Volume 12, Issue 2 (March & April 2021)
BCN 2021, 12(2): 233-242 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Yavari F, Oliazadeh P, Radfar M, Foroughipour M, Nikkhah K, Heidari Bakavoli A et al . Safety and Efficacy of Fingolimod in Iranian Patients with Relapsing-remitting Multiple Sclerosis. BCN 2021; 12 (2) :233-242
URL: http://bcn.iums.ac.ir/article-1-1412-en.html
URL: http://bcn.iums.ac.ir/article-1-1412-en.html
Fatemeh Yavari1 

 , Pardis Oliazadeh2
, Pardis Oliazadeh2 

 , Meisam Radfar3
, Meisam Radfar3 

 , Mohsen Foroughipour1
, Mohsen Foroughipour1 

 , Karim Nikkhah1
, Karim Nikkhah1 

 , Alireza Heidari Bakavoli4
, Alireza Heidari Bakavoli4 

 , Morteza Saeidi *
, Morteza Saeidi * 

 1
1


 , Pardis Oliazadeh2
, Pardis Oliazadeh2 

 , Meisam Radfar3
, Meisam Radfar3 

 , Mohsen Foroughipour1
, Mohsen Foroughipour1 

 , Karim Nikkhah1
, Karim Nikkhah1 

 , Alireza Heidari Bakavoli4
, Alireza Heidari Bakavoli4 

 , Morteza Saeidi *
, Morteza Saeidi * 

 1
1
1- Department of Neurology, Ghaem Hospital, Mashhad University of Medical Sciences, Mashhad, Iran.
2- School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
3- Department of Biotechnology and Plant Breeding, Gorgan University of Agricultural Sciences and Natural Recourses, Golestan, Iran.
4- Department of Cardiology, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
2- School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
3- Department of Biotechnology and Plant Breeding, Gorgan University of Agricultural Sciences and Natural Recourses, Golestan, Iran.
4- Department of Cardiology, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
Keywords: Multiple sclerosis, Fingolimod, Expanded Disability Status Scale (EDSS), Magnetic Resonance Imaging (MRI), Cardiovascular events, Therapy
Full-Text [PDF 791 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Multiple Sclerosis (MS) is a chronic disease that mainly affects young adults with a female predominance. It is characterized by inflammation, axonal loss, and demyelination in the Central Nervous System (CNS) white matter (Kamm, Uitdehaag, & Polman, 2014). Recent studies have also demonstrated the gray matter involvement in MS’s pathophysiology, resulting in irreversible disability (Lucchinetti et al., 2011; Geurts & Barkhof, 2008). Disease-Modifying Therapies (DMTs) mainly target acute inflammatory lesions of the nervous system, clinically known as relapse, and reduce the frequency and severity of attacks.
Interferon-beta (INF-β) preparations are the first-line therapy for MS in many countries (Wingerchuk & Carter, 2014). However, the injection route of administration and flu-like symptoms associated with interferons cause failure to drug adherence (Giovannoni, Southam, & Waubant, 2012). In recent years, a better understanding of the immunologic mechanisms involved in MS has offered wider therapeutic options (Mehling, Kappos, & Derfuss, 2011). Fingolimod is the first drug of a new class of immune-modulators that binds to Sphingosine 1-Phosphate Receptors (S1PRs), attenuating inflammatory damage through the reversible retention of auto-reactive lymphocytes in lymph nodes and the reduced lymphocyte infiltration into the CNS (Brinkmann et al., 2010; Chun & Hartung, 2010).
Fingolimod is the first oral DMT, which was approved by the Food and Drug Administration (FDA) in 2010 (US Food and Drug Administration, 2010) and the European Medicine Agency (EMA) in 2011 for the treatment of Relapsing-Remitting MS (RRMS) (0.5 mg daily) (European Medicines Agency Assessment Report, 2011). Clinical trials phase 2 and 3 have demonstrated that fingolimod therapy significantly reduces the Annualized Relapse Rate (ARR) and Magnetic Resonance Imaging (MRI) lesions compared to intramuscular interferon beta-1a or placebo in patients with MS (Cohen et al., 2010; Kappos et al., 2010). The results have also been sustained in further studies (Cohen et al., 2016; Kappos et al., 2015).
Epidemiological studies have revealed that the prevalence of MS is rapidly increasing among the Iranian population (Etemadifar et al., 2013). Because of the genetic and environmental differences between the Iranian people and other ethnicities in previous studies, fingolimod efficacy must be verified in Iran. Fingolimod is considered first-line therapy in the United States and as a second-line in European countries (Sorensen, 2014). According to the Iran Ministry of Health, Scientific Advisory Committee, it is also considered as a second-line treatment in the country. This clinical trial aimed to evaluate, for the first time, the efficacy, safety, and tolerability of fingolimod in patients with RRMS in the eastern part of Iran who did not respond to other therapies.
2. Methods
2.1. Patient selection
This clinical trial is a before-after study conducted at the university-based (tertiary) referral MS center at Ghaem Hospital, Mashhad University of Medical Sciences, Mashhad City, Iran. The sample size was calculated according to Alroughani, Ahmed, Behbehani, and Al-Hashel (2014) study (α=0.05, P=0.77, d=0.15p) based on the proportion of relapse-free patients. Patients eligible for the study were at the age of 18-50 years, diagnosed with RRMS according to the revised McDonald criteria (Polman et al., 2011). They either responded improperly to conventional interferon therapy or suffered from side effects. The key exclusion criteria were other chronic immune diseases, malignancies, varicella-zoster, Human T-Lymphotropic Virus 1 (HTLV1) or Tuberculosis (TB) infection, macular edema, clinically significant systemic disorders, and lymphopenia. Before initiation of the study, all the procedures were approved by the Ethics Committee of Mashhad University of Medical Sciences. Additionally, all the patients signed the written informed consent.
2.2. Study measures
The patients’ clinical features, prior treatments, and medical records for relapses within one year before the initiation of the trial were collected. The primary outcome of this trial was the proportion of relapse-free patients within the 12 months of fingolimod therapy. Relapse assessments were conducted during the study. A relapse was defined as a new neurological deficit that is not attributable to other etiologies rather than MS and lasts longer than 24 h. A certified neurologist assessed the patients’ disability progression through the Expanded Disability Status Scale (EDSS) at baseline and month 12. An MRI scan was also obtained from all the participants at baseline and month 12. Changes in EDSS scores and MRI lesions were regarded as the secondary endpoints.
Patients received 0.5 mg per day oral fingolimod purchased from the Iranian Osve pharmaceutical company, Iran. All the patients were monitored for cardiac arrhythmias within the first 24 h after the first dose administration of fingolimod at the hospital’s cardiology ward. Blood samples were obtained at months 1, 3, 6, and 12. The Complete Blood Count (CBC) and the liver profile were measured in these samples. All the laboratory measurements were done in the same place. The patients were examined for macular edema by an ophthalmologist at months 3, 6, and 12 of the study period. They were also routinely examined for possible side effects such as headache, lower respiratory tract infections, pharyngitis, and fatigue. The same neurologist did examinations for each patient.
2.3. Statistical analysis
To analyze our findings, we used the SPSS version 20. Considering the longitudinal evaluation of liver enzyme levels and lymphocyte count over the treatment period, their data were compared using repeated-measures analysis of variance. The study of the EDSS was done by comparing baseline scores with month 12 using the non-parametric Wilcoxon statistical hypothesis test due to lack of normality condition. Also, data related to the number of relapses and Gd-enhanced T1 and new/enlarged T2 lesions were assessed using the paired-sample t-test procedure. Differences were represented as statistically significant when P<0.05. All the data were expressed as Mean±SD.
3. Results
3.1. Baseline demographics
From the total of 50 patients with RRMS who participated in this clinical trial and received 0.5 mg of fingolimod per day, 37 people completed the study (more details in Figure 1).
.jpg)
Side effects were the main cause of discontinuation. Baseline demographic and clinical characteristics of the patients are shown in Table 1.
.jpg)
Briefly, 67.6% of the patients were women. The participants’ mean age and the disease mean duration were 33.68±5.12 and 8.38±4.02 years, respectively. All the patients were on different types of interferons before the study was started.
3.2. Clinical outcomes
Twenty-nine patients (78.4%) experienced no relapse during the 12-month treatment with fingolimod. The proportion of relapse-free patients markedly increased at the last follow-up (baseline vs. month 12, 5.4% vs. 78.4%). The mean number of relapses at baseline was 2.22±1.03. During the course of treatment with fingolimod, the mean number of relapses significantly decreased compared to the baseline (0.22±0.41, P<0.001), indicating that the fingolimod therapy can diminish the disease activity (Figure 2).
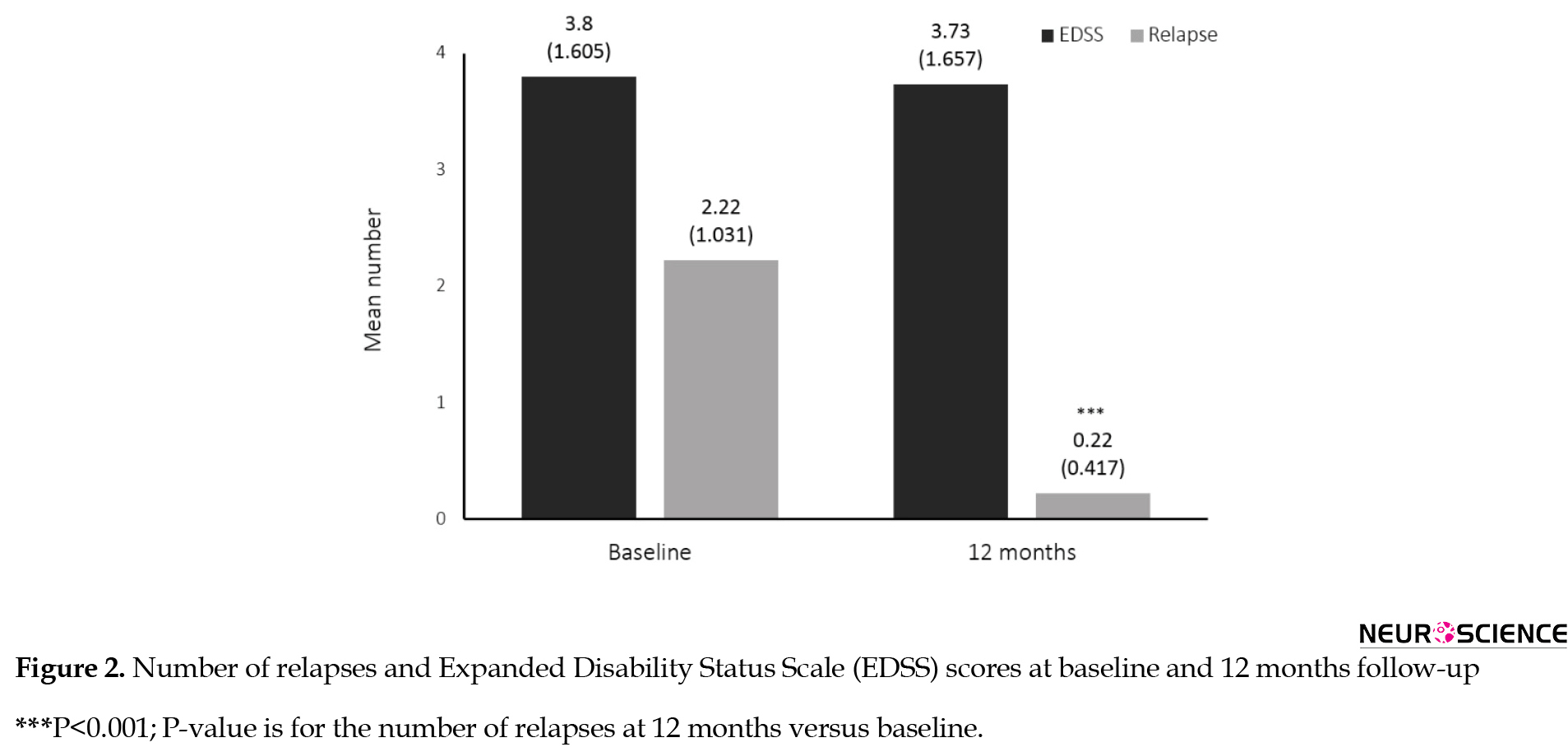
EDSS scores remained almost unchanged during the one year of the study (at baseline 3.80±1.60 and the end 3.73±1.65) (P=0.78) (Figure 2).
3.3. MRI results
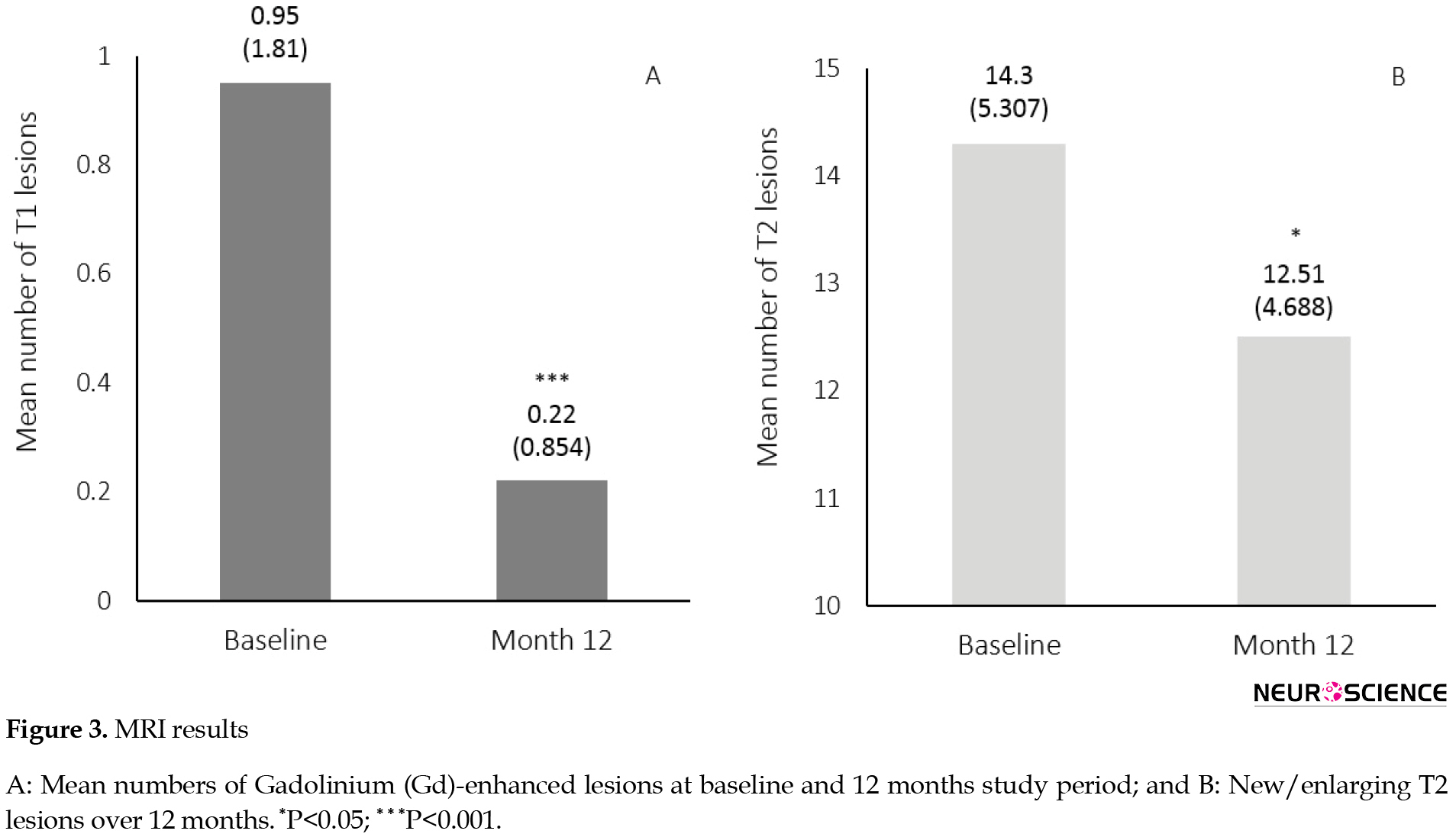
The proportion of patients free of Gd-enhanced lesions at month 12 was higher than the baseline (89% vs. 70%). Eighty-nine percent of the patients were free of new/enlarged T2 lesions over the 12 months of treatment. The patients had a reduced number of Gd-enhanced (T1) lesions at month 12 compared to the baseline (Mean±SD: 0.22±0.85 at month 12 and 0.95±1.81 at baseline, P=0.03) as well as reduced new/enlarged T2 lesions after 12 months (Mean±SD: 12.51±4.68 at month 12 and 14.30±5.30 at baseline, P<0.001) (Figure 3).
3.4. Safety and tolerability
As mentioned above, out of the 50 patients who enrolled in the study, 37 people continued the treatment for 12 months. The most frequent adverse event leading to study discontinuation was nausea, vomiting, and other gastrointestinal side effects (8%). Three patients experienced progressive clinical deterioration for several months despite fingolimod therapy. Following the further examinations, their disease was found to be progressive rather than relapsing-remitting. Early attacks led to study discontinuation in two patients. Severe lymphopenia was observed in two other patients leading to systemic infection and hospital admission in one person. In the liver function evaluations, one patient was found to have more than five times the normal level of Serum Glutamic Oxaloacetic Transaminase (SGOT) and Serum Glutamic Pyruvic Transaminase (SGPT). These two enzymes did not return to the normal range after the cessation of fingolimod. Skin rashes, which led to study discontinuation, was observed in one patient.
No arrhythmia, bradycardia, and other cardiac adverse events were recorded during the 24 h of cardiac monitoring in the hospital. No confirmed macular edema was reported among the patients during the study period. Neither minor nor major infection was reported in the patients who completed the 12-month course of the treatment. Only one serious event was observed in the participants (systemic infection), which led to study discontinuation. No serious event was reported among the patients who completed the treatment course.
Among the 37 patients who continued the study, the mean increase in the level of aminotransferases was observed at month 1 (Mean±SD for SGOT: 23.19±8.49 at baseline vs. 38.59±40.51 at month 1, P=0.003; for SGPT: 23.62±12.13 at baseline vs 36.27±36.17 at month 1) and reached a maximum level at month 3 for both SGOT and SGPT following the drug administration. Only in one patient was there an increase in aminotransferase levels at months 1 and 3, three times the normal level. Performing further analysis at months 6 and 12, the serum level of aminotransferases in almost all the patients returned to the normal values. Only in two patients (5%) at month 12, the levels were still high, about three times the normal range (Figure 4).
Multiple Sclerosis (MS) is a chronic disease that mainly affects young adults with a female predominance. It is characterized by inflammation, axonal loss, and demyelination in the Central Nervous System (CNS) white matter (Kamm, Uitdehaag, & Polman, 2014). Recent studies have also demonstrated the gray matter involvement in MS’s pathophysiology, resulting in irreversible disability (Lucchinetti et al., 2011; Geurts & Barkhof, 2008). Disease-Modifying Therapies (DMTs) mainly target acute inflammatory lesions of the nervous system, clinically known as relapse, and reduce the frequency and severity of attacks.
Interferon-beta (INF-β) preparations are the first-line therapy for MS in many countries (Wingerchuk & Carter, 2014). However, the injection route of administration and flu-like symptoms associated with interferons cause failure to drug adherence (Giovannoni, Southam, & Waubant, 2012). In recent years, a better understanding of the immunologic mechanisms involved in MS has offered wider therapeutic options (Mehling, Kappos, & Derfuss, 2011). Fingolimod is the first drug of a new class of immune-modulators that binds to Sphingosine 1-Phosphate Receptors (S1PRs), attenuating inflammatory damage through the reversible retention of auto-reactive lymphocytes in lymph nodes and the reduced lymphocyte infiltration into the CNS (Brinkmann et al., 2010; Chun & Hartung, 2010).
Fingolimod is the first oral DMT, which was approved by the Food and Drug Administration (FDA) in 2010 (US Food and Drug Administration, 2010) and the European Medicine Agency (EMA) in 2011 for the treatment of Relapsing-Remitting MS (RRMS) (0.5 mg daily) (European Medicines Agency Assessment Report, 2011). Clinical trials phase 2 and 3 have demonstrated that fingolimod therapy significantly reduces the Annualized Relapse Rate (ARR) and Magnetic Resonance Imaging (MRI) lesions compared to intramuscular interferon beta-1a or placebo in patients with MS (Cohen et al., 2010; Kappos et al., 2010). The results have also been sustained in further studies (Cohen et al., 2016; Kappos et al., 2015).
Epidemiological studies have revealed that the prevalence of MS is rapidly increasing among the Iranian population (Etemadifar et al., 2013). Because of the genetic and environmental differences between the Iranian people and other ethnicities in previous studies, fingolimod efficacy must be verified in Iran. Fingolimod is considered first-line therapy in the United States and as a second-line in European countries (Sorensen, 2014). According to the Iran Ministry of Health, Scientific Advisory Committee, it is also considered as a second-line treatment in the country. This clinical trial aimed to evaluate, for the first time, the efficacy, safety, and tolerability of fingolimod in patients with RRMS in the eastern part of Iran who did not respond to other therapies.
2. Methods
2.1. Patient selection
This clinical trial is a before-after study conducted at the university-based (tertiary) referral MS center at Ghaem Hospital, Mashhad University of Medical Sciences, Mashhad City, Iran. The sample size was calculated according to Alroughani, Ahmed, Behbehani, and Al-Hashel (2014) study (α=0.05, P=0.77, d=0.15p) based on the proportion of relapse-free patients. Patients eligible for the study were at the age of 18-50 years, diagnosed with RRMS according to the revised McDonald criteria (Polman et al., 2011). They either responded improperly to conventional interferon therapy or suffered from side effects. The key exclusion criteria were other chronic immune diseases, malignancies, varicella-zoster, Human T-Lymphotropic Virus 1 (HTLV1) or Tuberculosis (TB) infection, macular edema, clinically significant systemic disorders, and lymphopenia. Before initiation of the study, all the procedures were approved by the Ethics Committee of Mashhad University of Medical Sciences. Additionally, all the patients signed the written informed consent.
2.2. Study measures
The patients’ clinical features, prior treatments, and medical records for relapses within one year before the initiation of the trial were collected. The primary outcome of this trial was the proportion of relapse-free patients within the 12 months of fingolimod therapy. Relapse assessments were conducted during the study. A relapse was defined as a new neurological deficit that is not attributable to other etiologies rather than MS and lasts longer than 24 h. A certified neurologist assessed the patients’ disability progression through the Expanded Disability Status Scale (EDSS) at baseline and month 12. An MRI scan was also obtained from all the participants at baseline and month 12. Changes in EDSS scores and MRI lesions were regarded as the secondary endpoints.
Patients received 0.5 mg per day oral fingolimod purchased from the Iranian Osve pharmaceutical company, Iran. All the patients were monitored for cardiac arrhythmias within the first 24 h after the first dose administration of fingolimod at the hospital’s cardiology ward. Blood samples were obtained at months 1, 3, 6, and 12. The Complete Blood Count (CBC) and the liver profile were measured in these samples. All the laboratory measurements were done in the same place. The patients were examined for macular edema by an ophthalmologist at months 3, 6, and 12 of the study period. They were also routinely examined for possible side effects such as headache, lower respiratory tract infections, pharyngitis, and fatigue. The same neurologist did examinations for each patient.
2.3. Statistical analysis
To analyze our findings, we used the SPSS version 20. Considering the longitudinal evaluation of liver enzyme levels and lymphocyte count over the treatment period, their data were compared using repeated-measures analysis of variance. The study of the EDSS was done by comparing baseline scores with month 12 using the non-parametric Wilcoxon statistical hypothesis test due to lack of normality condition. Also, data related to the number of relapses and Gd-enhanced T1 and new/enlarged T2 lesions were assessed using the paired-sample t-test procedure. Differences were represented as statistically significant when P<0.05. All the data were expressed as Mean±SD.
3. Results
3.1. Baseline demographics
From the total of 50 patients with RRMS who participated in this clinical trial and received 0.5 mg of fingolimod per day, 37 people completed the study (more details in Figure 1).
.jpg)
Side effects were the main cause of discontinuation. Baseline demographic and clinical characteristics of the patients are shown in Table 1.
.jpg)
Briefly, 67.6% of the patients were women. The participants’ mean age and the disease mean duration were 33.68±5.12 and 8.38±4.02 years, respectively. All the patients were on different types of interferons before the study was started.
3.2. Clinical outcomes
Twenty-nine patients (78.4%) experienced no relapse during the 12-month treatment with fingolimod. The proportion of relapse-free patients markedly increased at the last follow-up (baseline vs. month 12, 5.4% vs. 78.4%). The mean number of relapses at baseline was 2.22±1.03. During the course of treatment with fingolimod, the mean number of relapses significantly decreased compared to the baseline (0.22±0.41, P<0.001), indicating that the fingolimod therapy can diminish the disease activity (Figure 2).

EDSS scores remained almost unchanged during the one year of the study (at baseline 3.80±1.60 and the end 3.73±1.65) (P=0.78) (Figure 2).
3.3. MRI results
The proportion of patients free of Gd-enhanced lesions at month 12 was higher than the baseline (89% vs. 70%). Eighty-nine percent of the patients were free of new/enlarged T2 lesions over the 12 months of treatment. The patients had a reduced number of Gd-enhanced (T1) lesions at month 12 compared to the baseline (Mean±SD: 0.22±0.85 at month 12 and 0.95±1.81 at baseline, P=0.03) as well as reduced new/enlarged T2 lesions after 12 months (Mean±SD: 12.51±4.68 at month 12 and 14.30±5.30 at baseline, P<0.001) (Figure 3).

3.4. Safety and tolerability
As mentioned above, out of the 50 patients who enrolled in the study, 37 people continued the treatment for 12 months. The most frequent adverse event leading to study discontinuation was nausea, vomiting, and other gastrointestinal side effects (8%). Three patients experienced progressive clinical deterioration for several months despite fingolimod therapy. Following the further examinations, their disease was found to be progressive rather than relapsing-remitting. Early attacks led to study discontinuation in two patients. Severe lymphopenia was observed in two other patients leading to systemic infection and hospital admission in one person. In the liver function evaluations, one patient was found to have more than five times the normal level of Serum Glutamic Oxaloacetic Transaminase (SGOT) and Serum Glutamic Pyruvic Transaminase (SGPT). These two enzymes did not return to the normal range after the cessation of fingolimod. Skin rashes, which led to study discontinuation, was observed in one patient.
No arrhythmia, bradycardia, and other cardiac adverse events were recorded during the 24 h of cardiac monitoring in the hospital. No confirmed macular edema was reported among the patients during the study period. Neither minor nor major infection was reported in the patients who completed the 12-month course of the treatment. Only one serious event was observed in the participants (systemic infection), which led to study discontinuation. No serious event was reported among the patients who completed the treatment course.
Among the 37 patients who continued the study, the mean increase in the level of aminotransferases was observed at month 1 (Mean±SD for SGOT: 23.19±8.49 at baseline vs. 38.59±40.51 at month 1, P=0.003; for SGPT: 23.62±12.13 at baseline vs 36.27±36.17 at month 1) and reached a maximum level at month 3 for both SGOT and SGPT following the drug administration. Only in one patient was there an increase in aminotransferase levels at months 1 and 3, three times the normal level. Performing further analysis at months 6 and 12, the serum level of aminotransferases in almost all the patients returned to the normal values. Only in two patients (5%) at month 12, the levels were still high, about three times the normal range (Figure 4).
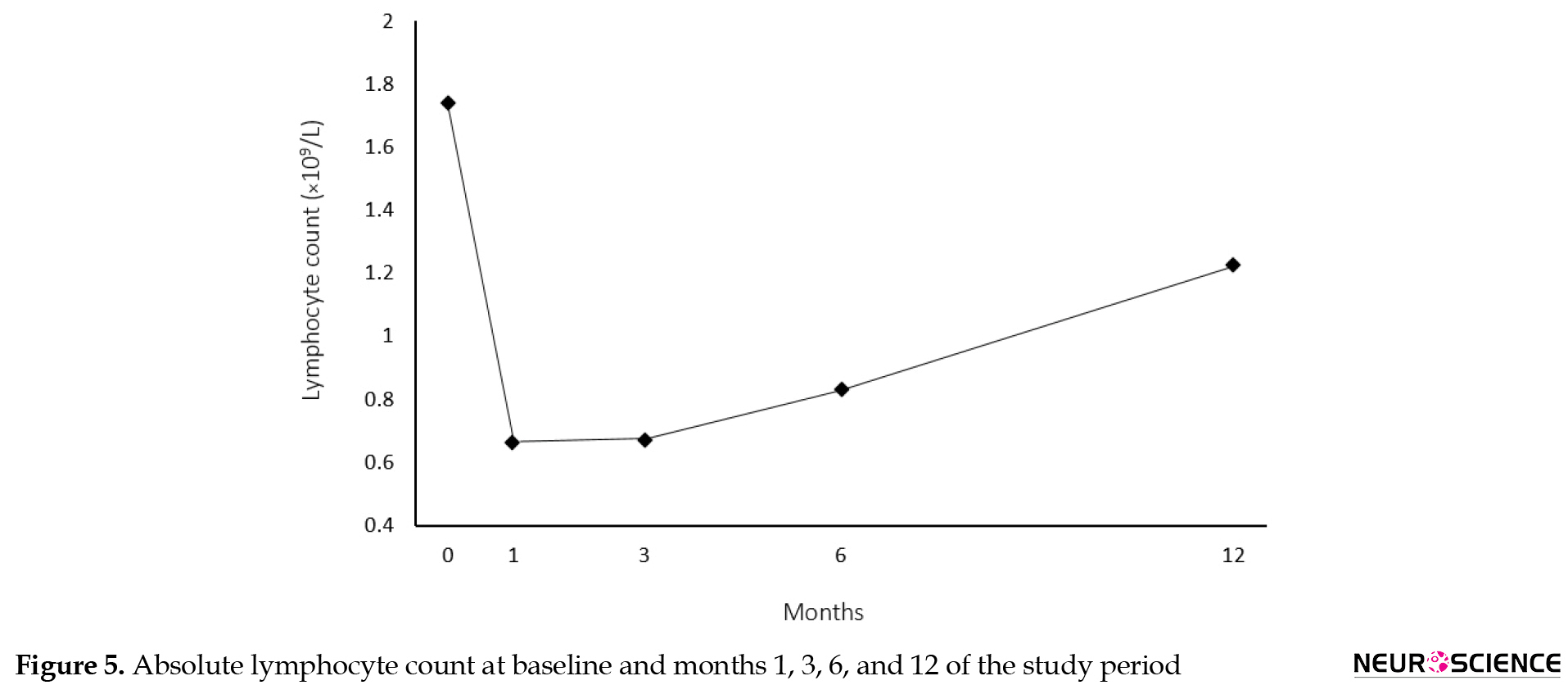
Absolute lymphocyte count reduced by 61% at month 1 (Mean±SD for lymphocyte count: 1740.19±724.11 cells/µL at baseline vs. 666.43±303.98 at month 1, P<0.001), but further assessments at months 3, 6, and 12 showed a gradual increase in the lymphocyte count but not up to the baseline levels (Mean±SD for lymphocyte count: 1226.08±1325.28 cells/µL at month 12) (Figure 5).
4. Discussion
The present investigation demonstrated that fingolimod therapy, the mean number of relapses reduced considerably after 12 months of treatment in the Iranian patients with RRMS who were previously on different types of interferons (P<0.001). Although there was a significant increase (73%) in the proportion of relapse-free patients over the treatment period, changes in EDSS score were not significant.
Fingolimod-received patients had significantly reduced Gd-enhanced (T1) and new/enlarged T2 lesions at the end of the treatment period compared to the baseline. Eighty-one percent of the patients were free from any new MRI activity (Gd-enhanced lesions and new/enlarged T2 lesions). The effects of fingolimod on MRI lesions and relapses were consistent with the previous studies (Cohen et al., 2015, 2010; Kappos et al., 2015, 2010). In the phase III FREEDOMS study, the clinical outcome and MRI activity were compared after 24 months among three groups (fingolimod [FTY] 0.5 mg, FTY 1.25 mg, and placebo). Annualized Relapse Rate (ARR), MRI lesions, and EDSS scores in both fingolimod-treated groups were significantly lower than placebo (Kappos et al., 2010). Devonshire et al. performed a subgroup analysis of the FREEDOMS study and assessed ARR and disability progression. The subgroups were selected based on demographics, treatment history, baseline disease characteristics, or disease activity in treatment-naive patients and previously treated patients. This study indicated that fingolimod significantly improved the relapse rate and disability progression in patients with different clinical characteristics and MRI features (Devonshire et al., 2012).
In the TRANSFORMS core study, the patients were randomly assigned to receive FTY 0.5 mg, FTY 1.25 mg, and the injectable interferon-beta 1-alpha (INFB-1a) for 12 months. There was a dramatic reduction in the ARR of the patients treated with fingolimod compared to INFB-1a. MRI activity lesions accompanied ARR, but there was no significant change in EDSS score among the groups (Cohen et al., 2010). The results of this study were similar to ours. In the extension phase of the TRANSFORMS study, the patients who previously received fingolimod continued their treatment, but those who were on INFB-1a were randomly allocated to receive FTY 0.5 or 1.25 mg. The within-group analysis demonstrated that ARR and MRI lesion activity in switched groups were significantly improved in months 13-24 compared to months 0-12. The continuous fingolimod treatment for 24 months showed persistent benefits in terms of ARR and MRI outcomes. On the other hand, the results of the between-group analysis revealed that the patients who were on fingolimod therapy for 24 months had lower relapses and MRI lesions relative to the patients who received INFB-1a during the first year and switched to fingolimod for the second year (Cohen et al., 2016). However, there was no effect on the disability progression, and EDSS scores did not change significantly.
The results of the TRANSFORMS extension phase study suggested that despite receiving other DMTs, delay in the start of fingolimod therapy could result in higher relapses and MRI lesion activities. Furthermore, according to an extension data analysis performed on the TRANSFORMS study, the early initiation of fingolimod therapy resulted in lower cost per relapse (Agashivala & Kim, 2012). A subgroup analysis of FREEDOMS and TRANSFORMS studies displayed that relapses and MRI activities diminished dramatically in the patients who experienced their first symptoms less than 3 years before the initiation of the study compared to those who experienced them more than 3 years (Agius, Meng, Chin, Grinspan, & Hashmonay, 2014). It shows that fingolimod could be more effective in patients with lower disease duration. Other investigations with smaller sample sizes have been consistent with our study and trials in Japan, Kuwait, and Italy (Alroughani et al., 2014; Saida et al., 2012). Also, our findings are consistent with the results of another Iranian study that investigated the efficacy of fingolimod in western Iran and demonstrated an 85% reduction in the ARR compared to baseline as well as a significant decline in EDSS (Ouspid et al., 2018).
Although fingolimod was well-tolerated in the previous studies (Cohen et al., 2016, 2010; Kappos et al., 2015, 2010), the number of patients who discontinued the therapy because of the adverse effects were higher in our study. After the exclusion of three people with symptoms, which were more similar to the progressive nature of the disease, 21% of the remaining 47 patients (n=10) suffered from different side effects and discontinued the study. However, the side effects were mild, and only one serious adverse effect (severe infection) occurred. There was no serious adverse event among the patients who completed the study for 12 months. In the FREEDOMS core study, 7.5% of the patients receiving fingolimod (0.5 mg daily) discontinued the treatment due to the adverse effects (Cohen et al., 2010). It has to be mentioned that not all the side effects observed in the excluded patients are necessarily attributed to the prescription of fingolimod. In other words, for some of these effects, which were not adverse, there was no logical explanation to justify leaving the study. However, they were let to feel free about their participation in the study. On the other hand, as a general consideration for immunomodulatory diseases like MS, possible side effects in different body organs, which can be due to the interaction of modulatory factors with the administrated drug, are not unexpected.
The effects of fingolimod are modulated through binding to Sphingosine-1-Phosphate (S1P) receptors. These receptors are also found in the cardiovascular system. Regarding the similarity between S1P and M2 receptor activation by the vagus nerve, it is predictable that the initiation of fingolimod therapy would decrease heart rate and blood pressure. In line with this, it was revealed that the first administration of fingolimod led to a transient decrease in heart rate (Simula et al., 2016). Although not very common, there are some reports of atrioventricular blocks among FTY-treated patients (Saida et al., 2012). In this study, no cardiovascular event such as bradycardia or other arrhythmias was reported, neither during the 24 h of cardiac monitoring in the hospital after the first administration of fingolimod nor over the 12-month follow-up. The incidence of bradycardia after the first administration of fingolimod varies in different studies. Although predispositions are not well understood, one study demonstrated that the baseline autonomic regulation of the heart could be regarded as a predictive factor for the magnitude of heart rate decrease (Simula et al., 2016). Despite having no cardiac side effects in our study, the cardiac safety of fingolimod in clinical practices is still a concern, especially because of the lack of long-term records.
As expected from the fingolimod mechanism of action, lymphocyte count reduced by 61% at month 1, similar to the previous studies (Cohen et al., 2016, 2010; Kappos et al., 2015, 2010). Further evaluations of follow-up sampling showed different results from the previously reported ones. FREEDOMS and TRANSFORMS core and extension studies and other investigations among Asian populations have revealed that the low lymphocyte count remains unchanged during the course of the study. Although the absolute lymphocyte count increased at months 6 and 12 in our experiment, it did not reach the normal level. The low incidence of infections in this trial (n=1) could be attributed to the increase in lymphocyte count during the treatment period. Unlike the previously reported data, no respiratory, urinary tract, or herpes virus infections were reported in this study (Cohen et al., 2016, 2010; Kappos et al., 2015, 2010). However, alteration in aminotransferases was similar to the previous studies.
There is no cure for MS, and as a chronic disease, long-term treatments are crucial for many patients. The effectiveness of therapy strongly depends on medication adherence. Investigations on tolerability and adherence to conventional DMTs for MS indicated that the discontinuation of treatment ranged from 17% to 36% due to the diverse types of injectable treatments (Hansen et al., 2015). Injection site reactions and flu-like symptoms were two of the most common reasons for discontinuation. Comparing fingolimod with other DMts, we can conclude that the persistence and adherence to oral fingolimod were higher than other injectable medications (Giovannoni et al., 2012). Wilson et al. evaluated the MS patients’ preference for different types of medications. They displayed that the patients preferred oral medicines to all injectable ones (Wilson et al., 2015). According to a retrospective cohort conducted using pharmacy data to describe the adherence to MS DMTs (interferon beta-1a IM [Avonex], interferon beta-1a SC [Rebif], interferon beta-1b SC [Betaferon], and glatiramer acetate SC [Copaxone]), it was revealed that only 30%-40% of the patients were adherent to the drugs after two years (Hansen et al., 2015).
One significant limitation in our study was the number of patients who did not complete the follow-up period for the reasons mentioned above. Most of the side effects were mild; however, those patients felt disappointed, perhaps because they were selected among those who had not responded to interferon/other therapies or suffered from different side effects before the study initiation. As told before, this study aimed to evaluate if any significant ameliorating effect could be demonstrated in RRMS patients who turned to fingolimod therapy after unsuccessful treatment with other medicines.
As the finishing statement, this 12-month study revealed, for the first time, the safety and efficacy of fingolimod therapy in Iranian patients with RRMS. Also, it was demonstrated that the patients preferred fingolimod to other available treatments. Concerning the reduced MRI lesions and diminished relapses observed in this study, fingolimod is preferred to other standard therapies and suggested as the first-line medication for the treatment of RRMS in Iran.
Ethical Considerations
Compliance with ethical guidelines
All procedures performed in this study were following the ethical standards of the Institutional Research Committee and with the 1964 Helsinki declaration and its comparable ethical standards.
Funding
This study was supported by the Research Vice-Presidency and the Referral MS Center of Mashhad University of Medical Sciences.
Authors' contributions
Conceptualization, methodology, investigation, final review, resources: All authors; Writing the original draft: Pardis Oliazadeh; Writing, review, and editing: Pardis Oliazadeh, Fatemeh Yavari; Funding acquisition: Morteza Saeidi, Mohsen Foroughipour; Supervision: Morteza Saeidi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank all the staff of Ghaem Hospital who helped us to conduct this investigation.
References
Agashivala, N., & Kim, E. (2012). Cost-effectiveness of early initiation of fingolimod versus delayed initiation after 1 year of intramuscular interferon beta-1a in patients with multiple sclerosis. Clinical Therapeutics, 34(7), 1583-90. [DOI:10.1016/j.clinthera.2012.06.012] [PMID]
Agius, M., Meng, X., Chin, P., Grinspan, A., & Hashmonay, R. (2014). Fingolimod therapy in early multiple sclerosis: an efficacy analysis of the TRANSFORMS and FREEDOMS studies by time since first symptom. CNS Neuroscience & Therapeutics, 20(5), 446-51. [DOI:10.1111/cns.12235] [PMID] [PMCID]
Alroughani, R., Ahmed, S. F., Behbehani, R., & Al-Hashel, J. (2014). Use of fingolimod in patients with relapsing remitting multiple sclerosis in Kuwait. Clinical Neurology and Neurosurgery, 119, 17-20. [DOI:10.1016/j.clineuro.2014.01.007] [PMID]
Brinkmann, V., Billich, A., Baumruker, T., Heining, P., Schmouder, R., & Francis, G., et al. (2010). Fingolimod (FTY720): Discovery and development of an oral drug to treat multiple sclerosis. Nature Reviews Drug Discovery, 9(11), 883-97. [DOI:10.1038/nrd3248] [PMID]
Chun, J., & Hartung, H. P. (2010). Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clinical Neuropharmacology, 33(2), 91. [DOI:10.1097/WNF.0b013e3181cbf825] [PMID] [PMCID]
Cohen, J. A., Barkhof, F., Comi, G., Hartung, H. P., Khatri, B. O., & Montalban, X., et al. (2010). Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. New England Journal of Medicine, 362(5), 402-15. [DOI:10.1056/NEJMoa0907839] [PMID]
Cohen, J. A., Khatri, B., Barkhof, F., Comi, G., Hartung, H. P., & Montalban, X., et al. (2016). Long-term (up to 4.5 years) treatment with fingolimod in multiple sclerosis: results from the extension of the randomised TRANSFORMS study. Journal of Neurology, Neurosurgery & Psychiatry, 87(5), 468-75. [DOI:10.1136/jnnp-2015-310597] [PMID] [PMCID]
Devonshire, V., Havrdova, E., Radue, E. W., O'Connor, P., Zhang-Auberson, L., & Agoropoulou, C., & FREEDOMS Study Group. (2012). Relapse and disability outcomes in patients with multiple sclerosis treated with fingolimod: subgroup analyses of the double-blind, randomised, placebo-controlled FREEDOMS study. The Lancet Neurology, 11(5), 420-8. [DOI:10.1016/S1474-4422(12)70056-X]
Etemadifar, M., Sajjadi, S., Nasr, Z., Firoozeei, T. S., Abtahi, S. H., & Akbari, M., et al. (2013). Epidemiology of multiple sclerosis in Iran: A systematic review. European neurology, 70(5-6), 356-63. [DOI:10.1159/000355140] [PMID]
European Medicines Agency. (2011). Assessment report: Gilenya. Doc Ref: EMA/108602/2011. Retrieved from http://www.ema.europa.eu/docs/ en_GB/document_library/EPAR_-_Public_assessment_report/human/ 002202/WC500104529.pdf.
Geurts, J. J., & Barkhof, F. (2008). Grey matter pathology in multiple sclerosis. The Lancet Neurology, 7(9), 841-51. [DOI:10.1016/S1474-4422(08)70191-1]
Giovannoni, G., Southam, E., & Waubant, E. (2012). Systematic review of disease-modifying therapies to assess unmet needs in multiple sclerosis: Tolerability and adherence. Multiple Sclerosis Journal, 18(7), 932-46. [DOI:10.1177/1352458511433302] [PMID]
Hansen, K., Schüssel, K., Kieble, M., Werning, J., Schulz, M., & Friis, R., et al. (2015). Adherence to disease modifying drugs among patients with multiple sclerosis in Germany: A retrospective cohort study. PloS One, 10(7), e0133279. [DOI:10.1371/journal.pone.0133279] [PMID] [PMCID]
. Kamm, C. P., Uitdehaag, B. M., & Polman, C. H. (2014). Multiple sclerosis: current knowledge and future outlook. European Neurology, 72(3-4), 132-41. [DOI:10.1159/000360528] [PMID]
Kappos, L., Radue, E. W., O'Connor, P., Polman, C., Hohlfeld, R., & Calabresi, P., et al. (2010). A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. New England Journal of Medicine, 362(5), 387-401. [DOI:10.1056/NEJMoa0909494] [PMID]
Kappos, L., O'Connor, P., Radue, E. W., Polman, C., Hohlfeld, R., & Selmaj, K., et al. (2015). Long-term effects of fingolimod in multiple sclerosis: The randomized FREEDOMS extension trial. Neurology, 84(15), 1582-91. [DOI:10.1212/WNL.0000000000001462] [PMID] [PMCID]
Lucchinetti, C. F., Popescu, B. F., Bunyan, R. F., Moll, N. M., Roemer, S. F., & Lassmann, H., et al. (2011). Inflammatory cortical demyelination in early multiple sclerosis. New England Journal of Medicine, 365(23), 2188-97. [DOI:10.1056/NEJMoa1100648] [PMID] [PMCID]
Mehling, M., Kappos, L., & Derfuss, T. (2011). Fingolimod for multiple sclerosis: Mechanism of action, clinical outcomes, and future directions. Current Neurology and Neuroscience Reports, 11(5), 492-7. [DOI:10.1007/s11910-011-0216-9] [PMID]
Ouspid, E., Razazian, N., Moghadasi, A. N., Moradian, N., Afshari, D., & Bostani, A., et al. (2018). Clinical effectiveness and safety of fingolimod in relapsing remitting multiple sclerosis in Western Iran. Neurosciences (Riyadh), 23(2), 129-134. [DOI:10.17712/nsj.2018.2.20170434] [PMID]
Polman, C. H., Reingold, S. C., Banwell, B., Clanet, M., Cohen, J. A., & Filippi, M., et al. (2011). Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Annals of Neurology, 69(2), 292-302. [DOI:10.1002/ana.22366] [PMID] [PMCID]
Saida, T., Kikuchi, S., Itoyama, Y., Hao, Q., Kurosawa, T., & Nagato, K., et al. (2012). A randomized, controlled trial of fingolimod (FTY720) in Japanese patients with multiple sclerosis. Multiple Sclerosis Journal, 18(9), 1269-277. [DOI:10.1177/1352458511435984] [PMID]
Simula, S., Laitinen, T., Laitinen, T. M., Tarkiainen, T., Hartikainen, P., & Hartikainen, J. E. (2016). Effect of fingolimod on cardiac autonomic regulation in patients with multiple sclerosis. Multiple Sclerosis Journal, 22(8), 1080-5. [DOI:10.1177/1352458515604384] [PMID]
Sorensen, P. S. (2014). New management algorithms in multiple sclerosis. Current Opinion in Neurology, 27(3), 246-59. [DOI:10.1097/WCO.0000000000000096] [PMID]
US Food and Drug Administration (FDA). (2010). Approves first oral drug to reduce MS relapses. Retreived from http://www.fda.gov/NewsEvents/ Newsroom/PressAnnouncements/ucm226755.htm
Wilson, L. S., Loucks, A., Gipson, G., Zhong, L., Bui, C., & Miller, E., et al. (2015). Patient preferences for attributes of multiple sclerosis disease-modifying therapies: Development and results of a ratings-based conjoint analysis. International Journal of MS Care, 17(2), 74-82. [DOI:10.7224/1537-2073.2013-053] [PMID] [PMCID]
Wingerchuk, D. M., & Carter, J. L. (2014). Multiple sclerosis: Current and emerging disease-modifying therapies and treatment strategies. Mayo Clinic Proceedings, 89(2), 225-40. [DOI:10.1016/j.mayocp.2013.11.002] [PMID]

4. Discussion
The present investigation demonstrated that fingolimod therapy, the mean number of relapses reduced considerably after 12 months of treatment in the Iranian patients with RRMS who were previously on different types of interferons (P<0.001). Although there was a significant increase (73%) in the proportion of relapse-free patients over the treatment period, changes in EDSS score were not significant.
Fingolimod-received patients had significantly reduced Gd-enhanced (T1) and new/enlarged T2 lesions at the end of the treatment period compared to the baseline. Eighty-one percent of the patients were free from any new MRI activity (Gd-enhanced lesions and new/enlarged T2 lesions). The effects of fingolimod on MRI lesions and relapses were consistent with the previous studies (Cohen et al., 2015, 2010; Kappos et al., 2015, 2010). In the phase III FREEDOMS study, the clinical outcome and MRI activity were compared after 24 months among three groups (fingolimod [FTY] 0.5 mg, FTY 1.25 mg, and placebo). Annualized Relapse Rate (ARR), MRI lesions, and EDSS scores in both fingolimod-treated groups were significantly lower than placebo (Kappos et al., 2010). Devonshire et al. performed a subgroup analysis of the FREEDOMS study and assessed ARR and disability progression. The subgroups were selected based on demographics, treatment history, baseline disease characteristics, or disease activity in treatment-naive patients and previously treated patients. This study indicated that fingolimod significantly improved the relapse rate and disability progression in patients with different clinical characteristics and MRI features (Devonshire et al., 2012).
In the TRANSFORMS core study, the patients were randomly assigned to receive FTY 0.5 mg, FTY 1.25 mg, and the injectable interferon-beta 1-alpha (INFB-1a) for 12 months. There was a dramatic reduction in the ARR of the patients treated with fingolimod compared to INFB-1a. MRI activity lesions accompanied ARR, but there was no significant change in EDSS score among the groups (Cohen et al., 2010). The results of this study were similar to ours. In the extension phase of the TRANSFORMS study, the patients who previously received fingolimod continued their treatment, but those who were on INFB-1a were randomly allocated to receive FTY 0.5 or 1.25 mg. The within-group analysis demonstrated that ARR and MRI lesion activity in switched groups were significantly improved in months 13-24 compared to months 0-12. The continuous fingolimod treatment for 24 months showed persistent benefits in terms of ARR and MRI outcomes. On the other hand, the results of the between-group analysis revealed that the patients who were on fingolimod therapy for 24 months had lower relapses and MRI lesions relative to the patients who received INFB-1a during the first year and switched to fingolimod for the second year (Cohen et al., 2016). However, there was no effect on the disability progression, and EDSS scores did not change significantly.
The results of the TRANSFORMS extension phase study suggested that despite receiving other DMTs, delay in the start of fingolimod therapy could result in higher relapses and MRI lesion activities. Furthermore, according to an extension data analysis performed on the TRANSFORMS study, the early initiation of fingolimod therapy resulted in lower cost per relapse (Agashivala & Kim, 2012). A subgroup analysis of FREEDOMS and TRANSFORMS studies displayed that relapses and MRI activities diminished dramatically in the patients who experienced their first symptoms less than 3 years before the initiation of the study compared to those who experienced them more than 3 years (Agius, Meng, Chin, Grinspan, & Hashmonay, 2014). It shows that fingolimod could be more effective in patients with lower disease duration. Other investigations with smaller sample sizes have been consistent with our study and trials in Japan, Kuwait, and Italy (Alroughani et al., 2014; Saida et al., 2012). Also, our findings are consistent with the results of another Iranian study that investigated the efficacy of fingolimod in western Iran and demonstrated an 85% reduction in the ARR compared to baseline as well as a significant decline in EDSS (Ouspid et al., 2018).
Although fingolimod was well-tolerated in the previous studies (Cohen et al., 2016, 2010; Kappos et al., 2015, 2010), the number of patients who discontinued the therapy because of the adverse effects were higher in our study. After the exclusion of three people with symptoms, which were more similar to the progressive nature of the disease, 21% of the remaining 47 patients (n=10) suffered from different side effects and discontinued the study. However, the side effects were mild, and only one serious adverse effect (severe infection) occurred. There was no serious adverse event among the patients who completed the study for 12 months. In the FREEDOMS core study, 7.5% of the patients receiving fingolimod (0.5 mg daily) discontinued the treatment due to the adverse effects (Cohen et al., 2010). It has to be mentioned that not all the side effects observed in the excluded patients are necessarily attributed to the prescription of fingolimod. In other words, for some of these effects, which were not adverse, there was no logical explanation to justify leaving the study. However, they were let to feel free about their participation in the study. On the other hand, as a general consideration for immunomodulatory diseases like MS, possible side effects in different body organs, which can be due to the interaction of modulatory factors with the administrated drug, are not unexpected.
The effects of fingolimod are modulated through binding to Sphingosine-1-Phosphate (S1P) receptors. These receptors are also found in the cardiovascular system. Regarding the similarity between S1P and M2 receptor activation by the vagus nerve, it is predictable that the initiation of fingolimod therapy would decrease heart rate and blood pressure. In line with this, it was revealed that the first administration of fingolimod led to a transient decrease in heart rate (Simula et al., 2016). Although not very common, there are some reports of atrioventricular blocks among FTY-treated patients (Saida et al., 2012). In this study, no cardiovascular event such as bradycardia or other arrhythmias was reported, neither during the 24 h of cardiac monitoring in the hospital after the first administration of fingolimod nor over the 12-month follow-up. The incidence of bradycardia after the first administration of fingolimod varies in different studies. Although predispositions are not well understood, one study demonstrated that the baseline autonomic regulation of the heart could be regarded as a predictive factor for the magnitude of heart rate decrease (Simula et al., 2016). Despite having no cardiac side effects in our study, the cardiac safety of fingolimod in clinical practices is still a concern, especially because of the lack of long-term records.
As expected from the fingolimod mechanism of action, lymphocyte count reduced by 61% at month 1, similar to the previous studies (Cohen et al., 2016, 2010; Kappos et al., 2015, 2010). Further evaluations of follow-up sampling showed different results from the previously reported ones. FREEDOMS and TRANSFORMS core and extension studies and other investigations among Asian populations have revealed that the low lymphocyte count remains unchanged during the course of the study. Although the absolute lymphocyte count increased at months 6 and 12 in our experiment, it did not reach the normal level. The low incidence of infections in this trial (n=1) could be attributed to the increase in lymphocyte count during the treatment period. Unlike the previously reported data, no respiratory, urinary tract, or herpes virus infections were reported in this study (Cohen et al., 2016, 2010; Kappos et al., 2015, 2010). However, alteration in aminotransferases was similar to the previous studies.
There is no cure for MS, and as a chronic disease, long-term treatments are crucial for many patients. The effectiveness of therapy strongly depends on medication adherence. Investigations on tolerability and adherence to conventional DMTs for MS indicated that the discontinuation of treatment ranged from 17% to 36% due to the diverse types of injectable treatments (Hansen et al., 2015). Injection site reactions and flu-like symptoms were two of the most common reasons for discontinuation. Comparing fingolimod with other DMts, we can conclude that the persistence and adherence to oral fingolimod were higher than other injectable medications (Giovannoni et al., 2012). Wilson et al. evaluated the MS patients’ preference for different types of medications. They displayed that the patients preferred oral medicines to all injectable ones (Wilson et al., 2015). According to a retrospective cohort conducted using pharmacy data to describe the adherence to MS DMTs (interferon beta-1a IM [Avonex], interferon beta-1a SC [Rebif], interferon beta-1b SC [Betaferon], and glatiramer acetate SC [Copaxone]), it was revealed that only 30%-40% of the patients were adherent to the drugs after two years (Hansen et al., 2015).
One significant limitation in our study was the number of patients who did not complete the follow-up period for the reasons mentioned above. Most of the side effects were mild; however, those patients felt disappointed, perhaps because they were selected among those who had not responded to interferon/other therapies or suffered from different side effects before the study initiation. As told before, this study aimed to evaluate if any significant ameliorating effect could be demonstrated in RRMS patients who turned to fingolimod therapy after unsuccessful treatment with other medicines.
As the finishing statement, this 12-month study revealed, for the first time, the safety and efficacy of fingolimod therapy in Iranian patients with RRMS. Also, it was demonstrated that the patients preferred fingolimod to other available treatments. Concerning the reduced MRI lesions and diminished relapses observed in this study, fingolimod is preferred to other standard therapies and suggested as the first-line medication for the treatment of RRMS in Iran.
Ethical Considerations
Compliance with ethical guidelines
All procedures performed in this study were following the ethical standards of the Institutional Research Committee and with the 1964 Helsinki declaration and its comparable ethical standards.
Funding
This study was supported by the Research Vice-Presidency and the Referral MS Center of Mashhad University of Medical Sciences.
Authors' contributions
Conceptualization, methodology, investigation, final review, resources: All authors; Writing the original draft: Pardis Oliazadeh; Writing, review, and editing: Pardis Oliazadeh, Fatemeh Yavari; Funding acquisition: Morteza Saeidi, Mohsen Foroughipour; Supervision: Morteza Saeidi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank all the staff of Ghaem Hospital who helped us to conduct this investigation.
References
Agashivala, N., & Kim, E. (2012). Cost-effectiveness of early initiation of fingolimod versus delayed initiation after 1 year of intramuscular interferon beta-1a in patients with multiple sclerosis. Clinical Therapeutics, 34(7), 1583-90. [DOI:10.1016/j.clinthera.2012.06.012] [PMID]
Agius, M., Meng, X., Chin, P., Grinspan, A., & Hashmonay, R. (2014). Fingolimod therapy in early multiple sclerosis: an efficacy analysis of the TRANSFORMS and FREEDOMS studies by time since first symptom. CNS Neuroscience & Therapeutics, 20(5), 446-51. [DOI:10.1111/cns.12235] [PMID] [PMCID]
Alroughani, R., Ahmed, S. F., Behbehani, R., & Al-Hashel, J. (2014). Use of fingolimod in patients with relapsing remitting multiple sclerosis in Kuwait. Clinical Neurology and Neurosurgery, 119, 17-20. [DOI:10.1016/j.clineuro.2014.01.007] [PMID]
Brinkmann, V., Billich, A., Baumruker, T., Heining, P., Schmouder, R., & Francis, G., et al. (2010). Fingolimod (FTY720): Discovery and development of an oral drug to treat multiple sclerosis. Nature Reviews Drug Discovery, 9(11), 883-97. [DOI:10.1038/nrd3248] [PMID]
Chun, J., & Hartung, H. P. (2010). Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clinical Neuropharmacology, 33(2), 91. [DOI:10.1097/WNF.0b013e3181cbf825] [PMID] [PMCID]
Cohen, J. A., Barkhof, F., Comi, G., Hartung, H. P., Khatri, B. O., & Montalban, X., et al. (2010). Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. New England Journal of Medicine, 362(5), 402-15. [DOI:10.1056/NEJMoa0907839] [PMID]
Cohen, J. A., Khatri, B., Barkhof, F., Comi, G., Hartung, H. P., & Montalban, X., et al. (2016). Long-term (up to 4.5 years) treatment with fingolimod in multiple sclerosis: results from the extension of the randomised TRANSFORMS study. Journal of Neurology, Neurosurgery & Psychiatry, 87(5), 468-75. [DOI:10.1136/jnnp-2015-310597] [PMID] [PMCID]
Devonshire, V., Havrdova, E., Radue, E. W., O'Connor, P., Zhang-Auberson, L., & Agoropoulou, C., & FREEDOMS Study Group. (2012). Relapse and disability outcomes in patients with multiple sclerosis treated with fingolimod: subgroup analyses of the double-blind, randomised, placebo-controlled FREEDOMS study. The Lancet Neurology, 11(5), 420-8. [DOI:10.1016/S1474-4422(12)70056-X]
Etemadifar, M., Sajjadi, S., Nasr, Z., Firoozeei, T. S., Abtahi, S. H., & Akbari, M., et al. (2013). Epidemiology of multiple sclerosis in Iran: A systematic review. European neurology, 70(5-6), 356-63. [DOI:10.1159/000355140] [PMID]
European Medicines Agency. (2011). Assessment report: Gilenya. Doc Ref: EMA/108602/2011. Retrieved from http://www.ema.europa.eu/docs/ en_GB/document_library/EPAR_-_Public_assessment_report/human/ 002202/WC500104529.pdf.
Geurts, J. J., & Barkhof, F. (2008). Grey matter pathology in multiple sclerosis. The Lancet Neurology, 7(9), 841-51. [DOI:10.1016/S1474-4422(08)70191-1]
Giovannoni, G., Southam, E., & Waubant, E. (2012). Systematic review of disease-modifying therapies to assess unmet needs in multiple sclerosis: Tolerability and adherence. Multiple Sclerosis Journal, 18(7), 932-46. [DOI:10.1177/1352458511433302] [PMID]
Hansen, K., Schüssel, K., Kieble, M., Werning, J., Schulz, M., & Friis, R., et al. (2015). Adherence to disease modifying drugs among patients with multiple sclerosis in Germany: A retrospective cohort study. PloS One, 10(7), e0133279. [DOI:10.1371/journal.pone.0133279] [PMID] [PMCID]
. Kamm, C. P., Uitdehaag, B. M., & Polman, C. H. (2014). Multiple sclerosis: current knowledge and future outlook. European Neurology, 72(3-4), 132-41. [DOI:10.1159/000360528] [PMID]
Kappos, L., Radue, E. W., O'Connor, P., Polman, C., Hohlfeld, R., & Calabresi, P., et al. (2010). A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. New England Journal of Medicine, 362(5), 387-401. [DOI:10.1056/NEJMoa0909494] [PMID]
Kappos, L., O'Connor, P., Radue, E. W., Polman, C., Hohlfeld, R., & Selmaj, K., et al. (2015). Long-term effects of fingolimod in multiple sclerosis: The randomized FREEDOMS extension trial. Neurology, 84(15), 1582-91. [DOI:10.1212/WNL.0000000000001462] [PMID] [PMCID]
Lucchinetti, C. F., Popescu, B. F., Bunyan, R. F., Moll, N. M., Roemer, S. F., & Lassmann, H., et al. (2011). Inflammatory cortical demyelination in early multiple sclerosis. New England Journal of Medicine, 365(23), 2188-97. [DOI:10.1056/NEJMoa1100648] [PMID] [PMCID]
Mehling, M., Kappos, L., & Derfuss, T. (2011). Fingolimod for multiple sclerosis: Mechanism of action, clinical outcomes, and future directions. Current Neurology and Neuroscience Reports, 11(5), 492-7. [DOI:10.1007/s11910-011-0216-9] [PMID]
Ouspid, E., Razazian, N., Moghadasi, A. N., Moradian, N., Afshari, D., & Bostani, A., et al. (2018). Clinical effectiveness and safety of fingolimod in relapsing remitting multiple sclerosis in Western Iran. Neurosciences (Riyadh), 23(2), 129-134. [DOI:10.17712/nsj.2018.2.20170434] [PMID]
Polman, C. H., Reingold, S. C., Banwell, B., Clanet, M., Cohen, J. A., & Filippi, M., et al. (2011). Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Annals of Neurology, 69(2), 292-302. [DOI:10.1002/ana.22366] [PMID] [PMCID]
Saida, T., Kikuchi, S., Itoyama, Y., Hao, Q., Kurosawa, T., & Nagato, K., et al. (2012). A randomized, controlled trial of fingolimod (FTY720) in Japanese patients with multiple sclerosis. Multiple Sclerosis Journal, 18(9), 1269-277. [DOI:10.1177/1352458511435984] [PMID]
Simula, S., Laitinen, T., Laitinen, T. M., Tarkiainen, T., Hartikainen, P., & Hartikainen, J. E. (2016). Effect of fingolimod on cardiac autonomic regulation in patients with multiple sclerosis. Multiple Sclerosis Journal, 22(8), 1080-5. [DOI:10.1177/1352458515604384] [PMID]
Sorensen, P. S. (2014). New management algorithms in multiple sclerosis. Current Opinion in Neurology, 27(3), 246-59. [DOI:10.1097/WCO.0000000000000096] [PMID]
US Food and Drug Administration (FDA). (2010). Approves first oral drug to reduce MS relapses. Retreived from http://www.fda.gov/NewsEvents/ Newsroom/PressAnnouncements/ucm226755.htm
Wilson, L. S., Loucks, A., Gipson, G., Zhong, L., Bui, C., & Miller, E., et al. (2015). Patient preferences for attributes of multiple sclerosis disease-modifying therapies: Development and results of a ratings-based conjoint analysis. International Journal of MS Care, 17(2), 74-82. [DOI:10.7224/1537-2073.2013-053] [PMID] [PMCID]
Wingerchuk, D. M., & Carter, J. L. (2014). Multiple sclerosis: Current and emerging disease-modifying therapies and treatment strategies. Mayo Clinic Proceedings, 89(2), 225-40. [DOI:10.1016/j.mayocp.2013.11.002] [PMID]
Type of Study: Original |
Subject:
Clinical Neuroscience
Received: 2020/01/23 | Accepted: 2020/12/1 | Published: 2021/03/1
Received: 2020/01/23 | Accepted: 2020/12/1 | Published: 2021/03/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






