Volume 14, Issue 6 (November & December 2023)
BCN 2023, 14(6): 727-740 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Abbaszadeh A, Pirzadroozbahani N, Moradkhani M R, Hasanvand A. The Antinociceptive Effects of Combined Treatment With Atorvastatin and Vitamin C in the Chronic Constriction Injury Model of Rats. BCN 2023; 14 (6) :727-740
URL: http://bcn.iums.ac.ir/article-1-1430-en.html
URL: http://bcn.iums.ac.ir/article-1-1430-en.html
1- Department of Plastic and Reconstructive Surgery, School of Medicine, Hazrat Fatemeh Hospital, Iran University of Medical Sciences, Tehran, Iran.
2- Student Research Committee, Lorestan University of Medical Sciences, Khorramabad, Iran.
3- Department of Anesthesia, Faculty of Medicine, Shohaday-e- Ashayer Hospital, Lorestan University of Medical Sciences, Khorramabad, Iran.
4- Department of Physiology and Pharmacology, Faculty of Medicine, Lorestan University of Medical Sciences, Khorramabad, Iran.
2- Student Research Committee, Lorestan University of Medical Sciences, Khorramabad, Iran.
3- Department of Anesthesia, Faculty of Medicine, Shohaday-e- Ashayer Hospital, Lorestan University of Medical Sciences, Khorramabad, Iran.
4- Department of Physiology and Pharmacology, Faculty of Medicine, Lorestan University of Medical Sciences, Khorramabad, Iran.
Full-Text [PDF 748 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Neuropathic pain (NP) refers to a chronic and disabling condition that can develop after an event that causes disease or damage to central or peripheral nerves. This suffering condition can affect the daily activities of millions of people worldwide (Hall et al., 2006). Allodynia and hyperalgesia are the two most prominent features of NP (Woolf & Mannion, 1999). Traumatic NP can be diagnosed by various symptoms, including persistent stimulus-independent pain or spontaneous pain, after abnormal sensory perception, such as hyperalgesia and dysesthesia, or abnormal pain, and eventually allodynia (Dworkin et al., 2003). Also, assessing the conduction velocity of the motor nerve (index of the rate of transmission of an electrical impulse) is one of the crucial criteria for the speed of transmission of pain signals that can be used in pain models (Chen et al., 2022; Richards et al., 2021). Several studies have indicated that a reduced nociceptive threshold is associated with increased inflammatory mediators, leading to symptoms of hyperalgesia and allodynia (Woolf & Mannion, 1999). Nerve damage induces a precipitate of inflammatory factors at the injury region, leading to the activation of the glial cells, responsible for increasing the inflammatory process by the production and release of proinflammatory factors (Tracey & Walker, 1995). Oxidative stress can decline the immune system by reducing the production of antioxidants, and in turn, increase the secretion of inflammatory factors in the DRG, nerve, and spinal cord (Li et al., 2014; Sandireddy et al., 2016). Statins, such as atorvastatin are known for their roles in lowering serum lipids. However, previous studies have shown that anti-inflammatory properties and their subsequent therapeutic effects are crucial in different diseases, including cancer, arthritis, and Alzheimer’s disease (Ghaisas et al., 2010). One of the anti-inflammatory activities of statins is associated with inhibiting mediators of inflammation, such as interleukins (IL-1, 2, 4, 5, 10, 12), C-reactive protein, and tumor necrosis factor-α (TNF-α) (Schonbeck & Libby, 2004; Taubes, 2002). Amongst statins, atorvastatin is a well-known drug capable of directly reducing inflammation (Grip et al., 2008). Furthermore, it is an efficient antioxidant (Li et al., 2010). Vitamin C is known as a neuroprotective agent (Pavlovic et al., 2009; Shah et al., 2015). Ascorbate in high doses (20 mM) interferes with the production of IL-6 and TNF-α by inhibiting the monocytes (Hartel et al., 2004). The inflammatory gene expression caused by phorbol myristate acetate (PMA)-activated neutrophils may be changed by vitamin C. It has been shown that vitamin C can be effective in inhibiting the production and expression of cytokines, such as cyclooxygenase-2 (COX-2), IL8, TNF-α, and nuclear factor-κβ (NFκβ) in response to phorbol myristate acetate (PMA) (Capó et al., 2015). Moreover, ascorbate exists in blood flow and normal circulating human neutrophils hold ascorbate in millimolar concentrations (Tauler et al., 2002).
Many studies have shown that ascorbic acid has a significant antioxidant effect by scavenging reactive oxygen species (ROS) (Bode, 1997). Vitamin C is considered an essential nutrient for all animals and plants, and it is required for the process of metabolism and also for protection against oxidative stress (Padayatty et al., 2003).
This study was conducted to evaluate the synergism effects of atorvastatin and vitamin C co-treatment for protection against neuropathic disorders caused by chronic constriction injury (CCI), while the behavioral tests and anti-inflammatory, as well as antioxidant properties, have been considered in rat models.
2. Materials and Methods
Materials
The chemicals needed to perform this study include atorvastatin (Sigma, St. Louis, MO, USA), vitamin C (L-ascorbic, Sigma), IL-6 and TNF-α, glutathione peroxidase (GPx), superoxide dismutase (SOD) enzyme-linked immunosorbent assay (ELISA) kits (Abcam, USA), malonaldehyde (MDA), and antibody NF-kB ELISA kits (Abcam, USA).
Animals and housing conditions
Seventy male adult Sprague–Dawley rats with a weight range of 220-240 g were purchased from the Animal House of Lorestan University of Medical Sciences (Khorramabad, Iran) and maintained in normal conditions, i.e. temperature at 22°C±2°C, the humidity of almost 45%, and 12 h light/dark cycle without any restriction to access water and standard food. The Ethical Committee confirmed all animal protocols for this study (Zimmermann, 1983).
Experimental design
The rats were randomly divided into 7 groups (10 rats in each group) as follows: 1: Sham-operated, 2: CCI vehicle-treated (CCI), 3: CCI+vitamin C (vit C) (500 mg/kg), 4: CCI+atorvastatin (ATOR) (5 mg/kg), 5: CCI+ator (10 mg/kg), 6: CCI+vit C (500 mg/kg)+ator (5 mg/kg), and 7: CCI+vit C (500 mg/kg)+ATOR (10 mg/kg).
Drug preparation
In this study, atorvastatin and vitamin C were solved by aqueous (distilled water), and both of these drugs were injected freshly and intraperitoneally (i.p.) daily for treatment (for 21 days.).
Surgery
The sciatic nerve injury was induced using the CCI model of NP (Bennett & Xie, 1988). After injecting thiopental for anesthesia (35 mg/kg), the considered nerve was found (sciatic nerve). Subsequently, without interrupting the blood flow, the sciatic nerve around four ligatures was loosened. According to the reference, the distance between these ligatures is 1 mm. The sterilization of the wound was carried out with normal saline (0.9%). Finally, for the surgical suture, the wound was closed in two layers with non-absorbable sutures 4-0 (face surface) and, if necessary, surgical skin staples were performed. Likewise, the same surgical procedure was performed for the sham-operated group except for the ligation.
Thermal stimulation tests
Behavioral assessments were done on the 3rd, 7th, 14th, and 21st days following the ligation in two steps. The tests began with heat hyperalgesia stimulation and then mechanical allodynia with an interval time of 120 minutes for two tests.
Heat hyperalgesia stimulation (hot plate test)
Eddy’s hot plate method was used to achieve the thermal hyperalgesia threshold. The rats were positioned on the plate while the temperature was set at 52.5°C±1.0°C. Then, withdrawal latency was noted regarding the licking of the hind paw in a few seconds. A period of 15 s was considered as the exposure time (Jain et al., 2009).
Mechanical hyperalgesia (pin prick)
Gauge needles (at 90°) were exposed to the hind paw with a certain intensity to penetrate the skin. A maximum cut-off time of 20 s was measured for the duration of withdrawing the paw (Park et al., 1995; Kukkar et al., 2013).
Cold allodynia (acetone test)
Initially, we located the rats’ hind paws on a wire mesh. Subsequently, the stimulation was induced by spraying 100 μL of acetone without any skin exposure. Then, the reactions of the rats to acetone were noted in 20 s and scored according to the Kukkar and Singh scale in their protocol (Kukkar et al., 2013).
Mechanical allodynia (von Frey test)
Von Frey filaments were used to induce mechanical allodynia (steeling, Wood Dale, IL, USA) in the following order: 0.6, 1.0, 1.4, 2.0, 4.0, 6.0, 8.0, 10.0, 15.0, 26.0, and 60 g. To complete the test, laboratory work was performed according to the protocol used in previous papers (Abed et al., 2015).
Motor nerve conduction velocity test (MNCV)
To study the conduction velocity, electrophysiological experiments were used 21 days after the induction of CCI. First, the rats were anesthetized using thiopental (35 mg/kg). Subsequently, the sciatic nerves situated near the sciatic notch and distal to the knee were stimulated by a Nicolet Viking Quest machine, while the body temperature was kept at 37°C during the experiment (Nicolet Biomedical, Madison, WI). The action potential was then measured using unipolar pin electrodes from the ankle (Hasegawa et al., 2006). Finally, MNCV (m/s) was calculated as Equation 1:
1. MNCV=D/(PL-DL).
(D=Distance in meters, PL=proximal latency in seconds, DL=distal latency in seconds).
Enzyme-linked immunosorbent assays (ELISA):
After spinal dislocation, the process continued with the removal of the fresh spinal cord, followed by the measurement of TNF-α, IL-6, GPx, SOD, and MDA levels. To begin, the fresh spinal cord between L4-L5 segments was removed right after MNCV measurements on the 21st day. Then, a glass homogenizer was used to homogenate the tissue with 0.9% saline at 2500 r/min for 10 minutes. Homogenate supernatant (10%,w/v) was separated for these tests (Li et al., 2008).
Histological examination of nerves:
To carry out this examination, sciatic nerves were separated after MNCV evaluation and their semithin sections (5 μm) were prepared and stained by hematoxylin and eosin. Subsequently, a pathologist who was unaware of the numbering of tissue samples analyzed the slides (Brummett et al, 2009).
Statistical analysis
The results were shown as Mean±SEM. The data obtained from behavioral tests were analyzed using a two-way analysis of variance (ANOVA). Furthermore, biochemical tests and MNCV test were examined using a one-way ANOVA, and GraphPad Prism software, version 5. A P˂0.05 was considered statistically significant. The sample size was calculated via power calculations using G*Power software, version .3.1.7. α error and power (1-β) were set at 0.05 and 0.8, respectively, and the total sample size needed for each was calculated as 8-10 animals. Hence, ten rats were selected in each group.
3. Results
Effects of the administration of vitamin C and atorvastatin on thermal hyperalgesia
One-way ANOVA analysis indicated significant differences between the experimental groups in levels of thermal hyperalgesia on days 7th (F(6, 60)=17.56, P˂0.00251), 14th (F(6, 60)=48.36, P˂0.001) and 21st (F(6, 60)=163.84, P˂0.001).
The hind paw reaction in the CCI group on the 7th day after the surgery showed a significant reduction in thermal hyperalgesia compared to the sham group indicating successful model induction. On this day, no significant results were observed between other groups compared to the sham. Moreover, it was found that on the 14th and 21st days after considering CCI, all the treatments significantly attenuated thermal hyperalgesia compared to the CCI group. Nevertheless, the best treatment was observed in the last group on the 21st day (Figure 1).

Effects of the administration of vitamin C and atorvastatin on mechanical hyperalgesia
One-way ANOVA analysis revealed significant differences between the experimental groups regarding the levels of thermal hyperalgesia on the 7th (F(6, 60)=23.54, P˂0.001), 14th (F(6, 60)=56.34, P˂0.001) and 21st (F(6, 60)=96.45, P˂0.001) days.
On the third day after CCI, a significant difference was observed between group II and the sham group, but other groups did not demonstrate a significant difference with the sham group. On the 14th and 21st days after considering CCI, the co-administration of vitamin C and atorvastatin could improve mechanical hyperalgesia compared to other treatment groups (Figure 2).
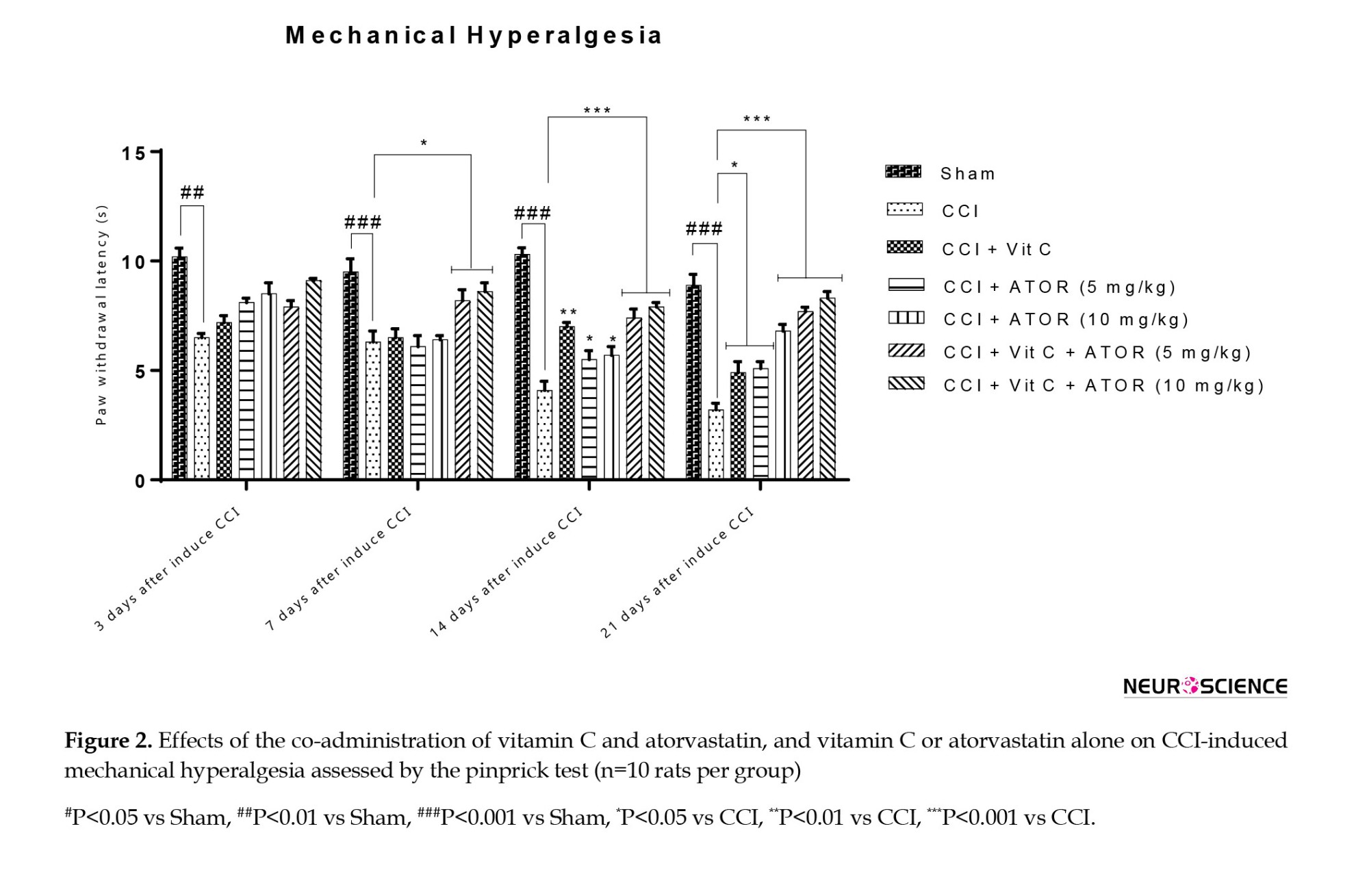
Effects of the administration of vitamin C and atorvastatin on cold allodynia
One-way ANOVA analysis showed a significant difference between the experimental groups regarding the levels of thermal hyperalgesia on the 7th (F(6, 60)=48.61, P˂0.001), 14th (F(6, 60)=85.96, P˂0.001) and 21st (F(6, 60)=128.64, P˂0.001) days.
Figure 3 shows that the increased response to the stimulus on a leg with neuropathy is characteristic of inducing NP in these animals. Vitamin C, atorvastatin 5, and 10 mg/kg in monotherapy attenuated sensitivity, and a significant difference was observed between this group and the CCI group. Moreover, the analysis showed that the treatment management strategies of the rats by combining these drugs for 21 days effectively reduced the number of times paw excreted into the non-noxious stimulus acetone.
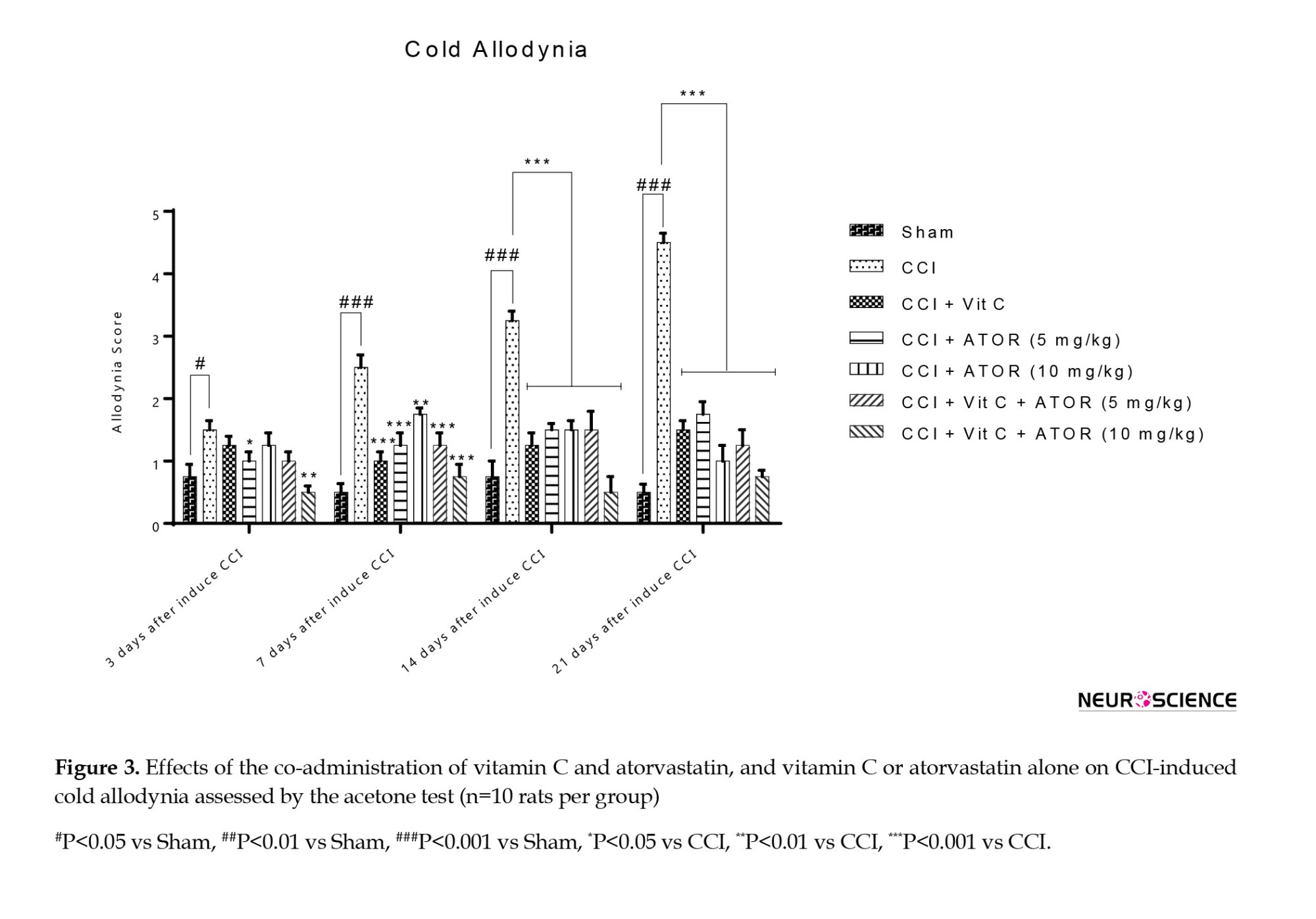
Effects of the administration of vitamin C and atorvastatin on mechanical allodynia
One-way ANOVA analysis revealed a significant difference between the experimental groups regarding the levels of thermal hyperalgesia on the 7th (F(6, 60)=7.51, P˂0.0025), 14th (F(6, 60)=95.31, P˂0.001) and 21st (F(6, 60)=98.54, P˂0.001) days.
Figure 4 shows the results of behavioral tests. Since the induction of CCI, the hind paw became sentient to mechanical stimuli, even with the feeble filament tested. The comparison of the CCI animals with the sham rats at the 3rd (P<0.01), 7th (P<0.001), 14th (P<0.001), and 21st (P<0.001) days showed a remarkably elevated response to the stimulus by the rats. On the 14th and 21st days after the induction of CCI, the rats treated with vitamin C or atorvastatin (5 or 10 mg/kg) exhibited a significant difference regarding withdrawal threshold compared to the sham rats. Furthermore, it was found that on the 7th, 14th, and 21st days after considering CCI, the co-administration of vitamin C and atorvastatin (10 mg/kg) in CCI animals improved mechanical allodynia better than other CCI groups.
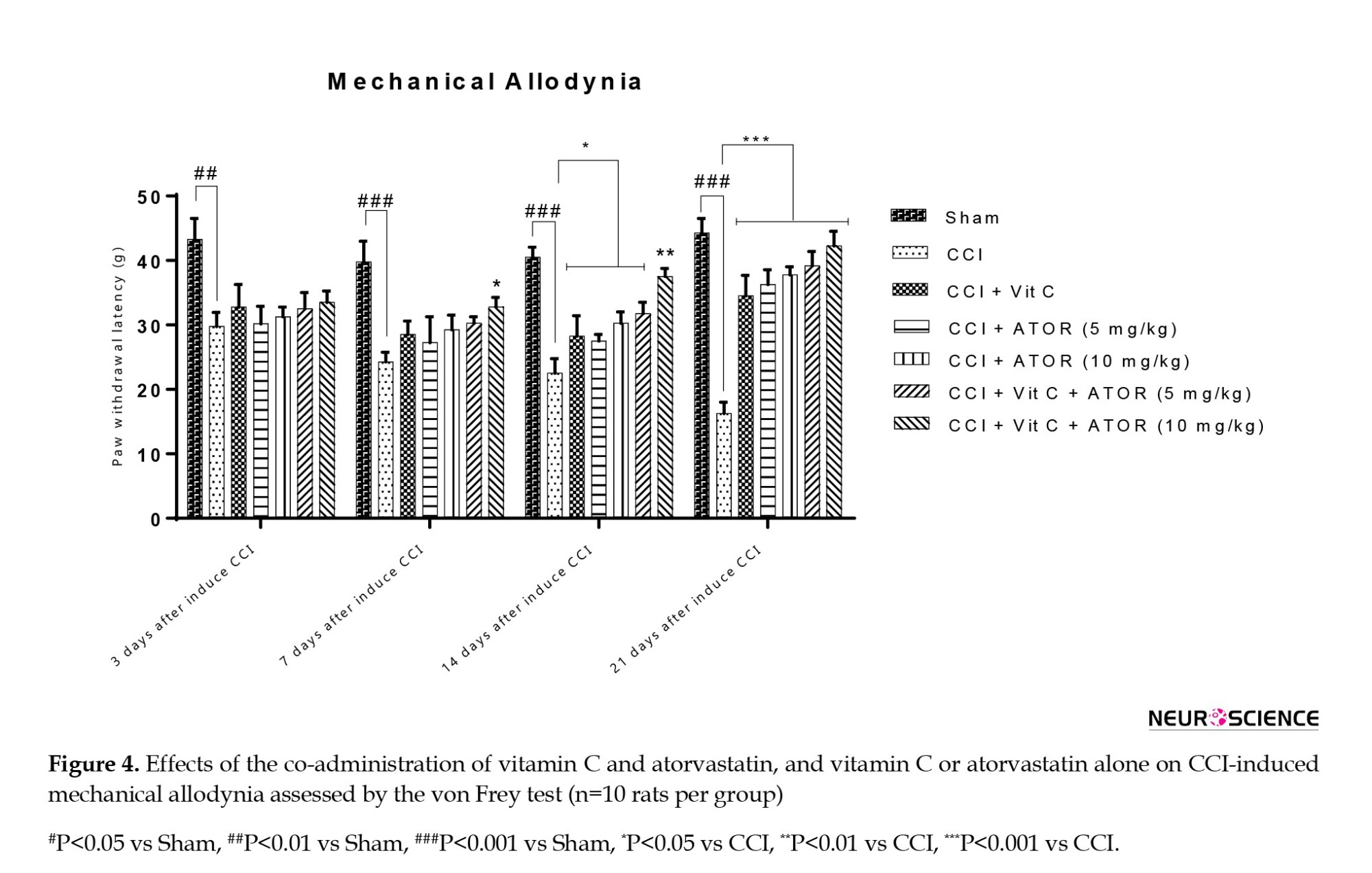
Effects of the administration of vitamin C and atorvastatin on MNCV
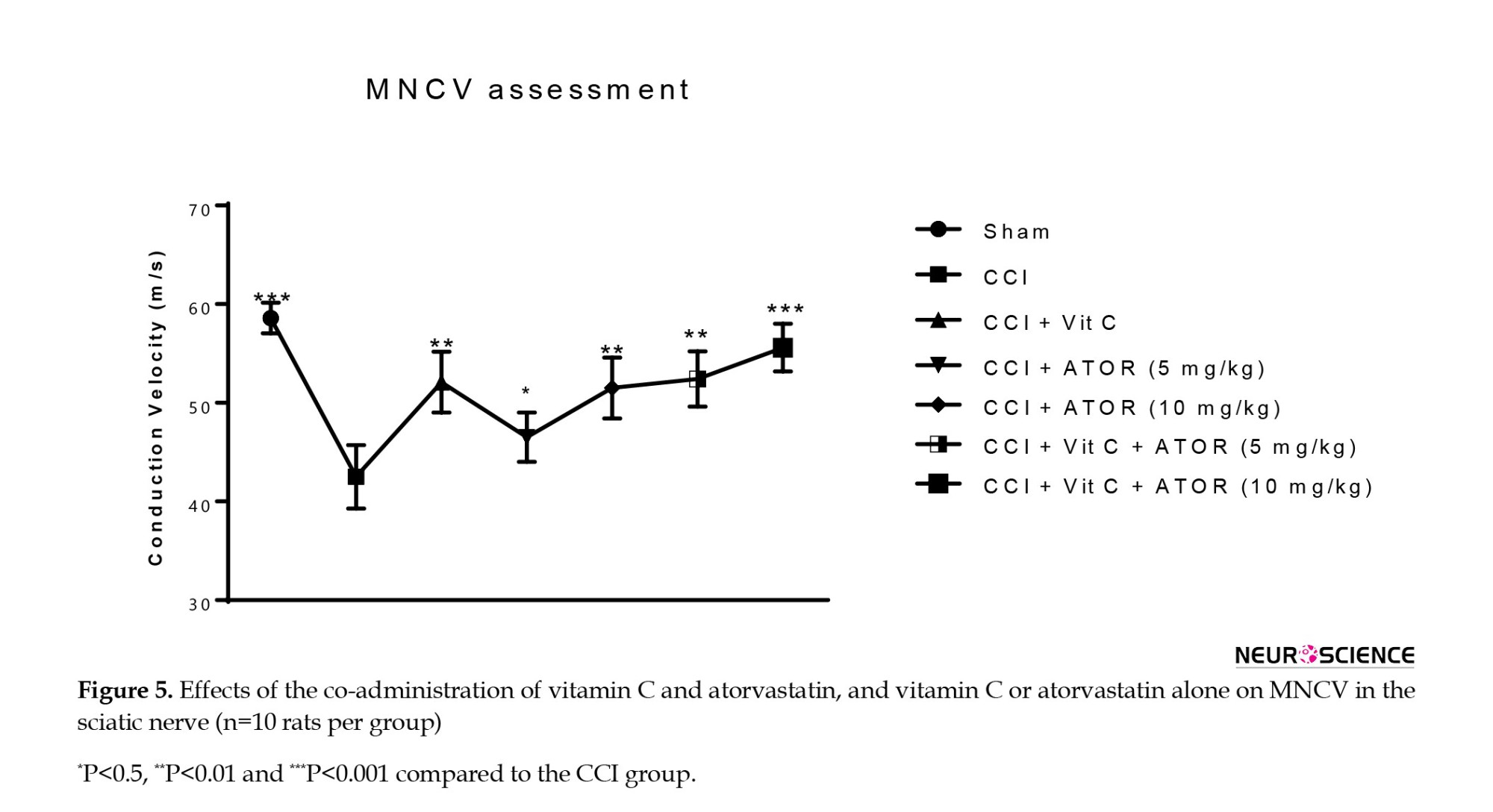
MNCV results showed a significant difference in the CCI group compared to the sham group (P<0.001). The CCI rats treated with vitamin C or atorvastatin for three weeks exhibited an improvement in their MNCVs as compared to group II. However, groups VI, and VII exhibited significant increases (respectively, P<0.01, P<0.001) compared to the CCI group. This result suggests that co-treatment vitamin C and atorvastatin can significantly improve the MNCV (Figure 5).
Assessment of the levels of TNF-α and interleukin-6 (IL-6)
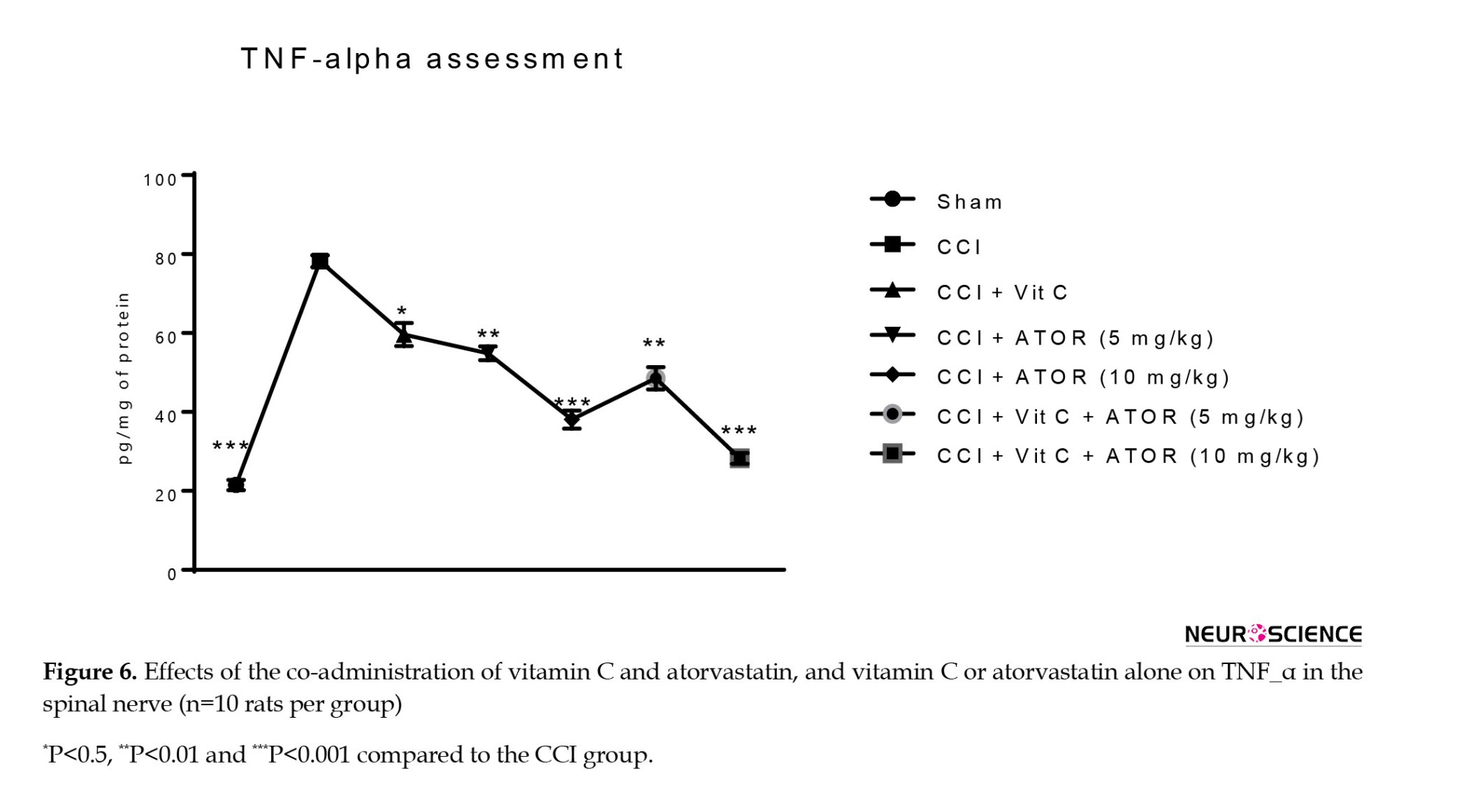
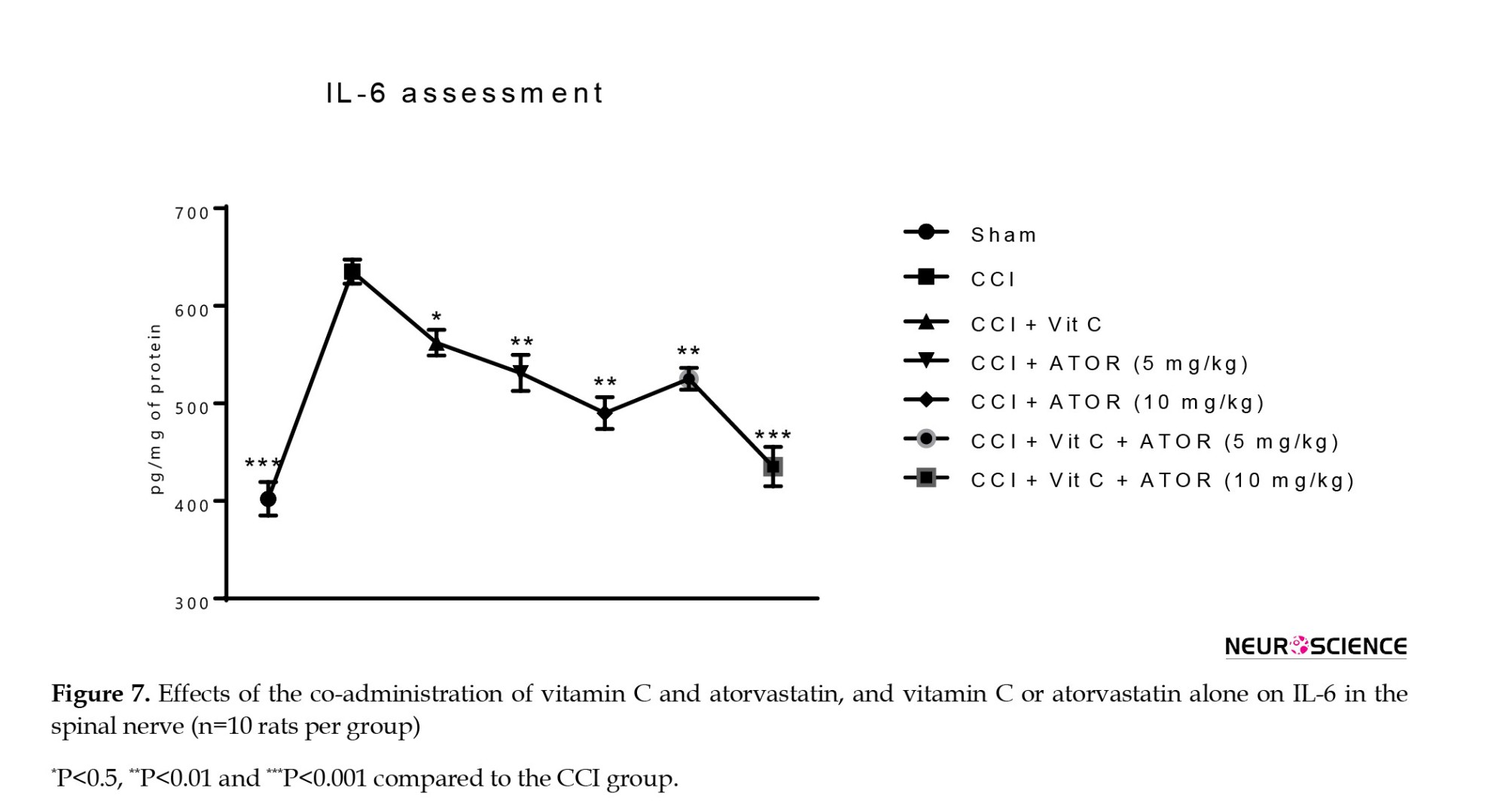
The CCI animals showed a significant increase in the serum levels of TNF-α (Figure 6) and IL-6 (Figure 7) compared to the sham group. All the treatments significantly reversed these changes and the efficacy of the treatment was dose-dependent.
Assessment of the levels of biochemical parameters in CCI-induced NP
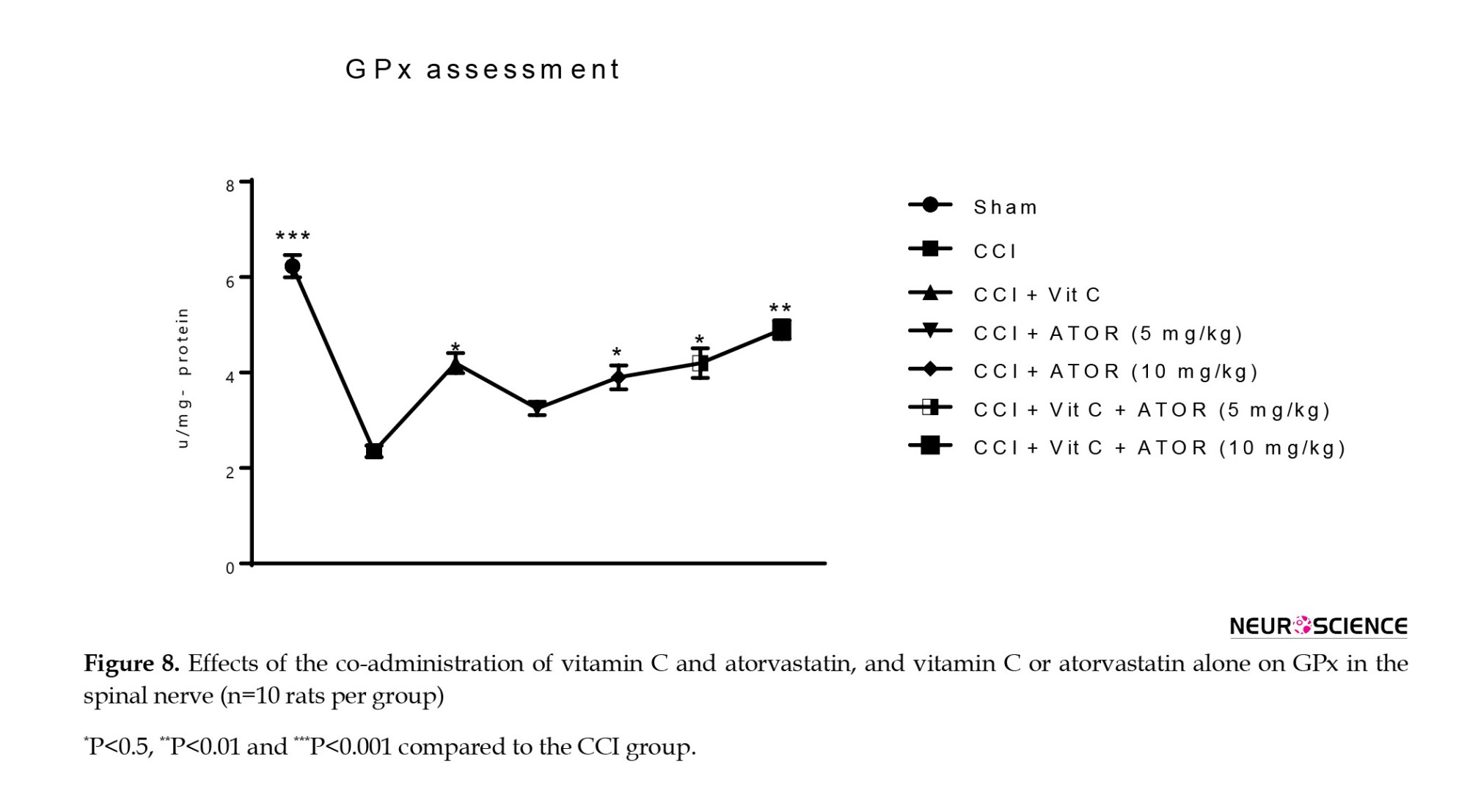
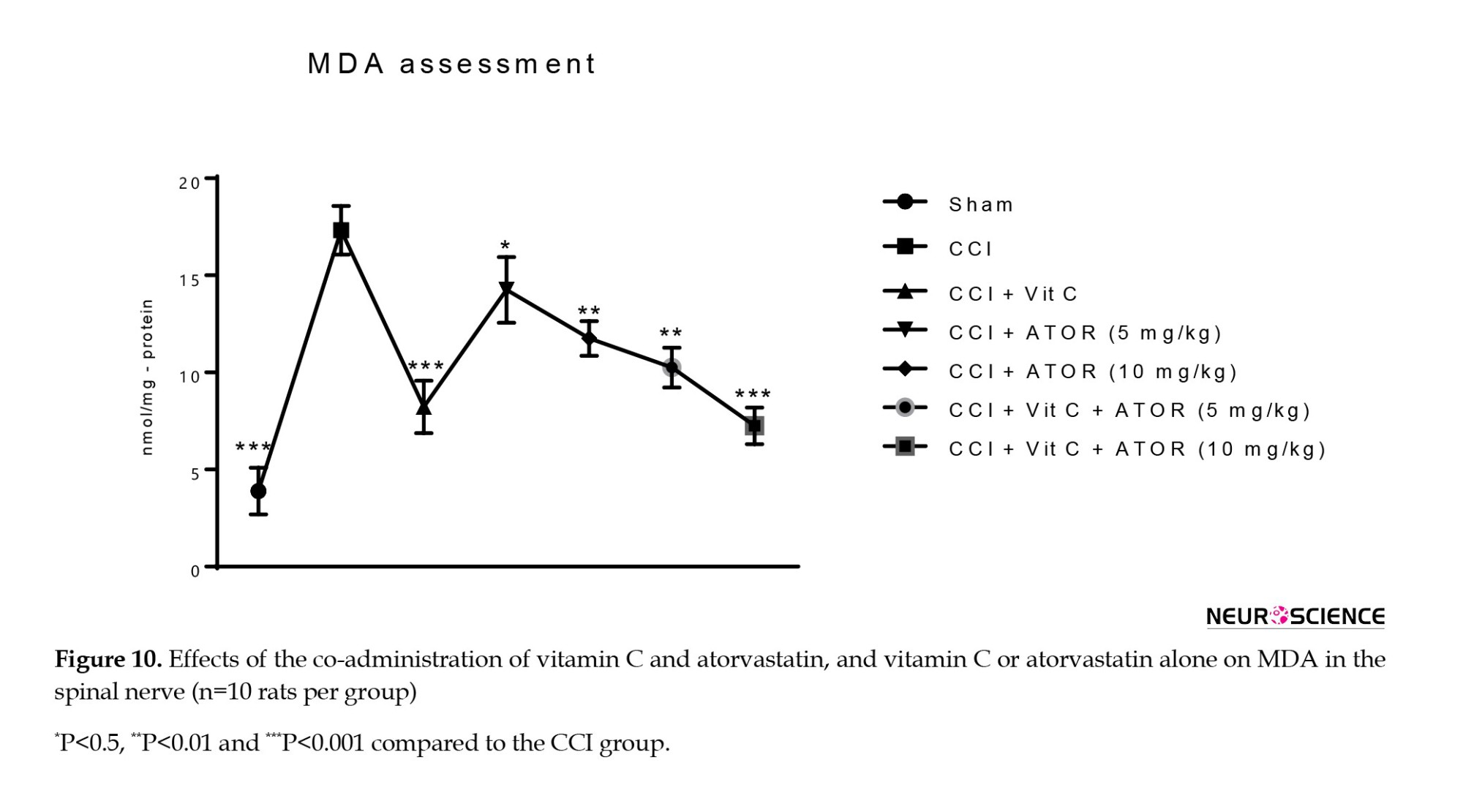
Biochemical parameters, including MDA as lipid peroxidation marker, GPx, and SOD as antioxidant enzymes were measured. CCI induced oxidative stress via significant decreases in the GPx (Figure 8) and activity of SOD (Figure 9), and a significant increase in the MDA level (Figure 10) in the spinal cord compared to the sham group. All the treatments significantly attenuated the levels of marked oxidative stress compared to the CCI group, and atorvastatin showed dose-dependent effects with vitamin C.

Perineural inflammation in the sciatic nerve
No signs of inflammation were observed in the sciatic nerve among the rats in the sham group. Moreover, histopathological examination showed that perineural inflammation was evident in the CCI groups, indicating that it causes NP in the sciatic nerve (score=3). All the treatment groups largely reversed these changes. Furthermore, histological examination indicated low levels of inflammation around the sciatic nerve in the CCI mice treated with the combination of vitamin C and atorvastatin (5 or 10 mg/kg) (score=1).
4. Discussion
The results of the present study revealed that thermal hyperalgesia was significantly reduced in all treated groups compared to the CCI group on the 14th and 21st days. Moreover, co-administration of vitamin C and atorvastatin was effective in decreasing the hind paw withdrawal threshold caused by CCI. It was also observed that the paw withdrawal rate to non-noxious stimulus acetone was effectively decreased in co-treated groups for 21 days. Likewise, the co-administration of vitamin C and atorvastatin resulted in a minor elevation of the withdrawal threshold on the 7th, 14th, and 21st days. MNCV was another parameter that was effectively enhanced after treatment of the rats with the combination of vitamin C and atorvastatin for 21 days in group VII compared to group II. Finally, the co-administration of atorvastatin and vitamin C improved the perineural inflammation around the sciatic nerve.
NP is a condition affecting the nervous system following an injury or disease, such as diabetes, cancer, multiple sclerosis, virus diseases, etc. Many people are affected by its harmful consequences all over the world (Amin & Hosseinzadeh, 2012). Allodynia and hyperalgesia are the two major indicators to describe the conditions of NP (Jensen & Finnerup, 2014). CCI is one of the main models used to induce and evaluate NP worldwide (Okamoto et al., 2001). One of the possible mechanisms that is highly effective in causing neurological pain, particularly the NP associated with diabetes, is the production of ROS (Nazıroğlu et al., 2012). This study was conducted to evaluate the reducing effect of co-treatment with atorvastatin and vitamin C on NP induced by CCI in mice through their anti-inflammatory, antioxidant, and neuroprotective effects. Several studies have evaluated the effects of compounds and medications on behavioral tests. One of them examined the effect of ethanolic saffron extract on thermal hyperalgesia. It was found in that research that chronic treatment can significantly reduce dose-dependent thermal hyperalgesia (Amin & Hosseinzadeh, 2012). It has been shown that reducing the blood flow inside the nerve can lead to oxidative stress and ultimately reduce the conduction nerve velocity (Sirisha et al., 2021), and this reduction in velocity can indicate nerve damage and consequent conduction disturbance. However, the results of this study showed that Co-treatment with vitamin C and atorvastatin can significantly improve MNCV by anti-inflammatory and antioxidant effects.
All the treatments significantly reduced these inflammatory markers mainly with hybrid groups. Today, the central role of pro-inflammatory cytokines, such as TNF-a, IL-1, and IL-6 in NP has been shown. This is a link between inflammation and hyperalgesia, particularly the withdrawal threshold (Sommer & Kress, 2004). Hence, therapeutic agents, such as atorvastatin can be efficient in reducing neuritis. Statins, particularly atorvastatin, in addition to their other effects, have anti-inflammatory effects. Atorvastatin has been reported to reduce inflammation through the downregulation of nuclear NFκB (Chu et al., 2015). A similar study showed that atorvastatin can significantly lower IL-1β levels after treatment (Pathak et al., 2013). Other treatments, such as pyrroloquinoline, quinone, and genistein were used to significantly reduce inflammatory cytokines, including TNF-α, IL-1β, and IL-6 (Gong et al., 2012; Valsecchi et al., 2008). Furthermore, all the treatments significantly reduced oxidative stress marker levels compared to the CCI group. The combination therapy significantly lowered MDA levels and increased GPx as well as SOD levels. It has been indicated that ROS are central to the prevention of heat hyperalgesia (Lee et al., 2007). Therefore, the presence of compounds that have activities similar to antioxidant enzymes is essential to prevent NP. Hence, atorvastatin as an antioxidant drug has anti-analgesic effects. Another study evaluated the effect of atorvastatin on reducing NP. It was shown that atorvastatin can reduce MDA levels. Atorvastatin also increased SOD, catalase (CAT), and glutathione-S-transferase (GST) activity. Moreover, glutathione (GSH) levels increased following atorvastatin therapy (Pathak et al., 2014). Also, histological studies have shown low levels of inflammation around the sciatic nerve in CCI mice treated with vitamin C or atorvastatin. A similar study found that treatment with amiloride and pralidoxime can reduce CCI-induced dysfunction and degeneration (Muthuraman et al., 2008). Another study indicated that acurus extract can significantly reduce CCI-induced fiber abnormalities, nerve fiber swelling, and neuroglial cell activation (Muthuraman & Singh, 2011).
5. Conclusion
This study showed that the anti-inflammatory, antioxidant, and neuroprotective properties of combined treatment with vitamin C and atorvastatin can improve the complications of CCI in an experimental neuropathic model in mice. The results of the present research revealed that concomitant treatment with vitamin C and atorvastatin can attenuate inflammatory cytokines (such as TNF-α and IL-6) and oxidative markers (such as GPx, SOD, and MDA), enhance nerve conduction quality and histopathological scores and improve the sciatic nerves. Since the protective properties of the combination of atorvastatin and vitamin C have remained largely unnoticed, this study opens a new horizon to understand the efficiency of this procedure.
Ethical Considerations
Compliance with ethical guidelines
The Ethics Committee of Lorestan University of Medical Sciences approved all animal protocols for this study (IR.LUMS.REC.1396287).
Funding
This study (as the complementary work) was financially supported by Lorestan University of Medical Sciences (Code: A-10-1551-4).
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank the Vice-Chancellery for Research and Technology of Lorestan University of Medical Sciences, and Razi Herbal Medicines Research Center affiliated to Lorestan University of Medical Sciences for their support.
References
Abed, A., Hajhashemi, V., Banafshe, H. R., Minaiyan, M., & Mesdaghinia, A. (2015). Venlafaxine attenuates heat hyperalgesia independent of adenosine or opioid system in a rat model of peripheral neuropathy. Iranian Journal of Pharmaceutical Research, 14(3), 843–850. [PMID] [PMCID]
Amin, B., & Hosseinzadeh, H. (2012). Evaluation of aqueous and ethanolic extracts of saffron, Crocus sativus L., and its constituents, safranal and crocin in allodynia and hyperalgesia induced by chronic constriction injury model of neuropathic pain in rats. Fitoterapia, 83(5), 888–895. [DOI:10.1016/j.fitote.2012.03.022] [PMID]
Bennett, G. J., & Xie, Y. K. (1988). A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain, 33(1), 87–107. [DOI:10.1016/0304-3959(88)90209-6] [PMID]
Bode A. M. (1997). Metabolism of vitamin C in health and disease. Advances in Pharmacology, 38, 21–47. [DOI:10.1016/S1054-3589(08)60977-1] [PMID]
Brummett, C. M., Padda, A. K., Amodeo, F. S., Welch, K. B., & Lydic, R. (2009). Perineural dexmedetomidine added to ropivacaine causes a dose-dependent increase in the duration of thermal antinociception in sciatic nerve block in rat. Anesthesiology, 111(5), 1111–1119. [DOI:10.1097/ALN.0b013e3181bbcc26] [PMID] [PMCID]
Capó, X., Martorell, M., Sureda, A., Tur, J. A., & Pons, A. (2015). Effects of docosahexaenoic supplementation and in vitro vitamin C on the oxidative and inflammatory neutrophil response to activation. Oxidative Medicine and Cellular Longevity, 2015, 187849. [DOI:10.1155/2015/187849] [PMID] [PMCID]
Chen, S. M., Wang, M. H., Soung, H. S., Tseng, H. C., Fang, C. H., & Lin, Y. W., et al. (2022). Neuroprotective effect of l-theanine in a rat model of chronic constriction injury of sciatic nerve-induced neuropathic pain. Journal of the Formosan Medical Association, 121(4), 802–814. [DOI:10.1016/j.jfma.2021.08.023] [PMID]
Chu, L. W., Chen, J. Y., Wu, P. C., & Wu, B. N. (2015). Atorvastatin prevents neuroinflammation in chronic constriction injury rats through nuclear NFκB downregulation in the dorsal root ganglion and spinal cord. ACS Chemical Neuroscience, 6(6), 889–898. [DOI:10.1021/acschemneuro.5b00032] [PMID]
Dworkin, R. H., Backonja, M., Rowbotham, M. C., Allen, R. R., Argoff, C. R., & Bennett, G. J., et al. (2003). Advances in neuropathic pain: Diagnosis, mechanisms, and treatment recommendations. Archives of Neurology, 60(11), 1524–1534. [DOI:10.1001/archneur.60.11.1524] [PMID]
Ghaisas, M. M., Dandawate, P. R., Zawar, S. A., Ahire, Y. S., & Gandhi, S. P. (2010). Antioxidant, antinociceptive and anti-inflammatory activities of atorvastatin and rosuvastatin in various experimental models. Inflammopharmacology, 18(4), 169–177. [DOI:10.1007/s10787-010-0044-6] [PMID]
Gong, D., Geng, C., Jiang, L., Aoki, Y., Nakano, M., & Zhong, L. (2012). Effect of pyrroloquinoline quinone on neuropathic pain following chronic constriction injury of the sciatic nerve in rats. European Journal of Pharmacology, 697(1-3), 53–58.[DOI:10.1016/j.ejphar.2012.09.052] [PMID]
Grip, O., Janciauskiene, S., & Bredberg, A. (2008). Use of atorvastatin as an anti-inflammatory treatment in Crohn's disease. British Journal of Pharmacology, 155(7), 1085–1092. [DOI:10.1038/bjp.2008.369] [PMID] [PMCID]
Hall, G. C., Carroll, D., Parry, D., & McQuay, H. J. (2006). Epidemiology and treatment of neuropathic pain: The UK primary care perspective. Pain, 122(1-2), 156–162. [DOI:10.1016/j.pain.2006.01.030] [PMID]
Härtel, C., Strunk, T., Bucsky, P., & Schultz, C. (2004). Effects of vitamin C on intracytoplasmic cytokine production in human whole blood monocytes and lymphocytes. Cytokine, 27(4-5), 101–106. [DOI:10.1016/j.cyto.2004.02.004] [PMID]
Hasegawa, T., Kosaki, A., Shimizu, K., Matsubara, H., Mori, Y., & Masaki, H., et al. (2006). Amelioration of diabetic peripheral neuropathy by implantation of hematopoietic mononuclear cells in streptozotocin-induced diabetic rats. Experimental Neurology, 199(2), 274–280. [DOI:10.1016/j.expneurol.2005.11.001] [PMID]
Jain, V., Jaggi, A. S., & Singh, N. (2009). Ameliorative potential of rosiglitazone in tibial and sural nerve transection-induced painful neuropathy in rats. Pharmacological Research, 59(6), 385–392. [DOI:10.1016/j.phrs.2009.02.001] [PMID]
Jensen, T. S., & Finnerup, N. B. (2014). Allodynia and hyperalgesia in neuropathic pain: Clinical manifestations and mechanisms. The Lancet. Neurology, 13(9), 924–935. [DOI:10.1016/S1474-4422(14)70102-4] [PMID]
Kukkar, A., Singh, N., & Jaggi, A. S. (2013). Neuropathic pain-attenuating potential of aliskiren in chronic constriction injury model in rats. Journal of the Renin-Angiotensin-Aldosterone system, 14(2), 116–123. [DOI:10.1177/1470320312460899] [PMID]
Lee, I., Kim, H. K., Kim, J. H., Chung, K., & Chung, J. M. (2007). The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain, 133(1-3), 9–17. [DOI:10.1016/j.pain.2007.01.035] [PMID] [PMCID]
Li, J., Sun, Y. M., Wang, L. F., Li, Z. Q., Pan, W., & Cao, H. Y. (2010). Comparison of effects of simvastatin versus atorvastatin on oxidative stress in patients with coronary heart disease. Clinical Cardiology, 33(4), 222–227. [DOI:10.1002/clc.20724] [PMID] [PMCID]
Li, S. S., Zhang, W. S., Ji, D., Zhou, Y. L., Li, H., & Yang, J. L., et al. (2014). Involvement of spinal microglia and interleukin-18 in the anti-nociceptive effect of dexmedetomidine in rats subjected to CCI. Neuroscience Letters, 560, 21–25. [DOI:10.1016/j.neulet.2013.12.012] [PMID]
Li, X. R., Long, Y. H., Fang, X., & Liu, X. G. (2008). [Effect of vitamin C and E on antioxidative enzyme, NOS activity and NO contents in hippocampus of rats with lead poisoning (Chinese)]. Zhejiang da xue xue bao. Yi xue ban, 37(2), 189–192. [DOI:10.3785/j.issn.1008-9292.2008.02.015] [PMID]
Muthuraman, A., Jaggi, A. S., Singh, N., & Singh, D. (2008). Ameliorative effects of amiloride and pralidoxime in chronic constriction injury and vincristine induced painful neuropathy in rats. European Journal of Pharmacology, 587(1-3), 104–111. [DOI:10.1016/j.ejphar.2008.03.042] [PMID]
Muthuraman, A., & Singh, N. (2011). Attenuating effect of Acorus calamus extract in chronic constriction injury induced neuropathic pain in rats: An evidence of anti-oxidative, anti-inflammatory, neuroprotective and calcium inhibitory effects. BMC Complementary and Alternative Medicine, 11, 24. [DOI:10.1186/1472-6882-11-24] [PMID] [PMCID]
Nazıroğlu, M., Dikici, D. M., & Dursun, S. (2012). Role of oxidative stress and Ca²+ signaling on molecular pathways of neuropathic pain in diabetes: Focus on TRP channels. Neurochemical Research, 37(10), 2065–2075. [DOI:10.1007/s11064-012-0850-x] [PMID]
Okamoto, K., Martin, D. P., Schmelzer, J. D., Mitsui, Y., & Low, P. A. (2001). Pro- and anti-inflammatory cytokine gene expression in rat sciatic nerve chronic constriction injury model of neuropathic pain. Experimental Neurology, 169(2), 386–391.[DOI:10.1006/exnr.2001.7677] [PMID]
Padayatty, S. J., Katz, A., Wang, Y., Eck, P., Kwon, O., & Lee, J. H., et al. (2003). Vitamin C as an antioxidant: Evaluation of its role in disease prevention. Journal of the American College of Nutrition, 22(1), 18–35. [DOI:10.1080/07315724.2003.10719272] [PMID]
Park, K. M., Max, M. B., Robinovitz, E., Gracely, R. H., & Bennett, G. J. (1995). Effects of intravenous ketamine, alfentanil, or placebo on pain, pinprick hyperalgesia, and allodynia produced by intradermal capsaicin in human subjects. Pain, 63(2), 163–172. [DOI:10.1016/0304-3959(95)00029-R] [PMID]
Pathak, N. N., Balaganur, V., Lingaraju, M. C., Kant, V., Latief, N., & More, A. S., et al. (2014). Atorvastatin attenuates neuropathic pain in rat neuropathy model by down-regulating oxidative damage at peripheral, spinal and supraspinal levels. Neurochemistry International, 68, 1–9. [DOI:10.1016/j.neuint.2014.01.014] [PMID]
Pathak, N. N., Balaganur, V., Lingaraju, M. C., More, A. S., Kant, V., & Kumar, D., et al. (2013). Antihyperalgesic and anti-inflammatory effects of atorvastatin in chronic constriction injury-induced neuropathic pain in rats. Inflammation, 36(6), 1468–1478. [DOI:10.1007/s10753-013-9688-x] [PMID]
Pavlovic, V., Pavlovic, D., Kocic, G., Sokolovic, D., Sarac, M., & Jovic, Z. (2009). Ascorbic acid modulates monosodium glutamate induced cytotoxicity in rat thymus. Bratislavske Lekarske Listy, 110(4), 205–209. [PMID]
Richards, J., Gechev, A., Alexander, J., Macedo, L., May, K. A., & Lindley, S. B. (2021). The effect of local cooling at the elbow on nerve conduction velocity and motor unit behaviour: An exploration of a novel neurological assessment. Sensors, 21(20), 6703. [DOI:10.3390/s21206703] [PMID] [PMCID]
Sandireddy, R., Yerra, V. G., Komirishetti, P., Areti, A., & Kumar, A. (2016). Fisetin imparts neuroprotection in experimental diabetic neuropathy by modulating Nrf2 and NF-κB pathways. Cellular and Molecular Neurobiology, 36(6), 883–892. [DOI:10.1007/s10571-015-0272-9] [PMID]
Schönbeck, U., & Libby, P. (2004). Inflammation, immunity, and HMG-CoA reductase inhibitors: Statins as antiinflammatory agents?. Circulation, 109(21 Suppl 1), II18–II26. [DOI:10.1161/01.CIR.0000129505.34151.23] [PMID]
Shah, S. A., Yoon, G. H., Kim, H. O., & Kim, M. O. (2015). Vitamin C neuroprotection against dose-dependent glutamate-induced neurodegeneration in the postnatal brain. Neurochemical Research, 40(5), 875–884. [DOI:10.1007/s11064-015-1540-2] [PMID]
Sirisha, A., Gaur, G. S., Pal, P., Bobby, Z., Balakumar, B., & Pal, G. K. (2021). Effect of honey and insulin treatment on oxidative stress and nerve conduction in an experimental model of diabetic neuropathy Wistar rats. plos One, 16(1), e0245395.[DOI:10.1371/journal.pone.0245395] [PMID] [PMCID]
Sommer, C., & Kress, M. (2004). Recent findings on how proinflammatory cytokines cause pain: Peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neuroscience Letters, 361(1-3), 184–187. [DOI:10.1016/j.neulet.2003.12.007] [PMID]
Taubes G. (2002). Cardiovascular disease. Does inflammation cut to the heart of the matter?. Science, 296(5566), 242–245. [DOI:10.1126/science.296.5566.242] [PMID]
Tauler, P., Aguiló, A., Cases, N., Sureda, A., Gimenez, F., & Villa, G., et al. (2002). Acute phase immune response to exercise coexists with decreased neutrophil antioxidant enzyme defences. Free Radical Research, 36(10), 1101–1107. [DOI:10.1080/1071576021000028334] [PMID]
Tracey, D. J., & Walker, J. S. (1995). Pain due to nerve damage: Are inflammatory mediators involved?. Inflammation Research, 44(10), 407–411. [DOI:10.1007/BF01757696] [PMID]
Valsecchi, A. E., Franchi, S., Panerai, A. E., Sacerdote, P., Trovato, A. E., & Colleoni, M. (2008). Genistein, a natural phytoestrogen from soy, relieves neuropathic pain following chronic constriction sciatic nerve injury in mice: Anti-inflammatory and antioxidant activity. Journal of Neurochemistry, 107(1), 230–240. [DOI:10.1111/j.1471-4159.2008.05614.x] [PMID]
Woolf, C. J., & Mannion, R. J. (1999). Neuropathic pain: Aetiology, symptoms, mechanisms, and management. Lancet, 353(9168), 1959–1964. [DOI:10.1016/S0140-6736(99)01307-0] [PMID]
Zimmermann M. (1983). Ethical guidelines for investigations of experimental pain in conscious animals. Pain, 16(2), 109–110.[DOI:10.1016/0304-3959(83)90201-4] [PMID]
Neuropathic pain (NP) refers to a chronic and disabling condition that can develop after an event that causes disease or damage to central or peripheral nerves. This suffering condition can affect the daily activities of millions of people worldwide (Hall et al., 2006). Allodynia and hyperalgesia are the two most prominent features of NP (Woolf & Mannion, 1999). Traumatic NP can be diagnosed by various symptoms, including persistent stimulus-independent pain or spontaneous pain, after abnormal sensory perception, such as hyperalgesia and dysesthesia, or abnormal pain, and eventually allodynia (Dworkin et al., 2003). Also, assessing the conduction velocity of the motor nerve (index of the rate of transmission of an electrical impulse) is one of the crucial criteria for the speed of transmission of pain signals that can be used in pain models (Chen et al., 2022; Richards et al., 2021). Several studies have indicated that a reduced nociceptive threshold is associated with increased inflammatory mediators, leading to symptoms of hyperalgesia and allodynia (Woolf & Mannion, 1999). Nerve damage induces a precipitate of inflammatory factors at the injury region, leading to the activation of the glial cells, responsible for increasing the inflammatory process by the production and release of proinflammatory factors (Tracey & Walker, 1995). Oxidative stress can decline the immune system by reducing the production of antioxidants, and in turn, increase the secretion of inflammatory factors in the DRG, nerve, and spinal cord (Li et al., 2014; Sandireddy et al., 2016). Statins, such as atorvastatin are known for their roles in lowering serum lipids. However, previous studies have shown that anti-inflammatory properties and their subsequent therapeutic effects are crucial in different diseases, including cancer, arthritis, and Alzheimer’s disease (Ghaisas et al., 2010). One of the anti-inflammatory activities of statins is associated with inhibiting mediators of inflammation, such as interleukins (IL-1, 2, 4, 5, 10, 12), C-reactive protein, and tumor necrosis factor-α (TNF-α) (Schonbeck & Libby, 2004; Taubes, 2002). Amongst statins, atorvastatin is a well-known drug capable of directly reducing inflammation (Grip et al., 2008). Furthermore, it is an efficient antioxidant (Li et al., 2010). Vitamin C is known as a neuroprotective agent (Pavlovic et al., 2009; Shah et al., 2015). Ascorbate in high doses (20 mM) interferes with the production of IL-6 and TNF-α by inhibiting the monocytes (Hartel et al., 2004). The inflammatory gene expression caused by phorbol myristate acetate (PMA)-activated neutrophils may be changed by vitamin C. It has been shown that vitamin C can be effective in inhibiting the production and expression of cytokines, such as cyclooxygenase-2 (COX-2), IL8, TNF-α, and nuclear factor-κβ (NFκβ) in response to phorbol myristate acetate (PMA) (Capó et al., 2015). Moreover, ascorbate exists in blood flow and normal circulating human neutrophils hold ascorbate in millimolar concentrations (Tauler et al., 2002).
Many studies have shown that ascorbic acid has a significant antioxidant effect by scavenging reactive oxygen species (ROS) (Bode, 1997). Vitamin C is considered an essential nutrient for all animals and plants, and it is required for the process of metabolism and also for protection against oxidative stress (Padayatty et al., 2003).
This study was conducted to evaluate the synergism effects of atorvastatin and vitamin C co-treatment for protection against neuropathic disorders caused by chronic constriction injury (CCI), while the behavioral tests and anti-inflammatory, as well as antioxidant properties, have been considered in rat models.
2. Materials and Methods
Materials
The chemicals needed to perform this study include atorvastatin (Sigma, St. Louis, MO, USA), vitamin C (L-ascorbic, Sigma), IL-6 and TNF-α, glutathione peroxidase (GPx), superoxide dismutase (SOD) enzyme-linked immunosorbent assay (ELISA) kits (Abcam, USA), malonaldehyde (MDA), and antibody NF-kB ELISA kits (Abcam, USA).
Animals and housing conditions
Seventy male adult Sprague–Dawley rats with a weight range of 220-240 g were purchased from the Animal House of Lorestan University of Medical Sciences (Khorramabad, Iran) and maintained in normal conditions, i.e. temperature at 22°C±2°C, the humidity of almost 45%, and 12 h light/dark cycle without any restriction to access water and standard food. The Ethical Committee confirmed all animal protocols for this study (Zimmermann, 1983).
Experimental design
The rats were randomly divided into 7 groups (10 rats in each group) as follows: 1: Sham-operated, 2: CCI vehicle-treated (CCI), 3: CCI+vitamin C (vit C) (500 mg/kg), 4: CCI+atorvastatin (ATOR) (5 mg/kg), 5: CCI+ator (10 mg/kg), 6: CCI+vit C (500 mg/kg)+ator (5 mg/kg), and 7: CCI+vit C (500 mg/kg)+ATOR (10 mg/kg).
Drug preparation
In this study, atorvastatin and vitamin C were solved by aqueous (distilled water), and both of these drugs were injected freshly and intraperitoneally (i.p.) daily for treatment (for 21 days.).
Surgery
The sciatic nerve injury was induced using the CCI model of NP (Bennett & Xie, 1988). After injecting thiopental for anesthesia (35 mg/kg), the considered nerve was found (sciatic nerve). Subsequently, without interrupting the blood flow, the sciatic nerve around four ligatures was loosened. According to the reference, the distance between these ligatures is 1 mm. The sterilization of the wound was carried out with normal saline (0.9%). Finally, for the surgical suture, the wound was closed in two layers with non-absorbable sutures 4-0 (face surface) and, if necessary, surgical skin staples were performed. Likewise, the same surgical procedure was performed for the sham-operated group except for the ligation.
Thermal stimulation tests
Behavioral assessments were done on the 3rd, 7th, 14th, and 21st days following the ligation in two steps. The tests began with heat hyperalgesia stimulation and then mechanical allodynia with an interval time of 120 minutes for two tests.
Heat hyperalgesia stimulation (hot plate test)
Eddy’s hot plate method was used to achieve the thermal hyperalgesia threshold. The rats were positioned on the plate while the temperature was set at 52.5°C±1.0°C. Then, withdrawal latency was noted regarding the licking of the hind paw in a few seconds. A period of 15 s was considered as the exposure time (Jain et al., 2009).
Mechanical hyperalgesia (pin prick)
Gauge needles (at 90°) were exposed to the hind paw with a certain intensity to penetrate the skin. A maximum cut-off time of 20 s was measured for the duration of withdrawing the paw (Park et al., 1995; Kukkar et al., 2013).
Cold allodynia (acetone test)
Initially, we located the rats’ hind paws on a wire mesh. Subsequently, the stimulation was induced by spraying 100 μL of acetone without any skin exposure. Then, the reactions of the rats to acetone were noted in 20 s and scored according to the Kukkar and Singh scale in their protocol (Kukkar et al., 2013).
Mechanical allodynia (von Frey test)
Von Frey filaments were used to induce mechanical allodynia (steeling, Wood Dale, IL, USA) in the following order: 0.6, 1.0, 1.4, 2.0, 4.0, 6.0, 8.0, 10.0, 15.0, 26.0, and 60 g. To complete the test, laboratory work was performed according to the protocol used in previous papers (Abed et al., 2015).
Motor nerve conduction velocity test (MNCV)
To study the conduction velocity, electrophysiological experiments were used 21 days after the induction of CCI. First, the rats were anesthetized using thiopental (35 mg/kg). Subsequently, the sciatic nerves situated near the sciatic notch and distal to the knee were stimulated by a Nicolet Viking Quest machine, while the body temperature was kept at 37°C during the experiment (Nicolet Biomedical, Madison, WI). The action potential was then measured using unipolar pin electrodes from the ankle (Hasegawa et al., 2006). Finally, MNCV (m/s) was calculated as Equation 1:
1. MNCV=D/(PL-DL).
(D=Distance in meters, PL=proximal latency in seconds, DL=distal latency in seconds).
Enzyme-linked immunosorbent assays (ELISA):
After spinal dislocation, the process continued with the removal of the fresh spinal cord, followed by the measurement of TNF-α, IL-6, GPx, SOD, and MDA levels. To begin, the fresh spinal cord between L4-L5 segments was removed right after MNCV measurements on the 21st day. Then, a glass homogenizer was used to homogenate the tissue with 0.9% saline at 2500 r/min for 10 minutes. Homogenate supernatant (10%,w/v) was separated for these tests (Li et al., 2008).
Histological examination of nerves:
To carry out this examination, sciatic nerves were separated after MNCV evaluation and their semithin sections (5 μm) were prepared and stained by hematoxylin and eosin. Subsequently, a pathologist who was unaware of the numbering of tissue samples analyzed the slides (Brummett et al, 2009).
Statistical analysis
The results were shown as Mean±SEM. The data obtained from behavioral tests were analyzed using a two-way analysis of variance (ANOVA). Furthermore, biochemical tests and MNCV test were examined using a one-way ANOVA, and GraphPad Prism software, version 5. A P˂0.05 was considered statistically significant. The sample size was calculated via power calculations using G*Power software, version .3.1.7. α error and power (1-β) were set at 0.05 and 0.8, respectively, and the total sample size needed for each was calculated as 8-10 animals. Hence, ten rats were selected in each group.
3. Results
Effects of the administration of vitamin C and atorvastatin on thermal hyperalgesia
One-way ANOVA analysis indicated significant differences between the experimental groups in levels of thermal hyperalgesia on days 7th (F(6, 60)=17.56, P˂0.00251), 14th (F(6, 60)=48.36, P˂0.001) and 21st (F(6, 60)=163.84, P˂0.001).
The hind paw reaction in the CCI group on the 7th day after the surgery showed a significant reduction in thermal hyperalgesia compared to the sham group indicating successful model induction. On this day, no significant results were observed between other groups compared to the sham. Moreover, it was found that on the 14th and 21st days after considering CCI, all the treatments significantly attenuated thermal hyperalgesia compared to the CCI group. Nevertheless, the best treatment was observed in the last group on the 21st day (Figure 1).

Effects of the administration of vitamin C and atorvastatin on mechanical hyperalgesia
One-way ANOVA analysis revealed significant differences between the experimental groups regarding the levels of thermal hyperalgesia on the 7th (F(6, 60)=23.54, P˂0.001), 14th (F(6, 60)=56.34, P˂0.001) and 21st (F(6, 60)=96.45, P˂0.001) days.
On the third day after CCI, a significant difference was observed between group II and the sham group, but other groups did not demonstrate a significant difference with the sham group. On the 14th and 21st days after considering CCI, the co-administration of vitamin C and atorvastatin could improve mechanical hyperalgesia compared to other treatment groups (Figure 2).

Effects of the administration of vitamin C and atorvastatin on cold allodynia
One-way ANOVA analysis showed a significant difference between the experimental groups regarding the levels of thermal hyperalgesia on the 7th (F(6, 60)=48.61, P˂0.001), 14th (F(6, 60)=85.96, P˂0.001) and 21st (F(6, 60)=128.64, P˂0.001) days.
Figure 3 shows that the increased response to the stimulus on a leg with neuropathy is characteristic of inducing NP in these animals. Vitamin C, atorvastatin 5, and 10 mg/kg in monotherapy attenuated sensitivity, and a significant difference was observed between this group and the CCI group. Moreover, the analysis showed that the treatment management strategies of the rats by combining these drugs for 21 days effectively reduced the number of times paw excreted into the non-noxious stimulus acetone.

Effects of the administration of vitamin C and atorvastatin on mechanical allodynia
One-way ANOVA analysis revealed a significant difference between the experimental groups regarding the levels of thermal hyperalgesia on the 7th (F(6, 60)=7.51, P˂0.0025), 14th (F(6, 60)=95.31, P˂0.001) and 21st (F(6, 60)=98.54, P˂0.001) days.
Figure 4 shows the results of behavioral tests. Since the induction of CCI, the hind paw became sentient to mechanical stimuli, even with the feeble filament tested. The comparison of the CCI animals with the sham rats at the 3rd (P<0.01), 7th (P<0.001), 14th (P<0.001), and 21st (P<0.001) days showed a remarkably elevated response to the stimulus by the rats. On the 14th and 21st days after the induction of CCI, the rats treated with vitamin C or atorvastatin (5 or 10 mg/kg) exhibited a significant difference regarding withdrawal threshold compared to the sham rats. Furthermore, it was found that on the 7th, 14th, and 21st days after considering CCI, the co-administration of vitamin C and atorvastatin (10 mg/kg) in CCI animals improved mechanical allodynia better than other CCI groups.

Effects of the administration of vitamin C and atorvastatin on MNCV
MNCV results showed a significant difference in the CCI group compared to the sham group (P<0.001). The CCI rats treated with vitamin C or atorvastatin for three weeks exhibited an improvement in their MNCVs as compared to group II. However, groups VI, and VII exhibited significant increases (respectively, P<0.01, P<0.001) compared to the CCI group. This result suggests that co-treatment vitamin C and atorvastatin can significantly improve the MNCV (Figure 5).

Assessment of the levels of TNF-α and interleukin-6 (IL-6)
The CCI animals showed a significant increase in the serum levels of TNF-α (Figure 6) and IL-6 (Figure 7) compared to the sham group. All the treatments significantly reversed these changes and the efficacy of the treatment was dose-dependent.


Assessment of the levels of biochemical parameters in CCI-induced NP
Biochemical parameters, including MDA as lipid peroxidation marker, GPx, and SOD as antioxidant enzymes were measured. CCI induced oxidative stress via significant decreases in the GPx (Figure 8) and activity of SOD (Figure 9), and a significant increase in the MDA level (Figure 10) in the spinal cord compared to the sham group. All the treatments significantly attenuated the levels of marked oxidative stress compared to the CCI group, and atorvastatin showed dose-dependent effects with vitamin C.



Perineural inflammation in the sciatic nerve
No signs of inflammation were observed in the sciatic nerve among the rats in the sham group. Moreover, histopathological examination showed that perineural inflammation was evident in the CCI groups, indicating that it causes NP in the sciatic nerve (score=3). All the treatment groups largely reversed these changes. Furthermore, histological examination indicated low levels of inflammation around the sciatic nerve in the CCI mice treated with the combination of vitamin C and atorvastatin (5 or 10 mg/kg) (score=1).
4. Discussion
The results of the present study revealed that thermal hyperalgesia was significantly reduced in all treated groups compared to the CCI group on the 14th and 21st days. Moreover, co-administration of vitamin C and atorvastatin was effective in decreasing the hind paw withdrawal threshold caused by CCI. It was also observed that the paw withdrawal rate to non-noxious stimulus acetone was effectively decreased in co-treated groups for 21 days. Likewise, the co-administration of vitamin C and atorvastatin resulted in a minor elevation of the withdrawal threshold on the 7th, 14th, and 21st days. MNCV was another parameter that was effectively enhanced after treatment of the rats with the combination of vitamin C and atorvastatin for 21 days in group VII compared to group II. Finally, the co-administration of atorvastatin and vitamin C improved the perineural inflammation around the sciatic nerve.
NP is a condition affecting the nervous system following an injury or disease, such as diabetes, cancer, multiple sclerosis, virus diseases, etc. Many people are affected by its harmful consequences all over the world (Amin & Hosseinzadeh, 2012). Allodynia and hyperalgesia are the two major indicators to describe the conditions of NP (Jensen & Finnerup, 2014). CCI is one of the main models used to induce and evaluate NP worldwide (Okamoto et al., 2001). One of the possible mechanisms that is highly effective in causing neurological pain, particularly the NP associated with diabetes, is the production of ROS (Nazıroğlu et al., 2012). This study was conducted to evaluate the reducing effect of co-treatment with atorvastatin and vitamin C on NP induced by CCI in mice through their anti-inflammatory, antioxidant, and neuroprotective effects. Several studies have evaluated the effects of compounds and medications on behavioral tests. One of them examined the effect of ethanolic saffron extract on thermal hyperalgesia. It was found in that research that chronic treatment can significantly reduce dose-dependent thermal hyperalgesia (Amin & Hosseinzadeh, 2012). It has been shown that reducing the blood flow inside the nerve can lead to oxidative stress and ultimately reduce the conduction nerve velocity (Sirisha et al., 2021), and this reduction in velocity can indicate nerve damage and consequent conduction disturbance. However, the results of this study showed that Co-treatment with vitamin C and atorvastatin can significantly improve MNCV by anti-inflammatory and antioxidant effects.
All the treatments significantly reduced these inflammatory markers mainly with hybrid groups. Today, the central role of pro-inflammatory cytokines, such as TNF-a, IL-1, and IL-6 in NP has been shown. This is a link between inflammation and hyperalgesia, particularly the withdrawal threshold (Sommer & Kress, 2004). Hence, therapeutic agents, such as atorvastatin can be efficient in reducing neuritis. Statins, particularly atorvastatin, in addition to their other effects, have anti-inflammatory effects. Atorvastatin has been reported to reduce inflammation through the downregulation of nuclear NFκB (Chu et al., 2015). A similar study showed that atorvastatin can significantly lower IL-1β levels after treatment (Pathak et al., 2013). Other treatments, such as pyrroloquinoline, quinone, and genistein were used to significantly reduce inflammatory cytokines, including TNF-α, IL-1β, and IL-6 (Gong et al., 2012; Valsecchi et al., 2008). Furthermore, all the treatments significantly reduced oxidative stress marker levels compared to the CCI group. The combination therapy significantly lowered MDA levels and increased GPx as well as SOD levels. It has been indicated that ROS are central to the prevention of heat hyperalgesia (Lee et al., 2007). Therefore, the presence of compounds that have activities similar to antioxidant enzymes is essential to prevent NP. Hence, atorvastatin as an antioxidant drug has anti-analgesic effects. Another study evaluated the effect of atorvastatin on reducing NP. It was shown that atorvastatin can reduce MDA levels. Atorvastatin also increased SOD, catalase (CAT), and glutathione-S-transferase (GST) activity. Moreover, glutathione (GSH) levels increased following atorvastatin therapy (Pathak et al., 2014). Also, histological studies have shown low levels of inflammation around the sciatic nerve in CCI mice treated with vitamin C or atorvastatin. A similar study found that treatment with amiloride and pralidoxime can reduce CCI-induced dysfunction and degeneration (Muthuraman et al., 2008). Another study indicated that acurus extract can significantly reduce CCI-induced fiber abnormalities, nerve fiber swelling, and neuroglial cell activation (Muthuraman & Singh, 2011).
5. Conclusion
This study showed that the anti-inflammatory, antioxidant, and neuroprotective properties of combined treatment with vitamin C and atorvastatin can improve the complications of CCI in an experimental neuropathic model in mice. The results of the present research revealed that concomitant treatment with vitamin C and atorvastatin can attenuate inflammatory cytokines (such as TNF-α and IL-6) and oxidative markers (such as GPx, SOD, and MDA), enhance nerve conduction quality and histopathological scores and improve the sciatic nerves. Since the protective properties of the combination of atorvastatin and vitamin C have remained largely unnoticed, this study opens a new horizon to understand the efficiency of this procedure.
Ethical Considerations
Compliance with ethical guidelines
The Ethics Committee of Lorestan University of Medical Sciences approved all animal protocols for this study (IR.LUMS.REC.1396287).
Funding
This study (as the complementary work) was financially supported by Lorestan University of Medical Sciences (Code: A-10-1551-4).
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank the Vice-Chancellery for Research and Technology of Lorestan University of Medical Sciences, and Razi Herbal Medicines Research Center affiliated to Lorestan University of Medical Sciences for their support.
References
Abed, A., Hajhashemi, V., Banafshe, H. R., Minaiyan, M., & Mesdaghinia, A. (2015). Venlafaxine attenuates heat hyperalgesia independent of adenosine or opioid system in a rat model of peripheral neuropathy. Iranian Journal of Pharmaceutical Research, 14(3), 843–850. [PMID] [PMCID]
Amin, B., & Hosseinzadeh, H. (2012). Evaluation of aqueous and ethanolic extracts of saffron, Crocus sativus L., and its constituents, safranal and crocin in allodynia and hyperalgesia induced by chronic constriction injury model of neuropathic pain in rats. Fitoterapia, 83(5), 888–895. [DOI:10.1016/j.fitote.2012.03.022] [PMID]
Bennett, G. J., & Xie, Y. K. (1988). A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain, 33(1), 87–107. [DOI:10.1016/0304-3959(88)90209-6] [PMID]
Bode A. M. (1997). Metabolism of vitamin C in health and disease. Advances in Pharmacology, 38, 21–47. [DOI:10.1016/S1054-3589(08)60977-1] [PMID]
Brummett, C. M., Padda, A. K., Amodeo, F. S., Welch, K. B., & Lydic, R. (2009). Perineural dexmedetomidine added to ropivacaine causes a dose-dependent increase in the duration of thermal antinociception in sciatic nerve block in rat. Anesthesiology, 111(5), 1111–1119. [DOI:10.1097/ALN.0b013e3181bbcc26] [PMID] [PMCID]
Capó, X., Martorell, M., Sureda, A., Tur, J. A., & Pons, A. (2015). Effects of docosahexaenoic supplementation and in vitro vitamin C on the oxidative and inflammatory neutrophil response to activation. Oxidative Medicine and Cellular Longevity, 2015, 187849. [DOI:10.1155/2015/187849] [PMID] [PMCID]
Chen, S. M., Wang, M. H., Soung, H. S., Tseng, H. C., Fang, C. H., & Lin, Y. W., et al. (2022). Neuroprotective effect of l-theanine in a rat model of chronic constriction injury of sciatic nerve-induced neuropathic pain. Journal of the Formosan Medical Association, 121(4), 802–814. [DOI:10.1016/j.jfma.2021.08.023] [PMID]
Chu, L. W., Chen, J. Y., Wu, P. C., & Wu, B. N. (2015). Atorvastatin prevents neuroinflammation in chronic constriction injury rats through nuclear NFκB downregulation in the dorsal root ganglion and spinal cord. ACS Chemical Neuroscience, 6(6), 889–898. [DOI:10.1021/acschemneuro.5b00032] [PMID]
Dworkin, R. H., Backonja, M., Rowbotham, M. C., Allen, R. R., Argoff, C. R., & Bennett, G. J., et al. (2003). Advances in neuropathic pain: Diagnosis, mechanisms, and treatment recommendations. Archives of Neurology, 60(11), 1524–1534. [DOI:10.1001/archneur.60.11.1524] [PMID]
Ghaisas, M. M., Dandawate, P. R., Zawar, S. A., Ahire, Y. S., & Gandhi, S. P. (2010). Antioxidant, antinociceptive and anti-inflammatory activities of atorvastatin and rosuvastatin in various experimental models. Inflammopharmacology, 18(4), 169–177. [DOI:10.1007/s10787-010-0044-6] [PMID]
Gong, D., Geng, C., Jiang, L., Aoki, Y., Nakano, M., & Zhong, L. (2012). Effect of pyrroloquinoline quinone on neuropathic pain following chronic constriction injury of the sciatic nerve in rats. European Journal of Pharmacology, 697(1-3), 53–58.[DOI:10.1016/j.ejphar.2012.09.052] [PMID]
Grip, O., Janciauskiene, S., & Bredberg, A. (2008). Use of atorvastatin as an anti-inflammatory treatment in Crohn's disease. British Journal of Pharmacology, 155(7), 1085–1092. [DOI:10.1038/bjp.2008.369] [PMID] [PMCID]
Hall, G. C., Carroll, D., Parry, D., & McQuay, H. J. (2006). Epidemiology and treatment of neuropathic pain: The UK primary care perspective. Pain, 122(1-2), 156–162. [DOI:10.1016/j.pain.2006.01.030] [PMID]
Härtel, C., Strunk, T., Bucsky, P., & Schultz, C. (2004). Effects of vitamin C on intracytoplasmic cytokine production in human whole blood monocytes and lymphocytes. Cytokine, 27(4-5), 101–106. [DOI:10.1016/j.cyto.2004.02.004] [PMID]
Hasegawa, T., Kosaki, A., Shimizu, K., Matsubara, H., Mori, Y., & Masaki, H., et al. (2006). Amelioration of diabetic peripheral neuropathy by implantation of hematopoietic mononuclear cells in streptozotocin-induced diabetic rats. Experimental Neurology, 199(2), 274–280. [DOI:10.1016/j.expneurol.2005.11.001] [PMID]
Jain, V., Jaggi, A. S., & Singh, N. (2009). Ameliorative potential of rosiglitazone in tibial and sural nerve transection-induced painful neuropathy in rats. Pharmacological Research, 59(6), 385–392. [DOI:10.1016/j.phrs.2009.02.001] [PMID]
Jensen, T. S., & Finnerup, N. B. (2014). Allodynia and hyperalgesia in neuropathic pain: Clinical manifestations and mechanisms. The Lancet. Neurology, 13(9), 924–935. [DOI:10.1016/S1474-4422(14)70102-4] [PMID]
Kukkar, A., Singh, N., & Jaggi, A. S. (2013). Neuropathic pain-attenuating potential of aliskiren in chronic constriction injury model in rats. Journal of the Renin-Angiotensin-Aldosterone system, 14(2), 116–123. [DOI:10.1177/1470320312460899] [PMID]
Lee, I., Kim, H. K., Kim, J. H., Chung, K., & Chung, J. M. (2007). The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain, 133(1-3), 9–17. [DOI:10.1016/j.pain.2007.01.035] [PMID] [PMCID]
Li, J., Sun, Y. M., Wang, L. F., Li, Z. Q., Pan, W., & Cao, H. Y. (2010). Comparison of effects of simvastatin versus atorvastatin on oxidative stress in patients with coronary heart disease. Clinical Cardiology, 33(4), 222–227. [DOI:10.1002/clc.20724] [PMID] [PMCID]
Li, S. S., Zhang, W. S., Ji, D., Zhou, Y. L., Li, H., & Yang, J. L., et al. (2014). Involvement of spinal microglia and interleukin-18 in the anti-nociceptive effect of dexmedetomidine in rats subjected to CCI. Neuroscience Letters, 560, 21–25. [DOI:10.1016/j.neulet.2013.12.012] [PMID]
Li, X. R., Long, Y. H., Fang, X., & Liu, X. G. (2008). [Effect of vitamin C and E on antioxidative enzyme, NOS activity and NO contents in hippocampus of rats with lead poisoning (Chinese)]. Zhejiang da xue xue bao. Yi xue ban, 37(2), 189–192. [DOI:10.3785/j.issn.1008-9292.2008.02.015] [PMID]
Muthuraman, A., Jaggi, A. S., Singh, N., & Singh, D. (2008). Ameliorative effects of amiloride and pralidoxime in chronic constriction injury and vincristine induced painful neuropathy in rats. European Journal of Pharmacology, 587(1-3), 104–111. [DOI:10.1016/j.ejphar.2008.03.042] [PMID]
Muthuraman, A., & Singh, N. (2011). Attenuating effect of Acorus calamus extract in chronic constriction injury induced neuropathic pain in rats: An evidence of anti-oxidative, anti-inflammatory, neuroprotective and calcium inhibitory effects. BMC Complementary and Alternative Medicine, 11, 24. [DOI:10.1186/1472-6882-11-24] [PMID] [PMCID]
Nazıroğlu, M., Dikici, D. M., & Dursun, S. (2012). Role of oxidative stress and Ca²+ signaling on molecular pathways of neuropathic pain in diabetes: Focus on TRP channels. Neurochemical Research, 37(10), 2065–2075. [DOI:10.1007/s11064-012-0850-x] [PMID]
Okamoto, K., Martin, D. P., Schmelzer, J. D., Mitsui, Y., & Low, P. A. (2001). Pro- and anti-inflammatory cytokine gene expression in rat sciatic nerve chronic constriction injury model of neuropathic pain. Experimental Neurology, 169(2), 386–391.[DOI:10.1006/exnr.2001.7677] [PMID]
Padayatty, S. J., Katz, A., Wang, Y., Eck, P., Kwon, O., & Lee, J. H., et al. (2003). Vitamin C as an antioxidant: Evaluation of its role in disease prevention. Journal of the American College of Nutrition, 22(1), 18–35. [DOI:10.1080/07315724.2003.10719272] [PMID]
Park, K. M., Max, M. B., Robinovitz, E., Gracely, R. H., & Bennett, G. J. (1995). Effects of intravenous ketamine, alfentanil, or placebo on pain, pinprick hyperalgesia, and allodynia produced by intradermal capsaicin in human subjects. Pain, 63(2), 163–172. [DOI:10.1016/0304-3959(95)00029-R] [PMID]
Pathak, N. N., Balaganur, V., Lingaraju, M. C., Kant, V., Latief, N., & More, A. S., et al. (2014). Atorvastatin attenuates neuropathic pain in rat neuropathy model by down-regulating oxidative damage at peripheral, spinal and supraspinal levels. Neurochemistry International, 68, 1–9. [DOI:10.1016/j.neuint.2014.01.014] [PMID]
Pathak, N. N., Balaganur, V., Lingaraju, M. C., More, A. S., Kant, V., & Kumar, D., et al. (2013). Antihyperalgesic and anti-inflammatory effects of atorvastatin in chronic constriction injury-induced neuropathic pain in rats. Inflammation, 36(6), 1468–1478. [DOI:10.1007/s10753-013-9688-x] [PMID]
Pavlovic, V., Pavlovic, D., Kocic, G., Sokolovic, D., Sarac, M., & Jovic, Z. (2009). Ascorbic acid modulates monosodium glutamate induced cytotoxicity in rat thymus. Bratislavske Lekarske Listy, 110(4), 205–209. [PMID]
Richards, J., Gechev, A., Alexander, J., Macedo, L., May, K. A., & Lindley, S. B. (2021). The effect of local cooling at the elbow on nerve conduction velocity and motor unit behaviour: An exploration of a novel neurological assessment. Sensors, 21(20), 6703. [DOI:10.3390/s21206703] [PMID] [PMCID]
Sandireddy, R., Yerra, V. G., Komirishetti, P., Areti, A., & Kumar, A. (2016). Fisetin imparts neuroprotection in experimental diabetic neuropathy by modulating Nrf2 and NF-κB pathways. Cellular and Molecular Neurobiology, 36(6), 883–892. [DOI:10.1007/s10571-015-0272-9] [PMID]
Schönbeck, U., & Libby, P. (2004). Inflammation, immunity, and HMG-CoA reductase inhibitors: Statins as antiinflammatory agents?. Circulation, 109(21 Suppl 1), II18–II26. [DOI:10.1161/01.CIR.0000129505.34151.23] [PMID]
Shah, S. A., Yoon, G. H., Kim, H. O., & Kim, M. O. (2015). Vitamin C neuroprotection against dose-dependent glutamate-induced neurodegeneration in the postnatal brain. Neurochemical Research, 40(5), 875–884. [DOI:10.1007/s11064-015-1540-2] [PMID]
Sirisha, A., Gaur, G. S., Pal, P., Bobby, Z., Balakumar, B., & Pal, G. K. (2021). Effect of honey and insulin treatment on oxidative stress and nerve conduction in an experimental model of diabetic neuropathy Wistar rats. plos One, 16(1), e0245395.[DOI:10.1371/journal.pone.0245395] [PMID] [PMCID]
Sommer, C., & Kress, M. (2004). Recent findings on how proinflammatory cytokines cause pain: Peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neuroscience Letters, 361(1-3), 184–187. [DOI:10.1016/j.neulet.2003.12.007] [PMID]
Taubes G. (2002). Cardiovascular disease. Does inflammation cut to the heart of the matter?. Science, 296(5566), 242–245. [DOI:10.1126/science.296.5566.242] [PMID]
Tauler, P., Aguiló, A., Cases, N., Sureda, A., Gimenez, F., & Villa, G., et al. (2002). Acute phase immune response to exercise coexists with decreased neutrophil antioxidant enzyme defences. Free Radical Research, 36(10), 1101–1107. [DOI:10.1080/1071576021000028334] [PMID]
Tracey, D. J., & Walker, J. S. (1995). Pain due to nerve damage: Are inflammatory mediators involved?. Inflammation Research, 44(10), 407–411. [DOI:10.1007/BF01757696] [PMID]
Valsecchi, A. E., Franchi, S., Panerai, A. E., Sacerdote, P., Trovato, A. E., & Colleoni, M. (2008). Genistein, a natural phytoestrogen from soy, relieves neuropathic pain following chronic constriction sciatic nerve injury in mice: Anti-inflammatory and antioxidant activity. Journal of Neurochemistry, 107(1), 230–240. [DOI:10.1111/j.1471-4159.2008.05614.x] [PMID]
Woolf, C. J., & Mannion, R. J. (1999). Neuropathic pain: Aetiology, symptoms, mechanisms, and management. Lancet, 353(9168), 1959–1964. [DOI:10.1016/S0140-6736(99)01307-0] [PMID]
Zimmermann M. (1983). Ethical guidelines for investigations of experimental pain in conscious animals. Pain, 16(2), 109–110.[DOI:10.1016/0304-3959(83)90201-4] [PMID]
Type of Study: Original |
Subject:
Behavioral Neuroscience
Received: 2019/02/15 | Accepted: 2022/05/22 | Published: 2023/11/1
Received: 2019/02/15 | Accepted: 2022/05/22 | Published: 2023/11/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








