BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://bcn.iums.ac.ir/article-1-1425-en.html


 , Robert D. E. Sewell2
, Robert D. E. Sewell2 

 , Zahra Rabiei3
, Zahra Rabiei3 
 , Fatemeh Forouzanfar4
, Fatemeh Forouzanfar4 

 , Sedigheh Kazemi Sheikhshabani1
, Sedigheh Kazemi Sheikhshabani1 

 , Mahmoud Rafieian-Kopaei *3
, Mahmoud Rafieian-Kopaei *3 


2- Cardiff School of Pharmacy and Pharmaceutical Sciences, Cardiff University, Cardiff, CF10 3NB. Wales, U.K.
3- Medical Plants Research Center, Basic Health Sciences Institute, Shahrekord University of Medical Sciences, Shahrekord, Iran.
4- Neuroscience Research Center, Mashhad University of Medical Sciences, Mashhad, Iran.
● Nitric oxide synthase types include endothelial (eNOS), neuronal (nNOS), and inducible (iNOS) types.
● Both nNOS and iNOS have deleterious effects on neurons in the ischemic brain.
● BDNFis a neurotrophic factor promoting synaptic plasticity and neuronal survival.
● Anchusa italic reduced iNOS but increased BDNF mRNA expression in a stroke model.
● A. italica is an anti-inflammatory/antioxidant herb with neuroprotective properties.
Plain Language Summary
Transient global cerebral ischemia can develop clinically. Cerebral blood vessel reperfusion may also augment brain damage and this is known as cerebral Ischemia-Reperfusion (I-R) injury. Nitric Oxide (NO) is a neurotransmitter involved in the formation of hippocampal memory and exerts its beneficial effects when it is synthesized at physiological concentrations in the brain. However, enhanced levels of NO can alter its physiological neuromodulator role to a nerve-damaging agent leading to induce inflammation. Antioxidants reduce the actions of unstable harmful oxygen-containing reactive molecules (reactive oxygen species) to protect nerve cells from I-R-induced damage in animal models. The medicinal plant, Anchusa italica, is a useful source of antioxidants that offers possible protection against global cerebral ischemia and reperfusion injury. Our findings showed that A. Italic decreases oxidative stress by down-regulating inducible nitric oxide synthase (iNOS) mRNA expression, and up-regulating the mRNA expression of Brain-Derived Neurotrophic Factor (BDNF), which boosts the survival of nerve cells during I-R.
1. Introduction
Ischemic stroke is the most prevalent type of stroke and a leading cause of adult disability and mortality worldwide (Bart van der Worp & van Gijn, 2007). Transient global cerebral ischemia can occur clinically, starving brain tissue of blood even temporarily to instigate the death of neuronal cells (Peng et al., 2012). Cerebral blood vessel reperfusion may also augment brain damage and this is known as cerebral Ischemia-Reperfusion (I-R) injury (Peng et al., 2012).
Multiple and complex factors, including oxidative, excitotoxic, and nitrosative stress, infarct depolarization, inflammation, and apoptosis-like mechanisms may well lead to more serious damage and cell death in cerebral ischemic stroke (Prentice, Modi, & Wu, 2015).
NO is a putative peripheral and central neurotransmitter, which is generated by three different subtypes of Nitric Oxide Synthases (NOSs), namely neuronal NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS) (Cui et al., 2013). Hippocampal nNOS and eNOS are plentiful and have an important role in learning and memory processes implicated in behavioral phenomena, such as inhibition of passive avoidance and spatial learning (Harooni, Aghdi, Sepehri, & Haeri Rohani, 2009). NO is involved in the formation of hippocampal short- and long-term memory and it has been shown that NOS inhibitors impair memory performance in both chicks and mice (Smith et al., 2016). Moreover, the NO generated after N-methyl-D-aspartate (NMDA) receptor stimulation also behaves as a retrograde messenger producing hippocampal long-term potentiation (Paul & Ekambaram, 2011). However, NO exerts all its positive learning and memory effects when it is generated at natural physiological concentrations (Paul & Ekambaram, 2011).
iNOS is unregulated after I-R injury and generates excessive NO, which reacts with superoxide to form peroxynitrite radical causing neuronal death. Due to its high rate of oxidative metabolic activity, the brain is vulnerable to oxidative stress, intense production of Reactive Oxygen Species (ROS), and a high content of polyunsaturated fatty acids. It also has a marginal antioxidant capacity, minimal reparative activity, and a non-replicating nature within neuronal cells (Liu, Li, Zhao, Wang, Qu, & Mu, 2015). Enhanced levels of NO may alter its physiological neuromodulatory role to neurotoxic agents inducing neuronal inflammation and demyelination, memory impairment and neurodegeneration (Paul & Ekambaram, 2011).
In contrast, eNOS confers beneficial effects during cerebral ischemia (Zeng et al., 2011). Thus, eNOS can lessen acute ischemic injury and promote recovery following cerebral ischemia by regulating cerebral blood flow, maintaining cerebral homeostasis and exerting an anti-inflammatory activity (Chen, Ivy, & Russo-Neustadt, 2006; Chen, Cui, Zacharek, Roberts, & Chopp, 2009). Moreover, eNOS up-regulates the expression of Brain-Derived Neurotrophic Factor (BDNF) (Cui et al., 2013). BDNF is a key neurotrophic factor, which not only promotes synaptic plasticity but also neuronal survival and it has an integral role in certain forms of learning and memory. Hence, up-regulating BDNF may well decrease or prevent cell damage following transient forebrain ischemia and reduce infarct size leading to improving outcomes (Jiang, Zuo, Wang, Lu, Yang, & Wang, 2017).
Animal models of cerebral ischemia antioxidants reduce ROS-mediated activity and protect neurons from I-R-induced neural loss (Mazza, Pomponi, Janiri, Bria, & Mazz, 2007). In this regard, Anchusa italicah as antioxidant properties decreasing the level of oxidative stress and also possesses free radical scavenging activity (Al-Snafi, 2014; Al-Snafi, 2015). This herb belongs to the Boraginaceae family and has been advocated as a folk medicine in temperate Mediterranean and Tropical regions. Phytochemical studies have revealed that A. Italica contains alkaloids, tannins, oil rich in vitamin E, triterpenes, and polyphenols (Alsabri et al., 2012; Al-Snafi, 2015). It exhibits several pharmacological properties, including anticancer, antioxidant, antiviral, antimicrobial, hypotensive, and antidiabetic activities (Al-Snafi, 2014).
In this context, the use of traditional medicine is widespread and plants still represent a significant source of natural antioxidants. Consequently, the aim of this study was to evaluate the effect of a hydroalcoholic extract of A. italica, (HEAI) as a useful source of antioxidants on possible protection against global cerebral ischemia and reperfusion using an animal model. In particular, we focused on the involvement of the nitrosative stress pathway and up-regulation of the BDNF gene as the possible mechanism (Al-Snafi, 2014; Blondeau, Lipsky, Bourourou, Duncan, Gorelick, & Marini, 2015).
2. Methods
2.1. Preparation of a hydro alcoholic extract of Anchusa italica
A. italica was purchased from a medicinal herb supplier in Shahrekord and authenticated taxonomically by a senior plant taxonomist in the Medical Plants Research Center, Shahrekord University of Medical Sciences. The flowers of A. italica were macerated with 70% ethanol at room temperature for one week. The extract was shaken, filtered, and evaporated in a rotary evaporator under reduced pressure for dryness. It was protected from light and stored at 4°C until tested and analyzed.
2.2. Radical scavenging assay
The antioxidant capability of HEAI was ascertained by the radical scavenging capacity for DPPH according to our previously established method. Accordingly, the IC50 values of HEAI were obtained for DPPH scavenging.
2.3. Total phenolic compounds in HEAI
The concentration of total phenolic compounds in HEAI was evaluated by an adapted Folin–Ciocalteu method described earlier.
2.4. Total flavonoids in HEAI
A colorimetric assay was employed to evaluate the total flavonoid content of HEAI. Total flavonoids were expressed in terms of Rutin equivalent (mg/g) (Molan & Mahdy, 2014).
2.5. Animals
Male Wister rats weighing 250-300 g sourced from the Razi Institute (Tehran, Iran) were housed for a minimum of 7 days in an air-conditioned environment before testing. They were maintained in controlled conditions of room temperature (25.0±3.0°C) and constant humidity (10%), on an alternating 12 h/12 h light/dark cycle and with ad libitum food plus water availability. The Ethics Committee of Shahrekord University of Medical Sciences approved the study (Ethics code: IR.SKUMS.REC.1394.256).
2.6. Global cerebral ischemia and preparation of experimental material
Animals were assigned in a random fashion to four groups (n=7) as follows: 1. sham-operated; 2. I-R (control), HEAI post-treatment at doses of; 3. 50 mg/kg (AI-50); and 4. 100 mg/kg (AI-100) administered daily for 10 days, 24 h after I-R induction. Also, the animals in the sham-operated group were managed identically to controls; however, they underwent surgery without global cerebral artery occlusion (Torki et al., 2018).
In all four groups, the rats were weighed and anesthetized with an intraperitoneal (i.p.) injection of chloral hydrate (Merck, Germany) (400 mg/kg), and then a midline ventral incision was made in the neck. In the case of induced global cerebral ischemia, the bilateral carotid arteries were occluded by small artery clips. Sixty minutes after ischemia, reperfusion was performed by releasing the artery clips. In the sham-operated group, the arteries were exposed for 60 min without occlusion.
During surgery, the rectal temperature was maintained at 37◦C by a heating pad. After reperfusion, animals were returned to their separate home cages. Twenty-four hours after surgery, AI-50 and AI-100 groups received 50 and 100 mg/kg HEAI for 10 days via i.p. injection, respectively. Doses were chosen from previously published data. The control and sham-operated groups received normal saline instead of HEAI for 10 days. Subsequently, animals were weighed and anesthetized with chloral hydrate. Blood was collected from the abdominal aorta, and then serum was separated and stored at -20°C. The rats were decapitated, their brains were removed immediately, and both hippocampi were dissected on ice-cold plates. One lateral hippocampus was stored at -70°C until the extraction of the mRNA content, whereas the other part was stored at -20°C until it was used in the malondialdehyde (MDA) assay.
2.7. Determination of lipid peroxide content in rat plasma
The formation of thiobarbituric acid reactive substances (TBARs) is used as an index of lipid peroxidation ascertained as MDA. This determination was carried out according to the method of Mihara and Uchiyama (Uchiyama & Mihara, 1978). Thus, 100 µl plasma or standard was mixed with 100 µL sodium dodecyl sulfate (8.1%) and 2.5 ml of Thiobarbituric Acid (TBA). The mixture was subsequently heated on a boiling water bath and the reaction was terminated 60 min later by placing the reaction tubes in ice. After centrifugation (4000 rpm x 10 min), the absorbance of the supernatant was measured at 535 nm by a flow-through spectrophotometer.
2.8. Determination of lipid peroxide content in rat hippocampus
Brain tissues were homogenized in ice-cold Tris-HCl buffer (0.05 mol/L, Ph=7.4) for 2 min at 5000 rpm. The homogenized solution was subsequently centrifuged at 4300 rpm for 60 min. Then, 100 µL of the supernatant was mixed with 10 µL butylated hydroxytoluene (0.5 mol/L), 200 µL sodium dodecyl sulfate (8.1%) and 1.5 mL TBA. This suspension was heated for 60 min in a boiling water bath. After cooling, 3.0 mL of butanol-pyridine was added to the suspension and centrifuged at 3900 rpm for 10 min. The organic layer was transferred to a fresh tube and its absorbance was measured at 535 nm using a spectrophotometer (Karatas, Karatepe, & Baysar, 2002).
2.9. Nitric oxide assays
Metabolites of NO in the serum were determined using the Griess reaction accompanied by the enzymatic reaction of nitrite and nitrate with copper-plated cadmium. Thus, the serum sample was deproteinated by adding ZnSO4 and NaOH solutions and activated cadmium granules were added to the pretreated deproteinated serum. Then, Griess reagents (sulfanilamide in 5% chloric acid) plus N-[1-napthyl] ethylene diamine dihydrochloride) were added to the sample. Sample absorbance was then measured using a spectrophotometer set at 540 nm. Concentrations of NO were measured based on a standard graph derived from a range of NaNO3 concentrations.
2.10. Quantitative analysis for eNOS, iNOS, and BDNF mRNA in hippocampal tissue using Real-Time-PCR
Total RNA was isolated from hippocampal tissue using total RNA extracted with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) adopting the manufacturer’s recommended procedure.
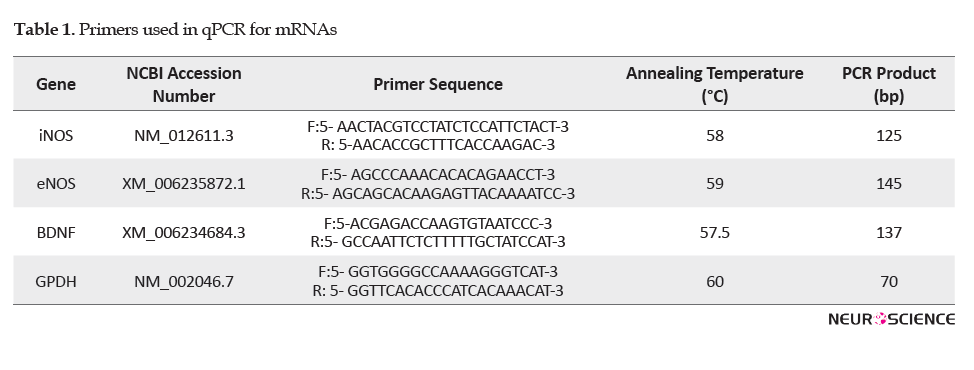
Reverse transcription was performed on an aliquot containing 500 ng of total RNA. The reaction was accomplished on the superscript first-strand cDNA synthesis system, based on the manufacturer’s recommended procedure (Takara, Japan). The sequences of target genes and the specific primers are shown in Table 1.
BDNF, iNOS, and eNOS mRNA levels were measured using a Rotor-Gene 6000 real-time PCR cycler (Corbett, Australia). The Real-time PCR reaction was also performed on 1 µl of synthesized cDNA solution, 5 µl of SYBR Premix Ex Taq II (TliRNase H Plus) (Takara, Japan), and 0.2 μl of each primer. Forty amplification cycles consisted of denaturation at 95°C for 15 s, annealing according to Table 1 for 30 s and an extension at 72°C for 30 s. Each sample was analyzed in triplicate. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was utilized as a loading control to normalize target gene expression. Relative expression levels of the target cDNAs were quantified by the 2ΔΔCts method.
2.11. Statistical analysis
Graph Pad software (San Diego, California, USA) was employed for data analysis. Statistical analysis of the data was performed employing the One-Way Analysis Of Variance (ANOVA) and Tukey post-hoc test to compare the differences between the control group and experimental groups (P<0.05 was considered significant).
3. Results
3.1. Standardization of HEAI
The total phenolic compound content in HEAI was 62.2±0.3 mg gallic acid equivalent/g of dried extract. Also, the total flavonoid compound content was 47.06±0.4 mg/g of dry matter.
3.2 Antioxidant activity of HEAI
The antioxidant activity of HEAI was evaluated using DPPH free radical scavenging and it possessed potent activity in this respect (IC50=90.5±0.3 μg/mL).
3.3. MDA content in rat serum after ischemia
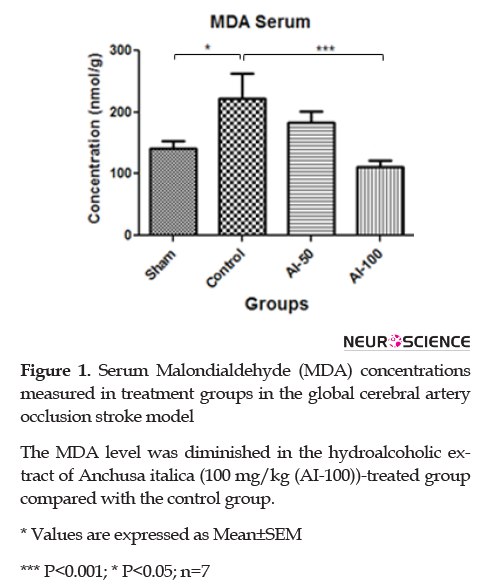
MDA levels in serum are shown in Figure 1. The MDA serum concentration in the global cerebral ischemia group was higher than the sham group and the MDA levels in the HEAI-treated groups decreased in a dose-dependent manner. HEAI, at the 100 mg/kg dose, significantly reduced (P<0.001) the MDA level than the controls.
3.4. MDA content in rat hippocampus after ischemia
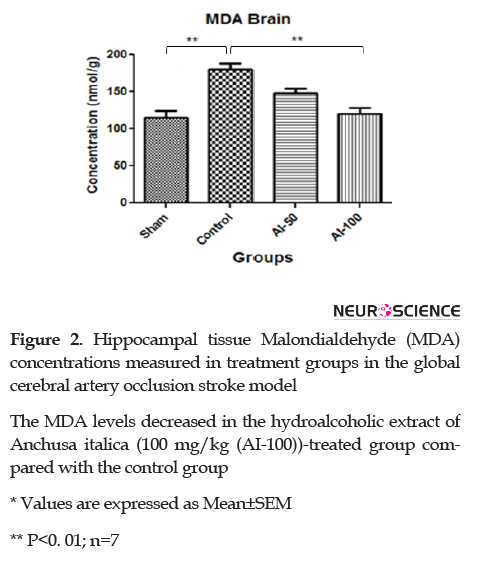
MDA levels in the hippocampus are shown in Figure 2. In the ischemic group, there was an elevation in MDA in comparison with the sham-operated group. Administration of HEAI resulted in a significant reduction (P<0.01) in lipid peroxidation (plasma MDA levels) at the 100 mg/kg/day dose (P<0.01) when compared with the control group. No significant difference in plasma MDA levels was observed between the AI-100 and AI-50 groups.
3.5. NO content in rat serum after ischemia
NO levels in the serum are shown in Figure 3. In the animal control group, there was a significantly higher level of NO compared with the sham-operated group (76.26 µM/L and 35.25 µM/L, respectively). The NO concentrations in the HEAI-treated groups decreased in comparison with controls; however, there was no significant difference (P<0.001) between NO levels in the AI-50 and AI-100 treatment groups (25.76 µM/L and 23.59 µM/L, respectively).
3.6. iNOS and eNOS mRNA expression in the hippocampus after ischemia
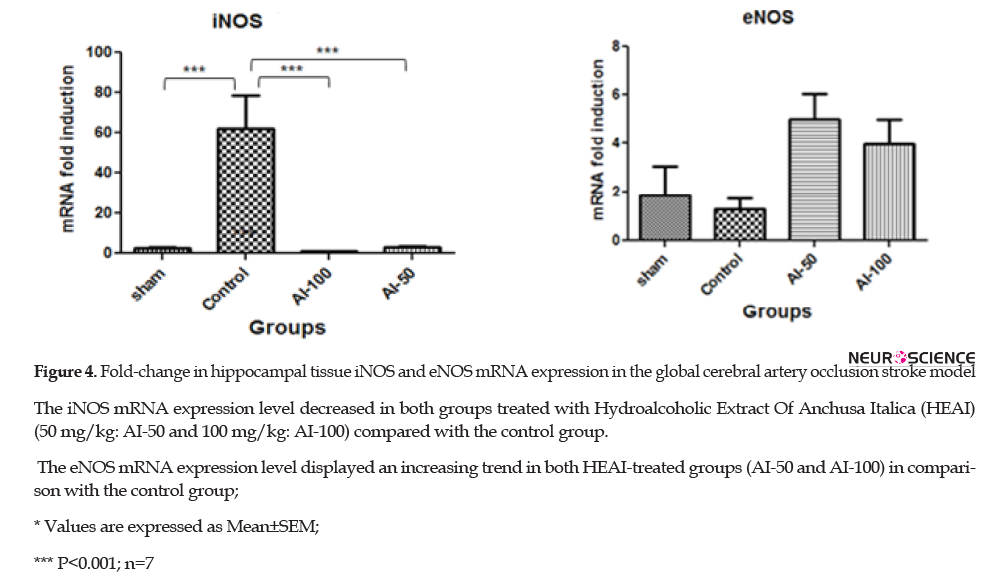
Global cerebral ischemia caused a decrease in iNOS mRNA expression in the sham-operated animals compared with the controls (P<0.001). Furthermore, HEAI at the doses of 50 mg/kg and 100 mg/kg both reduced the iNOS mRNA signal in the hippocampus compared with the control group (P<0.001); however, there was no difference in the reduction of iNOS mRNA between these two doses. However, the administration of HEAI produced an upward trend in eNOS mRNA expression in the hippocampus but it was not statistically significant and there was no difference between the effects of the two treatment groups (AI-50 and AI-100) (P<0.05) (Figure 4).
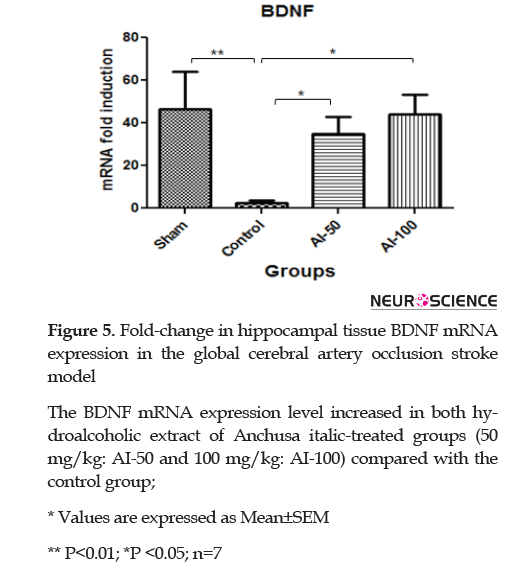
3.7. BDNF mRNA expression in the hippocampus after ischemia
Global cerebral ischemia resulted in significantly reduced BDNF mRNA hippocampal expression in the control group compared with sham-operated animals (P<0.01). HEAI at the doses of 50 and 100mg/kg both increased the BDNF mRNA signal in the hippocampus than controls; however, no difference was observed between the treated groups (A-50 and AI-100; (P<0.05) (Figure 5).
4. Discussion
This is the first report regarding a protective propensity of HEAI against global cerebral ischemia and reperfusion in an animal model via inhibition of the nitrosative stress pathway and up-regulation of the BDNF gene. During the breakdown of lipid hydroperoxides, MDA is formed. It is an established biomarker of free radical damage in pathologies related to oxidative stress. In the first phase of our study, we showed that post-cerebral ischemic treatment of rats with HEAI, at the doses of 50 and 100mg/kg, caused a significant decrease in MDA levels not only in serum but also in hippocampal brain tissue. HEAI at the higher dose appeared to be more effective than the lower dose (Figure 1).
In agreement with these findings, there are other reports concerning the modulatory effect of Borago officinales (Boraginaceae family) on lipid peroxidation in the hippocampus (Ghahremanitamadon, Shahidi, Zargooshnia, Nikkhah, Ranjbar, & Soleimani Asl, 2014). Besides, our results showed that the NO content in serum substantially decreased in the HEAI-treated animals compared with the I-R group, but this effect was not dose-dependent. Endogenous NO is generated by the NOS enzyme group. It has a wide range of physiological and pathophysiological actions and there are three NOS isoforms in the enzyme family, which are capable of producing NO (Blanco, Hernández, Franchelli, Ramos-Álvarez, & Peinado, 2017). NOS1 or neuronal NOS (nNOS) is constitutively expressed in neurons in the brain. NOS2 or inducible NOS (iNOS), is not present under normal physiological conditions but is produced in response to immunological stimulation. NOS3 or endothelial NOS (eNOS) has an important vasodilatory function and is constitutively expressed in brain endothelial cells (Blanco et al., 2017).
Endogenous NO released from nitrergic neurons regulates the release of several monoamine neurotransmitters, excitatory and inhibitory amino acids, and adenosine (Prast & Philippu, 2001). Furthermore, NO is not only a neuromodulator of neurotransmitter and neuropeptide function presynaptically but it also promotes synaptic plasticity. In addition, it is implicated in both short- and long-term memory in the hippocampus. In physiological conditions, NO activates soluble guanylate cyclase (sGC) producing cyclic guanosine monophosphate (cGMP). The NO/GC pathway is involved in hippocampal long-term memory formation and it has been shown that NOS inhibitors block long-term potentiation (Prast & Philippu, 2001).
NO either has a protective or detrimental influence on ischemic brain injury, which depends on the NOS isoform source and the cell type (Pluta, 2005). In our study, we discovered that iNOS mRNA was induced in hippocampal tissue following cerebral ischemia. Treatment with HEAI suppressed iNOS mRNA expression, which may conceivably be responsible for protective effects against ischemic stroke. Overexpression of NO from iNOS leads to neurodegeneration approved by a reduction in brain infarct volume and edema in iNOS knockout mice after transient focal ischemic injury (Hara, Huang, Panahian, Fishman, & Moskowitz, 1996).
We also observed that iNOS mRNA was overexpressed in hippocampal tissue as a result of cerebral ischemia, but after HEAI treatment, this overexpression was completely abolished. Although NO has positive effects, if its production becomes uncontrollably perturbed, this may be significantly harmful as a consequence of initiating neurotoxicity and apoptosis. In this context, NO and its superoxide combination product, peroxynitrite, have been reported to be conducive to the pathogenesis of neurodegenerative conditions (Anaeigoudari, Soukhtanloo, Reisi, Beheshti & Hosseini, 2016).
NO has been demonstrated to incite DNA fragmentation through a mitochondrial apoptotic pathway (Wang et al., 2010). It can also instigate oxidative DNA damage and break single-stranded DNA-triggering cellular injury through activation of poly (ADP-ribose) polymerase, a nuclear enzyme that depletes essential substrates and potentially contributes to neurotoxicity (Blanco et al., 2017). In this regard, iNOS expression may play a pathogenic role in the mechanisms of cerebral ischemic damage.
Treatment with HEAI displayed a trend towards up-regulation of eNOS mRNA expression in hippocampal tissue after global cerebral ischemic injury. Moreover, eNOS-deficient mice have been shown to exhibit a marked impairment of angiogenesis and increased infarct volume in the ischemic brain. In contrast, overexpressed eNOS in transgenic mice renders them resistant to stress-induced hypotension, inflammation, and stroke (Beck & Plate, 2009). It is well established that global ischemia, caused by reduced cerebral blood flow, initiates a cascade of mechanisms, including elevated levels of ROS, excitotoxicity, mitochondrial dysfunction and neuronal apoptosis (Tóthová, Kovalská, Kalenská, Tomašcová, & Lehotský 2018). Accordingly, eNOS has a neuroprotective role by increasing blood flow in the very early stages of cerebral ischemia (Hashiguchi et al., 2004).
BDNF expression is regulated by NO generated from eNOS in the brain (Cao et al., 2011). This highlights the potentially important role of neuronally-derived eNOS in modulating BDNF production and the need for further investigation of any favorable propensity of eNOS on neuronal survival following stroke. In this respect, eNOS-knockout-mice have shown a reduced expression of BDNF in the ischemic brain (Chen et al., 2005) and it has been postulated that a deficiency of eNOS associated with down-regulated BDNF expression, impairs neurogenesis after stroke. NO and BDNF appear to operate in a positive feedback loop promoting neurogenesis and eNOS may be a noteworthy target gene in new therapeutic approaches for central ischemia.
Our results showed that treatment with HEAI significantly up-regulated the expression of BDNF mRNA in brain tissue after ischemic injury. Furthermore, inhibition of NOS activity reduces the de novo synthesis of NO, leading to a decline in BDNF (Faraco et al., 2018) and this concurs with our observed down-regulated hippocampal BDNF mRNA expression in the I-R control group.
A. italic contains alkaloids, tannins, essential oils, triterpenes and polyphenols that possess antioxidant properties, and it is regarded as one of the best sources of γ-linolenic acid (GLA) and α-linolenic acid (ALA) (Conforti et al., 2011; Al-Snafi, 2014). ALA, in particular, is known to have beneficial effects on brain ischemia and post-stroke depression (Blondeau et al., 2015) and ALA increases BDNF mRNA and protein levels during cerebral ischemia. Additionally, recent studies have shown that ALA can increase neurogenesis, synaptogenesis, and synaptic function in the brain (Blondeau et al., 2015), which can be a useful characteristic in stroke.
It was observed that the administration of an A. italic extract decreased the levels of lipid peroxidation product, MDA, as a marker of oxidative stress. This suggested that A. italica reduces oxidative stress in global cerebral I-R injury. We also demonstrated that HEAI down-regulated iNOS mRNA expression induced an upward trend in eNOS mRNA, and up-regulated BDNF expression, which may be beneficial in the ischemic brain.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles were considered in this article. The study protocol was approved by the Ethics Committee of Shahrekord University of Medical Sciences (Ethics code: IR.SKUMS.REC.1394.256).
Funding
This research was financially supported by the Research Deputy of Shahrekord University of Medical Sciences, Shahrekord, Iran (Grant No.: 2052).
Authors' contributions
Conflict of interest
The authors declared no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Acknowledgments
The authors would like to offer their special appreciation to the Research Deputy of Shahrekord University of Medical Sciences, Shahrekord, Iran for financial support of this research.
References
Alsabri, S. G., Zetrini, A. E., Ermeli, N. B., Mohamed, S. B., Bensaber, S. M., & Hermann, A., et al. (2012). Study of eight medicinal plants for antioxidant activities. Journal of Chemical and Pharmaceutical Research, 4(8), 4028-31. https://www.researchgate.net/publication/233759560
Al-Snafi, A. E. (2014). The pharmacology of Anchusa italica and Anchusa strigosa. A review. International Journal of Pharmacy and Pharmaceutical Sciences, 6(4), 7-10. https://www.researchgate.net/publication/267033194
Al-Snafi, A. E. (2015). Therapeutic properties of medicinal plants: A review of plants with antioxidant activity (part 1). International Journal of Pharmacy & Therapeutics, 6(3), 159-82. https://www.researchgate.net/publication/313696720
Anaeigoudari, A., Soukhtanloo, M., Reisi, P., Beheshti F., & Hosseini, M. (2016). Inducible nitric oxide inhibitor aminoguanidine, ameliorates deleterious effects of lipopolysaccharide on memory and long term potentiation in rat. Life Sciences, 158, 22-30. [DOI:10.1016/j.lfs.2016.06.019] [PMID]
Asgharzade, S., Rabiei Z., & Rafieian-Kopaei, M. (2015). Effects of Matricaria chamomilla extract on motor coordination impairment induced by scopolamine in rats. Asian Pacific Journal of Tropical Biomedicine, 5(10), 829-33. [DOI:10.1016/j.apjtb.2015.06.006]
Asgharzade, S., Rafieian-Kopaei, M., Mirzaeian, A., Reiisi, S., & Salimzadeh, L. (2015). Aloe vera toxic effects: Expression of inducible Nitric Oxide Synthase (iNOS) in testis of wistar rat. Iranian Journal of Basic Medical Sciences, 18(10), 967-73. [PMID] [PMCID]
Bart van der Worp, H., & van Gijn, J. (2007). Acute ischemic stroke. The New England Journal of Medicine, 357(6), 572-9. [DOI:10.1056/NEJMcp072057] [PMID]
Beck, H., & Plate, K. H. (2009). Angiogenesis after cerebral ischemia. Acta Neuropathologica, 117(5), 481-96. [DOI:10.1007/s00401-009-0483-6] [PMID]
Blanco, S., Hernández, R., Franchelli, G., Ramos-Álvarez, M. M., & Peinado, M. Á. (2017). Melatonin influences NO/NOS pathway and reduces oxidative and nitrosative stress in a model of hypoxic-ischemic brain damage. Nitric Oxide, 62, 32-43. [DOI:10.1016/j.niox.2016.12.001] [PMID]
Blondeau, N., Lipsky, R. H., Bourourou, M., Duncan, M. W., Gorelick, P. B., & Marini, A. M. (2015). Alpha-linolenic acid: An omega-3 fatty acid with neuroprotective properties-ready for use in the stroke clinic? BioMed Research International, 2015, 519830. [DOI:10.1155/2015/519830] [PMID] [PMCID]
Cao, Y., Mao, X., Sun, C., Zheng, P., Gao, J., & Wang, X, et al. (2011). Baicalin attenuates global cerebral ischemia/reperfusion injury in gerbils via anti-oxidative and anti-apoptotic pathways. Brain Research Bulletin, 85(6), 396-402. [DOI:10.1016/j.brainresbull.2011.05.002] [PMID]
Chen, J., Cui, X., Zacharek, A., Roberts, C., & Chopp, M. (2009). eNOS mediates TO90317 treatment-induced angiogenesis and functional outcome after stroke in mice. Stroke, 40(7), 2532-8. [DOI:10.1161/STROKEAHA.108.545095] [PMID] [PMCID]
Chen, J., Zacharek, A., Zhang, C., Jiang, H., Li, Y., & Roberts, C., et al. (2005). Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. The Journal of Neuroscience, 25(9), 2366-75. [DOI:10.1523/JNEUROSCI.5071-04.2005] [PMID] [PMCID]
Chen, M. J., Ivy, A. S., & Russo-Neustadt, A. A. (2006). Nitric oxide synthesis is required for exercise-induced increases in hippocampal BDNF and phosphatidylinositol 3′ kinase expression. Brain Research Bulletin, 68(4), 257-68. [DOI:10.1016/j.brainresbull.2005.08.013] [PMID]
Conforti, F., Marrelli, M., Carmela, C., Menichini, F., Valentina, P., & Uzunov, D., et al. (2011). Bioactive phytonutrients (omega fatty acids, tocopherols, polyphenols), in vitro inhibition of nitric oxide production and free radical scavenging activity of non-cultivated Mediterranean vegetables. Food Chemistry, 129(4), 1413-9. [DOI:10.1016/j.foodchem.2011.05.085]
Cui, X., Chopp, M., Zacharek, A., Ning, R., Ding, X., & Roberts, C., et al. (2013). Endothelial nitric oxide synthase regulates white matter changes via the BDNF/TrkB pathway after stroke in mice. PLoS One, 8(11), e80358. [DOI:10.1371/journal.pone.0080358] [PMID] [PMCID]
Faraco, G., Wang, G., Santisteban, M. M., Chang, H., Segarra, S., & Anrather, J., et al. (2018). Abstract TMP94: Dietary salt impairs cognitive function through suppression of endothelial nitric oxide synthesis and hippocampal BDNF signaling. Stroke, 49(Suppl_1), ATMP94. [DOI:10.1161/str.49.suppl_1.TMP94]
Ghahremanitamadon, F., Shahidi, S., Zargooshnia, S., Nikkhah, A., Ranjbar, A., & Soleimani Asl, S. (2014). Protective effects of Borago officinalis extract on amyloid β-peptide (25-35)-induced memory impairment in male rats: A behavioral study. BioMed Research International, 2014, 798535. [DOI:10.1155/2014/798535] [PMID] [PMCID]
Hara, H., Huang, P. L., Panahian, N., Fishman, M. C., & Moskowitz, M. A. (1996). Reduced brain edema and infarction volume in mice lacking the neuronal isoform of nitric oxide synthase after transient MCA occlusion. Journal of Cerebral Blood Flow and Metabolism, 16(4), 605-11. [DOI:10.1097/00004647-199607000-00010] [PMID]
Harooni, H. E., Naghdi, N., Sepehri, H., & Haeri Rohani, A. (2009). The role of hippocampal Nitric Oxide (NO) on learning and immediate, short- and long-term memory retrieval in inhibitory avoidance task in male adult rats. Behavioural Brain Research, 201(1), 166-72. [DOI:10.1016/j.bbr.2009.02.011] [PMID]
Hashiguchi, A., Yano, S., Morioka, M., Hamada, J., Ushio, Y., & Takeuchi, Y., et al. (2004). Up-regulation of endothelial nitric oxide synthase via phosphatidylinositol 3-kinase pathway contributes to ischemic tolerance in the CA1 subfield of gerbil hippocampus. Journal of Cerebral Blood Flow and Metabolism, 24(3), 271-9. [DOI:10.1097/01.WCB.0000110539.96047.FC] [PMID]
Jiang, C., Zuo, F., Wang, Y., Lu, H., Yang,Q., & Wang, J. (2017). Progesterone changes VEGF and BDNF expression and promotes neurogenesis after ischemic stroke. Molecular Neurobiology, 54(1), 571-81. [DOI:10.1007/s12035-015-9651-y] [PMCID]
Karatas, F., Karatepe, M., & Baysar, A. (2002). Determination of free malondialdehyde in human serum by high-performance liquid chromatography. Analytical Biochemistry, 311(1), 76-9. [DOI:10.1016/S0003-2697(02)00387-1]
Liu, H., Li, J., Zhao, F., Wang, H., Qu, Y., & Mu, D. (2015). Nitric oxide synthase in hypoxic or ischemic brain injury. Reviews in the Neurosciences, 26(1), 105-17. [DOI:10.1515/revneuro-2014-0041] [PMID]
Mazza, M., Pomponi, M., Janiri, L., Bria, P., & Mazza, S. (2007). Omega-3 fatty acids and antioxidants in neurological and psychiatric diseases: An overview. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 31(1), 12-26. [DOI:10.1016/j.pnpbp.2006.07.010] [PMID]
Molan, A. L., & Mahdy, A. S. (2014). Iraqi medicinal plants: Total flavonoid contents, free-radical scavenging and bacterial beta-glucuronidase inhibition activities. IOSR Journal of Dental and Medical Sciences, 13(5), 72-7. [DOI:10.9790/0853-13527277]
Paul, V., & Ekambaram, P. (2011). Involvement of nitric oxide in learning & memory processes. Indian Journal of Medical Research, 133(5), 471-8. [PMID] [PMCID]
Peng, B., Guo, Q. L., He, Z. J., Ye, Z., Yuan, Y. J., & Wang, N., et al. (2012). Remote ischemic postconditioning protects the brain from global cerebral ischemia/reperfusion injury by up-regulating endothelial nitric oxide synthase through the PI3K/Akt pathway. Brain Research, 1445, 92-102. [DOI:10.1016/j.brainres.2012.01.033] [PMID]
Pluta, R. M. (2005). Delayed cerebral vasospasm and nitric oxide: Review, new hypothesis, and proposed treatment. Pharmacology & Therapeutics, 105(1), 23-56. [DOI:10.1016/j.pharmthera.2004.10.002] [PMID]
Prast, H., & Philippu, A. (2001). Nitric oxide as modulator of neuronal function. Progress in Neurobiology, 64(1), 51-68. [DOI:10.1016/S0301-0082(00)00044-7]
Prentice, H., Modi, J. P., & Wu, J. Y. (2015). Mechanisms of neuronal protection against excitotoxicity, endoplasmic reticulum stress, and mitochondrial dysfunction in stroke and neurodegenerative diseases. Oxidative Medicine and Cellular Longevity, 2015, 964518. [DOI:10.1155/2015/964518] [PMID] [PMCID]
Smith, A. E., Xu, Z., Lai, Y. Y., Kulkarni, P. M., Thakur, G. A., & Hohmann, A. G., et al. (2016). Source memory in rats is impaired by an NMDA receptor antagonist but not by PSD95-nNOS protein-protein interaction inhibitors. Behavioural Brain Research, 305, 23-9. [DOI:10.1016/j.bbr.2016.02.021] [PMID] [PMCID]
Torki, A., Khalaji-Pirbalouty, V., Lorigooini, Z., Rafieian-Kopaei, M., Sadeghimanesh, A., & Rabiei, Z. (2018). Anchusa italica extract: Phytochemical and neuroprotective evaluation on global cerebral ischemia and reperfusion. Brazilian Journal of Pharmaceutical Sciences, 54(1), e17251. [DOI:10.1590/s2175-97902018000117251]
Tóthová, B., Kovalská, M., Kalenská, D., Tomašcová, A., & Lehotský, J. (2018). Histone hyperacetylation as a response to global brain ischemia associated with hyperhomocysteinemia in rats. International Journal of Molecular Sciences, 19(10), 3147. [DOI:10.3390/ijms19103147] [PMID] [PMCID]
Uchiyama, M., & Mihara, M. (1978). Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Analytical Biochemistry, 86(1), 271-8. [DOI:10.1016/0003-2697(78)90342-1]
Wang, W., Xu, J., Li, L., Wang, P., Ji, X., & Ai, H., et al. (2010). Neuroprotective effect of morroniside on focal cerebral ischemia in rats. Brain Research Bulletin, 83(5), 196-201. [DOI:10.1016/j.brainresbull.2010.07.003] [PMID]
Received: 2019/02/11 | Accepted: 2019/07/20 | Published: 2020/05/1
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |