Volume 10, Issue 4 (July & August 2019)
BCN 2019, 10(4): 383-392 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Langhnoja J, Mustak M. Gamma-Radiation-Induced Endoplasmic Reticulum Stress and Downregulation of WFS1, Nectin 3, and Sostdc1 Gene Expression in Mice Hippocampus. BCN 2019; 10 (4) :383-392
URL: http://bcn.iums.ac.ir/article-1-1158-en.html
URL: http://bcn.iums.ac.ir/article-1-1158-en.html
1- Department of Applied Zoology, Faculty of Science & Technology, Mangalore University, Mangalagangothri, Karnataka State, India.
2- Department of Applied Zoology, Mangalore University, Karnataka, India
2- Department of Applied Zoology, Mangalore University, Karnataka, India
Full-Text [PDF 1823 kb]
| Abstract (HTML)
3.2. Protein expression analysis of CHOP and ERK½
CHOP gene has been commonly used as a hallmark for the ER stress-mediated apoptosis, and induction of this gene confirms ER stress-mediated apoptosis. Whole-body gamma radiation dose significantly increased (**P<0.01) expression of CHOP (Figure 3 a-b) protein level, suggesting the induction of ER-stress-mediated apoptosis in the isolated hippocampus. Significant increase in the CHOP protein expression (**P<0.01) at 10 Gy of dose gamma advocates profuse ER stress-mediated apoptosis. Also, significant (*P<0.05) increase of ERK½ protein expression at the dose range of 10 Gy dose of radiation are observed on the hippocampus (Figure 3 a, c) which further validate the involvement of ERK½ pathway, inducing ER-stress-mediated apoptosis in isolated hippocampus. Significant increase in the levels of CHOP and ERK½ suggests that IR induces hippocampal dysfunction by inducing ER-stress-mediated apoptosis, which might be further responsible for various IR-mediated hippocampal dysfunction.
4. Discussion
Understanding the molecular machinery responsible for radiation-induced cognitive dysfunction will provide insight into the molecular mechanism and neurobiology of stress-induced neurodegenerative disorders. Here we tried to understand how radiation onsets ER stress-induced dysfunction of the hippocampus of mice brain after whole body radiation. We observed a positive correlation with the upregulation of ER stress-specific genes and also the genes responsible for various physiological functions in the hippocampal regions. ER stress has been reported to disrupt neuronal functions and is responsible for various neurological disorders like Alzheimer disease, Parkinson disease, and Huntington disease (Siman, Flood, Thinakaran, Neumar, 2001). Radiation-induced ER stress has also been reported by us and others in various cell lines (Zhang et al., 2009).
In the present study, we found elevated expression of GRP78/BiP which has been reported earlier to have a dual role which activates under ER-stress condition as a self-defense mechanism in the cell, but under unresolved ER stress, this leads to cell death (Akutsu, Matsubara, Urashima, Komatsu, Sakata, Nishimori, et al, 2007). Another marker, CHOP, a downstream component of the ER stress pathway, is reported to get upregulated along with the induction of BiP signaling and lead the cell towards apoptotic pathway. CHOP overexpression has been linked with various neurodegenerative diseases and also targeted for development of therapeutic drugs against ER stress (Ohoka, Yoshii, Hattori, & Onozaki, Hayashi, 2005). In the present study, we observed significant upregulation with the increasing gamma radiation dose suggesting the 7 Gy gamma radiation exposure brings about significant induction of ER stress-mediated apoptosis in mice hippocampus.
Previous reports have suggested cells deficient in WFS1 are more susceptible to ER stress-mediated apoptosis. The present study we have observed a reduction of WFS1 gene with the increase of radiation dose in sync with an increase of BiP and CHOP, suggesting WFS1 plays an important role in mediating ER stress-mediated apoptosis. Also, the reduced level of WFS1 is linked with a genetic condition leading to Wolfram syndrome which causes severe depression, psychosis, or organic brain syndrome, as well as impulsive verbal and physical aggression (Takeda et al., 2001). In the current study, we observed a decrease in WFS1 gene expression with an increase of radiation dose, suggesting a link role of the WFS1 gene in inducing ER stress-mediated dysfunction in the hippocampus.
Previous reports have suggested the major role of CAM Nectin-3 in hippocampal-dependent learning and memory (Wang et al., 2013). Reduced level of nectin is associated with early life stress, disruption of synaptic contacts, and also hampered spatial memory. In our present study, we observed a dose-dependent decrease of Nectin-3 expression suggesting that 7- to 10-Gy gamma radiation have severe effect in destabilizing the hippocampal neurons, which might be disrupting hippocampal-dependent cognitive functions. Downregulation of Nectin-3 also suggests hampered episodic memories and loss of neurogenesis in the CA3 region of the hippocampus.
In conclusion, our present study suggests that a 7-Gy dose of whole-body gamma radiation in mice is sufficient to induce ER stress specific markers BiP and CHOP and also downregulates hippocampal genes WFS1, Sostdc1, and Nectrin3, which ultimately disrupt the hippocampal homeostasis. Significant increase of ERK½ also suggests cells innate response to overcome ER stress. As the hippocampus is involved in a wide array of physiological functions, radiation-induced damage in the hippocampus might lead to various neurodegenerative diseases such as AD, PD, etc. The present study may lead to the identification of ER stress and hippocampal genes as new markers to study radiation-induced neurodegenerative disorder induced by hippocampal dysfunction.
Ethical Considerations
Compliance with ethical guidelines
All experiments were conducted following the ethical guidelines by the Committee for the Purpose of Control and Supervision of Experiments on Animals, Government of India and cleared by the Institutional Animal Ethics Committee, Mangalore University, Karnataka, India. Supervision of animal protocols was carried out according to the research design.
Funding
This research was funded by a grant from the Department of Atomic Energy-Board Research in Nuclear Sciences (DAE-BRNS), Mumbai, Government of India, Sanction Number: 2013/34/5/BRNS/0444. Jit Chatterjee acknowledges the receipt of a fellowship from DAE-BRNS, Mumbai, India.
Authors' contributions
Study concept, design, student mentorship: Mohammed S Mustak; Acquisition of animal care and data generation: Jit Chatterjee; Animal dissection, RT PCR and western blot experiments: Jit Chatterjee &Jaldeep Langhnoja; Data analysis, interpretation of the findings: Mohammed S Mustak & Prakash Pillai; and Reviewing the manuscript and approving the final version for publication: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
We thank the Director of Centre for Application of Radiation and Radioisotope Technology (CARRT) and Microtome Centre Mangalore University for providing gamma radiation facility. We also thank DBT-MSUB-ILSPARE for the confocal microscopy facility and real-time PCR at Dr. Vikram Sarabhai Science Block, Faculty of Science, MSU, Vadodara.
References
Full-Text:
1. Introduction
In various clinical conditions, the brain gets exposed to ionizing radiation. Though radiotherapy is considered as one of the primary treatment modality in different pathological conditions, the potential injury to normal tissue is unavoidable. Radiation exposure often causes a wide array of cognitive dysfunctions in adult and pediatric tumor patients (Merchant, Pollack, & Loeffler, 2010). Ionizing Radiation (IR) has a wide plethora of effects in both young and adult brain. More histological changes are reported at higher doses of radiation, while low dose radiation causes cognitive dysfunctions in adult mammals (Raber et al., 2004). Repeated exposure to various stresses leads to the adverse effect in cognition across multiple life stages (Lupien, McEwen, Gunnar, & Heim, 2009).
Hippocampus is considered to be one of the major sites for active neurogenesis (Van Praag, 2005; Mizumatsu et al., 2003). Hippocampal CA1, CA2, CA3 areas play a significant role in maintaining CNS homeostasis and are involved in various physiological processes (Frederick Hitti, Siegelbaum, 2014; Jensen & Lisman, 1996; Dudek, Alexander, & Farris, 2016; Meyer et al., 2014). Nectin-3 an immunoglobulin-like cell adhesion molecule which is mainly localized in the CA3 pyramidal neurons plays an important role in the synaptic formation, maintenance, and remodeling (Mizoguchi et al., 2002; Honda et al., 2006; Thompson et al., 2008).
Studies indicate that exposure to ionizing radiation could induce ultrastructural modifications in the ER (Boraks, Tampelini, Pereira, Chopard, 2008). ER forms the major protein folding machinery in the cell. Cell homeostasis gets disrupted when the load of the unfolded protein increases and Unfolded Protein Response (UPR) pathway fails to repair the misfolded protein which leads to the accumulation of these proteins in the ER lumen (Taniguchi, Yoshida. 2015). Accumulation of these misfolded proteins has been proved to cause apoptosis (Oakes, Papa. 2015), which ultimately leads to neurodegenerative diseases. The UPR pathway usually is active as self-defense machinery in the cell, which increases the secretion of molecular chaperones such as BiP and GRP78, which belongs to the heat shock protein family and foldases. However, when misfolded proteins accumulate in the excessive amount, they may overwhelm the quality control machinery.
The mammalian UPR directs the cell to an apoptotic pathway, leading to cell death. C/EBP Homologous Protein (CHOP), also known as GADD153 (growth arrest- and DNA damage-inducible gene 153), is triggered by ER stress. CHOP overexpression triggers cell cycle arrest and apoptosis, down-regulates the pro-survival molecule Bcl-2, and promotes the production of reactive oxygen species (Marciniak et al., 2004). Quite the opposite, overexpression of the ER chaperone BiP reduces CHOP induction that is associated with ER stress and attenuates apoptosis (Wang et al., 2013).
Another player, WFS1, is a transmembrane protein present in the ER is shown to play a significant role in mitigating ER stress response in the cell (Takeda et al., 2001). Wolfram syndrome a genetic condition of diabetes, optic atrophy neurodegeneration, and psychiatric illness, is reported to be caused by mutation of WFS1 gene (Strom et al., 1998; Inoue et al., 1998). Reports also suggest that the increased level of ER-stress signaling leads to cell death, causing neuronal dysfunction in the Wolfram syndrome (Yamada et al., 2006; Riggs et al., 2005; Kakiuchi, Ishiwata, Hayashi, & Kato, 2006).
Sclerostin Domain Containing 1 (Sostdc1) belongs to a Bone Morphogenetic Protein (BMP) antagonist. In the development of cultured sympathetic and cerebellar neurons, the BMP family of proteins play a significant role by inducing synaptogenesis and dendritic growth (Lein et al., 2002). An altered level of Sostdc1 contributes to various disease conditions (Park et al., 2009). Sostdc1 gene has also been reported in thapsigargin-induced ER stress in mouse osteoblasts (Hamamura, Liu, & Yokota, 2008). However, the underlying mechanism is still unclear.
Cell Adhesion Molecules (CAM) are the principal constituent of synapses and also the modulators of synaptic activity and plasticity (Shapiro, Love, & Colman, 2007). Nectin-3 is a class of immunoglobulin-like CAM that presents in both postsynaptic and presynaptic and is connected to the actin cytoskeleton via L-afadin. The nectin-afadin complex coordinates with the cadherin-catenin junction and participates in synaptic formation, remodeling, and maintenance (Mizoguchi et al., 2002; Honda et al., 2006). Evidence also suggests impaired nectin-mediated damage in hippocampal development and mental retardation (Park et al., 2009). Nectin-3 is abundantly present in CA3 region of the hippocampus (Thompson et al., 2008) and is vulnerable to acute and chronic stress (Mizoguchi et al., 2002; Suzuki et al., 2000). Protein kinases play a crucial role in various signaling networks to maintain cell homeostasis and its functions.
Mitogen-Activated Protein Kinase (MAPK) has a conserved function and contributes to various hippocampus-mediated neurodegenerative diseases (Giovannini et al., 2008). Extracellular-signal-Regulated kinase½ (ERK½) is one of the members of the MAPK family and has been spotted in various disease conditions. Ultraviolet irradiation activates ERK½ in various primary immortalized and transformed cells (Tang et al., 2002). However, radiation-induced changes in hippocampal Nectrin3, WFS1, and Sostdc1 gene expression in ER stress condition via ERK½ pathway are still unclear. To the best of our knowledge, there is no report on how radiation induces ER stress-mediated alteration in the hippocampus of mice exposed to whole-body radiation. In the present study, we have tried to understand the ER stress-mediated changes in mice hippocampus after exposing to whole-body gamma radiation.
2. Methods
2.1. Study subjects
Adult Swiss albino mice (Mus musculus) were housed in pairs under standard laboratory conditions with artificial 12 h light/dark cycle at an ambient temperature of 25°C-27°C with free access to food and water. All experiments were conducted following the ethical guidelines by the Committee for the Purpose of Control and Supervision of Experiments on Animals, Government of India and cleared by the Institutional Animal Ethics Committee.
2.2. Gamma irradiation
For irradiating the samples, 60Co-gamma chamber-1200 supplied by Board of Radiation and Isotope Technology (BRIT), DAE, Mumbai was used in the Centre for Application of Radiation and Radioisotope Technology (CAART, Mangalore University). The dose rate of the above the gamma chamber was measured and found 10.2333 Gy/min using Fricke dosimetry system (Nairy, Bhat, Sanjeev, & Yerol, 2016). For the experiment, 6-8 weeks old matched (weighing: 25±2 g) male Swiss albino mice (Mus musculus) were used. All animals were supplied with standard mice food and water ad libitum.
Jagetia et al. (2003) reported, 6- to 12-Gy intensity of gamma radiation significantly increase lipid peroxidation and depletion of Glutathione (GSH) in mice exposed to whole-body radiation. In the present study, the mice were exposed to gamma radiation in dose ranges of 7 Gy, 8 Gy, 9 Gy, 10 Gy in a well ventilated restrained perplex box. After exposure, the animals were kept for 24 h and then sacrificed and their hippocampus were isolated for further analysis. The above experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) of Mangalore University.
2.3. Isolation of hippocampus
After killing the mice, their brains were immediately dissected out on the ice and placed in a pre-chilled stereotaxic brain block (Kopf, USA). One-millimeter thick sections of the hippocampus were serially cut out using Paxinos and Watson atlas. The parts of some sections of the hippocampus are stored in RNA Later Solution (Invitrogen) for gene expression studies and RIPA buffer (Himedia) for protein expression studies. For both Real-Time qPCR and western blot analysis, 5 adult Swiss albino mice were kept in each experimental group.
2.4. Real-time qPCR analysis
Total RNA was isolated from cells by TRIzol reagent (Invitrogen). The Qubit RNA assay kit (Invitrogen) was used for quantifying the isolated RNA. One microgram of the total RNA was used for a 20 μL reaction. The Verso cDNA synthesis kit (Applied Biosystems) was used for the Reverse Transcription (RT) reaction. For the quantitative RT-PCR, SYBR Select Master Mix (Applied Biosystems) was used in QuantStudio 12K (Life Technology) real-time PCR machine with primers specific to detect the target messenger RNA (mRNA).
2.5. Western blot
The western Blot analysis was carried out to understand the expression level of CHOP and ERK pathway on exposure to IR in mice hippocampus. The tissues were lysed using lysis buffer (Himedia) and stored at –20°C for further analysis. Qubit protein assay kit (Invitrogen) in Qubit 2.0 fluorometer (Invitrogen) was used to quantify the isolated protein from the hippocampus tissue homogenate. About 40 µg of the quantified protein sample was dissolved in 10% SDS polyacrylamide gel and further transferred to the nitrocellulose membrane. After transfer, the membrane was blocked using 3% BSA in Tris-buffered saline and Tween 20 mixture (0.2 %) and incubated in primary antibody overnight at 4°C. The primary antibodies of anti-GAPDH (1:1000, Abchem), anti-CHOP (1:1000, Cell signaling), and anti-ERK½ (1:1000, Pierce) were used. The bands were visualized in ChemiDoc (Bio-Rad) using corresponding horseradish peroxidase-conjugated secondary antibodies (Sigma). The bands were quantified using ImageJ software and graphs were plotted by GraphPad Prism-3 software.
2.6. Statistical analysis
Statistical analysis was performed by 1-way ANOVA followed by Dunnett’s multiple range test in Prism 3 software (GraphPad Software Inc.). The data were expressed as Mean±SD. P values less than 0.05 were considered statistically significant (*P<0.05; **P<0.01; ***P<0.001).
3. Results
3.1. Effect of radiation on ER stress and hippocampus specific gene expression
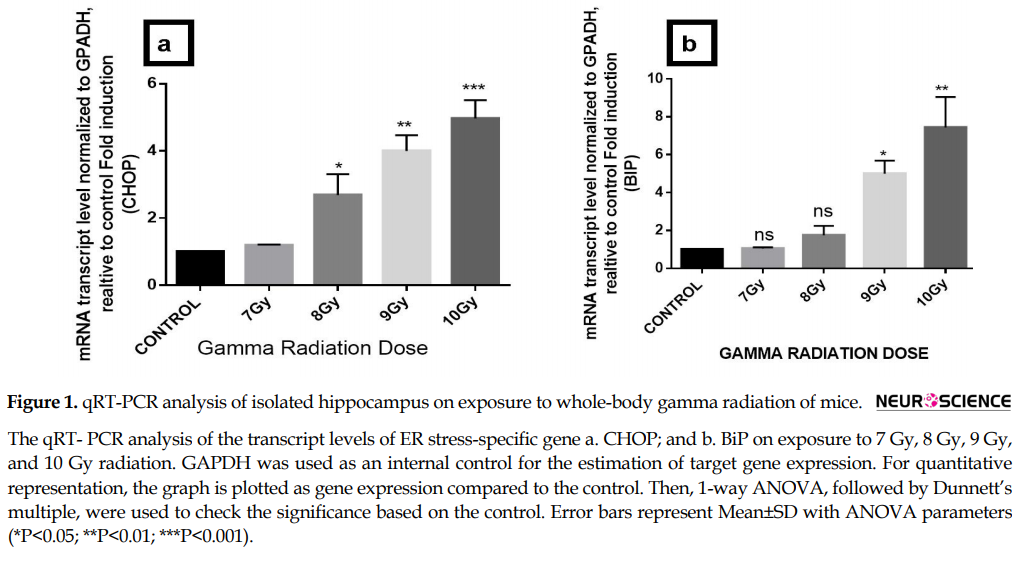
BiP and CHOP are well-known ER chaperones and get up-regulated under conditions of ER stress. We assessed the expression of BiP, CHOP, WFS1, Sostdc1, and Nectin3 by quantitative RT-qPCR on irradiated mice hippocampus. Results demonstrate increase in CHOP and BiP gene expression with increase in dose of 7 Gy to 10 Gy and also 4 fold increase of ER stress-specific gene CHOP (***P<0.001) and 6 fold increase in BiP (**P<0.01) at 10 Gy of gamma radiation dose with respect to control (Figure 1 A-b). Furthermore, 7-10 Gy dose of gamma radiation significantly downregulates the expression of Nectin3 gene (***P<0.001), WFS1 (***P<0.001) and Sostdc1 (*** P<0.001) by 1 fold which ultimately at the 10-Gy dose (Figure 2 a-c).
In various clinical conditions, the brain gets exposed to ionizing radiation. Though radiotherapy is considered as one of the primary treatment modality in different pathological conditions, the potential injury to normal tissue is unavoidable. Radiation exposure often causes a wide array of cognitive dysfunctions in adult and pediatric tumor patients (Merchant, Pollack, & Loeffler, 2010). Ionizing Radiation (IR) has a wide plethora of effects in both young and adult brain. More histological changes are reported at higher doses of radiation, while low dose radiation causes cognitive dysfunctions in adult mammals (Raber et al., 2004). Repeated exposure to various stresses leads to the adverse effect in cognition across multiple life stages (Lupien, McEwen, Gunnar, & Heim, 2009).
Hippocampus is considered to be one of the major sites for active neurogenesis (Van Praag, 2005; Mizumatsu et al., 2003). Hippocampal CA1, CA2, CA3 areas play a significant role in maintaining CNS homeostasis and are involved in various physiological processes (Frederick Hitti, Siegelbaum, 2014; Jensen & Lisman, 1996; Dudek, Alexander, & Farris, 2016; Meyer et al., 2014). Nectin-3 an immunoglobulin-like cell adhesion molecule which is mainly localized in the CA3 pyramidal neurons plays an important role in the synaptic formation, maintenance, and remodeling (Mizoguchi et al., 2002; Honda et al., 2006; Thompson et al., 2008).
Studies indicate that exposure to ionizing radiation could induce ultrastructural modifications in the ER (Boraks, Tampelini, Pereira, Chopard, 2008). ER forms the major protein folding machinery in the cell. Cell homeostasis gets disrupted when the load of the unfolded protein increases and Unfolded Protein Response (UPR) pathway fails to repair the misfolded protein which leads to the accumulation of these proteins in the ER lumen (Taniguchi, Yoshida. 2015). Accumulation of these misfolded proteins has been proved to cause apoptosis (Oakes, Papa. 2015), which ultimately leads to neurodegenerative diseases. The UPR pathway usually is active as self-defense machinery in the cell, which increases the secretion of molecular chaperones such as BiP and GRP78, which belongs to the heat shock protein family and foldases. However, when misfolded proteins accumulate in the excessive amount, they may overwhelm the quality control machinery.
The mammalian UPR directs the cell to an apoptotic pathway, leading to cell death. C/EBP Homologous Protein (CHOP), also known as GADD153 (growth arrest- and DNA damage-inducible gene 153), is triggered by ER stress. CHOP overexpression triggers cell cycle arrest and apoptosis, down-regulates the pro-survival molecule Bcl-2, and promotes the production of reactive oxygen species (Marciniak et al., 2004). Quite the opposite, overexpression of the ER chaperone BiP reduces CHOP induction that is associated with ER stress and attenuates apoptosis (Wang et al., 2013).
Another player, WFS1, is a transmembrane protein present in the ER is shown to play a significant role in mitigating ER stress response in the cell (Takeda et al., 2001). Wolfram syndrome a genetic condition of diabetes, optic atrophy neurodegeneration, and psychiatric illness, is reported to be caused by mutation of WFS1 gene (Strom et al., 1998; Inoue et al., 1998). Reports also suggest that the increased level of ER-stress signaling leads to cell death, causing neuronal dysfunction in the Wolfram syndrome (Yamada et al., 2006; Riggs et al., 2005; Kakiuchi, Ishiwata, Hayashi, & Kato, 2006).
Sclerostin Domain Containing 1 (Sostdc1) belongs to a Bone Morphogenetic Protein (BMP) antagonist. In the development of cultured sympathetic and cerebellar neurons, the BMP family of proteins play a significant role by inducing synaptogenesis and dendritic growth (Lein et al., 2002). An altered level of Sostdc1 contributes to various disease conditions (Park et al., 2009). Sostdc1 gene has also been reported in thapsigargin-induced ER stress in mouse osteoblasts (Hamamura, Liu, & Yokota, 2008). However, the underlying mechanism is still unclear.
Cell Adhesion Molecules (CAM) are the principal constituent of synapses and also the modulators of synaptic activity and plasticity (Shapiro, Love, & Colman, 2007). Nectin-3 is a class of immunoglobulin-like CAM that presents in both postsynaptic and presynaptic and is connected to the actin cytoskeleton via L-afadin. The nectin-afadin complex coordinates with the cadherin-catenin junction and participates in synaptic formation, remodeling, and maintenance (Mizoguchi et al., 2002; Honda et al., 2006). Evidence also suggests impaired nectin-mediated damage in hippocampal development and mental retardation (Park et al., 2009). Nectin-3 is abundantly present in CA3 region of the hippocampus (Thompson et al., 2008) and is vulnerable to acute and chronic stress (Mizoguchi et al., 2002; Suzuki et al., 2000). Protein kinases play a crucial role in various signaling networks to maintain cell homeostasis and its functions.
Mitogen-Activated Protein Kinase (MAPK) has a conserved function and contributes to various hippocampus-mediated neurodegenerative diseases (Giovannini et al., 2008). Extracellular-signal-Regulated kinase½ (ERK½) is one of the members of the MAPK family and has been spotted in various disease conditions. Ultraviolet irradiation activates ERK½ in various primary immortalized and transformed cells (Tang et al., 2002). However, radiation-induced changes in hippocampal Nectrin3, WFS1, and Sostdc1 gene expression in ER stress condition via ERK½ pathway are still unclear. To the best of our knowledge, there is no report on how radiation induces ER stress-mediated alteration in the hippocampus of mice exposed to whole-body radiation. In the present study, we have tried to understand the ER stress-mediated changes in mice hippocampus after exposing to whole-body gamma radiation.
2. Methods
2.1. Study subjects
Adult Swiss albino mice (Mus musculus) were housed in pairs under standard laboratory conditions with artificial 12 h light/dark cycle at an ambient temperature of 25°C-27°C with free access to food and water. All experiments were conducted following the ethical guidelines by the Committee for the Purpose of Control and Supervision of Experiments on Animals, Government of India and cleared by the Institutional Animal Ethics Committee.
2.2. Gamma irradiation
For irradiating the samples, 60Co-gamma chamber-1200 supplied by Board of Radiation and Isotope Technology (BRIT), DAE, Mumbai was used in the Centre for Application of Radiation and Radioisotope Technology (CAART, Mangalore University). The dose rate of the above the gamma chamber was measured and found 10.2333 Gy/min using Fricke dosimetry system (Nairy, Bhat, Sanjeev, & Yerol, 2016). For the experiment, 6-8 weeks old matched (weighing: 25±2 g) male Swiss albino mice (Mus musculus) were used. All animals were supplied with standard mice food and water ad libitum.
Jagetia et al. (2003) reported, 6- to 12-Gy intensity of gamma radiation significantly increase lipid peroxidation and depletion of Glutathione (GSH) in mice exposed to whole-body radiation. In the present study, the mice were exposed to gamma radiation in dose ranges of 7 Gy, 8 Gy, 9 Gy, 10 Gy in a well ventilated restrained perplex box. After exposure, the animals were kept for 24 h and then sacrificed and their hippocampus were isolated for further analysis. The above experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) of Mangalore University.
2.3. Isolation of hippocampus
After killing the mice, their brains were immediately dissected out on the ice and placed in a pre-chilled stereotaxic brain block (Kopf, USA). One-millimeter thick sections of the hippocampus were serially cut out using Paxinos and Watson atlas. The parts of some sections of the hippocampus are stored in RNA Later Solution (Invitrogen) for gene expression studies and RIPA buffer (Himedia) for protein expression studies. For both Real-Time qPCR and western blot analysis, 5 adult Swiss albino mice were kept in each experimental group.
2.4. Real-time qPCR analysis
Total RNA was isolated from cells by TRIzol reagent (Invitrogen). The Qubit RNA assay kit (Invitrogen) was used for quantifying the isolated RNA. One microgram of the total RNA was used for a 20 μL reaction. The Verso cDNA synthesis kit (Applied Biosystems) was used for the Reverse Transcription (RT) reaction. For the quantitative RT-PCR, SYBR Select Master Mix (Applied Biosystems) was used in QuantStudio 12K (Life Technology) real-time PCR machine with primers specific to detect the target messenger RNA (mRNA).
2.5. Western blot
The western Blot analysis was carried out to understand the expression level of CHOP and ERK pathway on exposure to IR in mice hippocampus. The tissues were lysed using lysis buffer (Himedia) and stored at –20°C for further analysis. Qubit protein assay kit (Invitrogen) in Qubit 2.0 fluorometer (Invitrogen) was used to quantify the isolated protein from the hippocampus tissue homogenate. About 40 µg of the quantified protein sample was dissolved in 10% SDS polyacrylamide gel and further transferred to the nitrocellulose membrane. After transfer, the membrane was blocked using 3% BSA in Tris-buffered saline and Tween 20 mixture (0.2 %) and incubated in primary antibody overnight at 4°C. The primary antibodies of anti-GAPDH (1:1000, Abchem), anti-CHOP (1:1000, Cell signaling), and anti-ERK½ (1:1000, Pierce) were used. The bands were visualized in ChemiDoc (Bio-Rad) using corresponding horseradish peroxidase-conjugated secondary antibodies (Sigma). The bands were quantified using ImageJ software and graphs were plotted by GraphPad Prism-3 software.
2.6. Statistical analysis
Statistical analysis was performed by 1-way ANOVA followed by Dunnett’s multiple range test in Prism 3 software (GraphPad Software Inc.). The data were expressed as Mean±SD. P values less than 0.05 were considered statistically significant (*P<0.05; **P<0.01; ***P<0.001).
3. Results
3.1. Effect of radiation on ER stress and hippocampus specific gene expression
BiP and CHOP are well-known ER chaperones and get up-regulated under conditions of ER stress. We assessed the expression of BiP, CHOP, WFS1, Sostdc1, and Nectin3 by quantitative RT-qPCR on irradiated mice hippocampus. Results demonstrate increase in CHOP and BiP gene expression with increase in dose of 7 Gy to 10 Gy and also 4 fold increase of ER stress-specific gene CHOP (***P<0.001) and 6 fold increase in BiP (**P<0.01) at 10 Gy of gamma radiation dose with respect to control (Figure 1 A-b). Furthermore, 7-10 Gy dose of gamma radiation significantly downregulates the expression of Nectin3 gene (***P<0.001), WFS1 (***P<0.001) and Sostdc1 (*** P<0.001) by 1 fold which ultimately at the 10-Gy dose (Figure 2 a-c).
3.2. Protein expression analysis of CHOP and ERK½
CHOP gene has been commonly used as a hallmark for the ER stress-mediated apoptosis, and induction of this gene confirms ER stress-mediated apoptosis. Whole-body gamma radiation dose significantly increased (**P<0.01) expression of CHOP (Figure 3 a-b) protein level, suggesting the induction of ER-stress-mediated apoptosis in the isolated hippocampus. Significant increase in the CHOP protein expression (**P<0.01) at 10 Gy of dose gamma advocates profuse ER stress-mediated apoptosis. Also, significant (*P<0.05) increase of ERK½ protein expression at the dose range of 10 Gy dose of radiation are observed on the hippocampus (Figure 3 a, c) which further validate the involvement of ERK½ pathway, inducing ER-stress-mediated apoptosis in isolated hippocampus. Significant increase in the levels of CHOP and ERK½ suggests that IR induces hippocampal dysfunction by inducing ER-stress-mediated apoptosis, which might be further responsible for various IR-mediated hippocampal dysfunction.
4. Discussion
Understanding the molecular machinery responsible for radiation-induced cognitive dysfunction will provide insight into the molecular mechanism and neurobiology of stress-induced neurodegenerative disorders. Here we tried to understand how radiation onsets ER stress-induced dysfunction of the hippocampus of mice brain after whole body radiation. We observed a positive correlation with the upregulation of ER stress-specific genes and also the genes responsible for various physiological functions in the hippocampal regions. ER stress has been reported to disrupt neuronal functions and is responsible for various neurological disorders like Alzheimer disease, Parkinson disease, and Huntington disease (Siman, Flood, Thinakaran, Neumar, 2001). Radiation-induced ER stress has also been reported by us and others in various cell lines (Zhang et al., 2009).
In the present study, we found elevated expression of GRP78/BiP which has been reported earlier to have a dual role which activates under ER-stress condition as a self-defense mechanism in the cell, but under unresolved ER stress, this leads to cell death (Akutsu, Matsubara, Urashima, Komatsu, Sakata, Nishimori, et al, 2007). Another marker, CHOP, a downstream component of the ER stress pathway, is reported to get upregulated along with the induction of BiP signaling and lead the cell towards apoptotic pathway. CHOP overexpression has been linked with various neurodegenerative diseases and also targeted for development of therapeutic drugs against ER stress (Ohoka, Yoshii, Hattori, & Onozaki, Hayashi, 2005). In the present study, we observed significant upregulation with the increasing gamma radiation dose suggesting the 7 Gy gamma radiation exposure brings about significant induction of ER stress-mediated apoptosis in mice hippocampus.
Previous reports have suggested cells deficient in WFS1 are more susceptible to ER stress-mediated apoptosis. The present study we have observed a reduction of WFS1 gene with the increase of radiation dose in sync with an increase of BiP and CHOP, suggesting WFS1 plays an important role in mediating ER stress-mediated apoptosis. Also, the reduced level of WFS1 is linked with a genetic condition leading to Wolfram syndrome which causes severe depression, psychosis, or organic brain syndrome, as well as impulsive verbal and physical aggression (Takeda et al., 2001). In the current study, we observed a decrease in WFS1 gene expression with an increase of radiation dose, suggesting a link role of the WFS1 gene in inducing ER stress-mediated dysfunction in the hippocampus.
Previous reports have suggested the major role of CAM Nectin-3 in hippocampal-dependent learning and memory (Wang et al., 2013). Reduced level of nectin is associated with early life stress, disruption of synaptic contacts, and also hampered spatial memory. In our present study, we observed a dose-dependent decrease of Nectin-3 expression suggesting that 7- to 10-Gy gamma radiation have severe effect in destabilizing the hippocampal neurons, which might be disrupting hippocampal-dependent cognitive functions. Downregulation of Nectin-3 also suggests hampered episodic memories and loss of neurogenesis in the CA3 region of the hippocampus.
Sostdc1 gene, which is predominantly found in the CA2 region of the hippocampus, is also reported to play a major role in the WNT receptor signaling pathway (Inestrosa et al., 2005). We have observed significant downregulation of Sostdc1 gene on the exposure of gamma radiation, which suggests radiation causes significant changes in the WNT signaling pathway, and this might ultimately lead to various hippocampus-induced neurodegenerative diseases.
Heat shock protein is said to activate various kinase pathways that control proliferation and survival like ERK½ and Akt (Mebratu & Tesfaigzi, 2009). ERK½ activation is reported to promote ER stress-induced cell death in neuroblastoma cell line (Arai et al., 2004; Mukerjee, McGinnis, Park, Gnegy, & Wang, 2000). The suppression of ERK½ or Akt activation during stress condition increases heat sensitivity. On the contrary, overexpression of wild-type ERK½ protects cells from stress (Gabai et al., 2000). In the present study, we have observed significant upregulation of ERK½ protein expression post 10-Gy exposure of gamma radiation suggesting ERK pathway mediated cell death, which might be caused via phosphorylation of pro-apoptotic signal of DAPK as also reported earlier byMebratu & Tesfaigzi (2009) (Figure 4).
In conclusion, our present study suggests that a 7-Gy dose of whole-body gamma radiation in mice is sufficient to induce ER stress specific markers BiP and CHOP and also downregulates hippocampal genes WFS1, Sostdc1, and Nectrin3, which ultimately disrupt the hippocampal homeostasis. Significant increase of ERK½ also suggests cells innate response to overcome ER stress. As the hippocampus is involved in a wide array of physiological functions, radiation-induced damage in the hippocampus might lead to various neurodegenerative diseases such as AD, PD, etc. The present study may lead to the identification of ER stress and hippocampal genes as new markers to study radiation-induced neurodegenerative disorder induced by hippocampal dysfunction.
Ethical Considerations
Compliance with ethical guidelines
All experiments were conducted following the ethical guidelines by the Committee for the Purpose of Control and Supervision of Experiments on Animals, Government of India and cleared by the Institutional Animal Ethics Committee, Mangalore University, Karnataka, India. Supervision of animal protocols was carried out according to the research design.
Funding
This research was funded by a grant from the Department of Atomic Energy-Board Research in Nuclear Sciences (DAE-BRNS), Mumbai, Government of India, Sanction Number: 2013/34/5/BRNS/0444. Jit Chatterjee acknowledges the receipt of a fellowship from DAE-BRNS, Mumbai, India.
Authors' contributions
Study concept, design, student mentorship: Mohammed S Mustak; Acquisition of animal care and data generation: Jit Chatterjee; Animal dissection, RT PCR and western blot experiments: Jit Chatterjee &Jaldeep Langhnoja; Data analysis, interpretation of the findings: Mohammed S Mustak & Prakash Pillai; and Reviewing the manuscript and approving the final version for publication: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
We thank the Director of Centre for Application of Radiation and Radioisotope Technology (CARRT) and Microtome Centre Mangalore University for providing gamma radiation facility. We also thank DBT-MSUB-ILSPARE for the confocal microscopy facility and real-time PCR at Dr. Vikram Sarabhai Science Block, Faculty of Science, MSU, Vadodara.
References
- Abayomi, O. K. (1996). Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncologica, 35(6), 659-63. [DOI:10.3109/02841869609083995] [PMID]
- Akutsu, Matsubara, Urashima, Komatsu, Sakata, Nishimori, et al. (2007). Combination of direct intratumoral administration of dendritic cells and irradiation induces strong systemic antitumor effect mediated by GRP94/gp96 against squamous cell carcinoma in mice. International Journal of Oncology. 31:509-15.
- Arai, K., Lee, S. R., Van Leyen, K., Kurose, H., & Lo, E. H. (2004). Involvement of ERK MAP kinase in endoplasmic reticulum stress in SH‐SY5Y human neuroblastoma cells. Journal of Neurochemistry, 89(1), 232-9. [DOI:10.1111/j.1471-4159.2004.02317.x] [PMID]
- Boraks, G., Tampelini, F. S., Pereira, K. F., & Chopard, R. P. (2008). Effect of ionizing radiation on rat parotid gland. Brazilian Dental Journal, 19:73-6.
- Chen, Y., Dubé, C. M., Rice, C. J., & Baram, T. Z. (2008). Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. Journal of Neuroscience, 28(11), 2903-11. [DOI:10.1523/JNEUROSCI.0225-08.2008] [PMID] [PMCID]
- Conrad, C. D. (2006). What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus? Behavioral and Cognitive Neuroscience Reviews, 5(1), 41-60. [DOI:10.1177/1534582306289043] [PMID] [PMCID]
- Dudek S. M., Alexander, G. M., & Farris, S. (2016). Rediscovering area CA2: unique properties and functions. Nature Reviews Neuroscience, 17(2), 89-102. [DOI:10.1038/nrn.2015.22] [PMID] [PMCID]
- Gabai, V. L., Yaglom, J. A., Volloch, V., Meriin, A. B., Force, T., Koutroumanis, M., et al. (2000). Hsp72-mediated suppression of c-Jun N-terminal kinase is implicated in development of tolerance to caspase-independent cell death. Molecular and Cellular Biology, 20(18), 6826-36. [DOI:10.1128/MCB.20.18.6826-6836.2000] [PMID] [PMCID]
- Giovannini, M. G., Cerbaim, F., Bellucci, A., Melani, C., Grossi, C., Bartolozzi, C., et al. (2008). Differential activation of mitogen-activated protein kinase signalling pathways in the hippocampus of CRND8 transgenic mouse, a model of Alzheimer’s disease. Neuroscience, 153(3):618-33. [DOI:10.1016/j.neuroscience.2008.02.061] [PMID]
- Hamamura, K., Liu, Y., & Yokota, H. (2008). Microarray analysis of thapsigargin- induced stress to the endoplasmic reticulum of mouse osteoblasts. Journal of Bone and Mineral Metabolism, 26(3), 231-40. [DOI:10.1007/s00774-007-0825-1] [PMID]
- Hitti, F. L., & Siegelbaum, S. A. (2014). The hippocampal CA2 region is essential for social memory. Nature, 508(7494), 88-92. [DOI:10.1038/nature13028] [PMID] [PMCID]
- Honda, T., Sakisaka, T., Yamada, T., Kumazawa, N., Hoshino, T., Kajita, M., et al. (2006). Involvement of nectins in the formation of puncta adherentia junctions and the mossy fiber trajectory in the mouse hippocampus. Molecular and Cellular Neuroscience, 31(2), 315-25. [DOI:10.1016/j.mcn.2005.10.002] [PMID]
- Inestrosa, N. C., Godoy, J. A., Quintanilla, R. A., Koenig, C. S., & Bronfman, M. (2005). Peroxisome proliferator-activated receptor γ is expressed in hippocampal neurons and its activation prevents β-amyloid neurodegeneration: Role of Wnt signaling. Experimental Cell Research, 304(1), 91-104. [DOI:10.1016/j.yexcr.2004.09.032] [PMID]
- Inoue, H., Tanizawa, Y., Wasson, J., Behn, P., Kalidas, K., Bernal-Mizrachi, E., et al. (1998). A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome). Nature Genetics, 20(2), 143-8. [DOI:10.1038/2441] [PMID]
- Jagetia, G. C., Baliga, M. S., Venkatesh, P., & Ulloor, J. N. (2003). Influence of ginger rhizome (Zingiber officinale rosc) on survival, glutathione and lipid peroxidation in mice after whole-body exposure to gamma radiation. Radiation Research, 160(5), 584-92. [DOI:10.1667/RR3057] [PMID]
- Jensen, O., & Lisman, J. E. (1996). Hippocampal CA3 region predicts memory sequences: accounting for the phase precession of place cells. Learning & Memory, 3(2-3), 279-87. [DOI:10.1101/lm.3.2-3.279]
- Kakiuchi, C., Ishiwata, M., Hayashi, A., & Kato, T. (2006). XBP1 induces WFS1 through an endoplasmic reticulum stress response element‐like motif in SH‐SY5Y cells. Journal of Neurochemistry, 97(2), 545-55. [DOI:10.1111/j.1471-4159.2006.03772.x] [PMID]
- Kim, J. S., Lee, H. J., Kim, J. C., Kang, S. S., Bae, C. S., Shin, T., et al. (2008). Transient impairment of hippocampus-dependent learning and memory in relatively low-dose of acute radiation syndrome is associated with inhibition of hippocampal neurogenesis. Journal of Radiation Research, 49(5), 517-26. [DOI:10.1269/jrr.08020] [PMID]
- Lein, P. J., Beck, H. N., Chandrasekaran, V., Gallagher, P. J., Chen, H. L., Lin, Y., et al. (2002). Glia induce dendritic growth in cultured sympathetic neurons by modulating the balance between Bone Morphogenetic Proteins (BMPs) and BMP antagonists. Journal of Neuroscience, 22(23), 10377-87. [DOI:10.1523/JNEUROSCI.22-23-10377.2002] [PMID]
- Lupien, S. J., McEwen, B. S., Gunnar, M. R., & Heim, C. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10(6), 434-45. [DOI:10.1038/nrn2639] [PMID]
- Mebratu, Y., & Tesfaigzi, Y. (2009). How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell cycle, 8(8), 1168-75. [DOI:10.4161/cc.8.8.8147] [PMID] [PMCID]
- Merchant, T. E., Pollack, I. F., & Loeffler, J. S. (2010). Brain tumors across the age spectrum: biology, therapy, and late effects. Seminars in Radiation Oncology, 20(1), 58-66. [DOI:10.1016/j.semradonc.2009.09.005] [PMID] [PMCID]
- Meyer, M. A. (2014). Highly expressed genes within hippocampal sector CA1: implications for the physiology of memory. Neurology international, 6(2), 32-5. [DOI:10.4081/ni.2014.5388]
- Mizoguchi, A., Nakanishi, H., Kimura, K., Matsubara, K., Ozaki-Kuroda, K., Katata, T., et al. (2002). Nectin: An adhesion molecule involved in formation of synapses. The Journal of Cell Biology, 156(3), 555-65. [DOI:10.1083/jcb.200103113] [PMID] [PMCID]
- Mizumatsu, S., Monje, M. L., Morhardt, D. R., Rola, R., Palmer, T. D., & Fike, J. R. (2003). Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Research, 63(14), 4021-7. [PMID]
- Mukerjee, N., McGinnis, K. M., Park, Y. H., Gnegy, M. E., & Wang, K. K. (2000). Caspase-mediated proteolytic activation of calcineurin in thapsigargin-mediated apoptosis in SH-SY5Y neuroblastoma cells. Archives of Biochemistry and Biophysics, 379(2), 337-43. [DOI:10.1006/abbi.2000.1889] [PMID]
- Nairy, R. K., Bhat, N. N., Sanjeev, G., & Yerol, N. (2017). Dose-response study using micronucleus cytome assay: A tool for biodosimetry application. Radiation Protection Dosimetry, 174(1), 79-87. [DOI:10.1093/rpd/ncw045] [PMID]
- Park, J. W., Park, E. S., Choi, E. N., Park, H. Y., & Jung, S. C. (2009). Altered brain gene expression profiles associated with the pathogenesis of phenylketonuria in a mouse model. Clinica Chimica Acta, 401(1-2), 90-9. [DOI:10.1016/j.cca.2008.11.019] [PMID]
- Raber, J., Rola, R., LeFevour, A., Morhardt, D., Curley, J., Mizumatsu, S., et al. (2004). Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiation Research, 162(1), 39-47. [DOI:10.1667/RR3206] [PMID]
- Riggs, A. C., Bernal-Mizrachi, E., Ohsugi, M., Wasson, J., Fatrai, S., Welling, C., et al. (2005). Mice conditionally lacking the Wolfram gene in pancreatic islet beta cells exhibit diabetes as a result of enhanced endoplasmic reticulum stress and apoptosis. Diabetologia, 48(11), 2313-21. [DOI:10.1007/s00125-005-1947-4] [PMID]
- Shapiro, L., Love, J., & Colman, D. R. (2007). Adhesion molecules in the nervous system: structural insights into function and diversity. Annual Review of Neuroscience, 30, 451-74. [DOI:10.1146/annurev.neuro.29.051605.113034] [PMID]
- Strom, T. M., Hörtnagel, K., Hofmann, S., Gekeler, F., Scharfe, C., Rabl, W., et al. (1998). Diabetes Insipidus, Diabetes Mellitus, Optic Atrophy and Deafness (DIDMOAD) caused by mutations in a novel gene (Wolframin) coding for a predicted transmembrane protein. Human Molecular Genetics, 7(13), 2021-8. [DOI:10.1093/hmg/7.13.2021] [PMID]
- Suzuki, K., Hu, D., Bustos, T., Zlotogora, J., Richieri-Costa, A., Helms, J. A., & Spritz, R. A. (2000). Mutations of PVRL1, encoding a cell-cell adhesion molecule/herpesvirus receptor, in cleft lip/palate-ectodermal dysplasia. Nature Genetics, 25(4), 427-30. [DOI:10.1038/78119] [PMID]
- Takeda, K., Inoue, H., Tanizawa, Y., Matsuzaki, Y., Oba, J., Watanabe, Y., et al. (2001). WFS1 (Wolfram syndrome 1) gene product: Predominant subcellular localization to endoplasmic reticulum in cultured cells and neuronal expression in rat brain. Human Molecular Genetics, 10(5), 477-84. [DOI:10.1093/hmg/10.5.477] [PMID]
- Tang, D., Wu, D., Hirao, A., Lahti, J. M., Liu, L., Mazza, B., et al. (2002). ERK activation mediates cell cycle arrest and apoptosis after DNA damage independently of p53. Journal of Biological Chemistry, 277(15), 12710-7. [DOI:10.1074/jbc.M111598200] [PMID]
- Thompson, C. L., Pathak, S. D., Jeromin, A., Ng, L. L., MacPherson, C. R., Mortrud, M. T., et al. (2008). Genomic anatomy of the hippocampus. Neuron, 60(6), 1010-21. [DOI:10.1016/j.neuron.2008.12.008] [PMID]
- Taniguchi, M., Yoshida, H, (2015) Endoplasmic reticulum stress in kidney function and disease. Curr Opin Nephrol Hypertens 24:345-50. [DOI: 10.1097/MNH.0000000000000141]
- Wang, X. D., Su, Y. A., Wagner, K. V., Avrabos, C., Scharf, S. H., Hartmann, J., et al. (2013). Nectin-3 links CRHR1 signaling to stress-induced memory deficits and spine loss. Nature Neuroscience, 16(6), 706-13. [DOI:10.1038/nn.3395] [PMID]
- Yamada, T., Ishihara, H., Tamura, A., Takahashi, R., Yamaguchi, S., Takei, D., et al. (2006). WFS1-deficiency increases endoplasmic reticulum stress, impairs cell cycle progression and triggers the apoptotic pathway specifically in pancreatic β-cells. Human Molecular Genetics, 15(10), 1600-9. [DOI:10.1093/hmg/ddl081] [PMID]
- Oakes, SA., Papa, FR. (2015) The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol Mech Dis 10:173–194. [DOI:10.1146/annurev-pathol-012513-104649]
- Van Praag, H. (2005) Exercise enhances learning and hippocampal neurogenesis in aged mice. Journal of Neuroscience, 25:8680-85. [DOI:10.1523/jneurosci.1731-05.2005]
- Marciniak, S. J. et al (2004) CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev, 18:3066-77. [DOI:10.1101/gad.1250704
- Siman, R., Flood, D. G., Thinakaran, G., Neumar, R. W. (2001). Endoplasmic reticulum stress-induced cysteine protease activation in cortical neurons: Effect of an alzheimer’s disease-linked presenilin-1 knock-in mutation. Journal of Biological Chemistry, 276:44736-43. [DOI: 10.1074/jbc.M104092200]
- Zhang, B. et al. (2010). ER stress induced by ionising radiation in IEC-6 cells. International Journal of Radiation Biology, 86:429-35. [DOI:10.3109/09553001003668014]
- Ohoka, Yoshii, Hattori, Onozaki, Hayashi. (2005). TRB3, a novel ER stress-inducible gene, is induced via ATF4– CHOP pathway and is involved in cell death. The EMBO Journal, 24:1243-55. [DOI:10.1038/sj.emboj.7600596]
Type of Study: Original |
Subject:
Behavioral Neuroscience
Received: 2018/04/23 | Accepted: 2018/12/25 | Published: 2019/07/1
Received: 2018/04/23 | Accepted: 2018/12/25 | Published: 2019/07/1
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |