebral cortex and cognitive mechanisms. Moreover, the cerebral cortex plays a significant role in the control of locomotion (Jacobs & Horak, 2007). Conventional physiotherapy protocols for neurological disease have a limited potential for neural repair rehabilitation and techniques that promote neuroplastic changes claim to have significant functional achievement in patient’s recovery (Dimyan & Cohen, 2011).
tDCS
Post-stroke patients exhibit changes in motor cortical excitability and disrupted inter-hemispheric inhibition from the unaffected to the affected motor cortex. This is based on the theory that following a focal lesion output from the lesioned hemisphere declines and the balance of interhemispheric communication interrupts (Di Pino et al., 2014). tDCS has gained growing attention as a promising neurorehabilitation tool in recent years (Nair et al., 2011). tDCS elicits regional neuroplasticity by induction of weak intracerebral ionic current between a positively charged anode and a negatively charged cathode (Stagg & Nitsche, 2011). Different mechanisms, such as calcium-dependent synaptic plasticity of glutamatergic neurons and impact on glutamatergic plasticity due to reducing gamma-aminobutyric acid neurotransmission could explain the therapeutic effect of tDCS (Nitsche et al., 2003). Accordingly, tDCS is considered a safe, portable, and inexpensive modality to alter cortical excitability (Bikson et al., 2016). Trials with tDCS applications have established motor skill learning enhancement and improved new motor skill learning to enhance execution and skills in chronic stroke patients (Reis et al., 2009, Kaminski et al., 2016). Previous studies suggest that daily tDCS stimulation is required to lead to significant cortical plasticity (Alonzo et al., 2012). Galea and Celnik observed anodal tDCS (a-tDCS) enhances the retention of motor memories (Galea & Celnik, 2009). Moreover, the effectiveness of tDCS in stroke patients has been reported in recent systematic reviews (Marquez et al., 2015, Bastani & Jaberzadeh, 2012). A more recent meta-analysis of tDCS interventions in stroke rehabilitation concluded that a-tDCS over the lesioned hemisphere can enhance motor cortex excitability and improve upper limb function with rehabilitation interventions (Butler et al., 2013). Many studies have focused on the effects of tDCS on upper limb rehabilitation. A few tDCS interventions on lower limb function are mostly investigated in healthy individuals. Jeffry et al. showed that the excitability of corticospinal tract projections to the tibialis anterior muscle increased after a-tDCS (Jeffery et al., 2007). Others have emphasized the utility of anodal tDCS in increasing maximum leg pinch force in healthy volunteers and knee extension force in individuals with hemiparetic stroke (Tanaka et al., 2009; Tanaka et al., 2011). Beck et al. demonstrated that the leg motor area is mainly involved in postural tasks and quiet standing (Beck et al., 2007; Tokuno et al., 2009). There is evidence supporting the utility of the effectiveness of tDCS applied to the leg area of the primary motor cortex in the improvement of balance performance in subacute stroke (Kaski et al., 2013).
Remarkably, studies that combined tDCS with motor recovery protocols have yielded promising results. A recent maintains the ability of a-tDCS over the M1 leg area to enhance dynamic balance learning in healthy young adults, suggesting that tDCS over M1 is capable of modulating adaptive motor control processes in young adults (Kaminski et al., 2016).
The evaluation of postural stability is necessary for planning effective balance rehabilitation. Postural stability is provided by multiple physiological systems interacting with one another throughout multiple scales of time series. This highly complex process provides the postural control system the ability to adapt to the various stressors of everyday life (Lipsitz, 2002). At this point, determining the impaired balance with conventional measures of the COP (COP surface area, COP velocity) to quantify postural control may yield an incomplete picture of postural control and have some limitations for the proper identification of the integrity of the postural control system. The loss of complexity hypothesis was first expressed by Lipsitz and Goldberger (2002). This theory states that systems that exhibit a reduction in the number of dynamic interactions involved in regulating physiological performance lead to a failure of physiological function associated with ageing and disease (Lipsitz, 2002). Individuals with stroke demonstrated overall more regular sway during quiet standing than controls. Reduced physiologic complexity of postural sway has been linked to deterministic patterns and a deficit in adaptability to intrinsic and external perturbations. They showed that sway regularity decreased in the course of rehabilitation (Roerdink et al., 2006).
Multiscale entropy (MSE) is a method used to evaluate the complexity of postural sway, as revealed by COP time series recorded by a force plate (Costa et al., 2005). Recently, the use of MSE for examining COP dynamics has received considerable attention, particularly in older adults (Duarte & Sternad, 2008; Manor et al., 2010; Zhou et al., 2015). However, the effects of tDCS on postural sway complexity in chronic stroke are currently unknown. Hence, there is a critical need to explore the therapeutic effects of tDCS paired with balance training targeting postural control in chronic stroke patients. We hypothesized that a-tDCS is capable of enhancing the leg motor area in its excitability and is a promising approach to balance recovery in chronic stroke. Moreover, we suppose that tDCS would alter the postural sway dynamics as computed by MSE. To the best of our knowledge, no study has analyzed the effects of a-tDCS with balance rehabilitation in chronic stroke patients. This is the first study to identify tDCS objective outcomes and investigate the impact of the intervention on the variability of postural stability in chronic stroke.
2. Materials and Methods
Study design
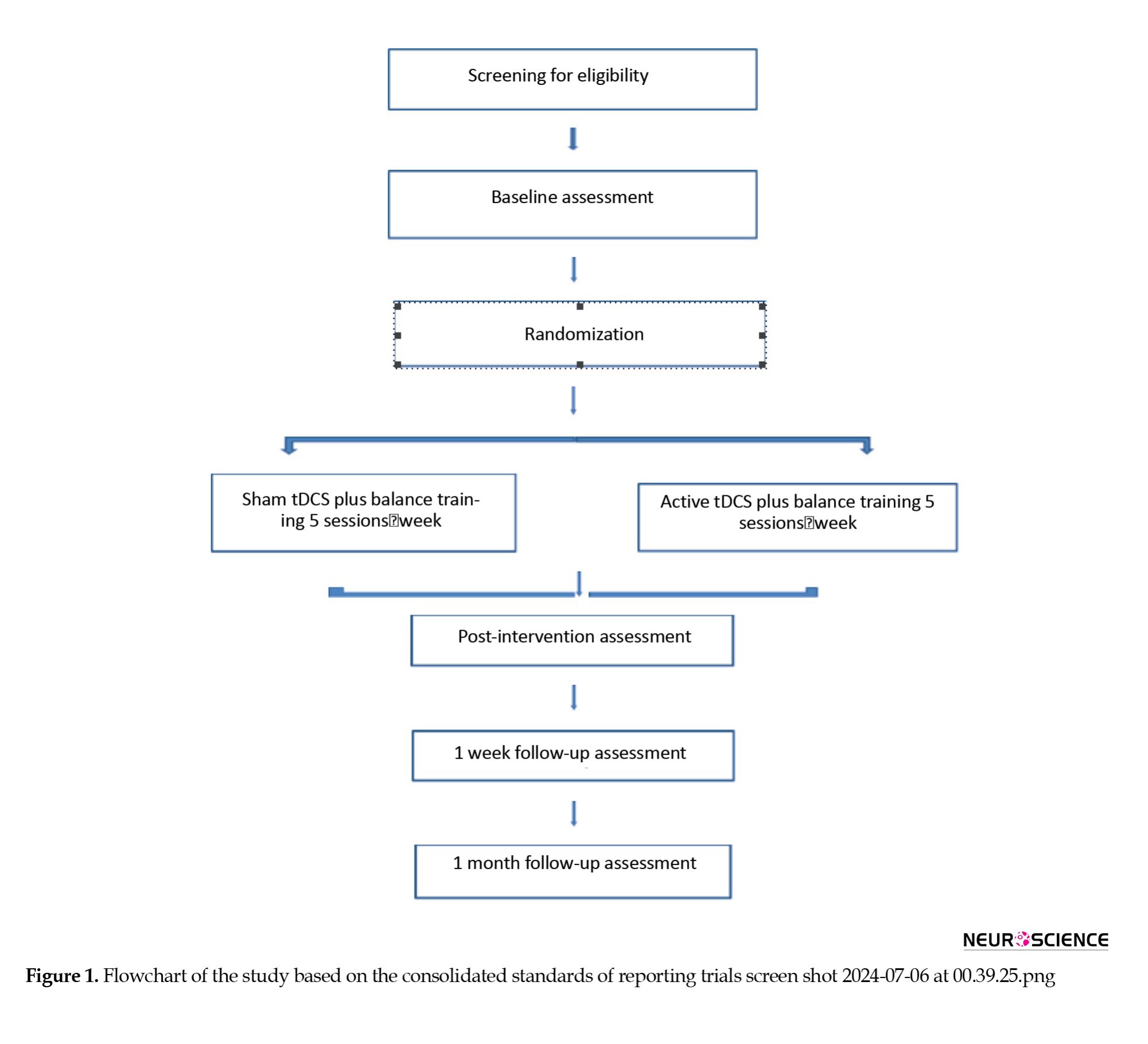
The study protocol follows the consolidated standards of reporting trial statements on randomized trials. In this randomized, sham-controlled, double-blinded study, the participants were randomly assigned to two groups: 1) a-tDCS plus balance training with the Biodex balance system, and 2) Sham tDCS plus Biodex balance training. All outcome measures were in 5 intervals: Pre-test, pos-test, after 5 sessions of intervention, after 1 week, and 1 month post intervention (Figure 1).
Study objectives and hypotheses
This study examines the efficacy of a-tDCS combined with Biodex balance training on postural control in chronic stroke patients using laboratory and clinical assessments. To analyze the postural behavior of standing stroke patients after the intervention, the changes in the amount and temporal structure of variability due to ischemic stroke by evaluating COP time series in chronic stroke patients were determined. It was hypothesized that subjects who undergo the anodal tDCS targeting primary leg motor cortex plus balance training program exhibit significant differences in the temporal structure and amount of variability in COP time series Berg balance scale, and timed up-and-go (TUG) test when compared to the control group.
Study participants
Chronic ischemic stroke patients with postural control impairments were recruited from the Multicenter University Hospitals within the healthcare system and through outpatient care programs. The inclusion criteria were as follows: age ≥18 years, first-ever unilateral ischemic stroke, chronic phase of recovery > 6 months, ability to walk 6 m supported or unsupported, ability to stand unsupported for at least 40 s with eyes closed, only ischemic stroke confirmed by computed tomography or magnetic resonance imaging. Meanwhile, the exclusion criteria were as follows: The use of any neuro- or psycho-active medications that alter the balance; any other neurological conditions or sensory disorders affecting postural control, such as brain tumor or substance abuse; orthopedic diseases; and ongoing/recent (within 3 months) balance rehabilitation. Patients with impaired ability to follow simple verbal instructions were also excluded from the study.
During the consent process, the investigator explained the benefits and risks of participation in the study and provided an informed consent form approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences. Only patients who provided written informed consent by signing the consent document were enrolled in the study. Safety was assessed daily throughout the study by monitoring adverse events during the active phase and at all follow-up time points and was routinely reviewed by the principal investigator.
Randomization and blinding procedure
Allocations were concealed in an opaque envelope and kept in a locked drawer. Using a sequence of computer-generated random numbers the number “1” or “2” was allocated to each group. They were opened by a research coordinator who was not involved in the data collection or analysis process. The participants and investigators (both trainers and assessors) were blinded to the group assignments.
In all sessions, the participants and the investigators were blinded to the intervention type. The experimenter who applied the intervention (active tDCS or sham) was different from the investigator who determined the outcome measures. The DC current was initially increasing in a ramp-like fashion over several seconds until it reached 2 mA which makes successful blinding of subjects possible. In the sham condition, the DC current was turned off slowly over a few seconds, out of the field of view of the patients. Double blinding is intended to minimize bias that could occur from participants’ perceptions of therapy or observer bias.
Sample size
Static balance, Berg balance scale, and TUG test served as primary outcome measures with all other assessments and time points serving as secondary outcome measures. Sample size and power calculations for the main study were based on repeated-measures analysis of variance with pre- to post-intervention changes in the Z score of the primary outcome from the initial pilot study. For each sample size calculation, power was set at 80% and a two-sided test at α=5%. Based on data from a previous study, the study involved 66 participants (Sohn et al., 2013) (Equation 1):

Intervention group
tDCS set up
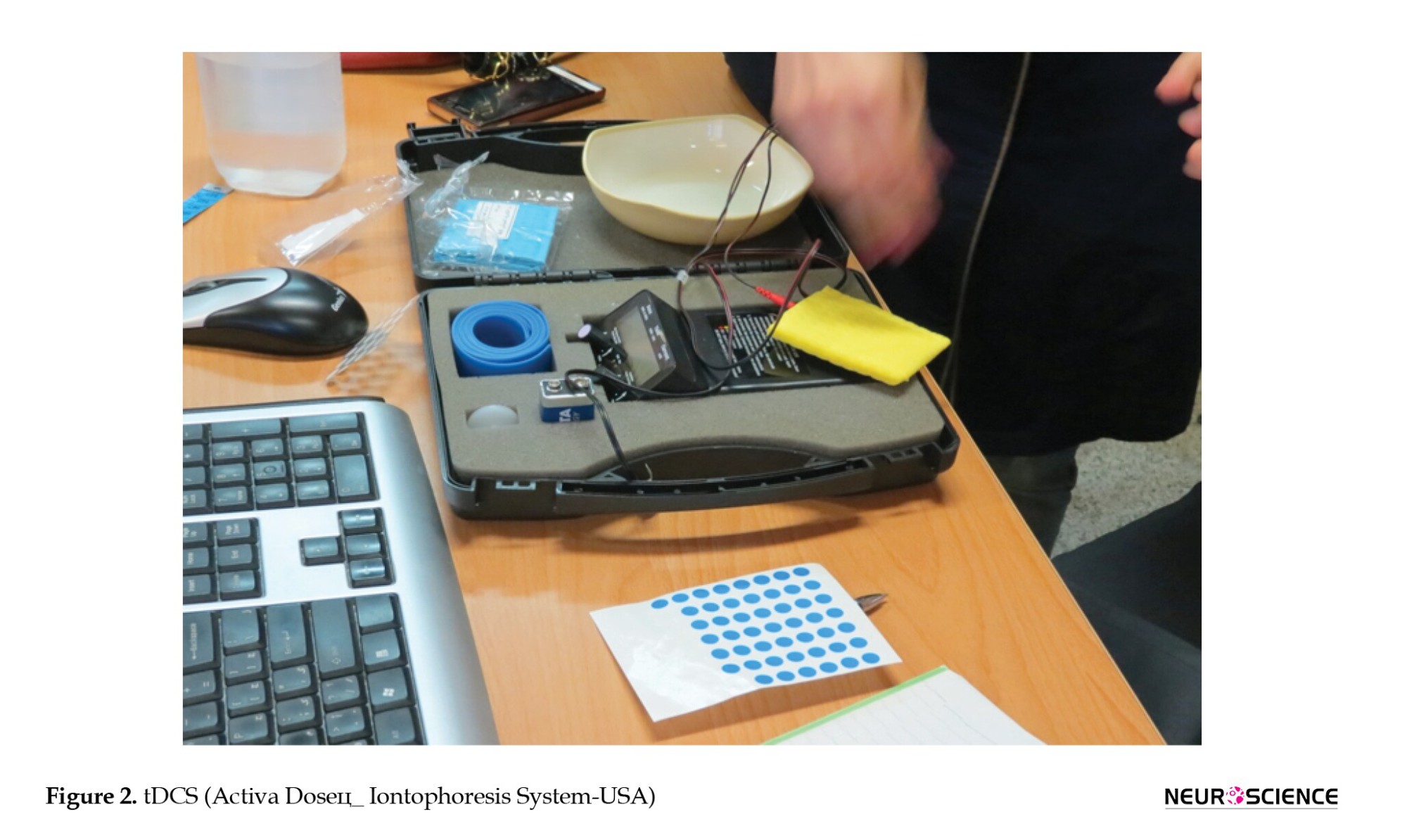
We used a battery-driven electrical stimulator (Activa DoseЦ_ Iontophoresis System-USA) connected to a 12 cm2 (3×4 cm) saline-soaked anode electrode and 35 cm2 saline-soaked reference electrodes placed on the contralateral supraorbital region for more focal current density (Bastani & Jaberzadeh, 2013) (Figure 2).

a-tDCS was delivered to the leg area (CZ) at a dose of 2 mA for 20 min for 5 sessions on consecutive days to elicit excitability of the leg motor area (Jeffery et al., 2007). The participants were exposed to daily balance training combined with active/sham tDCS. For all participants, the current was ramped up slowly at the onset of intervention to minimize abrupt tingling and maintain blinding.
Balance training
Once participants were randomly assigned to relevant groups, Biodex balance training was delivered under the supervision of the study’s principal investigator. The Biodex balance system uses a circular platform that is free to move in the anterior-posterior and medial-lateral axes simultaneously. The stability of the platform can be varied by adjusting the level of resistance given by the springs under the platform. The platform stability ranges from 1–8, with 1 representing the greatest instability. A lower resistance level indicates less stability in the platform. It provides visual feedback, on a screen at eye level, regarding the location of the participant’s COP. For example, if the participant’s weight shifts to the right, the cursor moves to the right. During the task, the participant attempts to maintain the cursor in a single position (static) or shift the cursor around the screen (dynamic), depending on the goal of the activity, mobility improving dynamic balance. The protocol of Biodex balance training discussed here was from the pilot study. All patients received balance training for 5 days which also included Biodex dynamic functional exercises, including a graded, feedback-driven approach combined with tDCS intervention. a-tDCS combined with Biodex balance training provides rich sensory stimuli with a modified excitability threshold of the leg M1 to enhance local synaptic efficacy and potentiate motor learning (Figure 3).
Control group
The participants in the control group received the Biodex balance training matched to the intervention group treatments with sham tDCS. During sham stimulation, the current ramped up for 30 s, came back down for 30 s and then remained off for the duration of the stimulation.
Outcome measures
This study compared two outcome measures as follows: Functional dynamic balance improvements according to the Berg scale and TUG test, and postural sway fluctuations according to linear and nonlinear analysis of force plate data. Functional scales and advanced laboratory systems are employed for assessing posture control. On the other hand, the complex behavior of standing postural control has been studied using different mathematical linear and nonlinear methods. In this study, we used both functional and advanced laboratory systems. The Berg balance scale was also used to get further information on functional posture deficits in participants. Furthermore, the analysis of COP dynamics and postural sway assessment could add information about the patient’s postural control.
Functional balance assessment
The Berg balance scale was used for the assessment of functional balance. In this study, we used the validated translated version of the Berg balance scale. It was a valid instrument used for the evaluation of the effectiveness of interventions and quantitative reports of function in research and clinical practice. This was a simple 14-item measure that addressed the performance of functional balance. Each item has a five-option ordinal scale ranging from 0 to 4 points, with a maximal overall score of 56. Scoring is based on both objective and subjective measures of the participant’s abilities to complete tasks, such as transfers, standing with feet together, and turning 360 degrees. The points are based on the time in which a position is maintained, the distance an upper limb can reach in front of the body and the time needed to complete the task (Salavati et al., 2012; Berg et al., 1995). The TUG test is widely used in the assessment of functional mobility and dynamic balance and measures the time (in seconds) necessary to stand up from a chair with armrests, walk 3 m, turn around, walk back to the chair, and sit down again.
Center of pressure analysis methods
The measures of the amount of variability included the range of COP displacement, which assessed the distance moved by the center of mass toward the outside of the base of support. COP data was obtained using a strain gauge Bertec 4060-10 force platform and Bertec AM-6504 amplifier (Bertec Corp., Columbus, OH). Postural sway was measured for 40 s while the participants stood on a force platform acquisition frequency (500 Hz). The patients were instructed to stand on the platform, barefoot, with feet shoulder-width apart and their arms relaxed at their sides, gazing fixed on a point in front of them. Foot position was marked to ensure consistency between trials. One trial was acquired with eyes open and one with closed eyes and between each trial participants were allowed to rest and sit down for 2 min. Postural measurements were obtained by the same rater in two sessions 48 h apart. The outputs of the force platform allowed us to compute the COP time series in the anterior/posterior direction COP and the medial/lateral direction COP. The first 10 s interval was discarded to avoid the transition phase in reaching the postural steady state. The antero-posterior and medio-lateral coordinates of the COP trajectory underwent post-acquisition filtering using a low-pass filter with a cut-off frequency based on our pilot study. Analyzed COP variables included ellipse area involving 95% of data (COP area), mean velocity (COP velocity), and amplitude displacement in both directions for anteroposterior and mediolateral directions, respectively, that were computed by the difference between maximal and minimum values obtained for each direction. We also analyzed the temporal structure of variability that included entropy analysis. It measures the self-similarity of the time series. Entropy analysis is a nonlinear measure that quantifies the predictability of a time series. It measures the probability that the distance between certain data point patterns will remain similar upon the next increment in time. Entropy-based methods have the potential as a valuable measure of detecting undetectable, subtle physiological changes after stroke. Several authors reported that entropy has the potential to assess specific postural behaviors induced by age, health conditions, and cognitive conditions (Busa et al., 2016; Chen & Jiang, 2014; Kang et al., 2009). In this study, the temporal structure was measured using nonlinear mathematical techniques, and the amount of variability was measured using linear mathematical techniques. With linear and nonlinear analysis, we could estimate which variable or variables change under different stance conditions to represent the clinical quantification of balance after intervention.
Complexity analysis of postural control
We estimated the degree of COP complexity, as defined by the presence of fluctuations existing over multiple timescales, using mean squared error (MSE) (Costa et al., 2005). Before MSE analysis, signals decomposition and reconstruction (EMD) was used to remove low-frequency trends and high-frequency noise in the raw time series, which was well-established previously (Gow et al., 2015). MSE uses sample entropy to quantify the degree of irregularity of a time series by employing the coarse-graining technique. Sample entropy reflects the negative natural logarithm of the conditional probability that a time series repeating itself within a tolerance of r for m points (pattern length), will also repeat itself for m + 1 points without self-matches. Thus, both the tolerance level r and pattern length m need to be set in the sample entropy algorithm for the MSE calculation. The coarse-grained time series for time scale n is the sequence of mean COP values provided by dividing the original time series into nonoverlapping windows with n data points and then computing the mean value for each window.
In this study, MSE will be computed for scale factors 1–20 to ensure sufficient samples (Richman & Moorman, 2000). Here, we used m=2 and r=15% of the standard deviation of the original signal (Equation 2):
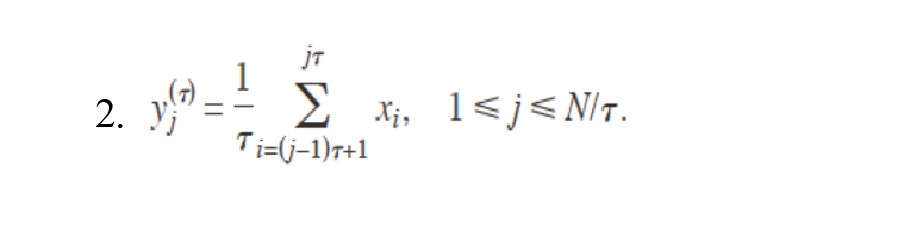
After plotting the sample entropy of each coarse-grained time series as a function of time scale, the COP complexity index (CI) was calculated. CI was identified as the area under the MSE resulting curve (Equation 3):

It provides us with an index for measuring the degree of the postural sway complexity. A larger area reflects higher greater complexity which means a more irregular and information-rich pattern while a lower CI value specifies poor adaptability. Then we compared the traditional COP analysis and CI (Costa et al., 2005; Duarte & Sternad, 2008, Jiang et al., 2013).
Adverse effects
tDCS is considered a safe non-invasive brain stimulation approach with a rare chance of adverse effects related to the procedure. At every tDCS session, all of the reported side effects related to tDCS, such as tingling, headache, itching, fatigue, pain, and problems concentrating, will be documented by the researcher who is applying the tDCS intervention.
Statistical data analyses
Patient characteristics were described using Means±SD, medians, and interquartile ranges (depending on whether data is normally distributed) and percentages. Group comparisons at baseline were performed using Student t-tests, Mann–Whitney U tests, and χ2 tests, where appropriate. Primary efficacy analysis was performed on an intention-to-treat basis. The effect of the two interventions (a-tDCS vs sham) on the outcome measures was determined using the two-way, repeated-measures analysis of variance with two factors: 1) Group (tDCS active vs sham group), and 2) Time (pre-training vs post-training and follow-ups). Paired sample t-tests with a 95% level of confidence were used to evaluate statistical differences between anterior/posterior and medial/lateral variables in each group. An α level of P<0.05 was set to determine the significance. Sensitivity analyses were employed for simulation and tested a range of scenarios assuming plausible arm-specific differences in outcomes for individuals who were lost to follow-up. Statistical analyses were performed using the SPSS software, version 22 (SPSS Inc., Chicago, IL).
3. Discussion
This study described the protocol of our ongoing clinical trial study in chronic stroke, where we test the efficacy and safety of balance rehabilitation combined with transcranial stimulation targeting the leg motor area in the affected hemisphere. Few exploratory studies have investigated the potential clinical efficacy of tDCS on balance and gait but not chronic stroke patients and follow-up (Kaminski et al., 2016; Sohn et al., 2013; Inukai et al., 2016; Kaminski et al., 2016; Sohn et al., 2013; Inukai et al., 2016). In our ongoing clinical trial, the effectiveness of tDCS cortical stimulation combined with Biodex balance training on a patient’s postural steadiness on chronic stroke with follow-up was tested. Because of the gap in balance rehabilitation in chronic stroke survivors, this proposed study is the first study that provides knowledge of the potential effects of tDCS intervention on postural control, including laboratory measurements and clinical tests. We enhance the current facility using a novel approach in balance rehabilitation by employing a-tDCS in chronic stroke with different electrode size that differs from the classical ones. COP fluctuation analysis provides information regarding the neuromuscular control of posture and therefore will reveal the intrinsic mechanisms responsible for maintaining balance, if there are problems with the intrinsic control of posture these will become apparent in the COP time series. In this study, functional and laboratory balance assessments were used. In summary, nonlinear measures along with linear measures to evaluate different aspects of the temporal structure and amount of variability in COP time series will offer a better paradigm to examine the effectiveness of interventions (Zhou et al., 2015; Fino et al., 2016). Using functional balance training which promotes rich multiple sensory stimuli will promote motor learning as motor learning depends on a change in the excitability of the cerebral cortex, a-tDCS stimulation is a way to modulate cortex activity, enhancing functional gains achieved with balance training. It has been suggested that balance rehabilitation intervention might exploit a crucial stage in which postural control and weight shifting are primed to be repaired, and this benefits walking late after a stroke (Yavuzer et al., 2006; Dimyan & Cohen, 2011).
4. Conclusion
The results have a strong contribution in rehabilitation settings which may even offer a new method to apply during long-term outpatient rehabilitation, and may eventually prime to reduce healthcare costs and improve mobility and QoL in these patients.
Study limitations
There were several limitations to our proposed study. Since no neuroimaging analysis is included, it would not be possible to estimate whether specific brain structures contributed to the intervention and also potential tDCS effects on neuronal networks. The result of this intervention can only be generalized to individuals with ischemic and chronic stroke. In our study, hemorrhagic patients and patients with cerebellar lesions are not recruited, so the effects of a-tDCS might be different in patients who also have defects in cerebellar regions or hemorrhagic stroke.
Ethical Considerations
Compliance with ethical guidelines
The study was conducted in line with the principles of the Declaration of Helsinki, and the Regulating Norms and Directives for Research Involving Human Subjects. The study protocol has been approved by the local and independent Ethics Committee of Shahid Beheshti University of Medical Sciences. The study is also registered by Iranian Registry of Clinical Trials (IRCT) (Code: IRCT2016121715840N1).
Funding
This paper was funded by Shahid Beheshti University of Medical Sciences.
Authors' contributions
Conceptualization: Fariba Yadolahi; Study design: Fariba Yadolahi and Alireza Akbarzadeh Baghban; Writing the original draft; Mohsen Roostayi, Minoo Khalkhali Zavieh, Abas Rahimi and Alireza Akbarzadeh Baghban; Review and editing: Alireza Akbarzadeh Baghban.
Conflict of interest
The authors declared no conflicts of interest.
Acknowledgments
The authors would like to thank the Physiotherapy Research Center, Shahid Beheshti University of Medical Sciences for their support.
References
Stroke is the main cause of long-term disability in adults which affects the independence, social participation, and quality of life (QoL) of survivors. Stroke in low-to-middle-income countries is increasing and these countries are the most affected (Strong et al., 2007). Based on the results of a study in 2010, the annual incidence of first-ever stroke is 139 per 10 000 among the Iranian population which is significantly higher compared to most Western countries and also occurs at lower age ranges. The majority of individuals with stroke have postural deficits, moderate to severe walking disability, and reduced gait speeds (Azarpazhooh et al., 2010).
Balance impairment is considered a challenging issue for healthcare providers due to its high prevalence of falling, in addition to its physical and financial burden on society. An increased postural sway and asymmetrical weight distribution with the center of pressure (COP) shift toward the unaffected side, increased spontaneous postural sway, is characteristic of postural impairment in hemiplegic stroke patients (Roerdink et al., 2006; Geurts et al., 2005). Individuals with stroke also have sensory deficits, abnormal sensory reweighting, and muscle weakness. Rehabilitation methods which improve balance and balance-recovery reactions are crucial for reducing the cost of long-term care of stroke patients and to prevent such a load on the healthcare system (Harris et al., 2005). Balance training improves walking through effects on weight-bearing after stroke (de Haart et al., 2004; Yavuzer et al., 2006).
Previous studies have confirmed the limited effectiveness of sensory stimulation by transcutaneous electrical nerve stimulation, functional electrical stimulation, electromyography feedback, or body weight-supported treadmill training on balance and related activities of daily living in patients with stroke (Verheyden et al., 2013; Brewer et al., 2012). However, no particular physiotherapy approach was more successful than any other in the recovery of postural control and lower limb function (Geurts et al., 2005).
Evidence revealed that postural control is extremely influenced by the cerebral cortex and cognitive mechanisms. Moreover, the cerebral cortex plays a significant role in the control of locomotion (Jacobs & Horak, 2007). Conventional physiotherapy protocols for neurological disease have a limited potential for neural repair rehabilitation and techniques that promote neuroplastic changes claim to have significant functional achievement in patient’s recovery (Dimyan & Cohen, 2011).
tDCS
Post-stroke patients exhibit changes in motor cortical excitability and disrupted inter-hemispheric inhibition from the unaffected to the affected motor cortex. This is based on the theory that following a focal lesion output from the lesioned hemisphere declines and the balance of interhemispheric communication interrupts (Di Pino et al., 2014). tDCS has gained growing attention as a promising neurorehabilitation tool in recent years (Nair et al., 2011). tDCS elicits regional neuroplasticity by induction of weak intracerebral ionic current between a positively charged anode and a negatively charged cathode (Stagg & Nitsche, 2011). Different mechanisms, such as calcium-dependent synaptic plasticity of glutamatergic neurons and impact on glutamatergic plasticity due to reducing gamma-aminobutyric acid neurotransmission could explain the therapeutic effect of tDCS (Nitsche et al., 2003). Accordingly, tDCS is considered a safe, portable, and inexpensive modality to alter cortical excitability (Bikson et al., 2016). Trials with tDCS applications have established motor skill learning enhancement and improved new motor skill learning to enhance execution and skills in chronic stroke patients (Reis et al., 2009, Kaminski et al., 2016). Previous studies suggest that daily tDCS stimulation is required to lead to significant cortical plasticity (Alonzo et al., 2012). Galea and Celnik observed anodal tDCS (a-tDCS) enhances the retention of motor memories (Galea & Celnik, 2009). Moreover, the effectiveness of tDCS in stroke patients has been reported in recent systematic reviews (Marquez et al., 2015, Bastani & Jaberzadeh, 2012). A more recent meta-analysis of tDCS interventions in stroke rehabilitation concluded that a-tDCS over the lesioned hemisphere can enhance motor cortex excitability and improve upper limb function with rehabilitation interventions (Butler et al., 2013). Many studies have focused on the effects of tDCS on upper limb rehabilitation. A few tDCS interventions on lower limb function are mostly investigated in healthy individuals. Jeffry et al. showed that the excitability of corticospinal tract projections to the tibialis anterior muscle increased after a-tDCS (Jeffery et al., 2007). Others have emphasized the utility of anodal tDCS in increasing maximum leg pinch force in healthy volunteers and knee extension force in individuals with hemiparetic stroke (Tanaka et al., 2009; Tanaka et al., 2011). Beck et al. demonstrated that the leg motor area is mainly involved in postural tasks and quiet standing (Beck et al., 2007; Tokuno et al., 2009). There is evidence supporting the utility of the effectiveness of tDCS applied to the leg area of the primary motor cortex in the improvement of balance performance in subacute stroke (Kaski et al., 2013).
Remarkably, studies that combined tDCS with motor recovery protocols have yielded promising results. A recent maintains the ability of a-tDCS over the M1 leg area to enhance dynamic balance learning in healthy young adults, suggesting that tDCS over M1 is capable of modulating adaptive motor control processes in young adults (Kaminski et al., 2016).
The evaluation of postural stability is necessary for planning effective balance rehabilitation. Postural stability is provided by multiple physiological systems interacting with one another throughout multiple scales of time series. This highly complex process provides the postural control system the ability to adapt to the various stressors of everyday life (Lipsitz, 2002). At this point, determining the impaired balance with conventional measures of the COP (COP surface area, COP velocity) to quantify postural control may yield an incomplete picture of postural control and have some limitations for the proper identification of the integrity of the postural control system. The loss of complexity hypothesis was first expressed by Lipsitz and Goldberger (2002). This theory states that systems that exhibit a reduction in the number of dynamic interactions involved in regulating physiological performance lead to a failure of physiological function associated with ageing and disease (Lipsitz, 2002). Individuals with stroke demonstrated overall more regular sway during quiet standing than controls. Reduced physiologic complexity of postural sway has been linked to deterministic patterns and a deficit in adaptability to intrinsic and external perturbations. They showed that sway regularity decreased in the course of rehabilitation (Roerdink et al., 2006).
Multiscale entropy (MSE) is a method used to evaluate the complexity of postural sway, as revealed by COP time series recorded by a force plate (Costa et al., 2005). Recently, the use of MSE for examining COP dynamics has received considerable attention, particularly in older adults (Duarte & Sternad, 2008; Manor et al., 2010; Zhou et al., 2015). However, the effects of tDCS on postural sway complexity in chronic stroke are currently unknown. Hence, there is a critical need to explore the therapeutic effects of tDCS paired with balance training targeting postural control in chronic stroke patients. We hypothesized that a-tDCS is capable of enhancing the leg motor area in its excitability and is a promising approach to balance recovery in chronic stroke. Moreover, we suppose that tDCS would alter the postural sway dynamics as computed by MSE. To the best of our knowledge, no study has analyzed the effects of a-tDCS with balance rehabilitation in chronic stroke patients. This is the first study to identify tDCS objective outcomes and investigate the impact of the intervention on the variability of postural stability in chronic stroke.
2. Materials and Methods
Study design
The study protocol follows the consolidated standards of reporting trial statements on randomized trials. In this randomized, sham-controlled, double-blinded study, the participants were randomly assigned to two groups: 1) a-tDCS plus balance training with the Biodex balance system, and 2) Sham tDCS plus Biodex balance training. All outcome measures were in 5 intervals: Pre-test, pos-test, after 5 sessions of intervention, after 1 week, and 1 month post intervention (Figure 1).

Study objectives and hypotheses
This study examines the efficacy of a-tDCS combined with Biodex balance training on postural control in chronic stroke patients using laboratory and clinical assessments. To analyze the postural behavior of standing stroke patients after the intervention, the changes in the amount and temporal structure of variability due to ischemic stroke by evaluating COP time series in chronic stroke patients were determined. It was hypothesized that subjects who undergo the anodal tDCS targeting primary leg motor cortex plus balance training program exhibit significant differences in the temporal structure and amount of variability in COP time series Berg balance scale, and timed up-and-go (TUG) test when compared to the control group.
Study participants
Chronic ischemic stroke patients with postural control impairments were recruited from the Multicenter University Hospitals within the healthcare system and through outpatient care programs. The inclusion criteria were as follows: age ≥18 years, first-ever unilateral ischemic stroke, chronic phase of recovery > 6 months, ability to walk 6 m supported or unsupported, ability to stand unsupported for at least 40 s with eyes closed, only ischemic stroke confirmed by computed tomography or magnetic resonance imaging. Meanwhile, the exclusion criteria were as follows: The use of any neuro- or psycho-active medications that alter the balance; any other neurological conditions or sensory disorders affecting postural control, such as brain tumor or substance abuse; orthopedic diseases; and ongoing/recent (within 3 months) balance rehabilitation. Patients with impaired ability to follow simple verbal instructions were also excluded from the study.
During the consent process, the investigator explained the benefits and risks of participation in the study and provided an informed consent form approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences. Only patients who provided written informed consent by signing the consent document were enrolled in the study. Safety was assessed daily throughout the study by monitoring adverse events during the active phase and at all follow-up time points and was routinely reviewed by the principal investigator.
Randomization and blinding procedure
Allocations were concealed in an opaque envelope and kept in a locked drawer. Using a sequence of computer-generated random numbers the number “1” or “2” was allocated to each group. They were opened by a research coordinator who was not involved in the data collection or analysis process. The participants and investigators (both trainers and assessors) were blinded to the group assignments.
In all sessions, the participants and the investigators were blinded to the intervention type. The experimenter who applied the intervention (active tDCS or sham) was different from the investigator who determined the outcome measures. The DC current was initially increasing in a ramp-like fashion over several seconds until it reached 2 mA which makes successful blinding of subjects possible. In the sham condition, the DC current was turned off slowly over a few seconds, out of the field of view of the patients. Double blinding is intended to minimize bias that could occur from participants’ perceptions of therapy or observer bias.
Sample size
Static balance, Berg balance scale, and TUG test served as primary outcome measures with all other assessments and time points serving as secondary outcome measures. Sample size and power calculations for the main study were based on repeated-measures analysis of variance with pre- to post-intervention changes in the Z score of the primary outcome from the initial pilot study. For each sample size calculation, power was set at 80% and a two-sided test at α=5%. Based on data from a previous study, the study involved 66 participants (Sohn et al., 2013) (Equation 1):

Intervention group
tDCS set up
We used a battery-driven electrical stimulator (Activa DoseЦ_ Iontophoresis System-USA) connected to a 12 cm2 (3×4 cm) saline-soaked anode electrode and 35 cm2 saline-soaked reference electrodes placed on the contralateral supraorbital region for more focal current density (Bastani & Jaberzadeh, 2013) (Figure 2).

a-tDCS was delivered to the leg area (CZ) at a dose of 2 mA for 20 min for 5 sessions on consecutive days to elicit excitability of the leg motor area (Jeffery et al., 2007). The participants were exposed to daily balance training combined with active/sham tDCS. For all participants, the current was ramped up slowly at the onset of intervention to minimize abrupt tingling and maintain blinding.
Balance training
Once participants were randomly assigned to relevant groups, Biodex balance training was delivered under the supervision of the study’s principal investigator. The Biodex balance system uses a circular platform that is free to move in the anterior-posterior and medial-lateral axes simultaneously. The stability of the platform can be varied by adjusting the level of resistance given by the springs under the platform. The platform stability ranges from 1–8, with 1 representing the greatest instability. A lower resistance level indicates less stability in the platform. It provides visual feedback, on a screen at eye level, regarding the location of the participant’s COP. For example, if the participant’s weight shifts to the right, the cursor moves to the right. During the task, the participant attempts to maintain the cursor in a single position (static) or shift the cursor around the screen (dynamic), depending on the goal of the activity, mobility improving dynamic balance. The protocol of Biodex balance training discussed here was from the pilot study. All patients received balance training for 5 days which also included Biodex dynamic functional exercises, including a graded, feedback-driven approach combined with tDCS intervention. a-tDCS combined with Biodex balance training provides rich sensory stimuli with a modified excitability threshold of the leg M1 to enhance local synaptic efficacy and potentiate motor learning (Figure 3).

Control group
The participants in the control group received the Biodex balance training matched to the intervention group treatments with sham tDCS. During sham stimulation, the current ramped up for 30 s, came back down for 30 s and then remained off for the duration of the stimulation.
Outcome measures
This study compared two outcome measures as follows: Functional dynamic balance improvements according to the Berg scale and TUG test, and postural sway fluctuations according to linear and nonlinear analysis of force plate data. Functional scales and advanced laboratory systems are employed for assessing posture control. On the other hand, the complex behavior of standing postural control has been studied using different mathematical linear and nonlinear methods. In this study, we used both functional and advanced laboratory systems. The Berg balance scale was also used to get further information on functional posture deficits in participants. Furthermore, the analysis of COP dynamics and postural sway assessment could add information about the patient’s postural control.
Functional balance assessment
The Berg balance scale was used for the assessment of functional balance. In this study, we used the validated translated version of the Berg balance scale. It was a valid instrument used for the evaluation of the effectiveness of interventions and quantitative reports of function in research and clinical practice. This was a simple 14-item measure that addressed the performance of functional balance. Each item has a five-option ordinal scale ranging from 0 to 4 points, with a maximal overall score of 56. Scoring is based on both objective and subjective measures of the participant’s abilities to complete tasks, such as transfers, standing with feet together, and turning 360 degrees. The points are based on the time in which a position is maintained, the distance an upper limb can reach in front of the body and the time needed to complete the task (Salavati et al., 2012; Berg et al., 1995). The TUG test is widely used in the assessment of functional mobility and dynamic balance and measures the time (in seconds) necessary to stand up from a chair with armrests, walk 3 m, turn around, walk back to the chair, and sit down again.
Center of pressure analysis methods
The measures of the amount of variability included the range of COP displacement, which assessed the distance moved by the center of mass toward the outside of the base of support. COP data was obtained using a strain gauge Bertec 4060-10 force platform and Bertec AM-6504 amplifier (Bertec Corp., Columbus, OH). Postural sway was measured for 40 s while the participants stood on a force platform acquisition frequency (500 Hz). The patients were instructed to stand on the platform, barefoot, with feet shoulder-width apart and their arms relaxed at their sides, gazing fixed on a point in front of them. Foot position was marked to ensure consistency between trials. One trial was acquired with eyes open and one with closed eyes and between each trial participants were allowed to rest and sit down for 2 min. Postural measurements were obtained by the same rater in two sessions 48 h apart. The outputs of the force platform allowed us to compute the COP time series in the anterior/posterior direction COP and the medial/lateral direction COP. The first 10 s interval was discarded to avoid the transition phase in reaching the postural steady state. The antero-posterior and medio-lateral coordinates of the COP trajectory underwent post-acquisition filtering using a low-pass filter with a cut-off frequency based on our pilot study. Analyzed COP variables included ellipse area involving 95% of data (COP area), mean velocity (COP velocity), and amplitude displacement in both directions for anteroposterior and mediolateral directions, respectively, that were computed by the difference between maximal and minimum values obtained for each direction. We also analyzed the temporal structure of variability that included entropy analysis. It measures the self-similarity of the time series. Entropy analysis is a nonlinear measure that quantifies the predictability of a time series. It measures the probability that the distance between certain data point patterns will remain similar upon the next increment in time. Entropy-based methods have the potential as a valuable measure of detecting undetectable, subtle physiological changes after stroke. Several authors reported that entropy has the potential to assess specific postural behaviors induced by age, health conditions, and cognitive conditions (Busa et al., 2016; Chen & Jiang, 2014; Kang et al., 2009). In this study, the temporal structure was measured using nonlinear mathematical techniques, and the amount of variability was measured using linear mathematical techniques. With linear and nonlinear analysis, we could estimate which variable or variables change under different stance conditions to represent the clinical quantification of balance after intervention.
Complexity analysis of postural control
We estimated the degree of COP complexity, as defined by the presence of fluctuations existing over multiple timescales, using mean squared error (MSE) (Costa et al., 2005). Before MSE analysis, signals decomposition and reconstruction (EMD) was used to remove low-frequency trends and high-frequency noise in the raw time series, which was well-established previously (Gow et al., 2015). MSE uses sample entropy to quantify the degree of irregularity of a time series by employing the coarse-graining technique. Sample entropy reflects the negative natural logarithm of the conditional probability that a time series repeating itself within a tolerance of r for m points (pattern length), will also repeat itself for m + 1 points without self-matches. Thus, both the tolerance level r and pattern length m need to be set in the sample entropy algorithm for the MSE calculation. The coarse-grained time series for time scale n is the sequence of mean COP values provided by dividing the original time series into nonoverlapping windows with n data points and then computing the mean value for each window.
In this study, MSE will be computed for scale factors 1–20 to ensure sufficient samples (Richman & Moorman, 2000). Here, we used m=2 and r=15% of the standard deviation of the original signal (Equation 2):
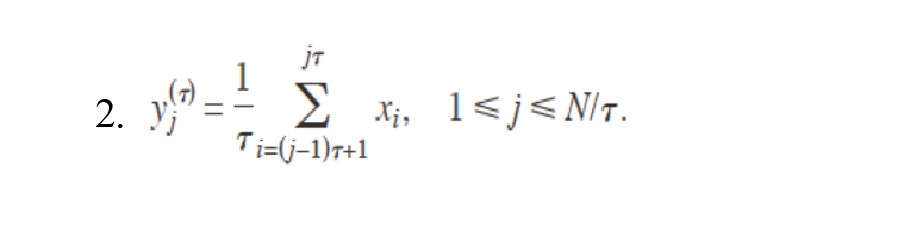
After plotting the sample entropy of each coarse-grained time series as a function of time scale, the COP complexity index (CI) was calculated. CI was identified as the area under the MSE resulting curve (Equation 3):

It provides us with an index for measuring the degree of the postural sway complexity. A larger area reflects higher greater complexity which means a more irregular and information-rich pattern while a lower CI value specifies poor adaptability. Then we compared the traditional COP analysis and CI (Costa et al., 2005; Duarte & Sternad, 2008, Jiang et al., 2013).
Adverse effects
tDCS is considered a safe non-invasive brain stimulation approach with a rare chance of adverse effects related to the procedure. At every tDCS session, all of the reported side effects related to tDCS, such as tingling, headache, itching, fatigue, pain, and problems concentrating, will be documented by the researcher who is applying the tDCS intervention.
Statistical data analyses
Patient characteristics were described using Means±SD, medians, and interquartile ranges (depending on whether data is normally distributed) and percentages. Group comparisons at baseline were performed using Student t-tests, Mann–Whitney U tests, and χ2 tests, where appropriate. Primary efficacy analysis was performed on an intention-to-treat basis. The effect of the two interventions (a-tDCS vs sham) on the outcome measures was determined using the two-way, repeated-measures analysis of variance with two factors: 1) Group (tDCS active vs sham group), and 2) Time (pre-training vs post-training and follow-ups). Paired sample t-tests with a 95% level of confidence were used to evaluate statistical differences between anterior/posterior and medial/lateral variables in each group. An α level of P<0.05 was set to determine the significance. Sensitivity analyses were employed for simulation and tested a range of scenarios assuming plausible arm-specific differences in outcomes for individuals who were lost to follow-up. Statistical analyses were performed using the SPSS software, version 22 (SPSS Inc., Chicago, IL).
3. Discussion
This study described the protocol of our ongoing clinical trial study in chronic stroke, where we test the efficacy and safety of balance rehabilitation combined with transcranial stimulation targeting the leg motor area in the affected hemisphere. Few exploratory studies have investigated the potential clinical efficacy of tDCS on balance and gait but not chronic stroke patients and follow-up (Kaminski et al., 2016; Sohn et al., 2013; Inukai et al., 2016; Kaminski et al., 2016; Sohn et al., 2013; Inukai et al., 2016). In our ongoing clinical trial, the effectiveness of tDCS cortical stimulation combined with Biodex balance training on a patient’s postural steadiness on chronic stroke with follow-up was tested. Because of the gap in balance rehabilitation in chronic stroke survivors, this proposed study is the first study that provides knowledge of the potential effects of tDCS intervention on postural control, including laboratory measurements and clinical tests. We enhance the current facility using a novel approach in balance rehabilitation by employing a-tDCS in chronic stroke with different electrode size that differs from the classical ones. COP fluctuation analysis provides information regarding the neuromuscular control of posture and therefore will reveal the intrinsic mechanisms responsible for maintaining balance, if there are problems with the intrinsic control of posture these will become apparent in the COP time series. In this study, functional and laboratory balance assessments were used. In summary, nonlinear measures along with linear measures to evaluate different aspects of the temporal structure and amount of variability in COP time series will offer a better paradigm to examine the effectiveness of interventions (Zhou et al., 2015; Fino et al., 2016). Using functional balance training which promotes rich multiple sensory stimuli will promote motor learning as motor learning depends on a change in the excitability of the cerebral cortex, a-tDCS stimulation is a way to modulate cortex activity, enhancing functional gains achieved with balance training. It has been suggested that balance rehabilitation intervention might exploit a crucial stage in which postural control and weight shifting are primed to be repaired, and this benefits walking late after a stroke (Yavuzer et al., 2006; Dimyan & Cohen, 2011).
4. Conclusion
The results have a strong contribution in rehabilitation settings which may even offer a new method to apply during long-term outpatient rehabilitation, and may eventually prime to reduce healthcare costs and improve mobility and QoL in these patients.
Study limitations
There were several limitations to our proposed study. Since no neuroimaging analysis is included, it would not be possible to estimate whether specific brain structures contributed to the intervention and also potential tDCS effects on neuronal networks. The result of this intervention can only be generalized to individuals with ischemic and chronic stroke. In our study, hemorrhagic patients and patients with cerebellar lesions are not recruited, so the effects of a-tDCS might be different in patients who also have defects in cerebellar regions or hemorrhagic stroke.
Ethical Considerations
Compliance with ethical guidelines
The study was conducted in line with the principles of the Declaration of Helsinki, and the Regulating Norms and Directives for Research Involving Human Subjects. The study protocol has been approved by the local and independent Ethics Committee of Shahid Beheshti University of Medical Sciences. The study is also registered by Iranian Registry of Clinical Trials (IRCT) (Code: IRCT2016121715840N1).
Funding
This paper was funded by Shahid Beheshti University of Medical Sciences.
Authors' contributions
Conceptualization: Fariba Yadolahi; Study design: Fariba Yadolahi and Alireza Akbarzadeh Baghban; Writing the original draft; Mohsen Roostayi, Minoo Khalkhali Zavieh, Abas Rahimi and Alireza Akbarzadeh Baghban; Review and editing: Alireza Akbarzadeh Baghban.
Conflict of interest
The authors declared no conflicts of interest.
Acknowledgments
The authors would like to thank the Physiotherapy Research Center, Shahid Beheshti University of Medical Sciences for their support.
References
Alnzo, A., Brassil, J., Taylor, J. L., Martin, D., & Loo, C. K. (2012). Daily transcranial direct current stimulation (tDCS) leads to greater increases in cortical excitability than second daily transcranial direct current stimulation. Brain Stimulation, 5(3), 208–213. [DOI:10.1016/j.brs.2011.04.006] [PMID]
Azarpazhooh, M. R., Etemadi, M. M., Donnan, G. A., Mokhber, N., Majdi, M. R., & Ghayour-Mobarhan, M., et al. (2010). Excessive incidence of stroke in Iran: Evidence from the Mashhad Stroke Incidence Study (MSIS), a population-based study of stroke in the Middle East. Stroke, 41(1), e3–e10. [DOI:10.1161/STROKEAHA.109.559708] [PMID]
Bastani, A., & Jaberzadeh, S. (2012). Does anodal transcranial direct current stimulation enhance excitability of the motor cortex and motor function in healthy individuals and subjects with stroke: A systematic review and meta-analysis. Clinical Neurophysiology : Official Journal of the International Federation of Clinical Neurophysiology, 123(4), 644–657. [DOI:10.1016/j.clinph.2011.08.029] [PMID]
Bastani, A., & Jaberzadeh, S. (2013). A-tDCS differential modulation of corticospinal excitability: The effects of electrode size. Brain Stimulation, 6(6), 932–937. [DOI:10.1016/j.brs.2013.04.005] [PMID]
Beck, S., Taube, W., Gruber, M., Amtage, F., Gollhofer, A., & Schubert, M. (2007). Task-specific changes in motor evoked potentials of lower limb muscles after different training interventions. Brain Research, 1179, 51–60. [DOI:10.1016/j.brainres.2007.08.048] [PMID]
Berg, K., Wood-Dauphinee, S., & Williams, J. I. (1995). The Balance Scale: Reliability assessment with elderly residents and patients with an acute stroke. Scandinavian Journal of Rehabilitation Medicine, 27(1), 27–36. [PMID]
Bikson, M., Grossman, P., Thomas, C., Zannou, A. L., Jiang, J., & Adnan, T., et al. (2016). Safety of transcranial direct current stimulation: Evidence based update 2016. Brain Stimulation, 9(5), 641–661. [DOI:10.1016/j.brs.2016.06.004] [PMID] [PMCID]
Brewer, L., Horgan, F., Hickey, A., & Williams, D. (2013). Stroke rehabilitation: Recent advances and future therapies. QJM: Monthly Journal of the Association of Physicians, 106(1), 11–25. [DOI:10.1093/qjmed/hcs174] [PMID]
Busa, M. A., Jones, S. L., Hamill, J., & van Emmerik, R. E. (2016). Multiscale entropy identifies differences in complexity in postural control in women with multiple sclerosis. Gait & Posture, 45, 7–11. [DOI:10.1016/j.gaitpost.2015.12.007] [PMID]
Butler, A. J., Shuster, M., O'Hara, E., Hurley, K., Middlebrooks, D., & Guilkey, K. (2013). A meta-analysis of the efficacy of anodal transcranial direct current stimulation for upper limb motor recovery in stroke survivors. Journal of Hand Therapy: Official Journal of the American Society of Hand Therapists, 26(2), 162–171. [DOI:10.1016/j.jht.2012.07.002] [PMID]
Chen, M. S., & Jiang, B. C. (2014). Resistance training exercise program for intervention to enhance gait function in elderly chronically ill patients: Multivariate multiscale entropy for center of pressure signal analysis. Computational and Mathematical Methods in Medicine, 2014, 471356. [DOI:10.1155/2014/471356] [PMID] [PMCID]
Costa, M., Goldberger, A. L., & Peng, C. K. (2005). Multiscale entropy analysis of biological signals. Physical Review. E, Statistical, Nonlinear, and Soft Matter Physics, 71(2 Pt 1), 021906. [DOI:10.1103/PhysRevE.71.021906] [PMID]
de Haart, M., Geurts, A. C., Huidekoper, S. C., Fasotti, L., & van Limbeek, J. (2004). Recovery of standing balance in postacute stroke patients: A rehabilitation cohort study. Archives of Physical Medicine and Rehabilitation, 85(6), 886–895.[DOI:10.1016/j.apmr.2003.05.012] [PMID]
Di Pino, G., Pellegrino, G., Assenza, G., Capone, F., Ferreri, F., & Formica, D., et al. (2014). Modulation of brain plasticity in stroke: A novel model for neurorehabilitation. Nature Reviews. Neurology, 10(10), 597–608. [DOI:10.1038/nrneurol.2014.162] [PMID]
Dimyan, M. A., & Cohen, L. G. (2011). Neuroplasticity in the context of motor rehabilitation after stroke. Nature Reviews. Neurology, 7(2), 76–85. [DOI:10.1038/nrneurol.2010.200] [PMID] [PMCID]
Duarte, M., & Sternad, D. (2008). Complexity of human postural control in young and older adults during prolonged standing. Experimental Brain Research, 191(3), 265–276. [DOI:10.1007/s00221-008-1521-7] [PMID]
Fino, P. C., Mojdehi, A. R., Adjerid, K., Habibi, M., Lockhart, T. E., & Ross, S. D. (2016). Comparing postural stability entropy analyses to differentiate fallers and non-fallers. Annals of Biomedical Engineering, 44(5), 1636–1645. [DOI:10.1007/s10439-015-1479-0] [PMID] [PMCID]
Galea, J. M., & Celnik, P. (2009). Brain polarization enhances the formation and retention of motor memories. Journal of Neurophysiology, 102(1), 294–301. [DOI:10.1152/jn.00184.2009] [PMID] [PMCID]
Geurts, A. C., de Haart, M., van Nes, I. J., & Duysens, J. (2005). A review of standing balance recovery from stroke. Gait & Posture, 22(3), 267–281. [DOI:10.1016/j.gaitpost.2004.10.002] [PMID]
Gow, B. J., Peng, C. K., Wayne, P. M., & Ahn, A. C. (2015). Multiscale entropy analysis of center-of-pressure dynamics in human postural control: Methodological considerations. Entropy, 17(12), 7926-7947. [DOI:10.3390/e17127849]
Harris, J. E., Eng, J. J., Marigold, D. S., Tokuno, C. D., & Louis, C. L. (2005). Relationship of balance and mobility to fall incidence in people with chronic stroke. Physical Therapy, 85(2), 150–158. [DOI:10.1093/ptj/85.2.150] [PMID]
Inukai, Y., Saito, K., Sasaki, R., Kotan, S., Nakagawa, M., & Onishi, H. (2016). Influence of transcranial direct current stimulation to the cerebellum on standing posture control. Frontiers in Human Neuroscience, 10, 325. [PMID]
Jacobs, J. V., & Horak, F. B. (2007). Cortical control of postural responses. Journal of Neural Transmission (Vienna, Austria: 1996), 114(10), 1339–1348. [DOI:10.1007/s00702-007-0657-0] [PMID] [PMCID]
Jeffery, D. T., Norton, J. A., Roy, F. D., & Gorassini, M. A. (2007). Effects of transcranial direct current stimulation on the excitability of the leg motor cortex. Experimental Brain Research, 182(2), 281–287. [DOI:10.1007/s00221-007-1093-y] [PMID]
Jiang, B. C., Yang, W. H., Shieh, J. S., Fan, J. Z., & Peng, C. K. (2013). Entropy-based method for COP data analysis. Theoretical Issues in Ergonomics Science, 14(3), 227-246. [DOI:10.1080/1463922X.2011.617109]
Kaminski, E., Steele, C. J., Hoff, M., Gundlach, C., Rjosk, V., & Sehm, B., et al. (2016). Transcranial direct current stimulation (tDCS) over primary motor cortex leg area promotes dynamic balance task performance. Clinical Neurophysiology, 127(6), 2455-2462. [DOI:10.1016/j.clinph.2016.03.018] [PMID]
Kang, H. G., Costa, M. D., Priplata, A. A., Starobinets, O. V., Goldberger, A. L., & Peng, C. K., et al. (2009). Frailty and the degradation of complex balance dynamics during a dual-task protocol. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences, 64(12), 1304–1311. [DOI:10.1093/gerona/glp113] [PMID] [PMCID]
Kaski, D., Dominguez, R. O., Allum, J. H., & Bronstein, A. M. (2013). Improving gait and balance in patients with leukoaraiosis using transcranial direct current stimulation and physical training: An exploratory study. Neurorehabilitation and Neural Repair, 27(9), 864-871. [DOI:10.1177/1545968313496328] [PMID]
Lipsitz L. A. (2002). Dynamics of stability: The physiologic basis of functional health and frailty. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 57(3), B115-B125. [DOI:10.1093/gerona/57.3.B115] [PMID]
Manor, B., Costa, M. D., Hu, K., Newton, E., Starobinets, O., & Kang, H. G., et al. (2010). Physiological complexity and system adaptability: Evidence from postural control dynamics of older adults. Journal of Applied Physiology (Bethesda, Md. : 1985), 109(6), 1786–1791. [DOI:10.1152/japplphysiol.00390.2010] [PMID] [PMCID]
Marquez, J., van Vliet, P., McElduff, P., Lagopoulos, J., & Parsons, M. (2015). Transcranial direct current stimulation (tDCS): Does it have merit in stroke rehabilitation? A systematic review. International Journal of Stroke, 10(3), 306–316.[DOI:10.1111/ijs.12169] [PMID]
Nair, D. G., Renga, V., Lindenberg, R., Zhu, L., & Schlaug, G. (2011). Optimizing recovery potential through simultaneous occupational therapy and non-invasive brain-stimulation using tDCS. Restorative Neurology and Neuroscience, 29(6), 411–420. [DOI:10.3233/RNN-2011-0612] [PMID] [PMCID]
Nitsche, M. A., Fricke, K., Henschke, U., Schlitterlau, A., Liebetanz, D., & Lang, N., et al. (2003). Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. The Journal of Physiology, 553(Pt 1), 293–301. [DOI:10.1113/jphysiol.2003.049916] [PMID] [PMCID]
Reis, J., Schambra, H. M., Cohen, L. G., Buch, E. R., Fritsch, B., & Zarahn, E., et al. (2009). Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proceedings of the National Academy of Sciences of the United States of America, 106(5), 1590–1595. [DOI:10.1073/pnas.0805413106] [PMID] [PMCID]
Richman, J. S., & Moorman, J. R. (2000). Physiological time-series analysis using approximate entropy and sample entropy. American Journal of Physiology-Heart and Circulatory Physiology, 278(6), H2039–H2049. [DOI:10.1152/ajpheart.2000.278.6.H2039] [PMID]
Roerdink, M., De Haart, M., Daffertshofer, A., Donker, S. F., Geurts, A. C., & Beek, P. J. (2006). Dynamical structure of center-of-pressure trajectories in patients recovering from stroke. Experimental Brain Research, 174(2), 256–269. [DOI:10.1007/s00221-006-0441-7] [PMID]
Salavati, M., Negahban, H., Mazaheri, M., Soleimanifar, M., Hadadi, M., & Sefiddashti, L., et al. (2012). The Persian version of the Berg Balance Scale: Inter and intra-rater reliability and construct validity in elderly adults. Disability and Rehabilitation, 34(20), 1695–1698. [DOI:10.3109/09638288.2012.660604] [PMID]
Sohn, M. K., Jee, S. J., & Kim, Y. W. (2013). Effect of transcranial direct current stimulation on postural stability and lower extremity strength in hemiplegic stroke patients. Annals of Rehabilitation Medicine, 37(6), 759–765. [DOI:10.5535/arm.2013.37.6.759] [PMID] [PMCID]
Stagg, C. J., & Nitsche, M. A. (2011). Physiological basis of transcranial direct current stimulation. The Neuroscientist : A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 17(1), 37–53. [DOI:10.1177/1073858410386614] [PMID]
Strong, K., Mathers, C., & Bonita, R. (2007). Preventing stroke: Saving lives around the world. The Lancet. Neurology, 6(2), 182–187. [DOI:10.1016/S1474-4422(07)70031-5] [PMID]
Tanaka, S., Hanakawa, T., Honda, M., & Watanabe, K. (2009). Enhancement of pinch force in the lower leg by anodal transcranial direct current stimulation. Experimental Brain Research, 196(3), 459–465. [DOI:10.1007/s00221-009-1863-9] [PMID] [PMCID]
Tanaka, S., Takeda, K., Otaka, Y., Kita, K., Osu, R., & Honda, M., et al. (2011). Single session of transcranial direct current stimulation transiently increases knee extensor force in patients with hemiparetic stroke. Neurorehabilitation and Neural Repair, 25(6), 565–569. [DOI:10.1177/1545968311402091] [PMID]
Tokuno, C. D., Taube, W., & Cresswell, A. G. (2009). An enhanced level of motor cortical excitability during the control of human standing. Acta Physiologica (Oxford, England), 195(3), 385–395. [DOI:10.1111/j.1748-1716.2008.01898.x] [PMID]
Verheyden, G. S., Weerdesteyn, V., Pickering, R. M., Kunkel, D., Lennon, S., & Geurts, A. C., et al. (2013). Interventions for preventing falls in people after stroke. The Cochrane Database of Systematic Reviews, 2013(5), CD008728. [DOI:10.1002/14651858.CD008728.pub2] [PMID] [PMCID]
Type of Study: Methodological Notes |
Subject:
Cognitive Neuroscience
Received: 2019/04/6 | Accepted: 2020/05/9 | Published: 2024/05/1
Received: 2019/04/6 | Accepted: 2020/05/9 | Published: 2024/05/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





